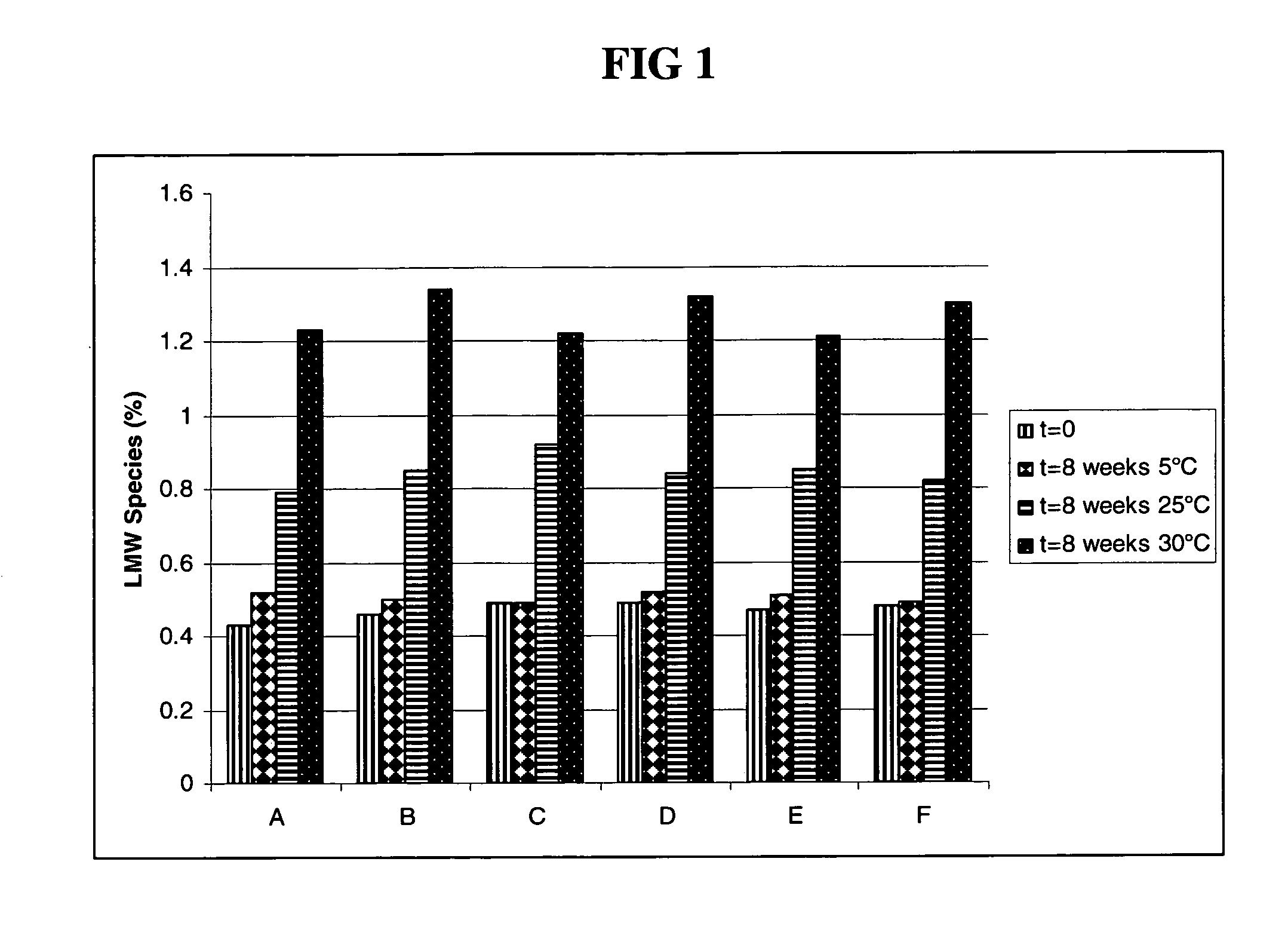
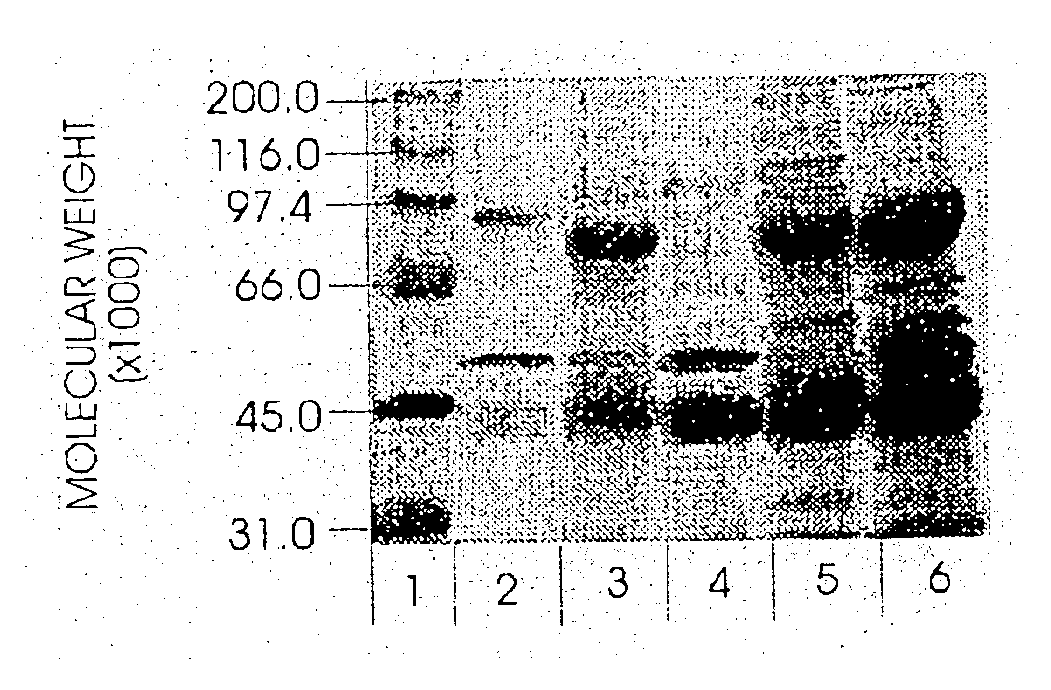
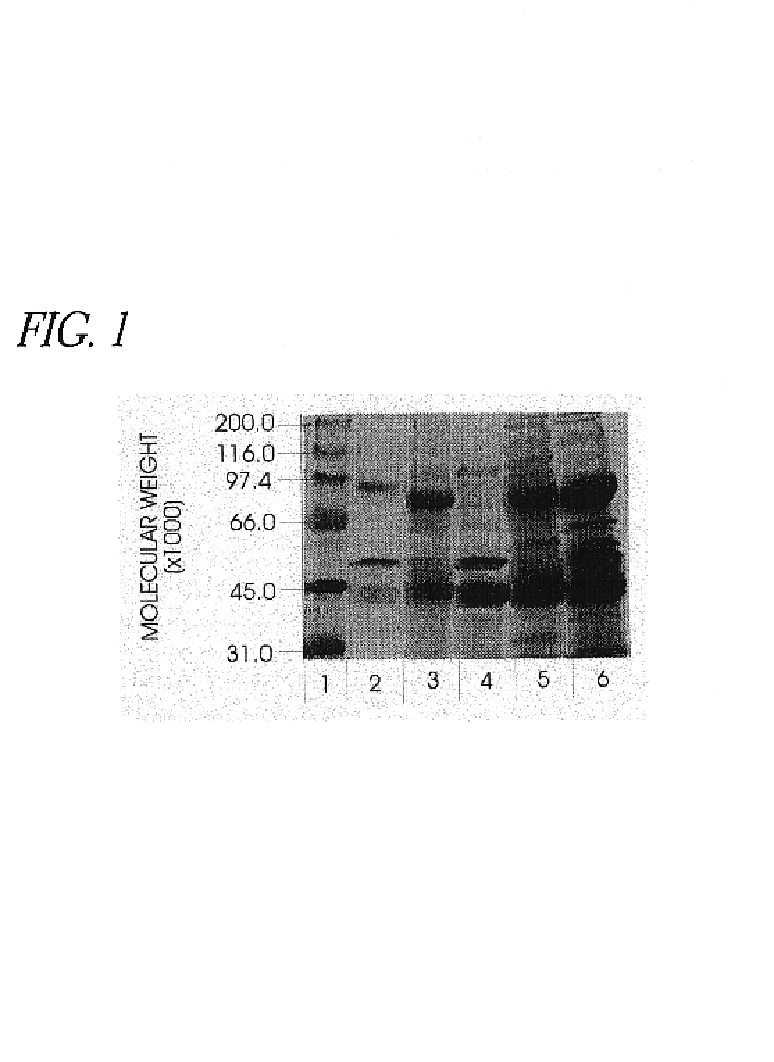
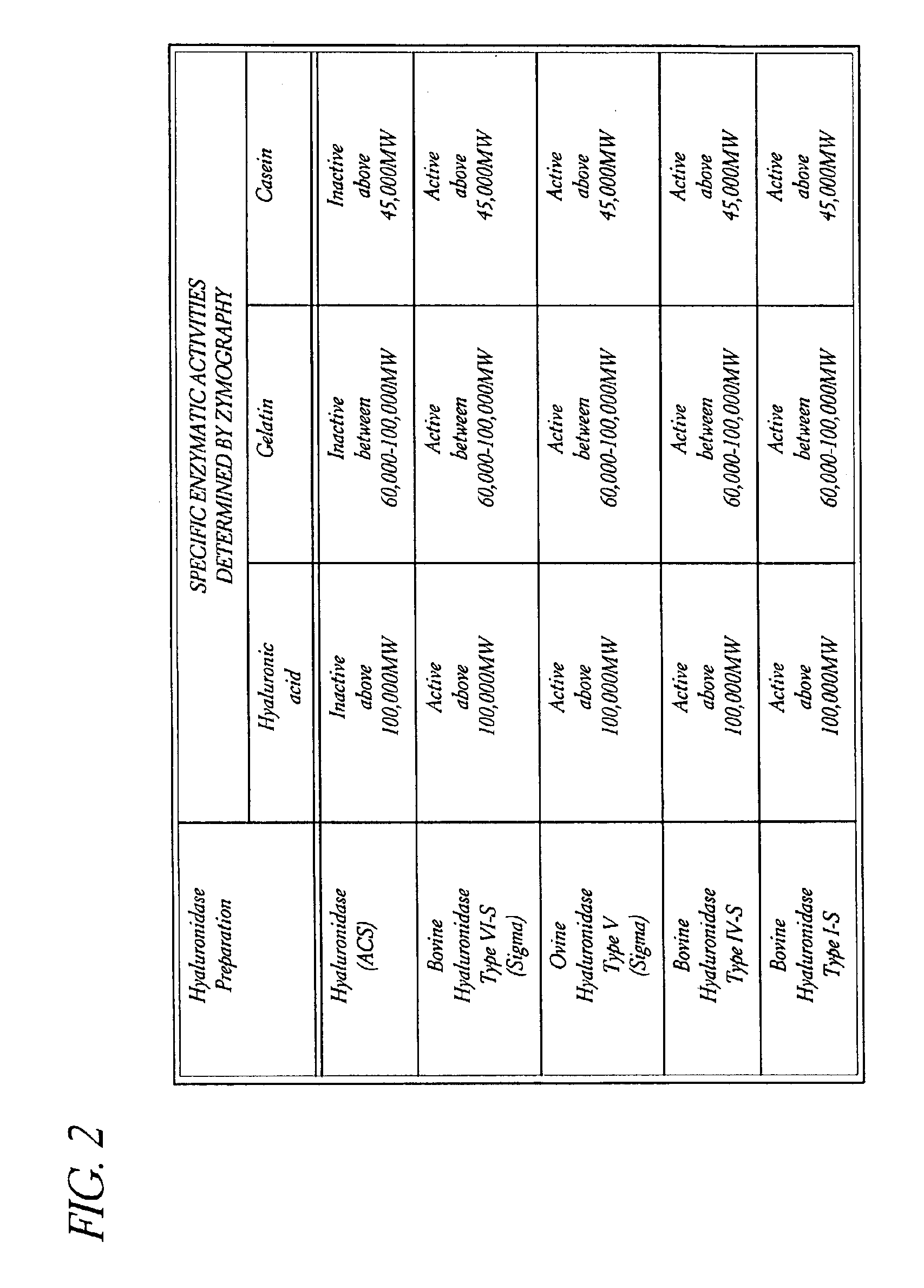
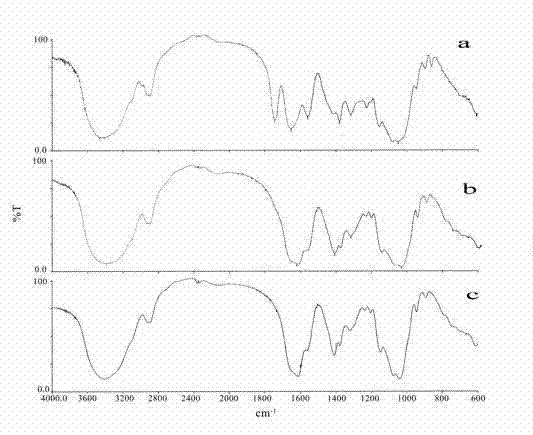
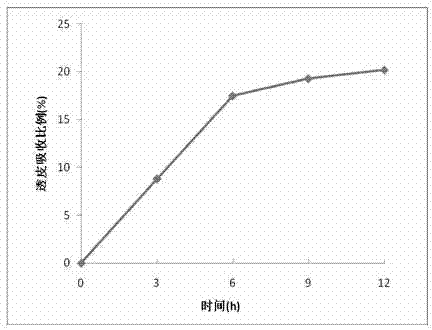
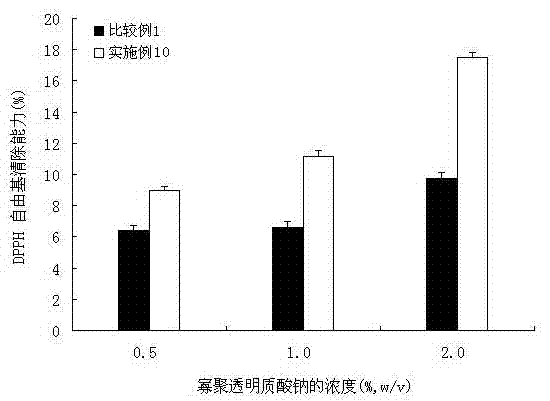
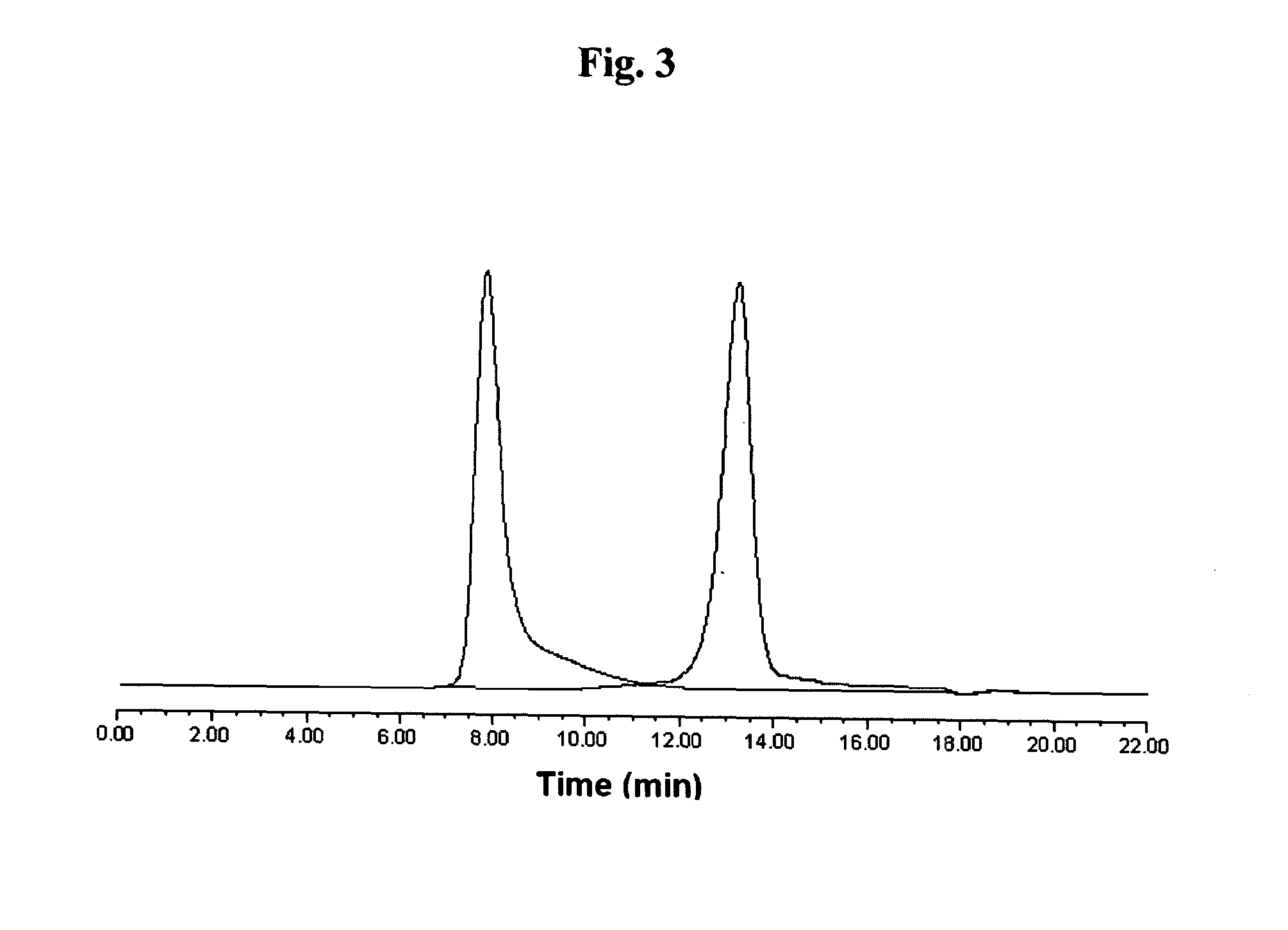
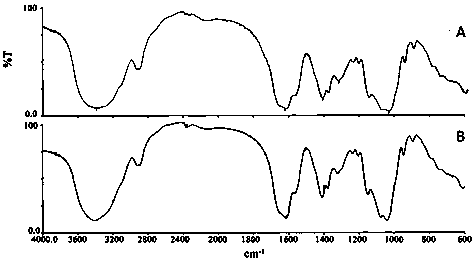
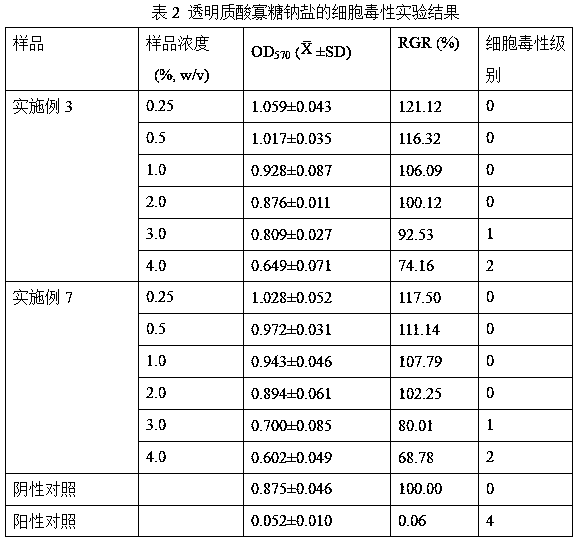
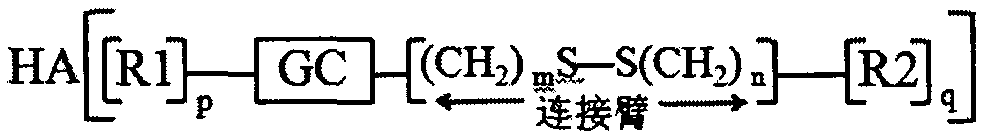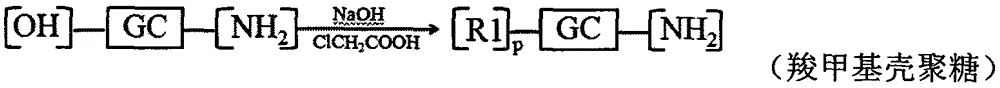Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
503 results about "Hyaluronidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
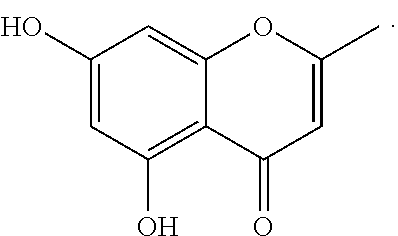
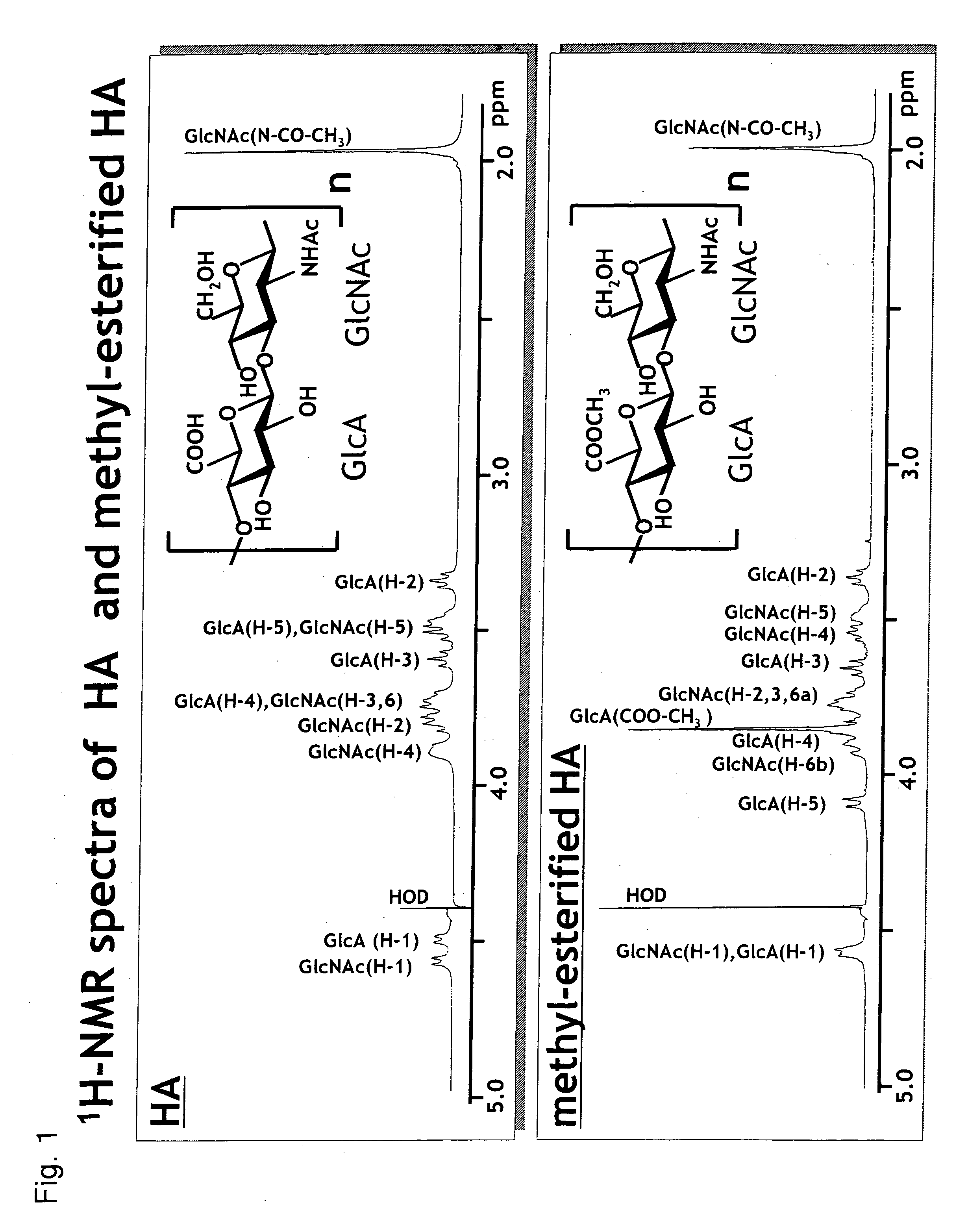
Hyaluronidases are a family of enzymes that catalyse the degradation of hyaluronic acid (HA). Karl Meyer classified these enzymes in 1971 into three distinct groups, a scheme based on the enzyme reaction products. The three main types of hyaluronidases are two classes of eukaryotic endoglycosidase hydrolases and a prokaryotic lyase-type of glycosidase.
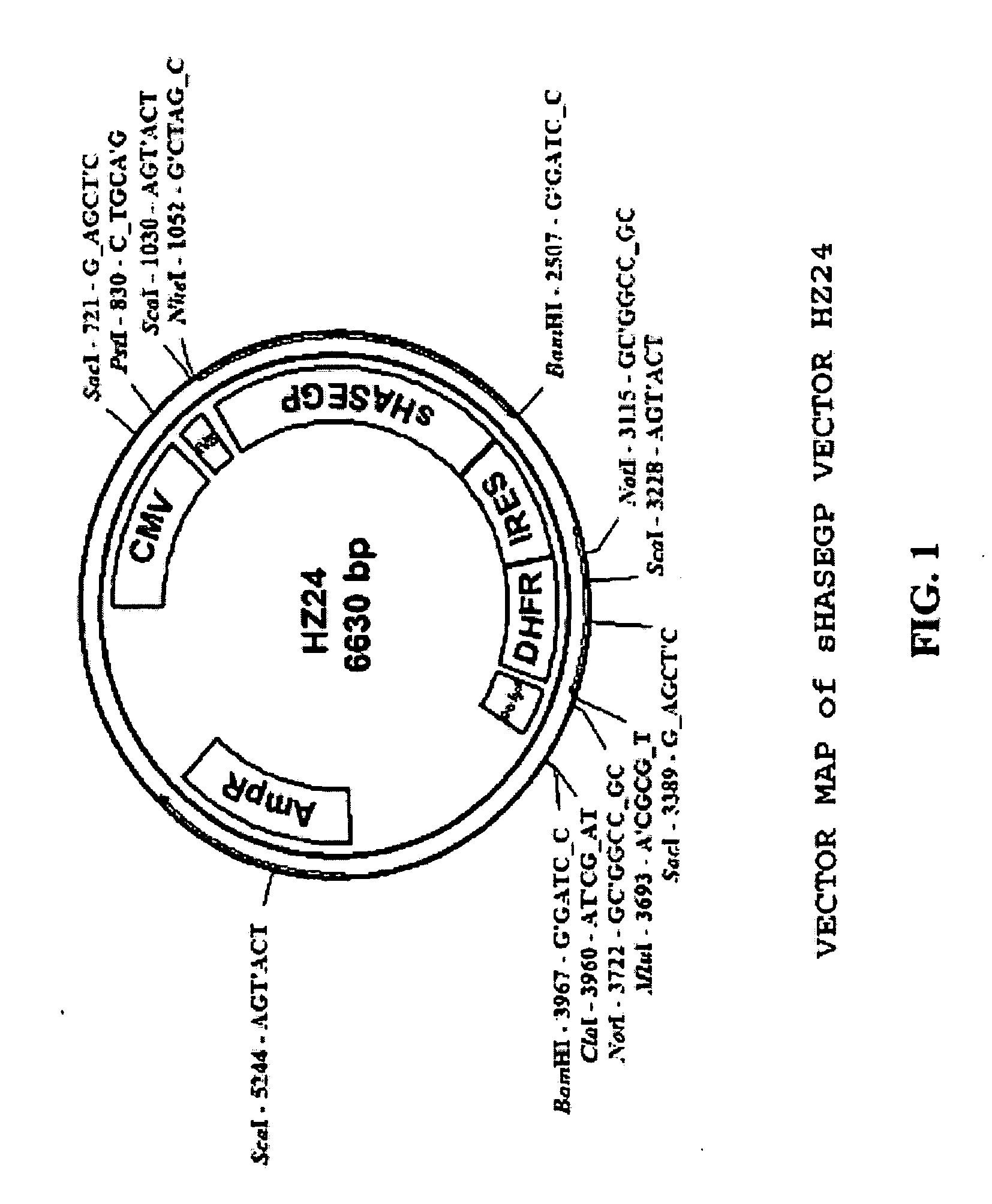
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminogly ycanases
PendingUS20060104968A1Improve extentIncrease ratingsSenses disorderNervous disorderHyaluronidaseRecombinant glycoprotein
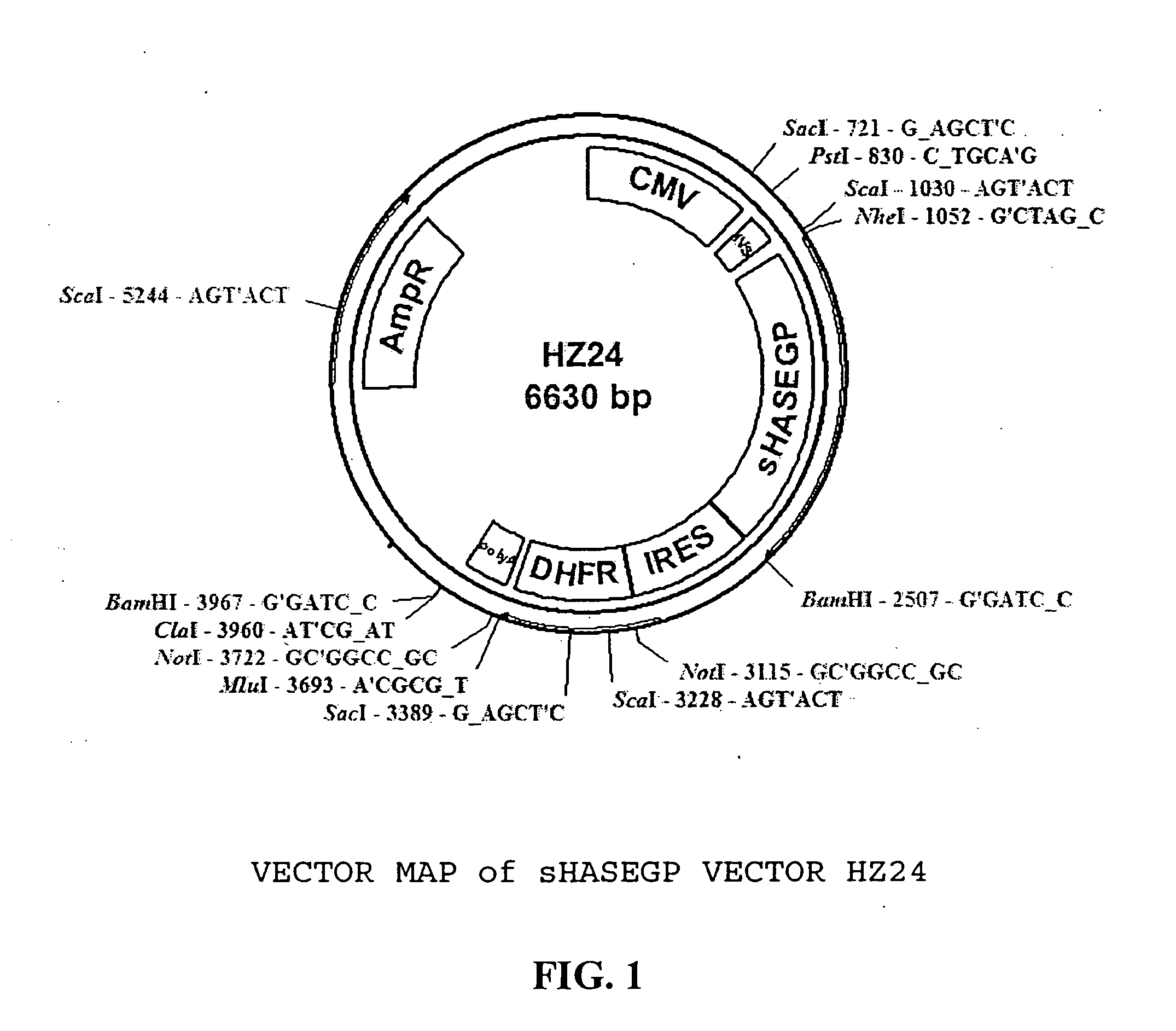
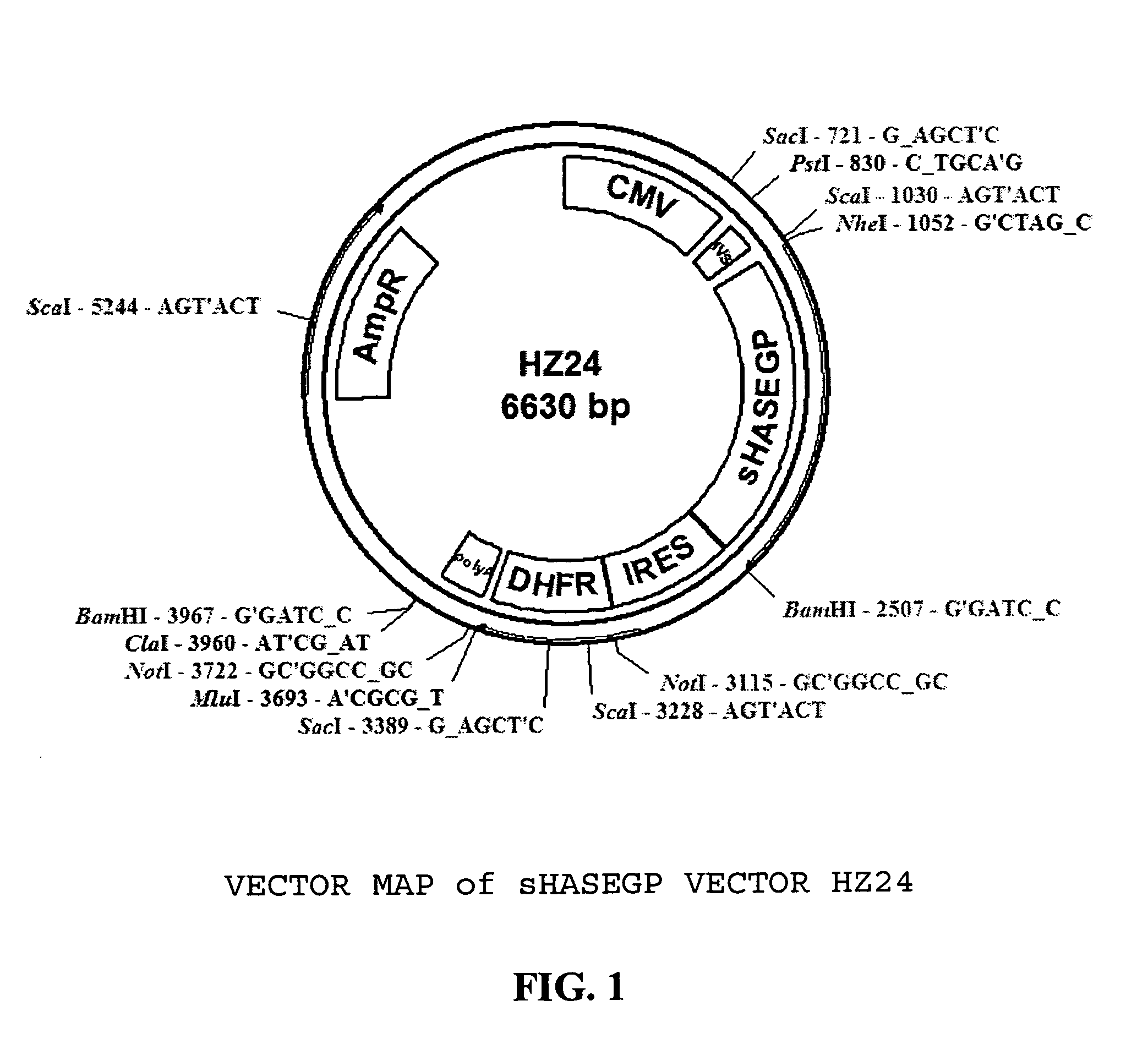
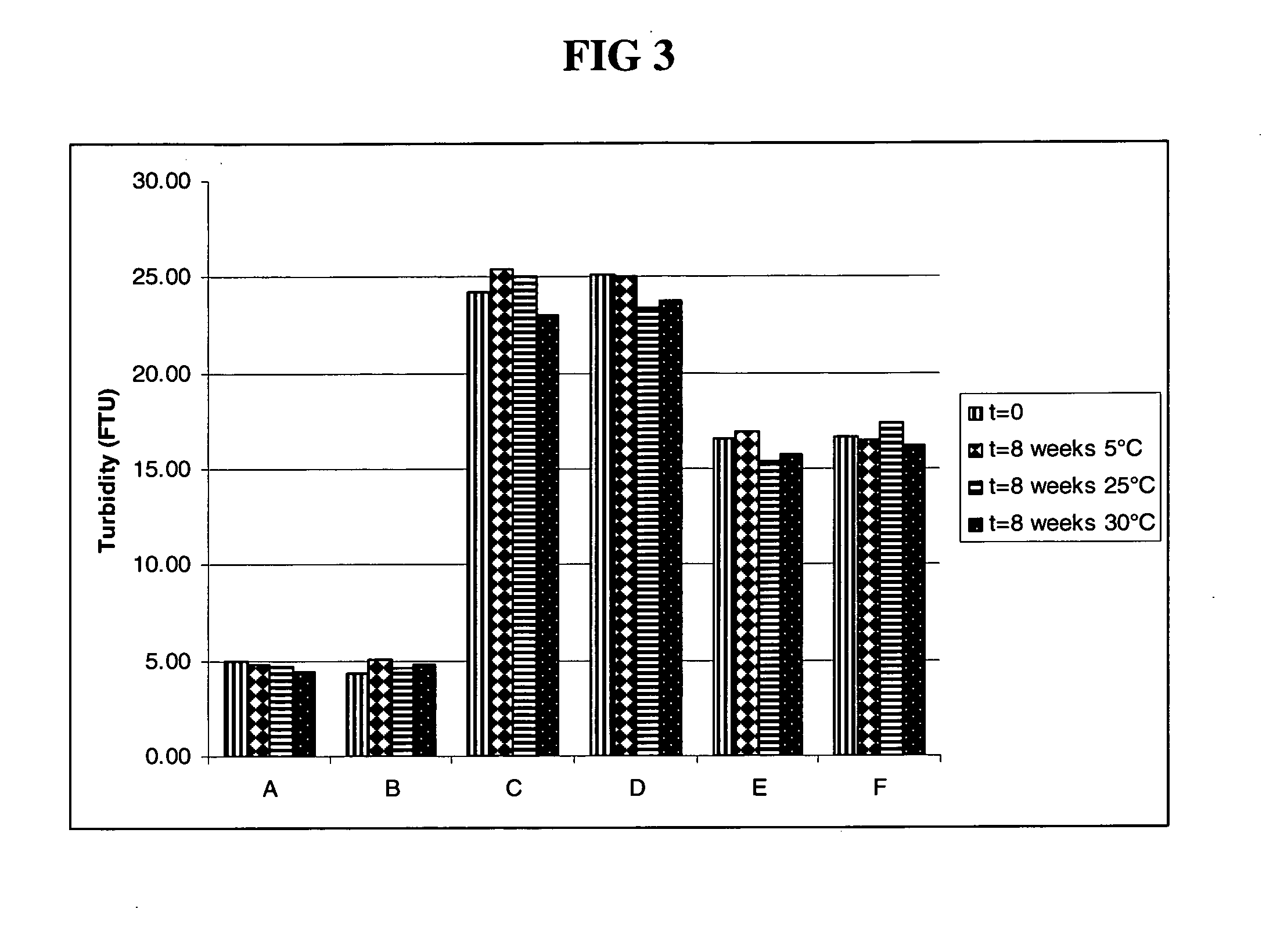
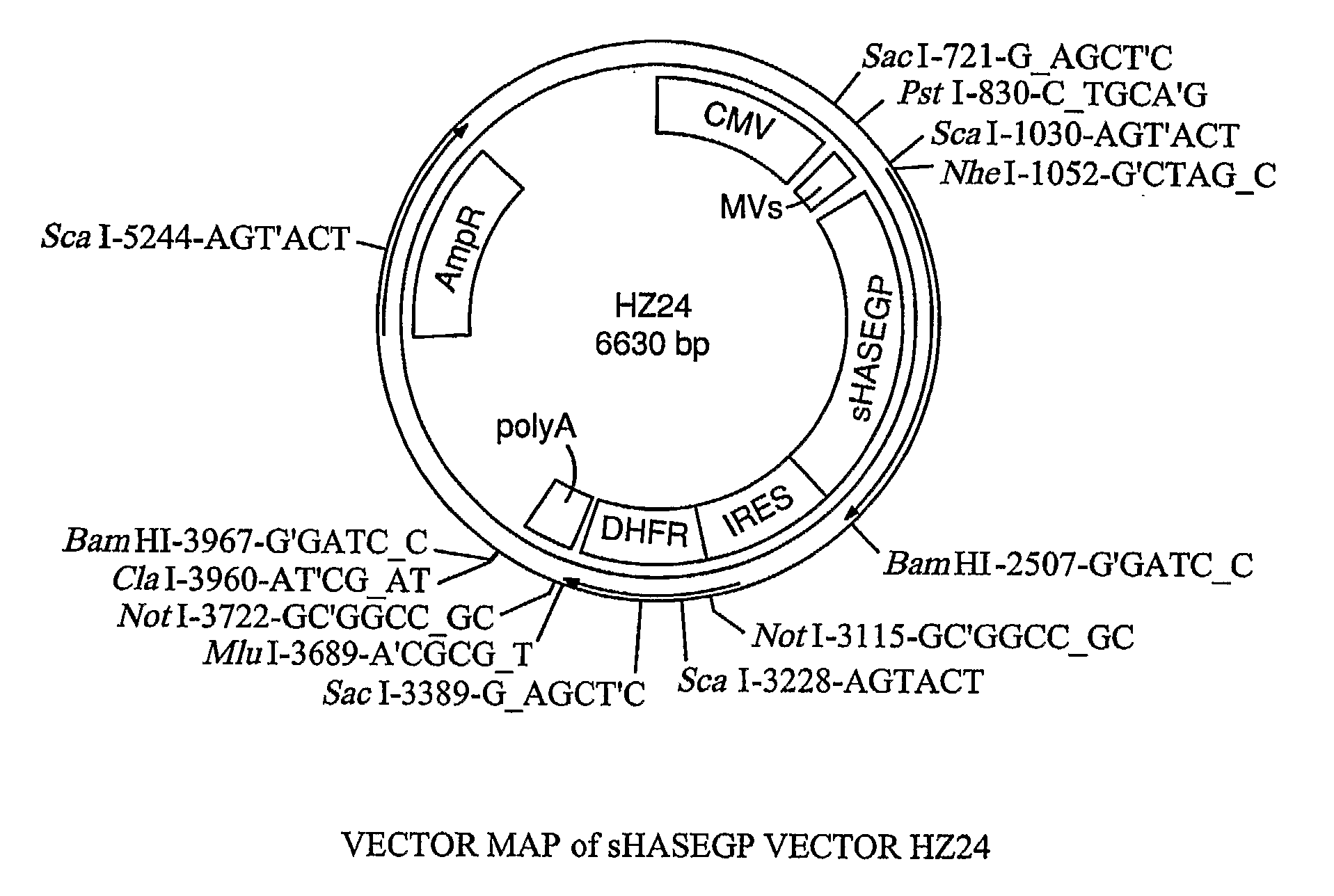
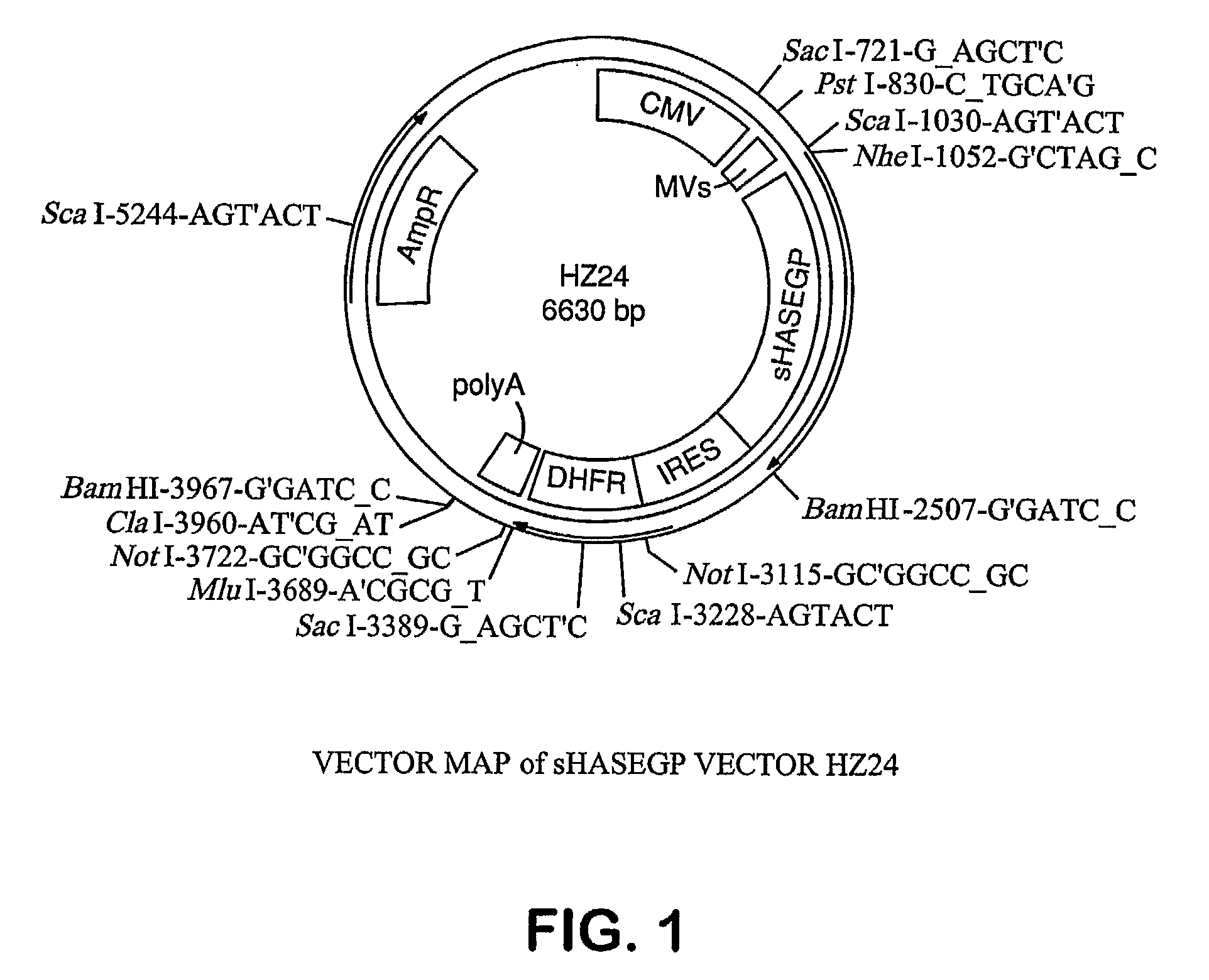
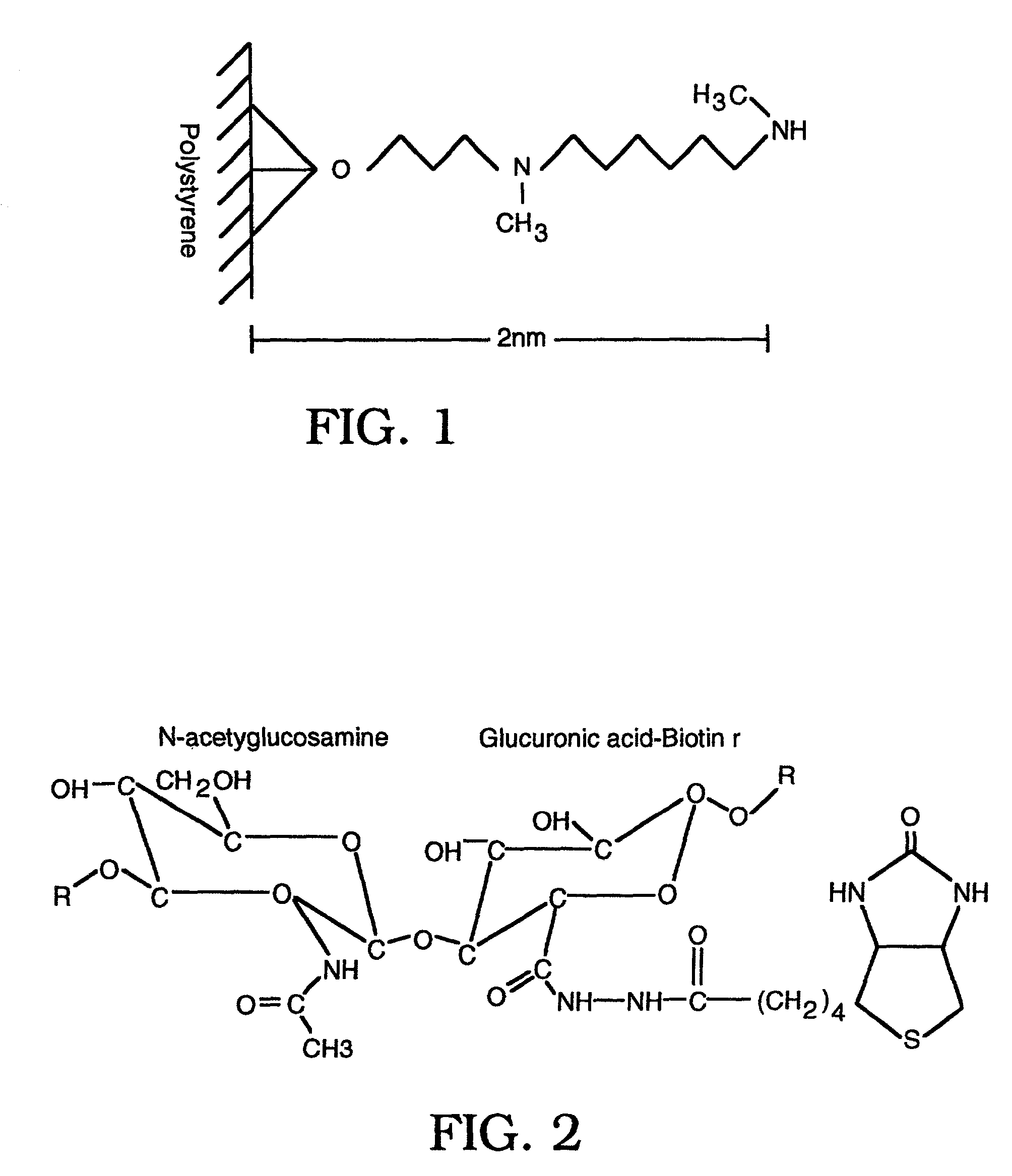
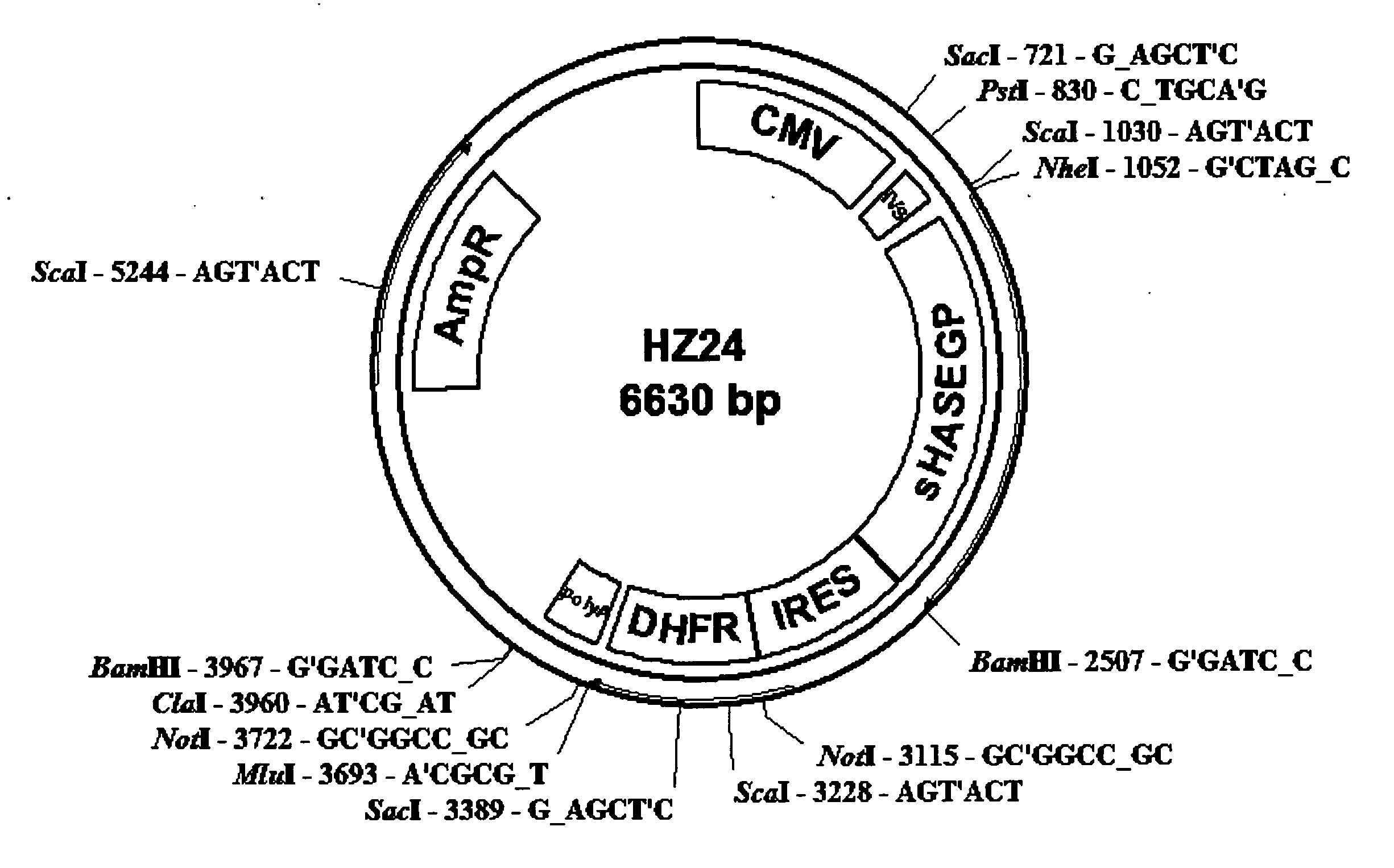
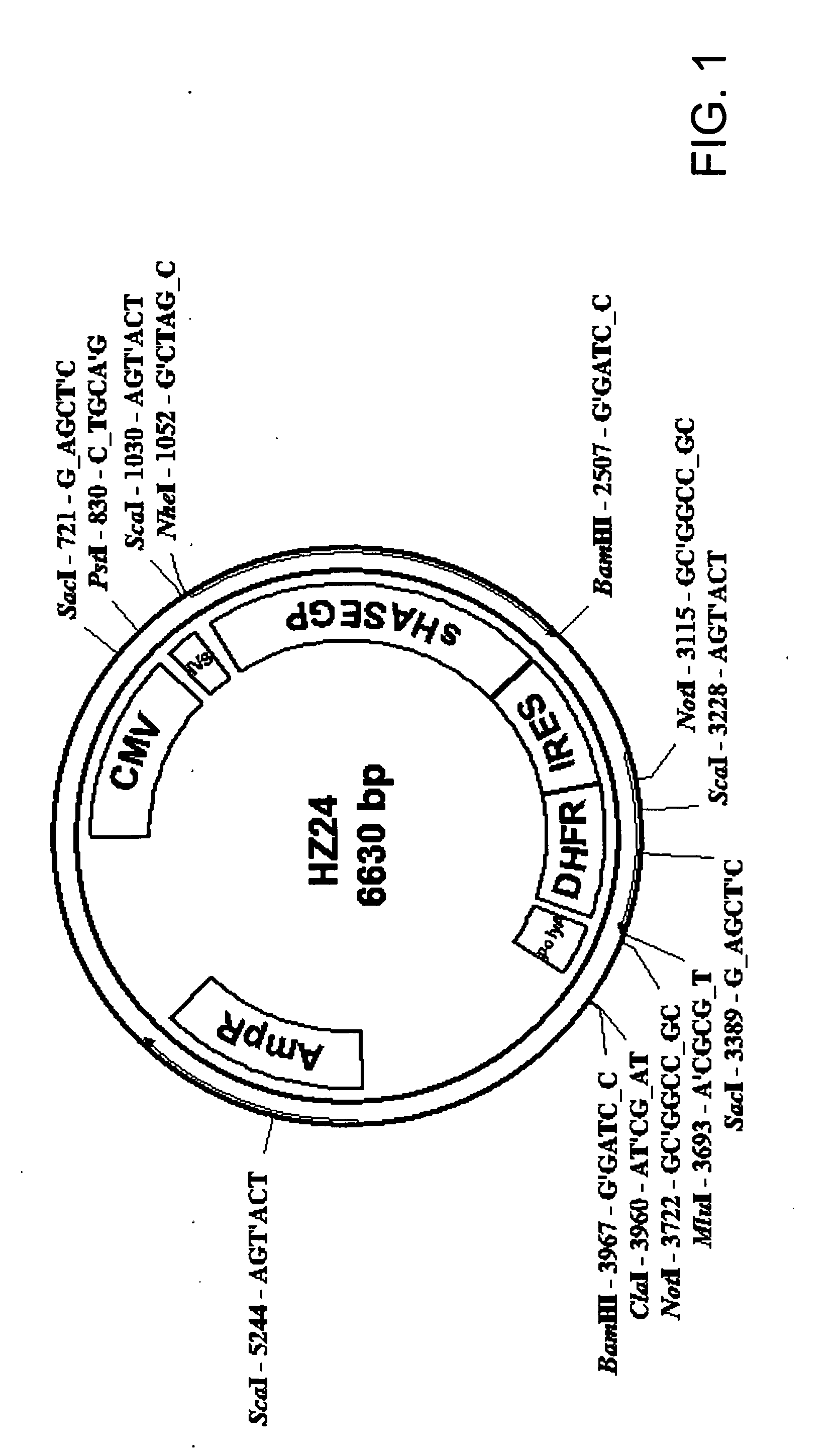
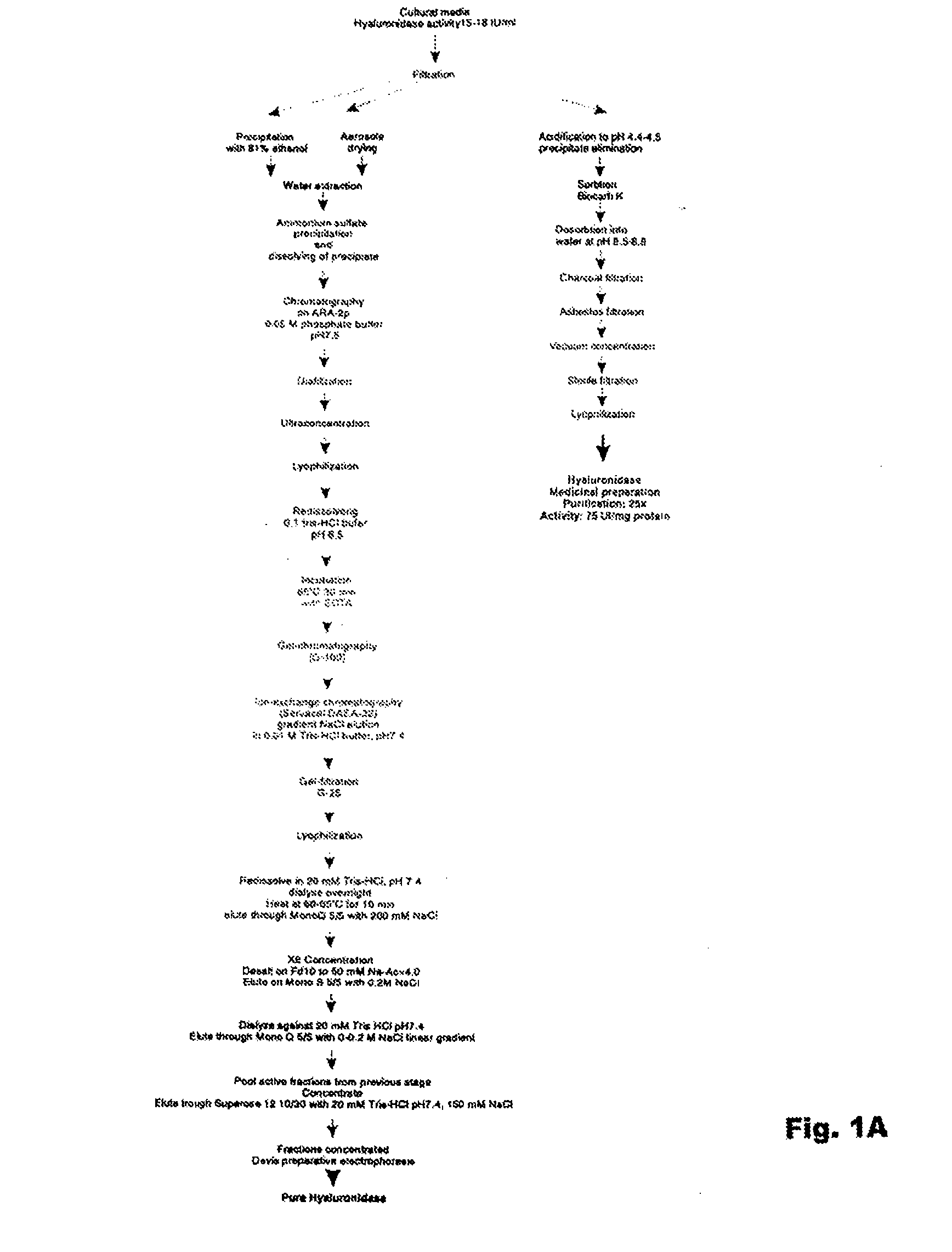
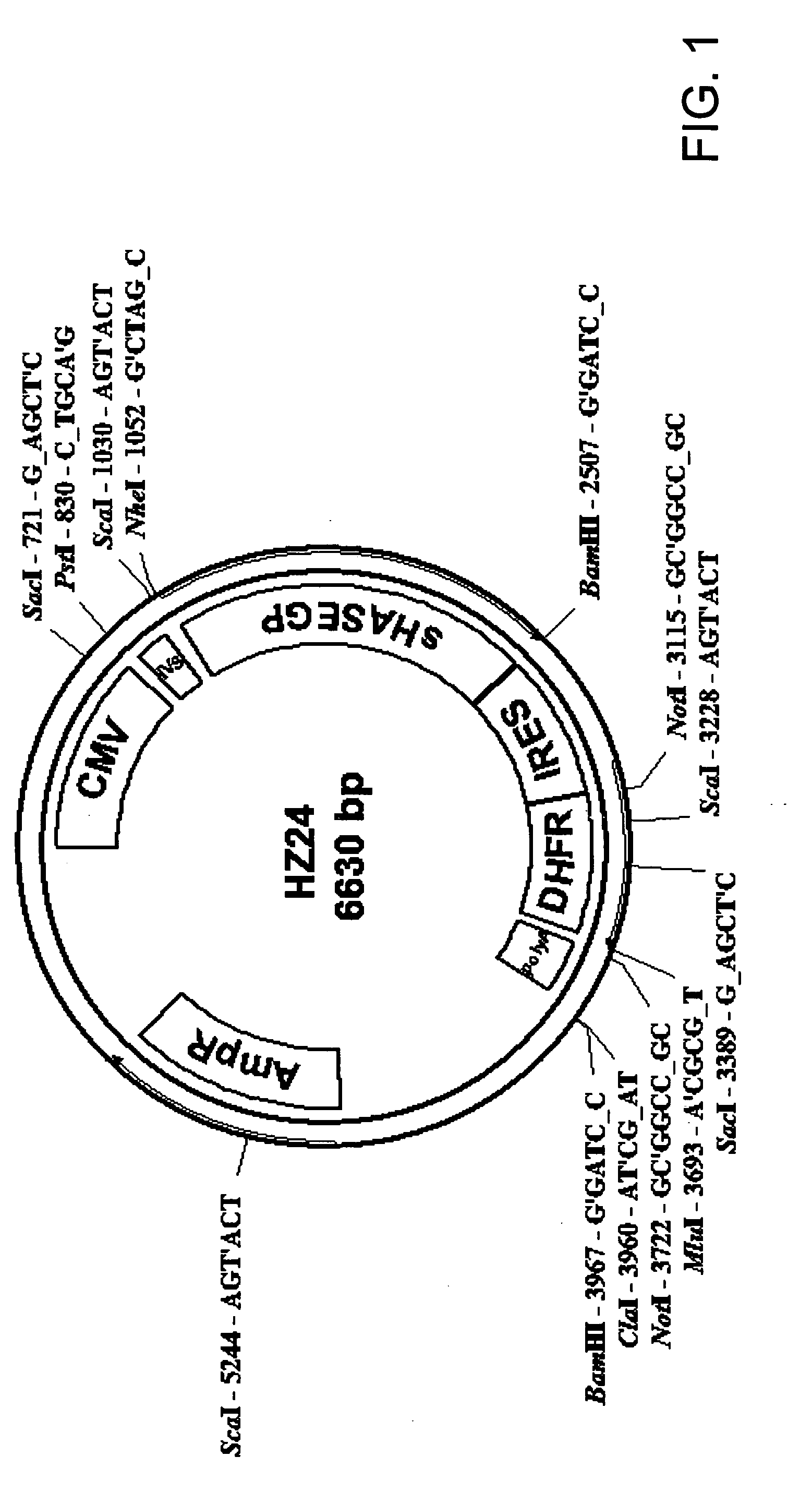
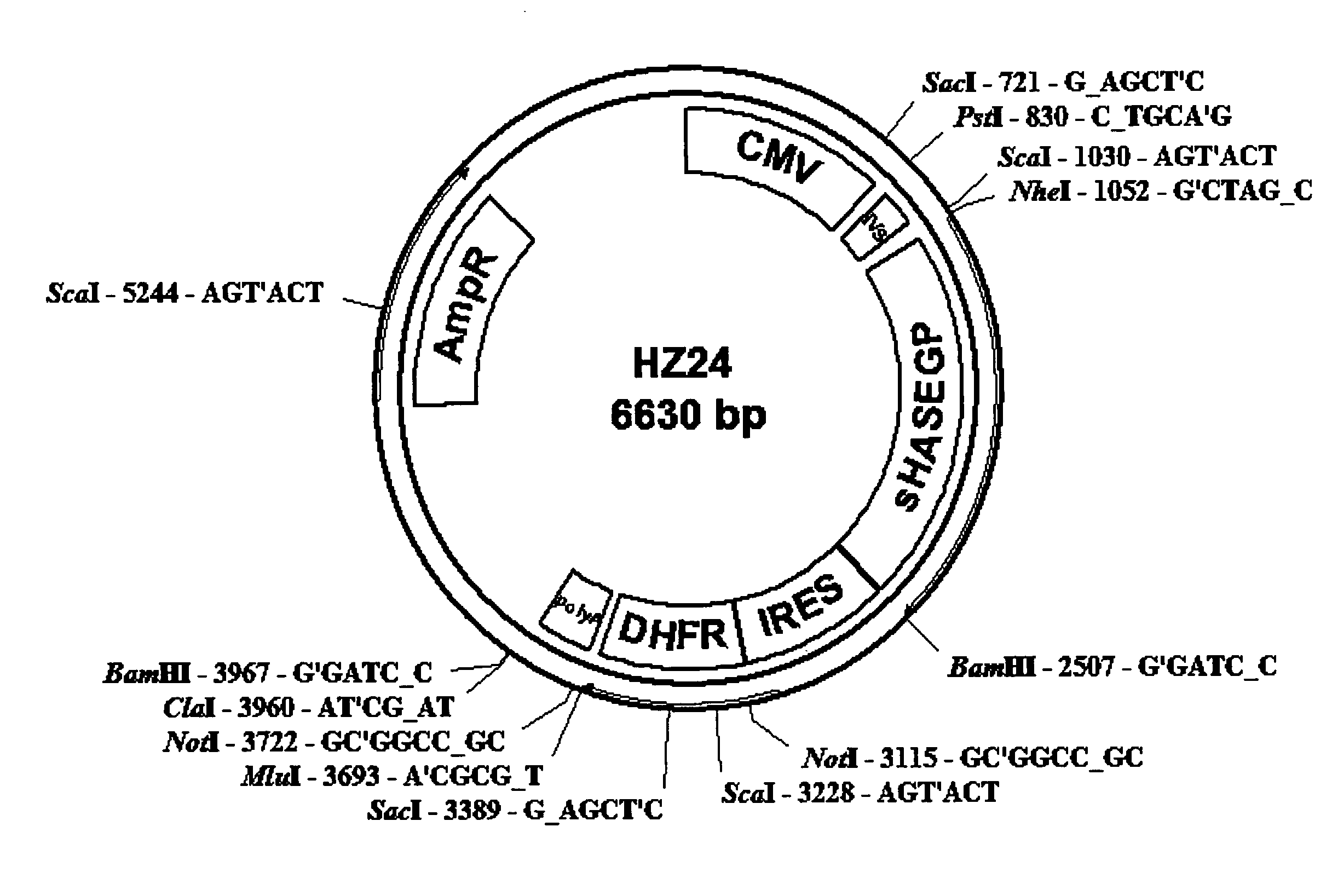
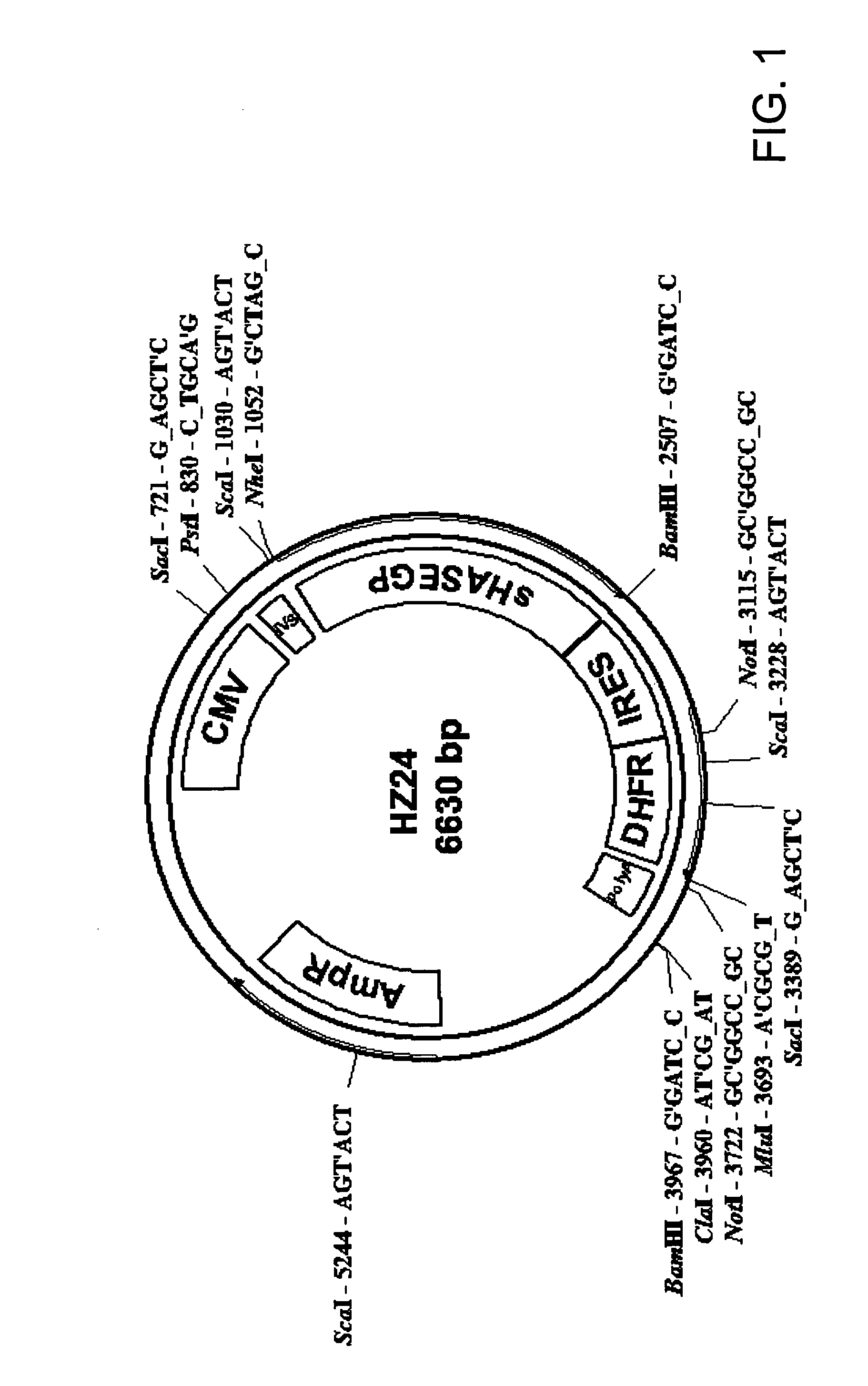
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20050260186A1Improve extentIncrease ratingsAntibacterial agentsSenses disorderHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Subcutaneous anti-HER2 antibody formulations and uses thereof
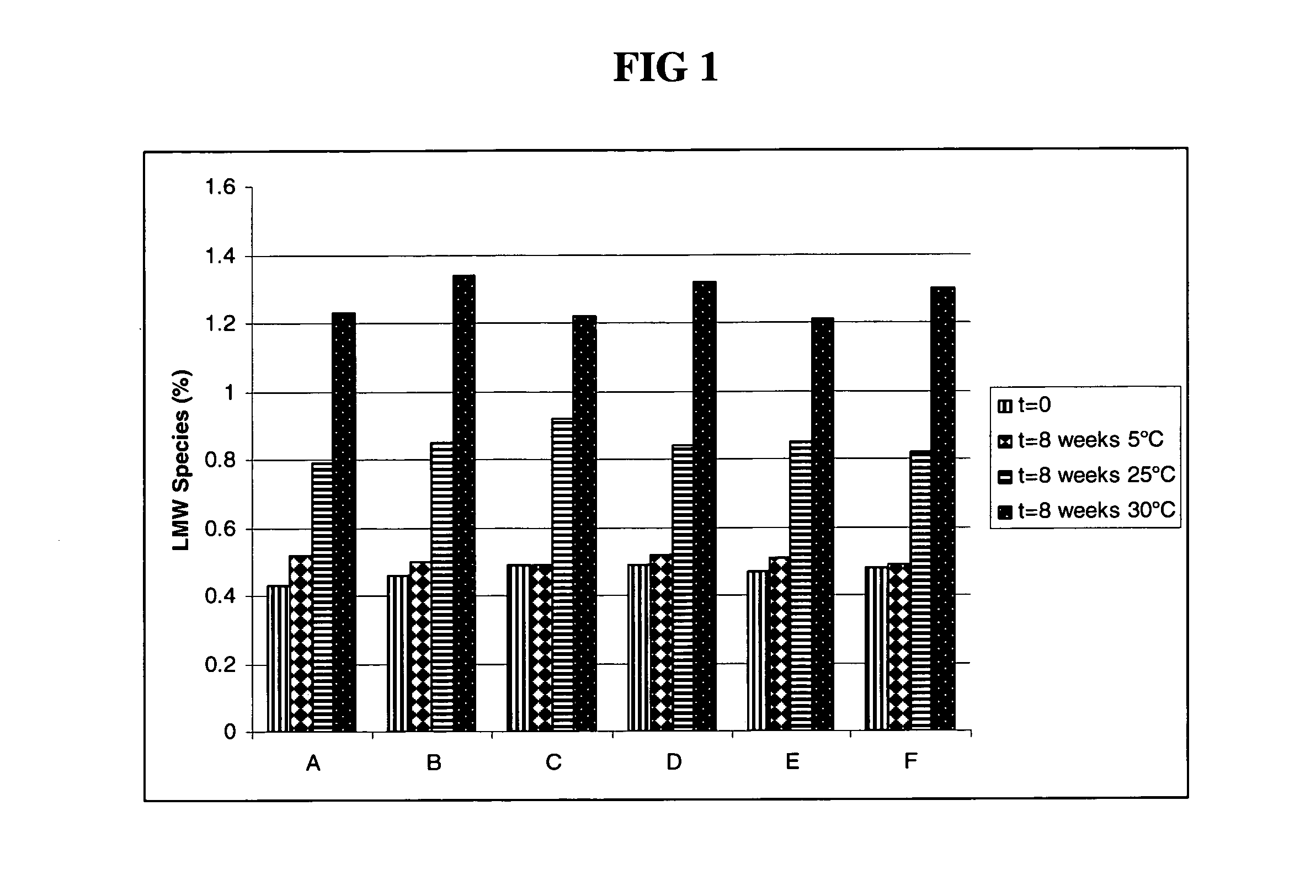
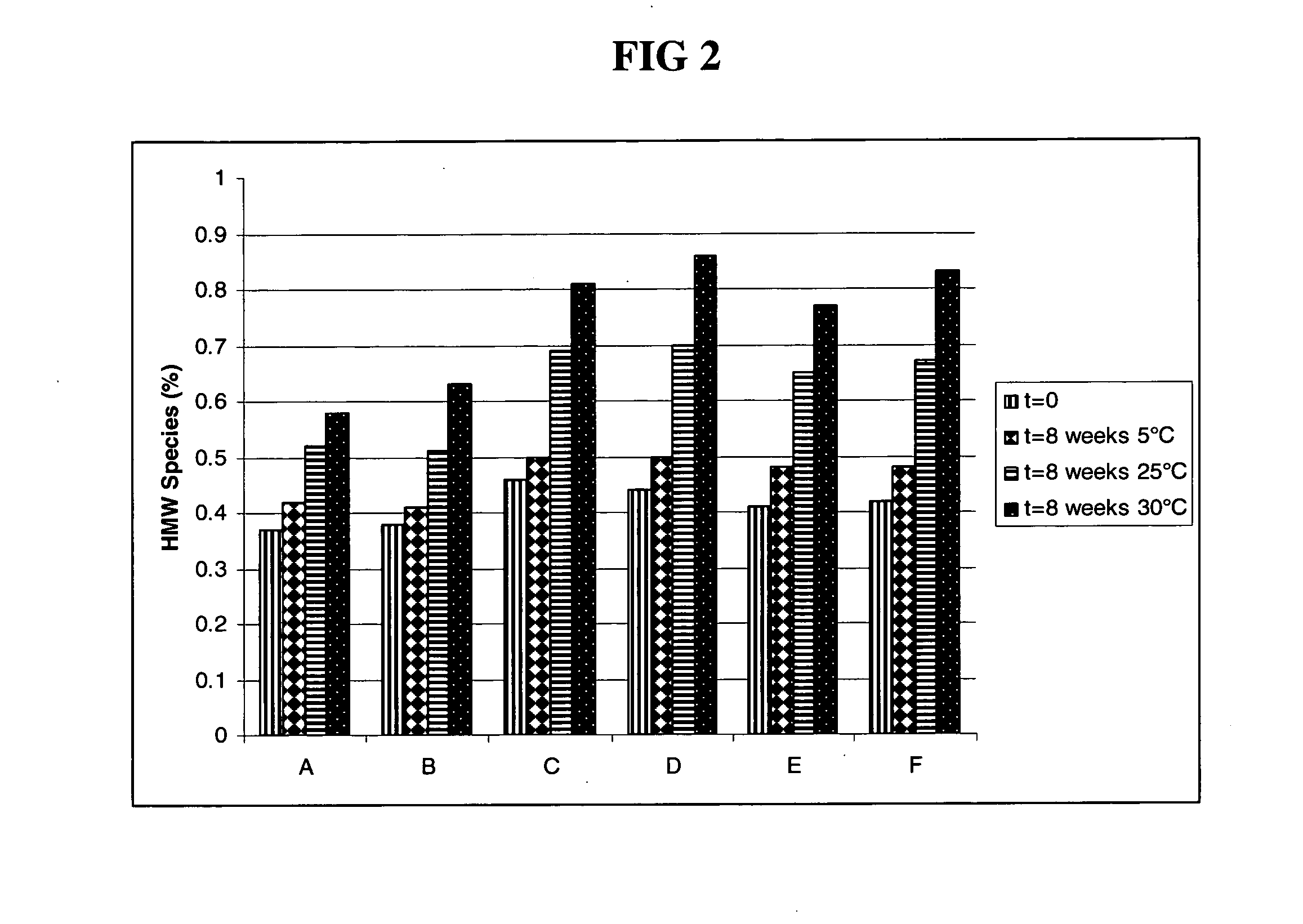
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
Soluble Glycosaminoglycanases and Methods of Preparing and Using Soluble Glycosaminoglycanases
InactiveUS20090123367A1Facilitated DiffusionEnhance convective transportBacterial antigen ingredientsPeptide/protein ingredientsHyaluronidaseNuclear chemistry
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME +6
Compositions for retarding skin aging
InactiveUS20040028643A1Convenient whiteningRetain tensionCosmetic preparationsBiocideHyaluronidaseActive oxygen
The present invention concerns a composition for retarding skin aging which contains an edible herb medicine or medicines round in Taiwan, in particular a plant extract or extracts having melanine formation-unhibiting, elastase-inhibiting, hyaluronidase-inhibiting, active oxygen-eliminating and / or radical-capturing type antioxidant activities, and a medicinally acceptable base and / or additives for external dermal application. The composition is useful in promoting skin whitening effects, maintaining the tension and elasticity of the skin, facilitating skin moistening and providing the skin with anti-inflammatory and / or anti-allergic properties.
Owner:YAKULT HONSHA KK
Use of human plasma hyaluronidase in cancer treatment
InactiveUS7148201B2Control of level of activityQuick filterBiocidePeptide/protein ingredientsPurification methodsScreening method
Owner:RGT UNIV OF CALIFORNIA
Human plasma hyaluronidase
InactiveUS7105330B2Less likely to induceControl of level of activityPeptide/protein ingredientsMicroorganism based processesPurification methodsScreening method
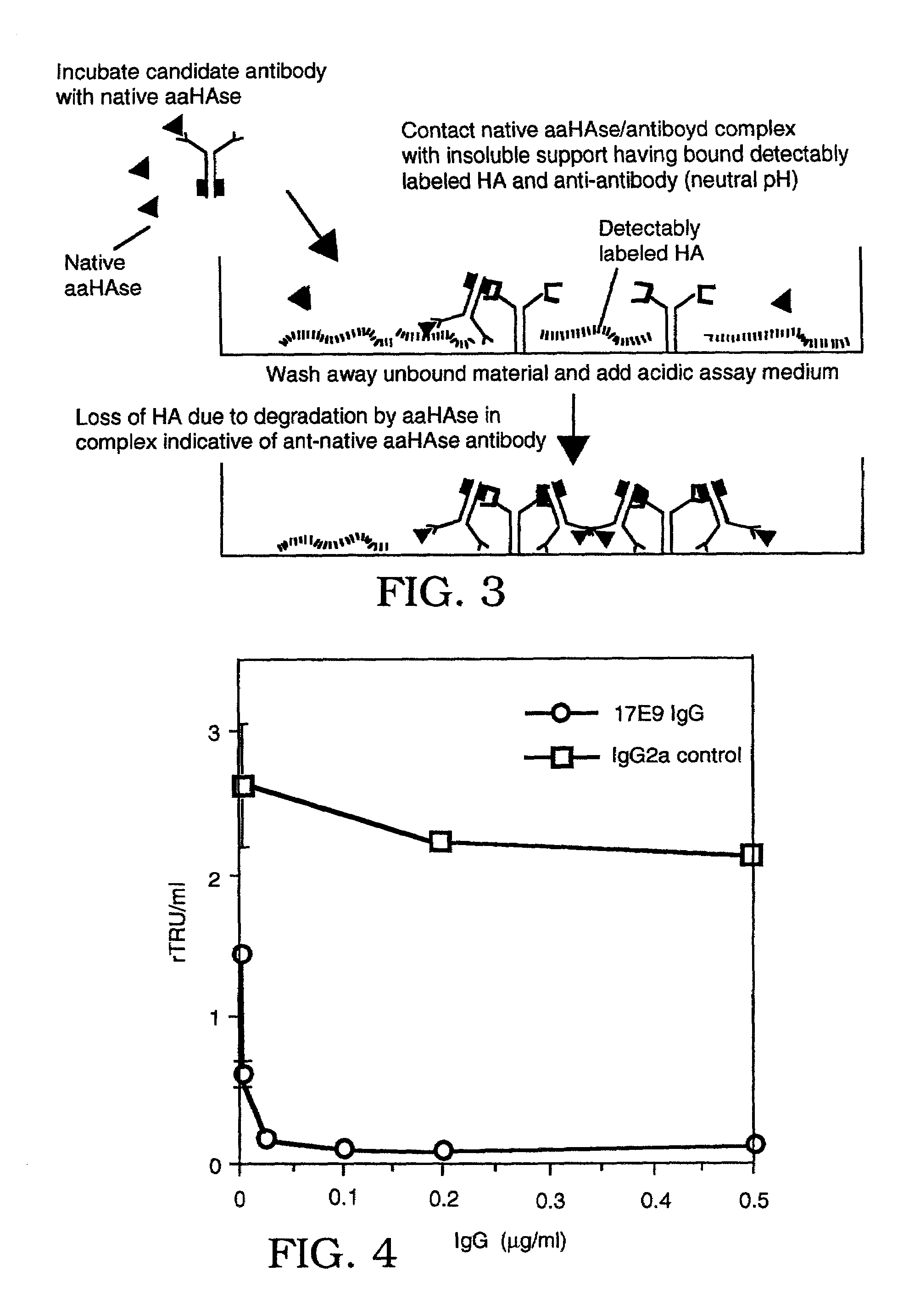
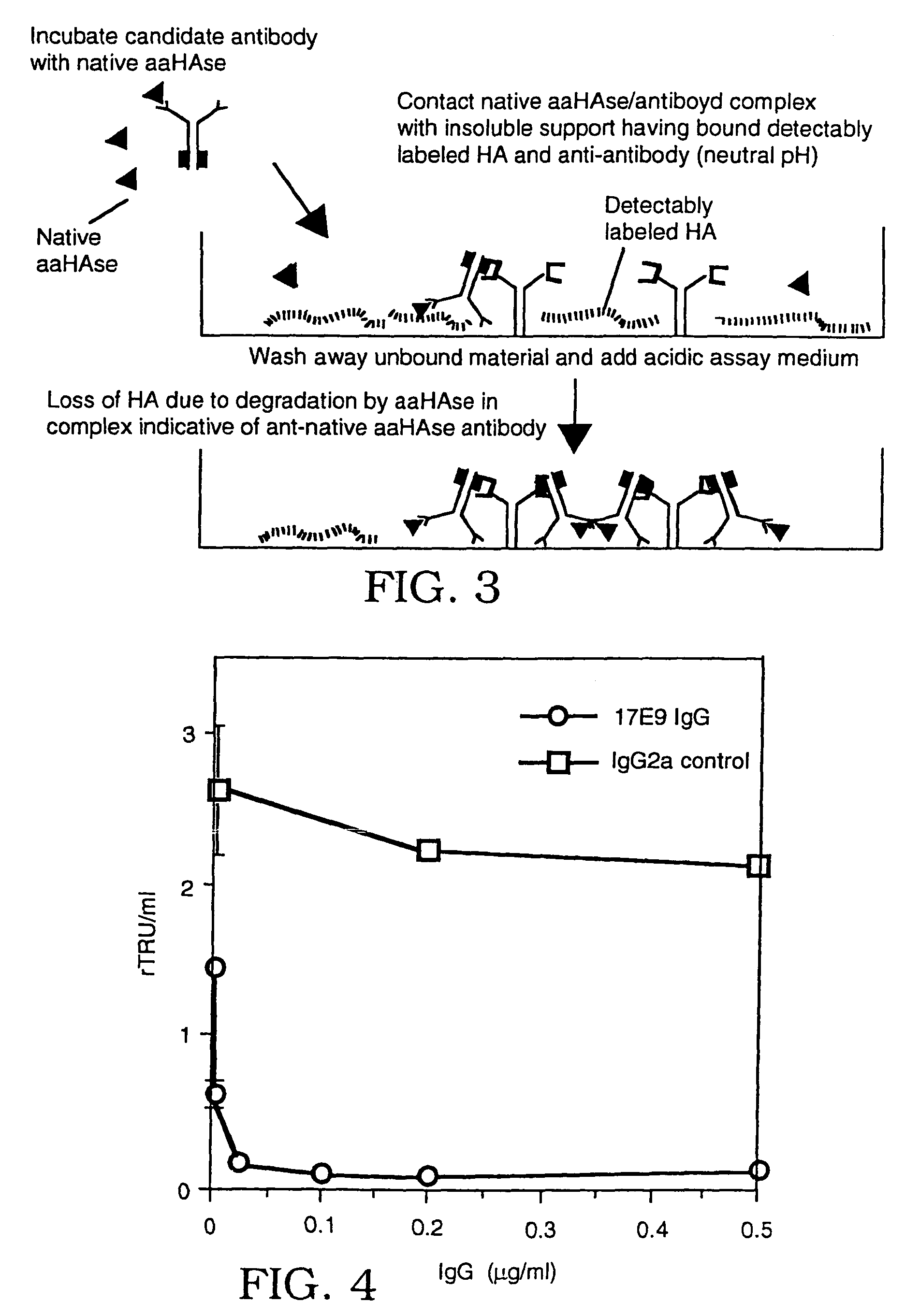
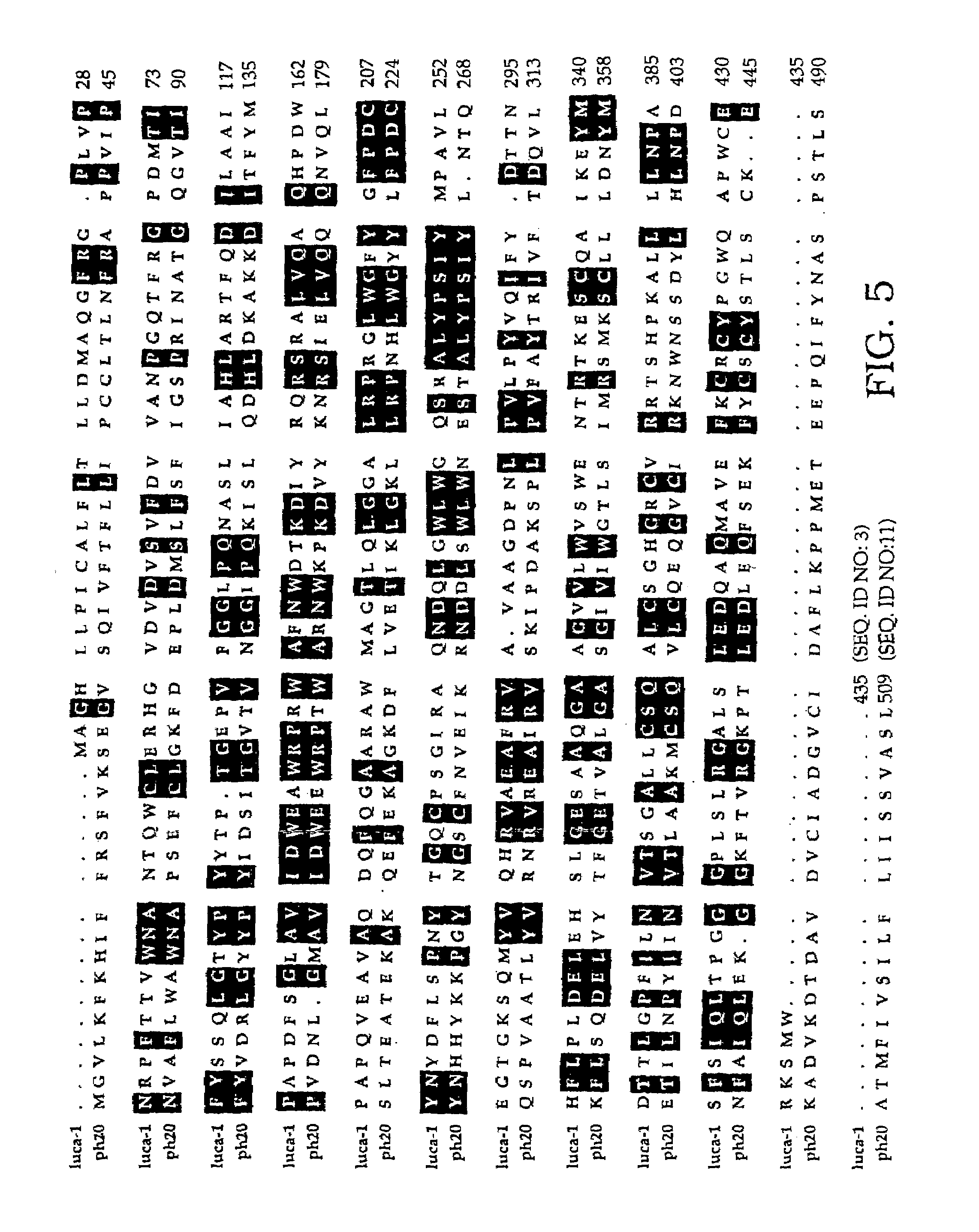
The invention is based on the discovery of methods for purification of an acid active hyaluronidase found in human plasma (hpHAse), including both biochemical and immunoaffinity purification methods. The method of immunoaffinity purification of the invention is based on the discovery of a method for identifying antibodies that specifically bind native hpHAse (anti-native hpHAse antibodies), and anti-native hpHAse antibodies identified by this screening method. The invention also features an assay for sensitive detection of HAse activity using biotinylated hyaluronic acid (bHA). Purification and characterization of hpHAse lead to the inventors' additional discovery that hpHAse is encoded by the LuCa-1 gene, which gene is present in the human chromosome at 3p21.3, a region associated with tumor suppression. The invention additionally features methods of treating tumor-bearing patients by administration of hpHAse and / or transformation of cells with hpHAse-encoding DNA.
Owner:RGT UNIV OF CALIFORNIA
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090214505A1Extended half-lifeOptimize allocationAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
Provided are soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. Sialated and pegylated forms of the sHASEGPs also are provided. Methods of treatment by administering sHASEGPs and modified forms thereof also are provided.
Owner:HALOZYME
Hyaluronidase and method of use thereof
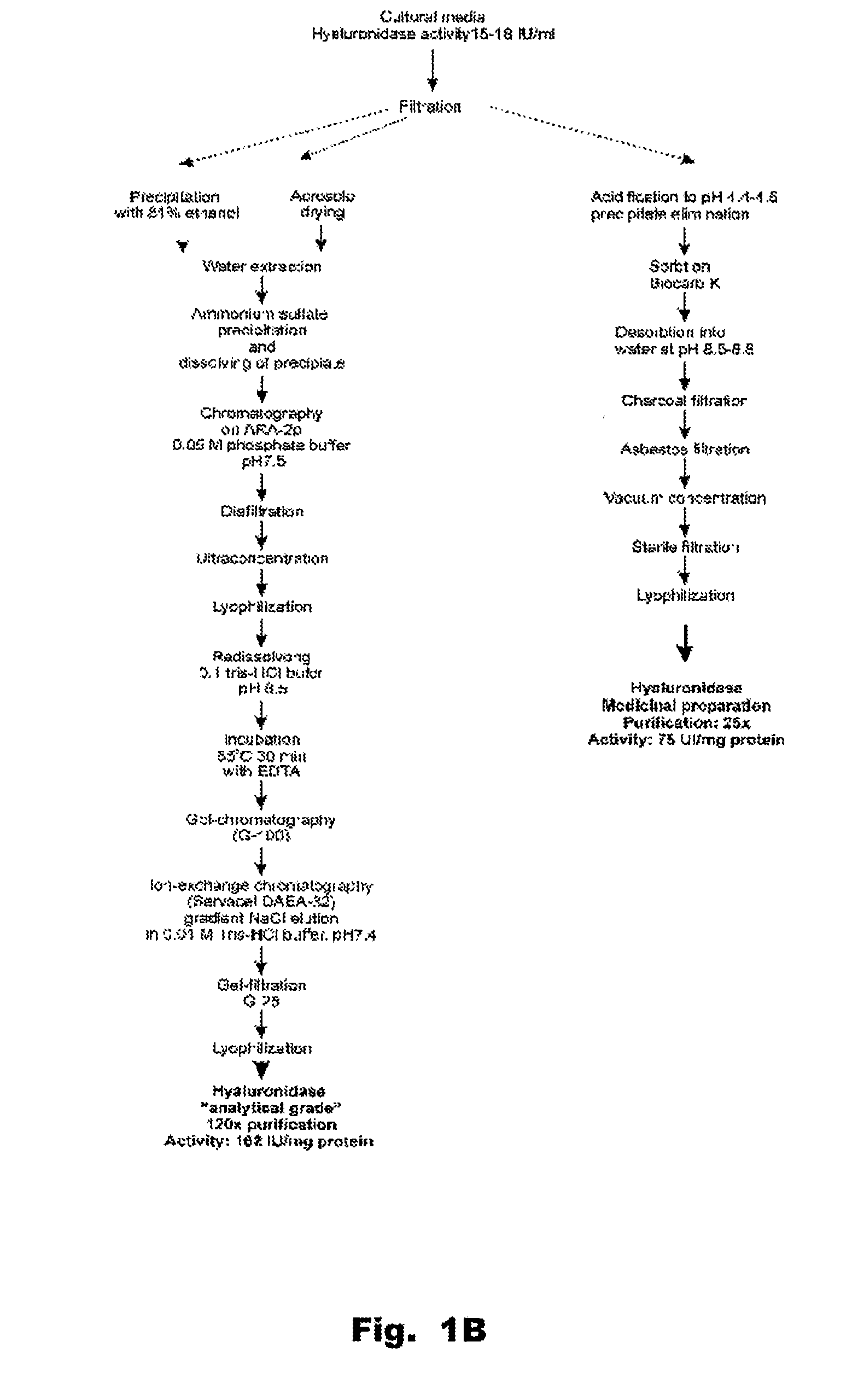
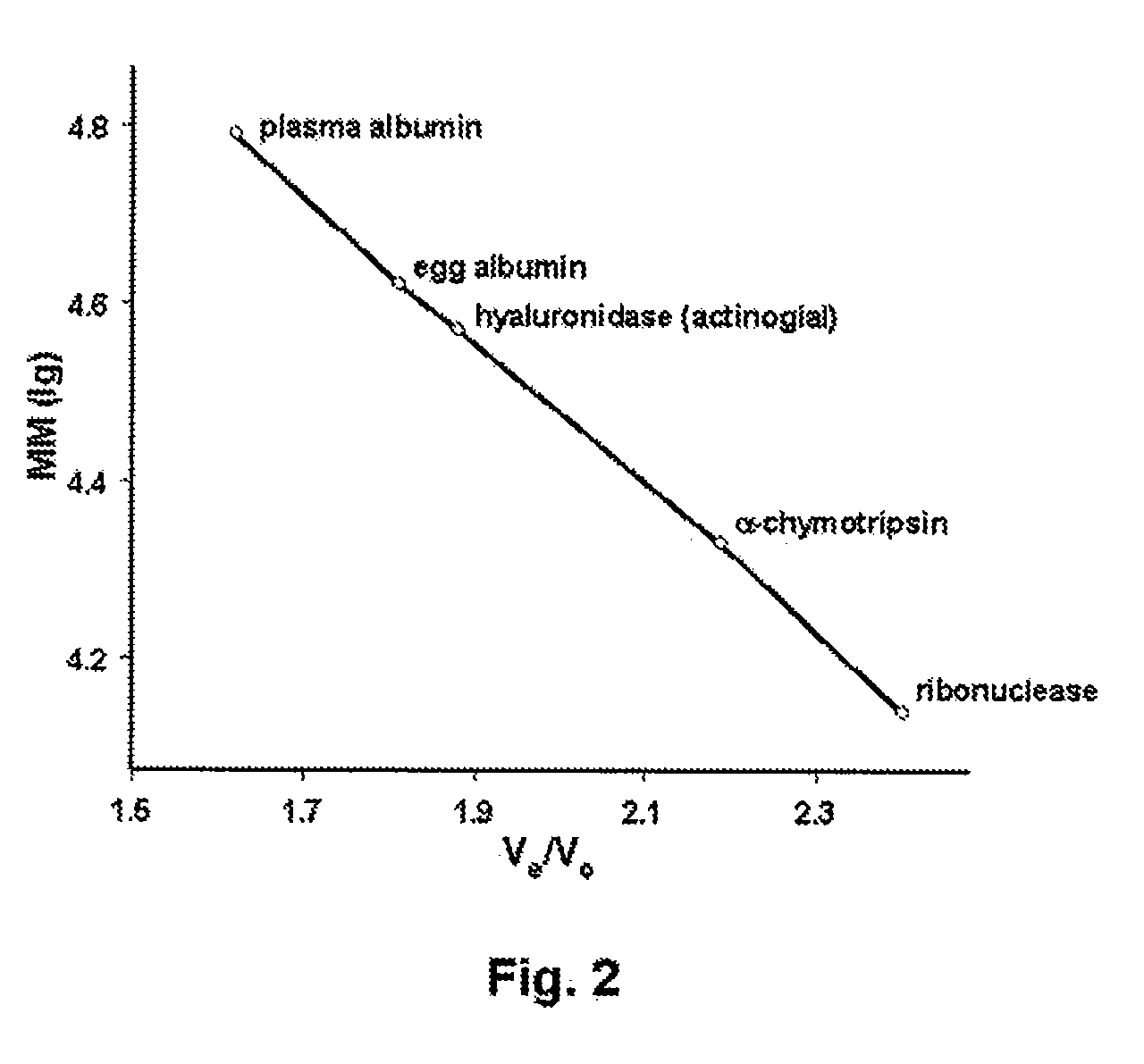
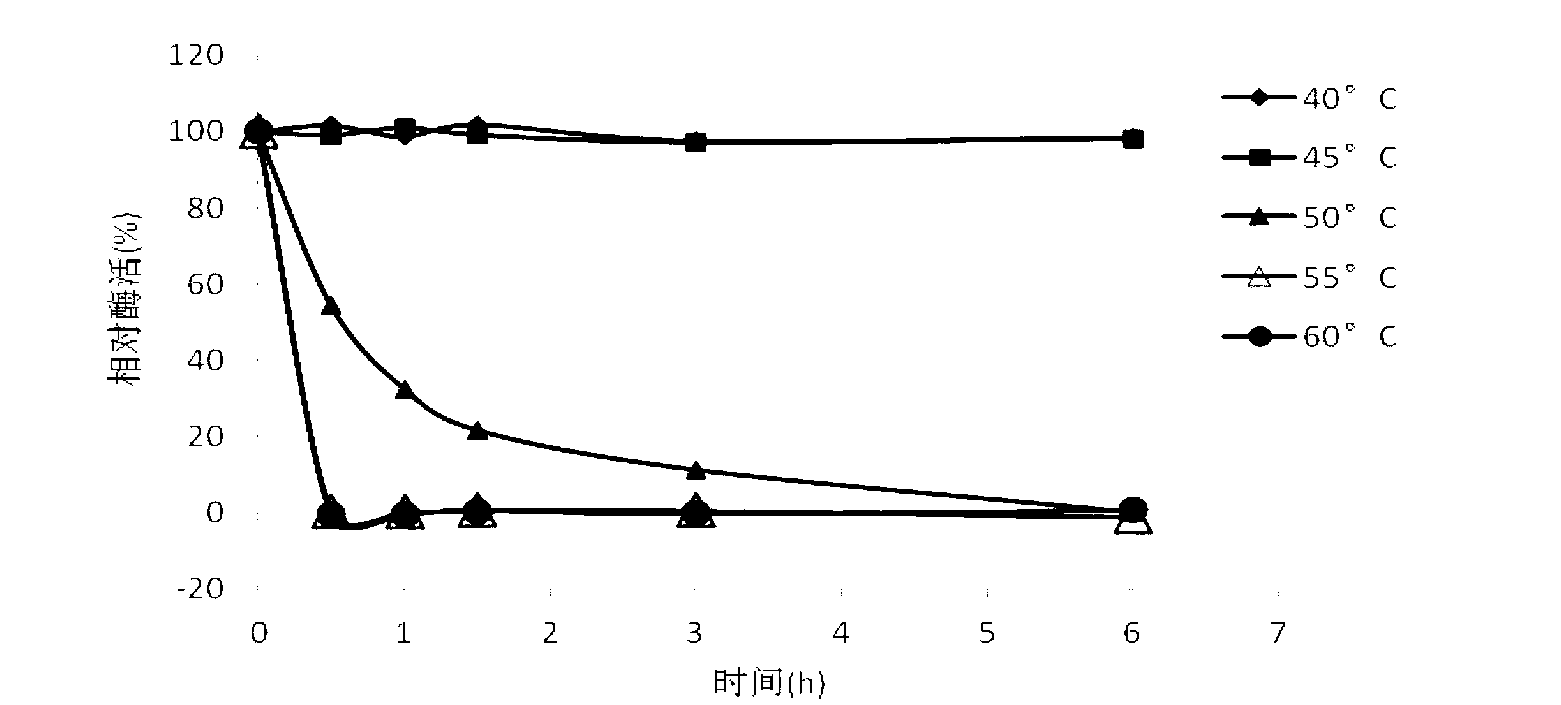
The present invention provides a tnaluronidase. The hyaluronidase can be produced by the strain Streptomyces aitinocidm 77, Exemplary characteristics of the hyaluronidase include specific C-terminal or other amino acid sequences, including full-length sequences, and improved physico-chemical and actix itj properties as compared to known h> alυronidase preparatkiiis. Described are also various uses of the hyaiurυnidasc, including topical administration of the h> aiuronidasc to improve skin penetration of a co-administered active substance.
Owner:UVARKINA TAMARA P +1
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090181032A1Extended half-lifeImprove distributionAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Cosmetic compositions
ActiveUS20120288478A1Reducing activity of hyaluronidaseSkin stimulationBiocideCosmetic preparationsTrifluoroacetic acidHyaluronidase
Disclosed are compositions and methods for their use that can be used in cosmetic applications. The composition can include an effective amount of a Centella asiatica stem cells to reduce the activity of hyaluronidase in skin, an effective amount of tetradecyl aminobutyroylvalylamino butyric urea trifluoroacetate or Alpinia galanga leaf extract to promote the production of hyaluronic acid in skin, an effective amount of tripeptide-1 to promote the production of fibronectin and laminin in skin, and a dermatologically acceptable vehicle.
Owner:MARY KAY INC
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090181013A1Reduce deliveryImprove usabilityAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
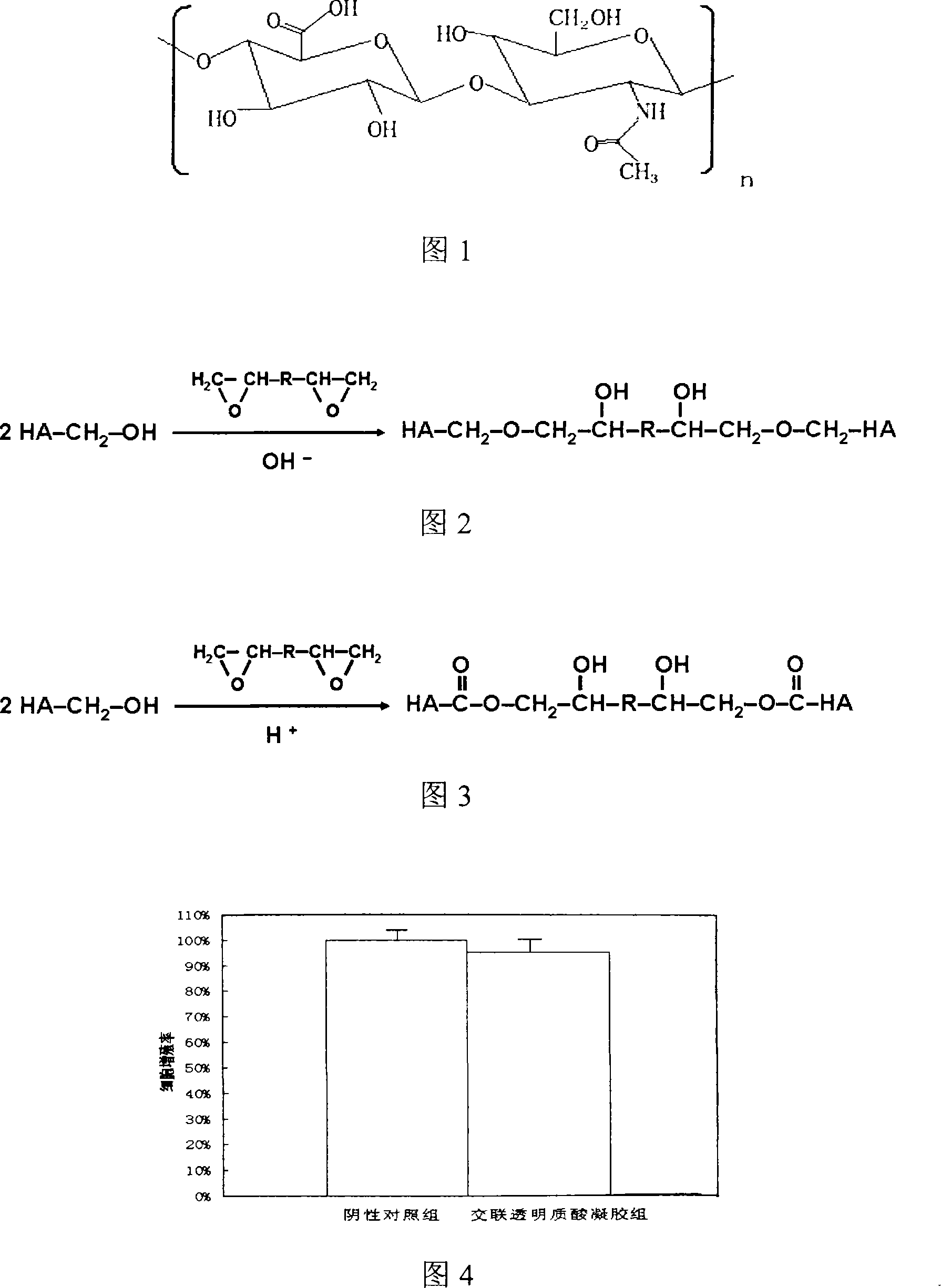
Method for preparing crosslinked hyaluronic acid microgel for tissue filling
The invention relates to a preparation method of cross-linking hyaluronic acid microgel for tissue filler; wherein, the monolithic gel is generated by the cross-linking reaction of the hyaluronic acid and the di-epoxide in the certain mold and processed by acid solution; the purification and dialysis by physiological balanced solution are processed; the microgel is made by extrusion of mechanical equipment. The cross-linking hyaluronic acid microgel has the advantages of (1) specific physical properties that the storage modulus (G') is 500-2000Pa, the loss modulus (G'') is 50-200Pa, the phase angle (Delta) is below 20 and the complex viscosity (Eta*) is 10-3500Pa x s as described by the dynamic viscoelasticity at frequency of 0.05-10Hz, (2) good stability that the performance of gel is independent of high pressure-high temperature treatment and the enzymolysis-resistant performance is excellent, (3) injectable property that the size of fine particle is 50-1000Mum, (4) good biocompatibility of no cytotoxicity, (5) biodegradability of being fully degraded by hyaluronidase, and (6) being suitable for tissue filler and bio-medical treatment.
Owner:SHANGHAI QISHENG BIOLOGICAL PREPARATION CO LTD
Novel topical skin care and nutraceutical applications of Glabridin or extracts containing a defined amount (4-90%) of Glabridin
InactiveUS20040121031A1Avoid damagePrevent photoagingCosmetic preparationsBiocideFine lineTyrosinase
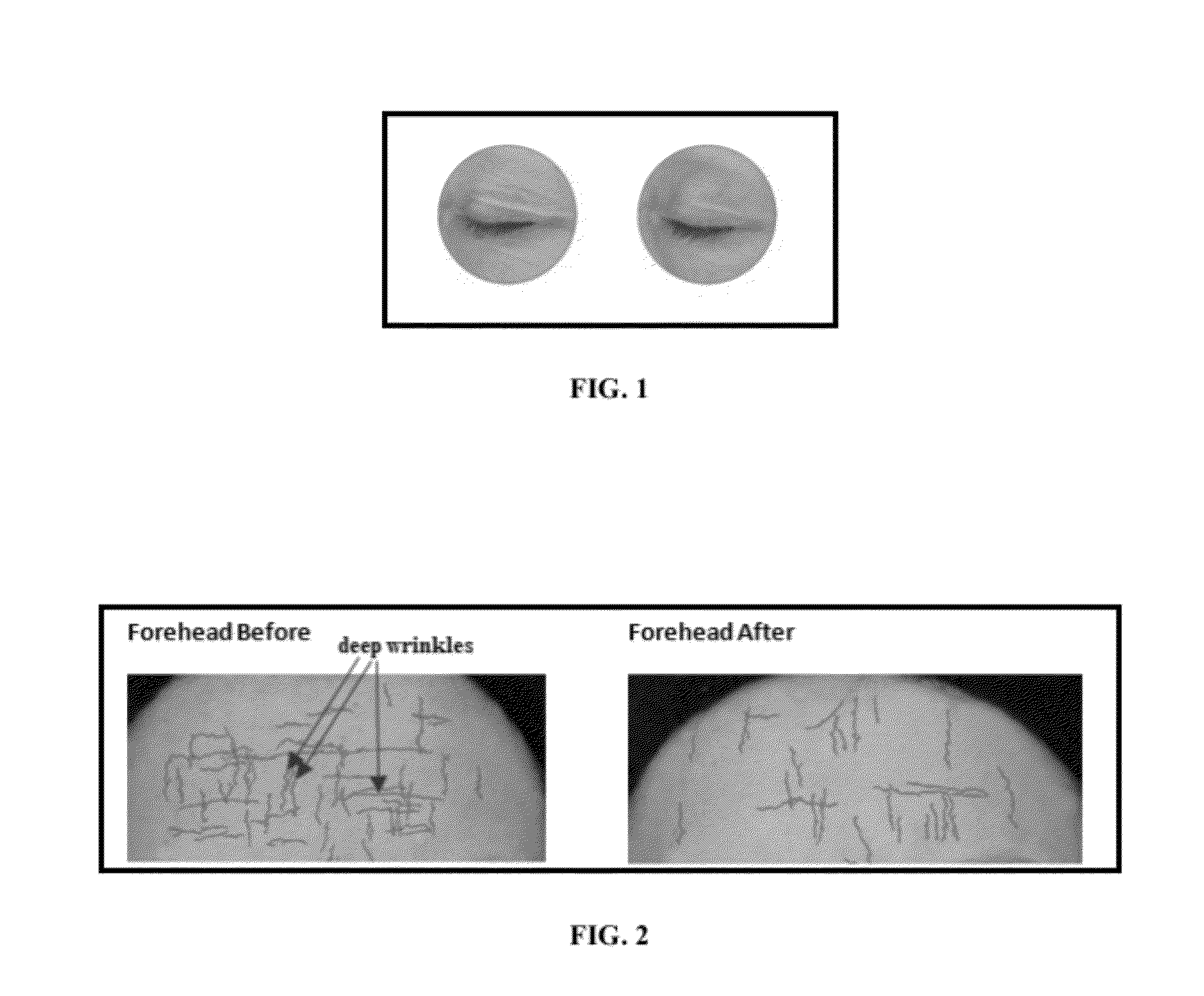
This application is a continuation-in-part of pending U.S. patent application Ser. No. 10 / 065,995 by the authors, filed on Dec. 9, 2002, for a Commercial Process for Isolation and Purification of Glabridin with High Tyrosinase Inhibitory Activity and its Cosmetic Compositions and Methods Of Use. The current invention discloses the use of Glabridin containing Licorice extract (4-90% Glabridin) as metalloprotease and hyaluronidase inhibiting component in cosmetic topical or oral formulations. These extracts and particularly Glabridin, are useful in anti-wrinkle and anti-aging products, providing elasticity, firmness, tone and texture to the skin, ameliorating fine lines and crows feet in under eye preparations, and prevent skin and hair damage due to UV rays, inflammation and itch, diaper rashes in baby products, as massage or toning oils or emulsion for babies.
Owner:SAMI LABS LTD
Subcutaneous anti-HER2 antibody formulations and uses thereof
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
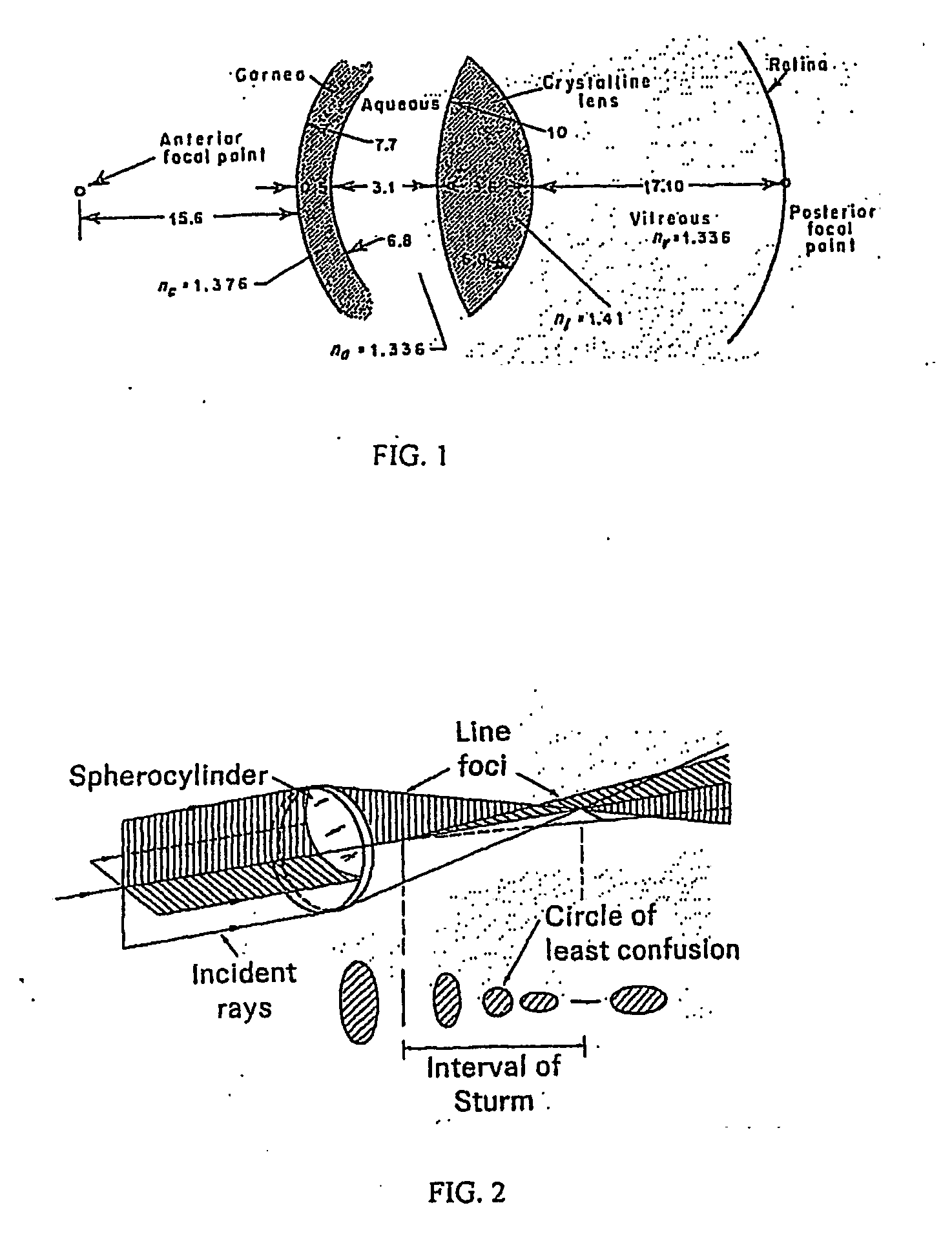
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
InactiveUS6863886B2Improve clearance rateIncreases rate of liquid exchangeBiocidePeptide/protein ingredientsDiseaseHyaluronidase
An enzymatic method is provided for treating ophthalmic disorders of the mammalian eye. Prevention of neovascularization and the increased rate of clearance from the vitreous of materials toxic to retina is accomplished by administering an amount of hyaluronidase effective to liquefy the vitreous humor of the treated eye without causing toxic damage to the eye. Liquefaction of the vitreous humor increases the rate of liquid exchange from the vitreal chamber. This increase in exchange removes those materials and conditions whose presence causes ophthalmological and retinal damage.
Owner:BAUSCH & LOMB PHARMA HLDG
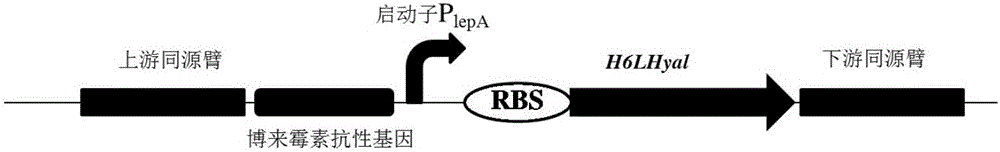
Construction method of recombinant bacillus subtilis capable of generating hyaluronic acid with specific molecular weight
ActiveCN105087456AIncrease productionReduce viscosityBacteriaTransferasesStreptococcus zooepidemicusHigh-Throughput Screening Methods
The invention discloses a construction method of recombinant bacillus subtilis capable of generating hyaluronic acid with specific molecular weight, belonging to the field of the bioengineering technology. According to the method, the production of hyaluronic acid is realized through integration of hyaluronic synthase (hasA) with streptococcus zooepidemicus source to bacillus subtilis; meanwhile, overexpression is realized on key genes of the synthetic route of HA, so that the high yield of bacillus subtilis is realized; on the basis, the hyaluronidase with the leech source is integrated to the genome of the bacillus subtilis, then mutant library construction is realized on ribosome bind sites (RBS) from the genetic expression translational level, and thus a series of mutants with different expression levels of hyaluronidases are obtained through high throughput screening; the yield of the hyaluronic acid achieves 19.38g / L, the molecular weight ranges from 103-106 Dalton, certain basis is laid for efficiently preparing micromolecule hyaluronic acids, and the construction method is suitable for industrial production and application.
Owner:JIANGNAN UNIV
Albumin-Free Botulinum Toxin Based Pharmaceutical Compositions Containing a Hyaluronidase and Methods of Use
InactiveUS20090324647A1Relieve symptomsReduce secretionBacterial antigen ingredientsNervous disorderSerum protein albuminMedicine
The present invention provides compositions that contain botulinum toxin and a hyaluronidase, and that lack human or recombinant serum albumin. The present invention also provides methods of administering the pharmaceutical composition to a subject in need thereof.
Owner:REVANCE THERAPEUTICS INC
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20100196423A1Reduce sensitivityGreater serum half-livesAntibacterial agentsSenses disorderHyaluronidaseNuclear chemistry
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated form of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Method for preparing oligomeric hyalurate by digestion method, and oligomeric hyalurate and application thereof
ActiveCN102876748ALow costImprove stabilityMicroorganism based processesFermentationAntioxidant capacityKetone
The invention discloses a method for preparing oligomeric hyalurate by a digestion method, and the oligomeric hyalurate and an application thereof. Bacillus hyaluronidase obtained through fermental cultivation of Bacillus sp. A50 CGMCC NO.5744 is used for degrading hyaluronic acid or a salt thereof, and the method comprises the steps of preparing the hyaluronic acid or the salt thereof, enzymolysis, inactivation, filtering, settling, dewatering and drying. According to the method, the hyaluronidase produced by the Bacillus is used for degrading the hyaluronic acid or the salt thereof, and the oligomeric hyalurate is prepared through dezymotizing, alcohol or ketone settling and dewatering and drying. The method is simple to operate and mild in condition, has no destroy on the product structure and no environmental pollution, the hyaluronidase for fermentation is low in cost and is suitable for large-scale industrial production, the prepared oligomeric hyalurate has the advantages of good percutaneous absorption capability, high purity, no cytotoxicity, strong oxidation resistance and the like, and can be used in fields such as cosmetics, foods and medicines.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Preparation and application of hyaluronic acid-modified amphipathic chitosan derivative carrier with tumor microenvironment specificity drug release effect
InactiveCN106581686AEvade captureImprove stabilityPharmaceutical non-active ingredientsEmulsion deliveryTreatment effectDrug release
The invention relates to a hyaluronic acid-modified amphipathic chitosan derivative carrier with tumor microenvironment specificity drug release effect. The hyaluronic acid-modified amphipathic chitosan derivative carrier is characterized in that firstly, a hydrophilic group is introduced into a chitosan skeleton; then, a hydrophobic group is introduced into a specifically degradable link arm containing disulfide bonds, so as to realize the amphipathic function; the amphipathic derivative is assembled into nanomicelle by self in a waterborne medium, and is further modified by a charge adsorbing principle to target the molecular hyaluronic acid; the nanomicelle can effectively load an anti-tumor drug, and the hyaluronic acid is targeted to the tumor microstructure and then is degraded by hyaluronic acid enzyme in focus cells, so that the drug can be quickly released from the nanomicelle to act on the focal part, thereby obviously improving the concentration, therapy effect and biological utilization degree of free drug on the focal part. The hyaluronic acid-modified amphipathic chitosan derivative carrier has the advantages that the carrier which carries pharmaceutical activity or pharmacological activity molecules can be applied to internal injection of blood vessels or muscles or oral administration, so as to obviously improve the anti-tumor activity of drug; the preparation method is simple, the technology is matured, and the preparation method is suitable for large-scale production.
Owner:CHINA PHARM UNIV
Use of Streptomyces hyalurolyticus enzyme in ophthalmic treatments
InactiveUS6902548B1Easy to useHigh purityPeptide/protein ingredientsPharmaceutical delivery mechanismProteinase activityStreptomyces
A highly specific and easily purified form of hyaluronidase is described for use in ophthalmic treatments. The enzyme, from Streptomyces hyalurolyticus is specific for hyaluronidase and carries out an elimination reaction that results in the production of double bonds at the nonreducing end of hyaluronic acid. Hyaluronidase from Streptomyces hyalurolyticus has a higher activity than comparable enzymes from other species. The enzyme is now capable of being purified in what is essentially a protease-free form making it applicable to medical treatments. The use of this source of hyaluronidase in ophthalmic treatments is now made possible by its high activity, specificity for hyaluronidase and purity.
Owner:SCHULER ED +1
Long acting hyaluronic acid - peptide conjugate
The invention relates to a novel bioconjugation protocol for peptide suitable for in vivo applications. Bioconjugation of the peptide to HA derivative increases its half life in circulation contributing for a high efficacy. More over, conjugate of HA derivative and peptide which is treated with hyaluronidase shows increased bioactivity. And also, in contrast to PEGylation, HA derivative can be conjugated with many numbers of peptide molecules per single HA derivative chain, which enables multiple action of peptide drugs.
Owner:POSTECH ACAD IND FOUND +1
Method for preparing ultra-low molecular weight hyaluronic acid oligosaccharides and salt thereof through combination of solid-liquid biphasic enzymolysis and ultrafiltration
ActiveCN108220364AReduce dosageIncrease enzyme activityFermentationHigh concentrationUltrafiltration
The invention discloses a method for preparing ultra-low molecular weight hyaluronic acid oligosaccharides and a salt thereof through combination of solid-liquid biphasic enzymolysis and ultrafiltration. The method comprises the following steps: degrading hyaluronic acid and a salt thereof by utilizing hyaluronidase produced by bacilli; preparing high-concentration hyaluronic acid enzymatic hydrolysate by adopting the method of combining a solid-liquid biphasic enzymolysis system and an ultrafiltration system, and then performing inactivating, activated carbon adsorption and impurity removal,filtering and spray-drying to obtain the ultra-low molecular weight hyaluronic acid oligosaccharides and the salt thereof with the molecular weight of less than or equal to 3 kDa. The product preparedby the method is high in stability, ultra-low in molecular weight, good in inflammation resistance and percutaneous absorptivity, high in purity, strong in oxidation resistance, does not have cytotoxicity, has the advantages of repairing skin cell damage and the like, and can be widely applied to the fields of cosmetics, food and medicines. According to the method, the process flow is easy to operate; conditions are mild; various alcohols are not used; the energy consumption is relatively low; the method does not have damage to a product structure, does not have environmental pollution, and is suitable for large-scale industrial production.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD +1
Treatment of ophthalmic conditions
ActiveUS20070122450A1Simple moldingIncrease changeLaser surgerySenses disorderRefractive errorAnterior cornea
Ophthalmic conditions such as presbyopia, myopia, and astigmatism can be corrected by the use of a molding contact lens in combination with a pharmaceutical composition suitable for delivery to the eye. The molding contact lenses are preferably commercially available and are not specifically designed for orthokeratology. The agents in the pharmaceutical compositions such as hyaluronase allow the cornea of the eye to be molded in order to correct the refractive error of the eye. The contact lenses and the pharmaceutical composition induce a change in the radius of curvature of the anterior surface of the cornea, thereby correcting the refractive error of the eye. One advantage of the inventive technique is that the patient with his or her own individual visual needs guides the treatment until the patient near and far visual needs are met. The present invention also provides for kits, which contain molding contact lenses, pharmaceutical composition suitable for delivery to the eye, and instructions, useful in the inventive system.
Owner:OSIO
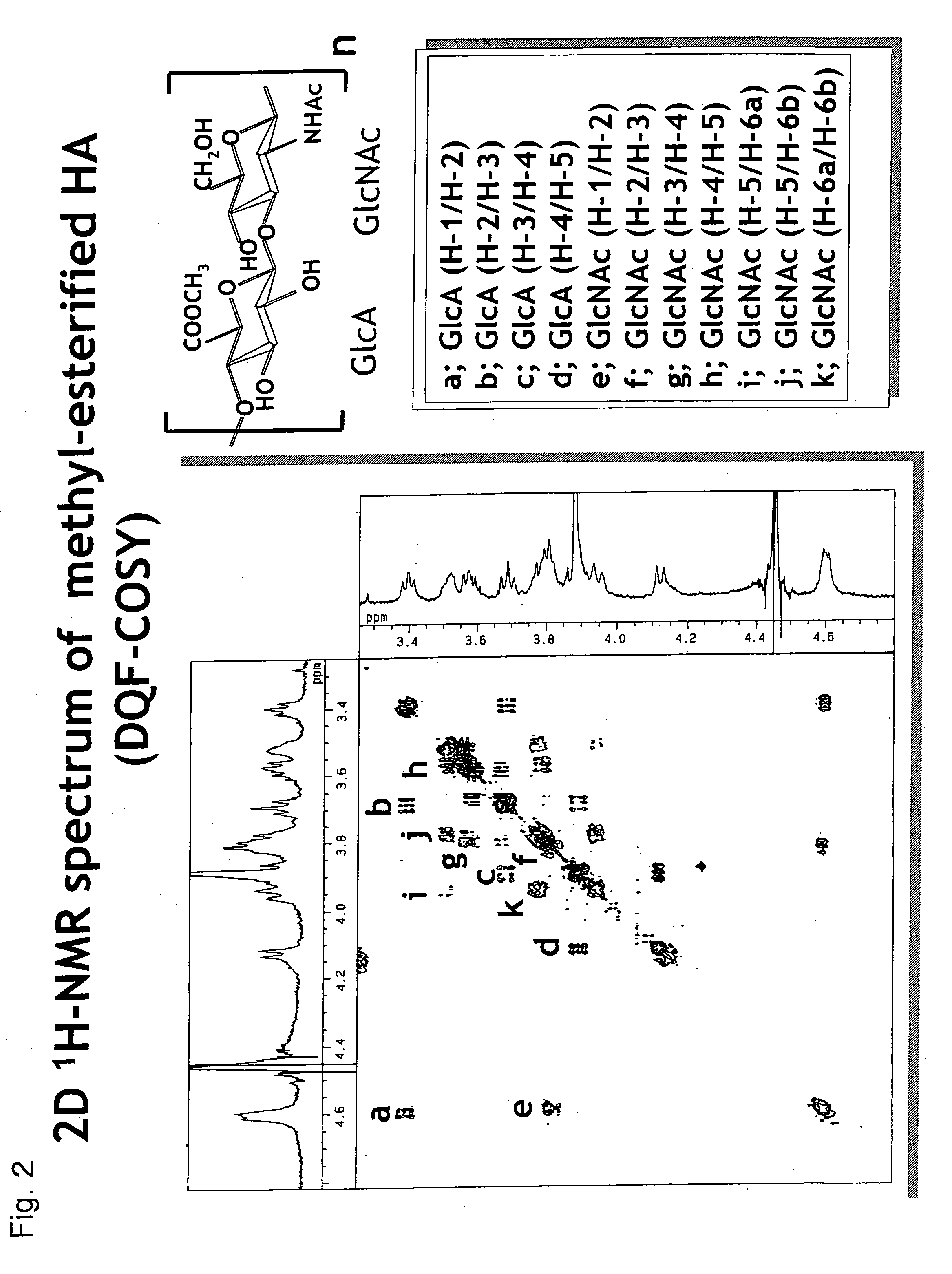
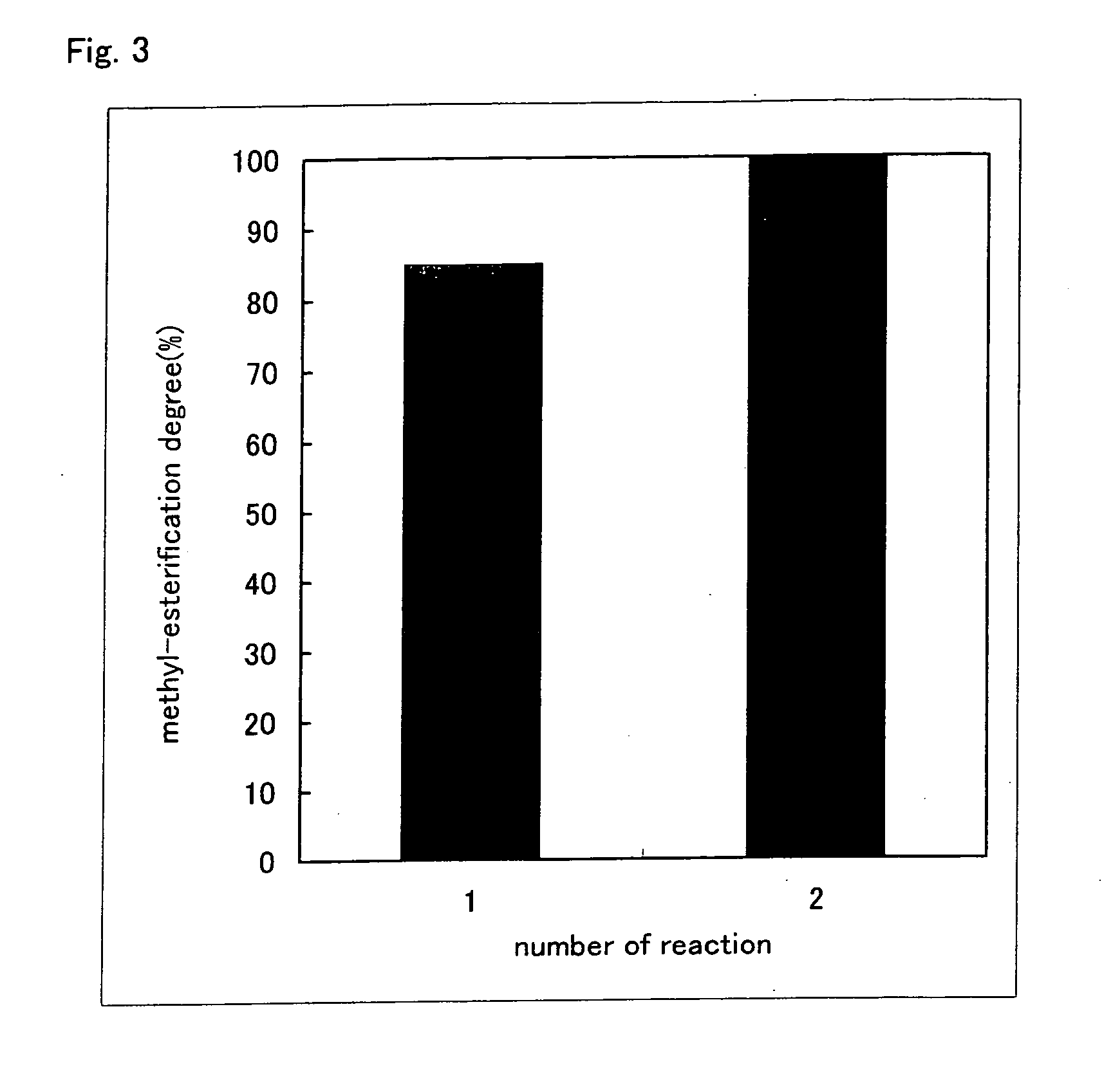
Method for producing alkyl-esterified glycosaminoglycan
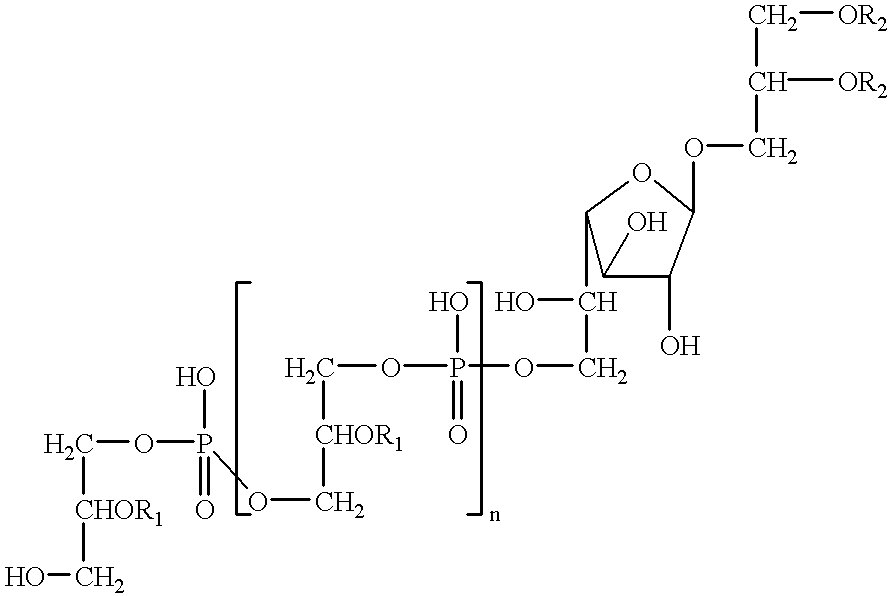
InactiveUS20060172967A1Easy to operateEasy to implementBiocideOrganic active ingredientsHyaluronidaseChemistry
Owner:SEIKAGAKU KOGYO CO LTD
Low molecular hyaluronate, preparation method and purpose thereof
ActiveCN103255187ALow costHigh purityOrganic active ingredientsCosmetic preparationsAntioxidant capacityHyaluronidase
The invention belongs to the fields of enzymology and pharmaceutical chemistry, and relates to the low molecular hyaluronate, preparation method and purpose thereof. Concretely, the preparation method comprises a degradation step of hyaluronic acid or salt thereof whose molecular weight is greater than 1000 kDa by hyaluronidase prepared by Bacillus spp whose preservation number is CGMCC No. 5744 or SEQ ID No:2 coded hyaluronidase. The preparation of low molecular hyaluronic acid or salts thereof by using hyaluronidase produced by Bacillus spp of the present invention has the advantages of simple operation, mild condition, non-destroy of the product structure and low cost. The product is suitable for large scale industrialized production. The low molecular hyaluronate prepared by the invention has the advantages of good transdermal absorbency, good purity, non-cytotoxicity, good antioxidation, etc., and can be used in the fields of cosmetic, foodstuff and medicine.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Anesthetic composition for topical administration comprising lidocaine, prilocaine and tetracaine
ActiveUS20070269465A9Rapid onsetImprove skinBiocidePharmaceutical delivery mechanismHigh concentrationHyaluronidase
Owner:FITA FERNANDO BOUFFARD
Antitumor and anticholesterol preparations containing a lipoteichoic acid from streptococcus
InactiveUS6214978B1Improve usabilityImprove liquidityBacteriaPeptide/protein ingredientsBackbone chainHyaluronidase
The invention concerns a new lipoteichoic acid which can be isolated from the new Streptococcus sp DSM 8747. The new LTA is called LTA-T. It has a lipid anchor, which is a galacto-furanosyl-beta-1-3-glycerol with different rests of fatty acids esterified in the two adjacent hydroxy groups in the glycerol moiety and a non-glycosylated, linear, unbranched GroP chain with an unusual short hydrophilic GroP chain. The hydrophilic backbone consists of only 10 glycerophosphate units esterified with D-alanine in an extent of 30%. The invention further concerns a pharmaceutical composition with the new LTA-T, optionally together with a monokine and / or hyaluronidase, a method of treating cancer comprising administration of an antitumor effective amount thereof, a method of producing the new compound and the new pharmaceutical composition, two degradation products of the new LTA-T and their use, and the new Streptococcus strain from which the new compound can be isolated.
Owner:LUNAMED
Method for inducing differentiation from human umbilical cord mesenchymai stem cells (hucMSCs) into neural cells
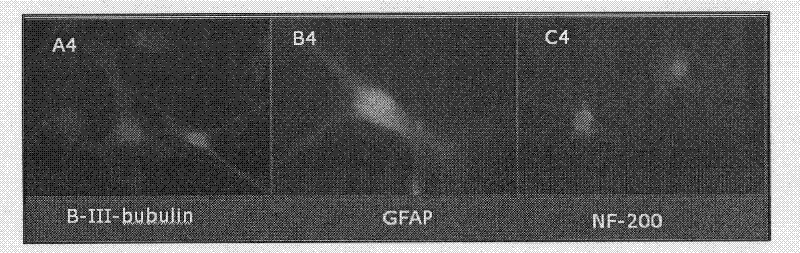
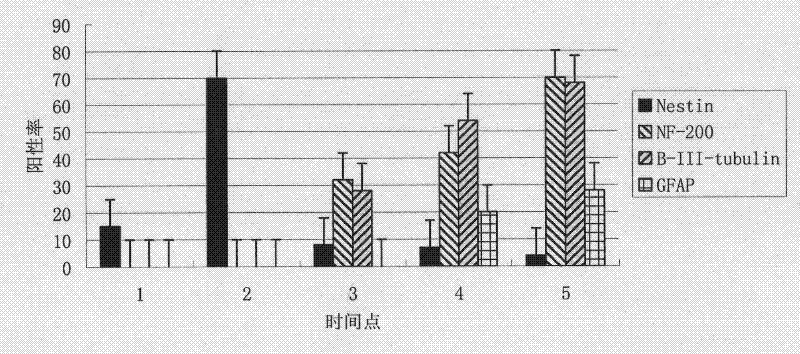
The invention belongs to the field of cell biology and relates to a method for inducing differentiation from human umbilical cord mesenchymai stem cells (hucMSCs) into neural cells. The method comprises that HUCMSCs and Schwann cells are putted in Transwell with apertures of 0.4 microns to be co-cultured separately; a Schwann cell culture medium is utilized as a cell culture medium and half or all of the cell culture medium is replaced every three days; and then neural cells can be obtained after two weeks of culturing. Through a separate co-culturing method utilized by the invention, an umbilical cord can be digested by a mixture of composite collagenase NB4 and hyaluronidase and thus a large amount of HUCMSCs can be separated. High purity HUCMSCs can be obtained in third generation passage cells. In the invention, HUCMSCs are induced and differentiate into neural cells, wherein a cell differentiation rate is over 70%. Expressing rate ranges of specific markers, such as NF-200, nestin, Betar-III-tubulin, etc., of neural cells are from 70 to 80%.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com