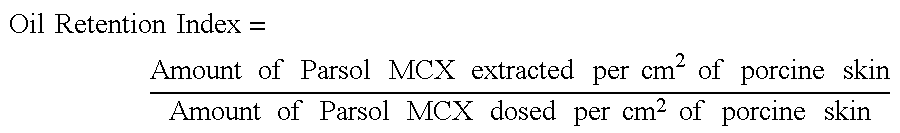Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6236results about How to "Fast absorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
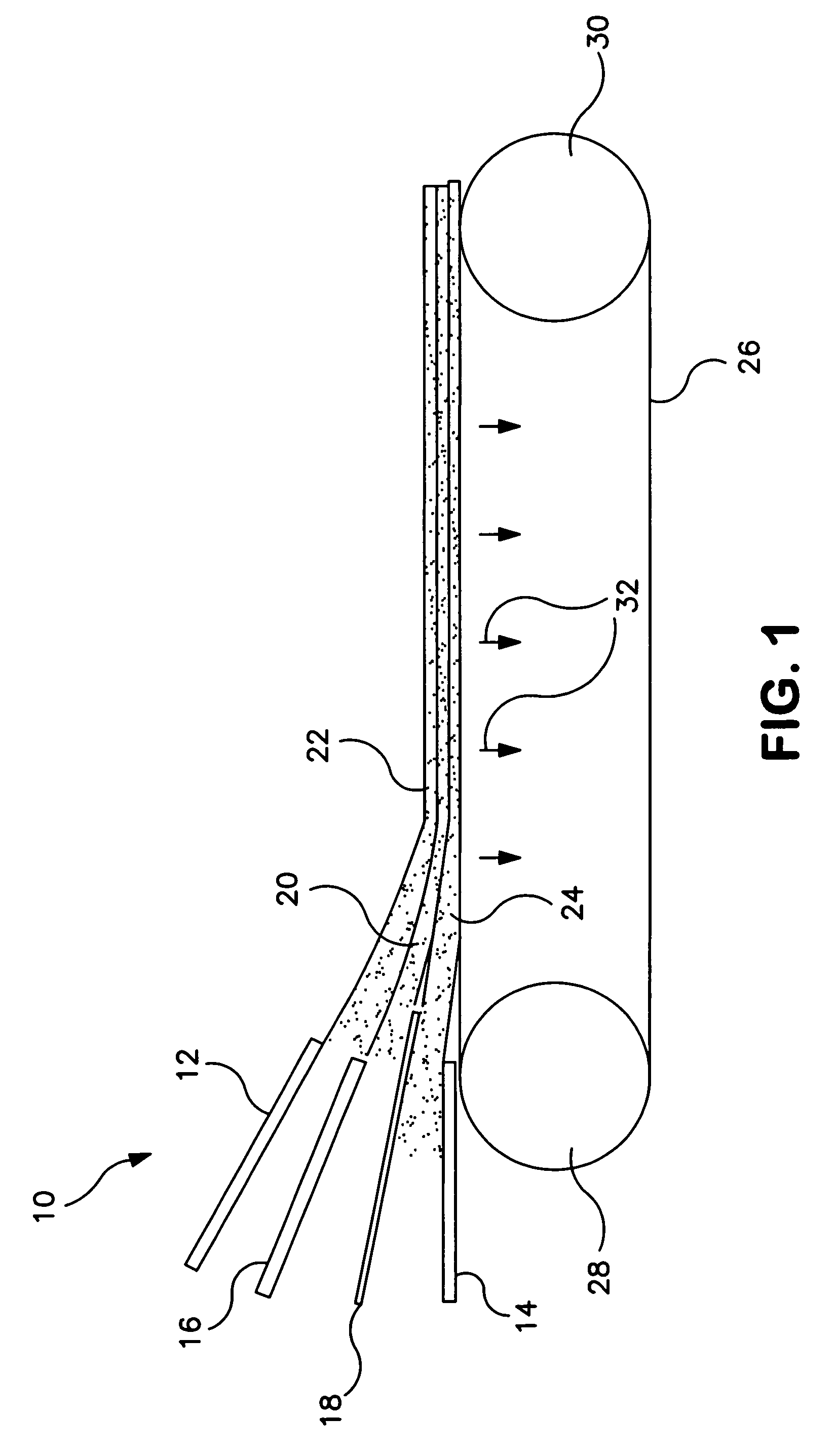
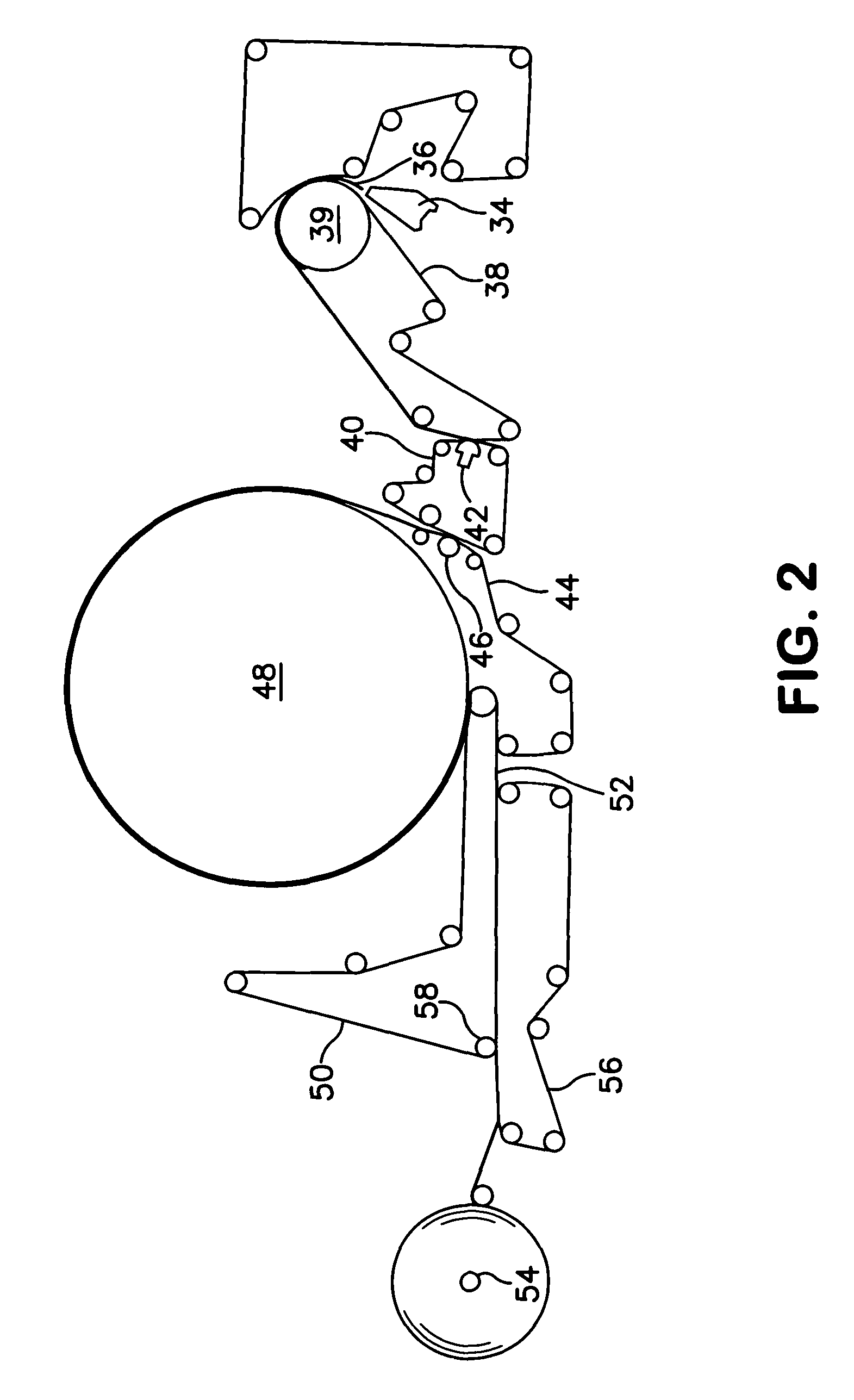
Malleable putty and flowable paste with allograft bone having residual calcium for filling bone defects
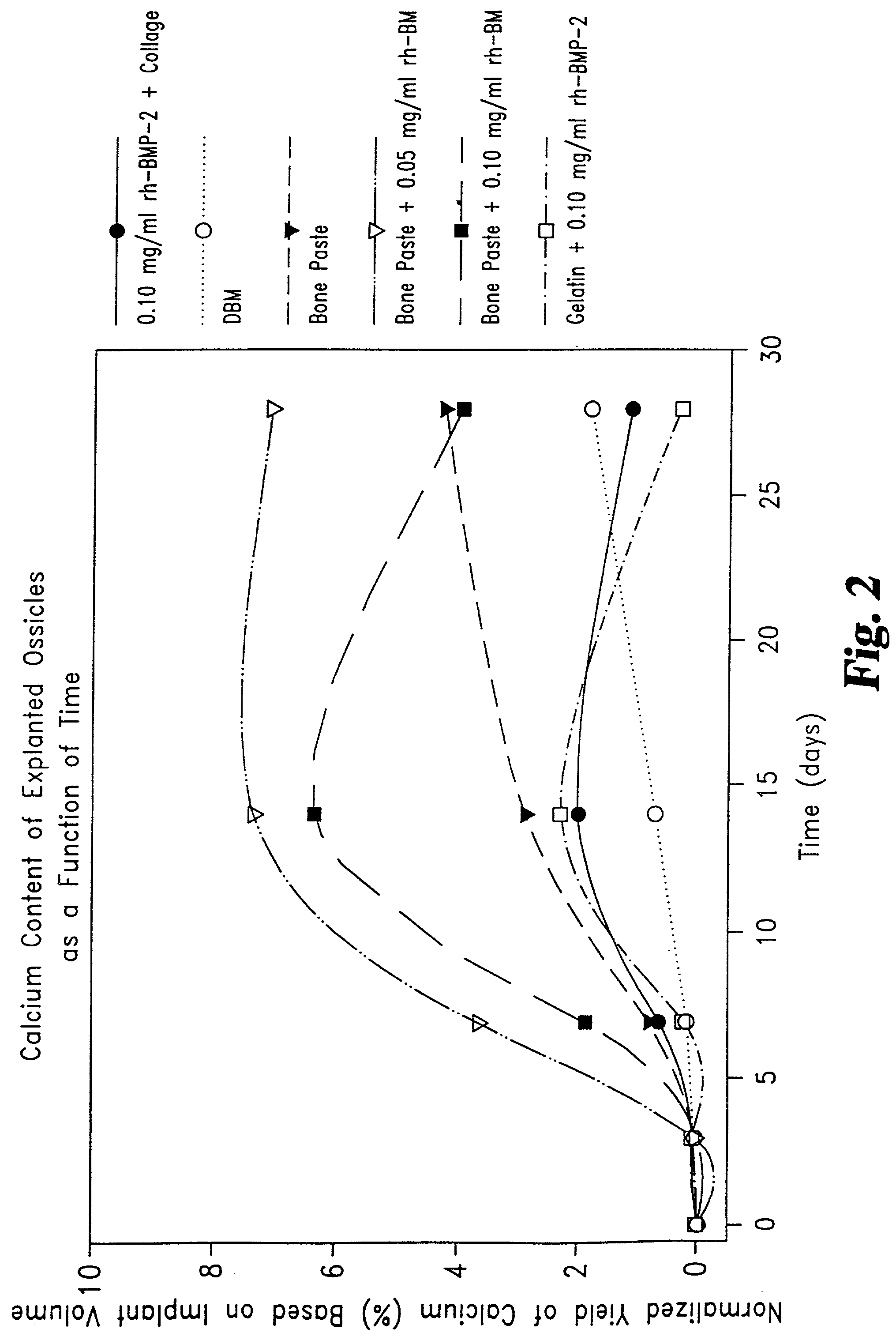
The invention is directed toward a malleable bone putty and a flowable pastel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of partially demineralized lyophilized allograft bone material having a residual calcium content ranging from 4 to 8% dry weight. The bone powder has a particle size ranging from about 100 to about 800 microns and is mixed in a high molecular weight hydrogel carrier containing a sodium phosphate saline buffer, the hydrogel component of the carrier ranging from about 1.00 to 50% of the composition and having a molecular weight of about at least 700,000 Daltons. The composition has a pH between 6.8-7.4 contains about 25% to about 35% bone powder and can be additionally provided with BMP's.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
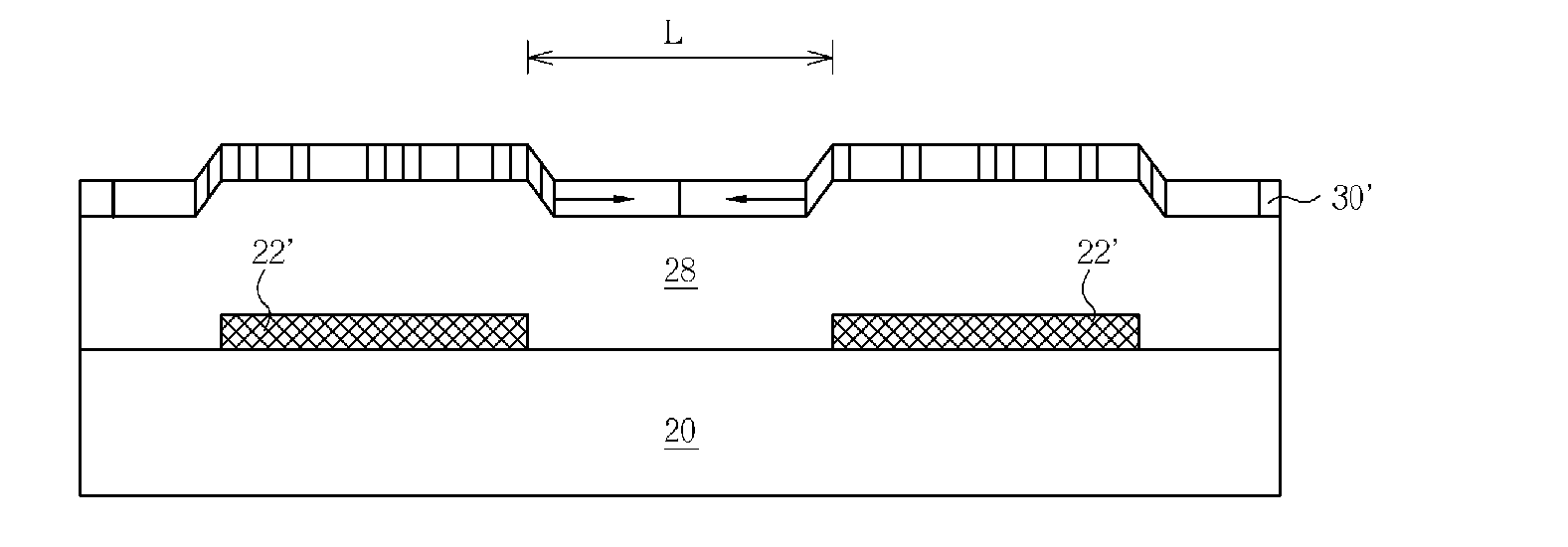
Semiconductor device and method of fabricating a low temperature poly-silicon layer
InactiveUS20060043367A1Promotes lateral growthHighly practicalTransistorSemiconductor/solid-state device detailsDevice materialAmorphous silicon
A method of fabricating a low temperature poly-silicon (LTPS). A plurality of semiconductor heat sinks are formed over a substrate. A buffer layer and an amorphous silicon layer are formed over the substrate and the semiconductor heat sinks. Following that, a laser crystallization process is performed to transform the amorphous silicon layer into a poly-silicon layer.
Owner:AU OPTRONICS CORP
Wet-skin treatment compositions
InactiveUS6645511B2Better behavedFast absorptionCosmetic preparationsToilet preparationsSkin treatmentsDermatology
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Drospirenone for hormone replacement therapy
InactiveUS20020132801A1Dissolve fastImprove bioavailabilityOrganic active ingredientsBiocideDrospirenoneBULK ACTIVE INGREDIENT
Drospirenone for Hormone Replacement Therapy A pharmaceutical composition comprising as a first active ingredient an estrogen, such as estradiol or estradiol valerate, in sufficient amounts to treat disorders and symptoms associated with deficient endogenous levels of estrogen in women, and as a second active ingredient 6beta,7beta; 15beta; 16beta-dimethylene-3-oxo-17alpha-preg-4-ene-21,17-carbolactone (drospirenone, DRSP) in sufficient amounts to protect the endometrium from the adverse effects of estrogen is useful for, amongst others, treating peri-menopausal, menopausal and post-menopausal women. This composition may be used for hormone replacement therapy and may be administered as a multi-phased pharmaceutical preparation. This combination therapy may comprise continuous, sequential or interrupted administration, or combinations thereof, of DRSP and estrogen, each optionally in micronized form.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Tissue repair devices of rapid therapeutic absorbency
ActiveUS8579990B2Avoid contactMinimize the possibilitySurgical drugsSurgeryTissue repairMedical device
Novel implantable tissue repair medical devices are disclosed. The devices have a central fabric member having anti-adhesion films on both opposed sides. The films have pores, and are arranged such that the pores on the opposed films are offset. The devices are useful in hernia repair procedures.
Owner:ETHICON INC
Self-emulsifying composition of OMEGA3 fatty acid
ActiveUS8618168B2Maintain good propertiesAvoid high concentrationsBiocideNervous disorderHydrophilic-lipophilic balanceSelf emulsifying
This invention provides a self-emulsifying composition comprising 50 to 95% by weight in total of at least one compound selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; and 5 to 50% by weight of an emulsifier having a hydrophilic lipophilic balance of at least 10. The composition has no or reduced ethanol content, and exhibits excellent self-emulsifying property, dispersibility in the composition, emulsion stability, and absorption property. The composition is adapted for use as a drug.
Owner:MOCHIDA PHARM CO LTD
Malleable paste for filling bone defects
InactiveUSRE38522E1Easy to packFast absorptionSurgical adhesivesPeptide/protein ingredientsBone defectBiomedical engineering
The invention is directed toward a malleable bone putty and a flowable gel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone powder. The bone powder has a particle size ranging from about 100 to about 850 microns and is mixed in a high molecular weight hydrogel carrier, the hydrogel component of the carrier ranging from about 0.3 to 3.0% of the composition and having a molecular weight of about at least 10,000 Daltons. The composition contains about 25% to about 40% bone powder and can be additionally provided with BMP's and a sodium phosphate buffer.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
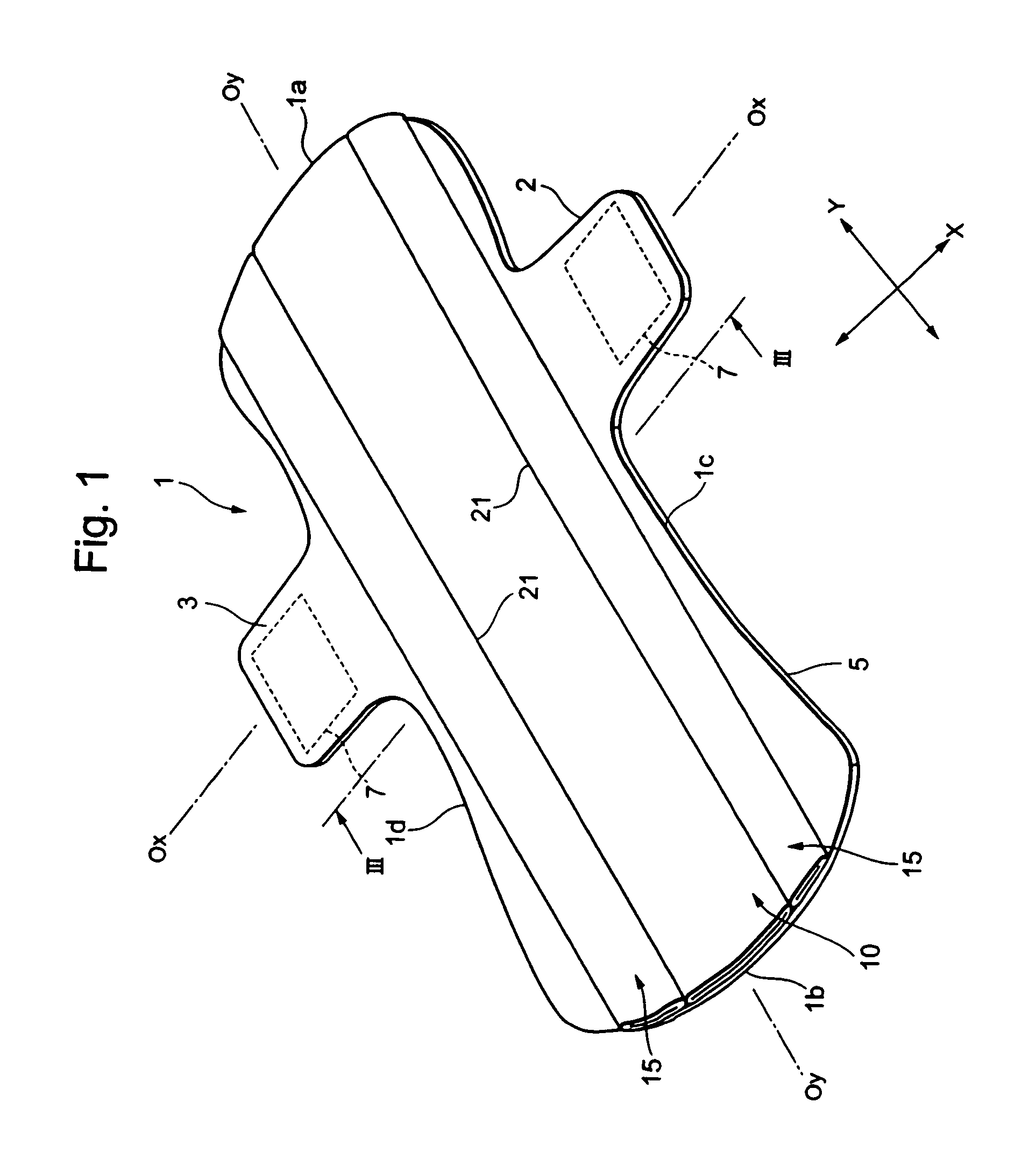
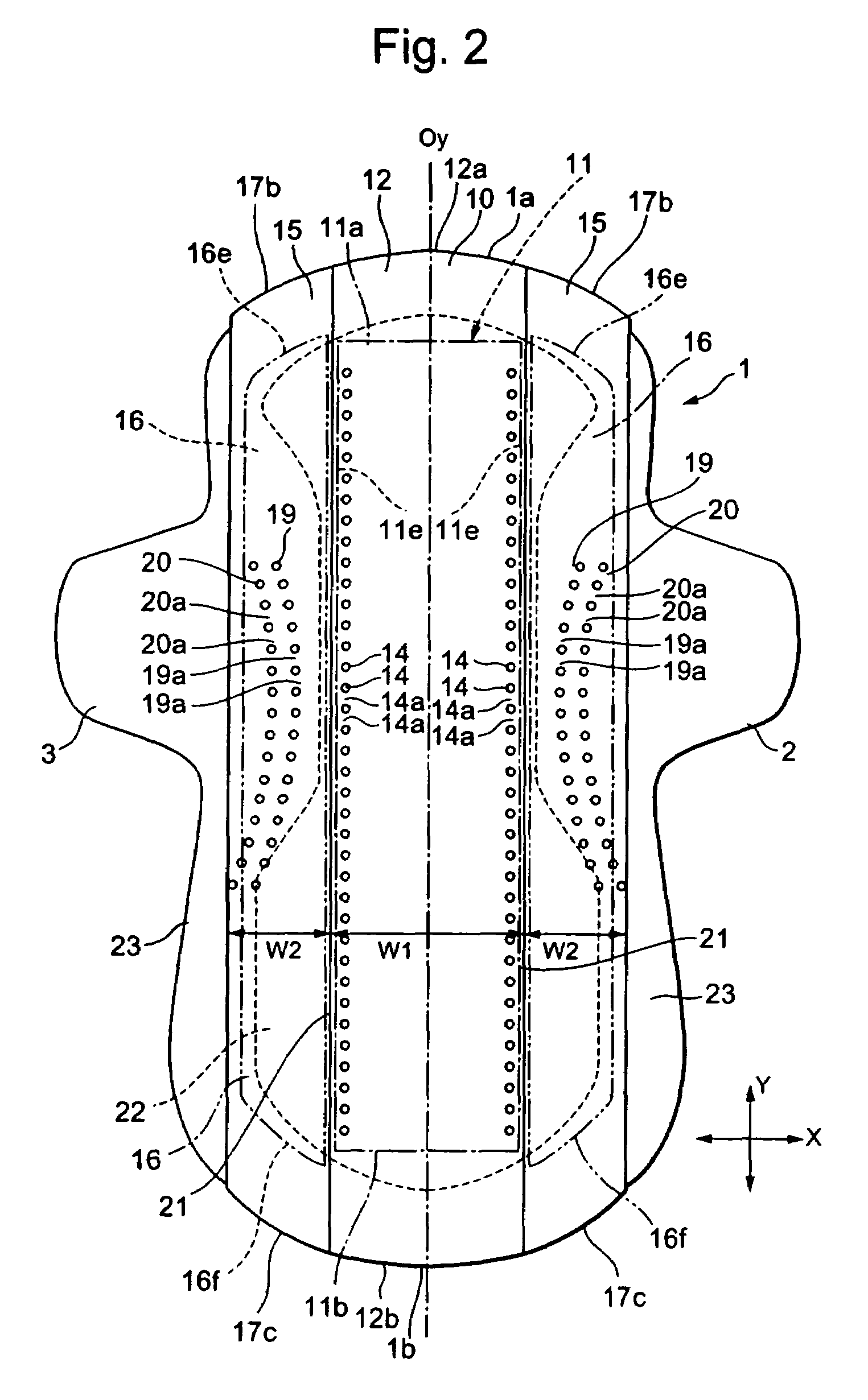
Absorbent article
InactiveUS7625363B2Effective preventionFast absorptionSanitary towelsBaby linensMechanical engineering
Owner:UNI CHARM CORP
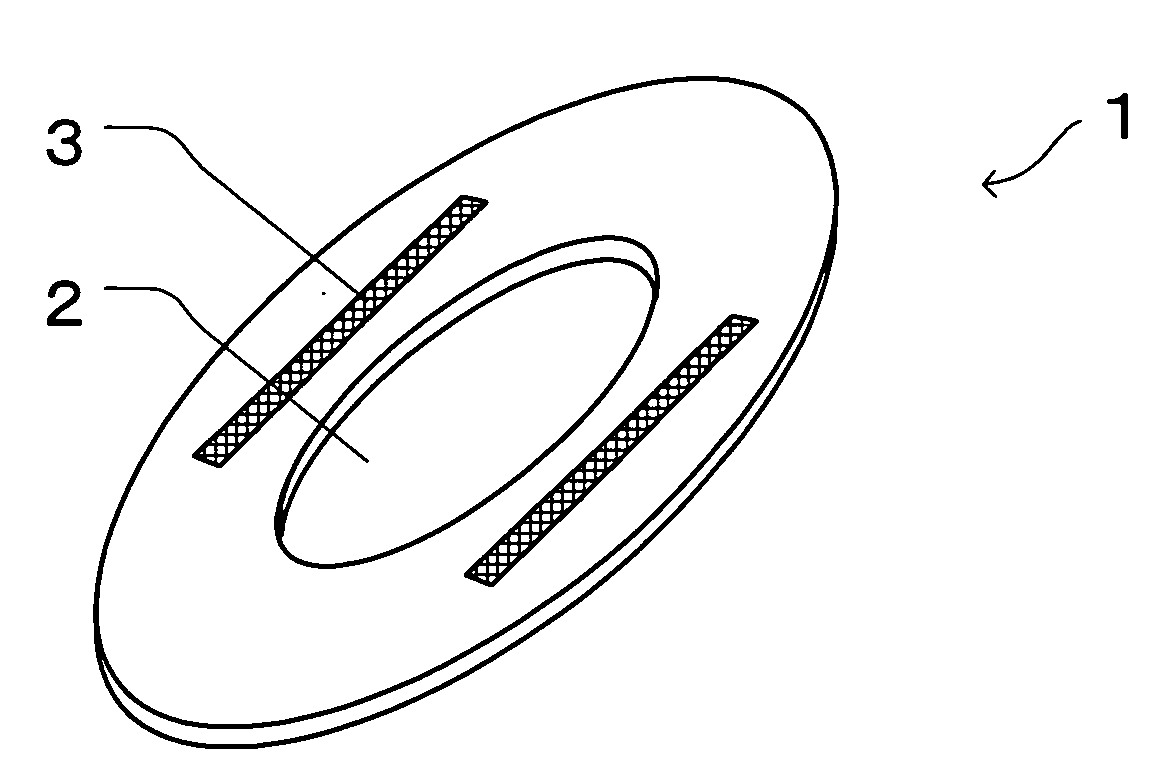
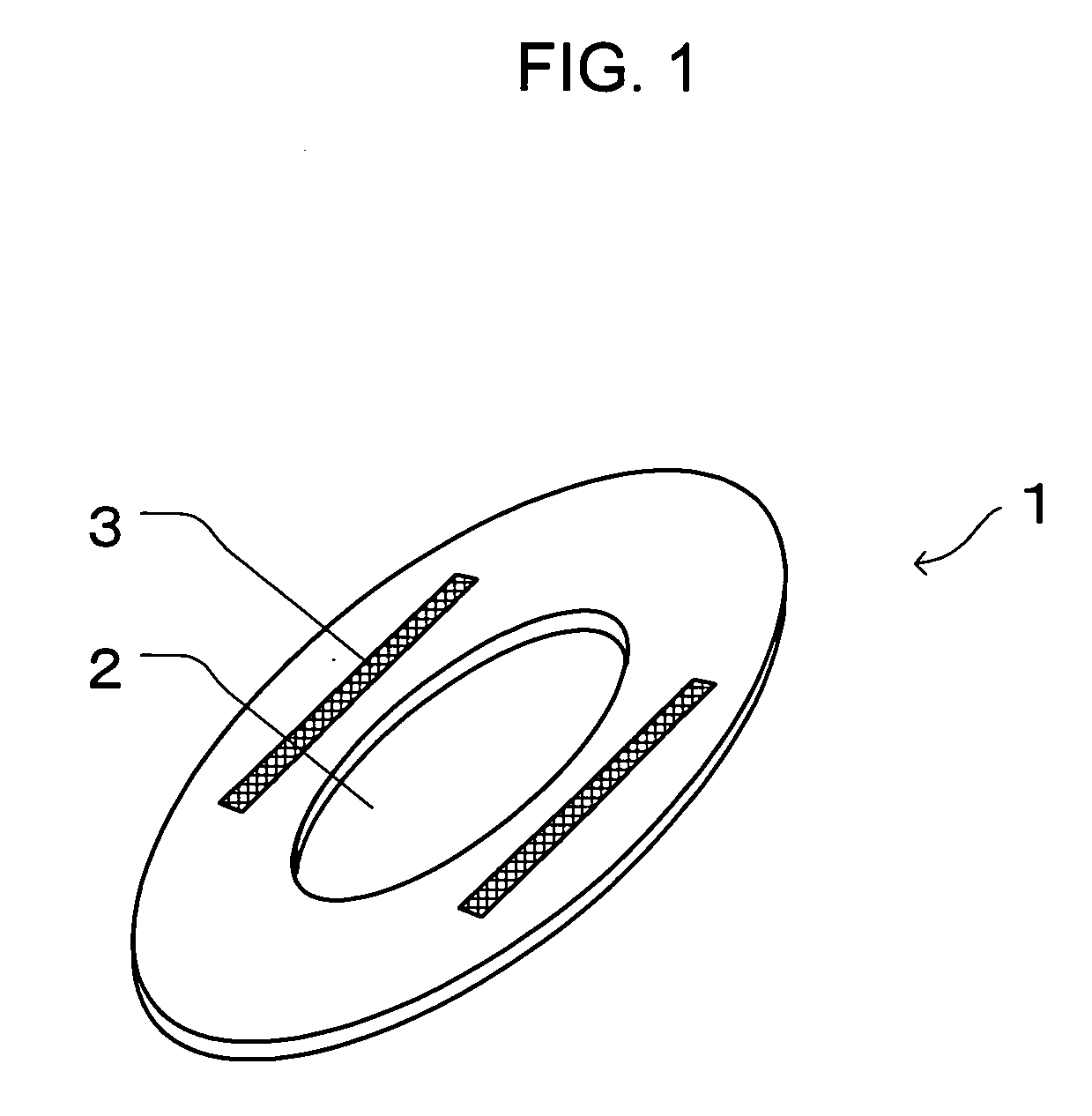
Auxiliary sheet for absorbable article
InactiveUS20050010185A1Fast absorptionDirect contact guaranteeSanitary towelsBaby linensBody fluidThigh musculature
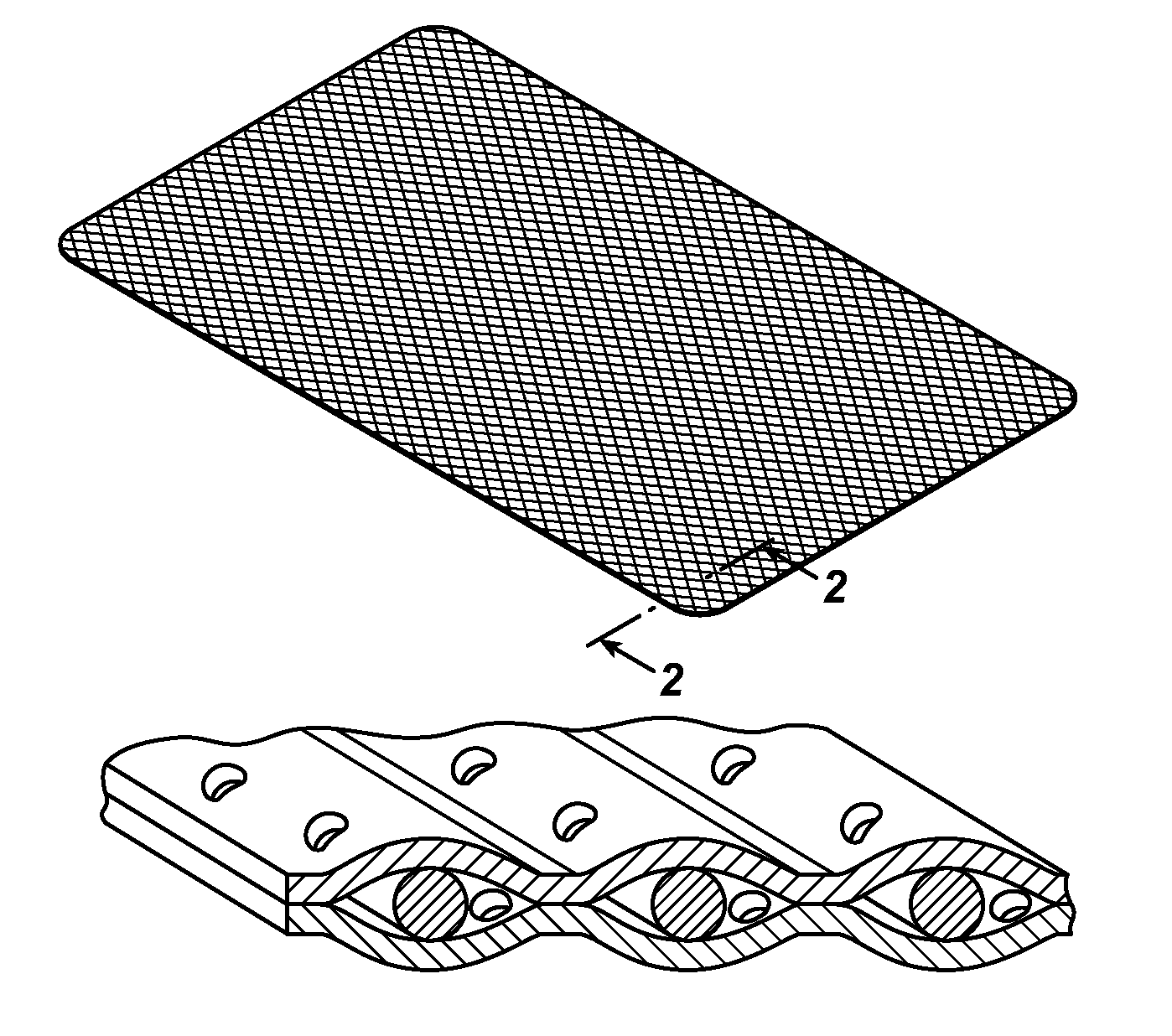
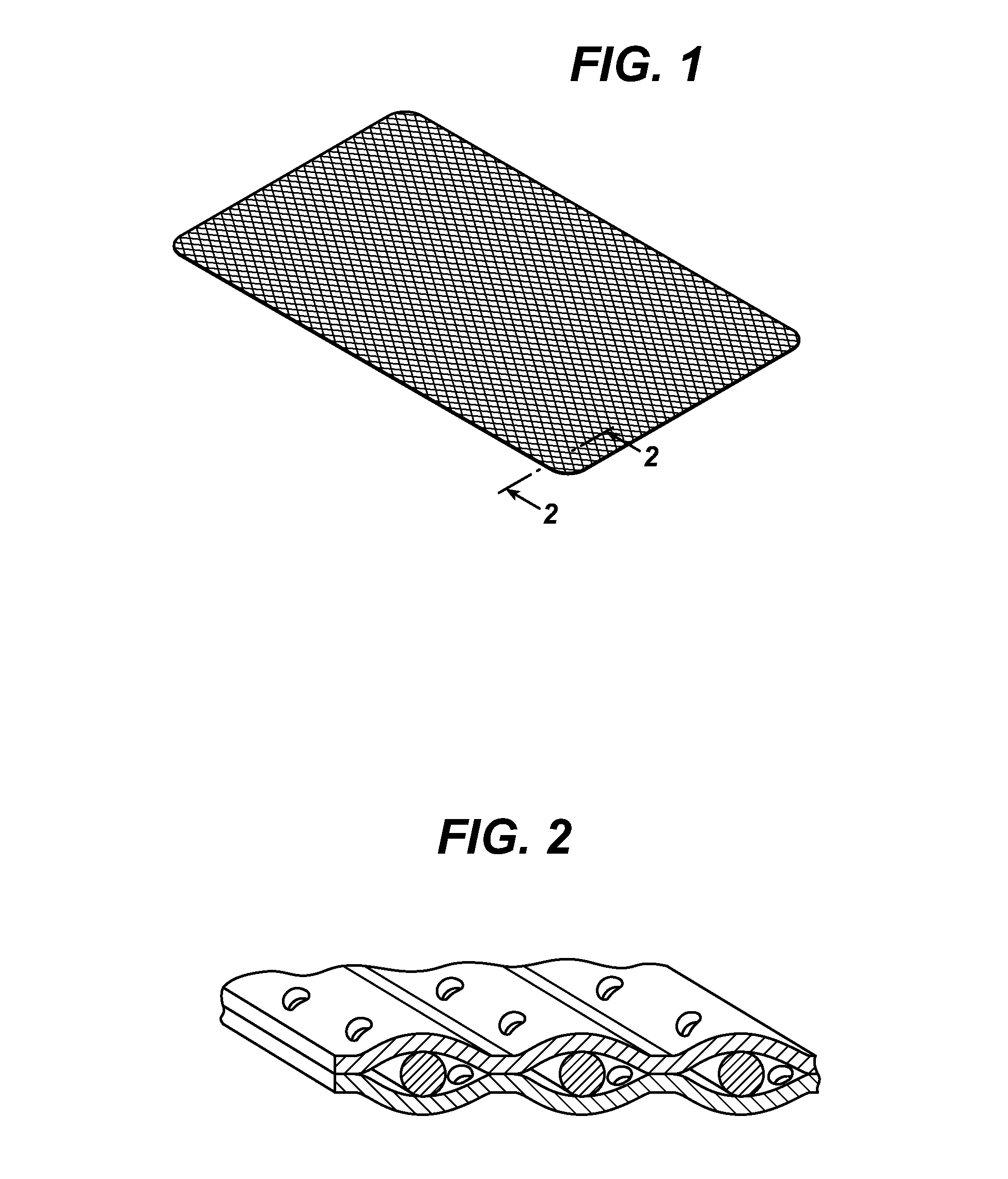
The present invention relates to an auxiliary sheet for an absorbent article which supplements function of a sheet-form absorbent article such as a sanitary napkin, or which adds new function to the sheet-form absorbent article, and provides an auxiliary sheet for an absorbent article which reduces a contact range between a body fluid absorbed in the sheet-form absorbent article and the body of a wearer and also prevents the body fluid from diffusing, and further prevents the skin from being re-wetted and also prevents the body fluid from being caused to leak out of the absorbent article. The auxiliary sheet for the absorbent article (1) provided with a through-hole (2) permitting to engage labia therein and an adhesive portion (3) for fixedly adhering to the body of the wearer in the neighborhood of the circumferential edge of this through-hole (2), is made to lie between a sanitary napkin (10) and the thighs of the wearer.
Owner:UNI CHARM CORP
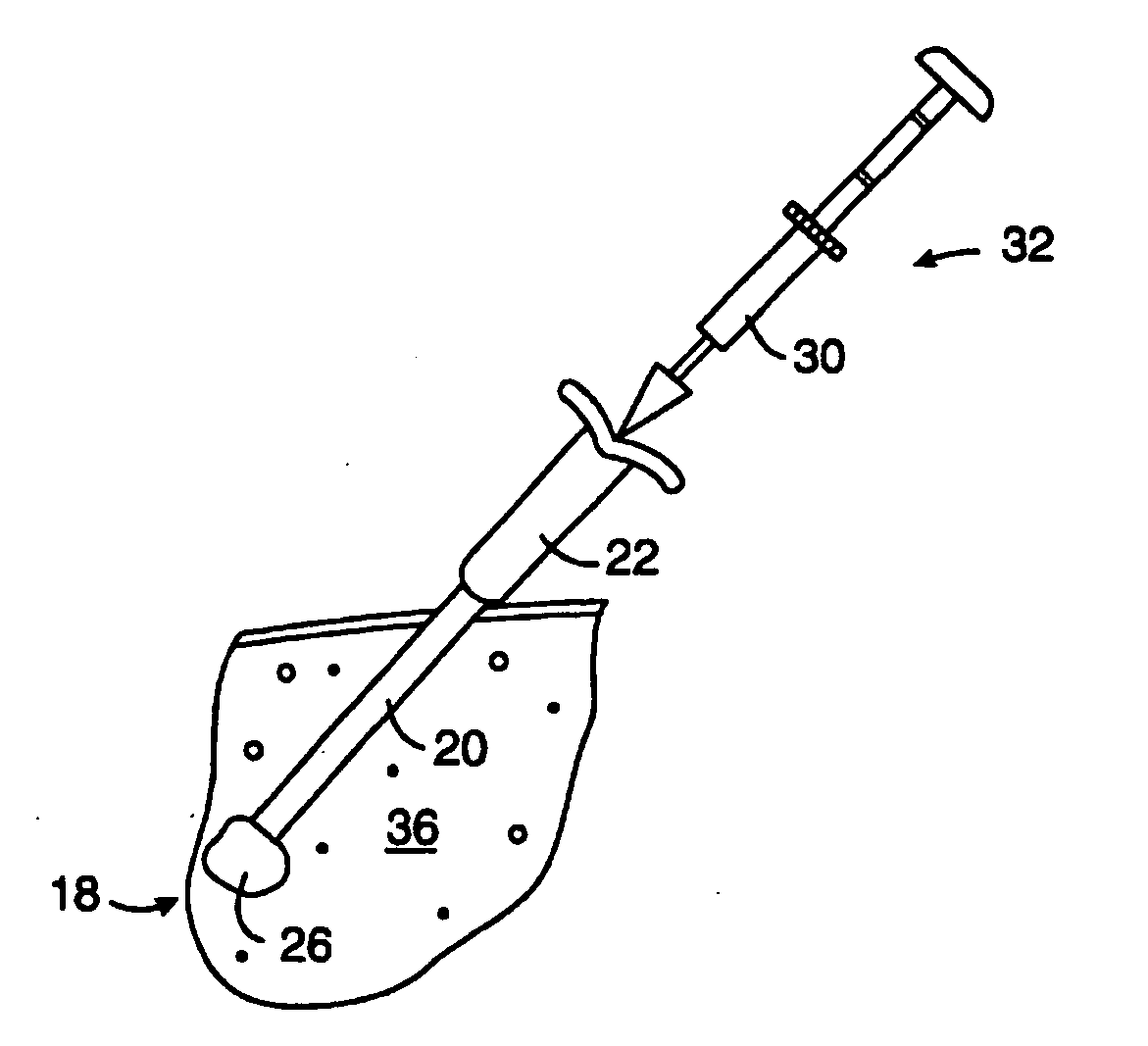
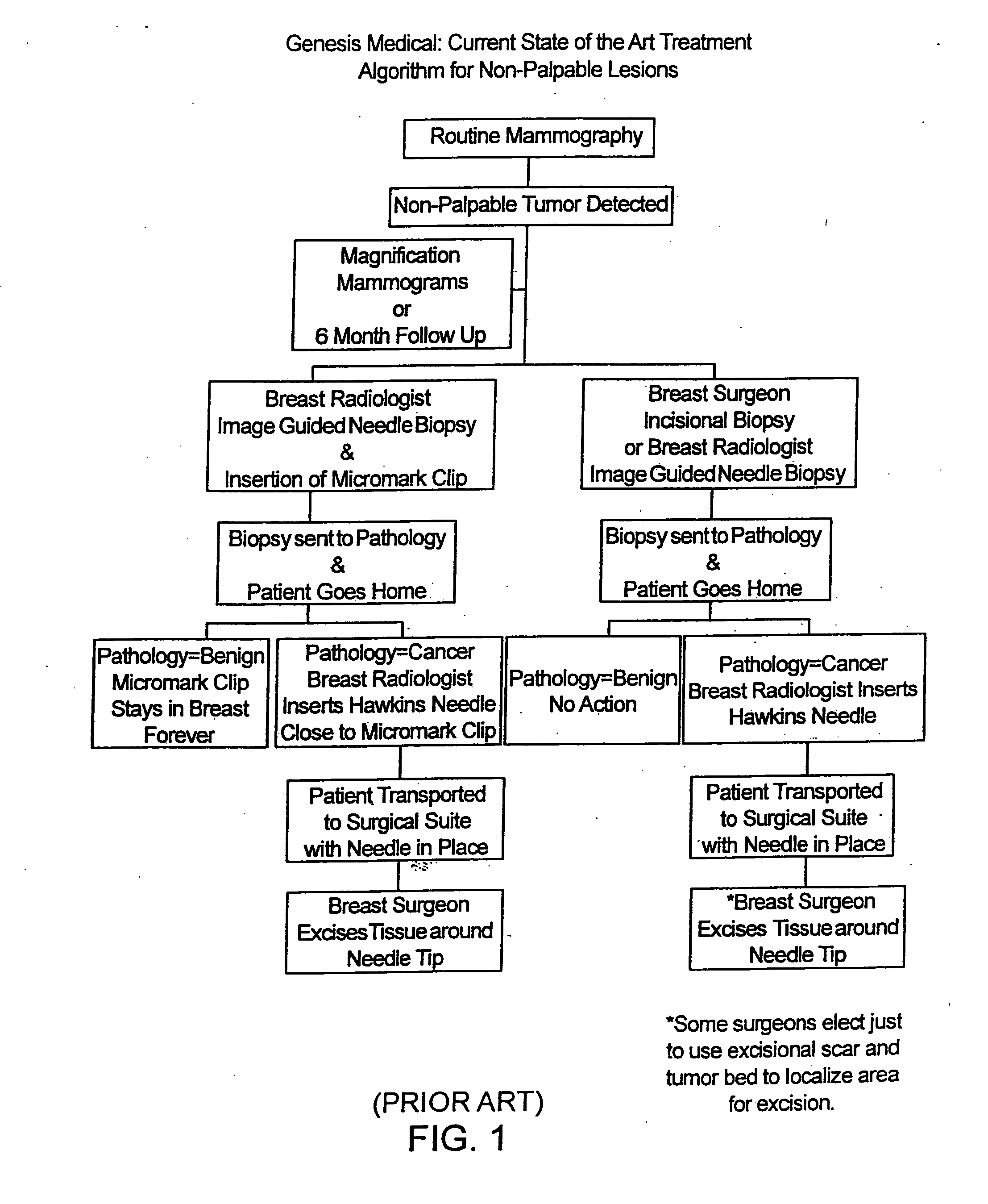
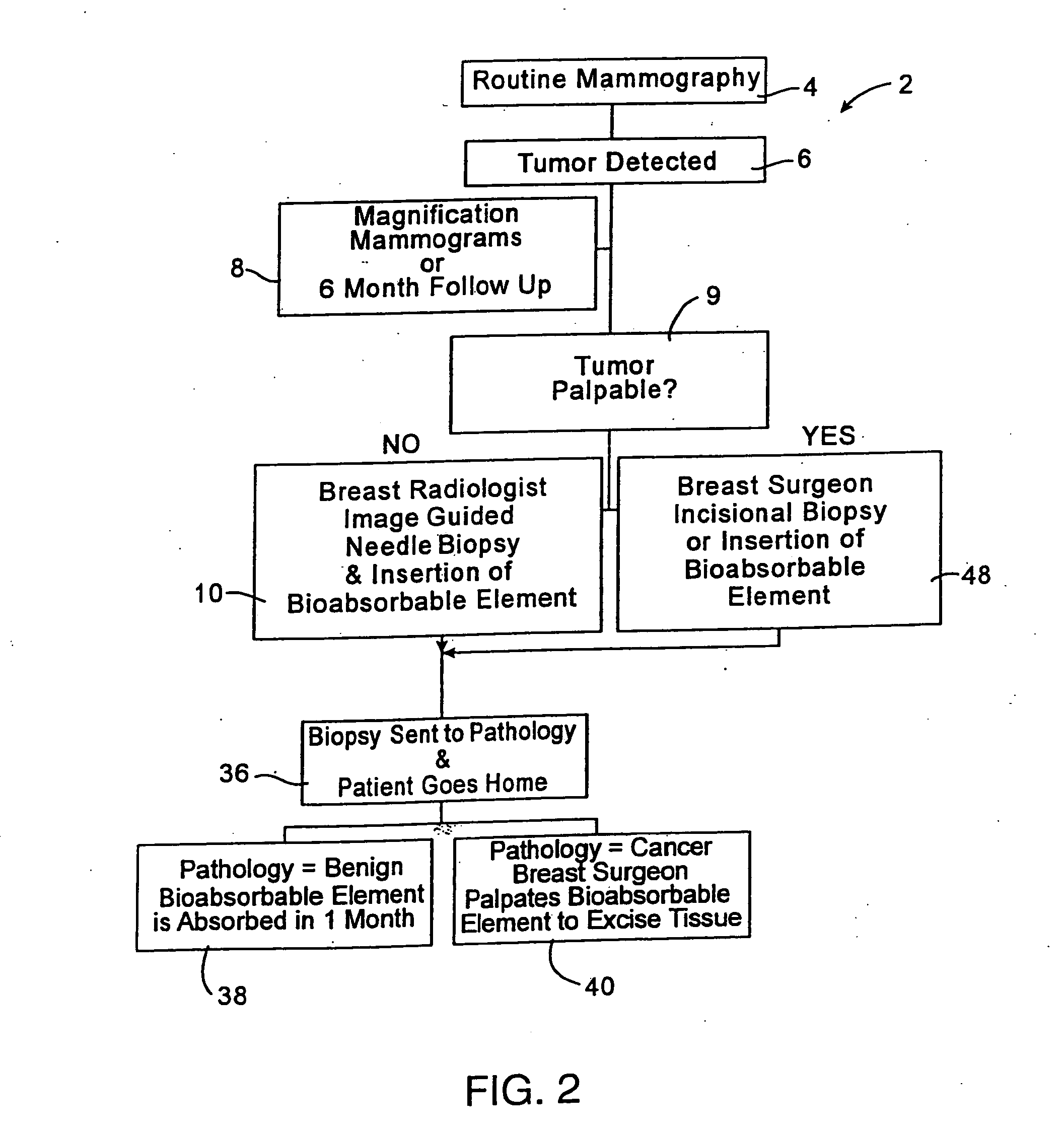
Biopsy localization method and device
InactiveUS20060079829A1Eliminate needEliminate useCannulasSurgical needlesSurgeryBiomedical engineering
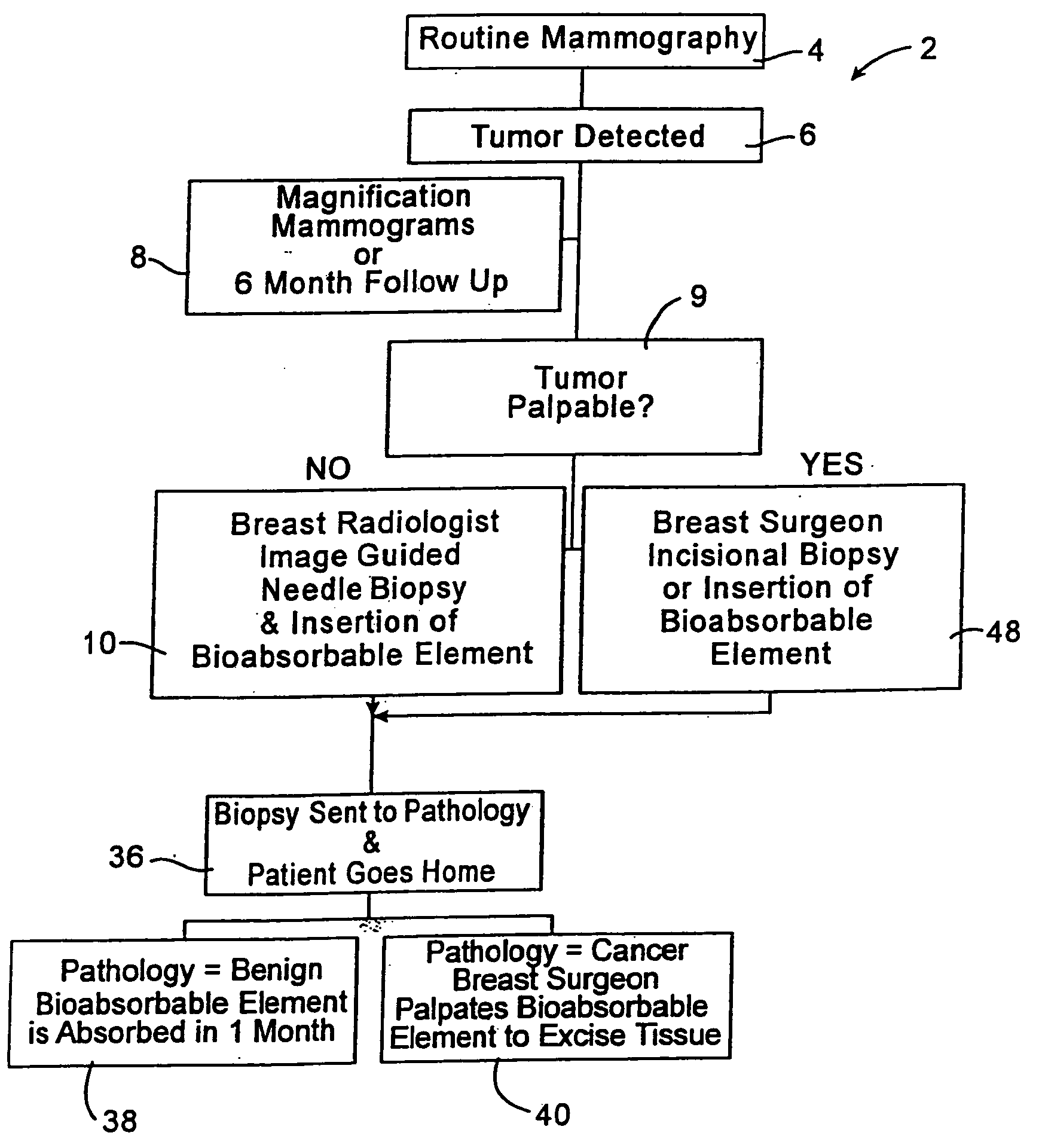
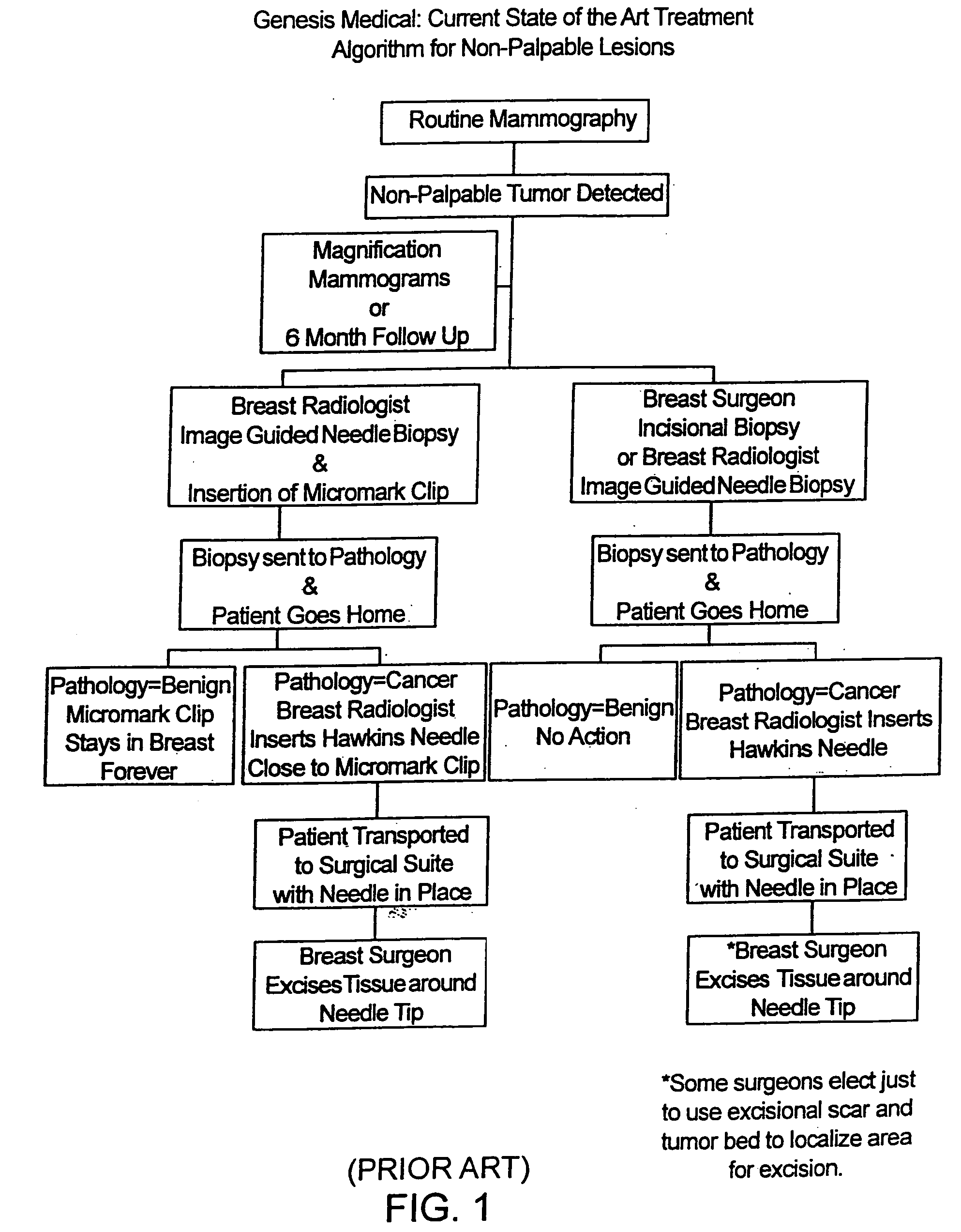
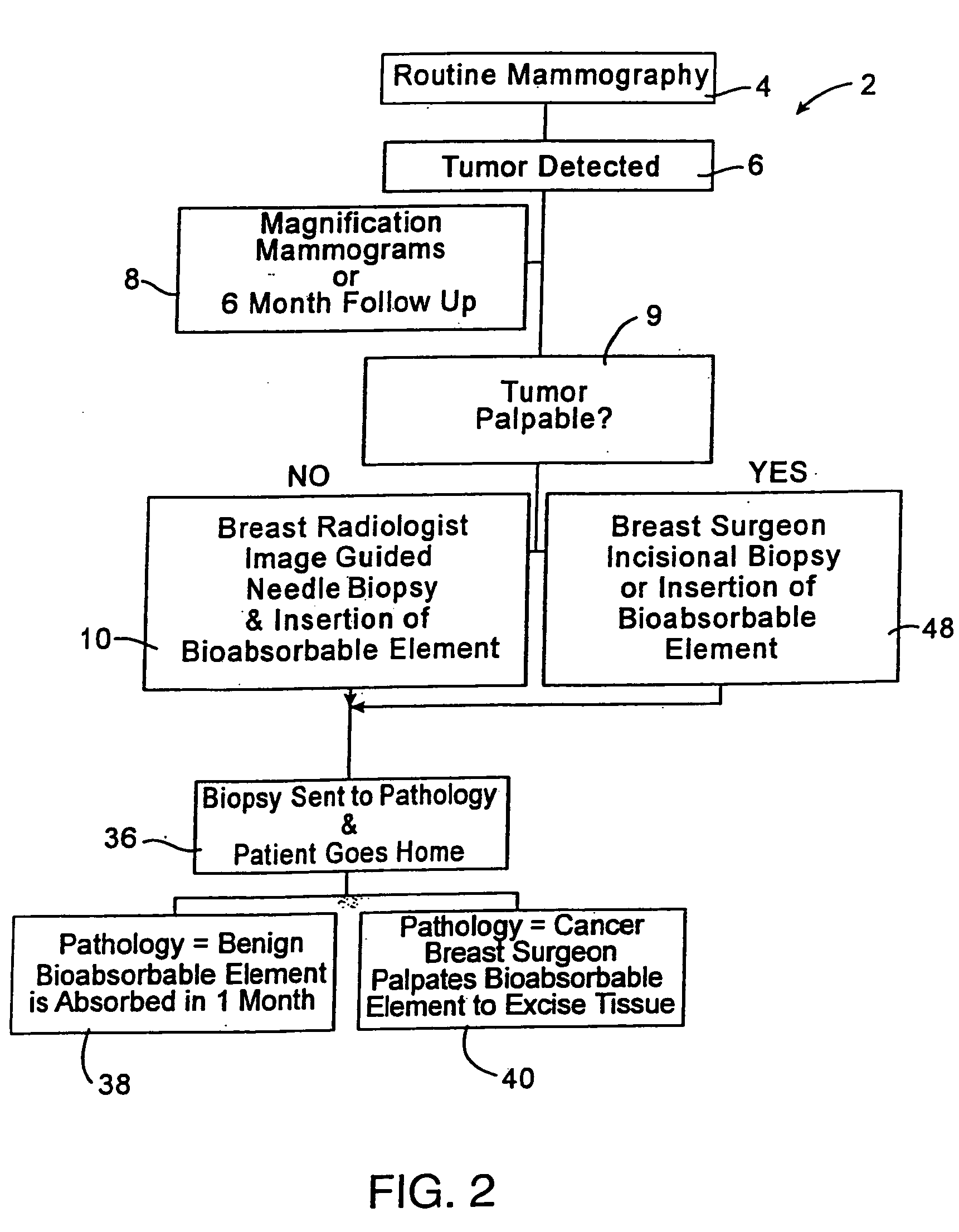
A biopsy localization device made according to the invention includes an intracorporeal delivery cannula and at least one marker disposed within an inner lumen of the delivery cannula. The marker includes an expandable fibrous body with at least one radiographically detectable marker element. The expandable fibrous body may also be bioabsorbable. Methods of use of the device are also described.
Owner:FULTON RICHARD E +1
Recording material for inkjet printing
InactiveUS6177187B1Crisp and brilliant imageFast absorptionDecorative surface effectsLayered productsPolymer chemistryInkjet printing
The present invention relates to a recording material for the inkjet method with aqueous inks, having at least one temporary substrate material and a porous ink absorption layer which is applied thereon, can be converted into a film and comprises from 60% by weight to 95% by weight of thermoplastic particles having a mean particle size between 1 mum and 40 mum, preferably from 5 to 20 mum, and 5-40% by weight of film-forming binder and, if required, conventional assistants and additives. After conversion of the recording layer into a self-supporting cohesive film by the action of heat and, if required, pressure, said film can be removed from the temporary substrate material at room temperature with a separation force of 10 cN / 50 mm strip width to 800 cN / 55 mm. In the case of further intermediate layers, the least adhesion of the laminated layers between one another in the laminate is that between temporary substrate material and adjacent layer.After film formation, the recording material according to the invention is particularly suitable for outdoor applications but also for transfer printing, for example on textiles.
Owner:SIHL
Biopsy localization method and device
Methods for localizing a biopsy site are disclosed. The method includes taking a tissue sample from a biopsy site and positioning a detectable, bioabsorbable element at the biopsy site at the time that the tissue sample was taken. The tissue sample is then tested. The biopsy site is then relocated by finding the bioabsorbable element. The bioabsorbable element may be made of collagen, gelatin, cellulose, polylactic acid, and / or polyglycolic acid. The detectable bioabsorbable element may be relocated using ultrasound or mammography. The bioabsorbable element may also swell upon contact with body fluid.
Owner:DEVICOR MEDICAL PROD
Absorbent compositions with clay aerogels and methods for forming absorbent compositions
InactiveUS20080132632A1Low densityComponents is relatively effectiveAnimal housingLitterPolymer composites
Lightweight, highly absorbent compositions including clay aerogels are suitable for use in the home as well as in industry and have particular suitability for use as animal litter. The compositions can further include components, for example as part of the mixture and / or as a coating on a surface of the clay aerogel component, that have properties, for example, that aid in malodor reduction, clumping, or the like, or combinations thereof. Also, clay aerogel polymer composites, formed from various types of clay and (co)polymers, which are relatively low density materials having myriad applications are disclosed. Numerous methods for preparing the clay aerogel polymer composites are disclosed.
Owner:CASE WESTERN RESERVE UNIV
Capsule Stable Against Mastication
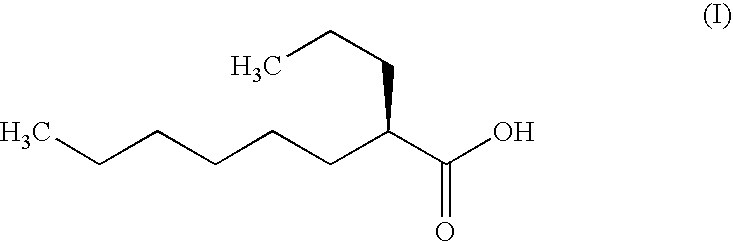
InactiveUS20080057115A1Good disintegrationExcellent pharmaceutical preparationAntibacterial agentsNervous disorderSlice thicknessMedicine
The present invention relates to a soft capsule which is easily disintegrated in the stomach, wherein the contents thereof are not easily leaked at the time of mastication, which is obtained by providing a soft capsule comprising (2R)-2-propyloctanoic acid or a salt thereof with at least one property, preferably all properties, selected from (A) wherein it has a strength of 150 to 400 N by a cracking test; (B) wherein it has a disintegration time of 3 to 10 minutes by the disintegration test stipulated in Japanese Pharmacopoeia; (C) wherein the capsule shell has a shell thickness of 0.05 to 0.50 mm; (D) wherein the capsule shell has a first seam thickness of 0.10 to 0.55 mm; (E) wherein the capsule shell has a second seam thickness of 0.05 to 0.50 mm; (F) wherein the capsule shell has a water content of 5.0 to 9.0%.
Owner:ONO PHARMA CO LTD
Human-body absorbable trauma dressing containing Yunnan white drug powder or Yunnan white drug powder extractive
InactiveCN101804218AIncrease usageImprove usabilityAbsorbent padsBandagesDressing changeCurative effect
The invention discloses a human-body absorbable trauma dressing containing Yunnan white drug powder or Yunnan white drug powder extractive, which is a novel medicine-carried dressing or a novel formulation of Yunnan white drug powder. The invention has the following remarkable characteristics: (1) the dressing can be absorbed by human bodies to lessen the pain added by dressing change and reduce the treatment cost; (2) the dressing can be made into a film solid dressing or an aquagel dressing so as to expand the use modes, the scope of applications and the drug effect of the Yunnan white drug powder; and (3) the curative effect of the dressing is enhanced by selecting a carrier material, auxiliary medicaments and functional accessories and adjusting the microstructure structure. The novel absorbable Yunnan white drug powder dressing overcomes the defects of the traditional Yunnan white drug powder in use and has economic and social values.
Owner:王艳
Creping process and products made therefrom
ActiveUS7883604B2Reduced strengthHigh strengthNatural cellulose pulp/paperMechanical working/deformationFiberPolymer science
Tissue products are disclosed containing an additive composition. The additive composition, for instance, comprises an aqueous dispersion containing an alpha-olefin polymer, an ethylene-carboxylic acid copolymer, or mixtures thereof. The alpha-olefin polymer may comprise an interpolymer of ethylene and octene, while the ethylene-carboxylic acid copolymer may comprise ethylene-acrylic acid copolymer. The additive composition may also contain a dispersing agent, such as a fatty acid. The additive composition may be incorporated into the tissue web by being combined with the fibers that are used to form the web. Alternatively, the additive composition may be topically applied to the web after the web has been formed. For instance, in one embodiment, the additive composition may be applied to the web as a creping adhesive during a creping operation. The additive composition may improve the strength of the tissue web without substantially affecting the perceived softness of the web in an adverse manner.
Owner:KIMBERLY-CLARK WORLDWIDE INC
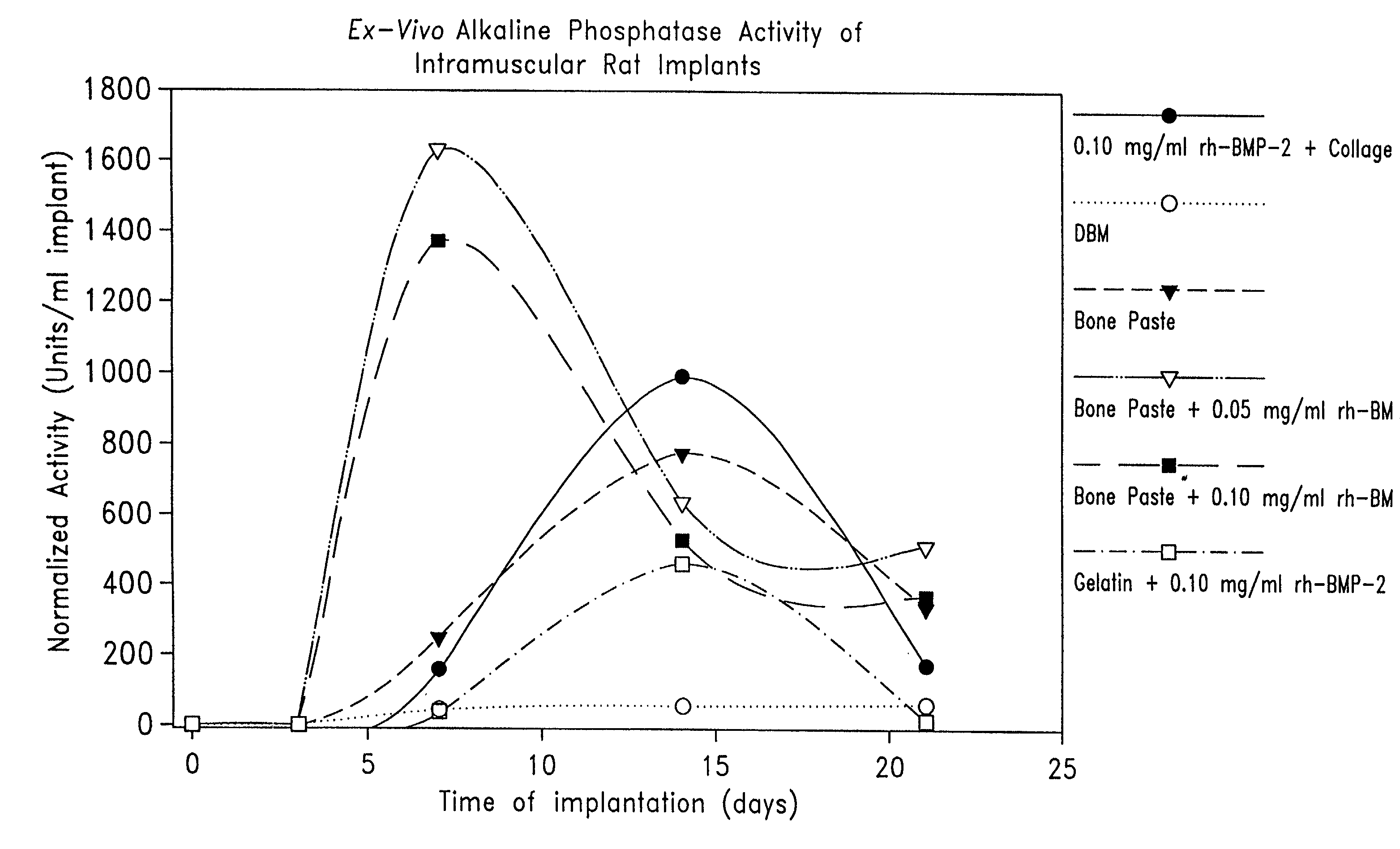
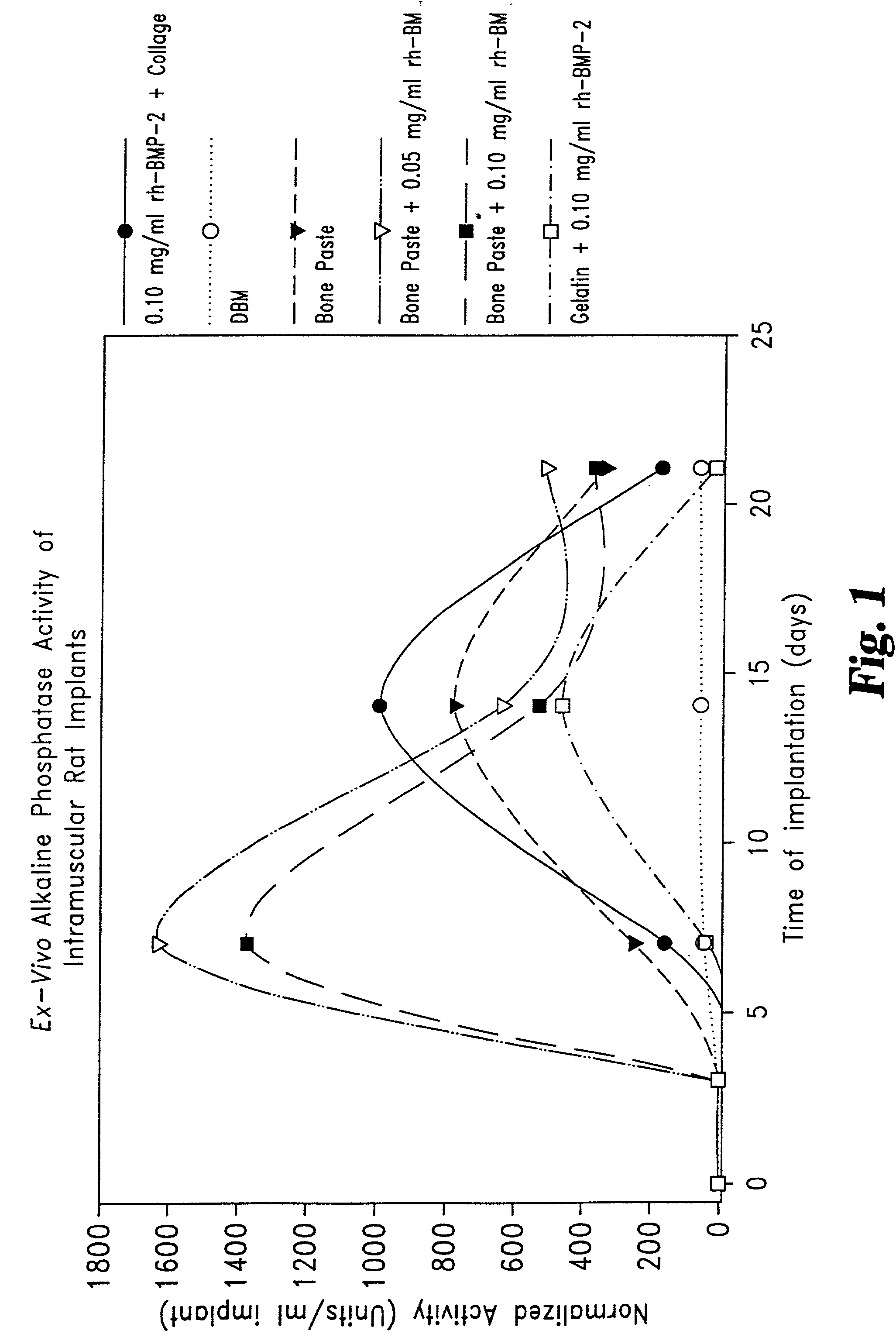
Osteogenic paste compositions and uses thereof
InactiveUS7172629B2Rapid and premature resorptionDiminish and eliminate capacityImpression capsSurgical adhesivesMineral matrixImplantation Site
Described are osteogenic paste compositions with enhanced osteoinductive properties for use in bone repair. Compositions comprising a quickly resorbable paste carrier, a more slowly resorbed mineral matrix, and Bone Morphogenetic Protein (BMP) or other osteogenic factor are described which enable increased osteoinductive activity while retaining a reliable scaffold for the formation of new bone at the implant site. Methods for making and methods for therapeutic use of the compositions are also disclosed.
Owner:WARSAW ORTHOPEDIC INC
Biopsy localization method and device
Owner:ARTEMIS MEDICAL
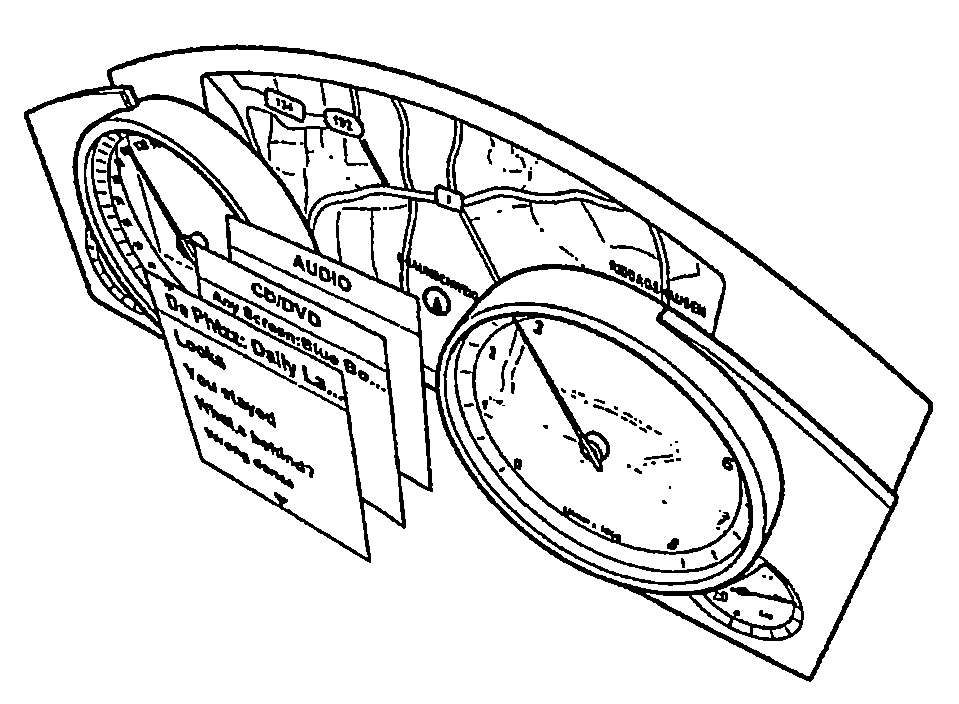
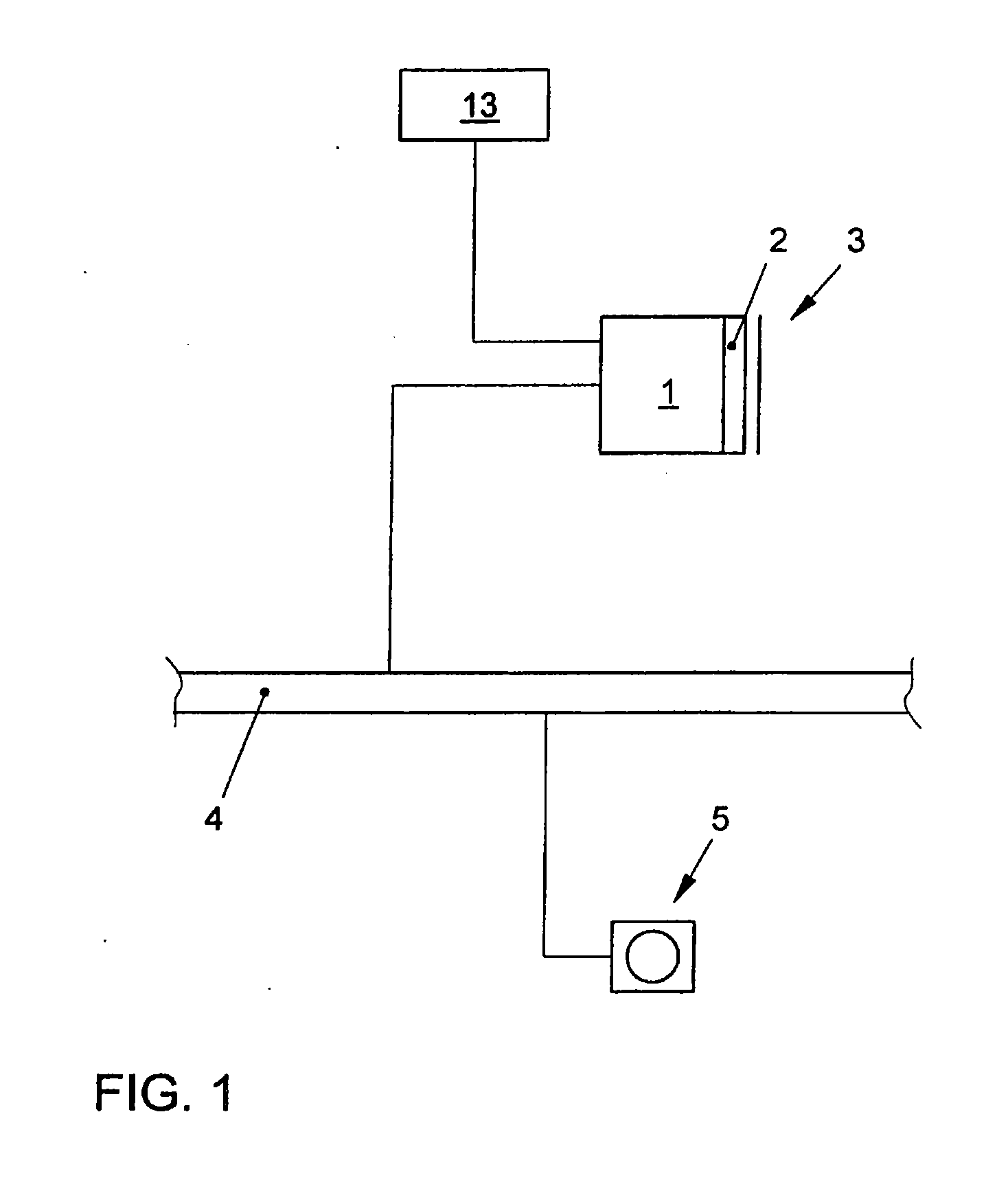
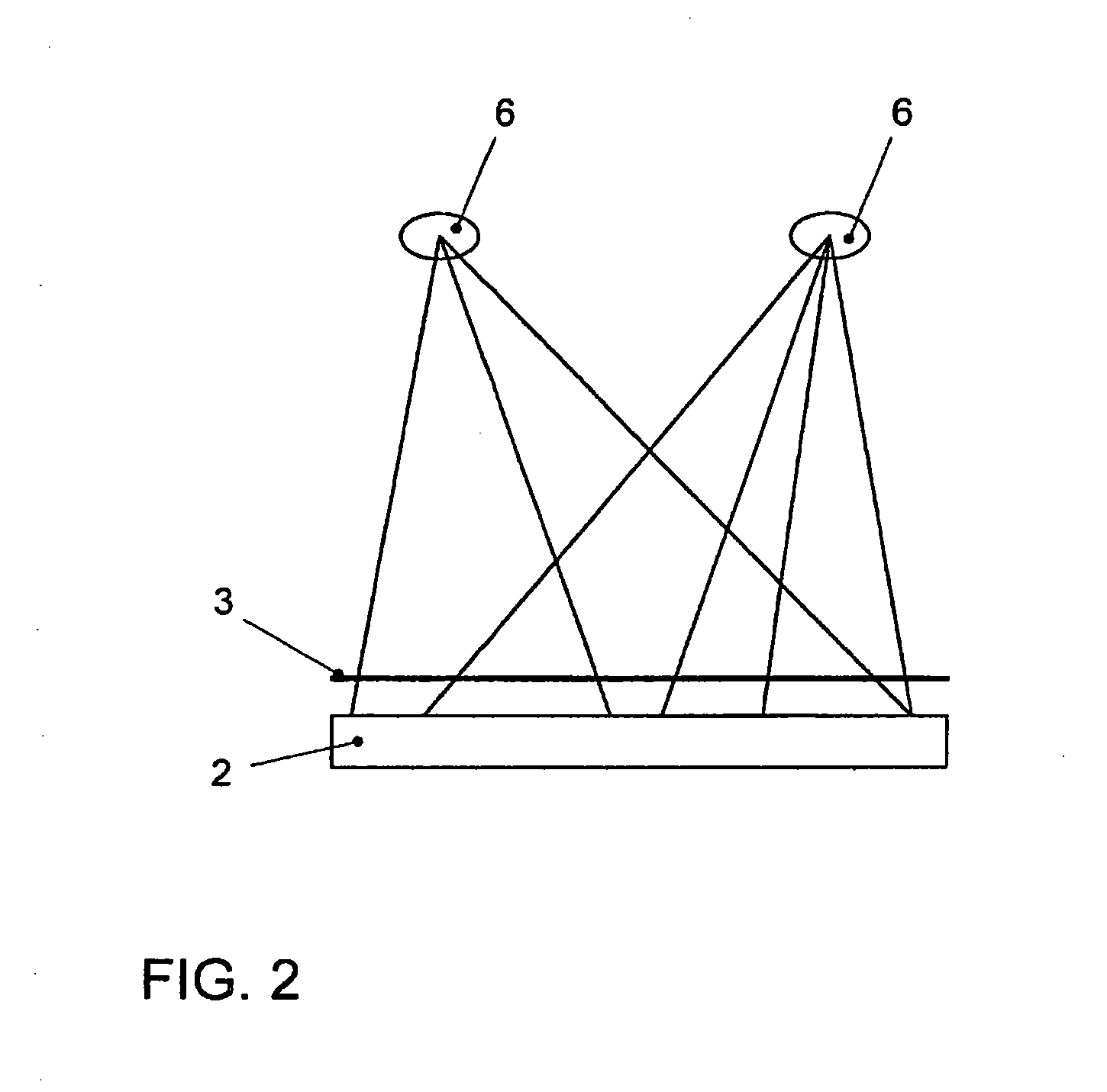
Method for Representing Items of Information in a Means of Transportation and Instrument Cluster for a Motor Vehicle
ActiveUS20080161997A1Easy to displaySpace minimizationDigital data processing detailsInstrument arrangements/adaptationsAutostereogramMobile vehicle
In a method for displaying items of information in a transportation device, the items of information are displayed in the form of hierarchical menu structures. A device for displaying items of information includes a control unit and a display unit for the stereoscopic and / or autostereoscopic display of items of information. In the method, the display is implemented stereoscopically, at least two different menus or menu items being displayed to the viewer at different distances. The instrument cluster includes the placement of a mask in front of the display of a display unit, the mask modifying the light emission of the light emitted by the display so as to result in a display of autostereoscopic images.
Owner:VOLKSWAGEN AG
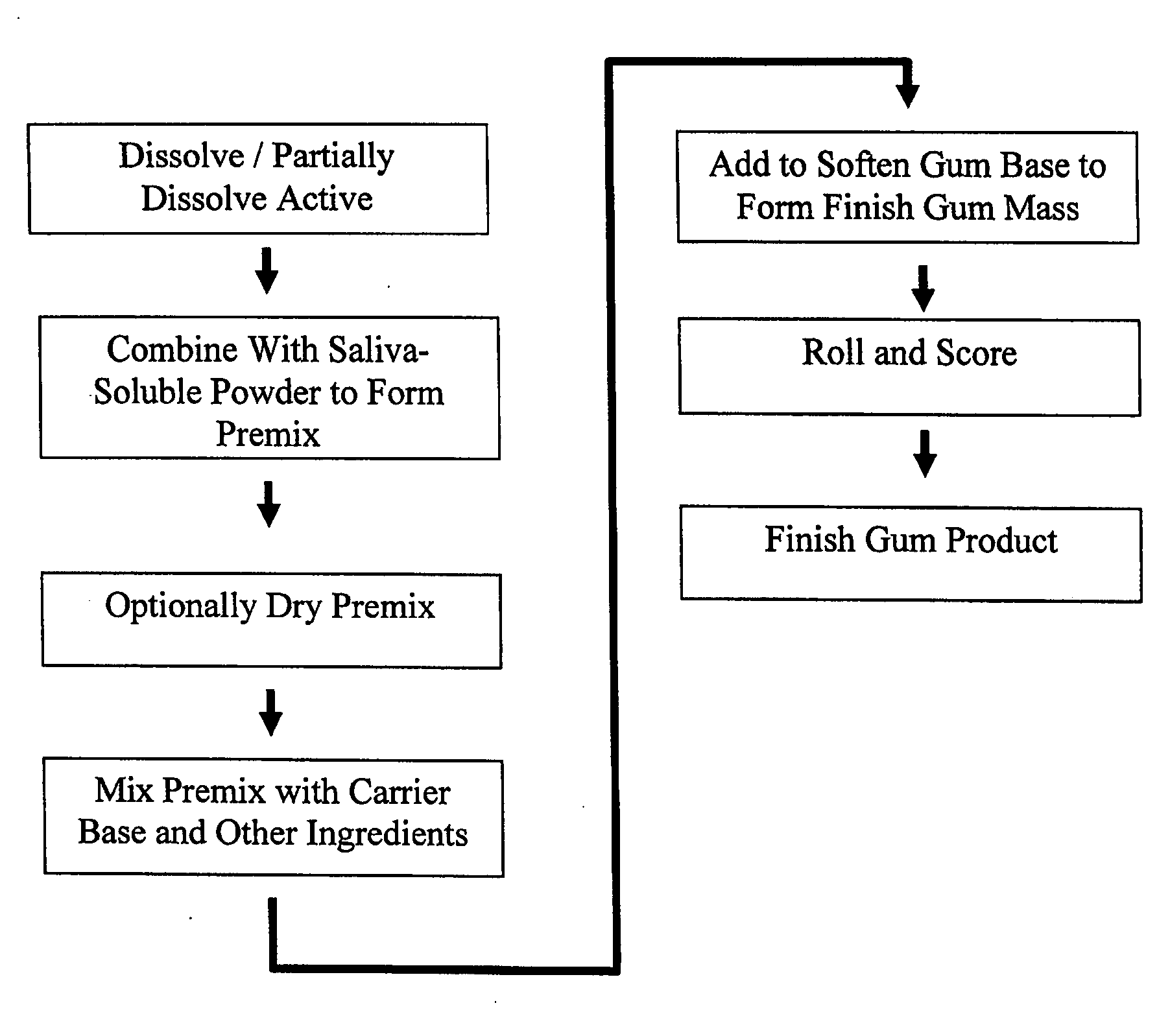
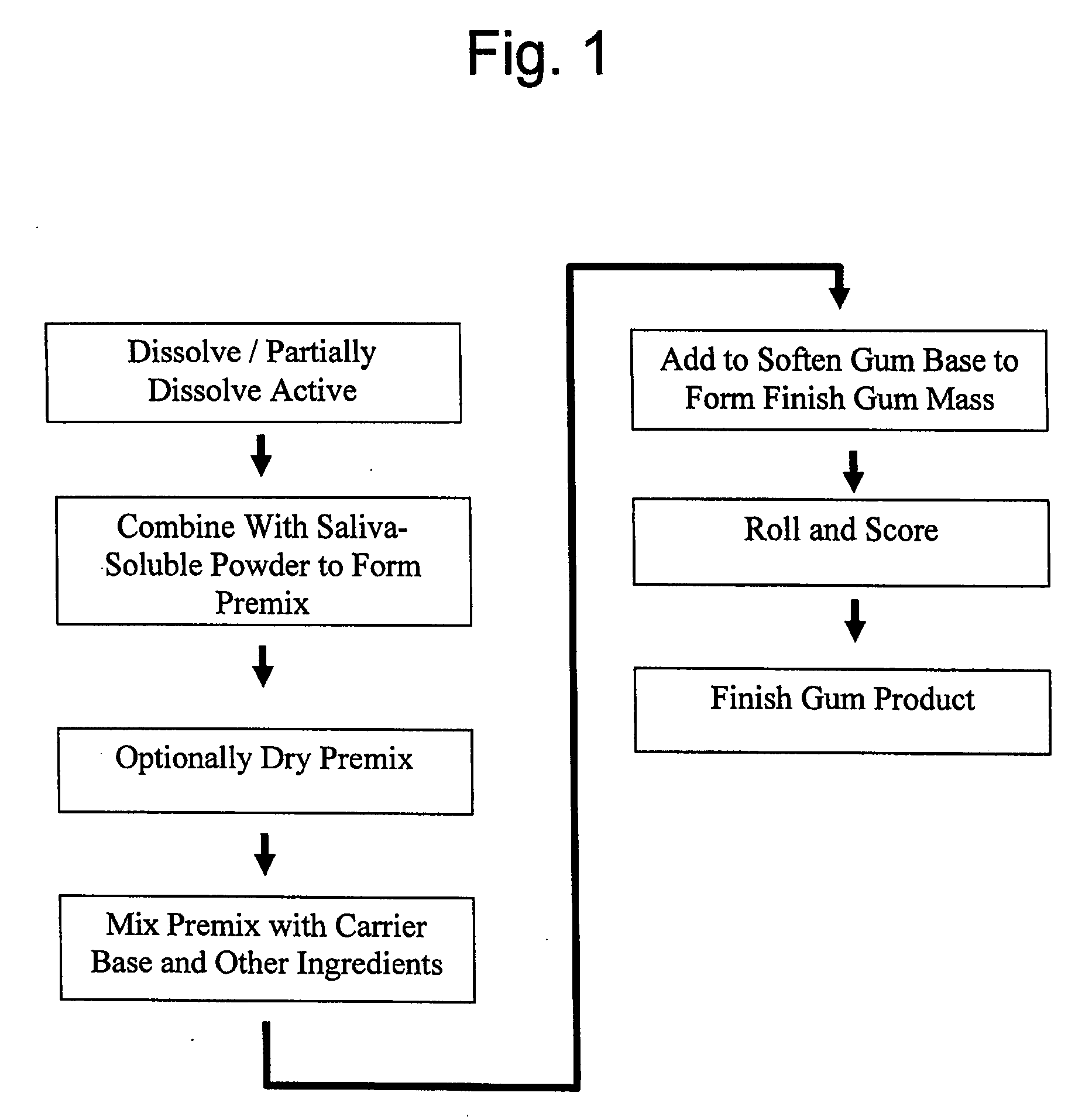
Medicinal delivery system, and related methods
ActiveUS20080020050A1Rapid and sustained reliefMedication quicklyPowder deliveryBiocideMedicineDelivery system
A method is provided of making a medicinal delivery system which satiates a craving in an individual when the medicinal delivery system is administered orally to the individual. A coating composition is applied on a saliva-soluble powder to establish a coated powder, the coating composition featuring an at least partially solubilized craving satiation medicinal compound. The coated powder is combined with an edible carrier base to establish a medicinal delivery system that rapidly releases medicine and buffer preferably followed by slower, sustained release.
Owner:JSR NTI
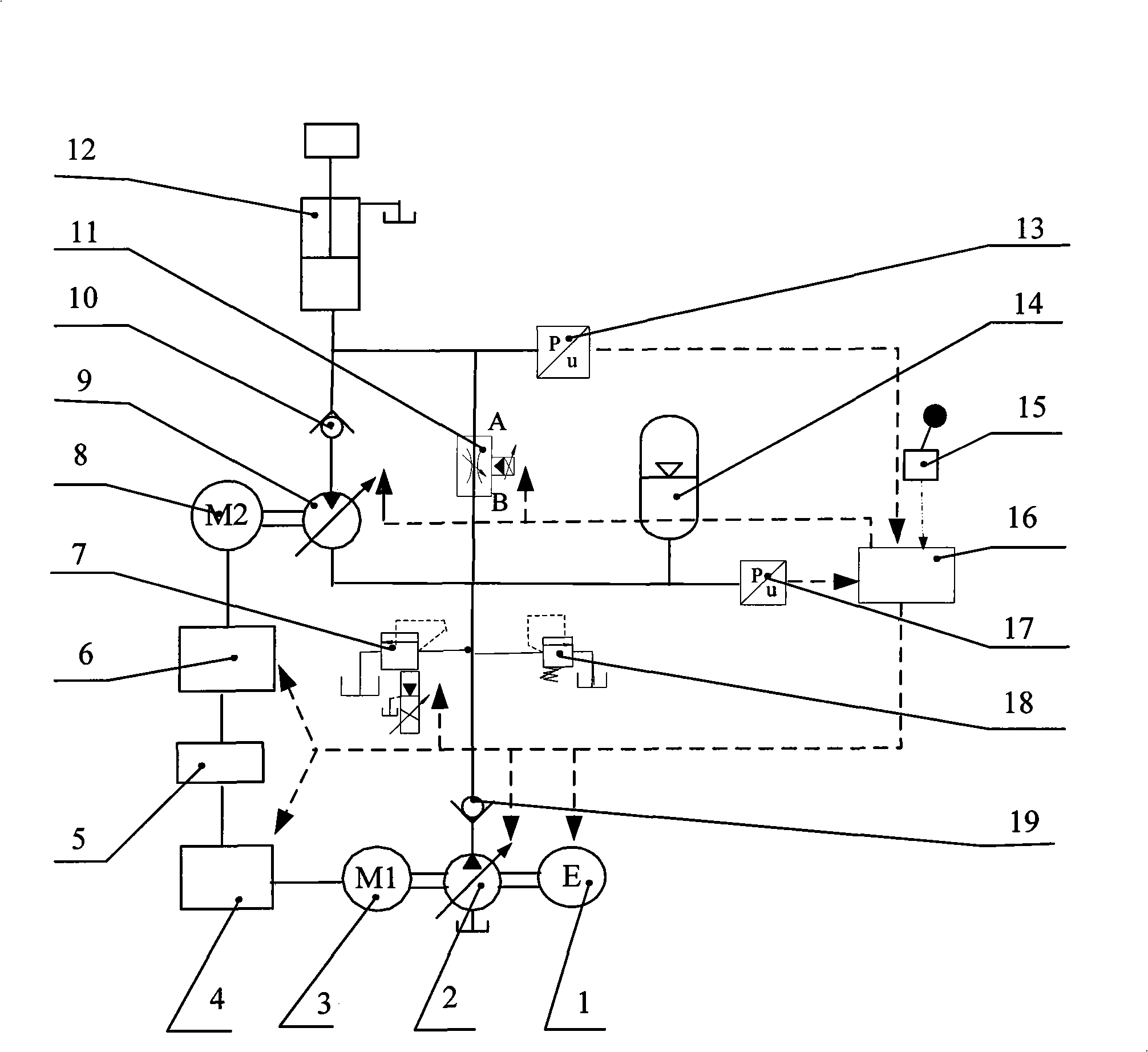
Energy recovery system of hybrid power engineering machinery energy accumulator-hydraulic motor
InactiveCN101408213AHigh specific powerFast absorptionAccumulator installationsGas pressure propulsion mountingHydraulic motorEnergy recovery
The invention discloses a hybrid power engineering mechanical accumulator-hydraulic motor energy recovery system. The system mainly comprises an engine, a variable pump, variable frequency motors, a variable motor, a signal control unit, an accumulator and a hydraulic implementation element, and the like. In the system, the variable motor is coaxially connected with a variable frequency motor M2 and forms a composite energy recovery system with the accumulator so as to recover the gravitational potential energy and braking energy in the lowering process of an implementation mechanism. The variable pump, the engine and a variable frequency motor M1 are coaxially connected; a hybrid power system which consists of the engine and the variable frequency motor M1 drives the hydraulic implementation mechanism to rise and lift heavy objects together with the accumulator. The system overcomes the defects that the variable motor-power generator energy recovery system responds slowly and the specific energy of the accumulator is low, enhances the dynamic response performance of the energy recovery system, improves the working conditions of power generation of an electric generator, and simultaneously can directly recover part of potential energy by the accumulator, thus raising the energy recovery efficiency of the system.
Owner:ZHEJIANG UNIV
Creping process and products made therefrom
ActiveUS20070137810A1High strengthImprove propertiesNatural cellulose pulp/paperMechanical working/deformationFiberPolymer science
Tissue products are disclosed containing an additive composition. The additive composition, for instance, comprises an aqueous dispersion containing an alpha-olefin polymer, an ethylene-carboxylic acid copolymer, or mixtures thereof. The alpha-olefin polymer may comprise an interpolymer of ethylene and octene, while the ethylene-carboxylic acid copolymer may comprise ethylene-acrylic acid copolymer. The additive composition may also contain a dispersing agent, such as a fatty acid. The additive composition may be incorporated into the tissue web by being combined with the fibers that are used to form the web. Alternatively, the additive composition may be topically applied to the web after the web has been formed. For instance, in one embodiment, the additive composition may be applied to the web as a creping adhesive during a creping operation. The additive composition may improve the strength of the tissue web without substantially affecting the perceived softness of the web in an adverse manner.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Intradermal delivery of substances
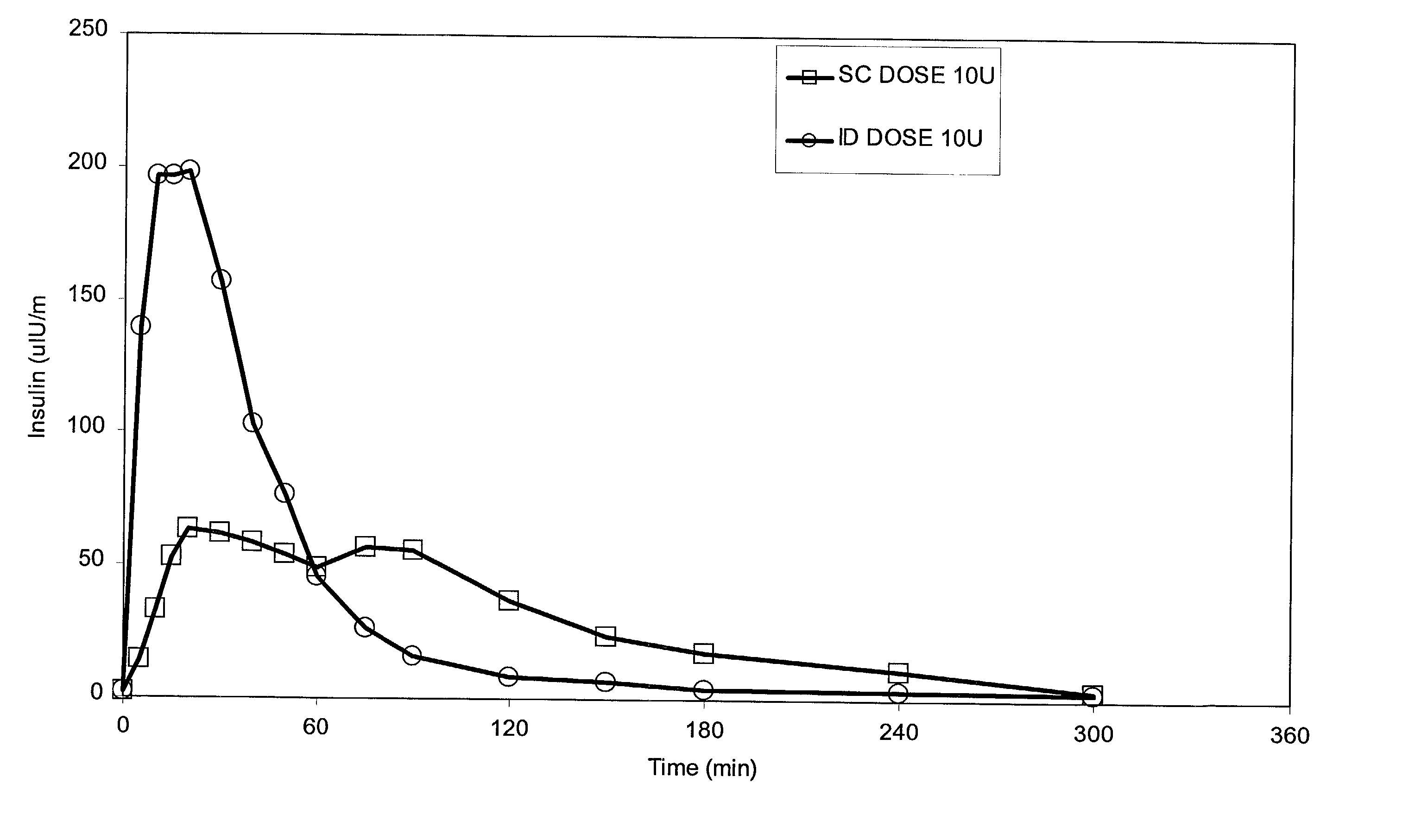
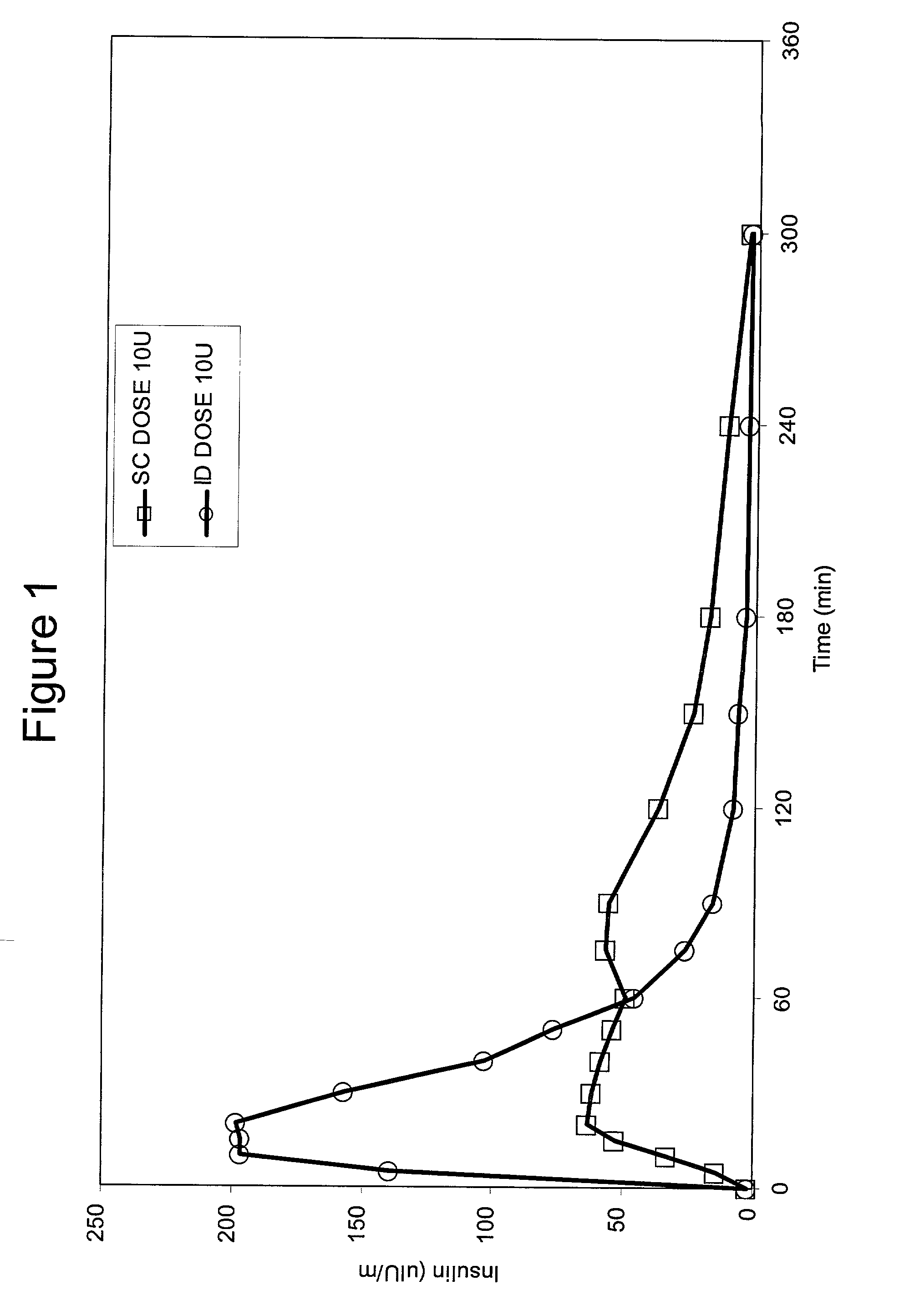
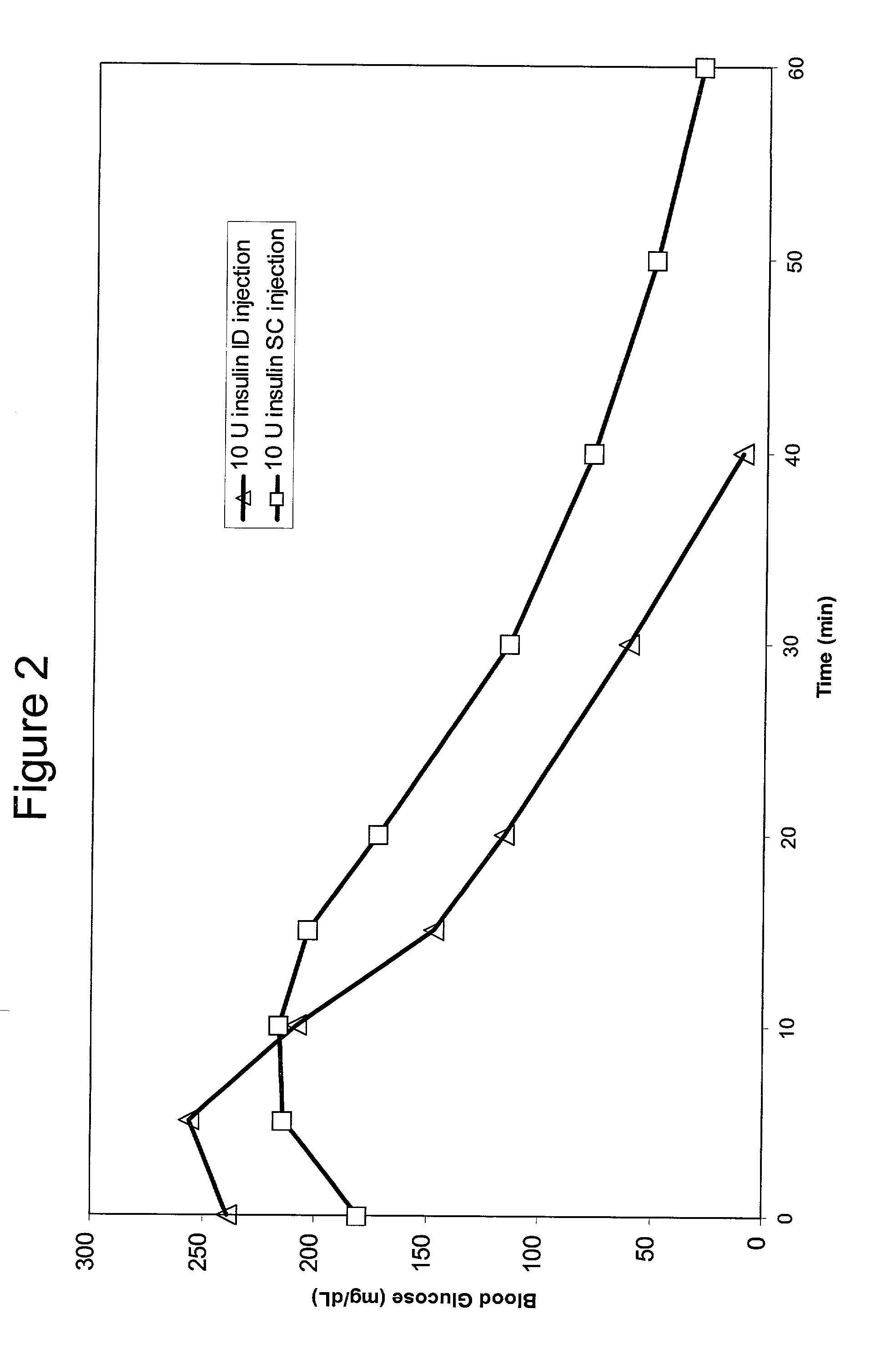
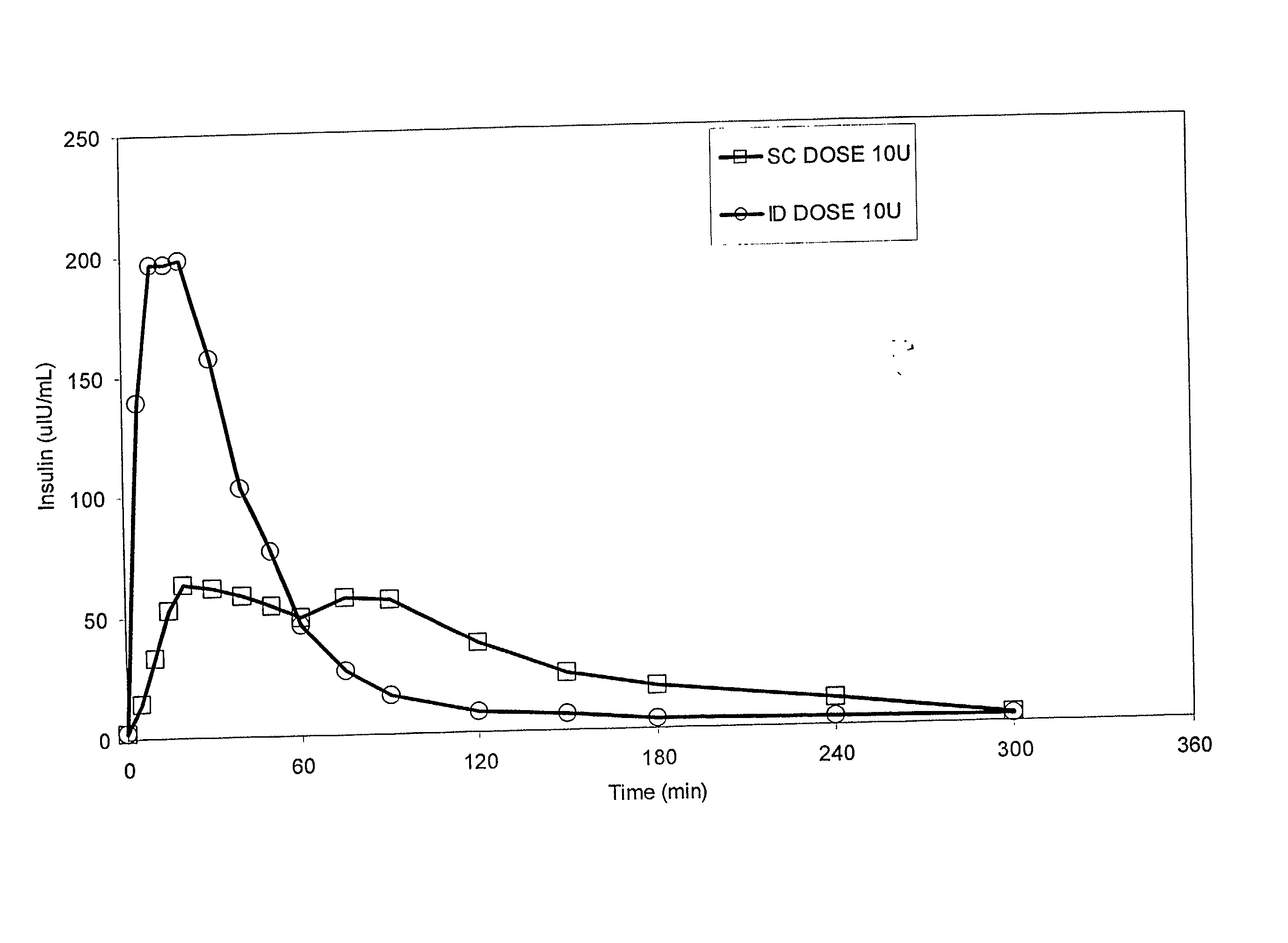
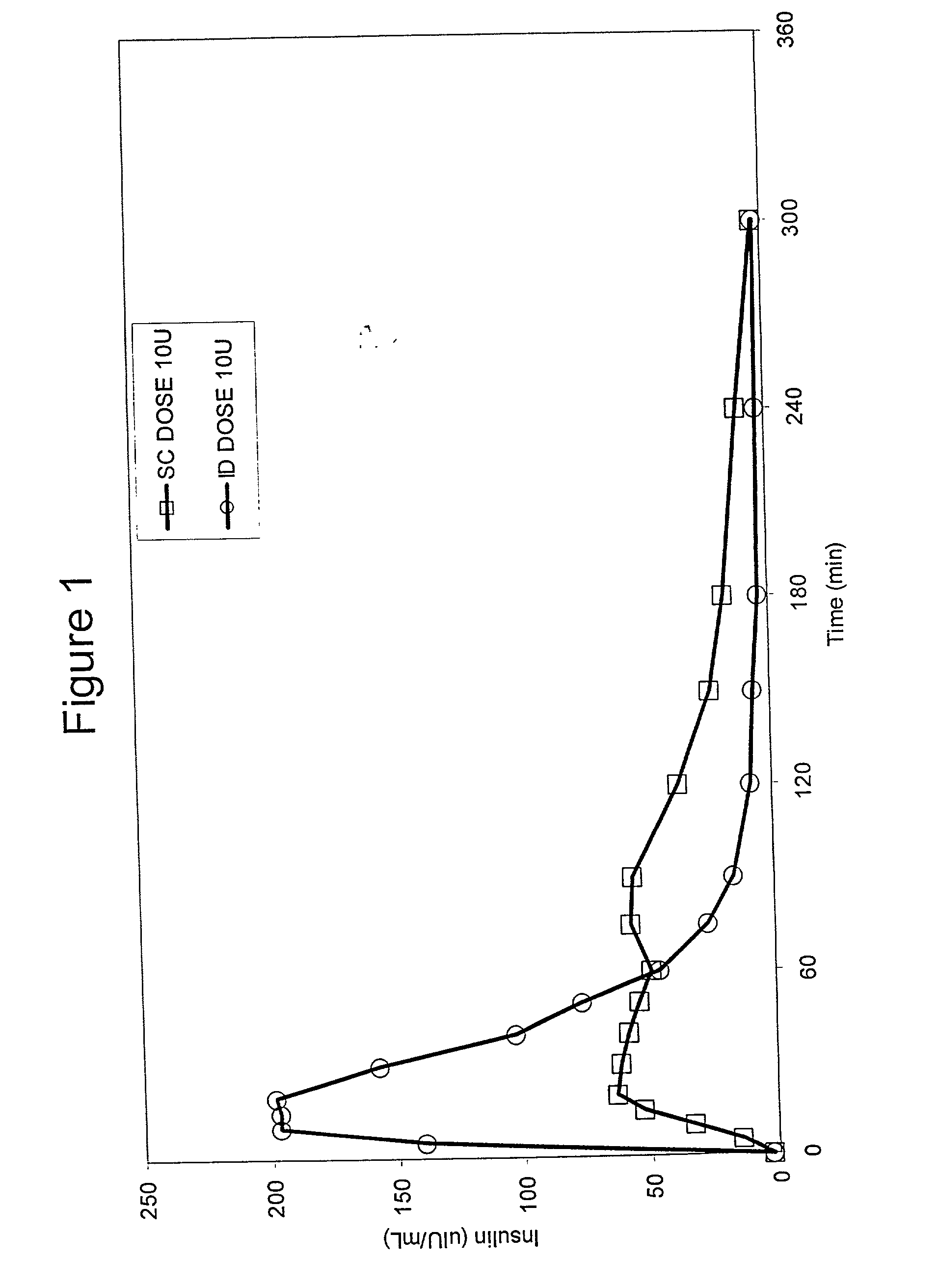
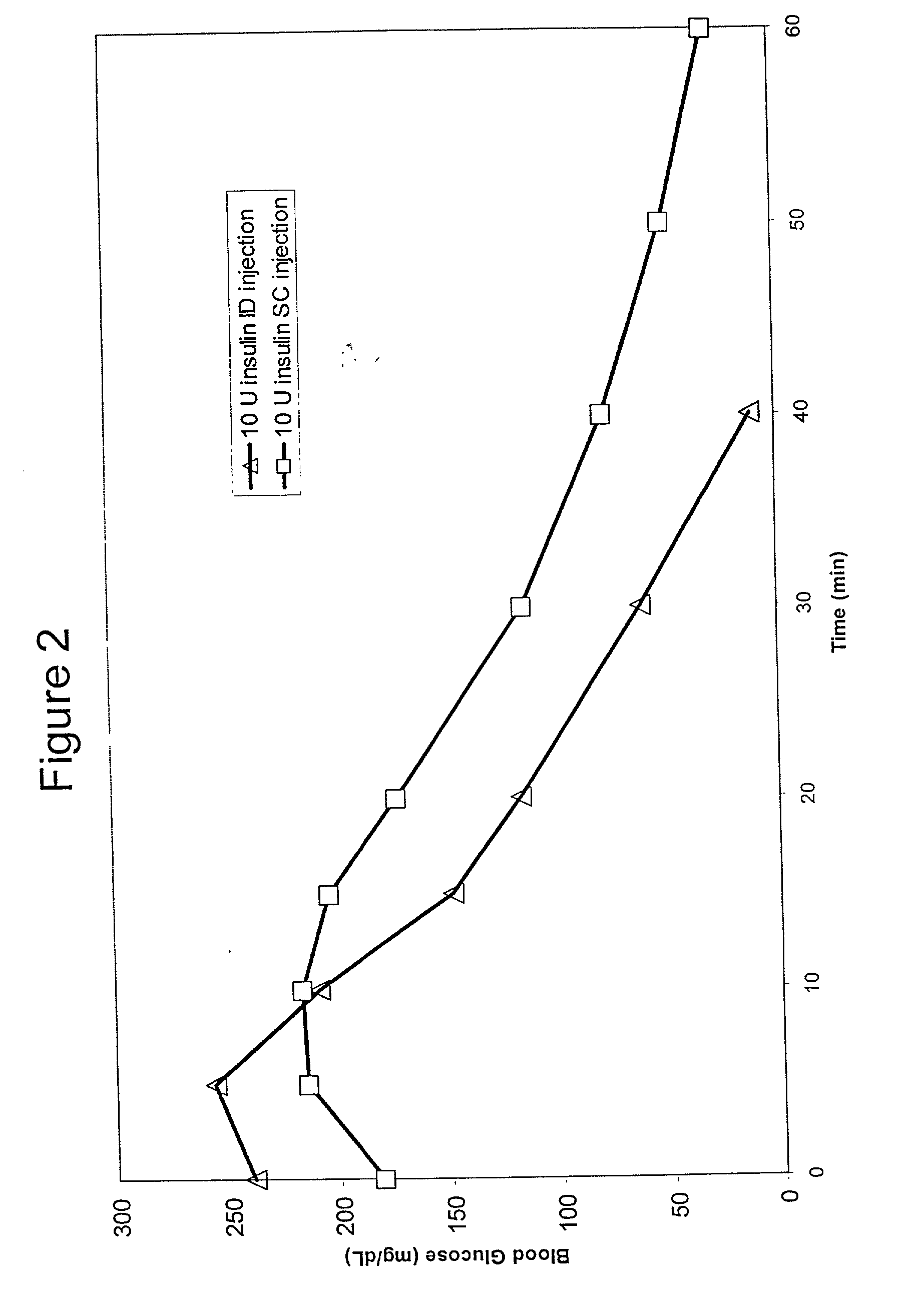
InactiveUS20040073160A1Increase uptakeRapid uptake rateJet injection syringesPeptide/protein ingredientsWhole bodyGrowth hormone
A method for administration of a substance into the dermis of a mammal is disclosed. The method involves administration into the dermis by injection which results in improved systemic absorption relative to that obtained upon subcutaneous administration of the substance. The substance administered may be a growth hormone, a low molecular weight heparin or a dopamine receptor agonist.
Owner:PHARMACIA CORP
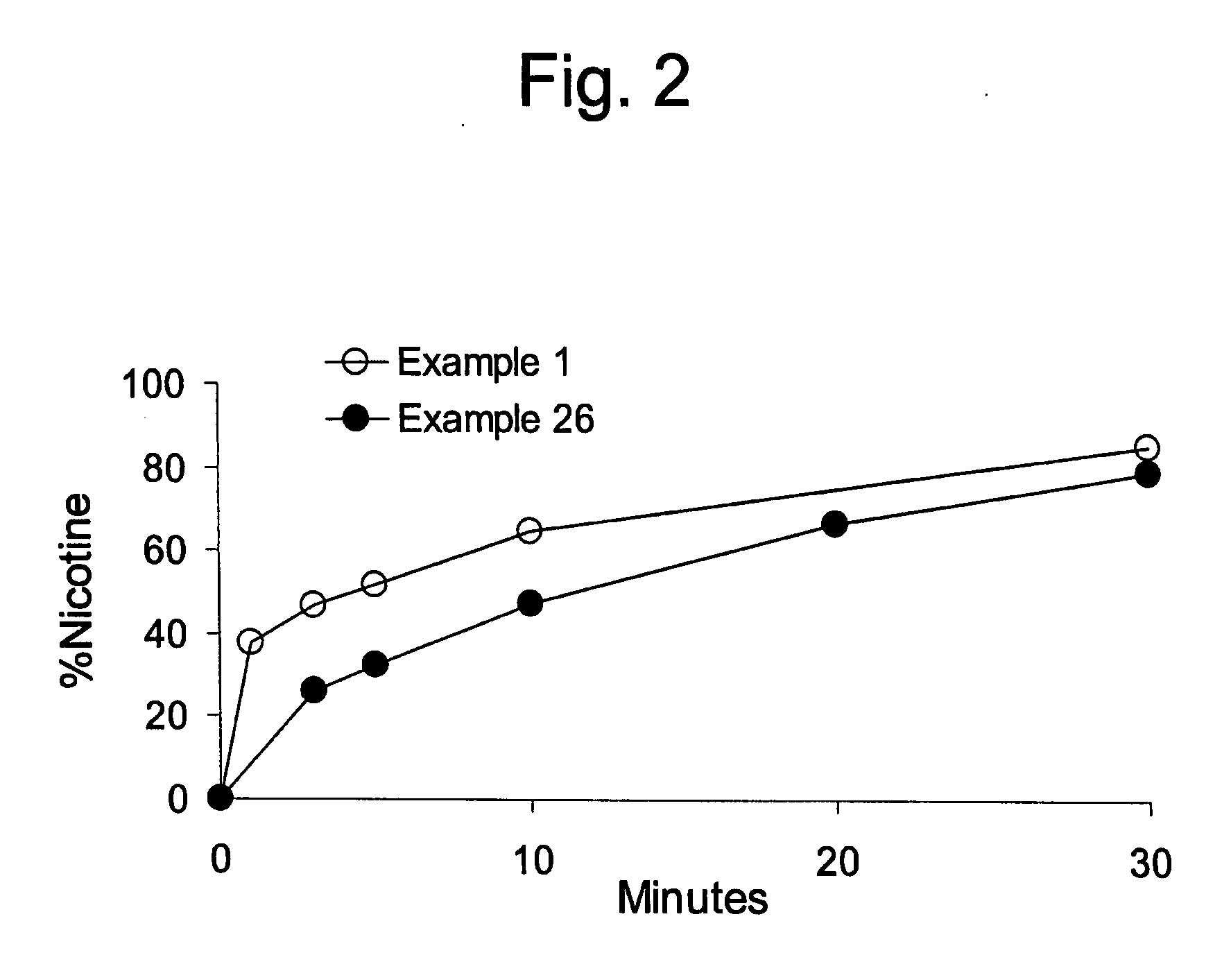
Pulmonary delivery devices
PendingUS20170157341A1Lower potentialFast absorptionRespiratorsMedical devicesMedicineBULK ACTIVE INGREDIENT
A pulmonary delivery apparatus (10) comprising: a first chamber (15) adapted to thermally vaporise a quantity of a first liquid to form a relatively warm first vapour (B) and a second chamber (16) adapted to atomize a quantity of a second liquid without heating of the second liquid to form a mist of a relatively cold second vapour (A), and an outlet (30) via which, in use, a user can inhale a mixture of the first and second vapours. An active ingredient such as nicotine is provided in the second chamber and an inert liquid in the first chamber.
Owner:TWENTY SIXTEEN 2016 PHARMA
Fibrillated polyolefin foam
ActiveUS20080076844A1Improve heat resistanceLow compression setAbsorbent padsBandagesThermoplasticFiber
A method for generating a thermoplastic foam from an aqueous dispersion, the aqueous dispersion comprising a thermoplastic resin, water, and a dispersion stabilizing agent, the method including: adding at least one froth stabilizing surfactant to the aqueous dispersion to form a mixture; adding a fiber to the mixture; and frothing the mixture to create a froth, removing at least a portion of the water in the froth to create a foam, wherein the foam generated has a non-cellular fibrillated morphology. In another aspect, embodiments disclosed herein relate to a foam having a thermoplastic-based, fibrillated, non-cellular structure, wherein the foam has an average density of about 0.02 g / cm3 to about 0.07 g / cm3. In certain embodiments, the foam may be used in an absorbent article.
Owner:DOW GLOBAL TECH LLC
Buccal, polar and non-polar spray containing ondansetron
InactiveUS20060198790A1Effect be very fastRapid absorptionOrganic active ingredientsNervous disorderSolventOral mucosa
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide ondansetron for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, ondansetron, and optional flavoring agent; formulation II: aqueous polar solvent, ondansetron, optionally flavoring agent, and propellant; formulation III: non-polar solvent, ondansetron, and optional flavoring agent; formulation IV: non-polar solvent, ondansetron, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, ondansetron, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, ondansetron, optional flavoring agent, and propellant.
Owner:DUGGER HARRY A III +1
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption
InactiveUS20030100885A1Increase uptakeRapid onsetOrganic active ingredientsAmpoule syringesWhole bodySystemic absorption
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption.
Owner:BECTON DICKINSON & CO
Glyceride compositions and methods of making and using same
InactiveUS20040209953A1Fast absorptionImprove stabilityOrganic active ingredientsBiocideMonoglycerideDiglyceride
Disclosed are glyceride compositions, methods of making the glyceride compositions, and nutritional formulations containing the glyceride compositions. The glyceride compositions contain predominantly monoglycerides and diglycerides carrying one or more long chain polyunsaturated fatty acids. Also disclosed are methods of using the glyceride compositions and nutritional formulations.
Owner:ABBOTT LAB INC
Aerosol and injectable formulations of nanoparticulate benzodiazepine
InactiveUS20060198896A1Easy doseReduce injection volumeBiocidePowder deliveryBenzodiazepinePolyethylene glycol
Described are nanoparticulate formulations of a benzodiazepine, such as lorazepam, that does not require the presence of polyethylene glycol and propylene glycol as stabilizers, and methods of making and using such formulations. The formulations are particularly useful in aerosol and injectable dosage forms, and comprise nanoparticulate benzodiazepine, such as lorazepam, and at least one surface stabilizer. The formulations are useful in the treatment of status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and as a pre-anesthesia medication.
Owner:ELAN PHRMA INT LTD
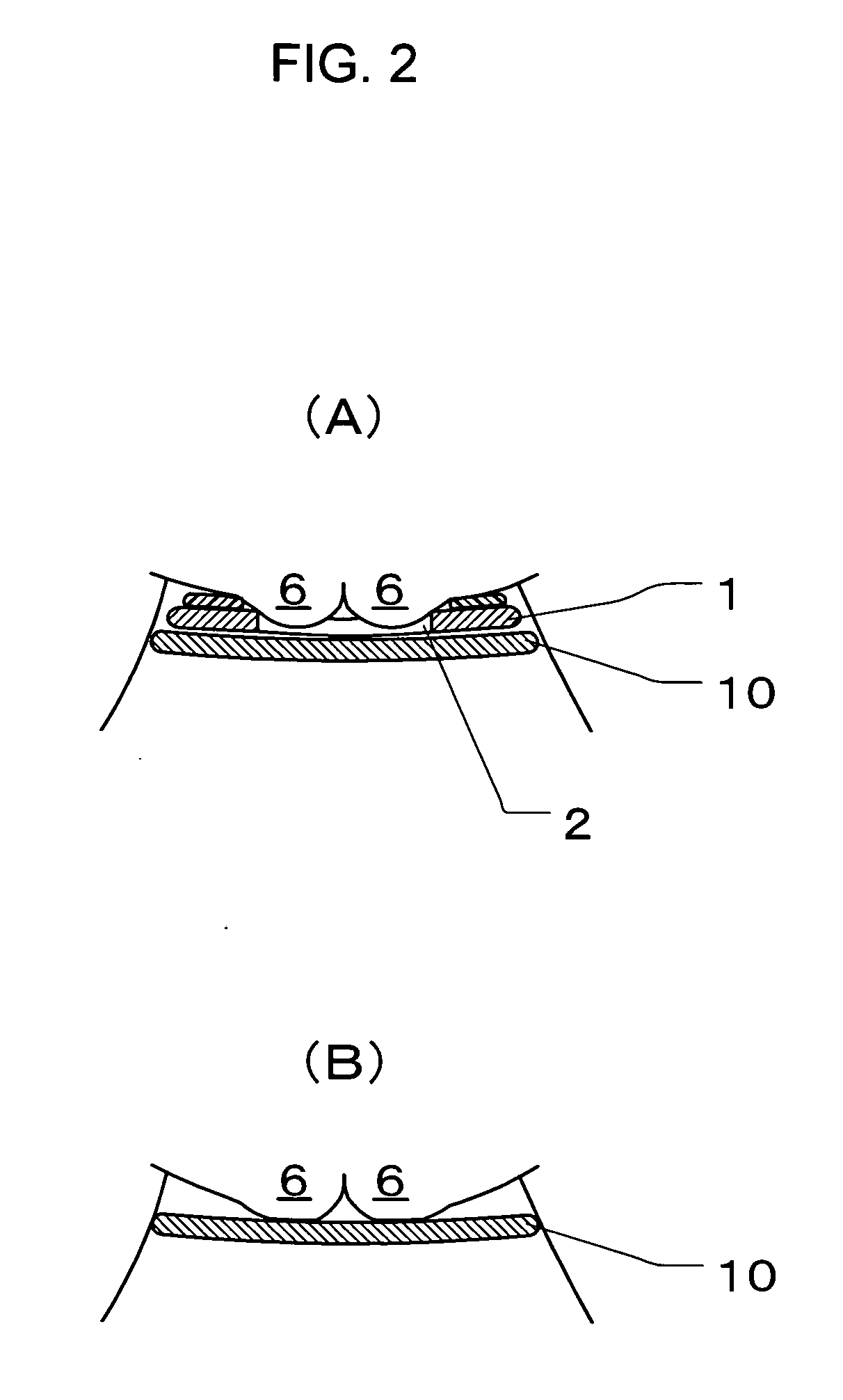
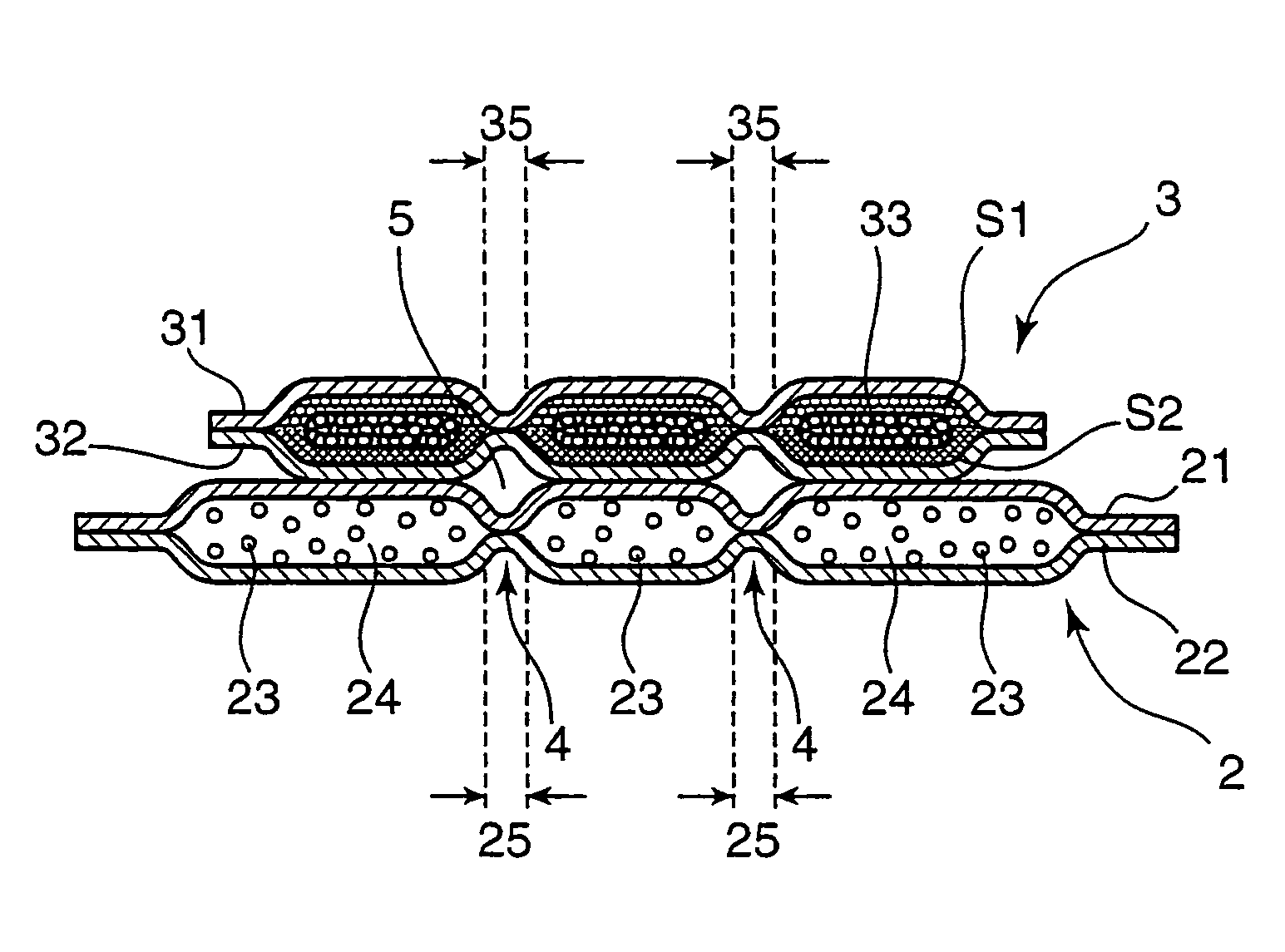
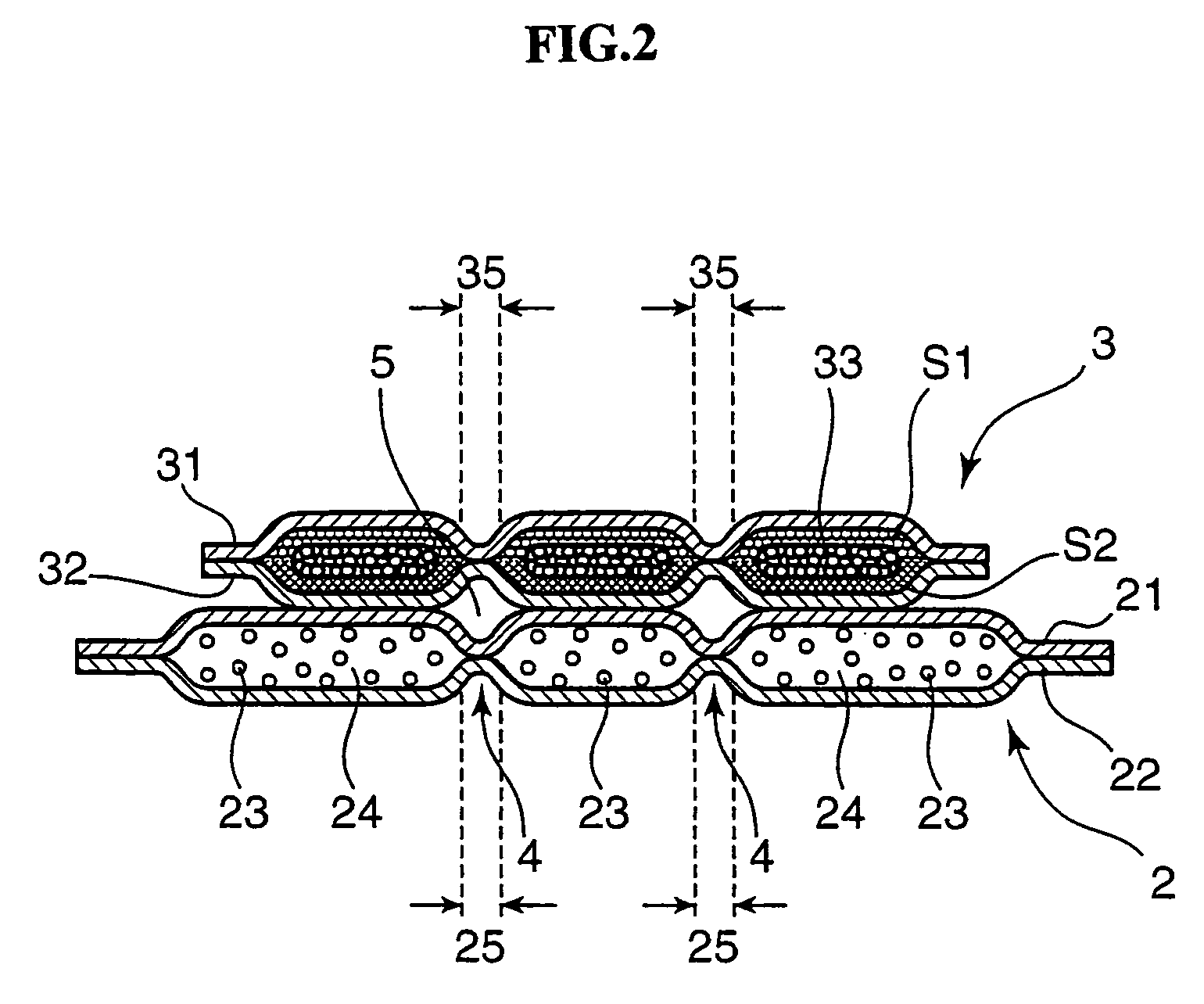
Disposable absorbent article
A disposable absorbent article includes an absorbent mat provided between a liquid-permeable top sheet and a liquid-impermeable back sheet. Herein, the absorbent mat includes a sheet-like water-absorbent layer that contains a water-absorbent resin powder but that does not contain pulp fibers, and a fiber assembly layer that contains both the water-absorbent resin powder and the pulp fibers, the sheet-like water-absorbent layer and the fiber assembly layer arranged sequentially in this order from a top sheet side. The fiber assembly layer includes a fiber presence region in which both the pulp fibers and the water-absorbent resin powder are present, and a fiber absence region in which both the pulp fibers and the water-absorbent resin powder are absent, the fiber presence region and the fiber absence region formed to be adjacent with each other.
Owner:LIVEDO CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com