Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1322 results about "Suppository" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A suppository is a solid dosage form that is inserted into the rectum (rectal suppository), vagina (vaginal suppository), or urethra (urethral suppository), where it dissolves or melts and exerts local or systemic effects. Suppositories are used to deliver medications that act both systemically and locally.
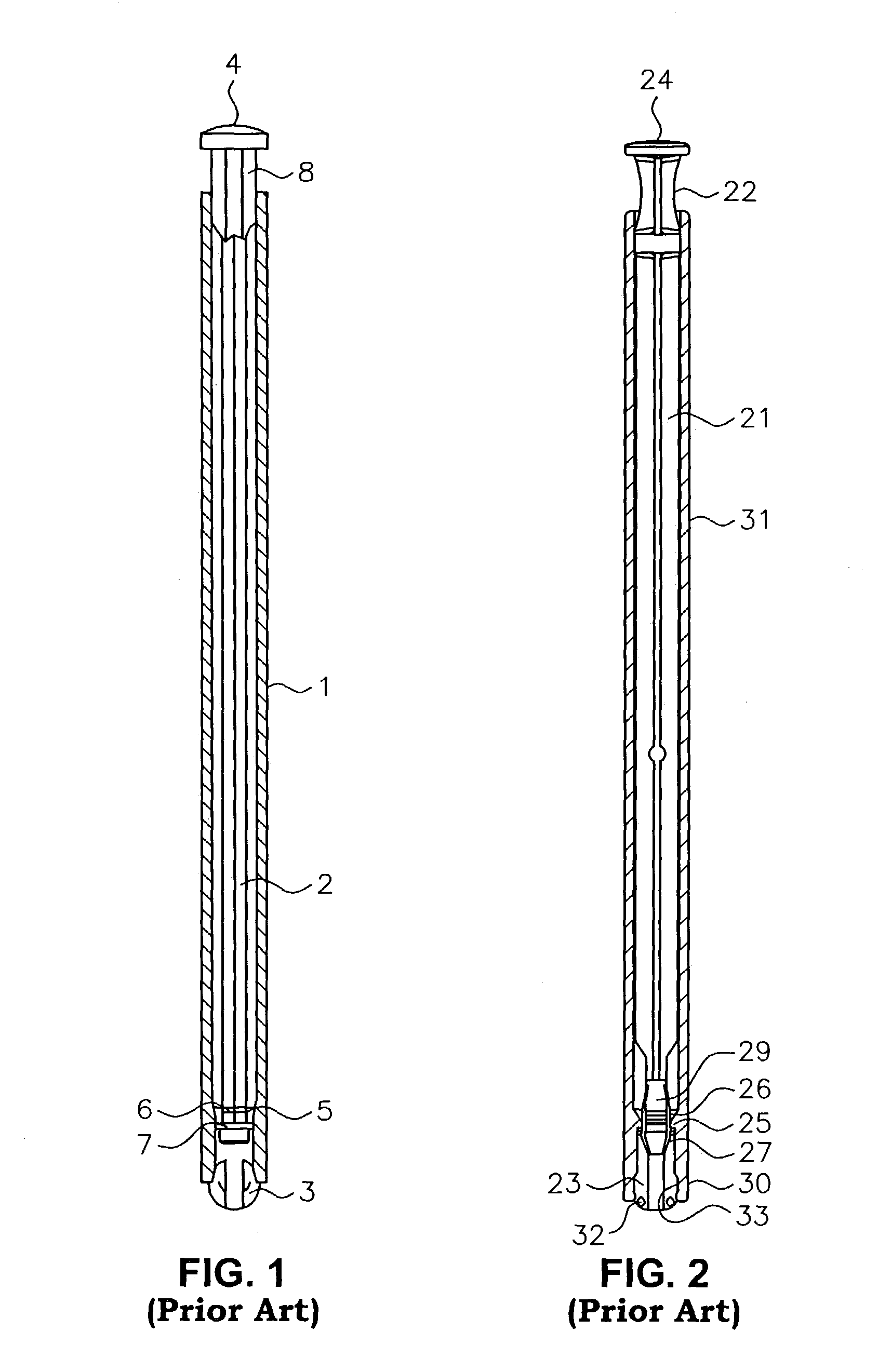
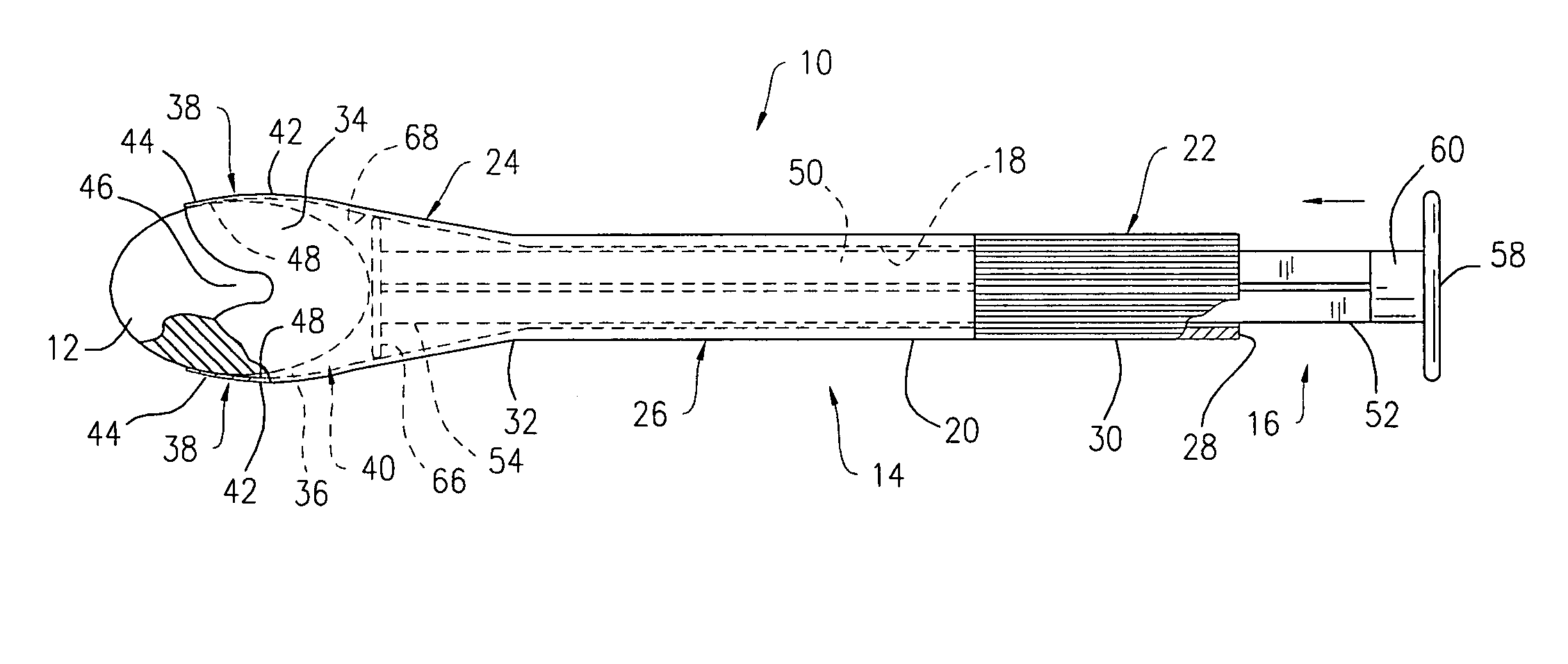
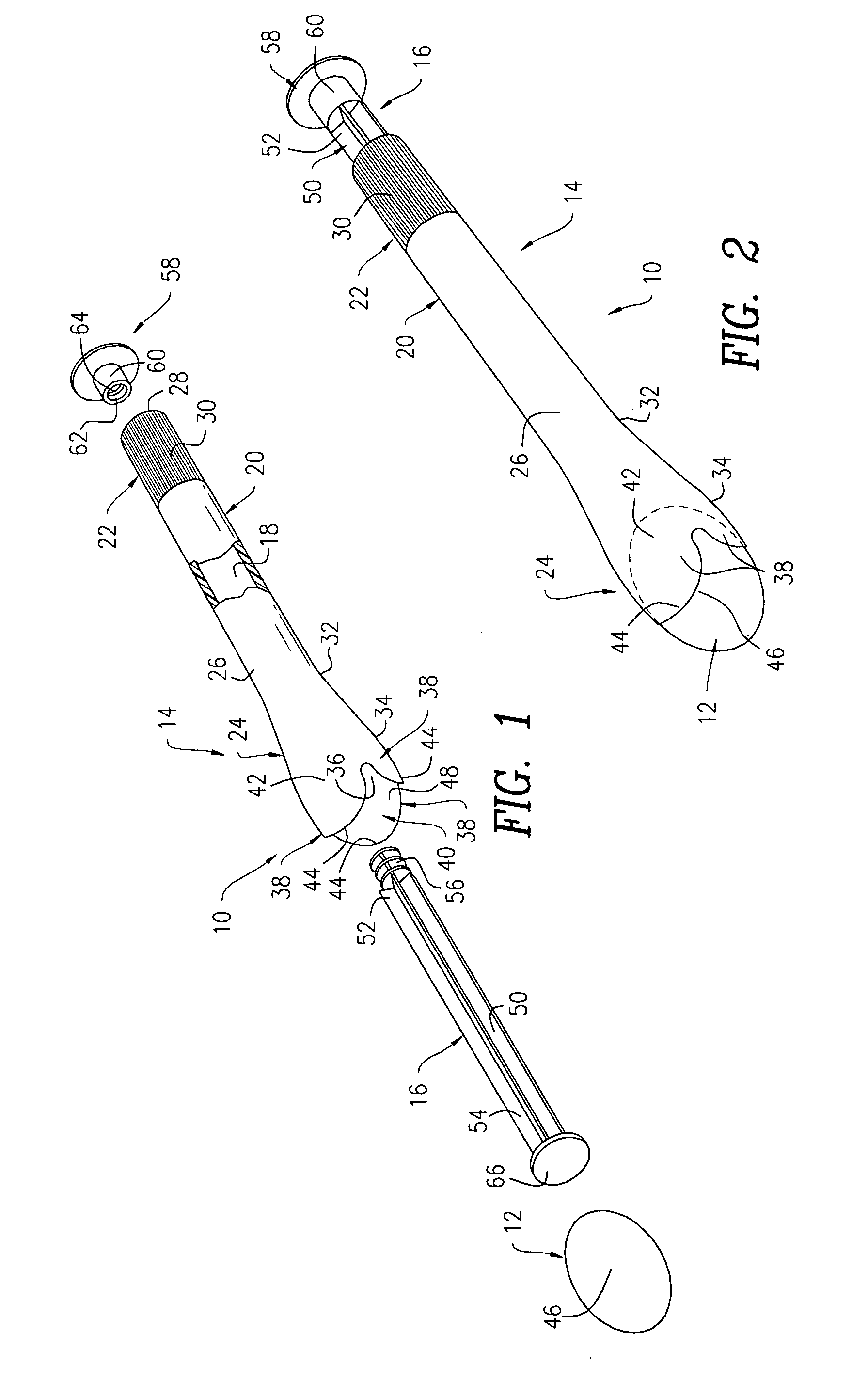
Applicator device for suppositories and the like
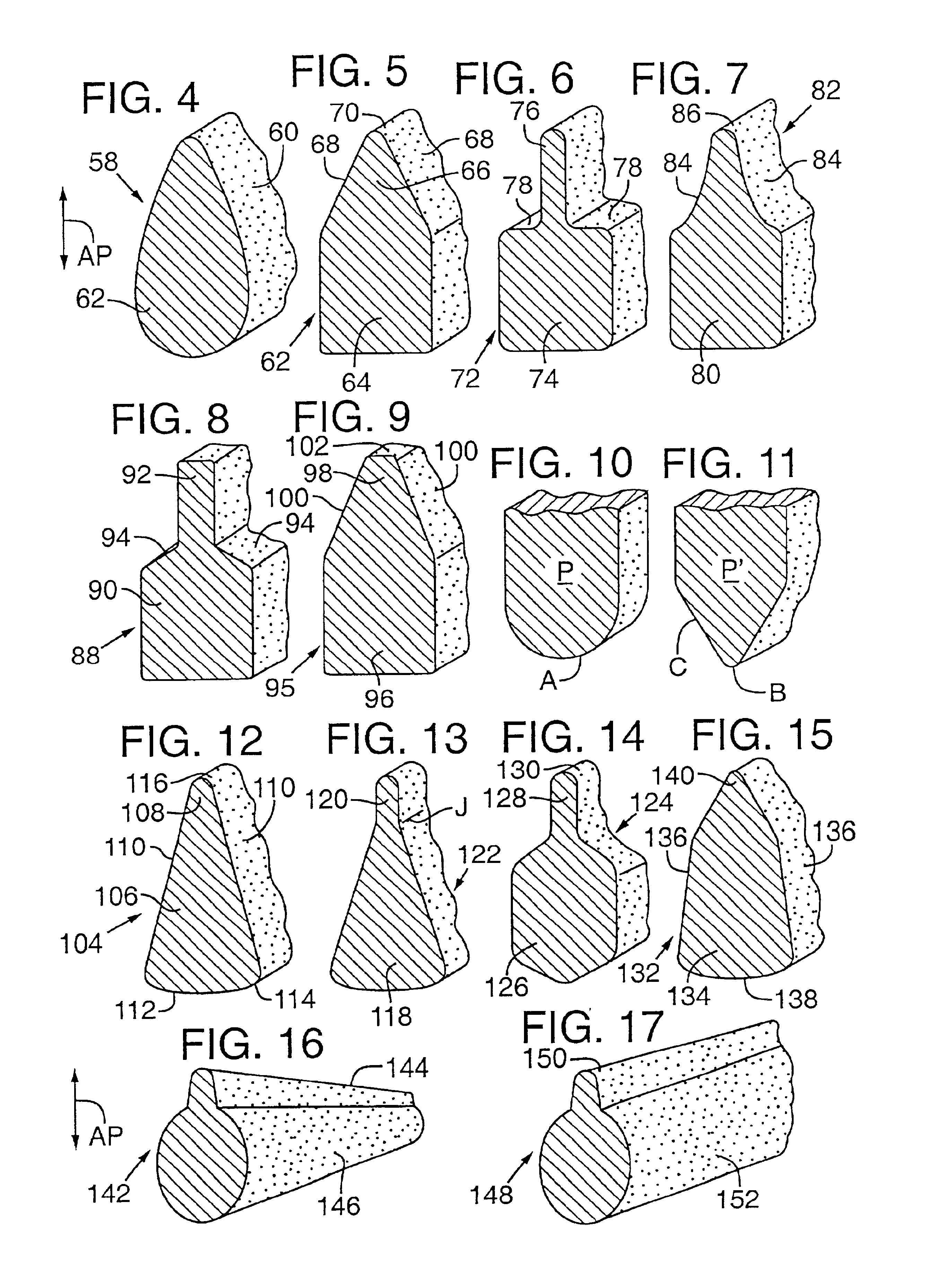
An applicator for delivering pharmaceutical products or the like to a bodily cavity includes a barrel member having a distal end which is equipped with an opening. The applicator also includes a plurality of petals extending outwardly from the distal end in a generally axial direction. The petals cooperate with the opening so as to form a receptacle for releasably receiving a pharmaceutical product in the distal end of the barrel member. Each of the petals has a truncated flexible tip sized and shaped so as to engage a substantially central portion of the pharmaceutical product such that a large section of the pharmaceutical product extends outwardly beyond the petals so as to facilitate the release of the pharmaceutical product from the receptacle. The device also includes a plunger member for releasing the pharmaceutical product from the receptacle. In accordance with the present invention, the device can be packaged in a package together with the pharmaceutical product received in the receptacle.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Anti-infection compound preparation and its preparation method
InactiveCN1380098AImprove immunityEffective excretionAntibody ingredientsUnknown materialsSide effectSuppository
The anti-infective medicine, including powder, mixture, aerosol, capsule, injection, suppository, ointment and microcapsule, is characterized by that on the theroretical basis of combining traditional Chinese medicine and modern immunology the immunoglobulin, effective components of Chinese medicinal materials which are extracted according to the compound prescription and auxiliary preparation are combined together organically, and undergone the fine preparation process to obtain a high-efficiency, safe, stable, environment-protecting type anti-infection medicine having no toxic side effect and having no drug resistance.
Owner:张勇飞 +2
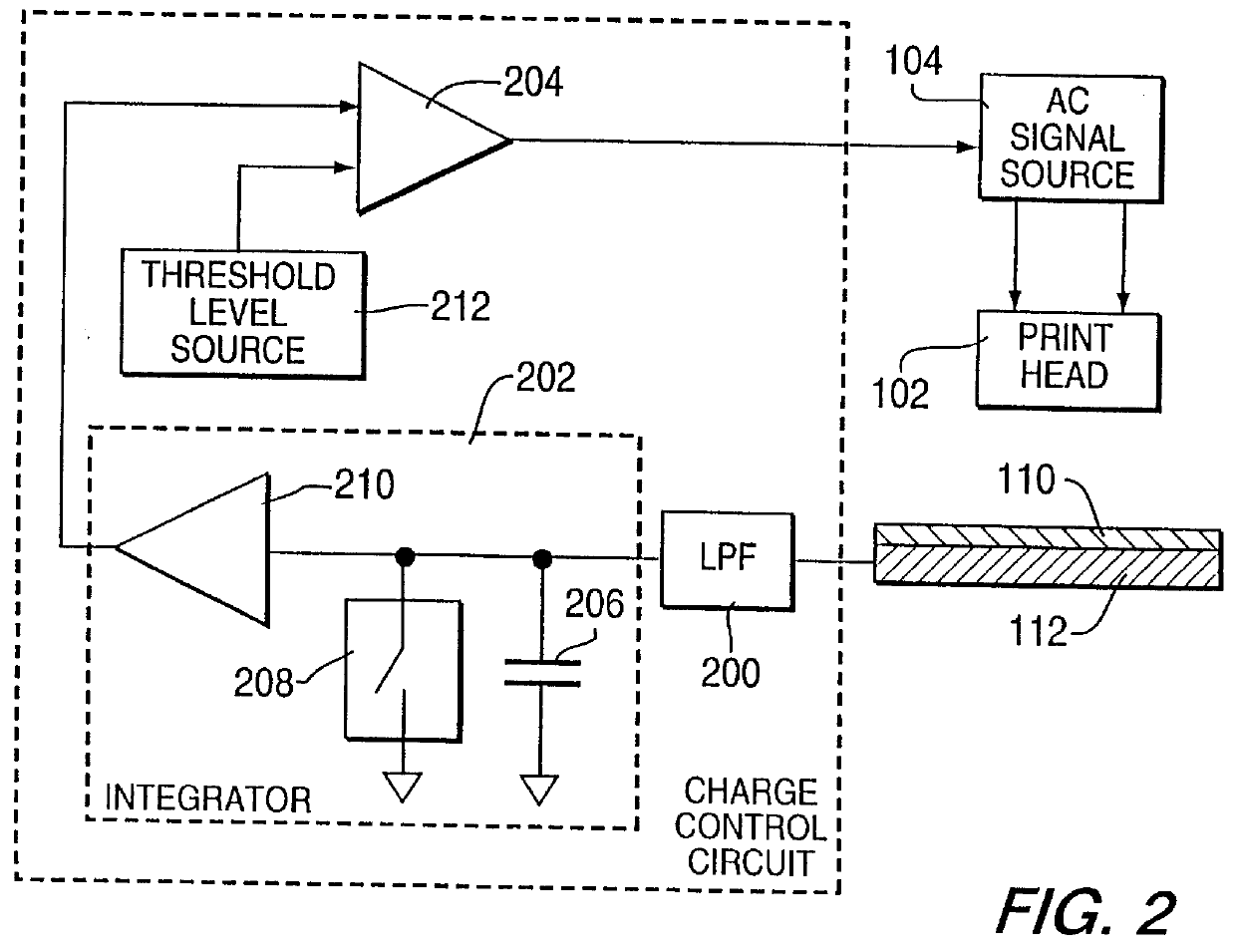
Method for electrostatically depositing a medicament powder upon predefined regions of a substrate
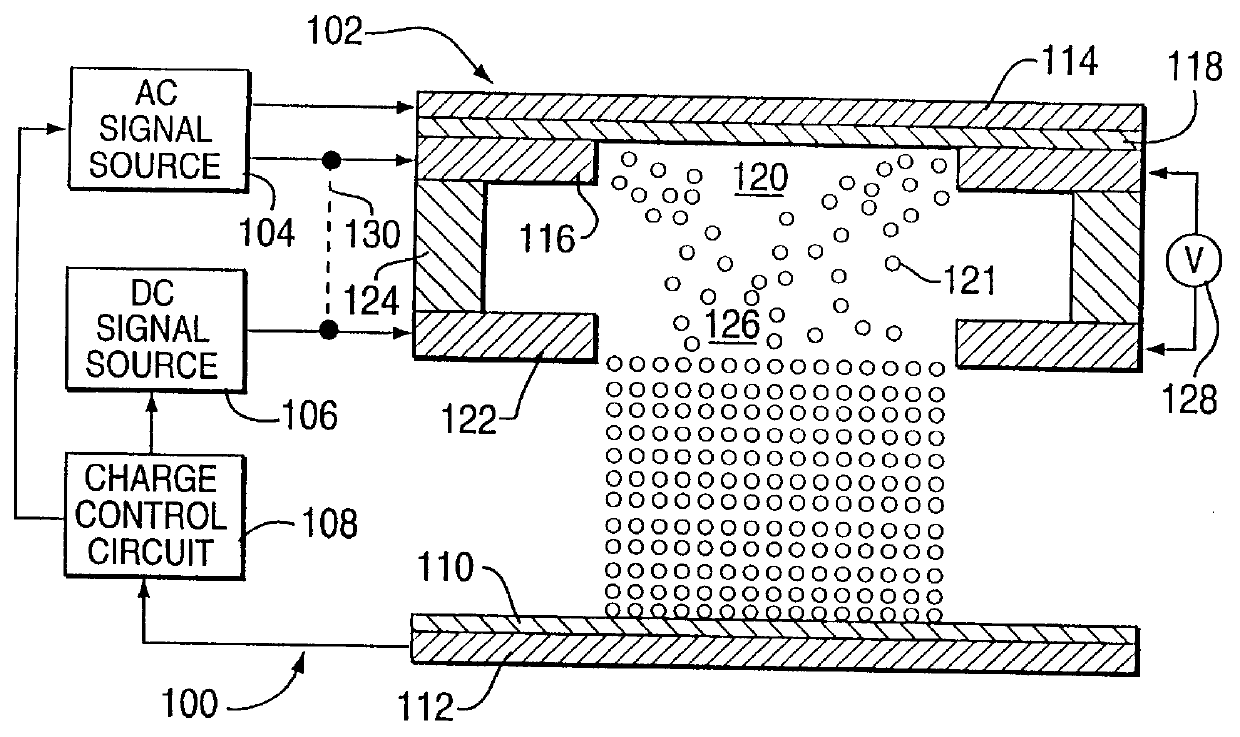
Method for electrostatically depositing select doses of medicament powder at select locations on a substrate. Specifically, the apparatus contains a charged particle emitter for generating charged particles that charge a predefined region of a substrate and a charge accumulation control circuit for computing the amount of charge accumulated upon the substrate and deactivating the emitter when a selected quantity of charge has accumulated. Additionally, a triboelectric charging apparatus charges the medicament powder and forms a charged medicament cloud proximate the charged region of the substrate. The medicament particles within the medicament cloud electrostatically adhere to the charged region. The quantity of charge accumulated on the substrate at the predefined region and the charge-to-mass ratio of the medicament powder in the cloud control the amount (dose) of medicament deposited and retained by the substrate. Consequently, this apparatus accurately controls both medicament dosage and deposition location. Furthermore, since the substrate can be of any dielectric material that retains an electrostatic charge, the apparatus can be used to deposit medicament on substrates that are presently used in oral medicament consumption, e.g., substrates that are used to fabricate suppositories, inhalants, tablets, capsules and the like.
Owner:DELSYS PHARMA
Composition and Method for Treating Infections and Promoting Intestinal Health
ActiveUS20110200570A1Improve floraPrevent bacterial growthAntibacterial agentsBiocideSuppositoryGlycerol
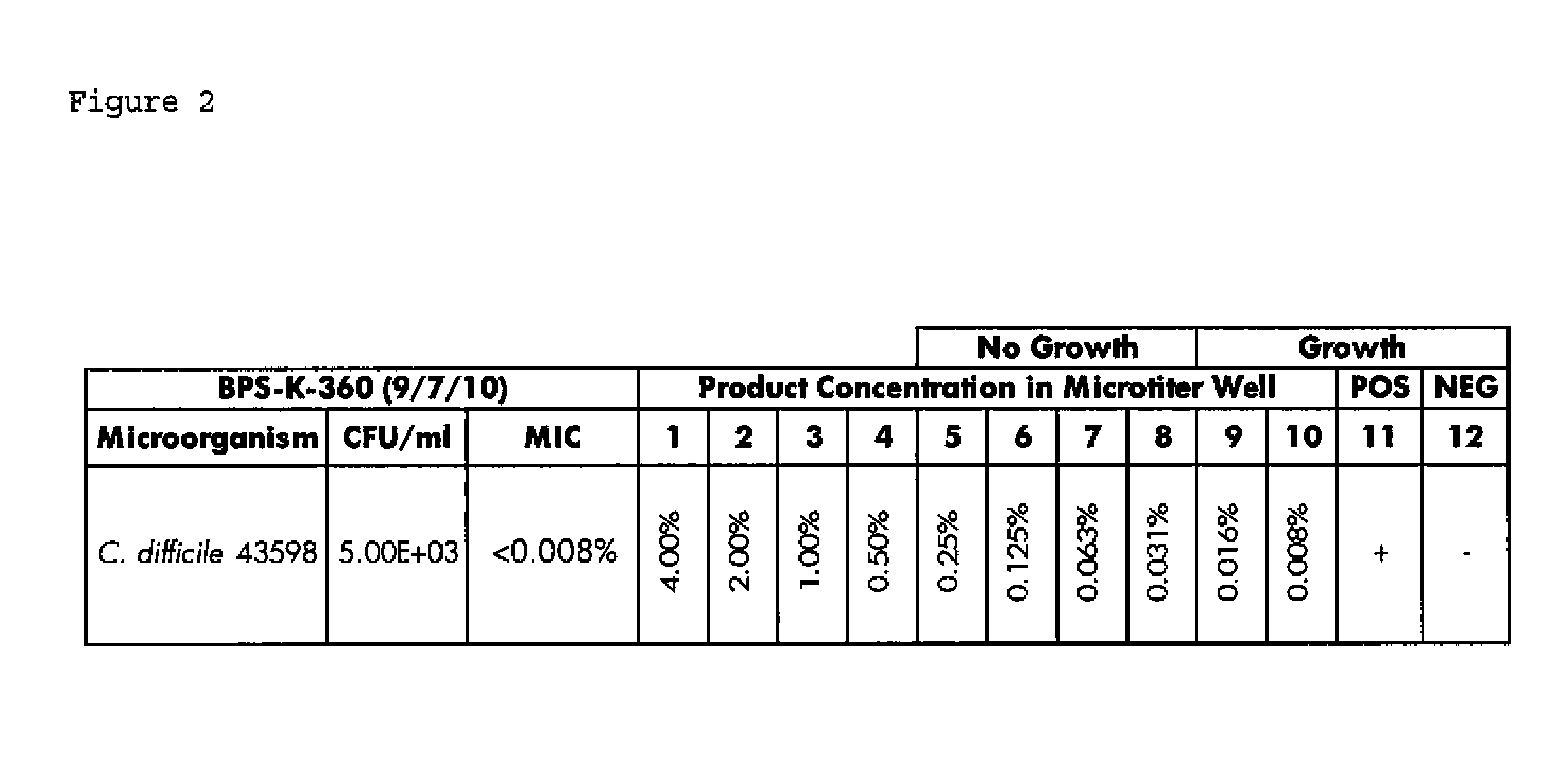
Compositions and methods for the treatment of intestinal infections. Compositions that include a liquid crystal mixture of an antimicrobial glycerol fatty acid ester and a polyhydric alcohol inhibit the growth of numerous deleterious intestinal pathogenic bacteria, including C. difficile. C. difficile is the causative agent in an increasing number of antibiotic-resistant bacterial infections. The formulations may be administered orally as capsules or soft gels, or alternatively as a enema, colonic, or rectal suppository. When combined with a probiotic supplement, the liquid crystal combinations reported here are able to treat an intestinal bacterial infection effectively and safely, thus promoting general intestinal health.
Owner:COPPERHEAD CHEM
Resilient device
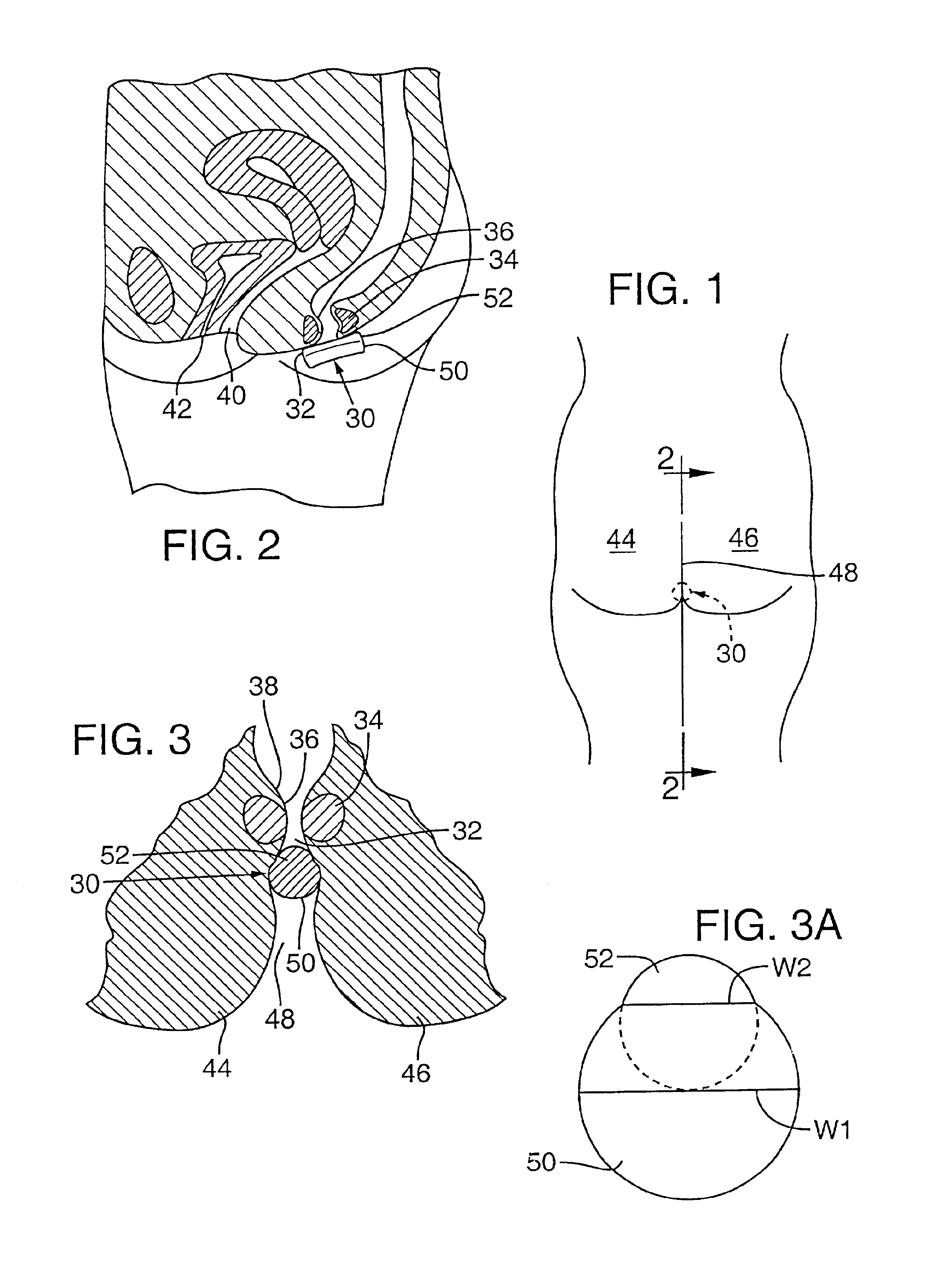
An intravaginal device has a working portion (e.g., intravaginal urinary incontinence device suppository, tampon) and an anchoring portion comprising at least one member extending beyond at least one end of the working portion to maintain the working portion in place during use.
Owner:FIRST QUALITY HYGIENIC
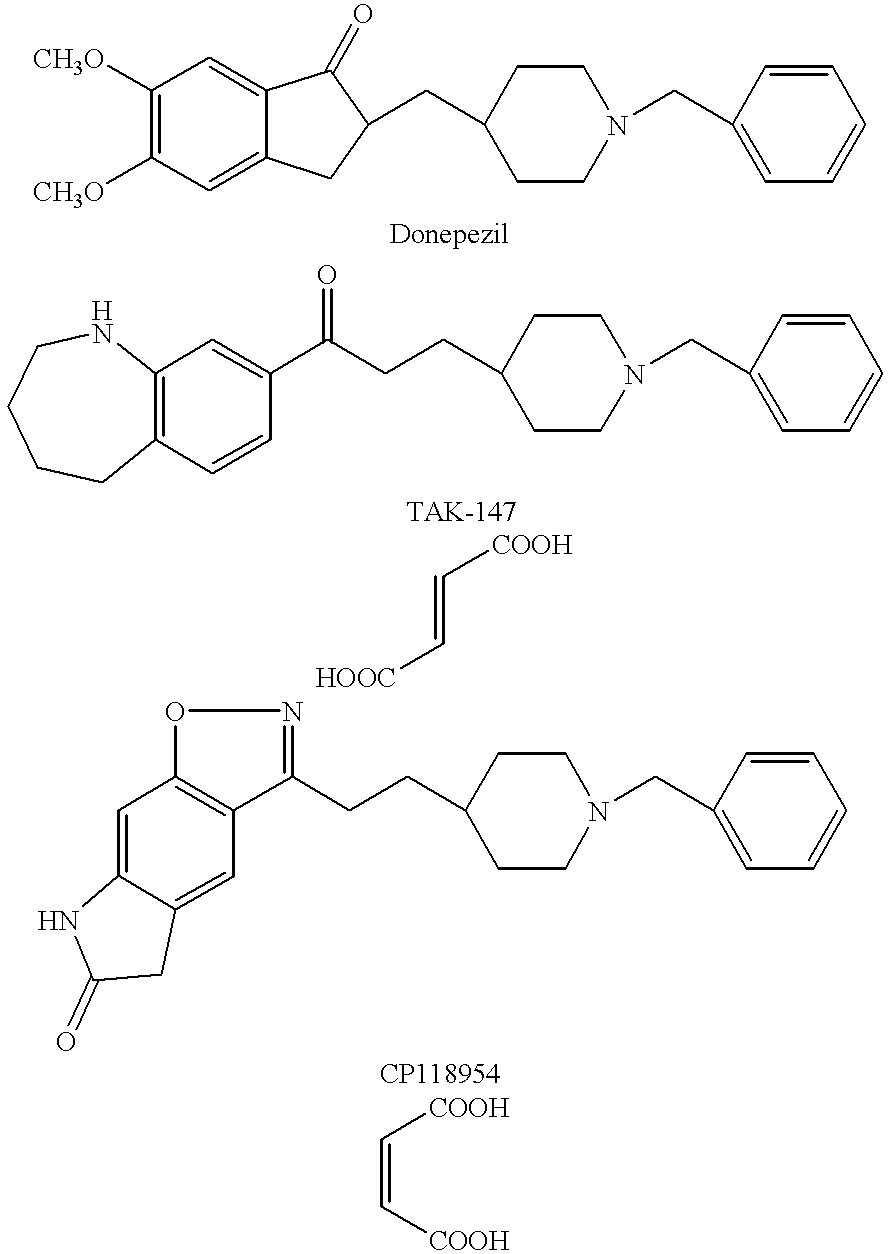
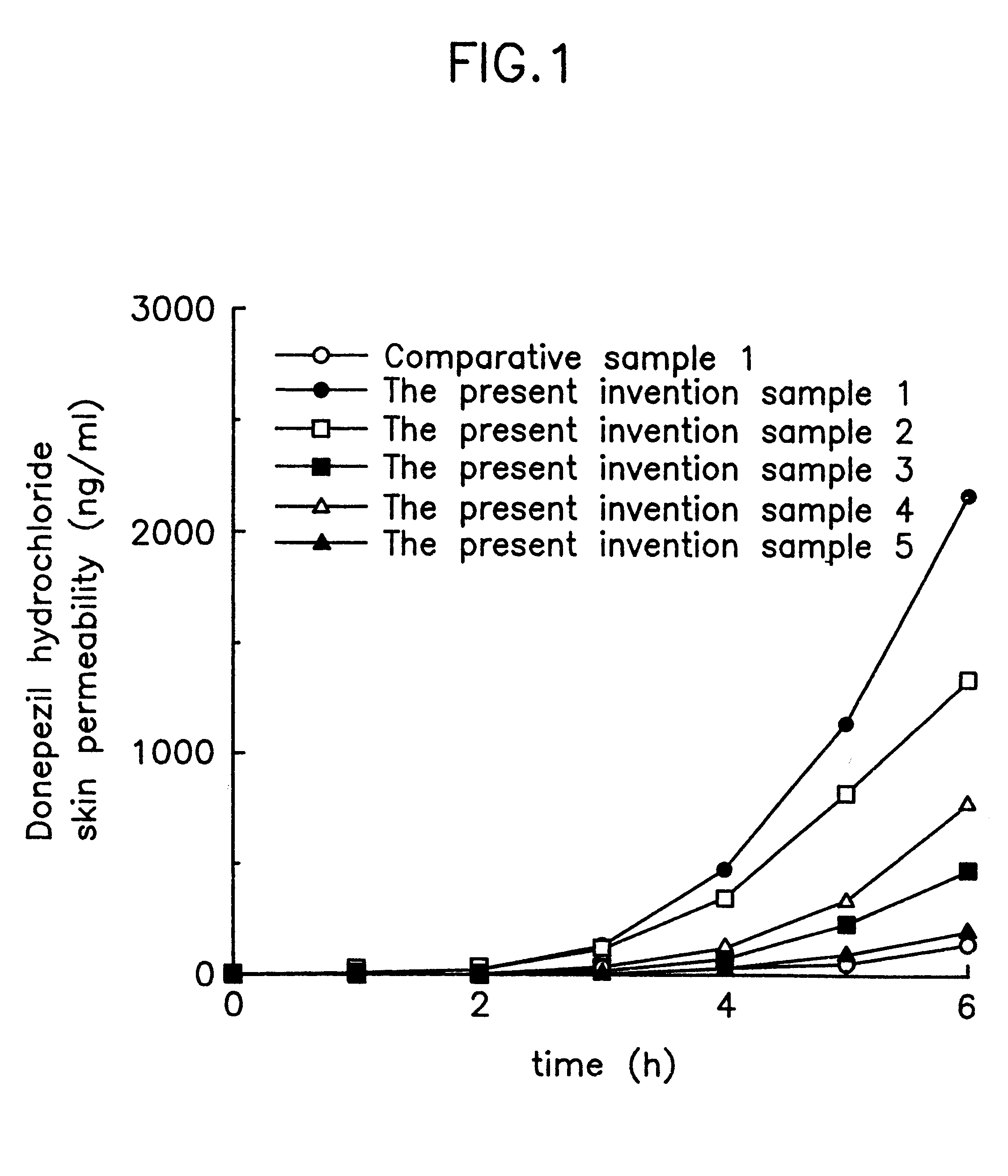
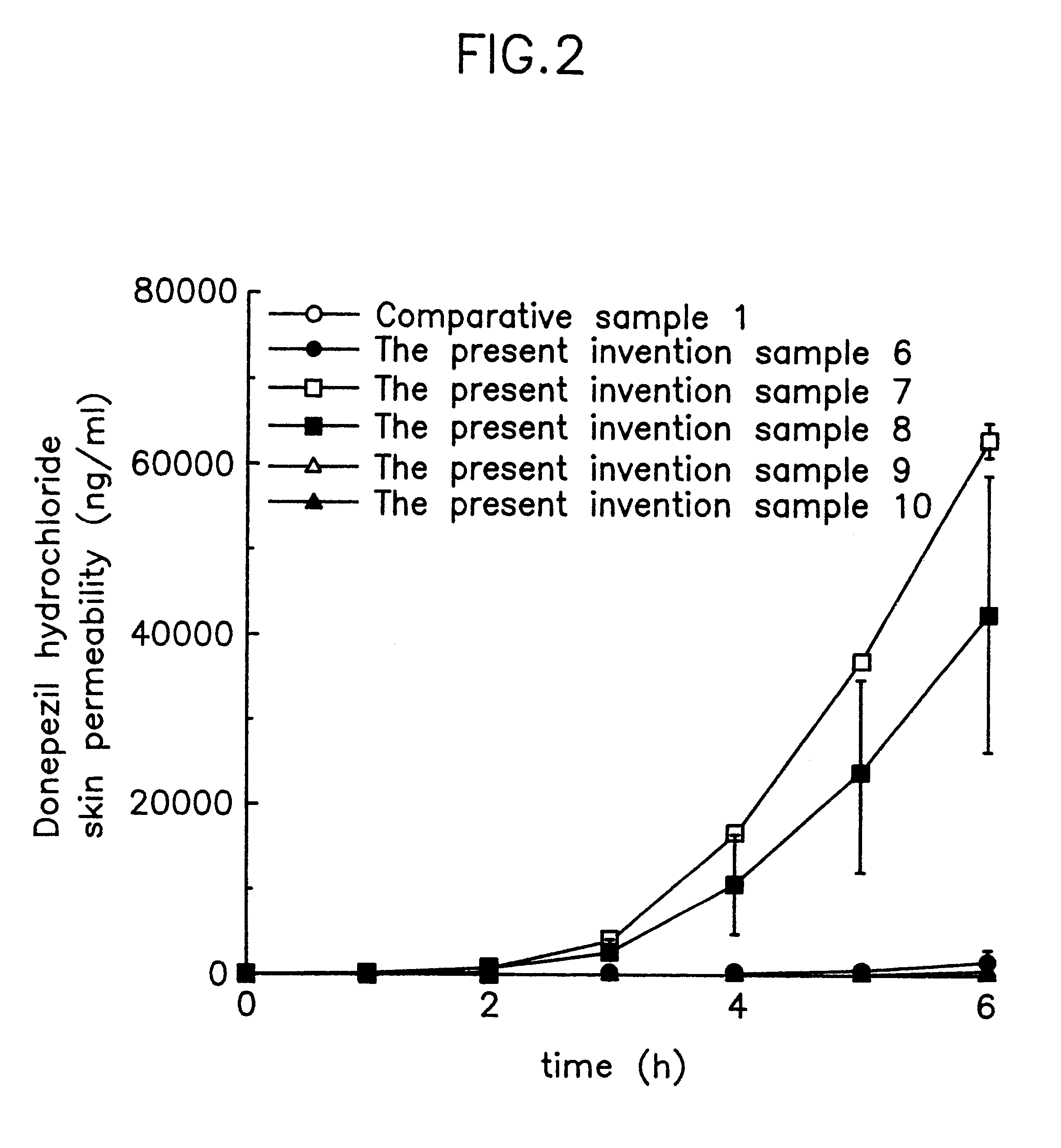
Suppository containing an antidementia medicament
The present invention provides a rectum applicable preparation containing an antidementia medicament, wherein the antidementia medicament is incorporated a triglyceride of a fatty acid and / or a water-soluble macromolecule.
Owner:EISAI CO LTD
Resilient device
An intravaginal device has a working portion (e.g., intravaginal urinary incontinence device suppository, tampon) and an anchoring portion comprising at least one member extending beyond at least one end of the working portion to maintain the working portion in place during use.
Owner:FIRST QUALITY HYGIENIC
Chinese herbal medicine combination for treating disease of disorder of bowels's function and its product
A Chinese medicine for treating irritable bowel syndrome (IBS) is prepared from 6 Chinese-medicinal materials including bupleurum root, white peony root, Chuan-xiong rhizome, liquorice root, etc. It may be particles, capsules, tablets, oral liquid injection, etc.
Owner:NANJING NORMAL UNIVERSITY
Method of treating gastrointestinal tract disease with purinergic receptor agonists
InactiveUS20020052336A1Increase secretionBiocideCarbohydrate active ingredientsDiseaseDisease irritable bowel
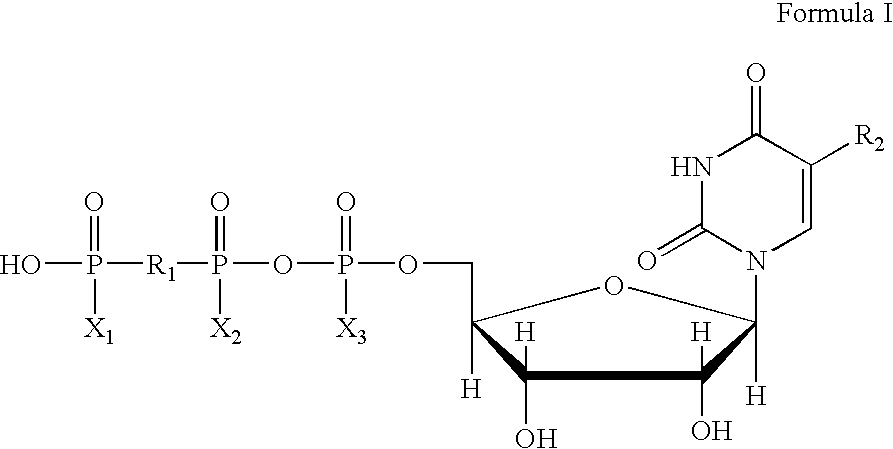
The invention provides a method of regulating water and mucin secretions and fluid transport in the gastrointestinal tract. The invention also provides a method for treating a gastrointestinal disease in which the mucosal barrier of the gastrointestinal system is impaired. The invention additionally provides a method for correcting disorders of fluid secretion or absorption in the gastrointestinal system. The method comprises administering to a patient a pharmaceutical composition comprising a purinergic P2Y receptor agonist, in an amount effective to regulate water and mucin secretions or to correct abnormal fluid transport in the gastrointestinal tract. The pharmaceutical composition used in this invention comprises a P2Y purinergic receptor agonist such as uridine 5'-diphosphate (UDP), uridine 5'-triphosphate (UTP), cytidine 5'-diphosphate (CDP), cytidine 5'-triphosphate (CTP), adenosine 5'-diphosphate (ADP), adenosine 5'-triphosphate (ATP), and their analogs; and dinucleotide polyphosphate compounds of general Formula (IV). Said compound is prepared in an oral form, an injectable form, or a suppository form, and administered to a patient.
Owner:INSPIRE PHARMA
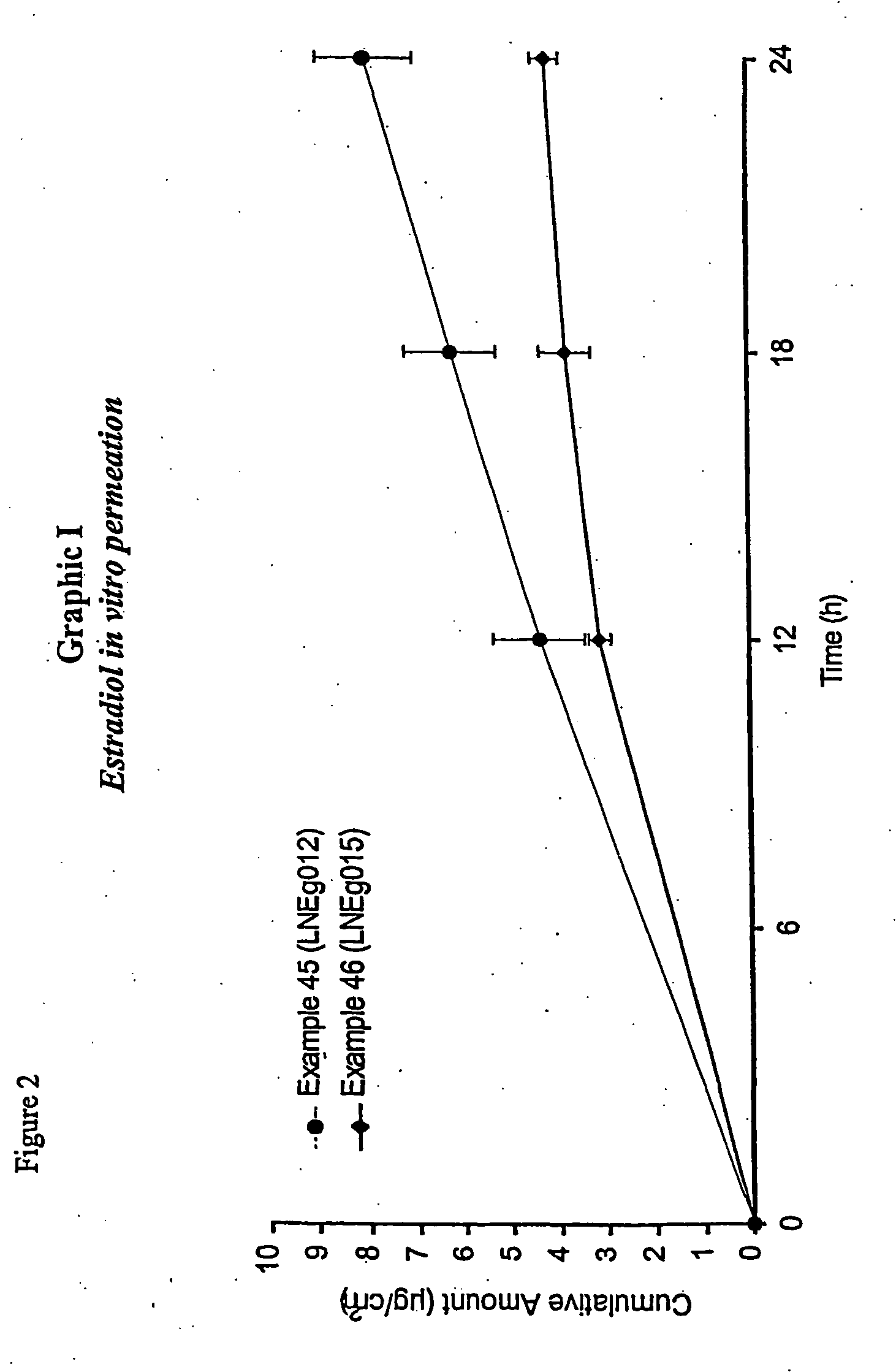
Treatment of Meconium Aspiration Syndrome with Estrogens
ActiveUS20100184736A1Reduce incidenceReduce morbidityOrganic active ingredientsPharmaceutical delivery mechanismSuppositoryNon invasive
One aspect of the present invention relates to the use of an estrogen in the treatment of Meconium Aspiration Syndrome (MAS) in a newborn infant, said treatment comprising administering an effective amount of estrogen to said newborn infant within 7 days after birth. The present treatment offers the advantage that estrogens can be administered using non-invasive modes of administration, e.g. oral or rectal administration. Other aspects of the present invention relate to a suppository for use in newborn infants comprising at least 1 μg of estrogen and to an oral applicator comprising a container holding an aqueous liquid containing micronised estetrol and a metering dispenser for metering the liquid into the oral cavity of a newborn infant.
Owner:ESTETRA SRL
Therapy for enteric infections
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
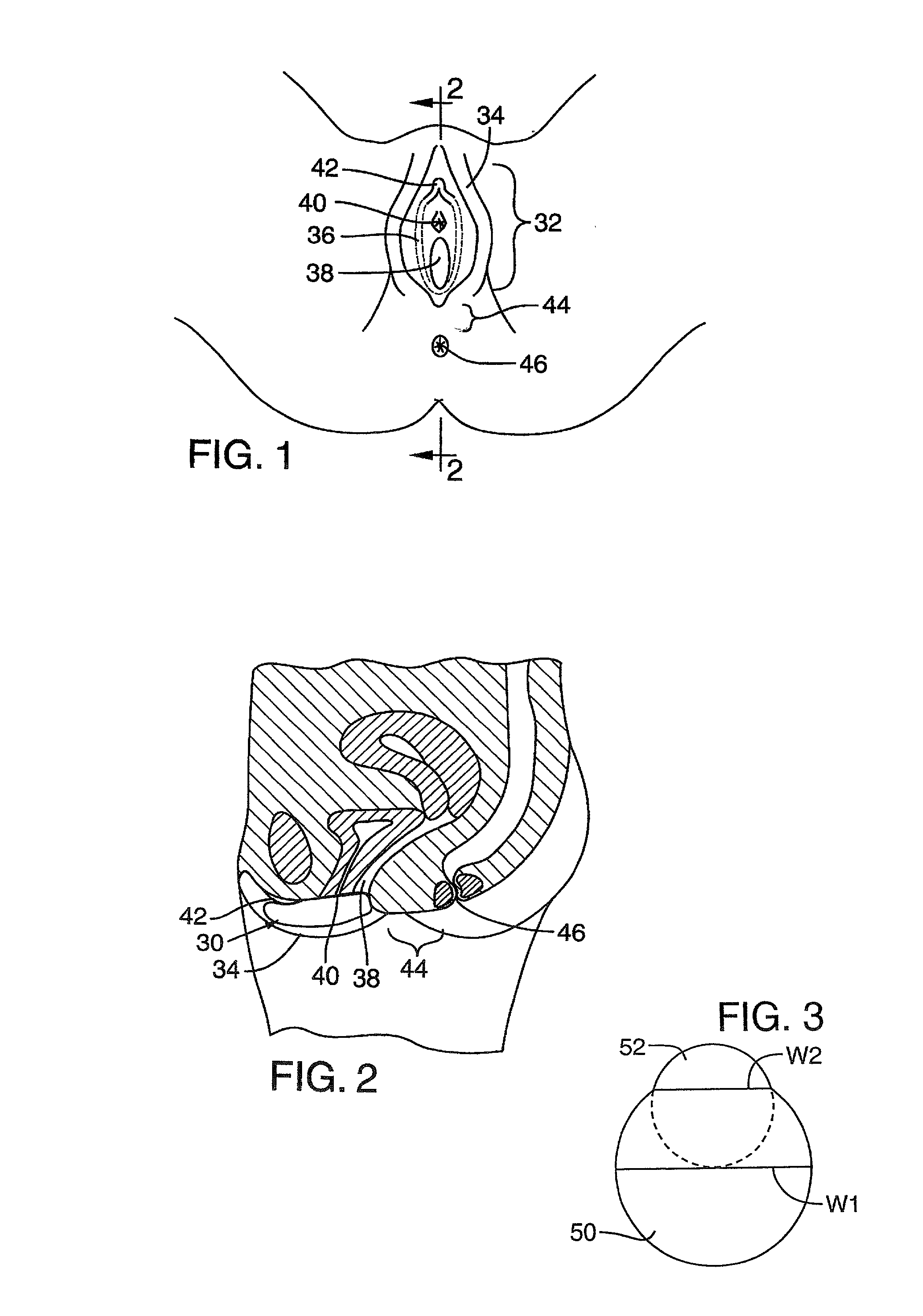
Administration of therapeutic or diagnostic agents using interlabial pad
InactiveUS20020115976A1Loss of some therapeutic efficacyDiscomfortSurgeryPharmaceutical delivery mechanismLeading edgeDiagnostic agent
A method is disclosed for administering an agent (for example delivering a drug or diagnostic agent) intravaginally or to the interlabial space by positioning an interlabial device, such as an absorbent pad, between the labia. The pad is retained between the labia for a sufficient period of time to deliver an active agent, or allow a reaction with a diagnostic agent. Alternatively, the pad is applied after medication is administered, for example intravaginally or labially or perilabially, to help reduce discomfort to the subject, or loss of medication. The active agent may be carried by the pad itself, or in an intravaginal extension of the pad, or separately in a suppository or other dosage form. In particular examples, the pad has a smaller minor portion superimposed on a larger major portion, and the smaller minor portion is inserted as a leading edge between the labia of the subject to facilitate interlabial insertion. In another example, the pad is placed interlabially after insertion of an agent (such as a medicated suppository) into the vagina, to inhibit loss of the medication from the vagina. The pad can have a variety of shapes, including major and minor portions that are portions of spheres or ellipsoids, or which are elongated and have cross-sections that are circular or ellipsoid, or pads which are folded.
Owner:PREPROGEN LLC
Preparation method and application of nanocomposite of silver, chitosan and/or derivative thereof
InactiveCN101618047AUniform particle sizeProtectiveAntibacterial agentsOrganic active ingredientsEquivalence ratioCervicitis
The invention discloses a nanocomposite of silver, chitosan and / or a chitosan derivative and a preparation method thereof. The preparation method comprises the steps of evenly mixing the chitosan and / or the chitosan derivative fine powder with silver nitrate fine powder and fully grinding for 1-3 hours with the weight proportion of the chitosan and / or the chitosan derivative to the silver nitrate of 100: 0.0005-0.5; adding a reducing agent with the reduction equivalence ratio of the reducing agent to the silver nitrate of 1: 1-2: 1 and fully grinding for 2-6 hours, thereby obtaining the nanocomposite of the silver, chitosan and / or the chitosan derivative with the silver particle size of 1-100nm. The nanocomposite has the effects of promoting wound healing, stopping bleeding, alleviating pain, anti-inflammation and sterilization, can be used for treating female vaginitis, cervicitis, cervical erosion, bedsores, skin ulcers, burns, scalds, trauma and other skin inflammation, tinea manus and pedis and the like, and can also be used for preparing a variety of trauma dressings, such as thin films, sponges, gynecological suppositories, patches and the like.
Owner:LIAOCHENG UNIV
HUMAN SECRETORY IgA FOR THE TREATMENT OF CLOSTRIDIUM DIFFICILE ASSOCIATED DISEASES
A composition for treating a subject is provided. The composition includes dimeric or polymeric IgA therapeutic. Formulating agents are mixed with the dimeric or polymeric IgA to yield a dosing form of a capsule, tablet, and a suppository. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided that the sequential modification of monomeric IgA with J chain and secretory component to form a dimeric or polymeric IgA therapeutic. The dimeric or polymeric IgA therapeutic is then mixed with formulating agents to create a capsule, tablet, or suppository dosing form. The therapeutic is amenable to enrobement directly through microeneapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SIMON MICHAEL R
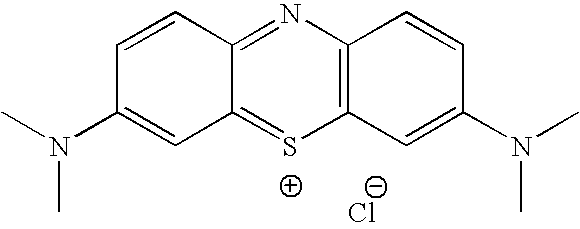
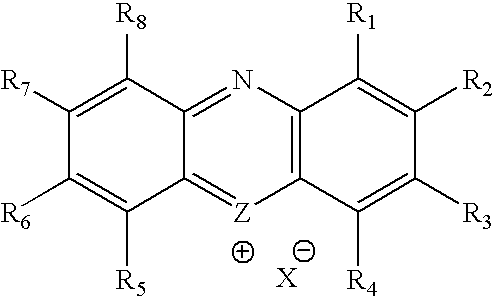
Methylene Blue Derivatives
InactiveUS20070116757A1Facilitated releaseRapid uptakeOrganic chemistryPill deliveryImmediate releaseFatty acid
Pharmaceutical compositions comprising a fatty acid salt, a dicarboxylic acid salt, an alkyl sulfate salt, an aryl sulfate salt or an alkyl aryl sulfonate salt of methylene blue or a derivative of methylene blue are described herein. The compositions are preferably administered orally and can be administered as tablets, soft or hard shell capsules (e.g. soft gelatin capsules), suspensions or solutions. The composition can also be formulated as a suppository or enema or rectal administration. The compositions further comprise a pharmaceutically acceptable carrier and optionally one or more pharmaceutically acceptable excipients. Suitable excipients include diluents, binders, plasticizers, lubricants, disintegrants, colorants, stabilizers, surfactants, and combinations thereof. The fatty acid salts, alkyl sulate salts, aryl sulfate salts or alkyl aryl sulfonate salts can be co-mixed or co-melted with one or more fatty acids to make more hydrophobic compositions, which may result in less staining formulations. The compositions can be formulated for immediate release, controlled release such as extended release, delayed release, and pulsatile release, or combinations thereof. In one embodiment, the derivative of methylene blue is methylene dodecylsulfate.
Owner:COLLEGIUM PHARMA INC
Medicinal composition for treating gynecological inflammation and application thereof
InactiveCN101648003AEase regenerationGood treatment effectPeptide/protein ingredientsHydroxy compound active ingredientsDiseaseUrethritis
The invention discloses a medicinal composition for treating gynecological inflammation, and belongs to the field of Chinese medicament. The medicinal composition consists of collagen and / or Chinese medicaments for releasing toxin and sterilizing such as collagen and lizardtail, glabrous greenbrier rhizome, Vietnamese sophora root and the like which are prepared into a plurality of dosage forms bycombining the prior art, and is mainly used for treating vaginitis, pelvic inflammation, urethritis and cervicitis diseases. The technical scheme overcomes the defects that the prior externally-applied preparations such as lotion, gel, suppository, ointment and the like have extremely slow response, longer disease course, repeated outbreak and the like, and creatively combines and applies the collagen and the Chinese medicament, can accelerate the healing of ulcer and erosion faces caused by the inflammation, and can greatly shorten the required time for tissue repair.
Owner:BEIJING HERUN INNOVATION PHARMA TECH DEV
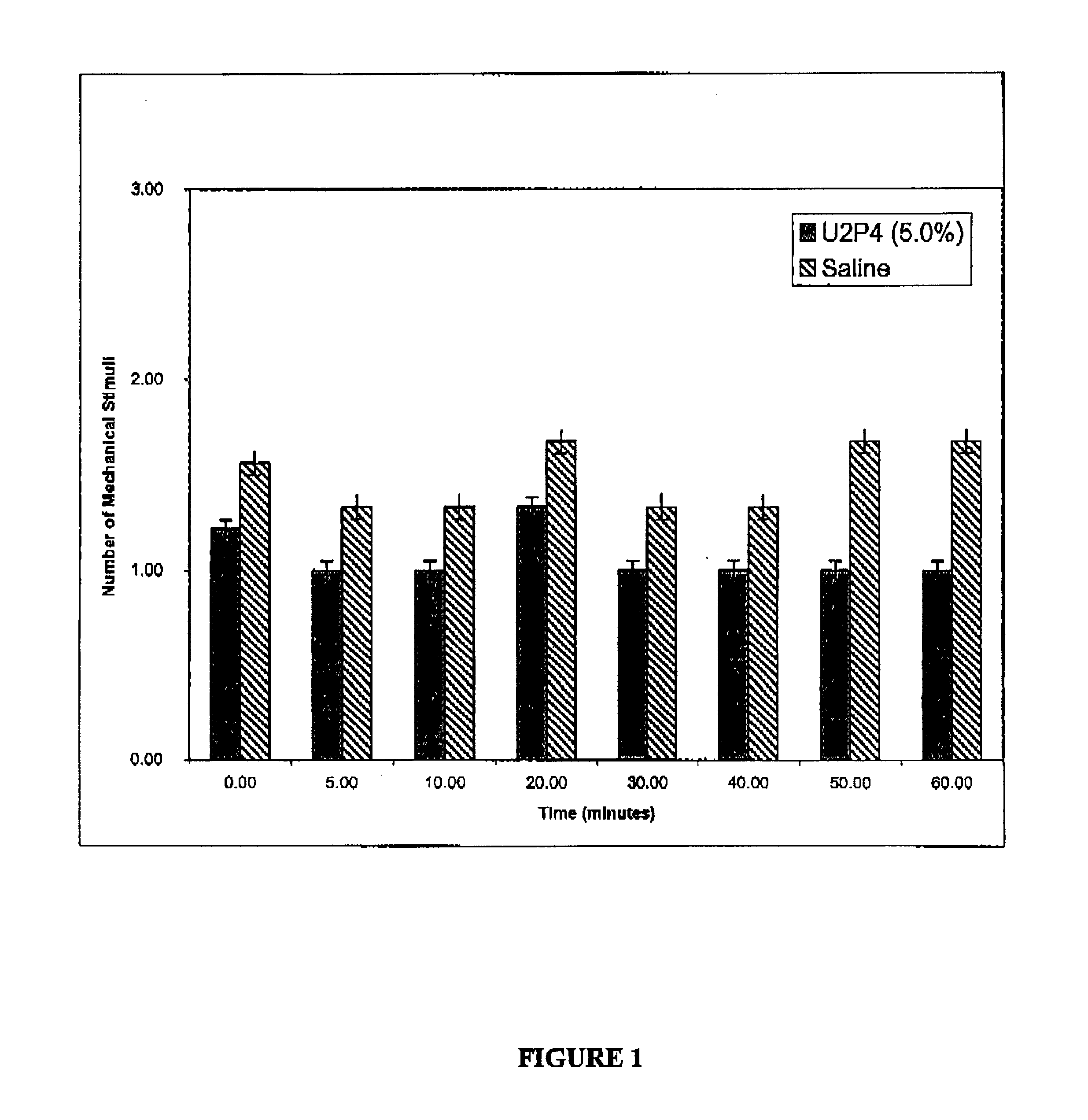
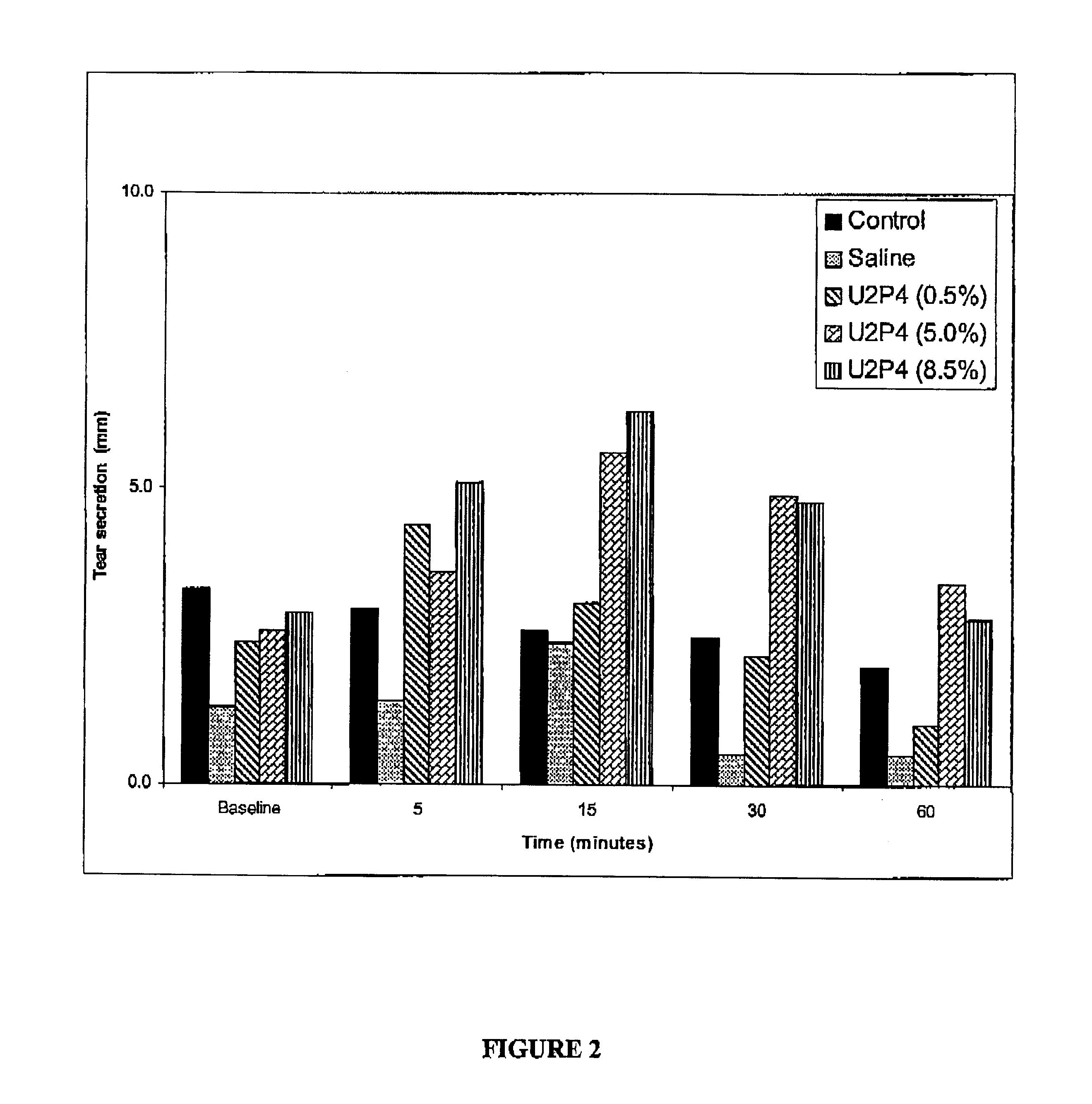
Method of treating dry eye disease with purinergic receptor agonists
A method and preparation for the stimulation of tear secretion in a subject in need of such treatment is disclosed. The method comprises administering to the ocular surfaces of the subject a purinergic receptor agonist such as uridine 5′-triphosphate [UTP], dinucleotides, cytidine 5′-triphosphate [CTP], adenosine 5′-triphosphate [ATP], or their therapeutically useful analogs and derivatives, in an amount effective to stimulate tear fluid secretion and enhance drainage of the lacrimal system. Pharmaceutical formulations and methods of making the same are also disclosed. Methods of administering the same would include: topical administration via a liquid, gel, cream, or as part of a contact lens or selective release membrane; or systemic administration via nasal drops or spray, inhalation by nebulizer or other device, oral form (liquid or pill), injectable, intra-operative instillation or suppository form.
Owner:MERCK SHARP & DOHME CORP
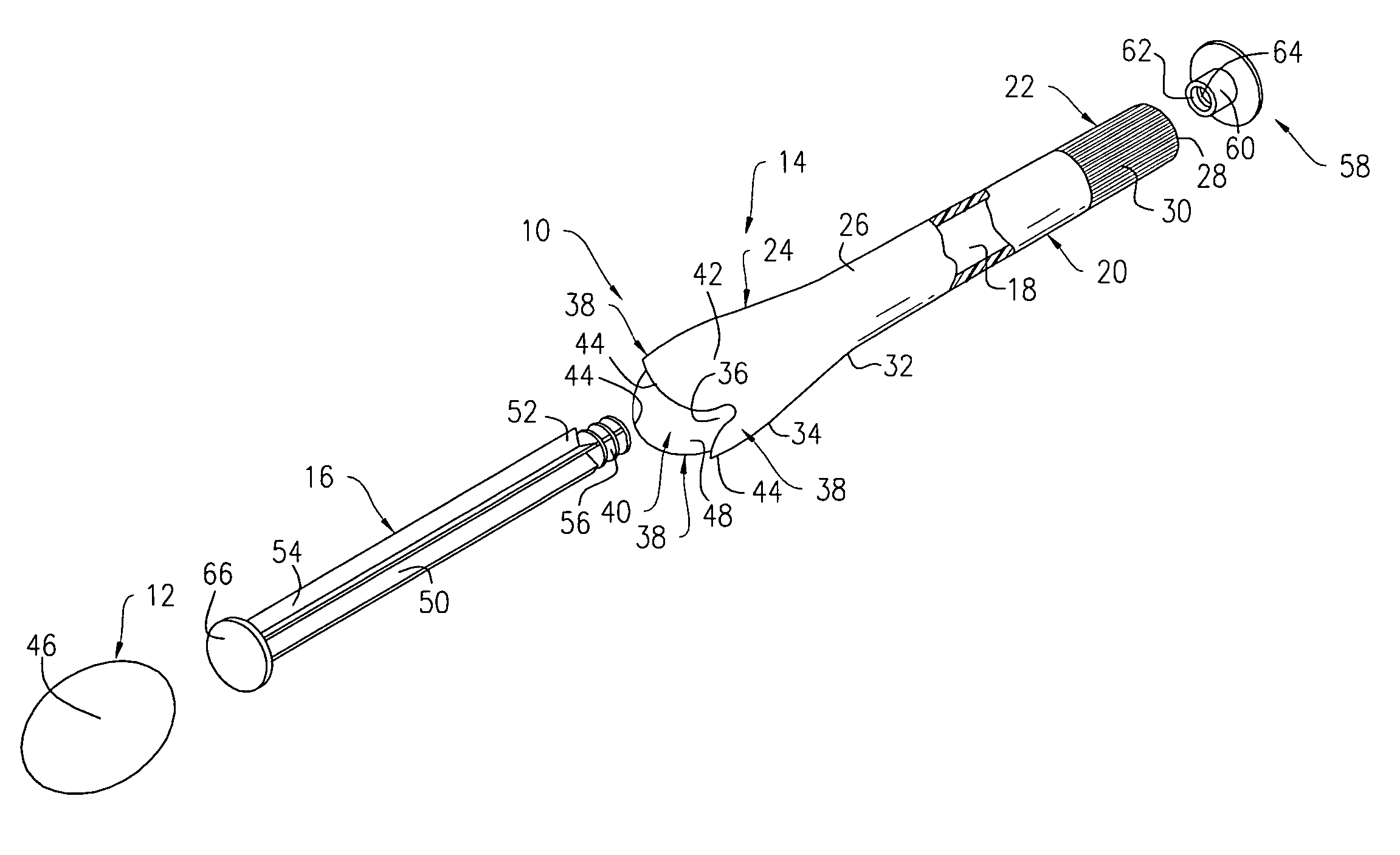
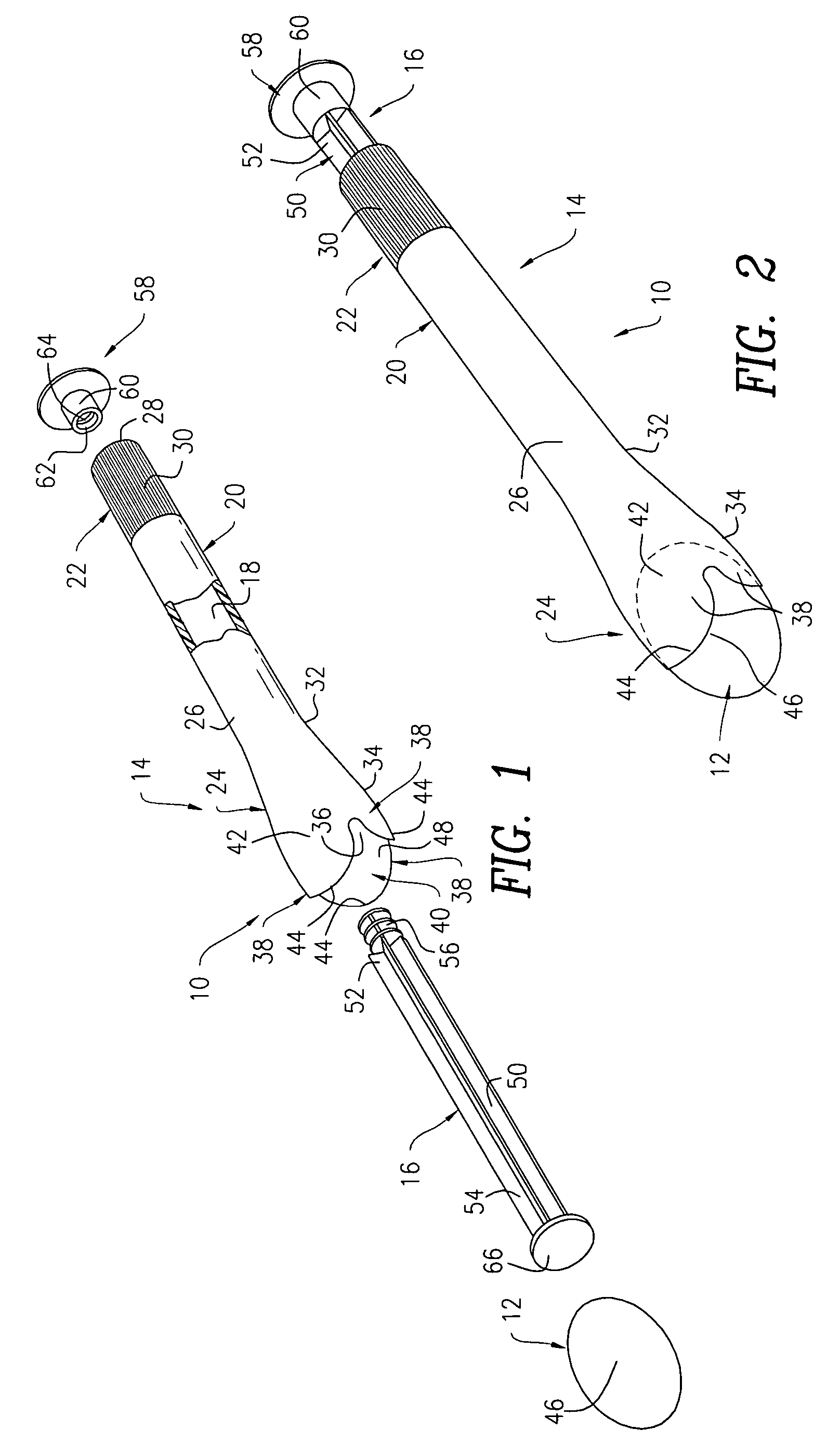
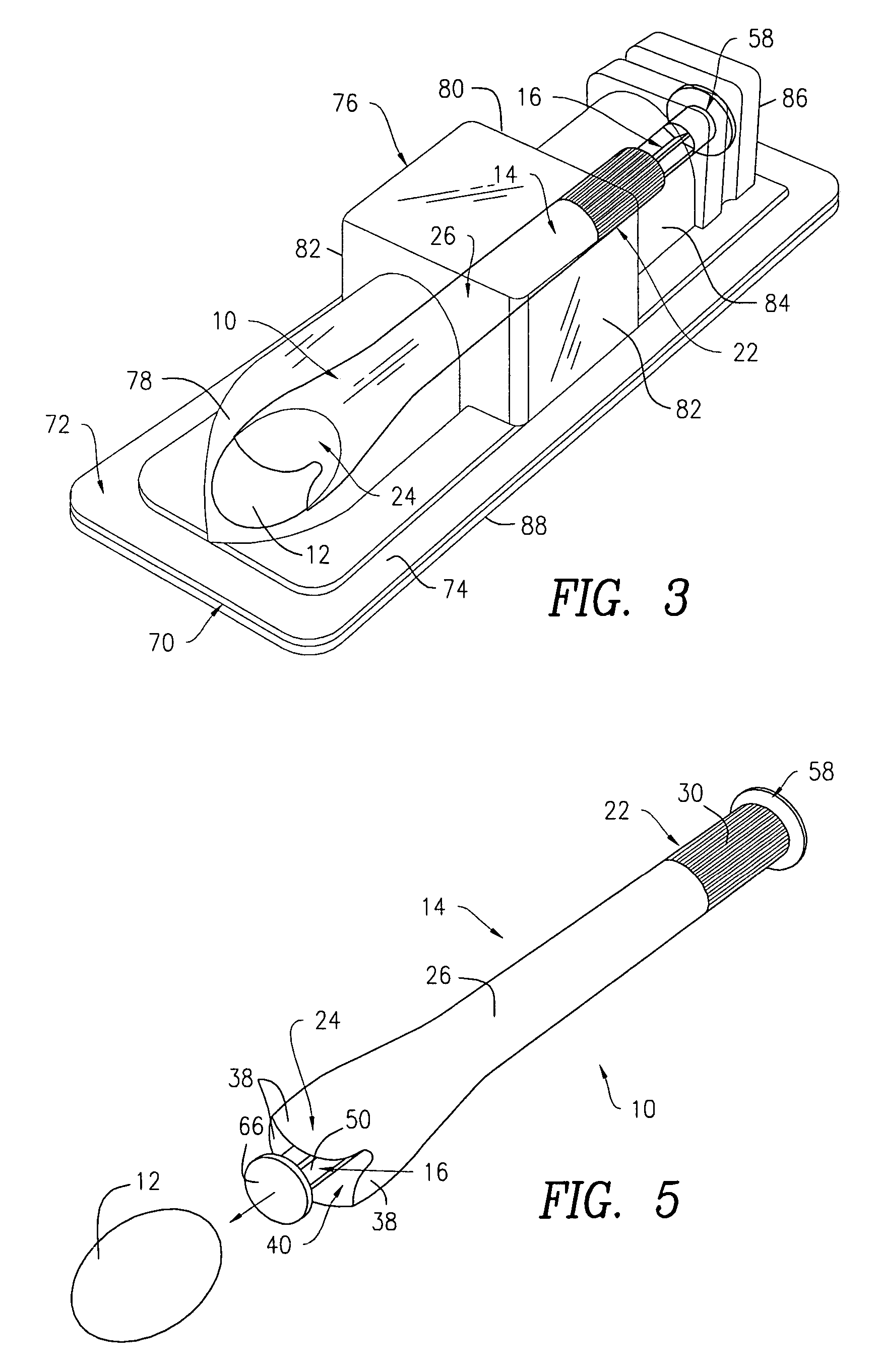
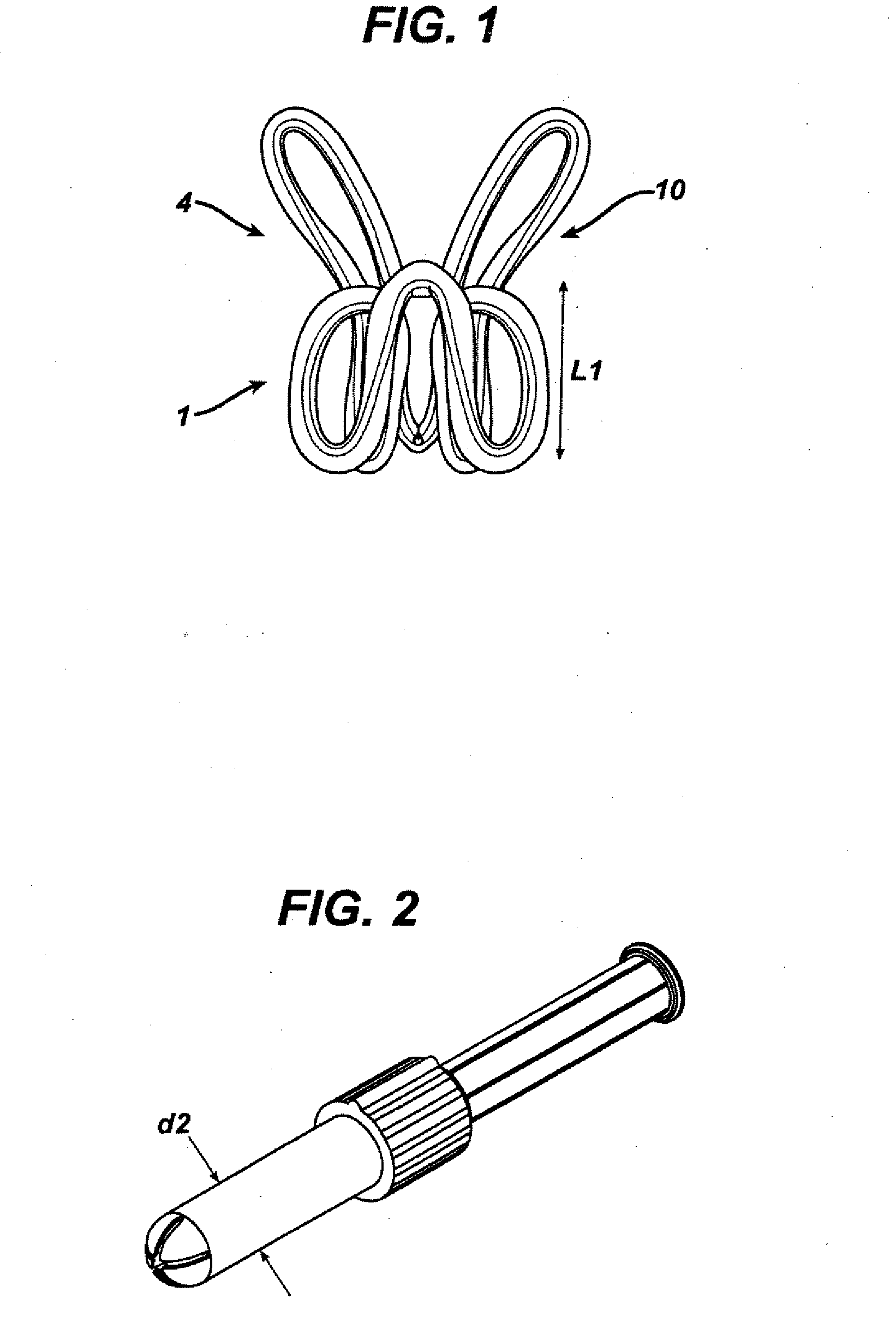
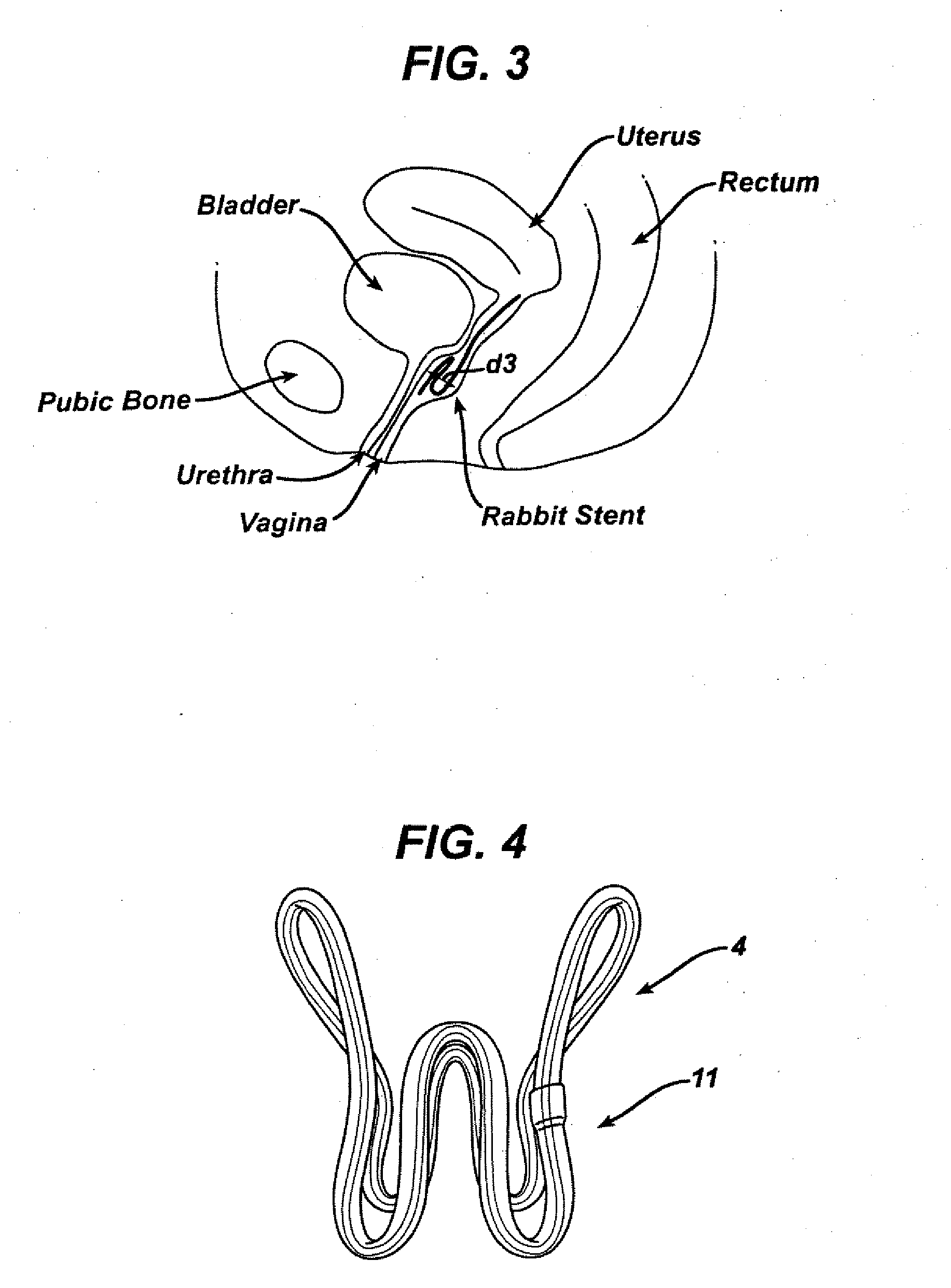
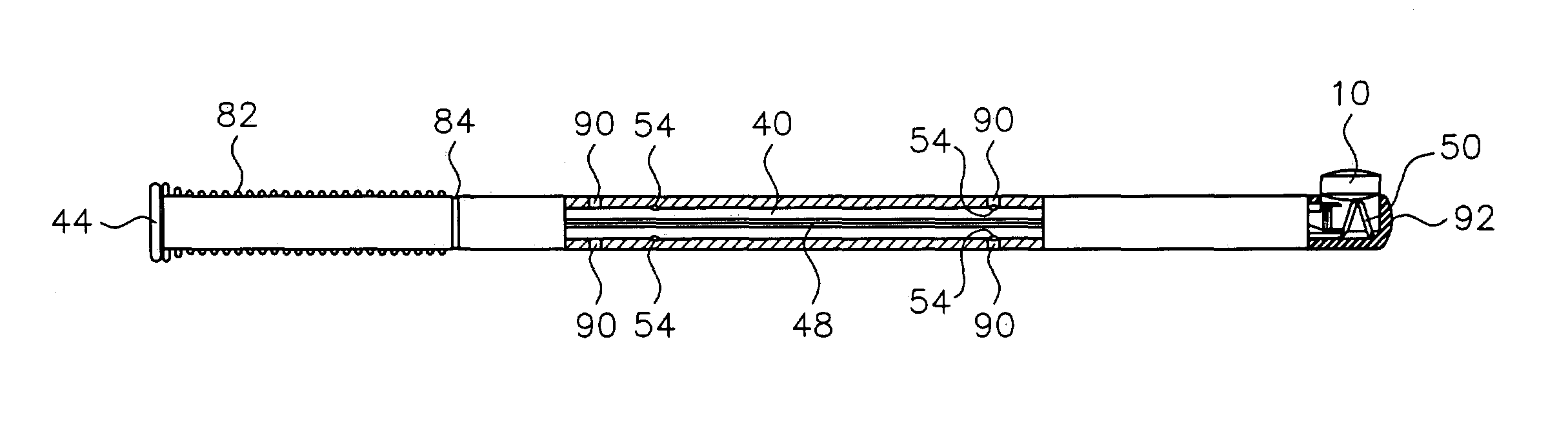
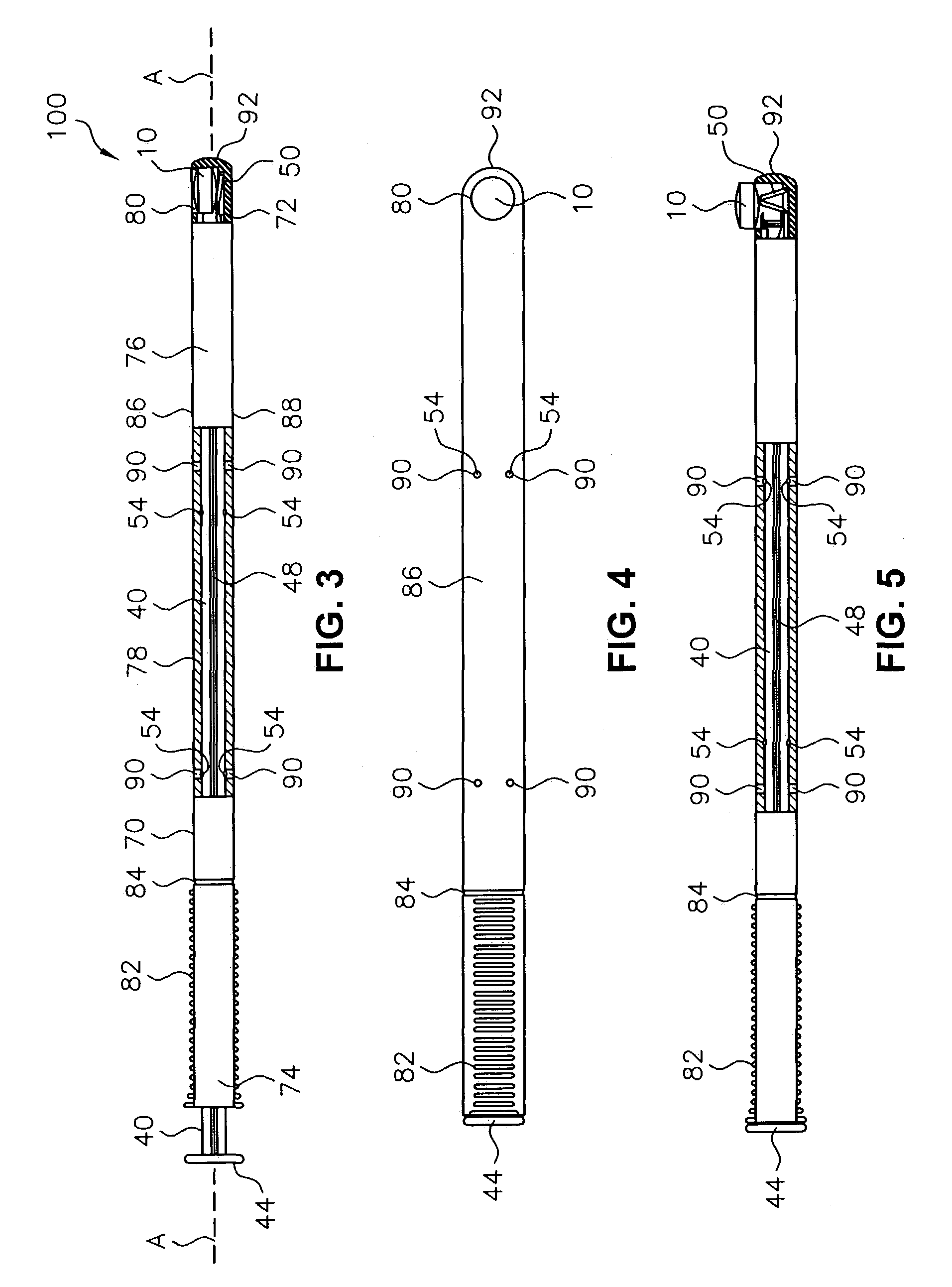
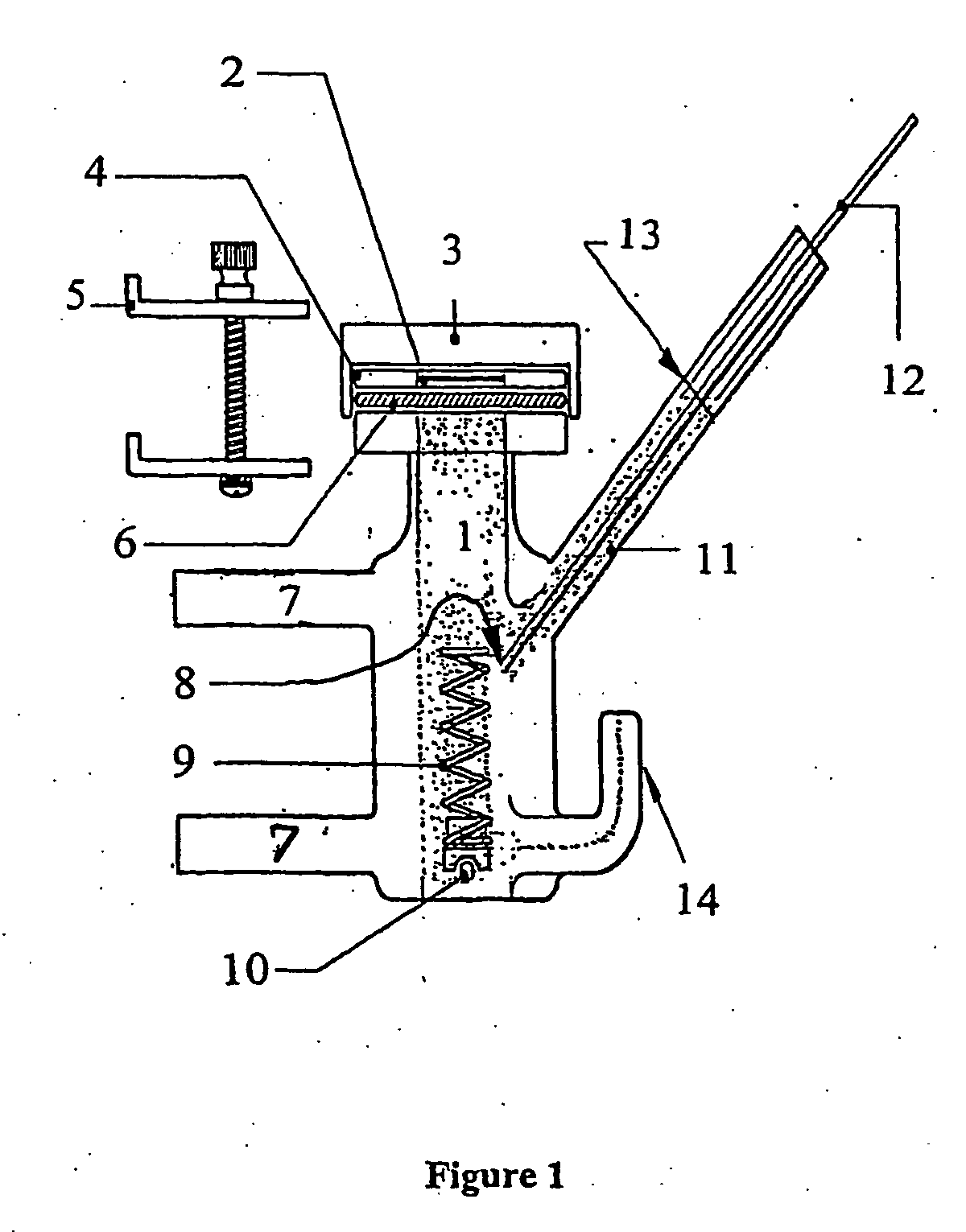
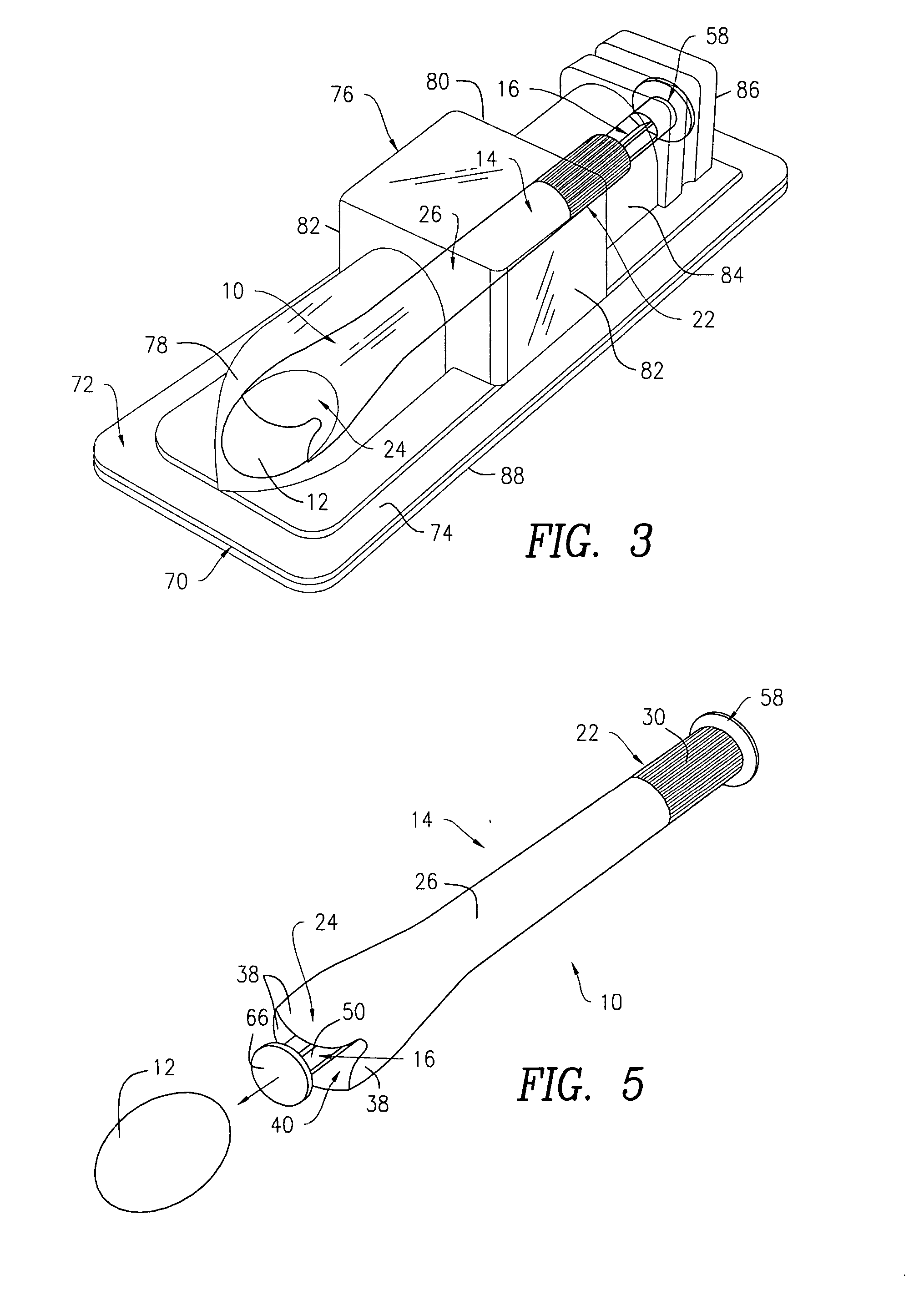
Side-delivery suppository dispenser
A dispenser for delivering a suppository within a body cavity. The dispenser has two main components: a barrel and a plunger. The barrel includes a head, a foot, and a body having a length and extending between the head and the foot. The barrel further defines an axial passage disposed along substantially the entire length of the body beginning at the foot and ending proximate the head, and includes a side aperture providing external access through the body of the barrel to the axial passage at the head of the barrel. The plunger includes a nose, a tail, and a frame extending between the nose and the tail. The plunger is sized to travel within the axial passage of the barrel between a relaxed position and a compressed position, in which a suppository is ejected from the side aperture, upon actuation.
Owner:B BRAUN MEDICAL
Novel composition for transdermal and/or transmucosal administration of active compounds that ensures adequate therapeutic levels
InactiveUS20070098775A1Easy to usePromote absorptionBiocideAerosol deliveryCompound (substance)Suppository
A pharmaceutical composition in the form of a solution, cream, lotion, spray, ointment, gel, aerosol, tablet, suppository or patch device for transdermal or transmucosal administration of alprazolam to a subject, which includes as a permeation enhancing mixture a fatty component in an amount of 0.1% to 20% by weight which is one of (a) a saturated fatty alcohol of formula CH3—(CH2)n—CH2OH, a saturated fatty acid of formula CH3—(CH2)n—CH2COOH, (b) an unsaturated fatty alcohol of formula CH3(CnH2(n−1))—OH, or (c) a fatty acid of formula CH3(CnH2(n−l))—COOH, wherein n is an integer of between 8 and 22; and a vehicle that includes a C1-C4 alkanol, a polyalcohol, and water.
Owner:ANTARES PHARMA IPL
Synthesis of human secretory IgA for the treatment of Clostridium difficile associated diseases
A composition for treating a subject is provided. The composition includes dimeric or polymeric secretory IgA therapeutic. Formulating agents are mixed with the dimeric or polymeric secretory IgA to yield a dosing form of a capsule, tablet, and a suppository. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided wherein dimeric or polymeric IgA is modified with secretory component to form a dimeric or polymeric secretory IgA therapeutic. The dimeric or polymeric secretory IgA therapeutic is then mixed with formulating agents to create a capsule, tablet, or suppository dosing form. The therapeutic is amenable to enrobement directly through microencapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SIMON MICHAEL R
Preparation method and application of chitosan and/or metal composite of chitosan derivative
InactiveCN101654529ASmall toxicityReduce metal contentOrganic active ingredientsAntimycoticsPolymer scienceRare earth metal compounds
The invention discloses a solid phase synthesis method and a liquid phase synthesis method of chitosan and / or zinc, silver, bismuth and rare earth metal composite of chitosan derivative. The solid phase synthesis comprises the following steps: (1) grinding chitosan and / or chitosan derivative to fine powder; (2) grinding the oxides of zinc and / or silver and / or bismuth and rare earth metal to fine powder of which content is 0.0005-2wt% of the weight of chitosan and / or chitosan derivative; (3) mixing the fine powders obtained in step (1) and step (2) and fully grinding for 1-6h to obtain the chitosan and / or metal composite of chitosan derivative. The product of the preparation method can be used to cure diseases such as female vaginitis, cervicitis, cervical erosion, bedsore, skin ulcer, fireburn, empyrosis, trauma, other dermatitis disease, tinea of feet and hands and the like and can be used to prepare various surgical dressings such as film, sponge, gynecological suppository, adhesivefilm and the like.
Owner:LIAOCHENG UNIV
Applicator device for suppositories and the like
An applicator for delivering pharmaceutical products or the like to a bodily cavity includes a barrel member having a distal end which is equipped with an opening. The applicator also includes a plurality of petals extending outwardly from the distal end in a generally axial direction. The petals cooperate with the opening so as to form a receptacle for releasably receiving a pharmaceutical product in the distal end of the barrel member. Each of the petals has a truncated flexible tip sized and shaped so as to engage a substantially central portion of the pharmaceutical product such that a large section of the pharmaceutical product extends outwardly beyond the petals so as to facilitate the release of the pharmaceutical product from the receptacle. The device also includes a plunger member for releasing the pharmaceutical product from the receptacle. In accordance with the present invention, the device can be packaged in a package together with the pharmaceutical product received in the receptacle.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Administration of therapeutic or diagnostic agents using interlabial pad
A drug or diagnostic agent) is delivered intravaginally or to the interlabial space by positioning an interlabial device, such as an absorbent pad, between the labia. The pad is retained between the labia to deliver an active agent, or allow a reaction with a diagnostic agent. Alternatively, the pad is applied after medication is administered, to help reduce discomfort to the subject, or loss of medication. The active agent may be carried by the pad itself, or in an intravaginal extension of the pad, or separately in a suppository or other dosage form. In particular examples, the pad has a smaller minor portion superimposed on a larger major portion, and the smaller minor portion is inserted as a leading edge between the labia of the subject to facilitate interlabial insertion. In another example, the pad is placed interlabially after insertion of an agent into the vagina.
Owner:PREPROGEN LLC
Beta-elemi alkene bulk medicament and method of preparing its preparations
InactiveCN101402543AHydrocarbon active ingredientsDistillation purification/separationVolatilesSelf emulsifying
The invention provides a method for preparing high-purity beta elemene from natural plants containing the beta elemene such as curcuma zedoary (earthnuts or tubers of the curcuma zedoary), cedronella (fresh leaves of the cedronella), yellowtop (roots, stems, leaves, flowers and seeds of the yellowtop) and so on, which can improve the production efficiency from starting materials to the high-purity beta elemene and reduce production cost. Compared with the prior art, the method is mainly different in that the roots, stems, leaves, flowers and seeds of the natural plants are taken as raw materials; oleum volatile of specific parts of the natural plants is obtained by methods for extracting different oleum volatiles, and is distilled by molecular distillation method to remove compositions with high boiling points and non-volatile compositions; impurity compositions are removed by the ethanol extraction method and silver nitrate complex extraction method; and finally the beta elemene with the content between 95.0 and 99.9 percent is obtained through reduced pressure distillation or rectification. The bulk pharmaceutical chemicals not only can be prepared into oral dosing preparation such as emulsion oral liquid, self-emulsifying / self-microemulsifying capsules, soft capsules and so on, but also can be prepared into non-alimentary dosing preparation such as emulsion injection, liquid drugs injection, transdermal absorbent, lung sprays, suppository and so on. The method has the advantages of novel design, concise operating steps, mild operating conditions and improvement of the production efficiency of the beta elemene.
Owner:沈阳万爱普利德医药科技有限公司
Toad peptide antibiotics separated from toad maggots and preparation method of antibacterial drugs thereof
ActiveCN102091318AAntibacterial agentsAnthropod material medical ingredientsDiseaseChromatographic separation
The invention provides toad peptide antibiotics separated from toad maggots and a preparation method of antibacterial drugs thereof. The preparation method comprises the following steps: taking toad maggots as raw materials; carrying out ultrafiltration and microfiltration, gel chromatography separation, ultrasonic standing wave liquid preparation chromatography and the like by adopting a solvent extraction method and an ion-exchange resin adsorption method; freeze-drying to prepare the toad peptide antibiotics; and combining the active components with appropriate excipients to prepare tablets, capsules, suppositories, ointments, enteric tablets and the like to be used for treating various diseases. The drugs prepared by the invention have the advantages of wide antibacterial range, specific drug components, controllable quality and stable performance and have industrial production values.
Owner:HARBIN INST OF TECH
Methods of thrombolytic organ treatment and repair
InactiveUS20020051779A1Improve survivabilityEffective therapyPeptide/protein ingredientsDead animal preservationStreptokinaseThrombus
The invention teaches methods and compositions for removing thrombi lodged in the microvasculature of an organ. To remove the thrombi, the organ may be perfused, flushed or washed with a suitable perfusion solution to which a sufficient amount of a thrombolytic agent, such as Streptokinase, has been added. The perfusing, flushing or washing process of the organ with the thrombolytic agent will promote thrombolysis on existing thrombi, prevent the formation of new thrombi in the organ, and / or open the vasculature of the organ thereby decreasing vascular resistance and increasing flow. The method of the invention may be practiced using an organ perfusion apparatus that would allow the viability of the organ to be sustained and / or restored upon perfusion with a thrombolytic agent.
Owner:ORGAN RECOVERY SYST
Method for preparing antibacterial material by combining nano silver and activated carbon
InactiveCN1480584AImprove the bactericidal effectRestore normal physiological self-cleaning functionBiocideFibre treatmentActivated carbonCarbon fibers
A process for preparing the antibacterial material which can be used to prepare the suppository for treating vaginal infections and cervical erosion with total effective rate up to 90% or more features that an ion exchange method is used to attach the combination of nano-class Ag and silver oxide to the surface of activated carbon fibres. Its advantage is high sterilizing power (15 mm for indirect sterilizing diameter).
Owner:刘维春
Cyclodextrin clathrate of breviscapine and its prepn
InactiveCN1739537AImprove solubilityImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismCyclodextrinSuppository
The present invention is cyclodextrin clathrate of breviscapine and its preparation. Cyclodextrin or its derivative is used to include breviscapine or added into breviscapine to raise the dissolubility of breviscapine in water, raise the stability of breviscapine and raise the bioavailability of breviscapine. The molar ratio between cyclodextrin or its derivative and breviscapine is 1-10. The present invention may be prepared into tablet, capsule, granule, dripping pill or suppository with relatively high bioavailability, and may be also prepared into oral liquid, injection or other preparation form with high stability.
Owner:张红军
Kangfu anti-inflammatory vaginal expansion suppository as well as preparation method and detection method thereof
ActiveCN103301295AWork quicklyImprove stabilityWeighing by removing componentSuppositories deliverySecondary InfectionsSuppository
The invention relates to a Kangfu anti-inflammatory vaginal expansion suppository. The expansion suppository comprises active ingredients prepared from raw material medicines such as radix sophorae flavescentis, patrinia and Chinese violet, a matrix and an expansion carrier. The suppository is fully expanded, so that the medicine-containing matrix is fully contacted with the inner wall of the vagina, and external flow of the liquid medicine is avoided; the medicine-containing matrix in the expansion suppository also comprises a dispersing carrier, so that release of the active ingredients can be promoted, and the dissolution rate is improved. The invention also provides a preparation method and a detection method of the expansion suppository. The provided Kangfu anti-inflammatory vaginal expansion suppository employs seven ingenious advanced technologies and has the beneficial effects that the external flow of the liquid medicine is avoided, the stability is high, the effect is fast, the drug action time is long, and secondary infection is avoided.
Owner:哈尔滨田美药业股份有限公司
Phosphatide compound of resveratrol compounds and method for preparing the same and the application thereof
InactiveCN101095664AImprove solubilityHigh dissolution rateAntibacterial agentsHydroxy compound active ingredientsSolubilitySuppository
The invention relates to resveratrol phosphatide compound, the preparation method and its applciaiton. The resveratrol mainly comprises stilbenes parent nucleus, and is slightly soluble or insoluble in water, and is not stable. So the biological utilization rate is low and medical effect is reduced and the parenteral administration is limited. The key is to increase its solubility, so the resveratrol phosphatide compound is needed by market. The resveratrol phosphatide compound comprises resveratrol and phosphatide, with the weight proportion being 1: 0.5-50.The product can be used to prepare tablet, capsule, granule, suppository, injection, or freeze dried or compound tablet.
Owner:HEILONGJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com