Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
288 results about "Antihistamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antihistamines are drugs which treat allergic rhinitis and other allergies. Typically people take antihistamines as an inexpensive, generic, over-the-counter drug that can provide relief from nasal congestion, sneezing, or hives caused by pollen, dust mites, or animal allergy with few side effects. Antihistamines are usually for short-term treatment. Chronic allergies increase the risk of health problems which antihistamines might not treat, including asthma, sinusitis, and lower respiratory tract infection. Consultation of a medical professional is recommended for those who intend to take antihistamines for longer-term use.
Dosage form containing multiple drugs
A pharmaceutical dosage form comprising a first drug and a second drug, both of which are selected from decongestants, antitussives, expectorants, analgesics and antihistamines. The dosage form provides a plasma concentration within a therapeutic range of the second drug over a period which is coextensive with at least about 70% of a period over which the dosage form provides a plasma concentration within a therapeutic range of the first drug. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Nanoparticulate corticosteroid and antihistamine formulations
InactiveUS20060216353A1Less liver toxicityUseful in prophylaxis and chronic treatment of asthmaBiocidePowder deliveryPediatric patientMicroparticle
Compositions comprising a nanoparticulate corticosteroid and an antihistamine are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining an antihistamine with a nanoparticulate corticosteroid in a single formulation results in improved efficacy.
Owner:ALKERMES PHARMA IRELAND LTD
Methods and compositions for improved non-viral gene therapy
Methods to prevent or reduce inflammation secondary to administration of a lipid-nucleic acid complex in a subject, that include administering to the subject a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunsuppressive agent with the lipid-nucleic acid complex are disclosed. Also disclosed are methods of screening for inhibitors of the inflammatory response associated with administration of a lipid-nucleic acid complex to a subject, including providing a candidate substance suspected of preventing or inhibiting the inflammation associated with administration of a lipid-nucleic acid complex to the subject. Also disclosed are compositions that include a lipid, a nucleic acid, and a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunosuppressive agent.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
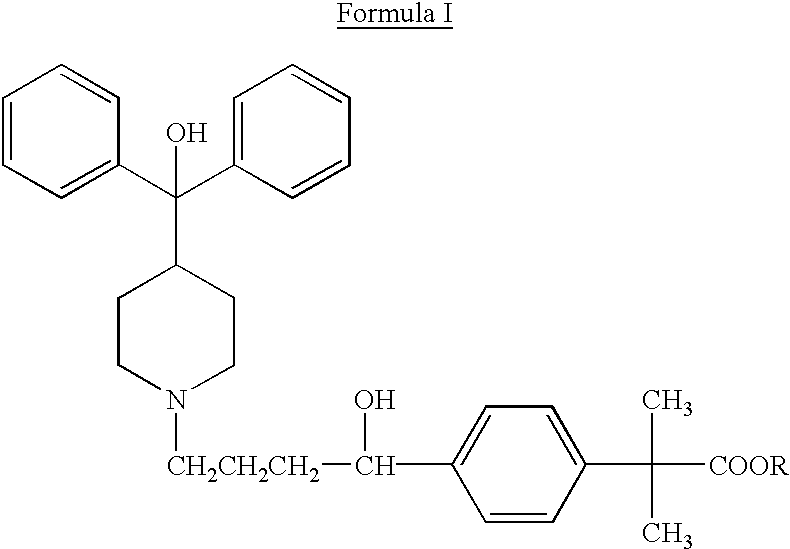
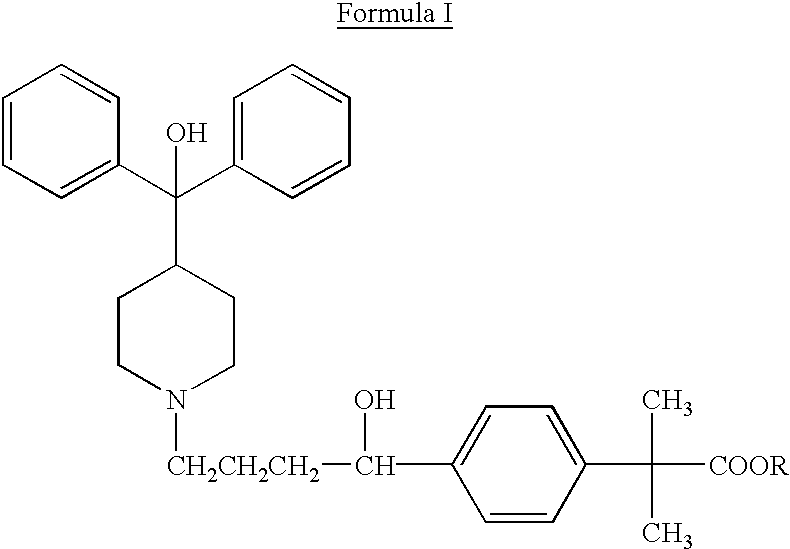
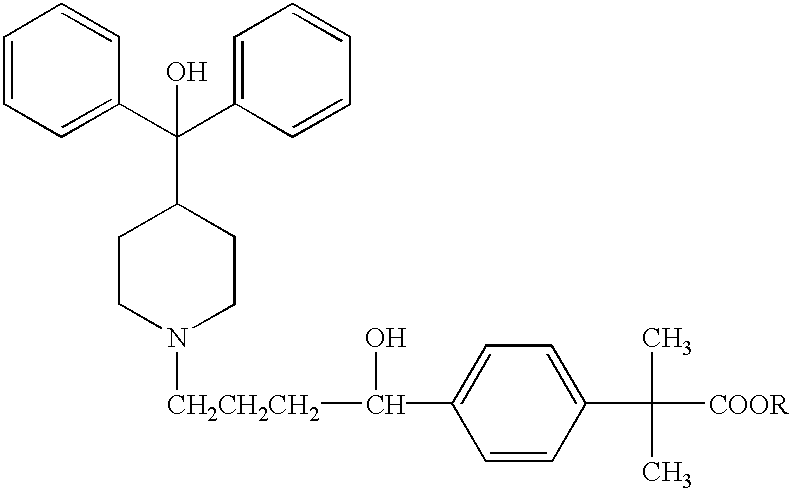
Method of enhancing bioavailability of fexofenadine and its derivatives
The present invention relates to a method of enhancing the bioavailability of a piperidinoalkanol antihistamine in a patient which comprises co-administering to said patient an effective antihistaminic amount of said piperidinoalkanol and an effective p-glycoprotein inhibiting amount of a p-glycoprotein inhibitor.
Owner:AVENTISUB II INC +1
Solution forms of cyclodextrins for nasal or throat delivery of essential oils
This invention further relates to a method for preventing or treating diseases or conditions of the oral cavity, throat or nose of warm-blooded animals including humans. More particularly, the invention pertains to a composition and method for spraying essential oils to the oral cavity, throat or nasal mucosa as cyclodextrin inclusion complexes. The spray composition includes a cyclodextrin in an amount of from about 0.1% w / v to about 20% w / v; at least one essential oil in an amount of from about 0.001% w / v to about 5.0% w / v; an effective amount of an antimicrobial preservative composition; and water. The composition may further comprise an alcohol co-solvent, a thickening agent, a sweetener, an antitussive, an anticholinergic, a decongestant, an antihistamine, an astringent, an anti-inflammatory steroid composition, a vitamin, a respiratory stimulant, a mucolytic agent, a bronchodilator, a beta-antagonist, an antidiarrheal agent, or combinations thereof.
Owner:QPHARMA
Sequential release pharmaceutical formulations
InactiveUS20070141147A1Efficient coordinationBiocideHydroxy compound active ingredientsControlled releaseImmediate release
A mixed-release tablet or capsule formulation including vehicles for the delivery of a plurality of drugs in various combinations of immediate release, extended release, and / or delayed release modes over a predetermined time period have been developed, which provide for controlled release not just of the drugs, but controlled release that is designed to create more effective coordination between the drugs being delivered. The drugs can be any medically and / or physiologically appropriate combination of drugs and active ingredients, preferably decongestant drugs, antihistamines, expectorants, antitussives, cough suppressants, and drying agents.
Owner:AURIGA LAB
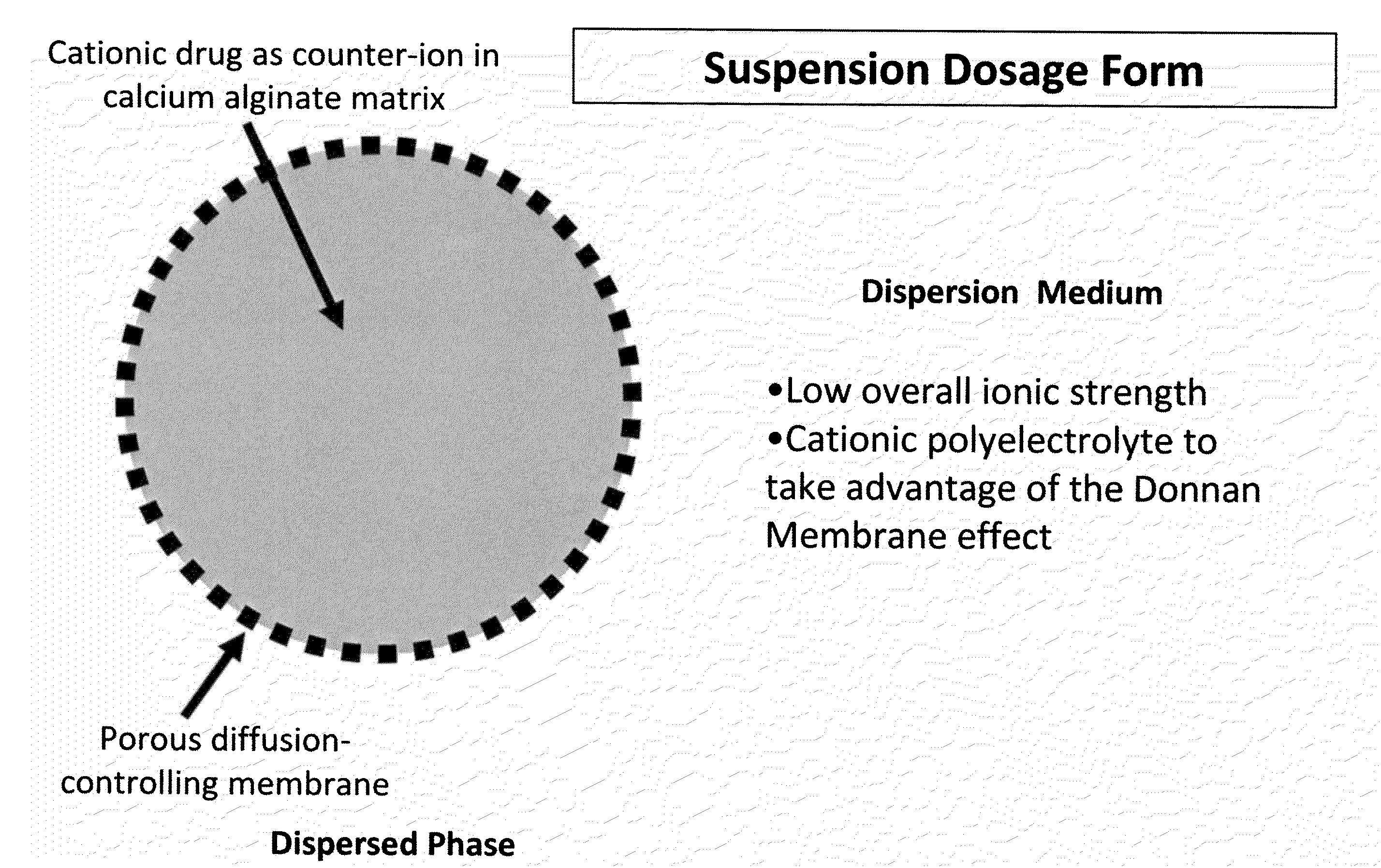
Sustained-release drug delivery compositions and methods
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
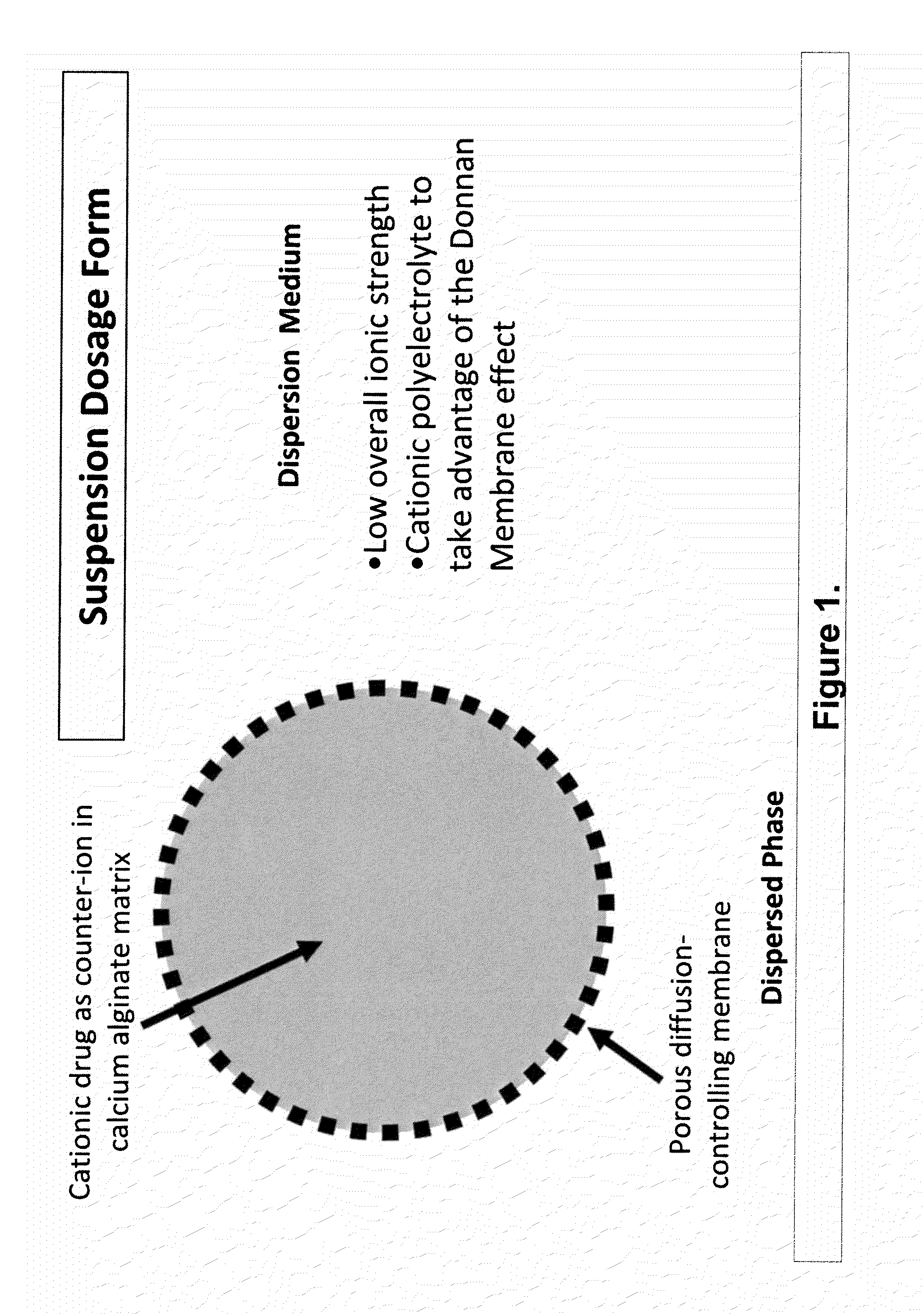
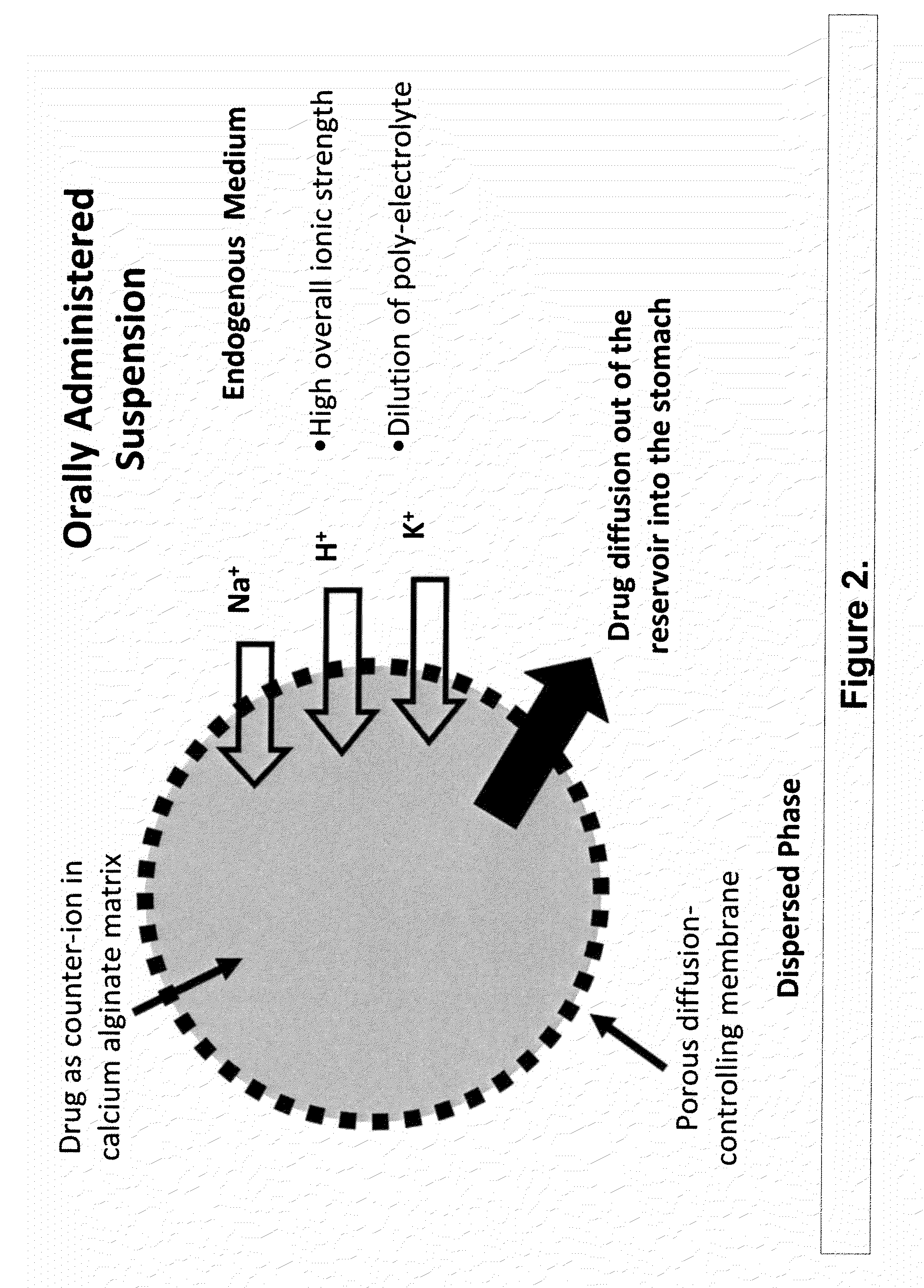
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
Methods of treating sleep disorders
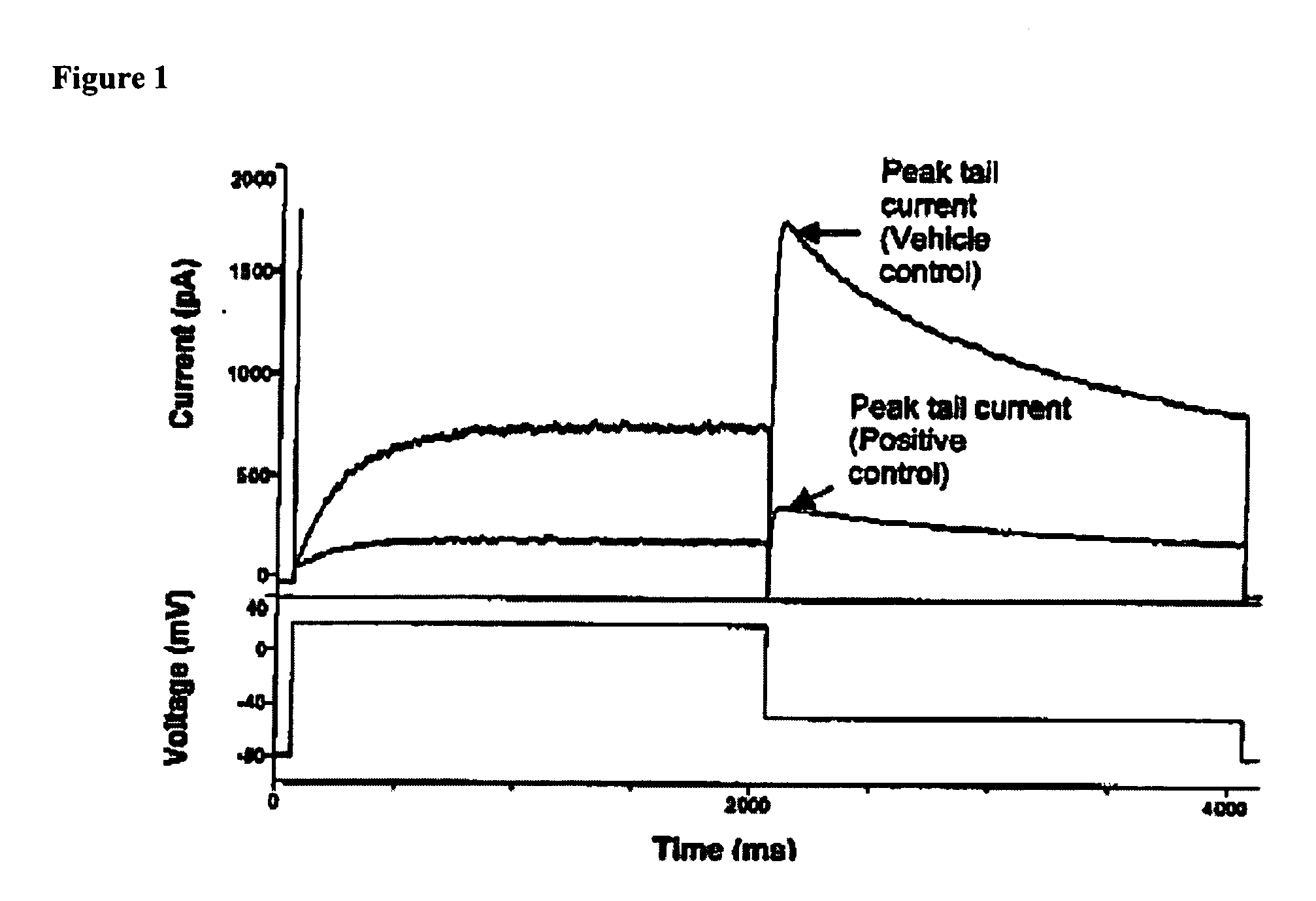
InactiveUS20050239838A1Shorten time to sleepIncrease the lengthBiocideOrganic chemistryDoxepin+MetabolitesSleep disorder
Owner:HYPNION INC
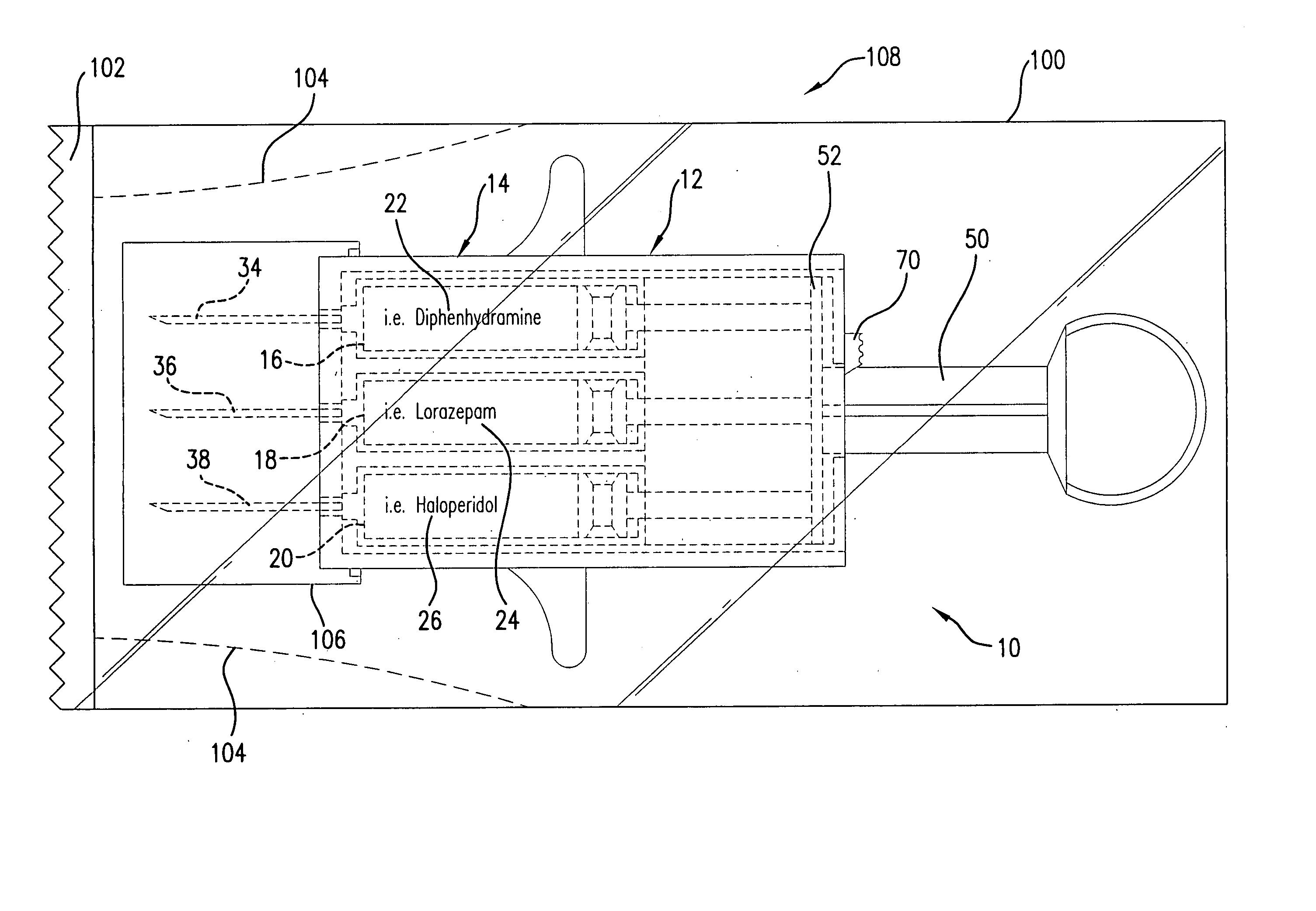
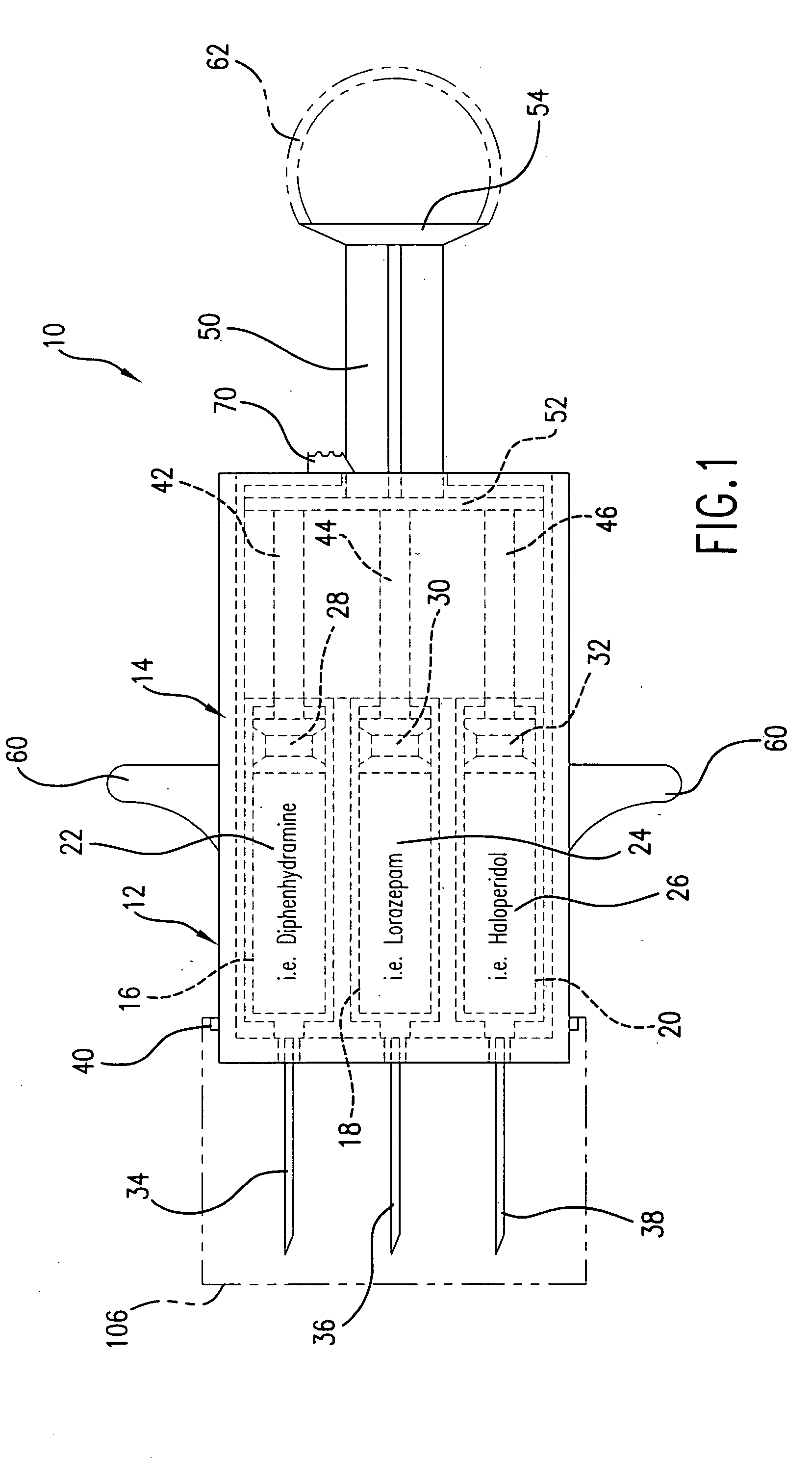
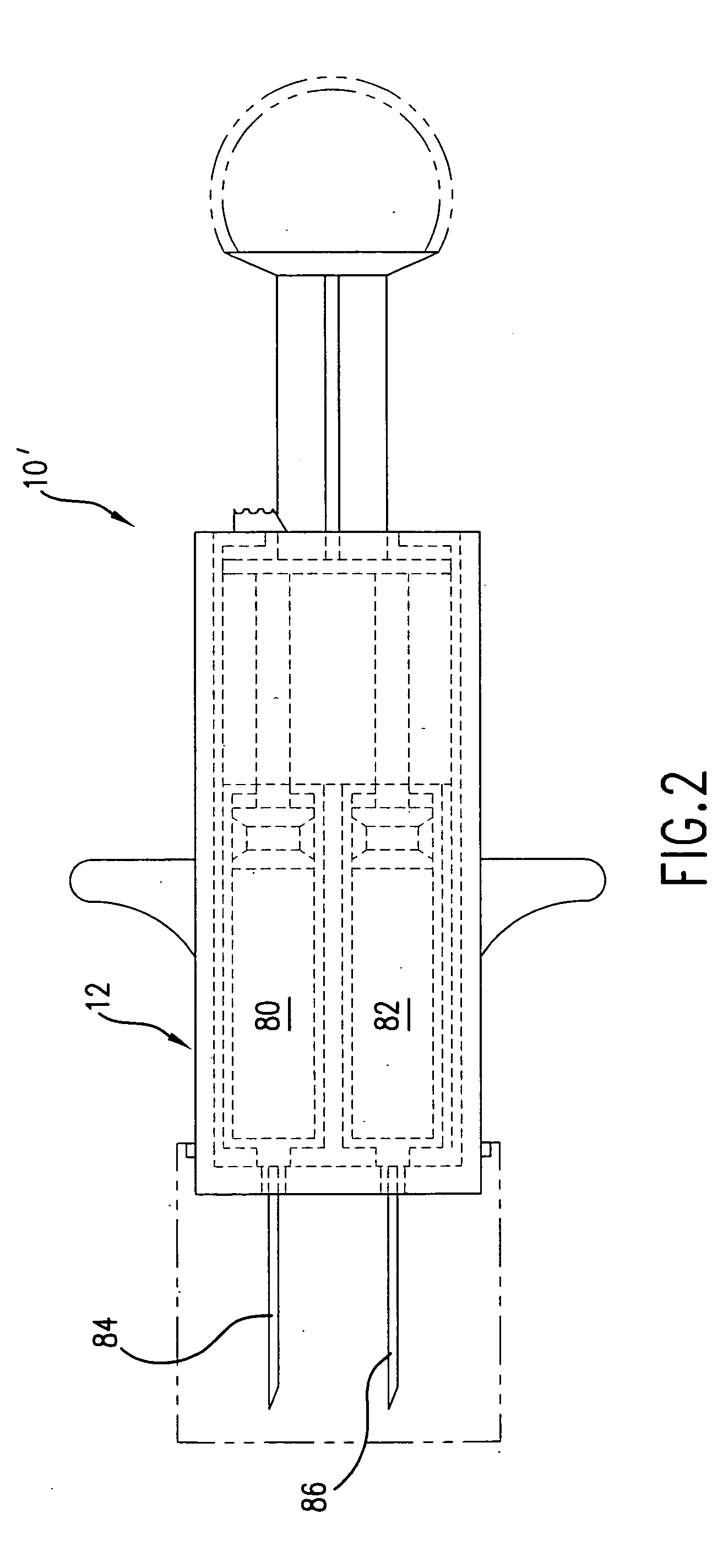
Hypodermic syringes with multiple needles and methods of calming psychiatric patients using such
Hypodermic syringes with multiple needles are used to practice a method of calming psychiatric patients. In accordance with one embodiment of the invention generally used to calm violent adult patients, the syringe has a first barrel containing an antipsychotic, a second barrel containing a sedating antihistamine and a third barrel containing an antianxiety sedative. Each barrel also has a separate projecting needle and contains a piston. A common operator, preferably in the form of a plunger simultaneously pushes all of the pistons so that the patient receives three injections simultaneously. For children, a second embodiment of the syringe includes two barrels instead of three, each barrel containing a separate medication. In each embodiment the syringe is packaged in a manually openable plastic envelope with a safety cap over the needles.
Owner:WILLIAMS ALTON
Compositions containing both sedative and non-sedative antihistamines
InactiveUS6827946B2Improve usabilityIncrease ratingsPowder deliveryGranular deliverySedating AntihistaminesHistamine
Compositions comprising both a sedative and a non-sedative antihistamine are disclosed as well as methods of inhibiting the release of histamines by administration of the compositions to a mammalian subject.
Owner:COLLEGIUM PHARMA INC
Use of an Inhibitor of TNFa Plus an Antihistamine to Treat Allergic Rhinitis and Allergic Conjunctivitis
InactiveUS20080254029A1Immediate and long-term reliefOvercomes drawbackBiocideSenses disorderNonallergic rhinitisAllergic conjunctivitis
Disclosed are methods of treating allergic conjunctivitis and allergic rhinitis in a subject that involve topically administering to the subject a composition comprising a pharmaceutically effective amount of an H1 antagonist and an anti-TNFα compound.
Owner:ALCON RES LTD
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833550B2Reduce morbidityNot impart bitter tastePowder deliverySenses disorderNasal cavityAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
Multi medication nasal spray device and method
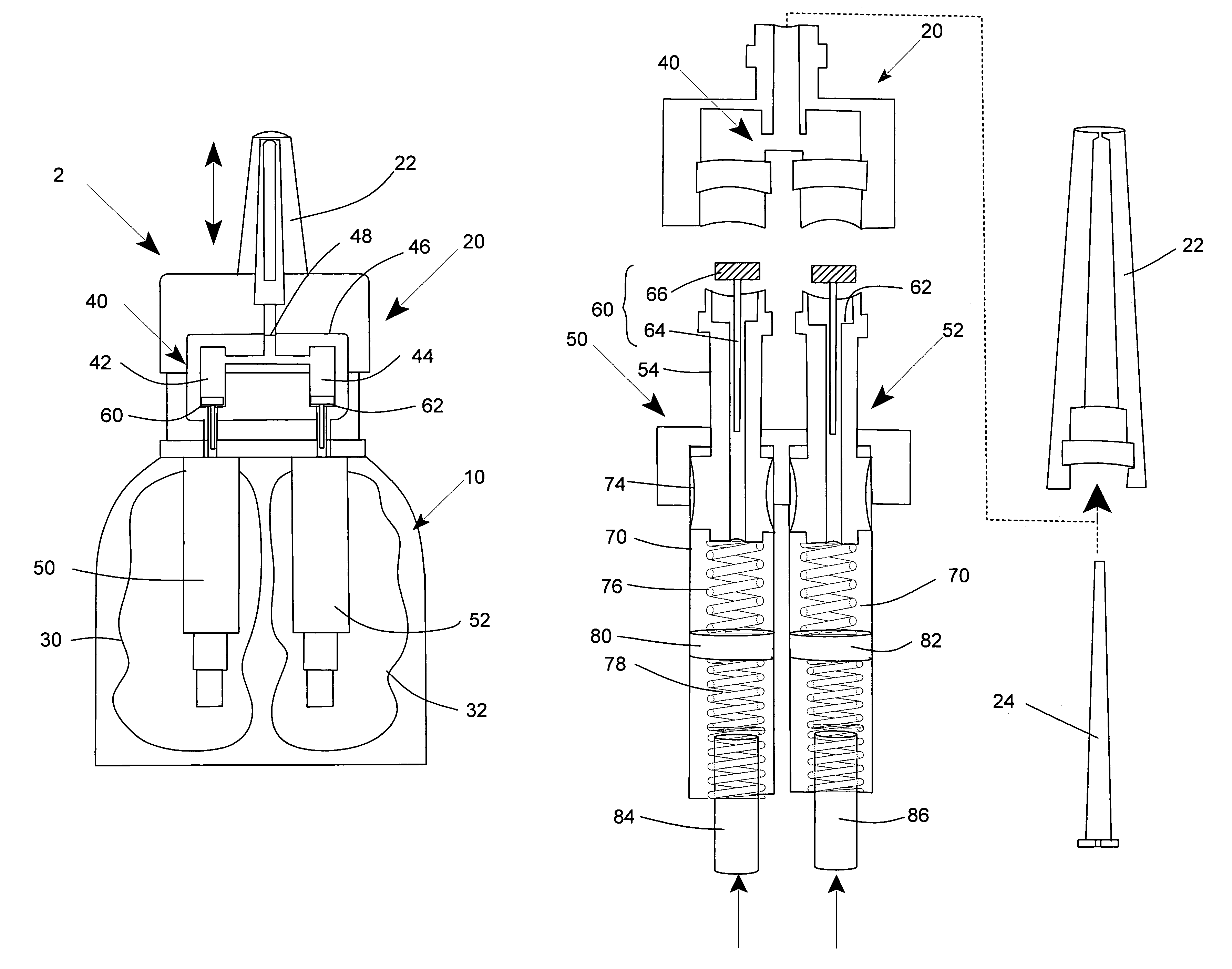
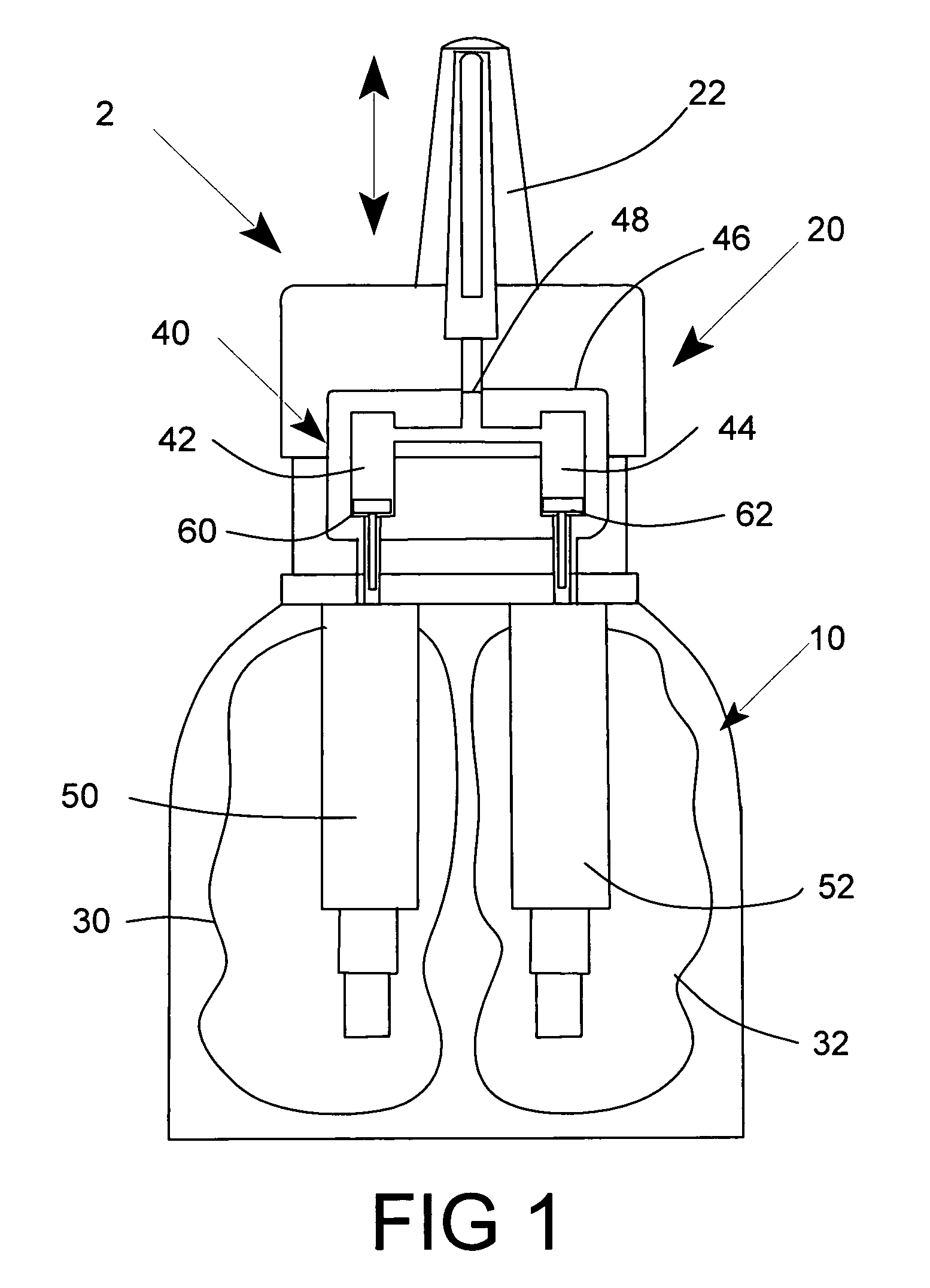
A nasal spray apparatus for simultaneously administrating metered amounts of multiple medicaments includes chambers for separately storing incompatible medicaments, such as an antihistamine and a steroid. Reciprocal piston pumps allow the medications to be sprayed into the user's nasal cavity. Two pumps can be used to separately transfer the medicaments to a receptacle where they can be initially mixed just prior to administration. A small volume receptacle is used to reduce the amount of mixture remaining after each stroke of the nasal spray apparatus. A check valve can be associated with each pump to further reduce medicament mixtures from cross contamination within storage chamber preparations. Collapsible components, including collapsible storage chambers or balloon capacitors can be employed to compensate for vacuums and back pressures as the medicaments are pumped to a spray nozzle.
Owner:MINOTTI AMERICO MICHAEL
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833549B2Reduce morbidityNot impart bitter tasteBiocidePowder deliveryNasal cavityAzelastine
Owner:MANNKIND CORP
Combination antihistamine and steroid medication
The invention provides a topical pharmaceutical composition for application to the nasal or ocular mucosa which comprises (1) a pharmaceutical excipient suitable for topical administration, (2) an antihistamine drug and (3) a mast cell stabilizer, a non-steroidal anti-inflammatory drug, a phosphodiesterase inhibitor, an anti-IgE agent, heparin, a topical steroid or a leukotriene blocker.
Owner:FAIRFIELD CLINICAL TRIALS
Methods and composition for treatment of migraine and symptoms thereof
Compositions, methods and kits are provided for the treatment of migraines. The compositions, methods and kits include an effective dose of trimethobenzamide and an ethanolamine antihistamine that, when administered to an individual suffering from migraine headaches, will alleviate symptoms associated with the migraine headaches. Compositions, methods, and kits for the treatment of migraines include pharmaceutical compositions of trimethobenzamide and diphenhydramine.
Owner:SALEHANI FOAD
Itching-relieving body wash and preparation method thereof
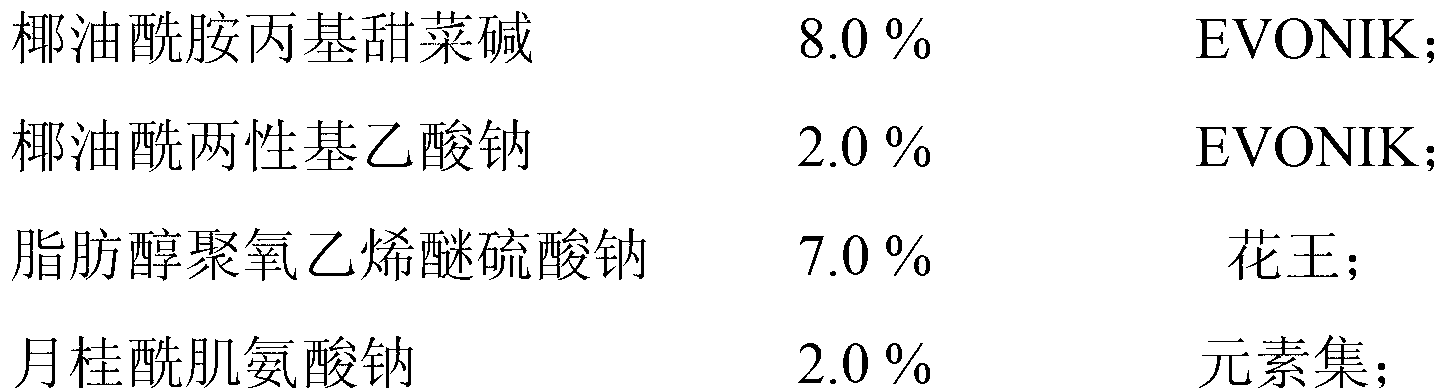
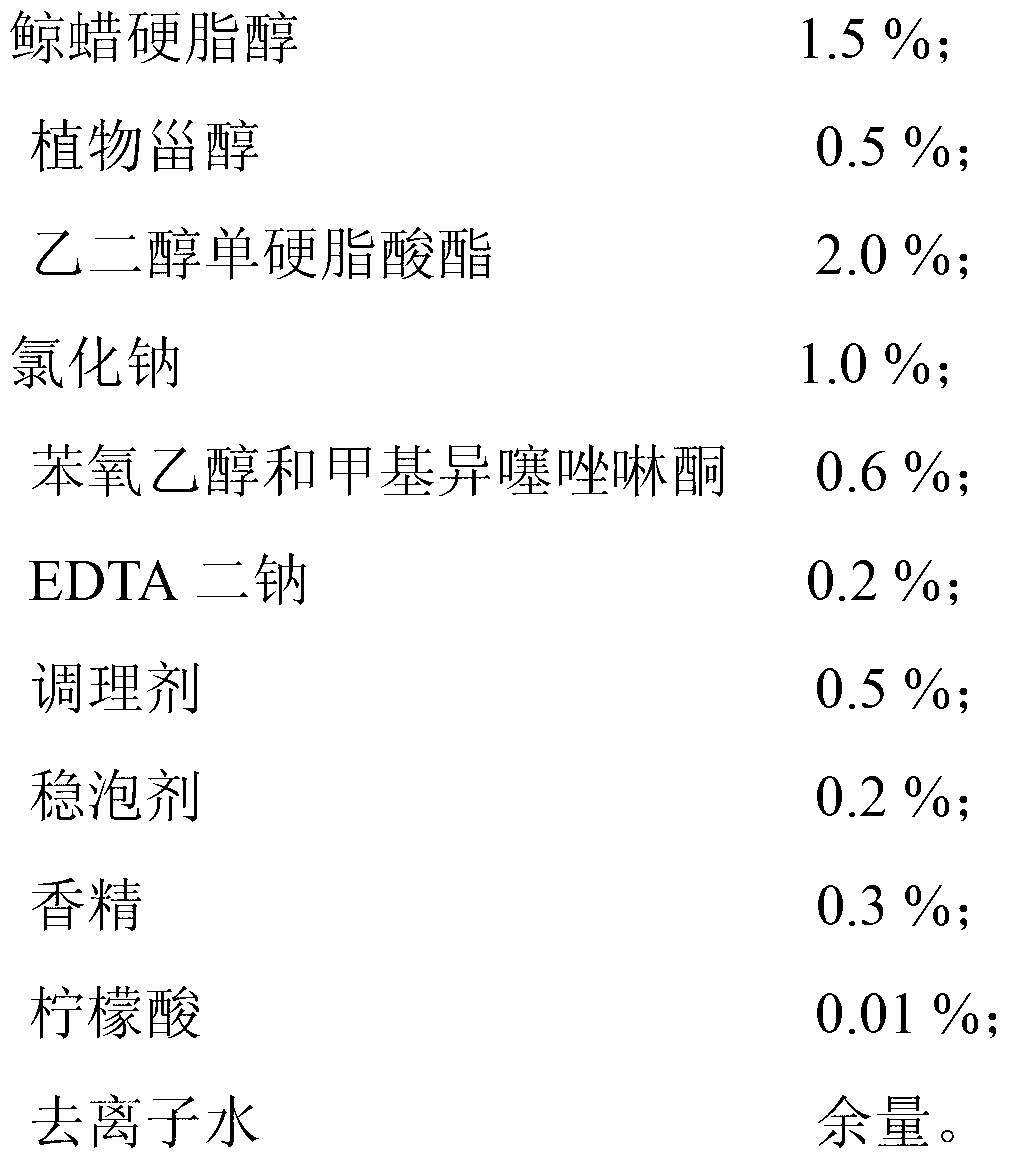
ActiveCN103315932AAntihistamine significantGood antibacterialCosmetic preparationsToilet preparationsPurslane extractCnidium
The invention discloses an itching-relieving body wash and a preparation method thereof. The itching-relieving body wash comprises the raw materials of, by mass, 1.0-20.0% of an amphoteric surfactant, 6.0-20.0% of an anionic surfactant, 0.5-8.0% of a non-ionic surfactant, 0.1-8.0% of a polyol humectant, 0.5-2.0% of a traditional Chinese medicine extract, 0.1-2.0% of a moisture, 0.01-2.0% of phytosterol, 0.01-0.5% of a chelating agent, 0-2.0% of a pearlescent agent, 0-2.0% of a viscosity adjusting agent, 0-0.5% of a conditioning agent, 0-0.5% of a foam stabilizing agent, 0-0.5% of an essence, 0-0.2% of a pH adjusting agent, 0-0.8% of a preservative, and balance of deionized water. The traditional Chinese medicine extract comprises a mixture of a purslane extract and a common cnidium fruit extract. The itching-relieving body wash has excellent antihistamine, antibacterial, bacteria-inhibiting, anti-inflammatory, and anti-allergic effects.
Owner:片仔癀(上海)生物科技研发有限公司
Antihistamine-and corticosteroid-containing lipsome composition and its use for the manufacture of medicament for treating rhinitis and related disorders
InactiveUS20090324699A1Reduce morbidityOrganic active ingredientsBiocideDiseaseObstructive Pulmonary Diseases
There is provided homogeneous pharmaceutical compositions for the treatment of, for example, rhinitis, asthma and / or chronic obstructive pulmonary disease comprising a corticosteroid and an antihistamine, a polar lipid liposome and a pharmaceutical-acceptable aqueous carrier.
Owner:MEDA AB
Combination of loteprednol and antihistamines
The present invention relates to a novel combination of a soft steroid, in particular loteprednol, and at least one antihistamine, such as, for example, azelastine and / or levocabastine, for simultaneous, sequential or separate administration in the local treatment of allergies and airway disorders, for example of allergic rhinitis (rhinoconjunctivitis).
Owner:VIATRIS GMBH & CO KG
Solid pharmaceutical dosage unit for alleviating symptoms of rhinorrhea
InactiveUS20080292699A1Reducing and eliminating over-dryingBiocidePill deliveryAnticholinergic agentsDecongestant
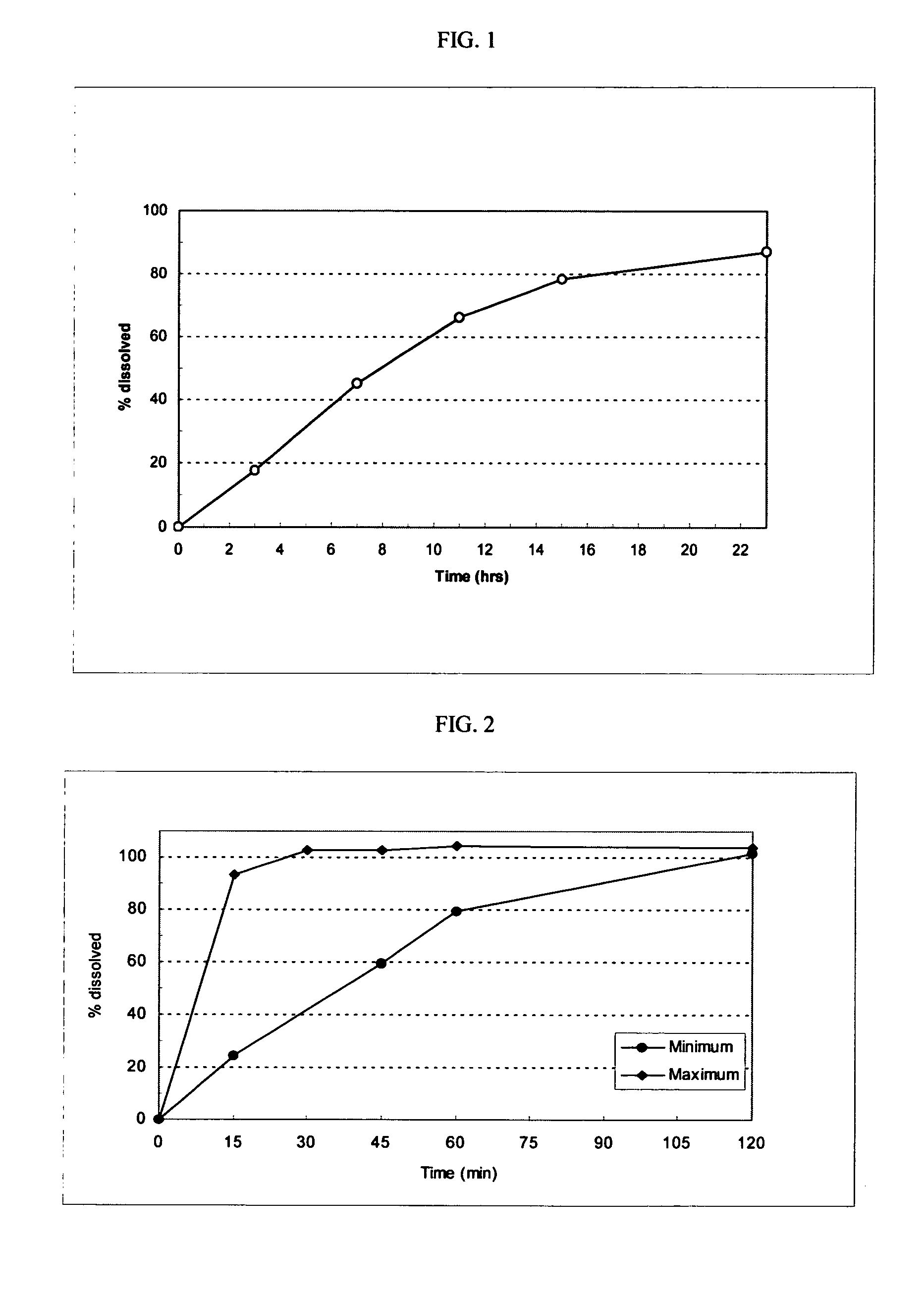
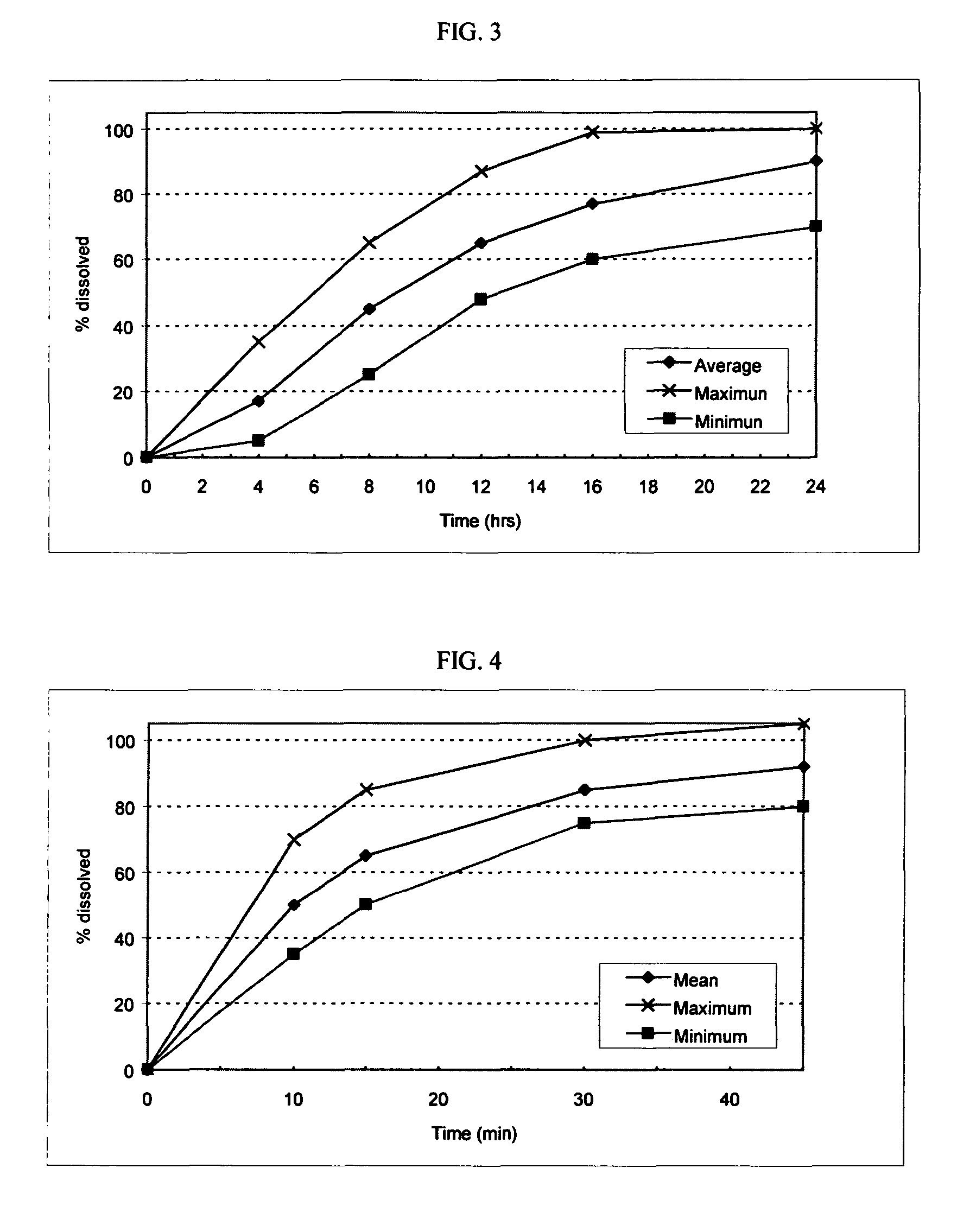
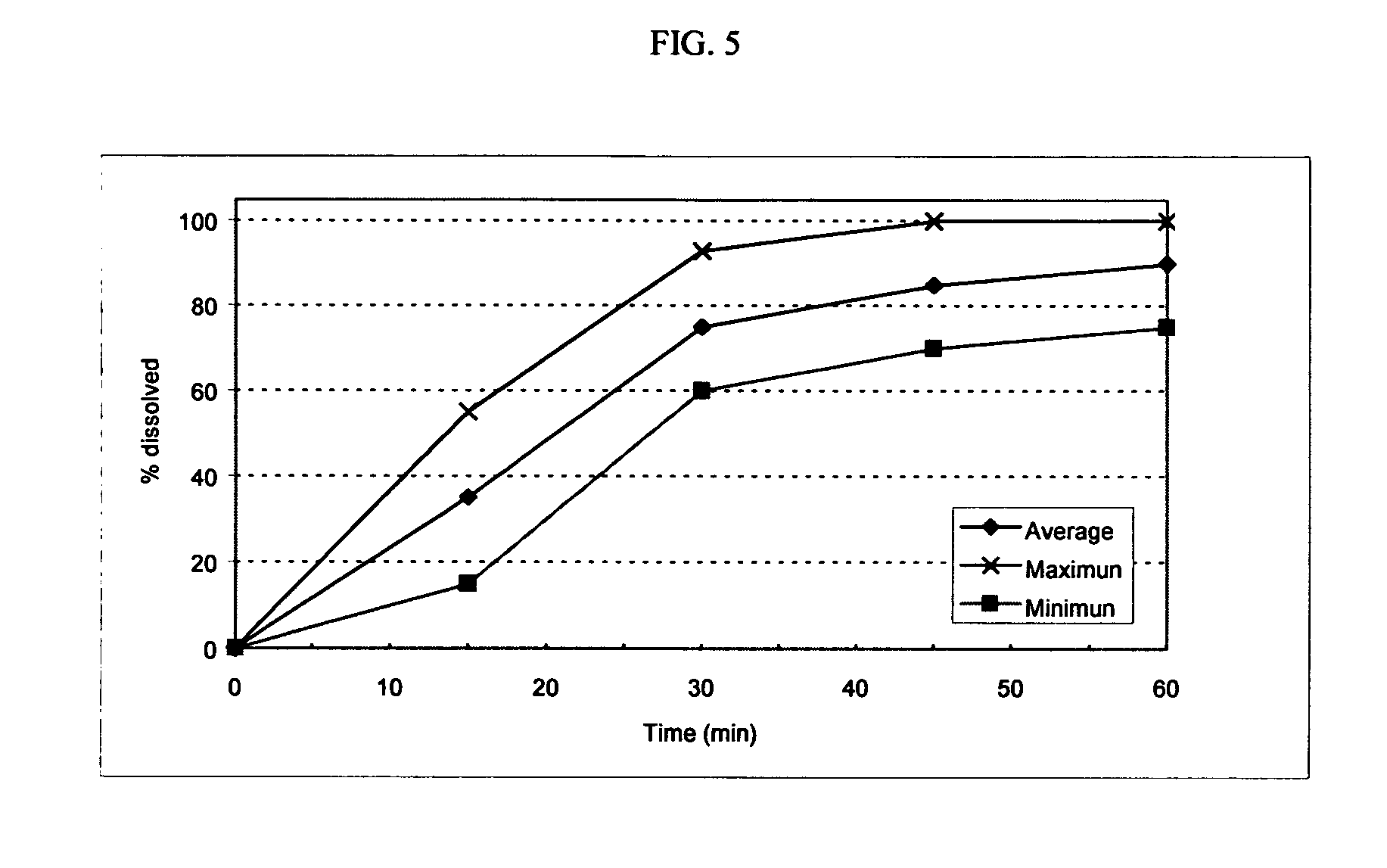
A solid pharmaceutical dosage unit for alleviating the symptoms of rhinorrhea. The dosage unit comprises an anticholinergic agent and an antihistamine and, optionally, a decongestant and, when placed in a basket in 500 ml of 0.01 N HCl of 37° C. which is stirred at 100 rpm, releases at least about 75% of the at least one anticholinergic agent within 45 minutes and releases the at least one antihistamine at a rate of from about 20% to about 60% after 2 hours, from about 45% to about 80% after 4 hours and at least about 75% after 8 hours. This Abstract is not intended to define the invention disclosed in the specification, nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Anti-allergic pharmaceutical composition containing at least one allergen and at least one antihistamine compound
InactiveUS7048928B2Good curative effectReduce concentrationOrganic active ingredientsBacterial antigen ingredientsActive agentAllergen
The present invention relates to an anti-allergic pharmaceutical composition containing at least two active agents chosen from among: (i) one allergen, (ii) one antihistamine compound, and (iii) one inhibitor of histamine synthesis. The active agents are associated in the composition with a pharmaceutically acceptable vehicle.
Owner:ANTIALIS
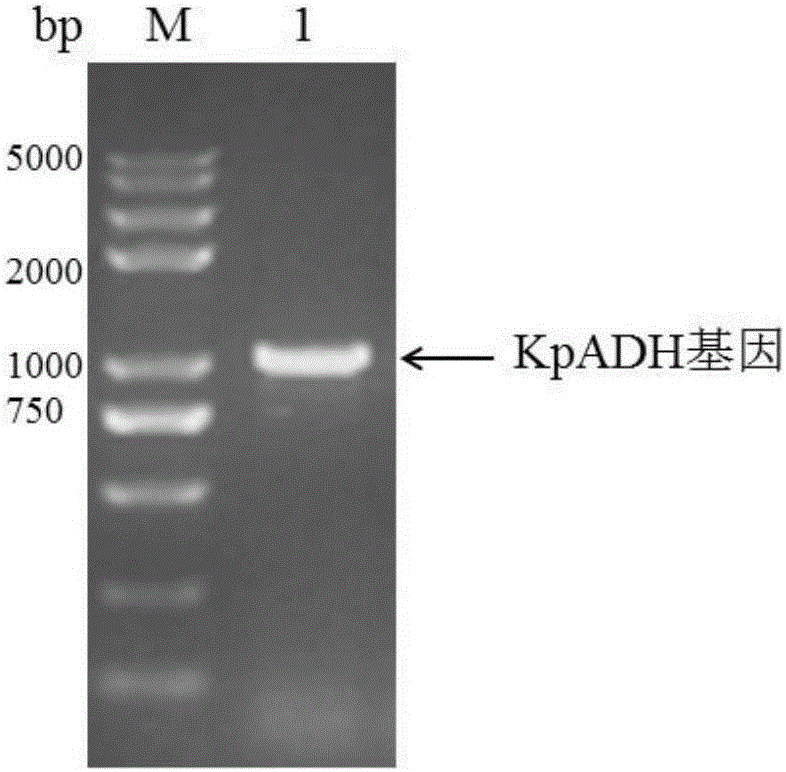
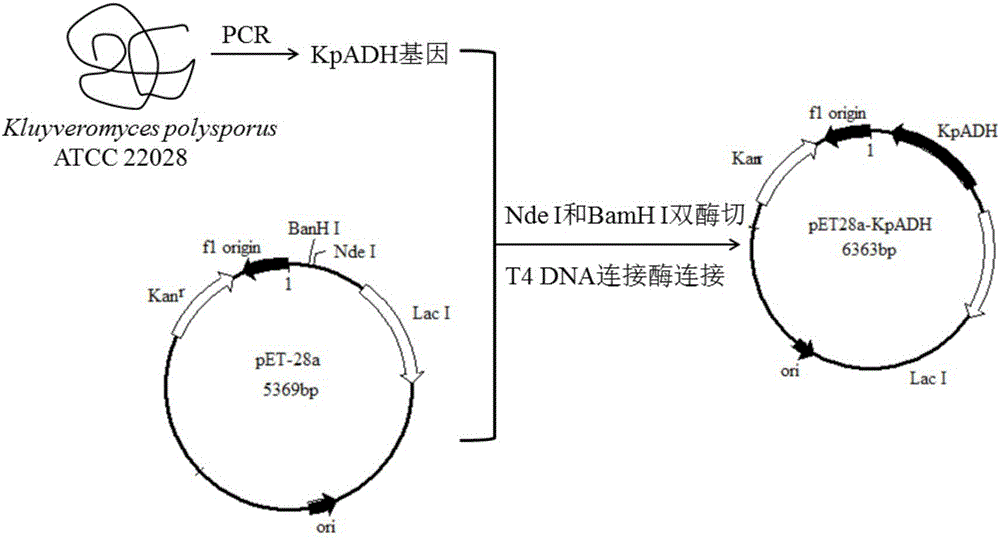
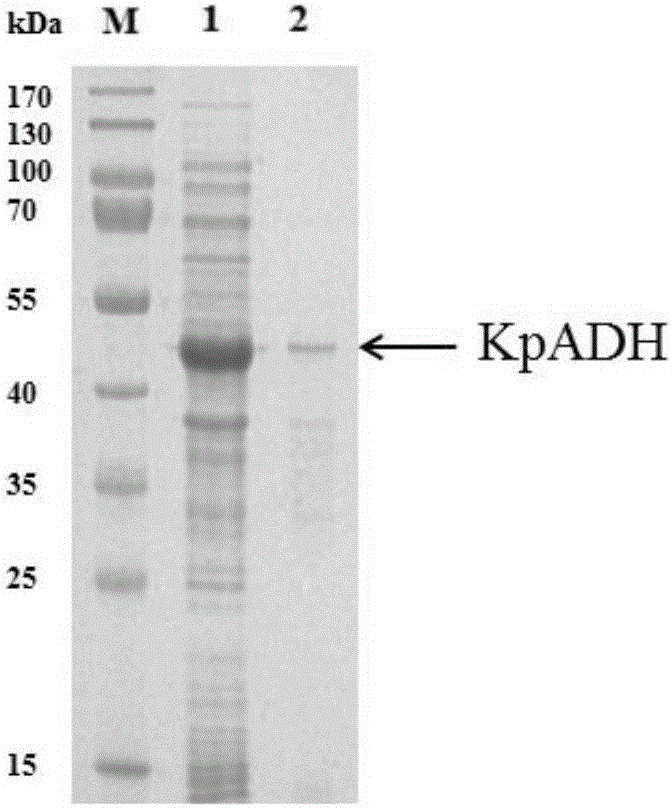
Alcohol dehydrogenase, gene and recombinase thereof, and application of alcohol dehydrogenase in synthesis of chiral diaryl secondary alcohol
InactiveCN105936909AImprove expression levelIncrease enzyme activityOxidoreductasesGenetic engineeringDiaryl ketoneGenus Kluyveromyces
The invention discloses an alcohol dehydrogenase, a gene thereof, a recombinant expression vector and a recombinant expression transformant respectively containing the gene, a recombinase of the alcohol dehydrogenase, and an application of the alcohol dehydrogenase in asymmetric reduction synthesis of chiral diaryl secondary alcohol as a catalyst, and belongs to the technical field of bioengineering. The alcohol dehydrogenase is from Kluyveromyces sp. CCTCCM2011385, has a carbonyl group reduction function, and also has a hydroxy group oxidation function. Extra addition of glucose dehydrogenase and other enzymes used for cofactor circulation is not needed when the alcohol dehydrogenase is used in the reduction of diaryl ketone into the chiral diaryl secondary alcohol as a biocatalyst, and the alcohol dehydrogenase has the advantages of high catalysis efficiency, mild reaction conditions, easy product recovery and low cost, so the alcohol dehydrogenase has very good application and exploitation prospect in the production of antihistamine medicines.
Owner:JIANGNAN UNIV
Kits for Prevention and Treatment of Rhinitis
Kits providing a combination of one or more pharmaceutical information comprising one or more agent(s) for the treatment or alleviation of symptoms commonly associated with a cold and an immunonutritional composition comprising immunonutritional agent and methods of using these kits are described . The kits provide both the pharmaceutical agent(s) and the immunonutritional agent in a convenient form for administration. The kit typically includes instruction for coordinating the administration of the pharmaceutical formulation with the administration of the immunonutritional composition. The preferred immunonutritional agents are compounds that contain a pharmaceutically acceptable form of zinc, such as zinc acetate, zinc gluconate, zinc gluconate glycine, and zinc sulfate. Preferably the kit contains multiple dosage forms containing the immunonutritional composition. In the most preferred embodiment, the immunonutritional composition is in the form of a lozenge. Suitable pharmaceutical agents include but are not limited to antihistamines, decongestants, anticholinergies, antitussives, analgestics, mucolytics, expectorants, and combinations thereof. The pharmaceutical formulations may be in any suitable dosage form, including forms which provide controlled release of the pharmaceutical agent, including immediate, sustained, modified, delayed or pulsed release pharmacokinetic mechanism or a combination thereof. The combined treatment requires administration of both the pharmaceutical formulation(s) for the treatment of symptoms commonly associated with a cold and the administration of the immunonutritional composition, which supplies nutritional support for the patient's innate immune response to the presence of infectious organisms.
Owner:AURIGA LAB
Antihistamine/Corticosteroid preparations for the treatment of atopic dermatitis
InactiveUS20090136430A1Relieve itchingBiocideAerosol deliveryCorticosteroid preparationAtopic dermatitis
Owner:DUGGER HARRY A
Acetaminophen compositions having minimized side effects including reduced hepatotoxicity
InactiveUS20080139654A1Promotes glutathione productionMitigate noxious smellBiocidePeptide/protein ingredientsSide effectOpiate
Solid tablets or gel capsules comprising acetaminophen and an agent that promotes glutathione production that mitigates adverse hepatic effects of acetaminophen. The glutathione production promoter is preferably n-acetylcysteine or other mercapto-2-amino alkyl carboxylic acid having glutathione production promoting properties. A preferred composition comprises acetaminophen (200 mg to 750 mg) and N-acetylcysteine (200 mg to 600 mg). Alternatively and / or in addition to N-acetylcysteine the composition can contain at least one of methionine and cysteine. Preferred compositions can contain at least one of an opiate (or synthetic equivalent), an antihistamine, an antiemetic, and a sedative. Physical encapsulation of the ingredients optionally with or in place of a fragrance is used to make the composition more acceptable to patients by mitigating noxious properties of the glutathione production promoter. Preferably, the compositions are prepared for patient self administration in tablet or gel capsule form wherein the patients can take the medication without the need for close oversight of a medical caregiver.
Owner:SODERLING ERIC MOTT
Drug delivery device containing neuraminidase inhibitor and an H1 antagonist
The present invention provides a dual release solid dosage form containing a first composition that releases a neuraminidase inhibitor, such as oseltamivir, zanamivir, or peramivir, in a controlled manner and a second composition that releases an H1 antagonist in a rapid and / or immediate manner. A wide range of H1 antagonist antihistamines, especially fexofenadine and loratadine, can be used in this device. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. The device is useful for the treatment of respiratory congestion and other viral infection associated symptoms.
Owner:ACELLA HLDG LLC +1
Antihistamine sprays and ointments for relief of delayed contact dermatitis
It has been found that certain antihistamines can mediate the delayed dermatitis and in particular that caused by poison ivy and poison sumac or poison oak. Especially useful are antihistamines having a high degree of intrinsic activity as shown by their low oral dosage as antihistamine (0.1-10, suitably 1-2 mg), which can be topically administered at a sufficiently high active concentration to be effective in the treatment of allergic reactions. Compositions and methods of utilizing such compositions for these purposes are disclosed.
Owner:FLEMINGTON PHARMA +1
Antihistamine and anti-nausea pharmaceutical compositions for topical application
InactiveUS20080255103A1Improve stabilitySuitable for useDigestive systemPharmaceutical delivery mechanismGel basedPromethazine
A topical pharmaceutical preparation suitable for use as an antihistamine or anti-nausea drug comprises an emu oil gel base and a therapeutic amount of promethazine.HCl. The preparation may further comprise an antioxidant. The preparation is preferably packaged as a single unit dose. A method for preparing the topical pharmaceutical preparation comprises the steps of combining a clear gel with an emu oil mixture and adding a therapeutic amount of promethazine.HCl solution.
Owner:BALLAY PHARMA
Pharmaceutical compositions including an antihistamine and a stimulant and method of use thereof
The present invention provides pharmaceutical compositions including a sedating antihistamine and a stimulant, and methods of use thereof. The stimulant reduces the sedation caused by the antihistamine, thereby allowing potent, but sedating, antihistamines to be used effectively.
Owner:PA2008
Medicinal composition for stabilizing delotadine in preparation
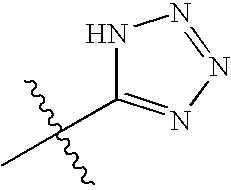
InactiveCN1552324ASolve the defect of brown-red substanceGuaranteed stabilityOrganic active ingredientsImmunological disordersLactoseActive ingredient
A composite medicine in which desloratadine is stabilized features that after desloratadine is mixed with medicinal auxiliaries, an antioxidizing agent is added to the mixture.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com