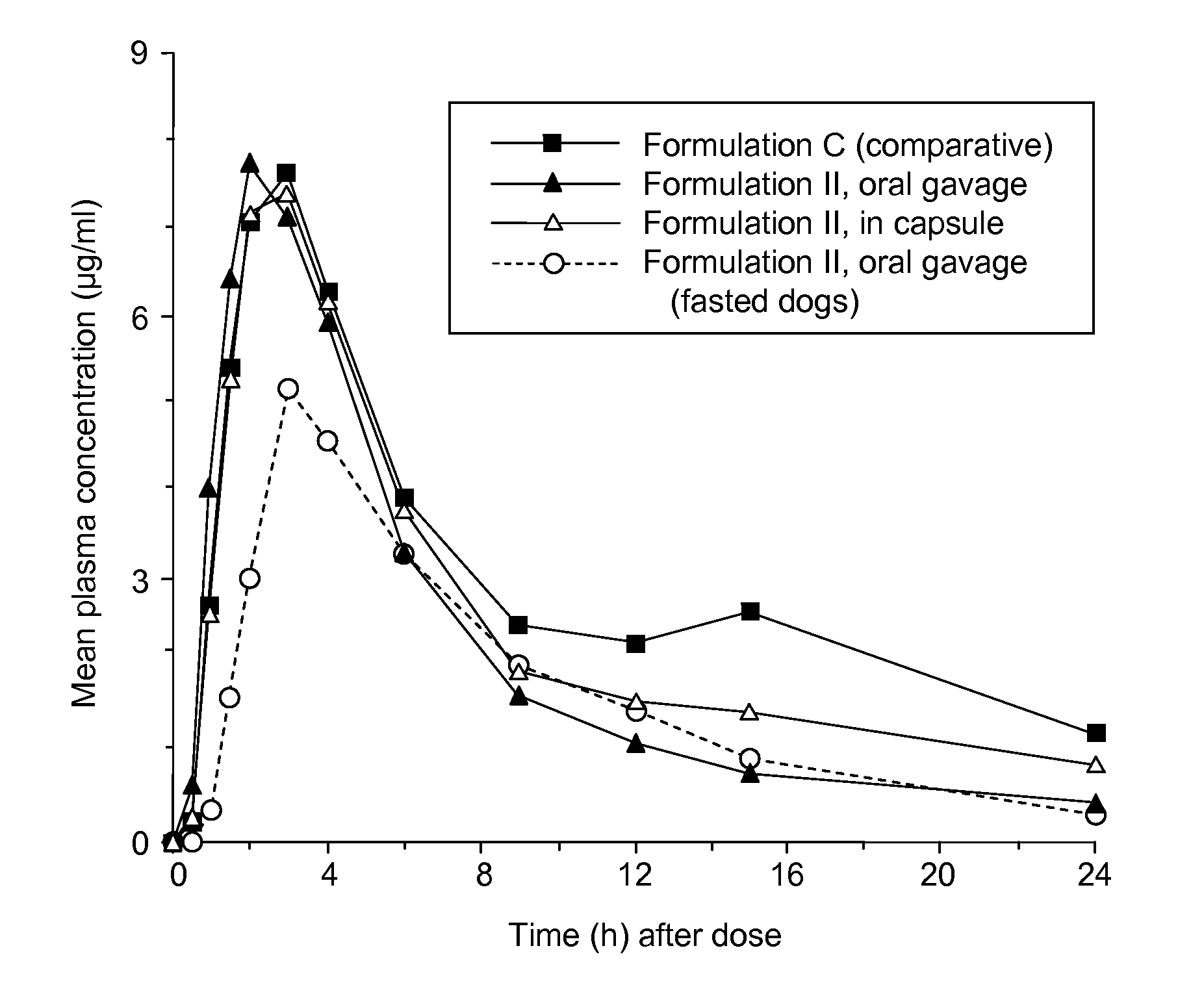
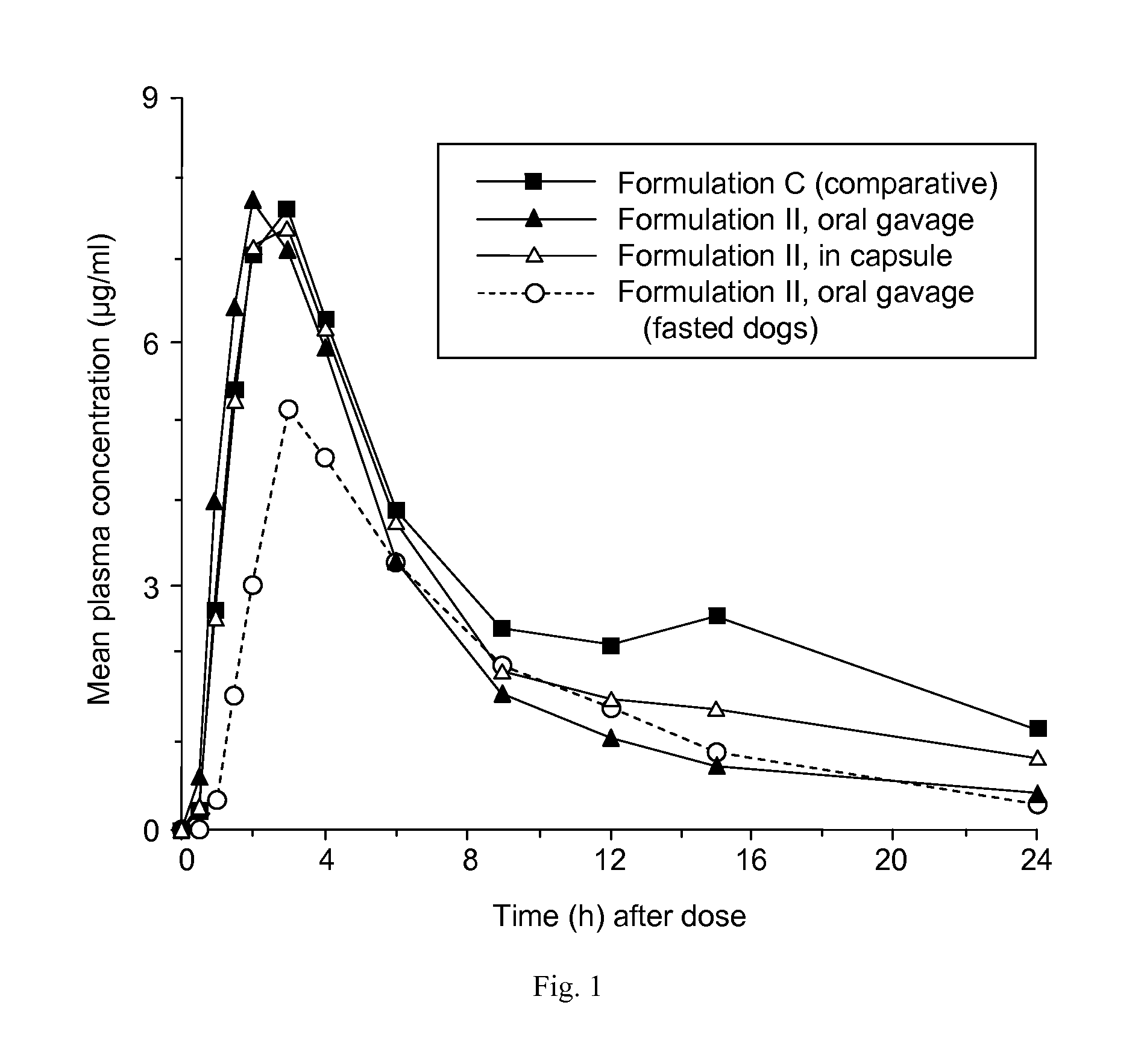
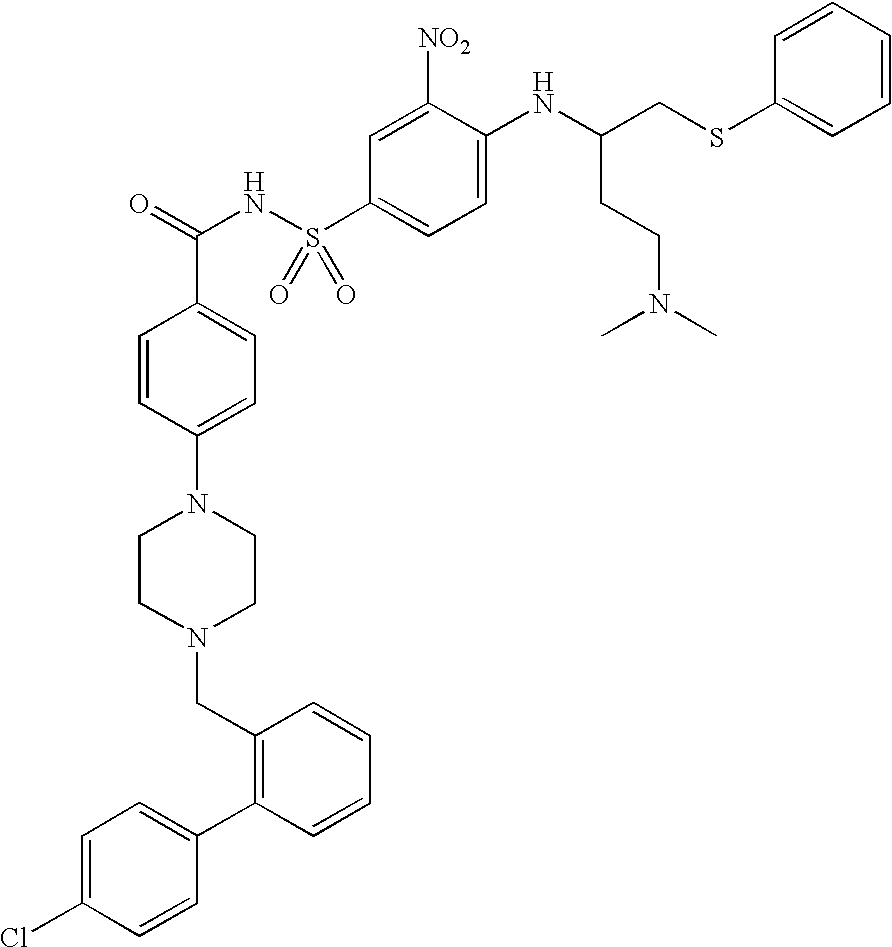
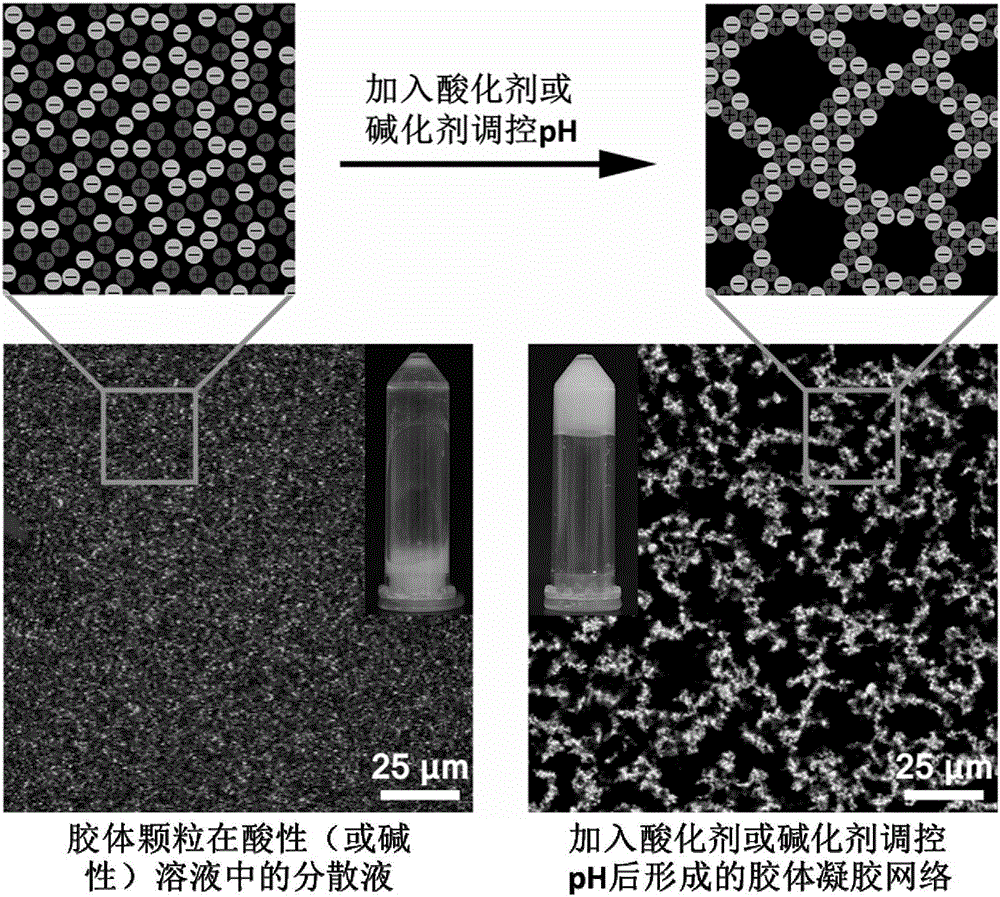
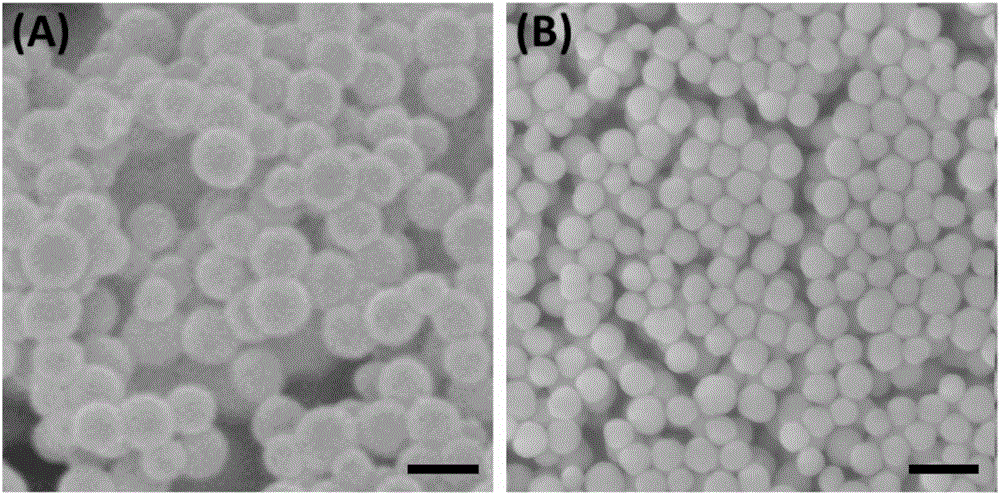
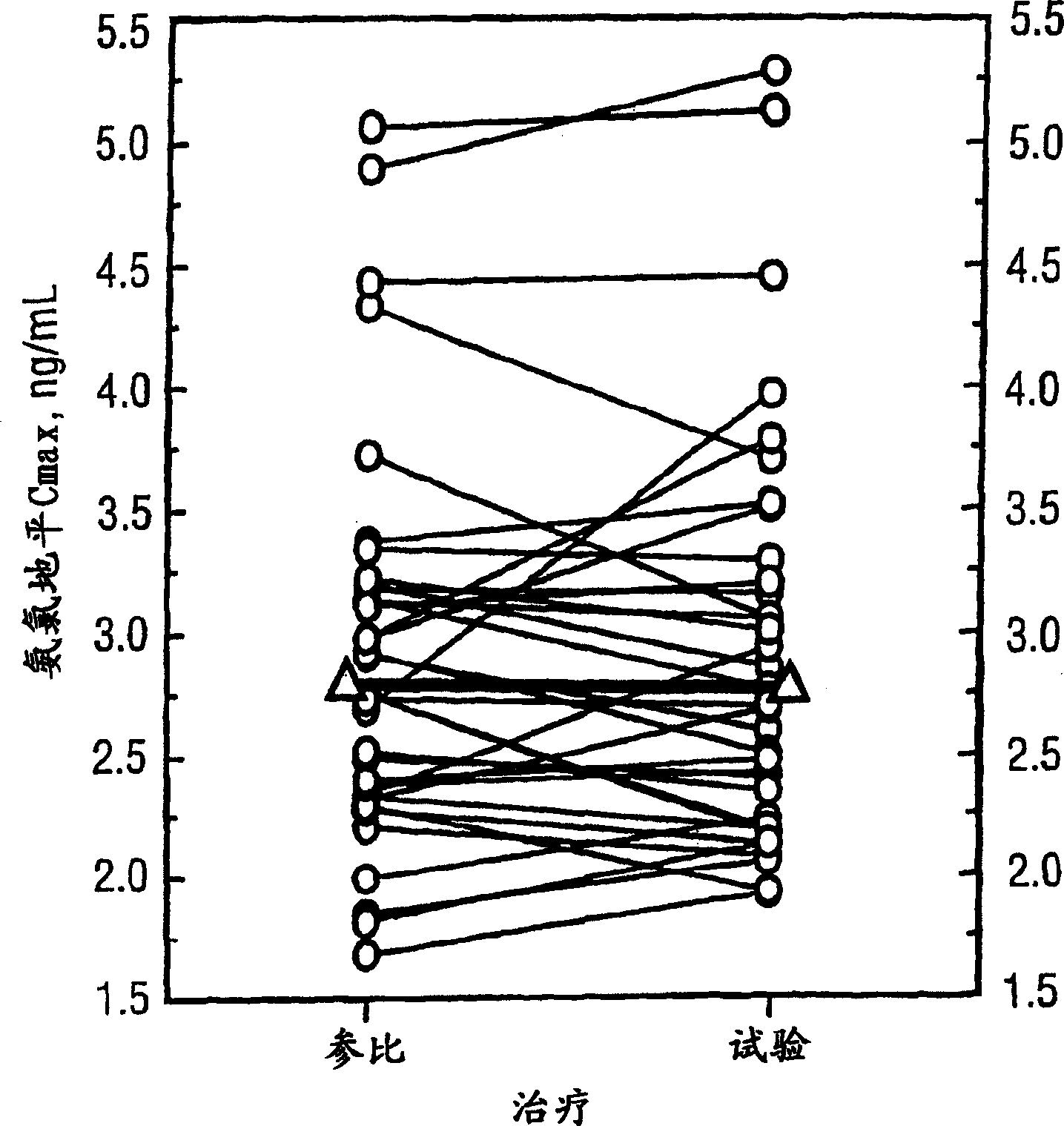
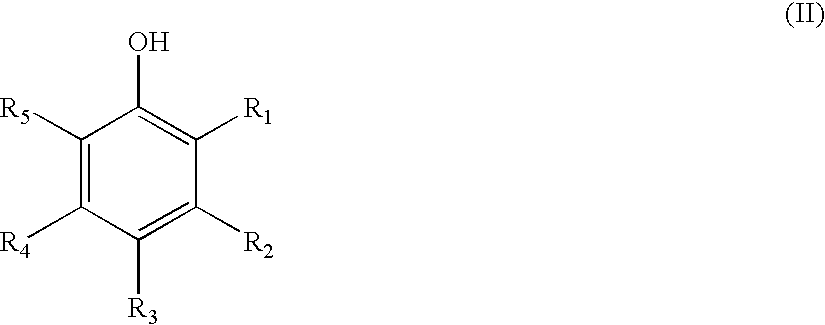Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
311 results about "Alkalizing agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
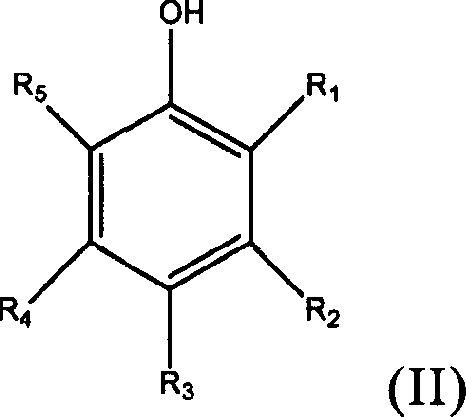
Alkalinizing agents are drugs used to manage disorders associated with low pH. For example, they may be used to treat acidosis due to renal failure. Used for oral or parenteral therapy, sodium bicarbonate is the commonly preferred alkalinizing agent.
Stable digestive enzyme compositions
InactiveUS20090117180A1Minimal loss of activityComposition is stablePowder deliveryHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 9% or less or 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 25%, about 20%, about 15% or about 10% after six months of accelerated stability testing and the titer level of a viral contaminant present in the pancreatin is at least about 1000 times less than the titer level of the viral contaminant present in a preparation from which the pancreatin is obtained.
Owner:APTALIS PHARMA
Anti-inflammatory and analgesic compositions and related methods
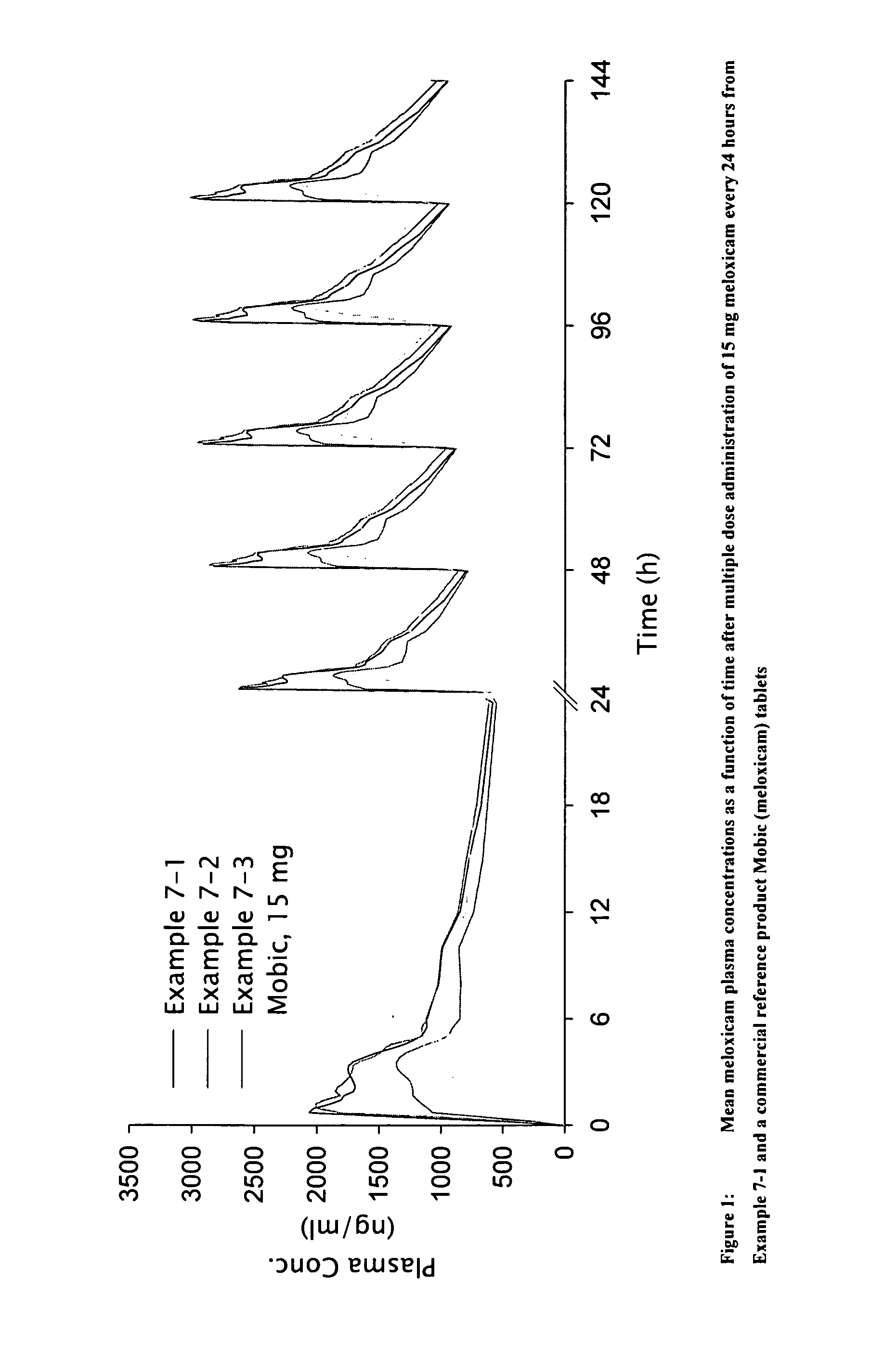
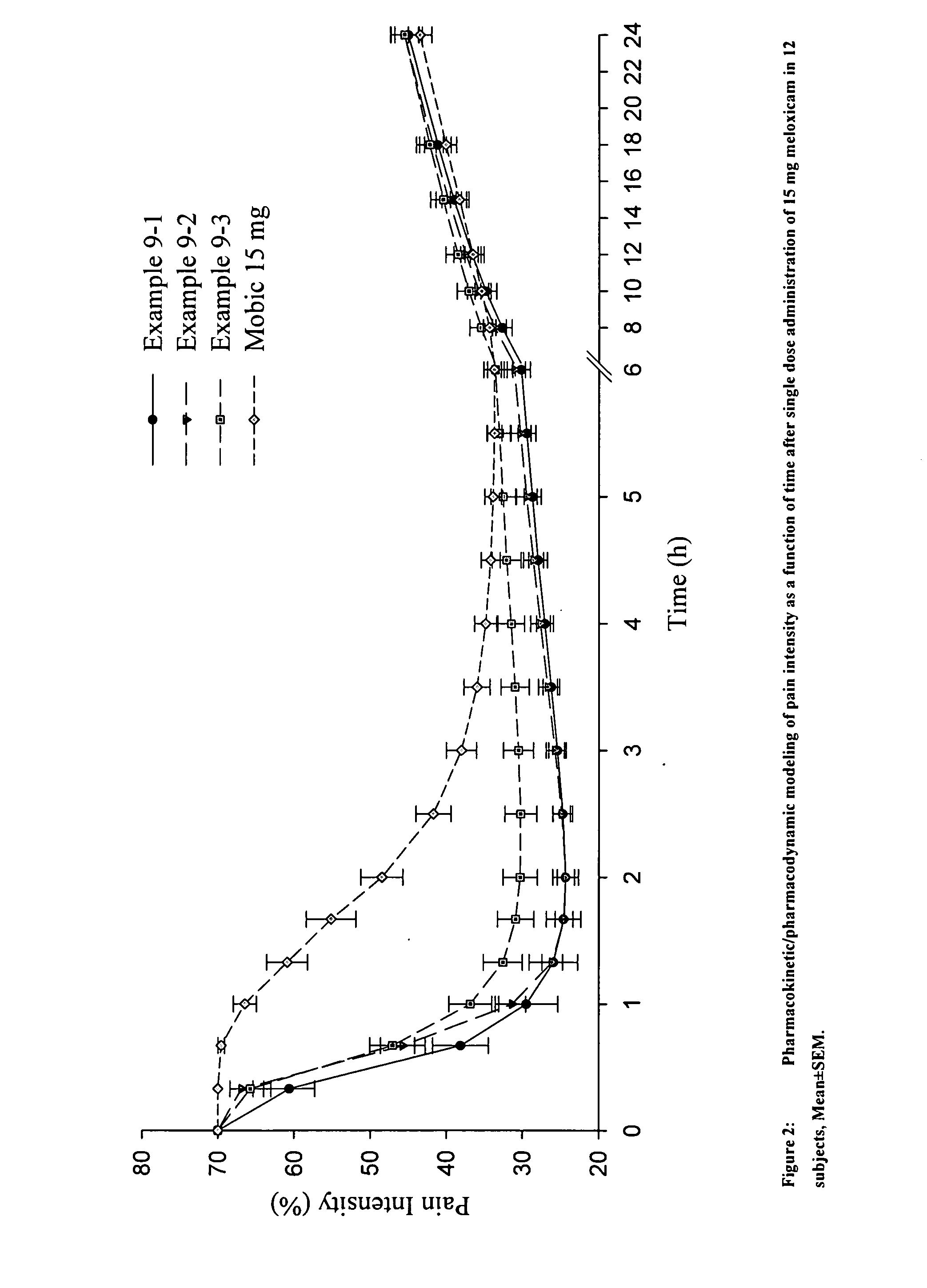
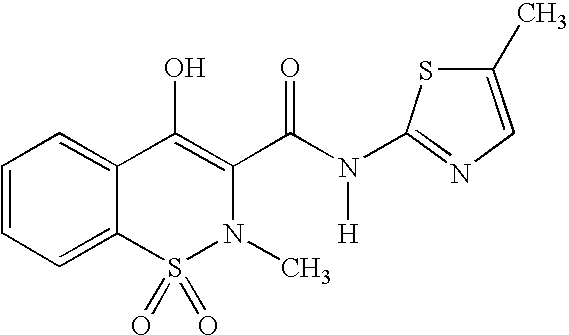
Methods and compositions for delivering a meloxicam compound are disclosed and described. In one aspect, a method may include perorally administering to a subject a therapeutically effective amount of a meloxicam compound that provides a meloxicam plasma concentration within 1 hour which is at least about 40% of the maximum plasma concentration attained by the formulation. In another aspect, a composition may include a therapeutically effective amount of a meloxicam compound in a pharmaceutically acceptable carrier including at least one of an alkalizer or a solubilizer, with the meloxicam compound having a solubility in the carrier that is greater than about 1.0 mg / gm.
Owner:LIPOCINE
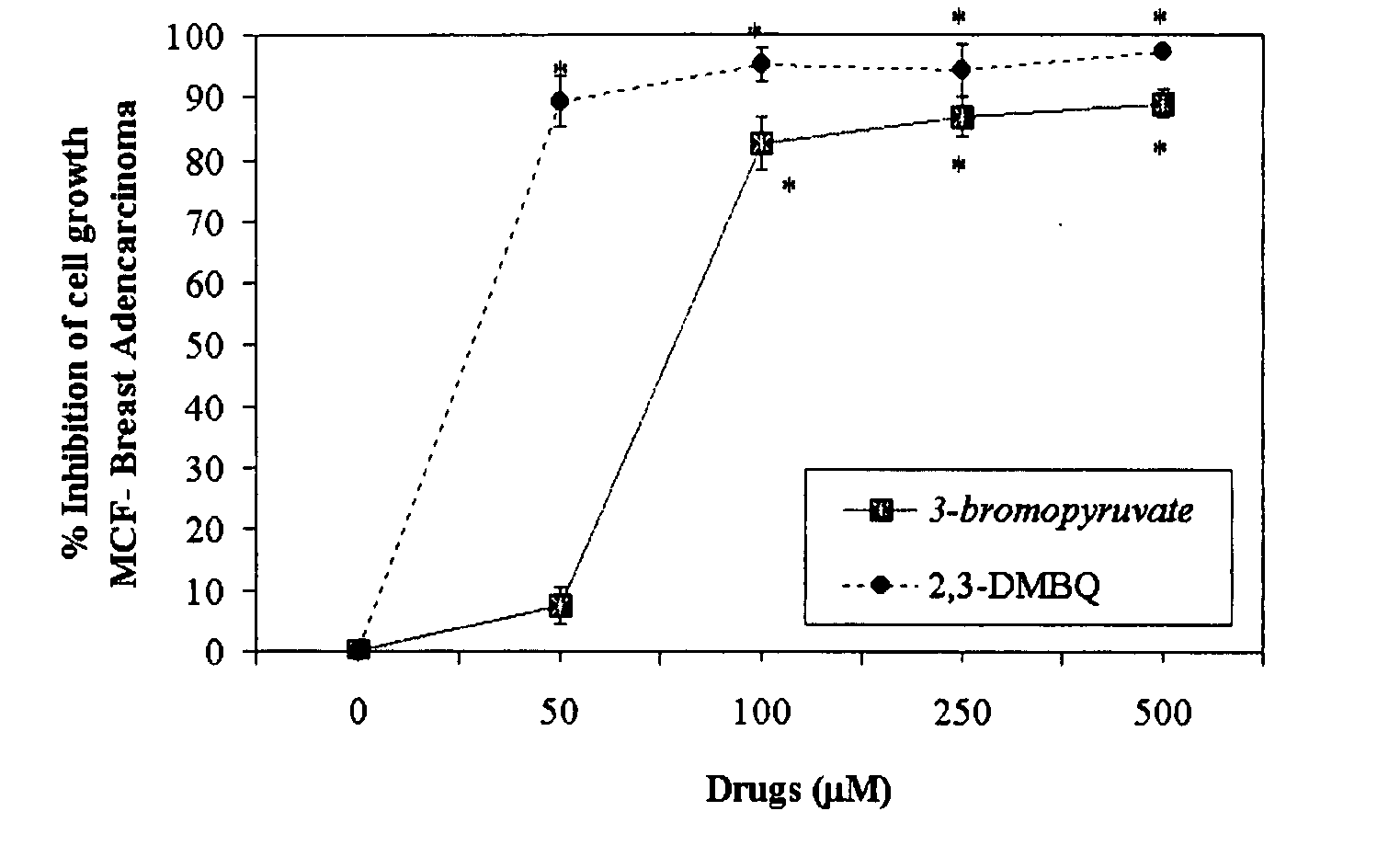
Nutraceutical composition and method of use for treatment / prevention of cancer
InactiveUS20070248693A1Function increaseAbility to createBiocideAlgae medical ingredients1,4-BenzoquinonePantothenic acid
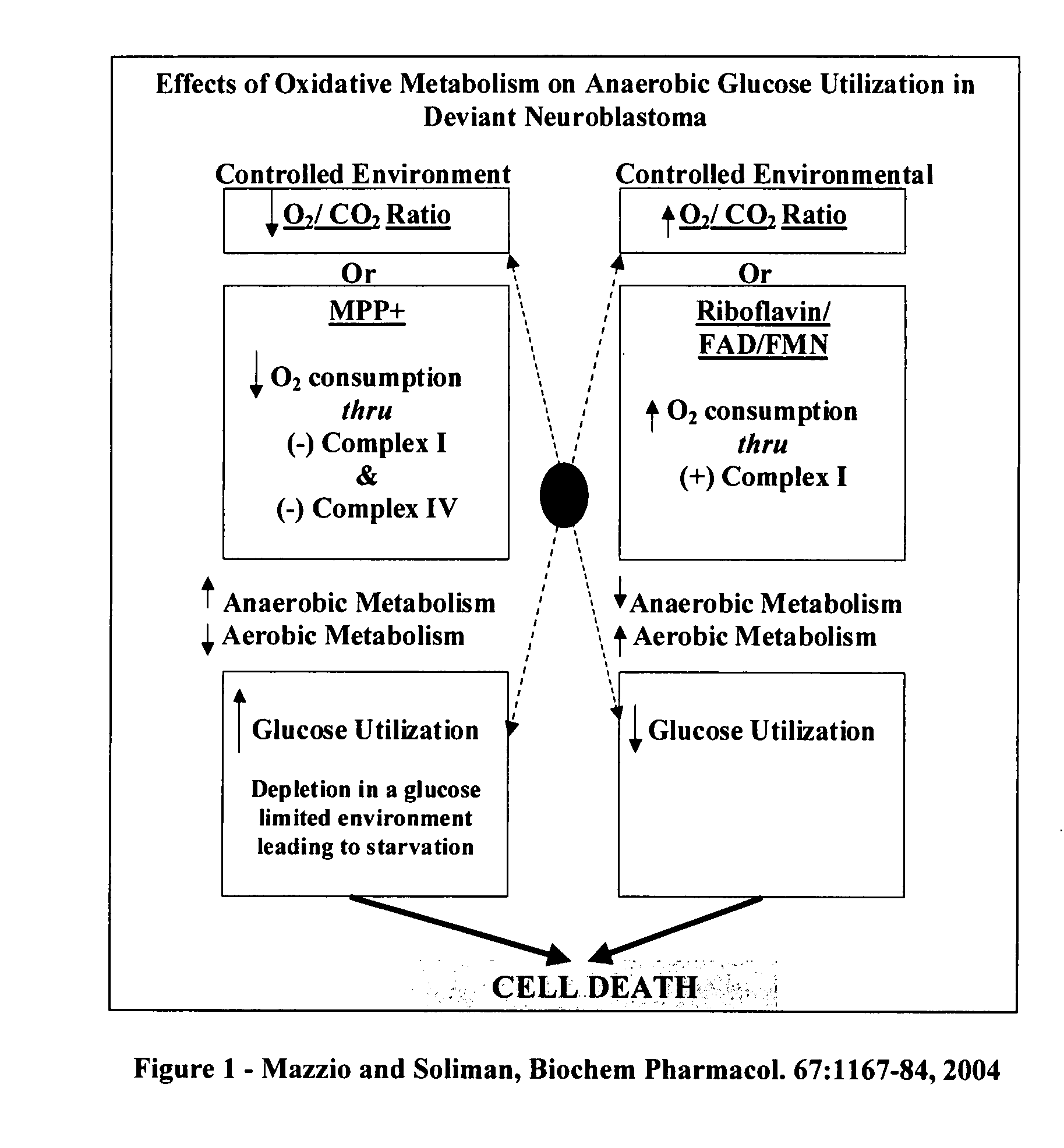
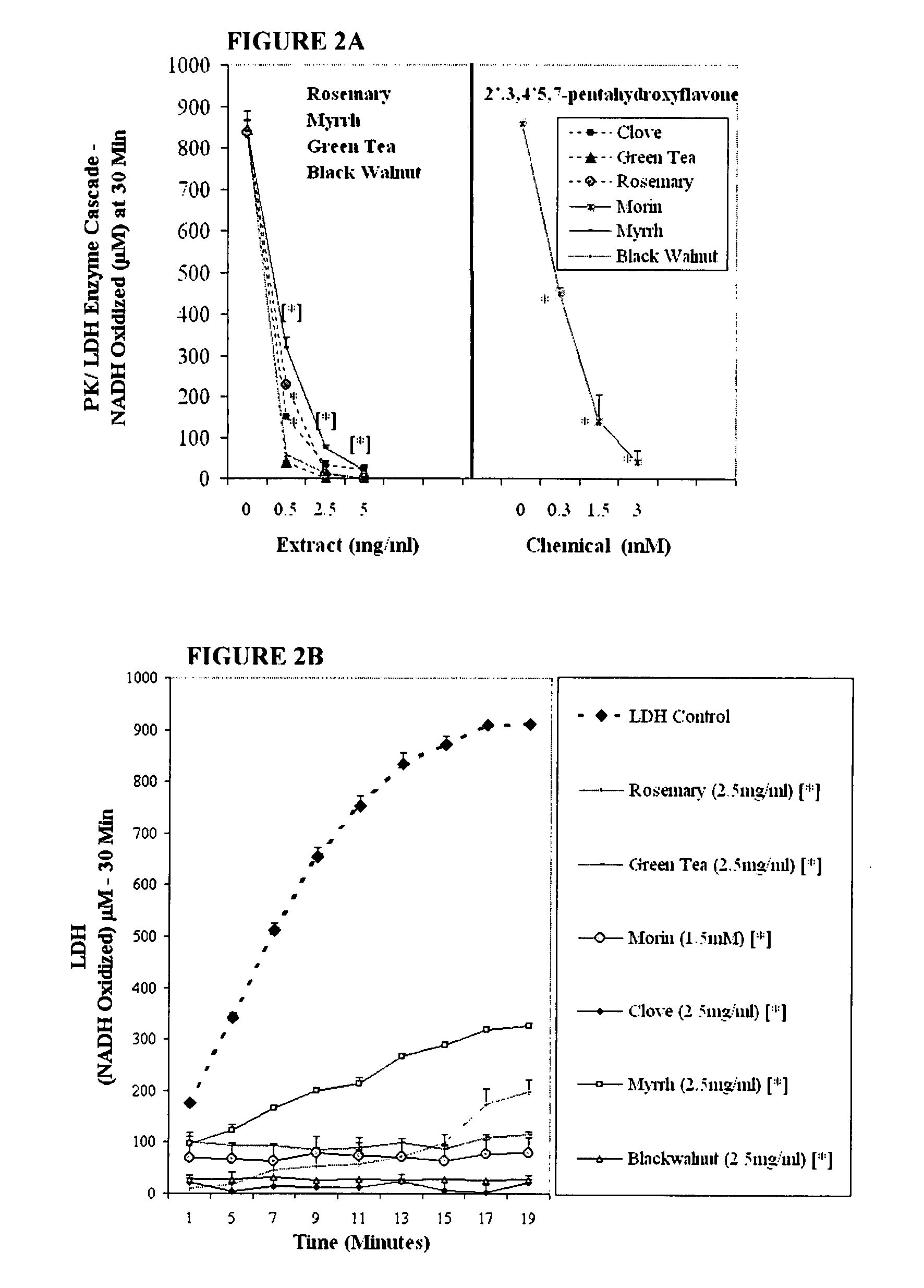
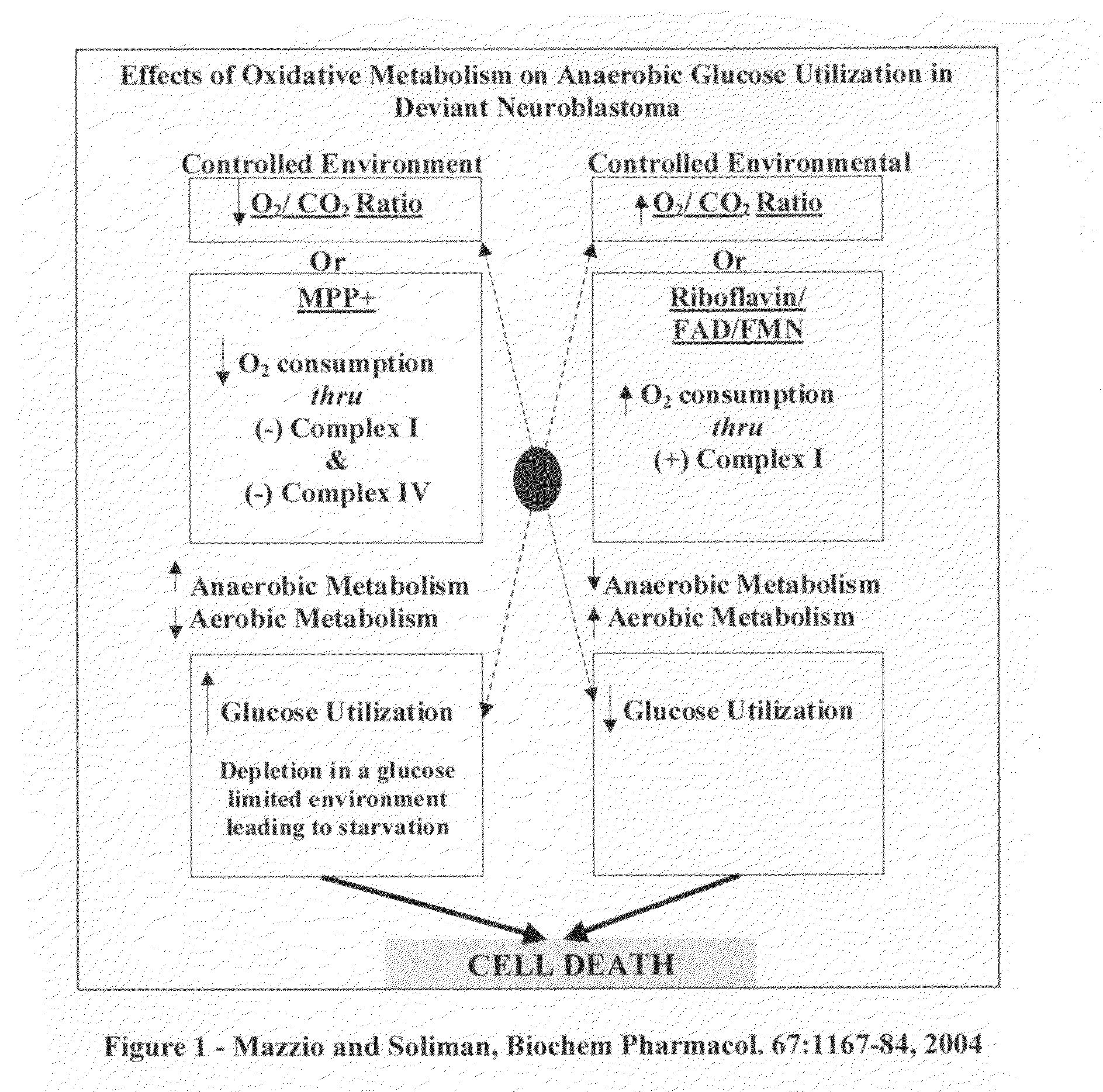
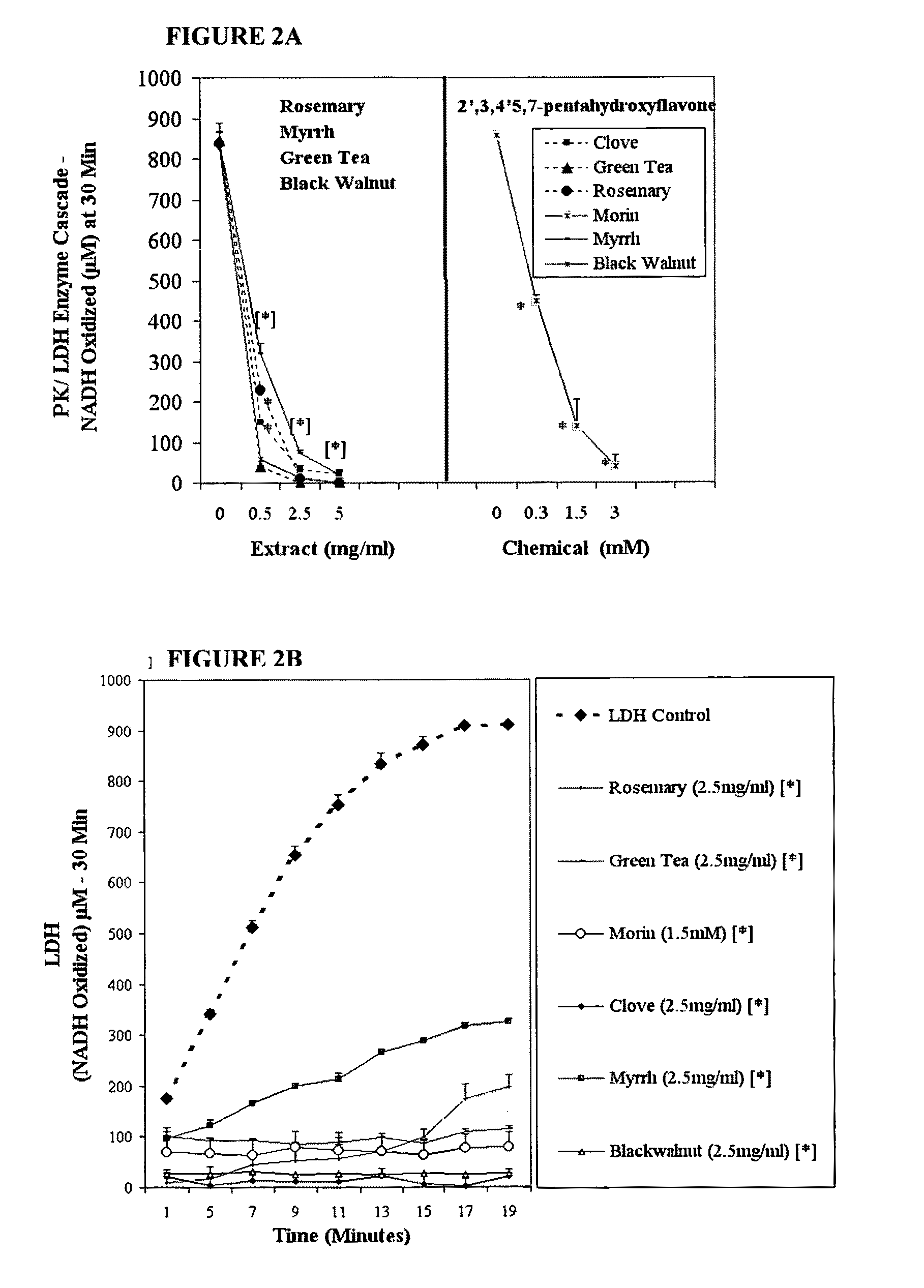
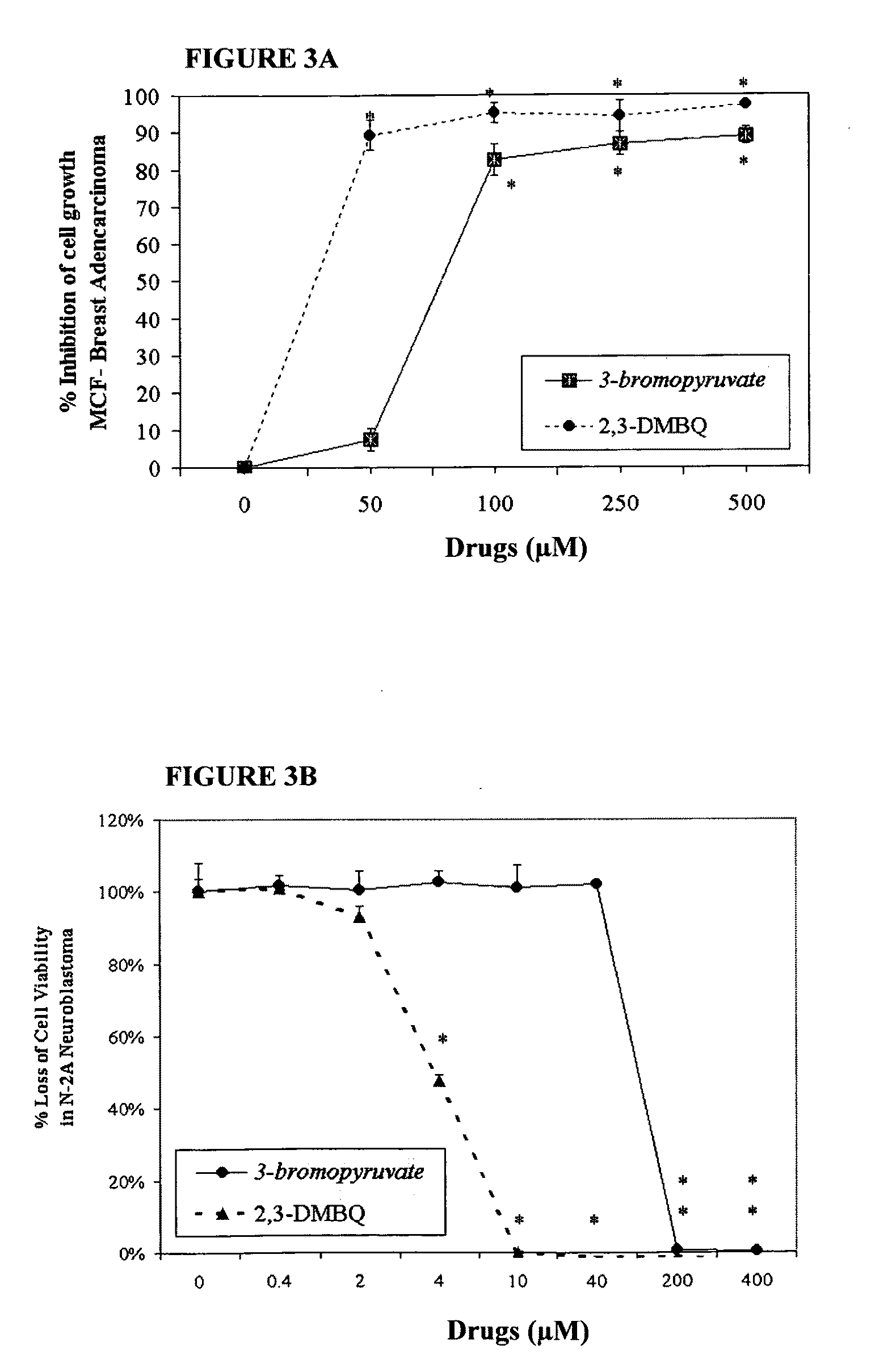
The invention describes a pharmaceutical composition and method for treating cancer comprised of A) 2,3-dimethoxy-5-methyl-1,4-benzoquinone and / or B) at least one of wild yam root, teasel root, balm of gilead bud, bakuchi seed, dichroa root, kochia seed, kanta kari, bushy knotweed rhizome, arjun, babul chall bark, opopanax and bhumy amalaki; optionally one or more of frankincense, garcinia fruit, vitex, dragons blood, mace, sage and red sandalwood with at least c) one compound capable of maximizing oxidative mitochondrial function preferably riboflavin or vitamin B2 derivatives, FAD, FMN, 5-amino-6-(5′-phosphoribitylamino)uracil, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, niacin, vitamin C and pantothenate and / or d) at least one lactic acid dehydrogenase inhibitor (preferably 2′,3,4′5,7-pentahydroxyflavone) and optionally f) an alkalizing agent (aloe vera, chlorella, wheat grass, sodium or potassium bicarbonate, potassium) g) an antiproliferative herb (speranskia or goldenseal) and h) a pharmaceutically acceptable carrier.
Owner:MAZZIO ELIZABETH +1
Stable pancreatic enzyme compositions
ActiveUS20080274174A1Stable digestive enzyme compositionThe dosage form is stablePeptide/protein ingredientsHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 15% after six months of accelerated stability testing.
Owner:SOC DES PROD NESTLE SA
Methods of producing stable pancreatic enzyme compositions
ActiveUS20080279953A1Minimal loss of activityComposition is stablePowder deliveryPeptide/protein ingredientsDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 15% after six months of accelerated stability testing.
Owner:SOC DES PROD NESTLE SA
Composition useful for the oxidation dyeing of human keratinous fibres
A composition useful for the oxidation dyeing of human keratinous fibers and in particular hair containing, in a cosmetically acceptable medium based on water and at a basic pH, at least one oxidation dye and an alkalinizing agent containing at least one alkali metal, alkaline-earth metal or ammonium metasilicate and at least one alkanolamine, and the dyeing method using this composition.
Owner:LOREAL SA
Nutraceutical composition and method of use for treatment / prevention of cancer
ActiveUS20100209388A1Easy to understandReduce weightOrganic active ingredientsBiocideMyrrh1,4-Benzoquinone
The invention describes a nutraceutical composition and method for preventing / treating cancer comprised of A) quinones (2,3-dimethoxy-5-methyl-1,4-benzoquinone, thymoquinone, tocopherolquinone) B) compounds capable of maximizing oxidative mitochondrial function preferably riboflavin, FAD, FMN, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, B3, C and pantothenate C) lactic acid dehydrogenase inhibitors; 2′,3,4′5,7-pentahydroxyflavone, epigallocatechin gallate, quercetin, citric acid, rosemary, black walnut, clove, nutmeg, licorice root, coriander, cinnamon, ginger root, myrrh gum and green tea D) alkalizing agents: aloe vera, chlorella, wheat grass, apple cider vinegar, burdock root, kudzu root, alfalfa, barley grass, spirulina, parsley leaf, calcium, magnesium, potassium or bicarbonate salts E) potent tumoricidal herbs; gromwell root, wild yam, beth root, teasel root, balm of gilead bud, frankincense, bakuchi seed, dichroa root, kochia seed, kanta kari, sweet myrrh, galbanum, garcinia fruit, mace, white sage and tumoricial plant derived constituents: gambogic acid, shikonin, diosmin or boswellic acid F) an antiproliferative herb (speranskia or goldenseal) and G) a pharmaceutically acceptable carrier.
Owner:FLORIDA A&M UNIVERSITY
Method for dyeing fibers containing keratin
InactiveUS20060265818A1Lightened uniformlyUniform lightening of keratin-containing fibers dyedCosmetic preparationsHair removalFiberEntire head
A method for dyeing keratin-containing fibers, in particular, human hair comprising the steps of: (A) contacting the hair with a dyeing composition, comprising color-imparting components for a contact time Z1; (B) rinsing the hair a to remove the dying composition; (C) optionally drying the rinsed hair; (D) contacting at least a portion of the hair from step (B) or (C) with a lightening or nuancing agent comprising, in a cosmetic carrier, at least one thickener, hydrogen peroxide and at least one alkalinizing agent for a contact time Z2; and (E) rinsing the hair to remove the adjusting agent; wherein the dyeing composition comprises a color-imparting component comprising, (a) at least two oxidation dye precursors, where at least one oxidation dye precursor must be of the developer type or (b) at least two oxo dye precursors, where at least one oxo dye precursor must be a reactive carbonyl compound. The method ensures a uniform lightening of keratin-containing fibers dyed with dyeing compositions, and produces uniform and natural-looking, lightened color reflections over the entire head hair area.
Owner:HENKEL KGAA
Method of rapid hair dyeing
The present invention relates to a method of hair colouring and bleaching compositions providing a composition comprising i) at least one source of peroxymonocarbonate ions, ii) at least one alkalizing agent, preferably a source of ammonium ions, and iii) at least one radical scavenger, wherein said composition has a pH of up to 9.5, and applying the composition to the hair and retaining on the hair for a period of less than 20 minutes, which provide a high level of lift and lightening and the required dye deposition and grey coverage whilst reducing the concentration of peroxide, the ammonia odour and reducing the hair fibre damage.
Owner:WELLA OPERATIONS US LLC
Stable nanoparticulate drug suspension
InactiveUS20100323020A1Convenient route of administrationEasy to doOrganic active ingredientsPowder deliverySodium bicarbonateDisease
A liquid pharmaceutical composition comprises an aqueous medium having suspended therein a solid particulate Bc1-2 family protein inhibitory compound such as ABT-263, having a D90 particle size not greater than about 3 μm; wherein the aqueous medium further comprises at least one pharmaceutically acceptable surfactant and at least one pharmaceutically acceptable basifying agent such as sodium bicarbonate in amounts that are effective together to inhibit particle size increase. The composition is suitable for oral or parenteral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bc1-2 family proteins, for example cancer.
Owner:ABBVIE INC
Composition for the treatment and prevention of ischemic events
InactiveUS7029701B2Permit slow releasePrevent and ameliorate associated side effect (ulceration)Powder deliveryOrganic active ingredientsSurface-active agentsAlkalizing agent
The invention relates to pharmaceutical compositions comprising omeprazole and aspirin wherein the combination is useful for the treatment and prevention of cardiovascular events including heart attacks and platelet aggregation leading to a potential cardiac event. A variety of drug delivery systems may be utilized to deliver the combination of active ingredients. The preferred delivery system utilizes a tablet or capsule containing an inert sugar core particle that is coated with subparticles of a coated omeprazole wherein the coating contains omeprazole, a binder, a surface active agent and a basifying agent along with a filler. The aspirin may be combined with this formulation to coat the sugar sphere or it may be part of a separate coating composition that forms a multilayer system that is ultimately coated with an enteric coating and then formed into the tablet or capsule by conventional means.
Owner:ANDRX PHARMA INC +1
Protecting colloid for gastroenteric mucosa
ActiveCN101933894ABlock digestionPromote healingOrganic active ingredientsDigestive systemCross-linkAlcohol
The invention relates to protecting colloid for gastroenteric mucosa, belonging to the field of biomedical preparation and comprising a collagenous substance, an acidifying or alkalizing agent, a cross-linking agent and an adhesive based on the weight ratio of 1:0.01-0.3:0.1-0.5, wherein the dosage of the acidifying or alkalizing agent is proper when the pH value is regulated to 6.5-8.5. Under the action of gastric acid, the cross-linking agent in the protecting colloid can be released so as to form a colloid membrane on the surface of ulcer, avoid the gastroenteric mucosa from being digested by gastric acid and pepsase and promote healing of the ulcer. Animal tests show that the protecting colloid can greatly lower the ulcer formation caused by absolute ethyl alcohol and hydrochloric acid, inhibit ulcer formation and have a protecting effect on injury of the mice gastroenteric mucosa caused by absolute ethyl alcohol and hydrochloric acid. Therefore, the protecting colloid is used for protecting and curing gastroenteric mucosa ulcer for patients.
Owner:北京瑞奇美德科技发展有限公司
Pharmaceutical composition for oral administration
Provided is a pharmaceutical composition for oral administration containing a 5-HT.3 receptor antagonist, which is suitable for self-medication because of good preservation stability, low synthesis, good uniformity and good external appearance, and good smoothness in throat, and easiness to be taken. Concretely, it is a jellied pharmaceutical composition for oral administration containing a 5-HT.3 receptor antagonist, a gelatinizing agent, and water; and having a pH of 7 or less. In particular, there is provided the pharmaceutical composition, where the gelatinizing agent is carrageenan, pectin, agar, alginic acid, sodium alginate, gelatin, manna, Kodak, konjakmannan, glucomannan, chitosan, xanthan gum, tamarind seed polysaccharide, gellan gum, karaya gum, or cassia gum, or preferably the pharmaceutical composition further containing a thickener.
Owner:NICHI IKO PHARMA FACTORY
Dye compositions containing quinolinium salts
InactiveUS6977001B2Uniformity and safetyHigh fastnessCosmetic preparationsHair cosmeticsFiberPolymer science
The dye composition (A) for dyeing fibers, especially keratin fibers, such as human hair, is made by mixing a first component (A1) and a second component (A2) and adding an alkalizing agent or an acidifying agent, as needed. The second component (A2) consists of at least one compound with a nucleophilic reaction center and the first component (A1) consists of at least one 1-alkyl quinolinium derivative of formula (Ia) or (Ib): A multicomponent kit consisting of the first and second components packaged separately is also described as well as a method of dyeing fibers using the multicomponent kit.
Owner:HFC PRESTIGE INT HLDG SWITZERLAND SARL
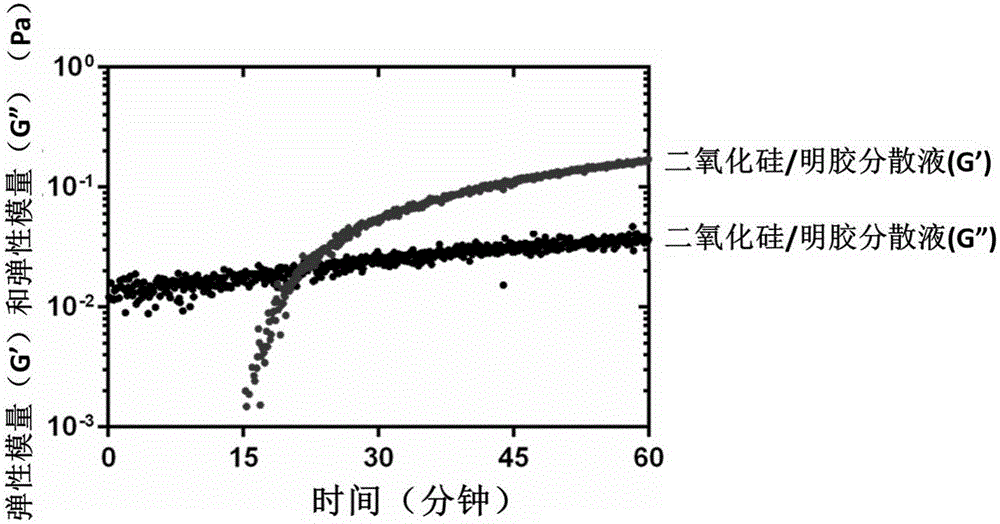
Nanometer colloid particle-assembled high-strength self-repairing injectable composite colloid gel material and preparation method and application thereof
InactiveCN105796478AHigh strengthImprove self-healing abilityAerosol deliveryInorganic non-active ingredientsBiological materialsSilicon dioxide
The invention discloses a nanometer colloid particle-assembled high-strength self-repairing injectable composite colloid gel material and a preparation method and application thereof. According to the preparation method, two-phase colloid particles with opposite electric charges are uniformly blended in an alkaline or acidic environment, an acidifying agent or alkalizing agent is added to induce the pH of the solution to recover the neutrality, electrostatic self-assembly among the two-phase colloid particles is initiated, and a uniformly-dispersed composite gel network is formed, wherein the gel is high in mechanical strength and wide in regulation and control range, the elasticity modulus of the gel can be regulated and controlled within the range of 10 Pa-100 kPa, and the self-repairing efficiency of the gel is higher than or equal to 100%. The silicon dioxide-gelatin composite colloid gel which has both the high mechanical strength and the self-repairing performance is finally and successfully prepared, and it is verified that the composite gel still can quickly recover the mechanical strength after being broken by shear force many times; in addition, the composite gel has the excellent injectable property and plasticity and can be used for an in-vitro cell culture matrix material by serving as an injectable biological material.
Owner:SHENZHEN HUA NOVA BIOTECH LTD
Dinotefuran insecticide having insect inducing effect
ActiveCN104472527AStrong chain killing effectGood moisturizing effectBiocidePest attractantsExcipientBalance water
The invention relates to an insecticide, in particular, relates to a dinotefuran insecticide having an insect inducing effect, and belongs to the technical field of sanitation and epidemic prevention. The dinotefuran insecticide comprises the following components by the weight percentage: 0.01-2% of dinotefuran, 0-2% of muscalure, 5-30% of sugar, 5-50% of a suggestive food ingredient, 5-30% of a moisturizing agent, 0.005-2.0% of a preservative agent, 0.01%-5.0% of an excipient, 0.5-1% of an acidifying or alkalizing agent, and the balance water. The preparation has the advantages that the preparation has a strong chain killing effect on cockroaches, has small drug application amount, has less pollution to the environment, and can penetrate into gaps for drug application. Moreover, after drug application, the cockroaches are not immediately dead after feeding but carry the attached drug back to a nest, with utilization of attributes that the cockroaches lick other cockroaches and like to eat other cockroach dead bodies, the drug is transferred to the other cockroaches, while the cockroaches are dead, other cockroach death is caused, and the whole nest of cockroaches are killed, so as to achieve the sustainable control effect.
Owner:江苏功成生物科技有限公司
Method and composition for permanently shaping hair
InactiveUS20080025939A1Improve odorHigh customer acceptanceCosmetic preparationsHair cosmeticsLithium hydroxideGuanidine
A composition for permanent hair shaping comprising: (i) about 1% by weight to about 30% by weight of an N-alkyl-2-mercaptoacetamide of formula or the salt thereof, wherein R1 is a straight chain alkyl residue with about 3 to about 6 carbon atoms or a straight chain hydroxyalkyl with about 3 to about 6 carbon atoms, R2 and R3 are, independently from each other, hydrogen or straight chain alkyl residues with about 1 to about 3 carbon atoms; (ii) about 0.1% by weight to about 15% by weight of at least one alkalizing agent selected from the group consisting of lithium hydroxide, earth alkali metal hydroxides; hydroxyalkyl substituted amines with about 1 to about 3 alkyl residues comprising about 1 to about 4 carbon atom in the alkyl residue and one or two hydroxyl groups on at least one of said alkyl residues; 2,2-imino-bis-ethanoliminourea (guanidine carbonate), tetrahydro-1,4-oxazine, 2-aminoethansulfonic acid and alkaline amino acids; and (iii) about 5% by weight to about 95% by weight of water, and the method of permanently shaping hair using said composition.
Owner:THE PROCTER & GAMBLE COMPANY
Nicotinic compound salt bagged buccal cigarette and preparation method thereof
InactiveCN107319629AEnjoy physical satisfactionGreat tasteTobacco treatmentAdditive ingredientAntioxidant
The invention discloses a nicotinic compound salt bagged buccal cigarette and a preparation method thereof. The cigarette comprises 30-60 parts of nicotinic compound salts, 10-30 parts of fillers, 1-20 parts of sweetening agents, 0.5-5 parts of acidifying or alkalizing agents, 0.5-5 parts of flavor corrective agents, 0.5-5 parts of edible essence, 0.5-5 parts of flavoring agents, 0.1-1 part of propylene glycol, 0.01-1 part of antioxidant and 0.01-1 part of preservative. Raw materials are mixed and subjected to heat-moisture treatment, seasoning, acidity and alkalinity regulating, balancing, sterilization and packaging, and the product is obtained. According to the nicotinic compound salt bagged buccal cigarette, tobacco powder ingredients in a conventional bagged buccal cigarette is replaced by the nicotinic compound salt, the taste of the bagged buccal cigarette can be remarkably improved, the nicotinic ingredients can be even and release continuously, the taste of the buccal cigarette is coordinated, the comfort is improved, and the stimuli for the oral cavity, the throat and the gastrointestinal tract by the buccal cigarette is reduced, so that consumers can better enjoy physiological satisfaction brought by nicotine; meanwhile, the appearance of the bagged buccal cigarette is optimized, and thus the consumers are more willing to try and accept.
Owner:CHINA TOBACCO YUNNAN IND
SOLID COMPOSITION FOR CONTROLLED RELEASE OF IONIZABLE ACTIVE AGENTS WITH POOR AQUEOUS SOLUBILITY AT LOW pH AND METHODS OF USE THEREOF
InactiveUS20100151019A1Reduces evacuationReduced bioavailabilityBiocideOrganic active ingredientsSolubilitySodium bicarbonate
A novel solid composition and methods for making and using the solid composition are provided. The solid composition comprises: (a) at least one active agent with a solubility of less than about 0.3 mg / ml in an aqueous solution with a pH of at most about 6.8 at a temperature of about 37° C.; and (b) a hydrophilic polymer matrix composition comprising: i) a hydrophilic polymer selected from the group consisting of METHOCEL™, POLYOX™ WSR 1105 and combinations thereof; and optionally ii) a hydrophobic polymer selected from the group consisting of Ethocel 20 premium; and (c) an alkalizer selected from the group consisting of calcium carbonate, magnesium oxide heavy and sodium bicarbonate; wherein the composition provides at least about 70% release of the active between about 7 to about 12 hours following oral administration.
Owner:ALEXION PHARMA INC
Brown-red plant ecological hair dye and preparation method thereof
InactiveCN102973467AImprove securityImprove dye uptakeCosmetic preparationsHair cosmeticsHair dyesAniline dye
The invention discloses a brown-red plant ecological hair dye which is composed of a staining agent and a dyeing auxiliary, wherein the staining agent comprises the components in parts by weight of vegetable dyes, fat, an emulsifier, a thickener, a humectant, a penetrating agent, a conditioner, an acidifying or alkalizing agent and deionized water; the dyeing auxiliary comprises the components of metal salt, the fat, the emulsifier, the thickener, the humectant, the penetrating agent, the conditioner, the acidifying or alkalizing agent and the deionized water. Natural plant red and black sorghum husks and black rice extract are adopted as dyes to replace chemical aniline dyes; the extracts are subjected to complex reaction together the metal salt which has no harm to a human body to generate colored complex compounds for dyeing. The brown-red plant ecological hair dye does not contain any aniline dye; no heavy metal is added; and the brown-red plant ecological hair dye is high in security, low in irritation, free of toxic and side effects, high in dyeing rate, and natural, bright and lasting in color and luster after being dyed, does not fade, is not dried, and is washable, lightfast, innocuous and non-irritant, thus being an ideal color hair dye.
Owner:贵州阿斯科科技开发有限公司
Hair colouring compositions
The present invention relates to hair colouring and bleaching compositions comprising i) at least one source of peroxymonocarbonate ions, ii) at least one alkalizing agent, preferably a source of ammonium ions, and iii) at least one radical scavenger, wherein said composition has a pH of up to 9.5, which provide a high level of lift and lightening and the required dye deposition and grey coverage whilst reducing the concentration of peroxide, the ammonia odour and reducing the hair fibre damage.
Owner:WELLA OPERATIONS US LLC
Hair coloring compositions comprising latex polymers
Disclosed is color base composition for coloring keratin fibers comprising: at least two latex polymers independently selected from acrylate latex polymers and polyurethane latex polymers; at least one alkalizing agent; at least one oxidative dye precursor; at least one organic solvent; and water; wherein the at least two latex polymers are partially or fully neutralized; and wherein the at least two latex polymers are present in a combined amount ranging from about 0.2% to about 2.5% by weight, relative to the weight of the composition.
Owner:LOREAL SA
Mild bleaching agents with increased lightening power
InactiveUS20060210499A1Improve lightening powerImprove compatibilityCosmetic preparationsHair cosmeticsFiberAlkaline earth metal
Bleaching agents for keratin-containing fibers, in particular, human hair, comprising (a) at least one peroxo compound and / or at least one alkalinizing agent chosen from ammonium, alkali metal and alkaline earth metal carbonates, hydrogencarbonates and carbamides (b) hydrogen peroxide, and (c) SiO2 compounds, where the last-mentioned compounds may be optionally hydrated. The agents have an effect of lightening the color of keratin-containing fibers, in particular, human hair. Preferably the bleaching agents are in a pH range of from 4.5 to 9.0. The bleaching agents have a particularly gentle effect on the keratin-containing fibers and the skin.
Owner:HENKEL KGAA
Building phase change energy storage insulating powder and preparation method thereof
InactiveCN103113852AGood compatibilityImprove heat transfer efficiencyHeat-exchange elementsArchitectural engineeringWater soluble
The invention discloses building phase change energy storage insulating powder and a preparation method thereof. The building phase change energy storage insulating powder is characterized by comprising the following components in parts by weight: 70-80 parts of an organic phase change material, 10-25 parts of a dispersing agent, 3-5 parts of water-soluble polymer powder, 1-2 parts of a modifier and 0.1-0.3 part of an acidifying or alkalizing agent. According to the modifier and the dispersing agent, the organic phase change material is changed into the powder type phase change energy storage insulating powder which can be rapidly dispersed in water through pressure reaction and mechanical chemical bridging, the powder can be added and used in concrete, mortar, putty, coatings and other building materials, the indoor environmental temperature can be induced, and the heat is continuously absorbed or released so as to regulate the temperature. The production process of the product is shortened, continuous operation can be performed, and the method is easy to implement and control and contributes to industrial production.
Owner:豫王建能科技股份有限公司
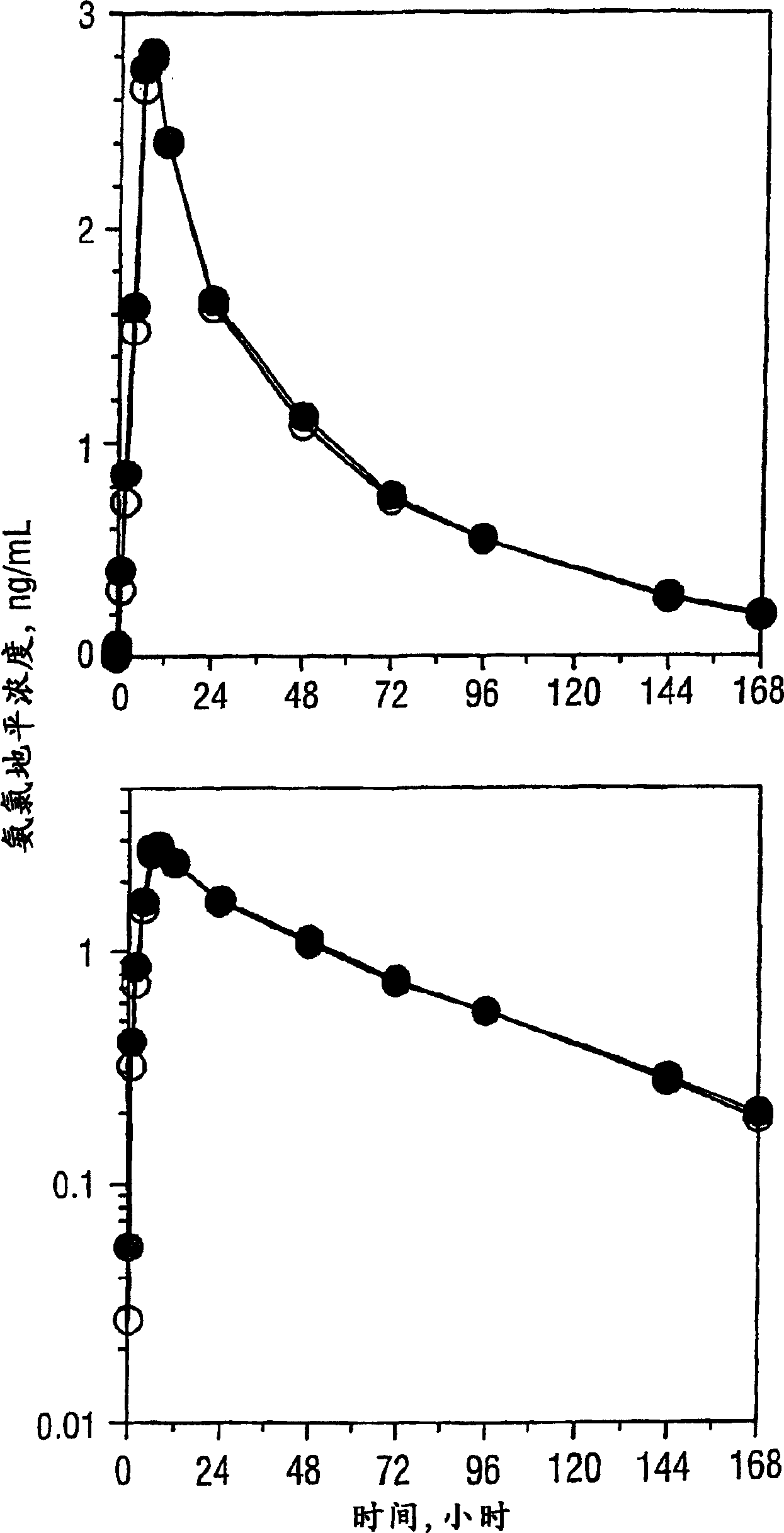
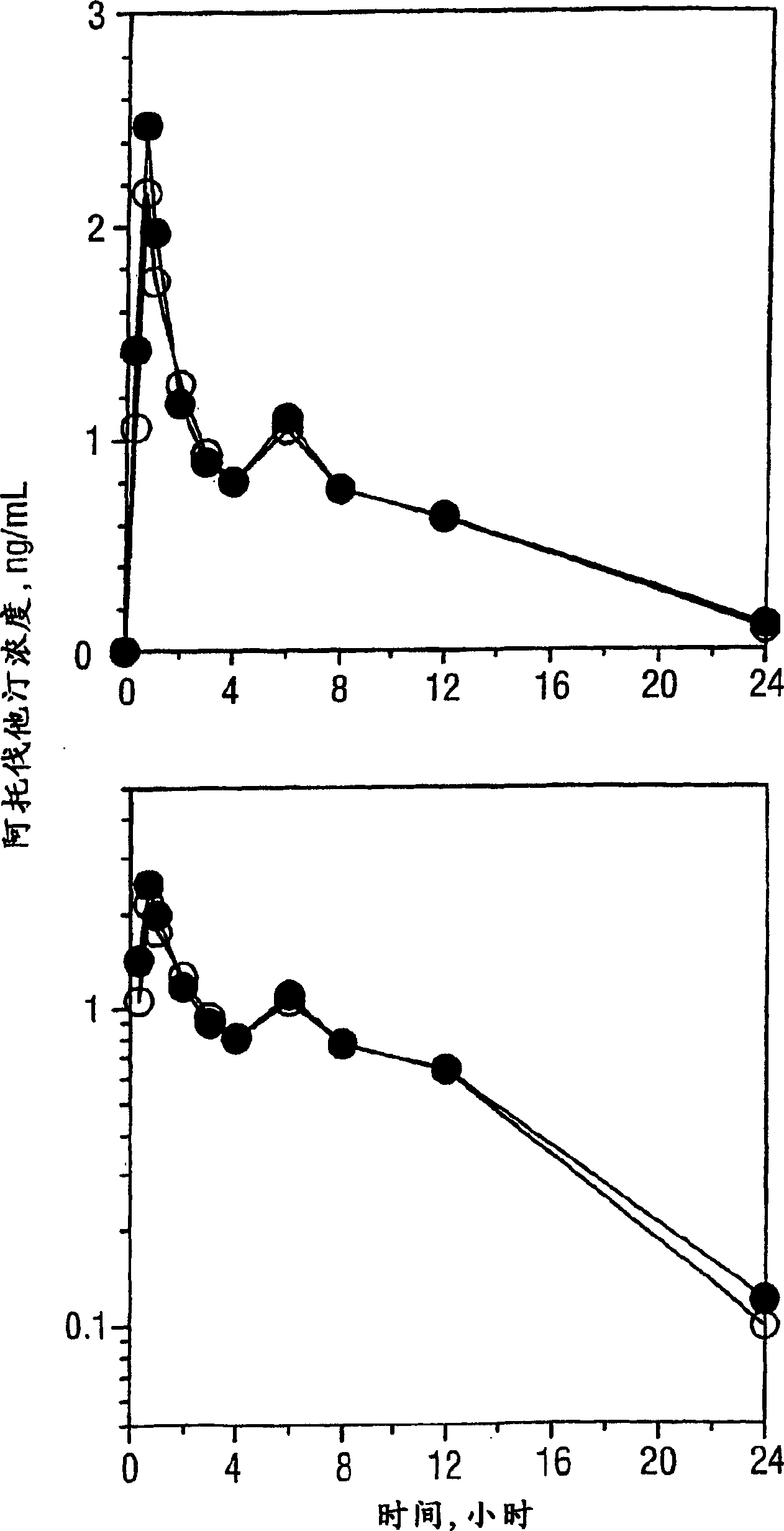
Pharmaceutical compositions of amlodipine and atorvastatin
InactiveCN1617717AMetabolism disorderInorganic non-active ingredientsPharmaceutical drugPharmaceutical medicine
The present invention describes a pharmaceutical composition comprising two components: (a) a component comprising granules of atorvastatin or a pharmaceutically acceptable salt thereof and a carrier, including an alkalizing agent to form a pH greater than 5; and (b) Two components comprising amlodipine or a pharmaceutically acceptable salt thereof and a carrier, excluding an alkalizing agent for forming a pH greater than 5, wherein the two components are mixed to form a final composition in solid dosage form, and a method of preparing the composition, A kit containing such a composition, and the use of a therapeutically effective amount of the pharmaceutical composition to treat angina pectoris, atherosclerosis, hypertension and hyperlipidemia complications and / or hypercholesterolemia and to treat heart disease risk signs.
Owner:WARNER LAMBERT CO LLC
Enteric film coating composition containing enteric polymer micronized with detackifier
Dry, enteric, film-coating compositions and aqueous dispersions containing the same are disclosed. When applied to orally-ingestible substrates such as oral solid dosage forms, the film coatings are capable of preventing the substrates from disintegrating in media with pH values from about 1 to about 4.5 or higher values. One preferred film-coating composition contains a micronized intermediate comprised of an acrylic resin and talc. Advantageously and surprisingly, the preferred film-coating composition does not contain an alkalizing agent.
Owner:FARRELL THOMAS P +5
Tobacco-containing areca catechu and preparation method thereof
ActiveCN102894465AEasy to useMeeting the Special Needs of NicotineTobacco treatmentNicotiana tabacumAdditive ingredient
The invention relates to tobacco-containing areca catechu and a preparation method thereof. The tobacco-containing areca catechu comprises edible areca catechu and bitter containing tobacco ingredients, wherein the bitter containing the tobacco ingredients comprises the following raw materials in percentage by weight: 20 to 70 percent of tobacco extract, 5 to 40 percent of acidifying or alkalizing agent, 5 to 50 percent of water, 0 to 35 percent of flavoring agent, 0 to 10 percent of essence and 0.5 to 3 percent of stabilizer. By the tobacco-containing areca catechu, the problem that the harm of smoke generated by the conventional tobacco product to passive smokers who are exposed in environment is solved; and the tobacco-containing areca catechu which can meet the special requirements of tobacco consumers on nicotine and can also reduce the influence of smoking on ambient environment and the preparation method are developed. By the tobacco-containing areca catechu, the obsession of the smoke generated by the conventional tobacco is overcome, the physiological needs of the tobacco consumers are met, and the consumption mode of the edible areca catechu is enriched.
Owner:CHINA TOBACCO HUNAN INDAL CORP
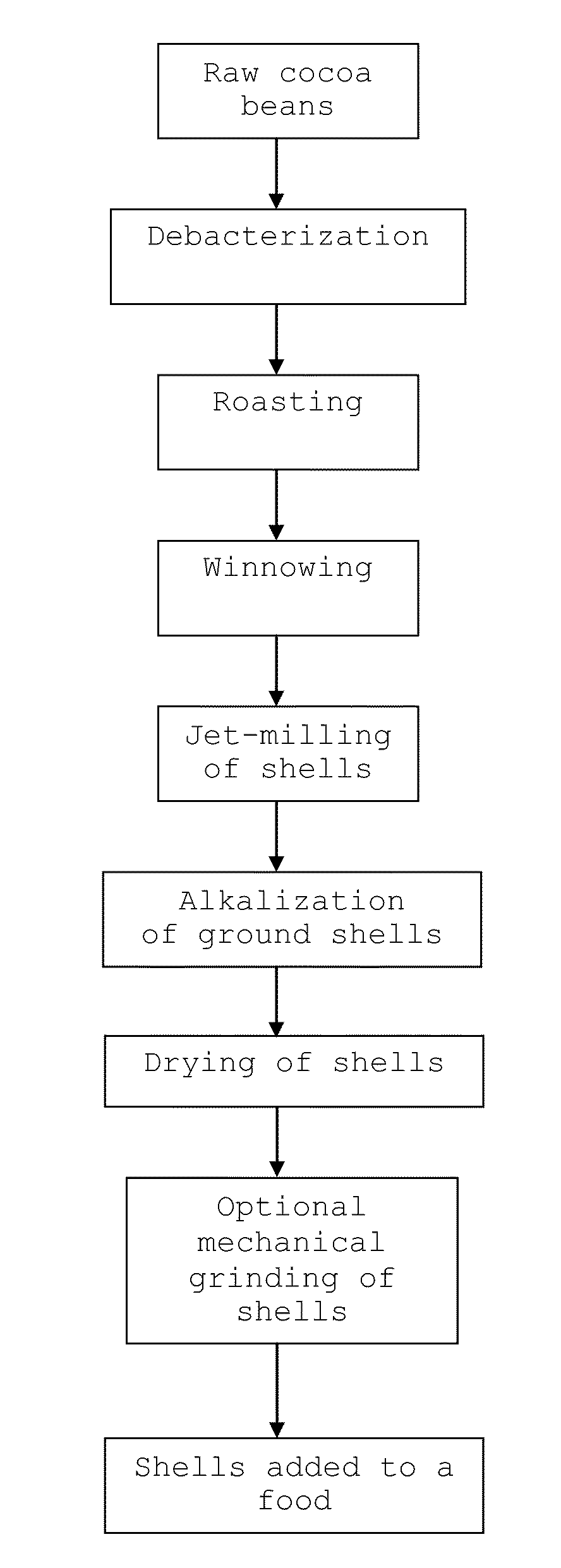
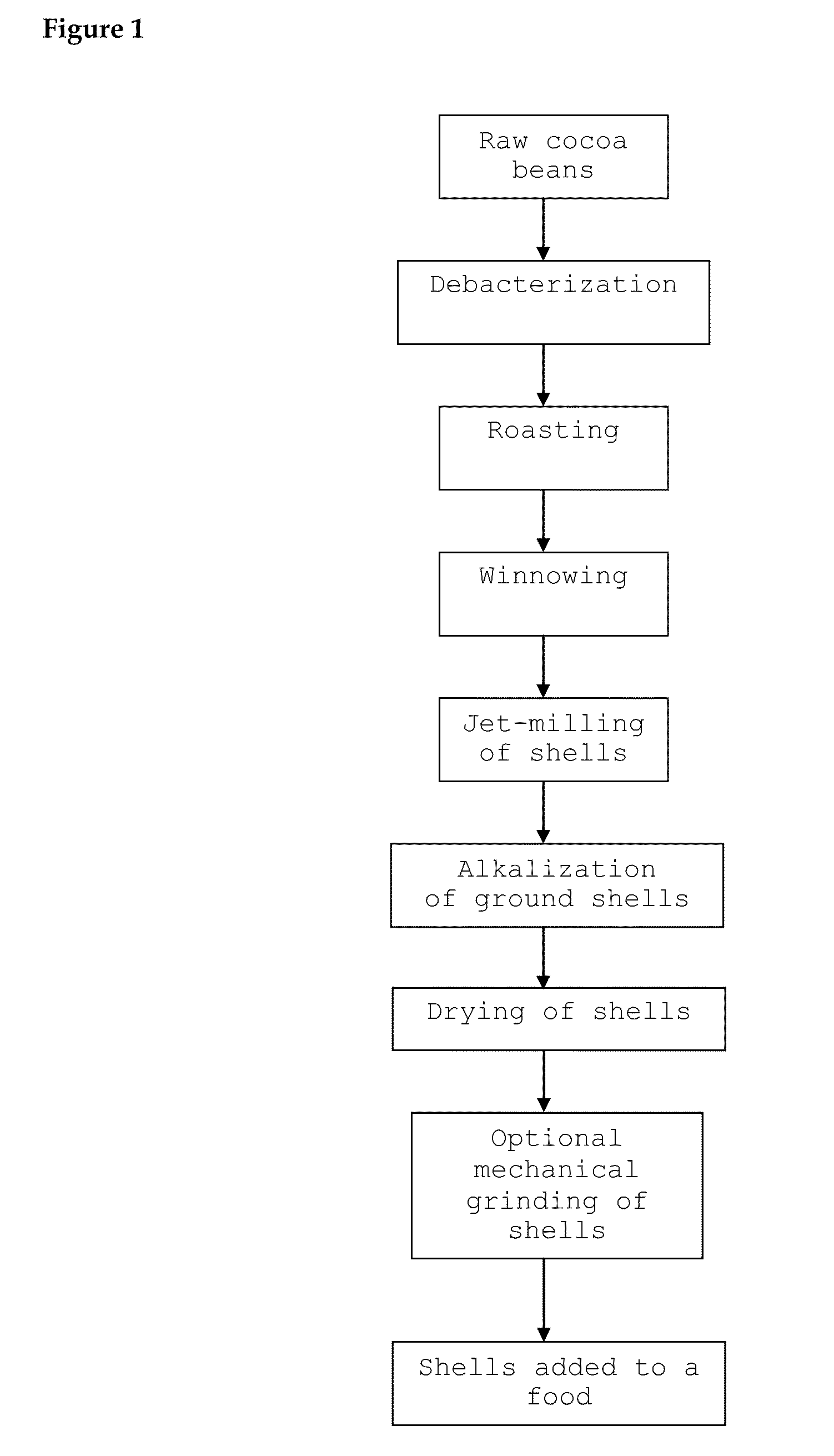
Food Comprising Alkalized Cocoa Shells And Method Therefor
InactiveUS20110151098A1Good chocolate flavourAvoid the tasteMilk preparationCocoaCocoa PowdersFood products
The present invention provides a food comprising at least 30 mass % alkalized cocoa shells based on the total mass of alkalized cocoa shells and cocoa powder in the food. Also provided is a method for manufacturing the food comprising (i) alkalizing cocoa shells which have been separated from cocoa nibs using an alkalizing agent, and (ii) adding the alkalized cocoa shells to a food.
Owner:KRAFT FOODS R & D INC
Method and composition for permanently shaping hair
A composition for permanent hair shaping comprising (i) 1 to 30 % by weight of an N-alkyl-2-mercaptoacetamide of formula (I), or the salt thereof, wherein R1 is a straight chain alkyl residue with 3 to 6 carbon atoms or a straight chain hydroxyalkyl with 3 to 6 carbon atoms, R2 and R3 are, independently from each other, hydrogen or straight chain alkyl residues with 1 to 3 carbon atoms, (ii) 0.1 to 15 % by weight of at least one alkalizing agent selected from the group consisting of lithium hydroxide, earth alkali metal hydroxides; hydroxyalkyl substituted amines with 1 to 3 alkyl residues comprising 1 to 4 carbon atom in the alkyl residue and one or two hydroxyl groups on at least one of said alkyl residues; 2,2-imino-bis-ethanoliminourea (guanidine carbonate), tetrahydro-1,4-oxazine, 2-aminoethansulfonic acid and alkaline amino acids; and (iii) 5 to 95 % by weight of water, and the method of permanently shaping hair using said composition.
Owner:THE PROCTER & GAMBLE COMPANY
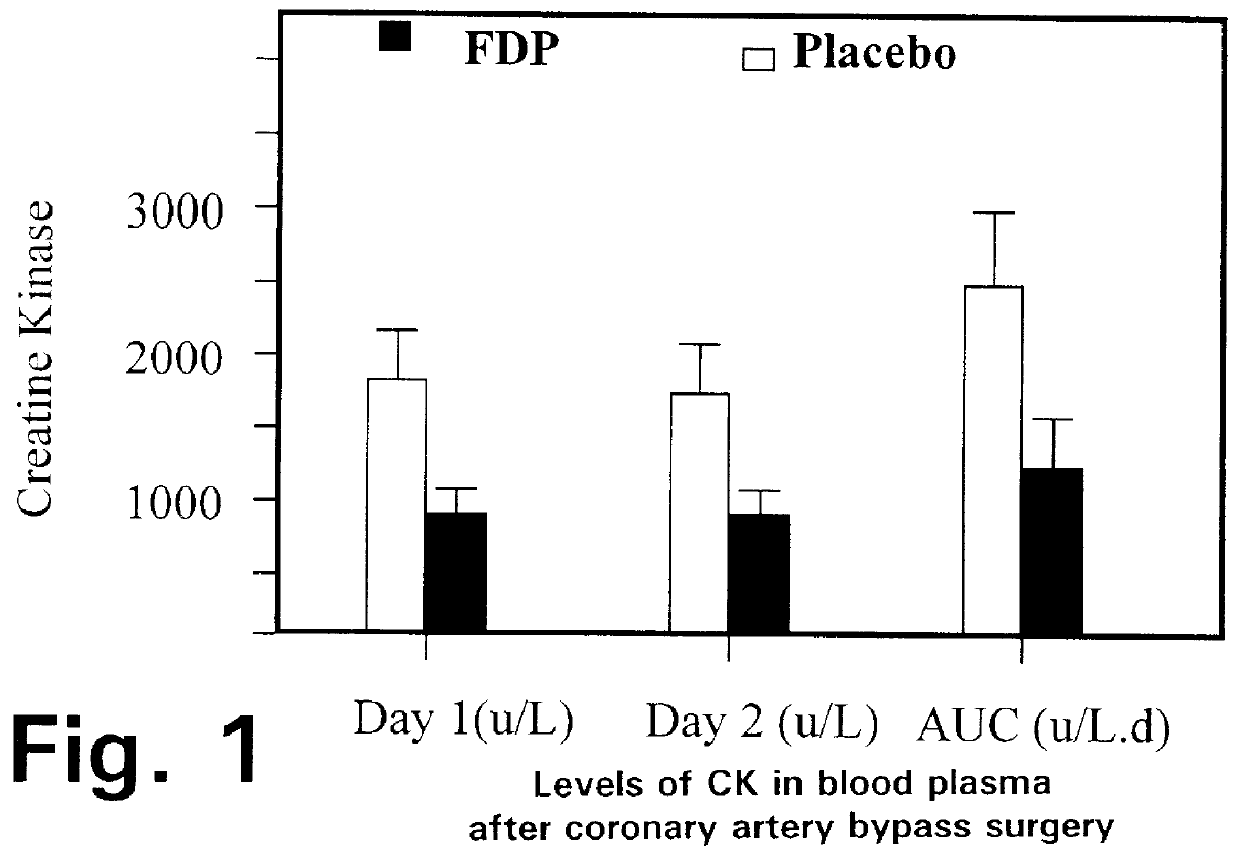
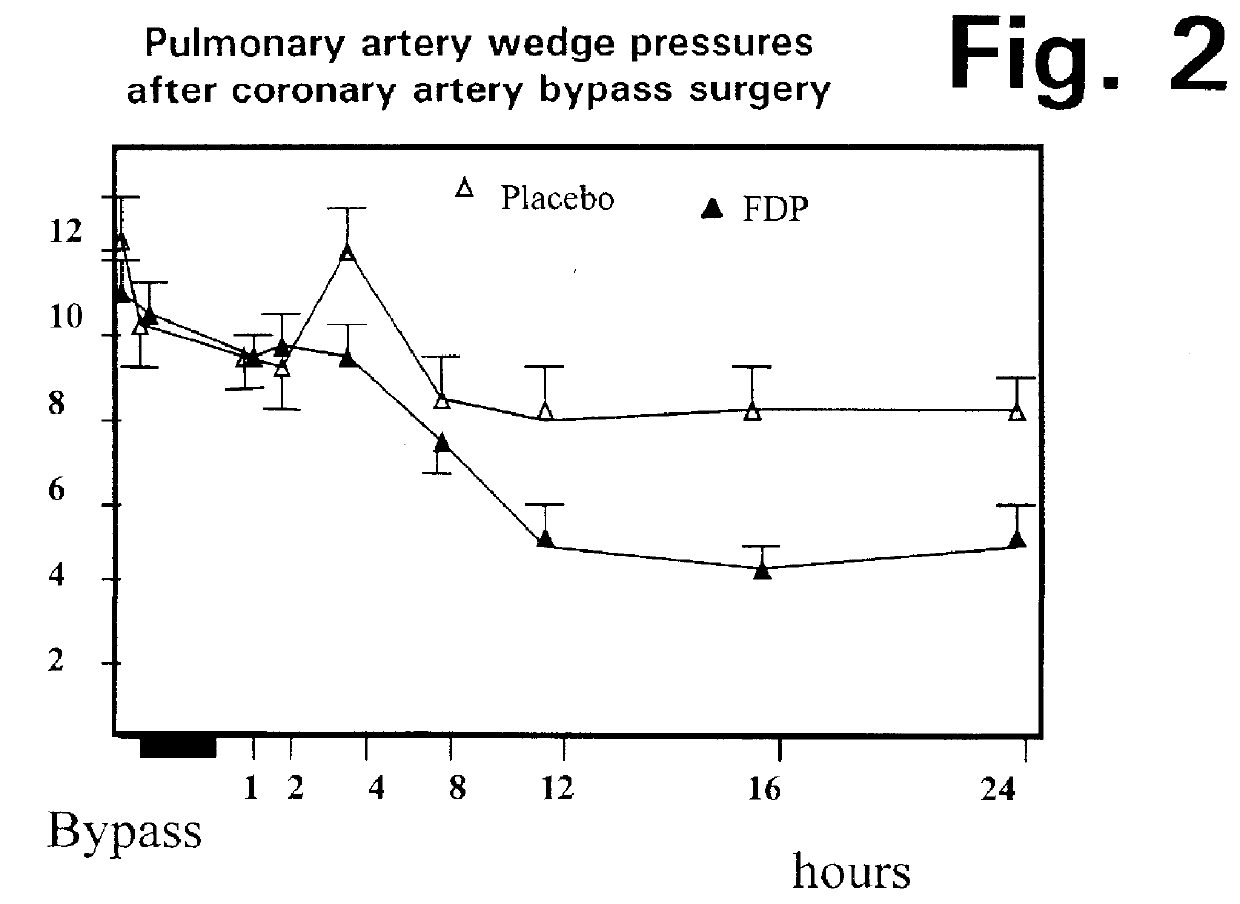
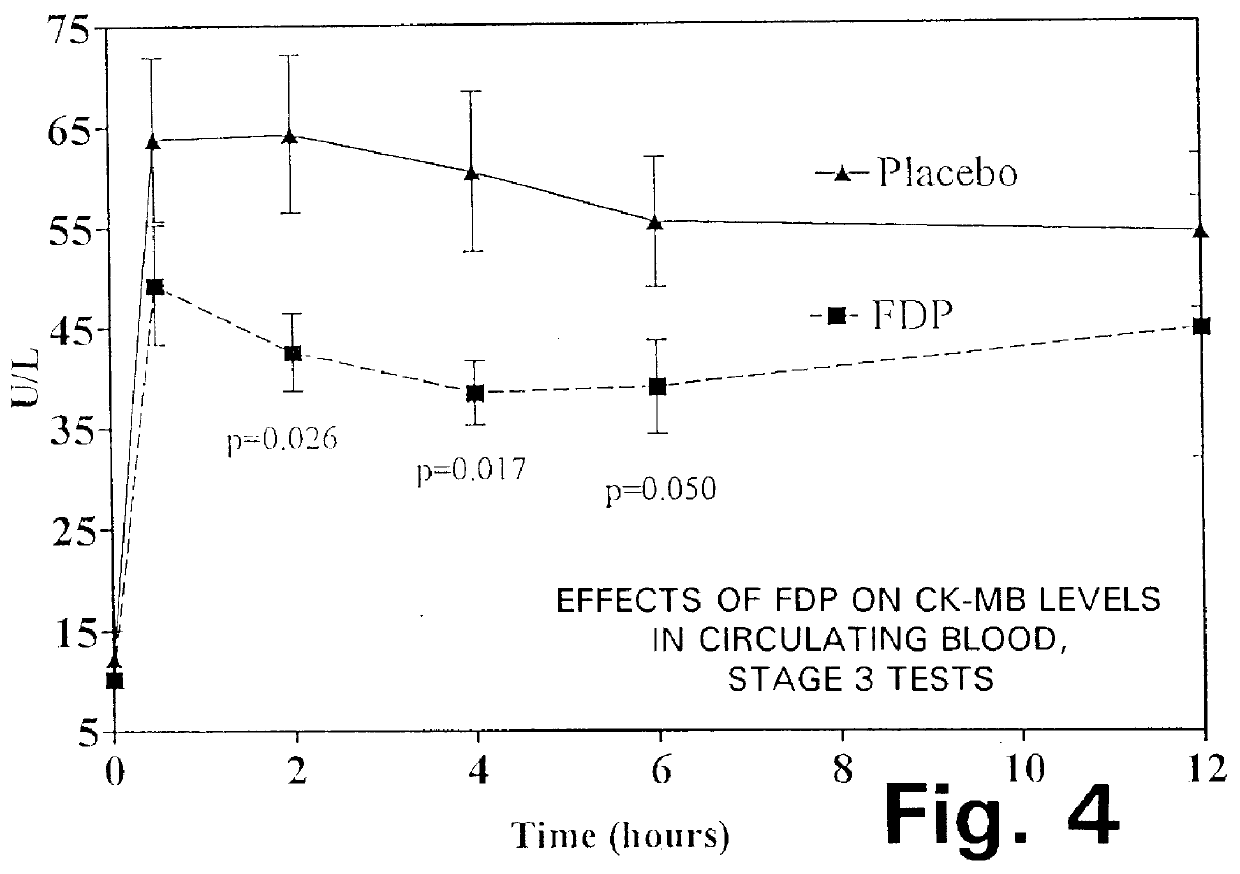
Method of reducing pulmonary hypertension and atrial fibrillation after surgery using cardiopulmonary bypass
A method is disclosed for using fructose-1,6-diphosphate (FDP) to reduce and prevent two very serious problems caused by surgery that requires cardiopulmonary bypass. Before bypass begins, a liquid that contains FDP is intravenously injected into the patient, preferably over a period such as about 10 to 30 minutes, to allow the FDP to permeate in significant quantity into the heart and lungs while the heart is still beating. FDP can be added to the cardioplegia solution that is pumped through the heart to stop the heartbeat, and / or during bypass. This treatment was found to reduce two very important and serious problems that have unavoidably plagued CPB surgery in the past, which are: (1) elevated levels of pulmonary vascular resistance (PVR), which includes pulmonary hypertension; and (2) high occurrence rates for atrial fibrillation. Prior to this discovery, there has never been any satisfactory treatment which could reduce the severity and occurrence rates for these two major problems. FDP also can be co-administered in this manner, along with (1) a buffering or alkalizing agent that counteracts acidosis, such as sodium bicarbonate or THAM, and / or (2) a drug that reduces the formation of lactic acid, such as dichloroacetate.
Owner:QUESTCOR PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com