Porous drug matrices and methods of manufacture thereof
a technology of porous drug matrix and matrices, which is applied in the field of drug formulations, can solve the problems of increasing the systemic toxicity affecting the bioavailability of the drug, and affecting the stability of the drug composition, so as to achieve the effect of enhancing the drug dissolution rate, low aqueous solubility, and fast dissolution ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
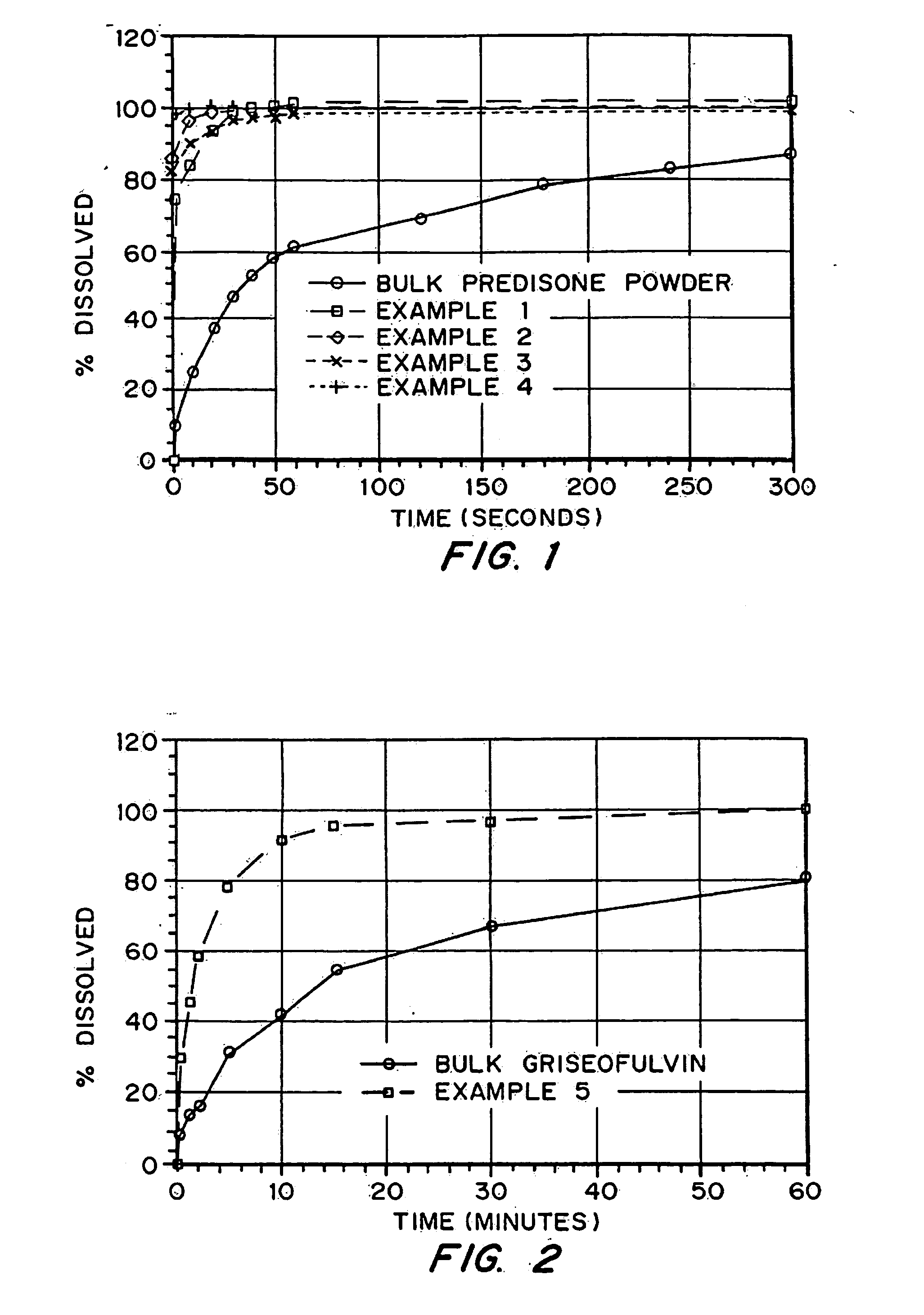
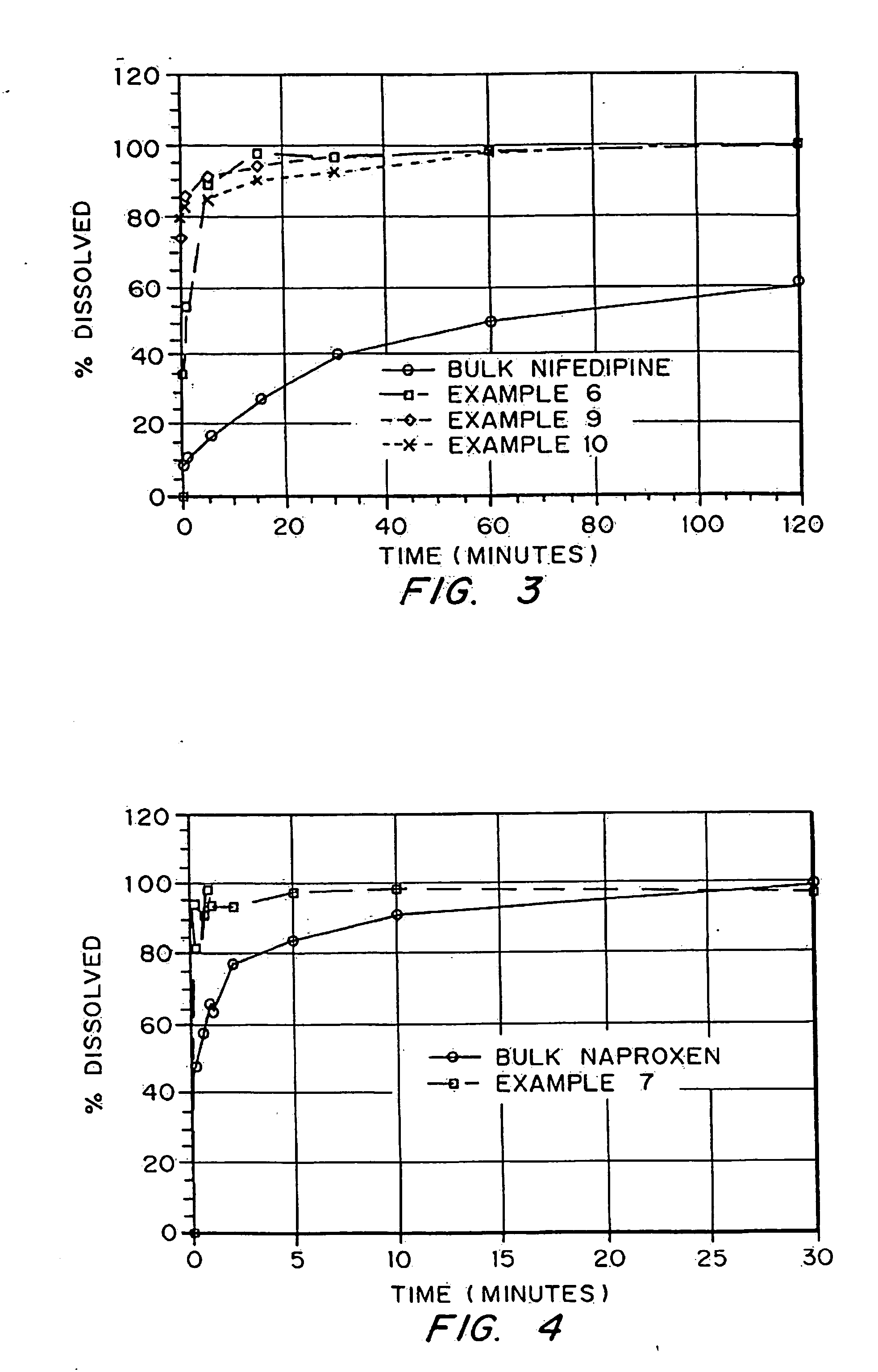
examples 1-10
demonstrate production of porous drug matrices using different pore forming agents, different drugs, and different solvents. Examples 1-8 use emulsion formulations to produce the matrices, whereas Examples 9 and 10 use solution formulations to produce the matrices.
examples 11-13
describe the analyses which were used to characterize the porous drug matrices produced in Examples 1-10. These characteristics include density, drug integrity, and dissolution properties. Example 14 describes particle size analysis and surface area analysis of low water solubility drug particles incorporated into the porous drug matrices.
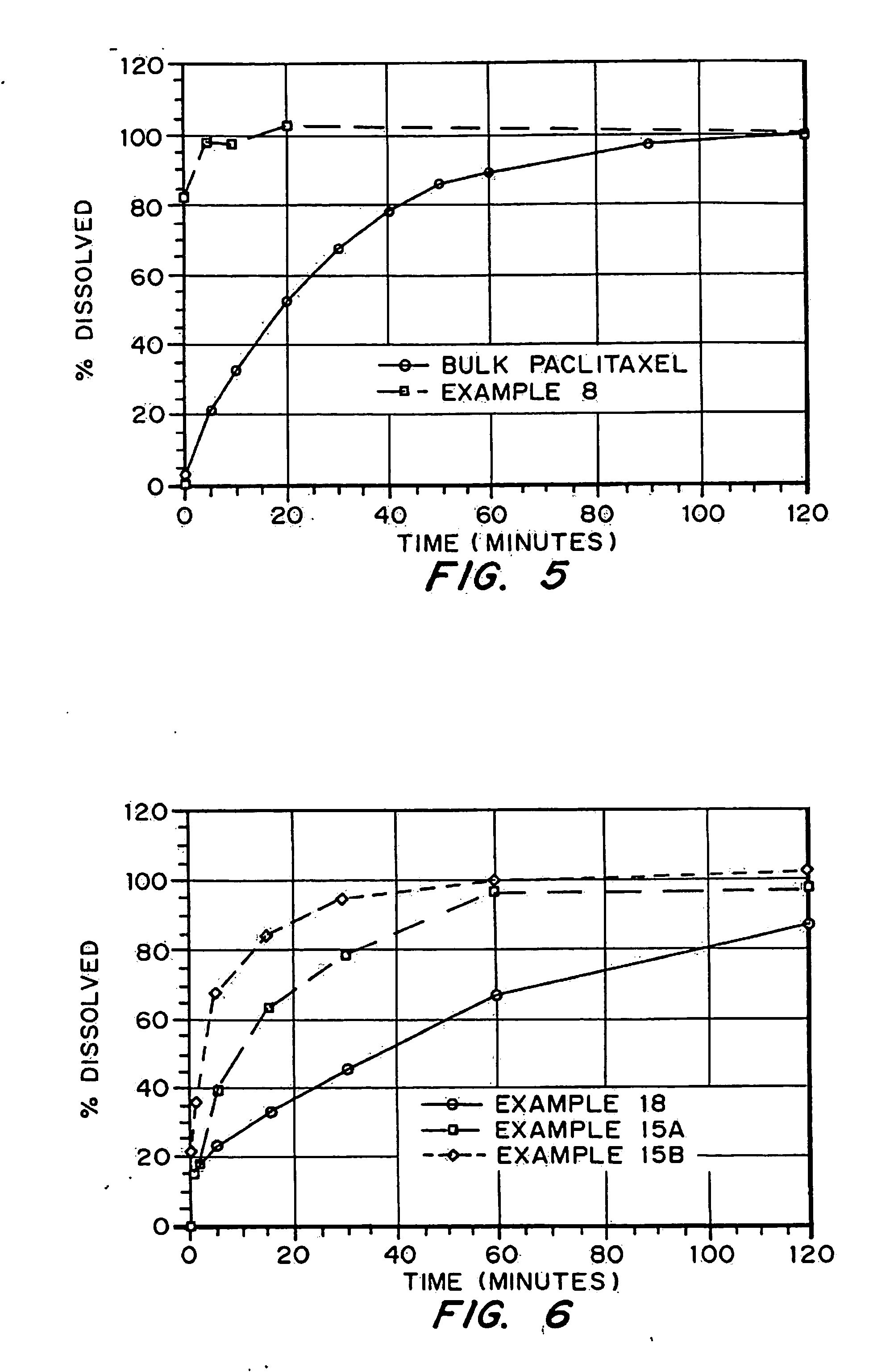
examples 15-17
describe experiments demonstrating the increased internal surface area of porous drug matrices produced with pore forming agents. Examples 18-21 describe experiments demonstrating the advantage or need to include a wetting agent as a component of the porous drug matrices.
PUM
| Property | Measurement | Unit |
|---|---|---|
| TAP density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com