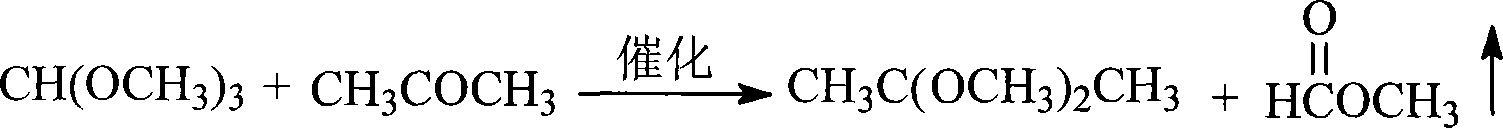Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
146 results about "Trimethyl orthoformate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trimethyl orthoformate is the simplest orthoester. It is a reagent used in organic synthesis for the introduction of a protecting group for aldehydes. The product of reaction of an aldehyde with trimethyl orthoformate is an acetal. In general cases, these acetals can be deprotected back to the aldehyde by using hydrochloric acid.
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Preparation method of azoxystrobin intermediate
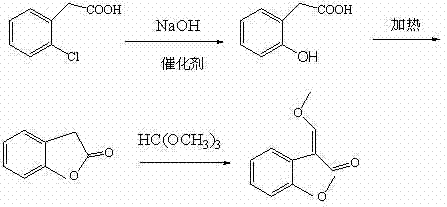
The invention relates to a preparation method of an azoxystrobin intermediate. The method comprises the following steps of: (1) synthesizing benzofuranone from o-hydroxyphenylacetic acid serving as a raw material; and (2) making benzofuranone react with trimethyl orthoformate to generate the azoxystrobin intermediate, wherein no solvent is used in the reaction in the step (1); and the reaction isperformed by the following steps of: putting o-hydroxyphenylacetic acid and 0-3 percent of catalyst into a reaction kettle; preserving heat in vacuum at the temperature 125-180 DEG C to react for 2-3hours; and evaporating water generated by the reaction in vacuum, wherein the residue in the reaction kettle is benzofuranone. The method has the advantages of simple process, high yield of the azoxystrobin intermediate and low production cost.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
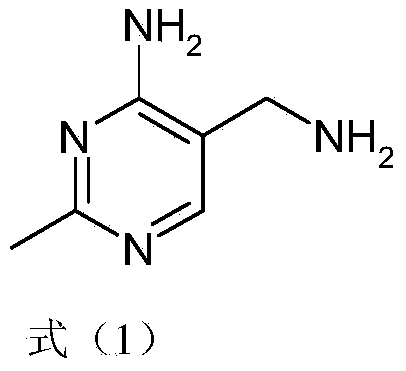
Simple and quick method for synthesizing improved vitamin B1 intermediate 2-methyl-4-amino-5-aminomethylpyrimidine
ActiveCN103435556AReduce concentrationReduce decompositionOrganic chemistryAminopropionitrilePropionitrile
The invention relates to a simple and quick method for synthesizing an improved vitamin B1 intermediate 2-methyl-4-amino-5-aminomethylpyrimidine. The method comprises the following steps: condensing 3-alkyl (aryl) formamido-propionitrile serving as a raw material with acetamidine in the catalytic action of lewis acid; cyclizing with trimethyl orthoformate, and hydrolyzing under an alkaline condition to prepare the vitamin B1 key intermediate 2-methyl-4-amino-5-aminomethylpyrimidine. The four reacting processes are performed in sequence by a one-pot reaction, and the product in each step does not need to be separated and purified. According to the method, highly carcinogenic o-chloroaniline or other small molecular aniline compounds are not used, residues of o-chloroaniline compounds in the vitamin B1 product can be eliminated. Furthermore, the preparation process is short and convenient in flow, small in wastewater amount and high in yield.
Owner:XINFA PHARMA
Para-(2-methoxyl) ethylphenol synthesis method
ActiveCN1800128AThe reaction route is simpleThree wastes lessEther preparation from oxiranesTyrosolMethyl carbonate
The invention discloses a method for synthesizing phenetyl, which uses p-chlorophehol or p-bromophenol as starting reaction raw material. It first uses methyl, benzyl or tert-butyl to protect the phenolic hydroxyl, the parivis which is protected by the phenolic hydroxyl reacts with the magnesium in the ether, tetrahydrofuran, tert-butyl methyl ether, isopropyl ether and its mixing solution to obtain the Grignard reagent, the Grignard reagent directly reacted with the etox to obtain the tyrosol which is protected by the phenolic hydroxyl, the tyrosol reacts with the dimethyl sulfate, dimethyl carbonate, trimethyl orthoformate to obtain the tyrosol ether which is protected by phenolic hydroxyl, which obtains the product in acid or hydrogenation protection.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
Method for preparing progesterone by taking 1,4-androstenedione as raw material
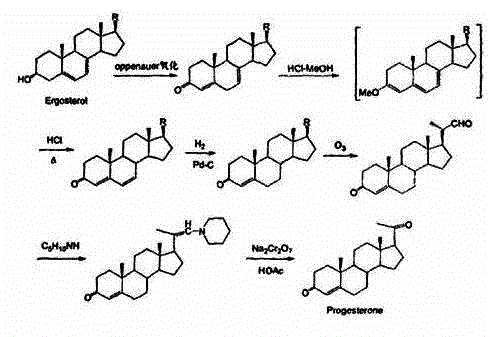
The invention discloses a method for preparing progesterone by taking 1,4-androstenedione as a raw material, which comprises the following steps: 1) dissolving 1,4-androstenedione into an organic solvent, adding the acid of trimethyl orthoformate or triethyl orthoformate, and introducing nitrogen to protect the 1,4-androstenedione to synthesize the enol ether of 1,4-androstenedione, namely 3-methoxy-androstane 3,5-diene-20-ketone; and 2) dispersing (1-methoxy ethyl)-triphenylphosphine salt in a reaction medium, an organic solvent, adding alkali at low temperature, performing a Wittig reaction of the 3-methoxy-androstane 3,5-diene-20-ketone synthesized in the step 1), and purifying and crystallizing to obtain progesterone. By adopting the 1,4-androstenedione as the raw material, the method solves the problem that of lack in raw materials for synthesizing steroid drugs such as progesterone, and improves the utilization rate of 1,4-androstenedione and the yield of progesterone; the preparation process is simple.
Owner:HUNAN KEYUAN BIO PRODS
Preparation method of high-purity palbociclib
ActiveCN106608876ARaw materials are cheap and easy to getShort processOrganic chemistryBulk chemical productionAcetoacetatesKetone
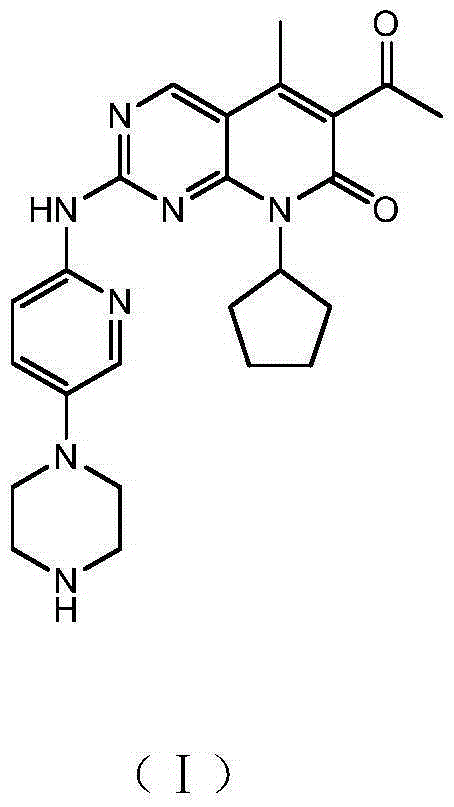
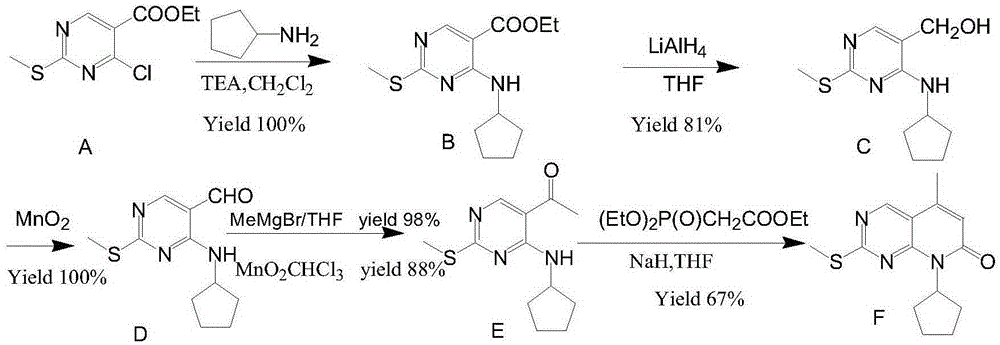
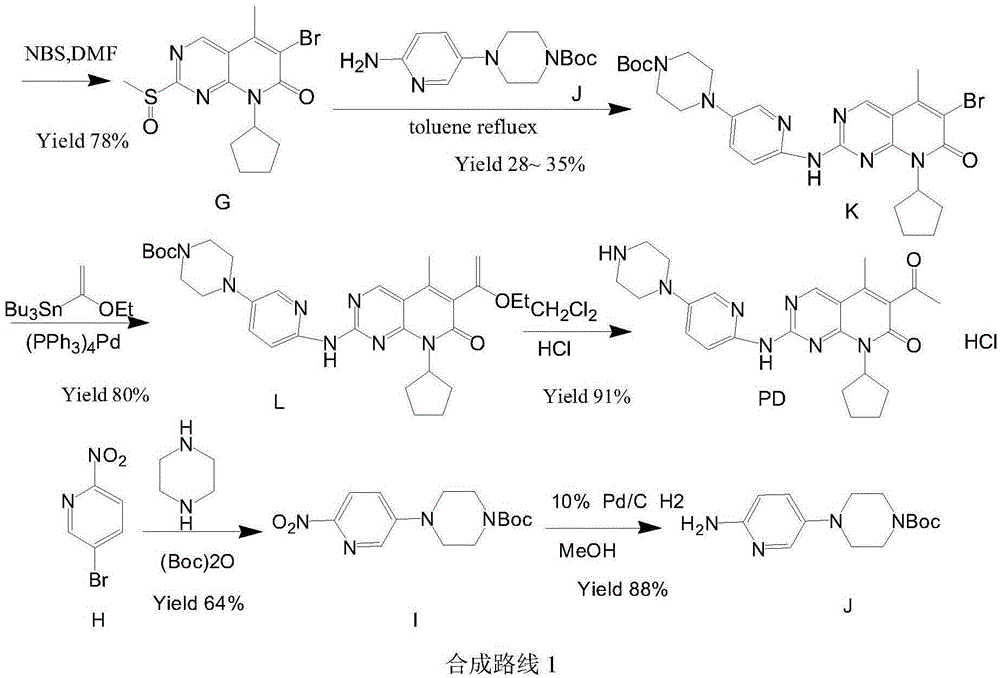
The invention relates to a preparation method of high-purity palbociclib. The method comprises using acetoacetate for reflux dehydration in the function of an acid catalyst to prepare 2-acetyl-3-methyl-2-diethyl (methyl) glutaconate (III), condensing the compound (III) and trimethyl orthoformate for methanol removal to obtain 2-acetyl-3-methyl-4-methoxymethylene-2-diethyl (methyl) glutaconate (IV), cyclizing the compound (IV) and N-(5-(4-tertbutoxycarbonyl-1-hexahydropiperazinyl)-2-pyridyl) guanidine (V) with pyrimidine to obtain 2-acetyl-3-[[5-(4-tertbutoxycarbonylpiperazinyl-1-yl) pyridine-2-yl] amino]-3H-4-one-dihydropiperazinyl-2-ethyl (methyl) butenyl ester (VI), and finally cyclizing the compound (VI) and cyclopentylamine to remove Boc protecting groups, thereby obtaining palbociclib. The preparation method is cheap and available in raw materials, short in technical process, simple and convenient to operate, good in reaction selectivity, and high in product yield and purity and is green and eco-friendly.
Owner:XINFA PHARMA
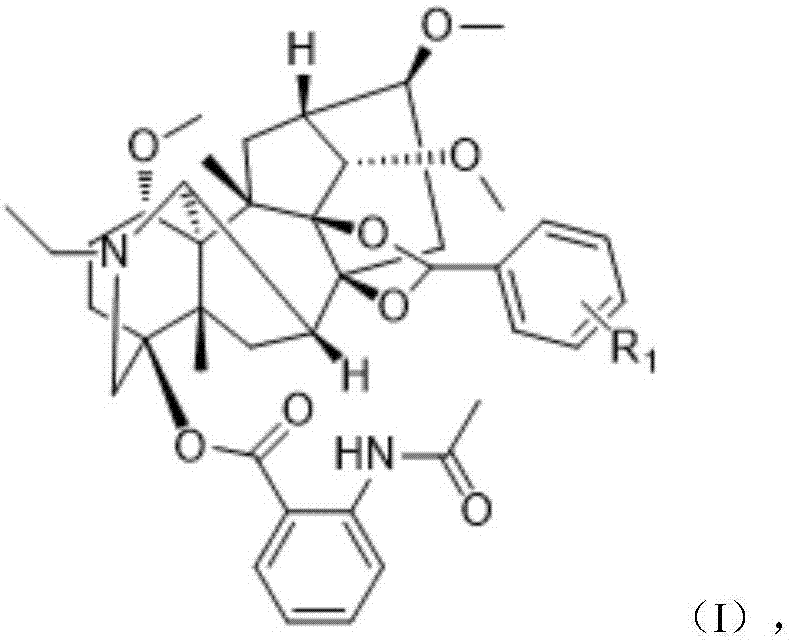
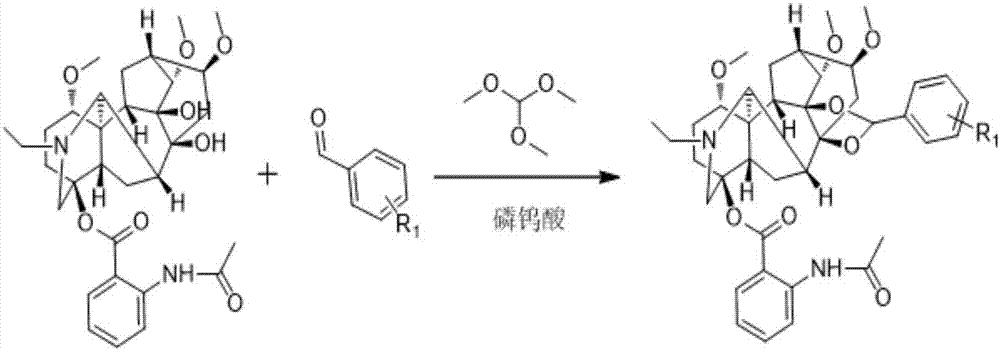
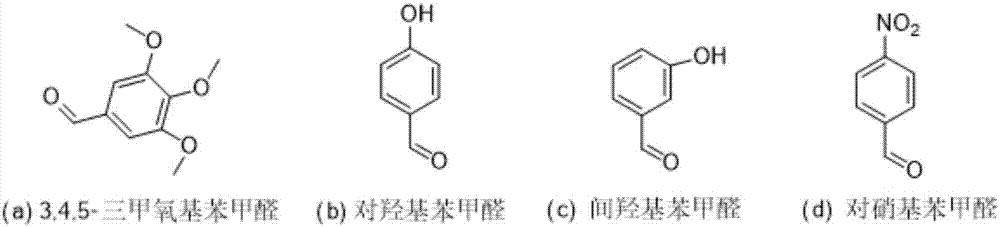
Lappaconitine acetal derivative with antineoplastic activity and synthetic method thereof
ActiveCN107540680ATake advantage ofImprove conversion rateOrganic chemistryAntineoplastic agentsOperational safetyDrug biological activity
A lappaconitine acetal derivative with antineoplastic activity and a synthetic method thereof are disclosed. By using lappaconitine and an aromatic aldehyde derivative as raw materials, an unreportedlappaconitine acetal derivative is synthesized under catalysis of phospho-tungstic acid and trimethyl orthoformate. The synthetic method of the lappaconitine acetal compound has high operational safety and mild reaction conditions, and is suitable for industrial production. It shows through preliminary biological activity tests that the compound has good antineoplastic activity and can be used inthe research on antineoplastic drugs.
Owner:SHAANXI UNIV OF SCI & TECH
Preparation method of trimethyl orthoformate
ActiveCN103130622AHigh purityLow content of triazine impuritiesOrganic chemistryOrganic compound preparationAlcoholTriazine
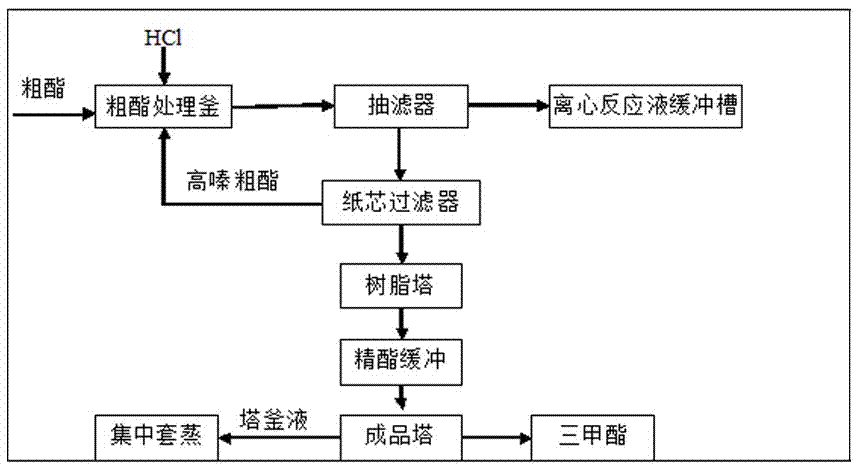
Disclosed are high-quality trimethyl orthoformate and a preparation method of the trimethyl orthoformate. The preparation method of the trimethyl orthoformate comprises the steps of obtaining amine salt by reaction between hydrocyanic acid and carbinol and hydrogen chloride and obtaining a trimethyl orthoformate coarse product through alcoholysis; and carrying out centrifugal separation on alcoholysis reaction liquid, and refining the obtained coarse product by adding aqueous alkali. The purity of the trimethyl orthoformate product obtained with the refining method can reach to 99.6%-99.8wt%, triazine impurity content is pretty small and is only 0.001-0.06%, and particularly purification yield of the refining method is more than 90% and can reach to 96% to the maximum degree. According to the trimethyl orthoformate and the preparation method of the trimethyl orthoformate, treating agents can be obtained easily, cost is low, reaction condition is moderate, and the trimethyl orthoformate and the preparation method of the trimethyl orthoformate are particularly suitable for industrial mass production.
Owner:重庆渝化新材料有限责任公司
Preparation method of polyalcohol acetal compound
The invention discloses a preparation method of a polyalcohol acetal compound. The preparation method comprises the following steps that polyalcohol, a solvent, a catalyst, a promoter and a water absorbent are added in a reaction vessel and stirred for 10-30 minutes, then substituted aldehyde is added into the mixture to be subjected to a reaction for 2-36 hours at the room temperature, stirring is conducted for 10-30 minutes after water is added, suction filtration, washing and swab-off are conducted, and the polyalcohol acetal compound is obtained, wherein polyalcohol is sorbitol, xylitol ormannitol, the solvent is dimethyl sulfoxide or a compound of dimethyl sulfoxide and methyl alcohol, the catalyst is sulfuric acid, hydrochloric acid, phosphoric acid or nitric acid, the promoter is phosphotungstic acid, the water absorbent is trimethyl orthoformate or triethyl orthoformate, and the substituted aldehyde is aromatic aldehyde or fatty aldehyde. The method is novel in route design, the reaction raw materials are easy to obtain, the product purity is good, post-treatment is simple, the applicability is high, and the method can be widely applied to synthesis of polyalcohol diacetaland polyalcohol triacetal compounds with different structures.
Owner:TAISHAN MEDICAL UNIV
Synthesis production process of 9-cyclohexadecylene lactone
InactiveCN101417992AReduce processing stepsNo pollutionOrganic chemistryChemistryTrimethyl orthoformate
The invention relates to a production process for synthesizing 9-cyclo-hexadecylene lactone, which comprises the following steps: (1) putting aleuritic acid and trimethyl orthoformate into a reactor according to weight ratio of between 1 to 0.5 and 1 to 4, and heating up the mixture to raise the temperature to finish a deshydroxy polyreaction at a temperature of between 60 and 180 DEG C; and (2) adding a depolymerization catalyst into the mixture to perform a depolymerization cyclization reaction under vacuum condition at a temperature of between 180 and 240 DEG C to generate the product of the 9-cyclo-hexadecylene lactone which is distilled out through adding an azeotropic agent to perform continuous azeotropic azeotropy, and rectifying and refining the distilled product to obtain the finished product of the 9-cyclo-hexadecylene lactone. The production process has the advantages that catalysts such as proton acid, low-carbon alkyl anhydride and the like are not used so that the cost is reduced, washing wastewater treatment processes are reduced, and no environmental pollution is caused; and the complex reaction processes are finished through simple process steps in a reaction pot, so that the operation is greatly simplified, and the industrial production is achieved.
Owner:INT FLAVORS & FRAGRANCES (HANGZHOU) CO LTD
Synthesis technology of trimethyl orthoformate
InactiveCN106946666AMild reaction conditionsLow costOrganic chemistryOrganic compound preparationDistillationCrystallization
The invention belongs to the technical field of trimethyl orthoformate, and discloses a synthesis technology of trimethyl orthoformate. The technology comprises the following steps: 1, proportioning; 2, crystallization salt formation, and alcoholysis; and 3, distillation. The synthesis technology has the advantages of good environmental protection property, energy saving, emission reduction, realization of large-scale industrial production, and high purity of the synthesized product.
Owner:临沭县华盛化工有限公司
4-aryl pyrimidine or 4-heterocyclic aryl pyrimidine compound luminescent material and preparation method thereof
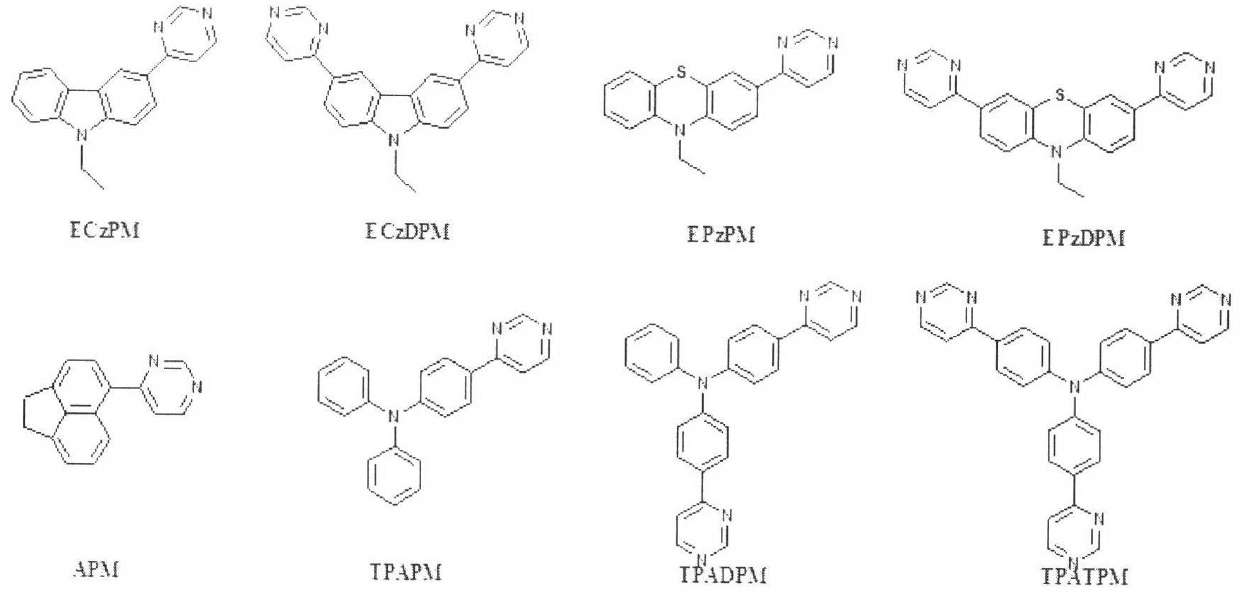
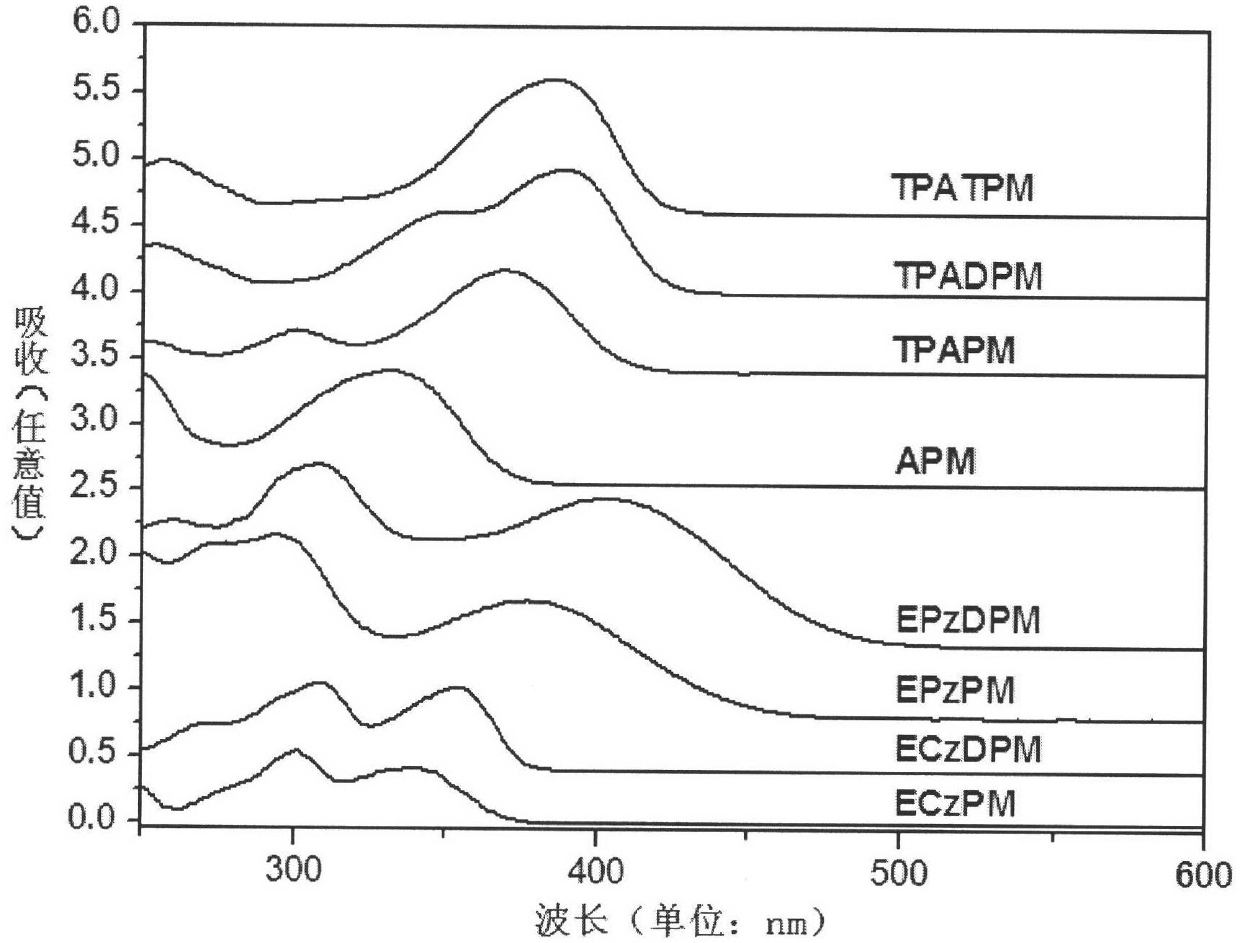
The invention relates to a 4-aryl pyrimidine or 4-heterocyclic aryl pyrimidine compound luminescent material and a preparation method thereof, and the structure can be represented as general formula (1), general formula (2), general formula (3), or general formula (4), wherein Ar represents an aryl group, a substituted aryl group, a heterocyclic aryl group, and a substituted heterocyclic aryl group. The method of the invention allows an acetyl-containing aryl or heterocyclic aryl compound to react with trimethyl orthoformate and ammonium acetate through a three-component coupling reaction under the action of a catalyst by using a one-pot method so as to obtain a series of aryl or heterocyclic aryl-containing 4-substituted pyrimidine compounds. The luminescent material of the invention has good dissolvability, film forming ability and stability, has high internal quantum efficiency, has a fluorescence emmission spectrum which can be adjusted from blue light to yellow light, can be used independently as a luminescent material, is expected to become a host material with excellent performance, and has important significance in the development of panel display devices with superior performance.
Owner:NANJING UNIV OF POSTS & TELECOMM
Method for synthesizing parent nucleus of cefroxadine
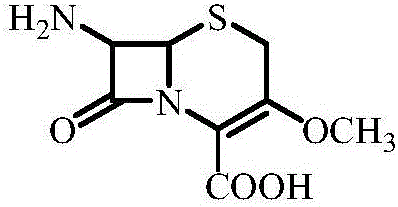
ActiveCN106632399AImprove protectionImprove operational safetyOrganic chemistryBulk chemical productionProtecting groupScandium
The invention discloses a method for synthesizing a parent nucleus of cefroxadine. The method includes taking 3-hydroxy-cepham as a starting material, and conducting methylation reaction and removal of an amino-protecting group-phenylacetic group at the 7 position and a protecting group-benzhydryl on the carboxyl at the 4 position sequentially so as to obtain the parent nucleus of the cefroxadine, wherein trimethyl orthoformate serves as a methylation reagent during the methylation reaction. The method has the advantages that the method is simple and safe to implement; during removal of the protecting group on the carboxyl at the 4 position, a scandium trifluoromethanesulfonate catalyst is reusable after being activated, and accordingly environmental protection is benefited; the method is high in product quality and suitable for industrial production. The parent nucleus of the cefroxadine produced by the method is white solid powder with the purity above 99.0%.
Owner:YANCHENG KAIYUAN MEDICINE CHEM
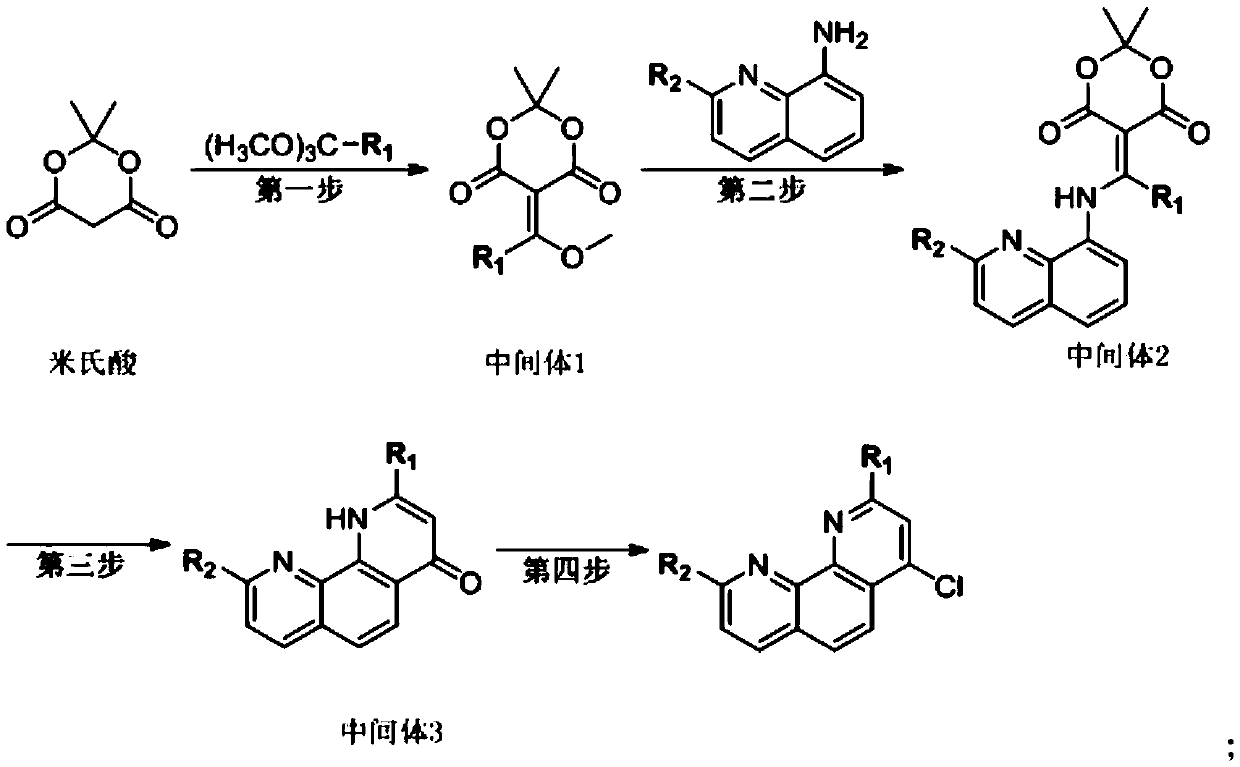
Synthesis method of 2, 9-substituted 4-halogenated-1, 10-phenanthroline
InactiveCN111087394AHigh yieldAvoid it happening againOrganic chemistryOrganic synthesisPhenanthroline
The invention discloses a synthesis method of 2, 9-substituted 4-halogenated-1, 10-phenanthroline, and belongs to the technical field of organic synthesis. The synthesis method comprises the followingsteps: S1, coupling mimic acid and trimethyl orthoformate to form alkene; S2, reacting a 8-aminoquinoline derivative with an intermediate alkene in the first step to generate secondary amine; S3, allowing secondary amine to be subjected to intramolecular cyclization to prepare a phenanthroline derivative of 4-keto carbonyl; and S4, synthesizing 4-halogenated-1, 10-phenanthroline of which the 2, 9positions are substituted through halogenation of phosphorus oxyhalide. According to the method, a four-step synthesis method is adopted, all the raw materials are strictly fed in sequence, corresponding purification treatment is carried out after each step of reaction, the conversion rate of each step of reaction is increased, finally, the yield of the product is effectively increased, and the problems that an existing synthesis method is difficult to synthesize and purify through adoption of a one-pot method, and the yield is low are solved; and the purification process is simple, generation of a large amount of waste acid is avoided, environment friendliness is achieved, and industrial production is facilitated.
Owner:XIAN RUILIAN NEW MATERIAL CO LTD
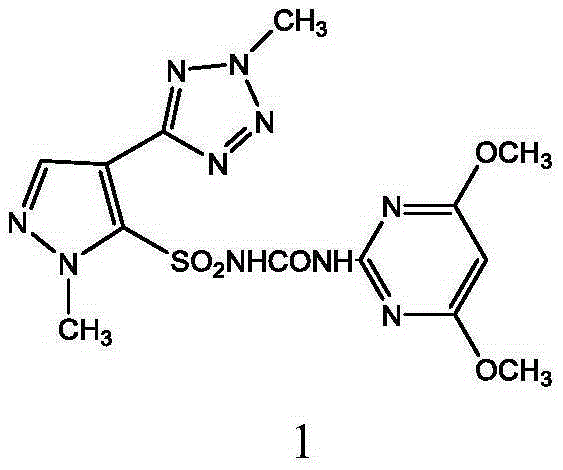
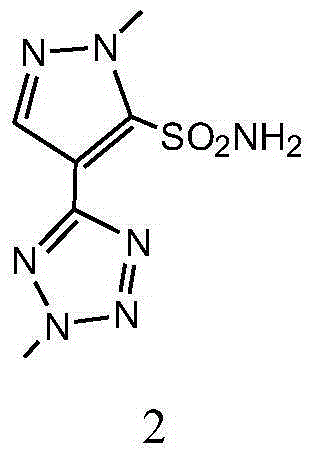
Preparation method and applications of azimsulfuron key intermediate
The present invention provides a preparation method of 1-methyl-4-(2-methyl-2H-tetrazole-5-yl)1H-pyrazole-5-sulfamide. The preparation method comprises that trimethyl orthoformate and malononitrile are adopted as starting raw materials, a three-step reaction is performed to obtain 1-methyl-5-amino-4-(tetrazole-5-yl)pyrazole, the 1-methyl-5-amino-4-(tetrazole-5-yl)pyrazole reacts with dimethylsulfate in the presence of an acid-binding agent to obtain 2-methyl-5-(1-methyl-5-amino-1H-pyrazole-4-yl)-2H-tetrazole, the 2-methyl-5-(1-methyl-5-amino-1H-pyrazole-4-yl)-2H-tetrazole is subjected to a sodium nitrite diazo-reaction under an acid condition, the obtained material reacts with a sodium sulfite-copper chloride-acetic acid mixture, and finally hydrolysis with ammonia is performed to obtain the target product. The present invention further provides a preparation method of azimsulfuron prepared from the 1-methyl-4-(2-methyl-2H-tetrazole-5-yl)1H-pyrazole-5-sulfamide. The preparation method of the present invention has characteristics of short reaction route, environmental protection raw materials, and high yield.
Owner:JIANGXI ANLIDA CHEM CO LTD
A method for preparing acetal with acrolein
The invention discloses a method for preparing an acetal from acrolein. The method comprises that acrolein and trimethyl orthoformate or triethyl orthoformate undergo a reaction under the acidic conditions to produce corresponding acrolein dimethyl acetal or acrolein diethyl acetal, and prepared acrolein dimethyl acetal or acrolein diethyl acetal is neutralized by alkalis and then is rectified toform a refined acetal. The method of the invention does not need a solvent. The method has the advantages of high reaction selectivity, good product quality, simple and convenient operation and the like.
Owner:荆州市新景化工有限责任公司
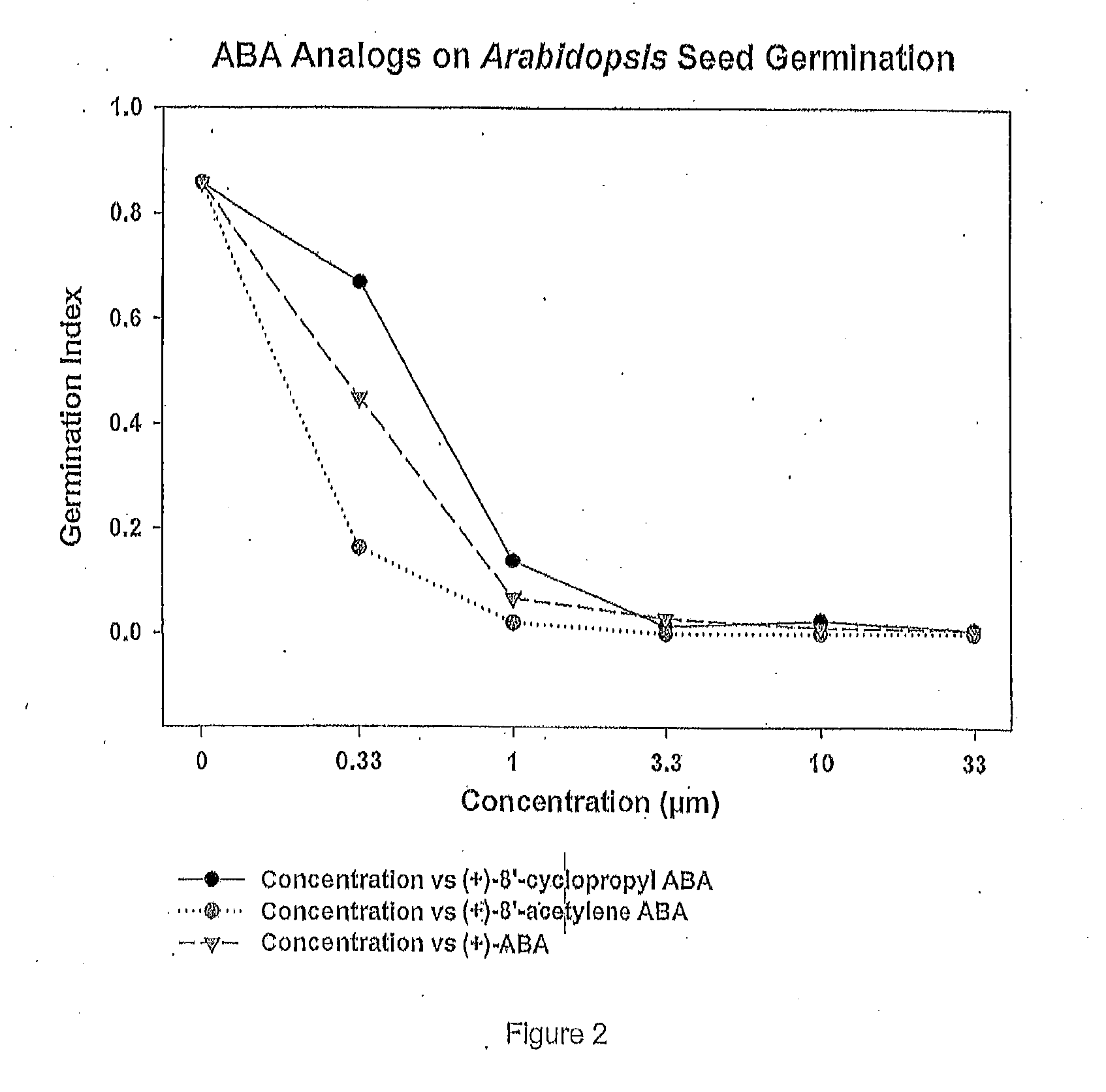
Efficient scalable syntheses of abscisic acid, 8'-acetylene abscisic acid and 8'-cyclopropyl abscisic acid
ActiveUS20170057899A1Reduce the amount requiredImprove consistencyOrganic compound preparationQuinone preparation by oxidation2,6-XylenolAbscisic acid
Methods are provided for synthesis of abscisic acid and 8′ analogues thereof (including an enantiopure 8′-acetylene analogue) including methods wherein the previously reported first step of oxidation of 2,6-dimethylphenol (VI) to 2,6-dimethylbenzoquinone, mono ketal (VII) is replaced by a novel two step process comprising (i) oxidation of 2,6-dimethylphenol (VI) using potassium peroxymonosulfate with a catalytic amount of iodobenzene to produce 2,6-dimethylbenzoquinone (XVI) and (ii) ketalization of 2,6-dimethylbenzoquinone (XVI) using ethylene glycol, trimethylorthoformate with a catalytic amount of p-toluenesulfonic acid to produce 2,6-dimethylbenzoquinone, mono ketal (VII).
Owner:NAT RES COUNCIL OF CANADA
Method for preparing known impurities of gefitinib
The invention discloses a method for preparing known impurities of gefitinib, comprising: mixing initial raw materials: 2-amino-4-methoxy-5-(3-morpholine propanolato) cyanophenyl, 3-fluorine-4-chloroaniline and acetate solvent with trimethyl orthoformate or triethyl orthoformate, simply preparing a crude product of the impurities of gefitinib, and recrystallizing to obtain the high purity known impurities of gefitinib, that is N-(4-chloro-3-fluorophenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine. The method has advantages of short synthesis route, simple operation, and high purity of obtained product, and can be used for reference substance research.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +2
Preparation method of erlotinib intermediate
The invention relates to a preparation method of an erlotinib intermediate. The method concretely comprises the following steps: 1, dissolving vanillin in an organic solvent I, adding aluminum trichloride, adding pyridine in a dropwise manner, and reacting to obtain ELTA; 2, dissolving the ELTA in an organic solvent II, adding 2-chloroethylmethyl ether, potassium carbonate and a phase transfer catalyst, and reacting to obtain ELTB; 3, adding the ELTB to water, adding an alkali and potassium permanganate, and reacting to obtain ELTC; 4, adding concentrated sulfuric acid to methanol in a dropwise manner, adding the ELTC to the above reaction system, and reacting to obtain ELTD; 5, adding concentrated sulfuric acid to concentrated nitric acid in a dropwise manner, dissolving the ELTD in an organic solvent IV, adding a prepared mixed acid to the reaction system, and reacting to obtain ELTE; 6, adding the ELTE, iron powder and ammonium chloride to an organic solvent V, adding concentrated hydrochloric acid in a dropwise manner, and reacting to obtain ELTF; and 7, adding the ELTF, trimethyl orthoformate and ammonium acetate to an organic solvent VI, and reacting to obtain the erlotinib intermediate.
Owner:CHONGQING WEIPENG PHARMA
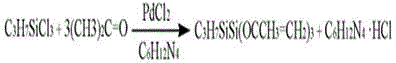
Preparation method of propyltriisoallyloxysilane
ActiveCN105601660ASuitable for industrial mass productionSilicon organic compoundsDistillationHexamethylenetetramine
The invention relates to a preparation method of propyltriisoallyloxysilane, belonging to the technical field of fine chemical engineering. The preparation method comprises the following steps: by using propyltrichlorosilane and acetone as raw materials, hexamethylenetetramine as a hydrochloric acid adsorbent, palladium chloride as a catalyst and trimethyl orthoformate as a dehydrator, carrying out reaction at 70-90 DEG C to synthesize a propyltriisoallyloxysilane-containing reaction product, filtering, and carrying out reduced pressure distillation to obtain the propyltriisoallyloxysilane product. The method has the advantages of simple technique and high reaction efficiency, and is suitable for industrial large-scale production. Compared with the existing propyltriisoallyloxysilane preparation method, the method provided by the invention shortens the reaction time, enhances the reaction efficiency, reduces the side reactions due to the use of the dehydrator, and enhances the product quality and yield.
Owner:JINGZHOU JIANGHAN FINE CHEM
Method for synthesizing 3-(Boc-aminomethyl)cyclobutanone
ActiveCN109053496ALow costUnique routeCarbamic acid derivatives preparationOrganic compound preparationSodium methoxideSynthesis methods
The invention relates to a method for synthesizing 3-(Boc-aminomethyl)cyclobutanone. The method for synthesizing 3-(Boc-aminomethyl)cyclobutanone provided by the invention mainly solves the technicalproblems of high raw material cost and relatively difficult post-processing according to the existing synthesis method. The method for synthesizing 3-(Boc-aminomethyl)cyclobutanone provided by the invention comprises the following steps: 3-oxocyclobutanecarboxylic acid and trimethyl orthoformate are reacted in the methanol solution to generate a compound 1; the compound 1 and benzylamine are reacted in the methanol solution under an action of sodium methoxide to generate a compound 2; the compound 2 and sodium bis(2-methoxyethoxy)aluminiumhydride are reacted in tetrahydrofuran solution to generate a compound 3; the compound 3 is subjected to a Pd / C debenzylation and hydrogenation operation in the methanol solution to obtain a compound 4; the compound 4 and Boc2O are reacted in the methanolsolution to obtain a compound 5; the compound 5 is reacted in a 0.05 M hydrochloric acid solution generate a target compound 6. By performing an enzymatic catalyzed deglandulation action of on the corresponding prochiral 3-substituted cyclobutanone, a series of gamma-butyrolactone derivatives, including some spiro derivatives, can be obtained; and all the derivatives are inhibitors having good biological activity.
Owner:GL BIOCHEM SHANGHAI
Method for synthesizing 2-amino-6-chloropurine
The invention discloses a method for synthesizing 2-amino-6-chloropurine, and belongs to the field of organic chemical synthesis. The synthesis method of the 2-amino-6-chloropurine comprises the following steps: enabling 6-hydroxyl-2,4,5-triaminopyrimidine to react with trimethyl orthoformate to generate guanine, and enabling the guanine to react with hydrogen chloride and hydrogen peroxide to generate 2-amino-6-chloropurine. The method has the advantages of short reaction time, high yield, no generation of a large amount of three wastes, simple operation, and facilitation of industrial production.
Owner:SHANGHAI LINKCHEM TECH CO LTD
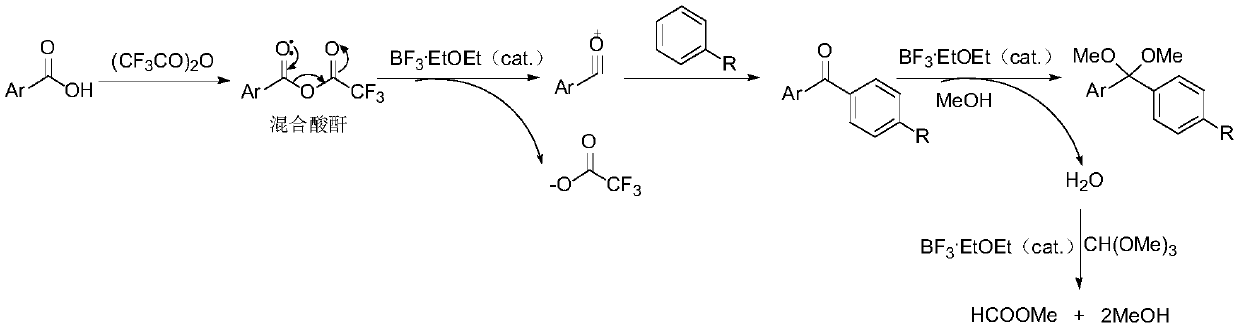
One-pot method used for synthesis of diaryl methyl ketal
ActiveCN110396040AImplement multi-step reactionsShort stepsOrganic chemistryOrganic compound preparationBenzeneAryl
Owner:SOUTHEAST UNIV +1
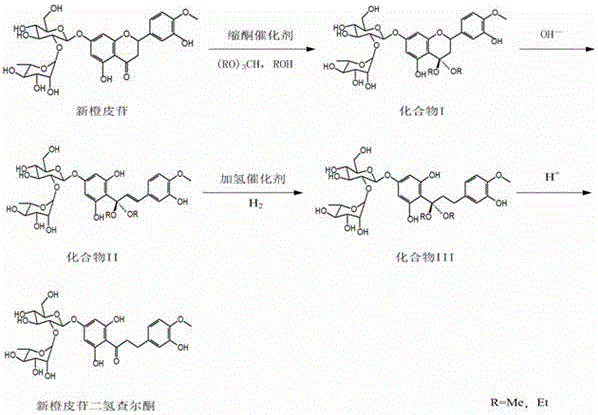
Preparation method of neohesperidin dihydrochalcone
ActiveCN106220694AIncrease profitSimple methodSugar derivativesChemical recyclingNitrogen gasNeohesperidine dihydrochalcone
The invention discloses a preparation method of neohesperidin dihydrochalcone. The method comprises the following steps: (1) carbonyl protection: dissolving the neohesperidin in an organic solvent or organic solvent aqueous solution, adding trimethyl orthoformate or triethyl orthoformate and ketal catalyst to perform the ketal reaction, reducing pressure and concentrating, cooling and crystallizing, filtering and drying to obtain a compound I; (2) hydrolysis reaction: dissolving the compound I in an aqueous alkali of the compound II; (3) hydrogenation reaction: adding the hydrogenation catalyst, introducing nitrogen to protect, and introducing the hydrogen to perform the hydrogenation reaction, filtering to obtain the aqueous alkali of the compound III; (4) acidification and crystallization: regulating pH value, cooling and crystallizing, filtering and drying to obtain the neohesperidin dihydrochalcone. The yield of the neohesperidin dihydrochalcone prepared through the method disclosed by the invention could reach 97.2%, the purity can reach 98.5%. The method disclosed by the invention is simple, mild in reaction condition and low in cost, and is capable of partially recycling the catalyst and suitable for the industrial production.
Owner:HUNAN HUACHENG BIOTECH
Preparation method of Azoxystrobin and intermediate thereof
Owner:PAPANNA BEIJING TECH
Method for synthesizing 2,2-dimethoxypropane
InactiveCN101157601AImprove conversion rateSimple equipment requirementsOrganic chemistryOrganic compound preparationDynamic balanceReactive distillation
The invention discloses a method of synthesizing a 2, 2-dimethyoxypropane. Under the effect of catalyst, acetone and trimethyl orthofomate are synthesized through reactive distillation; and the invention has the feeding mol ratio that the proportion between the acetone and the trimethyl orthofomate is 1:10 to 10:1. The reaction utilizes dynamics balance principle, and continuously transforms a product in reaction process by reaction distillation device, to lead the reaction to go along the direction of producing. The method, realizing high conversion, has the advantages of simple device and small corrosivity as well as being easy for large-scale producing and environment-friendly.
Owner:ZHEJIANG SCI-TECH UNIV
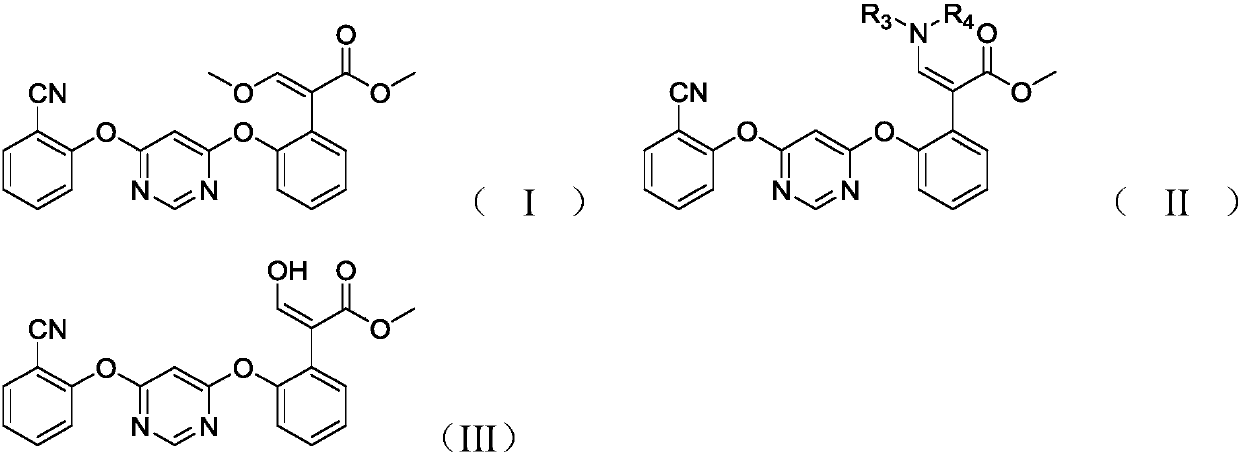
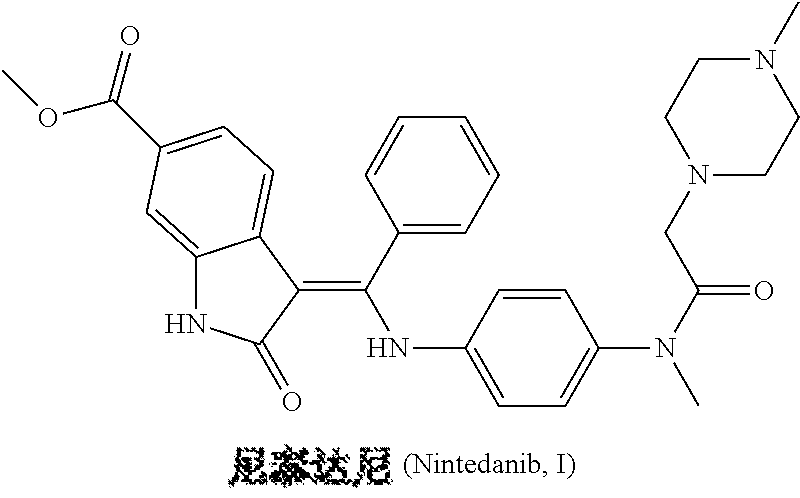
Preparation method of nintedanib
Disclosed is a preparation method of nintedanib (I), comprising the following steps: carrying out a condensation reaction on 4-(R acetate-2-yl)-3-nitrobenzoate (II) and trimethyl orthobenzoate to obtain (E)-4-[(2-methoxybenzylidene) R acetate-2-yl]-3-nitrobenzoate (III); carrying out a substitution reaction on the compound (EI) and N-(4-aminophenyl)-N-methyl-2-(4-methyl piperazine-1-yl) acetamide (IV) under the action of an acid-binding agent to generate (Z)-4-{[2-(N-methyl-2-(4-methyl piperazine-1-yl) acetamido-aniline) benzylidene] R acetate-2-yl}-3-nitrobenzoate (V); and sequentially carrying out reduction reactions and cyc-lization reactions on the compound (V) to prepare the nintedanib (I). The preparation method has an easily obtained raw material and a simple process, is economical and environmentally friendly, and is suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH
Synthetic technology of azoxystrobin
The invention provides a synthetic technology of azoxystrobin. The synthetic technology mainly comprises the following steps: adding hydroxy benzene acetic acid and trimethyl orthoformate butyl anhydride in a reaction still, and carrying out a heat preservation reaction for 20 hours; obtaining a kind of black solid through vacuum concentration, and recrystallizing after dissolving the black solid by using methyl alcohol; pumping nitrogen for protection, then adding 4,6-dichloro pyrimidine and methyl formate, slowly and dropwise adding a sodium methylate solution, and distilling after a reaction is ended, thus obtaining a residue mixture; adding a potassium hydroxide aqueous solution, reducing the temperature, standing after adding methylbenzene, separating an organic phase and a water phase, adding KHSO4 after desolventizing the organic phase, obtaining grease after the reaction is carried out by rising the temperature, continuously adding 2-cyano phenol and potassium carbonate, and cooling after the reaction; adding the methyl alcohol, recrystallizing after cooling a system to 0 DEG C, and obtaining the azoxystrobin after filtering and drying. Compared with the prior art, the synthetic technology disclosed by the invention has the advantages that the method is simple, raw materials are cheap and easy to obtain, the environment pollution is small, the yield of the azoxystrobin is high, and the industry prospect is good.
Owner:ANHUI GUANGXIN AGROCHEM
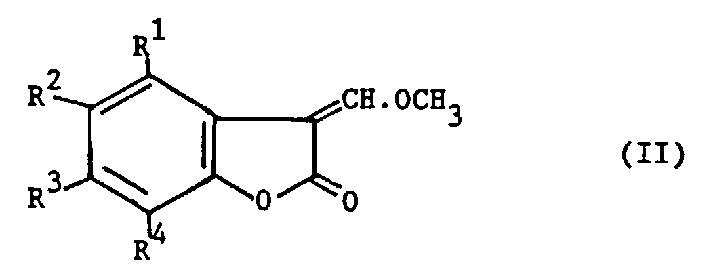
Process for preparing benzofuranone derivatives
Processes for the preparation of compounds (X), (XI) and (XII): <CHEM> <CHEM> <CHEM> where M is an alkali metal or alkaline earth metal and n is 1 or 2, and for obtaining compound (X) in a substantially pure form. These compounds are useful intermediates in the manufacture of fungicides.
Owner:ZENECA LTD
Synthetic method for azoxystrobin intermediate
InactiveCN107353255AReduce lossesHigh yieldOrganic chemistryChemical recyclingBenzeneAcetic anhydride
The invention discloses a synthetic method for an azoxystrobin intermediate. The method comprises the following steps: firstly, benzofuranone, trimethyl orthoformate and acetic anhydride are used as raw materials, reactive distillation operation is performed, and thus condensation substances are obtained by a one-pot method with a high yield; and secondly, a one-pot method reaction is performed on the condensation substances and 2,6-dichloropyrimidine in a methanol solution of sodium methylate; and finally, after the reaction is finished, the acidity is regulated, methanol is recycled, alkali washing is performed, a catalyst of p-toluenesulfonic acid is added to the condensed crude products, a reaction is performed, and thus the azoxystrobin intermediate (E)-2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxyacrylate is obtained. The method is simple in operation, high in yield, less in by-products, and suitable for industrialized production.
Owner:SHANGHAI INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)