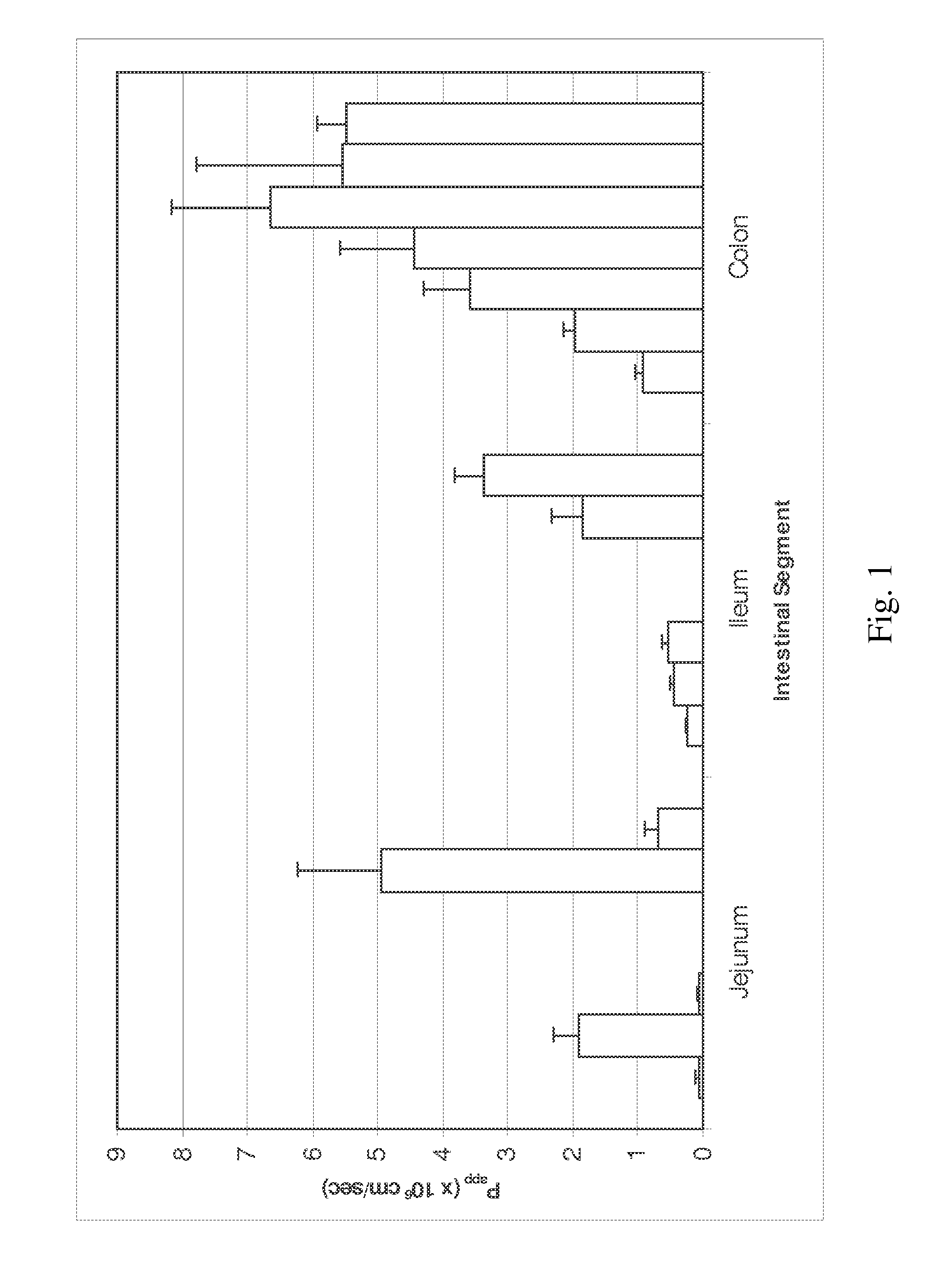
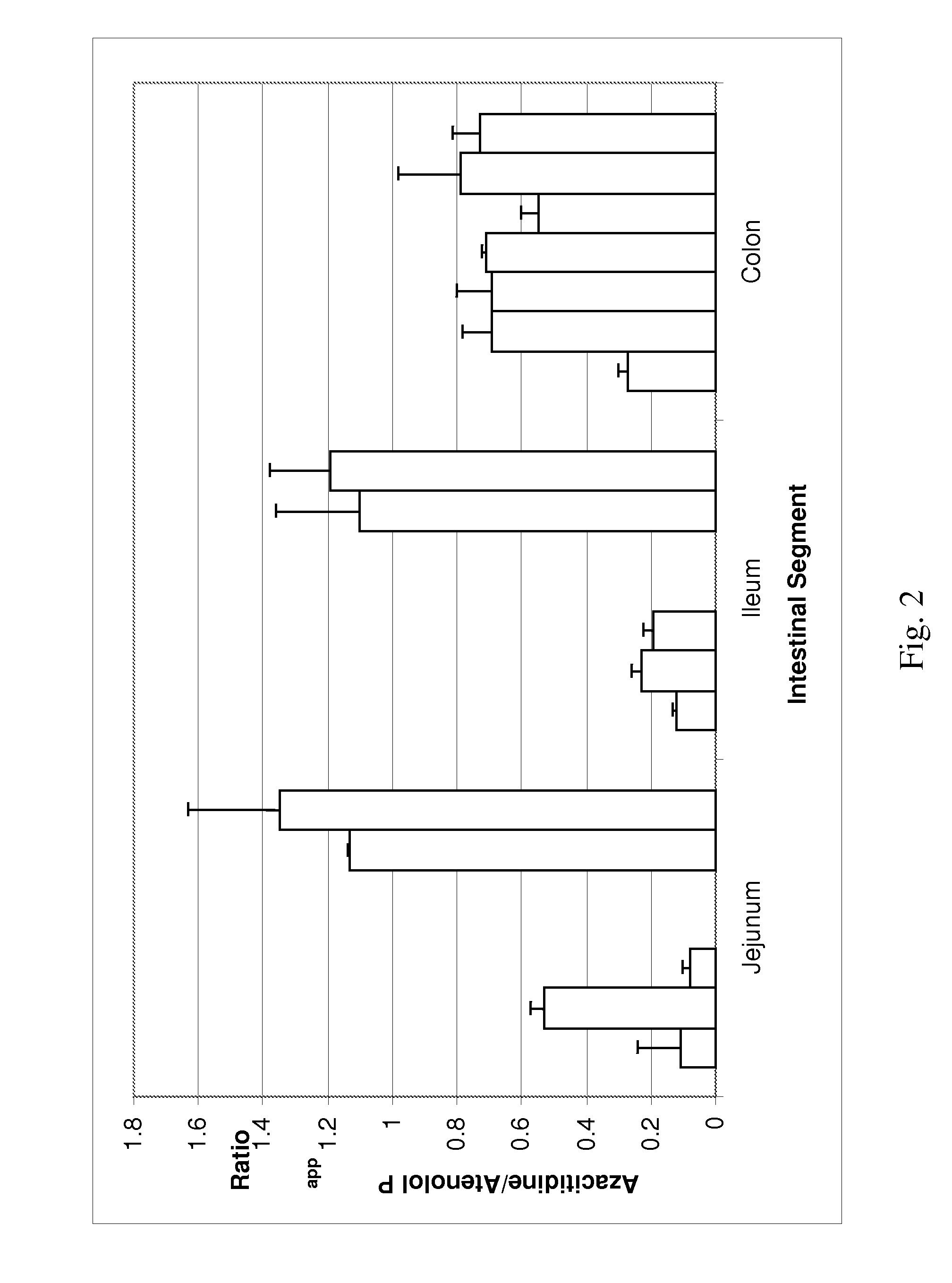
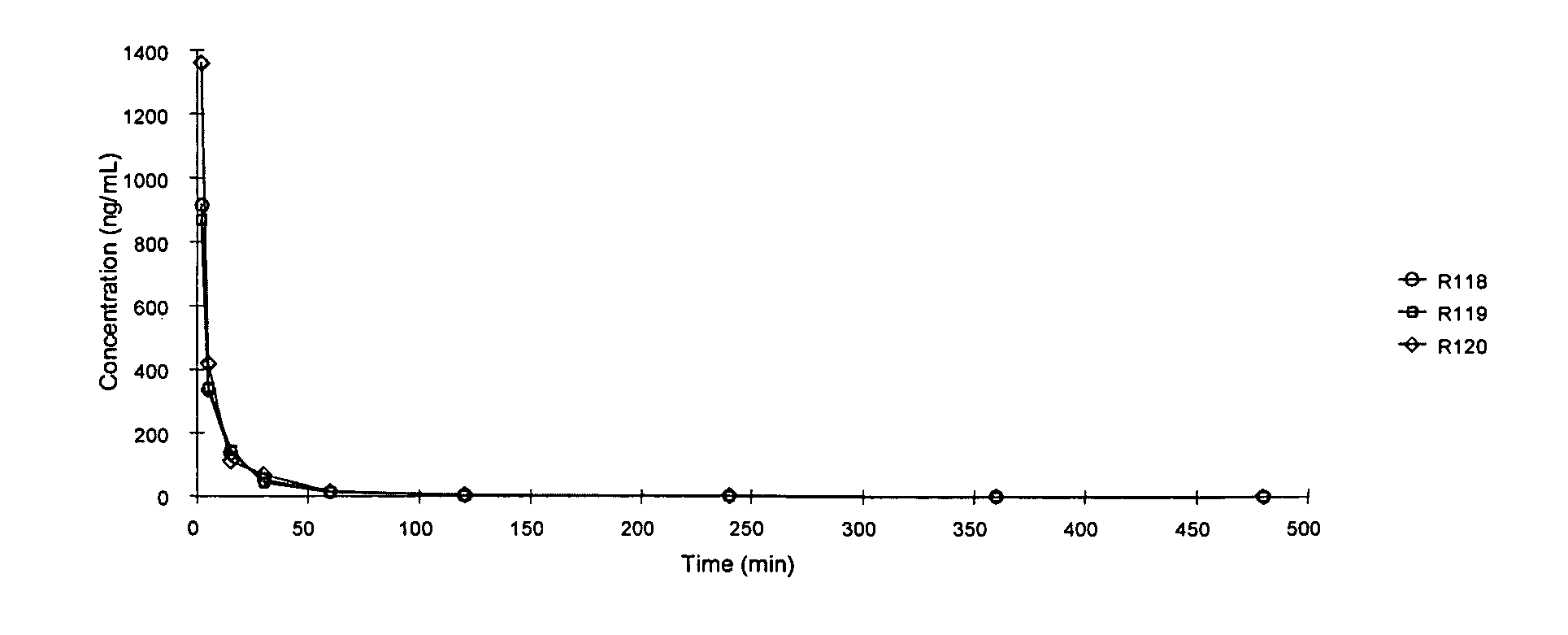
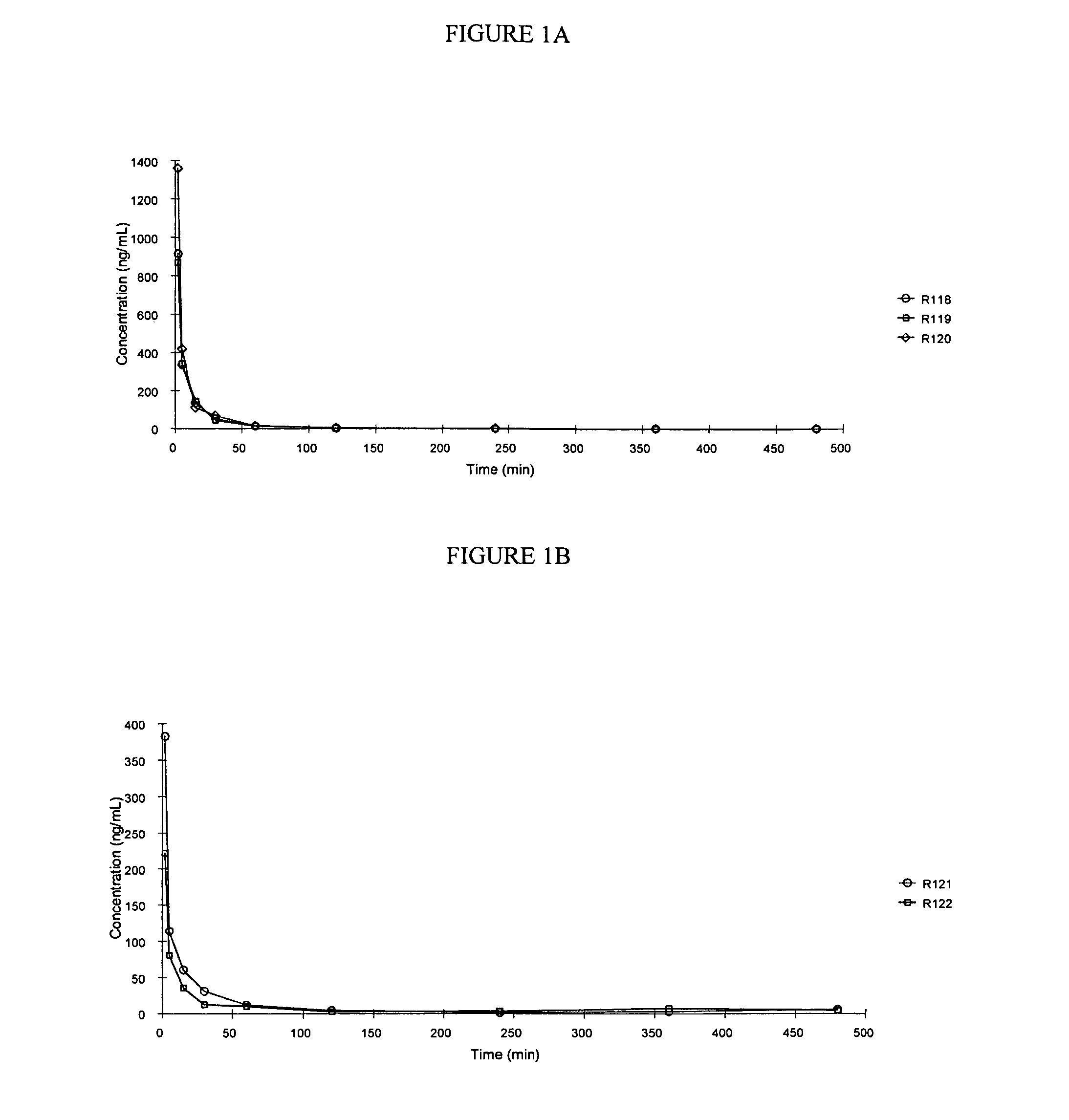
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
13390 results about "Bioavailability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
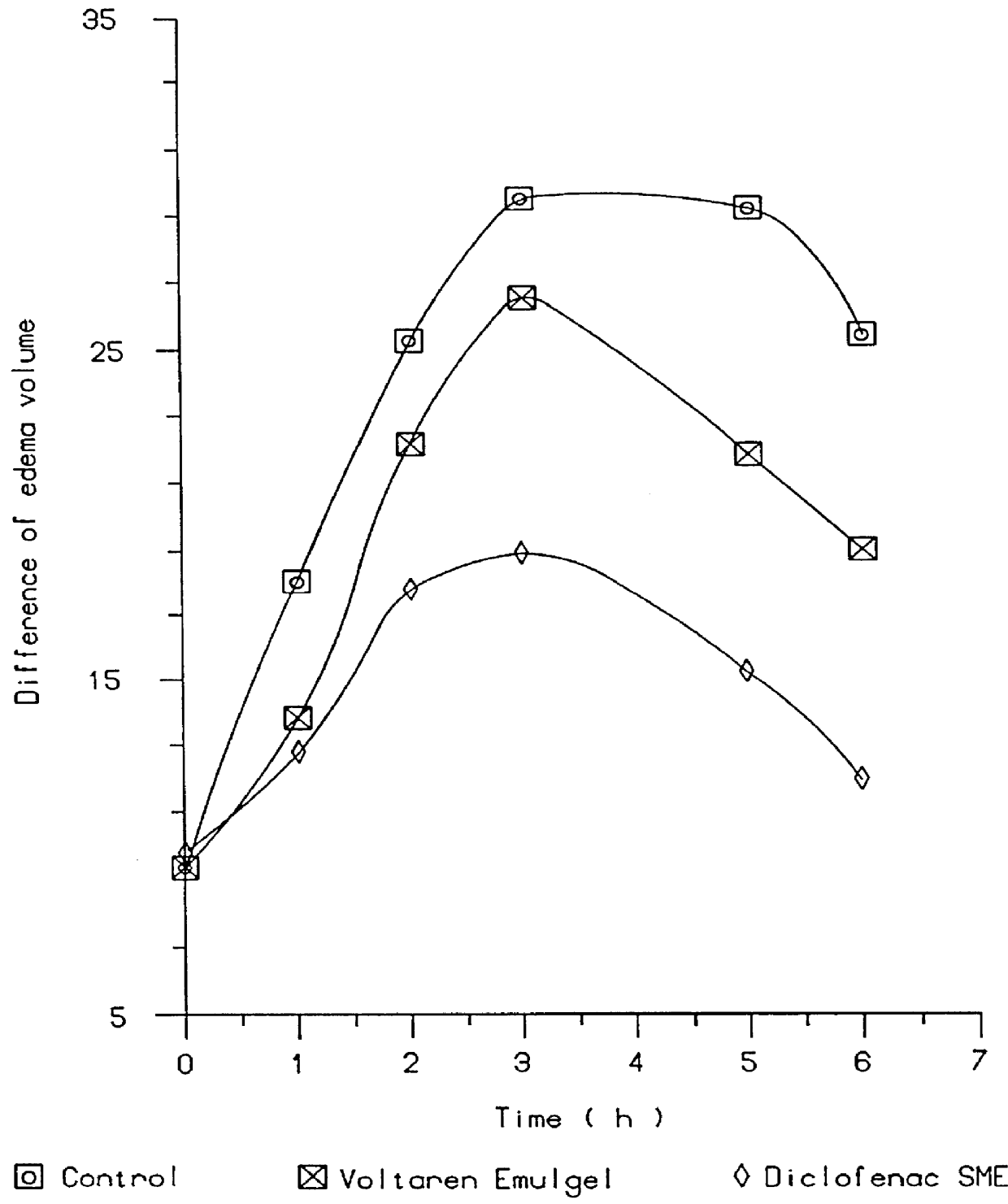
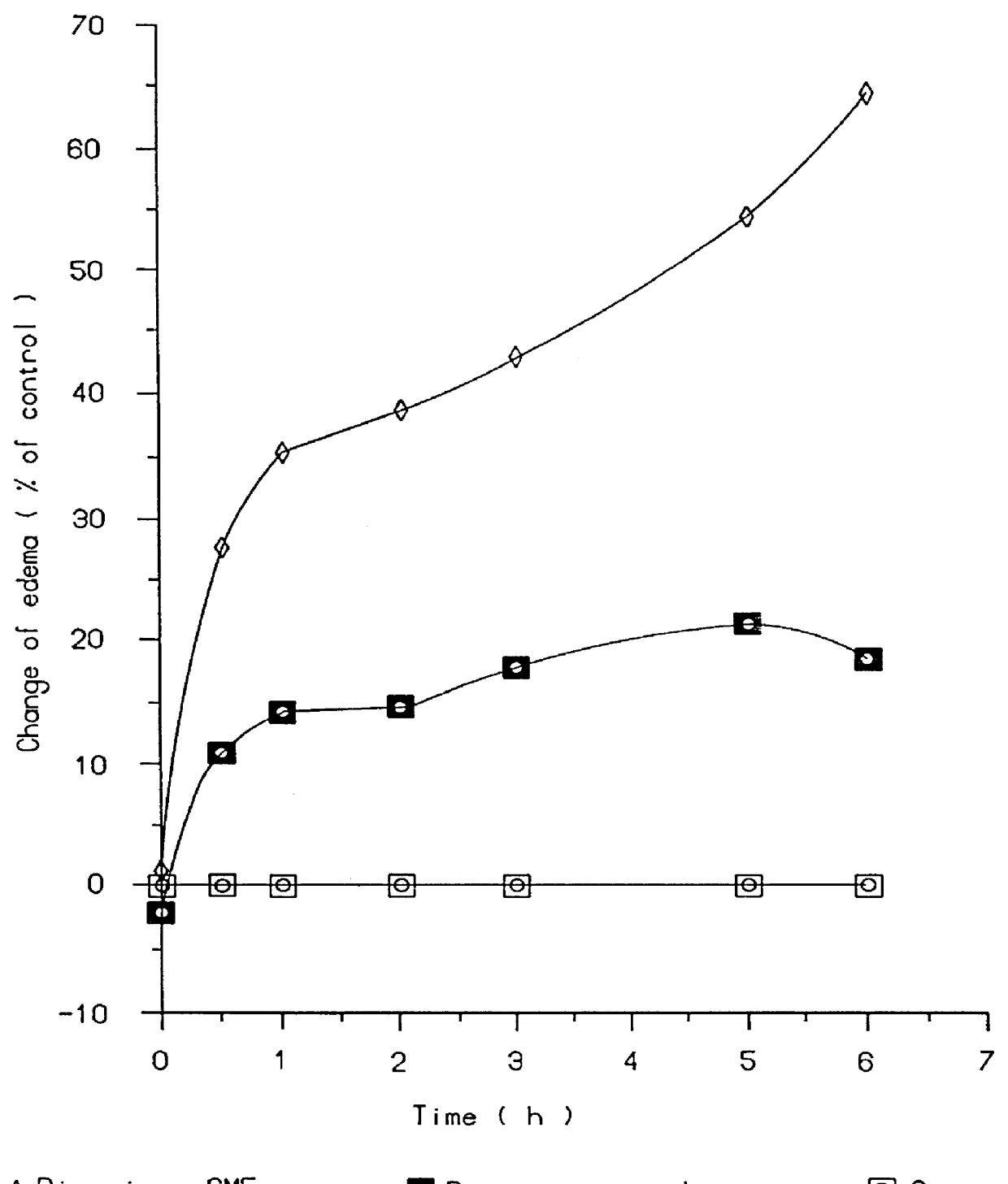
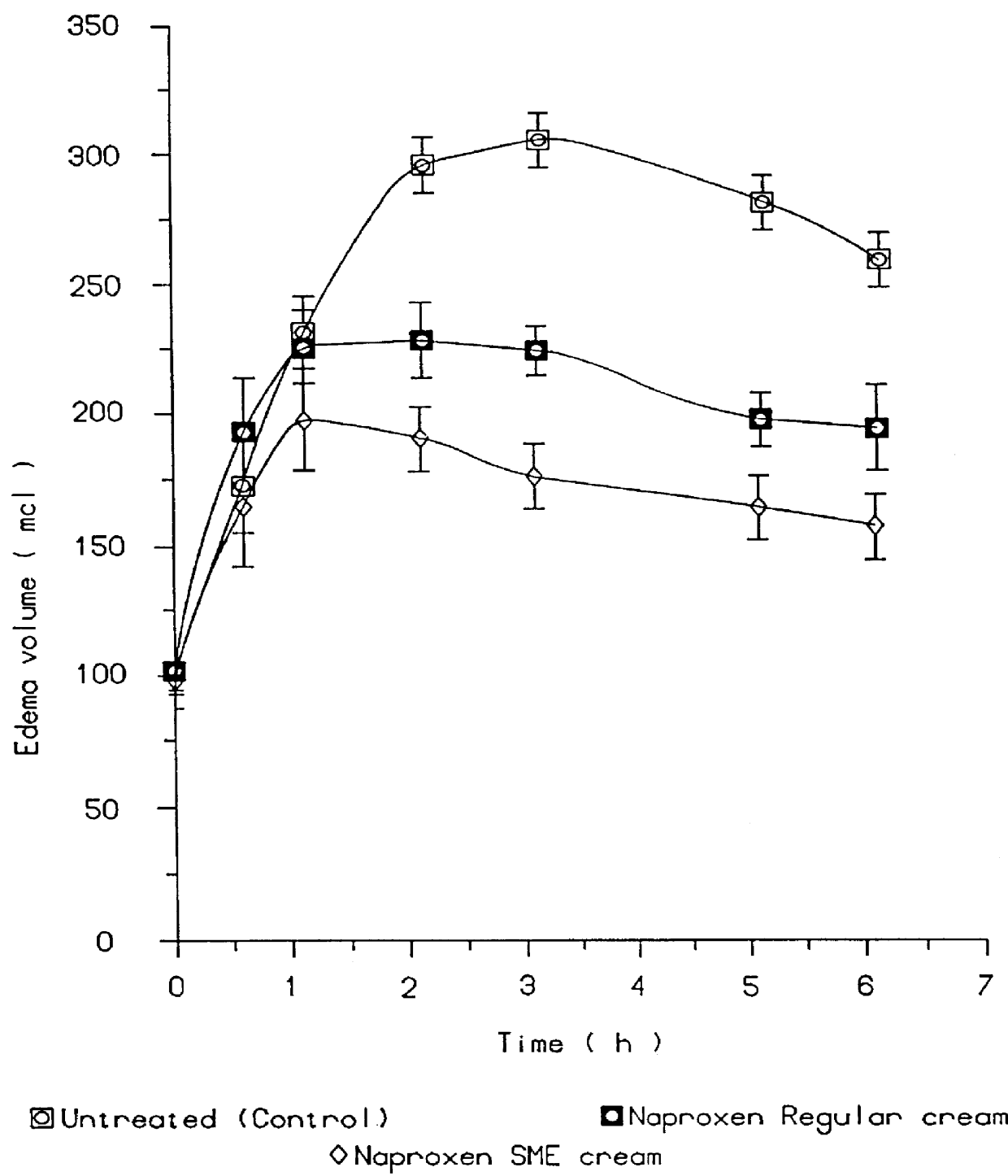
In pharmacology, bioavailability (BA or F) is a subcategory of absorption and is the fraction of an administered dose of unchanged drug that reaches the systemic circulation, one of the principal pharmacokinetic properties of drugs. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via other routes (such as orally), its bioavailability generally decreases (due to incomplete absorption and first-pass metabolism) or may vary from patient to patient. Bioavailability is one of the essential tools in pharmacokinetics, as bioavailability must be considered when calculating dosages for non-intravenous routes of administration.
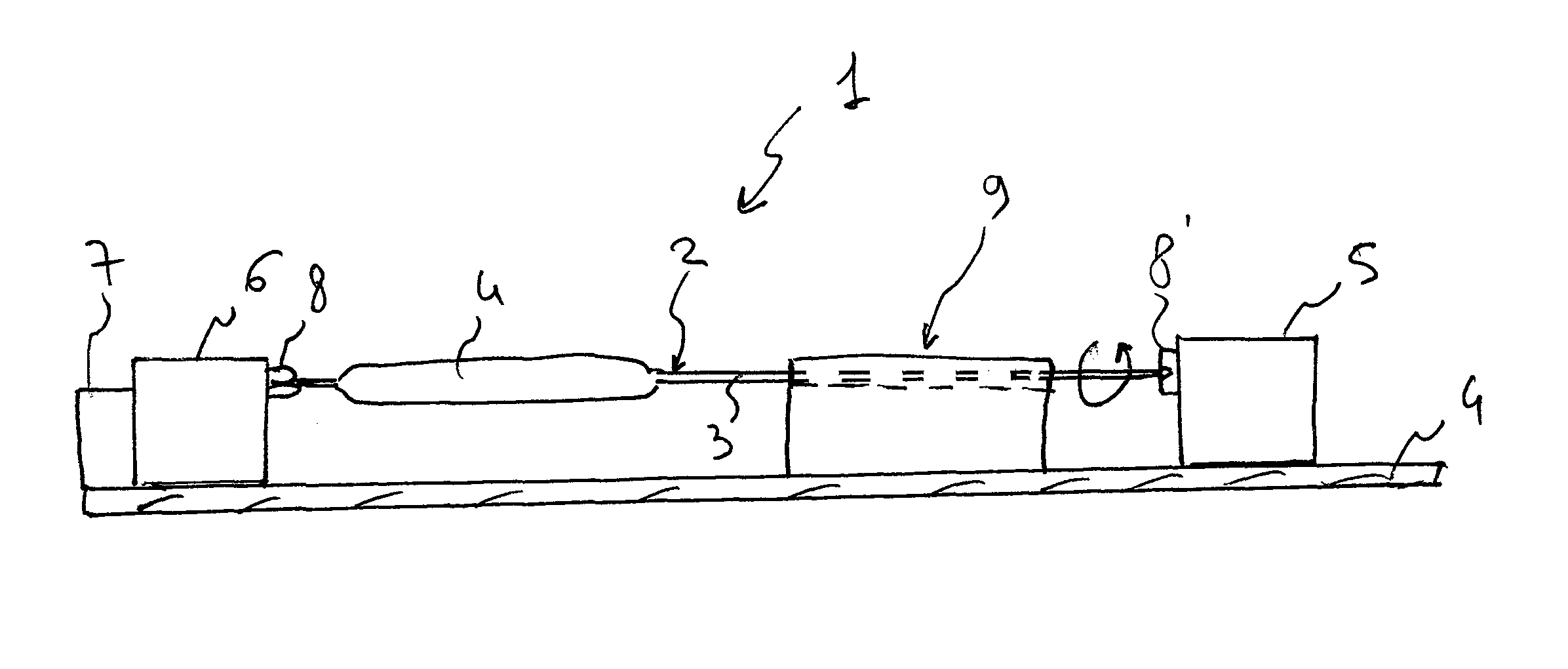
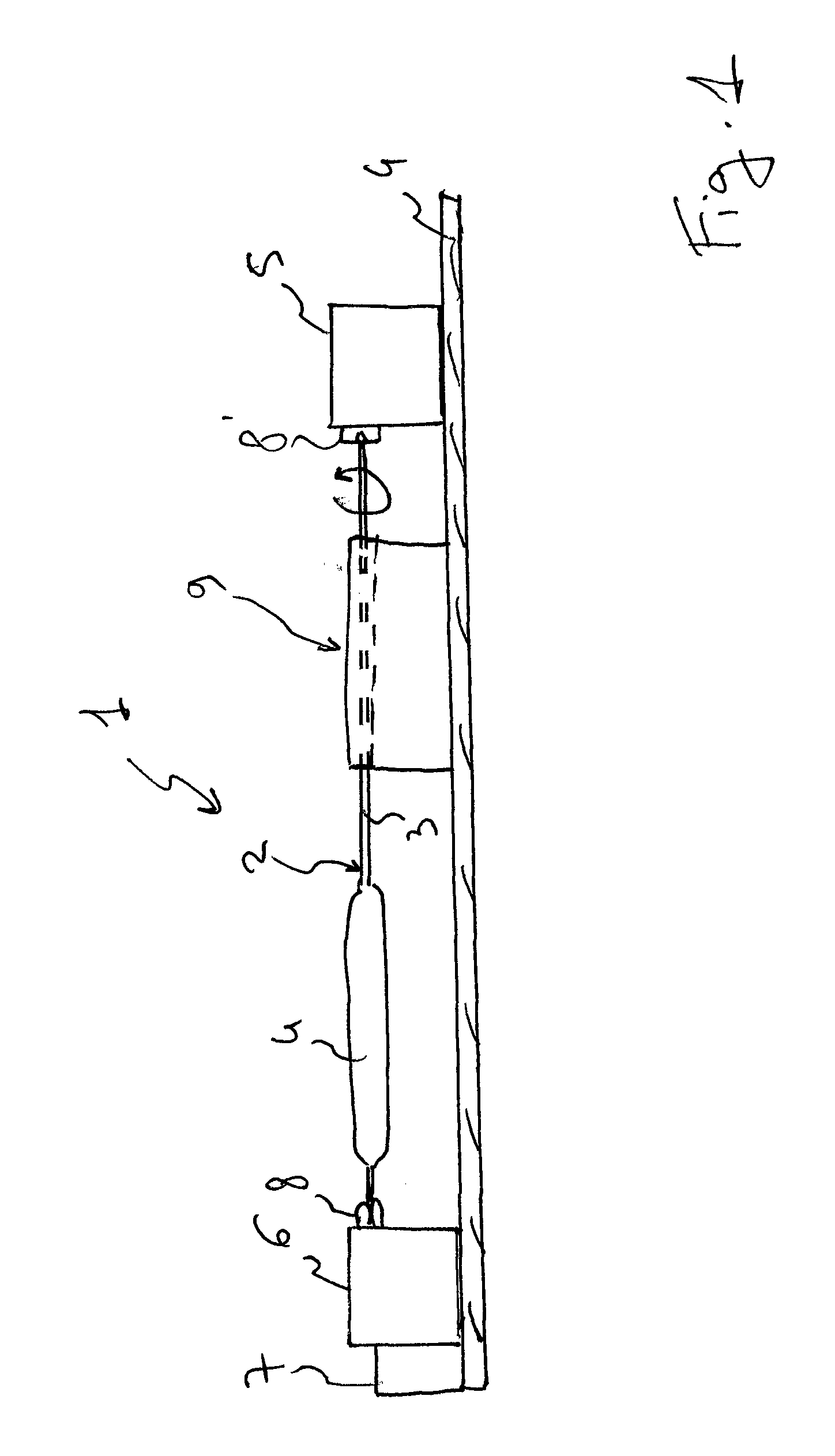
Method and Device for Ophthalmic Administration of Active Pharmaceutical Ingredients
InactiveUS20080233053A1Efficient transferImprove bioavailabilityOrganic active ingredientsPeptide/protein ingredientsAdditive ingredientBioavailability
Disclosed is the use of a mist of a pharmaceutical composition for ophthalmic delivery of a protein or peptide active pharmaceutical ingredient, a related method of treatment and a device useful in implementing the use and method. Disclosed is also the use of a mist for ophthalmic delivery of a pharmaceutical composition including a highly irritating penetration enhancer and an ophthalmically acceptable carrier, a related method of treatment and a device useful in implementing the use and method. Disclosed is also a device for ophthalmic administration configured to direct a mist of a pharmaceutical composition to the eye only when the eye is open. Disclosed is also a self-sterilizing device for ophthalmic administration. Disclosed is also a device and a method for increasing the bioavailability of an ophthalmically administered API in a pharmaceutical composition.
Owner:PHARMALIGHT
Effervescent green tea extract formulation
InactiveUS6299925B1Fast absorptionMaintain good propertiesAntibacterial agentsPre-extraction tea treatmentNatural productAdditive ingredient
A solid state water soluble formulation in granular or tablet form is provided. The formulation is a natural products formulation containing a green tea plant extract in combination with other ingredients which create an effervescent liquid composition upon dispensing the formulation in a liquid. The liquid form of administration, as well as the effervescent properties of the dissolved formulation increase bioavailability of the advantageous components of the green tea plants such as Polyphenols, by increasing absorption speed and amount in the human body. The formulation may include additional components such as, other plant extracts, vitamins, ionic minerals, and other substances purported to be of a health benefit.
Owner:XEL HERBACEUTICALS INC
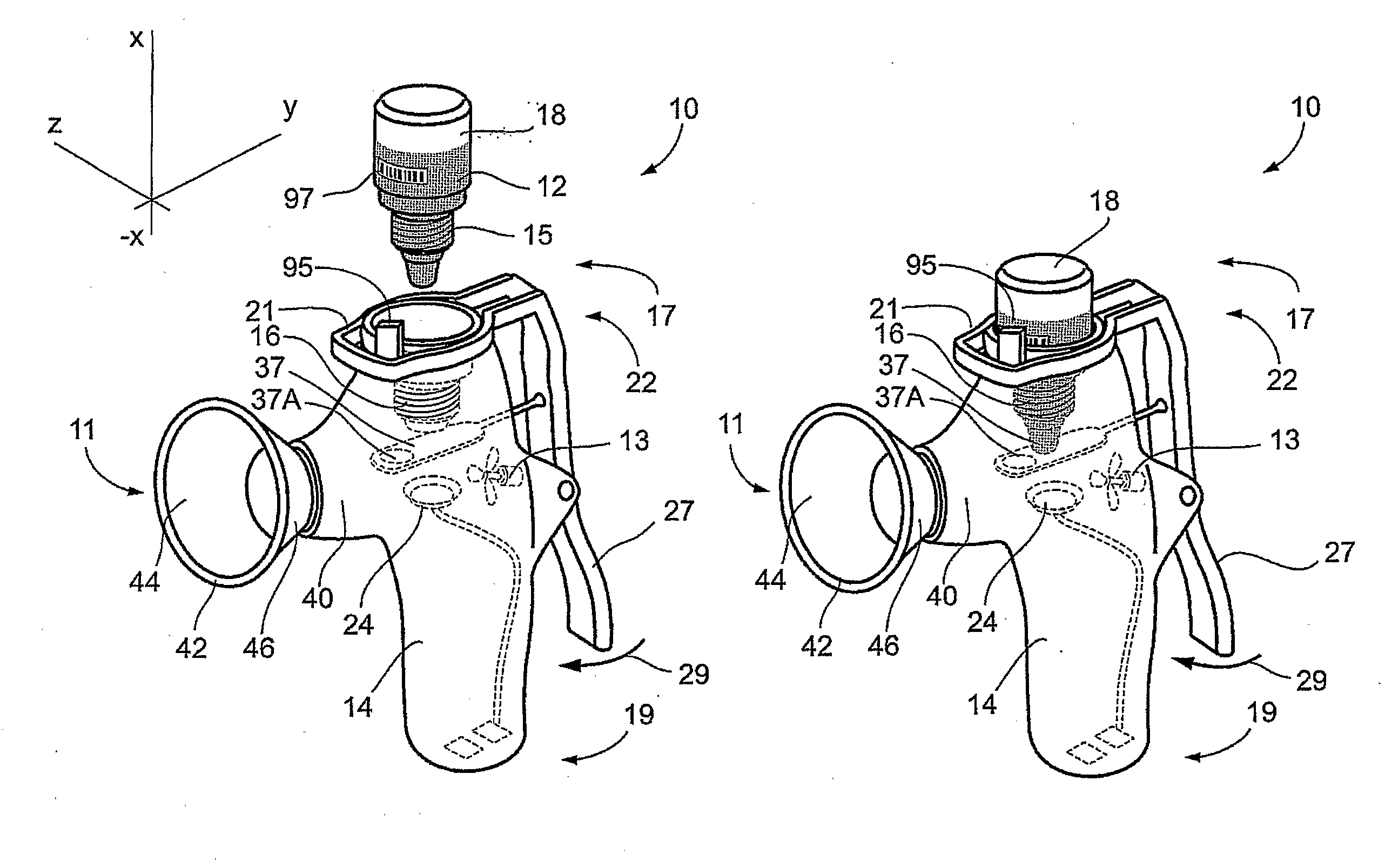
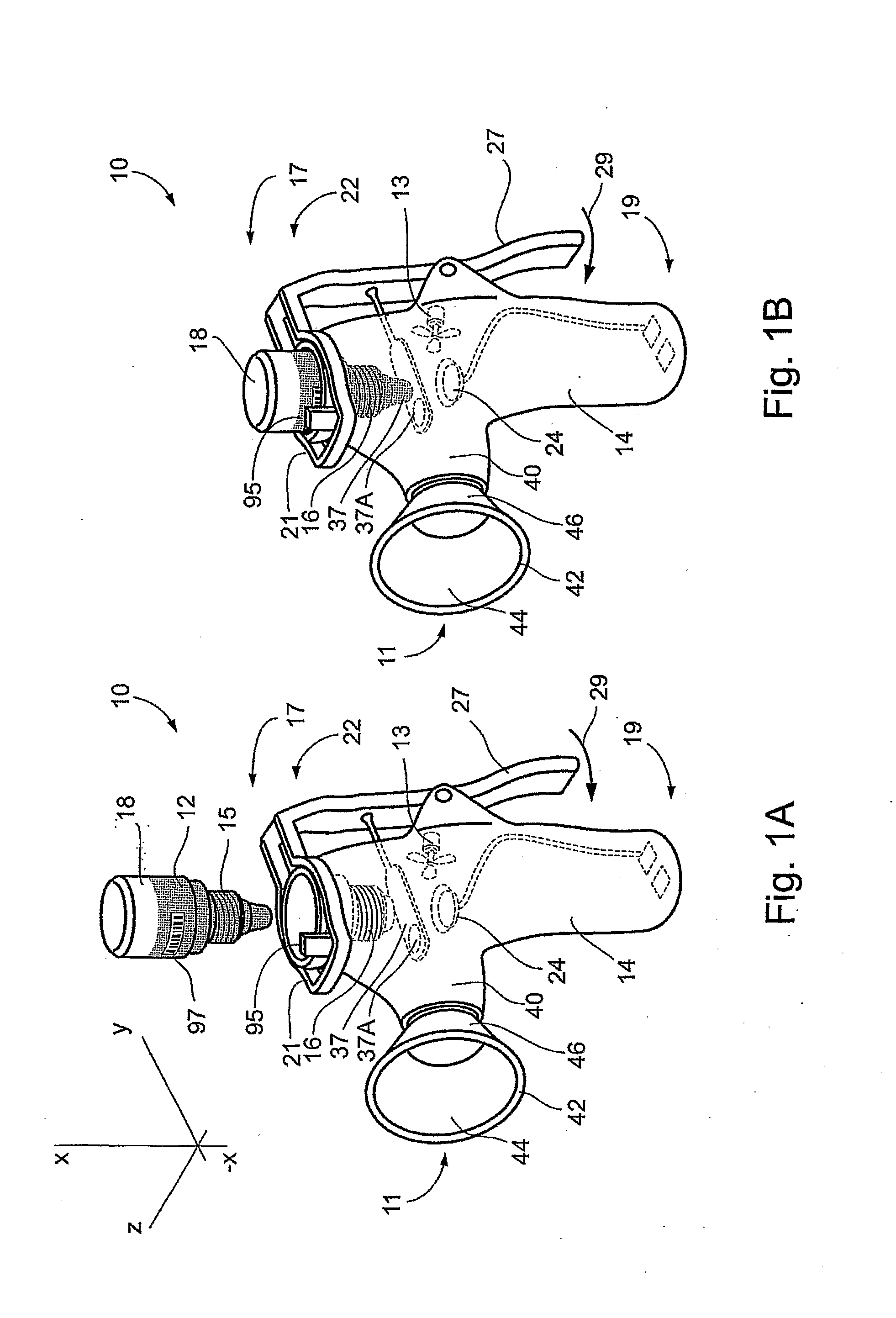
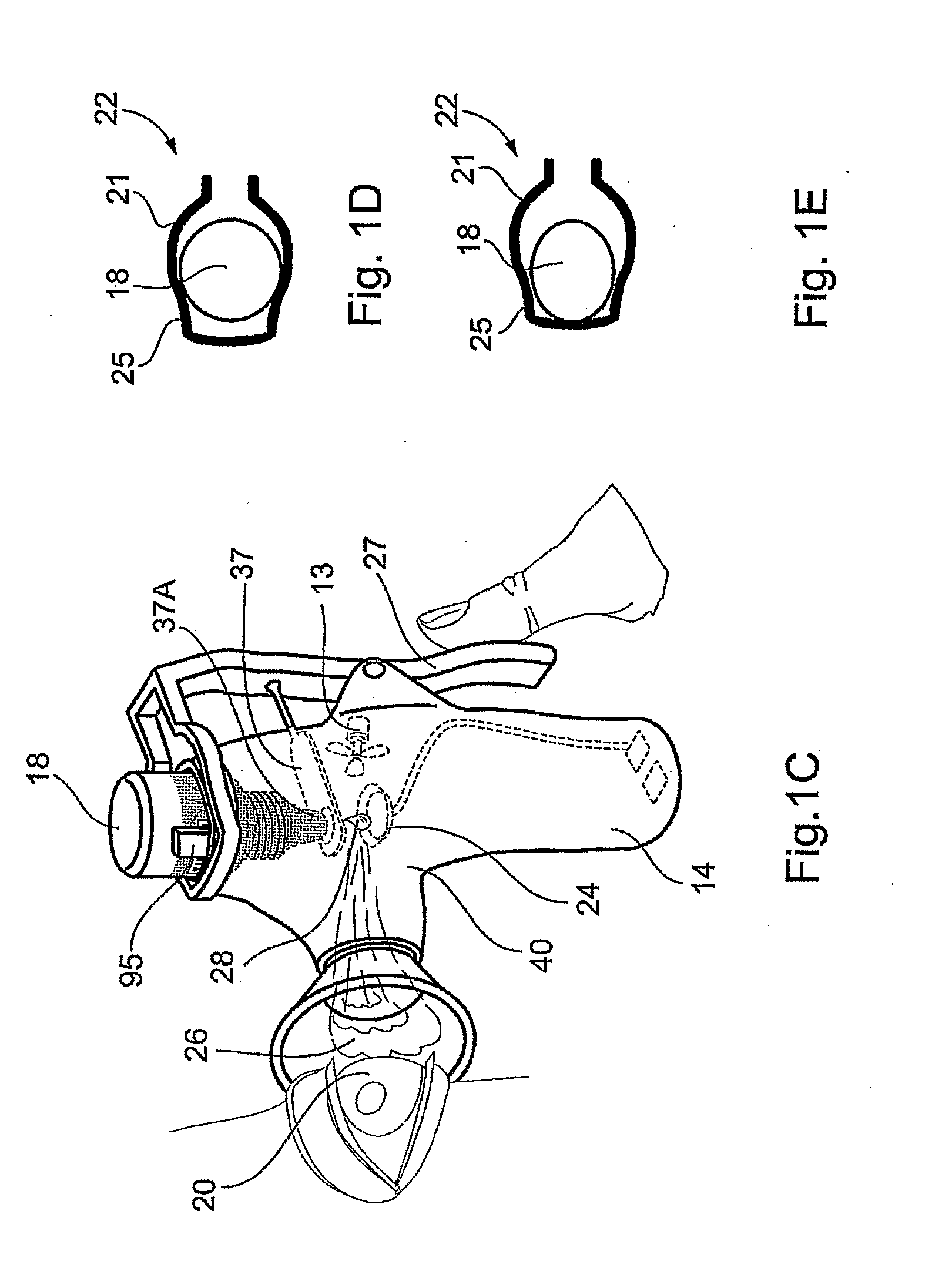
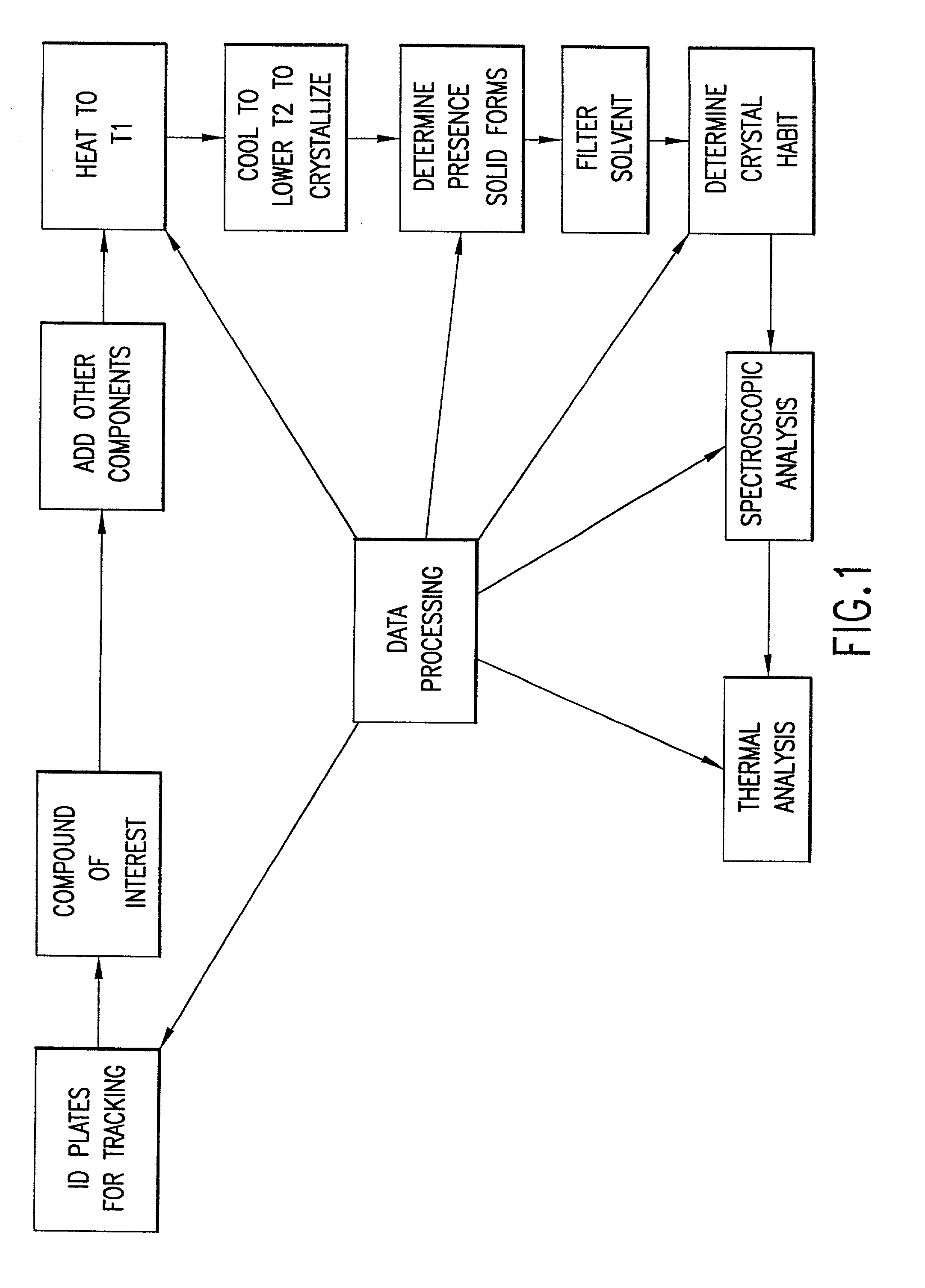
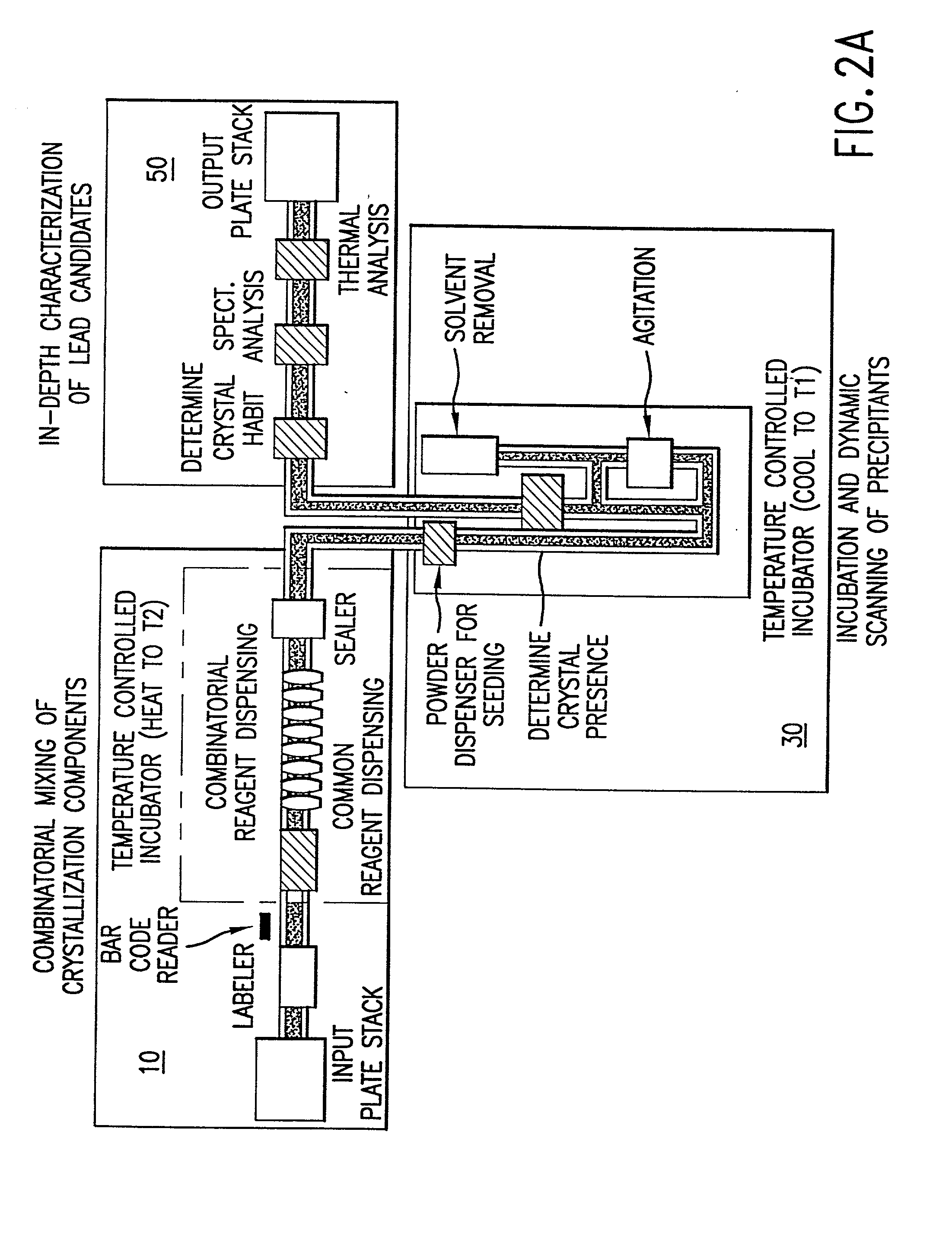
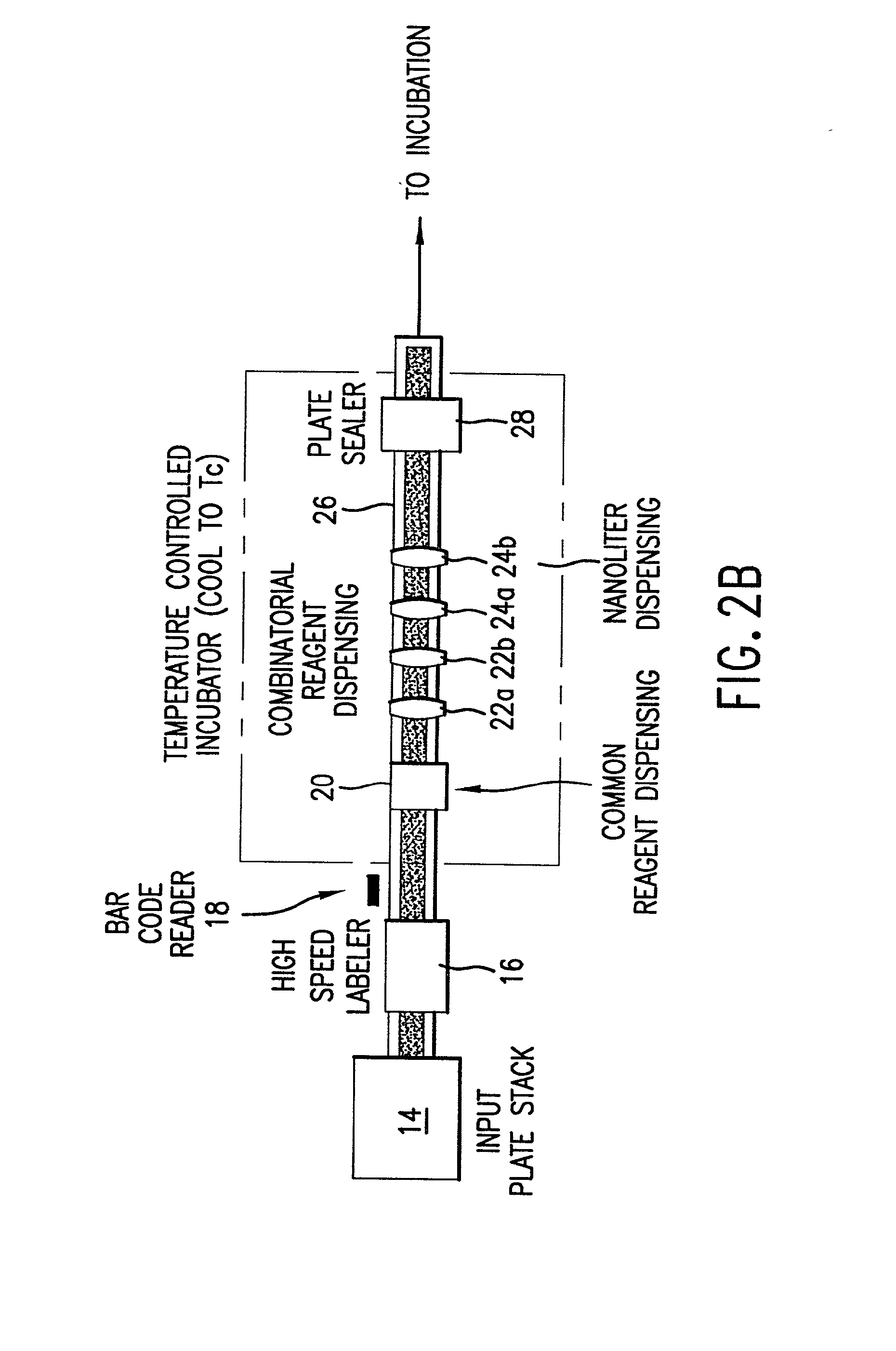
High-throughput formation, identification, and analysis of diverse solid-forms
InactiveUS20020048610A1Cost-effectiveImprove bioavailabilitySequential/parallel process reactionsFrom normal temperature solutionsSolubilitySolid mass
The invention concerns arrays of solid-forms of substances, such as compounds and rapid-screening methods therefor to identify solid-forms, particularly of pharmaceuticals, with enhanced properties. Such properties include improved bioavailability, solubility, stability, delivery, and processing and manufacturing characteristics. The invention relates to a practical and cost-effective method to rapidly screen hundreds to thousands of samples in parallel. The invention further provides methods for determining the conditions and / or ranges of conditions required to produce crystals with desired compositions, particle sizes, habits, or polymorphic forms. In a further aspect, the invention provides high-throughput methods to identify sets of conditions and / or combinations of components compatible with particular solid-forms, for example, conditions and / or components that are compatible with advantageous polymorphs of a particular pharmaceutical.
Owner:MILLENNIUM PHARMA INC +1
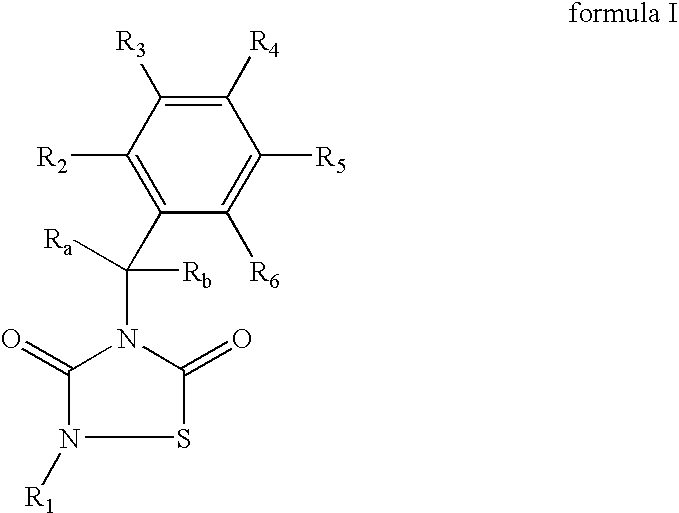
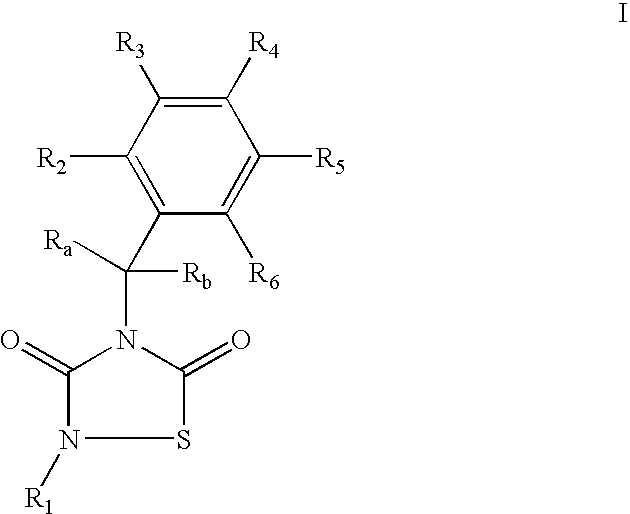
GSK-3 inhibitors
Provided are thiadiazolidine compounds of formula I wherein R1 is an organic group having at least 8 atoms selected from C or O, which is not linked directly to the N through a —C(O)— and comprising at least an aromatic ring, and their pharmaceutical compositions. These compounds are selective GSK-3 inhibitors and have improved bioavailability. They are useful for the treatment of GSK-3 mediated diseases, among others Alzheimer's disease, type II diabetes, depression and brain injury.
Owner:ASD THERAPEUTICS PARTNERS LLC
Controlled release preparation
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
Methods for preparing coated drug particles and pharmaceutical formulations thereof
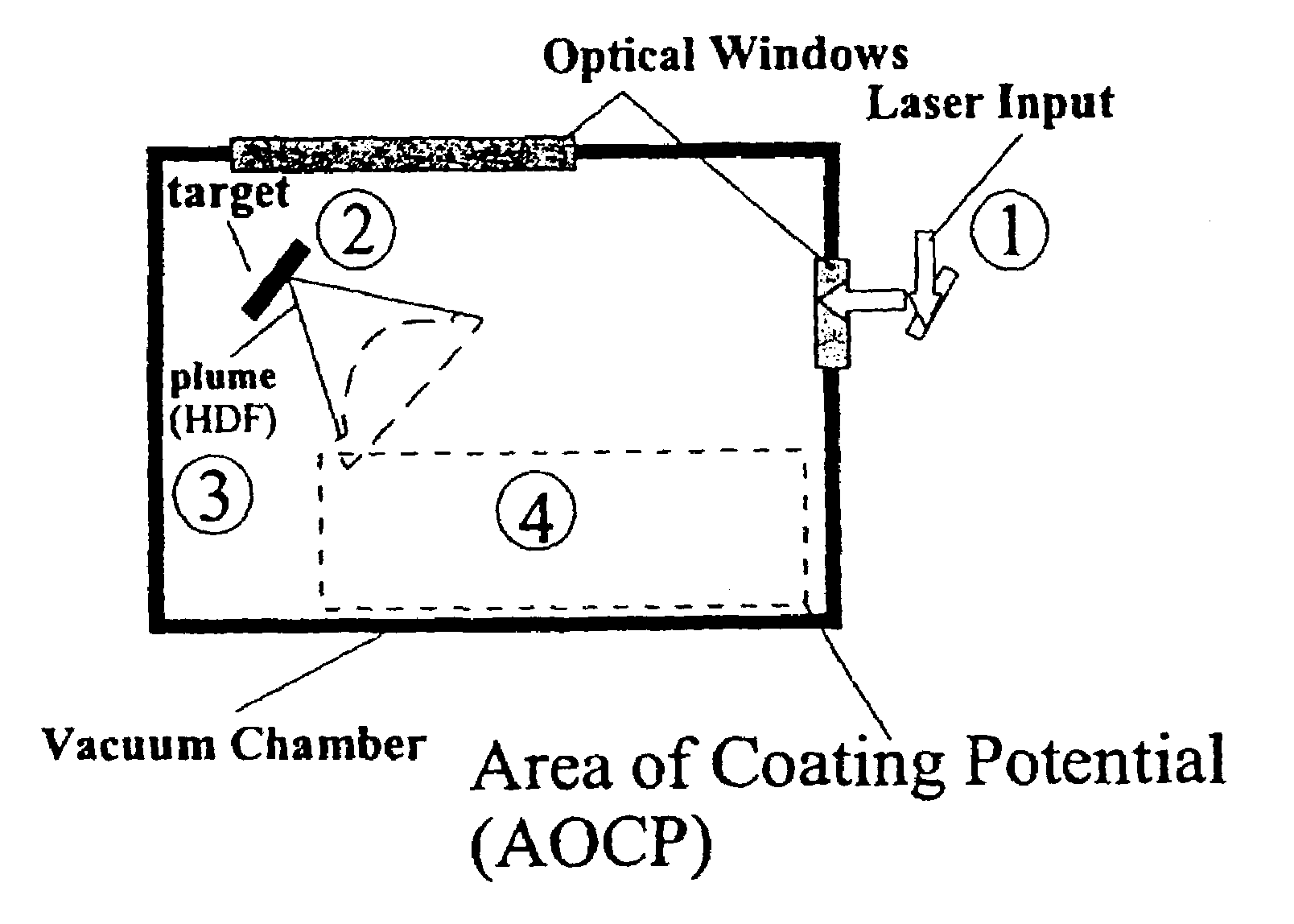
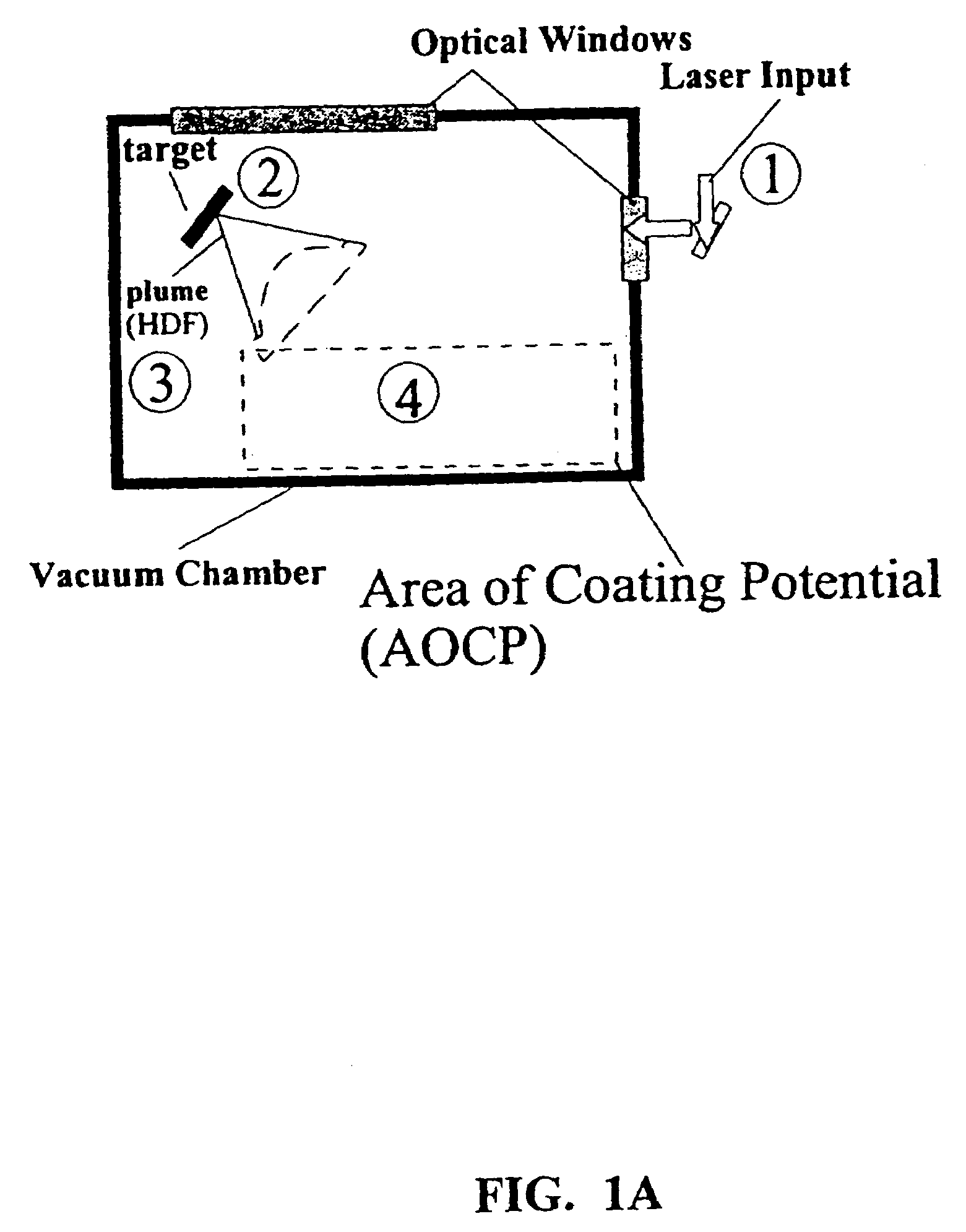
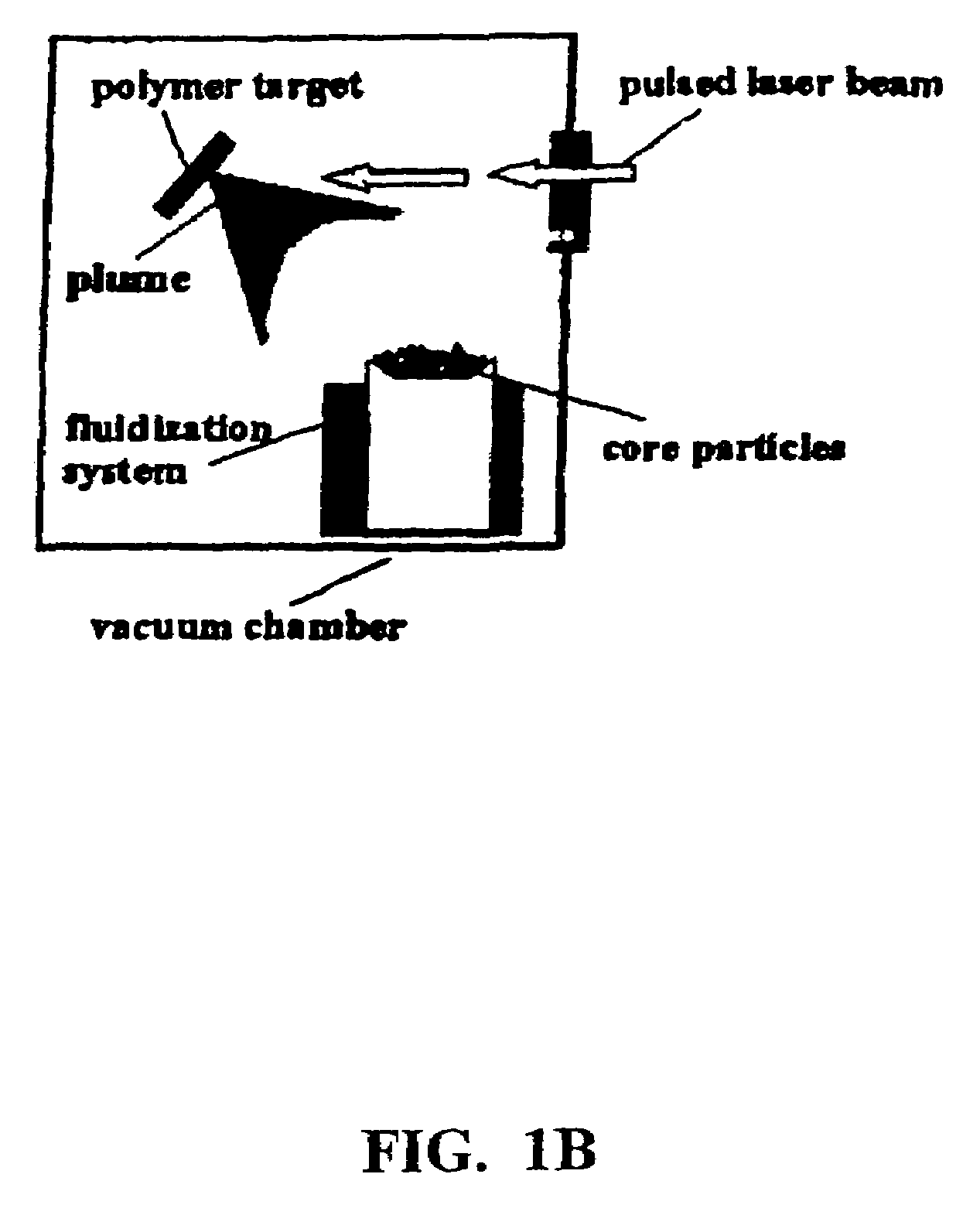
Disclosed are methods using pulsed laser ablation to prepare coated drug particles of uniform size and thickness. The coated drug particles ranged in size from several nanometers to several millimeters in diameter size, and were coated with organic polymer particle having average diameter sizes from about 1 to 50 nm. In illustrative embodiments, coated drug particles or drug delivery particles are disclosed comprising a biodegradable or biocompatible polymer coating having controlled thickness and controlled coating uniformity, that offer superior pharmaceutical properties for controlled delivery and increased bioavailability.
Owner:FLORIDA UNIV PF +2
Colon-targeted oral formulations of cytidine analogs
InactiveUS20080057086A1Improve bioavailabilityBiocideCarbohydrate active ingredientsDiseaseLower Gastrointestinal Tract
The present invention provides an oral formulation of a cytidine analog, including, 5-azacytidine, for delivery to the lower gastrointestinal tract, including, the large intestine; methods to treat diseases associated with abnormal cell proliferation by treatment with the oral formulations of the present invention; and methods to increase the bioavailability of a cytidine analog upon administration to a patient by providing an oral formulation of the present invention.
Owner:PHARMION
Compounds and methods for delivery of prostacyclin analogs
Owner:UNITED THERAPEUTICS CORP
Intra-dermal delivery of biologically active agents
InactiveUS20050163711A1Rapid uptakeFast shippingMetabolism disorderDigestive systemLymphatic vesselDiagnostic agent
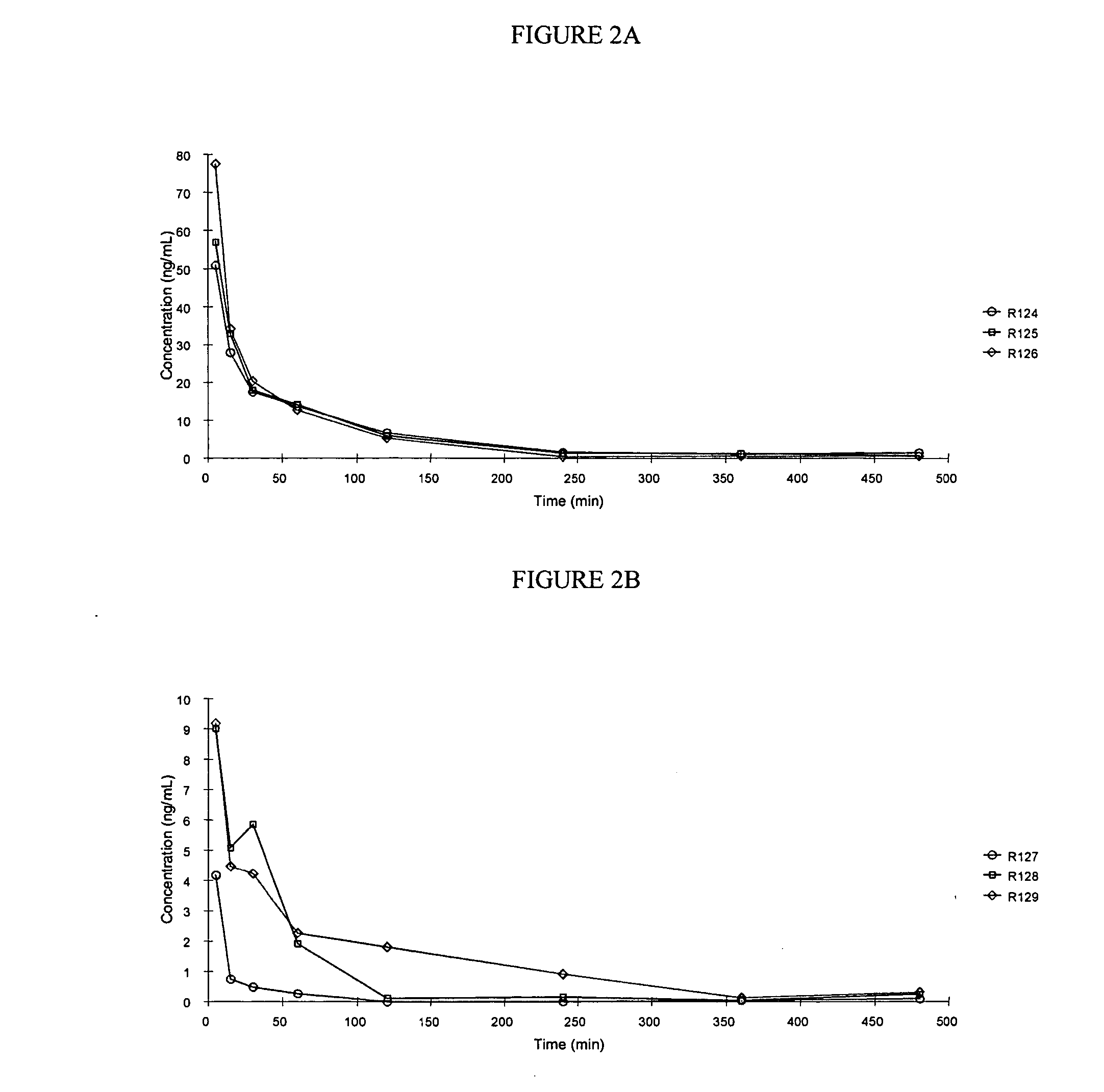
The present invention relates to methods and devices for delivering one or more biologically active agents, particularly a diagnostic agent to the intradermal compartment of a subject's skin. The present invention provides an improved method of delivery of biologically active agents in that it provides among other benefits, rapid uptake into the local lymphatics, improved targeting to a particular tissue, improved bioavailability, improved tissue bioavailability, improved tissue specific kinetics, improved deposition of a pre-selected volume of the agent to be administered, and rapid biological and pharmacodynamics and biological and pharmacokinetics. This invention provides methods for rapid transport of agents through lymphatic vasculature accessed by intradermal delivery of the agent. Methods of the invention are particularly useful for delivery of diagnostic agents.
Owner:BECTON DICKINSON & CO
Abuse-resistant amphetamine compounds
InactiveUS7105486B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsPeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Multi component controlled release system for oral care, food products, nutraceutical, and beverages
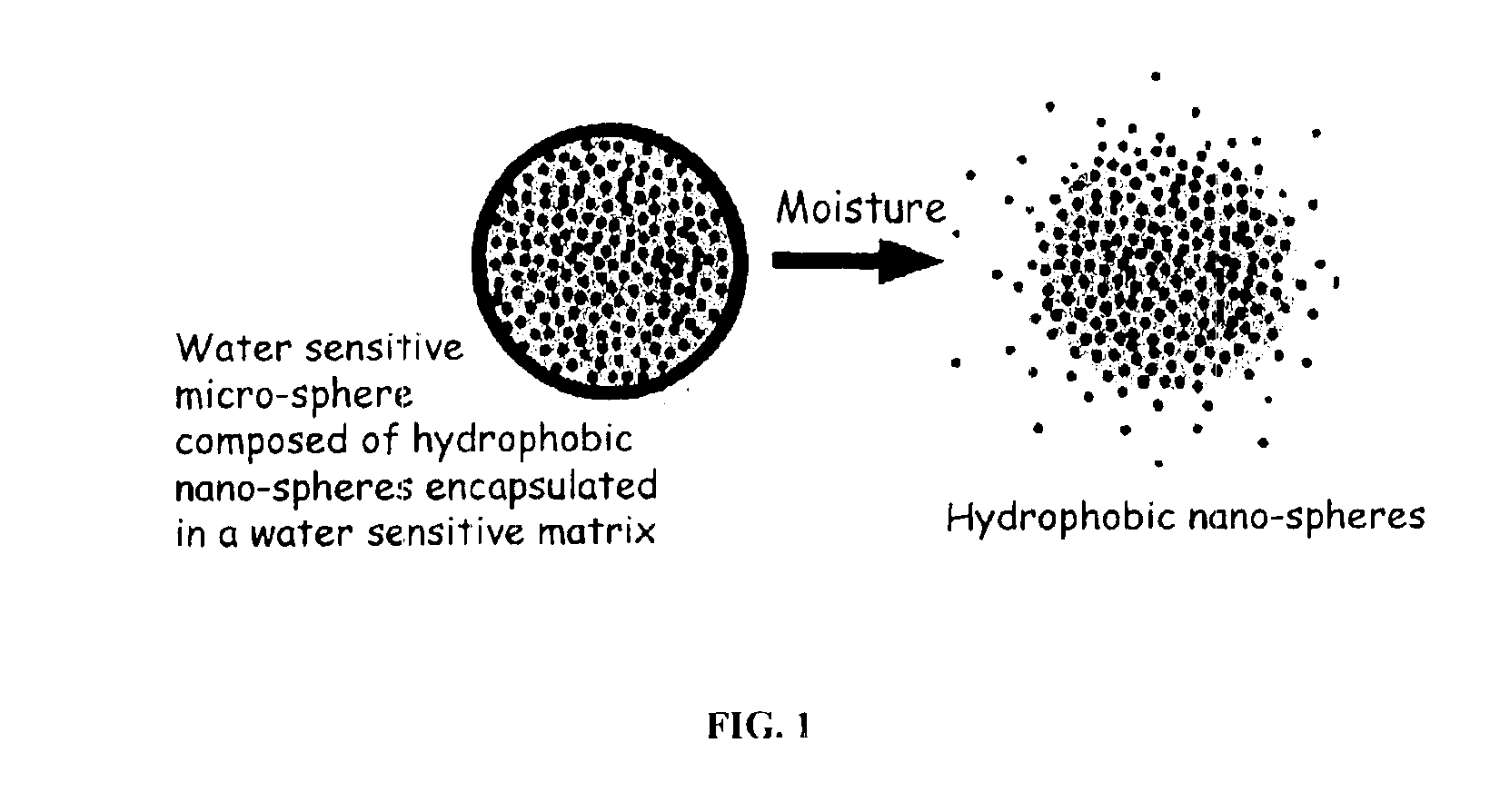
InactiveUS6887493B2Improve bioavailabilityImprove stabilityCosmetic preparationsPowder deliveryActive agentMicrosphere
The present invention relates to an improved controlled release system that can encapsulate different flavors, sensory markers, and active ingredients, or combinations of flavors, sensory markers and various active ingredients and release multiple active ingredients in a consecutive manner, one after the other. The controlled delivery system of the present invention is substantially free-flowing powder formed of solid hydrophobic nanospheres that are encapsulated in a moisture sensitive microspheres. The flavors, and active ingredients encapsulated in the hydrophobic nanospheres, in the water sensitive microsphere, or in both the nano and the microsphere. The flavors and active ingredients encapsulated in the nanospheres can be the same or different from those encapsulated in the microspheres. The encapsulation of different flavors or active agents in the various components of the system, such as nanospheres and microspheres, provides flavor transition (change in flavor character) during the use of the products. The controlled release system of the present invention enhances the stability and bioavailability of wide range of flavors, sensory markers, and other active ingredients, prolong their residence time in the oral cavity, control their release characteristics, and prolong the sensation of flavors and other sensory markers in the mouth to provide long lasting organoleptic perception or long lasting mouthfeel. The invention further relates oral care, food products, and beverages comprising the controlled release system of the present invention.
Owner:SHEFER ADI +1
Controlled release pharmaceutical compositions with improved bioavailability
PendingUS20070196396A1Efficient retentionImprove bioavailabilityHeavy metal active ingredientsBiocideControlled releaseActive agent
The present invention provides a controlled release oral pharmaceutical composition having a therapeutically effective amount of one or more pharmacologically active agent having low bioavailability; one or more solubilizers; one or more biocompatible swelling agents; and a swelling enhancer. The swelling agent, in combination with swelling enhancer, swells in the presence of water in gastric fluid such that the size of the dosage form is sufficiently increased to provide retention of the dosage form in the stomach of a patient, which gradually erodes within the gastrointestinal tract over a prolonged time period.
Owner:RUBICON RES PTY LTD
Compositions for oral drug administration
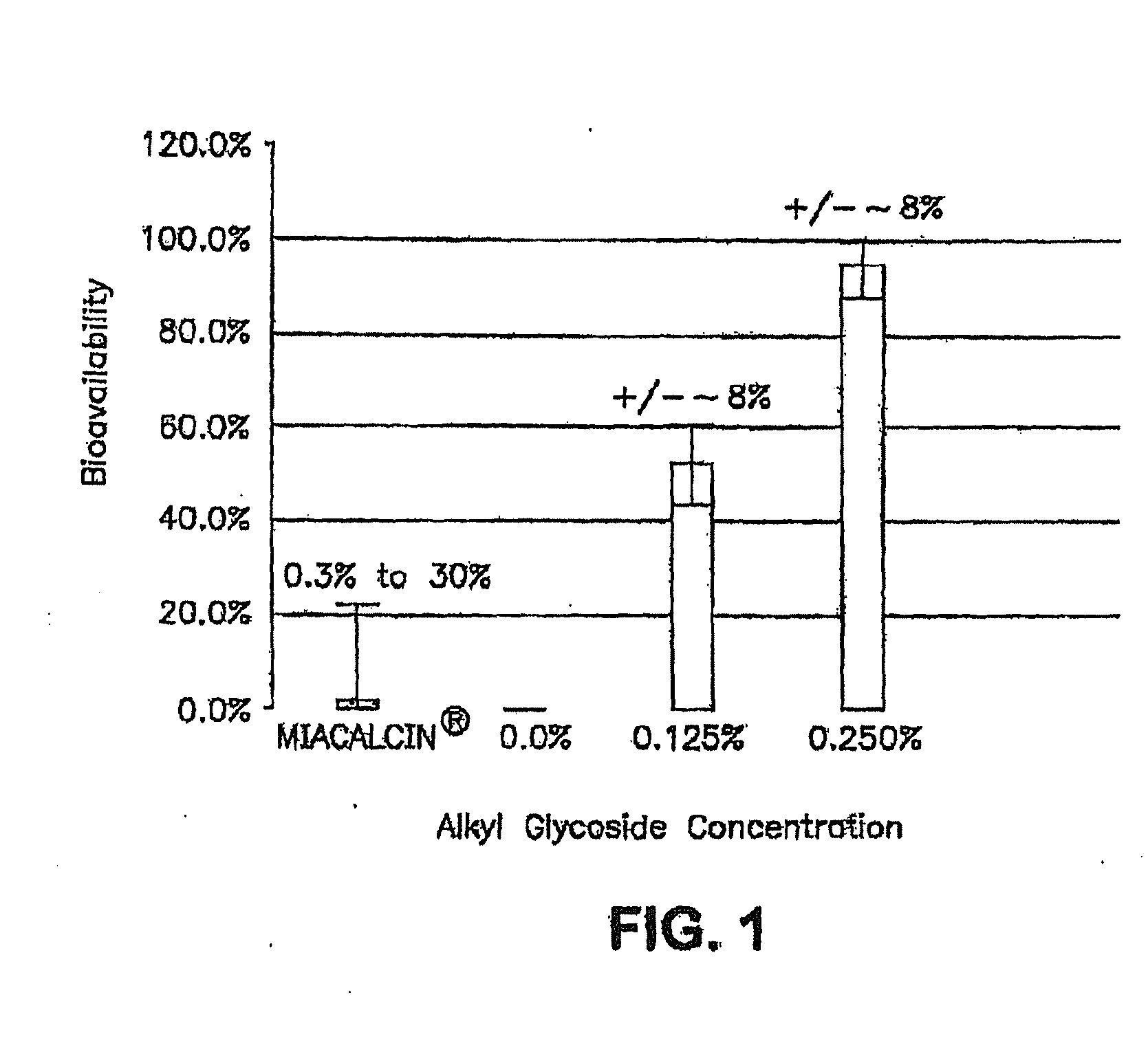
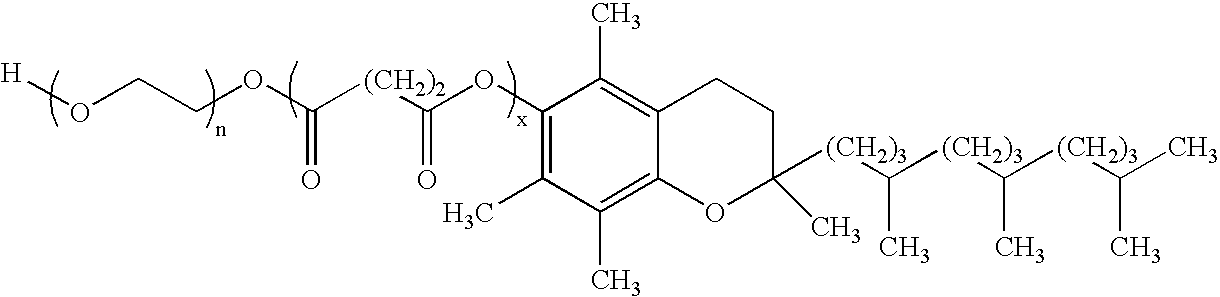
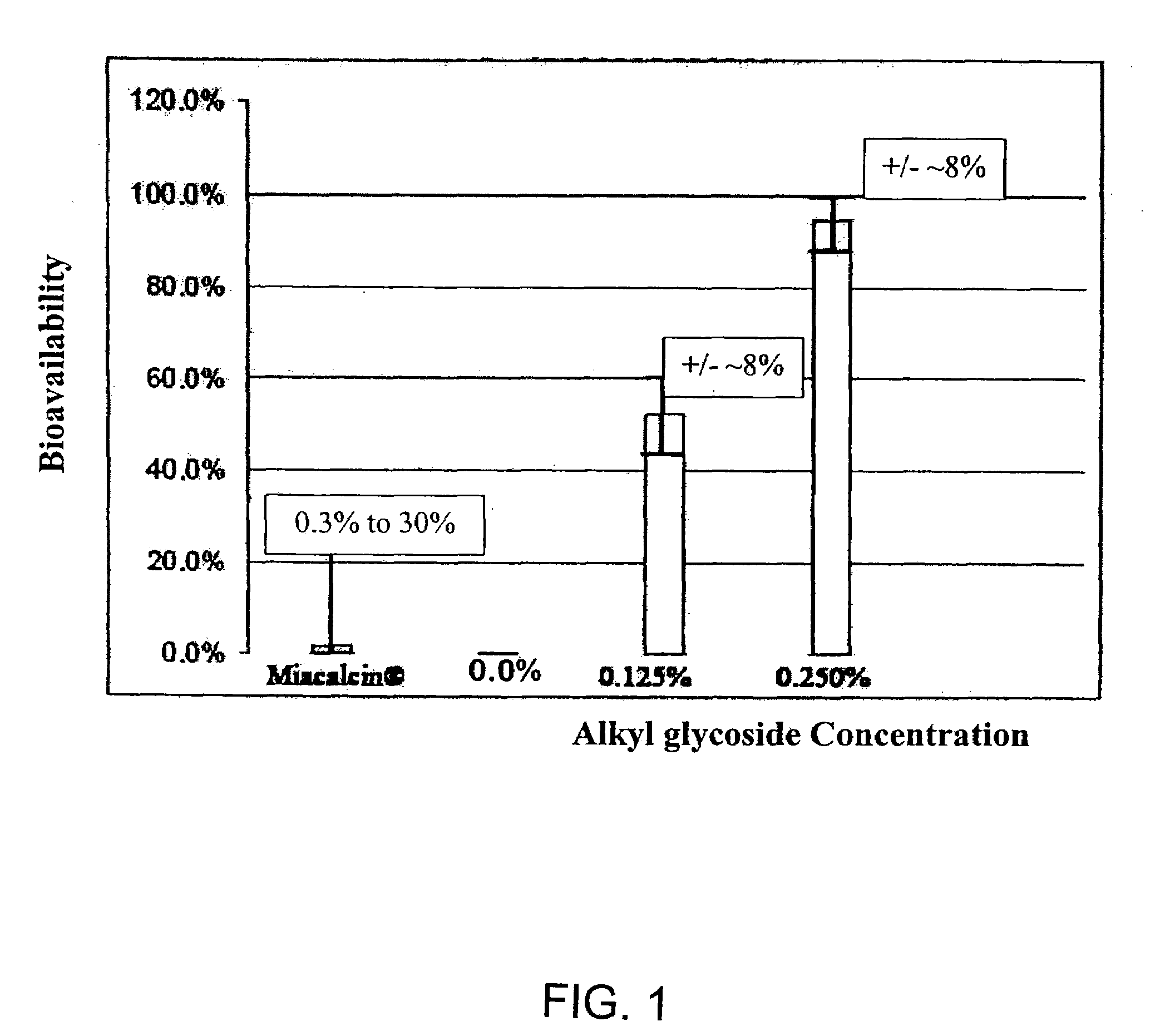
InactiveUS20140162965A1Promote absorptionImprove bioavailabilityBiocideCarbohydrate active ingredientsGlycosideOral medication
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms. In various aspects, the invention provides compositions and methods for oral delivery in the form of a tablet.
Owner:AEGIS THERAPEUTICS LLC
Bioavailability and improved delivery of alkaline pharmaceutical drugs
InactiveUS20050196418A1Easy to optimizeCosmetic preparationsPowder deliveryBioavailabilityDermatology
Embodiments of the invention relate to a composition, a process of making the composition, and to the use of the composition. The compositions include a molecular complex formed between an alkaline pharmaceutical drug and at least one selected from a hydroxyacid, a polyhydroxy acid, a related acid, a lactone, or combinations thereof. The compositions provide improved bioavailability and improved delivery of the drug into the cutaneous tissues.
Owner:YU RUEY J +1
Drug-eluting medical device
The present invention relates to a drug-eluting medical device, in particular a balloon for angioplasty catheters with drug elution to prevent the restenosis of the vessel subjected to angioplasty. More particularly, the present invention relates to a catheter balloon completely or partially coated with paclitaxel in hydrated crystalline form or in hydrated solvated crystalline form, having an immediate release and bioavailability of a therapeutically effective amount of paclitaxel at the site of intervention. The balloon can be made of a polyether-polyamide block copolymer, or a polyester amide, or polyamide-12.
Owner:INVATEC TECH CENT
Abuse resistant lysine amphetamine compounds
ActiveUS20050038121A1Prevents euphoriaLower potentialOrganic active ingredientsBiocideDiseaseAlternative treatment
The present invention describes compounds, compositions and methods of using the same comprising lysine covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Topical and transdermal delivery system utilizing submicron oil spheres
The present invention relates to a delivery system which includes a bioactive drug or cosmetic substance presented in the form of submicron oil spheres alone, or drugs or cosmetic substances in a combination with the oil spheres in an aqueous suspension or emulsion. Optionally, a skin penetration enhancer may be included in such formulations. Such preparations achieve improved bioavailability and exert larger pharmacological effects than an equivalent dose of the drug or cosmetic formulated in conventional creams, lotions or oleaginous bases.
Owner:PHARMOS
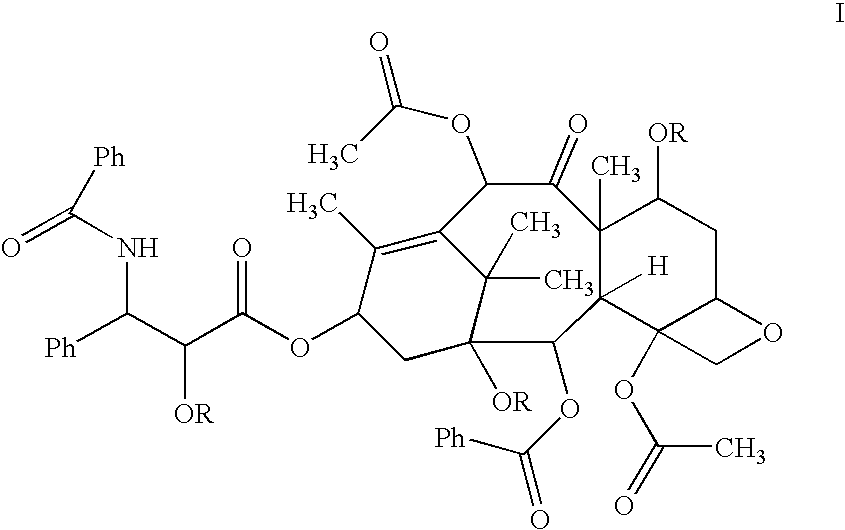
Oral pharmaceuticals formulation comprising paclitaxel, derivatives and methods of administration thereof
InactiveUS20040092428A1Improve bioavailabilityImprove oral bioavailabilityOrganic active ingredientsCyclic peptide ingredientsOral medicationBioavailability
The invention concerns excipients or combinations thereof suitable for preparing an oral formulation containing a pharmaceutical agent. More particularly, the invention is directed to stable, efficacious and bioavailable oral pharmaceutical formulations comprising paclitaxel, derivatives of paclitaxel and pharmaceutically acceptable salts thereof. The formulations of the invention increase bioavailability of paclitaxel when dissolved in the gastrointestinal system. The formulations of the invention are useful for administering paclitaxel, its derivatives, or pharmaceutically acceptable salts of such derivatives to patients in need thereof. The formulations of the invention are particularly suitable for oral administration to mammals including humans.
Owner:TRANSFORM PHARMACEUTICALS INC
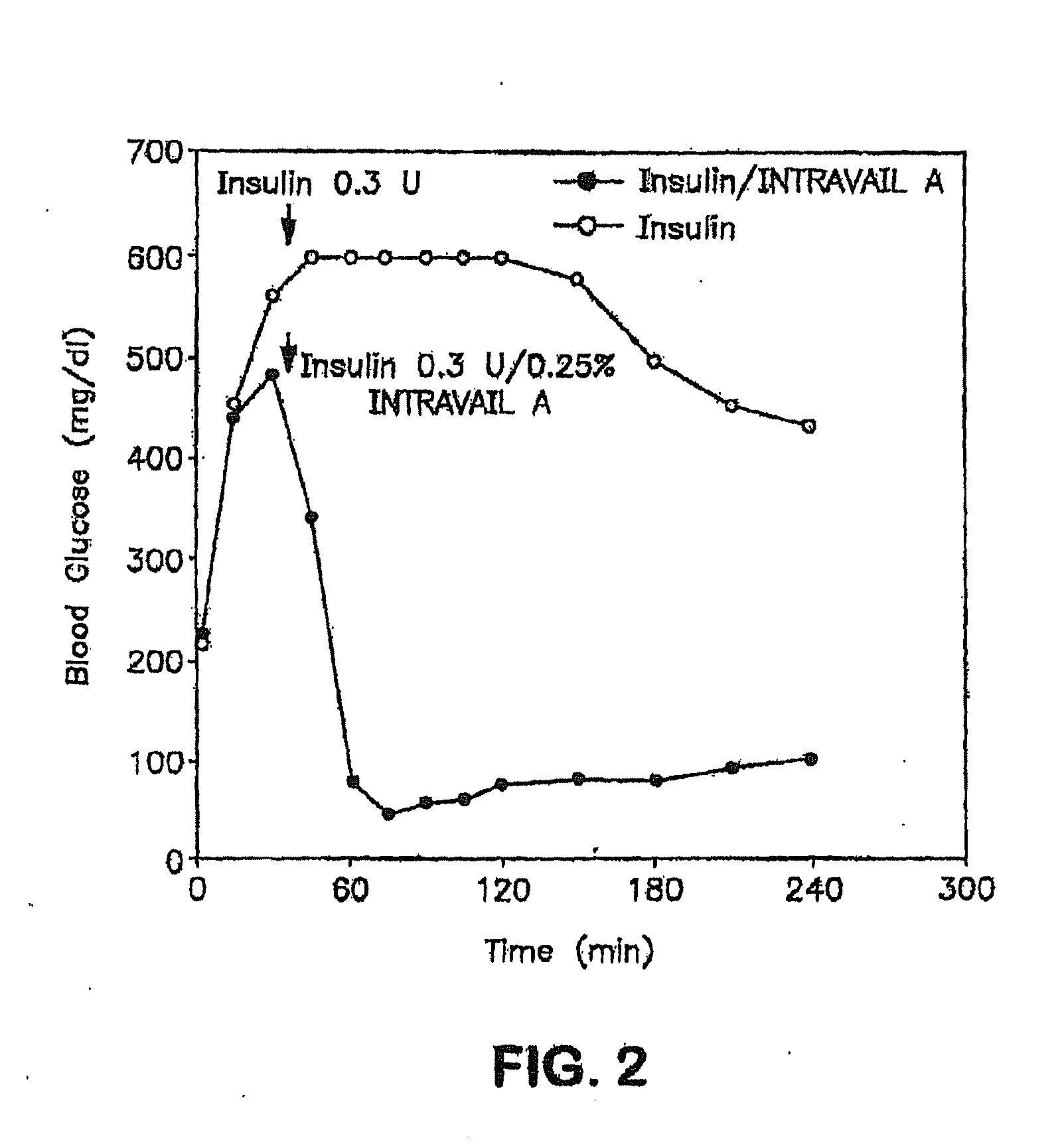
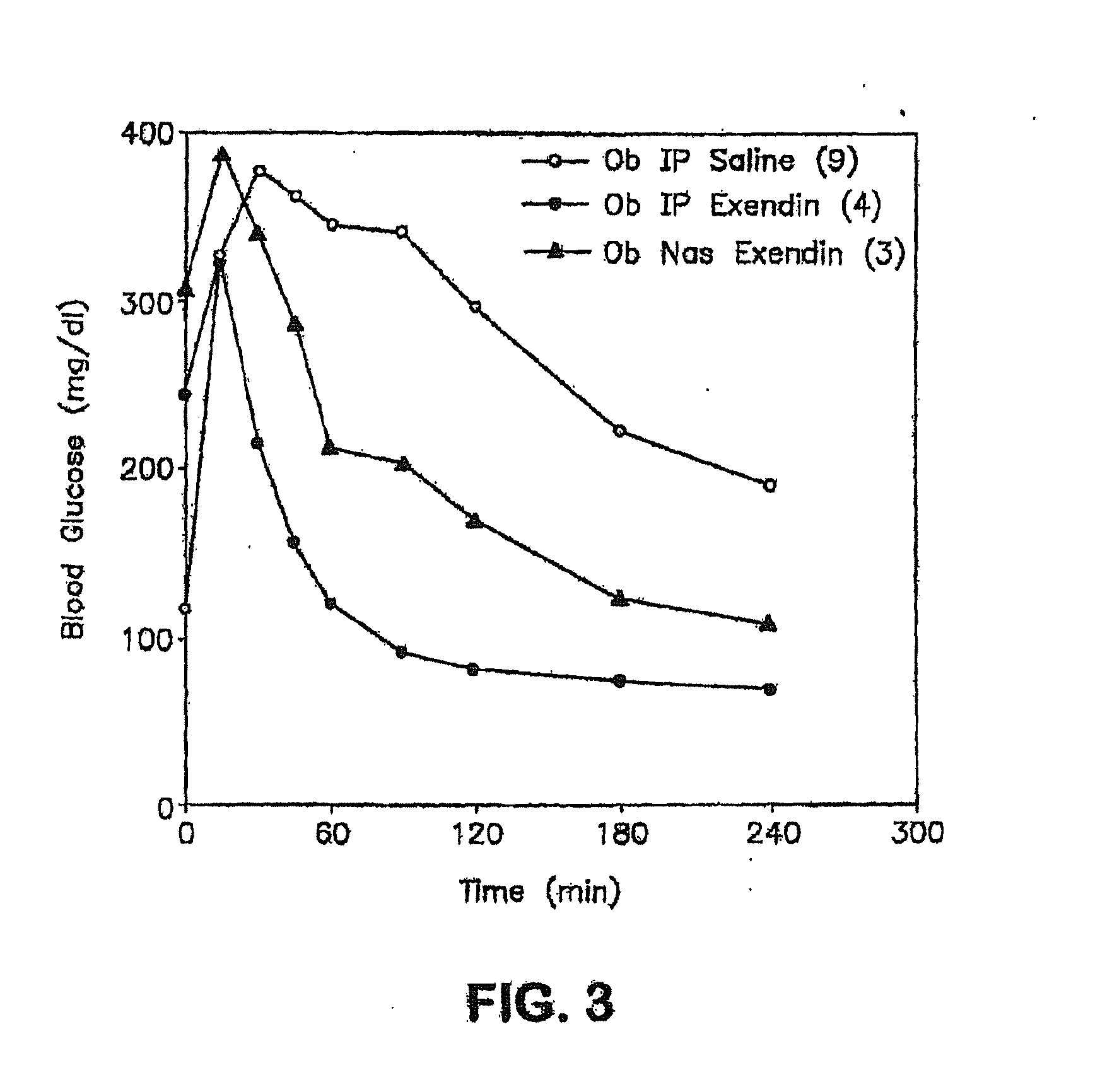
Absorption Enhancers for Drug Administration
ActiveUS20080299079A1Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderActive agentPancreatic hormone
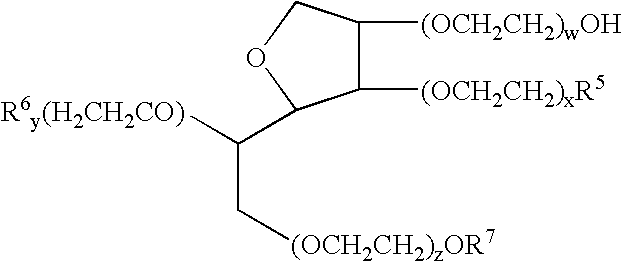
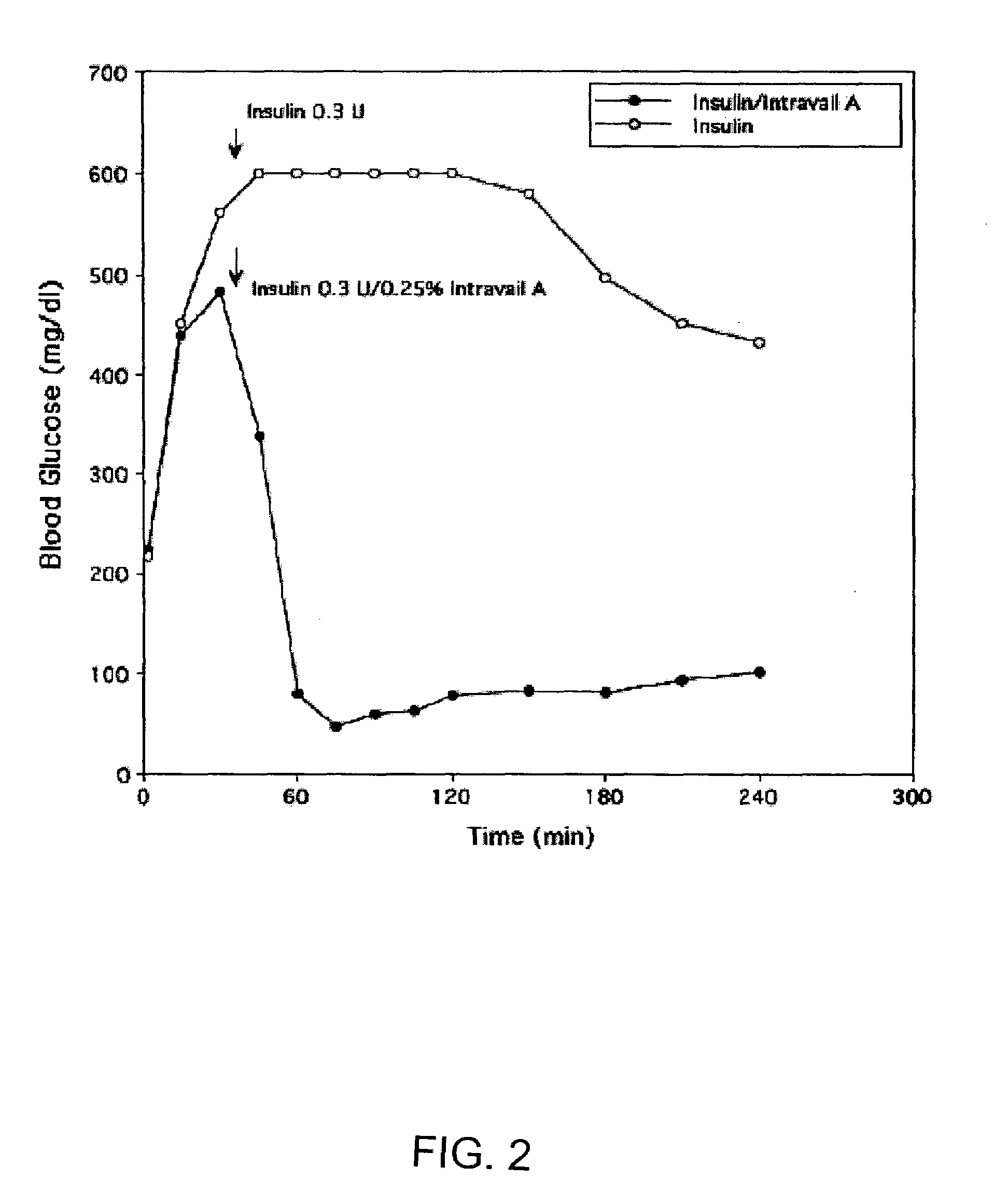
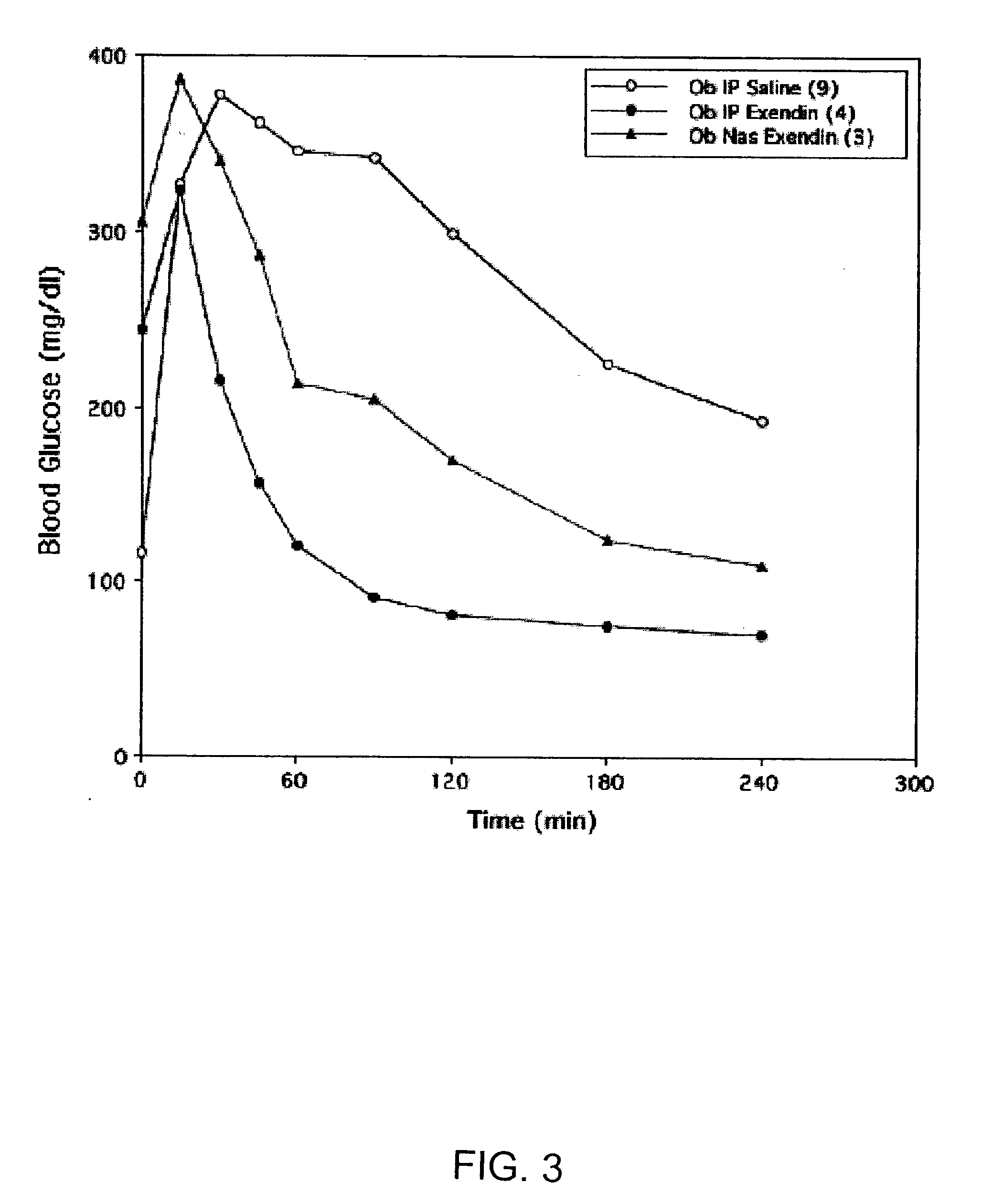
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC
Sulphonamide derivatives as prodrugs of aspartyl protease inhibitors
InactiveUS20050148548A1Good water solubilityImprove bioavailabilityBiocideSugar derivativesSulfur drugPatient compliance
The present invention relates to prodrugs of a class of sulfonamides which are aspartyl protease inhibitors. In one embodiment, this invention relates to a novel class of prodrugs of HIV aspartyl protease inhibitors characterized by favorable aqueous solubility, high oral bioavailability and facile in vivo generation of the active ingredient. This invention also relates to pharmaceutical compositions comprising these prodrugs. The prodrugs and pharmaceutical compositions of this invention are particularly well suited for decreasing the pill burden and increasing patient compliance. This invention also relates to methods of treating mammals with these prodrugs and pharmaceutical compositions.
Owner:VERTEX PHARMA INC
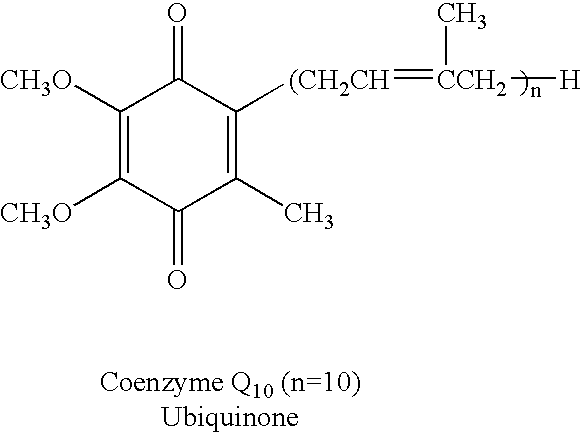
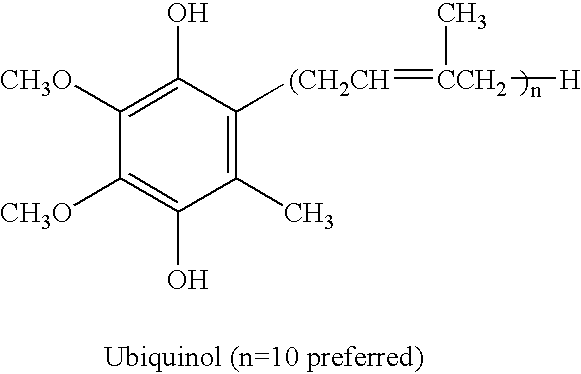
Reduced form of Coenzyme Q in high bioavailability stable oral dosage form
InactiveUS6740338B1Improve bioavailabilityReduced form requirementsBiocideEther/acetal active ingredientsOral medicationBioavailability
The present invention relates to a reduced form of Coenzyme Q also known as ubiquinol in oral dosage form such as a gelatin capsule, preferably a soft gelatin capsule. Compositions according to the present invention include storage stable compositions comprising effective amounts of ubiquinol in combination with an amount of a reducing agent effective to maintain ubiquinol in its reduced state when formulated in capsules, tablets and other orally administrable form. Methods of use are also disclosed.
Owner:QUTEN RES INST LLC
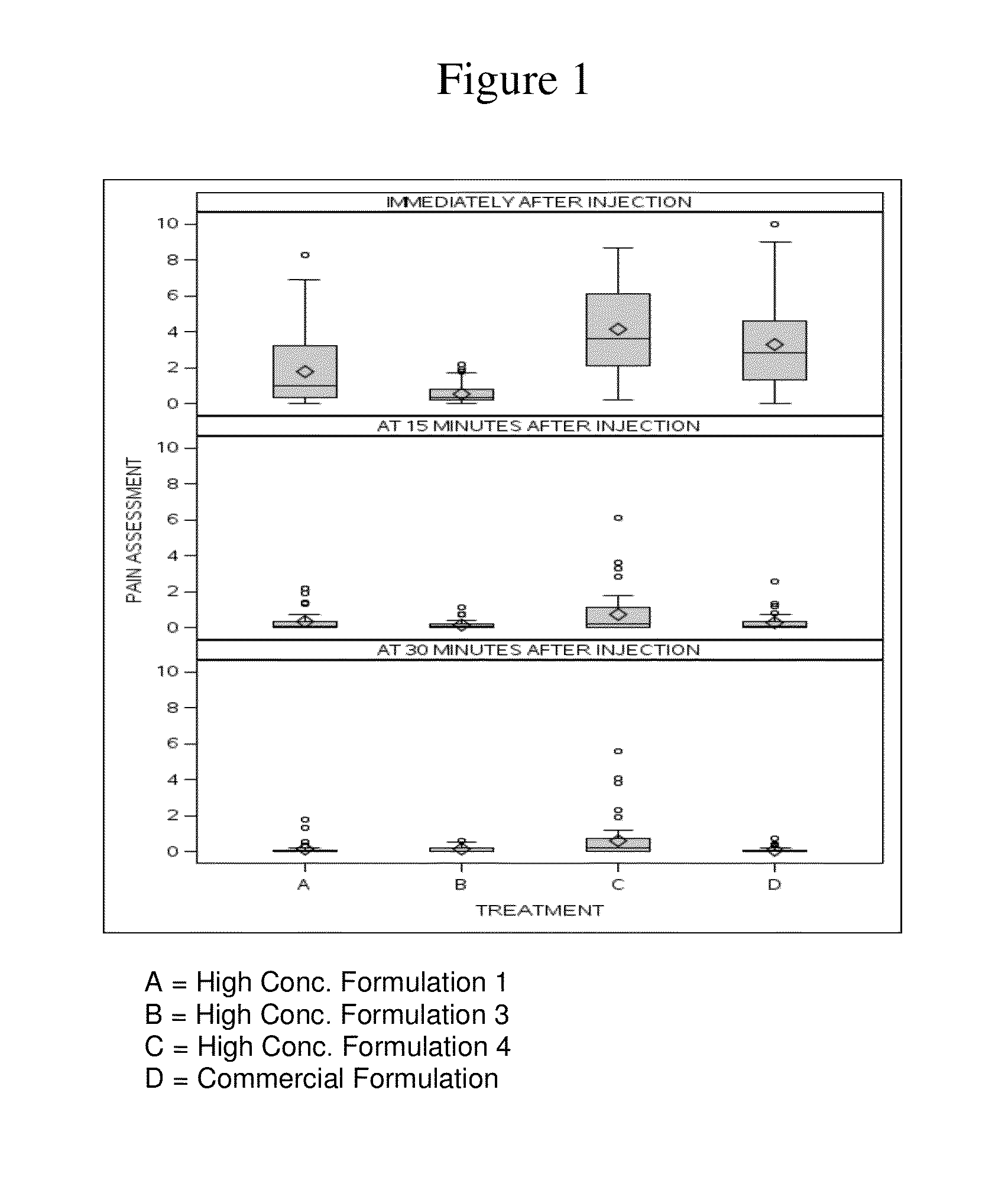
High concentration anti-TNFα antibody liquid formulations
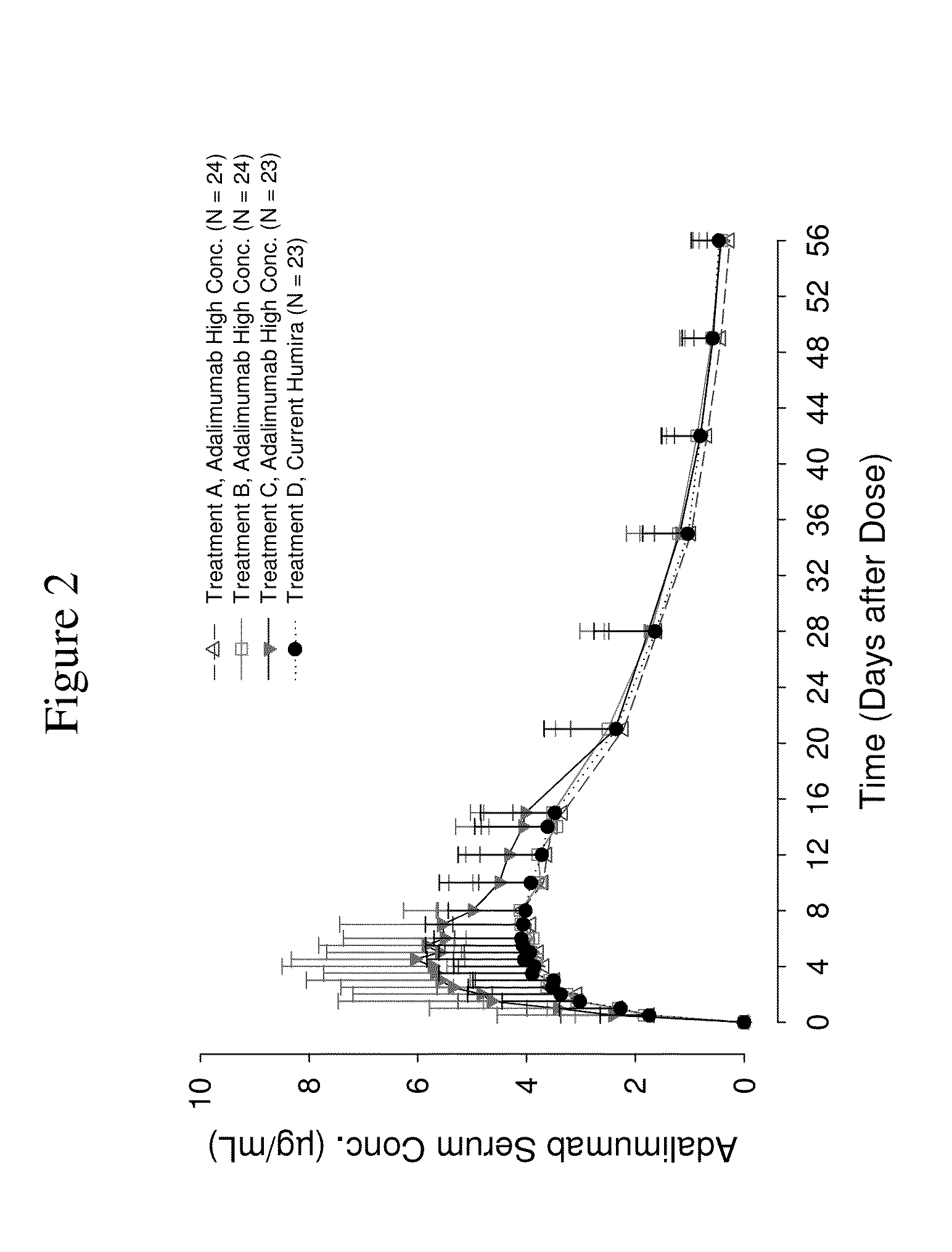
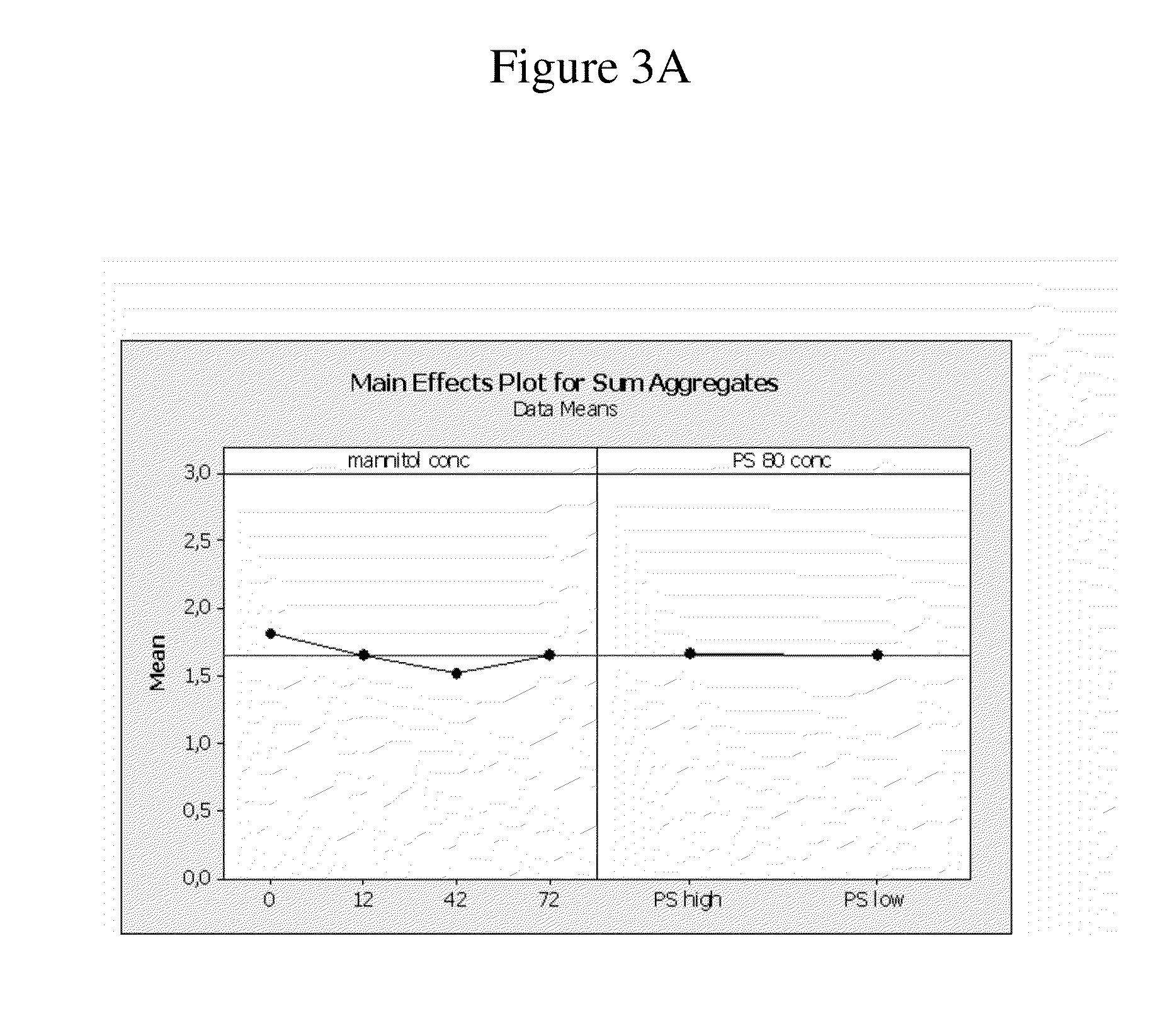
ActiveUS8821865B2Improve bioavailabilityRelieve painSenses disorderNervous disorderAntiendomysial antibodiesTherapeutic protein
The invention provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, which reduces pain associated with injection in a subject by at least about 50% when compared to injecting an otherwise identical formulation comprising at least one salt and / or at least one buffer. The invention also provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, having increased bioavailability upon subcutaneous administration into a subject. The formulation may comprise a therapeutic protein, such as a human anti-TNF-alpha antibody, or an antigen-binding portion thereof, or a biosimilar thereof.
Owner:ABBVIE BIOTECHNOLOGY LTD
Abuse-resistant amphetamine compounds
InactiveUS20050054561A1Prevents euphoriaLower potentialBiocidePeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Polymeric drug delivery system for hydrophobic drugs
InactiveUS20050249799A1Low oral bioavailabilityStable against aggregationAntibacterial agentsPowder deliveryHydrophobic polymerImmediate release
An oral delivery system for Class II drugs that have low oral bioavailability due to their insolubility in water and slow dissolution kinetics and method for making such a drug delivery system are disclosed herein. The formulation may be a controlled release or immediate release formulation. The immediate release formulation contains a Class II drug, together with a hydrophobic polymer, preferably a bioadhesive polymer. In one embodiment, the drug and polymer are co-dissolved in a common solvent. The solution is formed into small solid particles by any convenient method, particularly by spray drying. The resulting particles contain drug dispersed as small particles in a polymeric matrix. The particles are stable against aggregation, and can be put into capsules or tableted for administration. The controlled release formulations contain a BCS Class II drug and a bioadhesive polymer. The controlled release formulations may be in the form of a tablet, capsules, mini-tab, microparticulate, or osmotic pump. Enhancement of oral uptake of the drug from use of bioadhesive polymers occurs through (1) increased dissolution kinetics due to stable micronization of the drug, (2) rapid release of the drug from the polymer in the GI tract; and (3) prolonged GI transit due to bioadhesive properties of the polymers. The combination of these effects allows the preparation of a compact, stable dosage form suitable for oral administration of many class II drugs.
Owner:SPHERICS
Method for improving the bioavailability of orally delivered therapeutics
The disclosed invention is a method and composition for improving the bioavailability of a pharmaceutically active ingredient comprising an oral dosage form consisting essentially of a granulation of active ingredient, amino acid, and hydrophilic polymer, wherein the granulation is dispersed in an immediate release or extended release excipient.
Owner:SCOLR PHARMA
Bioadhesive polymers with catechol functionality
InactiveUS20050201974A1Good bioadhesionExtended stayNervous disorderPill deliveryArameHydrophobic polymer
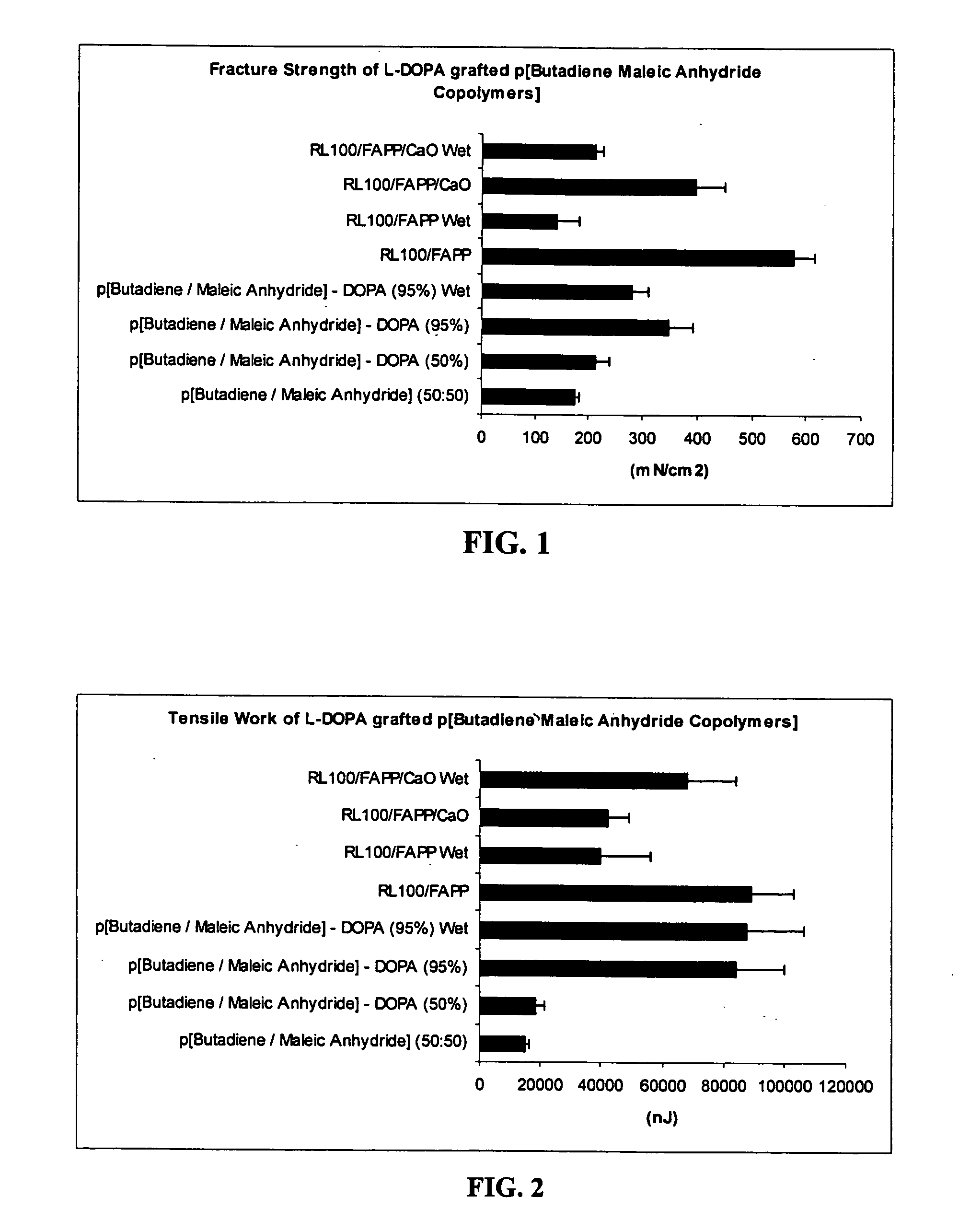
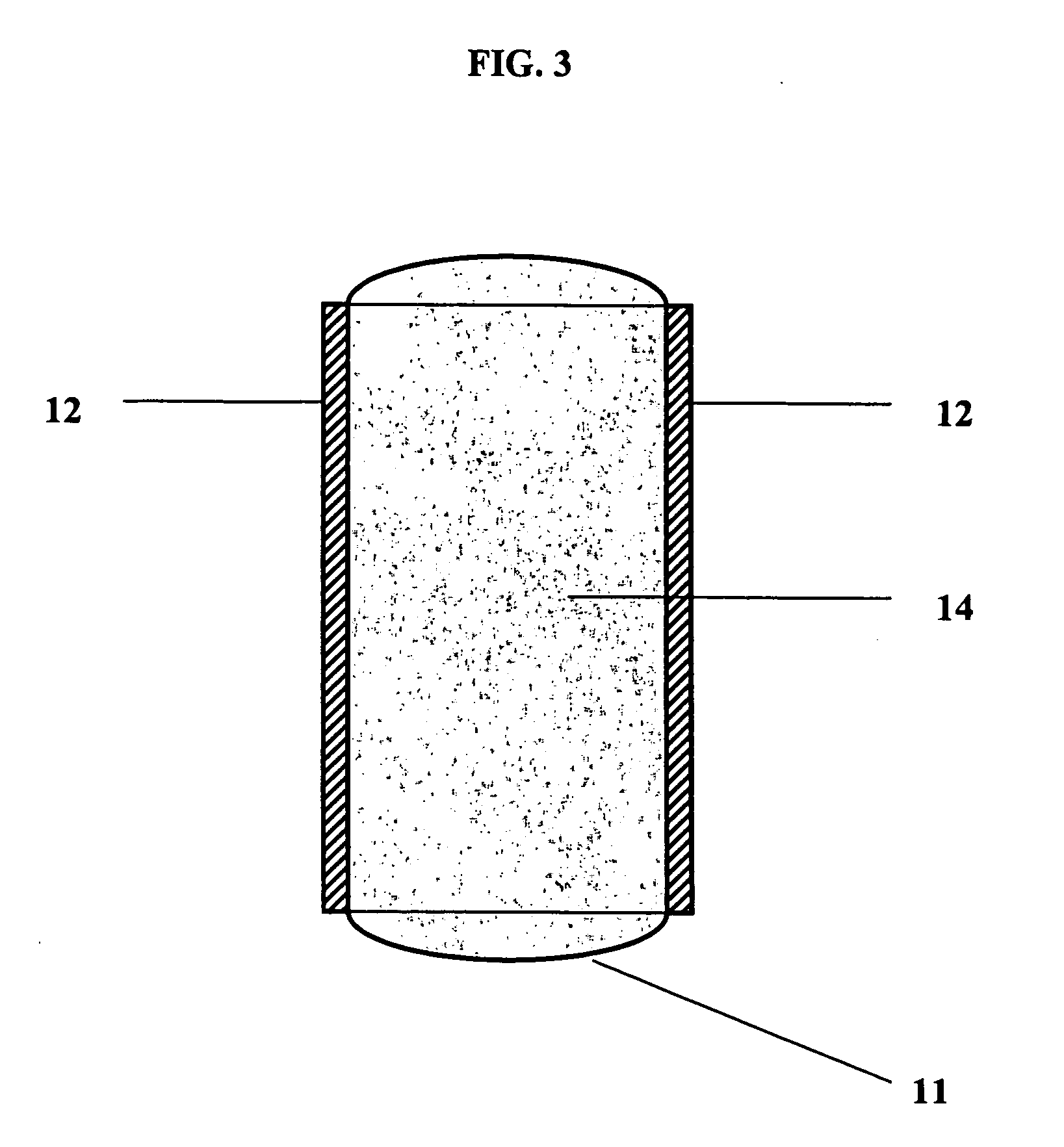
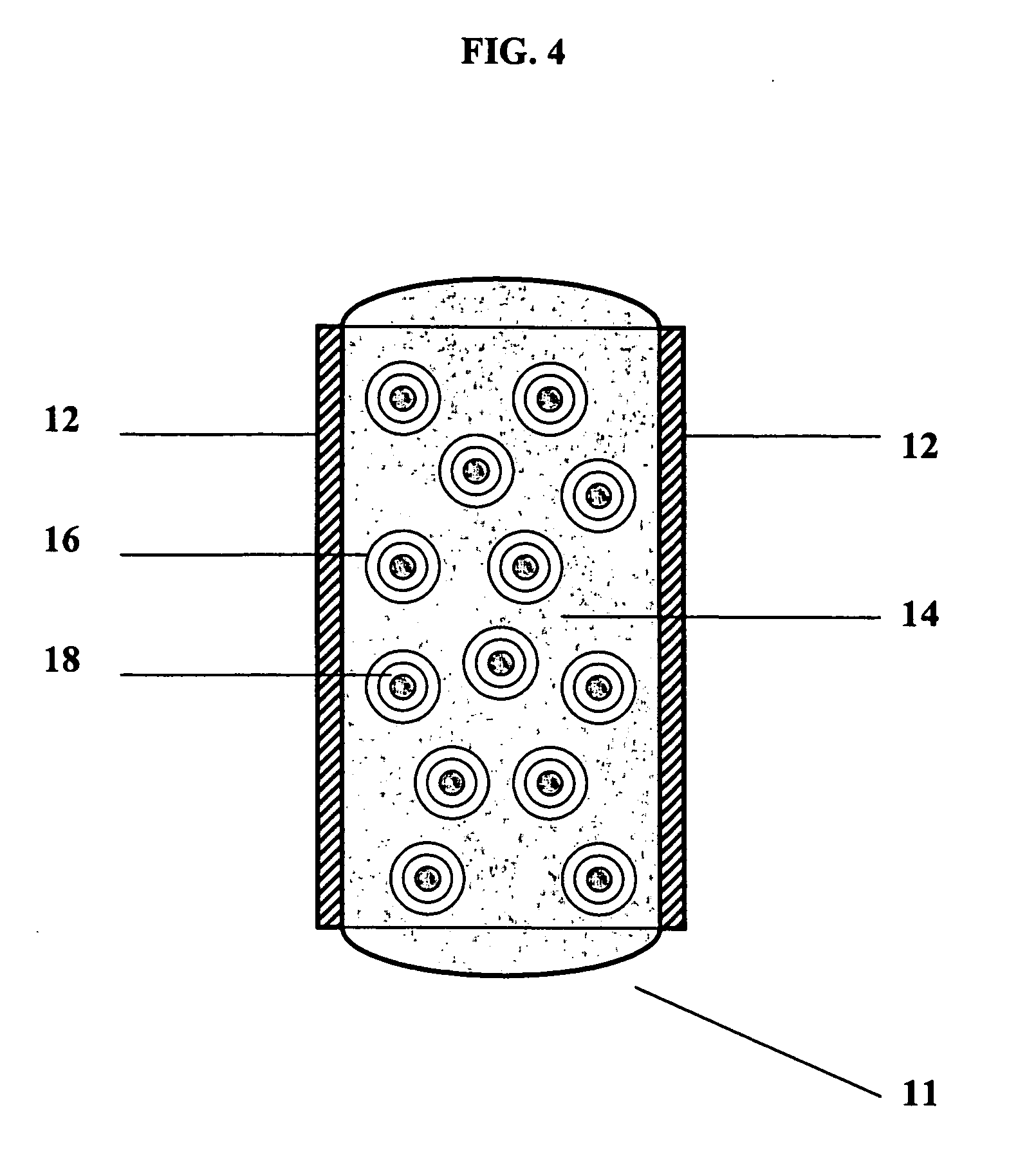
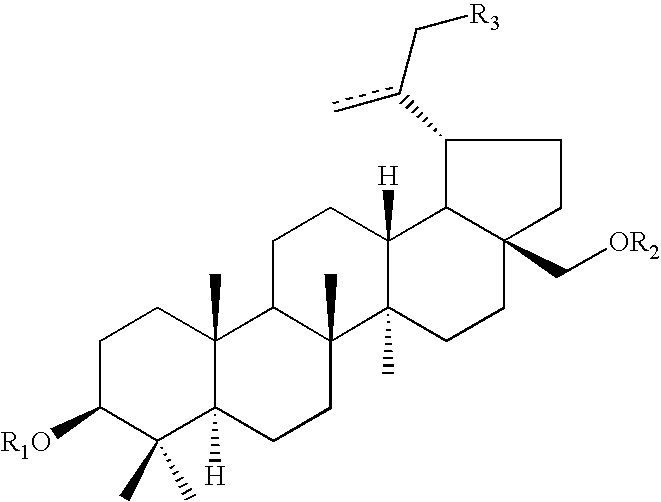
Polymers with improved bioadhesive properties and methods for improving bioadhesion of polymers have been developed. A compound containing an aromatic group which contains one or more hydroxyl groups is grafted onto a polymer or coupled to individual monomers. In one embodiment, the polymer is a biodegradable polymer. In another embodiment, the monomers may be polymerized to form any type of polymer, including biodegradable and non-biodegradable polymers. In some embodiments, the polymer is a hydrophobic polymer. In the preferred embodiment, the aromatic compound is catechol or a derivative thereof and the polymer contains reactive functional groups. In the most preferred embodiment, the polymer is a polyanhydride and the aromatic compound is the catechol derivative, DOPA. These materials display bioadhesive properties superior to conventional bioadhesives used in therapeutic and diagnostic applications. These bioadhesive materials can be used to fabricate new drug delivery or diagnostic systems with increased residence time at tissue surfaces, and consequently increase the bioavailability of a drug or a diagnostic agent. In a preferred embodiment, the bioadhesive material is a coating on a controlled release oral dosage formulation and / or forms a matrix in an oral dosage formulation.
Owner:SPHERICS
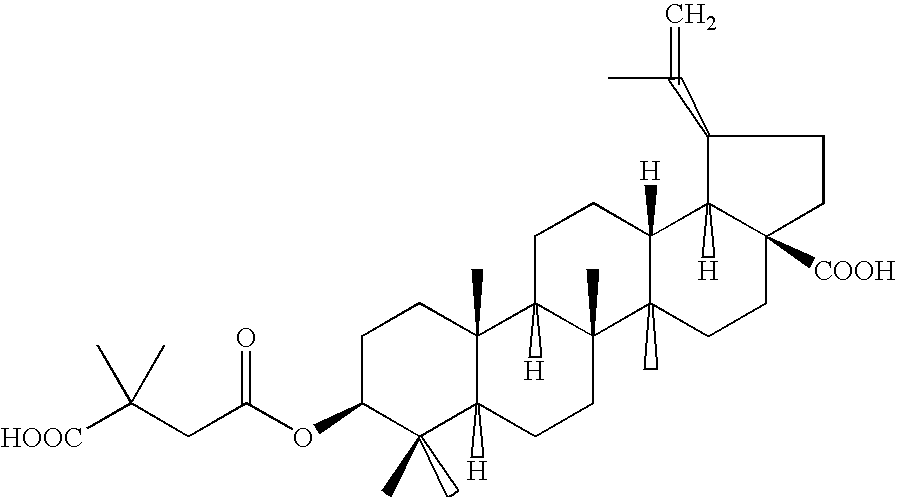
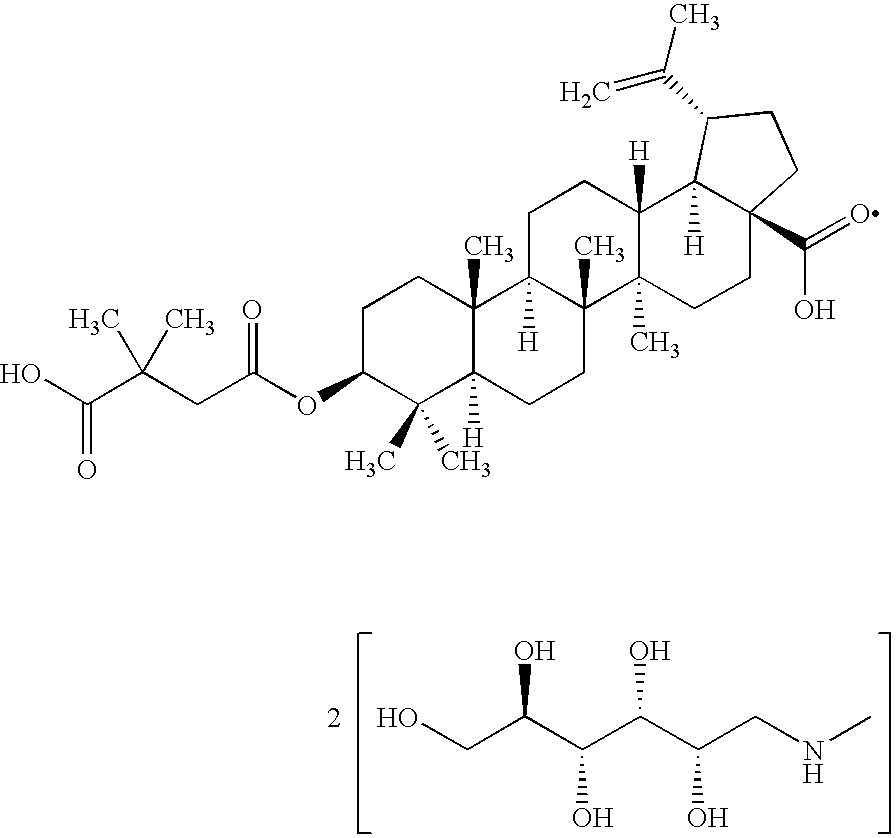
Pharmaceutical salts of 3-O-(3',3'-dimethylsuccinyl) betulinic acid
Salts of 3-O-(3′,3′-dimethylsuccinyl)Betulinic acid (DSB) are disclosed. Particularly, the preparation, pharmaceutical evaluation, and in vivo bioavailability evaluation of N-methyl-D-glucamine and alkali metal salt forms of DSB are disclosed. Pharmaceutical compositions including these salt forms are used in methods of treating HIV and related diseases. Methods of making the salts of DSB and the pharmaceutical compositions are also provided.
Owner:MYREXIS INC
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs
InactiveUS20100137271A1Improve bioavailabilitySuture equipmentsOrganic active ingredientsActive agentBioavailability
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs are provided. The pharmaceutical compositions include a therapeutically effective amount of a hydrophobic drug, preferably a steroid; a solubilizer, and a surfactant. The synergistic effect between the hydrophobic drug and the solubilizer results in a pharmaceutical formulation with improved dispersion of both the active agent and the solubilizer. As a result of the improved dispersion, the pharmaceutical composition has improved bioavailability upon administration. Methods of improving the bioavailability of hydrophobic drugs administered to a patient are also provided.
Owner:LIPOCINE
Oral GLP-1 formulations
InactiveUS20060286129A1Facilitate oral deliveryBioavailabilityPeptide/protein ingredientsMetabolism disorderBioavailabilityDrug
The present invention provides phamaceutical compositions comprising at least one delivery agent and GLP-1. These pharmaceutical compositions facilitate the oral delivery of GLP-1, providing improved (e.g. increased) bioavailability of GLP-1 compared to administration of GLP-1 without a delivery agent.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com