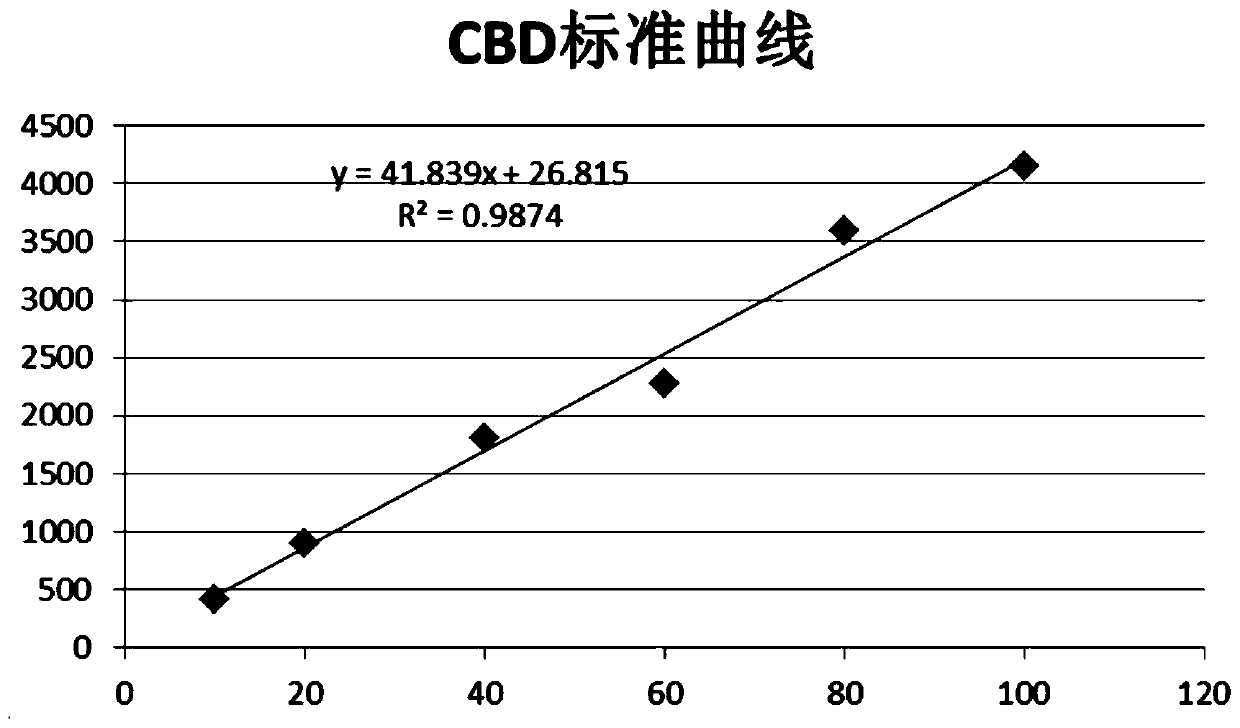
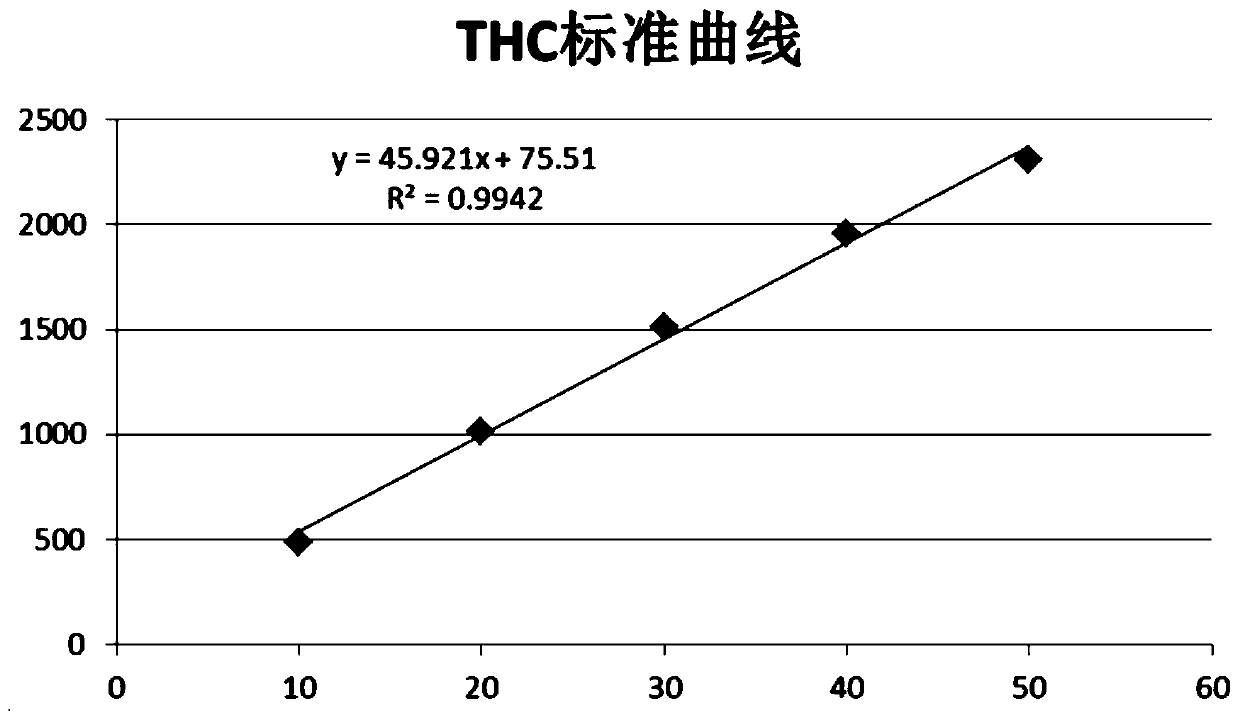
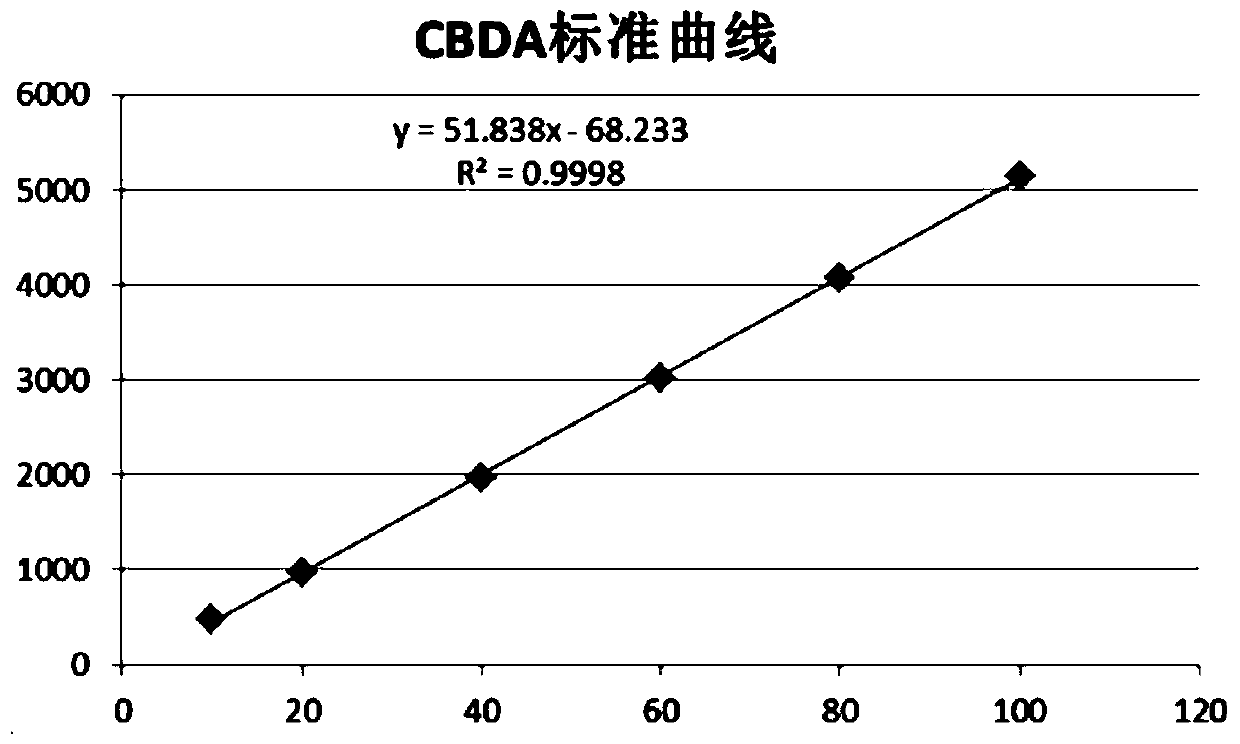
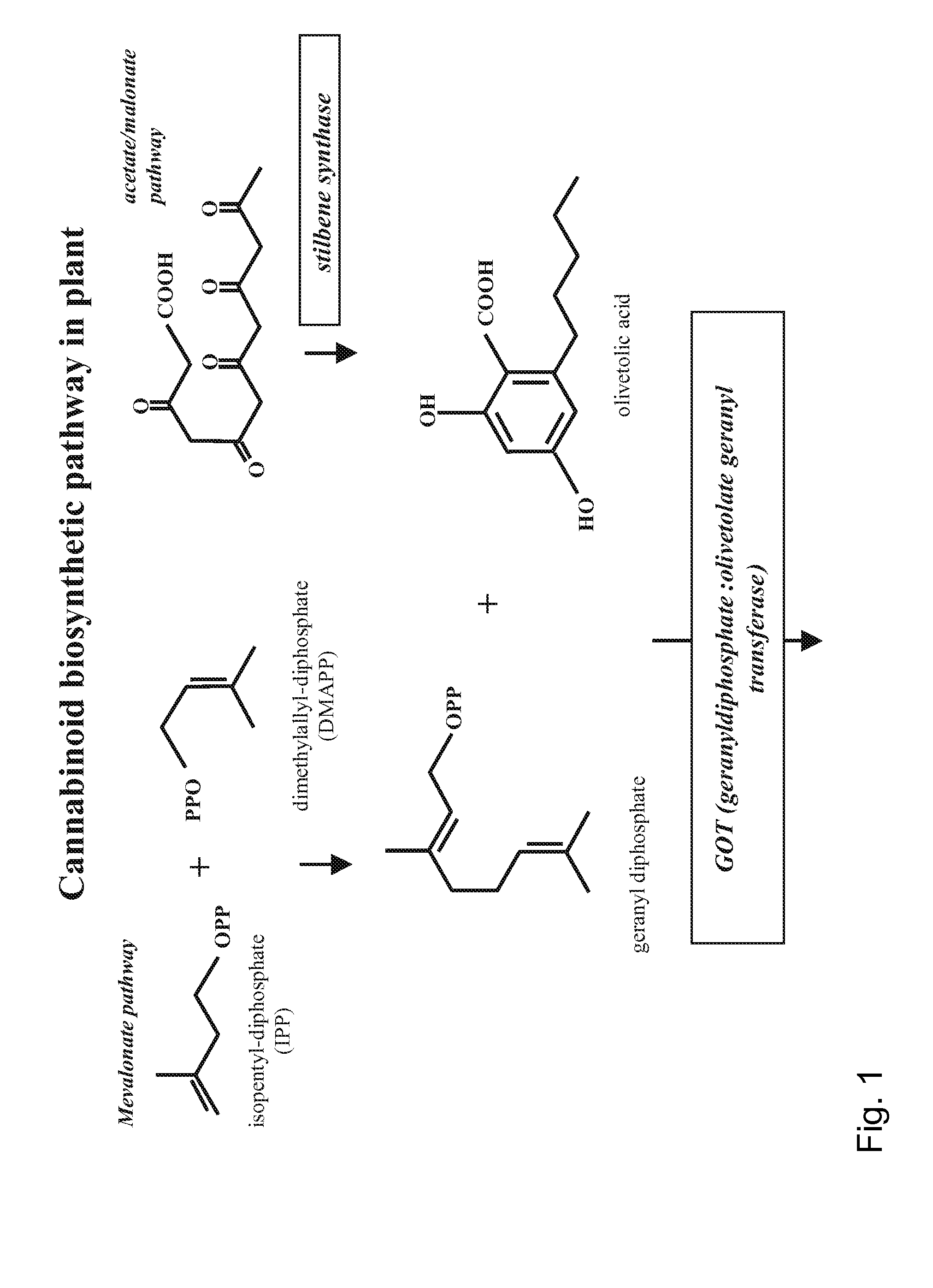
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48 results about "Cannabidiolic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
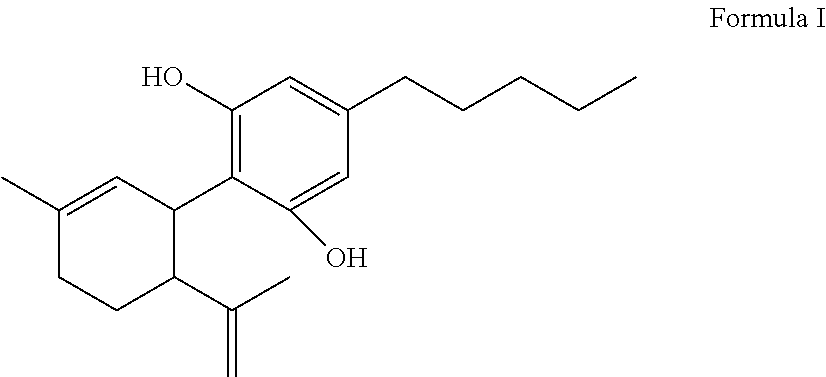
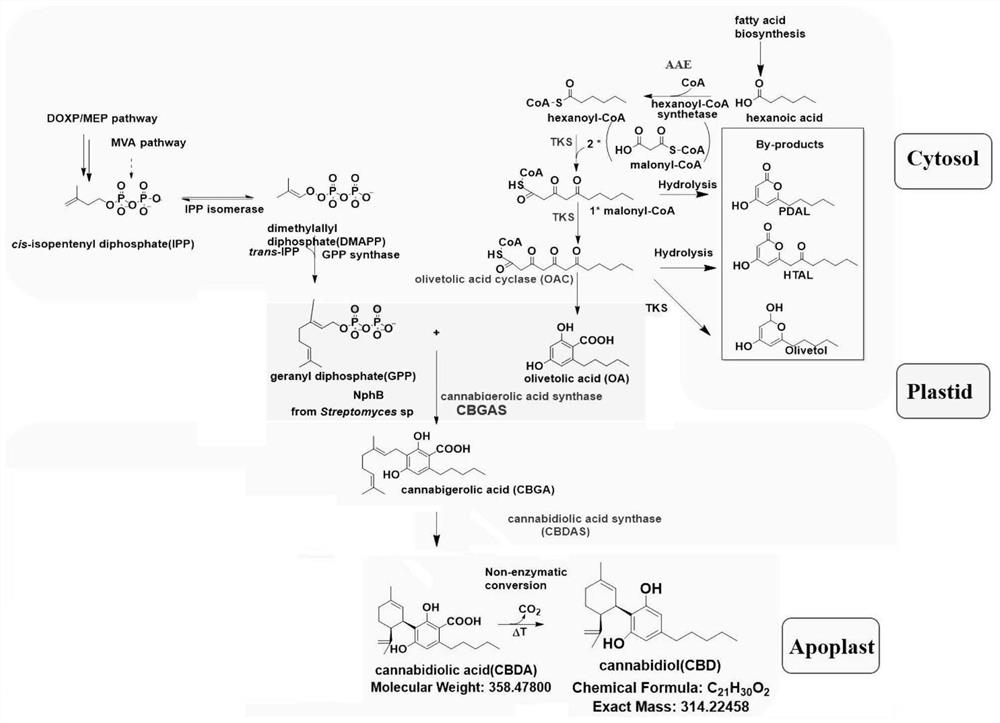
Cannabidiolic acid is a dihydroxybenzoic acid that is olivetolic acid in which the hydrogen at position 3 is substituted by a 3-p-mentha-1,8-dien-3-yl group. It is a phytocannabinoid, a member of resorcinols, a polyketide and a dihydroxybenzoic acid .
Purified cbd and cbda, and methods, compositions and products employing cbd or cbda
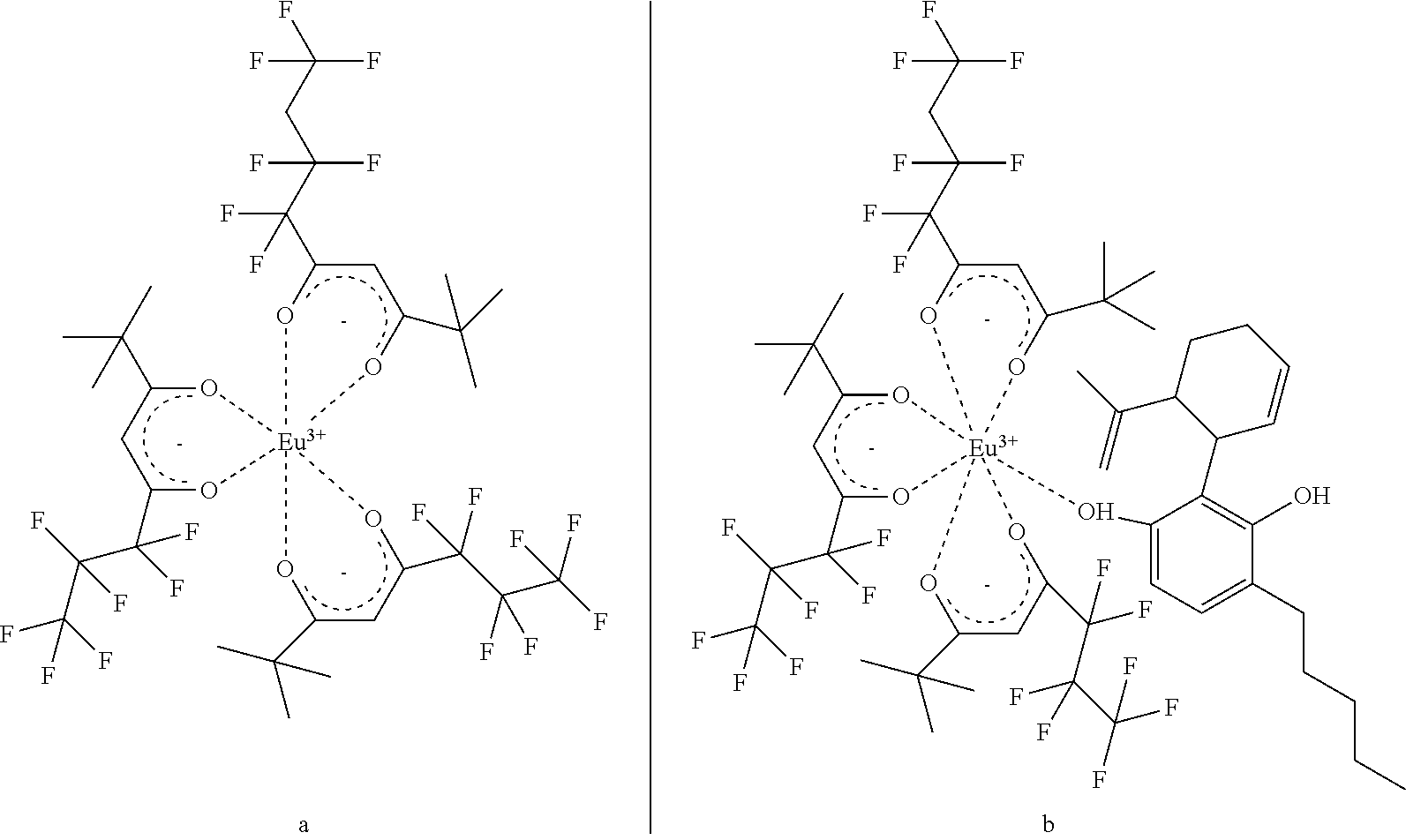
A purified cannabidiol (CBD) extract and / or cannabidiolic acid (CBDA) extract is isolated from industrial hemp and comprises less than 0.5 wt % organic impurities as measured by HPLC and 1H NMR spectroscopy exhibits no detectable peak at 4.07 ppm as measured by 1H NMR spectroscopy. The CBD and / or CBDA extract is in crystalline form. The CBD extract exhibits a melting point as measured by differential scanning calorimetry (DSC) of 69-70° C. Dry powder compositions comprise such extracts. Additional dry powder compositions comprise polyvinylpyrrolidone and an amorphous CBD extract. An adduct comprises CBD and / or CBDA bonded to a paramagnetic trivalent lanthanide (III) metal chelate.
Owner:COLORADO CAN LLC
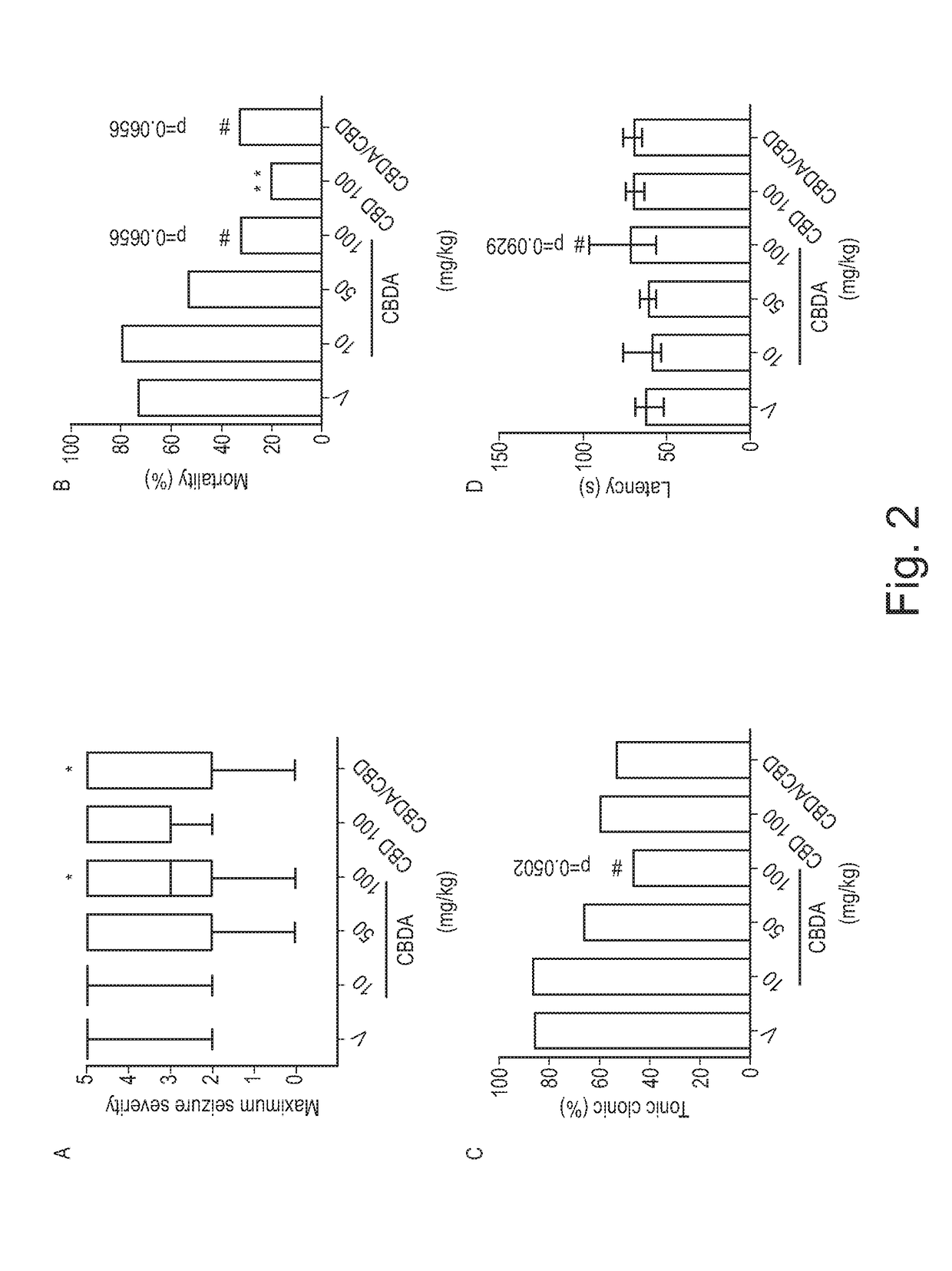
Use of cannabinoids in the treatment of epilepsy
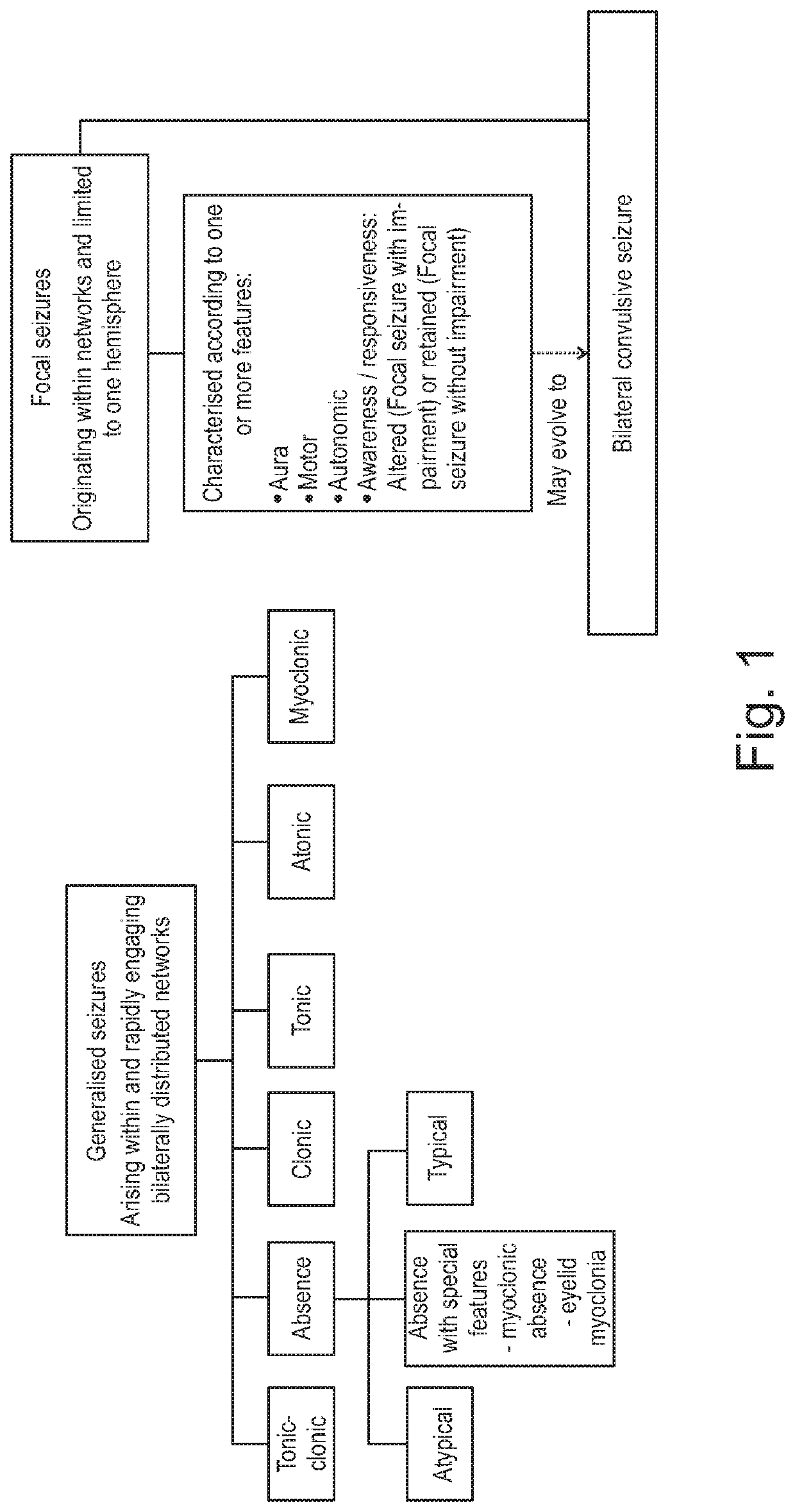
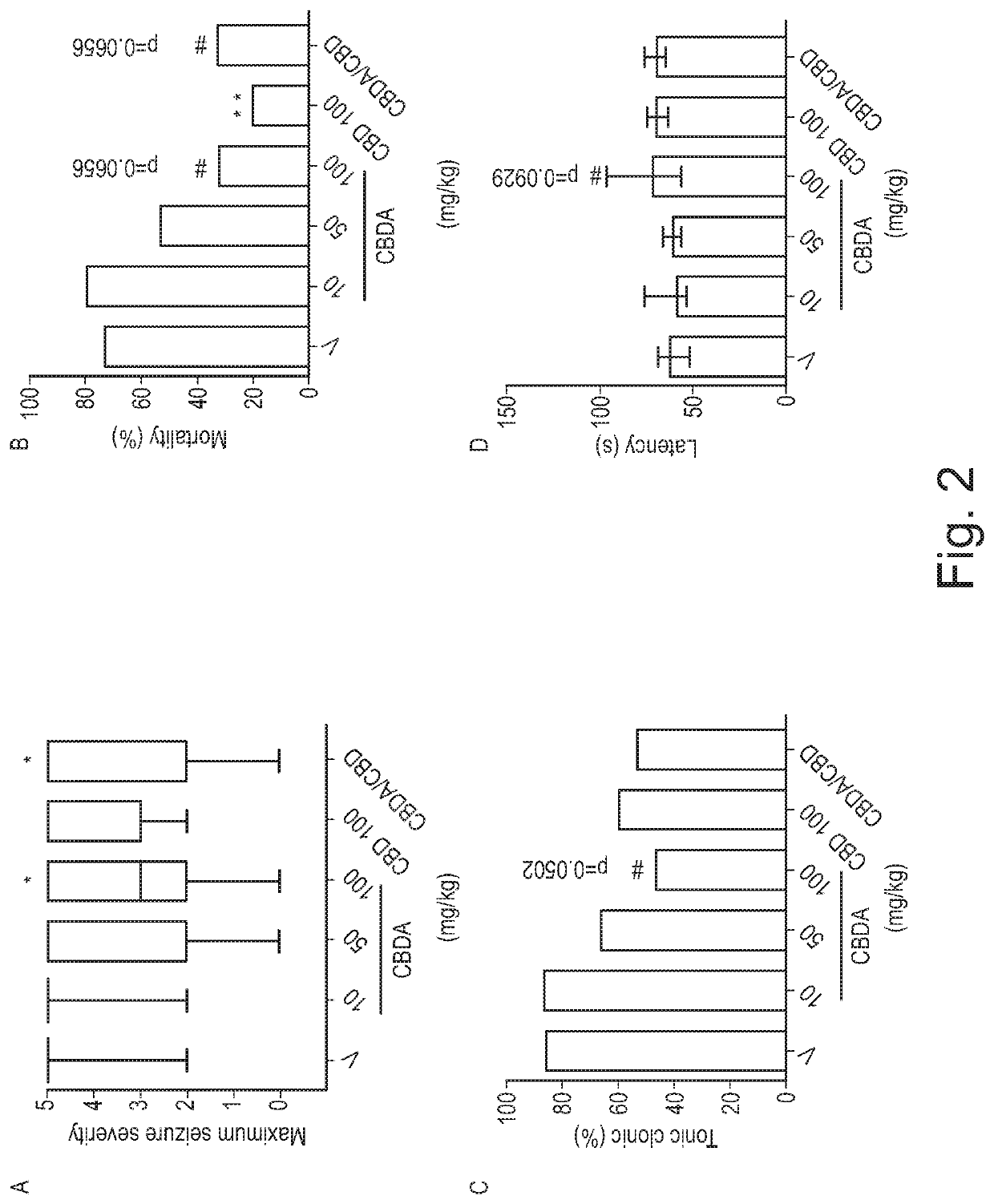
ActiveUS20180228751A1Reduce doseReduced dosNervous disorderHydroxy compound active ingredientsTonic-clonic seizuresEpileptic seizure
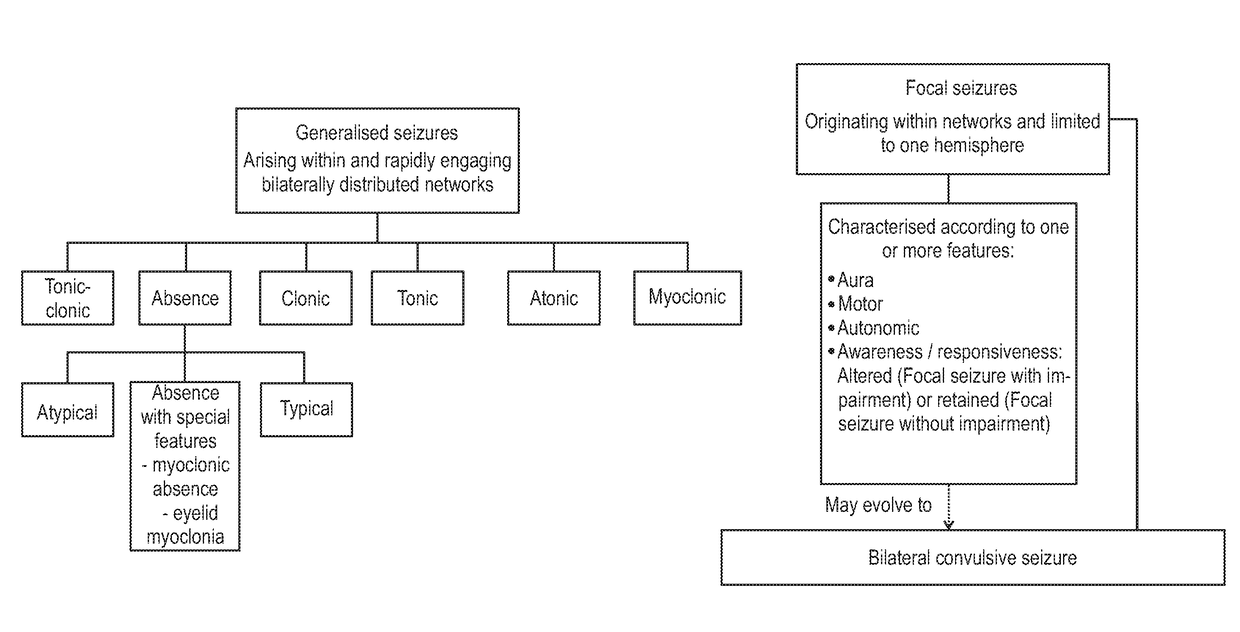
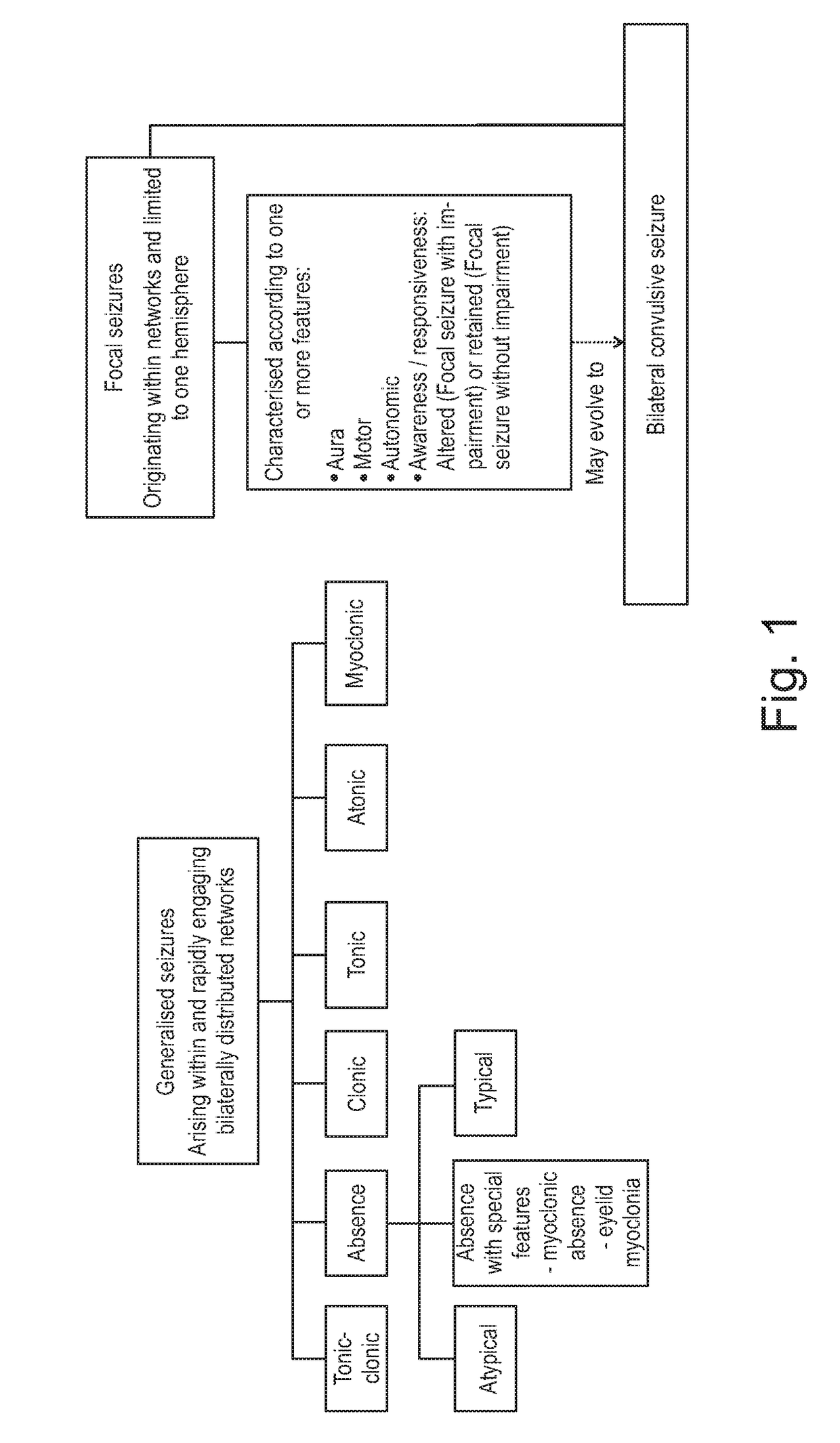
The present invention relates to the use of a therapeutically effective amount of cannabidiolic acid (CBDA) in the treatment of epilepsy. In one embodiment the CBDA is used in the treatment of generalised seizures, preferably tonic-clonic seizures. Preferably the CBDA used is in the form of a botanical drug substance in which the CBDA content is greater than 60%, and most preferably, it is a highly purified extract of cannabis such that the CBDA is present at greater than 95%, through 96% and 97% to most preferably, greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoids tetrahydrocannabinol (THC) or tetrahydrocannabinol acid (THCA) have been substantially removed. Alternatively, the CBDA may be synthetically produced.
Owner:GW RES LTD
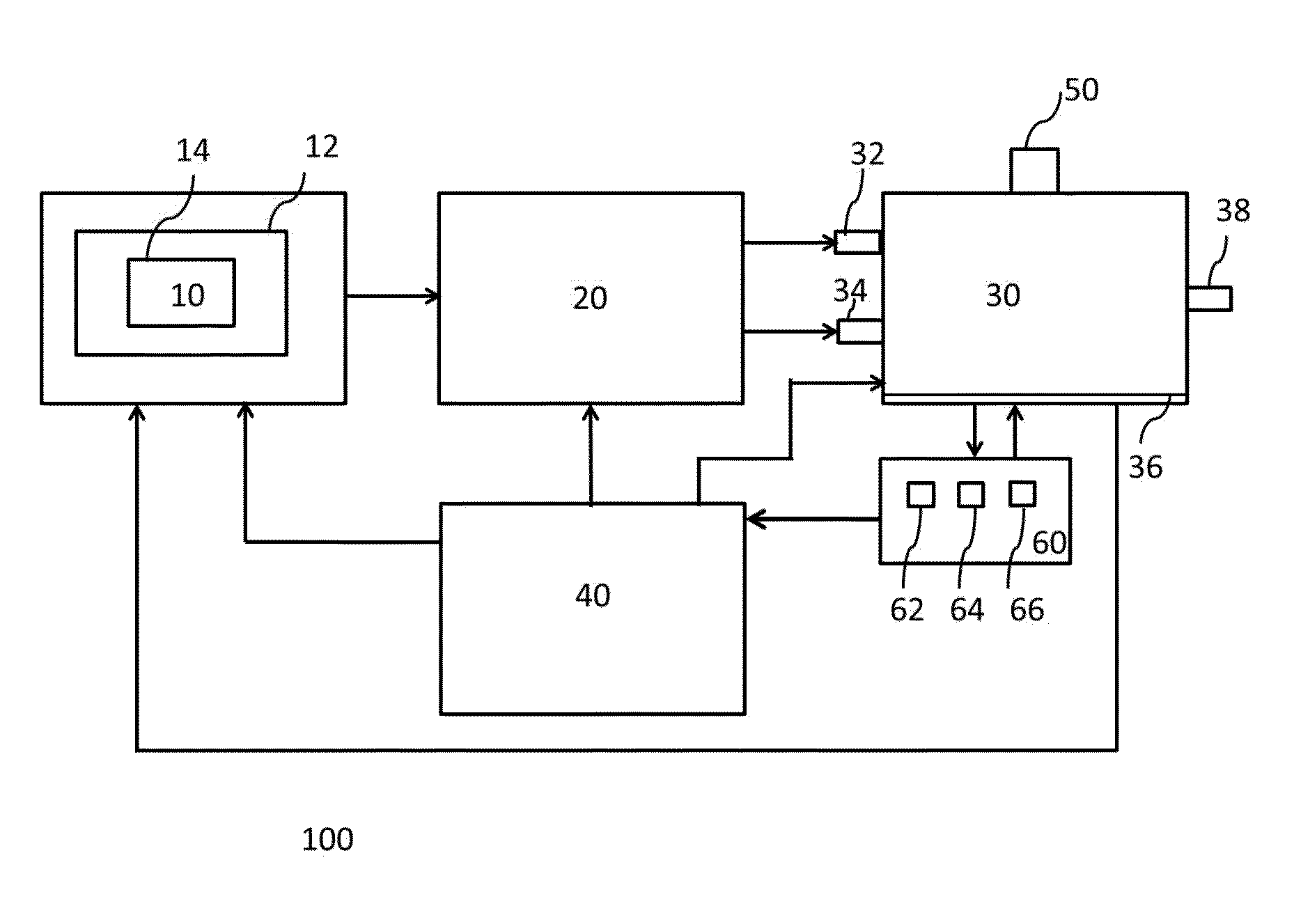
Apparatus and methods for the simultaneous production of compounds
ActiveUS9394510B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCannabielsoinEngineering
The present invention provides an apparatus for simultaneously producing different cannabinoids in different ratios. Specifically, the apparatus according to the invention produces tetrahydrocannabinolic acid (THCA) and cannabichormenic acid (CBCA) or cannabidiolic acid (CBDA) in different ratios, and it comprises a bioreactor that comprises a first automated supply system configured to deliver a substrate and a cannabinoid acid synthase, and a second automated system to cease the reaction; an extractor configured to recover the cannabionoids so produced; and a controller configured to modify at least on property of the reaction.
Owner:TEEWINOT TECH LTD
Parenteral formulations
ActiveUS20190314296A1Improve solubilityLow toxicityHydroxy compound active ingredientsPharmaceutical delivery mechanismSURFACTANT BLENDPharmacology
The present invention relates to parenteral cannabinoid formulations, and more particularly to cannabinoid containing intravenous (IV) formulations. Preferably the parenteral containing formulation comprises a cannabinoid; an isotonic agent; a surfactant; and one or more stability enhancers. Furthermore the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerolpropyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
Oral cannabinoid formulations
ActiveUS20190365667A1Ensure palatabilityEnsure stabilityNervous disorderDispersion deliveryNon ionicSURFACTANT BLEND
The present invention relates to an oral formulation containing one or more cannabinoids. Preferably one or more cannabinoids dissolved in a solvent system consisting essentially of: a non-ionic surfactant and water together with other components which ensure the cannabinoids stability and the formulations palatability. Furthermore, the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerol propyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
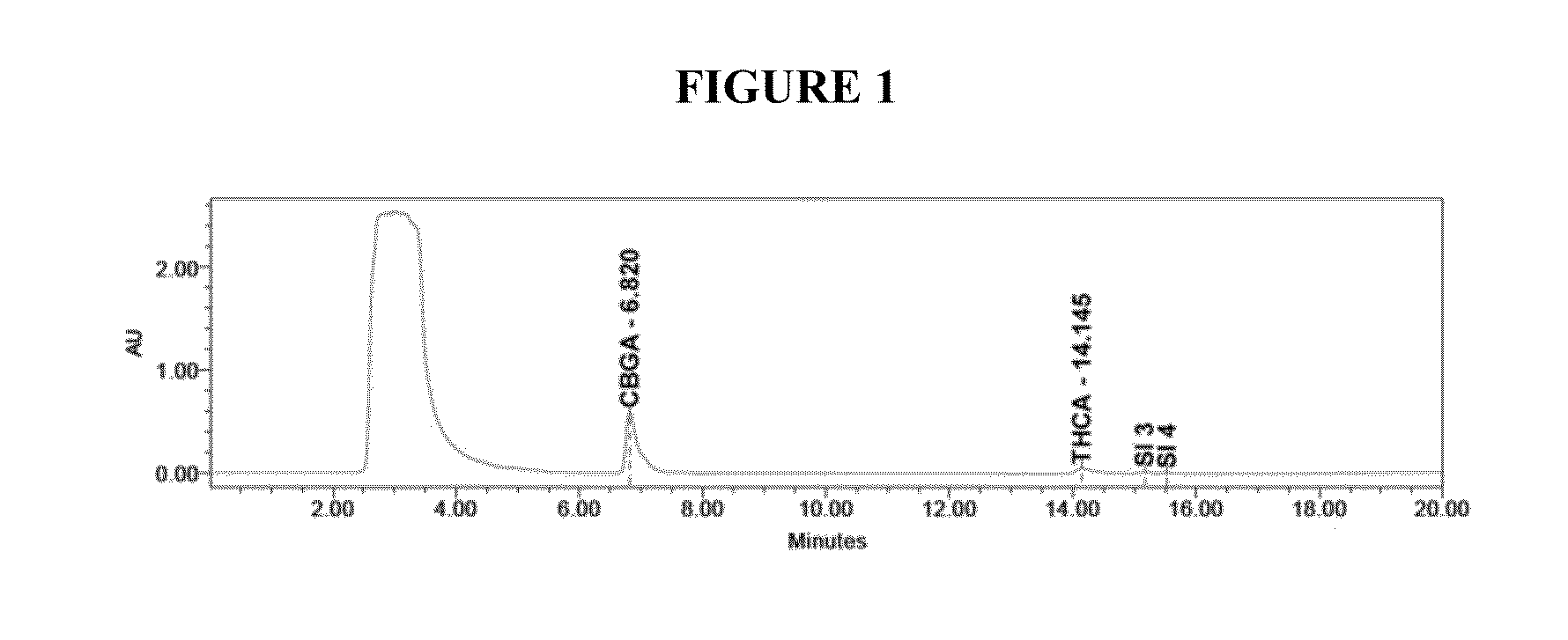
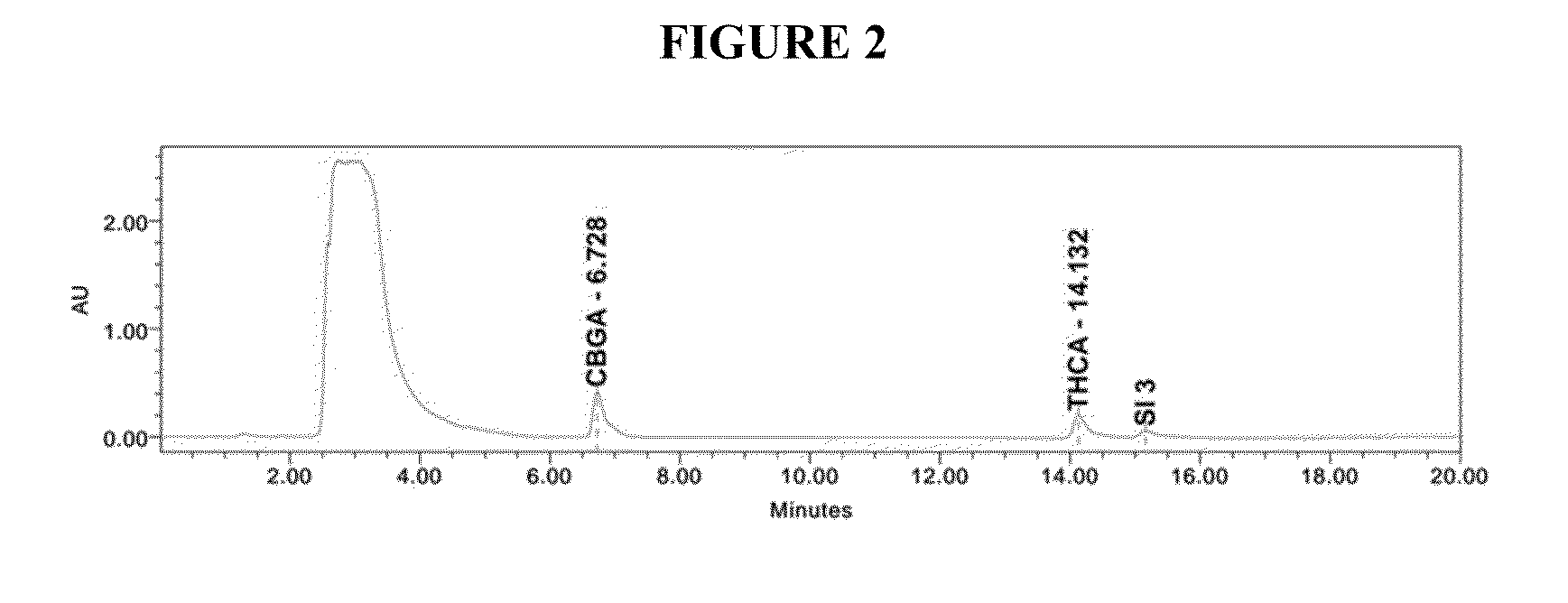
Qualitative and quantitative detection method of one or more of cannabidiol, cannabidiolic acid and tetrahydrocannabinol
InactiveCN109725080AAccurate detectionHigh sensitivityComponent separationTetrahydrocannabinolHplc mass spectrometry
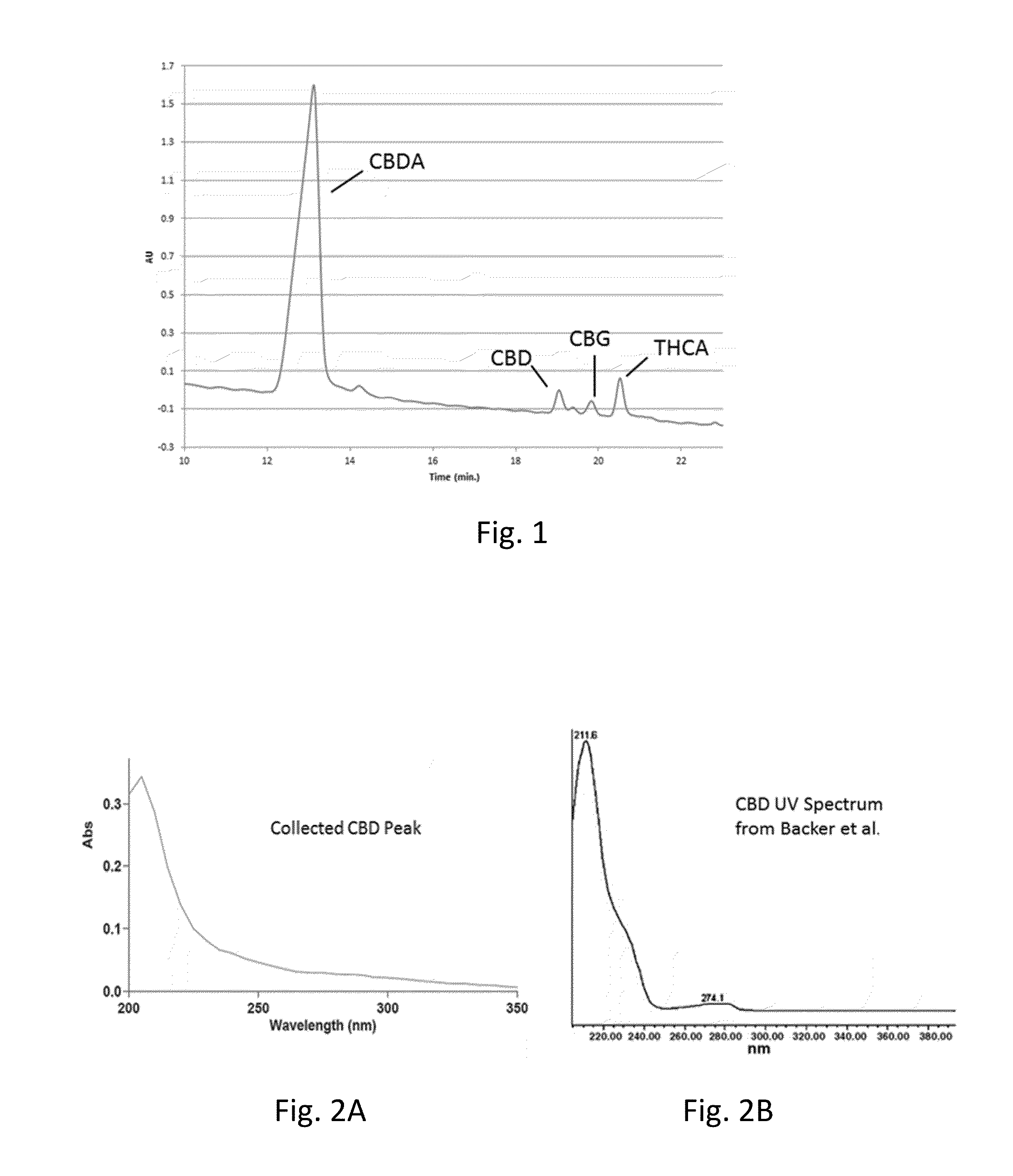
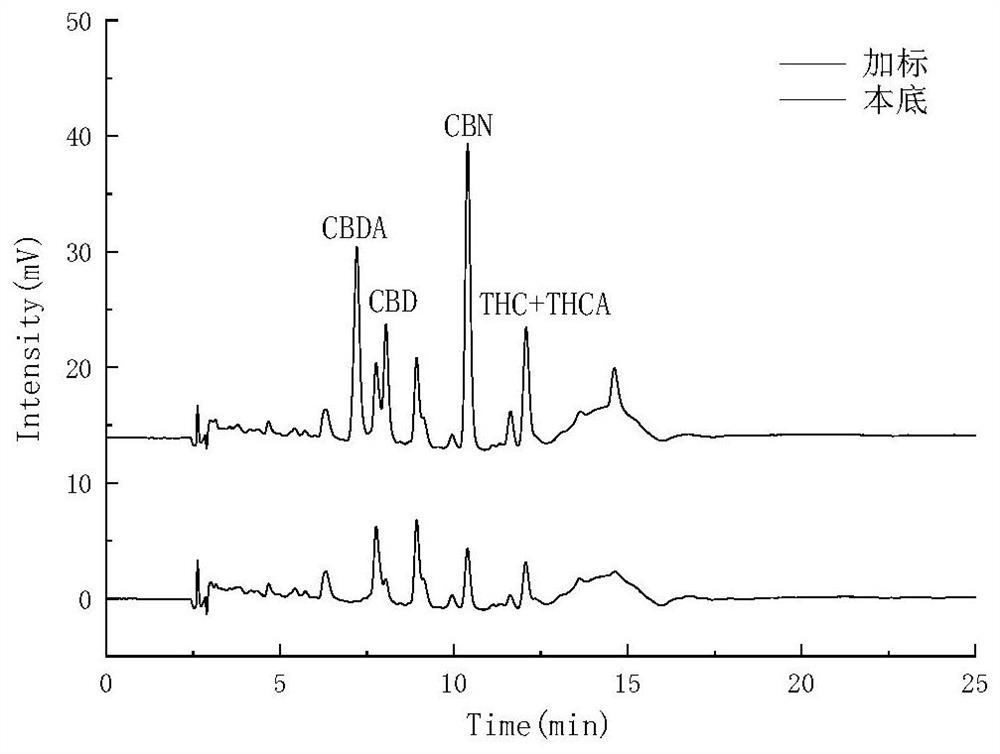
The invention relates to the technical field of detection, in particular to a qualitative and quantitative detection method of one or more of cannabidiol, cannabidiolic acid and tetrahydrocannabinol.The qualitative and quantitative detection method of one or more of the cannabidiol, the cannabidiolic acid and the tetrahydrocannabinol comprises the following steps that: ultrasound extraction is conducted on smashed hemp flower leaves through methyl alcohol, and a test solution is obtained; the test solution is separated and detected through high performance liquid chromatography, a moving phase of the high performance liquid chromatography is a mixture of acetic acid water solution and acetonitrile, and isocratic elution is conducted; and the content of one or more of the cannabidiol, thecannabidiolic acid and the tetrahydrocannabinol are detected through an external standard method. According to the qualitative and quantitative detection method of one or more of the cannabidiol, thecannabidiolic acid and the tetrahydrocannabinol, specific wave length and a moving phase are selected, the peak pattern is good, sensitivity is high, research is conveniently conducted, and qualitative and quantitative treatment of the cannabidiol, the cannabidiolic acid and the tetrahydrocannabinol can be detected quickly and accurately under treatment of the same sample.
Owner:INST OF BAST FIBER CROPS CHINESE ACADEMY OF AGRI SCI +1
The topical composition with active compounds from cannabis sativa and calendula officinalis for reduction of skin lesions
ActiveUS20190111093A1Deep hydrationReduces skin poreCosmetic preparationsOrganic active ingredientsCalendula officinalis extractUltraviolet
Disclosed is a topical composition including essential combination of synergistically acting phyto-active materials, non-psychotropic phytocannabinoids from the plant of Cannabis sativa: Cannabidiol, Cannabidiolic acid, Cannabivarin Cannabigerol in combination with extract of Calendula flower and the formulation of the base to ensure the features of anti-inflammation, anti-oxidation, emollient, and bactericidal components. The topical composition is an emollient dedicated for reduction of skin lesions caused by atopic dermatitis, urticaria, radiotherapy and UV induced skin damage and acne. In addition the topical composition could reduce secretion of fats, facilitate deep skin hydration, reduce pores and exert soothing effect.
Owner:UAB SATIMED
Medicinal Acidic Cannabinoids
InactiveUS20110021617A1Promote resultsReduce excretionBiocideNervous disorderOrganic chemistryMedical treatment
The invention relates to an acidic cannabinoid for medical use and to a cannabis extract comprising an acidic cannabinoid. The extract may comprise one or more compounds selected from the group consisting of cannabidiolic acid (CBD-A), cannabidiol (CBD), cannabigerolic acid (CBGA), cannabigerol (CBG), cannabinolic acid (CBN-A) and cannabinol. The invention further relates to a method for preparing a preparation comprising extracting an acidic cannabinoid from cannabis.
Owner:NEDERLANDSE ORG VOOR TOEGEPAST NATUURWETENSCHAPPELIJK ONDERZOEK TNO
Use of cannabinoids in the treatment of inflammatory skin diseases
ActiveUS20180263952A1Cosmetic preparationsHydroxy compound active ingredientsInflammatory skin diseaseCannabidiolic acid
The present invention relates to the use of one or more cannabinoids in the treatment of an inflammatory skin disease. Preferably the one or more cannabinoids are taken from the group consisting of: cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigervarin (CBGV) and tetrahydrocannabivarin (THCV). The inflammatory skin disease may be caused by one or more of the following: microbial infection-induced dermatitis; solar dermatitis; atopic dermatitis; and allergic contact dermatitis.
Owner:GW PHARMA LTD
Use of cannabinoids in the treatment of epilepsy
ActiveUS11147783B2Improve bioavailabilityAct more quicklyNervous disorderHydroxy compound active ingredientsClonic seizureCannabielsoin
The present invention relates to the use of a therapeutically effective amount of cannabidiolic acid (CBDA) in the treatment of epilepsy. In one embodiment the CBDA is used in the treatment of generalised seizures, preferably tonic-clonic seizures. Preferably the CBDA used is in the form of a botanical drug substance in which the CBDA content is greater than 60%, and most preferably, it is a highly purified extract of cannabis such that the CBDA is present at greater than 95%, through 96% and 97% to most preferably, greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoids tetrahydrocannabinol (THC) or tetrahydrocannabinol acid (THCA) have been substantially removed. Alternatively, the CBDA may be synthetically produced.
Owner:GW RES LTD
Pet food including cannabidiolic acid
ActiveUS20190091144A1Assure food safetyImprove stabilityHydroxy compound active ingredientsFrozen sweetsAntioxidantHash oil
A pet food product includes a food substrate manufactured by extrusion, baking or other process involving heat. The pet food product includes less than 10% of hemp oil by weight, and preferably less than 5% by weight. The hemp oil has cannabinoids, including acidiccannabinoids. The acidic cannabinoids have a concentration of less than 100 parts per million, and preferably between 10-40 parts per million in the hemp oil. The acidic cannabinoids include cannabidiolic acid (CBD-A) and cannabidiol (CBD) in a ratio of at least 1:1, and preferably 3:1, or greater. An antioxidant is added to the hemp oil to inhibit oxidation of the acidiccannabinoids including the (CBD-A). The pet food product includes a second oil blended with the hemp oil. The antioxidant and the second oil cooperate to inhibit oxidation of the acidic cannabinoids.
Owner:HIGH PLAINS NUTRITION LLC
Cannabis sativa plants rich in cannabichromene and its acid, extracts thereof and methods of obtaining extracts therefrom
InactiveUS20160360721A1Flowers cultivationOrganic active ingredientsCannabis sativa plantCannabichromene
The present invention relates to plants producing, as their major cannabinoid cannabichromenic acid (CBCA) or its neutral (decarboxylated) form cannabichromene (CBC), hereafter jointly referred to as CBC(A). It additionally relates to; a botanical material obtainable from said plants; a botanical raw material (BRM), an extract including a botanical drug substance (BDS) and a purified BDS; a formulation comprising the BRM, BDS, purified BDS or other extract; the use of the BRM, BDS, purified BDS or other extract in the manufacture of a medicament; a method of deriving plants yielding a high proportion of the in cannabinoid CBC(A) at the expense of other cannabinoids; a method of cultivating plants such that they yield a high proportion of the cannabinoid CBC(A) at the expense of other cannabinoids; and a method of extracting CBC(A) from said plants.
Owner:GW PHARMA LTD
Parenteral formulations
ActiveUS11229612B2Hydroxy compound active ingredientsPharmaceutical delivery mechanismActive agentSurface-active agents
The present invention relates to parenteral cannabinoid formulations, and more particularly to cannabinoid containing intravenous (IV) formulations. Preferably the parenteral containing formulation comprises a cannabinoid; an isotonic agent; a surfactant; and one or more stability enhancers. Furthermore the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerolpropyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
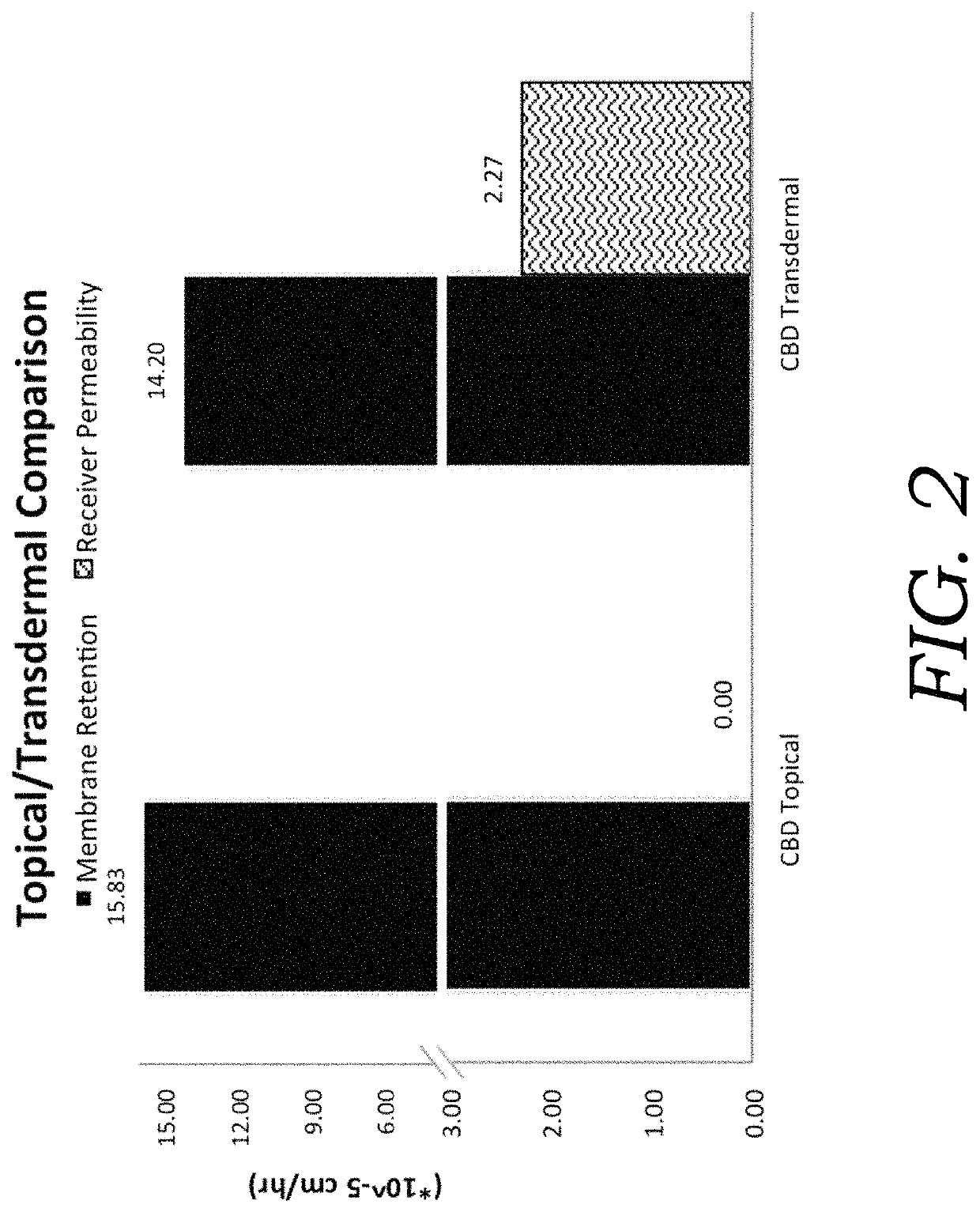
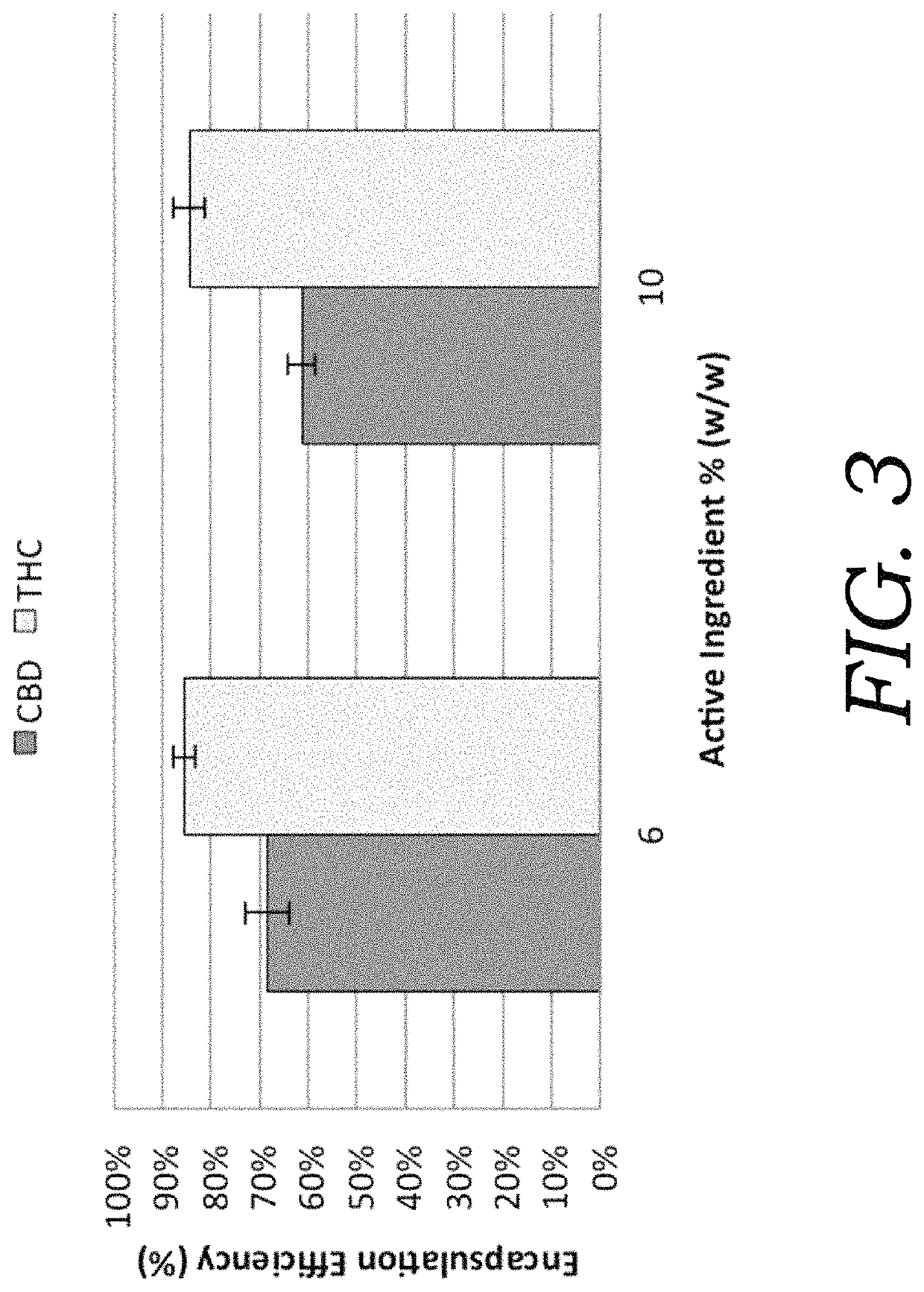
Encapsulated cannabinoid formulations for transdermal delivery
ActiveUS20190216870A1Improve bioavailabilityImprove emulsion stabilityPowder deliveryFood ingredient as thickening agentBioavailabilityCannabichromene
Preparation of cannabinoid formulations containing: Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), Δ9-tetrahydrocannabinolic acid (THCa), cannabidiol (CBD), cannabidiolic acid (CBDa), cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), either alone or in combinations henceforth known as cannabis, have been created using an emulsification process to encapsulate cannabinoids. The aqueous-based method involves micellular encapsulation of cannabinoids, a method that has been used to increase the bioavailability of poorly permeable, lipophilic drugs. The present invention demonstrates the viability of transdermal delivery with gels and patches for consistent and sustained cannabinoid dosing.
Owner:NUTRAE LLC
Compositions and methods for delivering cannabidiol and ketone bodies
ActiveUS10512615B1Shorten the durationReduce severityHydroxy compound active ingredientsPharmaceutical non-active ingredientsAcetoacetatesMammal
Disclosed herein are “ketonnabidiol” compositions including a combination of: (1) cannabidiol (CBD) and / or cannabidiolic acid (CBDA); (2) a ketone body component such as beta-hydroxybutyrate (BHB) and / or acetoacetate; and (3) a dietetically or pharmaceutically acceptable carrier. Also disclosed herein are methods of using such ketonnabidiol compositions for producing desired physiological effects, such as fat loss, in a mammal. The ketonnabidiol compositions beneficially enhance fat loss through ketosis while also reducing the duration and / or severity of unpleasant “keto flu” symptoms typically associated with ketosis.
Owner:AXCESS GLOBAL SCI LLC
Method for detecting five cannabinol compounds in cannabis oil by utilizing HPLC method
PendingCN112730696ASimple and fast operationEfficient separationComponent separationHplc methodGradient elution
The invention belongs to the field of analytical chemistry, and relates to a method for detecting five cannabinol compounds in cannabis oil by high performance liquid chromatography (HPLC). According to the method, a C18 chromatographic column is adopted, ultrapure water is used as a mobile phase A, acetonitrile is used as a mobile phase B for gradient elution, and a sample enters a detector for detection. The cannabinol compounds are delta 9-tetrahydrocannabinol, cannabidiol, cannabinol, tetrahydrocannabinolic acid and cannabidiolic acid. The technical scheme provided by the invention can lay a methodological foundation for the establishment of related detection method standards, and has important meanings for preventing cannabis oil with overproof cannabinol content from flowing into the market and promoting the effective supervision of industrial cannabis food at home and abroad.
Owner:CHINA NAT INST OF STANDARDIZATION
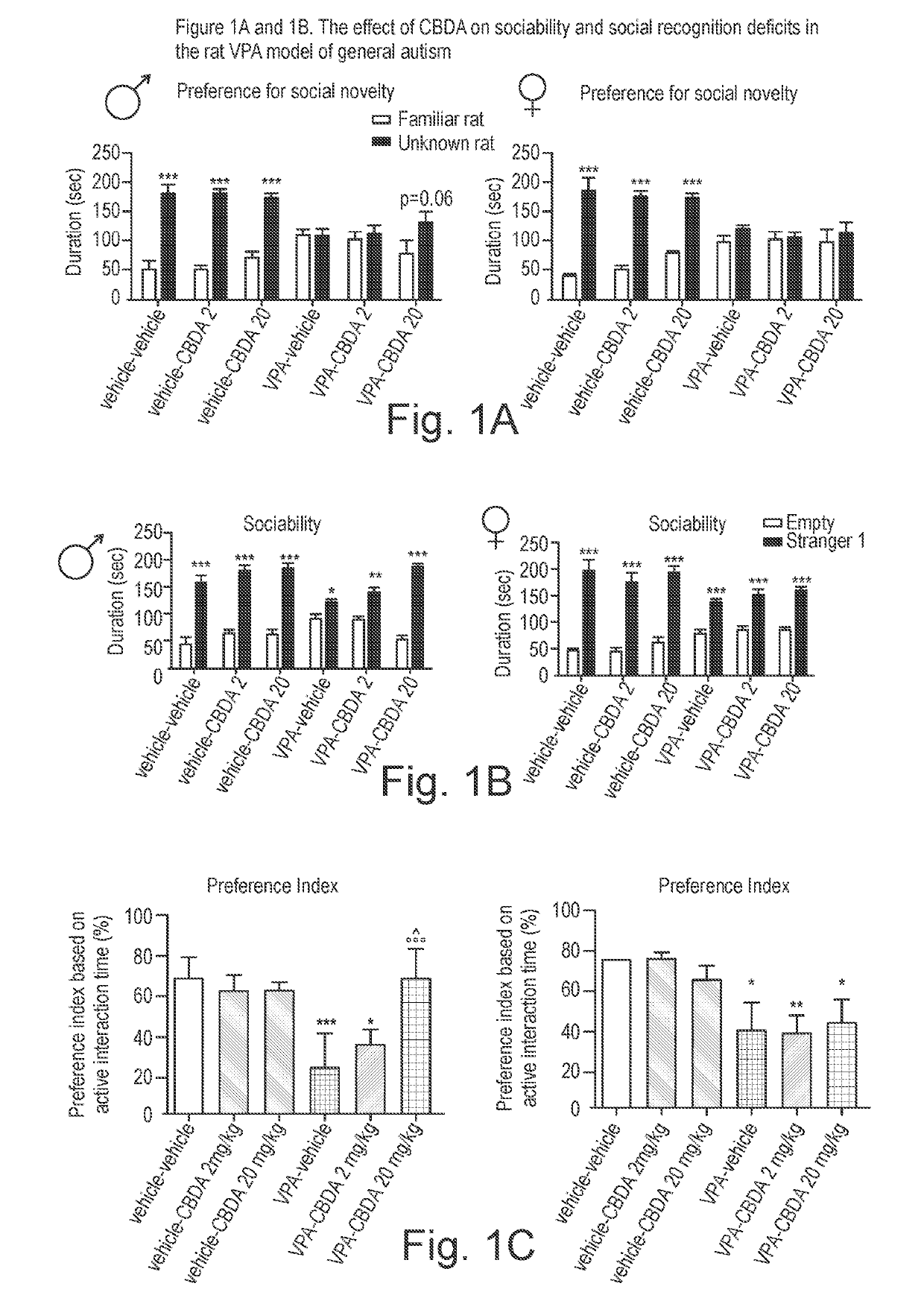
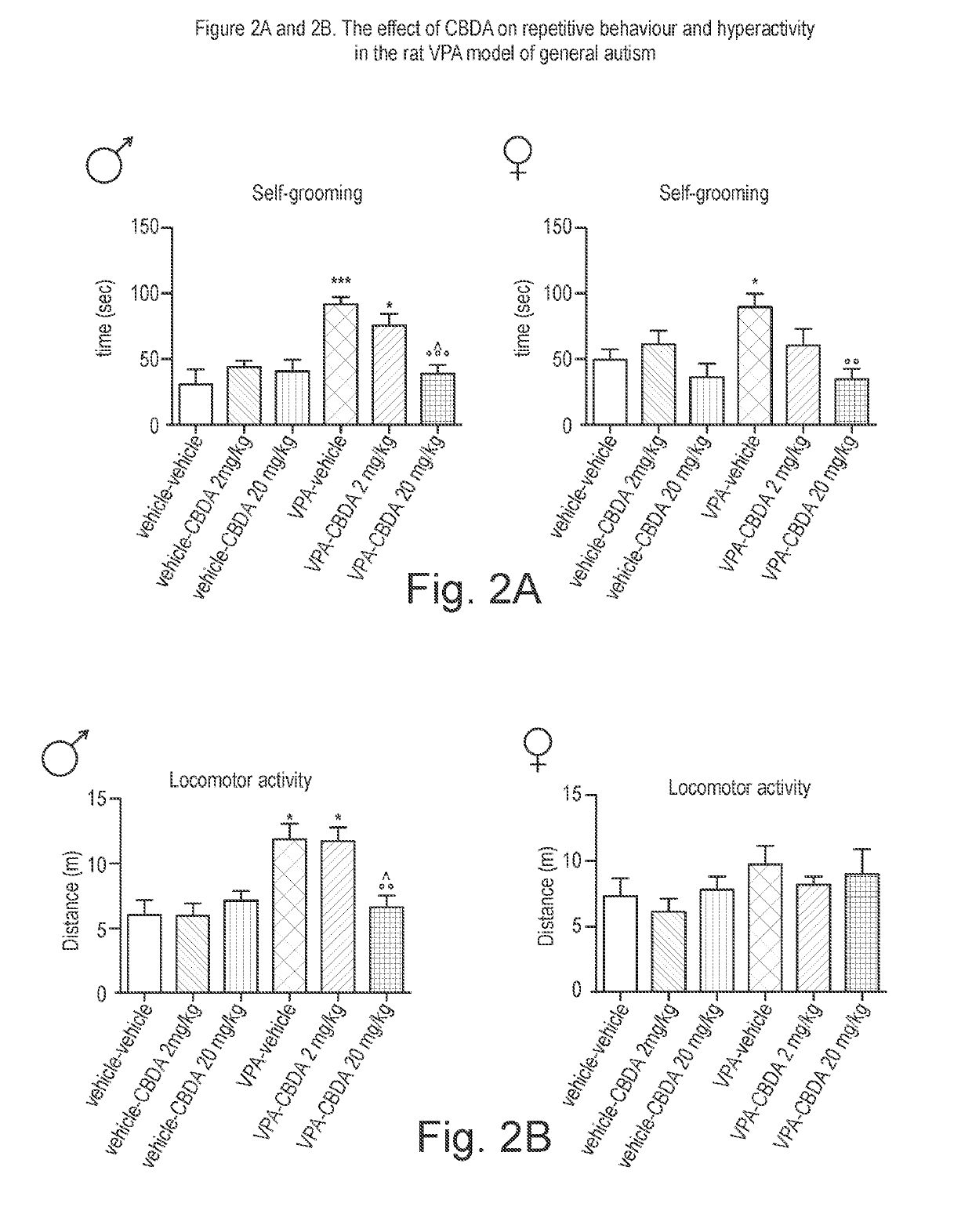
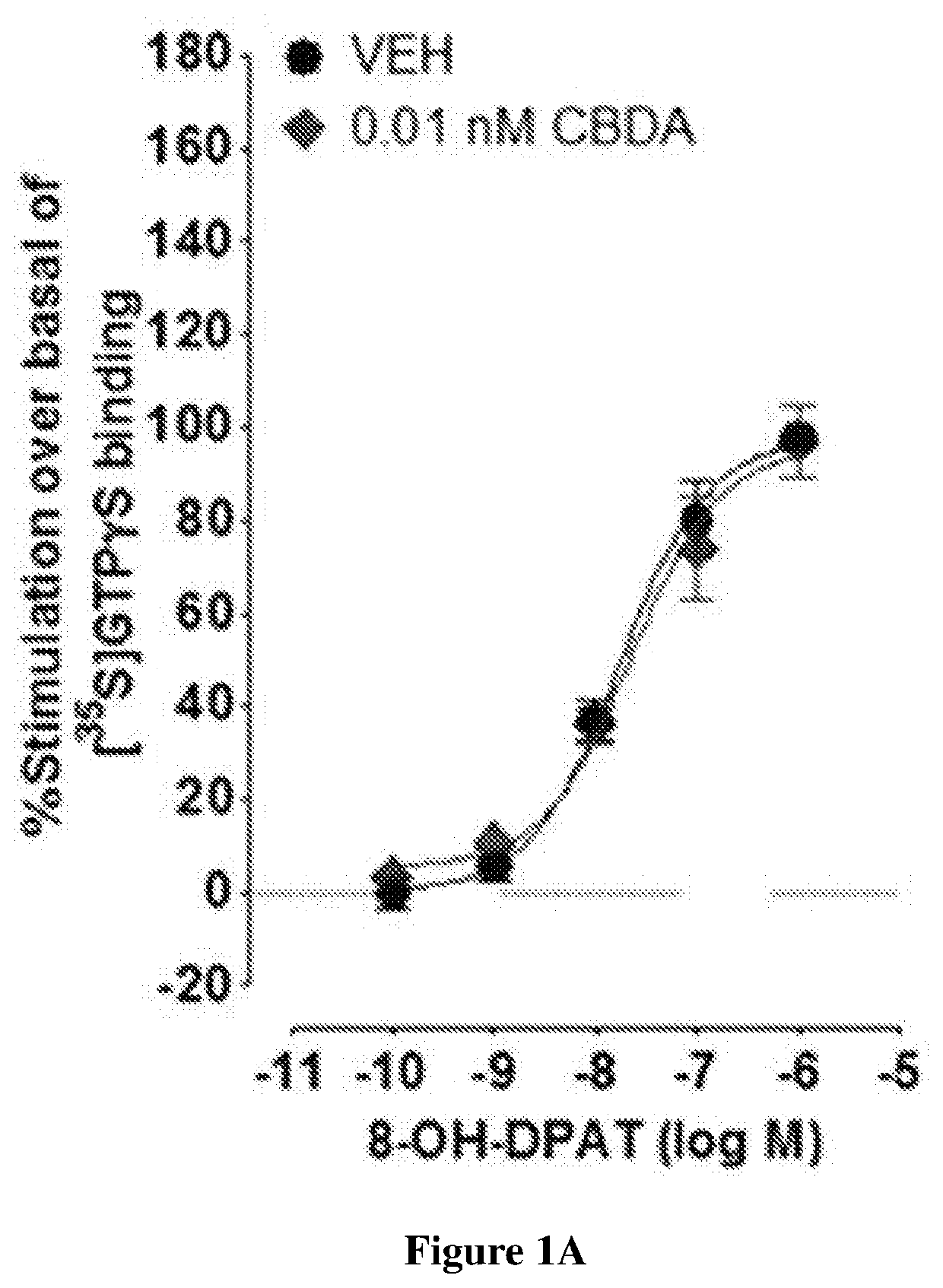
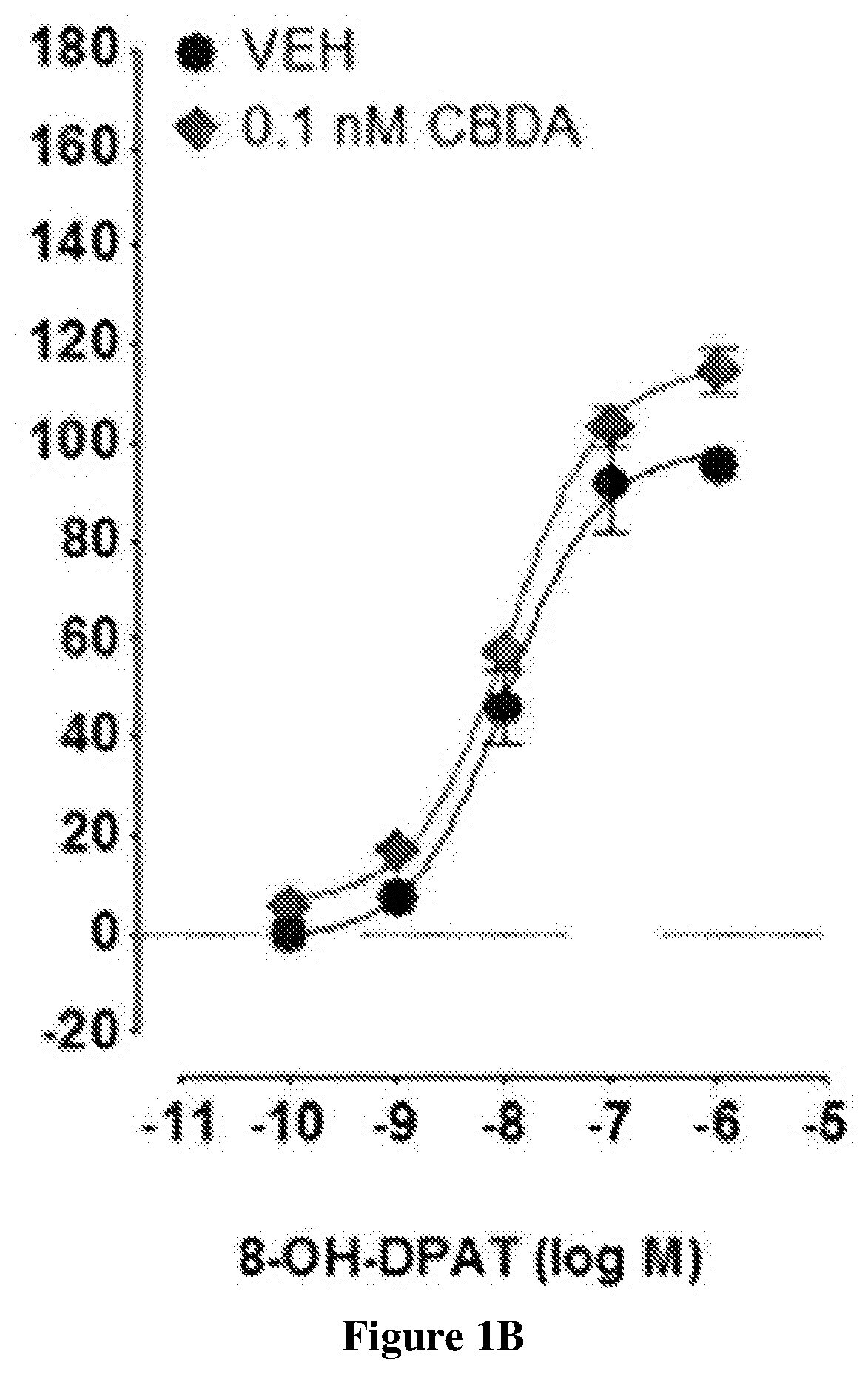
Use of cannabidiolic acid in the treatment of autism spectrum disorder and associated disorders
ActiveUS20190117619A1Symptoms improvedNervous disorderHydroxy compound active ingredientsDiseaseRodent model
The present invention relates to the use of cannabidiolic acid (CBDA) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders, such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). CBDA has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS and AS. The CBDA is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDA is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDA is synthetically produced.
Owner:GW RES LTD
Cannabidiolic acid esters compositions and uses thereof
Cannabidiolic acid esters, compositions comprising them and uses thereof in the treatment of various diseases, conditions and symptoms.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Method for simultaneously detecting five cannabinol compounds by using HPLC-MS/MS
PendingCN112730697AReduce difficultyHigh detection sensitivityComponent separationCannabielsoinGradient elution
The invention belongs to the field of analytical chemistry, and particularly relates to a method for simultaneously detecting five cannabinol compounds by using a high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). According to the method, a special chromatographic column for cannabinol is adopted, a formic acid aqueous solution is used as a mobile phase A, acetonitrile is used as a mobile phase B for gradient elution, and a sample enters a detector for detection. The cannabinol compound is tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), cannabinol (CBN), cannabidiol (CBD) and delta9-tetrahydrocannabinol (THC). The method is high in detection sensitivity, good in recovery rate and easy to operate.
Owner:CHINA NAT INST OF STANDARDIZATION
Pharmacological composition for preventing and treating epilepsy and preparation method thereof
ActiveCN108743571AReduce dosageEnhance the efficacy of antiepileptic drugsNervous disorderHydroxy compound active ingredientsAntiepileptic drugAdemetionine
The invention belongs to the technical field of medicine and specifically relates to pharmacological composition for preventing and treating epilepsy. The pharmacological composition is mainly prepared from the following raw materials in weight percentage: 55.00 to 75.00% of cannabidiol, 1.00 to 2.50% of cannabinol, 0.01 to 2.00% of cannabigerol, 0.10 to 5.00% of cannabidiolic acid and the balanceof hemp seed oil. A preparation method of the pharmacological composition comprises the steps: weighing all the raw materials according to the weight percentage and evenly mixing to obtain the pharmacological composition. The pharmacological composition disclosed by the invention has the advantages of strong antiepileptic pharmacological function and high safety.
Owner:YUNNAN HEMPSON BIO-TECH CO LTD
Oleo gel composition and delivery system with active compounds from cannabis sativa and mentha arvensis for reduction of inflammation and pain in deep tissues
ActiveUS10918686B2Reduce impactOrganic active ingredientsNervous disorderArthritis osteoarthritisPhenolic acid
Disclosed is an oleo gel composition including essential combination of synergistically acting phyto-active materials, non-psychotropic phytocannabinoids from the plant of Cannabis sativa: Cannabidiol, Cannabidiolic acid, Cannabivarin and Cannabigerol in combination with extract of Olive europaea Fruit Oil, Mentha arvensis leaf oil, and Silica colloidal anhydrous ensuring the delivery of cannabinoids to the deep tissues in order to reduce pain and inflammation of skeletal muscles and joints caused by trauma or / and induced by arthritis / osteoarthritis.
Owner:UAB SATIMED
Encapsulated cannabinoid formulations for oral delivery
ActiveUS10709747B2Improve bioavailabilityImprove emulsion stabilityPowder deliveryFood ingredient as thickening agentCannabichromenePharmaceutical Substances
Preparation of cannabinoid formulations containing: Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), Δ9-tetrahydrocannabinolic acid (THCa), cannabidiol (CBD), cannabidiolic acid (CBDa), cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), either alone or in combinations henceforth known as Cannabis, have been created using an emulsification process to encapsulate cannabinoids. The aqueous-based method involves micellular encapsulation of cannabinoids, a method that has been used to increase the bioavailability of poorly permeable, lipophilic drugs. These preparations demonstrates the viability of sublingual, buccal, or oral delivery using an aqueous-based encapsulation method, including as a beverage or drink.
Owner:NUTRAE LLC
Method for extracting cannabidiol acid from cannabis sativa
PendingCN114213237AQuick extractionHigh extraction rateNervous disorderAntipyreticOrganic chemistryMedicinal chemistry
The invention relates to a method for extracting cannabidiol acid, in particular to a method for extracting cannabidiol acid from cannabis sativa. The invention provides a method for extracting cannabidiol acid from cannabis sativa. The method comprises the following steps: carrying out supercritical CO2 extraction by using a supercritical extraction instrument by taking fresh whole cannabis sativa as a raw material, supercritical CO2 as a solvent and an alcoholic solution as an entrainer. The method has the advantages of simplicity in operation, short time consumption, high extraction rate of cannabidiol acid and few impurities. The method is mainly used for extracting cannabidiol acid from cannabis sativa.
Owner:哈尔滨市汉博生物科技有限公司
Encapsulated cannabinoid formulations for oral delivery
ActiveUS20190216869A1Improve bioavailabilitySmall particle sizePowder deliveryFood ingredient as thickening agentBioavailabilityCannabichromene
Preparation of cannabinoid formulations containing: Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), Δ9-tetrahydrocannabinolic acid (THCa), cannabidiol (CBD), cannabidiolic acid (CBDa), cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), either alone or in combinations henceforth known as Cannabis, have been created using an emulsification process to encapsulate cannabinoids. The aqueous-based method involves micellular encapsulation of cannabinoids, a method that has been used to increase the bioavailability of poorly permeable, lipophilic drugs. These preparations demonstrates the viability of sublingual, buccal, or oral delivery using an aqueous-based encapsulation method, including as a beverage or drink.
Owner:NUTRAE LLC
Encapsulated cannabinoid formulations for transdermal delivery
ActiveUS10709748B2Improve bioavailabilityImprove emulsion stabilityPowder deliveryFood ingredient as thickening agentBioavailabilityCannabichromene
Preparation of cannabinoid formulations containing: Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-tetrahydrocannabinol (Δ8-THC), Δ9-tetrahydrocannabinolic acid (THCa), cannabidiol (CBD), cannabidiolic acid (CBDa), cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), either alone or in combinations henceforth known as cannabis, have been created using an emulsification process to encapsulate cannabinoids. The aqueous-based method involves micellular encapsulation of cannabinoids, a method that has been used to increase the bioavailability of poorly permeable, lipophilic drugs. The present invention demonstrates the viability of transdermal delivery with gels and patches for consistent and sustained cannabinoid dosing.
Owner:NUTRAE LLC
Expression vector for synthesizing cannabidiol, heterologous expression method and application
InactiveCN113416748AEfficient productionReduce planting costsCarbon-sulfur lyasesBacteriaHeterologousSynthetic biology
The invention discloses an expression vector for synthesizing cannabidiol, a heterologous expression method and application, five gene expression cassette vectors are constructed through an in-vitro de novo design and five enzymes of hexanoyl-CoA synthase, polyketide synthase, oleinic acid synthase, isopentenyl transferase and cannabidiol acid synthase, and a transient co-transformation experiment in tobacco shows that in this way, high yield of CBD can be achieved. According to the invention, efficient production of CBD can be realized by using a synthetic biological technical means, the synthetic product does not contain THC, and the measured CBD content is high; in addition, as a conventional commercial crop for agricultural production, the tobacco has advantages in planting technology, field management, yield of harvested leaves and the like, and planting cost can be greatly reduced.
Owner:湖北碳元本草生物科技有限公司
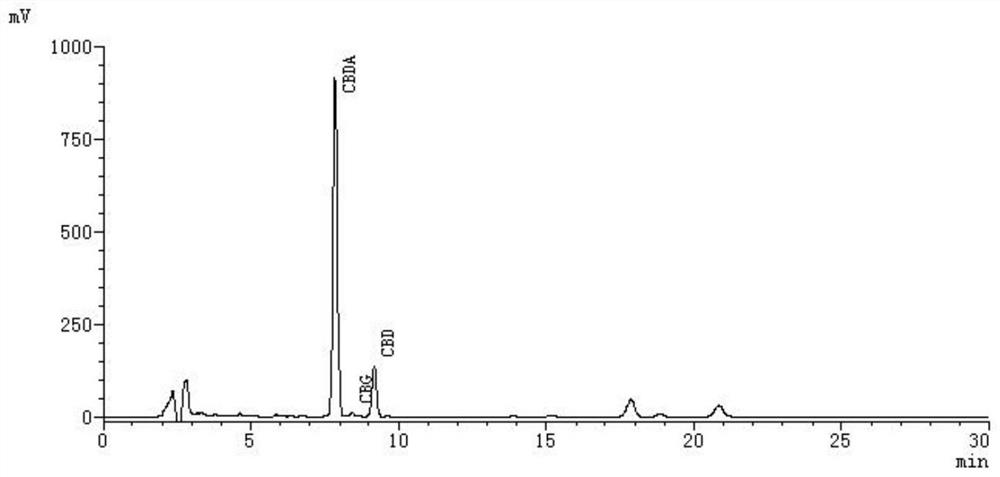
Method for preparing cannabidiol by decarboxylation of cannabidiolic acid in industrial hemp
PendingCN112266321AShorten the timeImprove efficiencyOrganic compound preparationCarboxylic compound separation/purificationCannabidiolic acidEnvironmental chemistry
The invention discloses a preparation method for decarboxylating cannabidiolic acid in industrial hemp, belongs to the technical field of plant component extraction and purification, and particularlyrelates to a pretreatment process method for preparing cannabidiol by decarboxylating cannabidiolic acid. A microwave radiation method is used for converting cannabidiolic acid into cannabidiol through cannabidiol acid decarboxylation. The optimal decarboxylation conditions are as follows: the microwave radiation temperature is 60 DEG C, the microwave power is 600W, the microwave radiation time is30 minutes. The decarboxylation rate of the cannabidiol acid is 98 percent or more through liquid chromatography analysis, the conversion rate of the cannabidiolic acid into the cannabidiol is 99 percent, and the cannabidiol structure is analyzed through mass spectrometry. The microwave radiation method is adopted for cannabidiolic acid decarboxylation, cannabidiol can be simply and efficiently obtained through convertion, compared with a heating decarboxylation method, the method has the advantages of being short in time, high in efficiency, low in energy consumption, small in environmentalpollution and the like, and the method is suitable for raw material pretreatment in the pharmaceutical field and industrial production.
Owner:DAQING BRANCH OF HEILONGJIANG ACAD OF SCI
Extraction method of cannabidiol
PendingCN113880697AFew stepsHigh purityOrganic chemistryOrganic compound preparationCannabielsoinOrganosolv
An extraction and purification refining method of cannabidiol belongs to the technical field of cannabidiol extraction. The method specifically comprises the following steps of: adding China-hemp flowers and leaves into water, heating to 30-70 DEG C, stirring for 1 hour, filtering and separating to obtain filtrate containing CDBA and other valuable cannabinol substances; adding beta-cyclodextrin or a derivative thereof into water to prepare an aqueous solution, and heating to 60 DEG C; adding a China-hemp extracting solution into the aqueous solution of beta-cyclodextrin or the derivative thereof while stirring to form an inclusion compound from beta-cyclodextrin and cannabidiolic acid, precipitating the inclusion compound from water, stirring for 1-2 hours, filtering to separate out the inclusion compound, and carrying out vacuum heating at 90 DEG C for 1 hour, so as to convert CBDA into CBD cannabidiol. The method does not need an organic solvent, the product purity is high, the extraction and refining of CBDA and the removal of THC are completed in one step, the investment is small, and the production cost is low. The inclusion compound of the Beta-cyclodextrin and the CBDA is stable, good in water solubility and high in biological activity.
Owner:于立群
Method for treating cannabis induced anxiety
InactiveUS10279000B1Good effectNervous disorderHydroxy compound active ingredientsApigeninANXIETY COMPLEX
Compositions and methods for the prevention and treatment of anxiety conditions associated with the use of cannabis are disclosed herein. The medicinal compositions include cannabidiol or cannabidiolic acid and one or more of the following compounds: bergamottin or dihydroxybergamottin, phloretin or a phloretin glycoside, piperine, apigenin or an apigenin glycoside and ursolic acid.
Owner:CANOPY GROWTH CORP
Topical composition with active compounds from Cannabis sativa and Calendula officinalis for reduction of skin lesions
ActiveUS11364273B2Reduce impactCut skinOrganic active ingredientsCosmetic preparationsCalendula officinalis FlowerSkin lesion
Disclosed is a topical composition including essential combination of synergistically acting phyto-active materials, non-psychotropic phytocannabinoids from the plant of Cannabis sativa: Cannabidiol, Cannabidiolic acid, Cannabivarin Cannabigerol in combination with extract of Calendula flower and the formulation of the base to ensure the features of anti-inflammation, anti-oxidation, emollient, and bactericidal components. The topical composition is an emollient dedicated for reduction of skin lesions caused by atopic dermatitis, urticaria, radiotherapy and UV induced skin damage and acne. In addition the topical composition could reduce secretion of fats, facilitate deep skin hydration, reduce pores and exert soothing effect.
Owner:UAB SATIMED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com