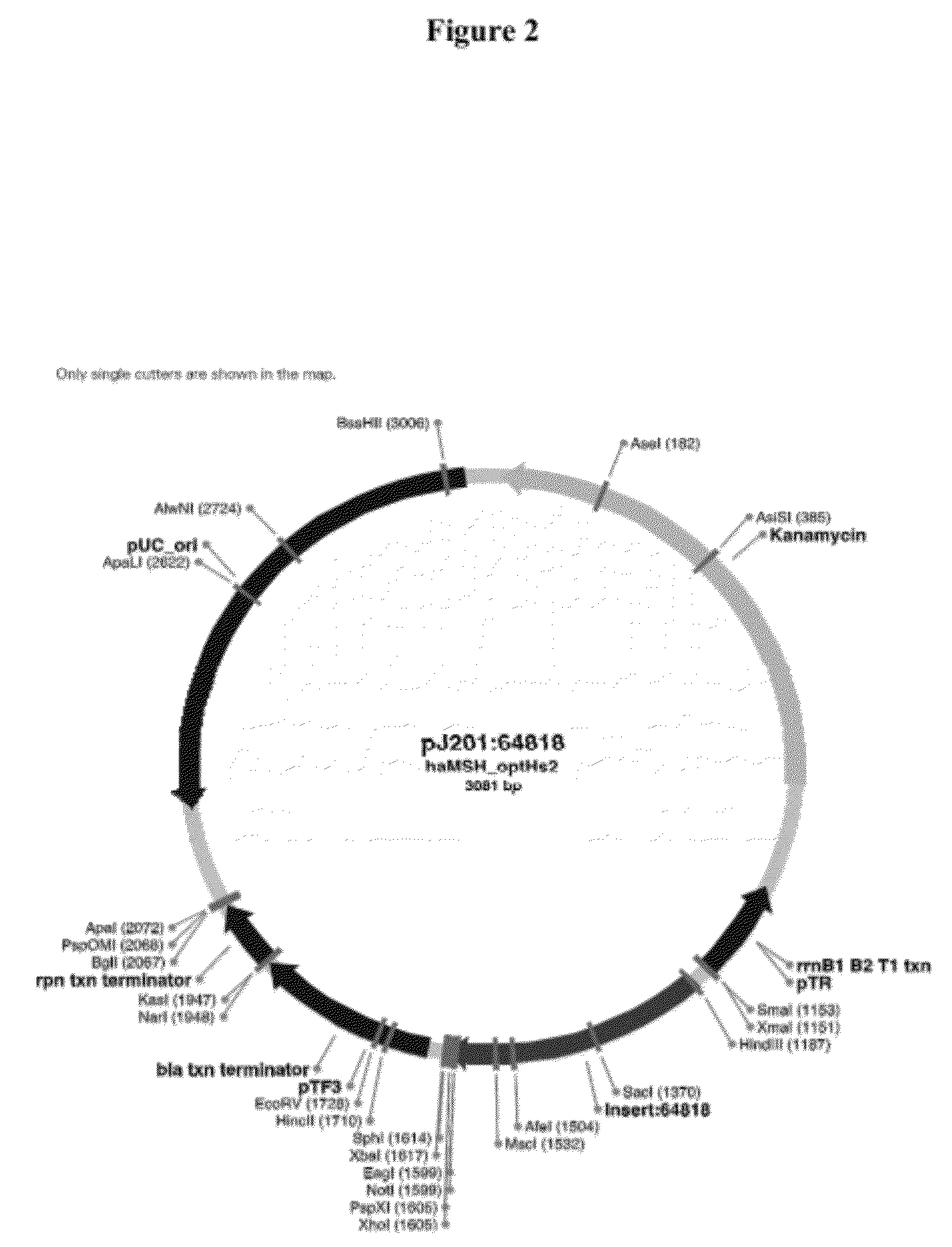
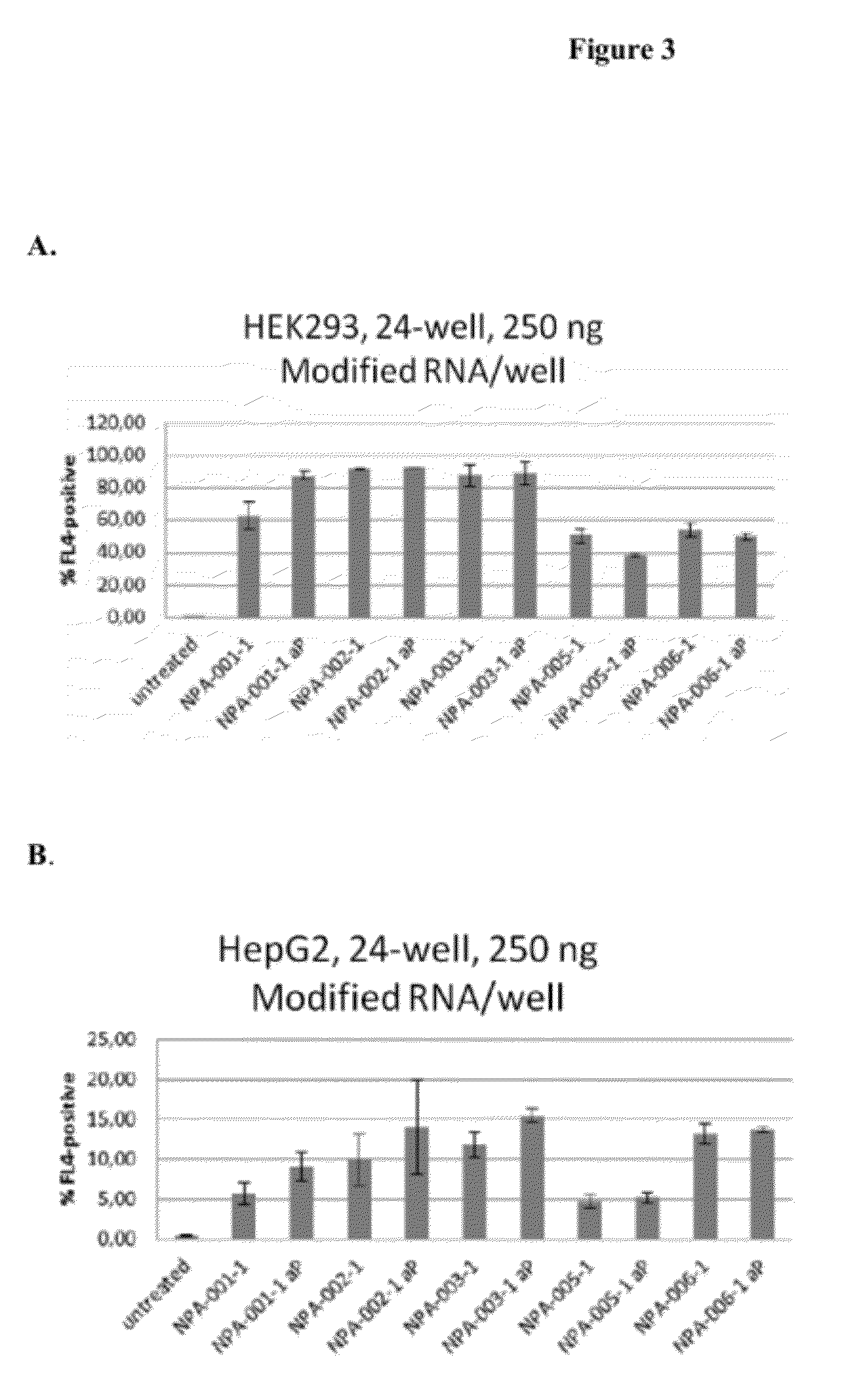
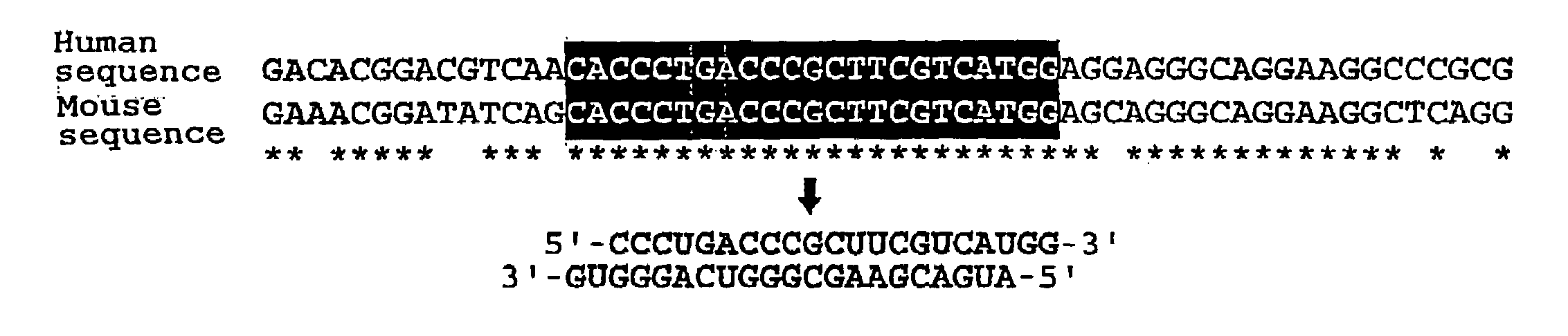
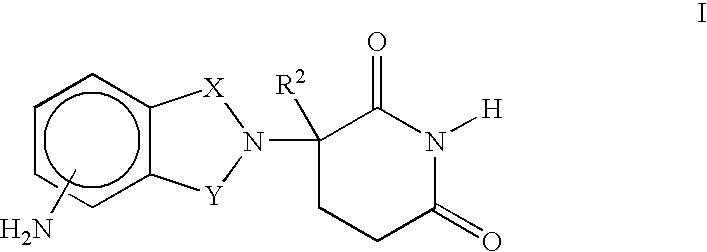
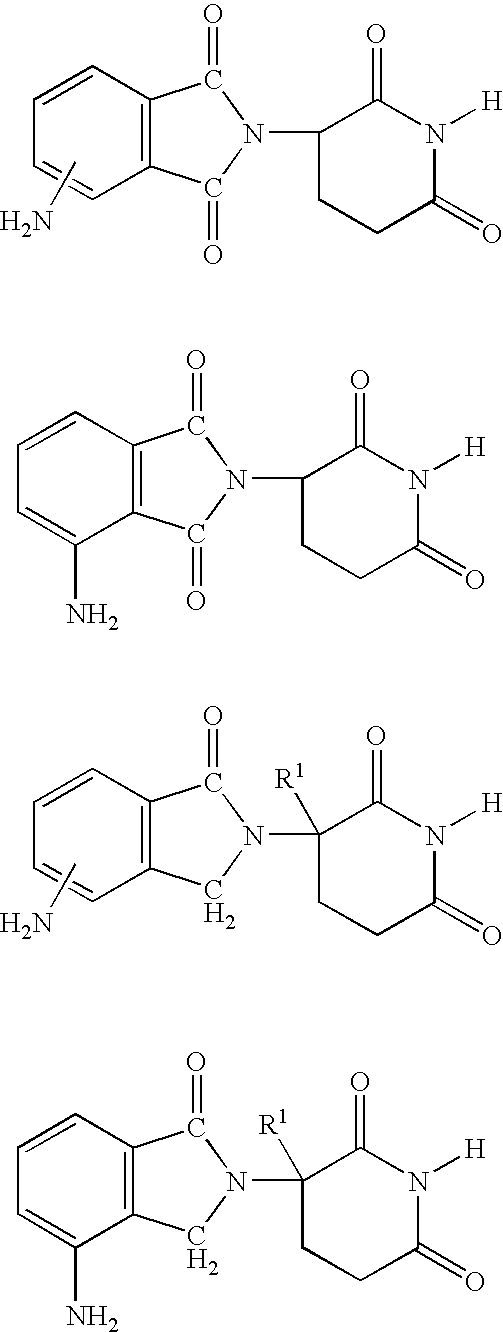
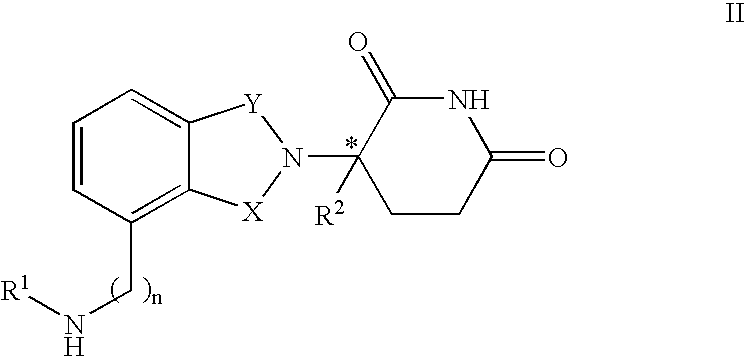
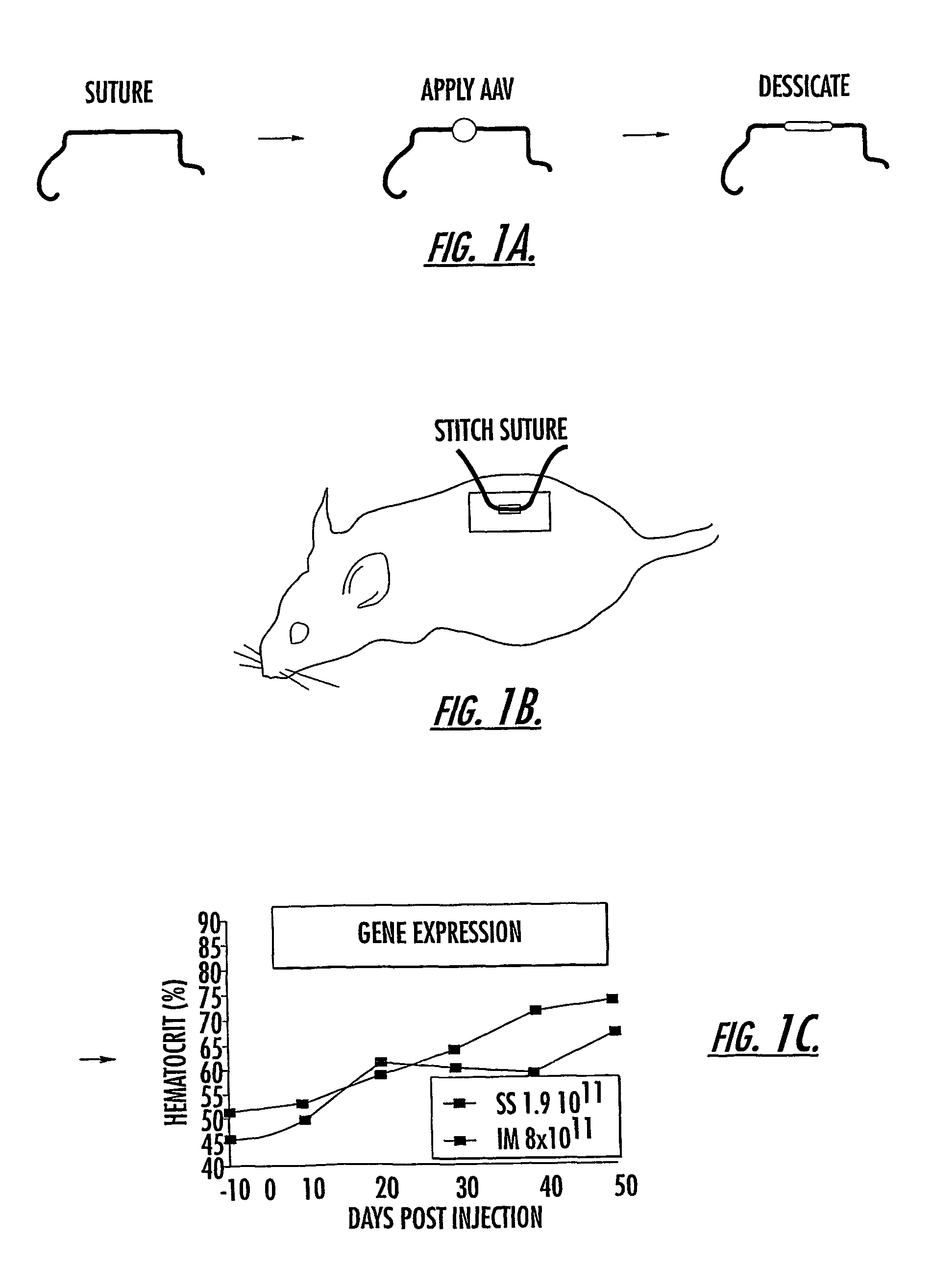
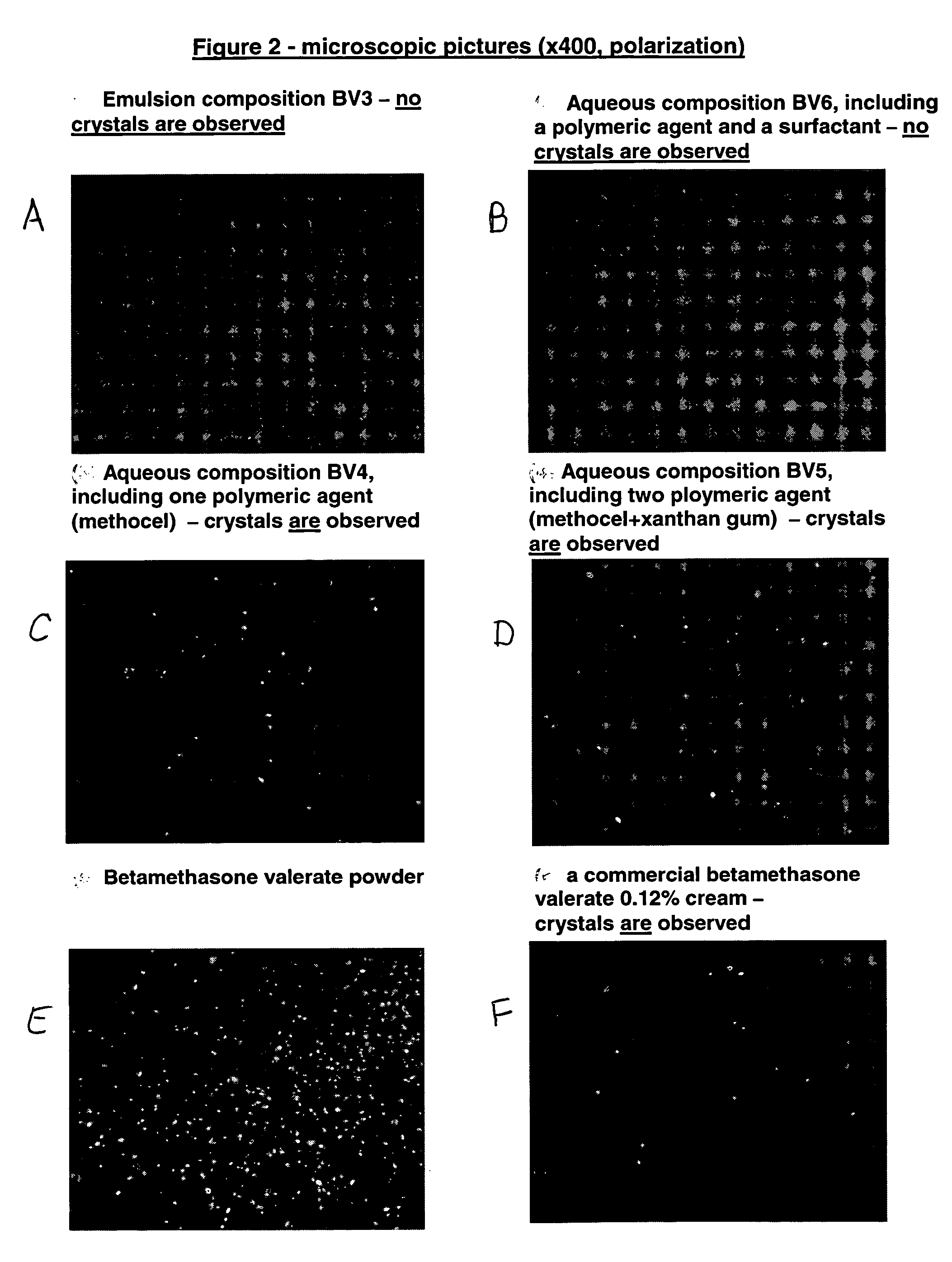
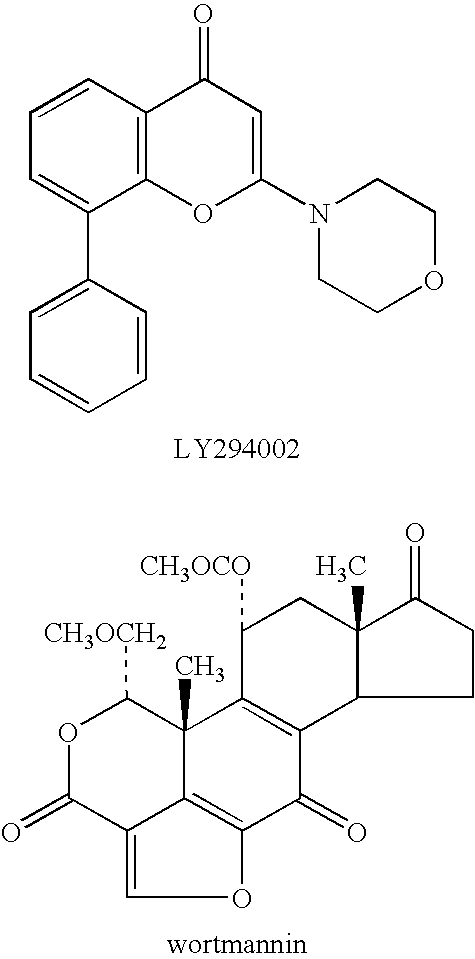
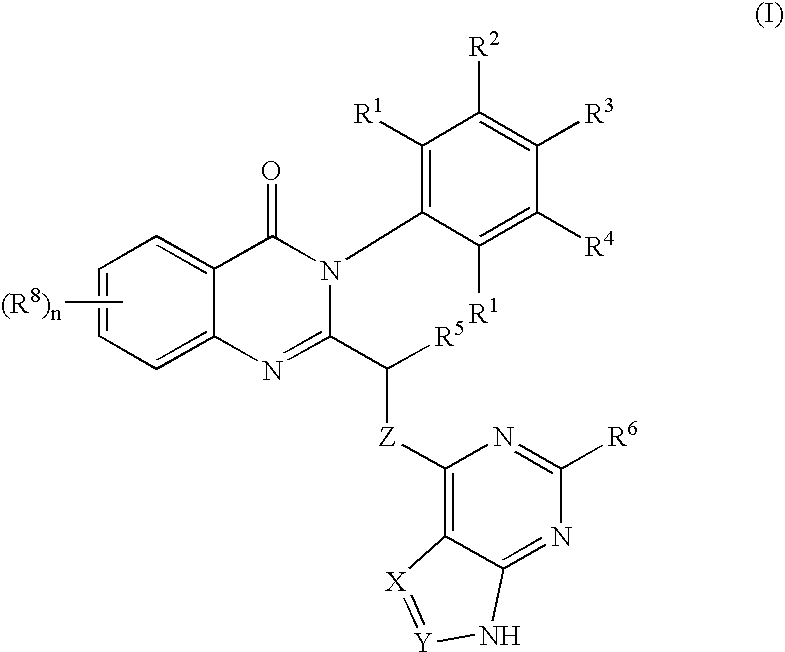
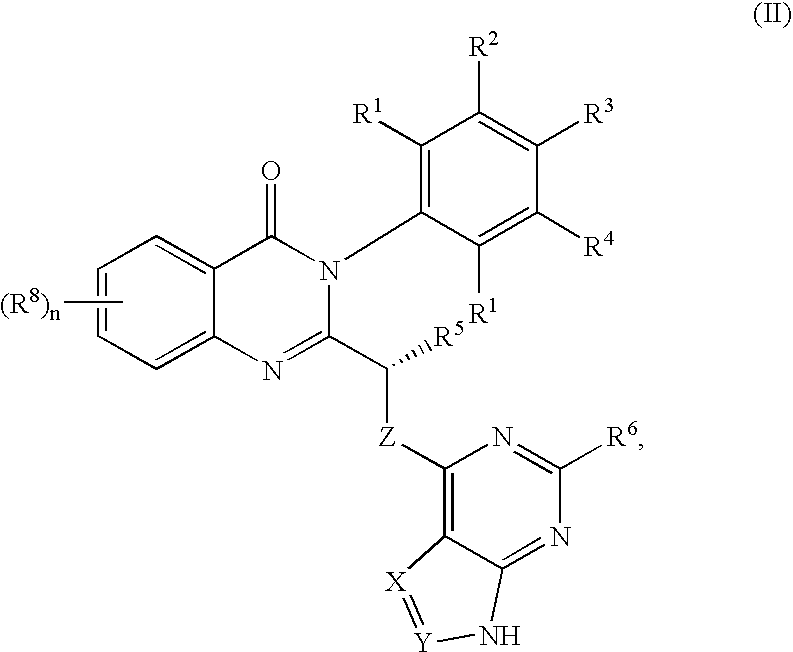
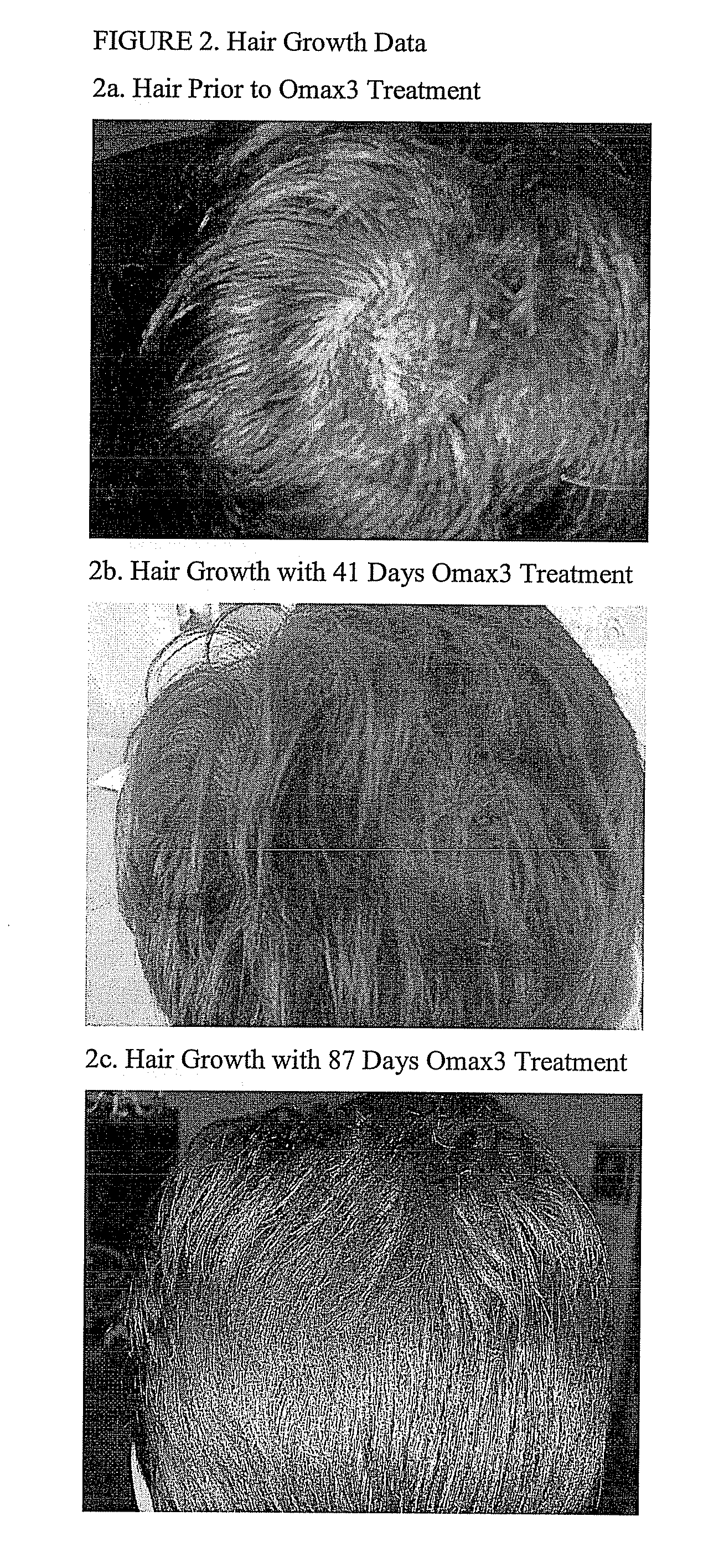
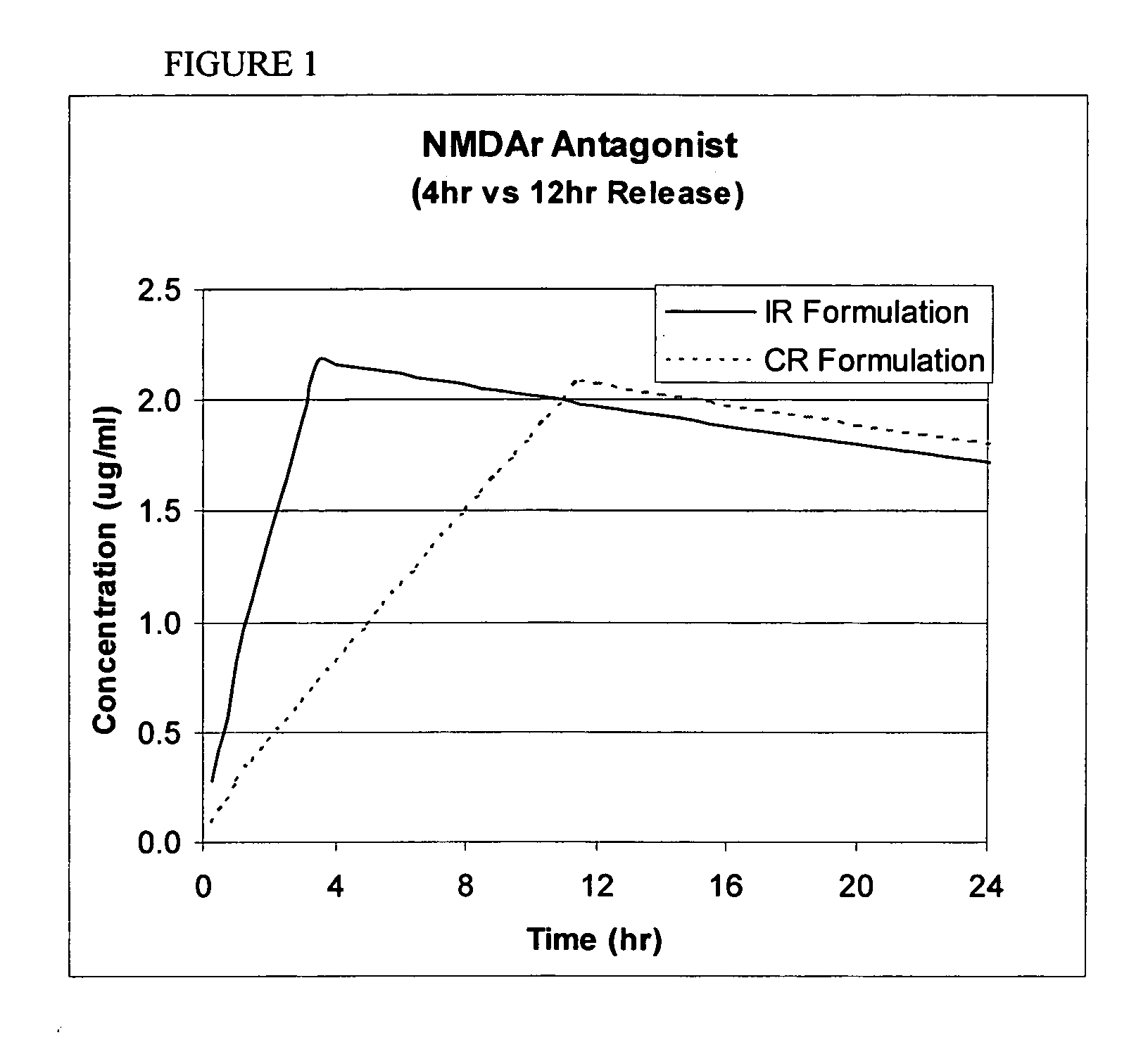
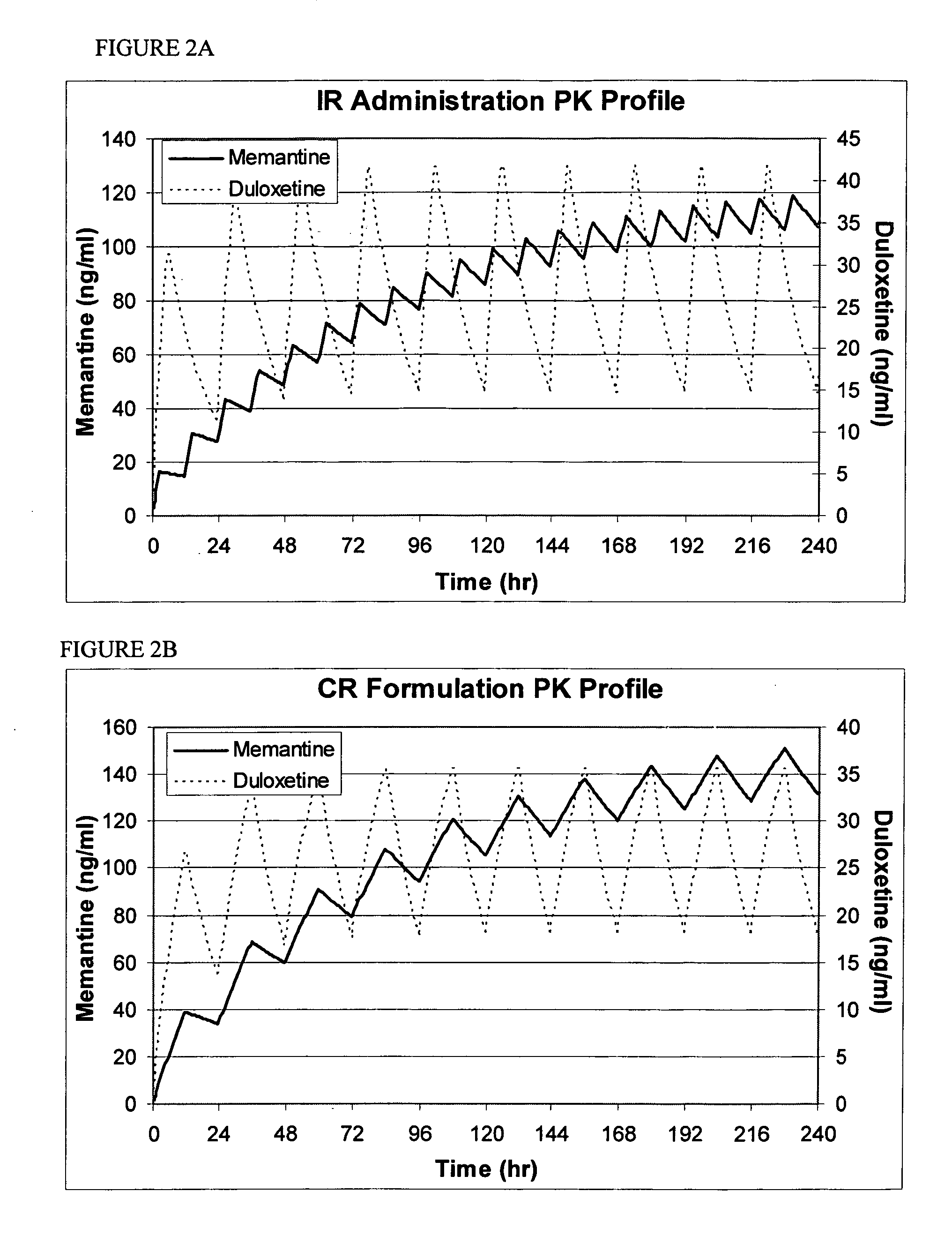
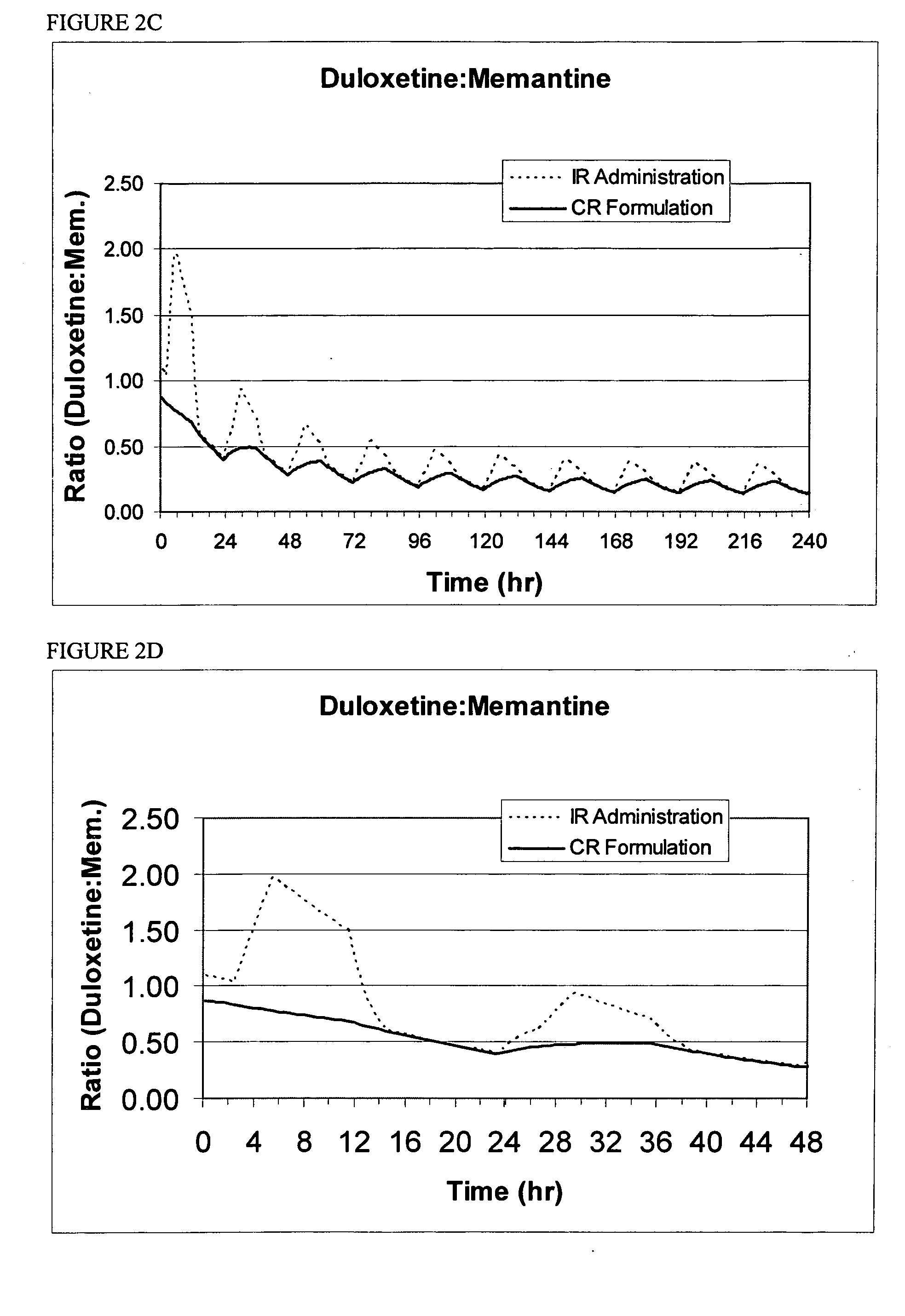
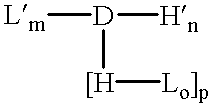Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
15211results about "Endocrine system disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method of producing sustained-release preparation
InactiveUS6267981B1Maintain good propertiesEnhancement of entrapmentPowder deliveryPeptide/protein ingredientsEntrapmentBiodegradable polymer
This invention provides a sustained-release preparation comprising a biodegradable polymer metal salt and broactive polypeptide, with enhanced entrapment of the bioactive polypeptides, a suppression of initial burst, and a constant long-term release of the bioactive polypeptides.
Owner:TAKEDA PHARMA CO LTD
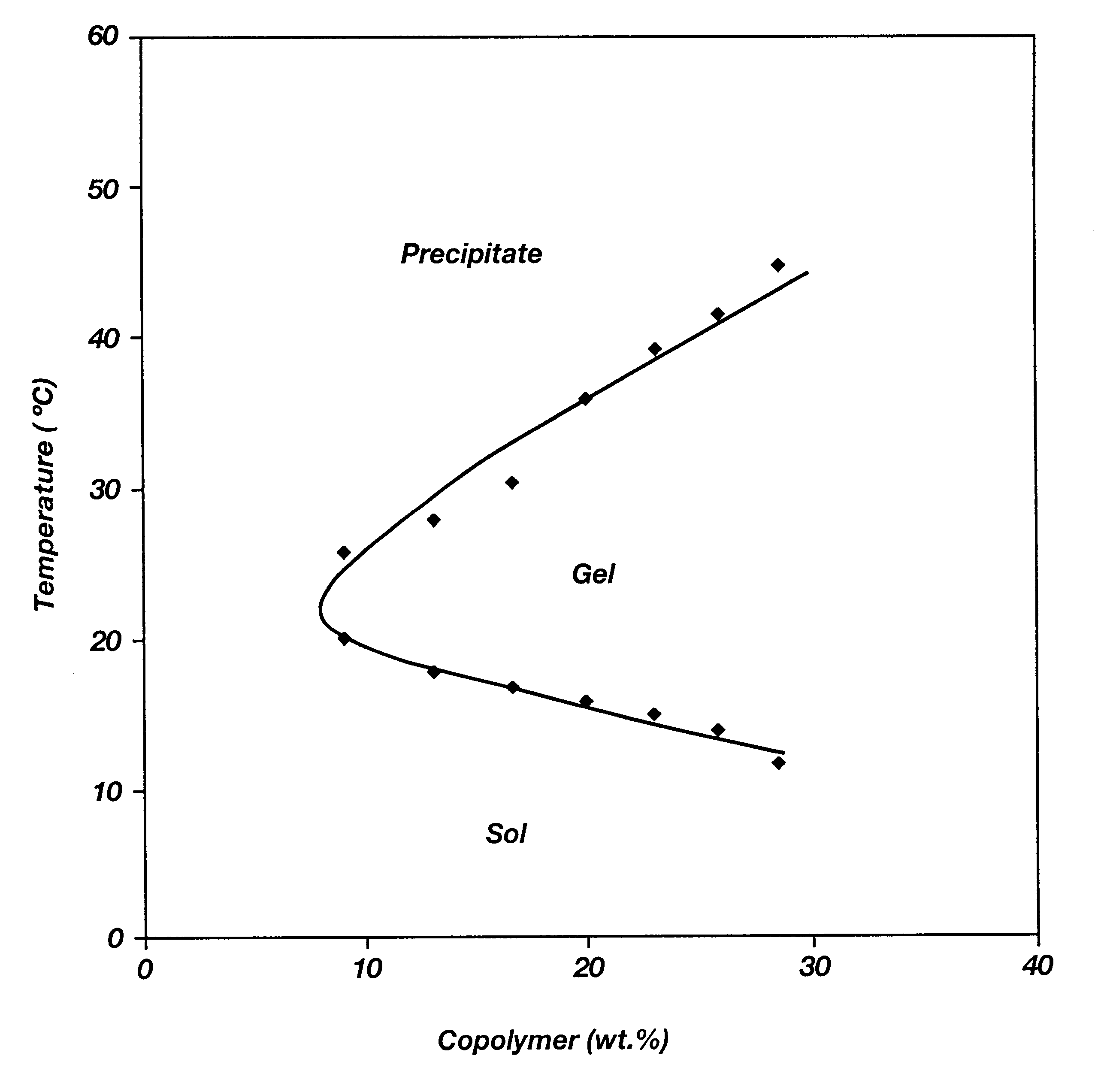
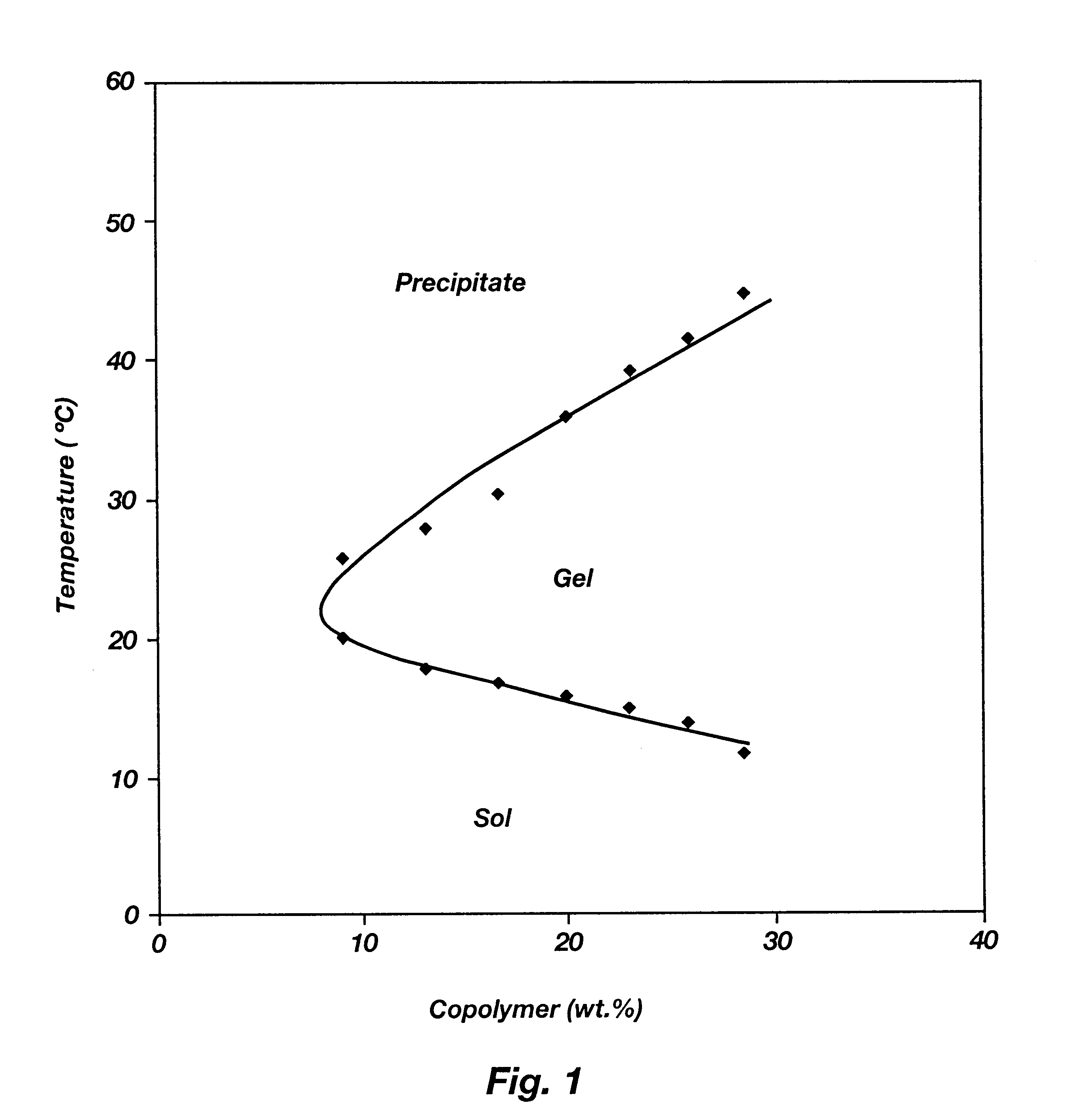
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
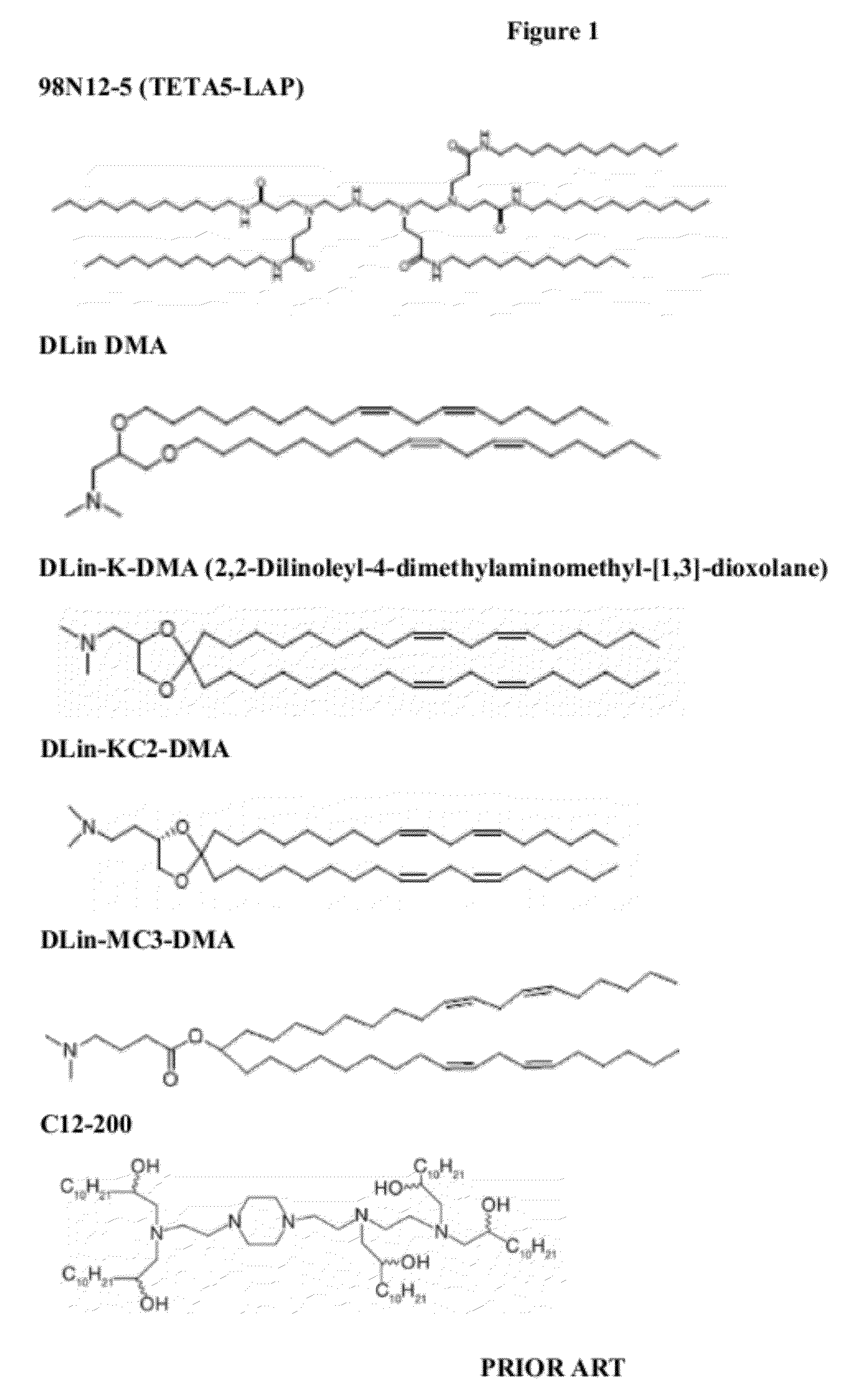
Delivery and formulation of engineered nucleic acids
ActiveUS20120251618A1Improve the level ofIncrease in level of polypeptideNervous disorderAntipyreticNucleic acidProtein expression
Provided are formulations, compositions and methods for delivering biological moieties such as modified nucleic acids into cells to modulate protein expression. Such compositions and methods include the delivery of biological moieties, and are useful for production of proteins.
Owner:MODERNATX INC
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
Polynucleotides for causing RNA interference and method for inhibiting gene expression using the same
InactiveUS20080113351A1High RNA interference effectLittle riskOrganic active ingredientsNervous disorderBase JNucleotide
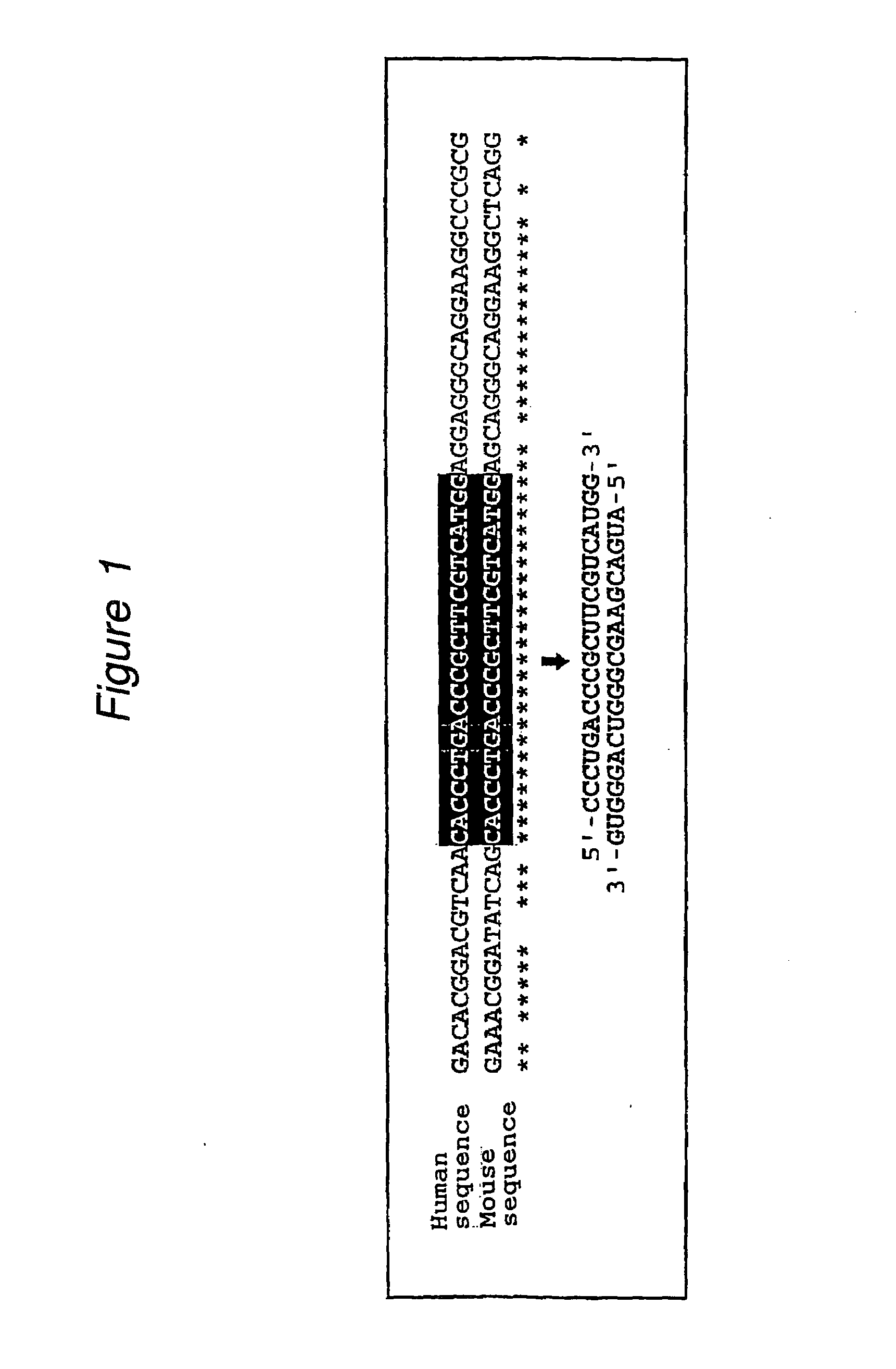
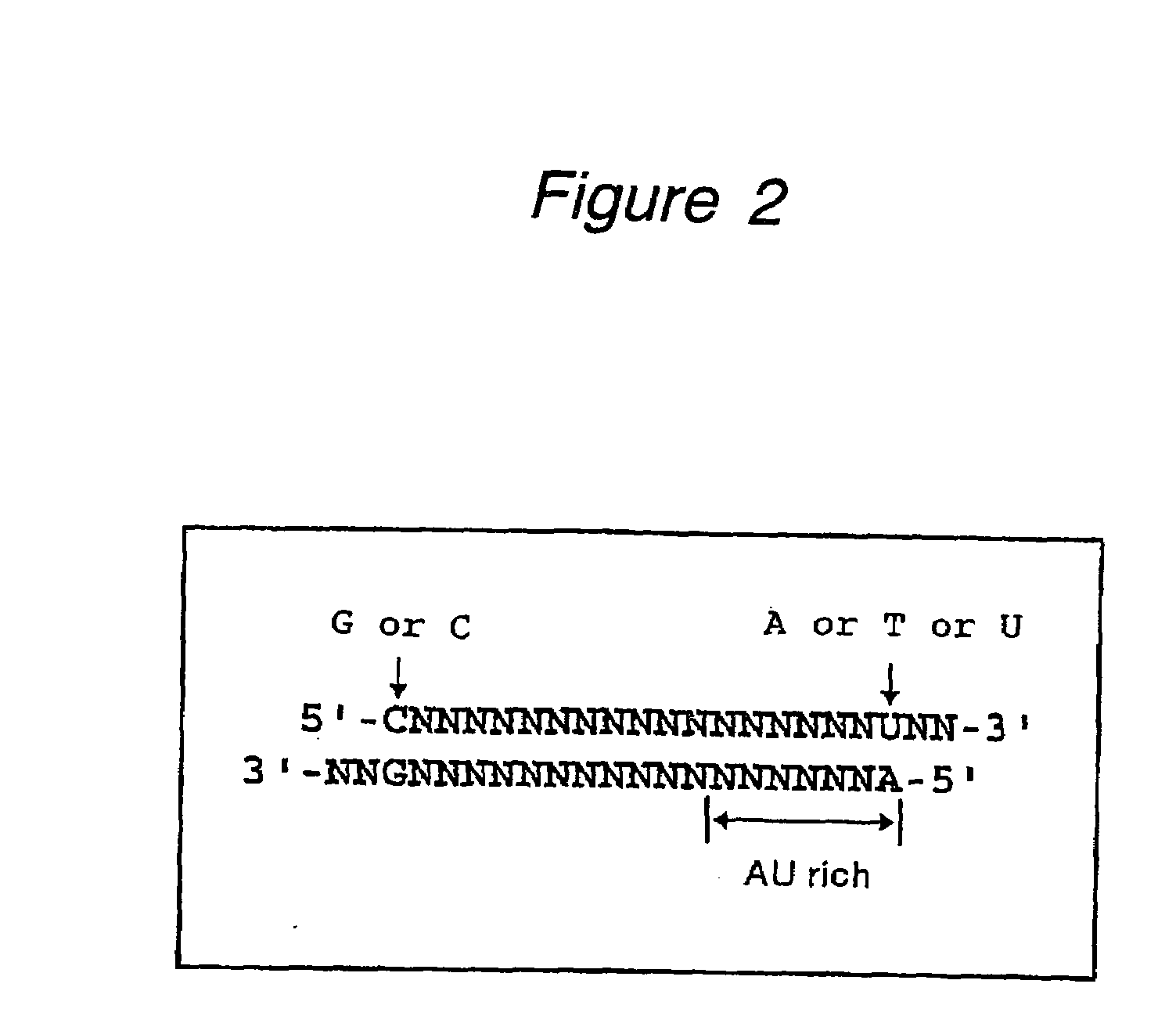
The present invention provides a polynucleotide that not only has a high RNA interference effect on its target gene, but also has a very small risk of causing RNA interference against a gene unrelated to the target gene. A sequence segment conforming to the following rules (a) to (d) is searched from the base sequences of a target gene for RNA interference and, based on the search results, a polynucleotide capable of causing RNAi is designed, synthesized, etc.:(a) The 3′ end base is adenine, thymine, or uracil,(b) The 5′ end base is guanine or cytosine,(c) A 7-base sequence from the 3′ end is rich in one or more types of bases selected from the group consisting of adenine, thymine, and uracil, and(d) The number of bases is within a range that allows RNA interference to occur without causing cytotoxicity.
Owner:ALPHAGEN
Modulation of stem and progenitor cell differentiation, assays, and uses thereof
InactiveUS20030235909A1Modulate their differentiationIncrease speedOrganic active ingredientsSenses disorderAssayPlacenta
The present invention relates to methods of modulating mammalian stem cell and progenitor cell differentiation. The methods of the invention can be employed to regulate and control the differentiation and maturation of mammalian, particularly human stem cells along specific cell and tissue lineages. The methods of the invention relate to the use of certain small organic molecules to modulate the differentiation of stem or progenitor cell populations along specific cell and tissue lineages, and in particular, to the differentiation of embryonic-like stem cells originating from a postpartum placenta or for the differentiation of early progenitor cells to a granulocytic lineage. Finally, the invention relates to the use of such differentiated stem or progenitor cells in transplantation and other medical treatments.
Owner:SIGNAL PHARMA LLC +2
Methods and compositions for the specific inhibition of gene expression by double-stranded RNA
The invention is directed to compositions and methods for selectively reducing the expression of a gene product from a desired target gene in a cell, as well as for treating diseases caused by the expression of the gene. More particularly, the invention is directed to compositions that contain double stranded RNA (“dsRNA”), and methods for preparing them, that are capable of reducing the expression of target genes in eukaryotic cells. The dsRNA has a first oligonucleotide sequence that is between 25 and about 30 nucleotides in length and a second oligonucleotide sequence that anneals to the first sequence under biological conditions. In addition, a region of one of the sequences of the dsRNA having a sequence length of at least 19 nucleotides is sufficiently complementary to a nucleotide sequence of the RNA produced from the target gene to trigger the destruction of the target RNA by the RNAi machinery.
Owner:CITY OF HOPE +1
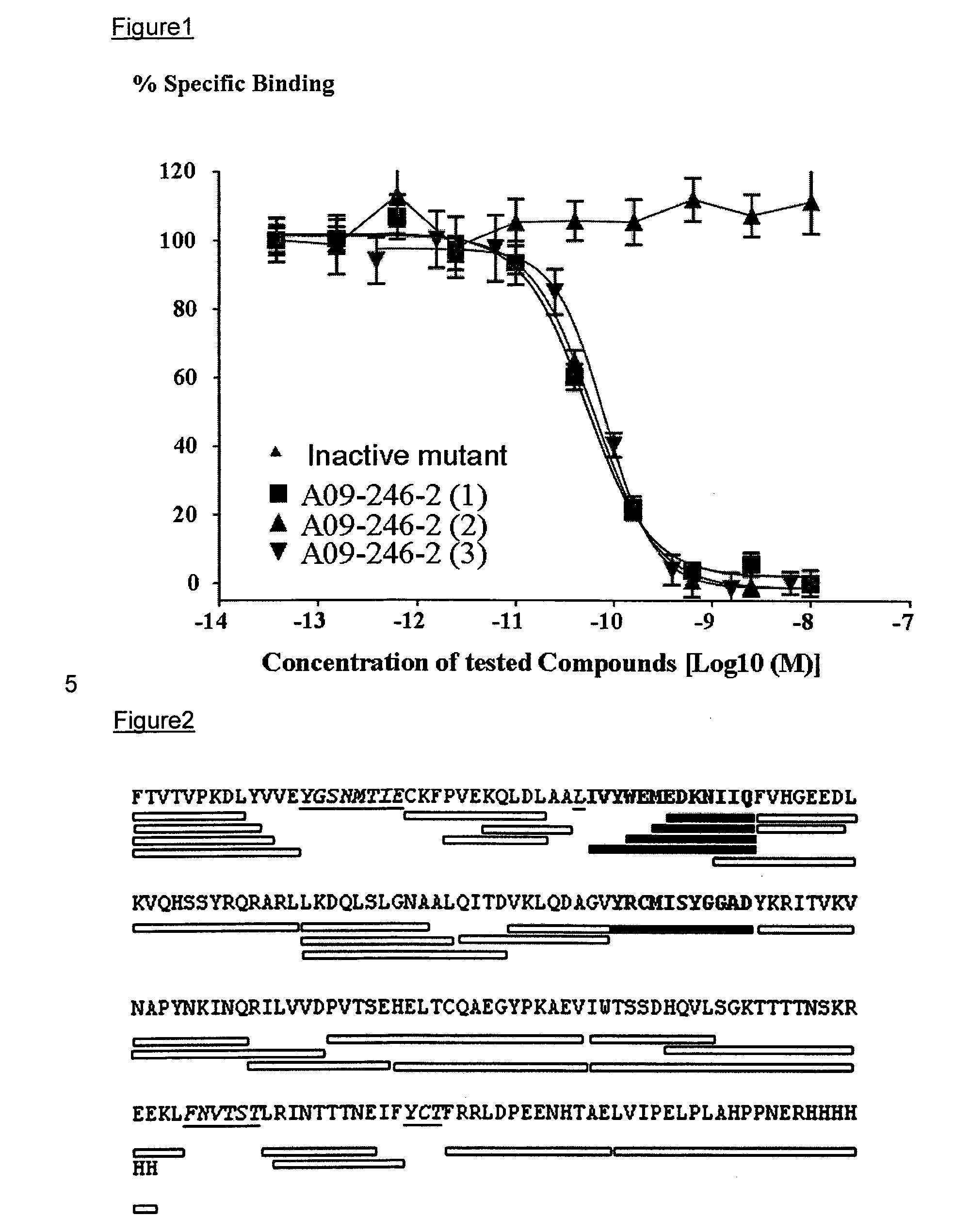
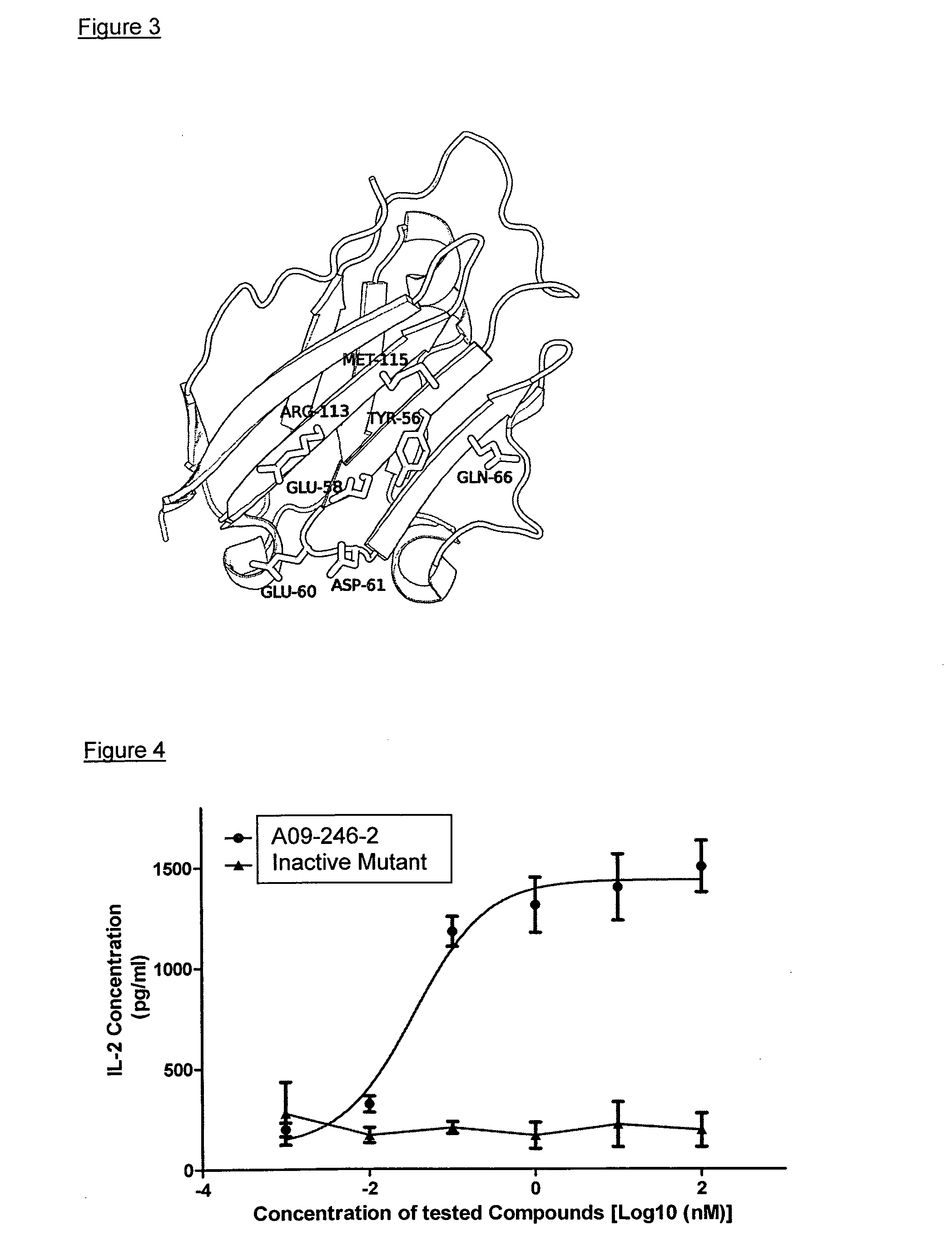
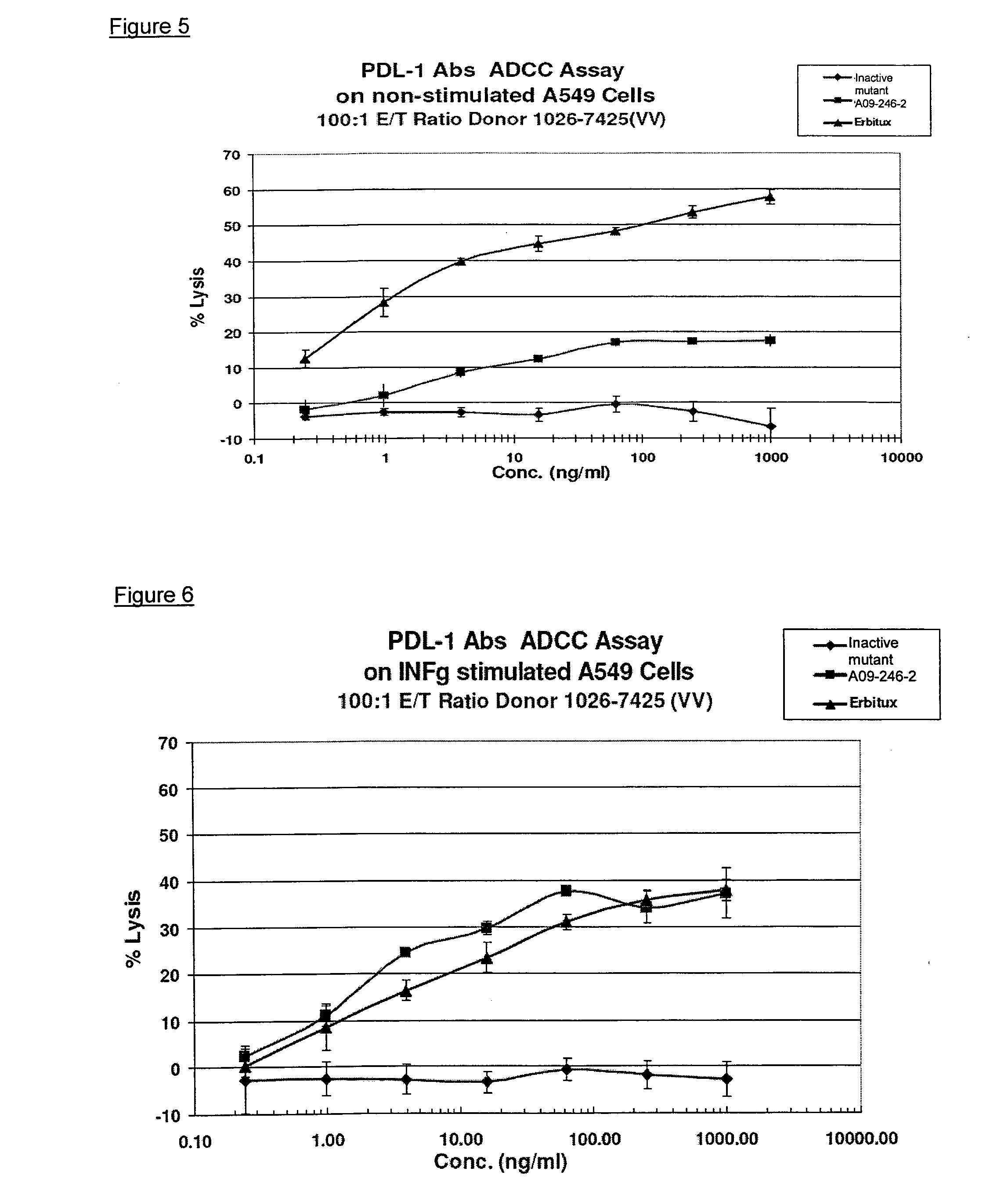
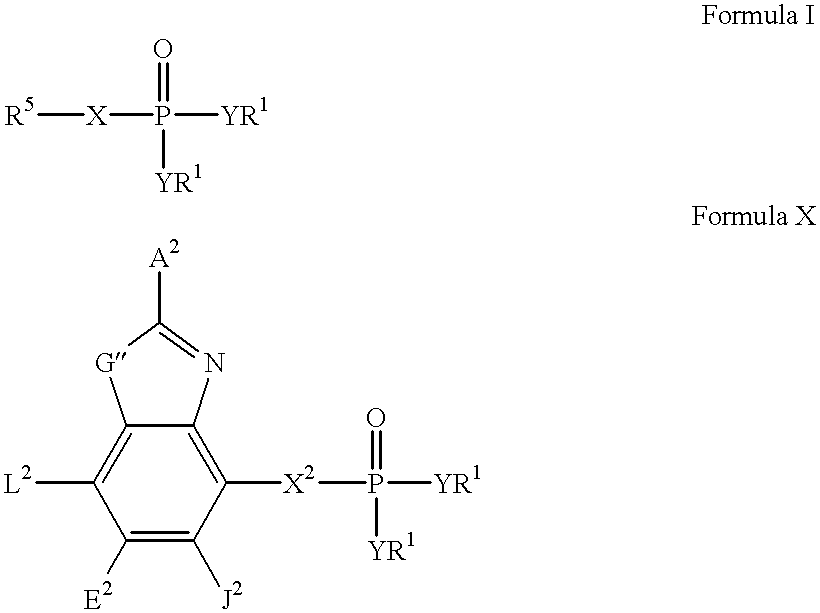
Anti-pd-l1 antibodies and uses thereof
ActiveUS20140341917A1Function increaseUpregulate cell-mediated immune responsesOrganic active ingredientsPeptide/protein ingredientsAntigen Binding FragmentAntigen binding
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
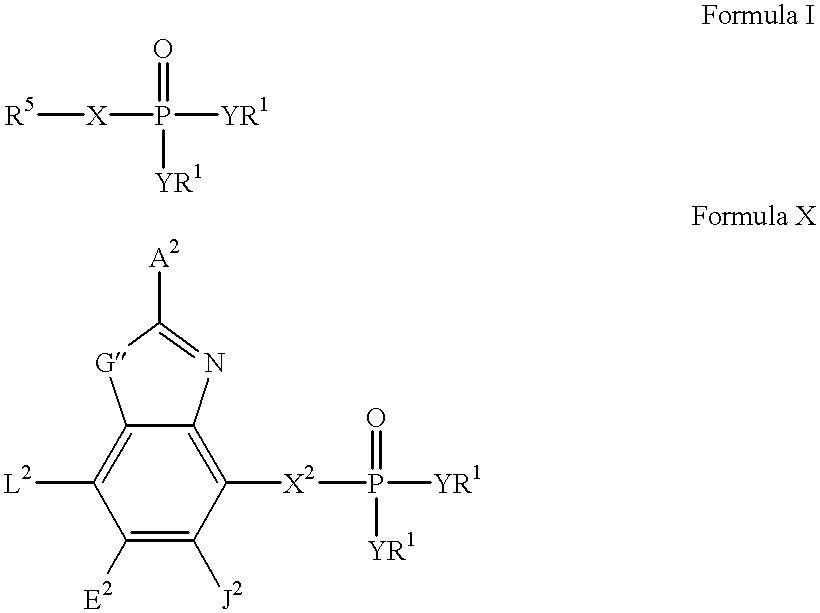
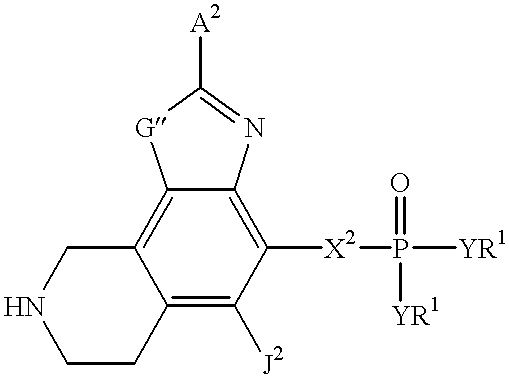
Heteroaromatic compounds containing a phosphonate group that are inhibitors of fructose-1,6-bisphosphatase
FBPase inhibitors of the formula I and Xare useful in the treatment of diabetes and other conditions associated with elevated blood glucose or excess glycogen storage.
Owner:METABASIS THERAPEUTICS INC
Method and composition for the treatment of diabetes
InactiveUS6153632AIncrease uptakeImprove utilizationBiocidePeptide/protein ingredientsIGT - Impaired glucose toleranceGlycosidase inhibitor
This invention is directed to a novel method and composition for the treatment of diabetes mellitus (Type I, Impaired Glucose Tolerance ["IGT"]and Type II). More specifically, this invention pertains to a novel method of treating diabetes mellitus by incorporating a therapeutic amount of one or more insulin sensitizers along with one or more of an orally ingested insulin, an injected insulin, a sulfonylurea, a biguanide or an alpha-glucosidase inhibitor for the treatment of diabetes mellitus.
Owner:RIEVELEY CHERYL ANNE
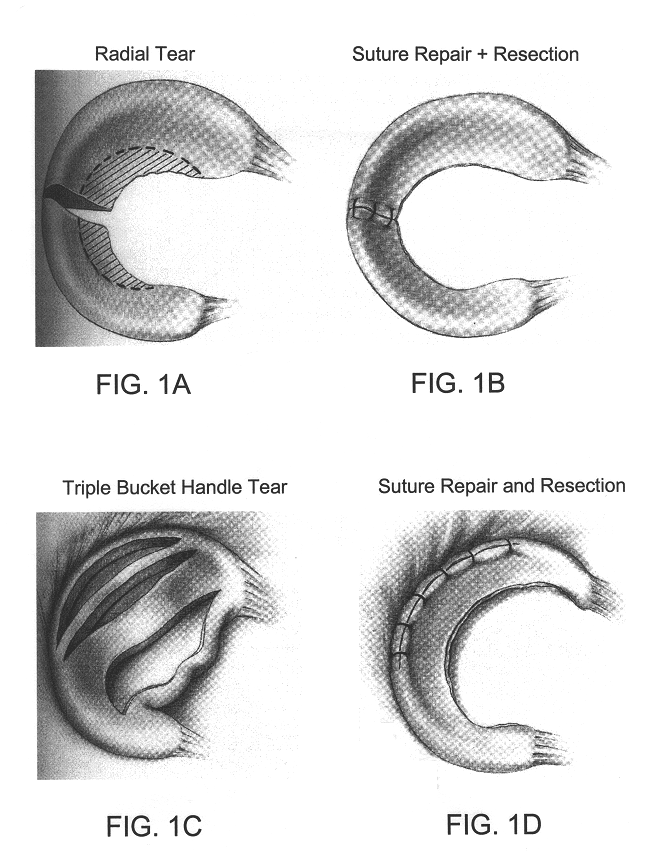
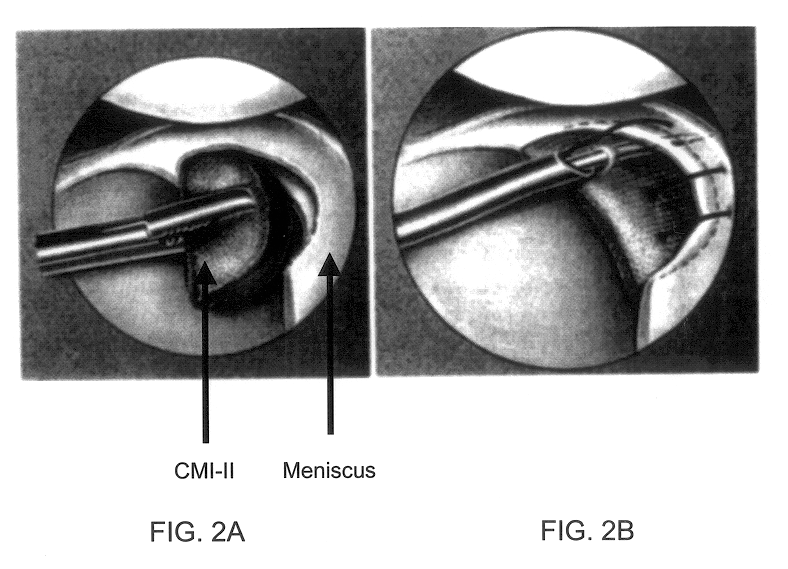
Compositions for regeneration and repair of cartilage lesions
InactiveUS6511958B1Increase ratingsImprove repair qualitySuture equipmentsPowder deliveryMedicineCartilage lesion
Disclosed is a cartilage repair product that induces both cell ingrowth into a bioresorbable material and cell differentiation into cartilage tissue. Such a product is useful for regenerating and / or repairing both vascular and avascular cartilage lesions, particularly articular cartilage lesions, and even more particularly mensical tissue lesions, including tears as well as segmental defects. Also disclosed is a method of regenerating and repairing cartilage lesions using such a product.
Owner:ZIMMER ORTHOBIOLOGICS
Anti-IGFR antibody therapeutic combinations
InactiveUS20050136063A1Inhibit growthPrevent proliferationNervous disorderAntipyreticAnti-CEA AntibodyAntibody
Owner:MERCK SHARP & DOHME CORP
Methods of using and compositions comprising immunomodulatory compounds for treatment and management of macular degeneration
InactiveUS20040091455A1Extension of timeUseful in treatingBiocideSenses disorderActive agentSurgical procedures
Methods of treating, preventing and / or managing macular degeneration are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or surgery. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Compositions and methods for treating a posterior segment of an eye
InactiveUS20050101582A1Improve abilitiesIncrease viscosity of compositionAntibacterial agentsOrganic active ingredientsShear ratePharmacology
Compositions, and methods of using such compositions, useful for injection into the posterior segments of human or animal eyes are provided. Such compositions include corticosteroid component-containing particles present in a therapeutically effective amount, a viscosity inducing component, and an aqueous carrier component. The compositions have viscosities of at least about 10 cps or about 100 cps at a shear rate of 0.1 / second. In a preferred embodiment, the viscosity is in the range of from about 140,000 cps to about 300,000 cps. The compositions advantageously suspend the particles for prolonged periods of time.
Owner:ALLERGAN INC
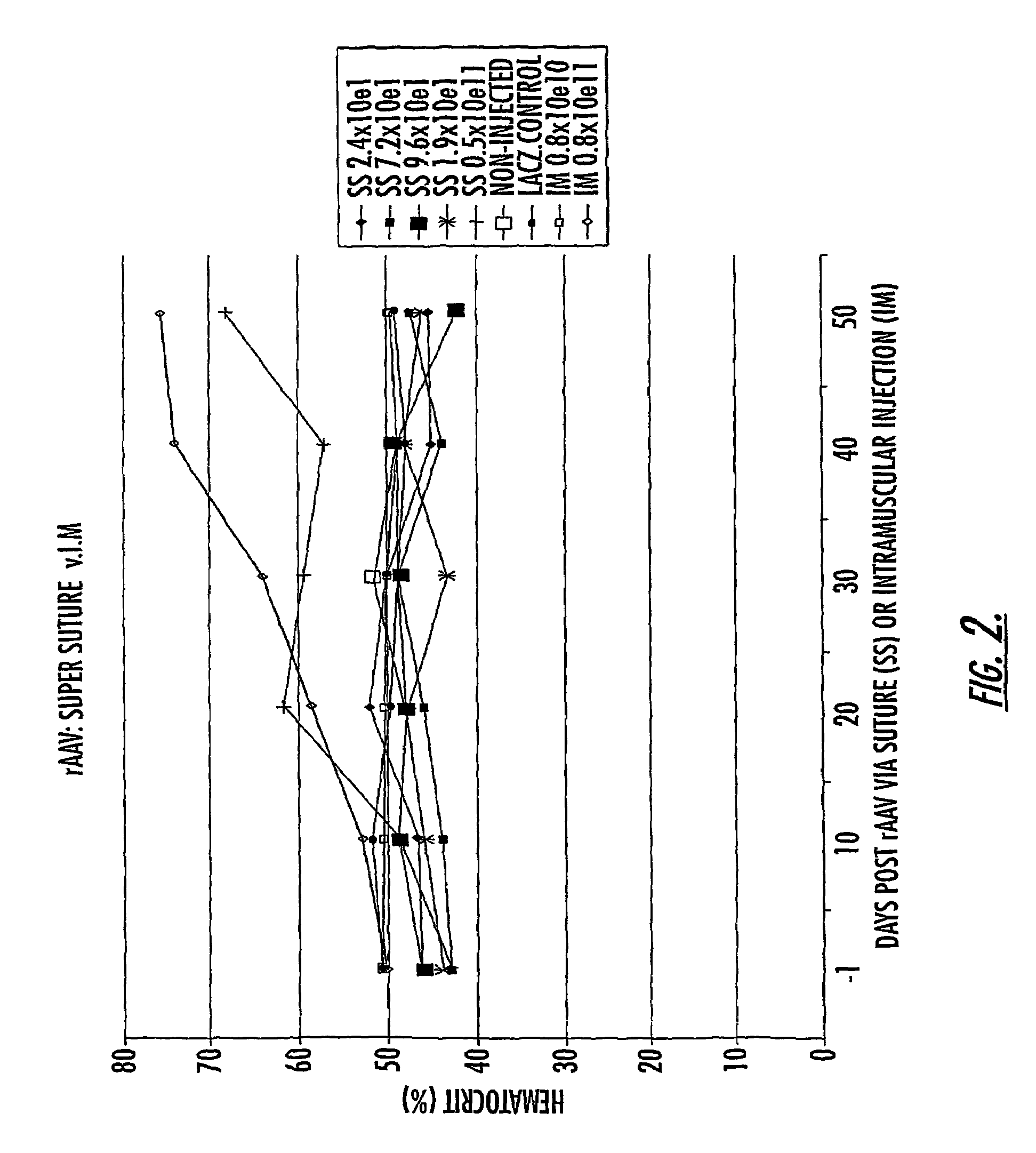
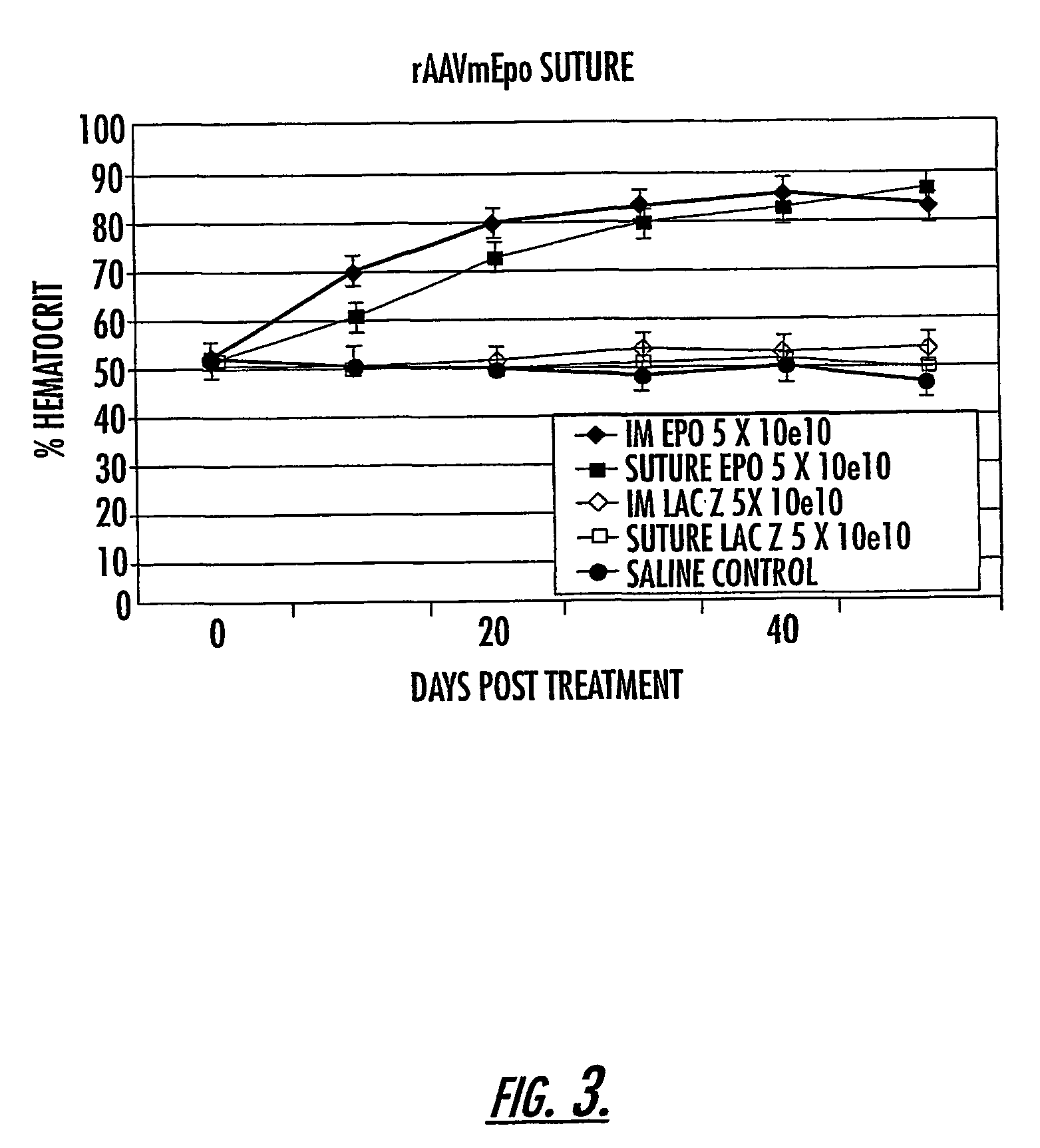
Methods and compounds for controlled release of recombinant parvovirus vectors
InactiveUS7201898B2Prevent relapseImproved pulmonary mechanicsSuture equipmentsAntibacterial agentsControlled releaseSupport matrix
The invention uses recombinant parvoviruses, and particularly recombinant adeno-associated virus (rAAV) to deliver genes and DNA sequences for gene therapy following manipulation of the therapeutic virus for packaging and transport. The invention delivers therapeutic viral vectors via rAAV affixed to support matrixes (i.e., sutures, surgically implantable materials, grafts, and the like).
Owner:NORTH CAROLINA AT CHAPEL HILL THE UNIV OF
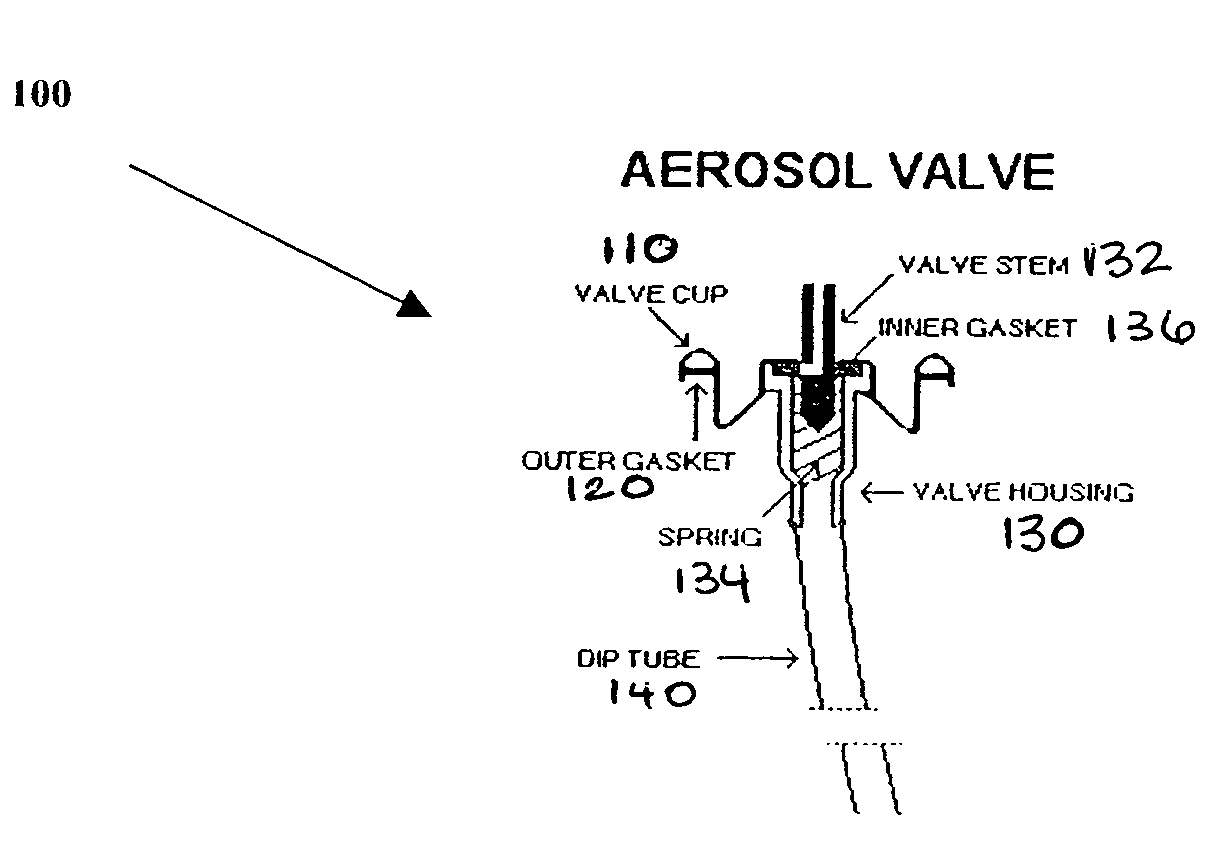
Steroid kit and foamable composition and uses thereof
InactiveUS20060018937A1Preventing and alleviatingCosmetic preparationsSenses disorderActive agentFilm-forming agent
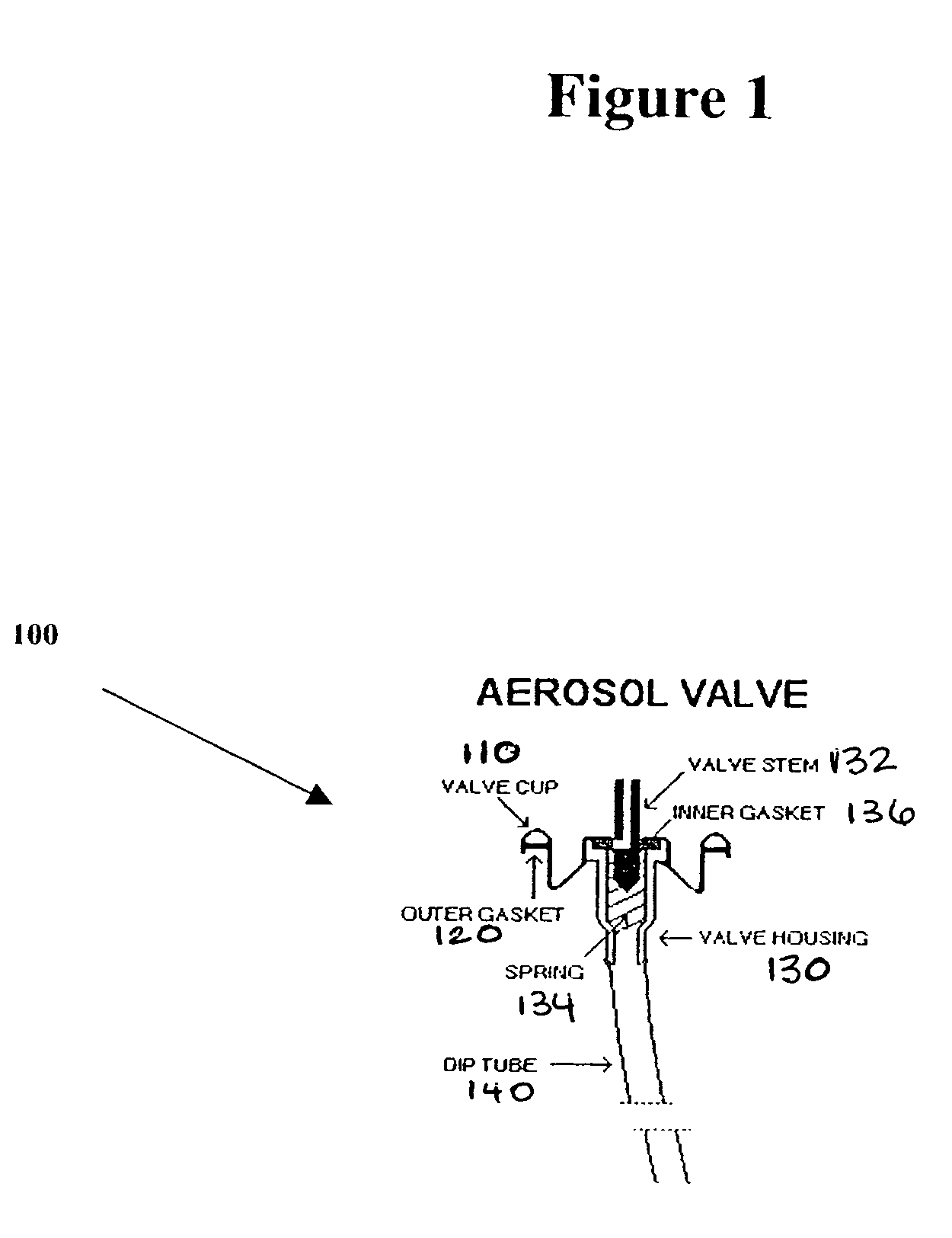
A composition and therapeutic kit including an aerosol packaging assembly including a container accommodating a pressurized product and an outlet capable of releasing a foamable composition, including a steroid as a foam. The pressurized product includes a foamable composition including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam; wherein the pressurized product comprises a foamable composition including: i. a steroid; ii. at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, a polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight; iii. a surface-active agent; iv. about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent; v. water; and vi. liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition. The composition further may include a therapeutically active foam adjuvant, selected from the group consisting of a fatty alcohol, a fatty acid, a hydroxyl fatty acid; and mixtures thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Cysteine variants of erythropoietin
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
Quinazolinones as inhibitors of human phosphatidylinositol 3-kinase delta
ActiveUS7932260B2Inhibit growth and proliferationInhibit growthBiocideSenses disorderLeukocyte functionWhite blood cell
The invention provides a class of substituted quinazolinone compounds and methods of treating diseases mediated by PI3Kδ activity. The disclosed compounds are useful in treating diseases such as bone-resorption disorders; and cancer, especially hematopoietic cancers, lymphomas, multiple myelomas and leukemia. The compounds are also useful in disrupting or inhibiting cellular processes such as leukocyte function or accumulation, neutrophils function, lymphocyte proliferation, and endogenous immune responses.
Owner:ICOS CORP
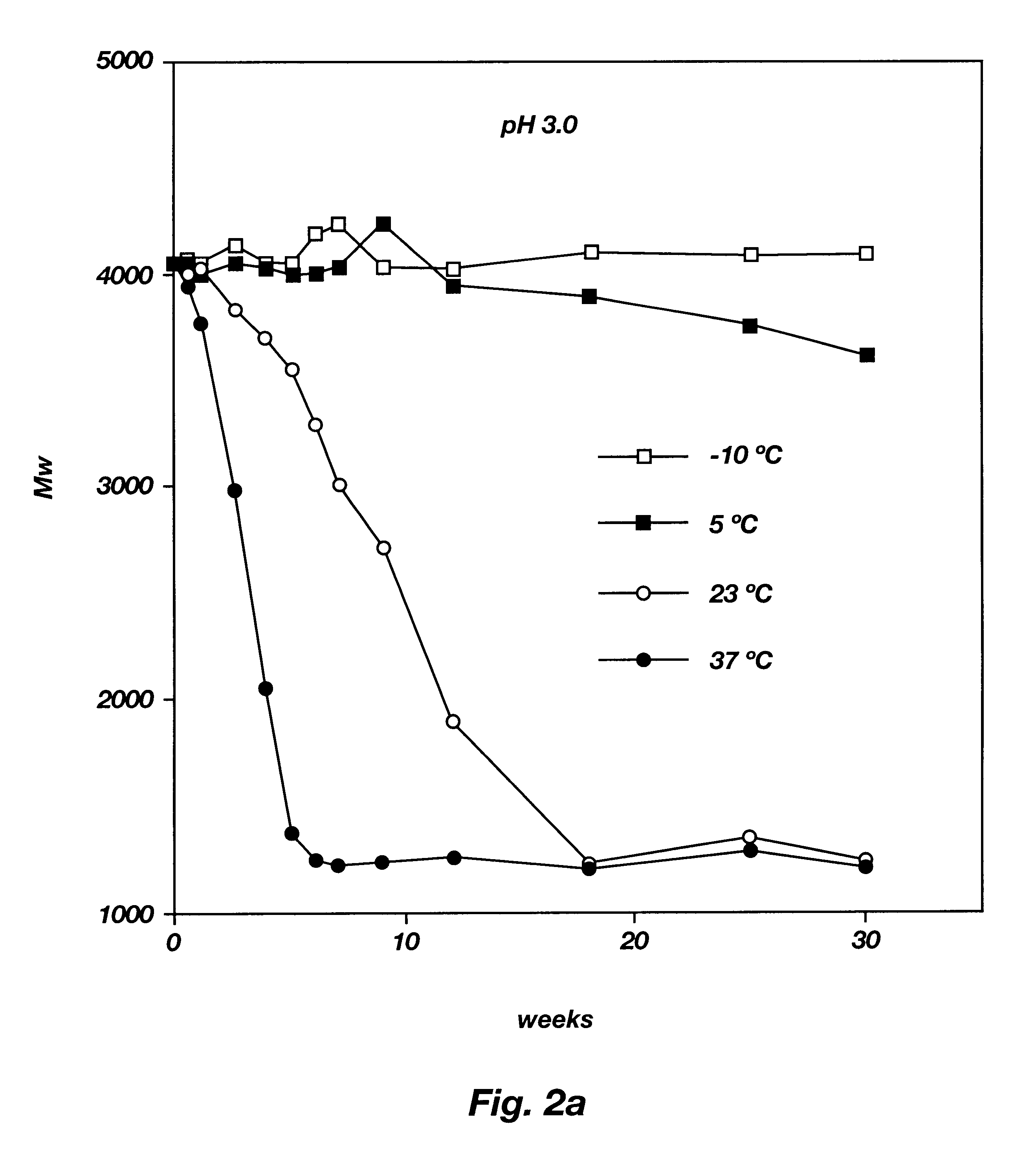
Sustained release pharmaceutical compositions for the parenteral administration of hydrophilic compounds
InactiveUS7157099B2Easy to prepareOrganic active ingredientsPeptide/protein ingredientsParenteral nutritionBULK ACTIVE INGREDIENT
Owner:ITALFARMACO SPA
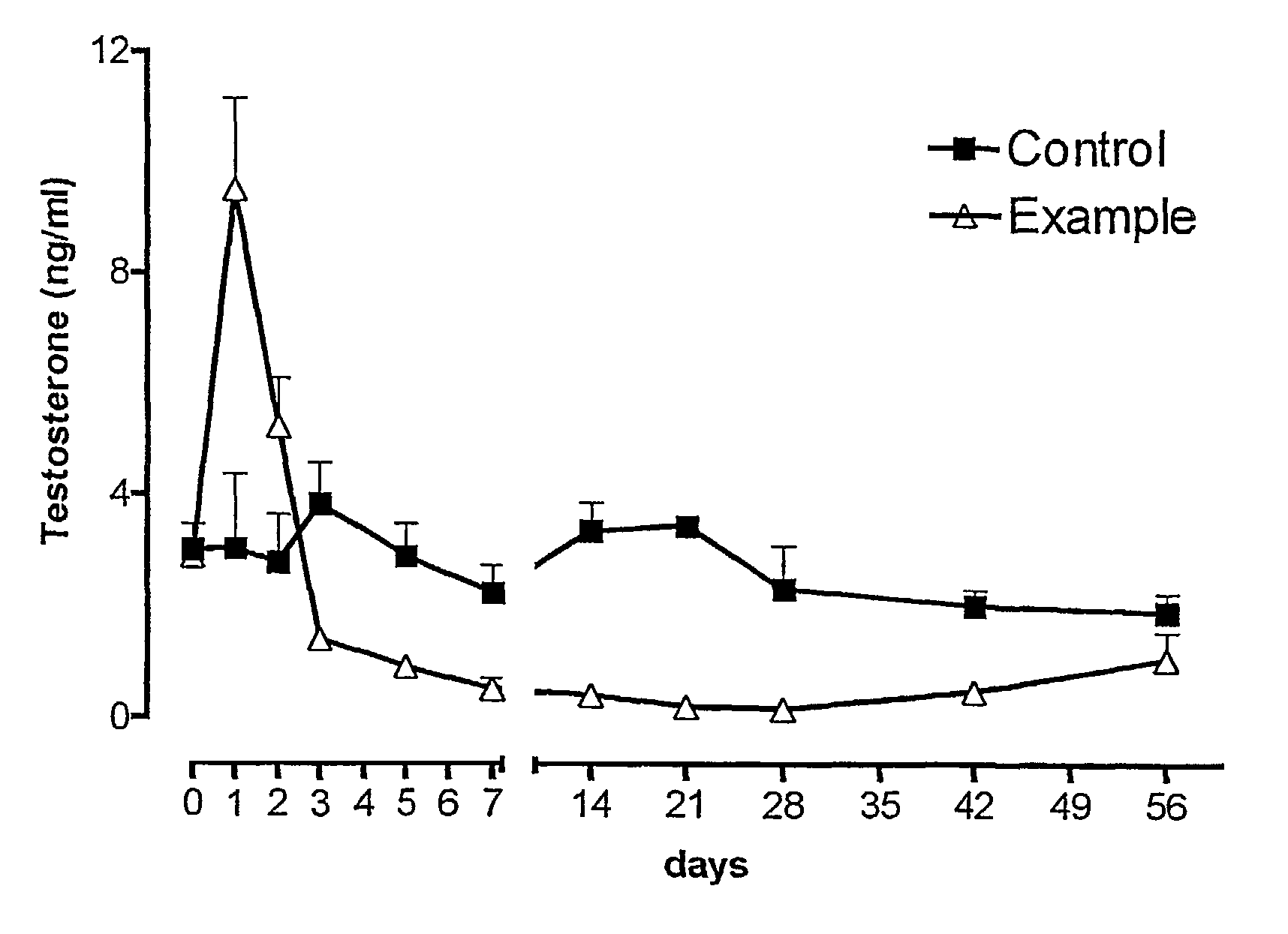
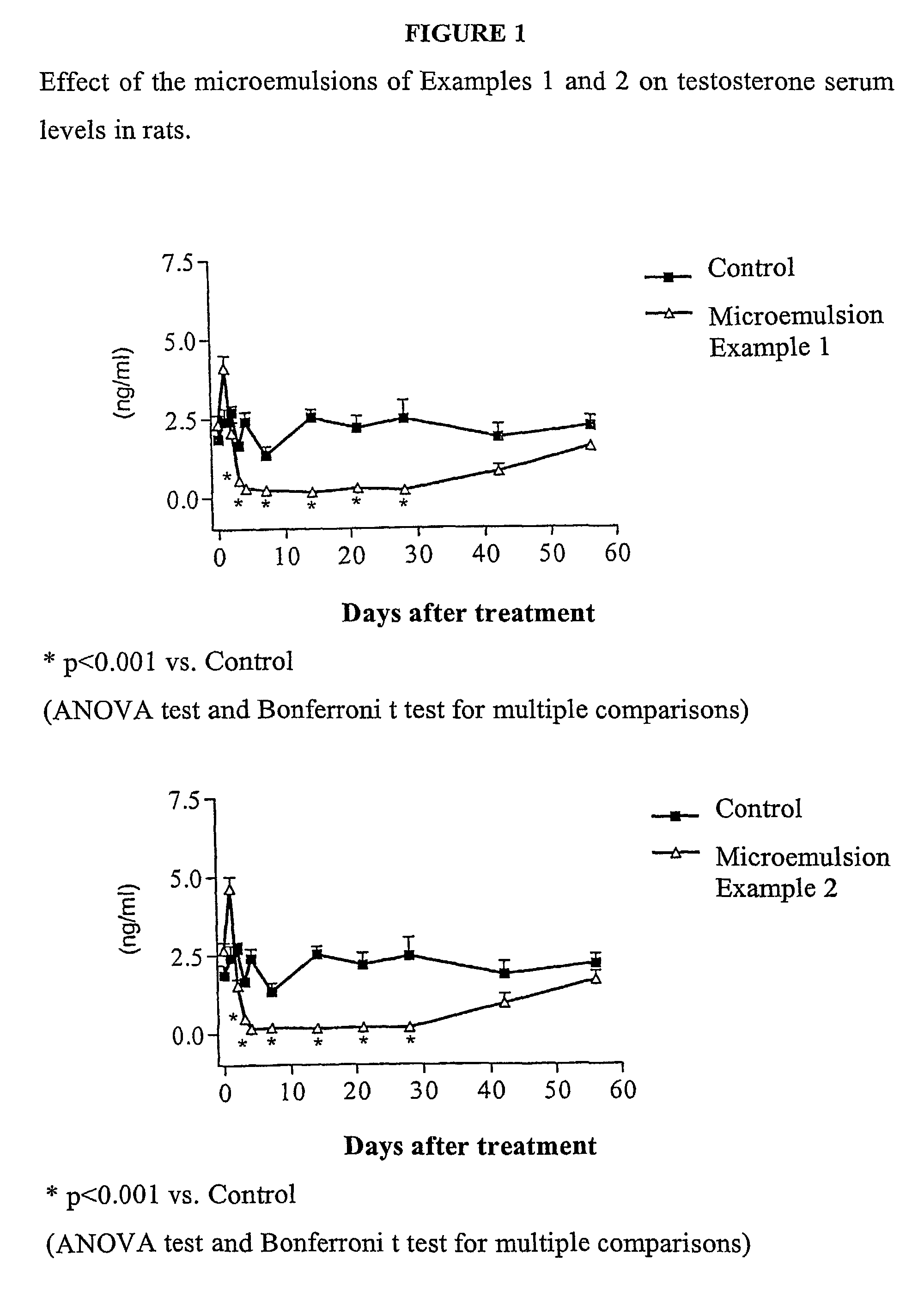
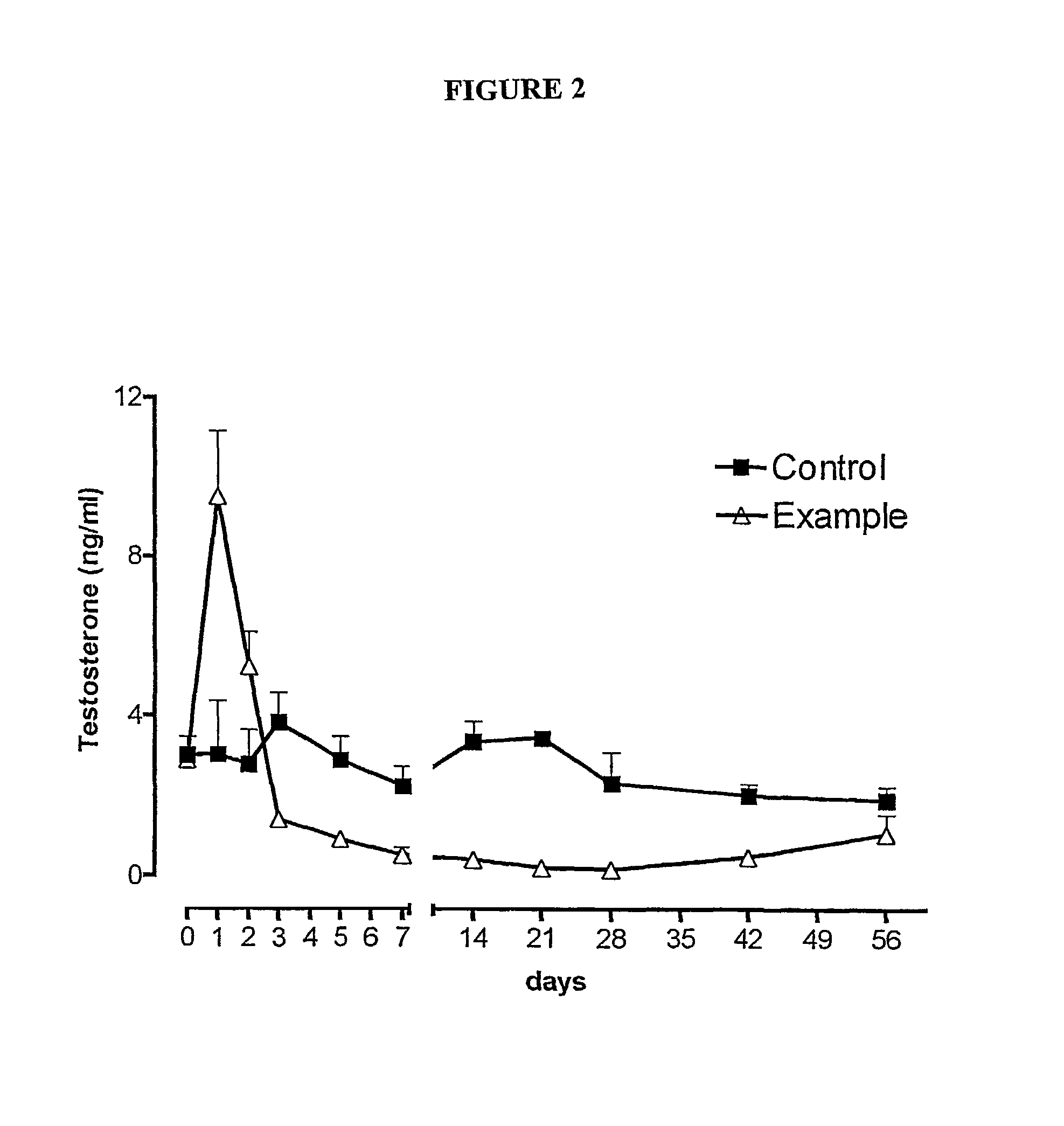
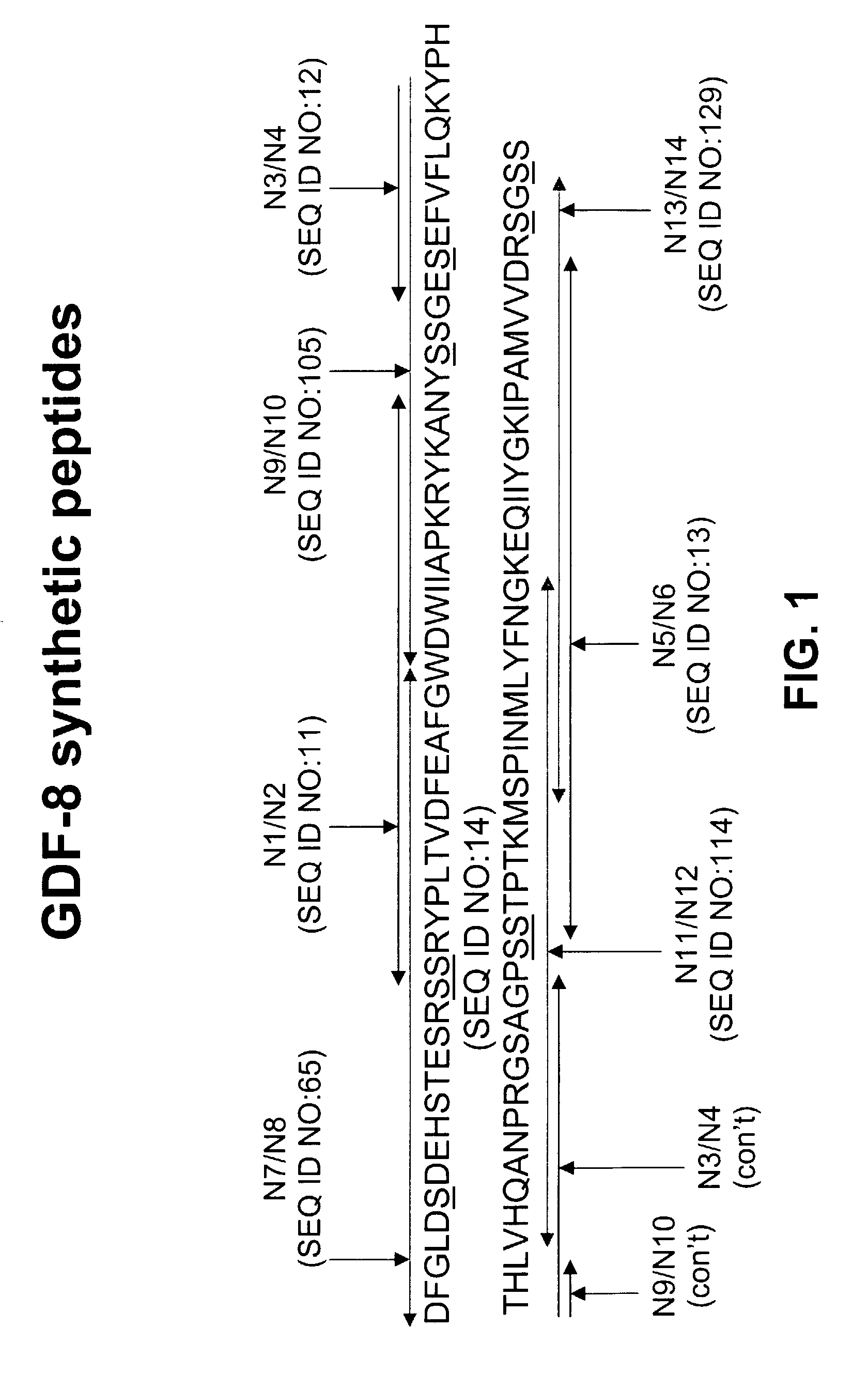
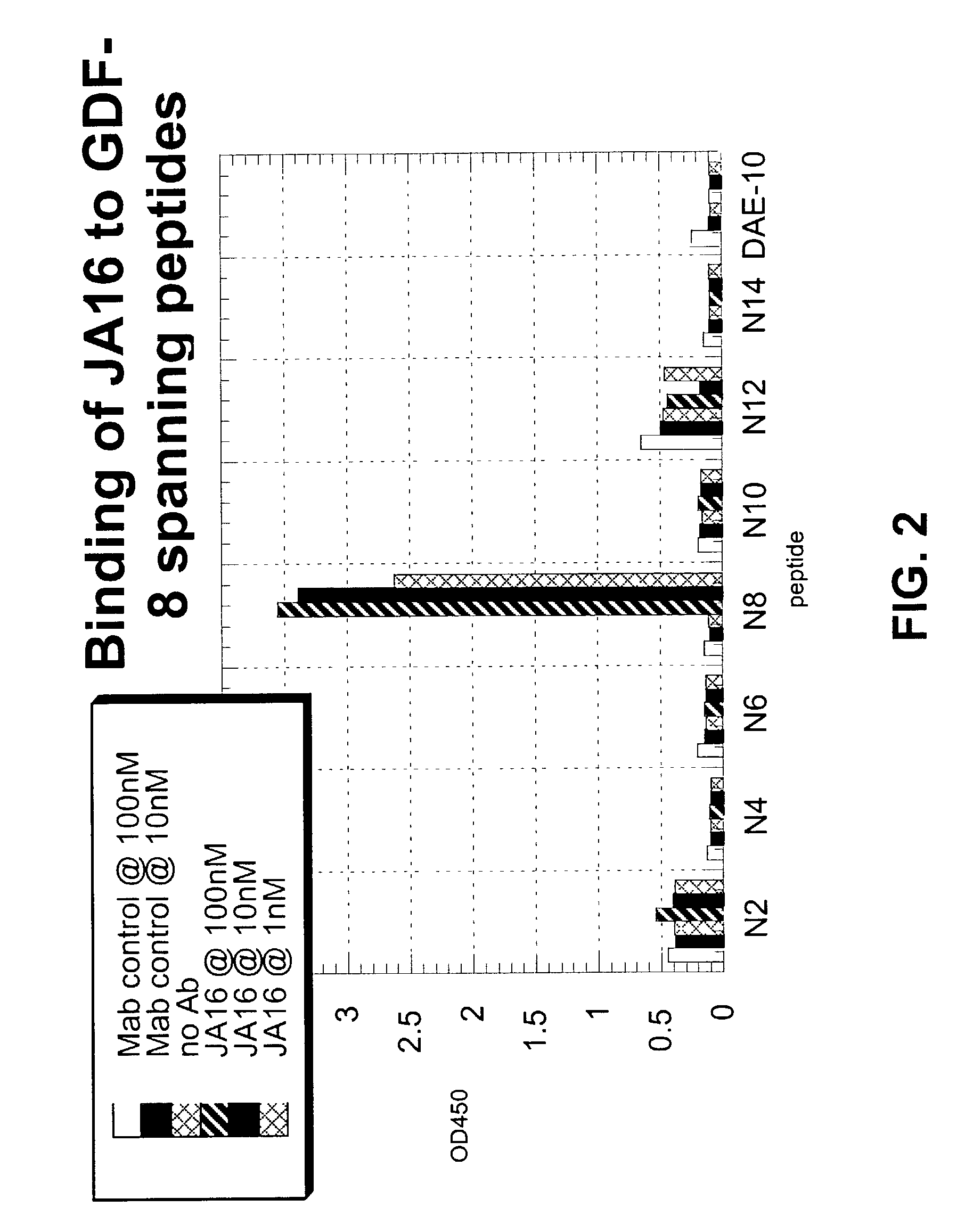
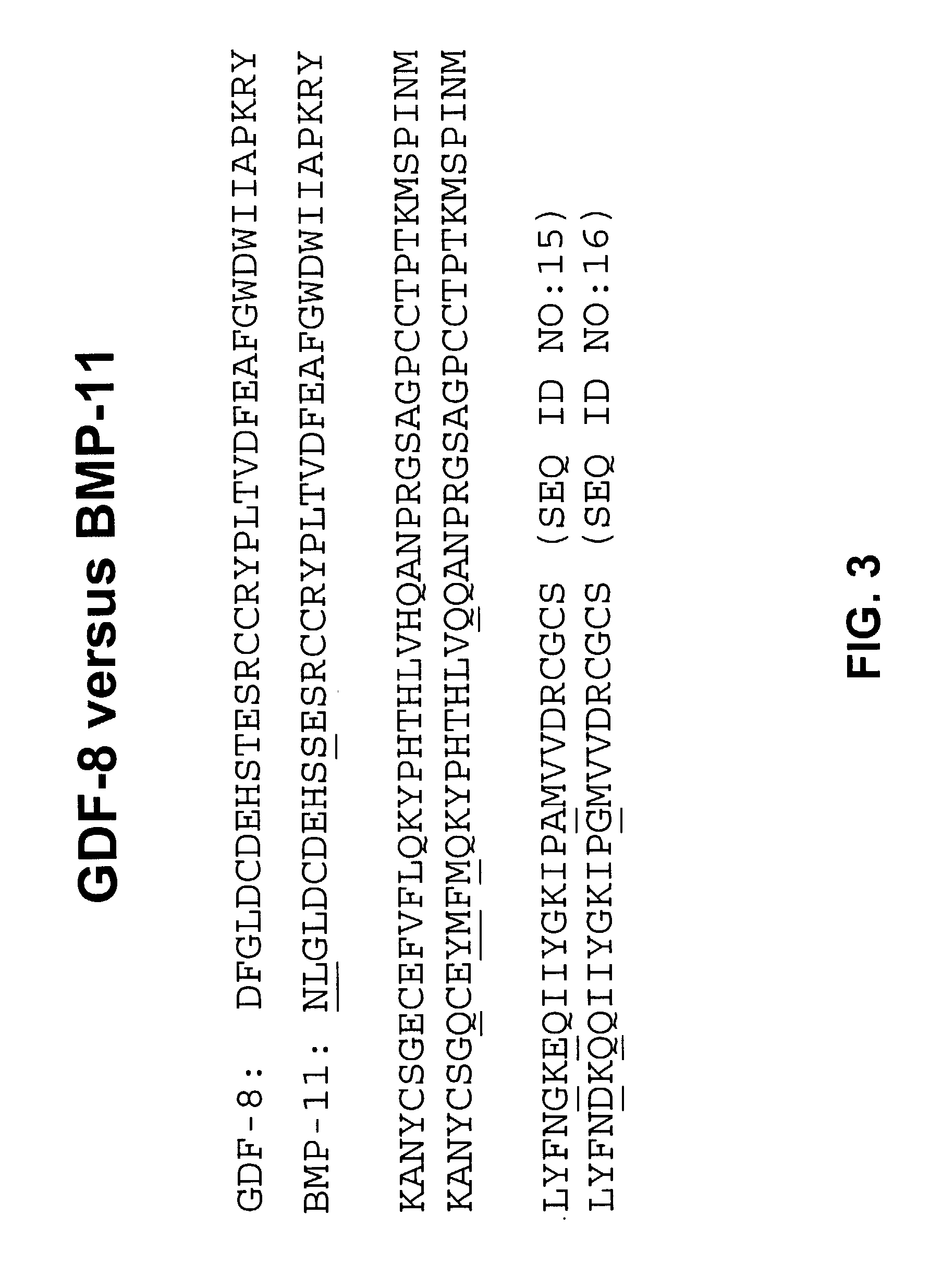
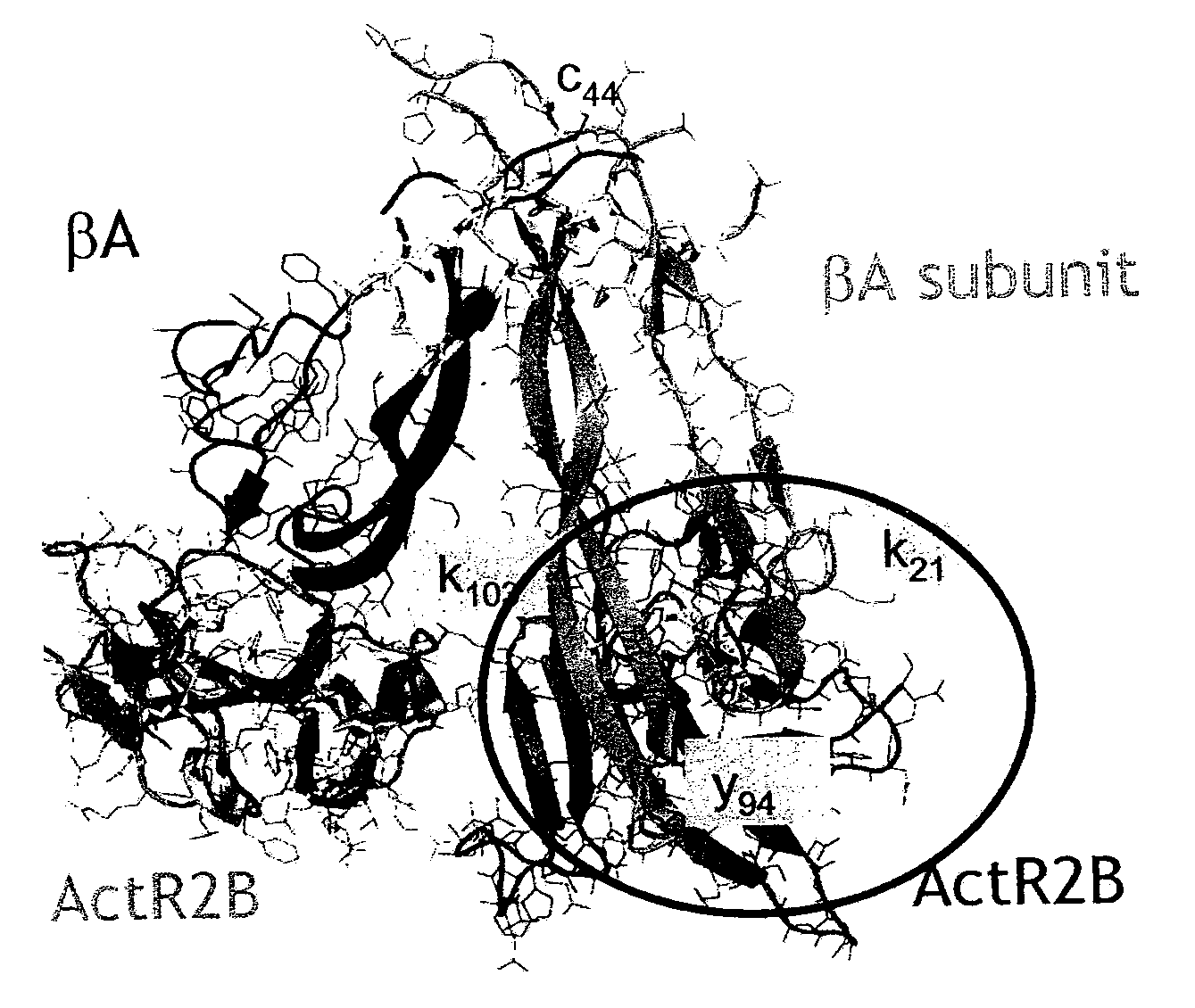
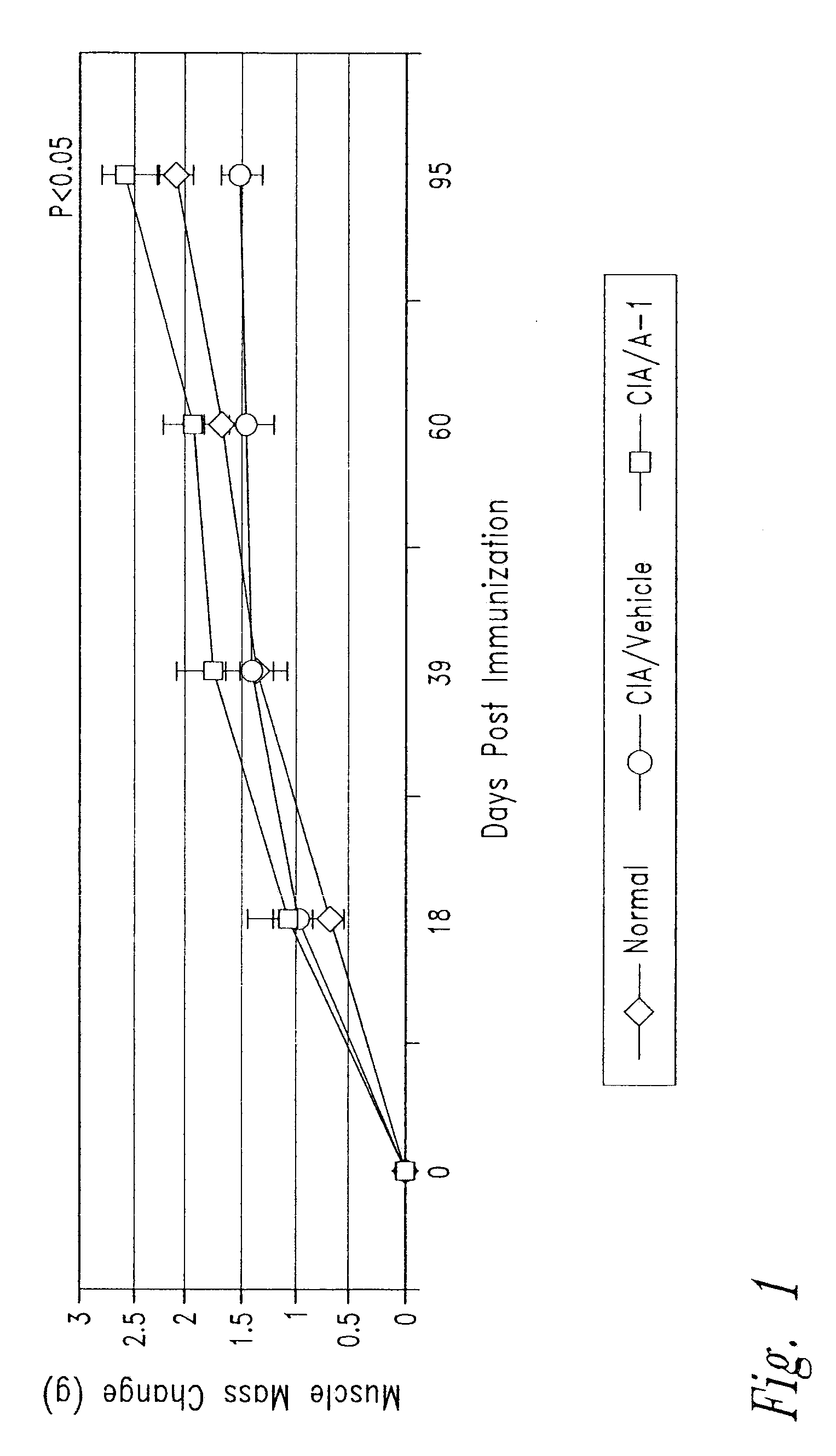
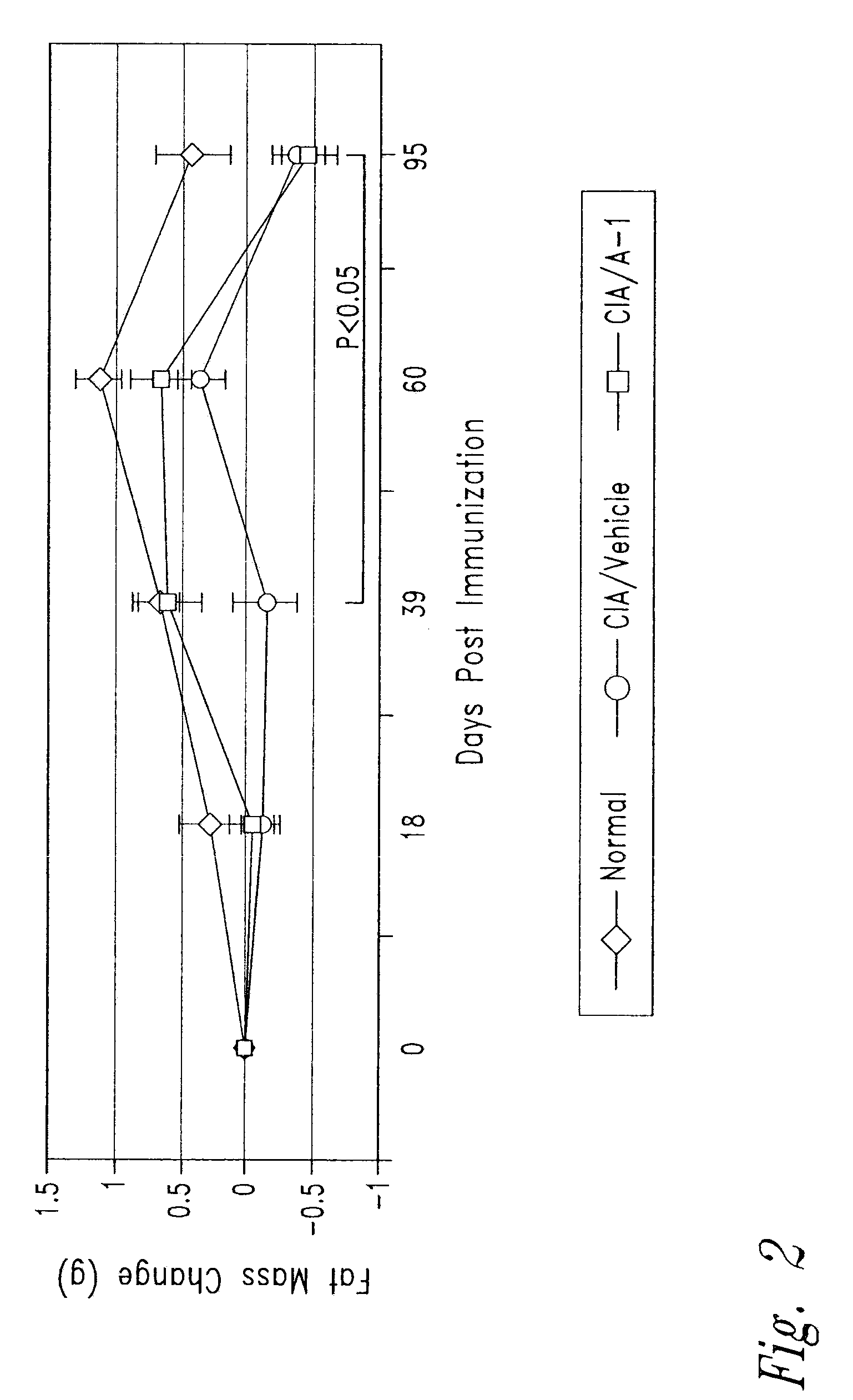
Antibody inhibitors of GDF-8 and uses thereof
ActiveUS7320789B2Reduction in one or more of the biological activitiesReduced activityNervous disorderMuscular disorderAntibody inhibitorAntibody fragments
The disclosure provides novel antibodies against growth and differentiation factor-8 (GDF-8), including antibody fragments, which inhibit GDF-8 activity in vitro and in vivo. The disclosure also provides methods for diagnosing, preventing, or treating degenerative disorders of muscle, bone, or insulin metabolism.
Owner:WYETH LLC
Degraded agonist antibody
InactiveUS20040242847A1Excellent antigen-binding propertyExcellent agonist activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseAntiendomysial antibodies
The invention relates to a modified antibody which contains two or more H chain V regions and two or more L chain V regions of monoclonal antibody and can transduce a signal into cells by crosslinking a cell surface molecule(s) to thereby serve as an agonist. The modified antibody can be used as a signal transduction agonist and, therefore, useful as a preventive and / or remedy for various diseases such as cancer, inflammation, hormone disorders and blood diseases.
Owner:CHUGAI PHARMA CO LTD
Oleaginous pharmaceutical and cosmetic foam
ActiveUS20050031547A1Pleasant and easy to spreadPatient compliance is goodAntibacterial agentsCosmetic preparationsActive agentNon ionic
The invention relates to stable oleaginous cosmetic or therapeutic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent selected from a hydrophobic solvent, a silicone oil, an emollient, a co-solvent, and mixtures thereof, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition; at least one gelling agent at a concentration of about 0.1% to about 5% by weight of the total composition; a therapeutically effective amount of at least one active agent; and at least one liquefied or compressed gas propellant, at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
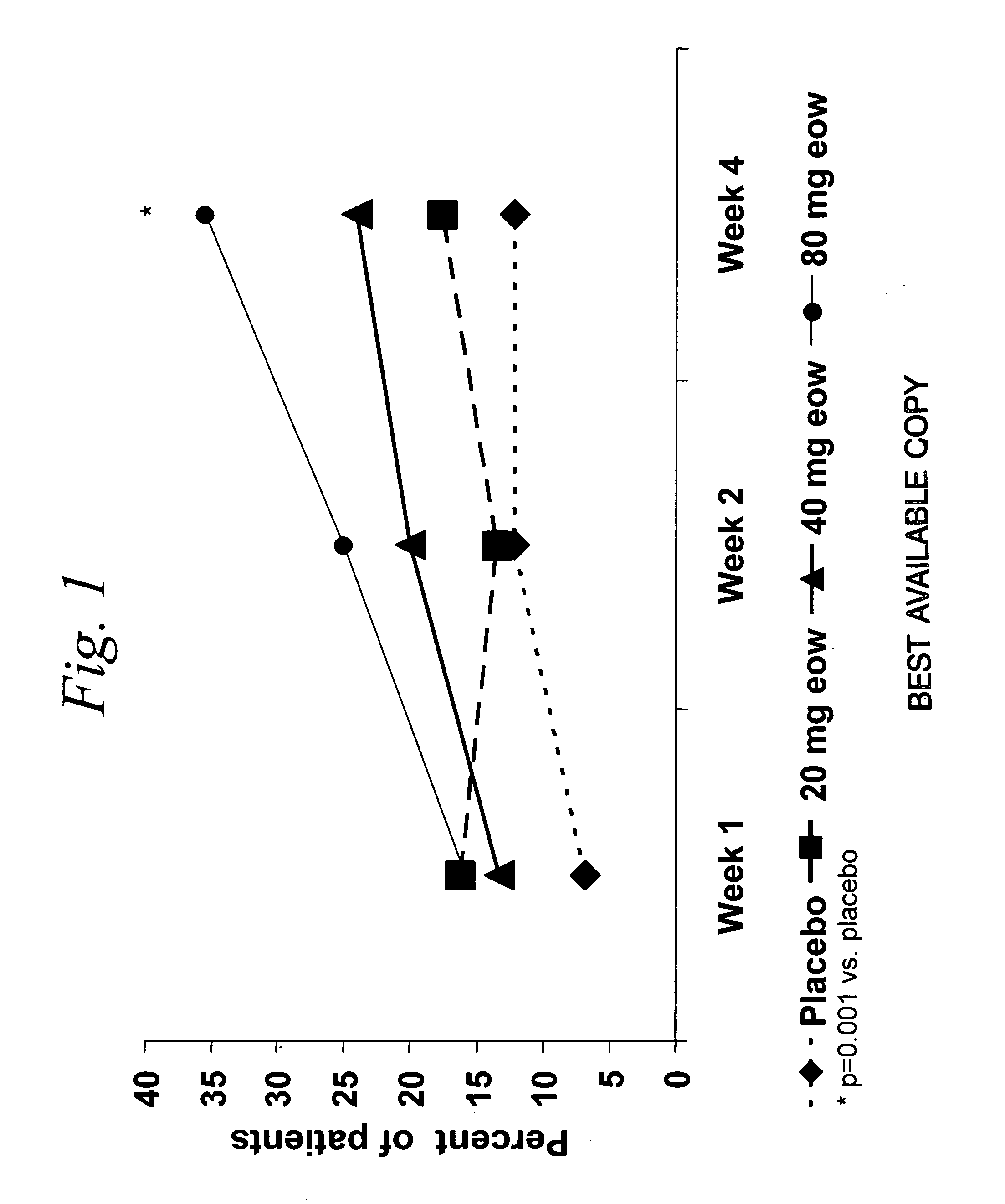
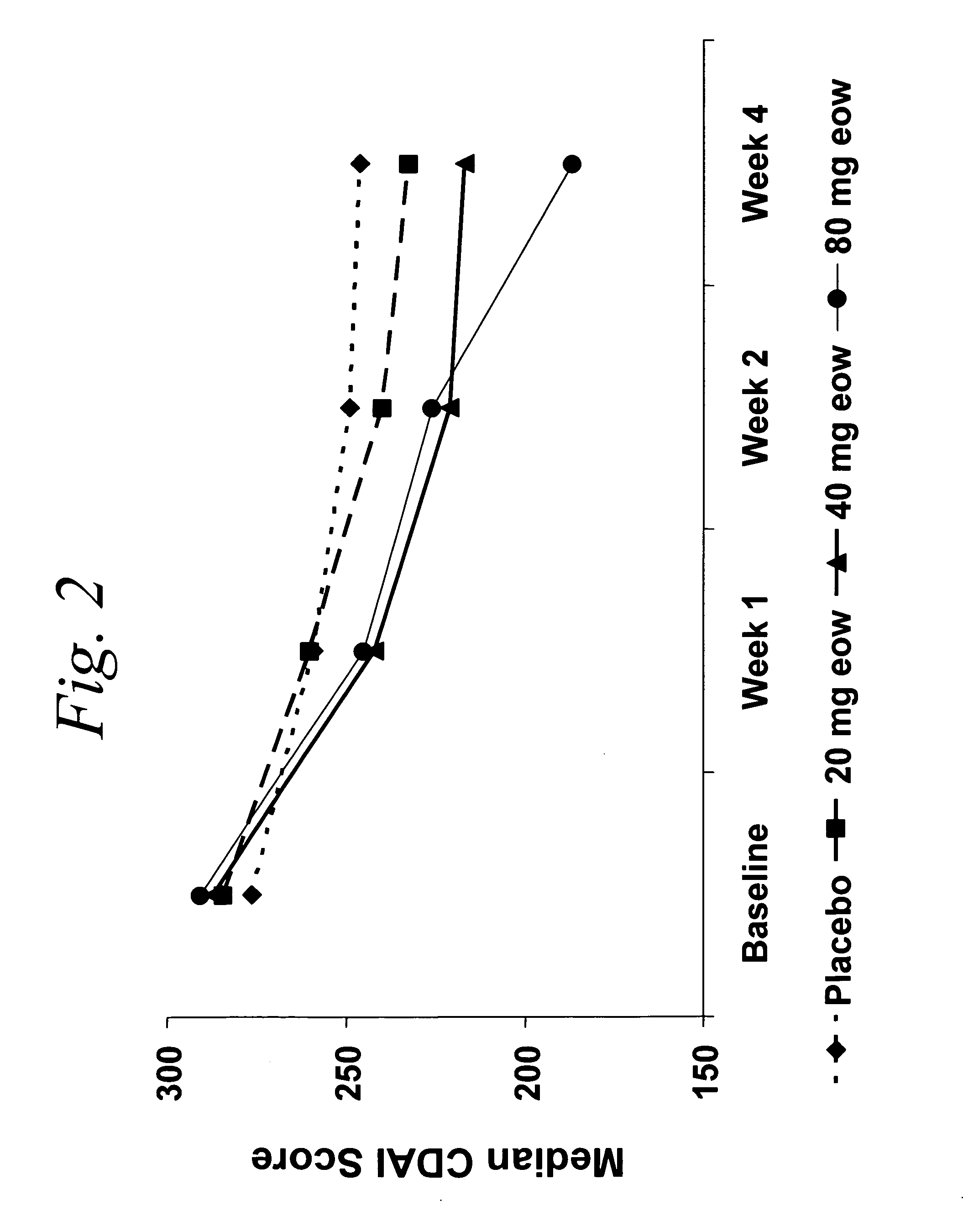
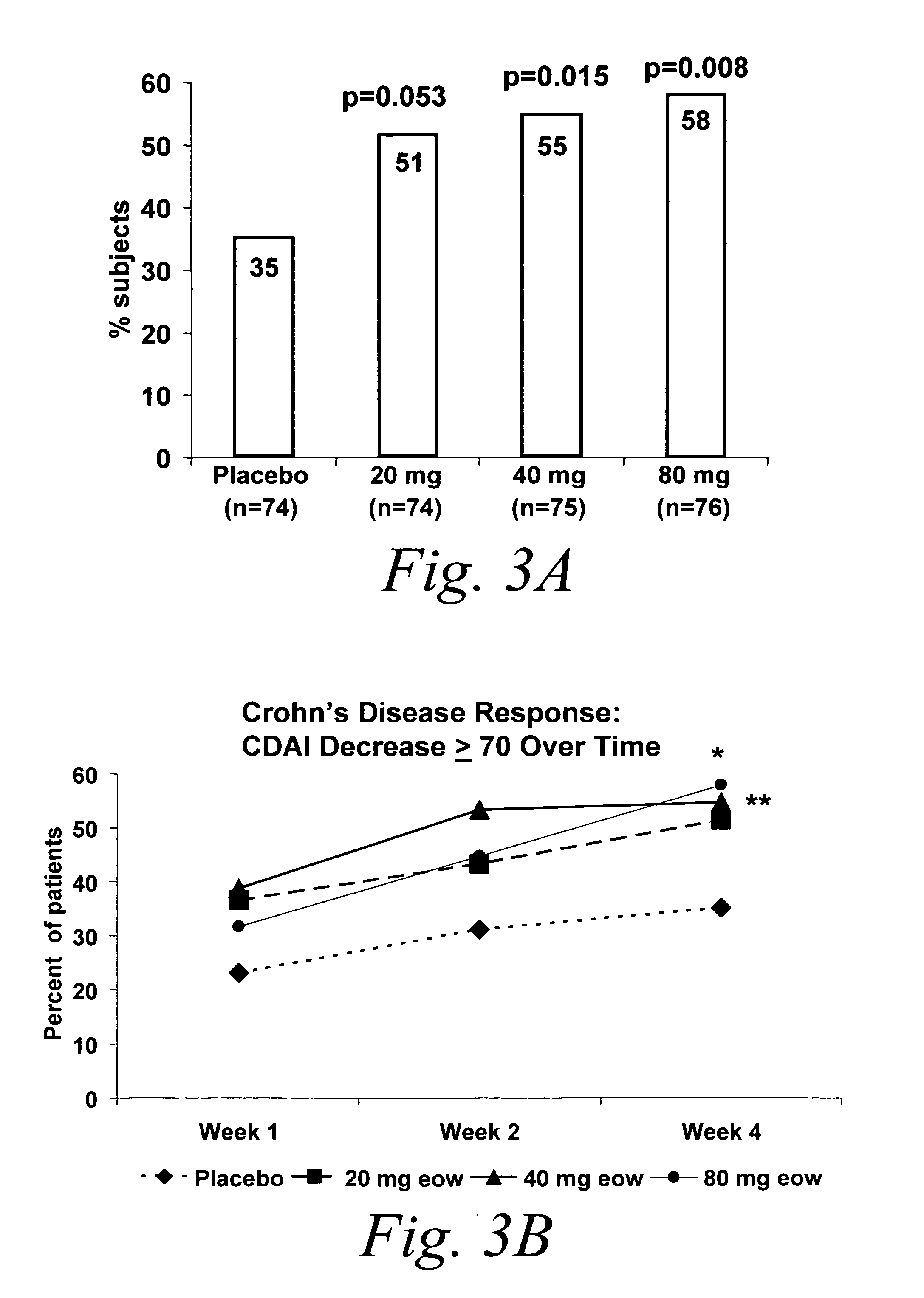
Multiple-variable dose regimen for treating TNFalpha-related disorders
ActiveUS20060009385A1Reduce decreaseIncrease the areaBiocideOrganic active ingredientsDosing regimenRegimen
Multiple-variable dose methods for treating TNFα-related disorders, including Crohn's disease and psoriasis, comprising administering TNFα inhibitors, including TNFα antibodies, are described. Multiple-variable dose methods include administration of a TNF-inhibitor in an induction or loading phase followed by administration of the agent in a maintenance or treatment phase, wherein the TNF-inhibitor is administered in a higher dosage during the induction phase.
Owner:ABBVIE BIOTECHNOLOGY LTD
Anti-activin a antibodies and uses thereof
ActiveUS20090234106A1Loss of body weightMassive lossAntipyreticAnalgesicsNucleotideAntibody fragments
The disclosure provides compositions and methods relating to or derived from anti-activin A binding proteins, including antibodies. In particular embodiments, the disclosure provides fully human, humanized, and chimeric anti-activin A antibodies that bind human activin A, activin A-binding fragments and derivatives of such antibodies, and activin A-binding polypeptides comprising such fragments. Other embodiments provide nucleic acids encoding such antibodies, antibody fragments and derivatives and polypeptides, cells comprising such polynucleotides, methods of making such antibodies, antibody fragments and derivatives and polypeptides, and methods of using such antibodies, antibody fragments and derivatives and polypeptides, including methods of treating or diagnosing subjects having activin A-related disorders or conditions including cachexia related to gonadal cancer, other cancers, rheumatoid arthritis, and other diseases.
Owner:AMGEN INC
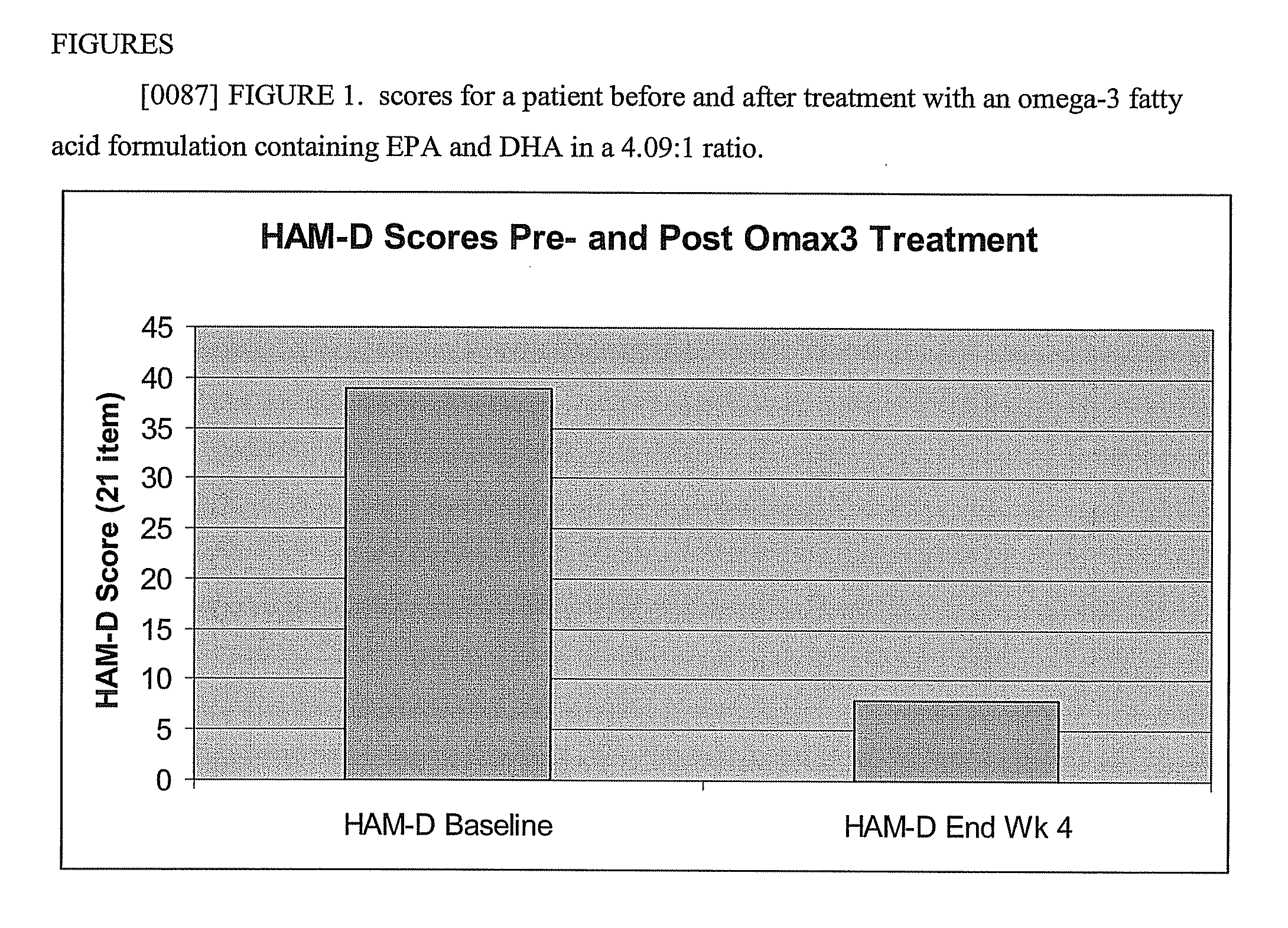
Omega 3 fatty acid formulations
The present invention provides highly purified omega-3 fatty acid formulations. Certain formulations provided herein have contain greater than 85% omega-3 fatty acids by weight. Certain other formulations provided herein contain EPA and DHA in a ratio of from about 4.01:1 to about 5:1. The invention also provides methods of using the dosage forms to treat a variety of cardiovascular, autoimmune, inflammatory, and central nervous system disorders by administering a formulation of the invention to a patient in need thereof.
Owner:CENESTRA
Methods and compositions for the treatment of psychiatric conditions
InactiveUS20050209218A1Low variabilityMaximizes therapeutic benefitBiocideNervous disorderDiseasePsychogenic disease
Owner:ADAMAS PHARMA INC
Albumin fusion proteins
InactiveUS20070048282A1Extended shelf lifeImprove stabilityAntibacterial agentsPeptide/protein ingredientsDiseaseAlbumin
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disorders or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
Vaccine Nanotechnology
ActiveUS20100233251A1Modulating immune systemEnhance and suppress and direct and immune responseNervous disorderAntipyreticDiseaseNanocarriers
Owner:MASSACHUSETTS INST OF TECH +4
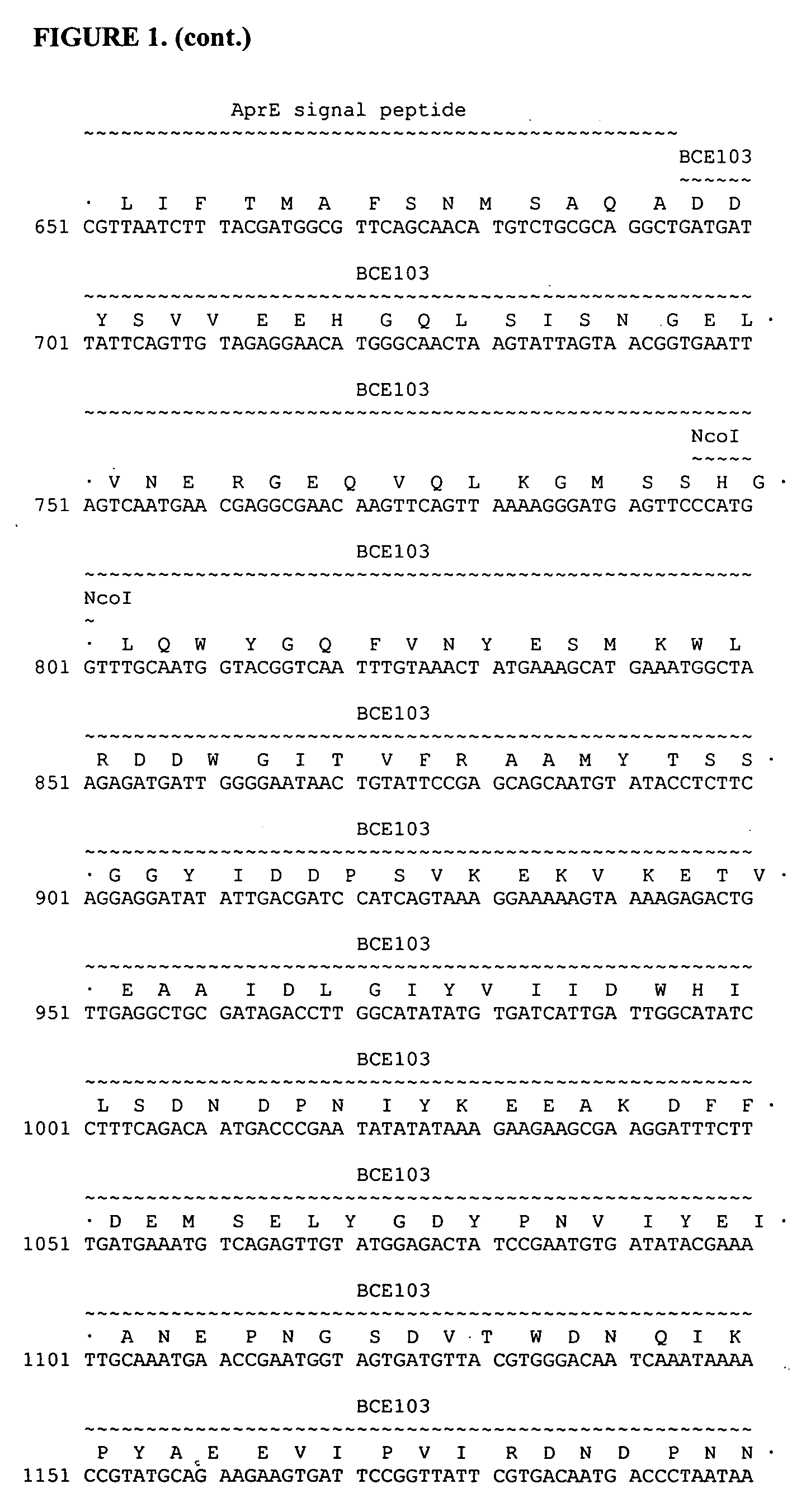
Bacterial expression of protease inhibitors and variants thereof
ActiveUS20050202535A1Reduce disulfide bondBacteriaPeptide/protein ingredientsProteinase activityProtease
Owner:GENENCOR INT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com