Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
105 results about "Pregnane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
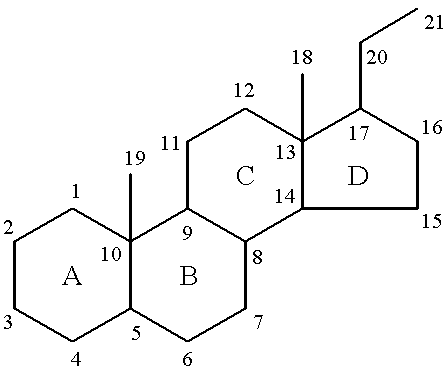
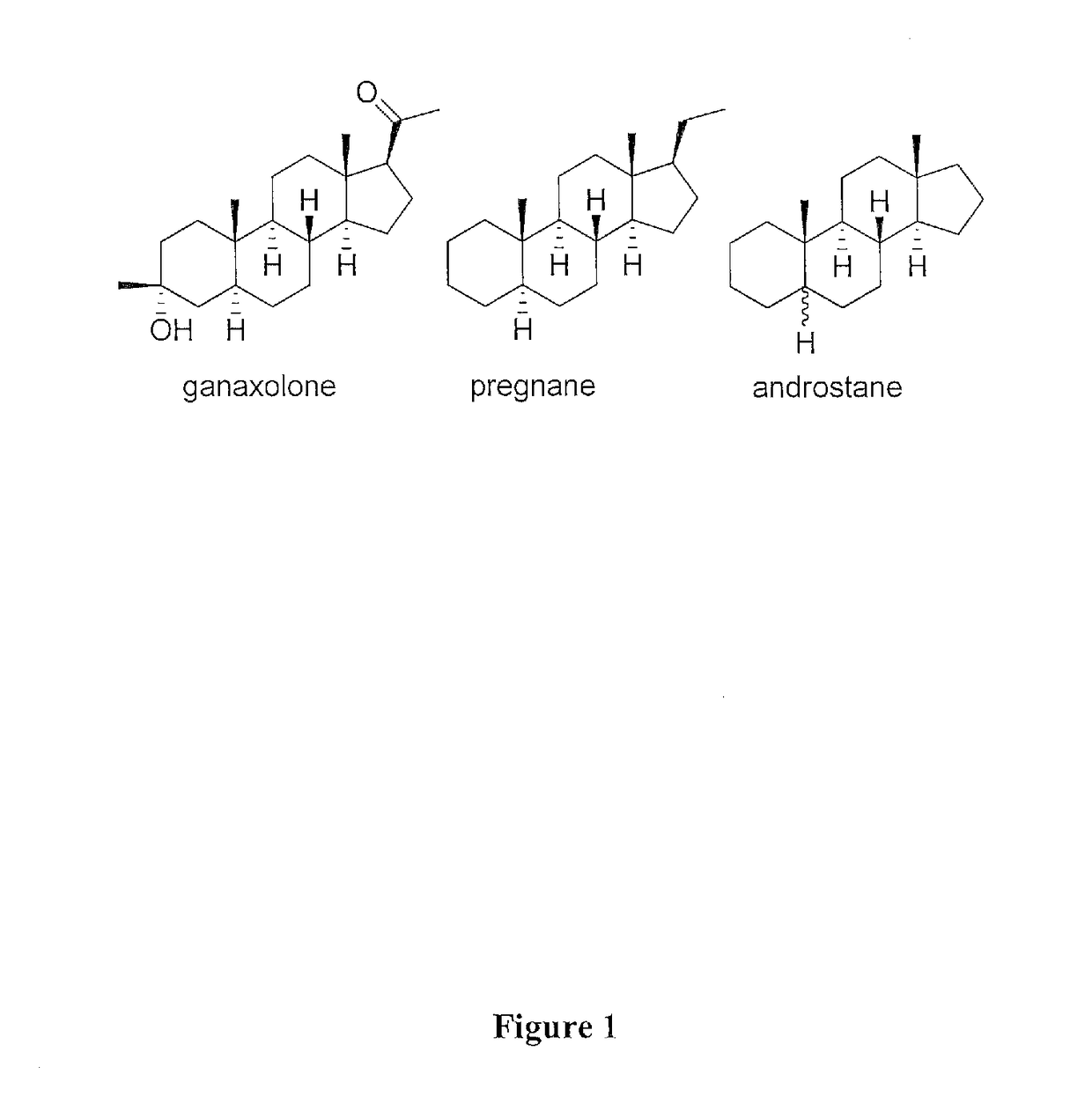
Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane (originally allopregnane) and 5β-pregnane (17β-ethyletiocholane). It has a gonane core.
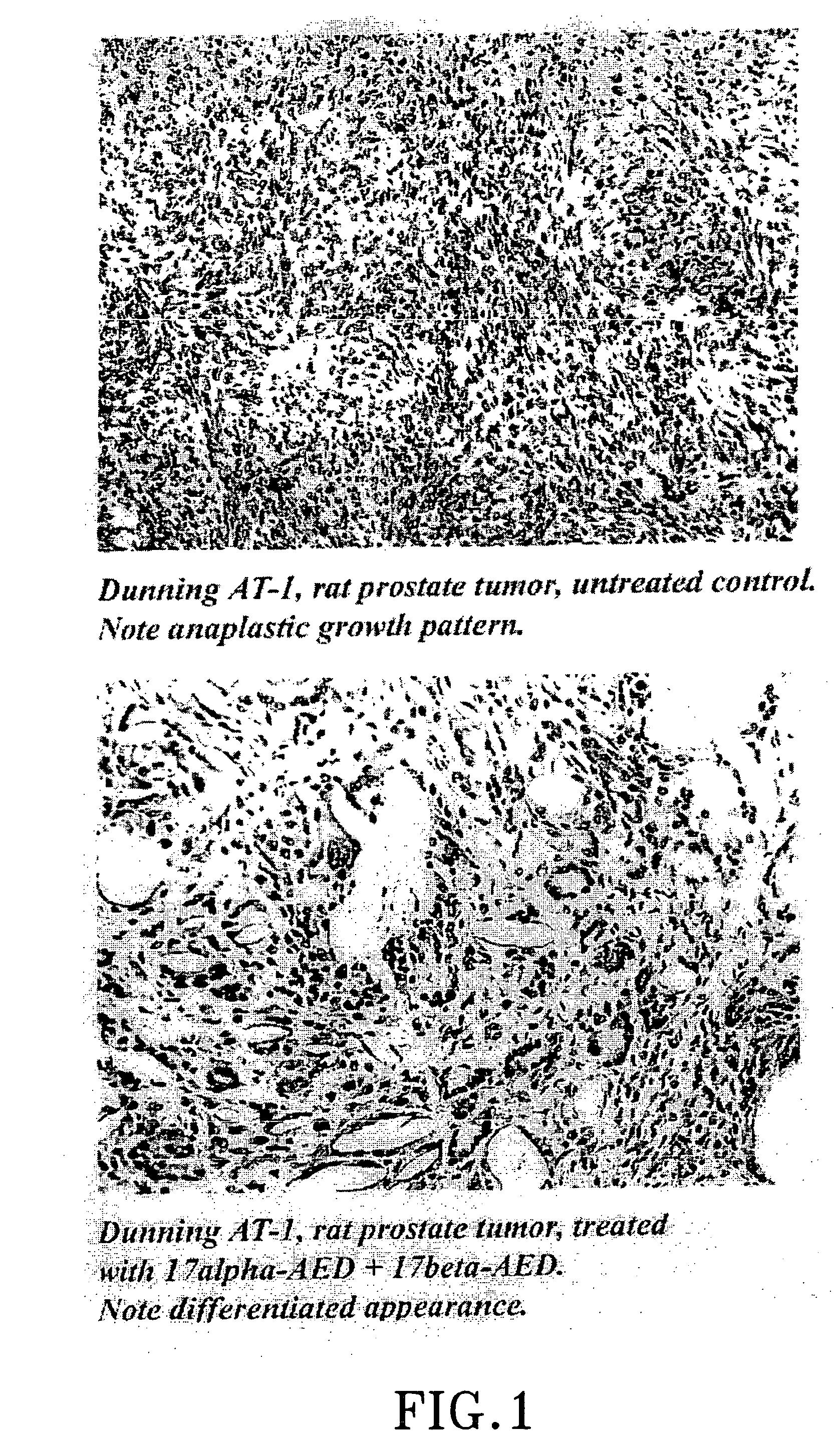
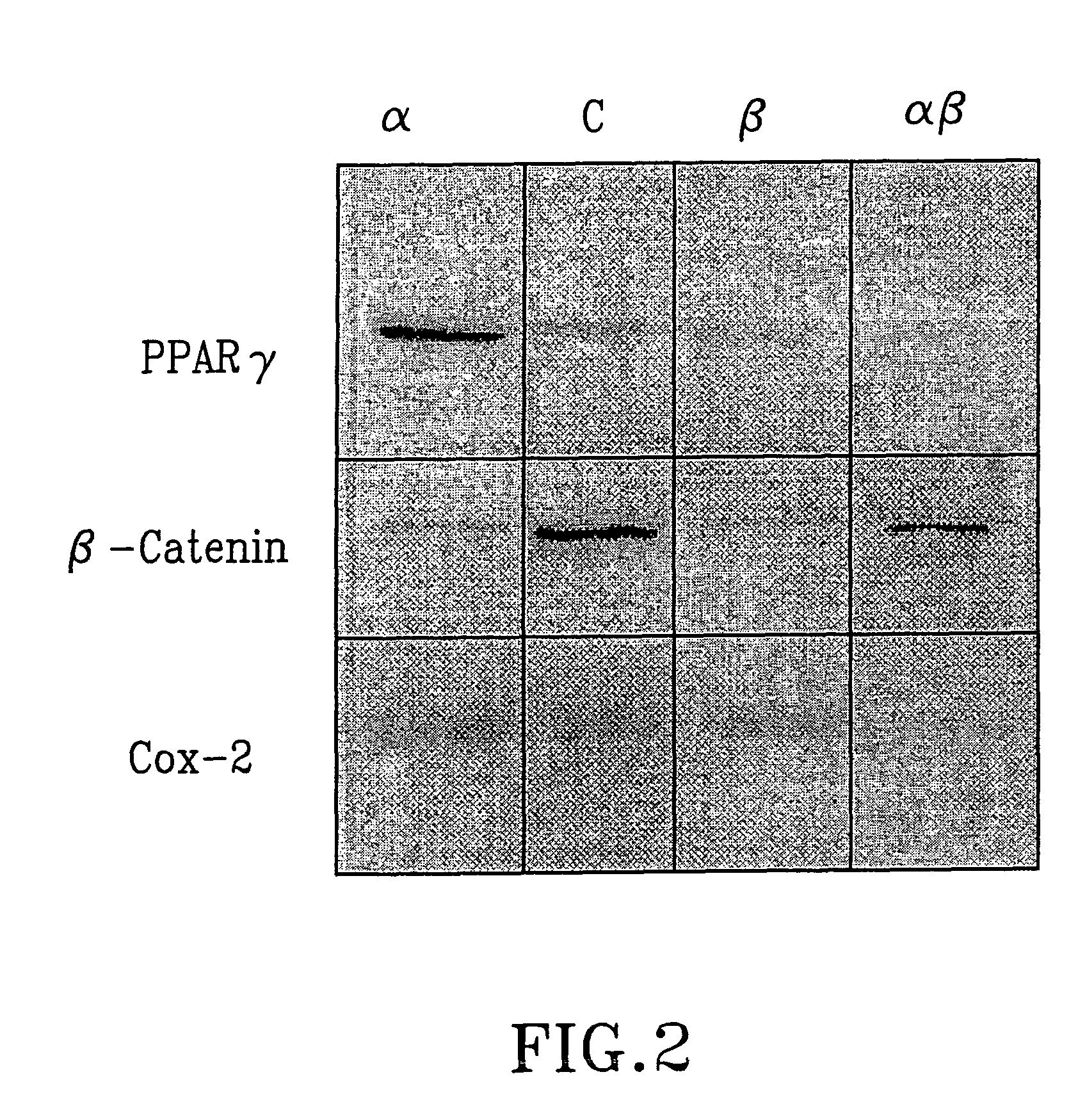
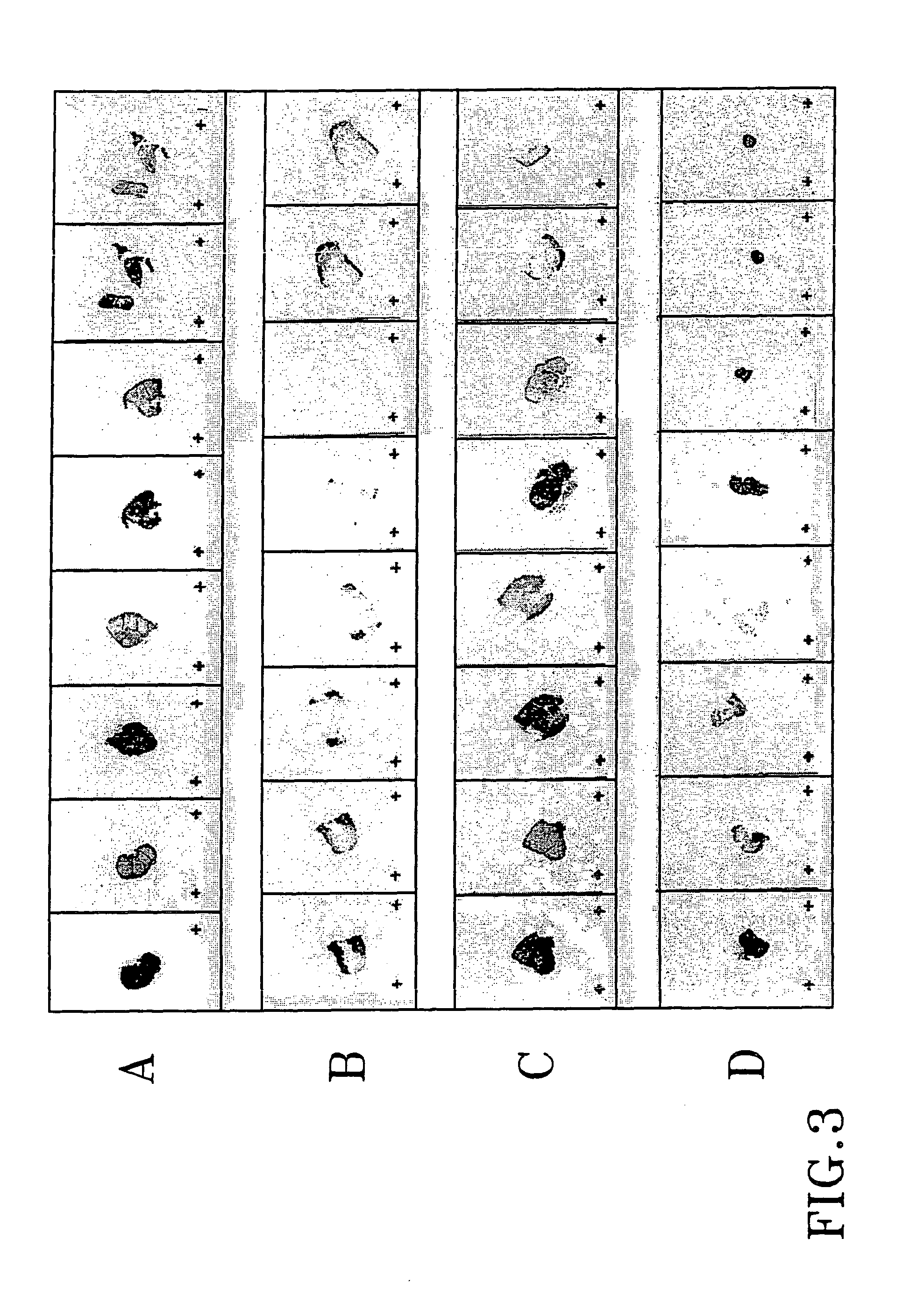
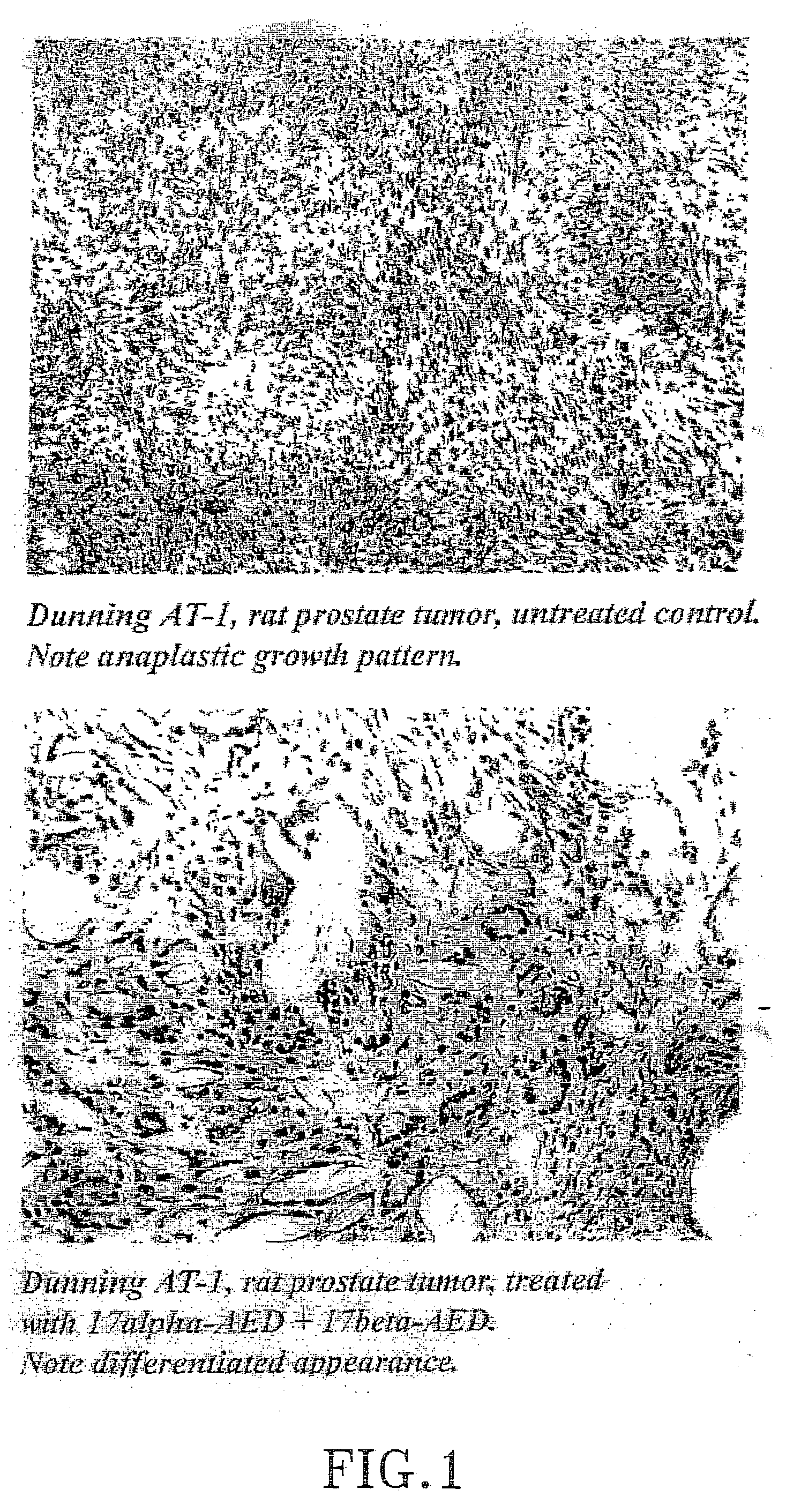
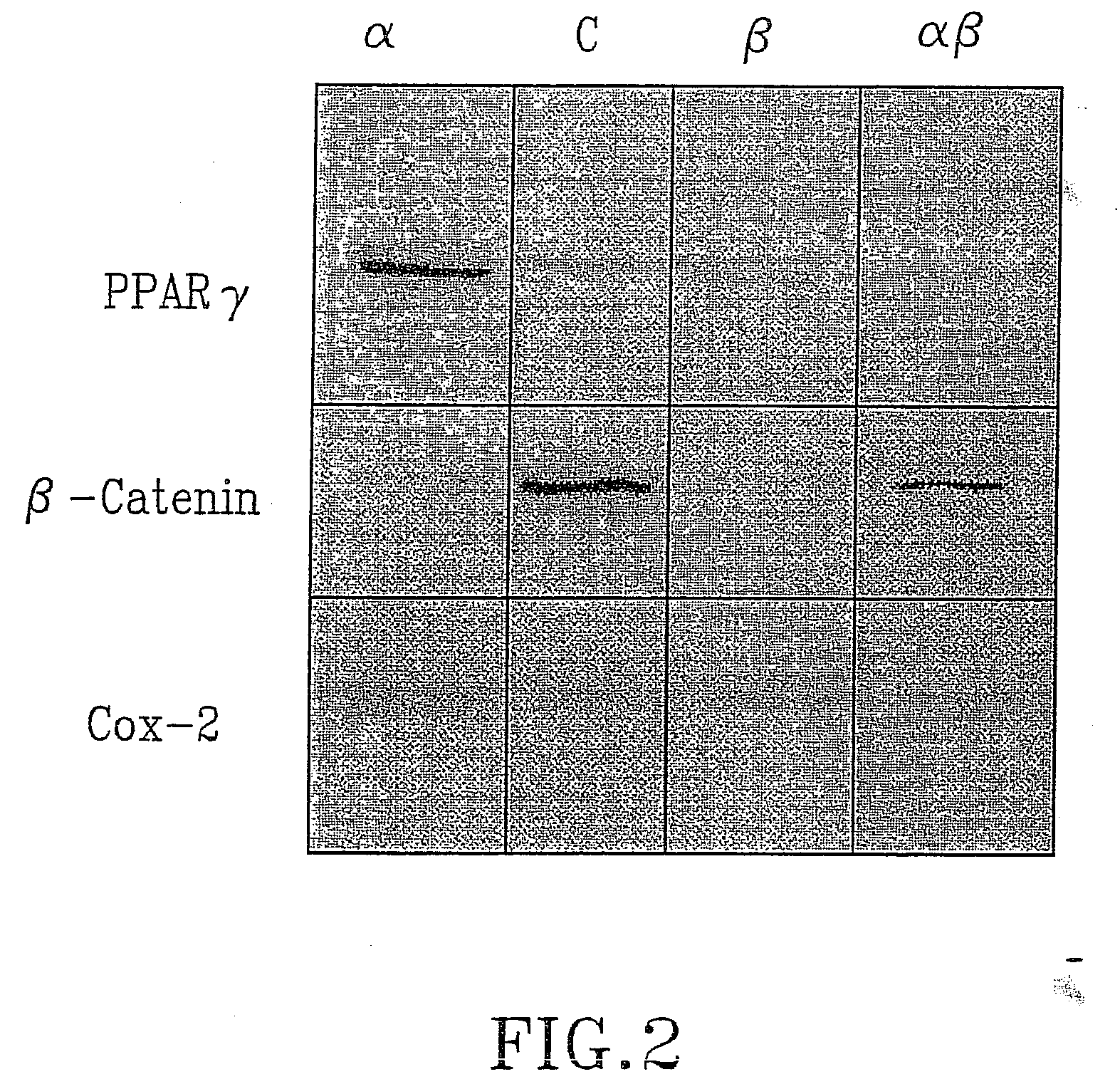
Treatment of tumours
InactiveUS20050192262A1Good curative effectSquelching unwanted PPARγ-activityOrganic active ingredientsSteroidsDiseaseAndrostane
The present invention refers to steroid derivatives for use as medicaments. More specifically, the invention also relates to the use of a steroid derivative of 5-androstene-, 5-pregnenolone or corresponding saturated derivatives (androstane- or pregnane-) in the manufacture of a medicament for the treatment of a benign and / or malignant tumour, which medicament is capable of interrupting disturbances in Wnt-signaling, such as cell-cycle arrest in G1-phase, and / or providing an angiostatic effect. Examples of such steroid derivatives are -5-androstene-17-ol, androstane-17-ol-pregnane-17-ol or pregnane-17-ol derivatives. In a further aspect, the invention relates to a method of producing a medicament for the treatment of a benign and / or malignant tumour and / or an inflammatory condition comprising the steps of contacting 5-androstane-3β,17-diol or androstane-3β-diol, an enzyme and a sulfotransferase to provide 5-androstene-17-ol-3β-sulfate or corresponding andros tane derivative (17-AEDS or 17-AADS); and mixing the 17-AEDS or 17-AADS so produced with a suitable carrier; whereby a medicament which is capable of acting as a ligand to peroxisome proliferators-activated receptor-(PPAR) is produced.
Owner:HAGSTROM TOMAS
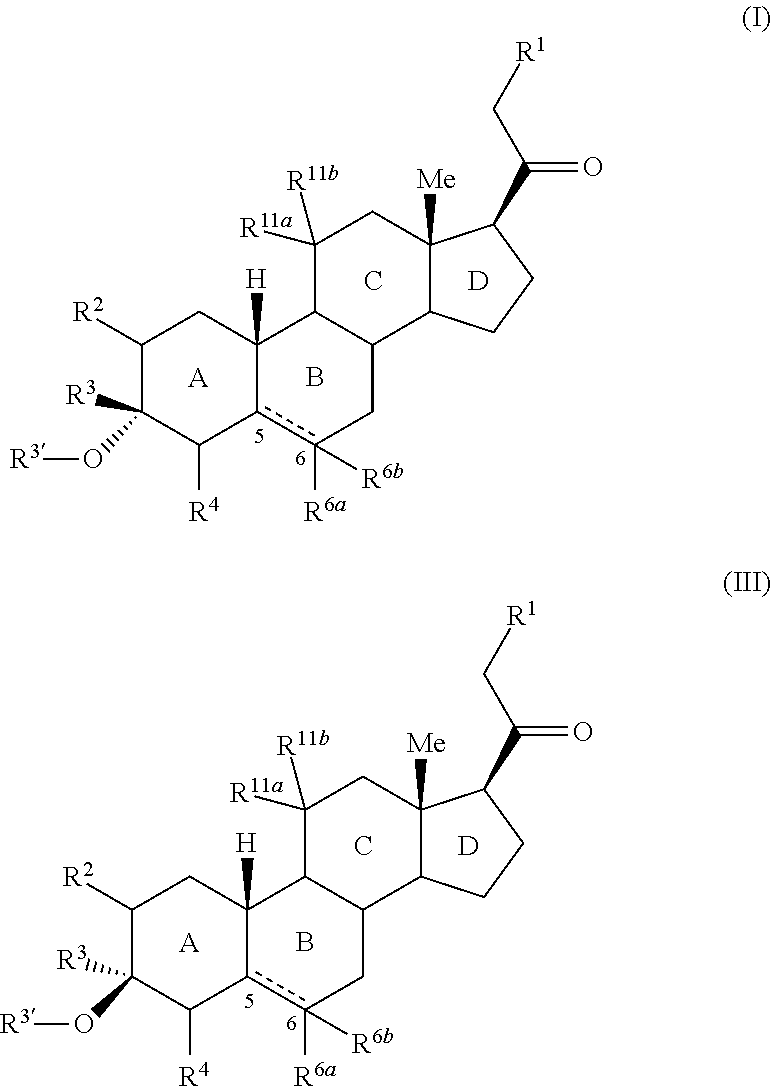
3,3 disubstituted 19-nor pregnane compounds, compositions, and uses thereof
InactiveUS20150291654A1Eliminate potentialInhibit metabolismSenses disorderNervous disorderInsomniaBrain traumas
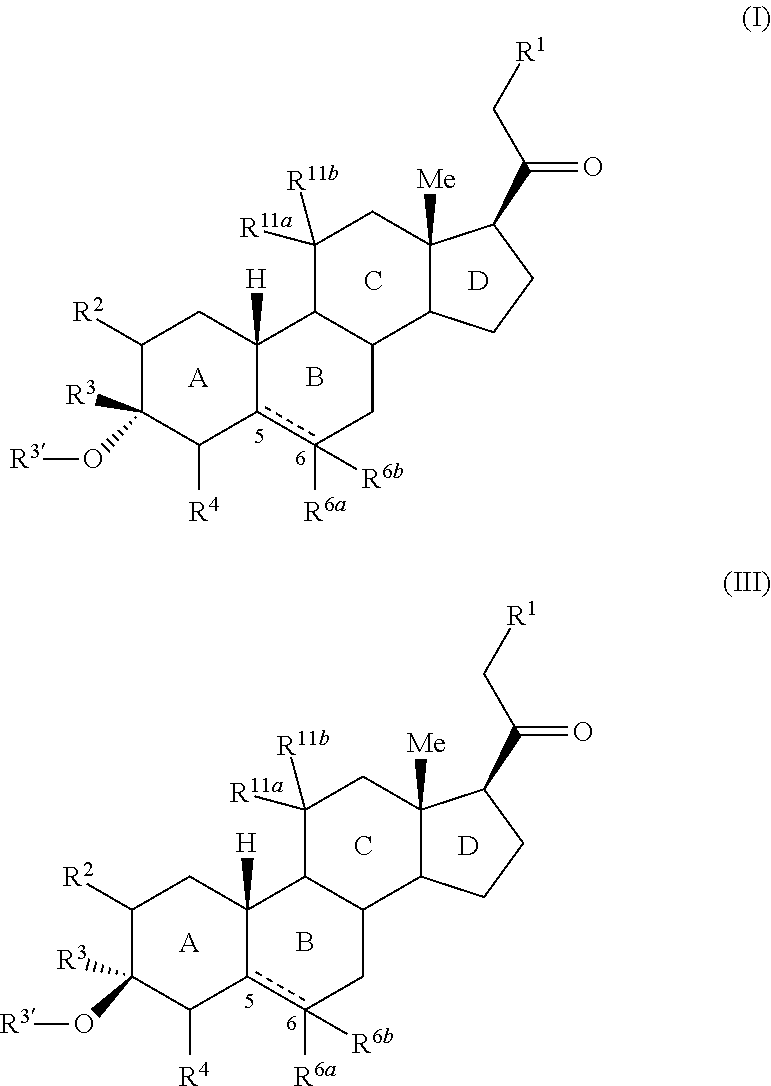
Provided herein are 3,3-disubstituted 19-nor-steroidal compounds according to Formula (I) and (III): where R1, R2, R3, R3′, R4, R6a, R6a, R11a, and R11b are as defined herein. Compounds of the present invention are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, insomnia, anxiety, depression, traumatic brain injury (TBI), stress, and epilepsy.
Owner:SAGE THERAPEUTICS
Reversing agent for drug-fast during treating tumor with multiple medicines
InactiveCN1911234AIncrease concentrationOrganic active ingredientsAntineoplastic agentsCancer cellTenacigenin B
Owner:广州中医药大学热带医学研究所
Isomorphic crystalline habits of 3alpha-hydroxy-21-(1'-imidazolyl)-3beta-methoxymethyl-5alpha-pregnane-20-one
The present invention provides stable particles of 3a-hydroxy-21-(1′-imidazolyl)-3β-methoxymethyl-5a-pregnan-20-one (Compound I), which possess and retain a shape and size appropriate for handling and manufacture of large-scale pharmaceutical preparations, even in the absence of further milling. Further provided is a method for obtaining such reproducible, stable particles by subjecting crude Compound I to controlled crystallization conditions comprising slow cooling of a solution of Compound I. Further provided is a pharmaceutical composition of unmilled crystalline Compound I, which does not require milling prior to formulation, and a method of modulating brain excitability using the same.
Owner:EURO-CELTIQUE SA
Inhibiting cyp3a4 induction
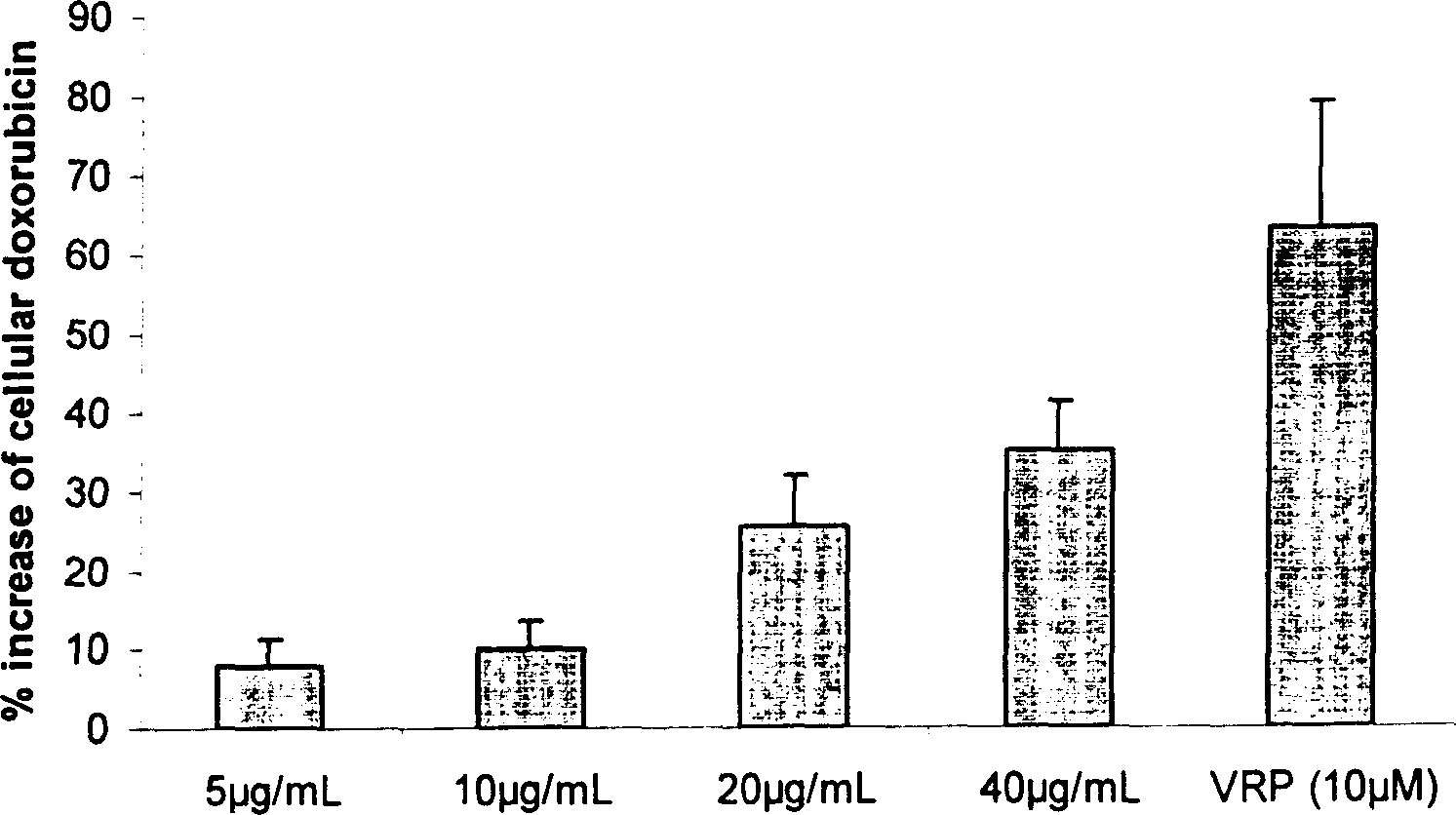
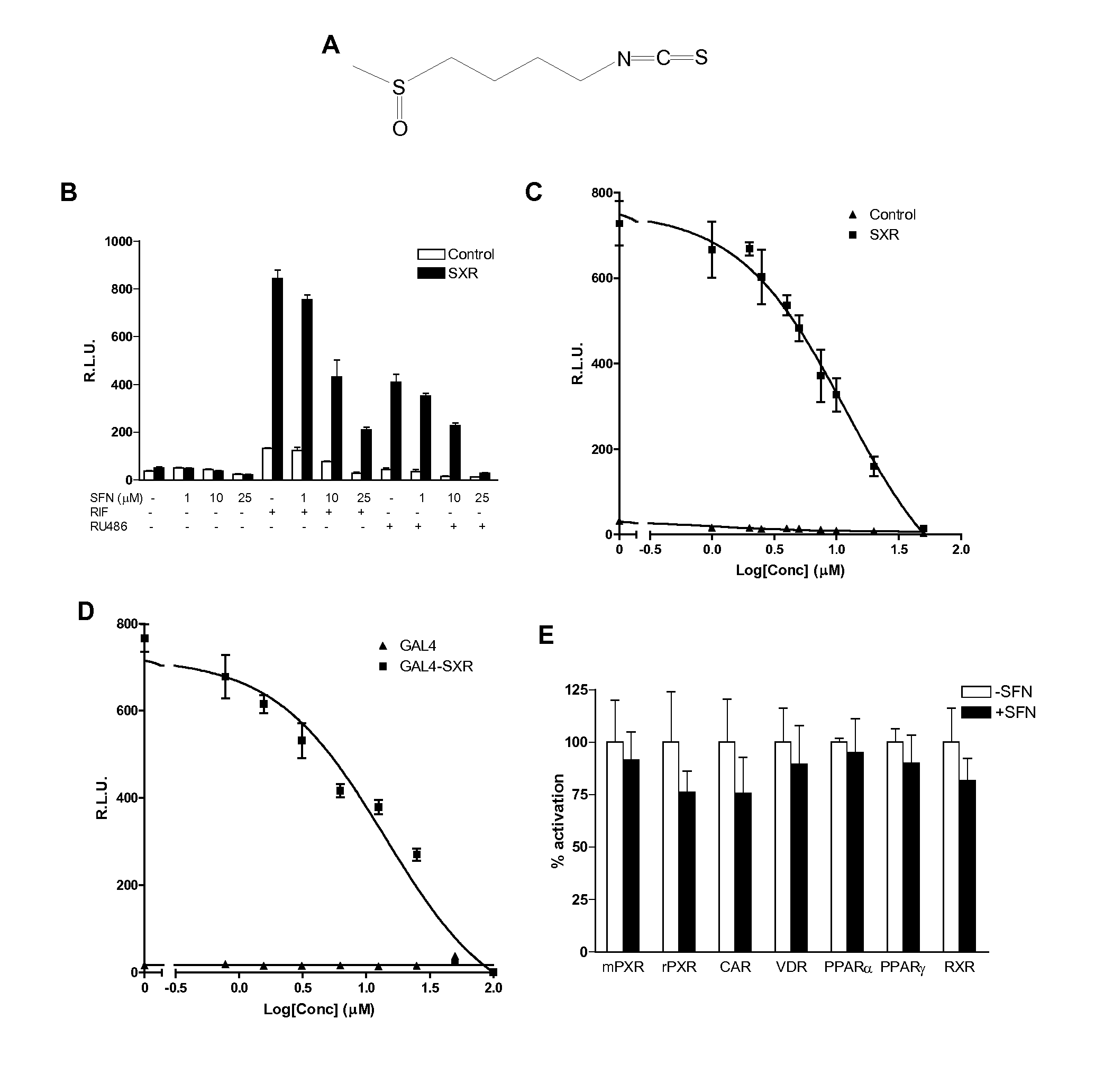
InactiveUS20080124407A1Prevent loss of efficacyInhibit expressionBiocideAnimal repellantsEfficacyPharmaceutical Substances
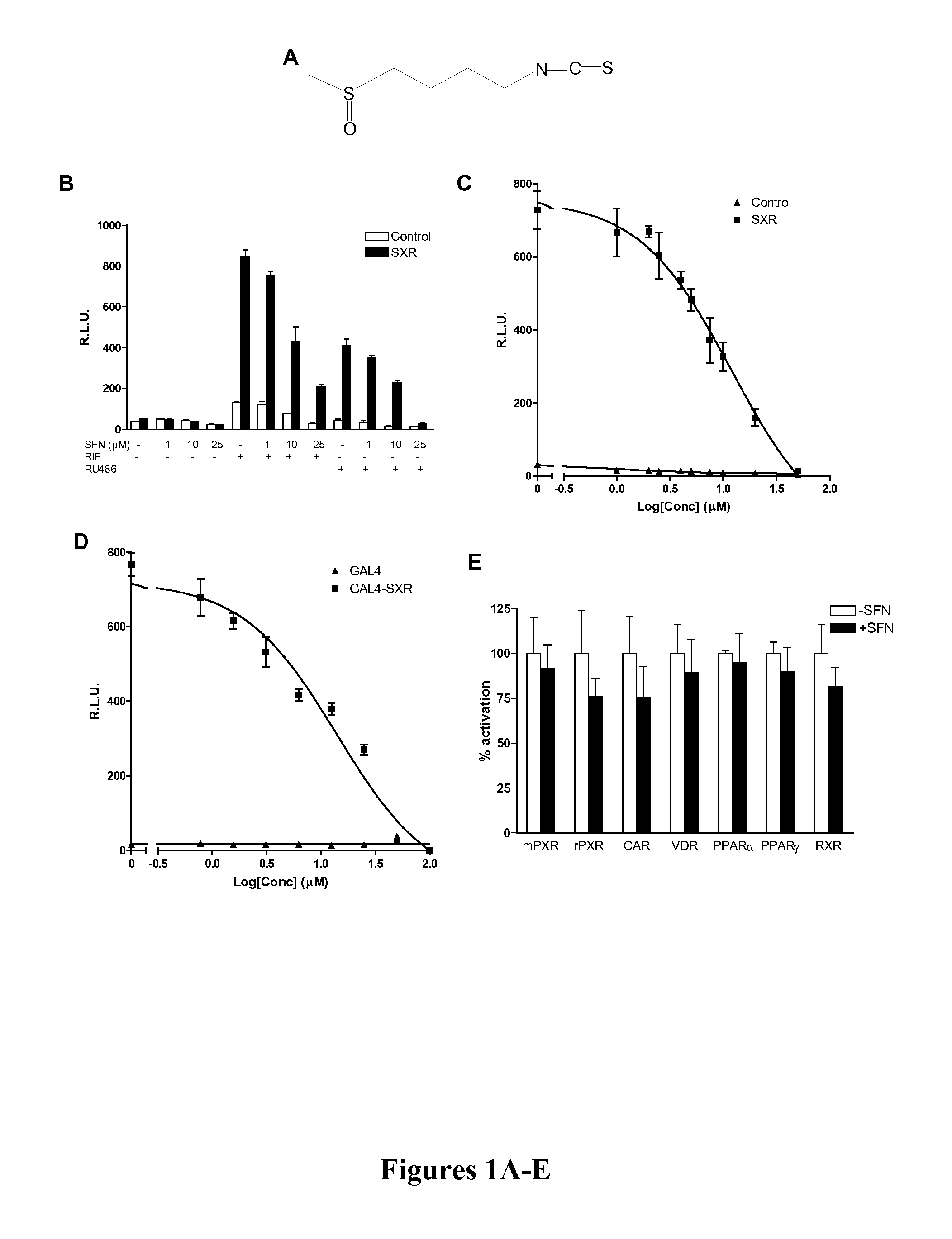
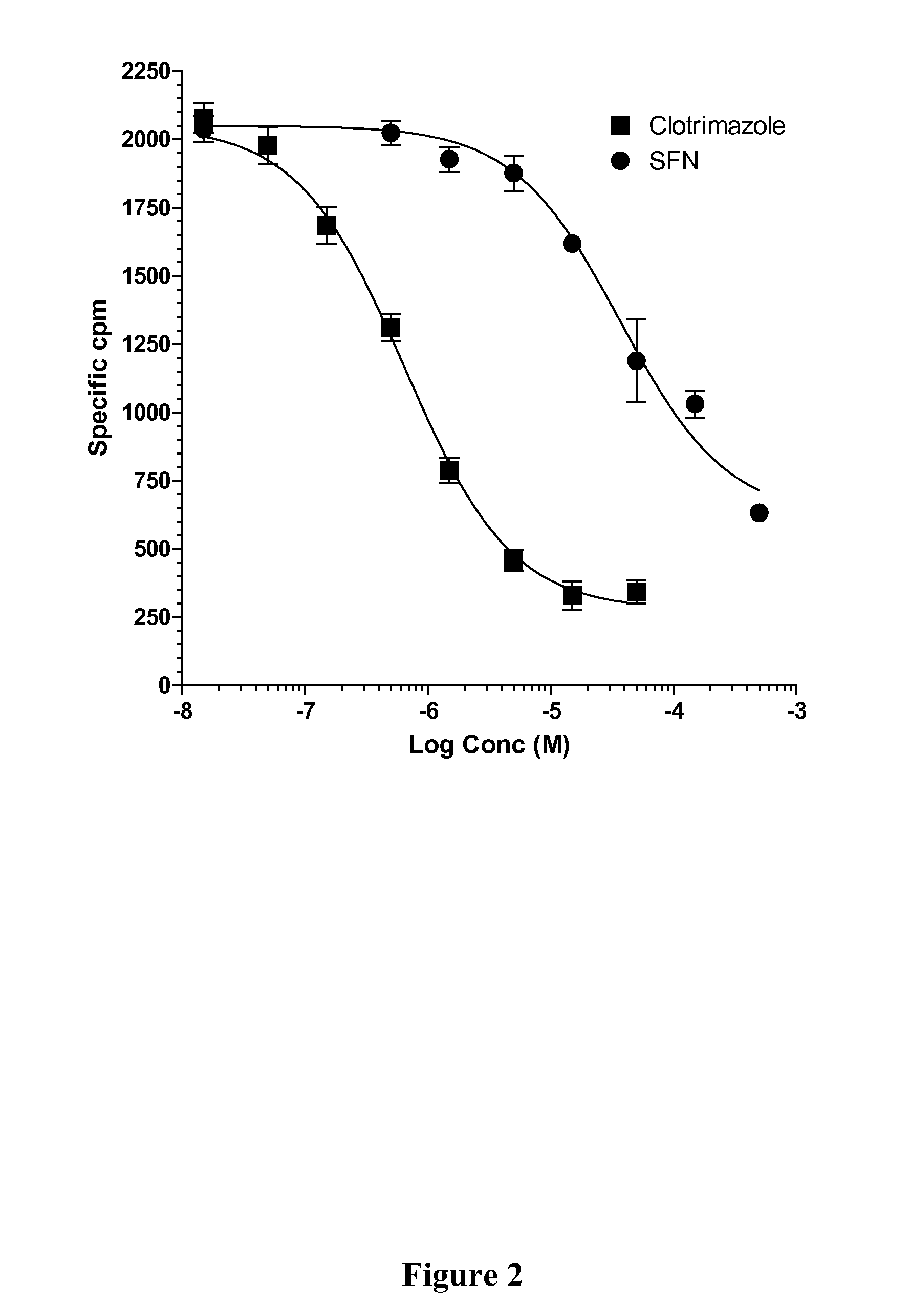
The present invention is directed to a method of inhibiting CYP3A4 induction. The method involves administering a compound of the following formula:R1—X—(CH2)n—N═C═Swith R1, X, and n defined herein, binds to a Pregnane X Receptor or Steroid and Xenobiotic Receptor (SXR or NR1I2) under conditions effective to inhibit CYP3A4 gene induction. The present invention also include a method of administering a compound, described herein, together with the CYP3A4 inducer to prevent a loss of efficacy in the subject to whom the CYP3A4 inducer is repeatedly administered. In addition, such compounds can be administered to block the interaction between a CYP3A4 inducer and another drug being administered that is a substrate of CYP3A4.
Owner:UNIV OF WASHINGTON
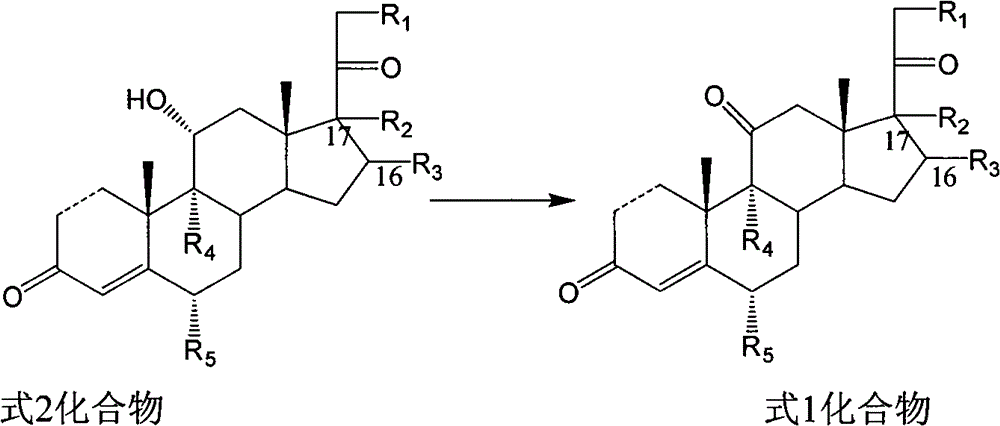
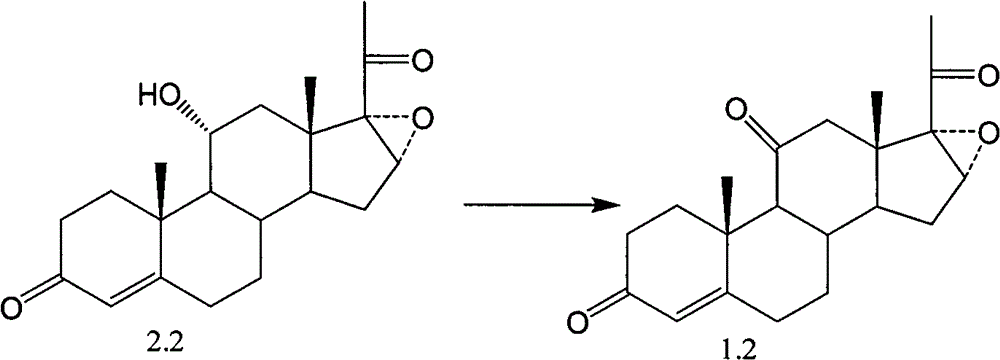
Novel technology for oxosynthesis of pregnane 11-bit ketonic group
The invention relates to a novel technology for oxosynthesis of pregnane 11-bit ketonic group, which is characterized in that a compound in a formula 2 uses piperidine nitroxide free-radical as an oxidation catalyst under the condition of an organic solvent, and uses positive valence halide as an oxidant to react to generate a compound in a formula 1.
Owner:TIANJIN JINYAO GRP
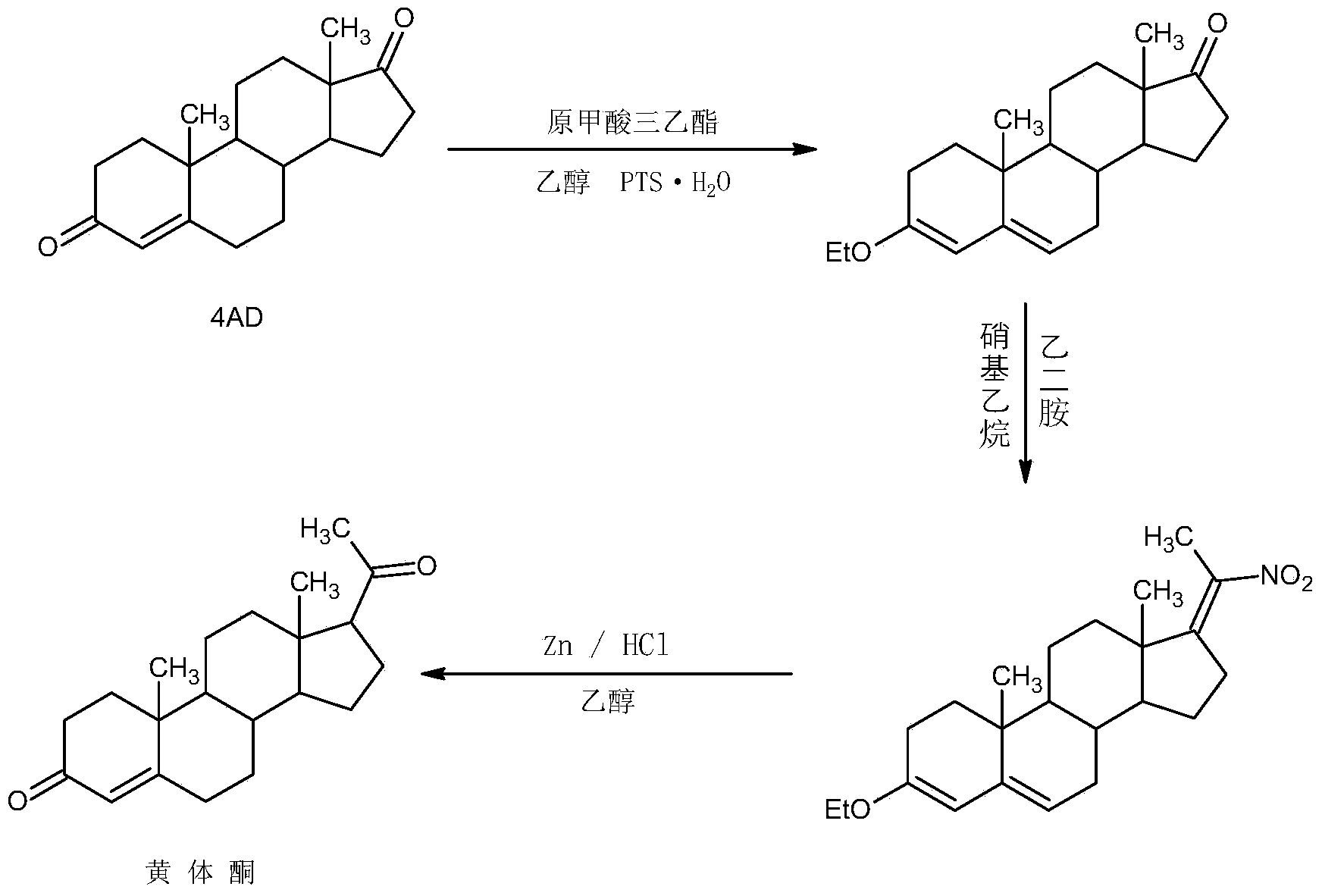
Preparation method for progestin
ActiveCN104262442AWide variety of sourcesProcess economy and environmental protectionSteroidsEthylenediamineKetone
The invention relates to a preparation method for progestin. 4-androstenedione is used as a raw material. The preparation method comprises the following steps: A, etherate is synthetized, wherein the 4-androstenedione and triethyl orthoformate perform an acid catalyzed reaction in organic solvents of dichloromethane, low-carbon alcohol and the like to obtain the etherate 3-ethoxy-androstane-3, 5-diolefin-17-ketone; B, a nitro substance is synthetized, wherein the etherate in the organic solvents and nitroethane perform 17-bit addition under the catalysis of ethylenediamine to obtain the nitro substance 3-ethoxy-20-nitro-pregnane-3, 5, 17 (20)-triene; and C, the progestin is synthetized, wherein the nitro substance is reduced by zinc powder in organic solvents of acetic acids, low-carbon alcohol and the like, acid hydrolysis is performed, so that semi-finished products of the progestin are obtained, the semi-finished products of the progestin are decolored and refined by alcohol and activated carbon to obtain the progestin, the content of HPLC is more than 99.5%, the melting point is 128-131 DEG C, and the total yield of synthetized weight is 83-87%. When the method disclosed by the invention is used for producing the progestin, the yield is high, the degree of purity is good, the quality is stable, the solvent recovering rate is high, and the method is economic and environment-friendly.
Owner:HUNAN KEREY BIOTECH
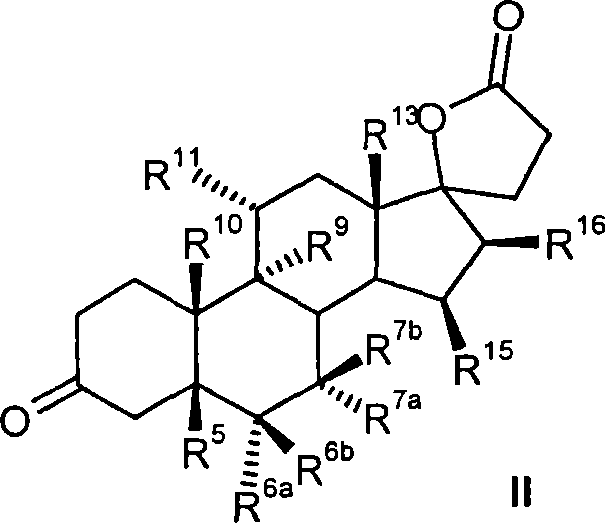
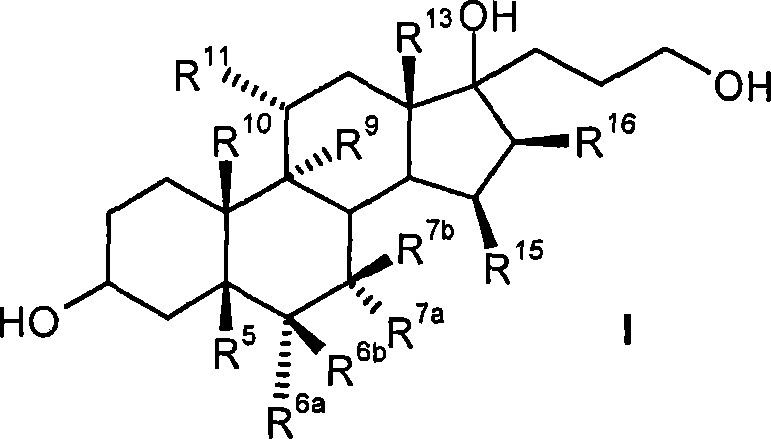
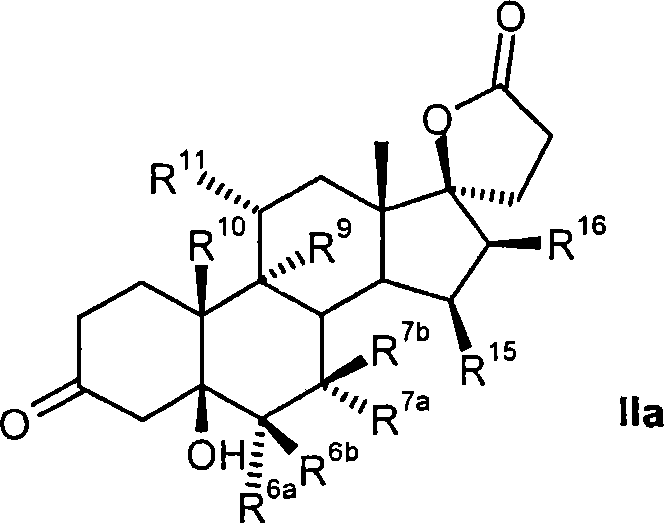
Process for the production of 3-oxo-pregn-4-ene-21,17-carbolactones by the metal-free oxidation of 17-(3-hydroxypropyl)-3,17-dihydroxyandrostanes
This invention relates to processes for the production of 3-oxo-pregnane-21,17-carbolactones of formula (II) as well as 3-oxo-pregn-4-ene-21,17-carbolactones of formula (III) by the metal-free oxidation of 17-(3-hydroxypropyl)-3,17-dihydroxyandrostanes of formula (I). In addition, the invention relates to the dichloromethane hemisolvate of 6ss ,7ss; 15ss,16ss-dimethylene-3-oxo-17a-pregnan-5ss-ol-21,17-carbolactone (IV) as such as well as to a process for the production of drospirenone.
Owner:BAYER PHARMA AG
Method for making 3α-hydroxy, 3β- substituted-5α-pregnan-20-ones
ActiveUS8362286B2Simple and cost-effectiveHigh yieldOrganic active ingredientsNervous disorderMethylating AgentRegioselectivity
Applicants have discovered a method for the stereoselective and regioselective synthesis of 3α-hydroxy, 3β-methyl-5α-pregnan-20-one (ganaxolone) comprising reacting 5α-pregnane-3,20-dione; with an organometallic methylating agent in an inert solvent to provide a compound of the formula
Owner:MARINUS PHARMA
3-Alpha-hydroxy 21-n-heteroaryl-pregnane derivatives for modulation of brain excitability and a process for the production thereof
The invention relates to a novel multi step process of making compounds of Formula I:wherein R1 is an alkoxy group and R2 is an optionally substituted, N-attached heteroaryl. The hydrogen at the 5-position can be α or β isomer, preferably α. Preferably the compound of Formula I is 17β isomer. The invention also relates to novel 3α-hydroxy-3β-substituted-17-substituted steroid compounds having GABAA receptor modulating activity, pharmaceutical compositions comprising these compounds, and the use of these compounds in a method of modulating brain excitability.
Owner:PURDUE PHARMA LP
Androstane and pregnane steroids with potent allosteric gaba receptor chloride ionophore modulating properties
This invention describes compounds of Structures 1, 2, and 3 and their use as allosteric modulators of the GABA receptor chloride ionophore complex to alleviate stress, anxiety, mood disorders, seizures, depression, treatment of drug and alcohol abuse, memory, premenstrual disorders, and neural system damage.
Owner:UNITED STATES OF AMERICA +1
Androstane and pregnane steroids with potent allosteric GABA receptor chloride ionophore modulating properties
This invention describes compounds of Structures 1, 2, and 3 and their use as allosteric modulators of the GABA receptor chloride ionophore complex to alleviate stress, anxiety, mood disorders, seizures, depression, treatment of drug and alcohol abuse, memory, premenstrual disorders, and neural system damage.
Owner:UNITED STATES OF AMERICA +1
Method for extracting compounds with pregnane mother nucleus structure from compositions
ActiveCN104198609AEfficient extractionEfficient removalComponent separationSolventComponents of crude oil
The invention particularly relates to a method for extracting compounds with a pregnane mother nucleus structure from compositions, belonging to the technical field of analytical chemistry. According to the method, inorganic salt is added into acetonitrile or an acetonitrile water solution which serves as a basic solvent so as to improve oil-water partition coefficients, the temperature is lowered to accelerate separation of auxiliary materials / matrixes in the compositions, and the separation of immiscible components such as oil and water is accelerated by centrifugation. The method has the beneficial effects that the universality is wide, the compounds can be effectively extracted without being damaged, and the subsequent analysis and detection cannot be interfered by the used solvent; the obtained solution can be directly utilized for carrying out high performance liquid phase analysis, and an obtained atlas has clean base lines, symmetric peak shapes and high number of theoretical plates; meanwhile, a sample solution is relatively stable, and more related substances can be detected; the method can be widely applied to the quality control of the compositions containing adrenocortical hormone components.
Owner:CHONGQING HUAPONT PHARMA
Use of pregnane-diones or diols as neuropathic analgesic agents
The present invention relates to the use of pregnanes in inducing analgesia, preferably without overt sedation, in a mammal in response to neuropathic pain, and compositions and kits therefore.
Owner:MONASH UNIV
19-norpregnane steroids as neurochemical initiators of change in human hypothalamic function
InactiveUS6242619B1Altered functionAffect brain functionOrganic active ingredientsNervous disorderHypothalamusNorpregnane
Several 19-nor-pregnanes are provided which have the capacity to neurochemically alter the hypothalamic function in an individual through nasal administration. These pharmaceutically active compounds can be administered by themselves or in the form of a pharmaceutical composition containing one or more pharmaceutically acceptable carriers to produce the desired effect.
Owner:PHERIN PHARMA INC
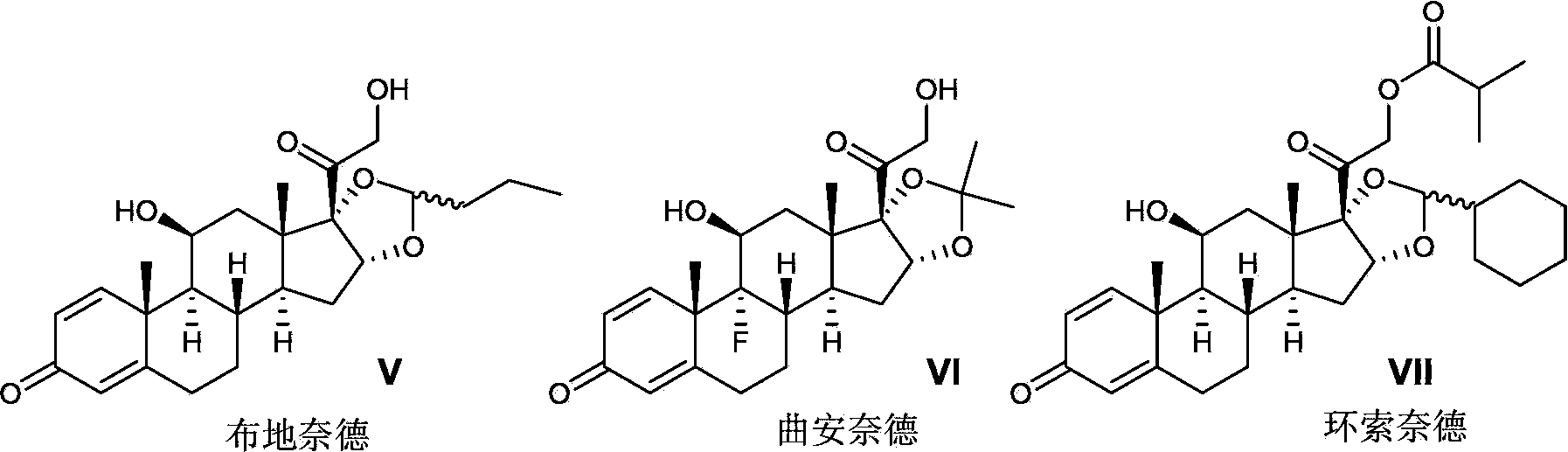
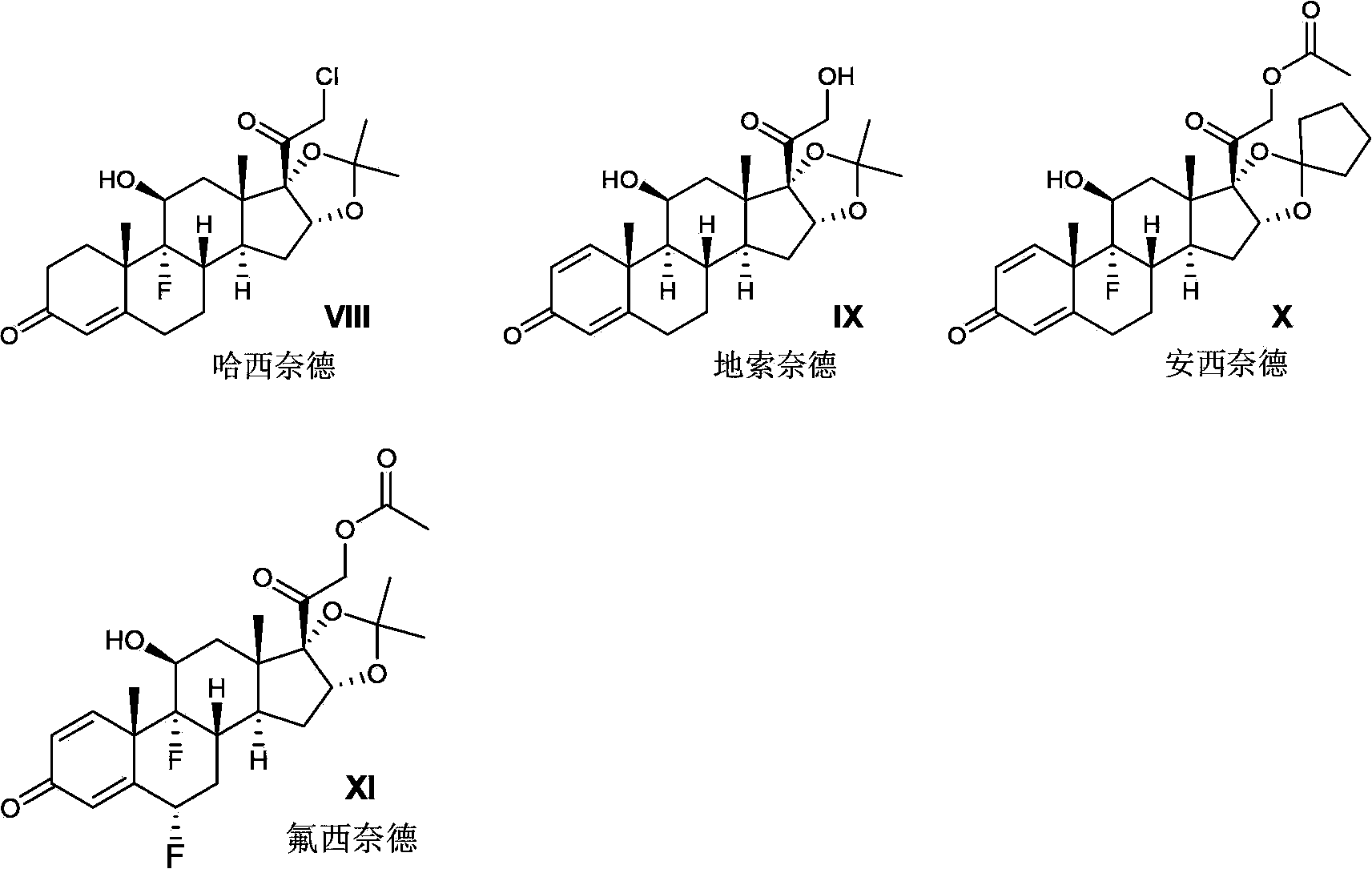
Novel process for preparation of glucocorticoid steroids
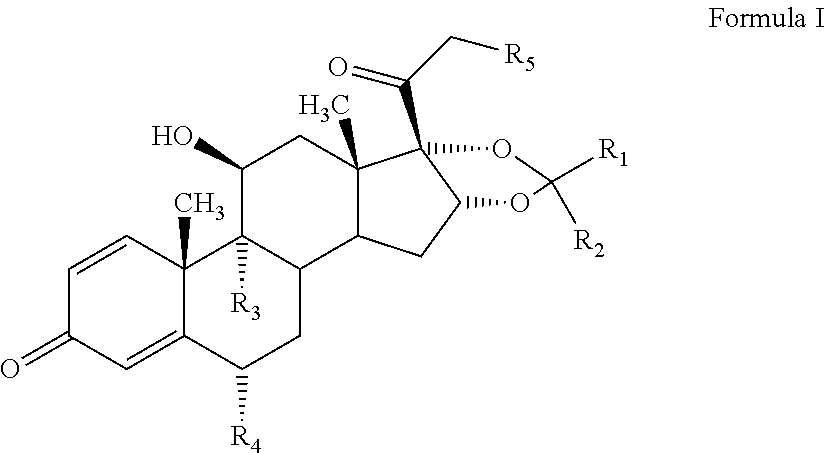
The present invention discloses a process for the preparation of 16, 17-acetals of pregnane derivatives having formula Iwherein each substituent is independently selected from;R1 is H or CH3;R2 is C1-C6 linear or branched alkyl, alkynyl group or cycloalkyl group; aryl or heteroaryl group; orR1 and R2 combine to form saturated, unsaturated C3-C6 cyclic or heterocyclic ring;R3 and R4 are same or different and each independently represents H or halogen;R5 is —OH or —OCOR wherein R represents H or C1-C6 linear, branched or cyclic alkyl group that may be substituted.
Owner:CORAL DRUGS PVT
Preparation method of pregnane derivatives 16,17-acetal (ketone)
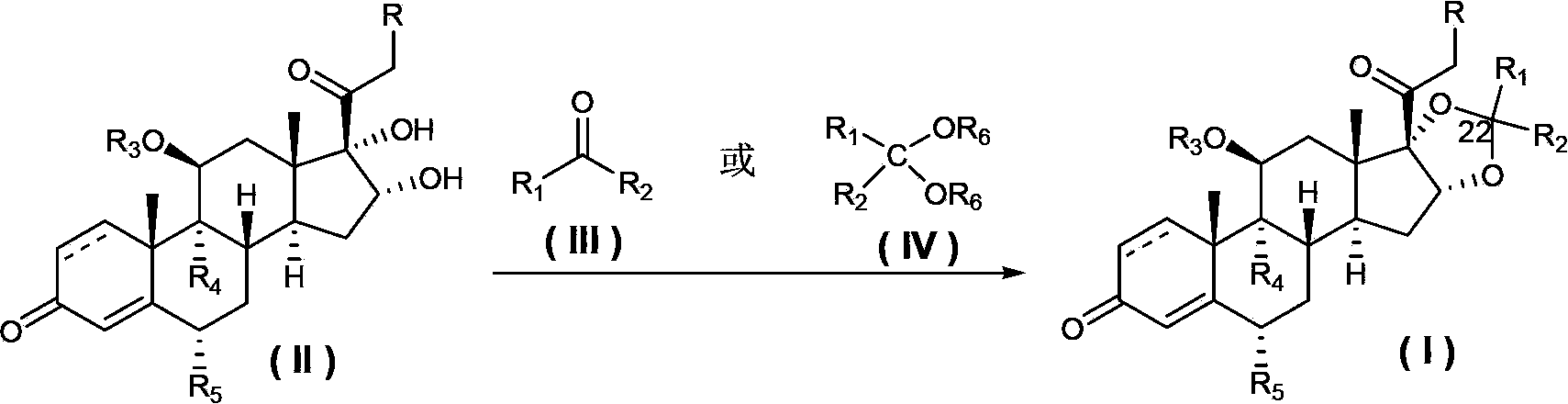
ActiveCN103421075AReduce pollutionQuality improvementOrganic active ingredientsSteroidsHydrogenHalogen
Disclosed is a method for preparing a pregnane derivative 16,17-acetal (ketal) compound shown in the general formula I, the method comprising the step of reacting a compound of a general formula II with a compound of a general formula III or a general formula IV in the presence of boron trifluoride, wherein the dotted line between site 1 and site 2 denotes a saturated or unsaturated bond; R is hydroxyl, halogen or -OCOR7, wherein R7 is a C1-C12 linear chain or branched alkyl, a C3-C10 cycloalkyl, a C2-C8 alkenyl or a C2-C8 alkynyl; R1 and R2 are each hydrogen, a C1-C12 linear chain or branched alkyl, a C3-C10 cycloalkyl, a C2-C8 alkenyl or a C2-C8 alkynyl, or R1, R2 and the carbon to which they are connected form a C3-C10 cycloalkyl together, with the provision that R1 and R2 are not hydrogen simultaneously; R3 is hydrogen or -OCOR8, wherein R8 is a C1-C12 linear chain or branched alkyl, or a C3-C10 cycloalkyl; R4 is hydrogen, fluorine or chlorine; R5 is hydrogen, fluorine, chlorine or methyl; and R6 is a C1-C12 linear chain or branched alkyl. Compared with current processes, the method causes little pollution to the environment, has relatively mild reaction conditions, ease of control, reduced energy consumption and low production costs.
Owner:TOPHARMAN SHANGHAI +1
Desogestrel preparation method and midbody compound
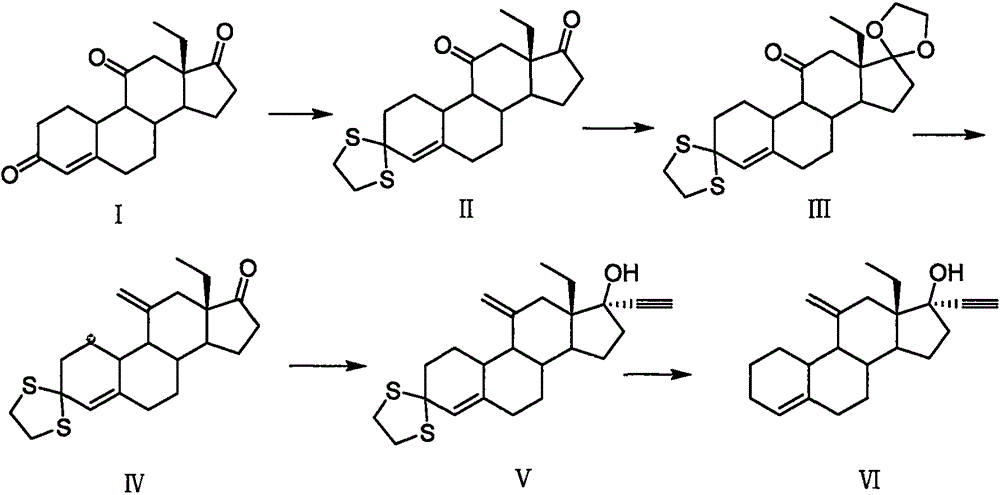
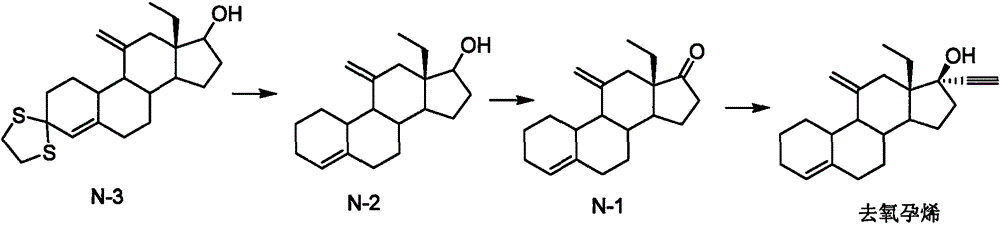
The invention relates to a novel steroidal compound and application of the novel steroidal compound in a desogestrel preparation process. The novel compound easy to separate is obtained through 17-bit selective ethynylation of 13beta-ethyl pregnane-4, 5-alkene-11, 17-diketone. When the compound is used for preparing desogestrel, simplicity and convenience in operation are achieved, and yield is high.
Owner:CHINA RESOURCES ZIZHU PHARMA
3,3 disubstituted 19-nor pregnane compounds, compositions, and uses thereof
ActiveUS20170342103A1Good potencyGood pharmacokineticSenses disorderNervous disorderInsomniaBrain traumas
Provided herein are 3,3-disubstituted 19-nor-steroidal compounds according to Formula (I) and (III):where R1, R2, R3, R3′, R4, R6a, R6a, R11a, and R11b are as defined herein. Compounds of the present invention are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, insomnia, anxiety, depression, traumatic brain injury (TBI), stress, and epilepsy.
Owner:SAGE THERAPEUTICS
Method for synthesizing 7,21-dyhydroxyl-20-methyl pregnane-4-alkene-3-ketone by microorganisms
ActiveCN109680032AHigh yieldImprove economyFungiMicroorganism based processesMicroorganismAspergillus fumigatus
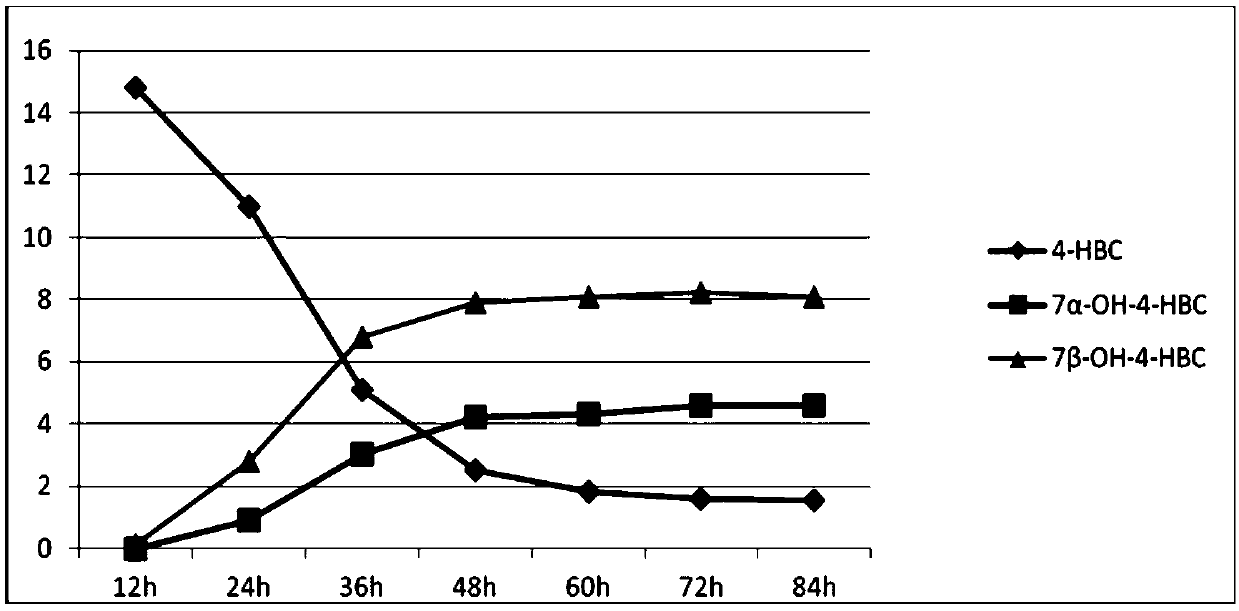
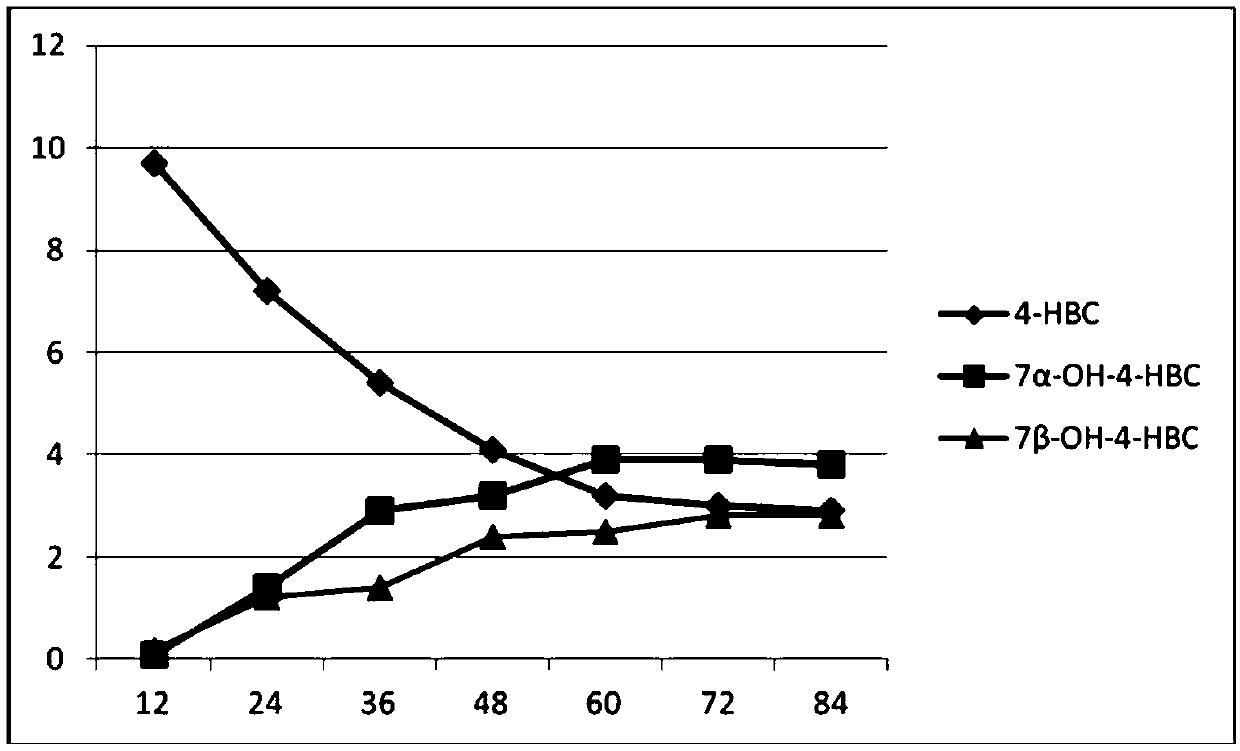
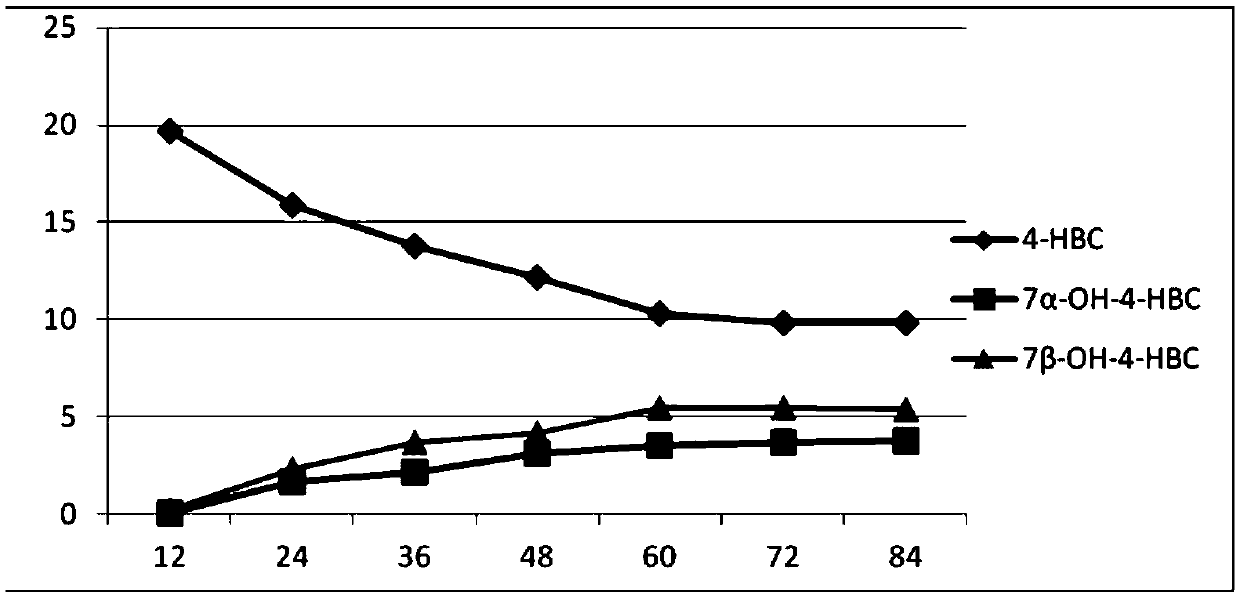
The invention provides a method for synthesizing 7,21-dyhydroxyl-20-methyl pregnane-4-alkene-3-ketone by microorganisms. Through a two-step fermentation method, one or more kinds of fermentation liquor including mucor circinelloides lusitanicus, acremonium strictum, mucor.racemosus and aspergillus fumigatus can be used for efficiently converting 4-HBC into 7 alpha-OH-4-HBC and 7beta-OH-4-HBC. Under a condition that the inventory of substrate 4-HBC is 10-20 g / L, a conversion rate is high, two products, including 7alpha and 7beta-hydroxy-4-HBC are separated from the product, and the total yieldof the product is 46.5% to 85.32%. The method disclosed by the invention is convenient in operation, low in cost and high in yield, and has good economic value, and a new method is provided for the development and the synthesis of steroid drugs.
Owner:北京泛球生物科技有限公司
16alpha-methyl-3-hydroxyl-19-norpregnane-1, 3, 5-triene-20-ketone and preparation method thereof
InactiveCN101538302AHigh stereoselectivityHigh yieldOrganic active ingredientsSteroidsEstroneMda mb 231
The invention relates to 16alpha-methyl-3-hydroxyl-19-norpregnane-1, 3, 5-triene-20-ketone and a preparation method thereof. In the invention, degraded product of natural diosgenine petunidin 3-acetoxyl group-pregnane-5, 16-diene-20-ketone is used as the raw material, the raw material is methylated by aluminum methide before hydrolization, 1-dehydrogenation and A aromaticcyclizationofparaflins to prepare 16alpha-methyl estrone steroid compound 16alpha-methyl-3-hydroxyl-19-norpregnane-1, 3, 5-triene-20-ketone. The compound has activity suppression effect for breast cancer cellbeads MDA-MB-231, and the preparation method is characterized by easily available raw material and high yield.
Owner:EAST CHINA NORMAL UNIV
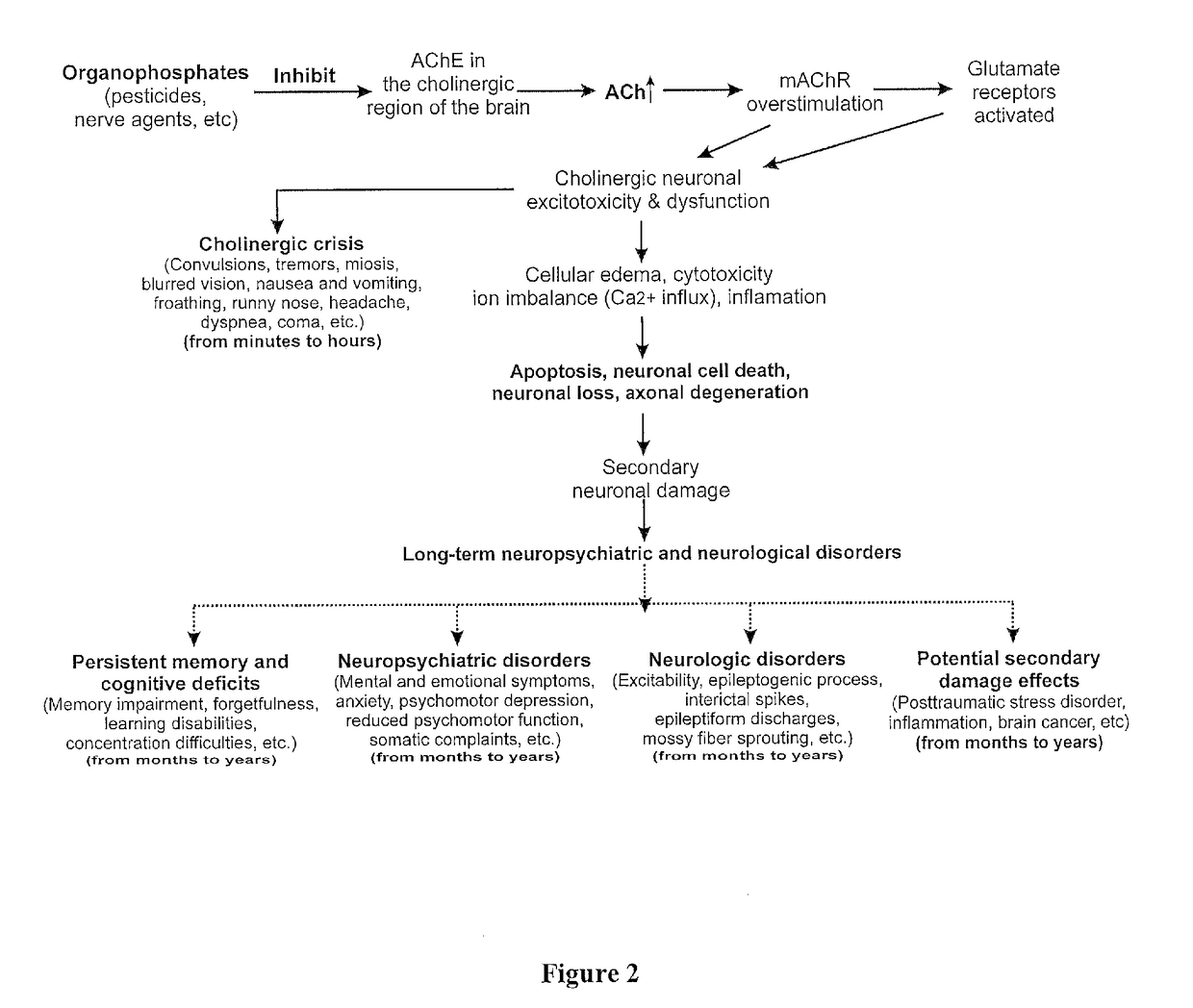
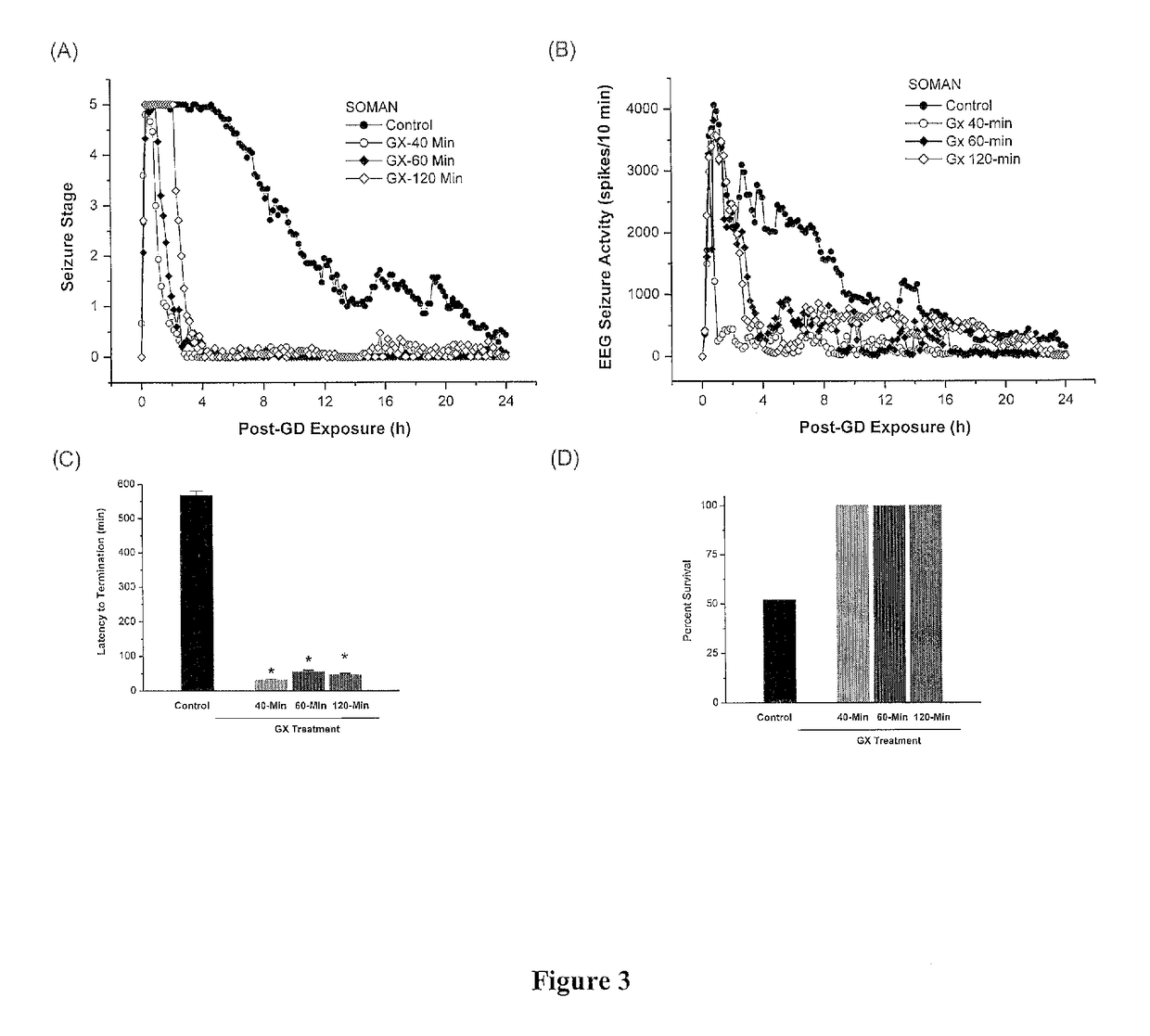
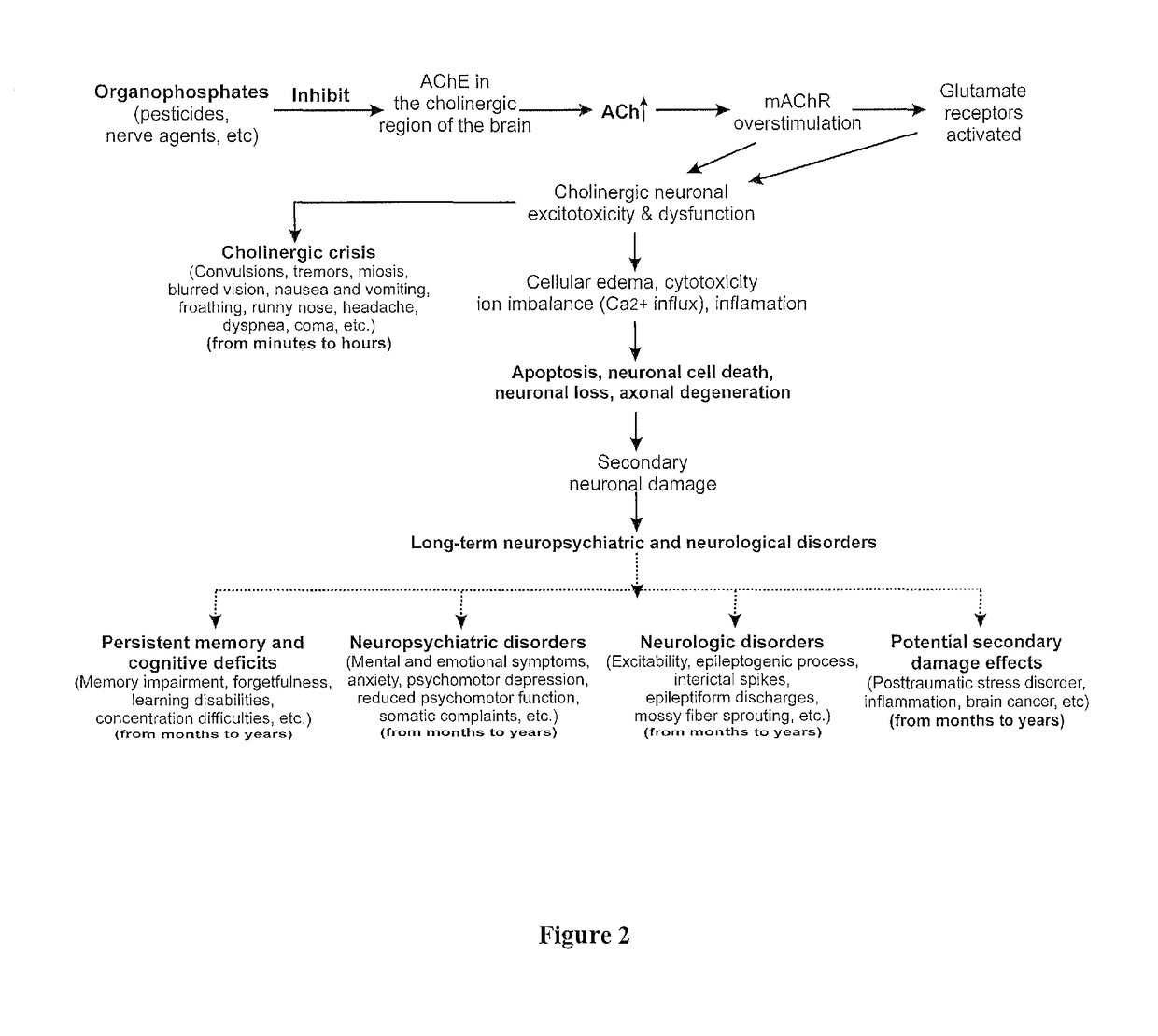
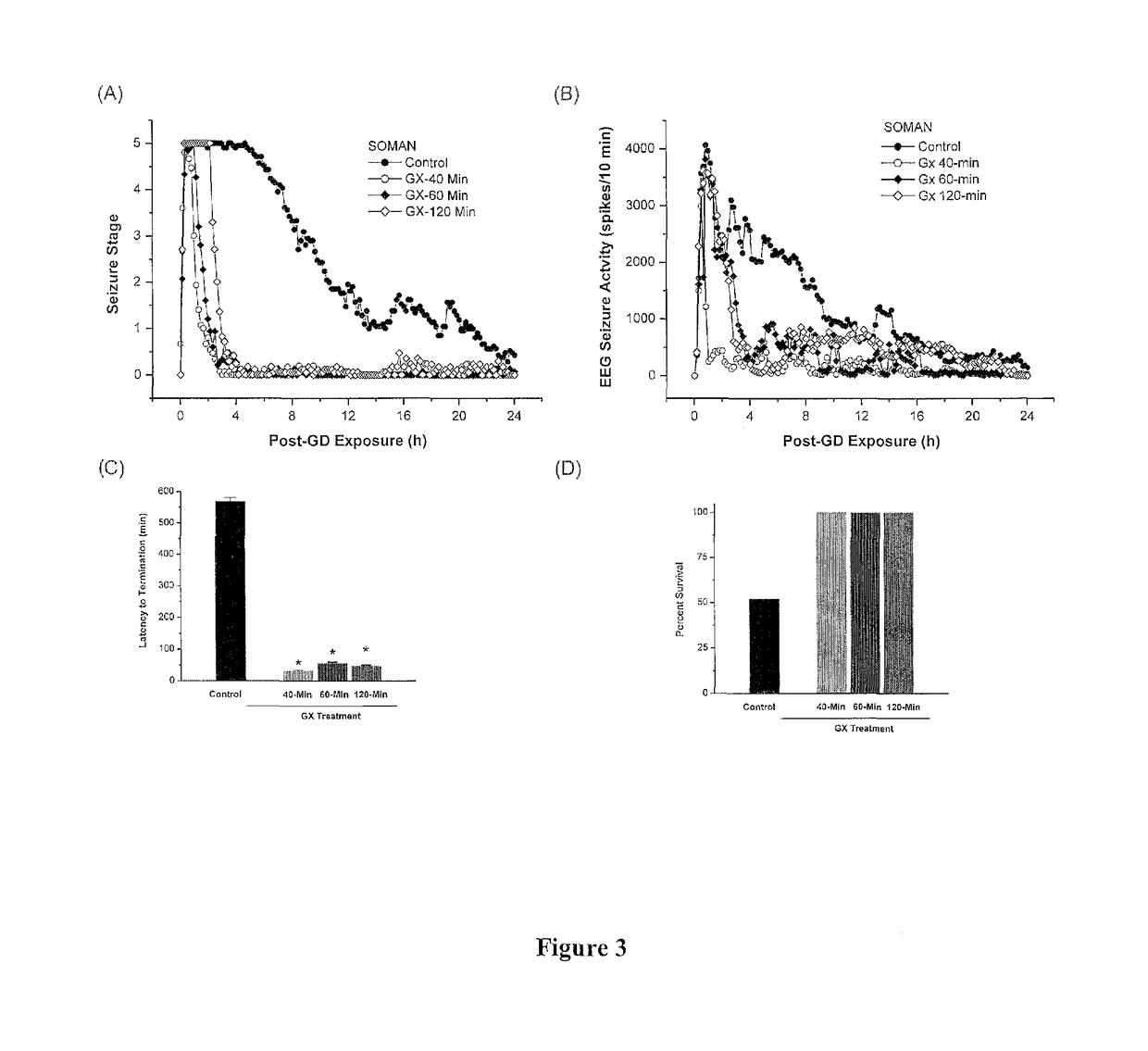
Method of treating organophosphate intoxication
ActiveUS20170246188A1Effective post-exposure treatmentPrevents subsequent neuronal injuryOrganic active ingredientsNervous disorderBenzodiazepineAntidote
The present invention provides new compositions and methods for treating and / or reversing organophosphate intoxication, manifested by both cholinergic and non-cholinergic crisis, in a mammal resulting from exposure to organophosphate compounds. The neurosteroidal compounds of this invention are those having the general structural formula of pregnane, androstane, 19-norandrostanes, and norpregnane with further moieties as defined herein. These compounds include, but are not limited to, ganaxolone, pregnanolone, and androstanediol and their analogs, salts and prodrugs. The present invention further relates to combining a therapeutically effective amount of a neurosteroidal compound with a standard organophosphate antidote (e.g. atropine, pralidoxime). The data suggests that neurosteroids are effective or more effective than benzodiazepines, whether given earlier or later than 40-min (up to several hours) after organophosphate compound exposure. Neurosteroids are effective to attenuate long-term neuropsychiatric deficits caused by organophosphate exposure.
Owner:TEXAS A&M UNIVERSITY
Process for preparing 6 alpha-methyl hydroprednisone
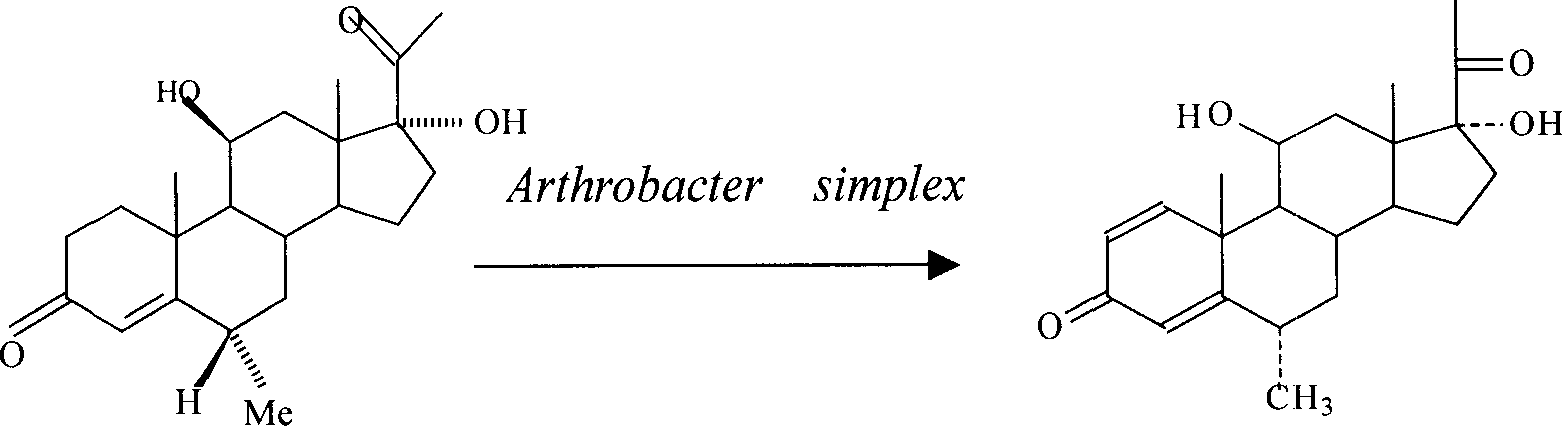
InactiveCN1966711AHigh purityRaw materials are easy to getMicroorganism based processesFermentationDiketoneVitamin K3
The invention provides a method for preparing 6alpha-medrol (1,4-diene-6alpha-methyl-3,20-diketone-11 beta,17alpha-dihydroxyl pregnane), and is characterized in the following method: adopting 4-alkene-6alpha-methyl-3,20-diketone-11 beta,17alpha-dihydroxyl pregnane as the substrate, fermentation enzyme solution obtained from the fermentation of Arthrobacter simplex as enzyme source, adding 0.01-0.08g / 100mL fermentation enzyme solution vitamin K3, carrying out a converting reaction at 25-35DEG C for 36-60h till complete reaction, separating and purifying to obtain the final product1,4-diene-6alpha-methyl-3,20-diketone-11 beta,17alpha-dihydroxyl pregnane. The beneficial effects of the biological preparation method of 1,4-diene-6alpha-methyl-3,20-diketone-11 beta,17alpha-dihydroxyl pregnane is mainly reflected in: (1) wide raw materials sources, and low cost, (2) good selectivity, simple process, industrial production, (3) mild reaction conditions, easy separation, highly pure product.
Owner:ZHEJIANG UNIV OF TECH
Pregnane alkaloid, and preparation method and application thereof to treatment of tumor
The present invention belongs to the pharmaceutical field, relates to a monomer compound containing pregnane alkaloid and separation preparation and application thereof to the treatment of tumor. The invention conducts systematic study on the chemical constituents and pharmacological effects of sarcococca and its affinis plant pachysandra axillaris belonging to Pachysandra, and obtains a pregnane alkaloid and a monomer compound thereof through separation. Pharmacological tests show that the obtained compound has good anti-tumor activity. The pregnane alkaloid monomer compound and combination thereof can be applied to preparation of antineoplastic drugs in the forms of a carrier or an excipient for systemic or topical administration, and can be used for the treatment of colon cancer, lung cancer, brain cancer and breast cancer.
Owner:FUDAN UNIV +2
Pregnane steroids for use in the treatment of cns disorders
Steroid compounds prossessing a hydrogen donor in 3beta position, either in the form of a hydroxy- or a sulfate group, function as efficient blockers of the 3alpha-hydroxy-pregnan-steroid action and thus have utility as therapeutic substances for the prevention and / or treatment of steroid related CNS disorders. Treatment methods based on the administration of these substances are disclosed, and these substances either alone or in combination are also suggested for the manufacture of pharmaceuticals for the treatment of many specific steroid induced CNS disorders.
Owner:UMECRINE AB
Process for obtaining steroidal phosphate compounds
ActiveCN101742987AHigh purityPromote degradationOrganic active ingredientsAntipyreticCompound aDisodium phosphate
A process for obtaining 21-disodium phosphate pregnane derivative compounds of formula (I), wherein X=R=H or X=F and R=alpha-CH3 or X=F and R=beta-CH3: comprises spray drying a solution comprising compound of formula (I).
Owner:HOVION ASSOC LTD
Method of treating organophosphate intoxication by administration of neurosteroids
ActiveUS10172870B2Effective post-exposure treatmentReverses organophosphate intoxication more effectivelyOrganic active ingredientsNervous disorderBenzodiazepineAntidote
The present invention provides new compositions and methods for treating and / or reversing organophosphate intoxication, manifested by both cholinergic and non-cholinergic crisis, in a mammal resulting from exposure to organophosphate compounds. The neurosteroidal compounds of this invention are those having the general structural formula of pregnane, androstane, 19-norandrostanes, and norpregnane with further moieties as defined herein. These compounds include, but are not limited to, ganaxolone, pregnanolone, and androstanediol and their analogs, salts and prodrugs. The present invention further relates to combining a therapeutically effective amount of a neurosteroidal compound with a standard organophosphate antidote (e.g. atropine, pralidoxime). The data suggests that neurosteroids are effective or more effective than benzodiazepines, whether given earlier or later than 40-min (up to several hours) after organophosphate compound exposure. Neurosteroids are effective to attenuate long-term neuropsychiatric deficits caused by organophosphate exposure.
Owner:TEXAS A&M UNIVERSITY
Method for synthesizing lithocholic acid by taking BA as raw material
The invention discloses a lithocholic acid synthesis method, which comprises the following steps: by using 21-hydroxy-20-methyl pregnane-4-ene-3-ketone (BA) as a raw material, carrying out an oxidation reaction and a Wittig reaction, and carrying out a reduction reaction to synthesize lithocholic acid; or after the oxidation reaction and the Wittig reaction, carrying out a hydrogenation reduction reaction, a carbonyl reduction reaction and a hydrolysis reaction to synthesize the lithocholic acid. The lithocholic acid synthesis method has the advantages of environmental protection, simple steps, few side reactions, high yield, cheap and easily available raw materials, and suitableness for industrial production; the method solves the problems of high synthesis cost, low yield and unsuitability for large-scale industrial production in the prior art.
Owner:JIANGSU JIAERKE PHARMA GRP CORP +1
Treatment of tumours
InactiveUS20070111973A1Good curative effectSquelching unwanted PPARγ-activityOrganic active ingredientsBiocideDiseaseAndrostane
The present invention refers to steroid derivatives for use as medicaments. More specifically, the invention also relates to the use of a steroid derivative of 5-androstene-, 5-pregnenolone or corresponding saturated derivatives (androstane- or pregnane-) in the manufacture of a medicament for the treatment of a benign and / or malignant tumour, which medicament is capable of interrupting disturbances in Wut-signaling, such as cell-cycle arrest in G1-phase, and / or providing an angiostatic effect. Examples of such steroid derivatives are -5-androstene-17-ol, androstane-17-ol-pregnane-17-ol or pregnane-17-ol derivatives. In a further aspect, the invention relates to a method of producing a medicament for the treatment of a benign and / or malignant tumour and / or an inflammatory condition comprising the steps of contacting 5-androstane-3B,17-dio 1 or androstane-3B-diol, an enzyme and a sulfotransferase to provide 5-androstene-17-ol-3B-sulfate or corresponding andros tane derivative (17-AEDS or 17-AADS); and mixing the 17-AEDS or 17-AADS so produced with a suitable carrier; whereby a medicament which is capable of acting as a ligand to peroxisome proliferators-activated receptor—(PPAR) is produced.
Owner:HAGSTROM TOMAS +2
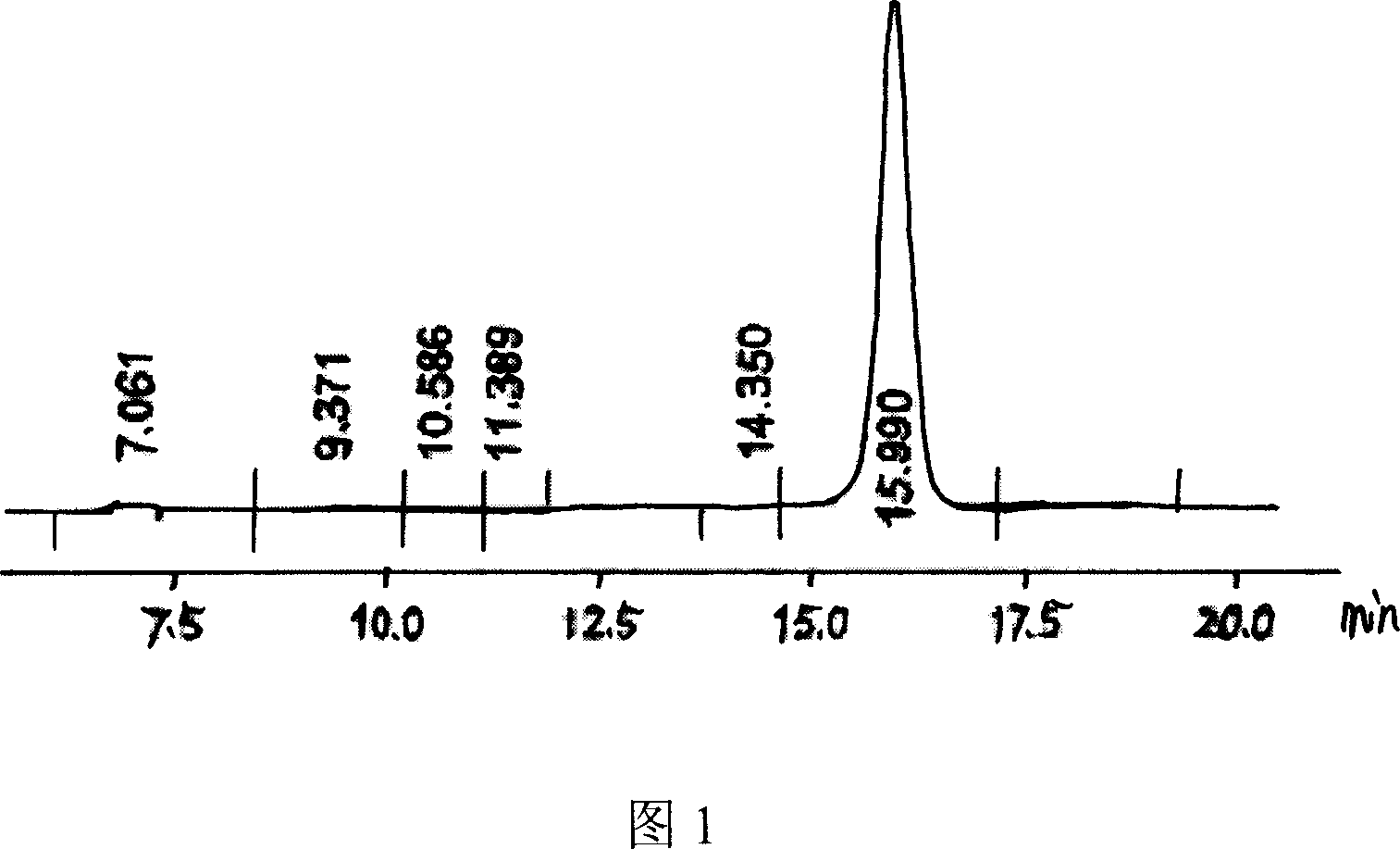
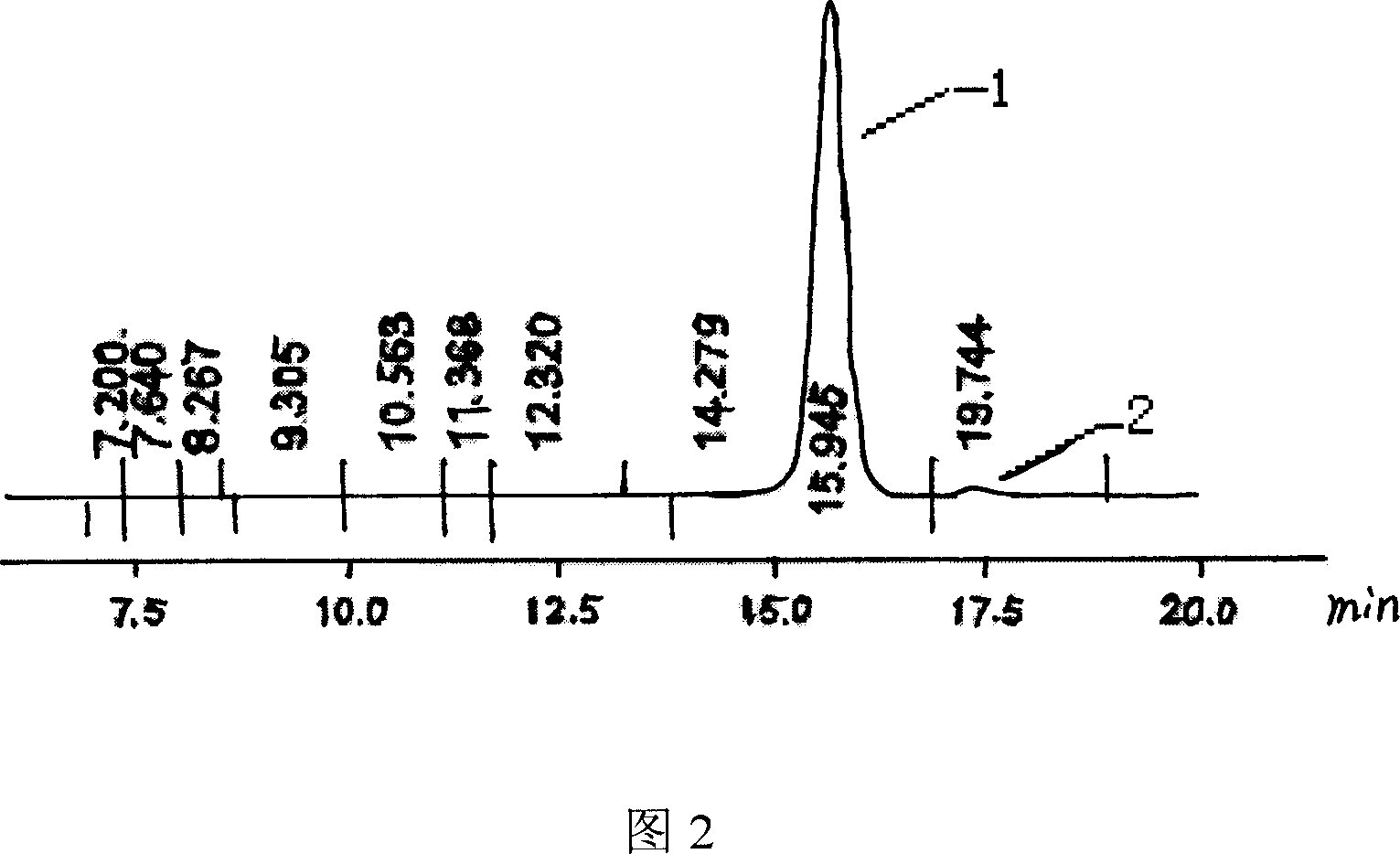
Preparation method for [17alpha, 16alpha-d] methyl oxazoline
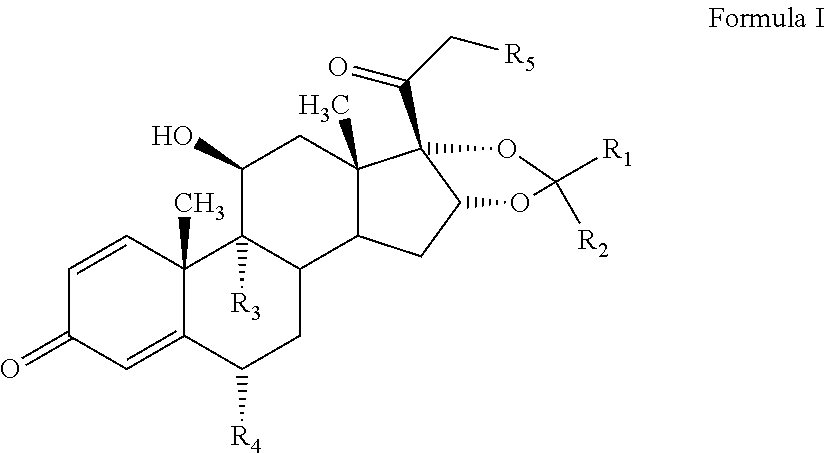
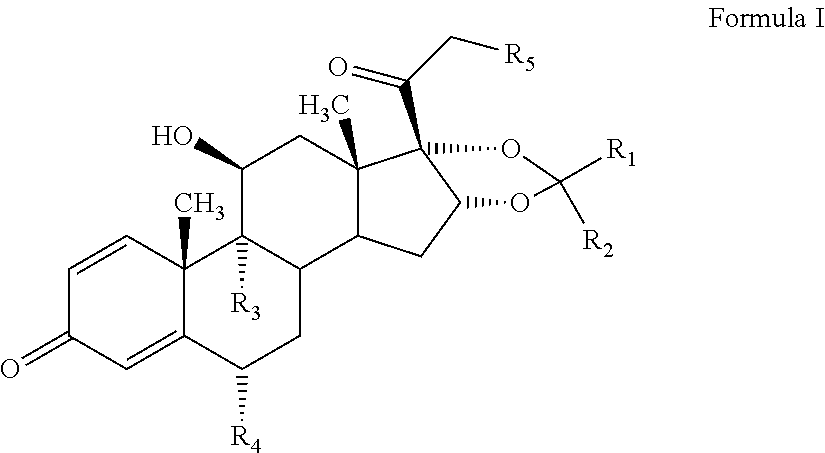
The invention discloses a preparation method for a denazacort key intermediate [17alpha, 16alpha-d] methyl oxazoline steroids. The steps include: dissolving [16, 17alpha-epoxy-pregnane-20-methyl formate hydrazine acetyl-1,4-diene-3,11-diketone] in trichloromethane, adding [16, 17alpha-epoxy-pregnane-20-methyl formate hydrazine acetyl-1,4-diene-3,11-diketone] and additives into a pressure reaction kettle, leading ammonia to the reaction kettle to a certain pressure under the condition of stirring, and reacting at certain temperature; and dissolving the obtained compound crude products in glacial acetic acid, adding a certain amount of anhydride under the condition of stirring, and controlling reaction temperature. After the reaction, reaction liquid is poured into 500mL of cold 10% sodium hydroxide solution and stirred for 1 hours, and the denazacort key intermediate [17alpha, 16alpha-d] methyl oxazoline steroids is obtained after suction filtration. The method achieves safe and clean production of denazacort, is favorable for reducing environment pollution, shortens a production period, reduces production cost for enterprises, improves production safety, and is remarkable in social benefit, environment benefit and economical benefit.
Owner:JIANGXI JUNYE BIOLOGICAL PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





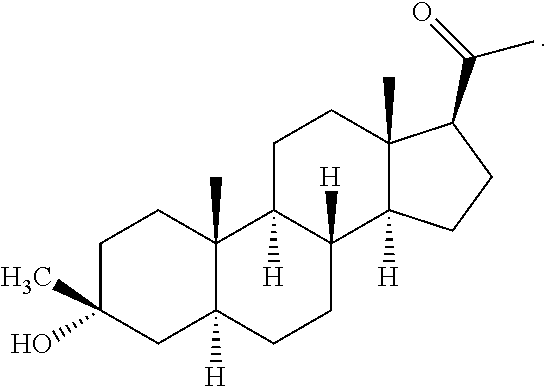

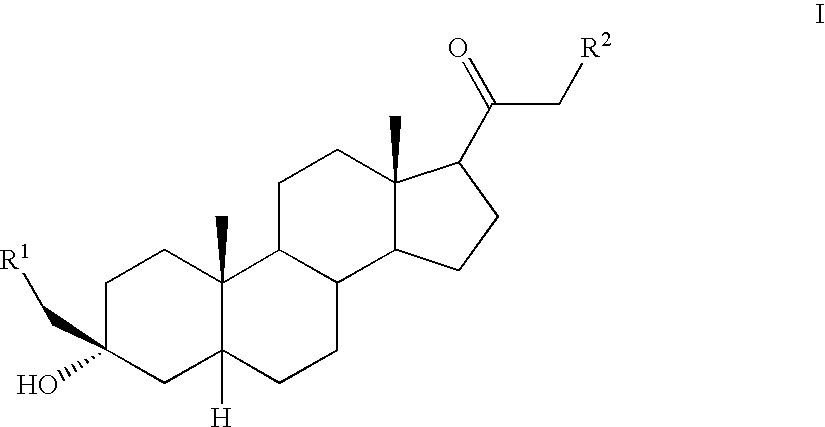
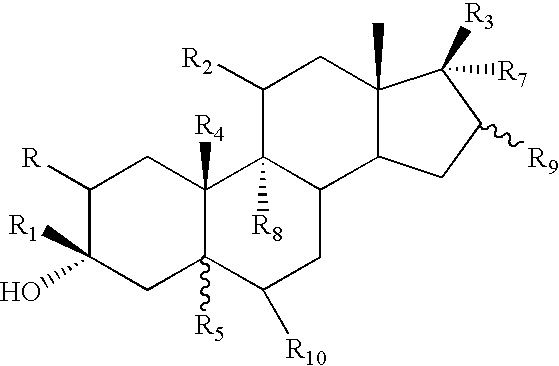
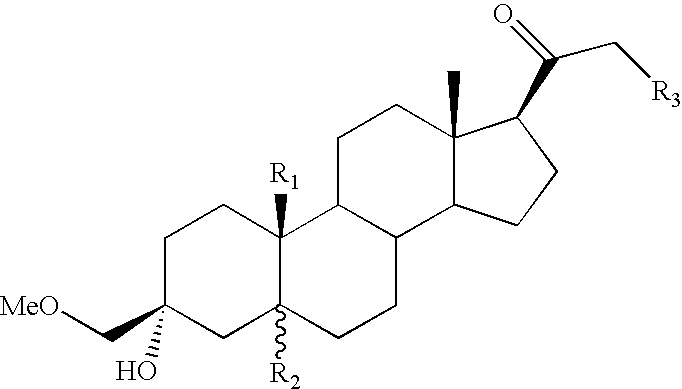



















![Preparation method for [17alpha, 16alpha-d] methyl oxazoline Preparation method for [17alpha, 16alpha-d] methyl oxazoline](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28dd9628-42fb-4f2b-802e-1bfb286190a9/88406DEST_PATH_IMAGE008.PNG)
![Preparation method for [17alpha, 16alpha-d] methyl oxazoline Preparation method for [17alpha, 16alpha-d] methyl oxazoline](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28dd9628-42fb-4f2b-802e-1bfb286190a9/899868DEST_PATH_IMAGE001.PNG)