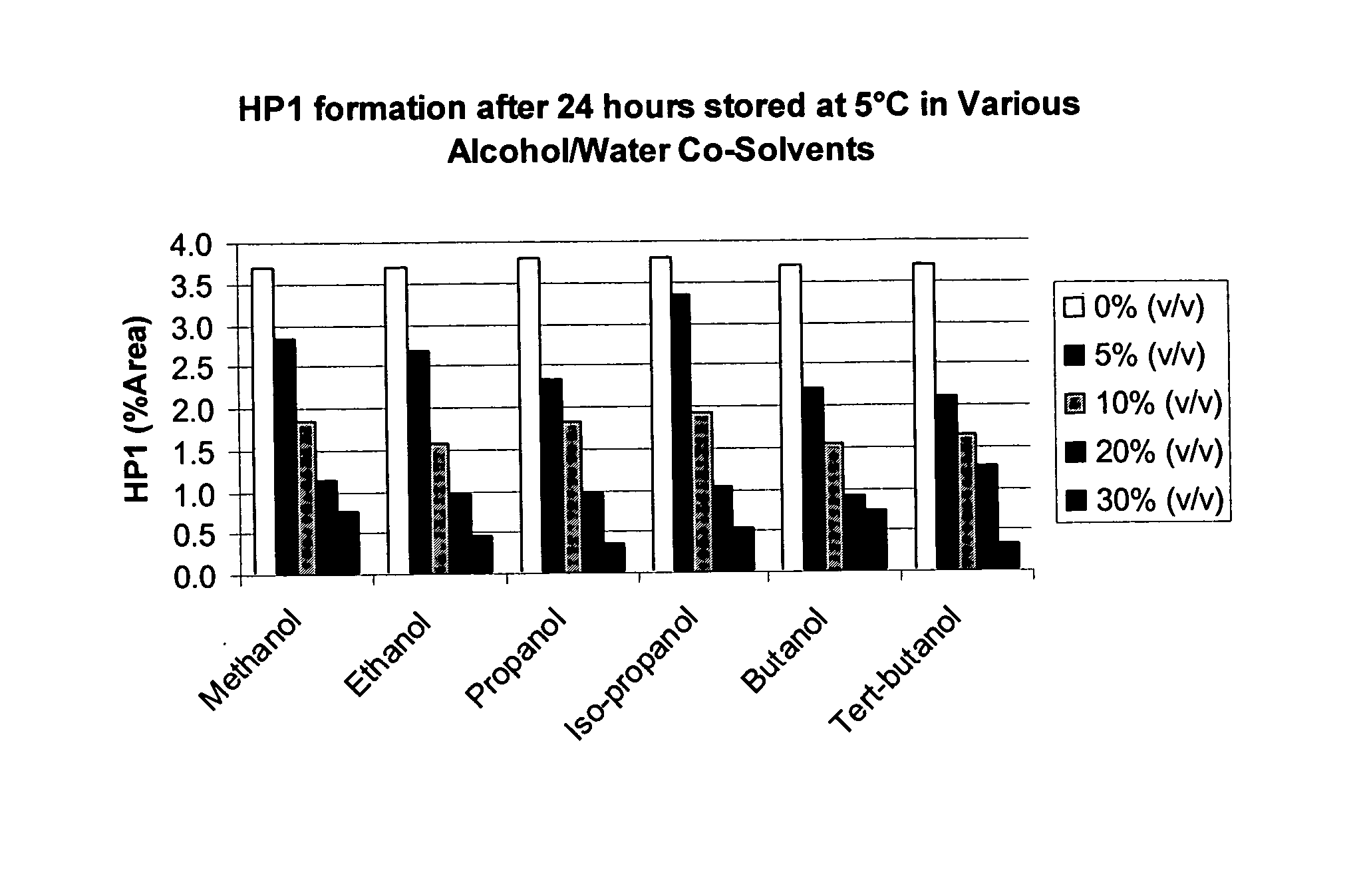
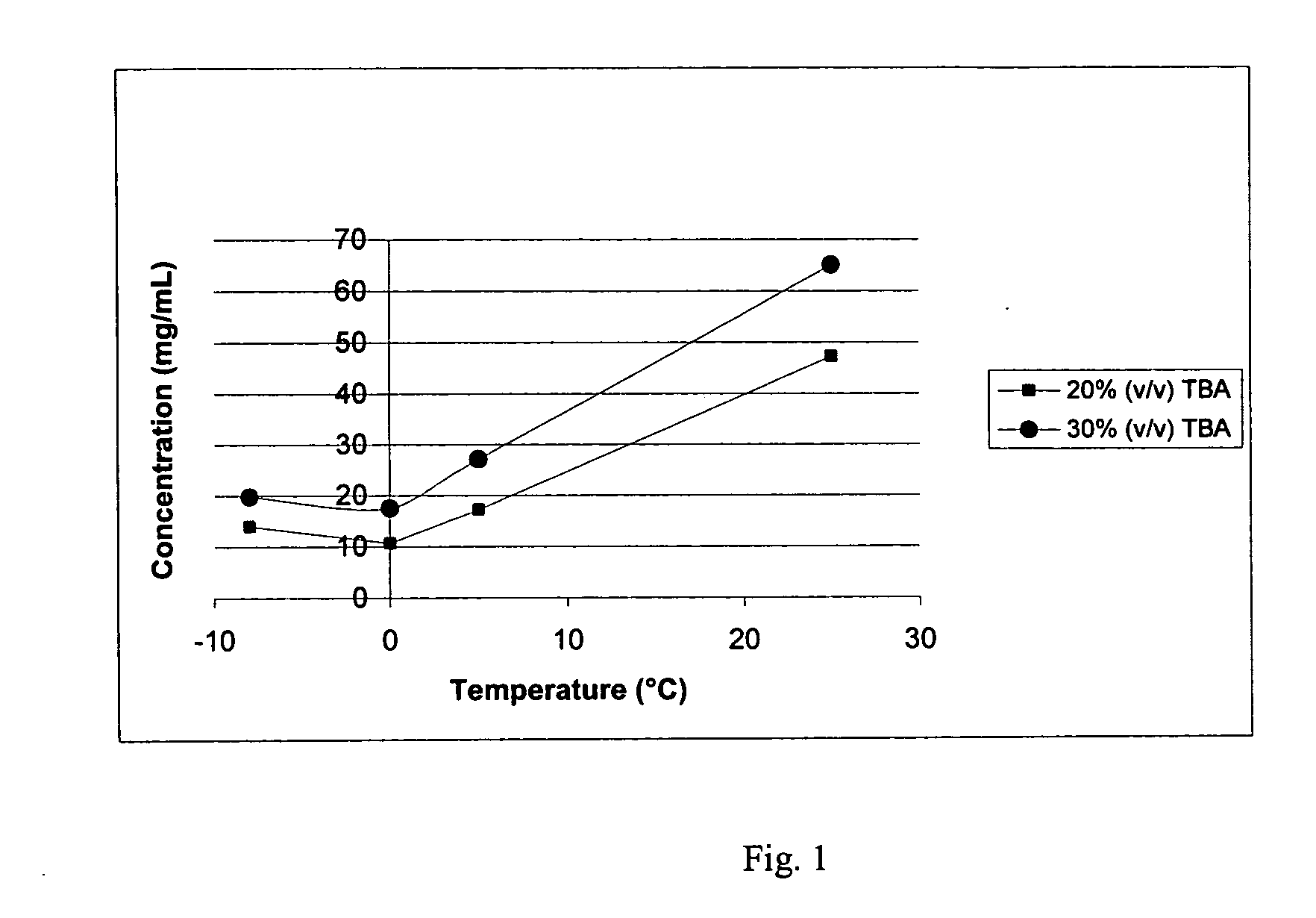
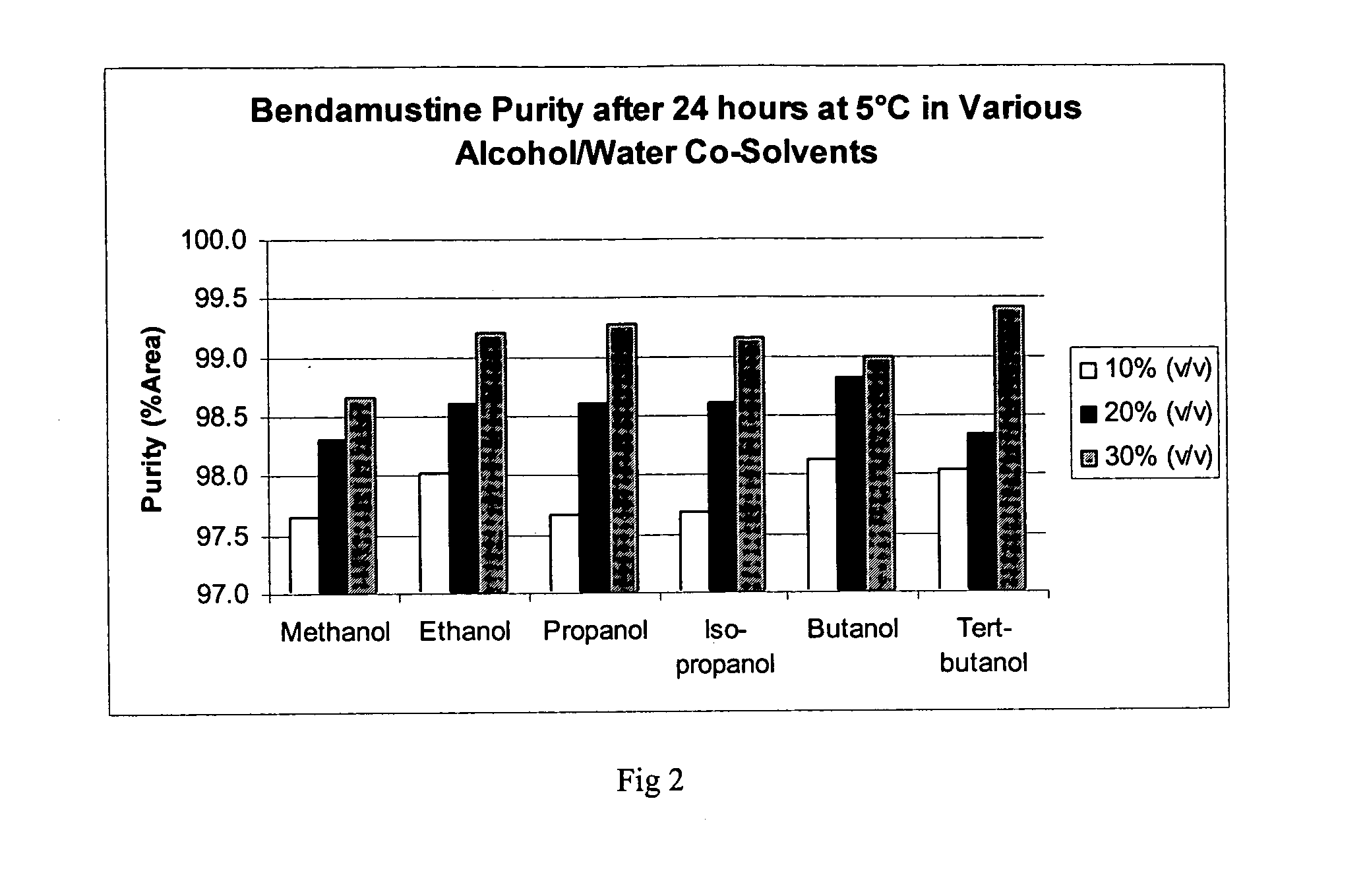
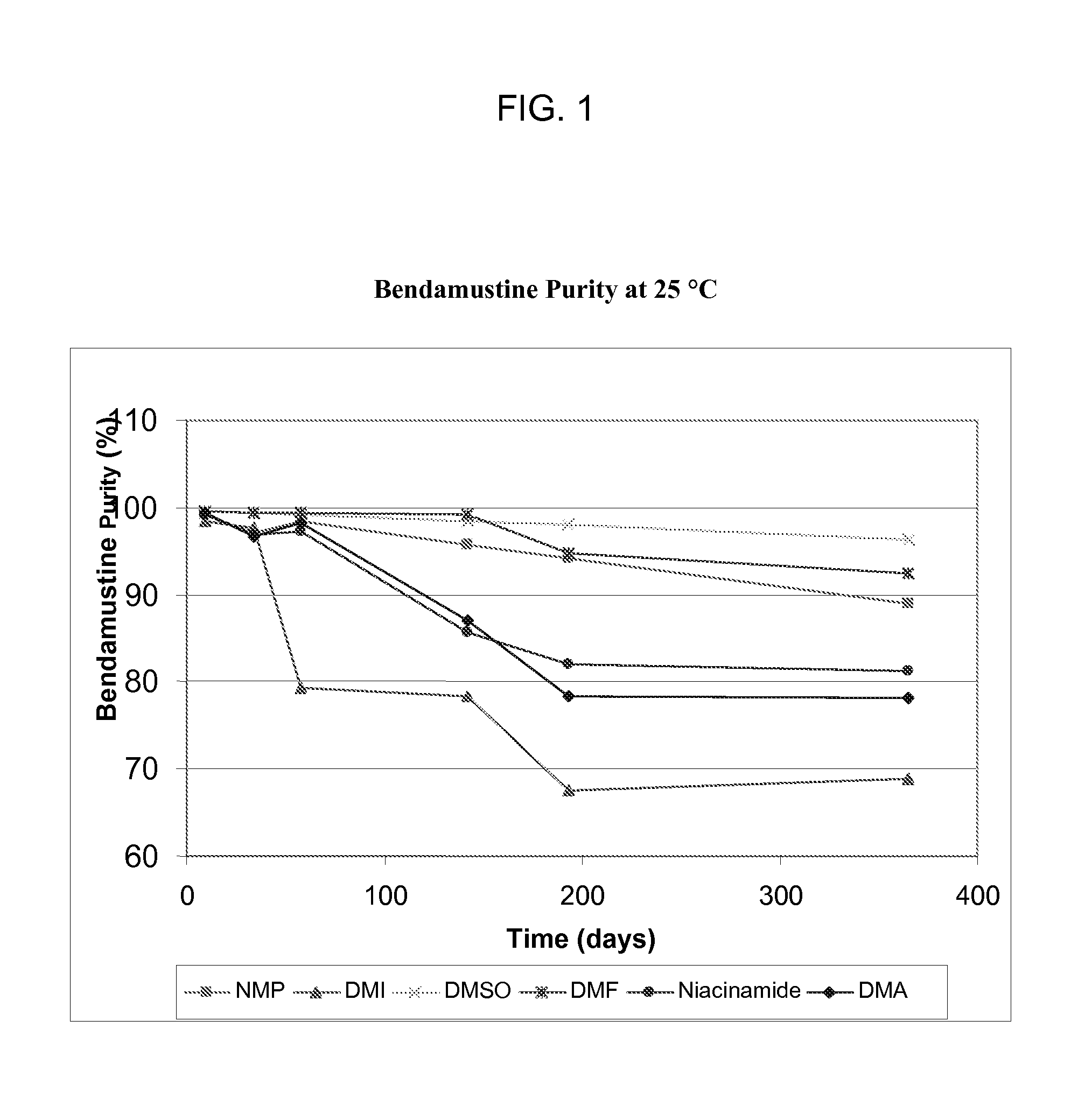
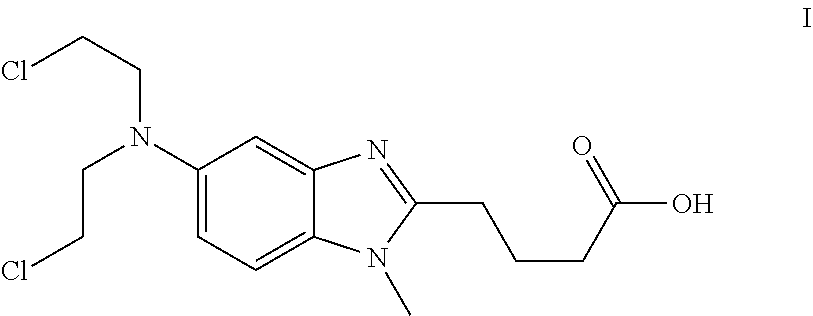
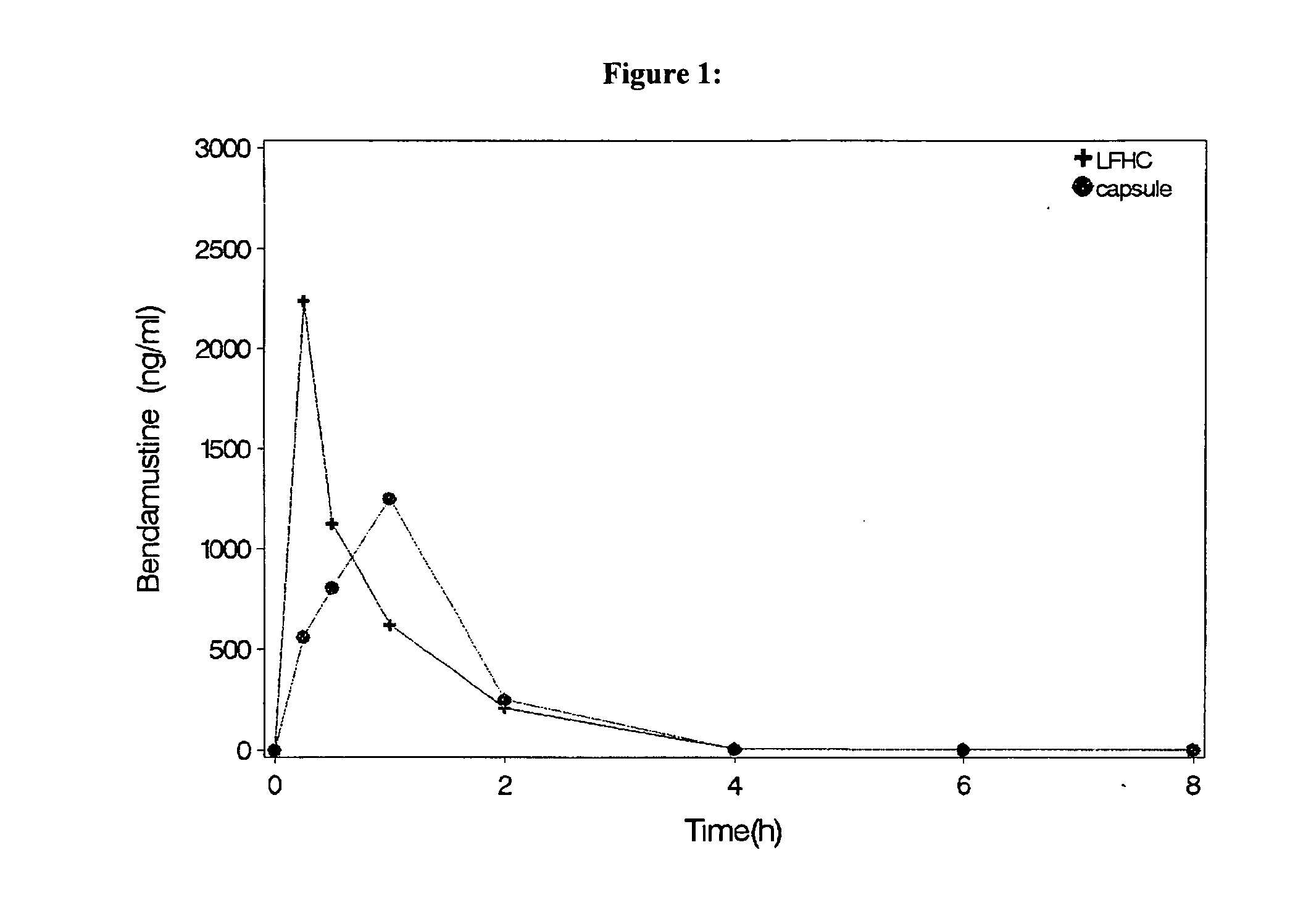
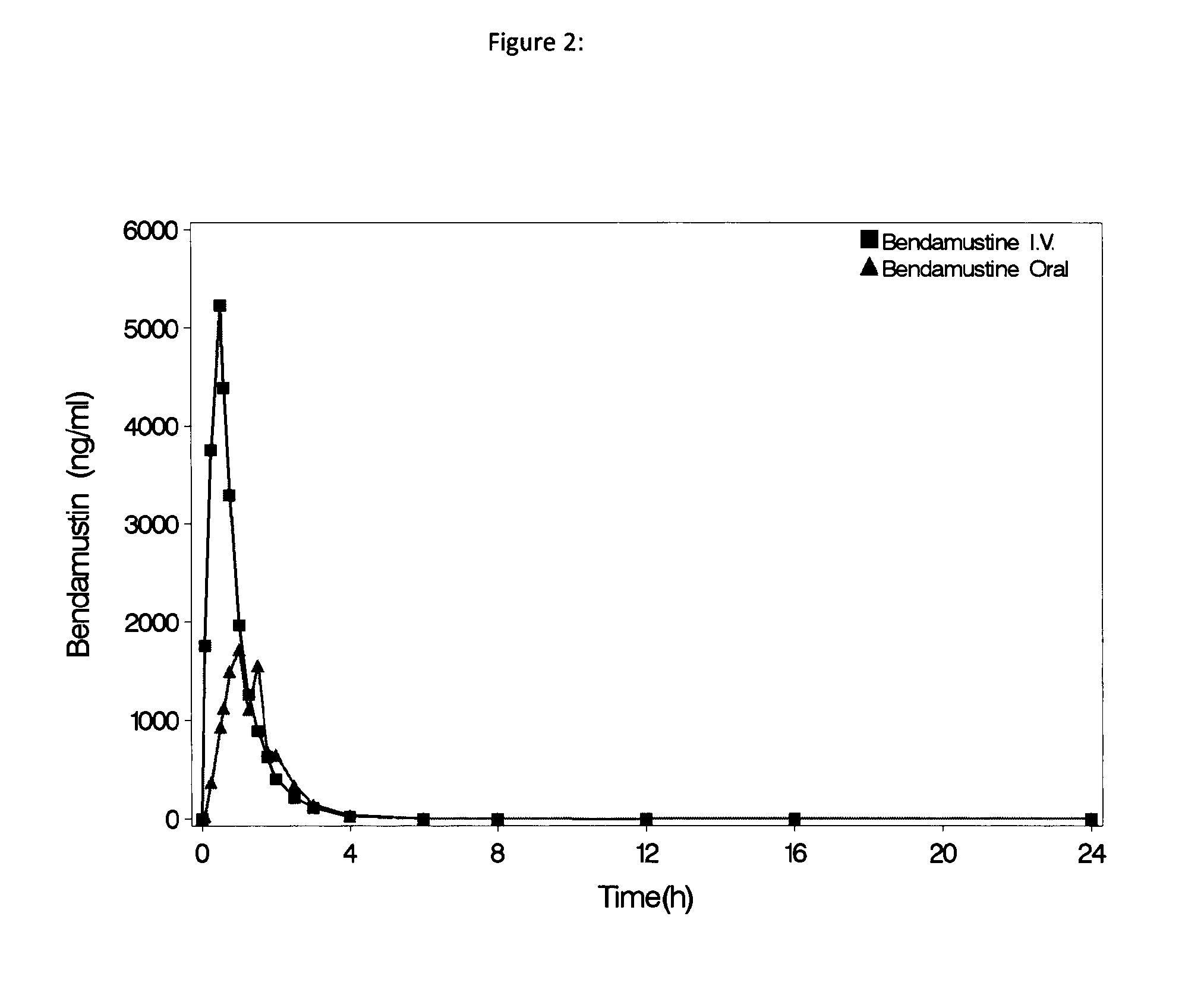
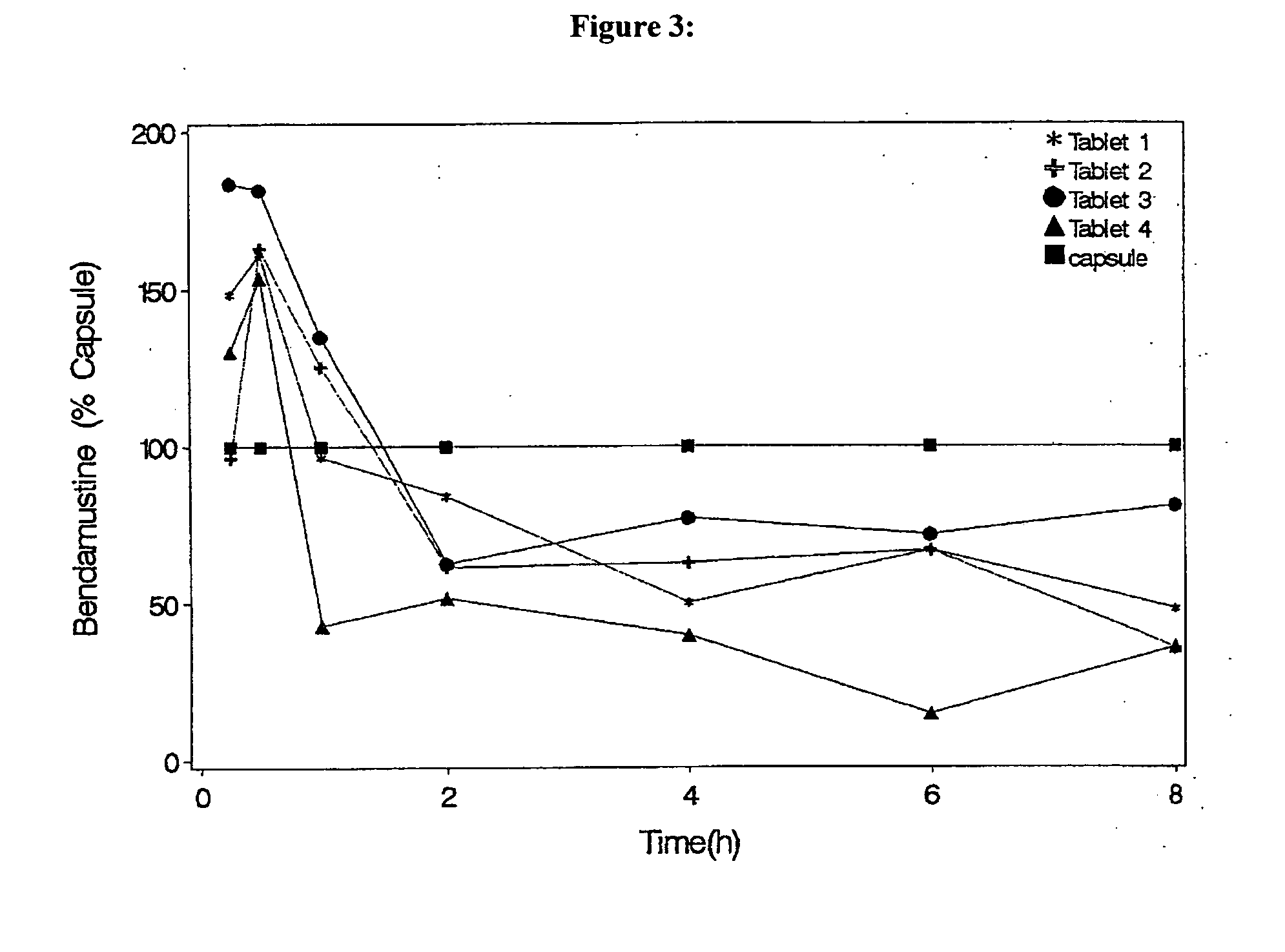
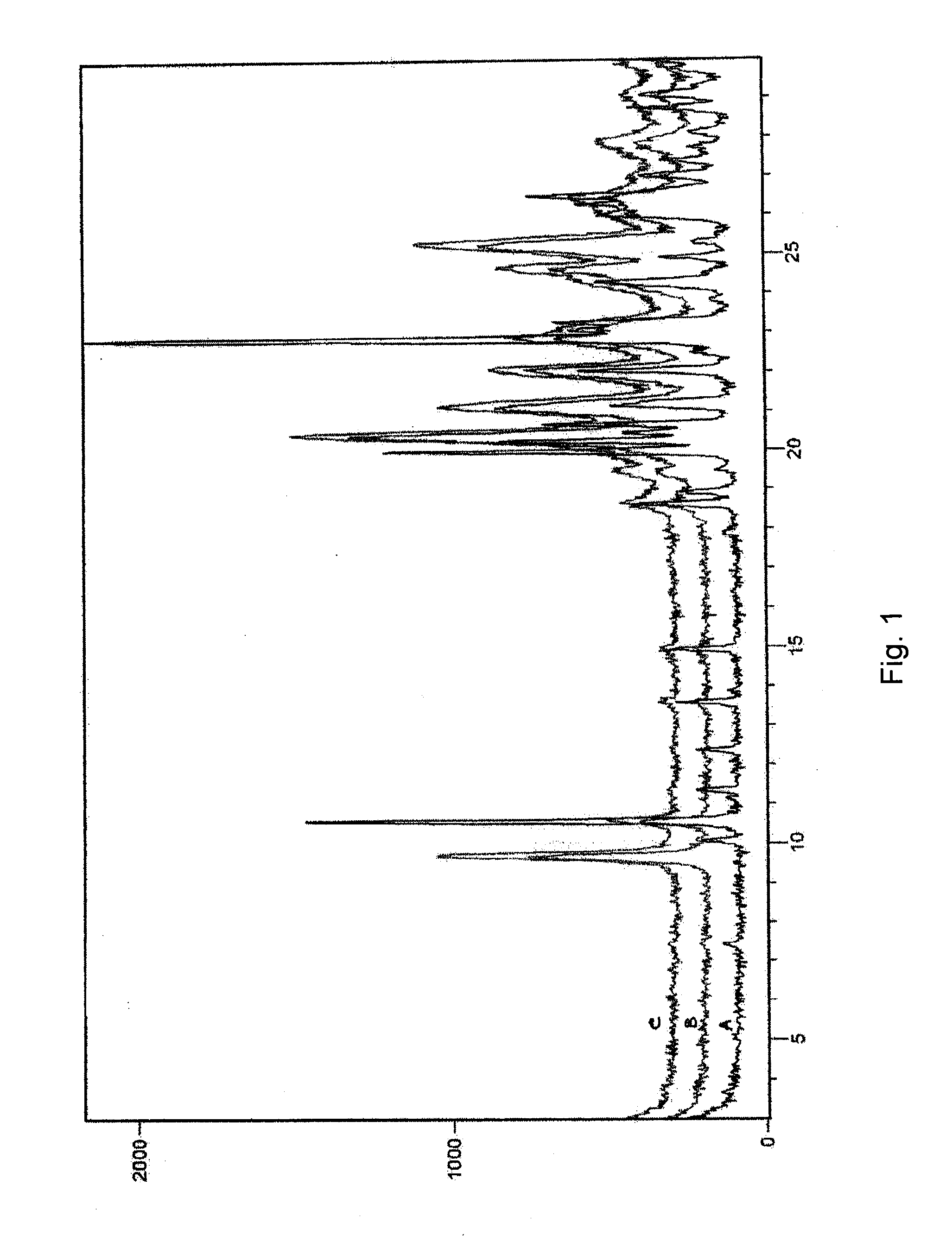
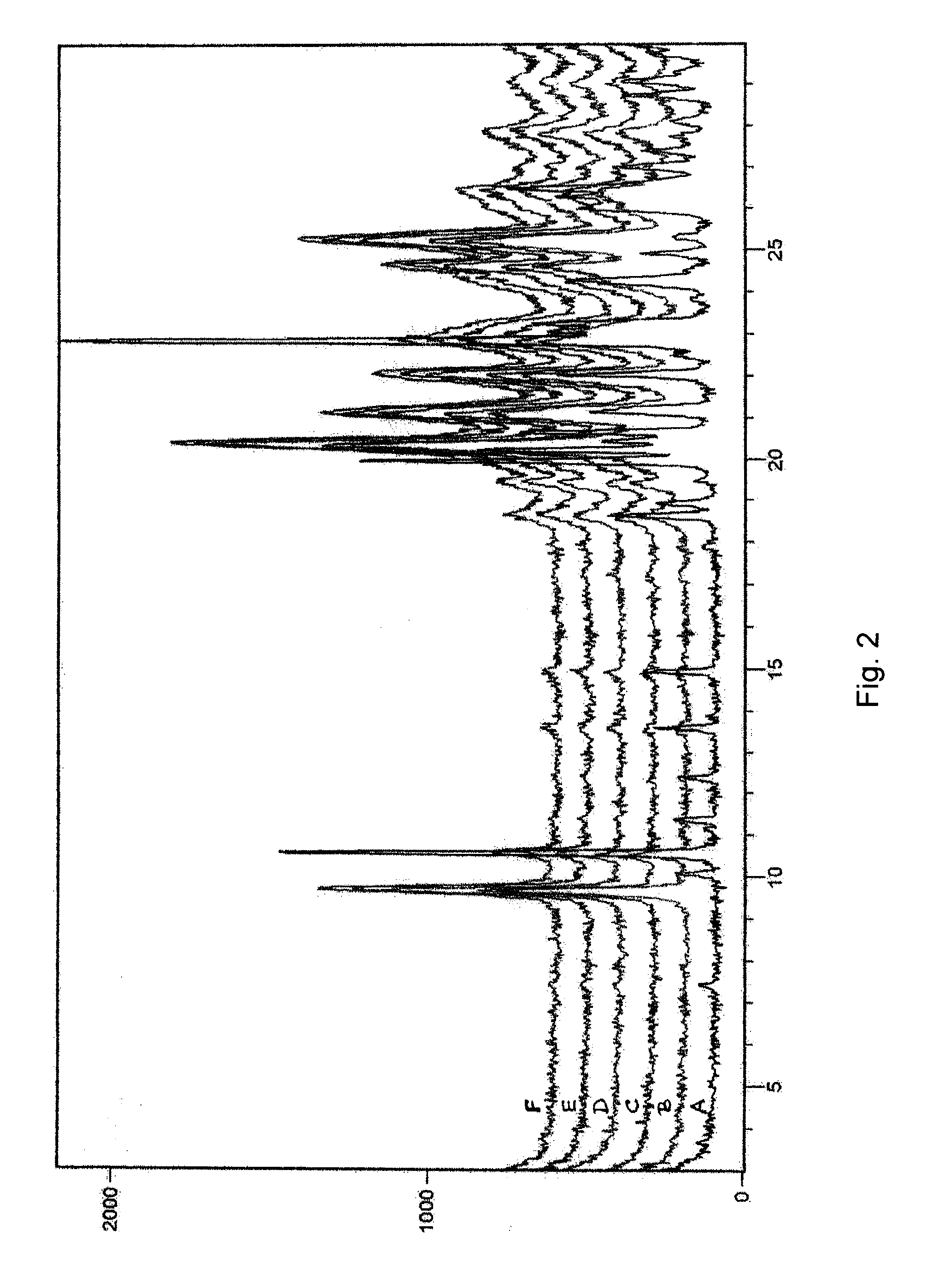
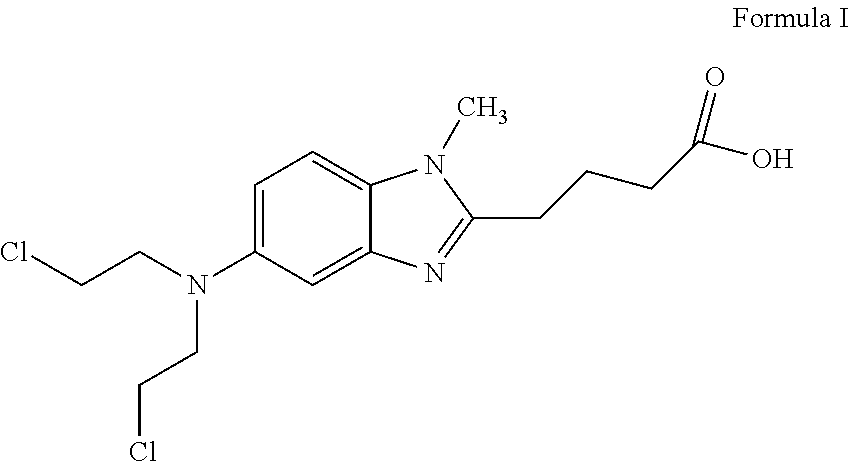
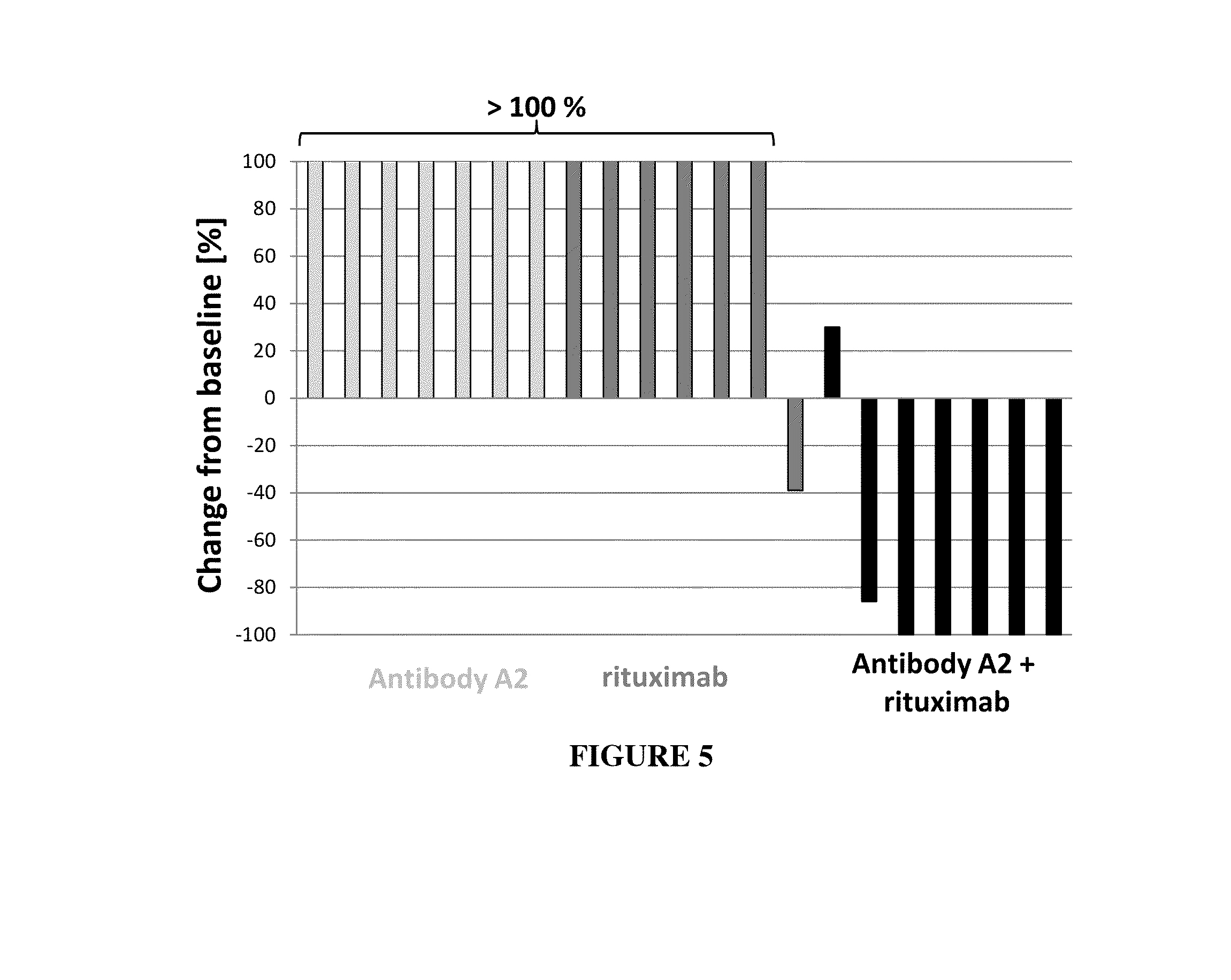
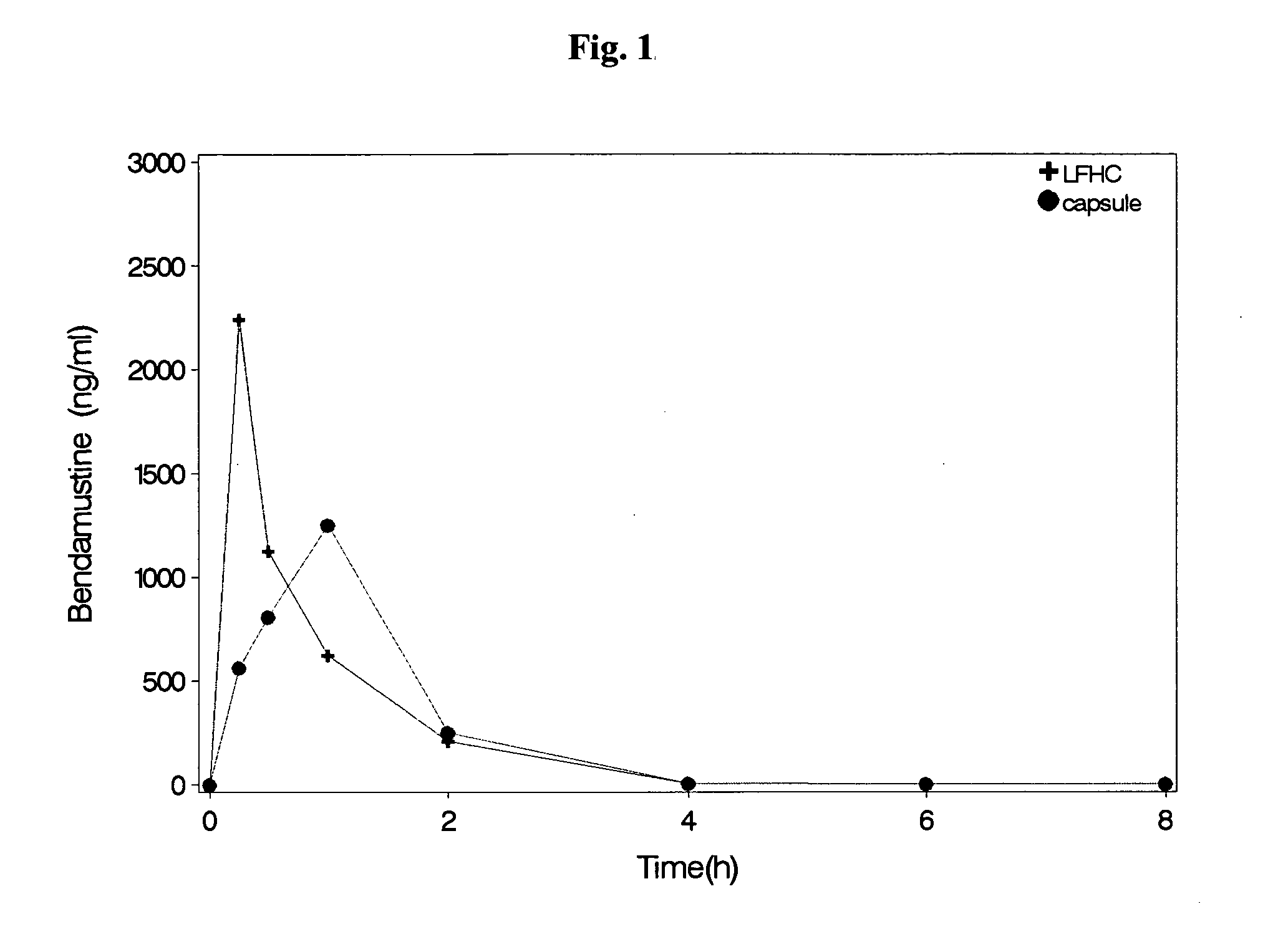
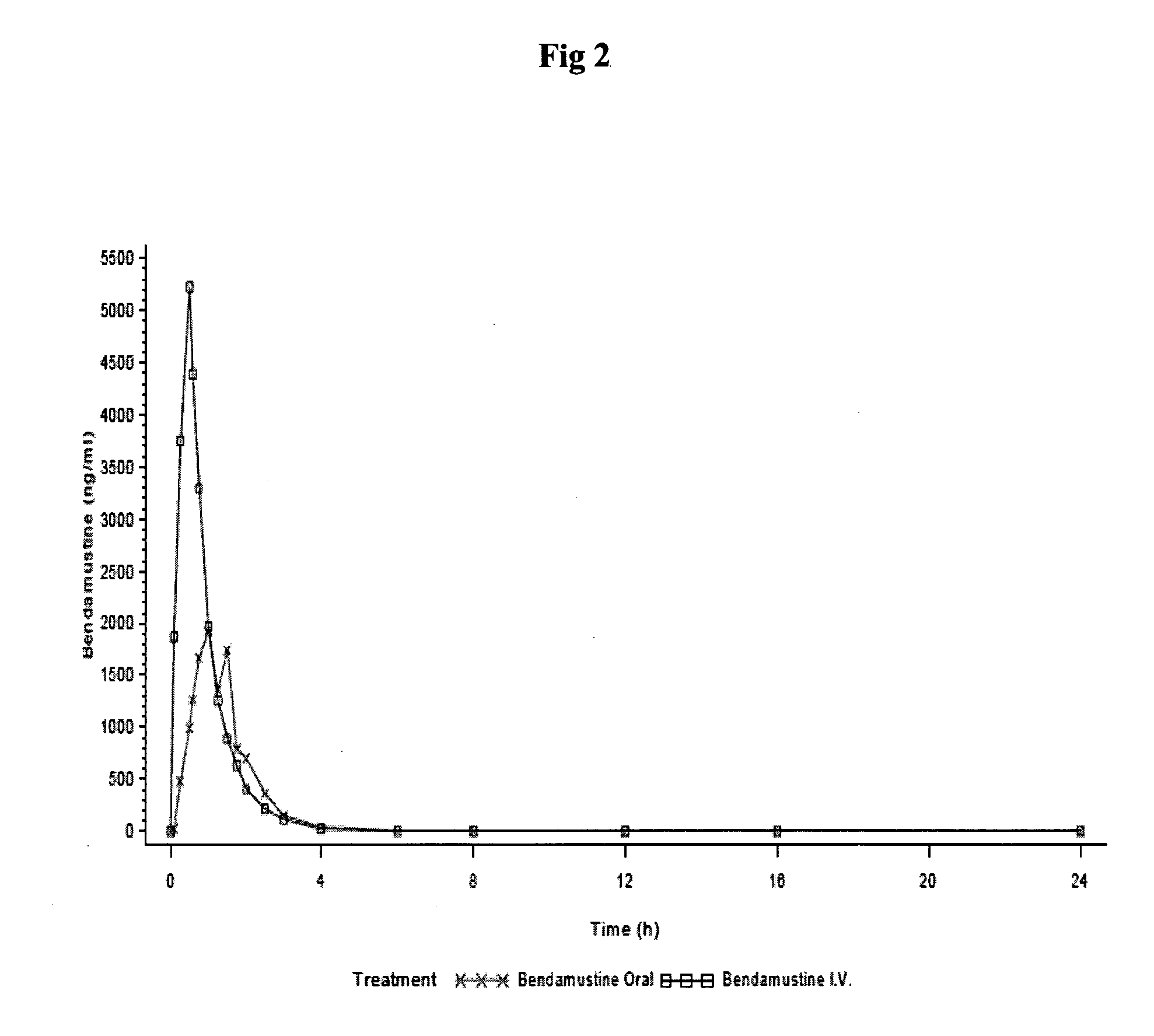
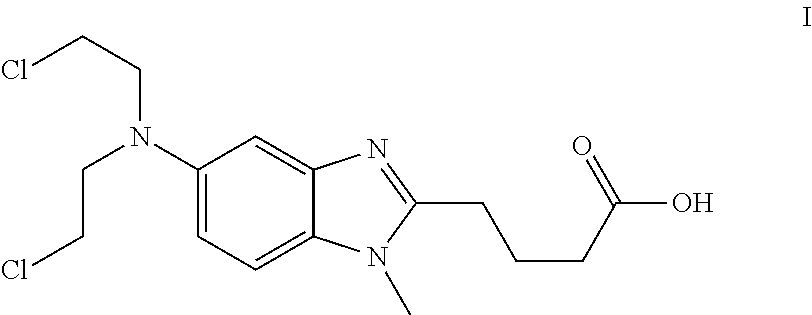
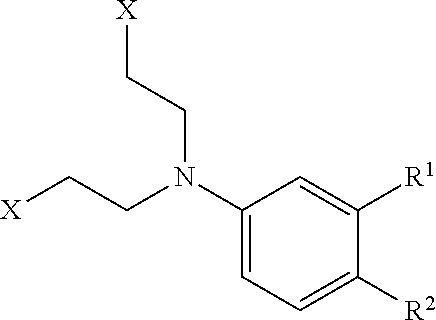
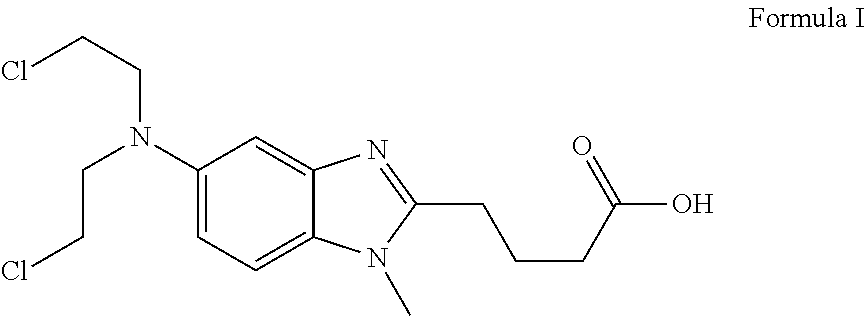
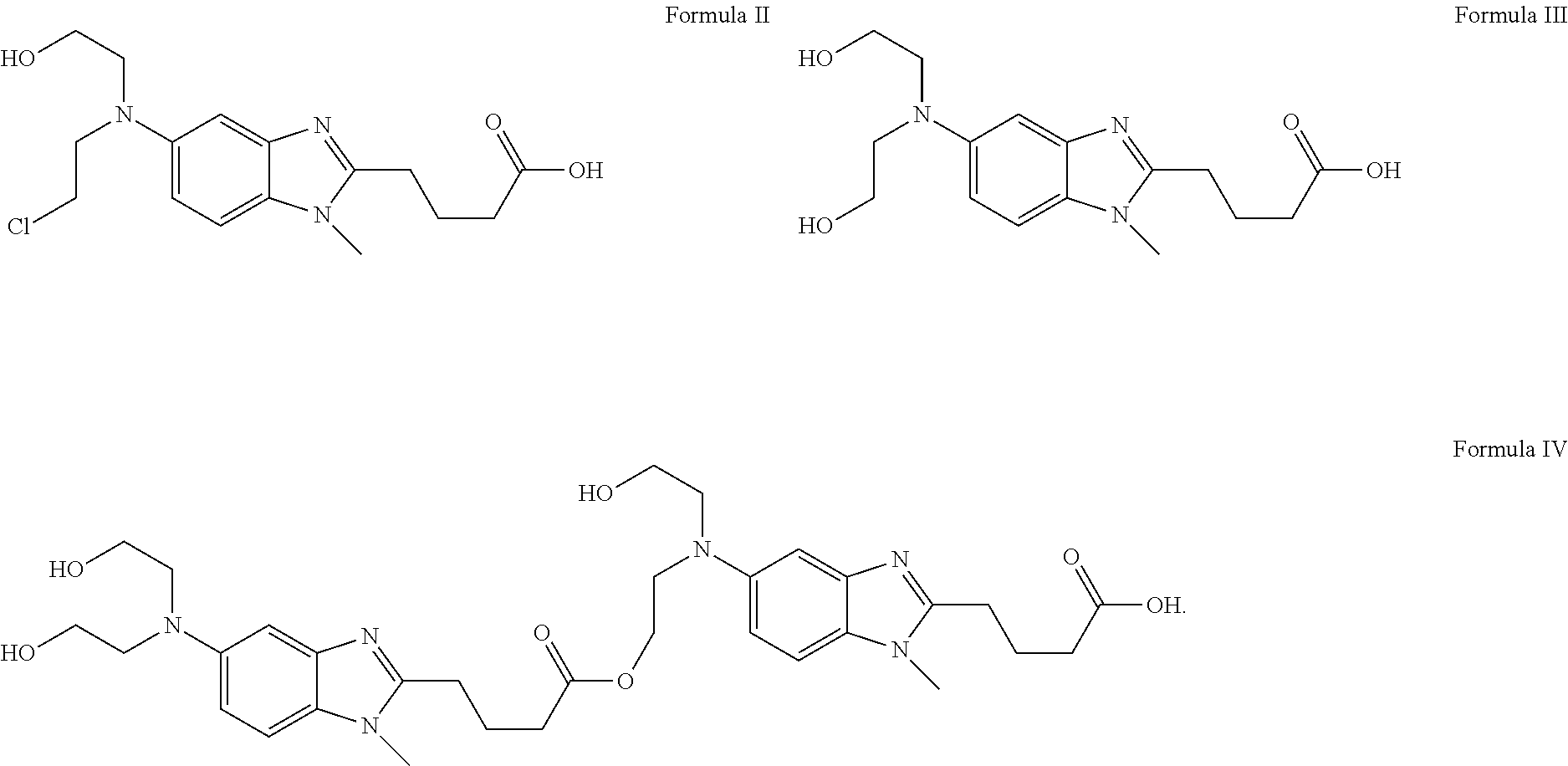
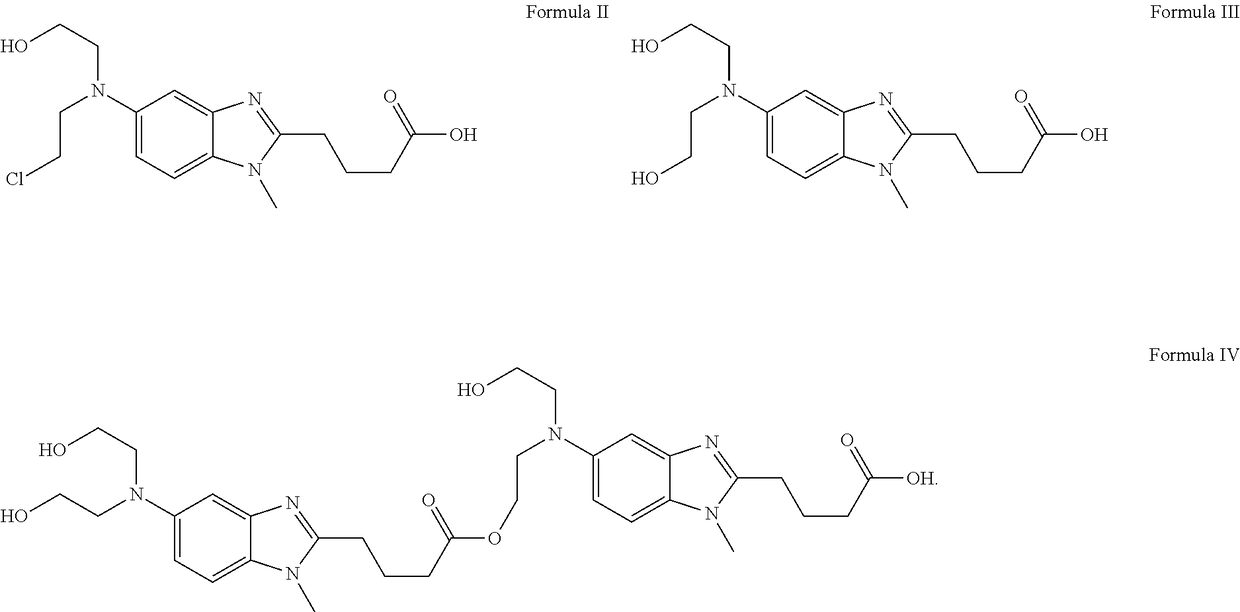
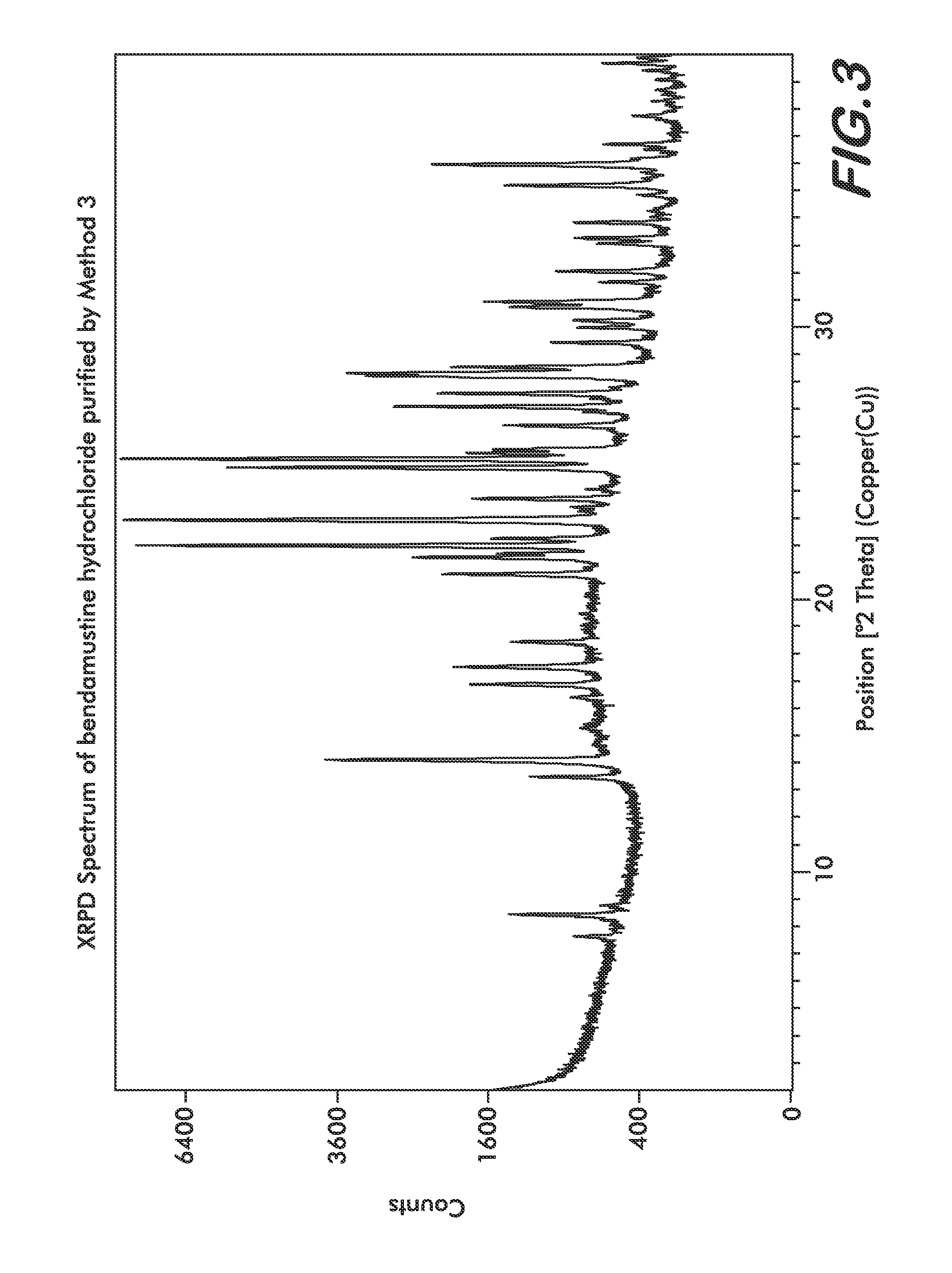
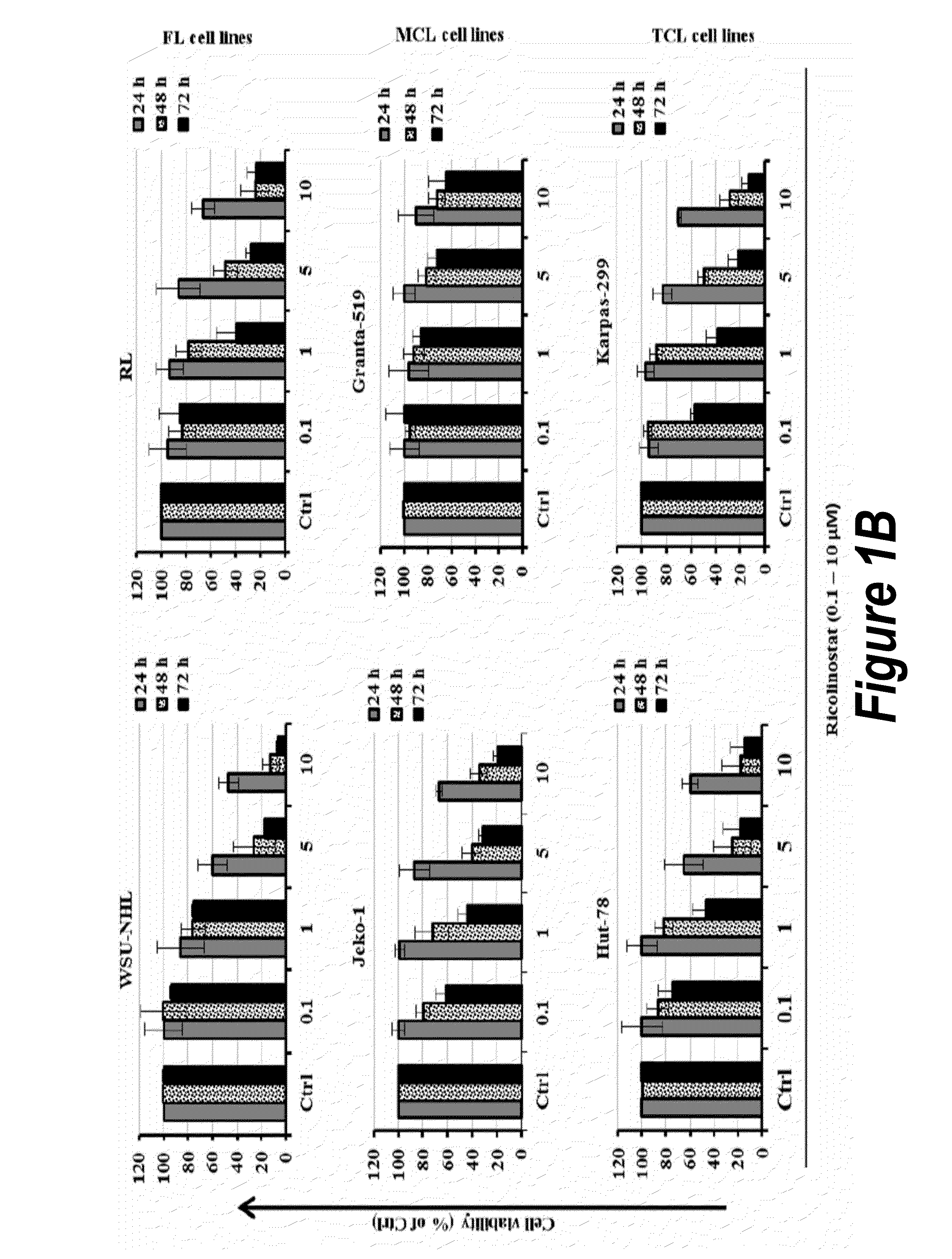
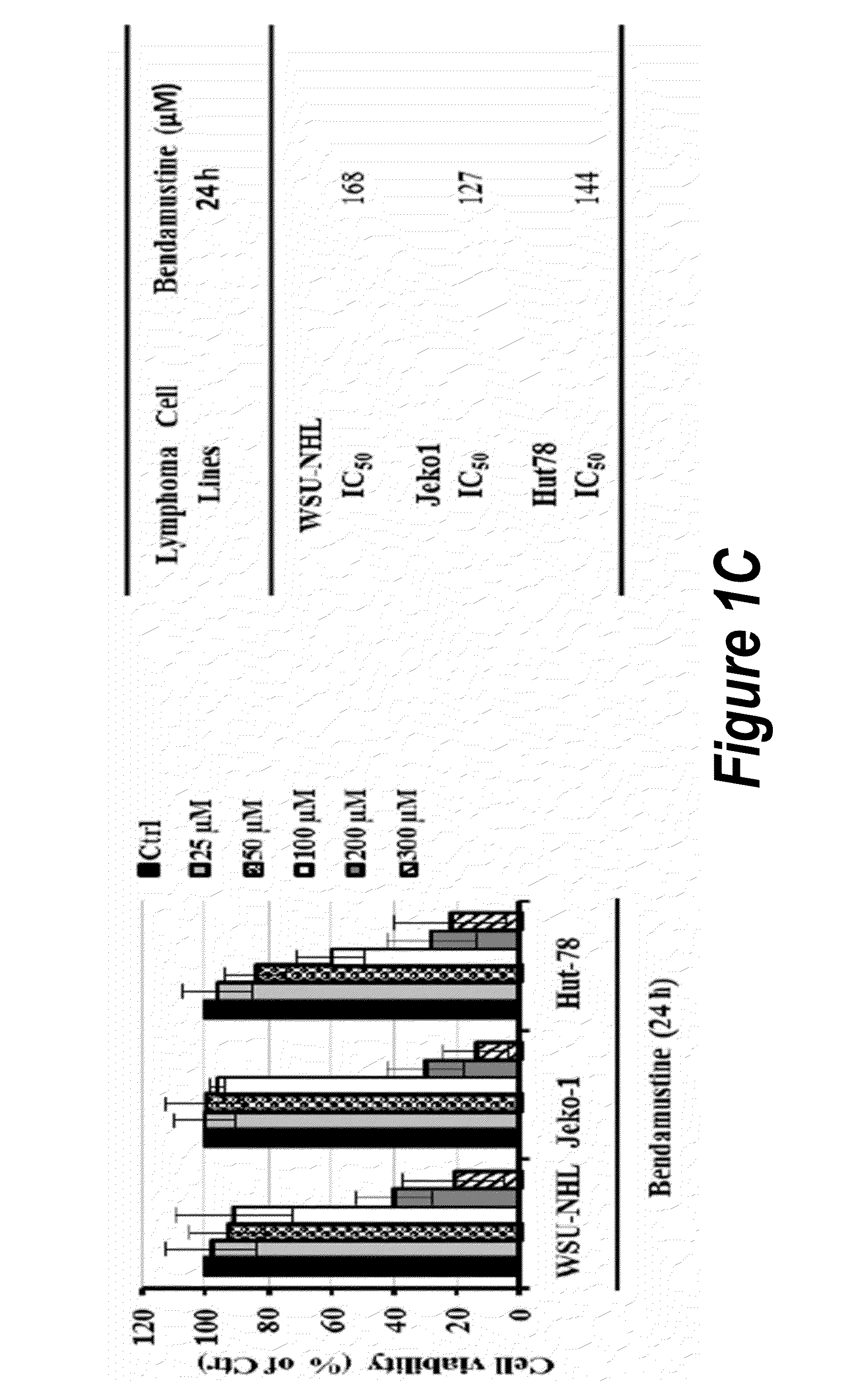
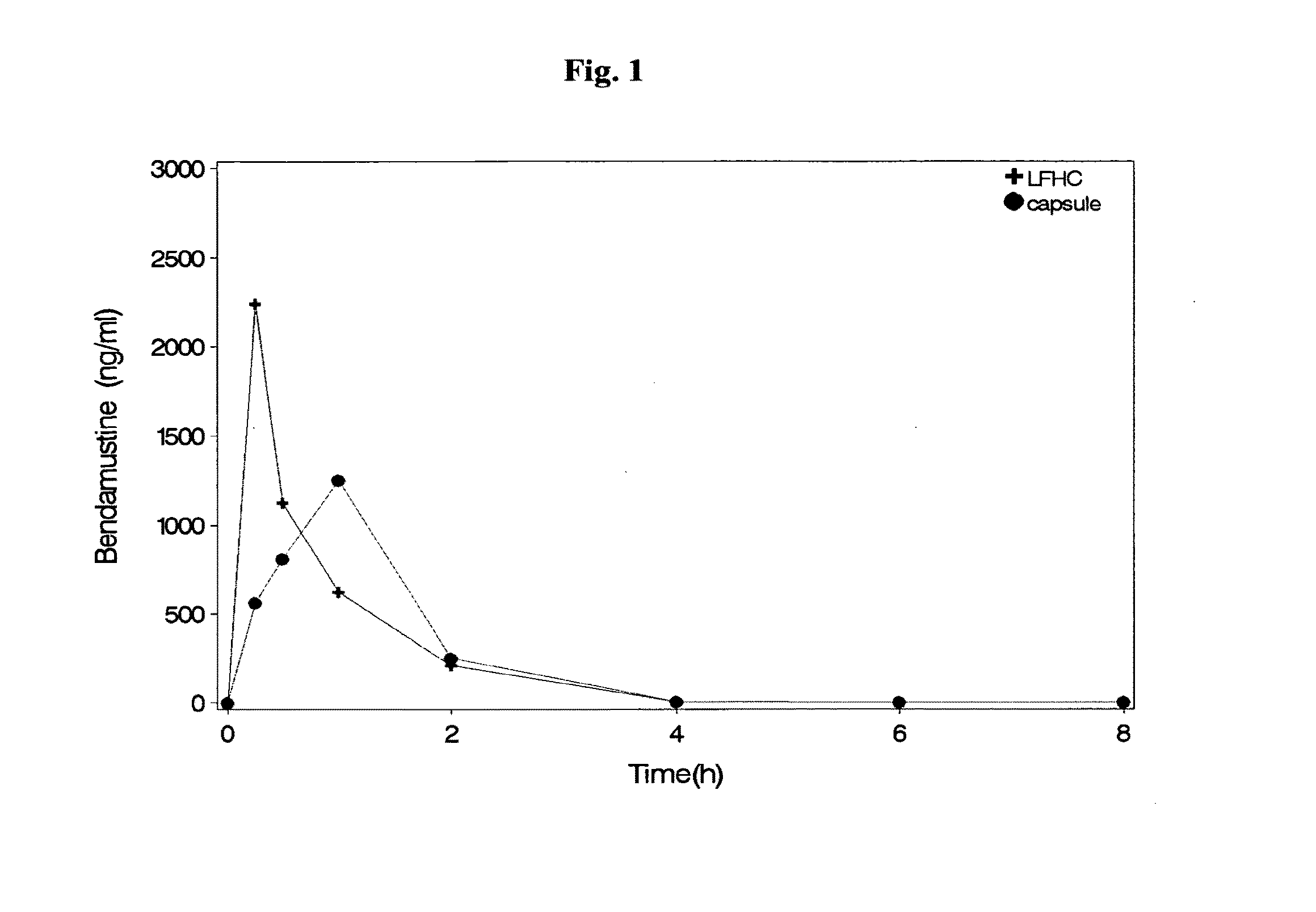
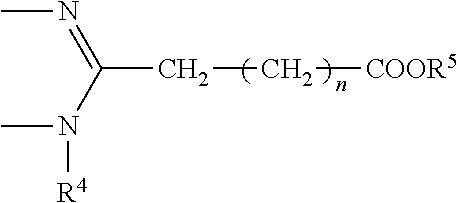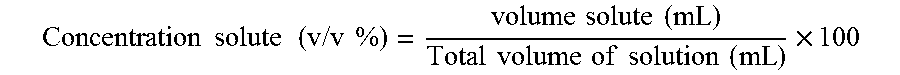Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "Bendamustine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
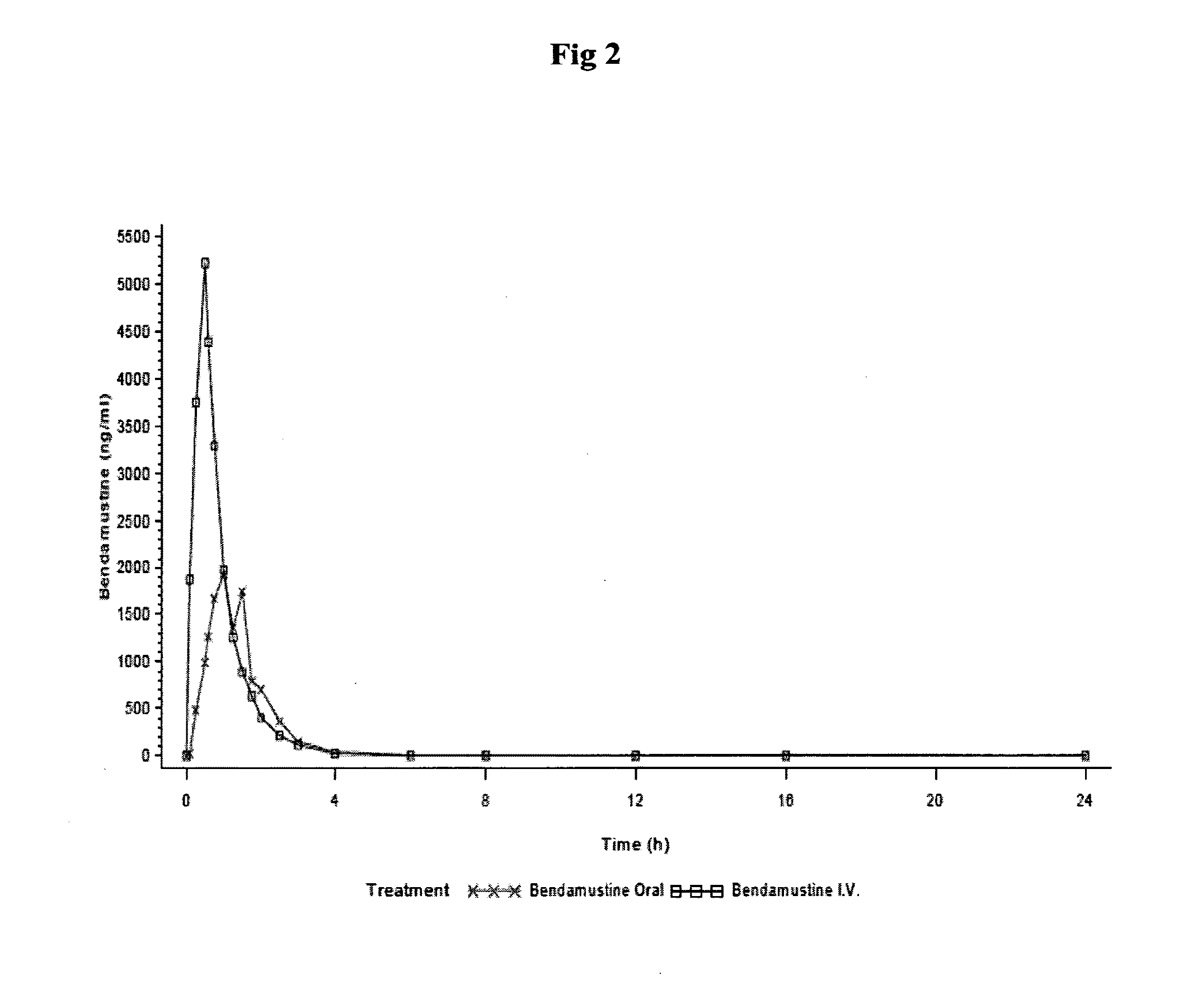
This medication is used to treat certain types of cancer (e.g., chronic lymphocytic leukemia-CLL, B-cell non-Hodgkin's lymphoma).
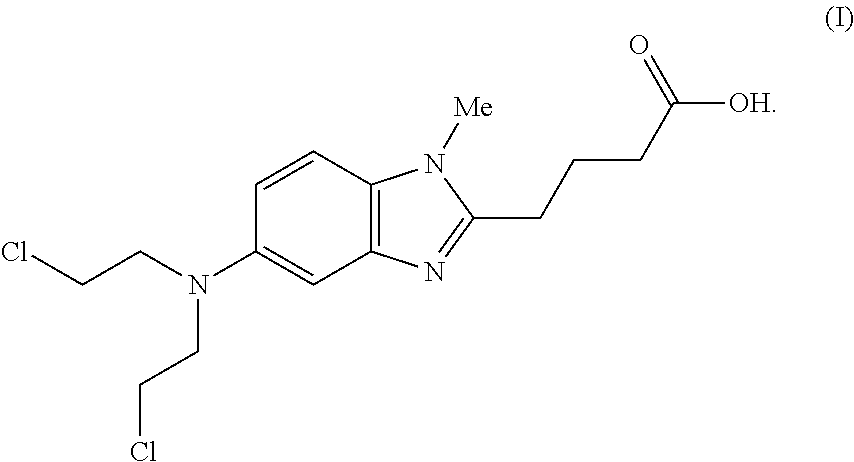
Bendamustine pharmaceutical compositions
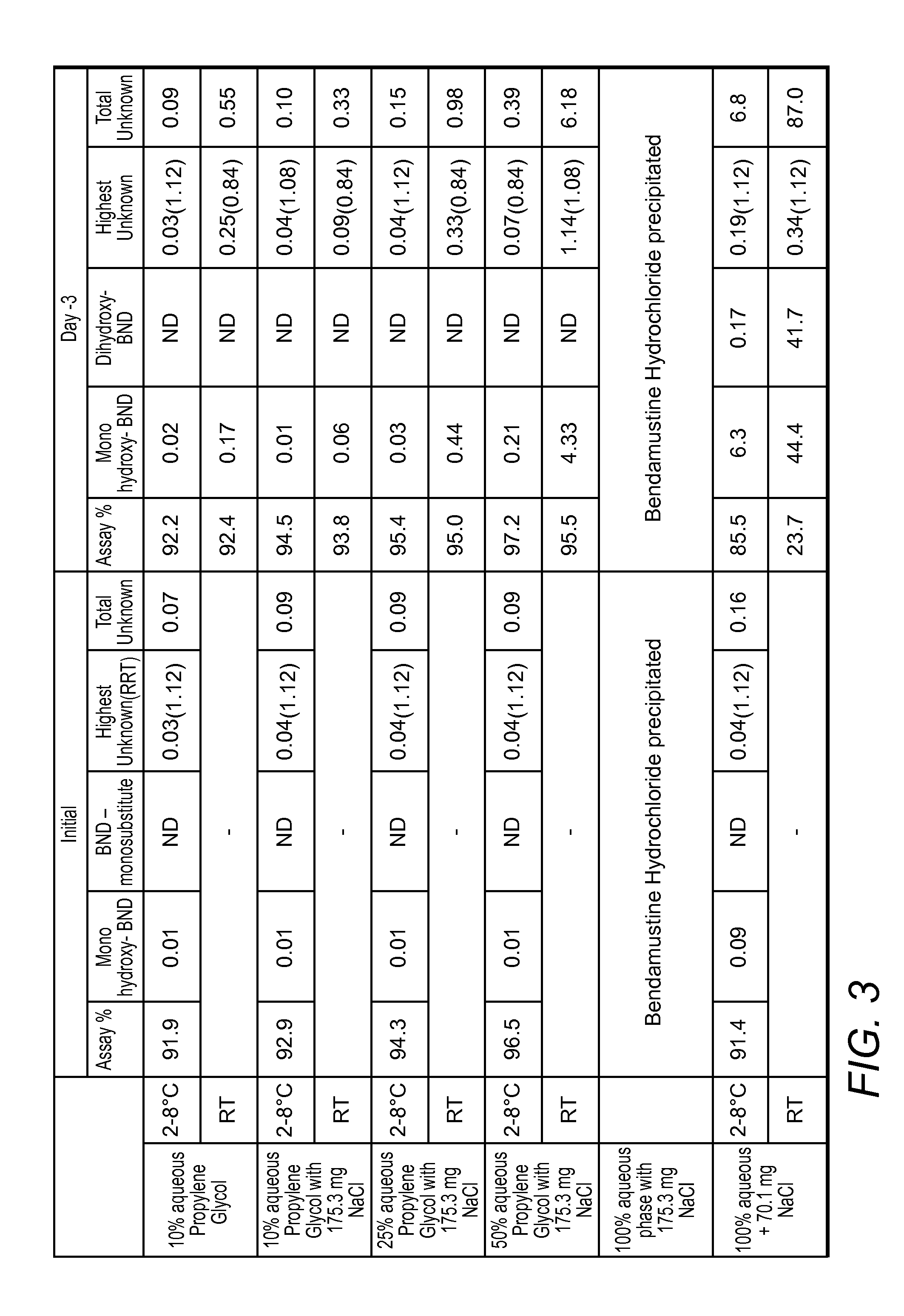
The present invention provides pharmaceutical formulations of lyophilized bendamustine suitable for pharmaceutical use. The present invention further provides methods of producing lyophilized bendamustine. The pharmaceutical formulations can be used for any disease that is sensitive to treatment with bendamustine, such as neoplastic diseases.
Owner:CEPHALON LLC
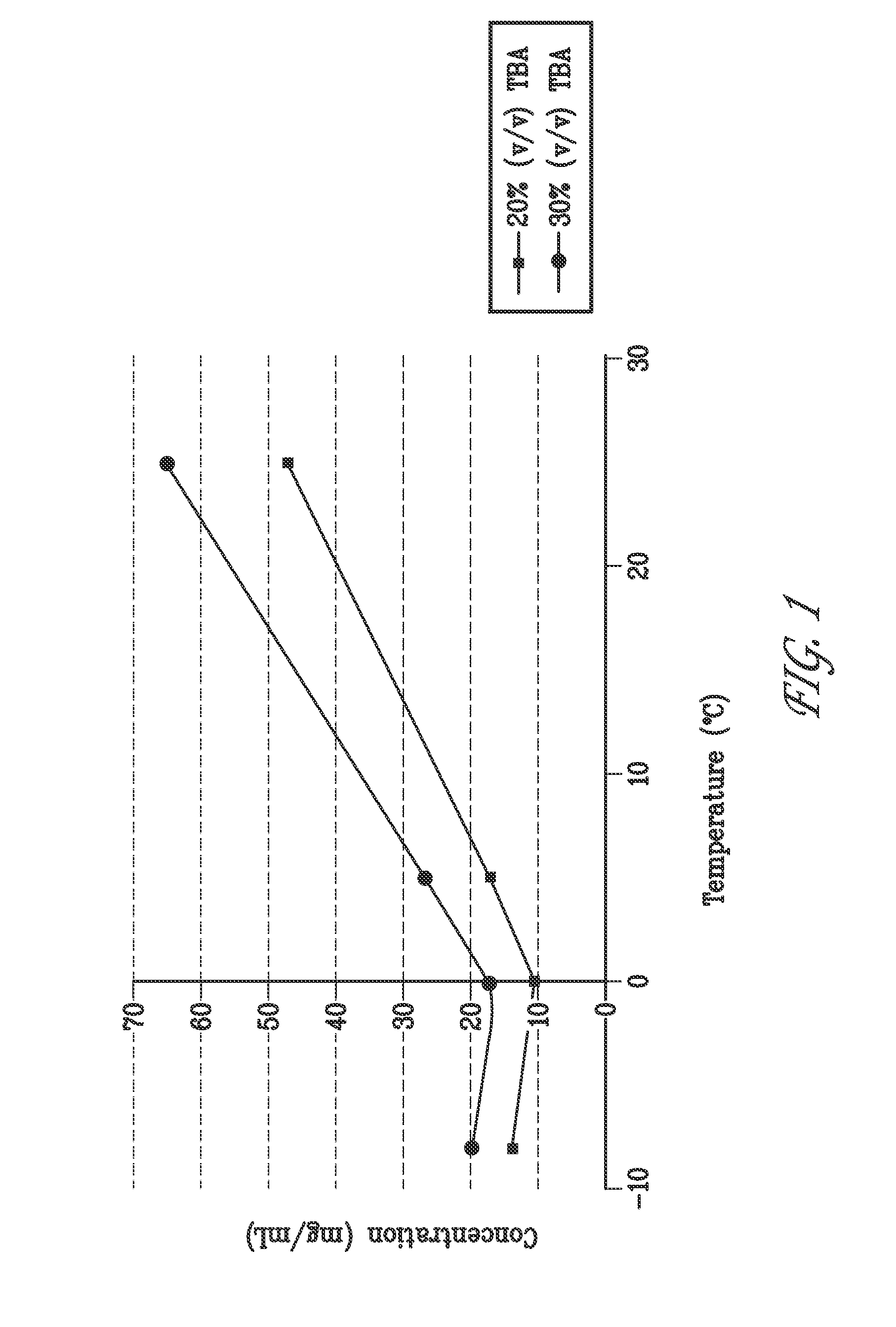
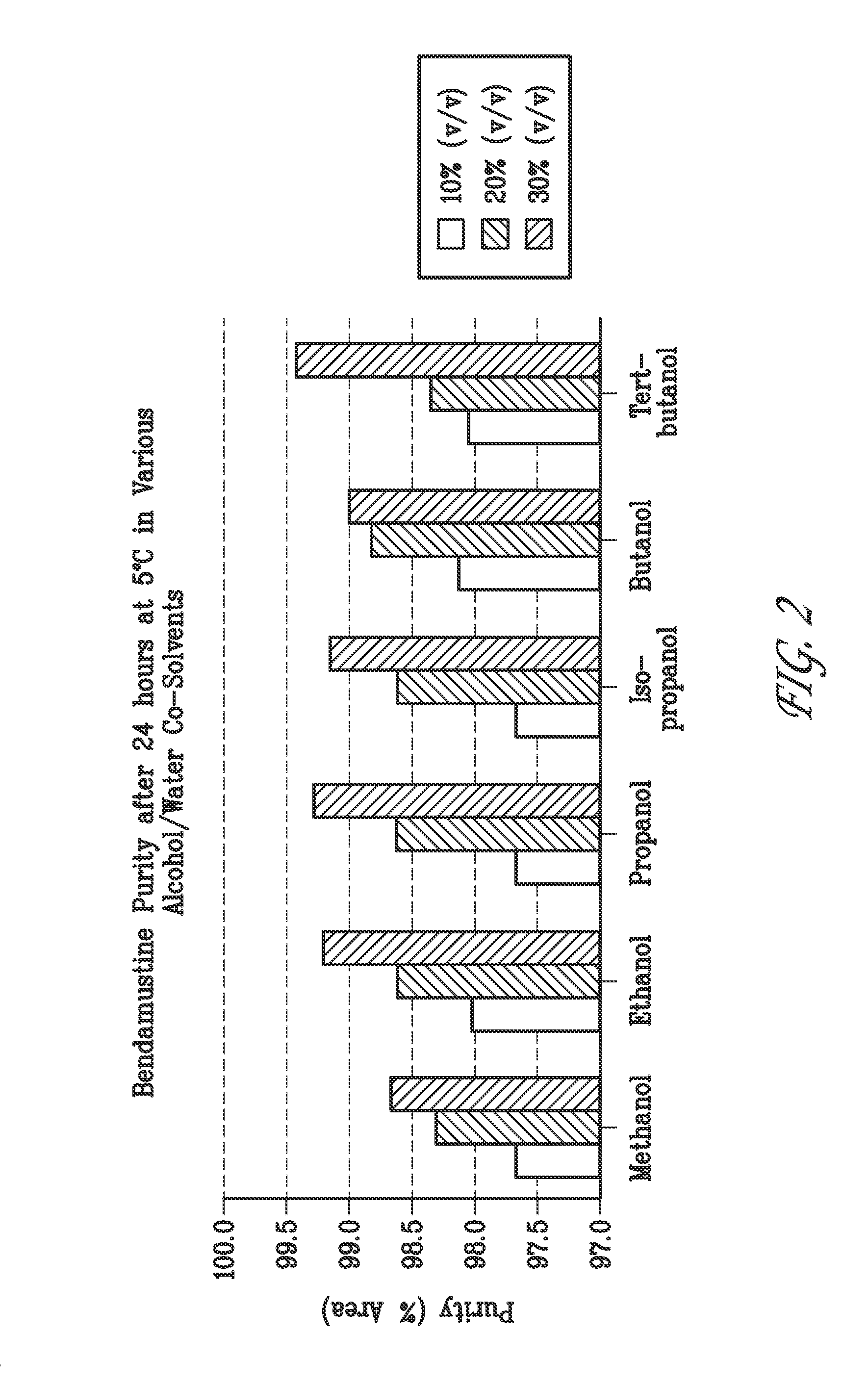
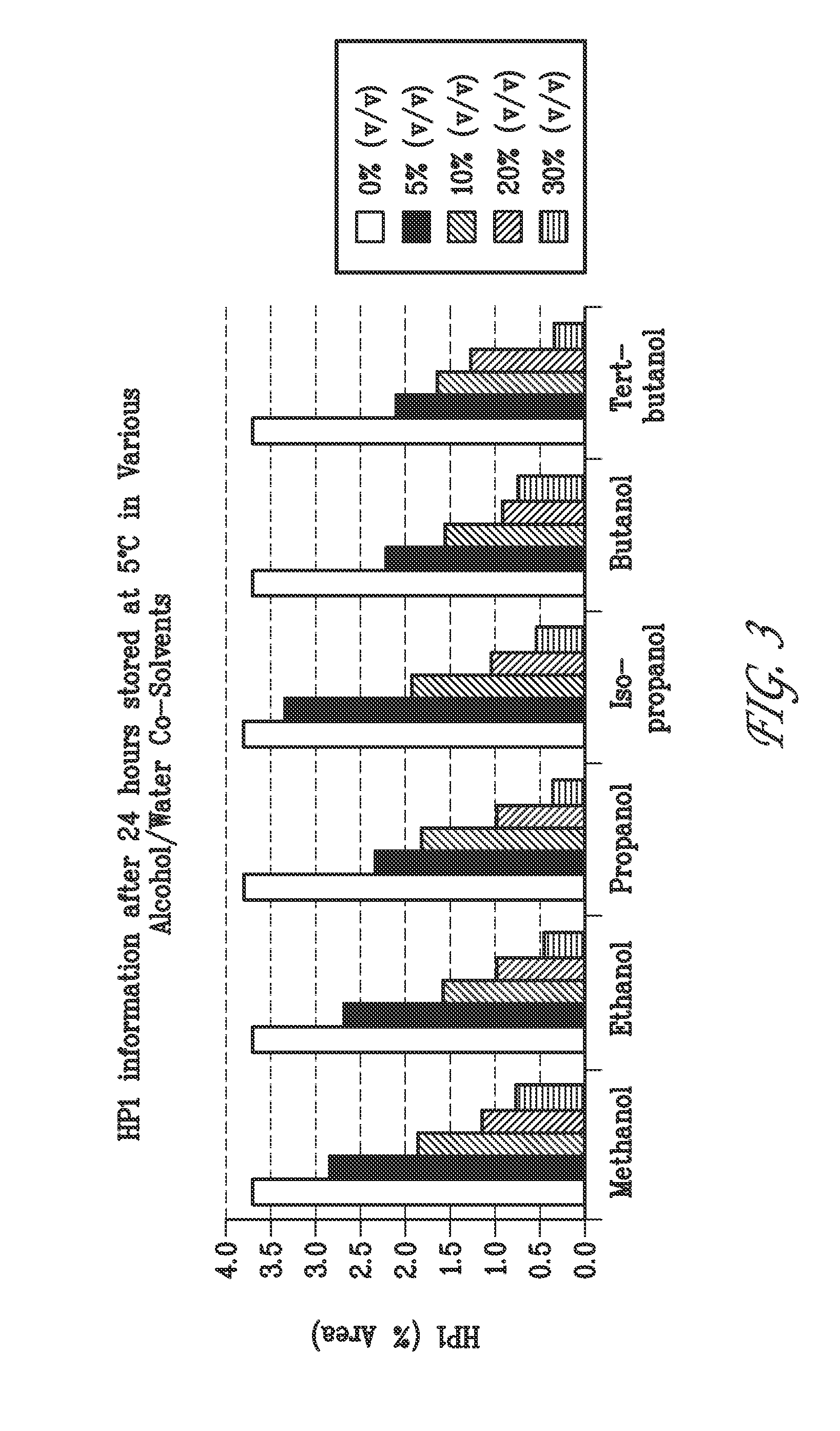
Liquid formulations of bendamustine
Stable liquid formulations of bendamustine, and pharmaceutically acceptable salts thereof, and polar aprotic solvents, are described.
Owner:CEPHALON INC
Novel uses
The present invention relates generally to the use of bendamustine in combination with an anti-CD20 antibody to treat cancer.
Owner:GLAXO SMITHKLINE LLC
Bendamustine cyclopolysaccharide compositions
The present invention is directed to pharmaceutical compositions comprising: (a) bendamustine, (b) a charged cyclopolysaccharide, and (c) a stabilizing agent having a charge opposite to that of the cyclopolysaccharide. Such composition provides unexpectedly desirable stability in reactive environments such as plasma, coupled with unexpectedly desirable anticancer activity. Such compositions are suitable for injection or infusion into patients in need for treatment with bendamustine.
Owner:SOFTKEMO PHARMA CORP
Formulations of bendamustine
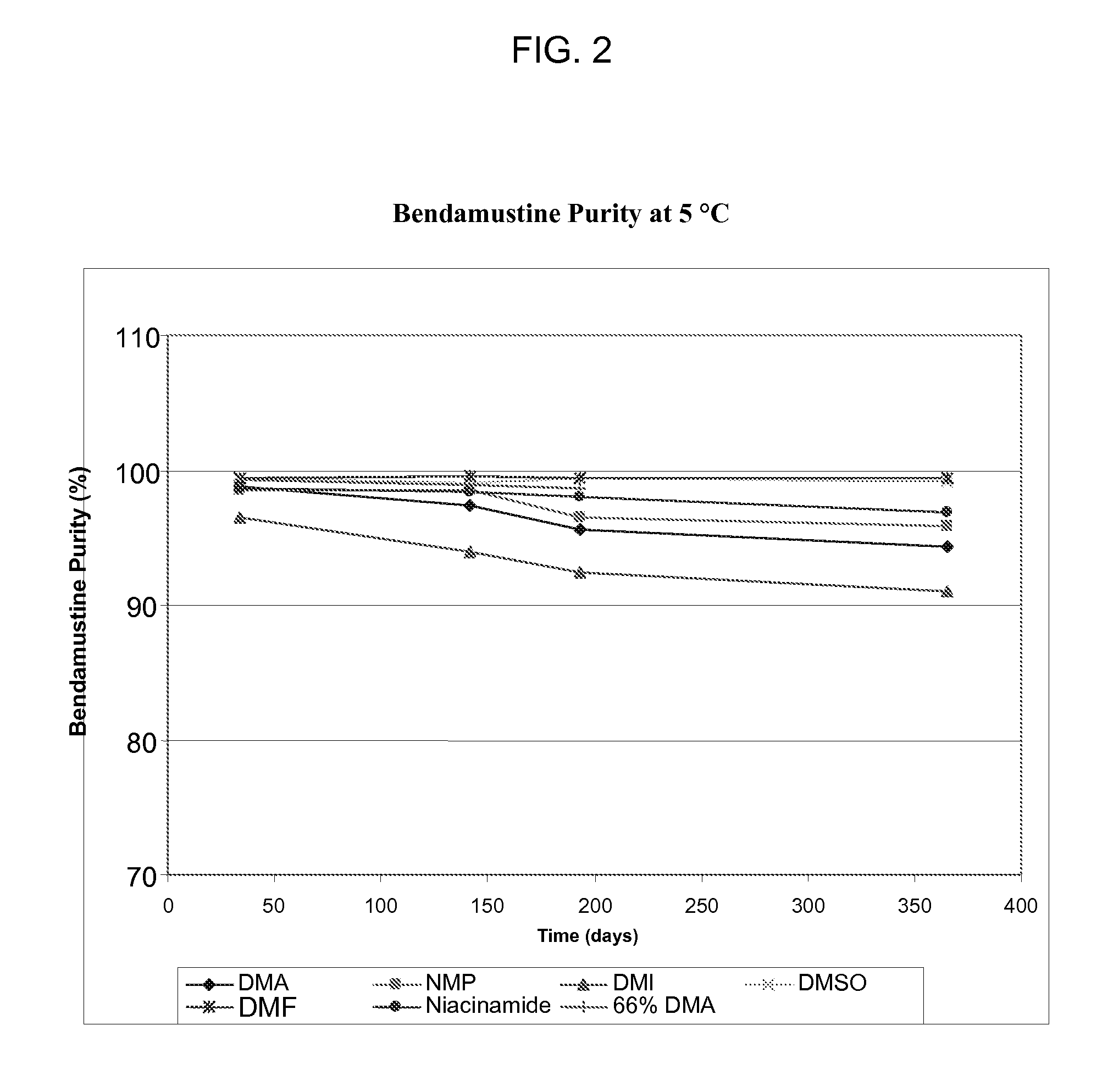
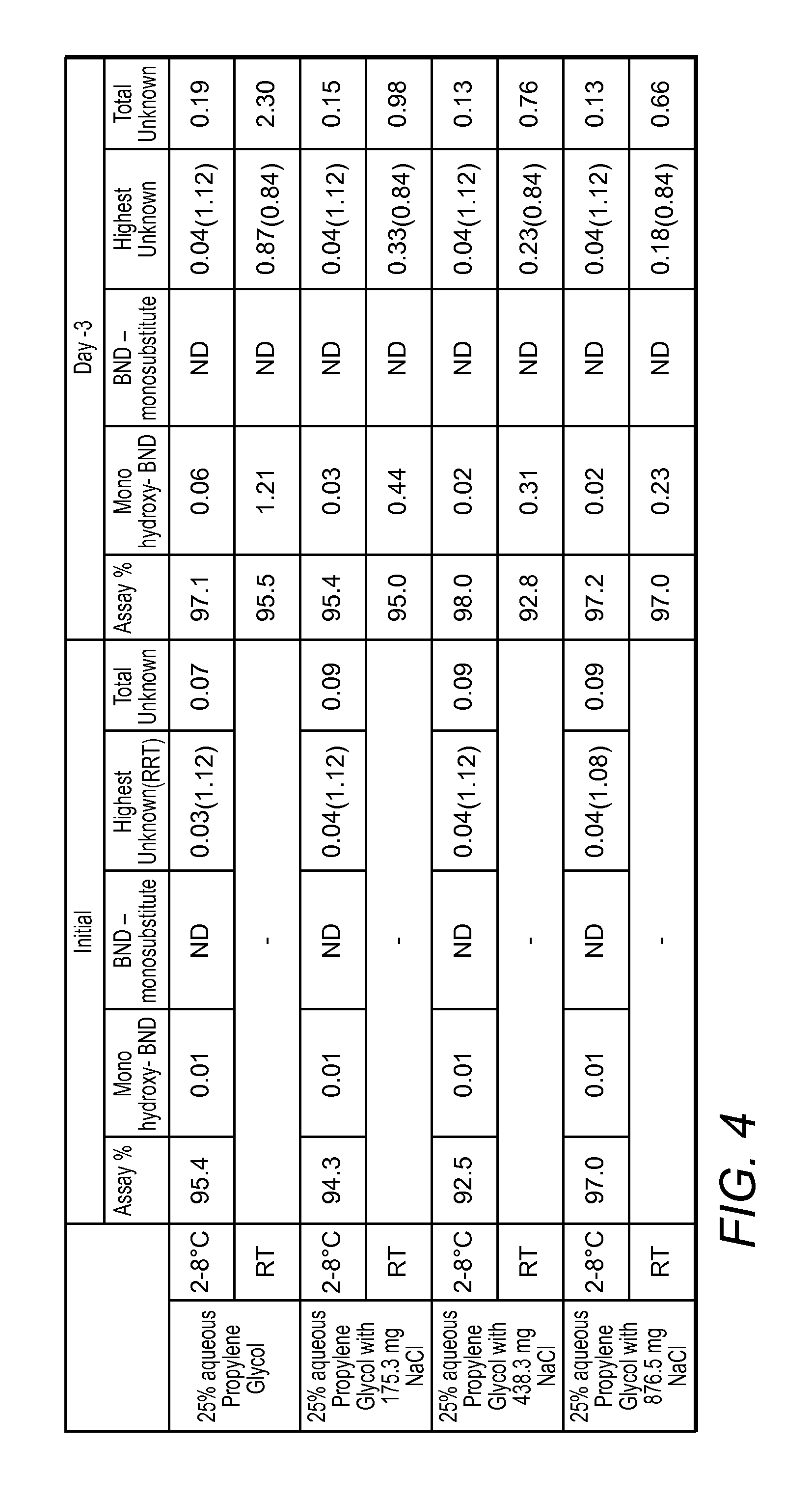
InactiveUS20130210879A1Improve long-term stabilityOrganic active ingredientsBiocideAntioxidantMedicine
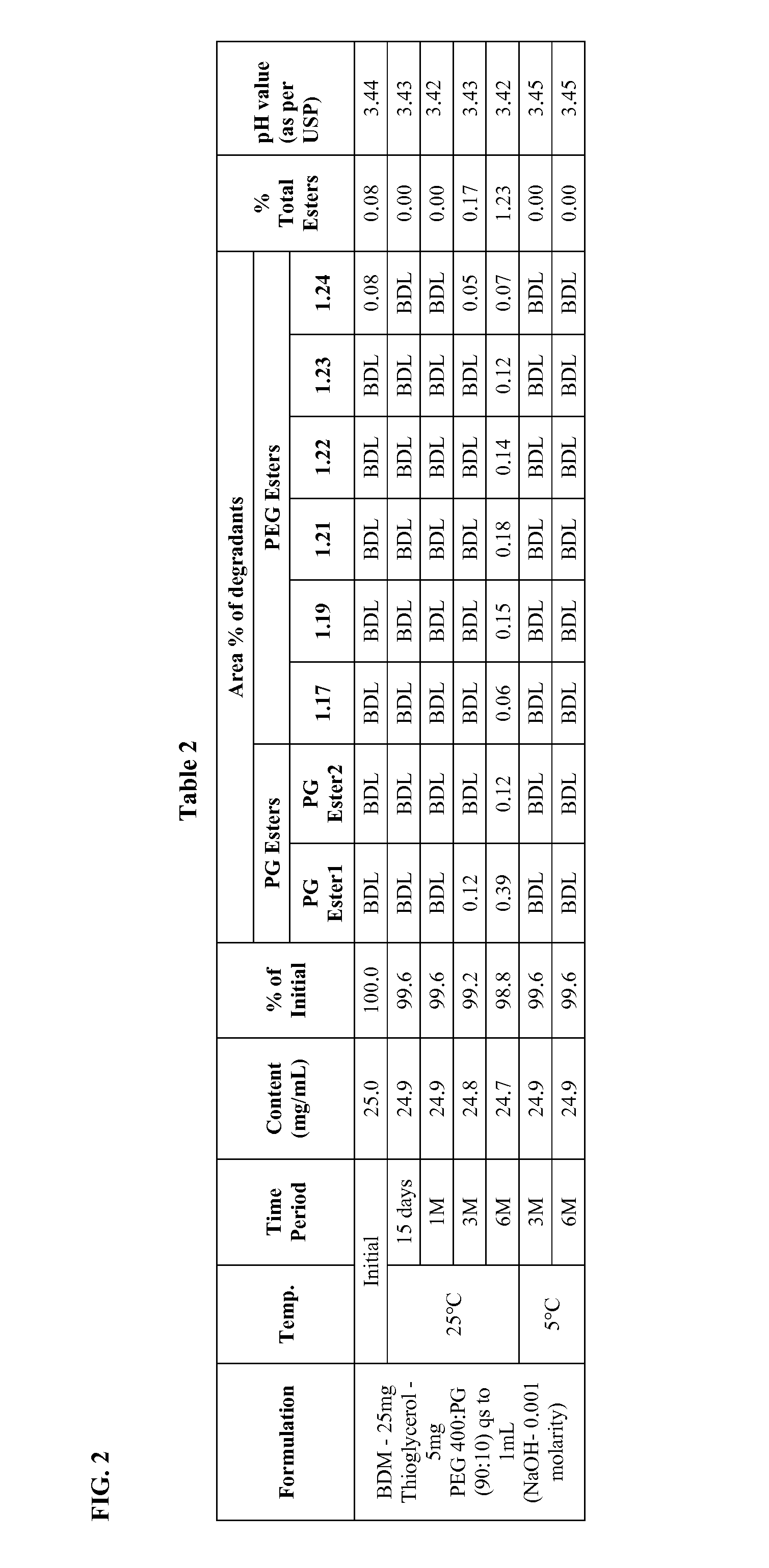
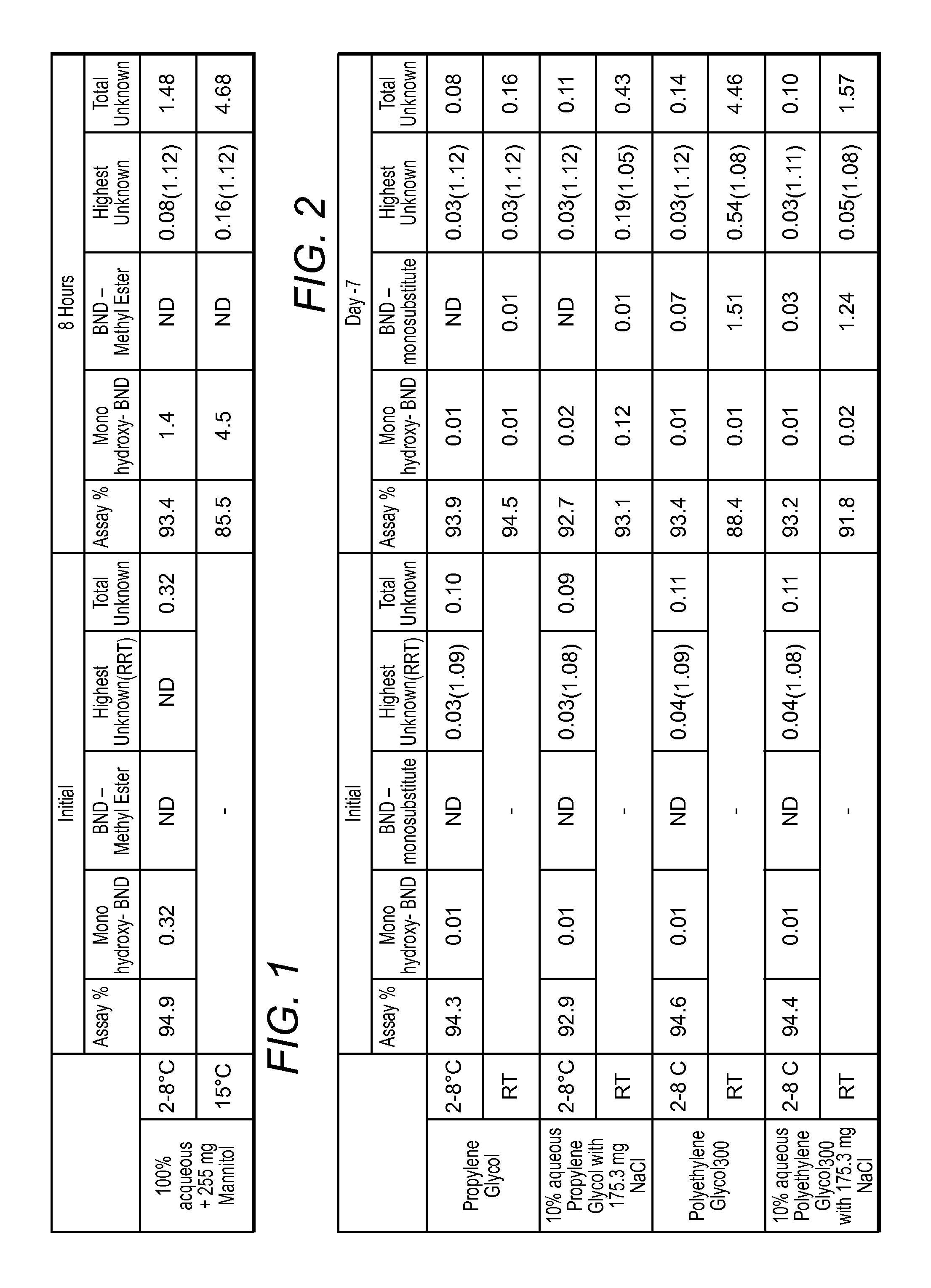
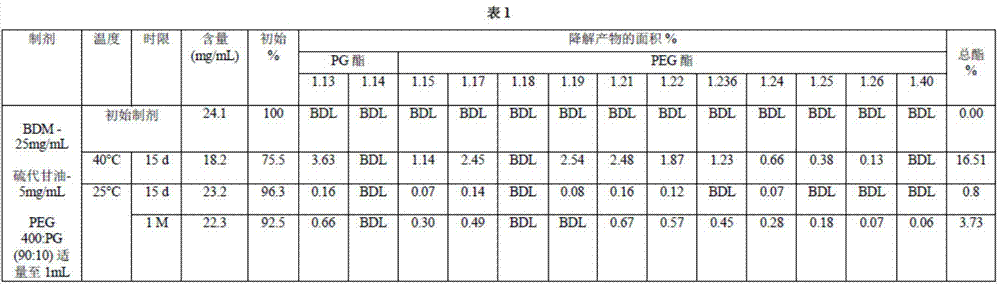
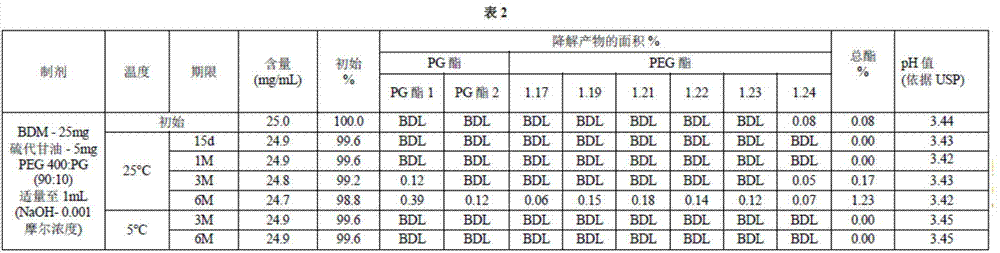
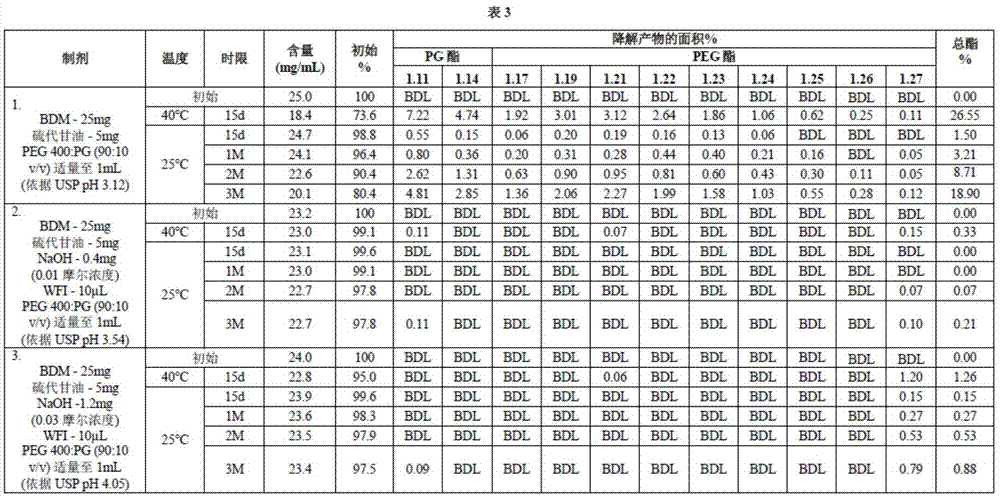
Long term storage stable bendamustine-containing compositions are disclosed. The compositions can include bendamustine or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable fluid contains a mixture of PEG and PG; an organic or inorganic compound in an amount sufficient to obtain a pH of from about 6.0 to about 11 for the polyethylene glycol, as measured using USP monograph for polyethylene glycol; and optionally an antioxidant. The bendamustine-containing compositions have less than about 5% total esters, on a normalized peak area response (“PAR”) basis as determined by high performance liquid chromatography (“HPLC”) at a wavelength of 223 nm, after at least about 15 months of storage at a temperature of from about 5° C. to about 25° C.
Owner:EAGLE PHARMACEUTICALS INC
Bendamustine compositions and methods therefore
InactiveUS20130210878A1Improve stabilityPrevent precipitationBiocideOrganic active ingredientsChlorideReady to use
Aqueous Bendamustine formulations with improved stability are disclosed. Especially preferred formulations are low-dose ready-to-use liquid formulations in which Bendamustine is in a non-aqueous vehicle in combination with an aqueous phase that contains significant quantities of chloride.
Owner:INNOPHARMA
Bendamustine Pharmaceutical Compositions
Owner:CEPHALON LLC
Formulations of bendamustine
ActiveUS8609707B2Improve long-term stabilityBiocideSulfur/selenium/tellurium active ingredientsAntioxidantChloride
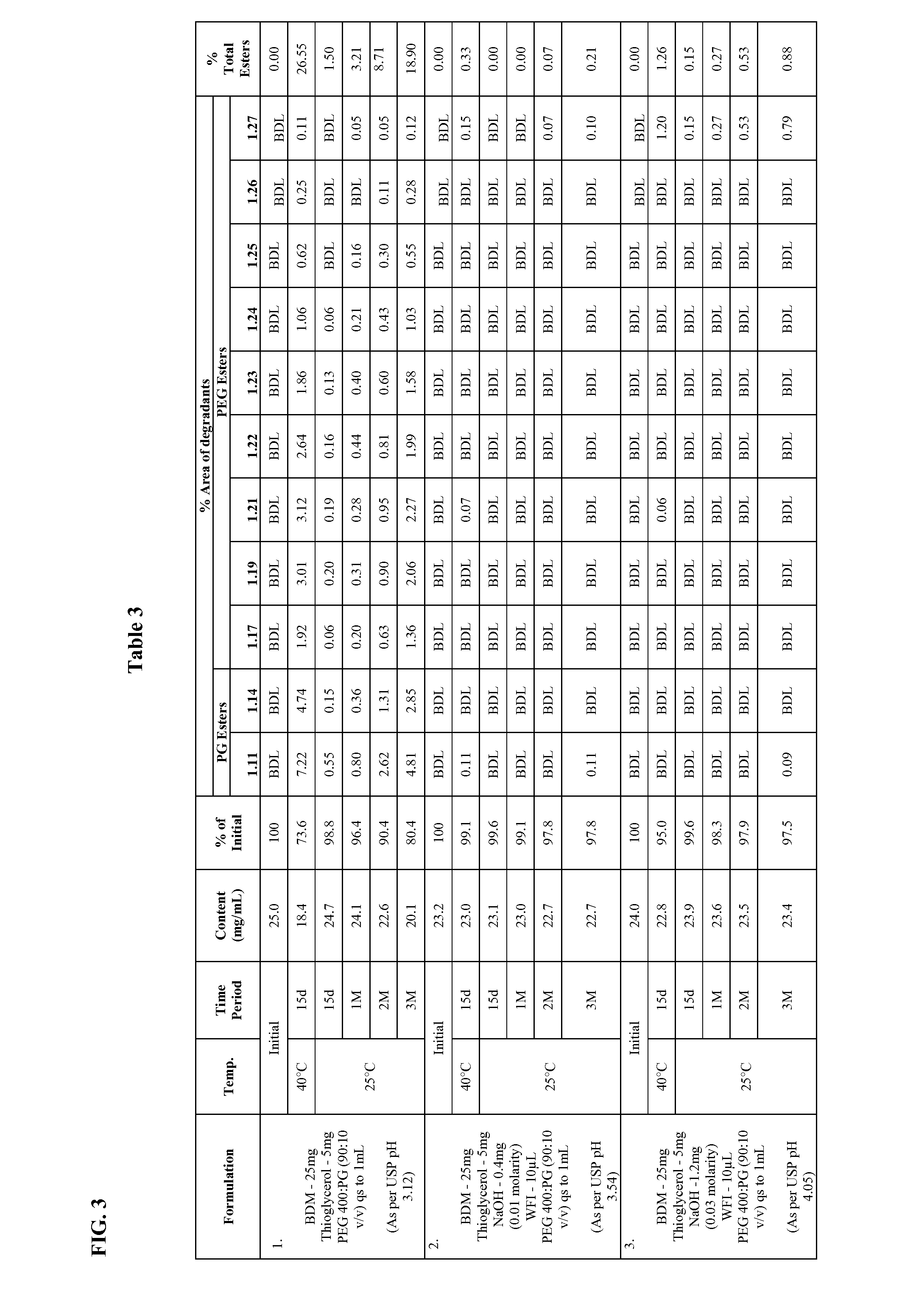
Long term storage stable bendamustine-containing compositions are disclosed. The compositions can include bendamustine or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable fluid which can include in some embodiments PEG, PG or mixtures thereof and an antioxidant or chloride ion source. The bendamustine-containing compositions have less than about 5% total impurities, on a normalized peak area response (“PAR”) basis as determined by high performance liquid chromatography (“HPLC”) at a wavelength of 223 nm, after at least about 15 months of storage at a temperature of from about 5° C. to about 25° C.
Owner:EAGLE PHARMACEUTICALS INC
Combination therapies for hematologic malignancies
InactiveUS20130064812A1Improved profileNo adverse effect on glucose and insulin levelBiocideDipeptide ingredientsHematologic malignancyBendamustine
The invention provides methods that relate to a novel therapeutic strategy for the treatment of hematological malignancies and inflammatory diseases. In particular, the method comprises administration of a compound of formula A,wherein R is H, halo, or C1-C6 alkyl; R′ is C1-C6 alkyl; ora pharmaceutically acceptable salt thereof; andoptionally a pharmaceutically acceptable excipient; andone or more additional therapeutic agents optionally selected from the group consisting of bendamustine, rituximab, and ofatumumab.
Owner:GILEAD CALISTOGA
Bendamustine amphiphilic anionic compositions
ActiveUS8389558B2Improve stabilityGood treatment effectBiocideOrganic chemistryMedicineTherapeutic effect
Owner:BENDARX CORP
Bendamustine amphiphilic anionic compositions
ActiveUS20110015244A1Improve stabilityGood treatment effectBiocideOrganic chemistryMedicineTherapeutic effect
The present invention is directed to pharmaceutical compositions comprising bendamustine and one or more amphiphilic anionic compounds and self assembled aggregates, which aggregates exhibit enhanced stability in aqueous solutions, including plasma, are disclosed. The unexpectedly enhanced stability afforded by such aggregates permits patients to be treated with bendamustine in lower and / or with less frequent dosages or to improve its therapeutic effect while using the same as presently used treatment protocol.
Owner:BENDARX CORP
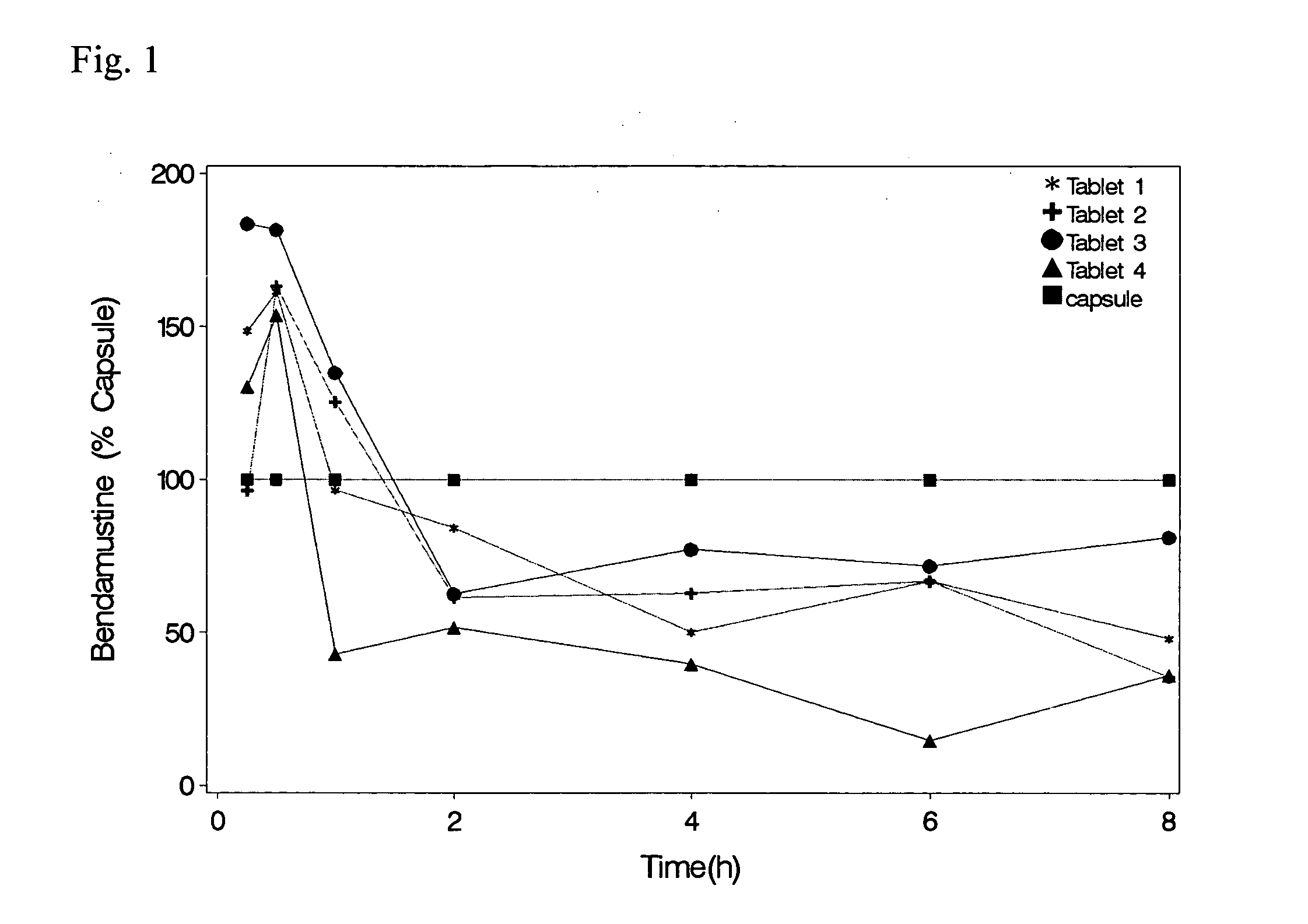
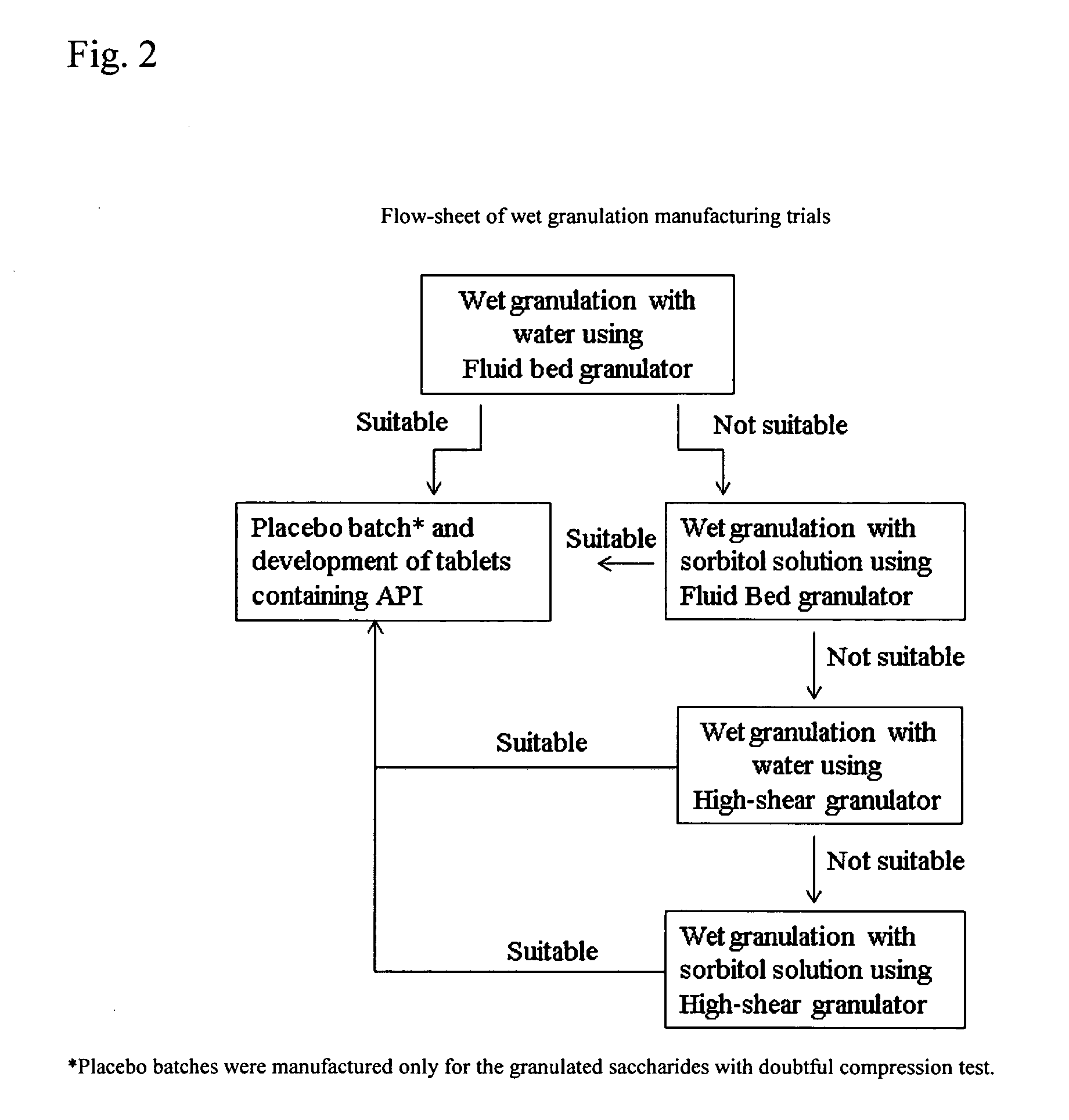
Solid Dosage Forms Of Bendamustine
InactiveUS20120003309A1High maximum concentrationBiocideHeavy metal active ingredientsBULK ACTIVE INGREDIENTExcipient
In the present invention there is provided a pharmaceutical composition in a solid dosage form suitable for oral administration, the composition comprising bendamustine or a pharmaceutically acceptable ester, salt or solvate thereof as an active ingredient, and at least one pharmaceutically acceptable excipient, which is a pharmaceutically acceptable saccharide selected from the group consisting of one or more of a monosaccharide, a disaccharide, an oligosaccharide, a cyclic oligosaccharide, a polysaccharide and a saccharide alcohol, wherein the ratio by weight of the active ingredient to the saccharide excipient(s) is in the range of 1:1-5.
Owner:ASTELLAS DEUTLAND
Formulations of bendamustine
ActiveUS20130253025A1Avoid side effectsSmall volumeBiocideOrganic active ingredientsBULK ACTIVE INGREDIENTBendamustine
Methods of treatment using bendamustine formulations designed for small volume intravenous administration are disclosed. The methods conveniently allow shorter administration time without the active ingredient coming out of solution as compared to presently available formulations.
Owner:EAGLE PHARMACEUTICALS INC
Oral Dosage Forms of Bendamustine and Therapeutic Use Thereof
InactiveUS20130209558A1Improve stabilitySuperior dissolution profileBiocideHeavy metal active ingredientsDiseaseOral treatment
In the present invention there is provided a pharmaceutical composition for oral administration which comprises bendamustine or a pharmaceutically acceptable, ester, salt or solvate thereof as an active ingredient, and a pharmaceutically acceptable excipient and which shows a dissolution of the bendamustine of at least 60% in 20 minutes, 70% in 40 minutes and 80% in 60 minutes, as measured with a paddle apparatus at 50 rpm according to the European Pharmacopoeia in 500 ml of a dissolution medium at a pH of 1.5, and wherein the pharmaceutically acceptable excipient is either a pharmaceutically acceptable non-ionic surfactant, selected from the group consisting of a polyethoxylated castor oil or derivative thereof and a block copolymer of ethylene oxide and propylene oxide or a pharmaceutically acceptable saccharide selected from the group consisting of one or more of a monosaccharide, a disaccharide, an oligosaccharide, a cyclic oligosaccharide, a polysaccharide and a saccharide alcohol, wherein the ratio by weight of the active ingredient to the saccharide excipient(s) is in the range of 1:1-5. The invention further relates to the above pharmaceutical composition for use for the oral treatment of a medical condition which is selected from chronic lymphocytic leukemia, acute lymphocytic leukaemia, chronic myelocytic leukaemia, acute myelocytic leukaemia, Hodgkin's disease, non-Hodgkin's lymphoma, multiple myeloma, breast cancer, ovarian cancer, small cell lung cancer and non-small cell lung cancer. The invention moreover relates to the above pharmaceutical composition for the above use wherein the dosage regimen comprises at least the administration of a dose of 100 to 600 mg / m2 / per person of bendamustine on day 1 and day 2, optionally a dose of 50 to 150 mg / m2 i.v. or orally of a corticosteroid on days 1 to 5, and optionally a suitable dose of a further active agent selected from the group consisting of an antibody specific for CD20, an anthracyclin derivative, a vinca alkaloid or a platin derivative; and the repetition of said dosage regimen 4 to 15 times after intervals of two to four weeks.
Owner:ASTELLAS DEUTLAND
Formulations of bendamustine
InactiveUS20140094496A1Avoid side effectsSmall volumeBiocideOrganic active ingredientsComing outBULK ACTIVE INGREDIENT
Methods of treatment using bendamustine formulations designed for small volume intravenous administration are disclosed. The methods conveniently allow shorter administration time without the active ingredient coming out of solution as compared to presently available formulations.
Owner:EAGLE PHARMACEUTICALS INC
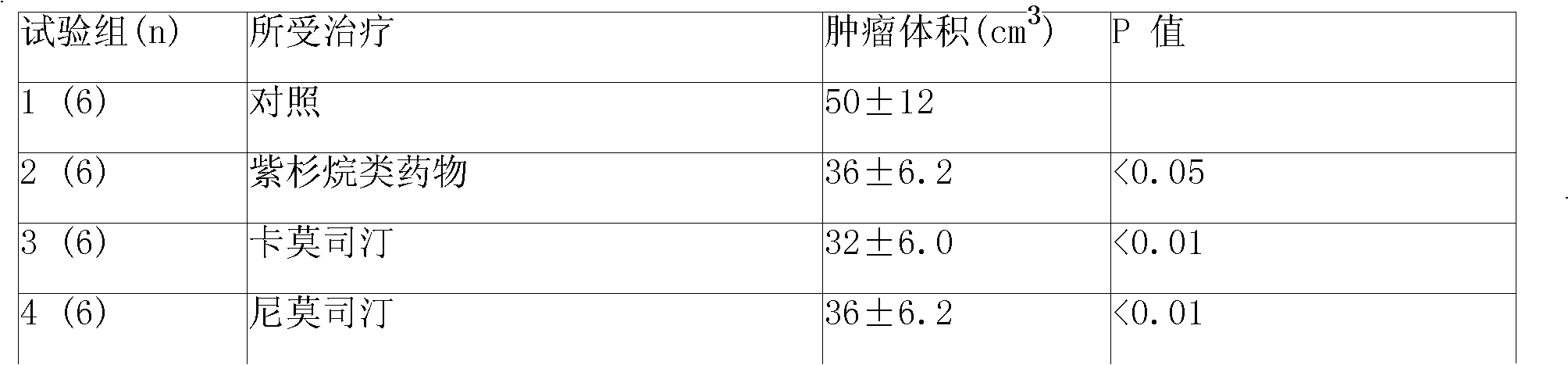
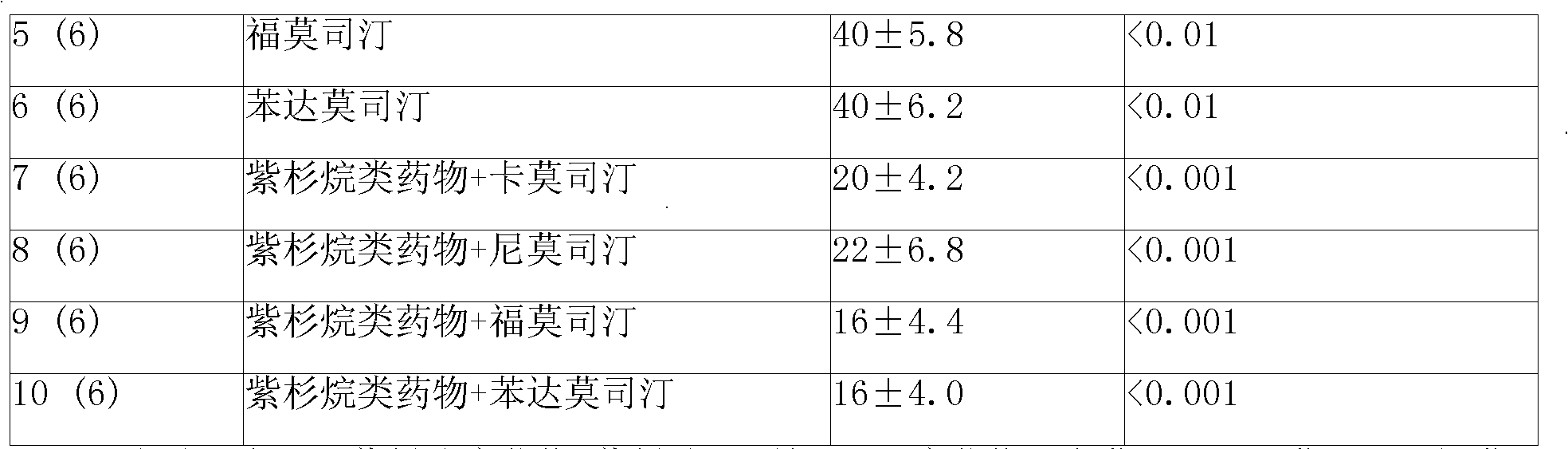
Anti-cancer composition containing bendamustine
An anti-cancer composition comprises anti-cancer effective component selected from tyrosine kinase inhibitor and / or bendamustine and slow releasing adjuvant, and can be prepared into slow releasing injection and slow releasing implantable agent. The slow releasing injection also comprises special dissolvant containing suspending agent. The Suspending agent has a viscocity of 100cp-3000cp (at 20deg.C-30deg.C) and is selected from sodium carboxymethylcellulose, and so on. the slow releasing adjuvant is selected from p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), p(BHET-EOP / TC), p(BHDPT-EOP / TC), p( BHDPT-EOP / TC), p(CHDM-HOP) or p(CHDM-EOP.Slow releasing injection and implantable agent can keep high medicinal concentration in tumour part through slow releasing for over 60 days after being injected or implanted in or around tumour. The anticancer composition may also be prepared into sustained-release implant. It can reduce systemic toxic reaction of anticancer drugs, and also selectively improve the therapeutic effect of non-operative therapy such as chemotherapy.
Owner:JINAN KANGQUAN PHARMA TECH
Bendamustine formulations
InactiveUS20140142153A1Improve responseReduce degradationOrganic active ingredientsBiocideMedicineEnantiomer
Aspects of the present appliction relate to pharmaceutical formulations comprising bendamustine or its pharmaceutically acceptable salts, isomers, racemates, enantiomers, hydrates, solvates, metabolites, polymorphs, and mixtures therof, suitable for phamaceutical use. Aspects further provide methods of producing stable bendamustine compositions.
Owner:DR REDDYS LAB LTD +1
Combination of cd37 antibodies with rituximab
InactiveUS20130309224A1Improve stabilityEasy to manageOrganic active ingredientsAntibody ingredientsDiseaseCD20
The present invention relates to immunotherapies that are based on depletion of CD37-positive cells such as B-cells. The present invention provides methods for reduction of CD37-positive cells such as B-cells in an individual / patient using a combination of CD37 antibody / antibodies and bendamustine. The combination of CD37 antibodies, CD20 antibodies and bendamustine is shown to have a synergistic effect. The application further provides materials and methods for treatment of diseases involving aberrant B-cell activity.
Owner:BOEHRINGER INGELHEIM INT GMBH
Combination of cd37 antibodies with bendamustine
InactiveUS20130287797A1Improve stabilityEasy to manageOrganic active ingredientsAntibody ingredientsDiseaseCD37
The present invention relates to immunotherapies that are based on depletion of CD37-positive cells such as B-cells. The present invention provides methods for reduction of CD37-positive cells such as B-cells in an individual / patient using a combination of CD37 antibody / antibodies and bendamustine. The combination of CD37 antibodies and bendamustine is shown to have a synergistic effect. The application further provides materials and methods for treatment of diseases involving aberrant B-cell activity.
Owner:BOEHRINGER INGELHEIM INT GMBH
Slow released compound anticancer injection containing bendamustine
InactiveCN1887261AEasy injectionIncrease drug concentrationOrganic active ingredientsSolution deliveryMicrospherePolyethylene glycol
The slow released compound anticancer injection containing bendamustine consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent. The effective anticancer component is the composition of bendamustine and bendamustine synergist selected from antitumor antibiotic and antimetabolite. The slow releasing supplementary material is polylactic acid and its copolymer, polifeprosan, EVAc, etc or their composition. The .suspending agent has viscosity of 80-3000 cp. The slow released microsphere may be also prepared into slow released implanting agent. The present invention can inhibit the growth of tumor and enhance the curative effect of chemotherapy, radiotherapy and other non-operative treatment.
Owner:JINAN SHUAIHUA PHARMA TECH
Oral Dosage Forms Of Bendamustine
InactiveUS20120003305A1Improve stabilityImproved profileBiocideHeavy metal active ingredientsNon ionicBULK ACTIVE INGREDIENT
In the present invention there is provided an oral pharmaceutical composition, comprising bendamustine or a pharmaceutically acceptable, ester, salt or solvate thereof as an active ingredient, and a pharmaceutically acceptable excipient, which is a pharmaceutically acceptable non-ionic surfactant, selected from the group consisting of polyethoxylated castor oil or derivative thereof and a block copolymer of ethylene oxide and propylene oxide.
Owner:ASTELLAS DEUTLAND
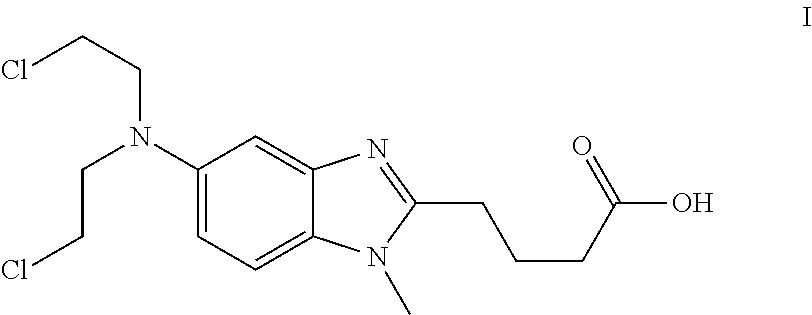
Process for the production of bendamustine alkyl ester, bendamustine, and derivatives thereof
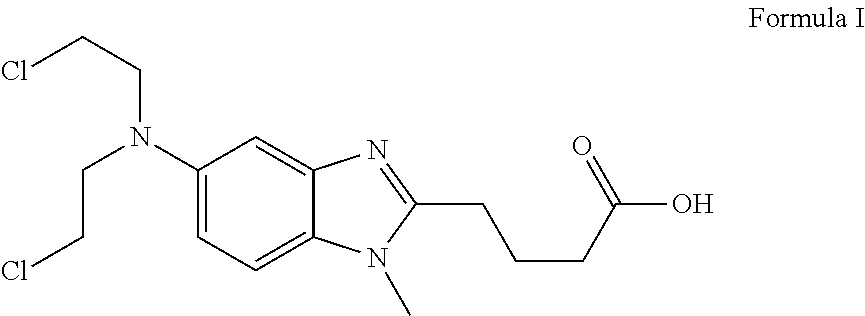
ActiveUS8481751B2Amino compound purification/separationOrganic compound preparationHalogenBendamustine
Methods are provided for the production of bendamustine alkyl ester, bendamustine, as well as derivatives thereof. With the methods the production of these compounds is possible in reproducibly high yields. To this end, hydroxyl-group-containing esters are used as the starting material, whose hydroxyl groups are substituted in a simple way by halogen groups. This substitution is possible in the presence of (i) oxalyl chloride and (ii) dialkylformamide, dialkyl acetamide or dimethyl sulfoxide. In a subsequent reaction, the resulting esters can be hydrolyzed to form the acid.
Owner:HERAEUS PRECIOUS METALS GMBH & CO KG
Liquid Bendamustine Formulation
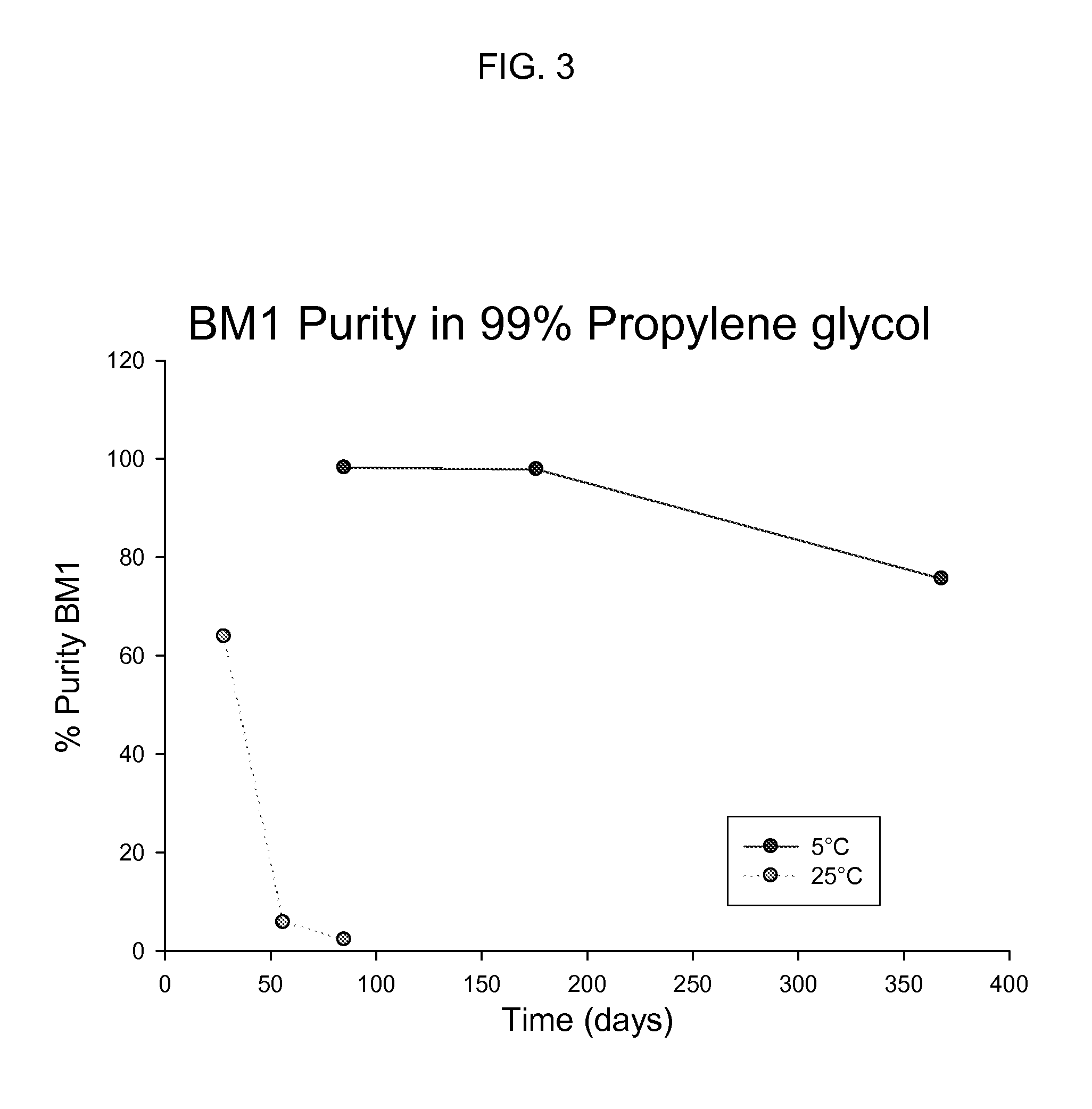
ActiveUS20160158362A1Good impurity profileImprove stabilityOrganic active ingredientsBiocideAntioxidantGlycerol
The present invention provides stable bendamustine-containing pharmaceutical compositions suitable for long term storage. The compositions include bendamustine, a pharmaceutically acceptable salt, and / or a hydrate form thereof, a solvent mixture of N,N-dimethylacetamide and glycerin, and an antioxidant. The bendamustine-containing compositions have less than about 2% of total impurities after two month storage at 25° C. / 60% RH. The pharmaceutical compositions may be used for treating neoplastic diseases.
Owner:NAVINTA
Formulations of bendamustine
ActiveCN104203235AOvercoming Stability DifferencesEasy to diluteOrganic active ingredientsInorganic non-active ingredientsAntioxidantInorganic compound
Long term storage stable bendamustine-containing compositions are disclosed. The compositions can include bendamustine or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable fluid contains a mixture of PEG and PG; an organic or inorganic compound in an amount sufficient to obtain a pH of from about 6.0 to about 11 for the polyethylene glycol, as measured using USP monograph for polyethylene glycol; and optionally an antioxidant. The bendamustine-containing compositions have less than about 5% total esters, on a normalized peak area response ("PAR") basis as determined by high performance liquid chromatography ("HPLC") at a wavelength of 223 nm, after at least about 15 months of storage at a temperature of from about 5° C. to about 25° C.
Owner:SCIDOSE
Liquid bendamustine formulation
ActiveUS9603930B2Improved profileImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismMedicineAntioxidant
The present invention provides stable bendamustine-containing pharmaceutical compositions suitable for long term storage. The compositions include bendamustine, a pharmaceutically acceptable salt, and / or a hydrate form thereof, a solvent mixture of N,N-dimethylacetamide and glycerin, and an antioxidant. The bendamustine-containing compositions have less than about 2% of total impurities after two month storage at 25° C. / 60% RH. The pharmaceutical compositions may be used for treating neoplastic diseases.
Owner:NAVINTA
Processes for the Preparation of Bendamustine
ActiveUS20110190509A1Simple methodOrganic chemistryAntineoplastic agentsBendamustineMedicinal chemistry
New methods for the preparation of bendamustine, and the pharmaceutical salts thereof, are described. Novel compounds useful for the preparation of bendamustine are also described.
Owner:CEPHALON INC
Fluorouracil containing anti-cancer sustained-release injection
InactiveCN101234084AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Combinations of histone deacetylase inhibitors and bendamustine
The invention relates to pharmaceutical combinations comprising an HDAC inhibitor and bendamustine; pharmaceutical compositions comprising the same; and methods of using such combinations and compositions for treating lymphoma in a subject in need thereof.
Owner:UNIVERSITY OF MODENA AND REGGIO EMILIA
Oral Dosage Forms of Bendamustine
InactiveUS20150335588A1Improve stabilityImproved profileBiocideHeavy metal active ingredientsMedicineBULK ACTIVE INGREDIENT
In the present invention there is provided an oral pharmaceutical composition, comprising bendamustine or a pharmaceutically acceptable, ester, salt or solvate thereof as an active ingredient, and a pharmaceutically acceptable excipient, which is a pharmaceutically acceptable non-ionic surfactant, selected from the group consisting of polyethoxylated castor oil or derivative thereof and a block copolymer of ethylene oxide and propylene oxide.
Owner:ASTELLAS DEUTLAND
Anticancer sustained-released gel injection and preparation method thereof
InactiveCN101336891APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTumor vesselSolvent
The invention relates to an anticancer slow-release gel injection and a preparation method thereof. The anticancer slow-release gel injection contains a taxane-like drug such as paclitaxel, docetaxel, hydroxypaclitaxel, epi-paclitaxel or deacetylpaclitaxel, a stine-like drug, an amphiphilic block polymer and a solvent. The composition exhibits the property of temperature-sensitive gelation, is a flowable liquid at the room temperature and turns into a stagnant and biodegradable water-insoluble gel in vivo in warm-blooded animals. The stine-like drug is selected from atrimustine, ambnmustine, nimustine, bendamustine, carmustine, elmustine, galamustine, fotemustine, estramustine, hesmustine, neptamustine, lomustine, semustine, ranimustine, semustine, tauromustine, tallimustine and spiromustine. The anticancer slow-release gel injection can release the drug slowly and locally around the tumor and retain the effective blood concentration for a plurality of weeks to a plurality of months, can not only kill tumor cells but also effectively inhibit tumor vessels, can reduce the general drug toxicity and can enhance the curative effect of chemotherapy particularly radioactive seed implantation.
Owner:济南基福医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com