Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Histamine Agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drugs used for their actions on histaminergic systems. Included are drugs that act at histamine receptors, affect the life cycle of histamine, or affect the state of histaminergic cells.
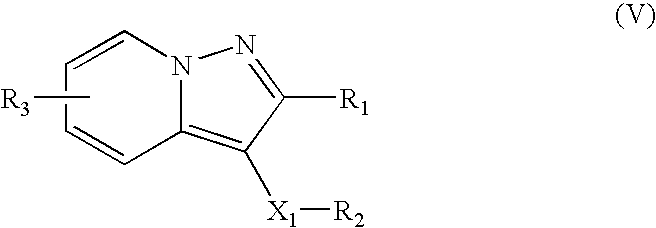
Dosage form containing multiple drugs
A pharmaceutical dosage form comprising a first drug and a second drug, both of which are selected from decongestants, antitussives, expectorants, analgesics and antihistamines. The dosage form provides a plasma concentration within a therapeutic range of the second drug over a period which is coextensive with at least about 70% of a period over which the dosage form provides a plasma concentration within a therapeutic range of the first drug. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Methods and compositions for improved non-viral gene therapy
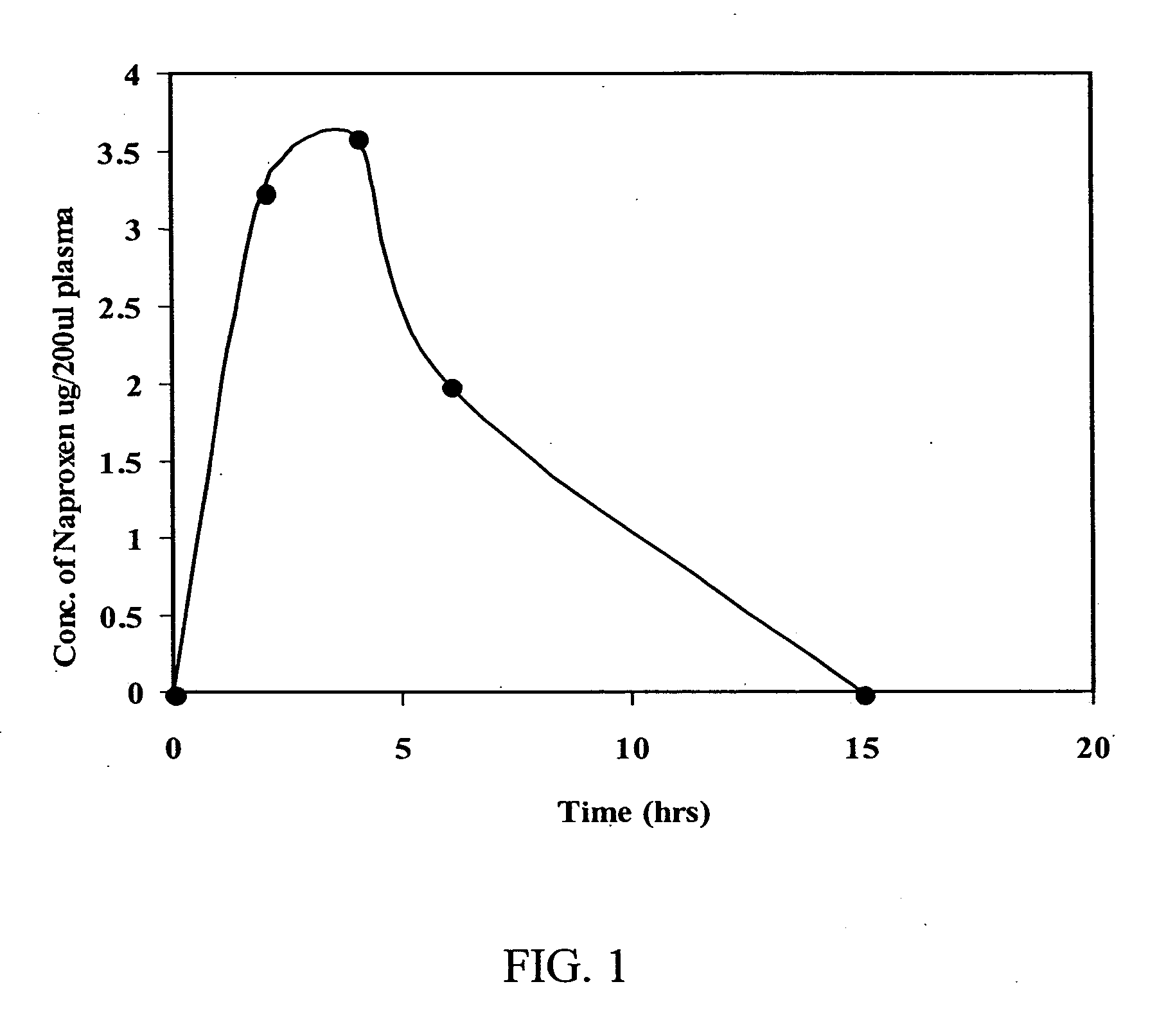
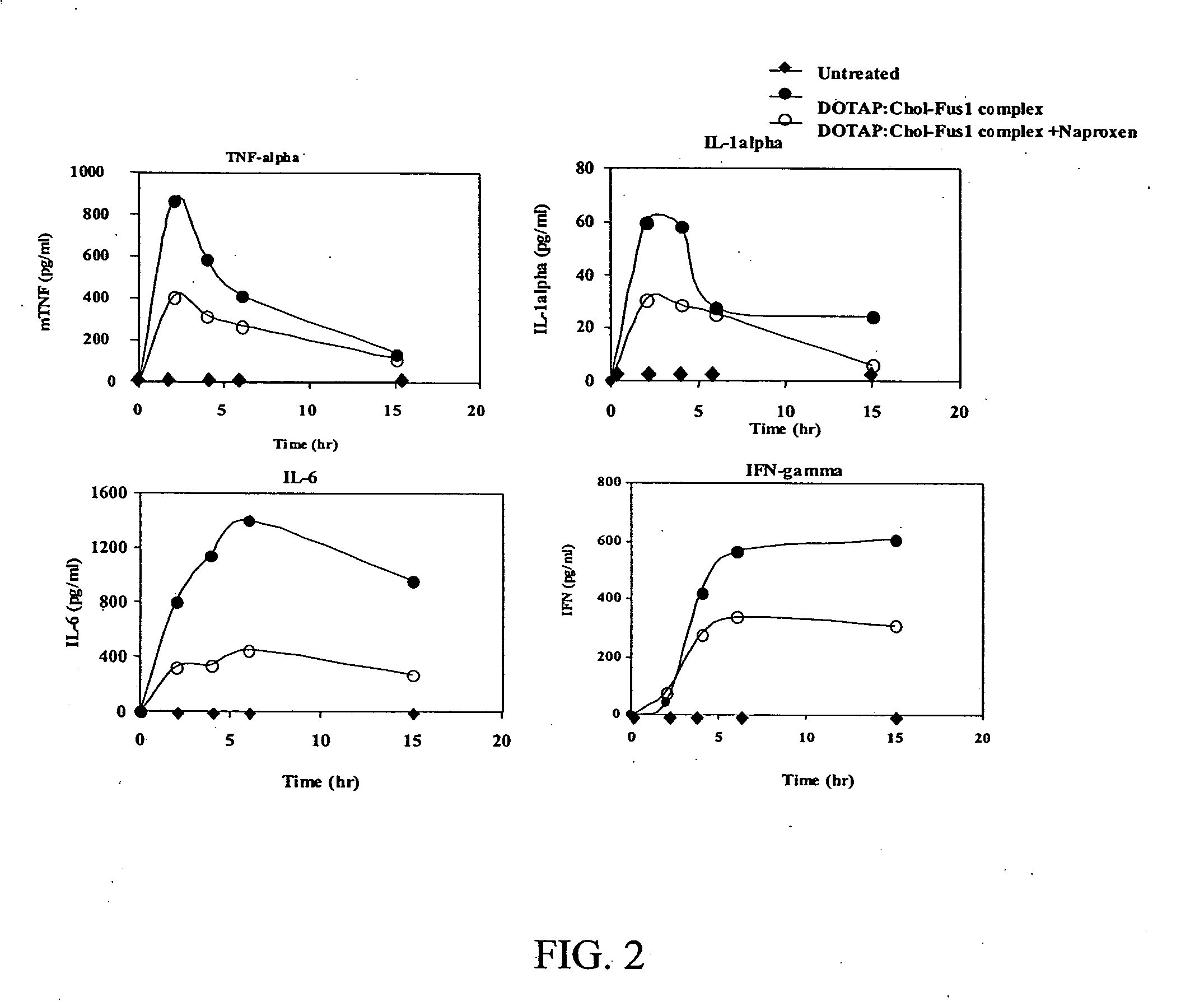
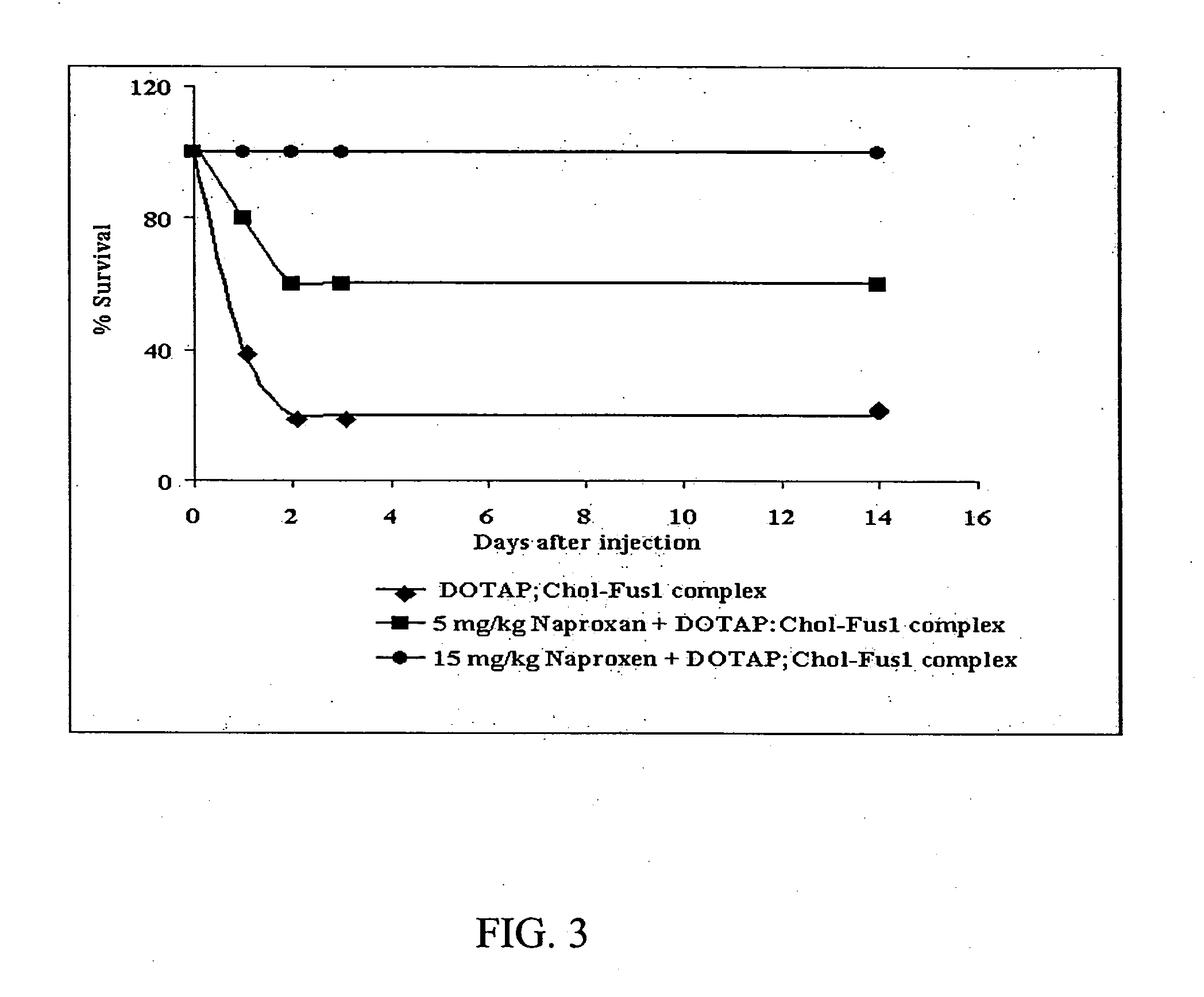
Methods to prevent or reduce inflammation secondary to administration of a lipid-nucleic acid complex in a subject, that include administering to the subject a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunsuppressive agent with the lipid-nucleic acid complex are disclosed. Also disclosed are methods of screening for inhibitors of the inflammatory response associated with administration of a lipid-nucleic acid complex to a subject, including providing a candidate substance suspected of preventing or inhibiting the inflammation associated with administration of a lipid-nucleic acid complex to the subject. Also disclosed are compositions that include a lipid, a nucleic acid, and a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunosuppressive agent.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods and reagents for the treatment of immunoinflammatory disorders
InactiveUS20050192261A1Great efficacyMore treatment satisfactionBiocideNervous disorderDrugAntihistamine
The invention features a method for treating a patient diagnosed with, or at risk of developing, an immunoinflammatory disorder by administering to the patient an antihistamine, either alone or in combination with one or more additional agents. The invention also features a pharmaceutical composition containing an antihistamine in combination with one or more additional agents.
Owner:COMBINATORX
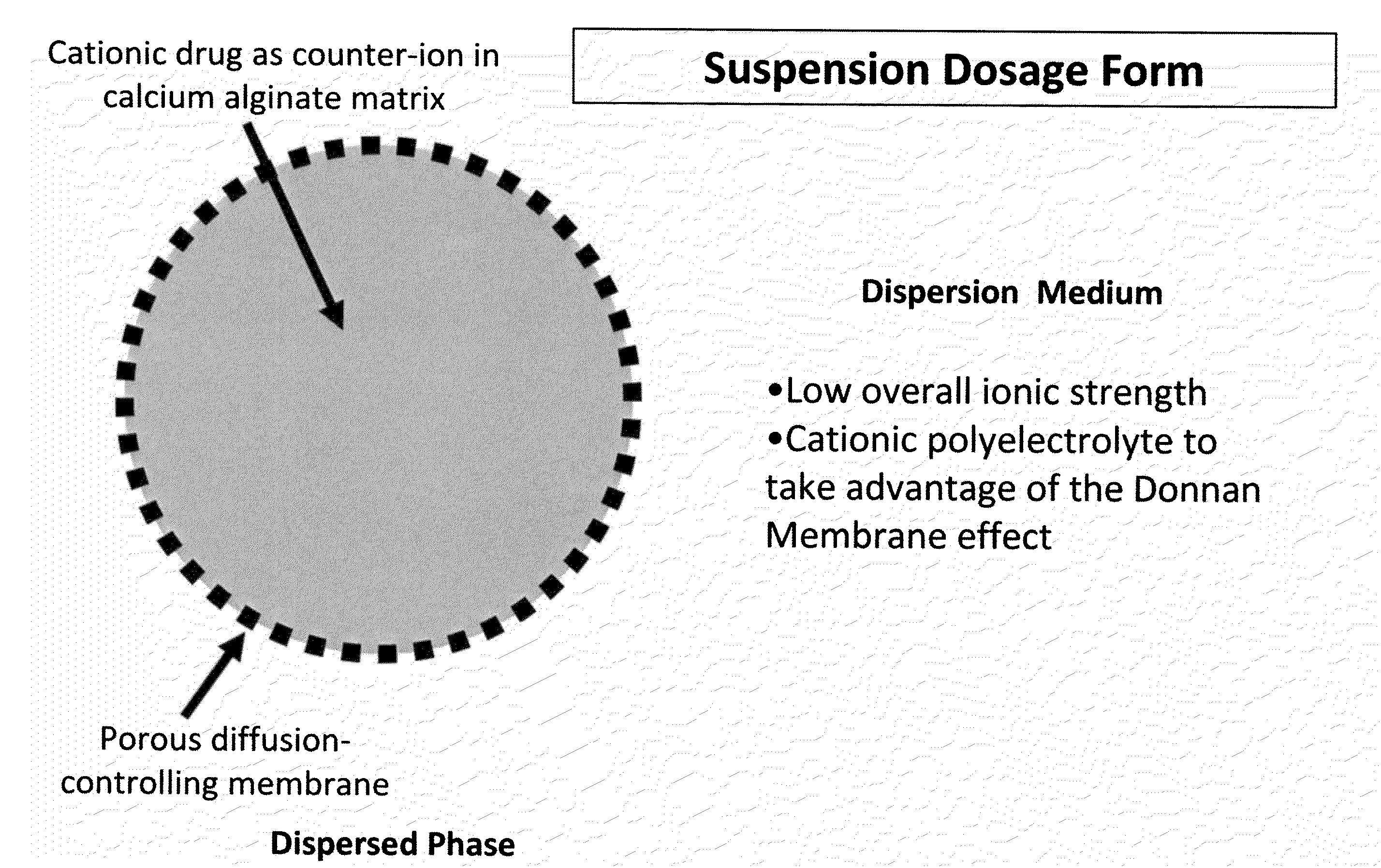
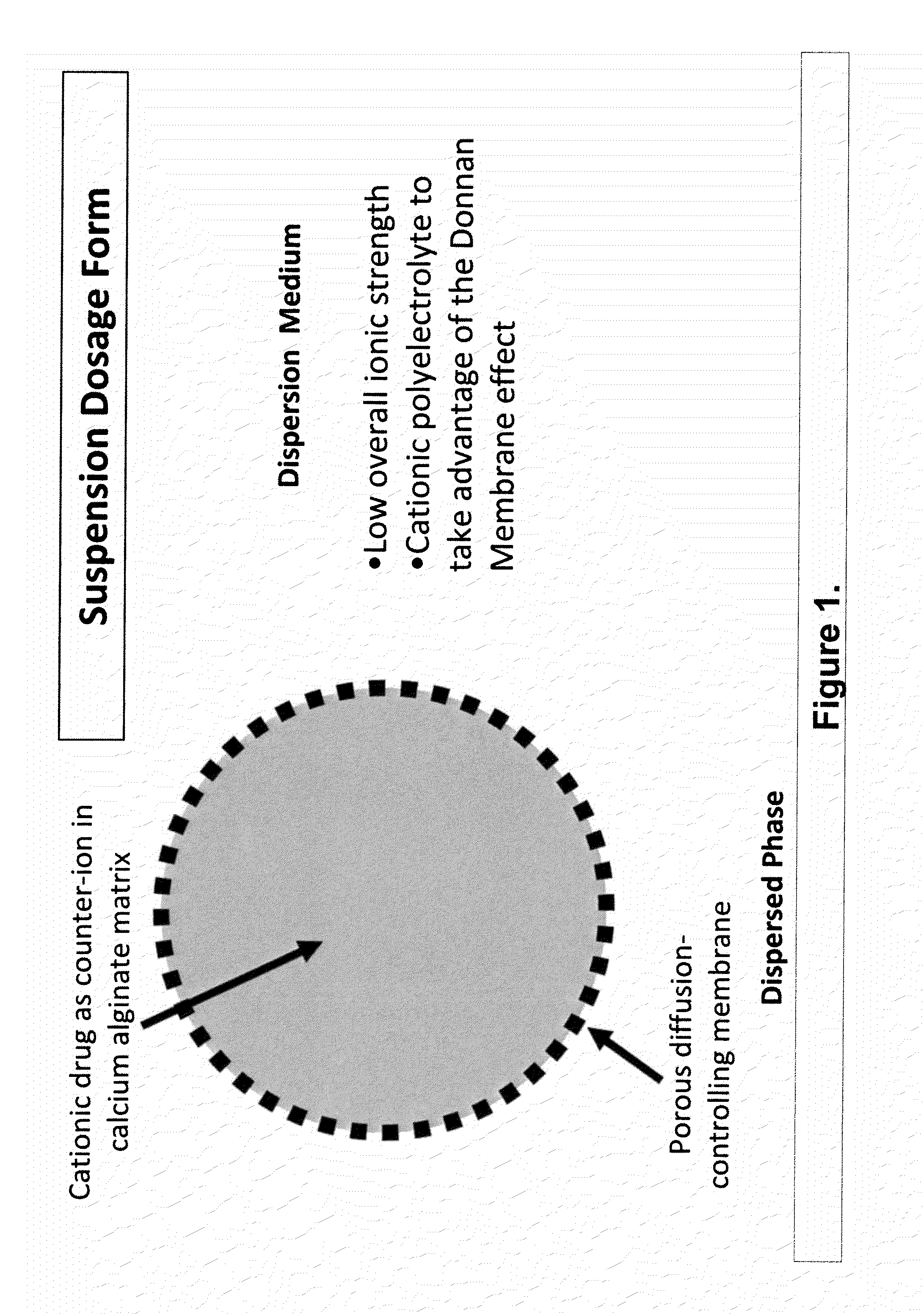
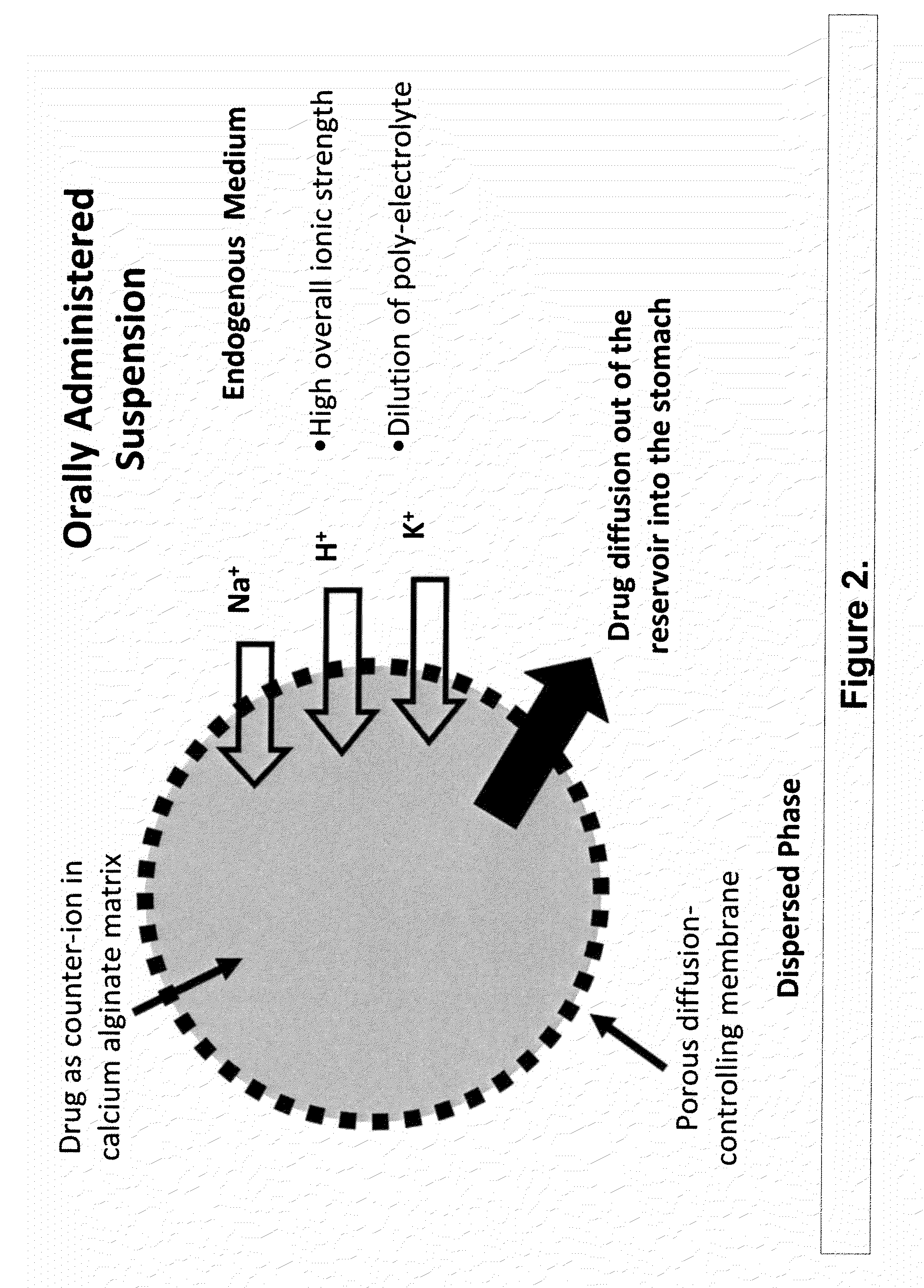
Sustained-release drug delivery compositions and methods
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
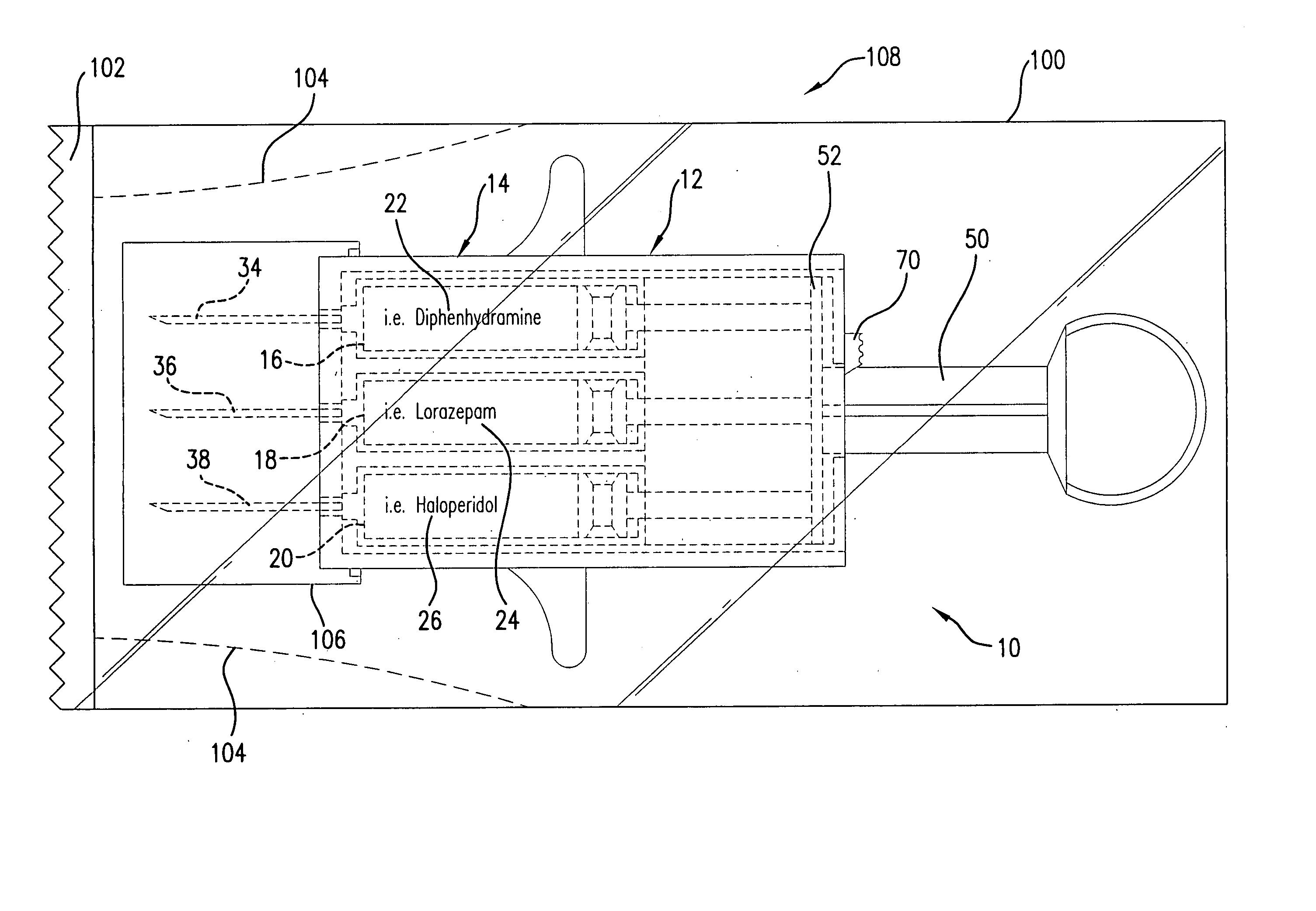
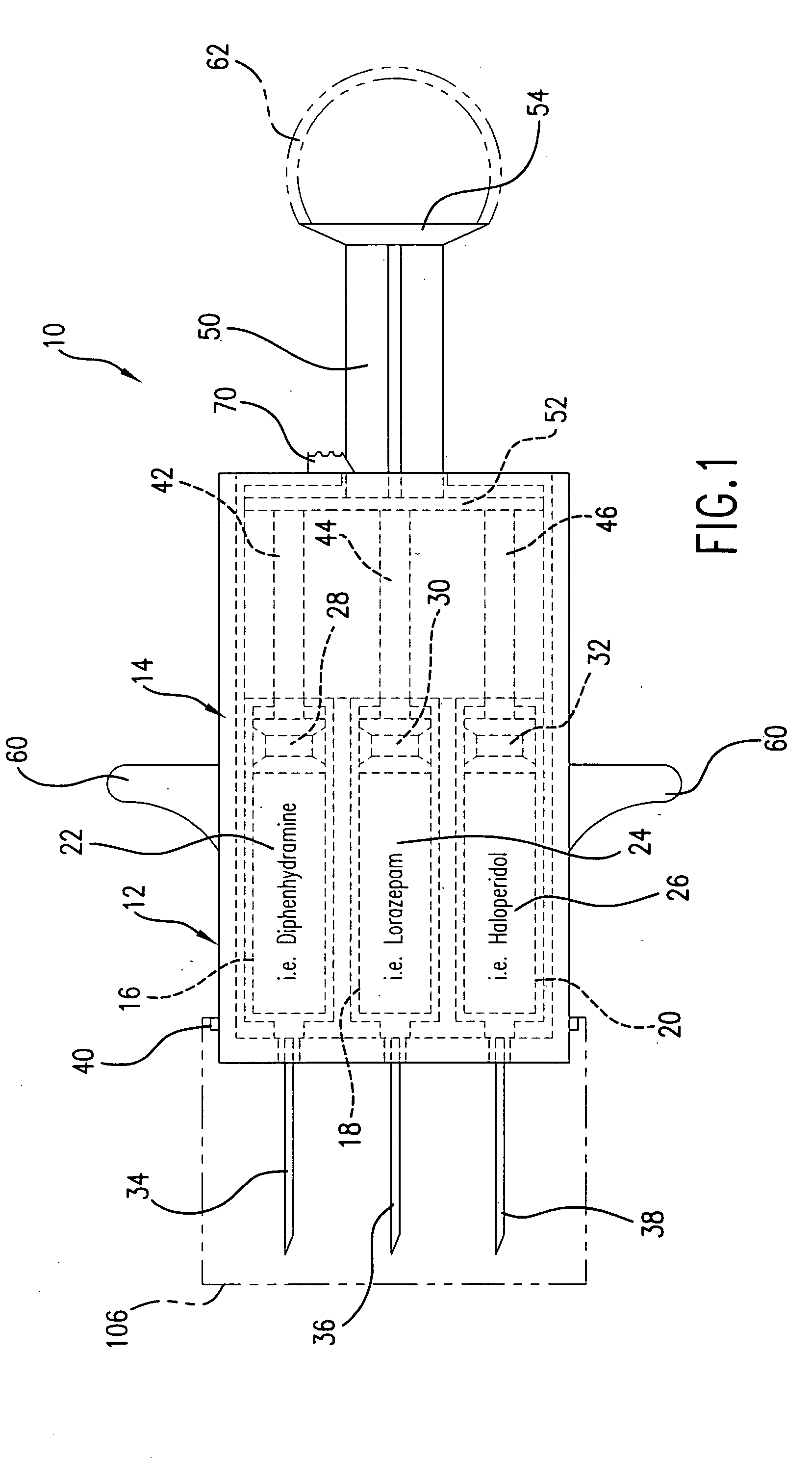
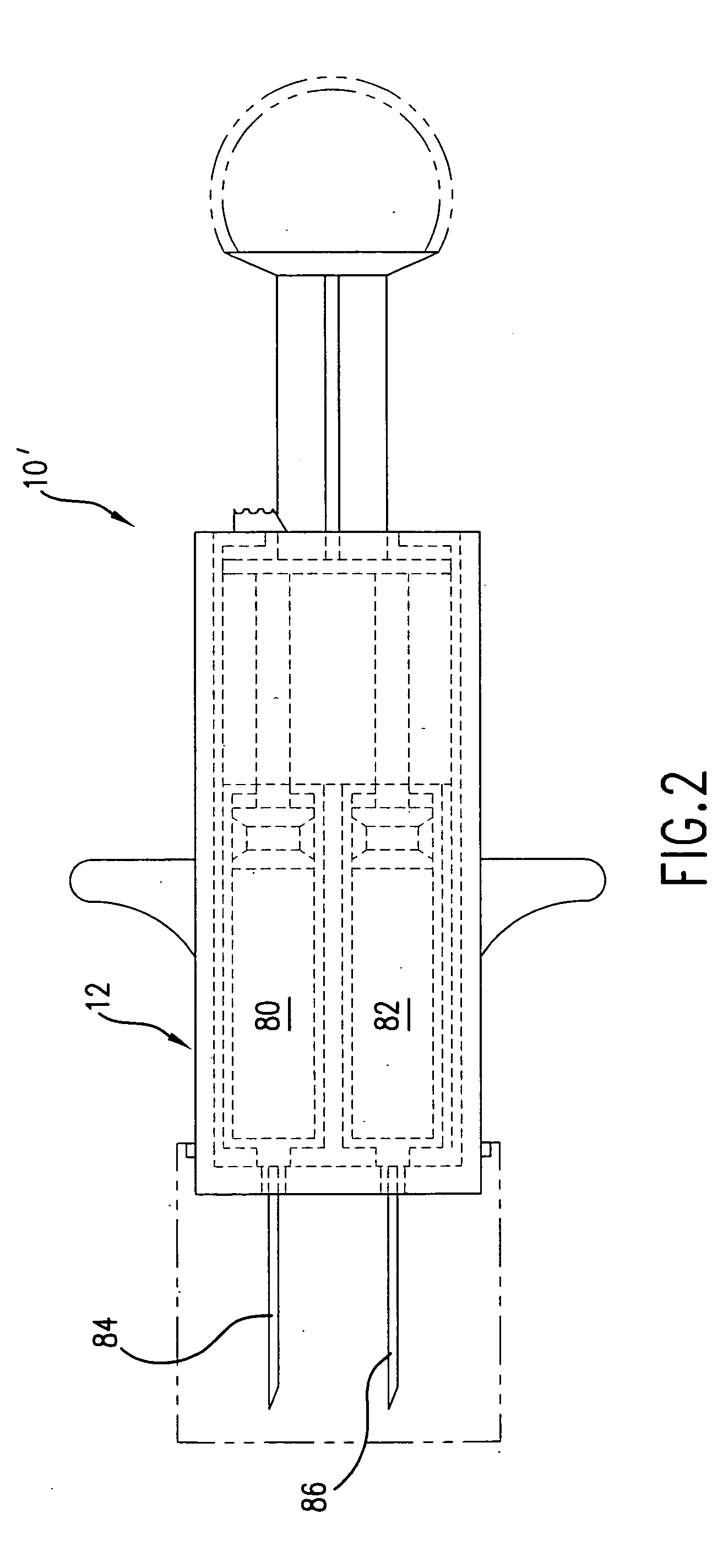
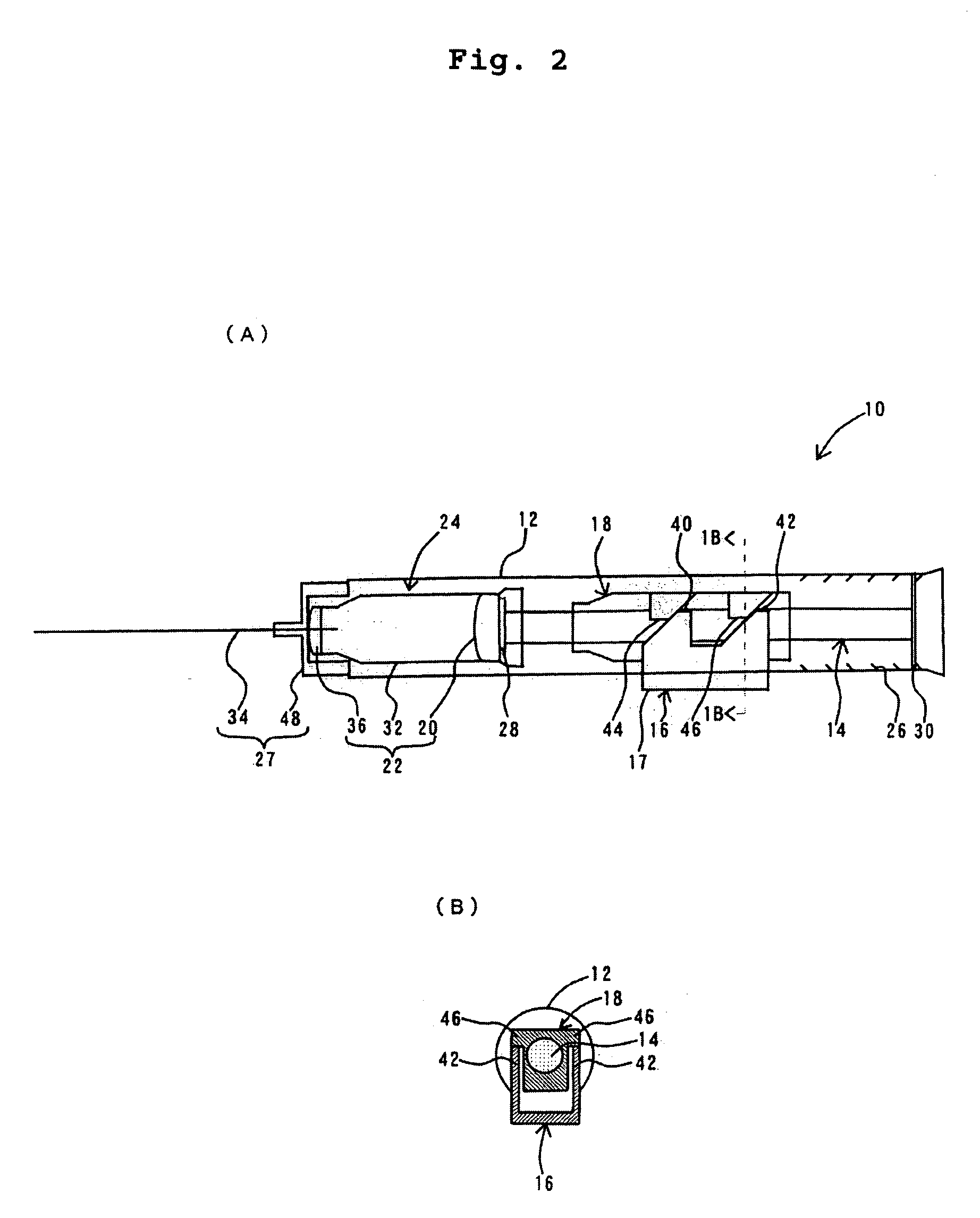
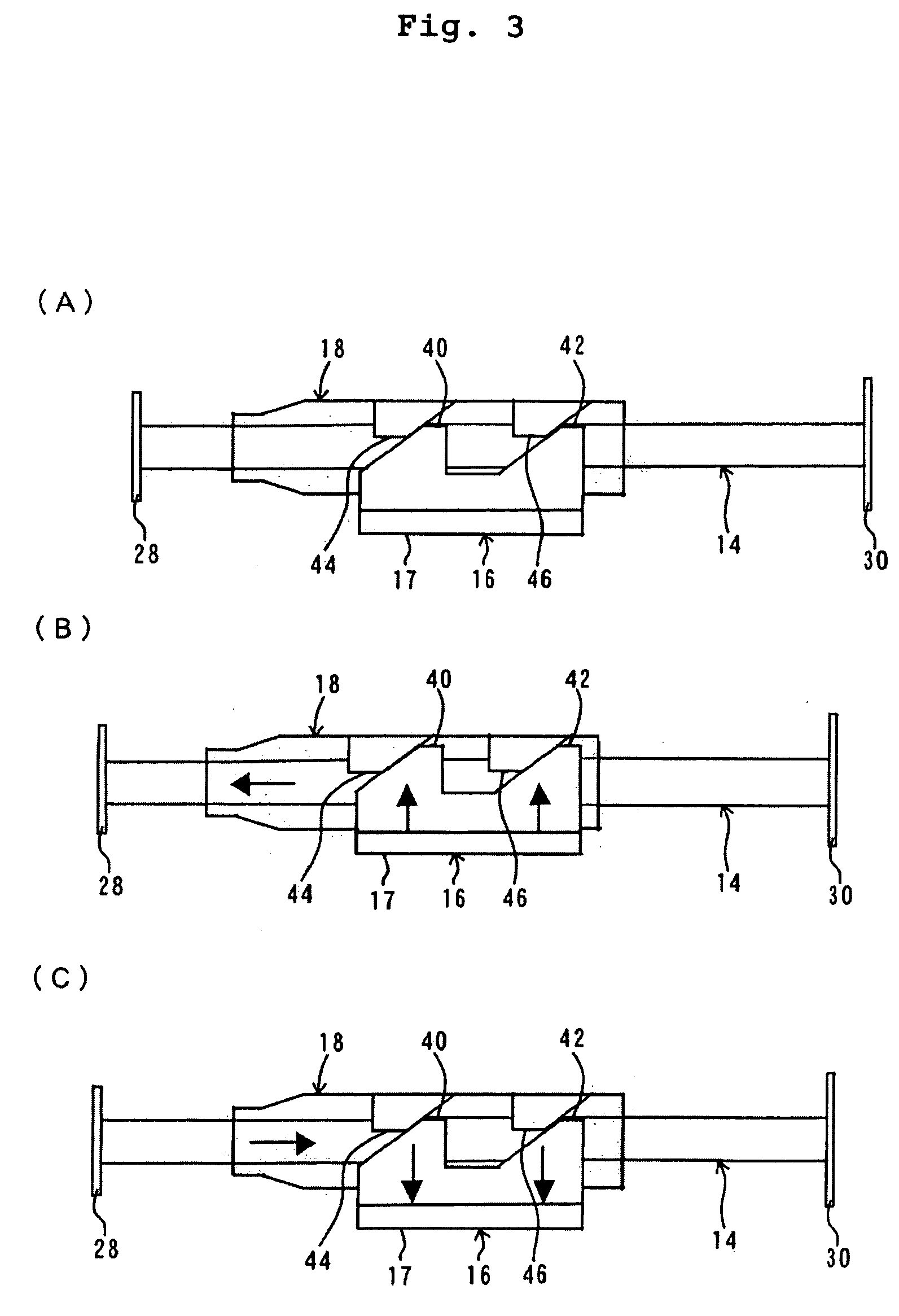
Hypodermic syringes with multiple needles and methods of calming psychiatric patients using such
Hypodermic syringes with multiple needles are used to practice a method of calming psychiatric patients. In accordance with one embodiment of the invention generally used to calm violent adult patients, the syringe has a first barrel containing an antipsychotic, a second barrel containing a sedating antihistamine and a third barrel containing an antianxiety sedative. Each barrel also has a separate projecting needle and contains a piston. A common operator, preferably in the form of a plunger simultaneously pushes all of the pistons so that the patient receives three injections simultaneously. For children, a second embodiment of the syringe includes two barrels instead of three, each barrel containing a separate medication. In each embodiment the syringe is packaged in a manually openable plastic envelope with a safety cap over the needles.
Owner:WILLIAMS ALTON
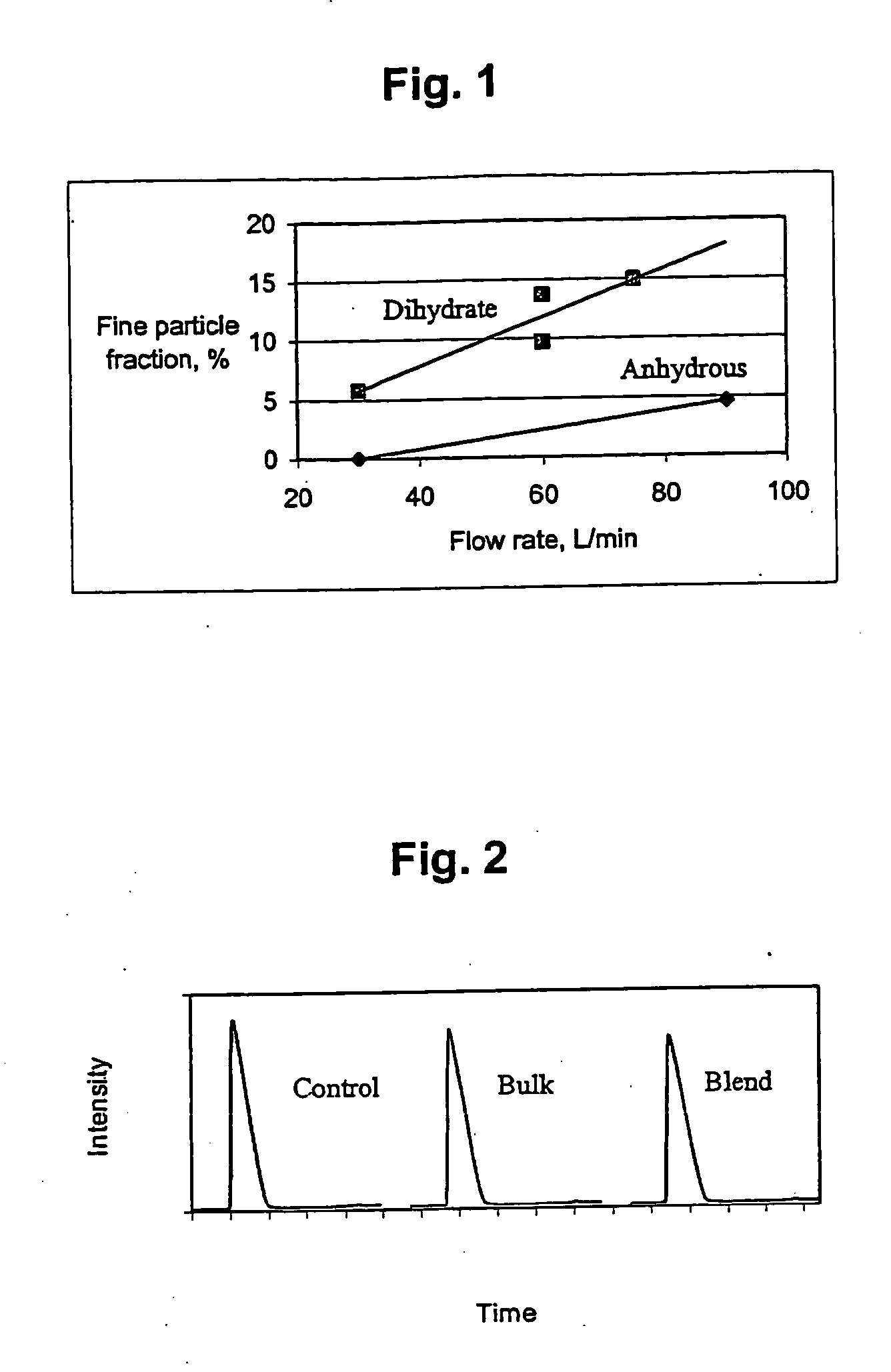
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833550B2Reduce morbidityNot impart bitter tastePowder deliverySenses disorderNasal cavityAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833549B2Reduce morbidityNot impart bitter tasteBiocidePowder deliveryNasal cavityAzelastine
Owner:MANNKIND CORP
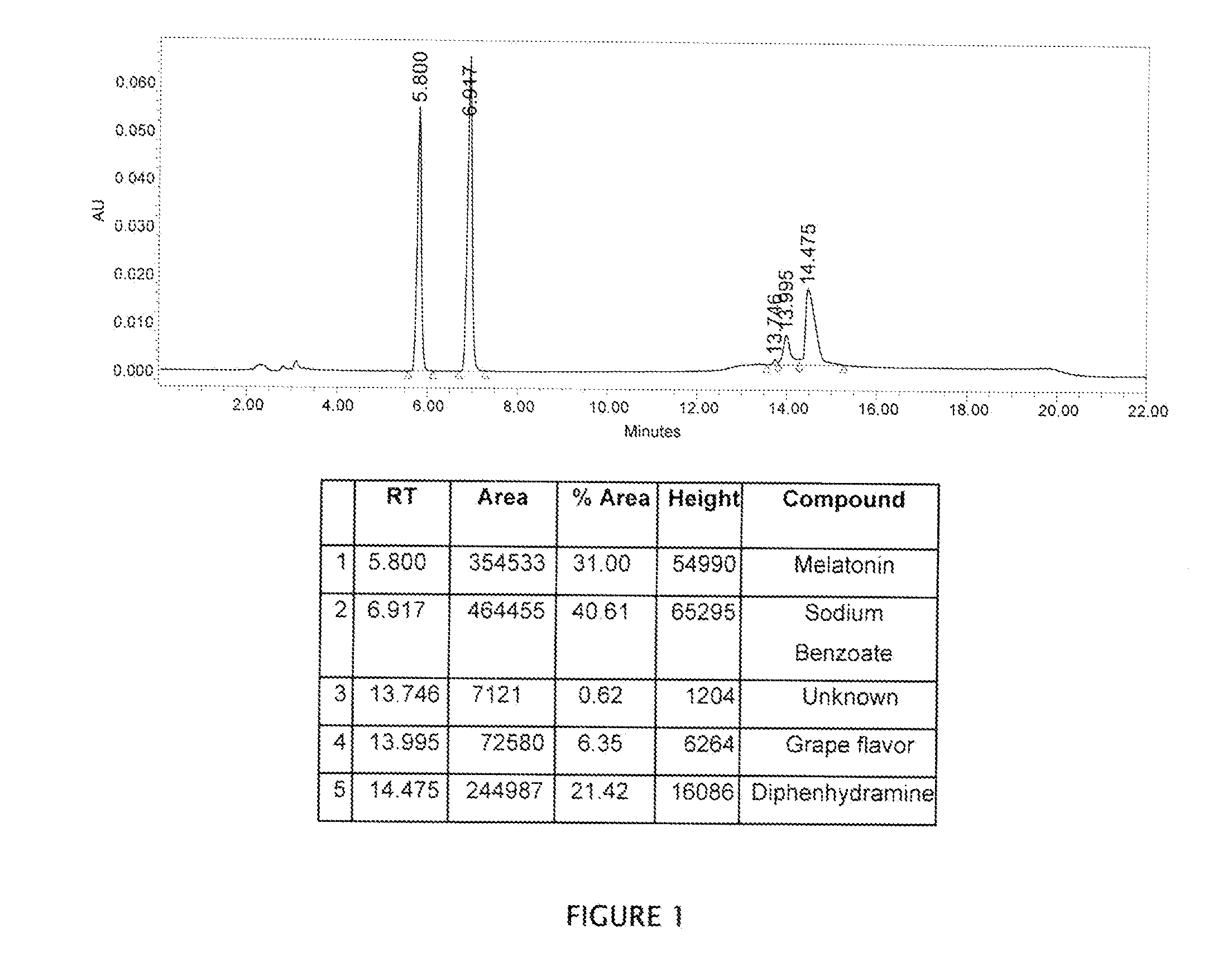
Stable combination oral liquid formulation of melatonin and an antihistaminic agent
InactiveUS20160166543A1Promote growthEasy to manageBiocideDispersion deliveryCyclodextrinPharmaceutical drug
A combination oral liquid formulation of melatonin with a antihistaminic drug has been proposed as a sleep-aid agent. The solubility and stability of melatonin was improved by using cyclodextrin and adjusting the pH to a suitable value. The combination drug is expected to have a dual mode of action. The antihistaminic agent may help patient to fall asleep quickly and melatonin may be effective subsequently providing a sound sleep to the patient.
Owner:JOSHI HEMANT N +1
Method and composition for dermatoses
The invention provides a method and composition for treating dermatoses by topical application of a composition containing an antihistamine, an NSAID and optionally a botanical medicinal compound. The compositions are useful for skin conditions such as inflammatory skin conditions, including eczema, atopic dermatitis, non-allergic dermatitis, psoriasis and rosacea, or any inflammation of the skin.
Owner:FAIRFIELD CLINICAL TRIALS
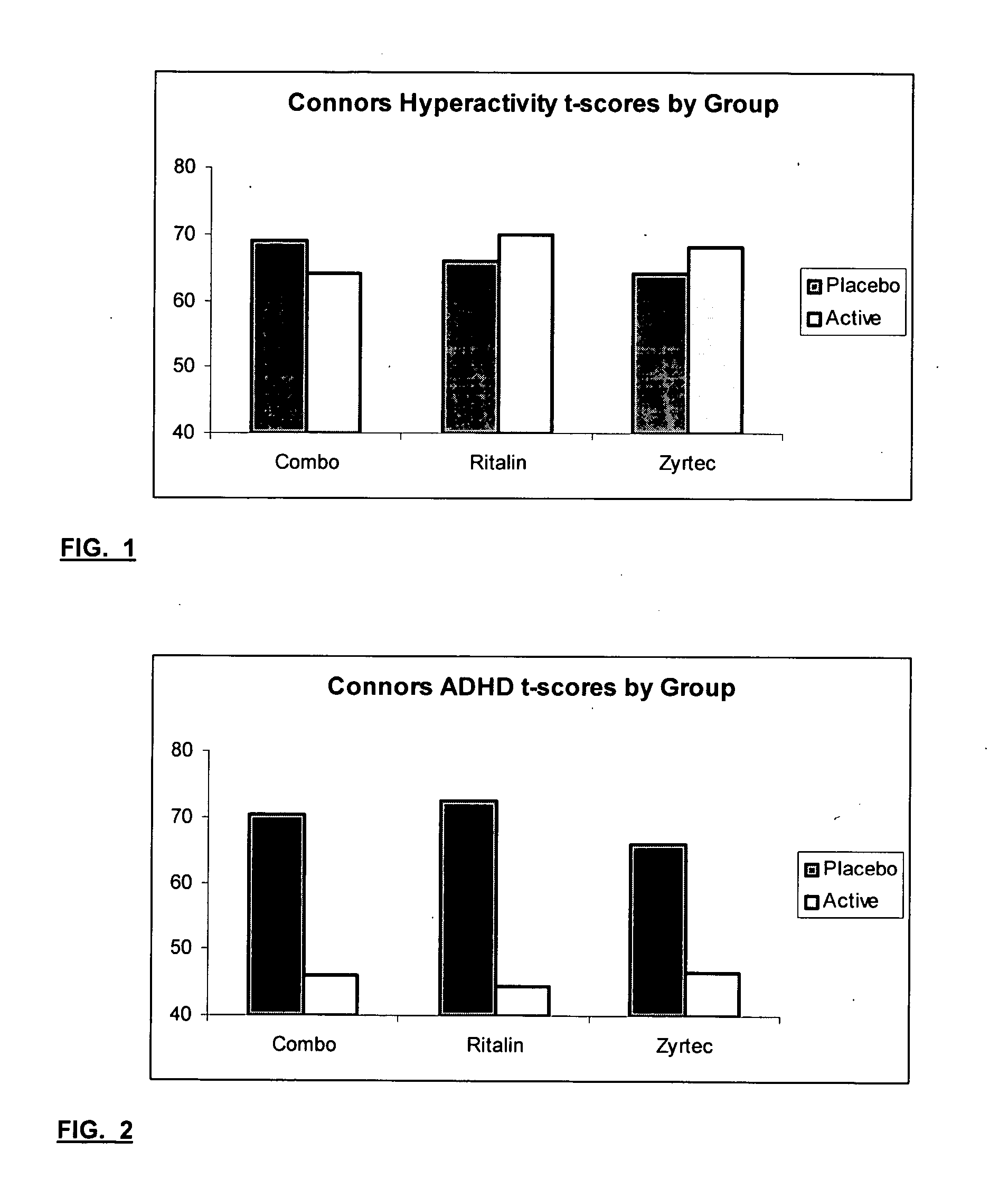
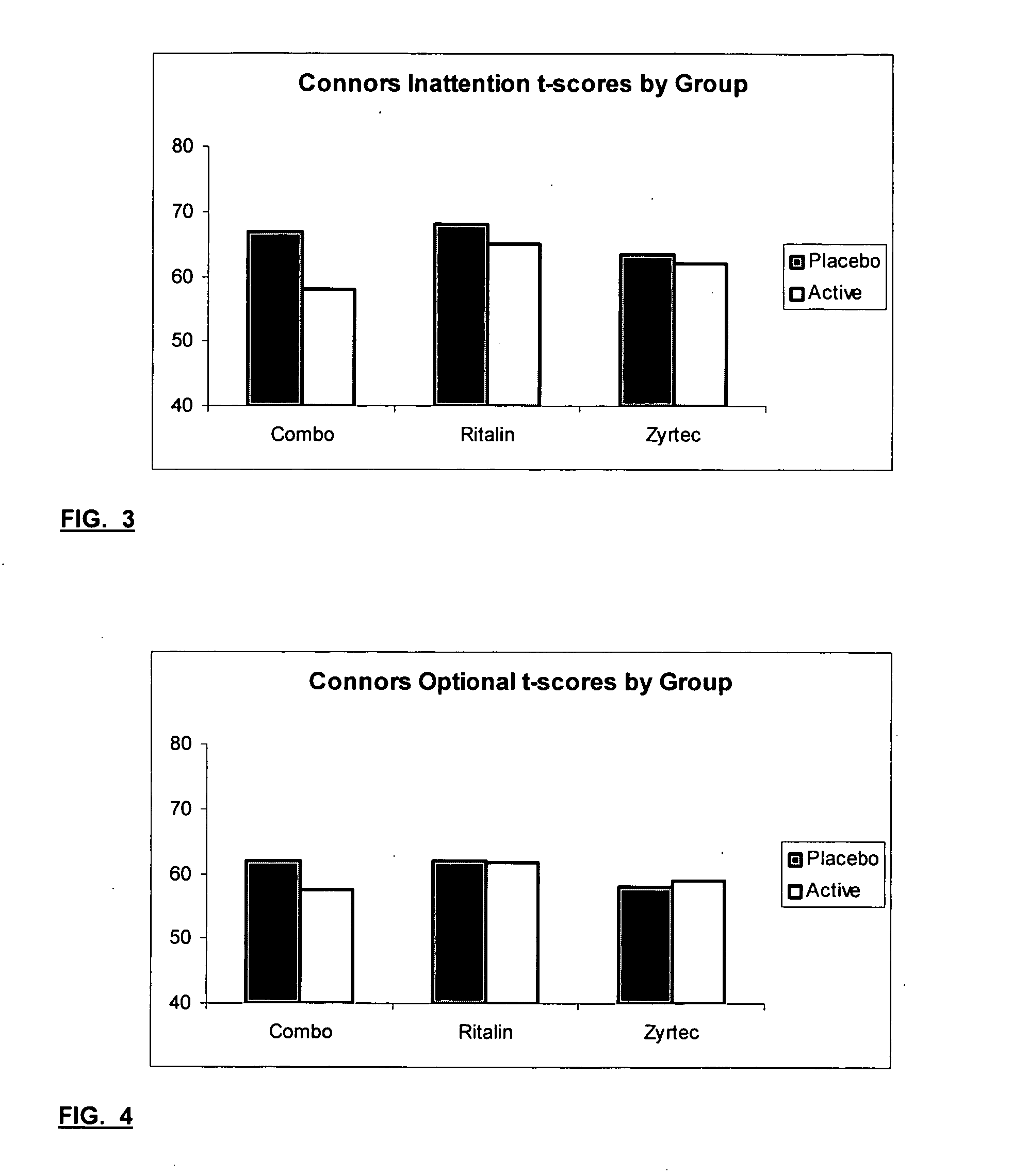
Treatment of behavioral disorders
InactiveUS20050192290A1Ameliorate behavioral disorderSufficient amountBiocideNervous disorderTherapeutic ACTHFexofenadine
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as ceterizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxieity, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Stable loratadine spill resistant formulation
The present invention provides for a storage stable pharmaceutical liquid suspension for oral administration having a pharmaceutically effective amount of an antihistamine. The storage stable suspension preferably contains loratadine. The present invention further provides a process of preparing the storage stable pharmaceutical liquid suspension as well as a method of treating a mammal with a therapeutically effective amount of loratadine in the stable pharmaceutical liquid suspension.
Owner:TARO PHARMA US INC
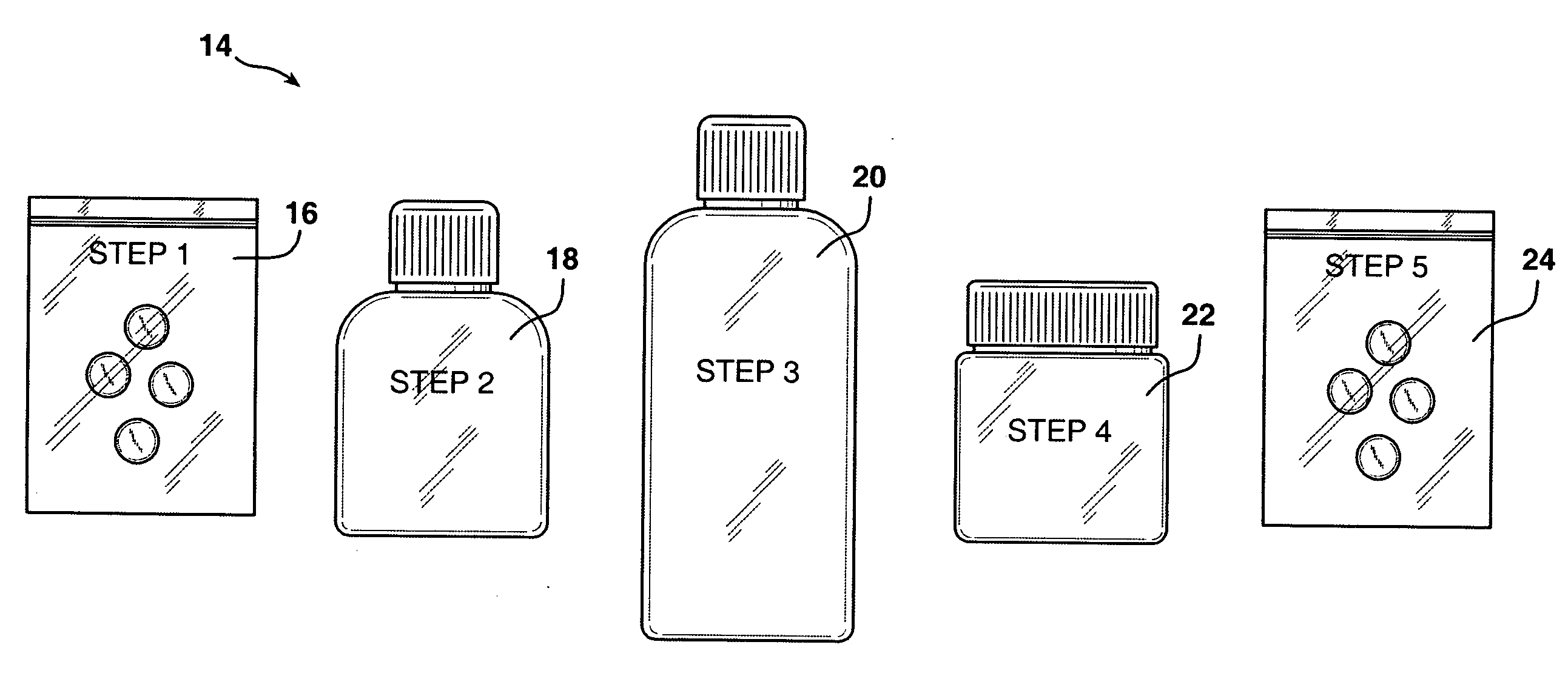
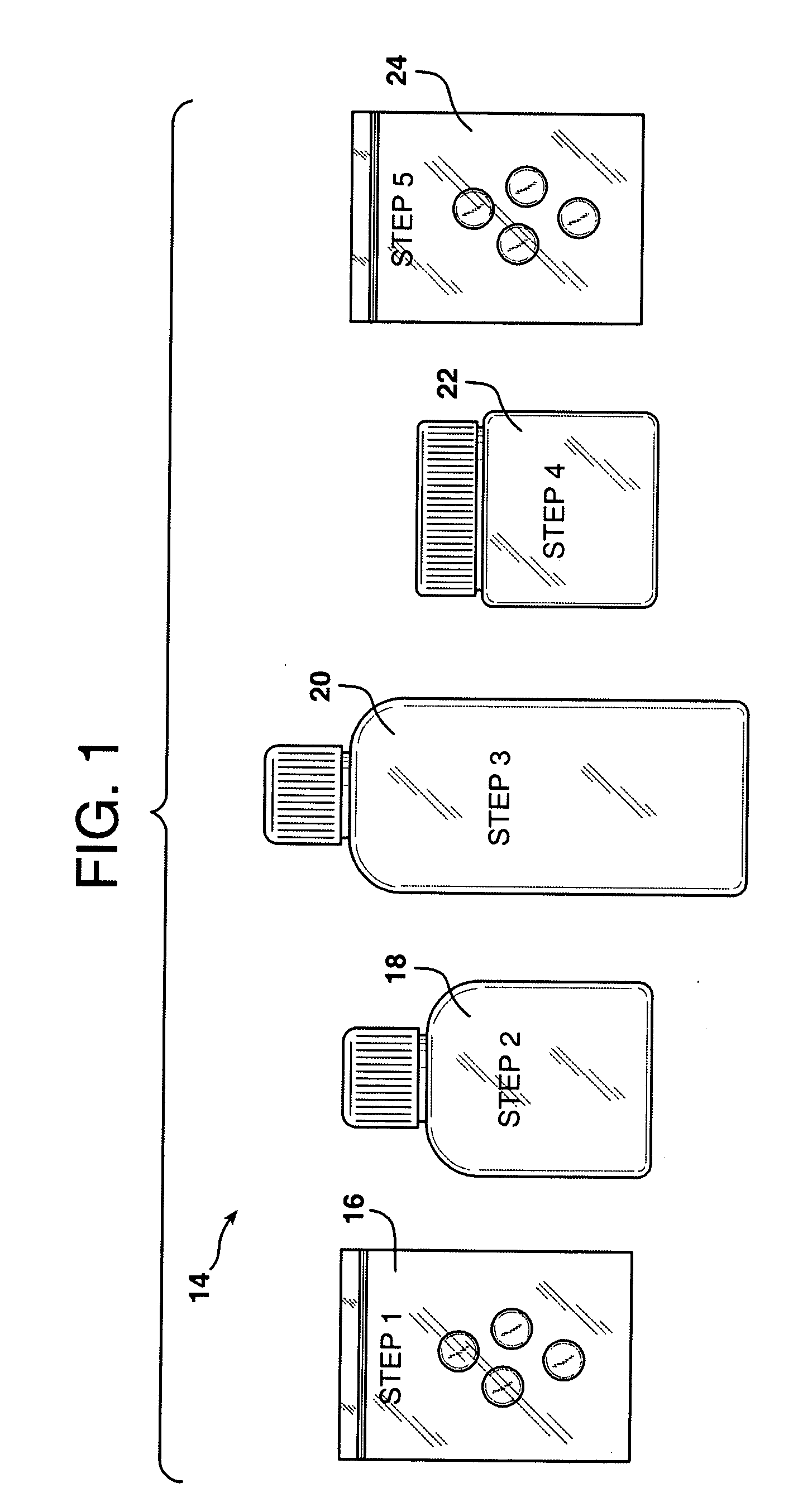
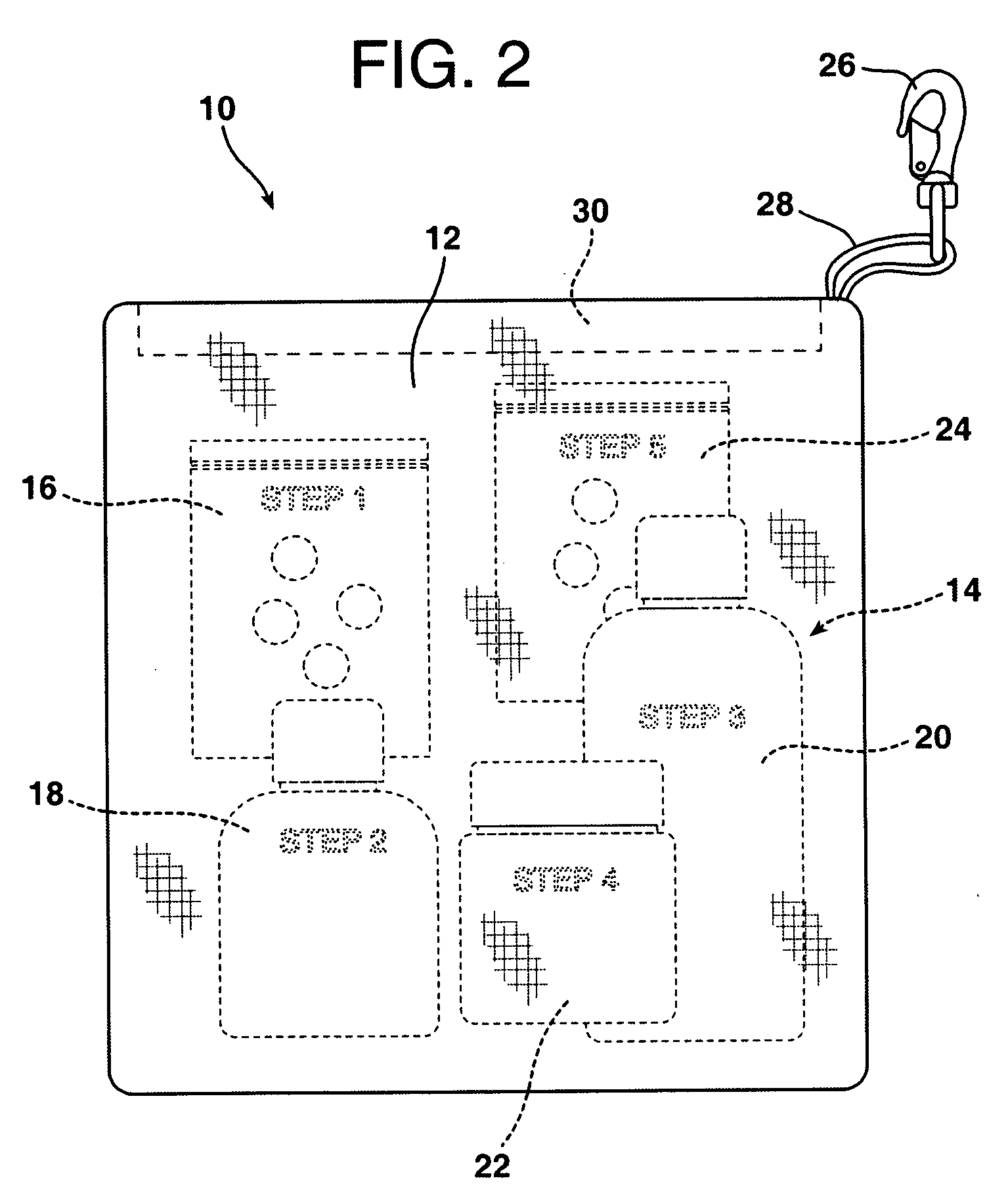
Personalized pharmaceutical kits, packaging and compositions for the treatment of allergic conditions
This invention relates to personalized pharmaceutical kits, packaging, compositions, and methods for treatment of a mammal, comprising at least one antihistamine for treating an allergic disease or condition in a mammal, in combination with at least one wakefulness promoting agent for preventing sedative effects during day time use, while promoting the antihistamine and sedating effect during evening use.
Owner:JDP THERAPEUTICS LLC
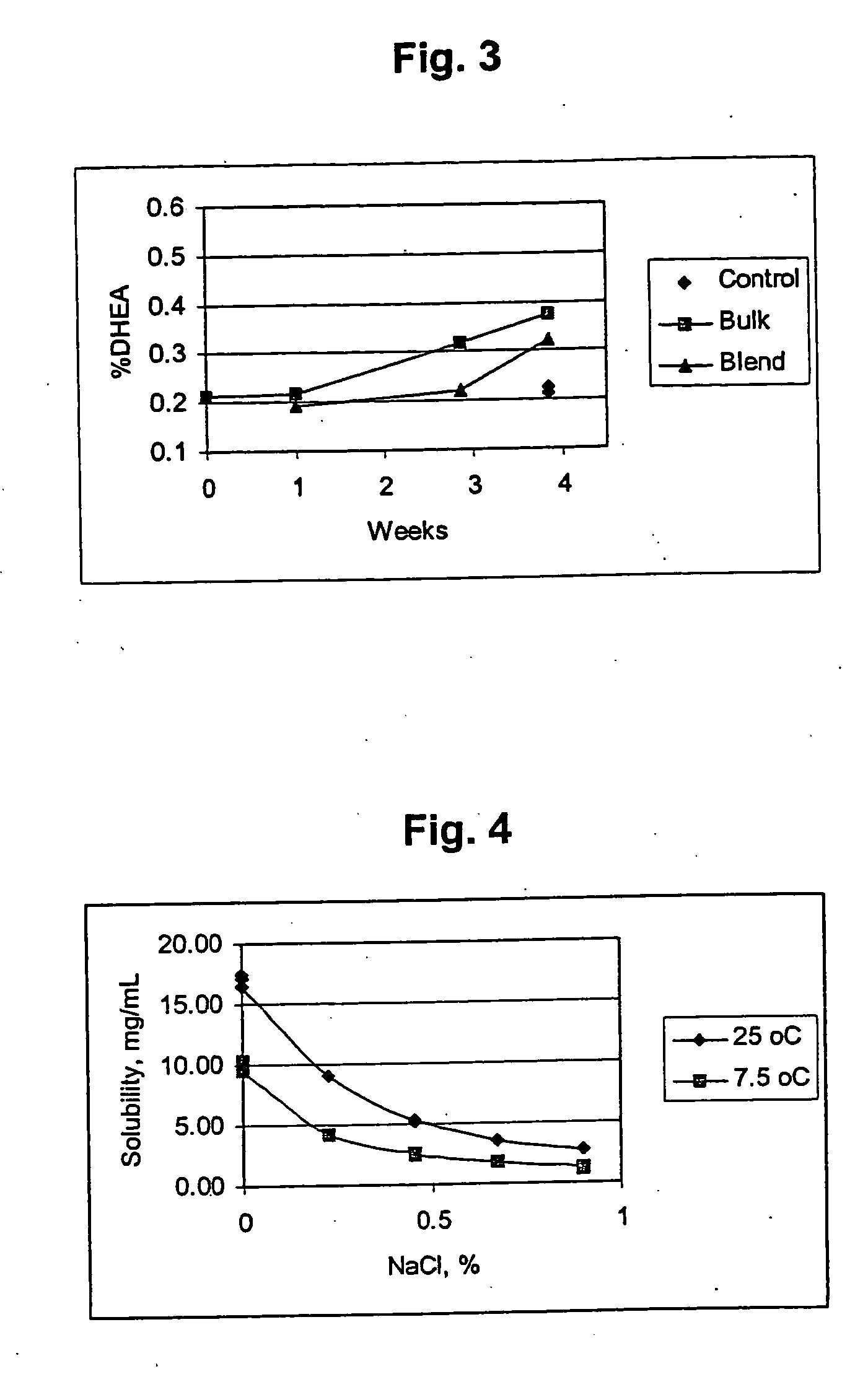
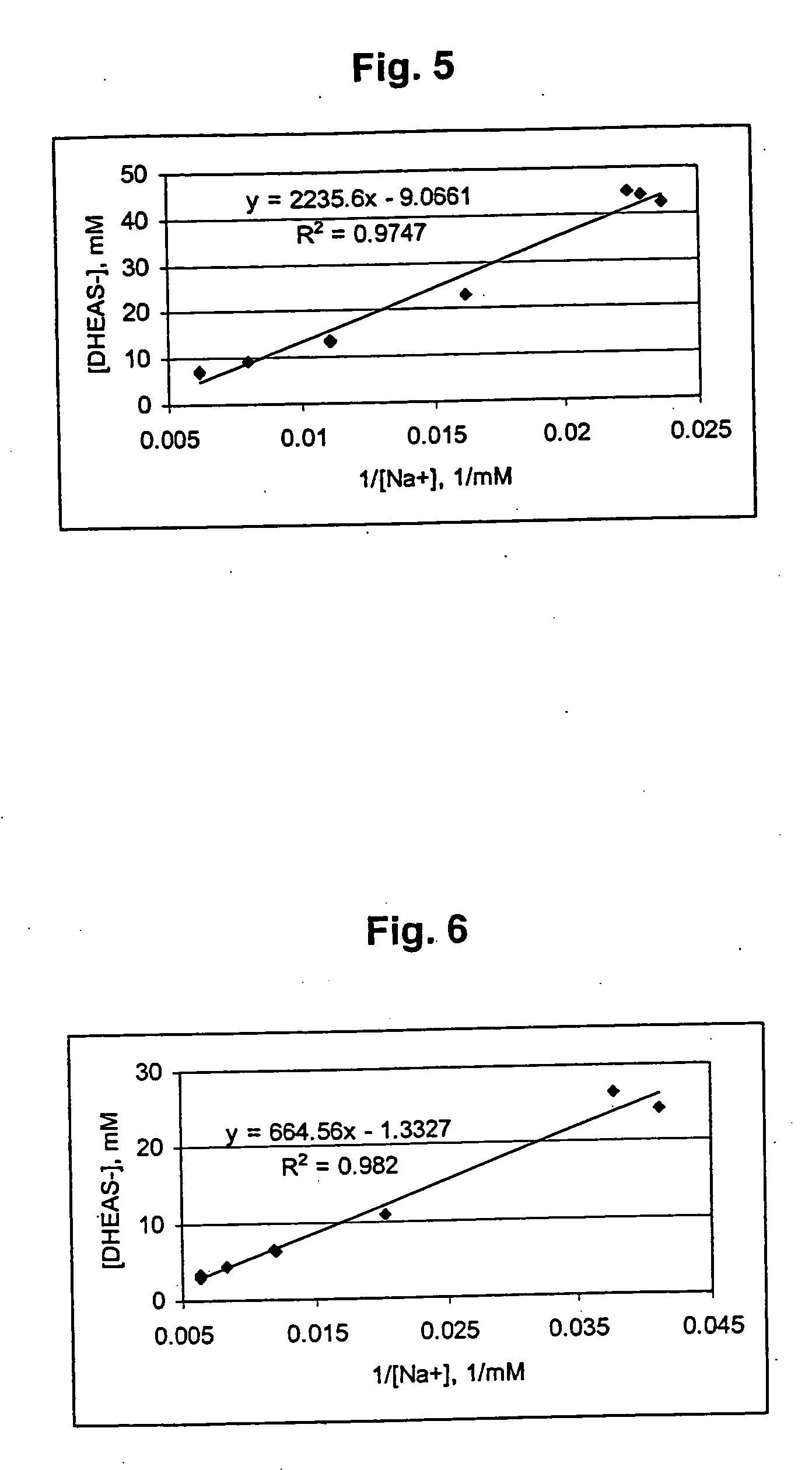
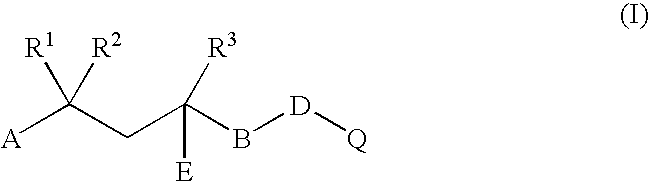
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with an antihistamine for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026890A1Alleviate different aspectConvenient treatmentBiocideOrganic active ingredientsDiseaseActive agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising an antihistamine for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Compositions and Methods for Treating, Controlling, Reducing, Ameliorating, or Preventing Allergy
InactiveUS20080064721A1Treating and controlling and reducing and ameliorating and preventing allergyBiocideSenses disorderAllergyPharmaceutical Substances
A composition for treating, controlling, reducing, ameliorating, or preventing allergy comprises a dissociated glucocorticoid receptor agonist (“DIGRA”), a prodrug thereof, a pharmaceutically acceptable salt thereof, or a pharmaceutically acceptable ester thereof. The composition can comprise an anti-allergic medicament and / or an additional anti-inflammatory agent and can be formulated for topical application, injection, or implantation. The anti-allergic medicament can comprise an antihistamine, a mast-cell stabilizer, a leukotriene inhibitor, an immunomodulator, an anti-IgE agent, or a combination thereof.
Owner:BAUSCH & LOMB INC
Phenylephrine pulsed release formulations and pharmaceutical compositions
The invention discloses a pulsed-release formulation or a pharmaceutical composition comprising phenylephrine. The pharmaceutical composition comprises an immediate-release component and an enteric-coated component formulated together either in solid form or in a suspension. The enteric-coated component comprises microcrystals seeded with phenylephrine as an active ingredient and coated with a pH sensitive coating to delay release of the phenylephrine. The pharmaceutical composition can further comprise at least one active selected from the group consisting of an antihistamine, an analgesic, anti-pyretic, non-steroidal anti-inflammatory and mixtures of two or more said actives.
Owner:SCHERING CORP
Method of treating snoring and other obstructive breathing disorders
InactiveUS20040258621A1Reduce eliminateRelieve symptomsBiocideImpression capsNasal cavityGastrointestinal reflux
A method of pharmaceutically managing snoring and impaired breathing is provided. This invention relates to treating snoring, sleep apnea, and other forms of sleep-disordered breathing in those with or without symptoms of or the diagnosis of gastro-intestinal reflux disease (GERD). It comprises administration of a therapeutically effective dose of Prevacid (Lansoprazole) or any other medication that can be used to treat symptoms of hyper-acidity or gastrointestinal reflux disease (GERD). The therapeutic medication may be used alone or in combination with other pharmacologic agents or mechanical modalities including but not limited to decongestants, antihistamines or mechanical nasal toilet. It also is of benefit in improving breathing disorders that are present while awake.
Owner:SOHNSTEARNS & STERN
Therapeutic causing contraction of mucosal tissue, method of treating diseases relating to mucosal tissues, injector and therapeutic set
InactiveUS20050187303A1Simple and safe processMinimal invasivenessBiocideHydroxy compound active ingredientsNoseMucosal inflammation
The invention provides for a therapeutic causing contraction of a mucosal tissue whereby various diseases relating to mucosal tissues can be easily, safely and treated with minimal invasiveness, a method of treating various diseases relating to mucosal tissues with the use of the therapeutic causing contraction of a mucosal tissue, and an injector and a therapeutic set usable in the treatment method. The invention also encompasses a therapeutic causing contraction of nasal mucosal tissue containing ethanol as the active ingredient preferably together with a steroid and / or an antihistaminic agent; a method of treating diseases with mucosal inflammation using the above therapeutic causing contraction of a mucosal tissue, and an injector and a therapeutic set usable in the treatment method.
Owner:JAPAN SCI & TECH CORP
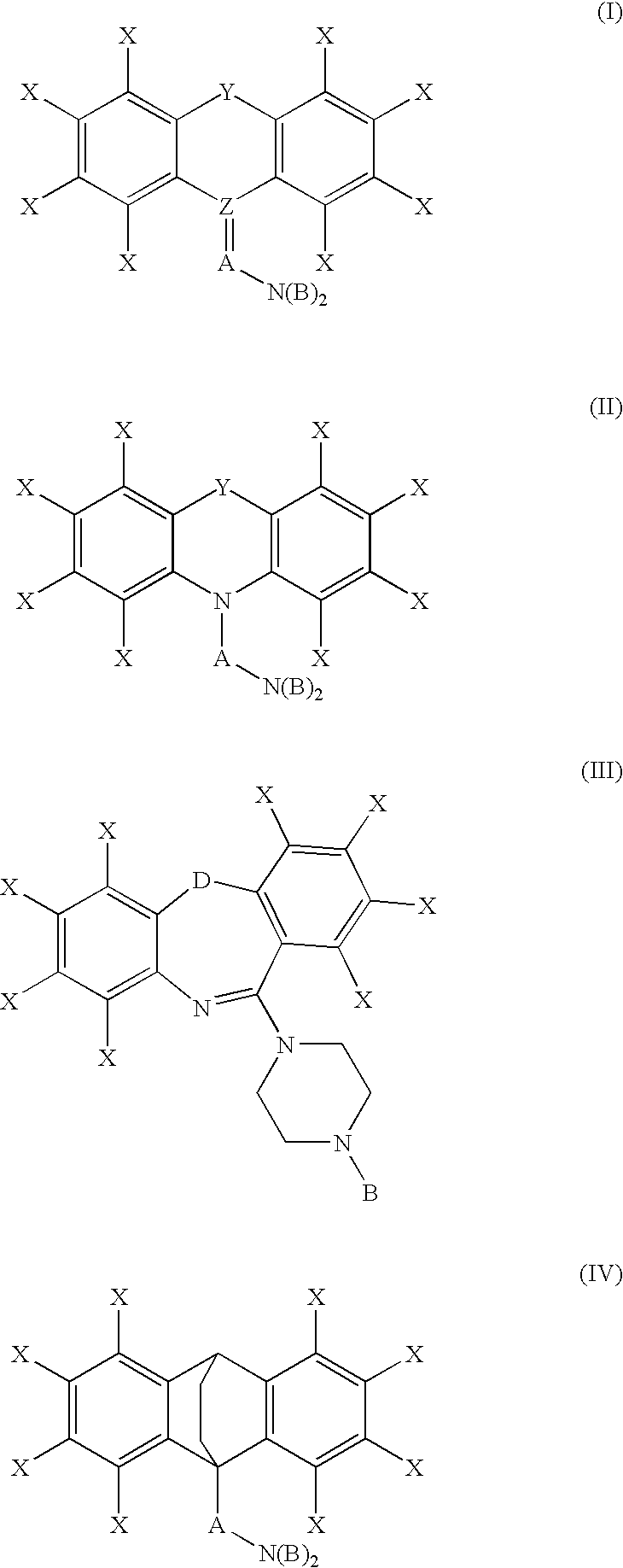
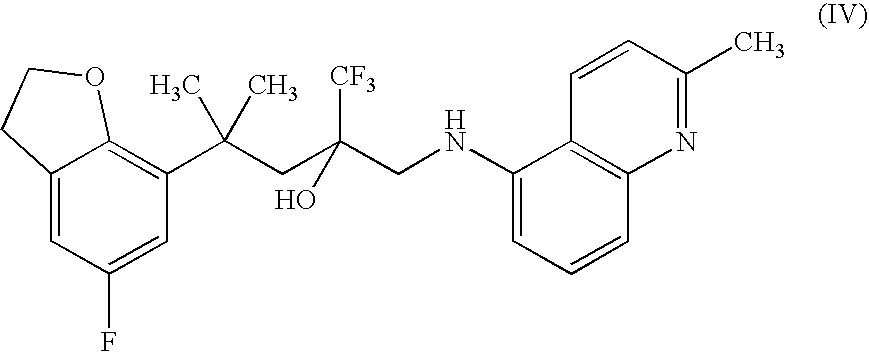
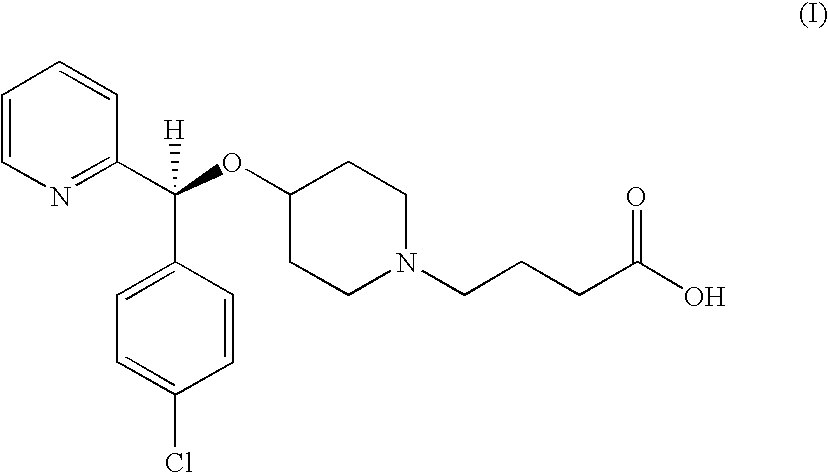
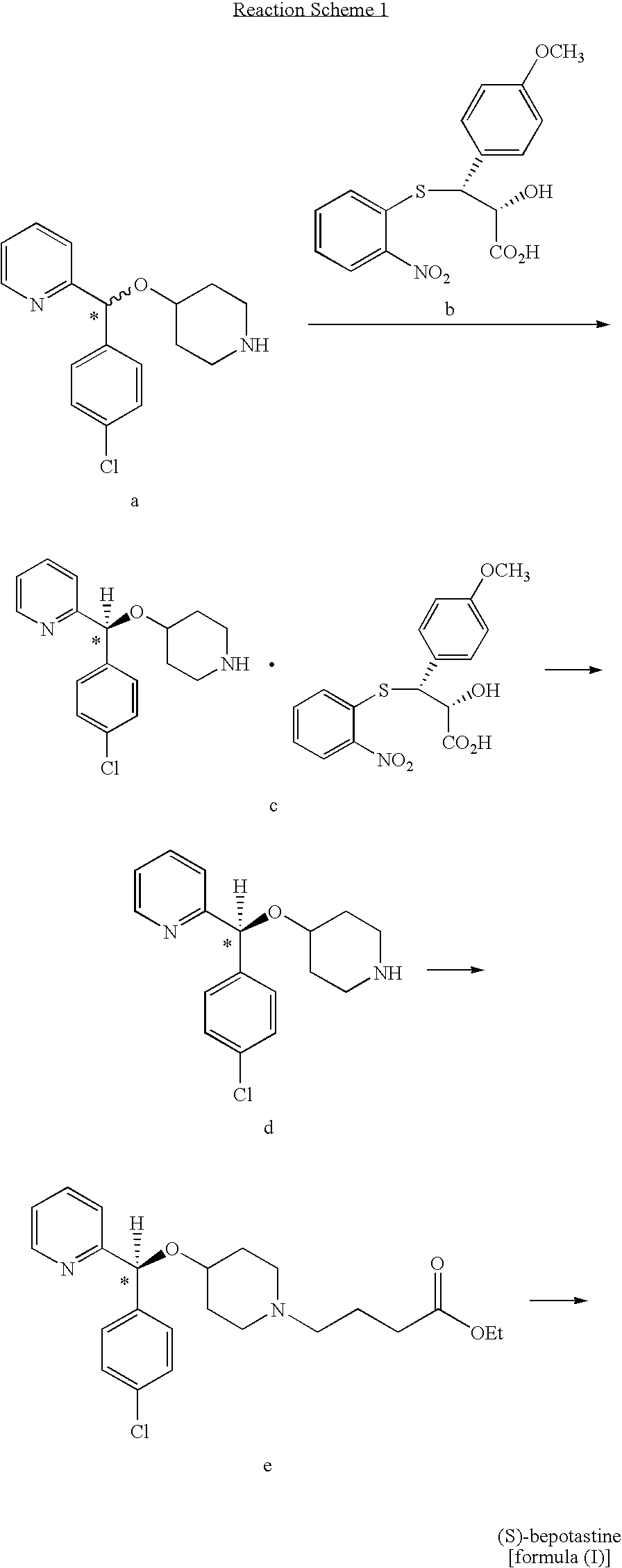
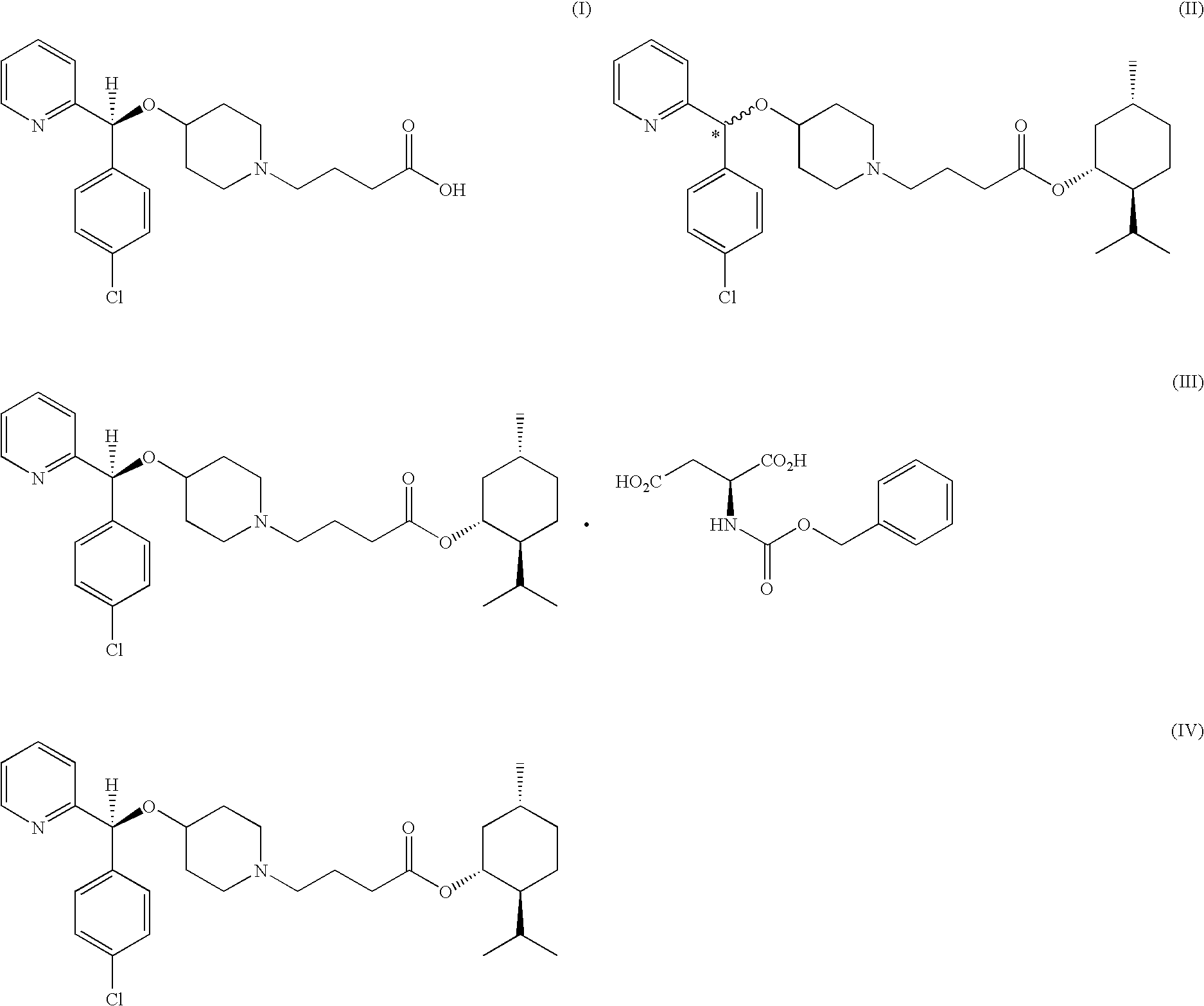
Process for preparing bepotastine and intermediates used therein
InactiveUS20100168433A1Efficient processingHighly stereospecificOrganic chemistryAnti-Allergic AgentsOrganic solvent
A process for stereospecific preparation of bepotastine of formula (I) and novel intermediates used therein having formulae (II) to (IV) are provided. The inventive process comprises subjecting (RS)-4-[(4-chlorophenyl)(2-pyridyl)methoxy]piperidine to a reaction with a 4-halobutanoic acid l-menthyl ester, halo being chloro, bromo or iodo, in an organic solvent in the presence of a base to produce (RS)-bepotastine l-menthyl ester of formula (II), conducting a reaction of the compound of formula (II) with N-benzyloxycarbonyl L-aspartic acid in an organic solvent to induce selective precipitation of bepotastine l-menthyl ester.N-benzyloxycarbonyl L-aspartate of formula (III), filtering the precipitates formed in step 2) to isolate the compound of formula (III), treating the compound of formula (III) with a base to liberate bepotastine l-menthyl ester of formula (IV), and hydrolyzing the compound of formula (IV) in the presence of a base. The inventive process can provide bepotastine having a high optical purity of not less than 99.5% in a high yield, and thus, is useful in the development of anti-histamines and anti-allergic agents.
Owner:HANMI SCI CO LTD
Lesion and ulcer medication
InactiveUS20020044956A1Pain reliefEffective antibioticBiocidePowder deliveryMedicineSecondary infection
Pharmaceutical compositions are provided which comprise effective amounts of antimicrobials, anti-inflammatories, and antihistamines, to provide an ulcer medication which prevents secondary infections and promotes healing while providing immediate relief from pain. The composition may be used to treat a variety of ulcers including but not limited to intraoral aphthous ulcers and non-oral lesions. The compositions of the present invention may also be combined with materials capable of forming seals over ulcers or lesions to further promote the healing process.
Owner:CAMPBELL PHILLIP
Novel use of antihistamine agents for the preventive or early treatment of inflammatory syndromes, in particular those triggered by togaviruses
The present invention relates to the use of at least one antihistamine agent for the preparation of a medicament for use in the preventive or early treatment of inflammatory syndromes of viral origin, in particular arthritis of the distal joints, more particularly those triggered by togaviruses. The invention also relates to a combination product of at least one antihistamine agent and of at least one antiserotonin agent for its simultaneous, separate or sequential use in preventive or early therapy for inflammatory syndromes of viral origin, in particular arthritis of the distal joints, more particularly those triggered by togaviruses.
Owner:PIERRE FABRE MEDICAMENT SAS
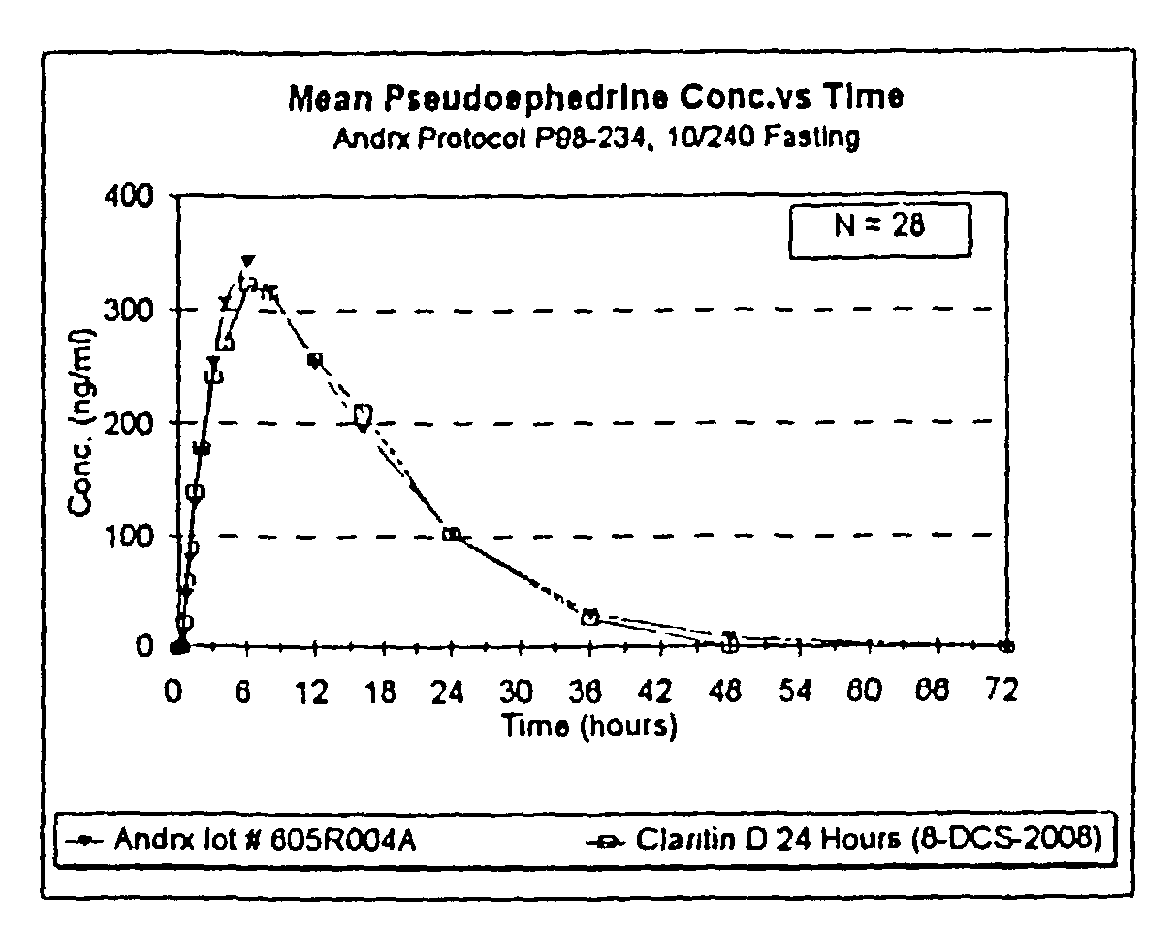
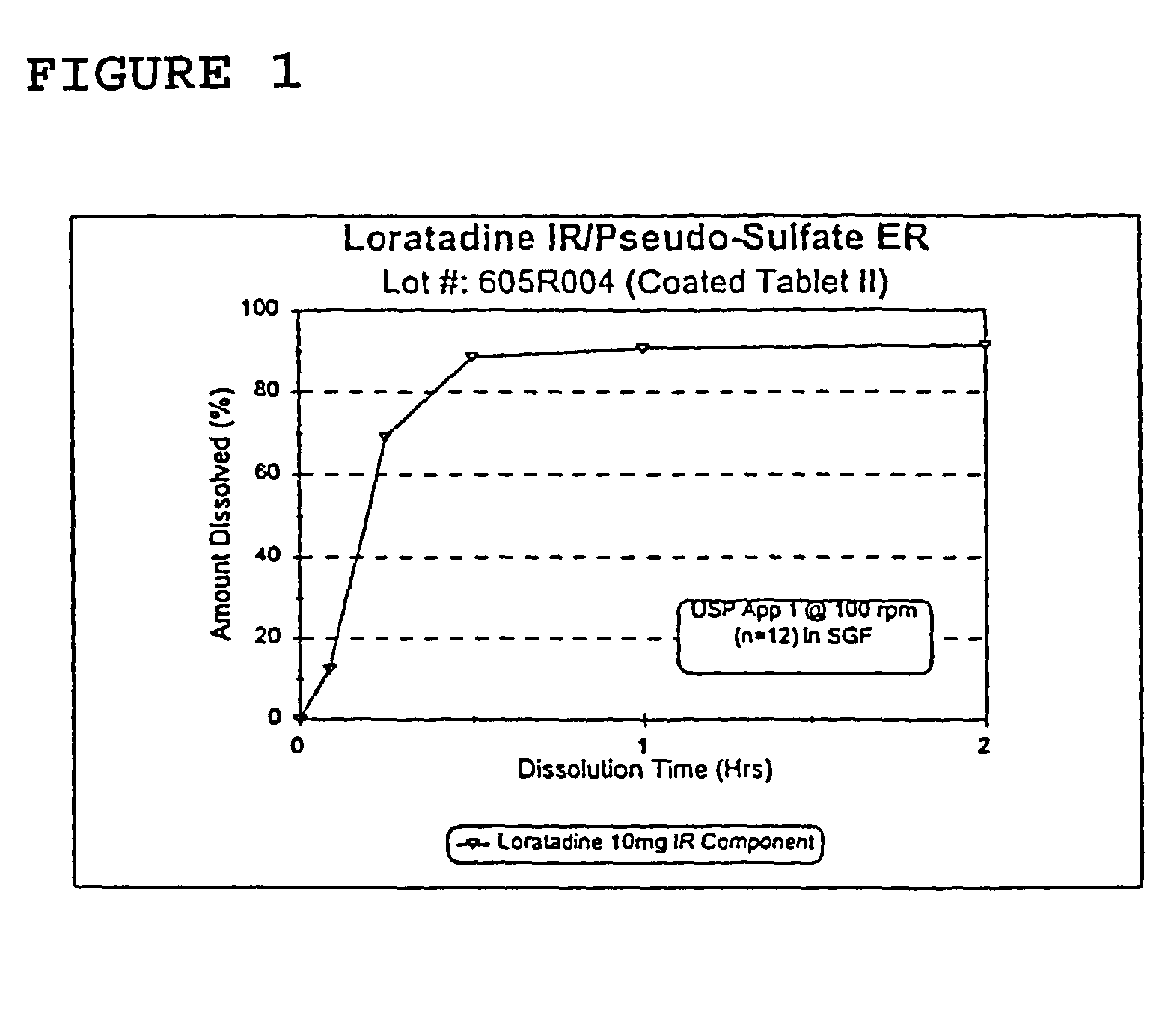
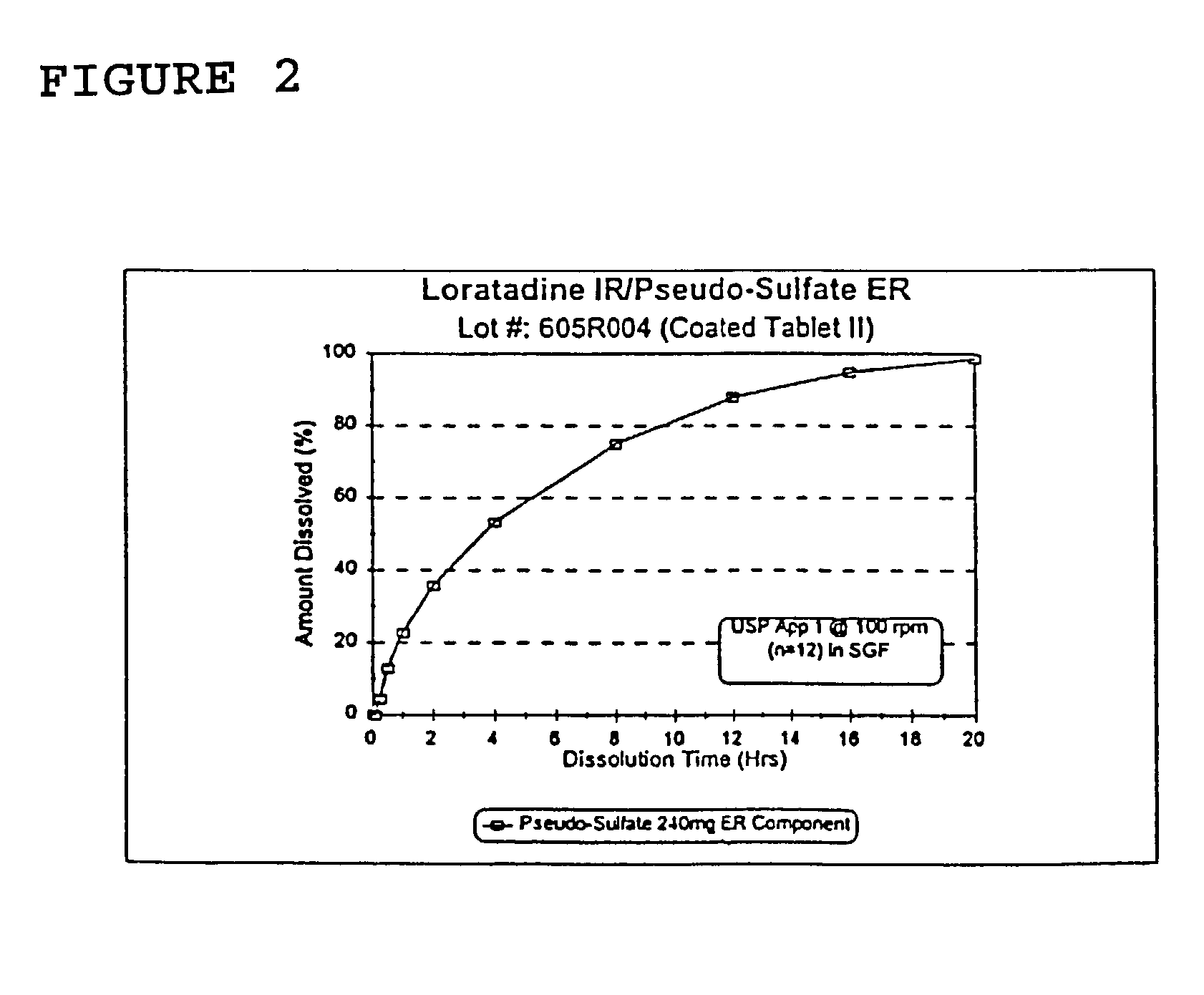
Once a day antihistamine and decongestant formulation
A controlled release pharmaceutical formulation for the administration of an antihistamine and decongestant to a patient wherein the formulation employs a compressed matrix core for the controlled release of a decongestant and an immediate release coating for the immediate release of the antihistamine.
Owner:ANDRX PHARMA INC
Compositions of non-steroidal anti-inflammatory drugs and decongestants or anti-histamines
The present invention relates to improved dosage forms of pharmaceuticals for treating rhinitis associated with allergies and colds. The dosage forms include an effective amount each of a non-steroidal anti-inflammatory drug, and a decongestant or an antihistamine wherein the effective amount of the decongestant or antihistamine is less than 75% of an amount present in an approved dose of the decongestant or the antihistamine relative to an amount of the NSAID corresponding to about 100% of the amount present in a normal strength dosage form of the NSAID.
Owner:WYETH LLC
Kit for insect bites
InactiveUS20090258044A1Reduce swellingReduce inflammationBiocideCosmetic preparationsMicroorganismNiacin
A travel kit used for inhibiting and reducing the incidence and effect of insect bites. The kit includes a pouch for enclosing a plurality of containers therein. The pouch has a closure means to maintain the containers in the pouch and a means for removably attaching the pouch to a person. The plurality of containers includes a first container containing at least one oral tablet comprising an effective amount of niacin (vitamin B-3) or its corresponding amide for inhibiting or reducing the incidence of insect bites. A second container is provided which contains a soap composition that includes an effective antimicrobial compound that when applied to a bite area of a person's skin that has an insect bite disinfects such bite area. A third container contains a mud composition that includes an effective amount of a clay that when applied to the bite area reduces the swelling, inflammation or itching of the bite area. A fourth container is provided which contains an effective sterilizing wash composition that when applied to the bite area sterilizes the bite area and assists in reducing the swelling, inflammation or itching of the bite area. Optionally, the kit further includes a fifth container containing at least one oral tablet comprising an effective amount of an antihistamine.
Owner:MOSHER JOHN
Compounds and methods for treatment of primary biliary cholangitis
The present invention relates to, inter alia, methods of treatment and combinations of (R)-2-(7-(4-cyclopentyl-3-(tri-fluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid (Compound 1) useful for the treatment of primary biliary cholangitis (PBC). In some embodiments, the methods further comprise administering Compound 1, or a pharmaceutically salt, solvate, or hydrate thereof, in combination with a compound selected from the group consisting of: an antihistamine (diphenhydramine), cholestyramine (questran, prevalite), rifampin, an opioid antagonist (naloxone), pilocarpine (isopto carpine, salagen), cevimeline (evoxac), calcium and / or vitamin D supplement, and vitamin A, D, E and / or K supplement. Other embodiments, relate to titration packages for enabling compliance with a regimen of changing dosage of a medication over a period of time for the treatment of primary biliary cholangitis (PBC).
Owner:ARENA PHARMA
Cooling adjunct for medications to treat disorders in the nasal cavity
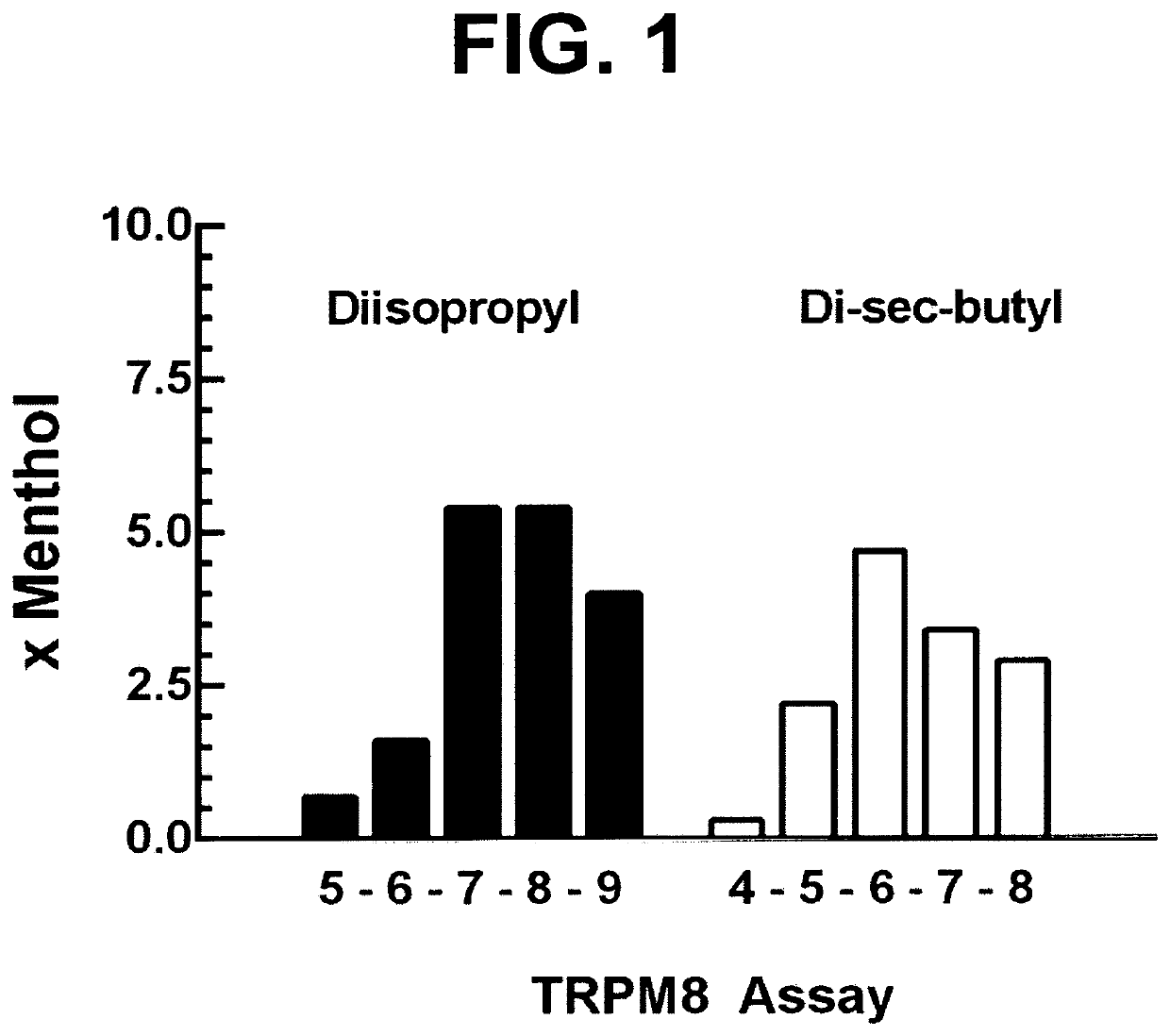
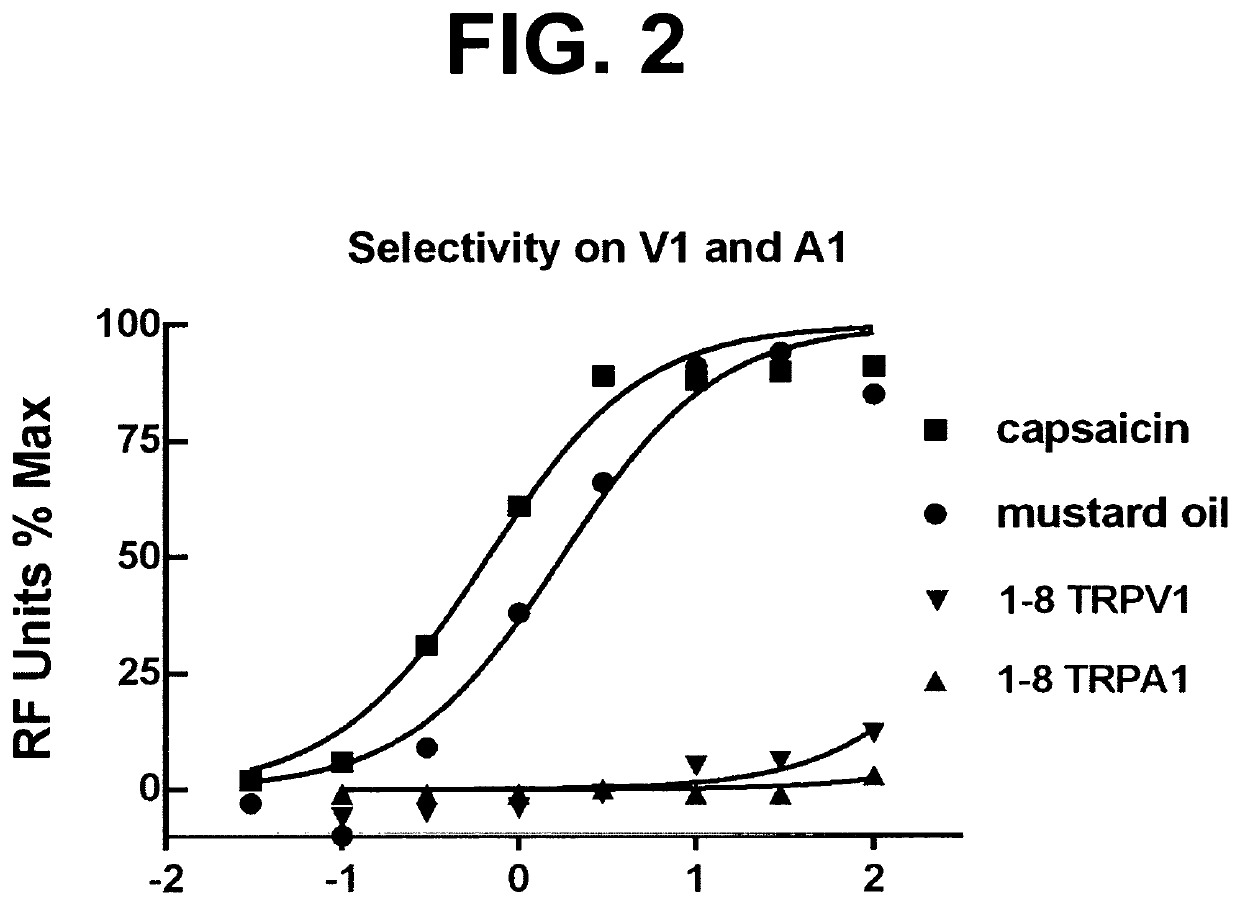
ActiveUS10722477B2Increase adherence/complianceRelief of nasal irritation and congestionInorganic non-active ingredientsMedical devicesDiseaseAlkane
The present discovery pertains generally to the field of therapeutic compounds. More specifically the present discovery pertains to certain di-alkyl-phosphinoyl-alkanes as described herein, DIPA-1-8 and DIPA-1-9, and 2-6 and 2-7 that are collectively referred to herein as “DAPA compounds”, that are useful in the treatment of disorders (e.g., diseases) including: sensory discomfort (e.g., caused by inflammation, irritation, itch, or pain) in the nasal cavity. The applicant has found that localized delivery of DAPA compounds in combination with an intranasal steroid or an intranasal antihistamine immediately relieves nasal discomfort and enhances patient adherence to the use of the nasal medications.
Owner:WEI EDWARD T
Compositions and methods for treating, controlling, reducing, ameliorating, or preventing allergy
InactiveUS20110104159A1BiocidePhosphorous compound active ingredientsAllergyPharmaceutical Substances
A composition for treating, controlling, reducing, ameliorating, or preventing allergy comprises a dissociated glucocorticoid receptor agonist (“DIGRA”), a prodrug thereof, a pharmaceutically acceptable salt thereof, or a pharmaceutically acceptable ester thereof. The composition can comprise an anti-allergic medicament and / or an additional anti-inflammatory agent and can be formulated for topical application, injection, or implantation. The anti-allergic medicament can comprise an antihistamine, a mast-cell stabilizer, a leukotriene inhibitor, an immunomodulator, an anti-IgE agent, or a combination thereof.
Owner:BAUSCH & LOMB INC
Treatment of sunburn using analgesics and antihistamines
ActiveUS20120225914A1Safe and effective and convenient treatmentTreat sunburnBiocideSmall article dispensingSunburnAnalgesic agents
Combination compositions and kits comprising an analgesic and an antihistamine are provided as well as methods of use in treating sunburn.
Owner:SEPHORIS PHARMA
A pharmaceutical composition for treatment of acute, chronic pain and/or neuropathic pain and migraines
Pharmaceutical compositions are disclosed for the treatment of acute, chronic and / or neuropathic pain. The pharmaceutical compositions are comprised of a therapeutically effective combination of a nicotine receptor partial agonist and an analgesic agent and a pharmaceutically acceptable carrier. The analgesic agent is selected from opioid analgesics, NMDA antagonists, substance P antagonists, COX 1 and COX 2 inhibitors, tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), capsaicin receptor agonists, anesthetic agents, benzodiazepines, skeletal muscle relaxants, migraine therapeutic agents, anti-convulsants, anti-hypertensives, anti-arrythmics, antihistamines, steroids, caffeine, and botulinum toxin. The method of using these compounds and a method of treating acute, chronic and / or neuropathic pain and migraine in a mammal including a human is also disclosed.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Novel pharmaceutical compositions for antihistaminic-decongestant combination and method of making such compositions
The present invention relates to pharmaceutical compositions of antihistamine-decongestant combination. Specifically the invention relates to bilayered tablet formulation comprising antihistaminic decongestant combination. More specifically present invention relates to the novel polymorph of fexofenadine or pharmaceutically accepted salts thereof, with at least one decongestant in the form of bilayered tablet. The preferred polymorphs are polymorph A and polymorph X of fexofenadine hydrochloride.
Owner:DR REDDYS LAB LTD
Method for Treating a Pulmonary Disease State in Mammals by Up Regulating Indigenous in vivo Levels of Inflammatory Agents in Mammalian Cells
The present invention provides novel methods for treating a pulmonary disease state in mammals by up-regulating indigenous in vivo levels of an inflammatory agent in mammalian cells comprising contacting the mammalian cells with a therapeutically effective amount of an inflammatory regulator and a pharmaceutical agent. The inflammatory agent is selected from the group consisting of cytokines, transforming growth factor-β, elastase, and white blood cells, and wherein the inflammatory regulator is selected from the group consisting of pyruvates and pyruvate precursors. The pharmaceutical agent is selected from the group comprising anti-bacterial agents, anti-virals, anti-fungals, anti-tumors, antihistamines, proteins, enzymes, hormones such as insulin, non-steroidal anti-inflammatories, cytokines, steroids, and nicotine.
Owner:CELLULAR SCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com