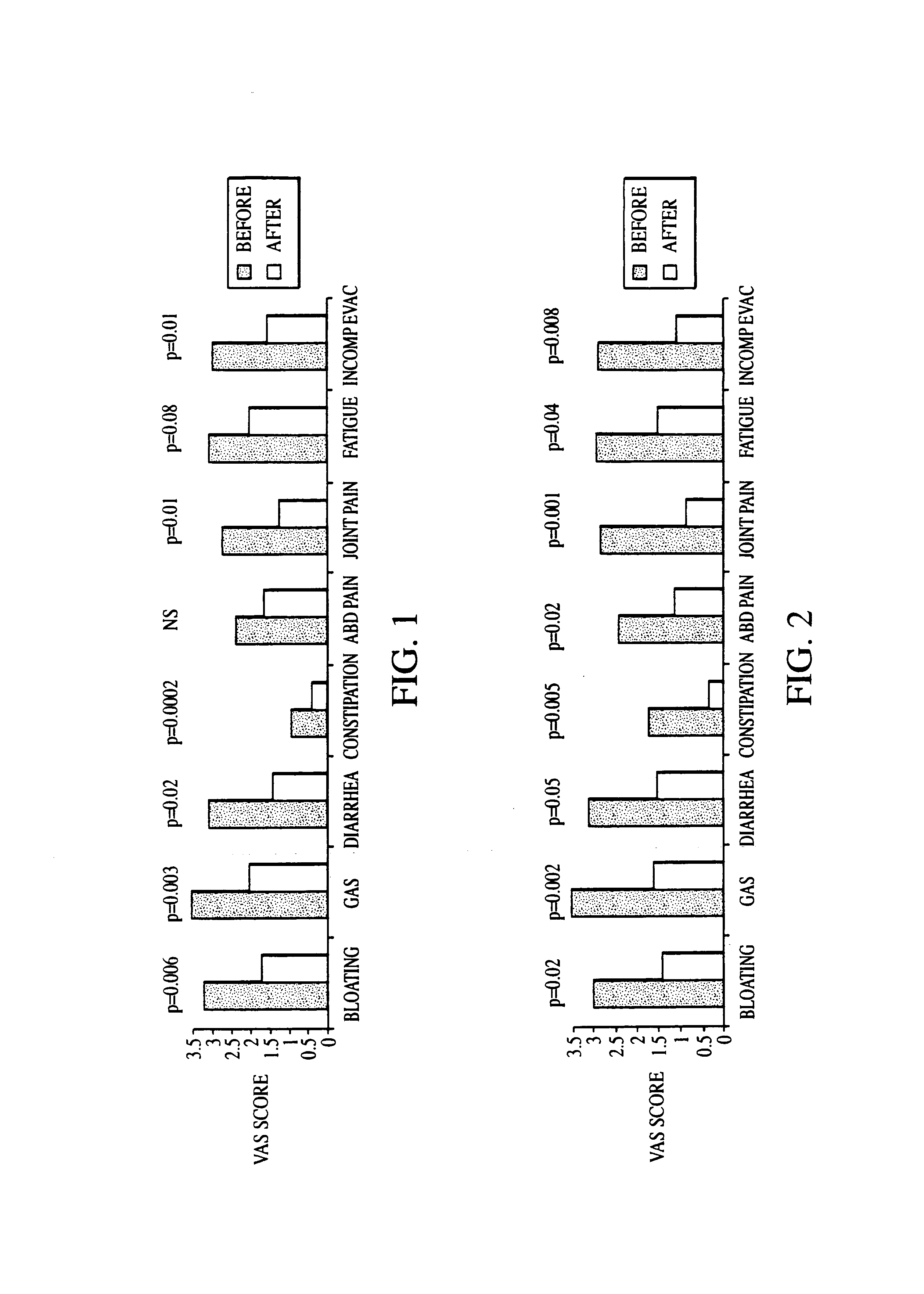
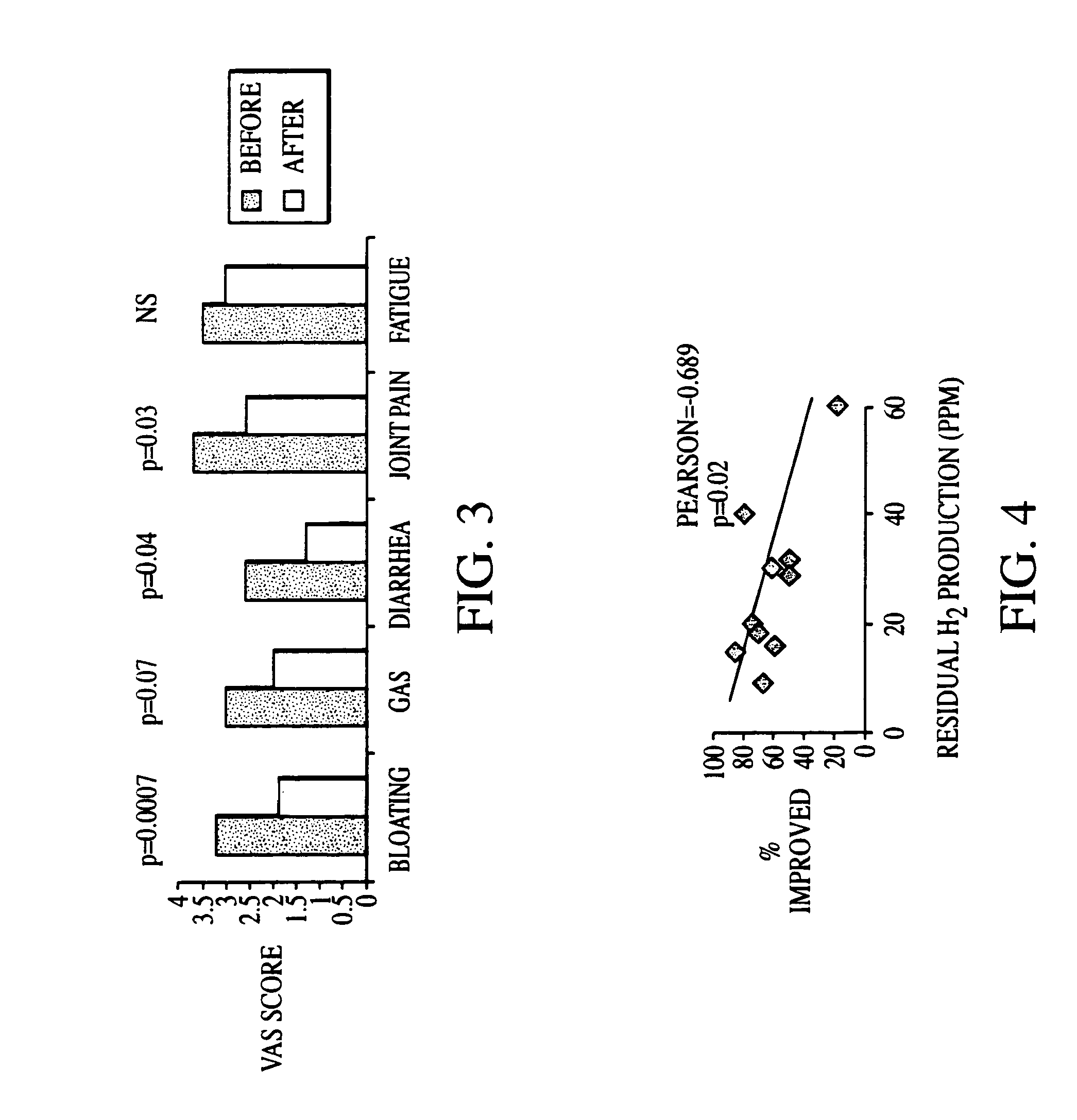
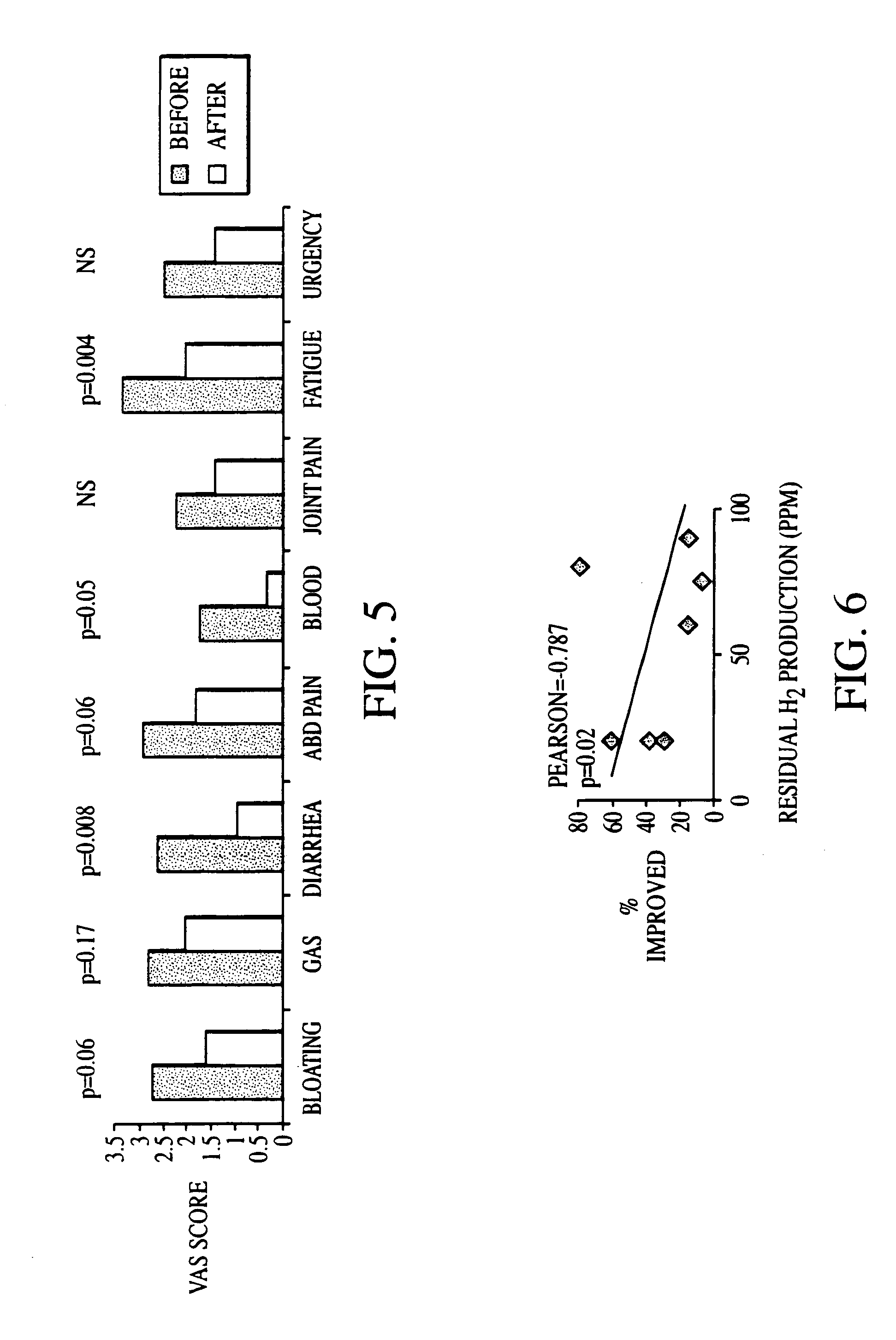
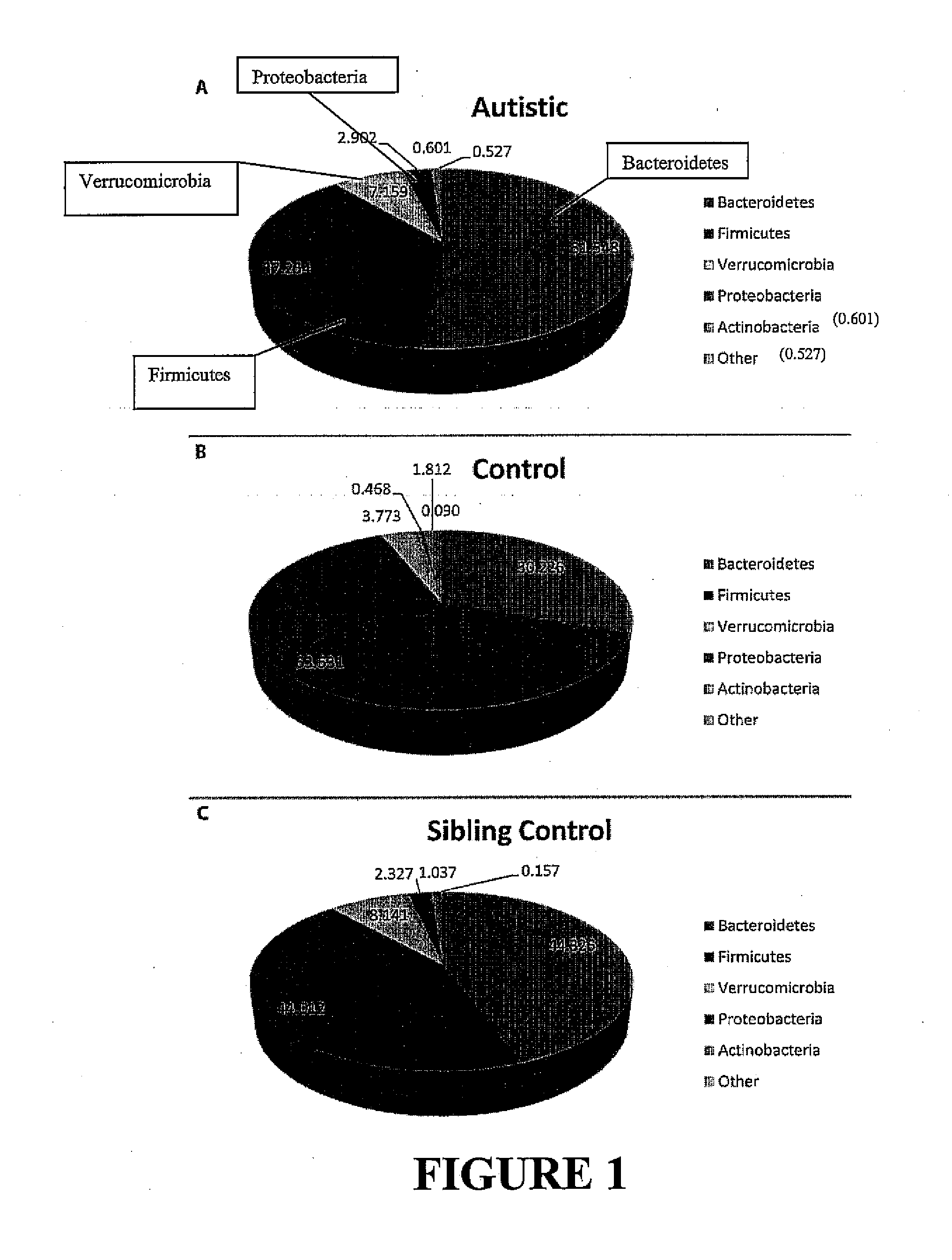
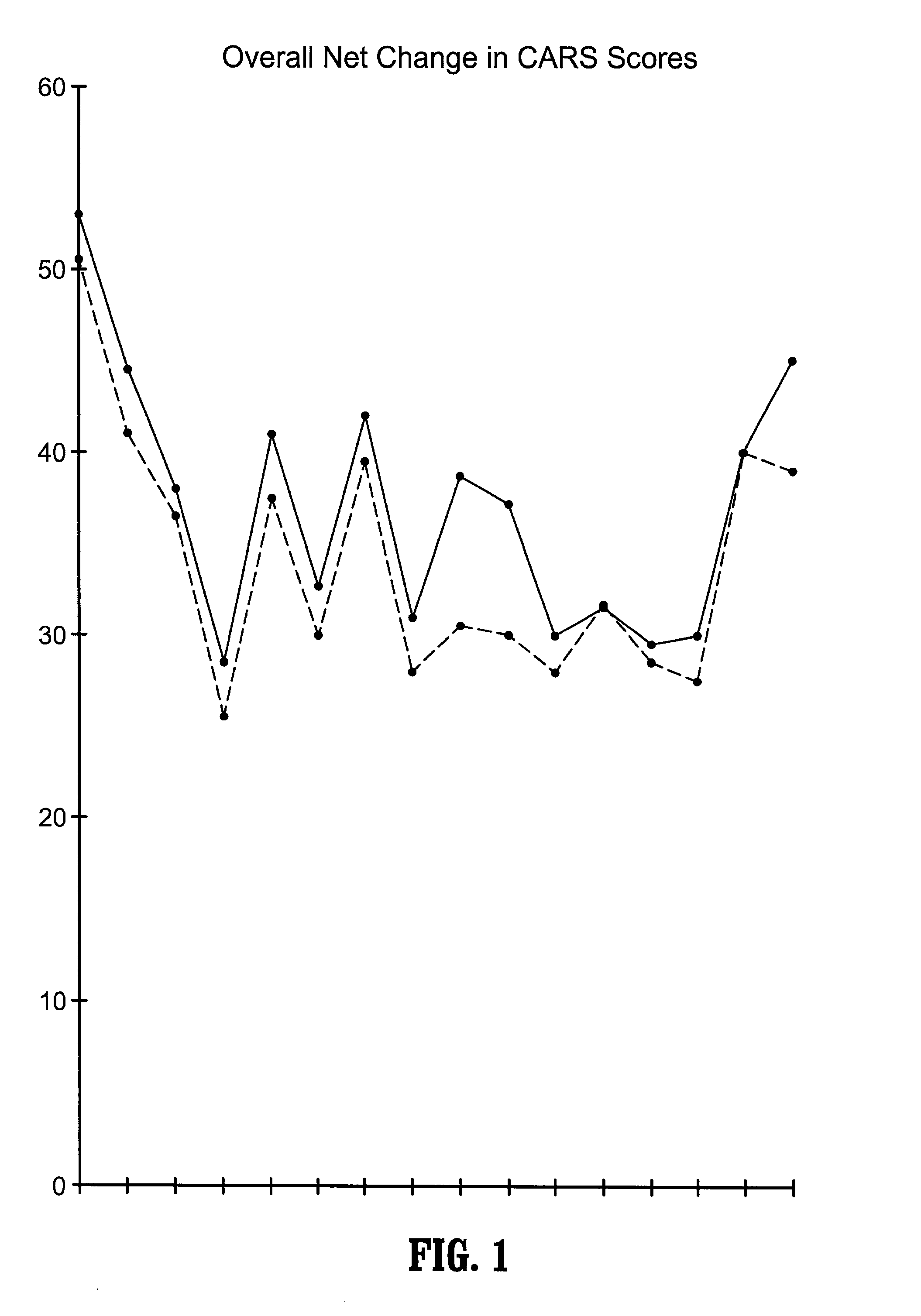Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
321 results about "Autism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Autism is a developmental disorder characterized by difficulties with social interaction and communication, and by restricted and repetitive behavior. Parents usually notice signs during the first three years of their child's life. These signs often develop gradually, though some children with autism reach their developmental milestones at a normal pace before worsening.
Methods of diagnosing and treating small intestinal bacterial overgrowth (SIBO) and SIBO-related conditions
InactiveUS7048906B2Prevent further growthReduced magnitudeAntibacterial agentsCompounds screening/testingImmunologic disordersPhysiology
Disclosed is a method of treating small intestinal bacterial overgrowth (SIBO) or a SIBO-caused condition in a human subject. SIBO-caused conditions include irritable bowel syndrome, fibromyalgia, chronic pelvic pain syndrome, chronic fatigue syndrome, depression, impaired mentation, impaired memory, halitosis, tinnitus, sugar craving, autism, attention deficit / hyperactivity disorder, drug sensitivity, an autoimmune disease, and Crohn's disease. Also disclosed are a method of screening for the abnormally likely presence of SIBO in a human subject and a method of detecting SIBO in a human subject. A method of determining the relative severity of SIBO or a SIBO-caused condition in a human subject, in whom small intestinal bacterial overgrowth (SIBO) has been detected, is also disclosed.
Owner:CEDARS SINAI MEDICAL CENT
Method for diagnosing, preventing, and treating neurological diseases
ActiveUS20120252775A1High activityLow systemic concentrationBiocideMicrobiological testing/measurementDiseaseClinical psychology
The invention provides for methods of treating autism associated with Desulfovibrio overgrowth in the gastrointestinal tract of a patient, said method comprising administering to the patient suffering from said autism a treatment course of aztreonam in an amount effective to treat autism in the patient, thereby treating autism.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
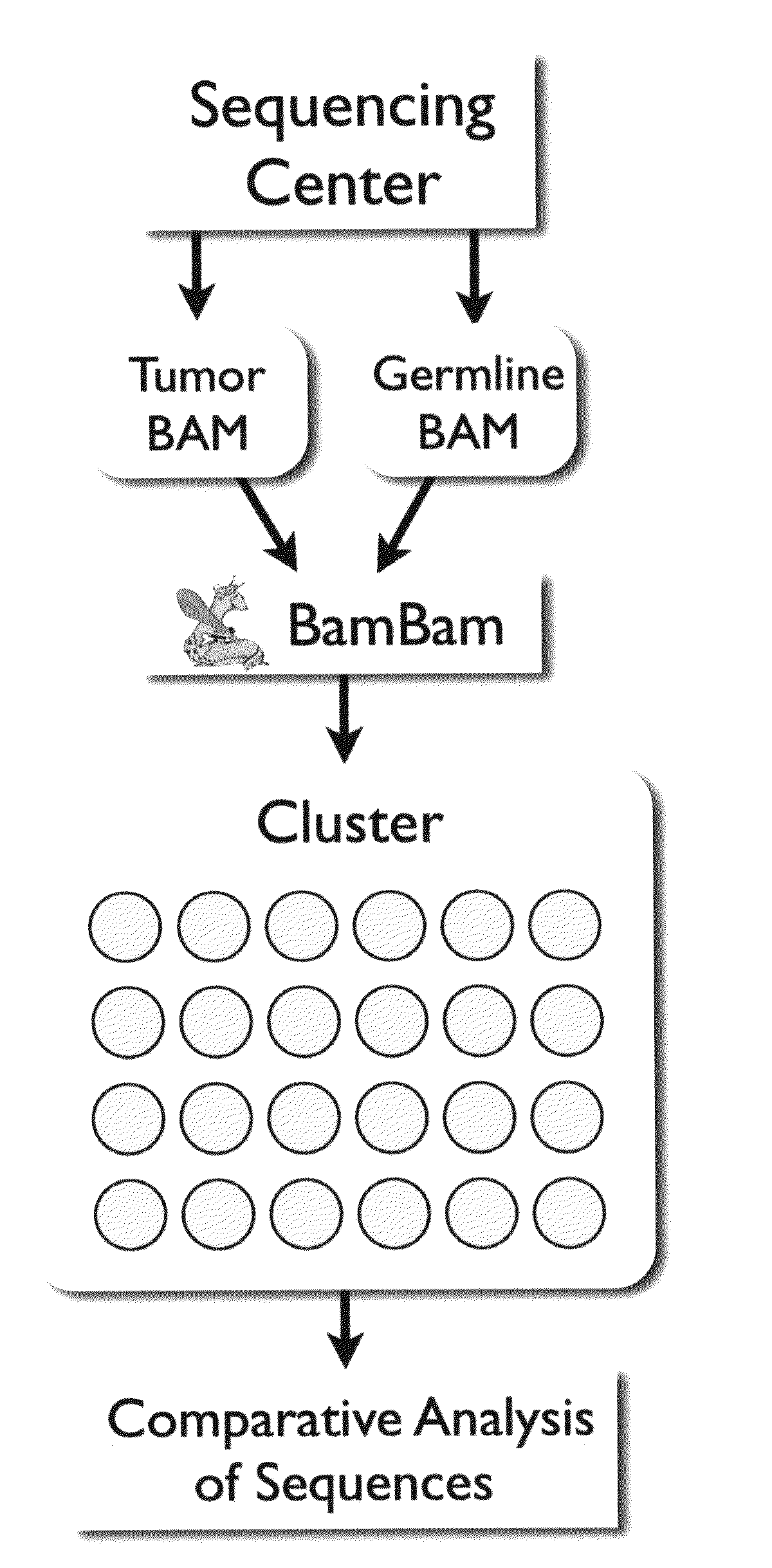
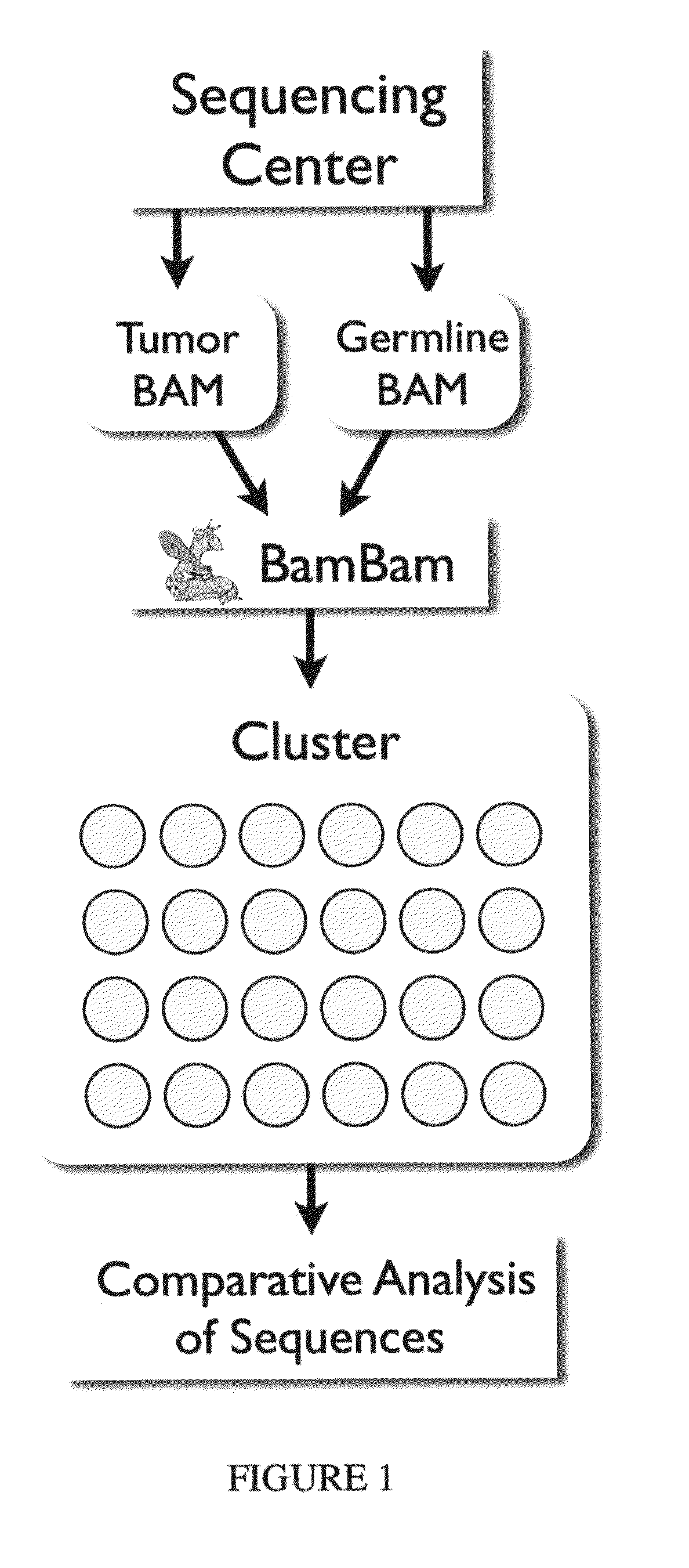
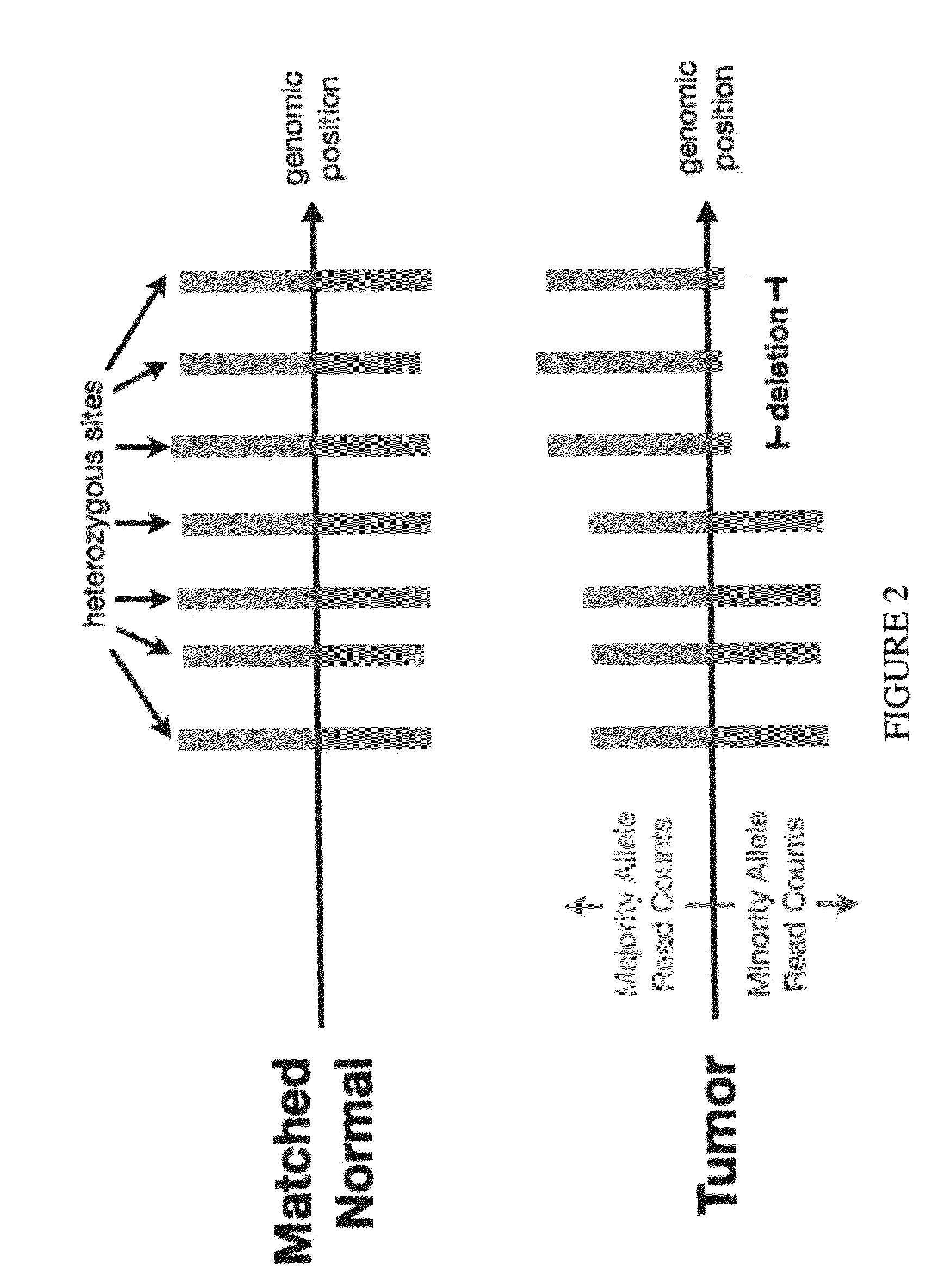
Bambam: parallel comparative analysis of high-throughput sequencing data
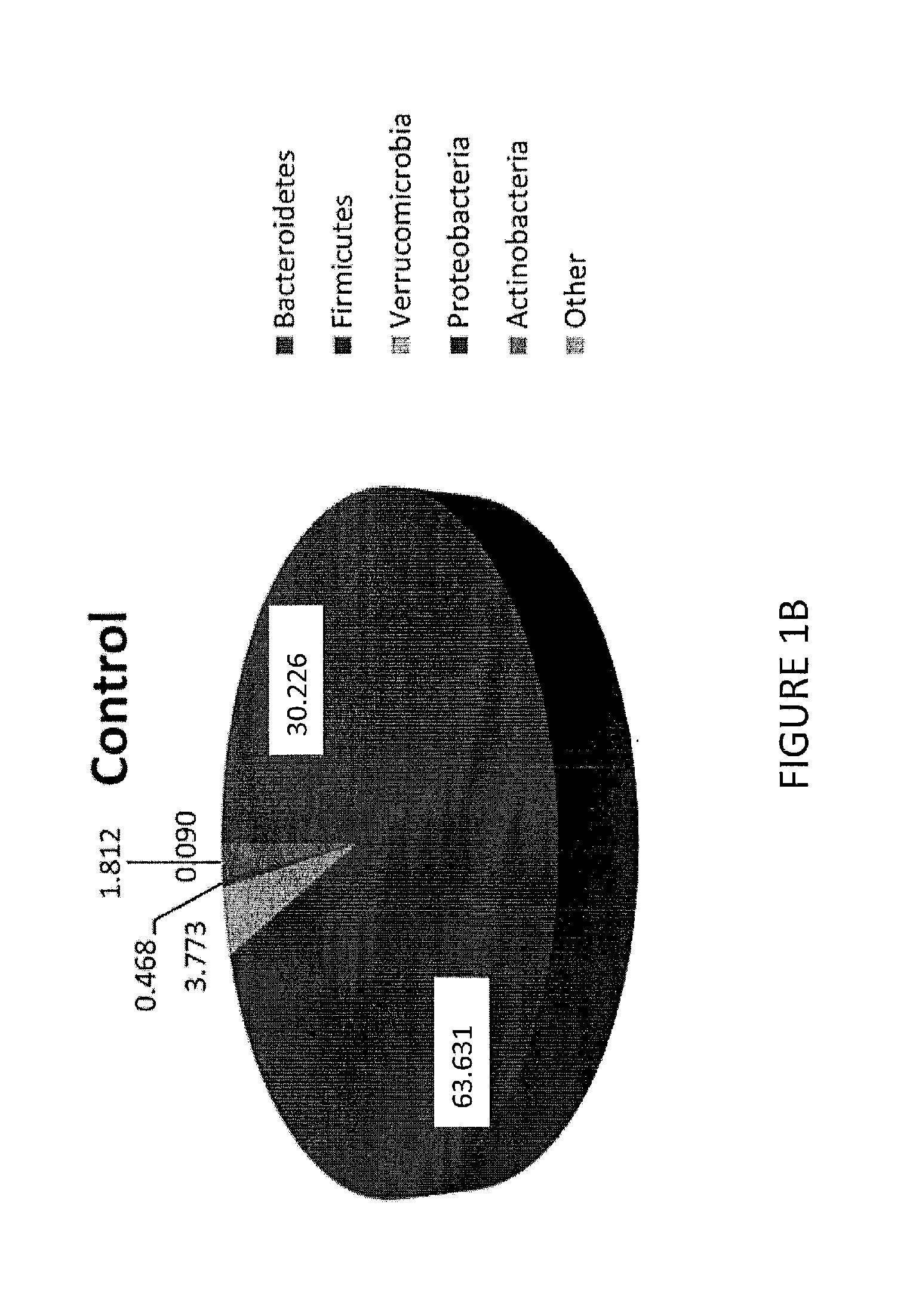
The present invention relates to methods for evaluating and / or predicting the outcome of a clinical condition, such as cancer, metastasis, AIDS, autism, Alzheimer's, and / or Parkinson's disorder. The methods can also be used to monitor and track changes in a patient's DNA and / or RNA during and following a clinical treatment regime. The methods may also be used to evaluate protein and / or metabolite levels that correlate with such clinical conditions. The methods are also of use to ascertain the probability outcome for a patient's particular prognosis.
Owner:RGT UNIV OF CALIFORNIA
Enzyme delivery systems and methods of preparation and use
ActiveUS20100260857A1Improve stabilityEnhanced administration propertyPowder deliveryNervous disorderDiseaseCystic fibrosis lungs
This invention relates to coated digestive enzyme preparations and enzyme delivery systems and pharmaceutical compositions comprising the preparations. This invention further relates to methods of preparation and use of the systems, pharmaceutical compositions and preparations to treat persons having ADD, ADHD, autism, cystic fibrosis and other behavioral and neurological disorders.
Owner:CUREMARK
Treatment of disease states and adverse physiological conditions utilizing anti-fungal compositions
InactiveUS20060177424A1Effectively ameliorateEffective regulationBiocideBacteria material medical ingredientsSinusitisVaginal Yeast Infections
A method for treatment or prophylaxis of a disease state or other physiological condition, e.g., autism, delayed development, acid reflux disease, vaginal yeast infections, impaired hearing, chronic ear infections, seasonal allergies, Fibromyalgia syndrome, Crohn's disease, colitis, irritable bowel syndrome, interstitial cystitis, acne, sinusitis, rheumatoid arthritis, chronic fatigue syndrome, asthma, attention deficit disorder, attention deficit / hyperactivity disorder, rosacea, multiple sclerosis, hyperglycemia, or Ménière's disease, by administration of an anti-fungal composition that includes at least one of the bacilli (1) Bacillus subtilis, (2) Lactobacillus sporogenes, and (3) Streptococcus faecalis.
Owner:COBB AND CO
Compositions and methods relating to reduction of symptoms of autism
InactiveUS6899876B2Relieve symptomsMany symptomPeptide/protein ingredientsOxidoreductasesNormorphineMedicine
Methods and compositions that can reduce the symptoms of autism in a human patient comprising administering a physiologically effective amount of one or both of a purified casomorphin inhibitor selected from the group consisting of a casomorphinase and a casomorphin ligand, and a physiologically effective amount of a purified gluteomorphin inhibitor selected from the group consisting of a gluteomorphinase and a gluteomorphin ligand, to a human patient in sufficient quantities to reduce the effects of the autism. In some embodiments, the compositions and methods further comprise a physiologically effective amount of an enkephalin inhibitor, preferably an enkephalinase, and a physiologically effective amount of an endorphin inhibitor, preferably an endorphinase.
Owner:PROTHERA
Methods of treating pervasive development disorders
InactiveUS20020090653A1Symptoms improvedPromote digestionPeptide/protein ingredientsMicrobiological testing/measurementDiseaseFeces
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADD, ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with a PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
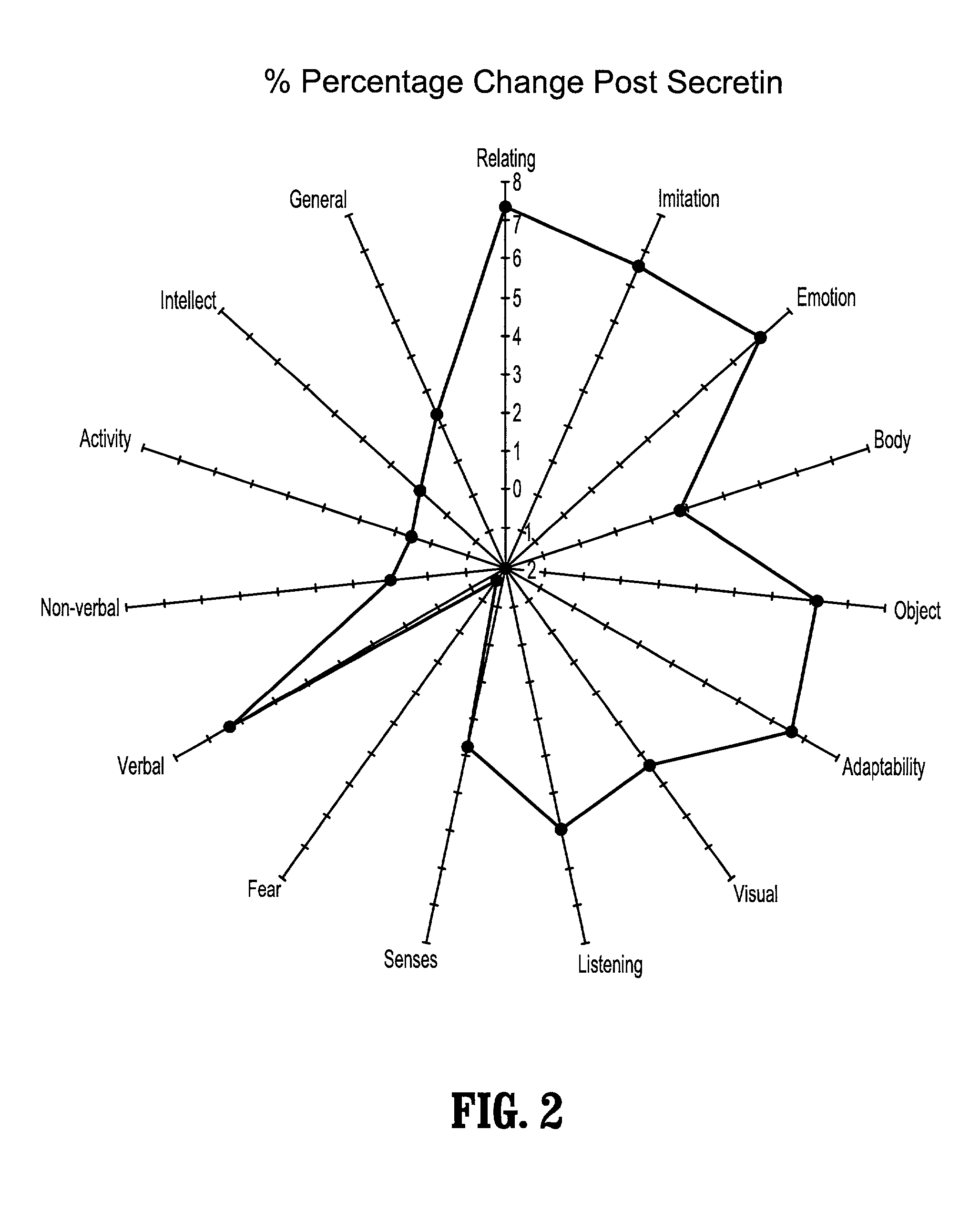
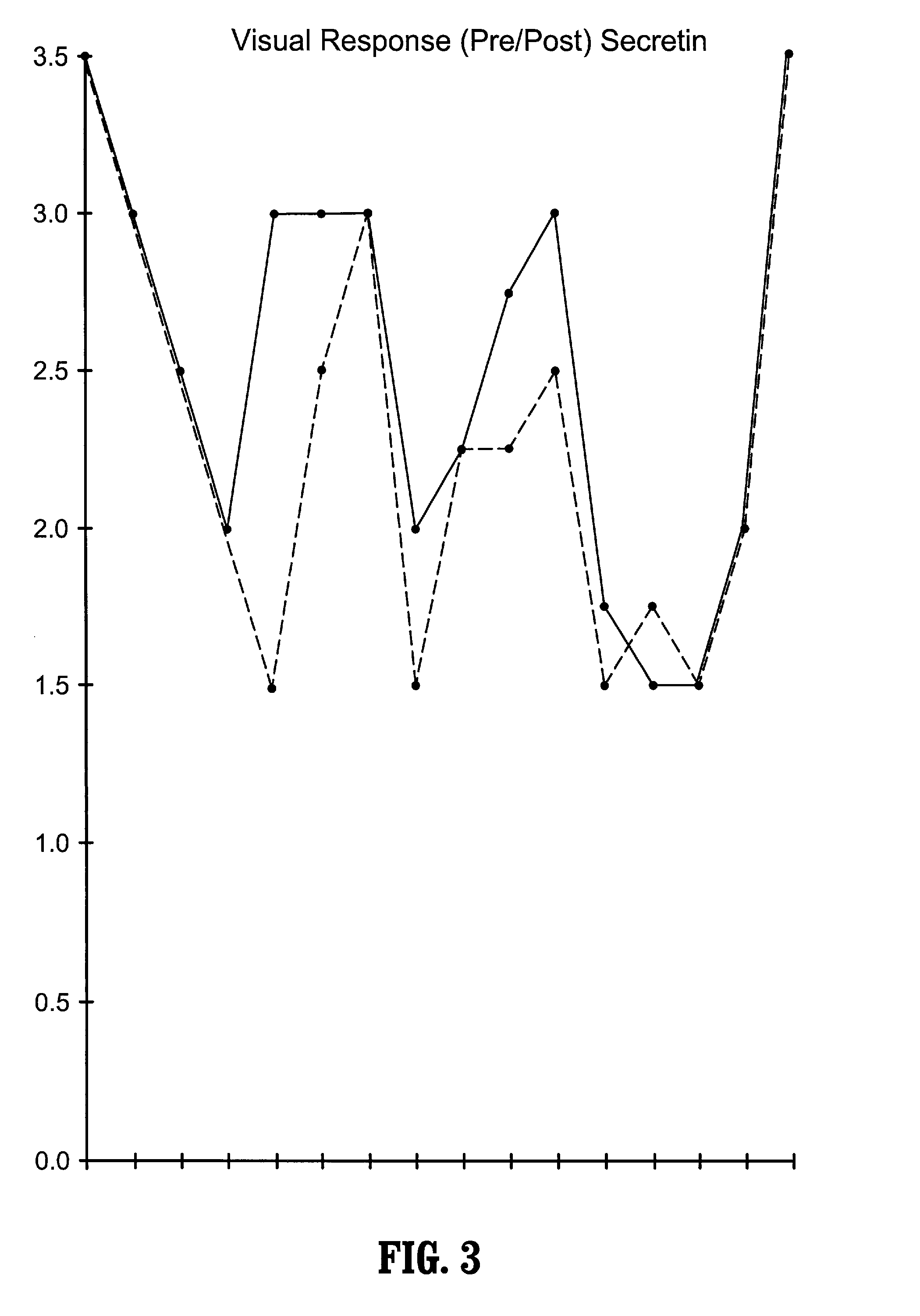
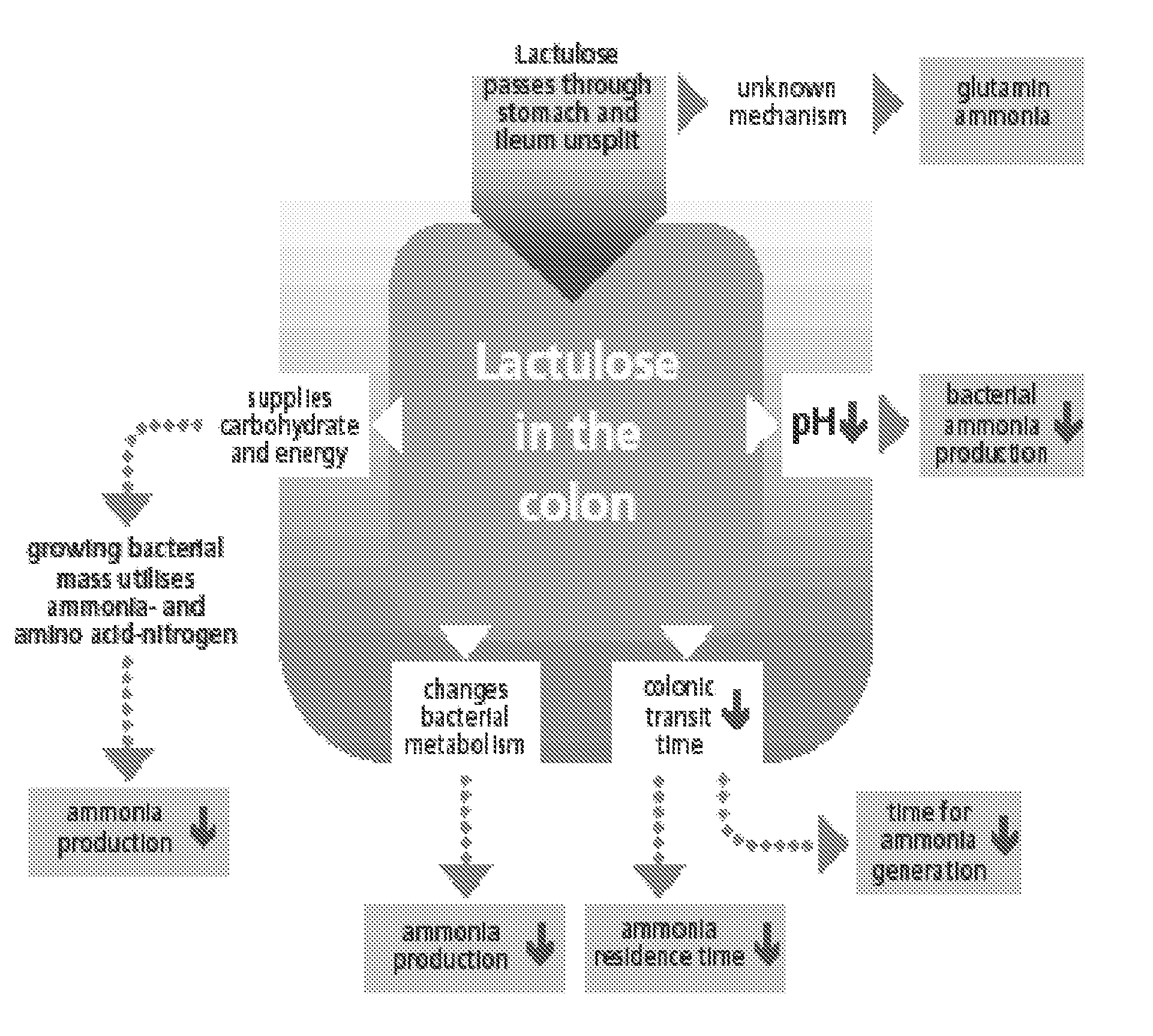
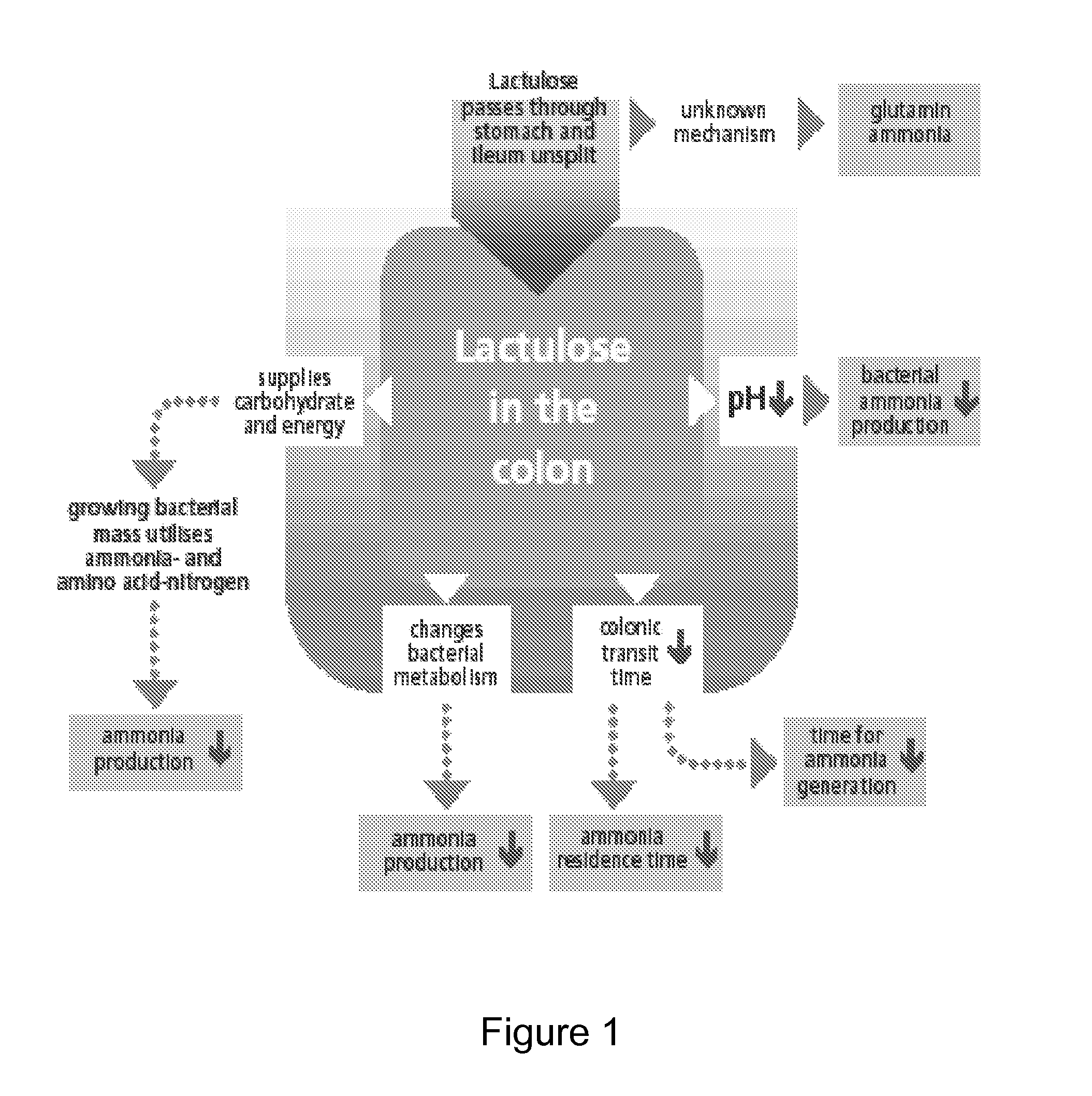
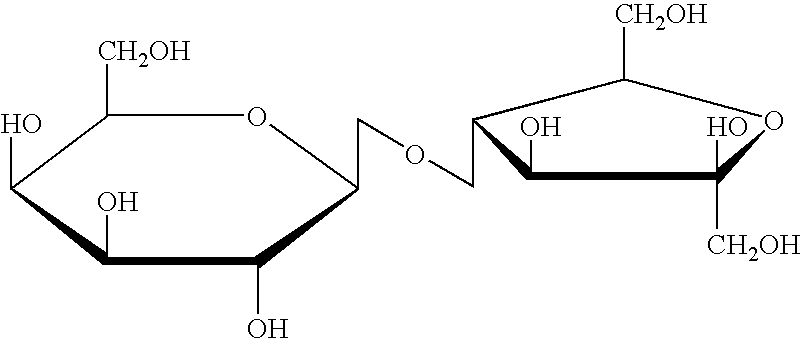
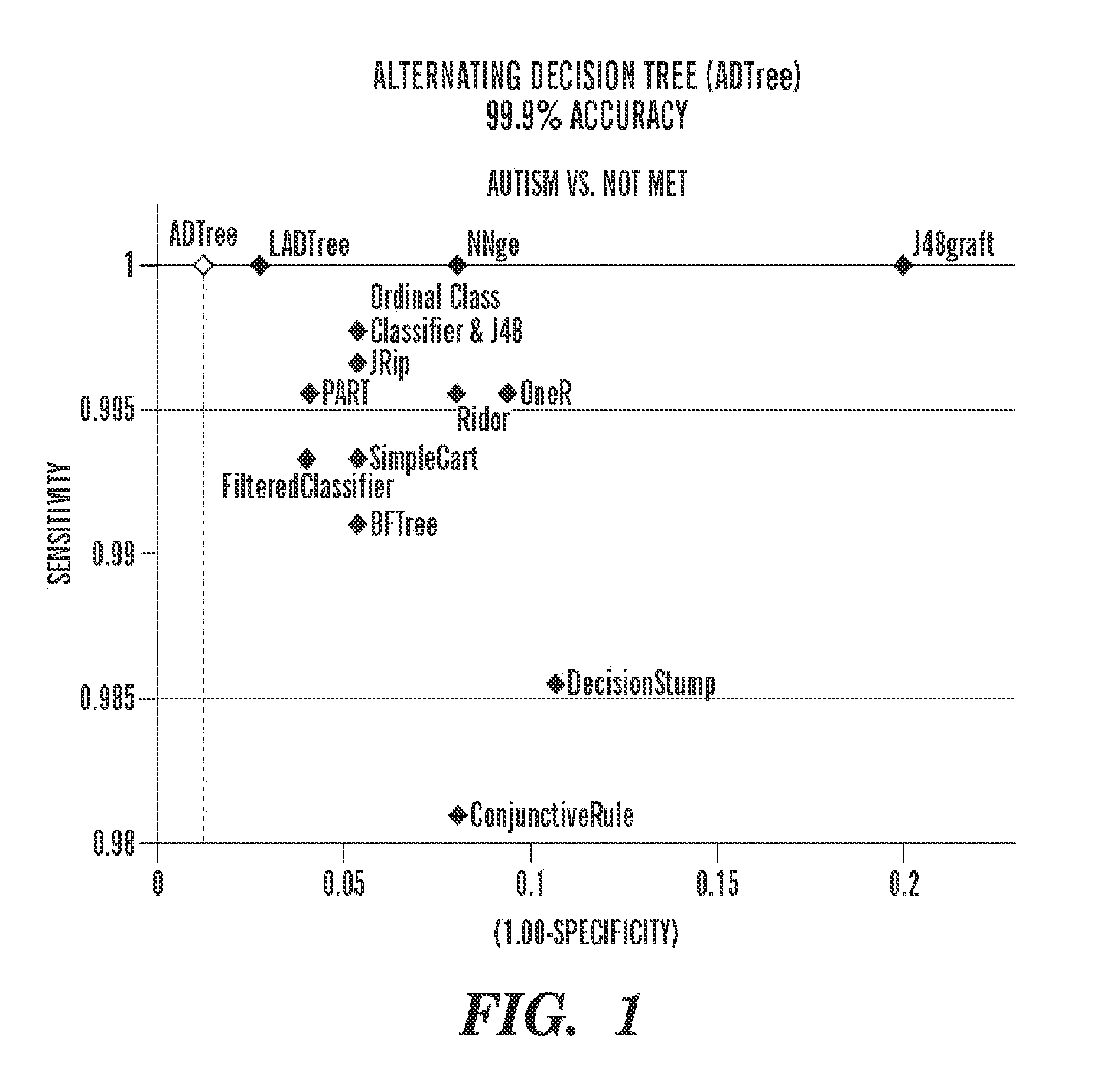
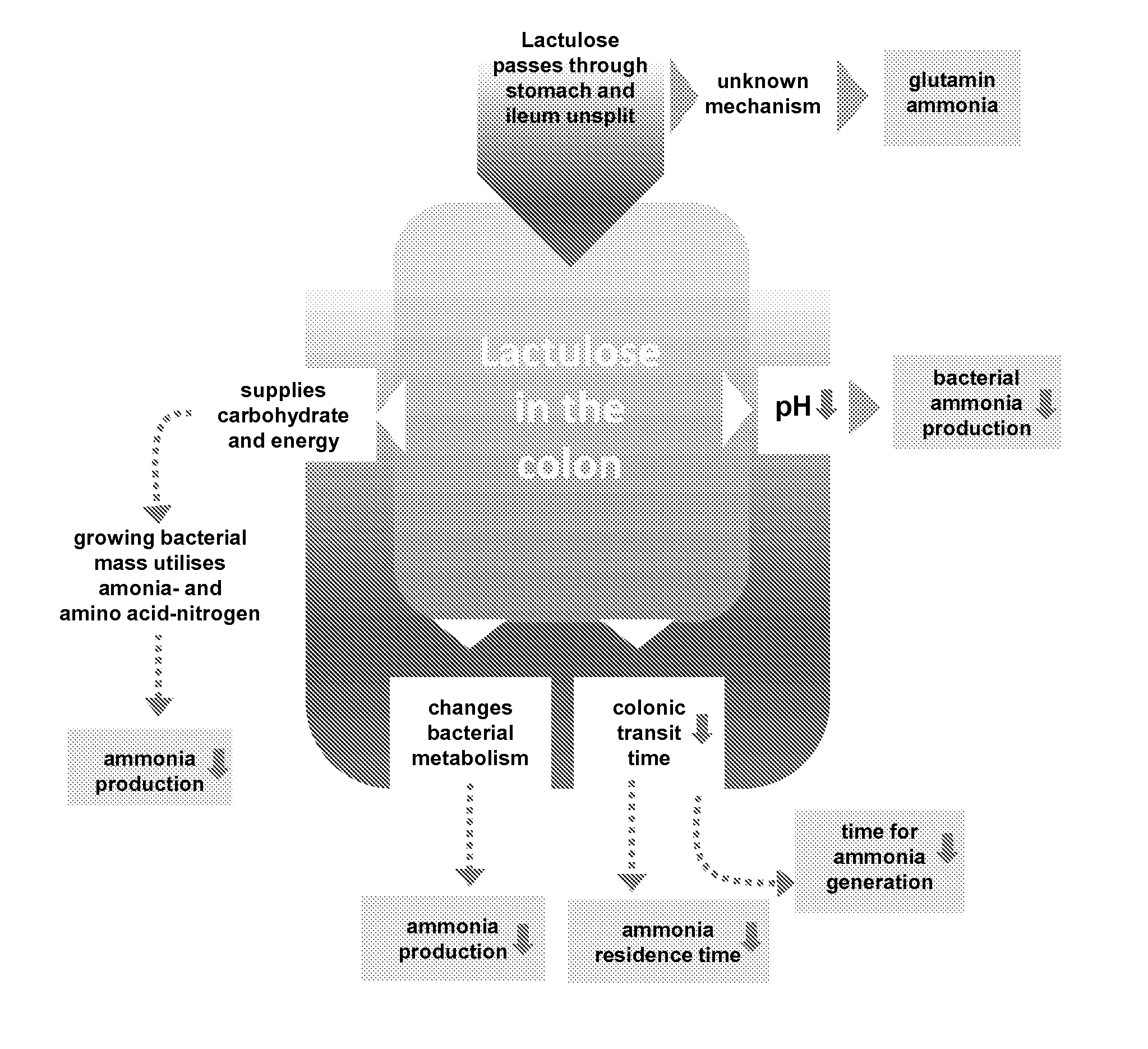
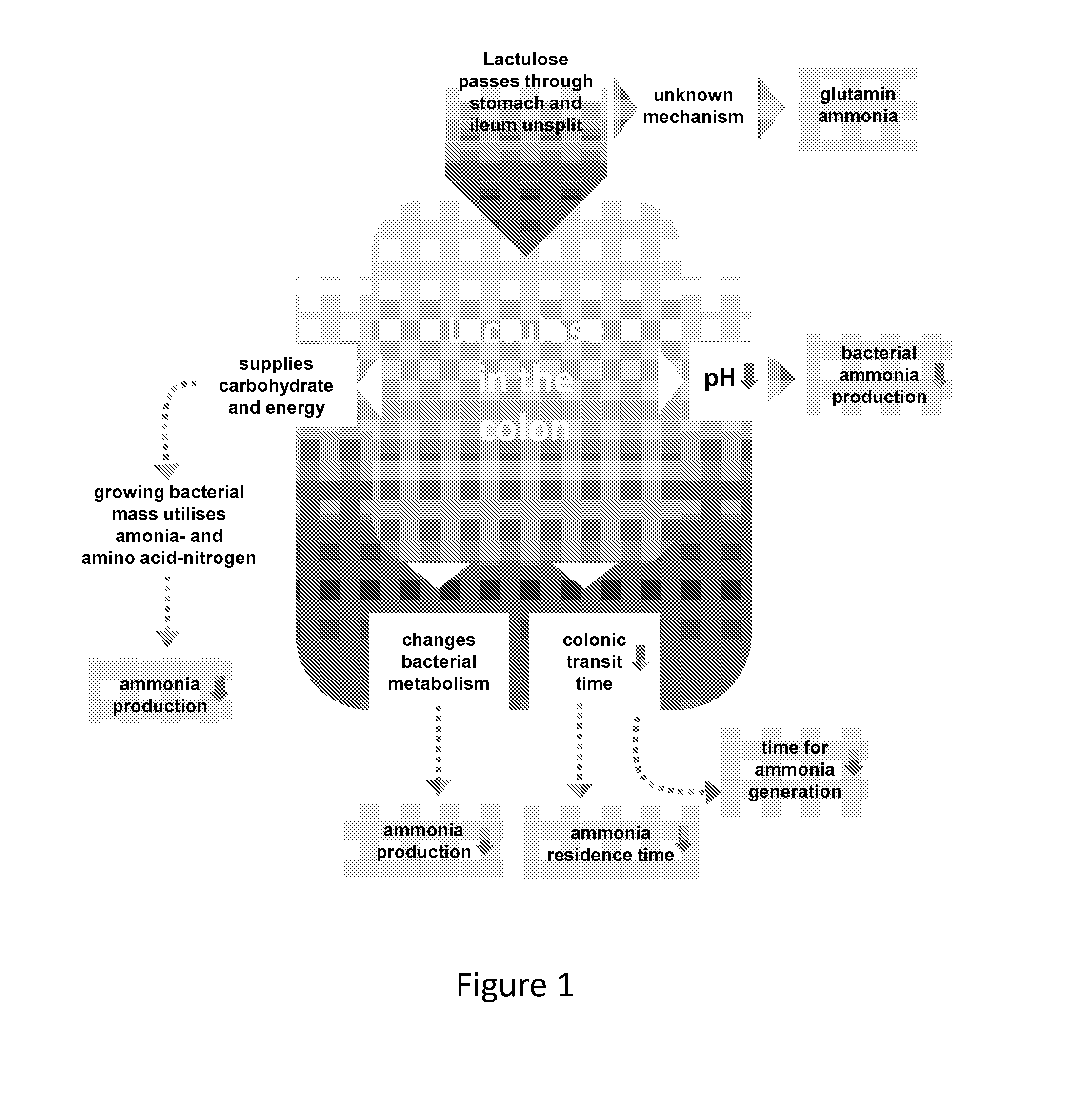
Use of lactulose in the treatment of autism
InactiveUS20080058282A1Avoid accumulationReverses effectBiocideNervous disorderNervous systemAntibiotic Y
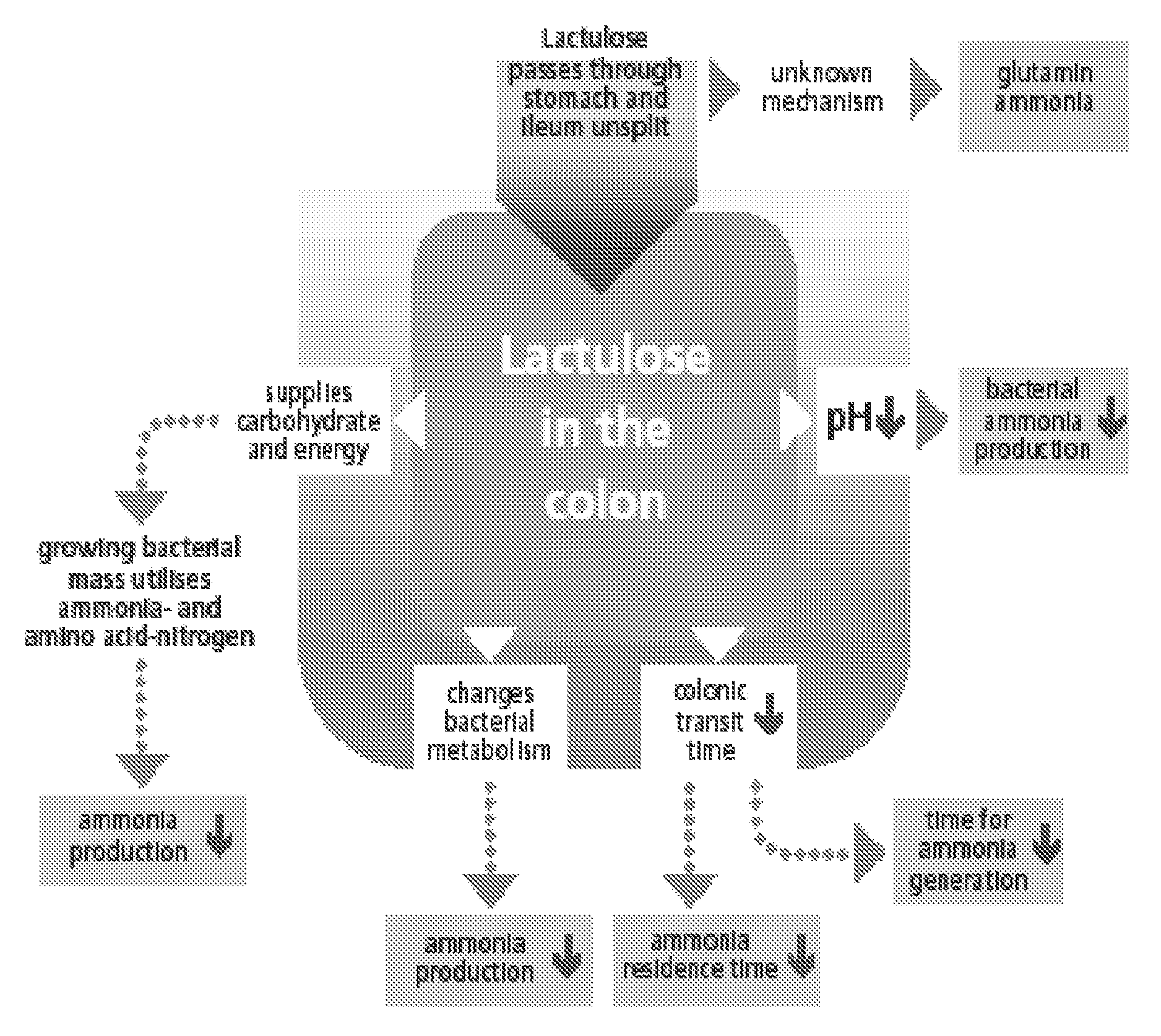
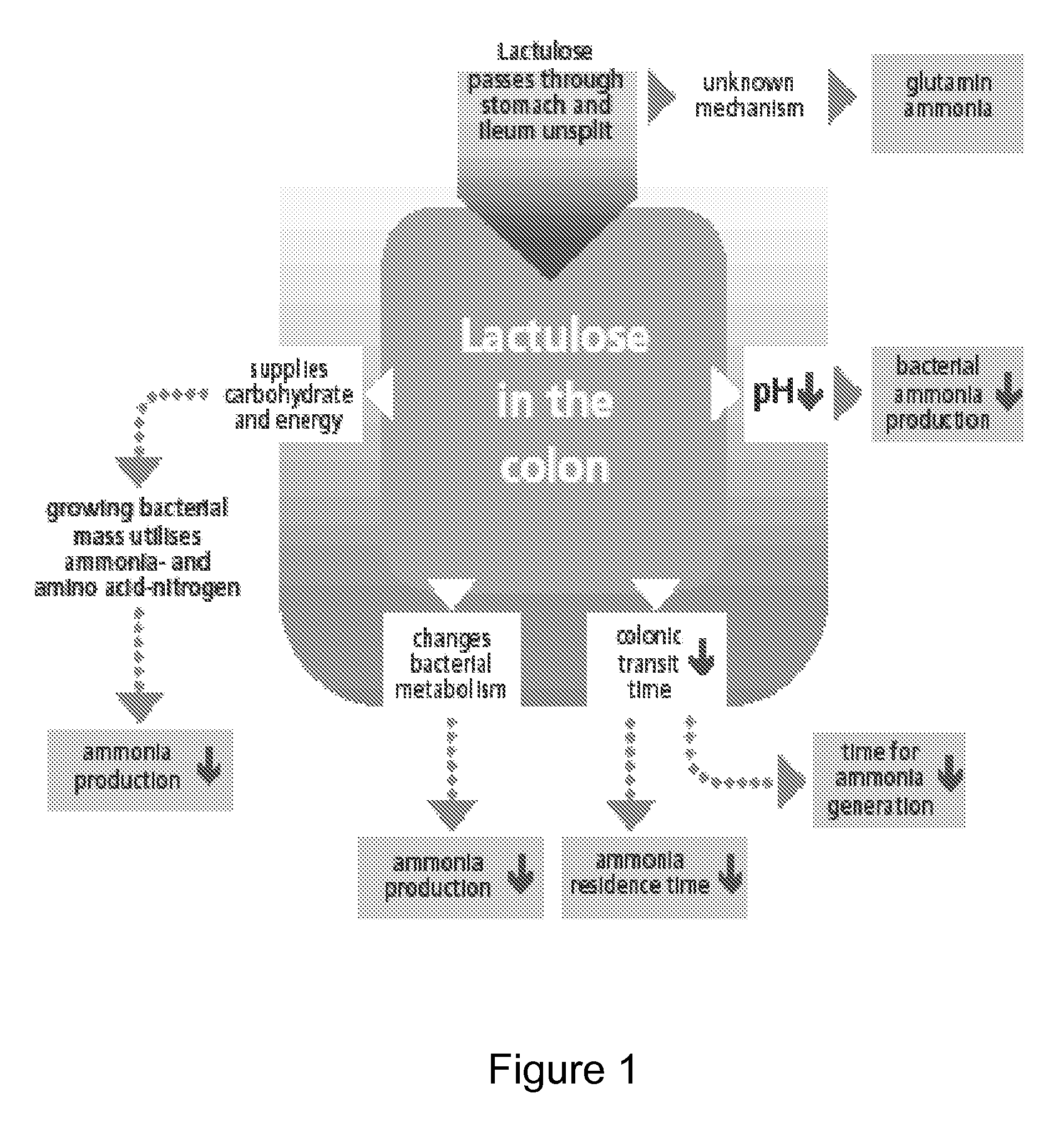
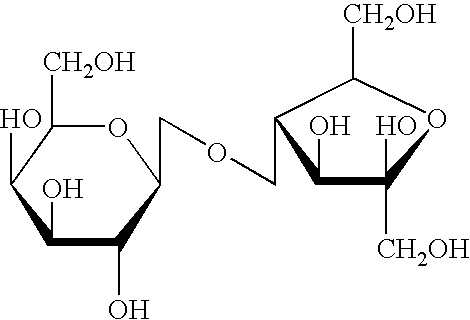
A treatment for autism in which an effective amount of lactulose is administered in order to bind excess ammonia in the gastrointestinal tract, the bloodstream, and the nervous system in order to prevent or reverse ammonia poisoning caused by the administration of certain antibiotics. Lactulose molecules in the colon are fermented by certain bacteria. The fermentation process lowers the colonic pH, and ammonia, in the form of ammonium ions, is used by the bacteria for amino acid and protein synthesis. This lowers the serum ammonia levels and reduces neurotoxicity.
Owner:CUREMARK
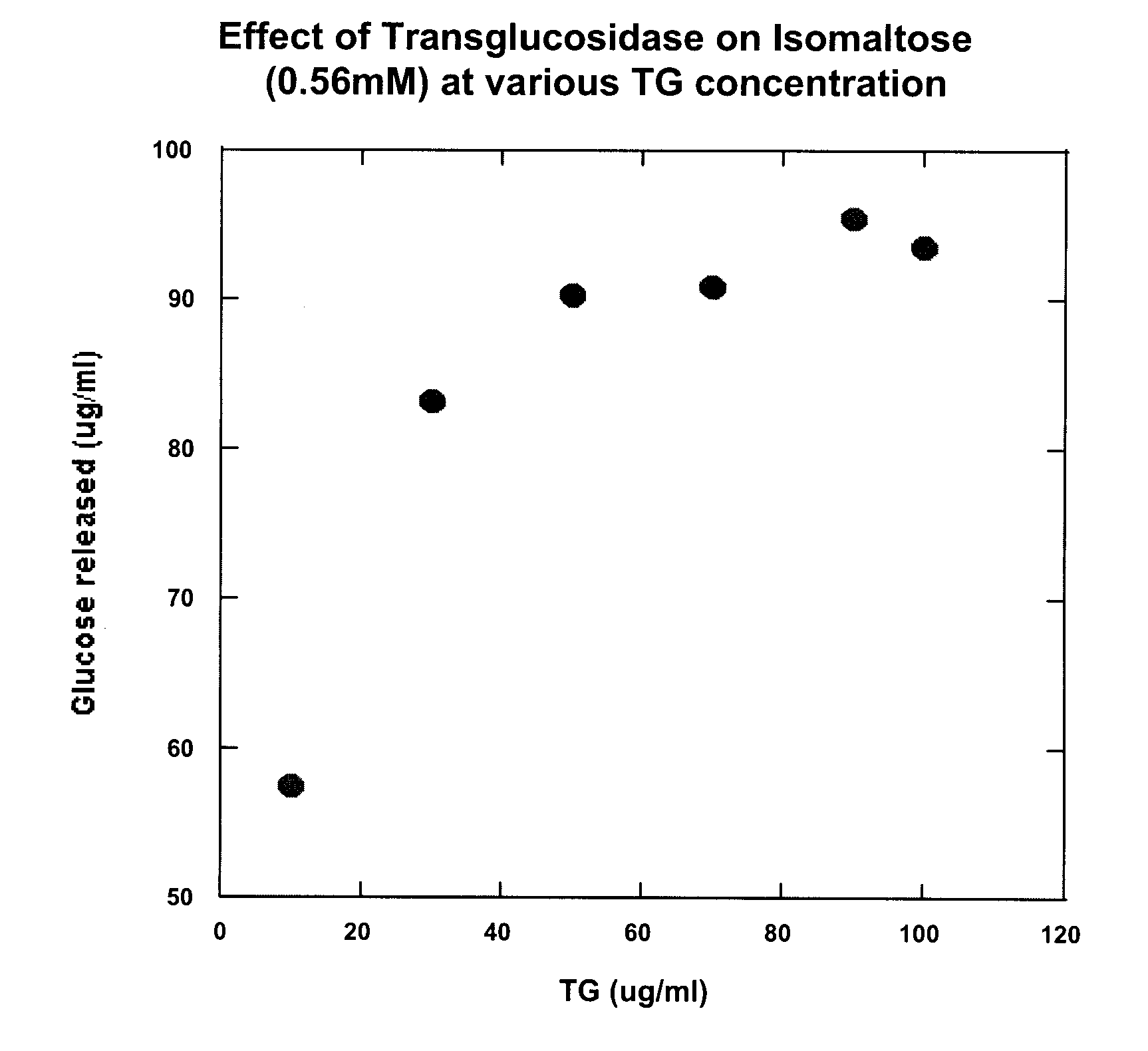
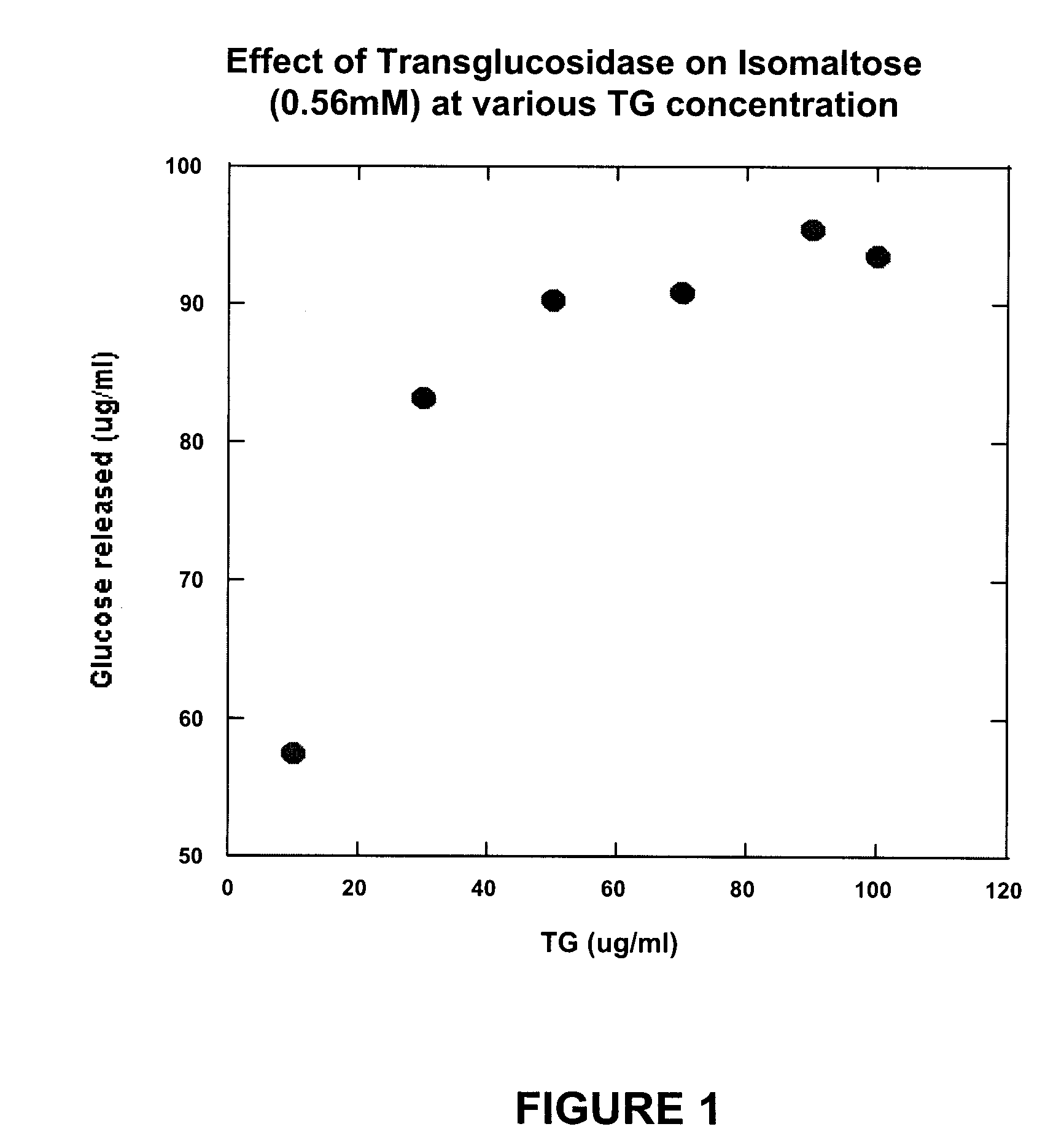
Compositions and methods for the treatment of autism
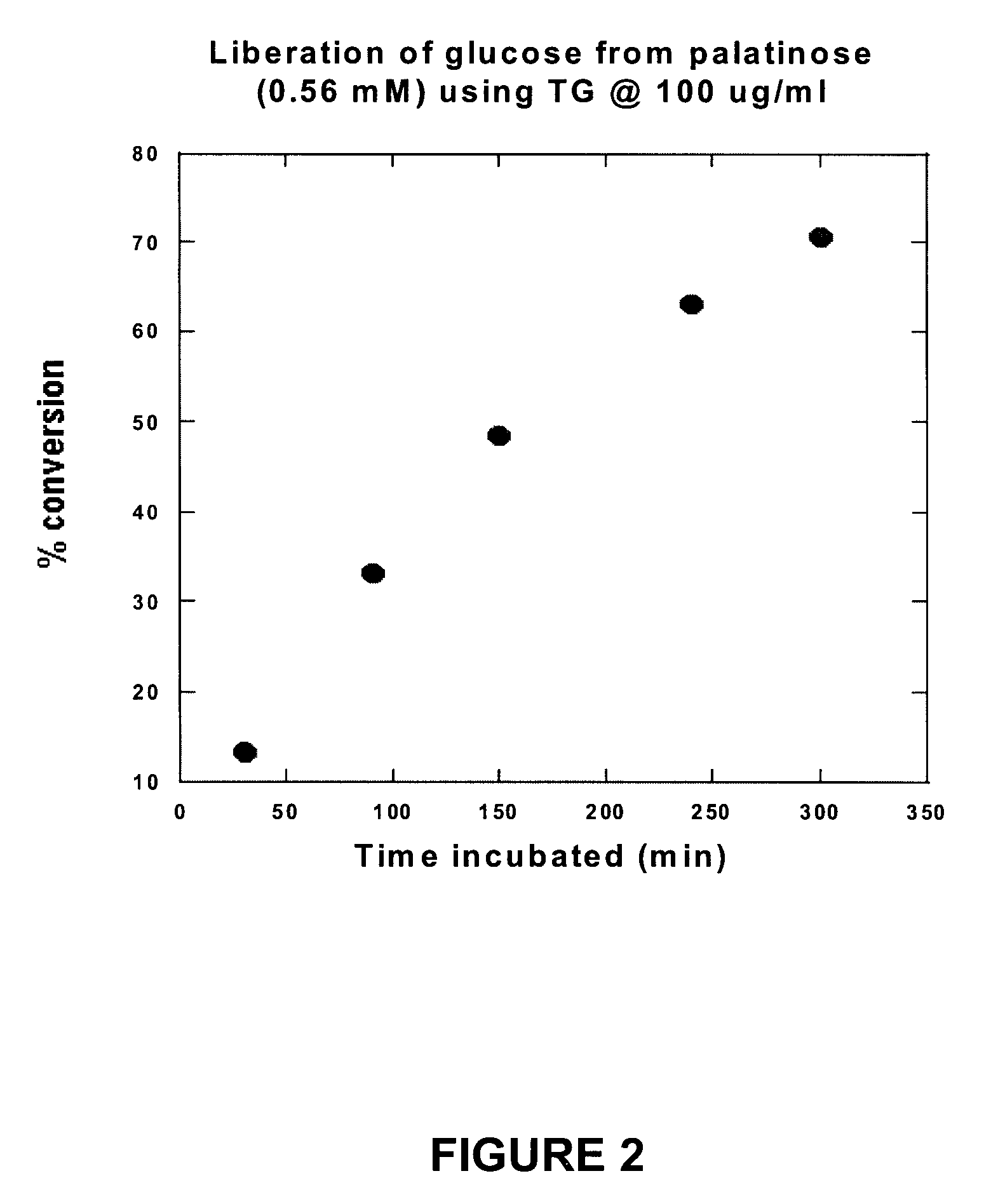
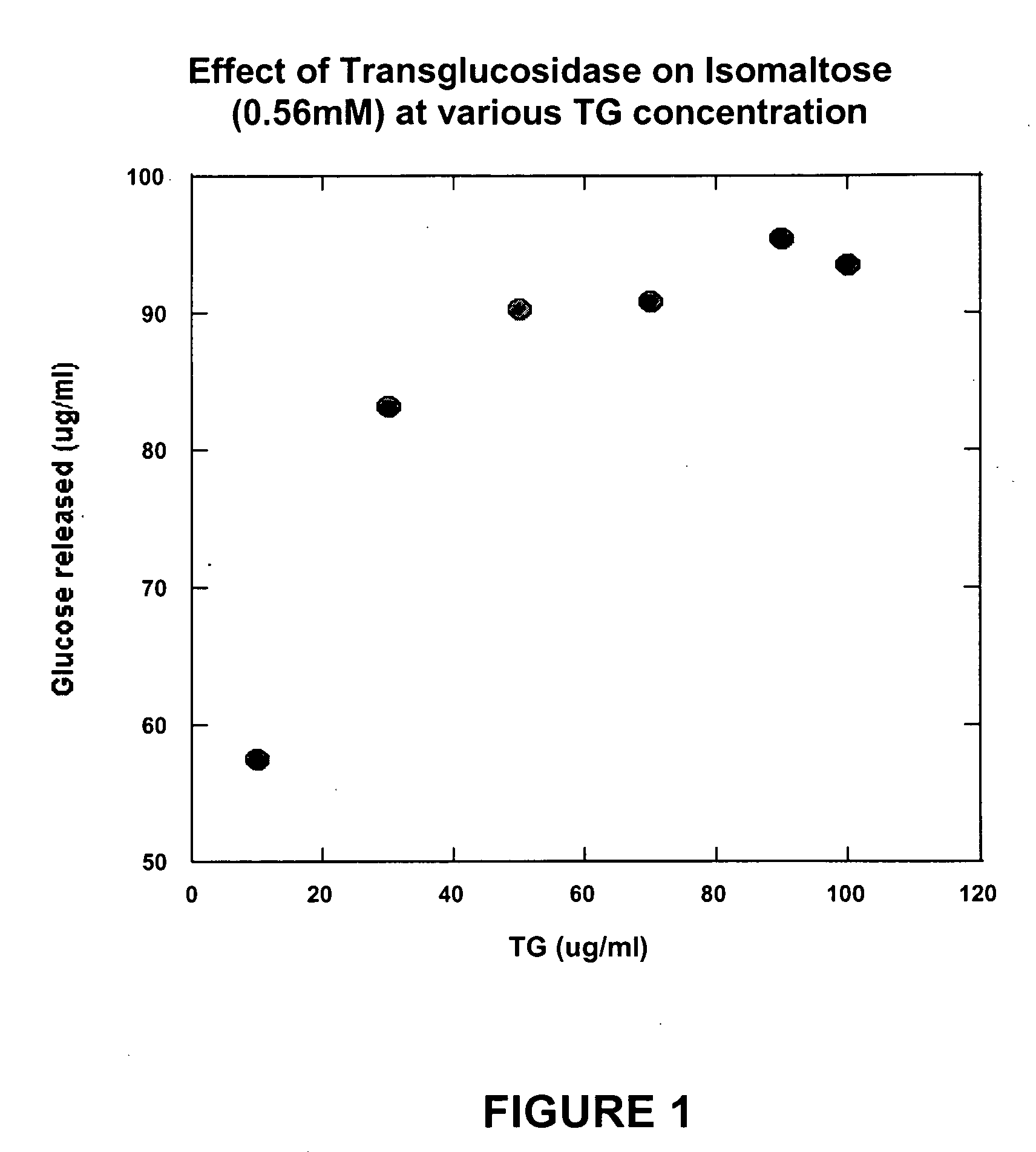
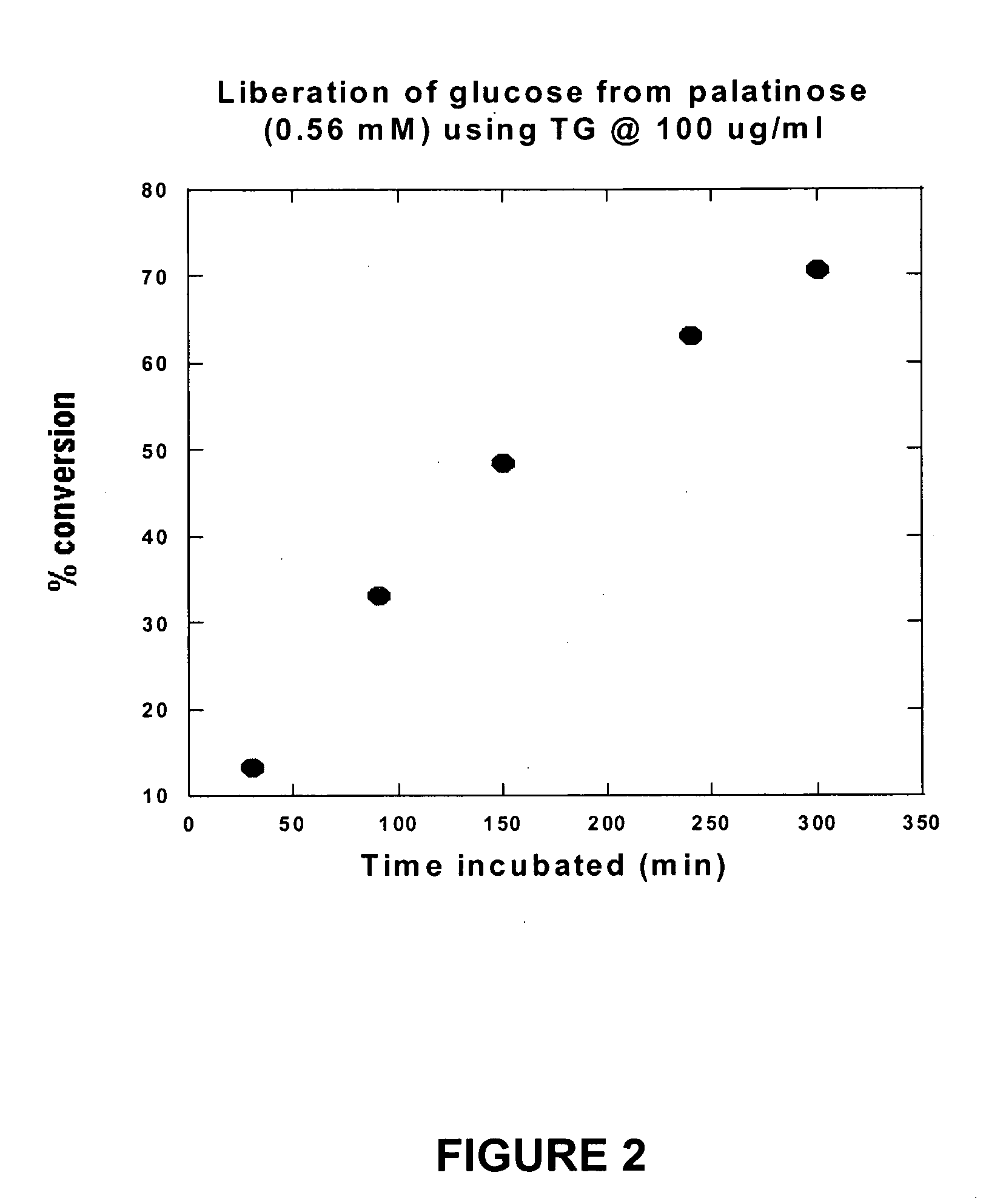
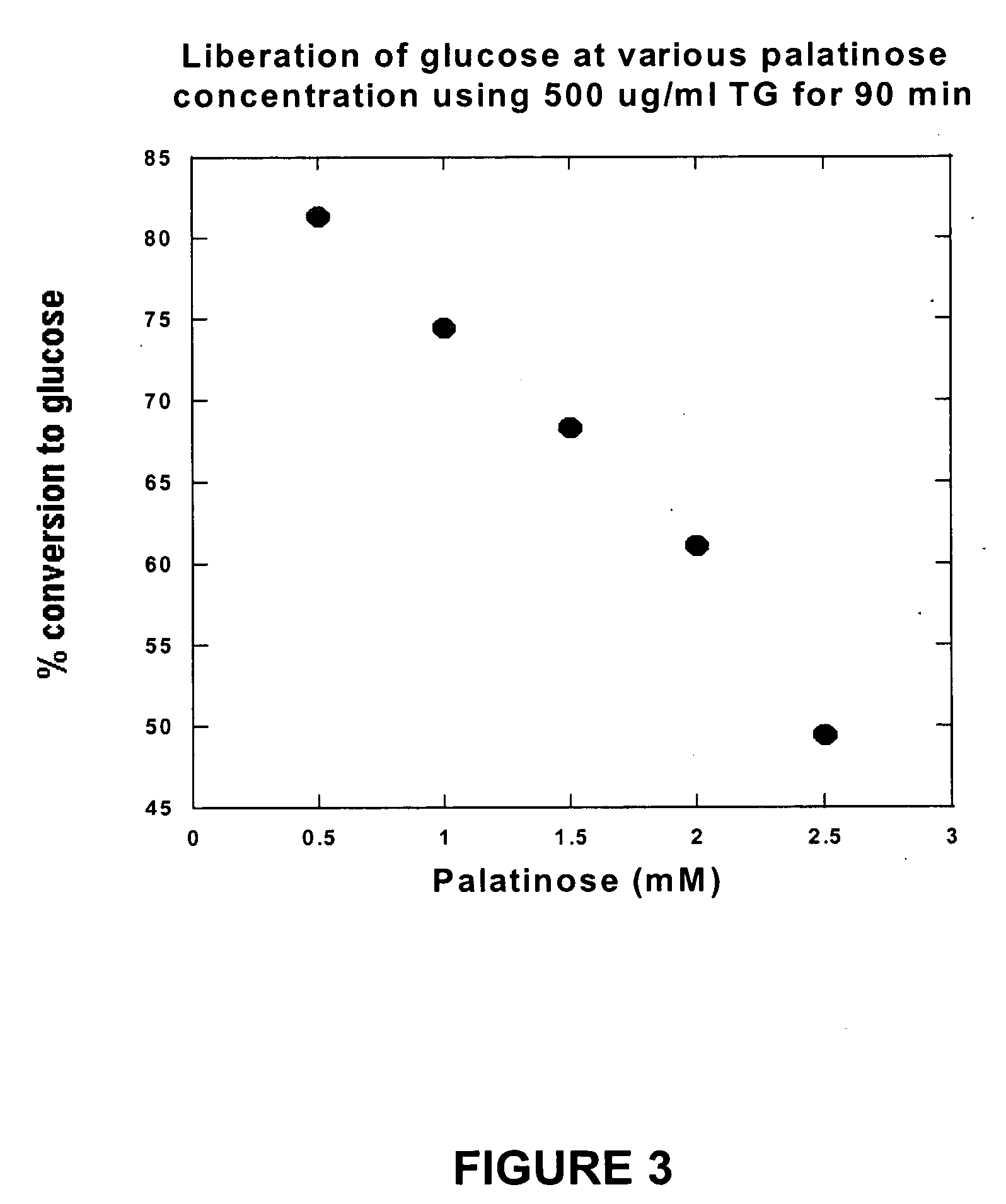
Compositions that may be usefully employed to alleviate symptoms resulting from deficiencies in carbohydrate enzymes, together with methods for the treatment of disorders that are characterized by such deficiencies, such as autism, are provided. The compositions preferably comprise transglucosidase isolated from A. niger.
Owner:KIRKMAN GROUP +1
Use of lactulose in the treatment of autism
InactiveUS20080161265A1Avoid accumulationReverses effectBiocideNervous disorderBacteroidesNervous system
A treatment for autism in which an effective amount of lactulose is administered in order to bind excess ammonia in the gastrointestinal tract, the bloodstream, and the nervous system in order to prevent or reverse ammonia poisoning caused by the administration of certain antibiotics. Lactulose molecules in the colon are fermented by certain bacteria. The fermentation process lowers the colonic pH, and ammonia, in the form of ammonium ions, is used by the bacteria for amino acid and protein synthesis. This lowers the serum ammonia levels and reduces neurotoxicity.
Owner:CUREMARK
Compositions and methods for the treatment of autism
Compositions that may be usefully employed to alleviate symptoms resulting from deficiencies in carbohydrate enzymes, together with methods for the treatment of disorders that are characterized by such deficiencies, such as autism, are provided. The compositions preferably comprise transglucosidase isolated from A. niger.
Owner:KIRKMAN GROUP +1
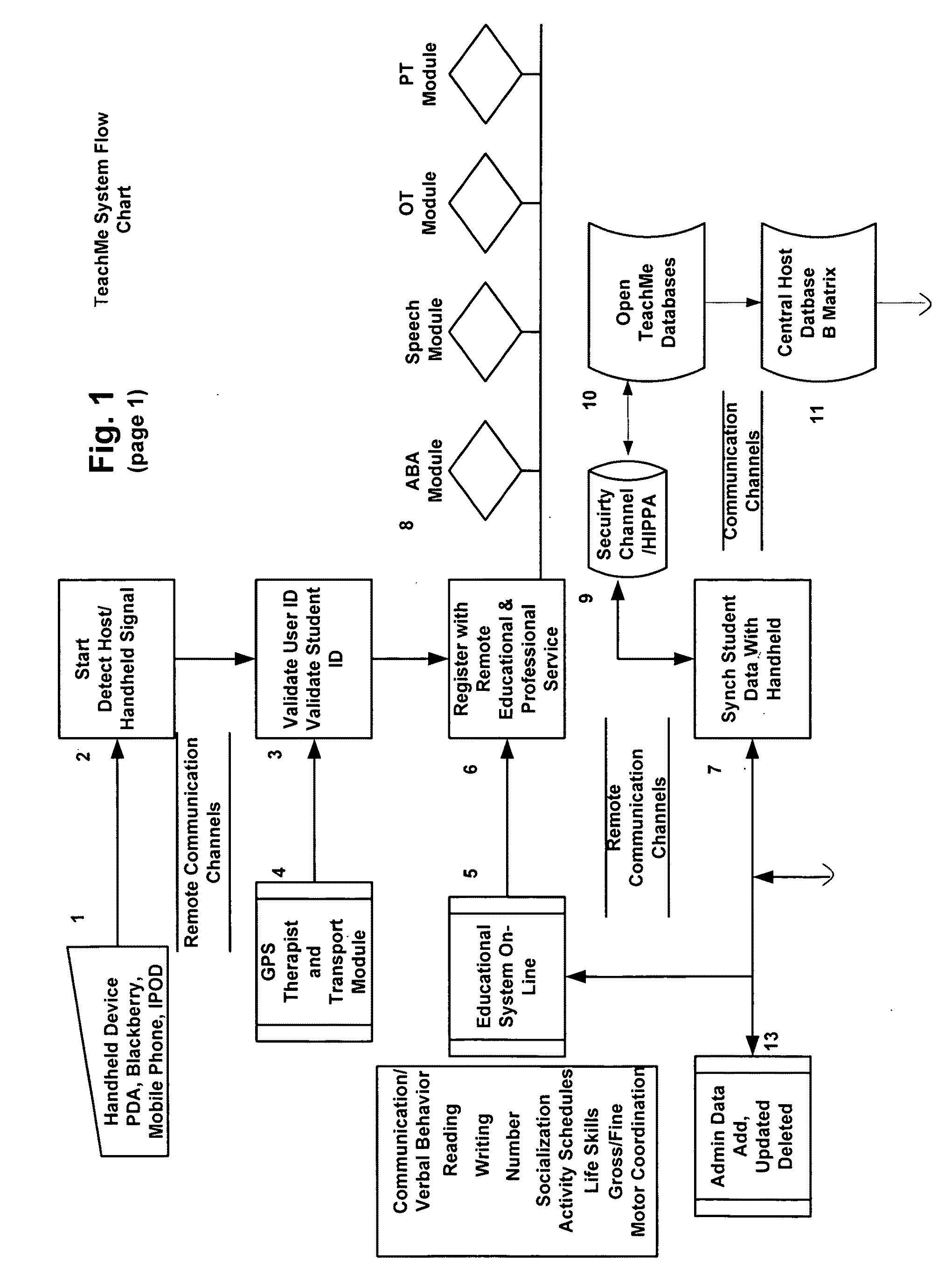
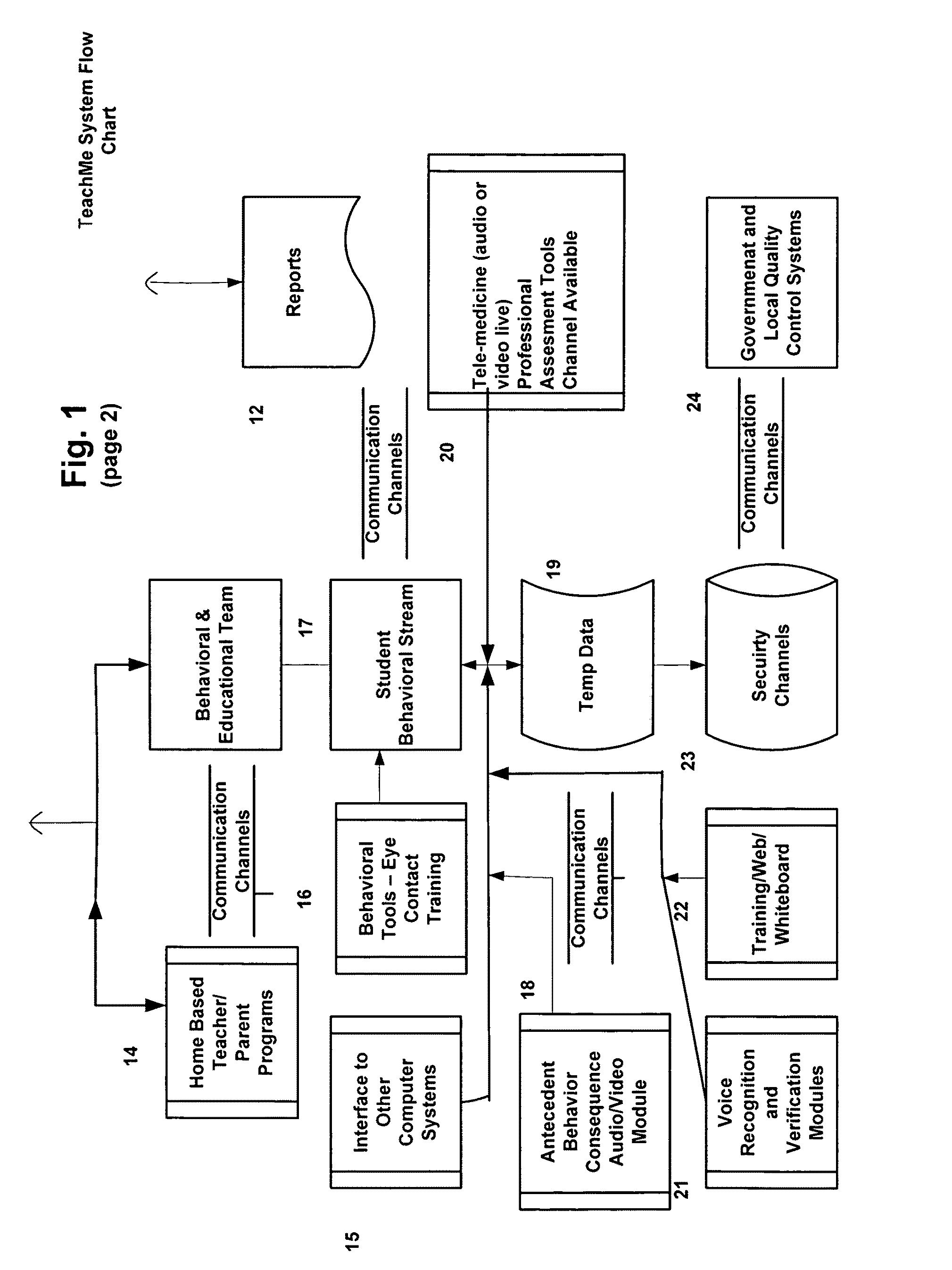
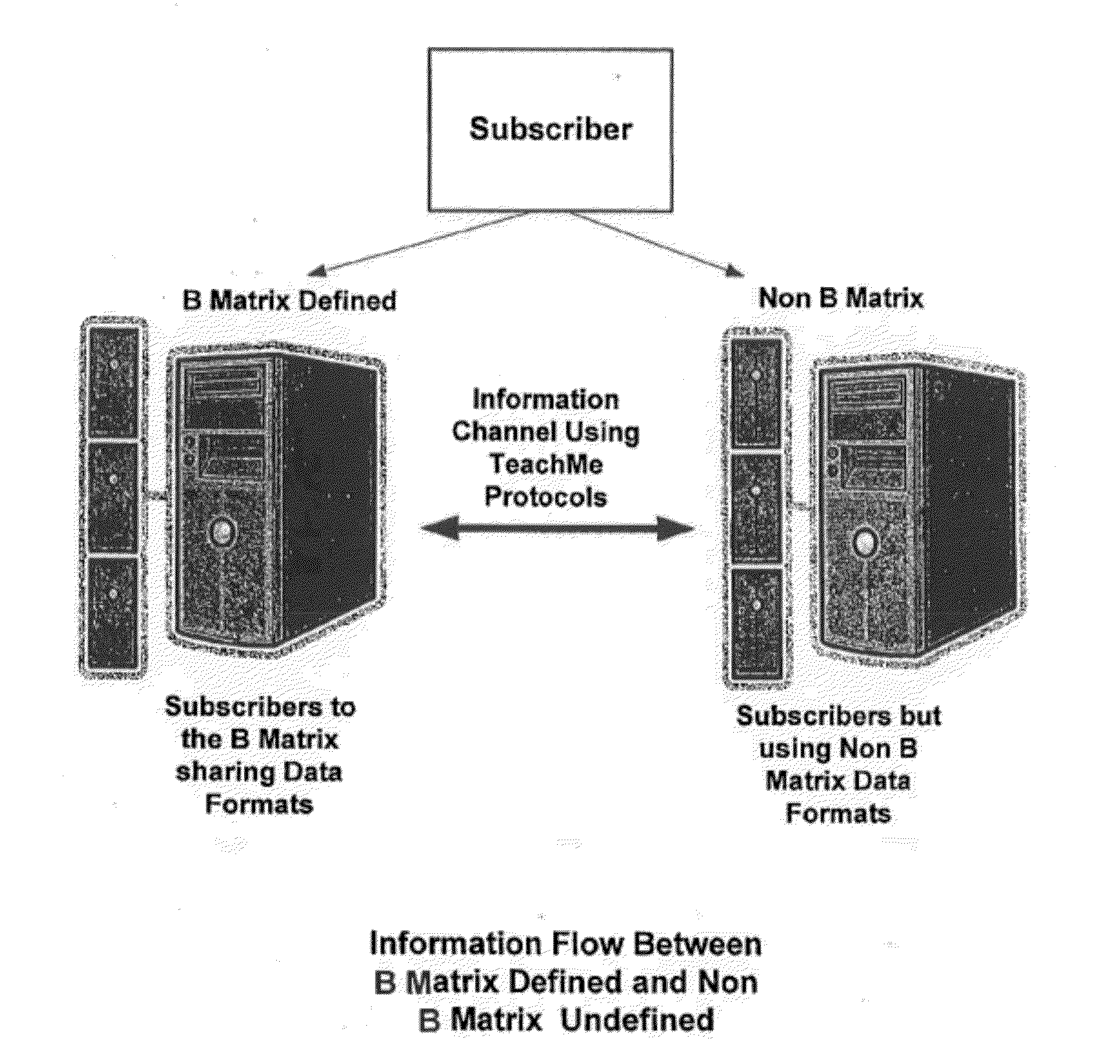
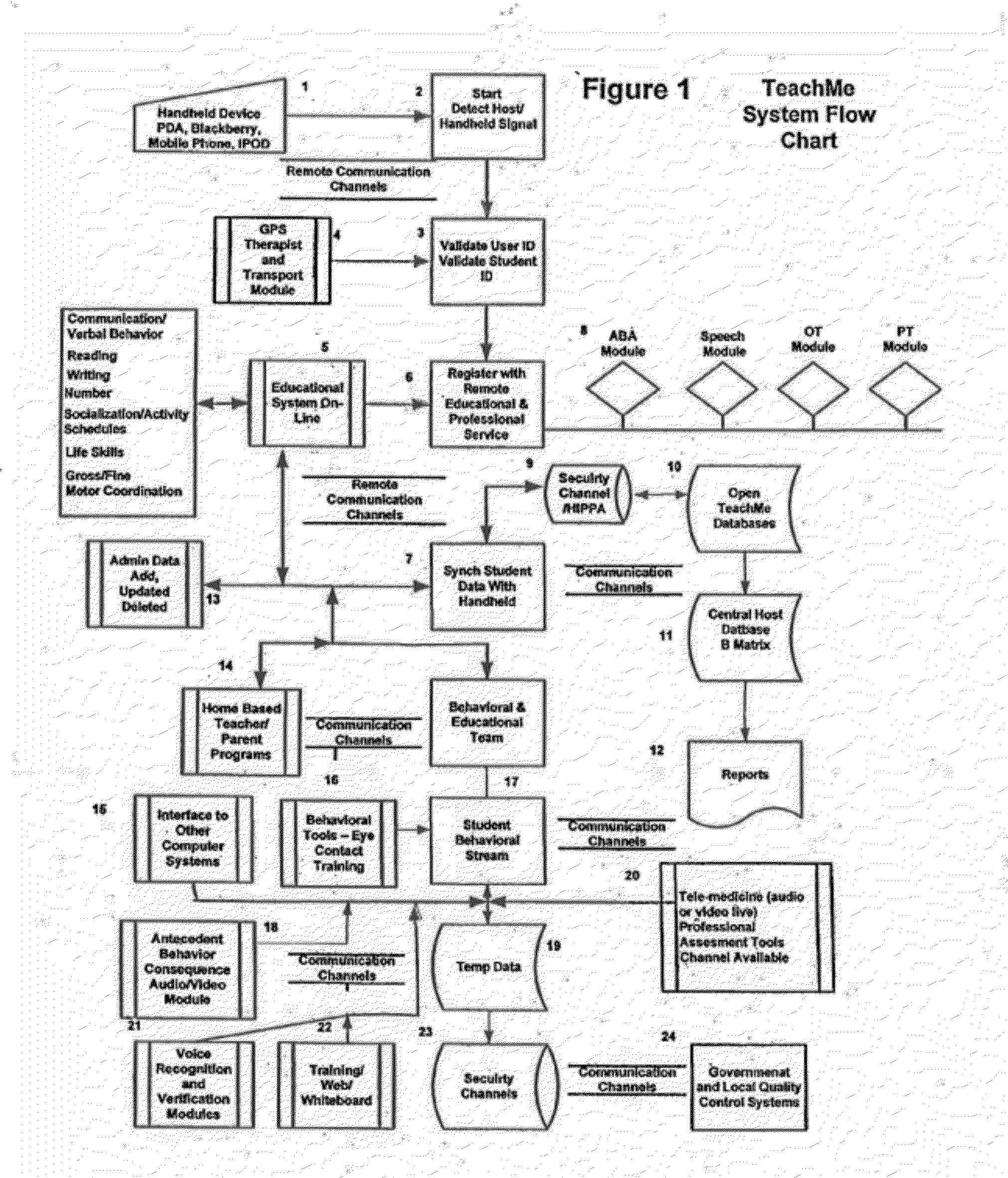
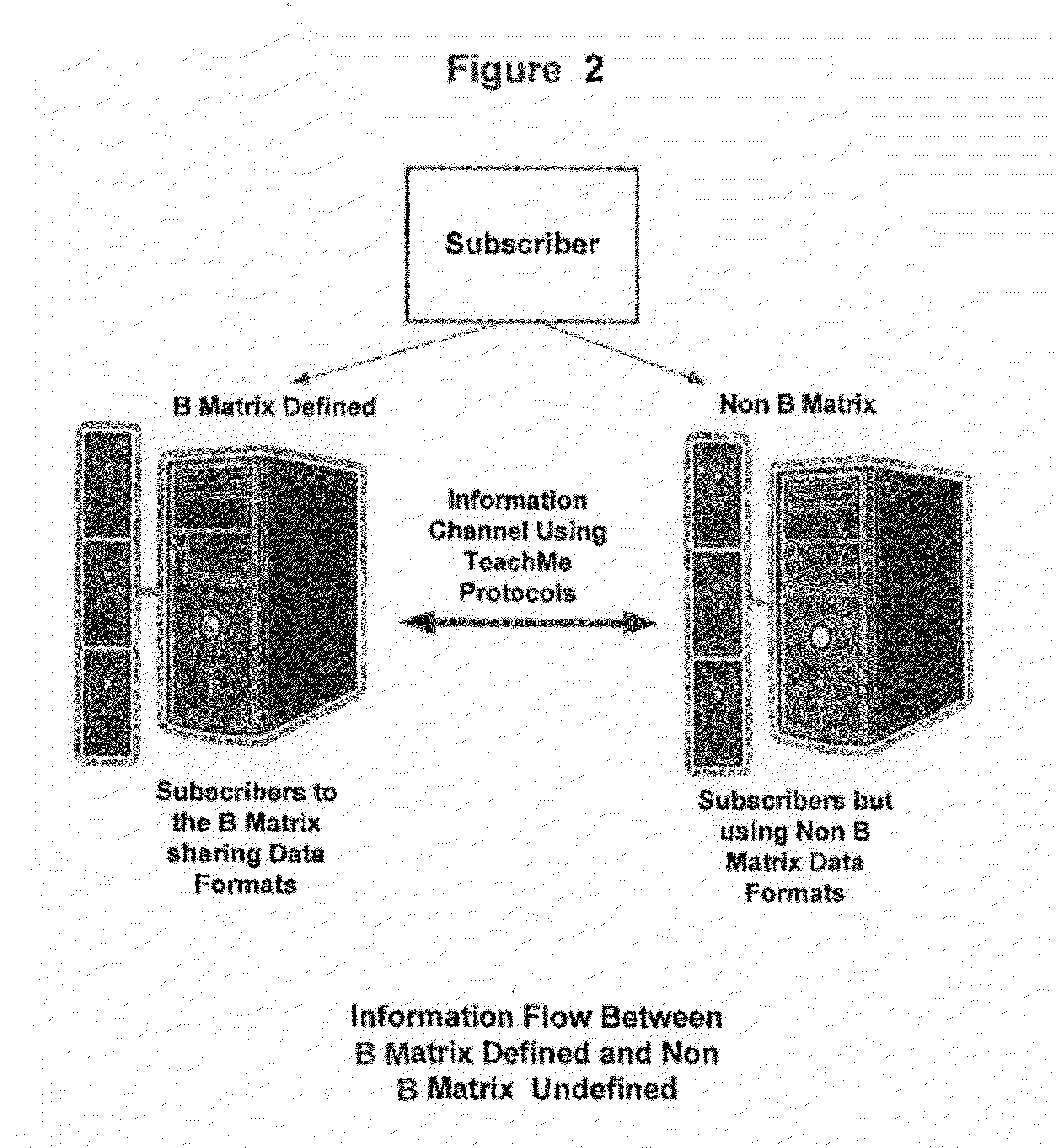
Response scoring system for verbal behavior within a behavioral stream with a remote central processing system and associated handheld communicating devices
InactiveUS20100145729A1Improve communication skillsFacilitate communicationRegistering/indicating time of eventsDigital computer detailsCommunication skillsSpecial needs
A system, method and related devices for monitoring and improving the training and social eye contact and communication skills of developmentally challenged individuals such as autistic individuals and other individuals with special needs in improving their interpersonal communicating and other skills. A so called, TeachMe component of the system allows the defining of a students / patients long-term behavior or academic or social goal treatment in a hierarchical relationship of skills in treatment plans. It captures behavioral event within a ‘behavioral stream’ and in real time stores the ‘behavioral stream’ in a centralized database that can be accessed anywhere and anytime using the Internet. Procedures and materials used during the treatment are identified and data is stored in a central depository and can be displayed in a therapist's handheld device. The WatchMe component of the system and method monitors and obtains qualitative and quantitative information about the eye contact habits of a subject being trained or interviewed. It provides stimuli that promotes and encourages improvements in the eye contact habits of the subject.
Owner:KATZ BARRY
Enhancing diagnosis of disorder through artificial intelligence and mobile health technologies without compromising accuracy
ActiveUS20140304200A1To offer comfortImprove impactDigital computer detailsMedical automated diagnosisDiseaseComputerized system
A computer system for generating a diagnostic tool by applying artificial intelligence to an instrument for diagnosis of a disorder, such as autism. For autism, the instrument can be a caregiver-directed set of questions designed for an autism classification tool or an observation of the subject in a video, video conference, or in person and associated set of questions about behavior that are designed for use in a separate autism classification tool. The computer system can have one or more processors and memory to store one or more computer programs having instructions for generating a highly statistically accurate set of diagnostic items selected from the instrument, which are tested against a first test using a technique using artificial intelligence and a second test against an independent source. Also, a computer implemented method and a non-transitory computer-readable storage medium are disclosed.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Autism-associated biomarkers and uses thereof
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
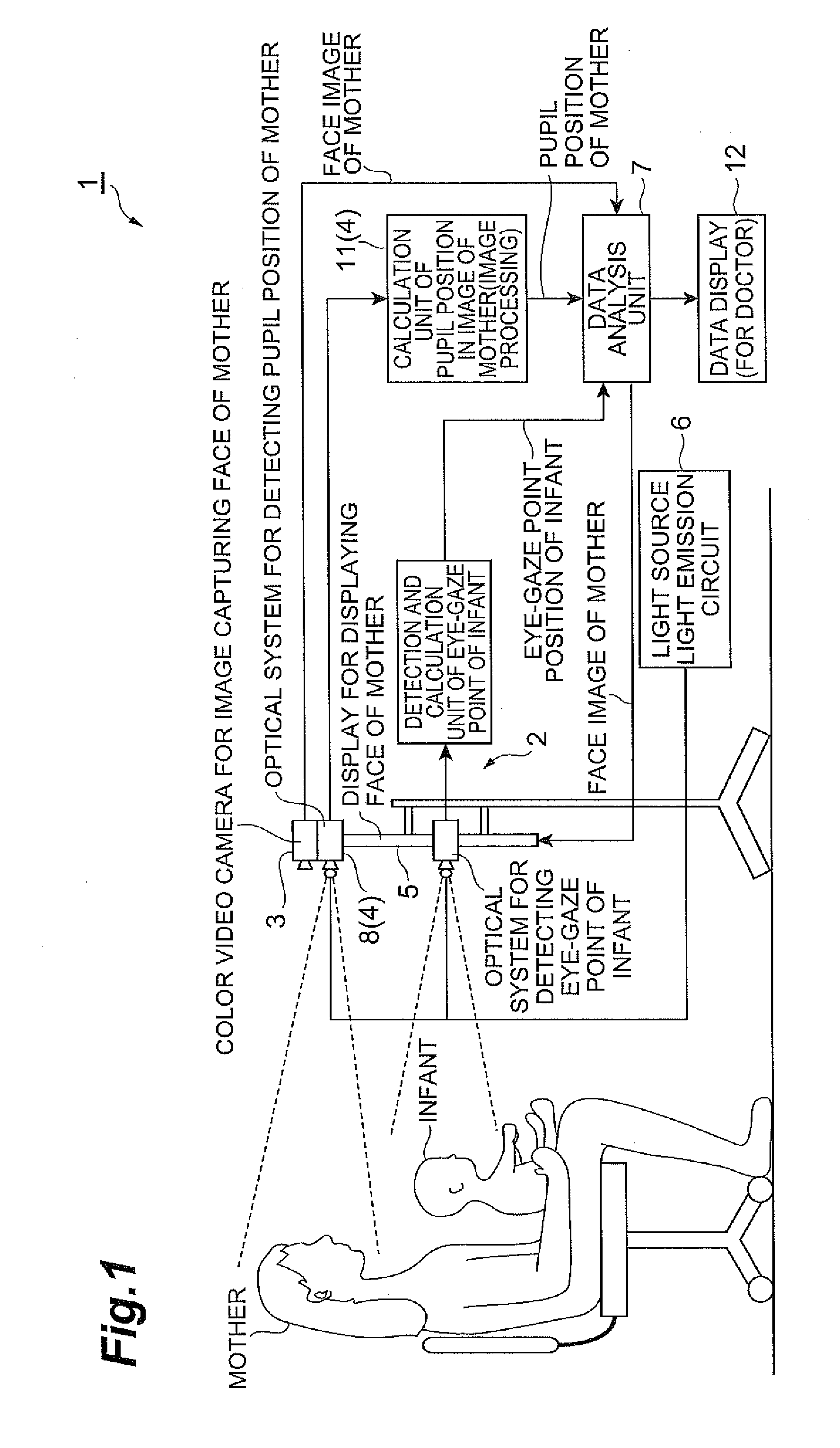
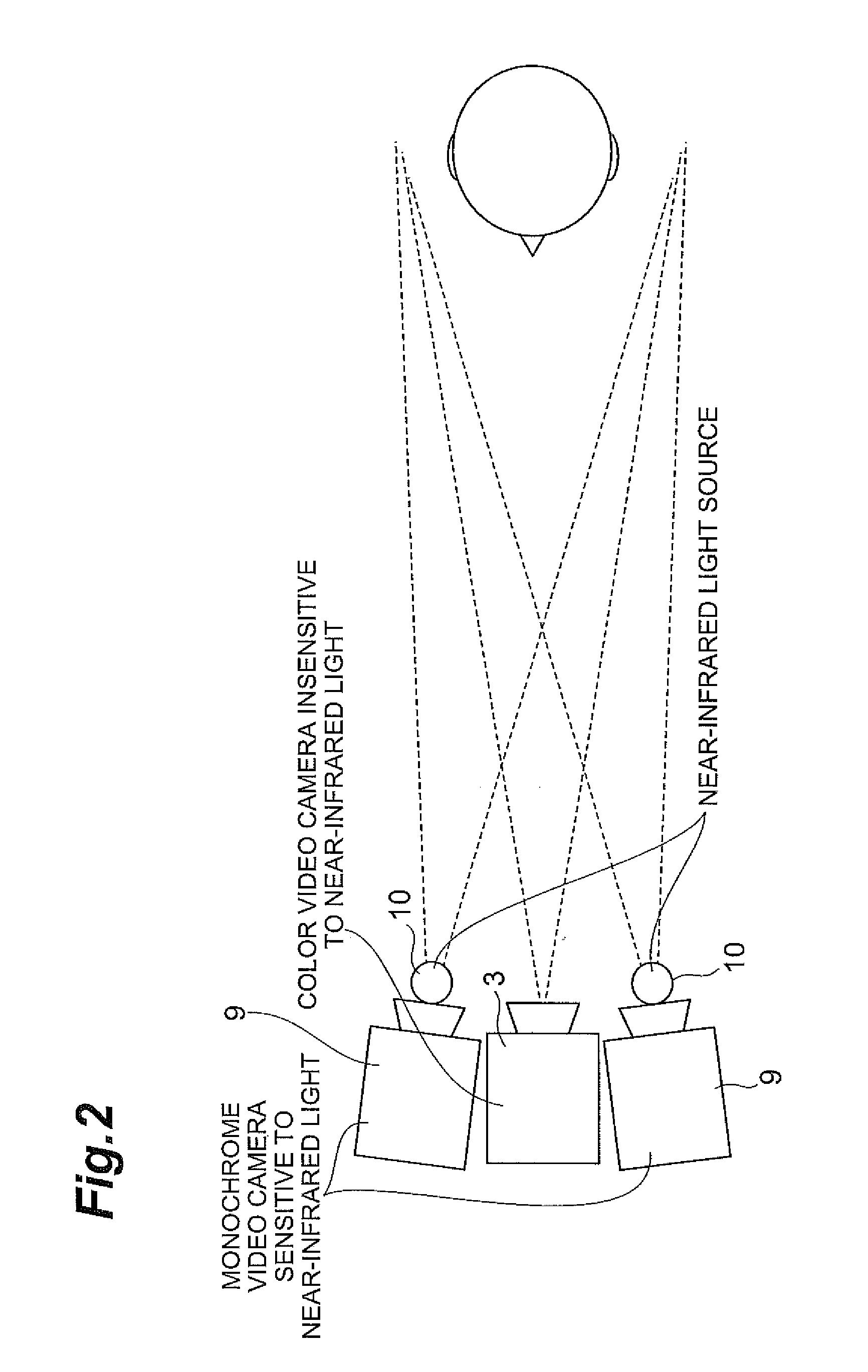
Autism diagnosis support apparatus
InactiveUS20110242486A1Easy accessReliable dataHealth-index calculationDiagnostic recording/measuringOphthalmologyPupil
An autism diagnosis support apparatus 1 according to the present invention is an autism diagnosis support apparatus that detects a symptom of autism based on a state of a subject looking at a target, including: an eye-gaze point detection unit 2 that detects a line-of-sight direction of the subject looking at the target; a color camera 3 that takes an image of the target; a pupil position detection unit 4 that measures a pupil coordinate of the target; and a data analysis unit 7 that calculates a relationship between the line-of-sight direction of the subject and a pupil position of the target using the line-of-sight direction and the pupil coordinate and outputs the relationship along with the image of the target.
Owner:NAT UNIV CORP SHIZUOKA UNIV
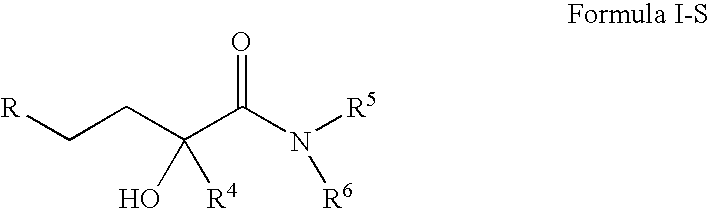
4-(p-QUINONYL)-2-HYDROXYBUTANAMIDE DERIVATIVES FOR TREATMENT OF MITOCHONDRIAL DISEASES
ActiveUS20090118257A1Good for healthRaise level of ATPBiocideSenses disorderKearn sayre syndromeHuntingtons chorea
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, and stroke (MELAS), Kearns-Sayre Syndrome (KSS), are disclosed, as well as compounds useful in the methods of the invention, such as 4-(p-quinolyl)-2-hydroxybutanamide derivatives. Methods and compounds useful in treating other disorders such as amyotrophic lateral sclerosis (ALS), Huntington's disease, Parkinson's disease, and pervasive developmental disorders such as autism are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
Therapy for enteric infections
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
Methods and Compositions for Autism Risk Assessment
InactiveUS20070248956A1Increased riskCell receptors/surface-antigens/surface-determinantsSugar derivativesGenetic riskGenetics
Methods and compositions are provided for evaluating an individual for relative genetic risk for autism, for identifying a form of a genetic polymorphism that is linked to autism, and for evaluating whether a compound affects autism.
Owner:BEATRICE & SAMUEL A SEAVER FOUND
Enhancing diagnosis of disorder through artificial intelligence and mobile health technologies without compromising accuracy
ActiveUS9443205B2To offer comfortImprove impactTelemedicineComputer-assisted medical data acquisitionDiseaseCare giver
A computer system for generating a diagnostic tool by applying artificial intelligence to an instrument for diagnosis of a disorder, such as autism. For autism, the instrument can be a caregiver-directed set of questions designed for an autism classification tool or an observation of the subject in a video, video conference, or in person and associated set of questions about behavior that are designed for use in a separate autism classification tool. The computer system can have one or more processors and memory to store one or more computer programs having instructions for generating a highly statistically accurate set of diagnostic items selected from the instrument, which are tested against a first test using a technique using artificial intelligence and a second test against an independent source. Also, a computer implemented method and a non-transitory computer-readable storage medium are disclosed.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Enzyme Delivery Systems and Methods of Preparation and Use
ActiveUS20120027848A1Improve stabilityImprove propertiesPowder deliveryNervous disorderDiseaseCystic fibrosis
This invention relates to coated digestive enzyme preparations and enzyme delivery systems and pharmaceutical compositions comprising the preparations. This invention further relates to methods of preparation and use of the systems, pharmaceutical compositions and preparations to treat persons having ADD, ADHD, autism, cystic fibrosis and other behavioral and neurological disorders.
Owner:CUREMARK
Use of lactulose in the treatment of autism
ActiveUS20120004192A1Avoid accumulationReverses effectBiocideNervous disorderBacteroidesNervous system
A treatment for autism in which an effective amount of lactulose is administered in order to bind excess ammonia in the gastrointestinal tract, the bloodstream, and the nervous system in order to prevent or reverse ammonia poisoning caused by the administration of certain antibiotics. Lactulose molecules in the colon are fermented by certain bacteria. The fermentation process lowers the colonic pH, and ammonia, in the form of ammonium ions, is used by the bacteria for amino acid and protein synthesis. This lowers the serum ammonia levels and reduces neurotoxicity.
Owner:CUREMARK
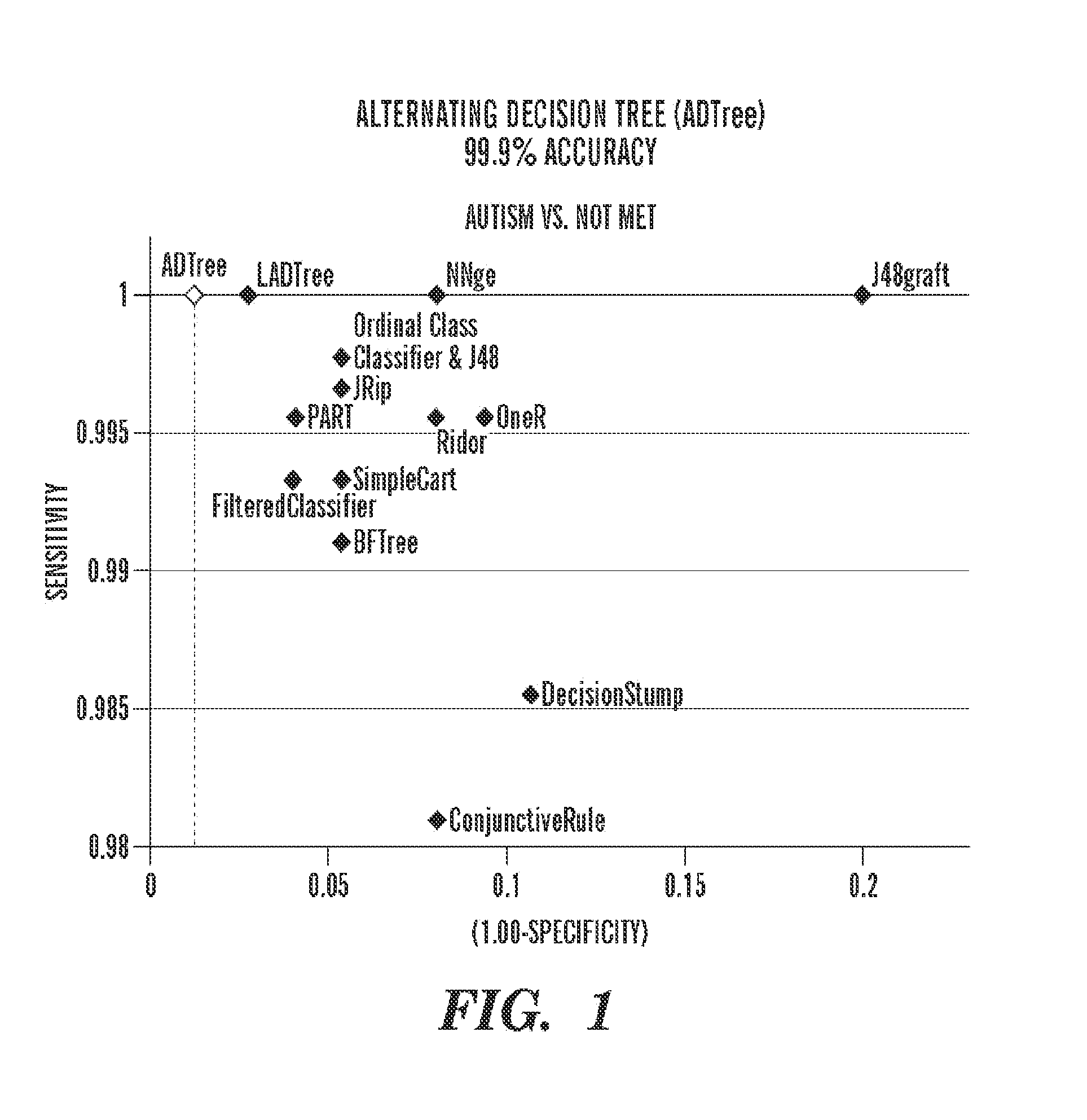
Machine learning-based method for evaluating and predicting ASD
ActiveCN105069304AAccurate and convenient assessmentImportantCharacter and pattern recognitionSpecial data processing applicationsLearning basedFeature extraction
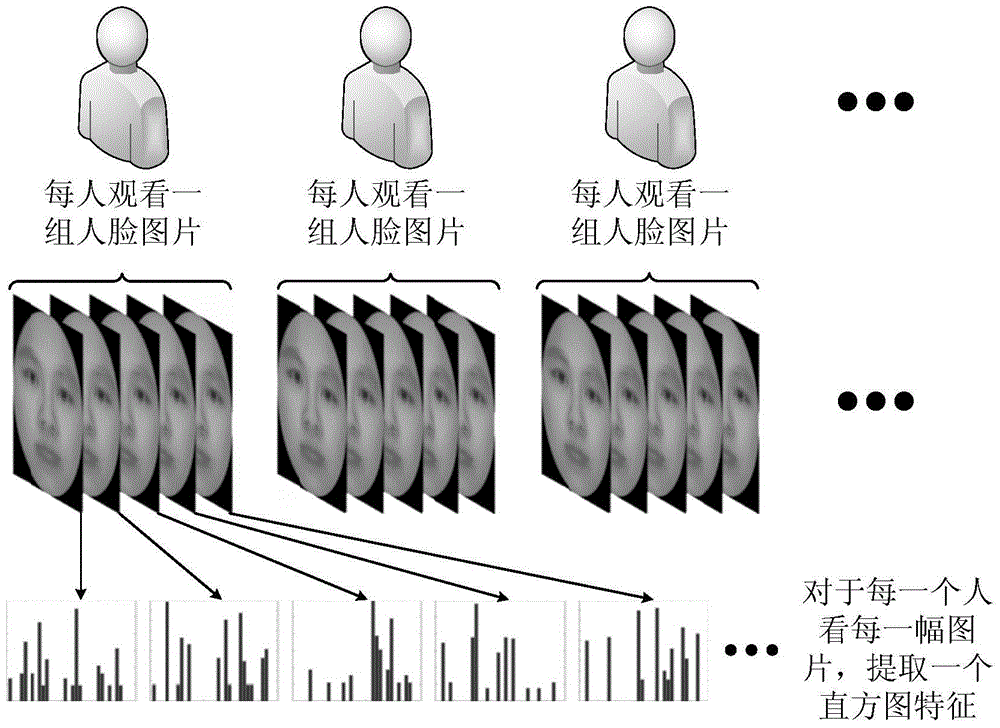
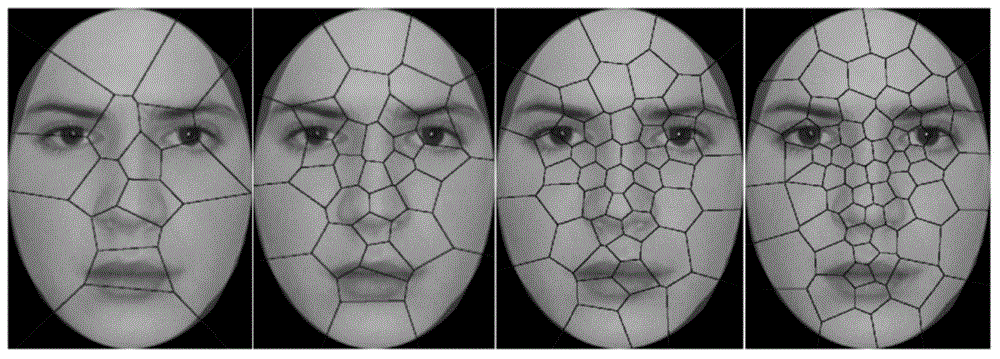
The present invention discloses a machine learning-based method for evaluating and predicting ASD, comprising the following steps: S1. data collection: using an eye tracker to separately collect eye movement data that an eye ball scans a human face when persons participating in a test watch a human face image, wherein the persons participating in the test comprise individuals who have ASD and normal individuals; S2. feature extraction: dividing the human face image into different areas according to collected eye movement coordinate data, extracting a feature from the original data collected by the eye tracker, and making a mark; S3. classifier training: training a classifier by using a marked feature, to obtain a classifier model for predicting ASD; and S4. prediction: testing on a test subject by using the classifier model for predicting ASD acquired in step S3, to evaluate and predict severity of autism of the test subject. The present invention may be considered as a supplementary method of ASD evaluation, so that ASD evaluation and prediction in an early stage is more accurate and convenient.
Owner:SYSU CMU SHUNDE INT JOINT RES INST
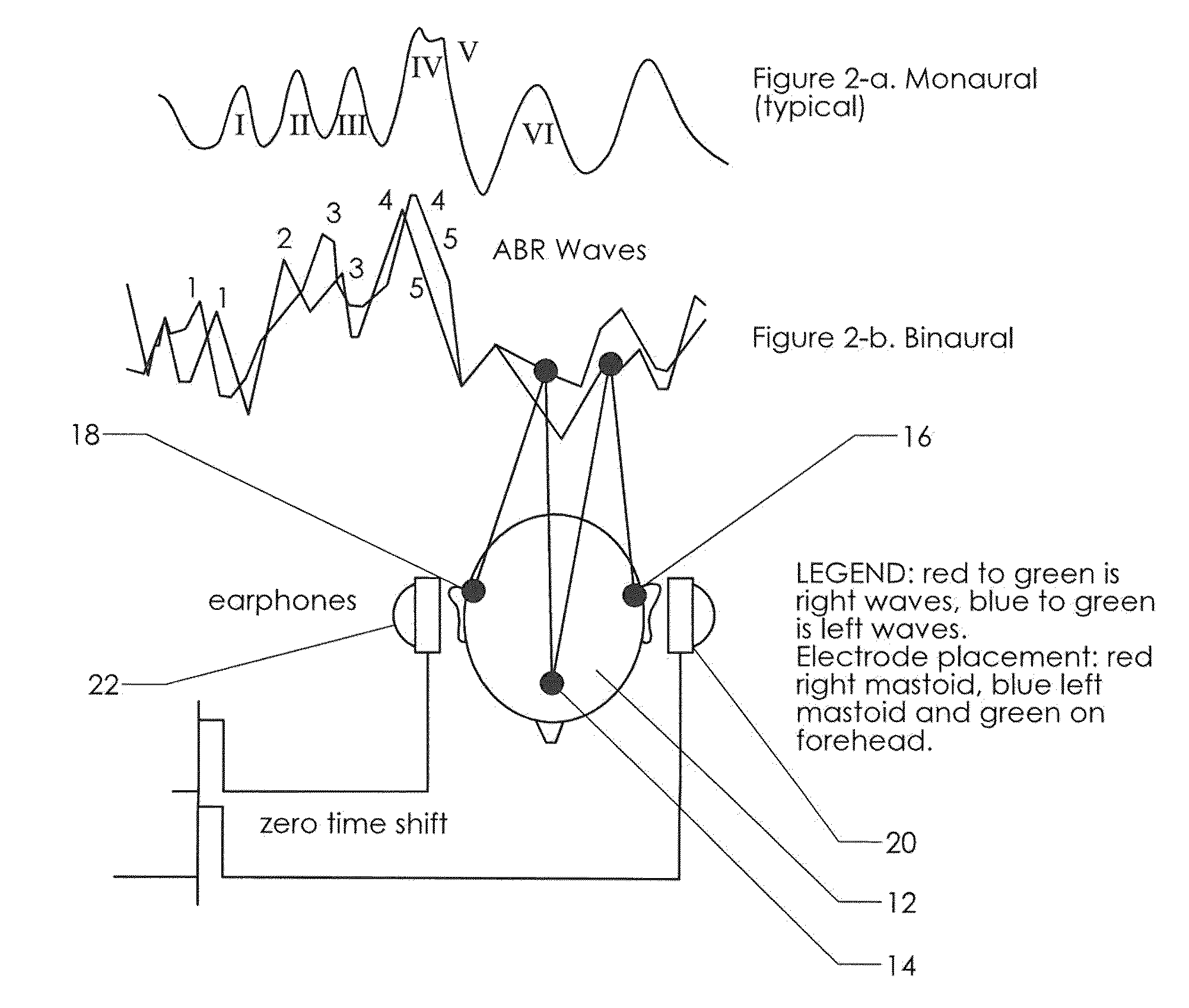
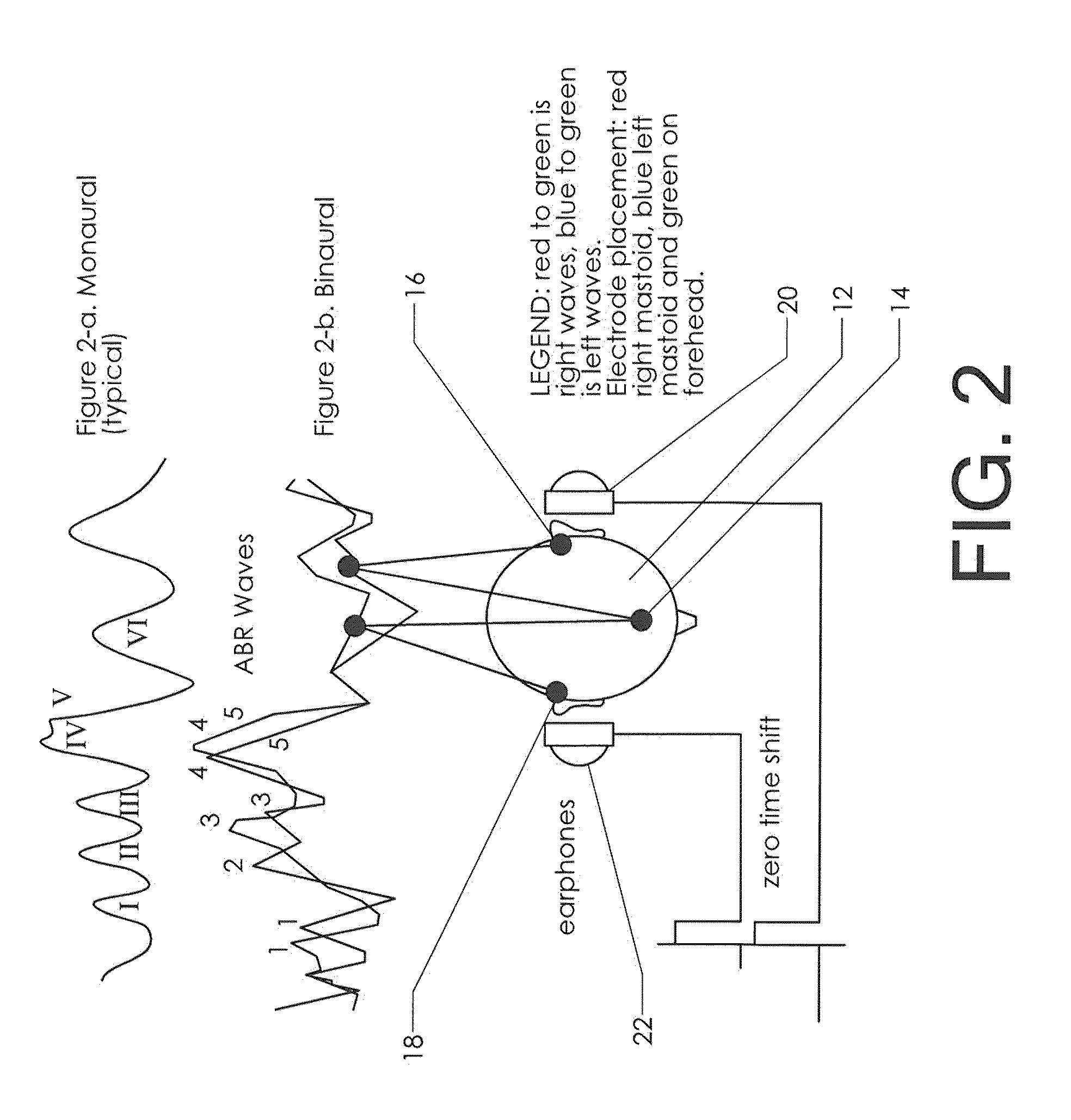
Neuroaudiological central auditory test apparatus and method of differentiation of the neural correlates in ptsd, tbi, autism, adhd, et al
An apparatus, software, procedures and technology that, beginning with a simple manipulative auditory paradigm, moves up the auditory neural pathways, modifying the basic stimulus from a unilateral routine audiology tool to a dichotic central auditory processing diagnostic tool that defines hearing loss along the entire auditory chain from the middle ear to the cortex. From the resultant data comes information related to the reduction of tinnitus employing parathreshold central procedures rather than the traditional suprathreshold masking or phase-shifting techniques. The new application modifies and applies known technology in new ways for both the top-down and bottom-up differential diagnosis of auditory disorders and the tinnitus reduction.
Owner:DICHONICS CORP
Treatment of autism using probiotic composition
A method of treating autism using a probiotic composition including the bacilli (1) Bacillus subtilis, (2) Bacillus coagulans, and (3) Enterococcus faecium. The composition may further include a carrier medium, such as fructo-oligo-saccharides (FOS), as incorporated in a dose form such as a pill, capsule, powder or sachet. The compositions of the invention may be usefully employed as health or nutritional supplements, food additives, or therapeutic agents for combating a wide variety of physiological disorders.
Owner:COBB & ASSOCS
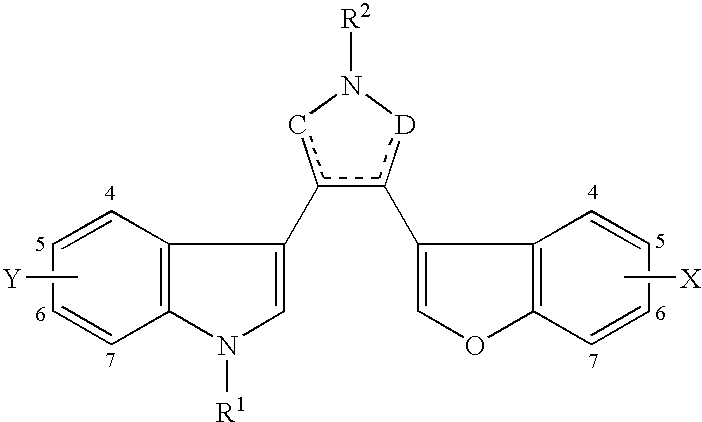
Benzofuran-3-yl(indol-3-yl) maleimides as potent GSK3 inhibitors
Compounds of formula:and pharmaceutically acceptable salts, esters and solvates thereof, where variables are defined in the specification, useful generally as inhibitors of protein kinases and particularly useful for inhibition of GSK-3.Pharmaceutically compositions and medicaments containing a compound of the invention are provided. The invention provides methods of treatment of protein kinase-related disease, disorders or conditions. The invention provides methods of treatment of GSK-3-related diseases, disorders or conditions. More specifically, methods of treatment of bipolar disorder, including mania, schizophrenia, stroke, epilepsy, motor neuron disease, cranial or spinal trauma, neurodegenerative disorders, including multiple sclerosis (MS), Alzheimer's disease, Fragile X syndrome, autism, Huntington's disease, Parkinson's disease, amylotrophic lateral sclerosis (ALS), AIDS-associated dementia, diabetes, particularly type II diabetes, cardiomycete hypertrophy, reperfusion / ischemia, cancer, particularly colorectal cancer, pancreatic cancer, allergies and / or asthma and hair loss or baldness.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Methods of treating alzheimer's disease, huntington's disease, autism, or other disorders
The disclosure relates, at least in part, to methods of treating autism in a patient in need thereof by administering an effective amount of a disclosed compound, e.g., a NMDA receptor glycine site partial agonist.
Owner:NORTHWEST UNIV
Use of memantine (namenda) to treat autism, compulsivity and impulsivity
The present invention relates to the treatment of compulsive, impulsive and pervasive developmental disorders. More particularly, the methods described herein comprise administration of memantine to an individual suffering from such a disorder in an amount effective to relieve one or more symptoms of said disorder. In particularly preferred aspects, the invention is directed to the use of memantine for the treatment of autism.
Owner:MT SINAI SCHOOL OF MEDICINE
Response scoring system for verbal behavior withina behavioral stream with a remote central processingsystem and associated handheld communicating devices
InactiveUS20120178064A1Facilitate communicationIncrease contactElectrical appliancesTeaching apparatusCommunication skillsThe Internet
A system, method and related devices for monitoring and improving the training and social eye contact and communication skills of developmentally challenged individuals such as autistic individuals. One component of the system allows the defining of a students / patients long-term behavior or academic or social goal treatment in a hierarchical relationship of skills in treatment plans. It captures behavioral events within a ‘behavioral stream’ and stores the ‘behavioral stream’ in a centralized database that can be accessed centrally, including with the Internet. Procedures and materials used during the treatment are identified and data is stored in a central depository and can be displayed in a therapist's handheld device. Another component provides stimuli that promotes and encourages improvements in the eye contact habits of the subject.
Owner:KATZ BARRY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com