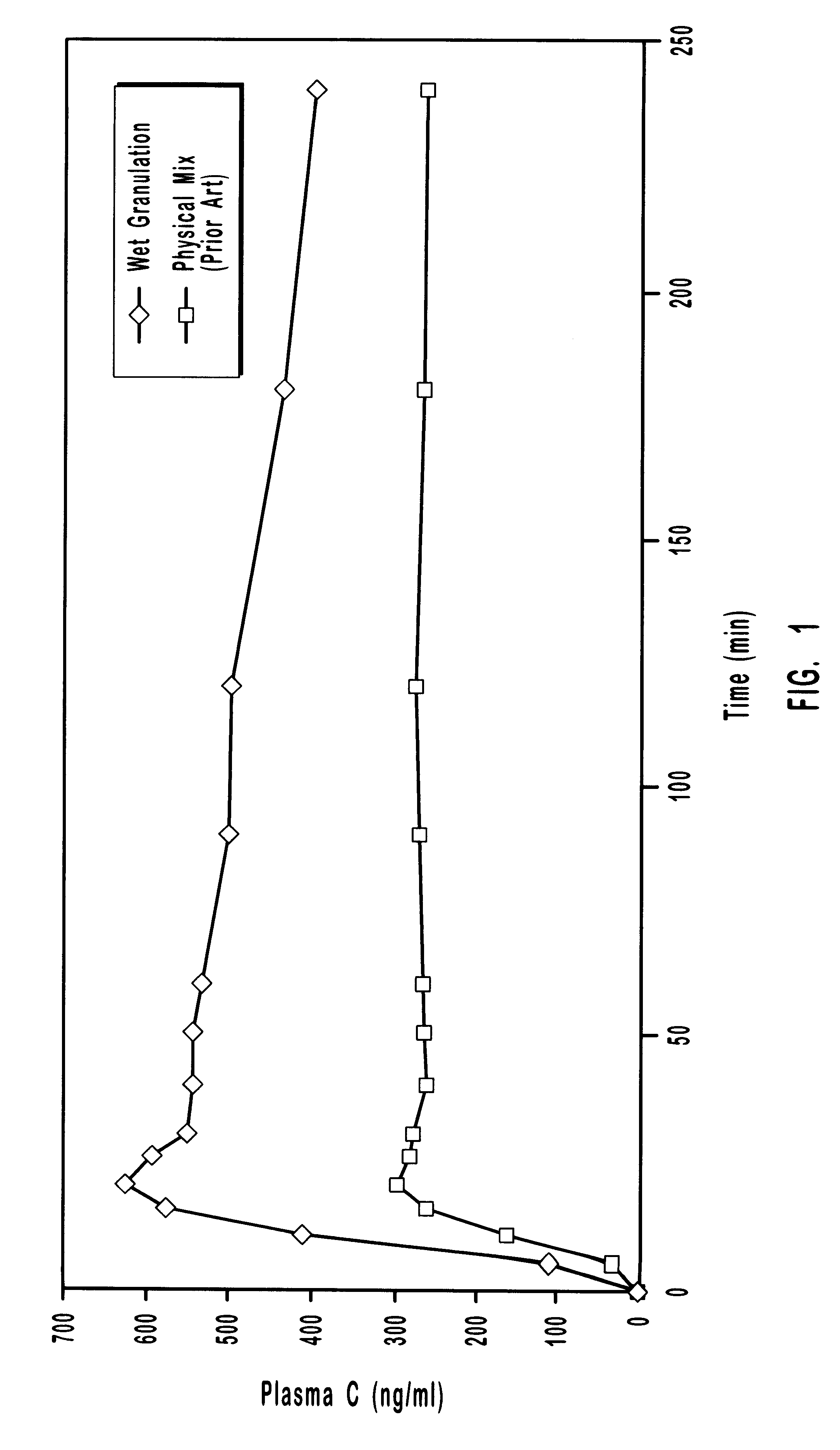
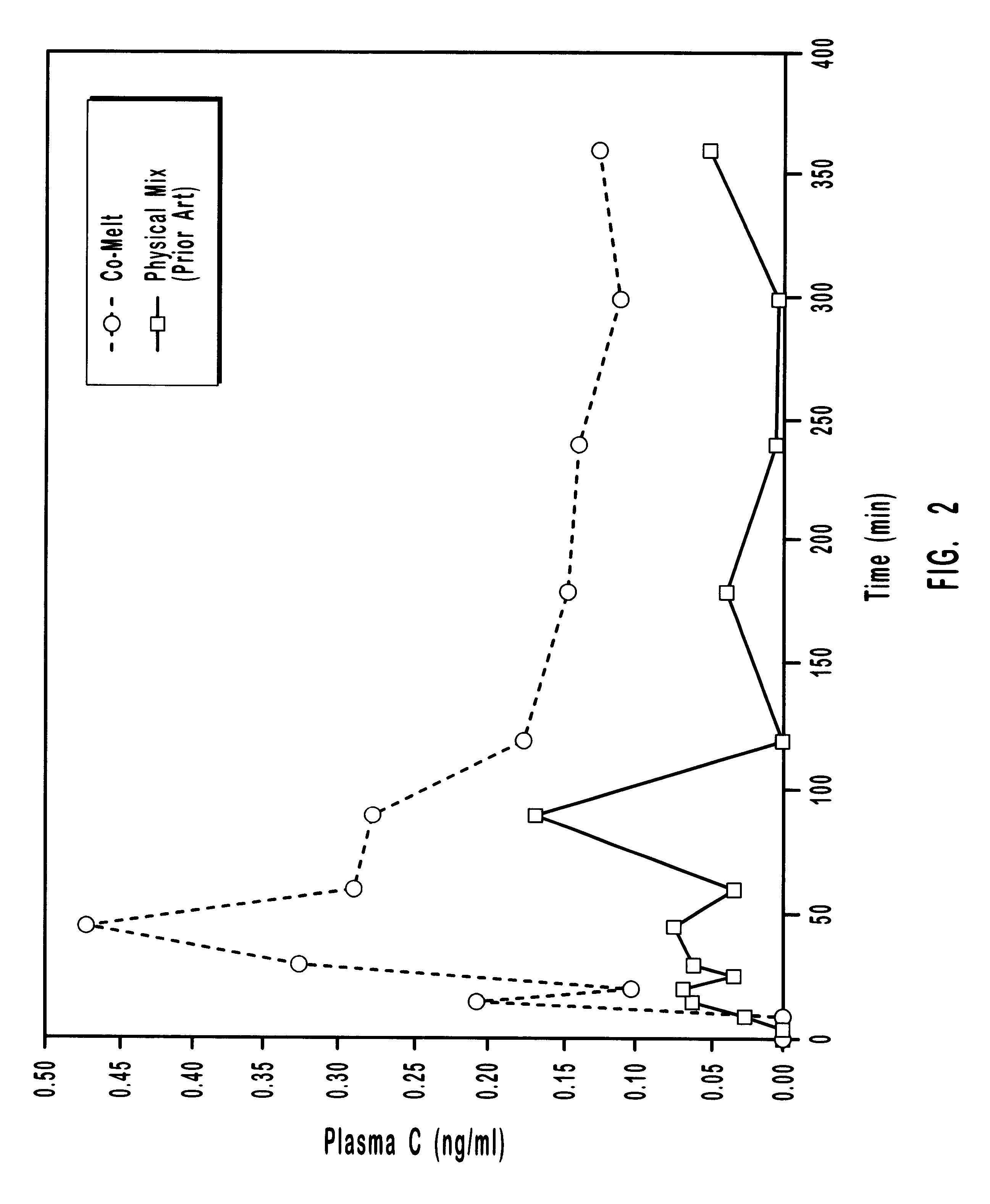
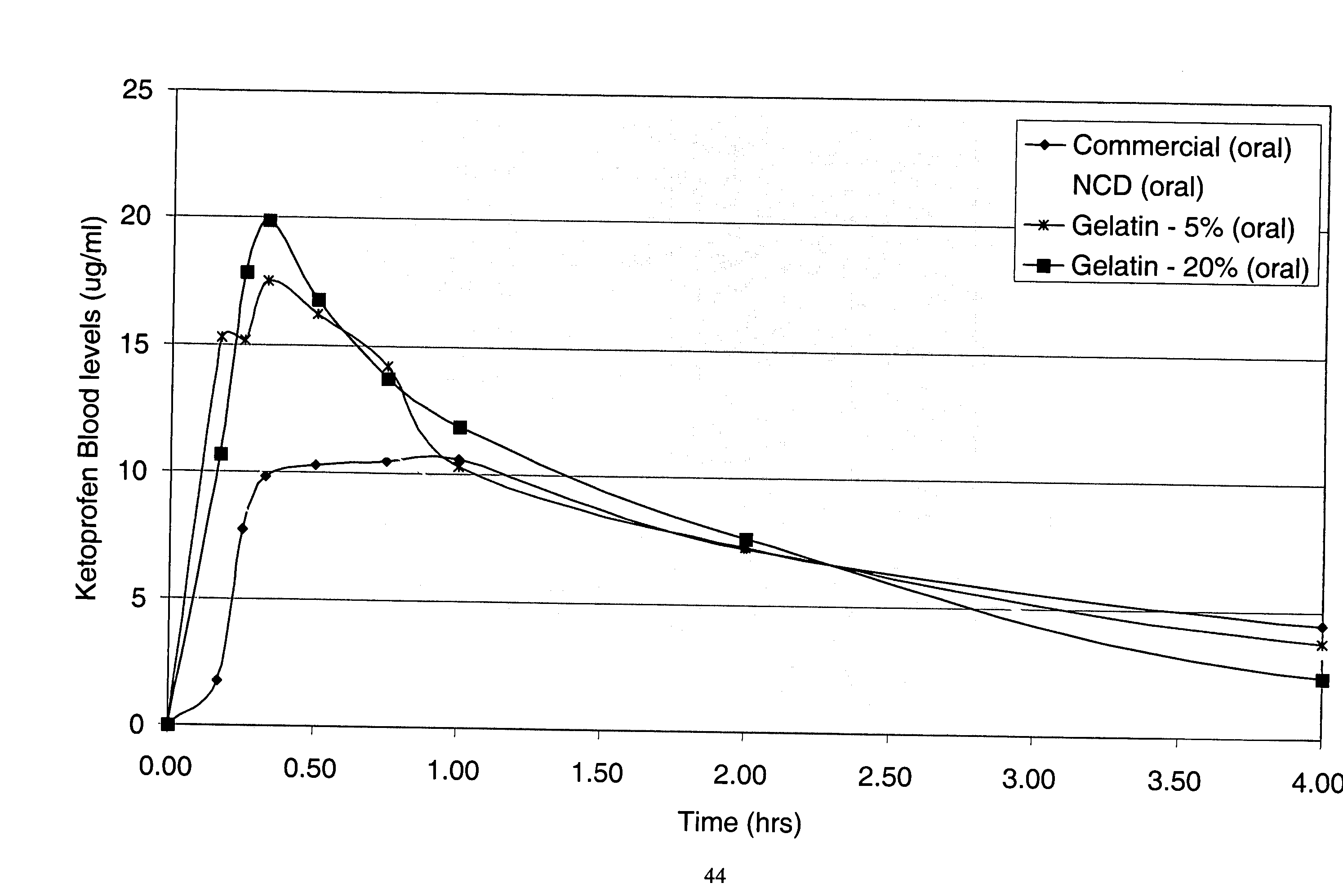
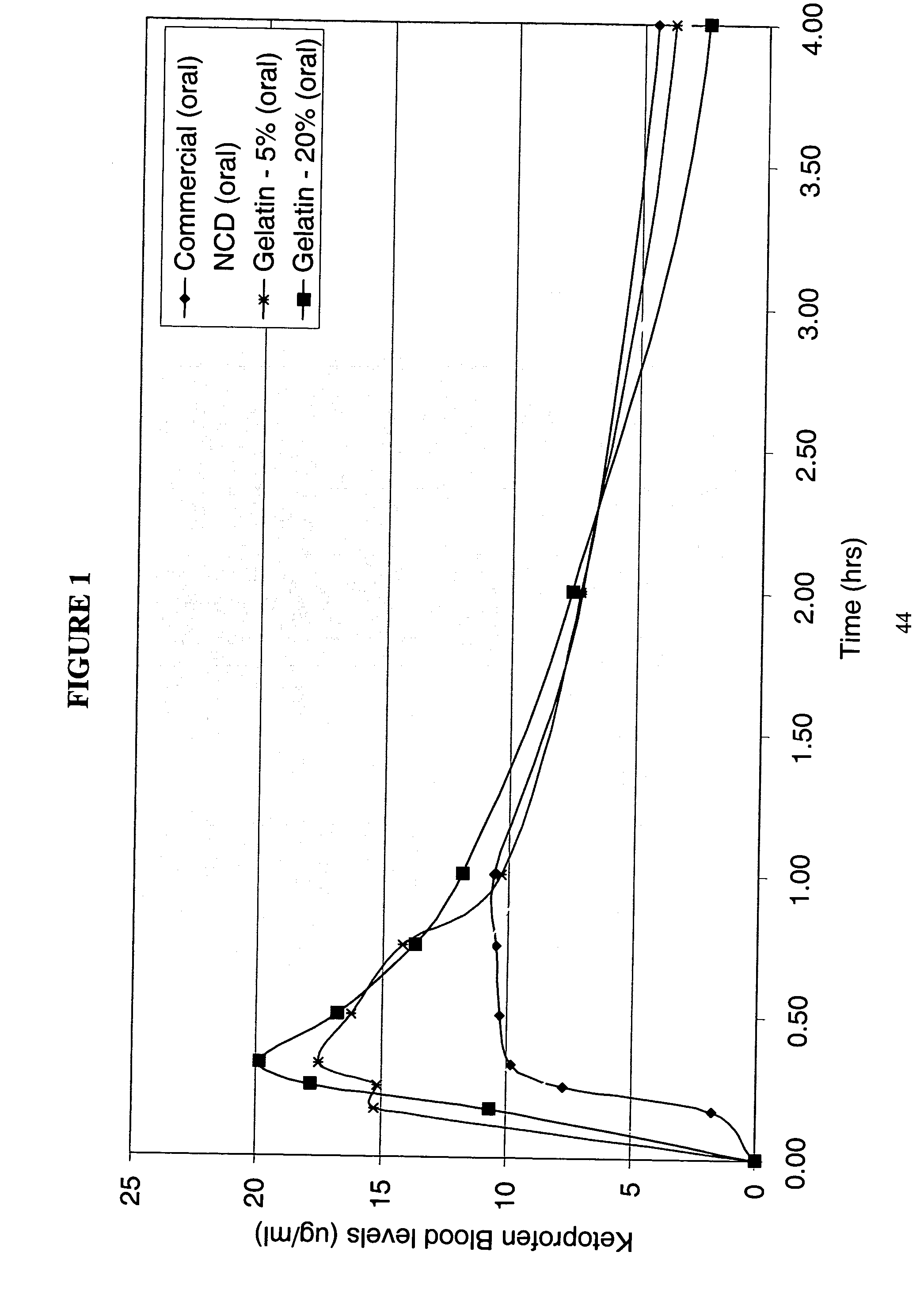
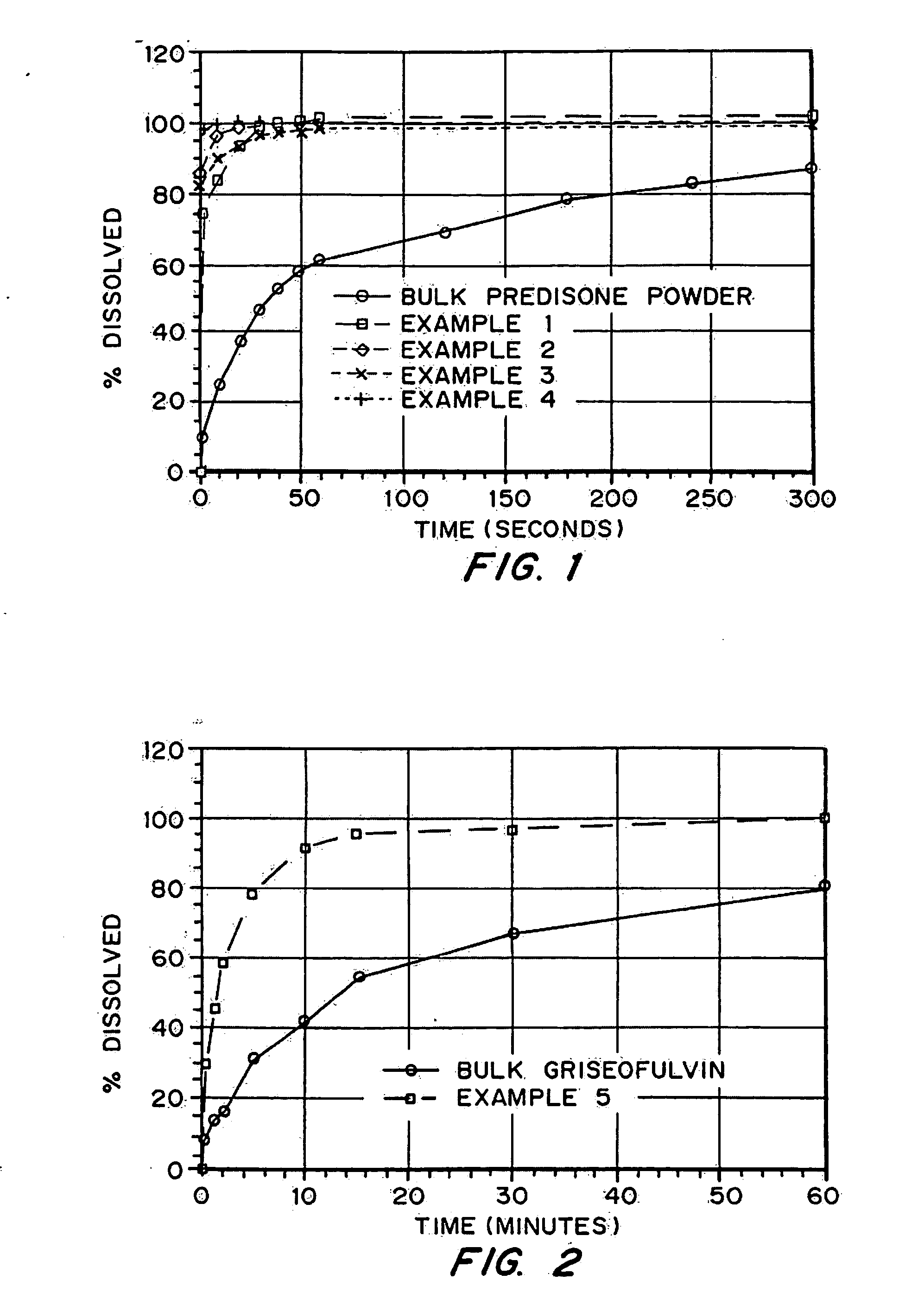
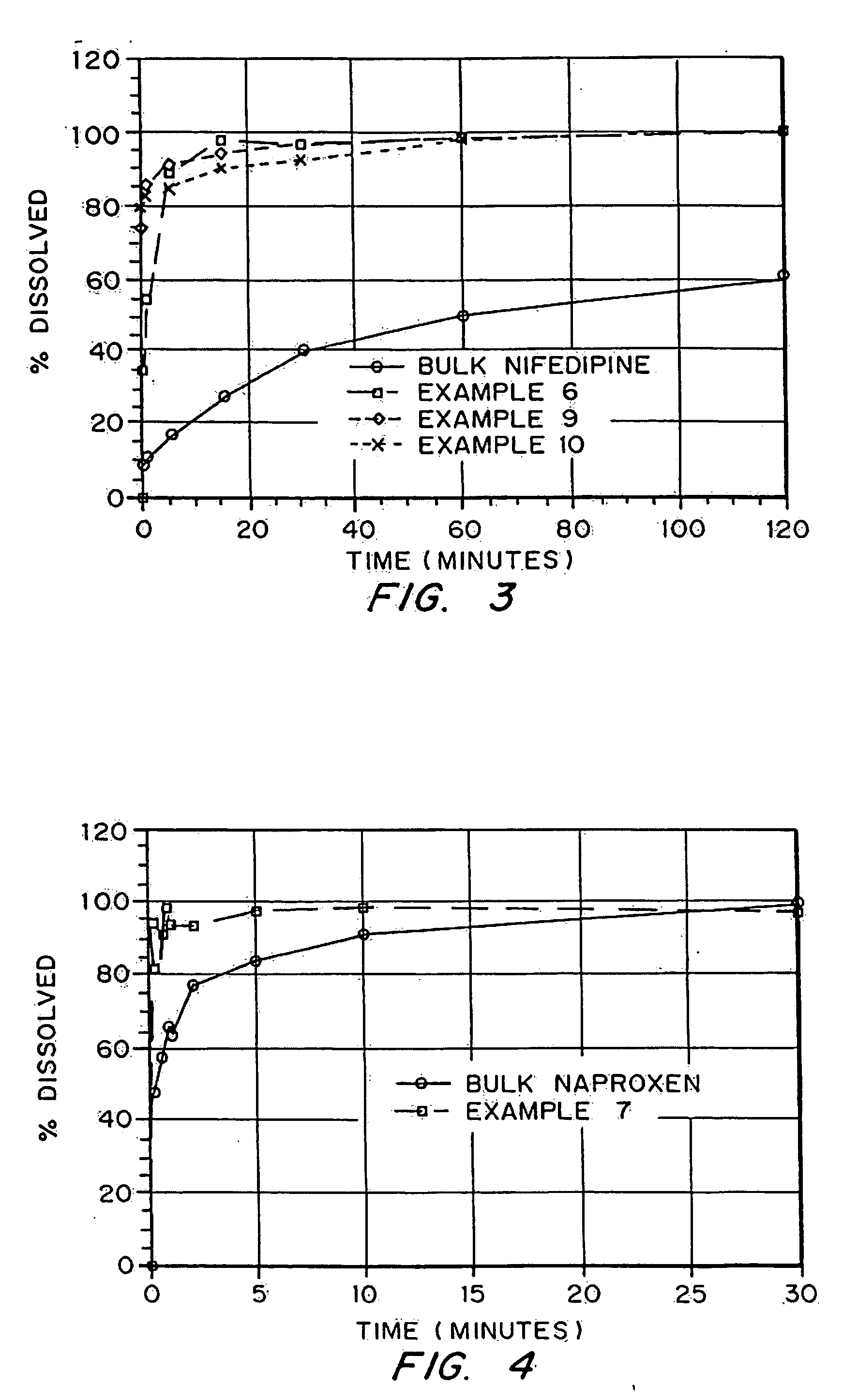
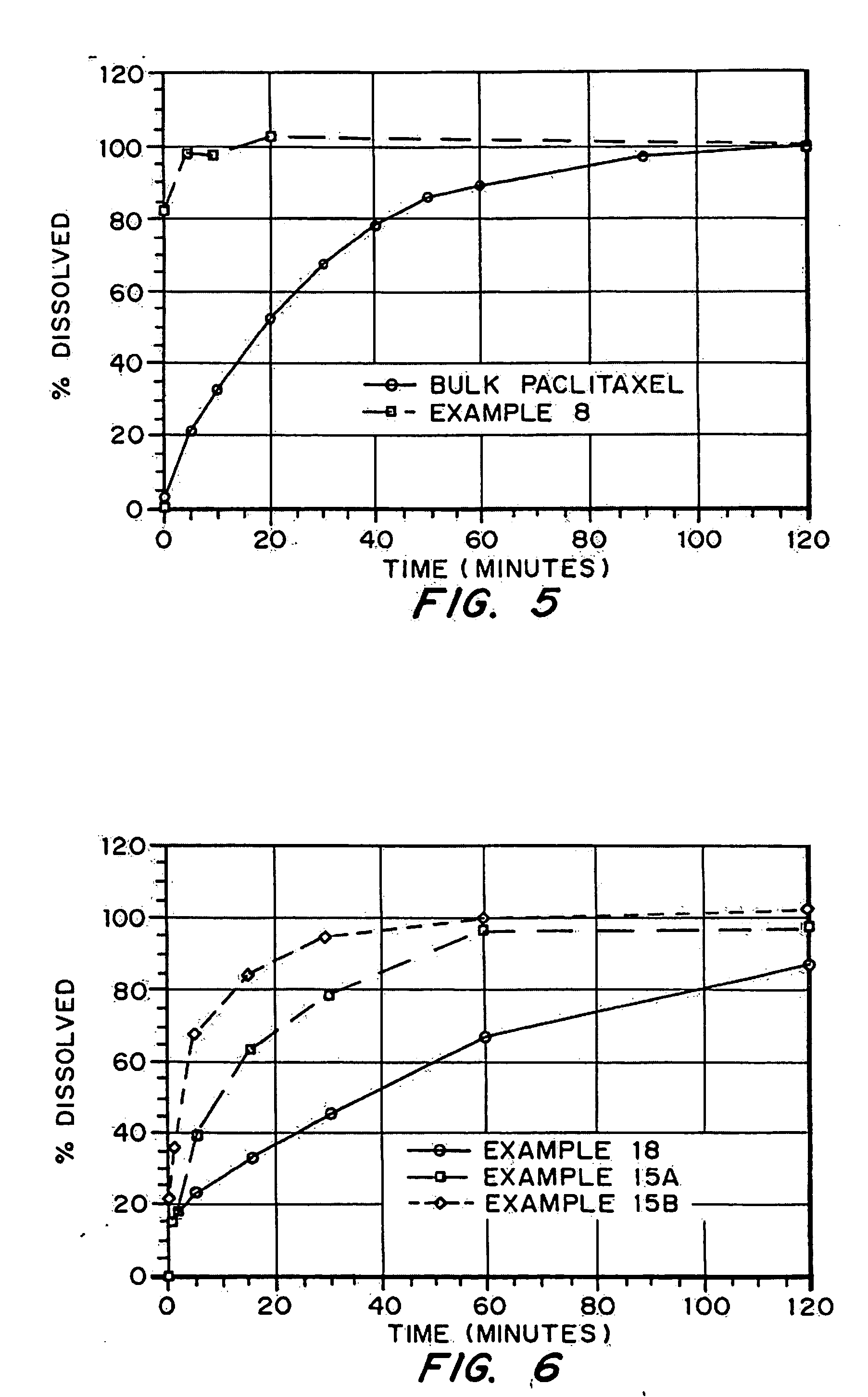
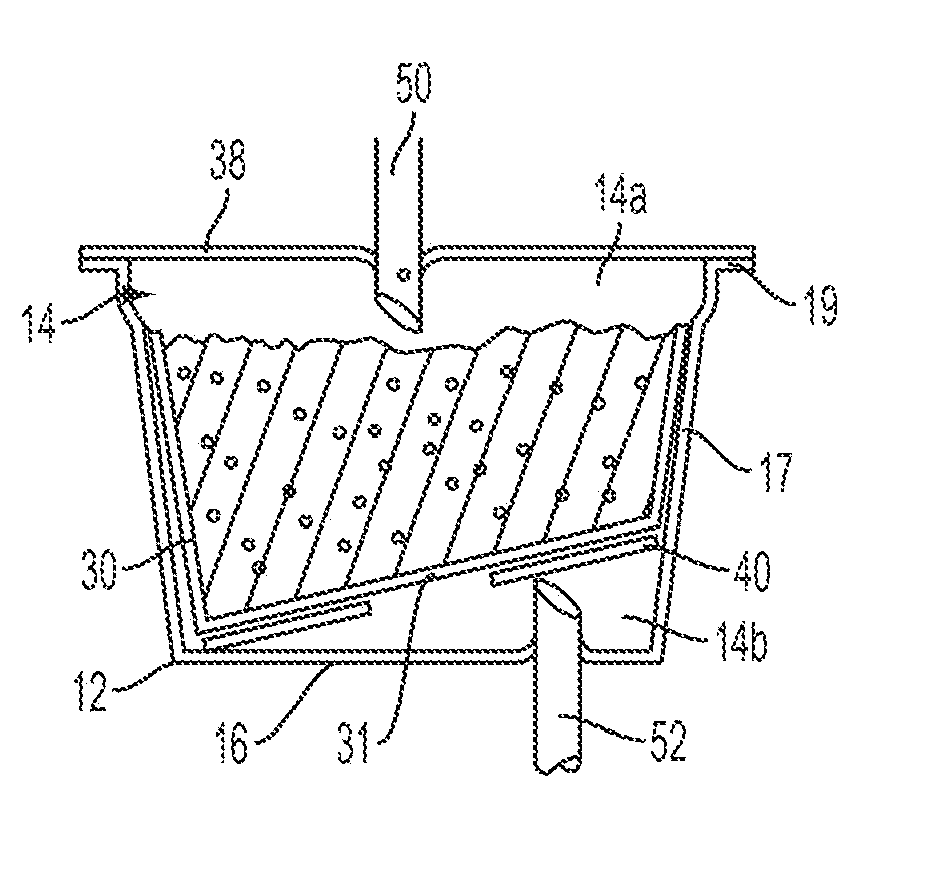
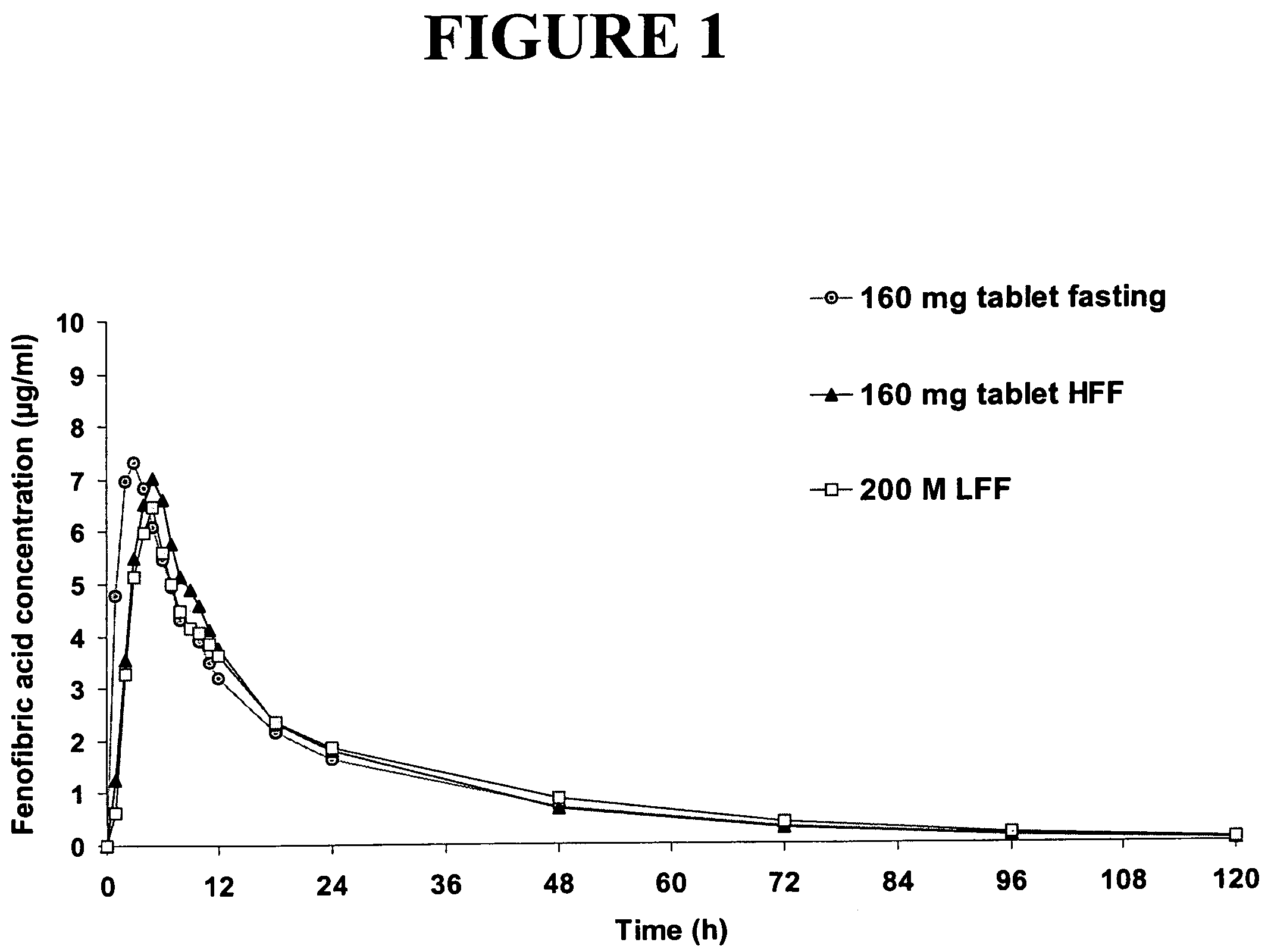
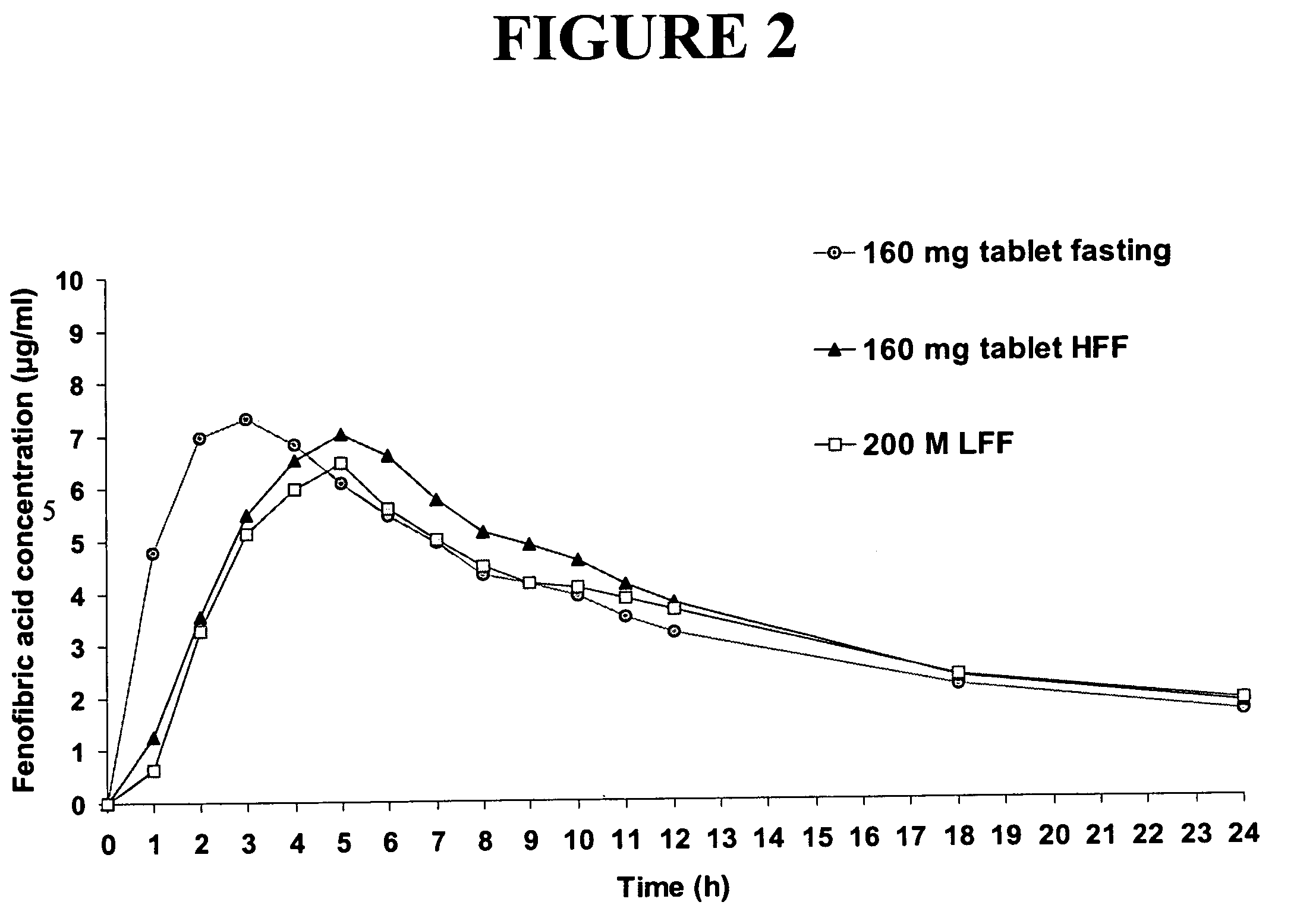
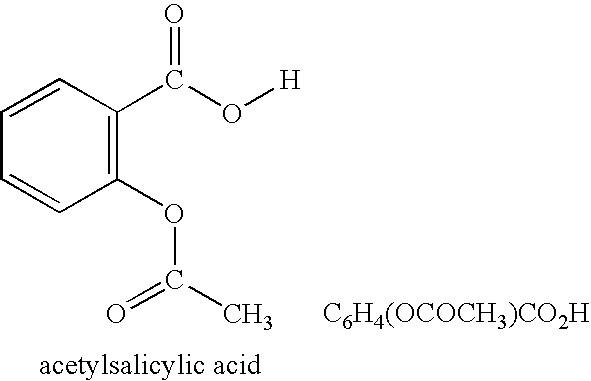
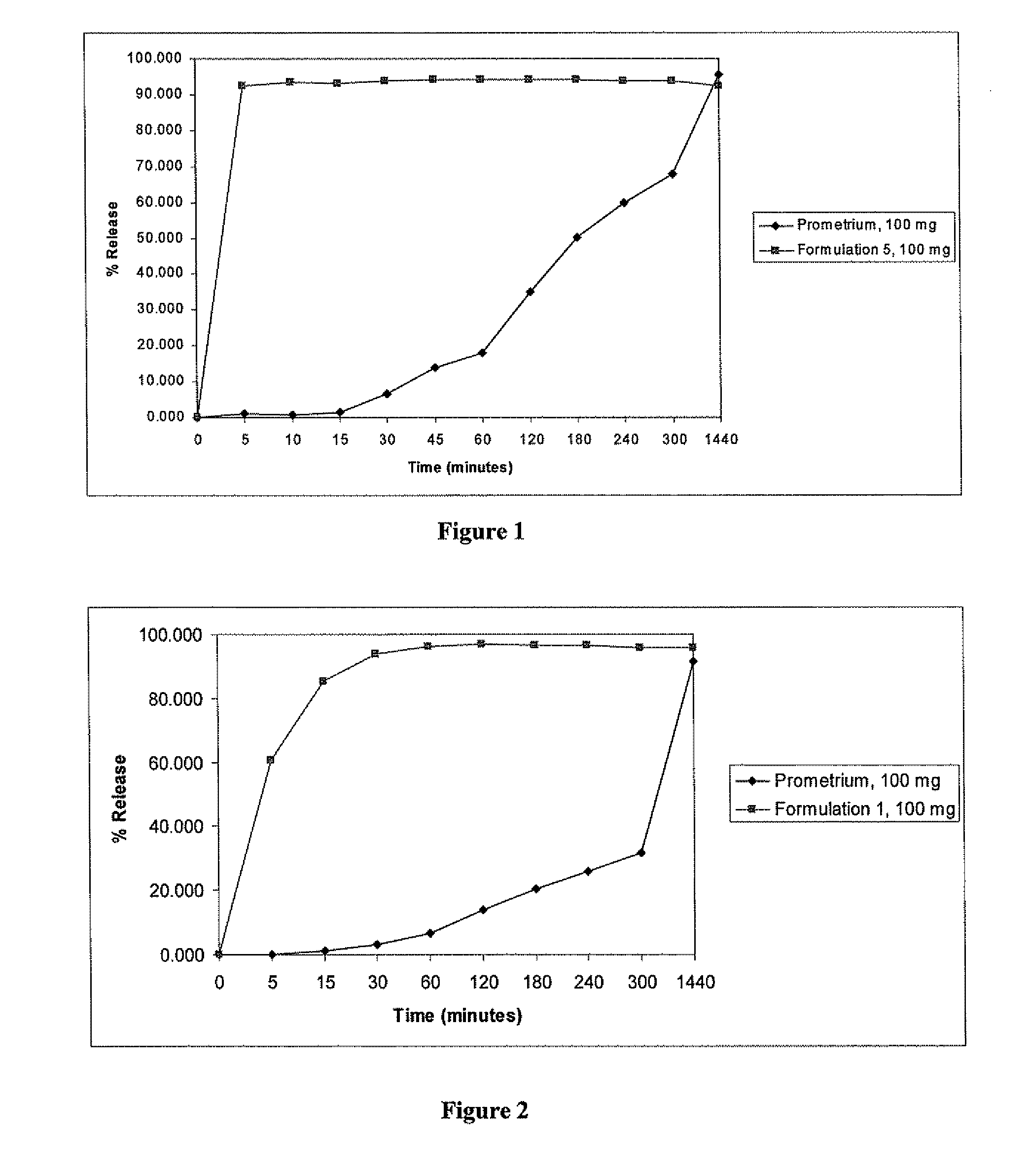
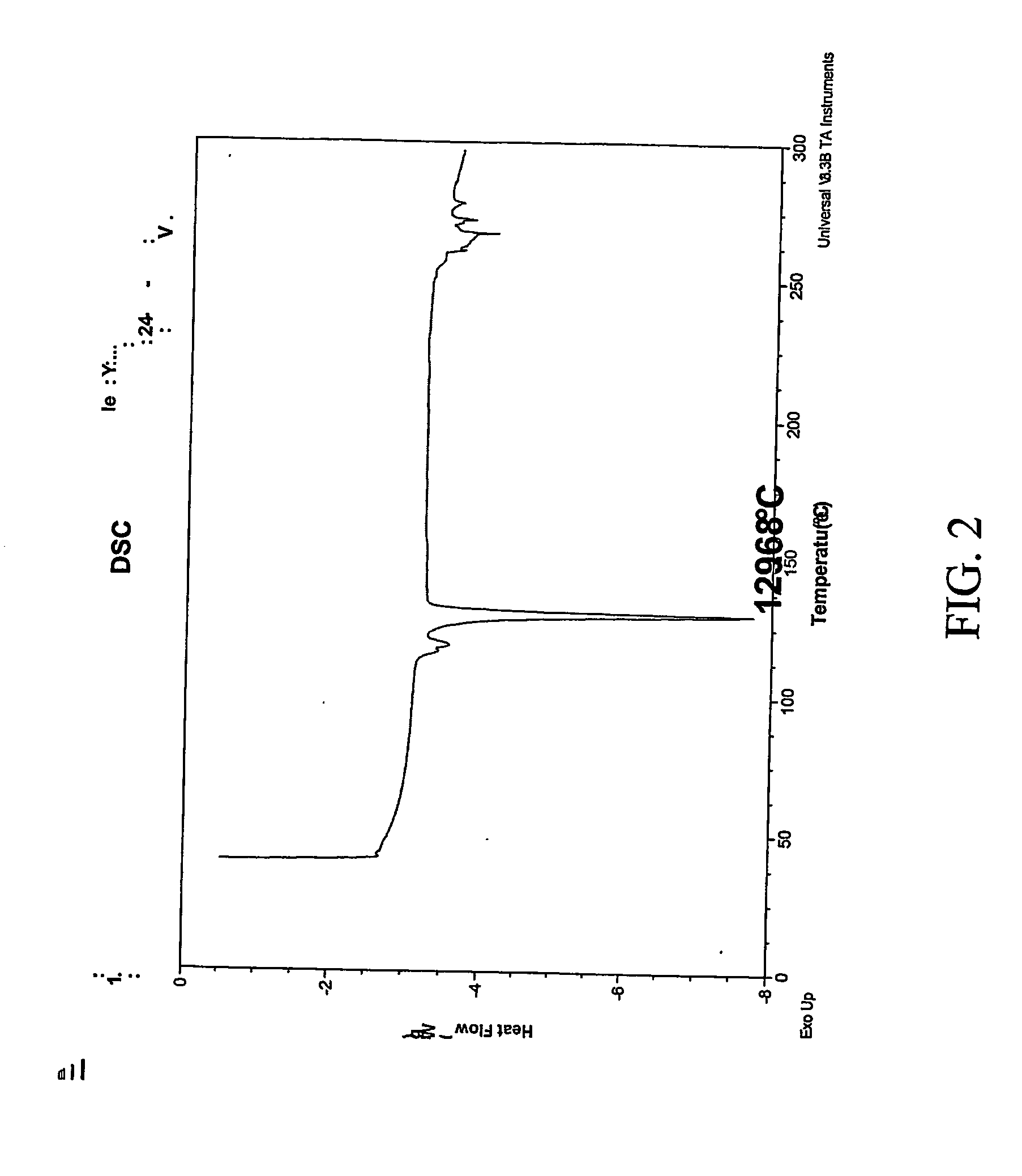
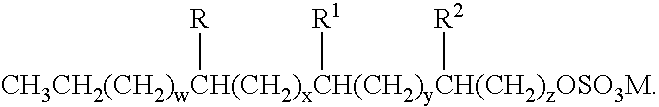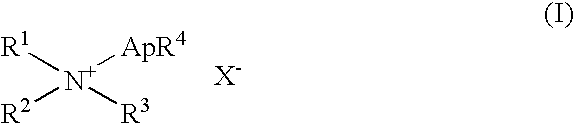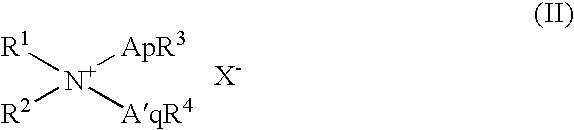Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4362results about How to "High dissolution rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
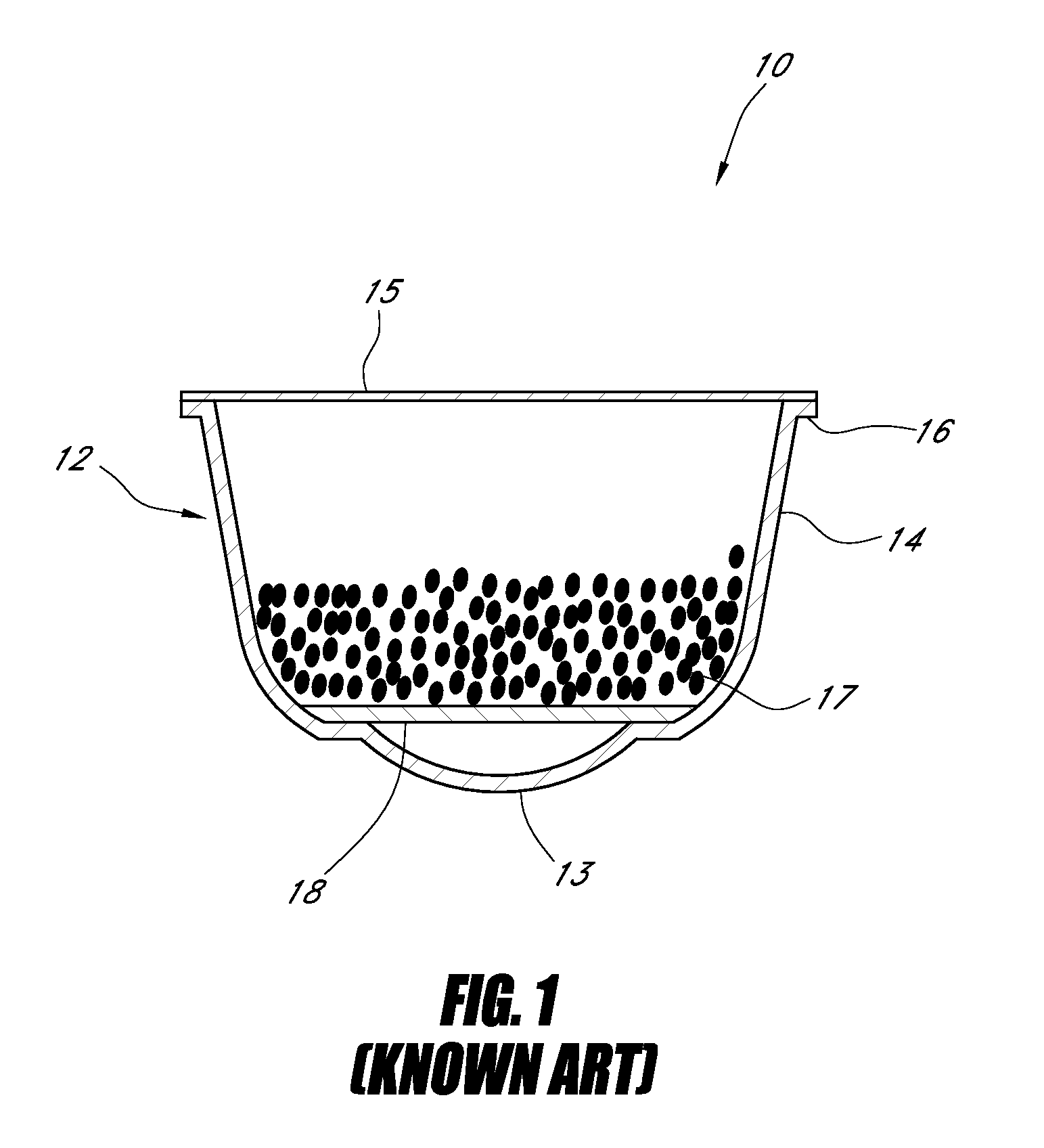
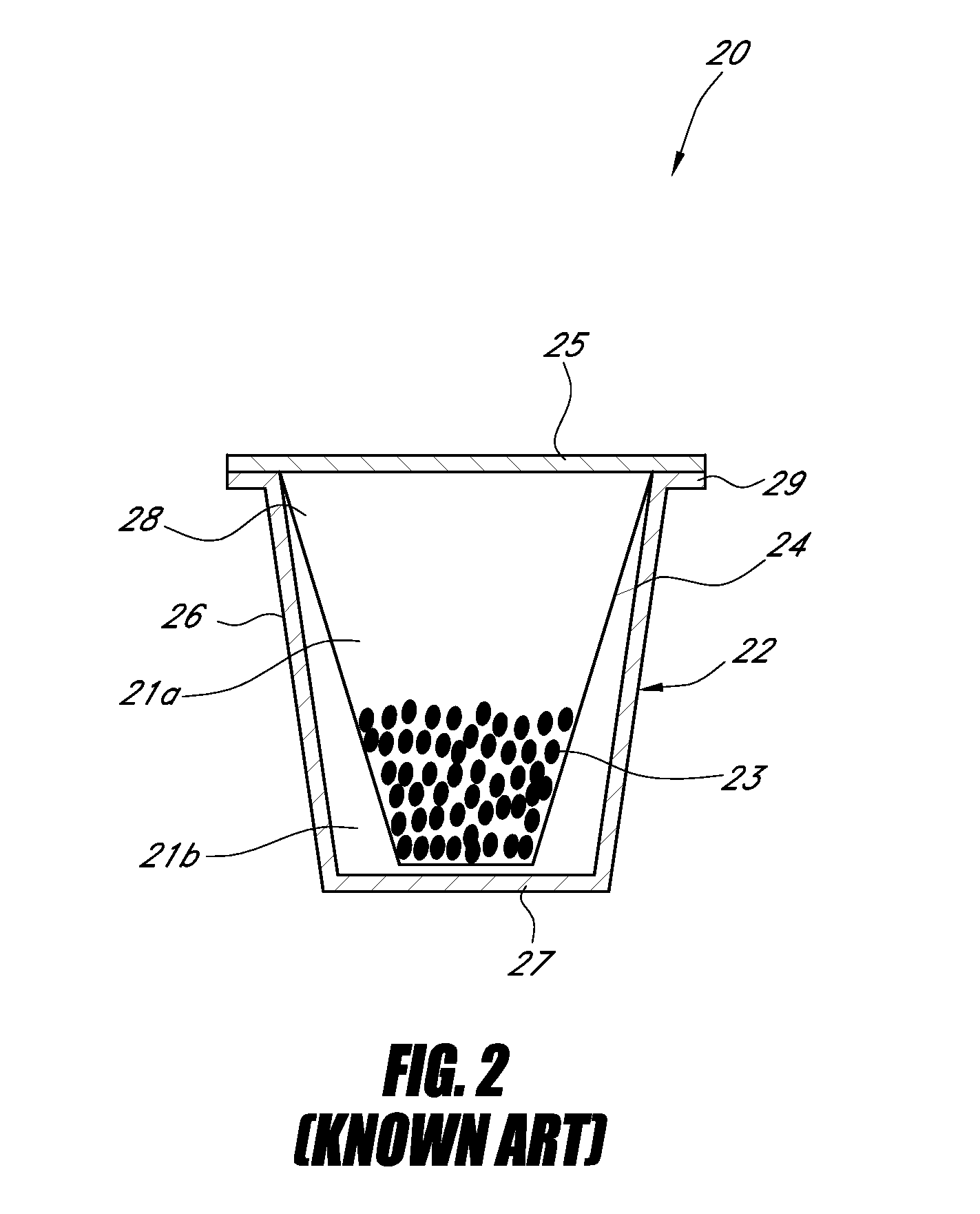
Controlling the dissolution of dissolvable polymer components in plural component fibers
The dissolution of dissolvable components in plural component polymer fibers is achieved by providing a polymer fiber including at least two sections, where at least one fiber section includes a dissolvable component. The rate at which at least part of the fiber dissolves is controlled by at least one of a fiber section having a non-round cross-sectional geometry, and at least two fiber sections including two different dissolvable components. In an exemplary embodiment, island-in-the-sea fibers are formed with non-round and elongated cross-sectional geometries. In another embodiment, sheath-core fibers are formed in which the sheath and core include different dissolvable components.
Owner:HILLS CO
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Gel stabilized nanoparticulate active agent compositions
ActiveUS20050031691A1Improve stabilityImprove dissolutionPowder deliveryBiocideActive agentAverage diameter
Disclosed is a solid or semi-solid gelatin nanoparticulate active agent dosage form comprising at least one nanoparticulate active agent and at least one gel forming substance which exhibits gelation sufficient to retain excess water in the solid or semi-solid gelatin form. The active agent particles have an effective average diameter prior to inclusion in the dosage form of less than about 2000 nm. The dosage form of the invention has the advantages of easy administration combined with rapid dissolution of the active agent following administration.
Owner:ALKERMES PHARMA IRELAND LTD
Pharmaceutical co-crystal compositions
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Beverage cartridge and method for beverage formation using filter aid
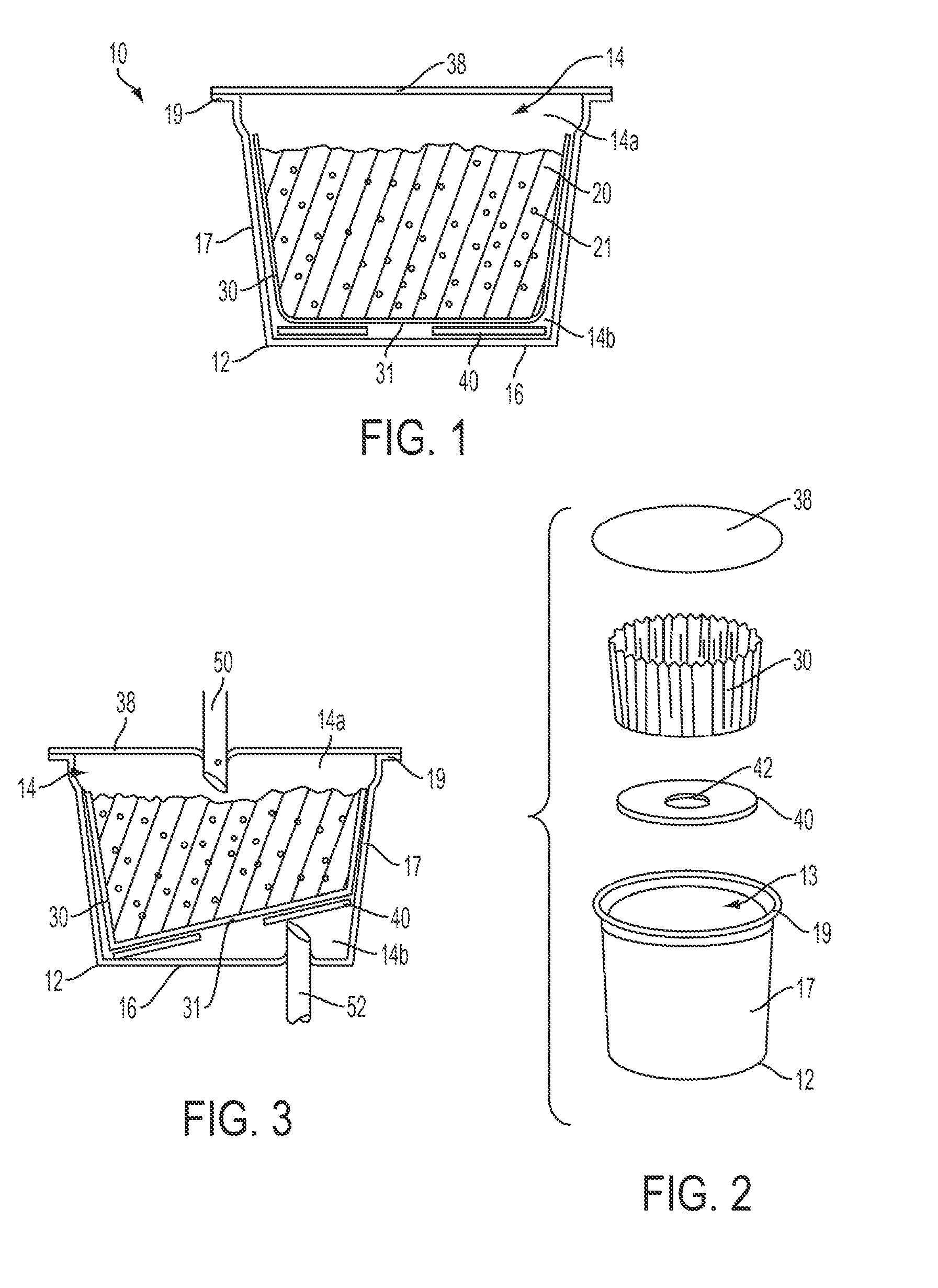
InactiveUS20110076361A1Speed up circulationReduce cloggingTea extractionBeverage vesselsParticulatesCellulose
A method and apparatus for forming a beverage involves the use of a filter aid, e.g., that is provided in a beverage cartridge with a beverage medium in a dry state. In one embodiment, a cartridge including a dried fruit material may also include a filter aid, such as perlite, diatomaceous earth or cellulose, that is mixed together with the fruit material. The filter aid may assist in flow through the beverage medium or through a filter, e.g., by helping prevent the clogging of pores of a filter used to remove particulate from a beverage formed by interaction of the beverage medium with water introduced into the cartridge. Thus, the filter aid may permit the use of some beverage media that would otherwise clog a filter and / or prevent proper dissolution of materials in the beverage media without the filter aid.
Owner:GREEN MOUNTAIN COFFEE ROASTERS INC
Film packaged product portion and method for producing the same
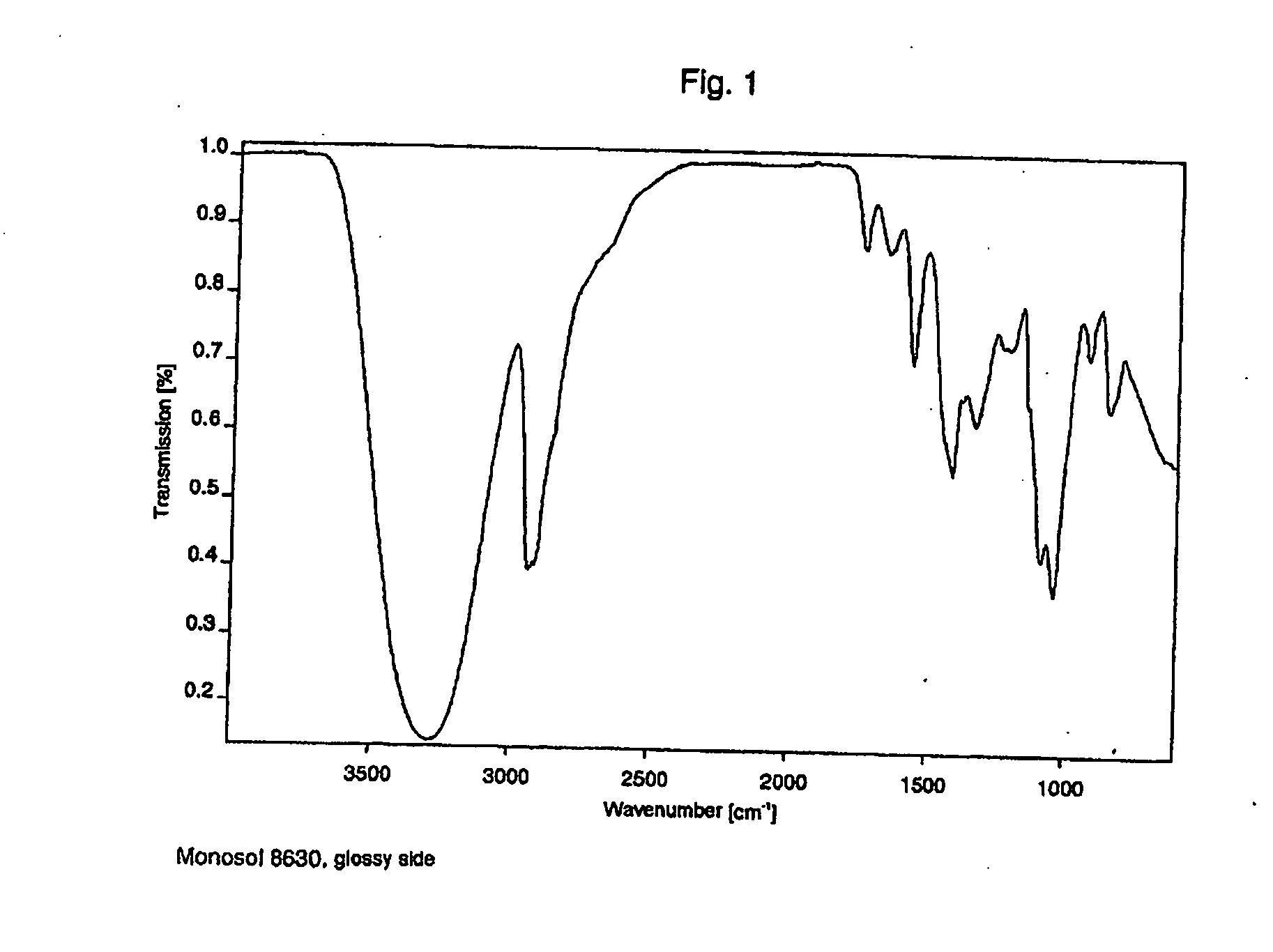
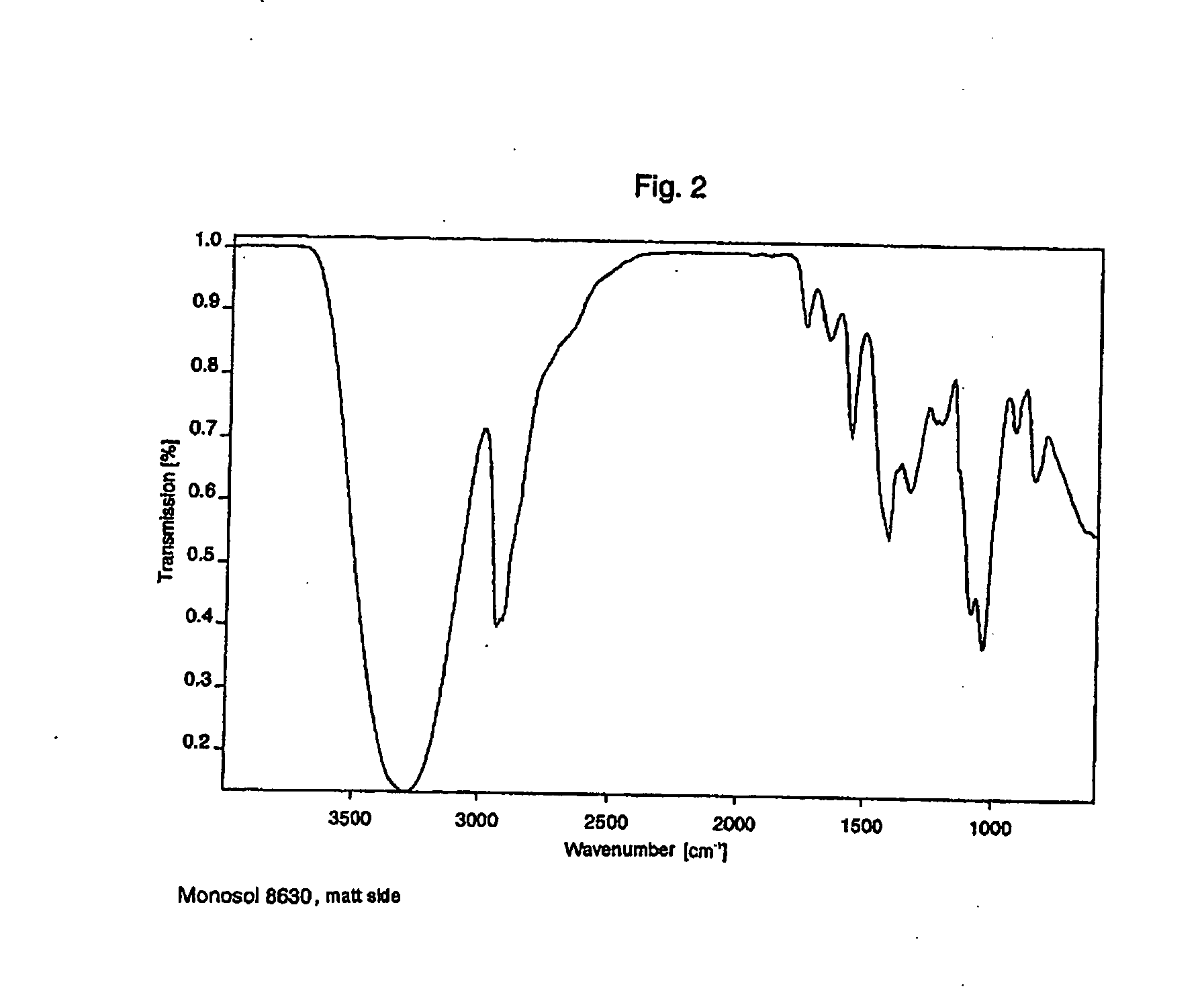
InactiveUS20060213801A1Improved film solubilityImprove dissolution rateSynthetic resin layered productsPackaging corrosive chemicalsEngineeringProduct Part
The invention relates to a package formed from at least two films, containing product portions, in particular washing, cleaning and / or care products, which are released when the package is opened, by dissolution or disintegration of at least one film. The package comprises at least one compartment for receiving the product. At least one film of the package is substantially plastically reshaped in order to provide a compartment to receive the product. The disintegration and / or dissolving times of the package measured in distilled water at a temperature of 10° C. are less than similar times for a conventional film package made, for example of a Mono-Sol-M-8630 or an equivalent film measured under the same conditions. The improved disintegration and / or dissolving time may be enhanced by providing a striated surface structure and / or small holes whose diameter ranges from 1 μm to 50 μm.
Owner:HENKEL KGAA
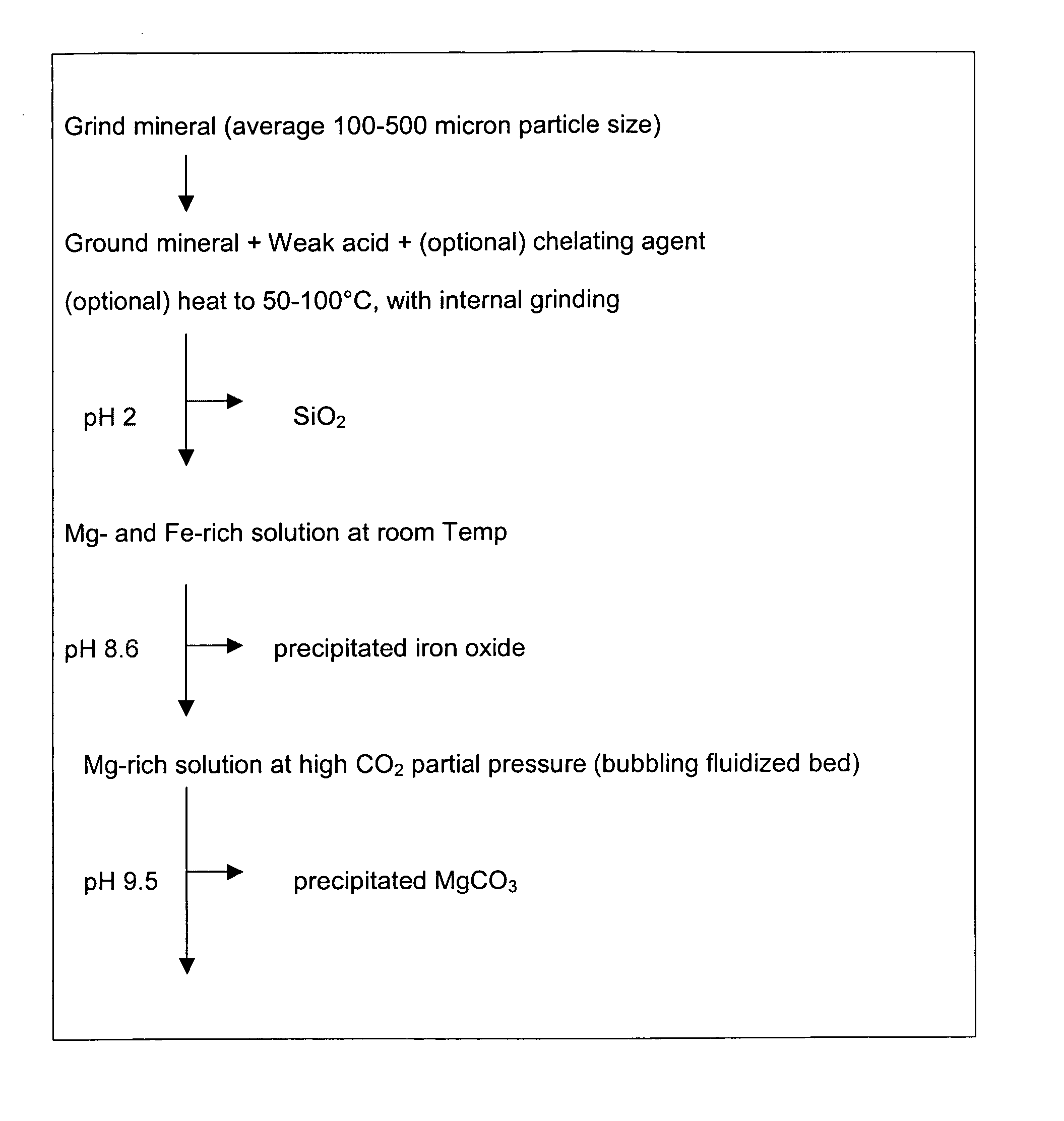
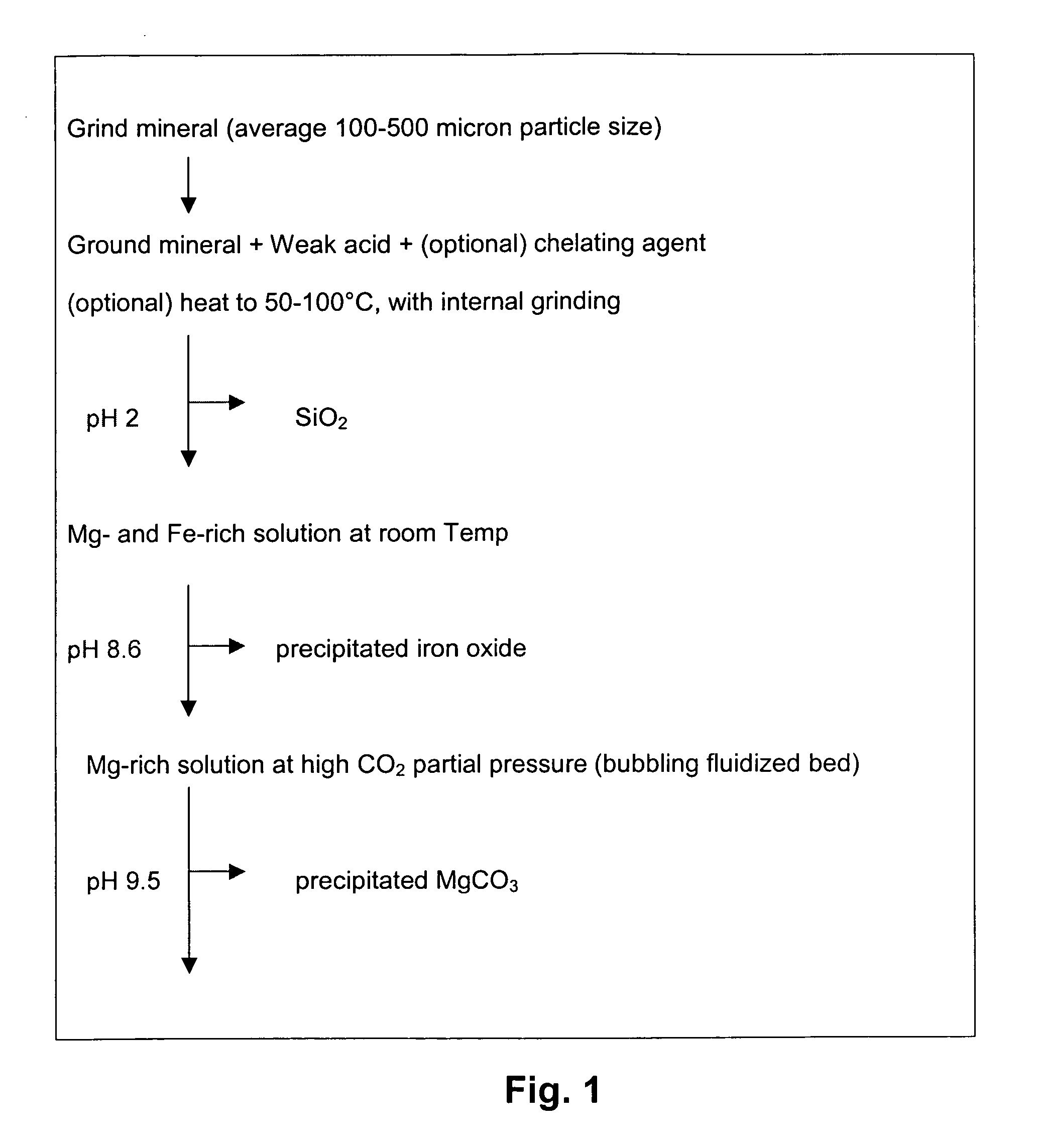
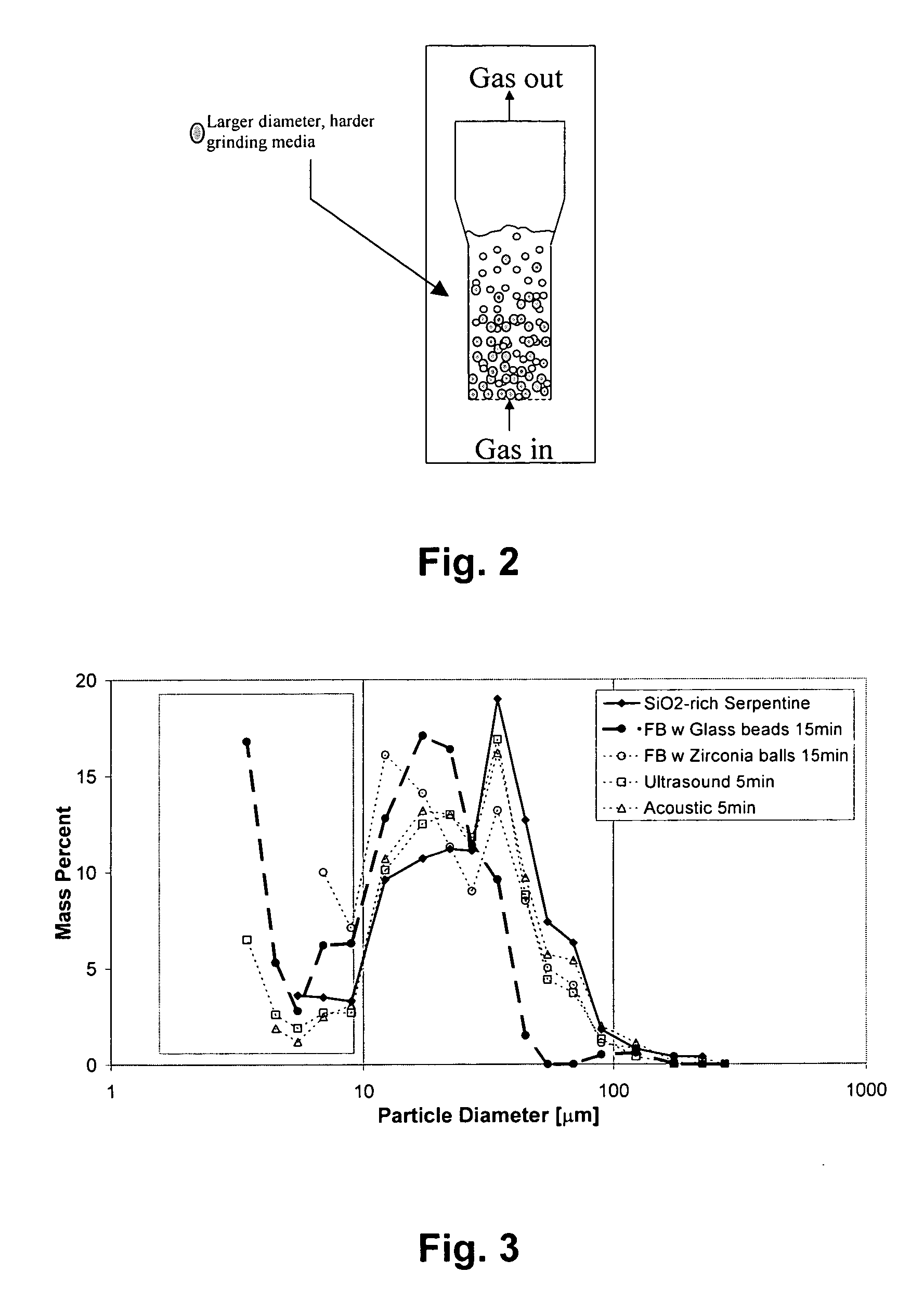
Carbon dioxide sequestration using alkaline earth metal-bearing minerals
ActiveUS20050180910A1High dissolution rateEfficient removalCalcium/strontium/barium carbonatesProductsParticulatesAlkaline earth metal
A method for mineral sequestration of pollutant gases resulting from the combustion of carbon-based fuels such as carbon and sulfur dioxides is provided and includes, providing a particulate magnesium-containing mineral and exposing the magnesium-containing mineral to a weak acid to dissolve magnesium from the mineral and form a magnesium-containing solution. The surface of the particulate magnesium-containing mineral is physically activated to expose and dissolve additional magnesium into the solution. Pollutant gases such as carbon dioxide are mixed with the magnesium-containing solution. When the pH of the magnesium-containing solution is increased, solid magnesium carbonate is formed.
Owner:THE OHIO STATES UNIV
Porous drug matrices and methods of manufacture thereof
InactiveUS20050058710A1Fast dissolutionExtended half-lifePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
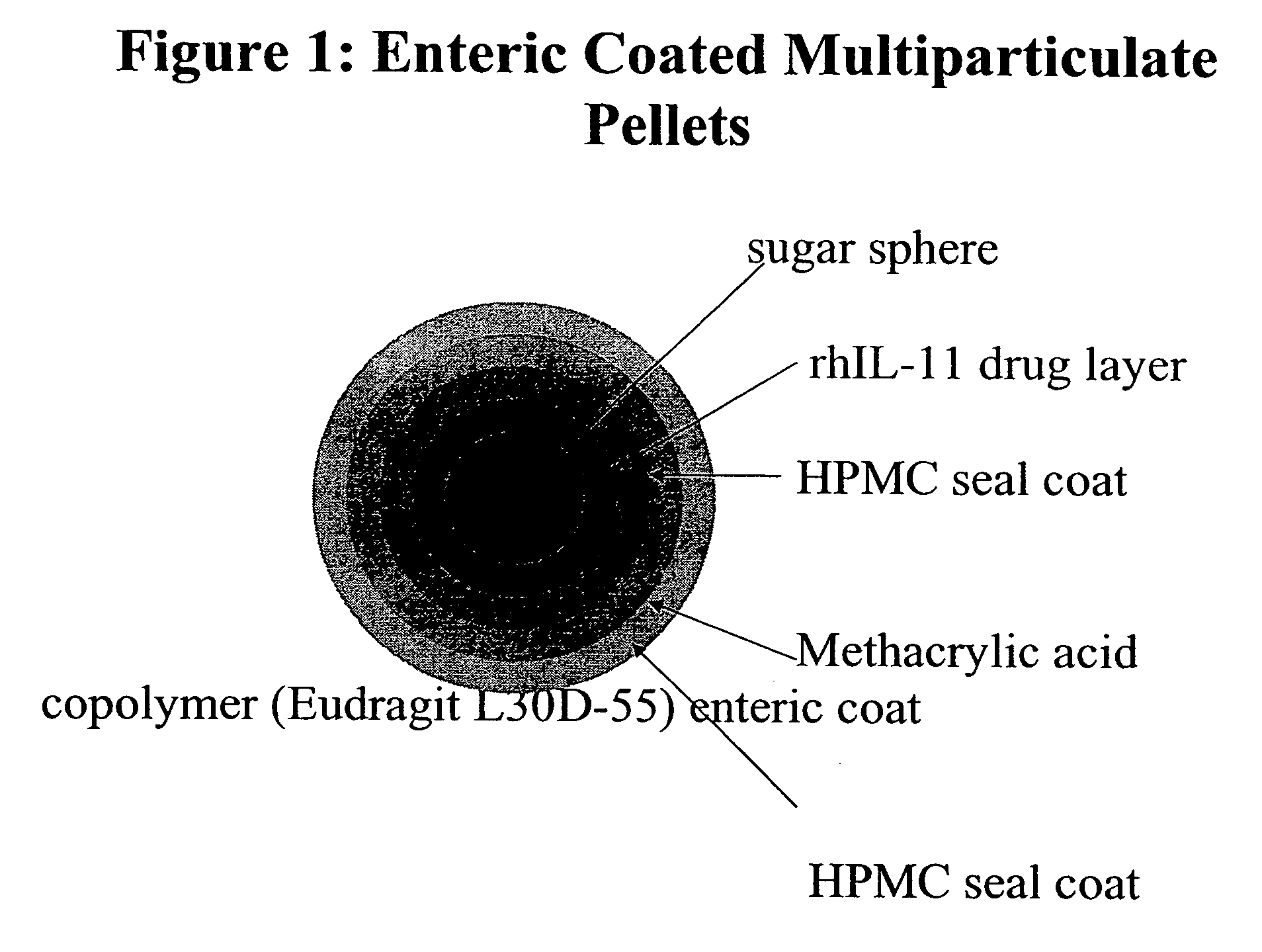
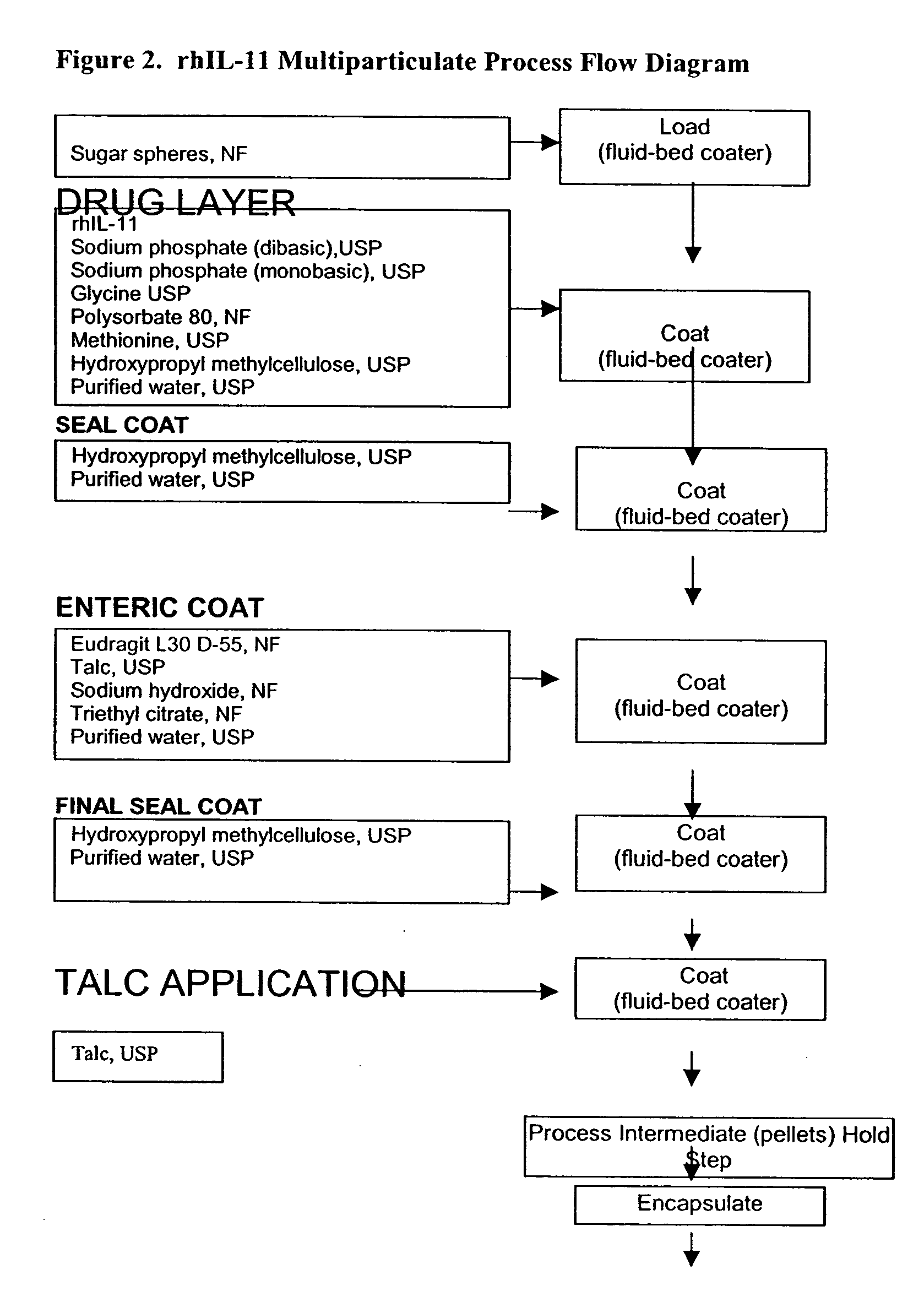
Delayed release formulations for oral administration of a polypeptide therapeutic agent and methods of using same
InactiveUS20040126358A1Increase ionic strengthReduced strengthAntipyreticAnalgesicsOral medicationWhite blood cell
The invention provides compositions containing polypeptides, including therapeutic polypeptides such as interleukin-11, that are suitable for oral administration.
Owner:WYETH LLC
Process for production of nanoparticles and microparticles by spray freezing into liquid
InactiveUS6862890B2Improve efficiencyImprove efficacyPowder deliveryNervous disorderPorosityNanoparticle
The present invention provides a system and a method for the production of microparticles and nanoparticles of materials that can be dissolved. The system and method of the present invention provide quicker freezing times, which in turn produces a more uniform distribution of particle sizes, smaller particles, particles with increased porosity and a more intimate mixing of the particle components. The system and method of the present invention also produce particles with greater surface area than conventional methods. One form of the present invention provides a method for the preparation of particles. An effective ingredient is mixed with water, one or more solvents, or a combination thereof, and the resulting mixture is sprayed through an insulating nozzle located at or below the level of a cryogenic liquid. The spray generates frozen particles.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
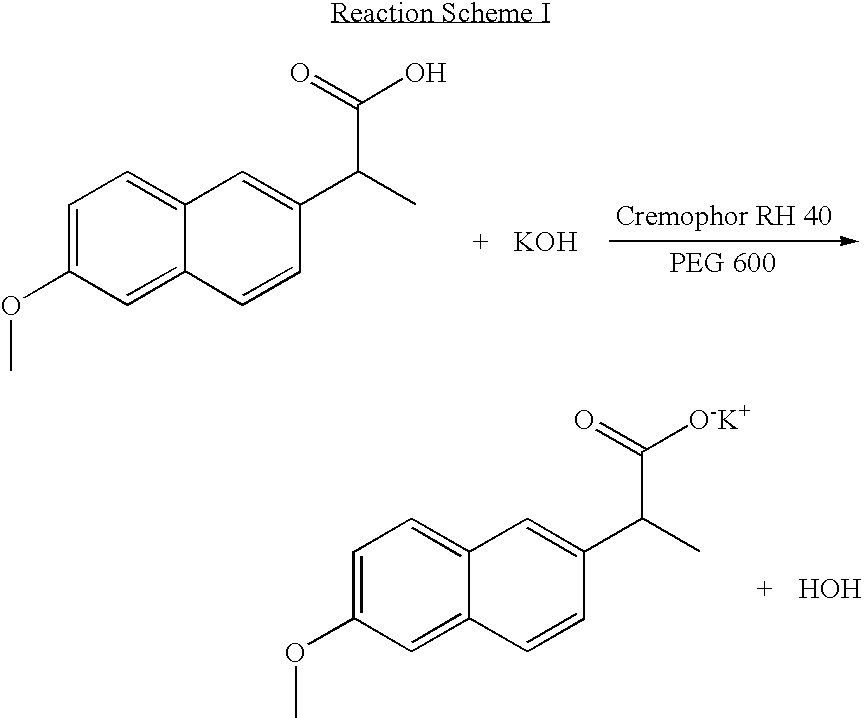
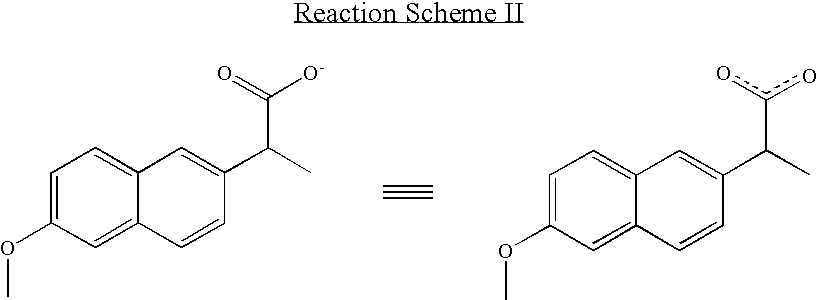
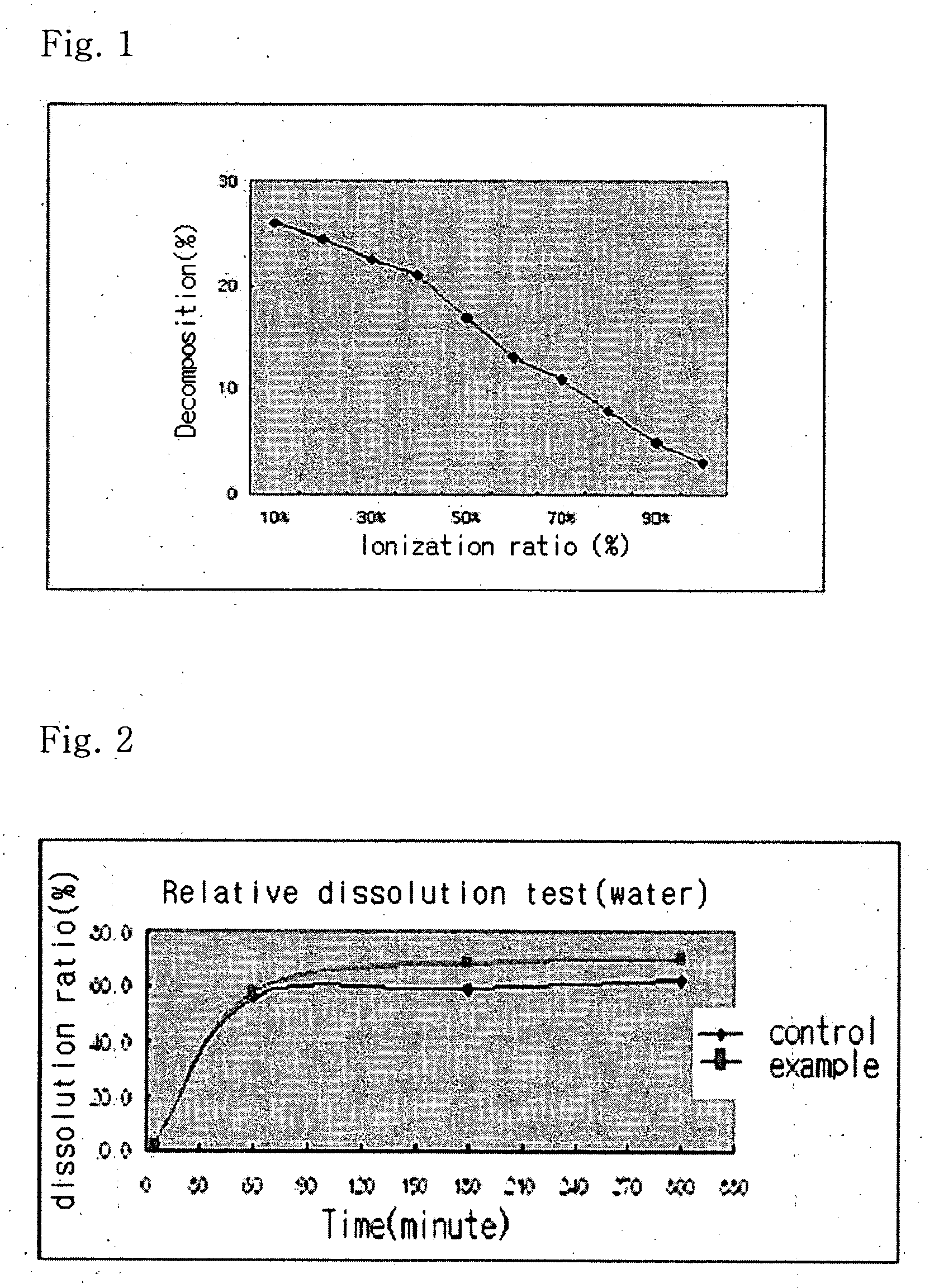
Solvent system of hardly soluble drug with improved dissolution rate
InactiveUS20040157928A1Good disintegrationPromote dissolutionBiocideAntipyreticDissolutionIonization
The present invention relates to a solvent system with improved disintegration degree and dissolution ratio of a hardly soluble drug by highly concentrating the drug through partial ionization, and by establishing optimal conditions for enhancing bioavailability of the drug, such as the co-relation between the acid drug and the accompanied components, ionization degree of a solvent system, use of an appropriate cation acceptance, water content, selection of optimal mixing ratio of the respective components and use of specific surfactants, and to a pharmaceutical preparation comprising the same. The solvent system of the invention has advantages in that it can enhance bioavailability by improving the disintegration degree and dissolution ratio of a hardly soluble drug and also provide a capsule with a sufficiently small volume to permit easy swallowing.
Owner:R & P KOREA
Instant beverage cartridges and methods
InactiveUS20120070542A1Improve convenienceReduce wasteMilk preparationAlcoholic beverage preparationEngineering
Present embodiments generally relate to a single-serve beverage cartridge for use with a single-serve coffee brewer. In some embodiments, the cartridge includes a cup, a lid, and a single serving of instant coffee or another instant beverage component. In some embodiments, the cartridge is configured to be pierced by one or more piercing members, which can provide an opening for liquid to flow into and / or out of the cartridge. In certain instances, the cartridge includes an insert configured to facilitate mixing of the instant beverage component with the liquid. In some embodiments, the instant beverage component is compressed. In certain embodiments, the cartridge does not include a filter or a barrier configured to retain non-liquid matter within the cartridge.
Owner:STARBUCKS
Pharmaceutical co-crystal compositions
InactiveUS20070026078A1Improve solubilityLow hygroscopicityBiocidePowder deliveryThioketoneHydroxamic acid
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
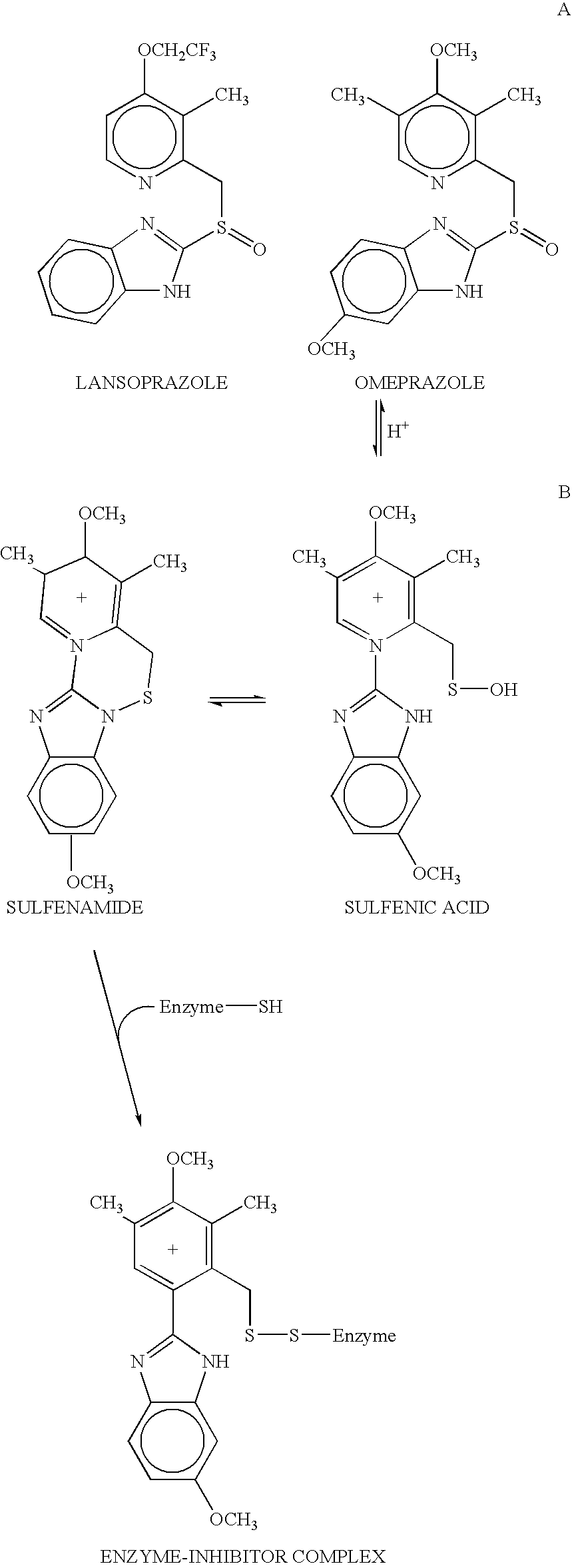
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20040048896A1Improve pharmacological activityGood effectBiocideOrganic chemistryDosage formBenzimidazole
Disclosed herein are compositions and methods for treating gastric acid disorders employing pharmaceutical compositions comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier.
Owner:UNIVERSITY OF MISSOURI
In vivo studies of crystalline forms of meloxicam
ActiveUS20090203680A1Improve bioavailabilityHigh dissolution rateOrganic chemistryAntipyreticMeloxicamDissolution
The invention is directed to novel crystalline forms of meloxicam. These novel crystalline forms of meloxicam have improved bioavailability, an enhanced rate of dissolution and shorter time to Cmax in blood, as compared to pure meloxicam.
Owner:THAR PHARMA
Matrix compositions for controlled delivery of drug substances
InactiveUS20070042044A1Improve solubilityImprove oral bioavailabilityBiocidePowder deliveryPolyethylene oxidePEG-PLGA-PEG
A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and / or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200° C., the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides, and the second polymer being selected form block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid)-b-ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide-polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and / or diagnostically active substance, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, wherein the active substance is released with a substantially zero order release.
Owner:EGALET LTD
Solid formulations of crystalline compounds
InactiveUS20100222311A1Fast dispersionFast dissolutionBiocidePowder deliverySolubilityAdditive ingredient
Described herein are formulations of active pharmaceutical ingredients, where the active pharmaceutical ingredients or drugs are included in a solid suspension with one or more solid additives. The formulations described herein are useful for formulating any drug or active pharmaceutical ingredient, including those that have limited solubility in organic and / or aqueous solvent systems.
Owner:PURDUE RES FOUND INC
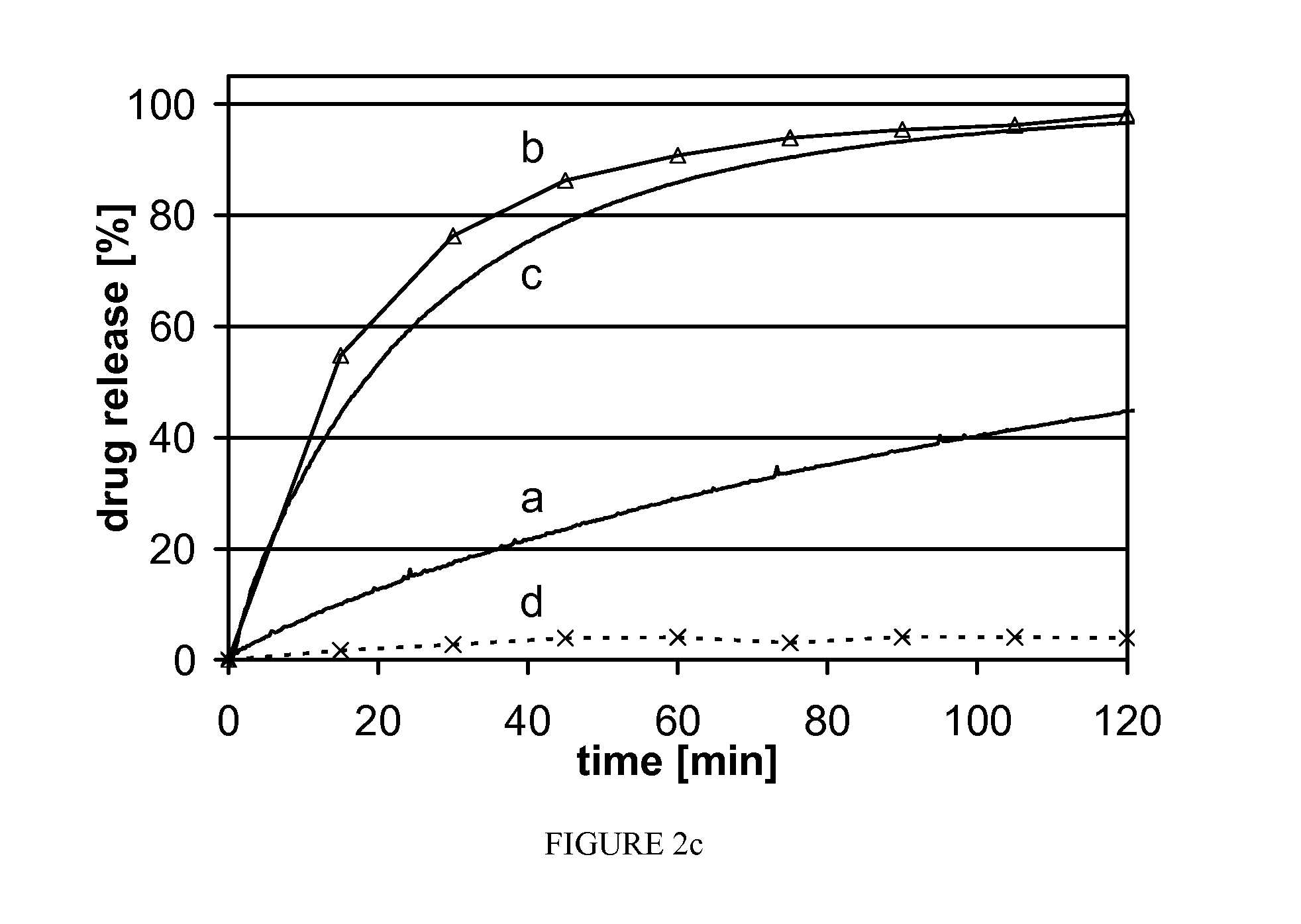
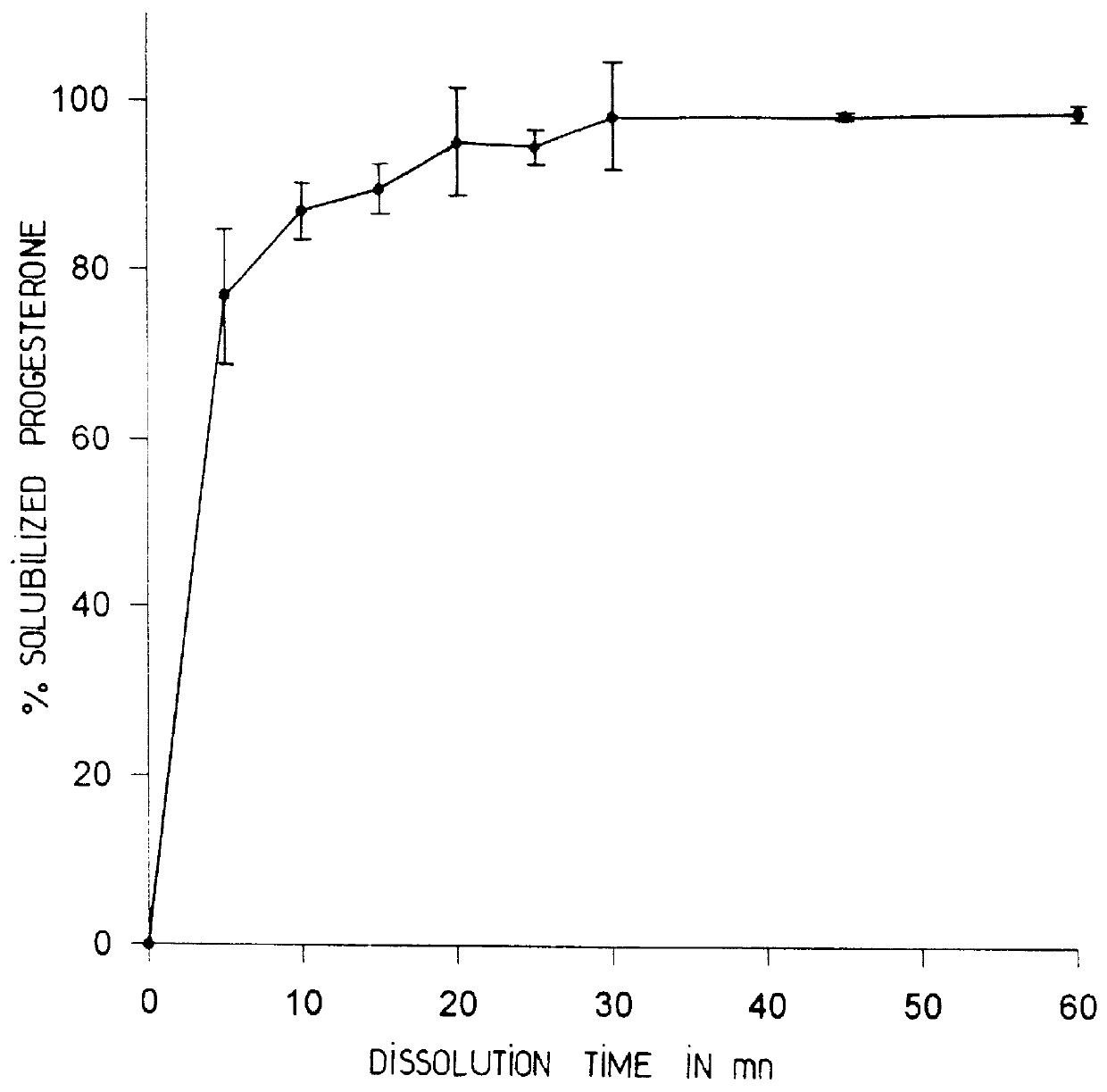
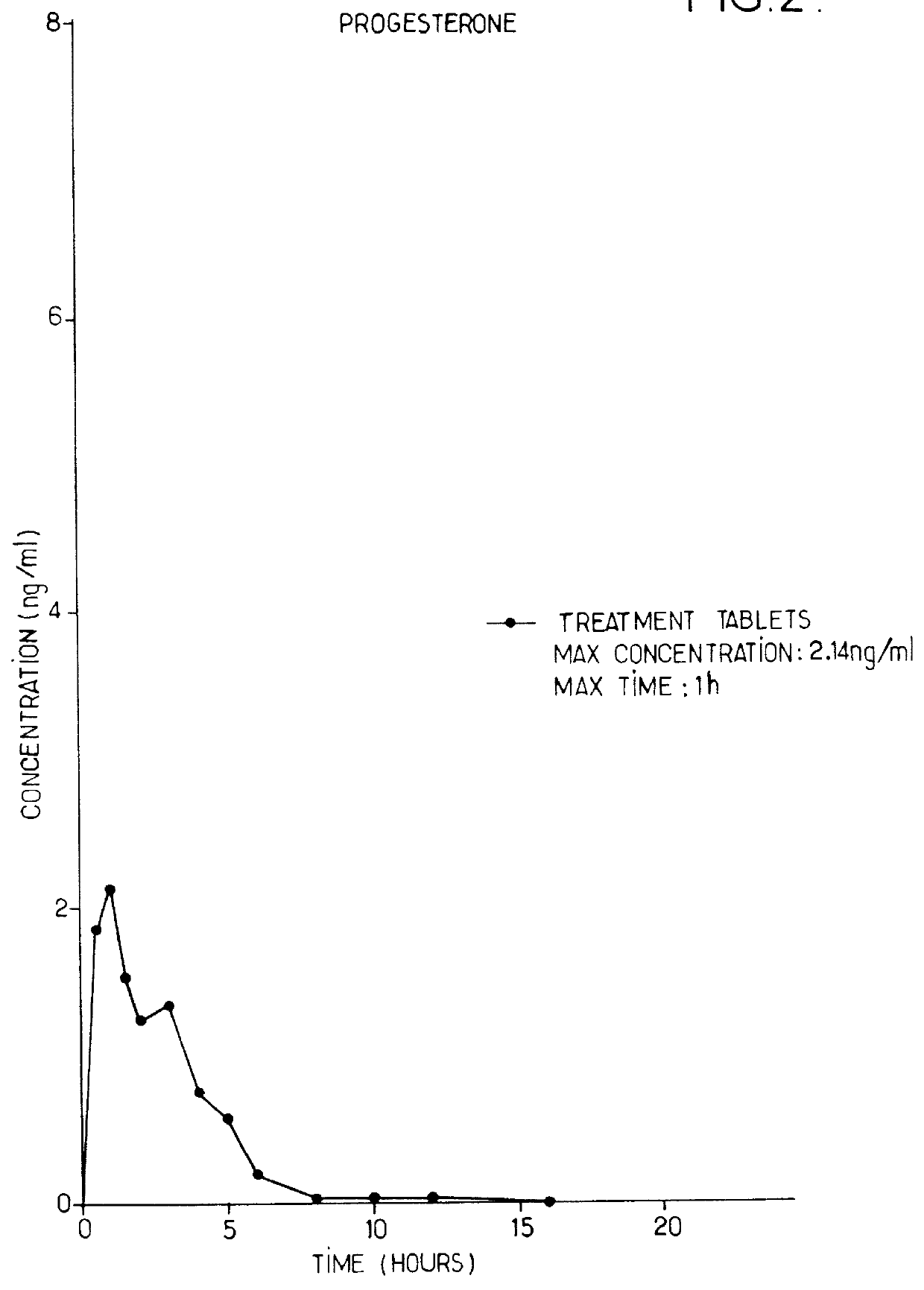
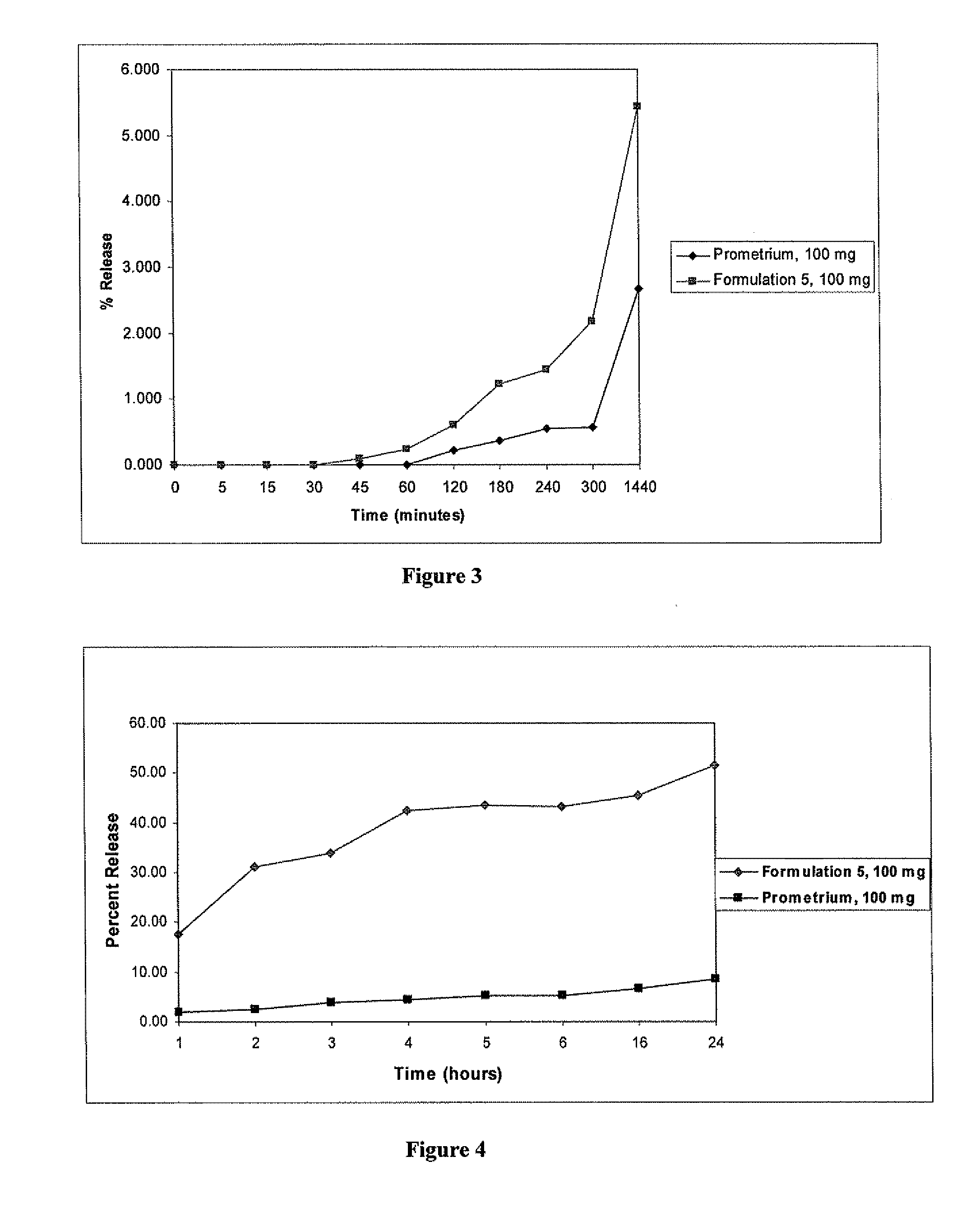
Progesterone tablet and its manufacturing process
InactiveUS6086916AImproved dissolution and self-lubricationGood flowing qualityOrganic active ingredientsPill deliveryDry matterChemistry
The invention concerns a progesterone tablet characterised by the fact that its excipient content is at most 45%, preferably at most 40%, and yet more preferably at most 38%, the percentages being expressed in weight relative to the total dry matter of the tablet.
Owner:BESINS HEALTHCARE SA
Method and means for capture and long-term sequestration of carbon dioxide
InactiveUS20090081096A1High heat of reactionHigh regeneration energyCombination devicesGas treatmentSolubilityAmbient pressure
The invention teaches a practical method of recovering CO2 from a mixture of gases, and sequestering the captured CO2 from the atmosphere for geologic time as calcium carbonate and provides a CO2 scrubber for carbon capture and sequestration. CO2 from the production of calcium oxide is geologically sequestered. A calcium hydroxide solution is produced from the environmentally responsibly-produced calcium oxide. The CO2 scrubber incorporates an aqueous froth to maximize liquid-to-gas surface area and time-of-contact between gaseous CO2 and the calcium hydroxide solution. The CO2 scrubber decreases the temperature of the liquid and the mixed gases, increases ambient pressure on the bubbles and vapor pressure inside the bubbles, diffuses the gas through intercellular walls from relative smaller bubbles with relative high vapor pressure into relative larger bubbles with relative low vapor pressure, and decreases the mean-free-paths of the CO2 molecules inside the bubbles, in order to increase solubility of CO2 and the rate of dissolution of gaseous CO2 from a mixture of gases into the calcium hydroxide solution.The CO2 scrubber recovers gaseous CO2 directly from the atmosphere, from post-combustion flue gas, or from industrial processes that release CO2 as a result of process. CO2 reacts with calcium ions and hydroxide ions in solution forming insoluble calcium carbonate precipitates. The calcium carbonate precipitates are separated from solution, and sold to recover at least a portion of the cost of CCS.
Owner:WESTEC ENVIRONMENTAL SOLUTIONS
Aerosol and injectable formulations of nanoparticulate benzodiazepine
InactiveUS20060198896A1Easy doseReduce injection volumeBiocidePowder deliveryBenzodiazepinePolyethylene glycol
Described are nanoparticulate formulations of a benzodiazepine, such as lorazepam, that does not require the presence of polyethylene glycol and propylene glycol as stabilizers, and methods of making and using such formulations. The formulations are particularly useful in aerosol and injectable dosage forms, and comprise nanoparticulate benzodiazepine, such as lorazepam, and at least one surface stabilizer. The formulations are useful in the treatment of status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and as a pre-anesthesia medication.
Owner:ELAN PHRMA INT LTD
Nanoparticulate fibrate formulations
InactiveUS7276249B2Improve bioavailabilityHigh dissolution ratePowder deliveryMetabolism disorderFibratePharmacology
Owner:ALKERMES PHARMA IRELAND LTD +1
Nanoparticulate clopidogrel and aspirin combination formulations
InactiveUS20070003615A1Reduced bioavailabilityHigh dissolution ratePowder deliveryBiocideControl releasePharmaceutical drug
The present invention is directed to compositions comprising a nanoparticulate clopidogrel and aspirin combination, or salts or derivatives thereof, having improved clopidogrel bioavailability. The nanoparticulate clopidogrel particles, and optionally the nanoparticulate aspirin particles, of the composition have an effective average particle size of less than about 2000 nm and are useful in the prevention and treatment of pathologies induced by platelet aggregation. The clopidogrel and aspirin particles may also be formulated as a controlled release polymeric coating or matrix drug delivery system.
Owner:ELAN PHRMA INT LTD
Method of preparing aluminum oxide from fly ash
ActiveCN1923695AHigh dissolution rateFewer separation stepsAluminium oxide/hydroxide preparationAluminium oxides/hydroxidesAluminium sulfateSlag
The invention discloses a preparing method of alumina through fly ash, which comprises the following steps: grinding fly ash; sintering; activating; stirring with H2SO4 evenly to sinter into dried slag; immersing through hot water; stripping aluminum sulfate; condensing; cooling to evolve aluminum sulfate; crystallizing; heating; dehydrating to obtain anhydrous aluminum sulfate; heating; decomposing to obtain gamma-Al2O3.
Owner:PINGSHUO INDAL
Progesterone Solutions for Increased Bioavailability
ActiveUS20100255085A1Improve bioavailabilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionProgesterones
Fill materials for hydrophobic drugs, such as progesterone, and methods of making and using thereof are described herein. The fill material contains the hydrophobic drug dissolved in one or more fatty acids. The concentration of the hydrophobic drug is typically from about 7% to about 50% by weight of the fill material. The concentration of the one or more fatty acids is from about 60% to about 95% by weight of the carrier. The formulation also contains an organic acid and one or both of one or more pharmaceutically acceptable alcohols and one or more pharmaceutically acceptable mono-, di-, or triesters of medium or long chain fatty acids. The fill material can be encapsulated in a hard or soft capsule. The formulations described herein have a higher dissolution rate and faster onset of dissolution compared to micronized progesterone suspended in an oil and thus should have increased bioavailability in vivo.
Owner:PATHEON SOFTGELS INC
Apertured Fibrous Structures and Methods for Making Same
ActiveUS20160101026A1High dissolution rateAppealing visualInorganic/elemental detergent compounding agentsCosmetic preparationsFiberActive agent
Owner:THE PROCTER & GAMBLE COMPANY
Nanoparticulate fibrate formulations
InactiveUS20050276974A1Improve bioavailabilityHigh dissolution ratePowder deliveryMetabolism disorderMicro particlesPharmacokinetics
Owner:ALKERMES PHARMA IRELAND LTD +1
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
Single compartment unit dose fabric treatment product comprising pouched compositions with non-cationic fabric softener actives
InactiveUS20040142841A1Disintegrate quickly and completelyEasy to cleanOrganic detergent compounding agentsLiquid soapsMedicineWater soluble
The present invention relates a unit dose fabric treatment product comprising a non-aqueous liquid fabric treatment composition contained in a single compartment water-soluble pouch. The inner space of said pouch comprises (A) a cleaning system comprising more than 5% by weight of the fabric treatment composition of at least one anionic surfactant; and (B) a fabric softening system comprising at least one non-cationic fabric softening active selected from the group consisting of fabric softening clays, fabric softening silicones, and mixtures thereof, wherein the fabric softening clay is added as a premix comprising the clay and a solvent; wherein the fabric softening silicone is added as a premix comprising the silicone and a solvent or wherein the fabric softening silicone is added as pure compound without any solvent. The invention further relates to a method of producing such compositions and to the use of such compositions to impart fabric-cleaning and fabric-softening benefits via single compartment water-soluble pouches to fabrics treated therewith.
Owner:THE PROCTER & GAMBLE COMPANY
Method for producing multicrystalline silicon substrate for solar cells
InactiveUS20050101153A1Promote oxidationHigh dissolution rateFinal product manufactureSemiconductor/solid-state device manufacturingMetal particleSolar cell
Disclosed is a method for producing a multicrystalline silicon substrate for solar cells comprising: a metal deposition step for depositing such metal particles as platinum and silver on the surface of the substrate by electroless-plating chloroplatinic acid or silver perchlorate; a boring step for subjecting the substrate surface to etching in a solution containing at least one of hydrofluoric acid and hydrogen peroxide; and a step for removing a stain layer by immersing the substrate into an alkaline solution. A multicrystalline silicon substrate having a lower reflectance is provided at a lower cost.
Owner:KANSAI TLO KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com