Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
278 results about "Botulinum toxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
There are different types of botulinum toxin products (toxin A and B) with different uses (eye problems, muscle stiffness/spasms, migraines, cosmetic, overactive bladder). Different brands of this medication deliver different amounts of medication. Your doctor will choose the correct product for you.
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
Pain treatment by peripheral administration of a neurotoxin
InactiveUS6869610B2Effective, long lasting, non-surgicalFunction increaseNervous disorderBacteriaMuscle spasmToxin
Methods for treating a non-spasm caused pain by peripheral administration to a patient of a therapeutically effective amount of a neurotoxin, such as a botulinum toxin.
Owner:ALLERGAN INC
Topically applied clostridium botulinum toxin compositions and treatment methods
InactiveUS20030113349A1Efficient use ofBacterial antigen ingredientsPeptide/protein ingredientsChromhidrosisSeborrheic dermatitis
Hyperactive glandular conditions are treated using topically formulated botulinum toxin compositions. In the preferred embodiment of the invention, topical botulinum preparations are applied directly to the skin by a patient as needed to suppress his or her hyperhidrosis, bromhidrosis, chromhidrosis, nevus sudoriferous, acne, seborrhiec dermatitis or other glandular condition. In other embodiments, topical botulinum toxins are applied with the aid of mechanical, electrical, and / or chemical transdermal delivery enhancers.
Owner:COLEMAN WILLIAM P III
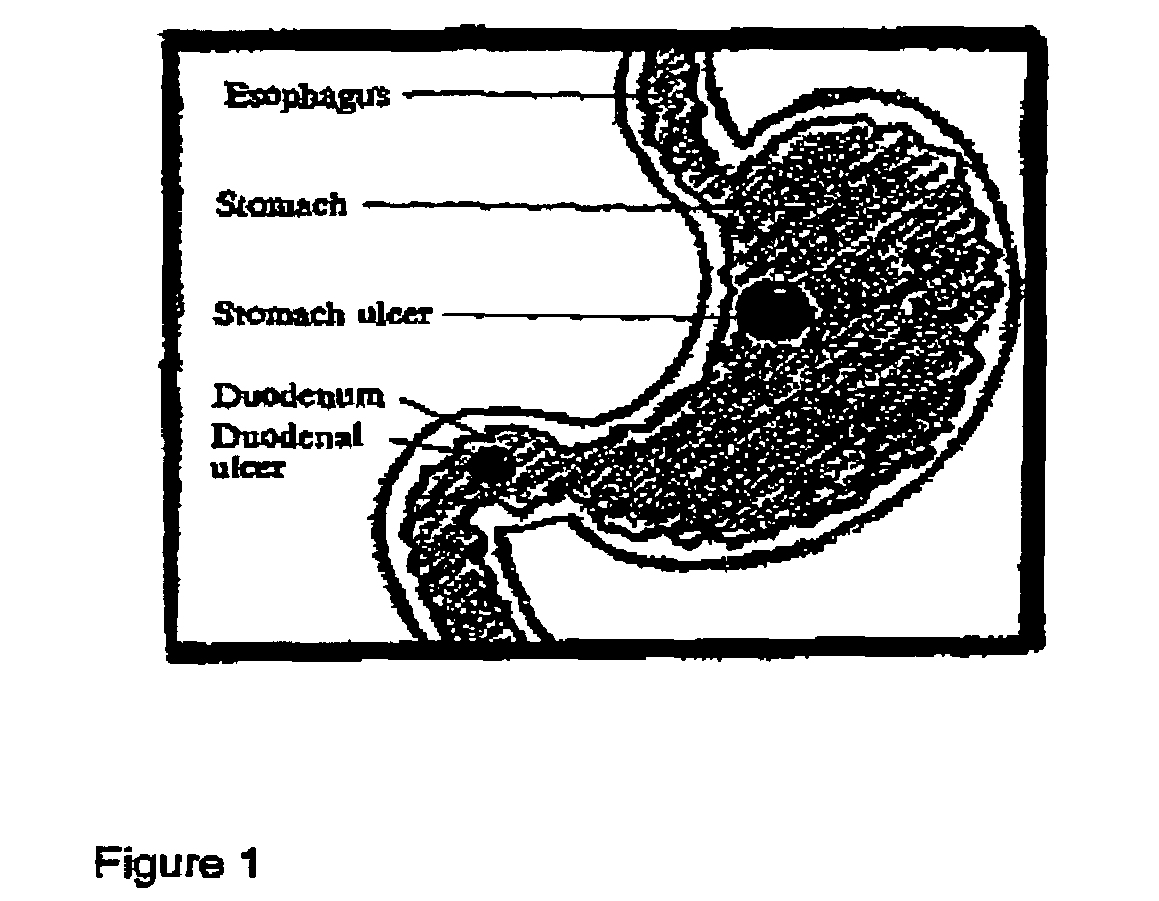
Methods for treating ulcers and gastroesophageal reflux disease
InactiveUS7238357B2Easy to changeChange effectBacterial antigen ingredientsPeptide/protein ingredientsRefluxOral medication
Methods for treating peptic ulcers and methods for treating gastroesophageal reflux disease by oral administration of a botulinum toxin.
Owner:ALLERGAN INC
Stabilized biodegradable neurotoxin implants
InactiveUS20050232966A1Patient compliance is goodReduce complicationsAntibacterial agentsBacterial antigen ingredientsOligomerTherapeutic effect
Biodegradable neurotoxin implants and methods of making and using such implants are provided. Biodegradable neurotoxin implants include a neurotoxin, a biodegradable polymer component, and an acidity regulating component. The biodegradable polymer component is effective in controlling the release of the neurotoxin from the implant when the implant is located in a patient's body. The acidity regulating component is effective in maintaining a pH of the implant in a desired range that may be effective in stabilizing the neurotoxin as the implant biodegrades when the implant is located in a patient's body. In one embodiment, an implant includes a botulinum toxin, a biodegradable polymer, and either monomers from which a biodegradable polymer is derived or oligomers including monomeric units substantially identical to a monomer from which a biodegradable polymer is derived, or a combination of such monomers and oligomers. The oligomers and biodegradable polymer may be derived from a single type of monomer. The implants disclosed herein may be administered to a human or animal patient in which a therapeutic effect is desired for prolonged periods of time.
Owner:ALLERGAN INC
Compositions and methods for topical diagnostic and therapeutic transport
Compositions and methods are provided that are useful for the delivery, including transdermal delivery, of biologically active agents, such as non-protein non-nucleotide therapeutics and protein-based therapeutics excluding insulin, botulinum toxins, antibody fragments, and VEGF. The compositions and methods are particularly useful for topical delivery of antifungal agents and antigenic agents suitable for immunization. Alternately, the compositions can be prepared with components useful for targeting the delivery of the compositions as well as imaging components.
Owner:REVANCE THERAPEUTICS INC
Botulinum toxin in the treatment or prevention of acne
InactiveUS20050074466A1Bacterial antigen ingredientsPeptide/protein ingredientsAnti-Androgen EffectSweat gland
Botulinum toxin may be used to inhibit the cascade of events leading to acne. Results in preliminary studies have been dramatic. Withoout wishing to be bound by this theory, it is believed that botulinum toxin achieves this result through parasympathetic effects, inhibiting sweat gland activity, stimulating keratinocyte locomotion, anti-inflammatory effects, and possibly anti-androgenic effects. Botulinum toxin can play an important role in decreasing and even preventing the formation of acne.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Dermal delivery
InactiveUS20110212157A1Not induce unwanted clinical effects inside and/orEnsure adequate treatmentOrganic active ingredientsPowder deliveryHypopigmentationChromhidrosis
The present invention describes systems and methods for treating disorders and / or conditions associated with the dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, and / or actinic keratosis. Methods generally involve administering nanoemulsions (e.g., nanoparticle compositions) comprising at least one therapeutic agent, such as botulinum toxin. In some embodiments, nanoemulsions are prepared, e.g., by high pressure microfluidization, and comprise a particle size distribution exclusively between 10 nm and 300 nm.
Owner:ANTERIOS INC
Clostridium botulinum toxin formulation and method for reducing weight
InactiveUS20060057165A1Reduced caloric intakeReduce weightBacterial antigen ingredientsPeptide/protein ingredientsSaxitoxinTaste cell
A method of altering taste sensation in an individual is provided, the method comprising administering an effective amount of botulinum toxin to the taste cells of the individual. The method can be used to effect reduced caloric consumption in an individual in need of reduced caloric consumption or to effect weight reduction in an individual in need of weight reduction.
Owner:DIMITRAKOUDIS DIMITRIOS +1
Modified clostridial neurotoxins with altered biological persistence
InactiveUS20020127247A1Increase and decrease persistenceIncrease or decrease stabilityNervous disorderPeptide/protein ingredientsDiseaseToxin
The present invention discloses modified neurotoxins with altered biological persistence. In one embodiment, the modified neurotoxins are derived from Clostridial botulinum toxins. Such modified neurotoxins may be employed in treating various conditions, including but not limited to muscular disorders, hyperhidrosis, and pain.
Owner:ALLERGAN INC
Rescue agents for treating botulinum toxin intoxications
ActiveUS20050106182A1Facilitates the nicking of the single chain toxinNervous disorderPeptide/protein ingredientsMedicineFood poisoning
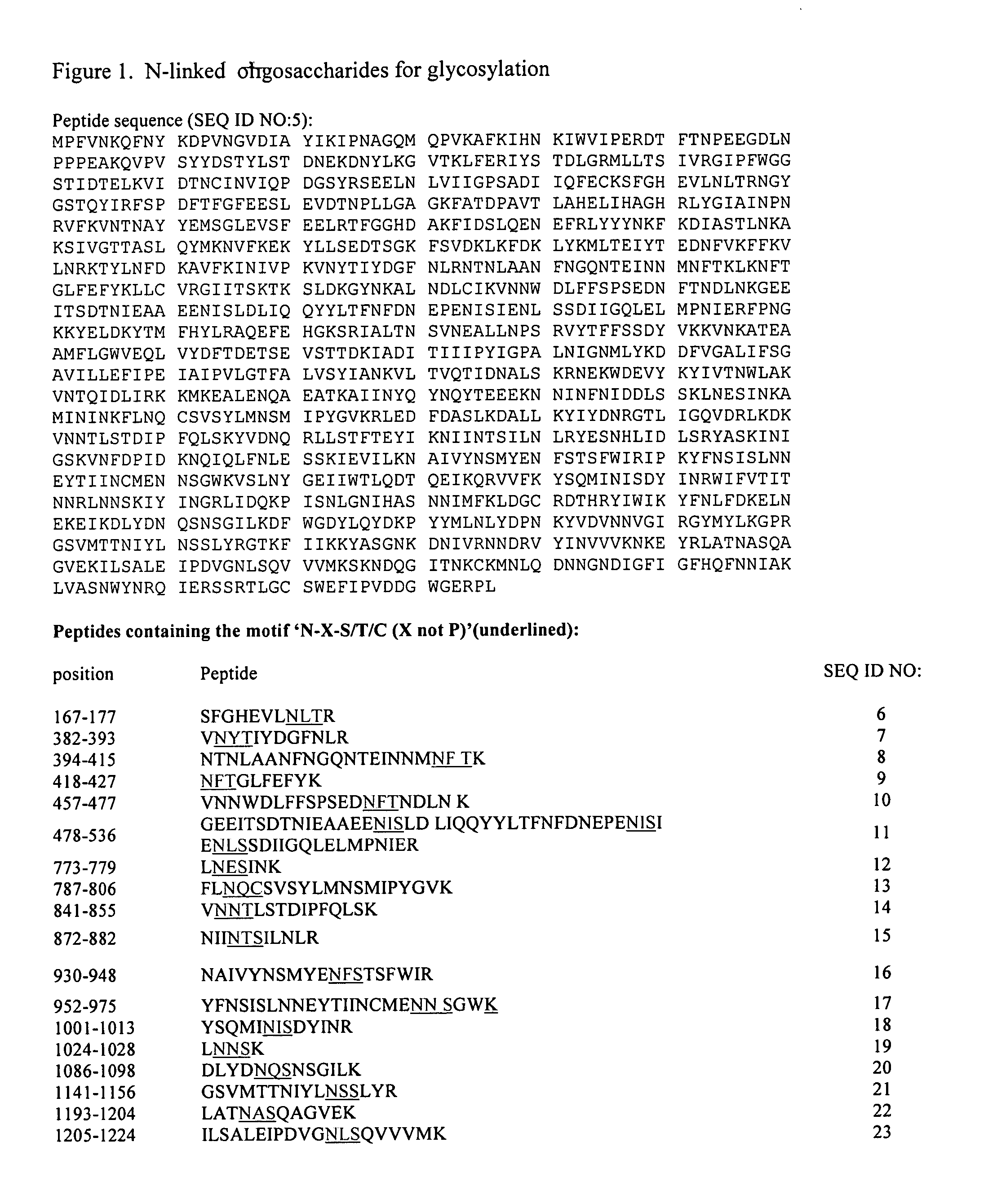
The present invention relates to rescue agents for use in the treatments of toxin intoxication—for example botulinum intoxication, which can result from food poisoning, an act of bioterrorism, or from accidental overdose in the course of treatment. In some embodiments, the rescue agents comprise at least one of an inactive botulinum toxin and a modified nontoxic nonhemagglutinin. The present invention also provides for glycosylated active and inactive toxins and methods of using same.
Owner:ALLERGAN INC
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
InactiveUS7563874B2Mitigate and eliminate symptomBiocidePeptide/protein ingredientsMedicineBotulinum Neurotoxin Type A
Owner:RGT UNIV OF CALIFORNIA
Incremental syringe
ActiveUS20160166772A1Easy to useGuaranteed to workInfusion syringesMedical devicesMultiple injectionBotulinum toxin
An incremental syringe useful for multiple injections of medications like botulinum toxin is provided. The syringe includes detents on the syringe plunger which provide a tactile feeling, a discrete audible sound or “click,” or preferably both, for every unit of medication aspirated or injected to or from an individual syringe. Hence, there is no need to look at the syringe, or bring it to the eye level, during use thereof. In some embodiments, a second set of detents is included, and in some embodiments a third set of detents is included. Syringe plungers useful for combining with a syringe body to produce such an incremental syringe are also described.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Albumin-free botulinum toxin formulations
This invention relates to botulinum toxin formulations that are stabilized without the use of any proteinaceous excipients. The invention also relates to methods of preparing and using such botulinum toxin formulations.
Owner:REVANCE THERAPEUTICS INC
Compositions and Methods for Enhancing Metal Ion Dependent Drug Therapies
ActiveUS20100330163A1Safely and effectively rapidly increaseImproved therapeutic outcomeBiocideOrganic active ingredientsPhytaseMedicine
Methods and compositions are provided for increasing responsiveness to therapeutic metalloproteases including increasing and / or maximizing responsiveness and preventing botulinum and tetanus toxin resistance due to a functional deficiency of zinc. Also provided are methods for zinc replacement or supplement in lacking individuals comprising the administration of a zinc supplement for a loading period and / or administration of a phytase supplement together with the zinc supplement. Also provided are methods for standardization of botulinum toxin potency assays that provide for greater certainty and margins of safety in the use of products from different manufacturers.
Owner:CNSS IP HLDG
Botulinum nanoemulsions
ActiveUS8318181B2Without significant irritationCosmetic preparationsNervous disorderToxinMuscle contracture
The embodiment described herein are related nanoemulsions comprising botulinum toxins. In one embodiment, the nanoemulsions are prepared by high pressure microfluidization and comprise a particle size distribution exclusively between 10 and 300 nm. The nanoemulsions contemplated by the present invention are useful for the cosmetic and medical treatment of muscular contracture states. For example, botulinum toxin may relax facial muscles such that skin wrinkles become smoother and less noticeable. Further, the present invention contemplates a cosmetic formulation that may be self-administered, for example, in the privacy of one's home and without medical supervision.
Owner:UNIVERSITY OF MASSACHUSETTS LOWELL
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS6822076B2Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsTetanusBasophilia
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-lIe-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Compositions, methods and devices for preparing less painful Botulinum toxin formulations
Devices, methods and kits are disclosed for preparing and administering less-painful formulations of Botulinum toxin. The devices, methods and kits of the present invention are comprised of or use an acidic formulation of Botulinum toxin, or, in certain embodiments, a freeze- or flash-dried composition of Botulinum toxin, having a long shelf-life which is subsequently mixed with an acid-neutralizing solution and, optionally a sequestration agent prior to administration to a patient in need thereof. The pH-neutralized formulation of Botulinum toxin is pharmaceutically acceptable for administration to a patient and is significantly less painful than acidic formulations of Botulinum toxin or formulations of Botulinum toxin having unnecessary antigens.
Owner:REVANCE THERAPEUTICS INC
Rescue agents for treating botulinum toxin intoxications
InactiveUS7172764B2Facilitates the nicking of the single chain toxinNervous disorderPeptide/protein ingredientsMedicineFood poisoning
Owner:ALLERGAN INC
Albumin-Free Botulinum Toxin Based Pharmaceutical Compositions Containing a Hyaluronidase and Methods of Use
InactiveUS20090324647A1Relieve symptomsReduce secretionBacterial antigen ingredientsNervous disorderSerum protein albuminMedicine
The present invention provides compositions that contain botulinum toxin and a hyaluronidase, and that lack human or recombinant serum albumin. The present invention also provides methods of administering the pharmaceutical composition to a subject in need thereof.
Owner:REVANCE THERAPEUTICS INC
Botulinum toxin and the treatment of primary disorders of mood and affect
ActiveUS20070009555A1Relieve symptomsReduce transmissionBacterial antigen ingredientsPeptide/protein ingredientsClinical psychologyAnxiety
The invention provides methods for treating primary disorders of mood and affect, including depressive disorders, anxiety and sleep disorders and CNS disorders comprising the administration of a neurotoxin.
Owner:REVANCE THERAPEUTICS INC
High-potency botulinum toxin formulations
InactiveUS7691394B2Reduce secretionPromote growthNervous disorderPeptide/protein ingredientsDiseaseNeuromuscular disease
The present invention provides improved formulations of botulinum toxin that increase delivery of the botulinum toxin to neural and associated tissues and exhibit a higher specific neurotoxicity and higher potency (in LD50 Units) than available formulations of botulinum toxins. These improved formulations enable physicians to treat a wide variety of pathological conditions with a lower toxin load that reduces the risk of inducing an immune response against the toxin and its associated proteins that may ultimately lead to the development of toxin resistance. These benefits are particularly important in the treatment of conditions that require high-dose or chronic administration of botulinum toxin. Additionally, the decreased in LD50 Unit doses of inventive formulations allows for controlled administration limits diffusion. The present invention also provides methods of treating neuromuscular diseases and pain, using low-dose botulinum toxin.
Owner:REVANCE THERAPEUTICS INC
Treating neoplasms with neurotoxin
InactiveUS7709440B2Inhibit transferSuppression of squeeze effectBiocidePeptide/protein ingredientsCancer cellAutoimmune responses
The present invention provides a method of treating a cancer using a neurotoxin, preferably Botulinum toxin (“BTX”). The application of a neurotoxin around a cancer acts to decrease the contractile forces of the muscles surrounding a neoplasm which normally squeeze cancer cells through efferent channels leaving the cancer vicinity to distant sites. Also, the application of the toxin at sites distant from the cancer enhances cellular and humoral immunologic functions which further contributes to cancer cell death and spread. Following administration of botulinum toxin around and distant to a cancer, it is noticed that local, regional, and distant spread is reduced or eliminated. Immunomodulation with botulinum toxin is also valuable in treating other disease that may or may not be associated with cancers, such as viral-induced growths, viral conditions, fungal disease, chronic wounds, graft versus host disease, autoimmune disease, and HIV.
Owner:TOXCURE
Methods and compositions for treating gastric disorders
Improved efficacy in treatment of gastric disorders with botulinum toxin is obtained using liposomal encapsulated botulinum formulations for topical administration of the botulinum toxin. The liposomes are typically administered in a physiologically acceptable carrier such as saline or phosphate buffered saline by application onto the surface of the tissue within into the gastrointestinal (GI) tract in need of treatment. Preferably, the formulation is applied via a roller, sponge or nozzle that is attached to an endoscope.
Owner:LIPELLA PHARMA
Compositions and methods for topical application and transdermal delivery of botulinum toxins
ActiveUS20070077259A1Reduce hypersecretionReduce sweatingCosmetic preparationsNervous disorderWrinkle skinPharmacology
Improved formulations for transdermal delivery of botulinum toxin are disclosed. The formulations include, for example, botulinum toxin non-covalently associated with a positively charged backbone having branching or efficiency groups. The formulations also include a partitioning agent, oligo-bridge, or polyanion bridge, and may optionally contain a viscosity modifying agent. The formulations are designed for topical application onto the skin of a patient and may be used to treat wrinkles, hyperhidrosis, and other health-related problems. Kits for administration are also described.
Owner:REVANCE THERAPEUTICS INC
Polysaccharide containing botulinum toxin pharmaceutical compositions and uses thereof
InactiveUS7758873B2High potencyImprove stabilityCosmetic preparationsOrganic active ingredientsToxinPolysaccharide
This invention relates to the use of a composition comprising a polysaccharide and a botulinum toxin for reducing a skin wrinkle. In some embodiments, the polysaccharide comprises disaccharides. In some embodiments, the average molecular weight of a disaccharide unit of the polysaccharide is between about 345 D and about 1,000 D.
Owner:ALLERGAN INC
Botulinum nanoemulsions
ActiveUS20120164182A1Without significant irritationCosmetic preparationsNervous disorderHigh pressureMimetic Muscles
The embodiment described herein are related nanoemulsions comprising botulinum toxins. In one embodiment, the nanoemulsions are prepared by high pressure microfluidization and comprise a particle size distribution exclusively between 10 and 300 nm. The nanoemulsions contemplated by the present invention are useful for the cosmetic and medical treatment of muscular contracture states. For example, botulinum toxin may relax facial muscles such that skin wrinkles become smoother and less noticeable. Further, the present invention contemplates a cosmetic formulation that may be self-administered, for example, in the privacy of one's home and without medical supervision.
Owner:UNIVERSITY OF MASSACHUSETTS LOWELL
Compositions and Methods for Topical Application and Transdermal Delivery of Botulinum Toxins
ActiveUS20090087457A1Reduce hypersecretionReduce sweatingCosmetic preparationsNervous disorderWrinkle skinPharmacology
Improved formulations for transdermal delivery of botulinum toxin are disclosed. The formulations include, for example, botulinum toxin non-covalently associated with a positively charged backbone having branching or efficiency groups. The formulations also include a partitioning agent, oligo-bridge, or polyanion bridge, and may optionally contain a viscosity modifying agent. The formulations are designed for topical application onto the skin of a patient and may be used to treat wrinkles, hyperhidrosis, and other health-related problems. Kits for administration are also described.
Owner:REVANCE THERAPEUTICS INC
Use of neurotoxin therapy for treatment of urologic and related disorders related to neurogenic bladder dysfunction
InactiveUS7449192B2Inexpensive and safeIncrease capacityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseUrethra
The present invention related to methods for treating neurological-urological conditions, including neurogenic bladder dysfunction. This is accomplished by administration of a botulinum toxin into the lower urinary tract of a patient with a neurogenic bladder dysfunction, including the bladder or urinary sphincter and the bladder wall.
Owner:ALLERGAN INC
Botulinum nanoemulsions
ActiveUS20140099342A1Cosmetic preparationsPeptide/protein ingredientsHigh pressureMuscle contracture
The embodiment described herein are related nanoemulsions comprising botulinum toxins. In one embodiment, the nanoemulsions are prepared by high pressure microfluidization and comprise a particle size distribution exclusively between 10 and 300 nm. The nanoemulsions contemplated by the present invention are useful for the cosmetic and medical treatment of muscular contracture states. For example, botulinum toxin may relax facial muscles such that skin wrinkles become smoother and less noticeable. Further, the present invention contemplates a cosmetic formulation that may be self-administered, for example, in the privacy of one's home and without medical supervision.
Owner:UNIVERSITY OF MASSACHUSETTS LOWELL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com