Nucleic acid film tape and kit for diagnosing alpha mediterranean anemia
A technology for thalassemia and nucleic acid membrane strips is applied in the field of diagnostic reagents for thalassemia, which can solve the problems of missed diagnosis, inability to detect alpha gene mutation, EB pollution, etc., and achieve the effect of reducing economic burden, family burden and social cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0100] Embodiment 2 is used for the design of the primer of DNA in the sample to be amplified
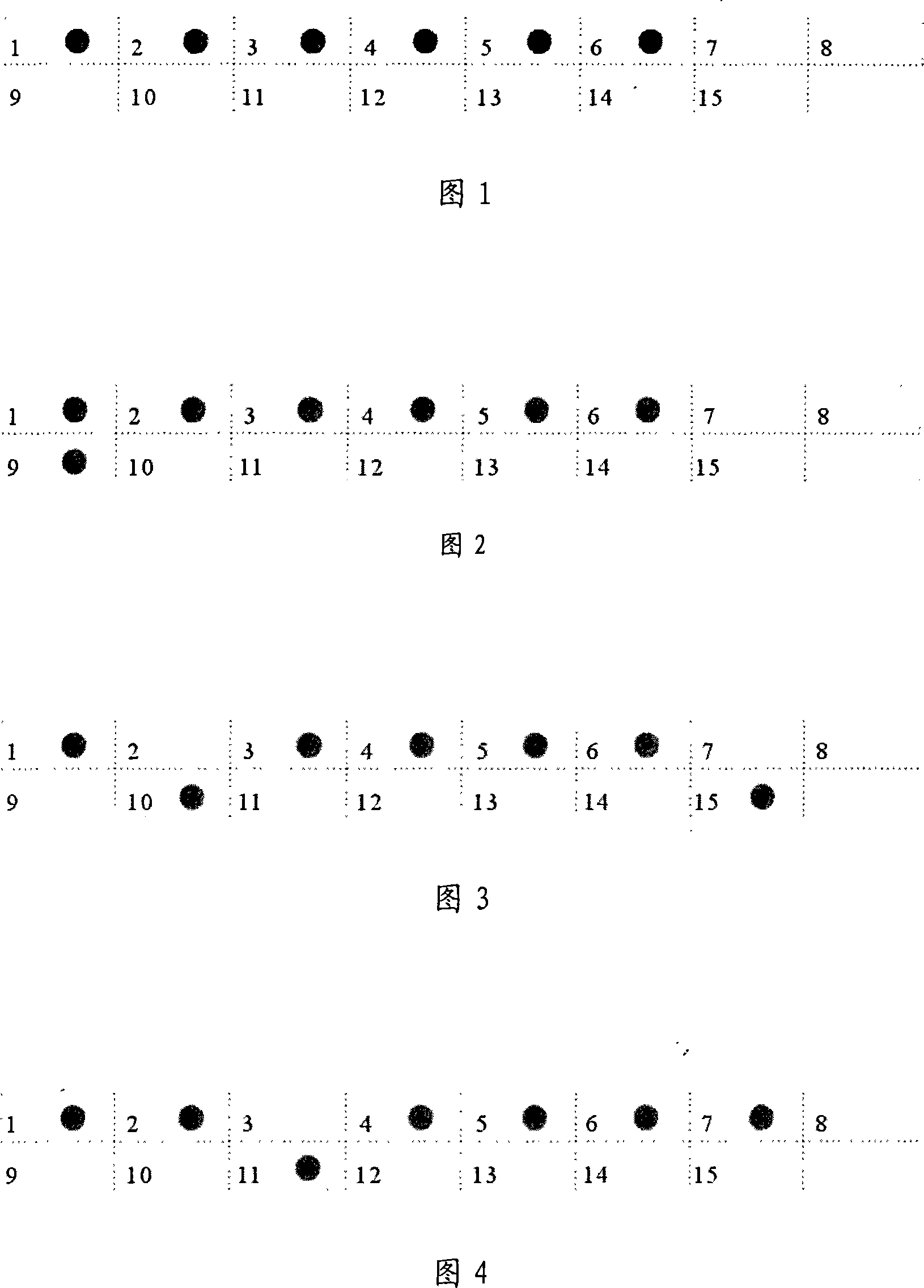
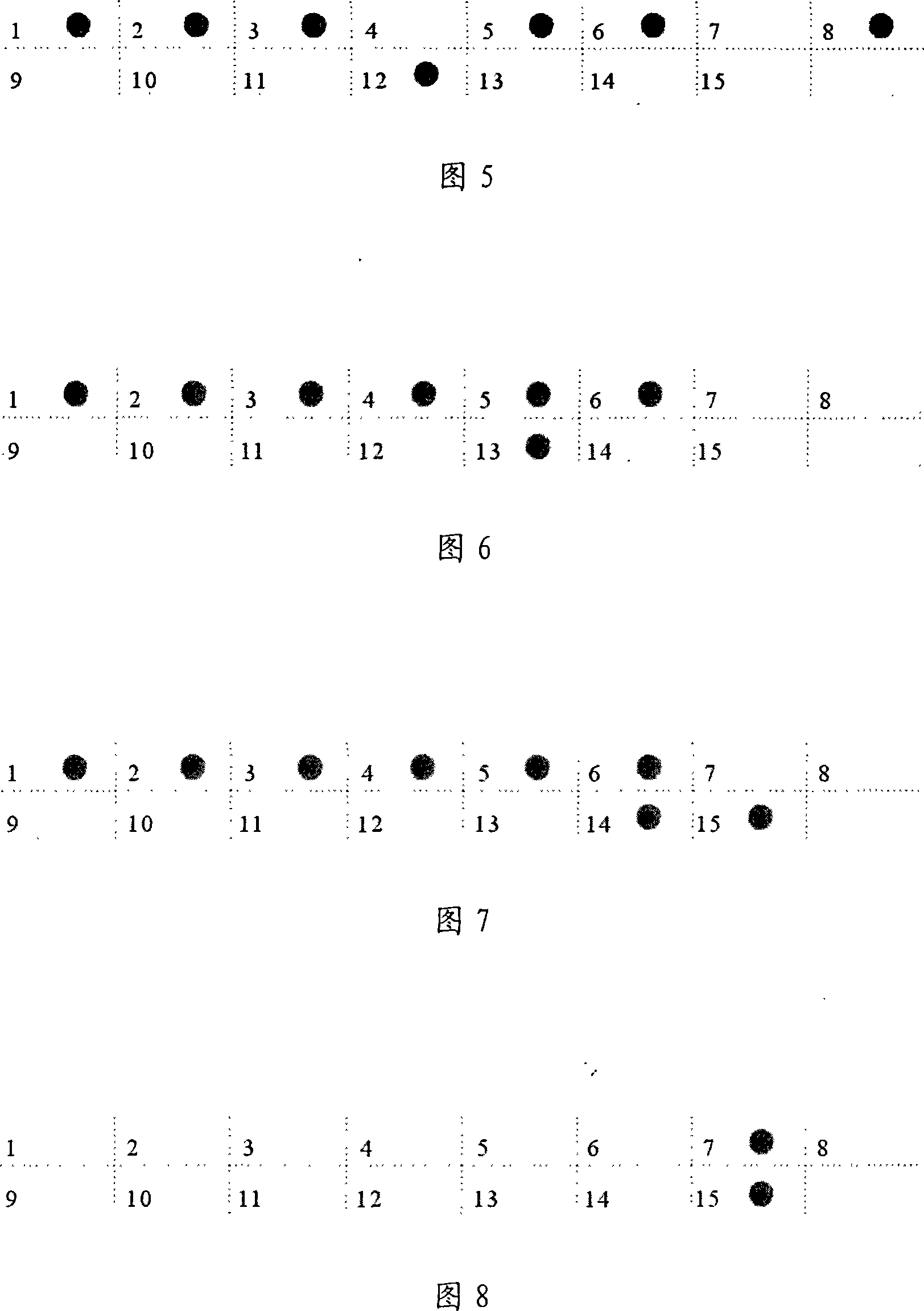
[0101] According to the α-thalassemia gene sequence data, 4 pairs (a total of 7, one shared by two pairs) were used to amplify α - a globin gene fragment. The nucleotide sequences of these seven primers are shown in SEQ ID NO.16-22. The amplified product of SEQ ID NO.16 and SEQ ID NO.22 can be used with-- SEA Probe hybridization, used to detect the presence of -- SEA Deletion; the amplification products of SEQ ID NO.17 and SEQ ID NO.20 can be combined with -α 4.2 Probe hybridization, used to detect the presence of -α in the sample 4.2 Deletion; the amplification products of SEQ ID NO.18 and SEQ ID NO.21 can be combined with -α 3.7 Probe hybridization, used to detect the presence of -α in the sample 3.7 Deletion; the amplified products of SEQ ID NO.18 and SEQ ID NO.19 can be hybridized with the αα probe to detect whether the two chromosome groups of the sample are missing (if t...
Embodiment 3
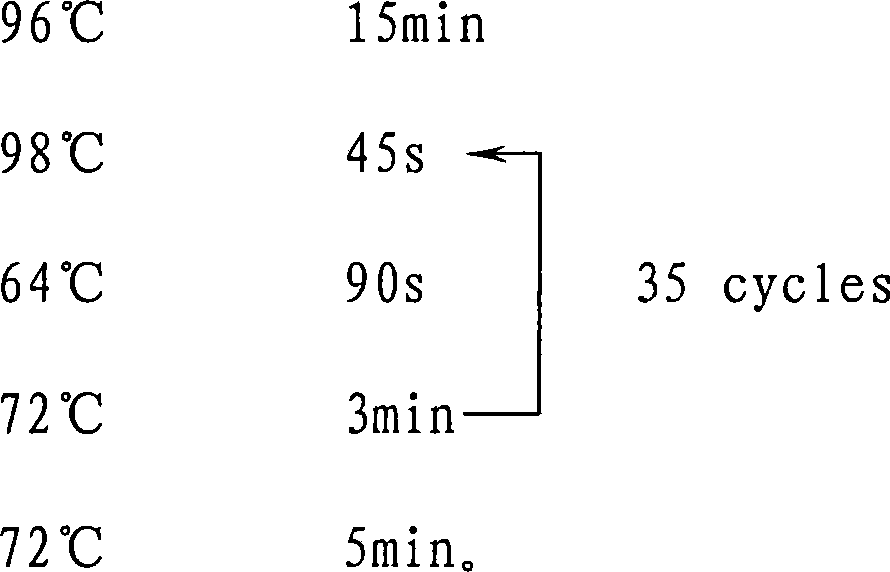
[0109] The detection of embodiment 3 to-be-checked sample DNA
[0110] 1. Experimental instruments and reagents
[0111] 1. Test equipment
[0112] PCR instrument, DNA electrophoresis instrument, molecular hybridization box, shaker
[0113] 2. Test reagents
[0114] (1) 3% H2O2
[0115] (2) 20×SSC (pH7.0): Take 175.3g of NaCl and 88.2g of sodium citrate, add H2O to dissolve to 1000mL, adjust the pH to 7.0 with a pH meter, and autoclave.
[0116] (3) 10% SDS (pH7.0): Dissolve 20g of SDS in 180mL of H2O, adjust the pH to 7.0 with HCl, and finally set the volume to 200mL.
[0117] (4) Liquid A (2×SSC, 0.1% SDS, pH7.4): Take 100mL of 20×SSC, 10mL of 10% SDS,
[0118] Add H2O to dissolve to 1000mL, and adjust the pH to 7.4.
[0119] (5) Solution B (0.5×SSC, 0.1% SDS, pH7.4): Take 25mL of 20×SSC, 10% SDS 10mL, add H2O to dissolve to 1000mL, and adjust the pH to 7.4.
[0120] (6) Solution C (0.1M sodium citrate, pH5.4): Dissolve 14.7g of sodium citrate in 450mL of water, adjus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com