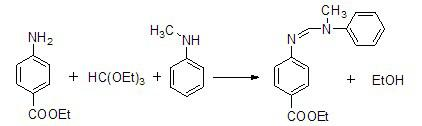
Method for preparing N-(4-ethyoxylcarbonylphenyl)-N'-methyl-N'-phenyl carbonamidine
A technology of ethoxycarbonylphenyl and phenylformamidine is applied in the field of preparation of N--N'-methyl-N'-phenylformamidine, which can solve the problems of reduced yield and product purity, cumbersome process routes, Low product purity and other issues, to achieve the effects of reducing production costs, reducing environmental pollution, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0017] ①Add 34.0mL of N-methylaniline (0.32mol), 49.5g of ethyl p-aminobenzoate (0.30mol) and 150.0mL of triethyl orthoformate (0.90mol) into a 250mL three-necked flask, and stir And slowly raise the temperature to 90°C.
[0018] ②Then add 17.1 mL of glacial acetic acid (0.3 mol) as a catalyst dropwise to the three-necked flask within 40 minutes to generate N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-benzene The reaction between methyl formamidine and ethanol, while distilling off the generated ethanol, kept the temperature of 90°C for 1h.
[0019] ③ Then slowly raise the temperature to 110°C, and at the same time reduce the pressure to 10mmHg, and continue the reaction until no ethanol is evaporated (about 10h).
[0020] 4. After the reaction finishes, underpressure distillation obtains the light yellow liquid N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenylformamidine of 76.7g, and purity is 99%, and productive rate is 91%.
Embodiment 2~ Embodiment 4
[0022] The preparation method of each embodiment is the same as that of Example 1, and the differences are shown in Table 1.
[0023]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com