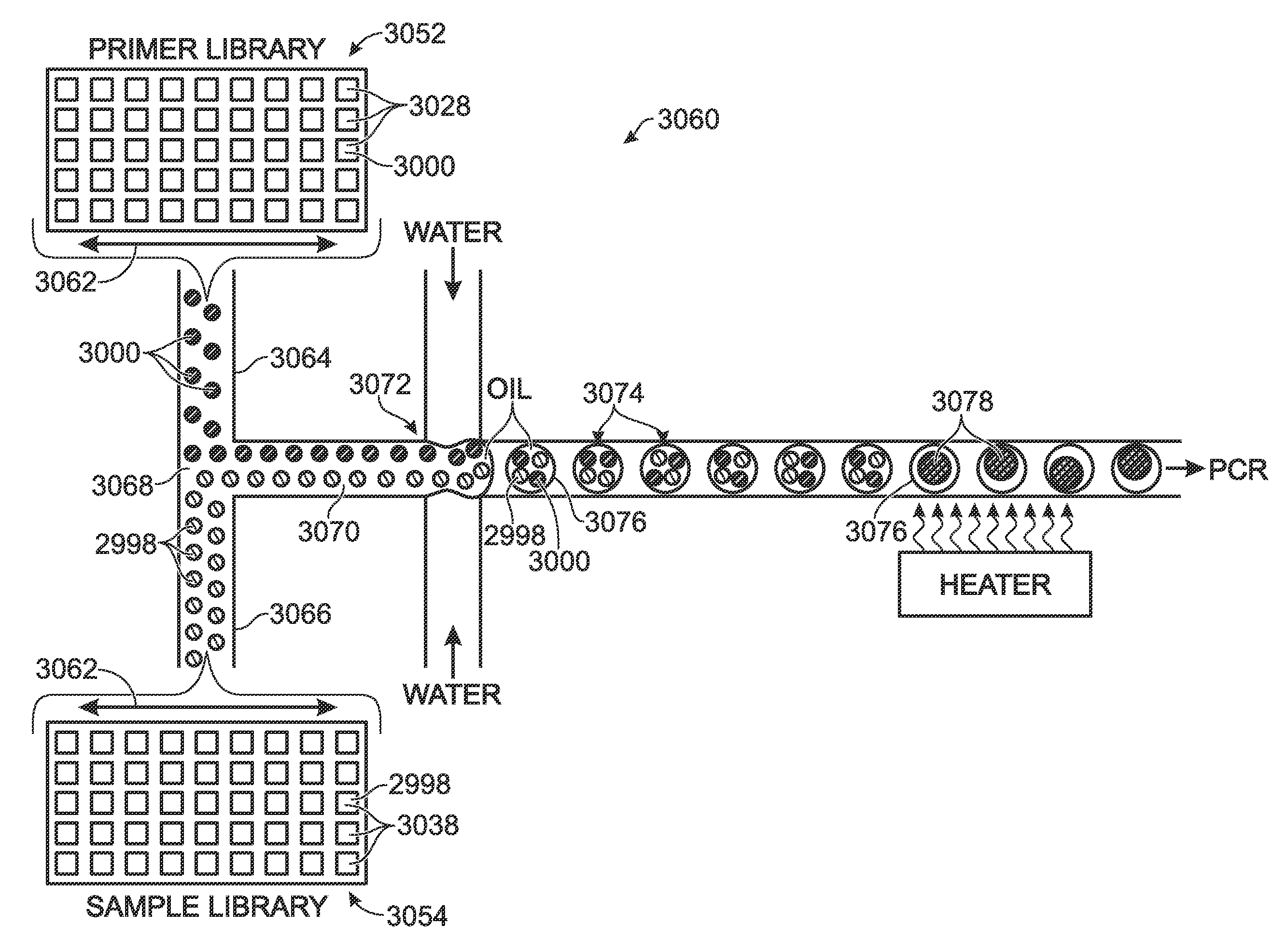
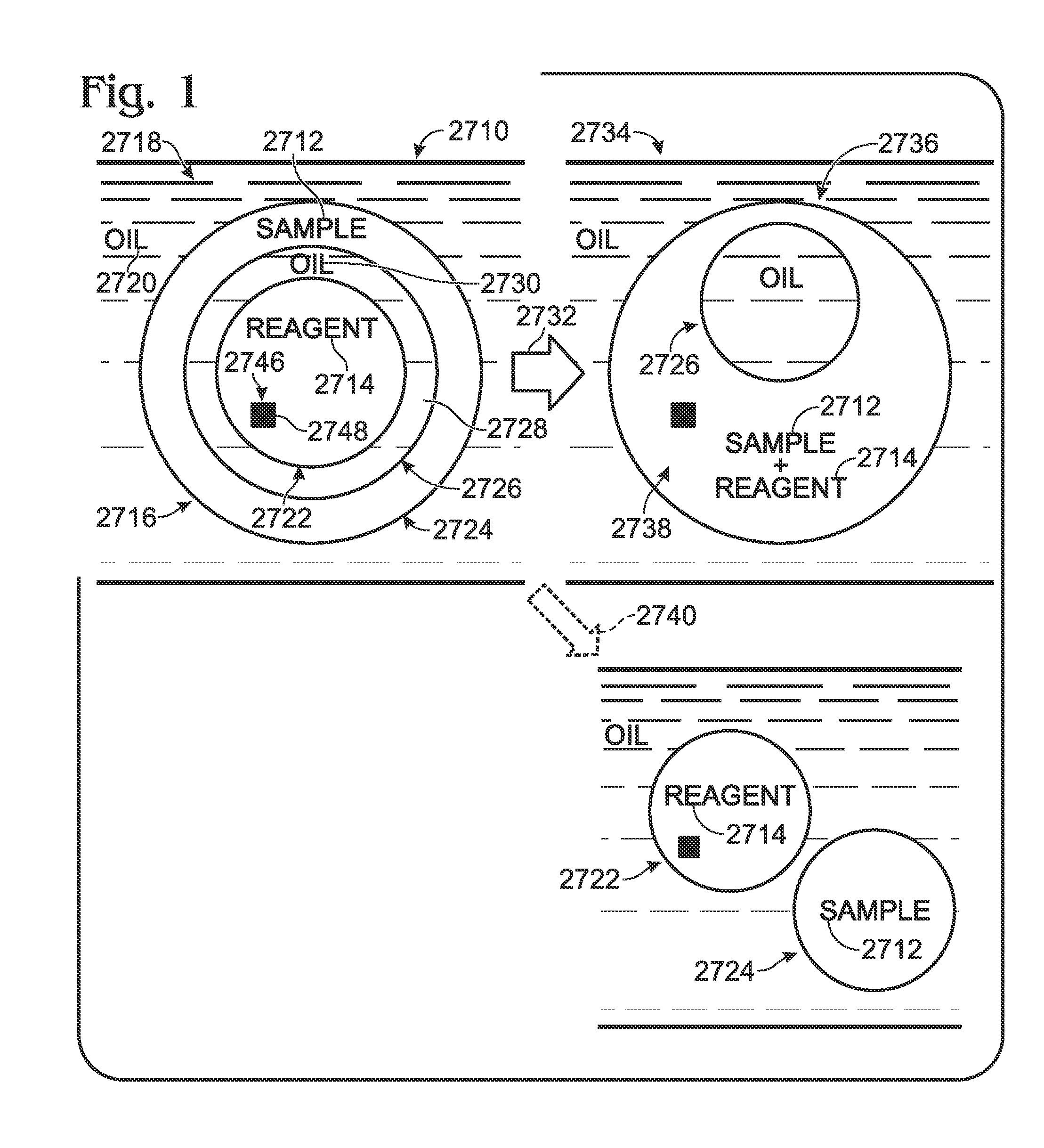
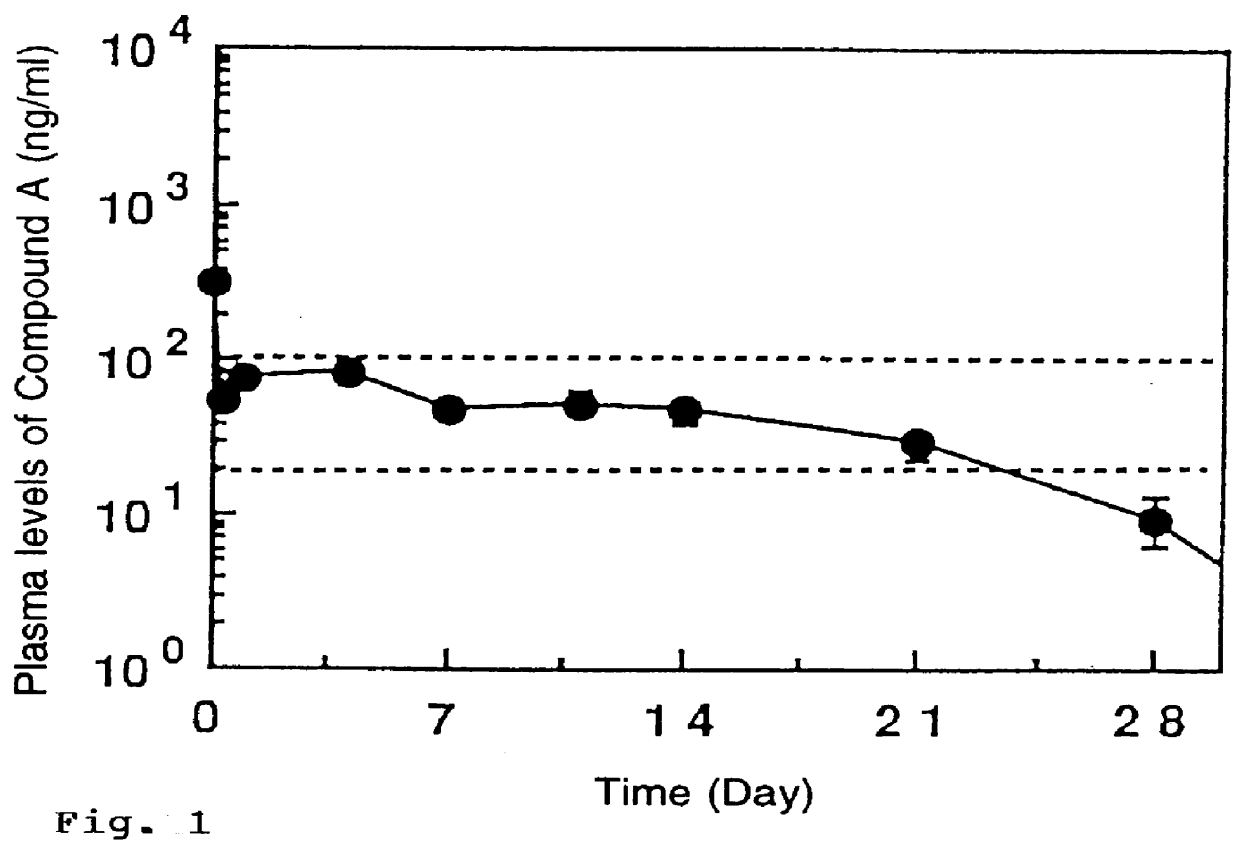
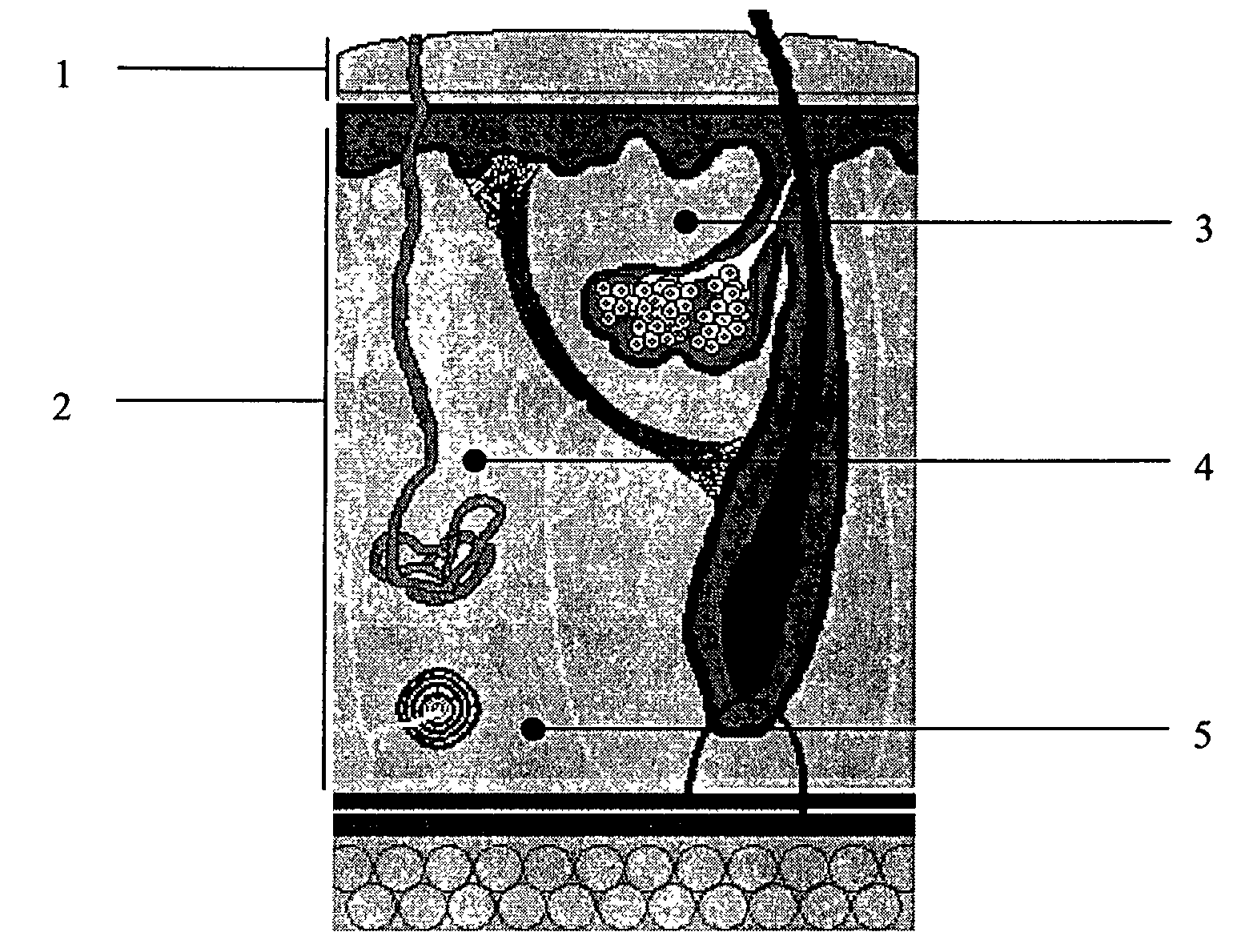
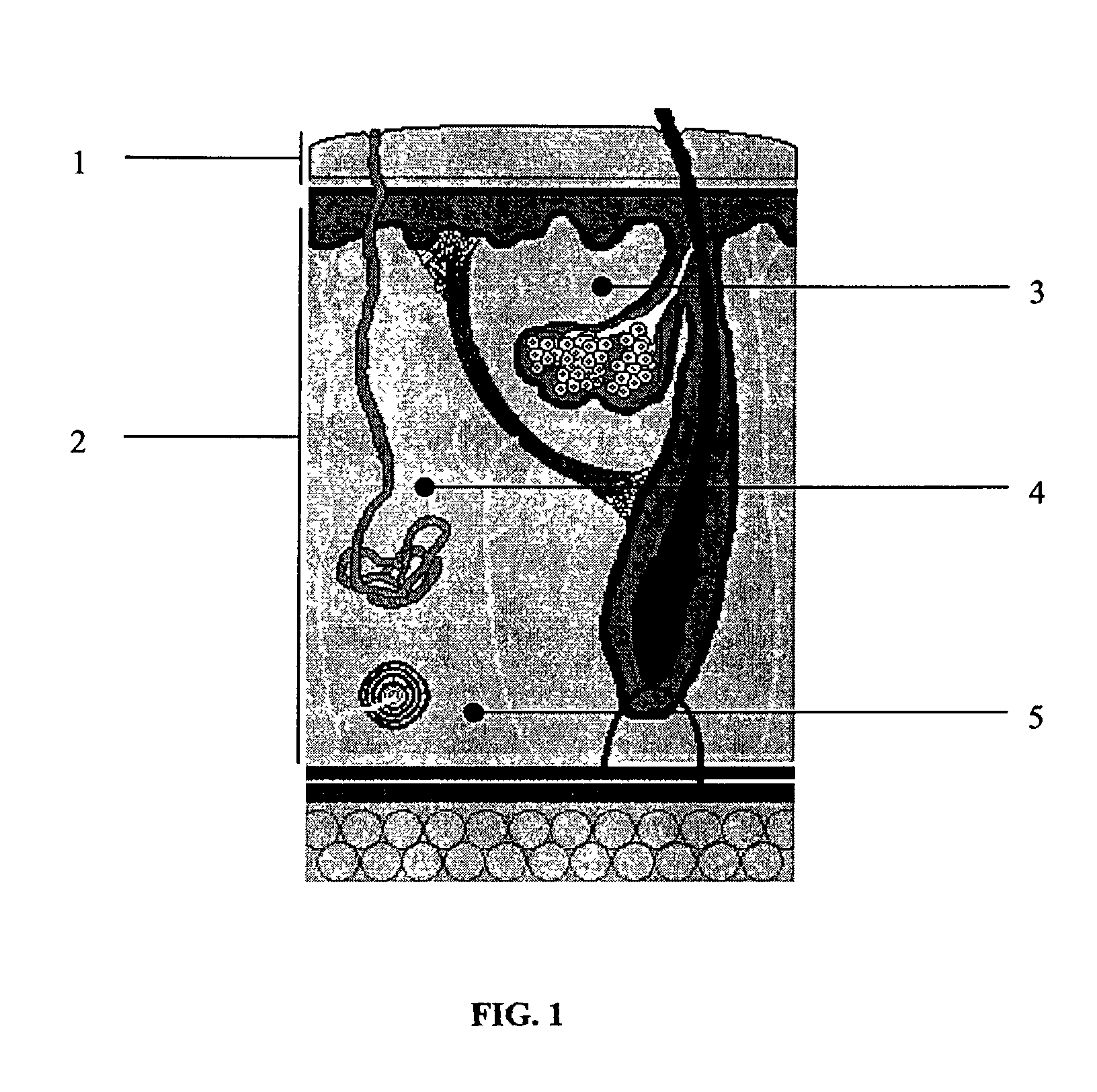
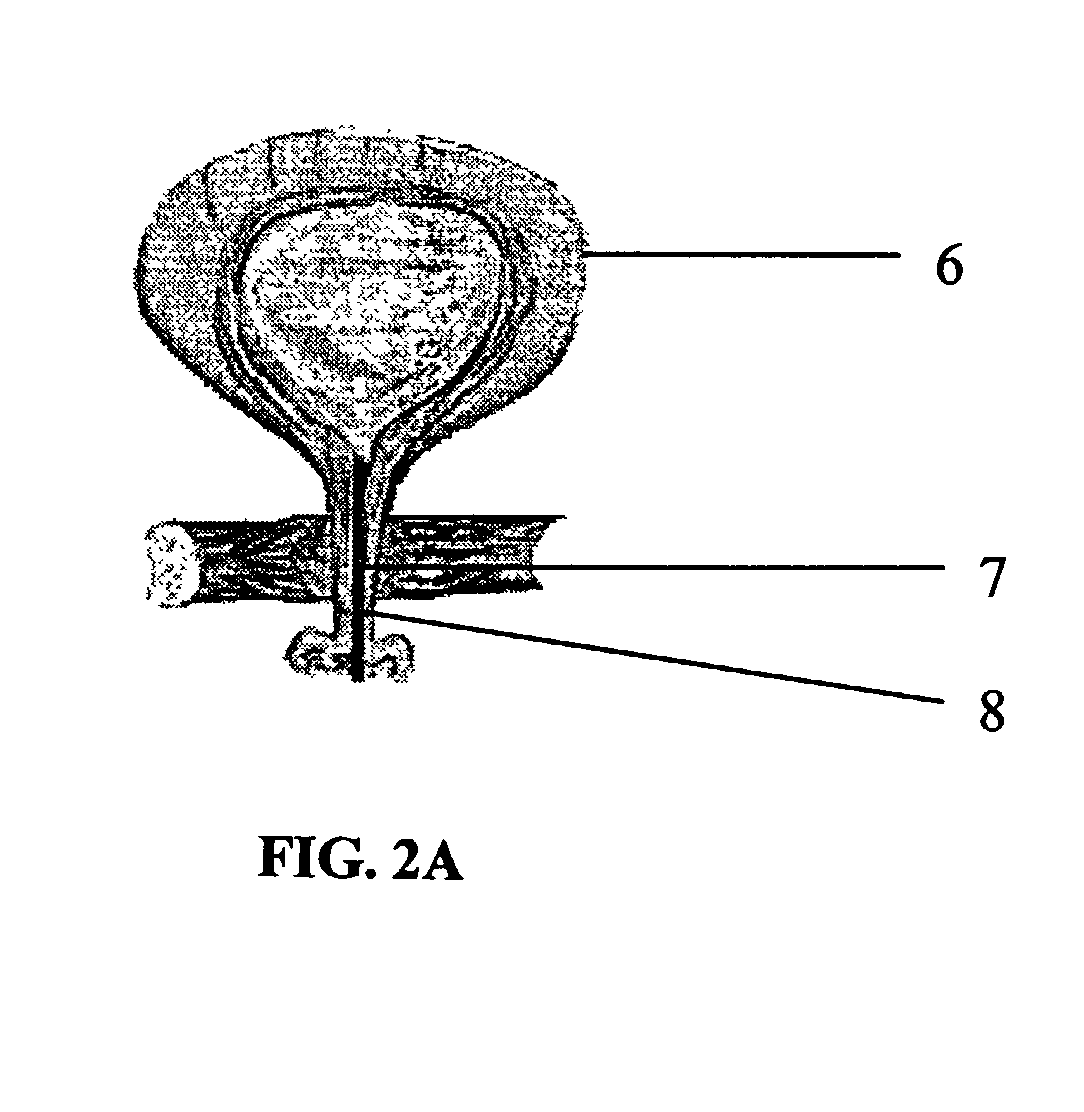
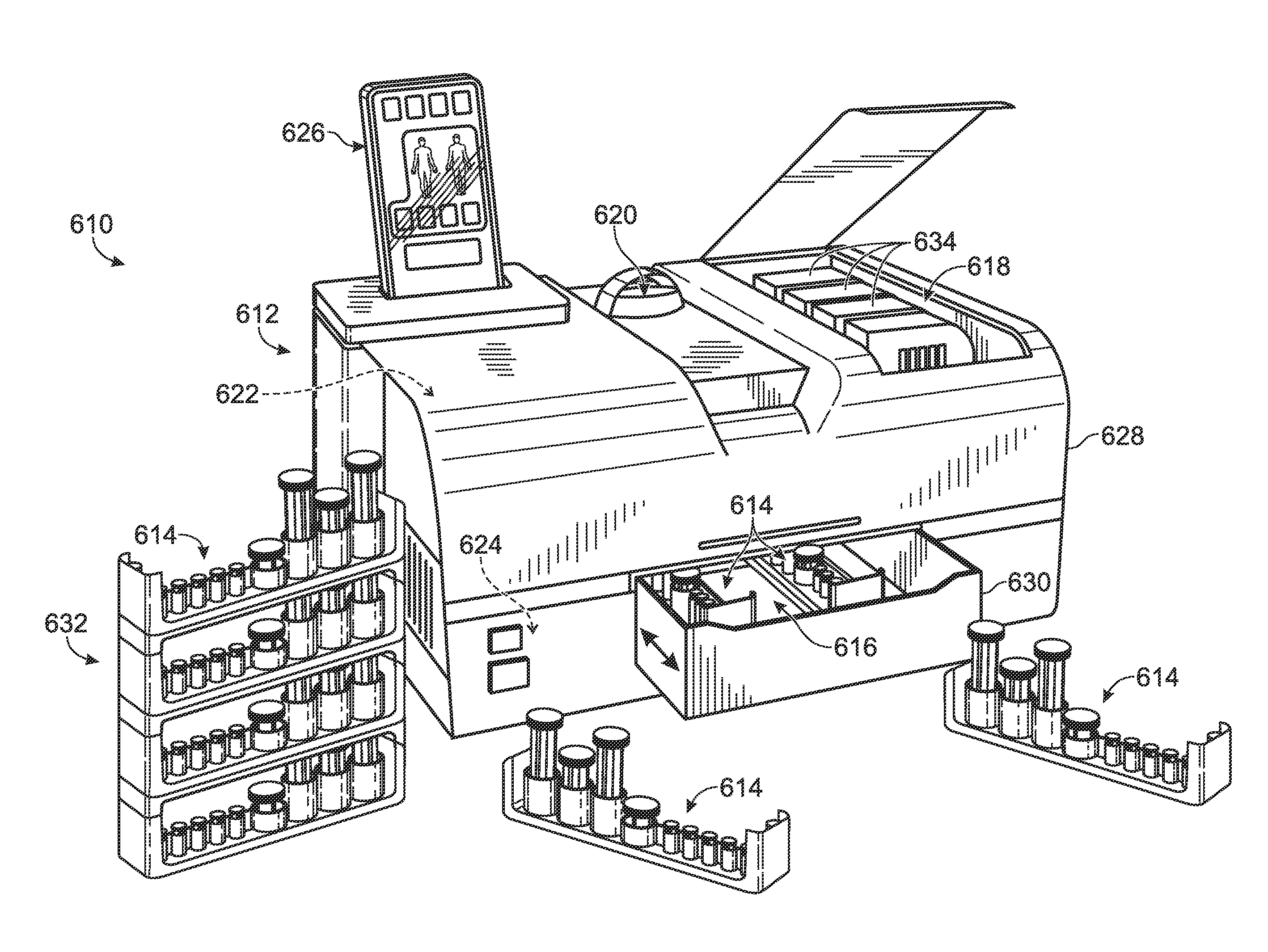
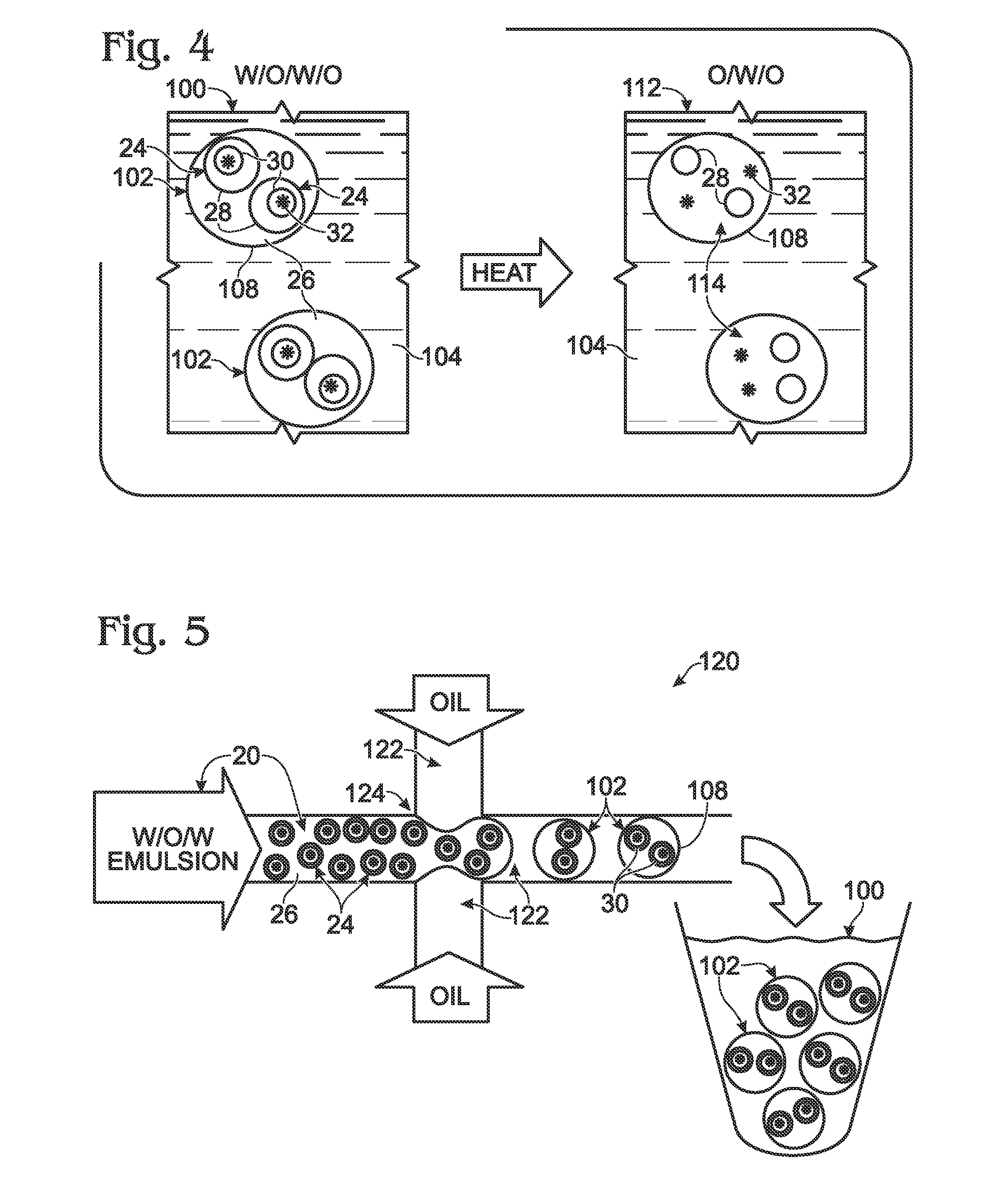
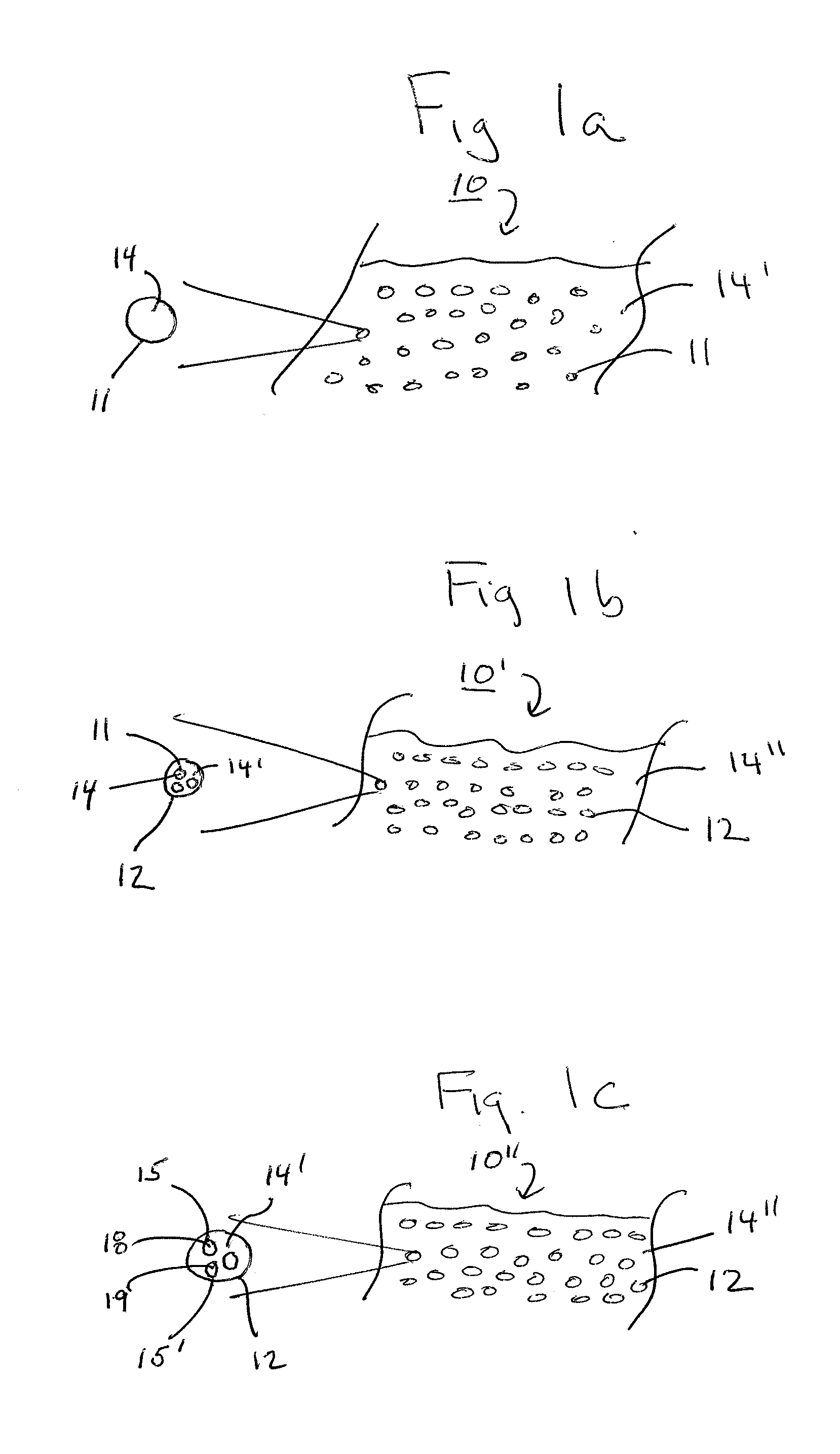
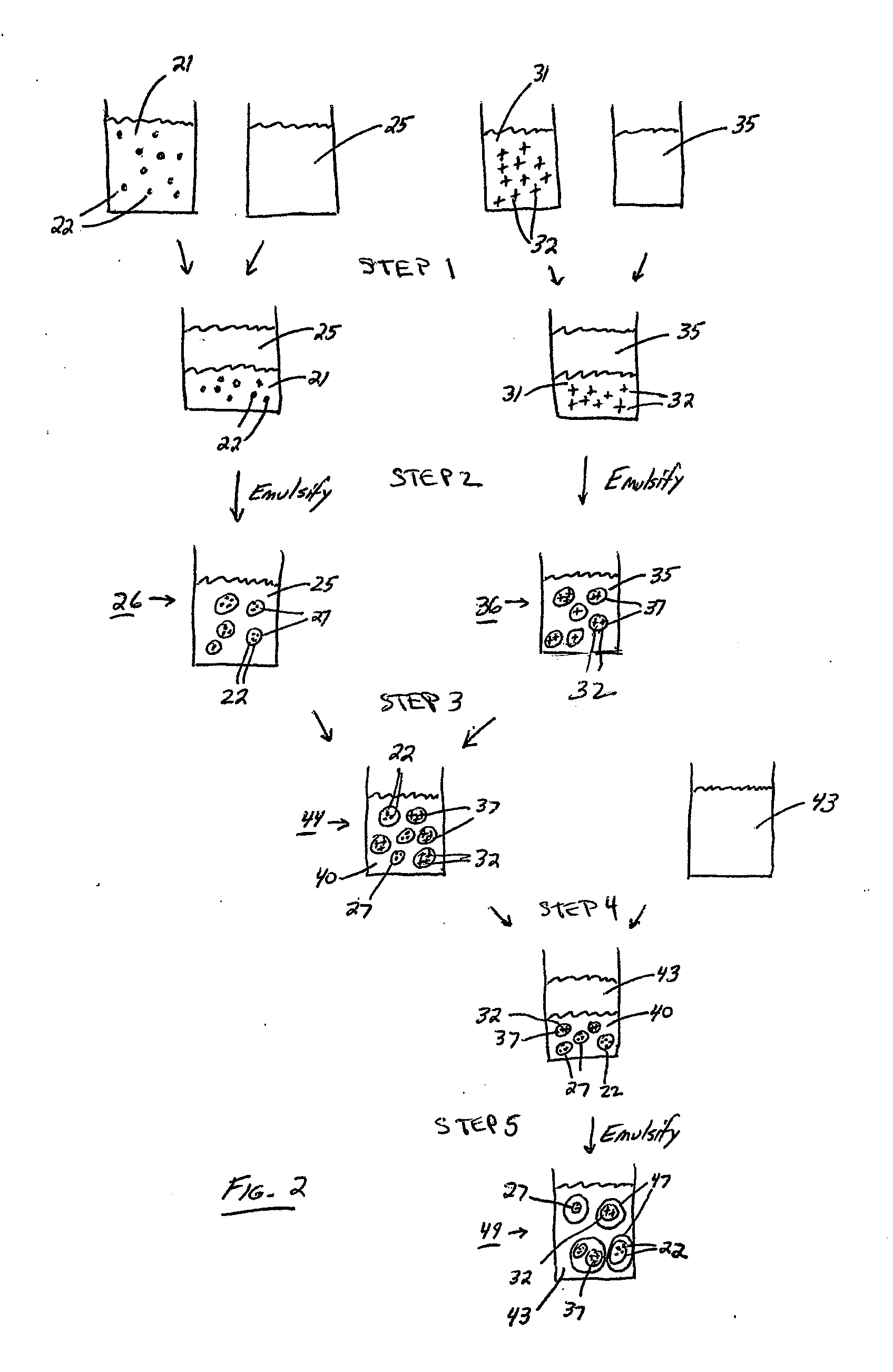
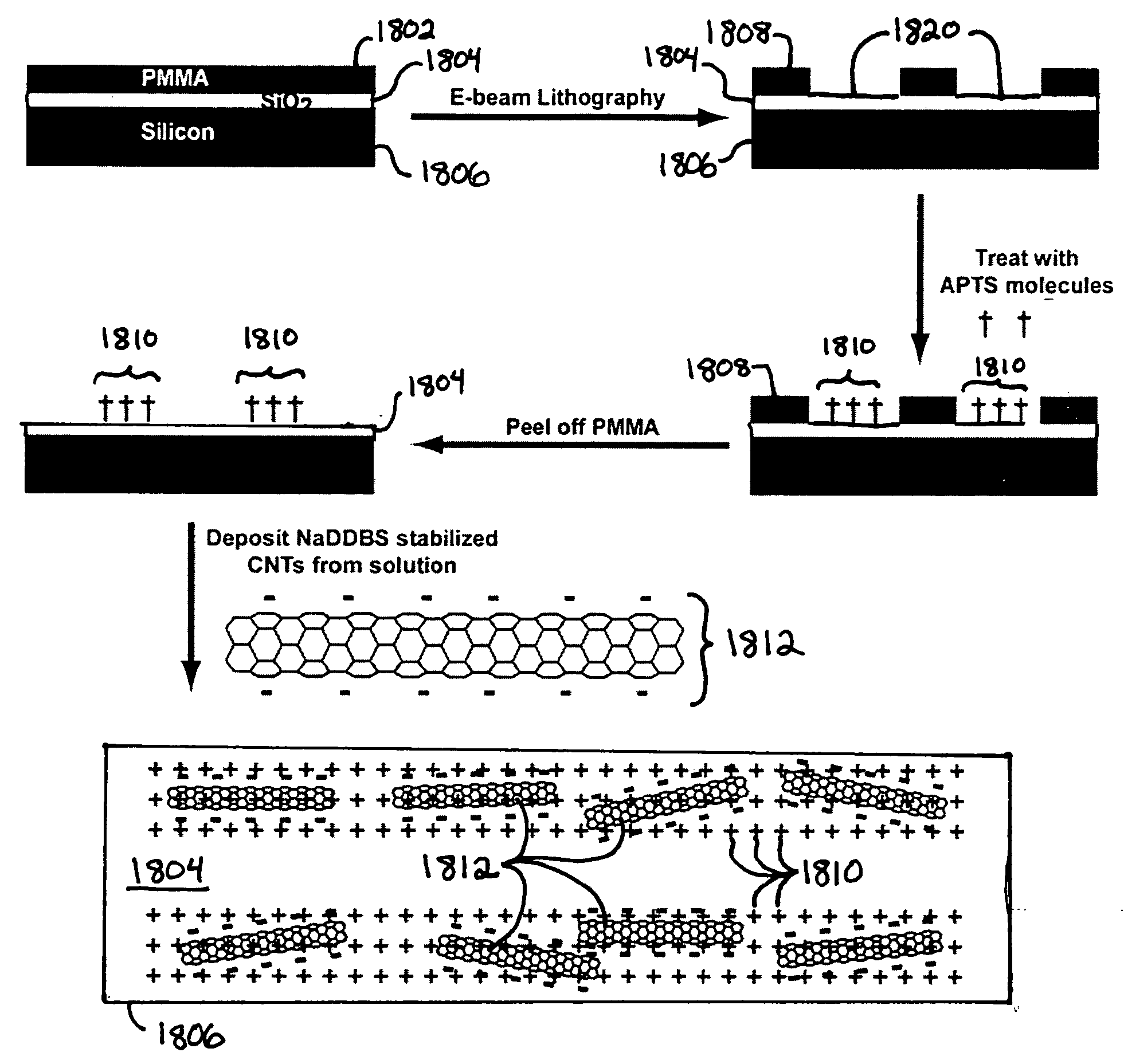
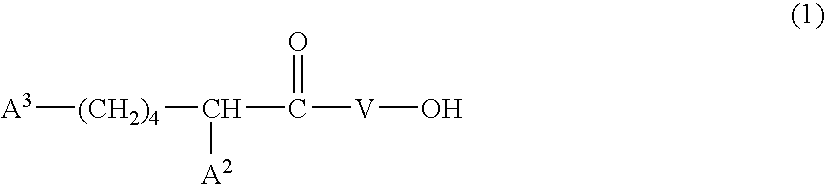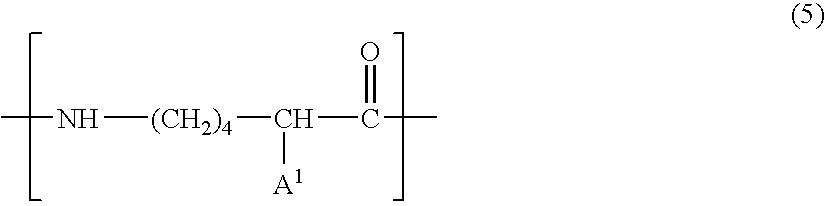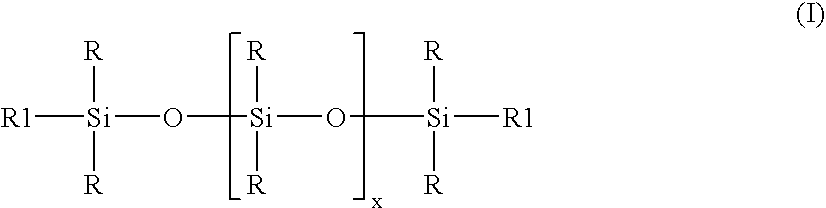Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59107 results about "Emulsion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable). Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase) is dispersed in the other (the continuous phase). Examples of emulsions include vinaigrettes, homogenized milk, and some cutting fluids for metal working.
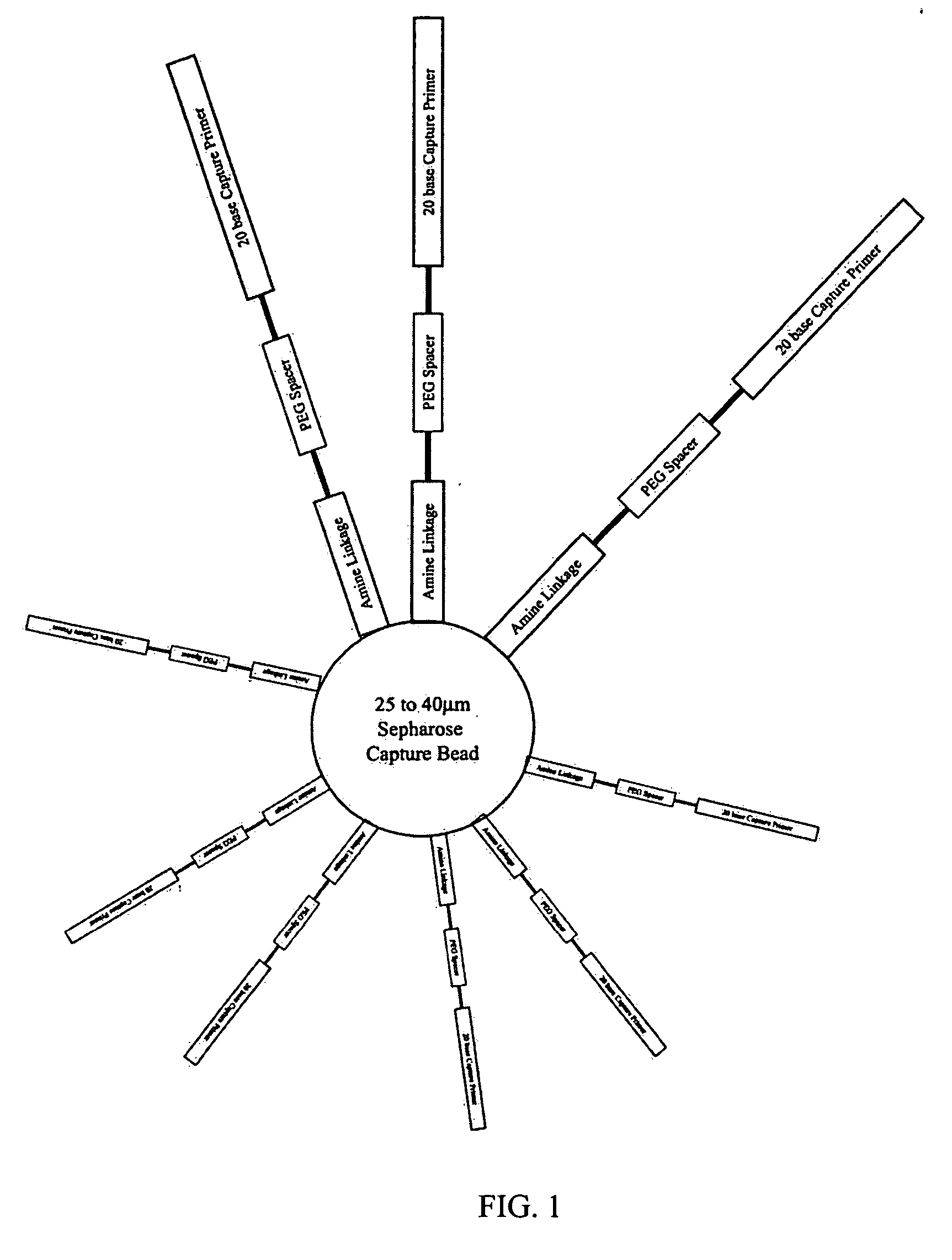
Bead emulsion nucleic acid amplification
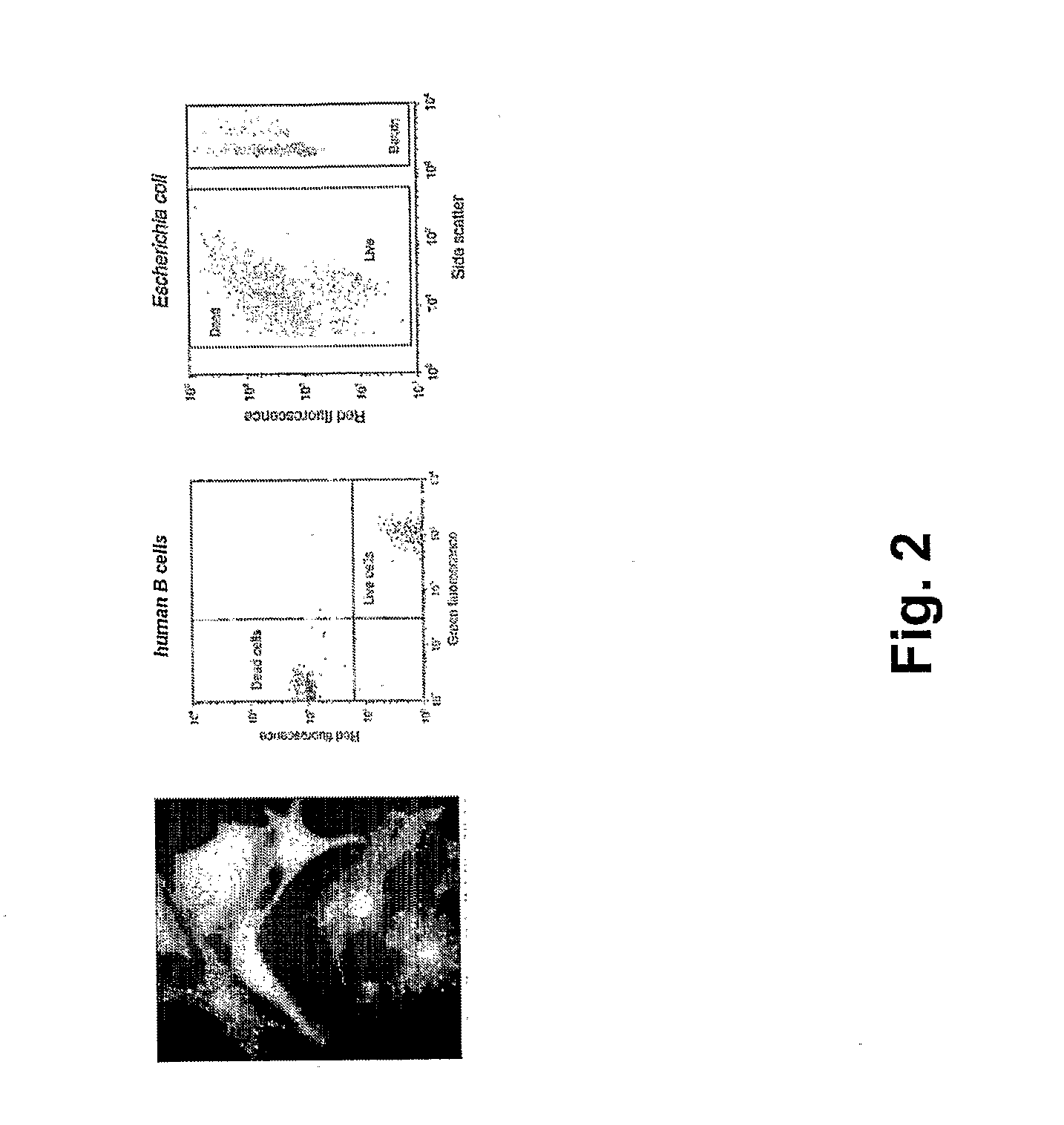
Disclosed are methods for nucleic acid amplification wherein nucleic acid templates, beads, and amplification reaction solution are emulsified and the nucleic acid templates are amplified to provide clonal copies of the nucleic acid templates attached to the beads. Also disclosed are kits and apparatuses for performing the methods of the invention.
Owner:454 LIFE SCIENCES CORP
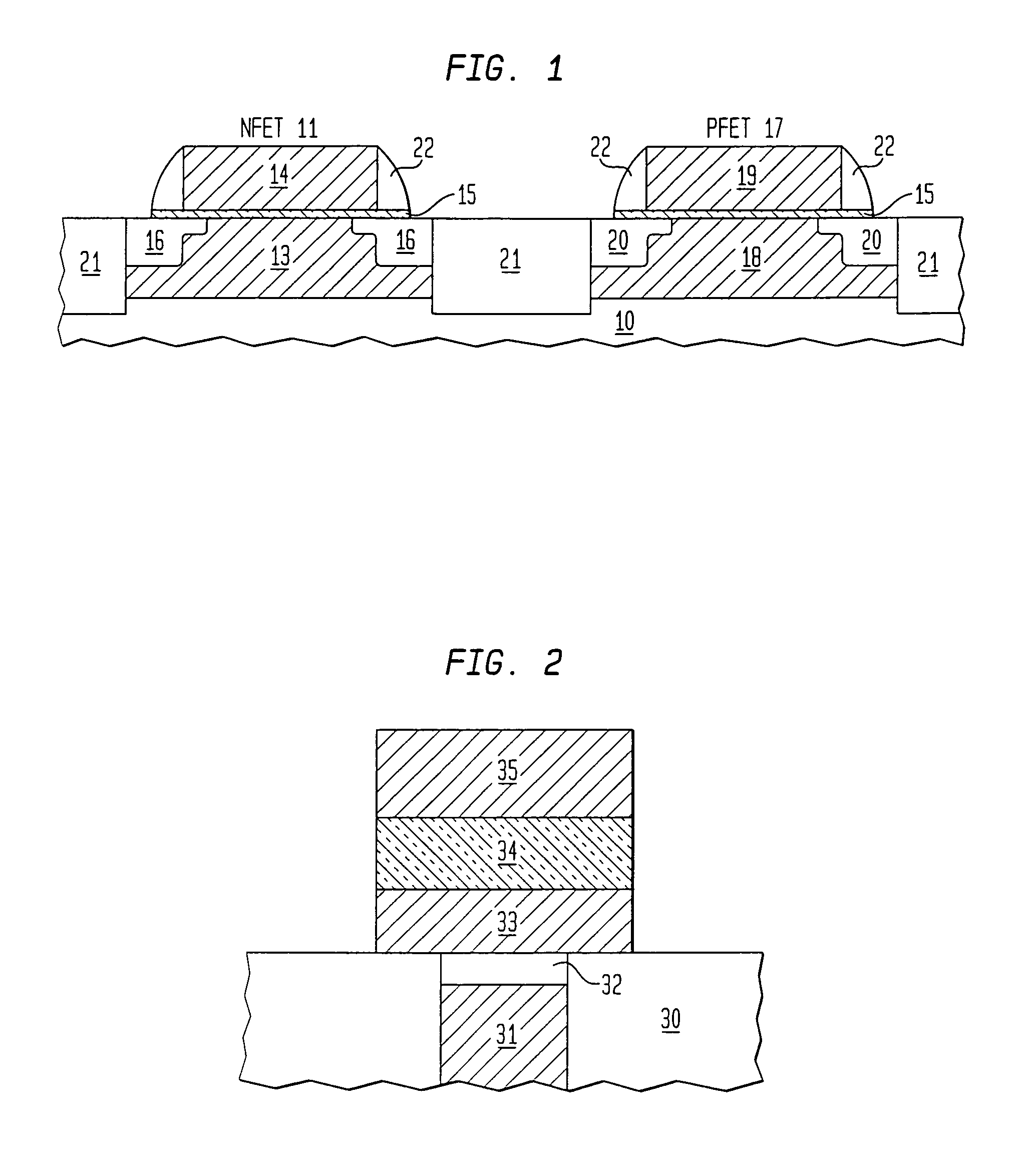
Precursor source mixtures
A precursor source mixture useful for CVD or ALD of a film comprising: at least one precursor composed of an element selected from the group consisting of Li, Na, K, Rb, Cs, Fr, Be, Mg, Ti, Zr, Hf, Sc, Y, La, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Hg, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, As, P, Sb and Bi, to which is bound at least one ligand selected from the group consisting of hydride, alkyl, alkenyl, cycloalkenyl, aryl, alkyne, carbonyl, amido, imido, hydrazido, phosphido, nitrosyl, nitryl, nitrate, nitrile, halide, azide, alkoxy, siloxy, silyl, and halogenated, sulfonated or silyated derivatives thereof, which is dissolved, emulsified or suspended in an inert liquid selected from the group consisting of aliphatic hydrocarbons, aromatic hydrocarbons, alcohols, ethers, aldehydes, ketones, acids, phenols, esters, amines, alkylnitrile, halogenated hydrocarbons, silyated hydrocarbons, thioethers, amines, cyanates, isocyanates, thiocyanates, silicone oils, nitroalkyl, alkylnitrate, and mixtures thereof. The precursor source mixture may be a solution, emulsion or suspension and may consist of a mixture of solid, liquid and gas phases which are distributed throughout the mixture.
Owner:GLOBALFOUNDRIES INC
Emulsion compositions
Owner:UK RES & INNOVATION LTD
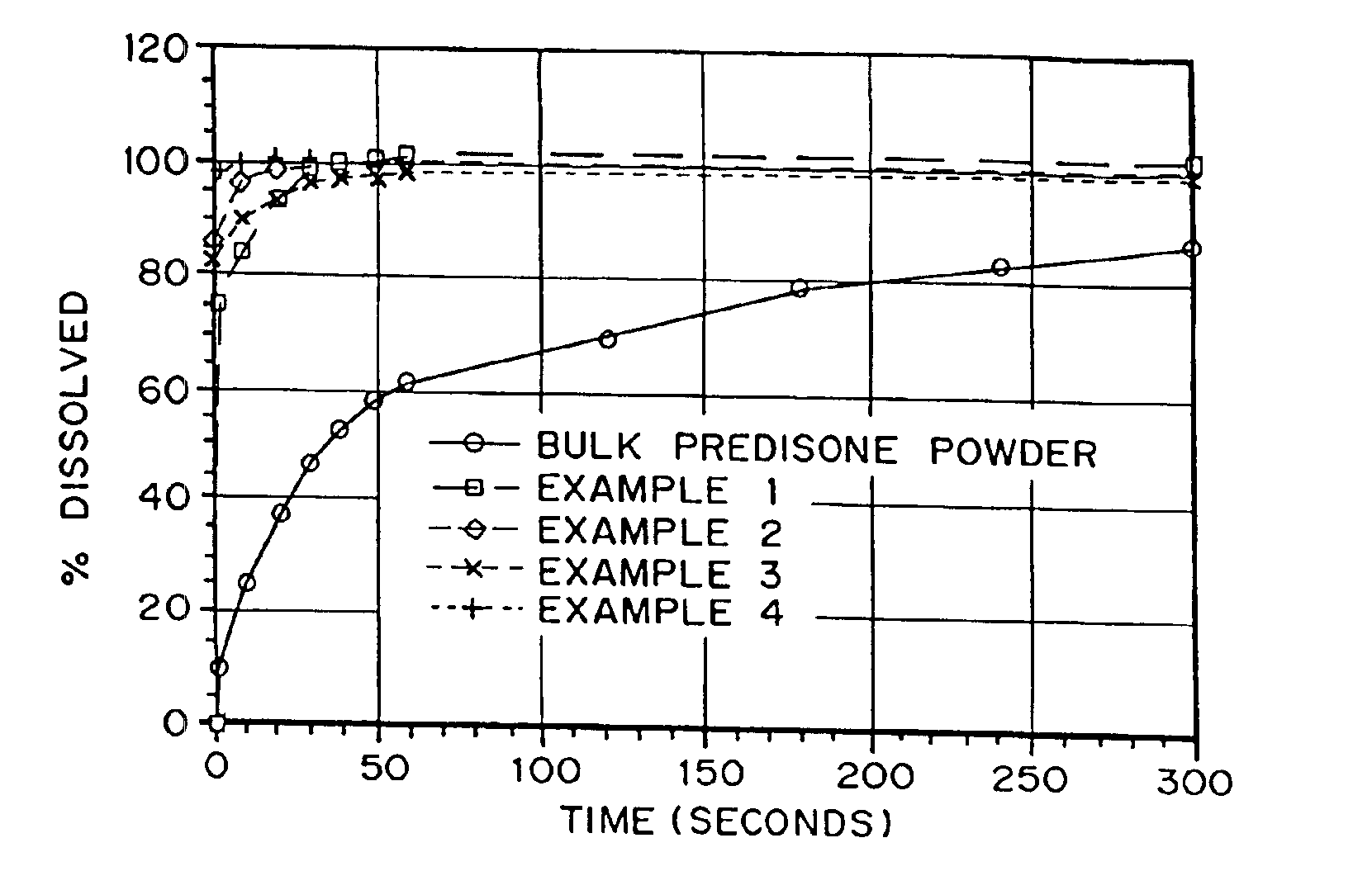
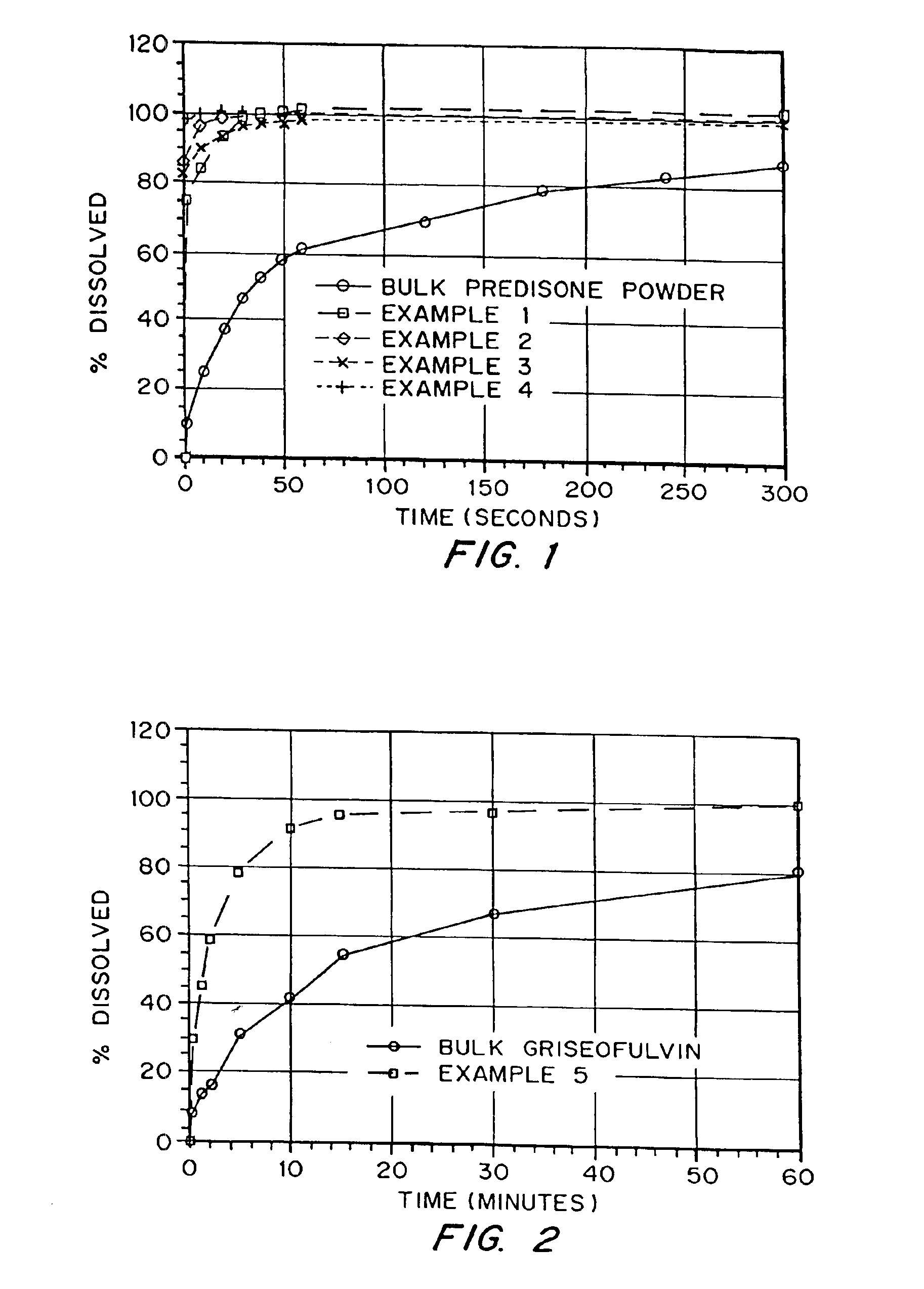
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
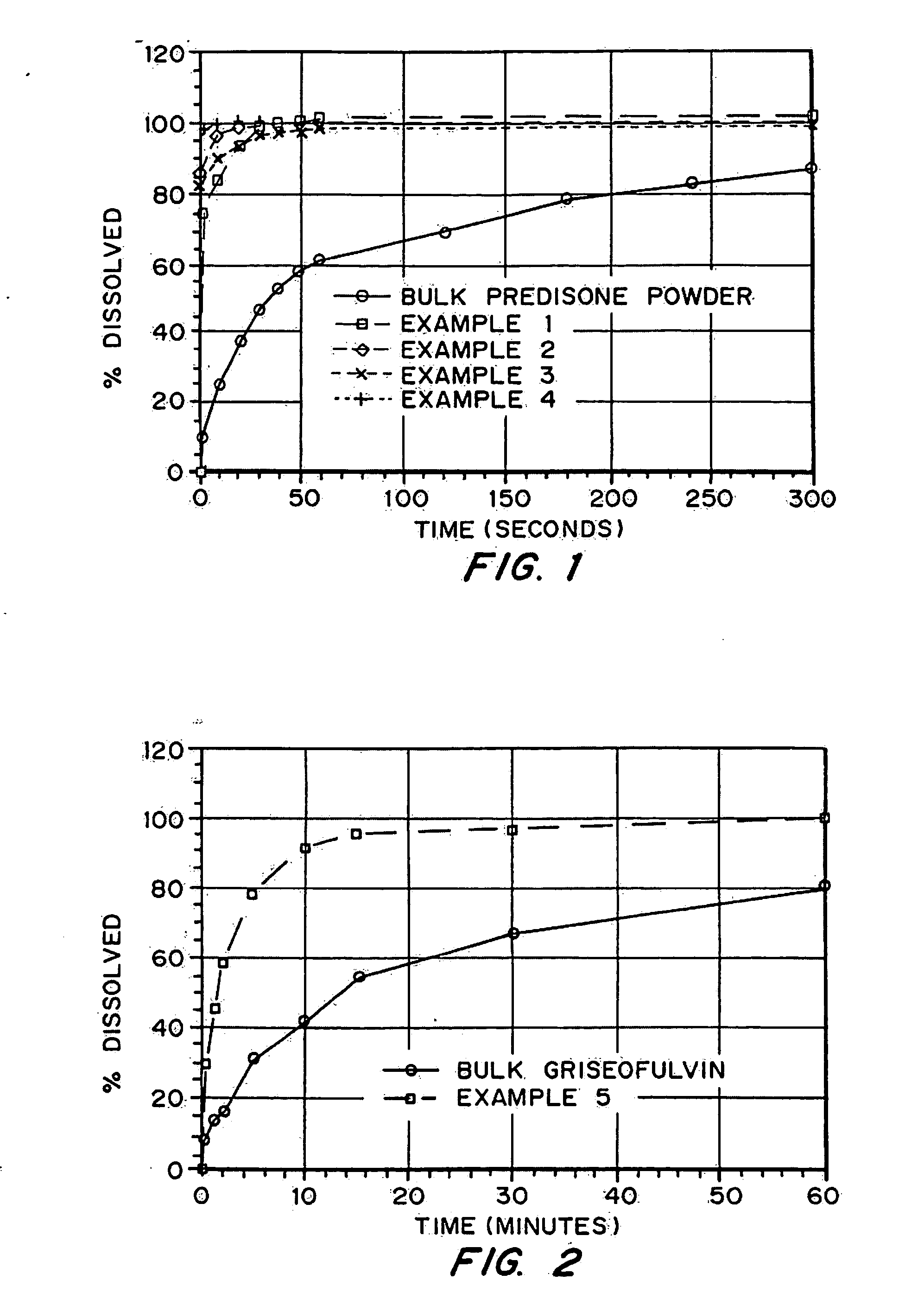
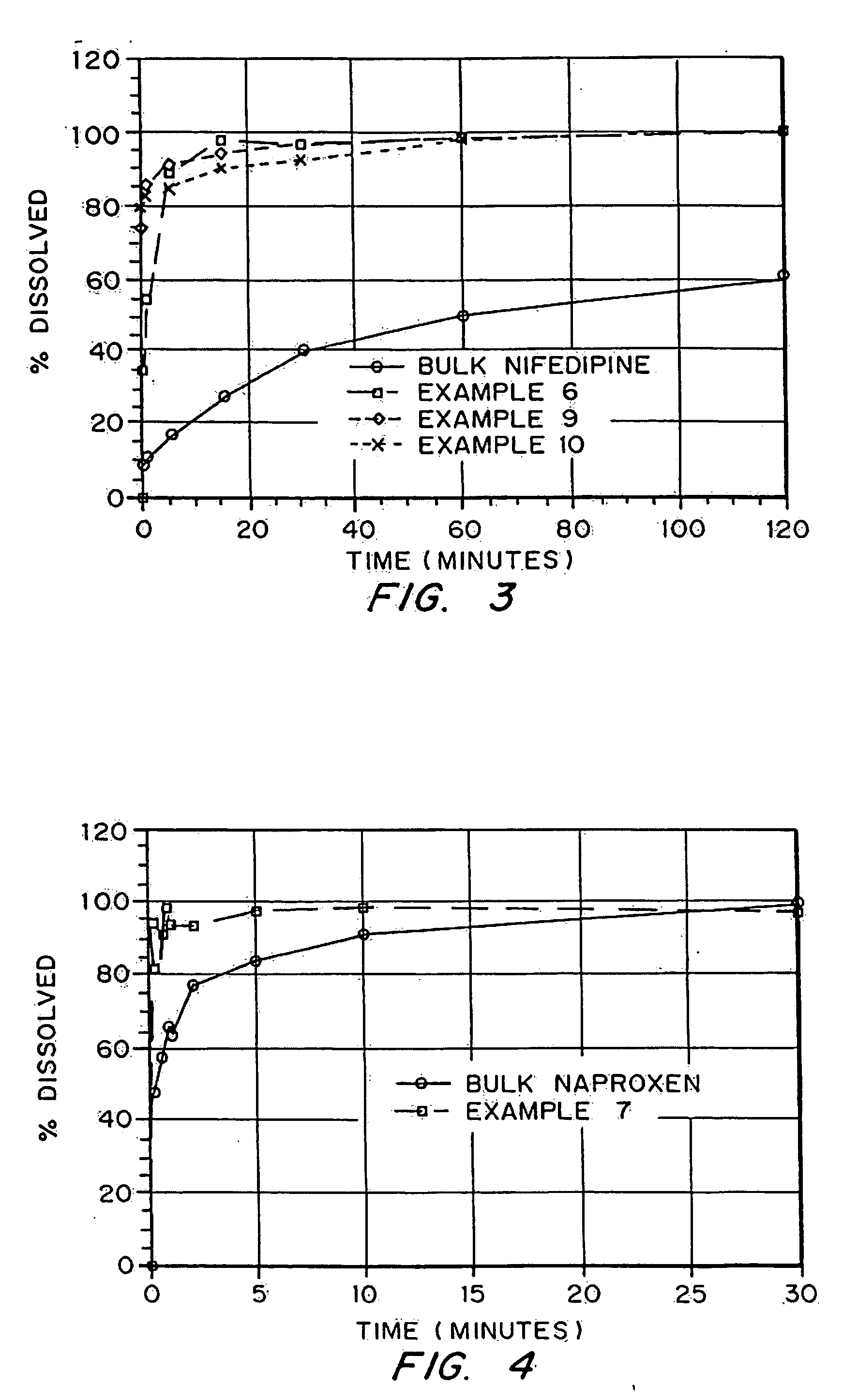
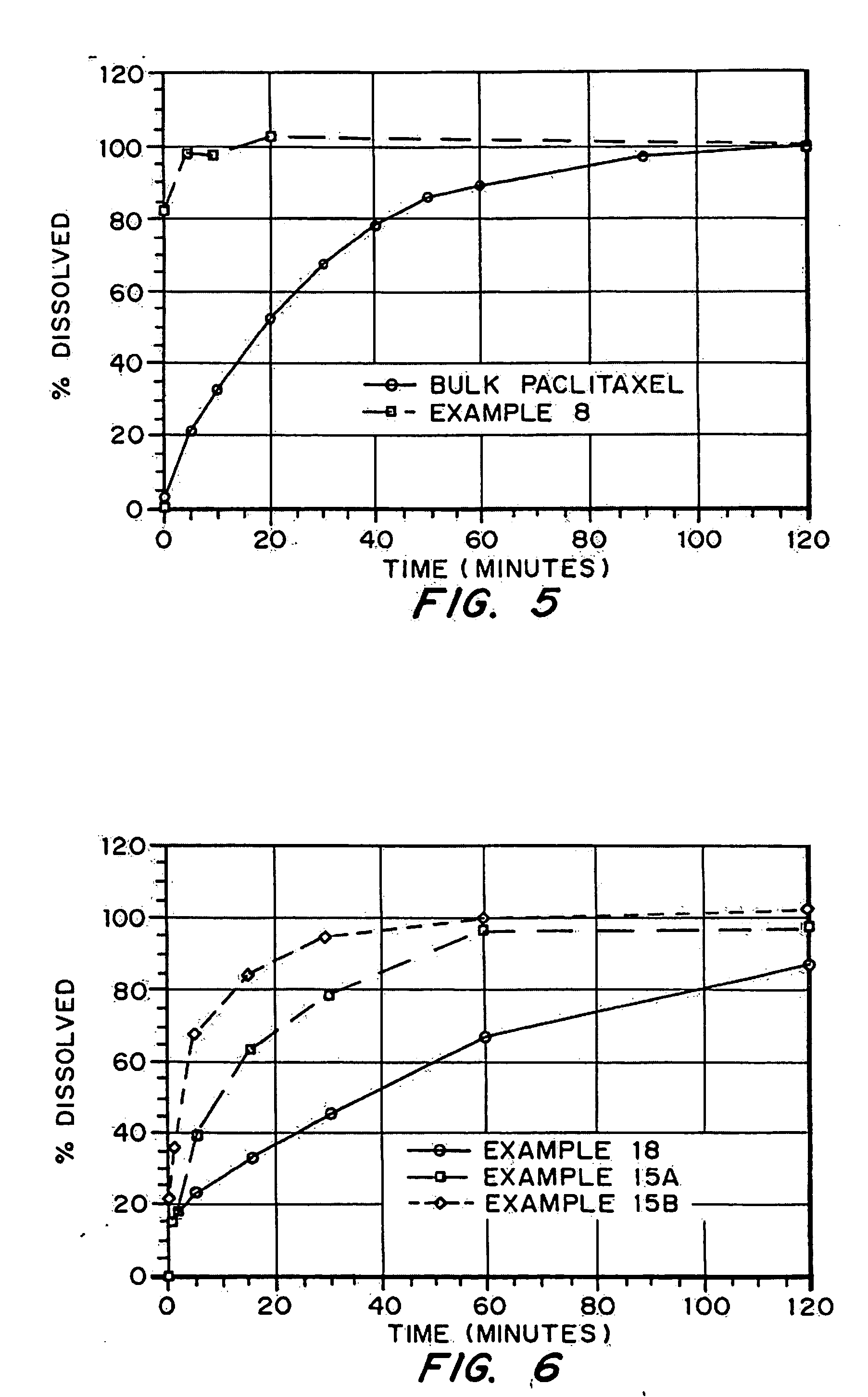
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Microfluidic Devices and Methods of Use in The Formation and Control of Nanoreactors
InactiveUS20100137163A1Material nanotechnologyCompound screeningHigh-Throughput Screening AssaysEmulsion
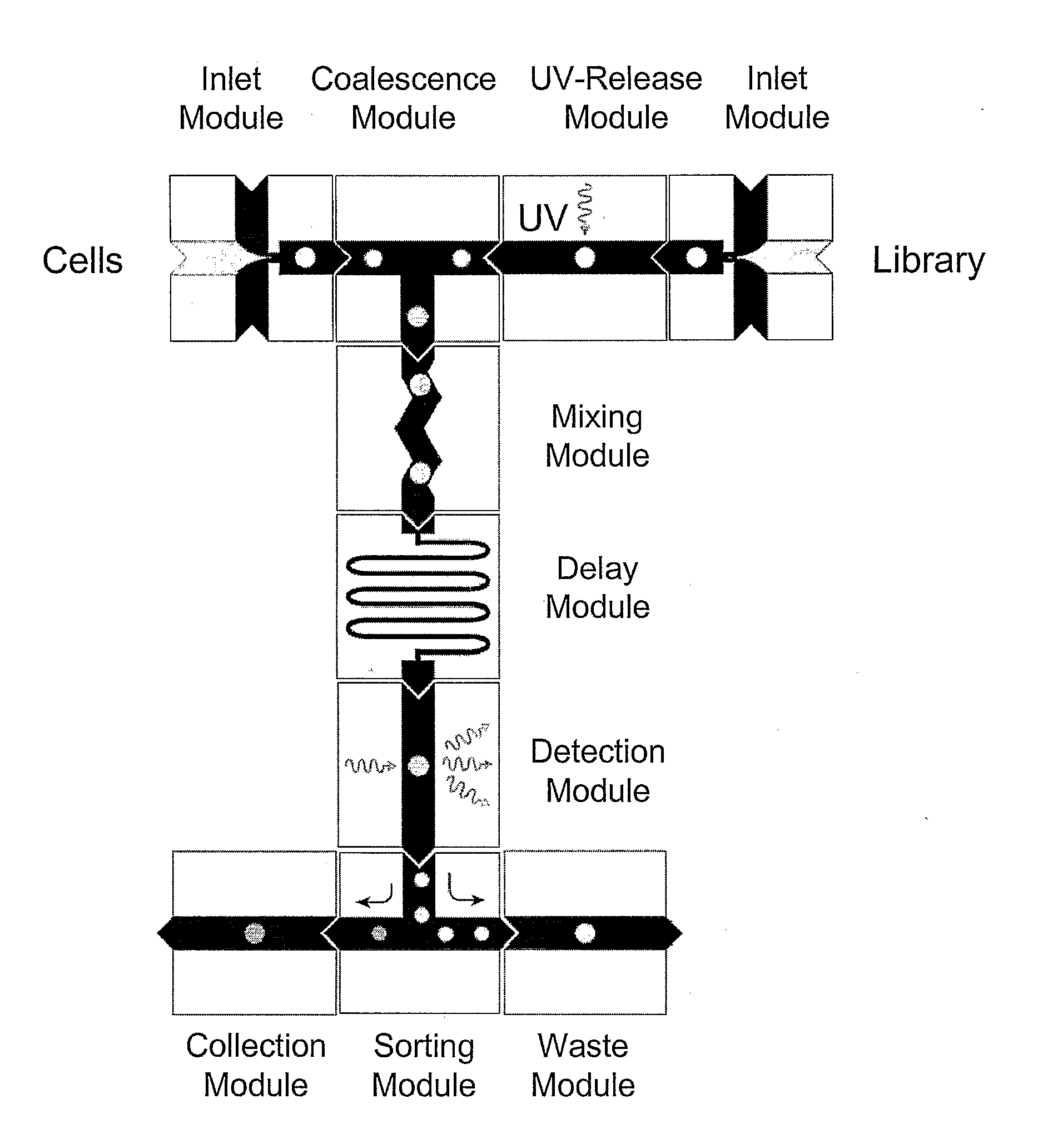
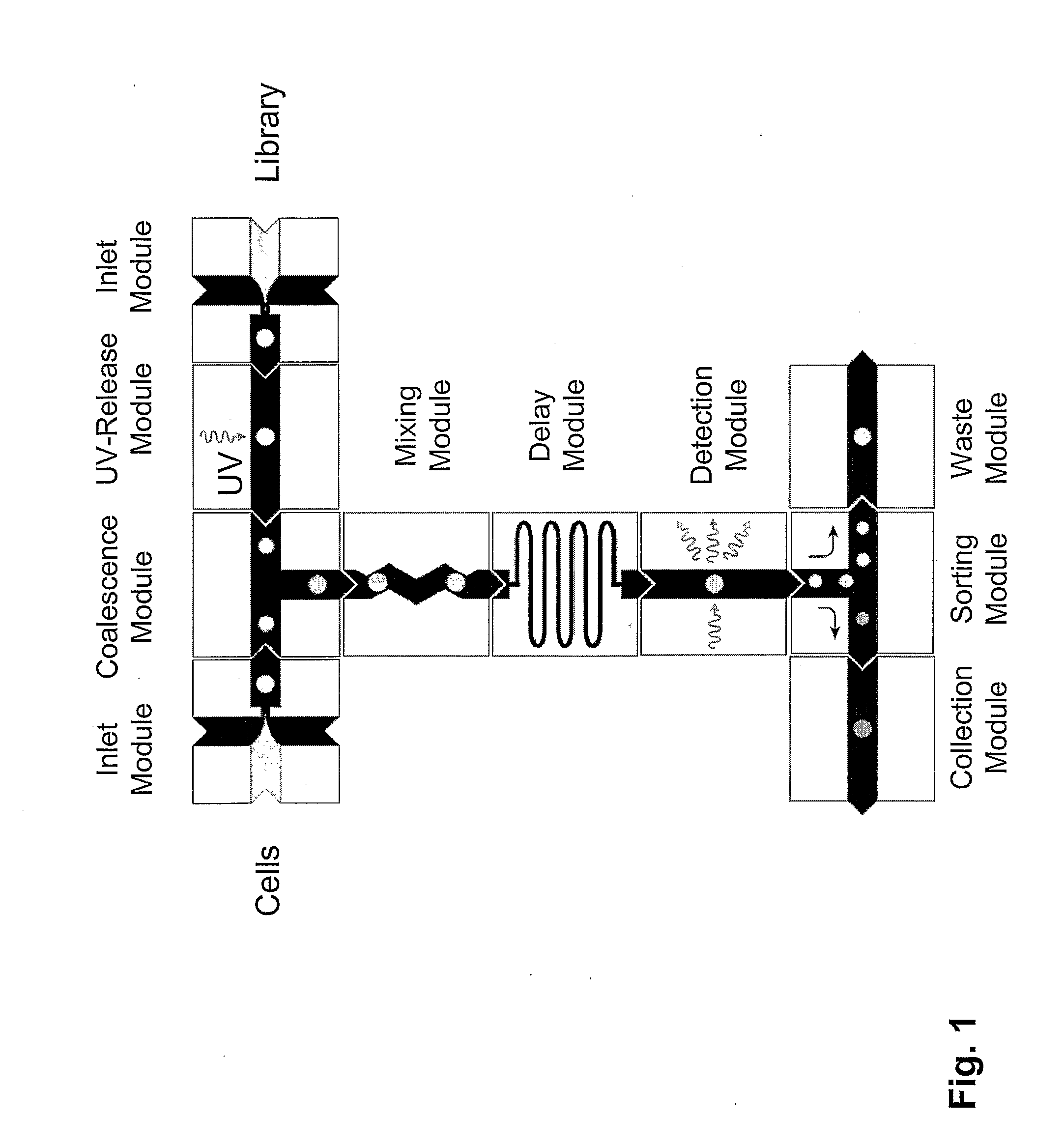
The present invention provides novel microfluidic devices and methods that are useful for performing high-throughput screening assays and combinatorial chemistry. Such methods can include labeling a library of compounds by emulsifying aqueous solutions of the compounds and aqueous solutions of unique liquid labels on a microfluidic device, which includes a plurality of electrically addressable, channel bearing fluidic modules integrally arranged on a microfabricated substrate such that a continuous channel is provided for flow of immiscible fluids, whereby each compound is labeled with a unique liquid label, pooling the labeled emulsions, coalescing the labeled emulsions with emulsions containing a specific cell or enzyme, thereby forming a nanoreactor, screening the nanoreactors for a desirable reaction between the contents of the nanoreactor, and decoding the liquid label, thereby identifying a single compound from a library of compounds.
Owner:BIO RAD LAB INC
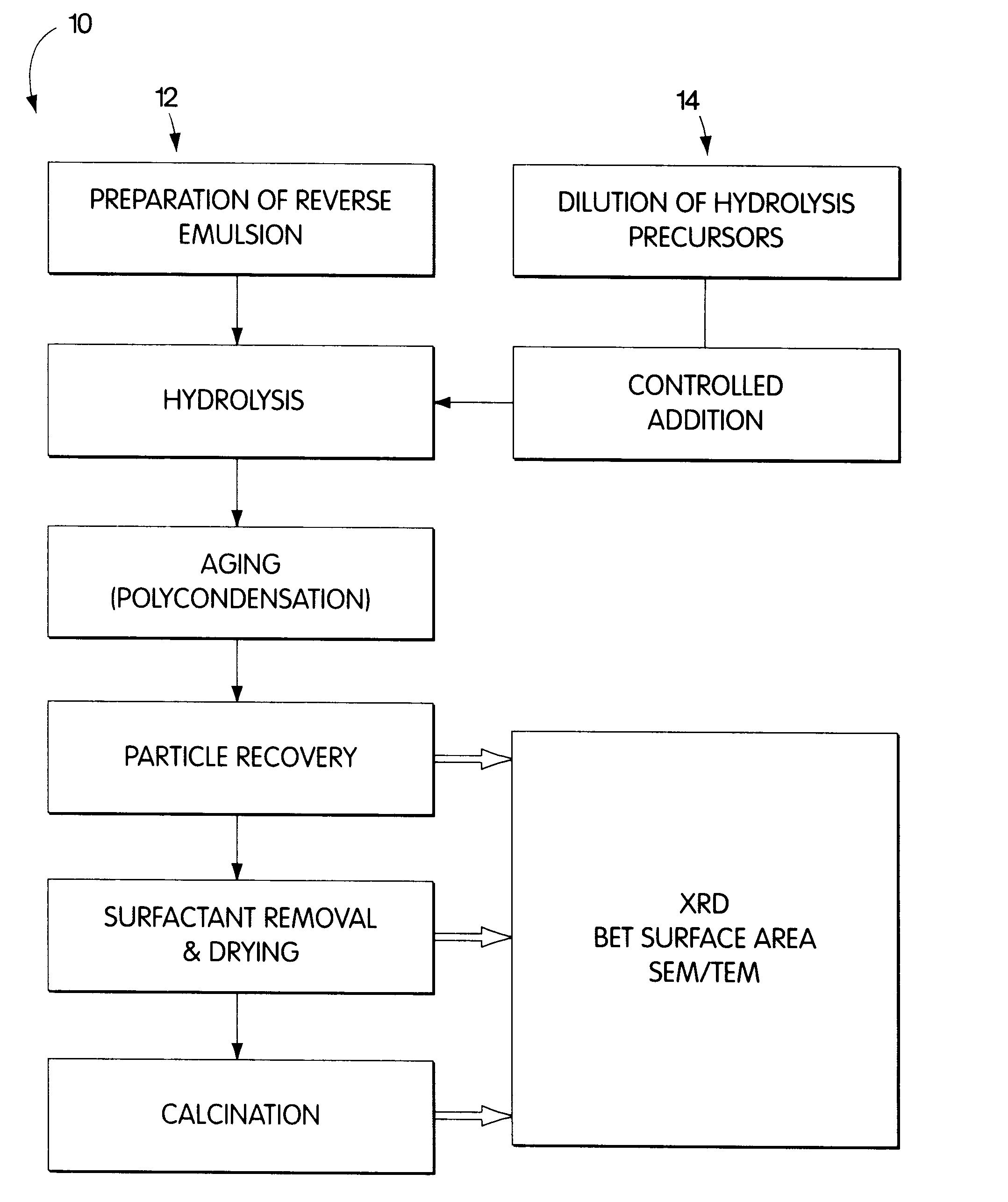
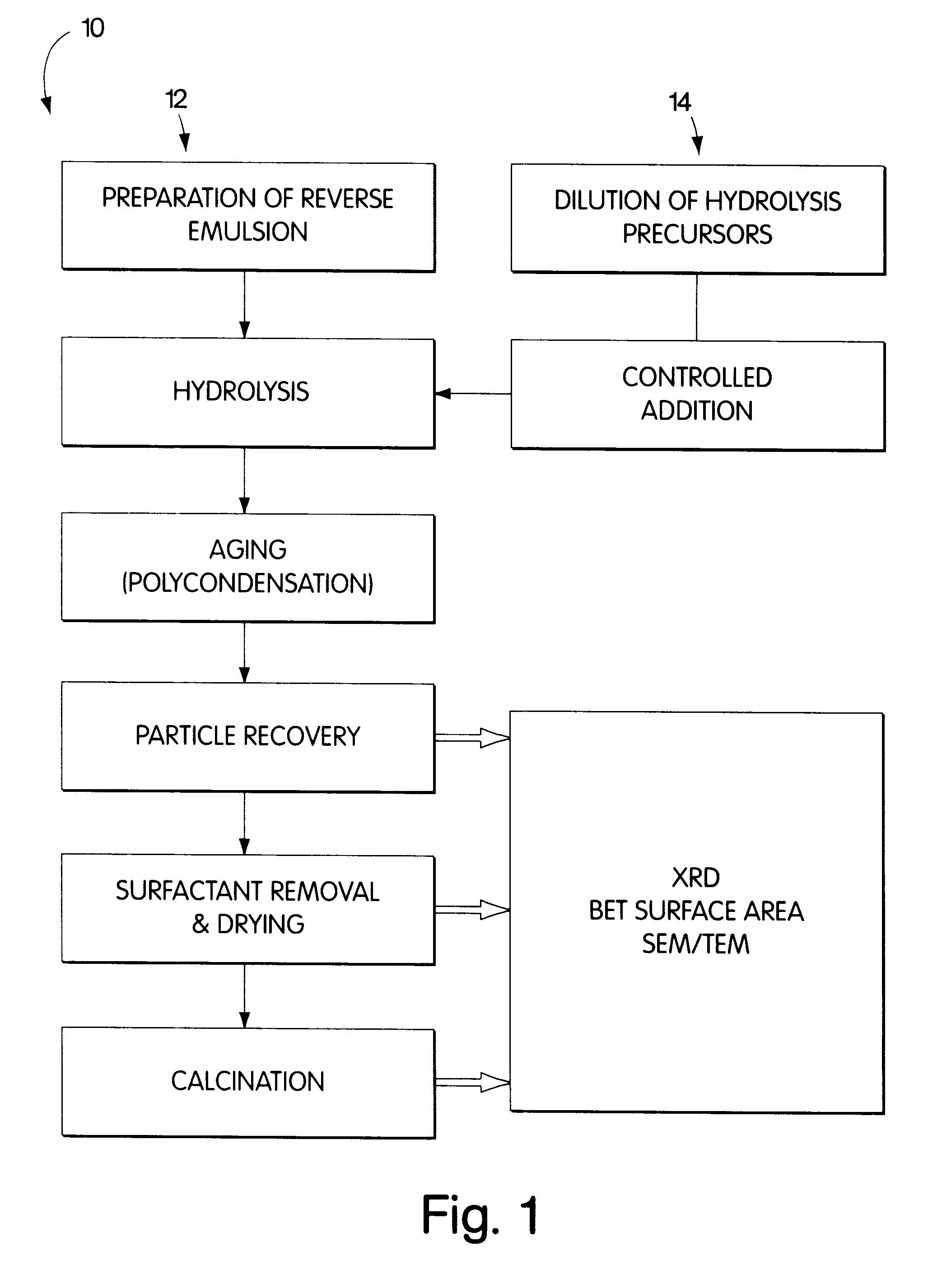
Synthesis of nanometer-sized particles by reverse micelle mediated techniques
The present invention relates to a method of producing particles having a particle size of less than 100 nm and surface areas of at least 20 m2 / g where the particles are free from agglomeration. The method involves synthesizing the particles within an emulsion having a 1-40% water content to form reverse micelles. In particular, the particles formed are metal oxide particles. The particles can be used to oxidize hydrocarbons, particularly methane.
Owner:MASSACHUSETTS INST OF TECH
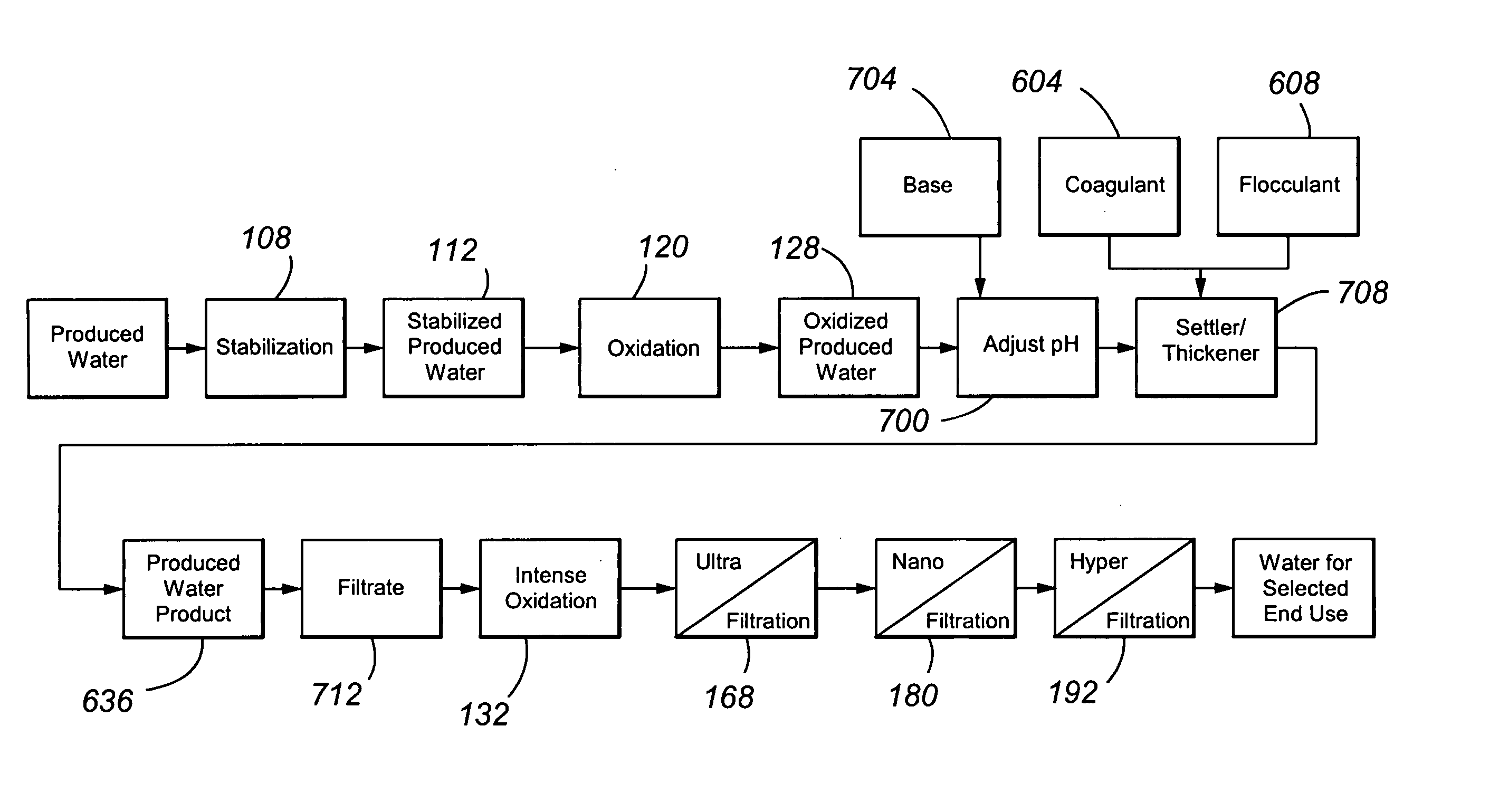
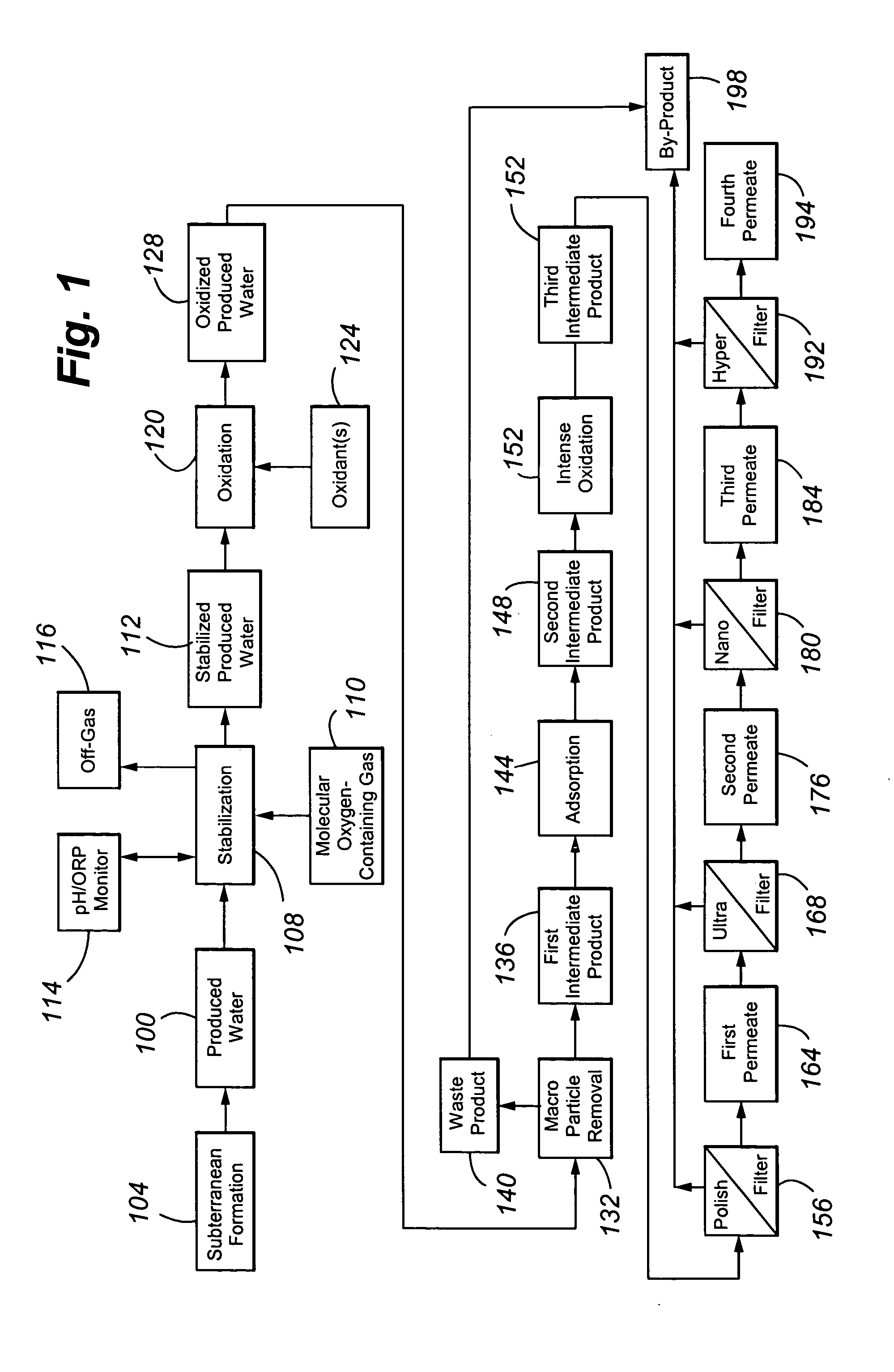
Treating produced waters
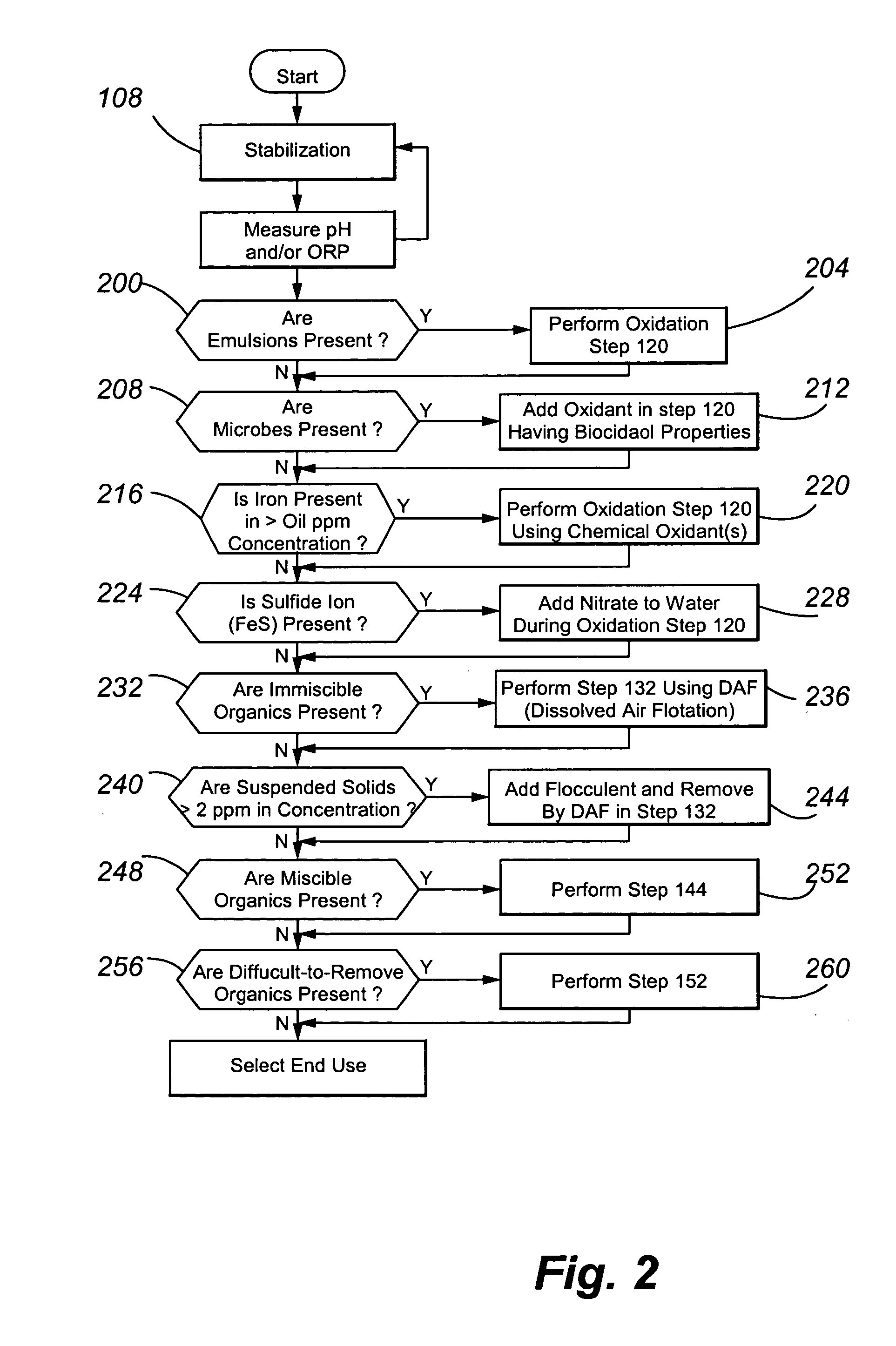
InactiveUS20070102359A1Complicate purificationIncrease ratingsUltrafiltrationTreatment involving filtrationEmulsionUnit operation
The present invention is directed to various sets of unit operations for treating aqueous effluents and logic for designing and effecting the treatment. The unit operations include stabilization of subterranean waters, sequential oxidation steps to alter selected target materials, oxidation to break up emulsions prior to removal of the emulsion components, and intense oxidation to break up difficult-to-remove organic target materials.
Owner:HW PROCESS TECH
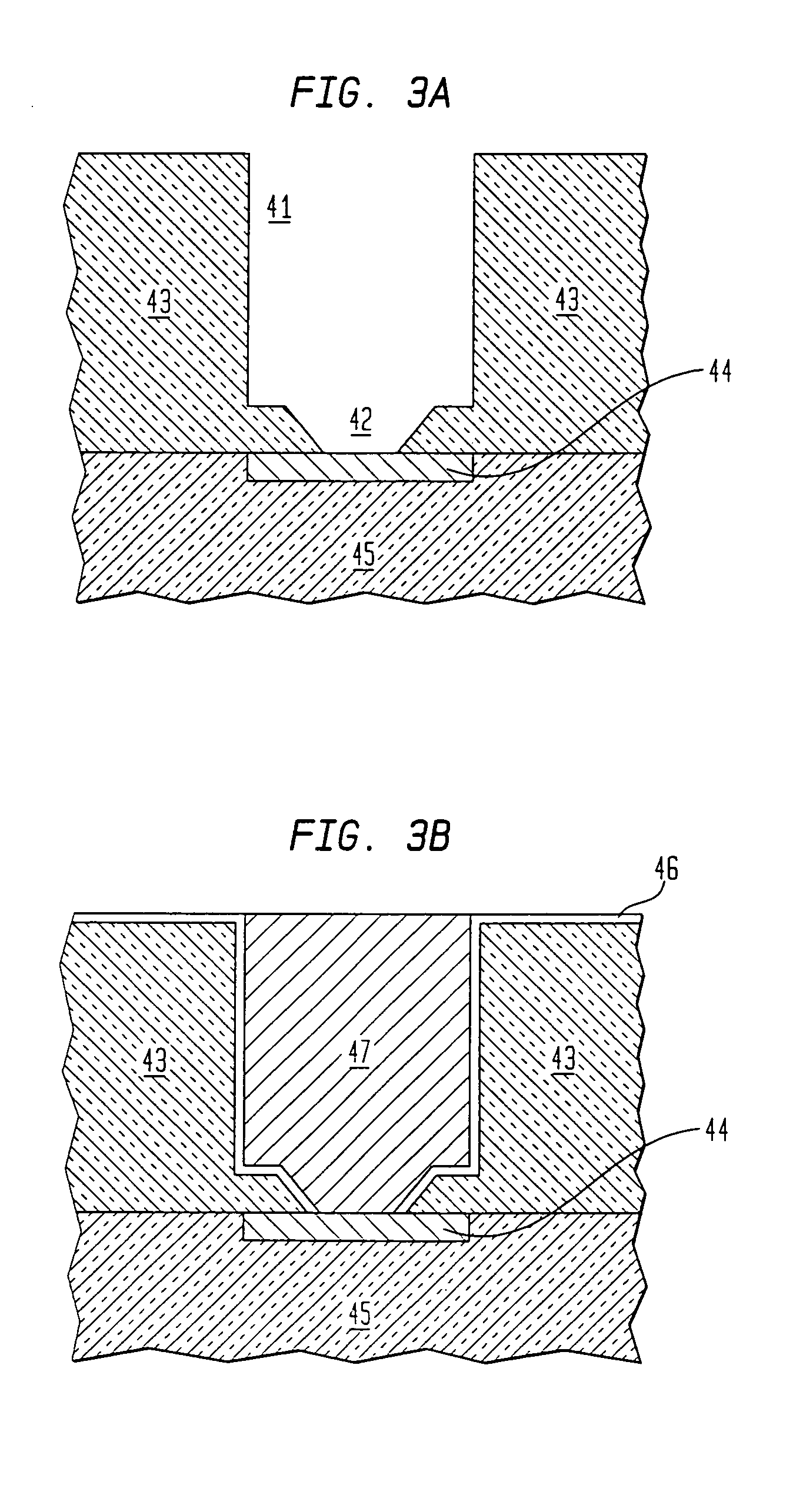
Process for producing emulsion and microcapsules and apparatus therefor
InactiveUS7268167B2Rapid productionSimple wayFlow mixersMixing methodsEmulsionMechanical engineering
Owner:JAPAN SCI & TECH CORP
Cosmetic composition and production thereof
InactiveUS20060018867A1Efficient use ofReduce the amount requiredCosmetic preparationsHair cosmeticsEpsilon-PolylysineEmulsion
It has been desired to develop a highly preservative and antibacterial cosmetic composition that can easily be applied to both emulsion and non-emulsion type cosmetics. It has also been desired to develop a method of improving a preservative and / or antibacterial effect(s) of a cosmetic composition comprising polyorganosiloxane-containing epsilon-polylysine and thereby reducing the amount of antibacterial preservative agent to be used. There is provided a cosmetic composition comprising one or a combination of two or more of polyorganosiloxane-containing epsilon-polylysine compounds obtained by reacting epsilon-polylysine with polyorganosiloxane or a physiologically acceptable salt thereof, and polyhydric alcohol.
Owner:CHISSO CORP
Pharmaceutical compositions for lipophilic drugs
InactiveUS7070802B1Good self-emulsifying performanceShelf-stableCyclic peptide ingredientsCapsule deliveryMonoglycerideCyclosporins
Stable solutions of lipophilic drugs, such as cyclosporin, forming a polar lipid self-emulsifying drug delivery system. The solutions can include lipophilic drugs, such as cyclosporin, dissolved in a polar lipid, such as having a C6-C12 fatty acid monoglyceride content of at least about 50%, surfactants and triglycerides. The composition forms a fine emulsion on exposure to water. The encapsulated dosage form of this composition needs neither a hydrophilic component nor air-tight blister packaging, and is particularly suitable for oral administration.
Owner:WATSON LAB INC
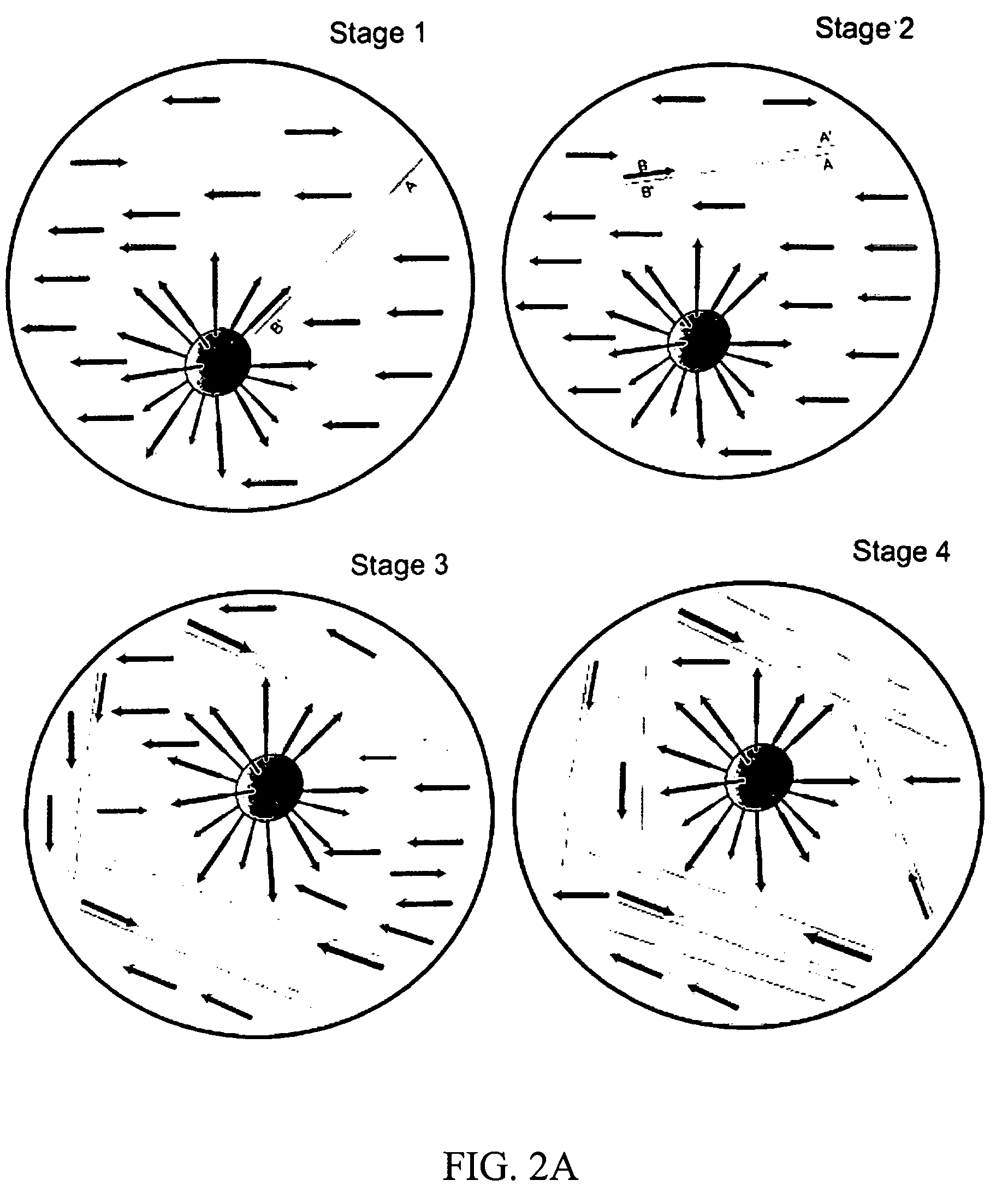
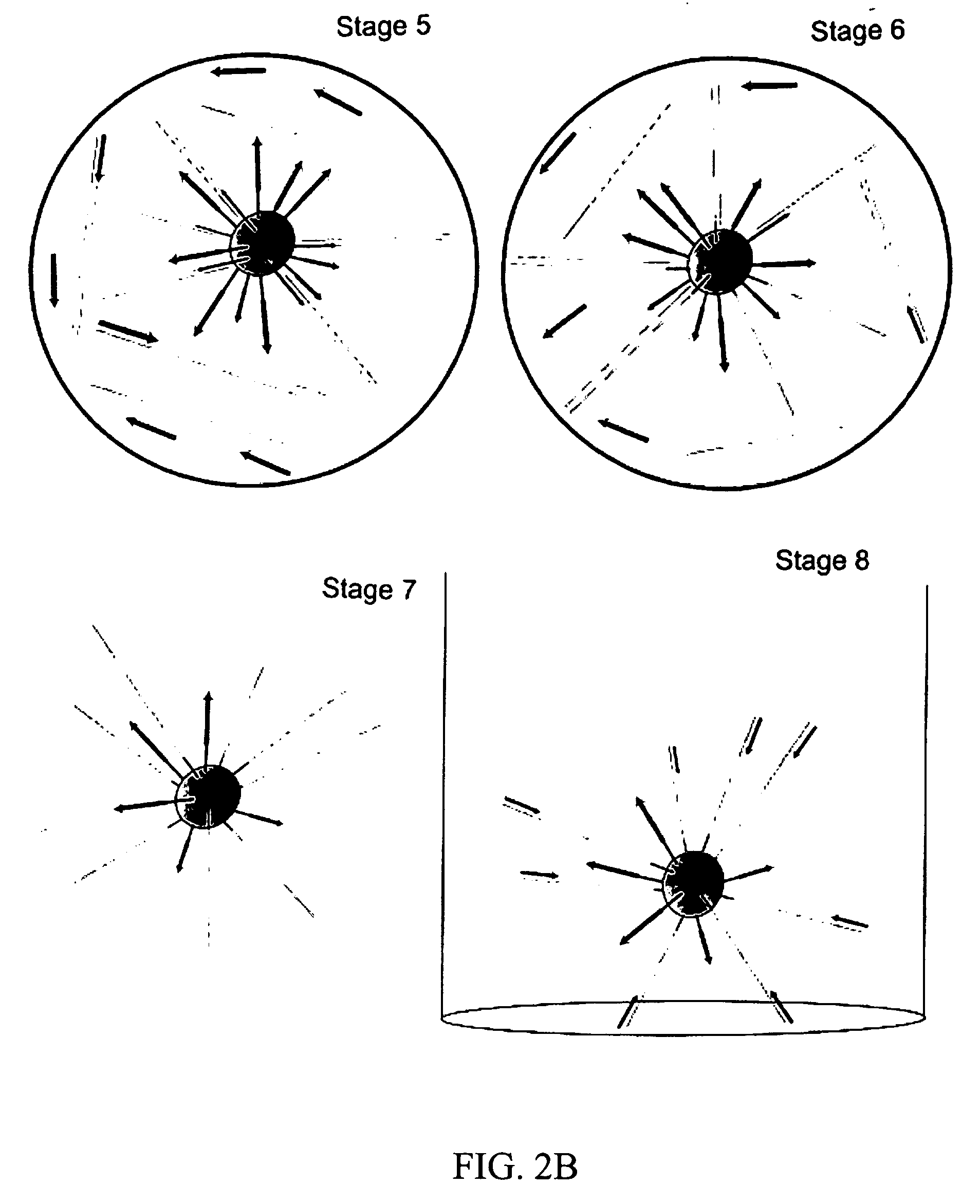
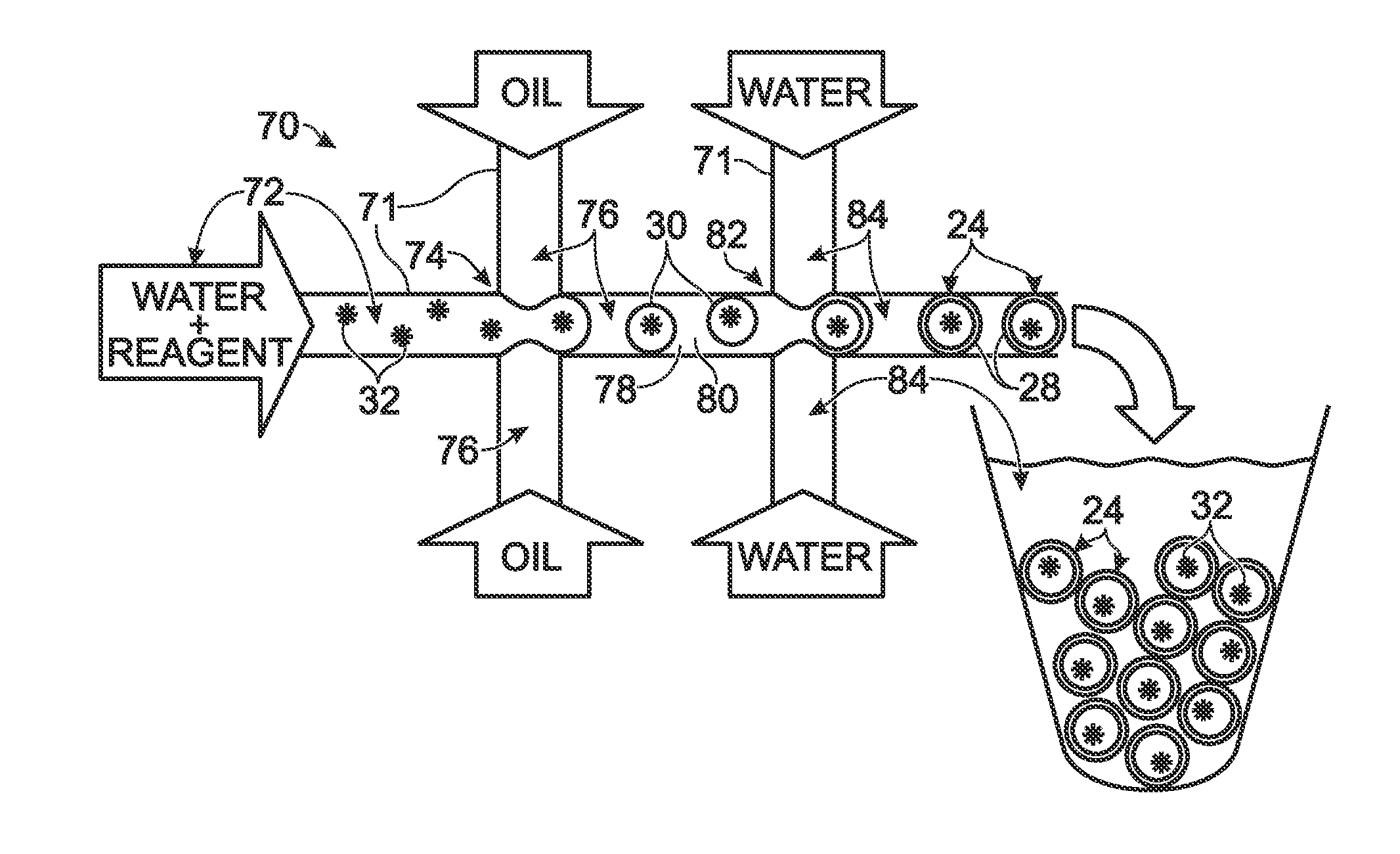
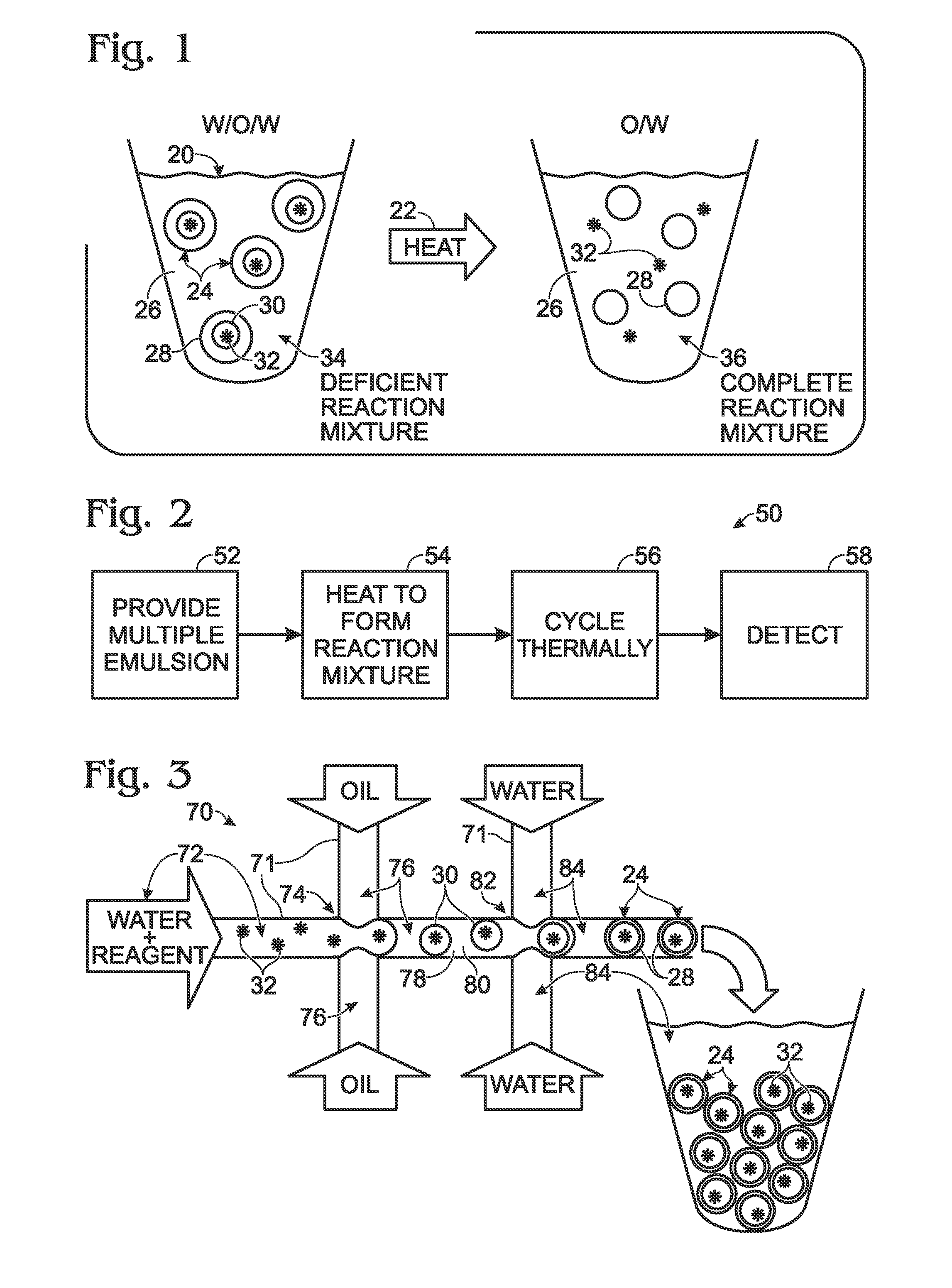
System for mixing fluids by coalescence of multiple emulsions
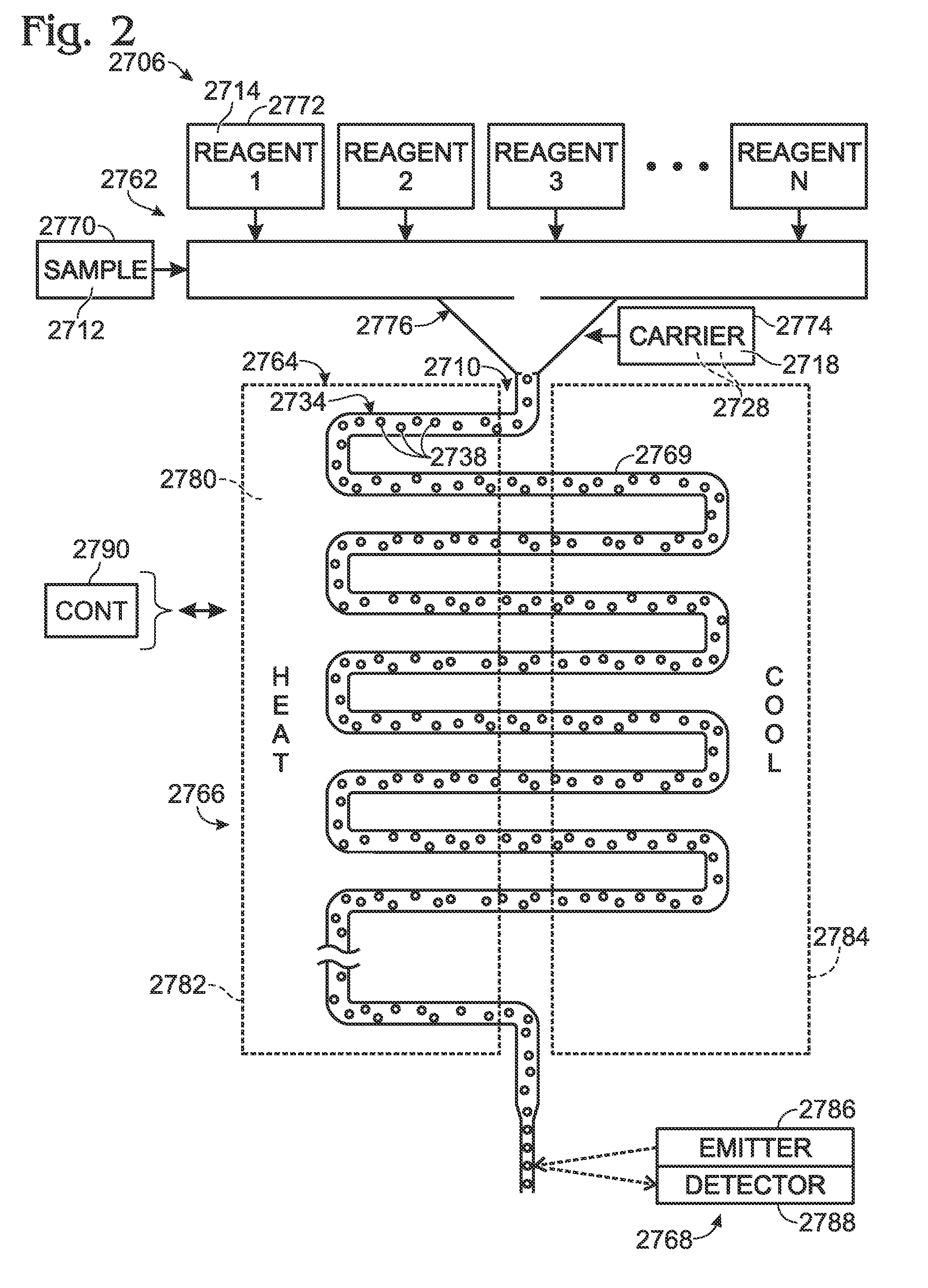
ActiveUS20110053798A1High-confidence resultLower the volumeSequential/parallel process reactionsHeating or cooling apparatusEmulsionChemistry
System, including methods, apparatus, compositions, and kits, for the mixing of small volumes of fluid by coalescence of multiple emulsions.
Owner:BIO RAD LAB INC
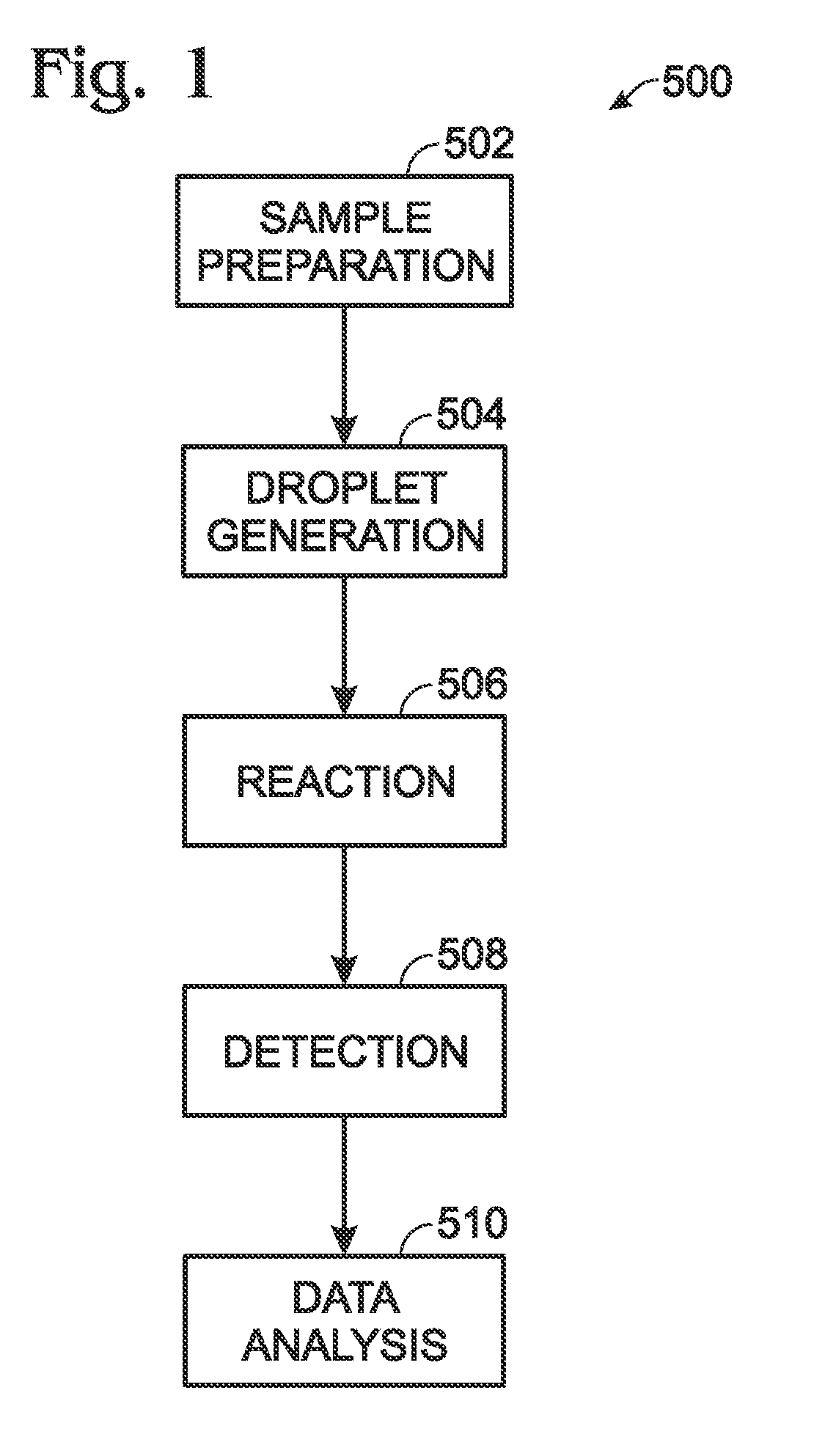
Droplet generation for droplet-based assays
ActiveUS20120190032A1High-confidence resultLower the volumeBioreactor/fermenter combinationsBiological substance pretreatmentsAssayEmulsion
A system, including method and apparatus, for generating droplets suitable for droplet-based assays. The disclosed systems may include either one-piece or multi-piece droplet generation components configured to form sample-containing droplets by merging aqueous, sample-containing fluid with a background emulsion fluid such as oil, to form an emulsion of sample-containing droplets suspended in the background fluid. In some cases, the disclosed systems may include channels or other suitable mechanisms configured to transport the sample-containing droplets to an outlet region, so that subsequent assay steps may be performed.
Owner:BIO RAD LAB INC
Preparation method of polymer/graphene composite material through in situ reduction
ActiveCN101864098AEvenly dispersedQuality improvementSpecial tyresNon-conductive material with dispersed conductive materialElectrical conductorVulcanization
The invention relates to a preparation method of a polymer / graphene composite material through in situ reduction, which is characterized by comprising the following steps: adopting ultrasonic wave or grinding to evenly disperse the graphite oxide prepared by a Hummers method into polymer dispersion; introducing reducing agent into the polymer dispersion for in situ reduction, enabling the graphite oxide to be reduced into the grapheme so as to obtain stable polymer / graphene composite emulsion; carrying out demulsification, agglomeration and drying to obtain the composite polymer / grapheme composite master batch; adding the dried polymer / grapheme composite master batch and various assistants into the polymeric matrix according to a certain ratio; and carrying out double-roller mixing, vulcanization, melt extrusion or injection molding to obtain the polymer / graphene composite material with excellent physical and mechanical properties.
Owner:成都创威新材料有限公司
Sustained-release microcapsule of amorphous water-soluble pharmaceutical active agent
InactiveUS6117455AHigh entrapment of a water-soluble drugSmall initial releaseNanotechGogglesEmulsionOrganic solvent
A sustained-release microcapsule contains an amorphous water-soluble pharmaceutical agent having a particle size of from 1 nm-10 mu m and a polymer. The microcapsule is produced by dispersing, in an aqueous phase, a dispersion of from 0.001-90% (w / w) of an amorphous water-soluble pharmaceutical agent in a solution of a polymer having a wt. avg. molecular weight of 2,000-800,000 in an organic solvent to prepare an s / o / w emulsion and subjecting the emulsion to in-water drying.
Owner:TAKEDA PHARMA CO LTD
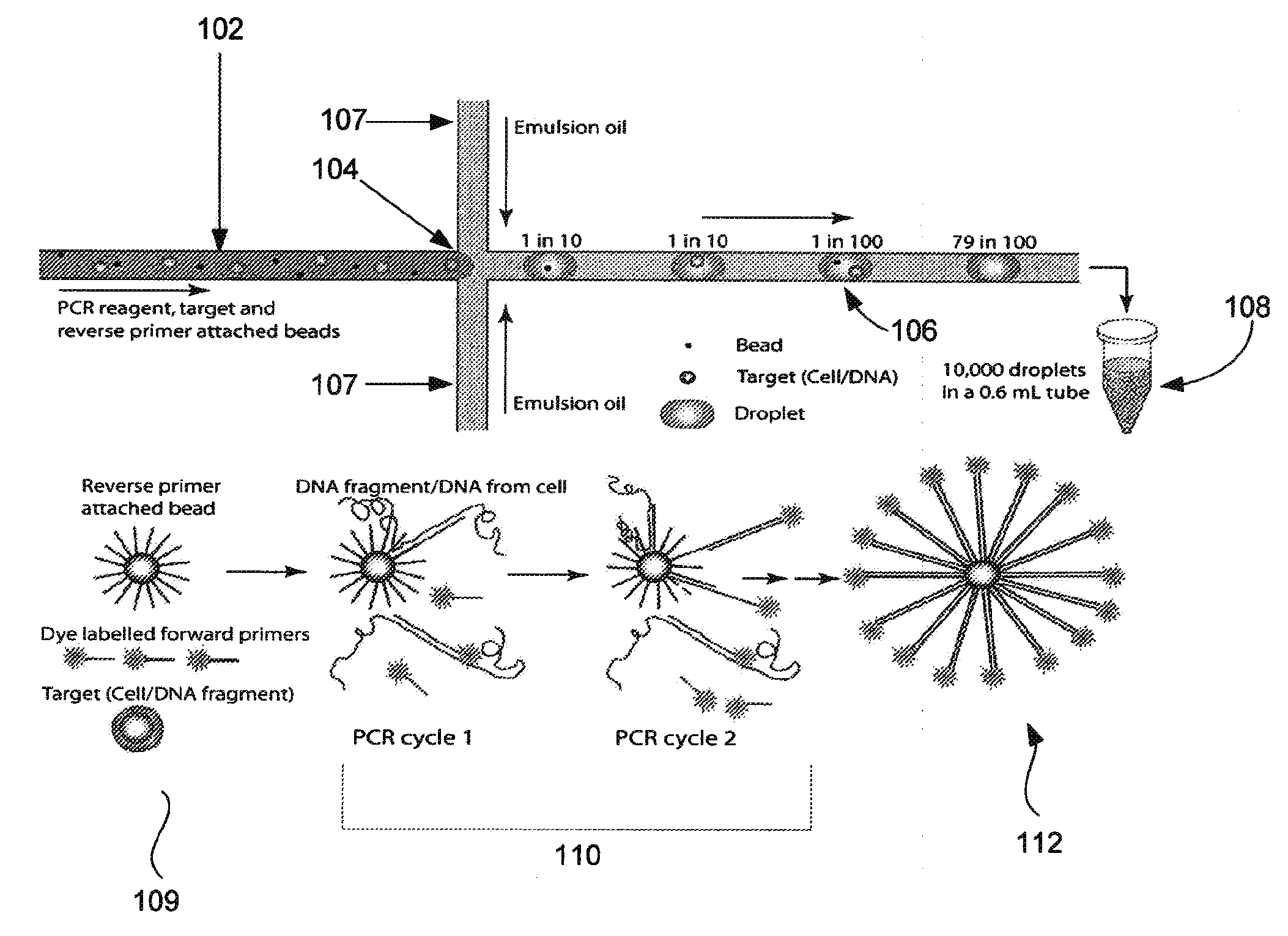
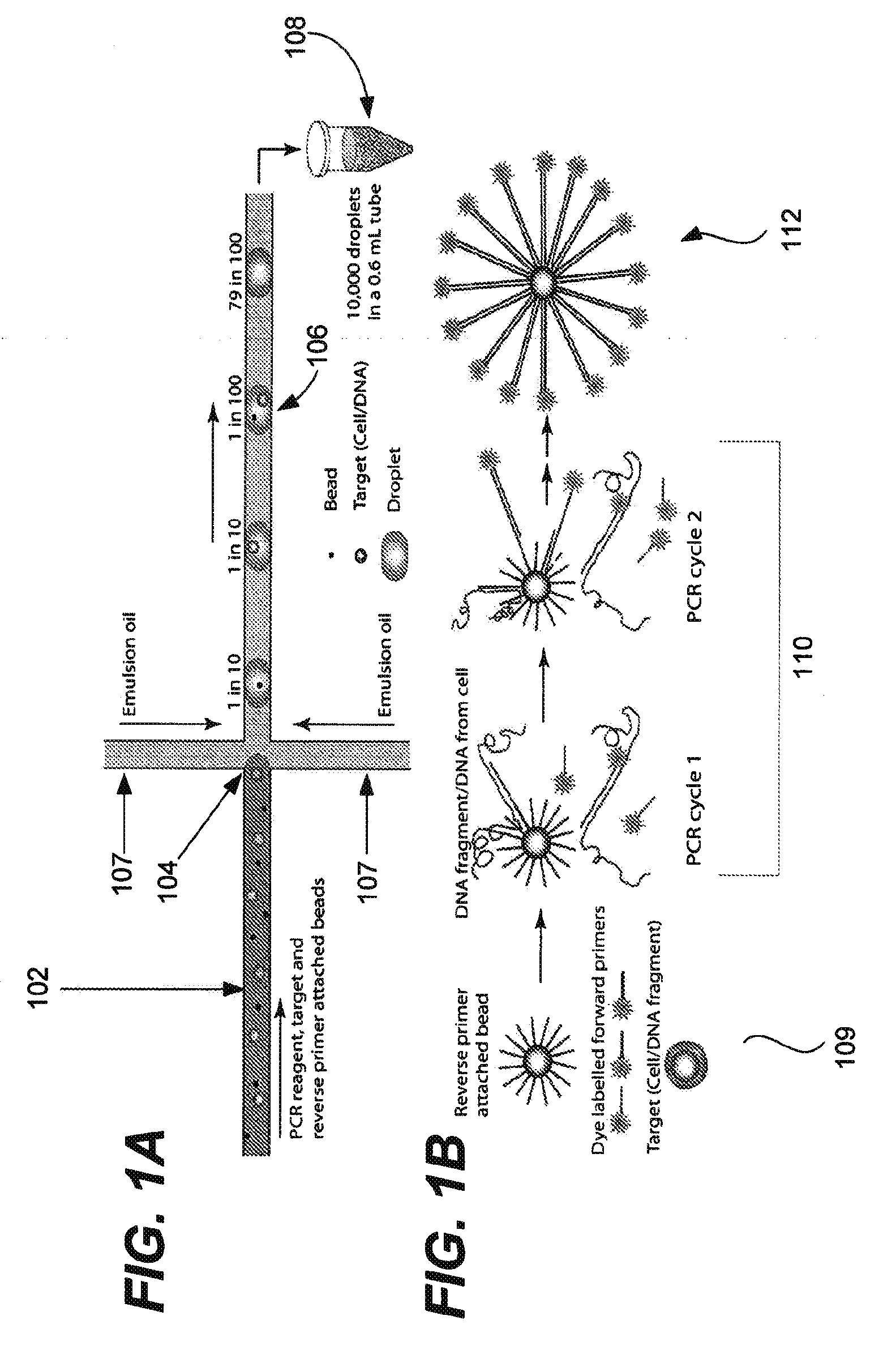
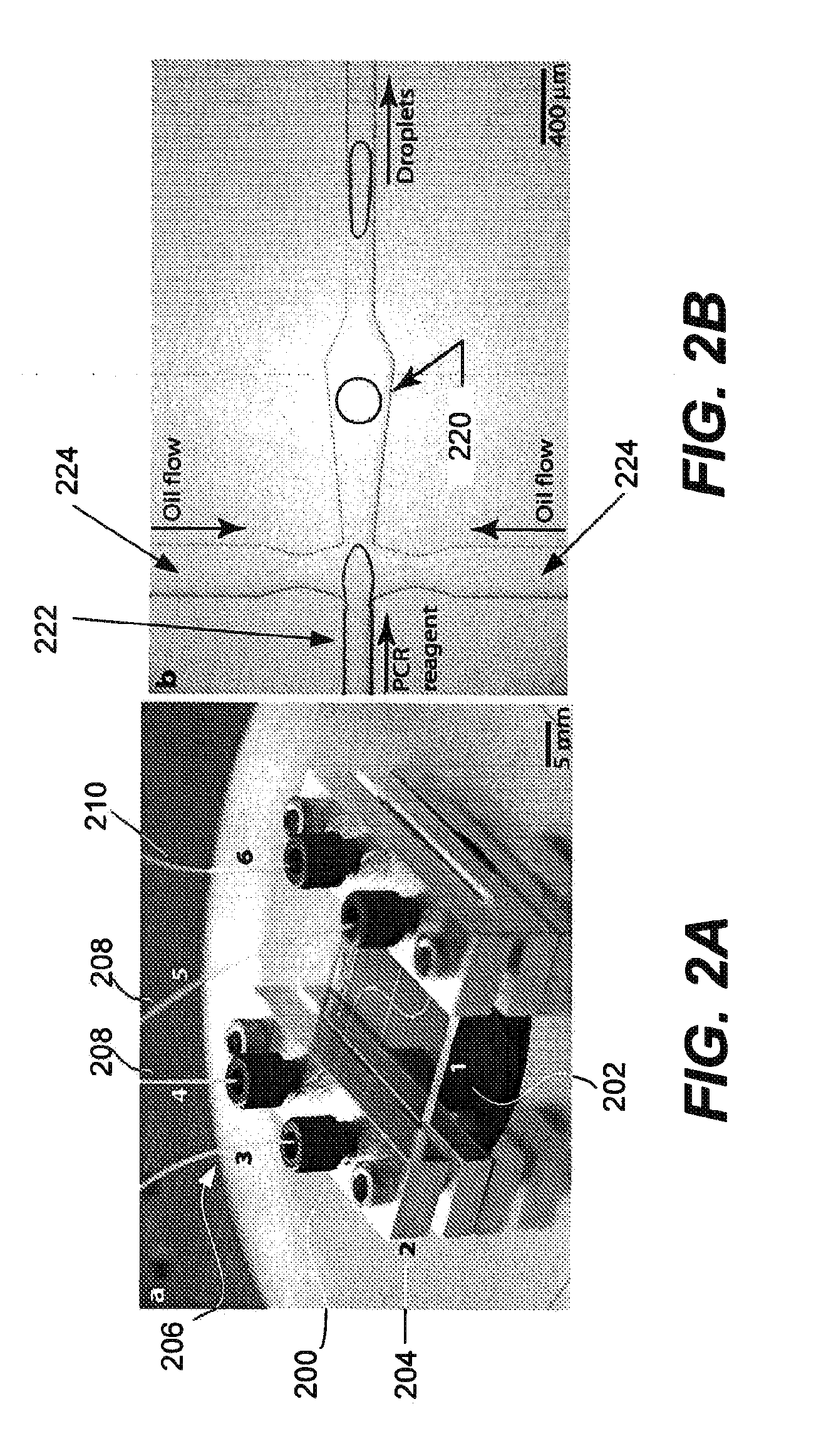
Microfabricated droplet generator for single molecule/cell genetic analysis in engineered monodispersed emulsions
ActiveUS20100285975A1Efficient routingEfficient single-molecule amplificationTransportation and packagingLibrary screeningEmulsionMicroparticle
Provided are microfluidic designs and methods for rapid generation of monodisperse nanoliter volume droplets of reagent / target (e.g., molecule or cell) mix in emulsion oil. The designs and methods enable high-throughput encapsulation of a single target (e.g., DNA / RNA molecules or cells) in controlled size droplets of reagent mix. According to various embodiments, a microfabricated, 3-valve pump is used to precisely meter the volume of reagent / target mix in each droplet and also to effectively route microparticles such as beads and cells into the device, which are encapsulated within droplets at the intersection of the reagent channel and an oil channel. The pulsatile flow profile of the microfabricated pumps provides active control over droplet generation, thereby enabling droplet formation with oils that are compatible with biological reactions but are otherwise difficult to form emulsions with.
Owner:RGT UNIV OF CALIFORNIA
Spontaneous emulsions containing cyclosporine
A pharmaceutical composition contains cyclosporine as the active ingredient. More specifically, the composition is an orally administered pharmaceutical formulation in the form of a spontaneous emulsion comprising cyclosporine, ethanol ethyl oleate and polyoxyethylene glycerol trioleate. A method for preparing an orally administered pharmaceutical composition involves first dissolving cyclosporine in ethanol. Polyoxyethylene glycerol trioleate and an oil component are then added, mixed and diluted in an aqueous media to form a spontaneous emulsion.
Owner:WOCKHARDT EU OPERATIONS SWISS
Biodegradable injectable implants containing glycolic acid
InactiveUS7314636B2Non-migratoryEasy to moveSolution deliveryPharmaceutical non-active ingredientsEmulsionGlycolic acid
Owner:MEDGRAFT MICROTECH
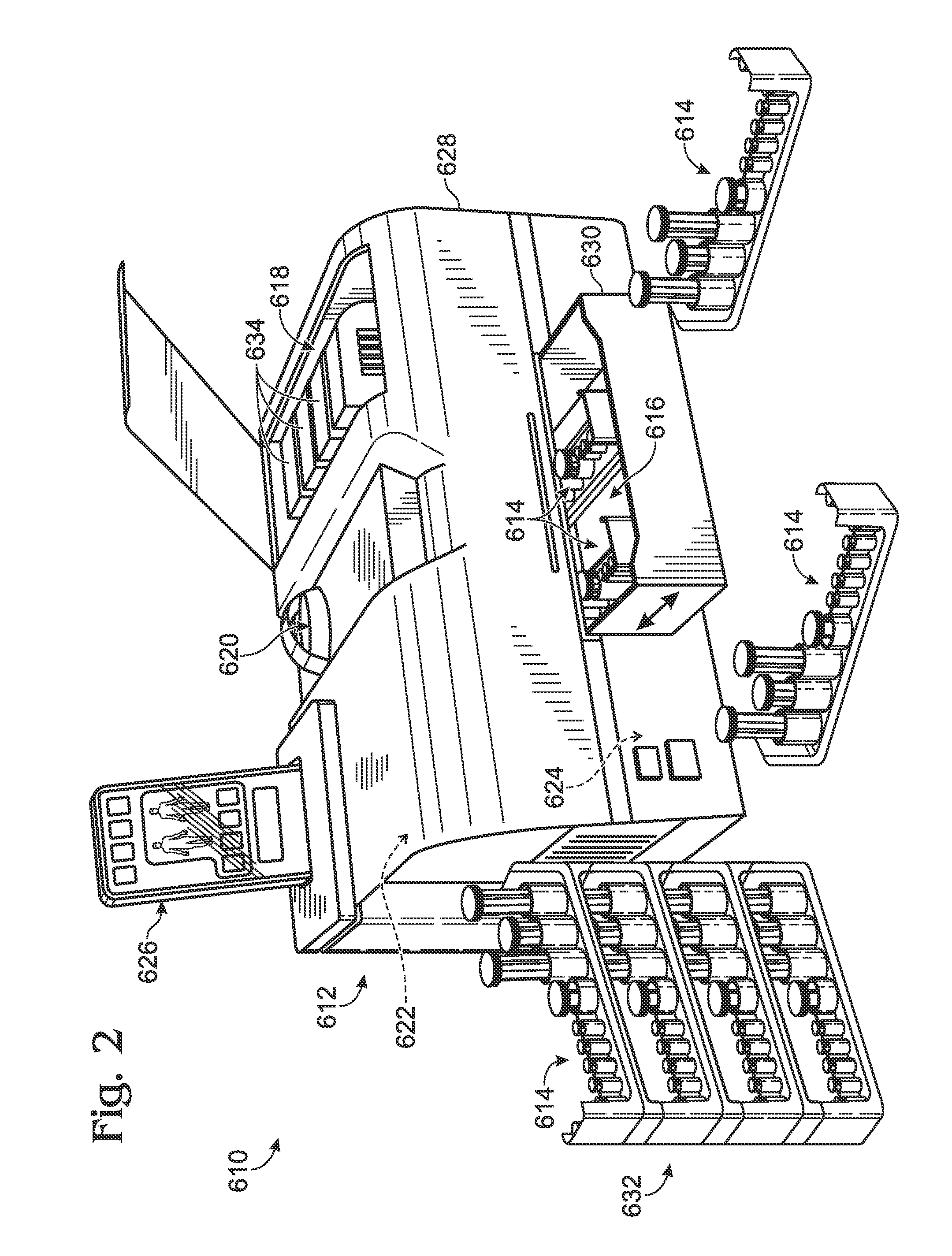
System for forming an array of emulsions
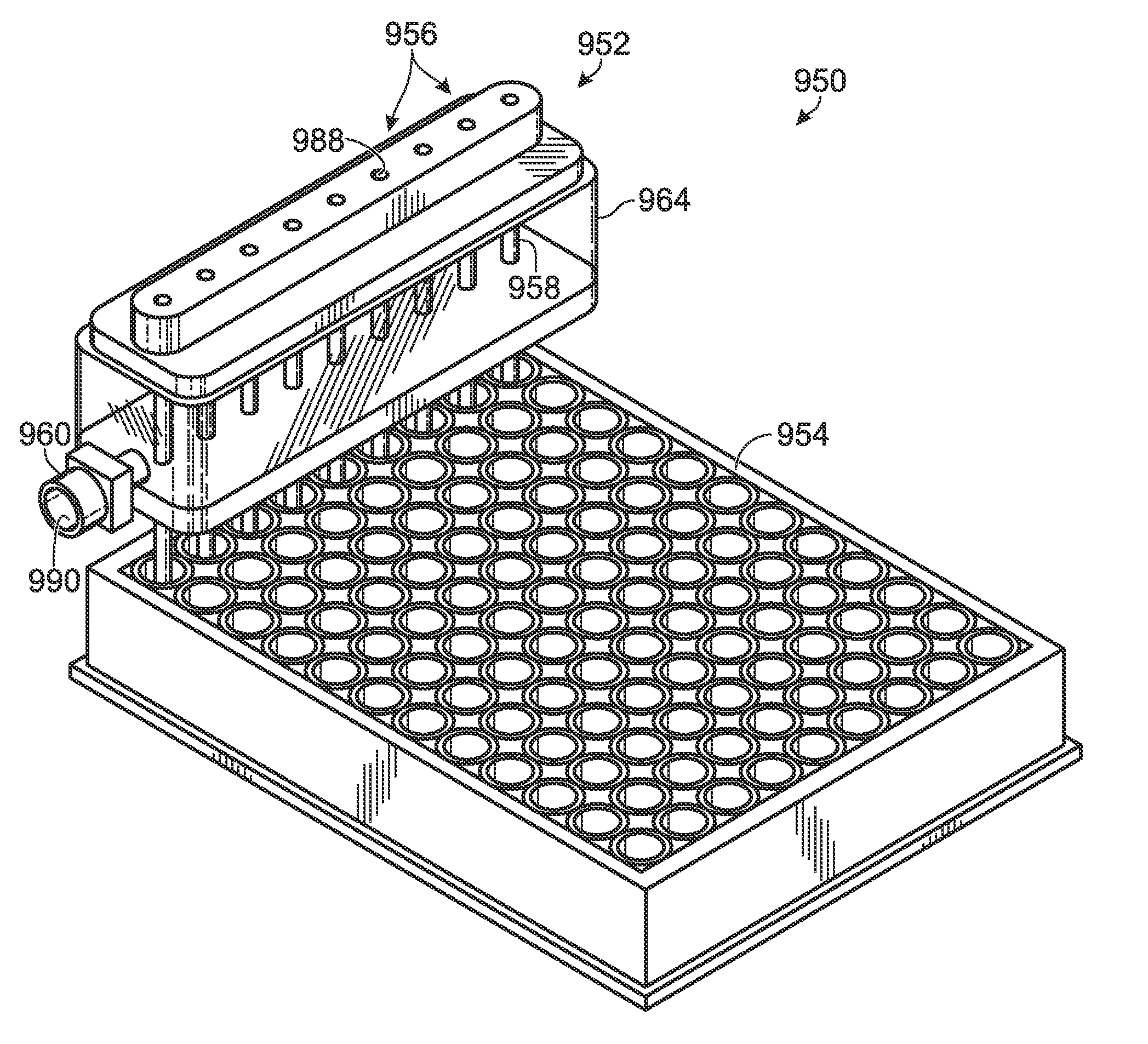
ActiveUS20110086780A1Easy CalibrationImprove accuracy and reliabilityHeating or cooling apparatusTransportation and packagingEmulsionEngineering
A system, including method and apparatus, for forming an array of emulsions. The system may include a plate providing an array of emulsion production units each configured to produce a separate emulsion and each including a set of wells interconnected by channels that intersect to form a site of droplet generation. Each set of wells, in turn, may include (1) at least one first input well to receive a continuous phase, (2) a second input well to receive a dispersed phase, and (3) an output well configured to receive from the site of droplet generation an emulsion of droplets of the dispersed phase disposed in the continuous phase.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Micro-emulsion type metal cutting liquor composition
InactiveCN101240217AEasy to handleImprove the lubrication effectAdditivesBase-materialsMaterials scienceCutting fluid
Disclosed is a microemulsifying metal-cutting-fluid composition, comprising base oil or oily agent, mixed alcohol-amine, boric acid, anionic surfactant, nonionic surfactant, antirust agent, copper alloy corrosion inhibitor, preservative, deionized water and the like. The invention has the advantages of excellent lubricity, cooling ability, cleaning ability and a long lifetime of metal cutting fluid, being suited to various metal processing technologies such as cutting, reaming, boring, grinding. Also, the wastewater is easy to treat.
Owner:益田润石(北京)化工有限公司
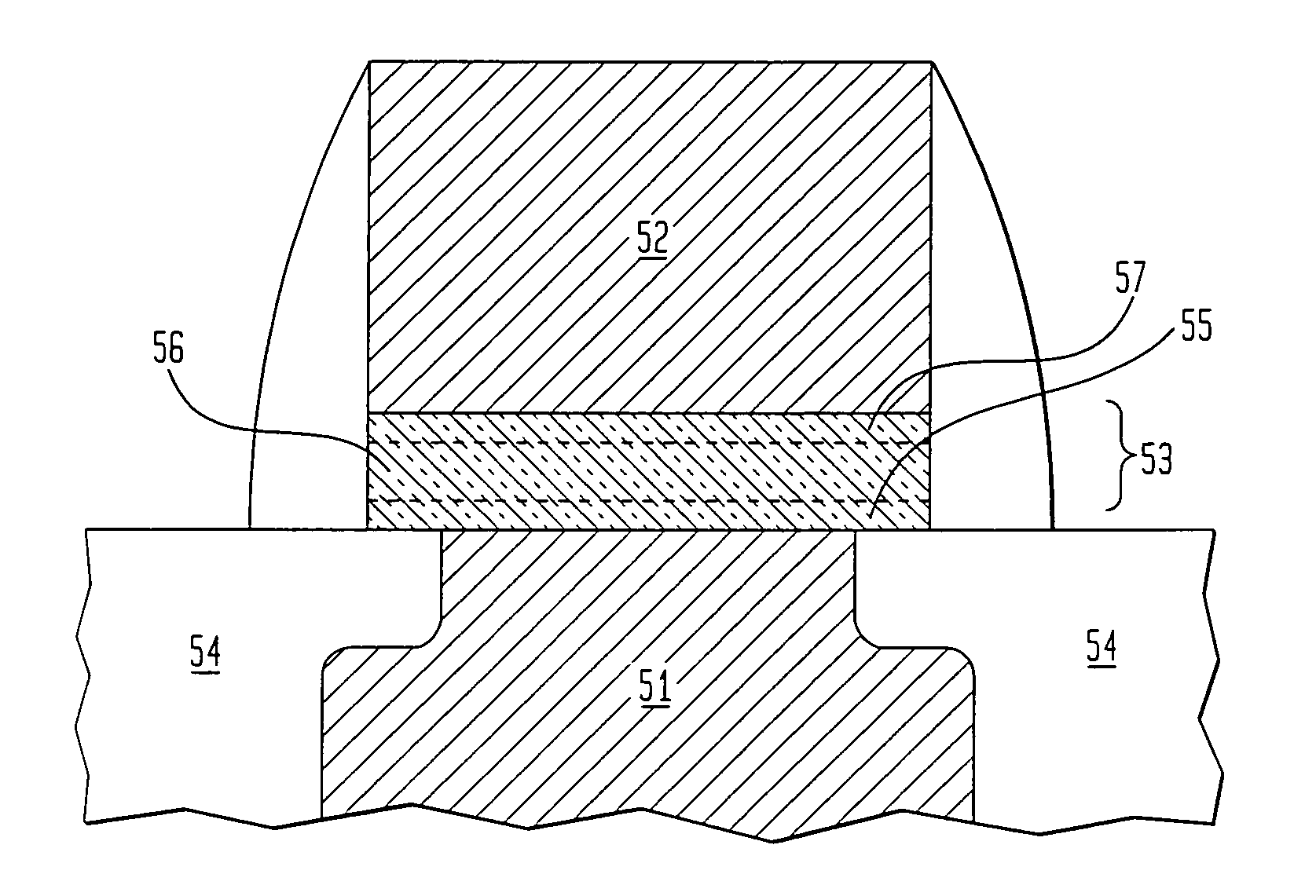
System for hot-start amplification via a multiple emulsion
System, including methods, apparatus, compositions, and kits, for making and using compound droplets of a multiple emulsion to supply an amplification reagent, such as a heat-stable DNA polymerase or DNA ligase, to an aqueous phase in which the compound droplets are disposed. The compound droplets may be induced to supply the amplification reagent by heating the multiple emulsion, to achieve hot-start amplification.
Owner:BIO RAD LAB INC
Oil-based settable spotting fluid
InactiveUS6524384B2Increase displacementHigh gel strengthDrilling compositionCoatingsEmulsionWater source
Oil-based settable spotting fluid compositions and methods of using the compositions are provided. The oil-based settable spotting fluid compositions are basically comprised of oil, an emulsifying surfactant for emulsifying the oil with water whereby an oil-external emulsion is formed, a de-emulsifying surfactant which in time de-emulsifies said oil-external emulsion when the emulsion is contacted with external water, a hydraulic settable component selected from the group consisting of ASTM Class C or the equivalent fly ash and ASTM Class F or the equivalent fly ash together with a source of calcium and water selected from the group consisting of fresh water and salt water.
Owner:HALLIBURTON ENERGY SERVICES INC
Emulsions and methods of making nanocarriers
This invention relates, in part, to methods of using emulsions for making synthetic nanocarriers and the synthetic nanocarriers formed by such methods.
Owner:SELECTA BIOSCI
Process and applications of carbon nanotube dispersions
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method for gas-solid contacting in a bubbling fluidized bed reactor
InactiveUS6894183B2Eliminate and drastically reduce bypassEffective contactThermal non-catalytic crackingCatalytic crackingForming gasSolid particle
Owner:COUNCIL OF SCI & IND RES
Microchannel apparatus and method of producing emulsions making use thereof
Owner:NORINSUISANSHO SHOKUHIN SOGO KENKYUSHOCHO +1
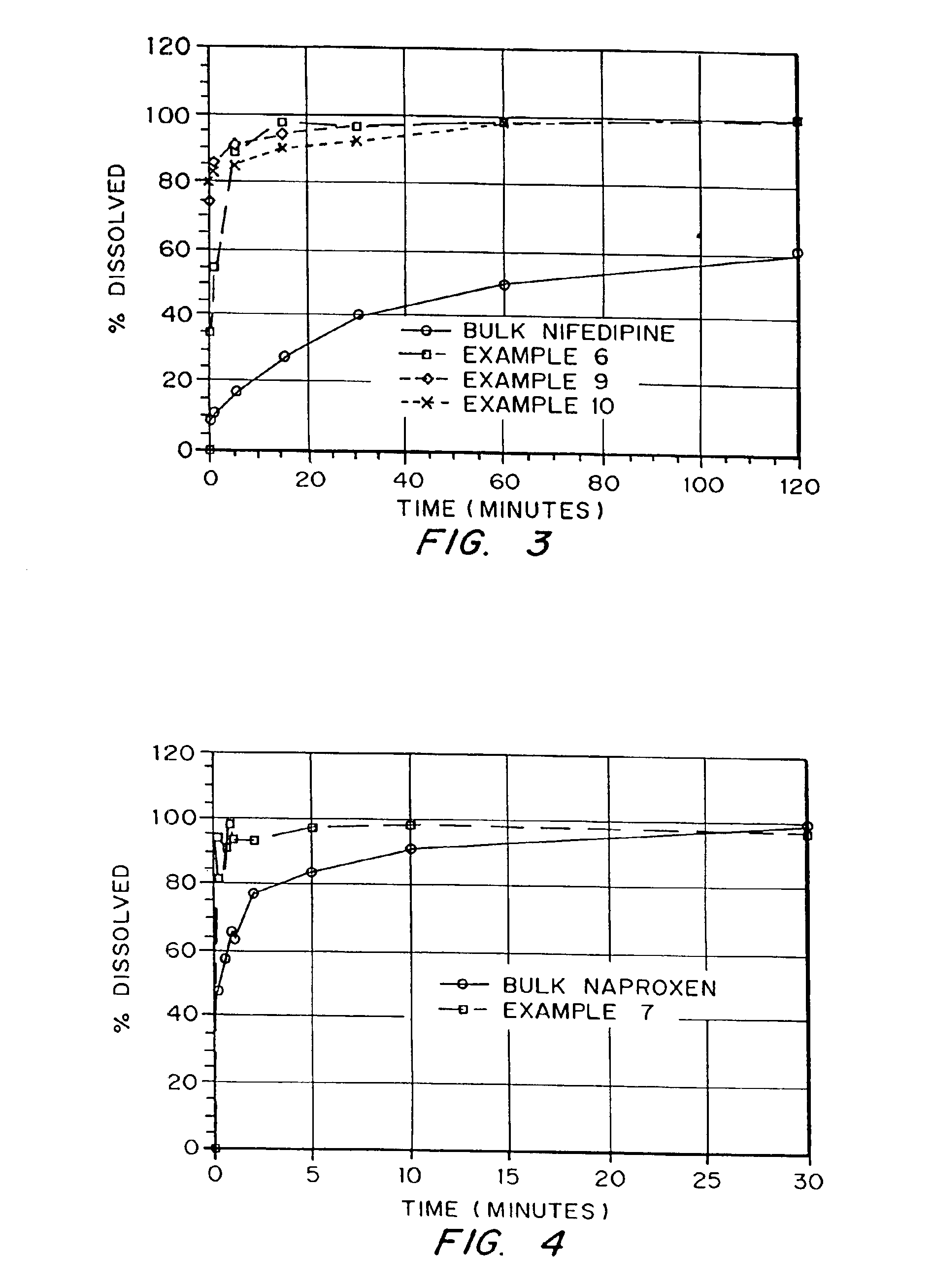
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Oil-based settable spotting fluid
InactiveUS6315042B1Increase displacementHigh gel strengthFlushingDrilling compositionEmulsionWater source
Oil-based settable spotting fluid compositions and methods of using the compositions are provided. The oil-based settable spotting fluid compositions are basically comprised of oil, an emulsifying surfactant for emulsifying the oil with water whereby an oil-external emulsion is formed, a de-emulsifying surfactant which in time de-emulsifies said oil-external emulsion when the emulsion is contacted with external water, a hydraulic settable component selected from the group consisting of ASTM Class C or the equivalent fly ash and ASTM Class F or the equivalent fly ash together with a source of calcium and water selected from the group consisting of fresh water and salt water.
Owner:HALLIBURTON ENERGY SERVICES INC
Manufacture of stable low particle size organopolysiloxane emuslion
ActiveUS20070276087A1Rapid emulsificationReduce polymerization timeMaterial nanotechnologyOther chemical processesPolymer scienceEmulsion
Stable high viscosity organopolysiloxane emulsions with particle sizes up to 150 nanometer may be made in a simple and cost-effective manner employing a standard homogenizer, and optional subsequent polymerization of the organopolysiloxan at controlled temperature. A combination of non-ionic emulsifier together with an at least one anionic emulsifier is employed, having an HLB value 12-15, while maintaining a temperature up to 50° C.
Owner:WACKER CHEM GMBH
Method of producing morphologically uniform microcapsules and microcapsules produced by this method
InactiveUS6294204B1Readily water-miscibleSpeed up concentrationPowder deliveryBiocidePolymer dissolutionEmulsion
The invention relates to a process for the production of morphologically uniform microcapsules that contain peptides, proteins or other water-soluble biologically active substances as active ingredients as well as microcapsules that are produced according to this process with a degree of concentration of between 3 to 30% by weight and a diameter<=8 mum.According to the invention, biodegradable polymers are dissolved in a halogen-free solvent or solvent mixture, and the buffered active ingredient solution, which has a pH of between 6.0 to 8.0, is dispersed into this solution. Then, an aqueous solution that contains a surface-active substance (W / O / W-emulsion) is added to this W / O-emulsion, and the solvent is removed.The microcapsules that are produced with this process do not show any tendency toward agglomeration. The encapsulation efficiency of the process is approximately 90 to 95%.
Owner:ALRISE BIOSYST
Solid matrix therapeutic compositions
The present invention is directed to a solid porous matrix comprising a surfactant in combination with a bioactive agent. The solid porous matrix may be prepared by combining a surfactant and a therapeutic, together with a solvent, to form an emulsion containing random aggregates of the surfactant and the therapeutic, and processing the emulsion by controlled drying, or controlled agitation and controlled drying to form the solid porous matrix.
Owner:IMARX THERAPEUTICS
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com