Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41results about How to "Rapid uptake" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
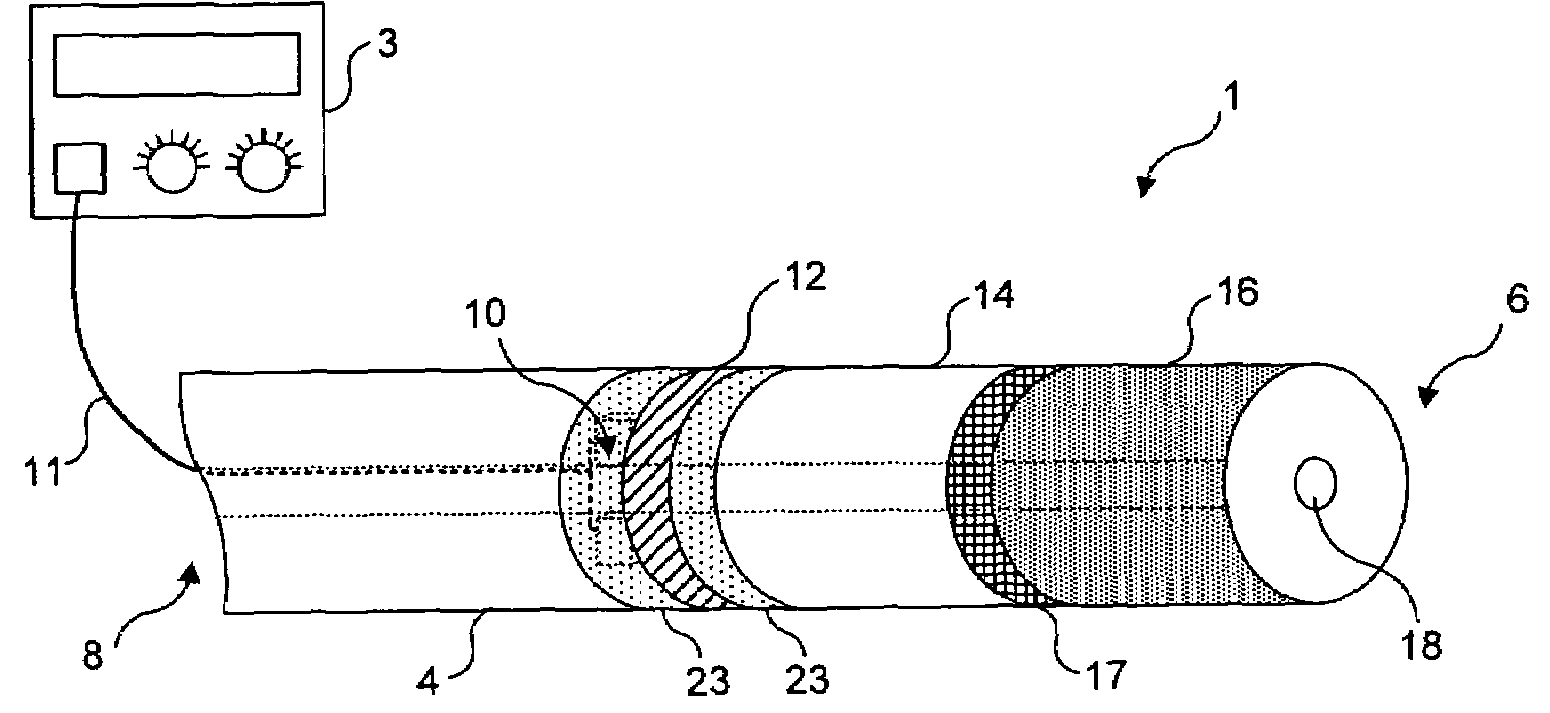
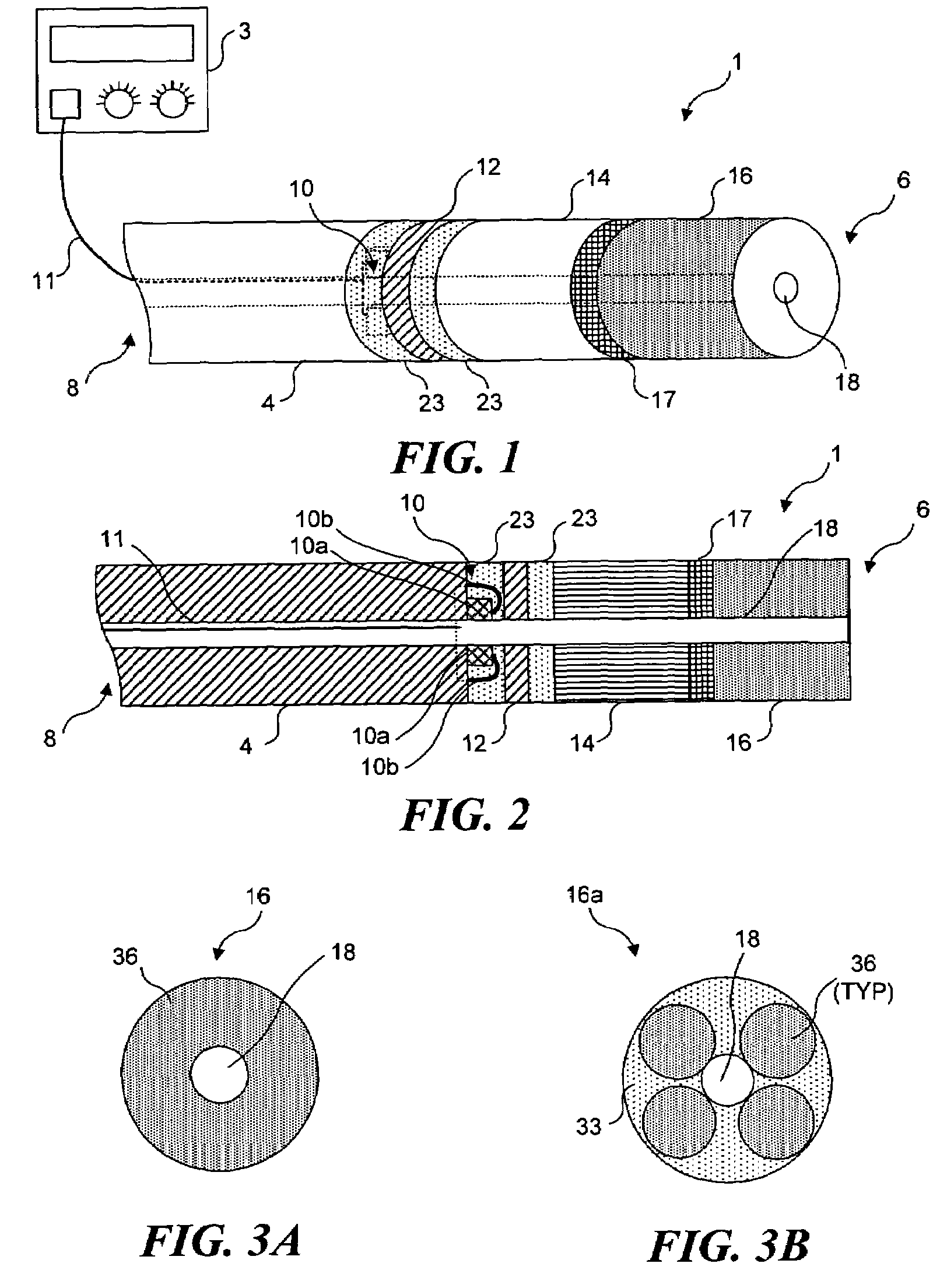
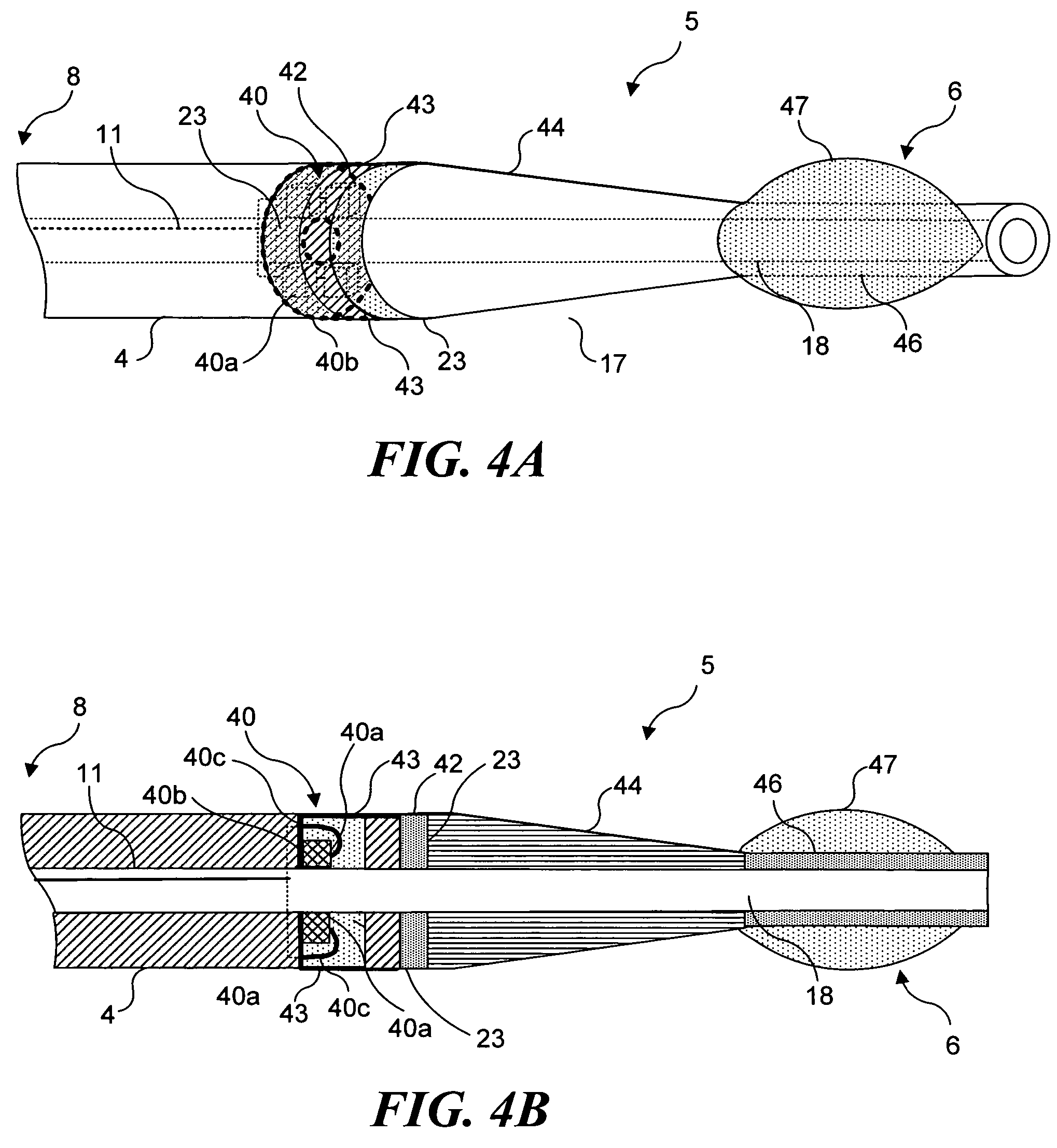
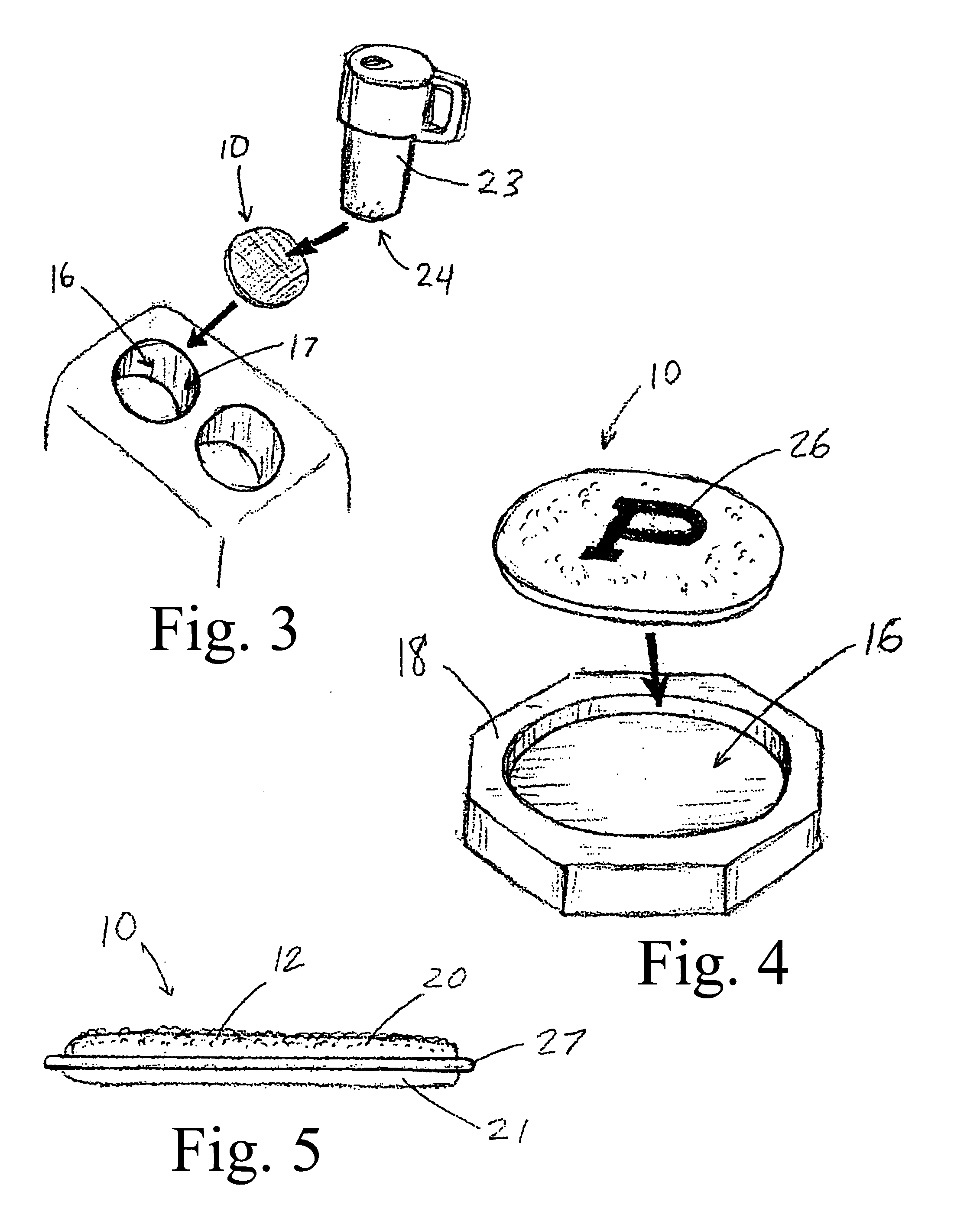
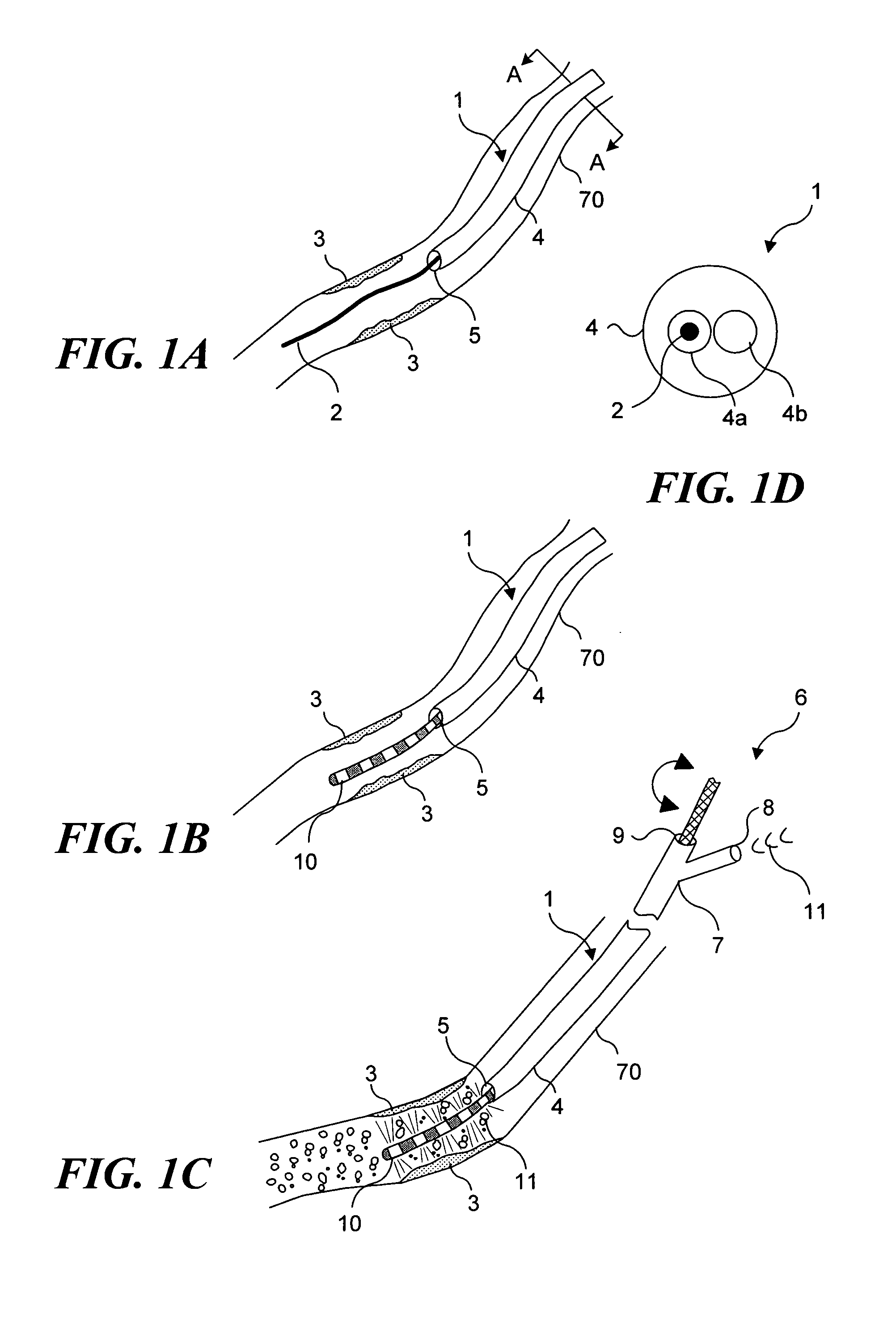
Light generating device to intravascular use
InactiveUS7252677B2High light transmittanceFacilitated DiffusionElectrotherapySurgical instrument detailsPhotodynamic therapyMedicine
Light generating devices for illuminating portions of vascular tissue, to render photodynamic therapy. In one embodiment, a light source array preferably including a plurality of light emitting diodes, a focusing lens, and a light diffusing element are included in a distal end of a catheter. A balloon is optionally provided to interrupt blood flow that can block the transmission of light, and to center the apparatus in a blood vessel. Optical fibers optionally direct light from the light source to the diffusing element. The light source array can have a radial or linear configuration and can produce more than one wavelength of light for activating different photoreactive agents. Linear light source elements are particularly useful to treat elongate portions of tissue in a vessel. One embodiment intended for use with a conventional balloon catheter integrates light sources into a guidewire.
Owner:LIGHT SCI ONCOLOGY
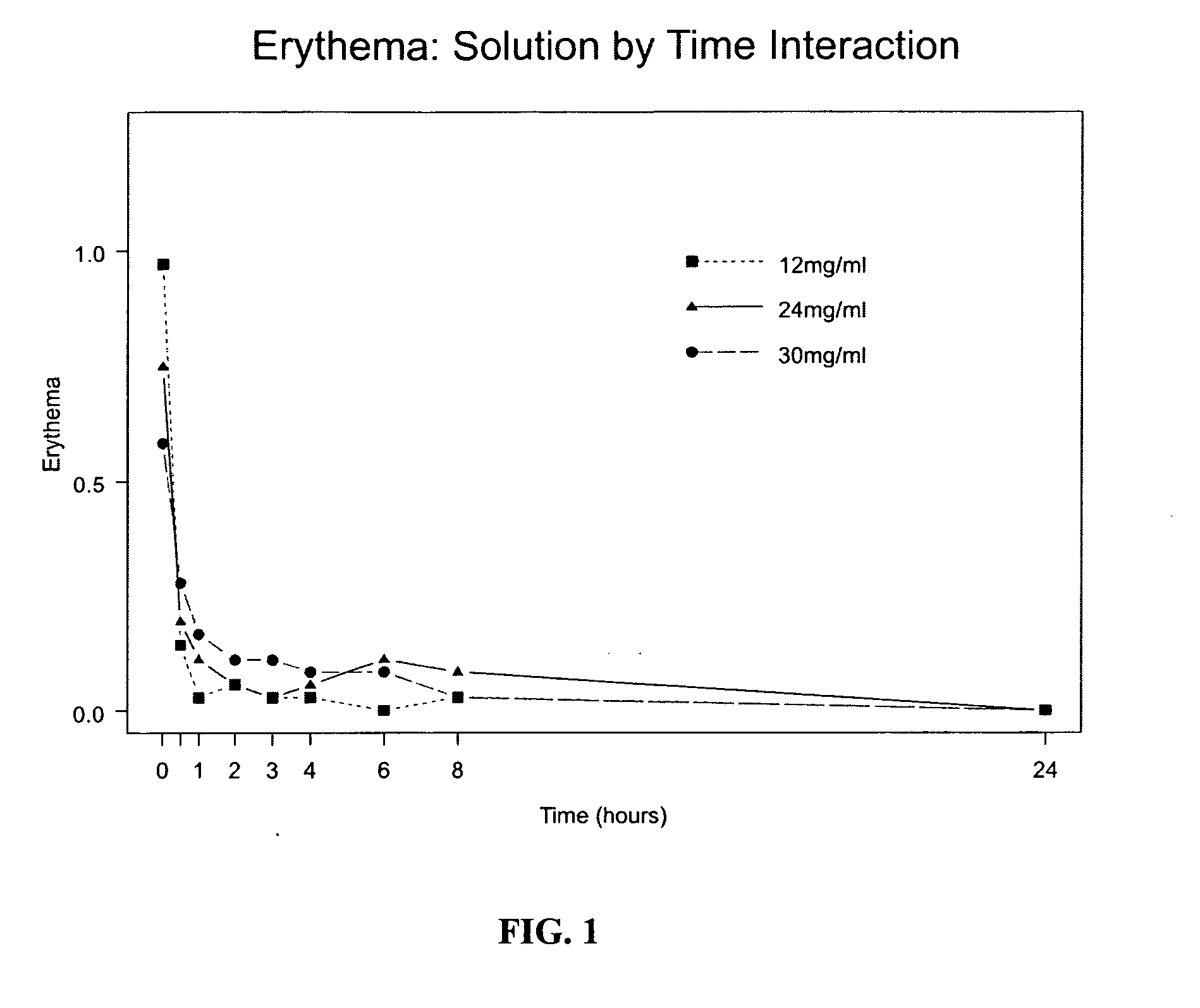
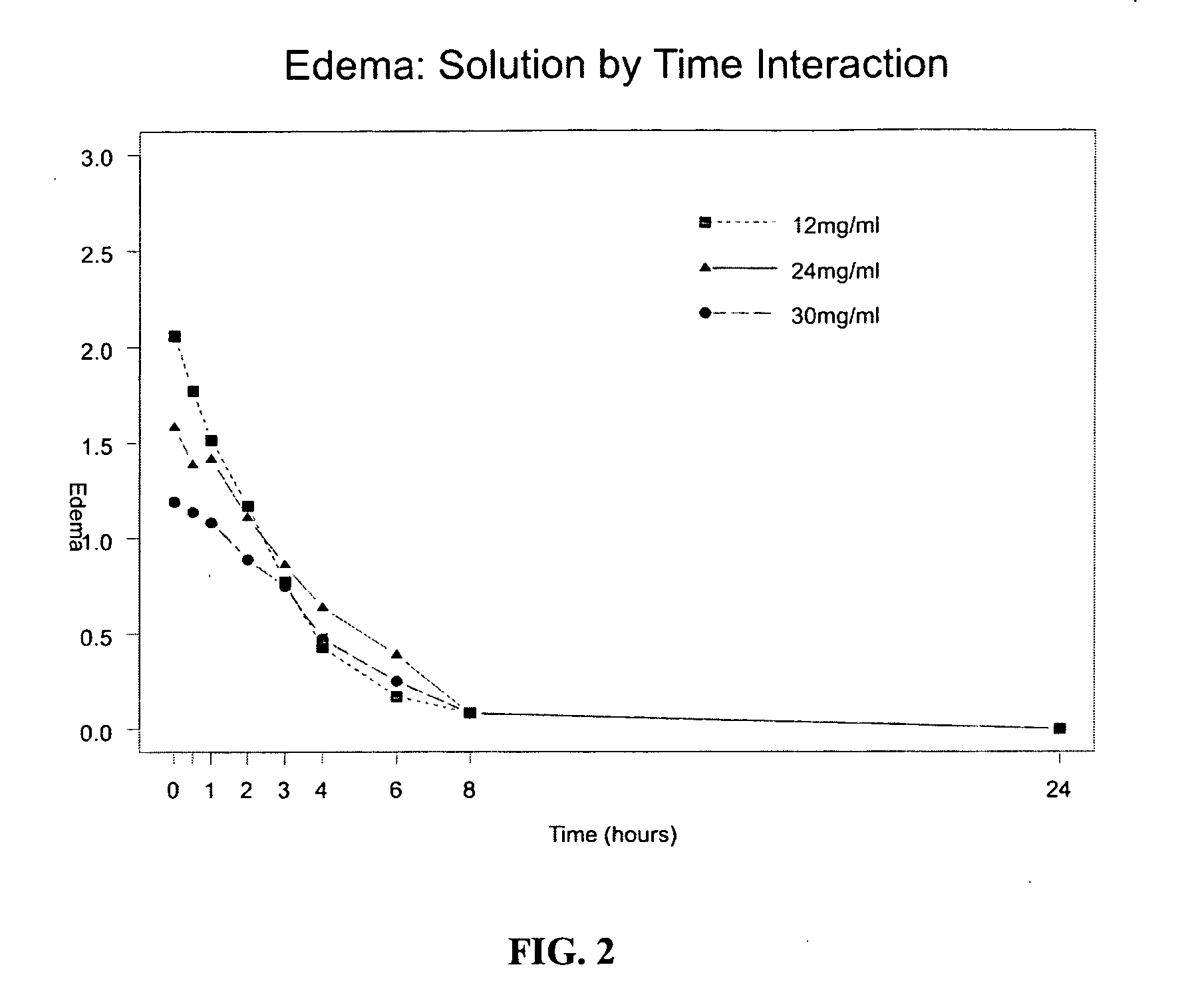
Analgesics for nasal administration
InactiveUS20050142072A1Rapid uptakeRapid onsetPowder deliveryBiocideNasal Cavity EpitheliumBlood plasma
An analgesic and a delivery agent are combined in a pharmaceutical composition such that, on introduction into the nasal cavity of a patient to be treated, the analgesic may be delivered to the bloodstream to produce within 30 minutes a therapeutic plasma concentration, Cther, of 0.2 ng / ml or greater which is maintained for a duration Tmaint of at least 2 hours. The analgesic may be an opioid analgesic or a non-steroidal anti-inflammatory drug.
Owner:IONIX PHARMA +1
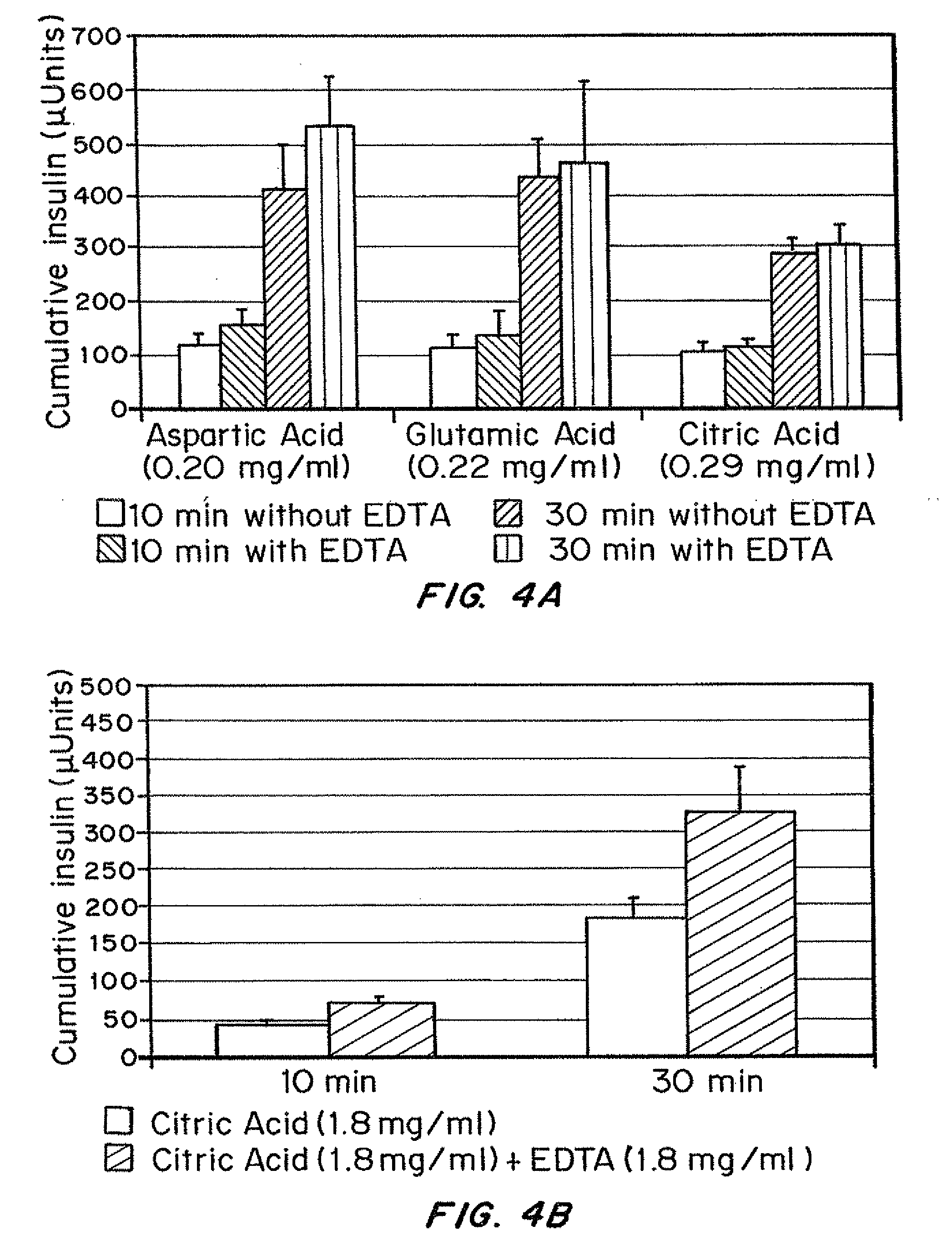
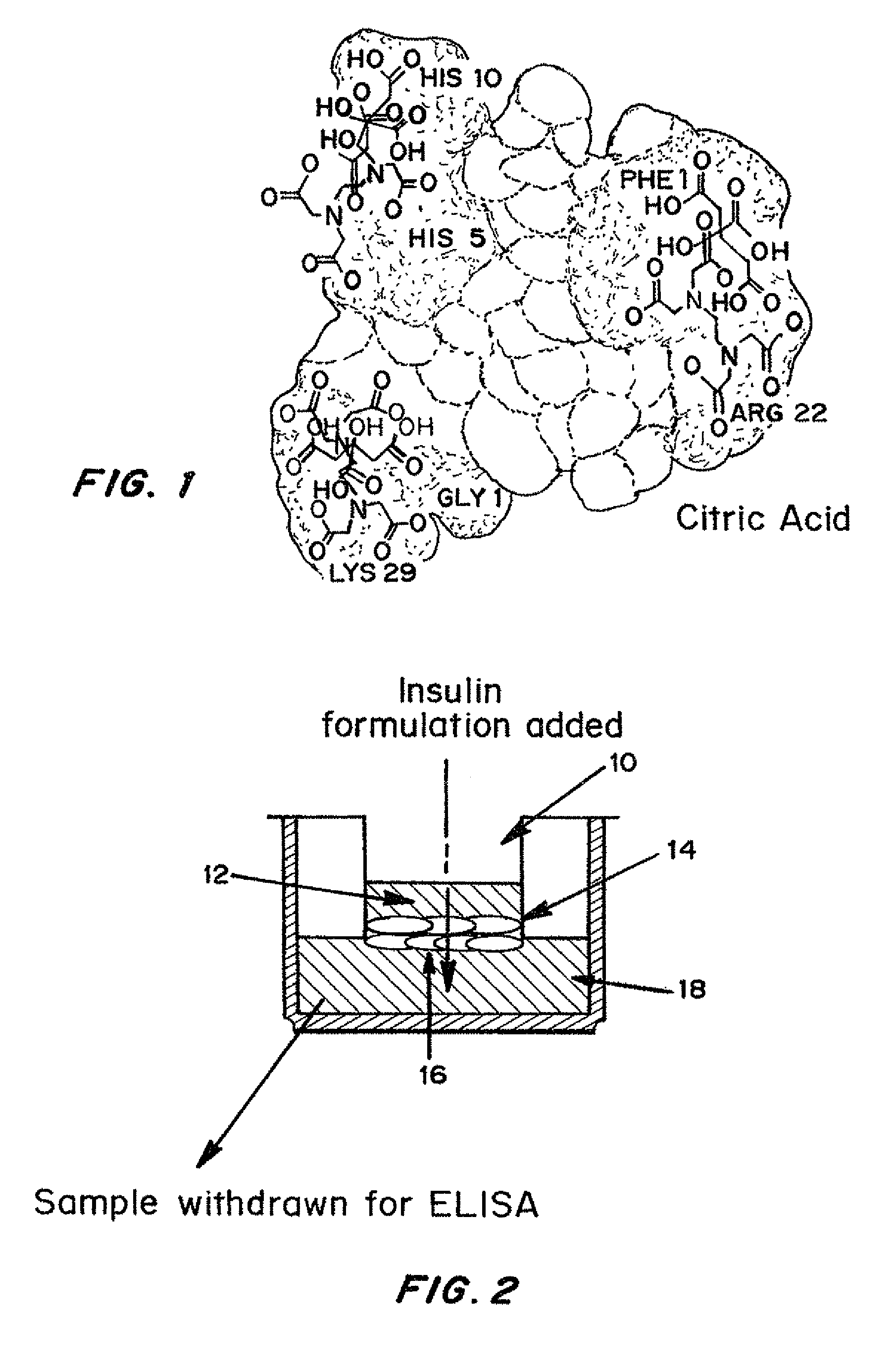
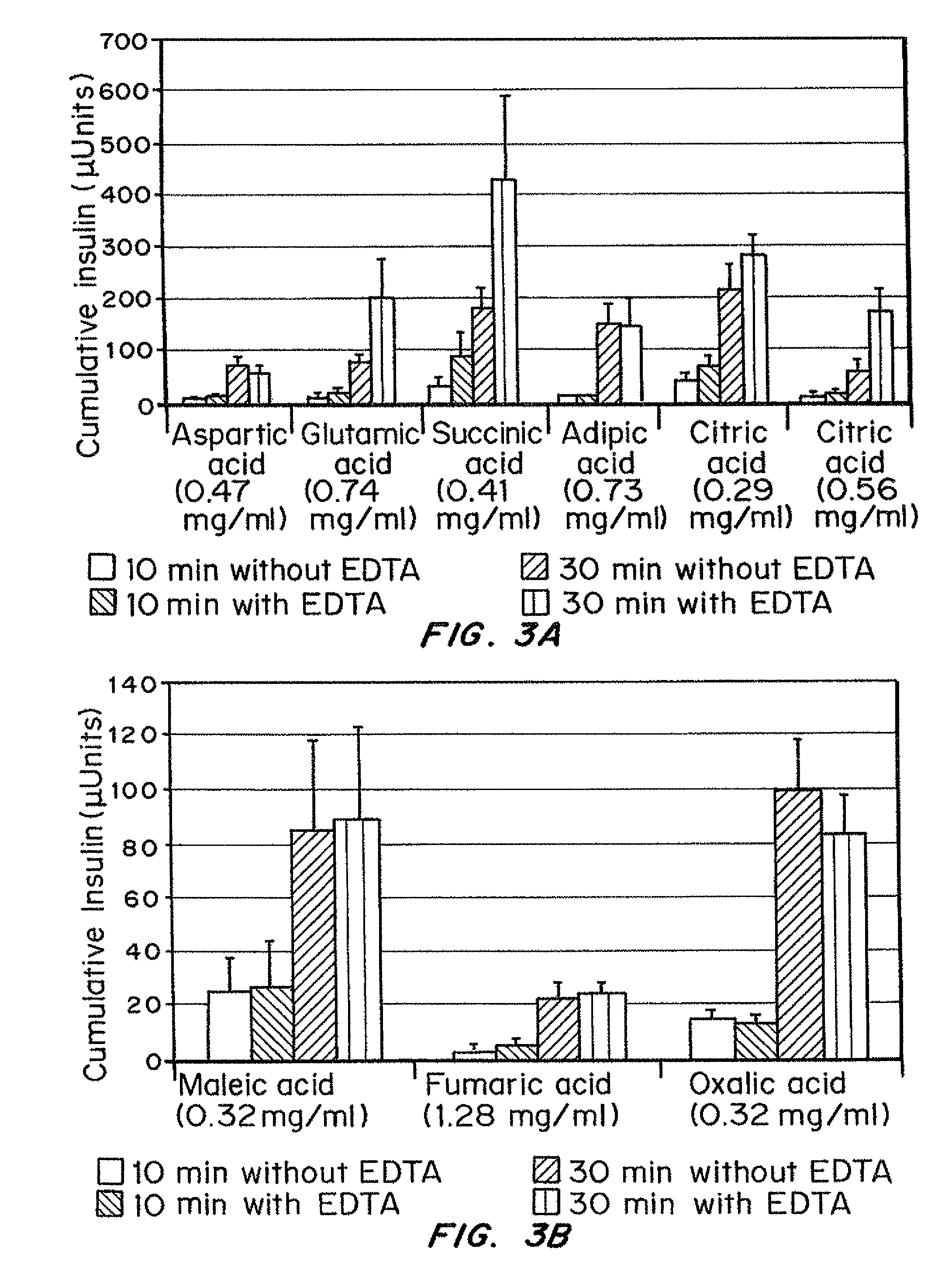
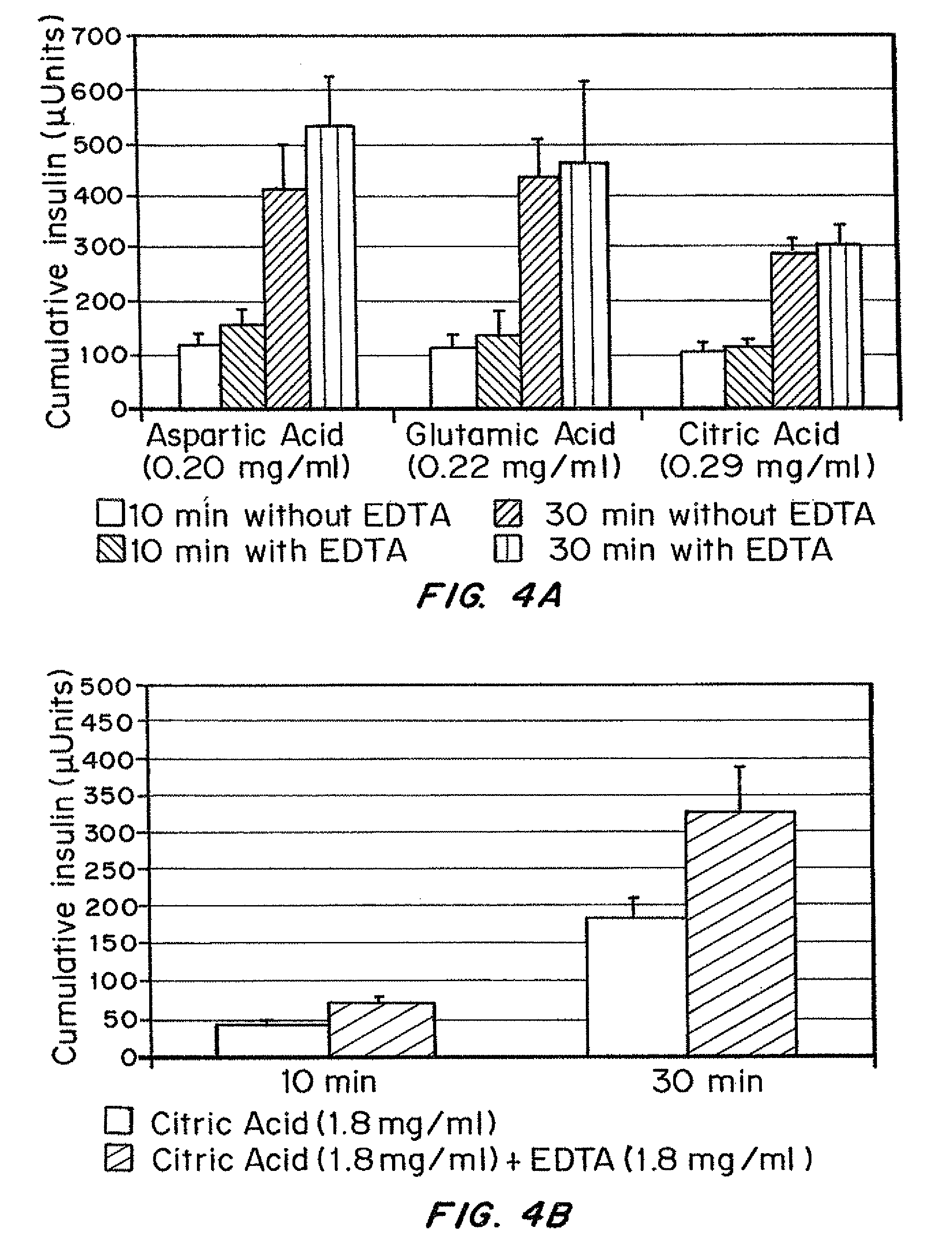
Insulin formulations for rapid uptake
ActiveUS20100227795A1Improve stabilityRapid actionOrganic active ingredientsPeptide/protein ingredientsVialTrisodium citrate
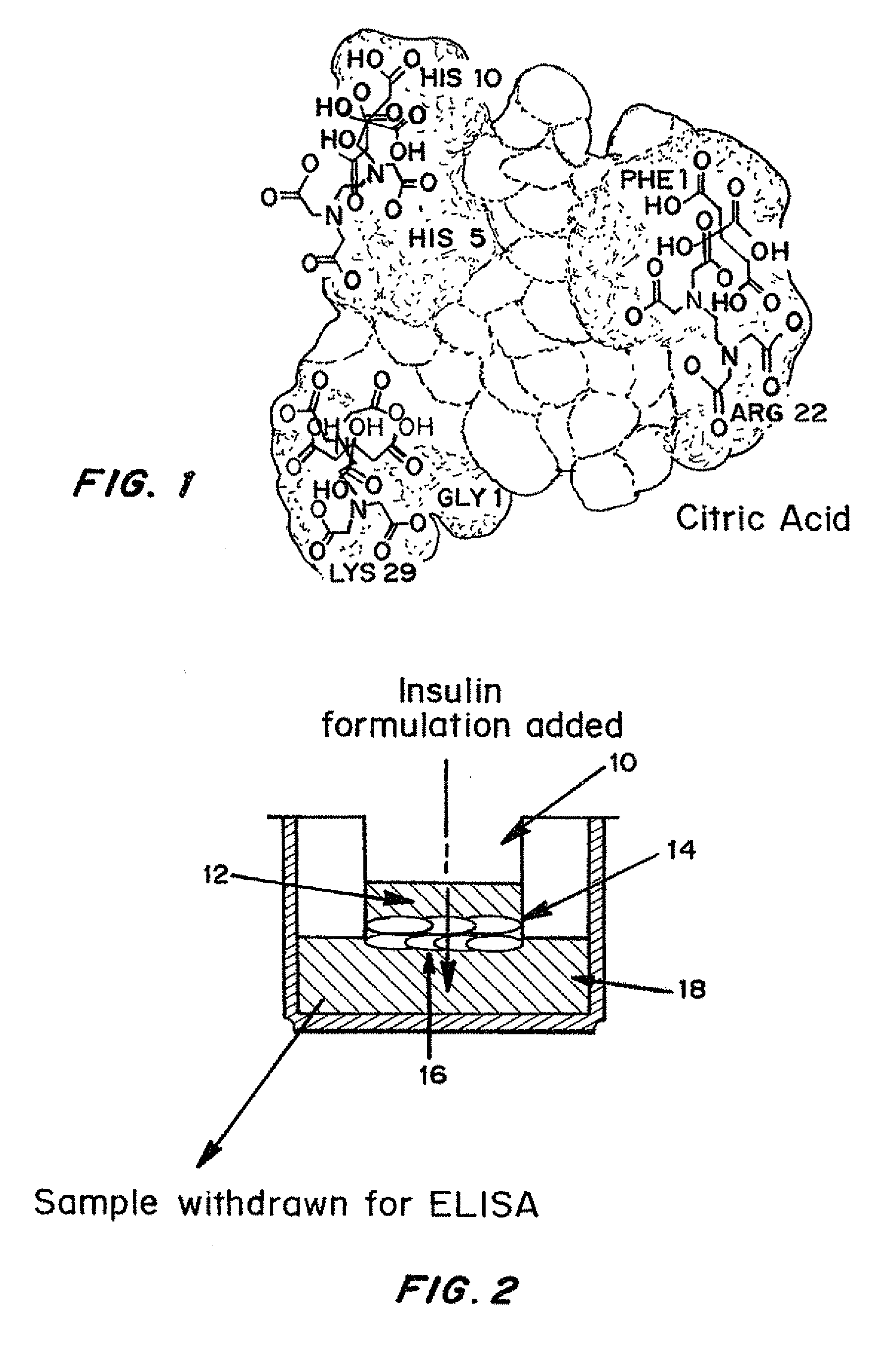
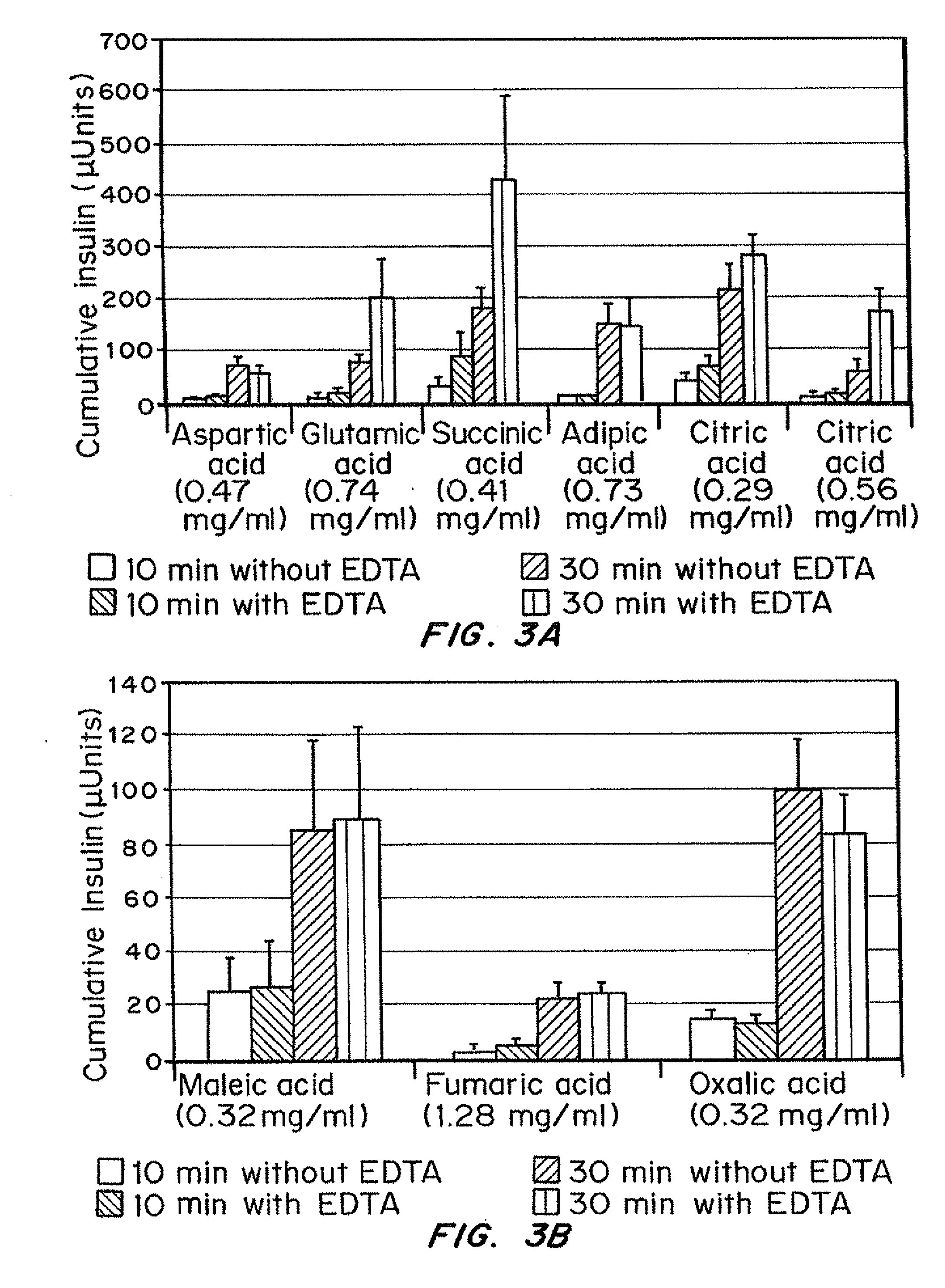
Injectable insulin formulations with improved stability and rapid onset of action are described herein. The formulations may be for subcutaneous, intradermal or intramuscular administration. In the preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid or sodium citrate. These formulations are rapidly absorbed into the blood stream when administered by subcutaneous injection. In the preferred embodiment, the insulin is provided as a clear liquid, neutral pH, in a multi-use sterile vial. In an alternative embodiment, the insulin is provided as a powder in a sterile vial. This is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water, a zinc chelator such as EDTA and a dissolution agent such as citric acid shortly before or at the time of administration. In another embodiment, the insulin is stored as a frozen mixture, ready for use upon thawing.
Owner:ELI LILLY & CO
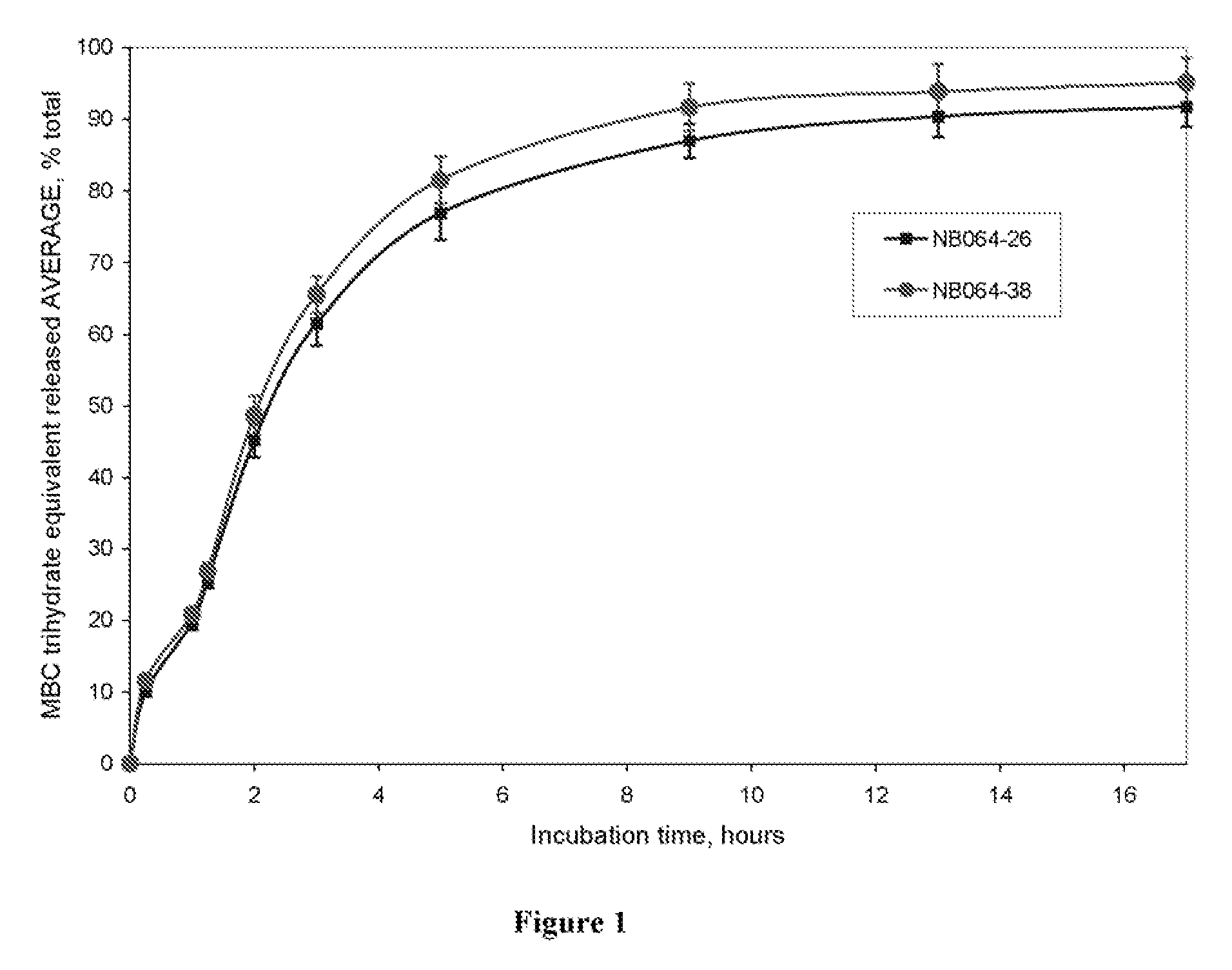
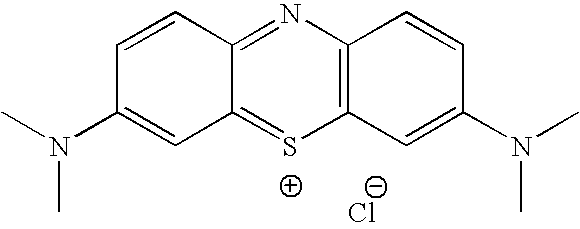
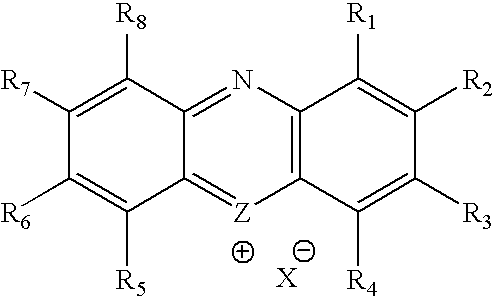
Methylene Blue Derivatives
InactiveUS20070116757A1Facilitated releaseRapid uptakeOrganic chemistryPill deliveryImmediate releaseFatty acid
Pharmaceutical compositions comprising a fatty acid salt, a dicarboxylic acid salt, an alkyl sulfate salt, an aryl sulfate salt or an alkyl aryl sulfonate salt of methylene blue or a derivative of methylene blue are described herein. The compositions are preferably administered orally and can be administered as tablets, soft or hard shell capsules (e.g. soft gelatin capsules), suspensions or solutions. The composition can also be formulated as a suppository or enema or rectal administration. The compositions further comprise a pharmaceutically acceptable carrier and optionally one or more pharmaceutically acceptable excipients. Suitable excipients include diluents, binders, plasticizers, lubricants, disintegrants, colorants, stabilizers, surfactants, and combinations thereof. The fatty acid salts, alkyl sulate salts, aryl sulfate salts or alkyl aryl sulfonate salts can be co-mixed or co-melted with one or more fatty acids to make more hydrophobic compositions, which may result in less staining formulations. The compositions can be formulated for immediate release, controlled release such as extended release, delayed release, and pulsatile release, or combinations thereof. In one embodiment, the derivative of methylene blue is methylene dodecylsulfate.
Owner:COLLEGIUM PHARMA INC
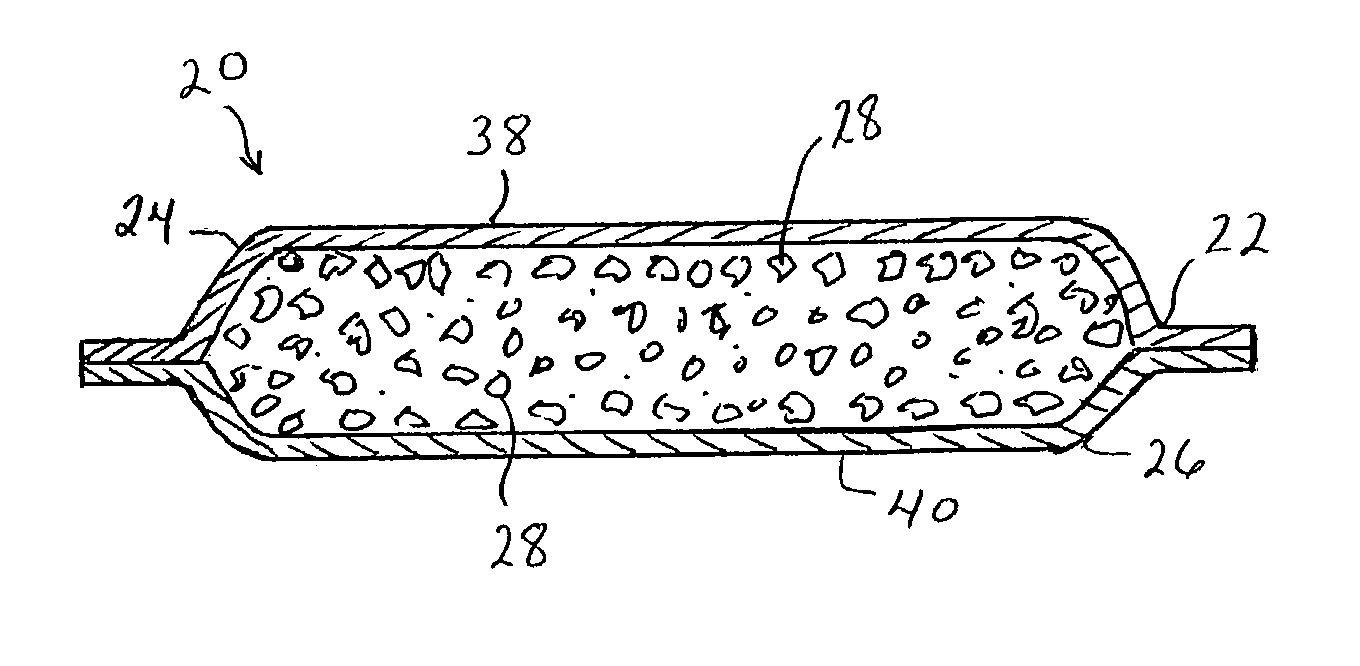
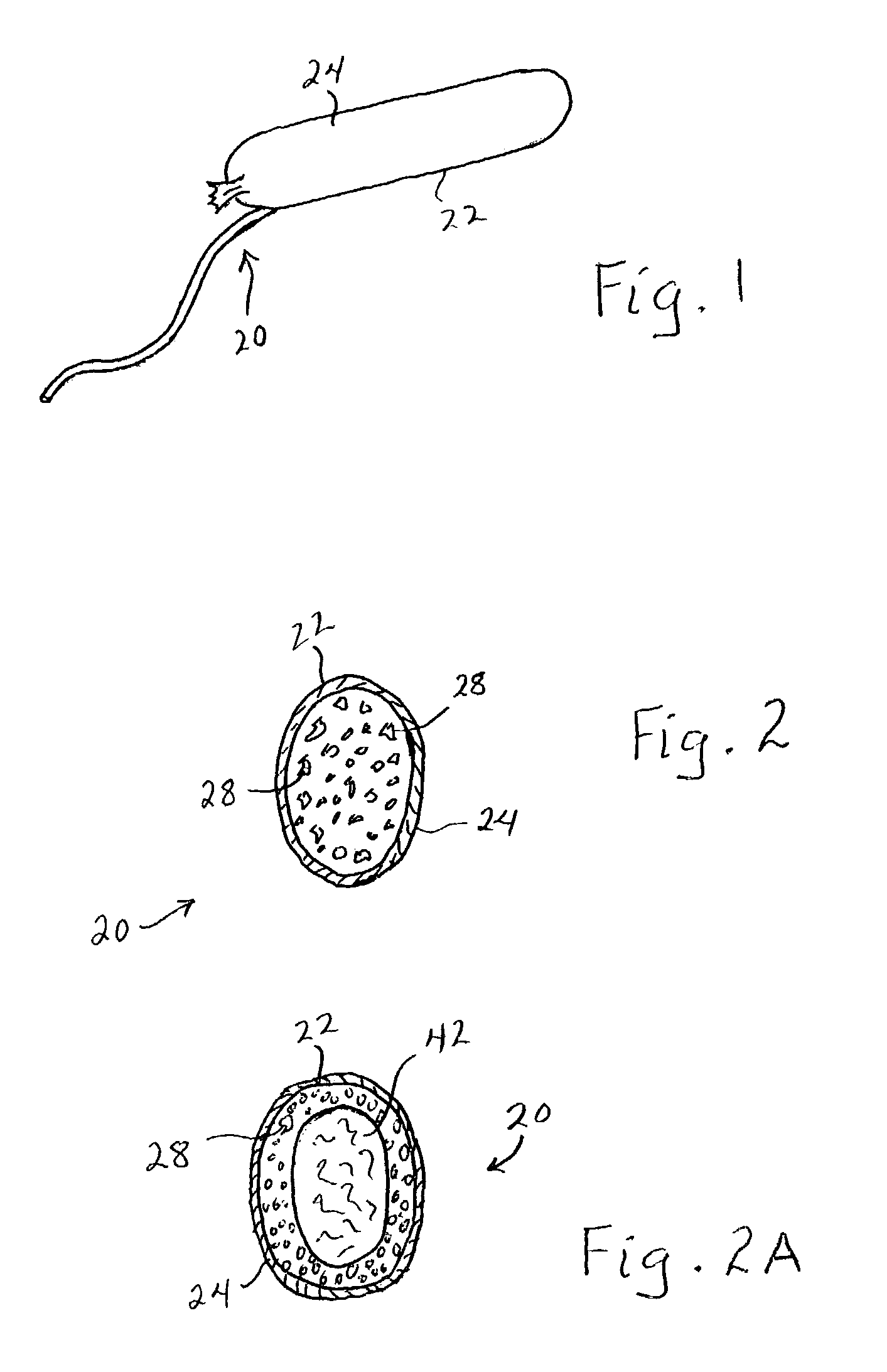
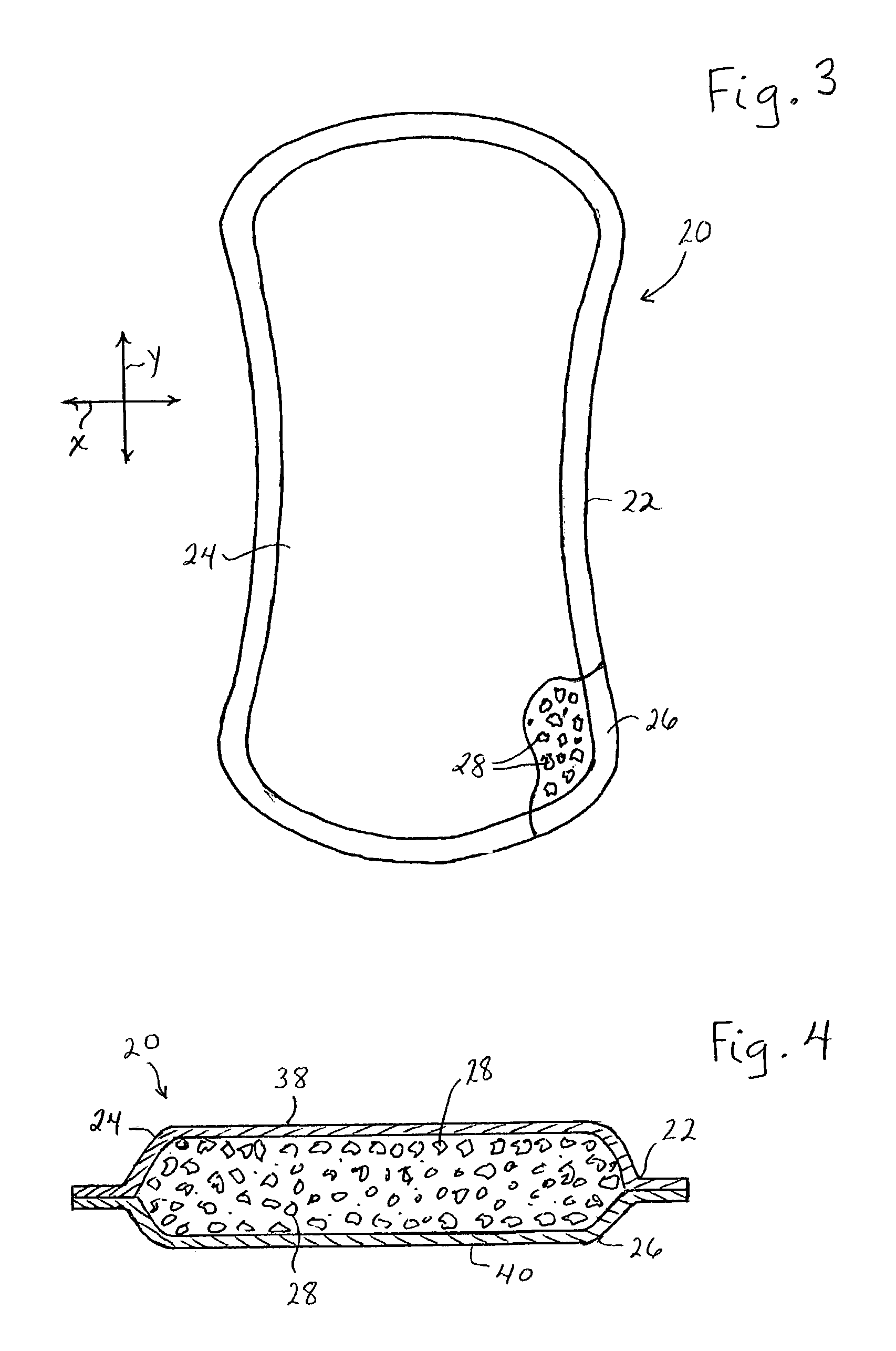
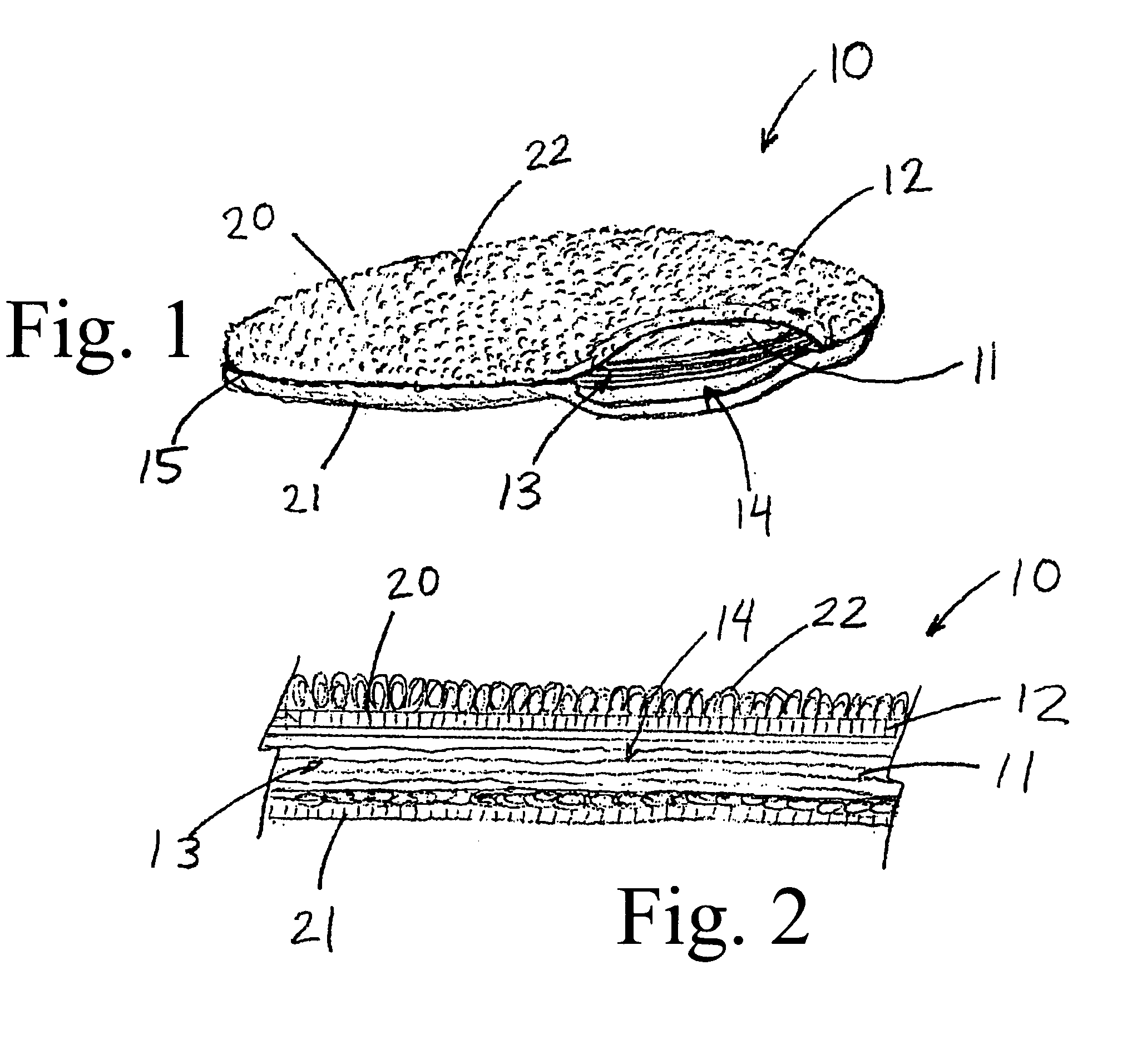
Enhanced body conformance with the use of free flowing particles
A body conformance system (22) includes at least one liquid-permeable, flexible containment layer (24), and an operative quantity of substantially free-flowing particulate material (28) constrained by the flexible containment layer (24). In a particular aspect, the substantially free-flowing particulate material (28) can exhibit a selected avalanche-time between avalanches. In another aspect, the particulate material can exhibit a selected, minimum retention capacity. In a further aspect, the system can exhibit a distinctive gap-protrusion area (104). In yet another aspect, the containment layer (24) can include a material that has a relatively high permeability to liquid, but a relatively high resistance to a passage of the particulate material.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Particulate polymeric material
InactiveUS20070043147A1Rapid uptakeDuplicating/marking methodsInksBiomedical engineeringSkeletal structures
A polymeric particulate material suitable for use in an ink-jet receiver is prepared by generating an emulsion comprising a first phase having a first carrier fluid and a second phase having a second carrier fluid, said first and second carrier fluids being immiscible; carrying out a first treatment to at least one component of the first phase to form and / or maintain a skeletal structure of the treated at least one component of the first phase; carrying out a second treatment to the second phase to substantially remove the carrier fluid thereby generating a large capacity porous structure defined by the skeletal structure; and mechanically dividing (e.g. milling) the skeletal structure. A coating of the particles is capable of rapid uptake of large quantities of ink, especially when formed using a high internal phase water-in-oil emulsion.
Owner:EASTMAN KODAK CO
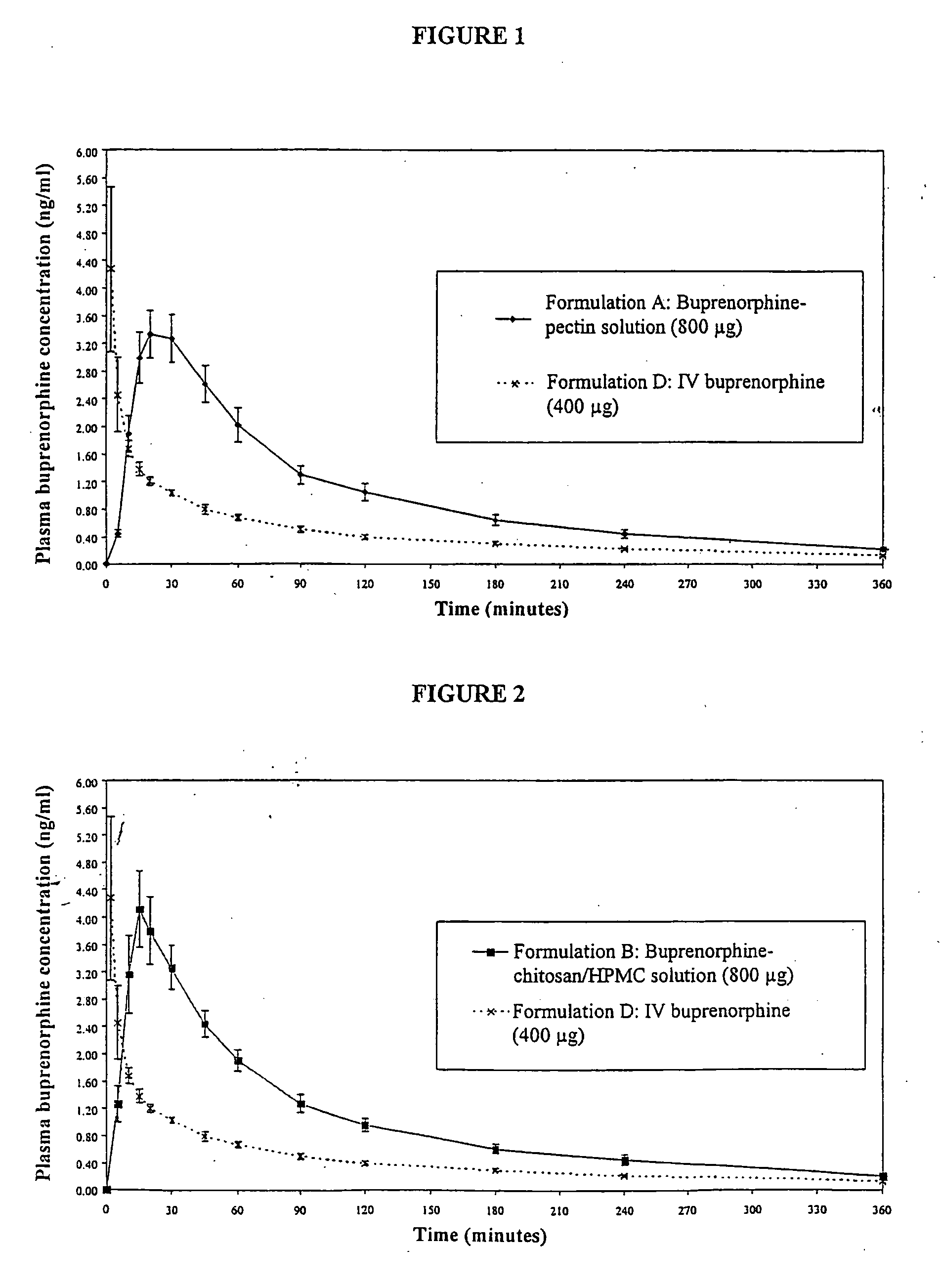
Formulation
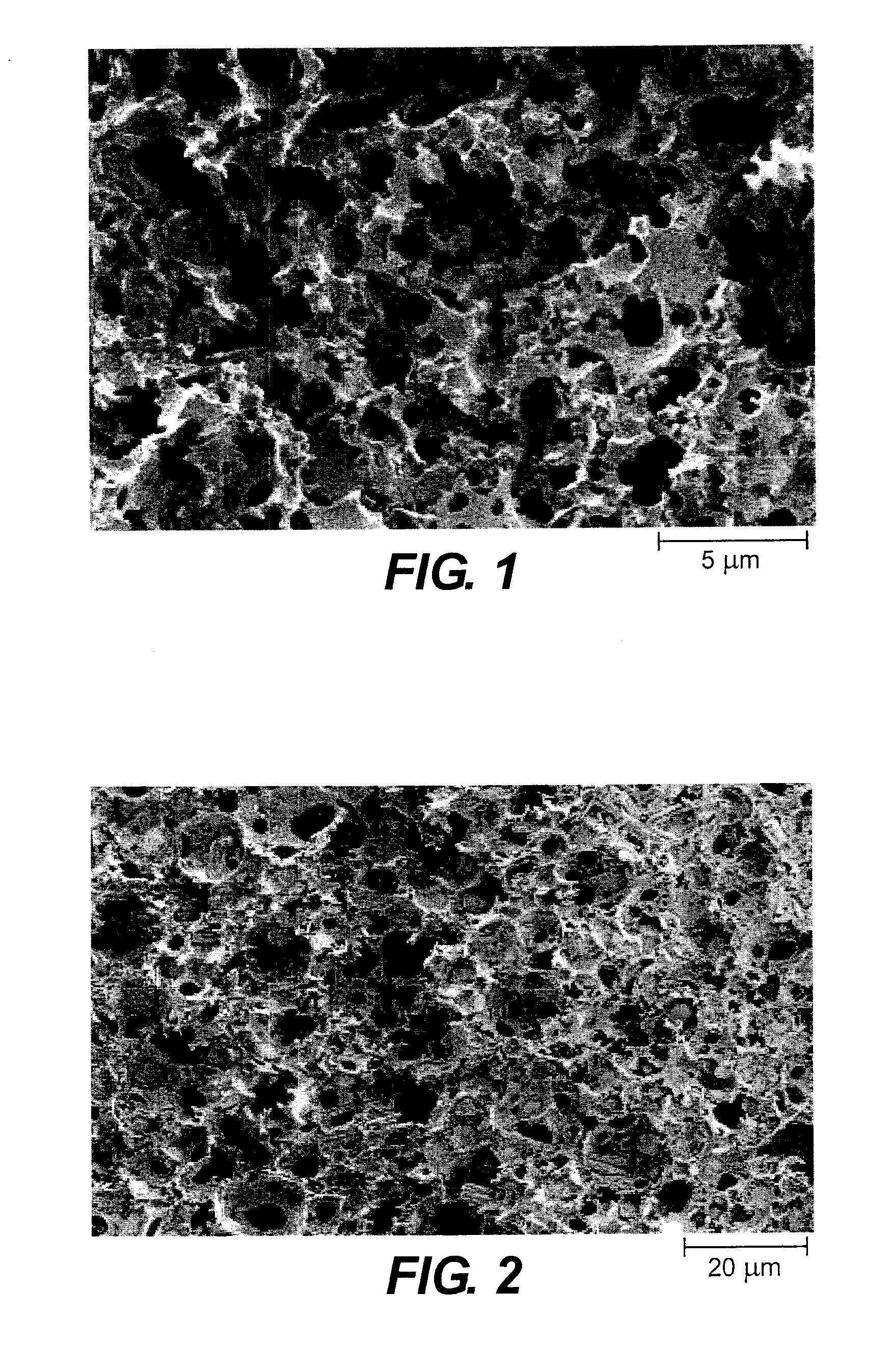
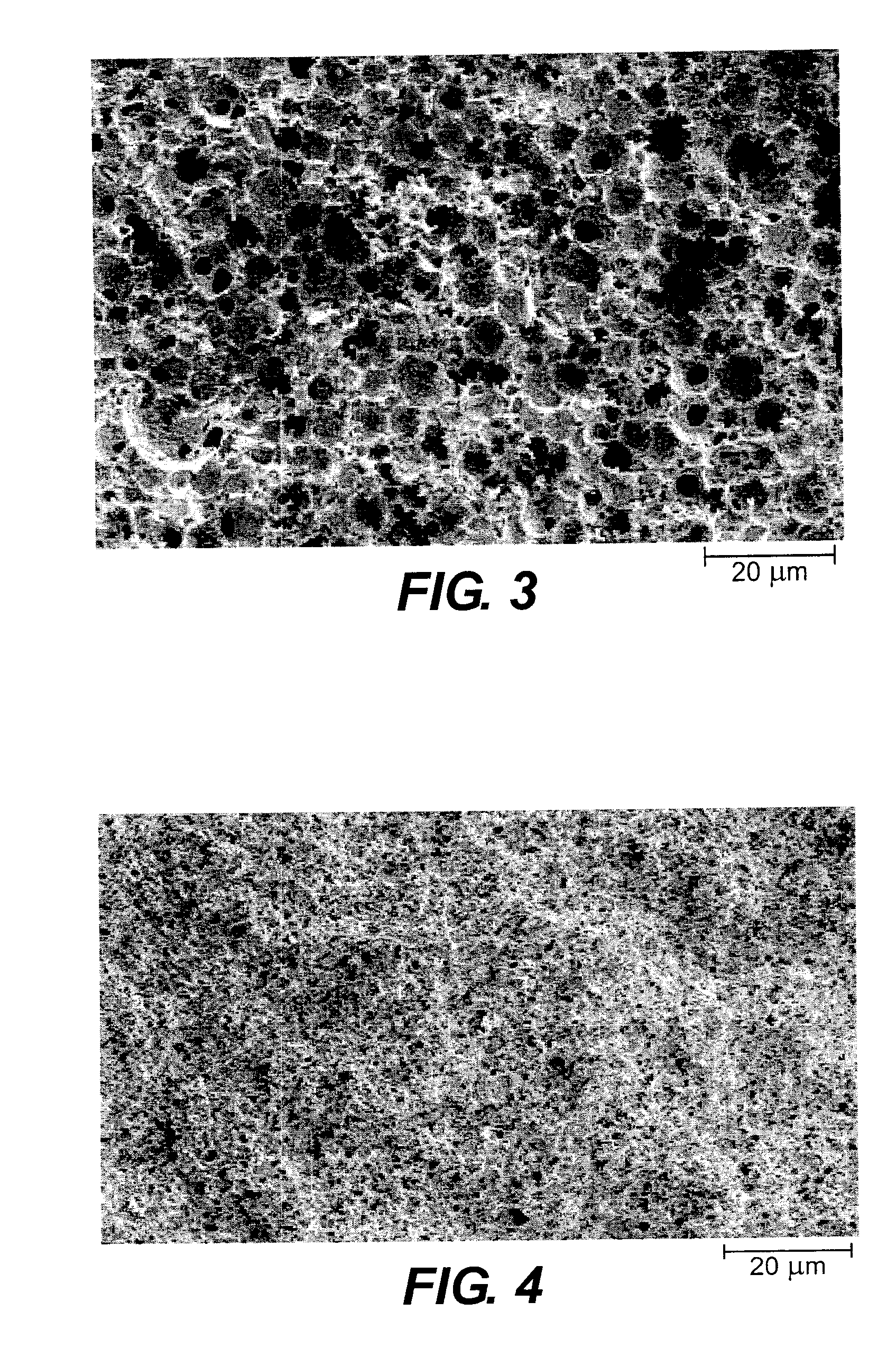
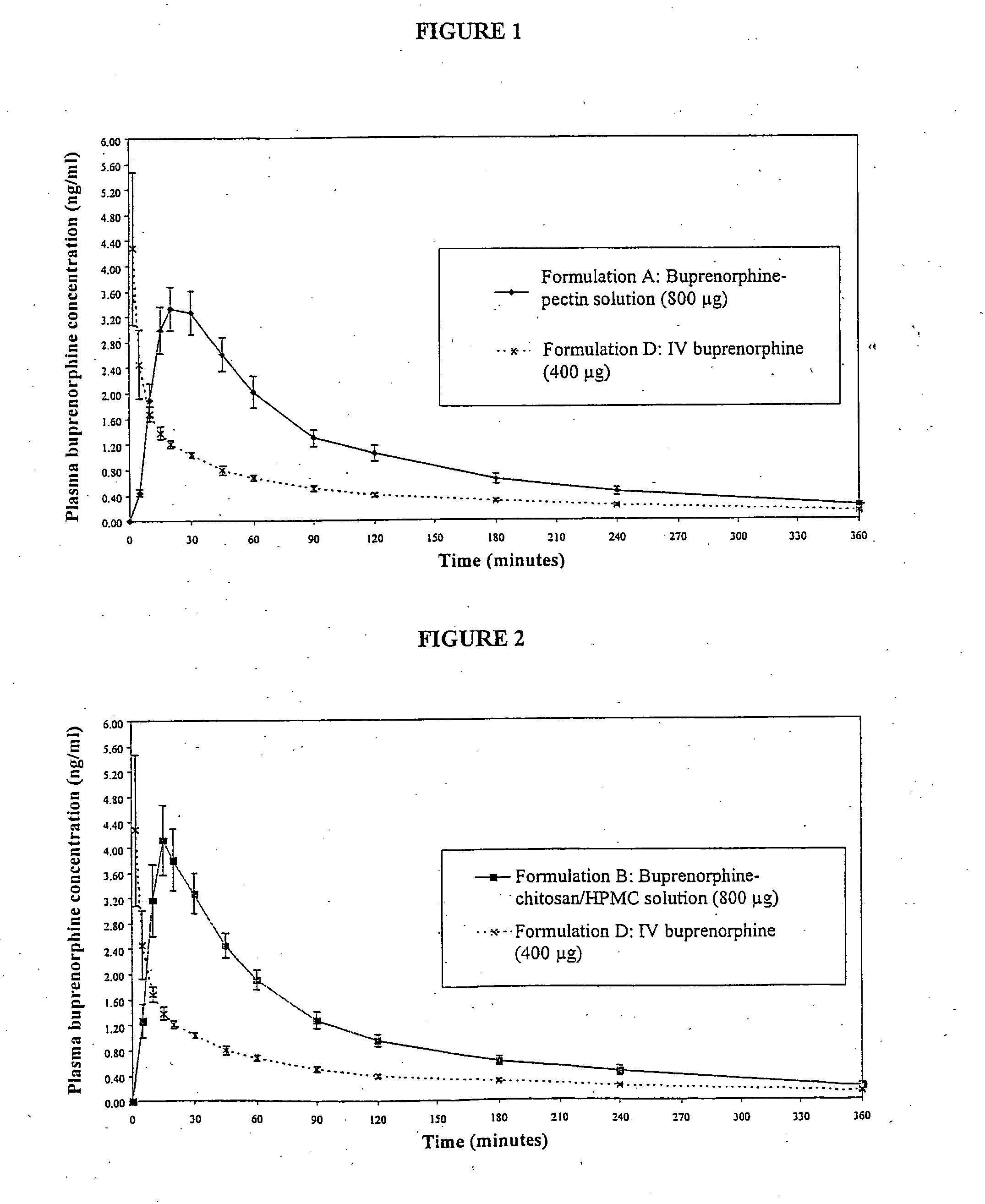
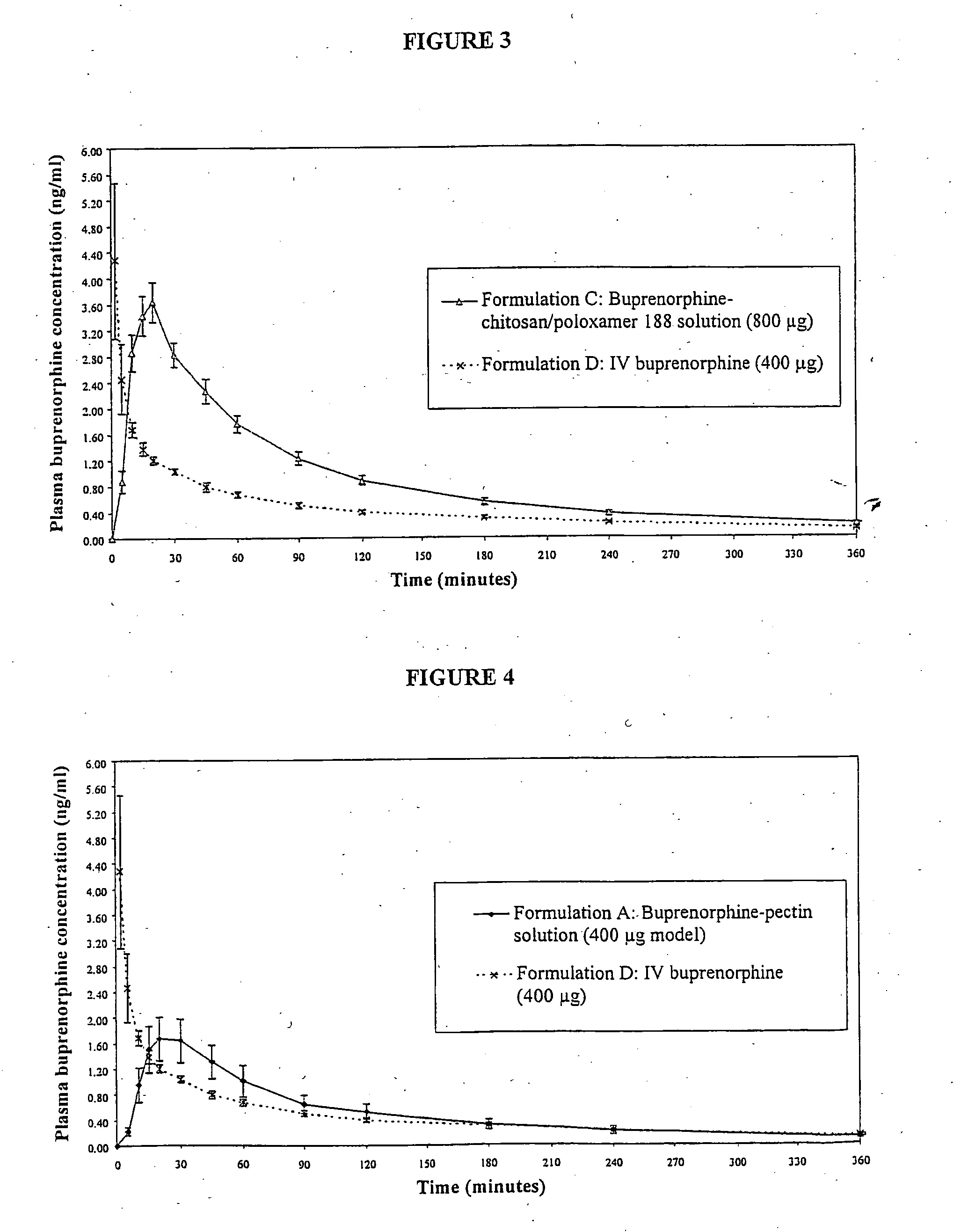
InactiveUS20050085440A1Rapid uptakeRapid onsetBiocideSenses disorderHydroxypropylmethyl celluloseBlood plasma
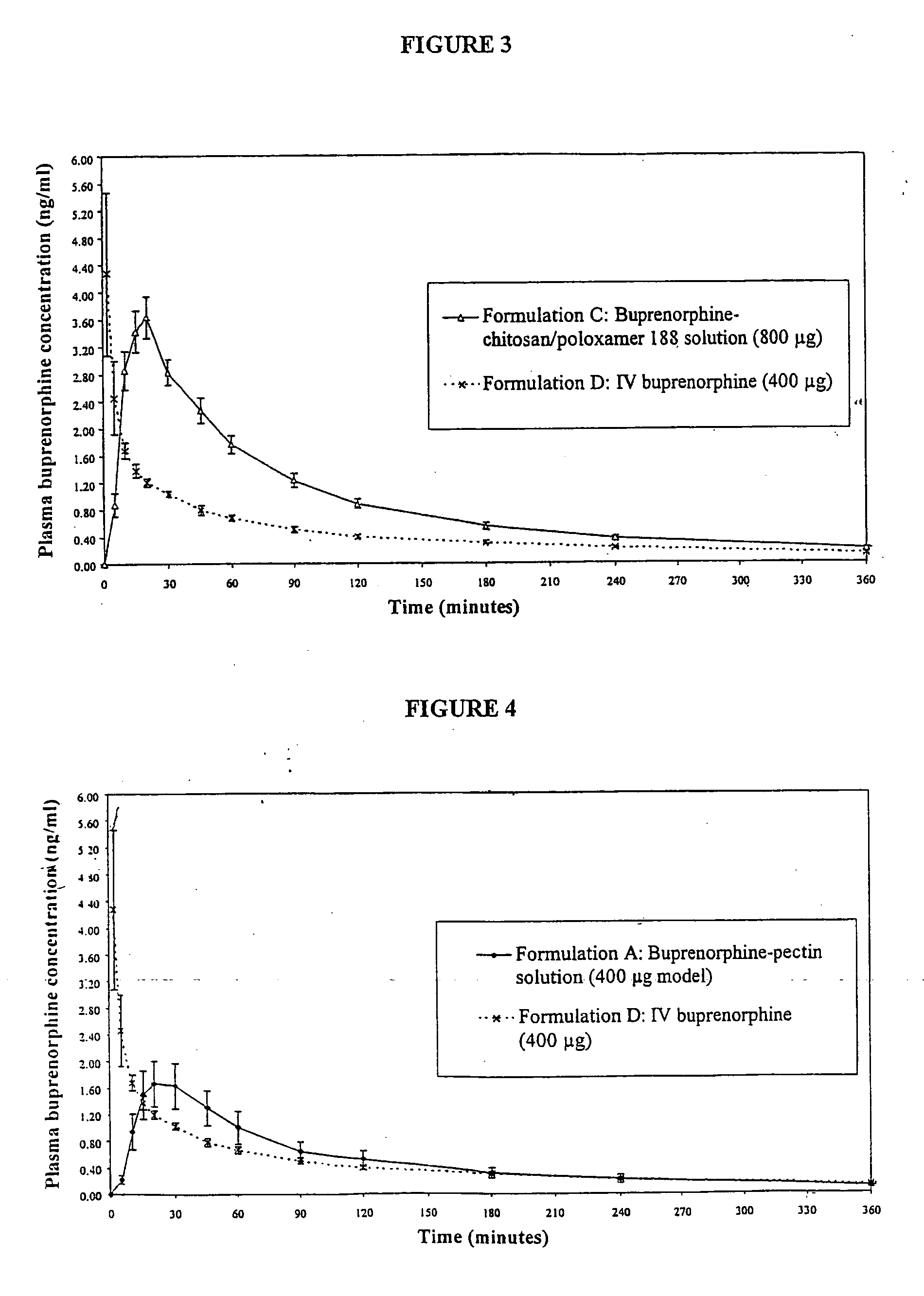
Aqueous formulations suitable for intranasal administration comprise buprenorphine or a physiologically acceptable salt or ester thereof and (a) a pectin having a degree of esterification of less than 50%, (b) chitosan and a polyoxyethylene-polyoxypropylene copolymer (poloxamer) or (c) chitosan and hydroxypropylmethylcellulose. Such formulations can induce rapid and prolonged analgesia when delivered intranasally to a patient. The buprenorphine or buprenorphine salt or ester may be delivered to the bloodstream to produce within 30 minutes a therapeutic plasma concentration of buprenorphine, Cther, of 0.2 ng / ml or greater which is maintained for a duration Tmaint of at least 2 hours.
Owner:ARCHIMEDES DEVMENT
Dehydrated hydrogel precursor-based, tissue adherent compositions and methods of use
InactiveUS8512749B2Rapid uptakeFacilitate cross-linkingPowder deliverySurgical adhesivesDrug deliveryBiomedical engineering
Compositions and methods are provided for forming tissue-adherent hydrogels using substantially dry precursors. The dehydrated precursors are premixed prior to in situ therapy and utilize naturally-occurring body fluids as an aqueous environment that initiates transformation, which causes dissolution and nearly simultaneous crosslinking of the precursors, thus forming an insoluble hydrogel implant. The dehydrated precursor-based hydrogels may be used as sealants for fluid leaks from tissue, as adherent drug delivery depots, as means for augmenting and / or supporting tissue, and as means for serving a variety of other useful medical and surgical purposes.
Owner:INCEPT LLC
Formulations of anti-pain agents and methods of using the same
InactiveUS20050256182A1Reduce skin irritationRelieve painBiocidePharmaceutical delivery mechanismSide effectDose sparing
The present invention relates to novel anti-pain formulations and methods of their delivery. Anti-pain agents delivered in accordance with the methods of the invention have an improved clinical utility and therapeutic efficacy relative to other drug delivery methods, including oral, intramuscular and subcutaneous delivery. The methods of the present invention provide benefits and improvements over conventional drug delivery methods including dose sparing, increased drug efficacy, reduced side effects.
Owner:BECTON DICKINSON & CO
Peptide clearing agents
InactiveUS20130053543A1Easy to removeReduce molecular weightPeptide sourcesNanomedicineDipeptideReactive site
A peptide clearing agent is provided for clearance of a conjugate of an enzyme and a binding molecule which binds specifically at a target location from a non-target location in a subject. The peptide clearing agent binds the active site of the enzyme. The peptide also binds to the asialoglycoprotein receptor expressed by hepatic cells to facilitate clearance through the liver. The peptide may be glycosylated to facilitate clearance through the liver by binding to hepatic cells expressing an asialoglyco-protein receptor. Typically, the peptide prevents or inhibits enzyme activity upon binding to the enzyme and is not substantially modified by the enzyme activity. The peptide may be based upon the dipeptide amino-naphthoic acid (ANA)-glutamate (GIu) and may comprise the amino acid sequence serine (Ser)-Alanine (Ala)-amino-naphthoic acid (ANA)-glutamate (GIu). In such cases, the enzyme of interest is typically CPG2.
Owner:MOLOGIC LTD
Tumor imaging compounds
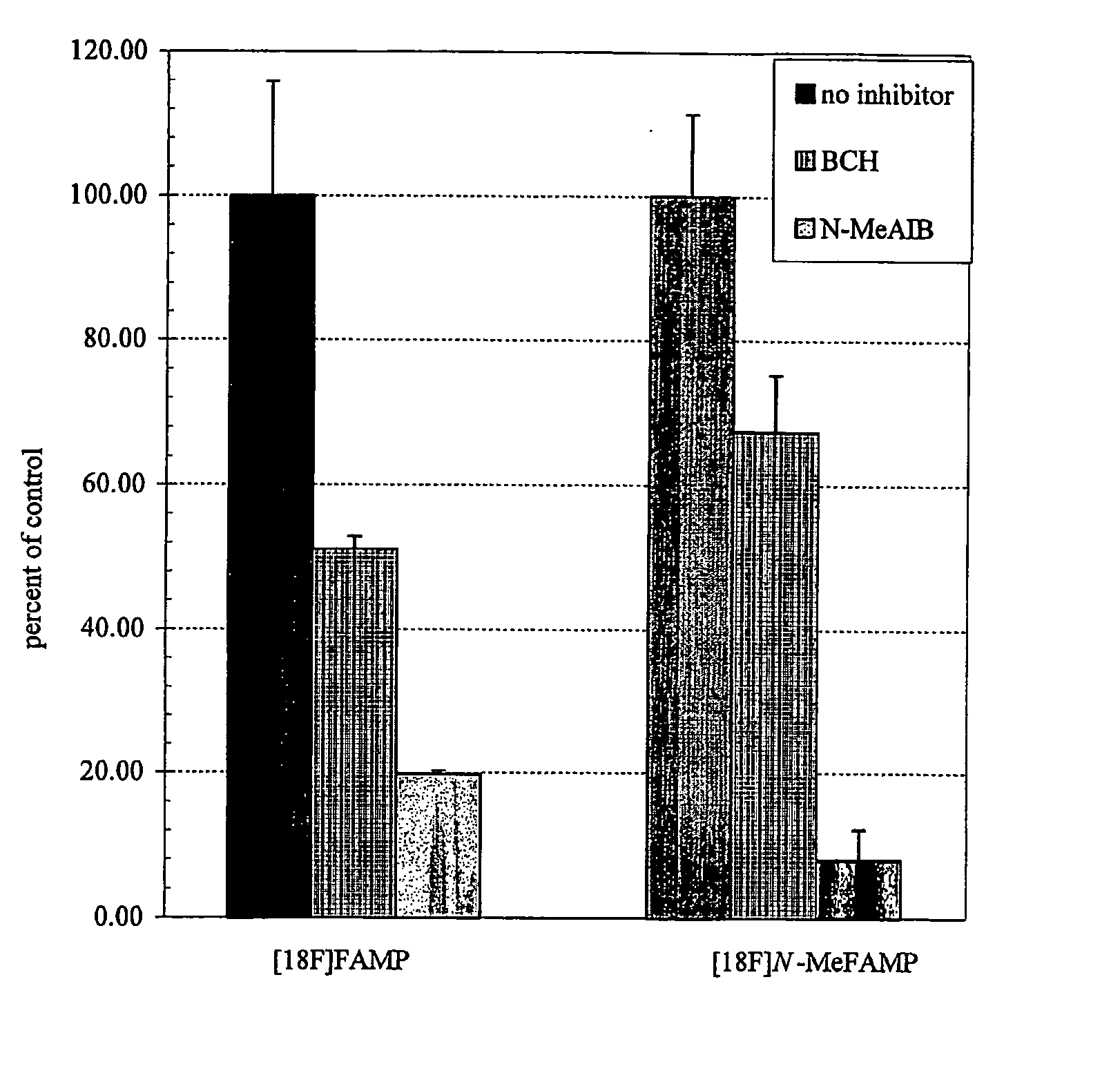
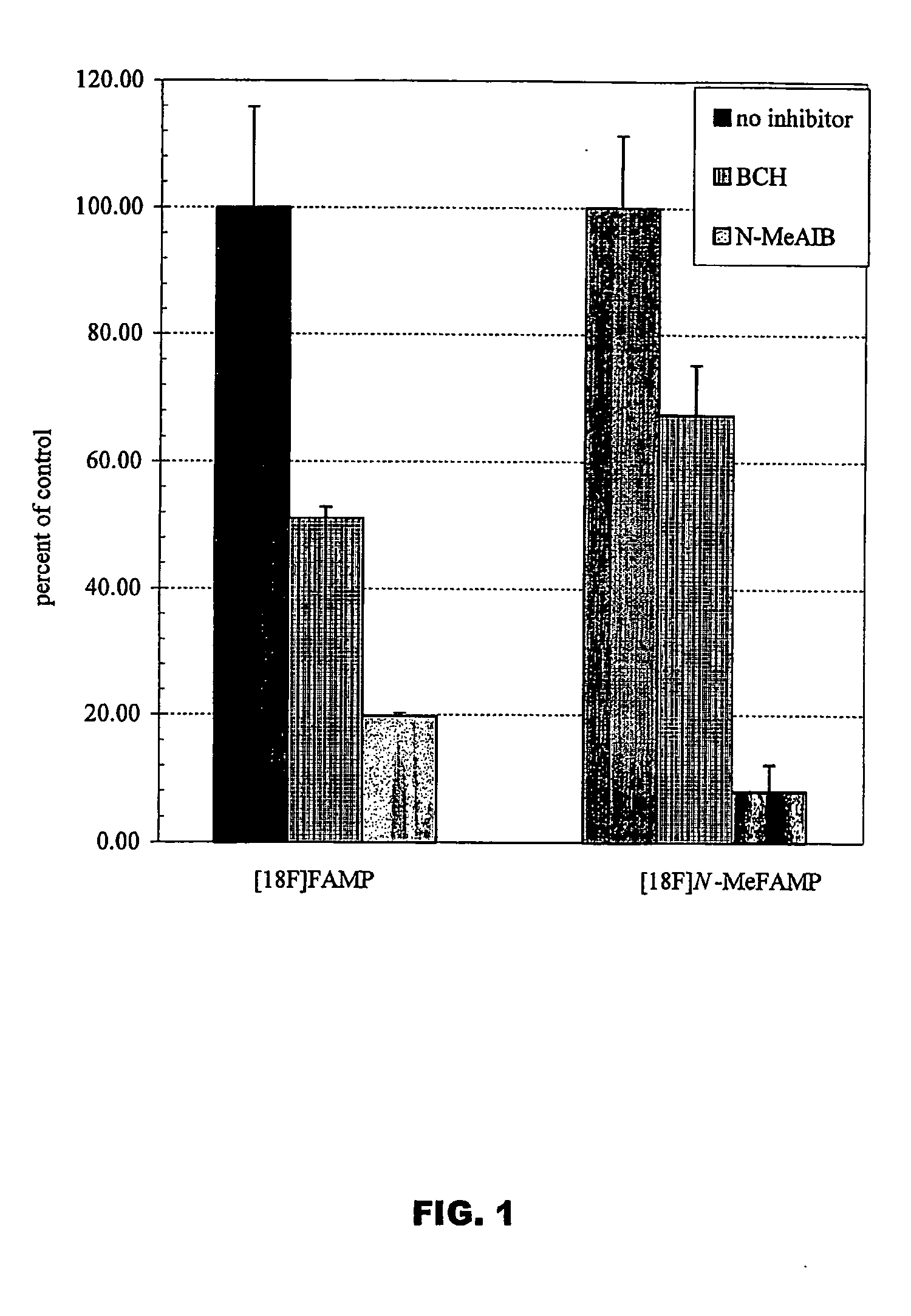
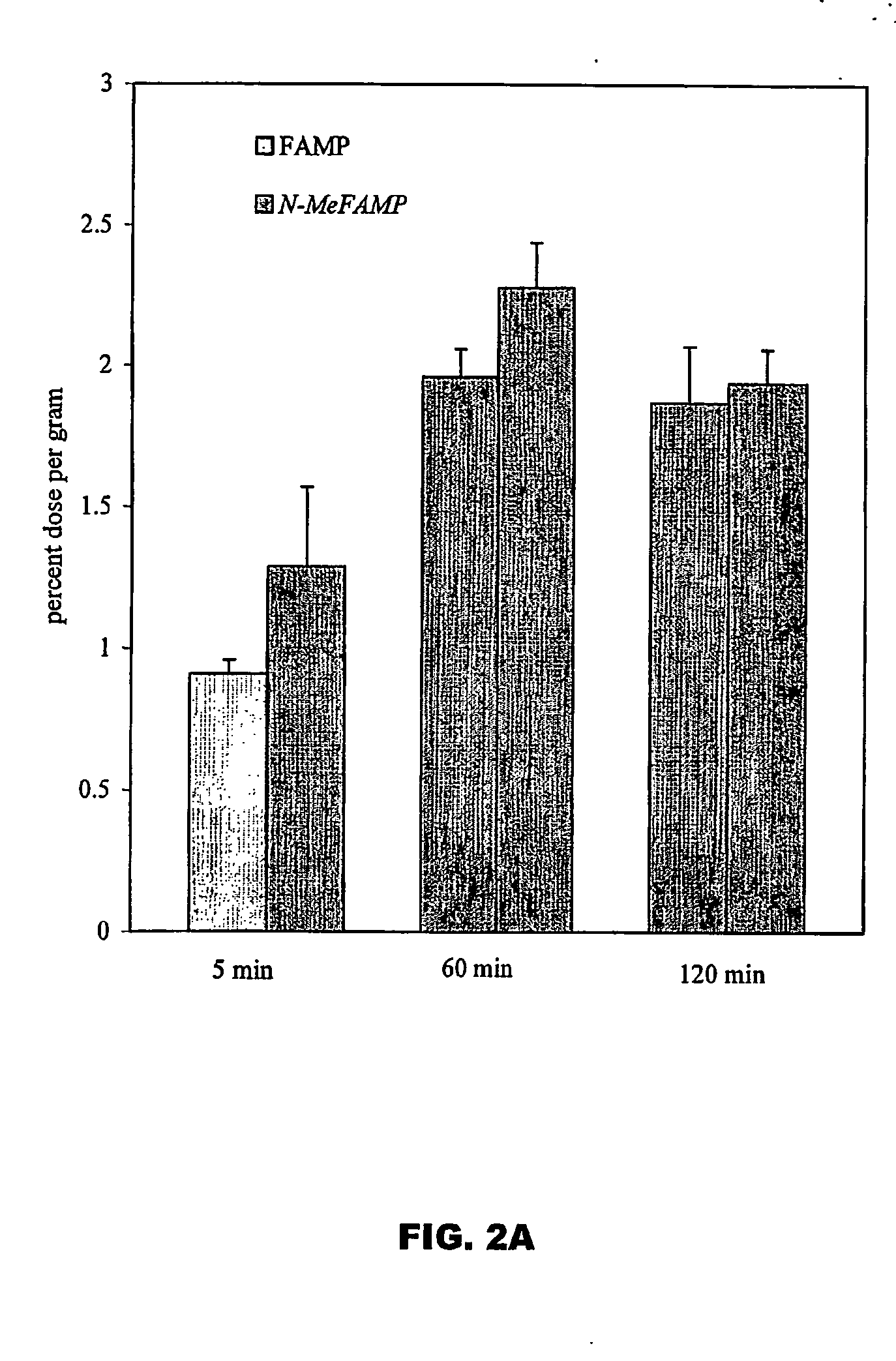
ActiveUS20050192458A1Rapid uptakeHigh retention rateBiocidePeptide/protein ingredientsAbnormal tissue growthRhenium
The invention provides novel amino acid compounds of use in detecting and evaluating brain and body tumors. These compounds combine the advantageous properties of α-aminoisobutyric acid (AIB) analogs namely, their rapid uptake and prolonged retention in tumors with the properties of halogen sustituents, including certain useful halogen isotopes such as fluorine-18, iodine-123, iodine-124, iodine-125, iodine-131, bromine-75, bromine-76, bromine-77, bromine-82, astatine-210, astatine-211, and other astatine isotopes. In addition the compounds can be labeled with technetium and rhenium isotopes using known chelation complexes. The amino acid compounds disclosed herein have a high specificity for target sites when administered to a subject in vivo. Preferred amino acid compounds include [18F] FAMP, ([18F]5a) and [18F]N-MeFAMP, ([18F]5b). The invention further features pharmaceutical compositions comprised of an α-amino acid moiety attached to eiher a four, five or a six member carbon-chain ring. The labeled amino acid compounds are useful as imaging agents in detecting and / or monitoring tumors in a subject by Positron Emission Tomography (PET) and Single Photon Emission Computer Tomography (SPECT).
Owner:EMORY UNIVERSITY
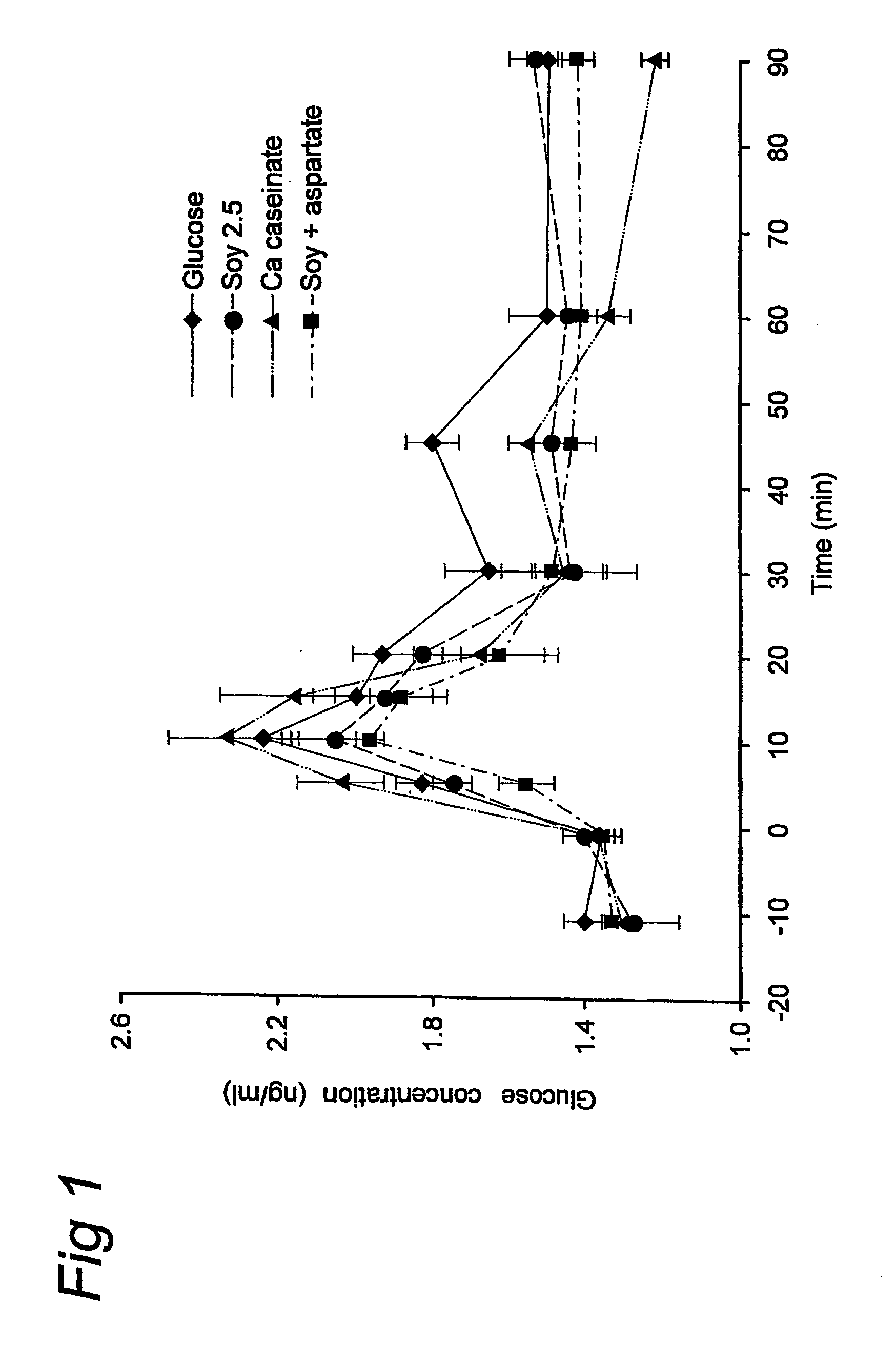
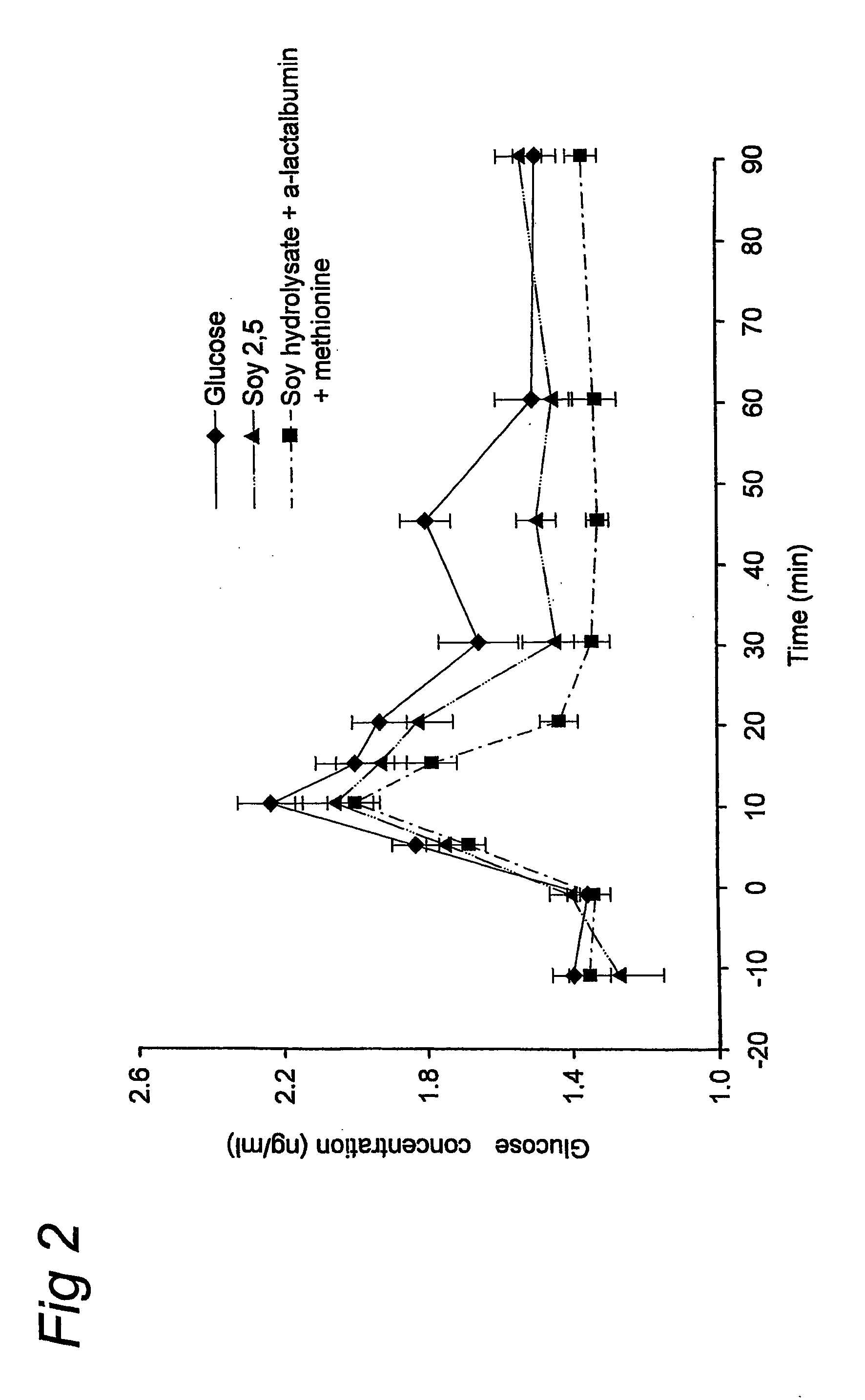
Preparation for Use of Aspartate for Regulating Glucose Levels in Blood
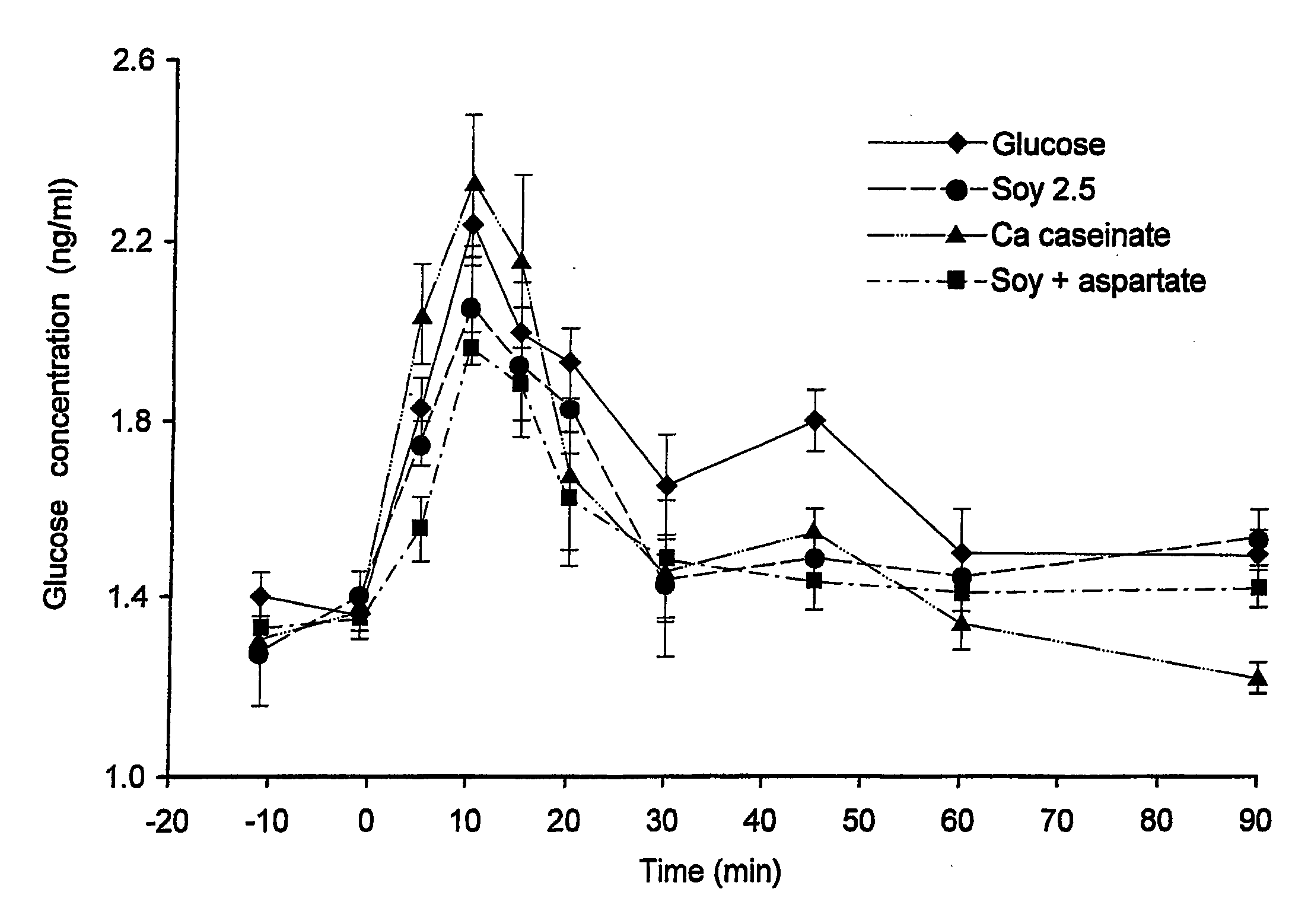
InactiveUS20080269117A1Increase glucose uptakeImprove responseOrganic active ingredientsSenses disorderPlasma glucose concentrationGlucose polymers
The invention relates to the use of specific protein and / or peptide fractions having a high aspartate content for regulating plasma glucose concentrations and increasing insulin sensitivity in a mammal. The invention relates to a complete food fortified with aspartate equivalents as well as a supplement rich in aspartate equivalents that is given simultaneously with or even minutes up to an hour prior to the consumption of a meal comprising glucose. The nutritional or pharmaceutical composition contains at least one protein having a high aspartate content, preferably of soy or dairy origin, which is further enriched with aspartate equivalents from another protein and / or free aspartate equivalents. The protein fraction comprises glutamate equivalents in a weight ratio of aspartate equivalents to glutamate equivalents (asp:glu) between 0.41:1 and 5:1.
Owner:NV NUTRICIA
Ink Receiving Material
InactiveUS20080160231A1Rapid uptakeCapablePretreated surfacesSpecial surfacesWater in oil emulsionCarrier fluid
A porous polymeric ink-jet receiver prepared by generating an emulsion comprising a first phase having a first carrier fluid and a second phase having a second carrier fluid, said first and second carrier fluids being immiscible; coating the emulsion onto a support; carrying out a first treatment to at least one component of the first phase to form and / or maintain a skeletal structure of the treated at least one component of the first phase; and carrying out a second treatment to the second phase to substantially remove the carrier fluid thereby generating a large capacity porous structure defined by the skeletal structure is capable of rapid uptake of large quantities of ink, especially when using a high internal phase water-in-oil emulsion.
Owner:EASTMAN KODAK CO
Compositions and methods for enhancing transmucosal delivery
InactiveUS20100086495A1Reduce deliveryRapid uptakeBiocideNervous disorderActive agentAdditive ingredient
The present invention provides transmucosal pharmaceutical or nutraceutical compositions and methods for enhancing transmucosal delivery of pharmaceutical and nutraceutical ingredients through use of methylsulfonylmethane (MSM) as a transmucosal delivery enhancer. In particular, the invention provides transmucosal compositions comprising an active agent selected from a non-steroid anti-inflammatory drug (NSAID); an analgesic; a migraine medication; a menopause medication; a sleep disorder medication; an erectile dysfunction medication, an appetite suppressant, a vitamin, a food supplement and a macromolecule. In certain particular exemplary formulations the appetite suppressant is DL-phenylalanine, and the vitamin is B12.
Owner:DERMA YOUNG LTD
Absorbent coaster insert
InactiveUS20080258031A1Rapid uptakeRapidly absorb and retainUnderlaysStands/trestlesMicrofiberPolypropylene
An absorbent coaster article for insertion into a cup holder that comprises an inner material including one or more layers of a superabsorbent polymeric material, such as a polypropylene microfiber fabric, that is enclosed by a protective outer covering comprising a fabric material effective for the passage of liquids therethrough to be absorbed by the superabsorbent inner material. The outer covering may be sewn to form an inner pocket containing the inner material, or the insert may include an outer ring member joining two halves of the outer material and / or to provide a means to grasp or shape the insert. The coaster article may further include a base member to hold the removable insert and protect the underlying surface from excess moisture.
Owner:CLEAVER CANDY J +1
Aerosolisation engine for liquid drug delivery background
InactiveUS20170143915A1Minimise amountReduces kinetic energySpray nozzlesMedical devicesDrugSpray nozzle
A spray device for generating an aerosol of a liquid such as a medicament. The device includes a perforate element comprising one or more nozzles, each nozzle having an inlet and an outlet. A drive mechanism causes, in use, liquid to be driven through the one or more nozzles, thereby forming a liquid spray having one or more streams of liquid. At least one impaction surface is provided onto which, in use, the liquid impacts, the impaction surface being located downstream of the nozzle outlet(s).
Owner:THE TECHNOLOGY PARTNERSHIP PLC
Pharmaceutical composition, method of preparation and methods of treating aches/pains
Provided are methods and compositions useful for treating / aches and / or pains. The compositions comprise an herbal therapeutic agent and an analgesic agent, wherein the composition is effective when delivered to the mucosal membrane.
Owner:DESICA NICHOLAS +1
Biomedical foams
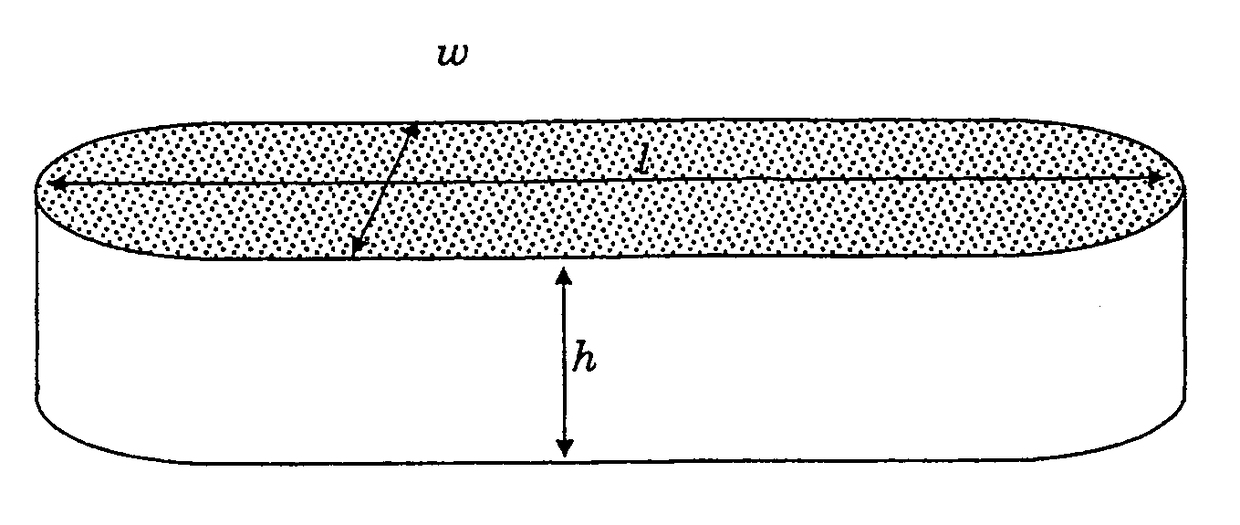
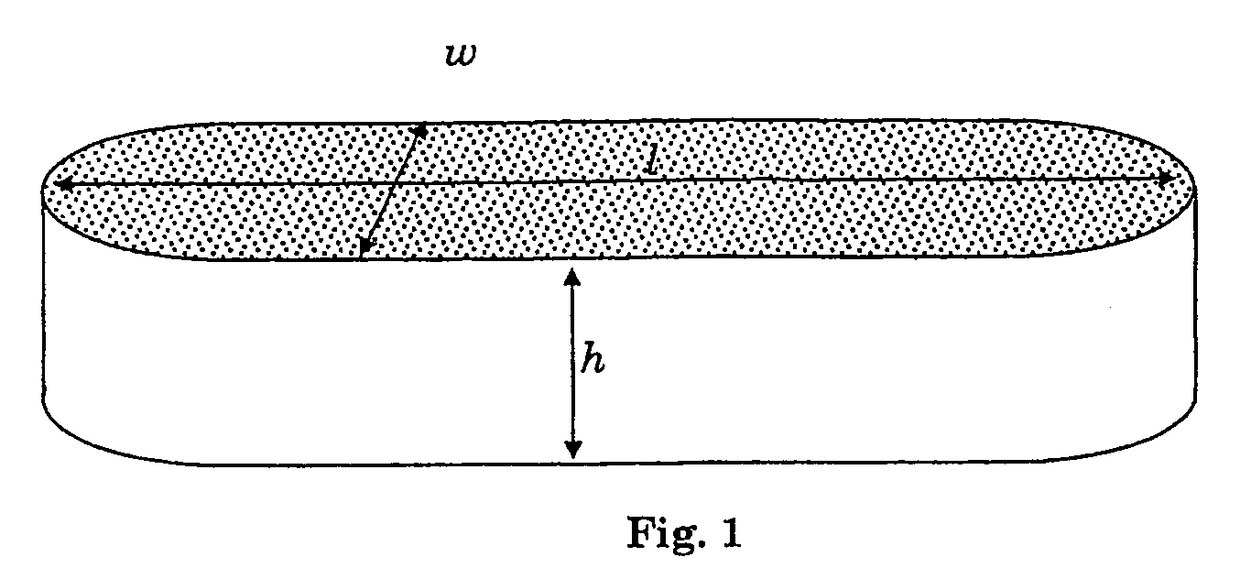
ActiveUS20150273102A1Reduce morbidityImprove mechanical propertiesImpression capsSurgical adhesivesPolyesterTamponade
The invention relates, generally, to porous absorbent materials which are suitable for packing antrums or other cavities of the human or animal body. More particularly, it relates to hydrophilic biodegradable foams, which may be used e.g. in the form of a plug or tampon, for instance for controlling bleeding, wound closure, prevent tissue adhesion and / or support tissue regeneration. The invention provides an absorbent foam, suitable for packing antrums or other cavities of the human or animal body, comprising a biodegradable synthetic polymer, which polymer preferably comprises —C(O)—O— groups in the backbone of the polymer, for instance polyurethane and / or polyester units combined with polyethers.
Owner:STRYKER EURO OPERATIONS HLDG LLC
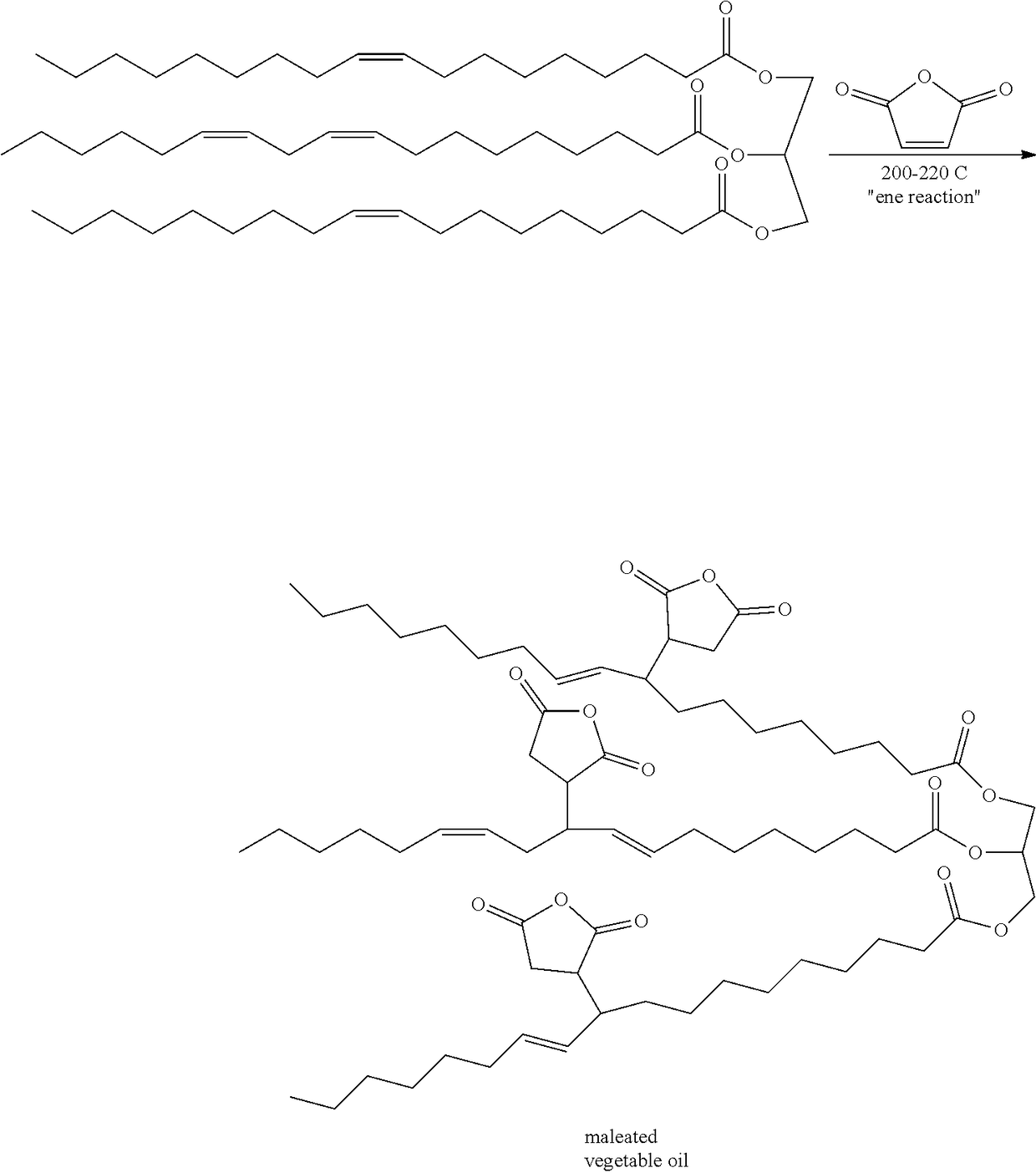
Maleated Natural Oil Derivatives as Agrochemical Inert Ingredients
The present disclosure provides an adjuvant composition that includes a maleated natural oil derivative. The adjuvant composition may be incorporated into agrochemical formulations and applied to target substrates to kill, inhibit, or repel pests.
Owner:INDORAMA VENTURES OXIDES LLC
Absorbent composition and extended use pet litter
InactiveUS20060124069A1Promote absorptionReduce maintenanceOther chemical processesAnimal housingLitterChemistry
Owner:IAMS
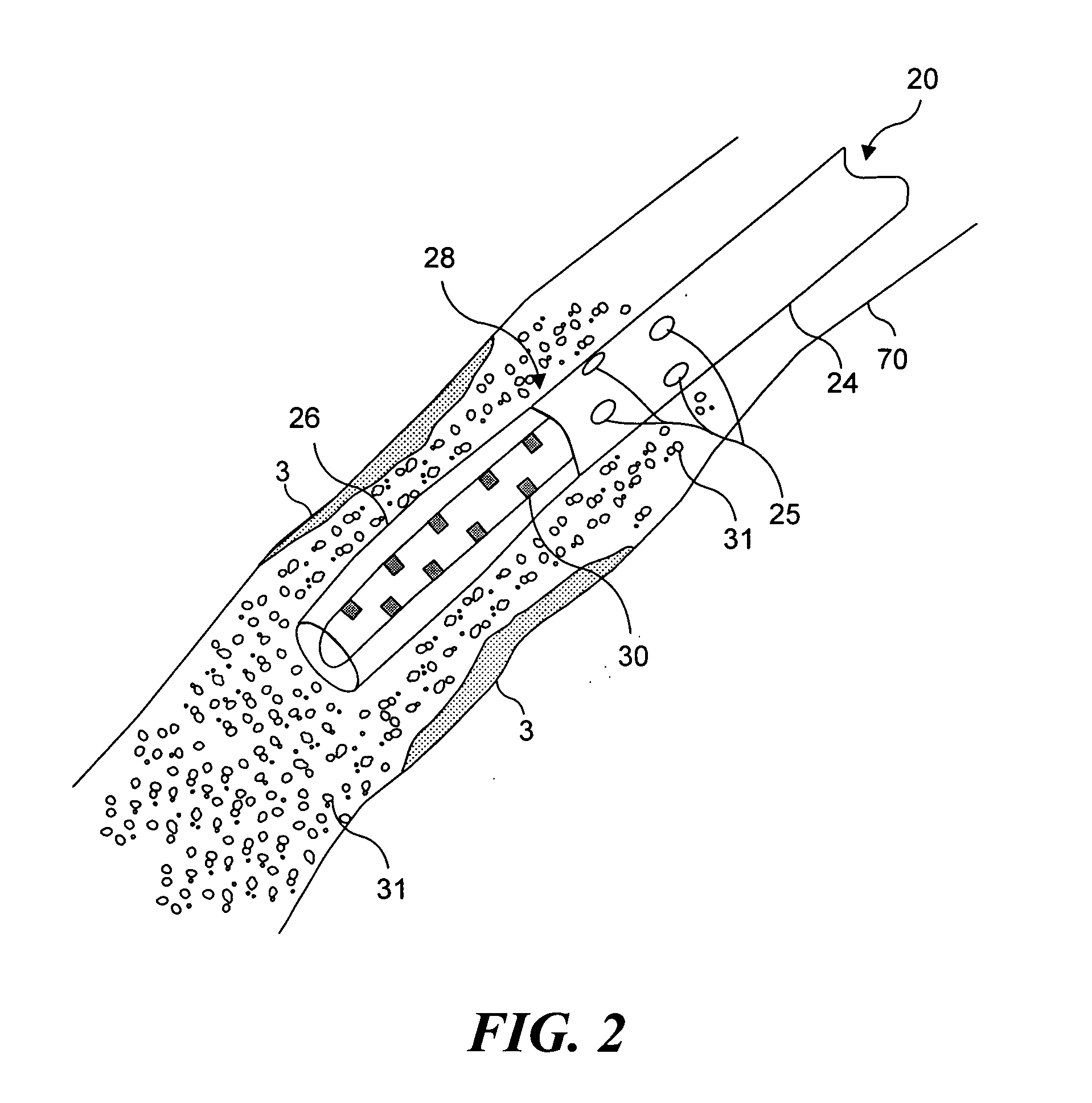
Light generating device that self centers within a lumen to render photodynamic therapy
InactiveUS20050128742A1Rapid uptakeUptake of the photoreactive agent into the target tisBalloon catheterElectric lightingBiomedical engineeringLight source
A light generating device for illuminating tissue adjacent to a body lumen while a distal end of the device is centered in the lumen, to render photodynamic therapy. The device can either occlude or displace bodily fluid, both without the use of a balloon. In one embodiment, a flushing lumen has a port adjacent to an array of light sources, to displace bodily fluid that might otherwise absorb light. Another embodiment employs a centering member that moves between a first position and a second position. The centering member centers the device in the lumen and preferably is formed of a shape memory material. In yet another embodiment, the device includes an outer sheath and an inner member that are independently positionable, enabling the centering member to be selectively positionable. The centering member can be non porous, such that the centering member also occludes fluid flow.
Owner:LIGHT SCI CORP
Pharmaceutical composition, method of preparation and methods of treating aches/pains
Provided are methods and compositions useful for treating / aches and / or pains. The compositions comprise an herbal therapeutic agent and an analgesic agent, wherein the composition is effective when delivered to the mucosal membrane.
Owner:DESICA NICHOLAS +1
Absorbent composition and extended use pet litter
InactiveUS7059273B2Promote absorptionReduce maintenanceOther chemical processesAnimal housingLitterChemistry
The invention provides compositions for use as pet litters and pet bedding, or for absorption of liquids and gases. These compositions are formed as aggregates or agglomerates of different components having different hydration capacities and hydration rates.
Owner:THE PROCTER & GAMBLE COMPANY +1
Biomedical foams
ActiveUS9610377B2Reduce morbidityImprove mechanical propertiesImpression capsSurgical adhesivesPolyesterAbsorbent material
Owner:STRYKER EURO OPERATIONS HLDG LLC
Insulin formulations for rapid uptake
ActiveUS9060927B2Improve stabilityQuick effectOrganic active ingredientsPeptide/protein ingredientsDissolutionInsulin humulin
Injectable insulin formulations with improved stability and rapid onset of action are described herein. The formulations may be for subcutaneous, intradermal or intramuscular administration. In the preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid or sodium citrate. These formulations are rapidly absorbed into the blood stream when administered by subcutaneous injection. In the preferred embodiment, the insulin is provided as a clear liquid, neutral pH, in a multi-use sterile vial. In an alternative embodiment, the insulin is provided as a powder in a sterile vial. This is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water, a zinc chelator such as EDTA and a dissolution agent such as citric acid shortly before or at the time of administration. In another embodiment, the insulin is stored as a frozen mixture, ready for use upon thawing.
Owner:ELI LILLY & CO
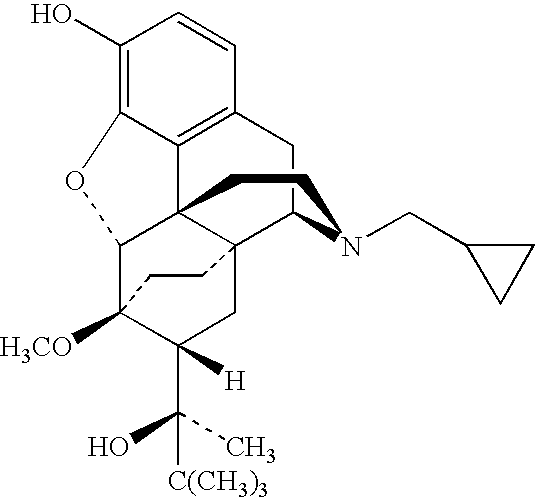
Cis-tetrahydro-spiro(cyclohexane-1, 1' -pyrido[3,4-b]indole)-4-amine Compounds
InactiveUS20160159787A1Impair pain sensationImproved profileBiocideNervous disorderChronic painVisceral pain
Cis-tetrahydro-spiro(cyclohexane-1,1′-pyrido[3,4-b]indole)-4-amine compounds which act on the nociceptin / ORL-1 receptor system as well as on the μ-opioid receptor system and which are distinguished in particular by selective effectiveness in the treatment of chronic pain, such as inflammatory pain, visceral pain, tumour pain, and neuropathic pain, without at the same time developing pronounced effectiveness against acute, nociceptive pain.
Owner:GRUNENTHAL GMBH
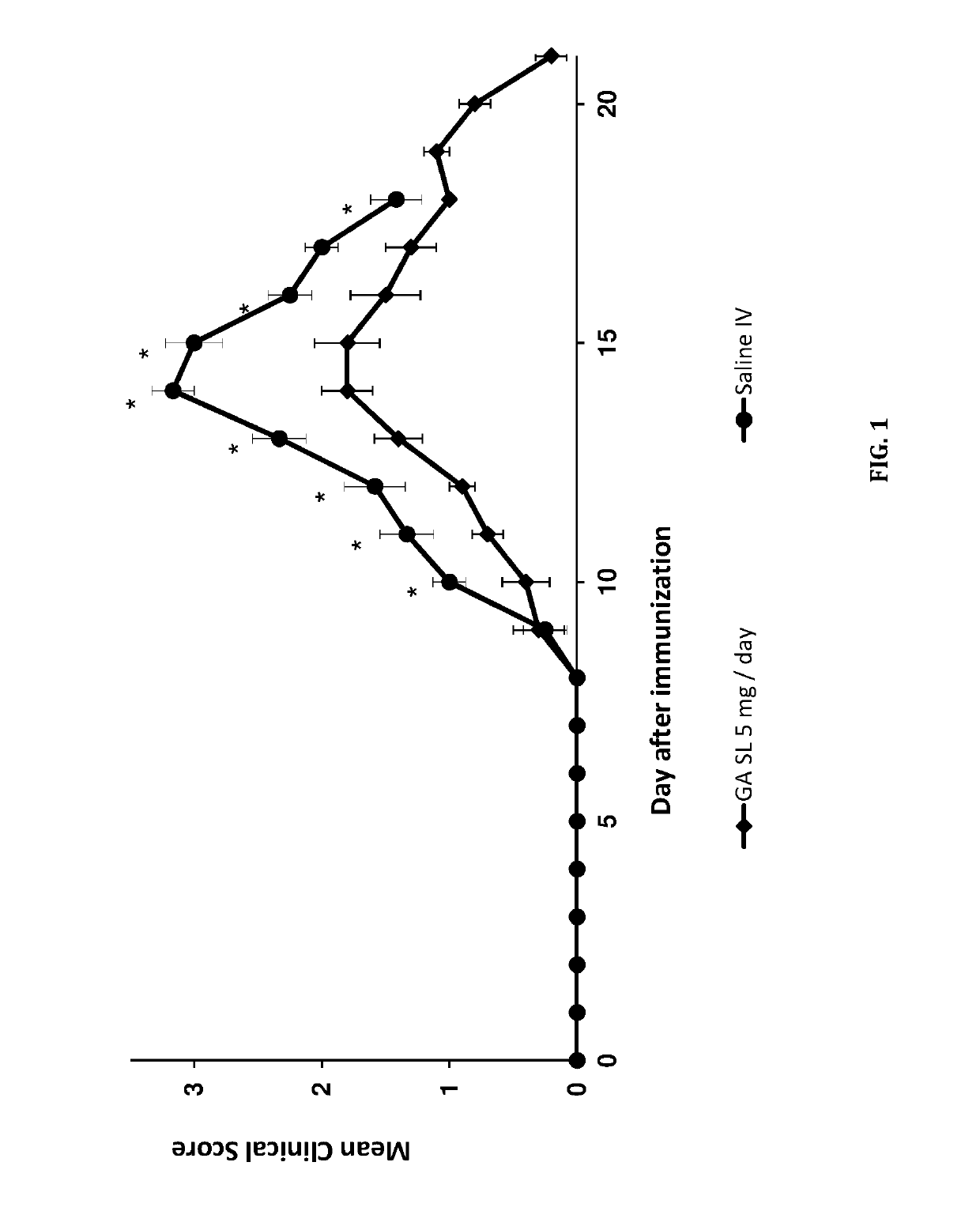
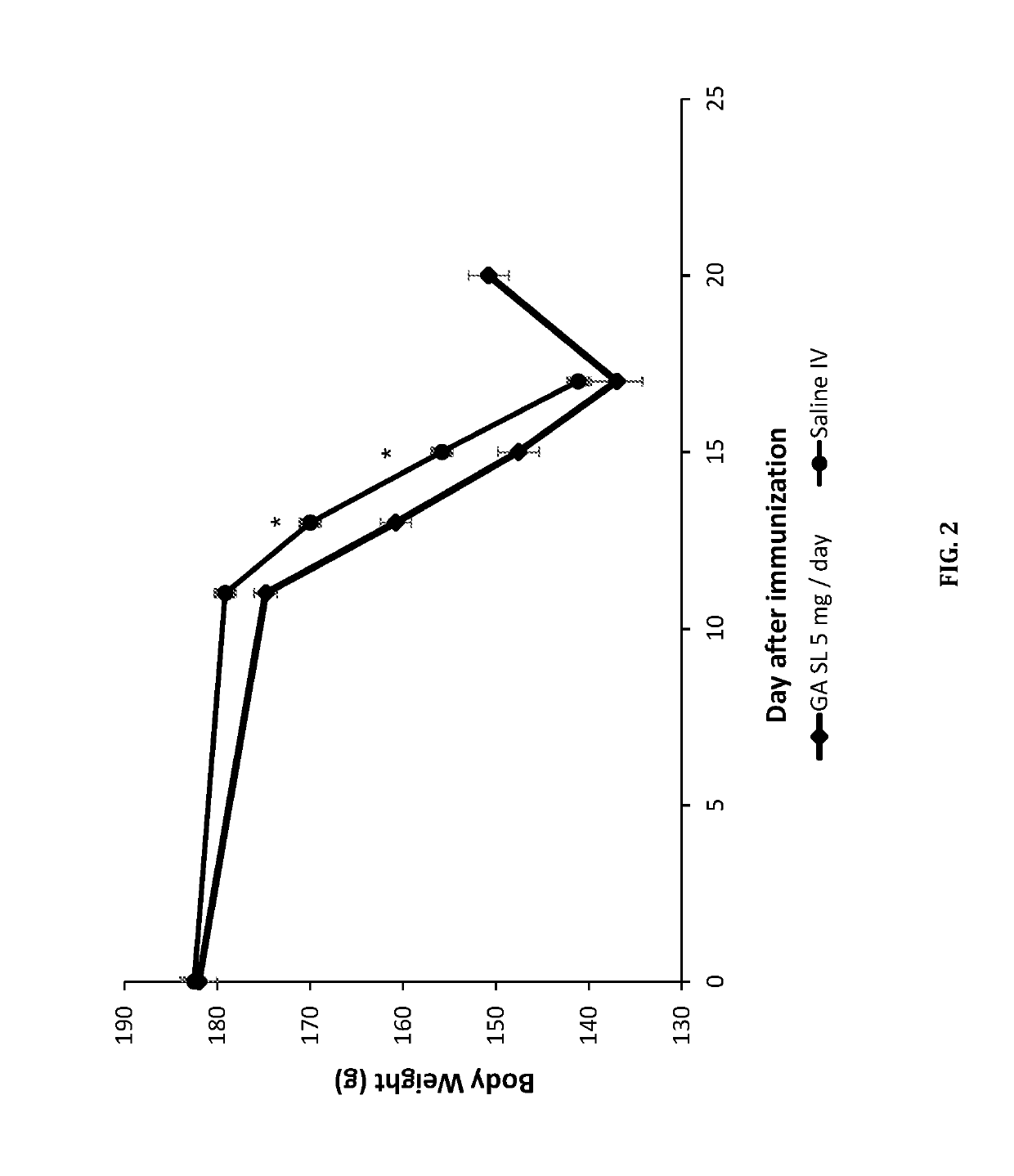
Sublingual delivery of glatiramer acetate
ActiveUS10493122B2Rapid uptakeQuick responsePeptide/protein ingredientsAerosol deliveryEthylic acidBiochemistry
Owner:MAPI PHARMA
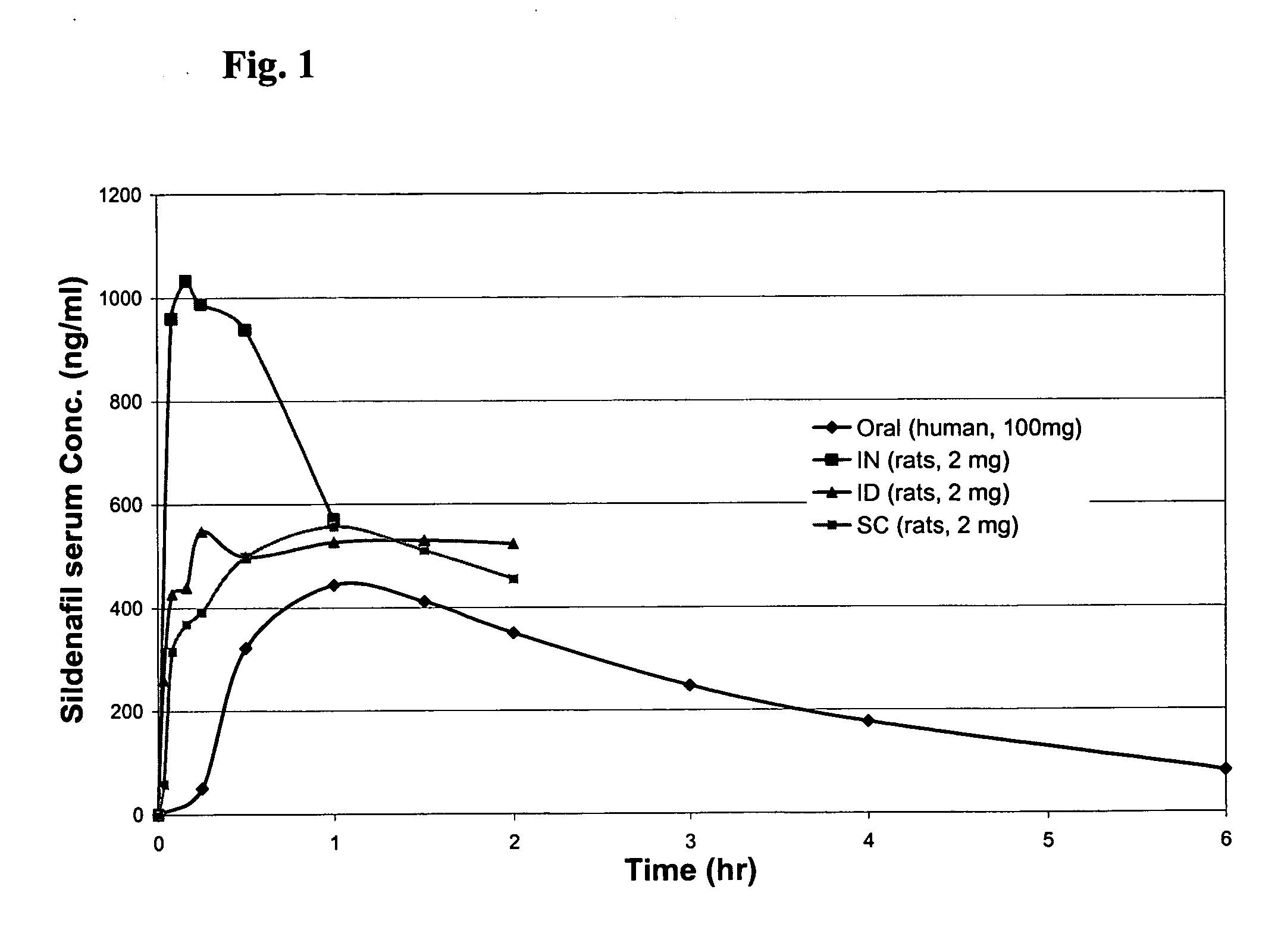
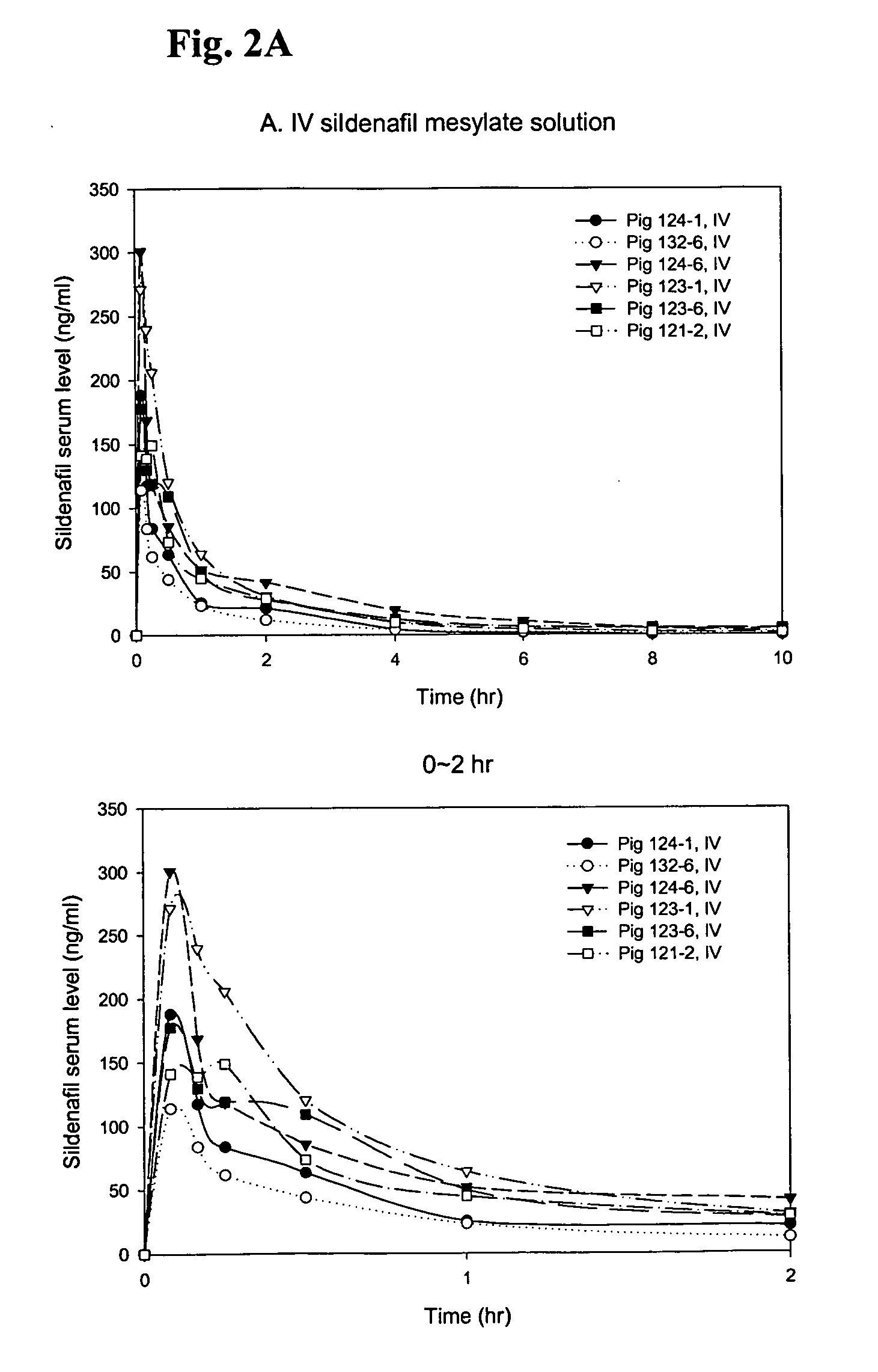
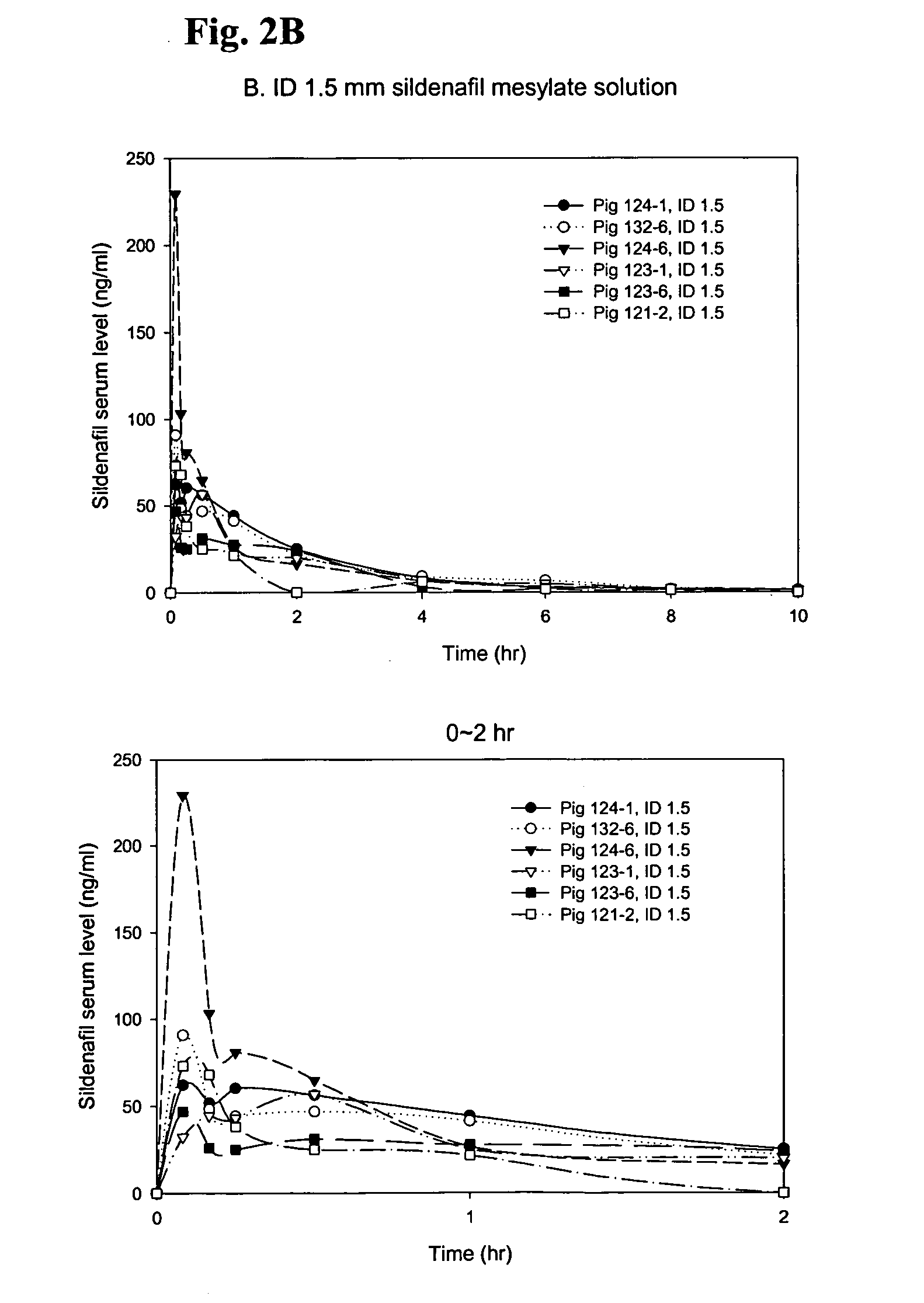
Formulations of phosphodiesterase 5 inhibitors and methods of use
InactiveUS20060035905A1Improve concentrationImprove solubilityOrganic active ingredientsLyophilised deliveryVardenafilPhosphodiesterase 5 inhibitor
The present invention provides compositions and delivery methods to enhance treatment for sexual dysfunction or hypofunction through the delivery of phosphodiesterase 5 inhibitors to a mammal. Phosphodiesterase 5 inhibitors are one example of a compound class used for this indication. Examples of compounds in this class include taldalafil and vardenafil and sildenafil. Furthermore, the present invention provides compositions and delivery methods to enhance the sildenafil concentration in solution, suspension and gel formulations and methods of parenteral, intradermal, sublingual, intranasal, and buccal sildenafil delivery.
Owner:BECTON DICKINSON & CO
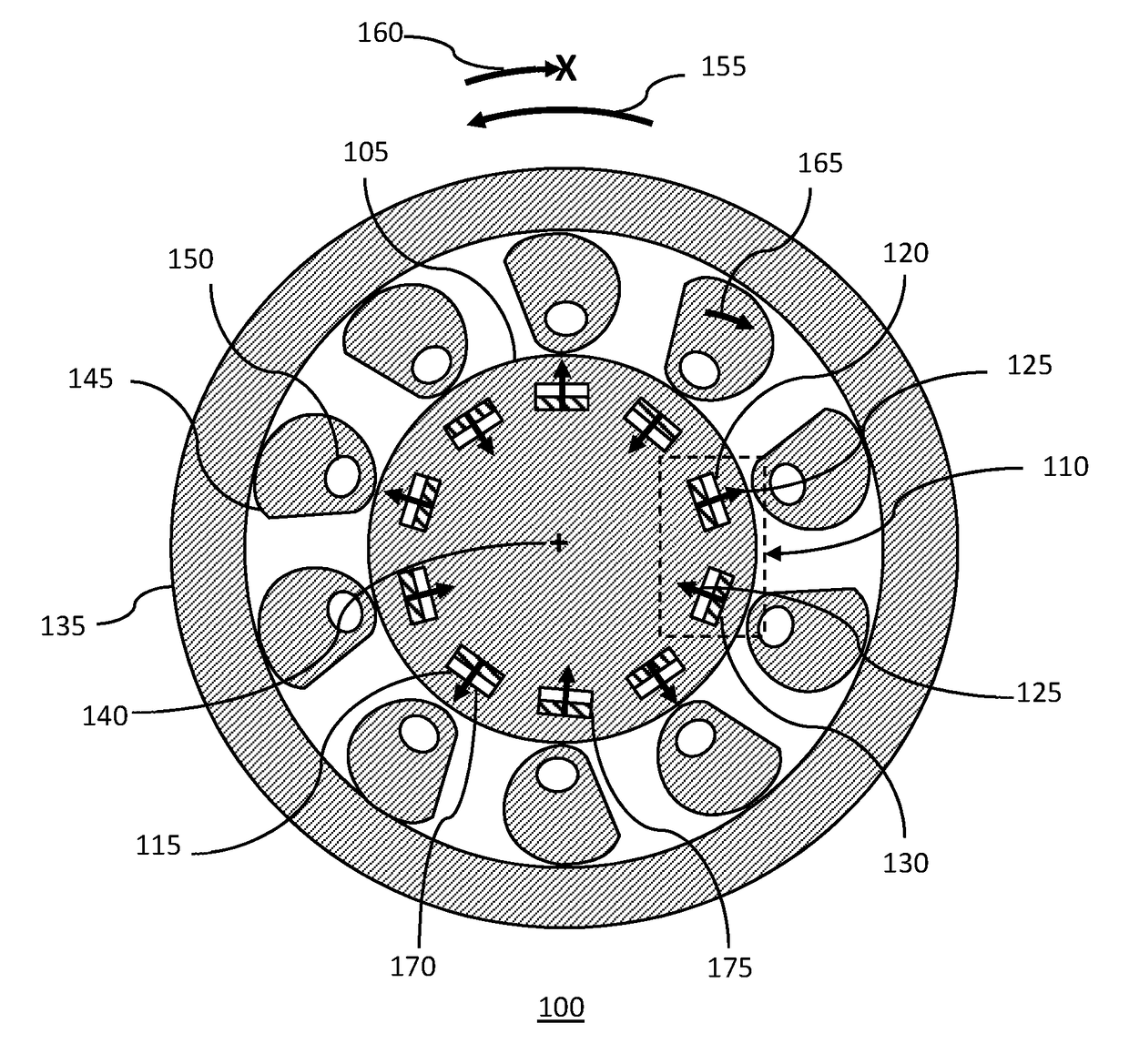
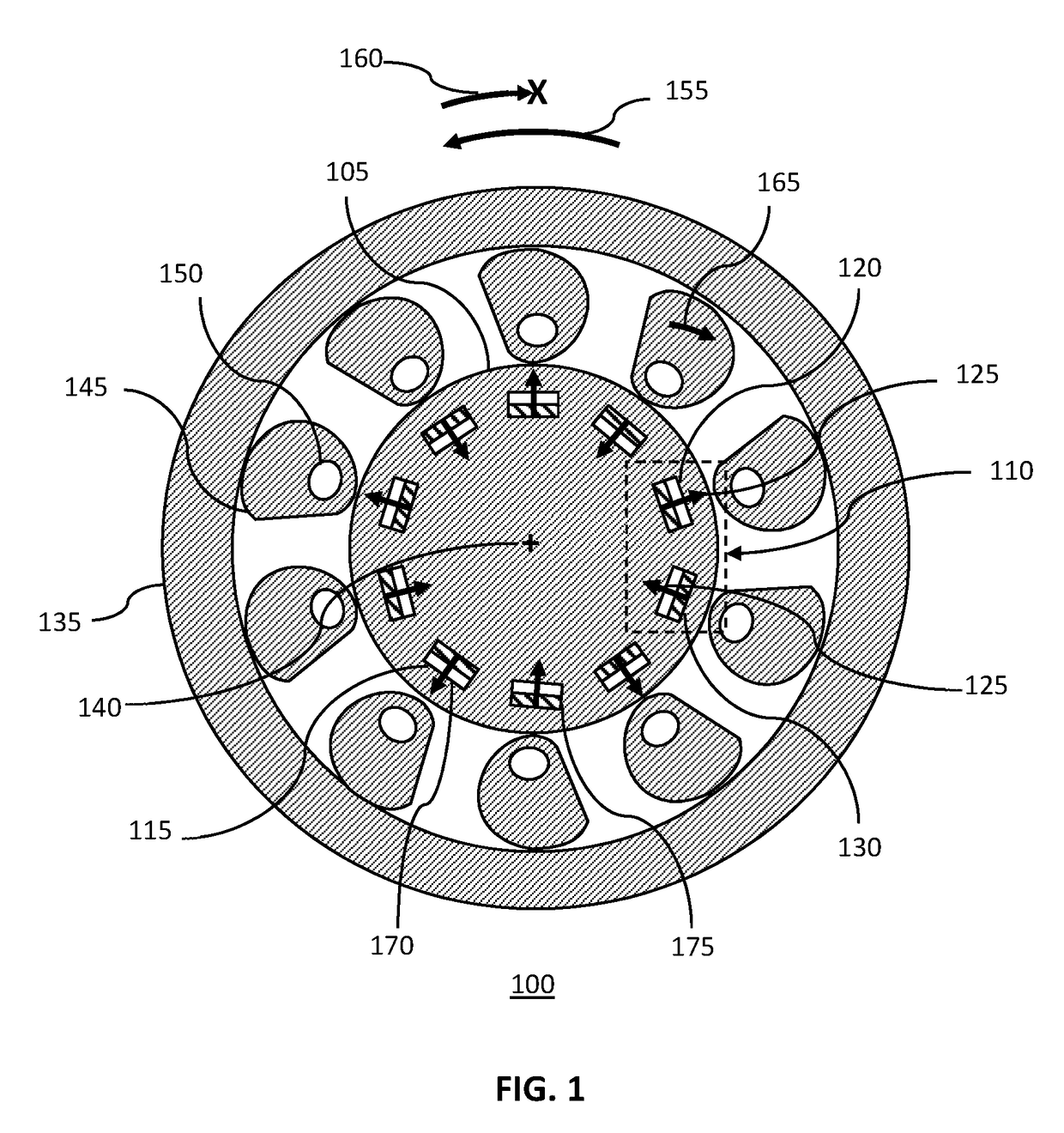
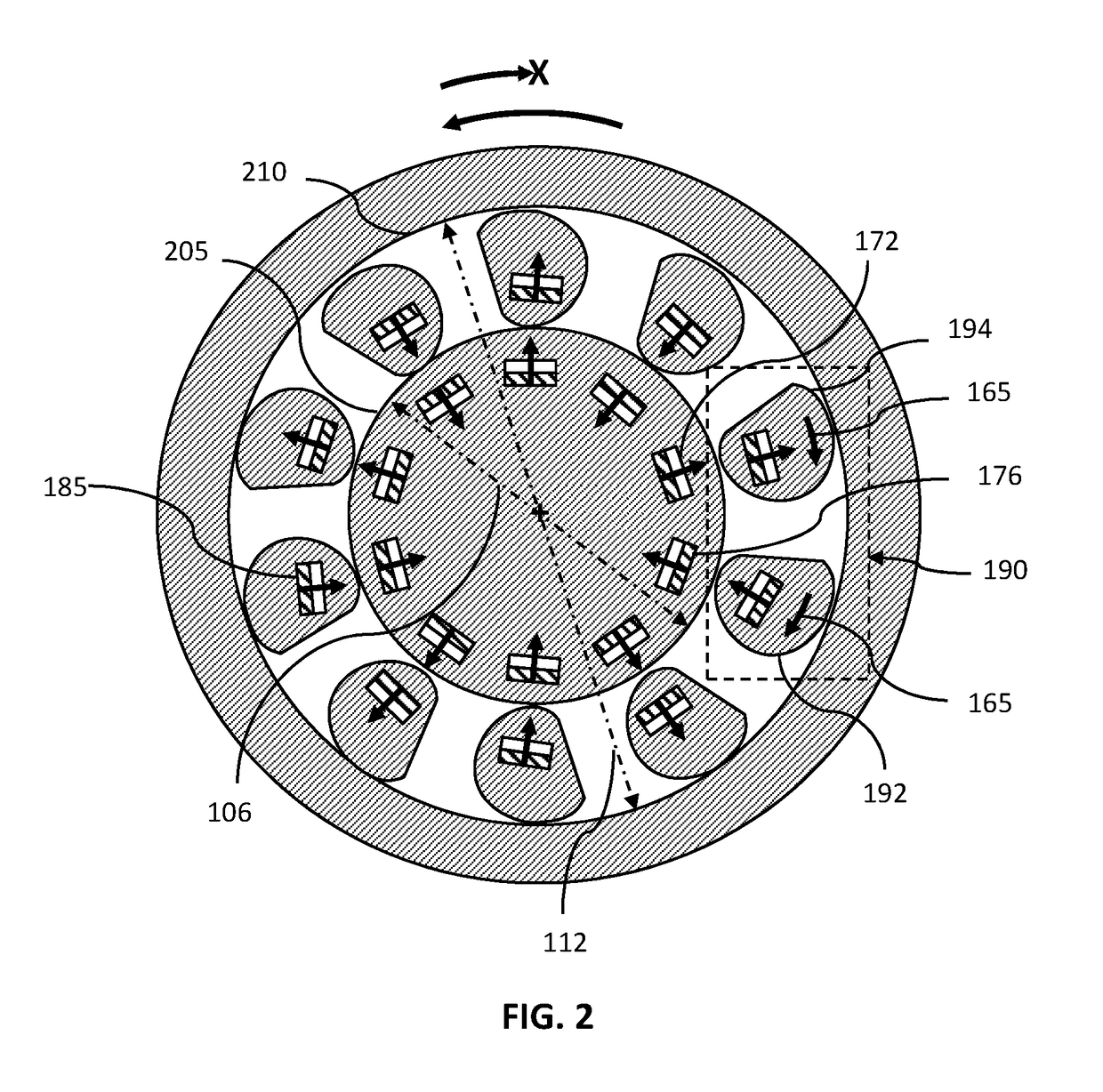
Magnetically Hinged Overrunning Clutch
InactiveUS20170343059A1Simple and effective and robust overrunningAvoid complex processMagnetically actuated clutchesFreewheel clutchesRare earthClutch
A magnetically hinged, overrunning clutch is disclosed. Sprags containing rare-earth permanent magnets, and arranged in pairs of opposite magnetic orientation, are located within the gap between the inner surface of a hollow, circularly cylindrical shaft and the external surface of a smaller diameter, second circularly cylindrical shaft. Pairs of rare-earth permanent magnets encircling the second cylindrical shaft are located at, or just beneath, the surface of the shaft and are arranged in pairs having alternating magnetic orientation. The sprags are cylinders having a pseudo-spiral cross-section and are sized, and the ferromagnetic region located, such that when the sprags are attracted to the shaft-magnets, the first shaft may be rotated with respect to the second shaft in a first, overrunning direction of rotation, but the first shaft does not rotate with respect to the second shaft in an opposite, or lock-up direction.
Owner:ROSSER SHEREE BERMAN
Adjuvant blend for pesticide formulations
InactiveUS20150181864A1Rapid uptakeHigh activityBiocidePharmaceutical non-active ingredientsAlcoholAdjuvant
The present invention provides an adjuvant blend that includes an alkyl ester obtained from the reaction of acidulated soap stock with a C3-C20 alcohol and a nonionic surfactant. The adjuvant blend may be incorporated into pesticide formulations and applied to target substrates to kill, inhibit, or repel pests.
Owner:HUNTSMAN PETROCHEMICAL LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

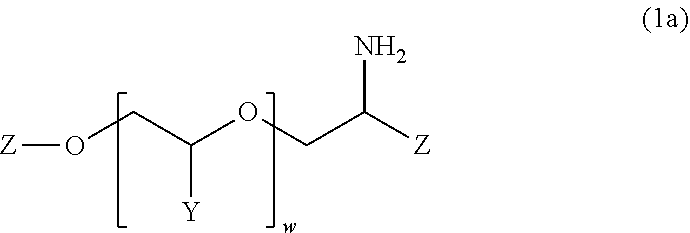
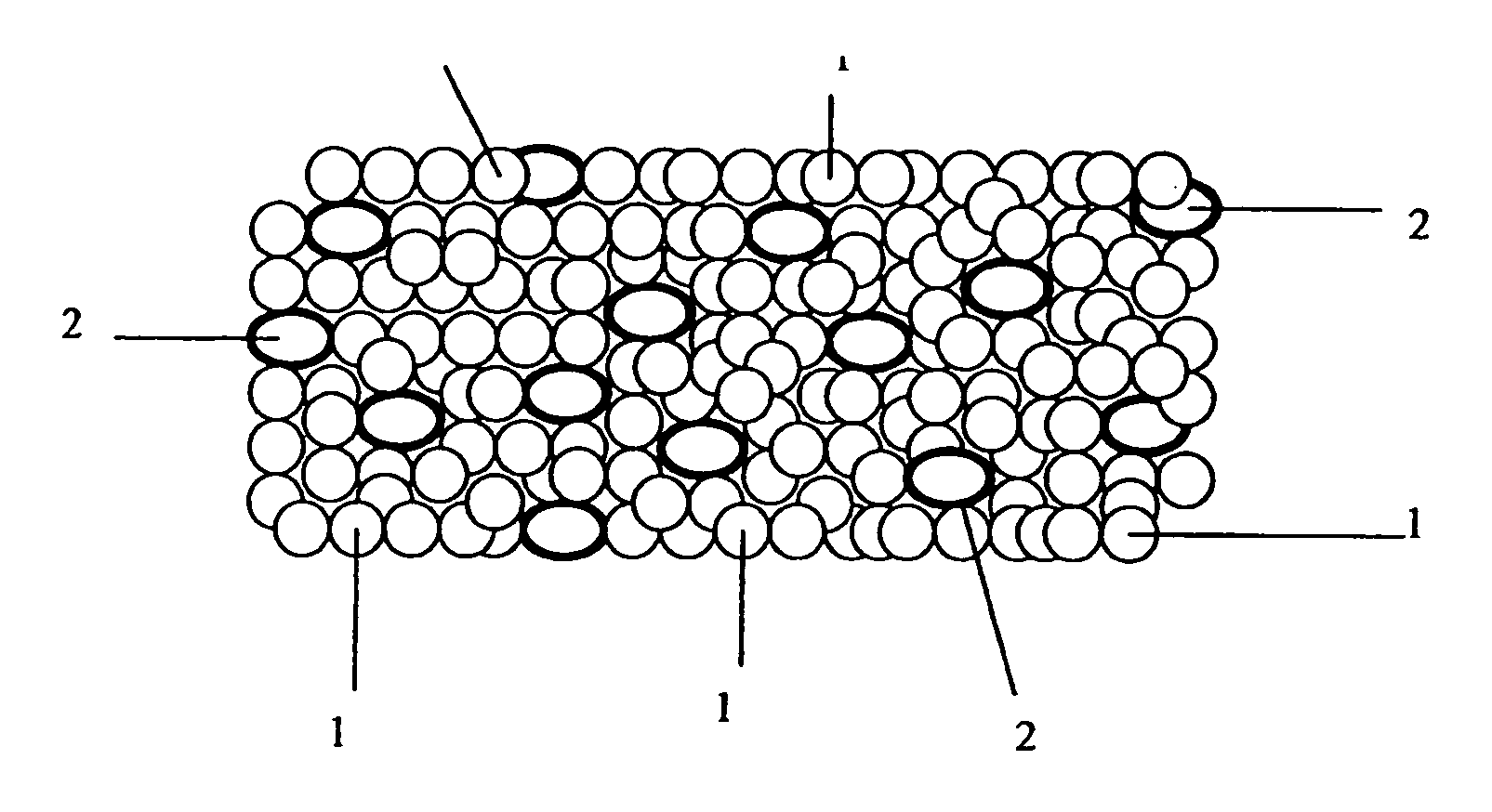
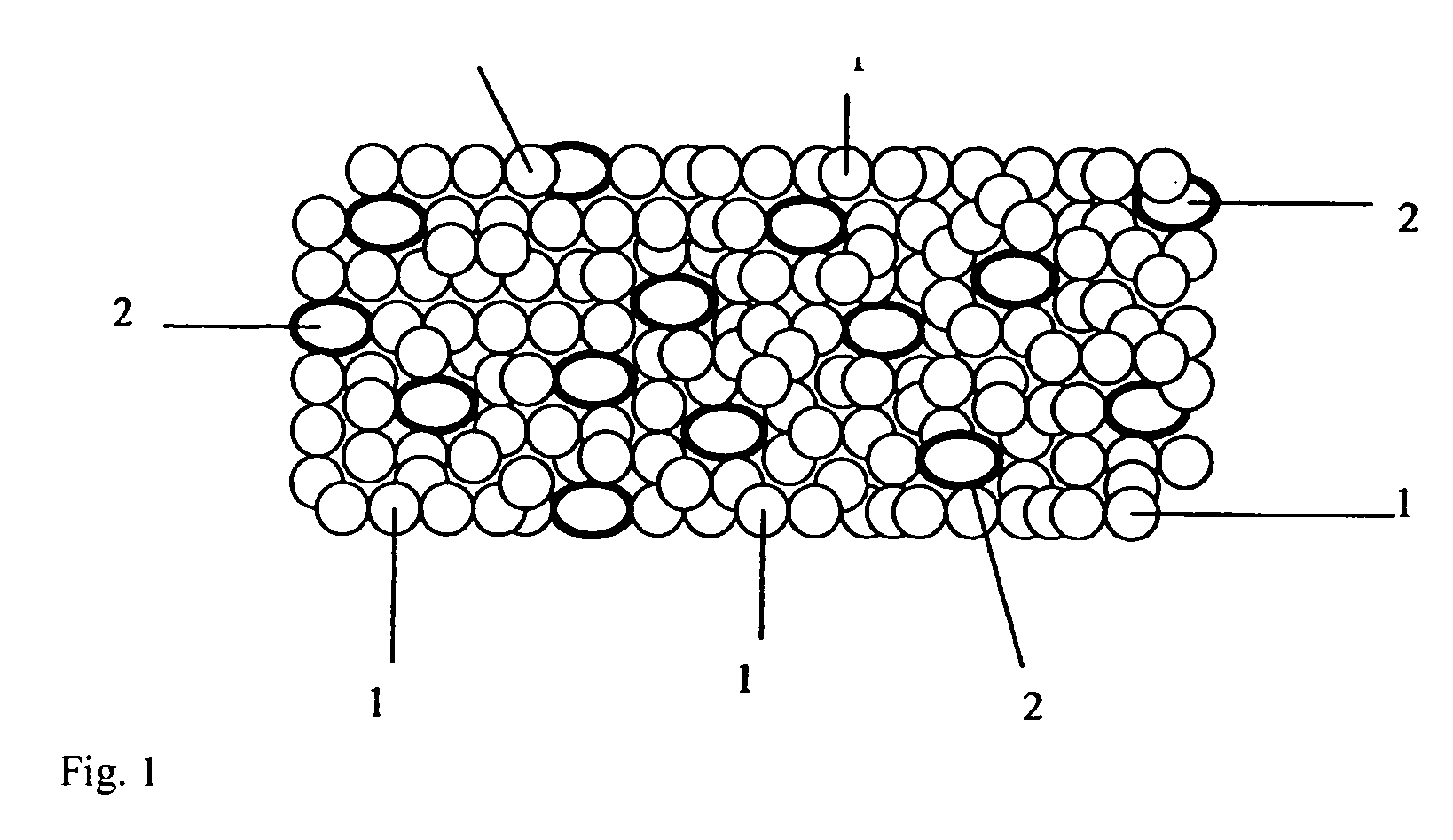
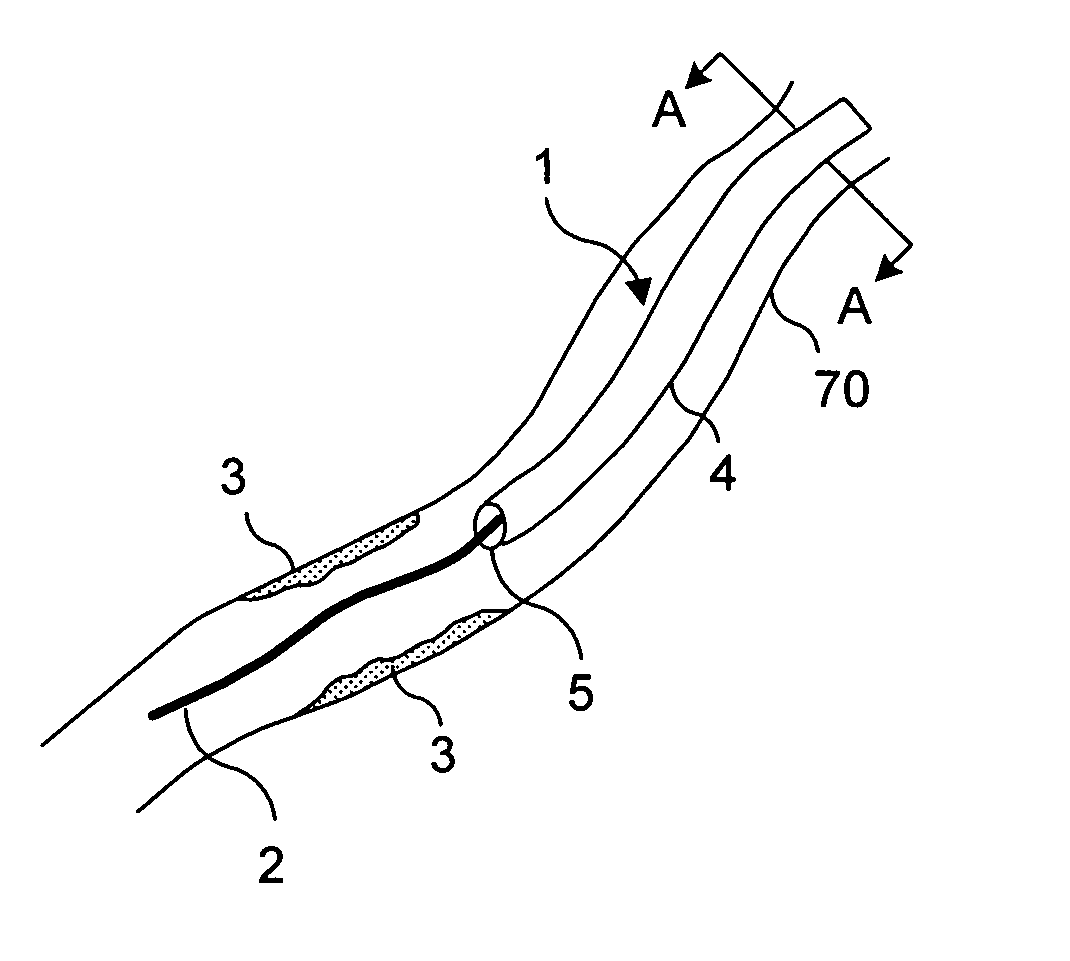
![Cis-tetrahydro-spiro(cyclohexane-1, 1' -pyrido[3,4-b]indole)-4-amine Compounds Cis-tetrahydro-spiro(cyclohexane-1, 1' -pyrido[3,4-b]indole)-4-amine Compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13f8d397-0497-41e7-a241-ba5781e87a1d/US20160159787A1-20160609-C00001.PNG)
![Cis-tetrahydro-spiro(cyclohexane-1, 1' -pyrido[3,4-b]indole)-4-amine Compounds Cis-tetrahydro-spiro(cyclohexane-1, 1' -pyrido[3,4-b]indole)-4-amine Compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13f8d397-0497-41e7-a241-ba5781e87a1d/US20160159787A1-20160609-C00002.PNG)
![Cis-tetrahydro-spiro(cyclohexane-1, 1' -pyrido[3,4-b]indole)-4-amine Compounds Cis-tetrahydro-spiro(cyclohexane-1, 1' -pyrido[3,4-b]indole)-4-amine Compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13f8d397-0497-41e7-a241-ba5781e87a1d/US20160159787A1-20160609-C00003.PNG)