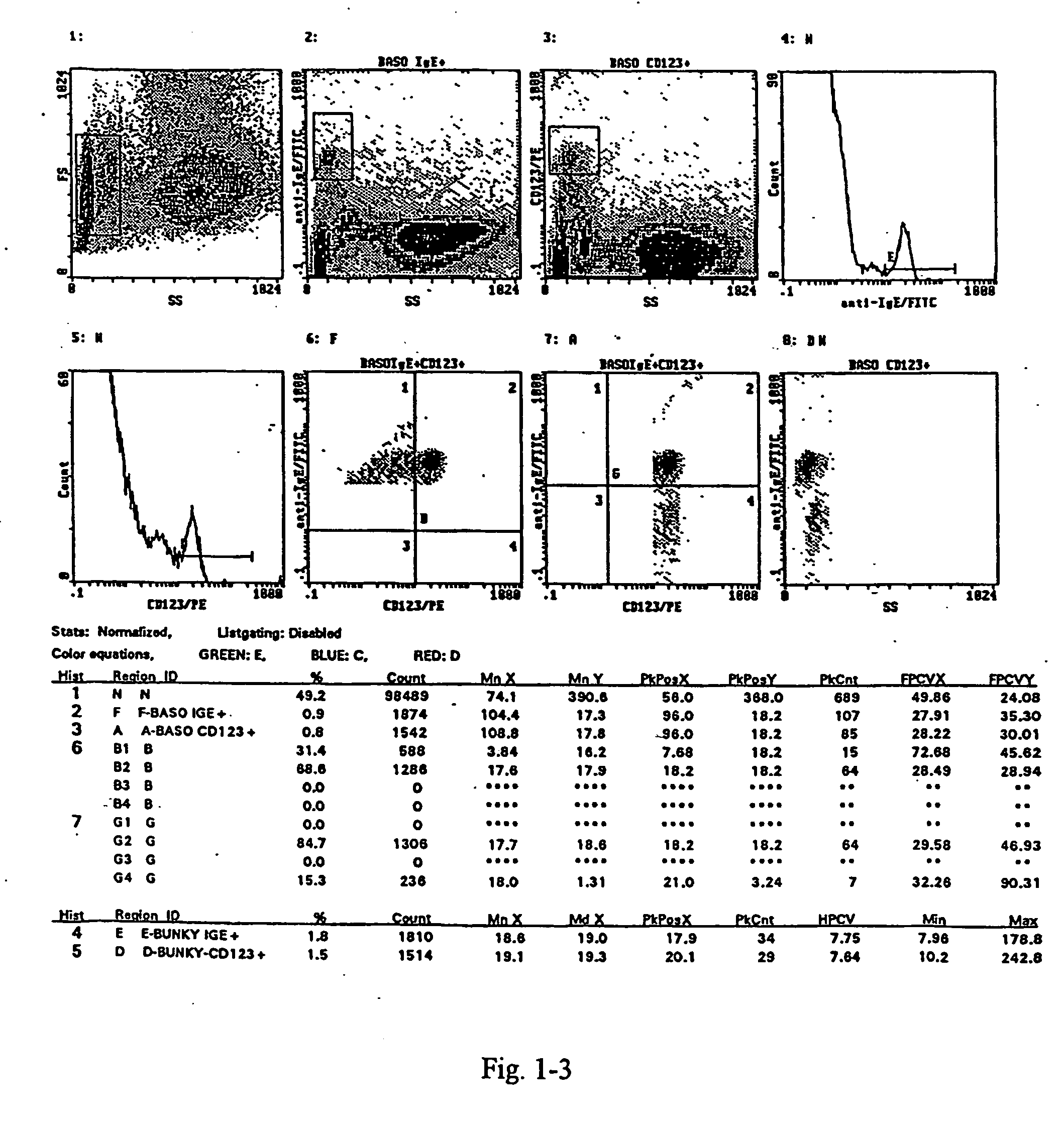
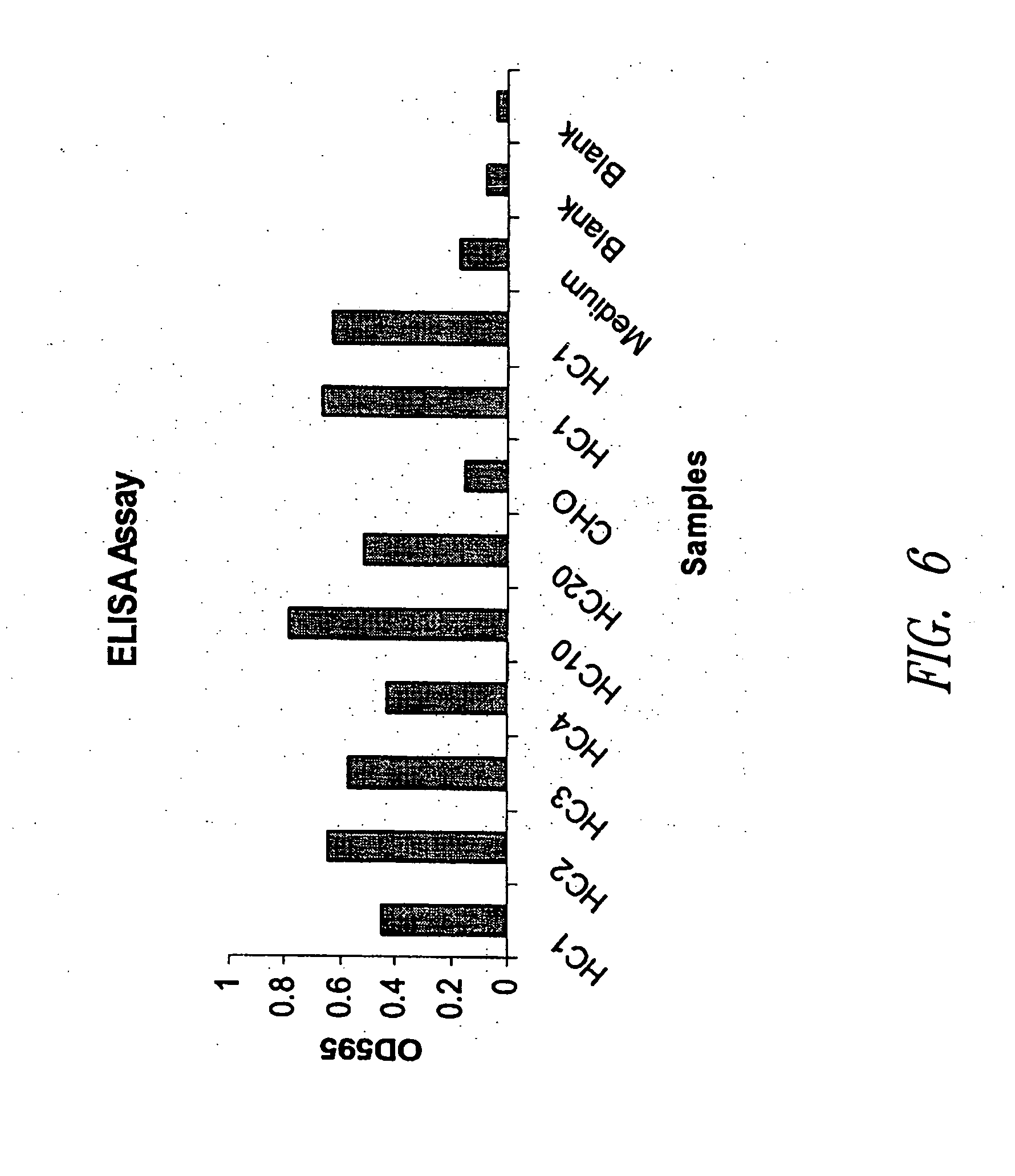
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Basophilia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Basophilia is the condition of having greater than 100 basophils/microliter in the peripheral blood.
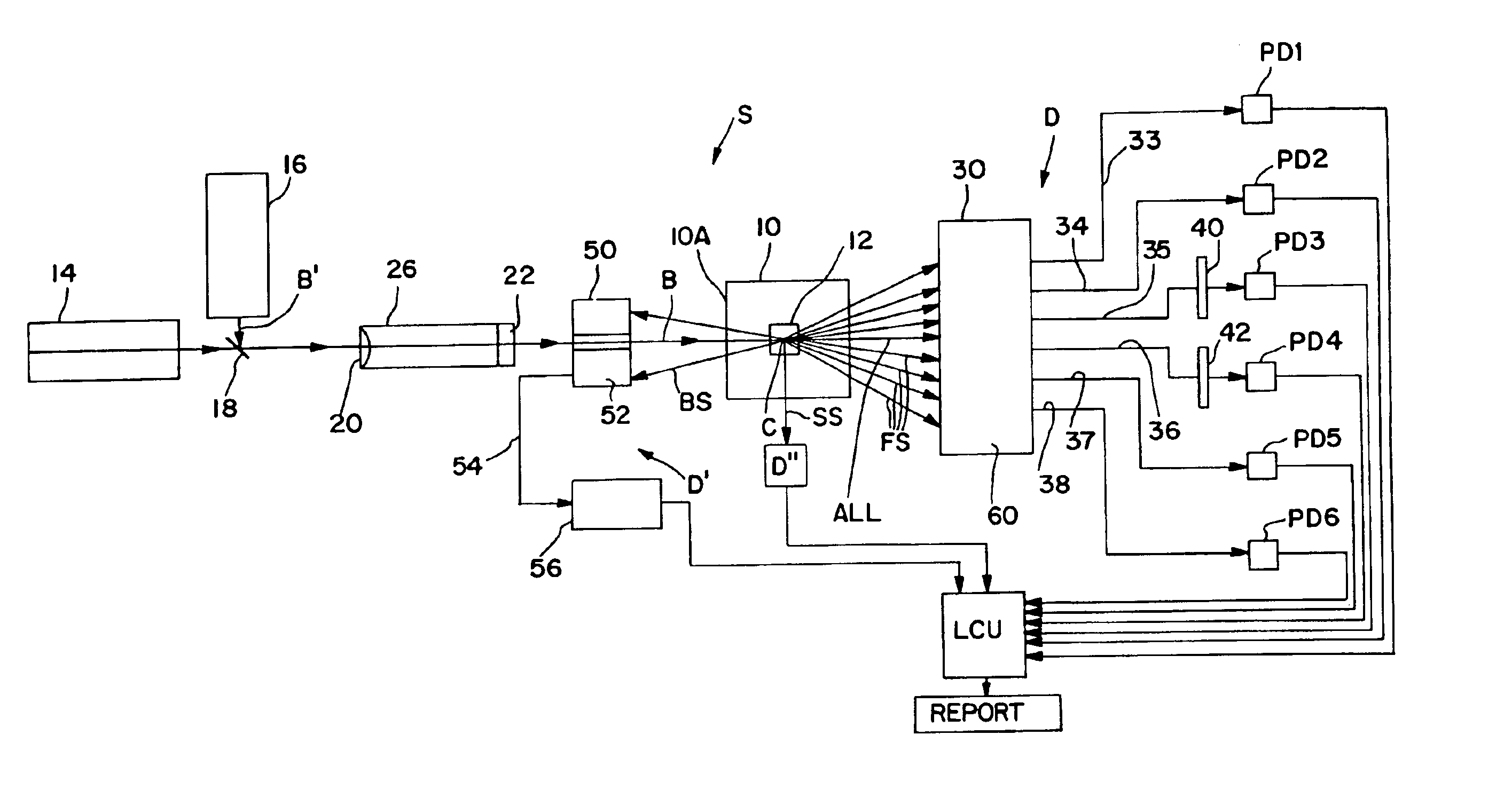
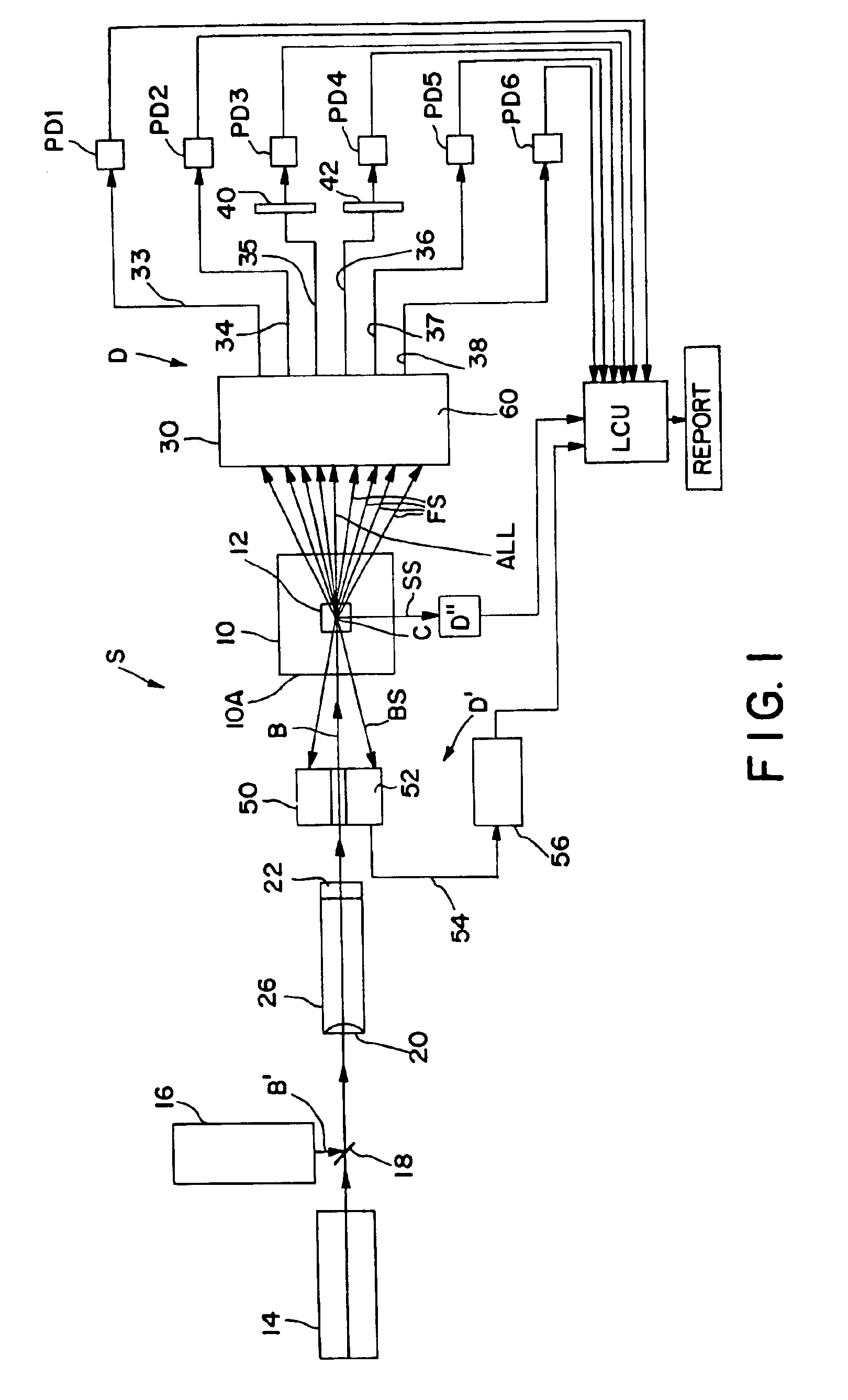
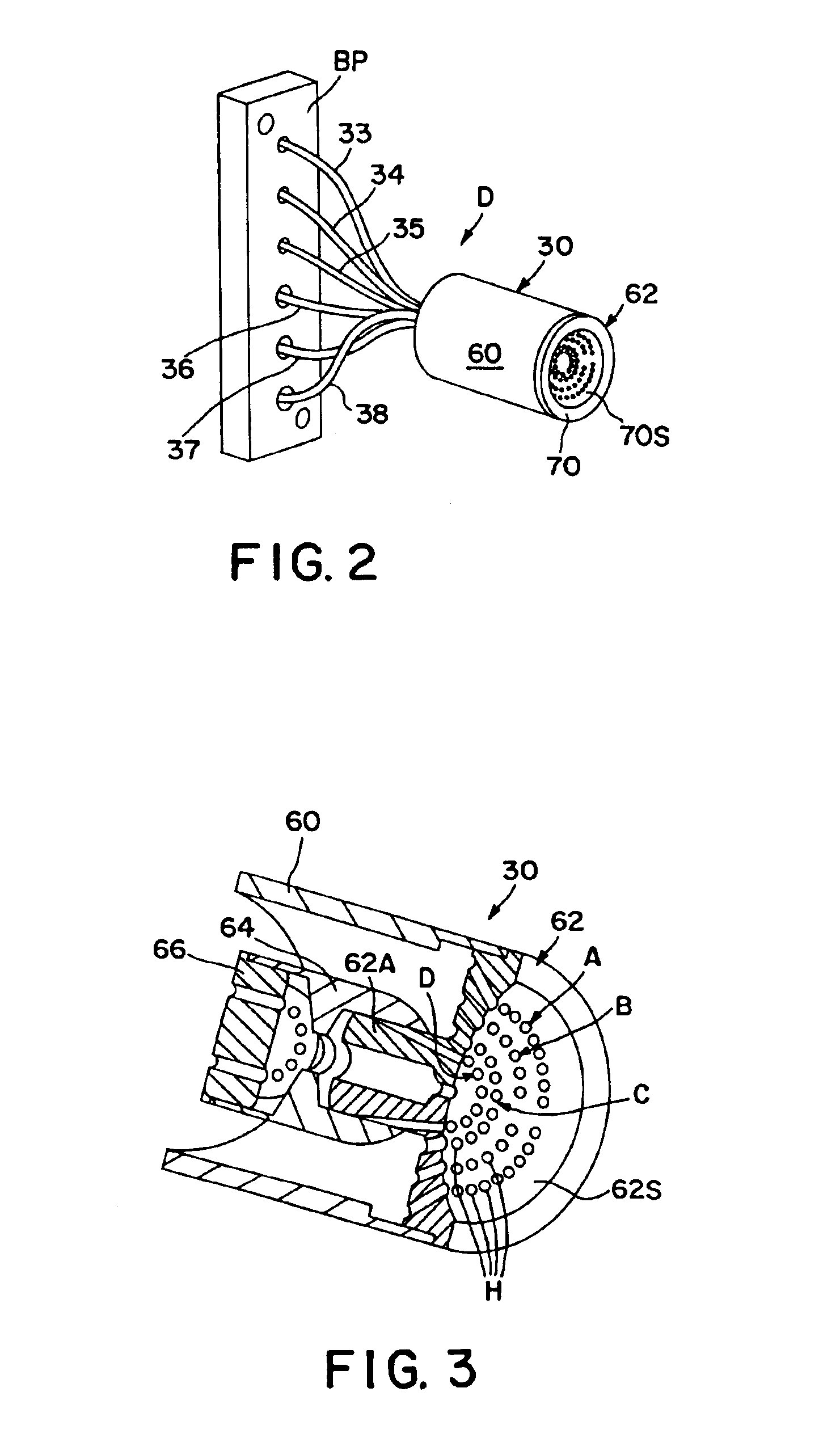
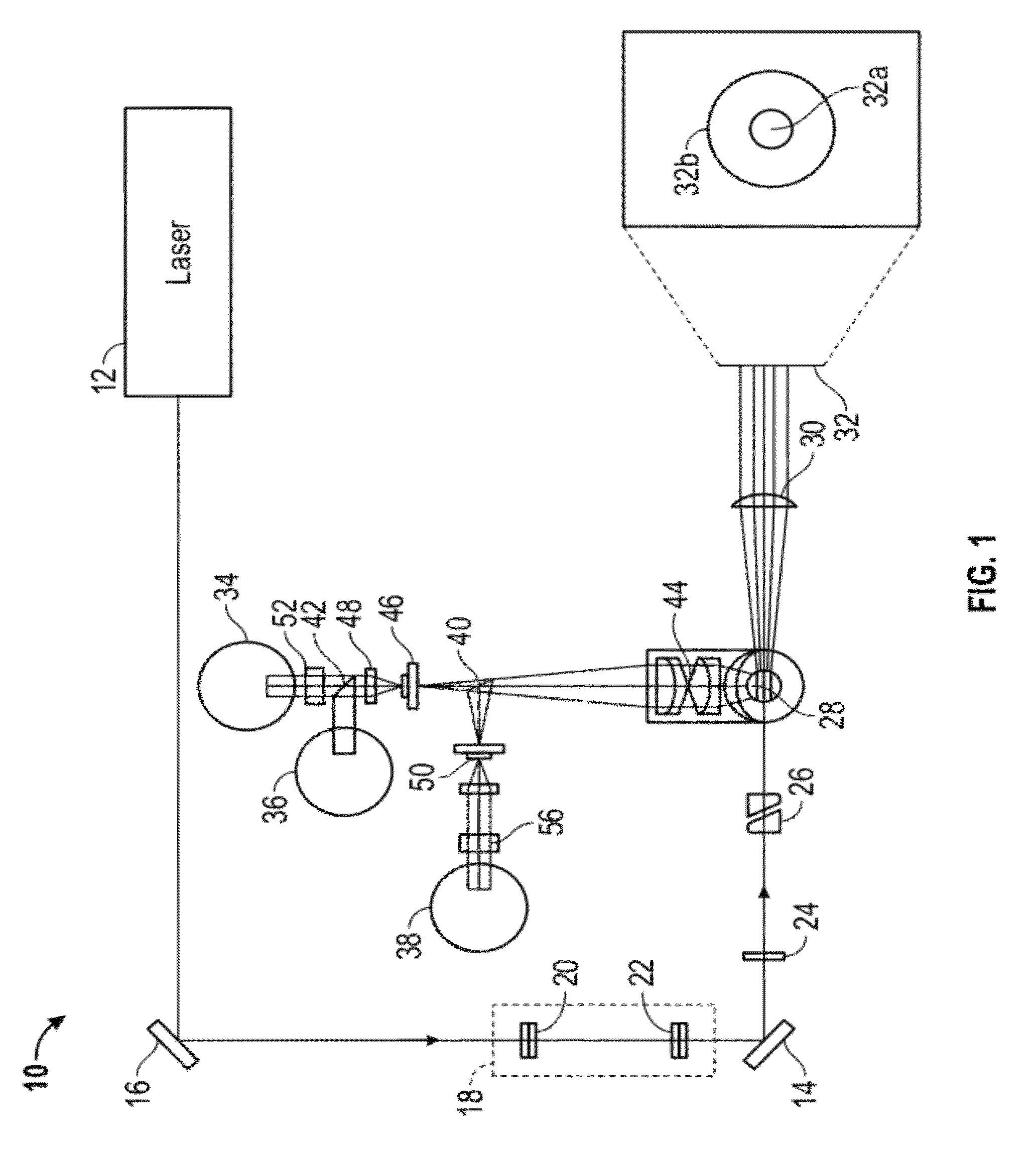
Apparatus for differentiating blood cells using back-scatter
InactiveUS6869569B2Material thermal conductivityMaterial analysis by electric/magnetic meansPhotodetectorRed blood cell
Blood cells of interest are readily distinguishable from other blood cells and look-a-like particles found in a blood sample by their back-scatter signature. A preferred method for differentiating platelets in a blood sample is to irradiate the cells and particles, one at a time, with a beam of radiation, and to detect back-scattered (reflected) radiation using a plurality of optical fibers to transmit the back-scattered radiation to a high-gain photodetector, e.g. a photomultiplier tube. Preferably, the back-scatter signal so obtained is combined with a second signal representing, for example, either the level of forward-scatter within a prescribed, relatively narrow angular range, or the level of side-scattered radiation, or the level of attenuation of the cell-irradiating beam caused by the presence of the irradiated cell or particle in the beam, or the electrical impedance of the irradiated cell or particle, to differentiate the cells of interest. The method and apparatus of the invention are particularly useful in differentiating platelets and basophils in a blood sample.
Owner:COULTER INTERNATIONAL CORPORATION
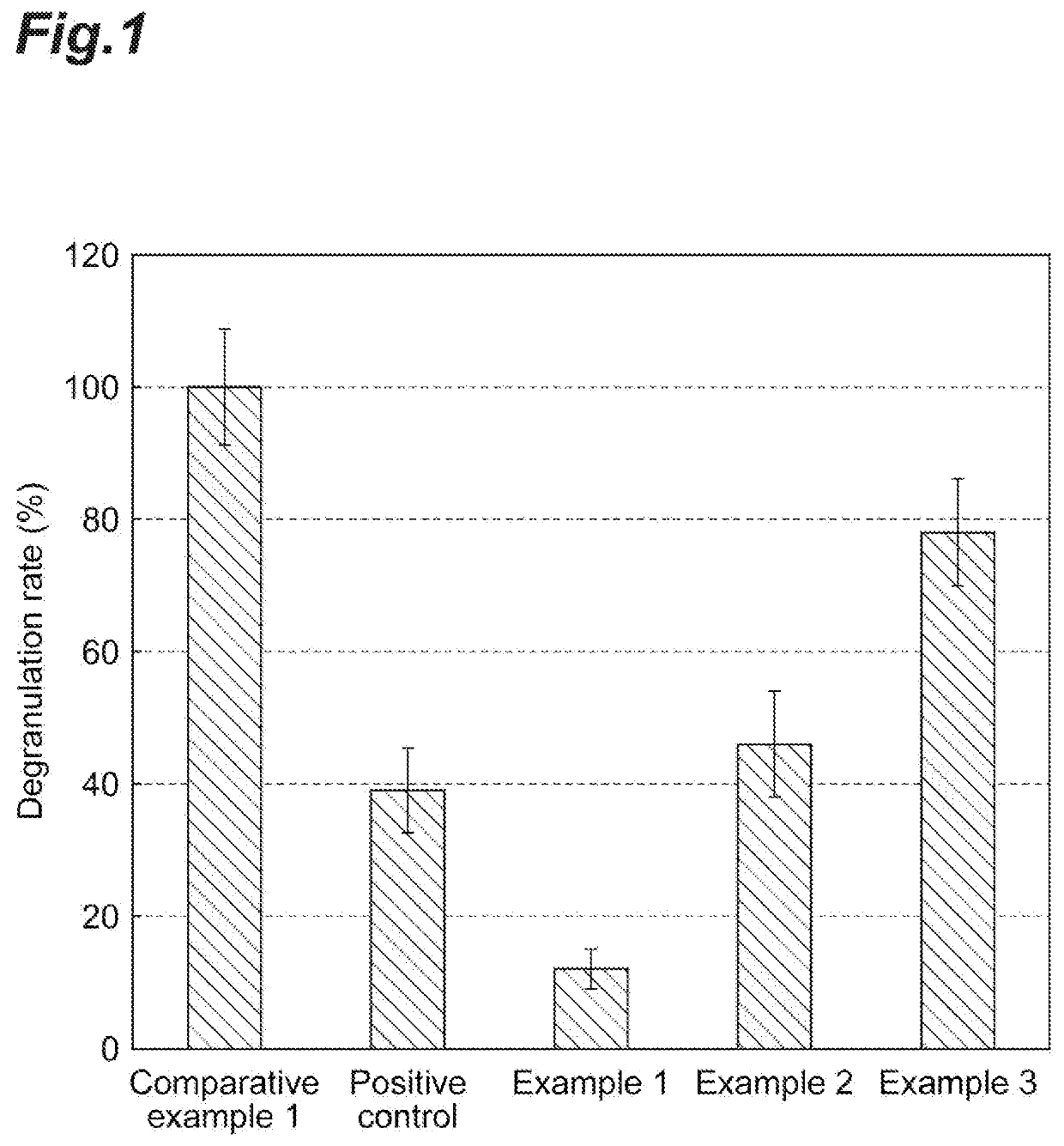
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS6822076B2Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsTetanusBasophilia
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-lIe-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Production of cultured human mast cells and basophils for high throughput small molecule drug discovery
InactiveUS7070996B2BiocideMicrobiological testing/measurementProgenitorHigh-Throughput Screening Methods
Provided are methods for producing and screening proliferated populations of CD34-negative progenitor cells, mucosal mast cells, connective tissue-type mast cells and basophil cells. The methods generate uniform proliferated populations of cells. The proliferated populations contain a uniform population of a size suitable for use in high throughput screening methods, for example, screening for agents that alter exocytosis. The invention includes screening the proliferated populations with at least one candidate bioactive agent, and evaluating the cells to detect a cell with an altered phenotype. The invention also includes isolating a candidate bioactive agent that causes the altered phenotype. Additionally, cells formed according to the described methods are also encompassed by the invention.
Owner:RIGEL PHARMA
Anaphylatoxins for detecting clinical conditions
InactiveUS20090226374A1Compounds screening/testingMicrobiological testing/measurementVasculitisBasophilia
Non-allergic hypersensitivity reactions can be observed in a sample of cells from a subject in response to anaphylatoxins. Accordingly, methods are provided for detecting clinical conditions such as cellular hyper-reactivity, non-allergic hypersensitivity, asthma, inflammation, chronic or acute infection, bacterial infection, viral infection, parasite infection, adverse drug reaction, organ rejection, vasculitis, mastocytosis, eosinophilia, basophilia, leukemia, and / or C3a or C5a receptor defects in a subject. Also provided are kits for detecting such clinical conditions in a subject.
Owner:HEALTH AIRE
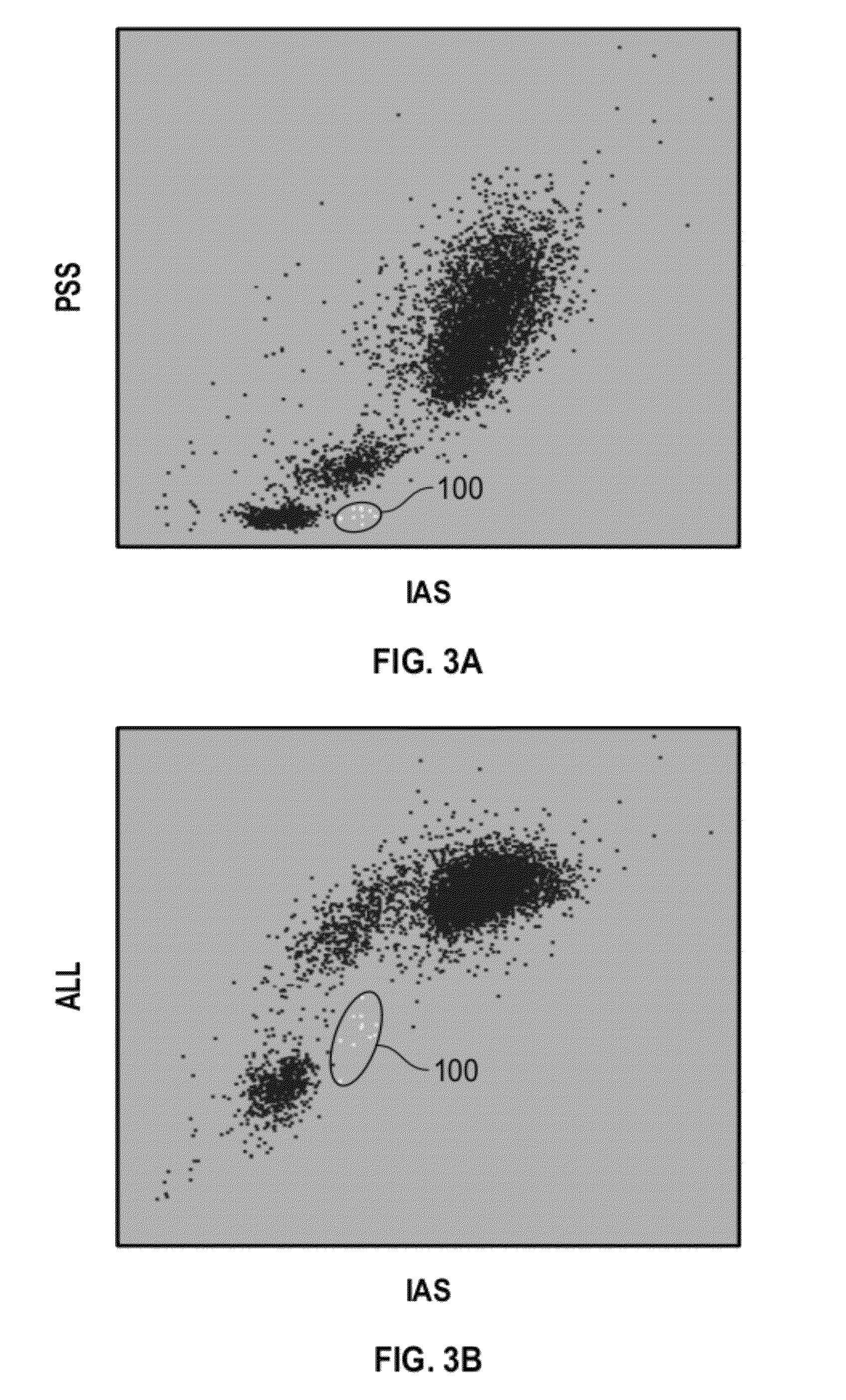
Method and kit for the measurement of the activation of basophils induced by allergen to determine hypersensitivity to some substances
InactiveUS20040265925A1Lower IgE levelsOvercome disadvantagesBiological material analysisAllergen ingredientsStainingCD63
A method for the measurement of the activation of the basophils following stimulation with an allergen to determine hypersensitivity to some substances, in which a blood sample with an addition of interleukin-3 in a quantity of 0.05 to 50 ng / ml based on the volume of the sample, and of appropriately diluted allergen in a quantity of 0.5 to 100 units / ml is incubated at the temperature corresponding to the physiological environment for 15 to 45 minutes, whereafter a staining with anti-CD63.antibody in an amount of 3 to 30 .mu.l / 100 .mu.l of blood and with the antibody against a receptor of the basophil in an amount of 3 to 30 .mu.l / 100 .mu.l of blood are added at a temperature of 0 to +10.degree. C. and the sample, after vortexing, is then incubated in an ice bath for 15 to 30 minutes, and then is lysed and subjected to flow cytometry.
Owner:HAVRANOVA MARIE
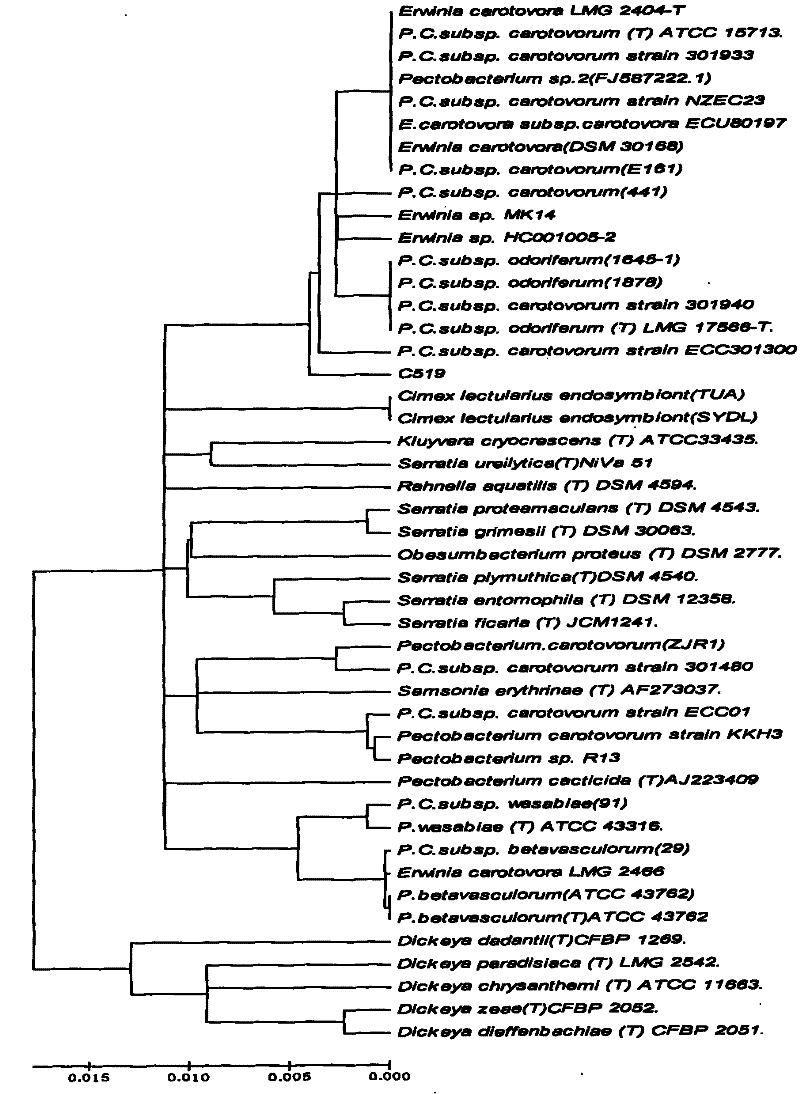
Pectobacterium carotovorum and applications thereof in bio-pulping
InactiveCN101899403APromote rapid proliferationDiffusion fastBacteriaMicroorganism based processesFiberPectobacterium carotovorum
The invention belongs to the field of papermaking and discloses pectobacterium carotovorum and applications thereof in bio-pulping. The pectobacterium carotovorum subsp. carotovorum C519 is preserved in China General Microbiological Culture Collection Center with a preservation number is CGMCC No. 3350. The strain can grow under the condition of high alkalinity (pH11), is suitable for degumming process of plant bast fibers and can be used for biological paper pulp. The C519 (CGMCC No. 3350) can grow under an alkaline condition (pH9 to pH 11), and secretes basophilia pectin with high activities (an appropriate pH value is between 8 and 10). Other general microorganisms are difficult to grow and breed in the range of alkalinity, so that the strain can avoid being polluted by other competitors. Moreover, cellulose has no activities under the alkaline condition, thus the strain greatly keeps the original performance of the bast fibers and improves yield.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Allergen vaccine proteins for the treatment and prevention of allergic diseases
InactiveUS20060292138A1Reduce riskAmeliorating and reducing risk of IgE-medicatedPeptide/protein ingredientsAntibody mimetics/scaffoldsFc(alpha) receptorCross-link
The present invention provides fusion proteins comprising an allergen sequence linked via an IgG hinge region to another polypeptide sequence capable of specifically binding to a native IgG inhibitory receptor containing an immune receptor tyrosine based inhibitory motif (ITIM). They are designed to cross-link an Fc receptor for IgE (e.g., FcεR1) and an IgG inhibitory receptor (e.g., FcγRIIb), thereby inhibiting the IgE-driven mediators released from mast cells and basophils. In addition, the present invention provides nucleic acid molecules encoding the fusion proteins, vectors and host cells for producing the fusion proteins, pharmaceutical compositions comprising the fusion proteins, and methods for ameliorating or reducing the risk of IgE-medicated allergic diseases.
Owner:CHEN HAIMING
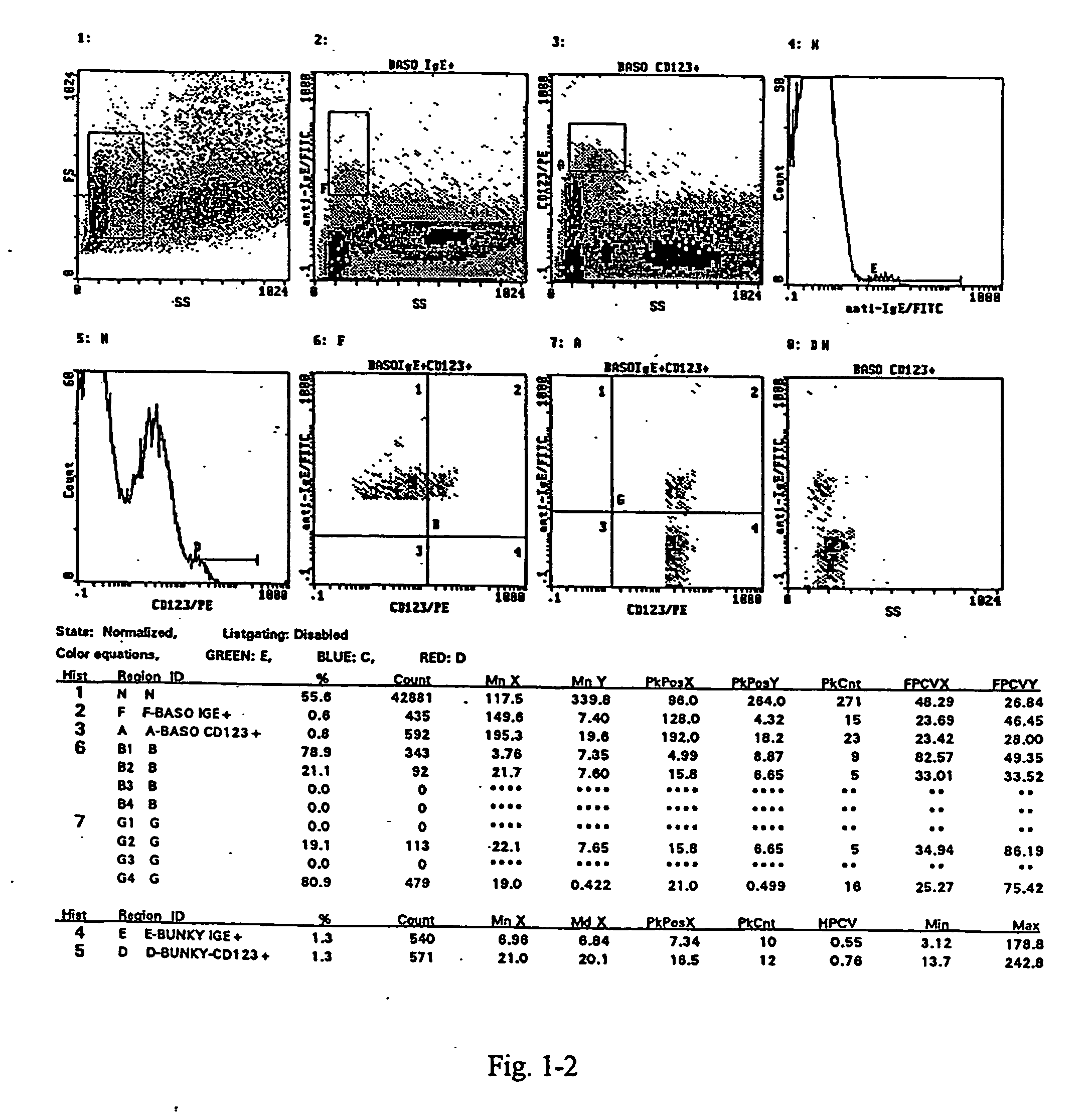
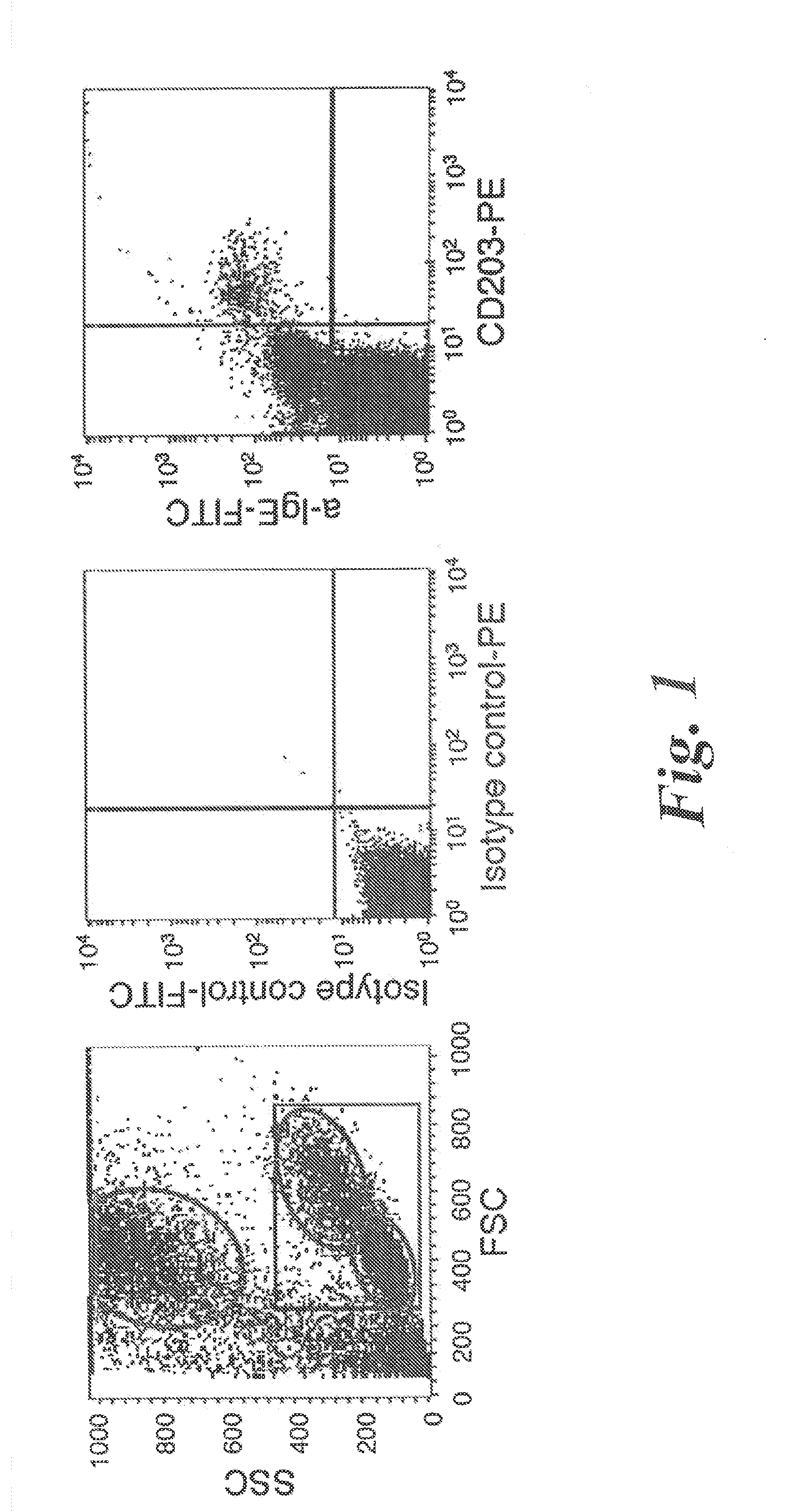
Basophil analysis system and method
InactiveUS9091625B2Bioreactor/fermenter combinationsBiological substance pretreatmentsSub populationsFluorescence
Provided herein are systems and methods for analyzing blood samples, and more specifically for performing a basophil analysis. In one embodiment, the systems and methods include: (a) staining a blood sample with an exclusive cell membrane permeable fluorescent dye; and then (b) using measurements of light scatter and fluorescence emission to distinguish basophils from other WBC sub-populations. In one embodiment, the systems and methods include performing a basophil cluster analysis of the blood sample, based on the combination of light scatter and fluorescence measurements.
Owner:ABBOTT LAB INC
Process for enriching basophils in a blood sample
Owner:INST PASTEUR
Method for identifying allergenic proteins and peptides
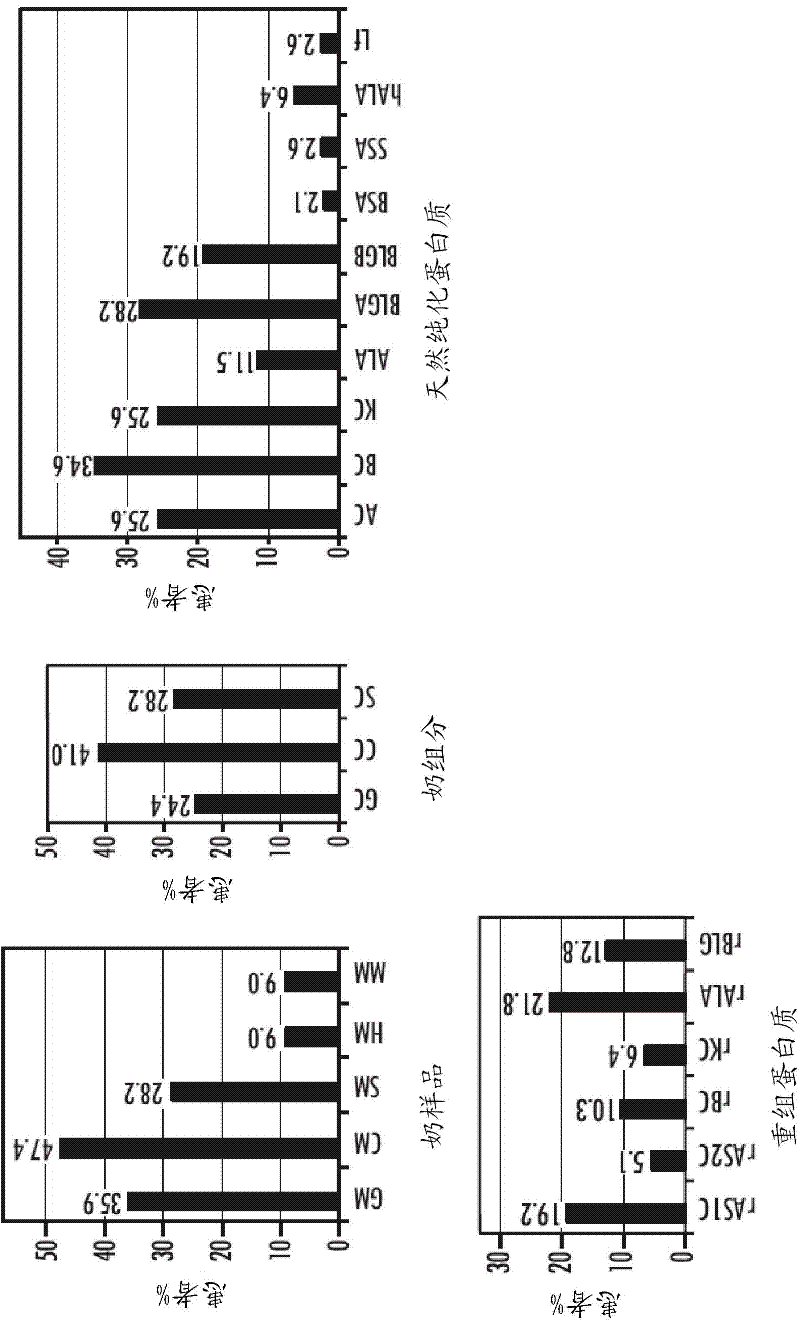
The present invention relates to a method for identifying allergenic proteins and peptides. More specifically, the present invention provides a method for identifying allergenic milk proteins and / or peptides comprising the steps of: providing at least one expression library comprising DNA or cDNA derived from the mammary gland tissue of a lactating mammal, preferably a lactating cow, expressing at least one protein or peptide encoded by said expression library, determining the binding capacity of said at least one protein or peptide to IgE of at least one serum of an individual who is sensitive to mammalian milk,preferably cow's milk, contacting the at least one protein or peptide exhibiting an IgE binding with basophil cells, eosinophil cells or mast cells and identifying the at least one protein or peptide as being allergenic when said basophil cells, eosinophil cells or mast cells release upon contact with at least one protein or peptide at least one mediator.
Owner:MJN U S HLDG LLC
Methods and kits for detecting basophil activation
InactiveUS20200011865A1Efficient detectionMethod is numerousDisease diagnosisBiological testingBasophiliaBasophil cell
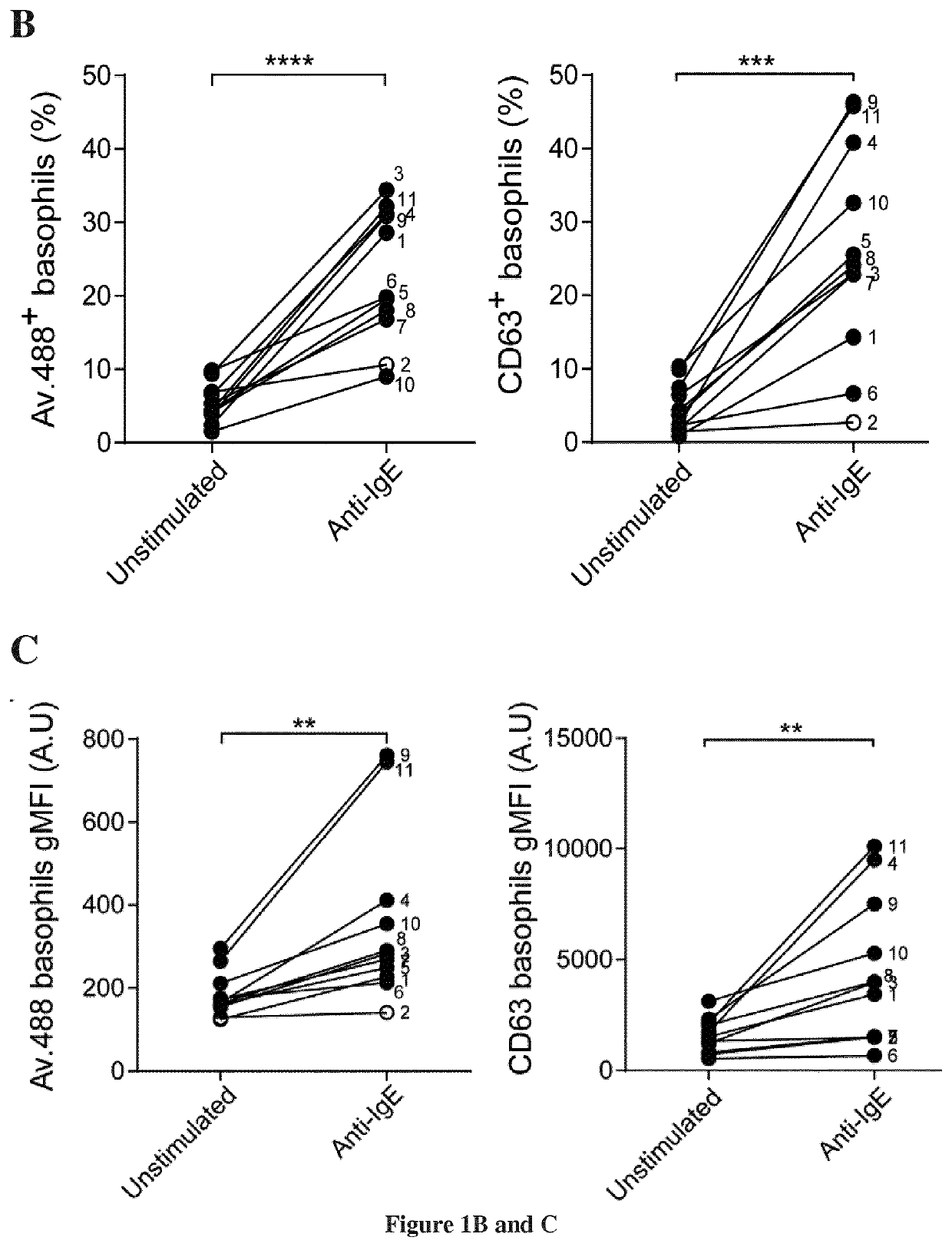
The present invention relates to methods and kits for detecting basophil cells activation. The inventors showed that fluorescent avidin binds to basophil cell surface upon degranulation and that this probe can be used to monitor basophil degranulation More specifically the present invention relates to methods for monitoring of basophil degranulation using avidin-based probes. The method of the invention described here allows to measure direct basophil degranulation following FcεRI crosslinking with allergen. This method provides a direct measurement of degranulation by staining exteriorized granules and unambiguously detects activated basophils degranulated. The extent of the degranulation can be directly deduced from the intensity of fluorescence of fluorochrome-labelled avidin measured on basophils. When applied to allergic patient samples the avidin-based method detected efficiently specific basophil responses.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +3
Whole-transcriptome expression profile of exosome, and construction method and application of whole-transcriptome expression profile
ActiveCN111172112ARealization of biological functionsCell dissociation methodsMicrobiological testing/measurementBasophiliaCell
The invention discloses a preparation method of an exosome released by activated basophil. The preparation method comprises the steps of preparing basophil from anticoagulant peripheral blood of a normal person by a negative selection method, performing activation through Anti-IgE stimulation, performing culture in an incubator containing 5% of CO2 at 37 DEG C through serum-free culture mediums for 12-48 hours, then collecting supernatant of cell culture fluid, performing centrifuging under the condition of 300g at room temperature, collecting culture fluid supernatant after the centrifuging,and performing ultracentrifugation so as to obtain the exosome released by activated basophil. The invention also discloses a whole-transcriptome expression profile of the exosome, and a constructionmethod of the whole-transcriptome expression profile. According to the preparation method disclosed by the invention, the exosome released by human basophil is successfully separated and identified for the first time, besides, the whole-transcriptome expression profile of the exosome released by the human basophil is built for the first time, a plurality of RNAs having difference expression afteractivation are identified, and a new target point is provided for further researching a molecular mechanism that the activated basophil acts with target cells during disease generation.
Owner:AFFILIATED HOSPITAL OF GUANGDONG MEDICAL UNIV
Application of nicotinamide mononucleotide and/or nicotinamide mononucleotide salt
InactiveCN110237088ASuppress immune responseReduce synthesisOrganic active ingredientsImmunological disordersMast cellAnti-allergy drugs
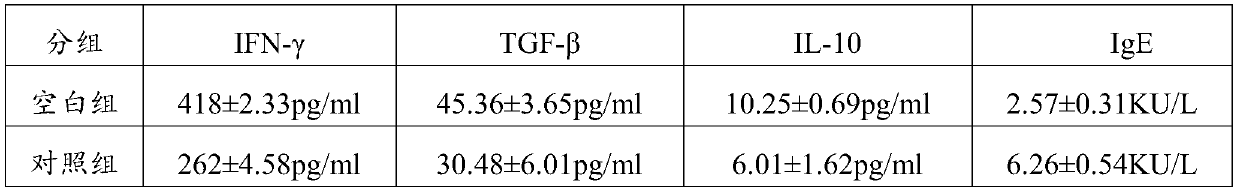
The invention belongs to the field of research and development of drugs and health-care products, and particularly relates to an application of nicotinamide mononucleotide and / or nicotinamide mononucleotide salt. The invention provides an application of the nicotinamide mononucleotide or the nicotinamide mononucleotide salt to anti-anaphylaxis drugs and / or anti-anaphylaxis health-care products. According to the technical scheme provided by the invention, after the nicotinamide mononucleotide or the nicotinamide mononucleotide salt (NMN) is used, Treg cells can be induced to secrete immunosuppression cytokine IL10, TGF-beta and the like, so that the immune reaction of TH2, blood basophilia and tissue mast cells can be restrained, sIgE synthesis is reduced, and finally the anaphylaxis symptoms can be alleviated; and further, through experimental determination, after the NMN is used, fewer adverse reactions exist, and the anaphylaxis recurrence rate is reduced. According to the application of the nicotinamide mononucleotide and / or the nicotinamide mononucleotide salt provided by the invention, the technical defects in the prior art that anti-anaphylaxis drugs have some adverse reactions, medication needs to be repeatedly performed, and the anaphylaxis recurs easily can be overcome.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
Basophilia mimic enzyme as well as preparation method and application thereof
ActiveCN107999128ASimple and fast operationIncreased peroxidase activityOrganic-compounds/hydrides/coordination-complexes catalystsDispersityPeroxidase
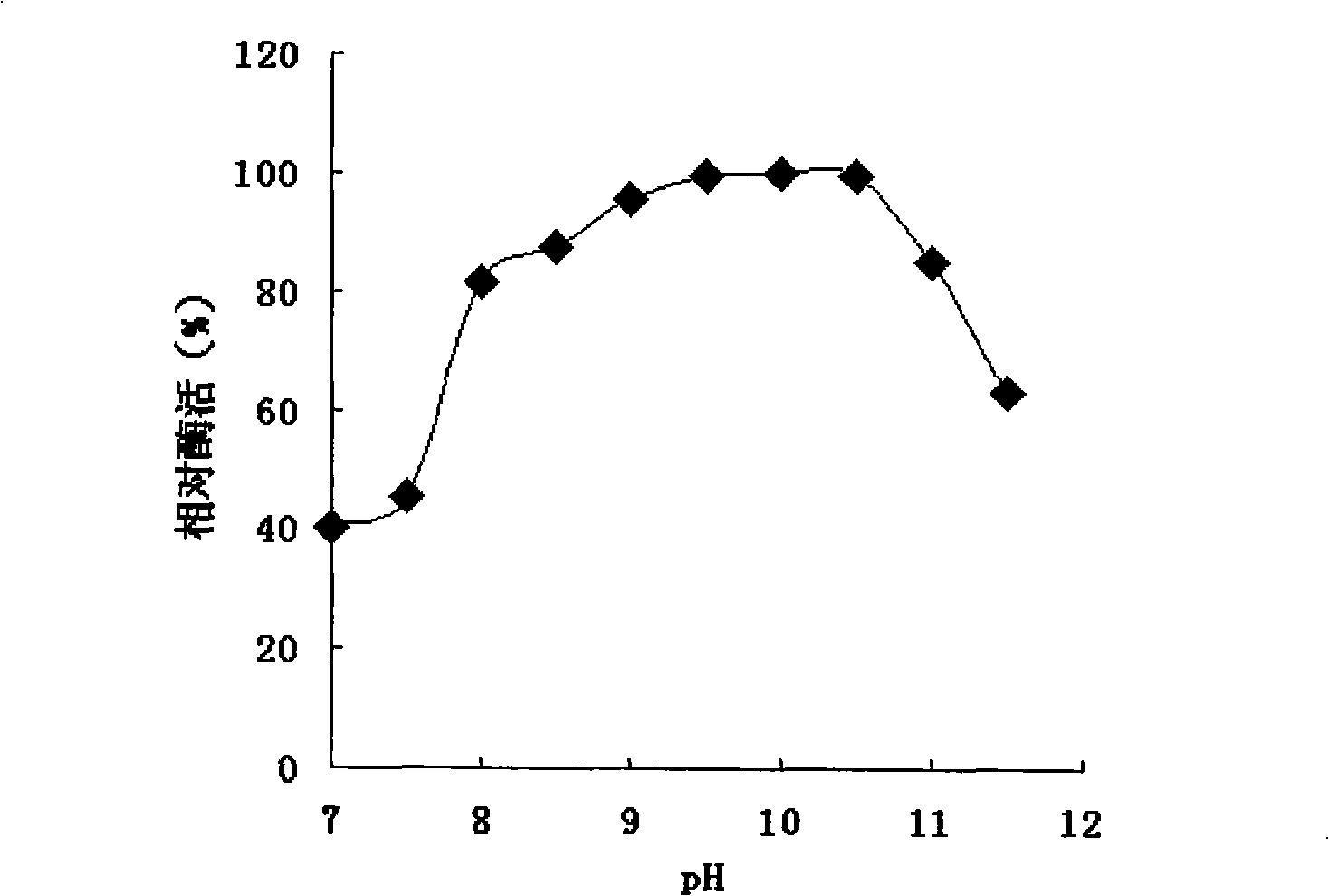
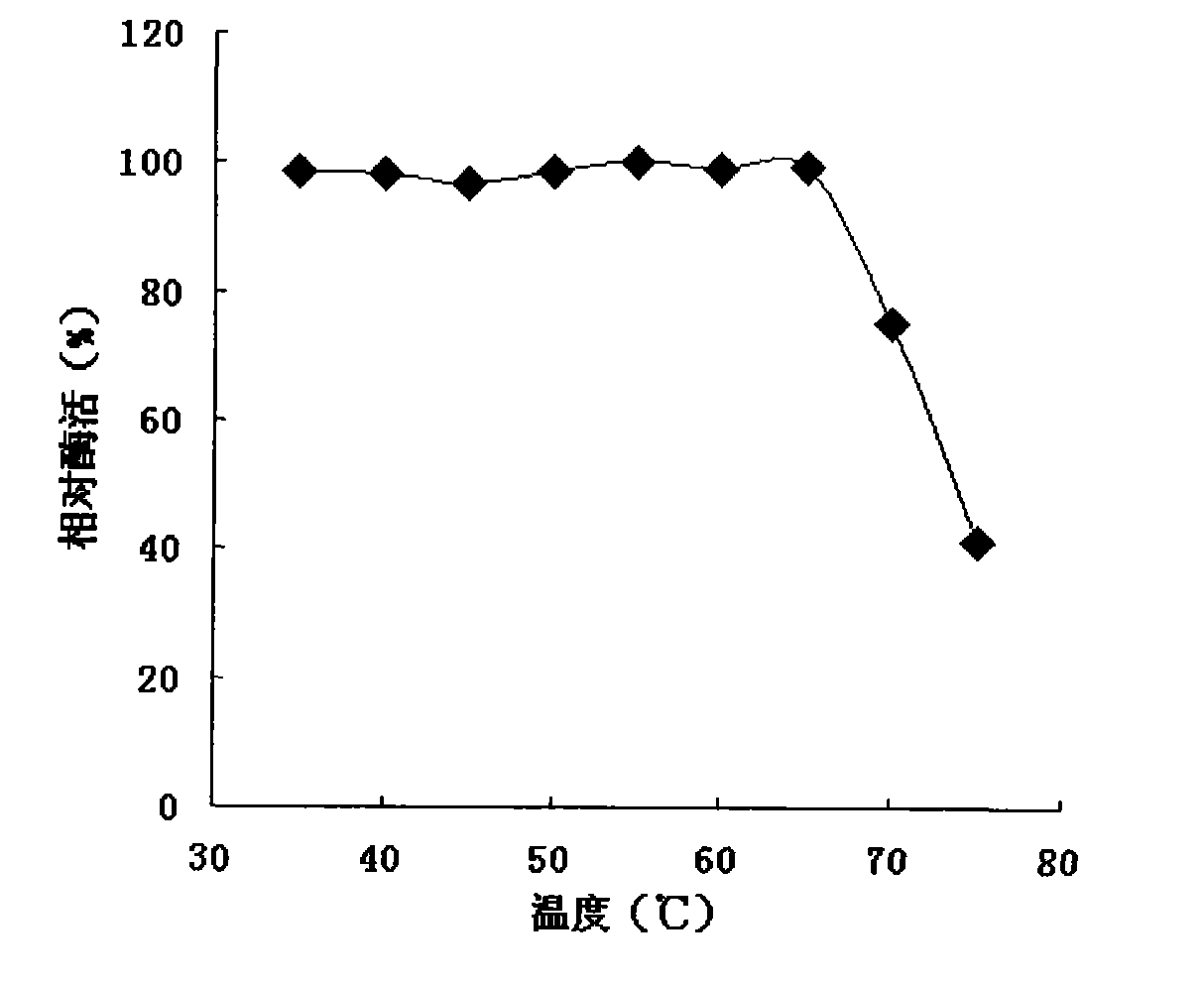
The invention discloses a basophilia mimic enzyme as well as a preparation method and application thereof. The preparation method comprises the following steps: pre-mixing a melamine aqueous solutionand a palladium salt aqueous solution, enabling melamine molecules to be combined with palladium ions by virtue of an electrostatic acting force, adding a reducing agent, stirring, and generating basophilia melamine protective nanopalladium, thus obtaining the basophilia mimic enzyme. The preparation method disclosed by the invention is simple in synthetic condition, low in price of raw materialsand easy in commercial development. The prepared basophilia mimic enzyme has good peroxidase activity under the extreme alkaline pH condition; and moreover, the dispersity in water is good, and the application prospect in the field such as pollutant treatment, detection under the extreme alkaline condition and the like is good.
Owner:广州市仟味食品有限公司
Methods of modulating m2 macrophage polarization and use of same in therapy
A method of treating a disease or disorder that can benefit from increasing an M2 / M1 macrophage ratio in a subject in need thereof is provided. The method comprising: (a) culturing basophils in the presence of IL33 and / or GM-SCF; and (b) administering to the subject a therapeutically effective amount of the basophils following the culturing, thereby treating the disease or disorder that can benefit from increasing an M2 / M1 macrophage ratio in the subject.
Owner:YEDA RES & DEV CO LTD
Cannabinoid-containing complex mixtures for the treatment of mast cell-associated or basophil-mediated inflammatory disorders
PendingCN110536681AReduce regulatory burdenReduce the ethical burdenHydroxy compound active ingredientsSurgical drugsContact dermatitisBasophilia
The present invention provides cannabinoid-containing complex mixtures suitable for use as active pharmaceutical ingredients. The complex mixtures are comprised of the major cannabinoid, cannabidiol,a first minor cannabinoid, which is cannabigerol, at least a first selected terpene, and optionally a second minor cannabinoid. Also provided are methods of making the complex mixtures, pharmaceuticalcompositions comprising the complex mixture, and methods of using the pharmaceutical compositions for the treatment of mast cell-related immune disorders, including allergy and atopy (allergic asthma, eczema, rhinitis), mast cell activation syndrome (MCAS), physical and chemical urticarias, idiopathic urticaria, Crohn's disease, inflammatory bowel disorder, dermatitis and contact dermatitis, arthritis and rheumatoid arthritis, canine mastocytosis, and allergy and inflammation in cattle, swine, etc. The methods of the present invention further relate to the treatment of various basophil-mediated immune disorders.
Owner:GBS GLOBAL BIOPHARMA INC
Method of treating an allergy with allergen-specific monoclonal antibodies
ActiveUS11352417B2Avoid allergic reactionsEasy to removeImmunoglobulins against animals/humansPharmaceutical delivery mechanismMast cellAntiendomysial antibodies
Owner:REGENERON PHARM INC
Alkali-fast bacillus cereus bacterial strain and produced pectinase thereof
InactiveCN101508967BImprove thermal stabilityGood substrate toleranceBacteriaHydrolasesHigh concentrationPectinase
The invention uses high-concentration pectin as a unique carbon source to screen an alkaline-resisting bacillus strain from soil; the strain is named Bacillus alcalophilus M29, the preservation date is January 6th, 2009, the full name of the preservation institution is China Center for Type Culture Collection, and the preservation number is CCTCC NO: M 209006; the invention further relates to pectase produced by alkaline-resisting Bacillus alcalophilus M29, having better thermal stability (45 to 65 DEG C), substrate tolerance and basophilia (8.5 to 11, the optimum pH value being 10) and belonging to heat-resisting basophilia pectase; and the invention also relates to the application of the alkaline-resisting Bacillus alcalophilus M29 strain in fermentation and preparation of pectase.
Owner:CHANGSHU INSTITUTE OF TECHNOLOGY
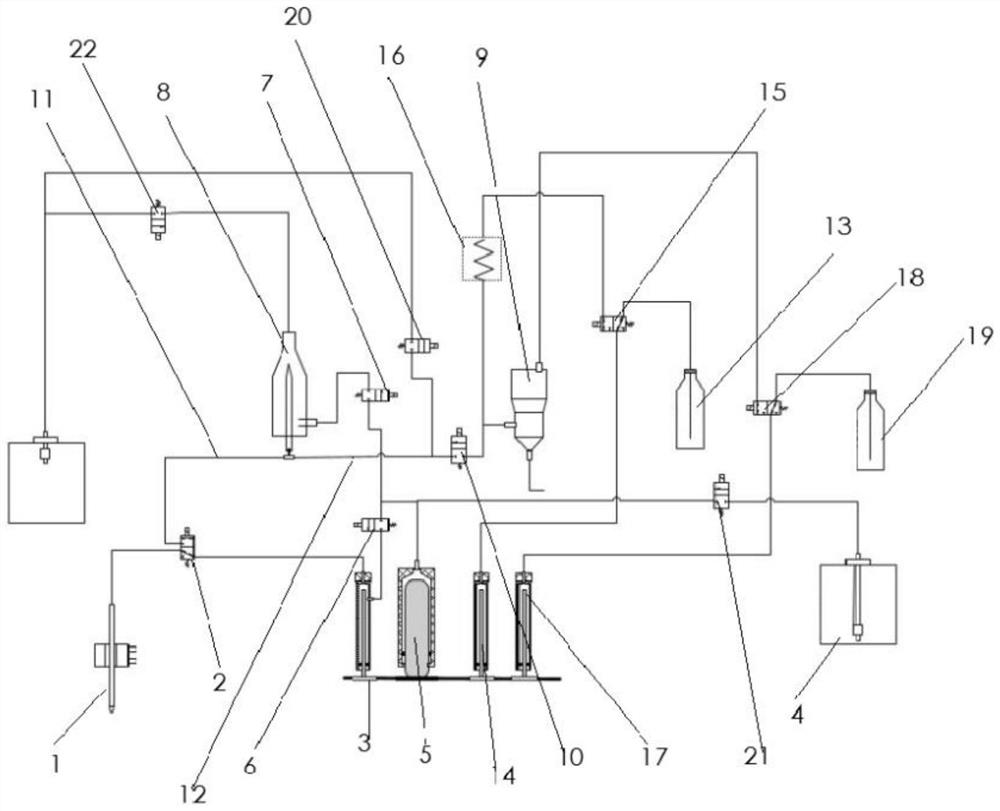
Liquid path system and hematology analyzer thereof
PendingCN112345433AThe test result is accurateSimple structureIndividual particle analysisBiological testingBasophiliaLymphocyte
The invention provides a liquid path system which comprises a liquid path of a pretreatment module, a liquid path of a reagent 1 input module, a liquid path of a reagent 2 input module, a liquid pathof a reagent 3 input module and a liquid path of a detection device cleaning module, wherein pipelines for detecting and analyzing lymphocytes, mononuclear cells, neutrophils, eosinophils and basophils are the same pipeline, the liquid path system is simplified, later installation and maintenance are facilitated, a solvent preheating device is added into the liquid path system, the reaction between reagents and samples can be accelerated, meanwhile, the invention also provides a hematology analyzer comprising the liquid path system, and the hematology analyzer is small in size.
Owner:ZYBIO INC
Application of NADH and/or NADPH in antiallergic drugs and/or antiallergic health products
InactiveCN110200985AHas an immunomodulatory effectSuppress immune responseOrganic active ingredientsDigestive systemAntiallergic drugsMast cell
The invention belongs to the field of research and development of medicines and health products, and particularly relates to an application of NADH and / or NADPH in antiallergic drugs and / or antiallergic health products. The present invention provides the application of NADH and / or NADPH in the antiallergic drugs and / or the antiallergic health products. In the present invention, NADH / NADPH can be administrated to induce Treg cells to secrete immunosuppressive cytokines IL10, TGF-beta and the like. which can inhibit the immune response of TH2, blood basophilia and tissue mast cells, reduces sIgEsynthesis, and increases sIgG and sIgA synthesis, and alleviates allergy symptoms; can stimulate the allergen-specific TH1 response, produces cytokines IFN-gamma and TGF-gamma, inhibits the immune response of TH2, blood basophilia and tissue mast cells, reduces sIgE synthesis and increases sIgG and sIgA synthesis, and alleviates allergy symptoms. The application solves the technical defects thatthe anti-allergic drugs have some adverse reactions, need repeated medication and allergic recurrence, and provides a long-acting, immunomodulatory natural anti-allergic drug or health care product.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
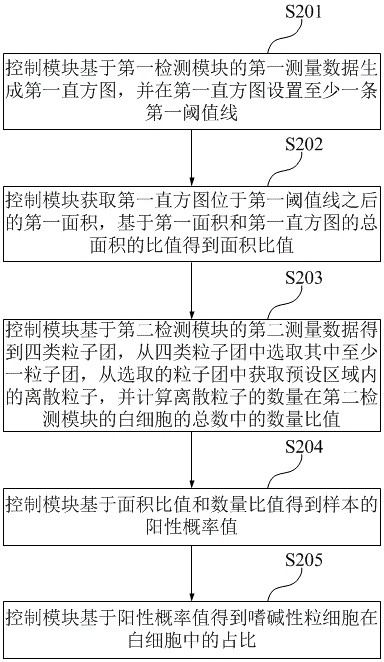
A sample analyzer and counting method thereof
ActiveCN114047109BReduce positive false positive rateImprove positive detection rateCharacter and pattern recognitionIndividual particle analysisComputer hardwareWhite blood cell
The application discloses a sample analyzer and its counting method. The sample analyzer includes a control module, a first detection module and a second detection module. The control module is respectively connected to the first detection module and the second detection module. The counting method includes: The control module generates a first histogram based on the first measurement data of the first detection module; the control module obtains an area ratio based on the ratio of the first area to the total area of the first histogram; the control module calculates the number of discrete particles in the second The number ratio of white blood cells in the detection module; the control module obtains the positive probability value of the sample based on multiple area ratios and number ratios; the control module obtains the proportion of basophils in white blood cells based on the positive probability value. Through the above method, the positive misjudgment rate of the sample can be reduced, the positive detection rate of the sample can be increased, and the reliability of the count value of basophils can be improved.
Owner:SHENZHEN DYMIND BIOTECH
Leukocyte classification reagent
InactiveCN111024563AChange brittlenessEfficient separationBiological particle analysisIndividual particle analysisBasophiliaWhite blood cell
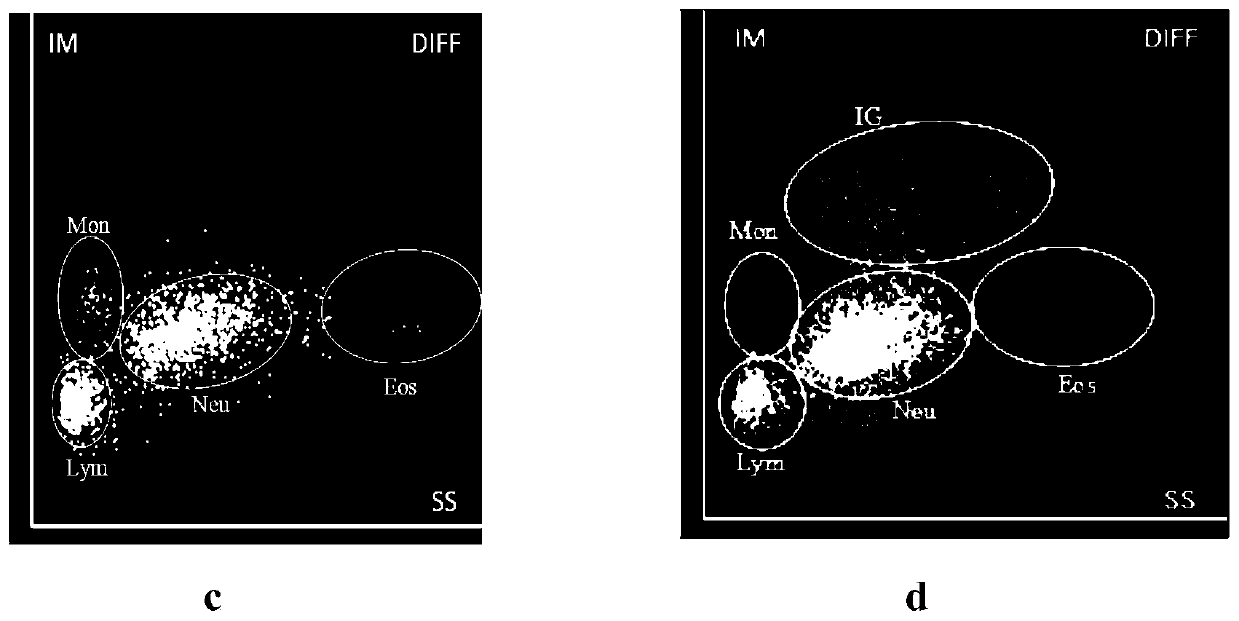
The invention relates to the technical field of in vitro diagnosis, and discloses a leukocyte classification reagent. The leukocyte classification reagent comprises a polyoxyethylene type nonionic surfactant, a polyol type nonionic surfactant, a buffer solution, a conductivity supplement, an osmotic pressure regulator and a preservative. The reagent provided by the invention is simple in formula and dye-free, the main components only comprise two specific nonionic surfactants, and the defects of excessive cell damage or too small size and too large distribution span of the same kind of cells and failure in effective classification which are possibly caused by other reagents are avoided. According to the present invention, with the reagent, leukocytes can be divided into monocytes, lymphocytes, neutrophils (including basophils) and eosinophils with clear boundaries, and juvenile granulocytes can be classified.
Owner:SONOSCAPE MEDICAL CORP
Method for identifying allergenic proteins and peptides
A method for identifying allergenic proteins and peptides. More specifically, a method for identifying allergenic milk proteins and / or peptides including the steps of: providing at least one expression library comprising DNA or cDNA derived from the mammary gland tissue of a lactating cow, expressing at least one protein or peptide encoded by said expression library, determining the binding capacity of said at least one protein or peptide to IgE of at least one serum of an individual who is sensitive to cow's milk, contacting the at least one protein or peptide exhibiting an IgE binding capacity as determined in step c) with basophil cells, eosinophil cells or mast cells and identifying the at least one protein or peptide as being allergenic when said basophil cells, eosinophil cells or mast cells release upon contact with at least one protein or peptide of step d) at least one mediator.
Owner:MJN U S HLDG LLC
A whole-transcriptome expression profile of exosomes and its construction method and application
ActiveCN111172112BRealization of biological functionsCell dissociation methodsMicrobiological testing/measurementDiseaseBasophilia
The invention discloses a method for preparing exosomes released by activated basophils, comprising the steps of: preparing basophils from anticoagulated peripheral blood of normal people by negative selection, passing Anti-IgE To stimulate activation, use serum-free culture at 37°C with 5% CO 2 Cultivate in an incubator for 12 to 48 hours, then collect the supernatant of the cell culture solution, centrifuge at room temperature at 300g, collect the supernatant of the culture solution after centrifugation, and obtain it by ultracentrifugation; the invention also discloses the full transcription of the above-mentioned exosomes Group expression profiles and methods for their construction. The present invention successfully isolated and identified the exosomes released by human basophils for the first time, and established the whole transcriptome expression profile of exosomes released by human basophils for the first time, and identified multiple post-activation differences The expressed RNA provides a new target for further research on the molecular mechanism of the interaction between activated basophils and target cells in the occurrence of diseases.
Owner:AFFILIATED HOSPITAL OF GUANGDONG MEDICAL UNIV
A method for identification of basophil activation and degranulation
ActiveCN104777303BNo need for separation and purificationGood repeatabilityDisease diagnosisIndividual particle analysisCD63Basophilic Granulocyte
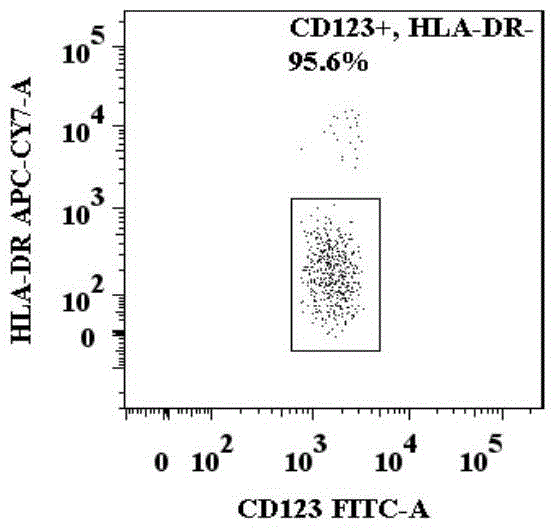
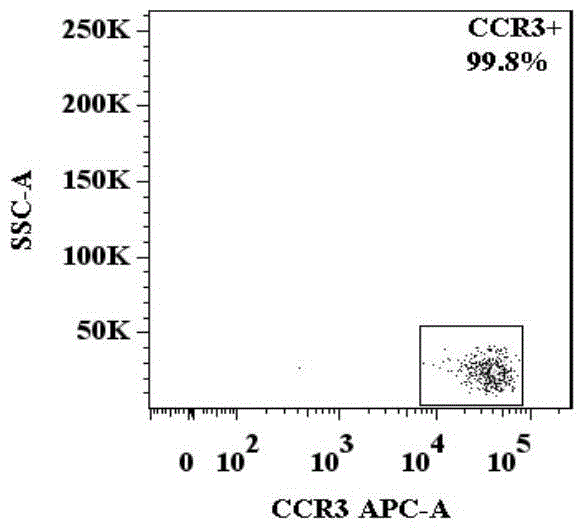
The invention provides a method for identifying basophil activation and degranulation, which includes the following steps: number the test samples in order, and mark the flow sample loading tubes required for the test; Add the required combination of anti-human CD123, anti-human CCR3, anti-human HLA‑DR, anti-human CD203c and / or anti-human CD63 fluorescently labeled flow cytometry antibodies; add the mixed whole blood to the labeled flow cytometry tube and keep at room temperature Incubate in the dark; add red blood cell lysis solution to each sample tube and incubate at room temperature in the dark; after the incubation, centrifuge to remove the supernatant; use a flow cytometer to detect and analyze the basophil count and average fluorescence intensity. The method of the present invention can distinguish 98-100% of basophils in peripheral blood from other types of cells without the need to separate and purify basophils, and can identify basophil activation and / or In the degranulated state, each sample to be tested requires no more than 100 μL of whole blood and has good repeatability.
Owner:HPY BIOTECH CO LTD
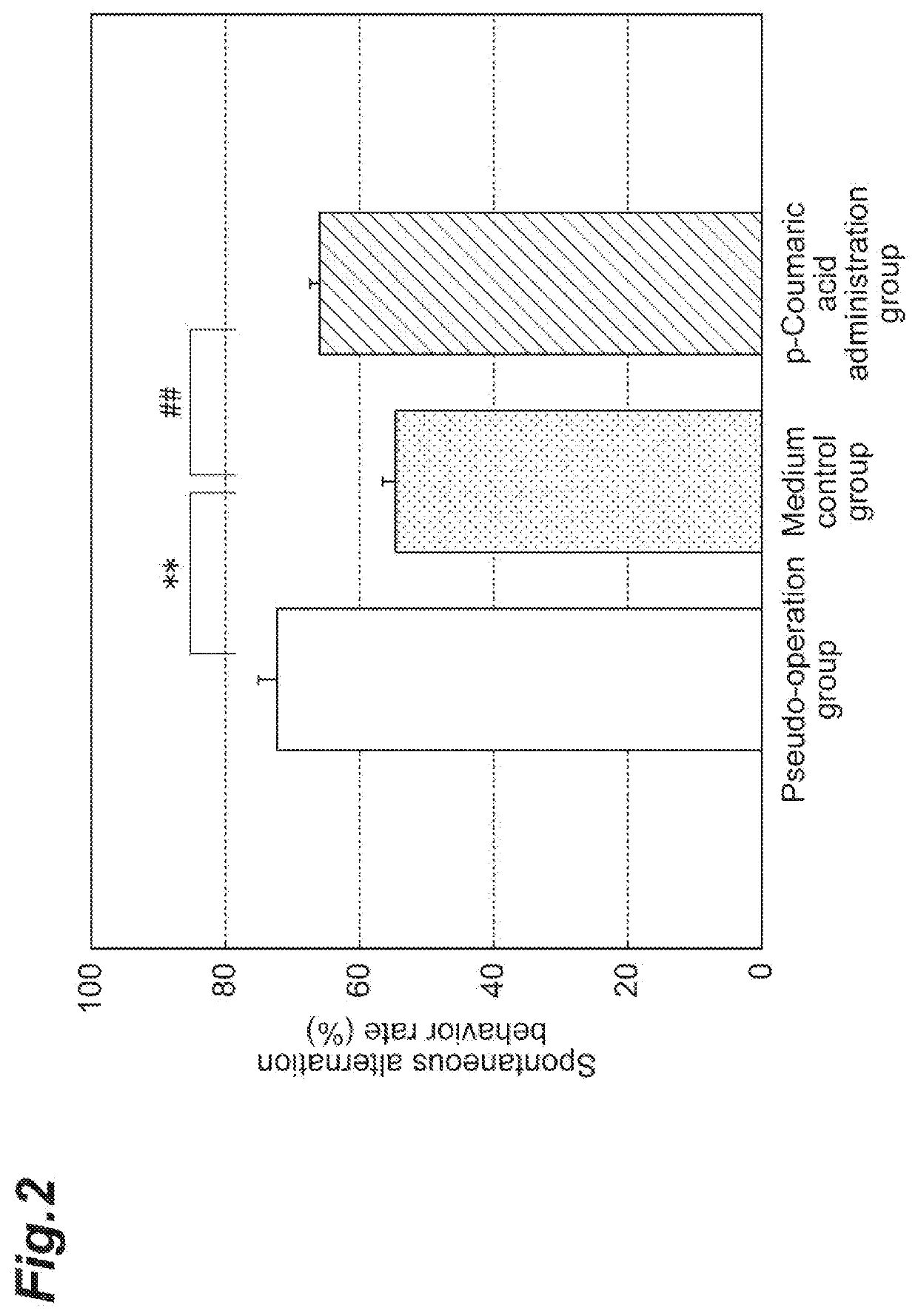
Anti-I-Type Allergy Agent, Degranulation Inhibitor for Basophils and Mast Cells, Anti-Dementia Agent, Agent for Improving/Inhibiting Short-Term Memory Impairment
PendingUS20210059964A1Improving/inhibiting short-term memory impairmentOrganic active ingredientsNervous disorderMast cellCoumaric acid
According to an aspect of the present invention, there is provided an anti-I-type allergy agent including: p-coumaric acid as an active ingredient. In addition, according to another aspect of the present invention, there is provided an anti-dementia agent including: p-coumaric acid as an active ingredient.
Owner:MITSUI SUGAR CO LTD
Skin epidermis repairing material and preparation method thereof
The invention discloses a skin epidermis repairing material and a preparation method thereof. The skin epidermis repairing material is mainly prepared from, by mass, 12-30 parts of keratin, 13-25 parts of eleidin, 8-25 parts of basophilia keratohyalin granules, 1-8 parts of melanin grains, 12-30 parts of glycosaminoglycans, 8-25 parts of hyaluronic acid, 6-26 parts of chitosan, 1-9 parts of fibronectin and 30-55 parts of deionized water. Compared with the prior art, the skin epidermis repairing material and the preparation method thereof have the advantages that by means of the prepared skin epidermis repairing material, the clinical requirements can be met, high fusion performance with human epidermis is achieved, and regeneration of the skin epidermis can be rapidly facilitated; in addition, toxic and side effects do not occur, use is safe, and healthy hidden troubles do not exist.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
Pectobacterium carotovorum and applications thereof in bio-pulping
InactiveCN101899403BPromote rapid proliferationDiffusion fastBacteriaMicroorganism based processesFiberAlkalinity
Owner:NANJING AGRICULTURAL UNIVERSITY +1
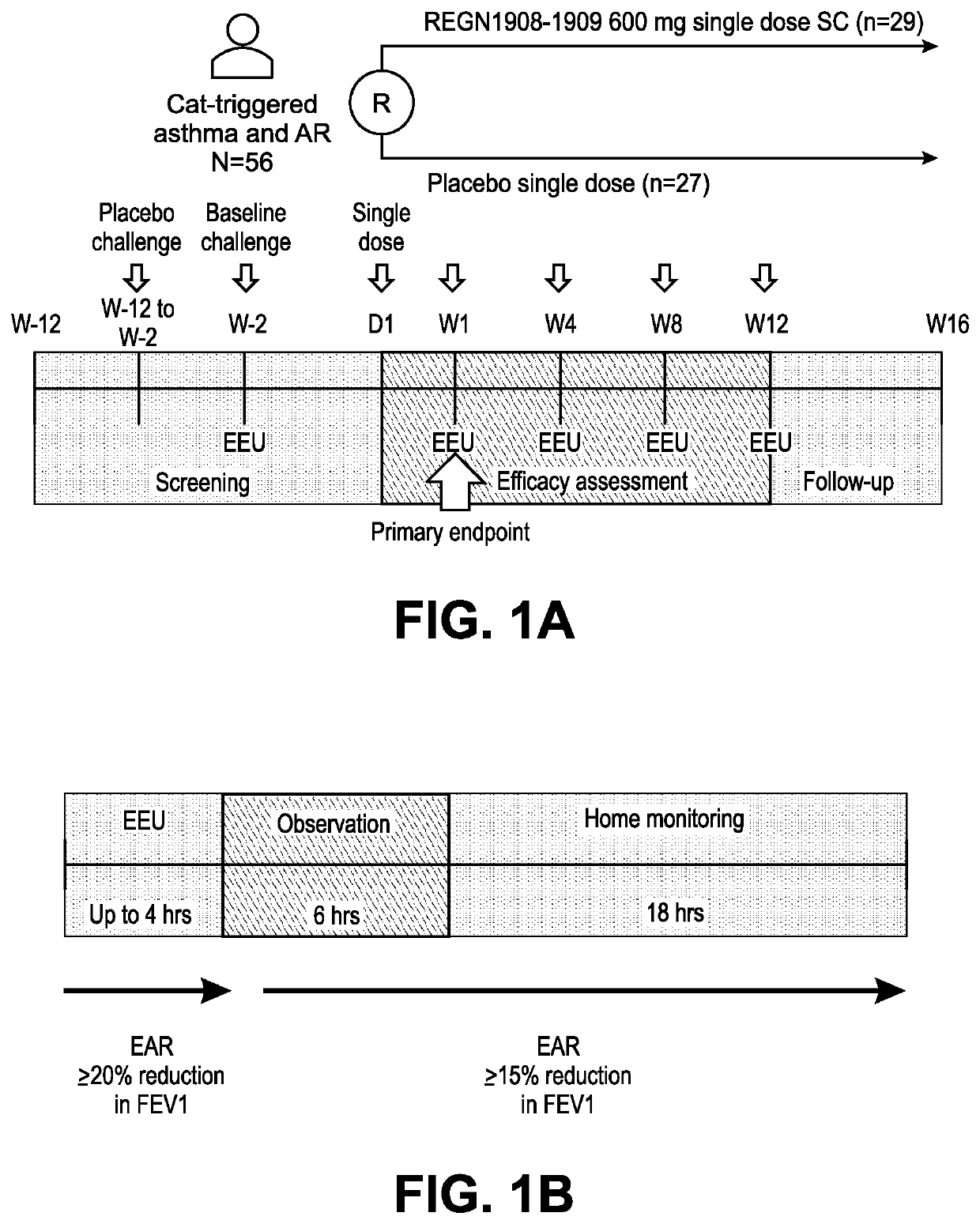
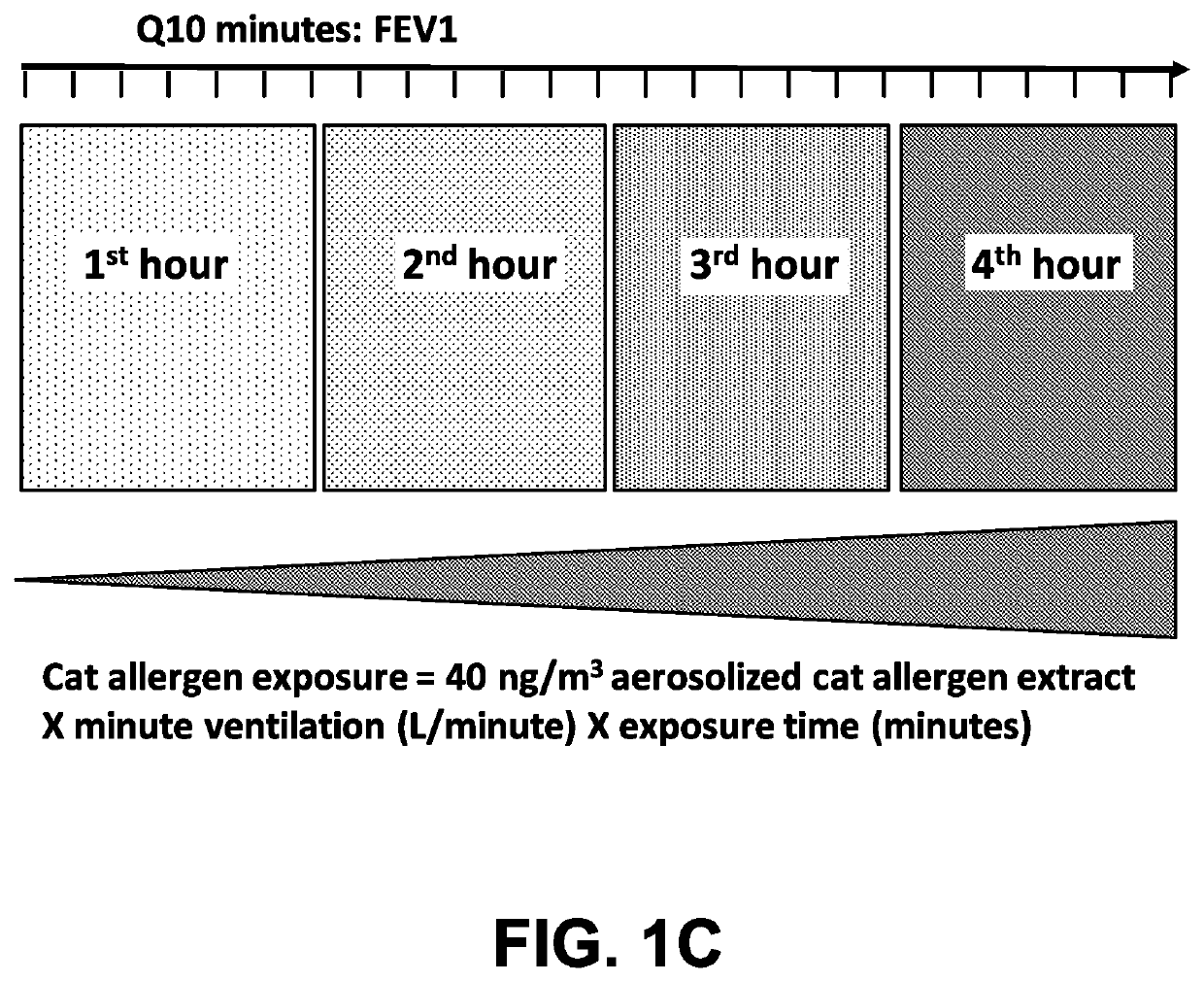
Method of treating an allergy with allergen-specific monoclonal antibodies
PendingUS20220064270A1Prevent allergen-inducedReduce severityImmunoglobulins against animals/humansAntibody ingredientsMast cellAntiendomysial antibodies
The present invention provides methods for treating an allergy by administering one or more antibodies that bind specifically to the allergen. In certain embodiments, the antibodies useful for treating an allergen, bind specifically to the cat allergen, Fel d1. According to certain embodiments of the invention, the antibodies are fully human antibodies that bind to Fel d1. The antibodies of the invention are useful for binding to the Fel d1 allergen in vivo, thus preventing binding of the Fel d1 allergen to pre-formed IgE on the surface of mast cells or basophils. In doing so, the antibodies act to prevent the release of histamine and other inflammatory mediators from mast cells and / or basophils, thus ameliorating the untoward response to the cat allergen in sensitized individuals.
Owner:REGENERON PHARM INC
Composition containing extract or fraction of genus Justicia plant
ActiveUS10688143B2Prevent, treat or improve allergic diseaseCosmetic preparationsSenses disorderBiotechnologyAntibody secretion
The present invention relates to a pharmaceutical composition, food composition and cosmetic composition for preventing, treating, or improving allergic diseases comprising extract or fraction of a plant of the Justicia genus as an active ingredient. The extract or a fraction of a plant of the Justicia genus according to the present invention can inhibit IgE antibody secretion and the degranulation of mast cells and basophils, and exhibits an excellent anti-allergic effect, and thus can effectively prevent, treat, or improve allergic diseases.
Owner:DONG WHA PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com