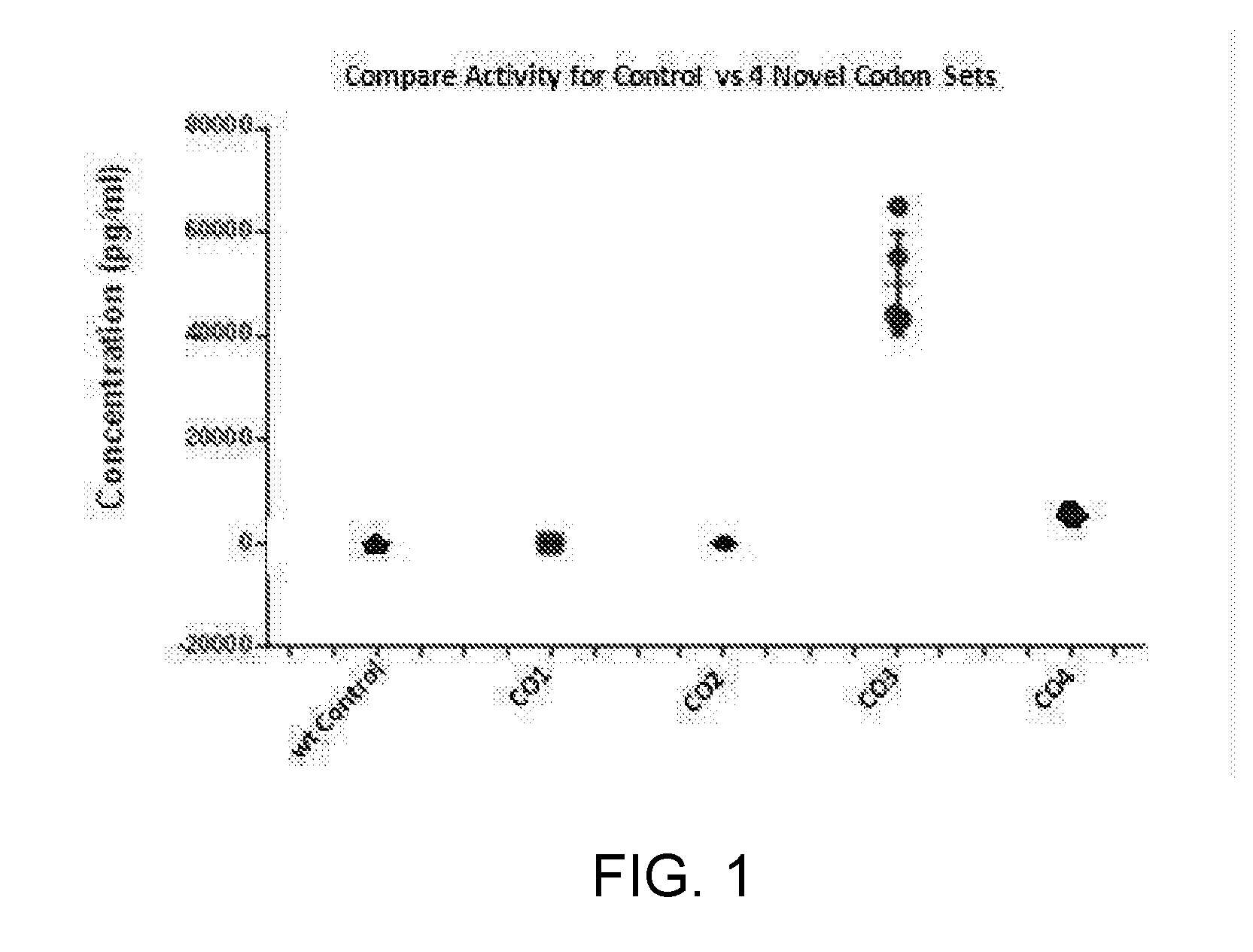
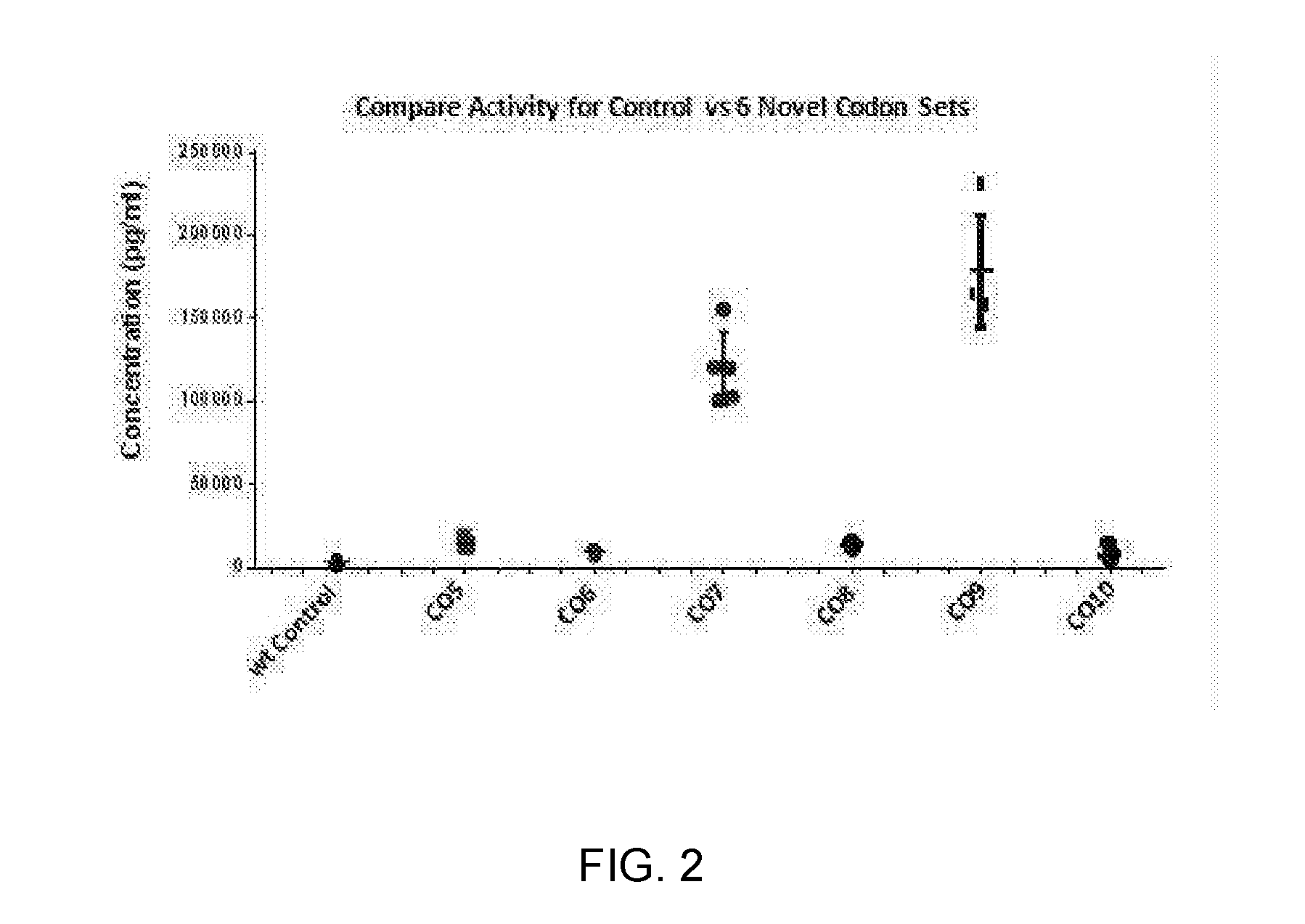
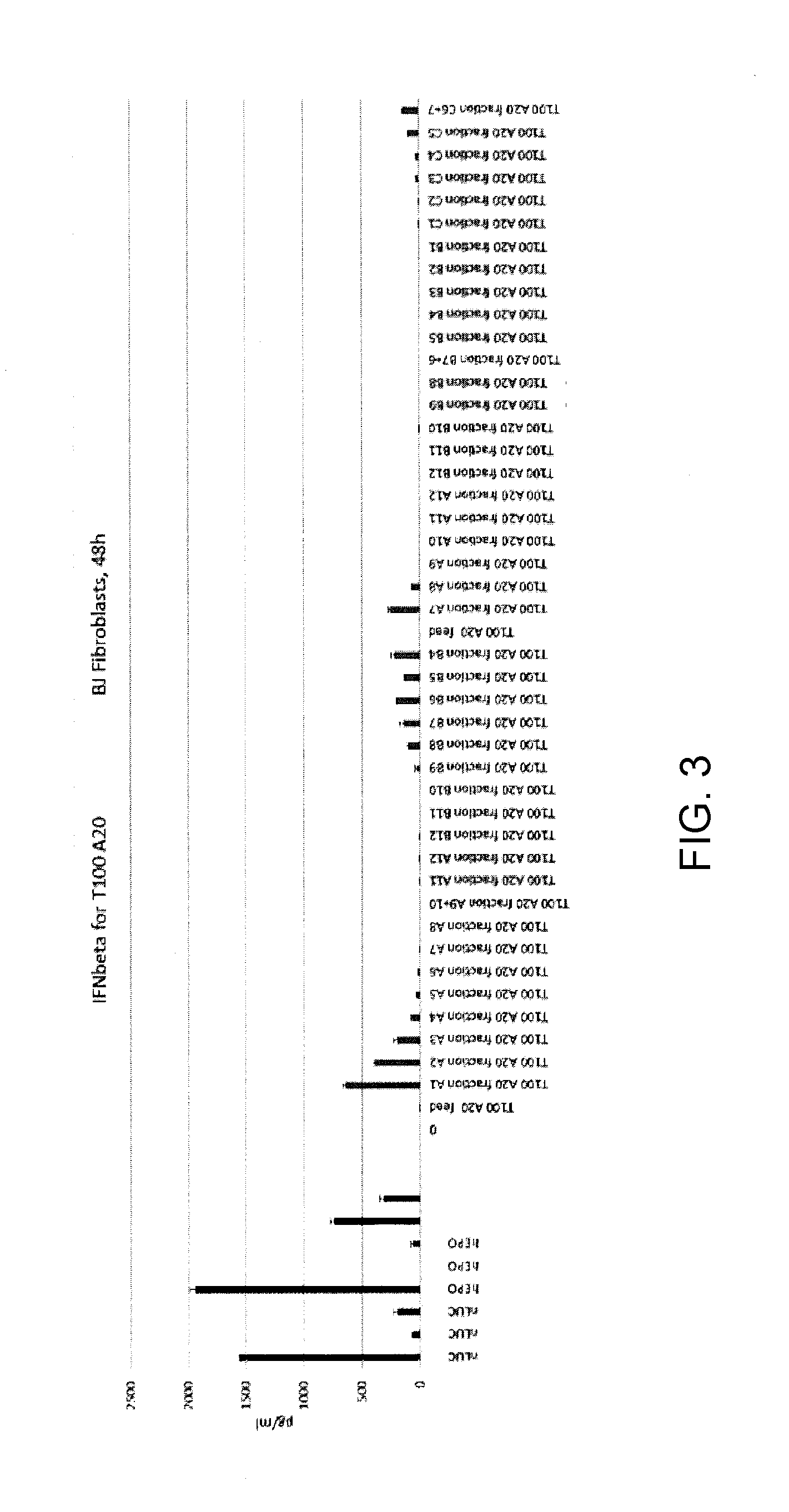
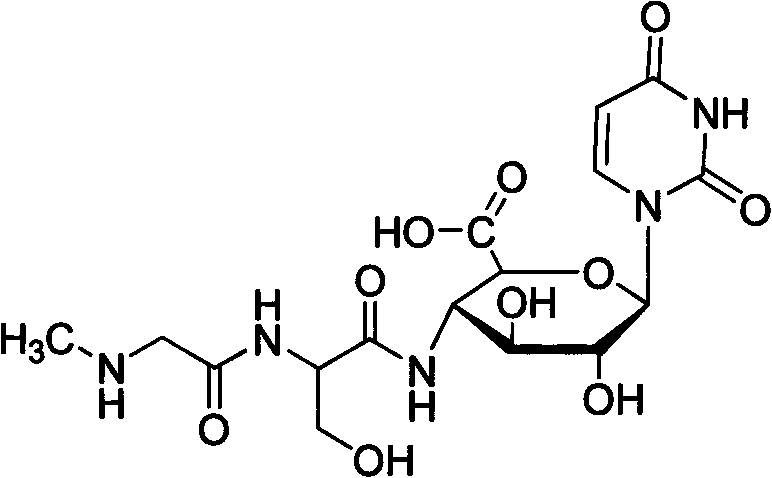
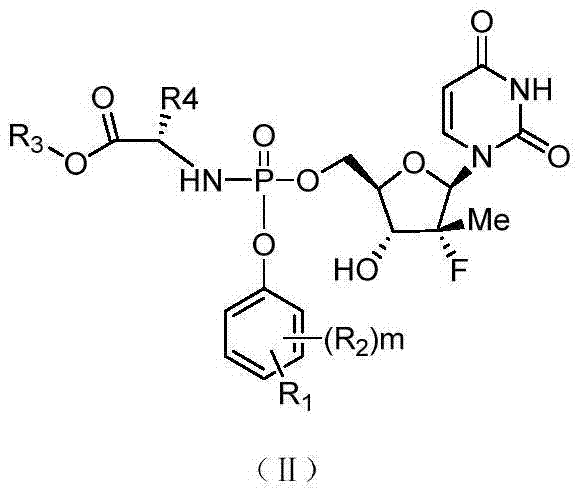
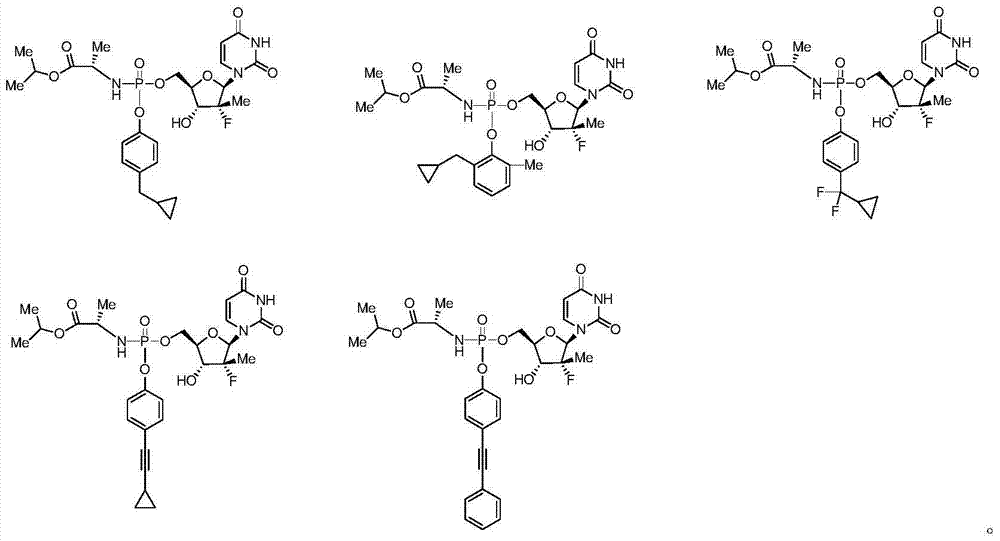
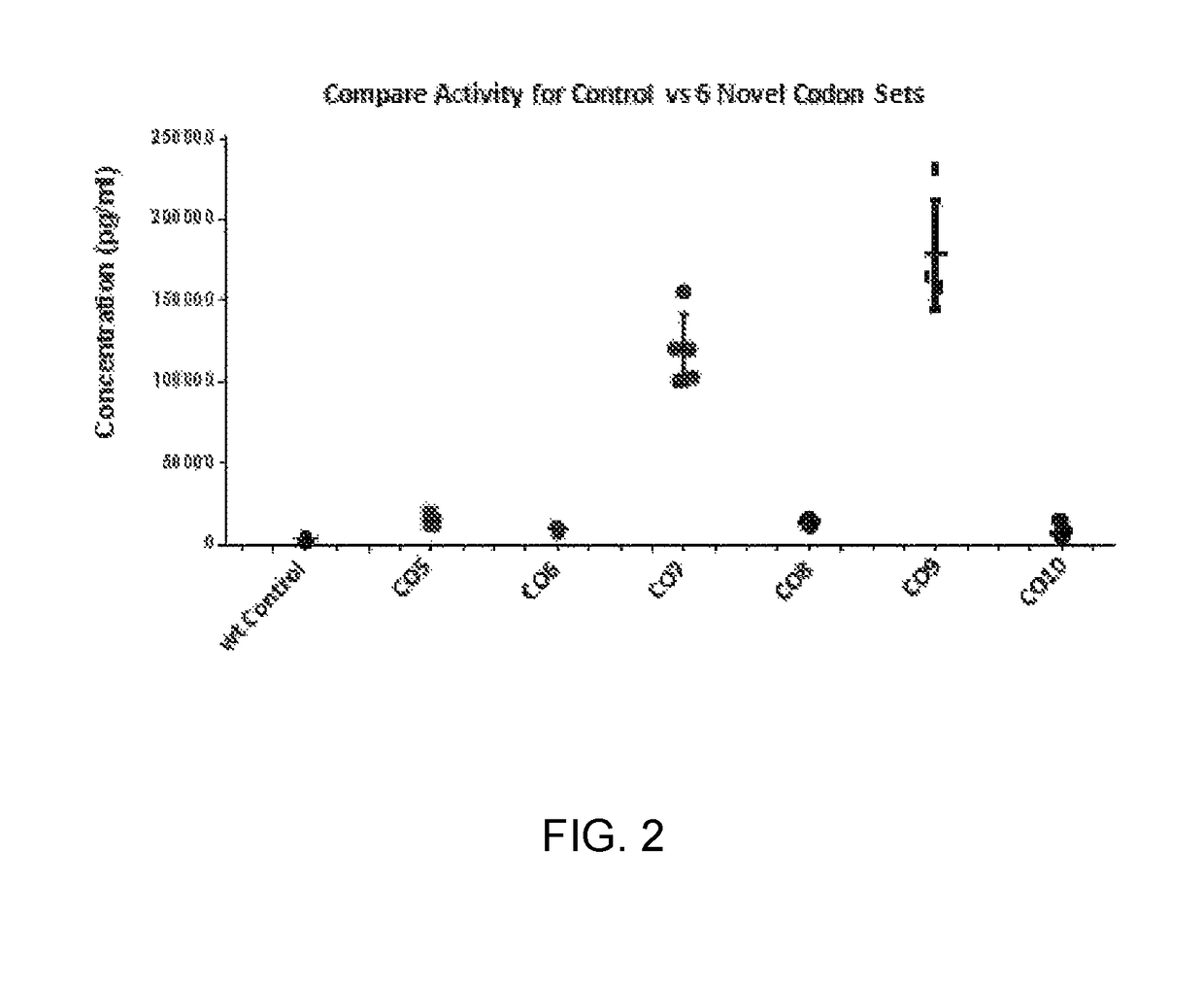
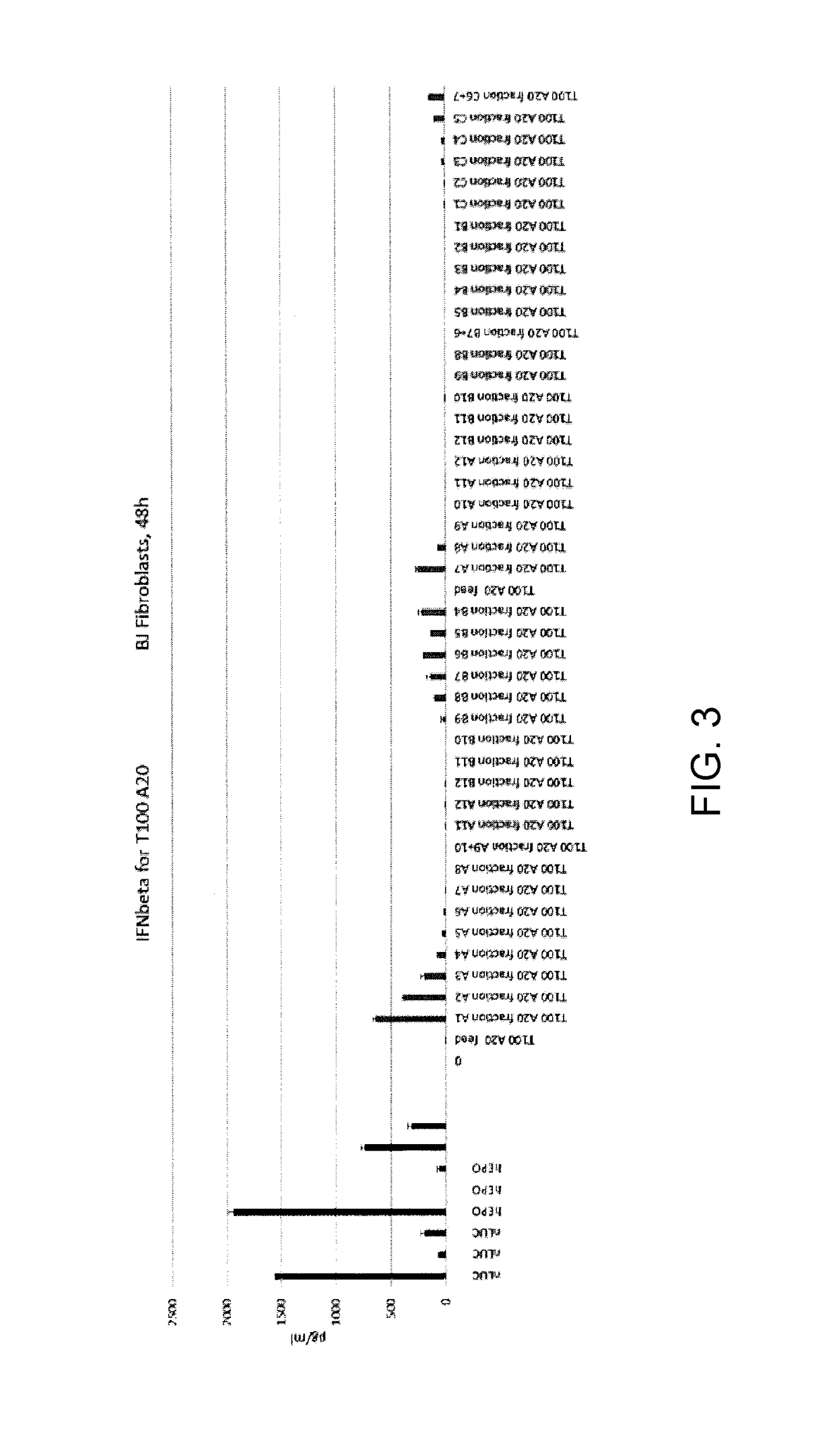
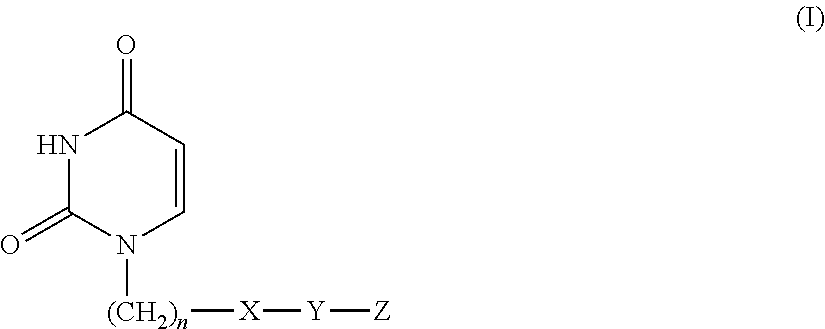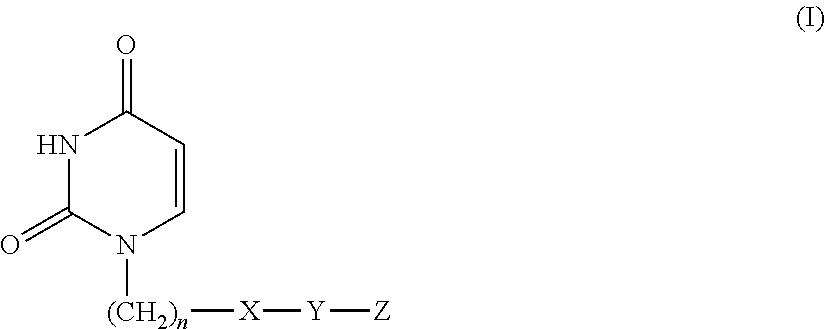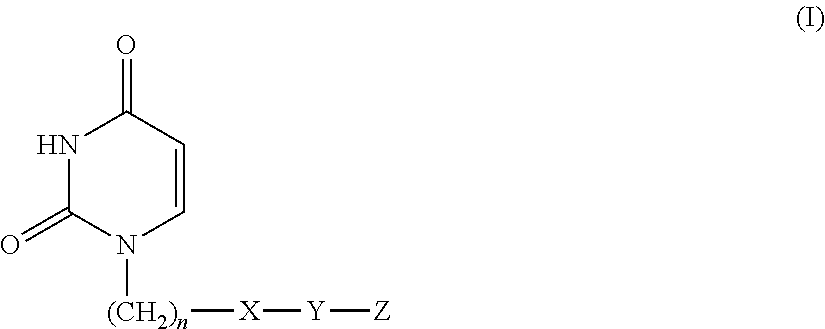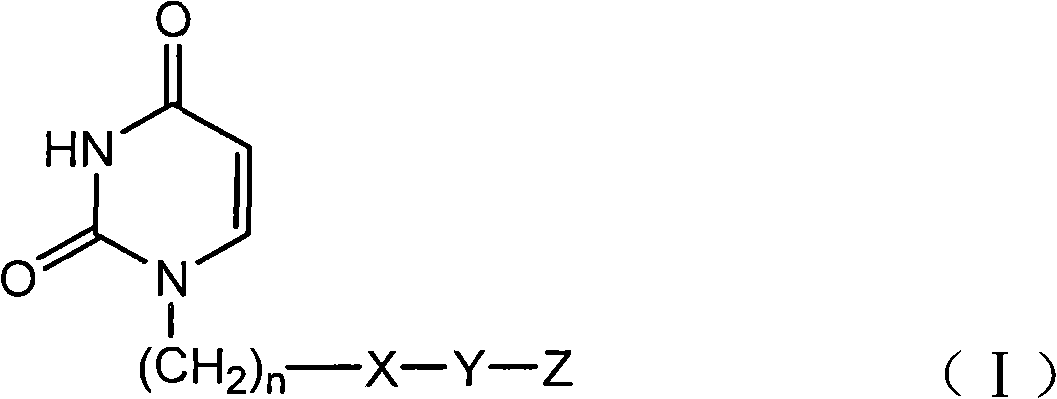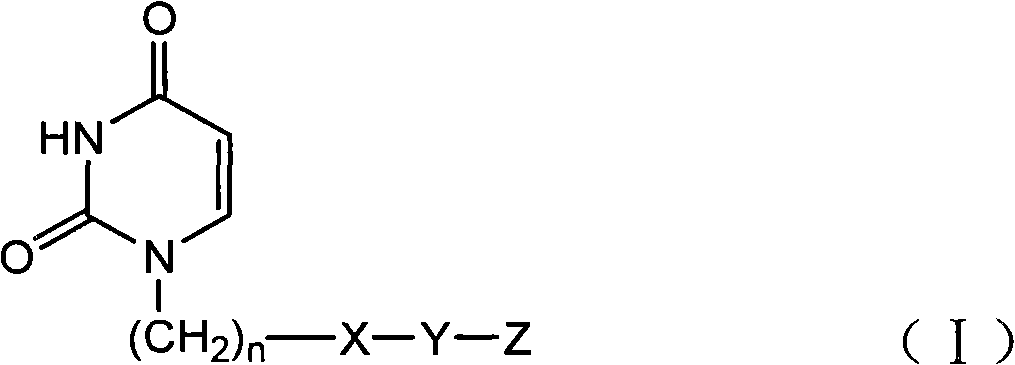Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
624 results about "Uracil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Uracil (/ˈjʊərəsɪl/; U) is one of the four nucleobases in the nucleic acid of RNA that are represented by the letters A, G, C and U. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine. Uracil is a demethylated form of thymine.
Polynucleotides for causing RNA interference and method for inhibiting gene expression using the same
InactiveUS20080113351A1High RNA interference effectLittle riskOrganic active ingredientsNervous disorderBase JNucleotide
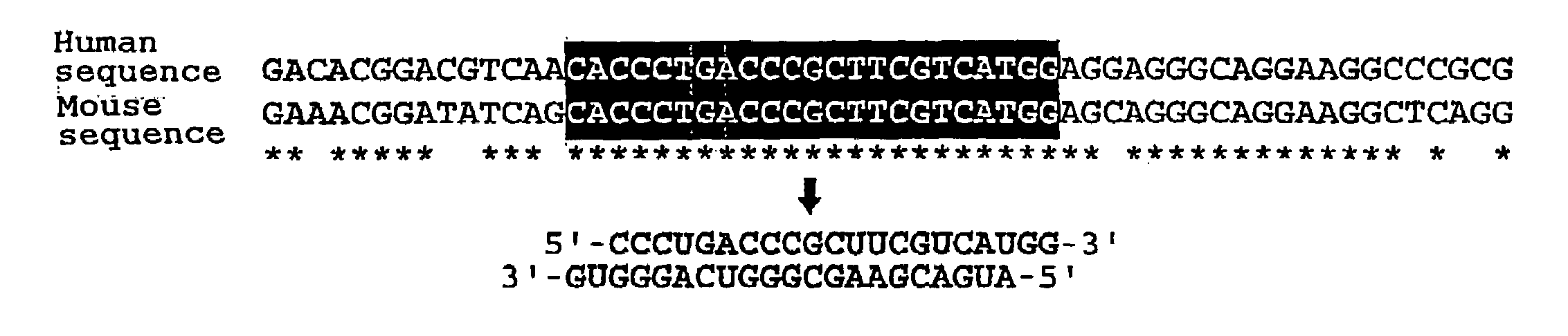
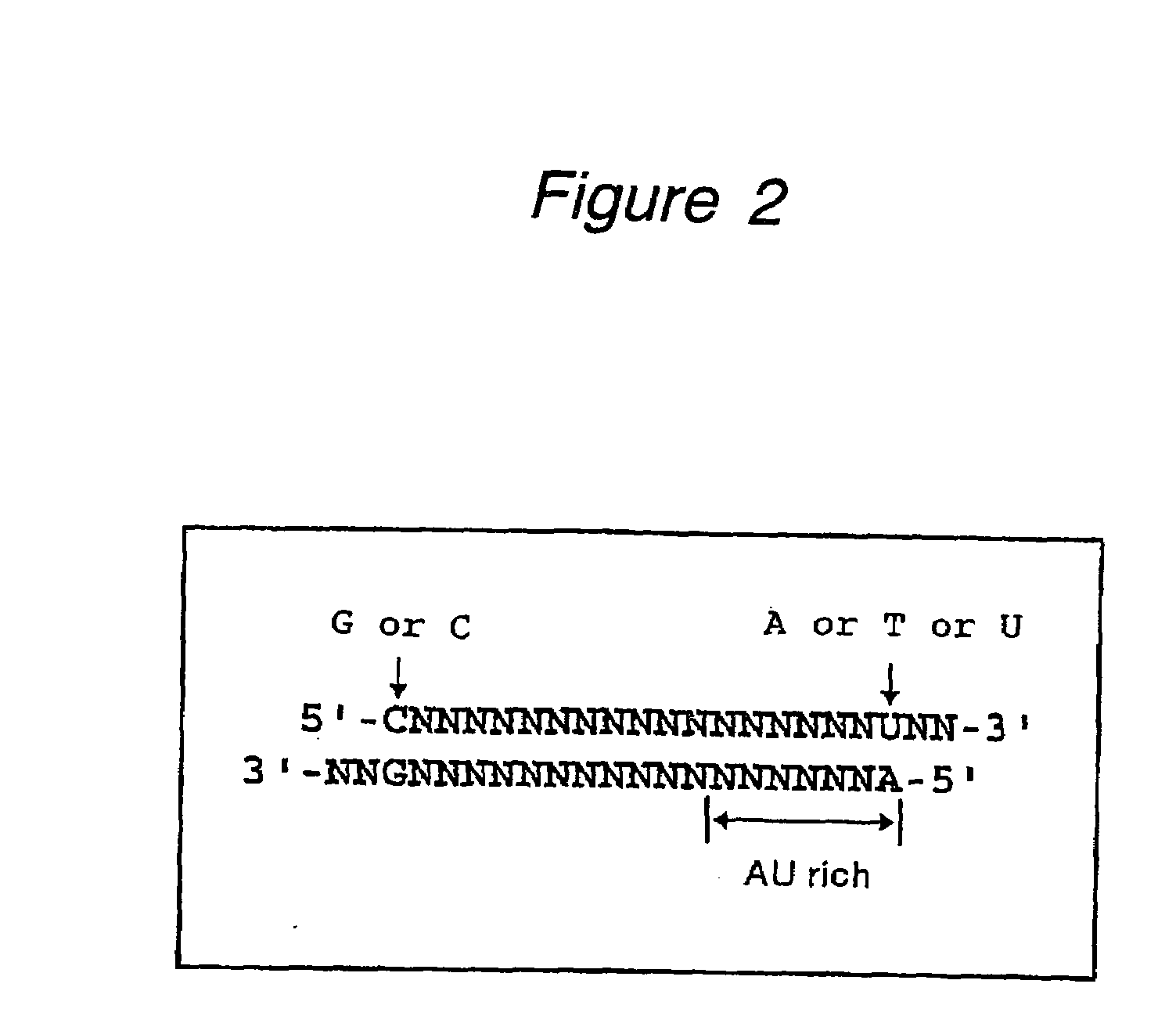
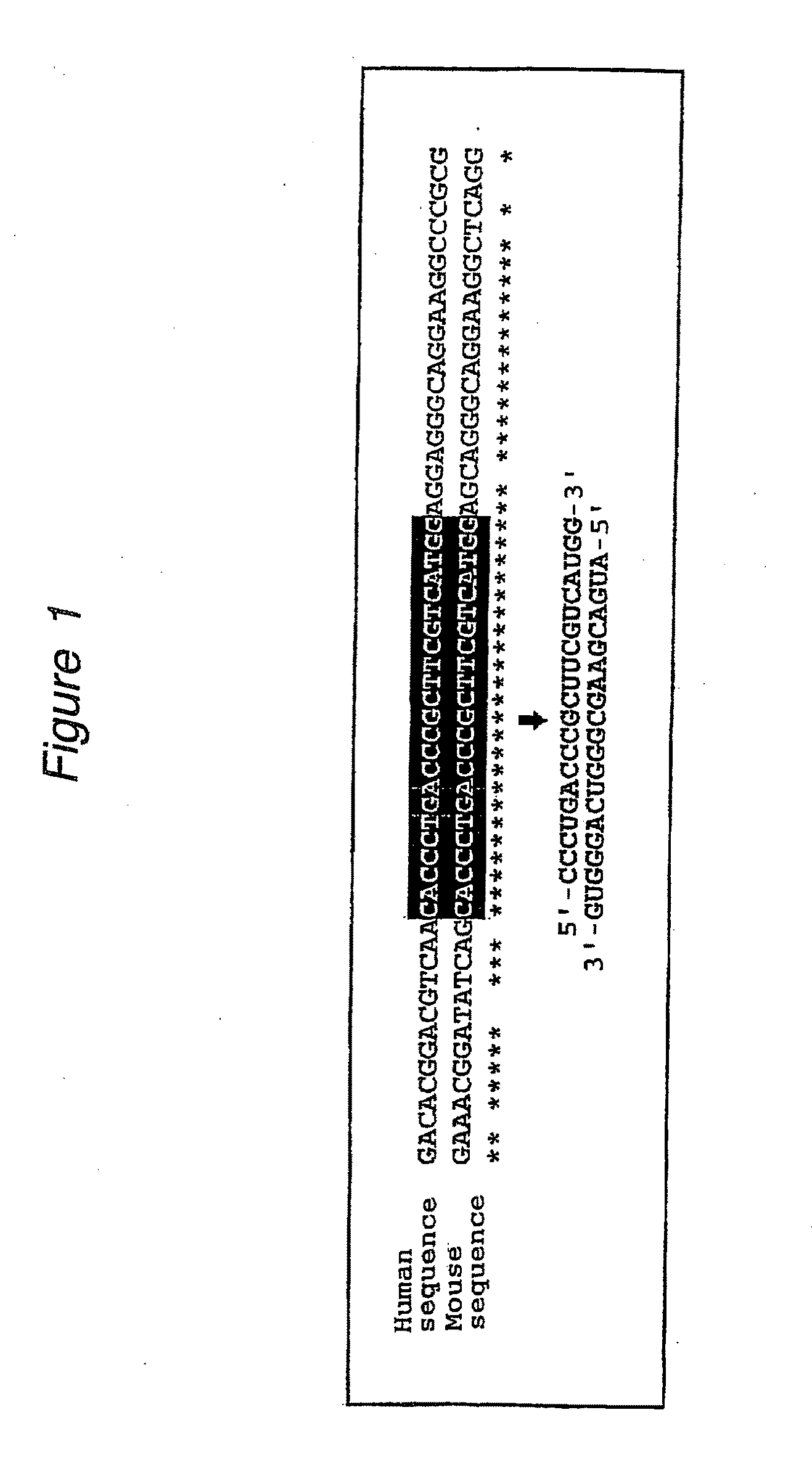
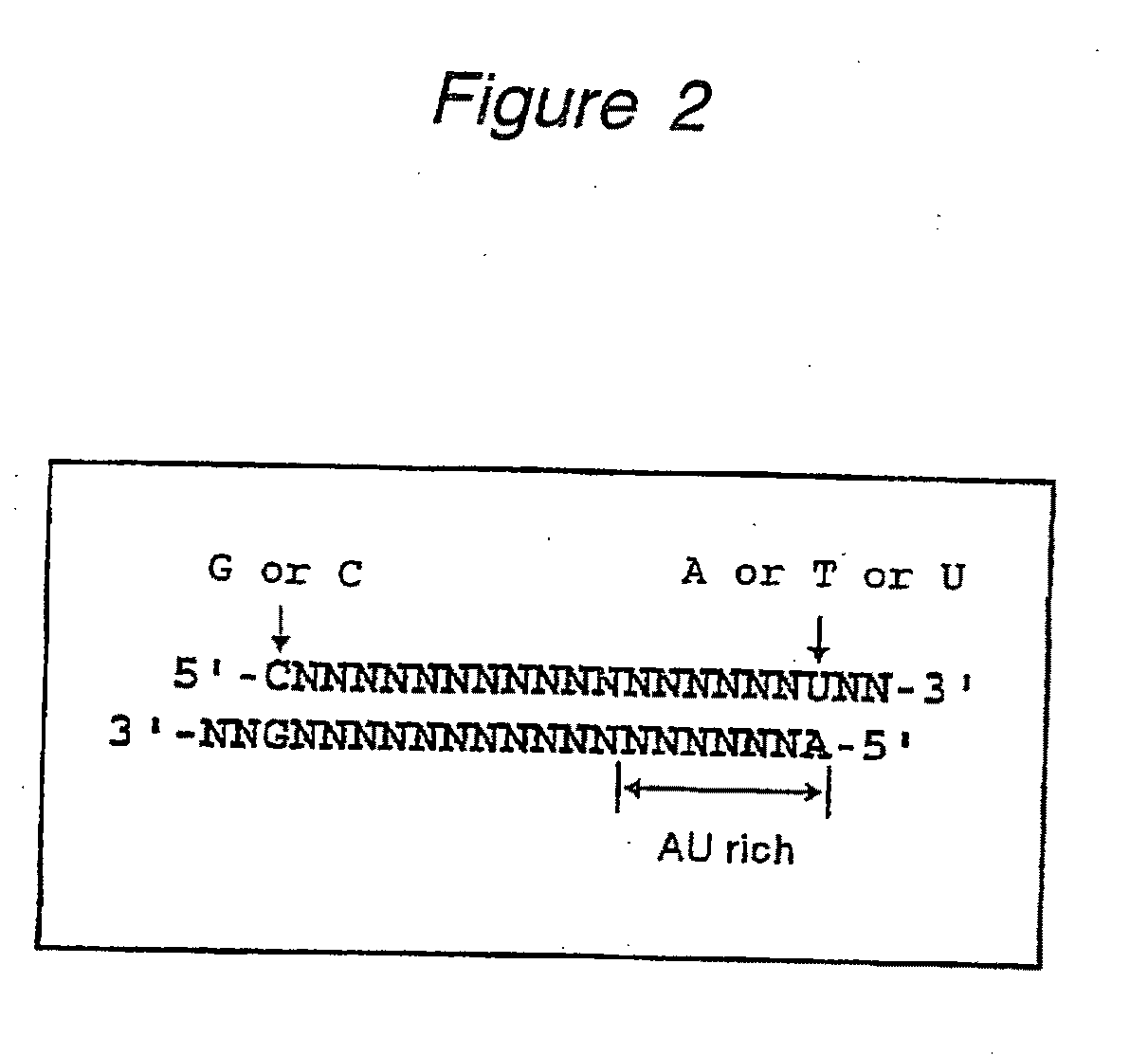
The present invention provides a polynucleotide that not only has a high RNA interference effect on its target gene, but also has a very small risk of causing RNA interference against a gene unrelated to the target gene. A sequence segment conforming to the following rules (a) to (d) is searched from the base sequences of a target gene for RNA interference and, based on the search results, a polynucleotide capable of causing RNAi is designed, synthesized, etc.:(a) The 3′ end base is adenine, thymine, or uracil,(b) The 5′ end base is guanine or cytosine,(c) A 7-base sequence from the 3′ end is rich in one or more types of bases selected from the group consisting of adenine, thymine, and uracil, and(d) The number of bases is within a range that allows RNA interference to occur without causing cytotoxicity.
Owner:ALPHAGEN
Method for producing complex DNA methylation fingerprints
InactiveUS6214556B1Accurately determineSugar derivativesMicrobiological testing/measurementFingerprintGenomic DNA
Method for characterizing, classifying and differentiating tissues and cell types, for predicting the behavior of tissues and groups of cells, and for identifying genes with changed expression. The method involves obtaining genomic DNA from a tissue sample, the genomic DNA subsequently being subjected to shearing, cleaved by means of a restriction endonuclease or not treated by either one of these methods. The base cytosine, but not 5-methylcytosine, from the thus-obtained genomic DNA is then converted into uracil by treatment with a bisulfite solution. Fractions of the thus-treated genomic DNA are then amplified using either very short or degenerated oligonucleotides or oligonuclcotides which are complementary to adaptor oligonucleotides that have been ligated to the ends of the cleaved DNA. The quantity of the remaining cytosines on the guanine-rich DNA strand and / or the quantity of guanines on the cytosine-rich DNA strand from the amplified fractions are then detected by hybridization or polymerase reaction, which quantities are such that the data generated thereby and automatically applied to a processing algorithm allow the drawing of conclusions concerning the phenotype of the sample material.
Owner:EPIGENOMICS AG
Substituted nucleosides, preparation thereof and use as inhibitors of RNA viral polymerases
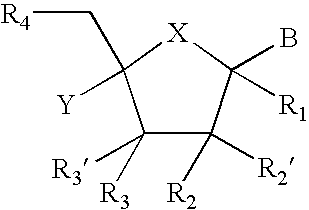
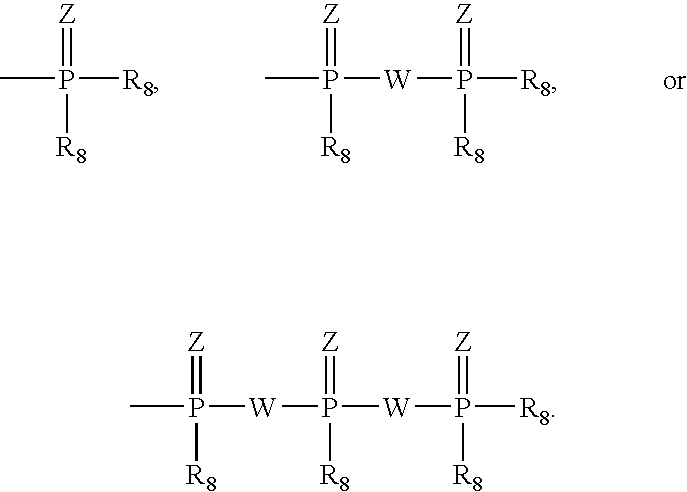
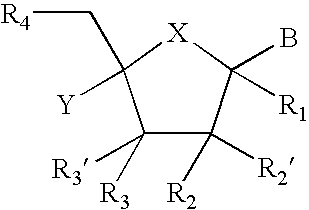
Provided are compounds represented by: X is O, S or NR6, R1 is H or (CH2)mR5, R2, R2', R3 and R3' are independently NO2, N3 or (CH2)mR5, OH R4 is H, OR6, SR6, NR6R6a, CN, C(O)OR6, C(O)NR6R6a, R6, OR7 or (CH2)nR7, R5 is H, halo, OR6, SR6, NR6R6a, CN, C(O)OR6, C(O)NR6R6a, R6, OR7 or (CH2)mR7, R6 and R6a are individually H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl or substituted aryl, R7 is: R8 is H, F, SR9 or OR9, R9 is H, alkyl, alkenyl, alkynyl, aryl or hydroxyprotecting group, Y is H, CH3 or (CH2)mR5, Z is O or S W is CH2, CF2, CHF or O, m is 0-4, B is adenine, guanine, cytosine, uracil, thymine, modified purines and pyrimidines substituted pyridines, five membered heterocycles substituted by at least one of amines, substituted amines, amides, substituted amides, esters, halogens, alkyls, ethers; and pharmaceutically acceptable salts thereof and prodrugs thereof. These ring systems may be substituted.
Owner:BIOCRYST PHARM INC
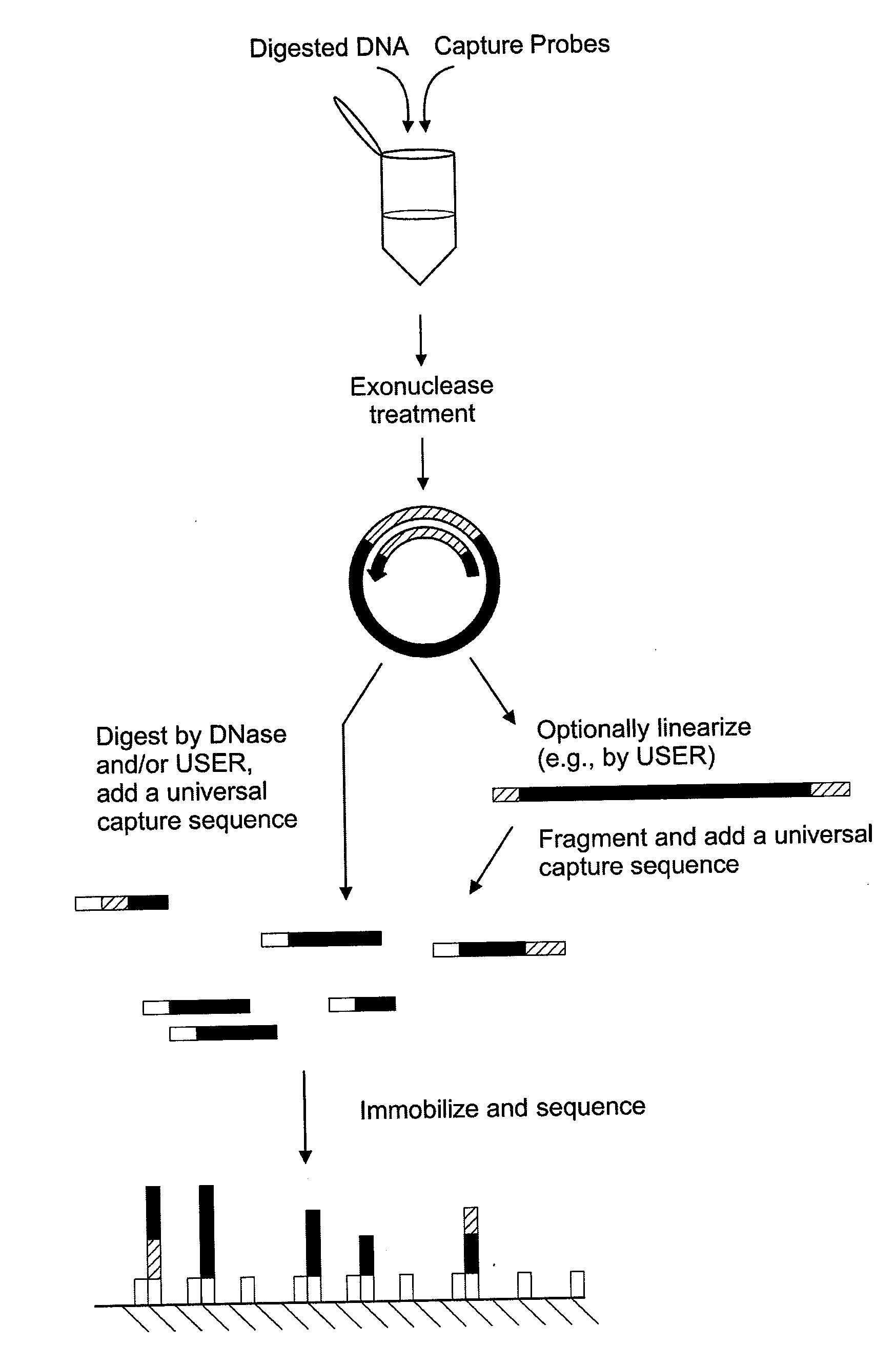
Surface-capture of target nucleic acids
The disclosure provides methods of capturing target nucleic acids (e.g., gene or gene fragments) onto a solid support for further analysis. The disclosed methods utilize a capture probe that selectively circularizes only the target nucleic acid. Following the circularization of the target, the linear, non-target, nucleic acids are removed from the sample. Next, the circularized target is linearized and bound to a solid support. To allow for linearization, the capture probe may include a cleavage site that can be a noncanonical nucleotide(s) (e.g., uracil in DNA) and / or a rare-cutter site (e.g., the Not I restriction site). In some embodiments, the target nucleic acid is captured onto a support without an intermediate amplification step.
Owner:FLUIDIGM CORP
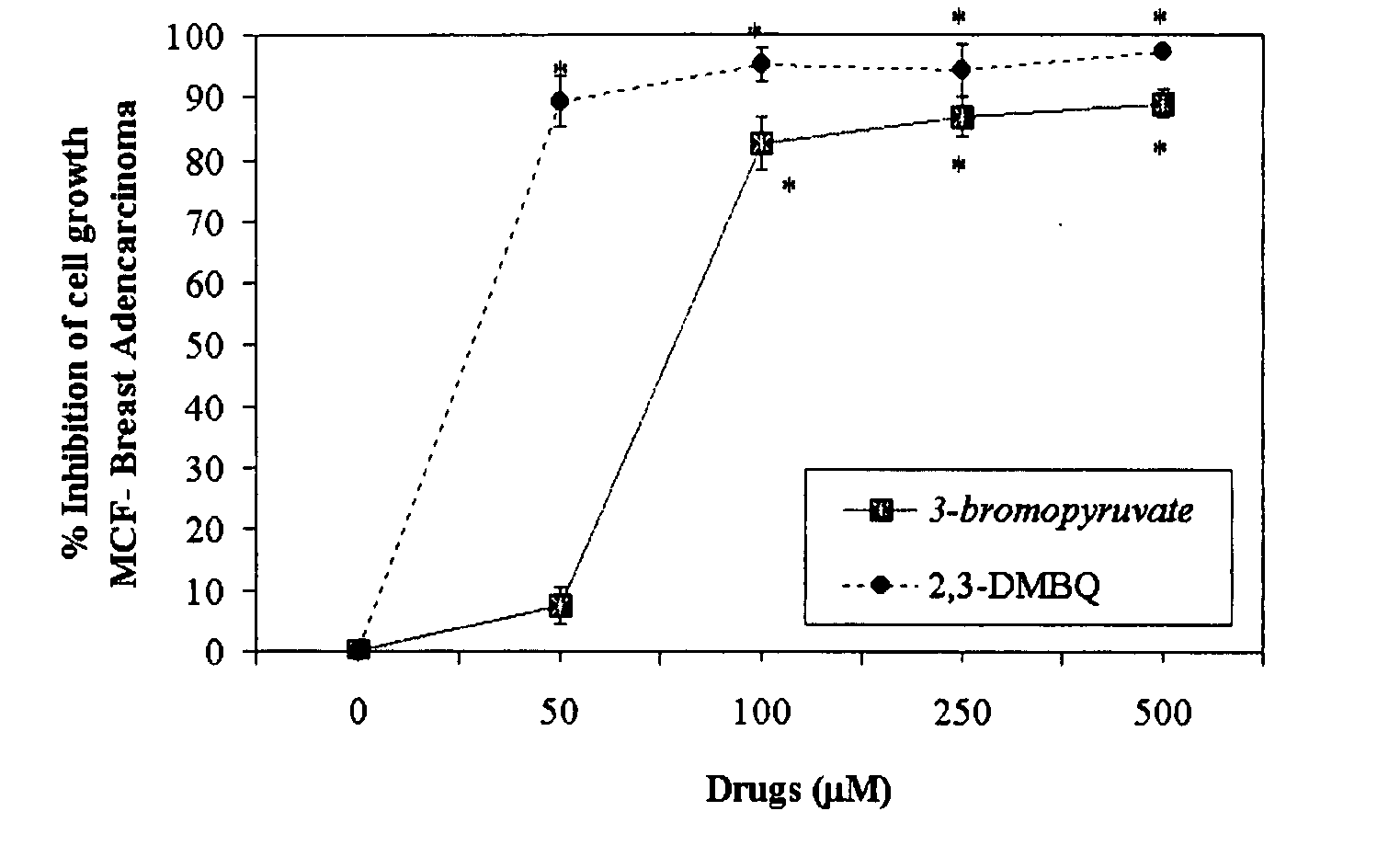
Nutraceutical composition and method of use for treatment / prevention of cancer
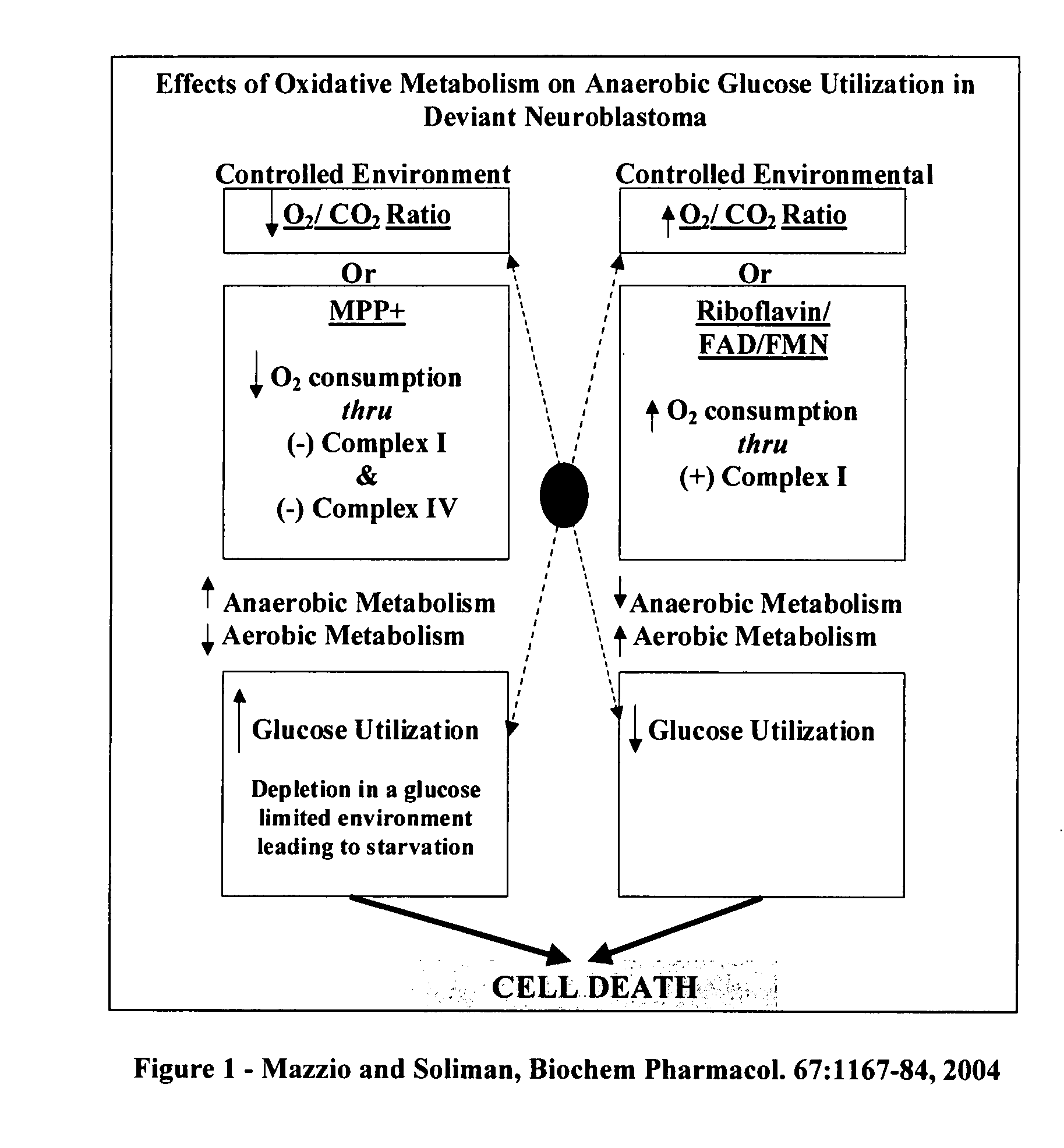
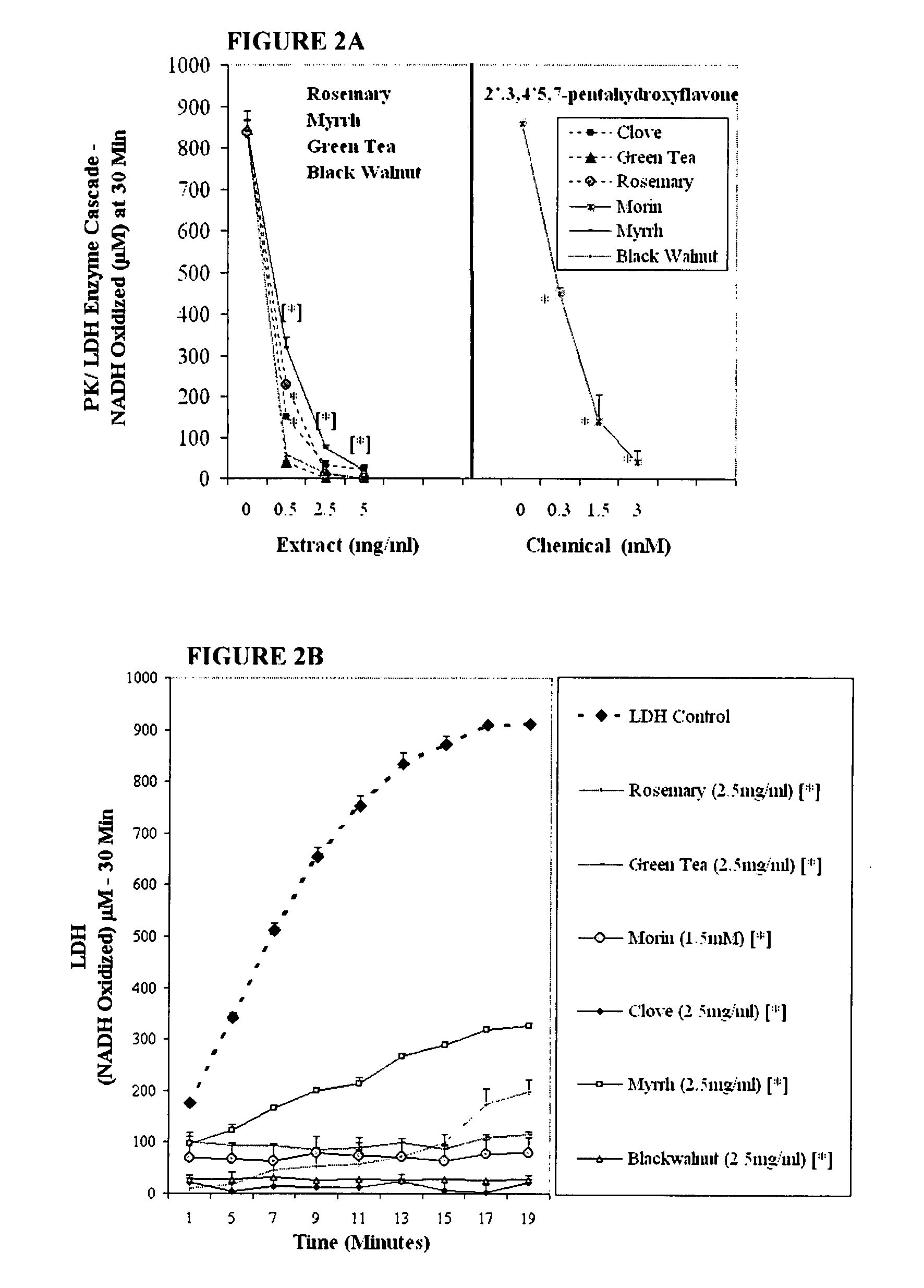
InactiveUS20070248693A1Function increaseAbility to createBiocideAlgae medical ingredients1,4-BenzoquinonePantothenic acid
The invention describes a pharmaceutical composition and method for treating cancer comprised of A) 2,3-dimethoxy-5-methyl-1,4-benzoquinone and / or B) at least one of wild yam root, teasel root, balm of gilead bud, bakuchi seed, dichroa root, kochia seed, kanta kari, bushy knotweed rhizome, arjun, babul chall bark, opopanax and bhumy amalaki; optionally one or more of frankincense, garcinia fruit, vitex, dragons blood, mace, sage and red sandalwood with at least c) one compound capable of maximizing oxidative mitochondrial function preferably riboflavin or vitamin B2 derivatives, FAD, FMN, 5-amino-6-(5′-phosphoribitylamino)uracil, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, niacin, vitamin C and pantothenate and / or d) at least one lactic acid dehydrogenase inhibitor (preferably 2′,3,4′5,7-pentahydroxyflavone) and optionally f) an alkalizing agent (aloe vera, chlorella, wheat grass, sodium or potassium bicarbonate, potassium) g) an antiproliferative herb (speranskia or goldenseal) and h) a pharmaceutically acceptable carrier.
Owner:MAZZIO ELIZABETH +1
Polynucleotides for causing RNA interference and method for inhibiting gene expression using the same
InactiveUS20110054005A1High RNA interference effectLittle riskOrganic active ingredientsNervous disorderBase JNucleotide
The present invention provides a polynucleotide that not only has a high RNA interference effect on its target gene, but also has a very small risk of causing RNA interference against a gene unrelated to the target gene. A sequence segment conforming to the following rules (a) to (d) is searched from the base sequences of a target gene for RNA interference and, based on the search results, a polynucleotide capable of causing RNAi is designed, synthesized, etc.:(a) The 3′ end base is adenine, thymine, or uracil,(b) The 5′ end base is guanine or cytosine,(c) A 7-base sequence from the 3′ end is rich in one or more types of bases selected from the group consisting of adenine, thymine, and uracil, and(d) The number of bases is within a range that allows RNA interference to occur without causing cytotoxicity.
Owner:BIO THINKTANK
Chemically cleavable 3'-o-allyl-DNTP-allyl-fluorophore fluorescent nucleotide analogues and related methods
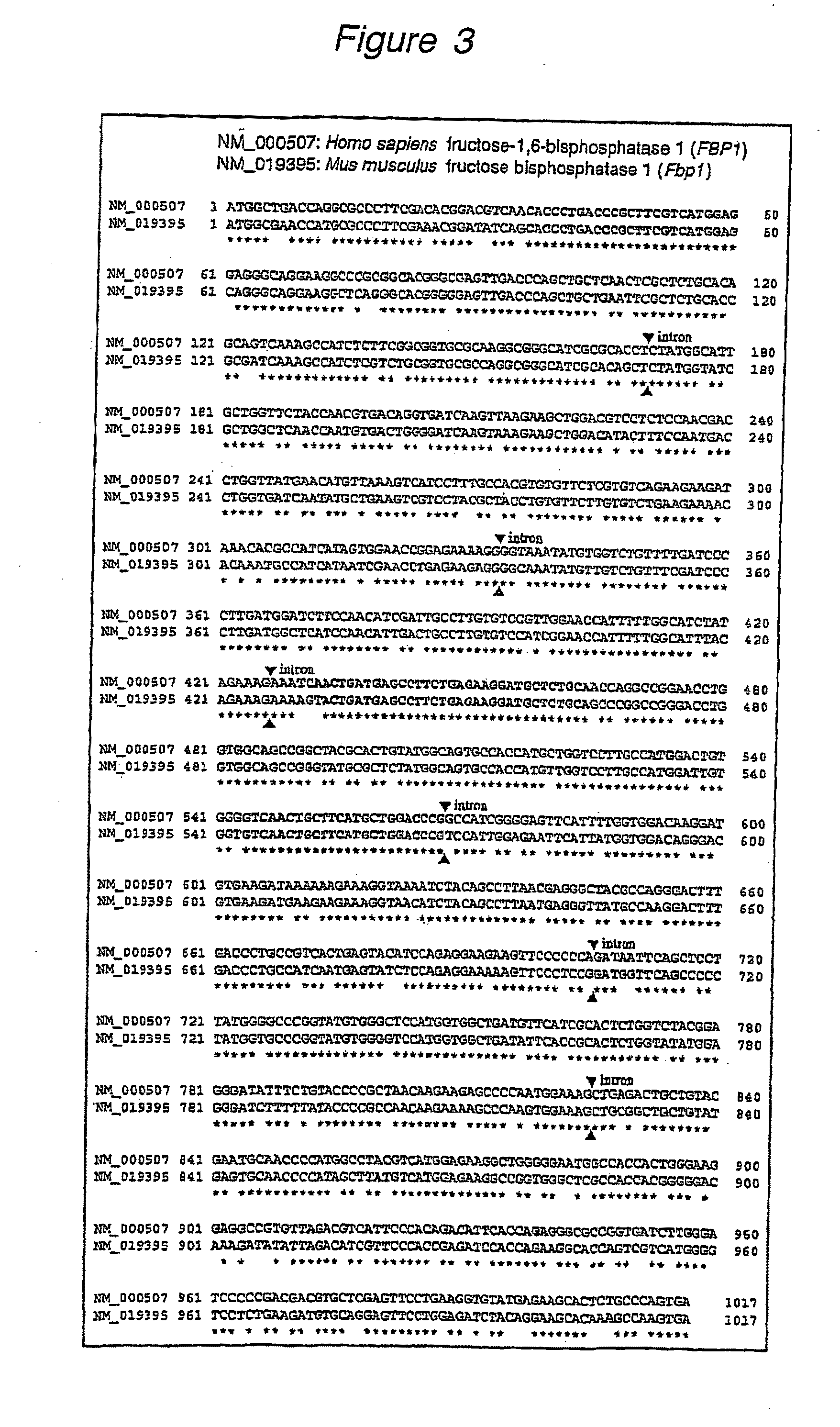
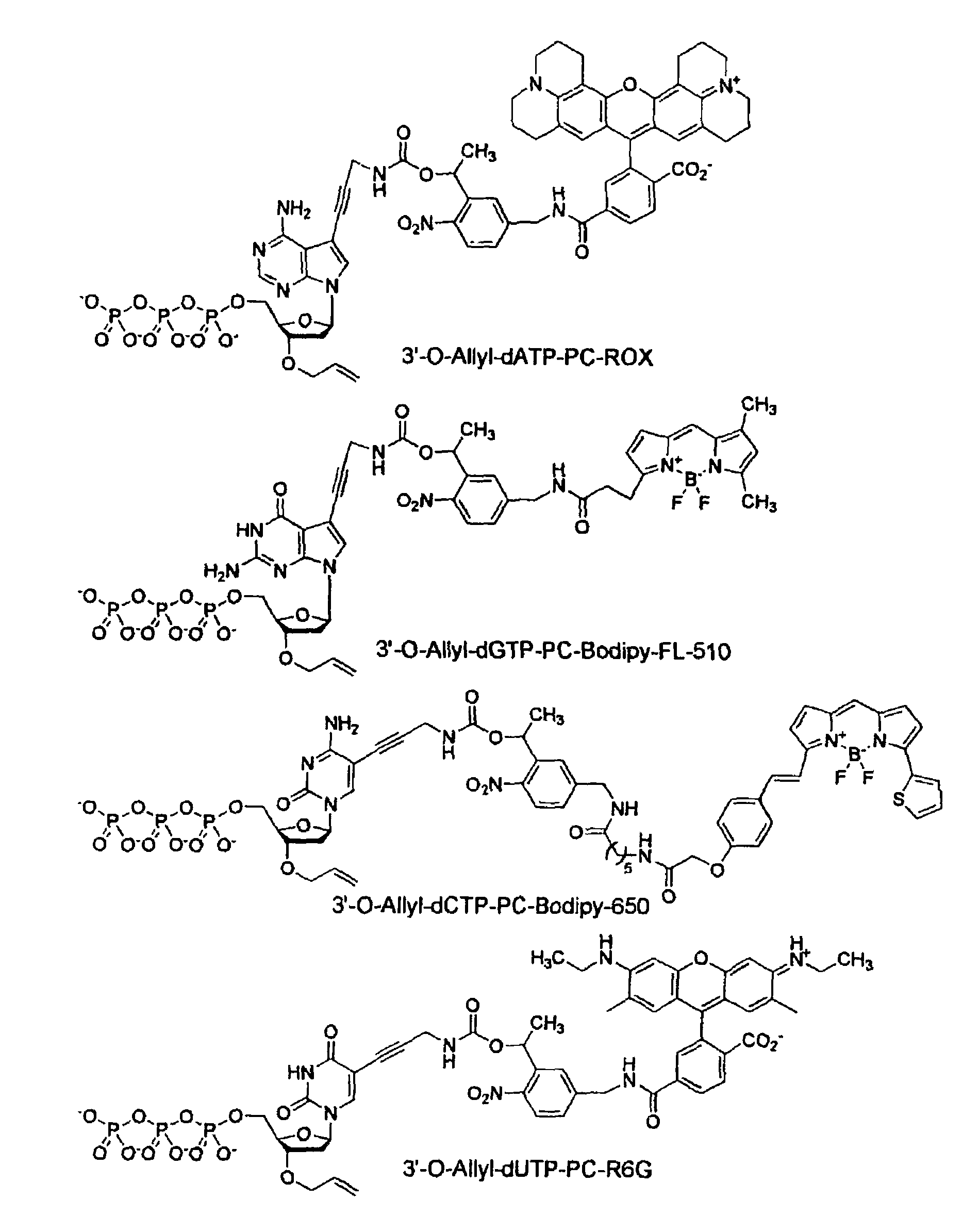
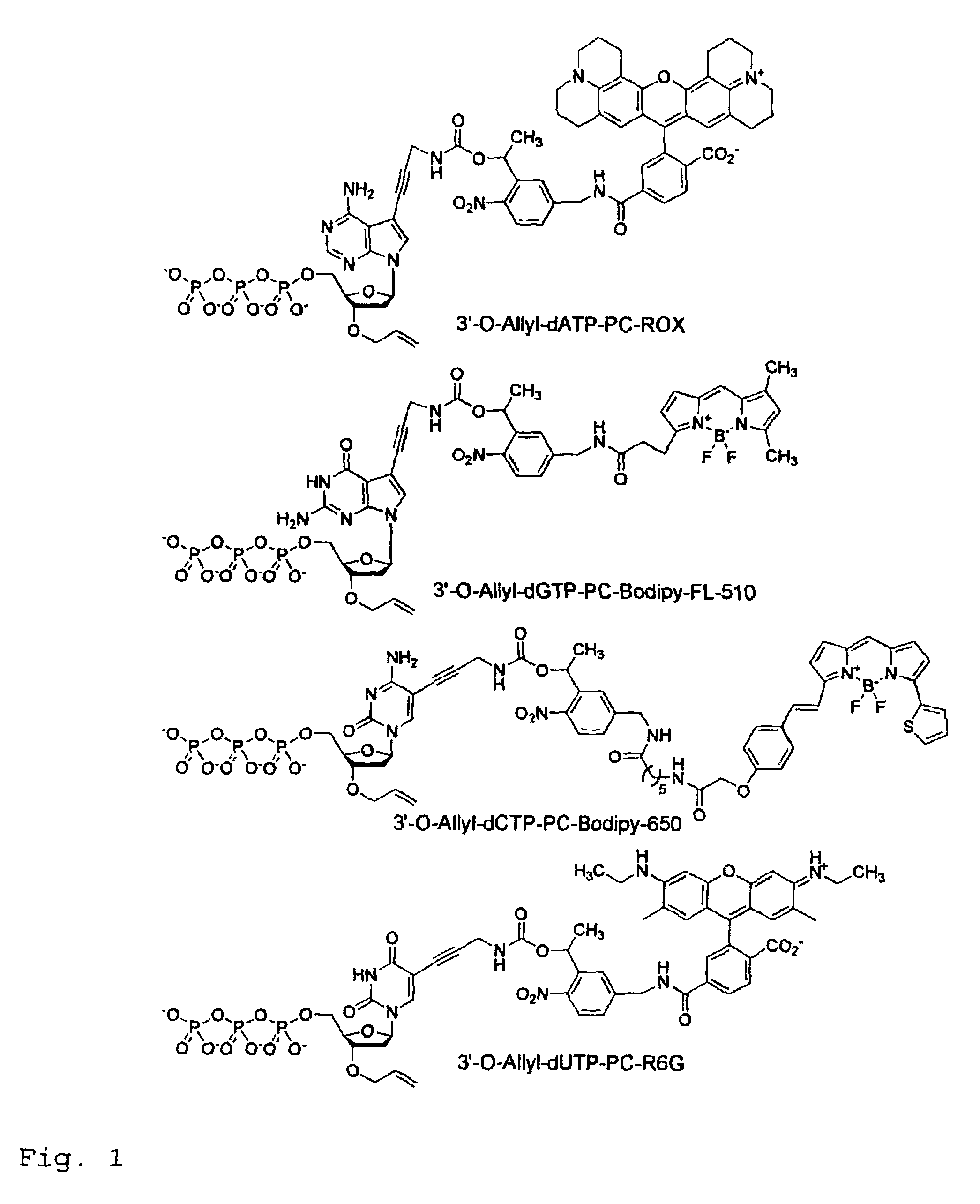
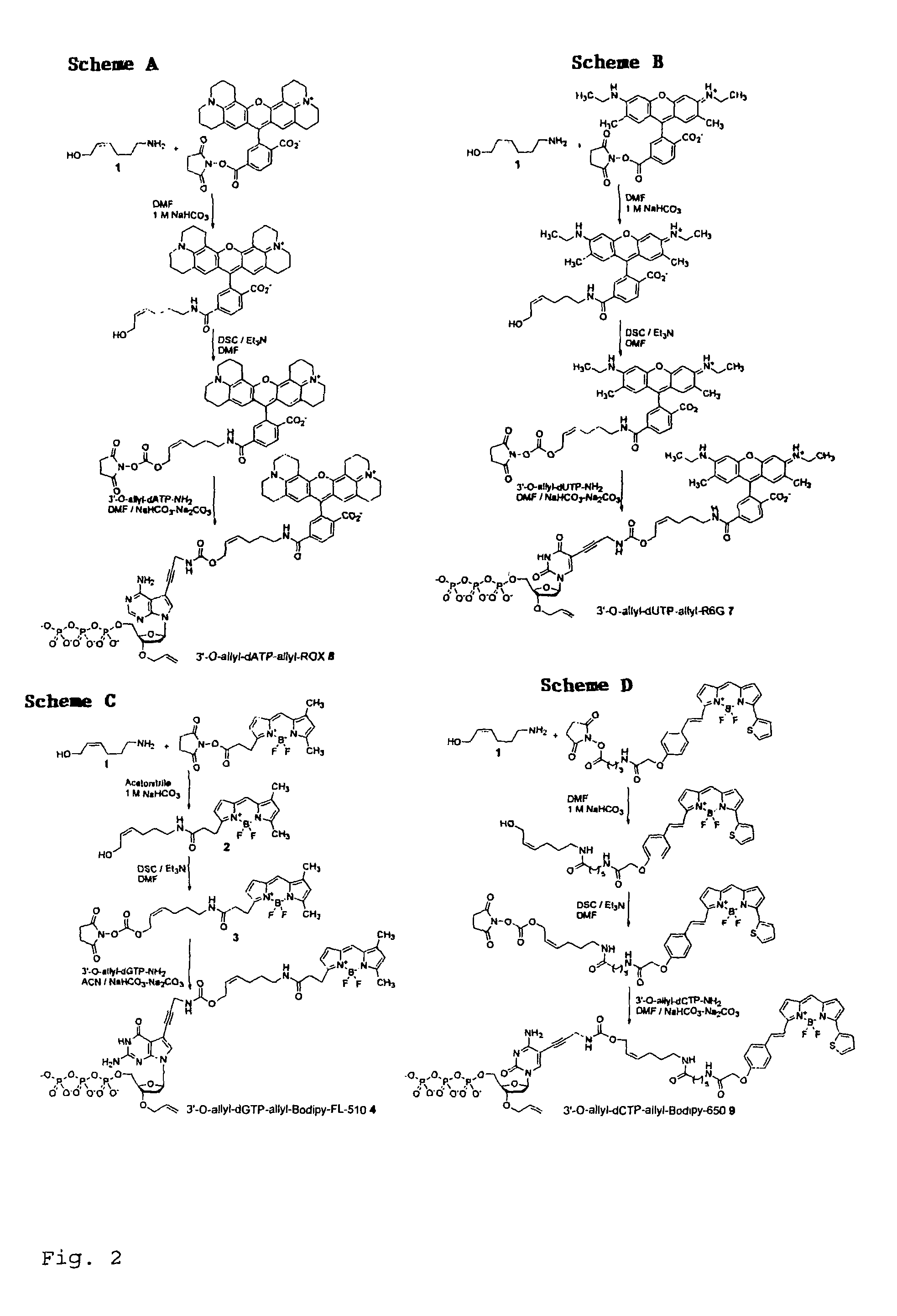
This invention provides a nucleotide analogue comprising (i) a base selected from the group consisting of adenine, guanine, cytosine, thymine and uracil, (ii) a deoxyribose, (iii) an allyl moiety bound to the 3′-oxygen of the deoxyribose and (iv) a fluorophore bound to the base via an allyl linker, and methods of nucleic acid sequencing employing the nucleotide analogue.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Alternative nucleic acid molecules containing reduced uracil content and uses thereof
ActiveUS20160237134A1Low immunogenicityEnhance protein expressionDepsipeptidesOxidoreductasesBiotechnologyUracil
The present disclosure provides alternative nucleosides, nucleotides, and nucleic acids, and methods of using them. In some aspects, the disclosure provides mRNA wherein the uracil content has been modified and which may be particularly effective for use in therapeutic compositions, because they may benefit from both high expression levels and limited induction of the innate immune response. In some aspects, the disclosure provides methods for the production of pharmaceutical compositions including mRNA without reverse phase chromatography.
Owner:MODERNATX INC
Application of CRISPR/nCas9 mediated site-directed base substitution in plant
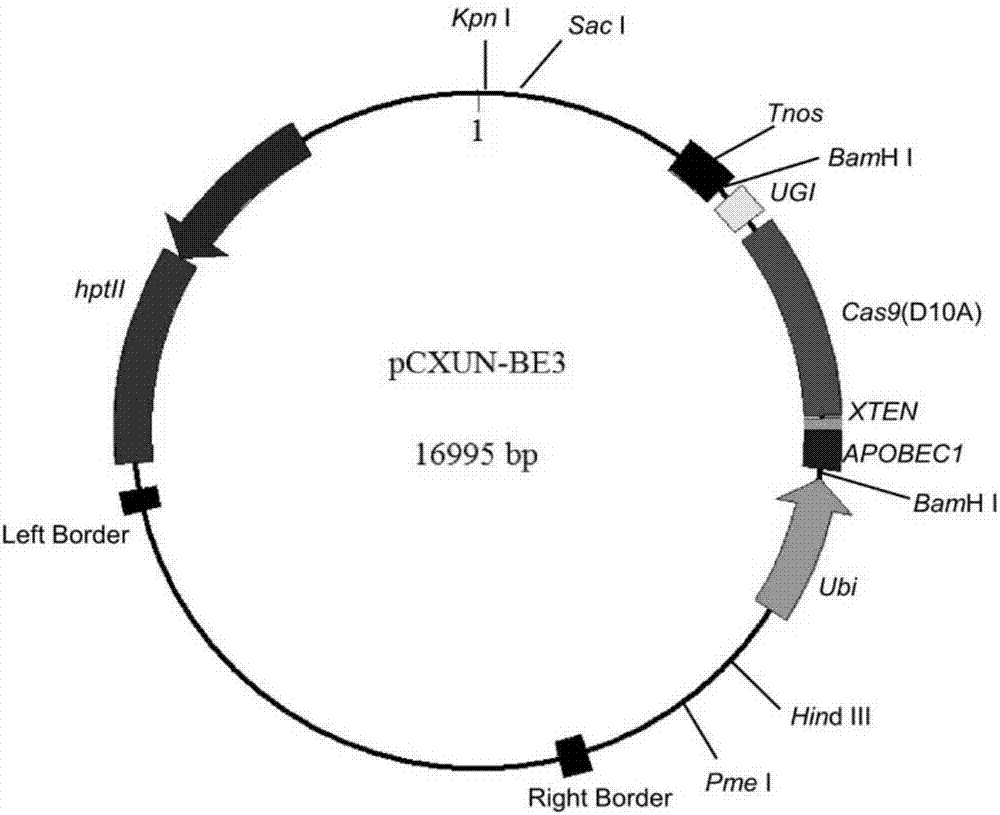
ActiveCN107043779ARapid improvement of agronomic traitsImproved agronomic traitsVectorsVector-based foreign material introductionUracil-DNA glycosylaseSubstitution method
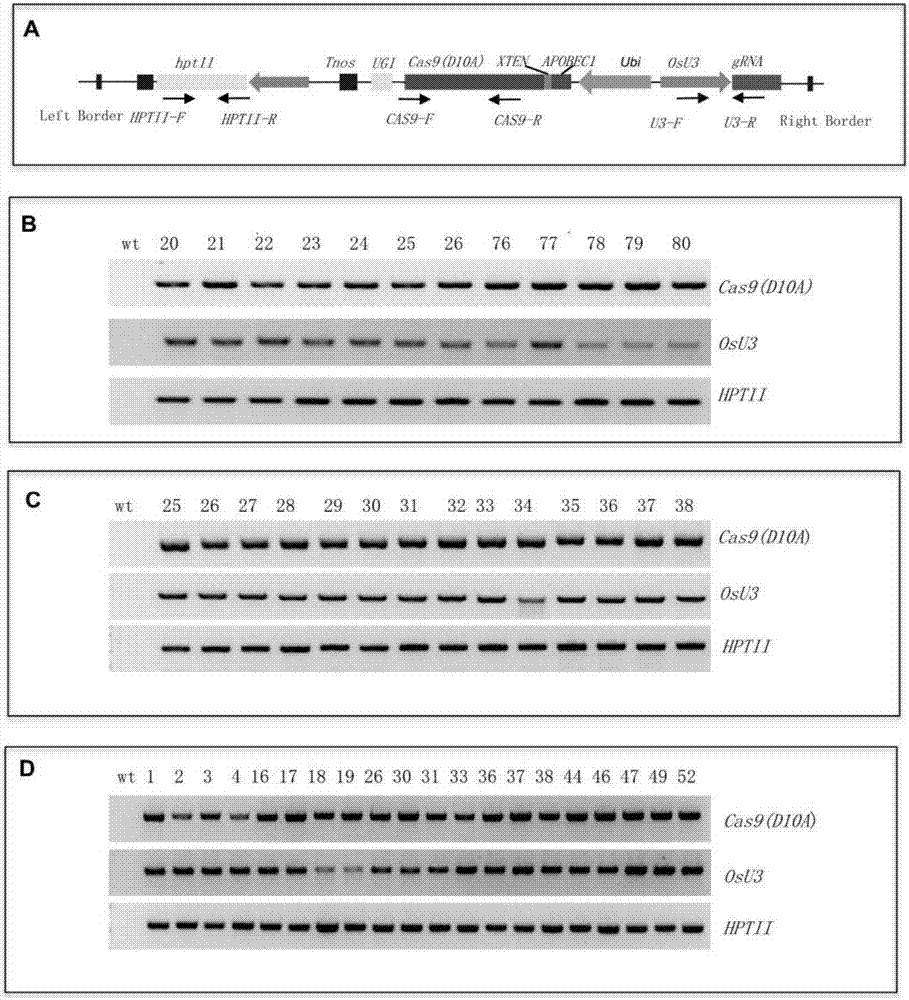
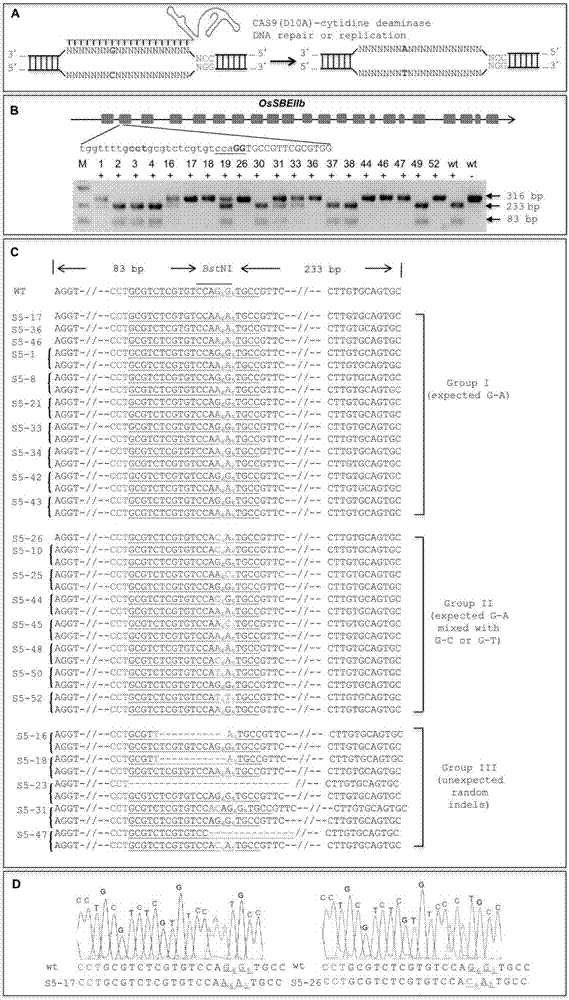
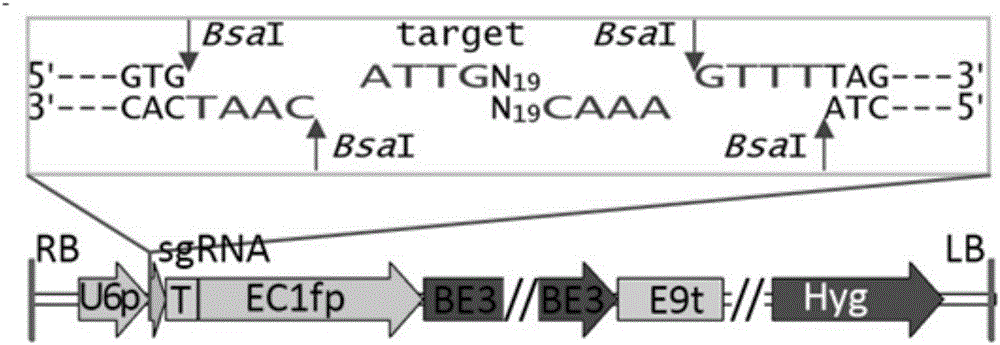
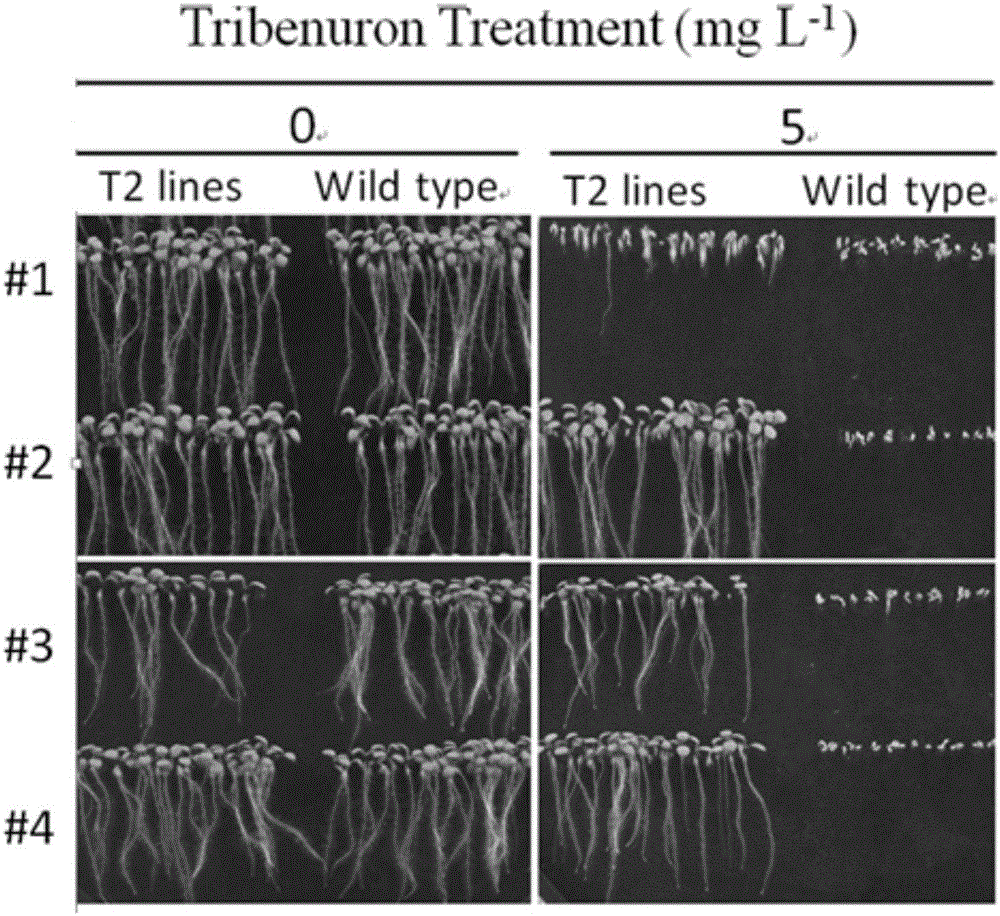
The invention discloses application of CRISPR / nCas9 mediated site-directed base substitution in a plant. The invention provides a plant genome site-directed edition system. The system comprises a BE3 plant expression carrier (expressing a fusion protein composed of nCas9(D10A), deaminase and a uracil DNA glycosylase inhibitory protein), and rice OsPDS and OsSBEIIb are taken as target genes for verifying the system. Results show that an expected site-directed mutant plant is respectively obtained in the three selected target spots, accurate site mutation of a base is realized in rice, and the highest efficiency reaches about 20%, so that a feasible and effective base substitution method is provided for crop breeding, the method has strong application potential in the aspect of agricultural breeding, and a foundation is provided for rapidly improving important agronomic traits of crops.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI
Method of preparing and using a cold extract from the leaves of nerium oleander
A method of preparing and using a sterile non-toxic pyrogen-free cold extract from the leaves of Nerium oleander as a supplementary medication to cancer chemo-, hormon and / or radiotherapy to restore and / or ameliorate the immune system of the patient and / or to decrease side effects and increase the antitumor effects of radiotherapy and chemotherapeutics, particularly when used in combination with taxol, adriamycin, cisplatin, 5-fluoro-uracil, alimta, cyclophosphamide, mitomycin-C, navelbine, taxotere and topotecan, respectively, and its use in the manufacture of a medicament for the treatment of one or more cancers of bladder, kidney, liver, ovary, pancreas, testicle, uterus, and vagina as well as pleuramesotheliomas and Hodgkin's lymphomas.
Owner:RASHAN JUAY JAMIL
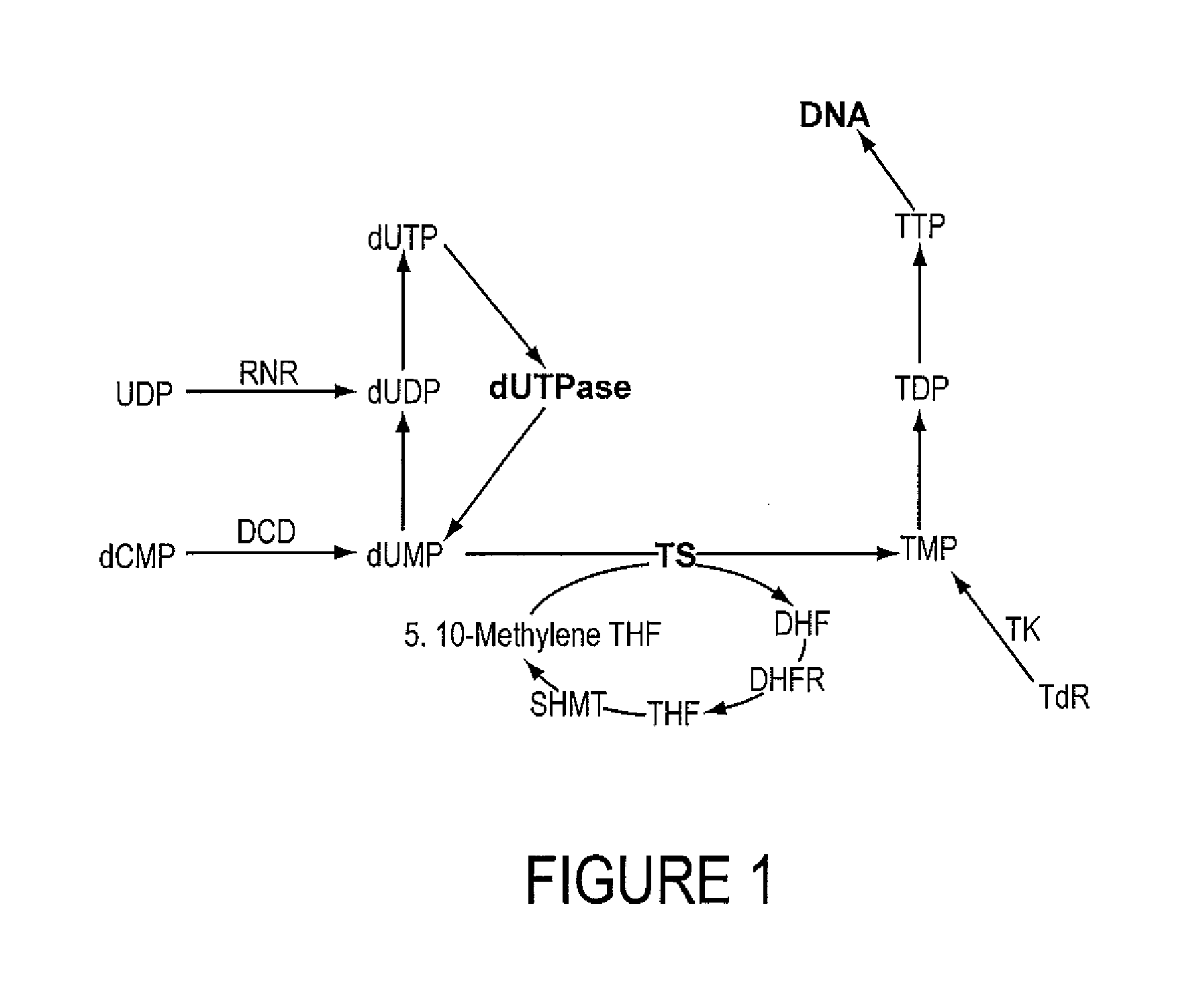
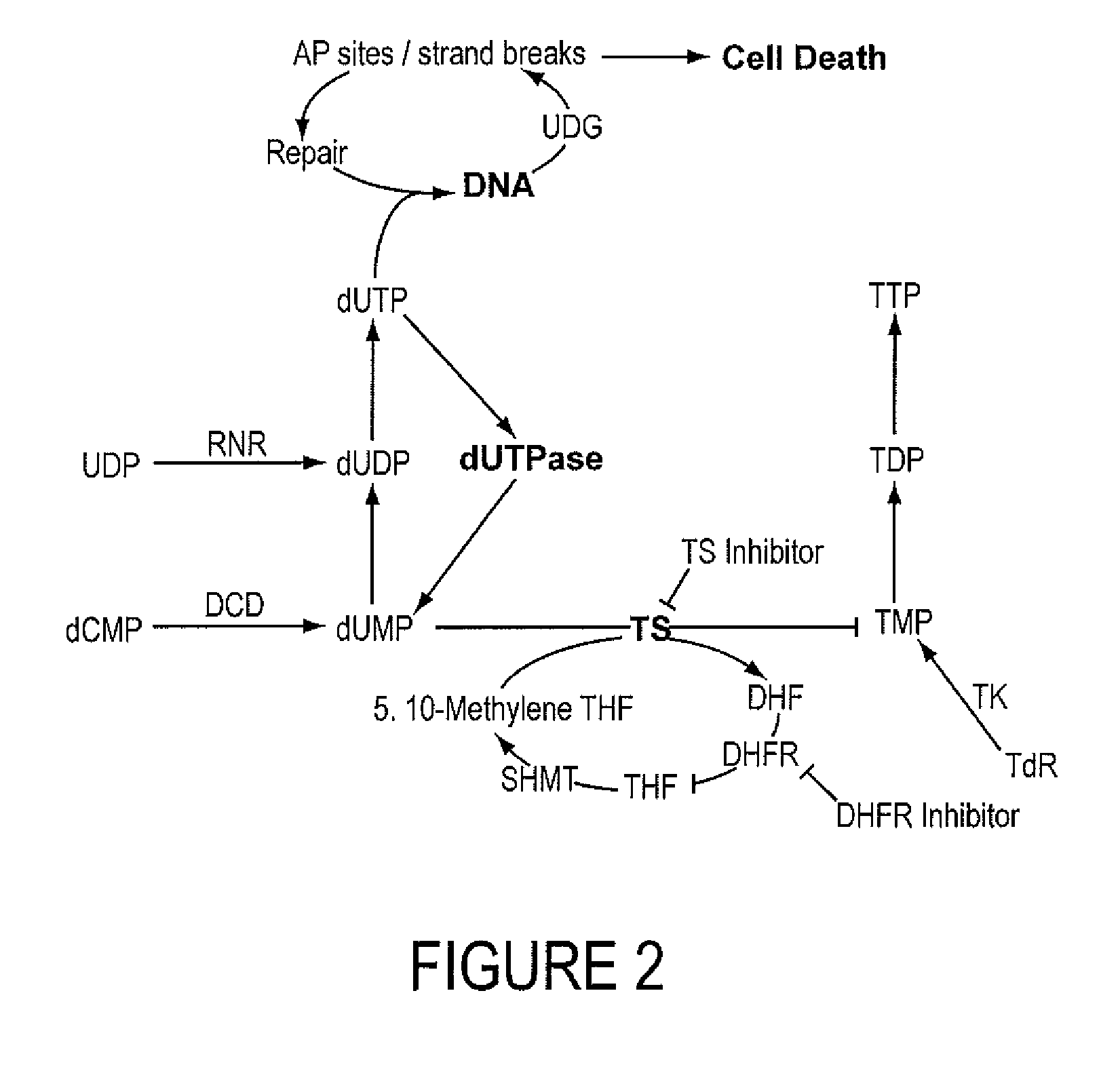
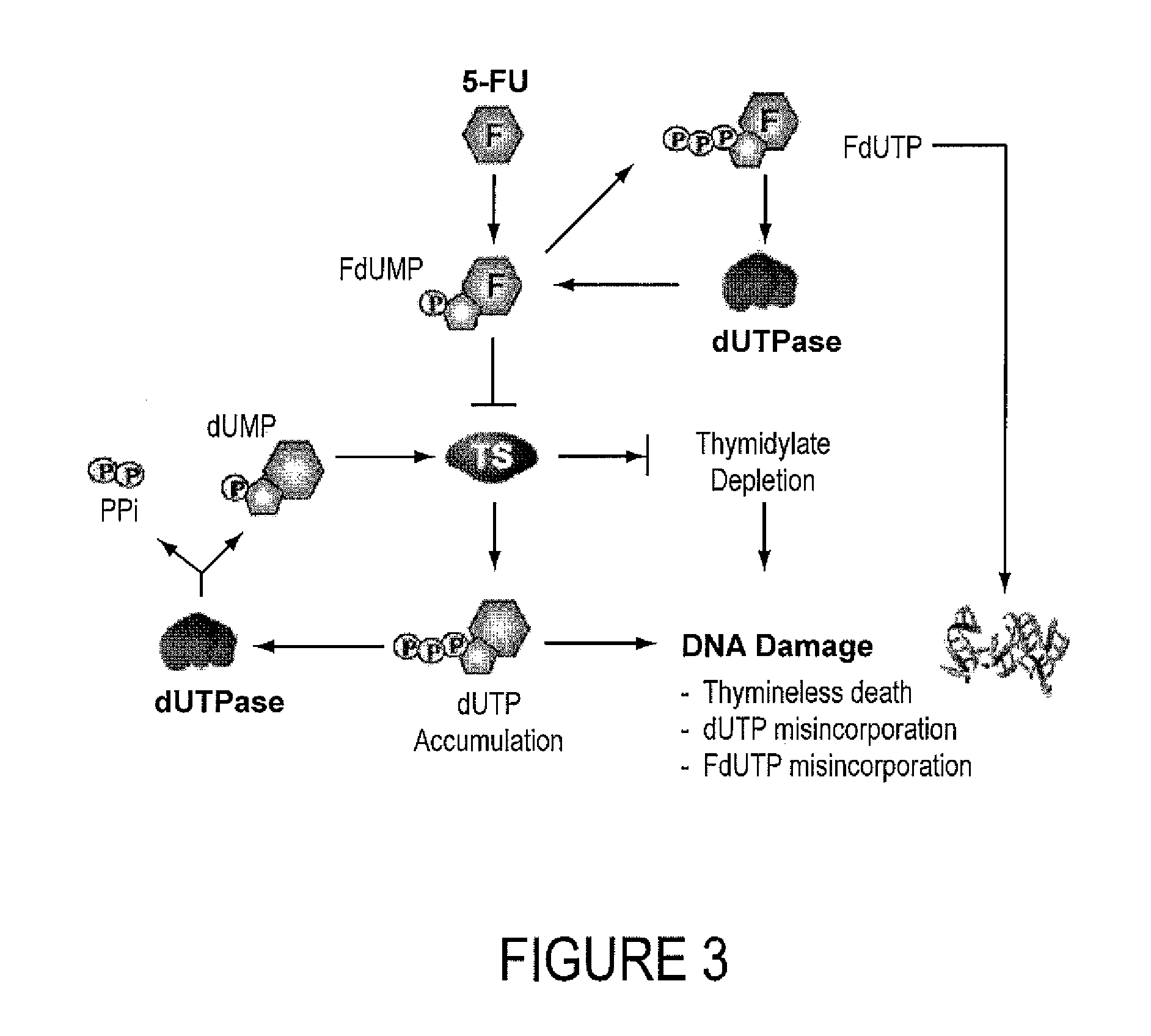
INHIBITORS OF dUTPase
InactiveUS20110212467A1Improve the immunitySuppresses dUTP poolOrganic active ingredientsOrganic chemistryClinical efficacyUracil
Evidence demonstrating that elevated expression of dUTPase protects breast cancer cells from the expansion of the intracellular uracil pool, translating to reduced growth inhibition following treatment with 5-FU is provided. The implementation of in silica drug development techniques to identify and develop small molecule inhibitors of dUTPase are reported. As 5-FU and the oral 5-FU pro-drug capecitabine remain central agents in the treatment of a variety of malignancies, the clinical utility of a small molecule inhibitor to dUTPase represents a viable strategy to improve the clinical efficacy of these mainstay chemotherapeutic agents.
Owner:UNIV OF SOUTHERN CALIFORNIA
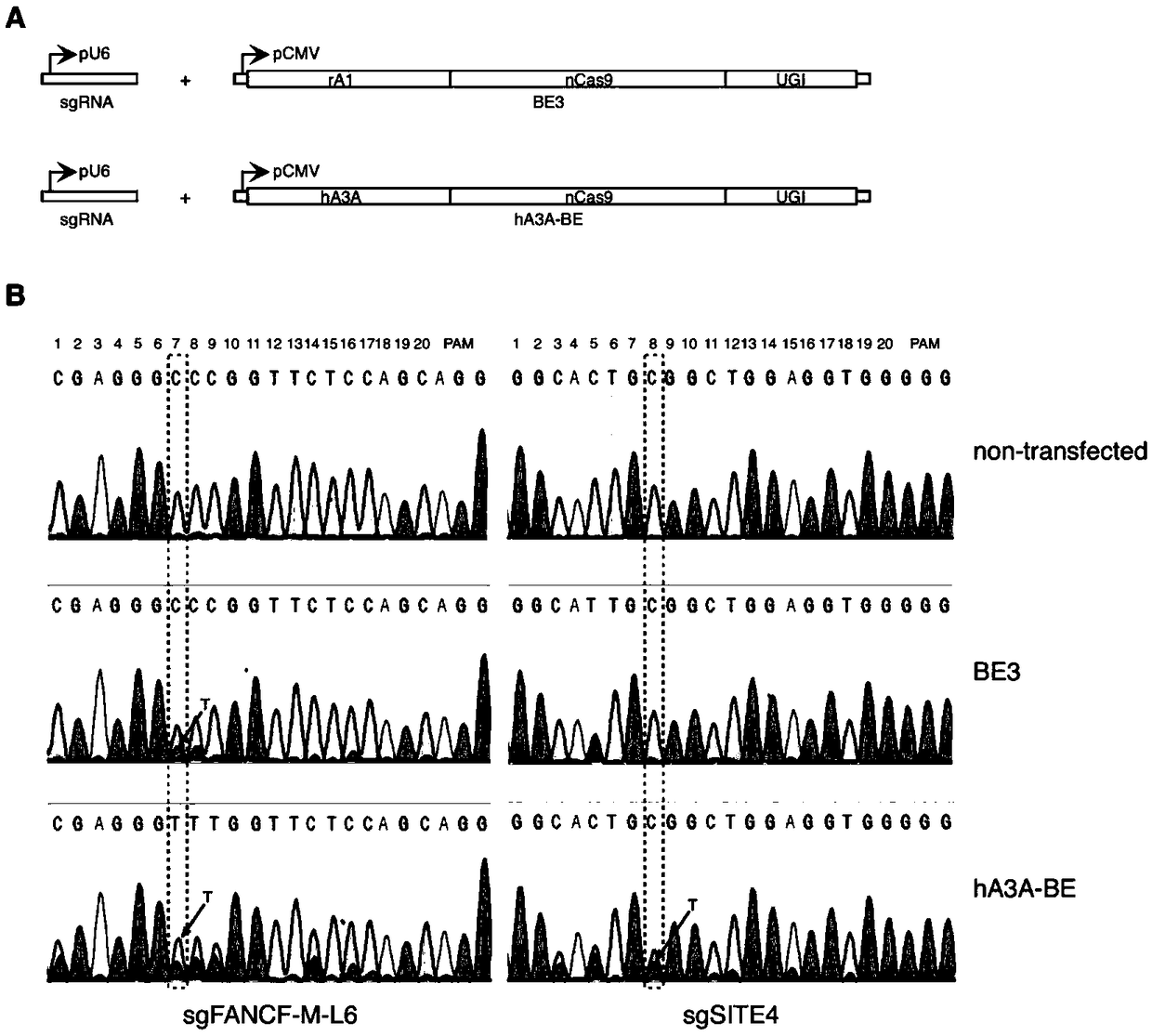
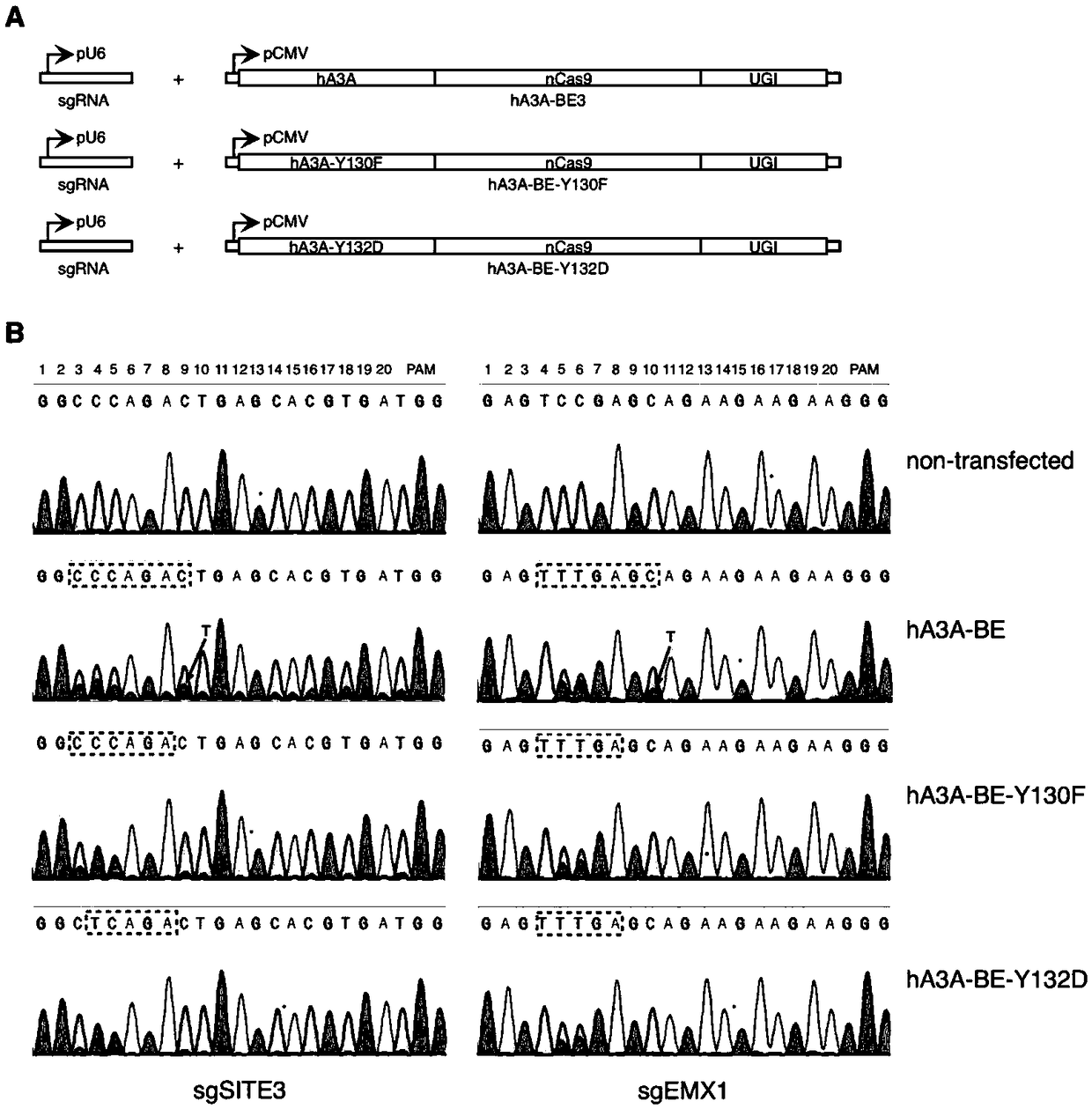
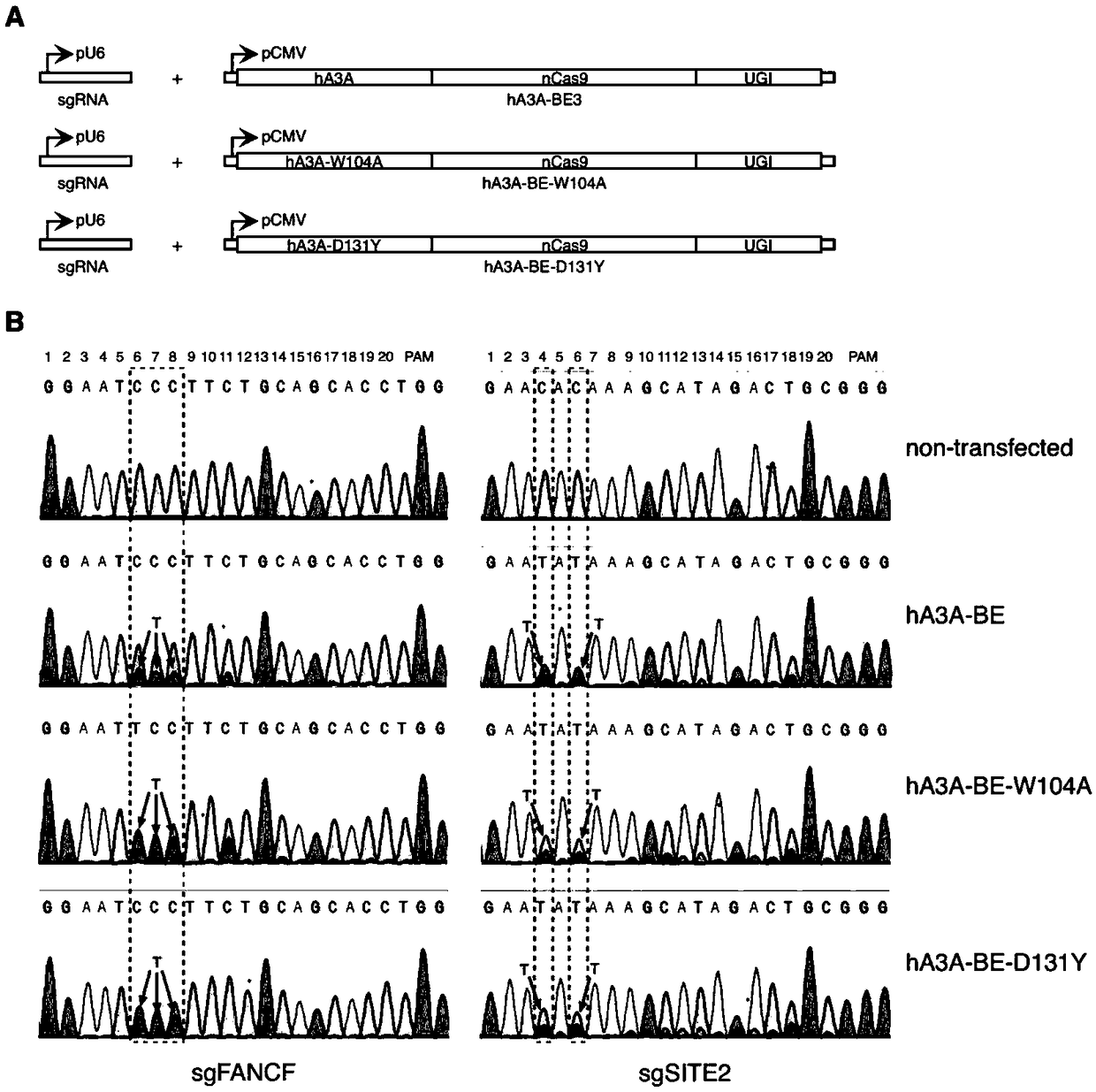
Gene base editor
The invention provides a gene base editor, in particular relates to fusion protein. The fusion protein is characterized by comprising two fragments. The first fragment comprises APOBEC3A and the second fragment comprises CRISPR-related Cas protein. The base editor is capable of performing base edition in DNA and performing deamination on cytosine to be uracil, and the base editor still has higherediting efficiency even if cytosine is located at a GpC site or in a hypermethylated state.
Owner:SHANGHAI TECH UNIV
Gene site-directed mutation vector as well as construction method and application thereof
ActiveCN106834341ANucleic acid vectorVector-based foreign material introductionCytosine deaminaseCytosine
The invention belongs to the technical field of bioengineering and in particular relates to a gene site-directed mutation vector as well as a construction method and application thereof. The invention provides the gene site-directed mutation vector due to lots of optimizations, cytosine deaminase is guided to be close to cytosine through a CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) system, the cytosine is changed into uracil, then uracil is changed into thymine through self-repair in plant cells, and finally, site-specific mutagenesis from C to T is realized in plants so as to generate resistance to herbicides. In addition, point mutation can be produced in a non-sgRNA targeted area, and novel herbicide-resistant great agronomic traits are brought.
Owner:CHINA AGRI UNIV
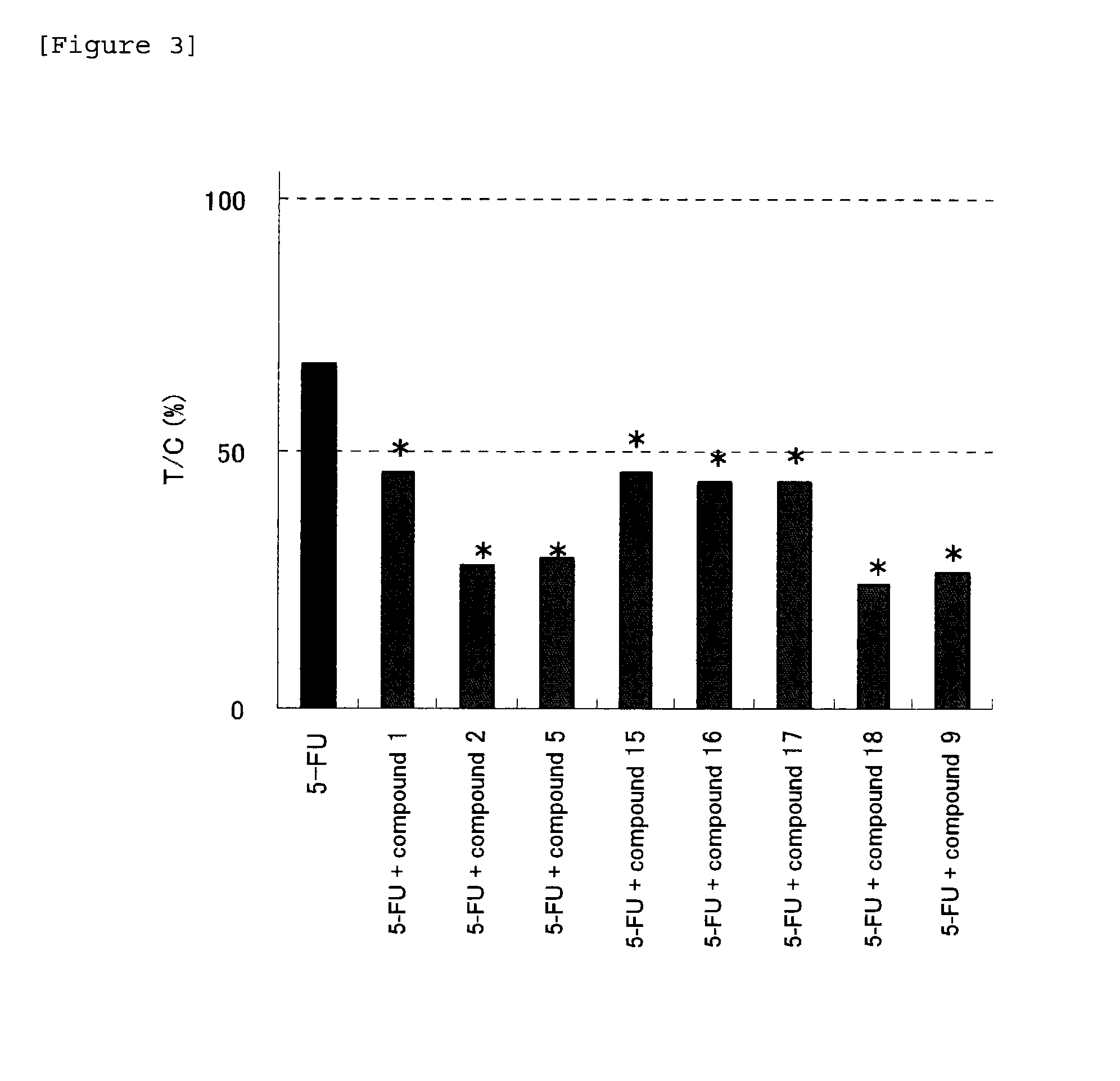
Anti-tumor effect potentiator
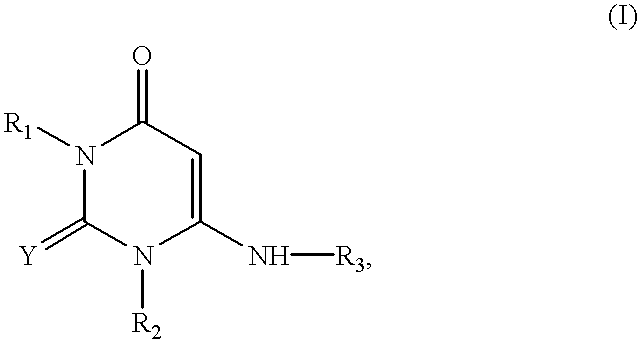
There is provided an agent for potentiating the effects of an anti-tumor agent.An anti-tumor effect potentiator containing, as an active ingredient, a uracil compound represented by the following formula (I) or a pharmaceutically acceptable salt thereof:wherein X represents a C1-5 alkylene group and one of methylene groups constituting the alkylene group is optionally substituted with an oxygen atom;R1 represents a hydrogen atom or a C1-6 alkyl group; R2 represents a hydrogen atom or a halogen atom; and R3 represents a C1-6 alkyl group, a C2-6 alkenyl group, a C3-6 cycloalkyl group, a (C3-6 cycloalkyl) C1-6 alkyl group, a halogeno-C1-6 alkyl group or a saturated heterocyclic group.
Owner:TAIHO PHARMA CO LTD
Processes for the preparation of uracil derivatives
The present invention relates to processes and intermediates for preparing Gonadotropin-Releasing Hormone (GnRH) receptor antagonists of structure (VI); and stereoisomers and pharmaceutically acceptable salts thereof.
Owner:NEUROCRINE BIOSCI INC
Novel uracil compound or salt thereof having human deoxyuridine triphosphatase inhibitory activity
ActiveUS20110082163A1Potent human dUTPase inhibitory activityAntibacterial agentsBiocideDeoxyuridinePharmaceutical Substances
Provided is a uracil compound or a salt thereof, which has potent human dUTPase inhibitory activity and is useful as, for example, an antitumor drug.A uracil compound represented by the general formula (I) or a salt thereof:wherein n represents an integer of 1 to 3; X represents a bond, an oxygen atom, a sulfur atom, or the like; Y represents a linear or branched alkylene group having 1 to 8 carbon atoms, or the like; and Z represents —SO2NR1R2 or —NR3SO2—R4, wherein R1 and R2 each represent an alkyl group having 1 to 6 carbon atoms, an aralkyl group which is optionally substituted, or the like; R3 represents an alkyl group having 1 to 6 carbon atoms, or the like; and R4 represents an aromatic hydrocarbon group, an unsaturated heterocyclic group, or the like.
Owner:TAIHO PHARMA CO LTD
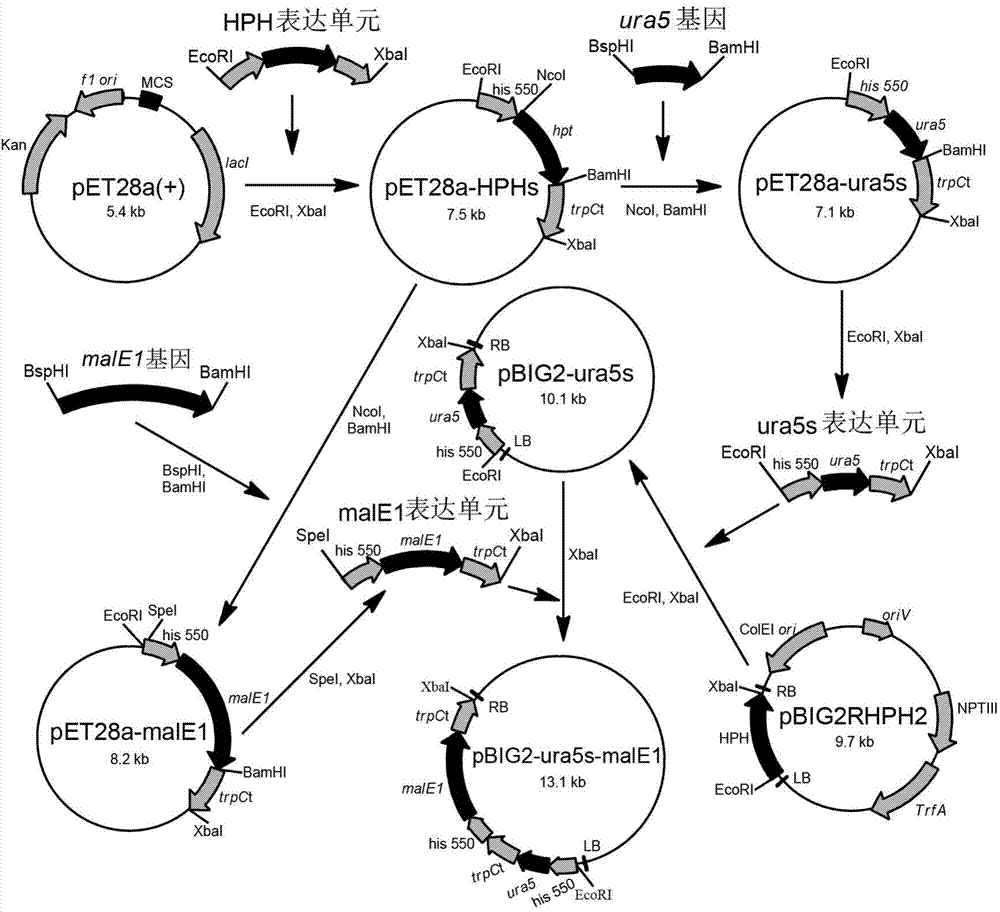
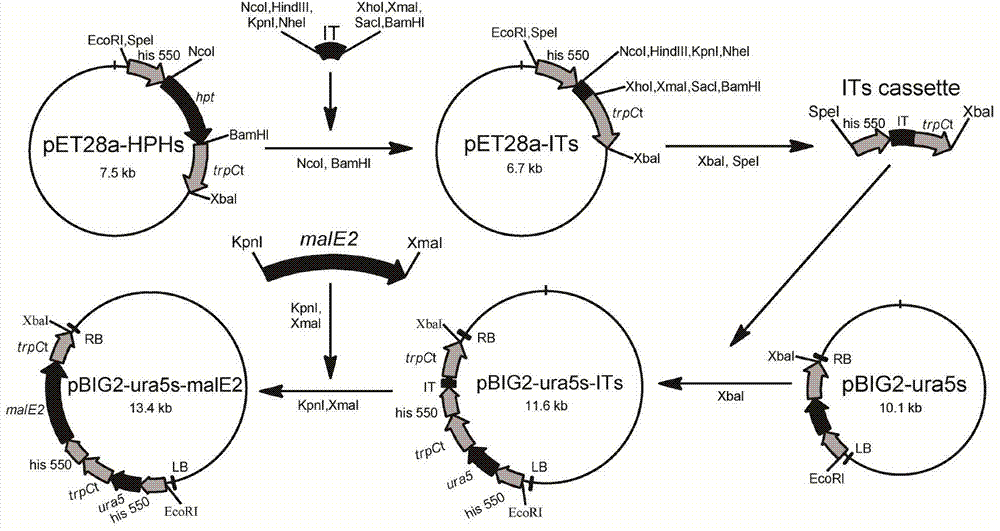
Recombination gene expression system of mortierella alpina and construction method and applications thereof
The invention relates to a recombination gene expression system of mortierella alpina (Mortierella alpina ATCC 32222) and applications thereof. According to the invention, by taking mortierella alpina ATCC 32222 uracil auxotroph strains as materials, a set of recombination gene expression system of mortierella alpina is constructed through carrying out genetic manipulation by using an agrobacterium tumefaciens mediated genetic manipulation technique; and an operation is operated by using the system, so that multiple high-yield polyunsaturated fatty acid mortierella alpina strains are obtained, therefore, the recombination gene expression system of mortierella alpina and construction method and applications thereof disclosed by the invention are of great importance in the basic theory study and product development of oil-producing fungi mortierella alpina ATCC 32222.
Owner:JIANGNAN UNIV
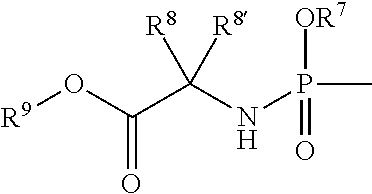
Uracyl cyclopropyl nucleotides
Compounds of the formula I:including any possible stereoisomers thereof, wherein:R1 is hydrogen or halo;R4 is a monophosphate, diphosphate or triphosphate ester; or R4 is a group of formulaR7 is optionally substituted phenyl; naphthyl; indolyl or N—C1-C6alkyloxycarbonyl-indolyl;R8 is hydrogen, C1-C6alkyl, benzyl;R8′ is hydrogen, C1-C6alkyl, benzyl; orR8 and R8′ together with the carbon atom to which they are attached form C3-C7cycloalkyl;R9 is C1-C10alkyl, benzyl, or optionally substituted phenyl;or a pharmaceutically acceptable salt or solvate thereof.pharmaceutical formulations and the use of compounds I as HCV inhibitors.
Owner:JANSSEN PROD +1
NH2-modified 6-aminouracils as stabilizers for halogenated polymers
A description is given of compounds of the general formula IwhereY is oxygen or sulfur, andR1 and R2 independently of one another are C1-C18-alkyl, C3-C6-alkenyl, unsubstituted or C1-C4-alkoxy-, C5-C8-cycloalkyl-, -OH- and / or Cl-substituted C1-C18-alkyl, C5-C8-cycloalkyl, phenyl or C7-C9-phenylalkyl which is unsubstituted or substituted on the phenyl ring by C1-C4-alkyl, C1-C4-alkoxy, C5-C8-cycloalkyl, -OH and / or Cl, andR3 is unsubstituted C9-C18-alkyl or is C1-C18-alkyl substituted by -OH, C1-C12-alkoxy, phenoxy, C6-C8-alkylphenoxy, C7-C9-alkoxyphenyl, -C=O(OR4) and / or -O-COR4, or is C3-C6-alkenyl, C5-C8-cycloalkyl, or mono- to tri-OH-, -C1-C4-alkyl-, -C1-C4-alkoxy-, -C=O(OR4)- and / or -O-COR4-substituted phenyl or naphthyl, and whereR4 is C1-C12-alkyl,which are suitable for stabilizing chlorine-containing polymers, especially PVC.
Owner:CHEMTURA VINYL ADDITIVES
Uracil compound or salt thereof having human deoxyuridine triphosphatase inhibitory activity
ActiveUS8530490B2Potent human dUTPase inhibitory activityAntibacterial agentsOrganic active ingredientsDeoxyuridinePharmaceutical Substances
Provided is a uracil compound or a salt thereof, which has potent human dUTPase inhibitory activity and is useful as, for example, an antitumor drug.A uracil compound represented by the general formula (I) or a salt thereof:wherein n represents an integer of 1 to 3; X represents a bond, an oxygen atom, a sulfur atom, or the like; Y represents a linear or branched alkylene group having 1 to 8 carbon atoms, or the like; and Z represents —SO2NR1R2 or —NR3SO2—R4, wherein R1 and R2 each represent an alkyl group having 1 to 6 carbon atoms, an aralkyl group which is optionally substituted, or the like; R3 represents an alkyl group having 1 to 6 carbon atoms, or the like; and R4 represents an aromatic hydrocarbon group, an unsaturated heterocyclic group, or the like.
Owner:TAIHO PHARMA CO LTD
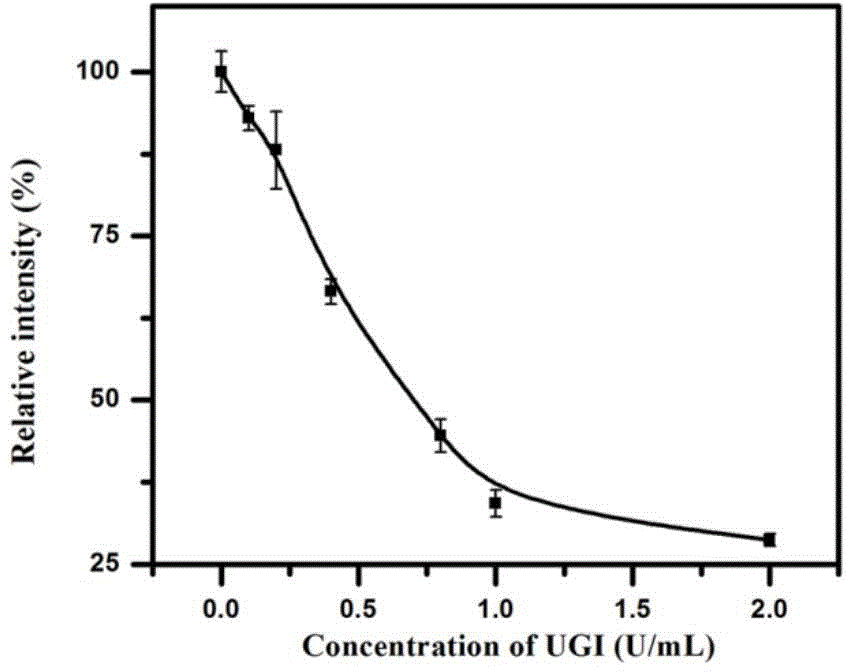
Method for detecting activity of uracil-DNA glycosylase (UDG) based on fluorescence amplification strategy of label-free non-enzyme DNA machines
InactiveCN104630363AImprove hybridization efficiencyEnhanced inhibitory effectMicrobiological testing/measurementFluorescenceNucleotide
The invention discloses a method for detecting the activity of uracil-DNA glycosylase (UDG) based on a fluorescence amplification strategy of label-free non-enzyme DNA machines, and the method comprises the following steps: (1) the preparation of a probe for the recognition and signal transduction of the UDG: firstly, designing an uracil base and initiation sequence containing double-stranded DNA probe P1-P2, wherein the P1 chain is an inhibition chain and the nucleotide sequence thereof is show in SEQ ID NO.1 in a sequence table; the P2 chain is a uracil-DNA sequence and initiation sequence containing chimeric conjugated chain and the nucleotide sequence thereof is show in SEQ ID NO.2 in the sequence table; and the P1 chain and the P2 chain are partially complemented so as to form the double-stranded DNA probe P1-P2; (2) the construction of a label-free non-enzyme DNA machine: according to an initiation sequence of the P2 chain, designing hairpin probes H1 and H2 which are partially complemented and used for constructing the label-free non-enzyme DNA machine, and grafting a G-quadruplet sequence to the tail end of the hairpin probe H2; and (3) the activity detection of UDG. The method disclosed by the invention successfully realizes background diminishing and signal amplification, and the LOD (limit of detection) is 0.00044 U / mL.
Owner:SHANDONG UNIV
Novel uracil compound having inhibitory activity on human deoxyuridine triphosphatase or salt thereof
ActiveCN102056905AExcellent human dUTPase inhibitory activityAntibacterial agentsOrganic active ingredientsUracilChemical compound
Disclosed is an uracil compound which has an excellent inhibitory activity on human dUTPase and is useful as an anti-tumor agent or the like or a salt of the uracil compound. Specifically disclosed is an uracil compound represented by general formula (I) or a salt thereof. [In general formula (I), n represents a number of 1 to 3; X represents a bond, an oxygen atom, a sulfur atom, or the like; Y represents a linear or branched alkylene group having 1 to 8 carbon atoms, or the like; Z represents -SO2NR1R2 or -NR3SO2-R4; R1 and R2 independently represent an alkyl group having 1 to 6 carbon atoms, an aralkyl group which may have a substituent, or the like; R3 represents an alkyl group having 1 to 6 carbon atoms, or the like; and R4 represents an aromatic hydrocarbon group, an unsaturated heterocyclic group, or the like].
Owner:TAIHO PHARMA CO LTD
5-Fluoro-uracil immunoassay
ActiveUS7205116B2Accurate monitoringReaction can be selectiveEnzymologyDepsipeptidesBiological fluidsFluorouracil
Owner:SALADAX BIOMEDICAL INC
Methods and means for treating DNA repeat instability associated genetic disorders
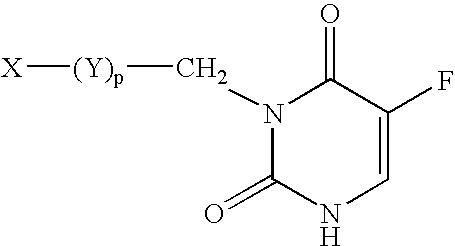
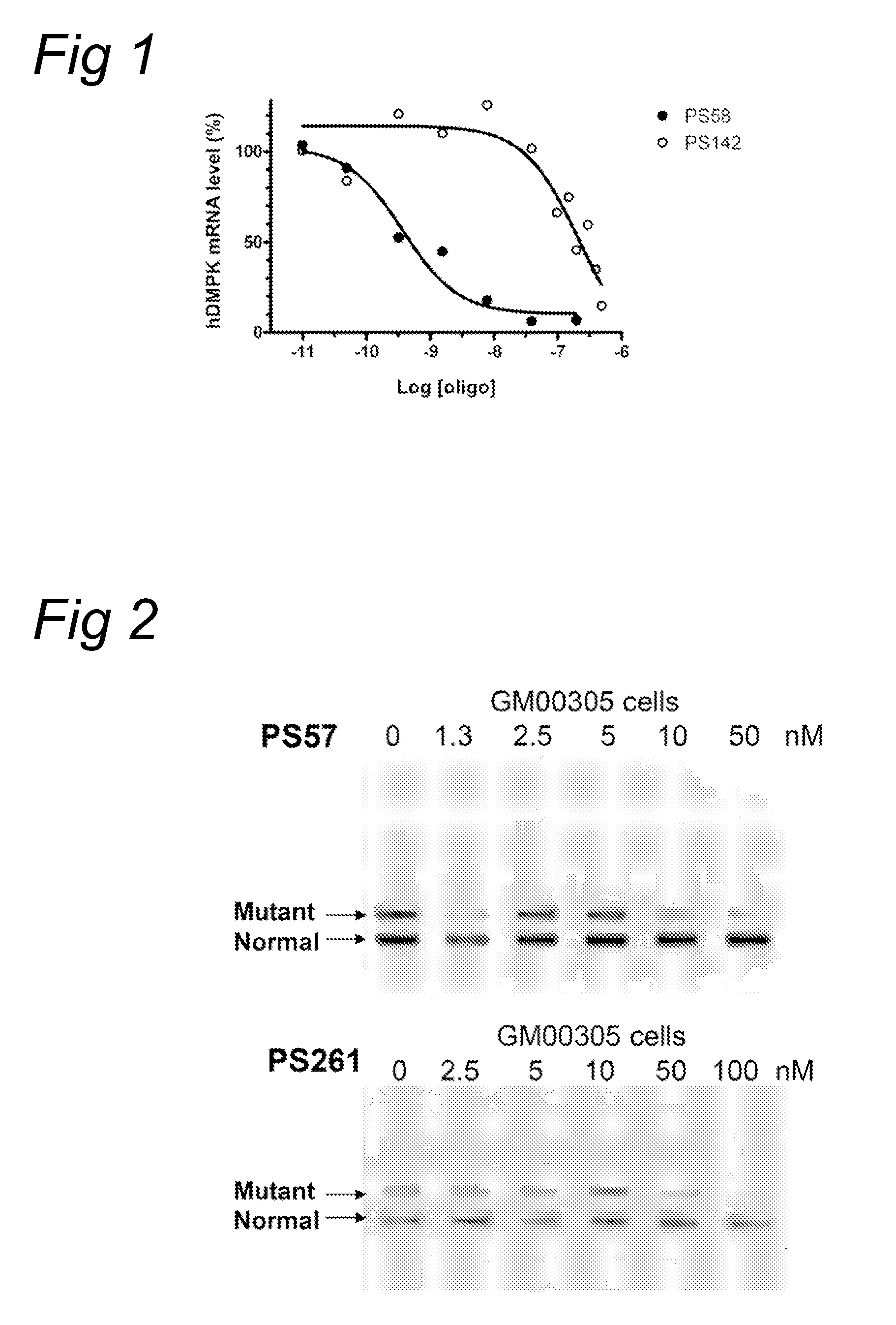
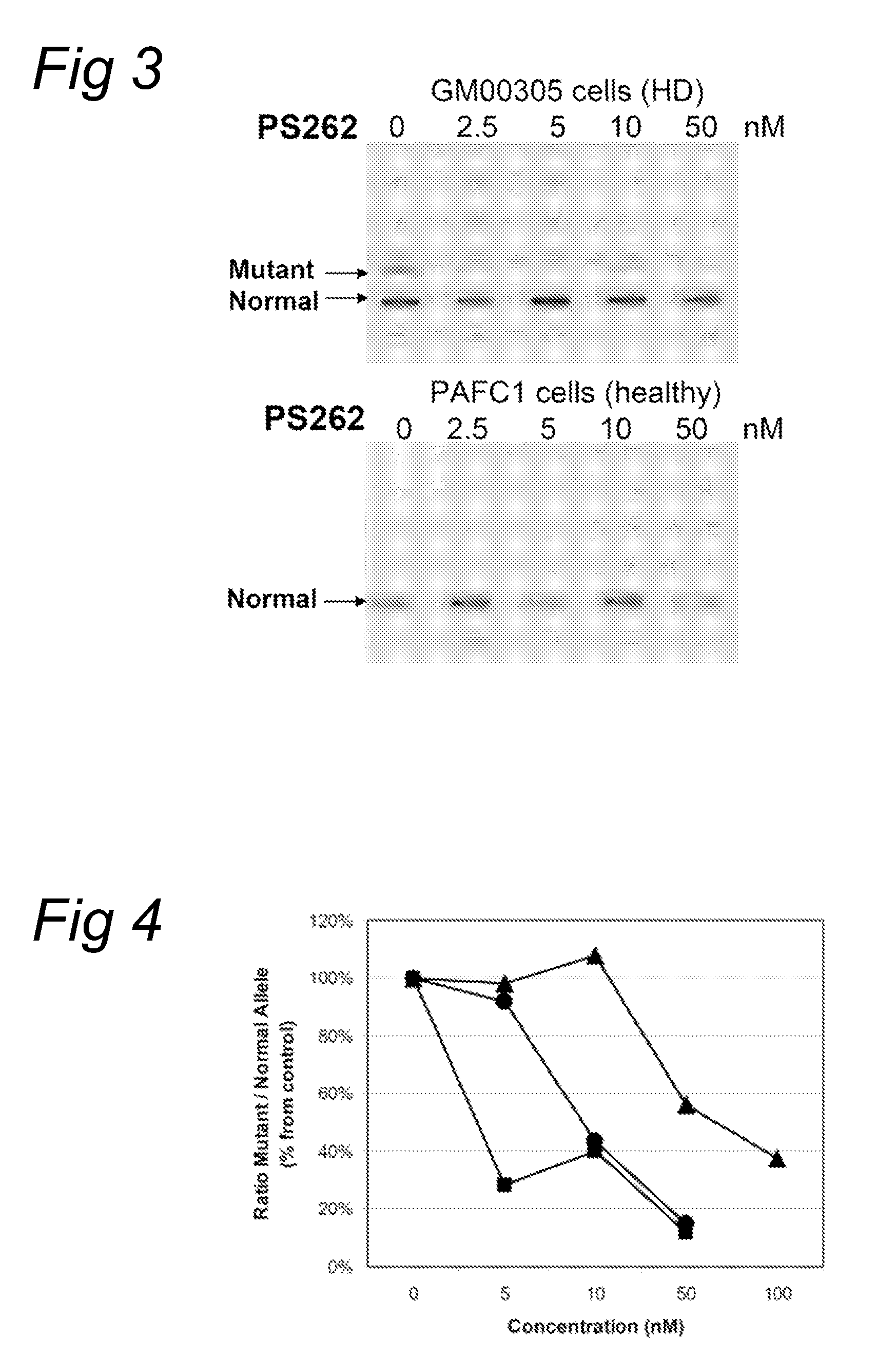
InactiveUS8263760B2Lower Level RequirementsReduced stabilityNervous disorderSugar derivativesUracilInstability
The current invention provides for methods and medicaments that apply an oligonucleotide comprising aninosine and / or an uracile and / or a nucleotide containing a base able to form a wobble base pair, said oligonucleotide being preferably RNAse H substantially independent and being complementary only to a repetitive sequence in a human gene transcript, for the manufacture of a medicament for the diagnosis, treatment or prevention of a cis-element repeat instability associated genetic disorders in humans. The invention hence provides a method of treatment for cis-element repeat instability associated genetic disorders. The invention also pertains to a modified oligonucleotide which can be applied in a method of the invention to prevent the accumulation and / or translation of repeat expanded transcripts in cells.
Owner:PROSENSA HLDG BV +1
Variant Forms of Urate Oxidase and Use Thereof
ActiveUS20090169534A1Retains uricolytic activityImprove stabilityBacteriaPeptide/protein ingredientsUracilVariant form
Owner:HORIZON THERAPEUTICS USA INC
Antimicrobial compound and preparation method and application thereof
InactiveCN101967182AIncrease synthesis rateIncrease productionAntibacterial agentsBiocideDipeptideD-Glucopyranose
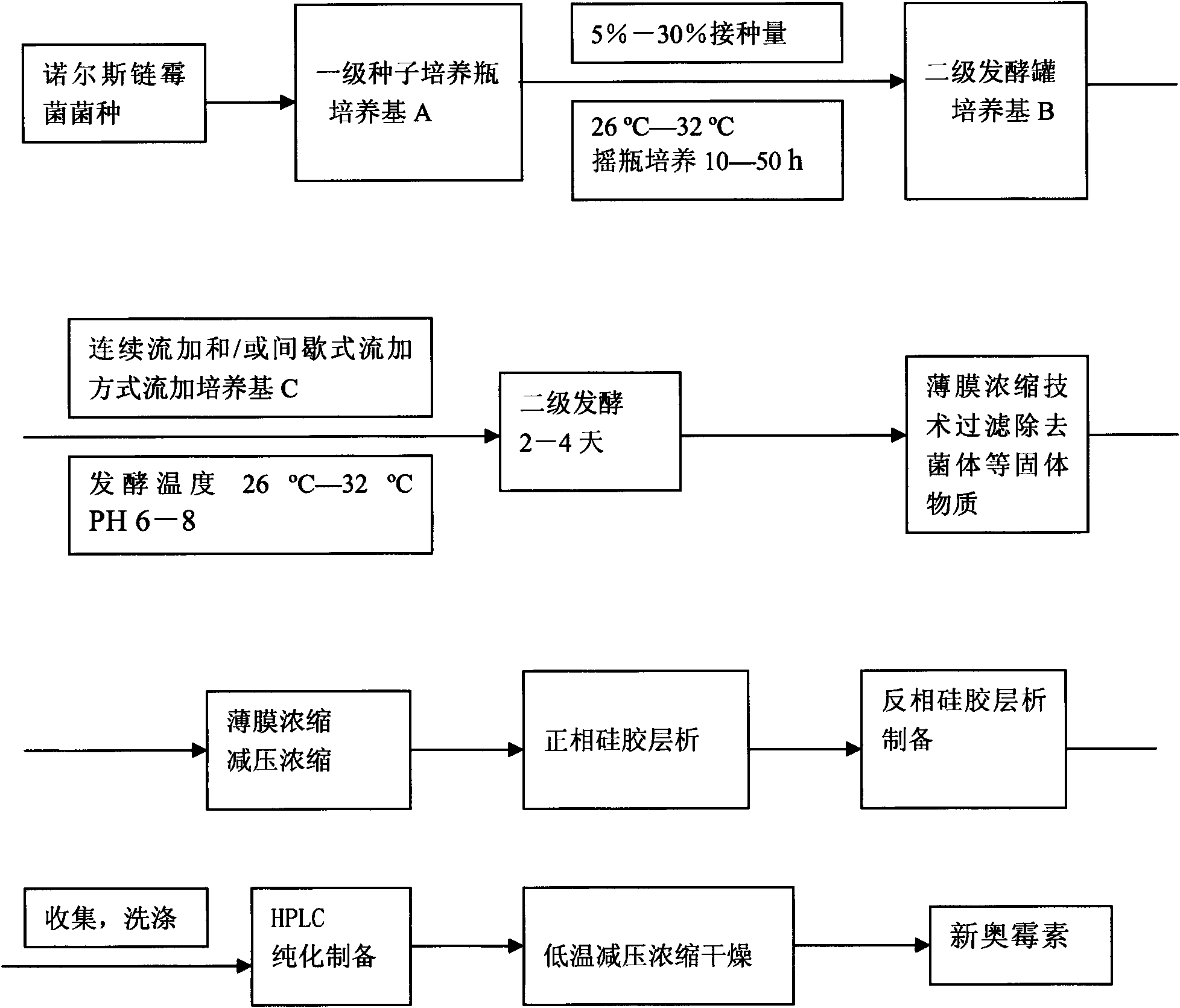
The invention belongs to the technical field of microbes, and in particular relates to an antimicrobial compound and a fermenting preparation method and application thereof. The molecular weight of the antimicrobial compound of the invention is 445, the molecular formula of the antimicrobial compound is C16H23N5O10, the chemical name of the antimicrobial compound is 1-uracil-4-sarkosyl-serylamido-1,4-dideoxy-beta-D-glucopyranosyl acid, and the antimicrobial compound belongs to uracil hexose dipeptide and is named xin'aomycin. The preparation method adopts actinomycetes Streptomyces noursei and genetically improved strains thereof, and comprises the following steps: culturing in a primary liquid culture medium as a seed liquid; inoculating the cultured seed liquid to a secondary liquid culture medium B for culturing, and carrying out fed-batch fermentation culture of fed-batch liquid C; and after fermentation is finished, separating and purifying the structural compound from the fermentation culture liquid. The antimicrobial compound is used for manufacturing pesticide or medicine products. The invention has the characteristics of high yield, simple process and easy industrialization.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
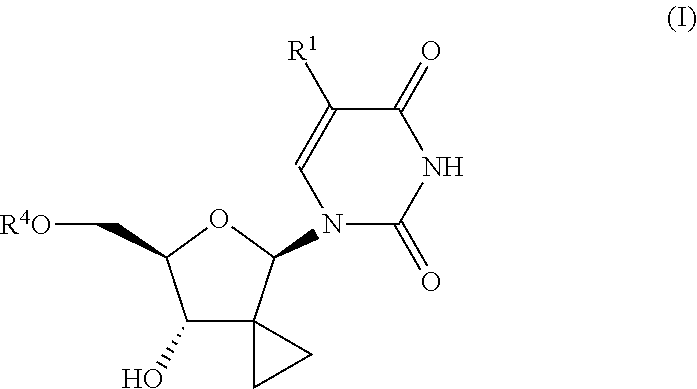
Uridine monophosphate analogue, and preparation method and applications thereof
The invention relates to uracil nucleotide analogs, preparation methods therefor and uses thereof. Specifically, the invention provides uracil nucleotide analogic compounds having the following formula (I), stereoisomers or pharmaceutically acceptable salts thereof, preparation methods therefor and uses thereof. These compounds are inhibitors of RNA-dependent RNA viral replication and are useful as inhibitors of HCV NS5B polymerase, as inhibitors of HCV replication, and for the treatment of hepatitis C infection in mammals. The compounds have a wide application prospect and are hopeful to be developed into a new generation of antiviral drugs.
Owner:JIANGSU HANSOH PHARMA CO LTD +1
Alternative nucleic acid molecules containing reduced uracil content and uses thereof
ActiveUS9751925B2Maximizes contentEnhanced ability to produceDepsipeptidesOxidoreductasesBiotechnologyUracil
The present disclosure provides alternative nucleosides, nucleotides, and nucleic acids, and methods of using them. In some aspects, the disclosure provides mRNA wherein the uracil content has been modified and which may be particularly effective for use in therapeutic compositions, because they may benefit from both high expression levels and limited induction of the innate immune response. In some aspects, the disclosure provides methods for the production of pharmaceutical compositions including mRNA without reverse phase chromatography.
Owner:MODERNATX INC
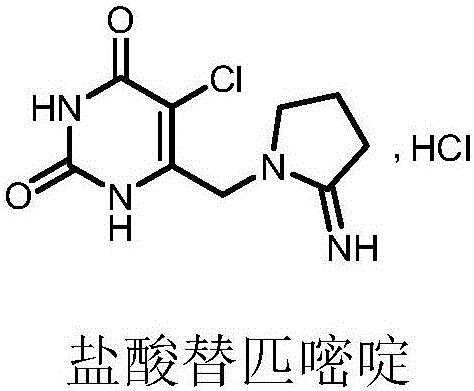
Preparation method of tipiracil
The invention discloses a preparation method of tipiracil. The method comprises the following steps: by using 6-chloromethyl uracil as the initial raw material, carrying out chlorination reaction to obtain an intermediate A, carrying out condensation reaction on the intermediate A and alpha-pyrrolidone in the presence of strong alkali to enhance the reaction selectivity, and finally, carrying out ammonolysis reaction on the intermediate B to obtain the tipiracil. The preparation method is simple, has the advantages of mild reaction conditions and high product purity, and is suitable for industrial production.
Owner:山东安信制药有限公司
Organic fuel cell anti-freeze cooling liquid with low conductivity and ultra-long acting and preparation method of anti-freeze cooling liquid
ActiveCN108102616AImprove conductivityReduce conductivityHeat-exchange elementsFuel cellsOrganic fuel8-Hydroxyquinoline
The invention discloses an organic fuel cell anti-freeze cooling liquid with low conductivity and ultra-long acting and a preparation method of the anti-freeze cooling liquid in the field of anti-freeze cooling fluids. The anti-freeze liquid is prepared from components in percentage by weight as follows: 10wt%-70wt% of ethylene glycol, 0.001wt%-0.01wt% of 8-hydroxyquinoline, 0.005wt%-0.02wt% of uracil, 0.01wt%-0.03wt% of 4-acetaminophen, 0.01wt%-0.05wt% of benzotriazole octadecylamine, 0.005wt%-0.05wt% of N-bromosuccinimide, 0.001wt%-0.01wt% of inosine and the balance of deionized water. The preparation method of the anti-freeze liquid comprises the steps as follows: all the components of the anti-freeze cooling fluid are put in a reaction kettle in percentage by mass; the components are stirred and mixed for 30-90 min, so that all components are fully dissolved and mixed uniformly, the mixed solution passes through anion and cation mixed exchange resin by the aid of a pressure pump, and the fuel cell anti-freeze cooling fluid is obtained. The organic fuel cell anti-freeze cooling liquid with low conductivity and ultra-long acting is weakly alkaline, is anti-freezing and anti-boiling and has performance of low conductivity, ultra-long acting and high corrosion inhibition.
Owner:扬州中德汽车零部件有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com