Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
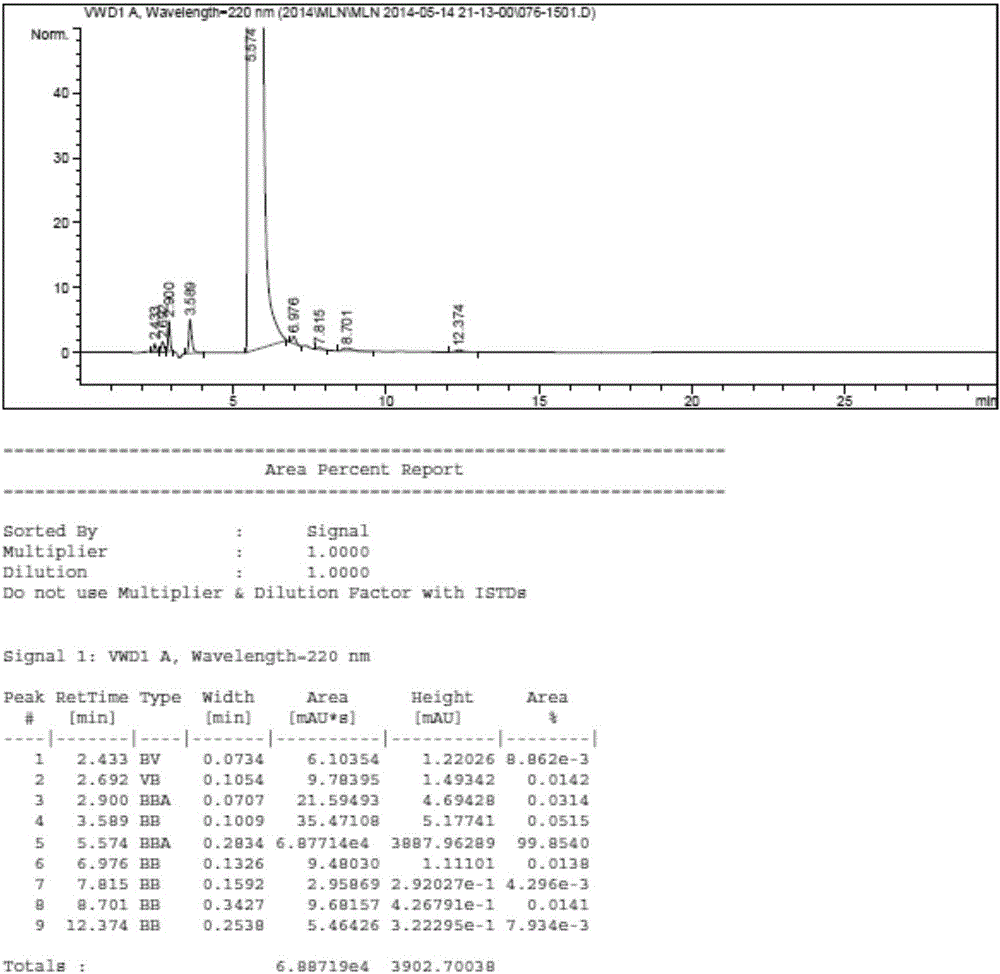
231 results about "Triethyl orthoformate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Triethyl orthoformate is an organic compound with the formula HC(OC₂H₅)₃. It is a colorless volatile liquid. It is orthoester of formic acid. It is commercially available. The industrial synthesis is from hydrogen cyanide and ethanol.
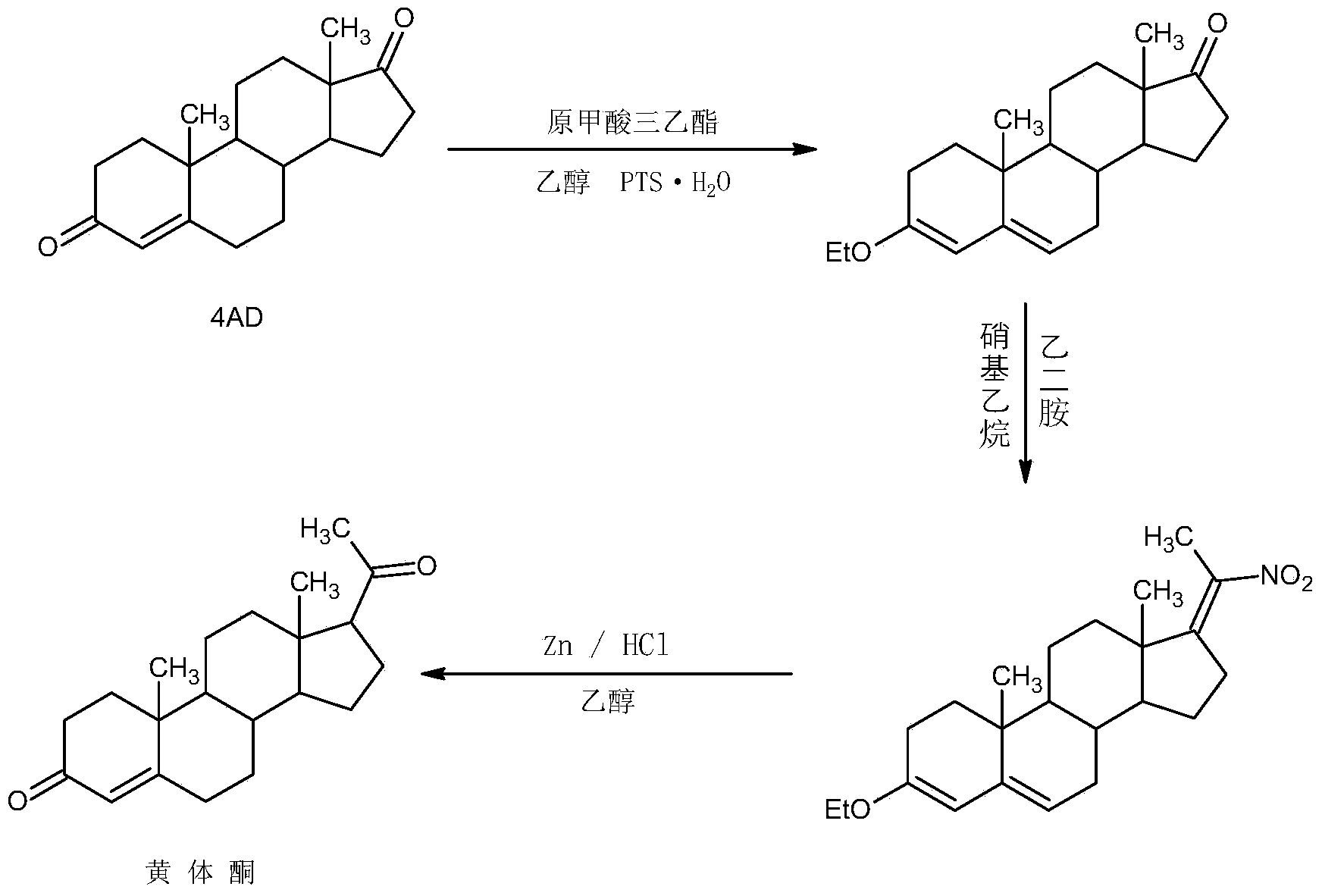
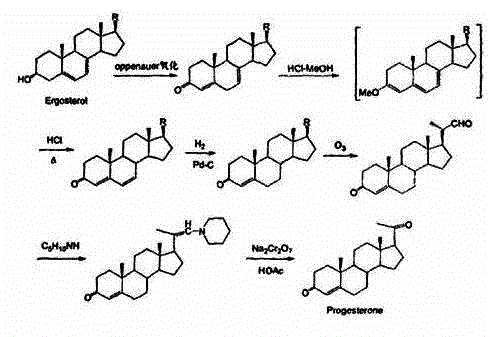
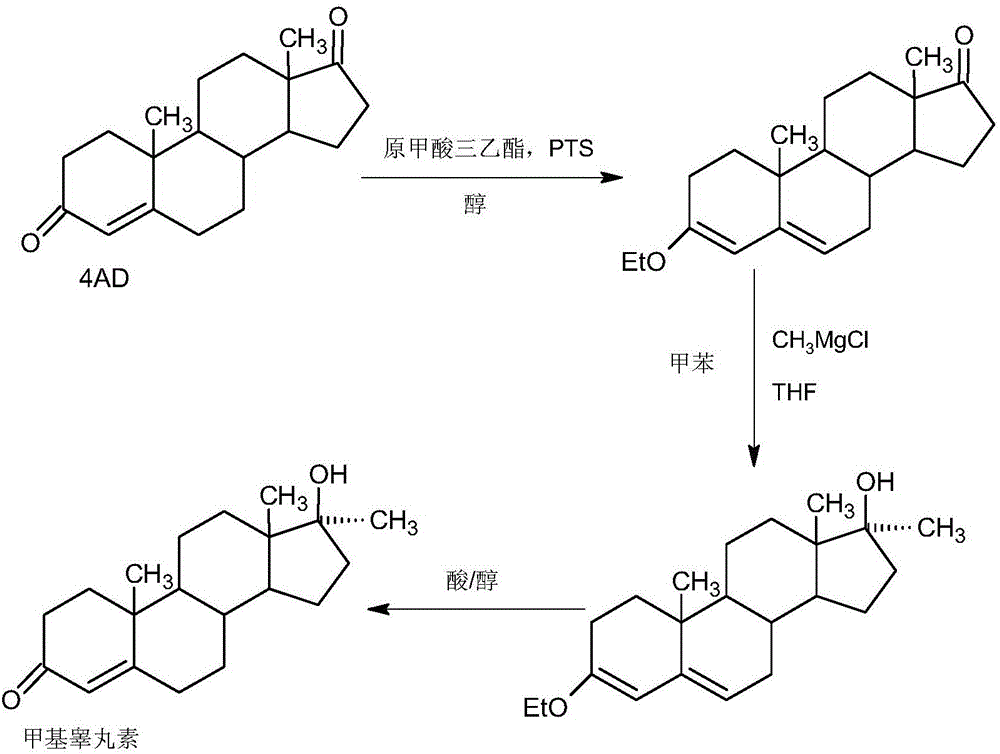
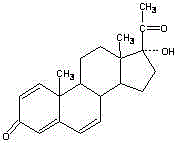
Synthesis method of 17alpha-hydroxyl progesterone
The invention discloses a synthesis method of 17alpha-hydroxyl progesterone, which comprises the steps of by taking 4-androstene-diketone as a starting raw material, carrying out vyanation via acetone cyanohydrin, protecting 3carbonyl by using triethyl orthoformate and ethyl alcohol, protecting 17hydroxy by using butyl vinyl ether, and carrying out hydrolysis after Grignard reaction to generate the 17alpha-hydroxyl progesterone. According to the synthesis method, the cost is reduced, the environment pollution is decreased, the reaction time is shortened, the aftertreatment process of the industrial production is simplified, the production time and cost are greatly saved, the productivity is improved and convenience is brought to the industrial implementation. Compared with the traditional process, the synthesis method has the characteristics of low raw material cost, simple and convenient method, high yield, good selectivity, mild reaction condition, small pollution and applicability to industrial production; and the method is stable and easy to realize.
Owner:ZHEJIANG PURUI PHARMA
Method for synthesizing cholesterol by using stigmasterol degradation products as raw materials
The invention provides a method for synthesizing cholesterol by using stigmasterol degradation products as raw materials. The method comprises the following steps: 1) performing an etherification reaction on 3-carbonyl-4-pregnene-22-aldehyde and triethyl orthoformate to obtain 3-ethyoxyl-3,5-pregnadiene-22-aldehyde; 2) preparing a 3-methylbutyltriphenyl phosphonium chloride solution; 3) adding potassium tert-butoxide into the 3-methylbutyltriphenyl phosphonium chloride solution, performing a wittig reaction on the 3-methylbutyltriphenyl phosphonium chloride solution and the 3-ethyoxyl-3,5-pregnadiene-22-aldehyde to obtain 3-ethyoxyl-3,5,22-triene cholestane; 4) catalyzing the 3-ethyoxyl-3,5,22-triene cholestane to perform a selective hydrogenation reaction to obtain 3-ethyoxyl-5-ene cholestane; 5) performing a reaction on the 3-ethyoxyl-5-ene cholestane and acetic anhydride to obtain 3-acetyl-5-ene cholestane; 6) performing a hydrolysis reaction on the 3-acetyl-5-ene cholestane to obtain the cholesterol. The synthesizing method is simple in process, and the mole yield of the cholesterol exceeds 85 percent by using the stigmasterol degradation products which are cheap and easily obtained as the raw materials; the production cost is low, the process is environmentally friendly, and the method is economical and environmentally friendly, and facilitates industrial implementation.
Owner:HUNAN KEREY BIOTECH
Environment-friendly siloxane-terminated polyurethane sealant and preparation method thereof
ActiveCN107216845ANo seepageNo pollutionNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesSealantMechanical property
The invention discloses an environment-friendly siloxane-terminated polyurethane sealant and a preparation method thereof. First, a NCO-terminated polyurethane prepolymer is prepared, hydroxypropyl siloxane is synthesized, the NCO-terminated polyurethane prepolymer is put in a reaction kettle, a silane coupling agent and the obtained hydroxypropyl methyldiethoxysilane are added into the reaction kettle for heating to 50 to 70 DEG C for reacting for 2-3H; NCO content is tested, and when the NCO content is close to zero, a small molecule volatile capping agent is added, and stirring is performed; triethyl orthoformate is added and stirred after cooling to room temperature, and a siloxane-terminated polyurethane prepolymer is discharged; a plasticizer and an inorganic filler are added, mixed and cooled to room temperature, and an additive and a catalyst are added, and mixed under vacuum conditions; and the obtained environment-friendly siloxane-terminated polyurethane sealant has good stability, can be fast cured at room temperature to obtain an elastic sealant with good mechanical properties, has a very good adhesion effect on glass, metal and other matrixes without a primer.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method for progestin
ActiveCN104262442AWide variety of sourcesProcess economy and environmental protectionSteroidsEthylenediamineKetone
The invention relates to a preparation method for progestin. 4-androstenedione is used as a raw material. The preparation method comprises the following steps: A, etherate is synthetized, wherein the 4-androstenedione and triethyl orthoformate perform an acid catalyzed reaction in organic solvents of dichloromethane, low-carbon alcohol and the like to obtain the etherate 3-ethoxy-androstane-3, 5-diolefin-17-ketone; B, a nitro substance is synthetized, wherein the etherate in the organic solvents and nitroethane perform 17-bit addition under the catalysis of ethylenediamine to obtain the nitro substance 3-ethoxy-20-nitro-pregnane-3, 5, 17 (20)-triene; and C, the progestin is synthetized, wherein the nitro substance is reduced by zinc powder in organic solvents of acetic acids, low-carbon alcohol and the like, acid hydrolysis is performed, so that semi-finished products of the progestin are obtained, the semi-finished products of the progestin are decolored and refined by alcohol and activated carbon to obtain the progestin, the content of HPLC is more than 99.5%, the melting point is 128-131 DEG C, and the total yield of synthetized weight is 83-87%. When the method disclosed by the invention is used for producing the progestin, the yield is high, the degree of purity is good, the quality is stable, the solvent recovering rate is high, and the method is economic and environment-friendly.
Owner:HUNAN KEREY BIOTECH
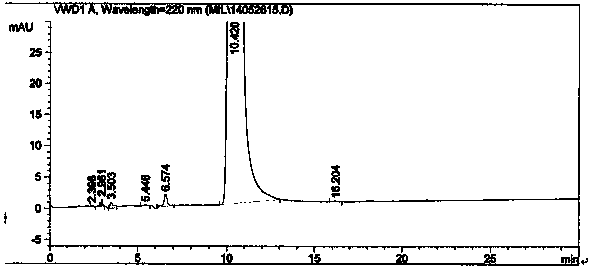
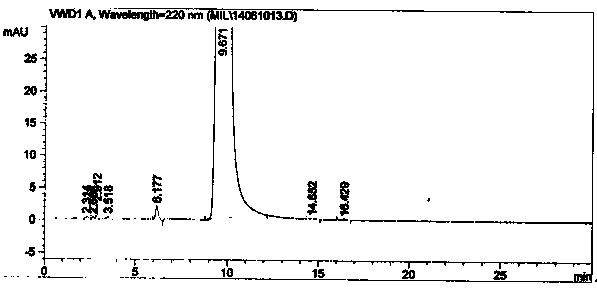
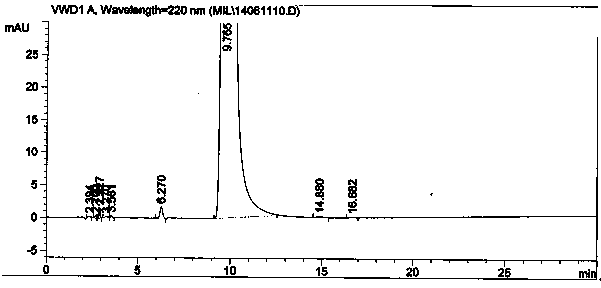
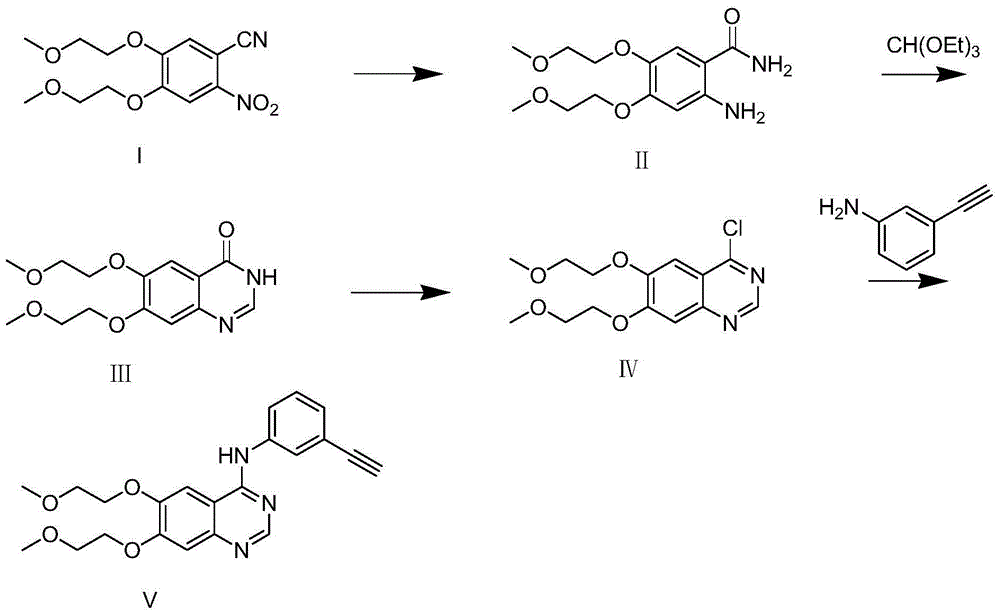
Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib
The invention discloses a method for synthesizing an important intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib. The method comprises the following steps: step one, condensing malononitrile and triethyl orthoformate and then carrying out a one-pot reaction with hydrazine hydrate to obtain 5-amino-4-cyano-pyrazole; step two, condensing the 5-amino-4-cyano-pyrazole with formamide to prepare a compound of formula (II) 4-amino-1H-pyrazolo[3,4-d]pyrimidine; step three, carrying out a bromination reaction of the compound of formula (II) and brominating agent to obtain a compound of formula (III) 4-amino-3-bromine-1H-pyrazolo[3,4-d]pyrimidine; step four, carrying out a Stille reaction on the compound of formula (III) and trimethyl p-phenoxy phenyltin under the catalyst effect of metal palladium to prepare a compound of formula (I) 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine. According to the method for synthesizing the important intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib provided by the invention, raw materials are low in price and easily obtained, the reaction conditions are moderate, the operation is simple and convenient, the method is suitable for industrial production, and a new way is provided for preparing Ibrutinib and intermediate.
Owner:HUAIHAI INST OF TECH
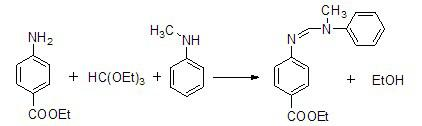
Method for preparing N-(4-ethyoxylcarbonylphenyl)-N'-methyl-N'-phenyl carbonamidine
The invention discloses a method for preparing N-(4-ethyoxylcarbonylphenyl)-N'-methyl-N'-phenyl carbonamidine. The method comprises the following steps of: (1) adding N-methylaniline, ethyl p-aminobenzoate and triethyl orthoformate into a reaction device, stirring and heating up to 80-90 DEG C; (2) adding propionic acid or glacial acetic acid used as a catalyst into the reaction device, meanwhile, evaporating out generated alcohol, and reacting for 1-3 hours at a temperature of 80-90 DEG C; (3) heating up to 100-110 DEG C, meanwhile, reducing the pressure to 1-20mmHg, continuously reacting until no alcohol is evaporated out; and (4) after the reaction ends, reducing the pressure and distilling to obtain the N-(4-ethyoxylcarbonylphenyl)-N'-methyl-N'-phenyl carbonamidine. The method has the advantages of simple process, moderate condition and high productivity and is suitable for large-scale industrial production.
Owner:大连新阳光材料科技有限公司 +1
Preparation method of 6a-methyl hydrocortisone
InactiveCN106518945AWide variety of sourcesProcess economy and environmental protectionSteroidsSolventMethyl group
The invention provides a preparation method of 6a-methyl hydrocortisone. The preparation method comprises the steps that hydrocortisone prepared from 4-androstene-3,17-dione (called as 4AD for short) is adopted as a raw material to generate an acid catalytic reaction with triethyl orthoformate in an organic solvent, and etherate 3-ether enol hydrocortisone is obtained; the etherate generates a Manlixi reaction with N-methylaniline and formaldehyde in an organic solvent, and a methylene product 6-methylene hydrocortisone is obtained; the methylene product generates a catalytic hydrogenation reaction in an organic solvent, and 6a-methyl hydrocortisone is obtained. Compared with a production method achieved by taking a mold removal product obtained by processing diosgenin as a raw material, the method for producing 6a-methyl hydrocortisone has the advantages that raw material sources are wide, the processes are economical and environmentally friendly, production operation is easy and convenient, the synthetic route is short, and the product yield is high; by producing 6a-methyl hydrocortisone through the method, the production cost is reduced by 40%-50% compared with a traditional method; the solvents used in production can be recycled and cyclically reused, and implementation of industrialized production is promoted.
Owner:HUNAN KEREY BIOTECH
Method for preparing progesterone by taking 1,4-androstenedione as raw material
The invention discloses a method for preparing progesterone by taking 1,4-androstenedione as a raw material, which comprises the following steps: 1) dissolving 1,4-androstenedione into an organic solvent, adding the acid of trimethyl orthoformate or triethyl orthoformate, and introducing nitrogen to protect the 1,4-androstenedione to synthesize the enol ether of 1,4-androstenedione, namely 3-methoxy-androstane 3,5-diene-20-ketone; and 2) dispersing (1-methoxy ethyl)-triphenylphosphine salt in a reaction medium, an organic solvent, adding alkali at low temperature, performing a Wittig reaction of the 3-methoxy-androstane 3,5-diene-20-ketone synthesized in the step 1), and purifying and crystallizing to obtain progesterone. By adopting the 1,4-androstenedione as the raw material, the method solves the problem that of lack in raw materials for synthesizing steroid drugs such as progesterone, and improves the utilization rate of 1,4-androstenedione and the yield of progesterone; the preparation process is simple.
Owner:HUNAN KEYUAN BIO PRODS
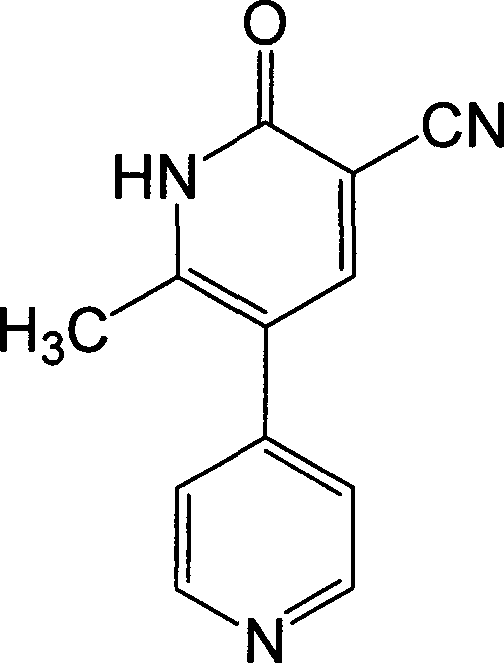
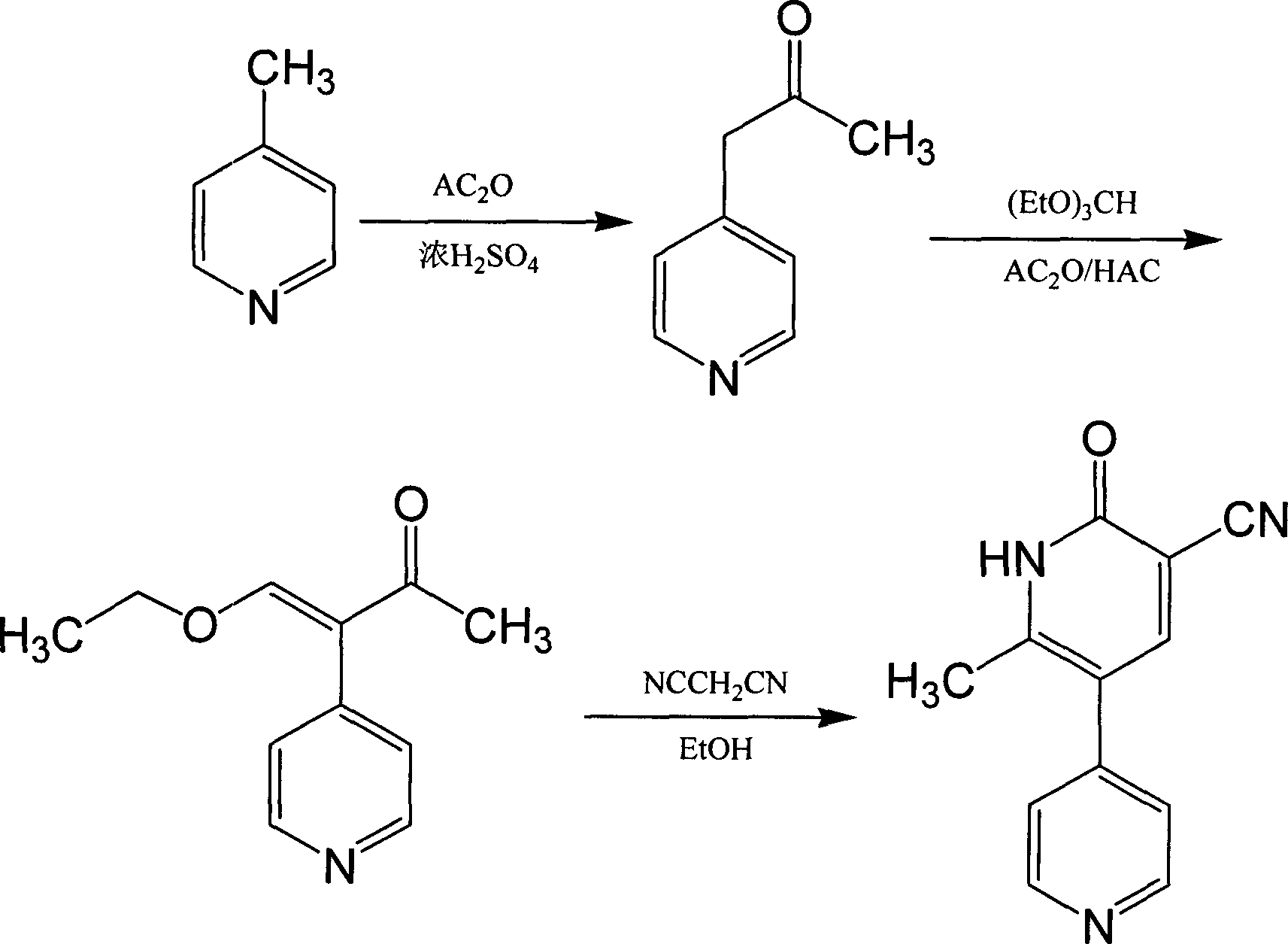
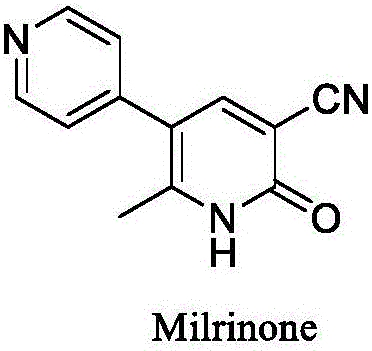
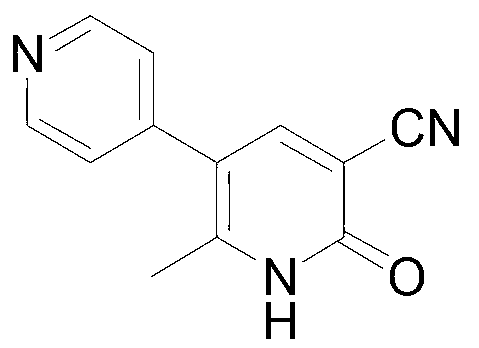
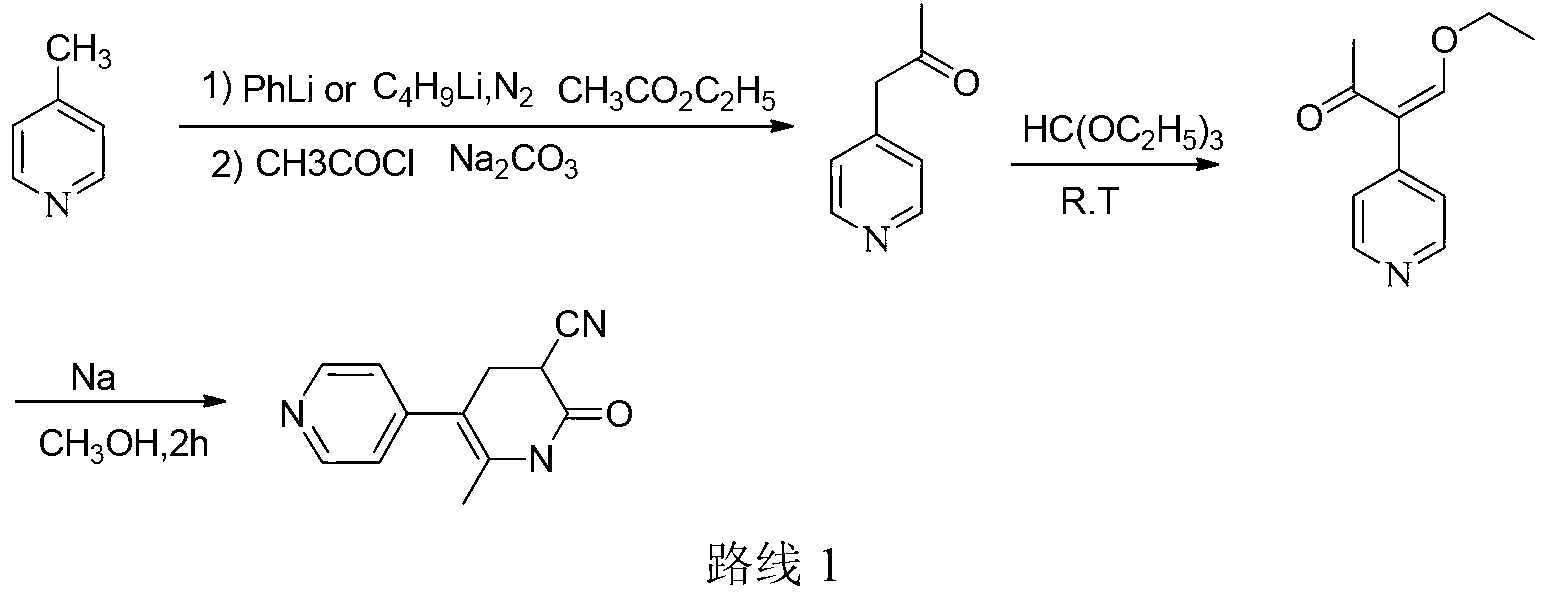
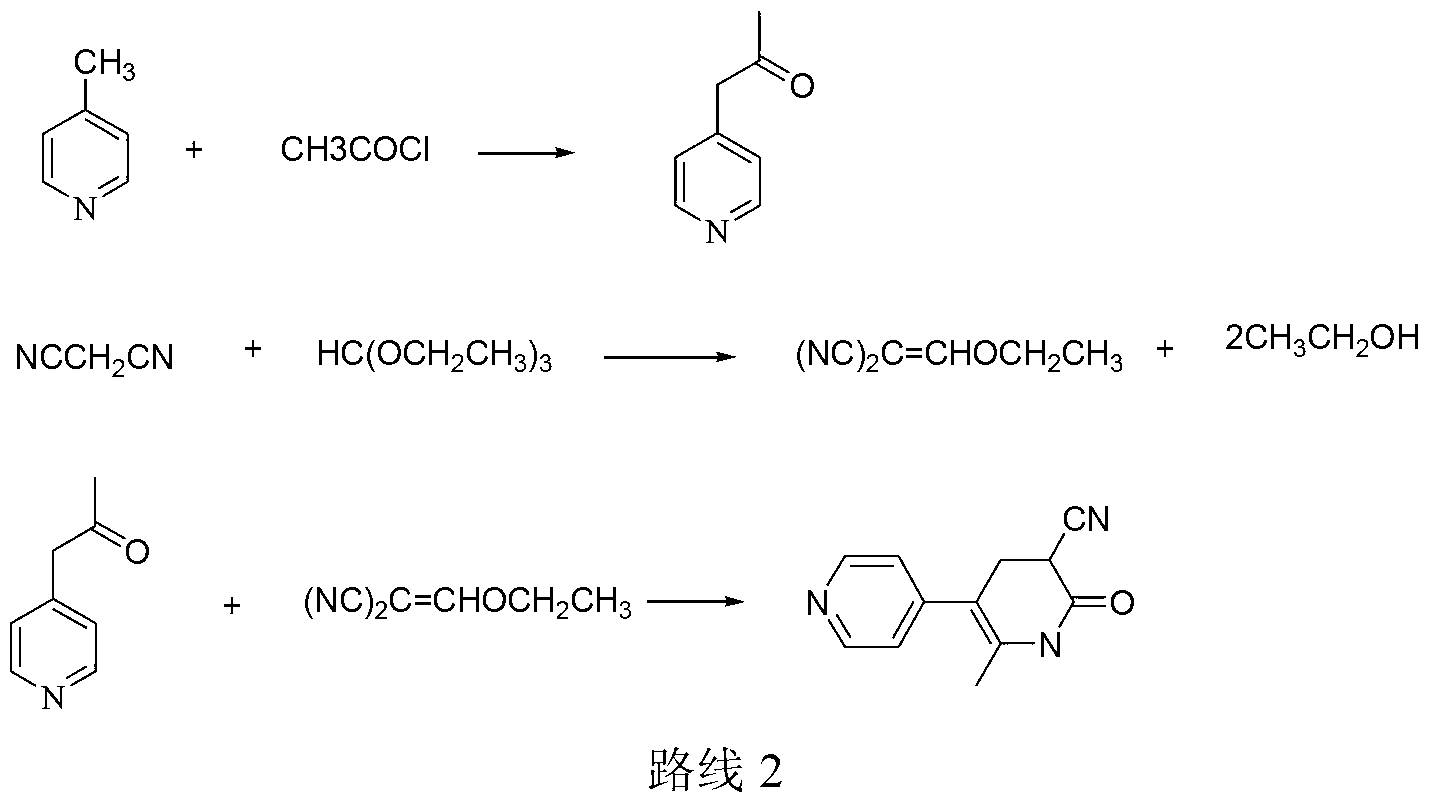
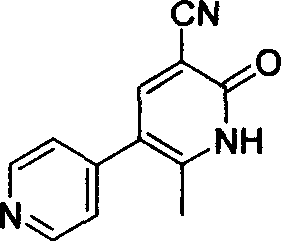
Method for synthesising milrinone
ActiveCN103288725ALow costThe number of times of extraction and decompression concentration is reducedOrganic chemistryAcetic anhydrideSodium bisulfate
The invention discloses a method for synthesising milrinone. The method comprises the following steps of: mixing 4-methylpyridine with acetylchloride in a solvent at a temperature below 10 DEG C; heating to react with or without a catalyst, and then adjusting the pH value of the reaction solution to 7-8 by sodium hydroxide aqueous solution; then directly adding saturated sodium hydrogen sulfite aqueous solution, and reacting; adjusting the pH value of the water layer obtained by the reaction by sodium hydroxide, and reacting to obtain a compound in formula (III); mixing the compound in formula (III) with glacial acetic acid, acetic anhydride and triethyl orthoformate, and then reacting at 50-100 DEG C to obtain a compound in formula (IV); cyclizing the compound in formula (IV) with alpha-cyanoacetamide in an alkaline condition to obtain a compound in formula (V). The process is more moderate in reaction conditions, simpler and more convenient to operate, and capable of greatly shortening the original reaction time, reducing cost and increasing yield simultaneously; the process is a preparation method for milrinone which is suitable for industrialized production.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Preparation method for high-purity milrinone
ActiveCN104387320AQuality improvementImprove crystal effectOrganic chemistryAcetic anhydrideSingle crystal
The invention discloses a preparation method for high-purity milrinone (shown as a formula (I), 1,6-dihydro-2-methyl-6-oxo-3,4-bipyridine-5-carbonitrile), and belongs to the field of chemical medicines. The method comprises: employing 4-methylpyridine as a raw material and acetylating with acetyl chloride, and hydrolyzing after the reaction is finished, so as to obtain a compound of a formula (III); mixing the compound of the formula (III) with glacial acetic acid, acetic anhydride and triethyl orthoformate, and reacting at 35 DEG C-45 DEG C, so as to obtain a compound of a formula (IV); performing cyclization on the compound of the formula (IV) and alpha-cyanoacetamide, so as to obtain a crude product of a compound of the formula (I); and refining the crude product of the formula (I) compound through an ethanol-water system, so as to obtain a high-purity refined product with the maximum interplanar spacing d of 8.39 + / - 0.02 Angstrom. The technology is relatively mild in reaction conditions and relatively simple in operation, and is capable of preparing the milrinone product with high purity and a single crystal form. The obtained milrinone crystal form is relatively excellent in solubility in normal saline or glucose, and is beneficial for improvement of the preparation quality.
Owner:HUZHOU ZHANWANG PHARMA
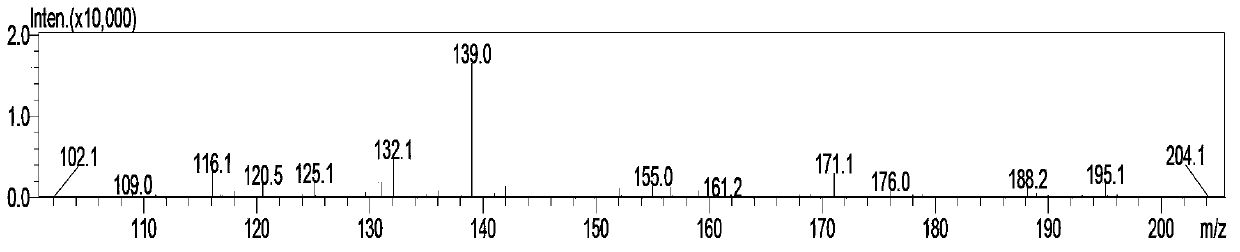
Simple and convenient preparation method of key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for vitamin B1
The invention relates to a simple and convenient preparation method of a key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for the vitamin B1. According to the method, lewis acid is used for catalyzing addition condensation reaction of acetamidine and 3-formyl amino ethyl cyanide and then catalyzing reaction of the condensation product and triethyl orthoformate to introduce a formyl chiral auxiliary, an imdo group and the formyl chiral auxiliary are subjected to ring formation to form 2- methyl-4-formyl amino-5- amino methyl pyrimidine, and finally basic hydrolysis is performed, so that 2-methyl-4-amino-5-amino methyl pyrimidine is obtained. The reaction procedures are carried out sequentially by adopting the one-pot method, the products in each step require no separation and purification, and the operation is simple and convenient. According to the method, highly toxic o-chloroaniline and other phenylamine compounds are not used, so that residue of o-chloroaniline compounds in the vitamin B1 product can be completely avoided. Meanwhile, the production wastewater is less and the yield is high.
Owner:XINFA PHARMA
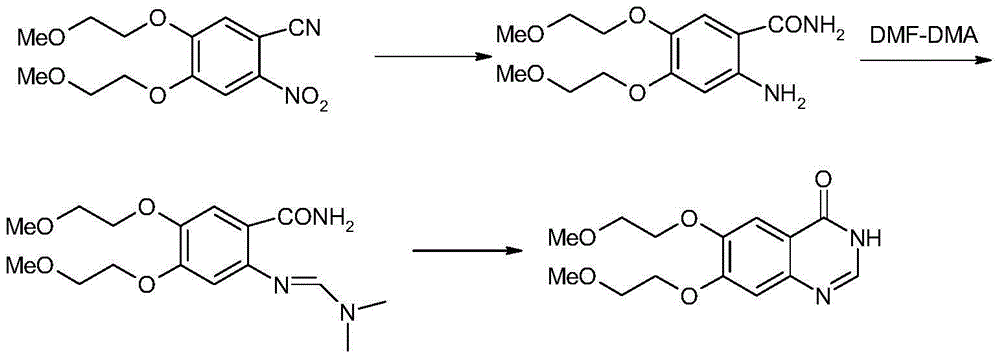
Preparation method of erlotinib
The invention discloses a preparation method of erlotinib and belongs to the technical field of medicament preparation. In the preparation method, 4,5-di(2-methoxylethoxy)-2-nitrobenzonitrile is used as a raw material and is subjected to reduction and hydrolysis to obtain an intermediate namely 2-amino-4,5-di(2-methoxylethoxy) benzamide which is subjected to cyclization with triethyl orthoformate directly to obtain an erlotinib key intermediate namely 6,7-di(2-methoxylethoxy)-3H-quinazoline-4-one, and a chlorinated product of quinazoline reacts with aminophenylacetylene to obtain erlotinib. By adopting the preparation method disclosed by the invention, the defects that an expensive catalyst is used by nitryl reduction in the conventional synthetic method and the temperature during cyclization is relatively high are overcome, a process with a formamidine intermediate is also avoided, the reaction step is shortened, the reaction cost is reduced, and the yield is improved. Moreover, all reaction conditions in the preparation method are very mild, so that the preparation method is particularly suitable for industrial production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
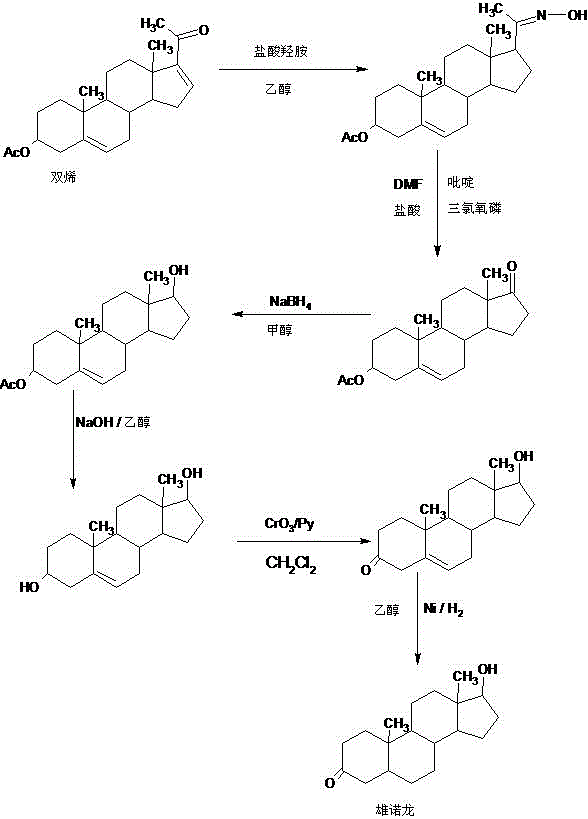
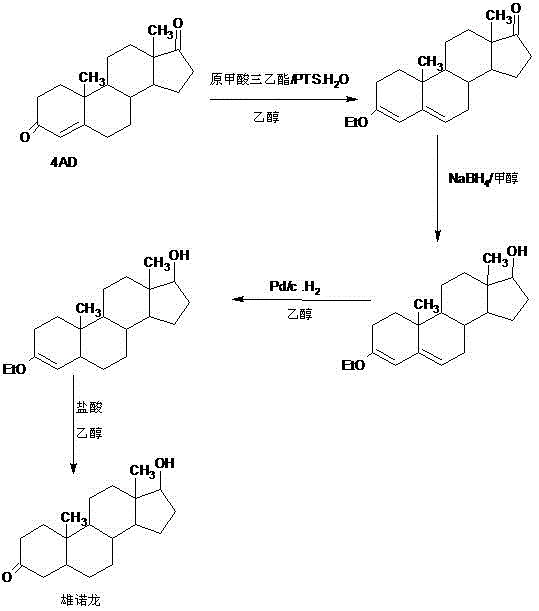
Preparation method of stanolone
InactiveCN106496297AWide variety of sourcesReduce manufacturing costEstrane derivativesDouble bondHydrolysis
A preparation method of stanolone comprises the following steps: with 4-androstenedione (shortened as 4AD) as a raw material, firstly performing an acid-catalyzed reaction on the 4AD and triethyl orthoformate in an organic solvent to obtain etherate 3-ethoxy-androst-3,5-diene-17-one; then, adding the etherate into the organic solvent, adding metal borohydride as a reducing agent, and reducing a 17-one group in a molecule of the etherate into a hydroxyl group to obtain the 3-ethoxy-androst-3,5-diene-17-ol; then, dissolving a reduced product into the organic solvent, adding a hydrogenation reaction catalyst, selectively catalyzing a 5-position double bond in the molecule of the reduced product to obtain hydride 3-ethoxy-androst-3-ene-17-ol; finally, performing acid-catalyzed hydrolysis on the hydride in the organic solvent to obtain the stanolone. Compared with the conventional production method, the preparation method provided by the invention has the advantages as follows: the raw material source is wide, the synthesis route is short, the preparation method is simple and environment-friendly in process, the product yield is high, the preparation method is economical and environment-friendly, the production cost is reduced by 35-40%, and the preparation method is very conductive to industrial production.
Owner:HUNAN KEREY BIOTECH
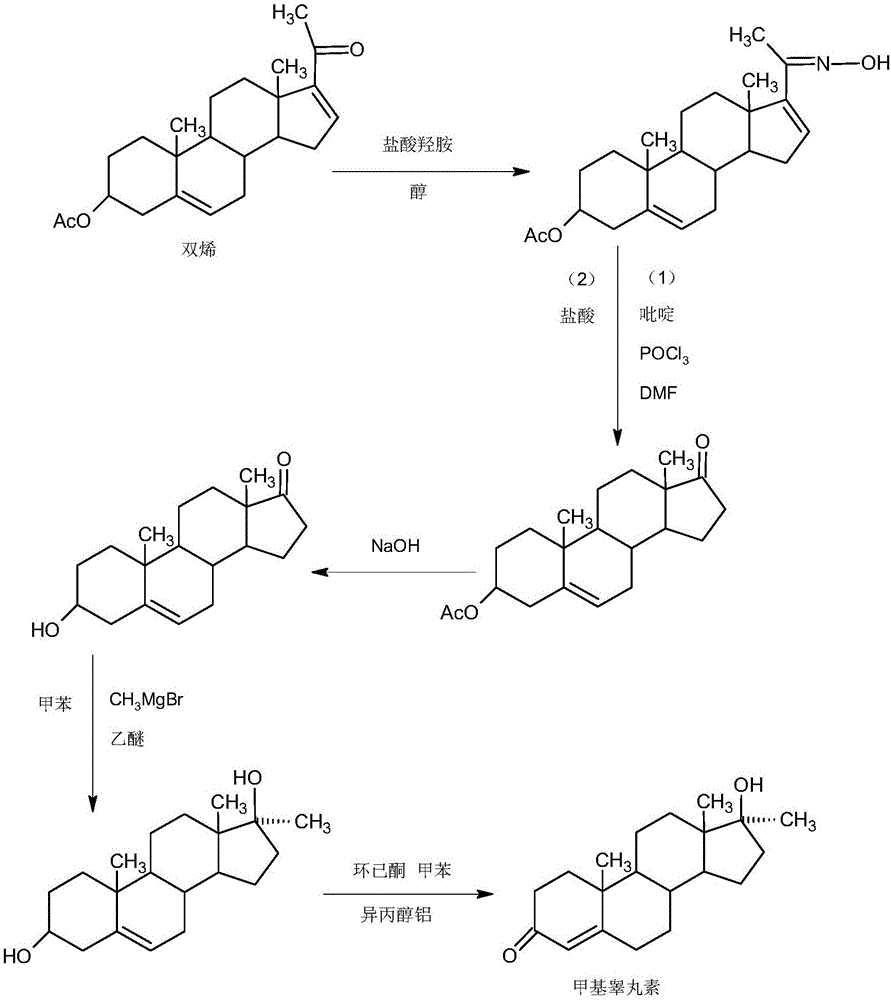
Preparation method of methyltestosterone
InactiveCN106397520AWide variety of sourcesProcess economy and environmental protectionSteroidsGrignard reagentKetone
The invention provides a preparation method of methyltestosterone. 4AD short for 4-androstenedione is taken as a raw material, and etherate is synthesized firstly as follows: 4AD and triethyl orthoformate are subjected to an acid catalyzed reaction in a low-carbon alcohol organic solvent, and 3-ethoxy-androst-3,5-diene-17-one as the etherate is obtained; then a Grignard product is synthesized as follows: a Grignard reagent methyl magnesium halide and the etherate are placed in an organic solvent, the 17-position ketone group of the etherate and the Grignard reagent are subjected to addition, and the Grignard product 3-ethoxy-17a-methyl-androst-3,5-diene-17-ol is obtained through hydrolysis; then the Grignard product is subjected to an acid catalyzed hydrolysis in an organic solvent, and crude methyltestosterone is obtained; the crude methyltestosterone is decolorized by activated carbon in C4-below low-carbon alcohol and recrystallized, the methyltestosterone is obtained, HPLC content is 99.0%-99.5%, and the total yield of synthesis weight is 75%-78%. According to the method, the raw materials are widely sourced, the process is simple and convenient to operate, the product yield is high, the purity is good, the solvent recovery rate is high in reaction and technological processing, and the method is economical and environment-friendly.
Owner:HUNAN KEREY BIOTECH
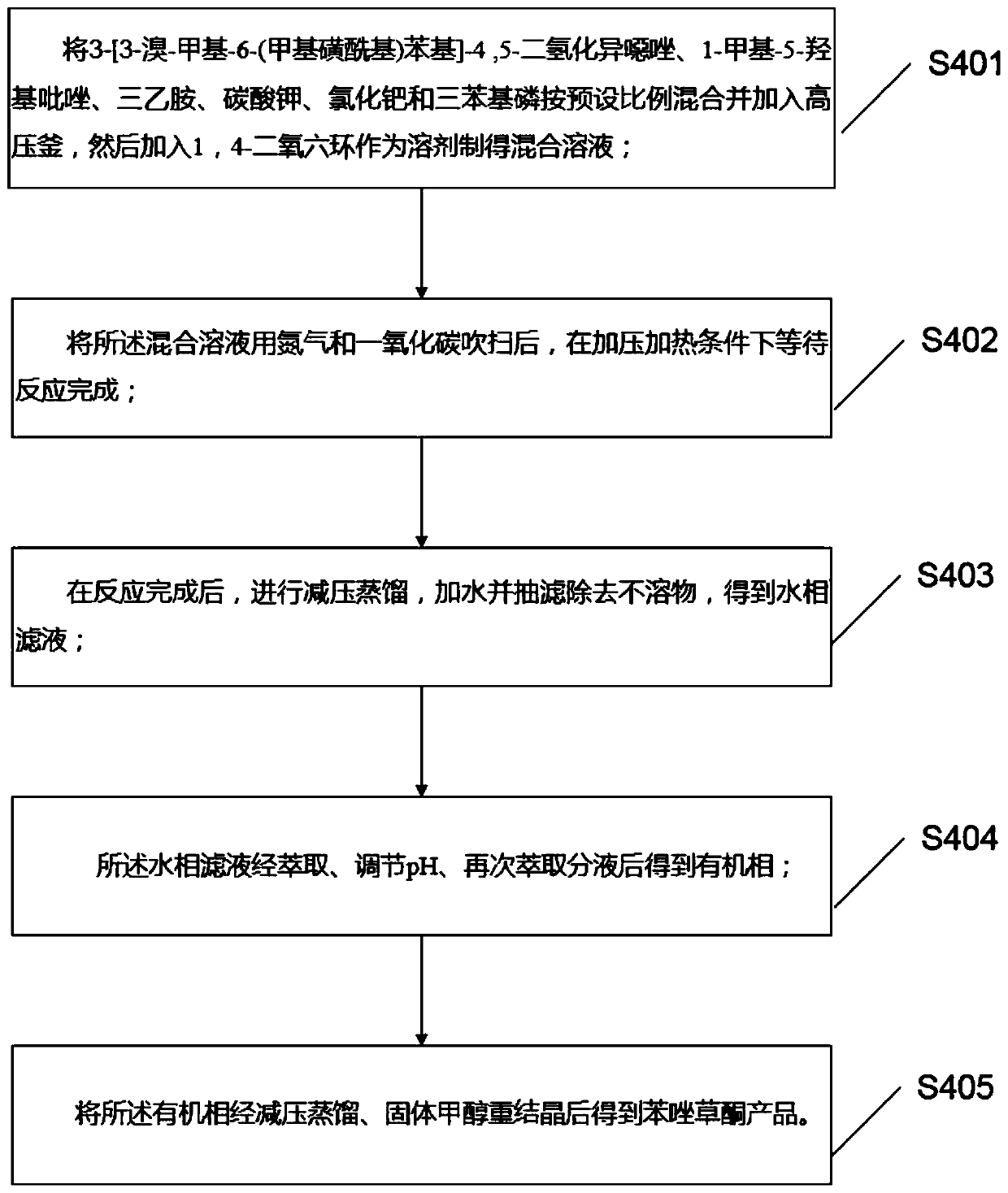
Preparation method and application of topramezone
PendingCN111440160AAvoid smelly problemsHigh yieldBiocideOrganic chemistryHydroxylamine HydrochloridePotassium carbonate
The invention discloses a preparation method and application of topramezone, and the preparation method comprises the following steps: taking 2-methylbenzaldehyde, a bromination reagent, a catalyst, hydroxylamine hydrochloride, an alkali, ethylene gas, a sulfonylation reagent and a preset solvent as reaction raw materials, and preparing 3-[3-bromo-methyl-6-(methylsulfonyl) phenyl]-4, 5-dihydroisoxazole through a first reaction process; taking diethyl malonate, triethyl orthoformate, nickel sulfate, monobasic saturated carboxylic acid, methylhydrazine, a hydrocarbon solvent, an ethanol solutionand hydrochloric acid as reaction raw materials, and carrying out a second reaction process to prepare 1-methyl-5-hydroxypyrazole; and taking the 3-[3-bromo-methyl-6-(methylsulfonyl) phenyl]-4, 5 dihydroisoxazole,-1-methyl-5-hydroxypyrazole, triethylamine, potassium carbonate, palladium chloride, triphenylphosphine, 1, 4-dioxane, water, a saturated NaHCO3 solution and a hydrochloric acid solutionas reaction raw materials, and carrying out a third reaction process to prepare the topramezone. The problems that a sulfur-containing intermediate can emit odor and the raw materials are difficult to obtain in the existing process are solved.
Owner:黑龙江省绥化农垦晨环生物制剂有限责任公司
Process for preparing milrinone
In accordance with the invention, 1-(4-pyridinyl)-propanone is used as raw material for condensation reaction with triethyl orthoformate, wherein the condensate directly reacts with the alpha-cyanoacetamide to obtain Milrinone without the need of purification. The prepared Milrinone is an important chemical product which can be used for preparing cardiotonic drugs.
Owner:LUNAN PHARMA GROUP CORPORATION
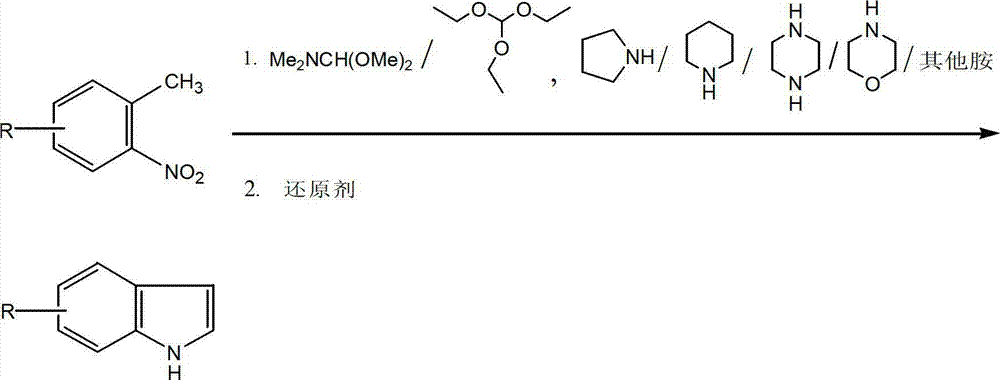
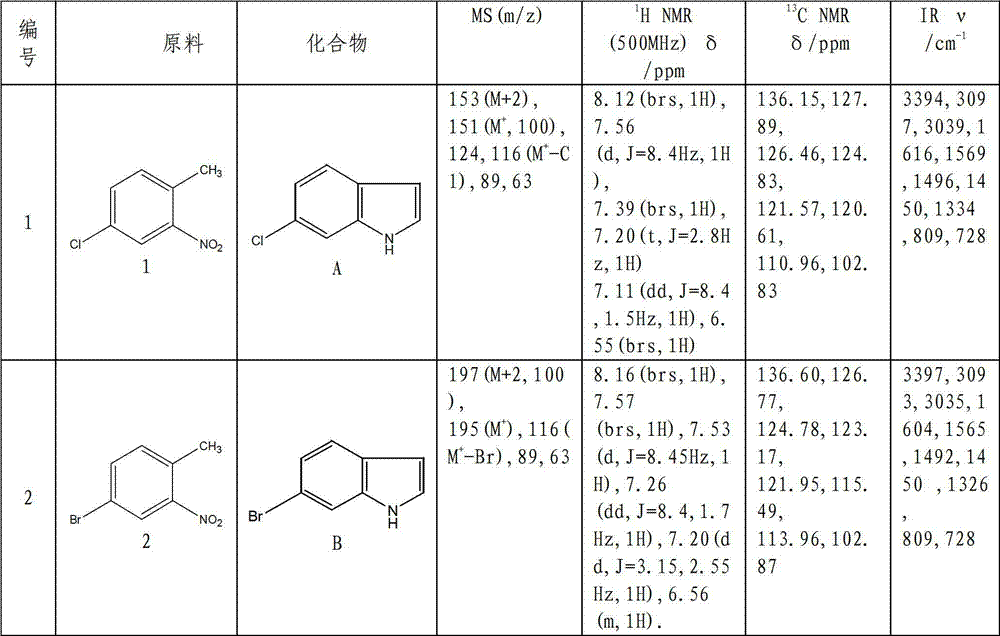
Method for synthesizing substituted indole compounds through one-pot method
The invention relates to a synthesis method of substituted indole compounds, and particularly relates to a method for synthesizing substituted indole compounds through a one-pot method. The method comprises the following steps: under alkaline and anaerobic conditions, reacting ortho-nitrotoluene derivatives and N,N-dimethylformamide dimethyl acetal or triethyl orthoformate used as raw materials in an organic solvent; and then, adding a reducer, and performing reduction and cyclization reaction to obtain indole derivatives, wherein R is a monosubstitution or polysubstitution located on site 4, 5, 6 or 7; and the R substituent is hydrogen, alkyl, substituted alkyl, alkoxy, amino or halogen atom. According to the invention, a one-pot method is adopted; the conventional and readily accessible ortho-nitrotoluene compounds are directly used as raw materials for reaction; separation and purification of intermediate compounds are not required; and the indole derivatives can be synthesized through the one-pot method by effectively controlling the reaction conditions, the charging sequence and the charging ratio. According to the invention, the technological operation procedure is simplified, the reaction time is shortened, the cost is saved, the total yield is improved, and better production and practical values can be achieved.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
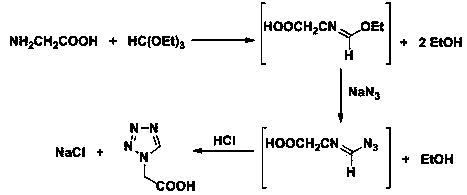
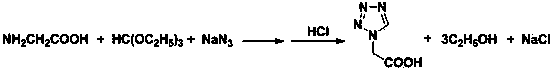
Post-processing method for preparing 1H-tetrazole-1-acetic acid through triethyl orthoformate method
ActiveCN103724288AGuaranteed water phase crystallizationHigh recovery rateOrganic chemistryTetrazoleOrganosolv
The invention discloses a post-processing method for preparing 1H-tetrazole-1-acetic acid through the triethyl orthoformate method by taking glycine, sodium azide and triethyl orthoformate as the raw materials. The method comprises the following steps: firstly, distilling a reaction liquid under the ordinary pressure gradient to sequentially recycle the by-product, namely, ethyl alcohol and acetic acid, a solvent; then adding excessive concentrated hydrochloric acid at the low temperature, and performing acidification and cyclization to remove sodium chloride; finally, performing activated carbon fading on the filter liquor, performing concentration to recycle hydrochloric acid, and cooling for crystallization to obtain 1H-tetrazole-1-acetic acid. The post-processing method has the advantages that the post-processing technology of ethyl acetate extraction, recrystallization of an organic solvent and the like of the traditional process is eliminated, and the purification strategy that a solvent is firstly recycled and then the acidification and salt removal are performed is created, so that the efficient separation and recovery of the by-product ethyl alcohol and the solvent acetic acid are realized, the use of the organic extraction reagent ethyl acetate is avoided, the crystallization and precipitation of the water phase of the 1H-tetrazole-1-acetic acid are ensured, the product purity reaches more than 99%, and the production cost is effectively lowered.
Owner:山东艾孚特科技有限公司
A kind of synthetic method of cyproterone acetate dehydrogenate
The invention provides a synthetic method of cyproterone acetate dehydrogenized substance, and belongs to the technical field of synthetic technologies of steroid agents. The synthetic method comprises the following processing steps of: 1) carrying out etherification reaction on 17 alpha-hydroxyl progesterone, absolute ethyl alcohol and triethyl orthoformate which serve as the raw materials, and carrying out acid-base neutralization after the reaction until reaching acidity; and 2) adding toluene to the reactant obtained in step 1) to be used as a reaction solvent; charging N2 for protecting; adding 2,3-fichloro-5,6-dicyano-1,4-benzoquinone to carry out dehydrogenation reaction; and sequentially filtering, concentrating and drying after the reaction, so as to obtain the cyproterone acetate dehydrogenized substance. According to the synthetic method, an efficient one-pot synthetic method is adopted, the double discharges in the conventional method are combined into one to be carried out, and 6s hydrogen ion in the raw materials is activated in the etherification process, so that the dual dehydrogenation reaction can be accomplished in one step, the product yield reaches 80 to 83%, the reaction period is shortened, the labor productivity is increased, the investment on equipment is decreased as well as the varieties and amount of used solvents, the environmental pollution is reduced, and the production cost of a plant is decreased.
Owner:ZHEJIANG XIANJU XIANLE PHARMA
Preparation method of one-class 1,3-dipole quinazoline compound
InactiveCN103172575AMild reaction conditionsLess side effectsOrganic chemistryChemical synthesisBenzaldehyde
The invention belongs to the technical field of organic chemical synthesis, and in particular relates to a preparation method of a novel 1,3-dipole quinazoline compound. The structure of the compound is represented and confirmed by using methods of 1HNMR, 13CNMR, IR, MS and the like. The novel 1,3-dipole quinazoline compound is prepared from a substituted benzaldoxime or a substituted benzylidene hydrazone compound and a triethyl orthoformate through cyclization reaction under the catalysis action of a lewis acid on mild conditions, wherein the substituted benzaldoxime or the substituted benzylidene hydrazone compound is prepared from a substituted benzaldehyde compound which has amino at the ortho-position. The preparation method for effectively preparing the 1,3-dipole quinazoline compound by using a serial method is mild in reaction condition, simple and convenient to operate, low in cost, less in side reaction, high in product purity, convenient to separate and purify, applicable to larger-scale preparation; and moreover the framework of the compound has broad biological activity and has good application prospect in pesticide and medicine research and development.
Owner:JIANGXI NORMAL UNIV
Preparation method for canrenone
The invention discloses a preparation method for canrenone. According to the preparation method, canrenone is obtained through an etherification reaction and a dehydrogenation reaction, wherein 17 beta-hydroxyl-4-alkene-3-ketone-17 alpha-pregnene-21-carboxylic acid-gamma-lactone is taken as a raw material; under the presence of a catalyst, the etherification reaction is carried out between 17 beta-hydroxyl-4-alkene-3-ketone-17 alpha-pregnene-21-carboxylic acid-gamma-lactone and triethyl orthoformate to generate 17 beta-hydroxyl-3,5-diene-3-ethoxy-17 alpha-pregnene-21-carboxylic acid-gamma-lactone; the catalyst is pyridine hydrobromide or pyridinium hydrochloride; under the presence of an organic solvent, the dehydrogenation reaction is carried out between an etherification reaction product and an oxidant to generate canrenone; the oxidant is tetrachloro-p-benzoquinone, tetrachloro-o-benzoquinone or 2,3-dichloro-5,6-dicyano-p-benzoquinone. Through the adoption of the preparation method, canrenone of which the purity is 99% or higher can be eventually obtained, and the total weight yield can reach 90% or higher. Therefore, the preparation method is suitable for industrialized production.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
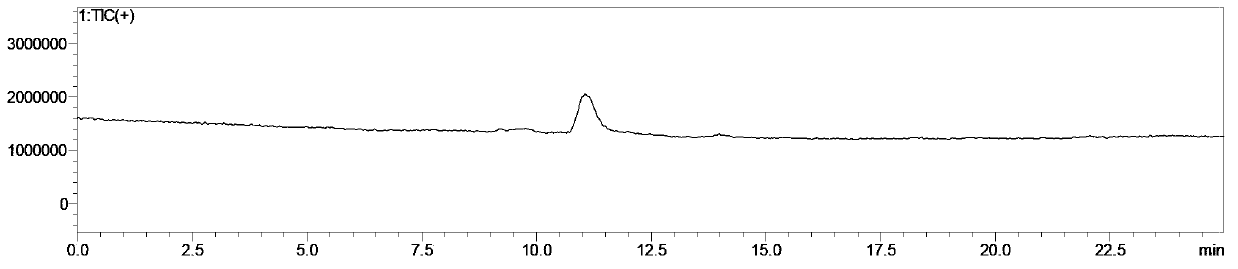
Method for preparing 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole
The invention relates to a method for preparing a herbicide isoxaflutole intermediate, namely, 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole. The method comprises the following steps: condensing 3-cyclopropyl-1-(2-methylthio-4-trifluoromethylphenyl)propyl-1,3-dione and triethyl orthoformate as starting materials to prepare 1-(4-trifluoromethyl-2-methylthio-phenyl)-3-cyclopropyl-2-ethoxymethylenepropane-1,3-dione (a compound II) and enabling the compound II to react with an aqueous solution of hydroxylamine hydrochloride and inorganic base in an organic solvent to prepare 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole (a compound I). The total yield of two steps is greater than 90.0%, the content of 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole is higher than the 98.0% and the content of impurity is less than 0.80%; and the preparation process has the advantages of simplicity in process, low cost, mild reaction conditions and less three wastes and is suitable for industrial production.
Owner:JIANGSU FLAG CHEM IND
Preparing method for pirfenidone
ActiveCN105330598ASimple and fast operationGood reaction selectivityOrganic chemistryAlkoxy groupAniline
The invention relates to a preparing method for pirfenidone. The method includes the steps that under the action of an acid catalyst, 2-pentenenitrile is used for reacting with trimethyl / triethyl orthoformate to generate 2-methyl-1-alkoxy-4-cyano-1,3-butadiene (II), and 2-methyl-1-alkoxy-4-cyano-1,3-butadiene (II) and aniline are condensed and then hydrolyzed to obtain pirfenidone. The used raw materials are low in price and easy to obtain, the process flow is short, and the preparing method is easy to operate, environmentally friendly, high in reaction selectivity and high in product yield and purity.
Owner:XINFA PHARMA
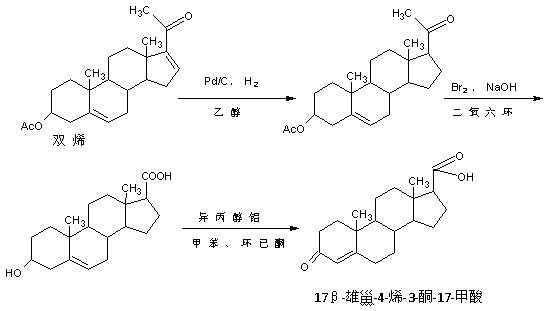
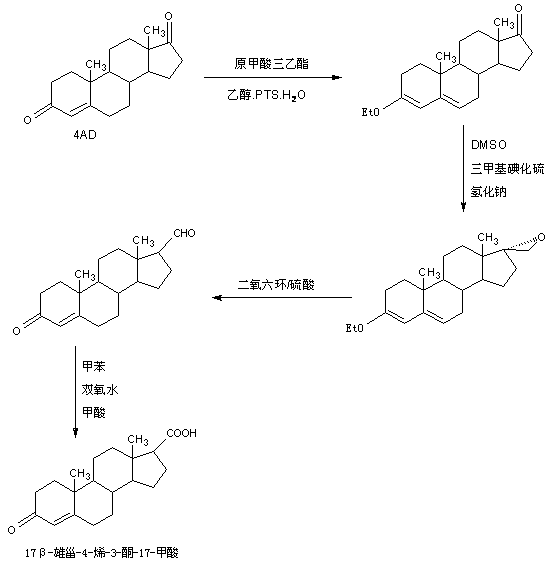
Preparation method of 17 beta-androst-4-ene-3-one-17-carboxylic acid
ActiveCN107629101ARaw materials are cheap and easy to getThe process is simple and environmentally friendlySteroidsCatalytic oxidationCarboxylic acid
The invention discloses a preparation method of 17 beta-androst-4-ene-3-one-17-carboxylic acid. The preparation method comprises that 4-androstenedione (called as 4AD for short) as a raw material andtriethyl orthoformate undergo a reaction in an organic solvent under acid catalysis, the reaction product is after-treated to form an etherate, the etherate is dissolved in an organic solvent and undergoes a reaction with trimethylsulfonium iodide under strong base catalysis, the product is after-treated to form an epoxide, the epoxide is dissolved in an organic solvent and then is subjected to rearrangement under acid catalysis to form an aldehyde, and aldehyde is dissolved in an organic solvent and undergoes a catalytic oxidation reaction with hydrogen peroxide acid to produce 17 beta-androst-4-ene-3-one-17-carboxylic acid. The 17 beta-androst-4-ene-3-one-17-carboxylic acid has a melting point of 244-246 DEG C, HPLC content of 99.0% or more and a reaction weight total yield of 70-72%. Compared with the traditional method, the preparation method utilizes cheap and easily available raw materials, has simple and environmental friendly processes and a high synthesis yield, produces highquality products, reduces a cost by 30-40% and is conducive to industrial production.
Owner:HUNAN KEREY BIOTECH
Synthesis method of milirinone
InactiveCN103804288AEasy to operateReduce pollutionOrganic chemistryChemical industry4-Methylpyridine
Belonging to the fields of chemical industry and chemical medicine, the invention discloses a synthesis method of milirinone. The method includes: adopting 4-methylpyridine as a raw material to undergo acetylation reaction with acetic anhydride, subjecting the acetylate to condensation with triethyl orthoformate directly without purification, and subjecting the condensation product and malononitrile to cyclization reaction in ethanol absolute, thus obtaining milirinone. The method is characterized by starting with relatively simple and easily available raw materials to directly obtain a complicated structure molecule without separation and purification of the intermediates. The milirinone synthesized by the method provided by the invention has the advantages of high yield and high sample purity. Milirinone is an important chemical product, and can be used as a cardiotonic drug and the like.
Owner:SICHUAN XINSIDUN PHARMA
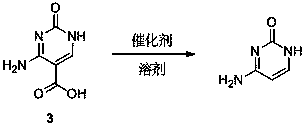
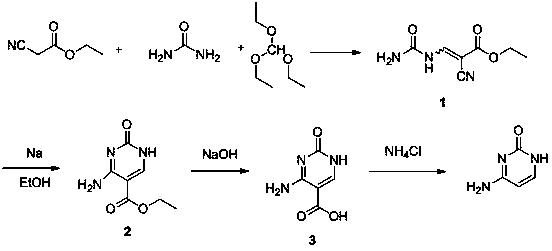
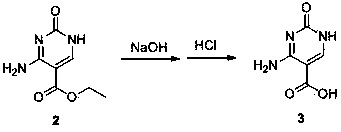
Synthesis method of cytosine
The invention discloses a synthesis method of cytosine. According to the method, ethyl cyanoacetate, urea and triethyl orthoformate are utilized as raw materials, ethyl 3-cyano-2-ureido-acrylate, 5-ethoxycarbonyl cytosine, and 5-carboxyl cytosine are sequentially synthesized, decarboxylation is performed to synthesize cytosine and refining, correction, perfection and other various process steps are sequentially performed. Therefore, the yield of the synthesis method is high, the highest total yield can achieve 75.14%, the comprehensive benefits are finally improved and the method has the advantages of simplicity in operation, small difficulty in actual production and convenience in large-scale application.
Owner:NANYANG NORMAL UNIV
Preparation method of danazol
The invention discloses preparation methods of danazol and an intermediate thereof. The preparation method of danazol is prepared by the steps of taking androstenedione as a starting raw material, and carrying out 3-site enol etherification, 17-site carbonyl ethinylation, 3-site hydrolysis, 2-site methylidynel hydroxylation and oximation to obtain danazol. The 3-site enol etherification comprises firstly carrying out a reaction of androstenedione and triethyl orthoformate for 4-10 h in the presence of absolute ethyl alcohol and p-toluenesulfonic acid and at the temperature of 30-50 DEG C, then adding triethylamine at the temperature of 0-10 DEG C, and continuing to carry out a reaction for 0.2-1 h; the 17-site carbonyl ethinylation comprises firstly carrying out a reaction of a potassium hydroxide powder for 1-2 h in an acetylene airflow and at the temperature of 5-10 DEG C, and then carrying out a reaction with the 3-site enol etherified product for 2-4 h in the presence of tetrahydrofuran and a catalyst, at the temperature of 15-30 DEG C and in the acetylene airflow. The 3-site enol etherification is mild in reaction conditions and relatively high in yield, and the 17-site carbonyl ethynylation is relatively high in reaction yield and relatively short in time.
Owner:佳尔科生物科技南通有限公司
Preparation method of polyalcohol acetal compound
The invention discloses a preparation method of a polyalcohol acetal compound. The preparation method comprises the following steps that polyalcohol, a solvent, a catalyst, a promoter and a water absorbent are added in a reaction vessel and stirred for 10-30 minutes, then substituted aldehyde is added into the mixture to be subjected to a reaction for 2-36 hours at the room temperature, stirring is conducted for 10-30 minutes after water is added, suction filtration, washing and swab-off are conducted, and the polyalcohol acetal compound is obtained, wherein polyalcohol is sorbitol, xylitol ormannitol, the solvent is dimethyl sulfoxide or a compound of dimethyl sulfoxide and methyl alcohol, the catalyst is sulfuric acid, hydrochloric acid, phosphoric acid or nitric acid, the promoter is phosphotungstic acid, the water absorbent is trimethyl orthoformate or triethyl orthoformate, and the substituted aldehyde is aromatic aldehyde or fatty aldehyde. The method is novel in route design, the reaction raw materials are easy to obtain, the product purity is good, post-treatment is simple, the applicability is high, and the method can be widely applied to synthesis of polyalcohol diacetaland polyalcohol triacetal compounds with different structures.
Owner:TAISHAN MEDICAL UNIV
Quinolone thiazole compound and preparation method and application thereof
ActiveCN104530034AHigh synthetic yieldEasy to prepareAntibacterial agentsOrganic active ingredientsThioureaSolvent
The invention discloses a quinolone thiazole compound as shown in the formula I and medicinal salt thereof, and further discloses a preparation method of the compound. Ethyl acetoacetate and triethyl orthoformate are used as raw materials to be subjected to multi-step reactions to obtain a quinolone ring with three-position acetyl, then bromization is conducted under the condition that acetic acid is used as solvent, and the acetic acid is reacted with thiourea so that the compound as shown in the formula I can be obtained. The quinolone thiazole compound has a certain inhibitory activity effect on gram positive bacteria and gram negative bacteria, and the method can be used for preparing antibacterial drugs (please see the formula in the specification).
Owner:SOUTHWEST UNIVERSITY
Method for preparing milrinone
The invention provides a method for preparing milrinone, wherein the method comprises the following steps: with 4-methyl pyridine (SM) as a raw material, in ethyl acetate, generating 1-(4-pyridyl)-2-acetone (represented by the formula I); then under action of triethyl orthoformate, acetic acid and acetic anhydride, generating 1-ethoxy-2-(4-pyridyl)vinyl methyl ketone (represented by the formula II); and finally, under an alkaline condition, carrying out a reaction of 1-ethoxy-2-(4-pyridyl)vinyl methyl ketone with cyanoacetamide to generate milrinone. The method has the advantages of simple and efficient operation, mild reaction conditions, strong safety, easy control and relatively high yield, and is suitable for industrialized production.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com










![Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2815104a-272d-435c-ad87-5493807452c7/BSA0000103894400000011.PNG)
![Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2815104a-272d-435c-ad87-5493807452c7/BSA0000103894400000012.PNG)
![Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2815104a-272d-435c-ad87-5493807452c7/BSA0000103894400000021.PNG)







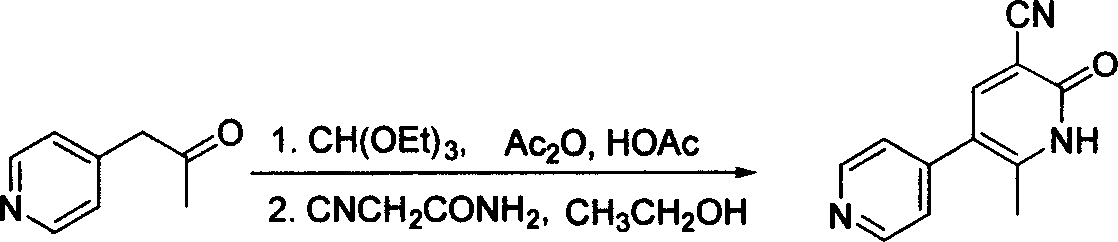
![Method for preparing 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole Method for preparing 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b8800c95-b8a4-4208-b2b7-eeffbe6b1924/375526DEST_PATH_IMAGE003.PNG)
![Method for preparing 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole Method for preparing 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b8800c95-b8a4-4208-b2b7-eeffbe6b1924/458074DEST_PATH_IMAGE001.PNG)
![Method for preparing 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole Method for preparing 5-cyclopropyl-4-[2-methylthio-4-(trifluoromethyl)benzoyl] isoxazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b8800c95-b8a4-4208-b2b7-eeffbe6b1924/529165DEST_PATH_IMAGE005.PNG)