Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Drospirenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
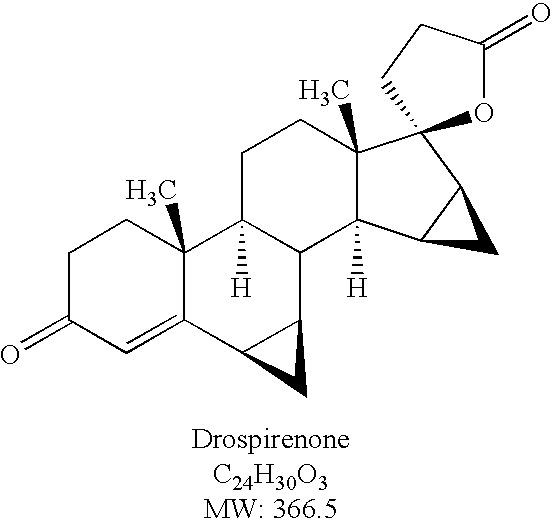
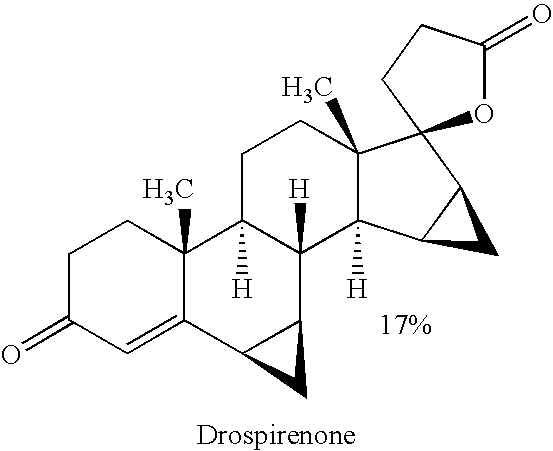
Drospirenone, sold under the brand names Yasmin and Angeliq among others, is a progestin medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses. It is available only in combination with an estrogen and is not available alone. The medication is taken by mouth.
Drospirenone for hormone replacement therapy
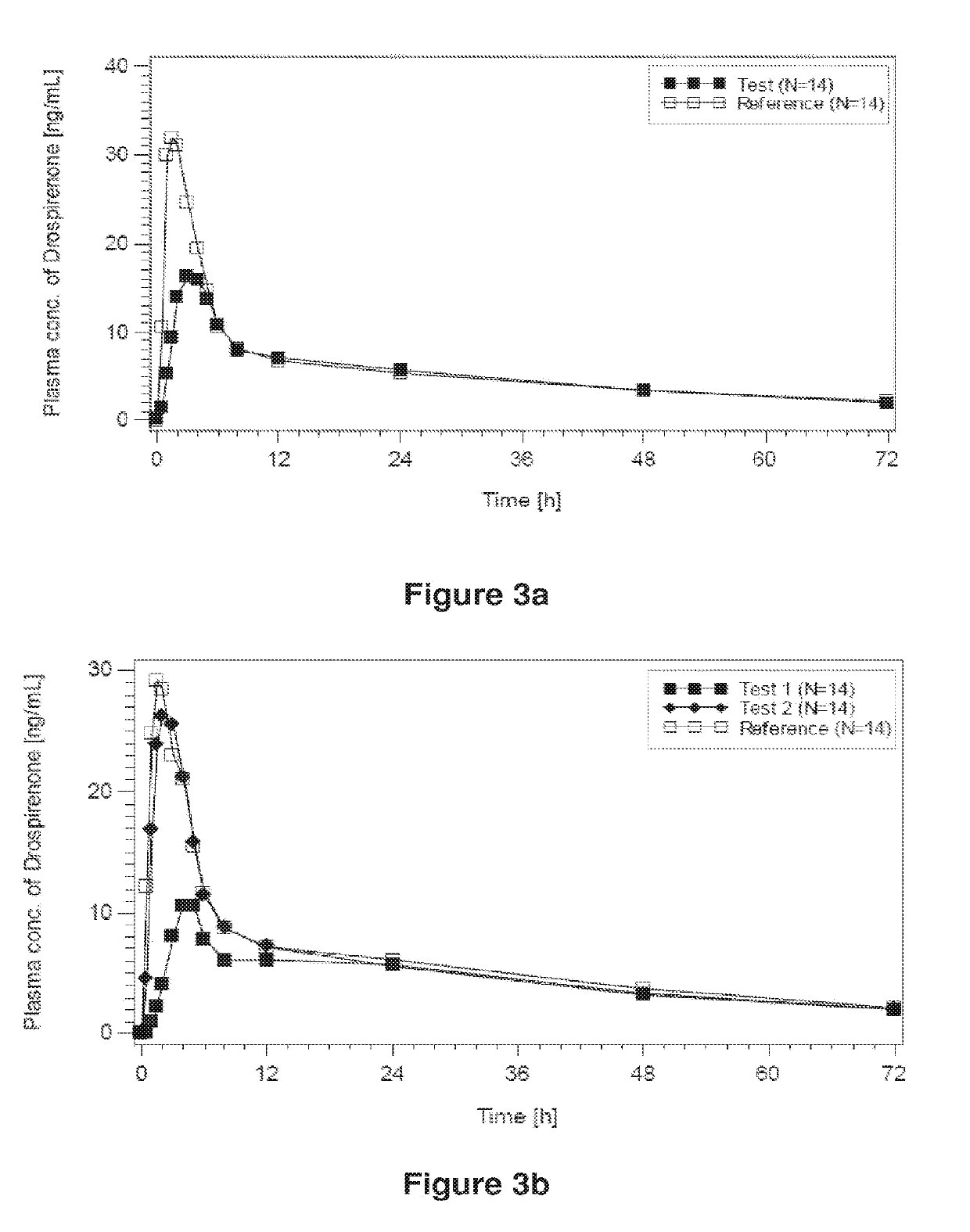
InactiveUS20020132801A1Dissolve fastImprove bioavailabilityOrganic active ingredientsBiocideDrospirenoneBULK ACTIVE INGREDIENT
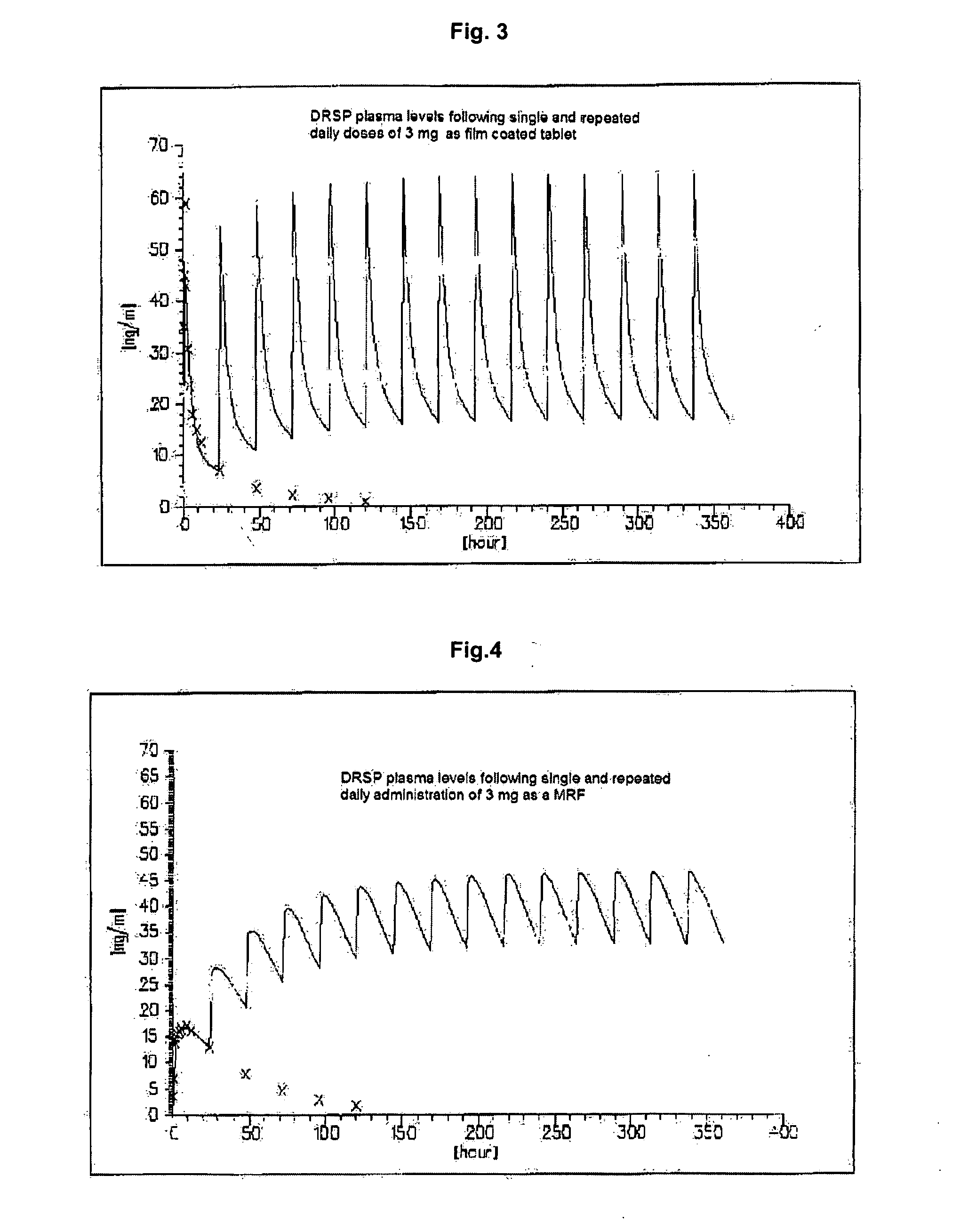
Drospirenone for Hormone Replacement Therapy A pharmaceutical composition comprising as a first active ingredient an estrogen, such as estradiol or estradiol valerate, in sufficient amounts to treat disorders and symptoms associated with deficient endogenous levels of estrogen in women, and as a second active ingredient 6beta,7beta; 15beta; 16beta-dimethylene-3-oxo-17alpha-preg-4-ene-21,17-carbolactone (drospirenone, DRSP) in sufficient amounts to protect the endometrium from the adverse effects of estrogen is useful for, amongst others, treating peri-menopausal, menopausal and post-menopausal women. This composition may be used for hormone replacement therapy and may be administered as a multi-phased pharmaceutical preparation. This combination therapy may comprise continuous, sequential or interrupted administration, or combinations thereof, of DRSP and estrogen, each optionally in micronized form.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Molecular dispersions of drospirenone
InactiveUS20050220825A1Improve bioavailabilityGood chemical stabilityPowder deliveryPill deliveryParticulatesDrospirenone
Described are pharmaceutical compositions comprising at least one steroidal drug such as a progestin (e.g. drospirenone, progesterone, eplerenone, etonogestrel) and / or an estrogen (estradiol and esters thereof) in molecularly dispersed form. The composition comprises a steroidal drug, preferably drospirenone, which is present in the composition in a non-particulate form. Preferably, the drug is present in a dissolved state in the excipient. The molecularly dispersed drug will be released very fast as dissolution takes place instantly when the dosage unit has disintegrated. Also described are methods for preparing the pharmaceutical compositions and methods of using the compositions.
Owner:BAYER SCHERING PHARMA AG
Drospirenone for hormone replacement therapy
InactiveUS20030144258A1Dissolve fastImprove bioavailabilityBiocideOrganic active ingredientsDrospirenoneBULK ACTIVE INGREDIENT
A pharmaceutical composition comprising as a first active ingredient an estrogen, such as estradiol or estradiol valerate, in sufficient amounts to treat disorders and symptoms associated with deficient endogenous levels of estrogen in women, and as a second active ingredient 6beta,7beta; 15beta; 16beta-dimethylene-3-oxo-17alpha-preg-4-ene-21,17-carbolactone (drospirenone, DRSP) in sufficient amounts to protect the endometrium from the adverse effects of estrogen is useful for, amongst others, treating peri-menopausal, menopausal and post-menopausal women. This composition may be used for hormone replacement therapy and may be administered as a multi-phased pharmaceutical preparation. This combination therapy may comprise continuous, sequential or interrupted administration, or combinations thereof, of DRSP and estrogen, each optionally in micronized form.
Owner:SCHERING AG
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
ActiveUS20120128733A1Inhibit ovulationBiocideOrganic active ingredientsDrospirenoneProgestogen-only contraception
The present invention relates to pharmaceutical compositions and kits comprising pharmaceutical compositions, and methods for administering pharmaceutical compositions comprising active contraceptive drugs in a patient. Specifically, the pharmaceutical compositions may comprise progestogen-only contraceptives (“POC”), such as Drospirenone.
Owner:LAB LEON FARMA
Drospirenone-containing preparations for transdermal use
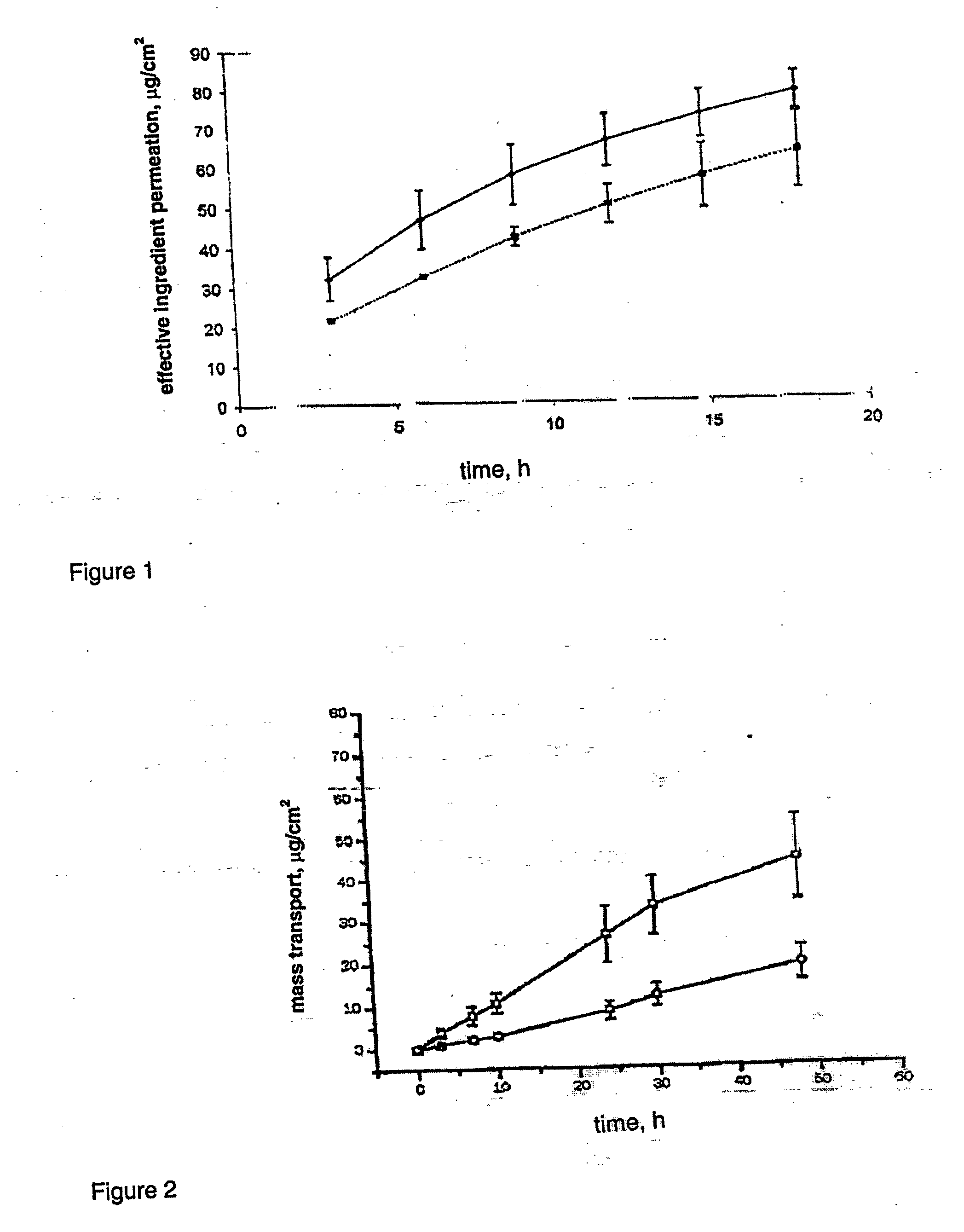
InactiveUS20050222106A1Increase supersaturationReduce dosageOrganic active ingredientsAerosol deliveryTransdermal patchDrospirenone
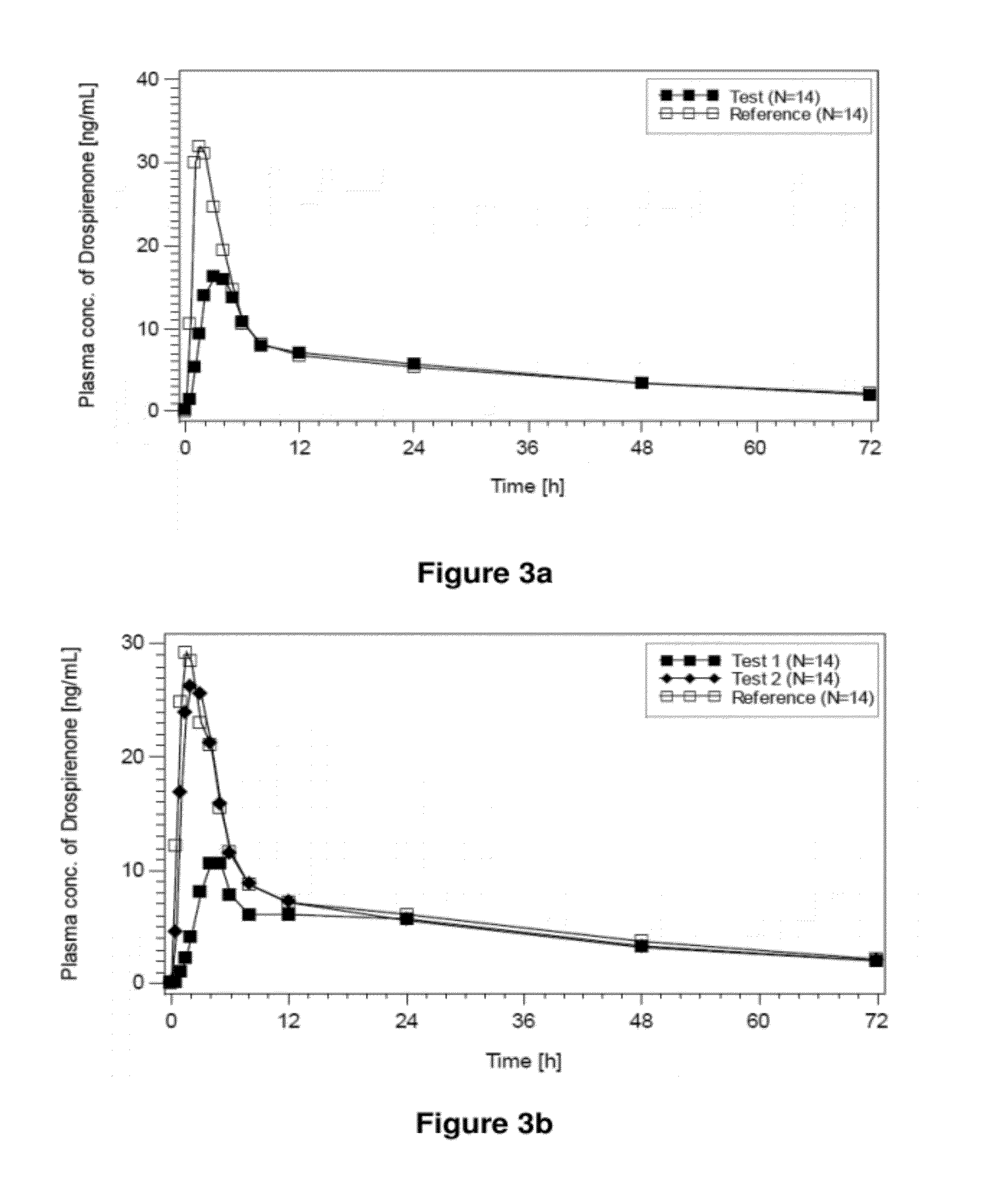
The pharmaceutical preparation for transdermal administration contains solvent ingredients, such as water and ethanol and / or propanol, and drospirenone. The drospirenone is contained in the preparation in an amount that is not above its saturation solubility in an initial state prior to application to skin. However after application to the skin the amount of drospirenone exceeds its saturation solubility due to escape or discharge of the solvent ingredients from the preparation. Preferably the saturation solubility is exceeded by at least a factor of five during application to the skin. The pharmaceutical preparation can also contain an estrogen, such as ethinyl estradiol. It can be in the form of a semi-solid or liquid preparation that is contained in a reservoir-type transdermal patch. A transdermal patch for contraception containing the pharmaceutical preparation including drospirenone and ethinyl estradiol is also disclosed.
Owner:SCHERING AG
Pharmaceutical composition containing a tetrahydrofolic acid
The present invention relates to solid pharmaceutical compositions, in particular to oral contraceptives, comprising a progestogen, such as drospirenone; an estrogen, such as ethinylestradiol; a tetrahydrofolic acid or a pharmaceutically acceptable salt thereof, such as calcium 5-methyl-(6S)-tetrahydrofolate; and at least one pharmaceutical acceptable excipient or carrier. The compositions of the invention provide good stability of the tetrahydrofolic acid upon storage while still ensuring a fast and reliable release of the estrogen and the progestogen present in the composition.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Oral modified release formulations
InactiveUS20100086599A1High dose of drugReduce doseBiocidePowder deliveryDrospirenoneImmediate release
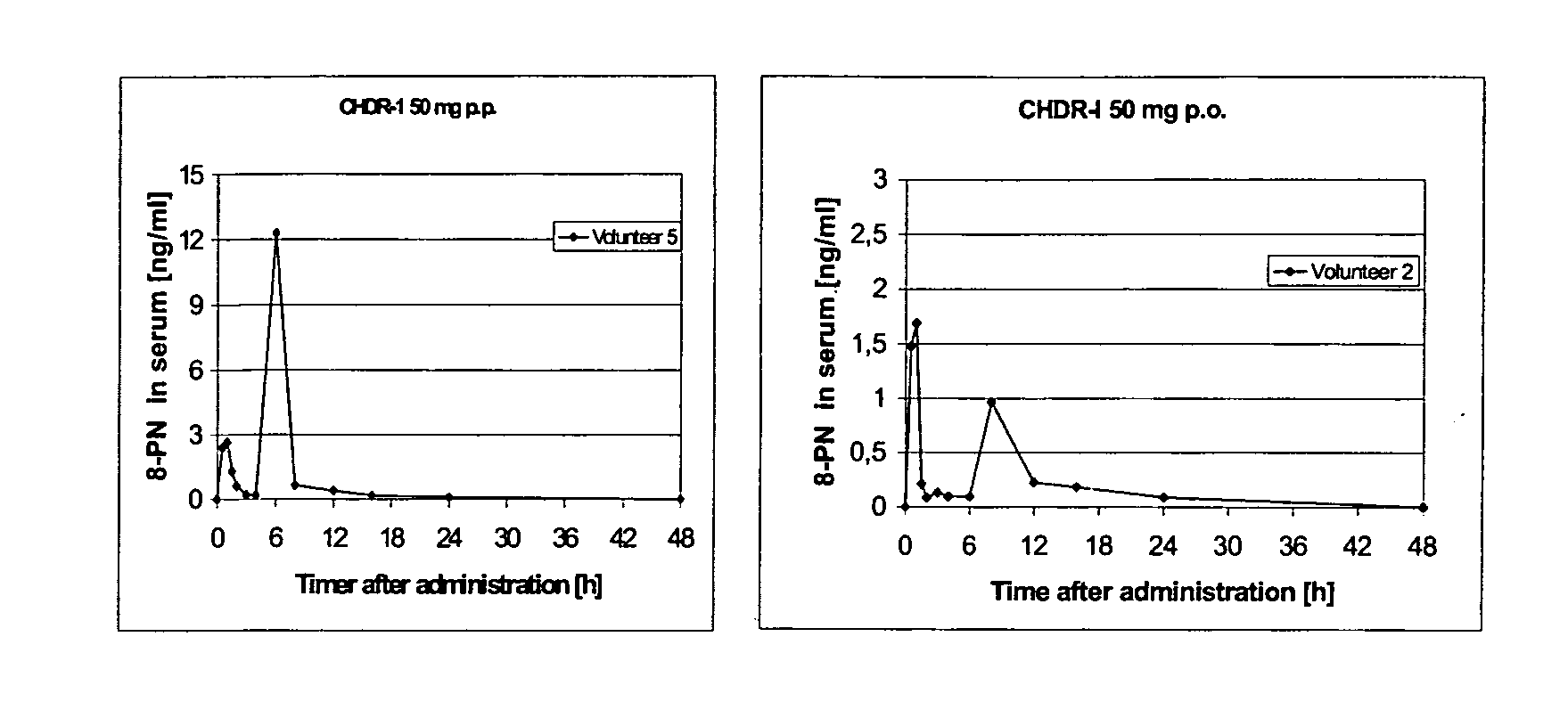
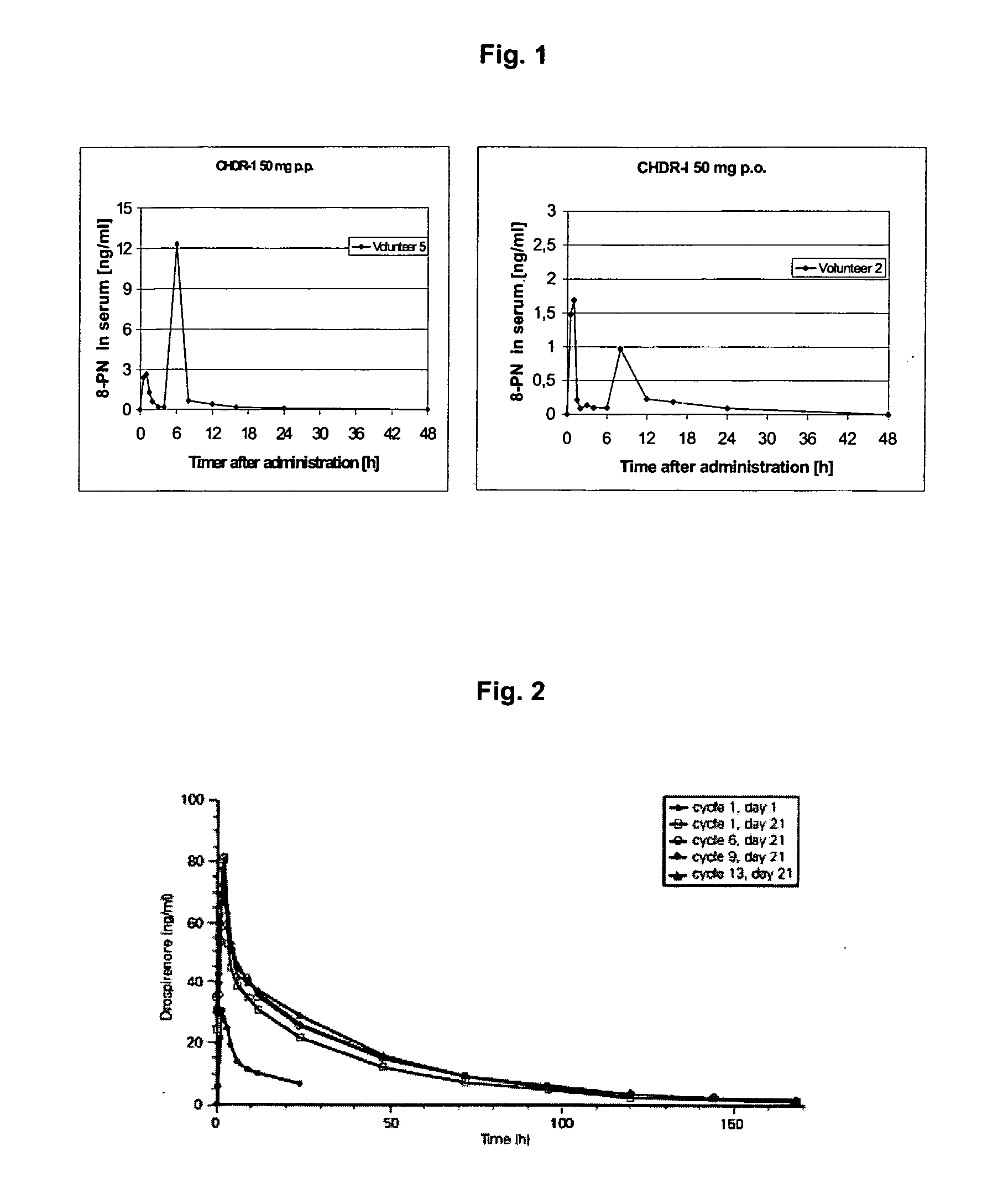
This invention is directed to an oral modified release formulation of the phytoestrogen 8-Prenylnaringenin in combination with a progestin, preferably with Drospirenone, and several uses thereof. In another aspect of the invention an oral modified formulation of 8-Prenylnaringenin with an immediately releasing progestin, like Drospirenone, is provided as well as several uses thereof.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Vaginal delivery system
InactiveUS20090142313A1Convenient and highly drug deliverySuitable for useAntibacterial agentsBiocideDiseaseControlled release
The present invention is related to an intravaginal delivery system for the controlled release of drospirenone and an estrogen, optionally also comprising one or more therapeutically active or a health-promoting substance capable of giving and / or enhancing protection against bacterial and fungal infections, and / or enhancing protection against sexually transmitted diseases. The delivery system consists of one or more compartments, one of each comprising a core and a membrane encasing the core, said core and membrane essentially consisting of a same or different polymer composition, wherein at least one compartment comprises drospirenone ant at least one compartment which may be the same or different from the one comprising drospirenone, comprises an estrogen or a mixture of drospirenone and an estrogen, and wherein the membrane or the surface of the membrane or at least one of the cores comprises said therapeutically active or a health-promoting substance.
Owner:BAYER OY
Vaginal delivery system
InactiveUS20100285097A1Convenient and highly drug deliverySuitable for useAntibacterial agentsBiocideDiseaseDrospirenone
The present invention is related to an intravaginal delivery system for the controlled release of drospirenone and an estrogen, optionally also comprising one or more therapeutically active or a health-promoting substance capable of giving and / or enhancing protection against bacterial and fungal infections, and / or enhancing protection against sexually transmitted diseases. The delivery system consists of one or more compartments, one of each comprising a core and a membrane encasing the core, said core and membrane essentially consisting of a same or different polymer composition, wherein at least one compartment comprises drospirenone ant at least one compartment which may be the same or different from the one comprising drospirenone, comprises an estrogen or a mixture of drospirenone and an estrogen, and wherein the membrane or the surface of the membrane or at least one of the cores comprises said therapeutically active or a health-promoting substance.
Owner:BAYER OY
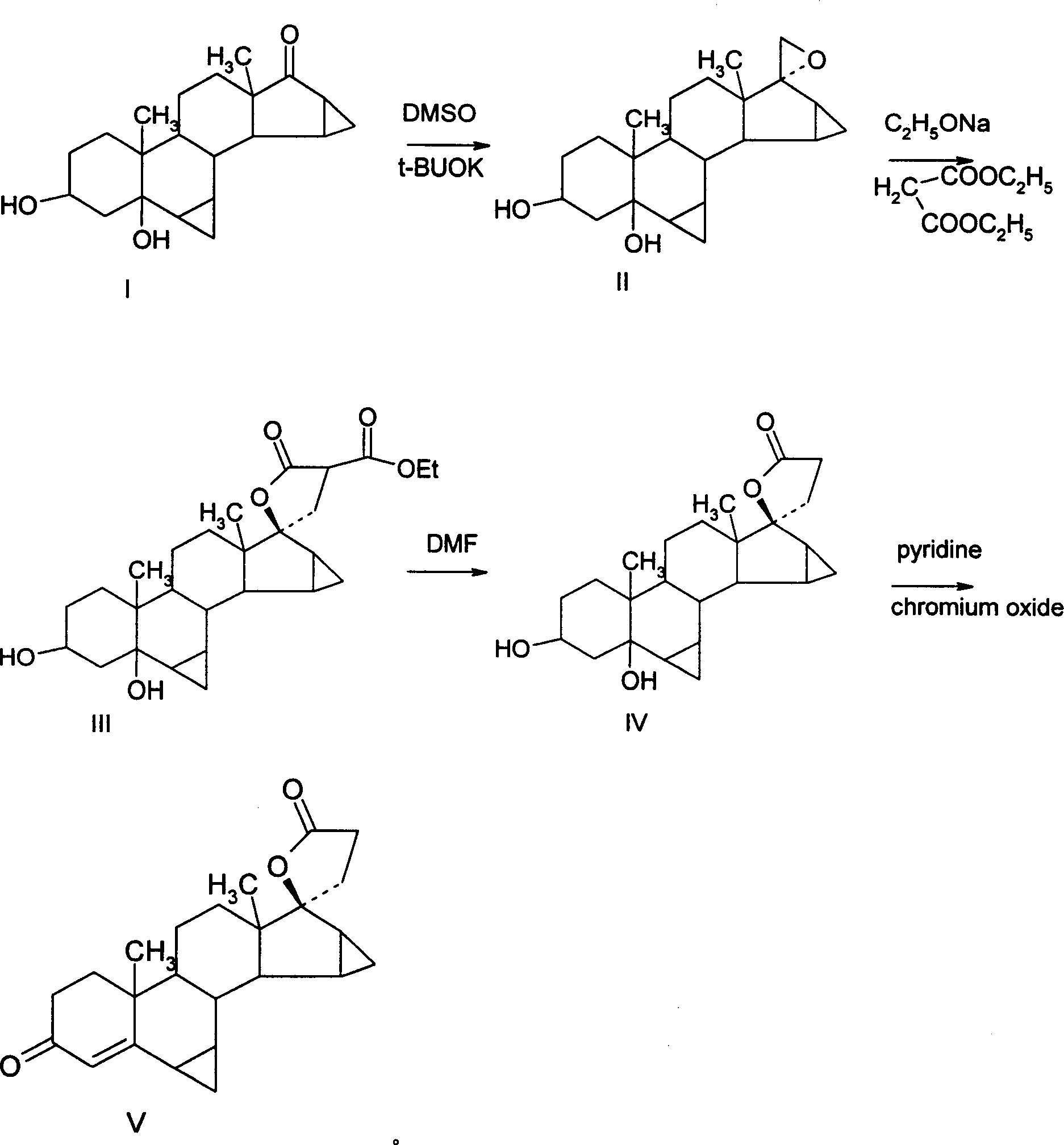
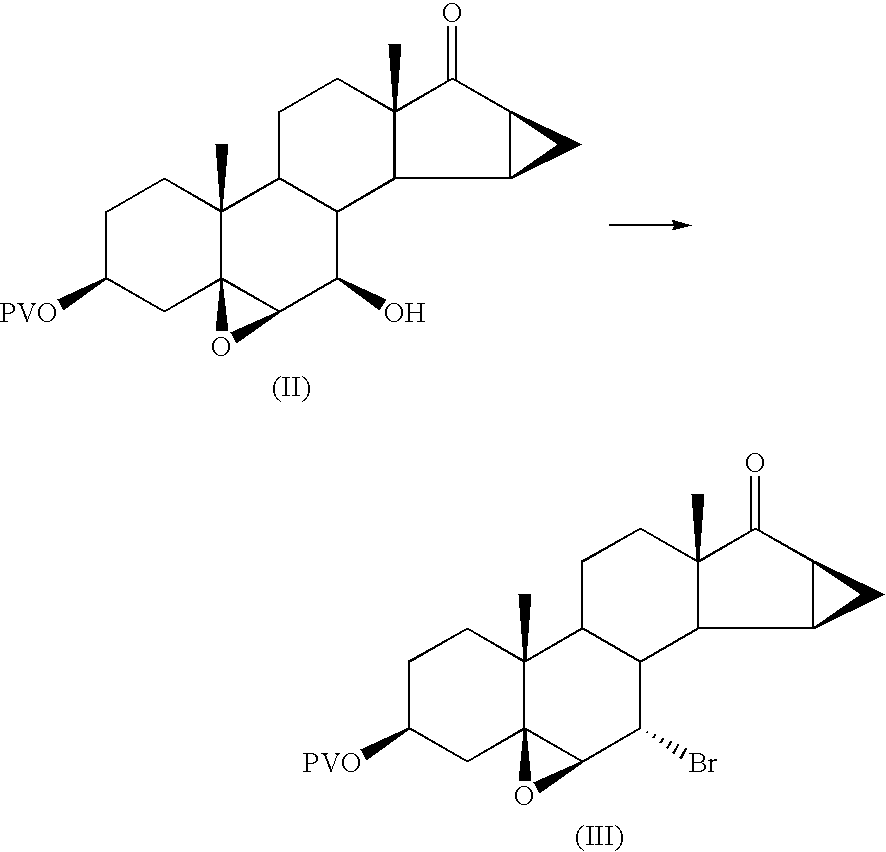
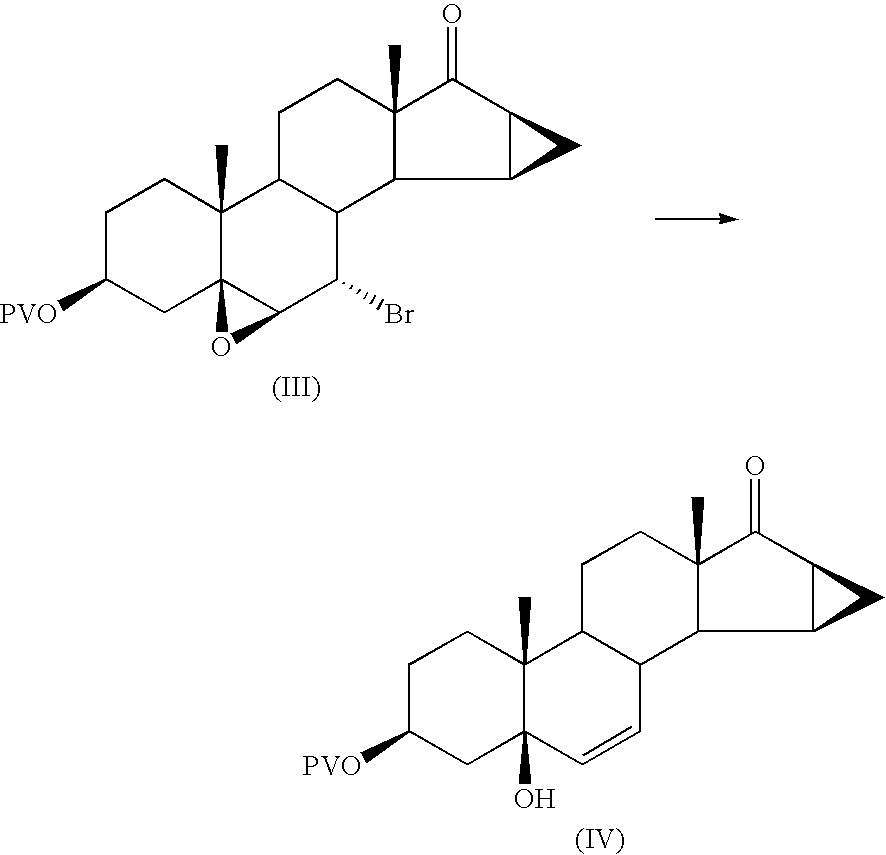
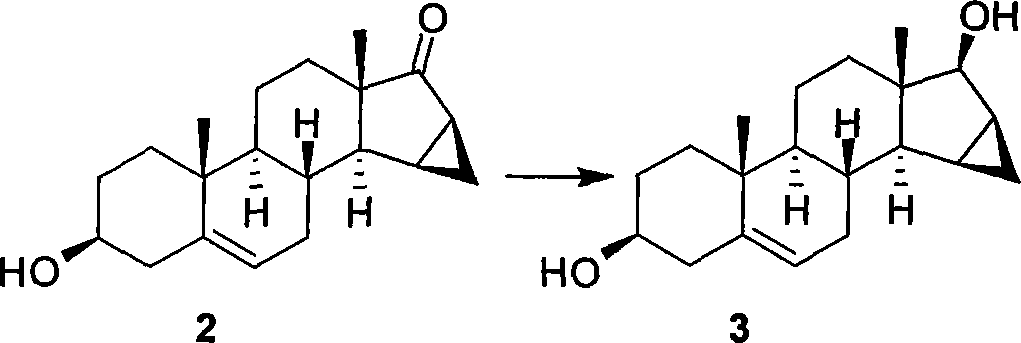
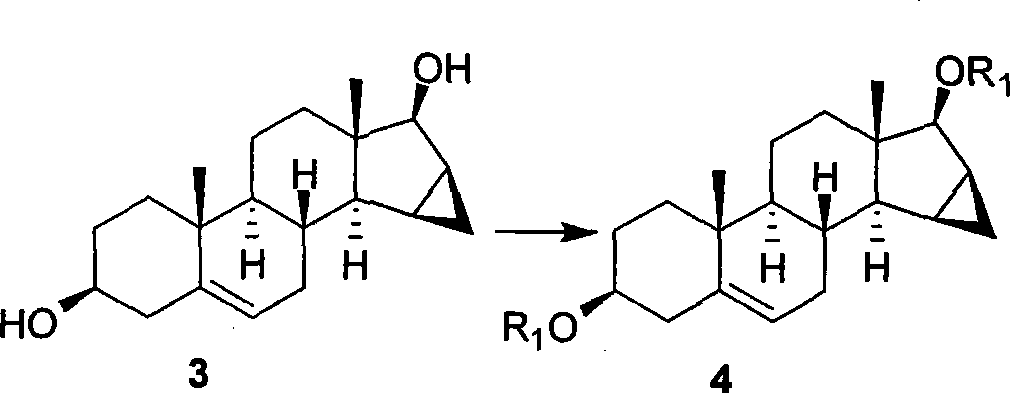
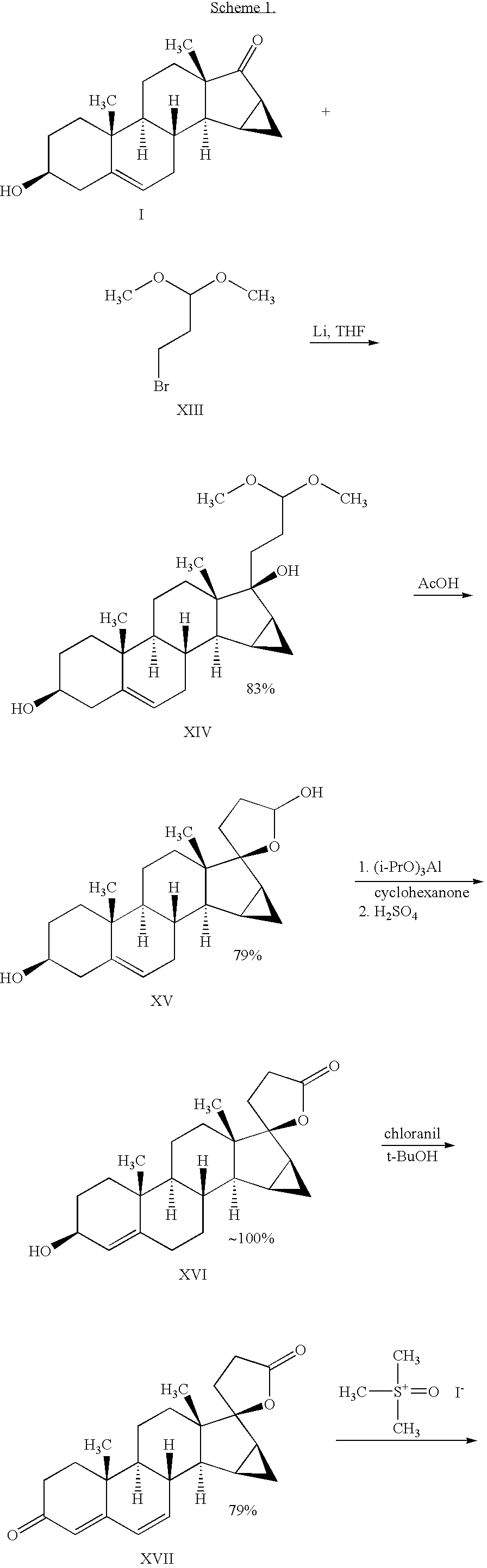
Method for synthesizing Drospirenone
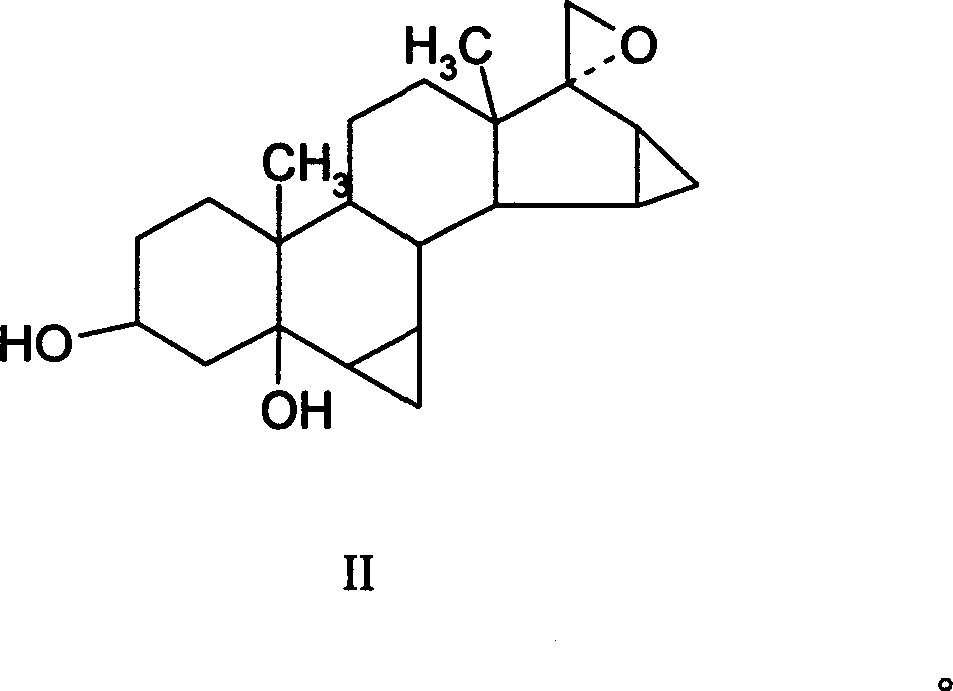
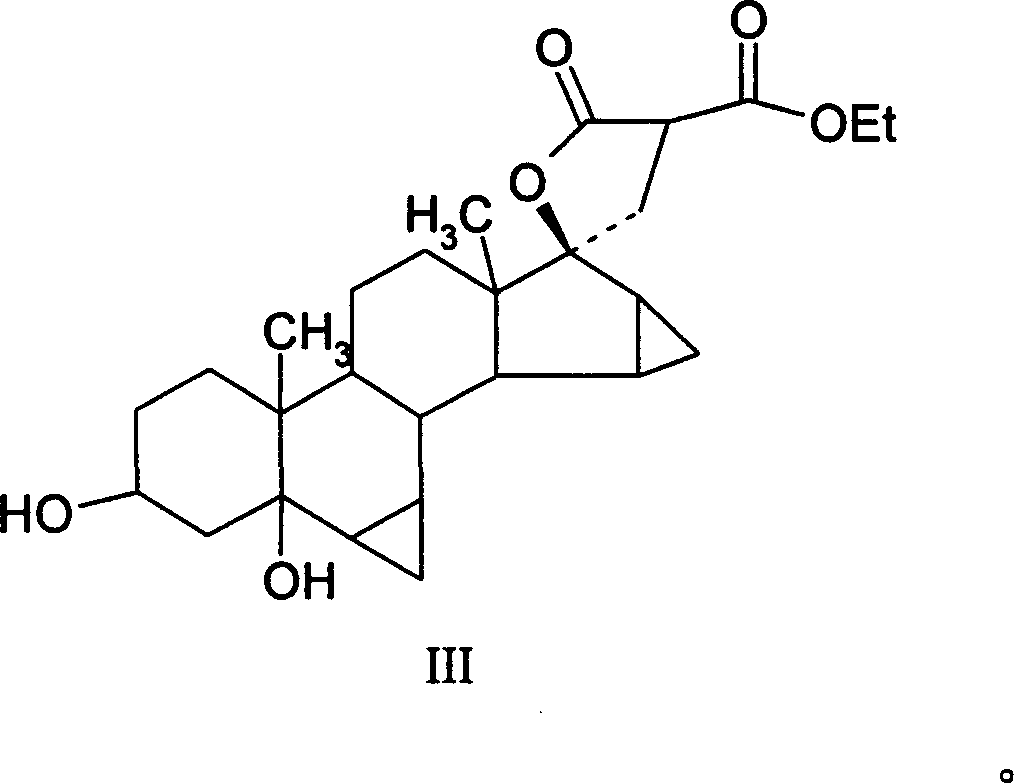
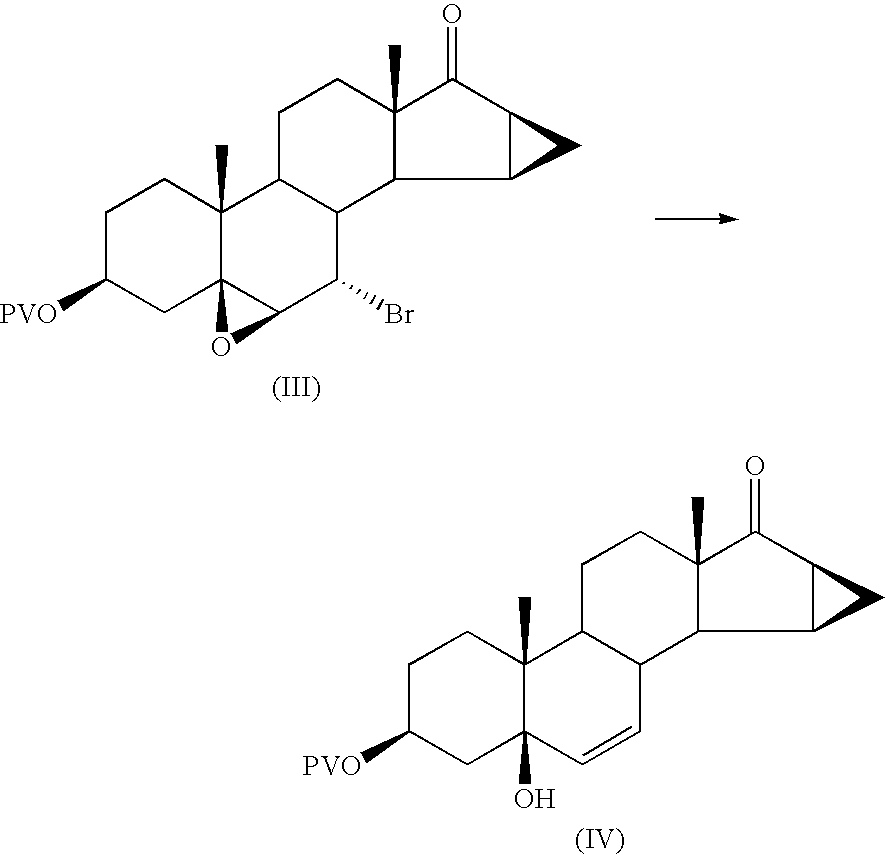
This invention provides a method for synthesizing steroidal progesterone drospirenone. The method comprises: utilizing 3beta, 5-dihydroxyl-6beta, 7beta, 15beta, 16beta-dimethylene-5beta-androstan-17-one (compound I) as the raw material, introducing epoxy at the 17 site, condensing, dehydrogenating, and oxidizing to obtain drospirenone. The method has such advantages as easy operation, high yield and is suitable for industrialization.
Owner:JIANGSU CHUANGUO PHARMA CO LTD
Cardiovascular protection using anti-aldosteronic progestins
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Process for the preparation of drospirenone
A process is described for preparing drospirenone, a synthetic steroid with progestogenic, antimineralocorticoid and antiandrogenic activity, useful for the preparation of pharmaceutical compositions with contraceptive action, starting from 5,6β-epoxy-7β-hydroxy-15β,16β-methylene-3β-pivaloyloxy-5β-androstan-17-one.
Owner:IND CHEM SRL
Method for preparing drospiroenonand intermediate
The invention relates to a new method for preparing drospirenone (6 beta, 7 beta; 15 beta, 16 beta- dimethylene-3-oxo-17 alpha- pregna-4-alkene-21, 17- carboxyl lactone) and an intermediate product obtained by the method, that is, 3- androstane protected by hydroxy group-5- alkene-15 beta, 16 beta- methylene-17-ketone. The invention uses the 3- androstane protected by hydroxy group-5- alkene-15 beta, 16 beta-methylene-17-ketone being available in the market as the starting material, which forms loop coils by the steps such as the hydroxy group protection, addition, hydrolysis, oxidation, and finally forms triatomic ring to prepare the drospirenone.
Owner:2Y CHEM
Process for the preparation of drospirenone
A process is described for preparing drospirenone, a synthetic steroid with progestogenic, antimineralocorticoid and antiandrogenic activity, useful for the preparation of pharmaceutical compositions with contraceptive action, starting from 5,6β-epoxy-7β-hydroxy-15β,16β-methylene-3β-pivaloyloxy-5β-androstan-17-one.
Owner:IND CHEM SRL
Novel method for stereo-selective chemosynthesis of drospirenone
The invention relates to a new method of stereo selectivity synthesis drospirenone and main intermediate in the synthesizing method; 15 Beta, 16 Beta-methylene dehydroepiandrosterone (3 Beta-hydroxy-15Beta, 16Beta-methylene -androstane -5-ene-17-ketone, 2) are adopted as starting raw material for the methods and the drospirenone is synthesized via fourteen steps. The invention has the advantages of higher yield and good stereo selectivity.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
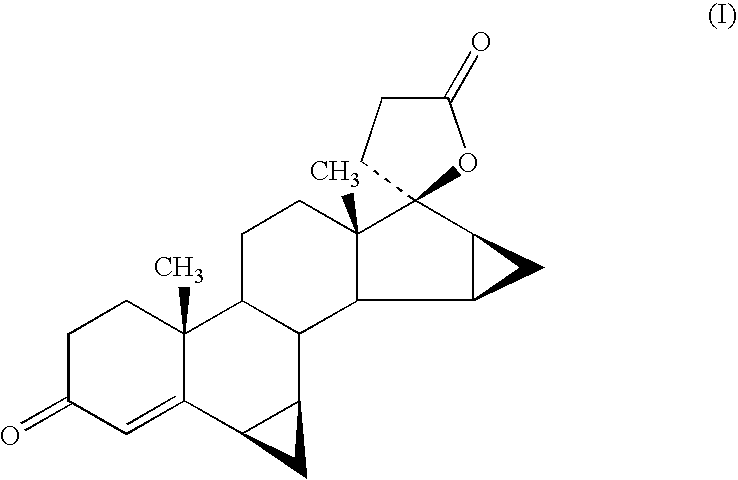
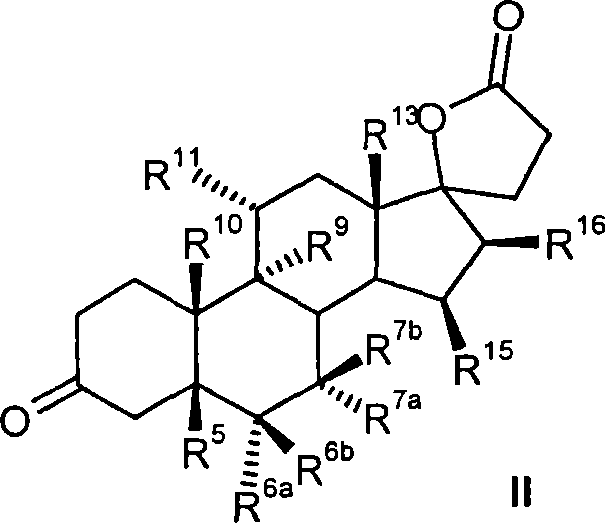
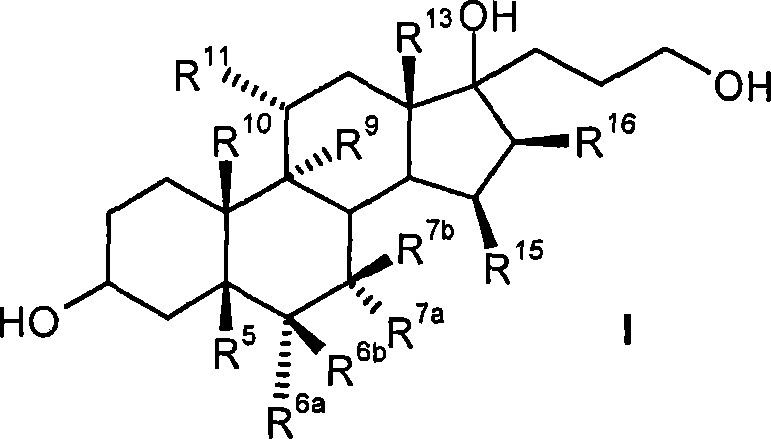
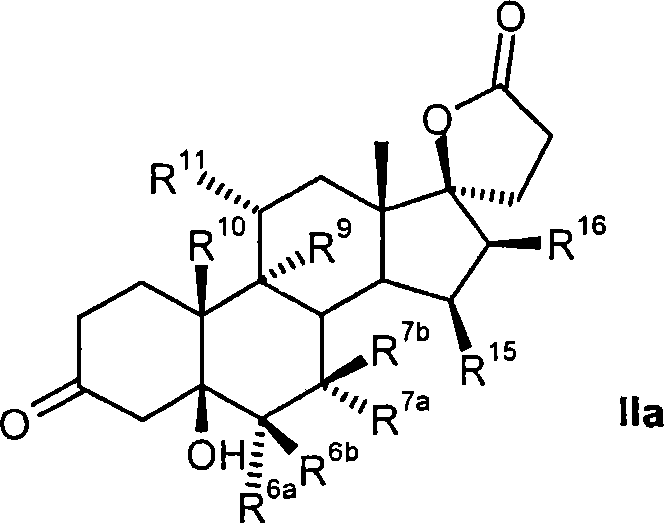
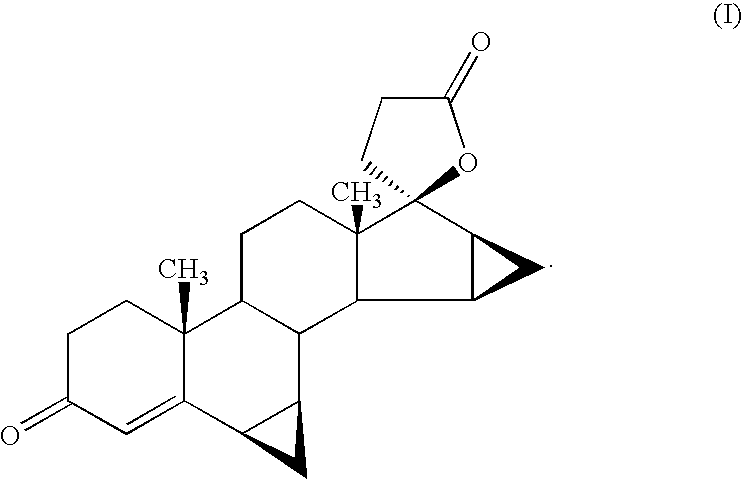
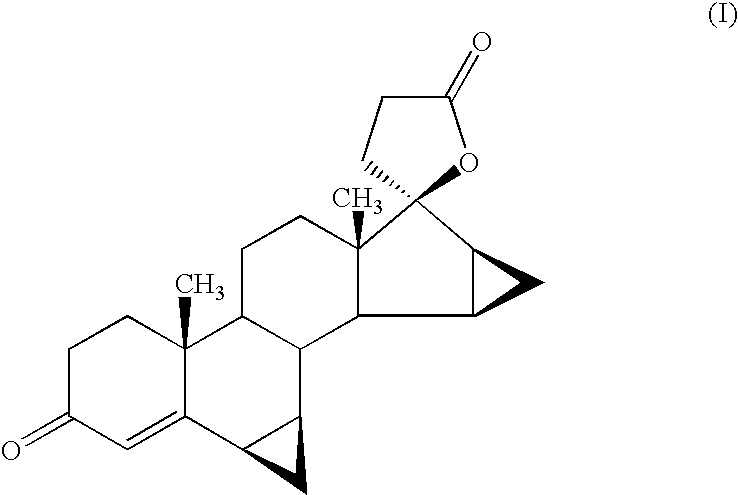
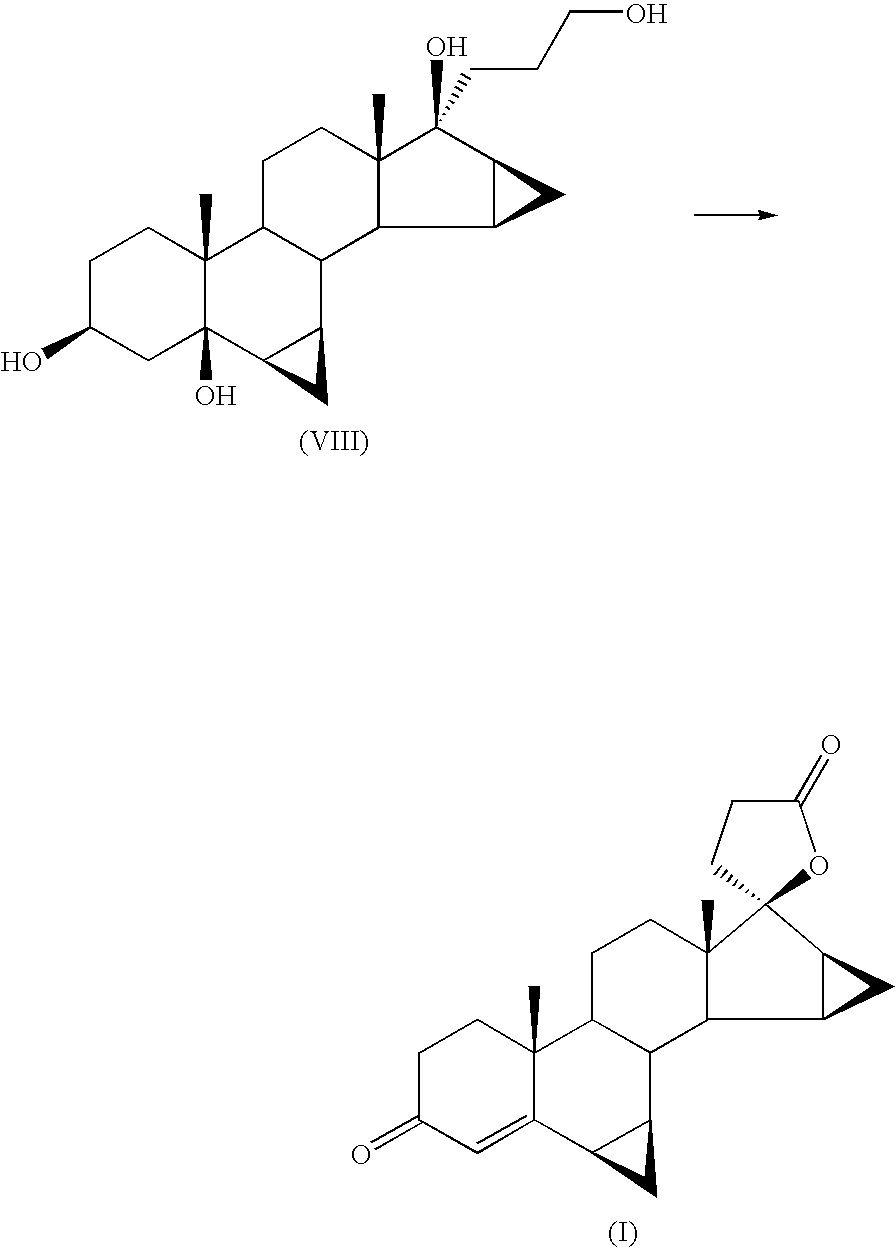
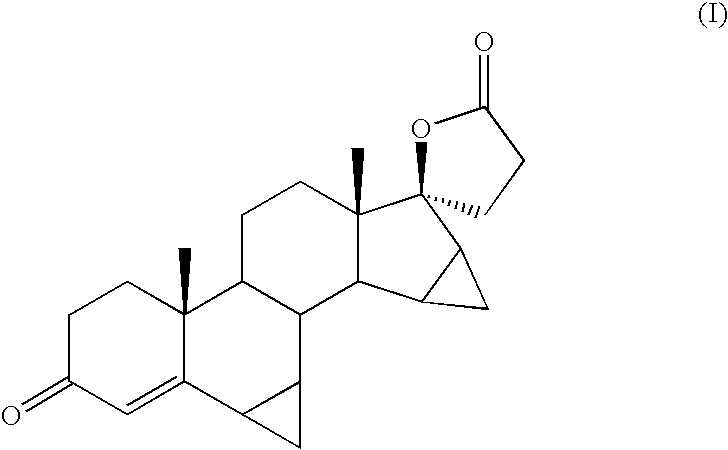
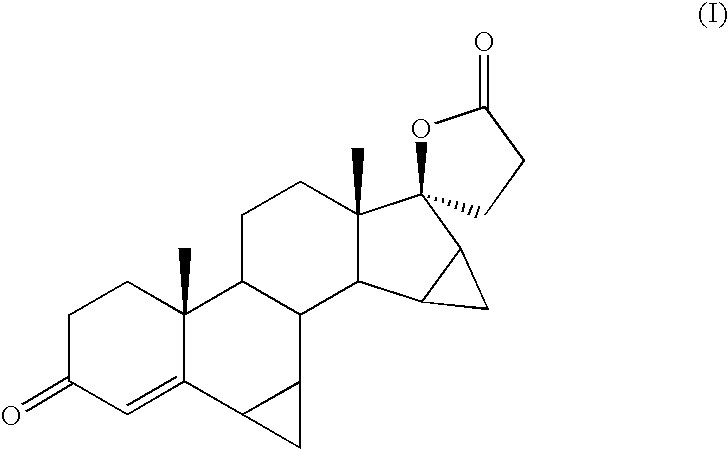
Process for the production of 3-oxo-pregn-4-ene-21,17-carbolactones by the metal-free oxidation of 17-(3-hydroxypropyl)-3,17-dihydroxyandrostanes
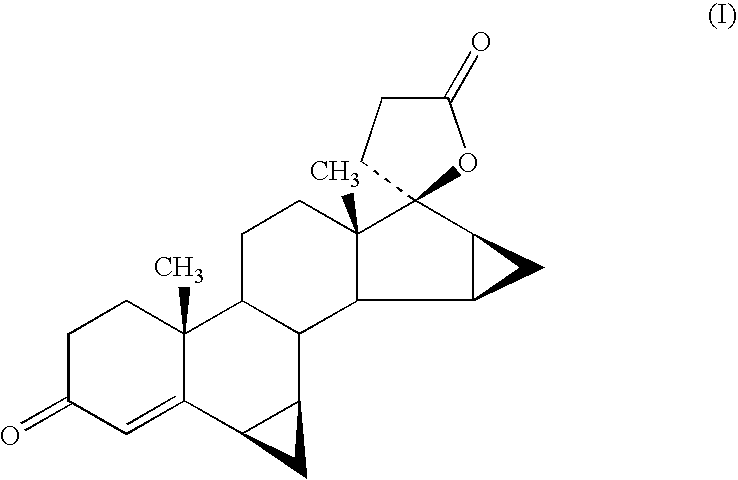
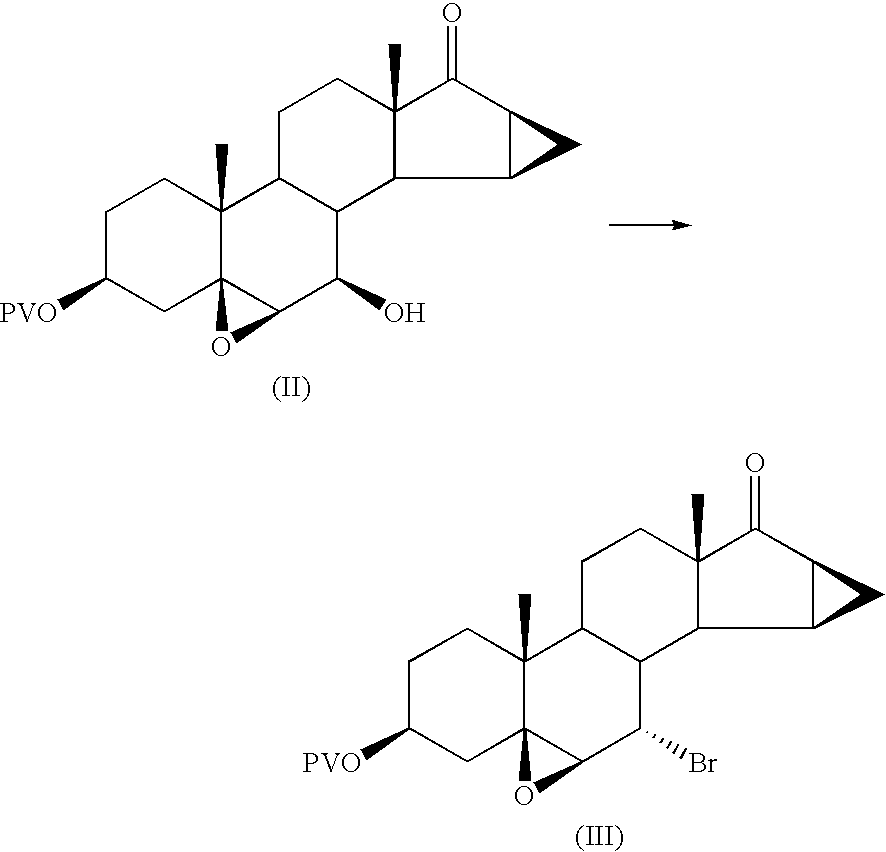
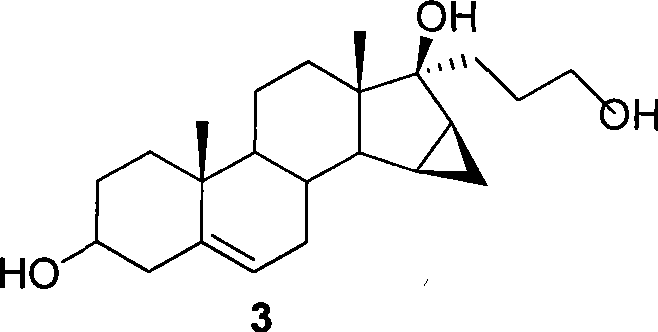
This invention relates to processes for the production of 3-oxo-pregnane-21,17-carbolactones of formula (II) as well as 3-oxo-pregn-4-ene-21,17-carbolactones of formula (III) by the metal-free oxidation of 17-(3-hydroxypropyl)-3,17-dihydroxyandrostanes of formula (I). In addition, the invention relates to the dichloromethane hemisolvate of 6ss ,7ss; 15ss,16ss-dimethylene-3-oxo-17a-pregnan-5ss-ol-21,17-carbolactone (IV) as such as well as to a process for the production of drospirenone.
Owner:BAYER PHARMA AG
Process For The Preparation Of Drospirenone
A process is described for the preparation of drospirenone, a synthetic steroid with progestogenic, antimineralocorticoid and antiandrogenic activity, useful for preparing pharmaceutical compositions with contraceptive action; comprising the oxidation of 17α-(3-hydroxypropyl)-6β,7β,15β,16β-dimethylene-5β-androstane-3β,5,17β-triol.
Owner:IND CHEM SRL
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
InactiveUS20190269620A1Inhibit ovulationBiocideOrganic active ingredientsDrospirenoneIUD with progestogen
The present invention relates to pharmaceutical compositions and kits comprising pharmaceutical compositions, and methods for administering pharmaceutical compositions comprising active contraceptive drugs in a patient. Specifically, the pharmaceutical compositions may comprise progestogen-only contraceptives (“POC”), such as Drospirenone.
Owner:LAB LEON FARMA
Cardiovascular protection using anti-aldosteronic progestins
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Pharmaceutical composition containing a tetrahydrofolic acid
The present invention relates to solid pharmaceutical compositions, in particular to oral contraceptives, comprising a progestogen, such as drospirenone; an estrogen, such as ethinylestradiol; a tetrahydrofolic acid or a pharmaceutically acceptable salt thereof, such as calcium 5-methyl-(6S)-tetrahydrofolate; and at least one pharmaceutical acceptable excipient or carrier. The compositions of the invention provide good stability of the tetrahydrofolic acid upon storage while still ensuring a fast and reliable release of the estrogen and the progestogen present in the composition.
Owner:BAYER IP GMBH
Process for preparing drospirenone and intermediate thereof
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
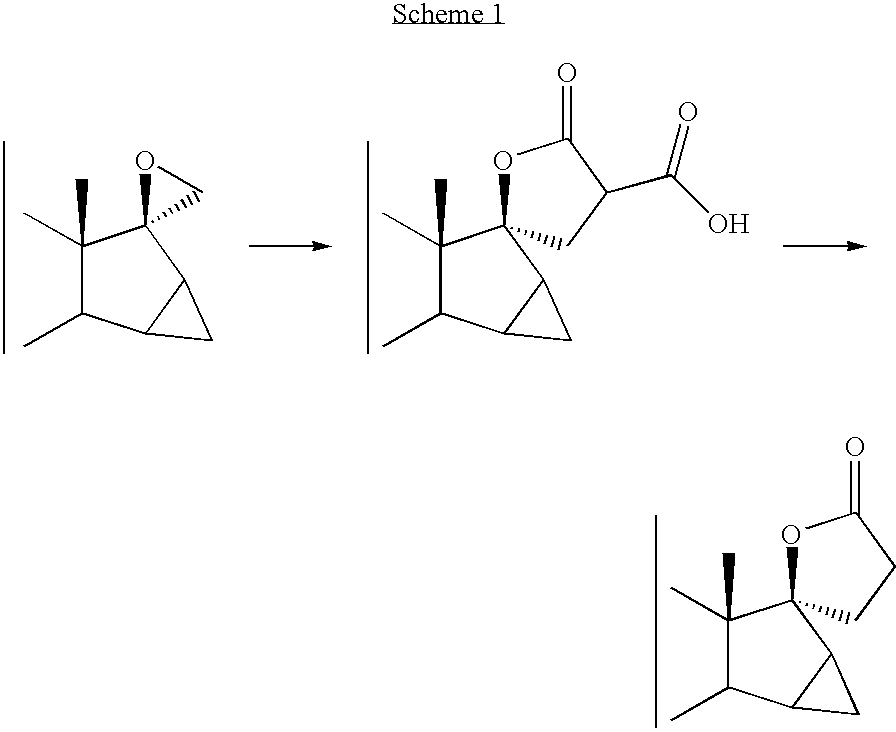
Epoxidation of 17-oxo-15,16-Methylene Steroids with Sulfoxonium Ylides
A process for the epoxidation of 17-oxo-15,16-methylene steroids, in particular of drospirenone precursors, comprising the use of sulfoxonium ylides, in particular of dimethylsulfoxonium methyl ylide. The process allows to prepare in good yields 17-spiro epoxides, which can be easily transformed into 17-spironolacto-steroids.
Owner:ANTIBIOTICOS SPA
Cyclodextrin-drospirenone inclusion complexes
InactiveUS6958326B2Improve stabilityShelf-life of an estrogen-containing product is improvedBiocideSugar derivativesDrospirenoneEthinylestradiol
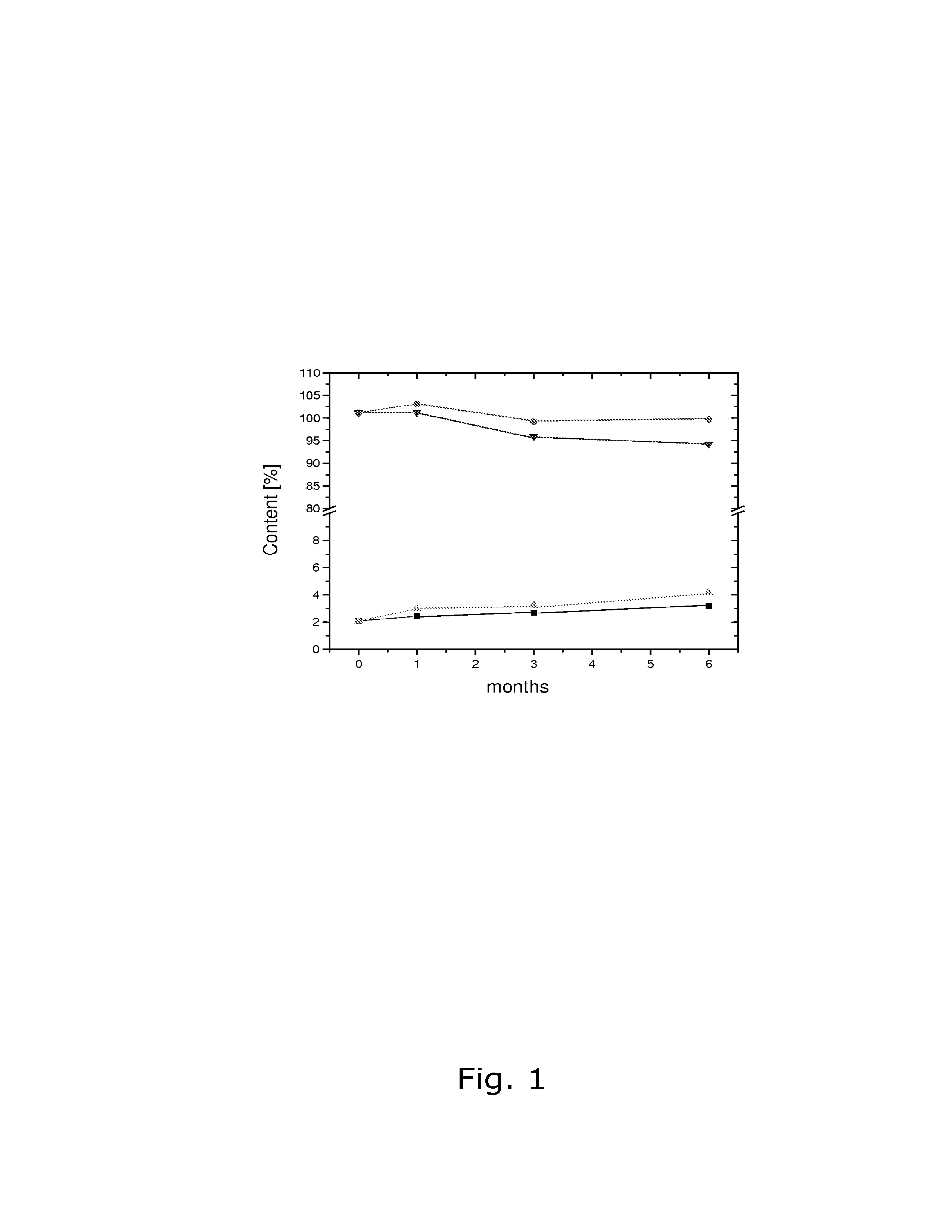
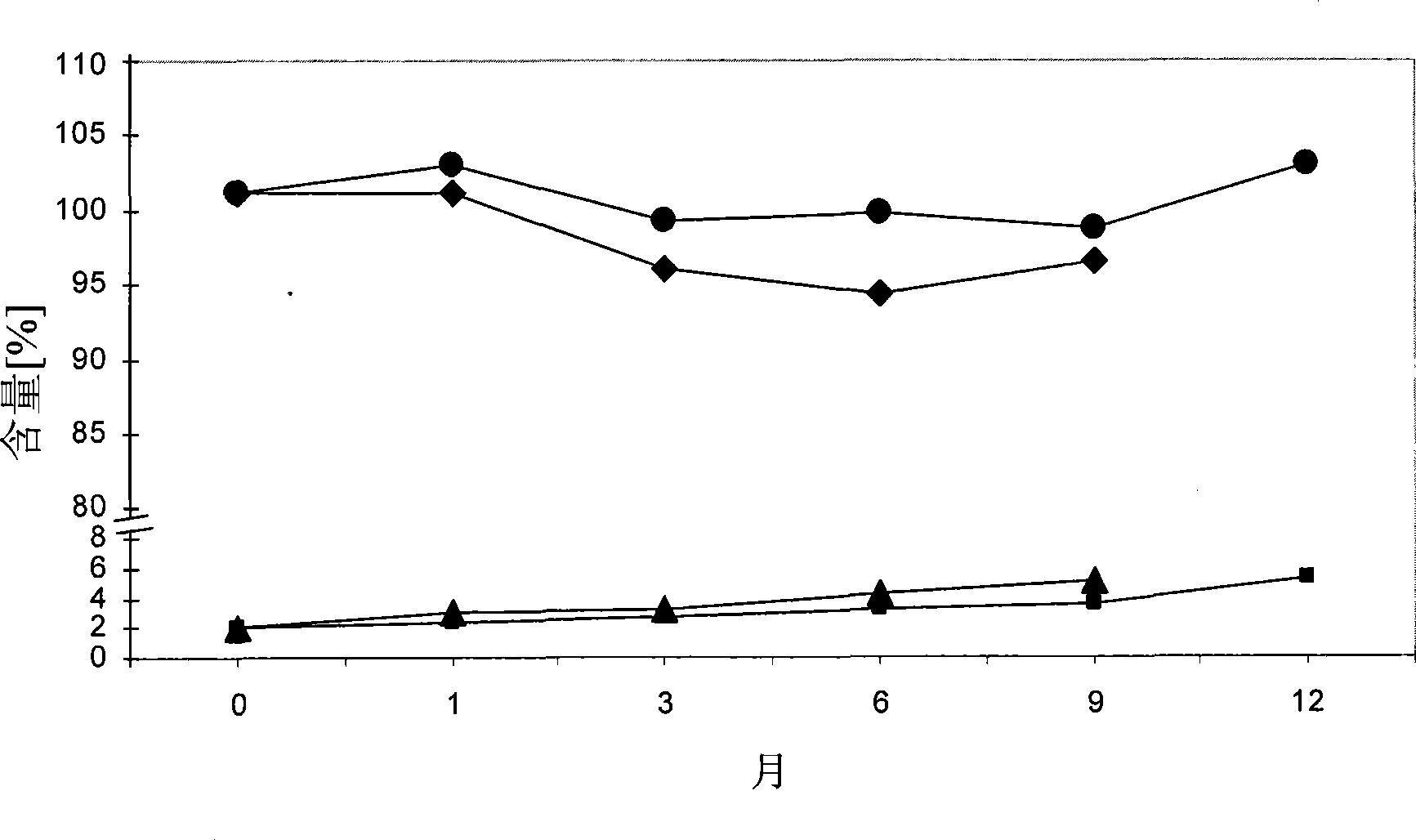
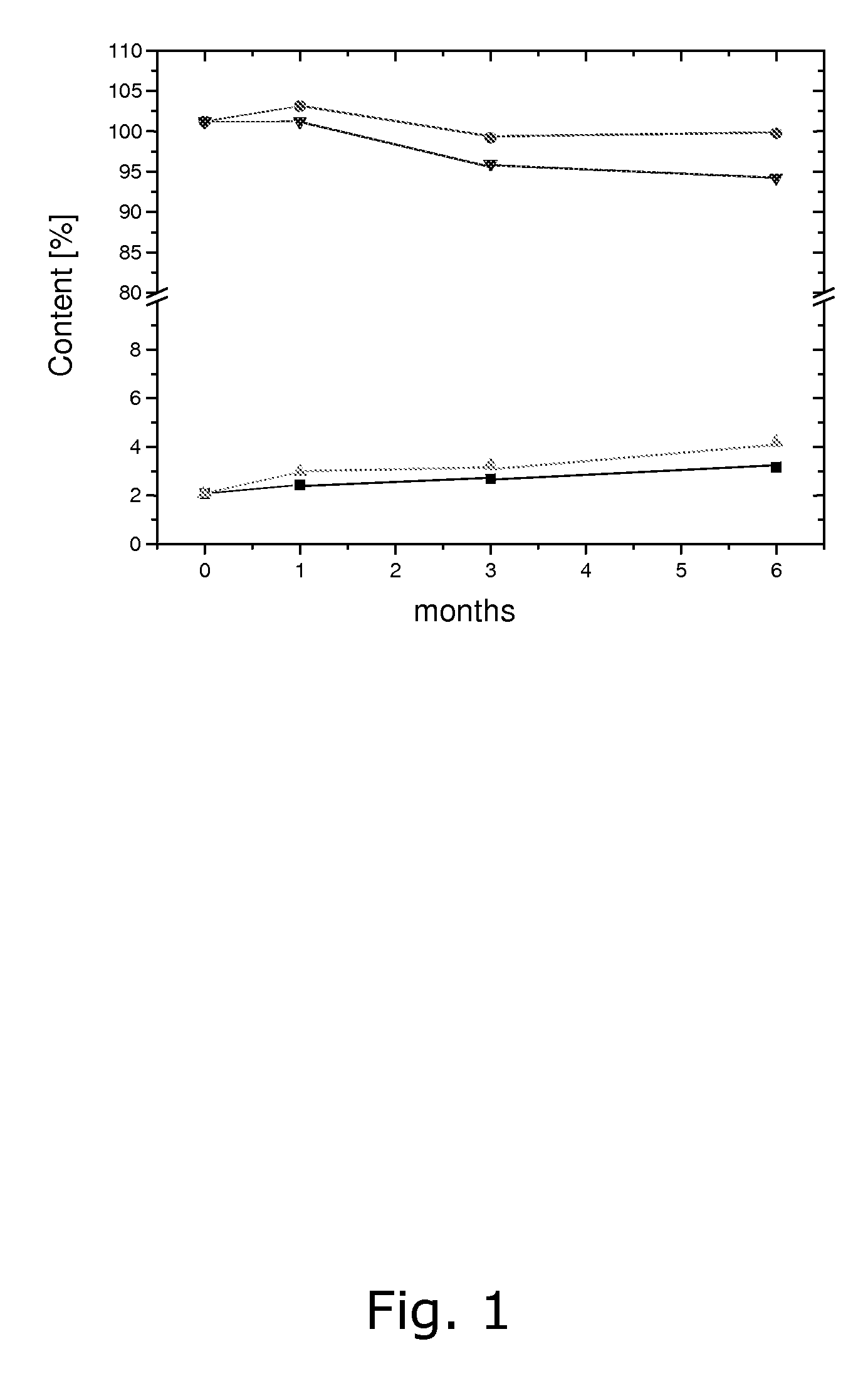
Pharmaceutical compositions comprising low doses of sensitive complexes between an estrogen and a cyclodextrin are provided with improved stability. In specific embodiments the composition comprises a complex between ethinyl estradiol and β-cyclodextrin in a granulate preparation and in yet another embodiment the composition comprises a limited amount of polyvinylpyrrolidone since this excipient was found to degrade ethinyl estradiol. Furthermore, a method for improving the stability of an estrogen in a composition and for manufacturing such a stable composition is provided. Essentially, the granulate preparation are manufactured under careful control of the relative humidity.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Combination of drospirenone and an estrogen sulphamate for HRT
InactiveUS6869941B2Poor memoryLoss of confidenceOrganic active ingredientsBiocideDrospirenoneEstriol
A pharmaceutical dosage unit comprising drospirenone and an estrogen sulphamate, such as an estradiol sulphamate or an estriol sulphamate for use in hormone replacement therapy is disclosed. This, combination therapy may comprise continuous or discontinuous administration of drospirenone and / or the estrogen sulphamate, such as weekly administration of both agents or weekly administration of the estrogen sulphamate and daily administration of the drospirenone.
Owner:BAYER SCHERING PHARMA AG
Method for synthesizing drospirenone
The invention relates to a method for synthesizing drospirenone and belongs to the field of pharmaceutical chemicals, which comprises: reacting a 3beta,5beta-dyhydroxy-6beta,7beta,15beta,16beta-imethylene-17alpha-(3'-hydroxypropyl)-androstene-17ol compound serving as a raw material in dichloromethane in the presence of dichlorodimethylhydantoin, potassium bicarbonate and crown ether, which serve as catalysts, to obtain an 3-oxo-5beta-hydroxy-6beta, 7beta,15beta,16beta-dimethylene-17alpha(spiro)butyrolactone intermediate; removing excessive oxidant by using a small amount of sodium sulfite, filtering the solution, adding a certain amount of phosphorus pentoxide into solution of dichloromethane for dehydration, adding water for washing the reaction product for one time at the end ( detected by thin-layer chromatography) of the reaction and washing the reaction product for one time with saturated solution of sodium bicarbonate; drying the reaction product with anhydrous sodium sulfate, filtering the reaction product, distilling and recovering solvent and crystallizing the solid with water solution (in a volume ratio of 3:1) of methanol; and finally, recrystallizing the obtained solid with isopropylacetate to obtain a qualified drospirenone product. The synthesis yield of the method is about 67 percent. The reaction is mild and easy to operate and consumes a small amount of organic solvent.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
Pharmaceutical composition containing a tetrahydrofolic acid
The present invention relates to solid pharmaceutical compositions, in particular to oral contraceptives, comprising a progestogen, such as drospirenone; an estrogen, such as ethinylestradiol; a tetrahydrofolic acid or a pharmaceutically acceptable salt thereof, such as calcium 5-methyl-(6S)-tetrahydrofolate; and at least one pharmaceutical acceptable excipient or carrier. The compositions of the invention provide good stability of the tetrahydrofolic acid upon storage while still ensuring a fast and reliable release of the estrogen and the progestogen present in the composition.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
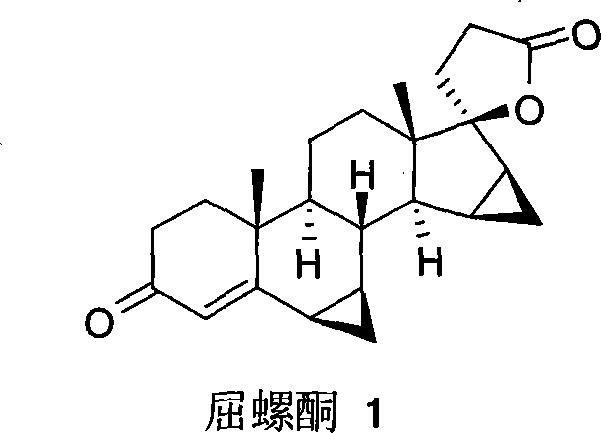
Whorl[(5 beta, 6 beta, 15 beta, 16 beta-dimethylene-androstane-14 beta-hydrogen-5, 7-diene-3-ketone)-17 alpha-2'-(1'-oxygen-cyclopentane-5'-ketone)] and synthesis process
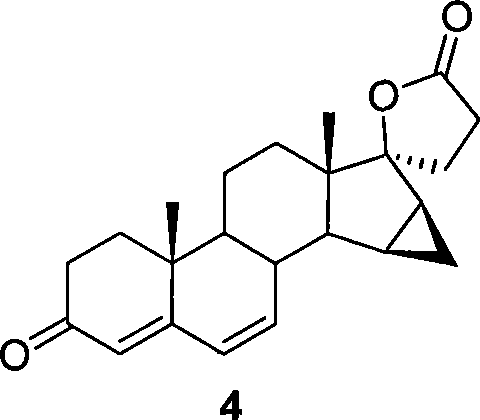
The invention relates to a spiro [(5beta, 6beta, 15beta, 16beta-dimethylene-androstane-14beta-hydrogen-5, 7-diene-3-keton)- 17alpha-2'-(1'-oxygen-cyclopentane-5'-ketone) and a synthetic method thereof. A structural formula of the compound is the right formula; the method begins from cheap and available dehydroepiandrosterone 1 and can fully obtain a product overturned by 14 H of a target product of drospirenone through an eight-step reaction, namely a C14beta-H isomer of the drospirenone; and the unique difference between the isomer and the drospirenone in the structure lies in that 14-H of the isomer is a beta configuration, while 14-H of the drospirenone is an alpha configuration. As the C14beta-H isomer of the drospirenone and the synthetic method are not reported in literature, the C14beta-H isomer of the drospirenone can be a new drug which has the same medicative effect as the drospirenone, has high development value and provides a new strategy for the synthesis of the drospirenone.
Owner:SHANGHAI UNIV
Preparation method of drospirenone
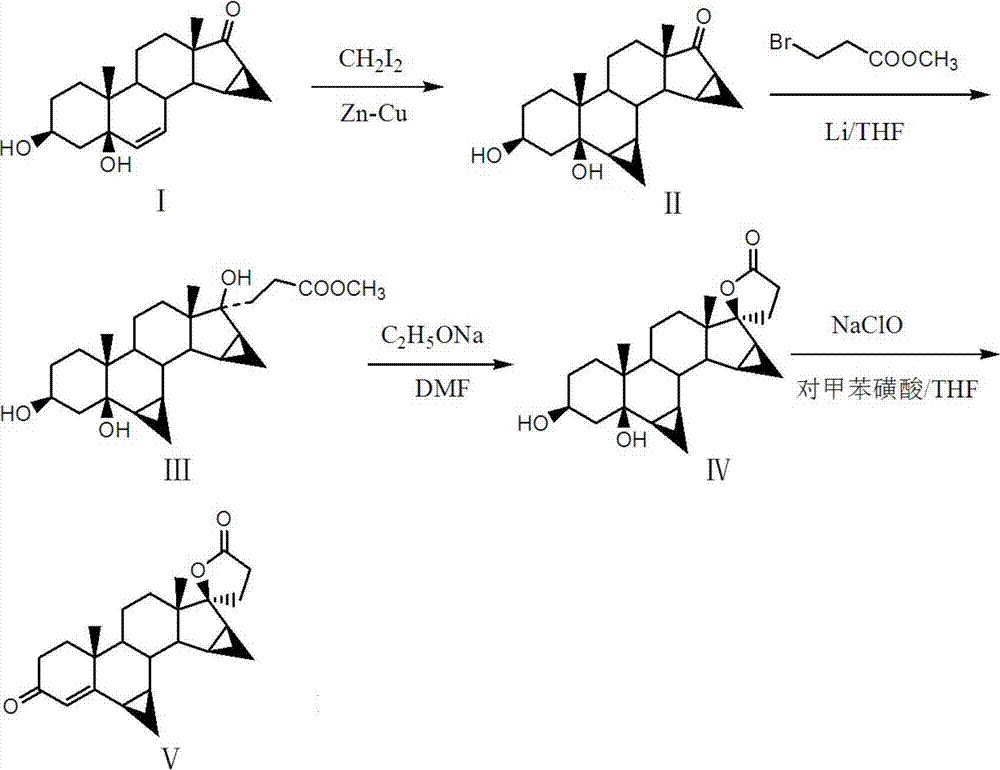
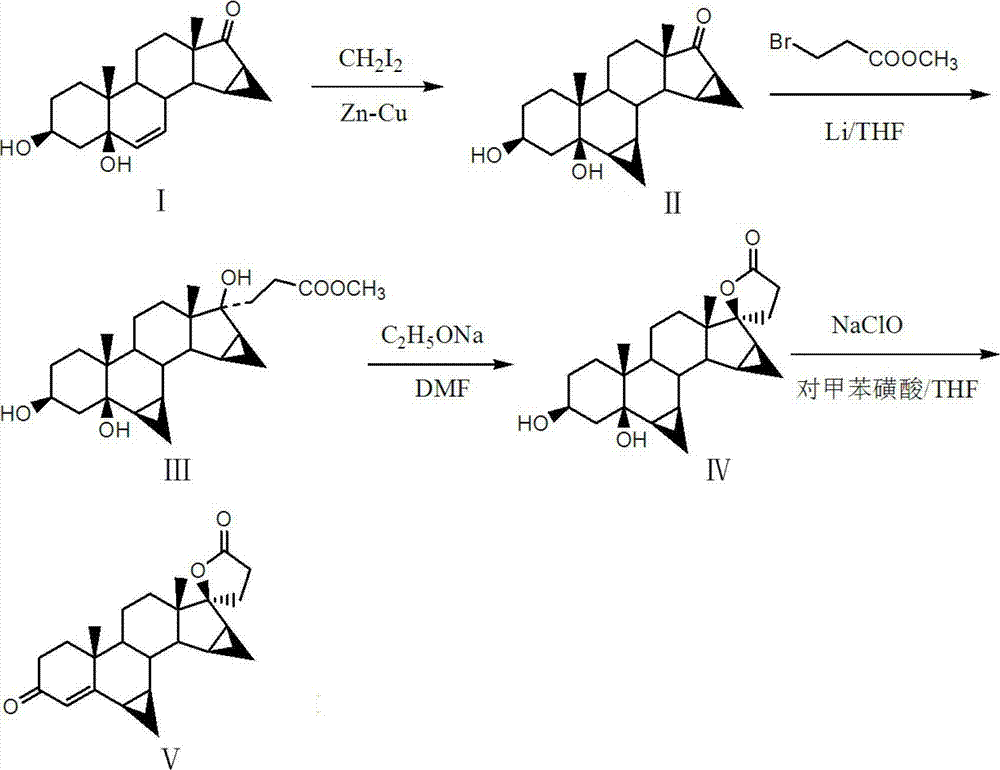
The invention discloses a preparation method of drospirenone, which aims at solving the problems of high production cost, low yield, and poor product quality in the existing preparation method of the drospirenone. The preparation method comprises the steps of: taking 3beta, 5-dyhydroxy-15beta, 16beta-methylene-5beta-androstane-6 alkene-17-ketone as materials, firstly, reacting with diiodomethane and zinc-copper couple, introducing 6beta and 7beta cyclopropane structures, then orderly carrying out condensation reaction and esterification reaction in THF (tetrahydrofuran) solution of lithium metal and 3-methyl bromopropionate and DMF (dimethyl formamide) solution of sodium ethoxide, finally oxidizing by sodium hypochlorite, and dewatering the toluenesulfonic acid to obtain the drospirenone. According to the preparation method, the efficiency is high, the production cost is low, by-products are few, special demands on production equipment do not exist, the reaction condition is mild, the process is stable, the operation is convenient, the environment is prevented from being damaged, and the large-scale industrial production is facilitated. The product obtained by the method is stable in quality, high in yield and purity and free of purification.
Owner:HANGZHOU FST PHARMA
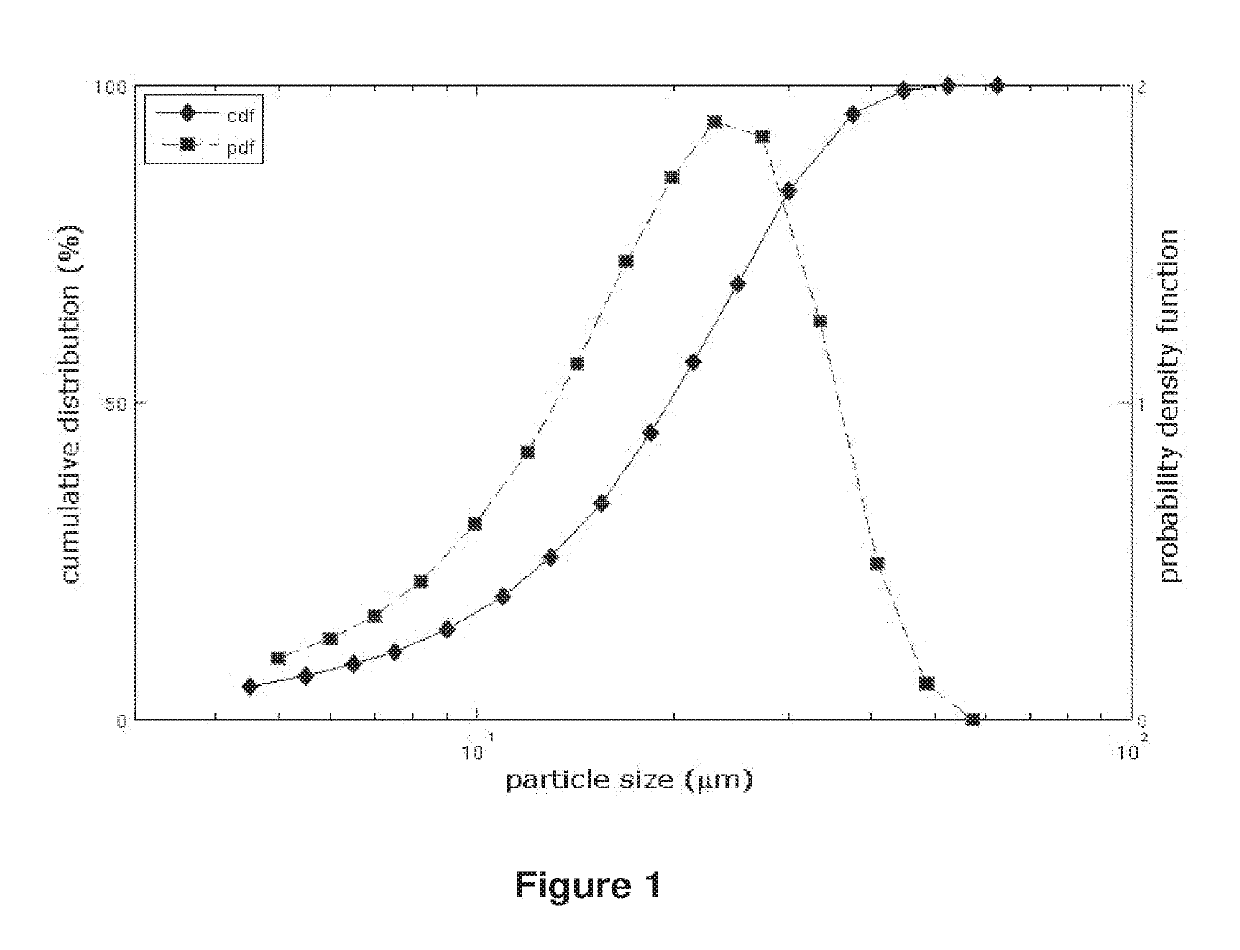
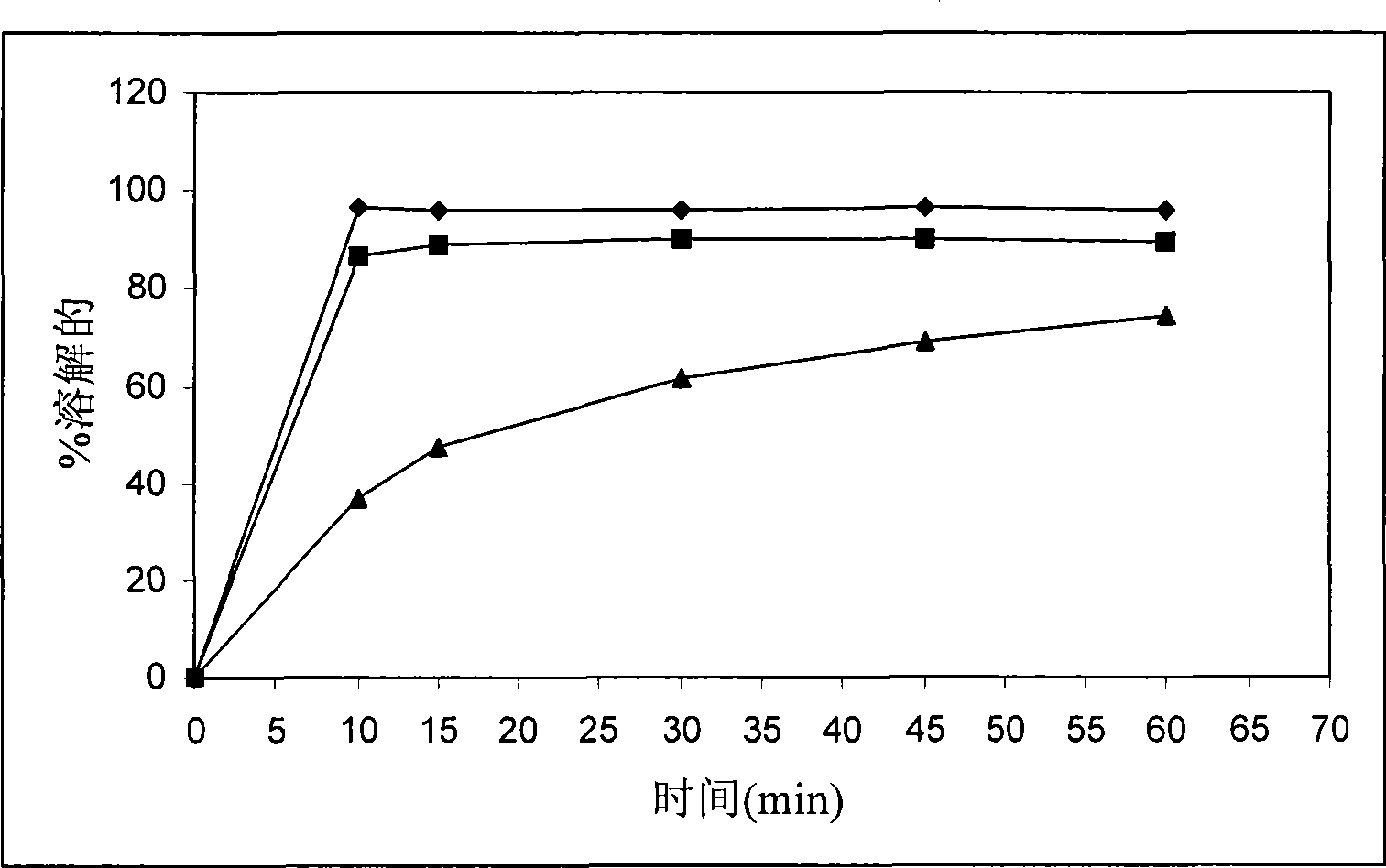
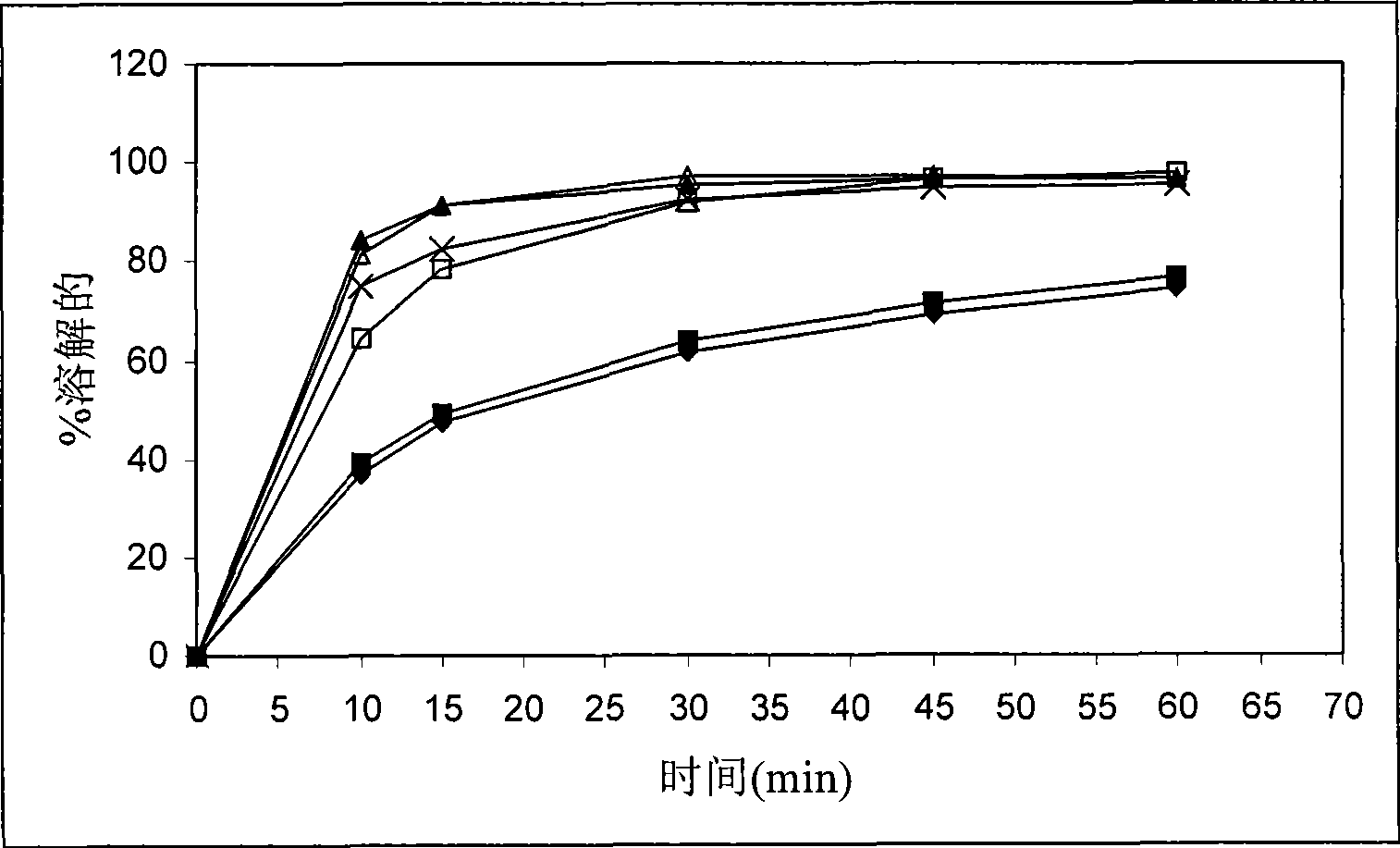
Method for modifying dissolution of drospirenone by using grinding and drospirenone solid dispersion
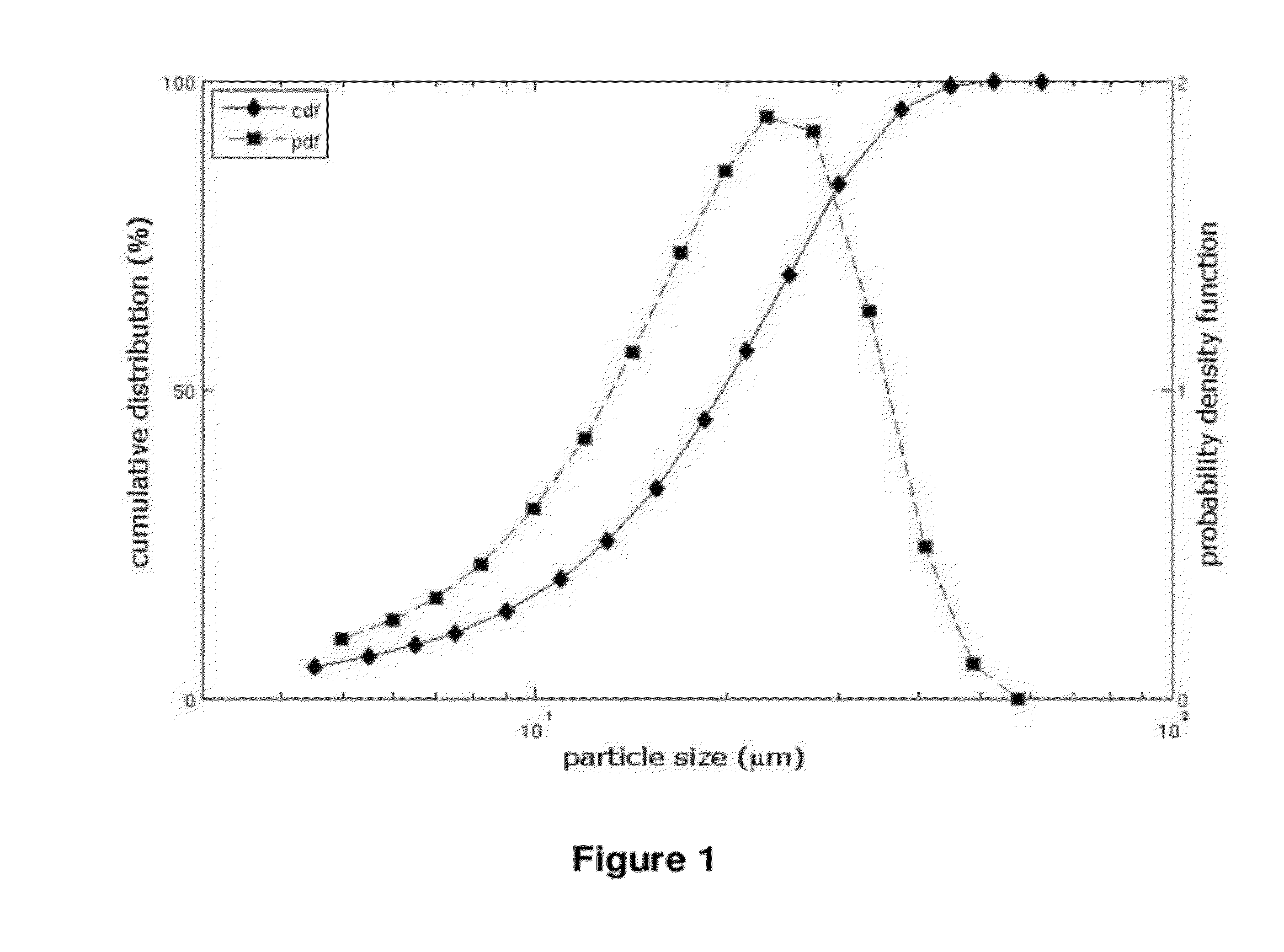
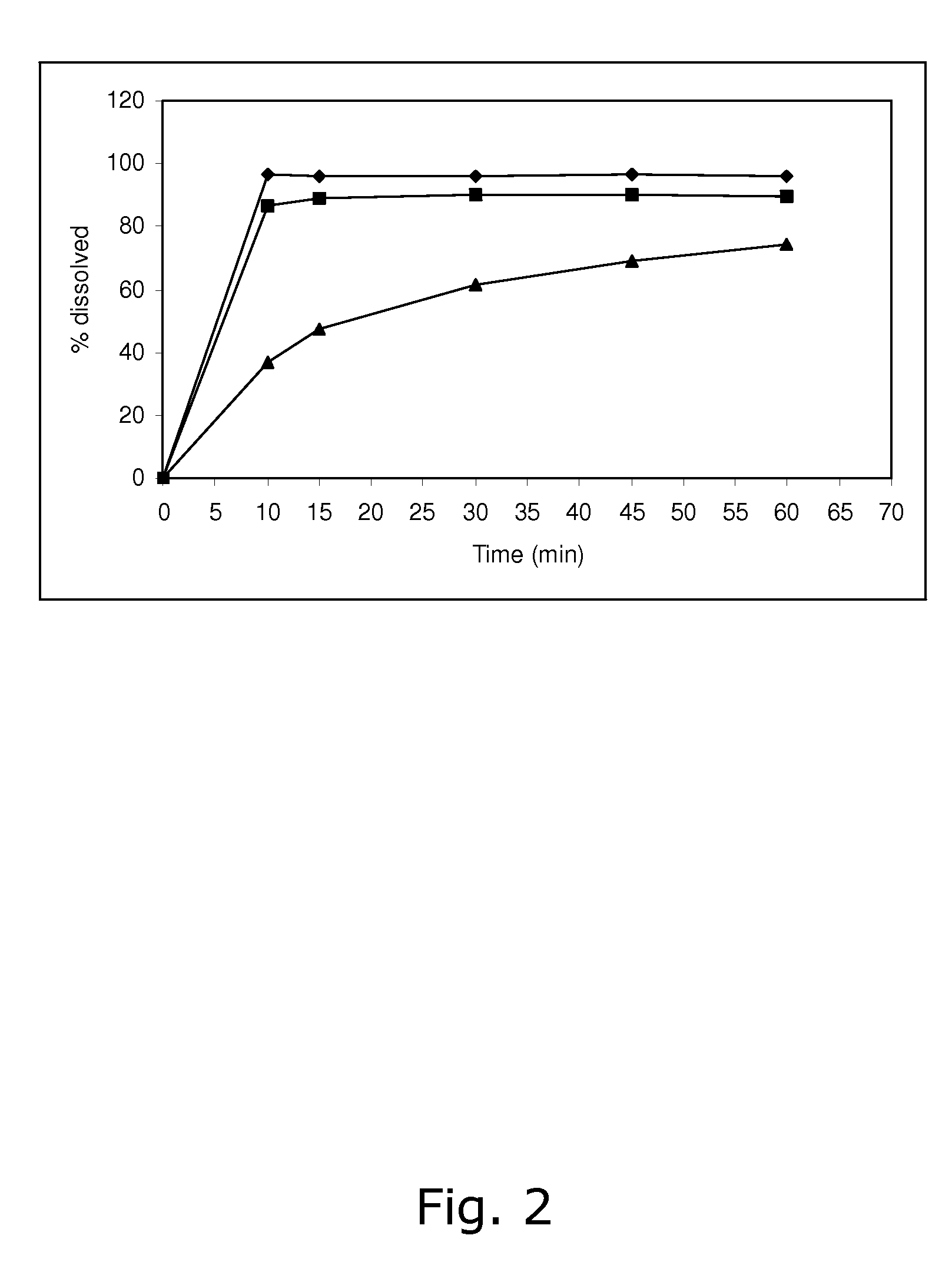
ActiveCN103040725ASmall granularityGuaranteed a high degree of dispersionOrganic active ingredientsPharmaceutical delivery mechanismOrganic solventDrospirenone
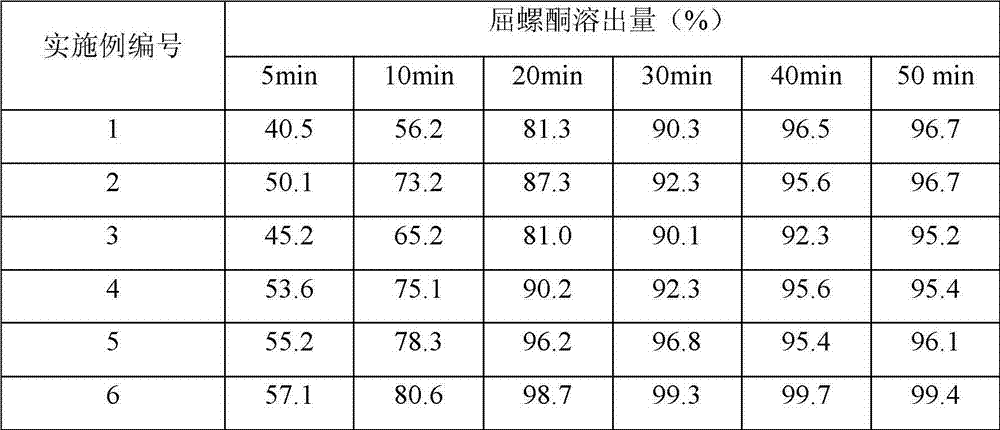
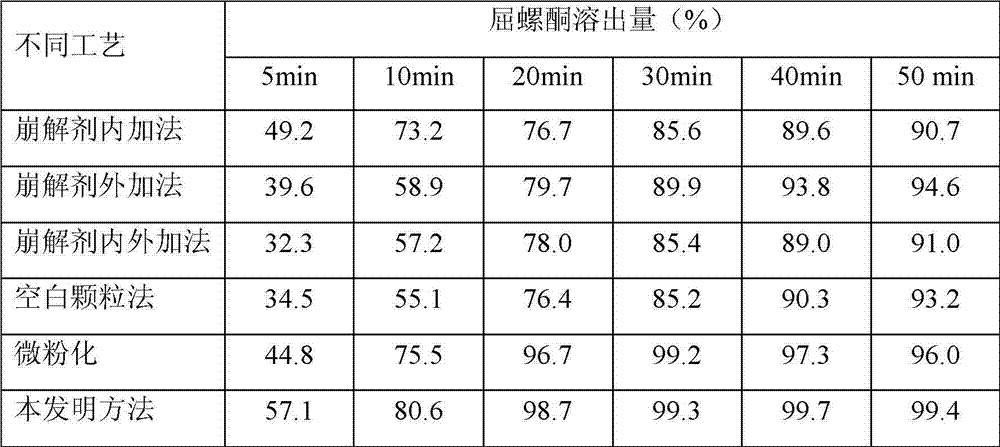
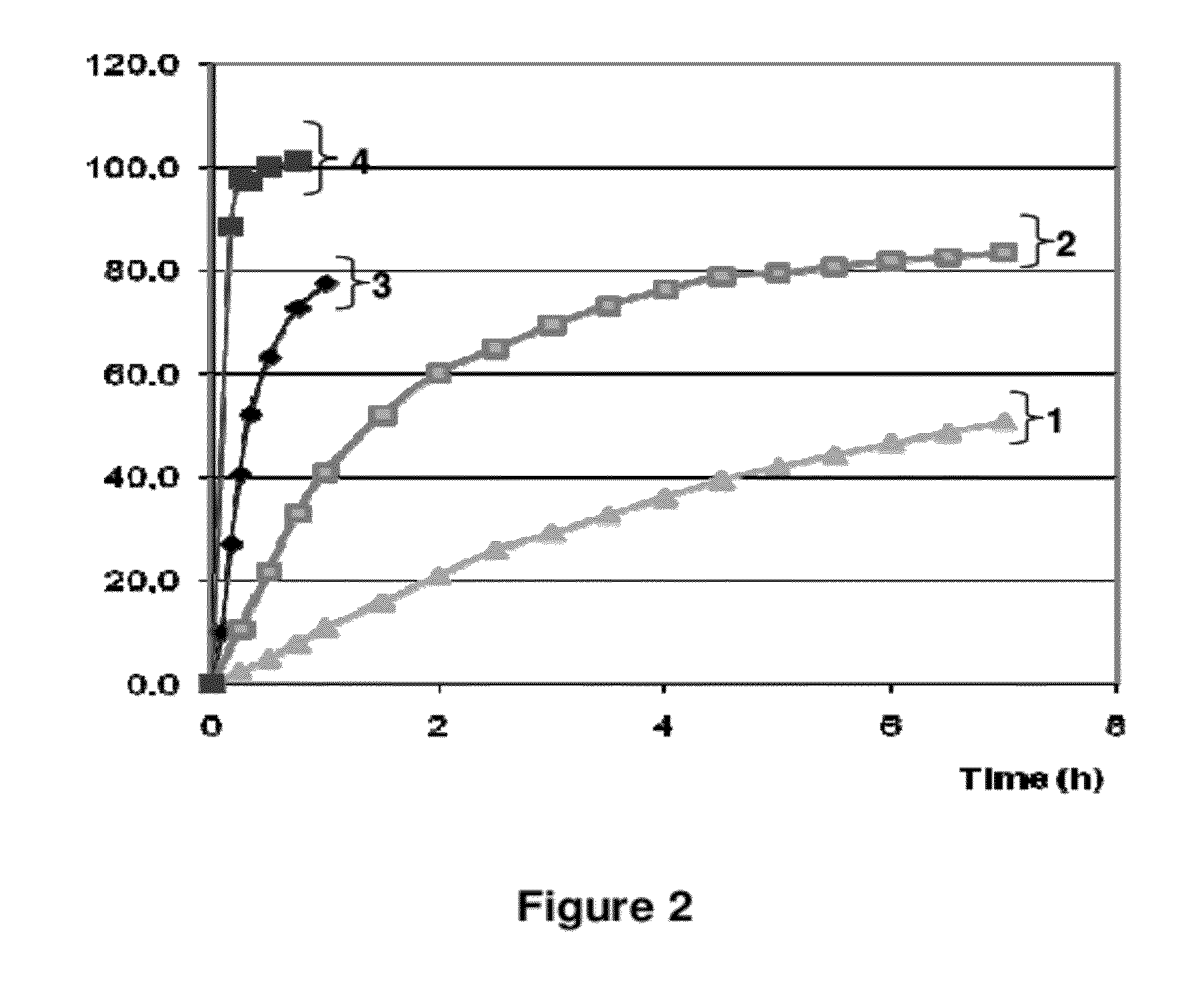
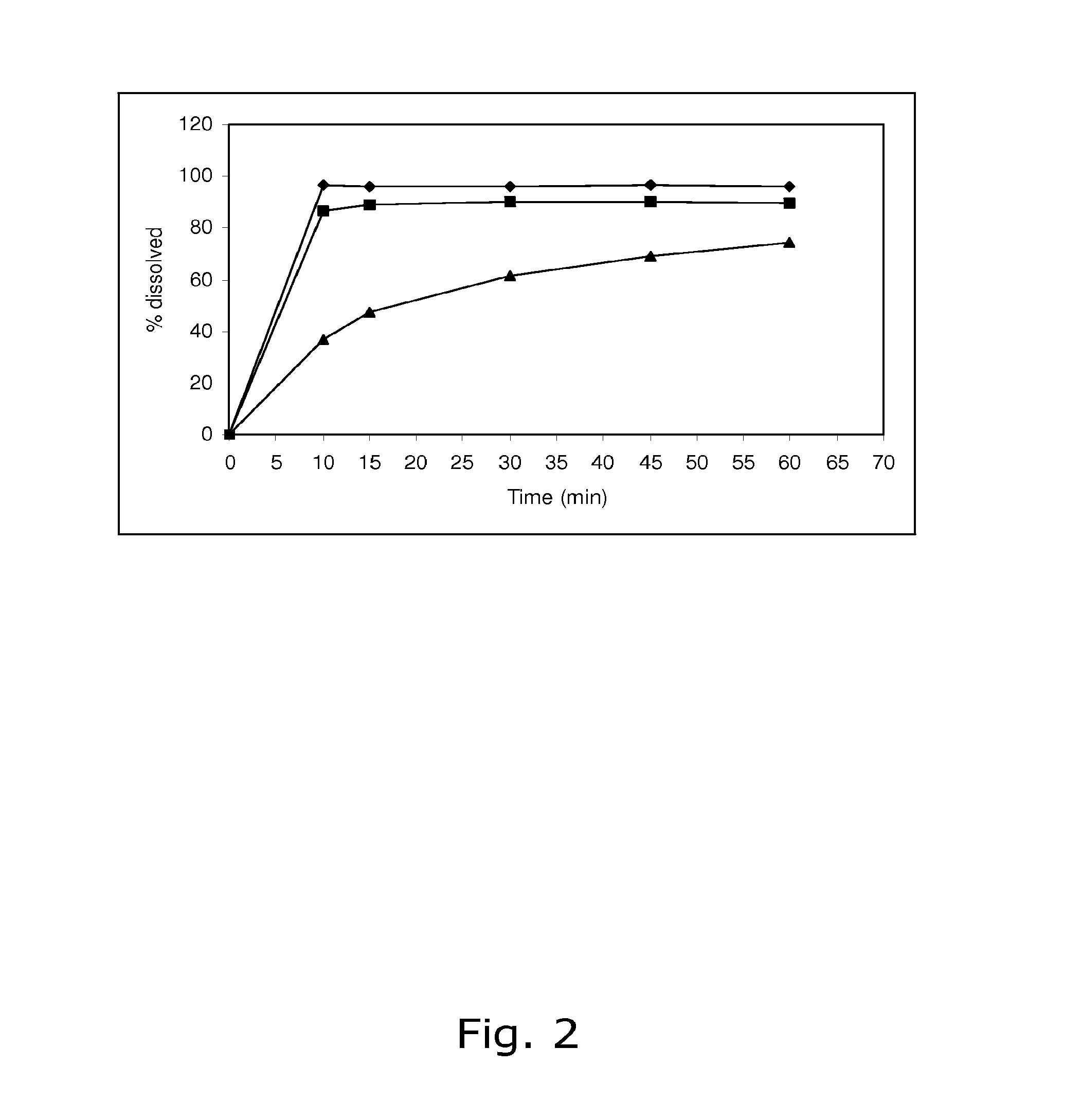
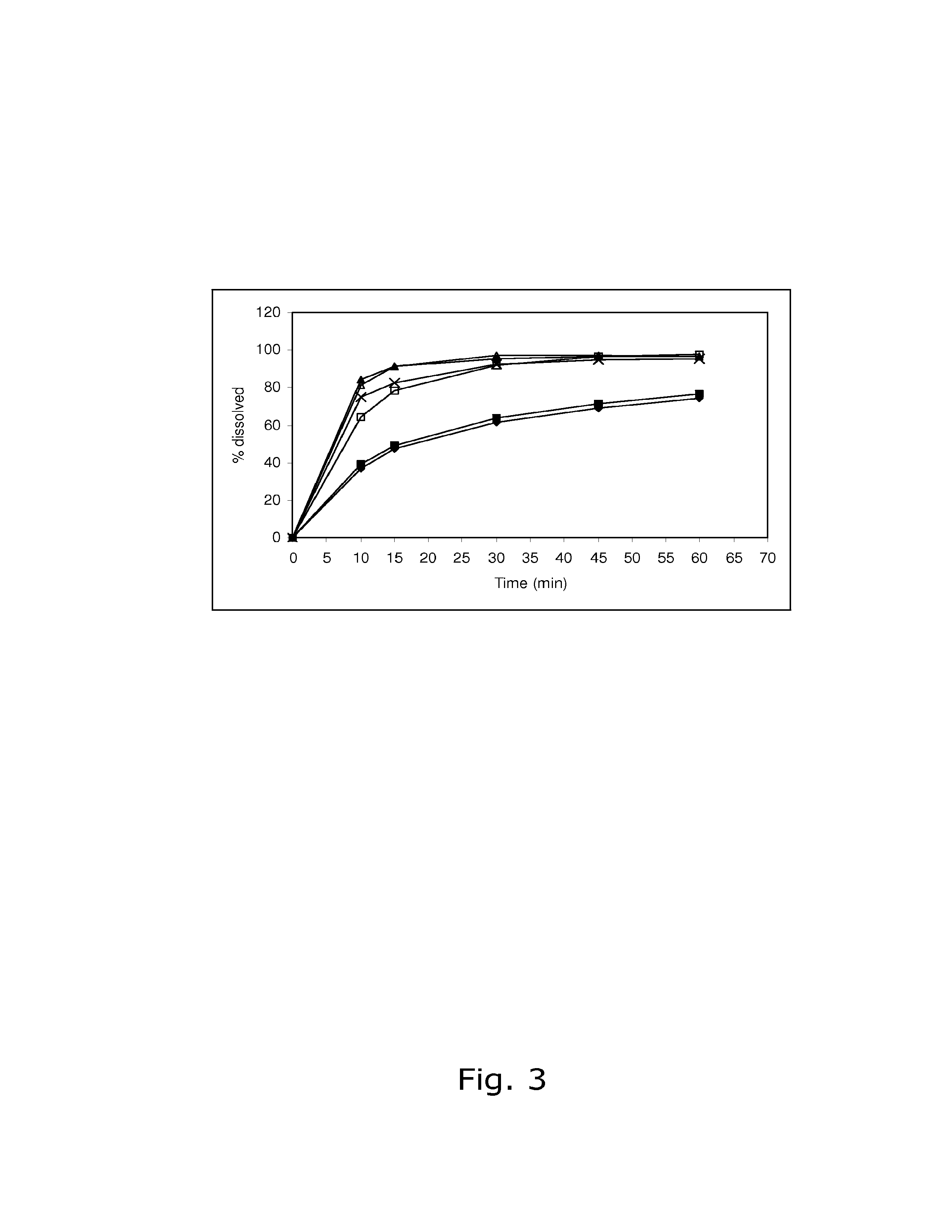
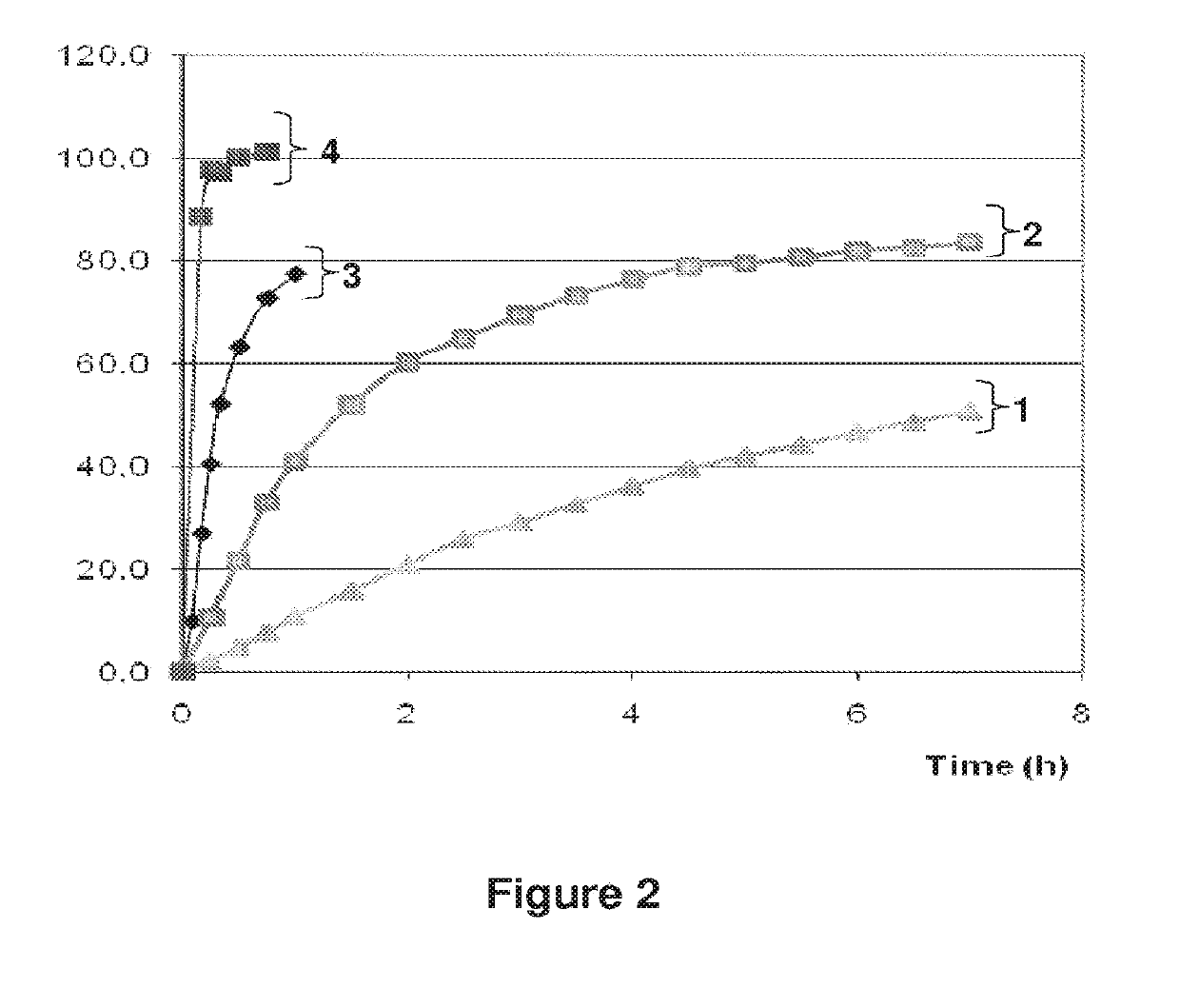
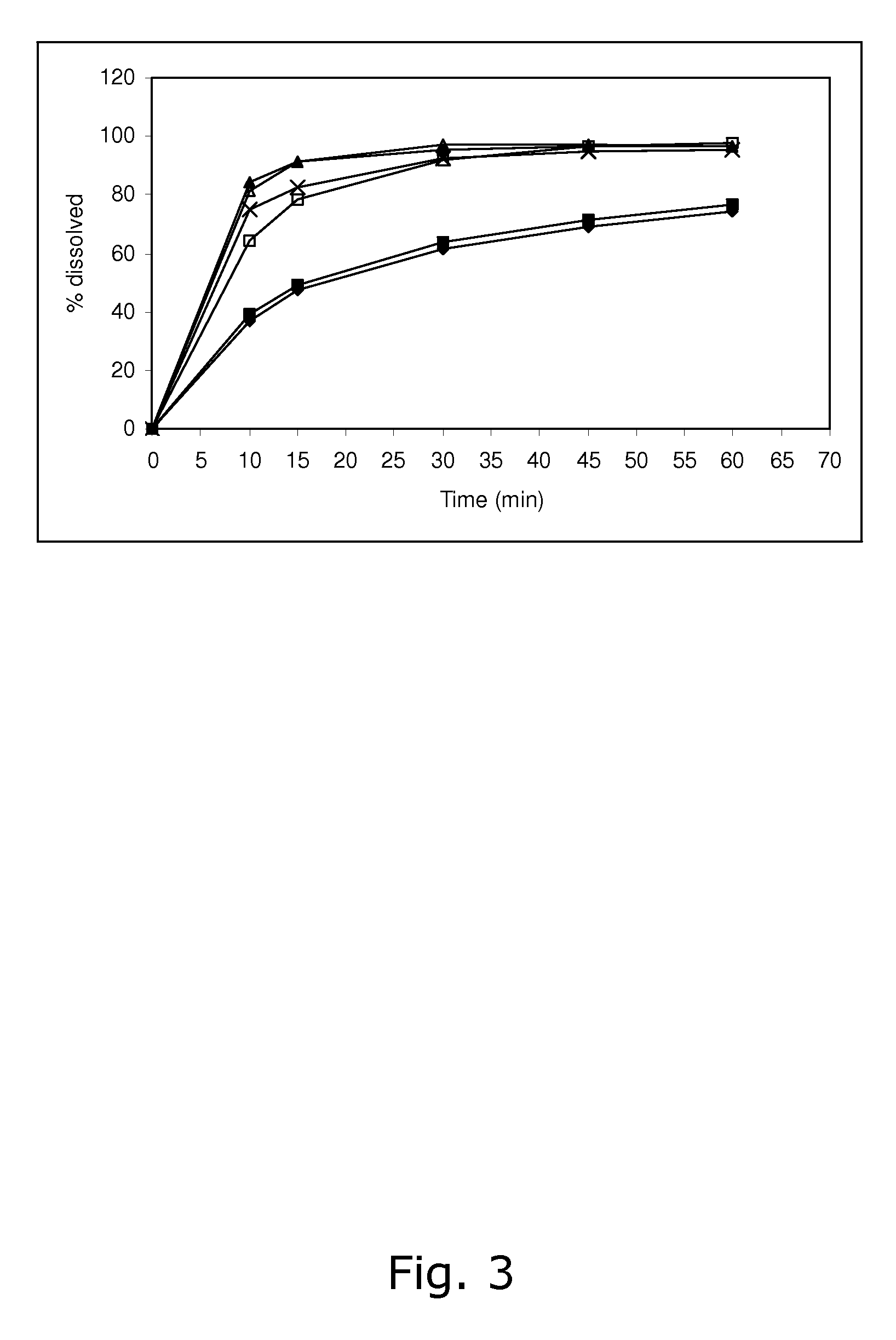
The invention belongs to the scientific field of pharmaceutic preparations, and in particular relates to a method for modifying dissolution of drospirenone by using grinding and drospirenone solid dispersion. The method comprises the following steps: (1) the drospirenone and hydrophilic non-polymer accessories or water soluble accessories are evenly mixed by the mass ratio of 1:(1-1):30; and (2) the evenly mixed materials are fully grinded or milled, and are screened. The method has the advantages of simple operation, low cost, simplicity, practicability and convenience in promotion; the preparation produced by drospirenone dispersion which is prepared by the method is quickly dissolved out; 80 percent of drospirenone can be furthest dissolved out in 10 minutes; and 98 percent of drospirenone can be furthest dissolved out in 20 minutes; the drospirenone is quickly dissolved out; the biological utilization rate is high; no organic solvent is remained; the stability is high; and the method is suitable for preparing solid preparation.
Owner:武汉九珑人福药业有限责任公司
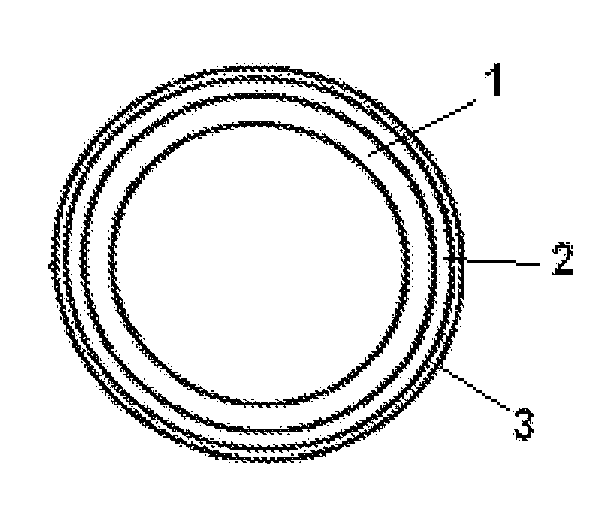
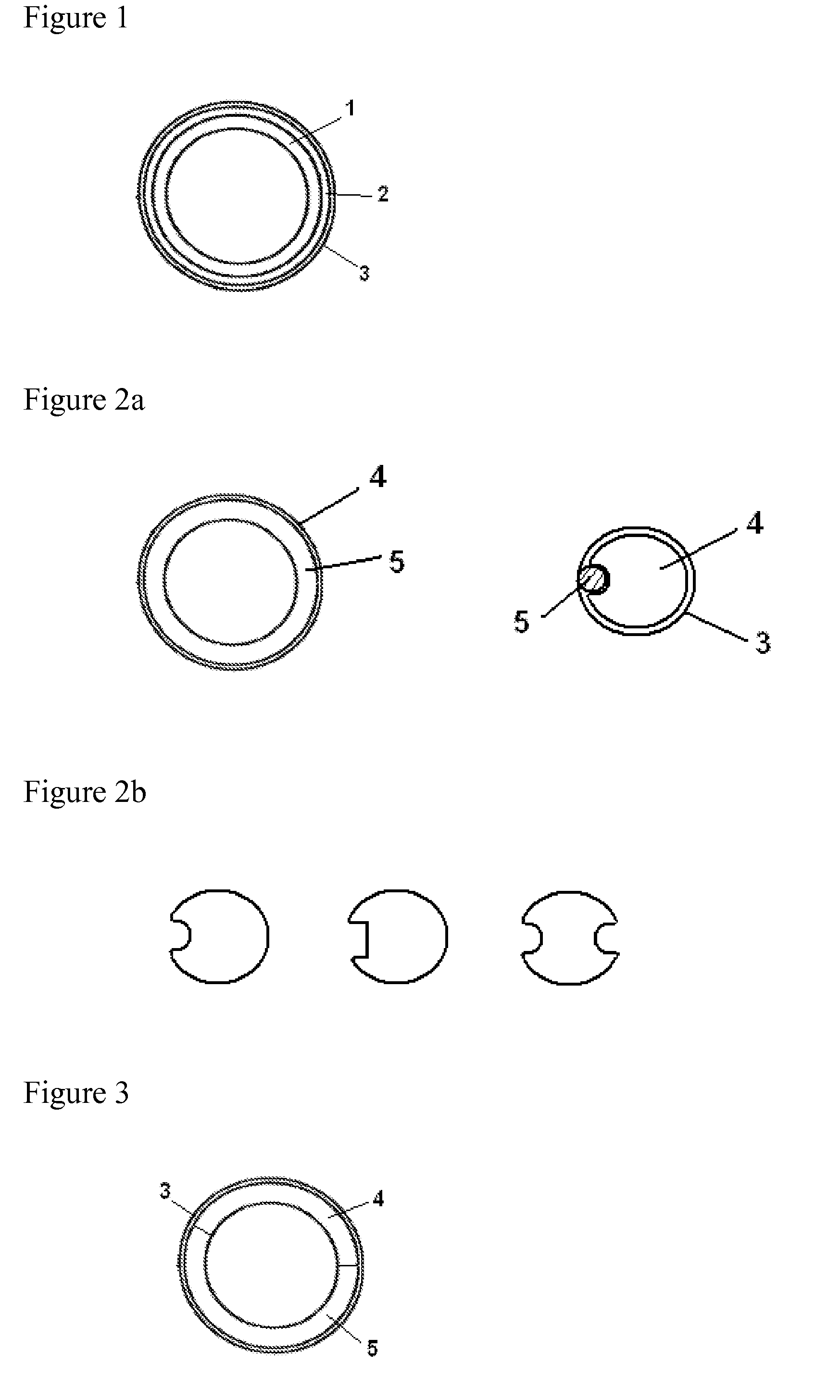
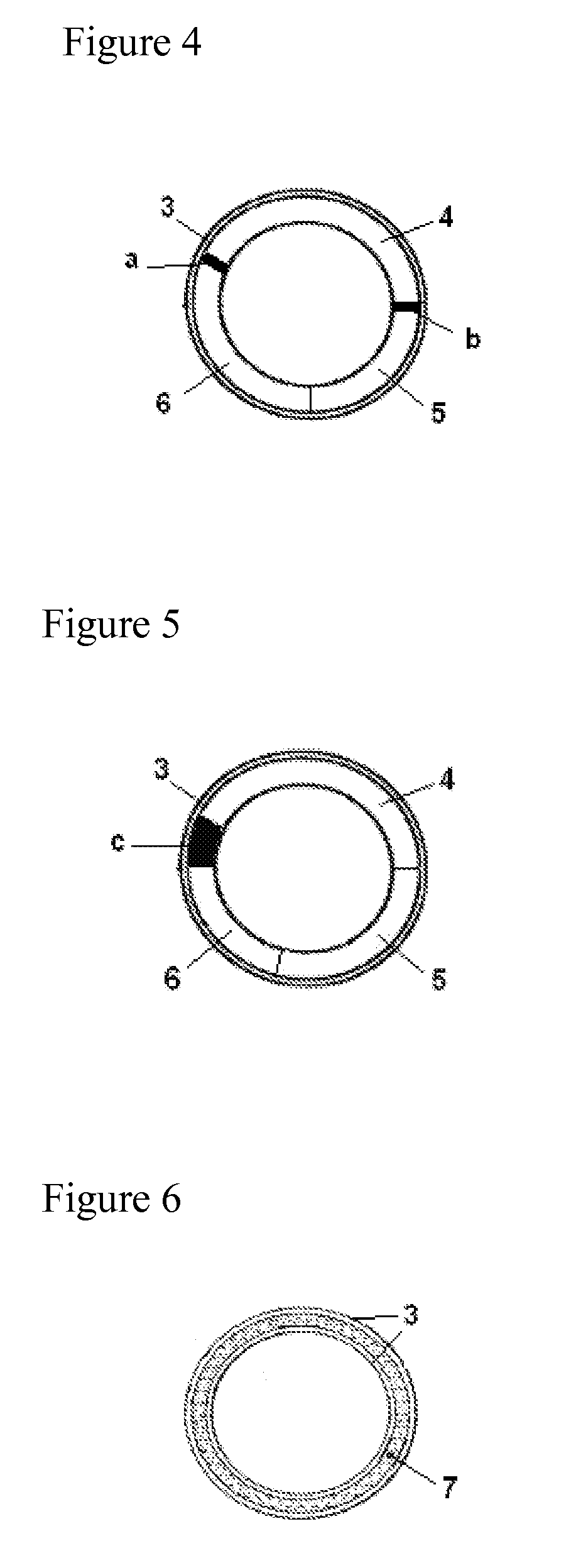
Pessulum preparation containing drospirenone or drospirenone and estrogen
InactiveCN103372015AOrganic active ingredientsPharmaceutical delivery mechanismDrospirenoneControl release
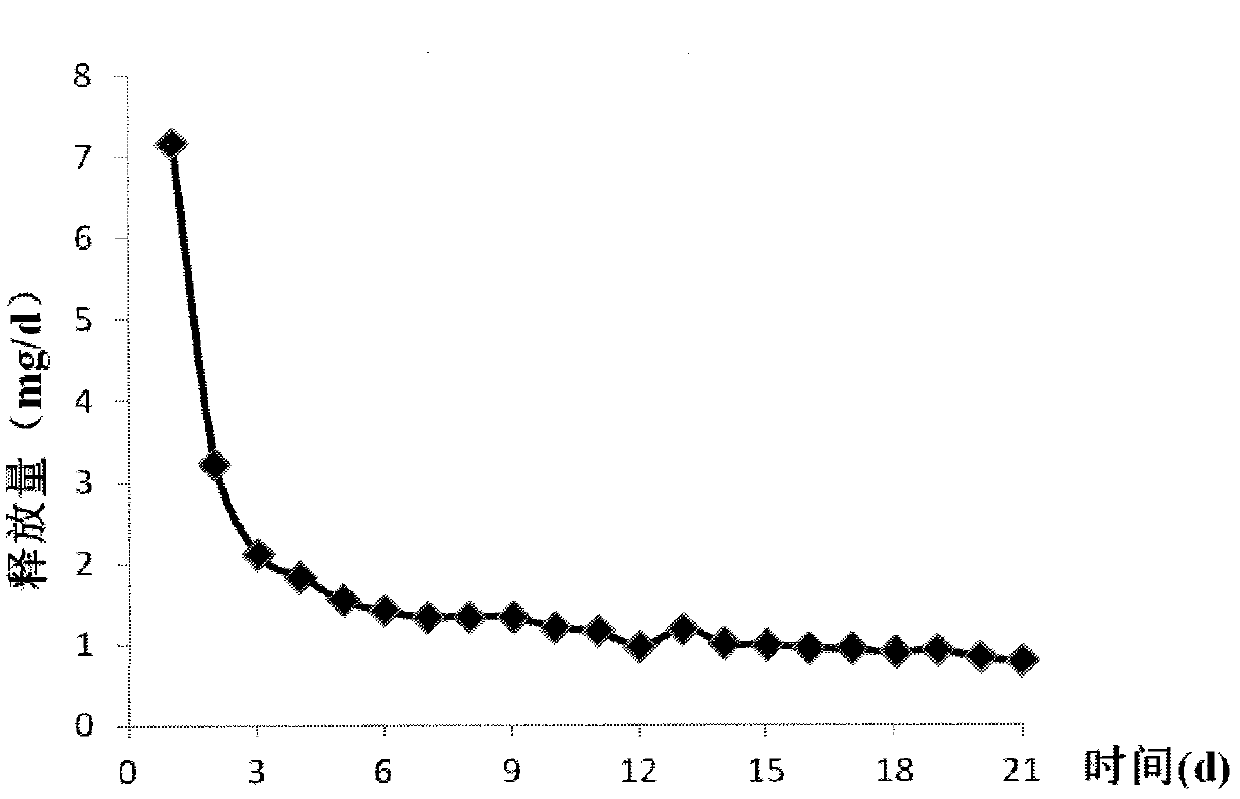
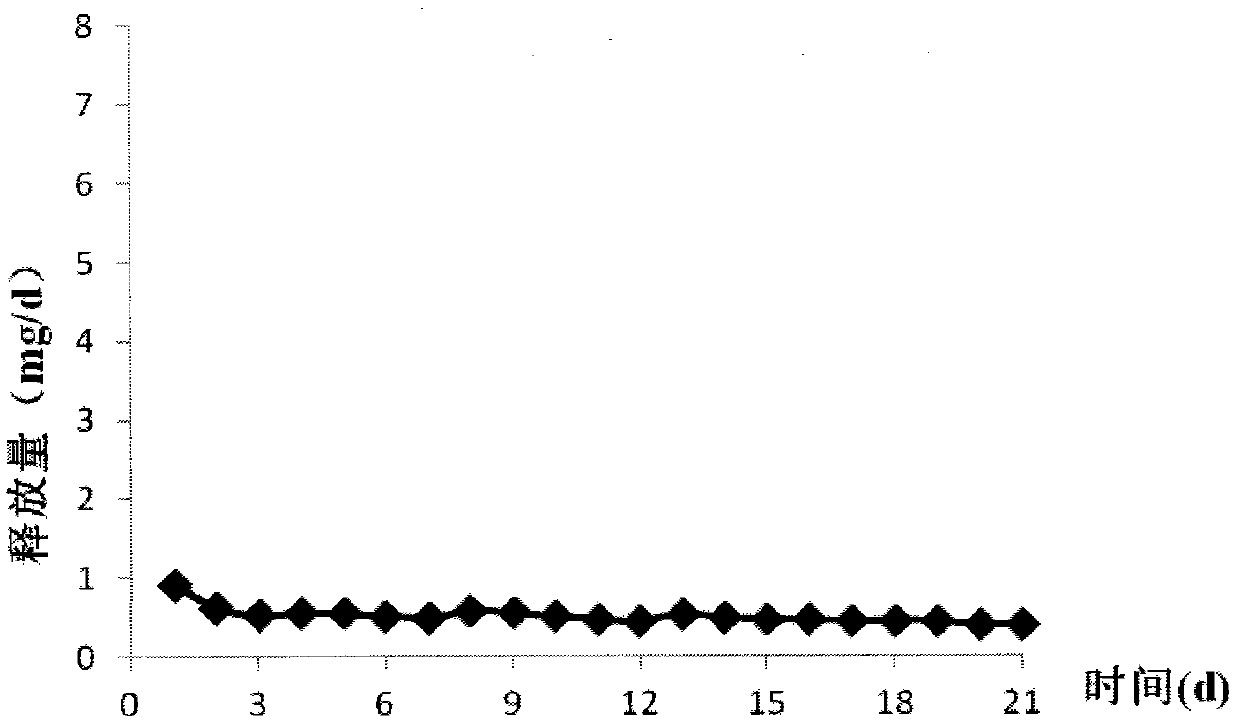
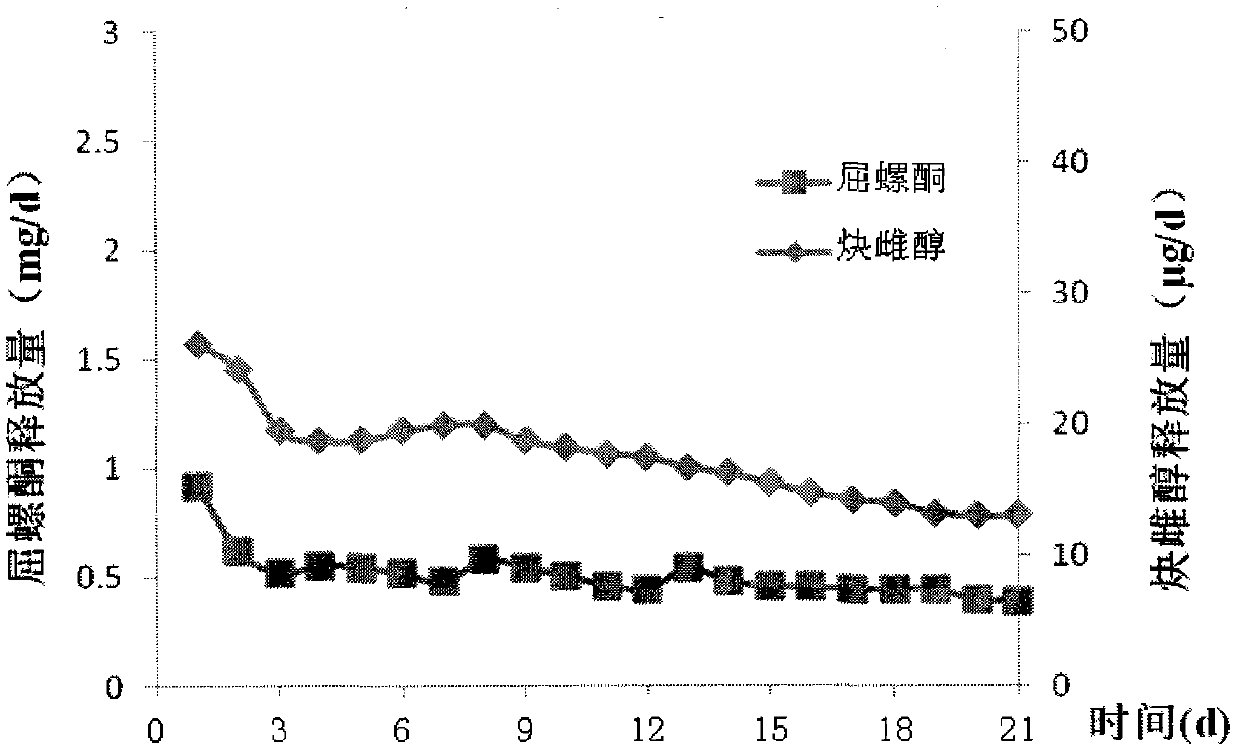
The invention relates to a contraceptive which is a pessulum preparation containing drospirenone or drospirenone and estrogen. The pessulum comprises a controlled release membrane and one or more framework sections; and at least one framework section contains the drospirenone which can slowly release acyeterion at a constant speed within 21 days, so that the zero-order release characteristic is realized. The controlled release medicinal preparation containing the drospirenone or drospirenone and estrogen can overcome the inconvenience caused by an oral contraceptive pill and improve the compliance in taking the medicine.
Owner:NAT RES INST FOR FAMILY PLANNING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
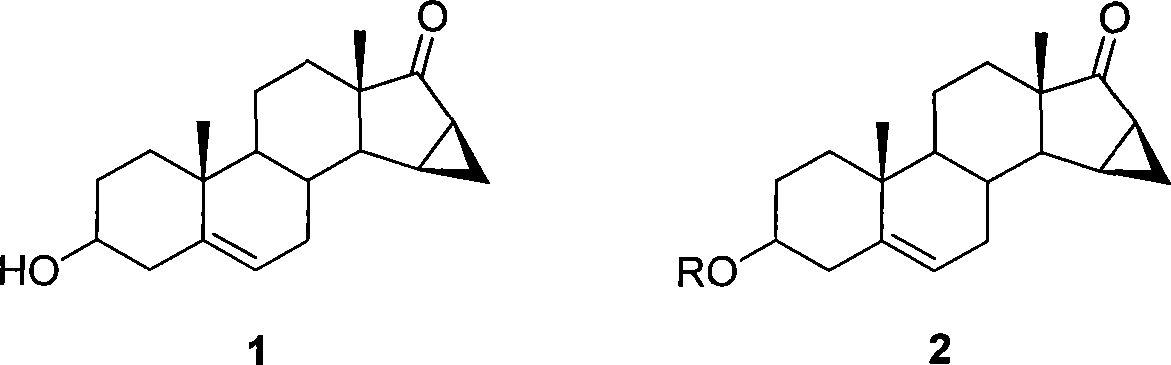
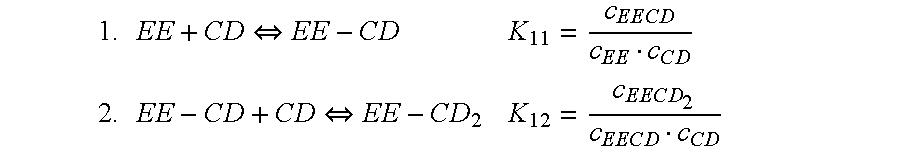
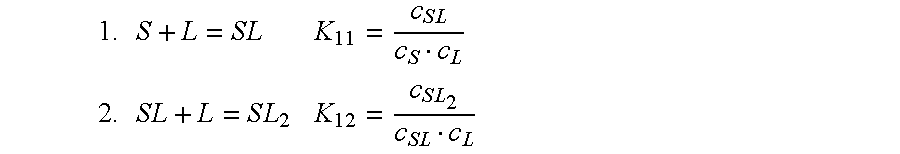

![Whorl[(5 beta, 6 beta, 15 beta, 16 beta-dimethylene-androstane-14 beta-hydrogen-5, 7-diene-3-ketone)-17 alpha-2'-(1'-oxygen-cyclopentane-5'-ketone)] and synthesis process Whorl[(5 beta, 6 beta, 15 beta, 16 beta-dimethylene-androstane-14 beta-hydrogen-5, 7-diene-3-ketone)-17 alpha-2'-(1'-oxygen-cyclopentane-5'-ketone)] and synthesis process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8e1b0445-409f-4477-a415-259012f80aa6/a2008100363740002c1.PNG)
![Whorl[(5 beta, 6 beta, 15 beta, 16 beta-dimethylene-androstane-14 beta-hydrogen-5, 7-diene-3-ketone)-17 alpha-2'-(1'-oxygen-cyclopentane-5'-ketone)] and synthesis process Whorl[(5 beta, 6 beta, 15 beta, 16 beta-dimethylene-androstane-14 beta-hydrogen-5, 7-diene-3-ketone)-17 alpha-2'-(1'-oxygen-cyclopentane-5'-ketone)] and synthesis process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8e1b0445-409f-4477-a415-259012f80aa6/a20081003637400061.PNG)
![Whorl[(5 beta, 6 beta, 15 beta, 16 beta-dimethylene-androstane-14 beta-hydrogen-5, 7-diene-3-ketone)-17 alpha-2'-(1'-oxygen-cyclopentane-5'-ketone)] and synthesis process Whorl[(5 beta, 6 beta, 15 beta, 16 beta-dimethylene-androstane-14 beta-hydrogen-5, 7-diene-3-ketone)-17 alpha-2'-(1'-oxygen-cyclopentane-5'-ketone)] and synthesis process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8e1b0445-409f-4477-a415-259012f80aa6/a20081003637400062.PNG)