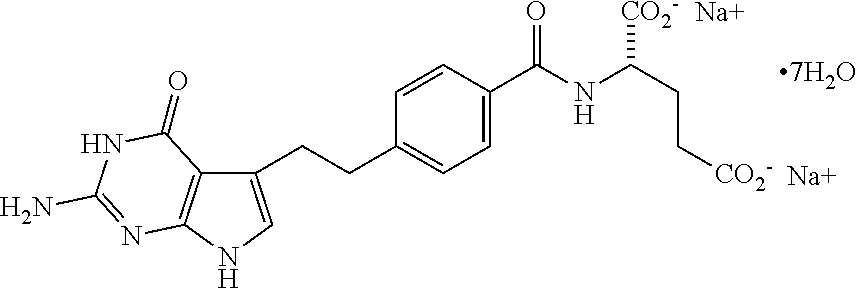
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3394 results about "High-performance liquid chromatography" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
High-performance liquid chromatography (HPLC; formerly referred to as high-pressure liquid chromatography) is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column.
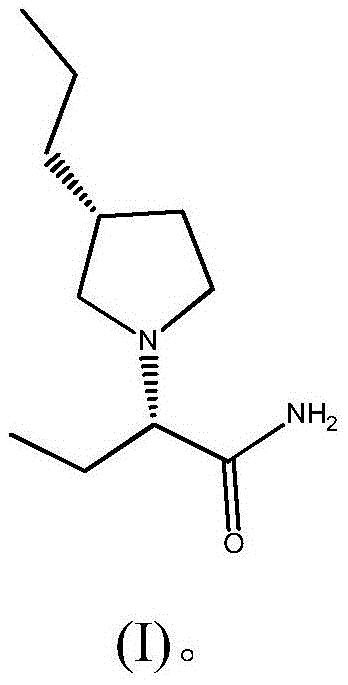
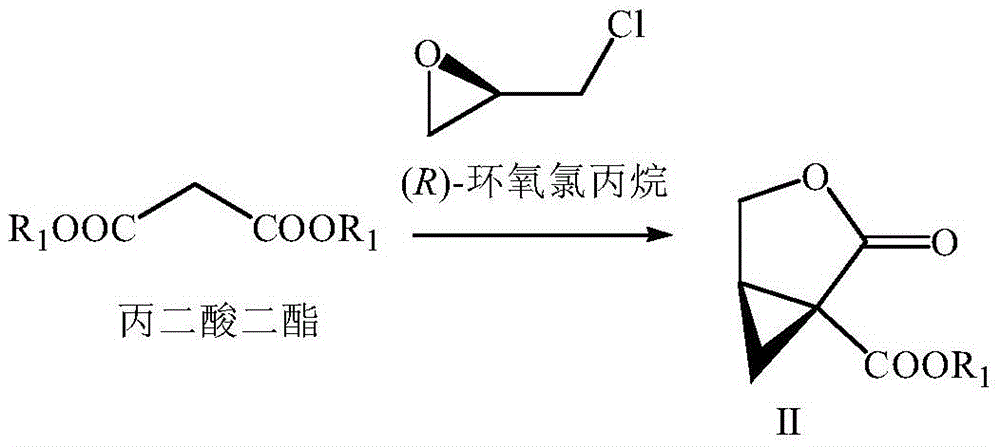
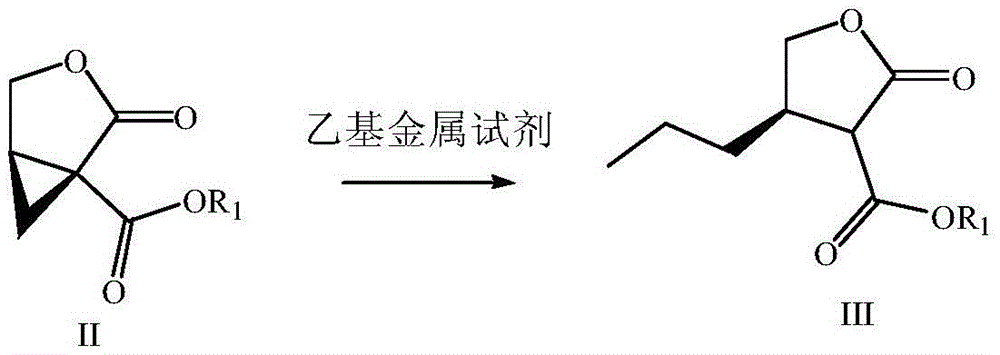
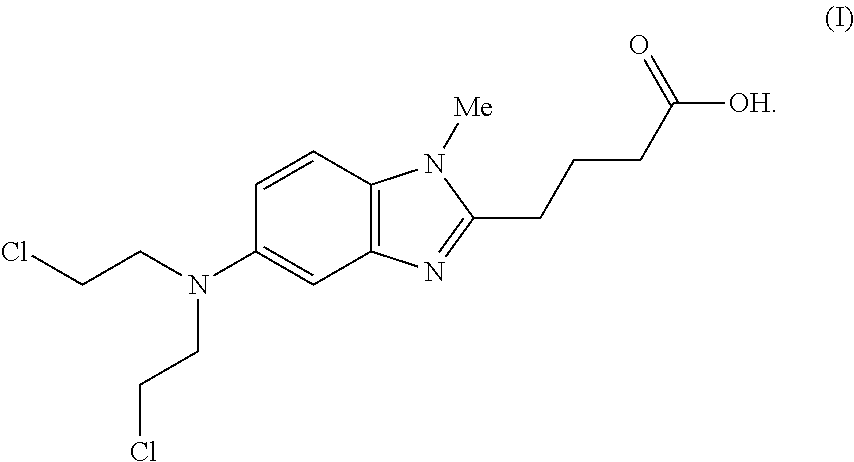
Preparation method of brivaracetam
The invention provides a preparation method of brivaracetam. The preparation method of brivaracetam comprises the following steps of in an alkaline reagent, reacting malonate ester and (R)-epichlorohydrin to obtain lactone (II), reacting the lactone (II) and an ethyl metal-based reagent to obtain an intermediate (III), removing carboxylase to obtain (R)-4-propyl-dihydrofuran-2-ketone (IV), performing ring-opening reaction under the action of a halogenated ring-opening reagent to obtain (R)-3-halogenarated methyl hexanoate or (R)-3- halogenarated methyl hexyl acetate (V), and reacting with (S)-2-aminobutanamide or an acceptable salt, so as to obtain the brivaracetam. The preparation method has the advantages that the high-purity brivaracetam (HPLC (high performance liquid chromatography: greater than 96%) and stereo rotary brivaracetam (chirality HPLC: greater than 98%) can be directly prepared; the silicagel column separation and purification or the chirality preparation column separation and purification is not used, so that the complicated separation and purification step is not performed, the cost is saved, and the preparation method is more suitable for industrial production.
Owner:佛山市隆信医药科技有限公司
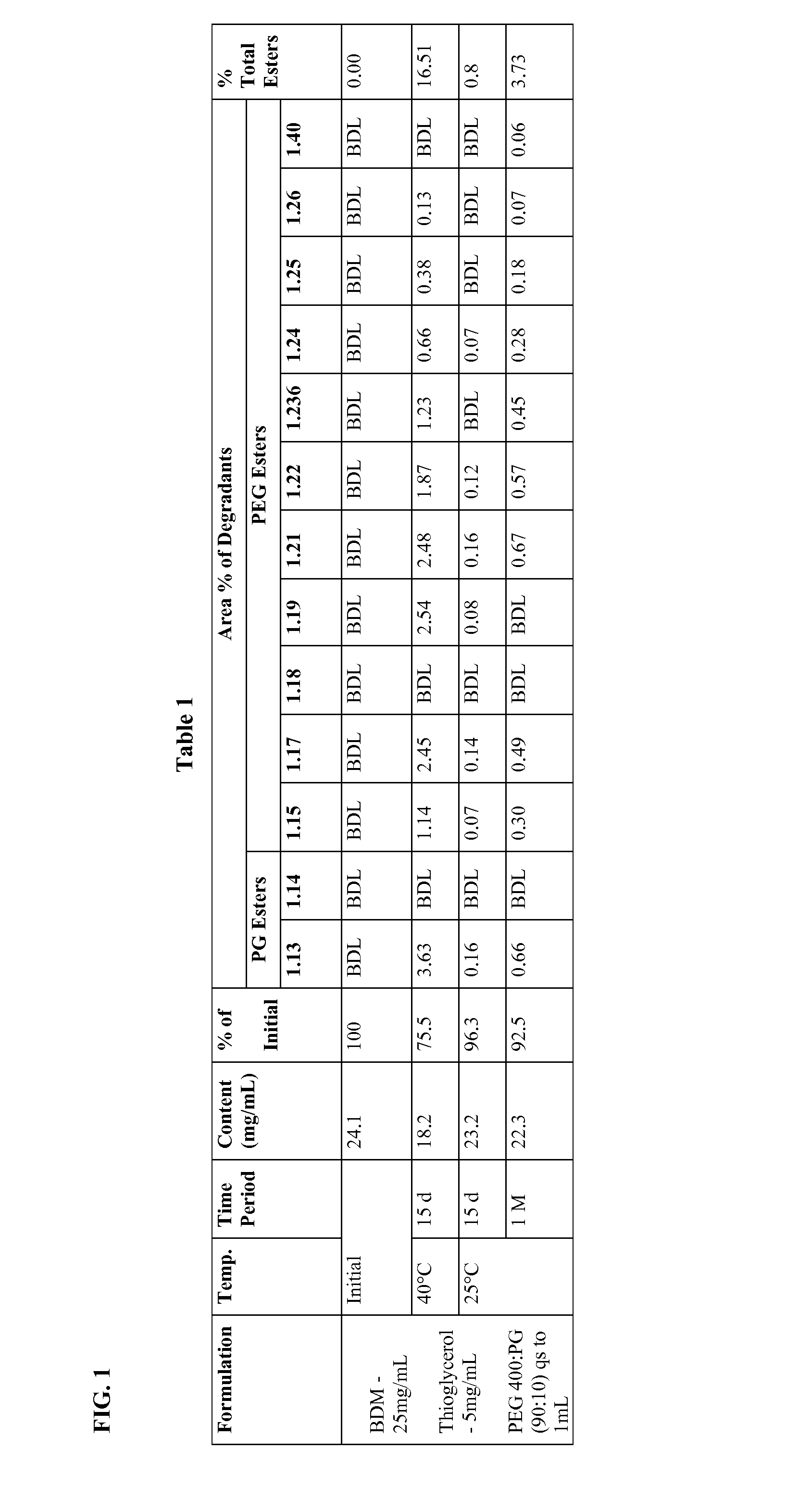
Formulations of bendamustine
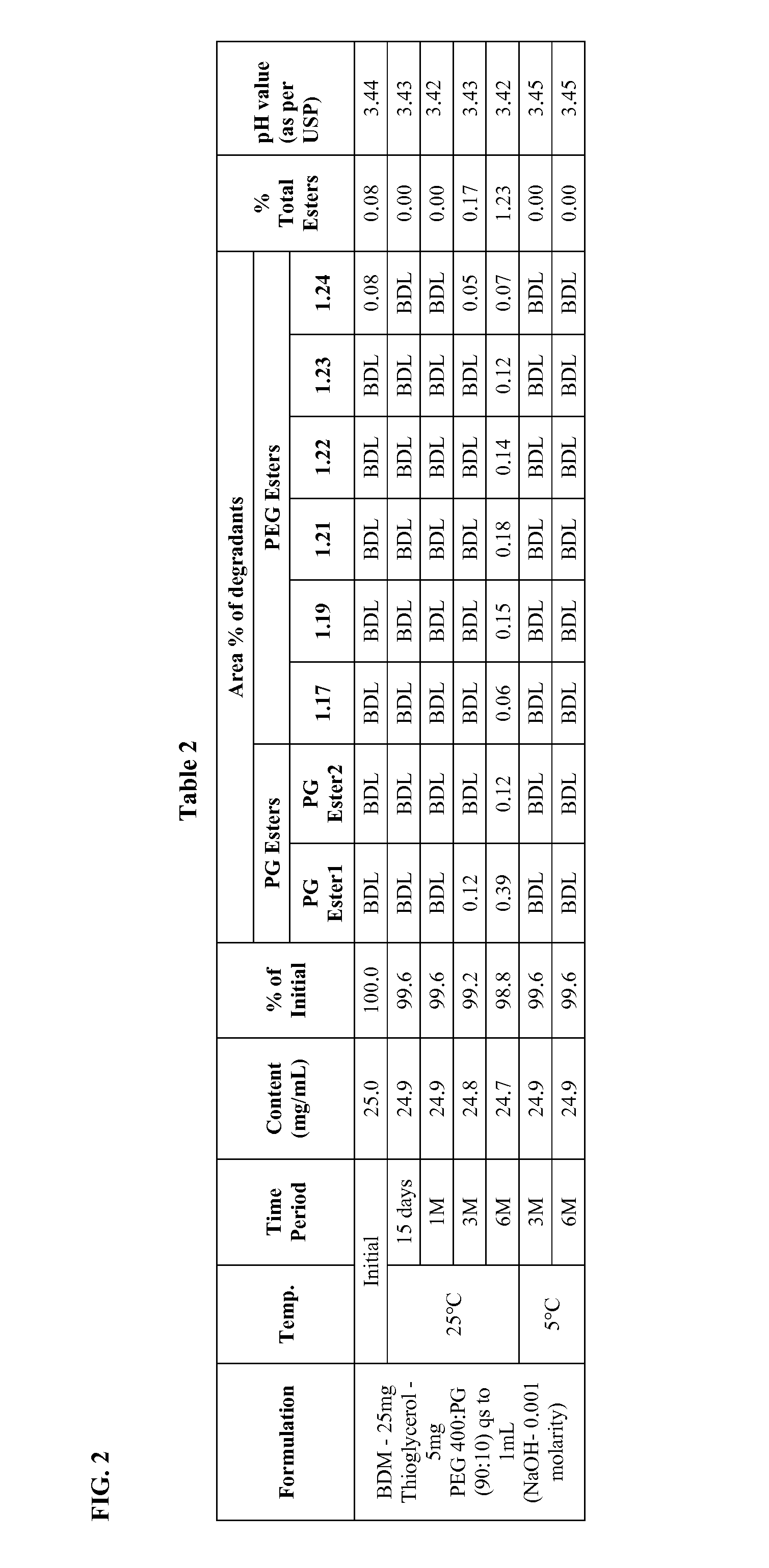
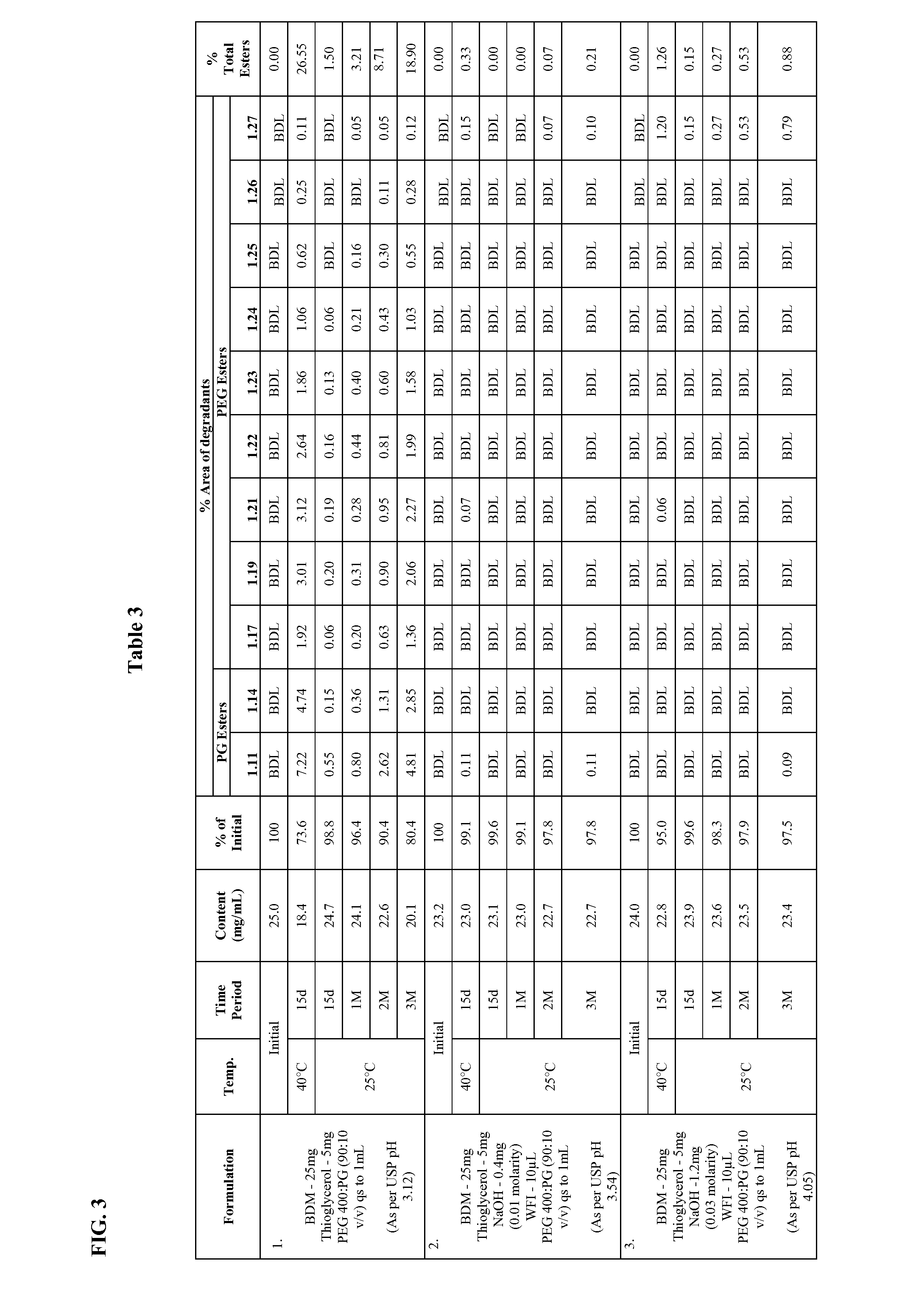
InactiveUS20130210879A1Improve long-term stabilityOrganic active ingredientsBiocideAntioxidantMedicine
Long term storage stable bendamustine-containing compositions are disclosed. The compositions can include bendamustine or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable fluid contains a mixture of PEG and PG; an organic or inorganic compound in an amount sufficient to obtain a pH of from about 6.0 to about 11 for the polyethylene glycol, as measured using USP monograph for polyethylene glycol; and optionally an antioxidant. The bendamustine-containing compositions have less than about 5% total esters, on a normalized peak area response (“PAR”) basis as determined by high performance liquid chromatography (“HPLC”) at a wavelength of 223 nm, after at least about 15 months of storage at a temperature of from about 5° C. to about 25° C.
Owner:EAGLE PHARMACEUTICALS INC
Formulations of bendamustine
Long term storage stable bendamustine-containing compositions are disclosed. The compositions can include bendamustine or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable fluid which can include in some embodiments PEG, PG or mixtures thereof and an antioxidant or chloride ion source. The bendamustine-containing compositions have less than about 5% total impurities, on a normalized peak area response (“PAR”) basis as determined by high performance liquid chromatography (“HPLC”) at a wavelength of 223 nm, after at least about 15 months of storage at a temperature of from about 5° C. to about 25° C.
Owner:EAGLE PHARMACEUTICALS INC
Method for purifying solid-phase synthetic coarse liraglutide
ActiveCN102584982ASolid sorbent liquid separationPeptide preparation methodsAqueous solutionSolid-phase synthesis
The invention relates to the field of biomedicine, in particular to a method for purifying solid-phase synthetic coarse liraglutide. The method comprises the following steps of: dissolving solid-phase synthetic coarse liraglutide into acetonitrile aqueous solution to obtain coarse peptide solution; and purifying through four-step HPLC (High Performance Liquid Chromatography) to obtain the liraglutide. The method has the advantages of high purity and high yield.
Owner:HYBIO PHARMA
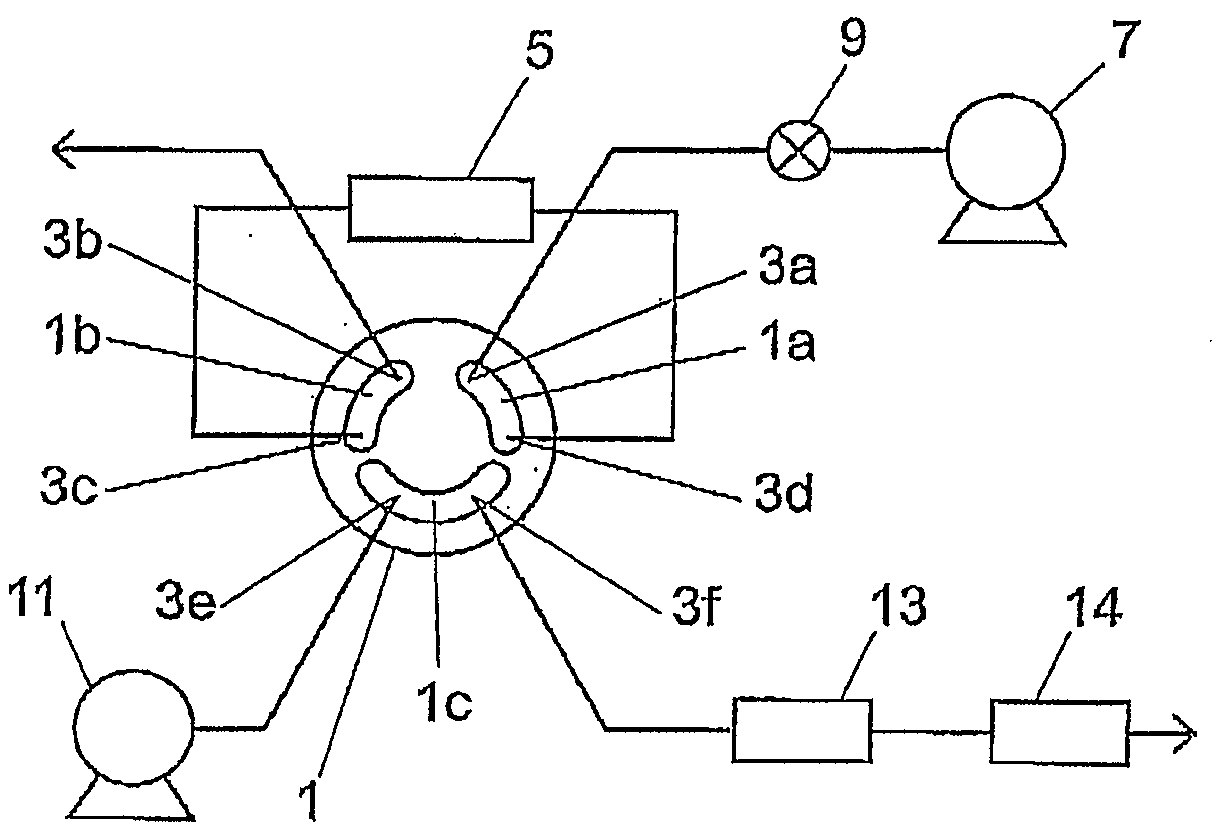
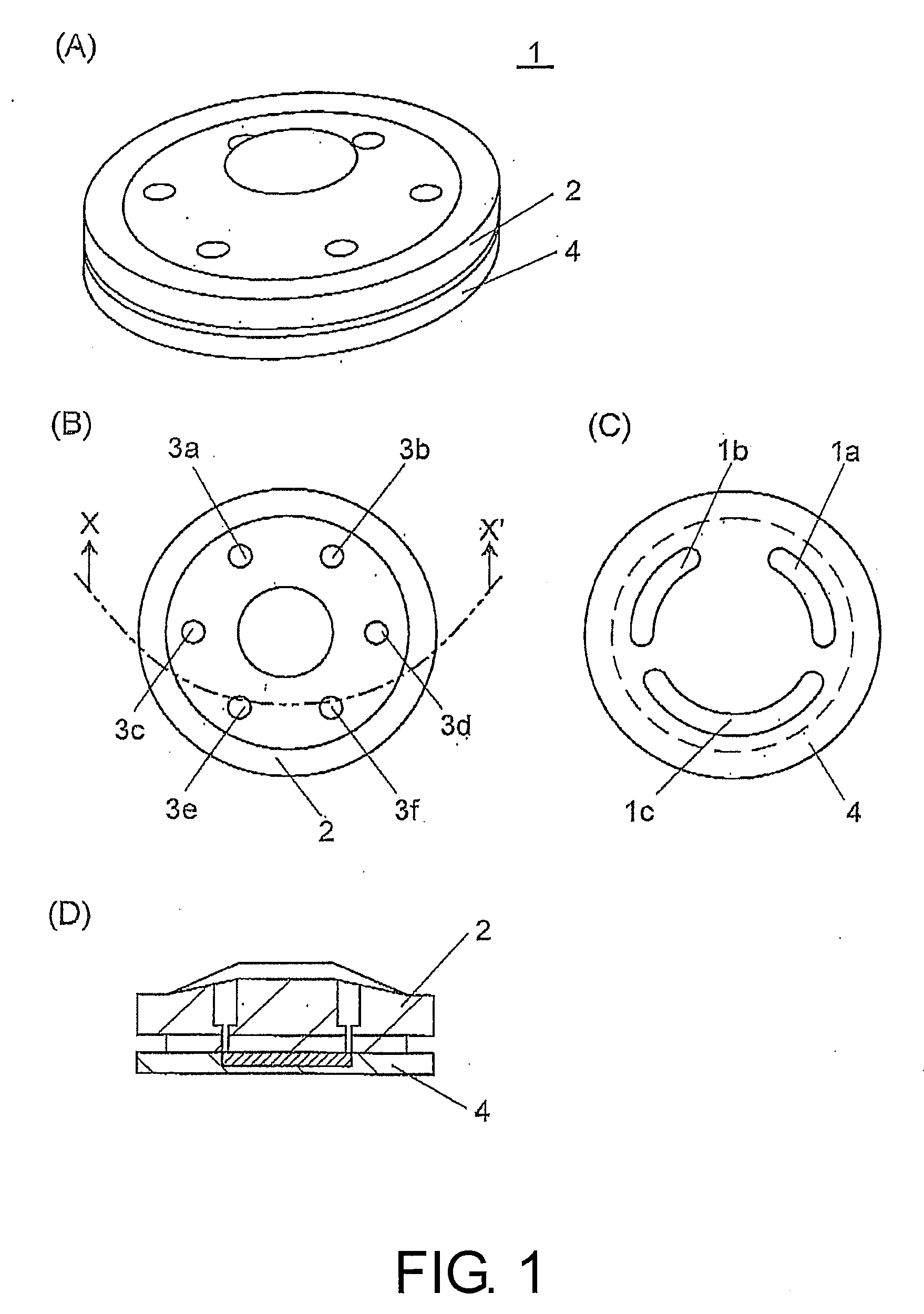
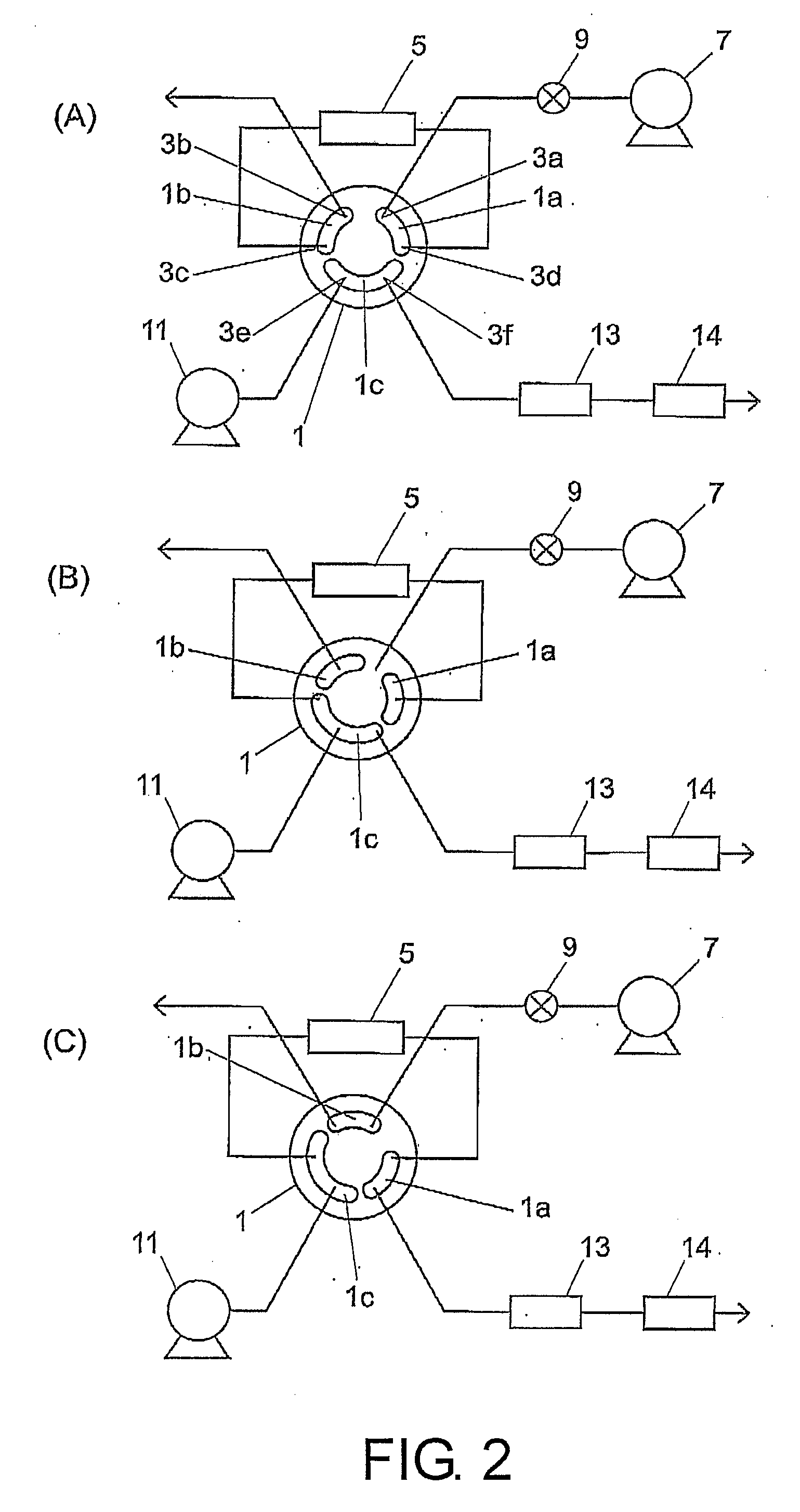
Flow path switching valve, high performance liquid chromatography using the same and analytical method thereof
InactiveUS20070251302A1Good reproducibilityEliminate differential pressureSamplingComponent separationEngineeringPath switching
A flow path switching valve is provided, in which an impact due to the pressure change when a flow path is switched is prevented from being generated. (A) A rotor slot 1c allows an analysis infusion pump 11 to be connected to an analytical column 13, so as to form a flow path (condensing procedure). (B) The rotor of the flow path switching valve 1 is rotated clockwise for 30 degrees, the rotor slot 1c allows the analysis infusion pump 11, the analytical column 13, and a trap column 5 be connected. After the pressure in the trap column 5 is raised to the same pressure level as that of the analytical column 13, the pressure is stabilized, and the pressure difference between the two columns 5 and 13 is counteracted (high-pressure procedure). (C) After the pressure between the two columns 5 and 13 has been stabilized sufficiently, the rotor is further rotated for 30 degrees, and the trap column 5 and the analytical column 13 are connected in series, so the sample analysis can be performed (dissolution procedure and detection procedure).
Owner:SHIMADZU CORP
Formulations of bendamustine
ActiveUS8609707B2Improve long-term stabilityBiocideSulfur/selenium/tellurium active ingredientsAntioxidantChloride
Long term storage stable bendamustine-containing compositions are disclosed. The compositions can include bendamustine or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable fluid which can include in some embodiments PEG, PG or mixtures thereof and an antioxidant or chloride ion source. The bendamustine-containing compositions have less than about 5% total impurities, on a normalized peak area response (“PAR”) basis as determined by high performance liquid chromatography (“HPLC”) at a wavelength of 223 nm, after at least about 15 months of storage at a temperature of from about 5° C. to about 25° C.
Owner:EAGLE PHARMACEUTICALS INC
Metformin hydrochloride enteric-coated tablets quality control method
ActiveCN101339178AFacilitated releaseGuaranteed to dissolveComponent separationColor/spectral properties measurementsPhosphateMetformin Hydrochloride
The invention discloses a quality control method of metformin hydrochloride enteric coated tablet, comprising the aspects of character, identification, examination and content measurement; wherein, release examination comprises the release quantity examination of acid in hydrochloric acid solution of 0.1 mol / l and the release quantity examination in phosphate buffer with the pH value of 6.8; the examination of relevant substances comprises the following steps: dicyandiamide is taken as reference, sulfonic group cation exchange bonded silica is taken as filler, ammonium dihydrogen phosphate solution of 1.7 percent with the pH value of 3 is mobile phase and the high performance liquid chromatography is used for examining the relevant substances. The invention controls the release quantity of the metformin hydrochloride enteric coated tablet in gastric juice strictly, reduces the adverse reaction of patients effectively, improves the release quantity of the metformin hydrochloride enteric coated tablet in the buffer solution (simulated intestinal juice) and ensures the dissolution of the enteric coated tablet in the intestinal juice effectively; the invention also adds the examination of dicyandiamide impurity under the examination item and enhances the safety of the medicine.
Owner:贵州天安药业股份有限公司
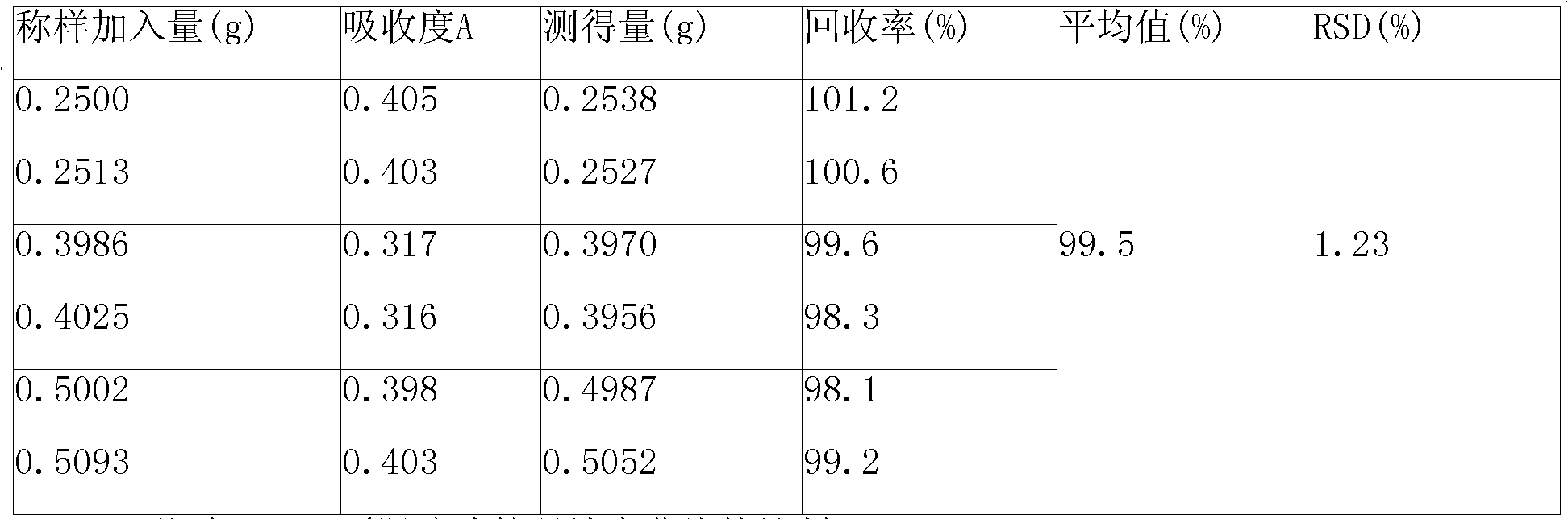
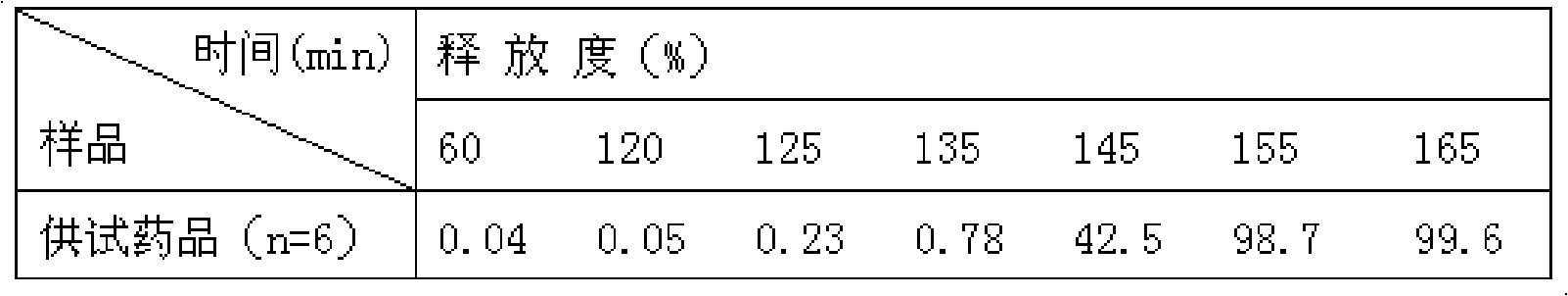
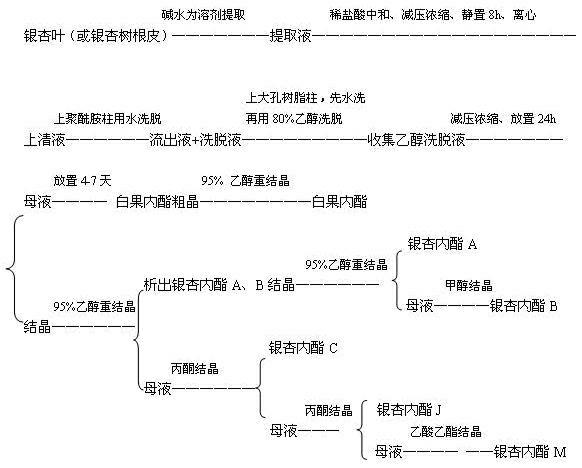
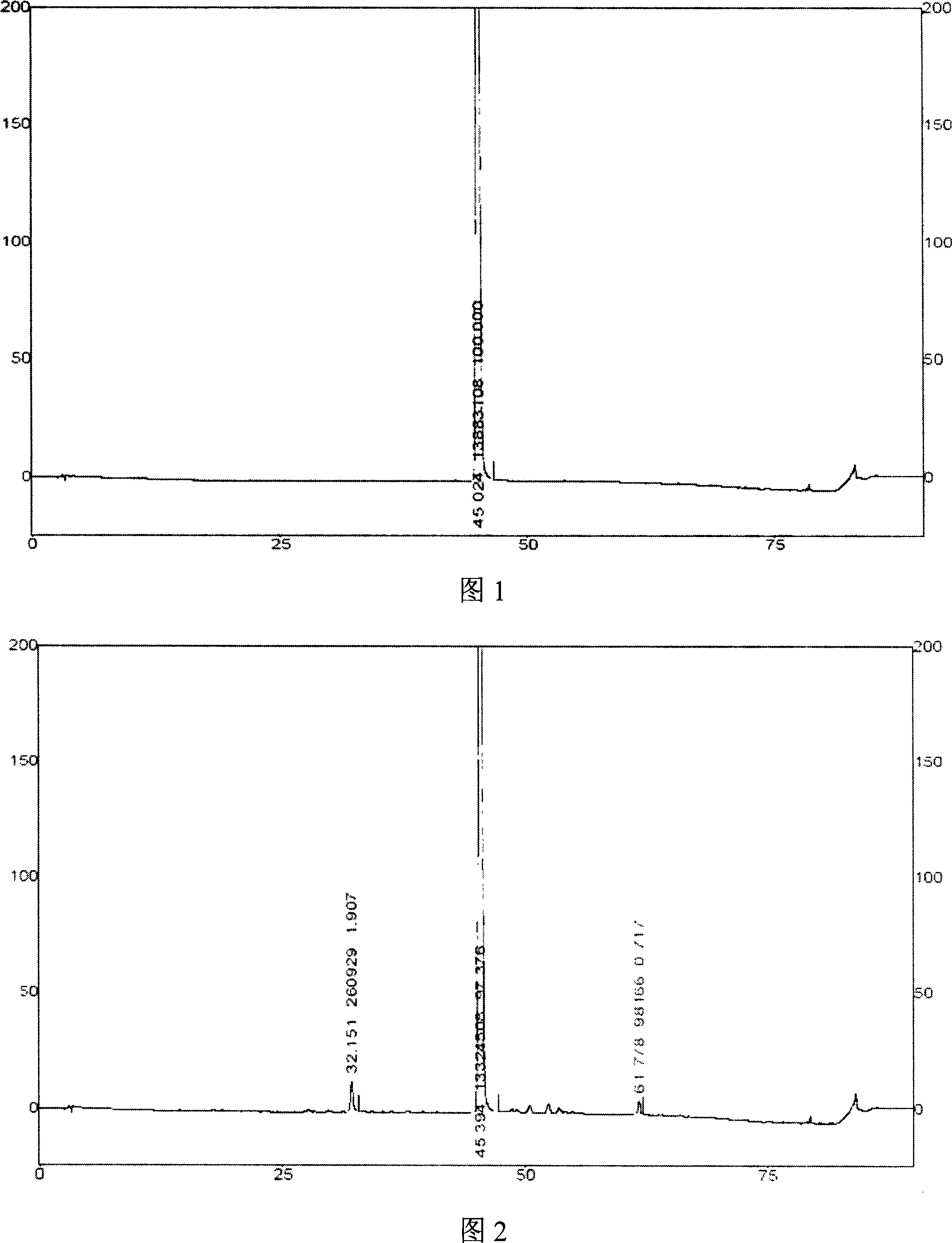
Process for extraction and separation of ginkgolides A, B, C, J, M and bilobalide
InactiveCN102627656AHigh yieldLow costOrganic chemistryGinkgophyta medical ingredientsTree rootAlkaline water
Owner:合肥创新医药技术有限公司
Red sage root salvianolic acid A injection formulation for treating cardiovascular diseases and preparation process thereof
The invention discloses a salvianolic acid A for injection and its preparing process, wherein high efficiency liquid chromatography method is employed to determine salvianolic acid A in the raw material medicaments, the content of salvianolic acid A is between 92% and 100%, two foreign substances are present. The mass ratio of salvianolic acid A and anti-oxidizing agent in the preparation is 2-50:1, the pH of the injection is controlled to 3-7.
Owner:CHIATAI QINGCHUNBAO PHARMA
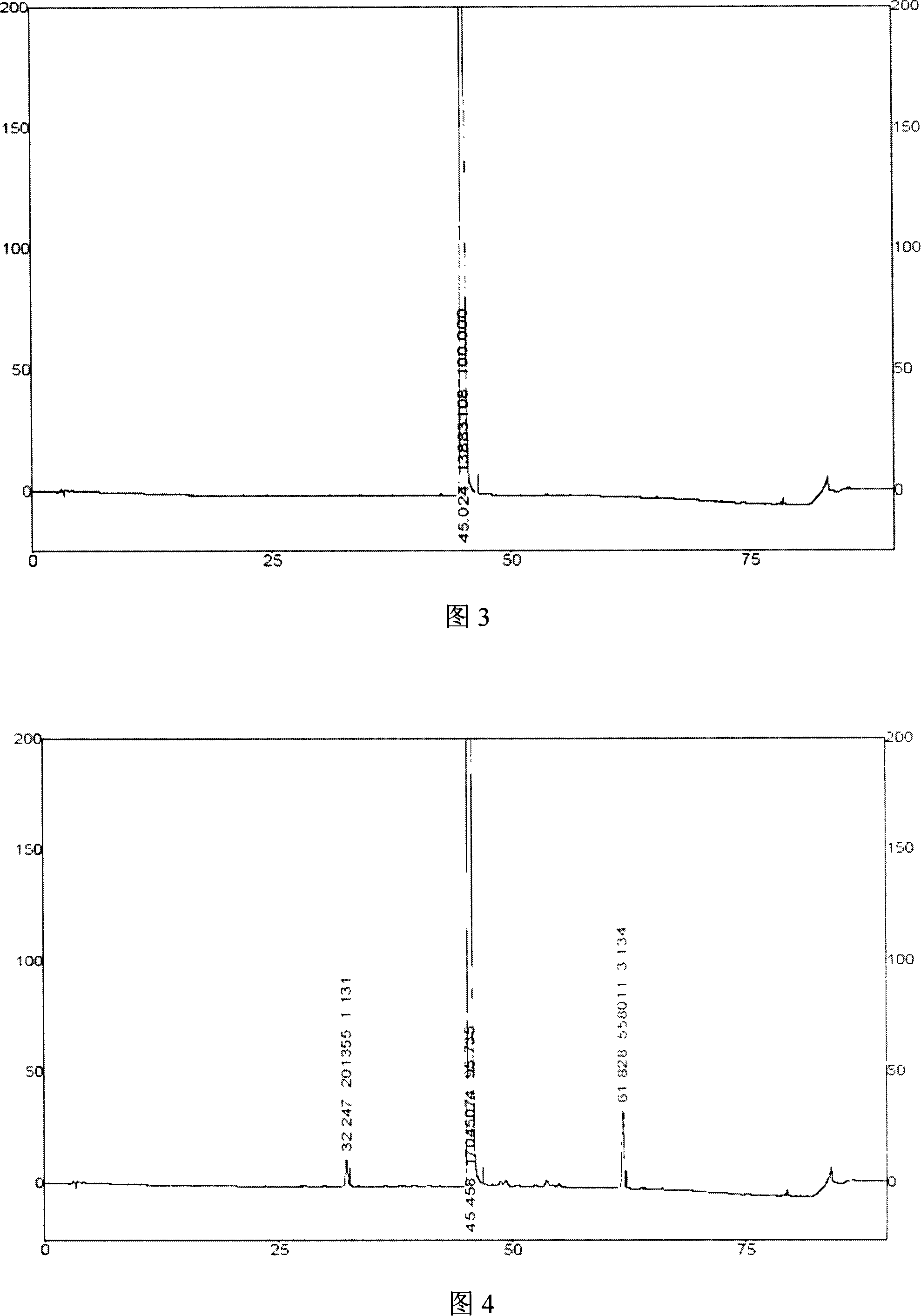
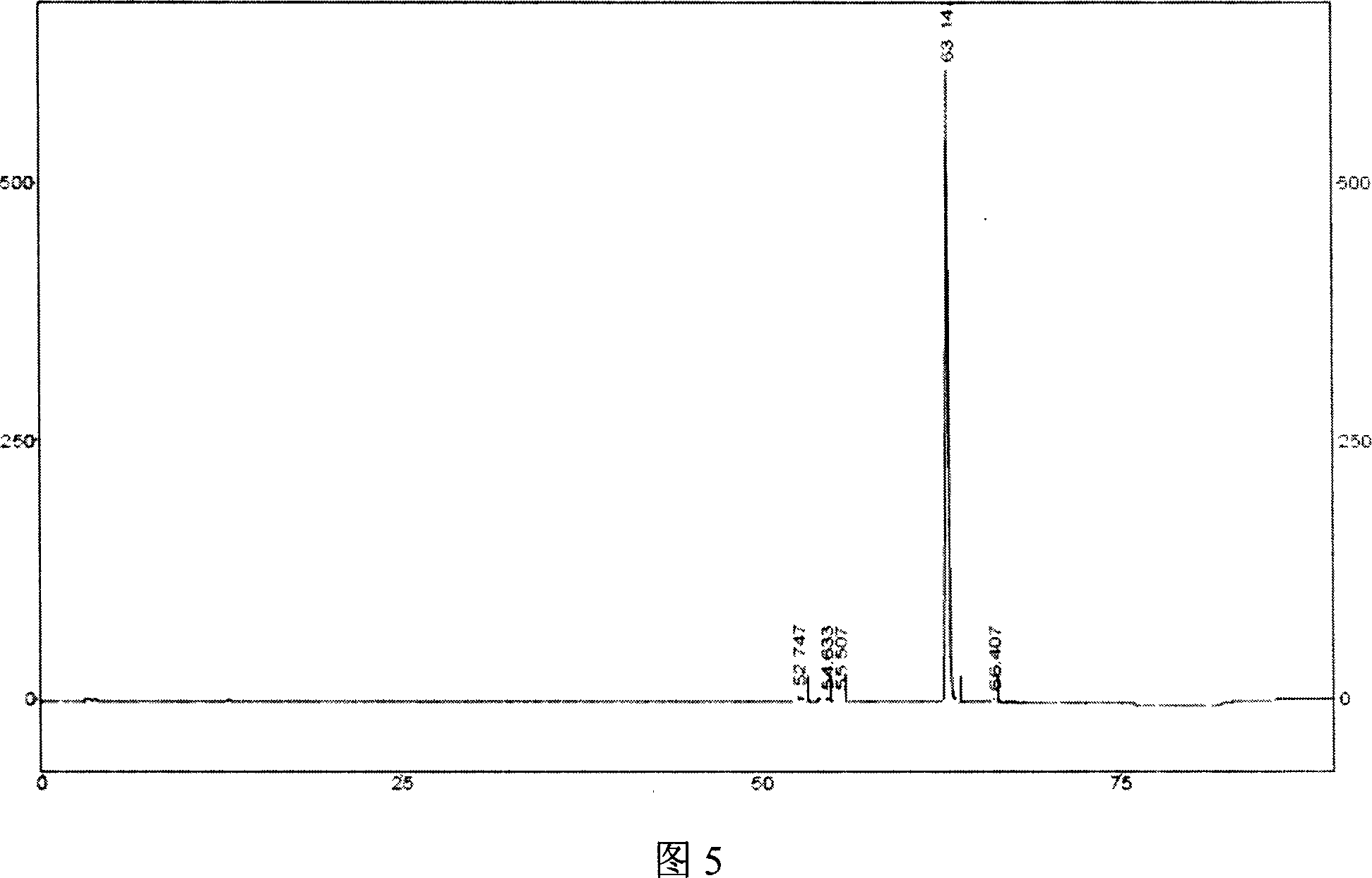
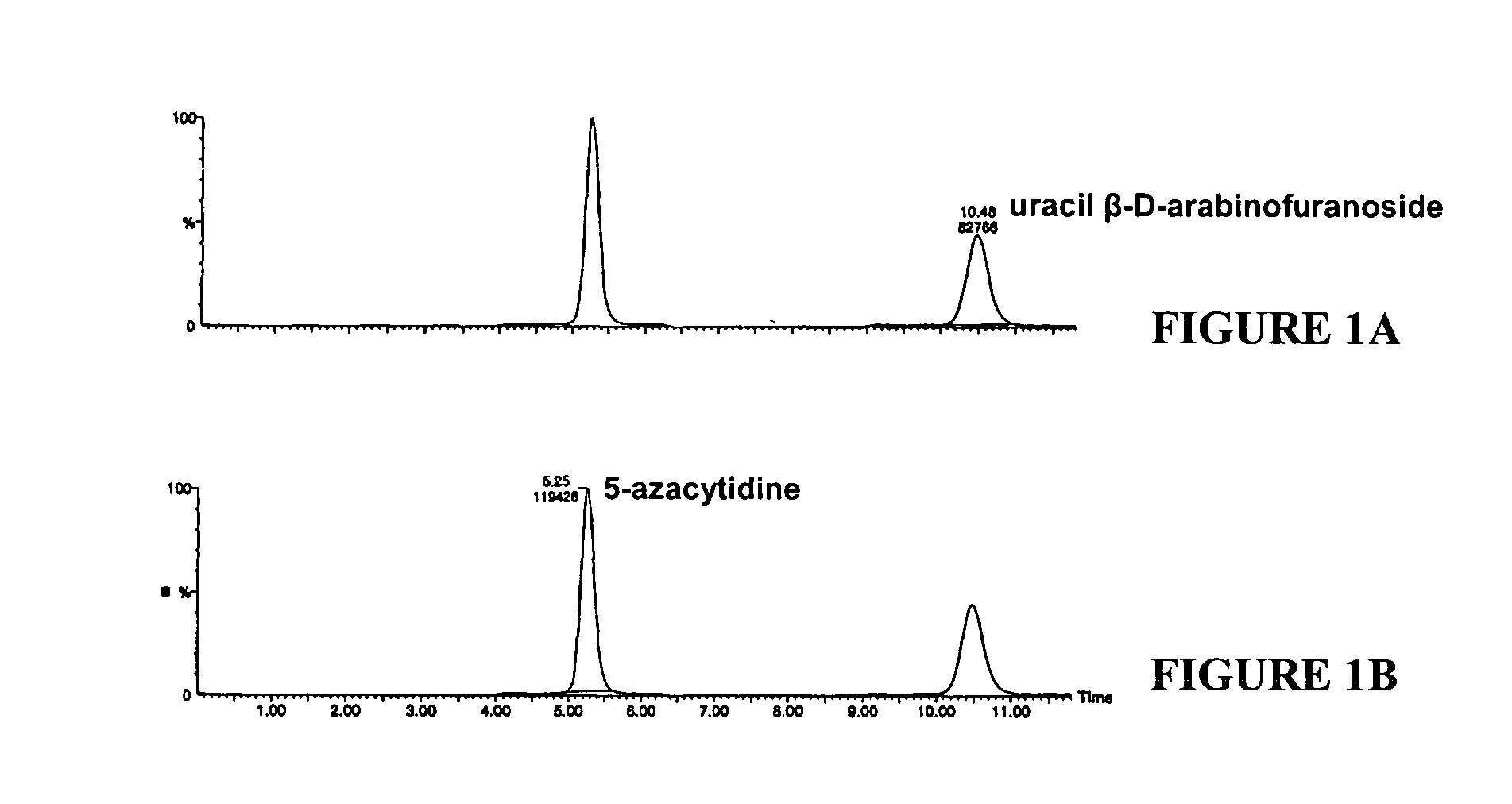
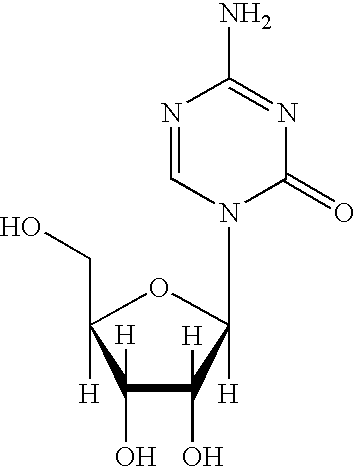
Methods for stabilizing 5-azacytidine in plasma
Owner:PHARMION
Method for detecting release situation of nicotine in buccal tobacco products
ActiveCN102156178AThe detection method is accurateGood repeatabilityComponent separationFluid phaseArtificial salivas
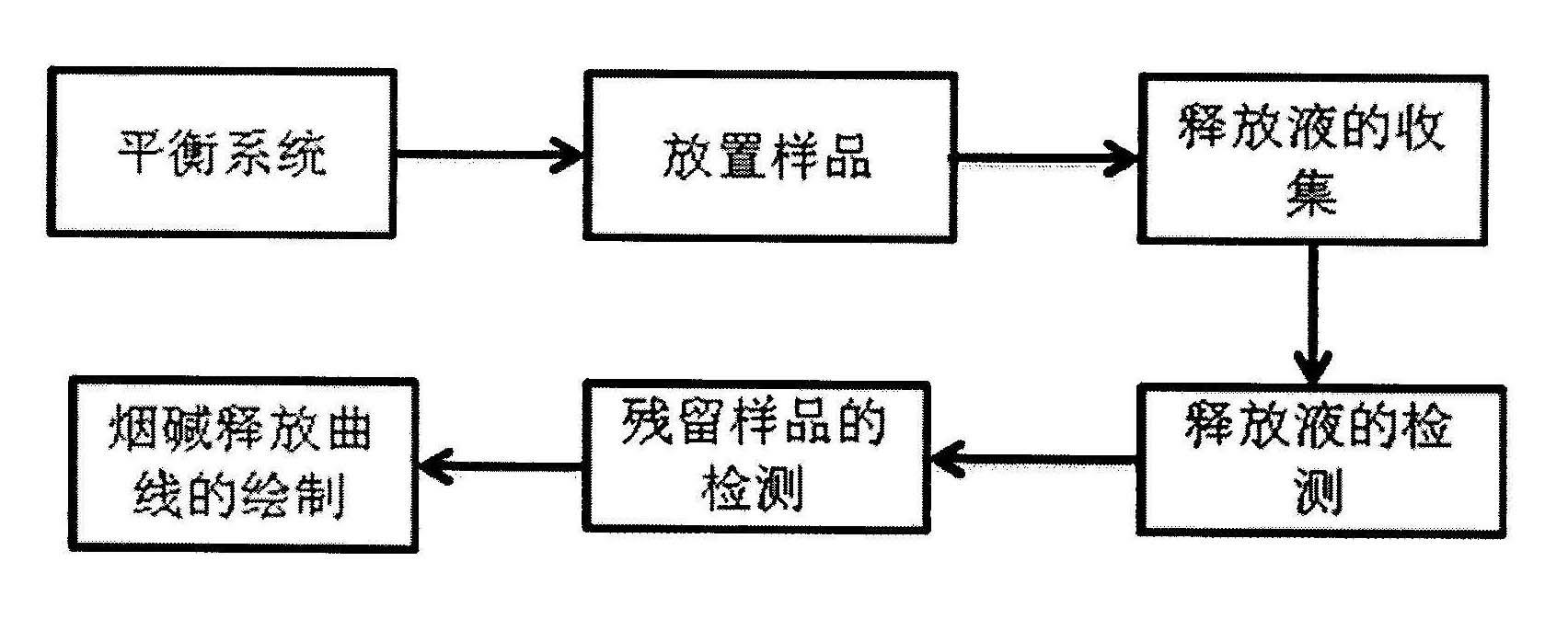
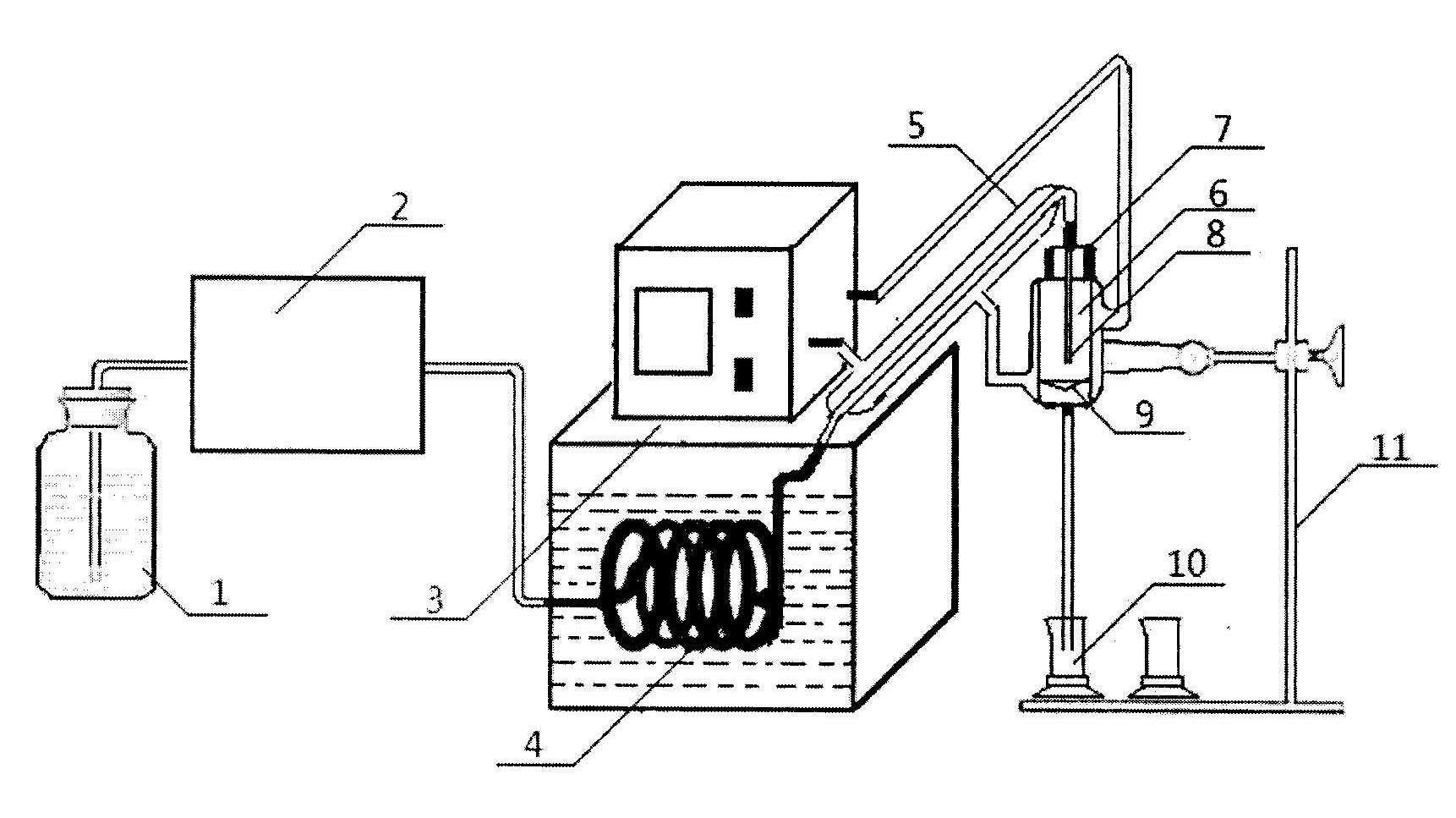
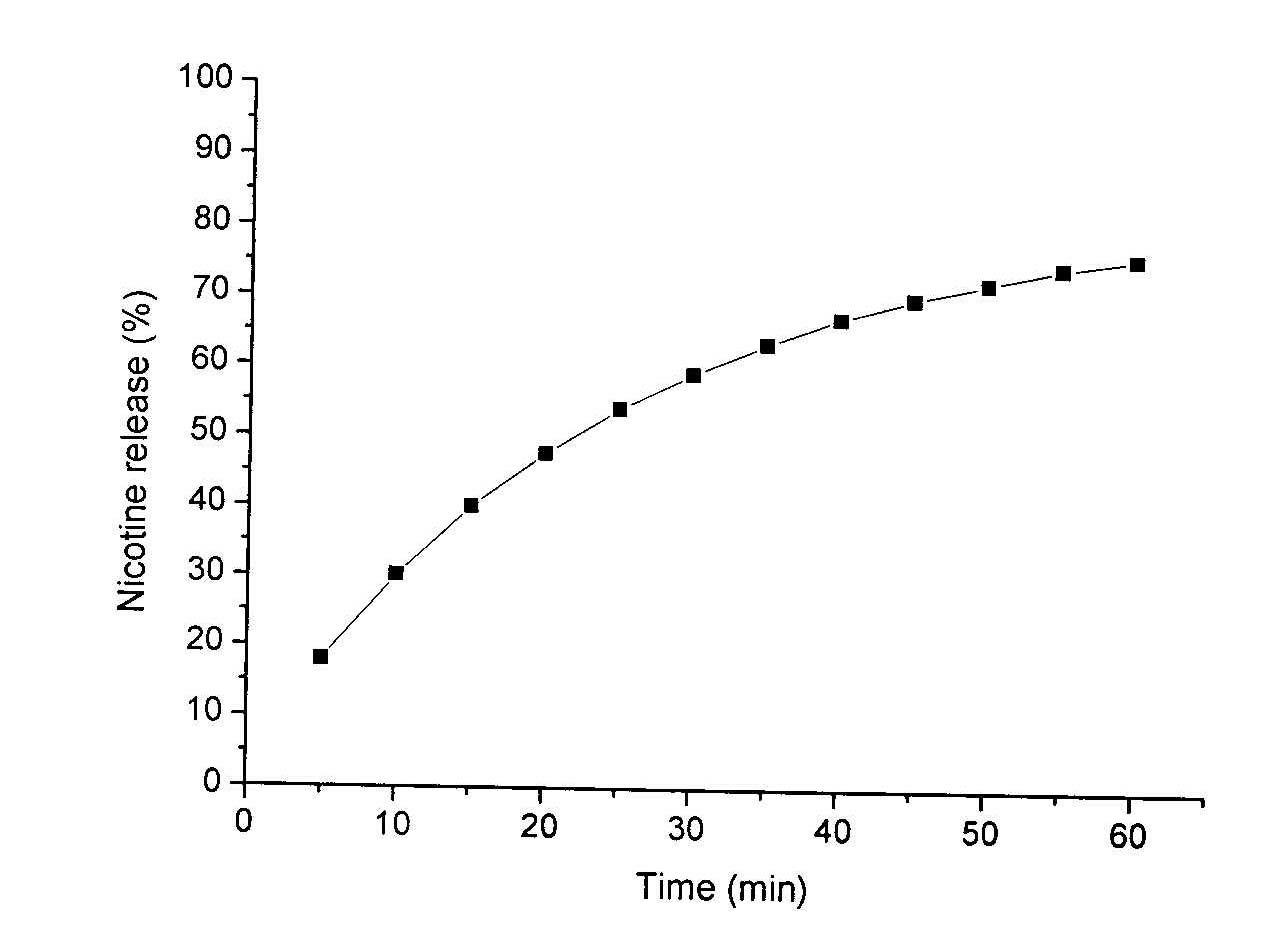
The invention relates to a method for detecting a release situation of nicotine in buccal tobacco products, which is characterized by comprising the steps of simulating the release situation of the nicotine in the buccal tobacco products in the human mouth, instantly quantifying the release quantity and the release speed and then detecting the release speed and the release degree of the nicotine in the buccal tobacco products. The release and the collection of the nicotine are realized through a release and collection device in vitro; by means of the device, the release process of the nicotine in the buccal tobacco products in the human mouth can be simulated and nicotine release solutions can be the instantly collected, wherein the release process is a process that the nicotine is continuously extracted from the buccal tobacco products by utilizing artificial saliva at a constant temperature; and the detection of the nicotine content in the release solution is realized through a high performance liquid chromatography. The method has the advantages: the release speed and the release degree of the nicotine in various buccal tobacco products can be rapidly and accurately detected with good repeatability, high sensitivity and short detection period and is suitable for the rapid analysis of mass samples for different industries and different purposes.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Method for rapidly detecting bisphenol A and bisphenol AF in food
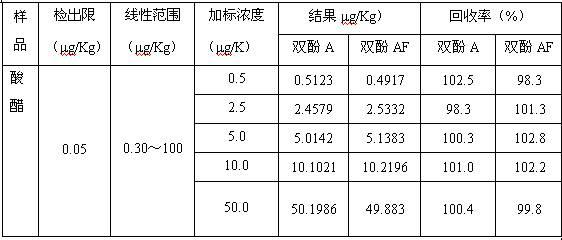
The invention relates to a method for detecting bisphenol A and bisphenol AF in food, belonging to technical field of analytical chemistry determination method. With ionic liquid (1-butyl-3-methylimidazole hexafluorophosphate) as an extractant and TritonX-100 or acetone as an dispersant, ultrasonic oscillation is carried out to form emulsion; after centrifugal separation, extraction drops at the lower layer are directly subjected to HPLC (high performance liquid chromatography) quantitative analysis. The method disclosed by the invention has the advantages of simpleness in detection steps, easiness in operation, low detection limit and high enrichment multiple, greatly reduced detection cost. The method disclosed by the invention is a rapid, efficient and environmentally friendly pre-treatment technology and has a wider application prospect in the field of food analysis.
Owner:KUNMING UNIV OF SCI & TECH TECH IND SALES MANAGEMENT
Method for simultaneously detecting seven slimming chemical components which are illegally added to traditional Chinese medicine, health food or cosmetics
InactiveCN102901780AEasy to separateQualitatively accurateComponent separationPhenolphthaleinEphedrine
The invention relates to a method for simultaneously detecting seven slimming chemical components which are illegally added to traditional Chinese medicine, health food or cosmetics. According to the method, an appropriate mobile phase and a reasonable elution gradient are adopted during liquid-phase separation, so that the slimming components needing to be detected are effectively separated within 12 min, the analysis time of a sample instrument is shortened by 88%, and the work efficiency is greatly increased. Additionally, the advantages of high separation ability of the HPLC (High Performance Liquid Chromatography) technology, high sensitivity and stronger qualitative ability of the mass spectrum and the like are combined, so screening and confirmation can be simultaneously completed during one operation. By using the method disclosed by the invention, the seven chemical components including sibutramine, fenfluramine, phenolphthalein, ephedrine, caffeine, N,N-bi-demethylation sibutramine and furosemide can be effectively separated, the inspection time can be shortened, and the slimming chemical components which are illegally added to the traditional Chinese medicine, the health food or the cosmetics can be accurately and effectively distinguished.
Owner:HUNAN INST FOR FOOD & DRUG CONTROL
Mass spectrometer autosampler
InactiveUS20030222007A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSmall sampleAutosampler
The present invention relates to an autosampler device useful in high pressure liquid chromatography (HPLC), and more particularly to a device useful for the automated introduction of small sample volumes into a HPLC system. Methods of analyzing low abundant protein samples using such a device are also included.
Owner:BRISTOL MYERS SQUIBB CO
Method for preparing teriparatide
InactiveCN104017064APromote resultsLow purityPeptide preparation methodsParathyroid hormonesDipeptideSide chain
The invention relates to a method for preparing teriparatide, which comprises the following steps: A) in the presence of an activator system, coupling a resin solid-phase carrier and Fmoc-Phe-OH to obtain a Fmoc-Phe-resin; B) sequentially coupling amino acids with N terminal Fmoc protection and side chain protection according to the teriparatide main chain peptide sequence by solid-phase synthesis, wherein the 16th-17th amino acids in the peptide sequence are coupled by using pseudoproline dipeptide Fmoc-Asn(Trt)-Ser(PsiMe,MePro)-OH; and C) carrying out peptide resin cracking by using a TFA (trifluoroacetic acid) cracking solution containing NH4I / Me2S, purifying the crude product by HPLC (high performance liquid chromatography), and freeze-drying to obtain the teriparatide. The content of the technical impurity Met8(O)-teriparatide is lower than 0.1%. The technique for preparing teriparatide has the advantages of high purity and low cost, is suitable for large-scale production, and can effective control the Met8(O)-teriparatide content without influencing the total yield of the teriparatide.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
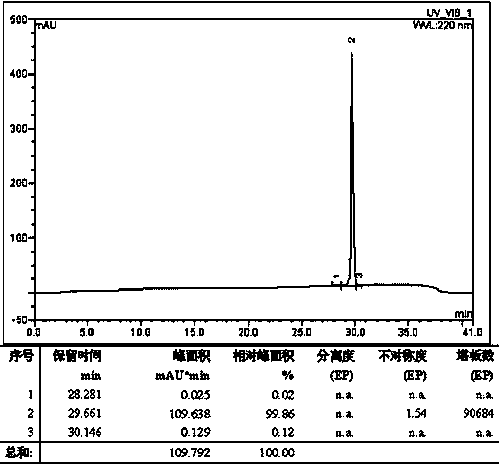
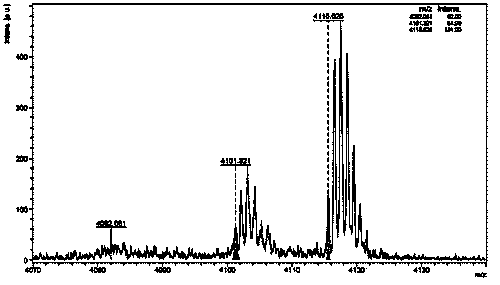
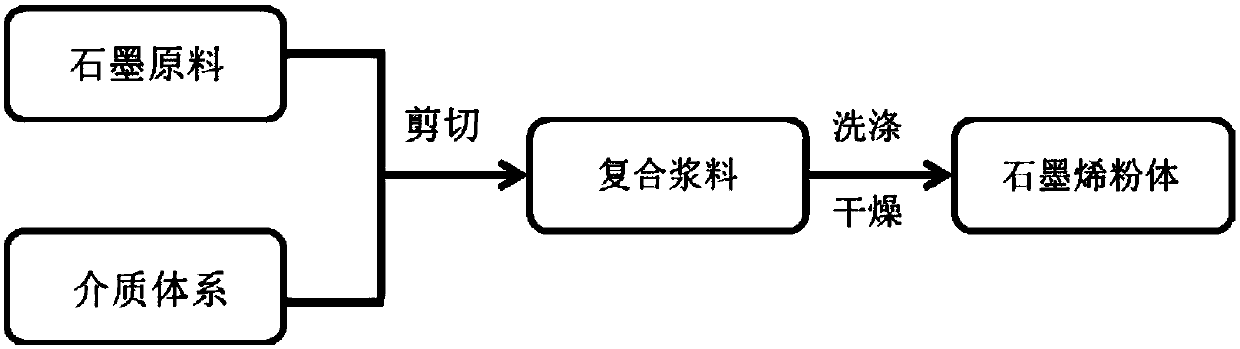
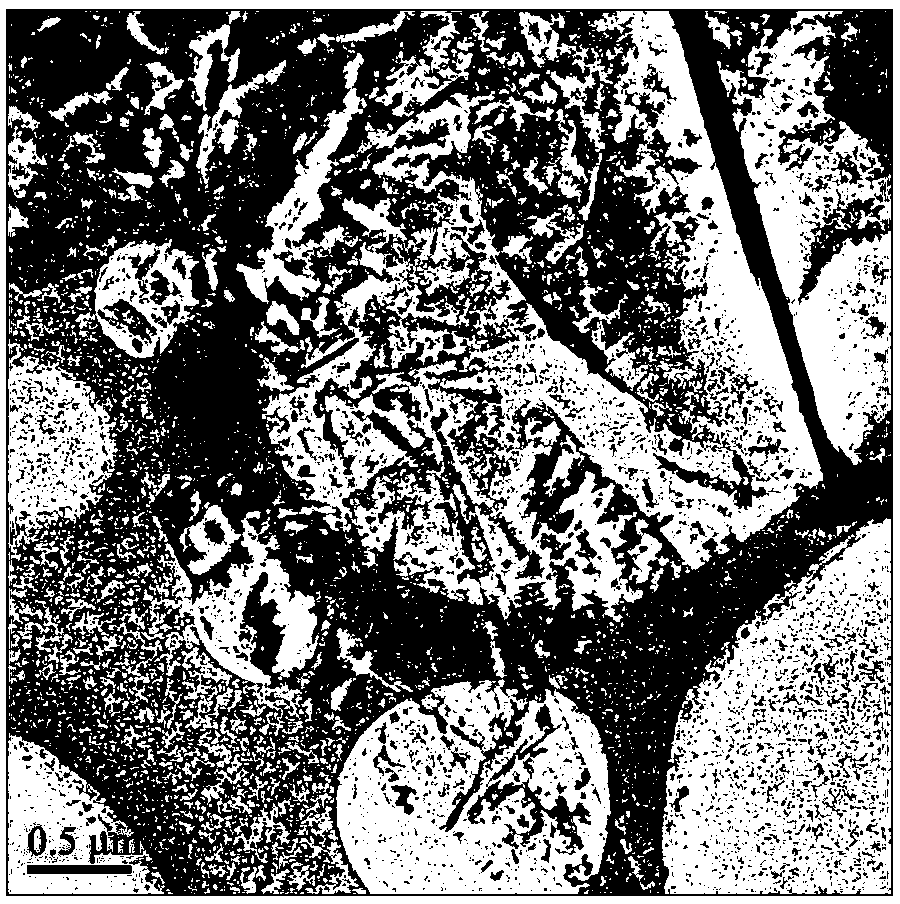
Method for preparing graphene from graphite through HPLC (high performance liquid chromatography) stripping
The invention provides a method for preparing graphene from graphite through HPLC (high performance liquid chromatography) stripping. The method comprises steps as follows: 1), preparation of a medium system: a), the medium system is prepared from macromolecules and an organic solvent which are polymerized; or b), the medium system is prepared from macromolecule prepolymers; 2), shearing and stripping: graphite raw materials are uniformly dispersed in the prepared medium system, a mixture is formed and then is sheared, graphite stripping is realized, and composite slurry is obtained; 3), separation: the composite slurry is separated, and graphene powder can be obtained. According to the method, the stripping efficiency is substantially improved by the aid of medium viscosity, wettability between graphite and medium is improved through matching with interface energy, and the problem of dispersion of the graphene is solved. The stripping efficiency of a common solvent is far lower than 1%, but the stripping efficiency of the invention can reach 100%. The method is simple and efficient, and high-yield and large-scale industrial production of high-quality graphene can be realized with the method.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
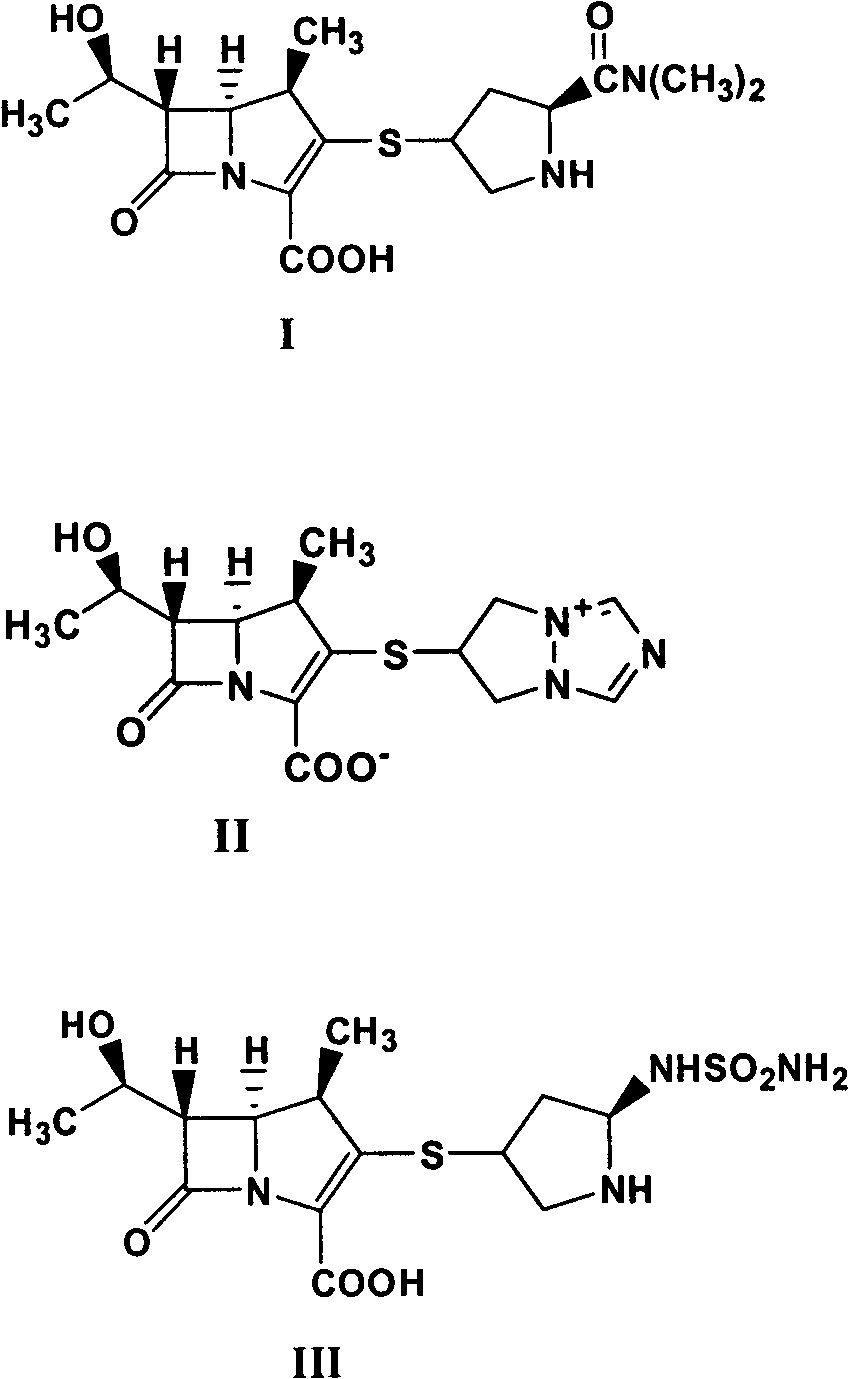
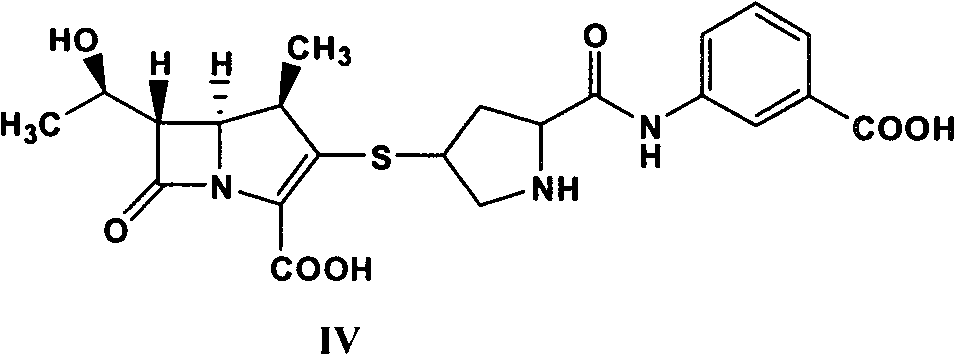
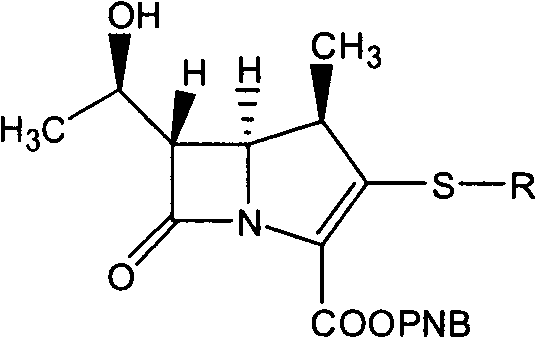
Method for synthesizing 1 beta methyl carbapenem antibiotic
InactiveCN101935321AEasy to operateEase of mass productionOrganic chemistryBulk chemical productionOrganic baseReaction temperature
The invention relates to a method for synthesizing 1 beta methyl carbapenem antibiotic, comprising the following steps of: (1) adding an organic solvent, water, pivaloyloxymethyl ester, organic base and a catalyst to a reactor, introducing hydrogen, and then reacting for above 15 minutes, wherein the pressure of the hydrogen is controlled in 10.0 MPa, and the reaction temperature is controlled below 70 DEG C; (2) monitoring by using a HPLC (High Performance Liquid Chromatography), removing the hydrogen after finishing reaction, and filtering and recovering the catalyst (palladium charcoal or platinum charcoal); and (3) cooling a filtrate, adding a crystallizing solvent, stirring and devitrifying for above 10 minutes, and then filtering to obtain a corresponding target compound. The method has convenient operation, and a reaction liquid can be directly added to the solvent for crystallization without purification.
Owner:SHENZHEN HAIBIN PHARMA
HPLC (High Performance Liquid Chromatography)-based carrier electric meter intelligent management method and device
ActiveCN110113078AIncrease collection rateIncrease success rateUtility meters data arrangementsPower distribution line transmissionMaster stationCarrier signal
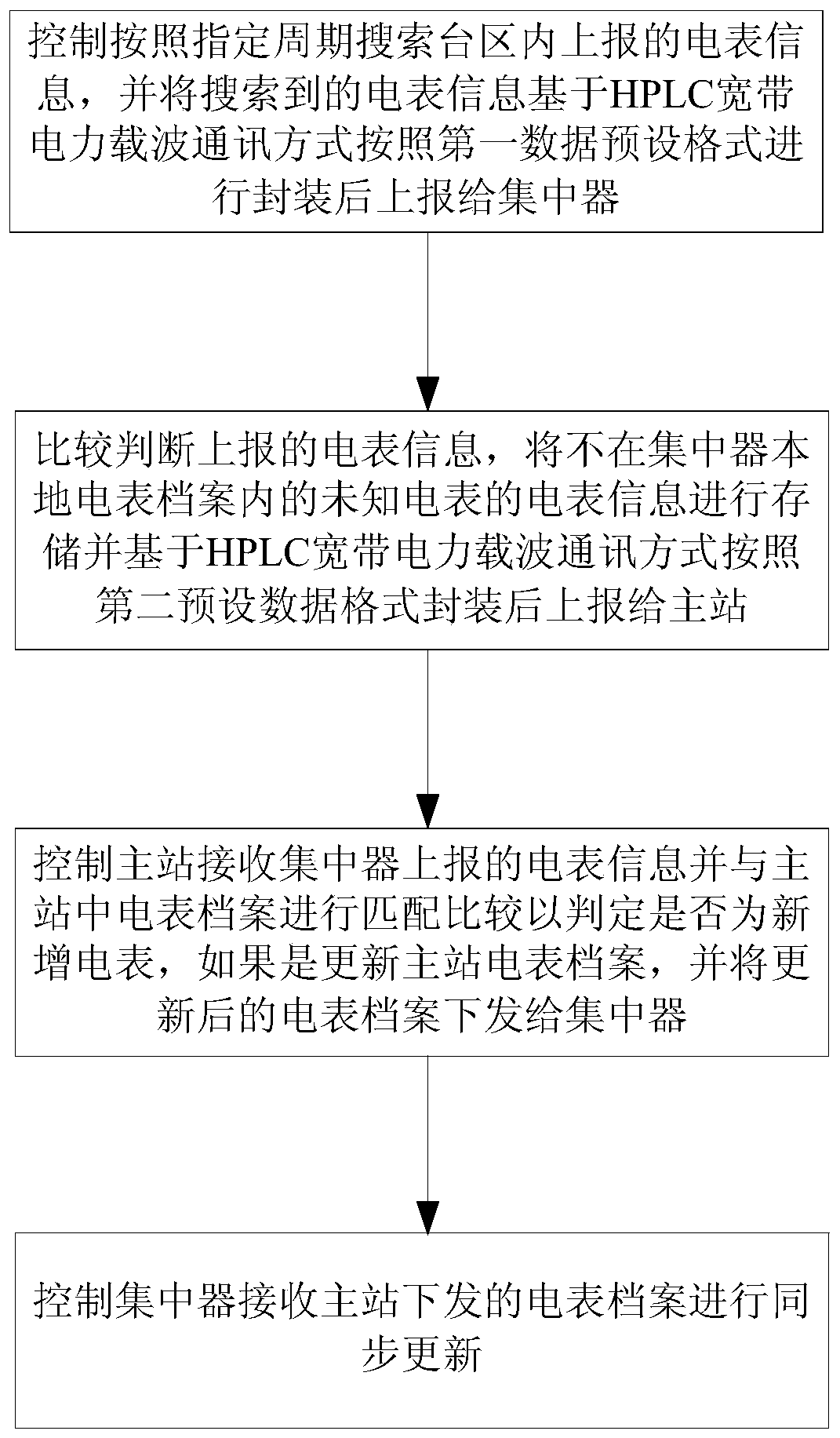
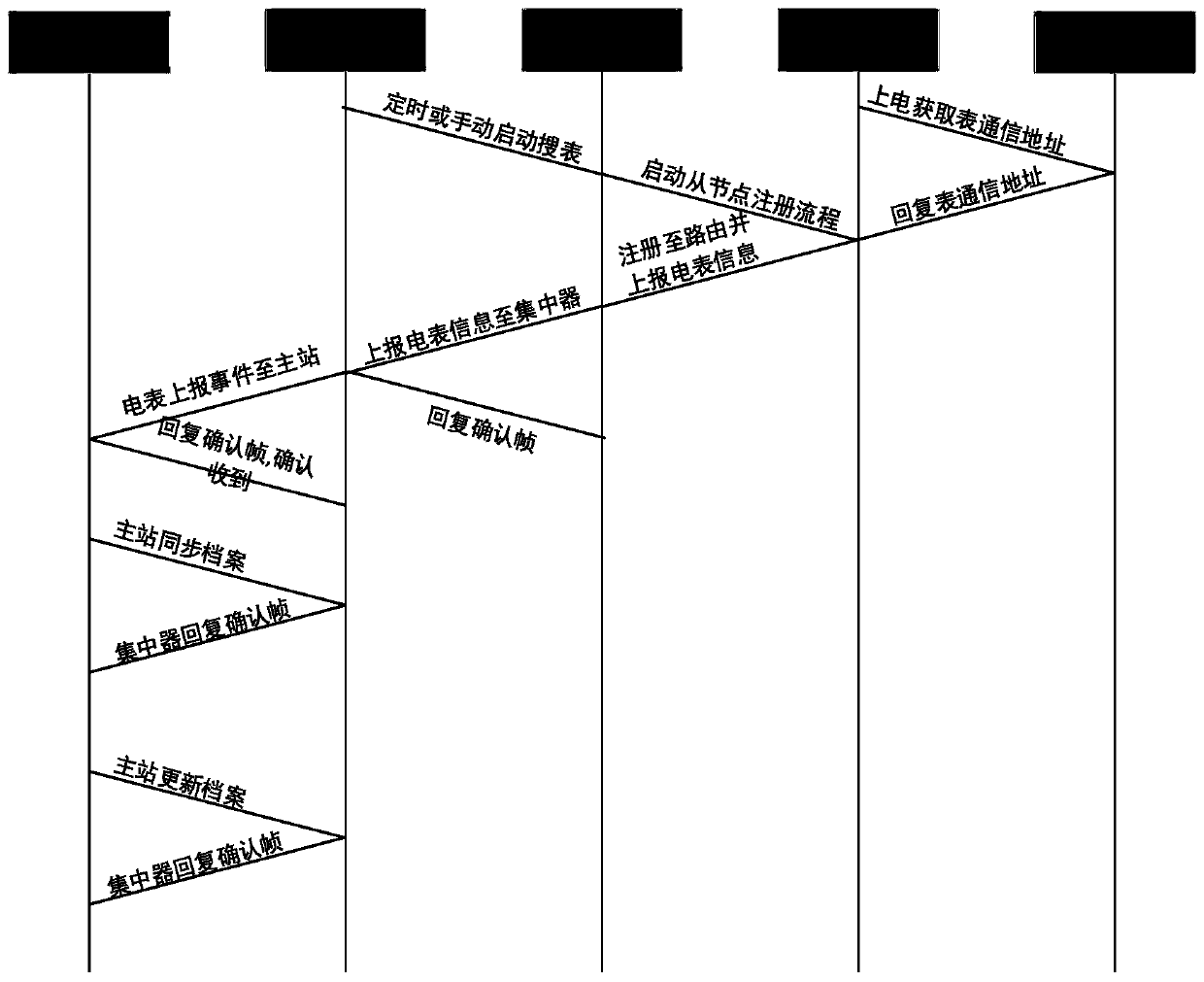
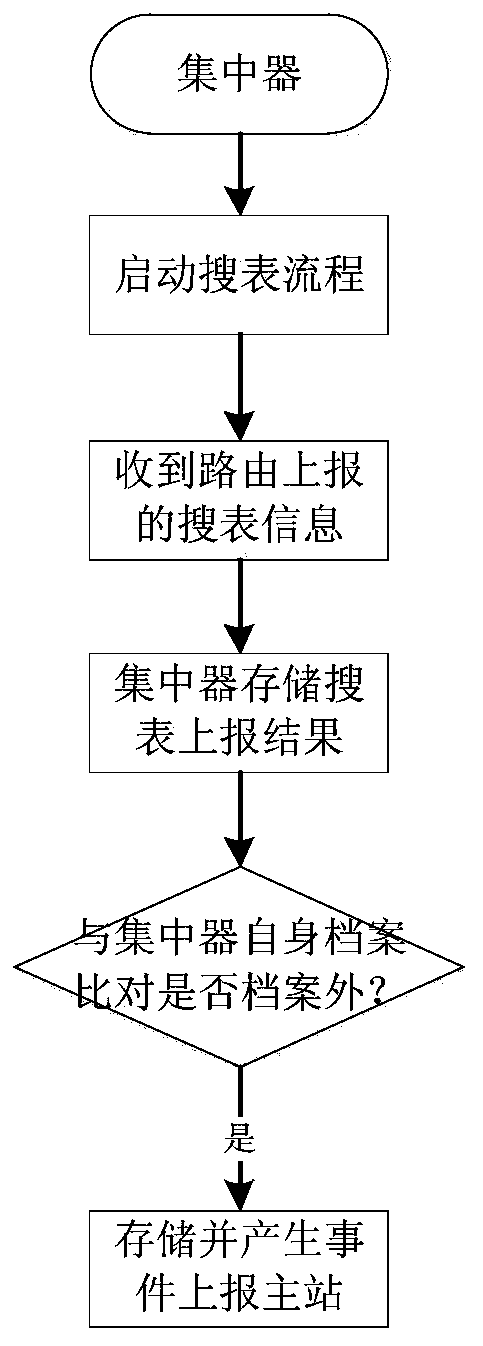
The invention discloses an HPLC (High Performance Liquid Chromatography)-based carrier electric meter intelligent management method and device, and the method comprises the following steps: S1, searching electric meter information reported in a transformer area according to a specified period, packaging the searched electric meter information based on HPLC according to a first data preset format,and reporting the packaged electric meter information to a concentrator; S2, comparing and judging the reported electric meter information, storing the electric meter information which is not in the concentrator local electric meter file, packaging the electric meter information based on HPLC according to a second preset data format, and reporting the packaged electric meter information to the main station; S3, enabling the master station to receive the electric meter information reported by the concentrator, match and compare the electric meter information with the electric meter file in themaster station, update the electric meter file of the master station, and issue the updated electric meter file to the concentrator; and S4, enabling the concentrator to receive the electric meter file issued by the master station and synchronously update the electric meter file. According to the invention, intelligent management of the carrier electric meter can be realized based on HPLC power line carrier communication, so that the power utilization data acquisition efficiency and the success rate are greatly improved.
Owner:STATE GRID HUNAN ELECTRIC POWER +2
Method for detecting quality of compound capsule prepared from 8 kinds of amino acids and 11 kinds of vitamins
ActiveCN102072846AQuality improvementGuaranteed clinical efficacyWeighing by removing componentComponent separationVitamin b6Vitamin C
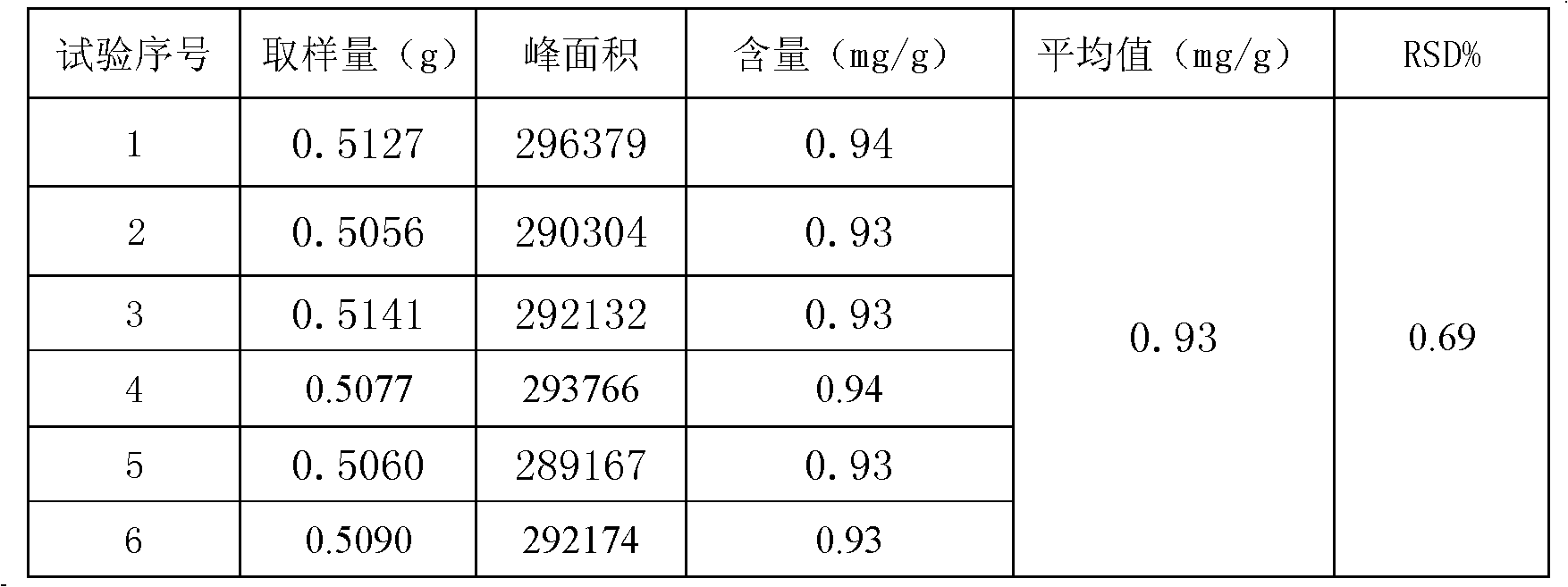
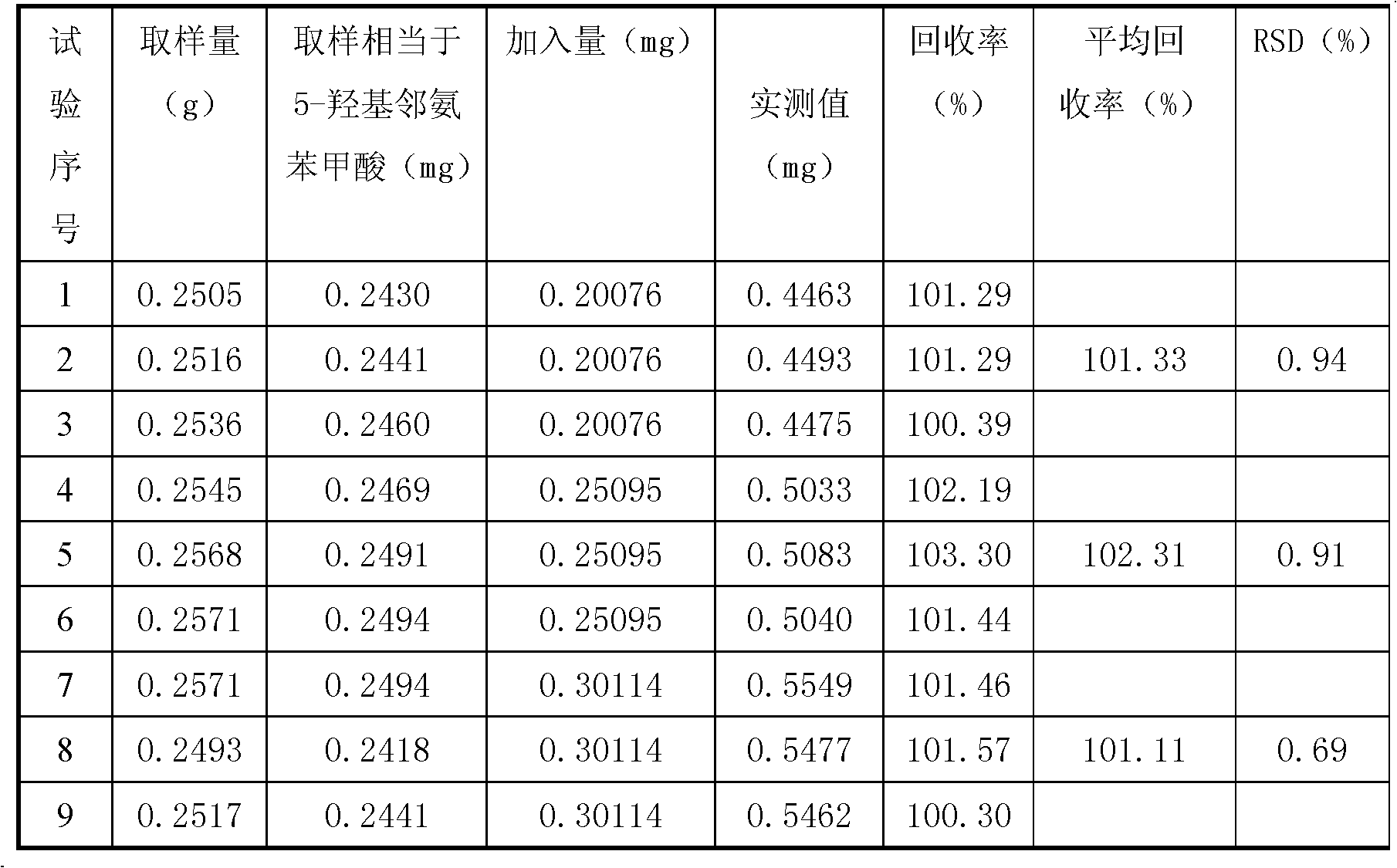
The invention discloses a method for detecting the quality of a compound capsule prepared from 8 kinds of amino acids and 11 kinds of vitamins, mainly comprising the steps of detecting whether the inclusion of the capsule is yellow or tangerine powder or particles, differentiating, checking the loss on drying and measuring the contents of amino acids, vitamin C, niacinamide, vitamin B1, vitamin B6, vitamin B2, calcium pantothenate, folic acid, vitamin A, vitamin D2, vitamin E and 5-hydroxy o-aminobenzoic acid in the capsule by adopting the high-performance liquid chromatography. The method for detecting the quality of the compound capsule prepared from 8 kinds of amino acids and 11 kinds of vitamins is scientific and reasonable, has high degree of accuracy, good repeatability, can comprehensively and effectively control the quality of the compound capsule prepared from 8 kinds of amino acids and 11 kinds of vitamins, and can ensure the clinical efficacy of the preparation.
Owner:GUIZHOU MAQIKA PHARMA
Crystal form F of ibrutinib and preparation method
InactiveCN105646498AImprove stabilityHigh purityOrganic active ingredientsOrganic chemistry methodsSolubilitySolvent
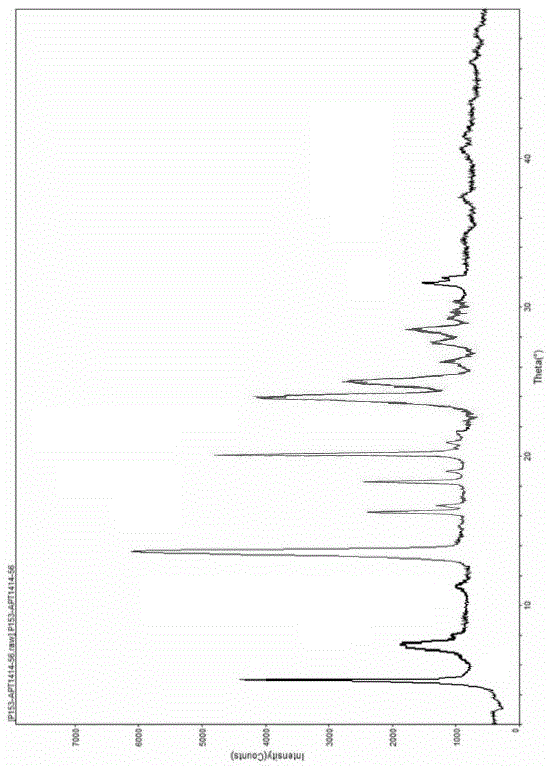
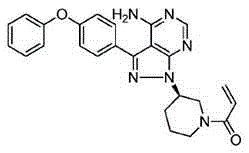
The invention discloses a crystal form F of ibrutinib. The crystal form F is characterized in that X-ray powder diffraction (X-RPD) which adopts Cu-Kalpha radiation and is represented with a 2theta angle has diffraction peaks in positions at angles of 3.7 degrees plus or minus 0.2 degrees, 6.7 degrees plus or minus 0.2 degrees, 13.2 degrees plus or minus 0.2 degrees, 16.1 degrees plus or minus 0.2 degrees, 19.1 degrees plus or minus 0.2 degrees, 20.0 degrees plus or minus 0.2 degrees, 23.8 degrees plus or minus 0.2 degrees and 24.6 degrees plus or minus 0.2 degrees. Related solvents in a preparation process of the crystal form F are cheap, the conditions are mild, the operation is simple, good controllability and reproducibility are realized, further, the prepared crystal form has great stability, the HPLC (high performance liquid chromatography) purity is higher than 99%, and the phenomenon of crystal transformation can be avoided; besides, the solubility is high, the dissolubility is good, and the bioavailability is high.
Owner:孙霖
Method for extracting purified dendrobine from dendrobium stem
The invention mainly relates to a method for extracting purified dendrobine from dendrobium stem. The method comprises the following steps of: firstly, extracting with acid alcohol, enriching and purifying extract by cation exchange resin, stripping with an organic solvent, and separating and preparing by HPLC (High Performance Liquid Chromatography). The stable extract with the dendrobine content not less than 90% is prepared by the method, the extract is furthered prepared by the HPLC, and the dendrobine with the purity not less than 95% is obtained. Compared with the traditional process, the invention has the advantages of high selectivity, moderate reaction condition, simple process, short production period, and high dendrobine yield and purity. By utilizing the HPLC to separate and prepare, the dendrobine loss can be reduced, the relevant matters with similar structure can be obtained, the practical value is high, and a new path by utilizing the dendrobium stem plants is provided.
Owner:ZUNYI MEDICAL UNIVERSITY
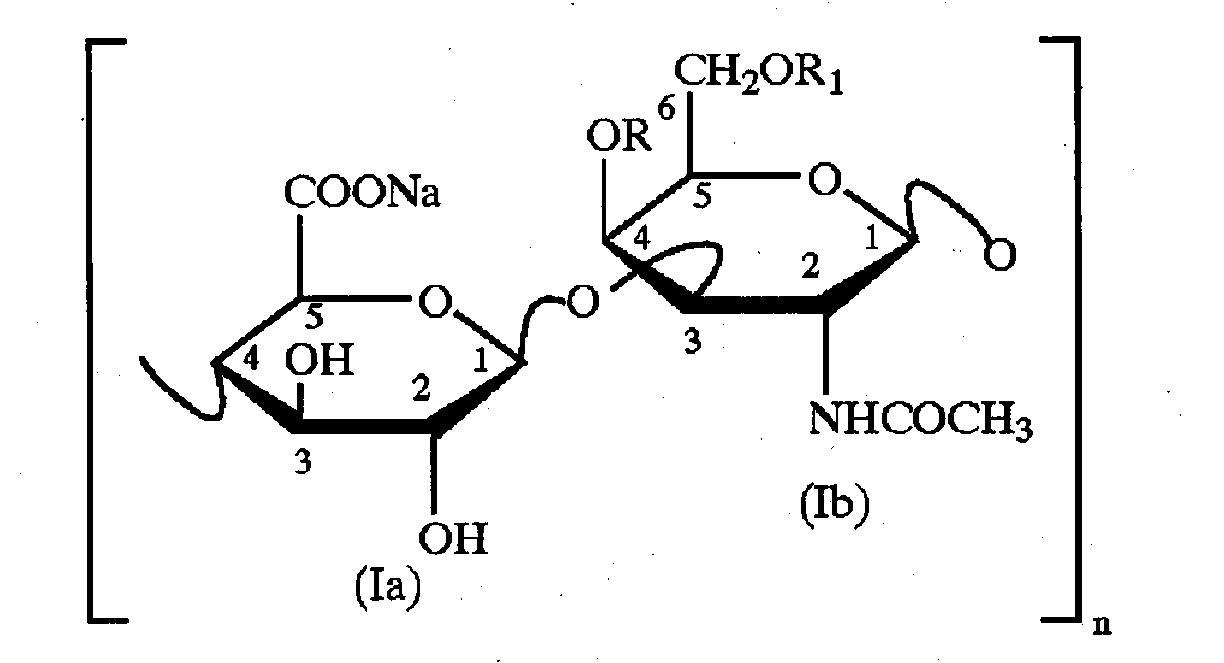
Method for quantitatively detecting hyaluronic acid fragment structure change
ActiveCN102323344AComponent separationMaterial analysis by observing effect on chemical indicatorCarbazoleFluid phase
The invention relates to a method for quantitatively detecting the structure change of a hyaluronic acid fragment produced by degrading a polymer sodium hyaluronate. A high performance liquid chromatography and a carbazole method are used for simultaneously measuring the content of the hyaluronic acid fragment, and a difference between the contents obtained by the two methods is used for quantifying the structure change of the hyaluronic acid fragment prepared by different methods. The method provided by the invention is suitable for the hyaluronic acid fragment prepared by different methods,can evaluate the damage degree to the hyaluronic acid structure caused by the degradation method and preliminarily judge the production method of the hyaluronic acid fragment according to the measurement result, and is an important measure for evaluating the quality of the hyaluronic acid fragment.
Owner:SHANDONG BLOOMAGE HYINC BIOPHARM CORP LTD
Composition of oral medicine preparation and its preparing method
A medicinal composition for the orally taken medicines in the form of tablet, capsule, particle, micropill, dripping pill, etc contains danshinolic acid A and dashinolic acid B. Its preparing process and quality control method are also disclosed.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Preparation method of (Lactobacillus planetarium subsp. plantarum)Zhang-LL and its listeria monocytogene-resistant bacteriocin
ActiveCN104531562ASource securityStable sourceBacteriaMicroorganism based processesFood borneListeria murrayi
The invention relates to a preparation method of (Lactobacillus planetarium subsp.plantarum)Zhang-LL and its listeria monocytogene-resistant bacteriocin. The preparation method is suitable for preservation and fresh-keeping of meat and meat products, milk and milk products, fruits and vegetable, and instant foods. The (Lactobacillus planetarium subsp.plantarum)Zhang-LL (CGMCC No.6936) is selected from bacon on the Fujian farmer's market. A bacteriocin production broth is obtained by fermentation of a Zhang-LL strain, and the Zhang-LL strain bacteriocin is extracted and purified by a pH-dependent adsorption-desorption method, a cation exchange chromatography and a reversed phase high-performance liquid chromatography so that titer is improved by 32 times and purity is improved by 36.65 times. The bacteriocin can inhibit a plurality of food-borne pathogenic bacteria such as listeria monocytogenes, has high bacteriostatic activity, good heat, acid and base stability, can be degraded by human protease and is a natural and safe biological preservative. The preparation method has the advantages of simple processes, stability, high efficiency, source convenience, low cost and industrial production feasibility.
Owner:BEIJING BEINONG HONGZE BIOTECH CO LTD
Determination method of benzo(a)pyrene in Camellia oleifera seed oil
InactiveCN102288720AAccurate analysisSusceptible to outside interferenceComponent separationEnvironment effectFood safety
The invention discloses a method for detecting benzo(a)pyrene in Camellia oleifera seed oil, belonging to the technical field of food safety detection. It includes steps such as sample extraction, purification and qualitative and quantitative analysis. The invention adopts a stable high-performance liquid chromatography method, is less affected by the external environment, can accurately quantitatively analyze, and has a detection limit of 1 μg / kg. The invention adopts a saponification method to change the properties of the oil matrix, and then purifies it with an alumina purification column, so that a better purification effect can be achieved even if the sampling amount is increased.
Owner:RES INST OF SUBTROPICAL FORESTRY CHINESE ACAD OF FORESTRY
Pharmaceutical compositions containing pemetrexed having extended storage stability
ActiveUS20130231357A1Improve long-term stabilityBiocideOrganic active ingredientsDiseasePharmaceutical medicine
Long term storage stable pemetrexed-containing liquid pharmaceutical compositions are disclosed. The compositions can include pemetrexed or pharmaceutically acceptable salts thereof; an antioxidant selected from lipoic acid, dihydrolipoic acid, methionine and mixtures thereof; a chelating agent selected from lactobionic acid, sodium citrate, tribasic and mixtures thereof; and a pharmaceutically acceptable fluid. The pH of the compositions is in a range of about 8 to about 9.5. The pemetrexed-containing compositions have less than about 5% total impurities, on a normalized peak area response (“PAR”) basis as determined by high performance liquid chromatography (“HPLC”) at a wavelength of 227 nm, after at least about 18 months of storage at a temperature of from about 5° C. to about 25° C. Methods of preparing the formulation as well as methods of treatment of pemetrexed-susceptible diseases using the same are also disclosed.
Owner:EAGLE PHARMACEUTICALS INC
L-d-glutamic oxidase with substrate specificity and alpha-oxoglutarate produced by catalysis of same
InactiveCN102994467ANo pollution in the processSimple production processMicroorganism based processesEnzymesSodium GlutamateIsopropyl alcohol
The invention discloses L-d-glutamic oxidase with substrate specificity and alpha-oxoglutarate produced by catalysis of the same, and belongs to the field of enzyme-method catalytic production of fine chemicals. The invention has the advantages that the L-d-glutamic oxidase which only has the substrate specificity to L-glutamic acid, L-sodium glutamate and glutamine is utilized, 20g / L L-sodium glutamate solution is added in fermented liquid which is fermented for 60 hours and contains the L-d-glutamic oxidase, carrying out conversion for 12 hours under the ventilating condition and the conditions that the temperature is 30 DEG C, the pH is 8.5 and the 5% (v / v) isopropyl alcohol, and measured by a high-performance liquid chromatography, the content of alpha-oxoglutarate reaches 14.5g / L.
Owner:JIANGNAN UNIV
Non-interpenetrating chiral MOF stationary phase, its preparation method and application in enantiomer separation in HPLC
InactiveCN103331151ARaw materials are cheap and easy to getEasy to operateAmino compound purification/separationOther chemical processesEnantiomerStructural formula
The invention relates to a non-interpenetrating chiral MOF (metal organic framework) stationary phase, its preparation method and application in enantiomer separation in HPLC (high-performance liquid chromatography). The stationary phase is a non-interpenetrating chiral three-dimensional porous framework complex with a structural formula as {[ZnL].H2O}n. An asymmetric structural unit {[ZnL].H2O} of the complex is composed of a Zn<2+>, an L ligand and a guest water molecule. The L ligand is -NH- containing chiral pyridine carboxylic acid, its chemical composition is [(N-(4-pyridylmethyl)-L-leucine.HBr)], and its molecular formula is C12H19BrN2O2. Chiral amino acid and 4-pyridylaldehyde are selected as raw materials to synthesize the-NH- containing pyridine carboxylic acid chiral ligand by a one-step process. The ligand and zinc acetate are adopted as raw materials to undergo room temperature diffusion so as to obtain the MOF stationary phase. The material provided in the invention has uniform chiral helical channel, uniform aperture and orifice, and can be used for separation of chiral drugs and other enantiomers. The separation is selectively dependent on the size of a separated enantiomer molecular size, but is not dependent on the functional group of the separated enantiomer. Thus, the non-interpenetrating chiral MOF stationary phase has the characteristics of traditional zeolite molecular sieve separation.
Owner:SHANDONG NORMAL UNIV
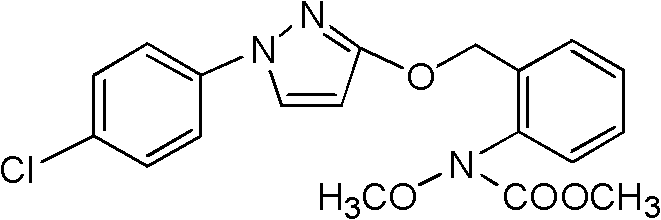
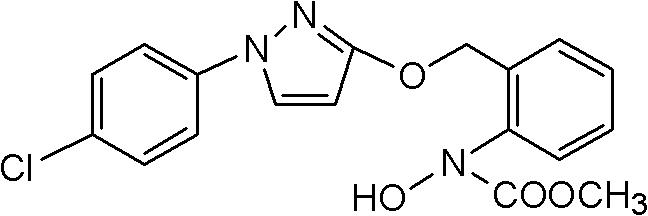
Pyraclostrobin and method for economically synthesizing same
InactiveCN102399190AIncrease profitReduce energy consumptionBiocideOrganic chemistryCarbamateSolvent
The invention aims to provide pyraclostrobin and a method for economically synthesizing the same. High purity pyraclostrobin can be synthesized at low cost. The method comprises the following steps of: adding N-hydroxy-N-2-[(N-parachlorobenzyl)-3-pyrazoloxymethyl]phenyl carbamate and an acid bonding agent into a polar solvent or non-polar solvent, uniformly mixing, adding dimethyl sulfate, and reacting at the temperature of between 20 and 30DEG C; and after the reaction is monitored to be finished through high performance liquid chromatography (HPLC), adding water to neutralize until the pH=7, removing the solvent, immersing in a recrystallization solvent, performing recrystallization, and removing a recrystallization solution to obtain the pyraclostrobin, wherein a molar ratio of the N-hydroxy-N-2-[(N-parachlorobenzyl)-3-pyrazoloxymethyl]phenyl carbamate to the acid bonding agent to the dimethyl sulfate is 1:(1-1.8):(1-1.8). By the invention, the energy consumption is low, the utilization rate of materials is high, the operation is simple, and the yield and purity of the product are high; and compared with the traditional method, the method is more energy-saving, environment-friendly and economic, and has great industrial application value.
Owner:HENAN UNIV OF CHINESE MEDICINE
Screening method for producing chondroitin sulfate bacterial strain and application of bacterial strain fermentation method in production of chondroitin sulfate
The invention relates to a screening method for producing chondroitin sulfate bacillus subtilis and application of a bacterial strain fermentation method in the production of chondroitin sulfate, belonging to the technical field of biological engineering. The bacterial strain disclosed by the invention is bacillus subtilis which is obtained by the following steps of: diluting and coating fermented soya beans subjected to a boiling water bath, dyeing and carrying out microscopic examination on an obtained soporiferous strain, then carrying out primary fermentation and shaking culture one by one on screened bacterial strains, adding chondroitin sulfate standard substance of sharks in a formation liquor to be used as an internal standard, carrying out qualitative analysis and screening by adopting a high performance liquid chromatography, and carrying out morphological, physiological and biochemistric and molecular biological identification on the screened bacterial strains to obtain thebacillus subtilis for producing the chondroitin sulfate. Meanwhile, the invention discloses a method for producing the chondroitin sulfate by using the bacterial strain; and the method is used for fermenting the chondroitin sulfate for 24h, wherein the yield of the chondroitin sulfate is 177mg / L.
Owner:HUNAN WUXING BIOLOGICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com