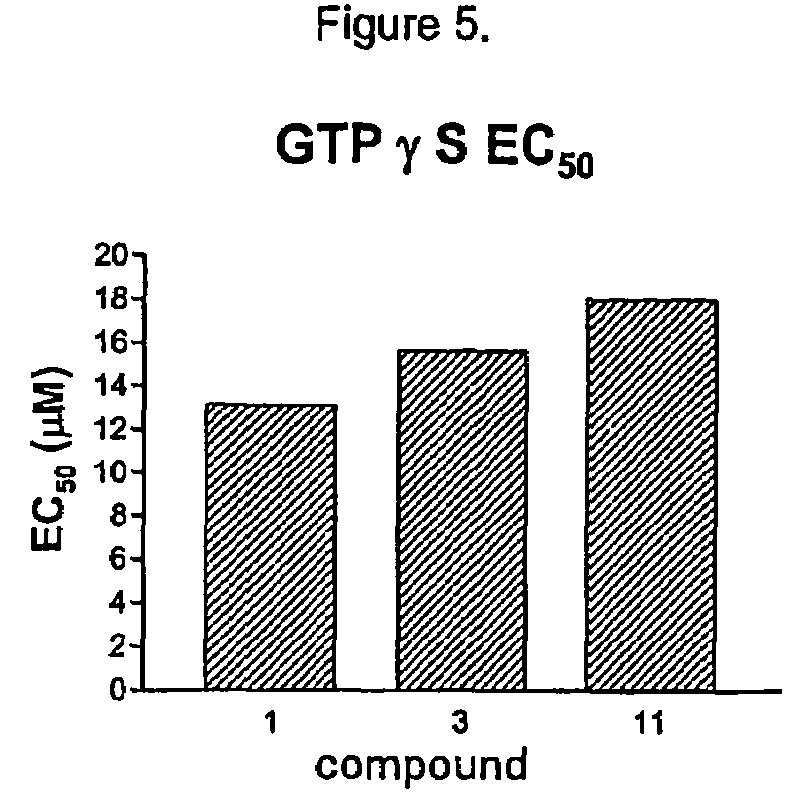
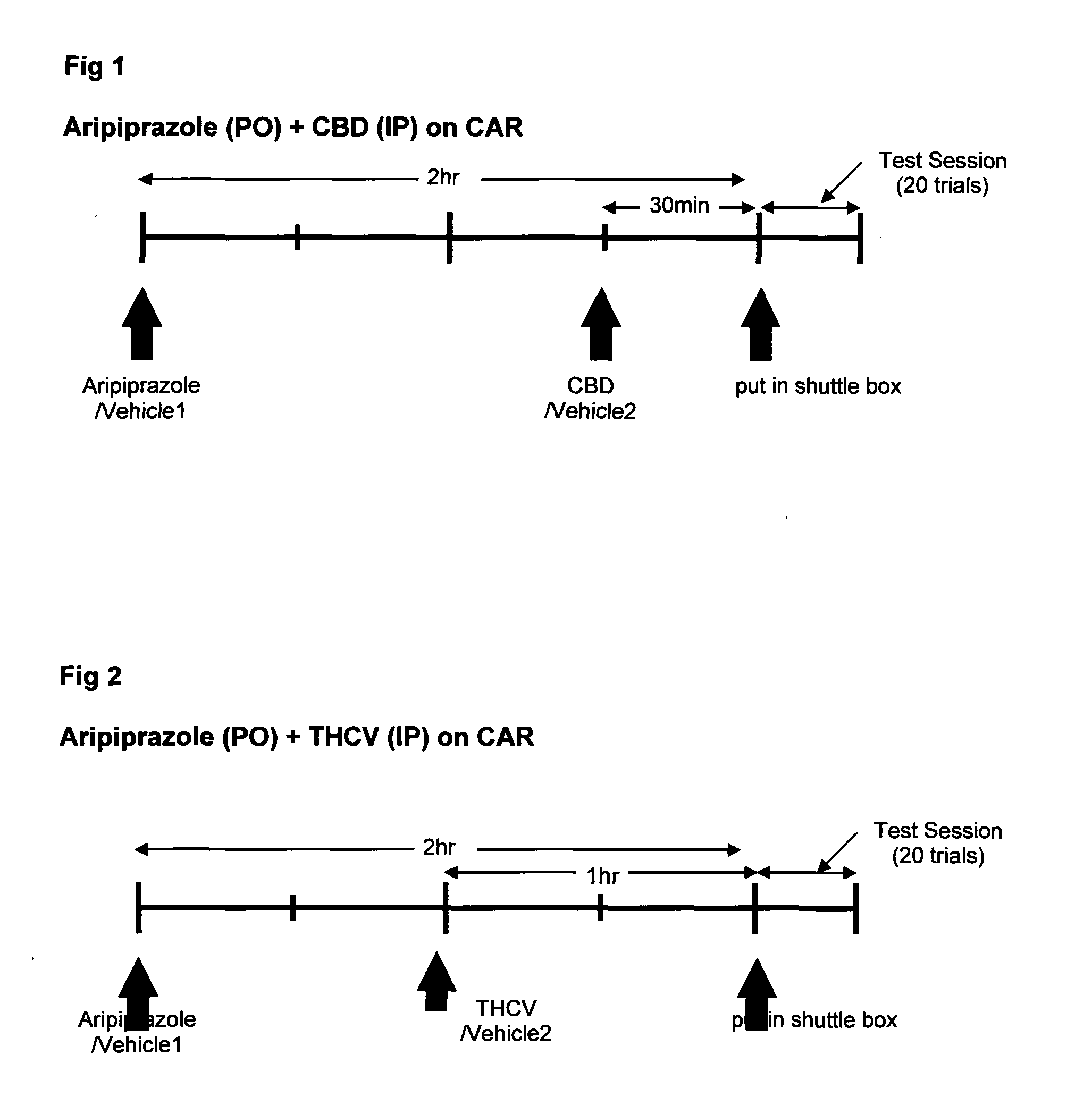
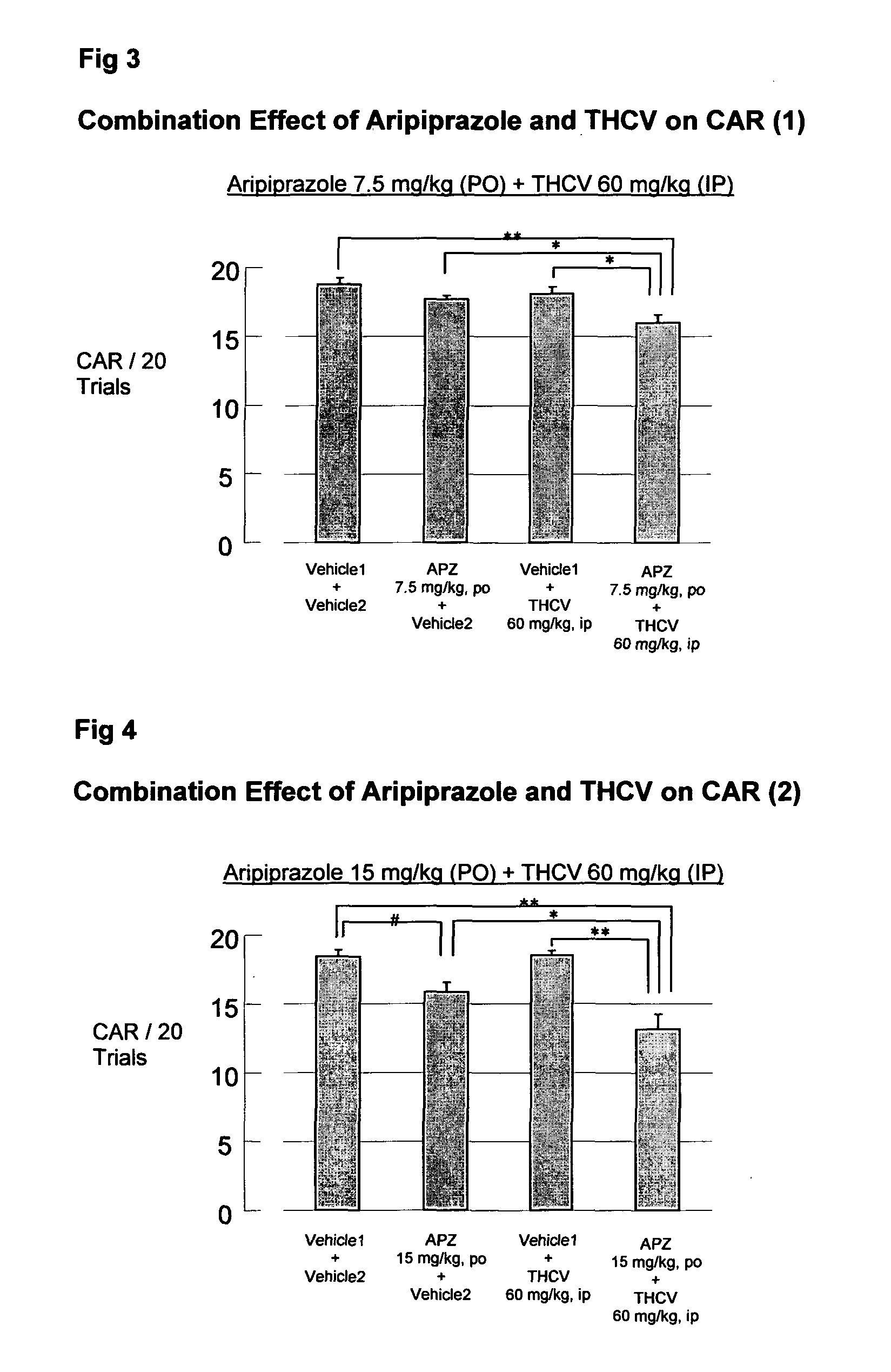
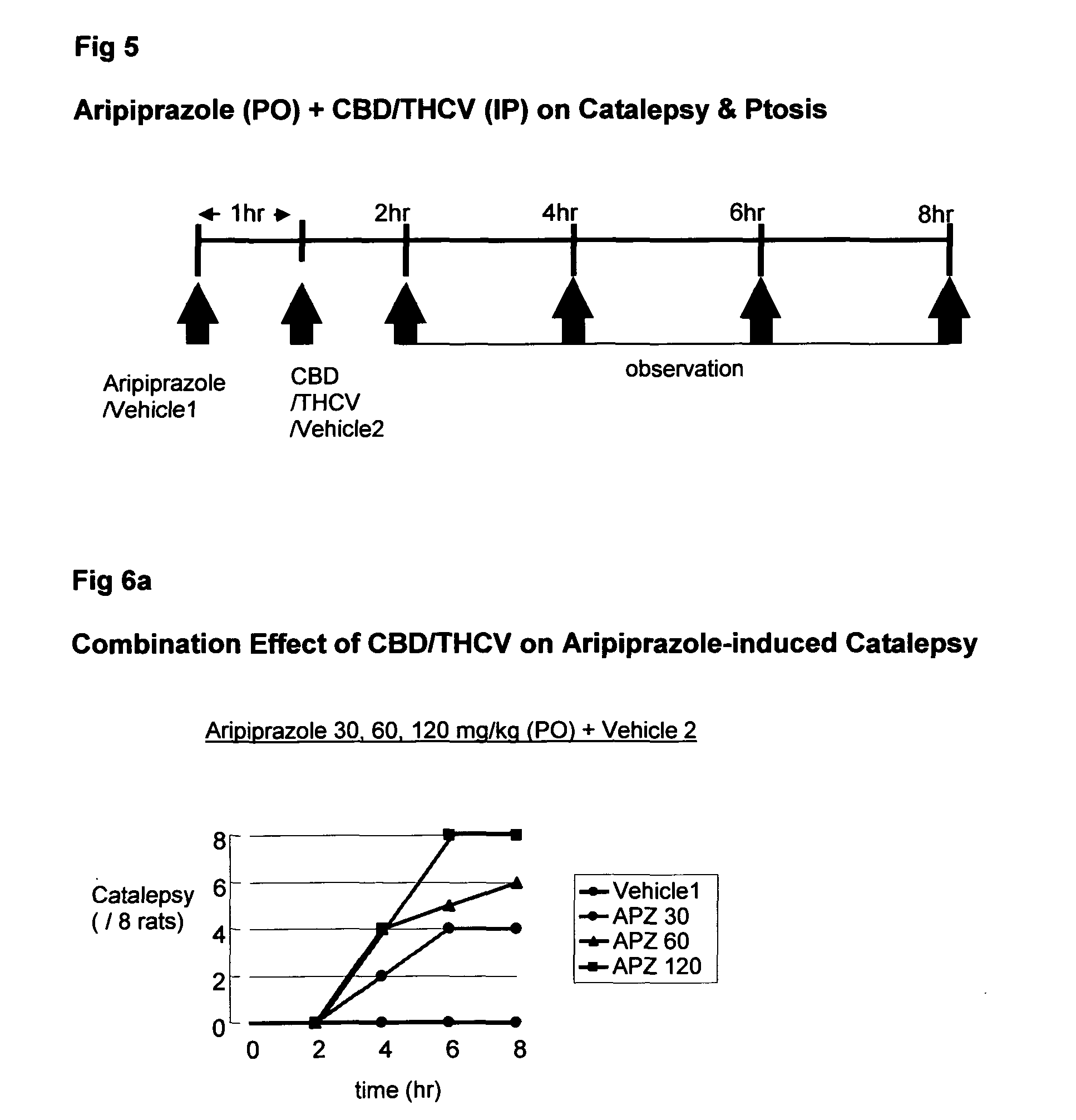
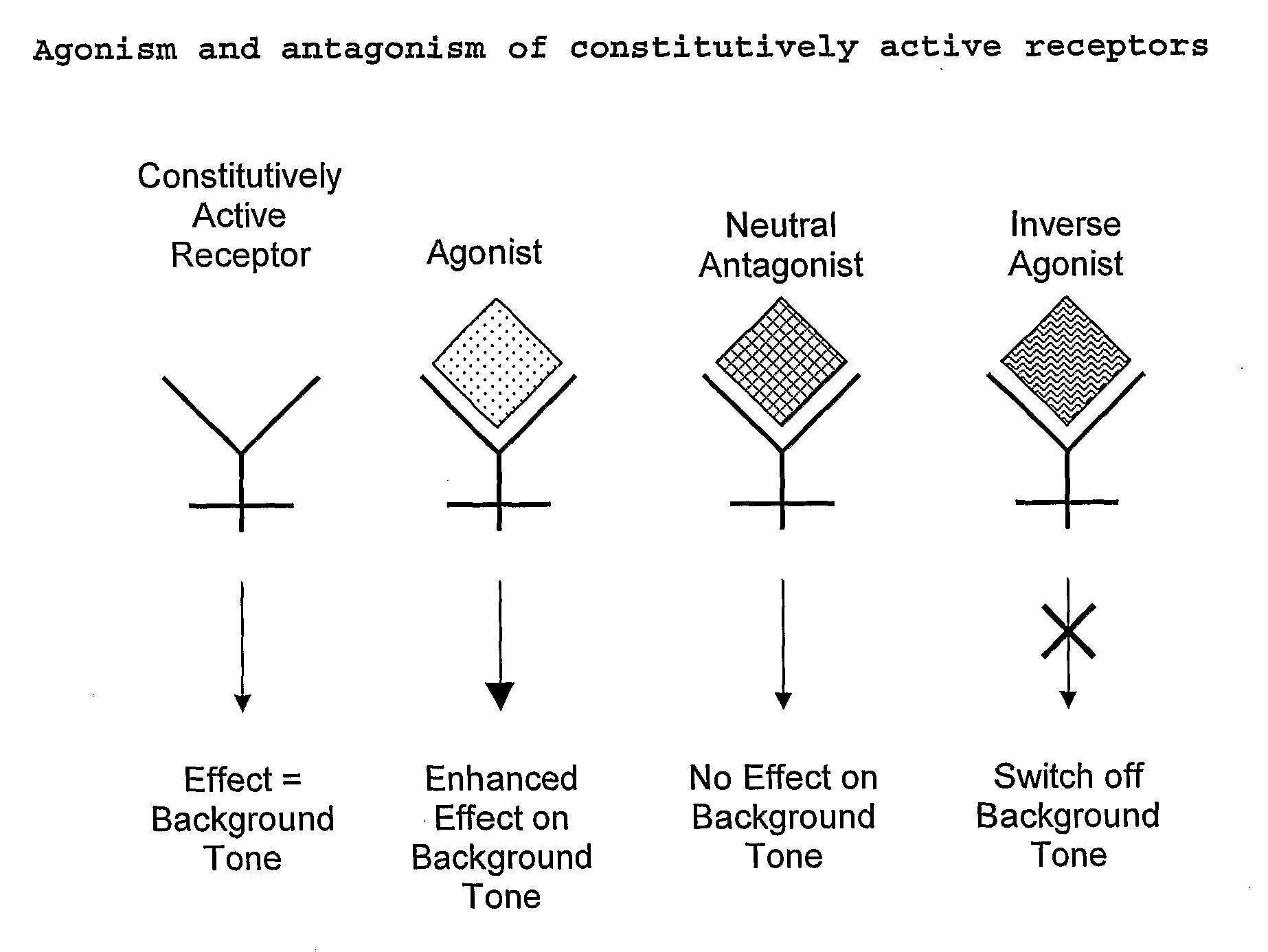
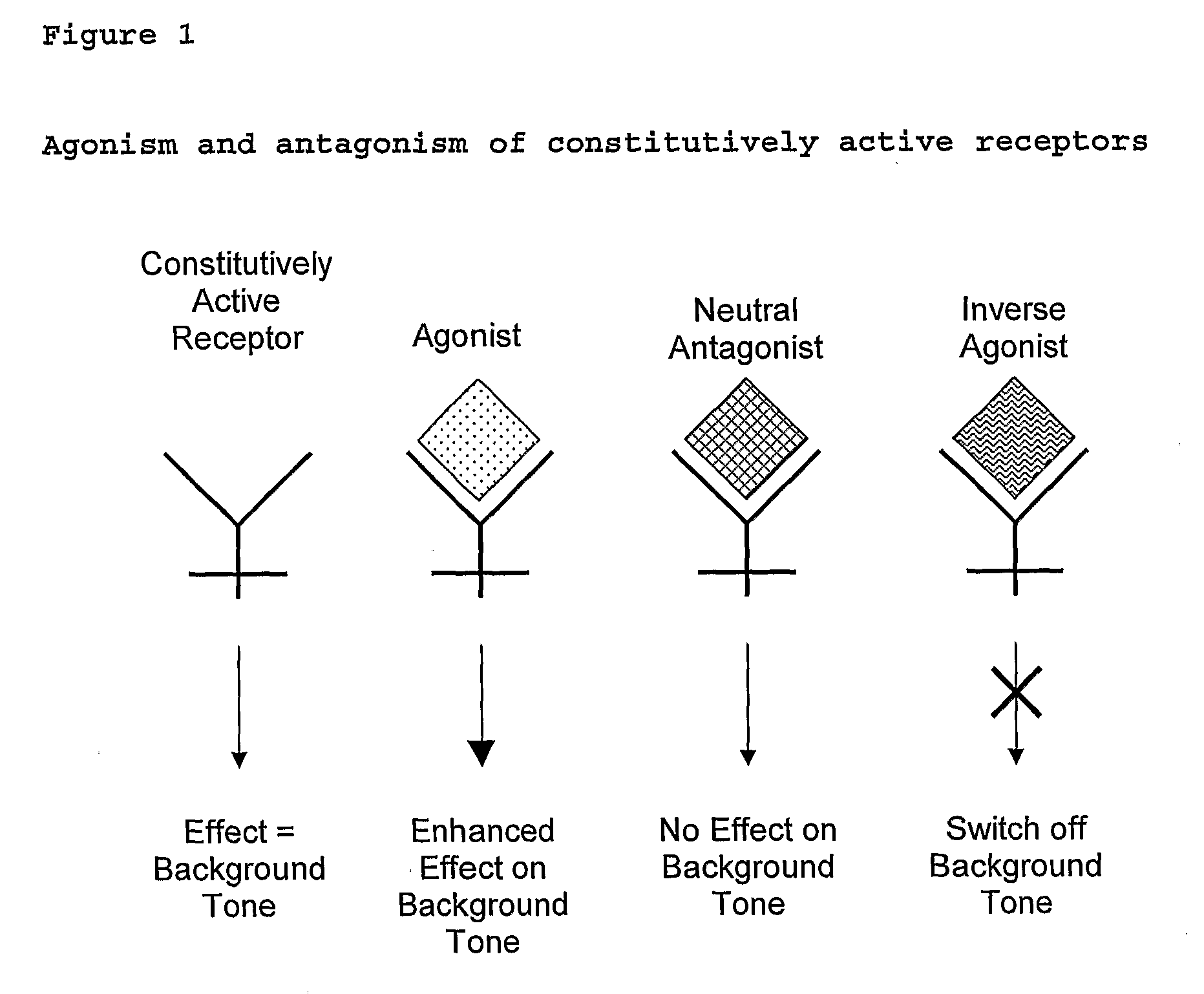
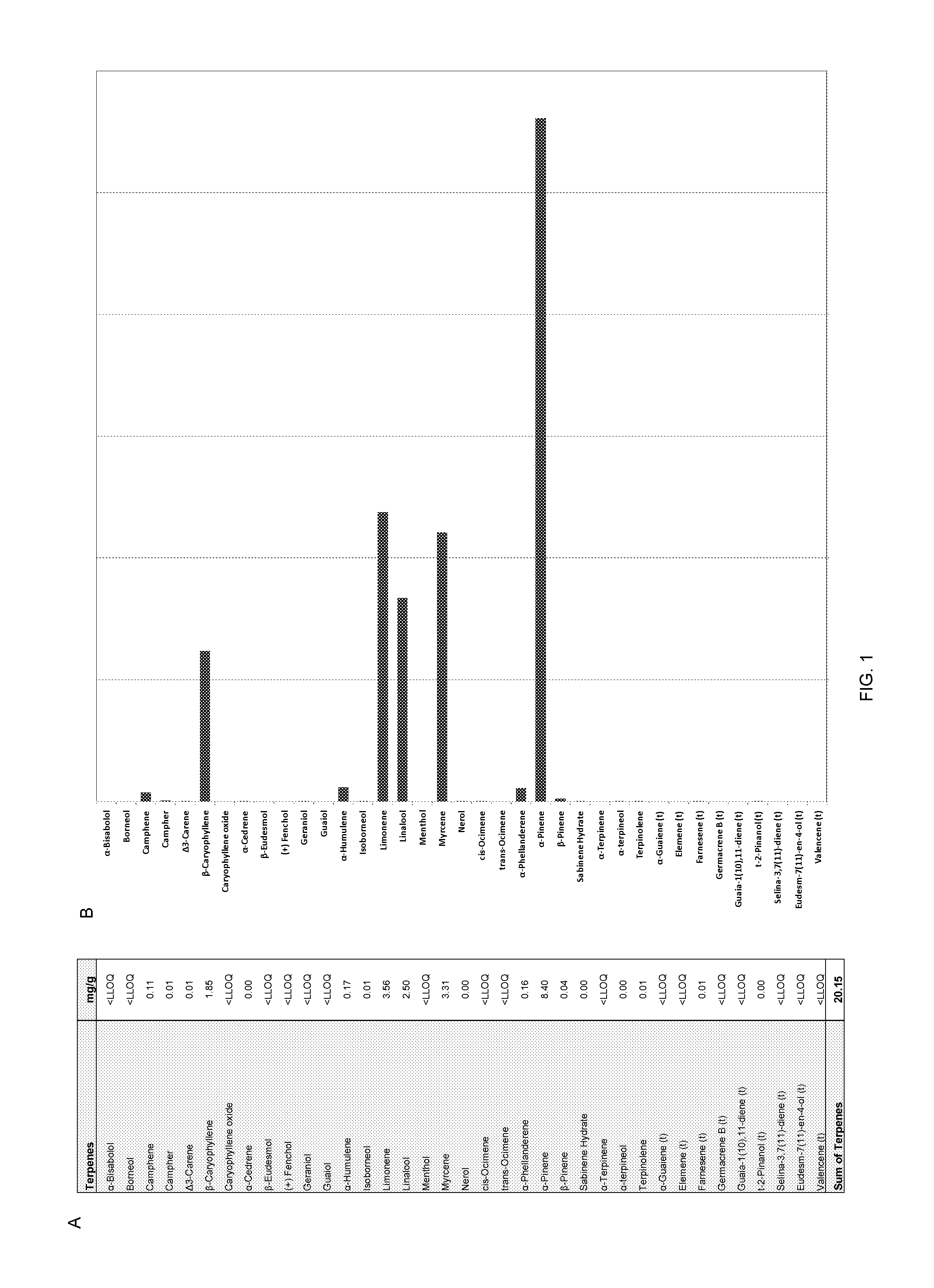
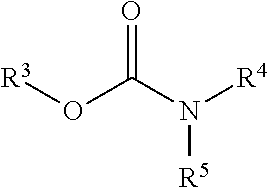
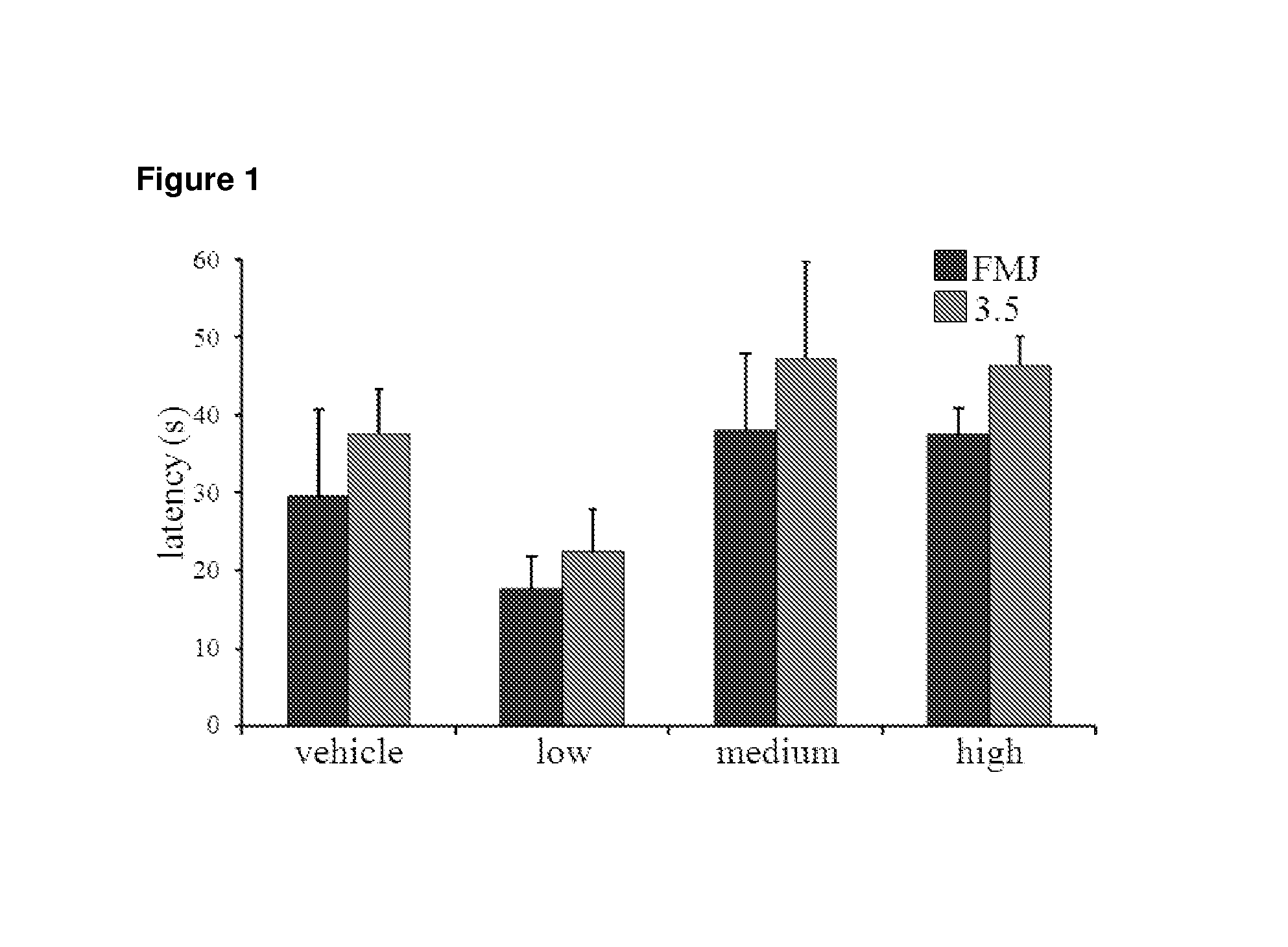
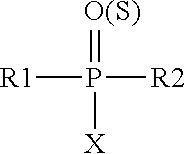Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
708 results about "Cannabinoid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
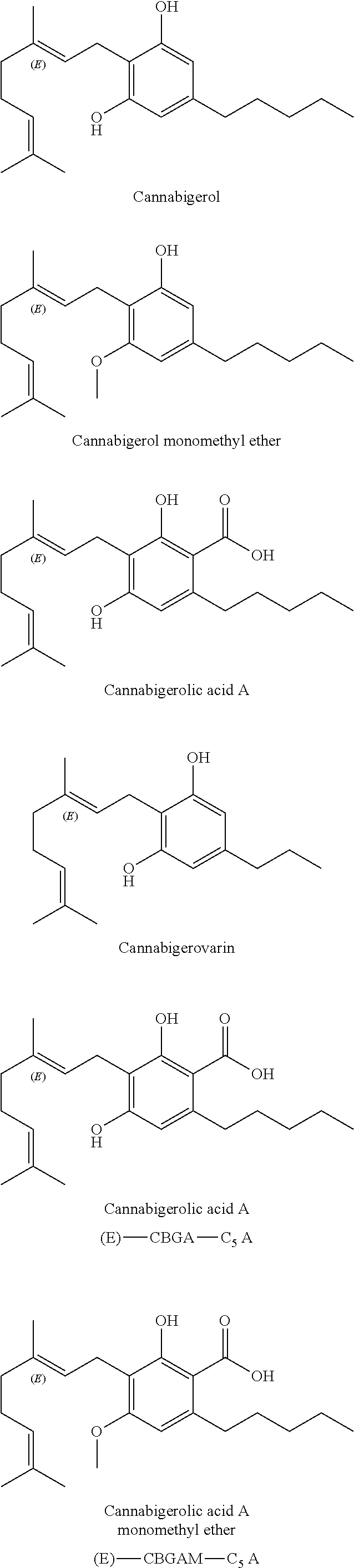
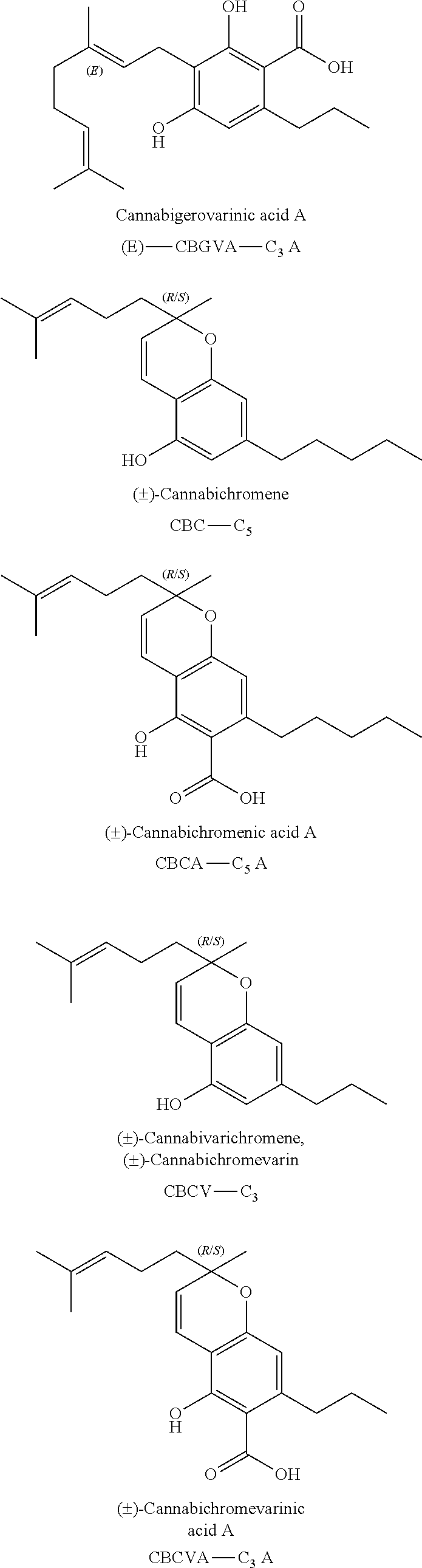
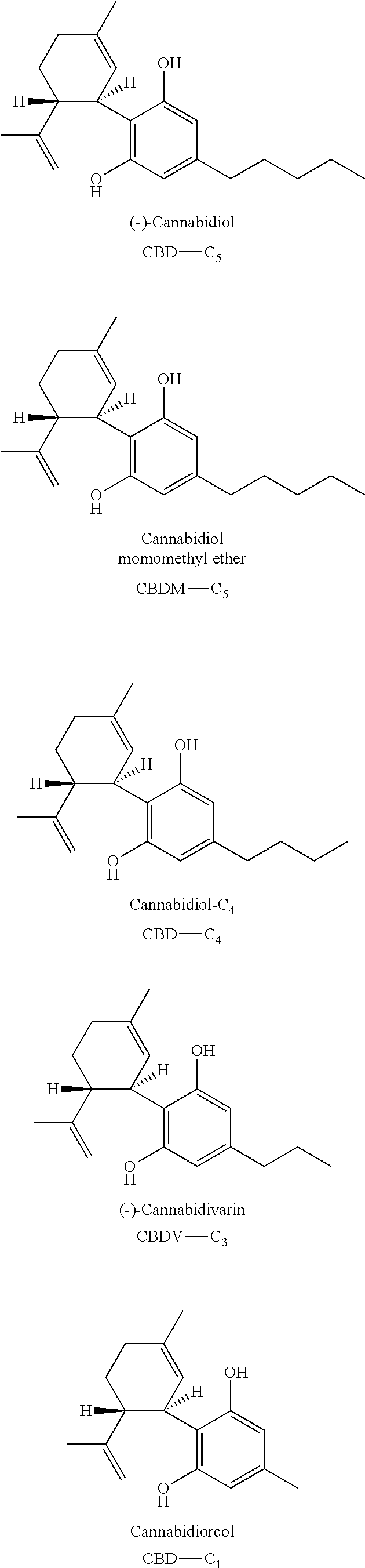
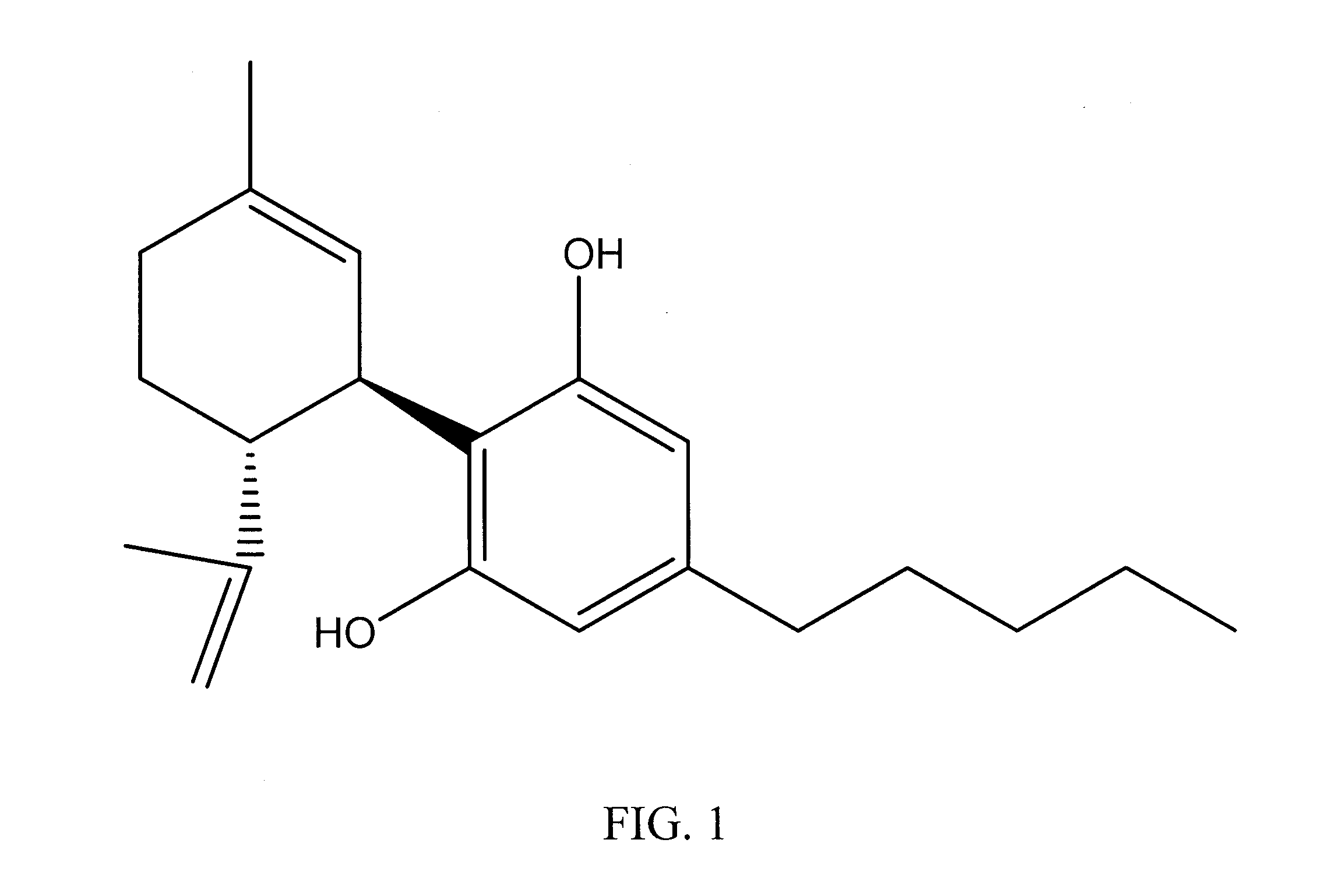
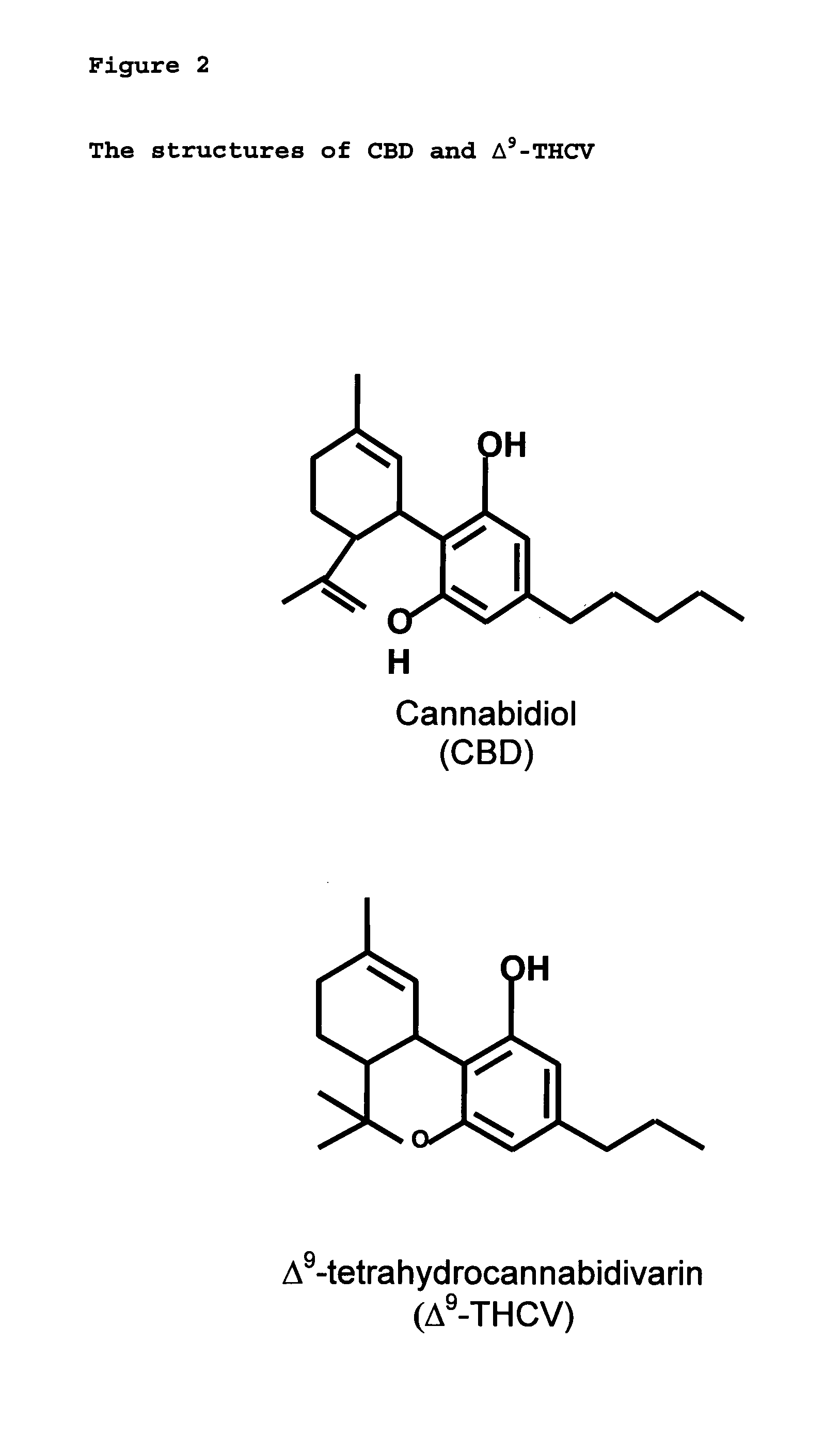
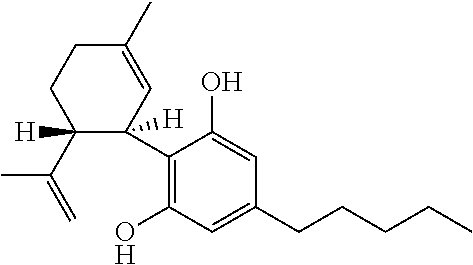
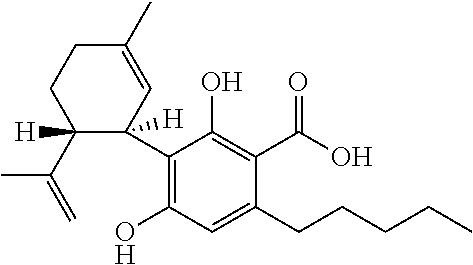
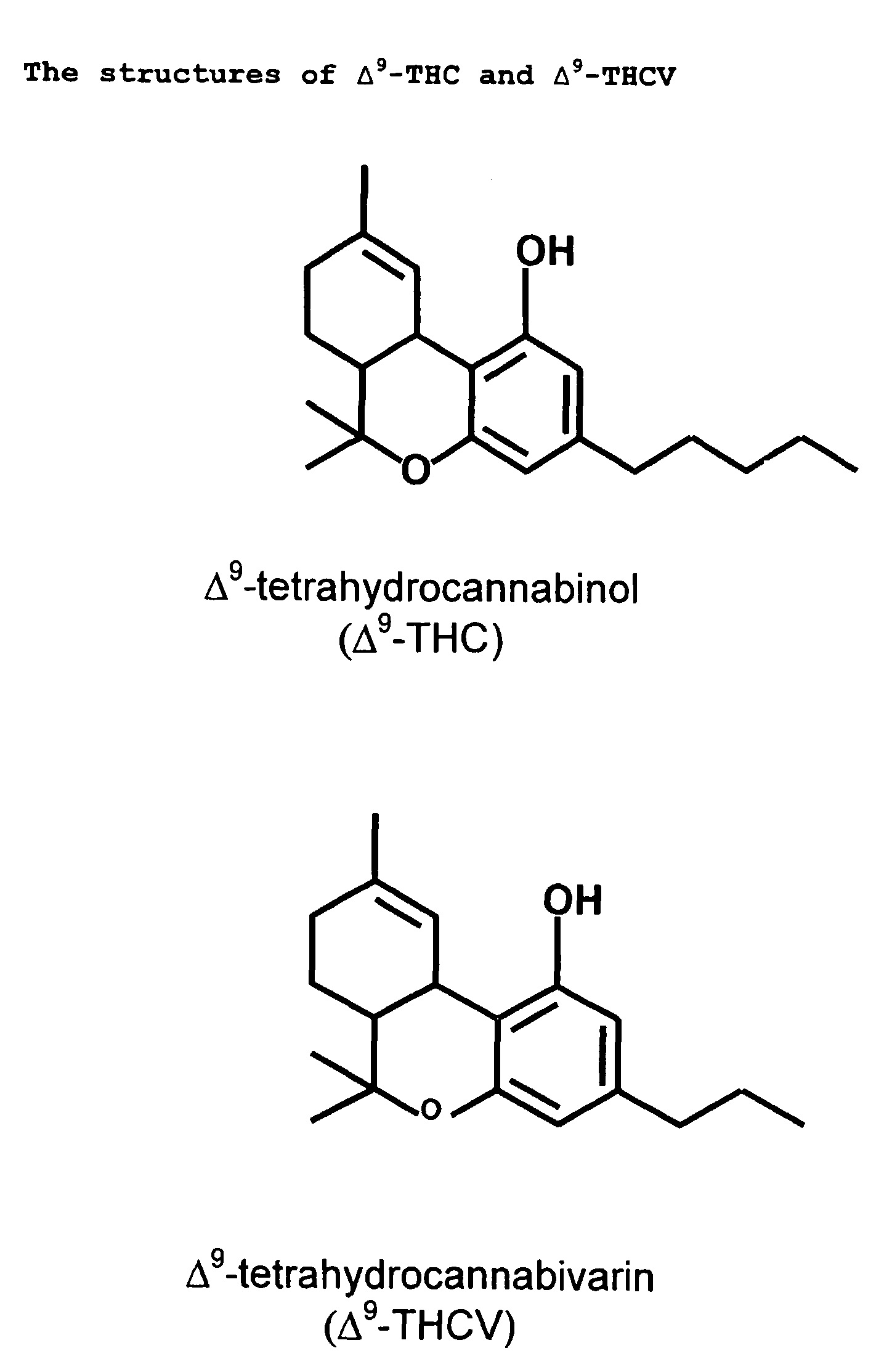
A cannabinoid is one of a class of diverse chemical compounds that acts on cannabinoid receptors, also known as the endocannabinoid system in cells that alter neurotransmitter release in the brain. Ligands for these receptor proteins include the endocannabinoids produced naturally in the body by animals; phytocannabinoids, found in cannabis; and synthetic cannabinoids, manufactured artificially. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is another major constituent of the plant. There are at least 113 different cannabinoids isolated from cannabis, exhibiting varied effects.
Bioactive concentrates and uses thereof
The present invention relates to concentrates obtained from extraction from Cannabis, preferably cannabinoid and / or terpene concentrates, and formulation of the concentrates, particularly for use for direct vaporization, infusion into edible matrices, in electronic inhalation devices, and as nutraceuticals.
Owner:PURPLE MUNDO INC
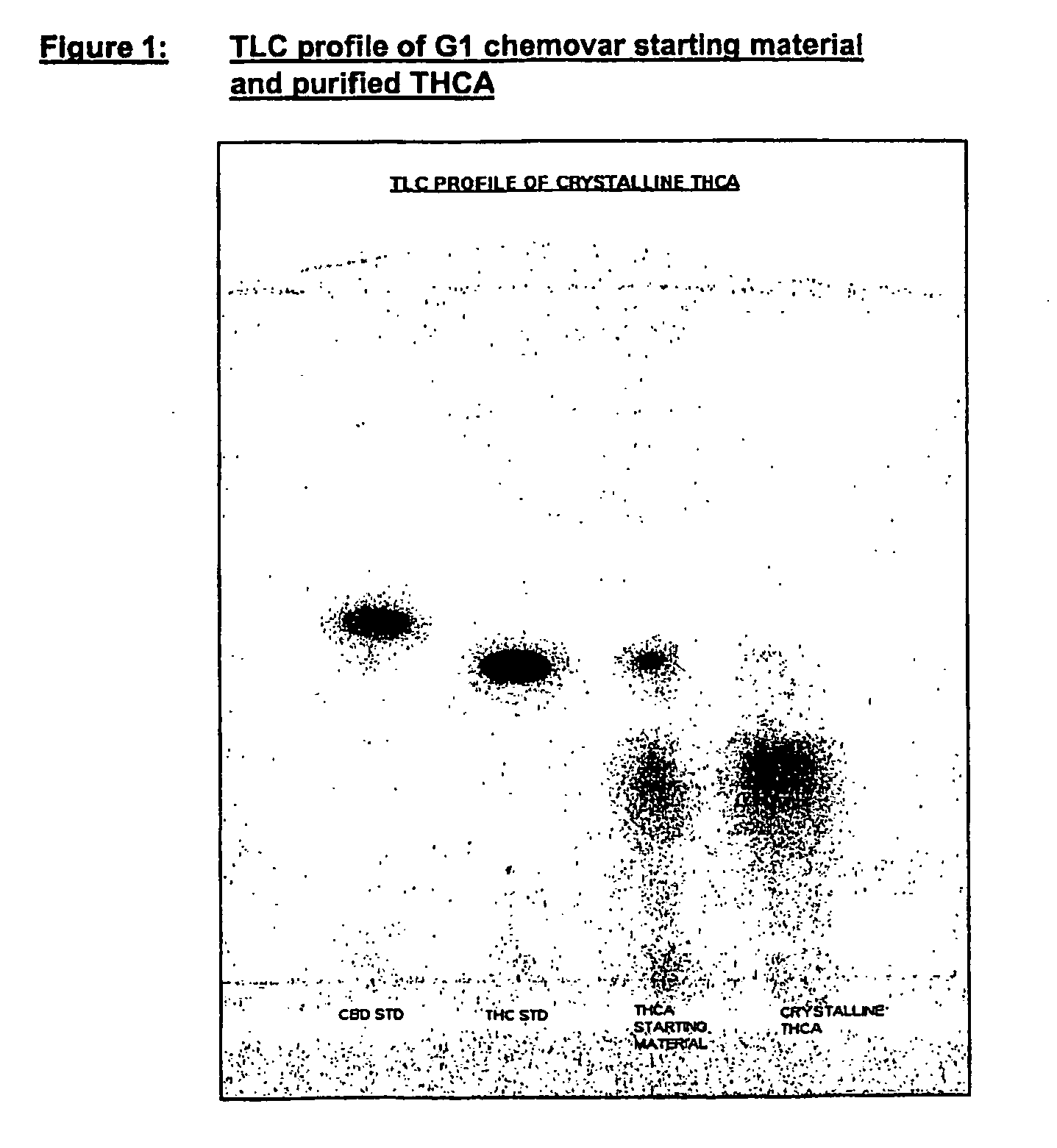
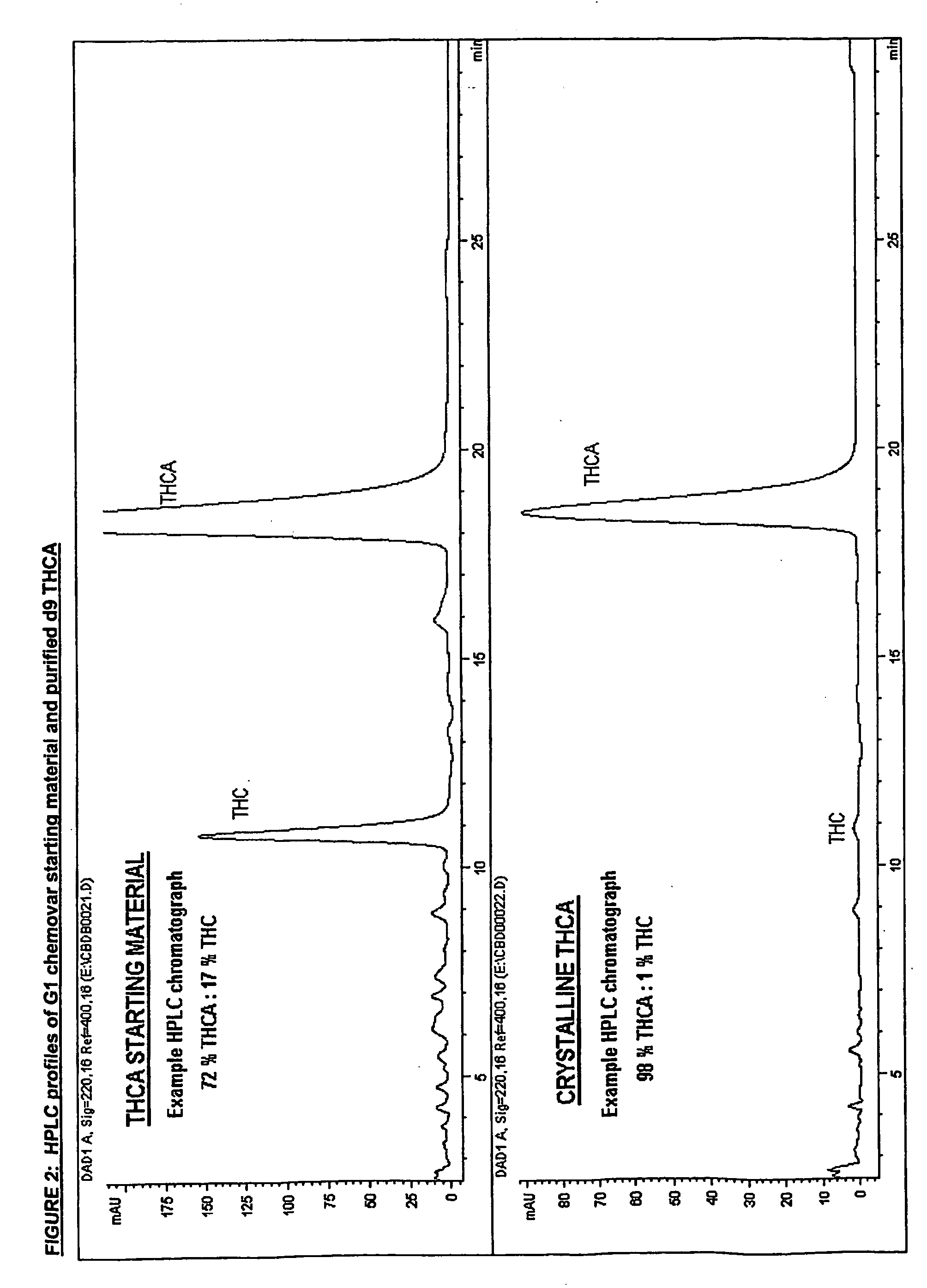
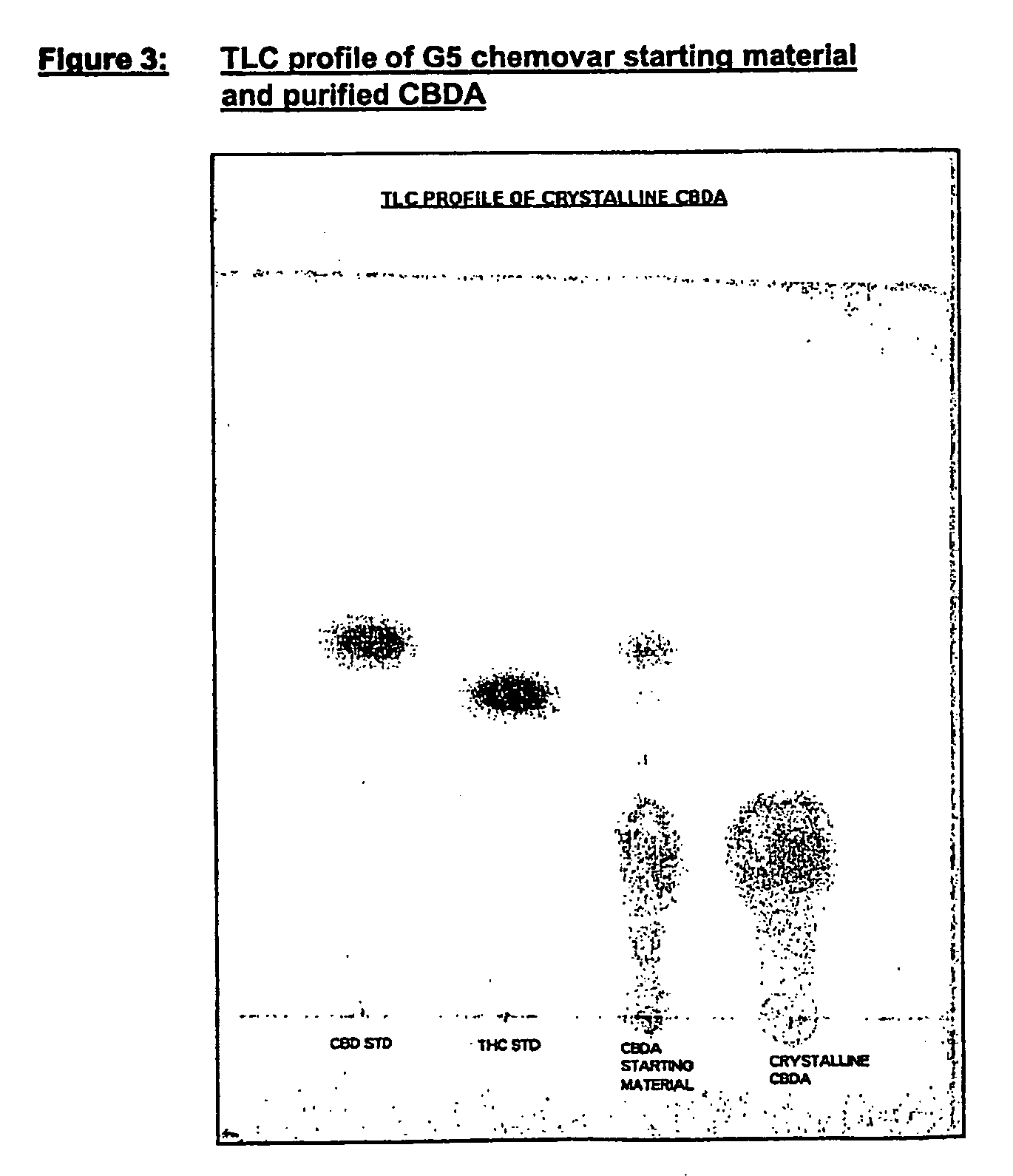
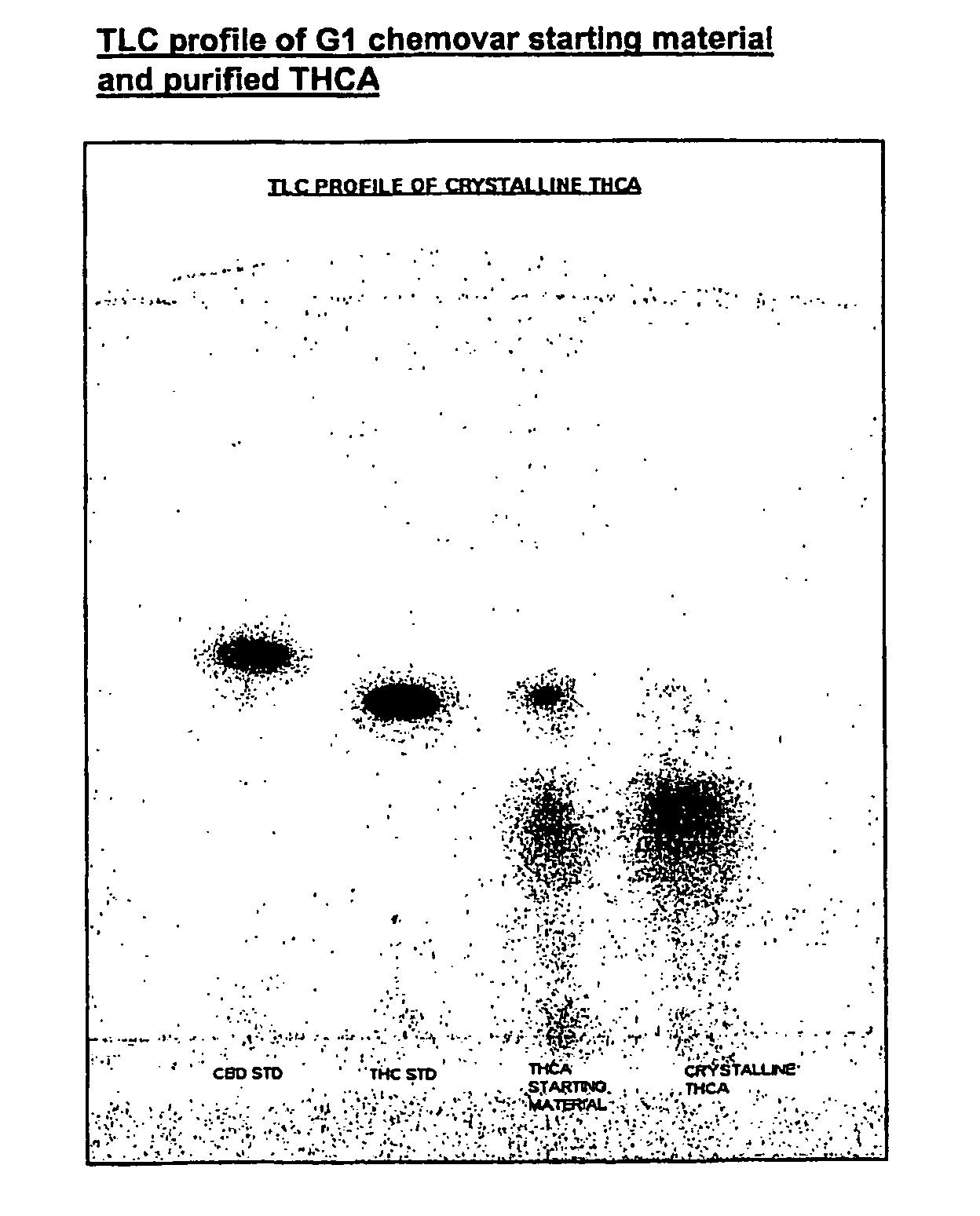
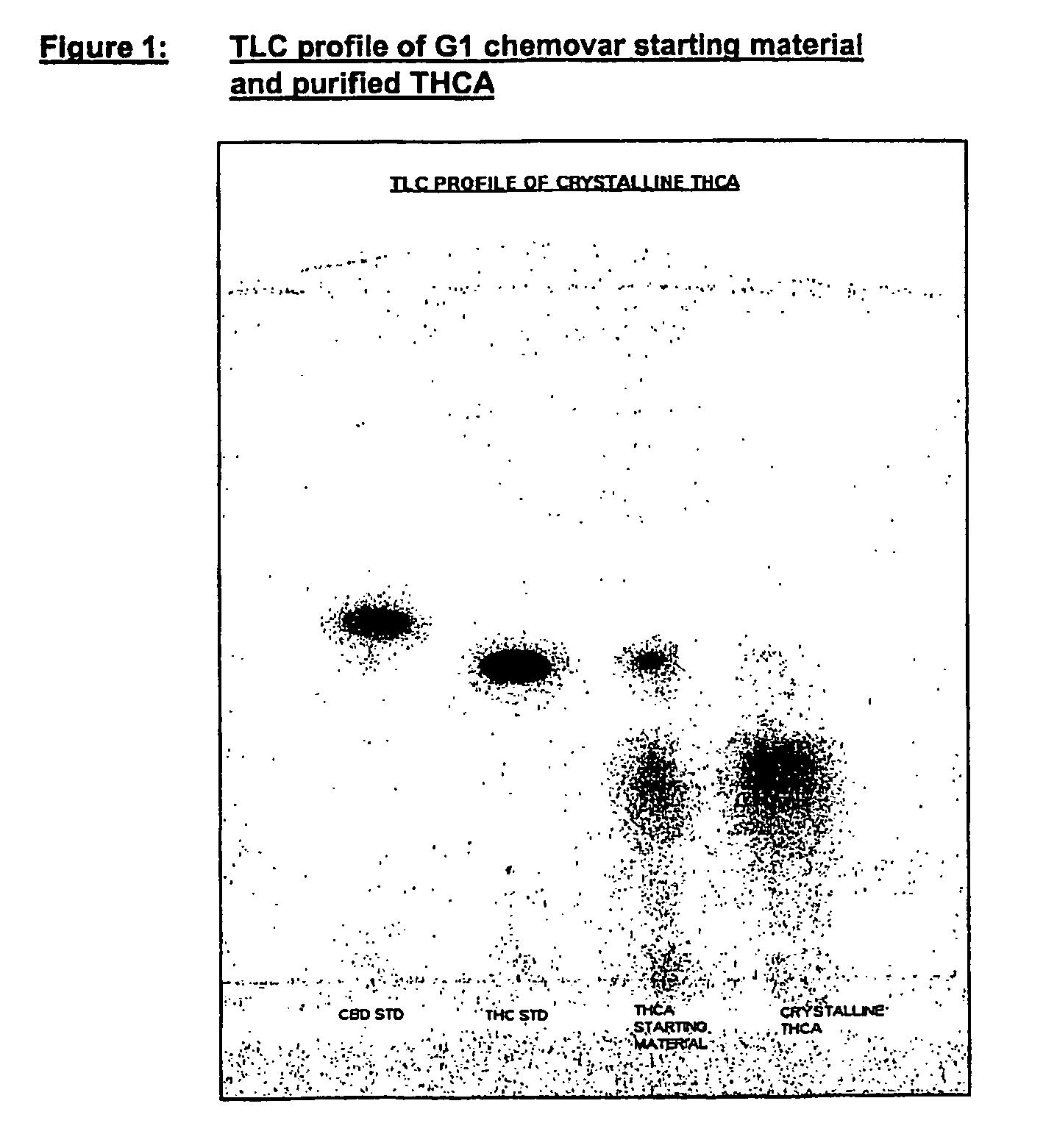
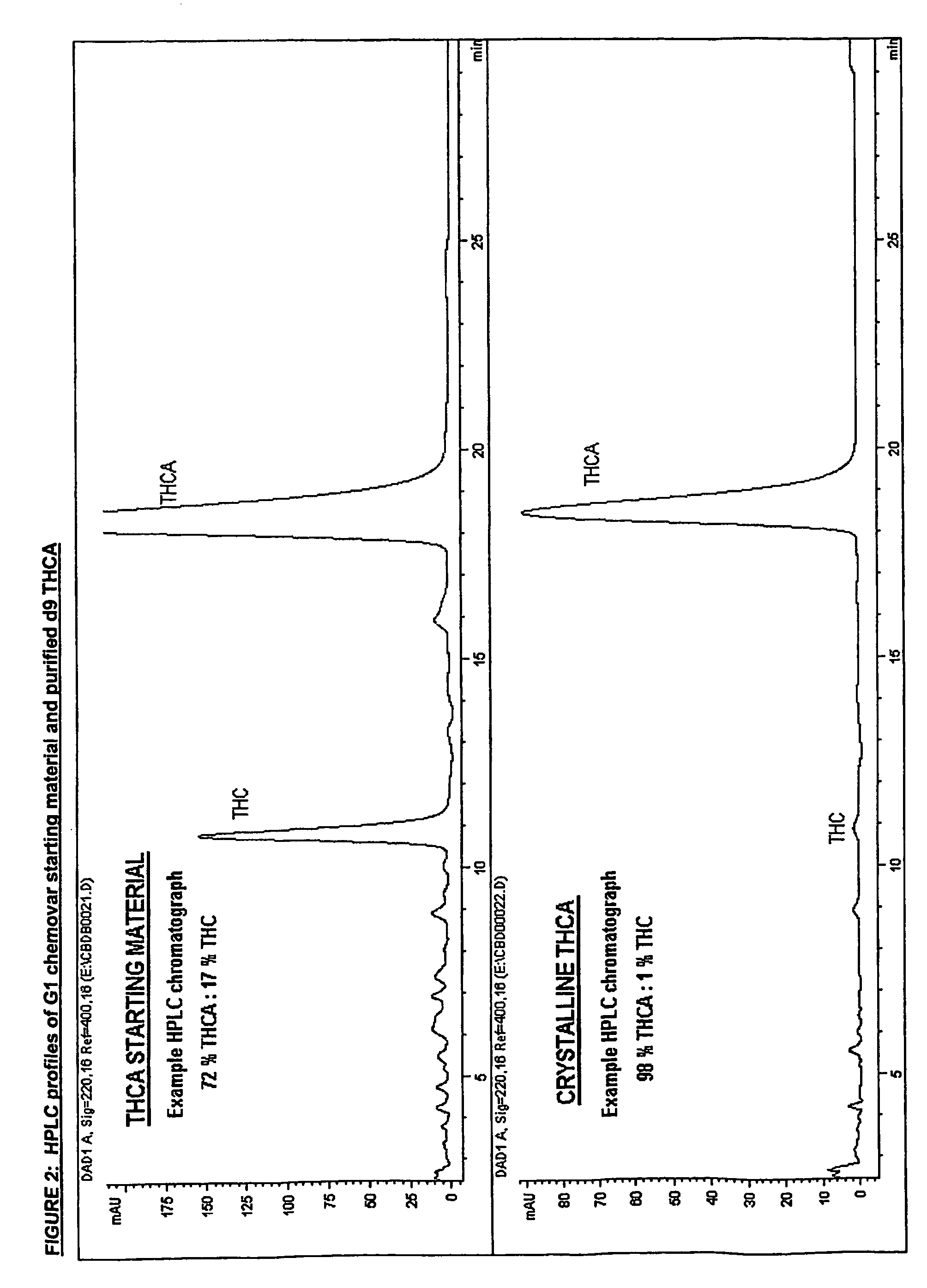
Methods of purifying cannabinoids from plant material
The invention relates to methods of preparing cannabinoids in substantially pure form starting from plant material. Also described are substantially pure preparations of various cannabinoids and cannabinoid acids, and also extracts enriched in cannabinoids and cannabinoid acids.
Owner:GW PHARMA LTD
Sustained release cannabinoid medicaments
The present invention provides a medicament which results in delivery of a therapeutic level of one or more cannabinoids during a clinically relevant therapeutic window. The therapeutic window is a longer window than provided by an immediate release medicament such as Marinol containing an equivalent amount of the cannabinoid. Oral administration of the present compositions provides therapeutic dosing while maintaining safe, side effect sparing, levels of a cannabinoid. The present invention also provides methods of treating cannabinoid-sensitive disorders.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
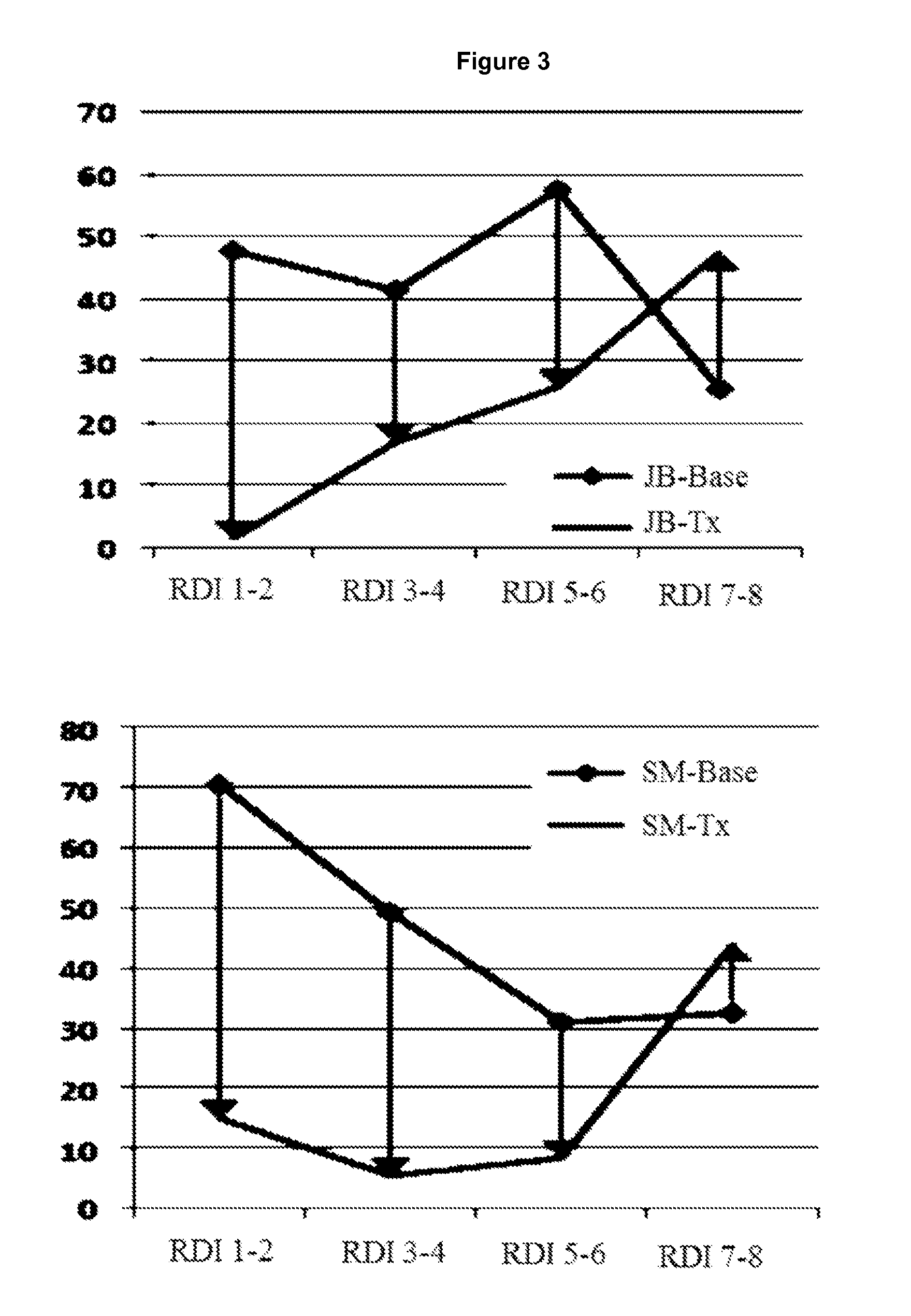
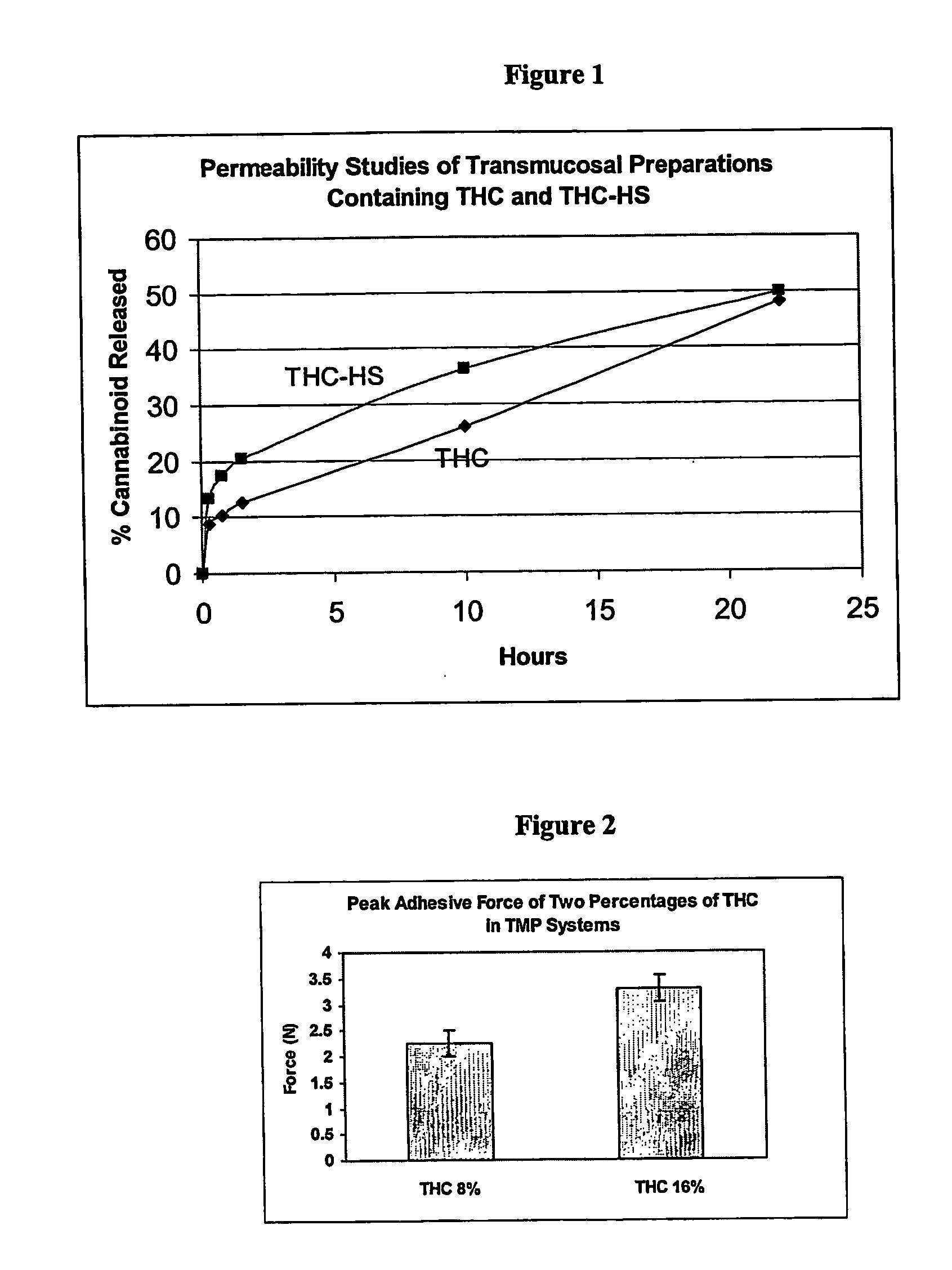
Transmucosal delivery of cannabinoids
InactiveUS20060257463A1Strong tendencyReduced bioavailabilityBiocidePharmaceutical non-active ingredientsCannabisCannabinoid
A method of transmucosally delivering a cannabinoid to a subject in need of such treatment comprising the steps of: administering to the subject a transmucosal preparation containing the cannabinoid wherein said transmucosal preparation is made by incorporating an effective amount of the cannabinoid via hot-melt extrusion technology, hot-melt molding, admixing or a solvent cast technique into a film matrix or a reservoir containing the cannabinoid, and attaching said transmucosal preparation to the mucosa of the subject.
Owner:UNIVERSITY OF MISSISSIPPI
Heteroindanes: a new class of potent cannabimimetic ligands
One aspect of the invention is concerned with cannabimimetic heteroindane analogs having affinities and / or selectivities for a cannabinoid receptor. A further aspect of the invention is concerned with pharmaceutical preparations employing the inventive analogs and methods of administering therapeutically effective amounts of the inventive analogs to provide a physiological effect.
Owner:UNIV OF CONNECTICUT
Use of cannabinoids in combination with an Anti-psychotic medicament
ActiveUS20110038958A1High activityLess degree of activityBiocideSenses disorderPsychosis drugTypical antipsychotic
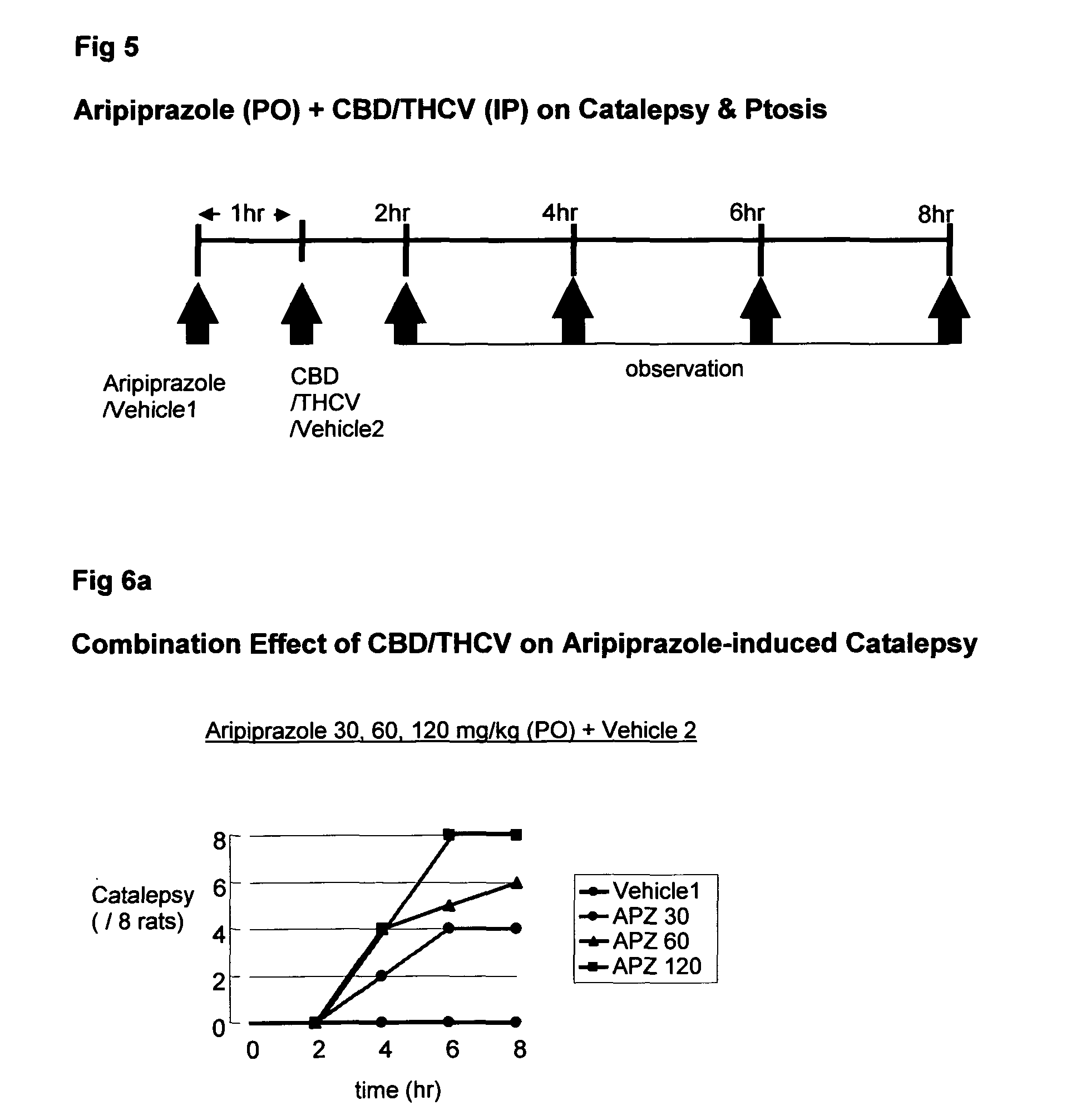
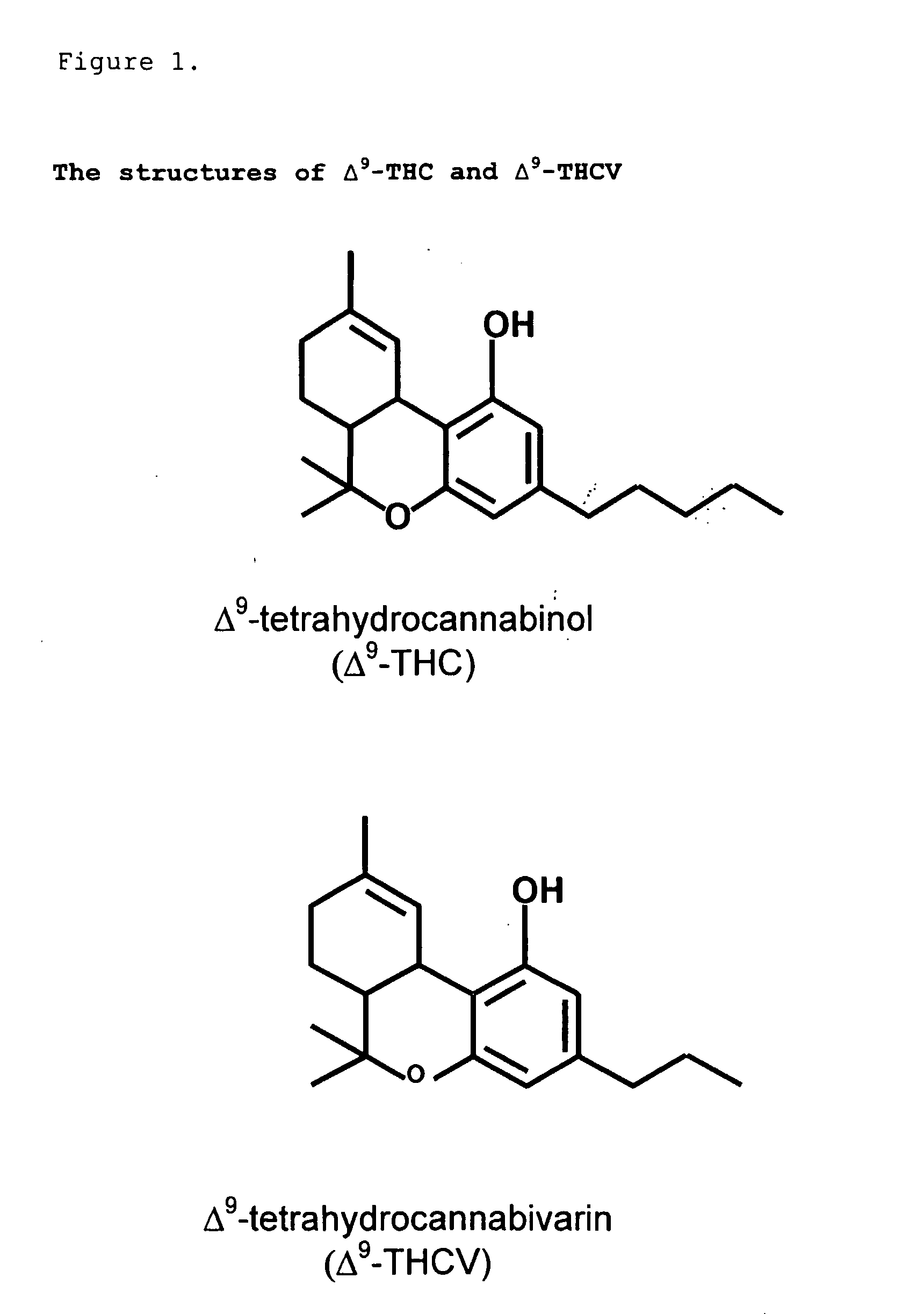
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
Cannabis extracts and methods of preparing and using same
The invention relates to the extraction of pharmaceutically active components from plant materials, and more particularly to the preparation of a botanical drug substance (BDS) for incorporation in to a medicament. It also relates to a BDS, for use in pharmaceutical formulations. In particular it relates to BDS comprising cannabinoids obtained by extraction from cannabis
Owner:BLACKMON EARNEST VINSON MR
Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances
ActiveUS20100008985A1Disperse fastEfficient packagingBiocidePowder deliveryWater insolubleProphylactic treatment
One aspect of the invention relates to a pharmaceutical dosage unit for sublingual, buccal, pulmonary or oral administration, said dosage unit having a weight of 20-500 mg and comprising 1-80 Wt. % of a microgranulate that is distributed throughout a solid hydrophilic matrix; said microgranulate being characterised in that it: has a volume weighted average diameter of 5-100 m; contains at least 0.01 wt. %, preferably at least 0.1 wt. % of one or more water-insoluble pharmaceutically active substances; contains at least 10 wt. %, preferably at least 20 wt. % of an emulsifier component; and is capable of forming a micro-emulsion upon contact with saliva or water. The dosage units of the present invention achieve the inherent benefits of oral delivery whilst at the same time realising a high transmucosal absorption rate of the cannabinoids contained therein. Other aspects of the present invention relate to the use of the aforementioned dosage units in the therapeutic or prophylactic treatment and to a process for the manufacture of said dosage units.
Owner:ECHO PHARM BV (NL)
Cannabinoid-containing plant extracts as neuroprotective agents
The invention relates to the use of cannabinoid-containing plant extracts in the prevention or treatment of neural degeneration. In particular, the invention relates to use of one or more cannabinoid-containing plant extracts in the prevention or treatment of neural degeneration, wherein the one or more cannabinoid-containing plant extracts comprise: i) a cannabinoid-containing fraction; and ii) a non-cannabinoid containing fraction.
Owner:GW RES LTD
Methods of purifying cannabinoids from plant material
ActiveUS7700368B2Less polarHigh chromatographic puritySenses disorderNervous disorderCannabinoidOrganic chemistry
The invention relates to methods of preparing cannabinoids in substantially pure form starting from plant material. Also described are substantially pure preparations of various cannabinoids and cannabinoid acids, and also extracts enriched in cannabinoids and cannabinoid acids.
Owner:GW PHARMA LTD
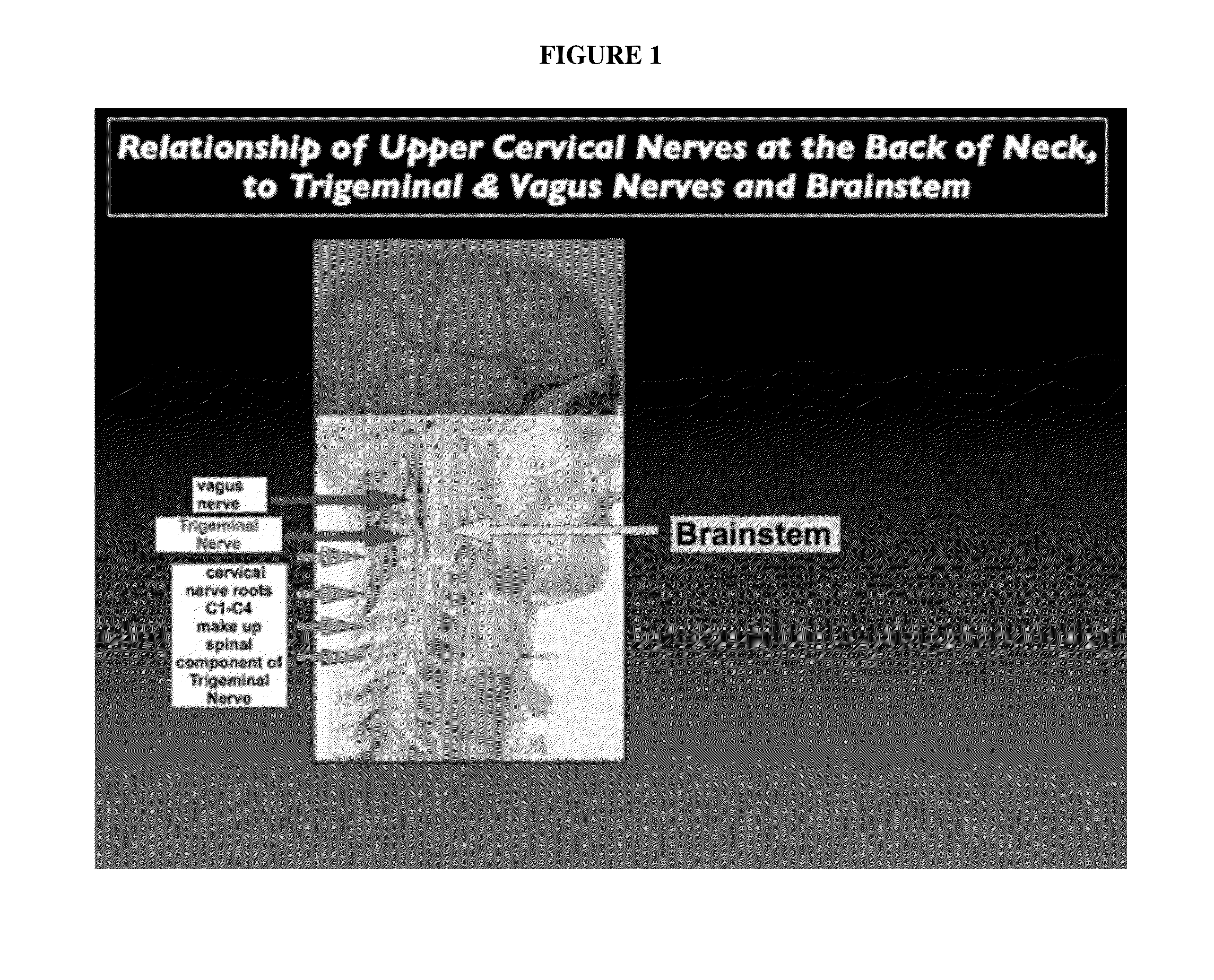
Topical regional neuro-affective therapy with cannabinoids
ActiveUS20160256411A1Modulate afferent neural inputGood effectSenses disorderNervous disorderDiseaseCannabinoid
A method of treating a disease state or condition in humans via topical brainstem afferent stimulation therapy via the administration of a cannabinoid drug(s) to the back of the neck of a human patient to provide regional neuro-affective therapy is disclosed. In certain preferred embodiments, the cannabinoid drug(s) are not psychoactive or substantially not psychoactive. In certain embodiments, the cannabinoid drug(s) are incorporated into a pharmaceutically acceptable topical carrier, e.g., a cream. In certain preferred embodiments, the cannabinoid drug(s) comprises cannabidiol.
Owner:DEF LLC
Chewing gum compositions comprising cannabinoids
The present invention relates to a chewing gum composition comprising 0.01 to 15% by weight a cannabinoid or a derivative thereof, based on the total weight of the chewing gum composition, and to chewing gums and blistering packages comprising said chewing gums.
Owner:CANCHEW BIOTECH LLC
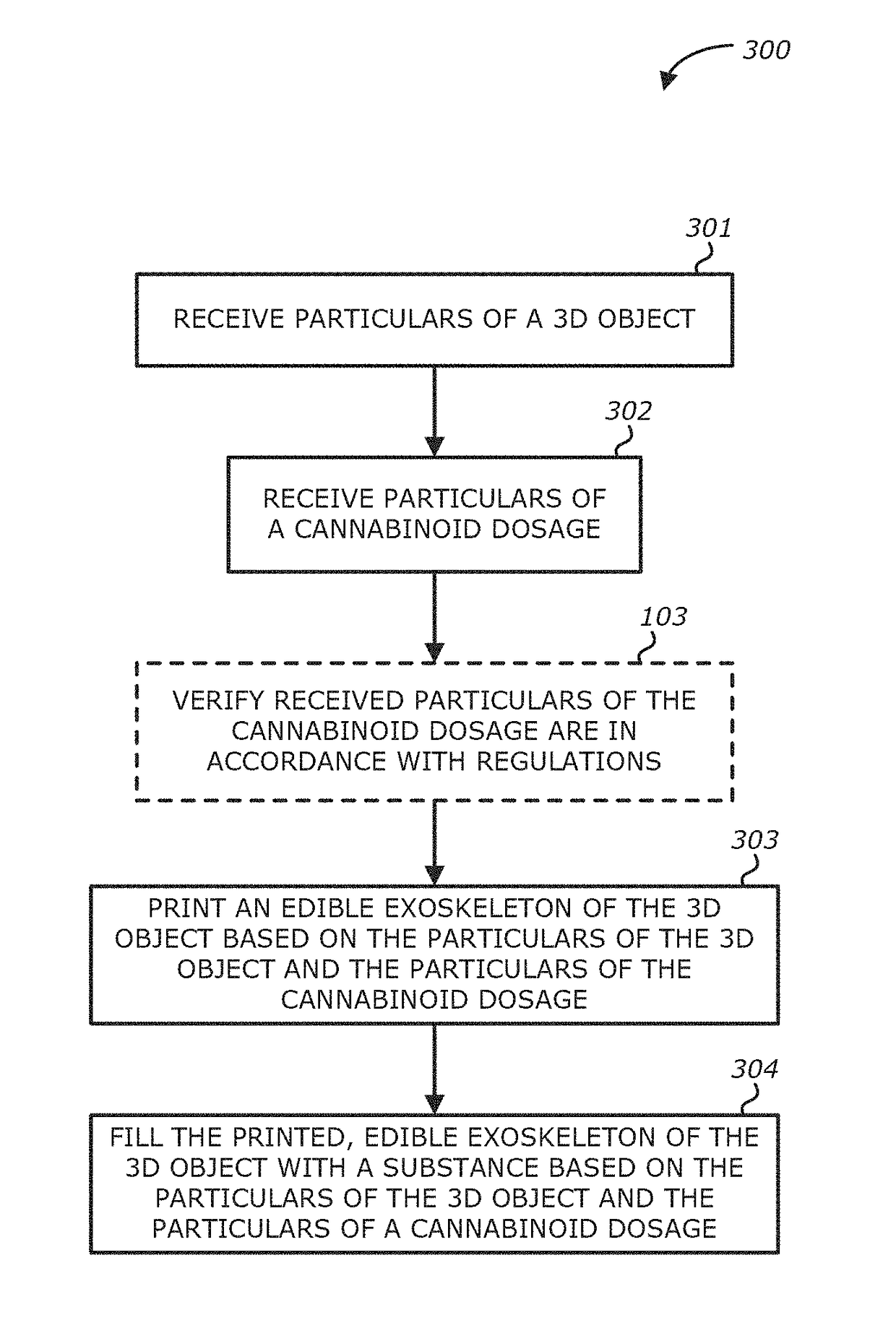
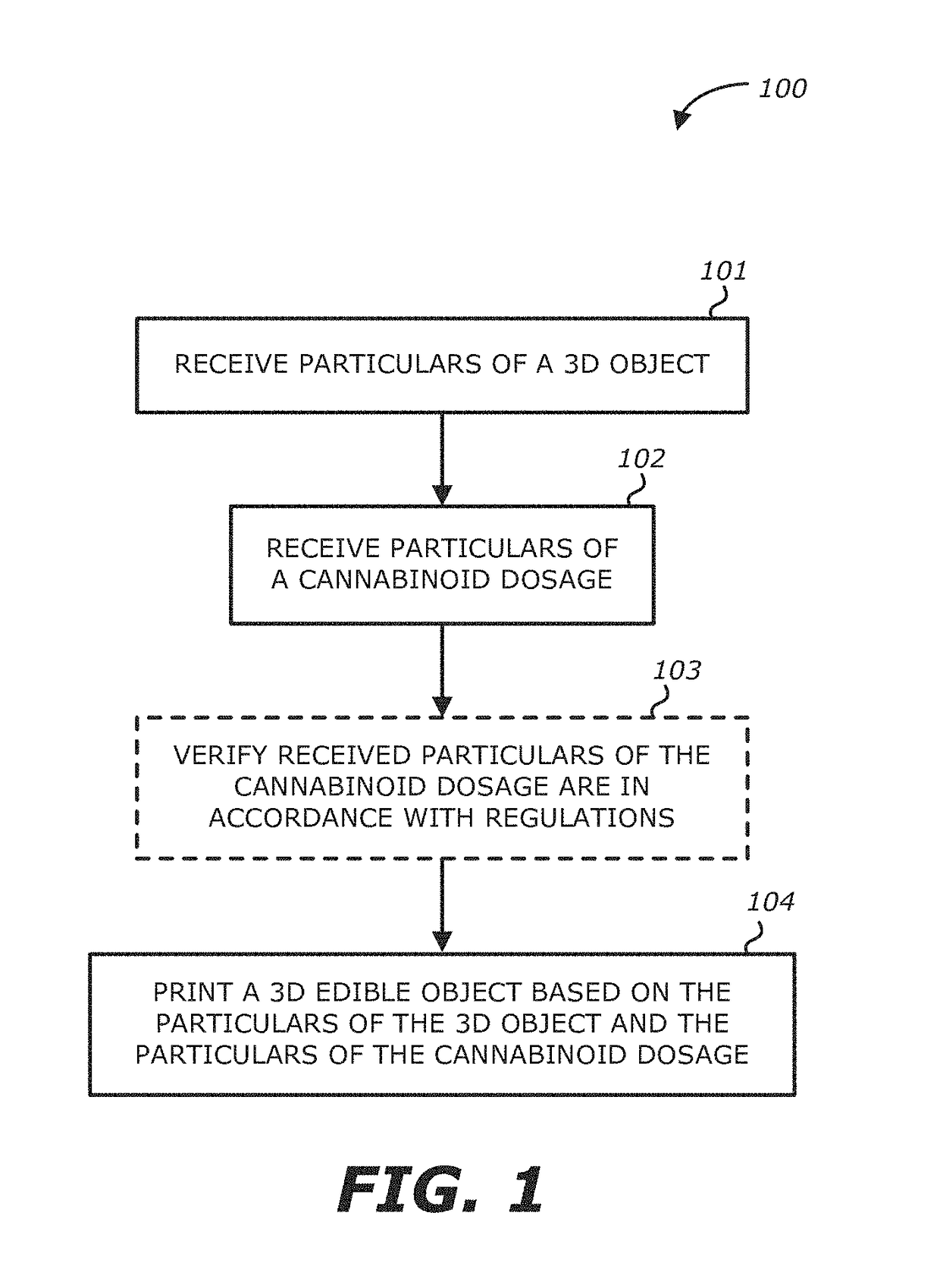
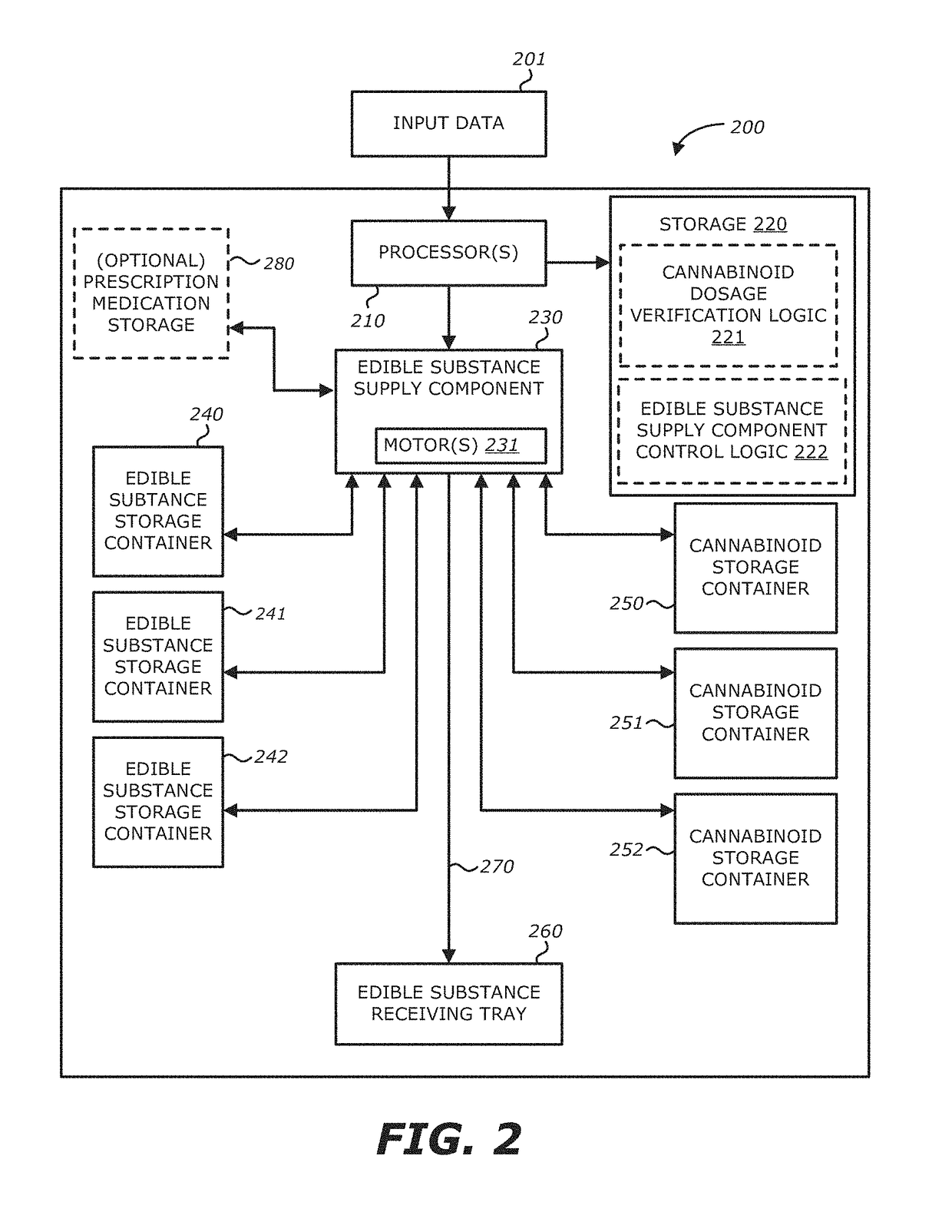
Method, system and apparatus for creating 3D-printed edible objects
InactiveUS9854828B2Additive manufacturing apparatusHydroxy compound active ingredientsObject basedCannabinoid
According to one embodiment, a system of using a three-dimensional (3D) printer to include a cannabinoid comprising: one or more extruders; one or more processors; and a storage module communicatively coupled to the one or more processors, the storage module comprises logic, upon execution by the one or more processors, that receives particulars of a 3D object; receives particulars of a cannabinoid dosage; and instructs the one or more extruders to print the 3D object based on the particulars of the 3D object and the particulars of the cannabinoid dosage, wherein at least a portion of the printed 3D object contains the type of cannabinoid described in the instructions of the particulars of the cannabinoid dosage, and the printed 3D object is edible is provided.
Owner:LANGELAND WILLIAM
Cannabis extracts and methods of preparing and using same
The invention relates to the extraction of pharmaceutically active components from plant materials, and more particularly to the preparation of a botanical drug substance (BDS) for incorporation in to a medicament. It also relates to a BDS, for use in pharmaceutical formulations. In particular it relates to BDS comprising cannabinoids obtained by extraction from cannabis.
Owner:BLACKMON EARNEST VINSON MR
Use of cannabinoids in combination with an anti-psychotic medicament
ActiveUS9017737B2High activityLess degree of activityBiocideSenses disorderTypical antipsychoticPsychosis drug
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
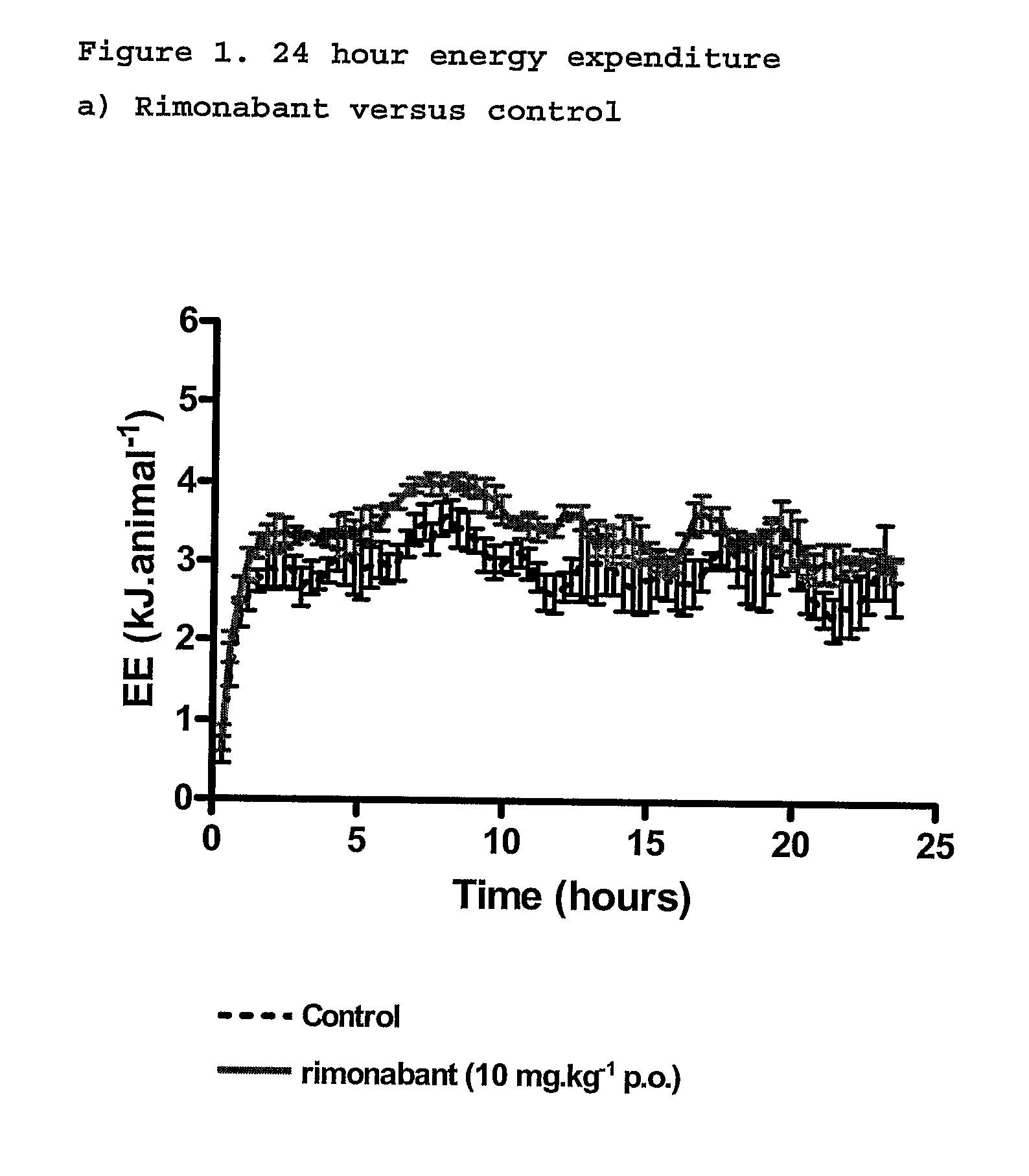
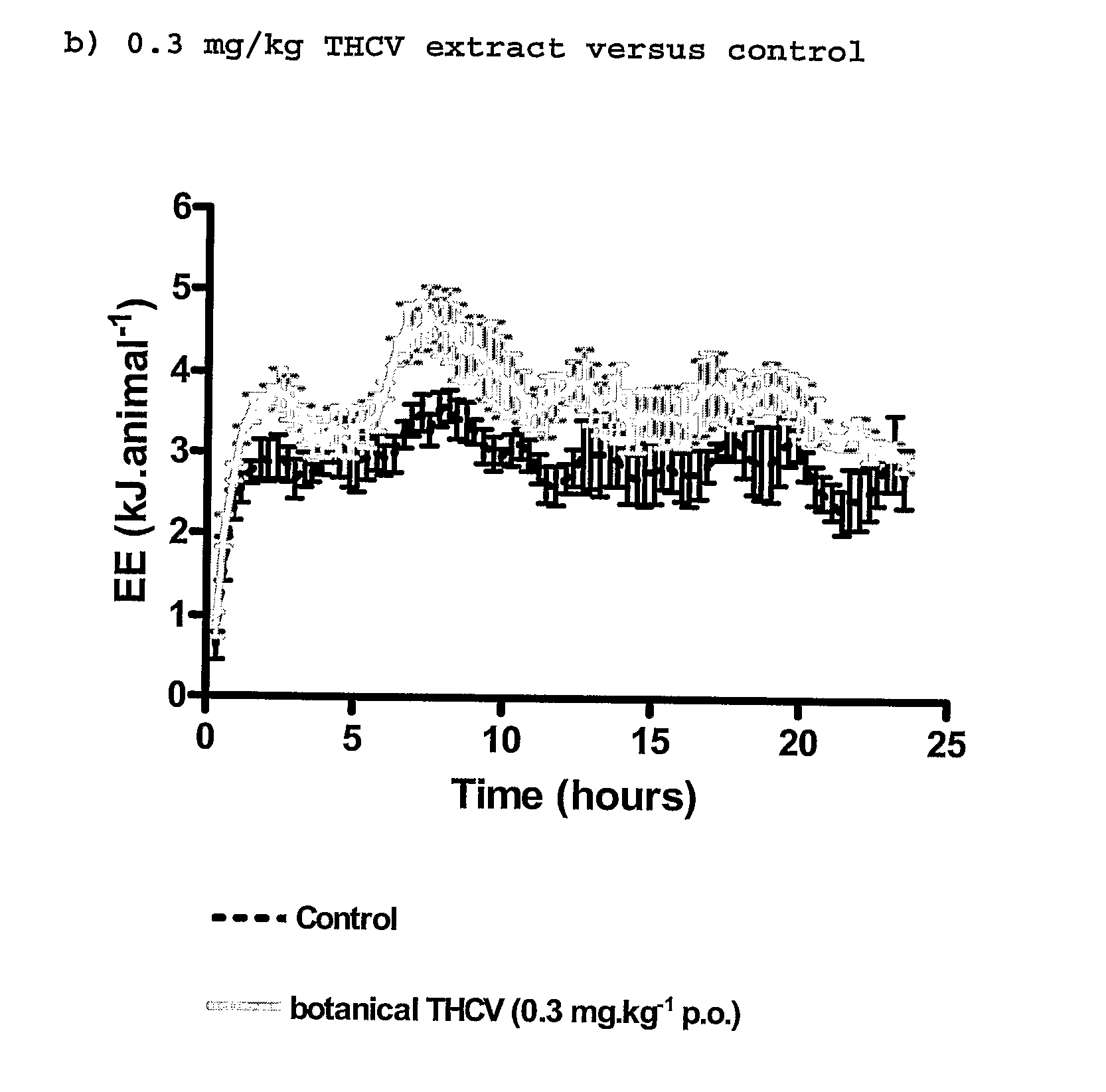
New use for cannabinoids
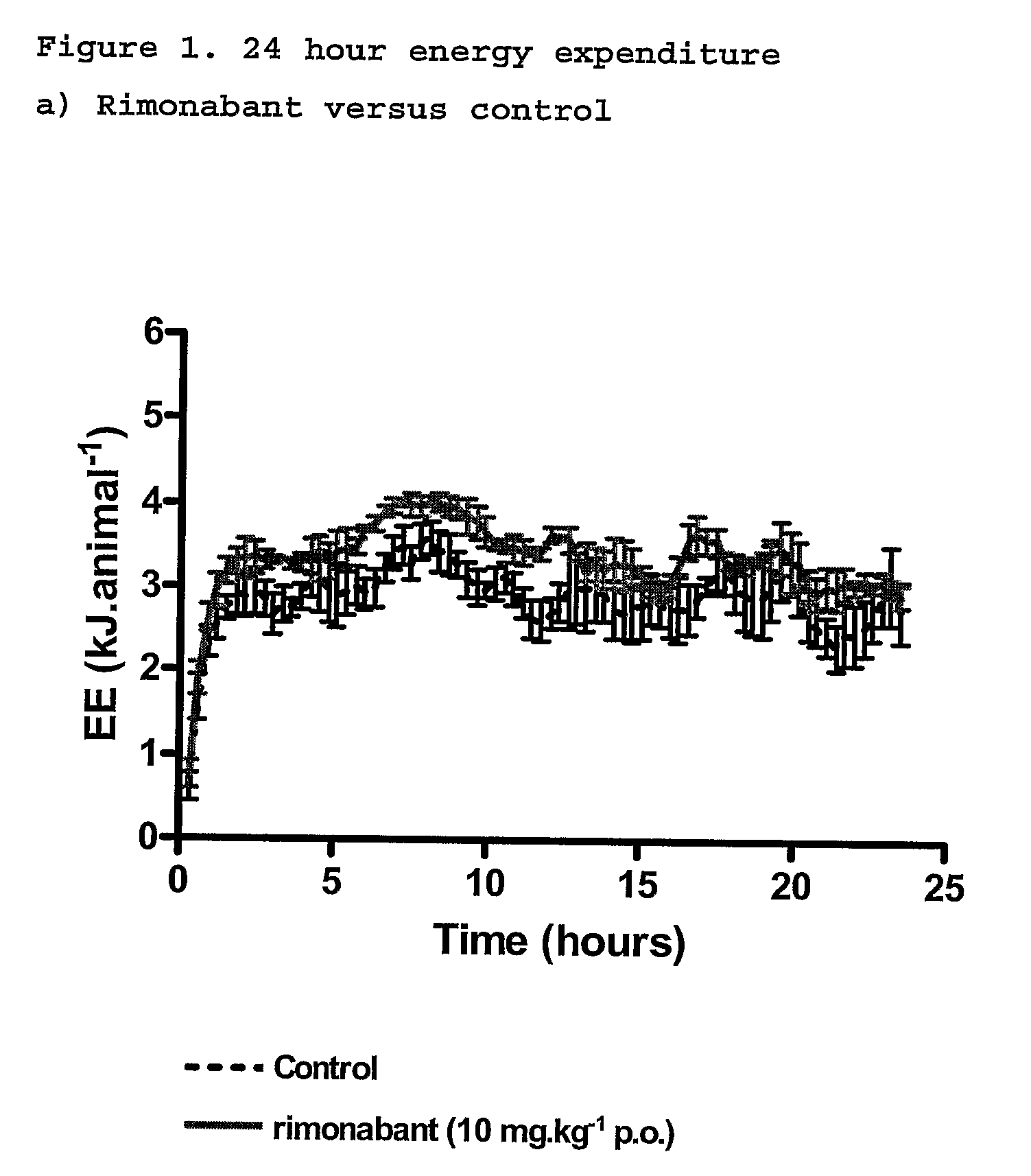
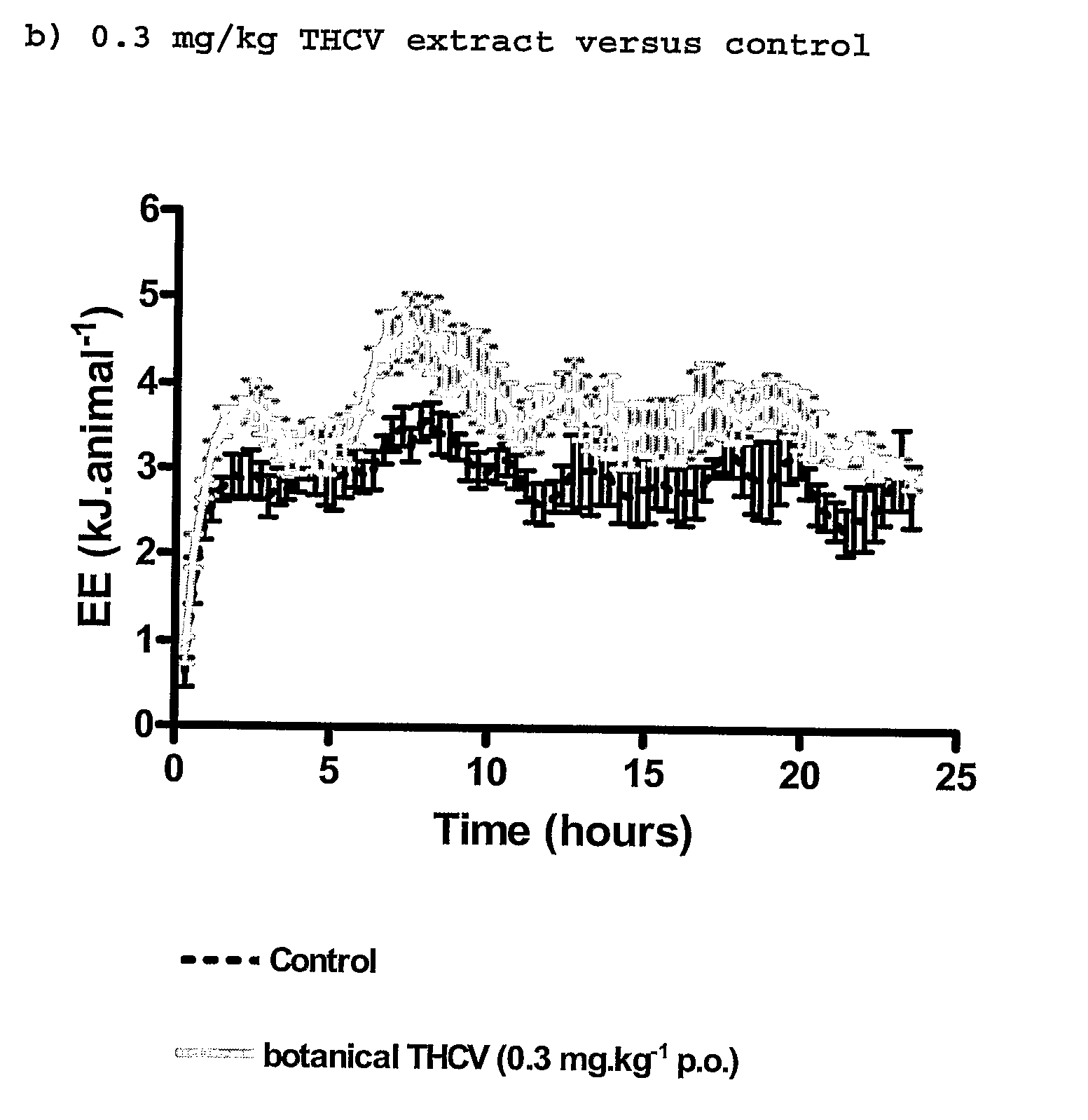
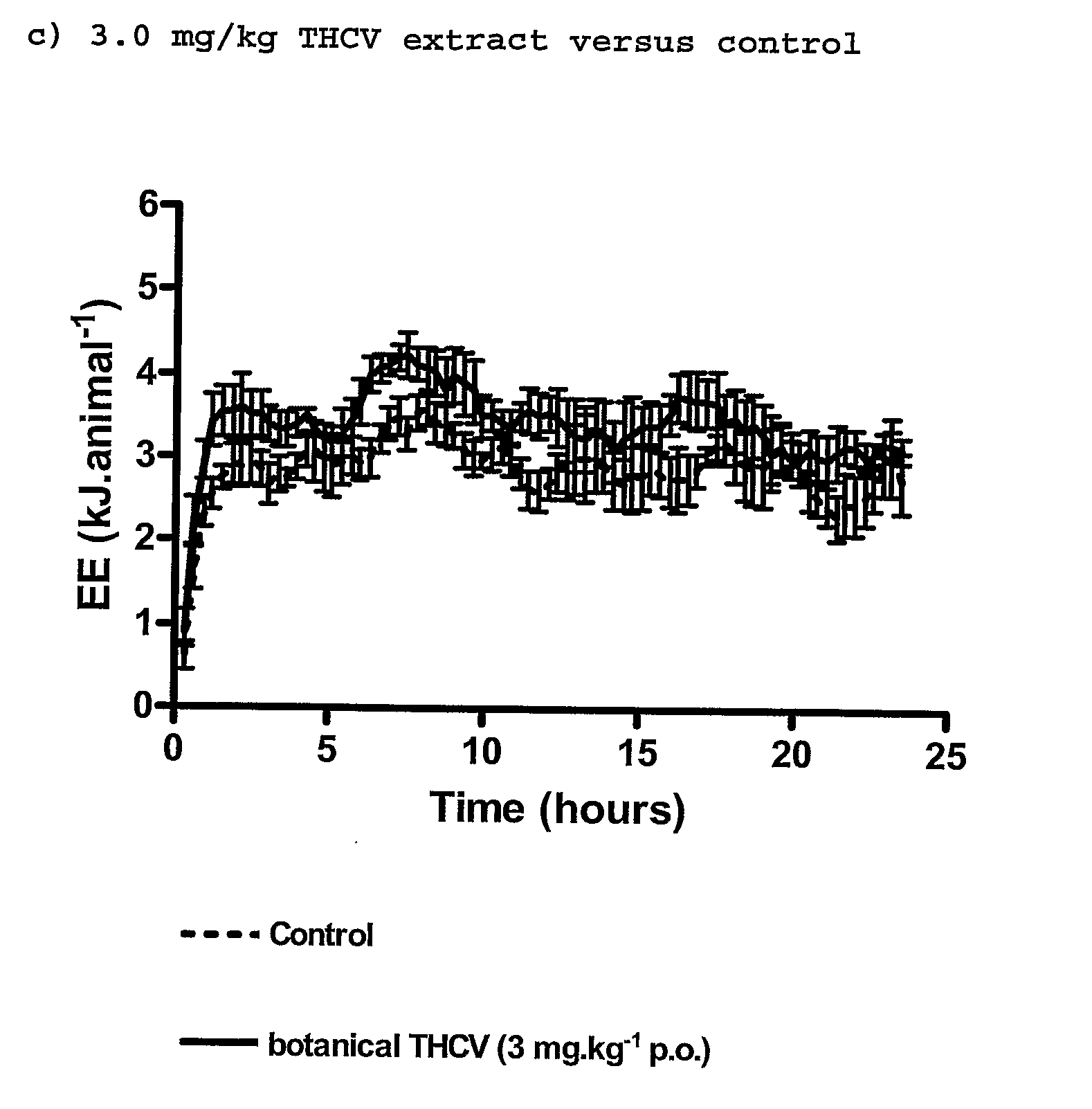
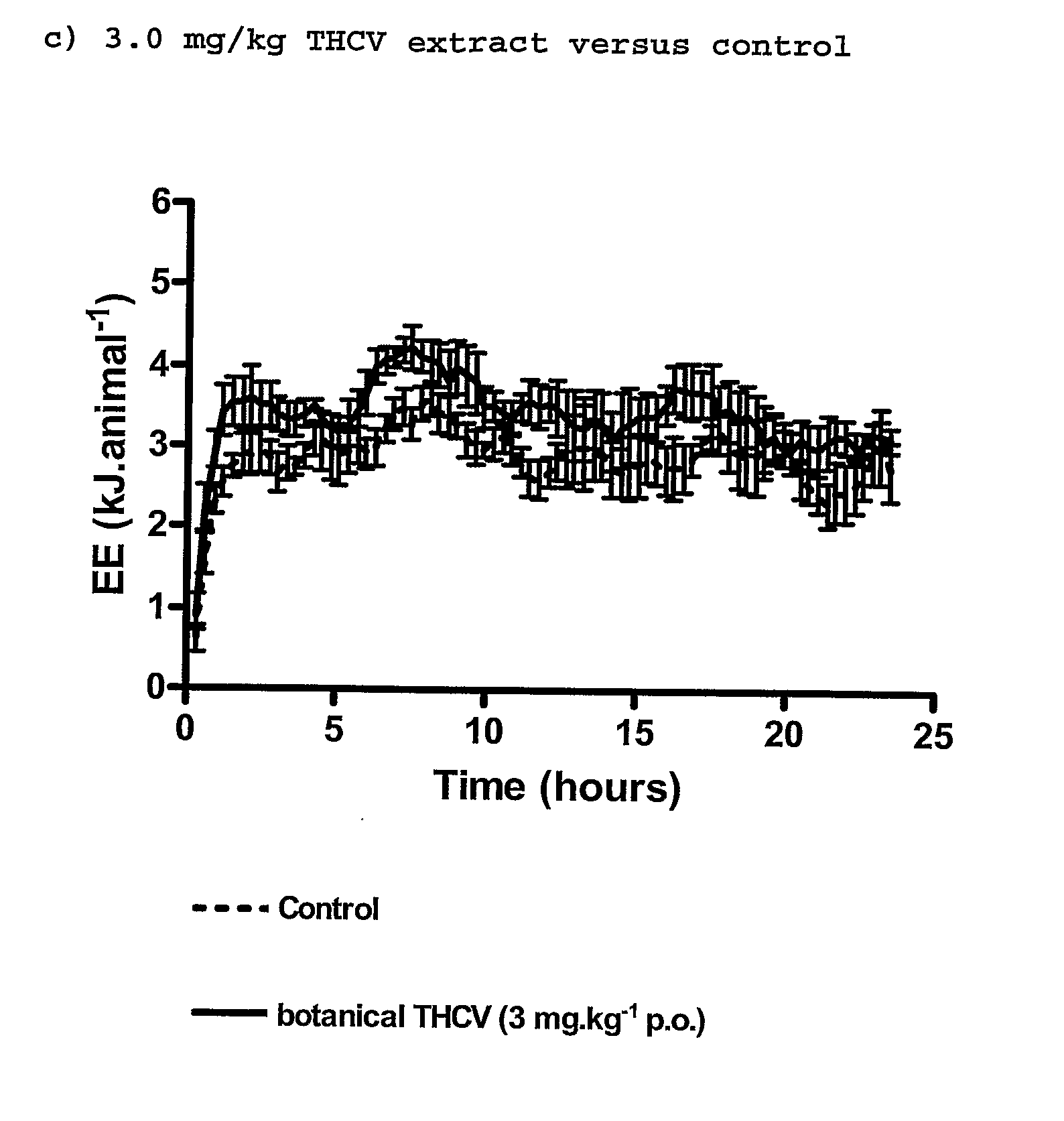
InactiveUS20110082195A1Increased energy expenditureHigh activityBiocideOrganic chemistryDyslipidemiaCannabinoid
The present invention relates to the use of CBD alone or in combination with another cannabinoid, in the manufacture of a pharmaceutical or neutraceutical formulation for use in controlling cholesterol levels in a subject. It also relates to the use of THCV alone or in combination with another cannabinoid, in the manufacture of a pharmaceutical or neutraceutical formulation for use in increasing energy expenditure in a subject. Furthermore the CBD alone or in combination with another cannabinoid or the THCV alone or in combination with another cannabinoid are used as part of a regime to manage or treat type I or II diabetes, obesity, dyslipidaemia, related metabolic disorders and cardiovascular disease.
Owner:GW PHARMA LTD
Cannabinoid-Containing Compositions and Methods for Their Use
InactiveUS20120202891A1Improve clinical efficacyReduce inflammationBiocideHydroxy compound active ingredientsInjury causeCannabis
This invention relates to cannabinoid-containing compositions, particularly cannabinoid-containing gel formulations and methods for the treatment of traumatic injury, e.g., strains, sprains and contusions, and disease conditions, e.g., arthritis, particularly osteoarthritis. The methods involve topically applying a cannabinoid or a cannabinoid-containing composition to a subject's skin near, or distant from, the area of the injury or the area affected by the disease condition, e.g., an arthritic joint. The cannabinoid-containing composition is preferably a pharmaceutically acceptable gel containing a therapeutically effective amount of a cannabinoid sufficient to alleviate the symptoms associate with the injury or disease condition.
Owner:KENTUCKY ECONOMIC DEV FINANCE AUTHORITY +1
New pharmaceutical formulation comprising cannabidiol and tetrahydrocannabidivarin
InactiveUS20100317729A1Enhanced treatment optionGood for weight lossBiocideNervous disorderCannabinoidPharmaceutical formulation
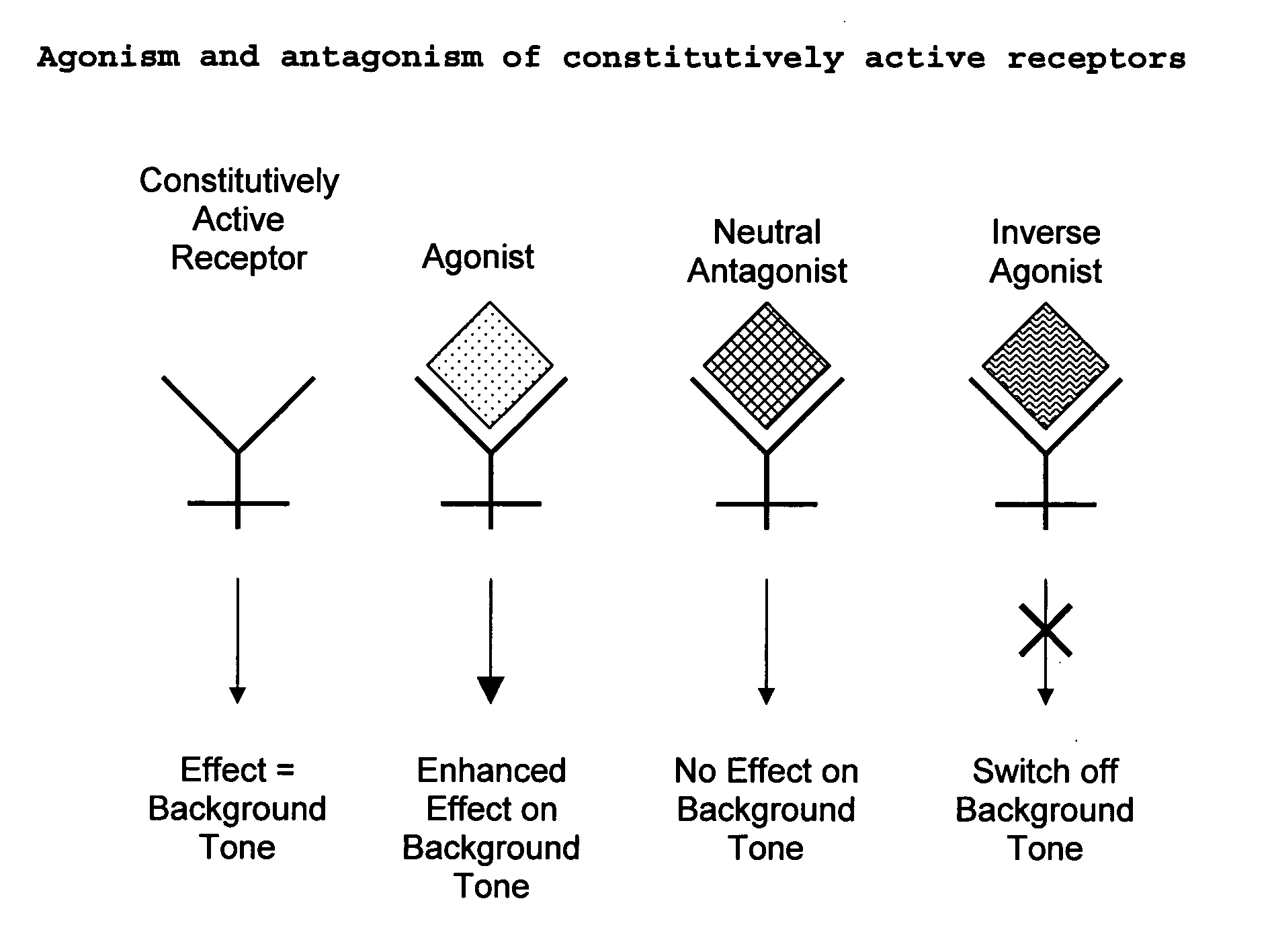
The present invention relates to a novel pharmaceutical formulation comprising a ratioed mix of: (i) one or more compounds that acts as an inverse agonist of the CB1 and / or CB2 receptor; and (ii) one or more compounds that acts as a neutral antagonist of the CB1 and / or CB2 receptor. Preferably both the inverse agonist of the CB1 and / or CB2 receptor and the neutral antagonist of the CB1 and / or CB2 receptor are cannabinoids. Preferably the cannabinoids are tetrahydrocannabidivarin (THCV) and cannabidiol (CBD).
Owner:GW PHARMA LTD
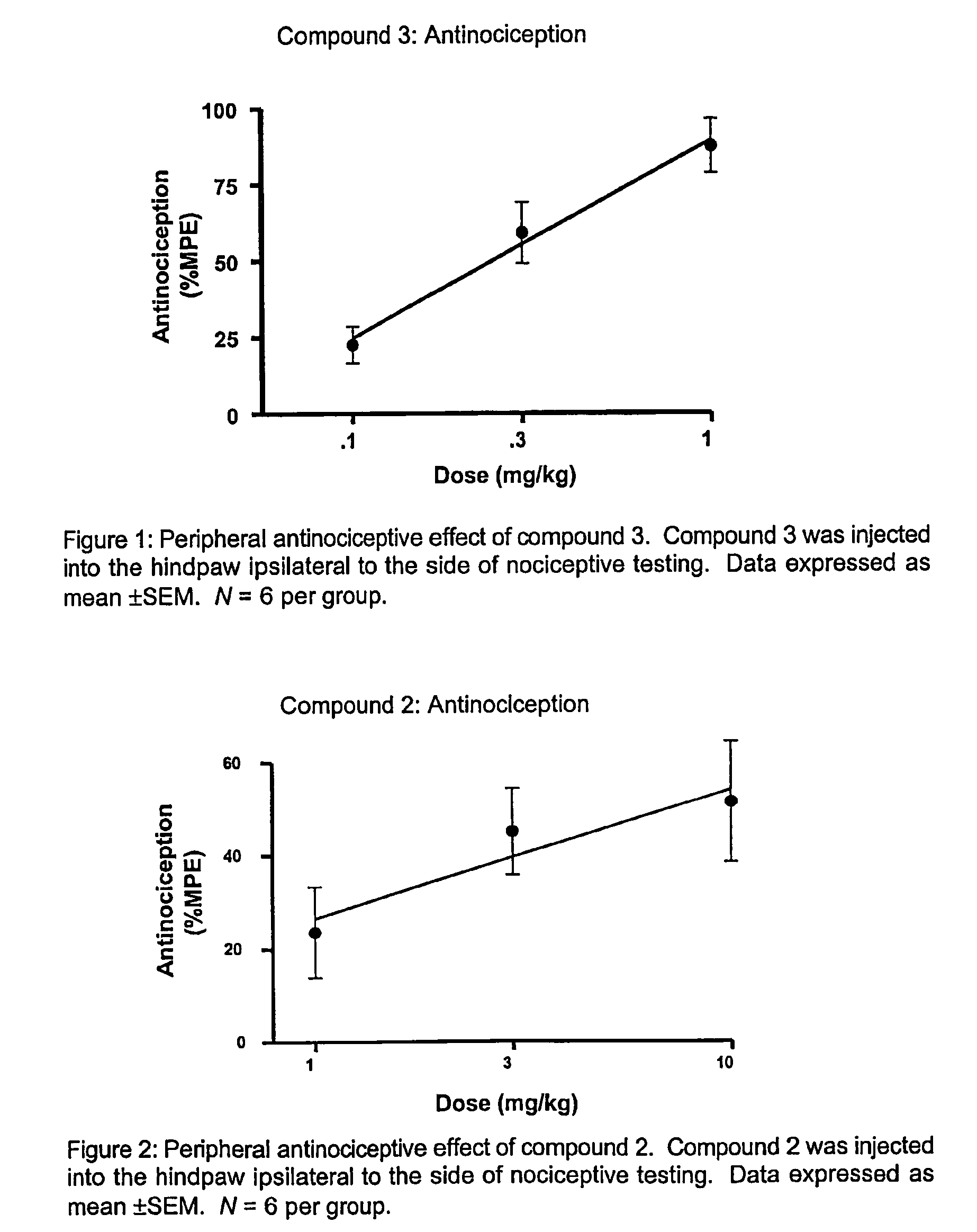
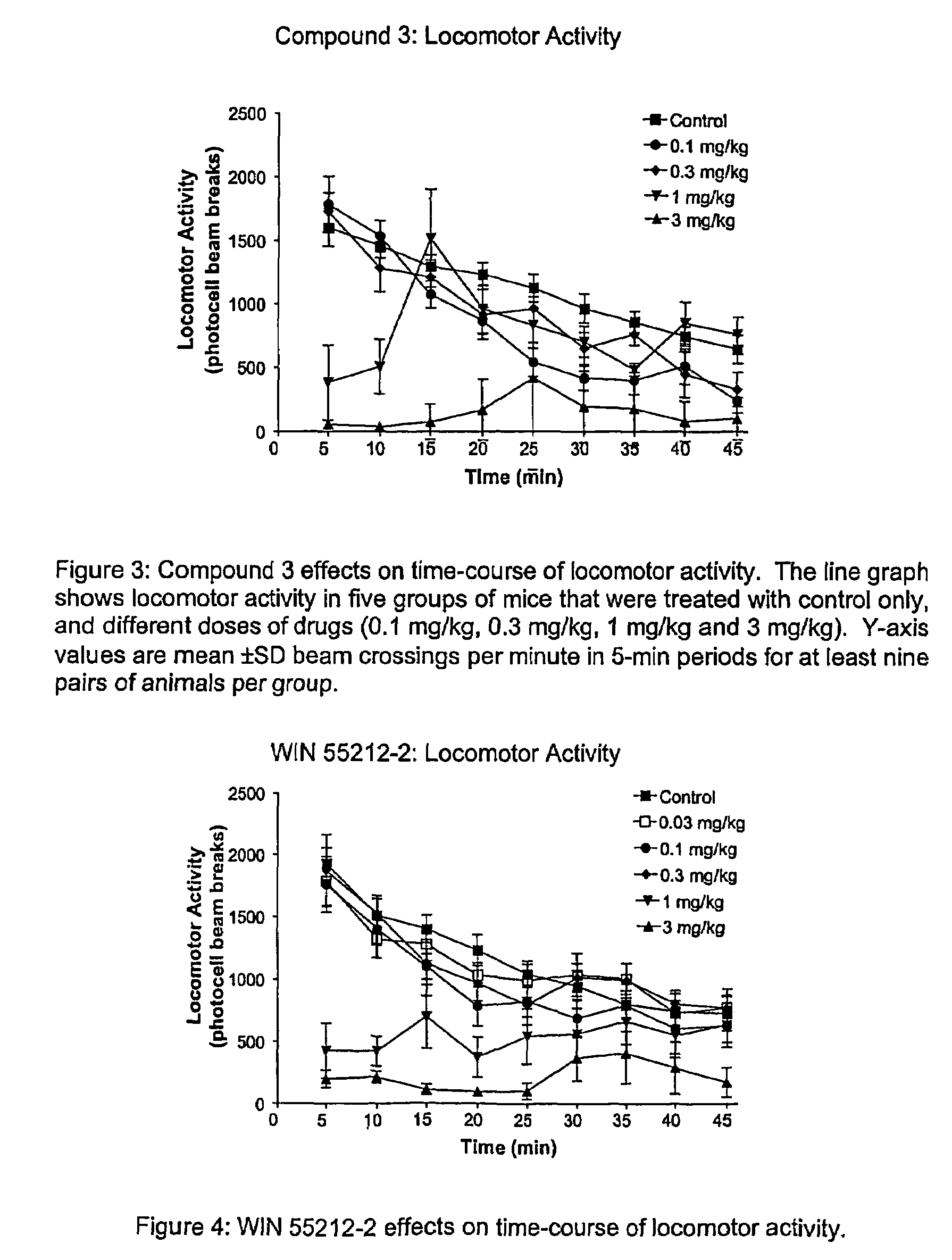
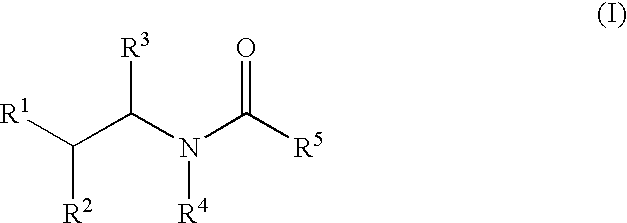
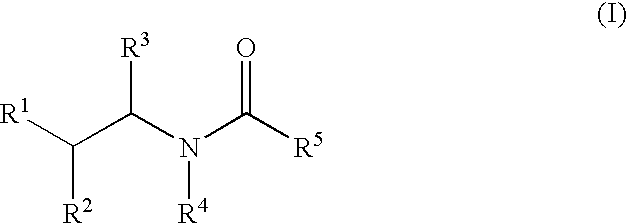
Substituted amides
Novel compounds of the structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as centrally acting drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Use for Cannabinoid
InactiveUS20090306221A1High activityLess degree of activityBiocideNervous disorderDiseaseCannabinoid
The present invention relates to the use of one or more cannabinoids in the manufacture of medicaments for use in 0 the treatment of diseases and conditions benefiting from inverse agonism of the CB1 and / or the CB2 cannabinoid receptor. Preferably the cannabinoid is a cannabidiol (CBD) type compound or derivative thereof.
Owner:GW PHARMA LTD
Cannabinoid-containing plant extracts as neuroprotective agents
The invention relates to the use of cannabinoid-containing plant extracts in the prevention or treatment of neural degeneration. In particular, the invention relates to use of one or more cannabinoid-containing plant extracts in the prevention or treatment of neural degeneration, wherein the one or more cannabinoid-containing plant extracts comprise: i) a cannabinoid-containing fraction; and ii) a non-cannabinoid containing fraction.
Owner:GW RES LTD
Methods for preparation of cannabis oil extracts and compositions
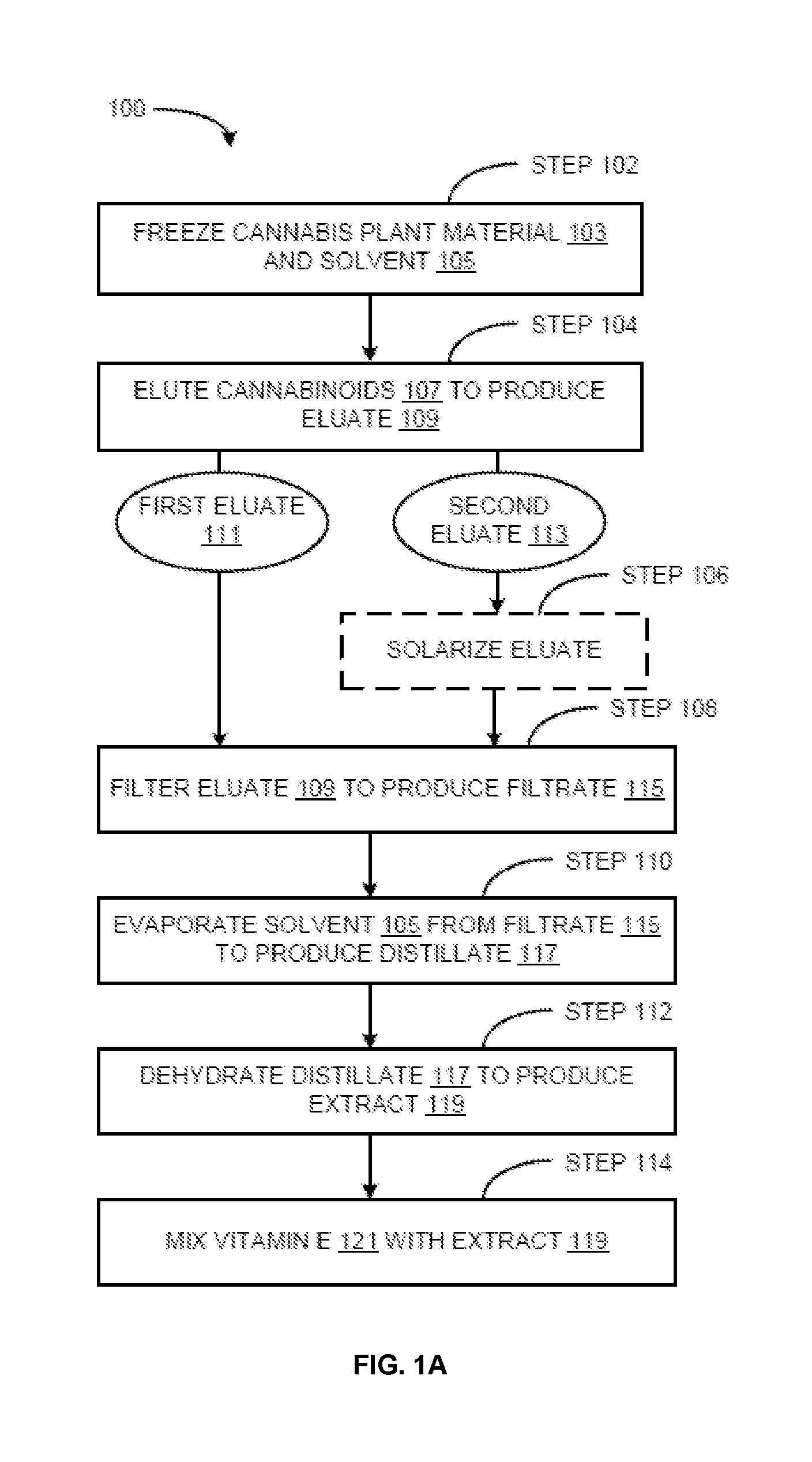
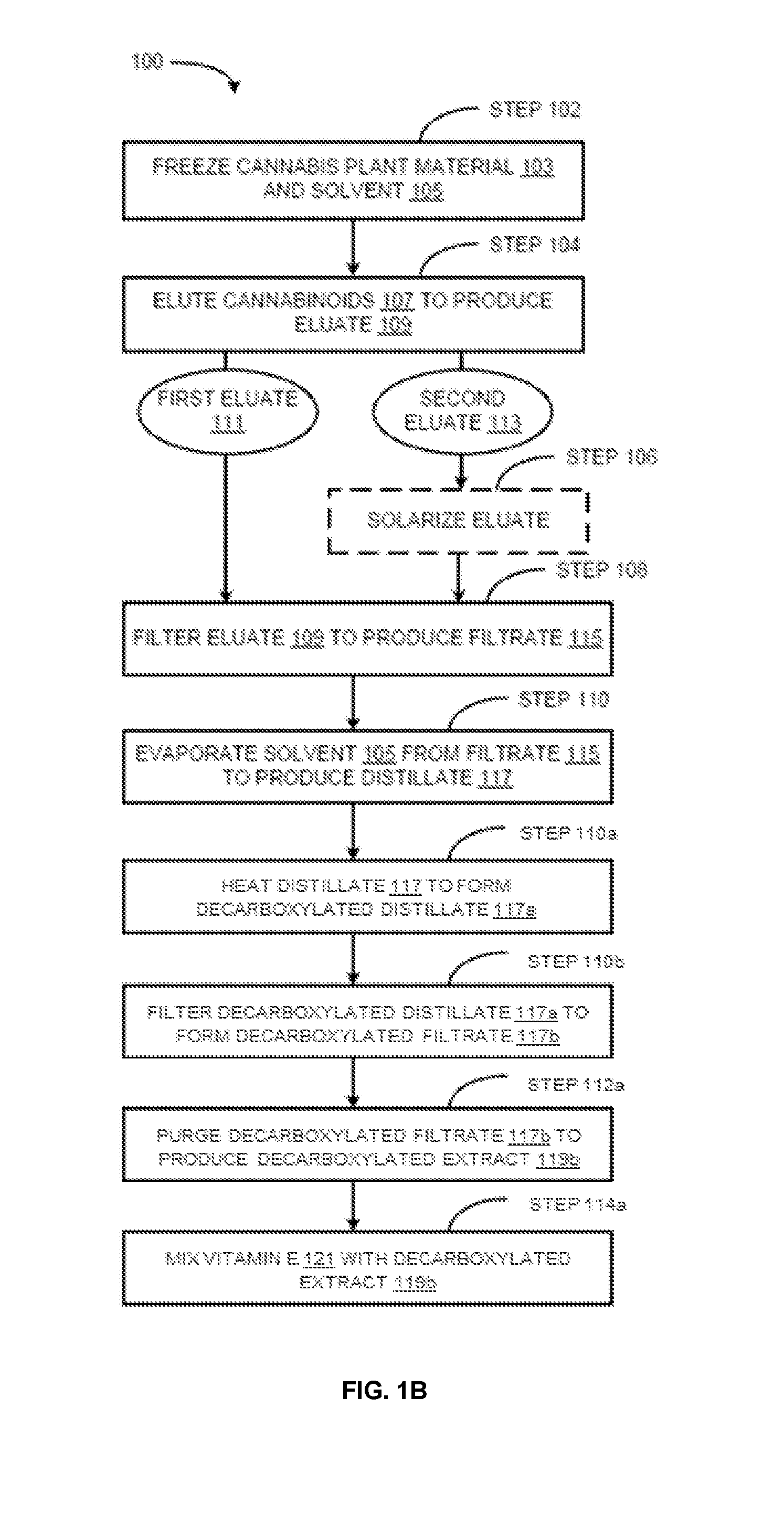
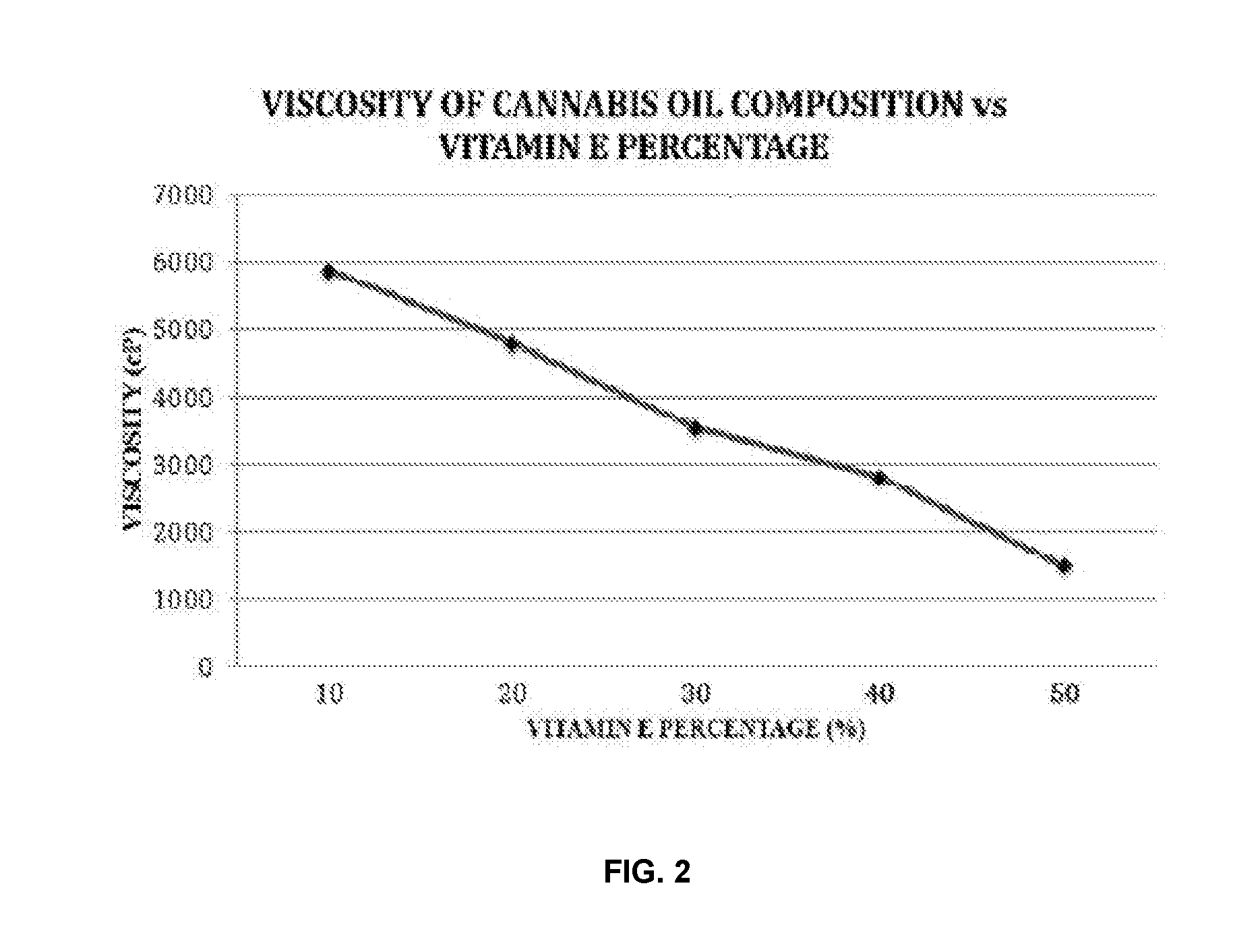
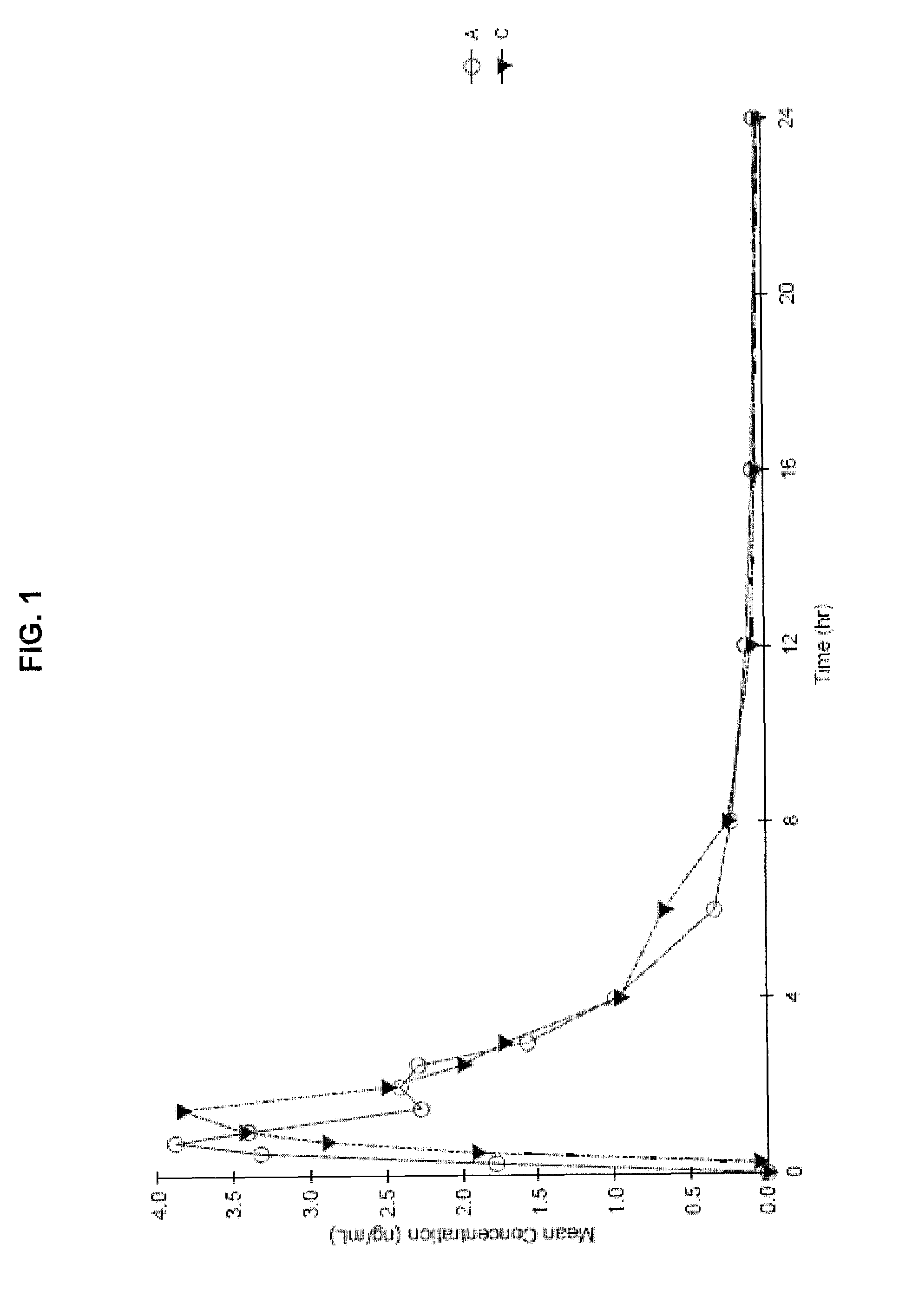
The present invention provides cannabis oil extracts and compositions thereof, including cannabis oil compositions containing vitamin E, and methods for preparing the extracts and compositions. In some embodiments, the present invention provides a method for preparing a cannabis oil extract comprising eluting cannabinoids from cannabis plant material with a solvent to produce an eluate, filtering the eluate with a filter to produce a filtrate, evaporating the solvent from the filtrate with a distiller to produce a distillate, and purging the distillate under conditions sufficient to remove residual solvent, thereby preparing the extract. In some embodiments, the method further includes mixing a quantity of vitamin E with the extract to produce a cannabis oil composition.
Owner:CONSTANCE THERAPEUTICS INC
Liquid cannabinoid formulations
Oral cannabinoid formulations, including an aqueous-based oral dronabinol solution, that are stable at room or refrigerated temperatures and may possess improved in vivo absorption profiles with faster onset and lower inter-subject variability.
Owner:BENUVIA OPERATIONS LLC
Terpene and cannabinoid formulations
InactiveUS20160279073A1Readily apparentHydrocarbon active ingredientsDispersion deliveryCannabinoidLiposome
The present invention provides stable, fast-acting liposome and micelle formulations of terpenes, hemp oil, cannabinoids, or mixtures of a cannabinoid and terpenes or hemp oil and cannabinoids that are suitable for pharmaceutical and nutraceutical applications. Also provided are methods for the manufacture of micelle and liposomal formulations.
Owner:TEEWINOT TECH LTD
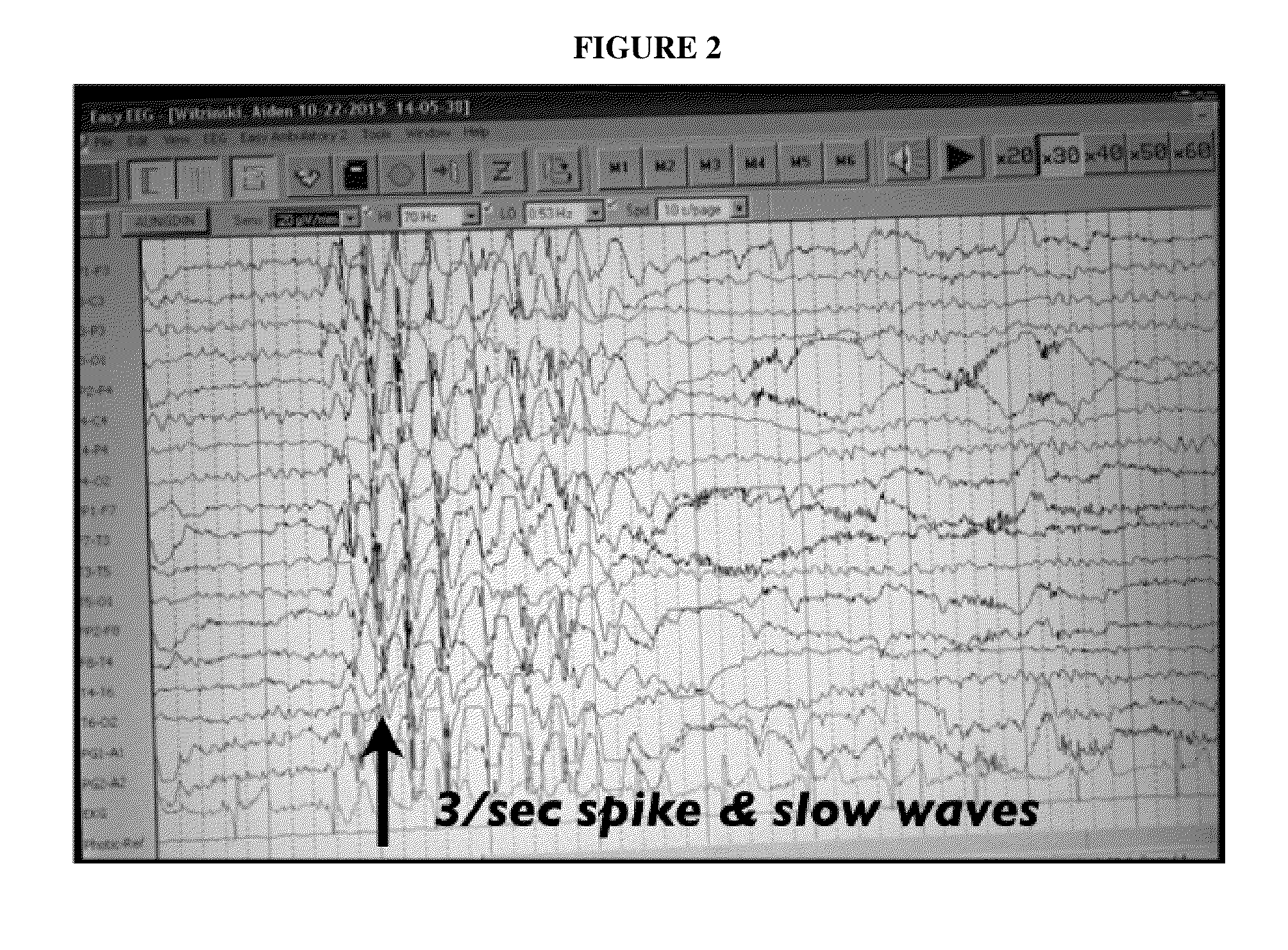
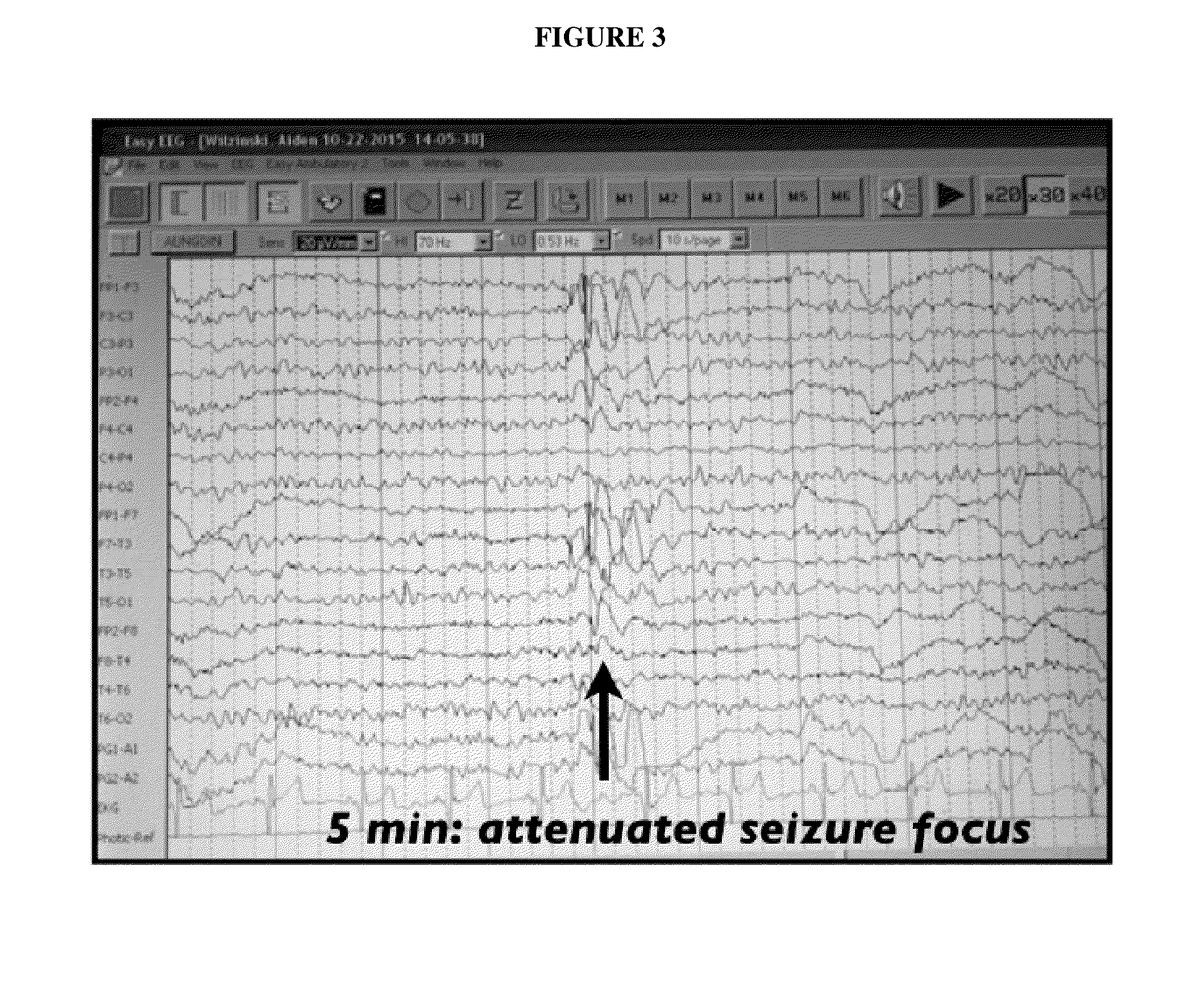
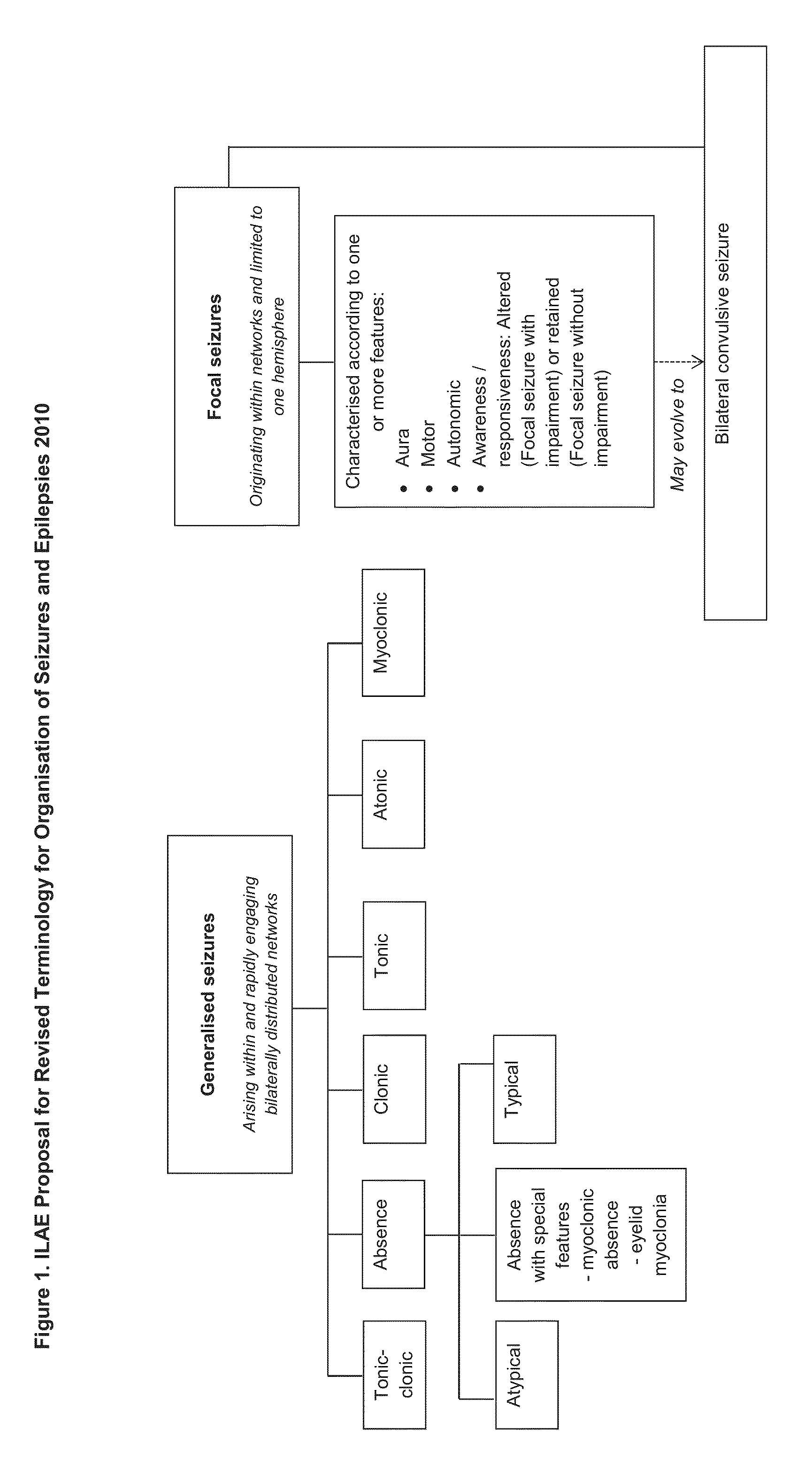
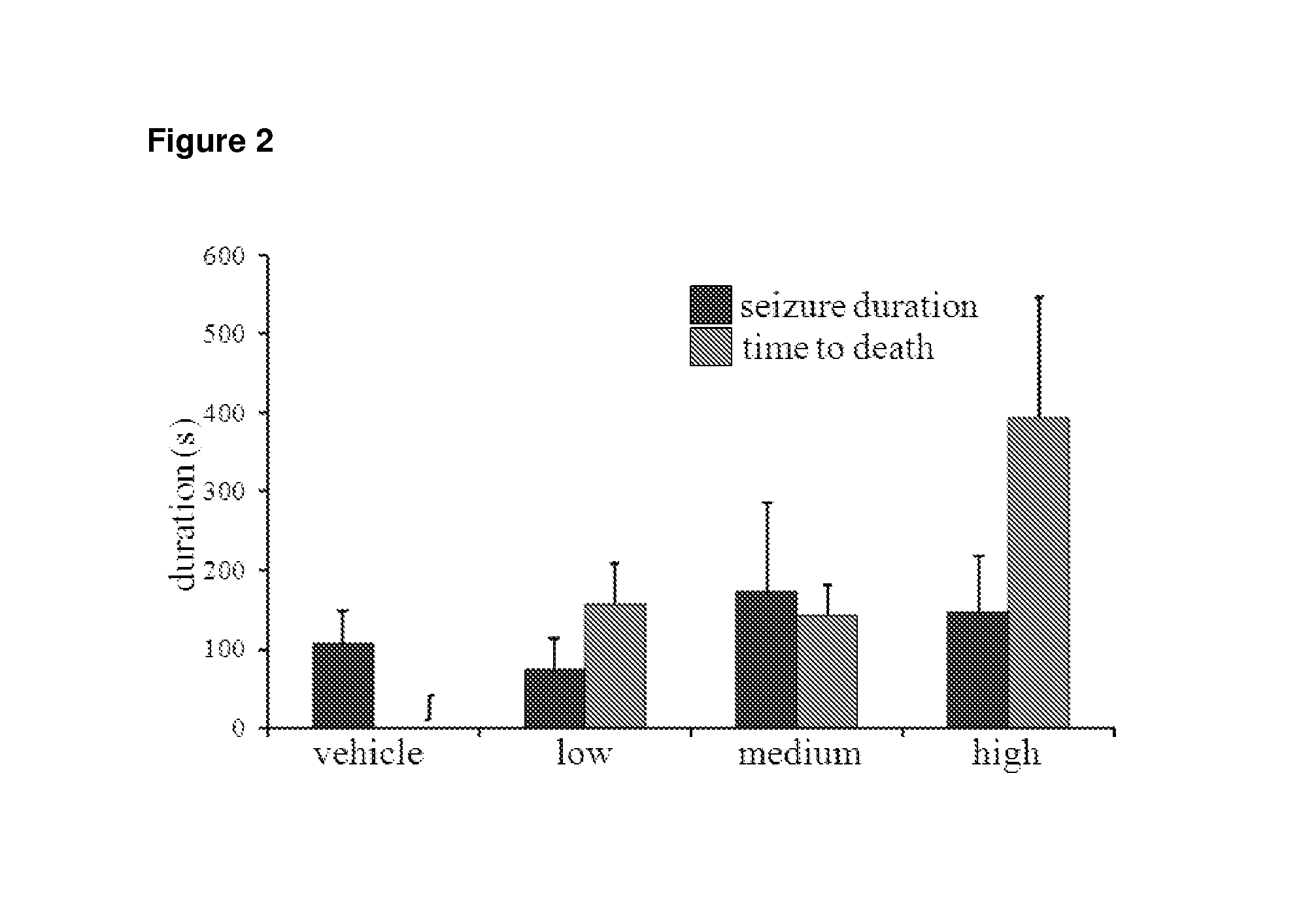
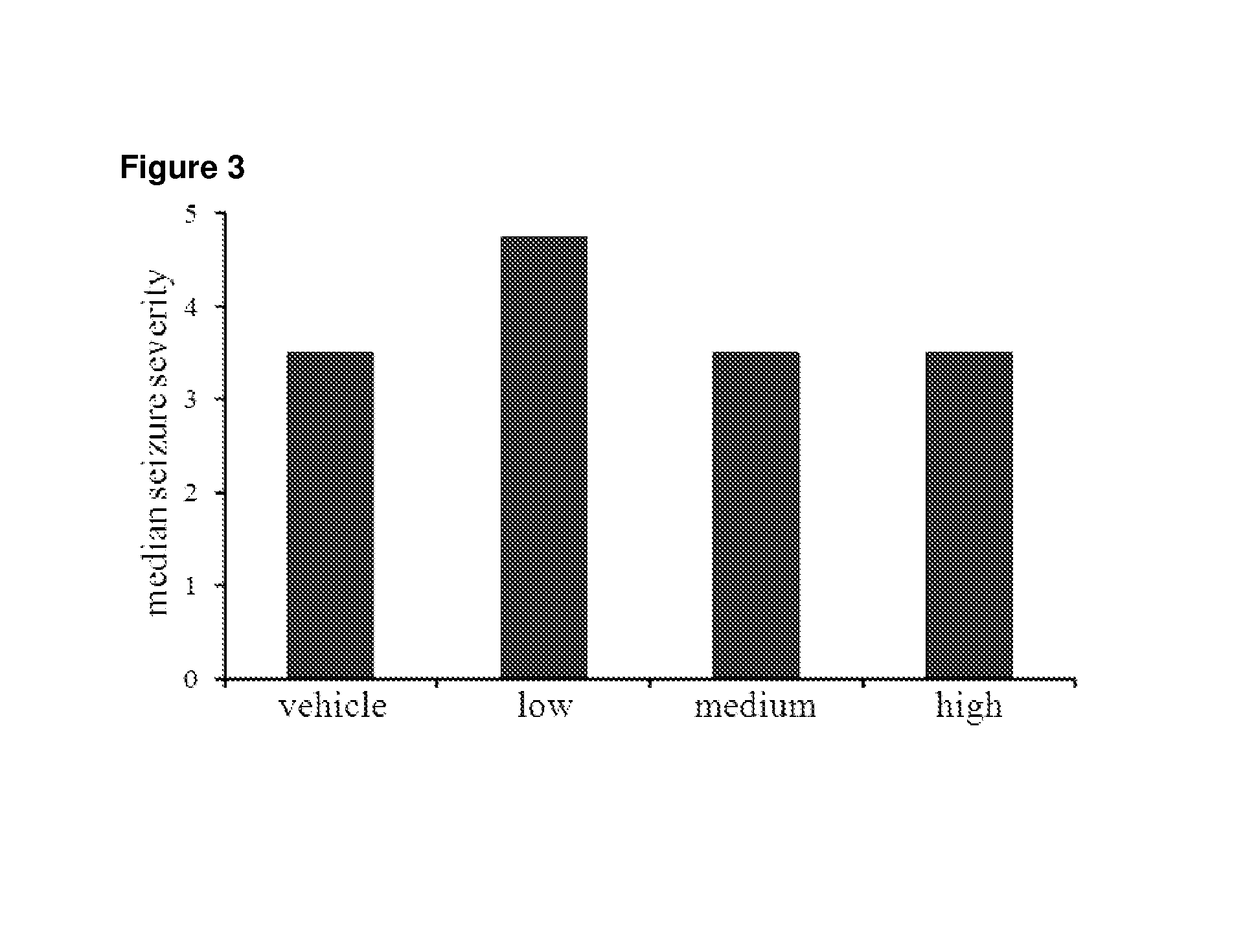
Use of cannabinoids in the treatment of epilepsy
The present disclosure relates to the use of cannabidiol (CBD) in the treatment of absence seizures. In particular, the disclosure relates to the use of CBD for reducing absence seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; Doose Syndrome; CDKL5; Dup15q; Jeavons syndrome; Myoclonic Absence Epilepsy; Neuronal ceroid lipofuscinoses (NCL) and brain abnormalities. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
Use for Cannabinoid
The invention relates to the use of one or more cannabinolds in the manufacture of medicaments for use in the treatment of diseases and conditions benefiting from neutral antagonism of the CB, cannabinoid receptor. Preferably the cannabinoid is tetrahydrocannabivarin (THCV). Preferably the diseases and conditions to be treated are taken from the group: obesity, schizophrenia, epilepsy, cognitive disorders such as Alzheimer's, bone disorders, bulimia, obesity associated with type n diabetes (non-insulin dependant diabetes) and in the treatment of drug, alcohol and nicotine abuse or dependency.
Owner:GW PHARMA LTD
Use of cannabinoids and terpenes for treatment of organophosphate and carbamate toxicity
InactiveUS20150313868A1Prevent crashExtended duration of actionBiocideHydrocarbon active ingredientsDiseaseCarbamate
Pharmaceutical compositions in which isolated cannabinoid receptor modulators are optionally combined with terpene blends in a pharmaceutically acceptable carrier. Methods for treating or preventing a disease, disorder, dysfunction or condition caused by exposure to an organophosphate or carbamate acetylcholineesterase inhibitor with the inventive compositions are also disclosed.
Owner:KOTZKER CONSULTING
Use of one or a combination of phyto-cannabinoids in the treatment of epilepsy
This invention relates to the use of one or more cannabinoids in the treatment of epilepsy and more particularly to the use of one or a combination of cannabinoids in the treatment of generalized or partial seizure. In one embodiment it relates to the use of the cannabinoid THCV, as a pure or isolated compound, or as a plant extract in which significant amounts of any THC naturally present has been selectively removed. In another embodiment the phytocannabinoid is CBD.
Owner:GW PHARMA LTD
Use for cannabinoids
InactiveUS20130245110A1High activityLess degree of activityBiocideOrganic chemistryDyslipidemiaCholesterol
The present invention relates to the use of CBD alone or in combination with another cannabinoid, in the manufacture of a pharmaceutical or neutraceutical formulation for use in controlling cholesterol levels in a subject. It also relates to the use of THCV alone or in combination with another cannabinoid, in the manufacture of a pharmaceutical or neutraceutical formulation for use in increasing energy expenditure in a subject. Furthermore the CBD alone or in combination with another cannabinoid or the THCV alone or in combination with another cannabinoid are used as part of a regime to manage or treat type I or II diabetes, obesity, dyslipidaemia, related metabolic disorders and cardiovascular disease.
Owner:GW PHARMA LTD
Use for cannabinoid
ActiveUS9168278B2High activityLess degree of activityBiocideNervous disorderDiseaseCannabinoid receptor
The invention relates to the use of one or more cannabinoids in the manufacture of medicaments for use in the treatment of diseases and conditions benefiting from neutral antagonism of the CB, cannabinoid receptor. Preferably the cannabinoid is tetrahydrocannabivarin (THCV). Preferably the diseases and conditions to be treated are taken from the group: obesity, schizophrenia, epilepsy, cognitive disorders such as Alzheimer's, bone disorders, bulimia, obesity associated with type II diabetes (non-insulin dependant diabetes) and in the treatment of drug, alcohol and nicotine abuse or dependency.
Owner:GW PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com