Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
331 results about "Rapid onset" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Description. Rapid-onset dystonia parkinsonism is a rare movement disorder. "Rapid-onset" refers to the abrupt appearance of signs and symptoms over a period of hours to days. Dystonia is a condition characterized by involuntary, sustained muscle contractions. Parkinsonism can include tremors, unusually slow movement (bradykinesia), rigidity,...
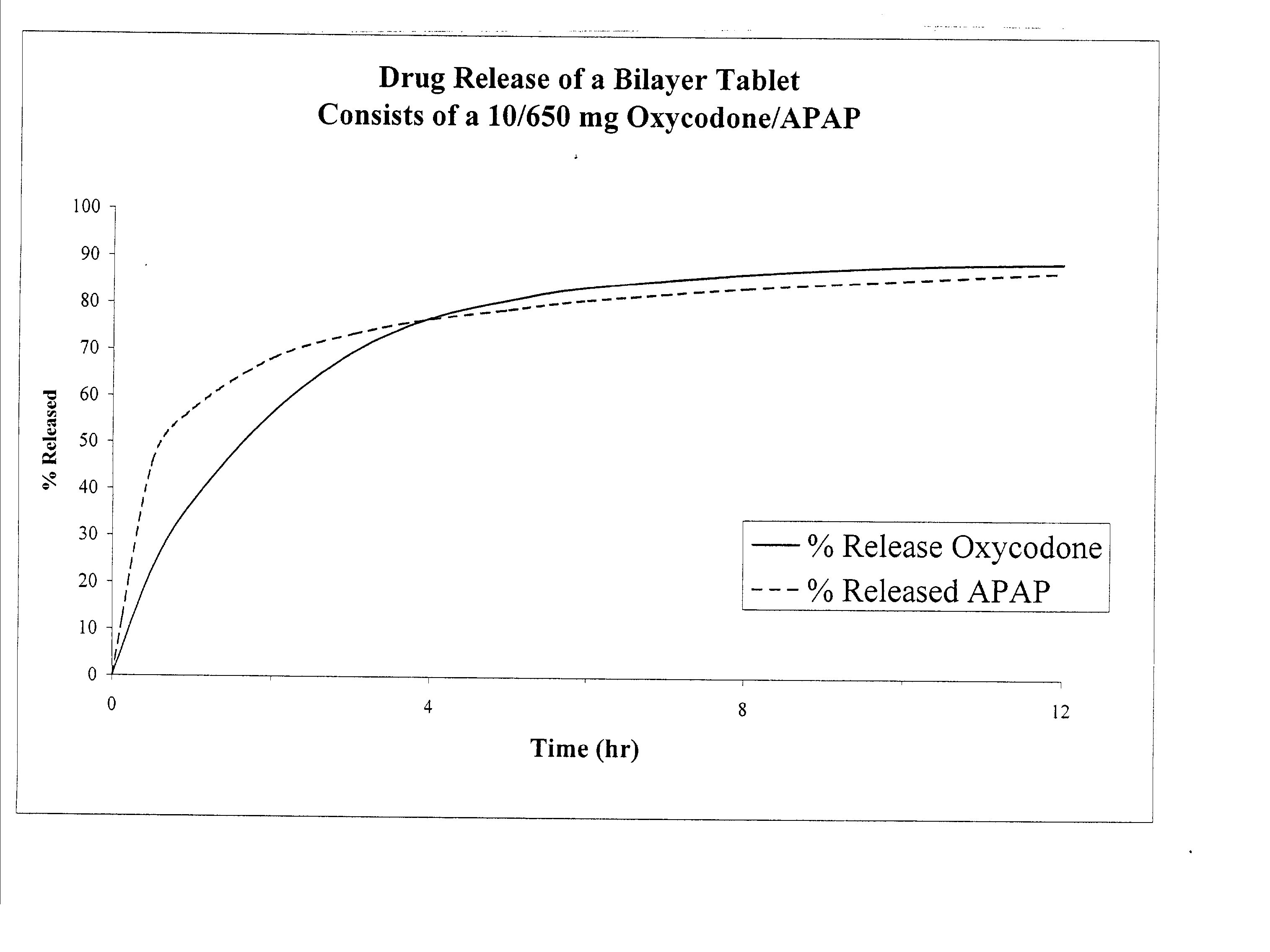
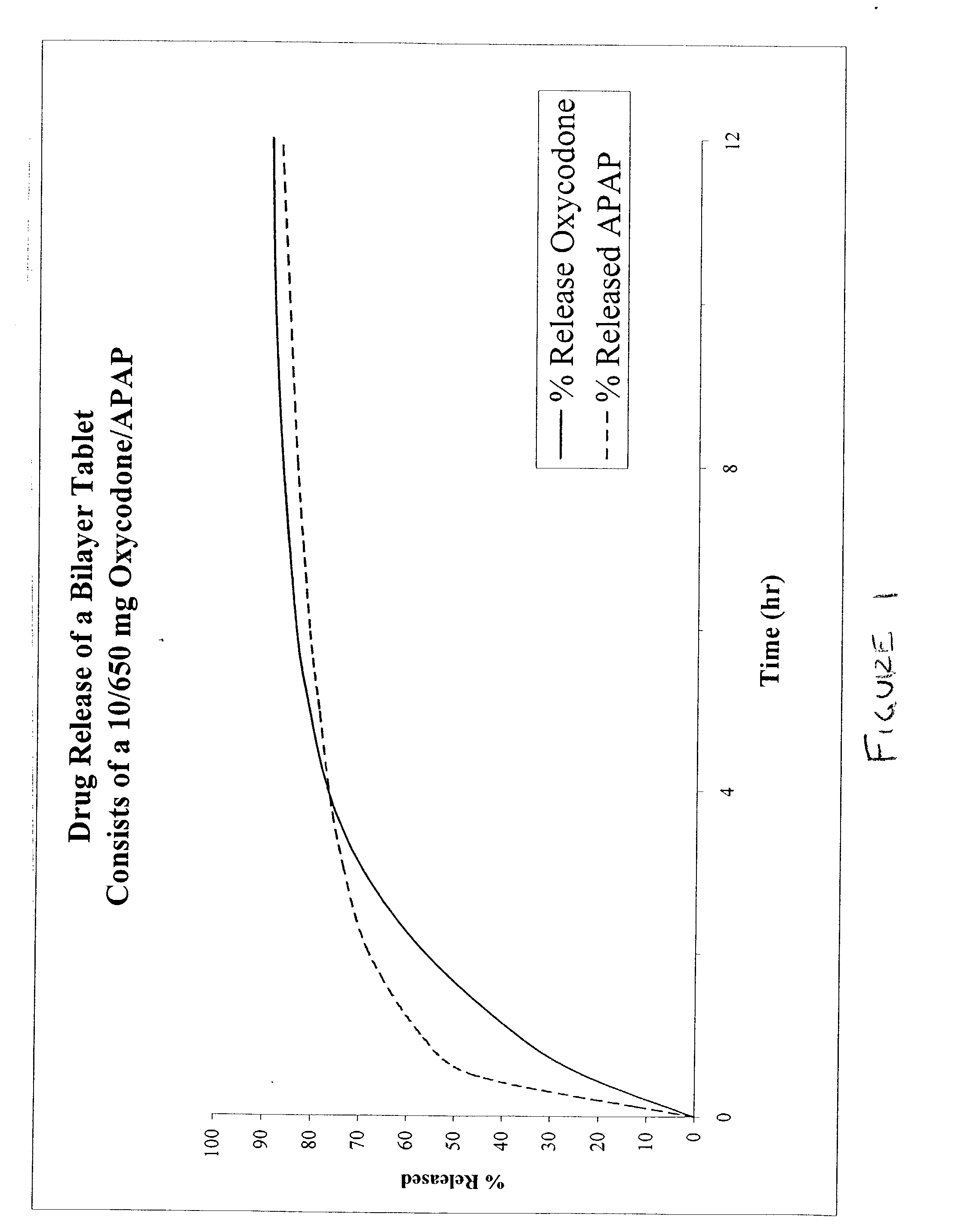
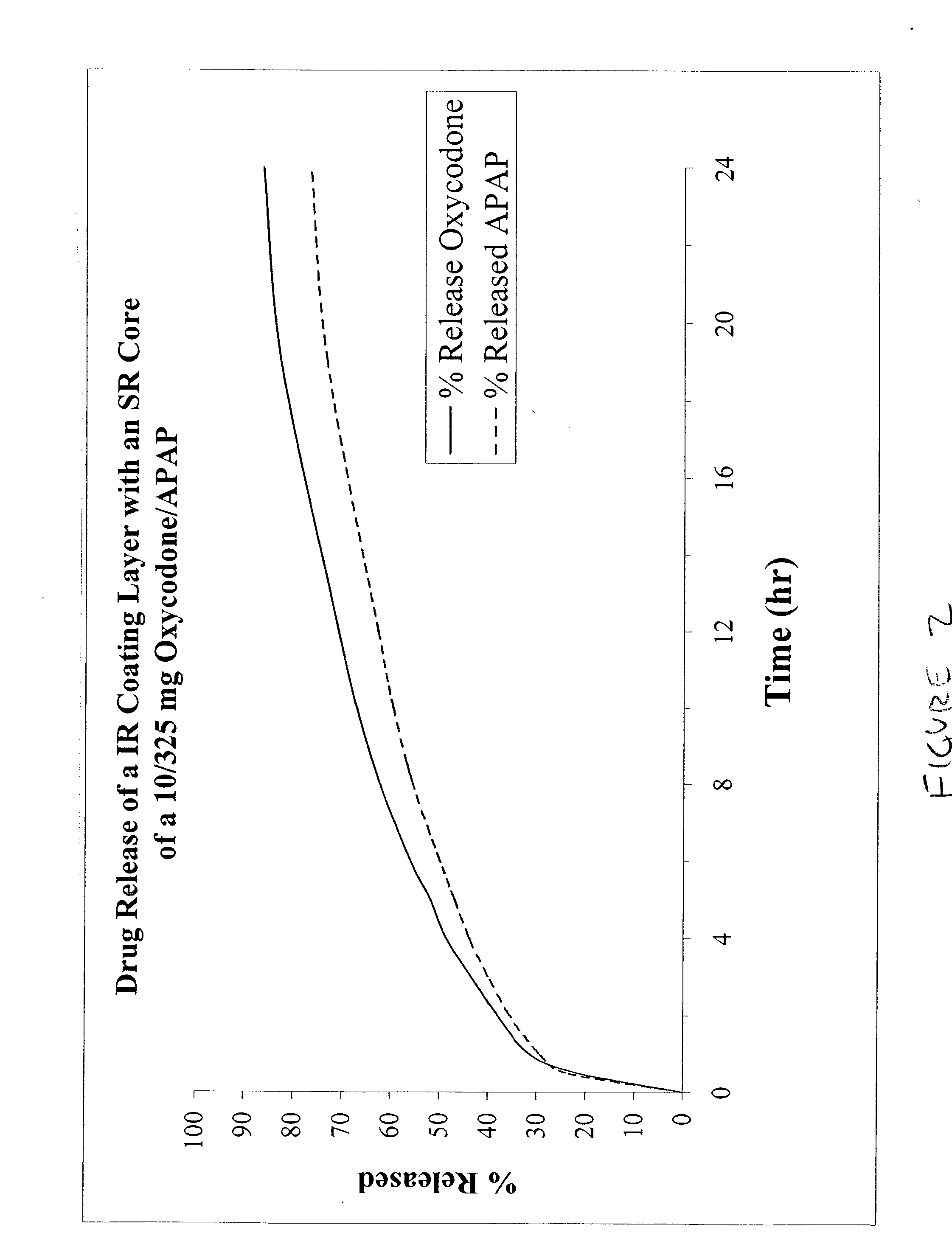
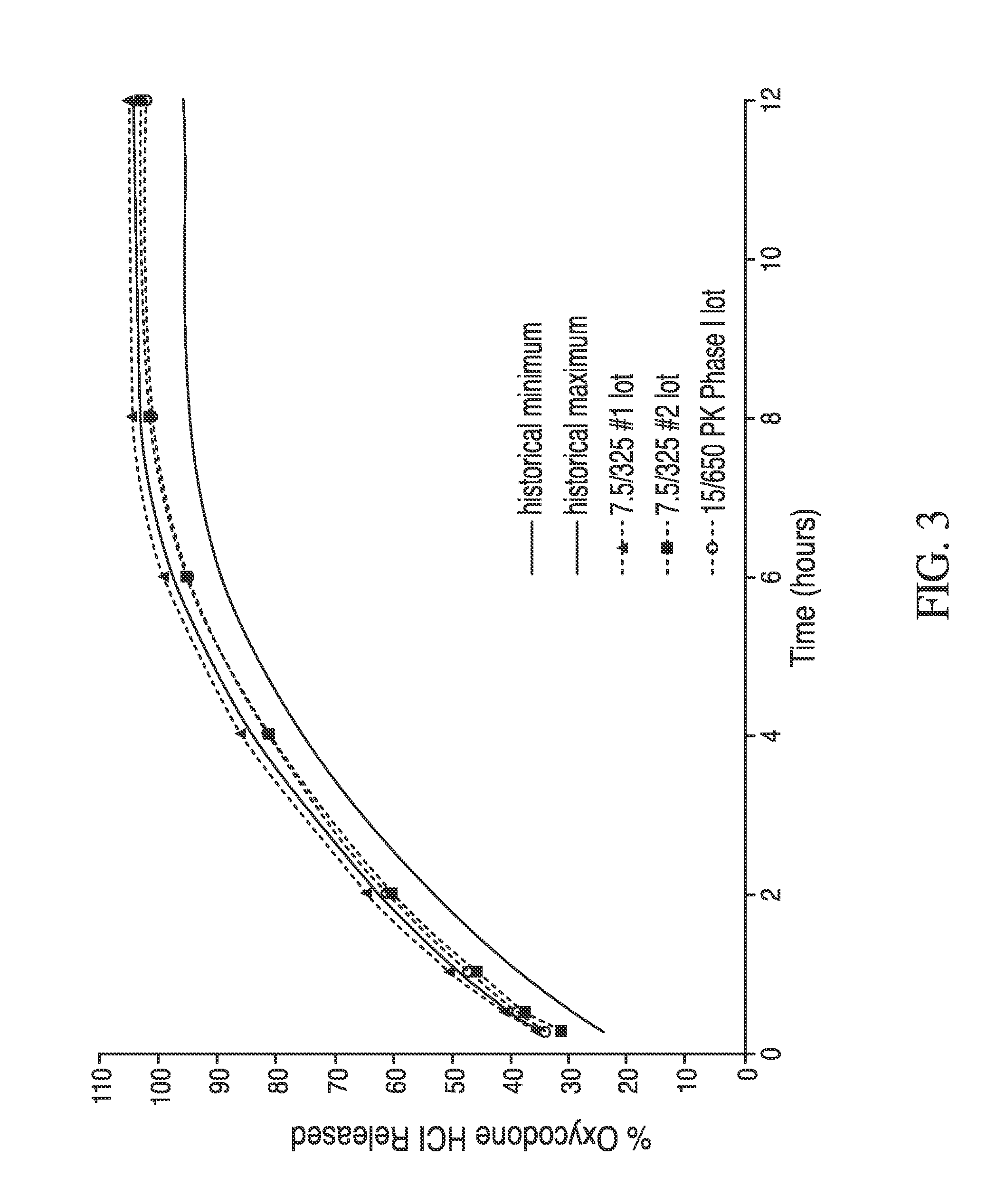
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
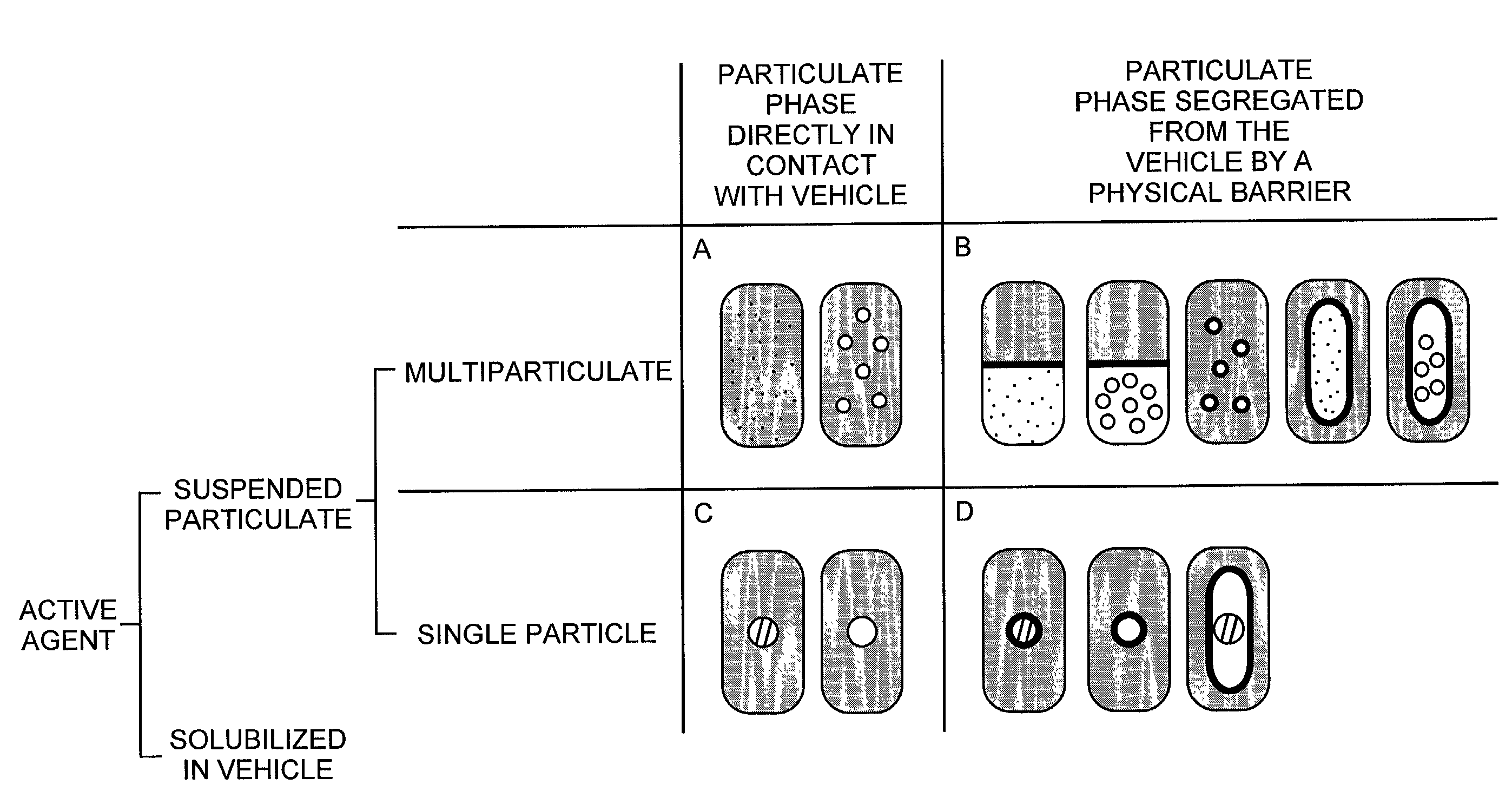
Pharmaceutical formulations and systems for improved absorption and multistage release of active agents
InactiveUS7374779B2Improve bioavailabilityLow variabilityPowder deliveryOrganic active ingredientsActive agentFast release
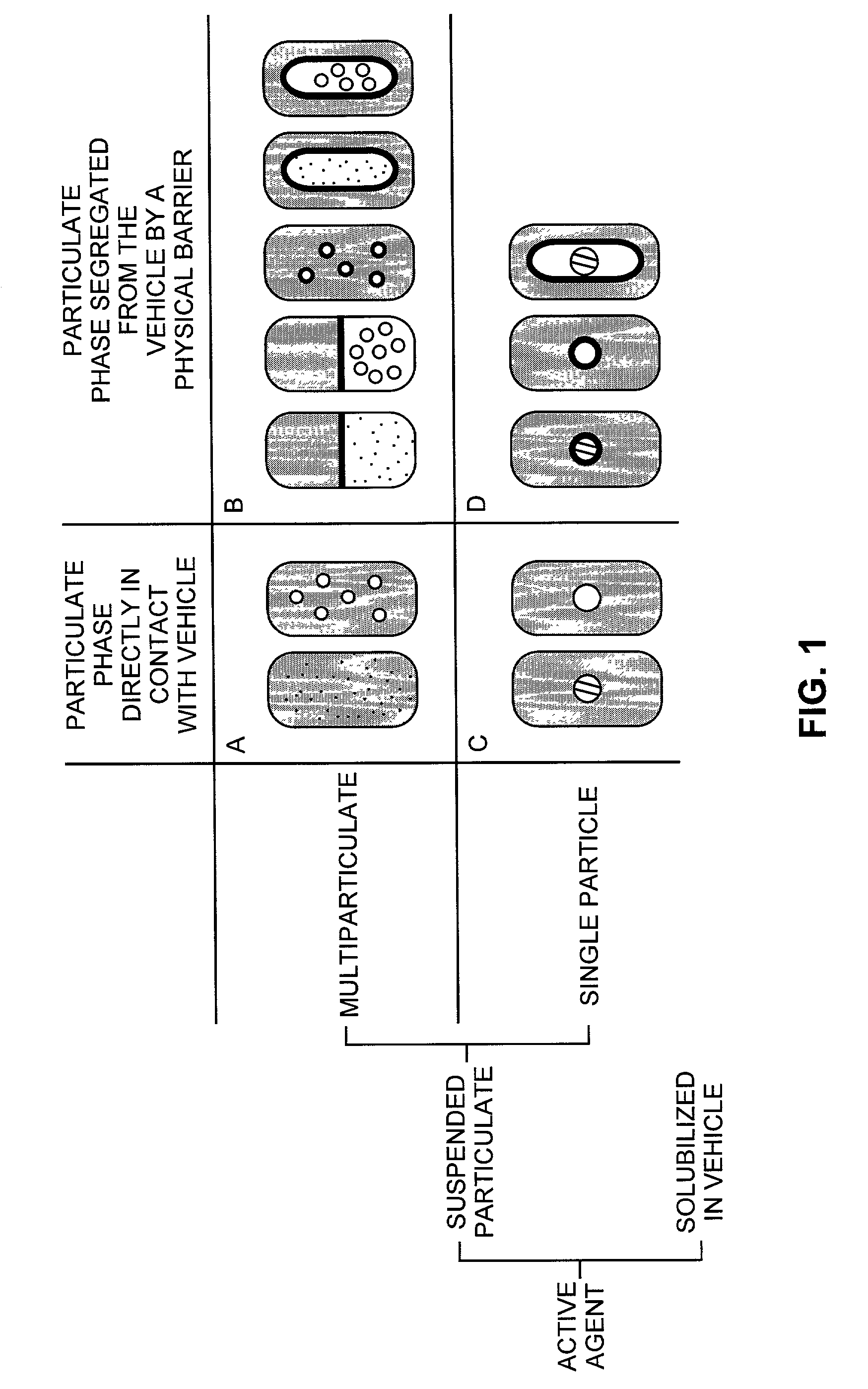
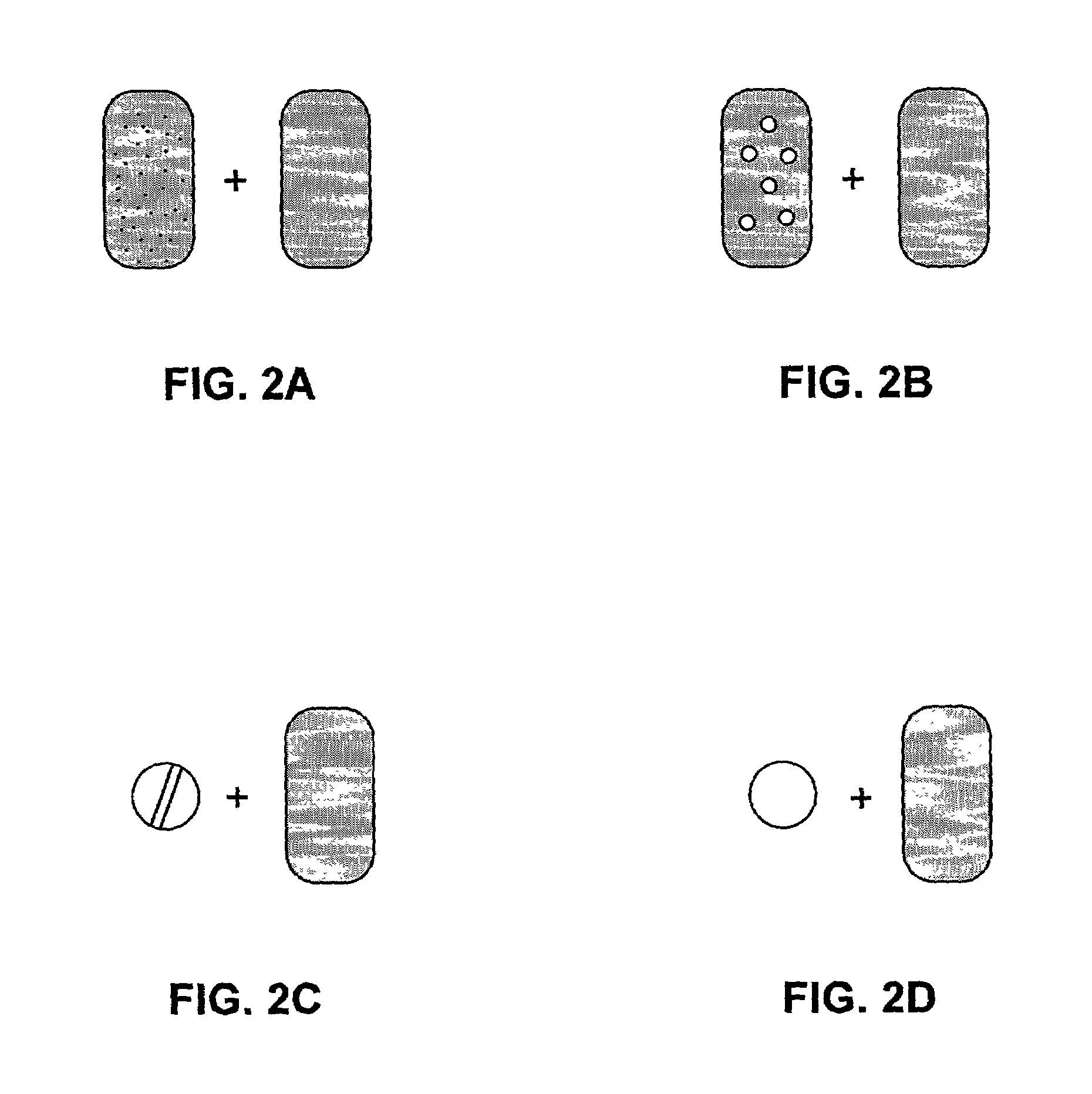
The present invention pertains to pharmaceutical formulations and systems for delivery of active agents, wherein a first fraction of an active agent is suspended in a vehicle and a second fraction of active agent is solubilized in the vehicle, with the suspended fraction representing about 5 wt. % to about 80 wt. % of the active agent and the second fraction representing about 20 wt. % to about 95 wt. % of the active agent. One or more additional active agents, which may be fully solubilized, partially solubilized, or suspended, may also be present. The first and second fractions of the active agent may or may not have different release profiles. Generally, a significant fraction of the solubilized drug will release rapidly, providing for rapid onset, while the suspended drug may be formulated for delayed and / or sustained release.
Owner:LIPOCINE
Rapid response system for the detection and treatment of cardiac events
InactiveUS6985771B2Minimize damageIncrease ratingsElectrotherapyElectrocardiographyRapid response systemHeart pacemakers
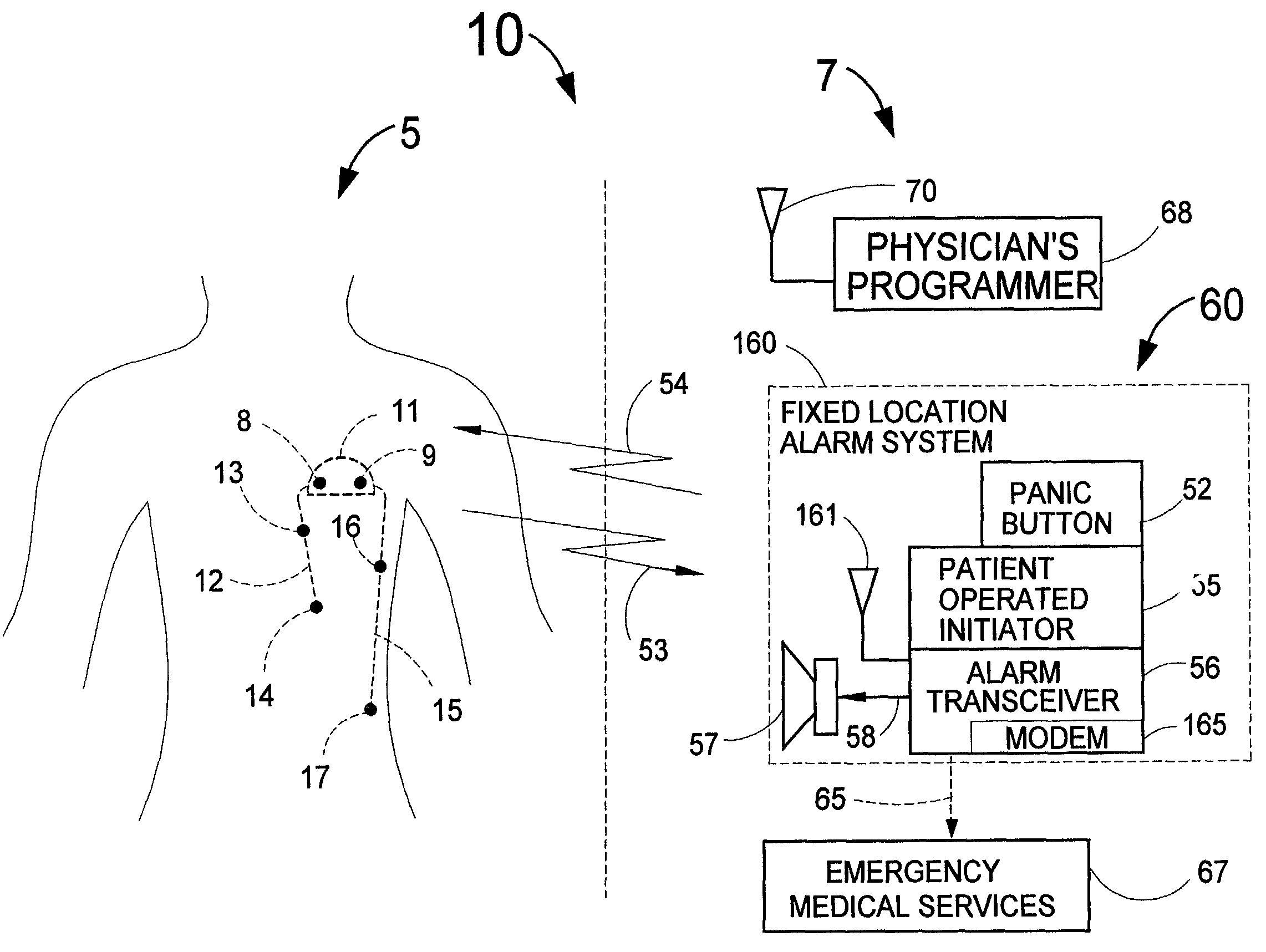
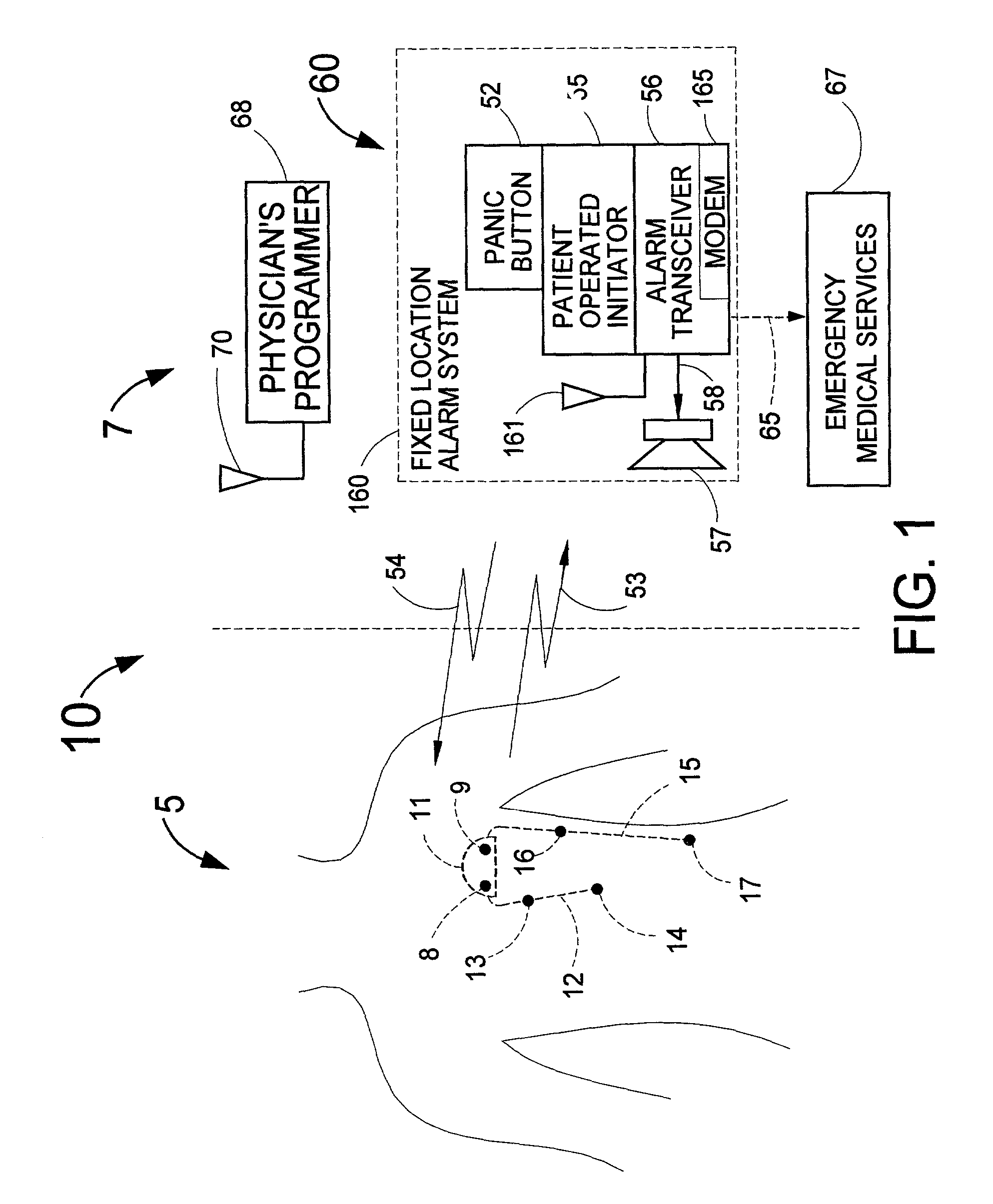
Disclosed is a system for detecting an acute myocardial infarction (i.e., a heart attack) at the earliest possible time and promptly warning the patient that he should immediately seek medical care. The present invention includes an implantable electronic system that can sense a change in the patient's electrogram that is indicative of a heart attack. If a heart attack is sensed, the device would then cause an implantable and / or externally located alarm to be actuated to warn the patient of his condition and a medical practitioner at a remote diagnostic center would receive the patient's electrogram for analysis. The patient or a caretaker would then be informed to self-inject medication through a subcutaneous, pass-through drug port that can be a separate device or integrated into the implanted device that is designed for the early detection of a heart attack. Since an implantable heart pacemaker or defibrillator already has within its structure many of the elements required for the device to recognize a heart attack, it would be expeditious to add a capability to these existing devices to detect a heart attack, have a pass-through drug port and provide an implantable and / or external alarm means to inform the patient to take appropriate action.
Owner:ANGEL MEDICAL SYST
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS6419960B1Patient compliance is goodGood retarding effectPowder deliveryOrganic active ingredientsImmediate releasePlasma drug concentration
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
Taste-masked pharmaceutical compositions
ActiveUS20060078614A1Effective taste-maskingRapid/complete releasePill deliveryAdditive ingredientOrally disintegrating tablet
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity in about 60 seconds forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active) applied with a taste-masking membrane comprising a combination of water-insoluble and gastrosoluble polymers release not less than about 60% of the dose is in the stomach in about 30 minutes, thus maximizing the probability of achieving bioequivalence to the reference IR product having rapid onset of action (short Tmax). A process for preparing such compositions for oral administration using conventional fluid-bed equipment and rotary tablet press is also disclosed.
Owner:ADARE PHARM INC
Oral pharmaceutical composition with delayed release of active ingredient for pantoprazole
An oral pharmaceutical composition comprises an acid-labile irreversible proton pump inhibitor in pellet or tablet form, wherein the irreversible proton pump inhibitor is at least partly in slow-release form. On combined administration with an anti-microbially-active ingredient, the composition is distinguished by imparting an enhanced action of rapid onset against disorders caused by Helicobacter.
Owner:TAKEDA GMBH
Rapid acting drug delivery compositions
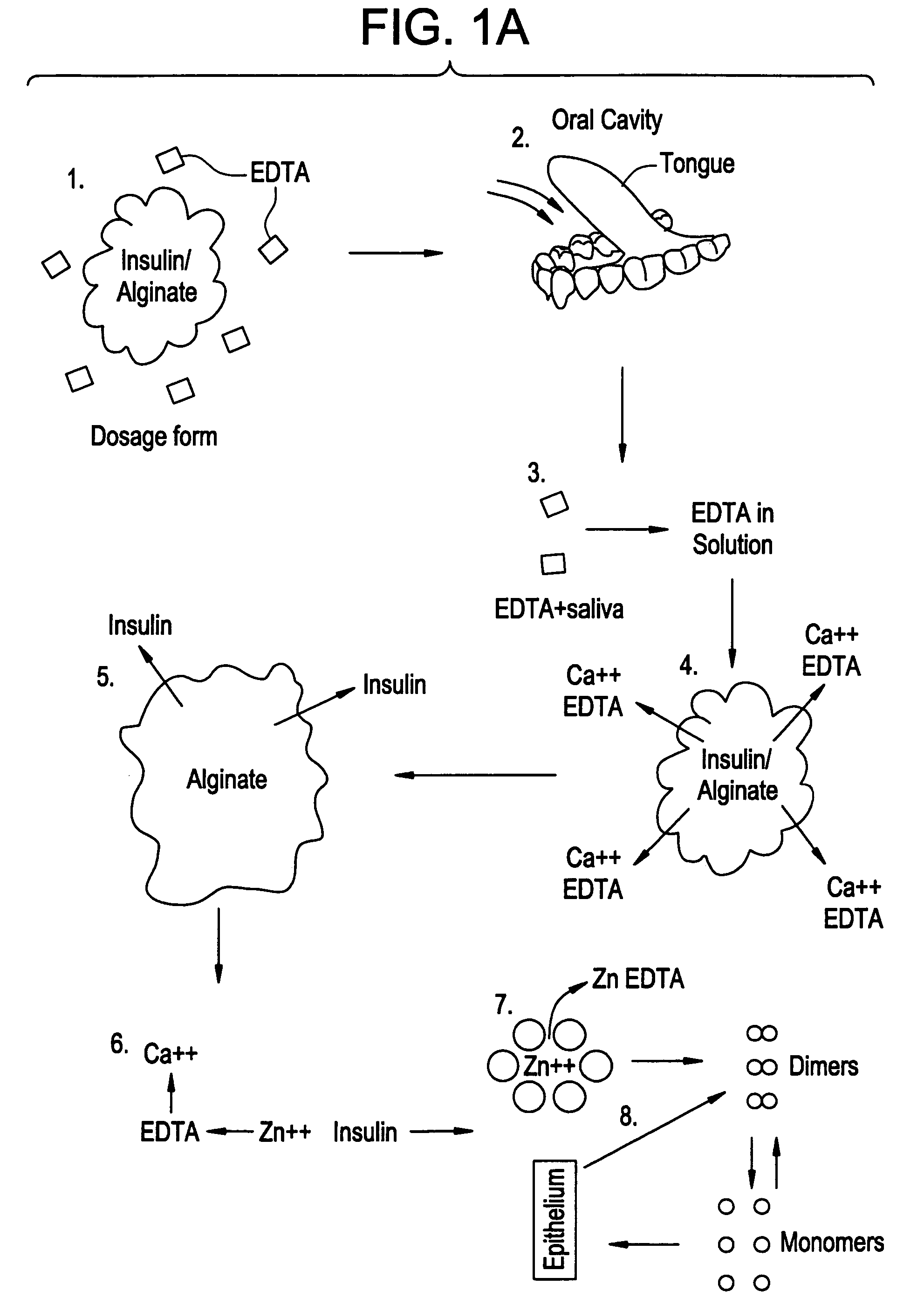
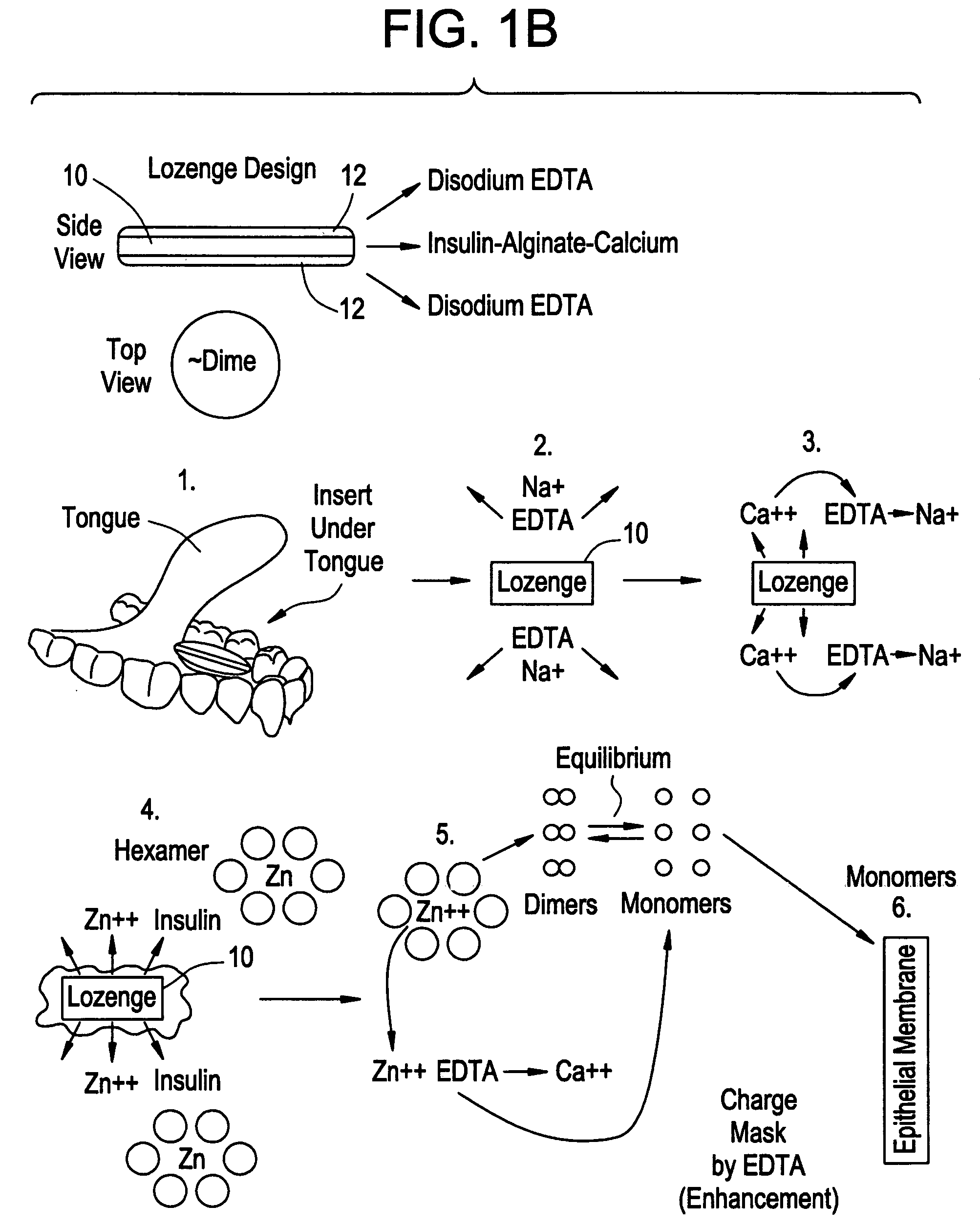
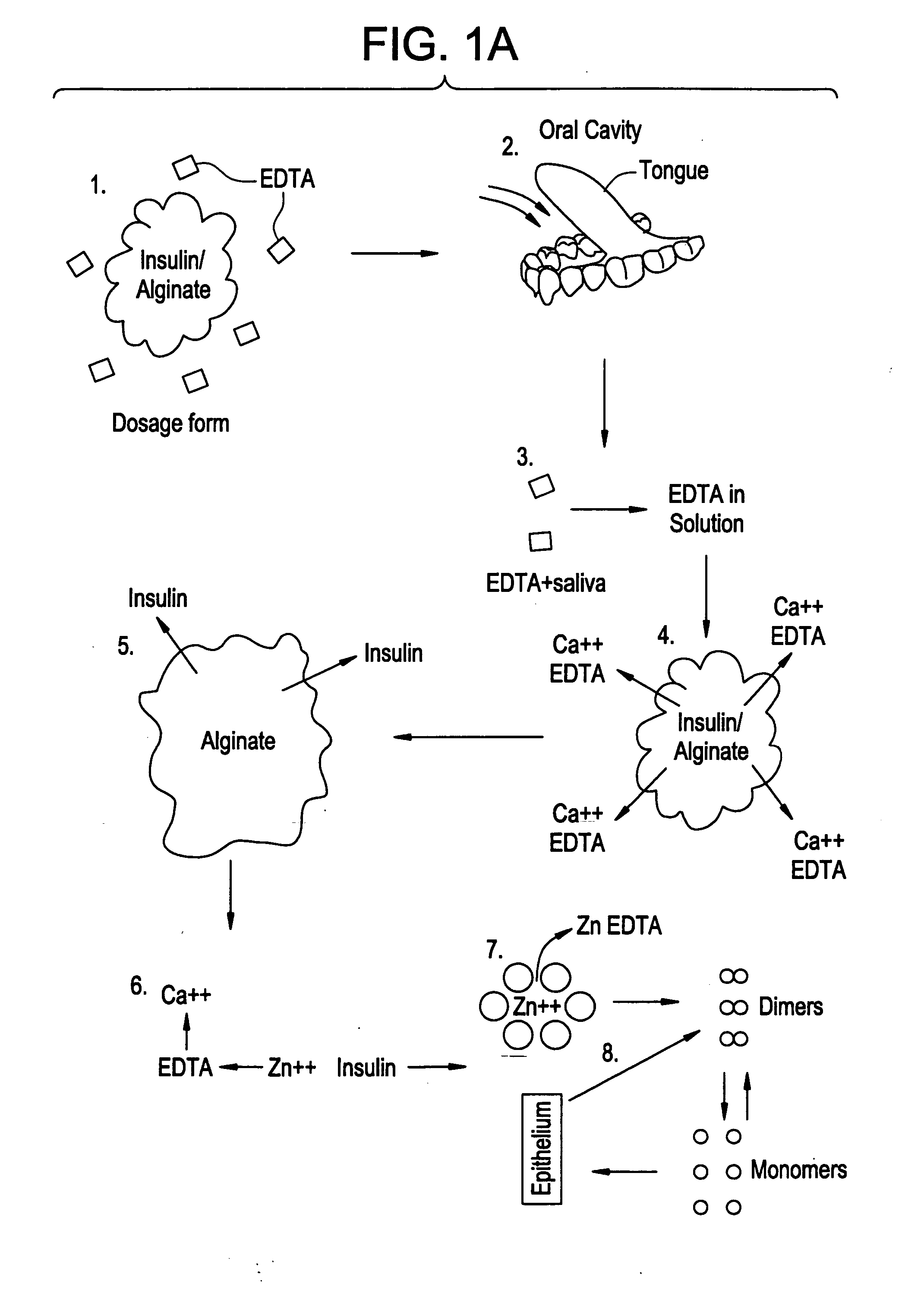
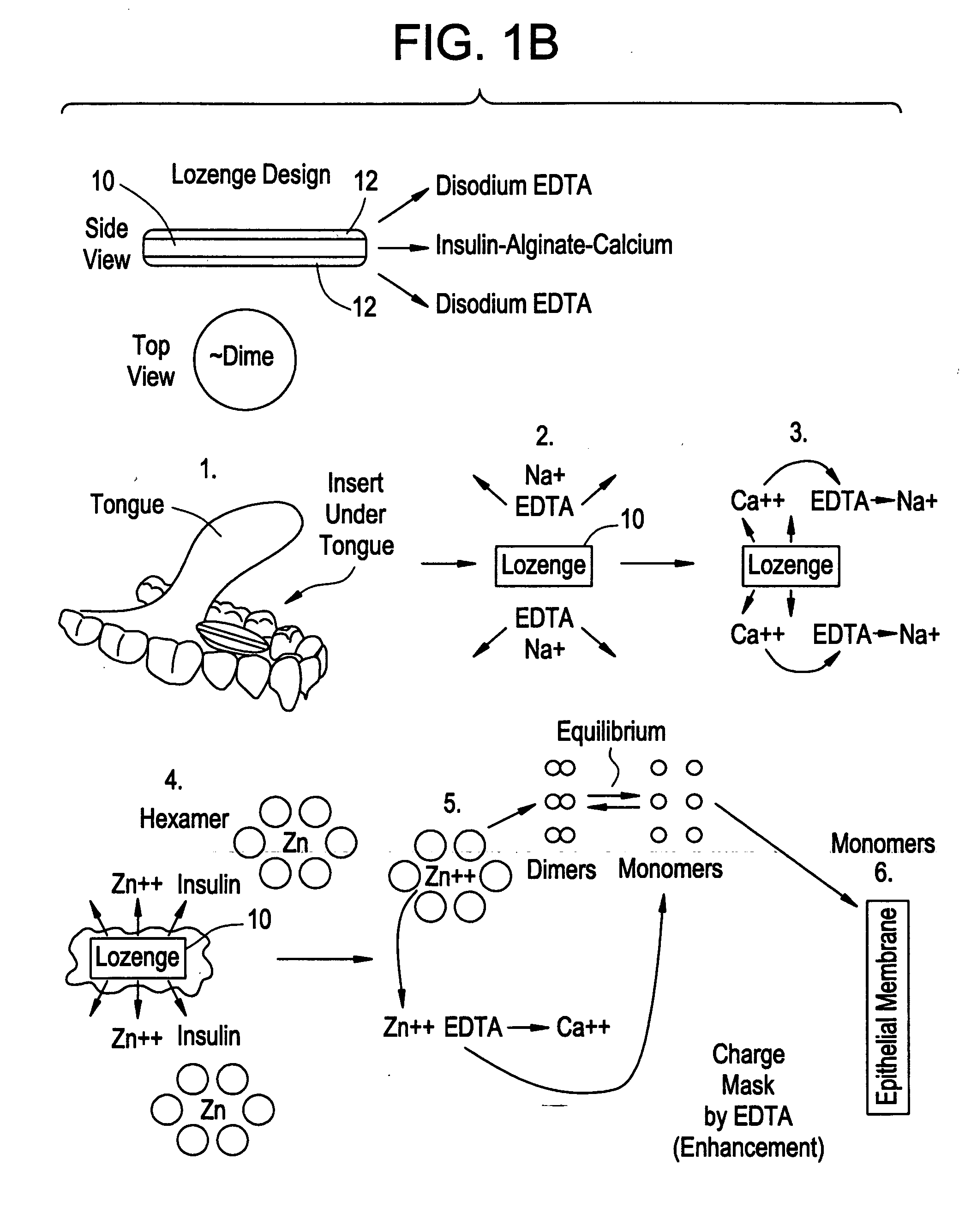
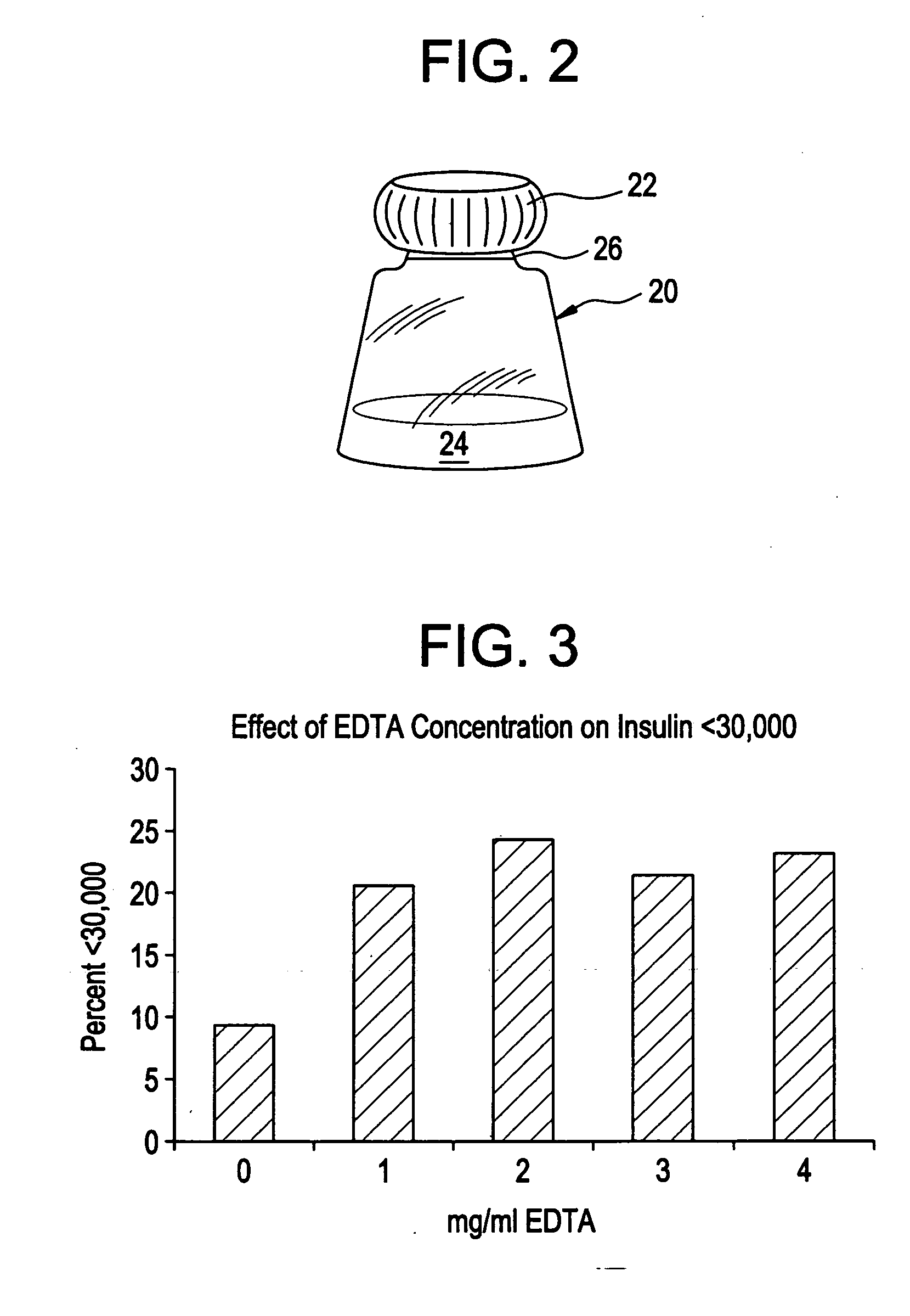
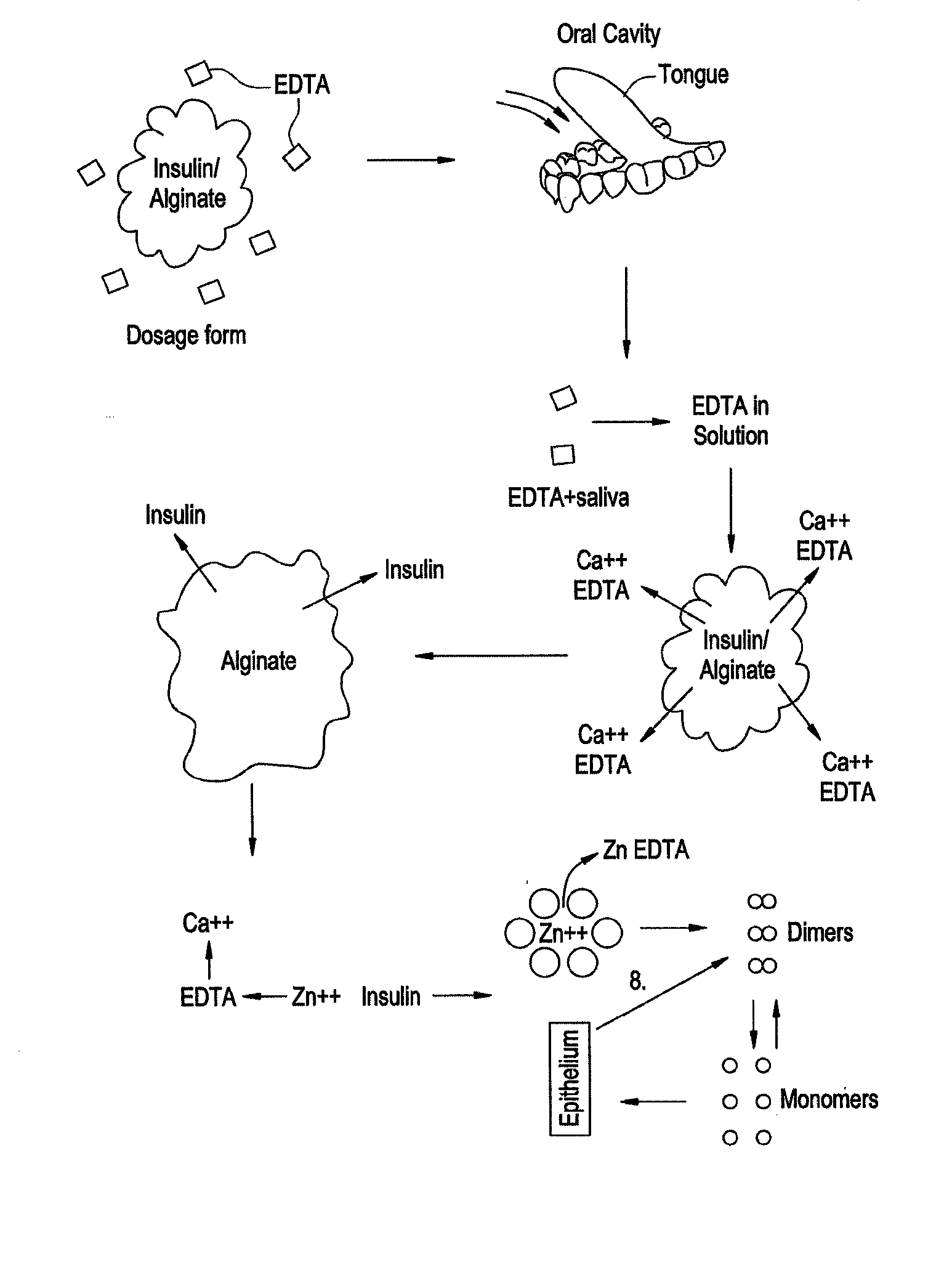
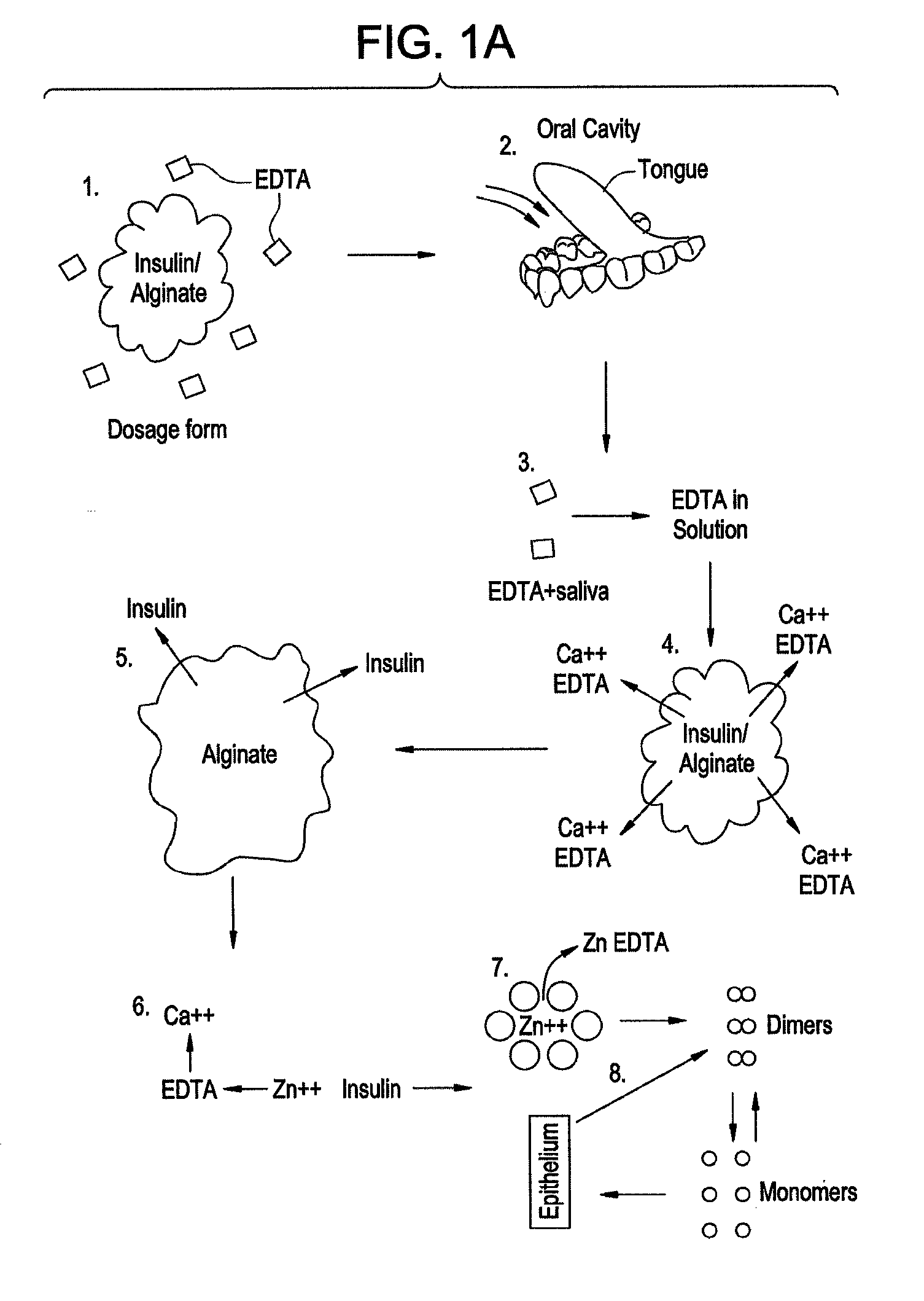
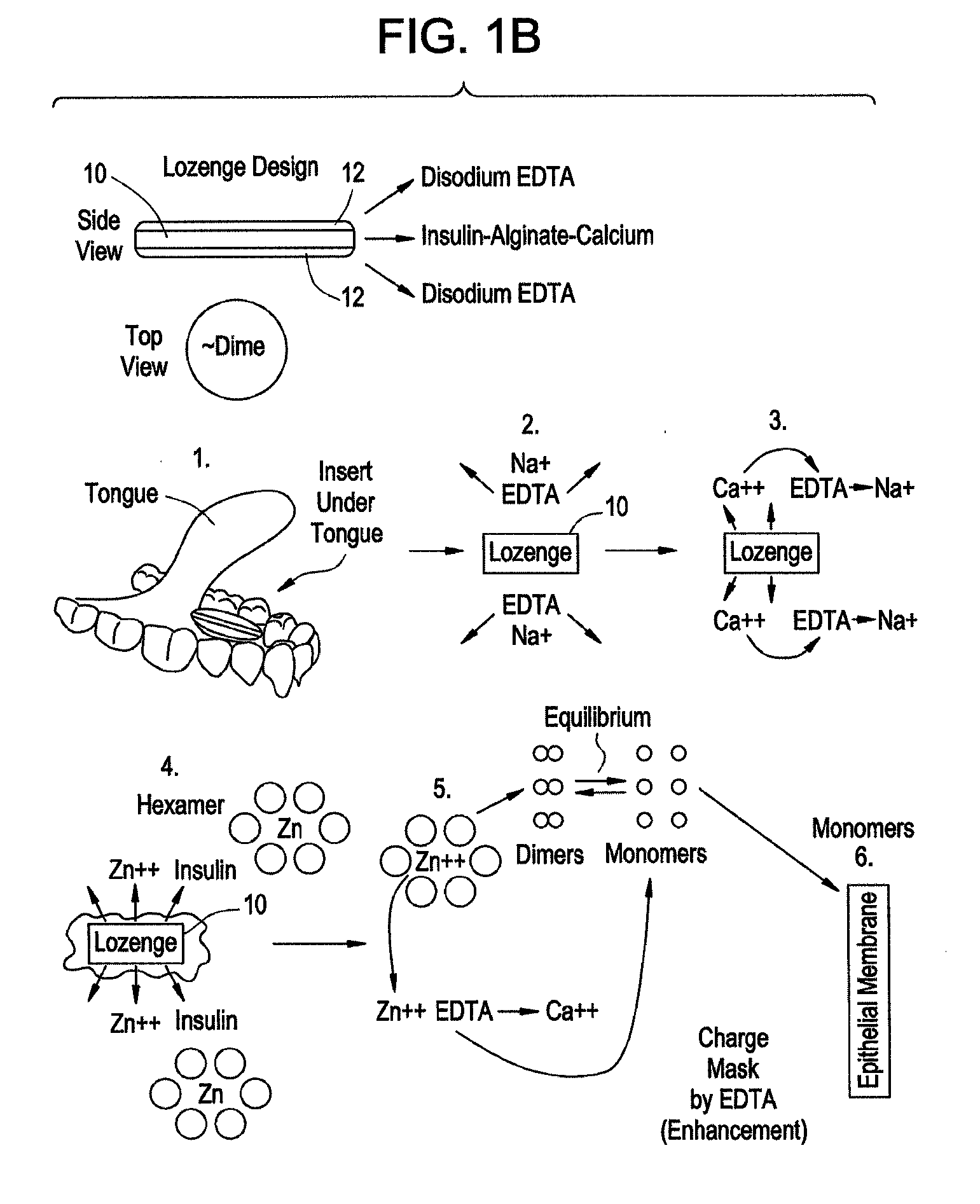
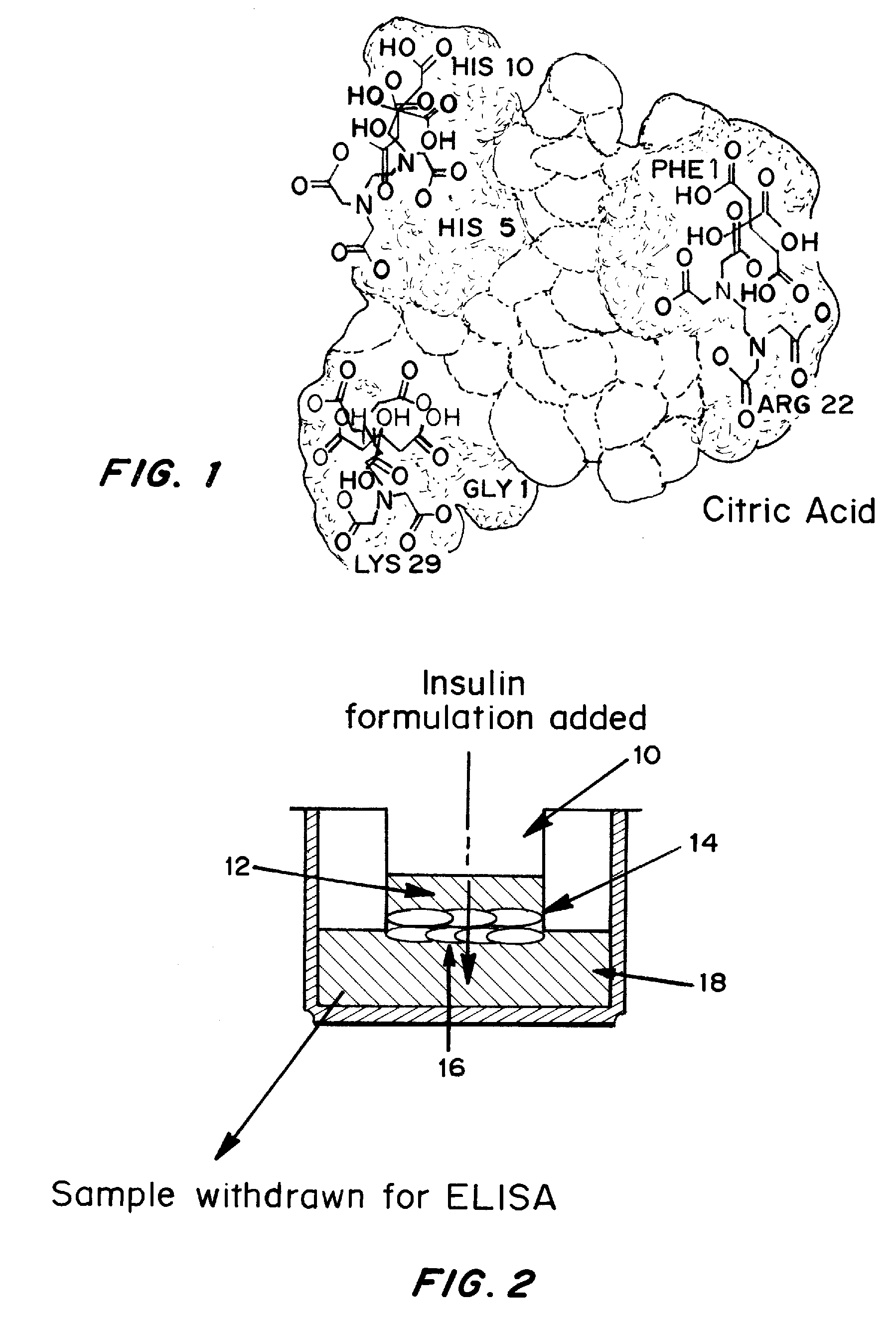
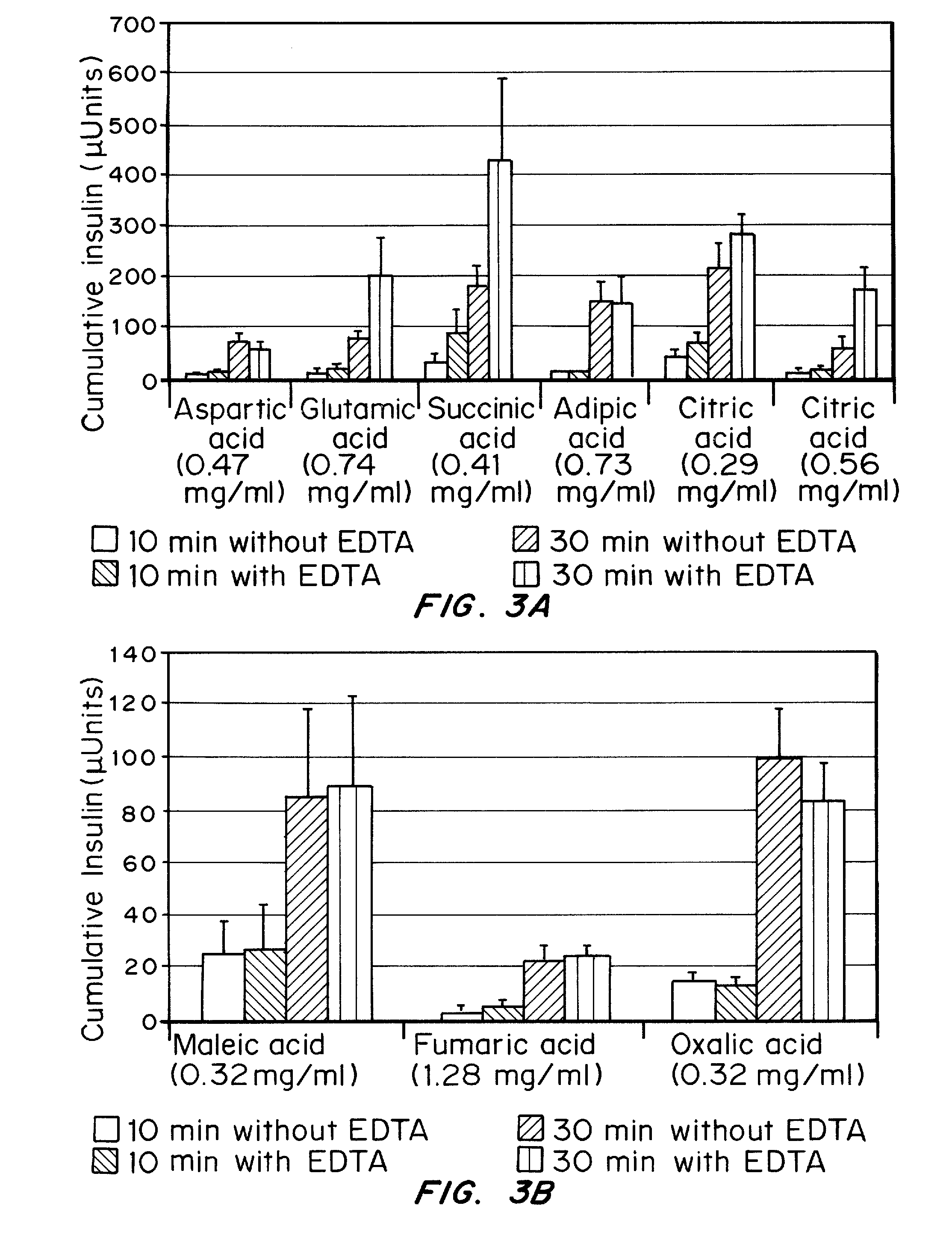
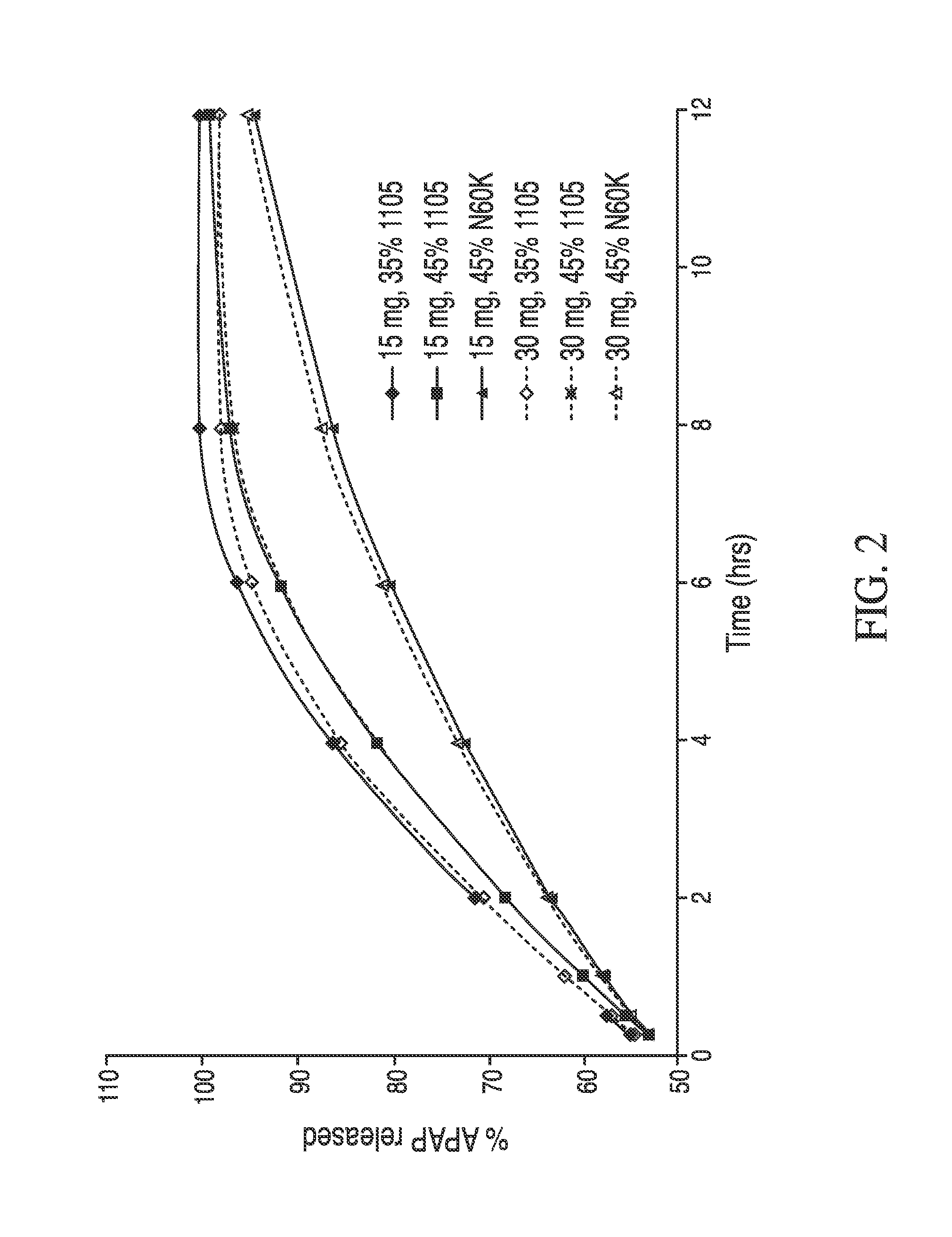
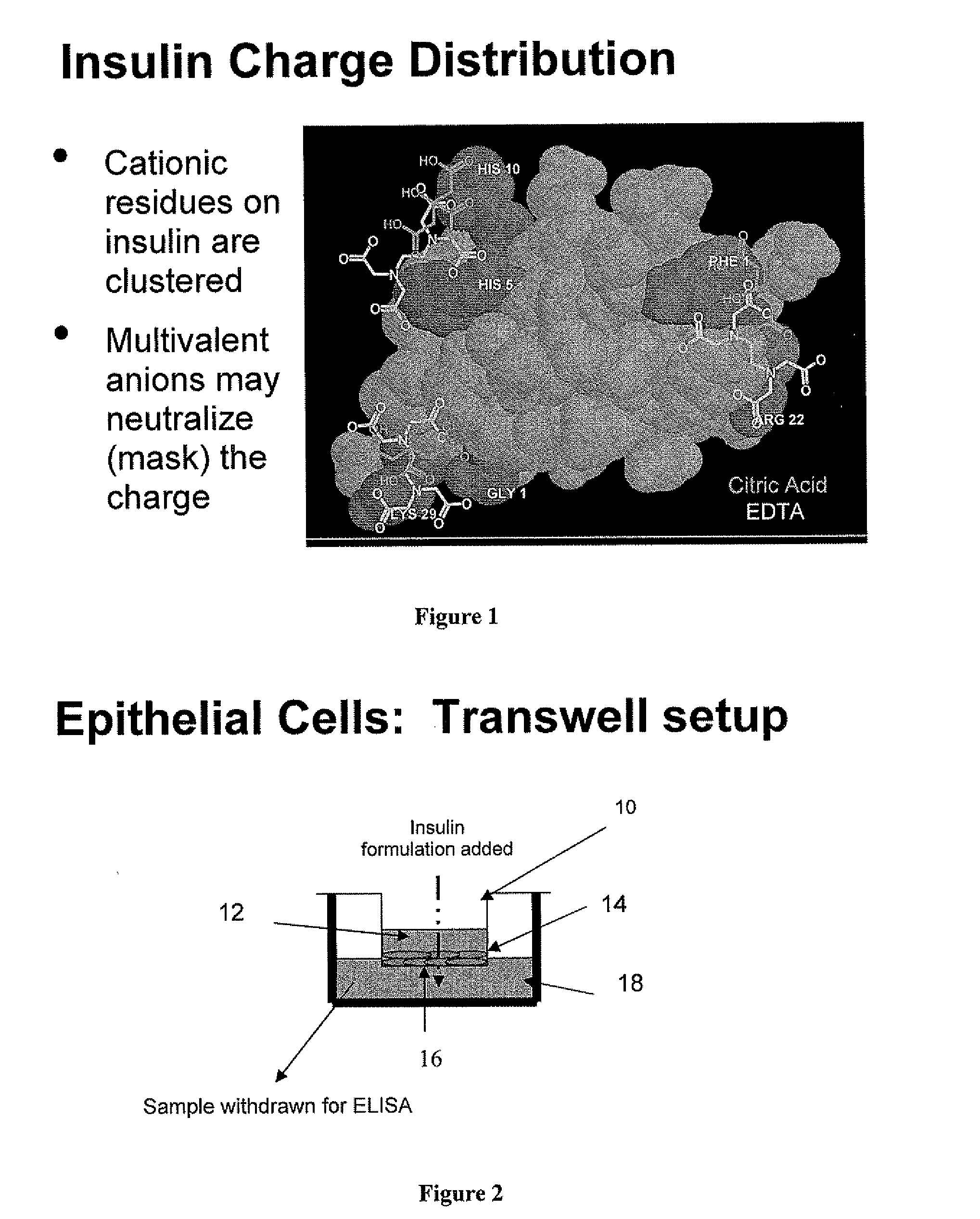
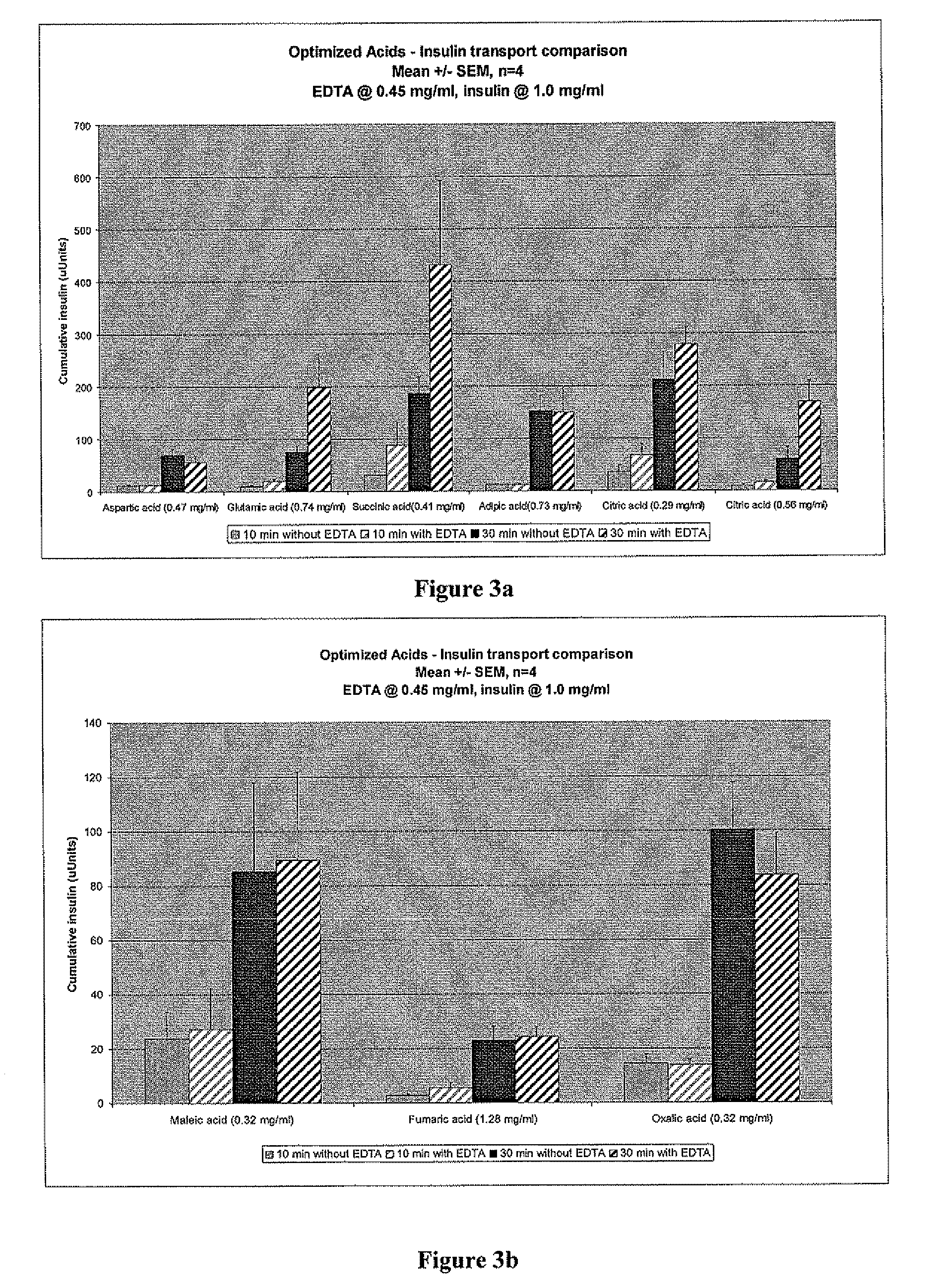
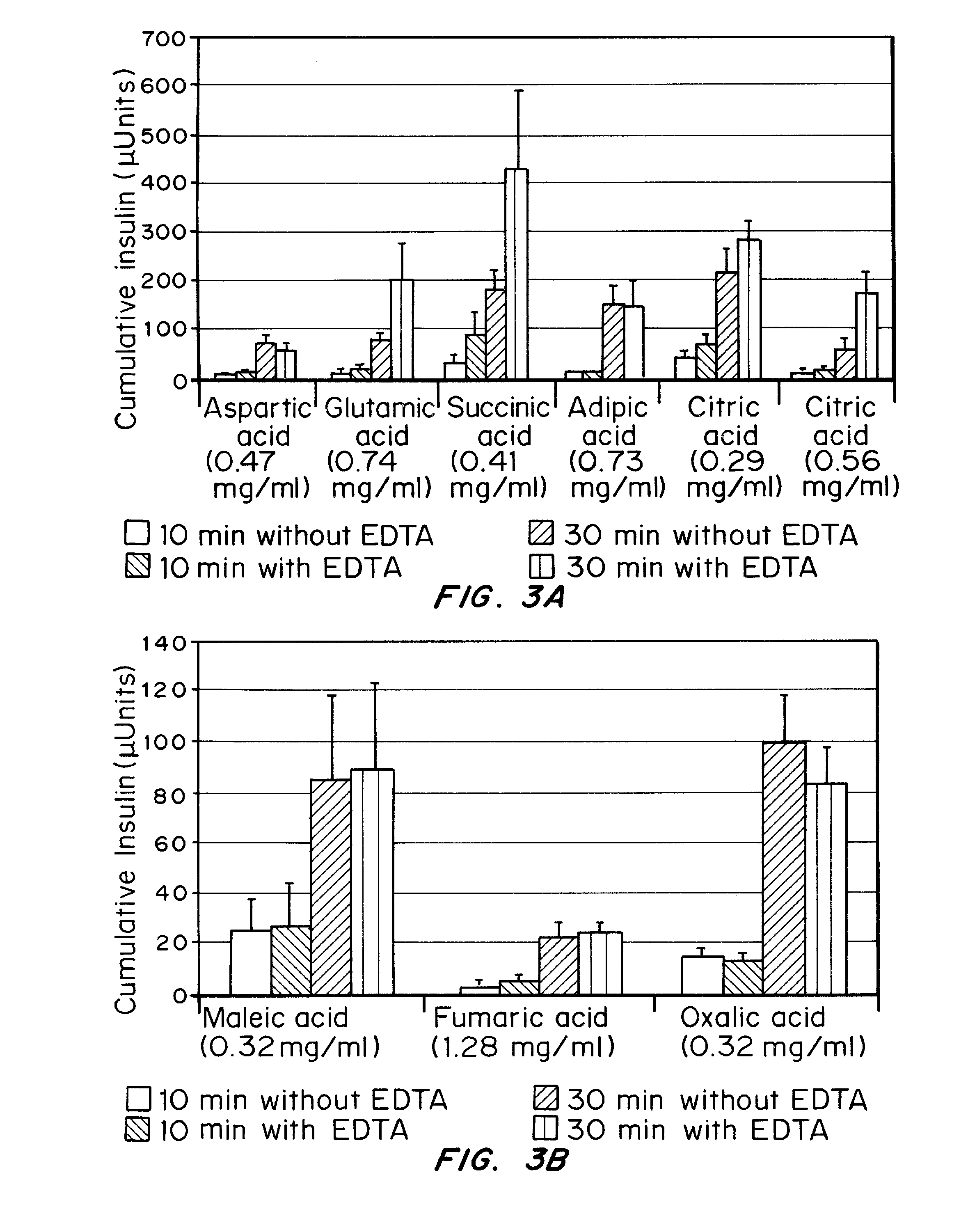
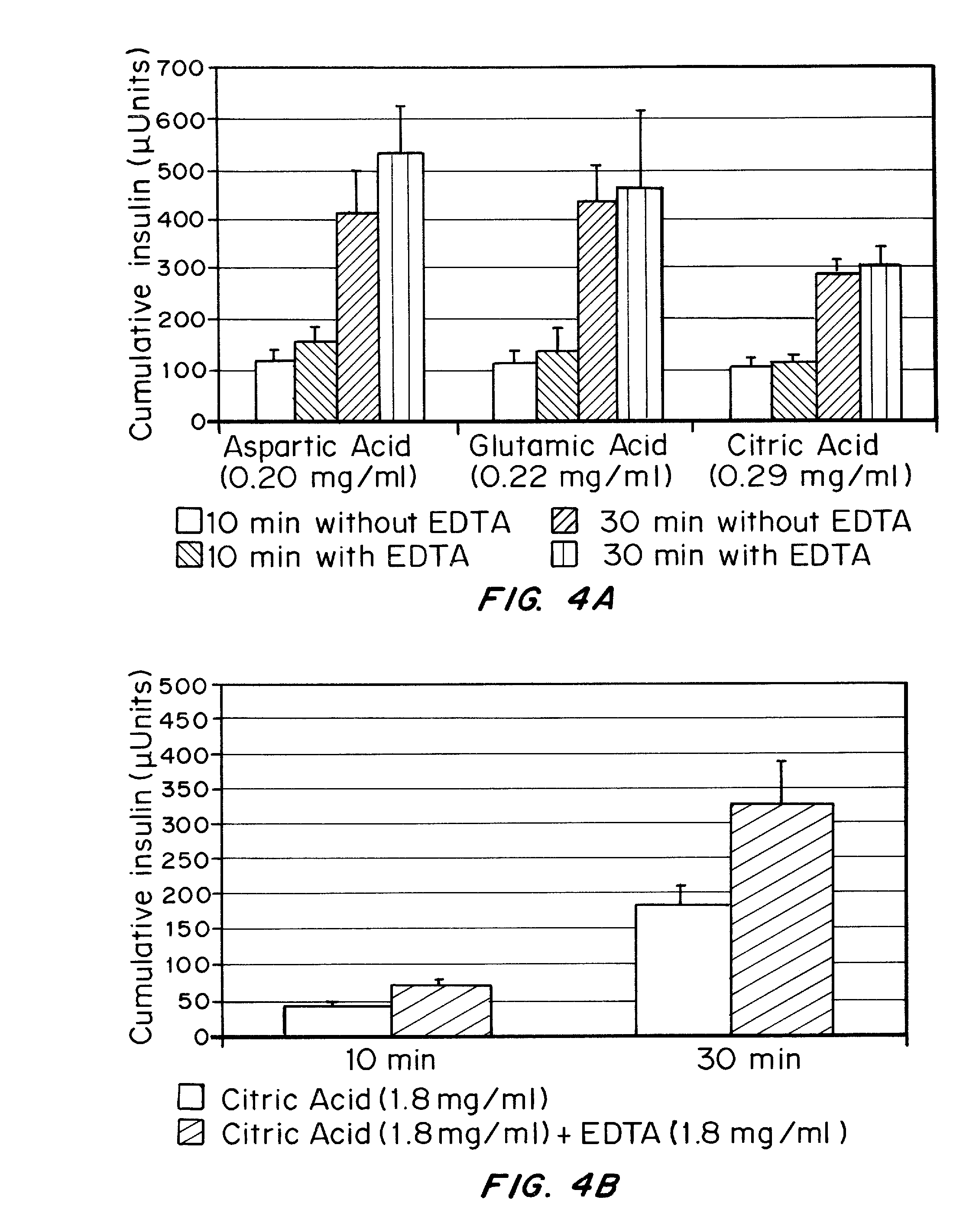
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
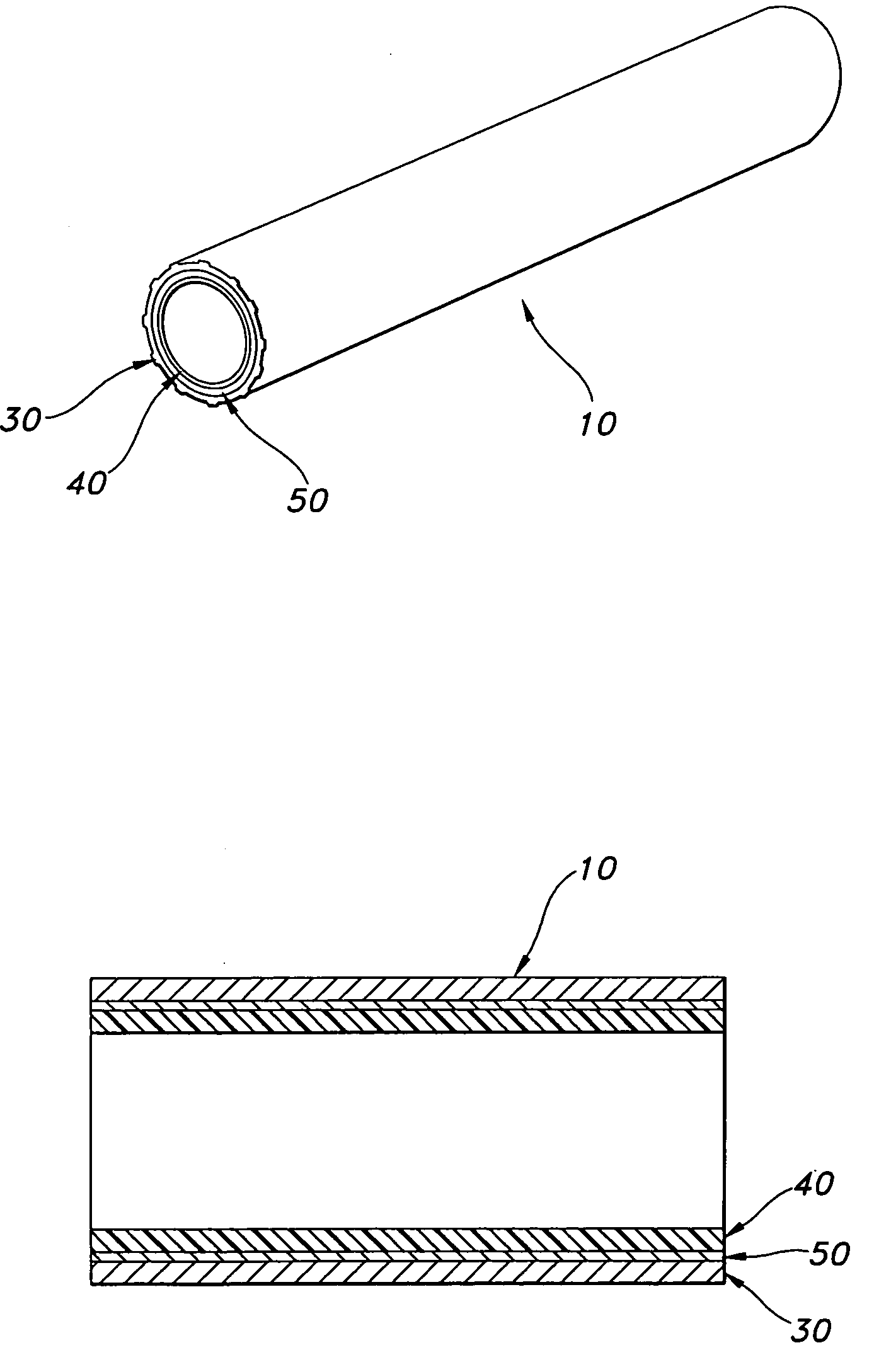
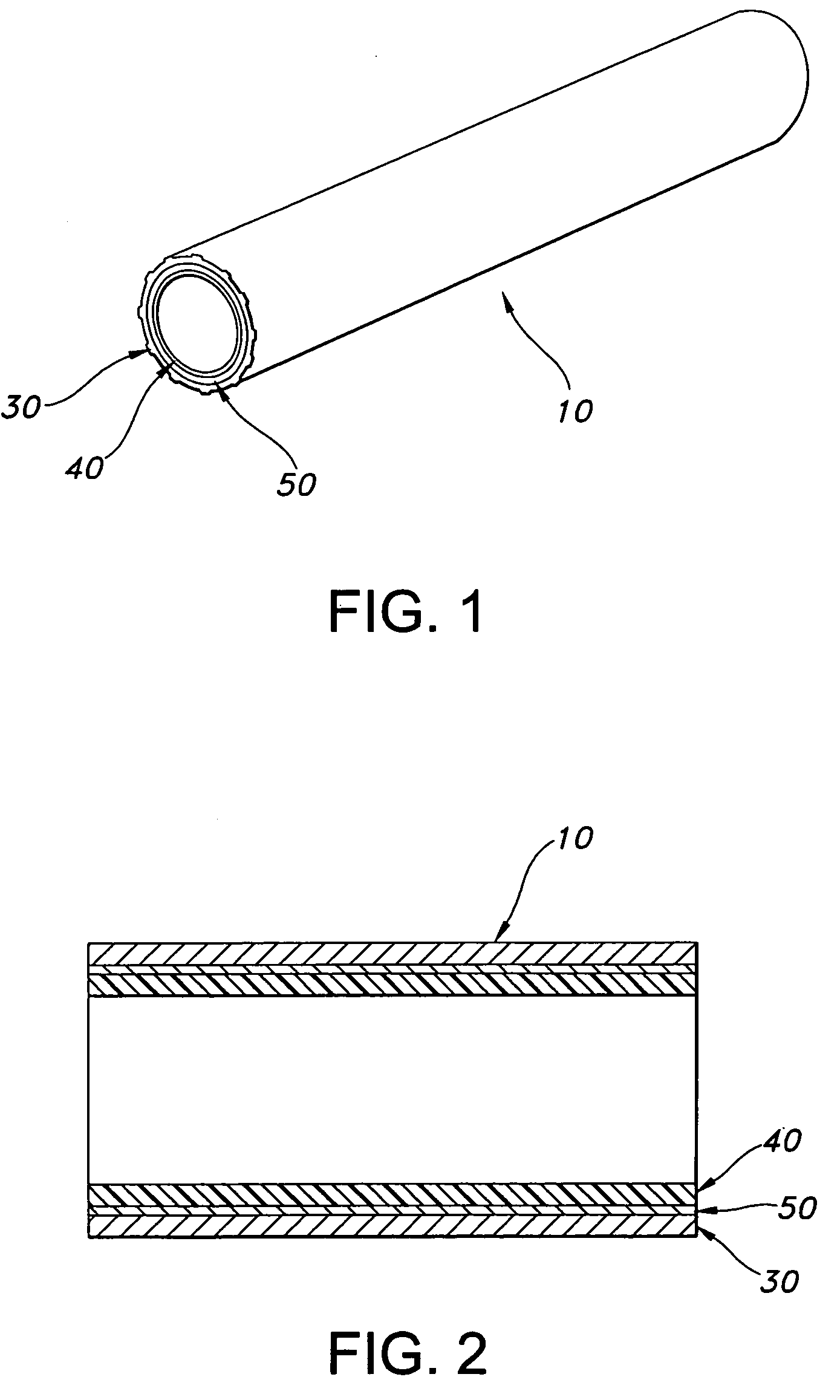
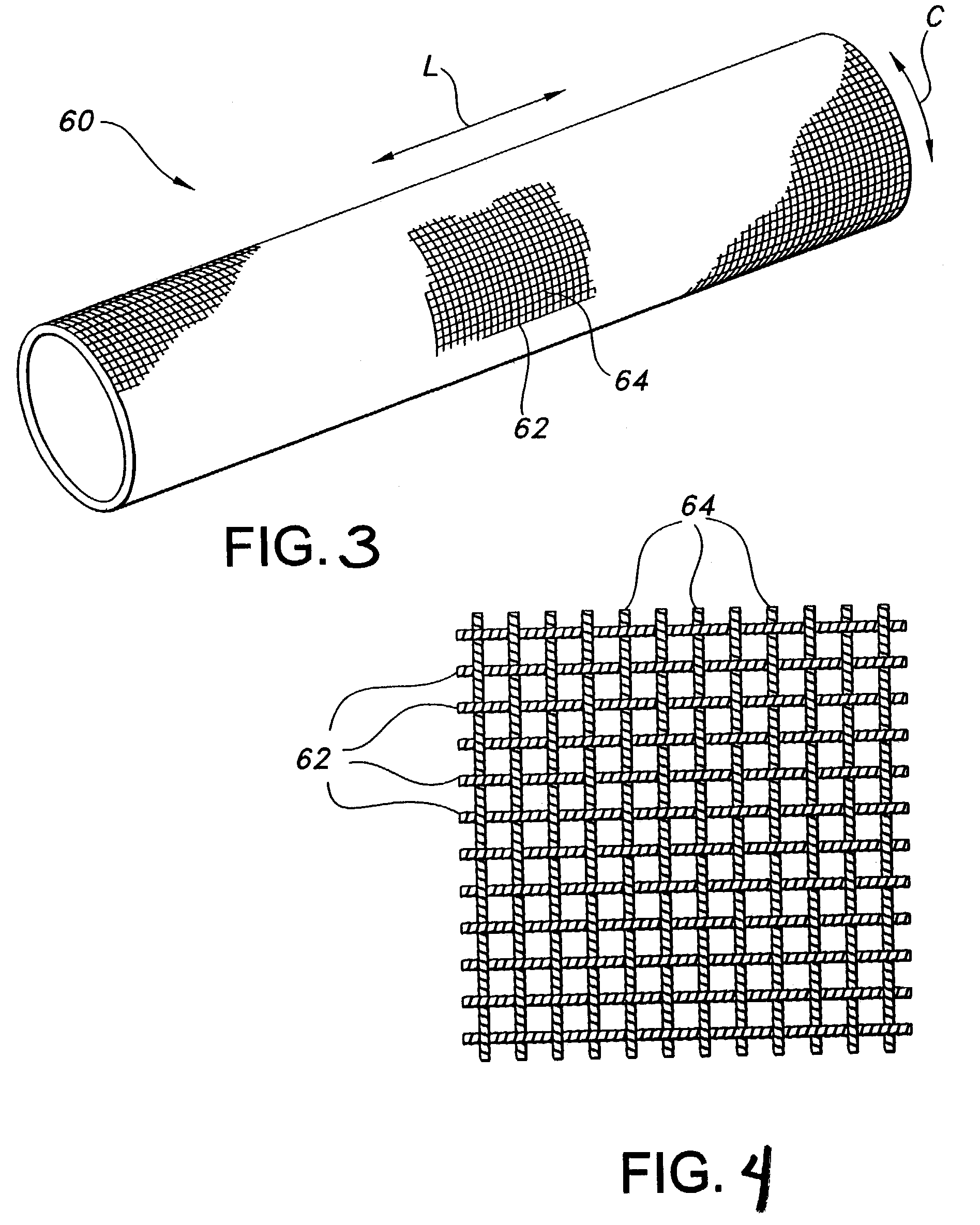
AV grafts with rapid post-operative self-sealing capabilities
The invention provides a self-sealing arteriovenous graft including a tube having a polymeric inner layer defining a lumen through which blood may flow and an outer textile layer. The outer textile layer is concentrically disposed about the inner layer. Furthermore, an intermediate self-sealing layer is concentrically positioned between the inner and outer layers. The self-sealing layer includes a biocompatible polymer.
Owner:MAQUET CARDIOVASCULAR LLC
Combinations of 5-ht2a inverse agonists and antagonists with antipsychotics
InactiveUS20090053329A1Achieve effectQuick effectCompounds screening/testingBiocideSide effectAntipsychotic drug therapy
Combinations of 5-HT2A inverse agonists or antagonists such as pimavanserin with antipsychotics such as risperidone are shown to induce a rapid onset of antipsychotic action and increase the number of responders when compared to therapy with the antipsychotic alone. These effects can be achieved at a low dose of the antipsychotic, thereby reducing the incidence of side effects. The combinations are also effective at decreases the incidence of weight gain and increased glucose or prolactin levels caused by the antipsychotic.
Owner:ACADIA PHARMA INC
Novel formulations for treatment of migraine
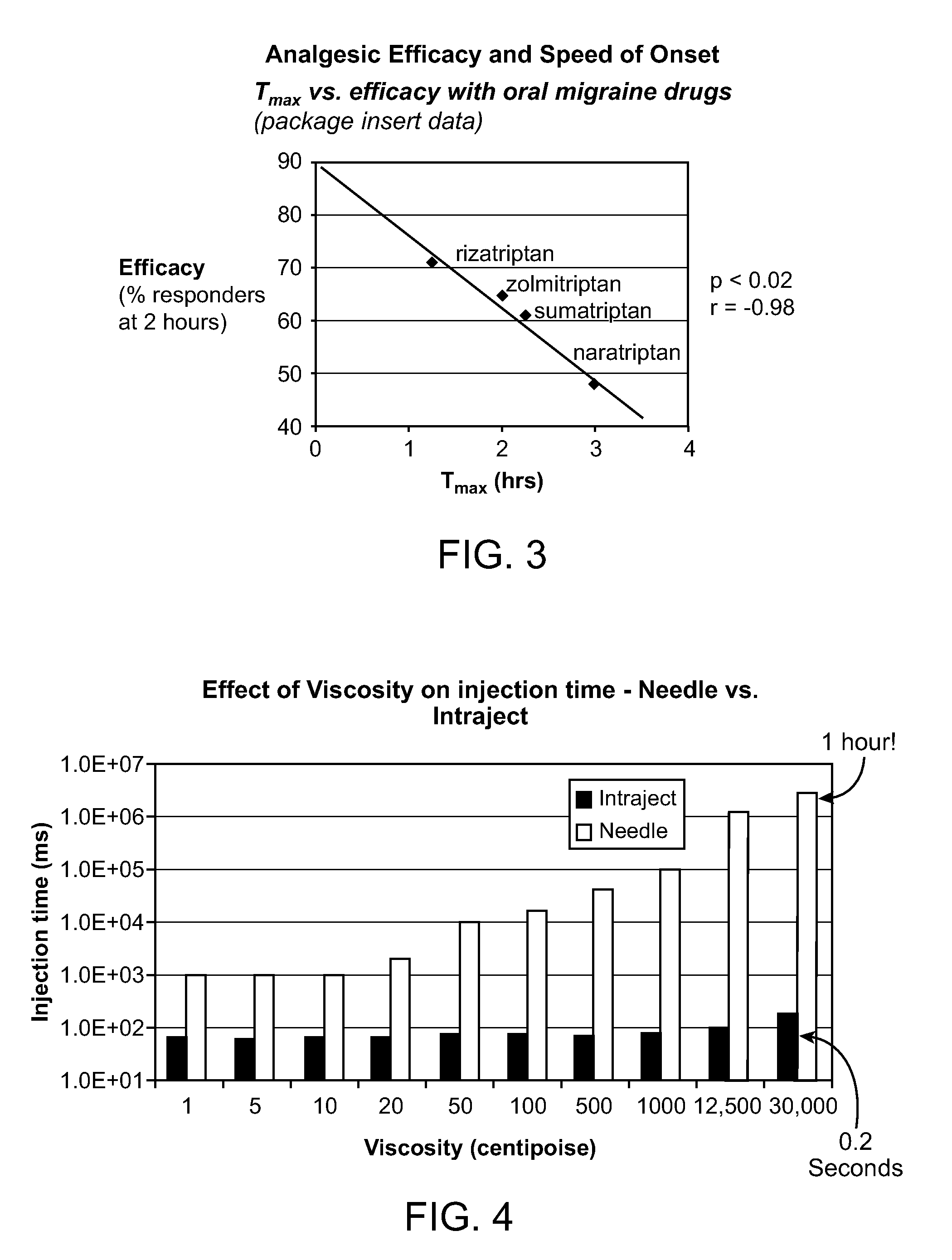
InactiveUS20110118189A1Immediate pharmacological effectLonger effectBiocideSenses disorderNeedle freeHeadaches
Systems and methods are described for treating un-met medical needs in migraine and related conditions such as cluster headache. Included are treatments that are both rapid onset and long acting, which include sustained release formulations, and combination products. Also included are treatments for multiple symptoms of migraine, especially headache and nausea and vomiting. Systems that are self contained, portable, prefilled, and simple to self administer at the onset of a migraine attack are disclosed, and preferably include a needle-free injector and a high viscosity formulation, to eliminate such issues as fear of self administration with needles, and needle stick and cross contamination.
Owner:ZOGENIX INC
Rapid Acting Drug Delivery Compositions
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:BIODEL INC
Compositions and delivery systems for administration of a local anesthetic agent
InactiveUS20050152957A1Good effectLocal effectBiocideAdhesive dressingsActive agentHydrophobic polymer
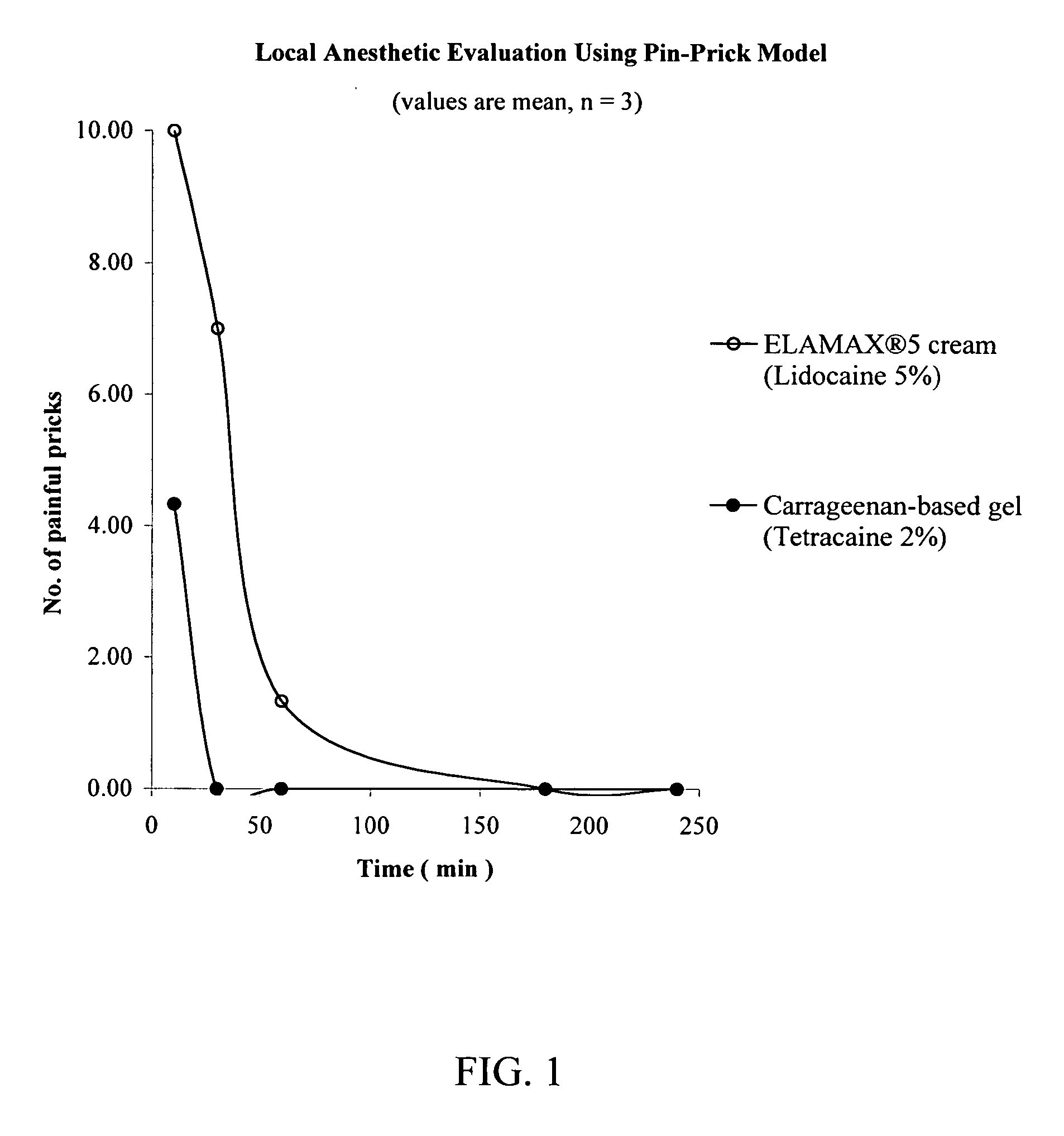
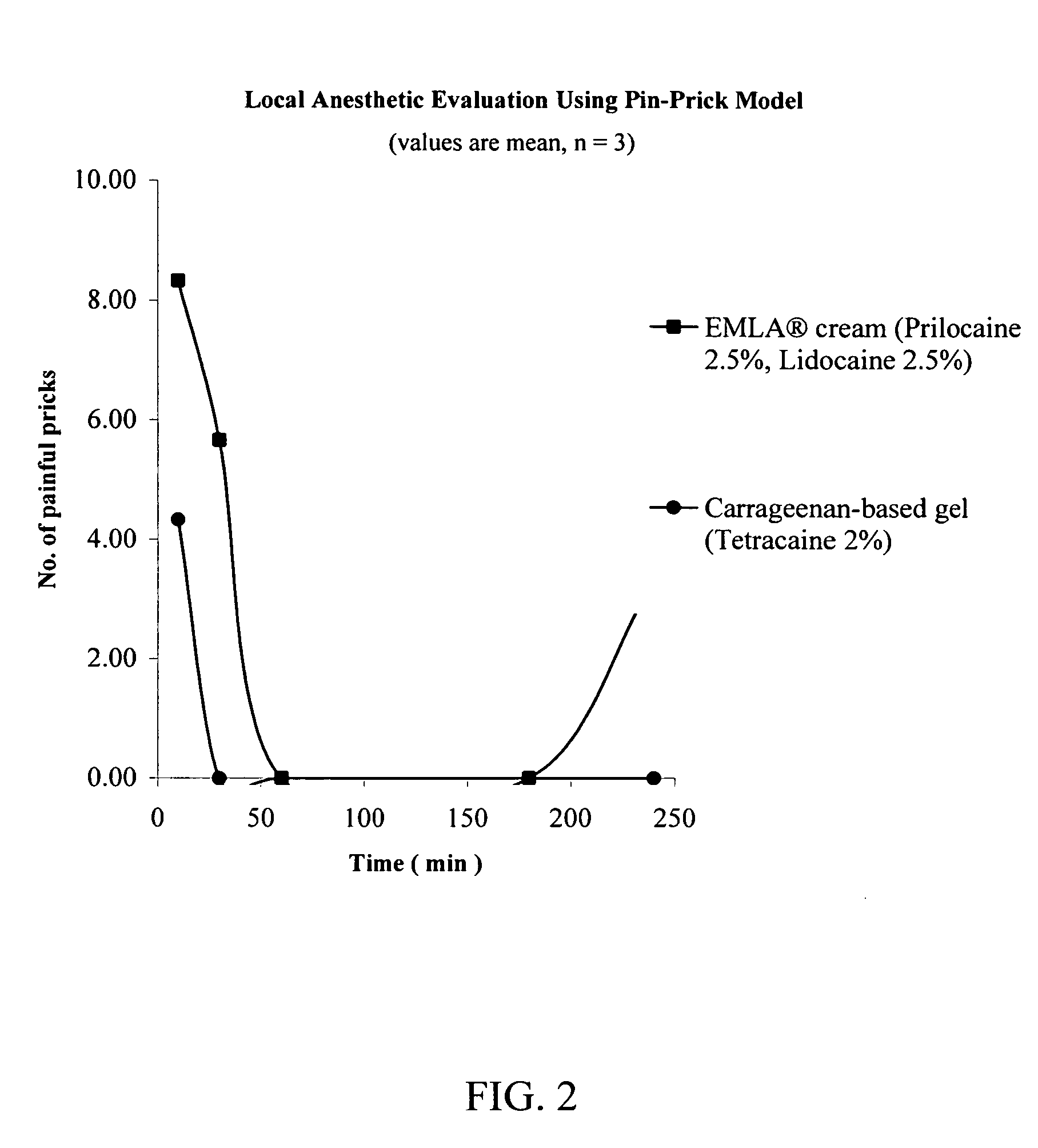
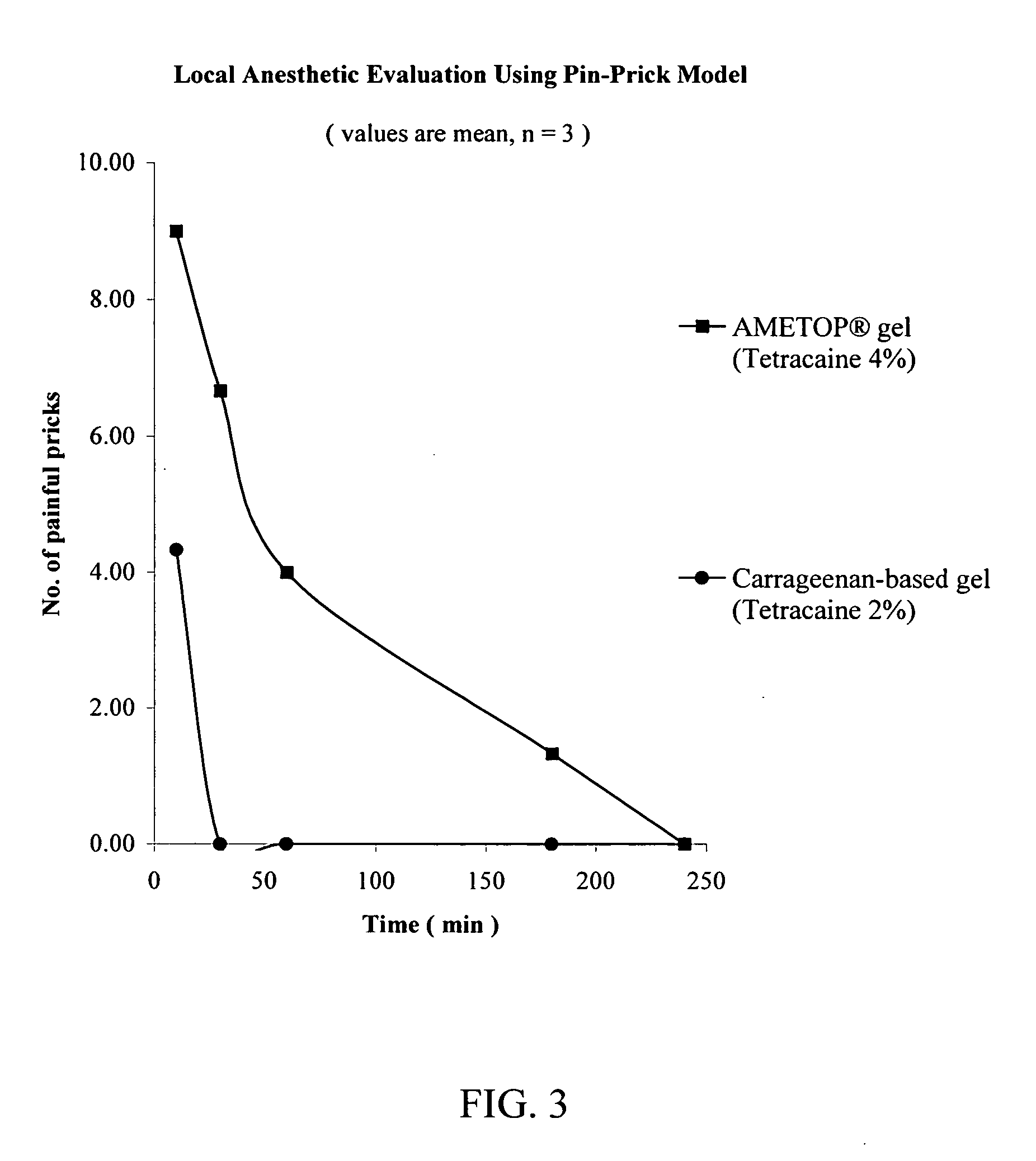
A pharmaceutical composition is provided for topical administration of a local anesthetic agent. The composition comprises (a) a therapeutically effective amount of a local anesthetic agent and (b) a pharmaceutically acceptable, nonliposomal carrier comprised of a monohydric alcohol, a penetration enhancer, and polymer, which may be a hydrophilic polymer, a hydrophobic polymer or a combination thereof. The composition can be in the form of a gel, or it may form a film following application to a patient's body surface and evaporation of the monohydric alcohol. The composition provides rapid onset of local anesthesia as well as penetration of the active agent into the skin. Methods and drug delivery systems for administration of local anesthetic agents are also provided.
Owner:CORIUM INT
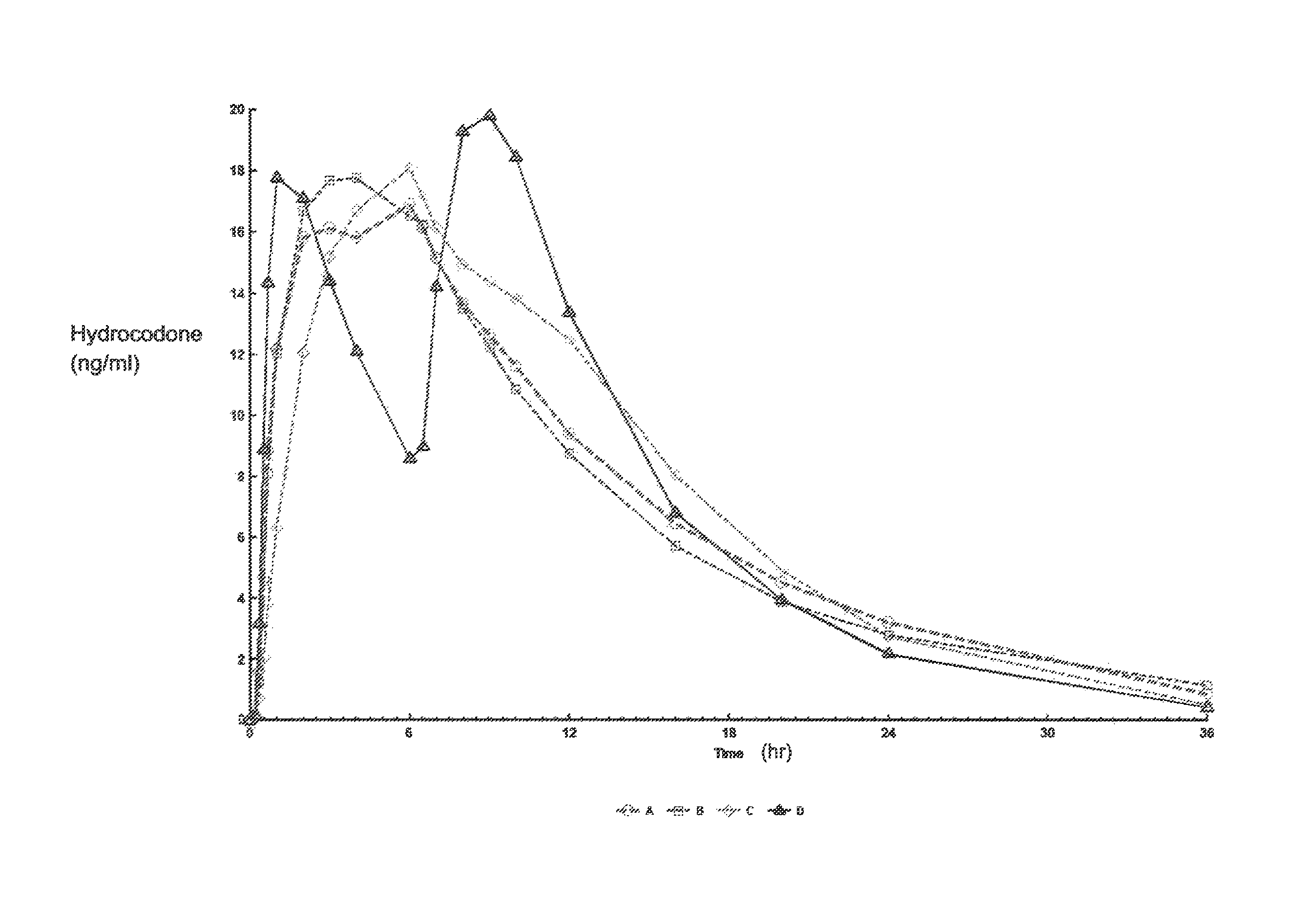
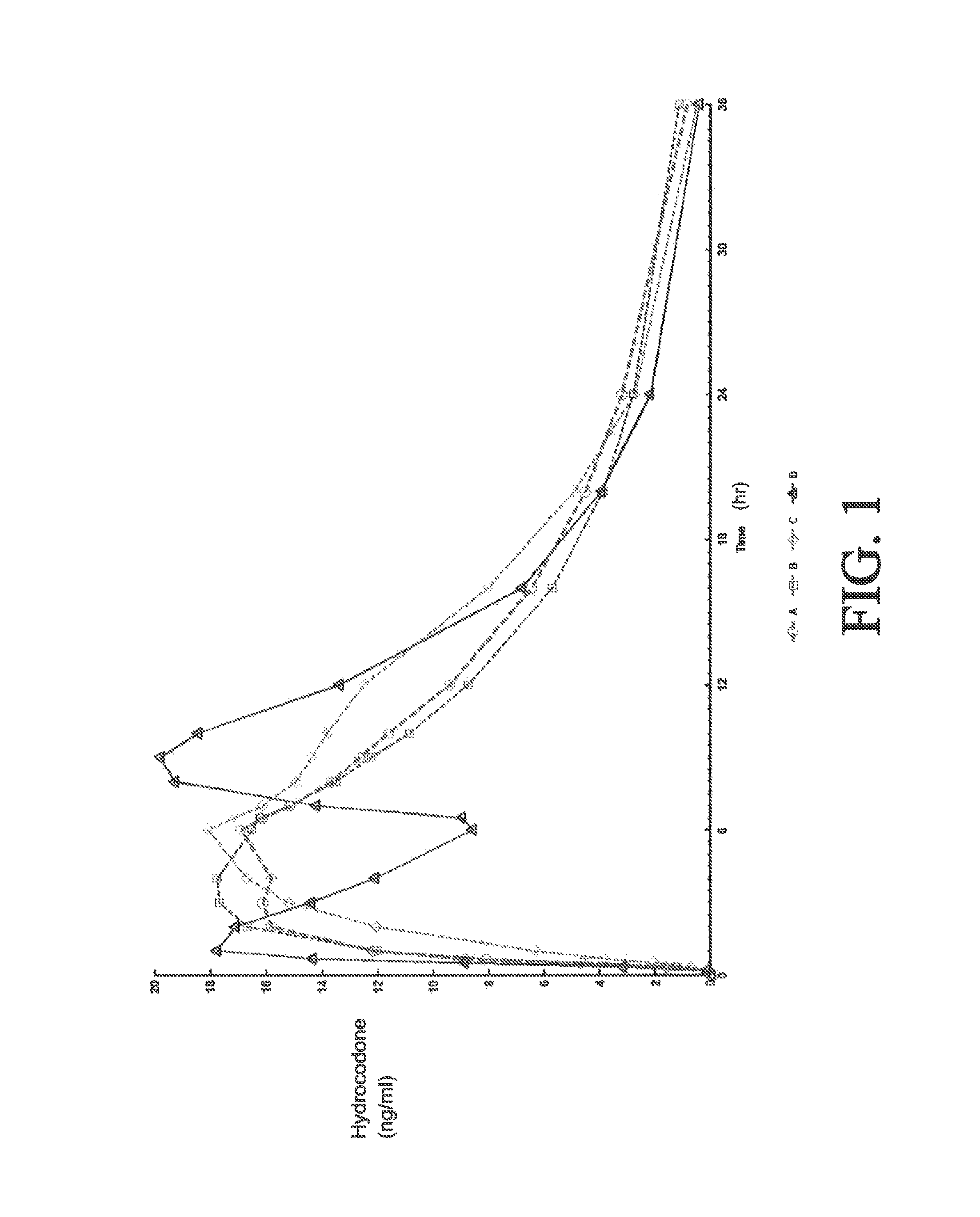
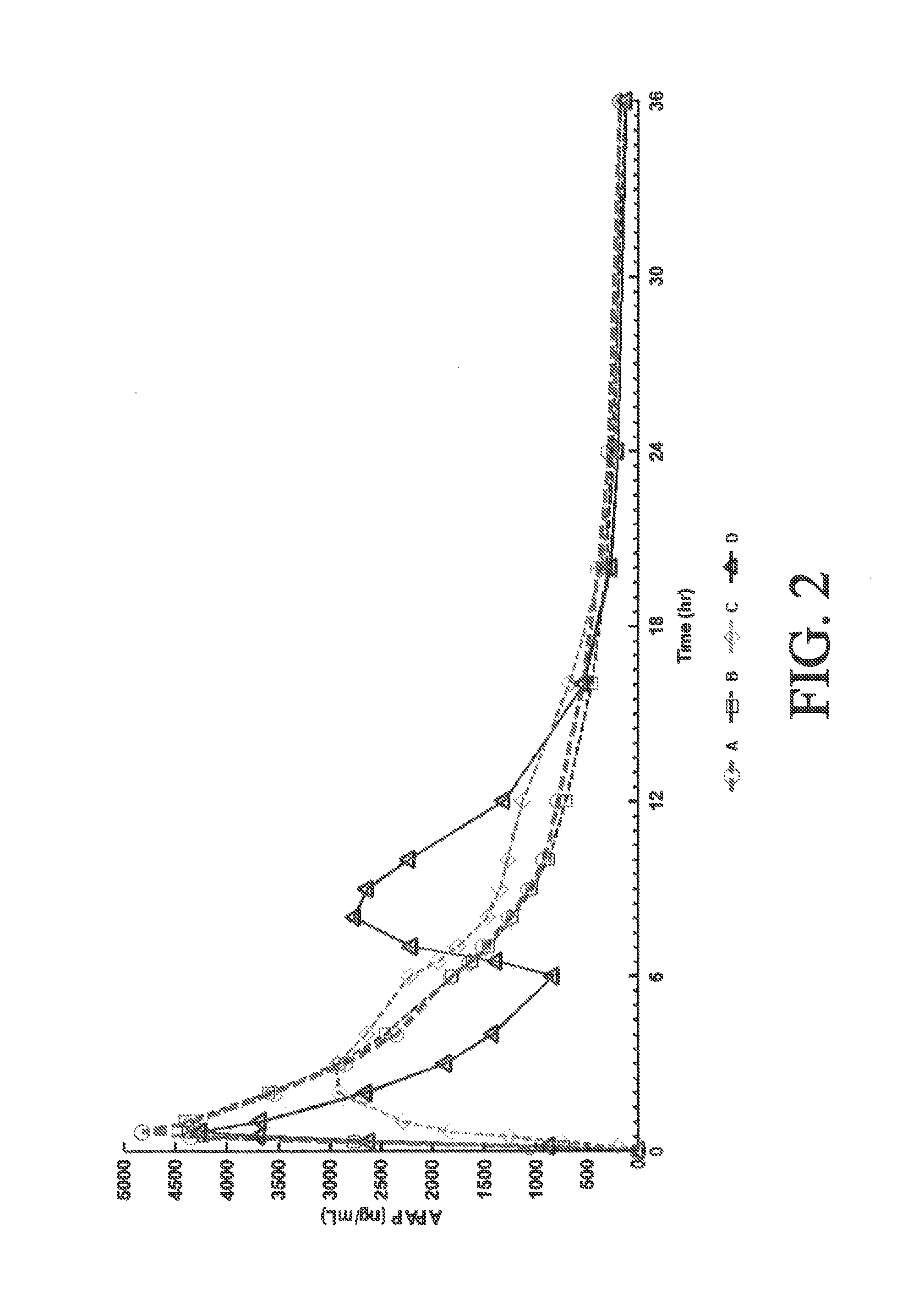
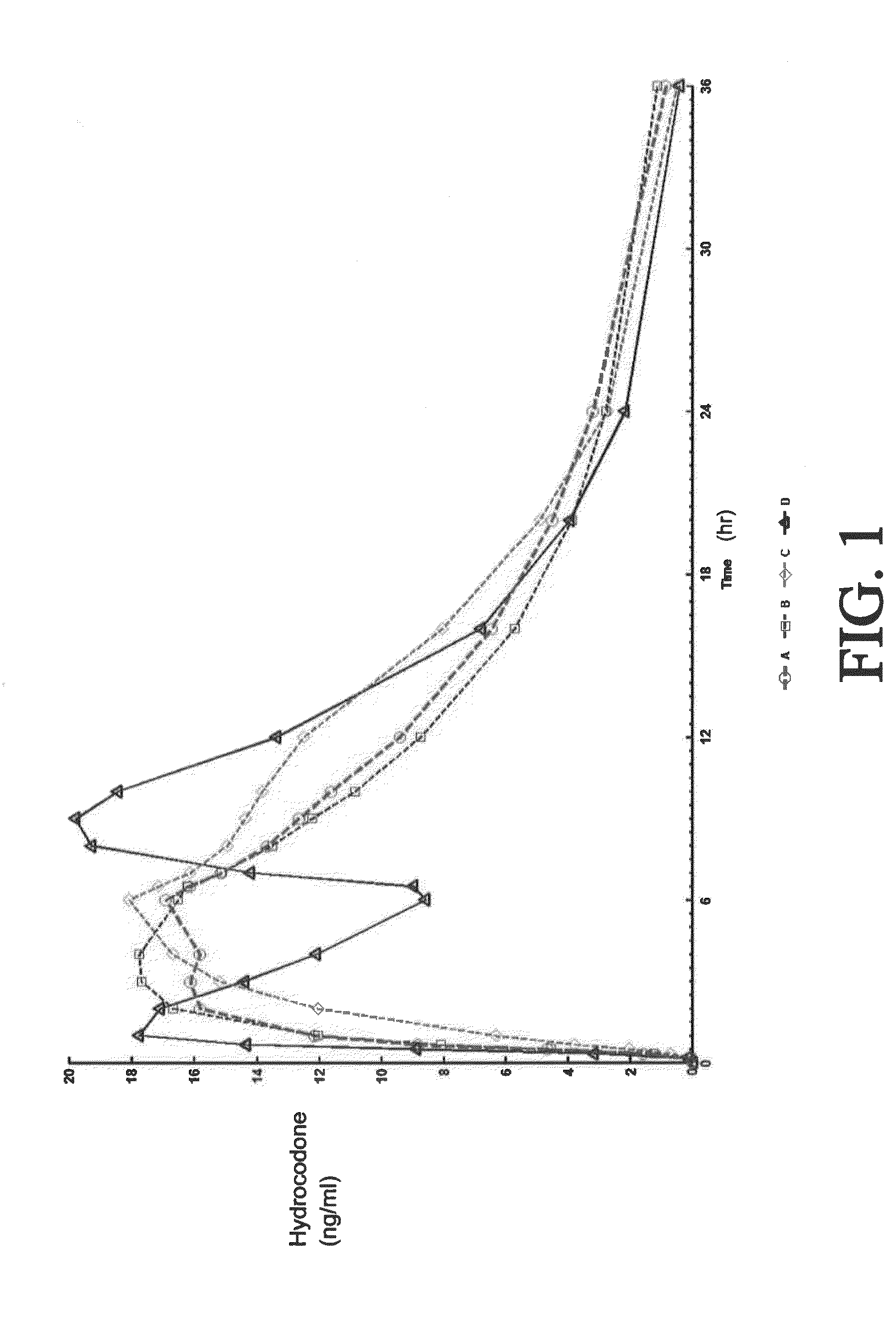
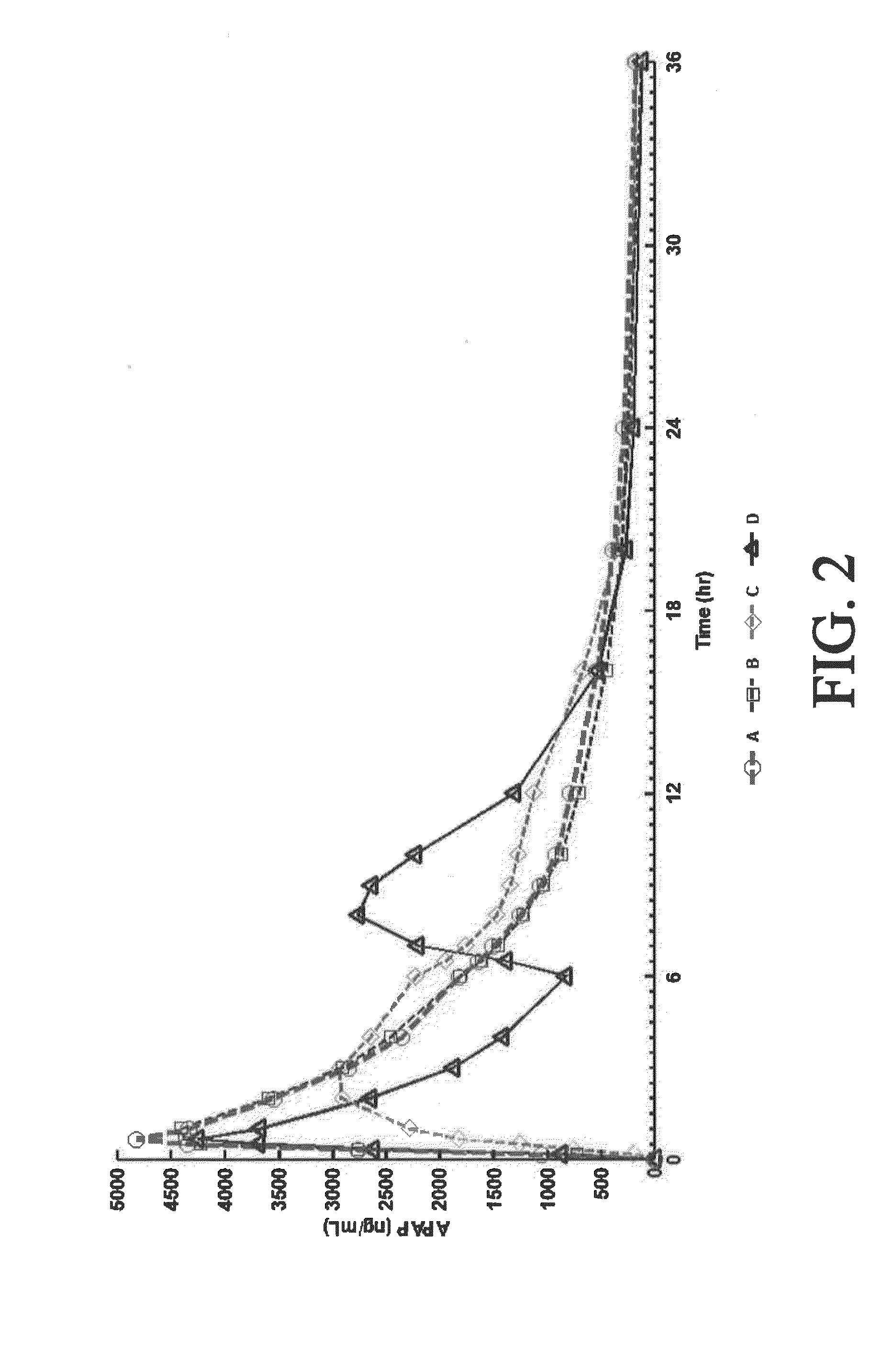
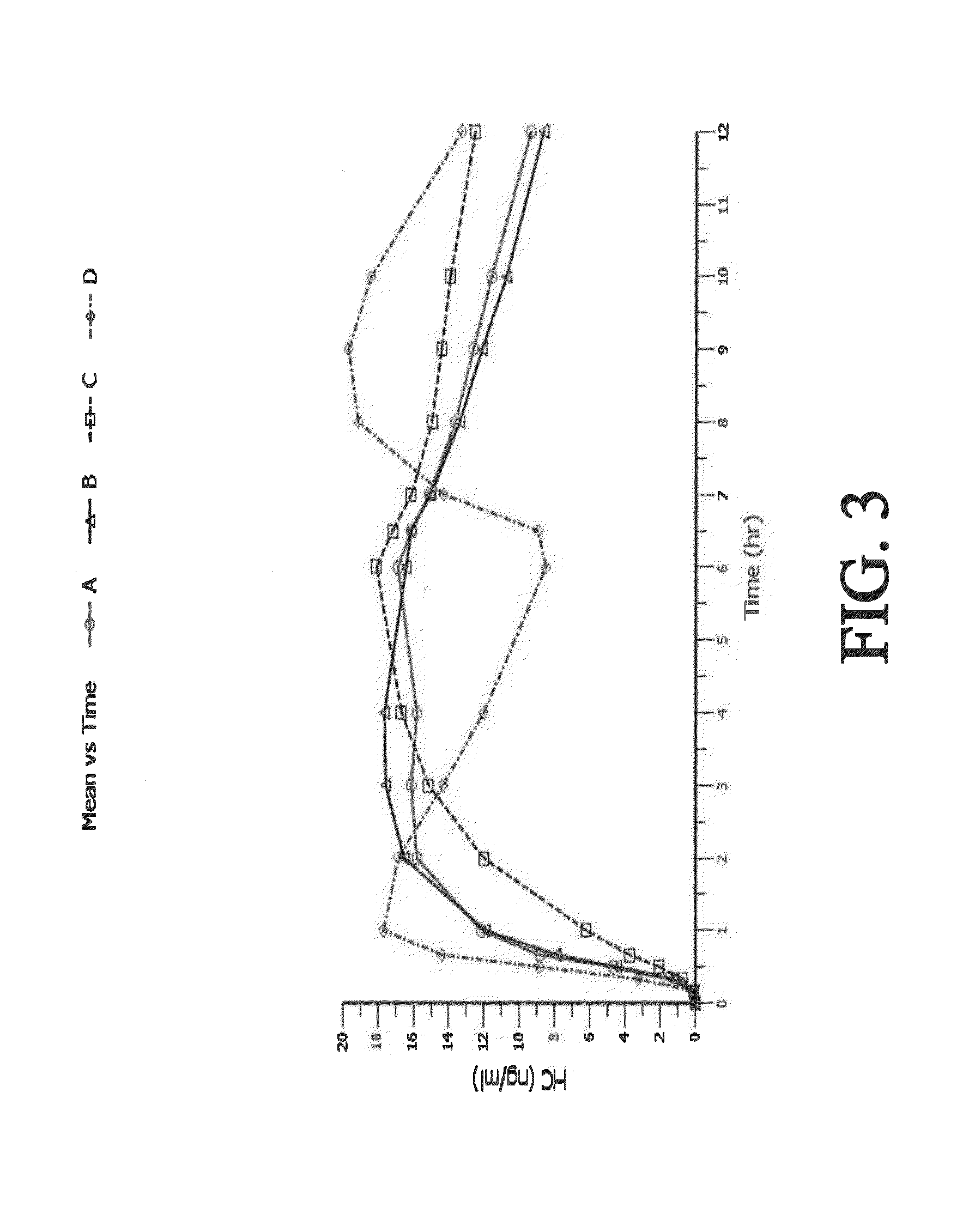
Tamper resistant composition comprising hydrocodone and acetaminophen for rapid onset and extended duration of analgesia
The present disclosure provides an extended release pharmaceutical composition comprising hydrocodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
Rapid mucosal gel or film insulin compositions
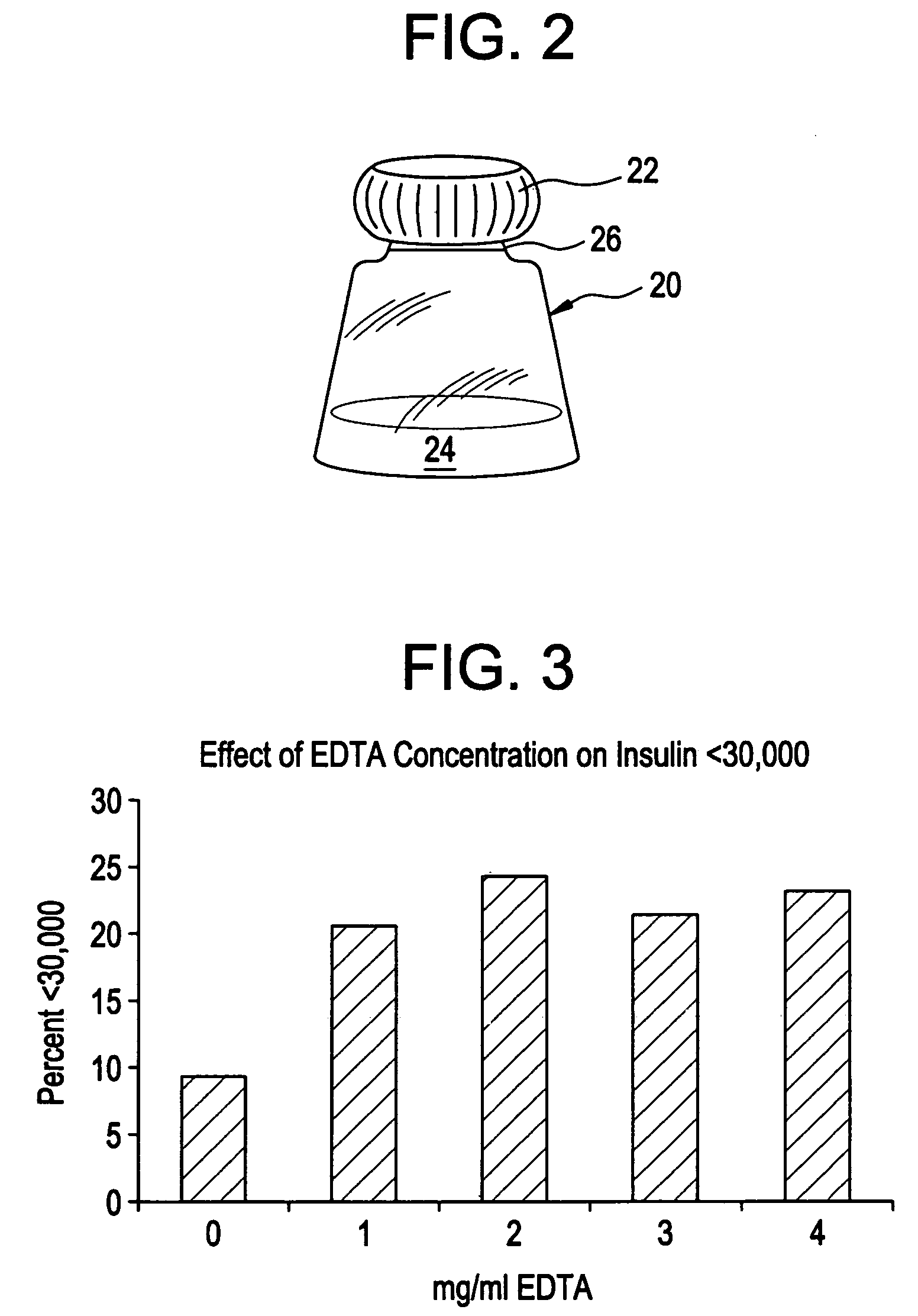
InactiveUS20080096800A1Improve stabilityRapid onsetPowder deliveryPeptide/protein ingredientsWhole bodyDissolution
Gel, powder, suspension, emulsions or film formulations for systemic delivery of insulin with improved stability and rapid onset of action are described herein. The formulations are preferably absorbed to a mucosal surface, most preferably via buccal or sublingual administration, although rectal, vaginal, nasal or ocular administration is possible. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. Following administration, these formulations are rapidly absorbed into the blood stream. The formulation is preferably a polymeric gel, powder or film which adheres to the mucosal surface, thereby enhancing uptake of the incorporated drug. In the preferred embodiment, this formulation is administered sublingually, most preferably before a meal or after a meal.
Owner:BIODEL INC
Tamper Resistant Composition Comprising Hydrocodone And Acetaminophen For Rapid Onset And Extended Duration Of Analgesia
The present disclosure provides an extended release pharmaceutical composition comprising hydrocodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
Compositions Comprising An Opioid And An Additional Active Pharmaceutical Ingredient For Rapid Onset And Extended Duration Of Analgesia That May Be Administered Without Regard To Food
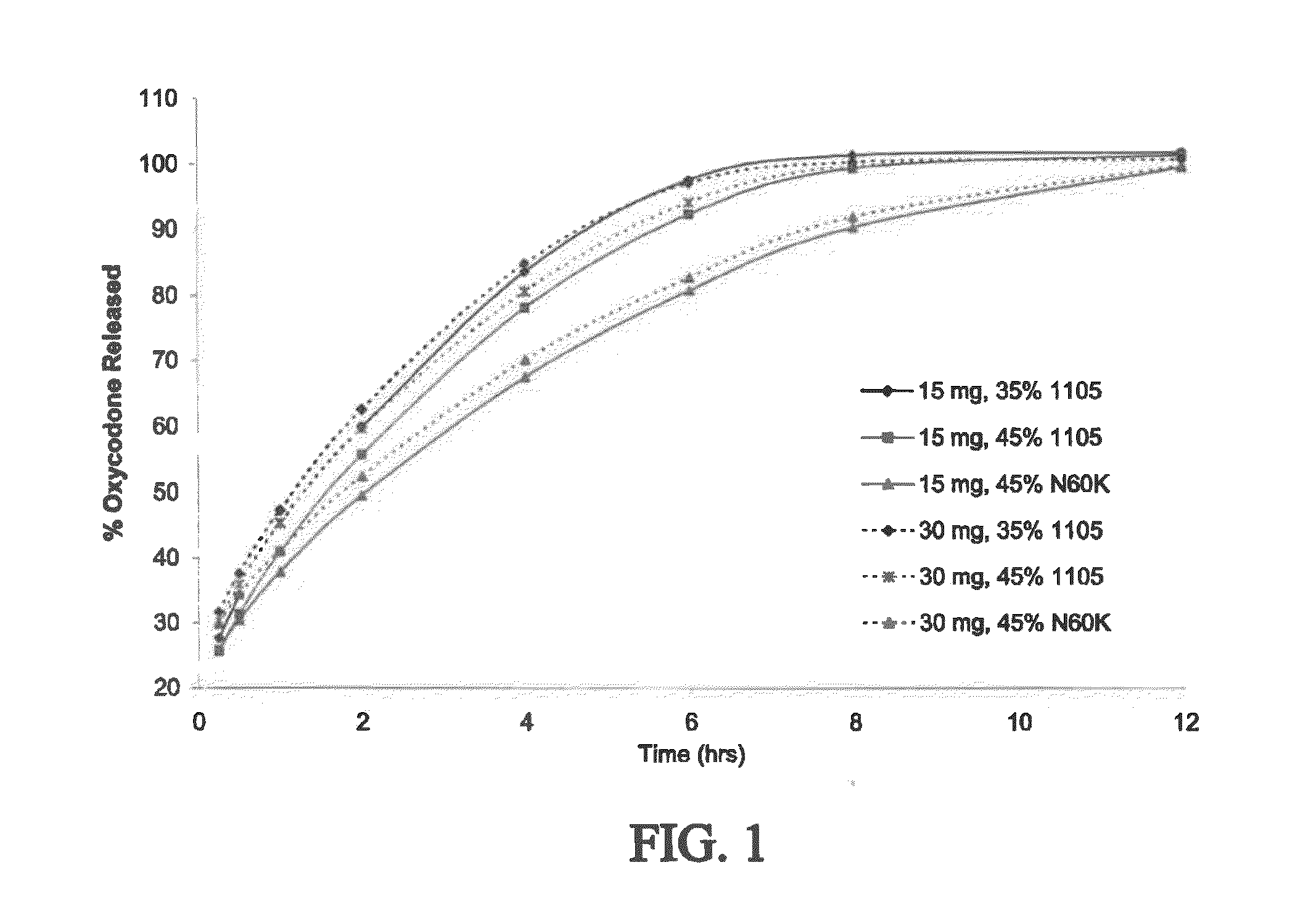
The present disclosure provides pharmaceutical compositions comprising an opioid and an additional active pharmaceutical ingredient, wherein the composition exhibits gastric retentive properties which are achieved by a combination of a physical property of the composition and release of the opioid, wherein upon administration to a subject, the composition has at least one pharmacokinetic parameter that differs by less than about 30% when the subject is in a fasted state as compared to a fed state. The present disclosure further provides pharmaceutical composition comprising oxycodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are an extended release pharmaceutical composition comprising oxycodone and acetaminophen that provides reduced abuse potential.
Owner:MALLINCKRODT INC
Rapid Acting Injectable Insulin Compositions
InactiveUS20080090753A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsDissolutionExcipient
Injectable insulin formulations with improved stability and rapid onset of action are described herein. The formulations may be for subcutaneous, intradermal or intramuscular administration, In the preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. These formulations are rapidly absorbed into the blood stream when administered by subcutaneous injection. In the preferred embodiment, the insulin is provided as a dry powder in a sterile vial. This is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water, a zinc chelator such as EDTA and a dissolution agent such as citric acid shortly before or at the time of administration. In another embodiment, the insulin is stored as a frozen mixture, ready for use upon thawing.
Owner:BIODEL
Drug delivery system for conscious sedation
InactiveUS20030233086A1Increase oxygenationRelief the painHalogenated hydrocarbon active ingredientsNervous disorderAmnesiaSedation
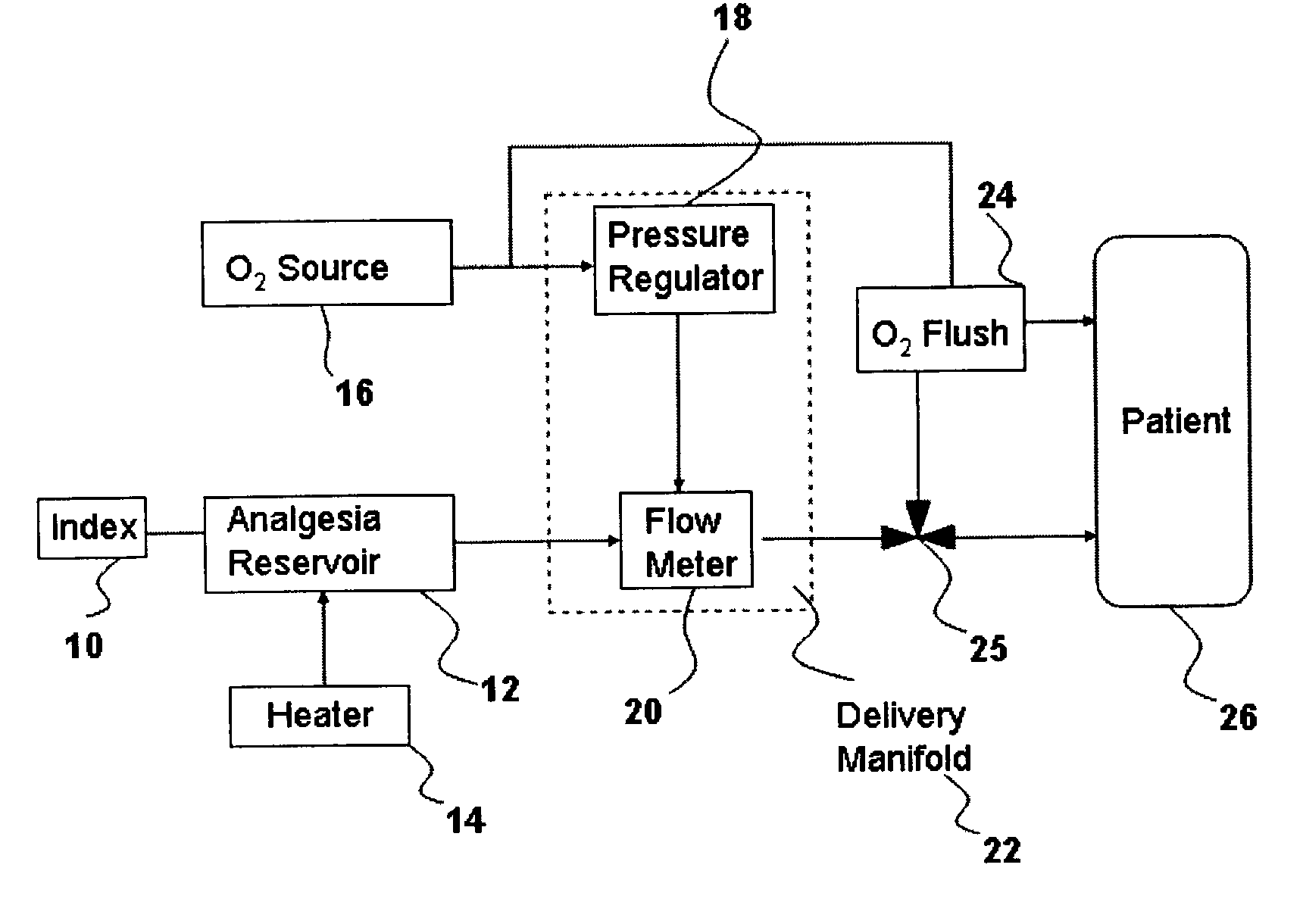
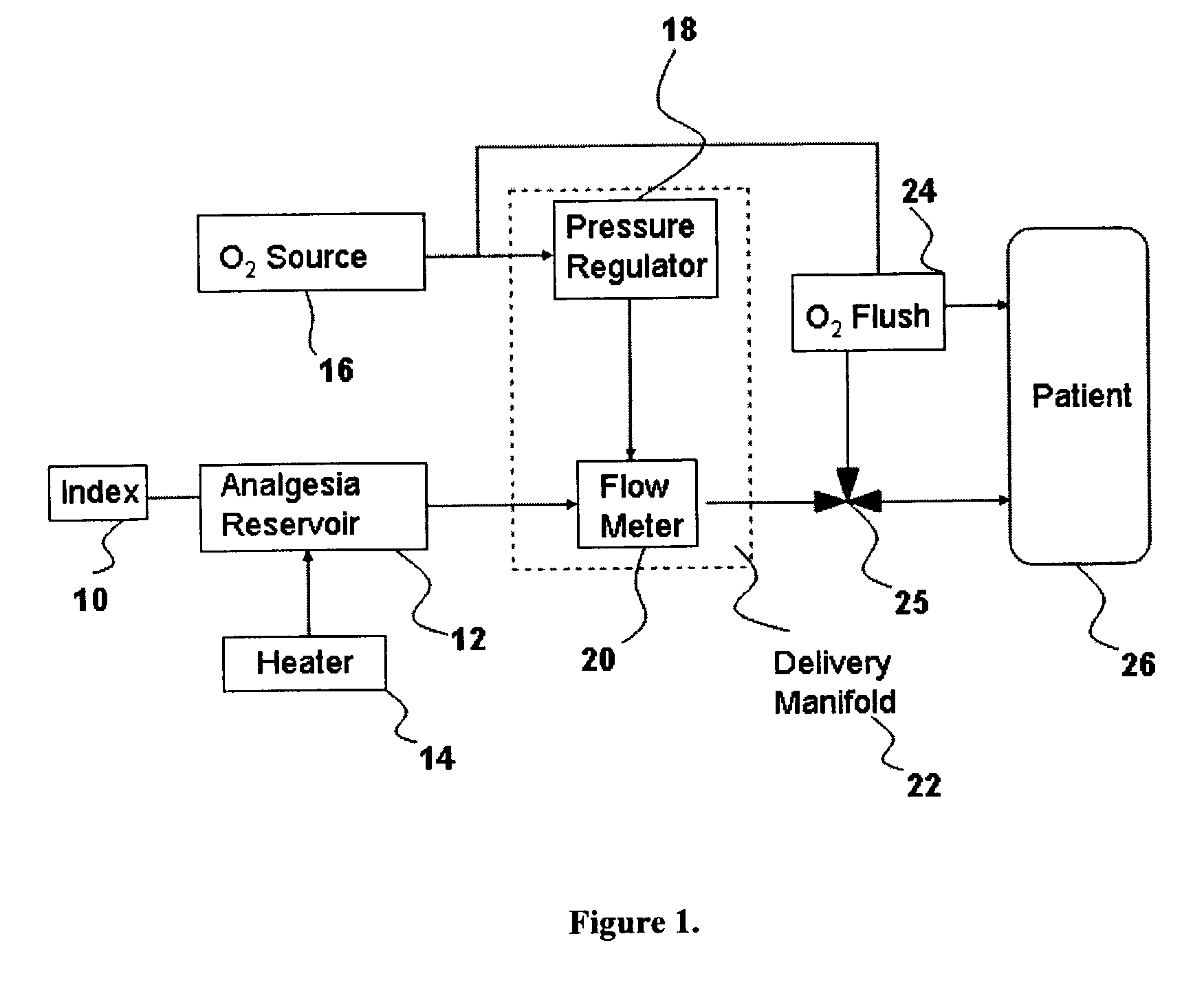
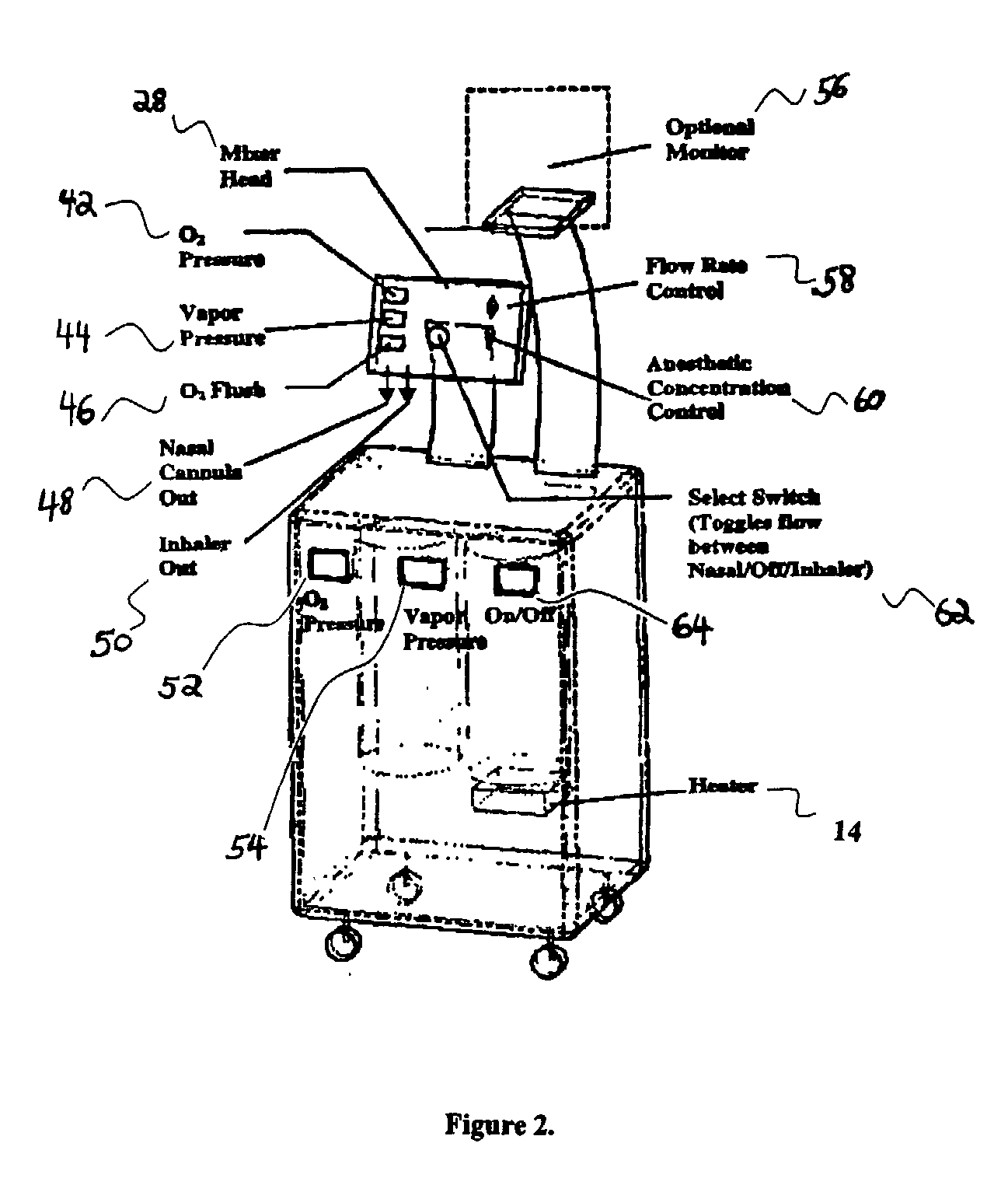
Inhalant anesthetics are developed with a number of properties including rapid onset and recovery, controllability, and, ideally, a broad safety profile. The efficacy of these agents is measured by their ability to create anesthesia within the framework of the other desirable properties. The instant invention focuses on the dosage level where analgesia occurs but amnesia or lack of consciousness does not. In addition to identifying the dosage level where pain is sharply reduced or eliminated but awareness remains, a delivery system for safe and effective delivery of the agent is described.
Owner:FIRST NIAGARA BANK
Rapid Mucosal Gel or Film Insulin Compositions
InactiveUS20080085298A1Improve stabilityRapid onsetPowder deliveryPeptide/protein ingredientsNasal cavityWhole body
Gel, powder, suspension, emulsions or film formulations for systemic delivery of insulin with improved stability and rapid onset of action are described herein. The formulations are preferably absorbed to a mucosal surface, most preferably via buccal or sublingual administration, although rectal, vaginal, nasal or ocular administration is possible. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. Following administration, these formulations are rapidly absorbed into the blood stream. The formulation is preferably a polymeric gel, powder or film which adheres to the mucosal surface, thereby enhancing uptake of the incorporated drug. In the preferred embodiment, this formulation is administered sublingually, most preferably before a meal or after a meal.
Owner:BIODEL
Quaternary ammonium salt compound and preparation method and application thereof
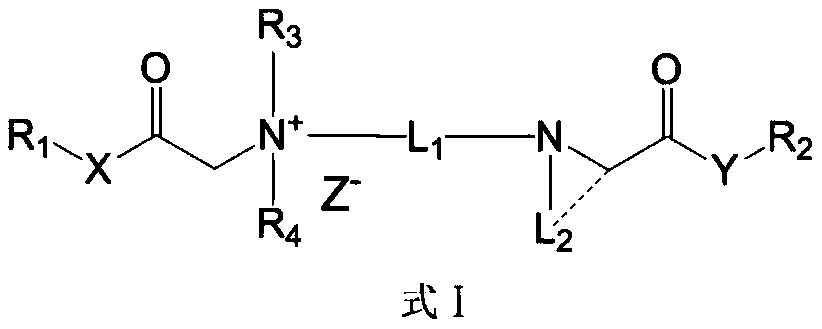
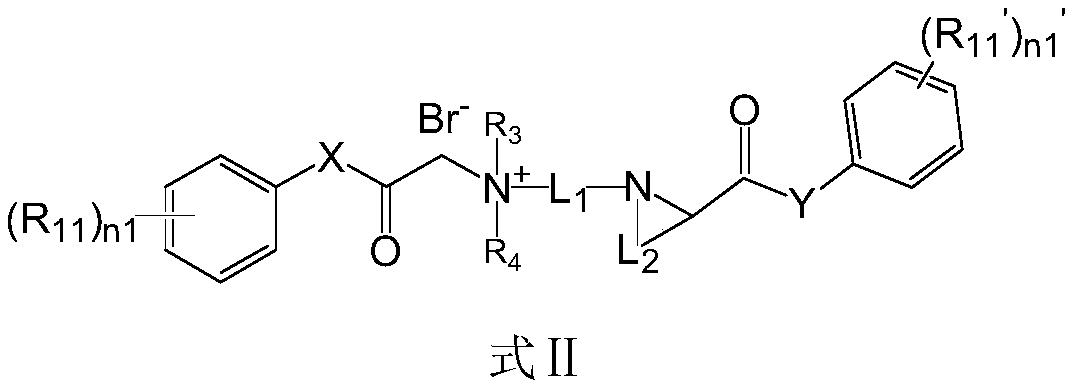
ActiveCN110156665AQuick effectImprove securityOrganic compound preparationAnaestheticsMetaboliteSide effect
The invention discloses a quaternary ammonium salt compound and a preparation method and application thereof. The invention provides a compound shown in a formula I with a novel structure, or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a solvate thereof, or a prodrug thereof, or a metabolite thereof. The compound has the advantages of rapid onset of action, long-termlocal anesthesia effect after single administration, sensory nerve block time greater than motor nerve block time, and both long-term local anesthesia effect and selective local anesthesia effect, remarkably reduces side effects of QX314 and QX314 compositions and quaternary ammonium salt compounds with surfactant structural characteristics, and has better safety. Namely, the compound of formula Iand the pharmaceutically acceptable salt thereof can be used for preparing safe drugs with long-term local anesthesia and selective local anesthesia effects, and have the advantages of long-term local anesthesia, good local anesthesia selectivity, less nerve damage and high safety.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Method of treating alcoholism or alcohol abuse
The present invention relates to a method of treating alcoholism or alcohol abuse by administering to a subject a pharmaceutically effective amount of an opioid antagonist before imminent drinking. Particularly, the present invention relates to a method of treating alcoholism or alcohol abuse by administering transmucosally to a subject a pharmaceutically effective amount of an opioid antagonist before imminent drinking. Preferably, the opioid antagonist used in the method is nalmefene or a pharmaceutically acceptable salt thereof. The invention also relates to a method of treating alcoholism or alcohol abuse by administering to a subject before imminent drinking a transmucosal preparation comprising a pharmaceutically effective amount of an opioid antagonist, wherein the transmucosal preparation has rapid onset of action. Advantageously, a FAH+ subject is treated. Further, the invention relates to a method of treating alcoholism or alcohol abuse of a FAH+ subject, comprising extinguishing an alcohol-drinking response by administering to the FAH+ subject a pharmaceutically effective amount of nalmefene or a pharmaceutically acceptable salt thereof.
Owner:CONTRAL PHARMA
An aromatic essential oil drug for preventing and treating depression and neurosis, boosting mood and improving brain function and its composition
InactiveCN102258546AAvoid first pass effectEasy to useNervous disorderHydroxy compound active ingredientsSide effectBULK ACTIVE INGREDIENT
The invention discloses an aromatic essential oil drug for preventing and treating depression, boosting mood and improving brain function and its composition, which contains main pharmacological active ingredients: vanillin, vanillin salt or isomers of vanillin Any one, two or three mixtures can also be added with other natural spices and native plant essential oils such as rosewood, clove, benzoin essential oils and so on. The medicine of the present invention stimulates the olfactory nerve through the nose and the oral cavity to regulate the olfactory center of the brain and then affect different brain regions, so as to improve brain function, relieve depression, improve mood, and reduce suicide rate, and has quick onset, low concentration, no toxic side effect.
Owner:李光武 +1
Combination composition comprising oxycodone and acetaminophen for rapid onset and extended duration of analgesia
The present disclosure provides an extended release pharmaceutical composition comprising oxycodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
Anesthetic composition for topical administration comprising lidocaine, prilocaine and tetracaine
ActiveUS20070269465A9Rapid onsetImprove skinBiocidePharmaceutical delivery mechanismHigh concentrationHyaluronidase
Owner:FITA FERNANDO BOUFFARD
Gastric acid secretion inhibiting composition
InactiveUS20060115530A1Delayed and extended releaseExtended releaseAntibacterial agentsBiocideDepressantSecreted substance
An oral pharmaceutical dosage form comprises pharmacologically effective amounts of an acid-susceptible proton pump inhibitor and an H2 receptor antagonist in combination with at least on pharmacologically acceptable excipient which causes a delayed release and / or an extended release of the proton pump inhibitor. The H2 receptor antagonist is included in the dosage form in such a way that it is rapidly released after administration. This dosage form is suitable for the treatment of conditions associated with an excessive secretion of gastric acid and provides a suitable combination of a rapid onset and a long-lasting duration of the effect. The invention also relates to a method for manufacturing such a dosage form and to a method for the treatment of conditions associated with the secretion of gastric acid.
Owner:OREXO AB
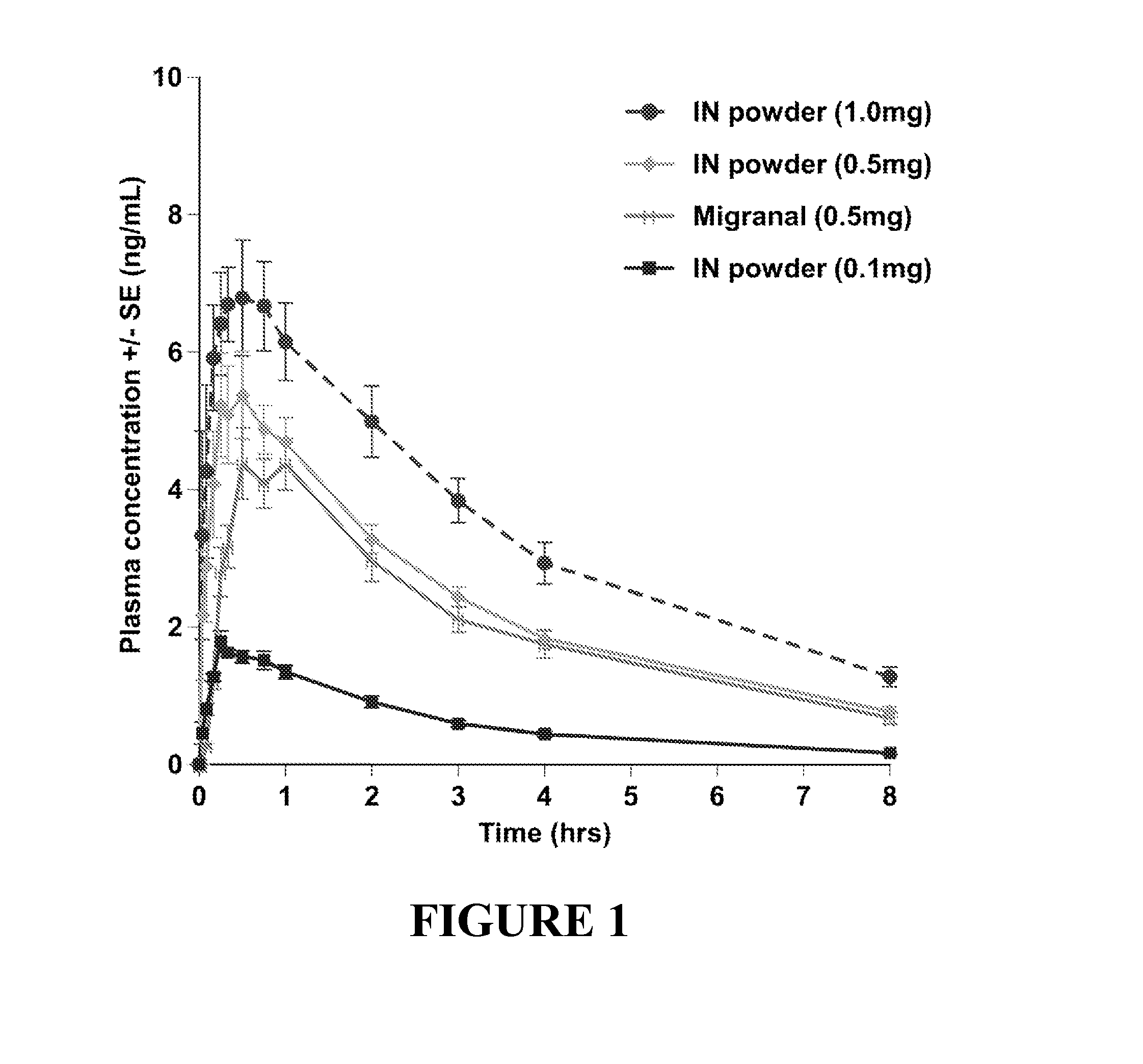
Intranasal dhe for the treatment of headache
Presented herein are powder formulations comprising dihydroergotamine (DHE), or a pharmaceutically acceptable salt thereof. In addition to such formulations, also presented herein are methods comprising intranasally administering powder formulations comprising dihydroergotamine, or a pharmaceutically acceptable salt thereof. The presented methods can be used for treating headache, for example, for rapid onset treatment of headache, including migraine, e.g. acute treatment of migraine with or without aura.
Owner:SATSUMA PHARMA INC
Rapid Onset and Short Term Modafinil Compositions and Methods of Use Thereof
InactiveUS20090123545A1Good conditionRapid onsetOrganic active ingredientsBiocideEnantiomerIntensive care medicine
Compositions are described that comprise a modafÊnil component that is a combination of the d- and l-enantiomers of modafinil and wherein the modafÊnil component is greater than 50% by weight d-modafÊnil for use in promoting or enhancing the state of wakefulness, alertness, and / or central nervous system stimulation in an individual.
Owner:NEUROHEALING PHARMA INC
Peptide boronic acid compounds useful in anticoagulation
InactiveUS20050282757A1Reduce the amount of solutionImprove consistencyBiocideDipeptide ingredientsThrombin activityBoronic acid
A method for preventing thrombosis in a setting where rapid onset and / or rapid offset of anticoagulation is required, comprising administering a compound selected from the group consisting of boronic acids which have a neutral thrombin P1 domain linked to a hydrophobic moiety capable of binding to the thrombin S2 and S3 subsites, and pharmaceutically acceptable salts, prodrugs and pharmaceutically acceptable prodrug salts of such acids.
Owner:PAION GMBH
Compound betamethasone suspension injection
InactiveCN101167730A"block" does not appearGood injectabilityOrganic active ingredientsComponent separationSolubilityCollagen disease
The invention provides a compound betamethasone suspension injection, which is a compound preparation composed of low-solubility betamethasone dipropionate and high-solubility betamethasone sodium phosphate. Long-lasting, it can be used for the treatment of musculoskeletal and cartilage tissue diseases, allergic diseases, skin diseases, collagen diseases, tumors and other clinical diseases.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com