Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
828 results about "Water activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Water activity (aw) is the partial vapor pressure of water in a substance divided by the standard state partial vapor pressure of water. In the field of food science, the standard state is most often defined as the partial vapor pressure of pure water at the same temperature. Using this particular definition, pure distilled water has a water activity of exactly one. As temperature increases, aw typically increases, except in some products with crystalline salt or sugar.
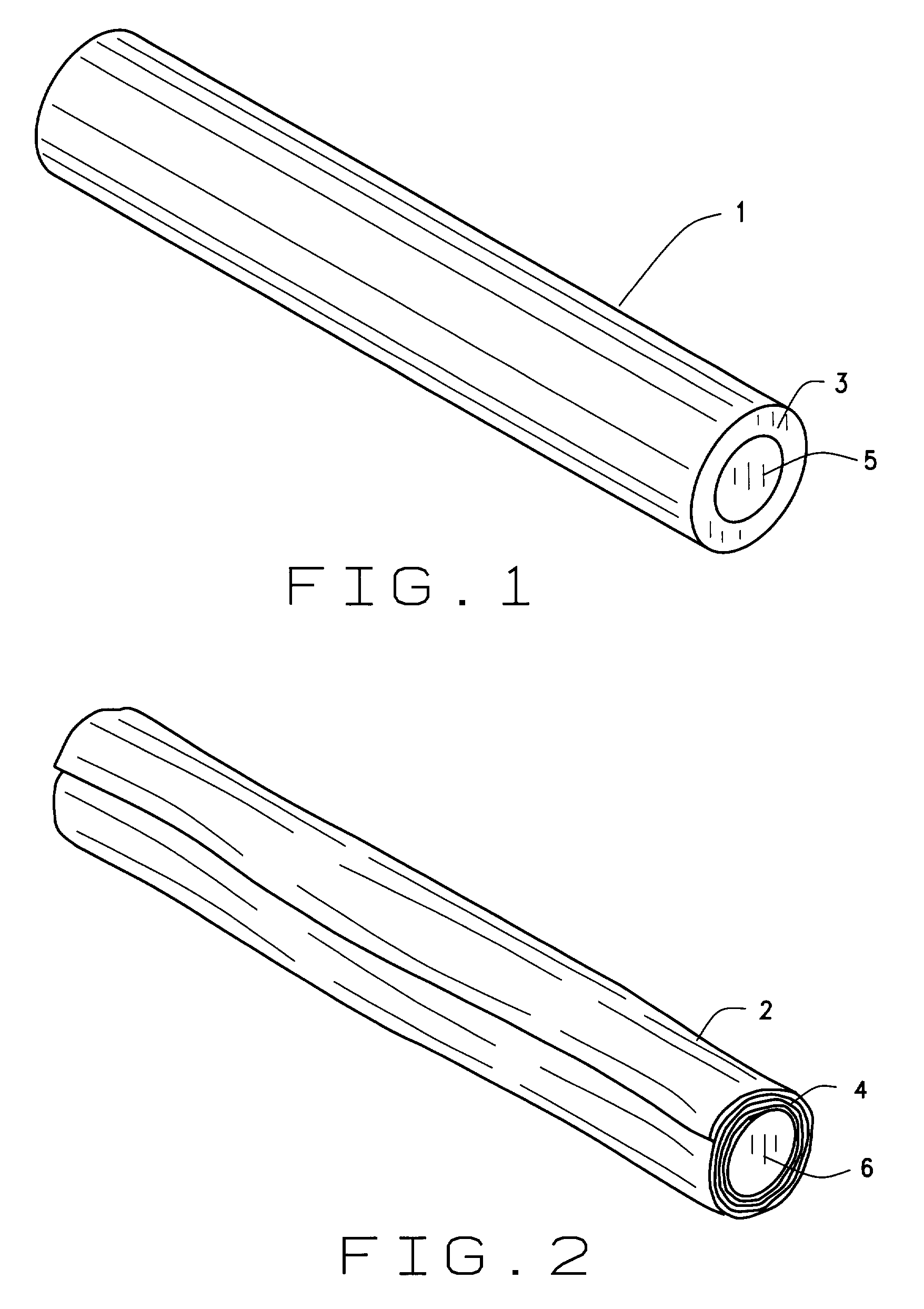
Combination rawhide and formulated food pet chew
InactiveUS6277420B1Limited toughnessChew life increaseProtein composition from fishMeat/fish preservationWater activityCapillary action
A highly palatable and long lasting dog chew for pets has been developed by combining a formulation and processing sequence which results in a highly palatable meat based filling being incorporated into the center of a preformed rawhide stick or rawhide roll. Such outside rawhide fraction is extremely tough and chewy which results in a dog chew which takes a long period of time for the dog to consume. The inside meat filling is highly palatable which results in the animal maintaining interest in the treat until nearly the entire chew has been consumed. The interior meat filling is preserved by reduced water activity to below 0.85 as a result of incorporating of salt, sugars and natural humectants. Said filling is formulated and processed in such a manner that the water phase is bound within the filling and does not pass by capillary action to the outside rawhide fraction. This results in the outer rawhide shell maintaining a tough and chewable texture until such point as the dog is offered the finished chew.
Owner:ANDERSEN DAVID B +1
Method and system for using a cellular phone in water activities
InactiveUS20090017884A1Devices with multiple display unitsDevices with GPS signal receiverWater activityCellular telephone
The present invention relates to a method and system for carrying out water activities under or above water, comprising: (a) a cellular phone for enabling a user to carry out water activities; (b) a waterproof case having a front section and a back section, said waterproof case being suitable to contain said cellular phone, said back section comprising a control button(s) panel, including one or more control buttons, for remotely controlling said cellular phone; and (c) an electronic circuit, programmed according to the cellular phone type, for processing data for controlling said cellular phone by means of said one or more control buttons and for forwarding said data to said cellular phone, said electronic circuit communicating with both said cellular phone and said control button(s) panel.
Owner:ROTSCHILD CARMEL
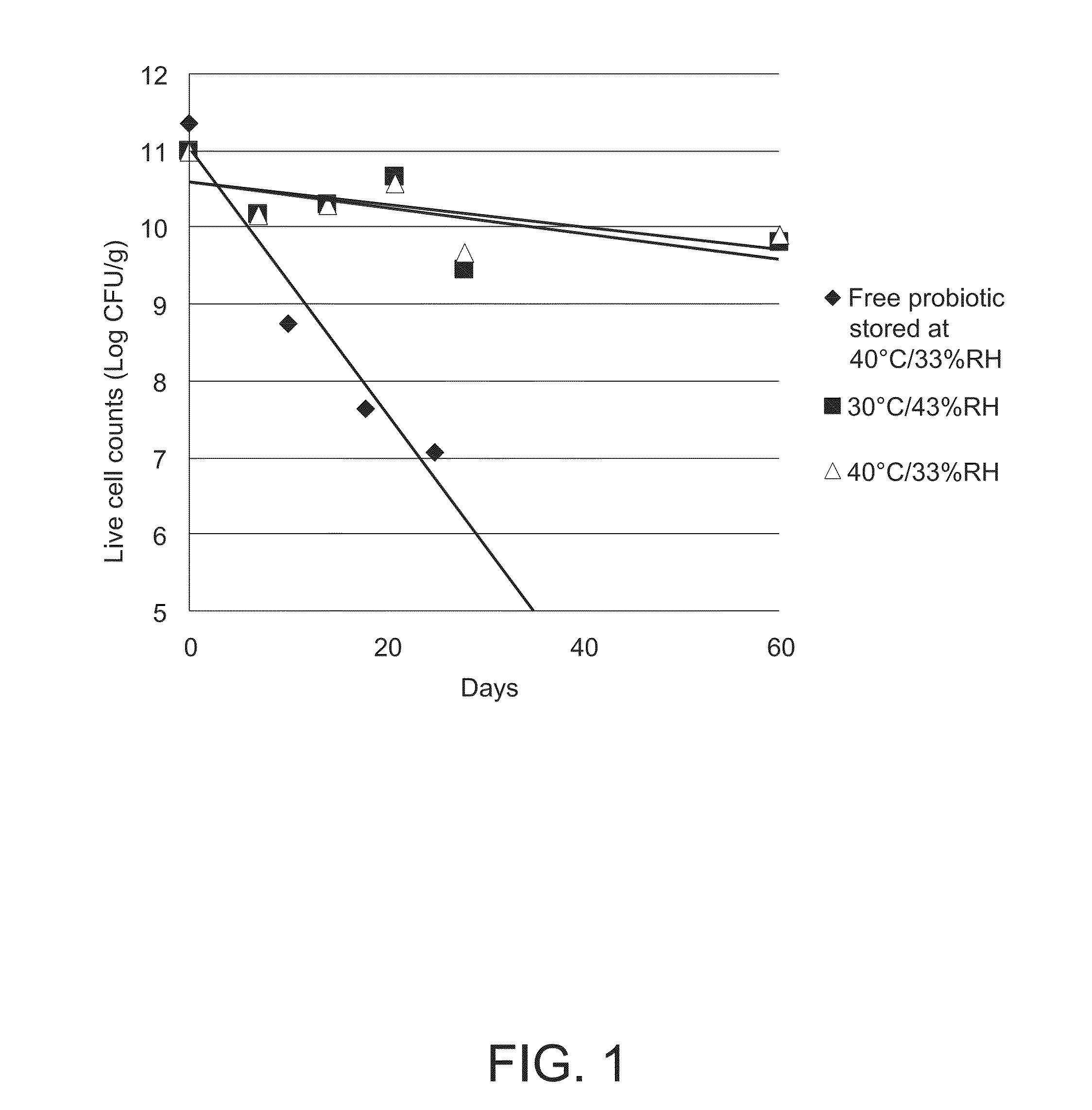
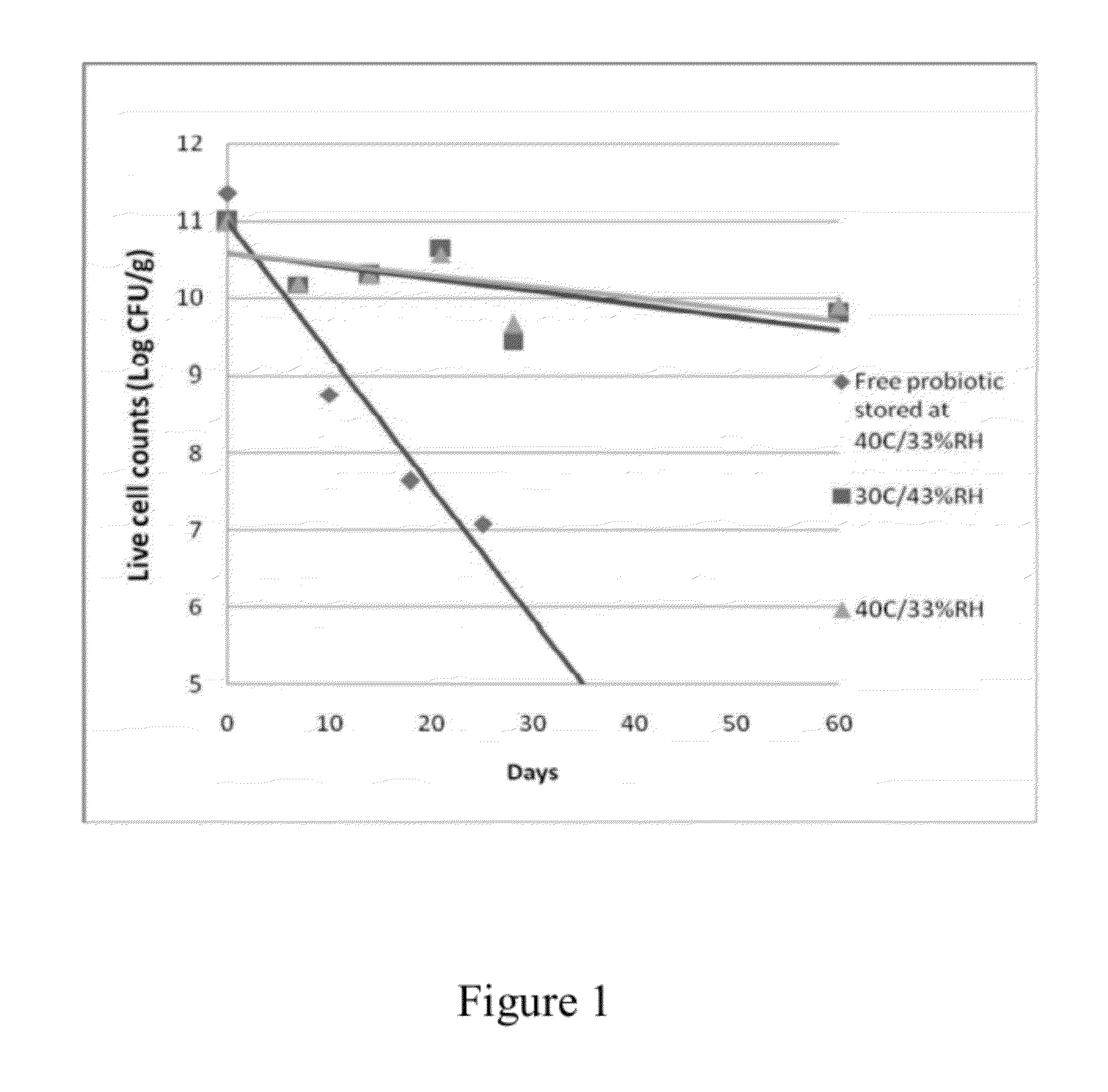
Stable probiotic microsphere compositions and their methods of preparation
The invention relates to viable and stable probiotic formulations for intestinal targeting made of microspheres comprising each a core of one or more probiotic bacteria, microcrystallline cellulose with a degree of polymerization from 165-365 and mean diameter from 45 to 180 μm, a disintegrant and a stabilizer, the core being coated with a non-enteric coating and further coated with an enteric coating. Each probiotic microsphere has a residual moisture level of less than 5% and a water activity (aw) between 0.1 and 0.5. Such a probiotic microsphere shows no reduction in viable bacteria after one hour in simulated gastric fluid. The present invention also relates to the process of preparing such formulation.
Owner:CANACURE CORP
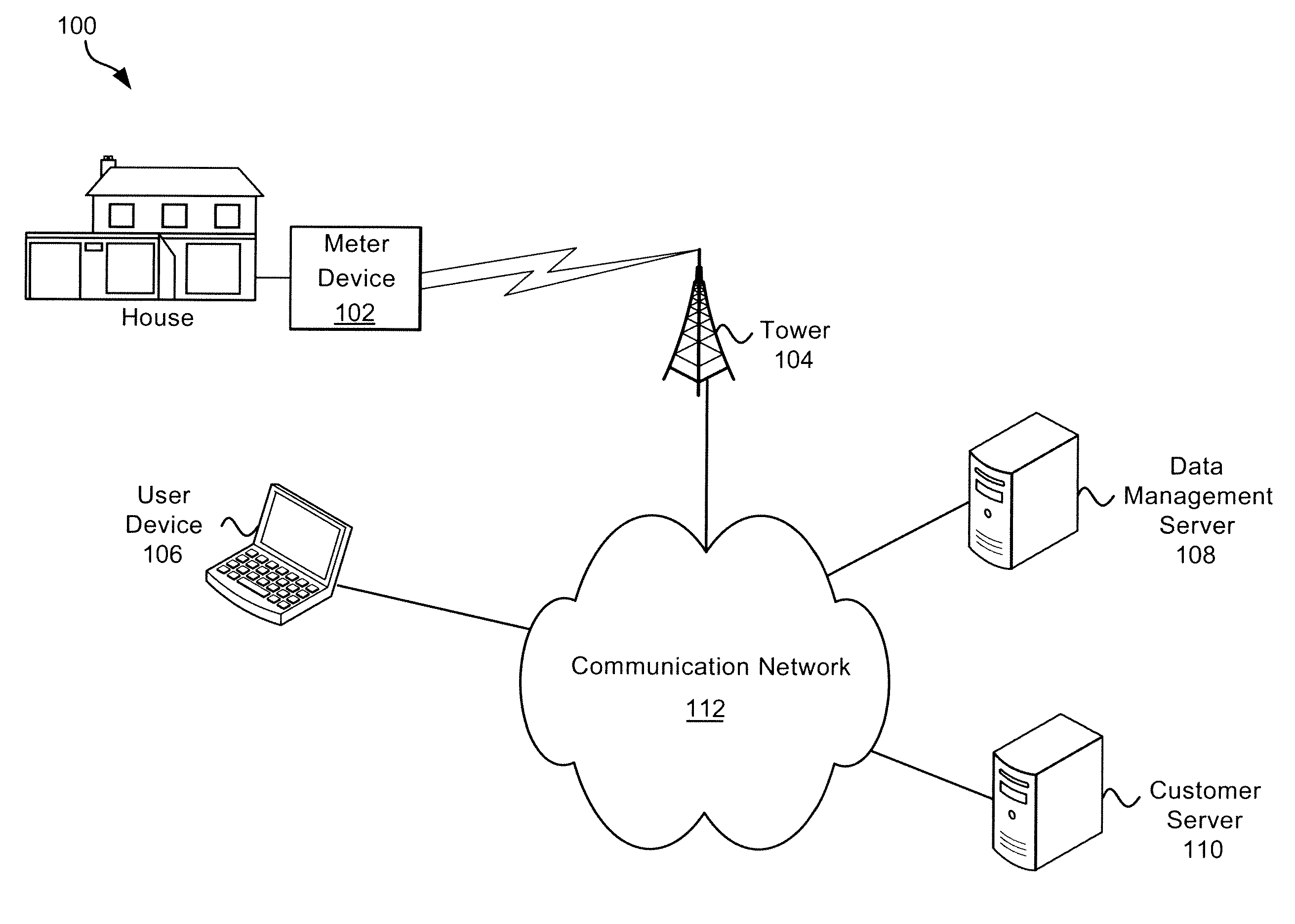
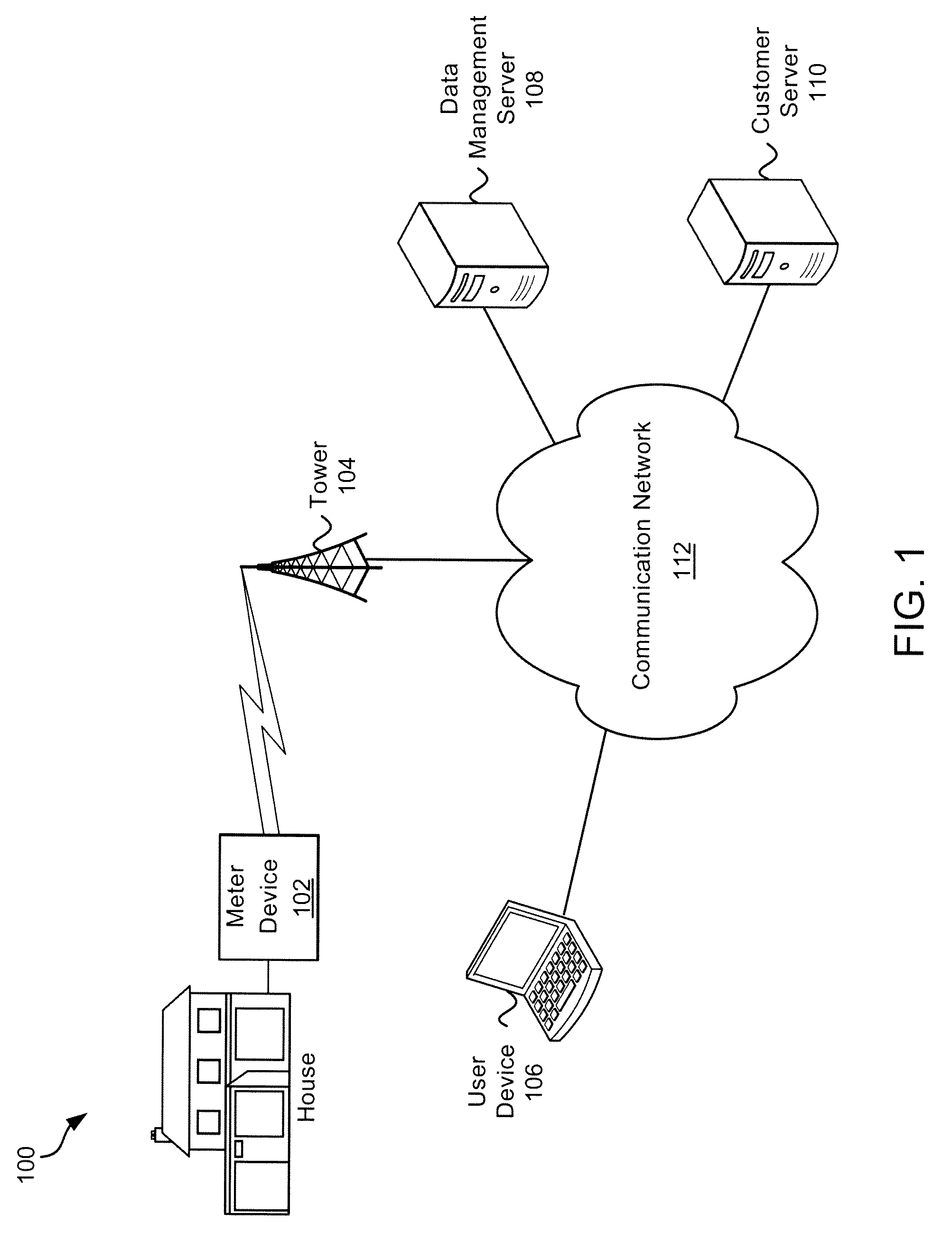
Systems and Methods of Interaction with Water Usage Information
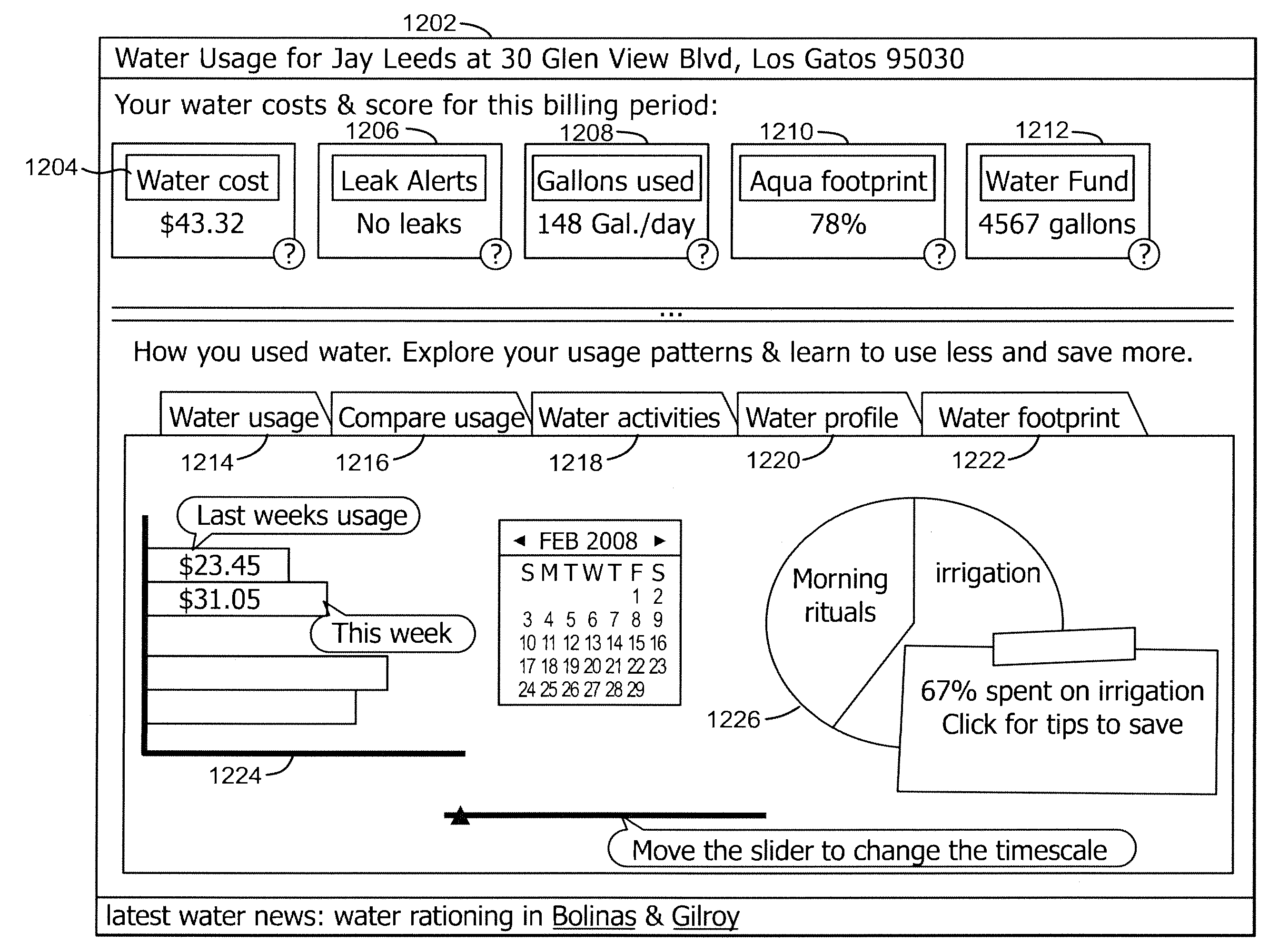
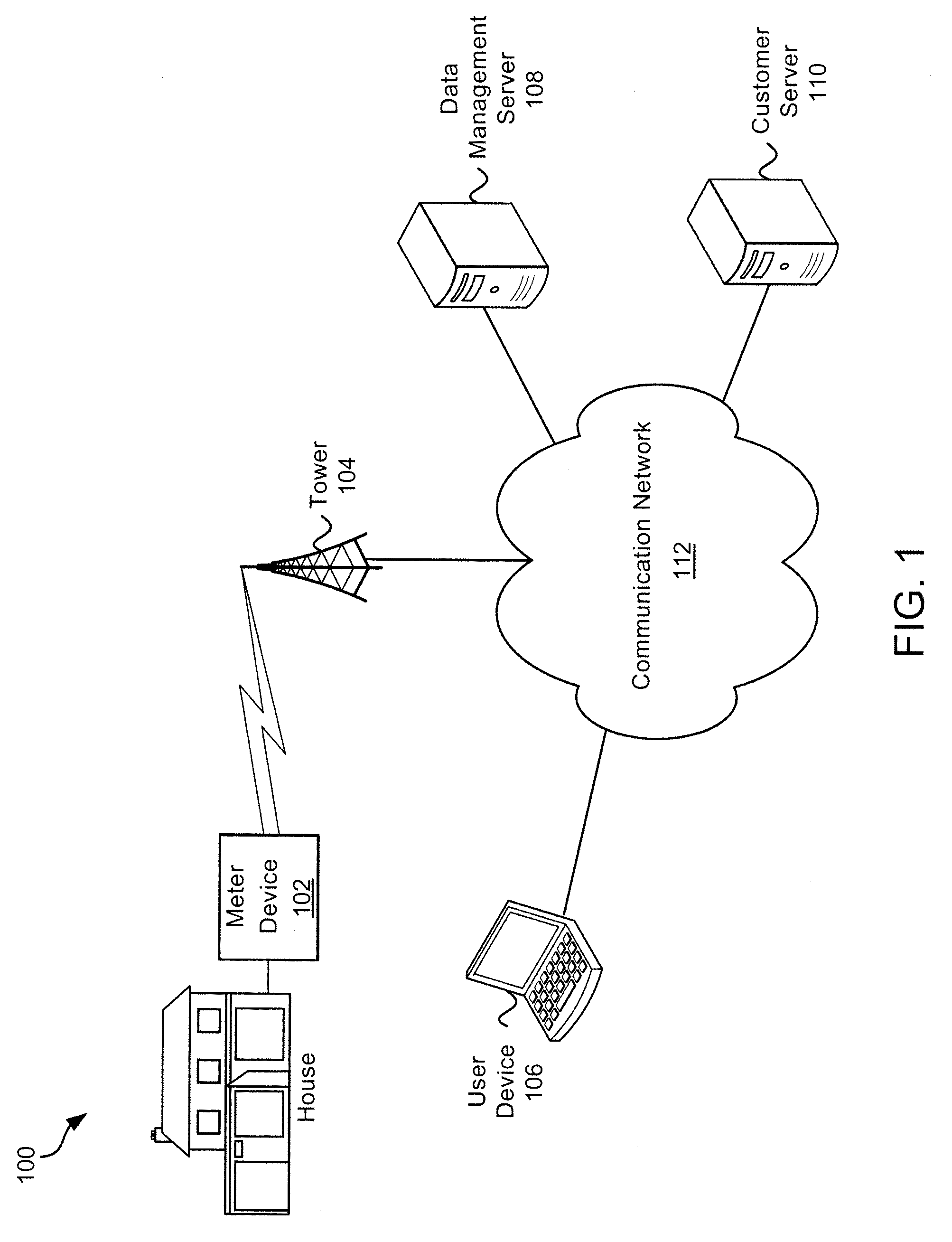
ActiveUS20100289652A1Conserve waterElectric signal transmission systemsUtility meters data arrangementsWater activityHuman–computer interaction
Exemplary systems and methods for interaction with water usage information are provided. In various embodiments, a method comprises receiving water usage data from a meter device, receiving an identifier from a user associated with the meter device, providing an interactive interface to the user, the interactive interface conveying at least some water usage information based on the water usage data, receiving a first characterization of a first water activity from the user, generating a visualization based on the water usage information and the first characterization of the first water activity, and displaying the visualization.
Owner:BADGER METER
Stable digestive enzyme compositions
InactiveUS20090117180A1Minimal loss of activityComposition is stablePowder deliveryHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 9% or less or 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 25%, about 20%, about 15% or about 10% after six months of accelerated stability testing and the titer level of a viral contaminant present in the pancreatin is at least about 1000 times less than the titer level of the viral contaminant present in a preparation from which the pancreatin is obtained.
Owner:APTALIS PHARMA
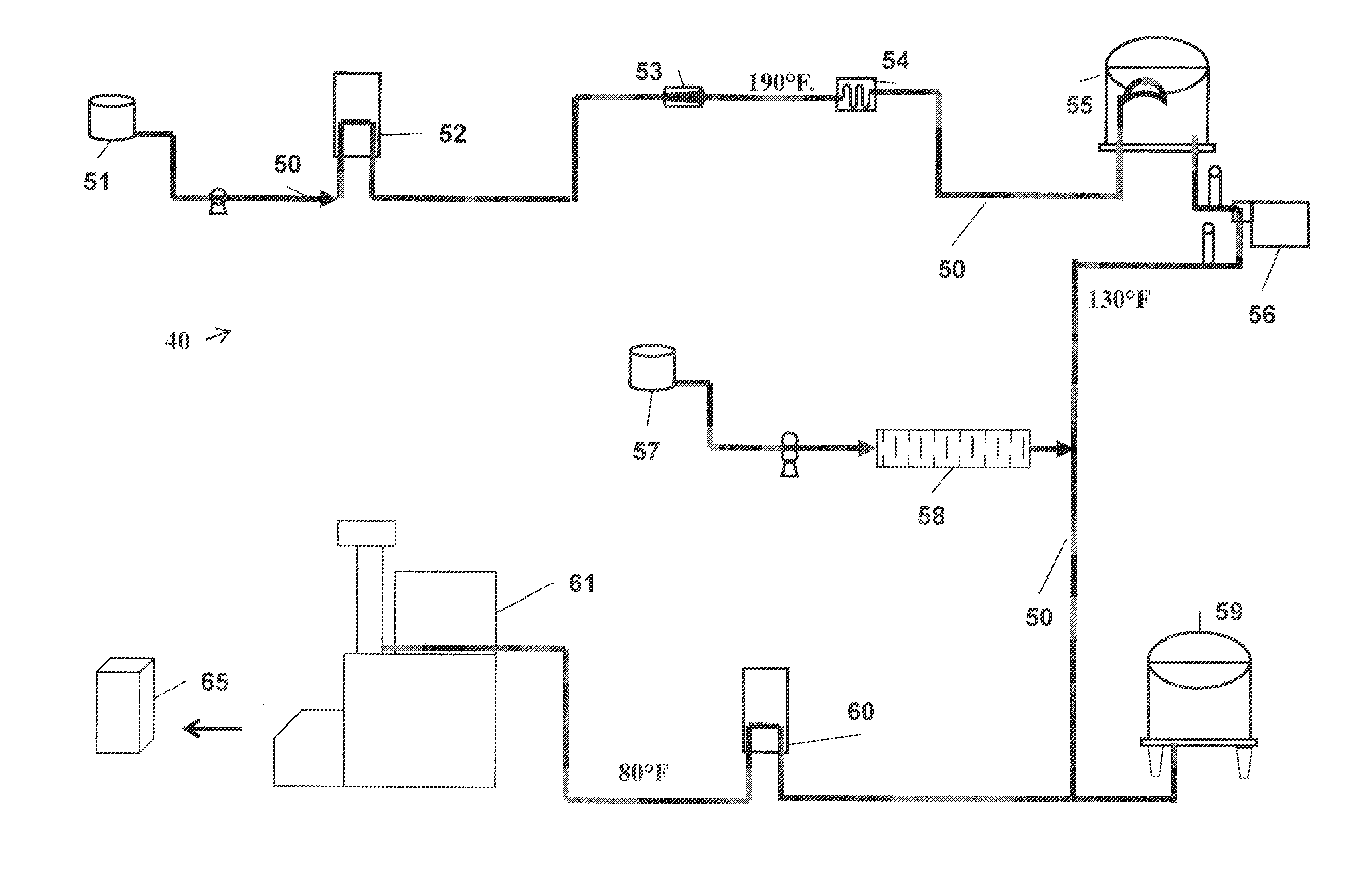
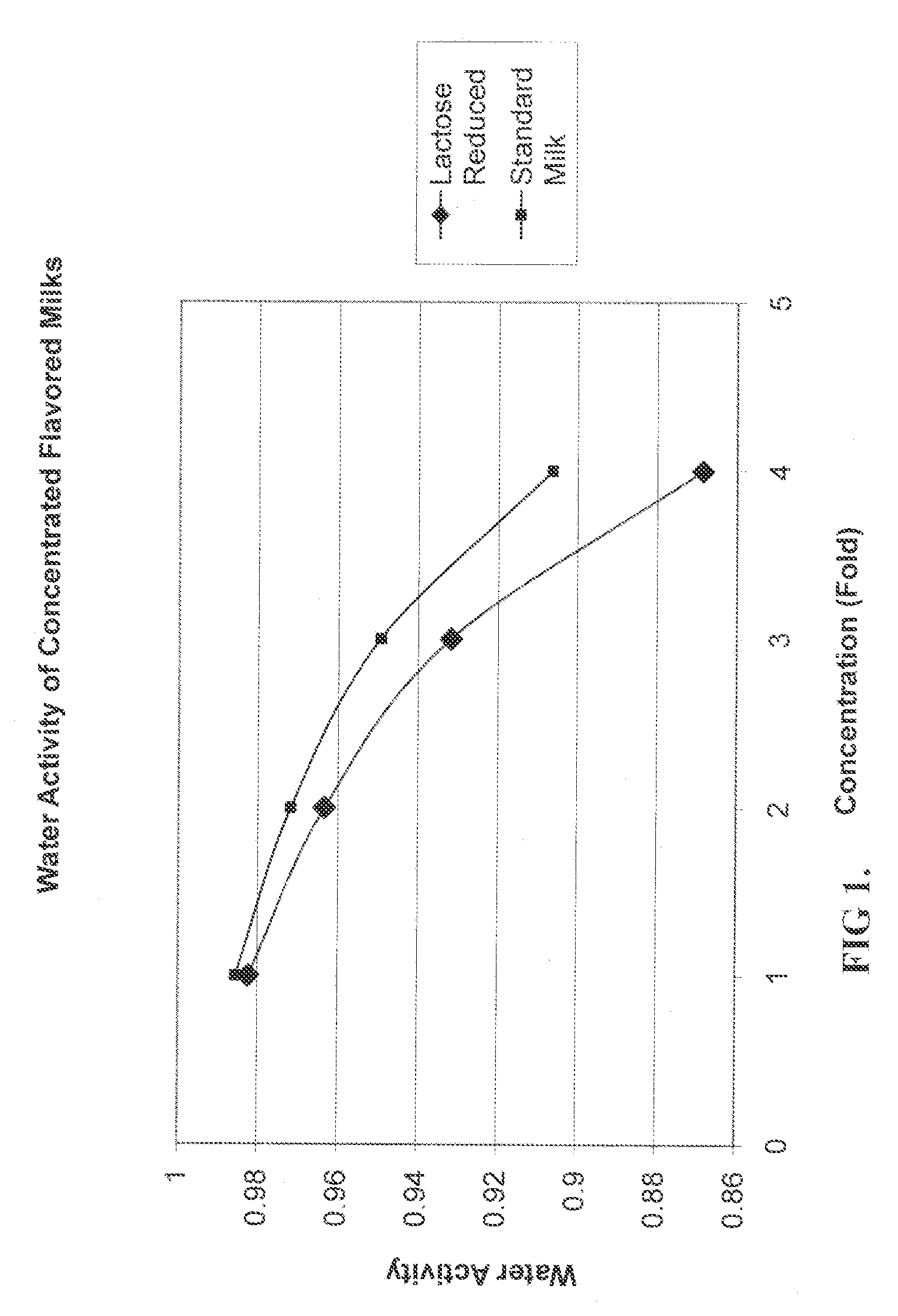
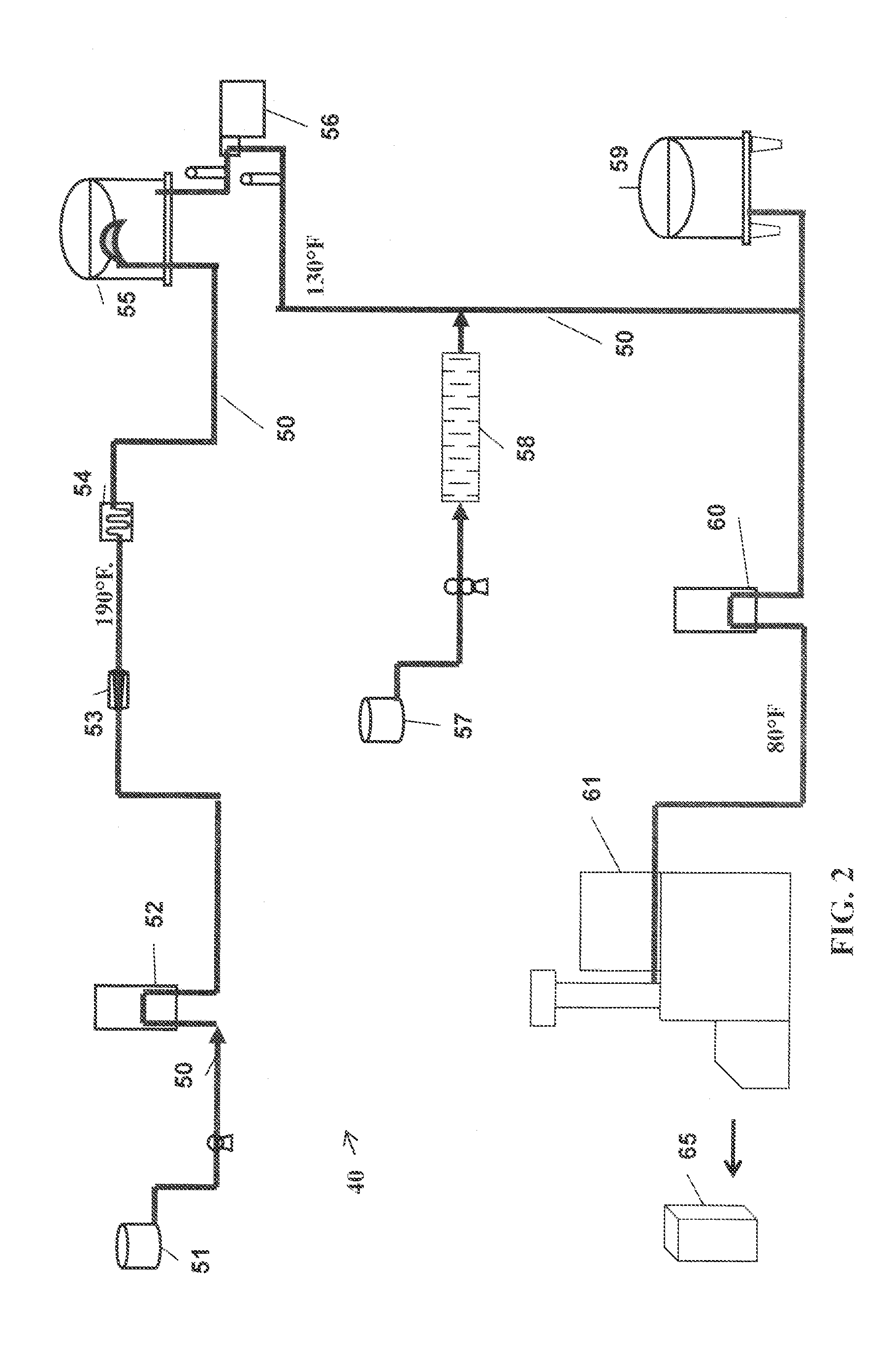
Process For Making A Shelf-Stable Milk Based Beverage Concentrate
InactiveUS20090317514A1Minimizes thermal exposureLower water activityMilk preparationMilk preservationLactaseWater activity
A concentrate, system and low-temperature process for preparing a shelf-stable milk concentrate that does not require ultra-high temperature thermal processing for control of the microbiology of the product is disclosed herein. The method preferably incorporates aseptic technology and the enzymatic reduction of lactose to control water activity. The method preferably includes the enzymatic conversion of the lactose in the milk to its component sugars glucose and galactose, which preferably changes the colligative properties of the concentrate, decreases the amount of free water, and reduces the osmolarity.
Owner:DAIRYVATIVE TECH
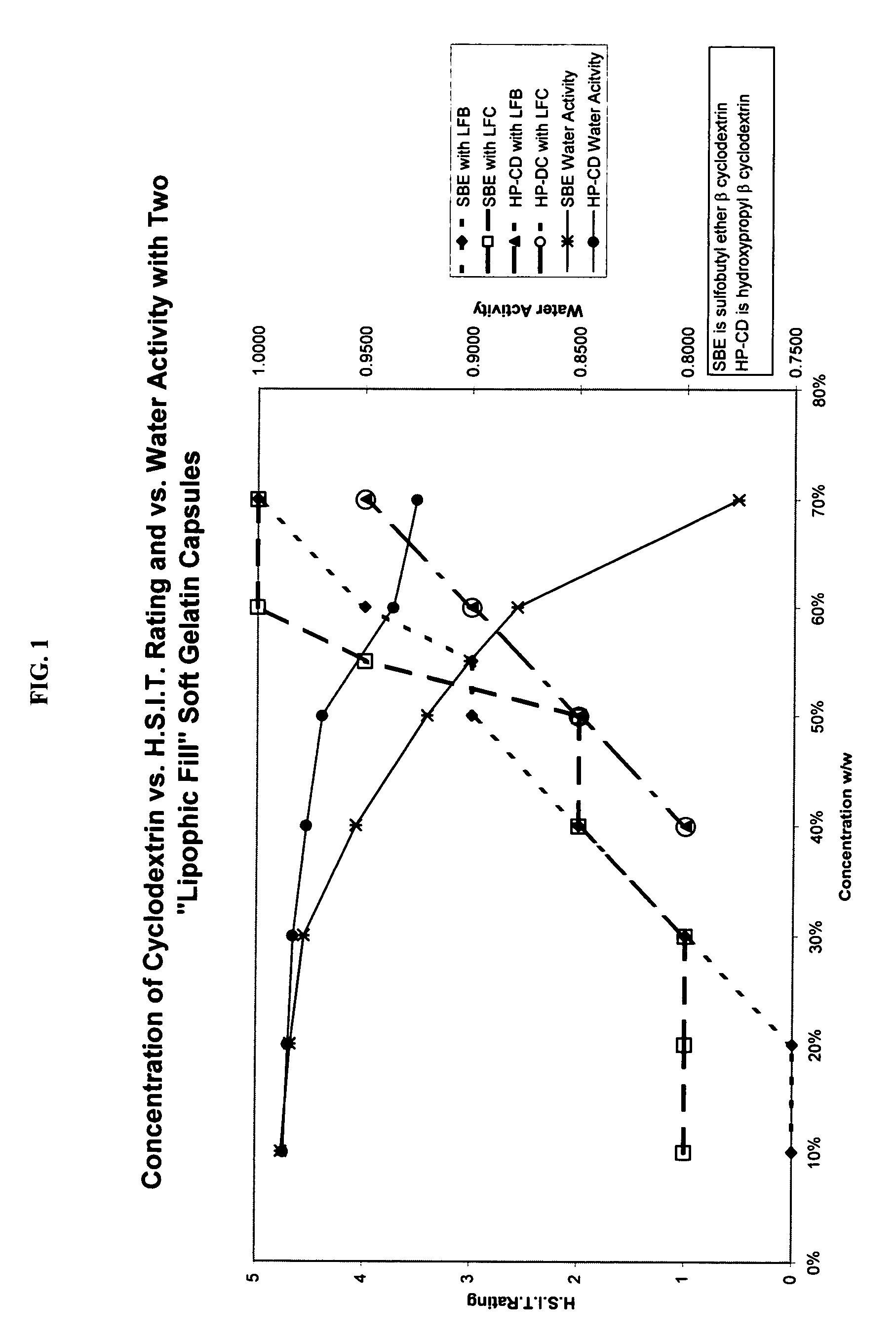
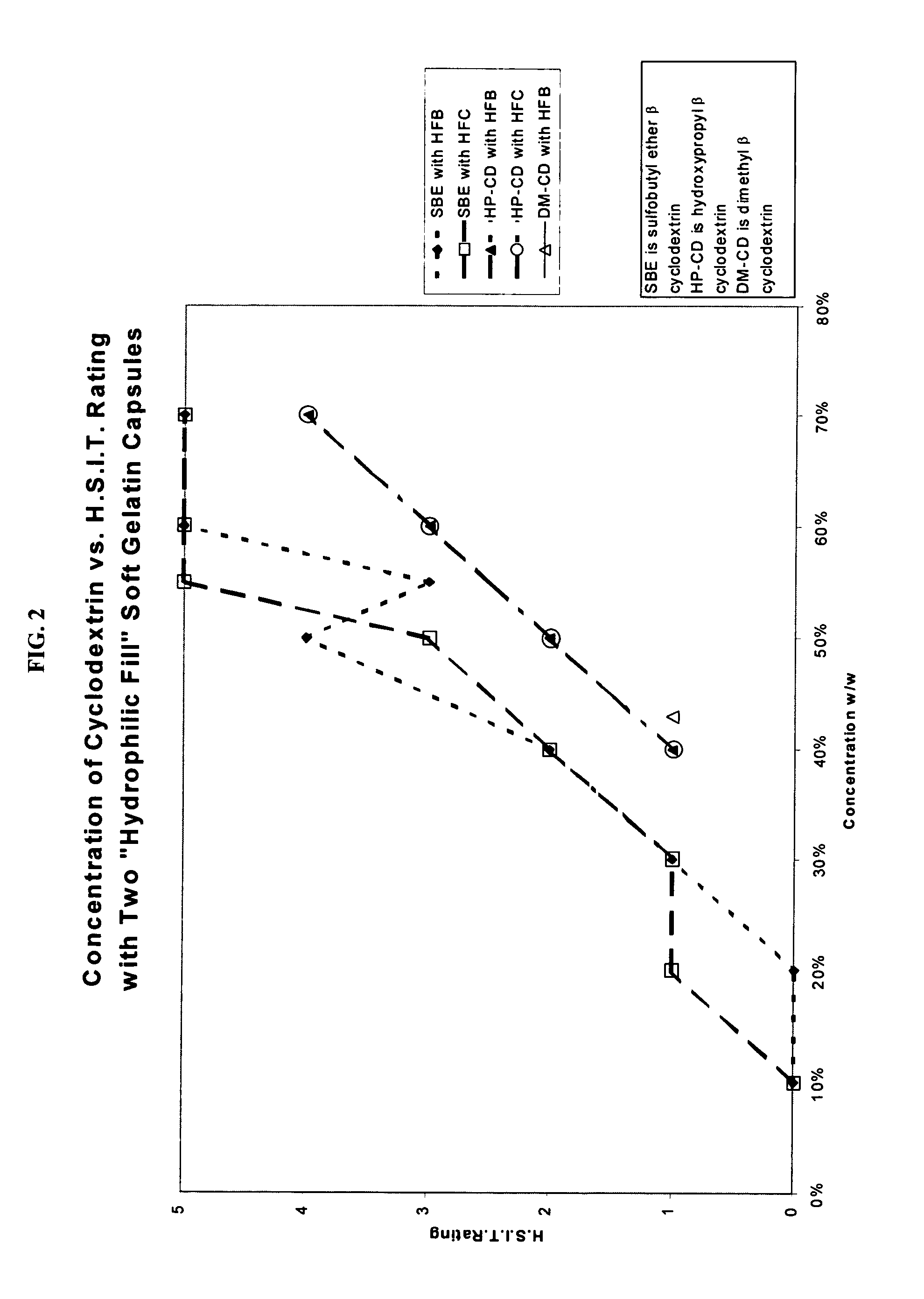
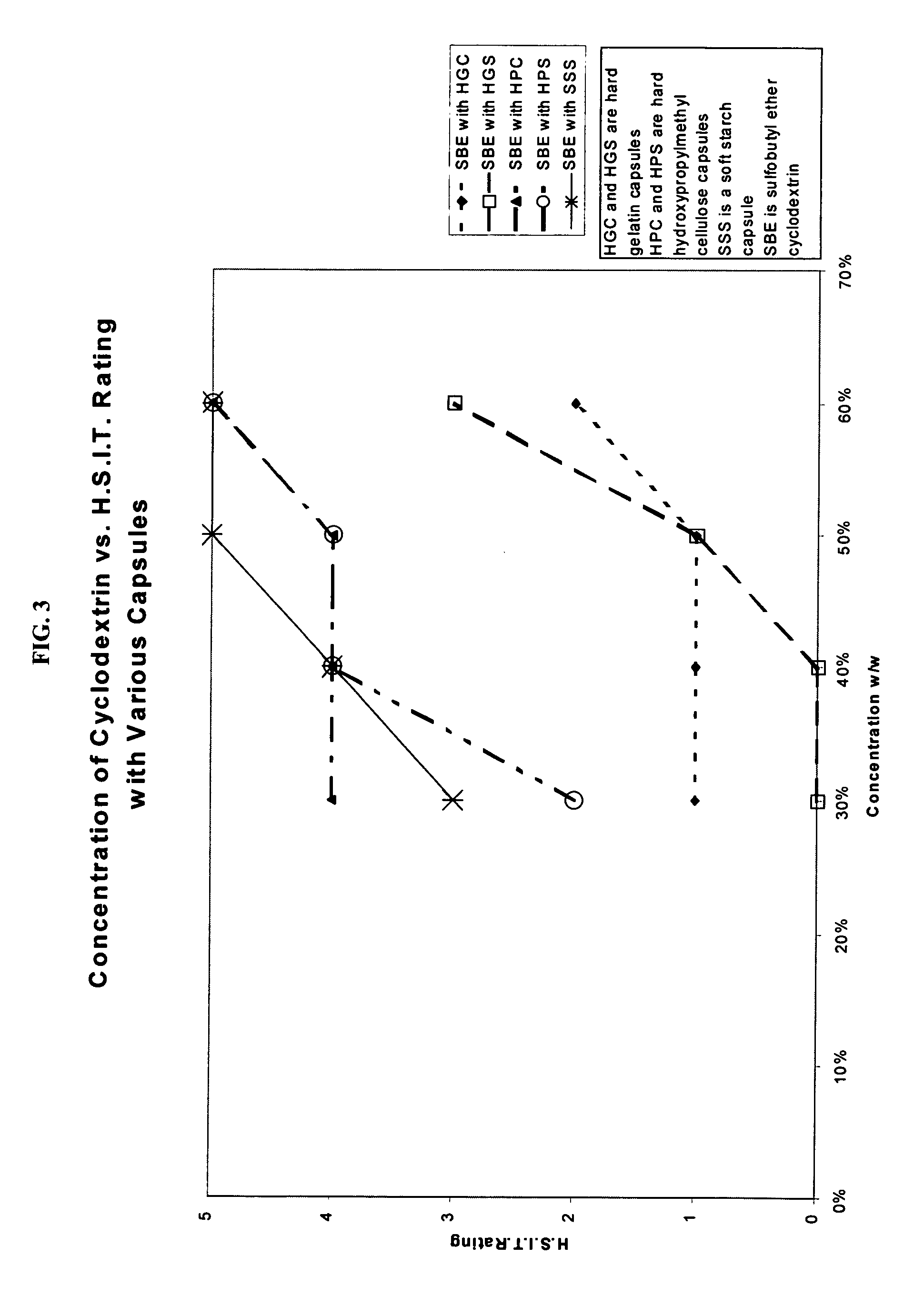
Capsules containing aqueous fill compositions stabilized with derivatized cyclodextrin
ActiveUS20050186267A1Reduce and stop dissolutionReduce and stop and erosionBiocideOrganic active ingredientsActive agentWater activity
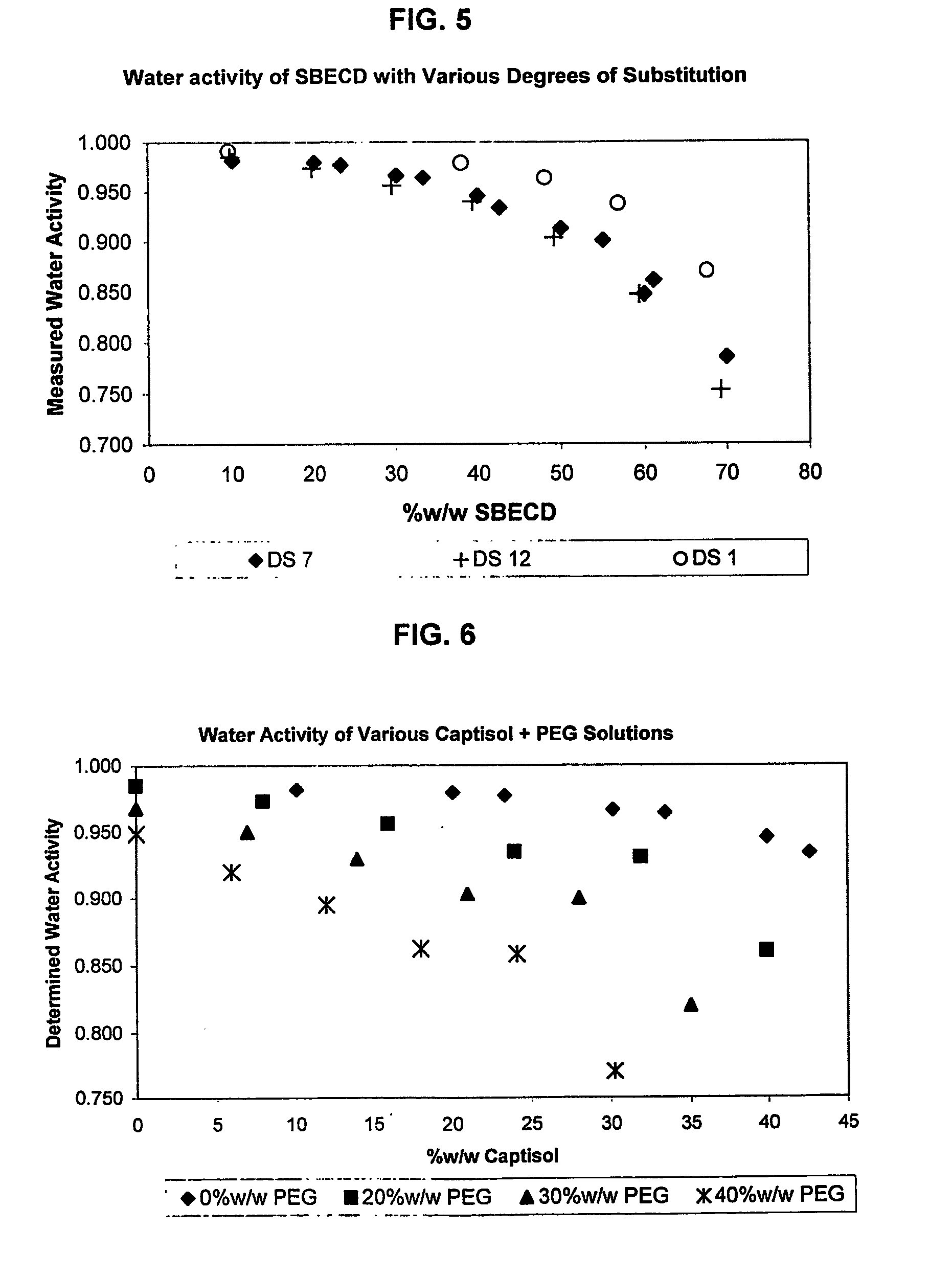
A capsule containing an aqueous fill composition that comprises water, a derivatized cyclodextrin, such as sulfoalkyl ether cyclodextrin (SAE-CD) or hydroxypropyl cyclodextrin (HPCD), optionally one or more active agents and optionally one or more excipients is stabilized from degradation, erosion, swelling or dissolution of its shell during storage. The derivatized cyclodextrin is present in an amount sufficient to reduce, eliminate or inhibit degradation, erosion, swelling and / or dissolution of the shell by water present in the fill composition. Alternatively, the derivatized cyclodextrin and another shell-stabilizing material together stabilize the shell from degradation, erosion, swelling and / or dissolution by water present in the fill composition. The derivatized cyclodextrin can reduce the water activity of the fill composition.
Owner:CYDEX PHARMACEUTICALS INC
Stable pancreatic enzyme compositions
ActiveUS20080274174A1Stable digestive enzyme compositionThe dosage form is stablePeptide/protein ingredientsHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 15% after six months of accelerated stability testing.
Owner:SOC DES PROD NESTLE SA
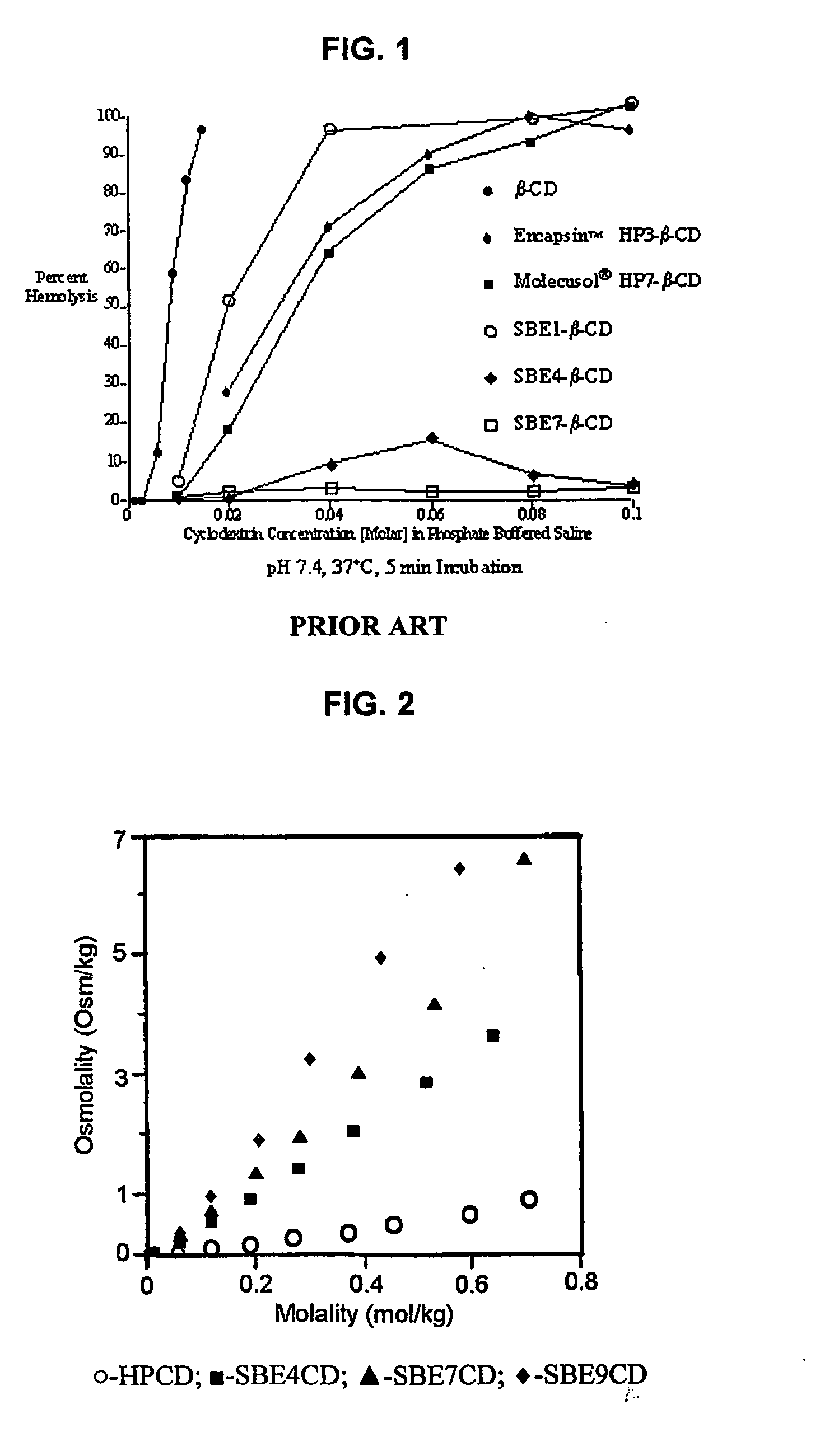
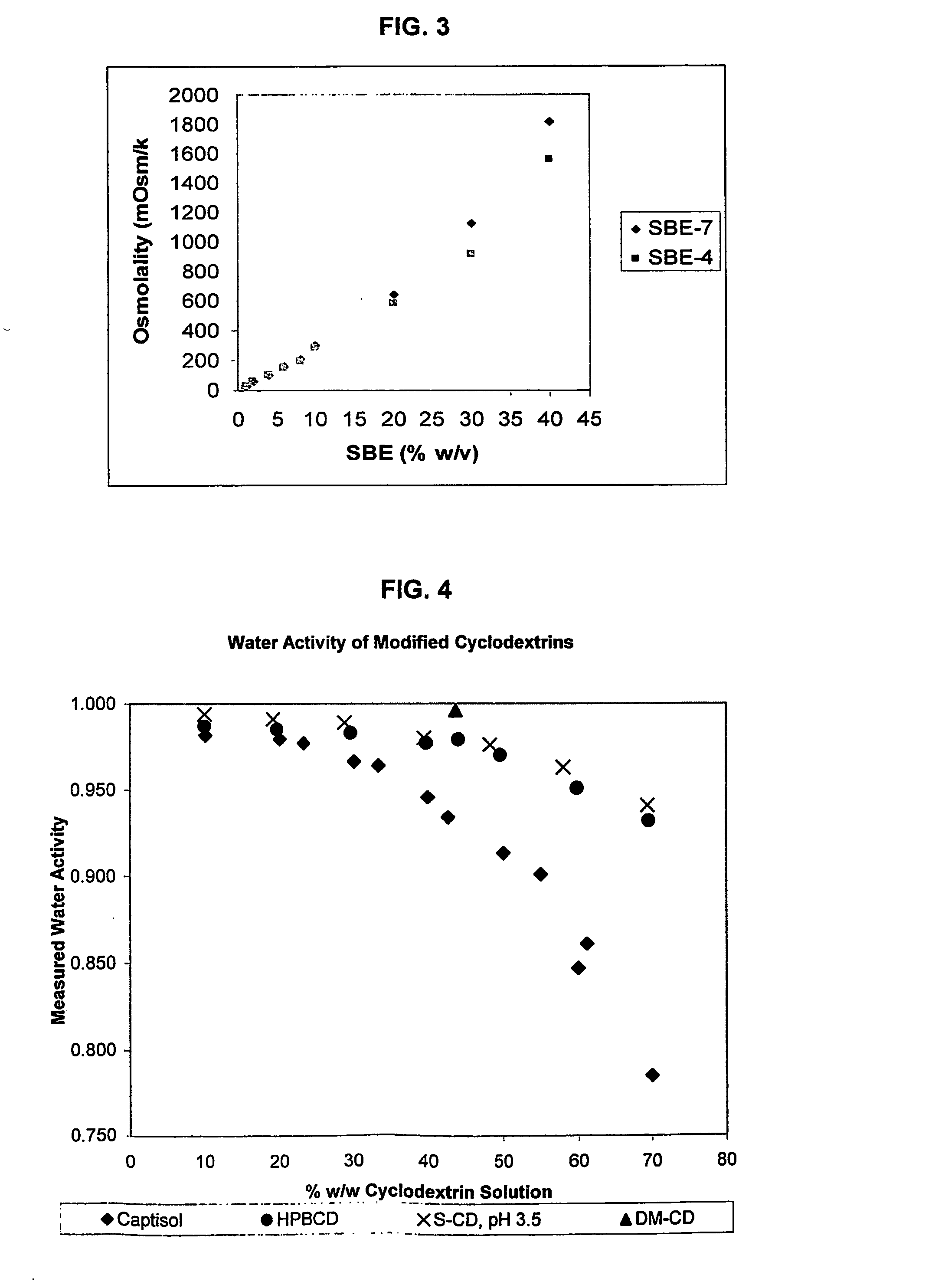
Use of sulfoalkyl ether cyclodextrin as a preservative
A method of preserving formulations is provided. The method includes the step of including a derivatized cyclodextrin in a formulation capable of sustaining microbial growth. One embodiment of the formulation employs a sulfoalkyl ether cyclodextrin as a preservative and optionally as a solubilizing and complexing agent. A suitable cyclodextrin is the CAPTISOL” brand cyclodextrin (sulfobutyl ether R-cyclodextrin). Whether or not the formulation includes a conventional preservative, the formulation will remain preserved for at least a minimum predetermined period. Specific embodiments of the invention include a carrier, a derivatized cyclodextrin and optionally one or more active agents, one or more water activity-reducing agents, and / or one or more complexation-enhancing agents. The derivatized cyclodextrin reduces the water activity of the formulation. A liquid formulation can be lyophilized or otherwise dried to yield a solid formulation that is optionally reconstitutable.
Owner:CYDEX INC
Soft capsules
The present invention provides soft capsules comprising a capsule shell having a water activity lower than that of a capsule filling; and a production process of the soft capsules. The present invention makes it possible to prepare soft capsules without lowering the water activity of an active ingredient of a medicament or the like, which activity varies widely, depending on the active ingredient employed, leading to the provision of the soft capsules having original unimpaired properties or stability, and moreover, palatability and texture.
Owner:WAKUNAGA PHARMA CO LTD
Methods of producing stable pancreatic enzyme compositions
ActiveUS20080279953A1Minimal loss of activityComposition is stablePowder deliveryPeptide/protein ingredientsDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 15% after six months of accelerated stability testing.
Owner:SOC DES PROD NESTLE SA
Probiotic food, process for its preparation and dietary regimen
InactiveUS20070160589A1Easy maintenanceStrong feelingBiocideMetabolism disorderWater activityDietary regimen
A probiotic food item containing a beneficial amount of dry active probiotic cultures is provided. The food item also contains a substantial continuous fat-based coating with an effectively low water activity level. The food item is packaged in a substantially moisture impermeable package marked with a use by or sell by date, so as to ensure a desired minimal amount of probiotic colony forming units (CFUs) on the use by or sell by date. Methods for manufacturing the probiotic food product are also provided.
Owner:ATTUNE FOODS
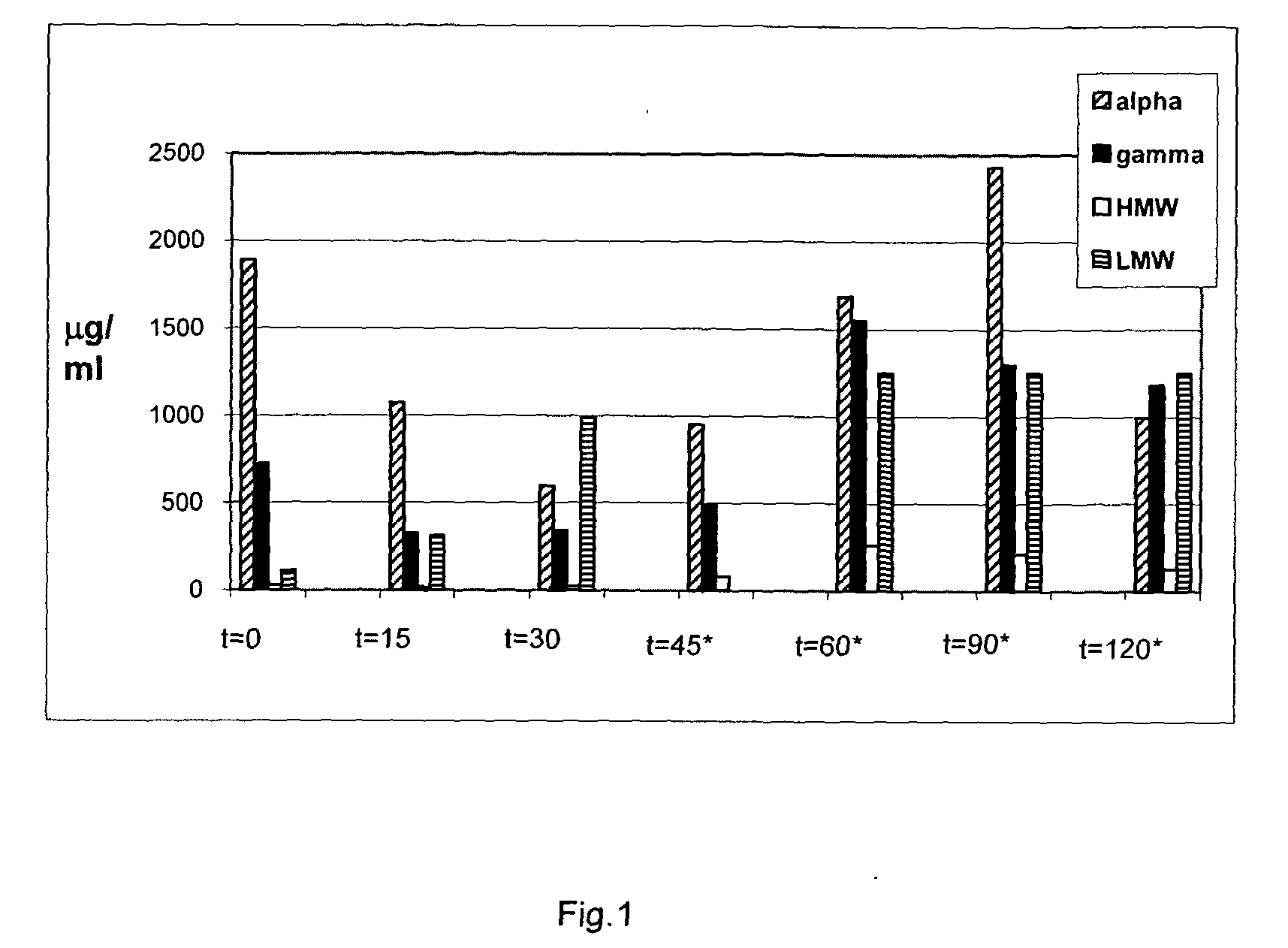
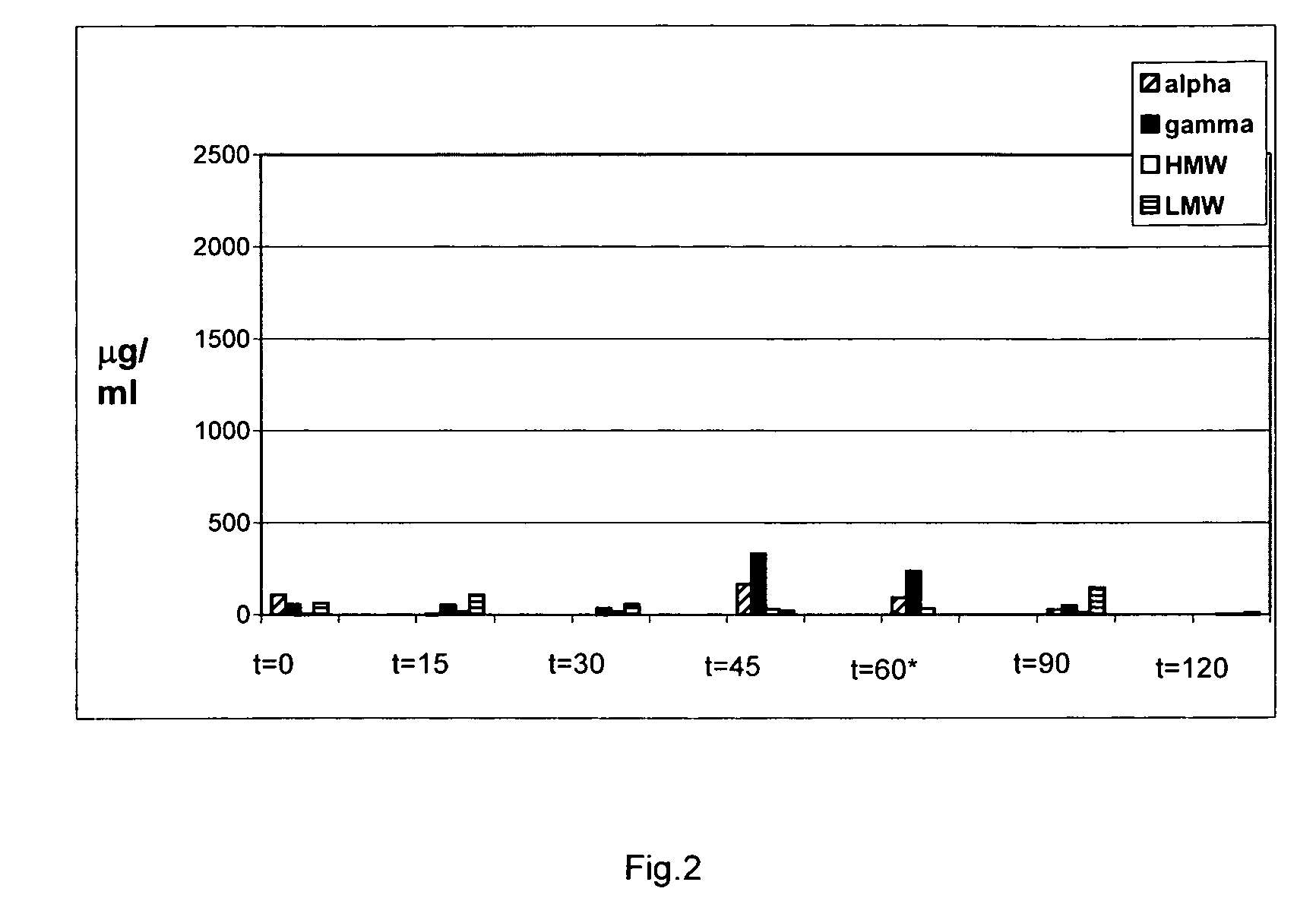
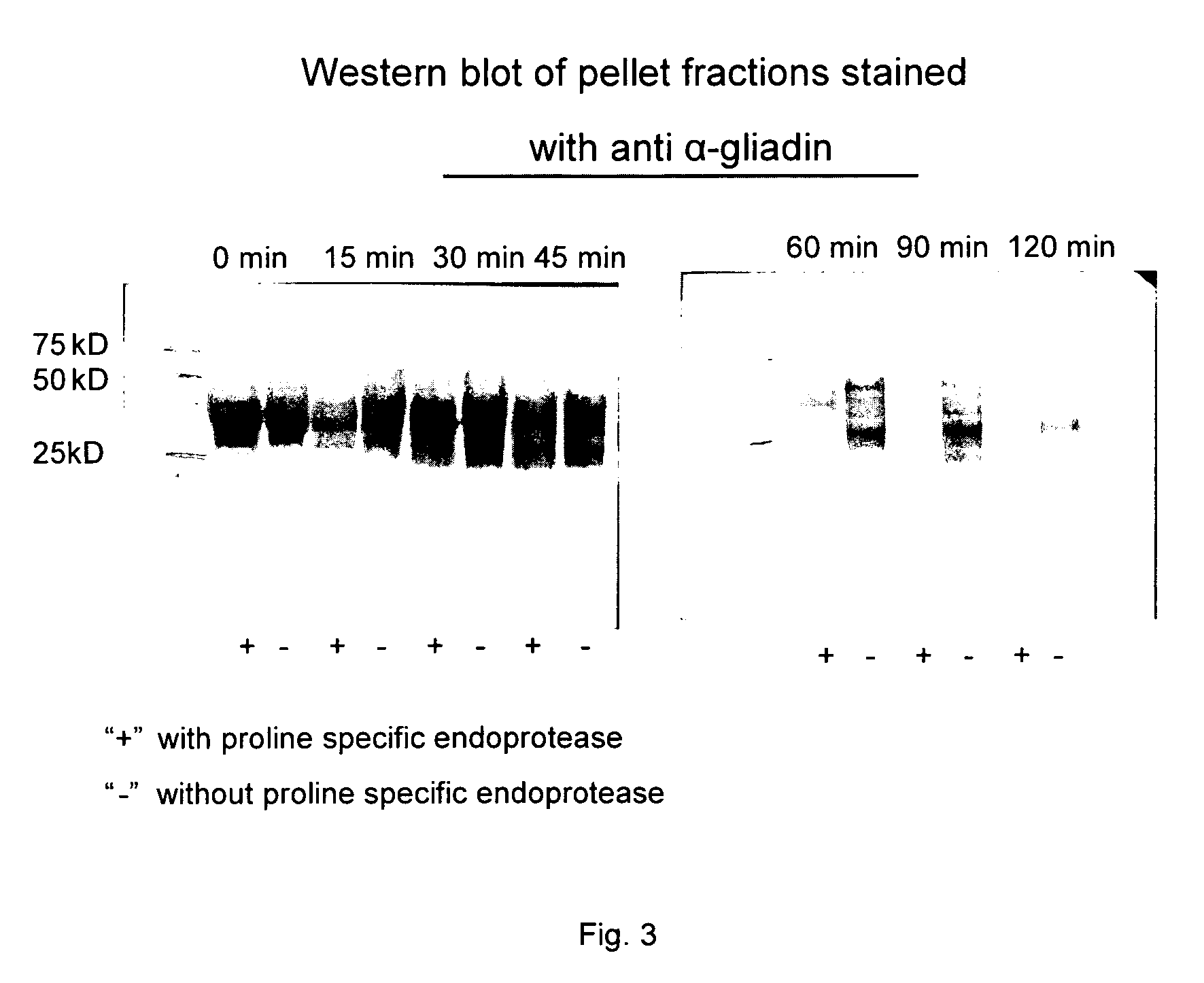
Food product comprising a proline specific protease, the preparation thereof and its use for degrading toxic or allergenic gluten peptides
InactiveUS20090304670A1Extended shelf lifeIntense and long interaction periodSpread compositionsPeptide/protein ingredientsProteinase activityWater activity
The present invention relates to a pasteurized food product having a water activity of at least 0.80, preferably at least 0.85 and containing a proline specific protease.
Owner:UPONOR INNOVATION AB +1
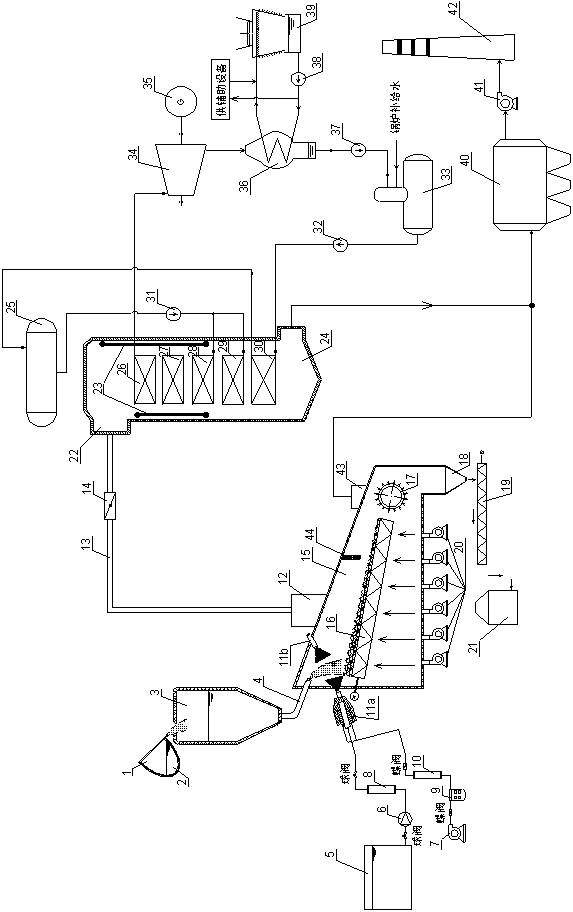
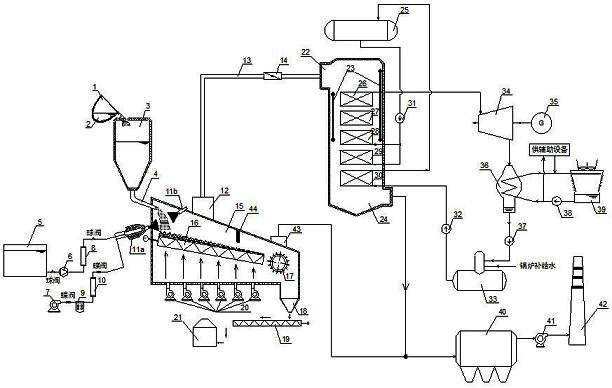
Melting furnace slag quenching dry type granulation and sensible heat recovery generating system and method using same
ActiveCN102433401AReduce cooling air volumeReduce power consumptionIncreasing energy efficiencyRecycling and recovery technologiesSlagHigh pressure water
The invention provides a melting furnace slag quenching dry type granulation and sensible heat recovery generating system and a method using the same and belongs to the technical field of steel metallurgy furnace slag treatment and complementary energy recovery. The melting furnace slag quenching dry type granulation and sensible heat recovery generating system provided by the invention comprisesa slag-receiving device, a high-pressure water-air pulverization furnace slag quenching and granulating device, a furnace slag slow-cooling device, a sensible heat recovery generating device and a waste gas purification treatment device. According to the invention, the melting furnace slag is quenched and granulated by utilizing the high-pressure water-air pulverization device, so that the melting furnace slag is rapidly cooled to be in a glassy state; and then through a water-cooled type vibration grid plate, the melting furnace slag in the glassy state is further subjected to heat exchange with cold air so as to facilitate the subsequent recovery of the sensible heat. By the system provided by the invention, the melting furnace slag is quenched and granulated, and the high-temperature sensible heat resource of the furnace slag is sufficiently recovered and is used for generation on the basis that the water activity quality of the furnace slag is not influenced.
Owner:NANJING KESEN KENEN ENVIRONMENT & ENERGY
Sustained-release drug delivery compositions and methods
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
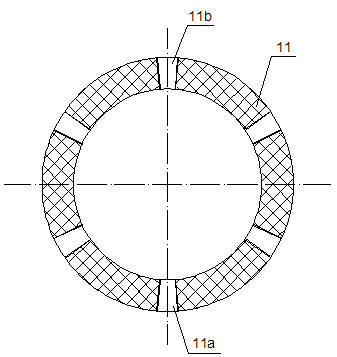
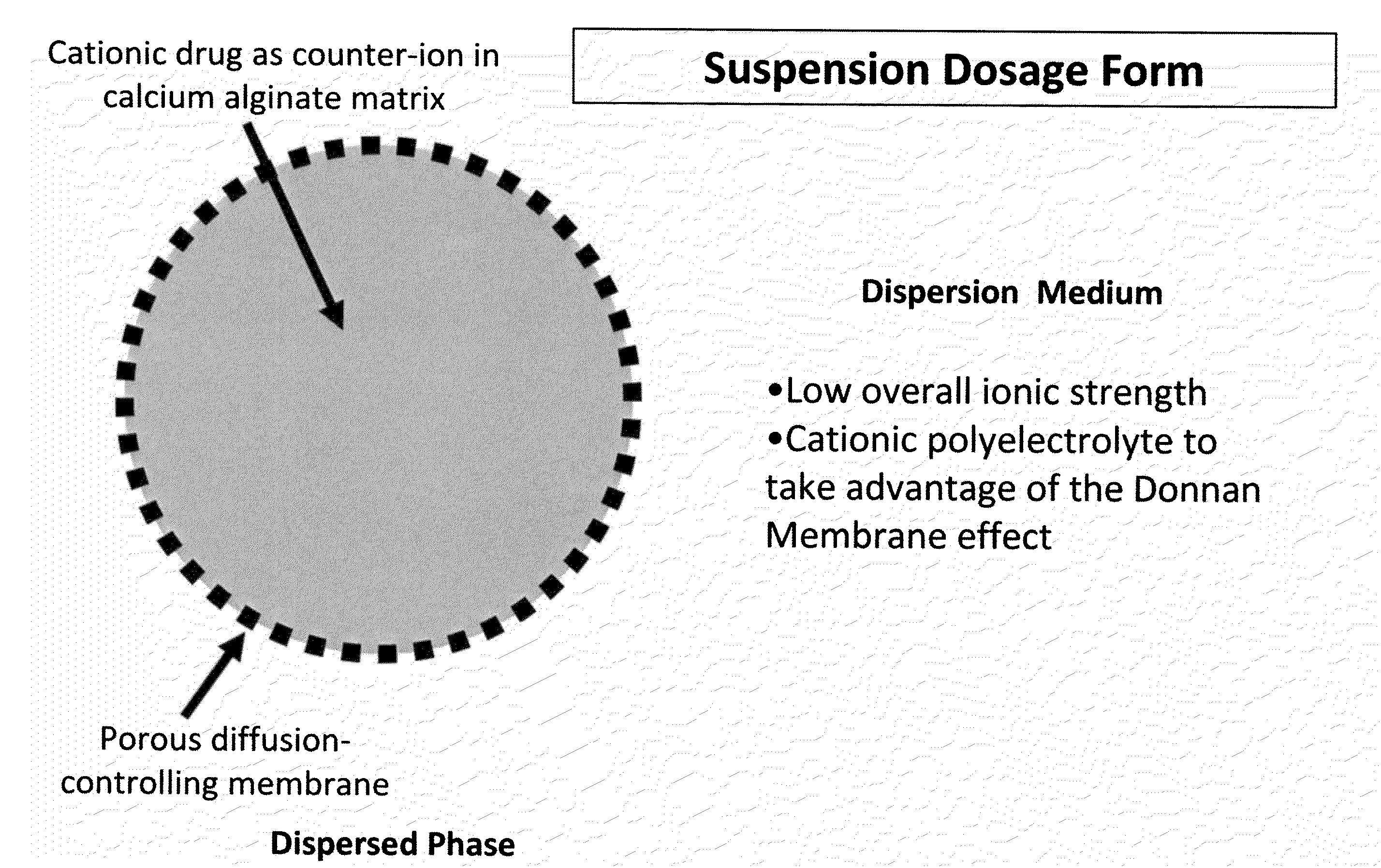
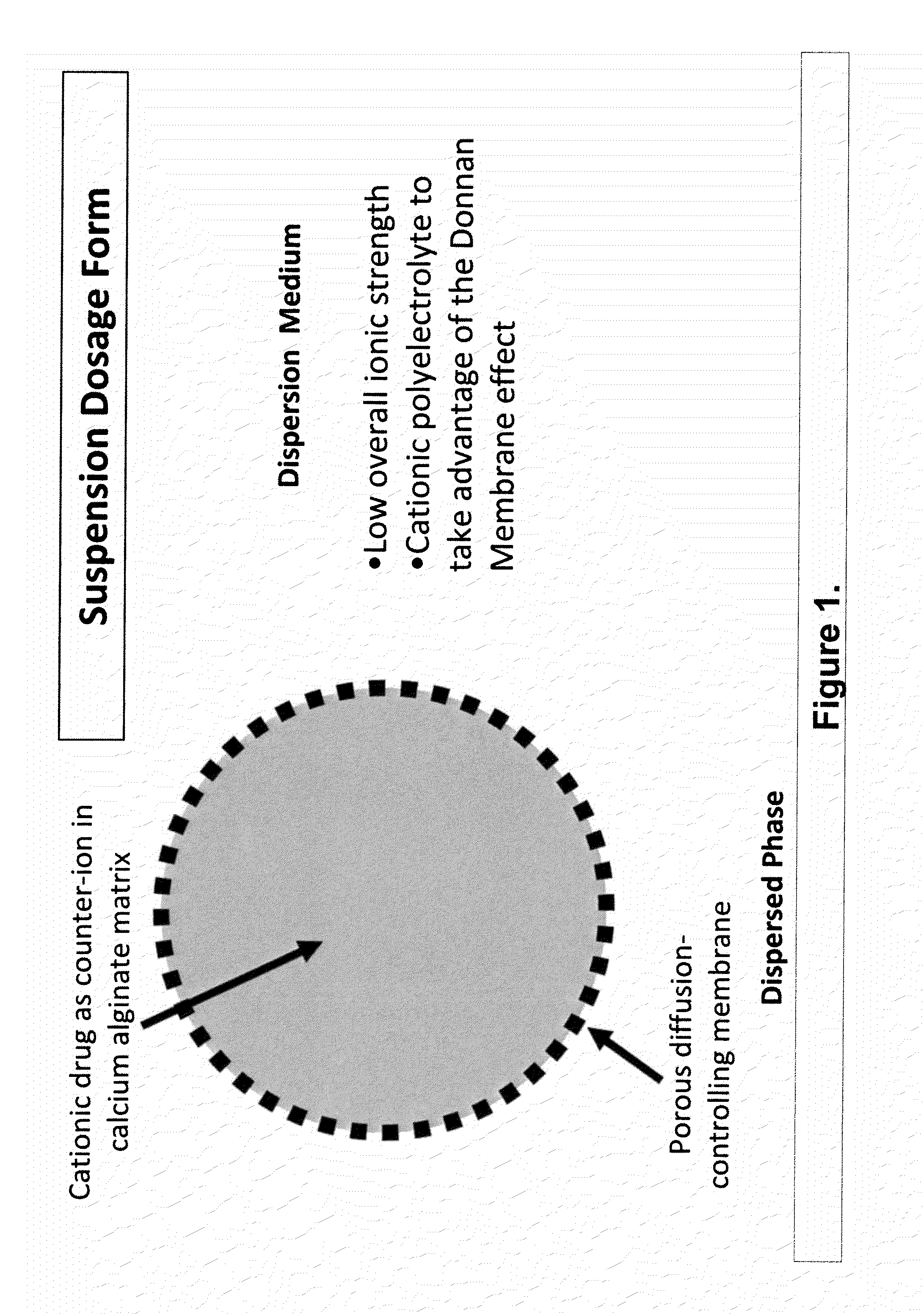
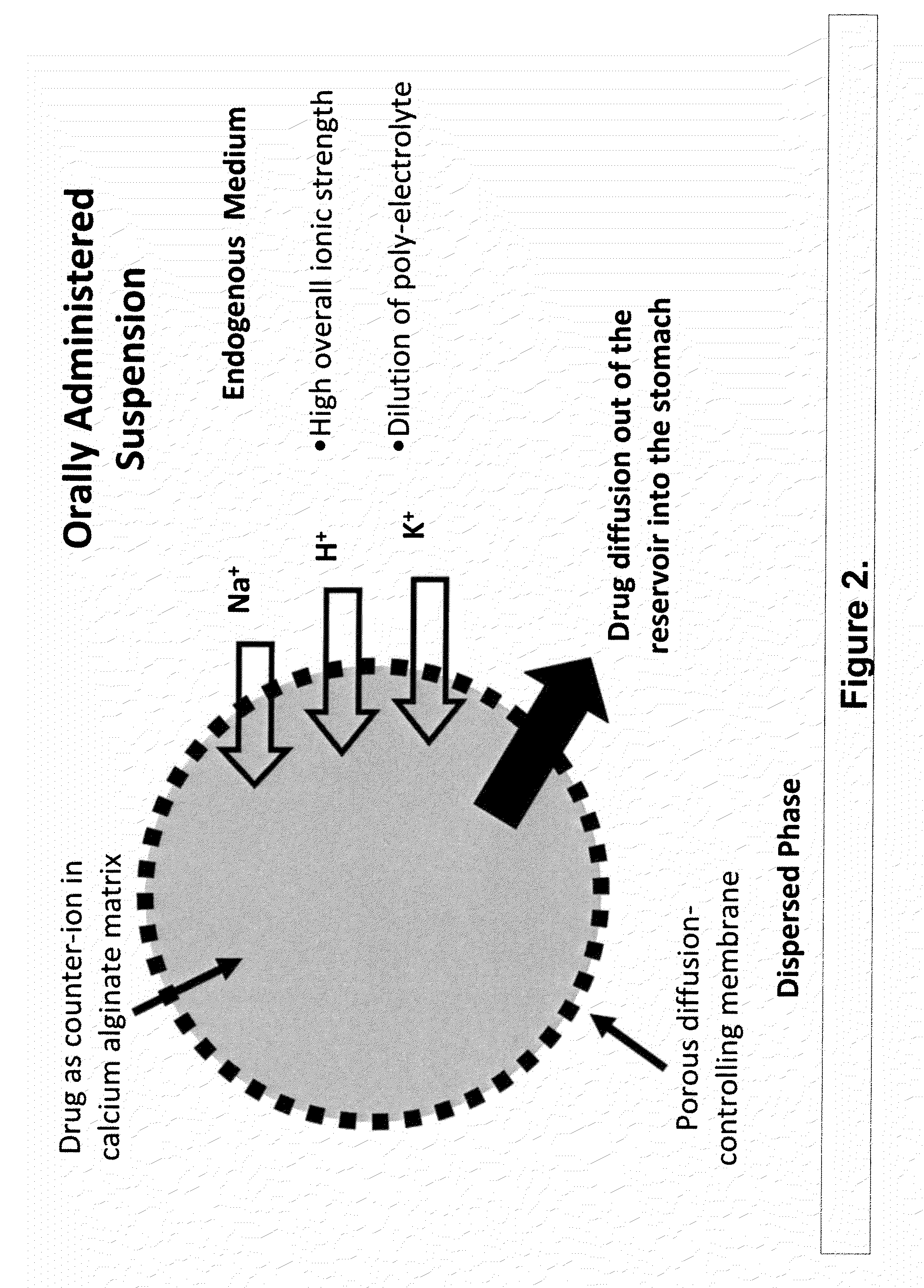
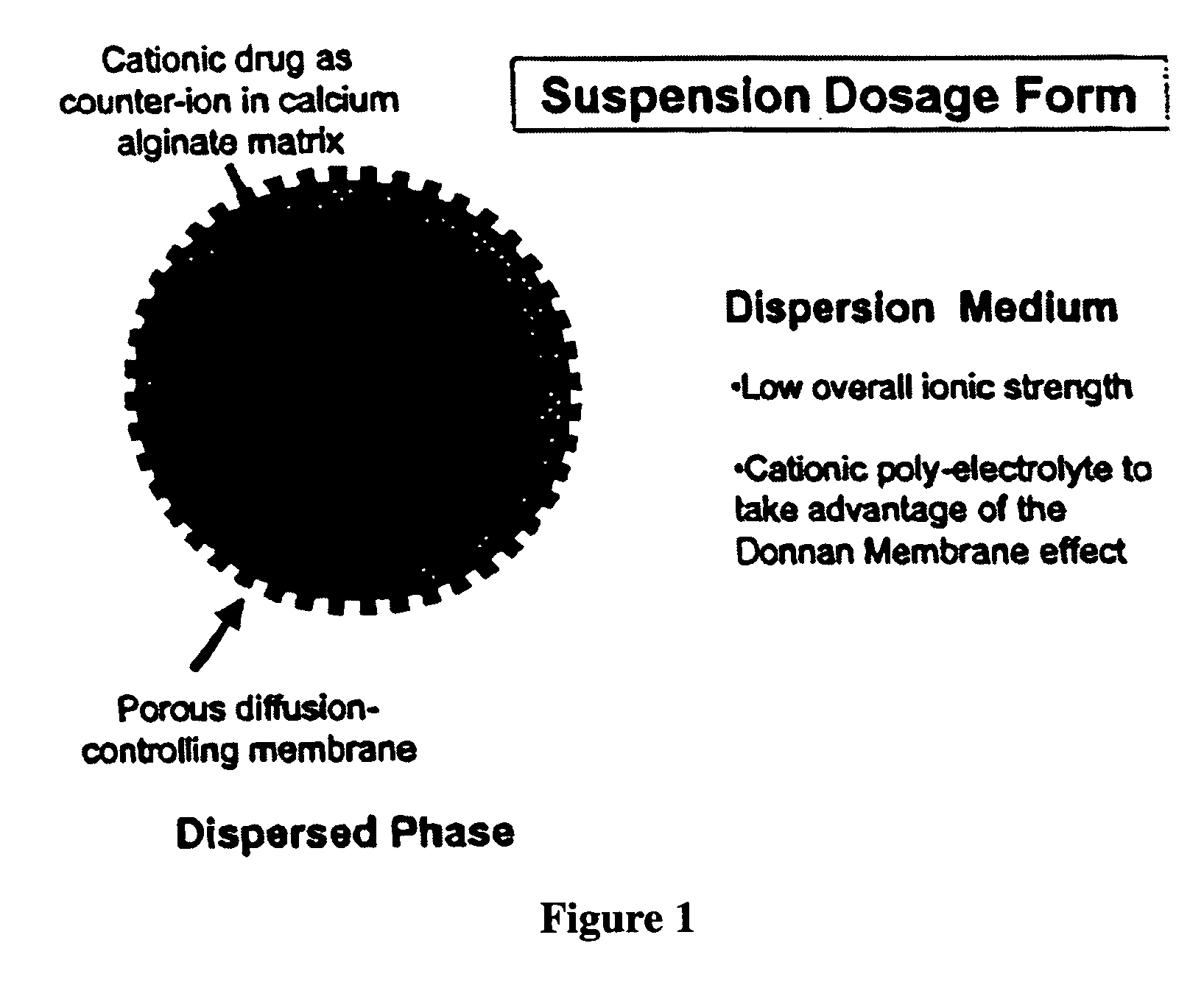
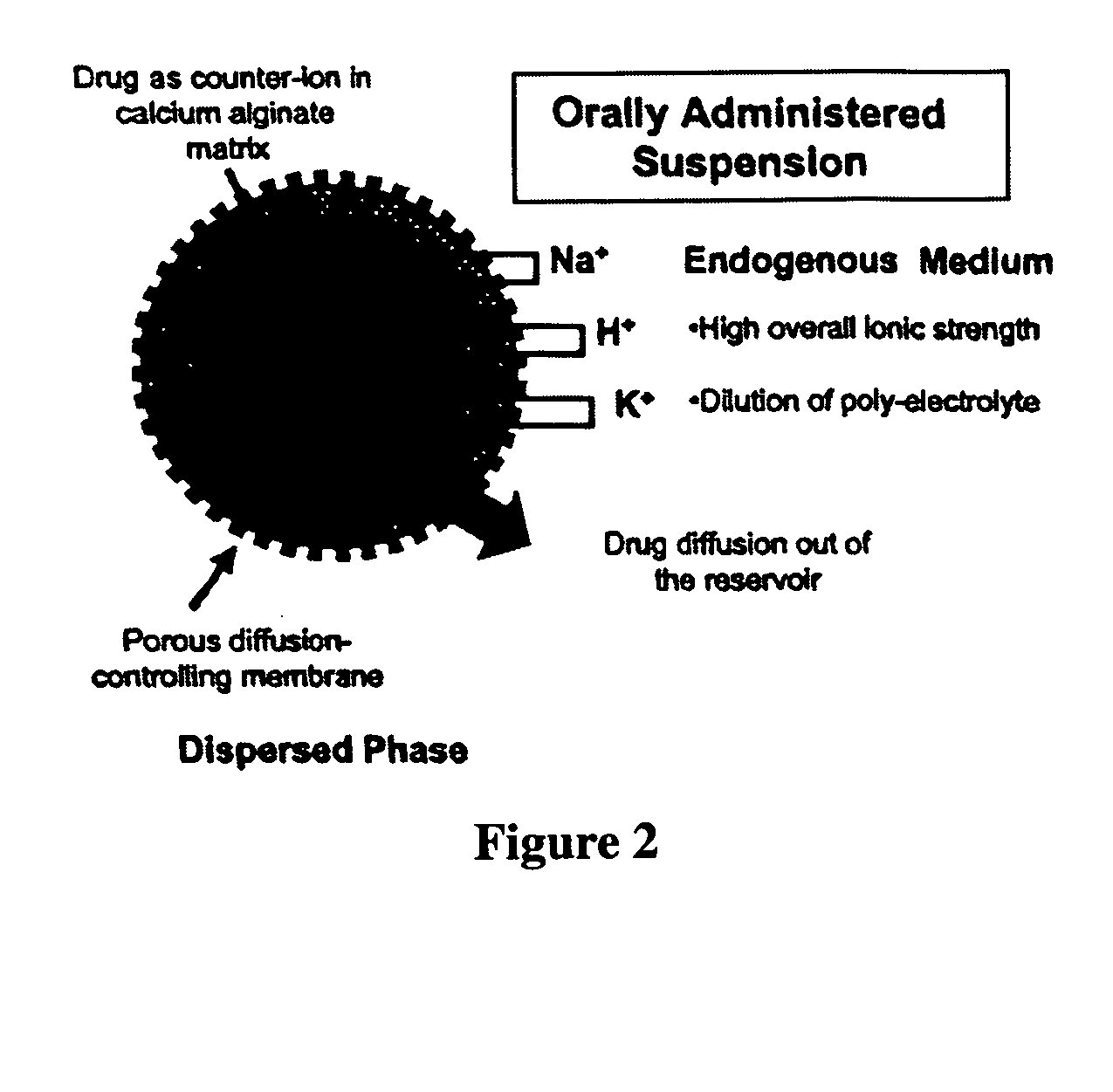
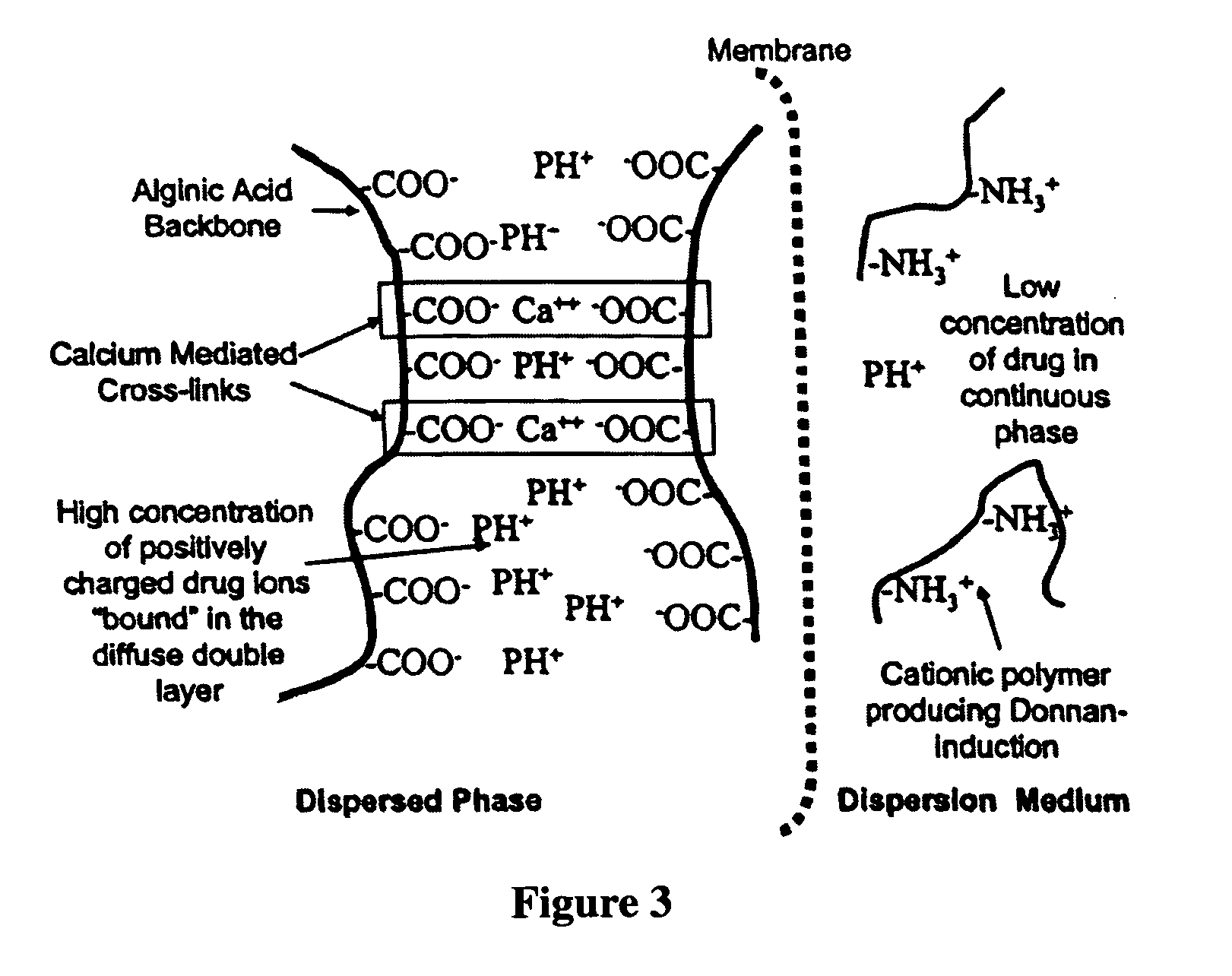
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
Method for delivering hot and cold beverages on demand in a variety of flavorings and nutritional additives
The invention relates to a method for delivering a flavored and / or nutritionally enhanced non-carbonated beverage on premise. The method comprises a step of providing at least one packaged source of a liquid premix base comprising at least one microbiologically, physically, enzymatically and / or chemically sensitive beverage component which is not solely carbohydrate, a water activity lowering component. The liquid premix based is formulated with a water activity and / or solid content effective to render it shelf stable at room temperature. The method comprises a step of separately providing a plurality of flowable additive packaged sources adapted to tailor the flavor, aroma, body and / or nutritional value of the beverage. The method comprises a step in which liquid premix base is mixed to hot or cold non-carbonated water to provide a beverage base which is dispensed to the cup and wherein at least one additive is delivered and mixed with the beverage base into the cup.
Owner:NESTEC SA
Aqueous sustained-release drug delivery system for highly water-soluble electrolytic drugs
InactiveUS20060134148A1Reduce molecular weightQuick releasePowder deliveryPharmaceutical non-active ingredientsElectrolysisIon exchange
Owner:HOLLENBECK R GARY
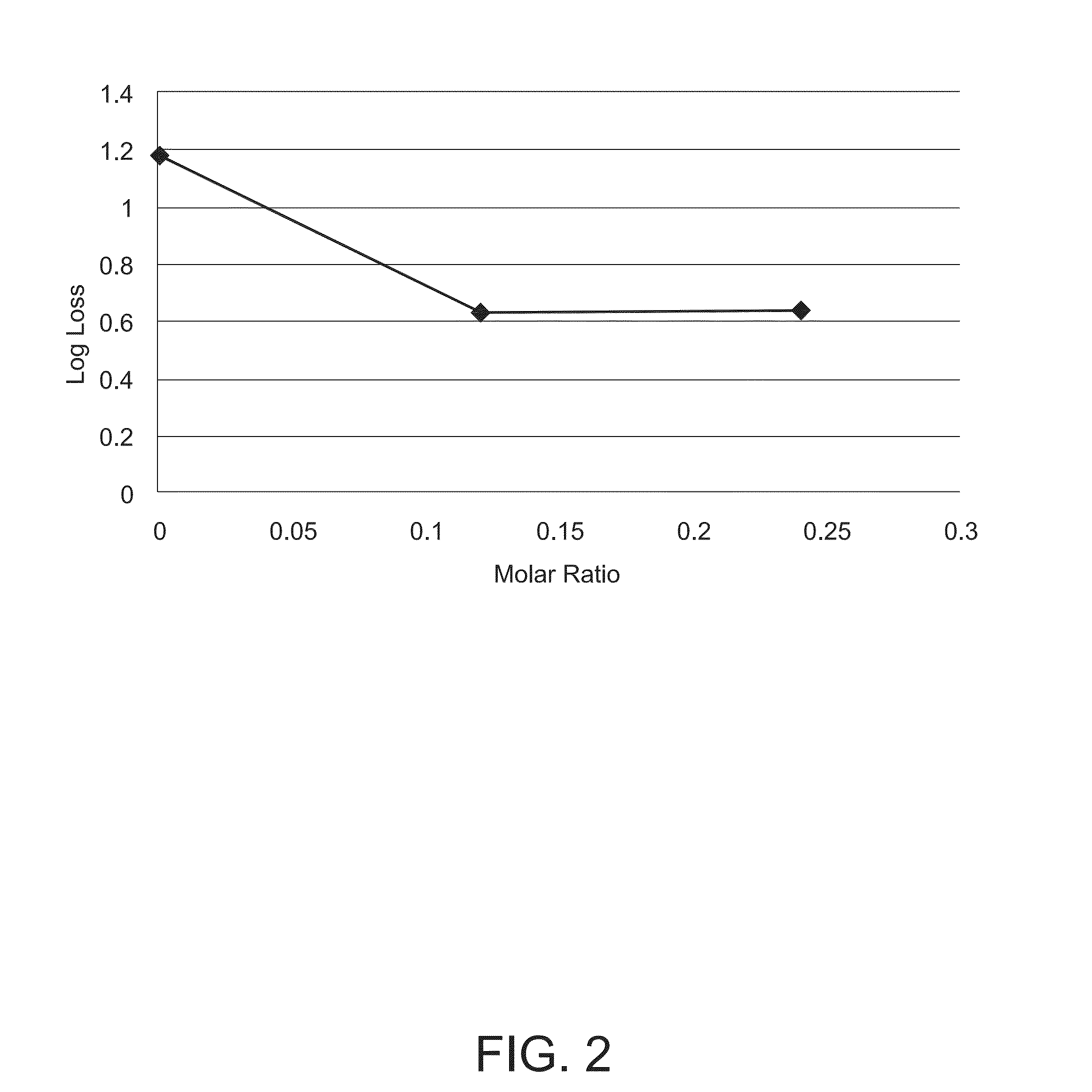
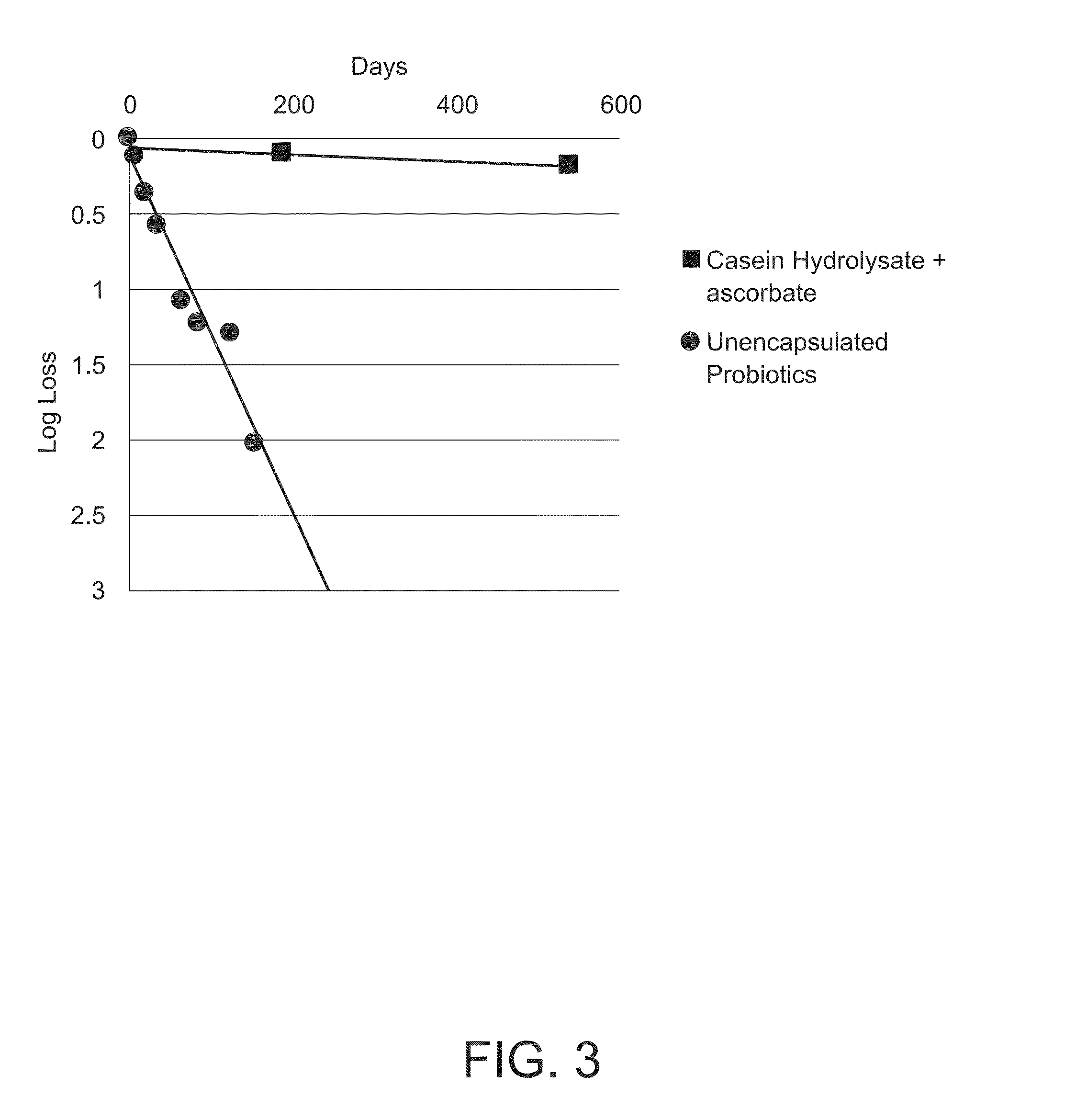
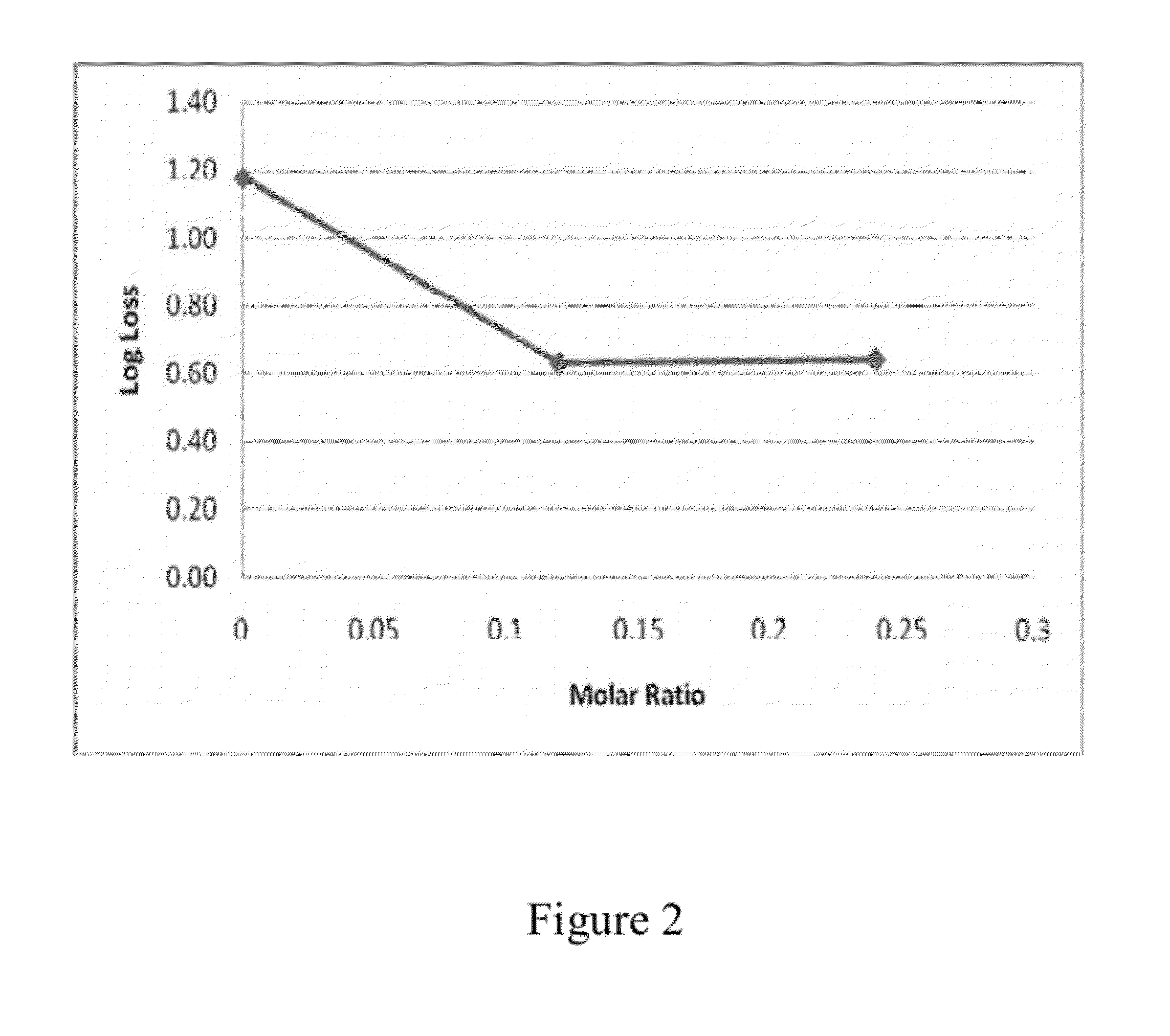
Stabilizing Composition for Biological Materials
Dry stabilizing compositions for bioactive materials include sugars and hydrolyzed proteins, and may be formed into tablets or other forms providing enhanced stability for the bioactive material. Compositions containing the bioactive materials may be produced by a method that includes (a) combining the bioactive material with other ingredients in an aqueous solvent to form a viscous slurry; (b) snap-freezing the slurry in liquid nitrogen to form solid frozen particles, beads, droplets or strings; (c) primary drying by water removal under vacuum of the product of step (b) while maintaining it at a temperature above its freezing temperature; and (d) secondary drying of the product of step (c) at maximum vacuum and a temperature of 20° C. or higher for a time sufficient to reduce the water activity to below 0.3 Aw.
Owner:ADVANCED BIONUTRITION CORP
Dried product and a drying process
InactiveUS6268012B1Minimize impactNot to damageFruits/vegetable preservation by dehydrationMeat/fish preservation by dryingWater activityFresh air
A dried fruit or vegetable has a water content in the range 4% to 7%, and has a water activity of 0.4. Substantially all of the cells of the dried product are undamaged. An air drying process is gentle and contains four phases, during which the temperature of the drying air is maintained at 60° C. In a first phase the relative humidity of the drying air is allowed to rise to between 50% and 55%, and is maintained substantially constant at this value during a second phase by maintaining exchange of the drying air with fresh air substantially constant. In a third phase of the process, the relative humidity of the drying medium is permitted to decrease relatively rapidly until the fourth phase commences, at which stage the relative humidity is permitted to asymptotically approach a predetermined relative humidity value. During the drying process, excessive temperature differences and relative humidity differences between the temperature and relative humidity, respectively of the drying medium and the product are avoided in order to minimize damage to the cellular structure of the product.
Owner:DTL
Electro-powder
A method and a process are disclosed for preparation of medical electro-powders. The electro-powder results from preparations of chemical and biological substances to form electro-powders suitable for electrostatic charging and dosing for functionality in a dry powder inhaler device. The electro-powder resulting from the method and process forms an active powder substance or a dry powder medical formulation with a fine particle fraction representing of the order 50% or more of the content having a size ranging between 0.5-5 mum and provides electrostatic properties with an absolute specific charge per mass after charging of the order 0.1x10<-6 >to 25x10<-6 >C / g and presenting a charge decay rate constant Q50>0.1 sec with a tap density of less than 0.8 g / ml and a water activity aw of less than 0.5. In the processing the active substance is a generally pharmaceutical active chemical or biological substance, for instance a polyeptide or any other corresponding substance selected alone or mixed or blended together with one or more excipients being a compound to improve electrostatic properties of the medical dry powder substance or dry powder medical formulation. Further the electro-powder may even be formed as a micro-encapsulation by coating micronized powder with the excipient in such a way that the active substance is capsulated, whereby the powder electrostatic properties mainly comes from the excipient.
Owner:MEDERIO AG
Food products containing whole chia seed or a gluten-free agglutinant derived therefrom and methods of making same
Food products containing whole chia seeds or a gluten-free agglutinant derived therefrom are made by mixing a food material with water, adding whole chia seeds or an agglutinant derived therefrom in an agglutinating amount, and reducing the water activity of the mixture. Other ingredients such as honey, syrups, and sprouted grains can also be mixed with the chia seeds. The gluten free varieties are of especial value for those individuals who are allergic to the gluten in wheat and other grains.
Owner:FITZPATRICK MICHAEL
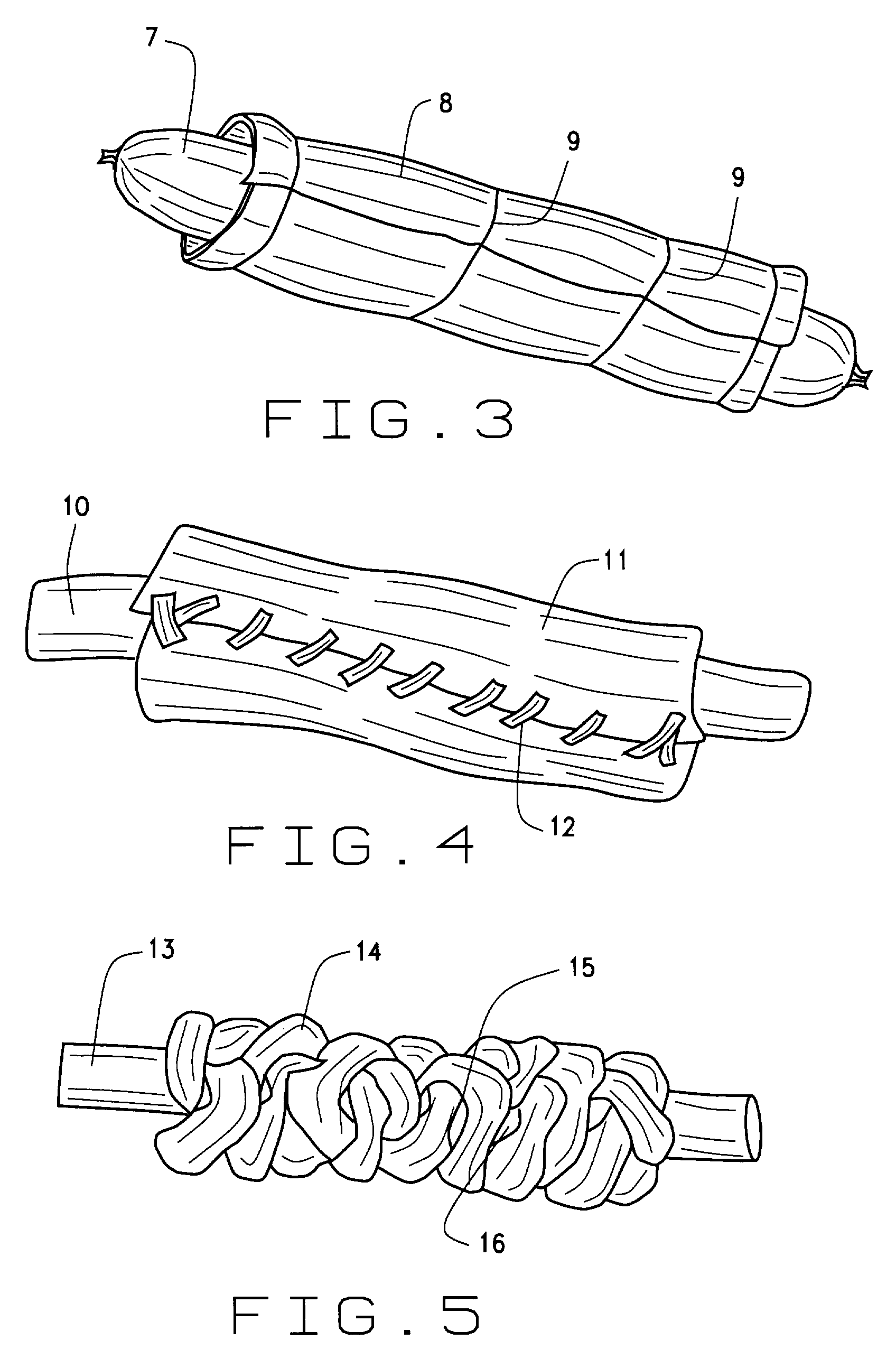
Foodstuff containing a moist meaty filling
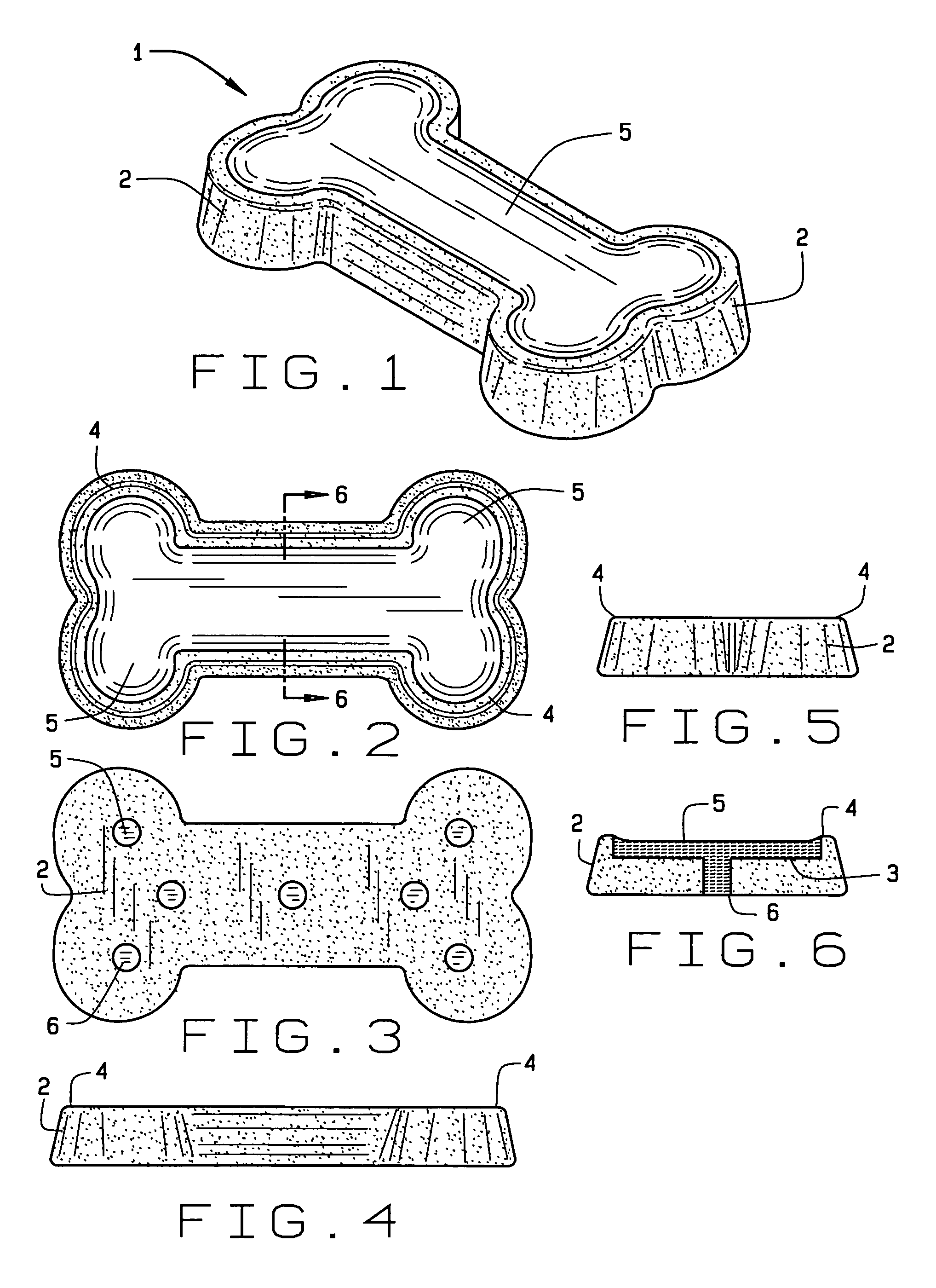
ActiveUS7485330B2Improve palatabilityEnhanced edgeMeat/fish preservationBaking mixturesBrixWater activity
A biscuit treat for dogs has improved palatability by incorporating a highly palatable shelf stable meaty filling into a cavity formed upon the top surface of the biscuit. The meaty filling includes agar as a gelling agent in combination with sufficient soluble solids to yield a high Brix number and reduced water activity. Precise quantities of agar and of a soluble solids concentration exceeding 65% eliminate capillary transfer of moisture from the highly aqueous meaty filling to the low moisture dry biscuit. The resulting highly palatable two phase biscuit has a long shelf life suitable for commercialization in the pet treat market.
Owner:REDBARN PET PRODS
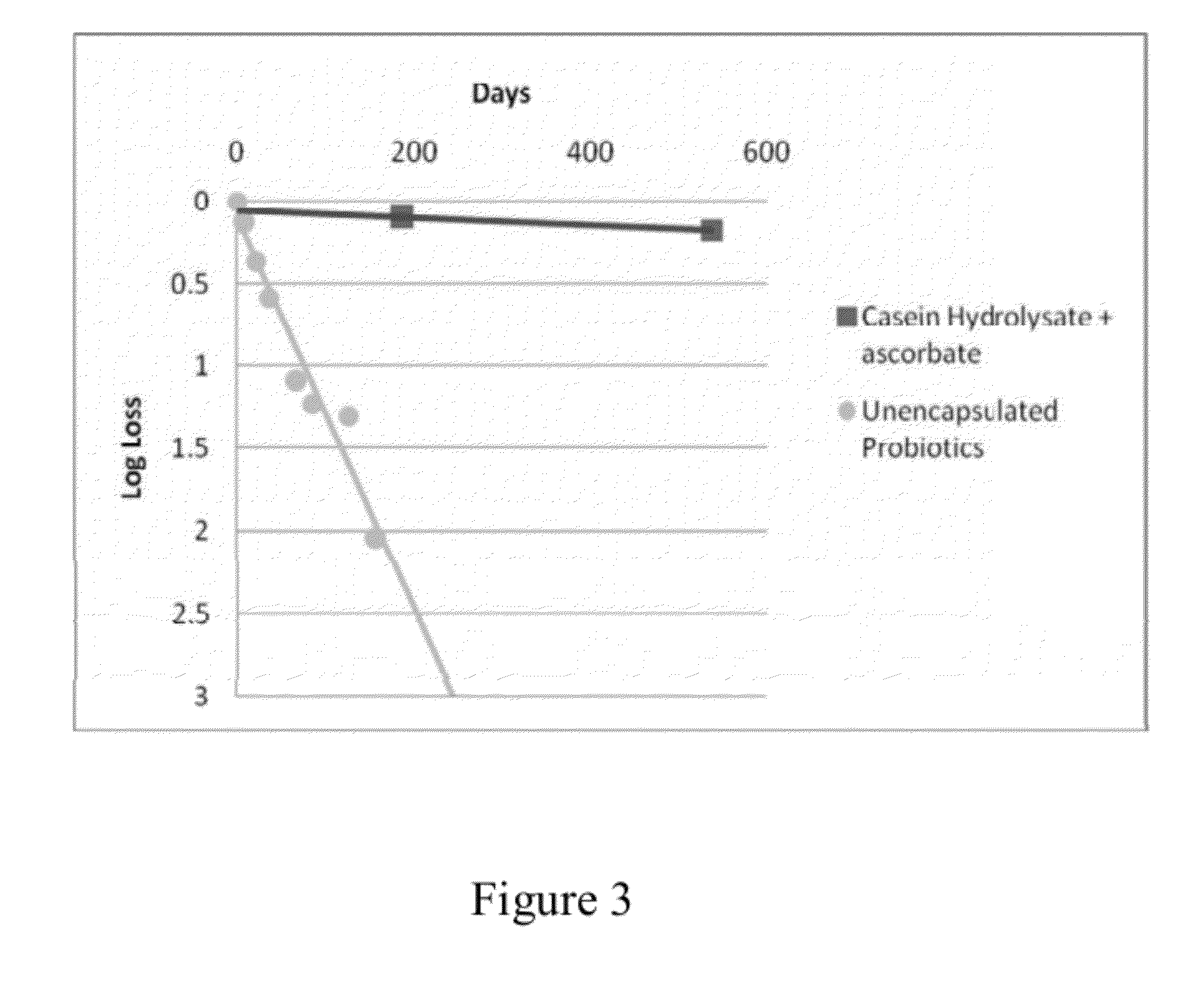
Dry storage stabilizing composition for biological materials
ActiveUS20120039956A1Improve biostabilityBenefit of stabilizingPowder deliveryVirusesBiotechnologyBiological materials
The present invention includes compositions and drying methods for preserving sensitive bioactive materials, such as peptides, proteins, hormones, nucleic acids, antibodies, drugs vaccines, yeast, bacteria (probiotic or otherwise), viruses and / or cell suspensions, in storage. The compositions include a carbohydrates component and a glass enhancer component, wherein the carbohydrate component includes a mixture of di-, oligo- and polysaccharides and the glass enhancer includes ions of organic acid and protein hydrolysates. The composition is prepared by dispersing all the solid components in a solution and then snap-frozen to form small beads, strings or droplets. The preferred drying method of the frozen beads, strings or droplets is initiated by a short purging and structure stabilizing step of the frozen particles under a vacuum pressure of less than <2000 mTORR followed by a primary drying step under vacuum pressure of more than >2000 mTORR and at a desired temperature. During the secondary and final drying step of the material a full vacuum pressure and elevated temperature are applied, to achieve a final desirable water activity of the dry material.
Owner:ADVANCED BIONUTRITION CORP
Granule with hydrated barrier material
InactiveUS20080206830A1Moderate and high water activityOrganic detergent compounding agentsHydrolasesWater activityChemistry
A granule having high stability and low dust is described. The granule includes a hydrated barrier material having moderate or high water activity. Also described are methods of producing the granules.
Owner:GENENCOR INT INC
Flavored yogurt products and methods of making same
InactiveUS20060068075A1Reduced activityNot adversely microbial stabilityMilk preparationOther dairy technologyWater activityLactic acid fermentation
The invention provides fermented dairy products composed of fermented dairy base containing active cultures and a low water activity sweet brown base component admixed within the fermented dairy base. Further, the invention provides methods for preparing fermented dairy products including steps of fermenting a dairy base by lactic fermentation to a pH of 4.7 to 5.3 to provide a fermented dairy base; cooling the fermented dairy base; admixing a sweet brown base component with the fermented dairy base to form a sweet brown flavored fermented dairy product; and packaging the sweet brown flavored fermented dairy product. Methods of formulating yogurt compositions are also described.
Owner:GENERAL MILLS INC
Cultures Encapsulated With Compound Fat Breakfast Cereals Coated With Compound Fat and Methods of Preparation
InactiveUS20080305210A1Improve the level ofSufficient amountMilk preparationEdible oils/fats ingredientsWater activityDrying
Food products are provided comprising a food base and the compound fat encapsulated pro-biotic as a coating or portion or phase of the food product. The food base can include the compound fat encapsulated pro-biotic as a topical coating or phase or portion. The food base or foodstuff is dried and has a water activity ranging from about 0.1 to about 0.35. The weight ratio of food base to compound fat encapsulated pro-biotic ranges from about 100:1 to about 100:400. The pieces of the coated food base can be admixed with pieces of uncoated dried food base of the same or different composition to provide desired levels of pro-biotic fortification
Owner:GENERAL MILLS INC
Systems and methods of interaction with water usage information
ActiveUS8618941B2Electric signal transmission systemsUtility meters data arrangementsWater activityHuman–computer interaction
Exemplary systems and methods for interaction with water usage information are provided. In various embodiments, a method comprises receiving water usage data from a meter device, receiving an identifier from a user associated with the meter device, providing an interactive interface to the user, the interactive interface conveying at least some water usage information based on the water usage data, receiving a first characterization of a first water activity from the user, generating a visualization based on the water usage information and the first characterization of the first water activity, and displaying the visualization.
Owner:BADGER METER
Stable dry powder composition comprising biologically active microorganisms and/or bioactive materials and methods of making
InactiveUS20120135017A1Faster primary dryingImprove stabilityPowder deliveryFungiMicroorganismSolid component
The present invention relates to embedding live or dead microorganisms and / or bioactive materials in a protective dry formulation matrix, wherein the formulation includes the bioactive microorganism or material, a formulation stabilizer agent, and a protective agent. The formulation is prepared by dispersing all the solid components in a solution, with or without a vacuum, and cooling the solution to a temperature above its freezing temperature. The methods include a primary drying step of the formulation at a desired temperature and time period, and an accelerated secondary drying step under maximum vacuum and elevated temperature, to achieve a final desirable water activity of the dry material.
Owner:ADVANCED BIONUTRITION CORP
Damp cleansing wipe
A disposable substantially damp cleansing article is disclosed having a cleansing composition impregnated onto a flexible substrate such as a non-woven cloth. The impregnated compositions include lathering surfactants and water, and a water-binding agent resulting in a composition having a water activity less than 0.977 but no lower than 0.001. Amounts of water range from greater than 15% to no higher than about 40% by weight of the total article. Speed of lather formation and foam volume increases within the window of the stated water activity and water range.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Snack having a soft edible layer and method of making
The present invention is directed to a method of making a shelf stable edible snack. The method comprises the steps of: (A) providing an edible core having an outside surface; and (B) applying at least one soft edible layer that substantially covers the outside surface of the edible core; wherein the outer layer is applied by a method comprising the steps of: (a) applying a base liquid onto the outside surface of the edible core, thereby forming a liquid coated component; (b) applying a dry powder to the liquid coated component, thereby forming an edible layered component; and (c) optionally, (i) drying the liquid coated core after step (a), (ii) drying the edible layered component after step (b), or (iii) drying the liquid coated core after step (a) and drying the edible layered component after step (b); wherein the soft edible layer has a water activity of about 0.2 to about 0.8 at 25° C.
Owner:MARS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com