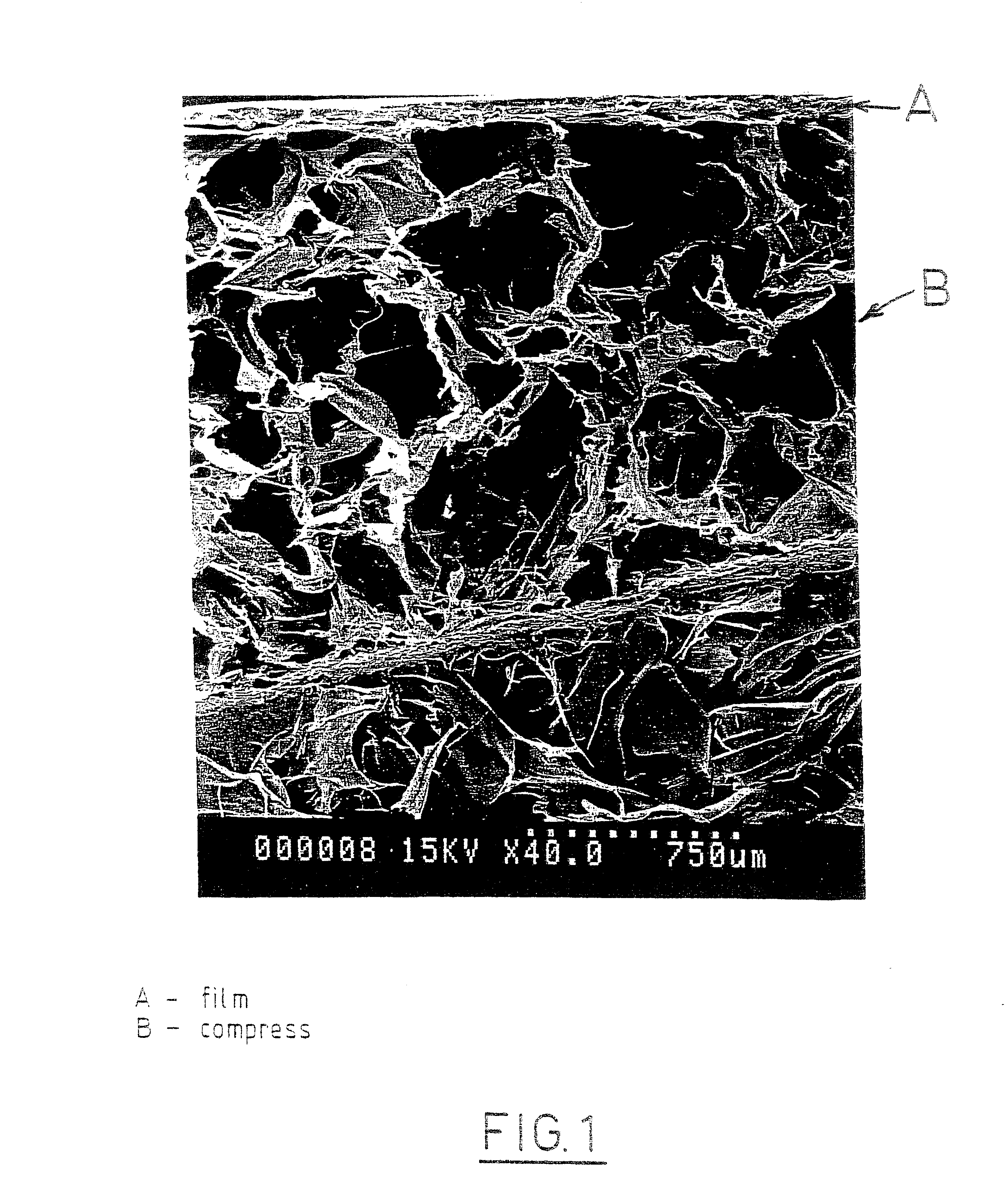
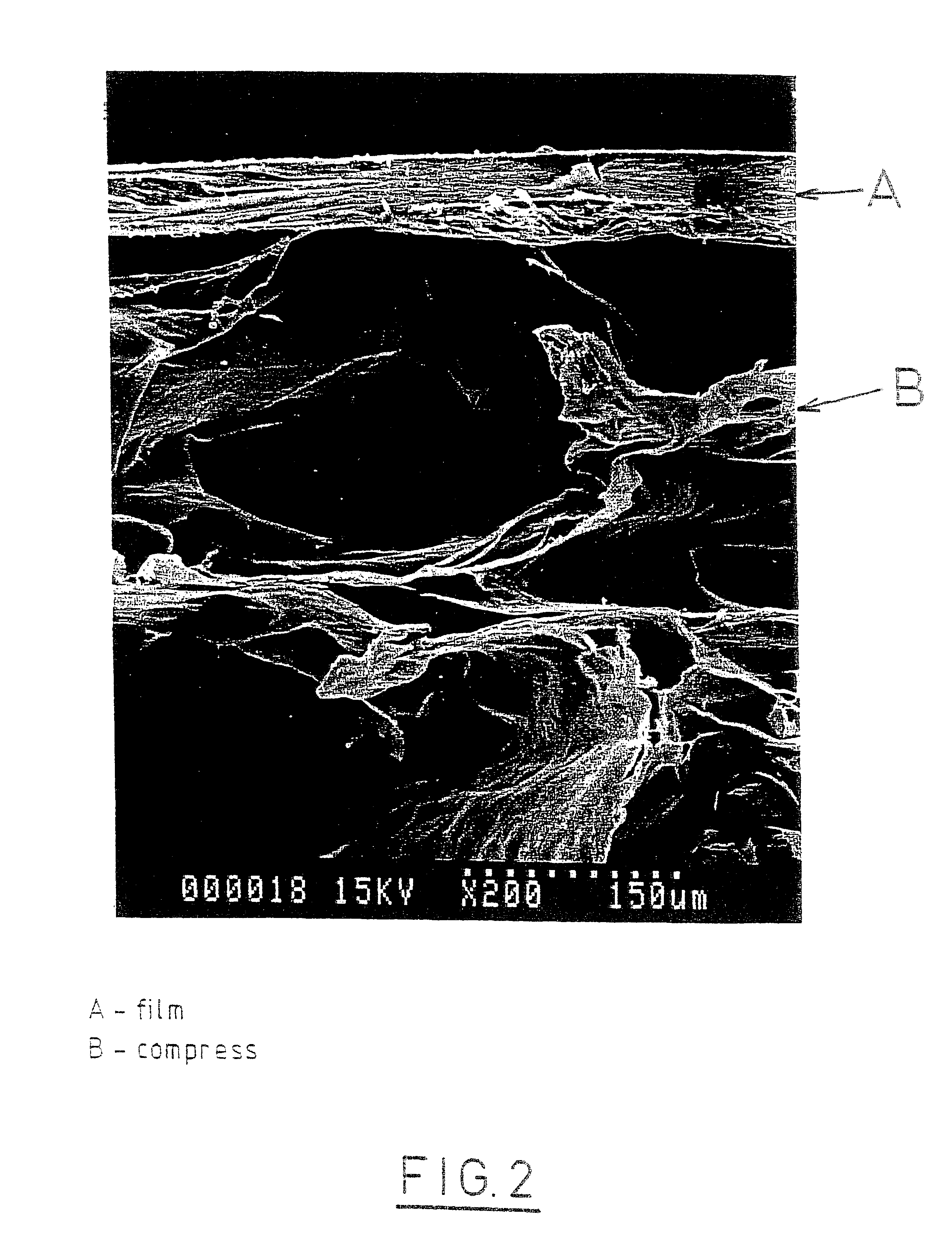
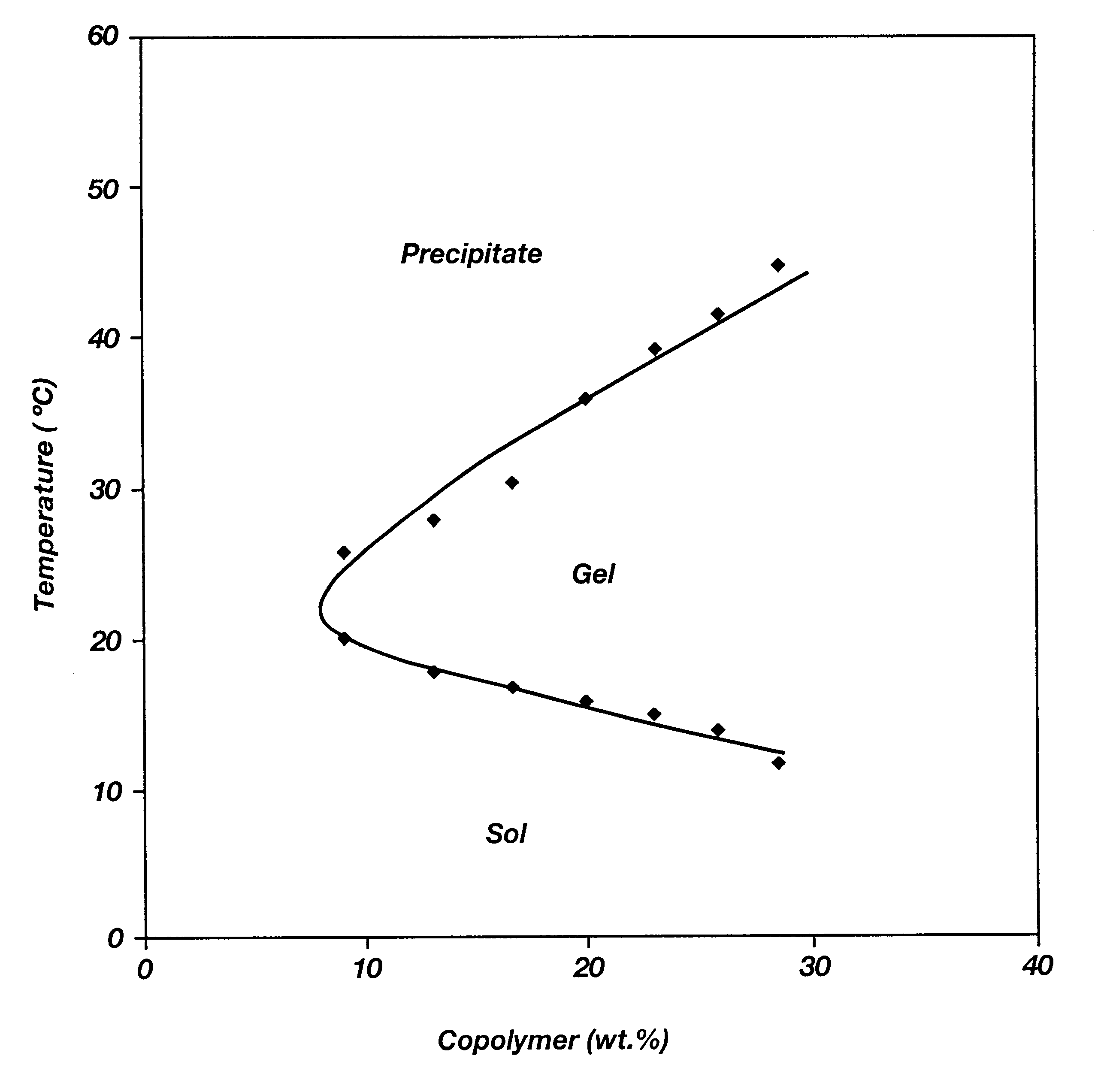
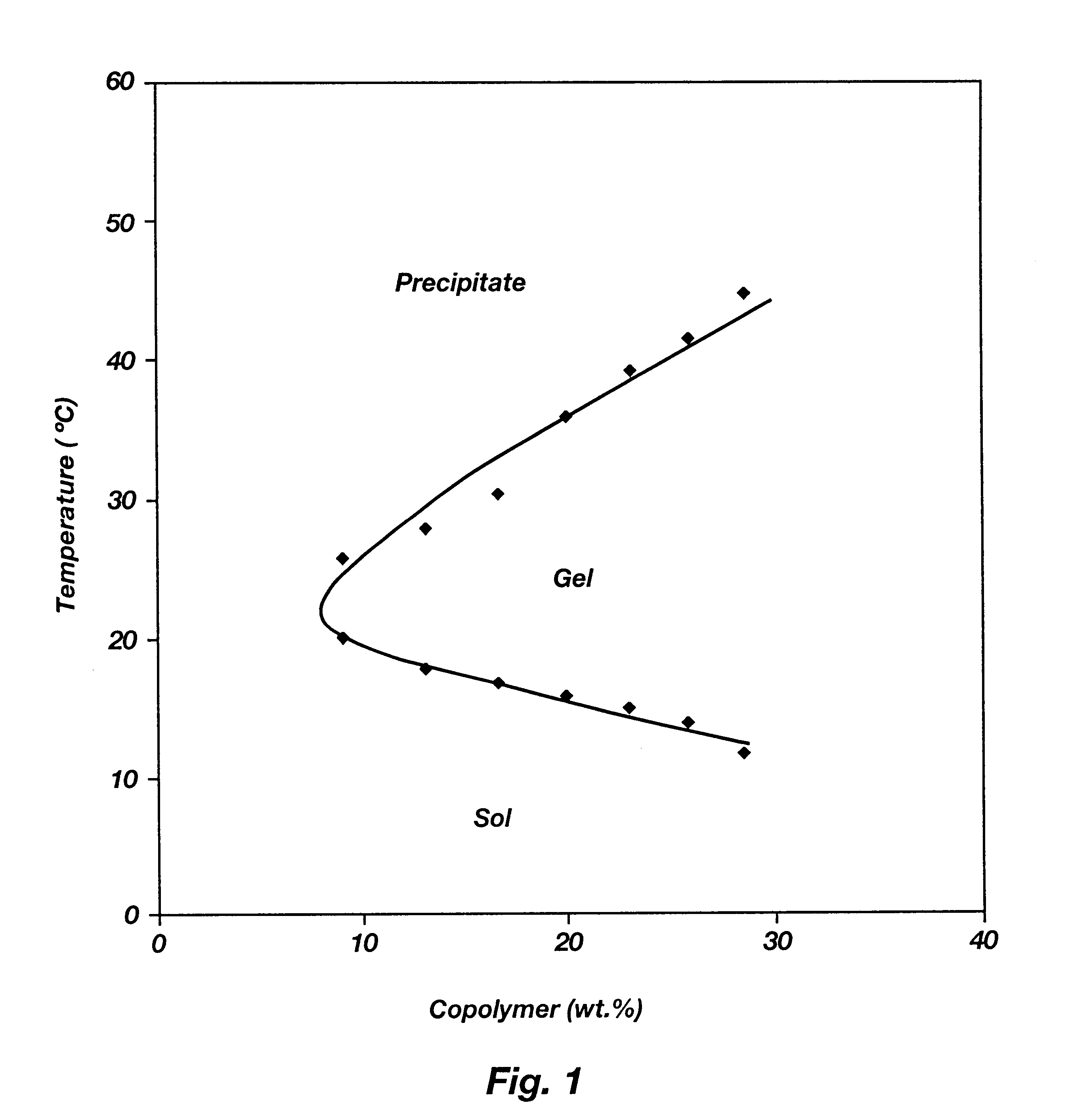
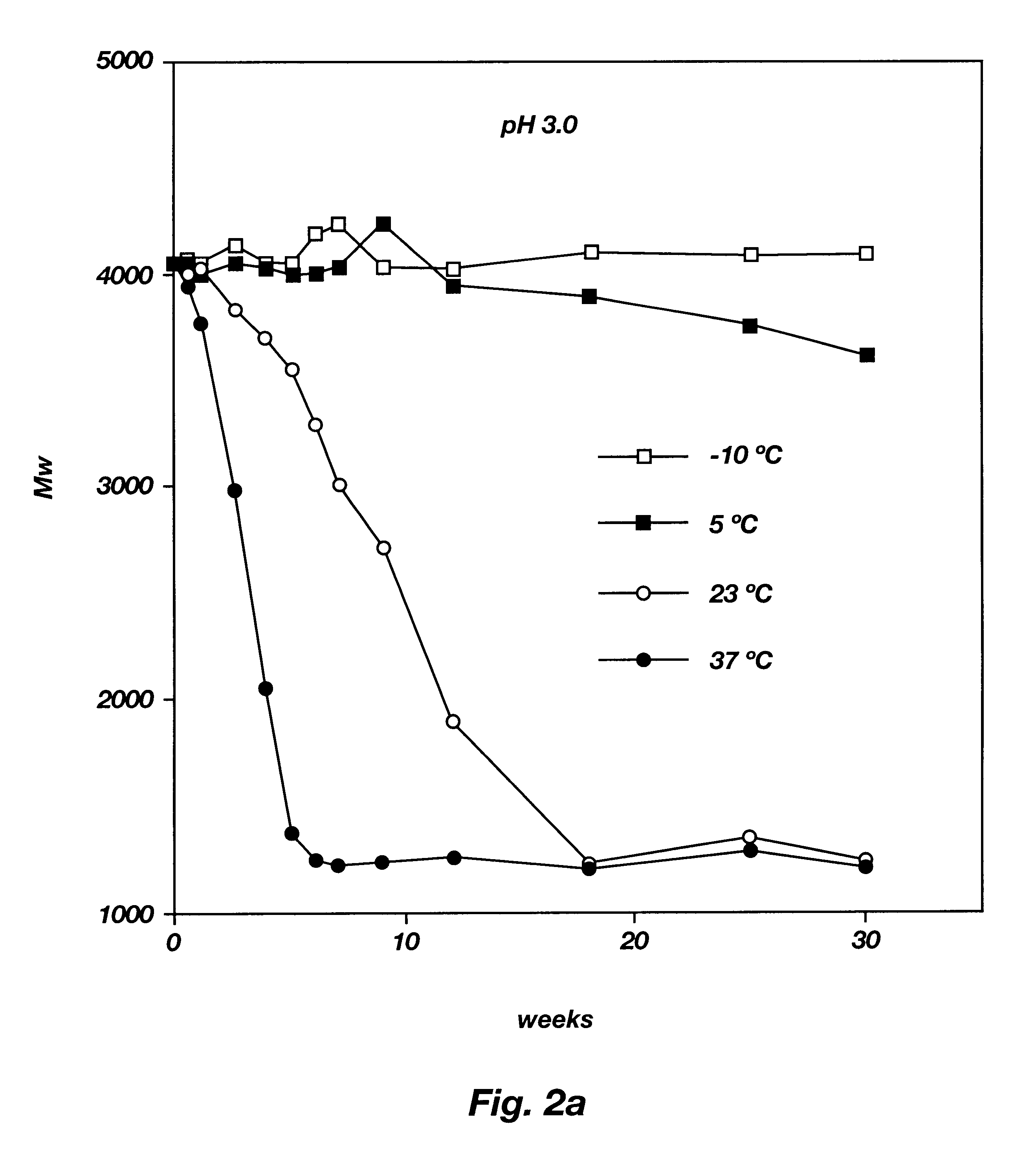
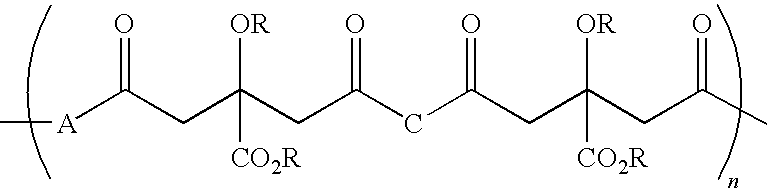
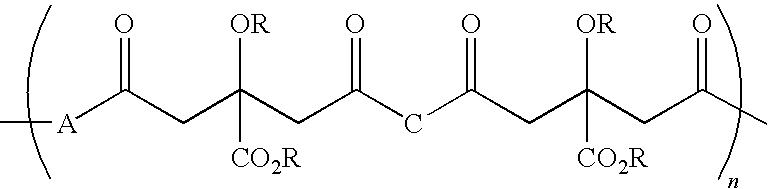
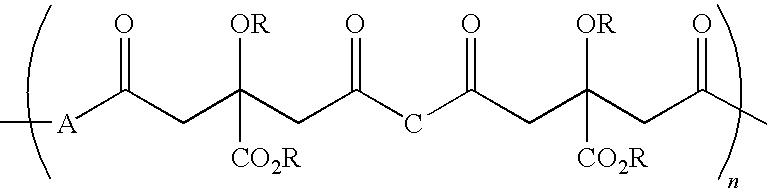
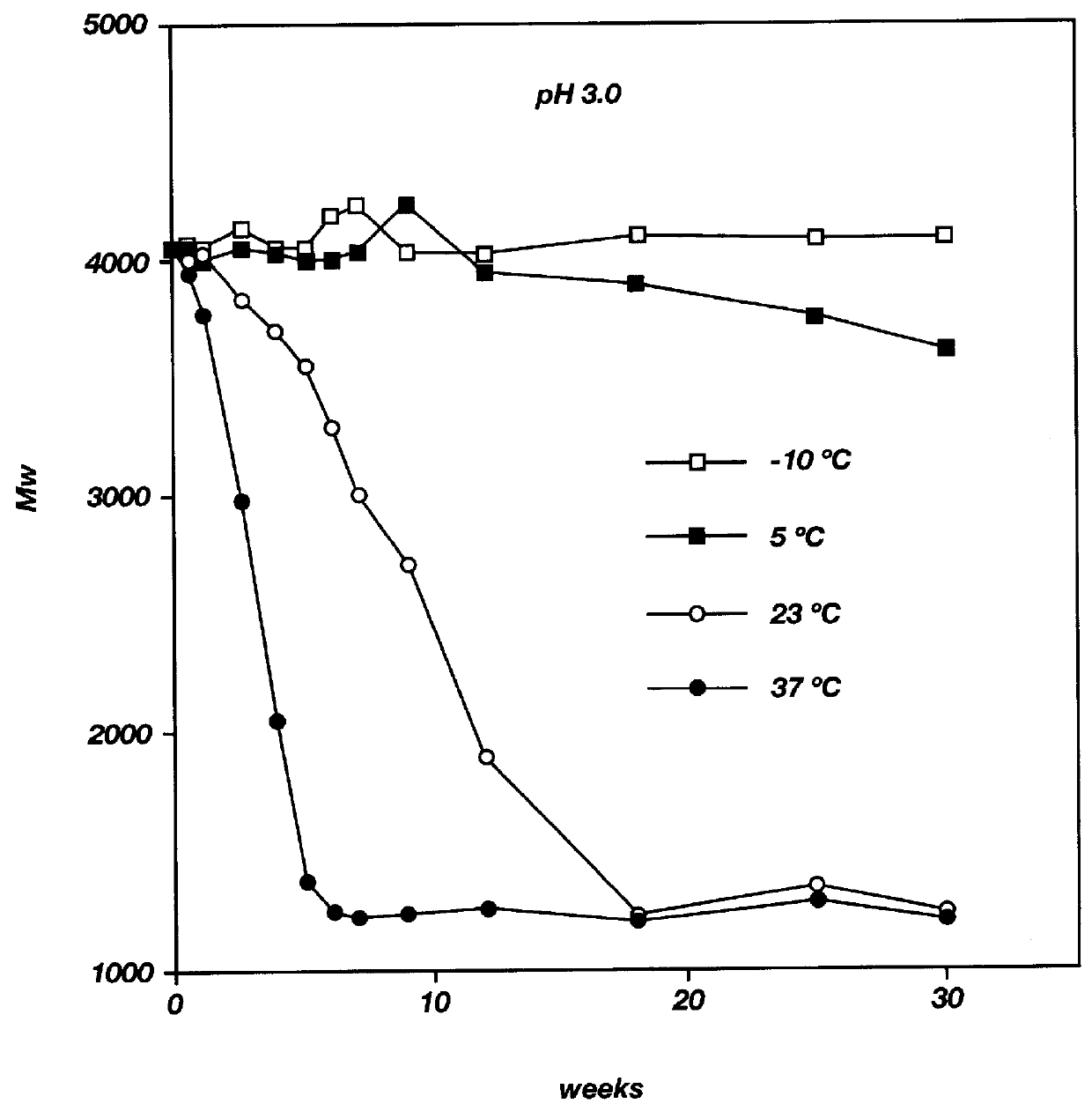
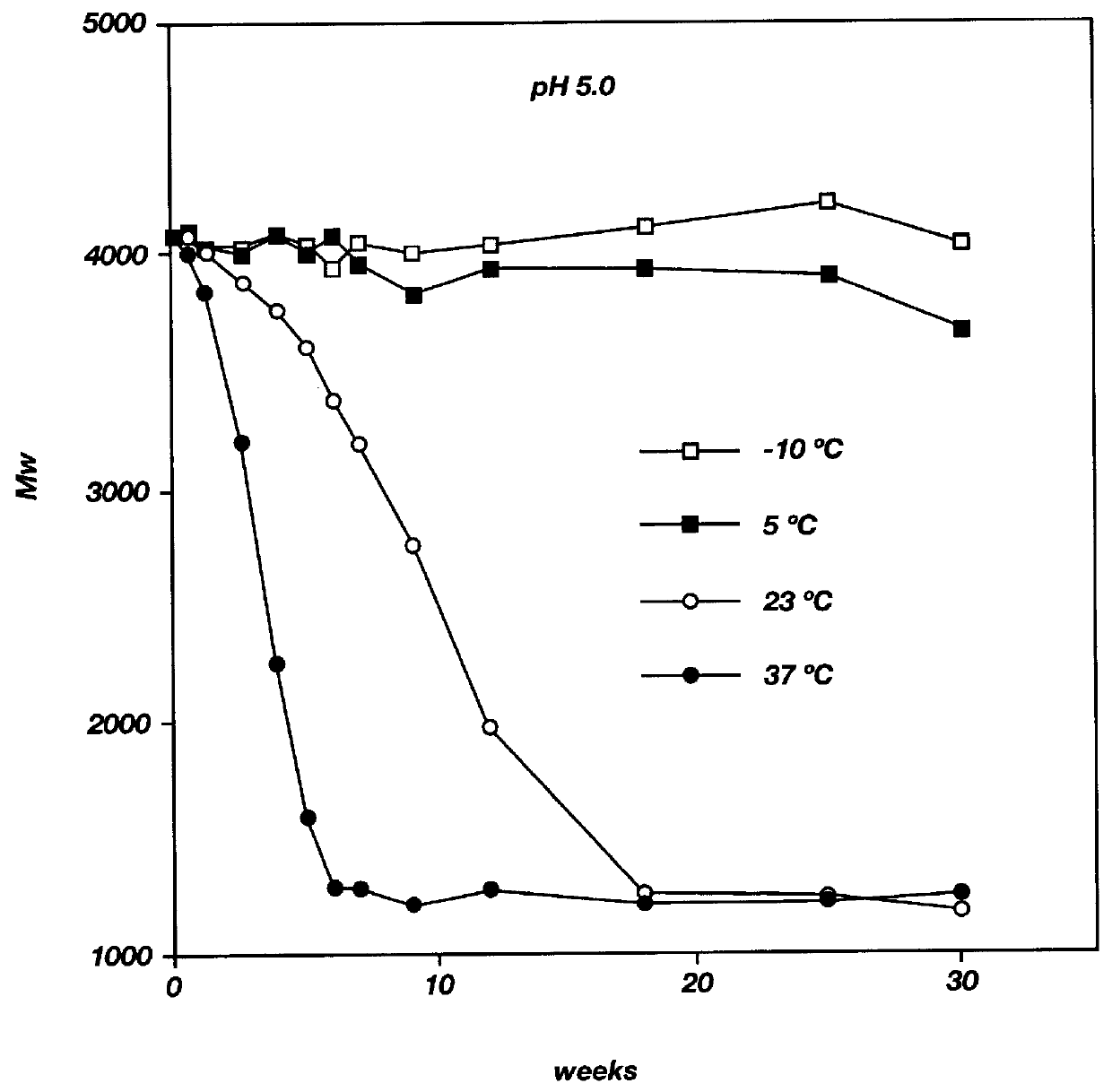
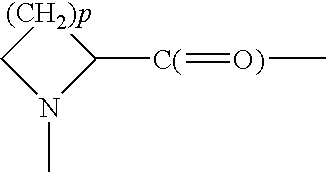
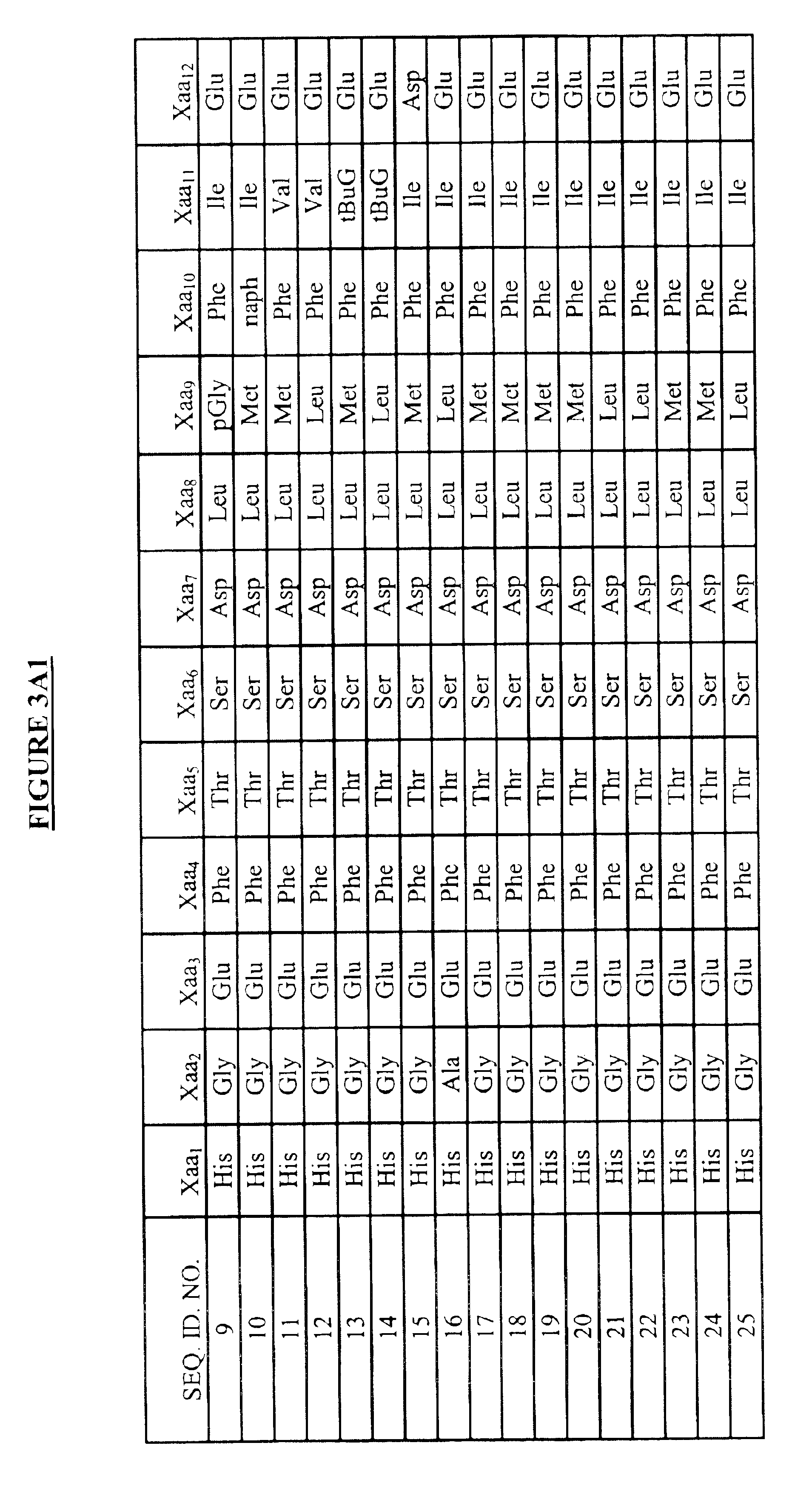
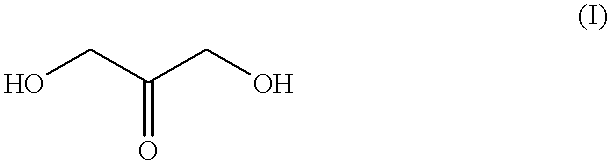
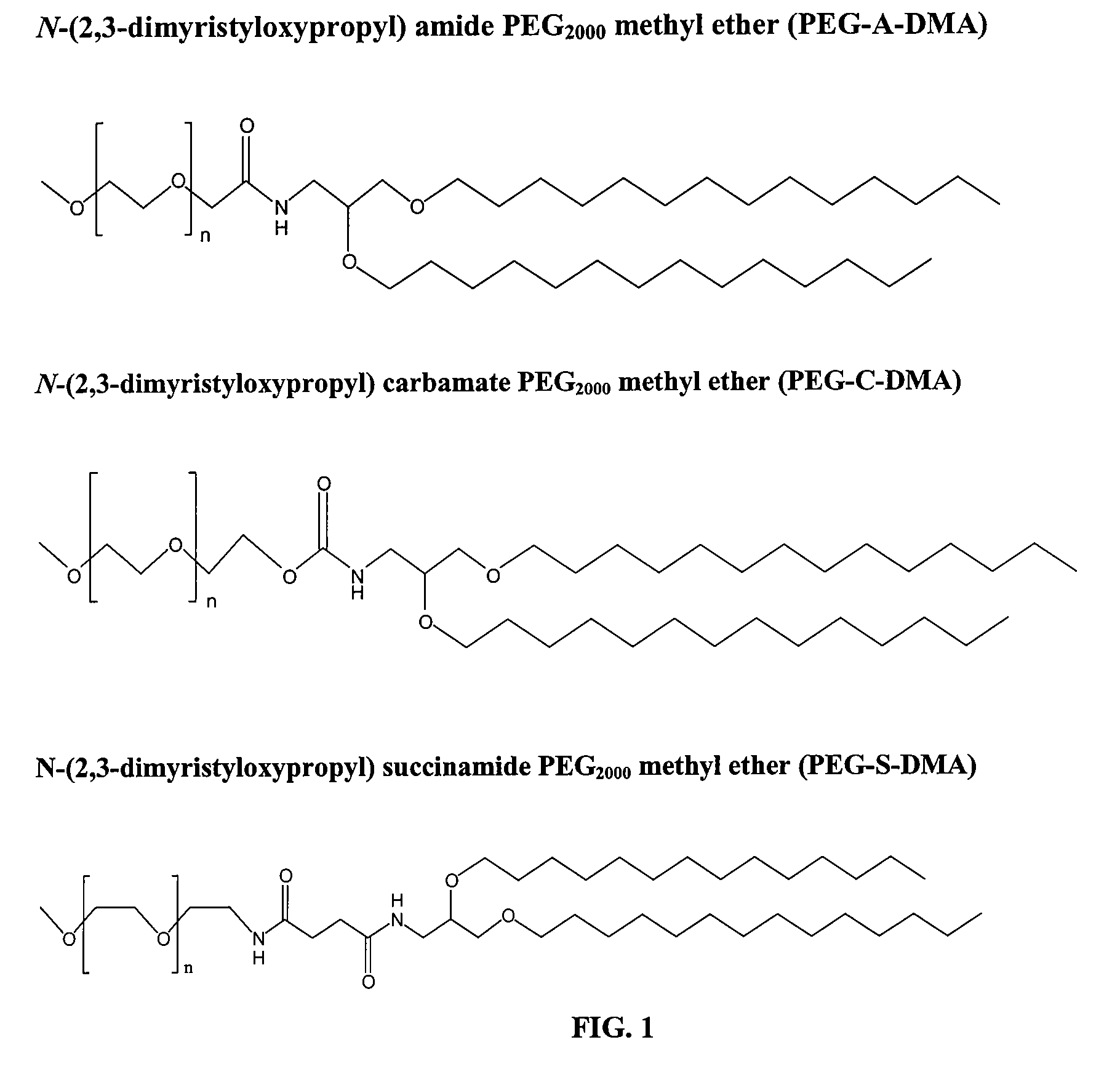
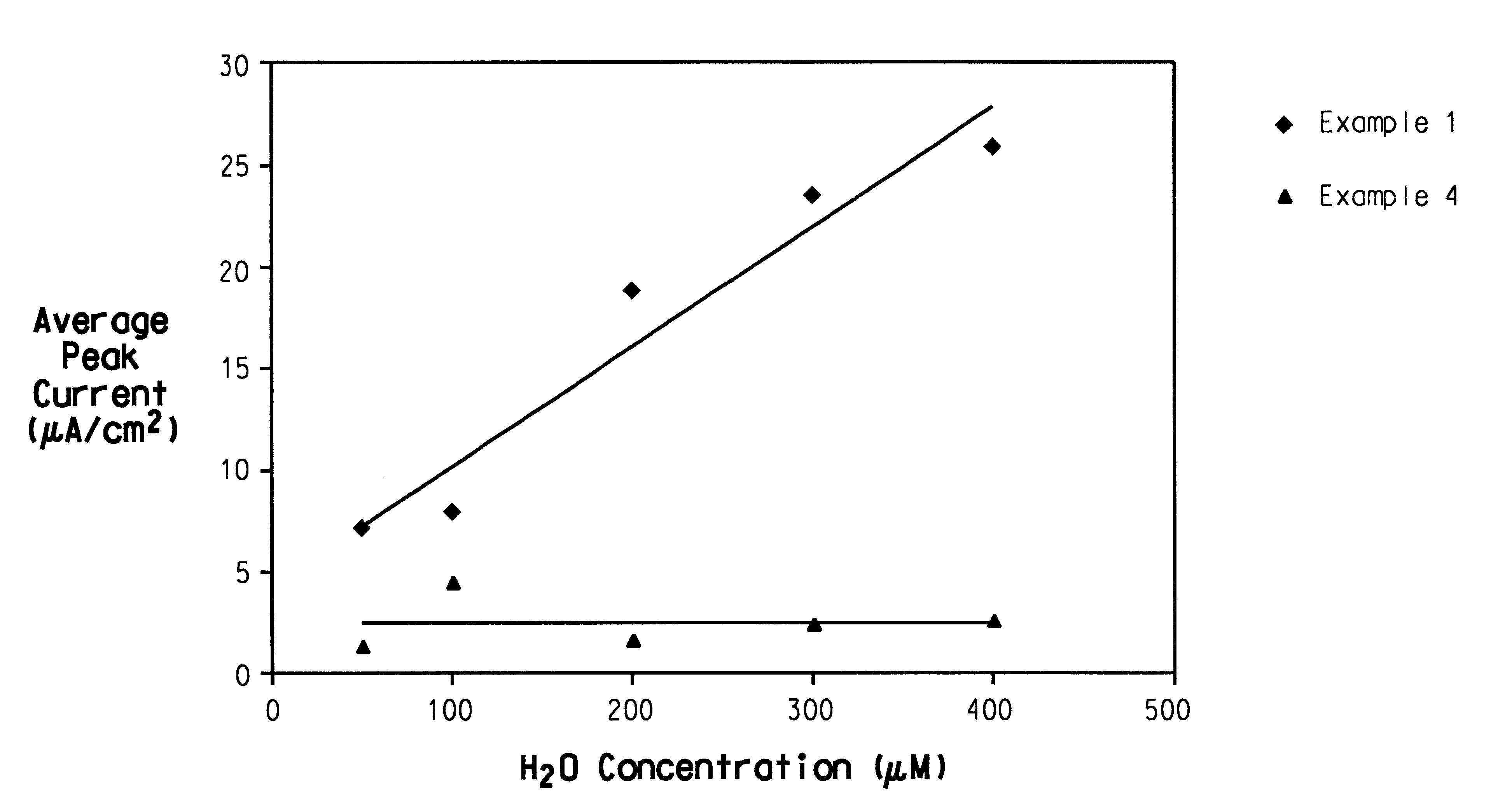
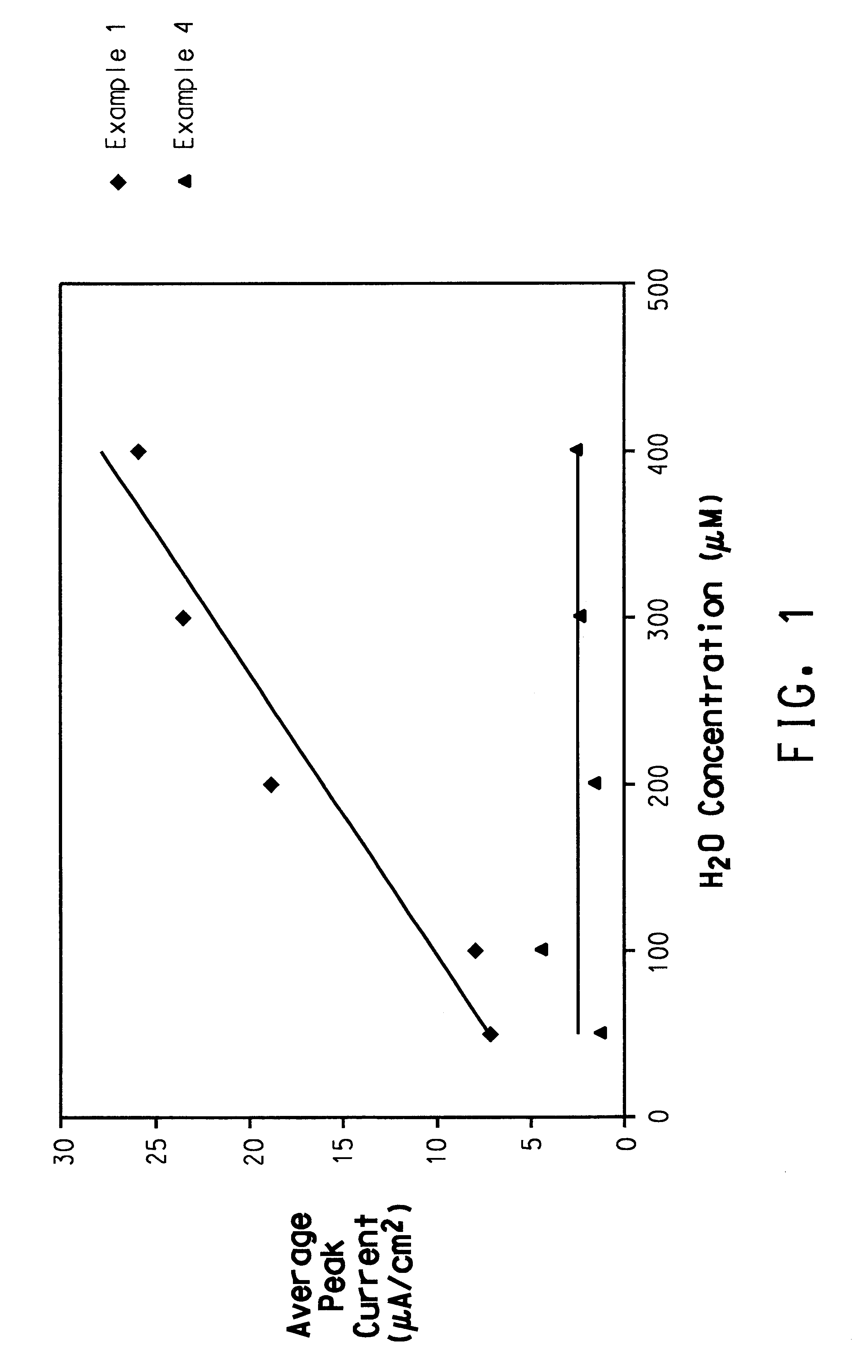
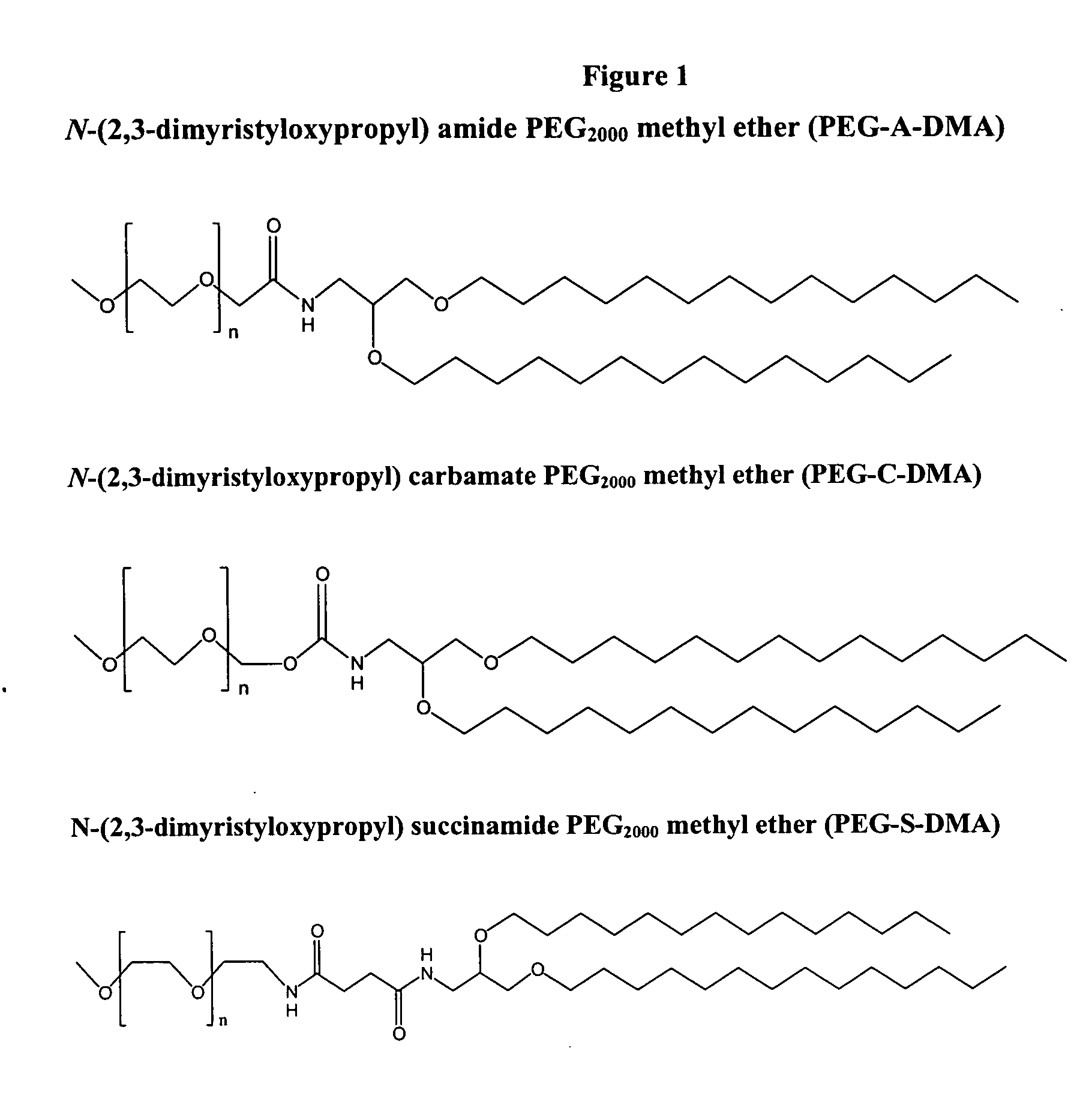
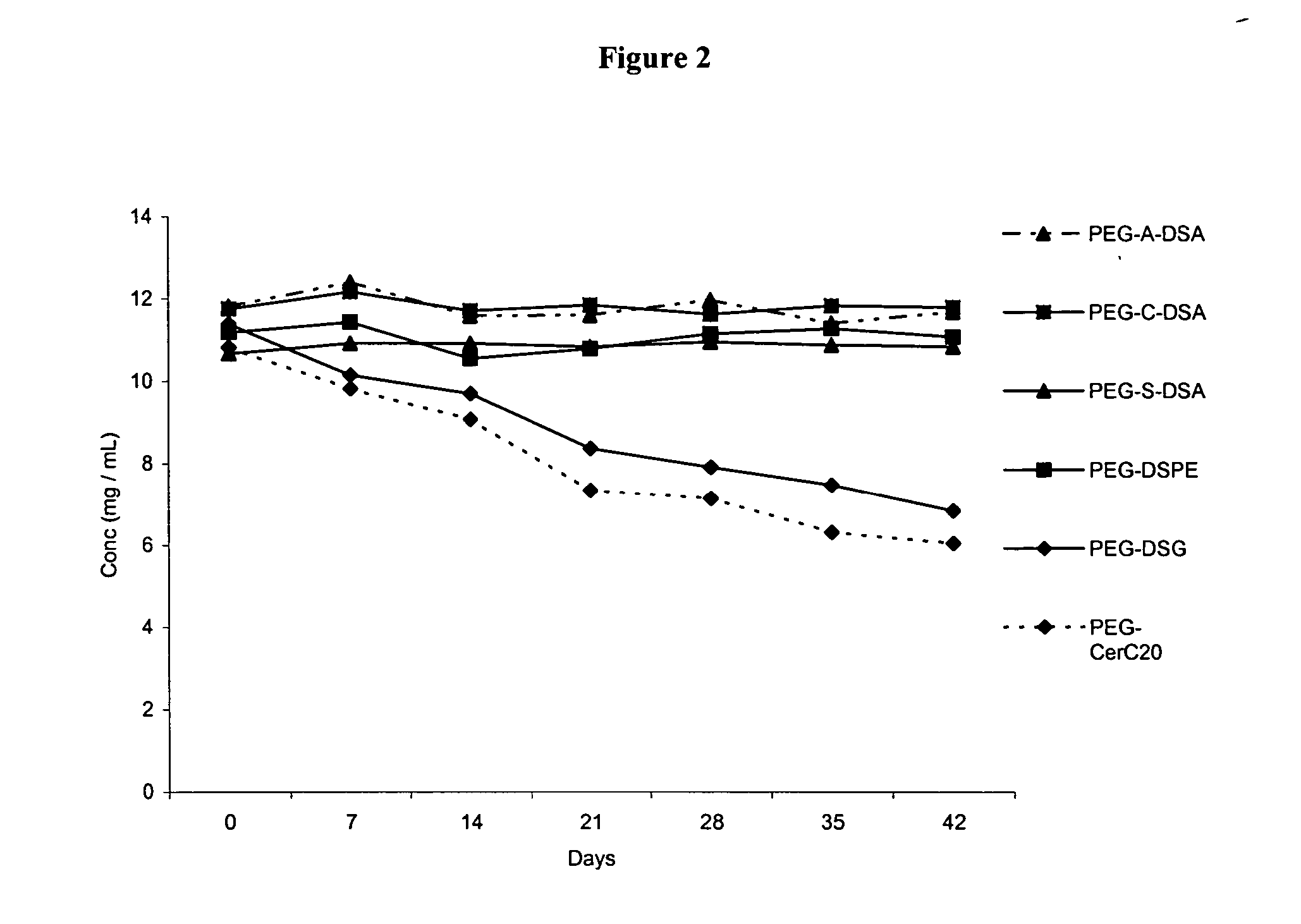
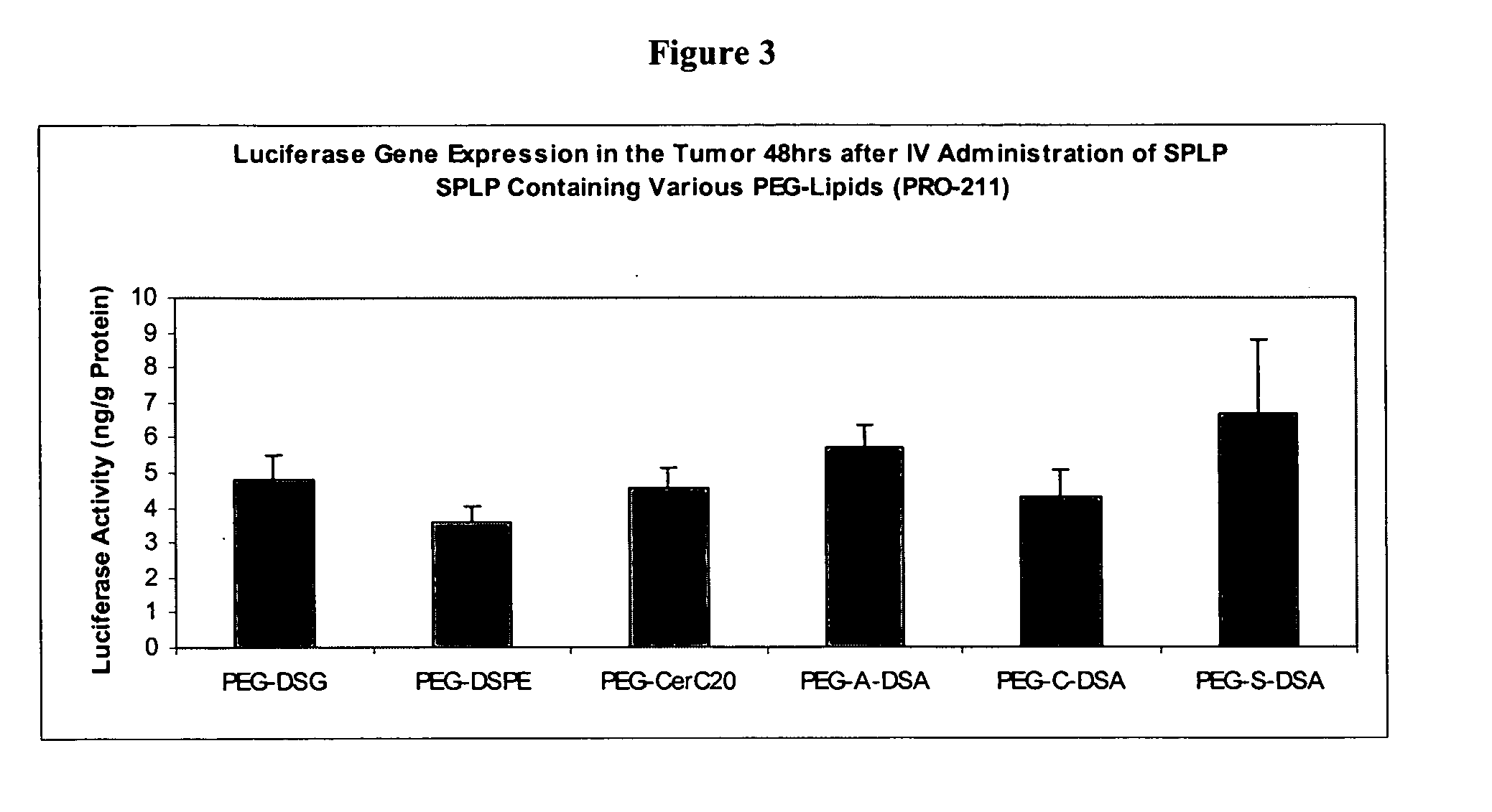
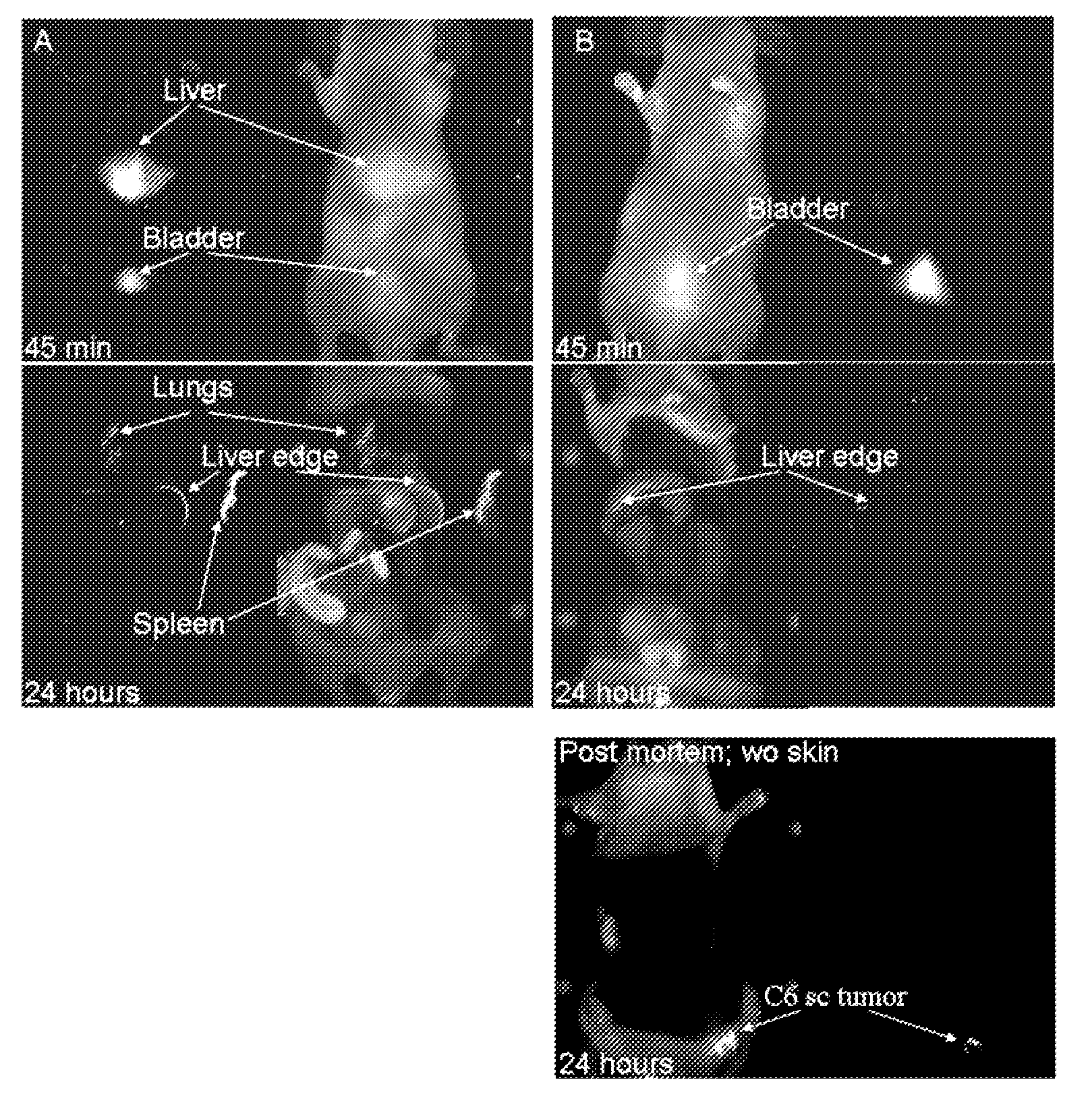
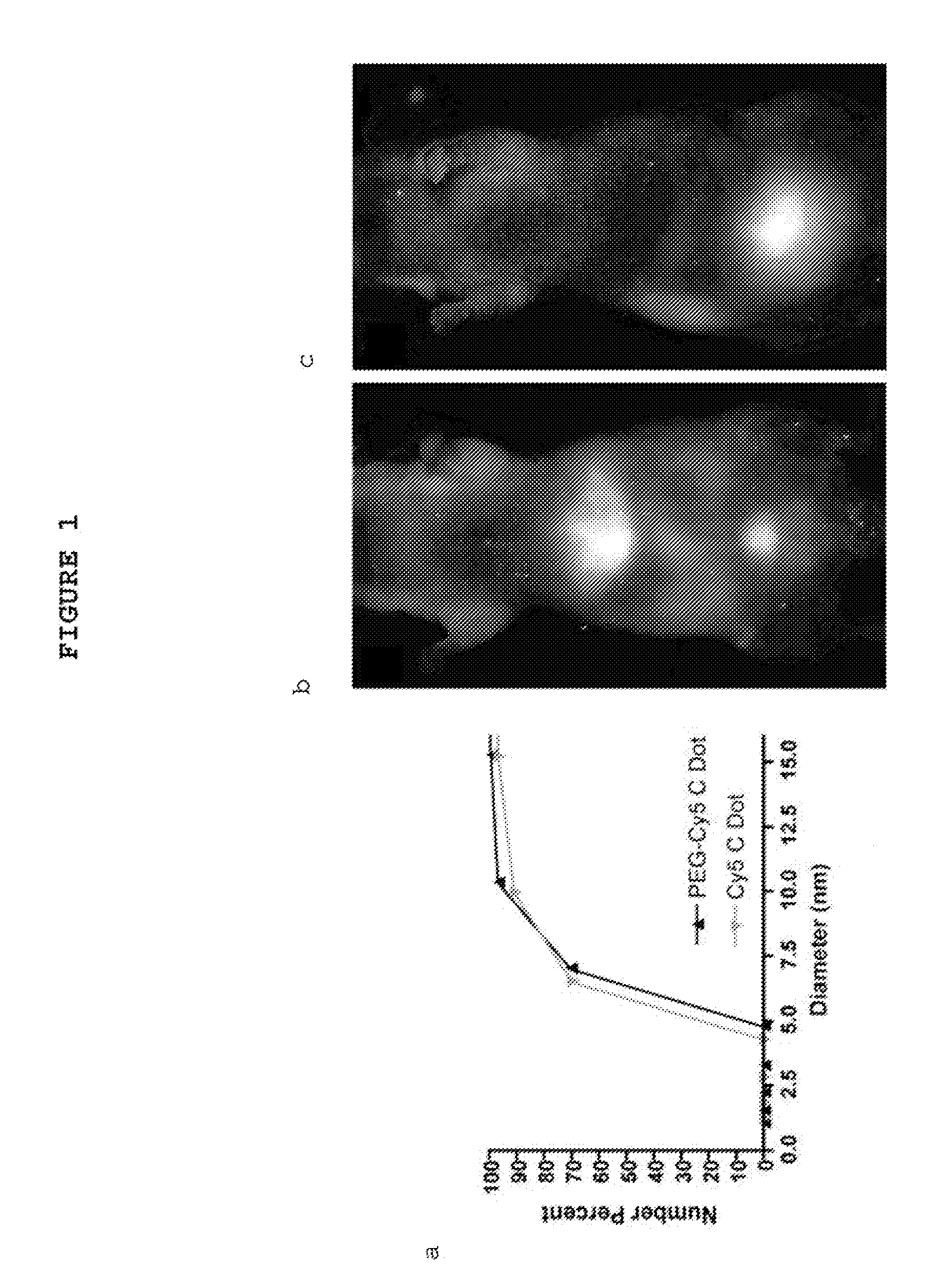
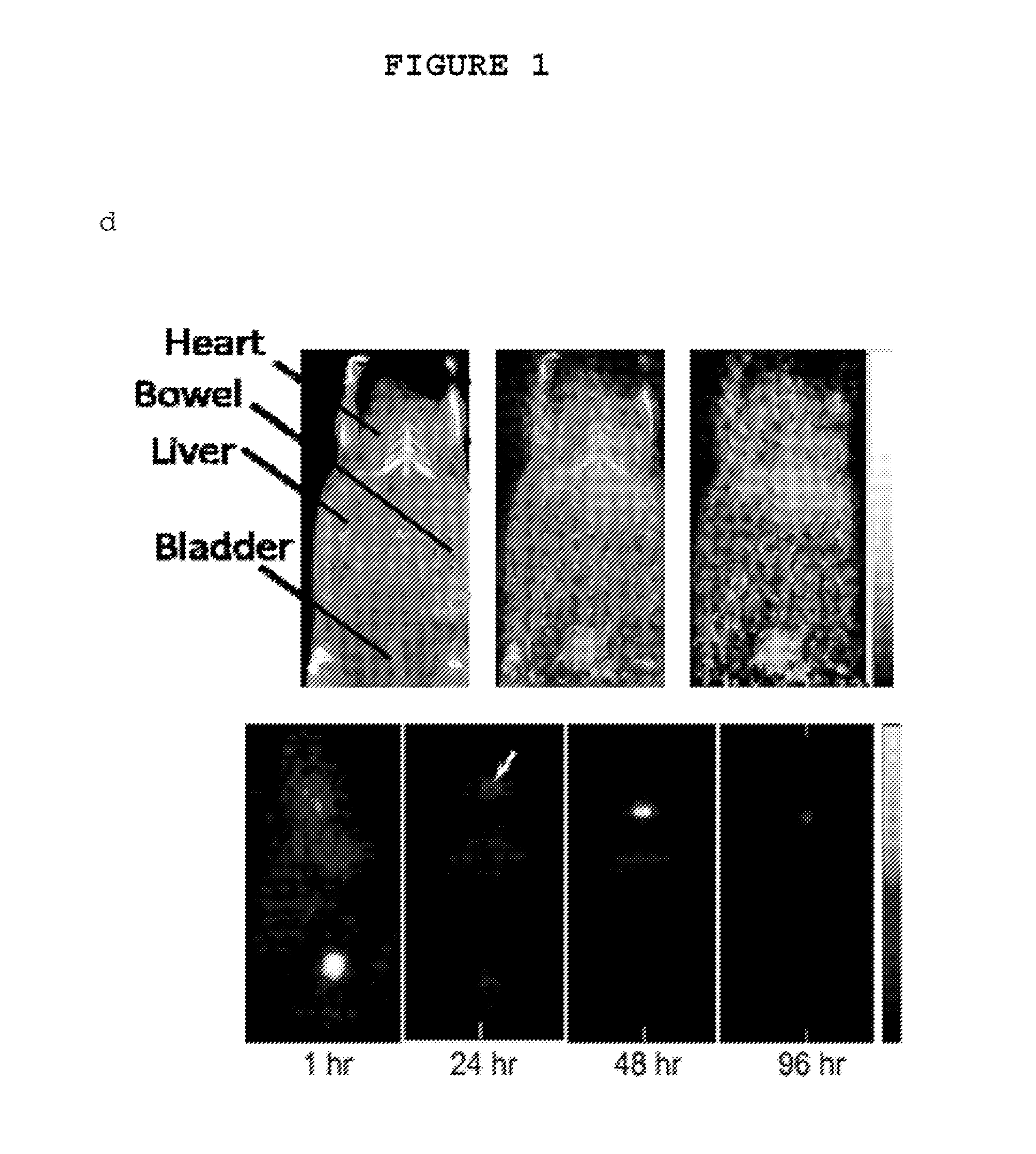
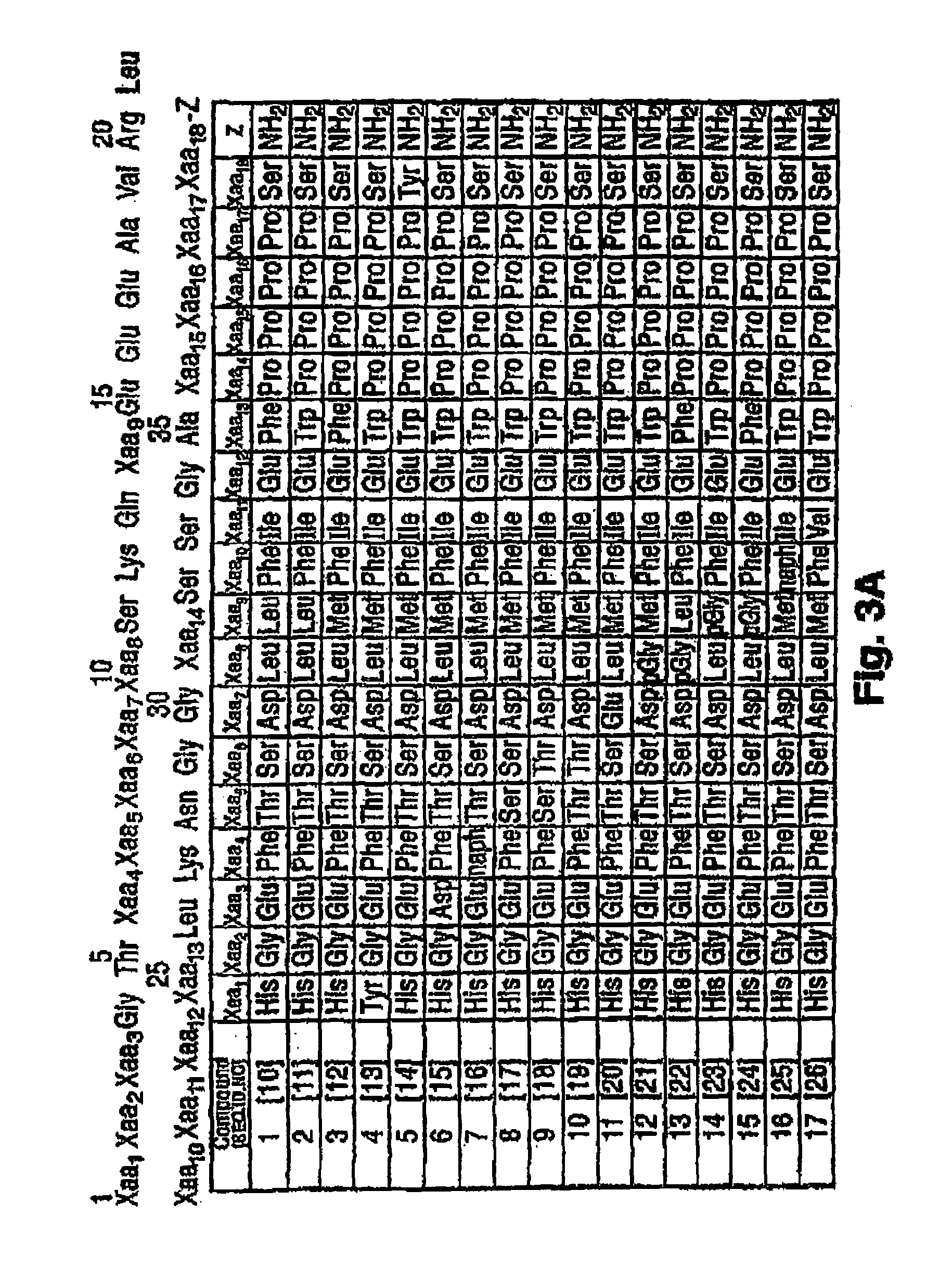
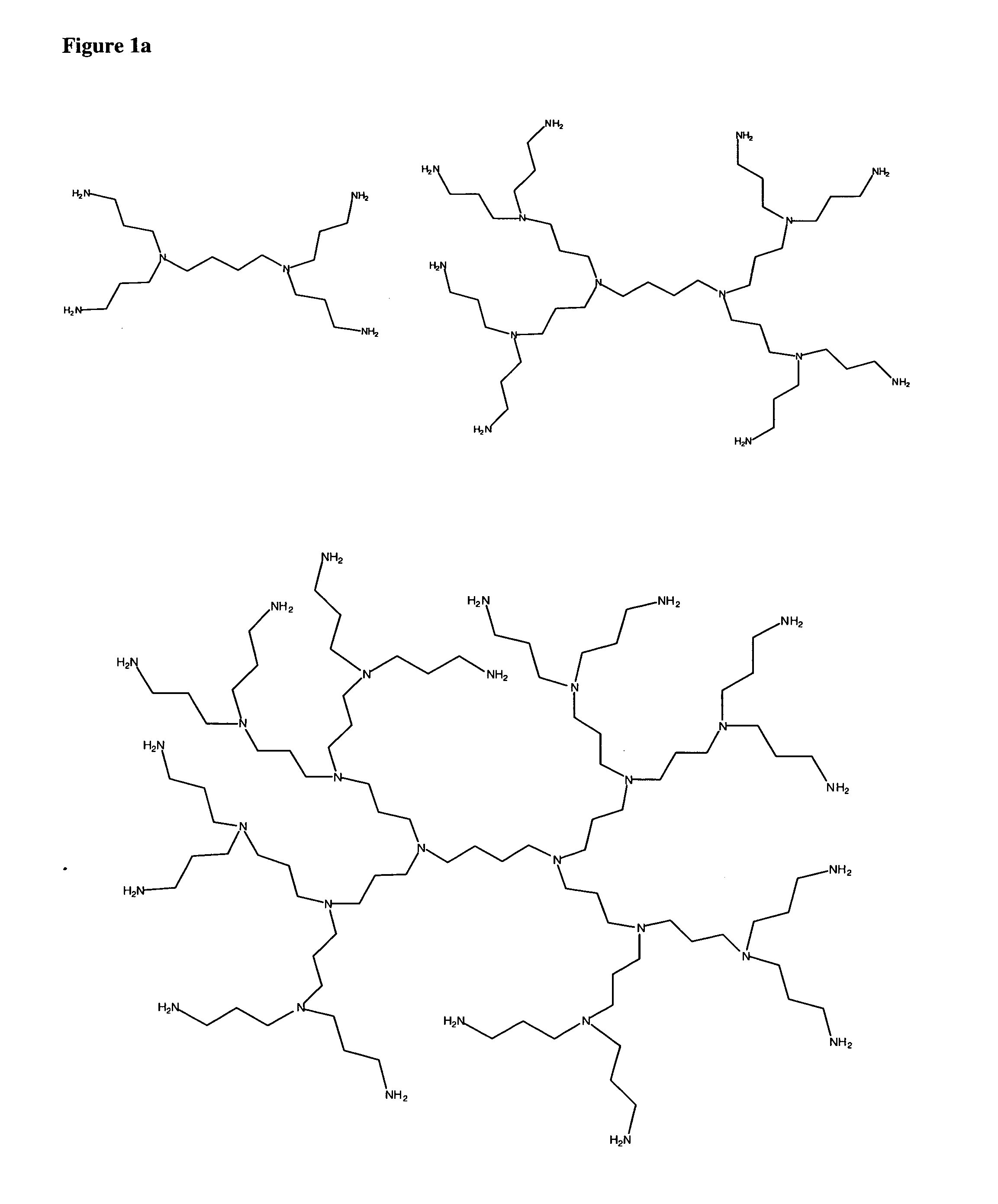
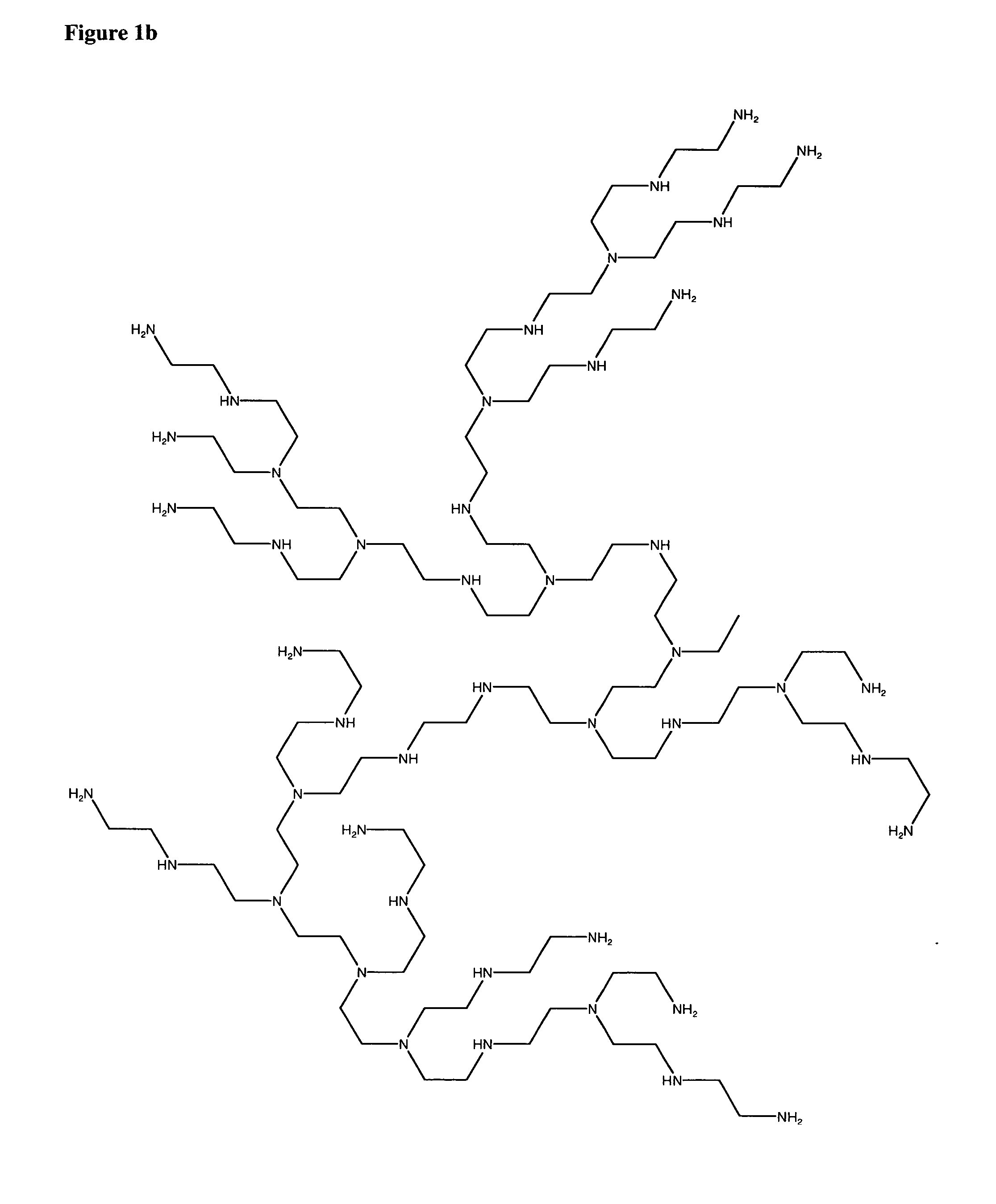
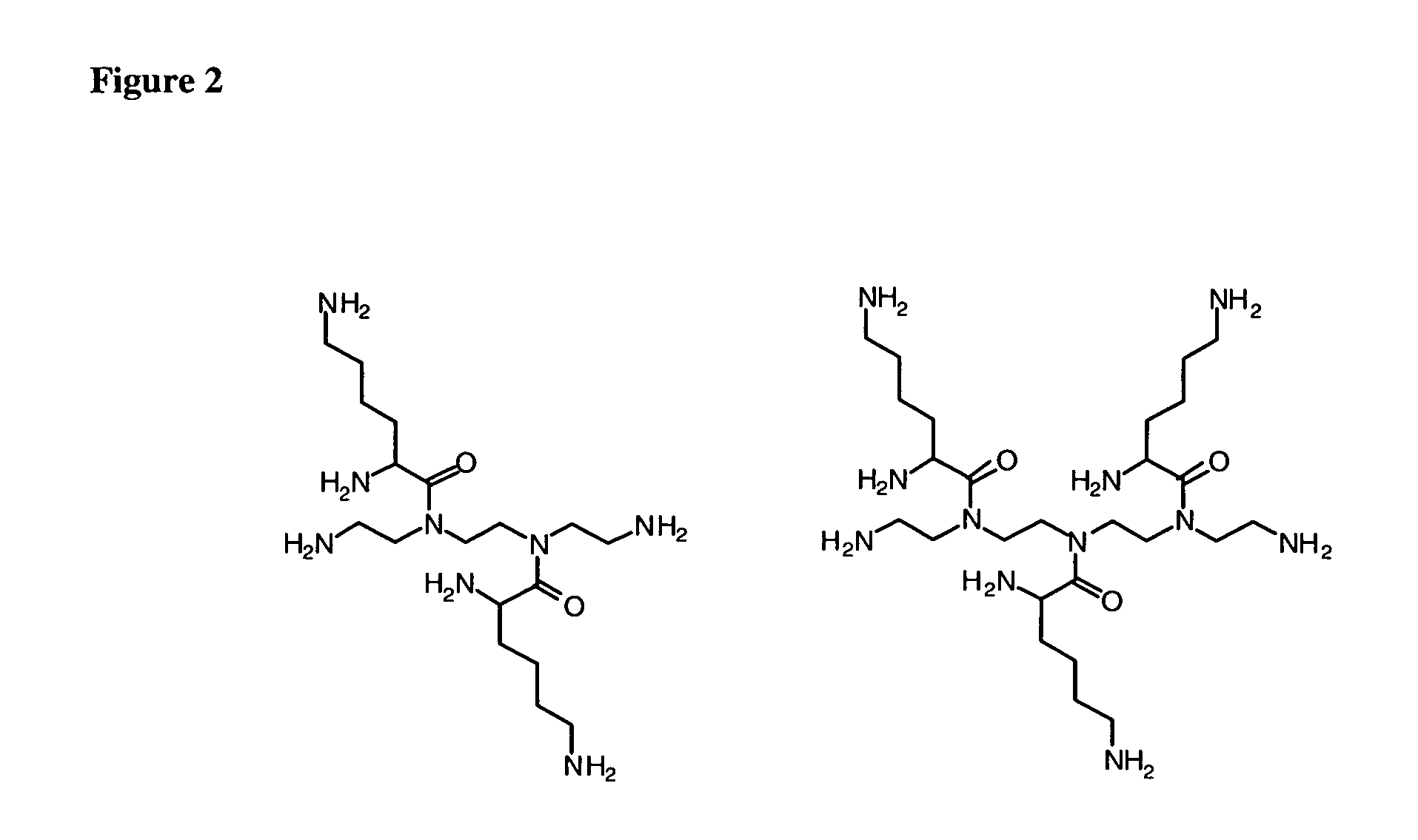
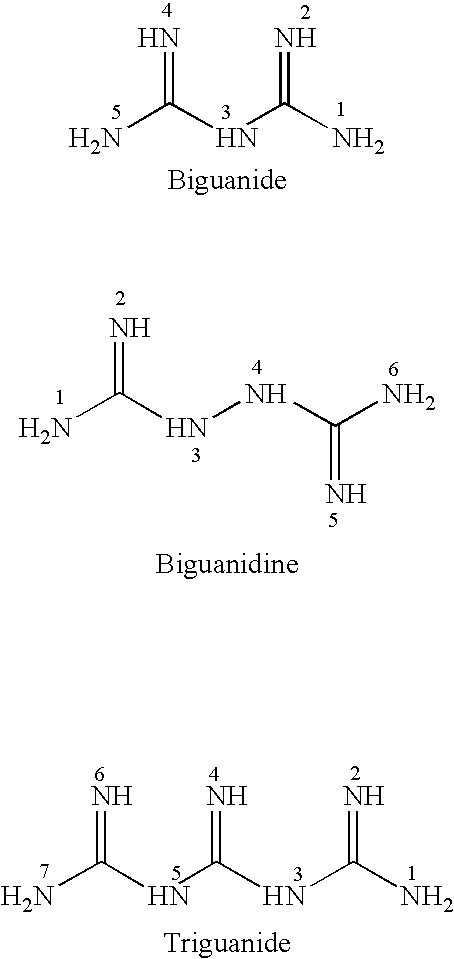
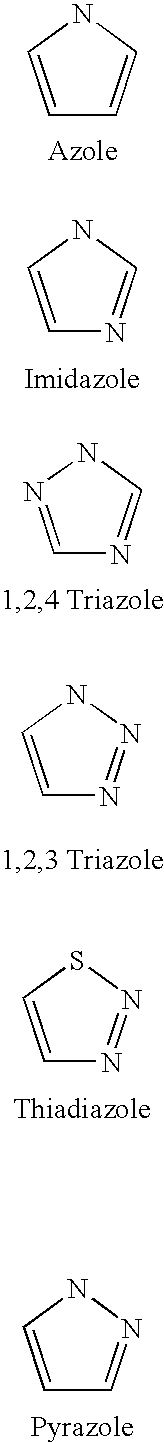
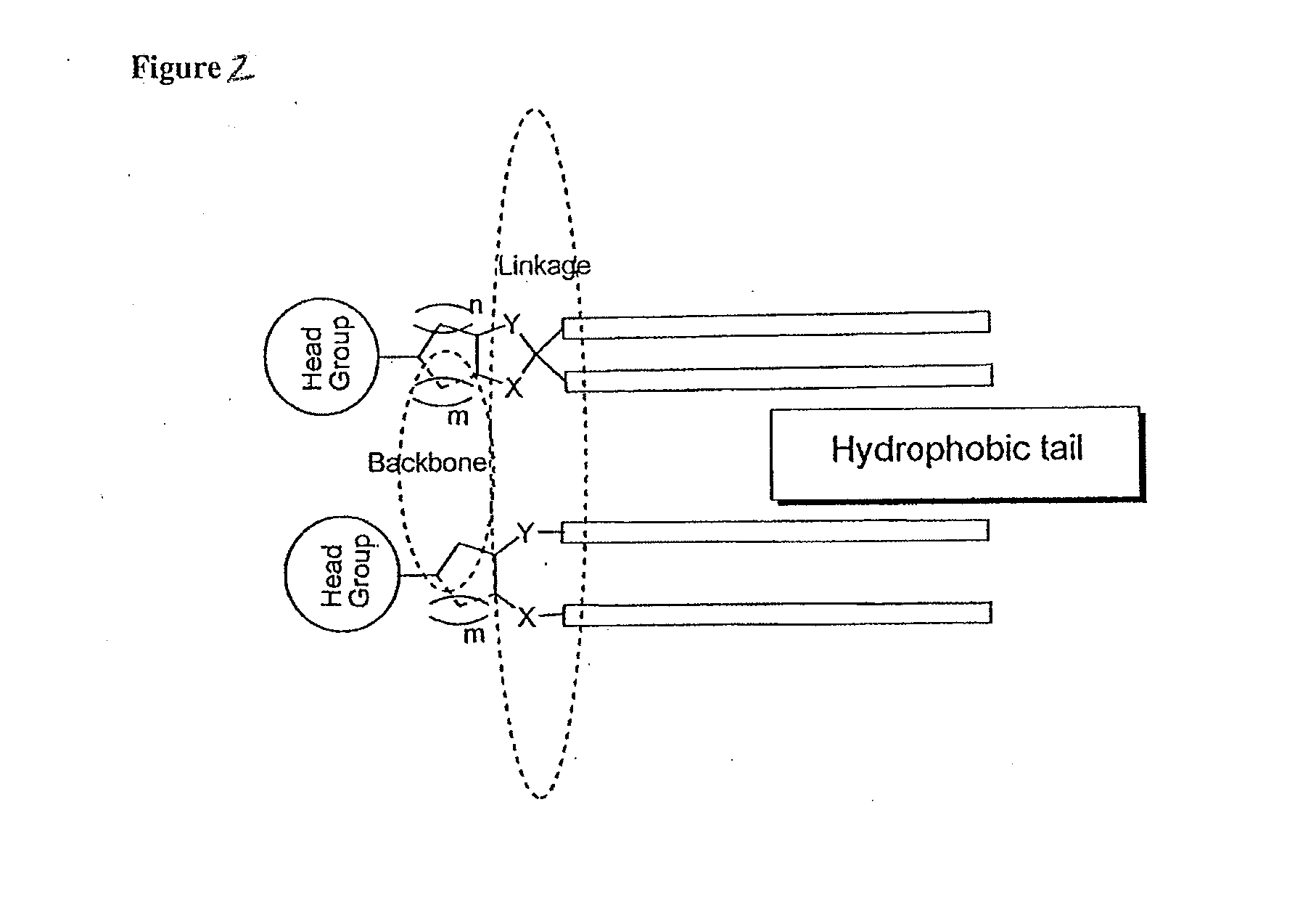
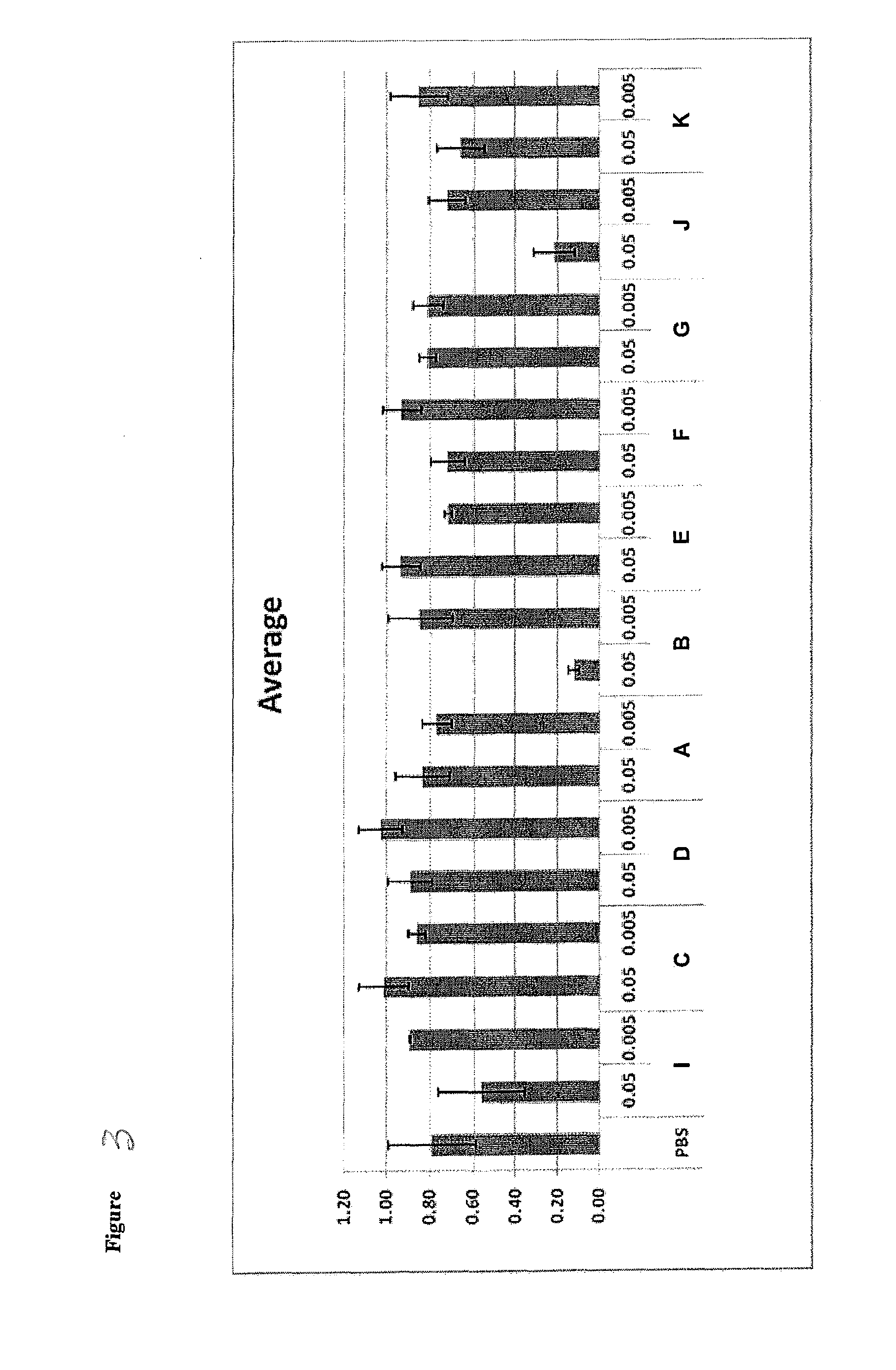
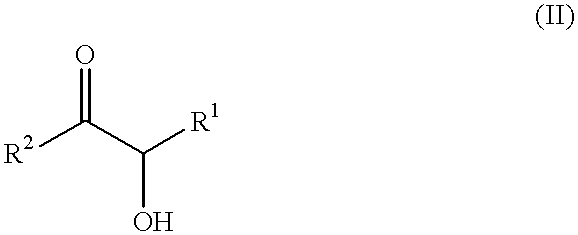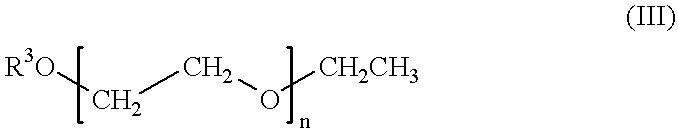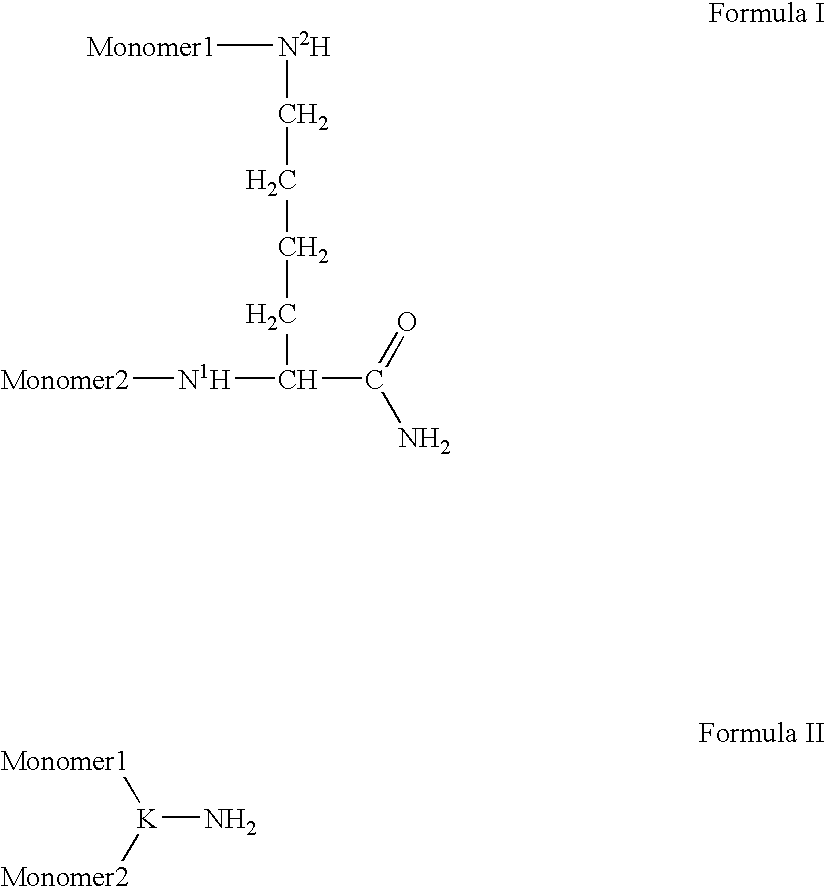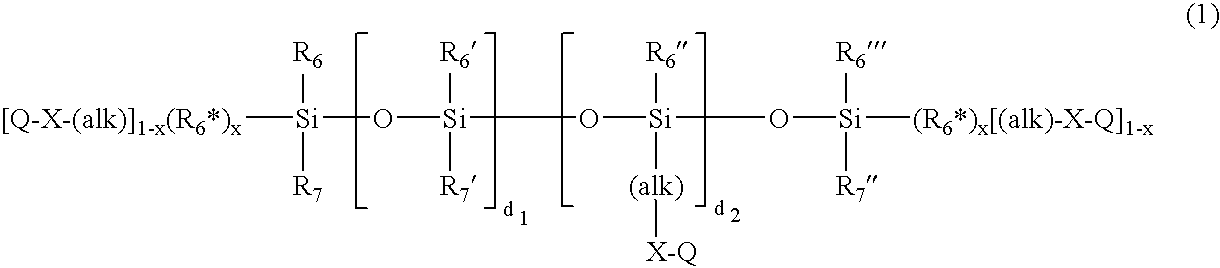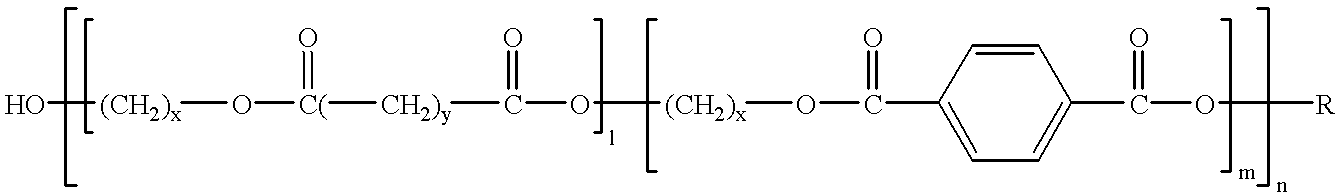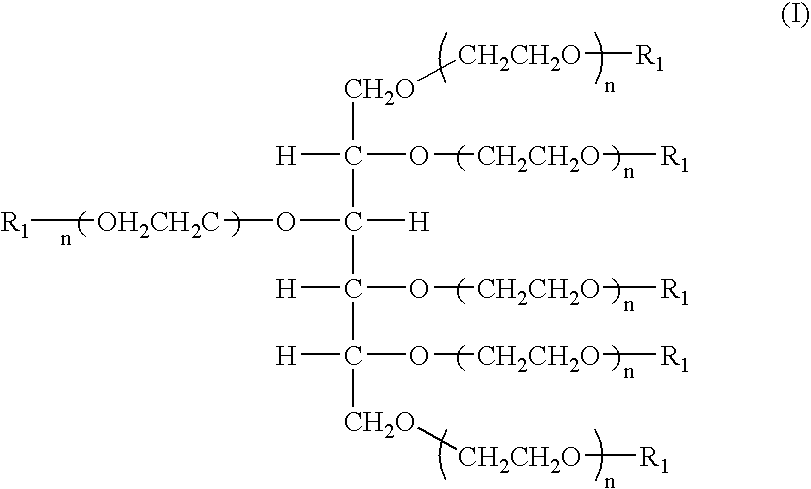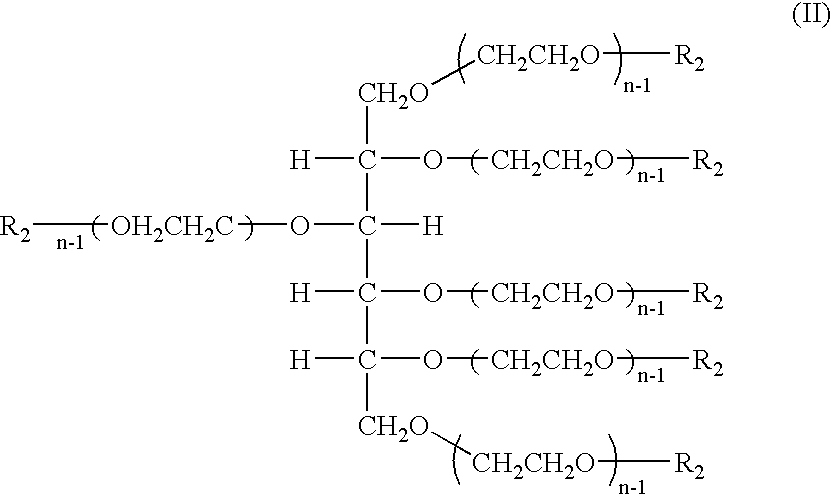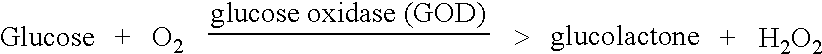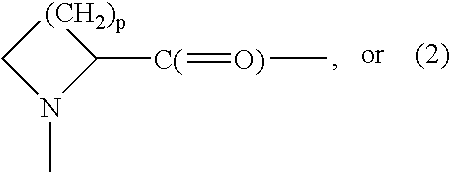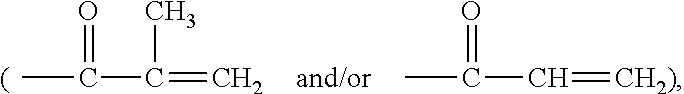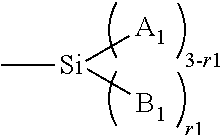Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34653 results about "Polyethylene glycol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
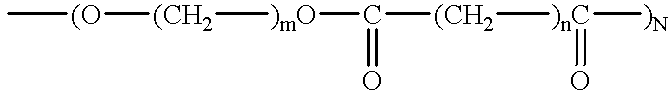
Polyethylene glycol (PEG; /ˌpɒliˈɛθəlˌiːn ˈɡlaɪˌkɒl, -ˌkɔːl/) is a polyether compound with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH₂−CH₂)ₙ−OH.
Method for preparing two-layer bicomposite collagen material for preventing post-operative adhesions
InactiveUS6596304B1Improve propertiesAvoid stickingPeptide/protein ingredientsSurgerySurgical operationPost operative
A bicomposite material based on collagen is prepared which has two closely bound layers and is biocompatible, non-toxic, hemostatic and biodegradable in less than a month, and can be used in surgery to achieve hemostasis and prevent post-surgical adhesion. To prepare the material, a solution of collagen or gelatin, which may contain glycerine and a hydrophilic additive such as polyethylene glycol or a polysaccharide, is poured onto an inert support to form a layer 30 .mu.m to less than 100 .mu.m thick. Then a polymeric porous fibrous layer is applied during gelling of the collagen or gelatin, and the resultant material is dried. The polymeric porous fibrous layer may be made of collagen or a polysaccharide, and have a density of not more than 75 mg / cm.sup.2, a pore size from 30 .mu.m to 300 .mu.m and a thickness of 0.2 cm to 1.5 cm.
Owner:IMEDEX BIOMATERIAUX CHAPONOST
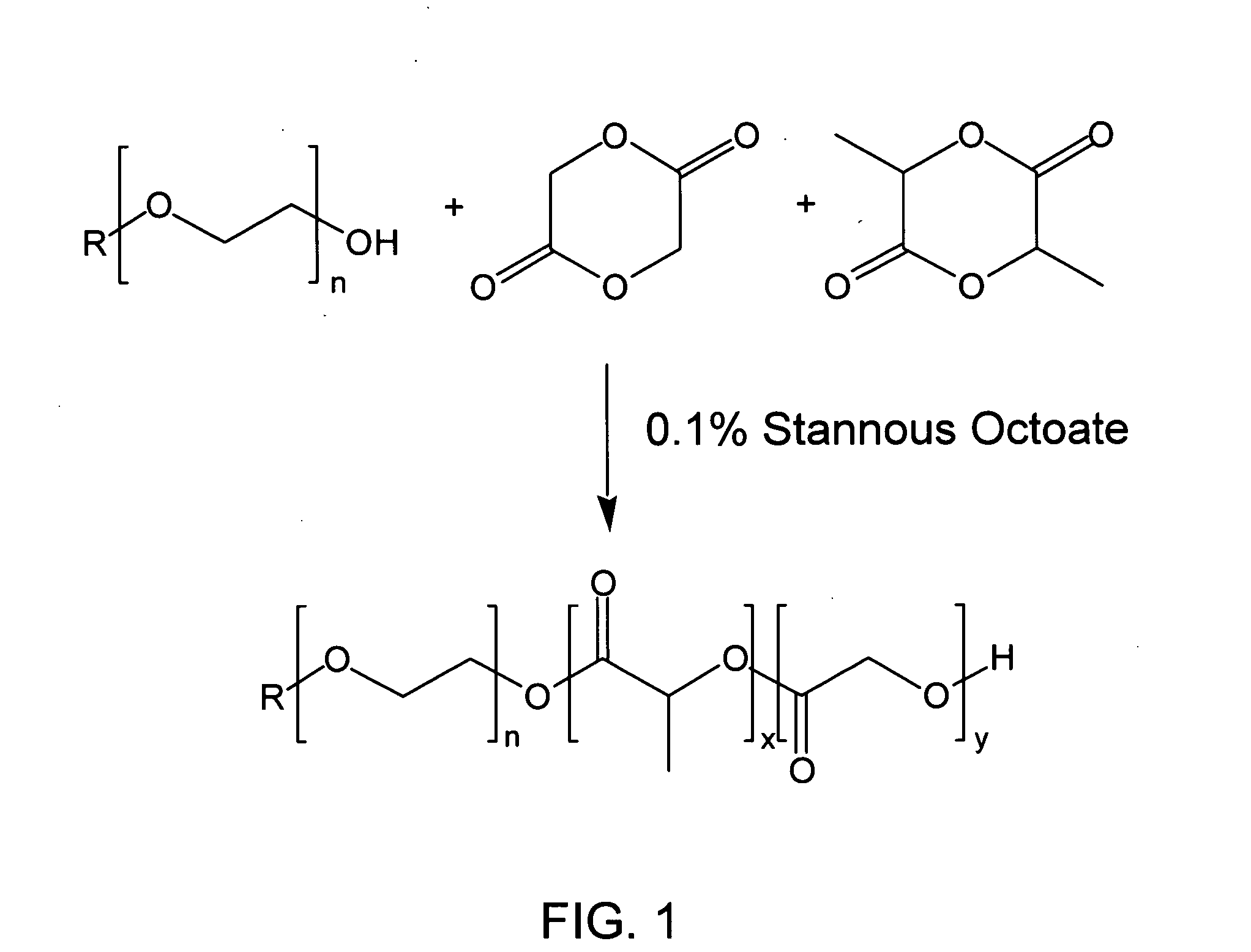
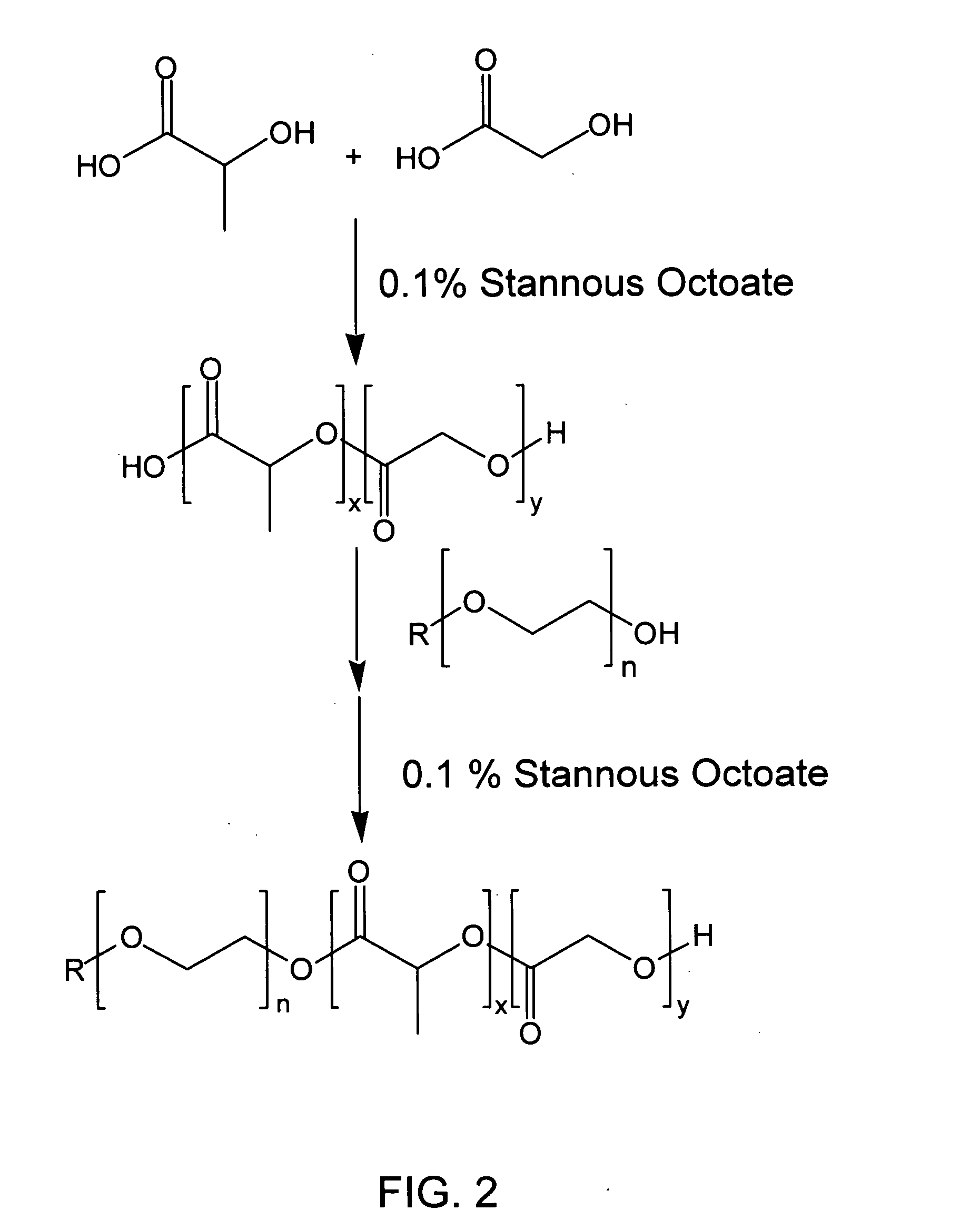
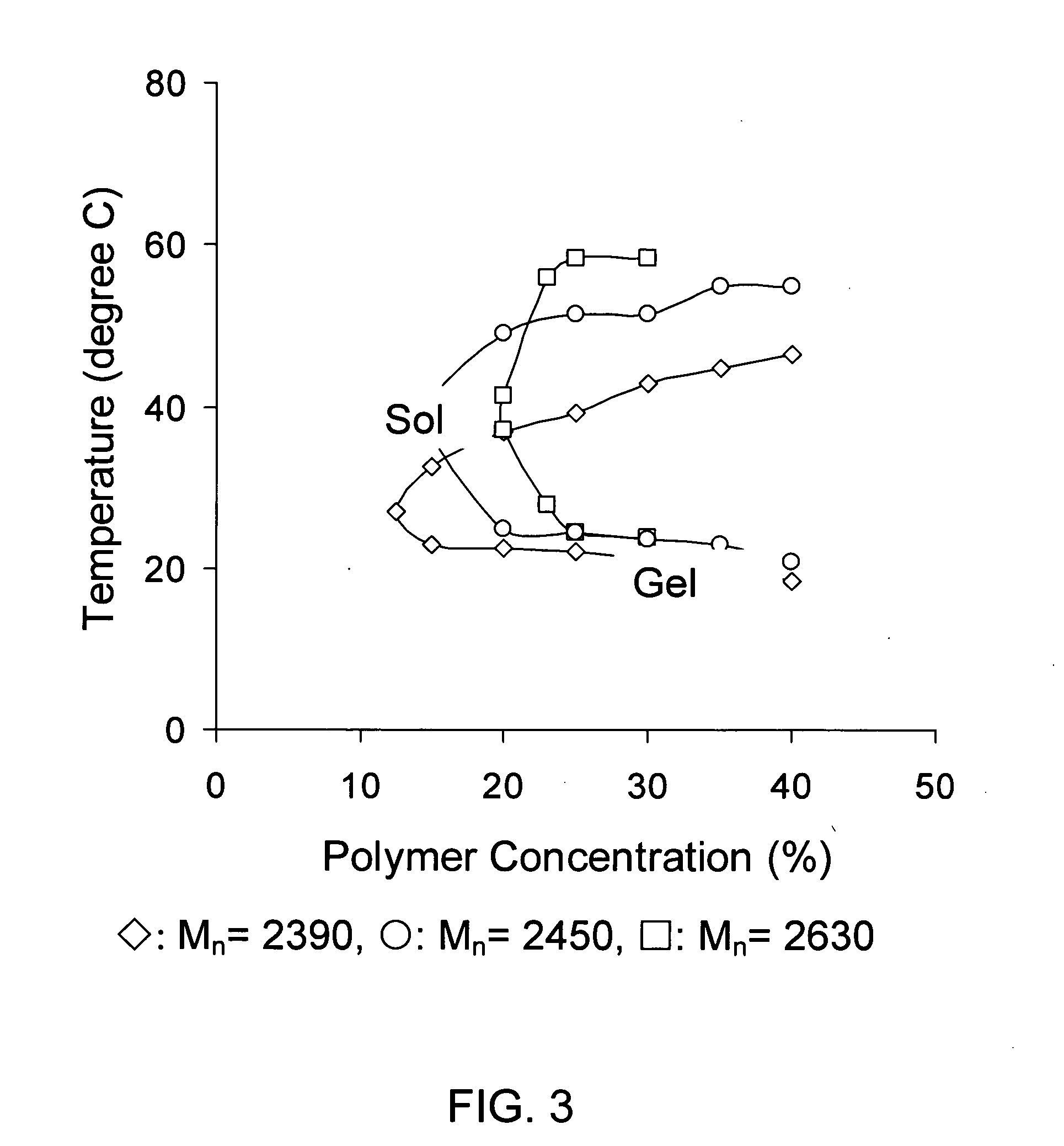
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Citric acid polymers
InactiveUS20090325859A1Peptide/protein ingredientsGenetic material ingredientsPoly(ethylene glycol) dimethyl etherPolyethylene glycol
The present invention provides polymers (e.g., elastomeric citric acid polymers) and methods of making and using these polymers (e.g., as a biologically active molecule delivery platform). In certain embodiments, the polymer has adsorbed biologically active molecules. In particular embodiments, the polymer comprises pores that are between about 7 and 15 nanometers in diameter. In other embodiments, the polymer comprises poly(1,8 octanediol-co-ctric acid). In certain embodiments, the polymers are made by employing polyethylene glycol dimethyl ether (PEGDM).
Owner:NORTHWESTERN UNIV
Nucleic Acid-Lipopolymer Compositions
InactiveUS20090042829A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsCholesterolFiller Excipient
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
Modified exendins and exendin agonists
InactiveUS6924264B1Increase ratingsDecrease amount of potassiumMetabolism disorderSaccharide peptide ingredientsGastric emptyingPolyethylene glycol
Novel modified exendins and exendin agonist analogs having an exendin or exendin agonist analog linked to one or more polyethylene glycol polymers, for example, and related formulations and dosages and methods of administration thereof are provided. These modified exendins and exendin agonist analogs, compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
Self-tanning dihydroxyacetone formulations having improved stability and providing enhanced delivery
A composition is provided which is useful for self-tanning skin coloring and is characterized by improved stability, which comprises from about 0.5% to about 20.0% by weight, based on total weight of said composition, of a self-tanning skin coloring agent subject to chemical instability, which is preferably dihydroxyacetone; from about 2.0% to about 40.0% by weight of a polyethoxyglycol, which is preferably ethoxydiglycol; and from about 0.1% to about 15.0% by weight of a polyol comprising a polyhydric compound having at least three hydroxyl groups and at least three carbon atoms, which is preferably D-sorbitol. The self-tanning composition may further optionally contain from about 0.1% to about 8.0% by weight of a water soluble dihydroxyl compound having at least two, and up to eight carbon atoms, which is preferably ethylene glycol; and the self-tanning composition may still further optionally contain an acidifying agent in amount sufficient to maintain the pH of said total composition at from about 3.5 to about 4.5, which is preferably sorbic acid. Cosmetologic products and methods of tanning are also provided.
Owner:SCHERING PLOUGH HEALTHCARE PRODUCTS INC
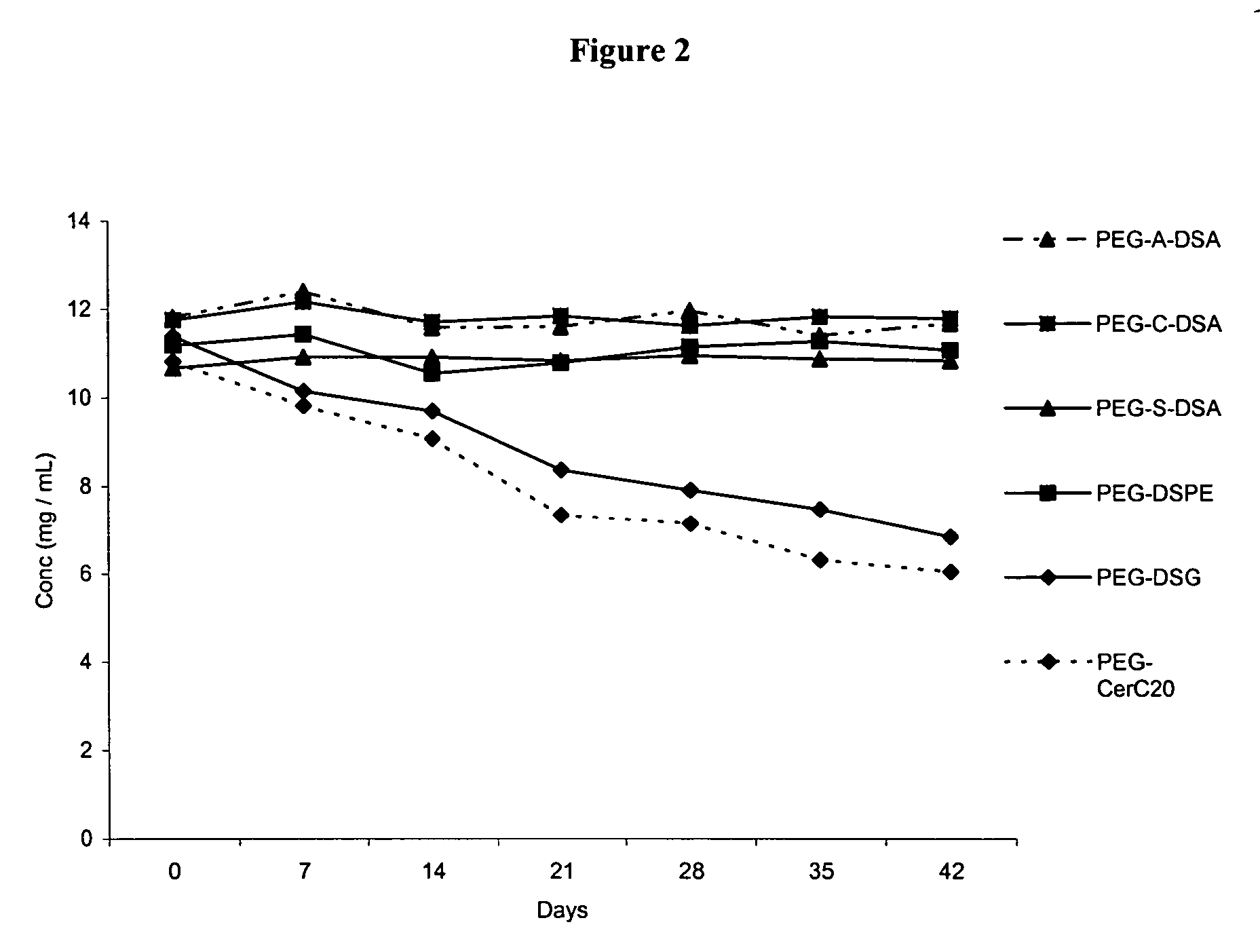
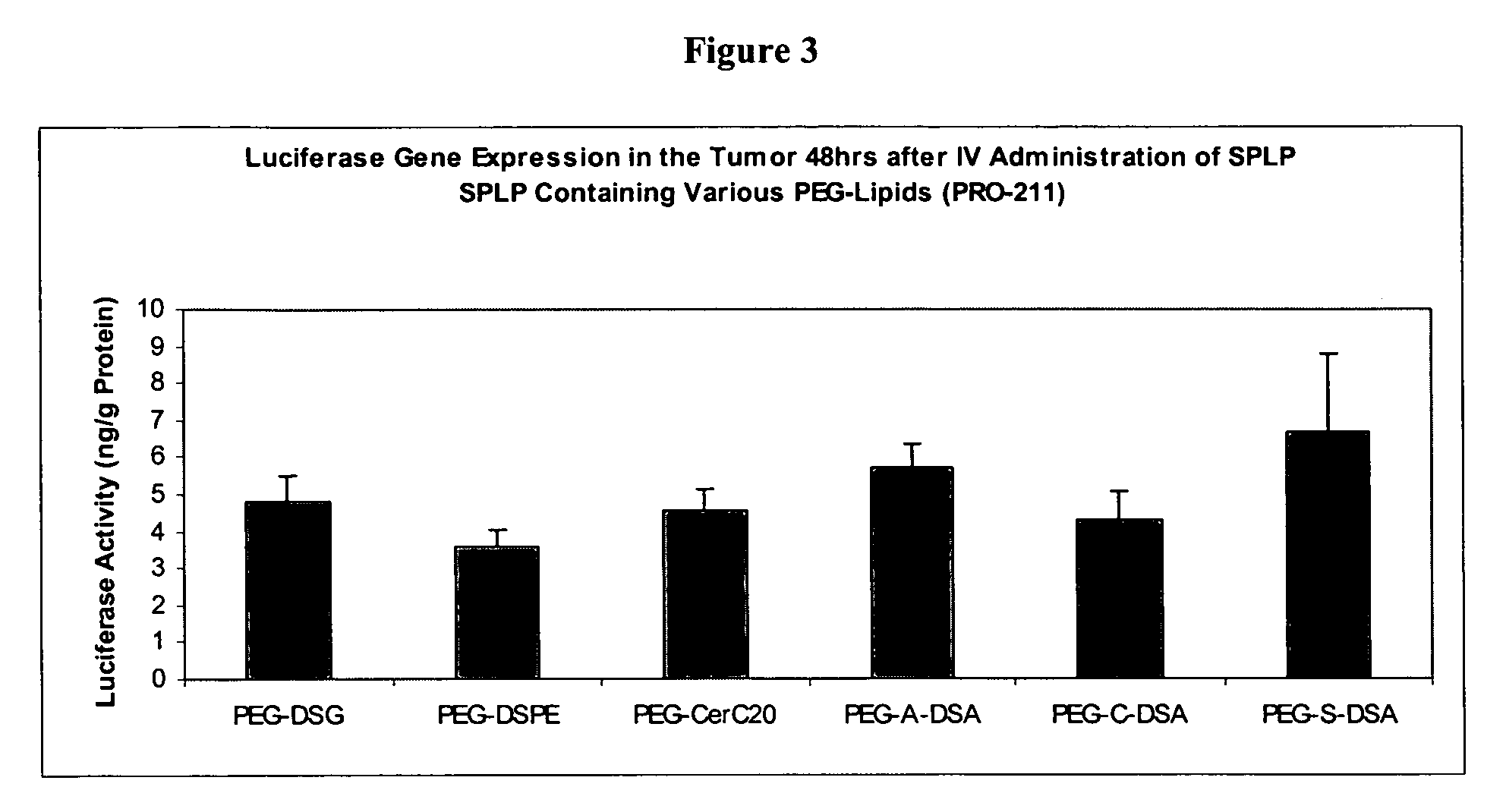
Polyethyleneglycol-modified lipid compounds and uses thereof
ActiveUS7803397B2Improve stabilityImprove propertiesNervous disorderOrganic chemistryPolyethylene glycolLiposome
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Lyophilization process and products obtained thereby
ActiveUS20070116729A1Dissolve fastSuitable for useBiocidePowder deliveryHigh concentrationFreeze-drying
A lyophilization process which comprises dissolving a material in one or more solvents for said material to form a solution; forcing said material at least partially out of solution by combining the solution and a non-solvent for the material, which non-solvent is miscible with the solvent or solvents used and wherein said non-solvent is volatilizable under freeze-drying conditions. In addition, for hydrophobic and / or lipophilic materials, the anti-solvent can be omitted, and the solution of the material in the solvent can be subjected directly to freeze drying. The lyophilizates can then be reconstituted with typical aqueous diluent in the case of hydrophilic materials. Hydrophobic and or lipophilic materials can be initially reconstituted with propylene glycol and / or polyethyleneglycol to form a high concentration solution therein and this is further diluted for use with a diluent of Intralipid, plasma, serum, or even whole blood.
Owner:SCIDOSE PHARMA +1
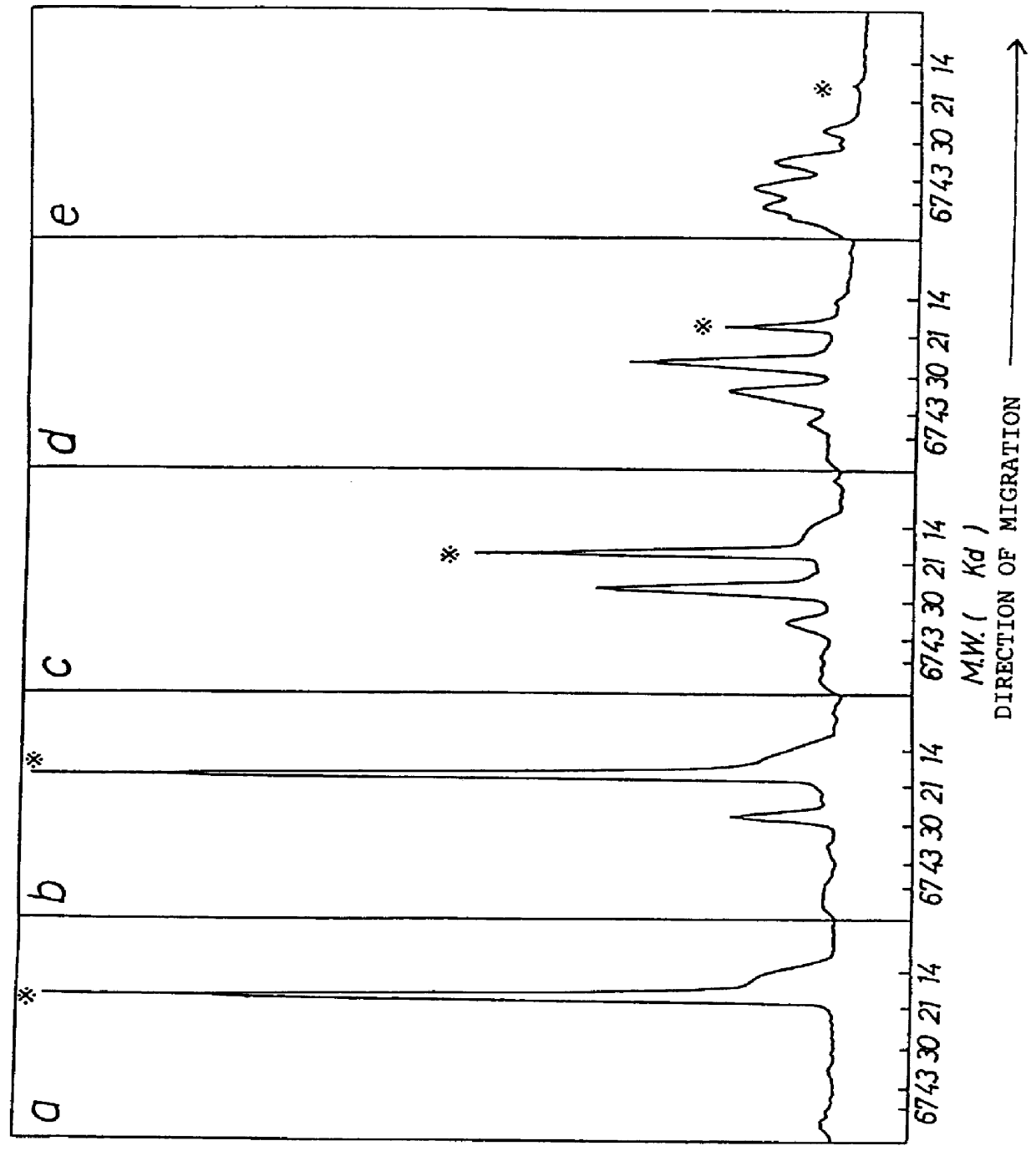
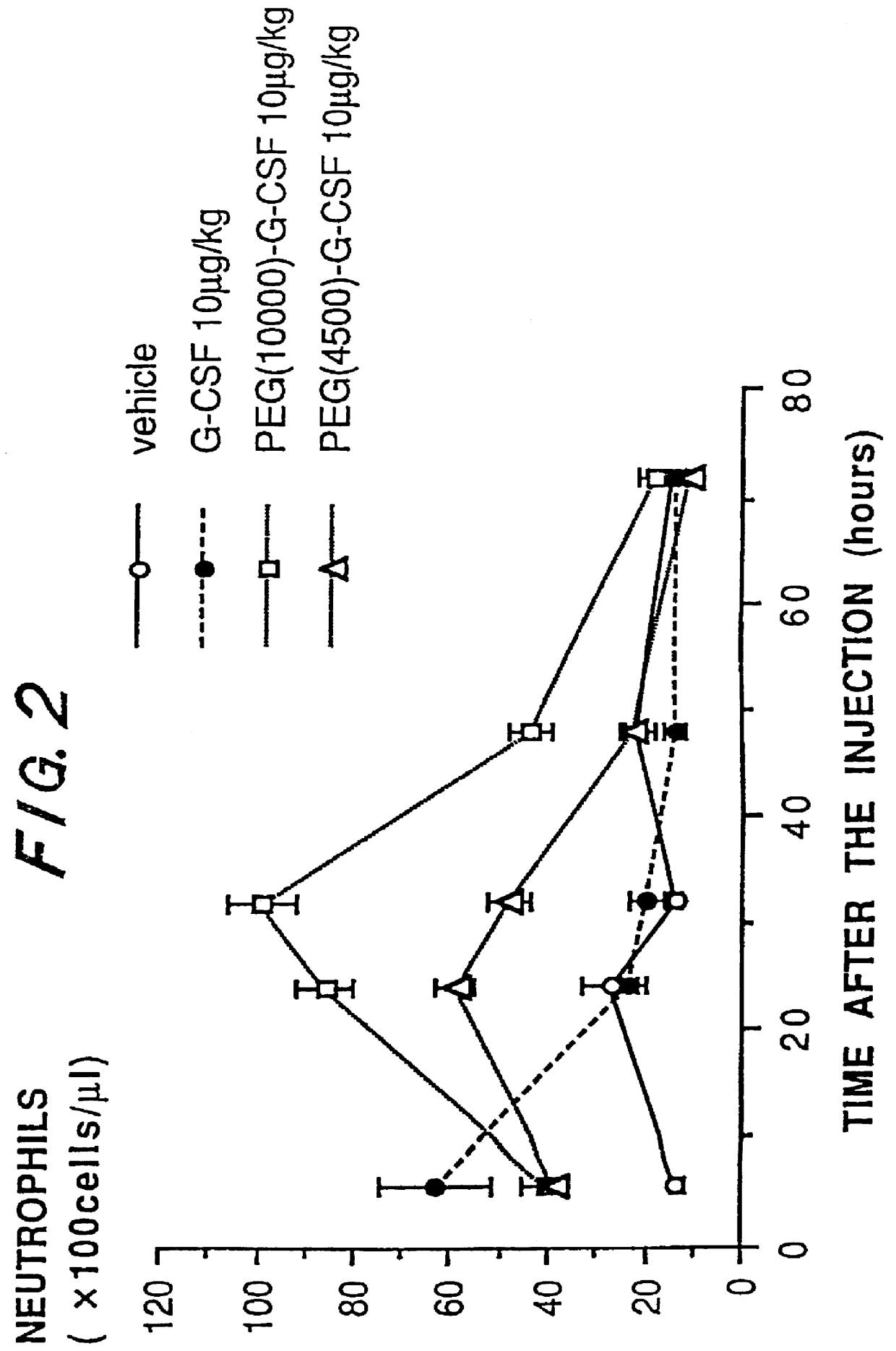
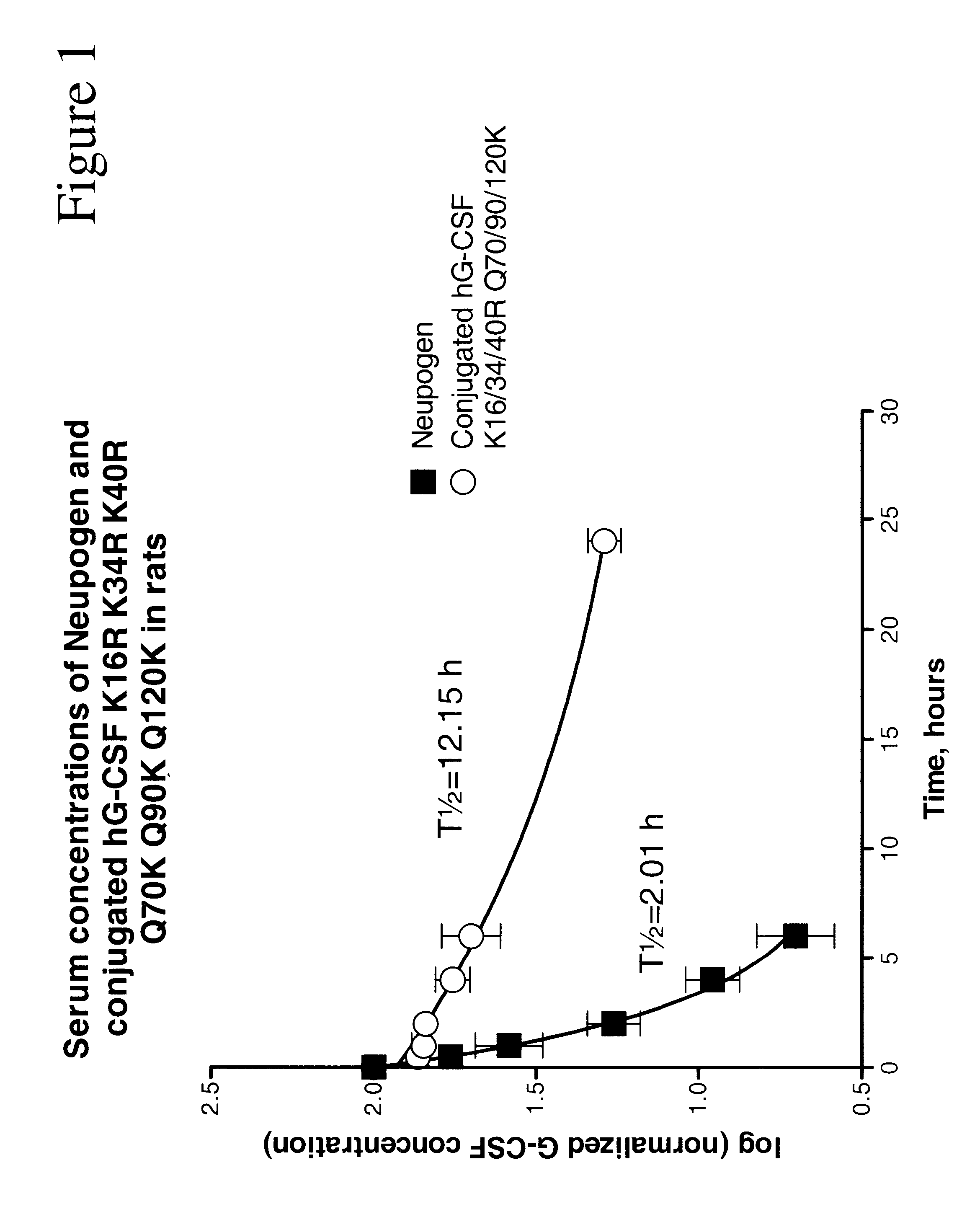
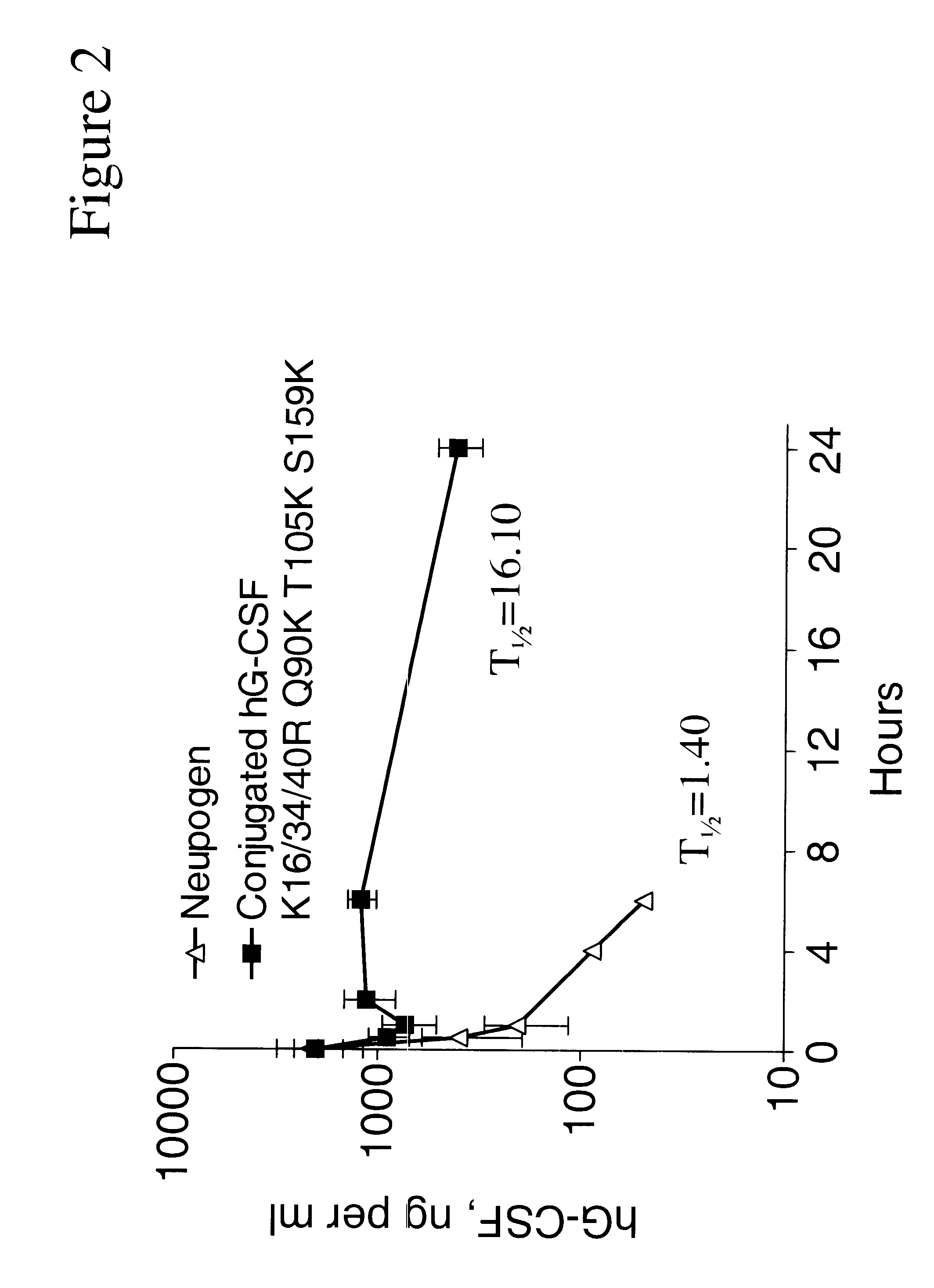
Chemically-modified G-CSF
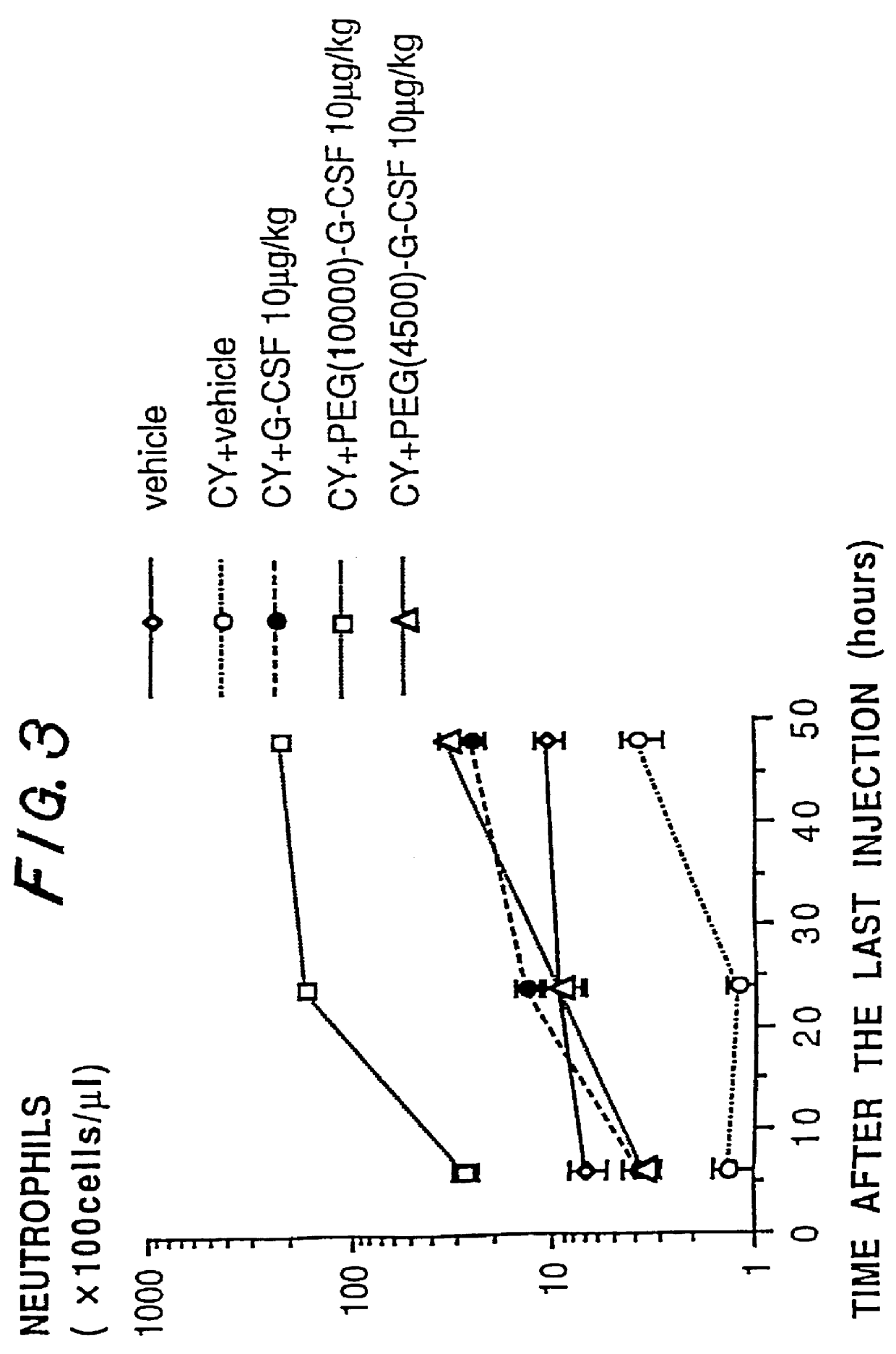
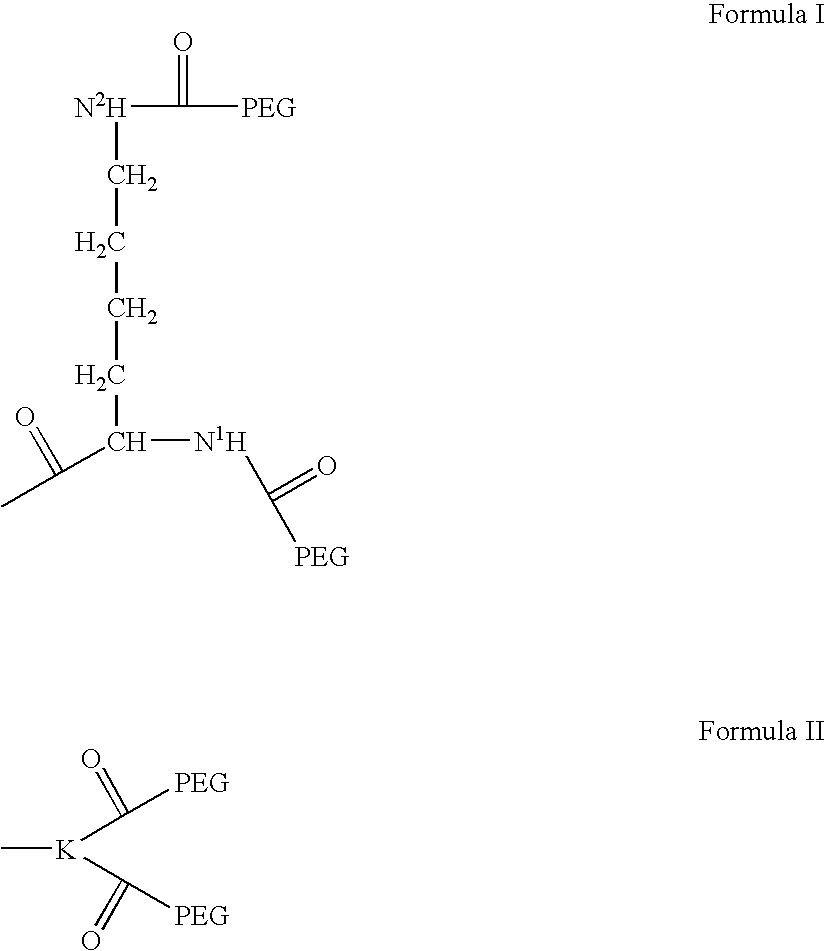
InactiveUS6166183APharmacological effectPromote recoveryDepsipeptidesPeptide preparation methodsExogenous DNAPolyethylene glycol
The present invention provides a chemically-modified protein prepared by binding polyethylene glycol to a polypeptide characterized by being the product of expression by a host cell of an exogenous DNA sequence and substantially having the following amino acid sequence: - - (Het)n - - Thr Pro Leu Gly Pro Ala Ser Ser Leu Pro Gln - - Ser Phe Leu Leu Lys Cys Leu Glu Gln Val Arg - - Lys Ile Gln Gly Asp Gly Ala Ala Leu Gln Glu - - Lys Leu Cys Ala Thr Tyr Lys Leu Cys His Pro - - Glu Glu Leu Val Leu Leu Gly His Ser Leu Gly - - Ile Pro Trp Ala Pro Leu Ser Ser Cys Pro Ser - - Gln Ala Leu Gln Leu Ala Cly Cys Leu Ser Gln - - Leu His Ser Gly Leu Phe Leu Tyr Gln GIY Leu - - Leu Gln Ala Leu Glu Gly Ile Ser Pro Glu Leu - - Gly Pro Thr Leu Asp Thr Leu Gln Leu Asp Val - - Ala Asp Phe Ala Thr Tbr Ile Trp Gln Gln Het - - Glu Glu Leu Gly Het Ala Pro Ala Leu Gln Pro - - Thr Gln Gly Ala Het Pro Ala Phe Ala Ser Ala - - Phe Gln Arg Arg Ala Gly Gly Val Leu Val Ala - - Ser His Leu Gln Ser Phe Leu Glu Val Scr Tyr - - Arg Val Leu Arg His Leu Ala Gln Pro - (n = 0 or 1) - The chemically-modified protein according to the present invention has a neutrophils-increasing activity much more lasted than that of the intact human G-CSF, enabling fewer numbers of administration with a lower dose.
Owner:KIRIN AMGEN
Novel poly(ethylene glycol) modified compounds and uses thereof
InactiveUS20050107297A1Good antigenicityIncreased durabilityPeptide/protein ingredientsDepsipeptidesPolyethylene glycolGlycol synthesis
The present invention relates to a peptide-based compound comprising a peptide moiety and a poly(ethylene glycol) moiety wherein the poly(ethylene glycol) moiety (preferably linear) has a molecular weight of more than 20 KDaltons (preferably from 20 to 60 KDaltons). The peptide moiety may be monomeric, dimeric or oligomeric. Such peptide-based compounds may optional include a linker moiety and / or a spacer moiety.
Owner:AFFYMAX
Oleaginous pharmaceutical and cosmetic foam
InactiveUS20070292461A1Pleasant and easy to spreadPatient compliance is goodCosmetic preparationsMetabolism disorderActive agentPolyethylene glycol
The invention relates to stable pharmaceutical or cosmetic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent comprising polyethylene glycol (PEG) or PEG derivative and mixtures thereof, or comprising propylene glycol, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Novel Polymers
ActiveUS20080015315A1Sufficient amountReduce the amount requiredOrganic compound preparationCarboxylic acid esters preparationHydrophilic monomerPolymer science
The invention relates to novel crosslinkable copolymers which are obtainable by (a) copolymerizing at least two different hydrophilic monomers selected from the group consisting of N,N-dimethyl acrylamide (DMA), 2-hydroxyethyl acrylate (HEA), glycidyl methacrylate (GMA), N-vinylpyrrolidone (NVP), acrylic acid (AA) and a C1-C4-alkoxy polyethylene glycol (meth)acrylate having a weight average molecular weight of from 200 to 1500, and at least one crosslinker comprising two or more ethylenically unsaturated double bonds in the presence of a chain transfer agent having a functional group; and (b) reacting one or more functional groups of the resulting copolymer with an organic compound having an ethylenically unsaturated group.
Owner:ALCON INC
Compositions and methods for manufacturing thermoplastic starch blends
InactiveUS6235816B1Improved chemical and physical propertyPromote degradationFireproof paintsPaper coatingPolyesterPolymer science
A biologically degradable polymer mixture containing at least one biopolymer made from renewable raw materials and a polymer selected from the following materials: an aromatic polyester; a polyester-copolymer with both aliphatic and aromatic blocks; a polyesteramide; a polyglycol; a polyester urethane; and / or mixtures of these components. The preferred renewable raw material is starch, more preferably native starch, most preferably native starch that has been predried.
Owner:BIO TEC BIOLOGISCHE NATURVERPACKUNGEN
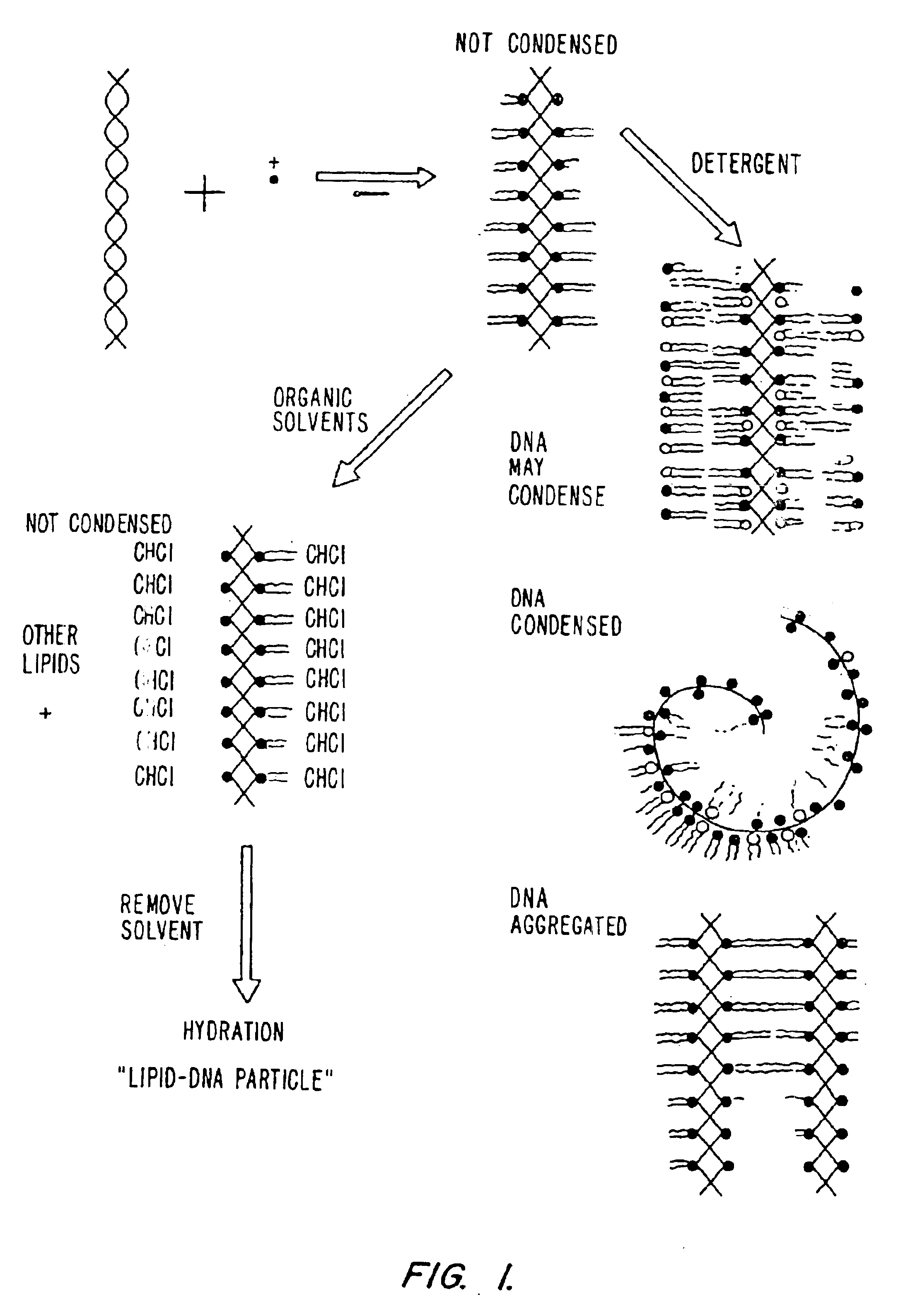
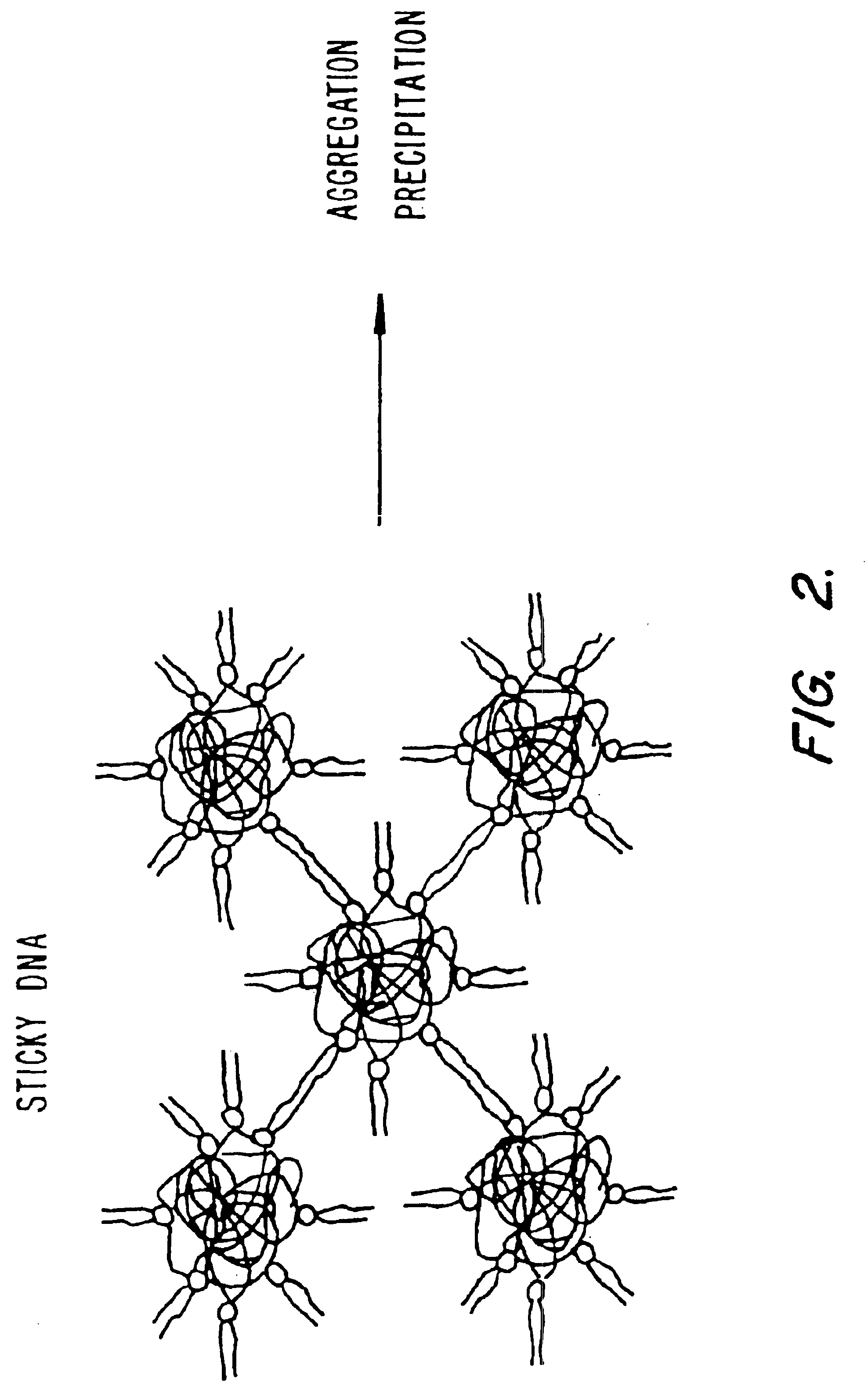
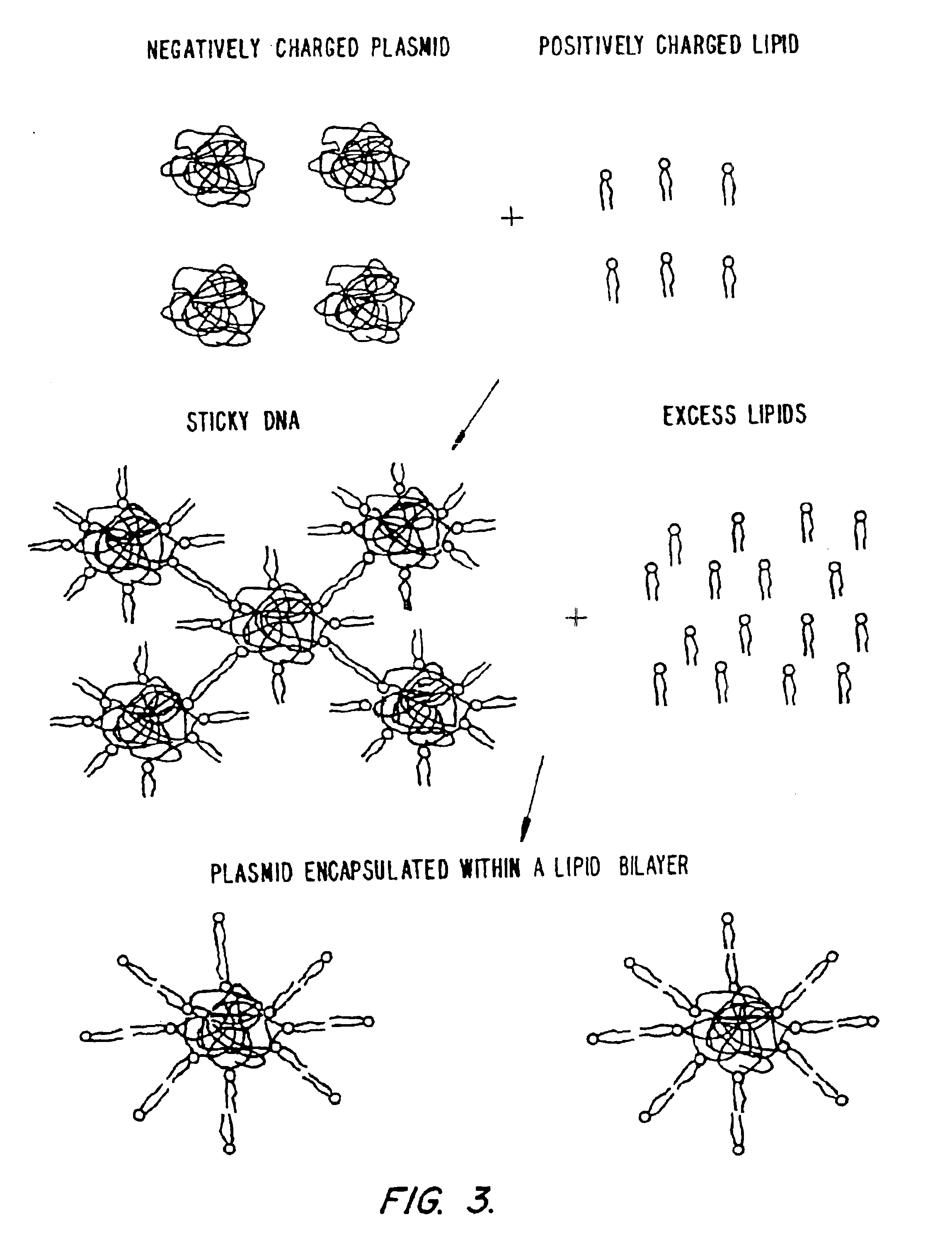
Method of preventing aggregation of a lipid:nucleic acid complex
InactiveUS6858224B2Microencapsulation basedGenetic material ingredientsLipid formationPolyethylene glycol
Particle aggregation of lipid:nucleic acid complex particles is prevented by incorporating a non-cationic lipid into lipid:nucleic acid complex particles containing a cationic lipid and a nucleic acid polymer. The non-cationic lipid is a polyethylene glycol-based polymer.
Owner:TEKMIRA PHARMA CORP +1
Biodegradable diblock copolymers having reverse thermal gelation properties and methods of use thereof
InactiveUS20060034889A1Good drug release propertiesPowder deliveryPharmaceutical non-active ingredientsPolymer sciencePolyethylene glycol
A water soluble, biodegradable AB type diblock copolymer which comprises 61 to 85% by weight of a biodegradable, hydrophobic A block comprising a biodegradable polyester, and 15 to 39% by weight of a biocompatible, hydrophilic B block comprising a monofunctional polyethylene glycol(PEG) having a number average molecular weight less than 5000, and wherein said diblock copolymer has a number average molecular weight less than 15000 and possesses reverse thermal gelation properties.
Owner:PROTHERICS SALTLAKE CITY INC
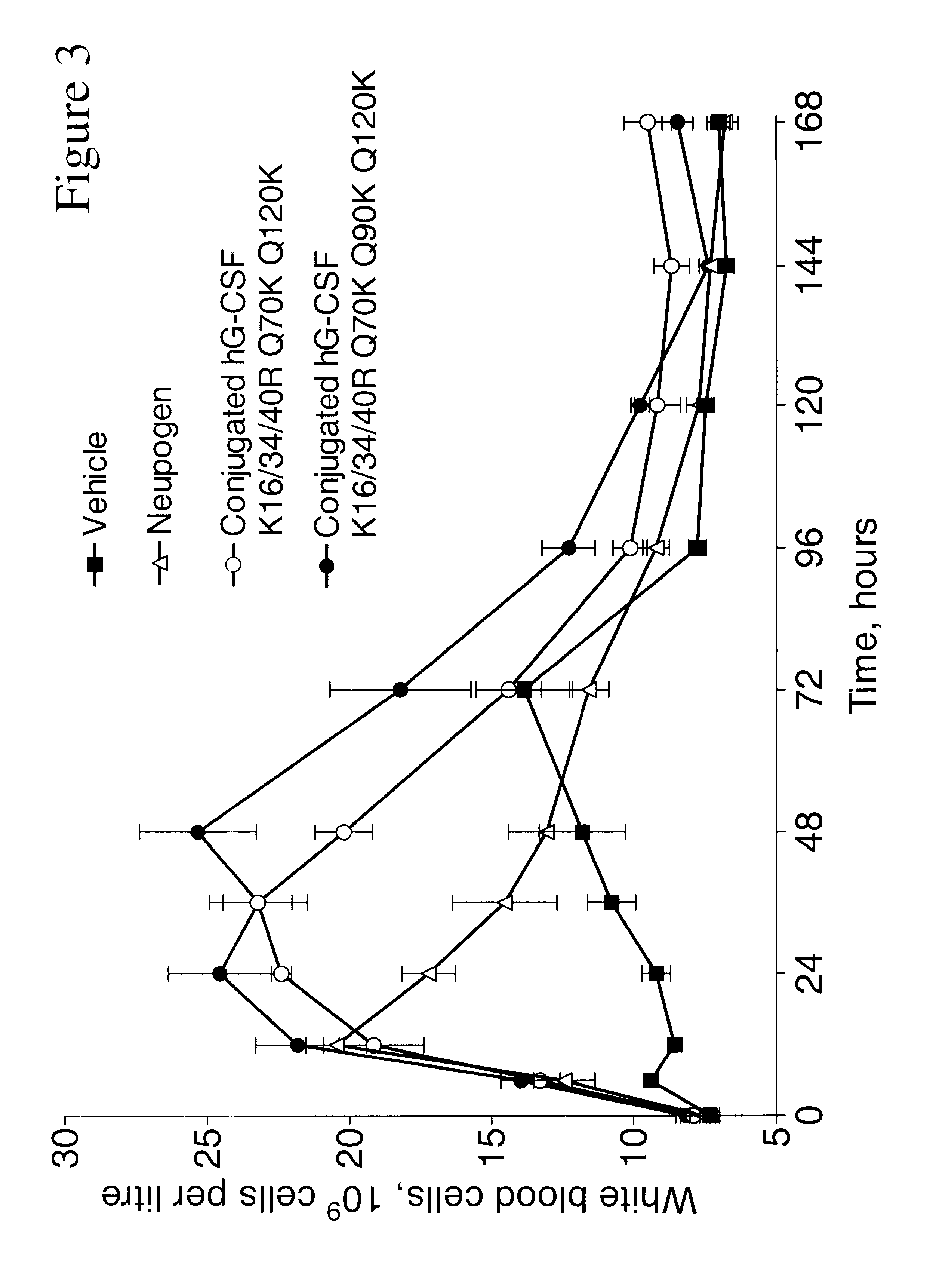
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
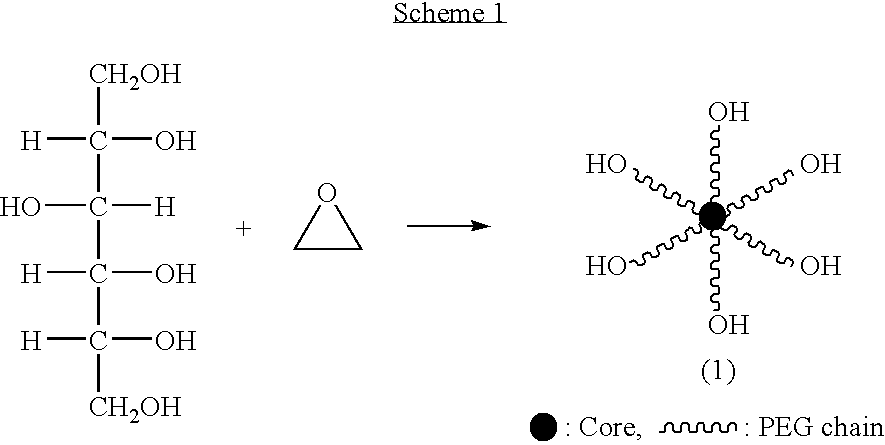
Hexa-arm polyethylene glycol and its derivatives and the methods of preparation thereof
The present invention relates to novel hexa-arm polyethylene glycol (6-arm PEG) and its derivatives. The core of 6-arm PEG derivatives is sorbitol and the end groups can be derivatized into many different reactive functionalities that are useful in conjugating with many different targets. The present invention also provides a biodegradable polymeric hydrogel-forming composition comprising the 6-arm PEG and its derivatives, and methods of using such 6-arm PEG derivatives as surgical or biological implants or sealants.
Owner:SUN BIO INC
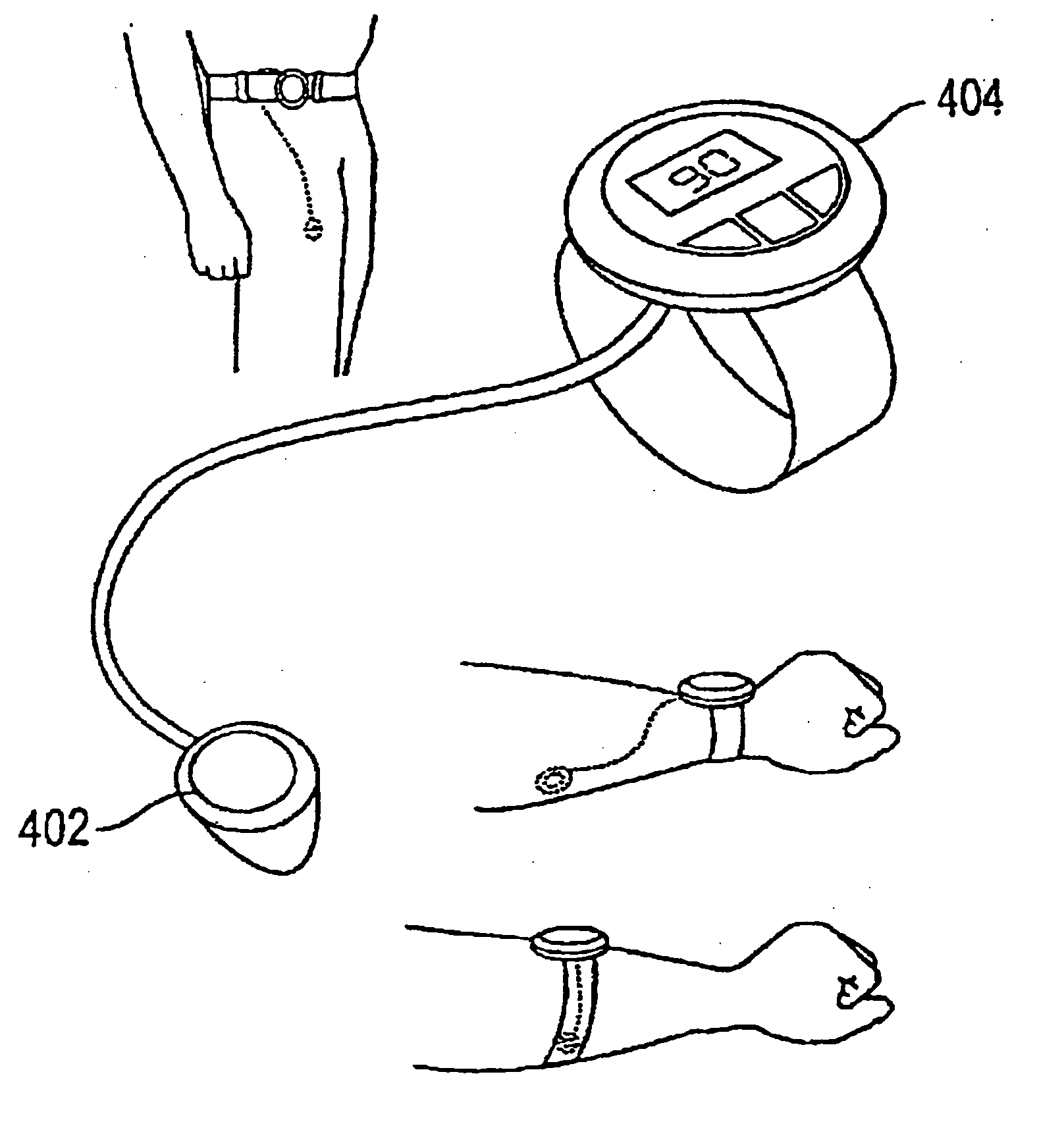
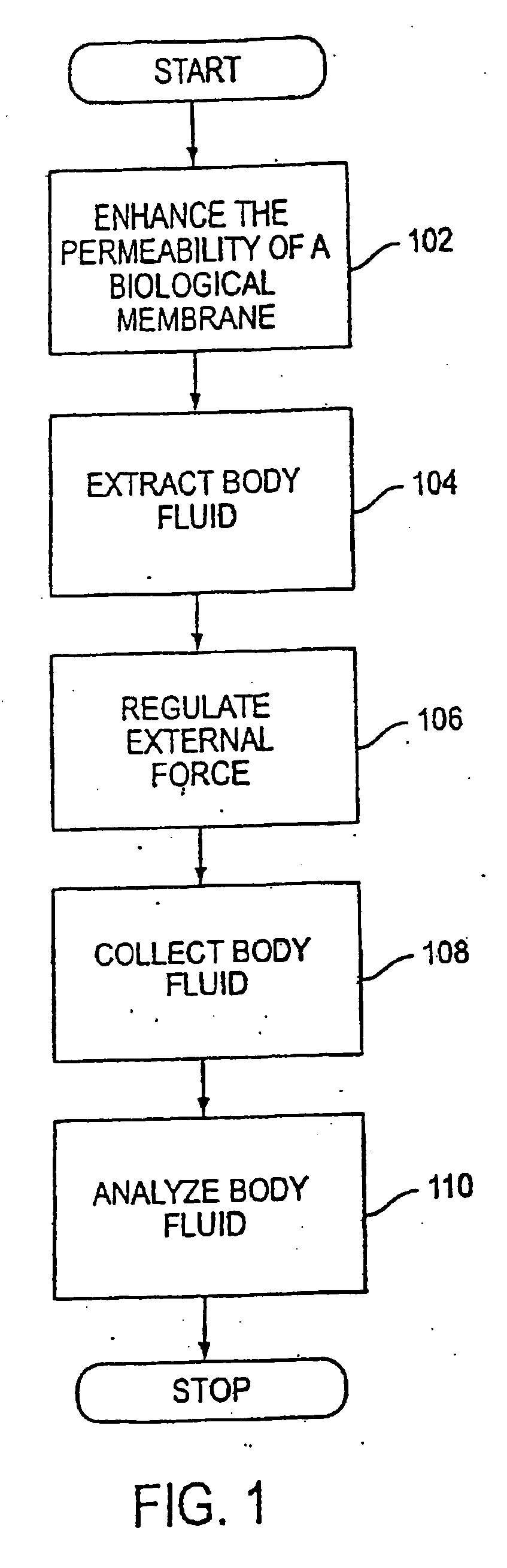
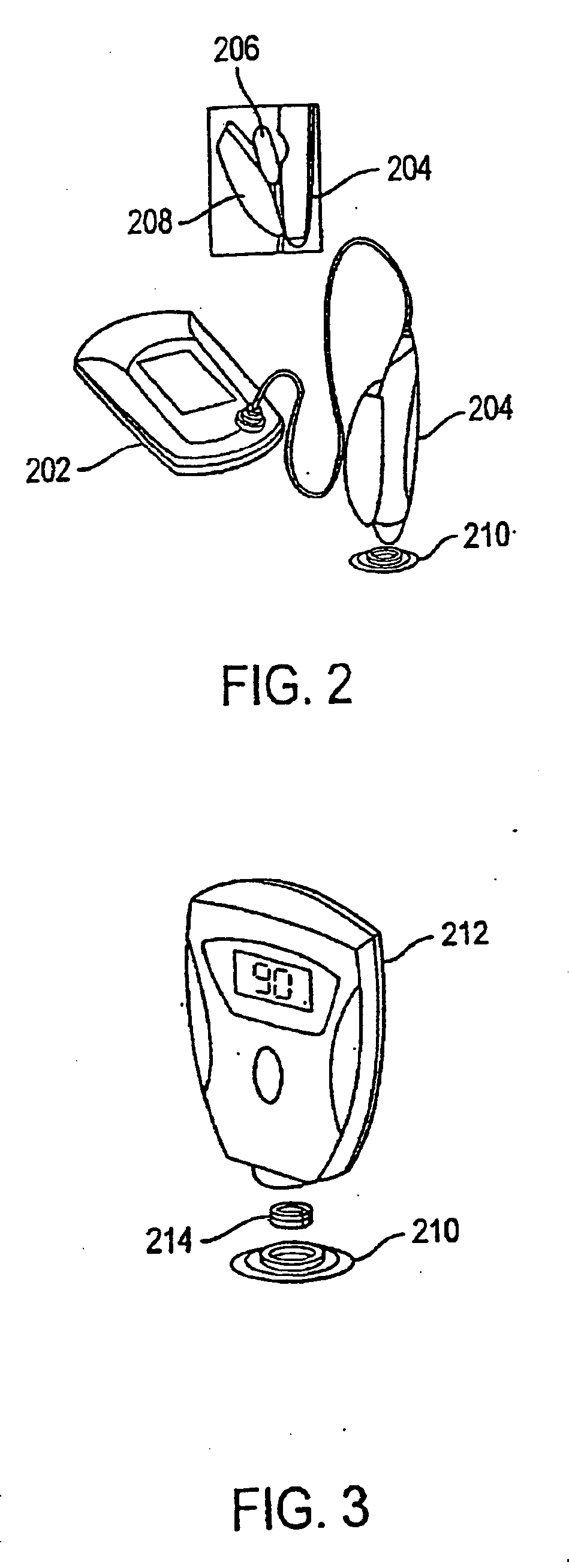
System and method for analyte sampling and analysis with hydrogel
InactiveUS20060094946A1Microbiological testing/measurementVolume/mass flow measurementPolyethylene glycolCorrection method
The invention relates to a transdermal analyte monitoring system comprising a medium adapted to interface with a biological membrane and to receive an analyte from the biological membrane and an electrode assembly comprising a plurality of electrodes, wherein the medium is adapted to react continuously with the analyte, an electrical signal is detected by the electrode assembly, and the electrical signal correlates to an analyte value. The analyte value may be the flux of the analyte through the biological membrane or the concentration of the analyte in a body fluid of a subject. The medium may comprise a vinyl acetate based hydrogel, an agarose based hydrogel, or a polyethylene glycol diacrylate (PEG-DA) based hydrogel, for example. The surface region of the electrode may comprise pure platinum. The system may include an interference filter located between the biological membrane and the electrode assembly for reducing interference in the system. The system may comprise a processor programmed to implement an error correction method that corrects for sensor drift.
Owner:ECHO THERAPEUTICS INC
Thick film conductor composition for use in biosensors
InactiveUS6627058B1Immobilised enzymesBioreactor/fermenter combinationsElectrical conductorPolyethylene glycol
This invention is directed to a composition comprising: (a) platinum group metal powder, alloys, or mixtures thereof as a powder or deposited on graphite supports; (b) poly(glycol ether), derivatives, or mixtures thereof; (c) carbon-based electrically conductive filler; and (d) thermoplastic polymer or mixtures thereof. The invention is further directed to a process for dispersing platinum group metal powder, alloys, or mixtures thereof in poly(glycol ether), derivatives, or mixtures thereof. The invention is further directed to the above composition wherein the platinum group metal powder has been dispersed according to the above process.
Owner:EI DU PONT DE NEMOURS & CO
Polyethyleneglycol-modified lipid compounds and uses thereof
ActiveUS20050175682A1Improve stabilityImprove propertiesOrganic active ingredientsNervous disorderLipid formationPolyethylene glycol
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Fluorescent silica-based nanoparticles
ActiveUS20130039848A1Ultrasonic/sonic/infrasonic diagnosticsAntibacterial agentsDiseaseCellular component
The present invention provides a fluorescent silica-based nanoparticle that allows for precise detection, characterization, monitoring and treatment of a disease such as cancer The nanoparticle has a fluorescent compound positioned within the nanoparticle, and has greater brightness and fluorescent quantum yield than the free fluorescent compound To facilitate efficient urinary excretion of the nanoparticle, it may be coated with an organic polymer, such as polyethylene glycol) (PEG) The small size of the nanoparticle, the silica base and the organic polymer coating minimizes the toxicity of the nanoparticle when administered in vivo The nanoparticle may further be conjugated to a ligand capable of binding to a cellular component associated with the specific cell type, such as a tumor marker A therapeutic agent may be attached to the nanoparticle Radionuclides / radiometals or paramagnetic ions may be conjugated to the nanoparticle to permit the nanoparticle to be detectable by various imaging techniques.
Owner:CORNELL UNIVERSITY +1
Methods for glucagon suppression using modified exendins
InactiveUS7153825B2Saccharide peptide ingredientsVasoactive intestinal peptideDiseaseBiological half-life
We claim a method of lowering plasma glucagon in a subject in need thereof comprising administering to the subject a composition comprising a modified exendin or modified exendin analog, wherein said modification comprises one or more molecule linked to an exendin or the exendin analog wherein said molecule is selected from the group consisiting of polyethylene glycol, gelatin and / or albumin. The modified exendin or the modified exendin analog has activity of suppressing glucagon secretion and / or lowering glucagon levels in the subject and possesses increased biological half-life compared to unmodified exendin or unmodified exendin analog. The method is useful in treating hyperglucagonemia and other disorders that would be benefited by lowering plasma glucagon or suppressing glucagon secretion.
Owner:AMYLIN PHARMA INC
Crosslinked gels comprising polyalkyleneimines, and their uses as medical devices
ActiveUS20070196454A1Promote cell growthSoft tissue growthIn-vivo radioactive preparationsSurgical adhesivesCross-linkCysteine thiolate
One aspect of the present invention generally relates to methods of sealing a wound or tissue plane or filling a void splace. In a preferred embodiment, the wound is an ophthalmic, pleural or dural wound. In certain instances, the compositions used to seal the wound or tissue plane comprises a polyalkyleneamine. In a preferred embodiment, the polyalkyleneamine is polyethyleneimine. Treatment of the polyethyleneimine with a cross-linking reagent causes the polyethyleneimine polymers to polymerize forming a seal. In certain instances, the cross-linking reagent is a polyethylene glycol having reactive terminal groups. In certain instances, the reactive terminal groups are activated esters, such as N-hydroxy succinimide ester. In certain instances, the reactive terminal groups are isocyanates. In certain instances, the polyethyleneimine has a lysine, cysteine, isocysteine or other nucleophilic group attached to the periphery of the polymer. In certain instances, the polyethyleneimine is mixed with a second polymer, such as a polyethylene glycol containing nucleophilic groups. In certain instances, the compositions used to seal the wound or tissue plane are formed by reacting a polyalkyleneamine bearing electrophilic groups with a cross-linking reagent containing nucleophilic groups. In certain instances, the electrophilic groups on the polyalkyleneamine are activated esters, such as N-hydroxy succinimide ester. In certain instances, the compositions used to seal the wound or tissue plane are formed by reacting a polyalkyleneamine bearing photopolymerizable groups with ultraviolet or visibile light. Compositions used to seal the wound which contain PEI or a derivative of PEI are found to adhere tightly to the tissue. Other aspects of the present invention relate to methods of filling a void of a patient or adhering tissue. In certain instances, the methods use a polyalkyleneamine. In a preferred embodiment, the polyalkyleneamine is polyethyleneimine. Another aspect of the present invention relates to a polymeric composition formed by exposing a polyalkyleneamine to an activated polyalkylene glycol. In certain instances, the composition is attached to mammalian tissue.
Owner:SQUARE 1 BANK
Alcohol-free transdermal analgesic composition and processes for manufacture and use thereof
InactiveUS7052715B2Reduced shelf lifeImprove permeabilityOrganic active ingredientsBiocideAlkaneAlcohol free
The instant invention is directed toward a dermal delivery system composition comprising an aqueous base vehicle including American Emu oil, Isopropyl Palmitate (PROTACHEM IPP), PEG-8 (a polyethylene glycol available under the tradename PROTACHEM 400), methylsulfonylmethane (MSM) and SEPIGEL 305 (a combination including polyacrylamide / C13–C14 Iso-paraffin and Laureth-7), in combination with an analgesic composition, such as ibuprofen, and to processes for the manufacture and use thereof.
Owner:ALL NATURAL FMG
Oleaginous pharmaceutical and cosmetic foam
InactiveUS20080063607A1Pleasant and easy to spreadPatient compliance is goodBiocideCosmetic preparationsActive agentPolyethylene glycol
The invention relates to stable pharmaceutical or cosmetic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent comprising polyethylene glycol (PEG) or PEG derivative and mixtures thereof, or comprising propylene glycol, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition.
Owner:FOAMIX PHARMACEUTICALS LIMITED
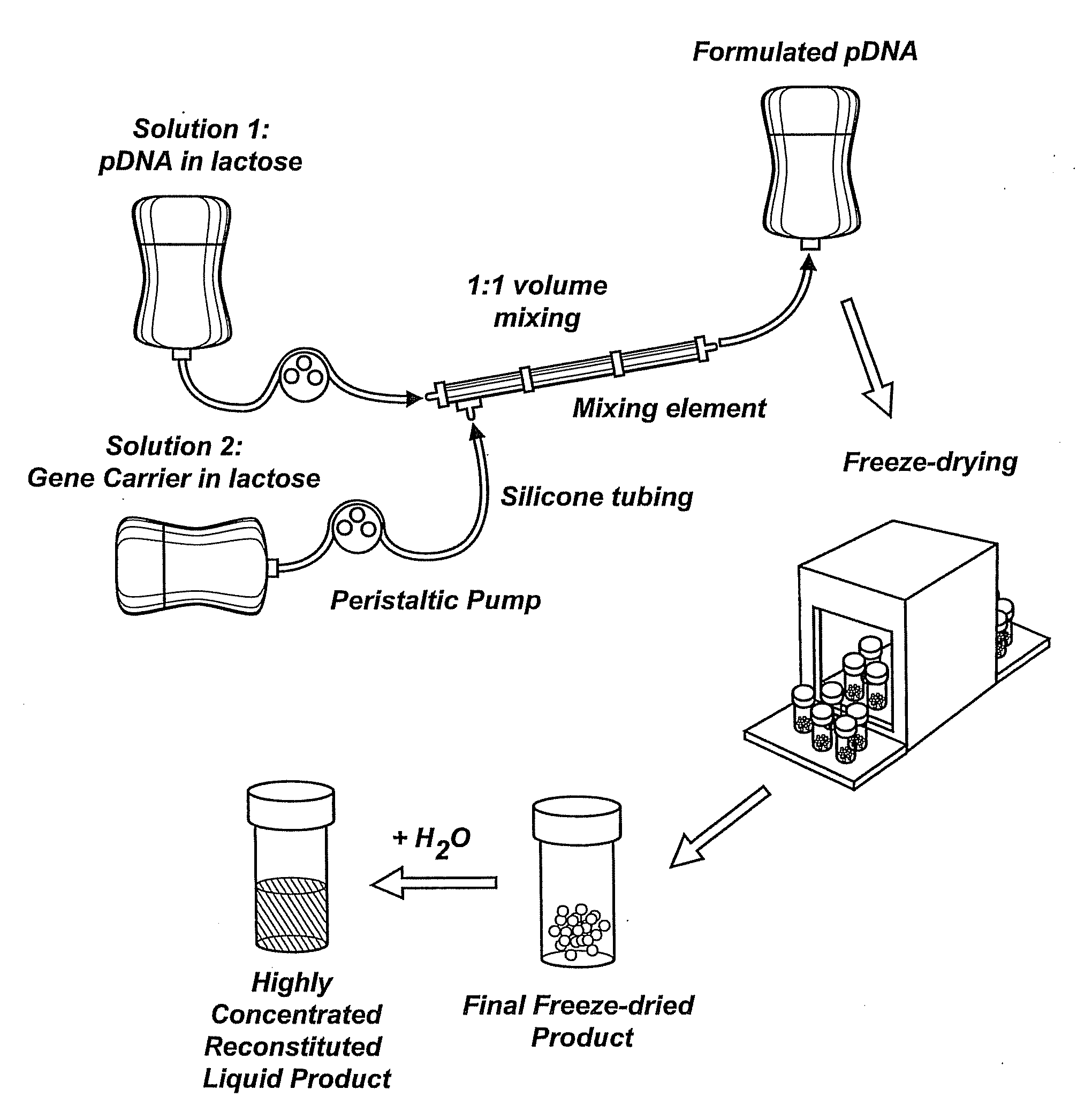
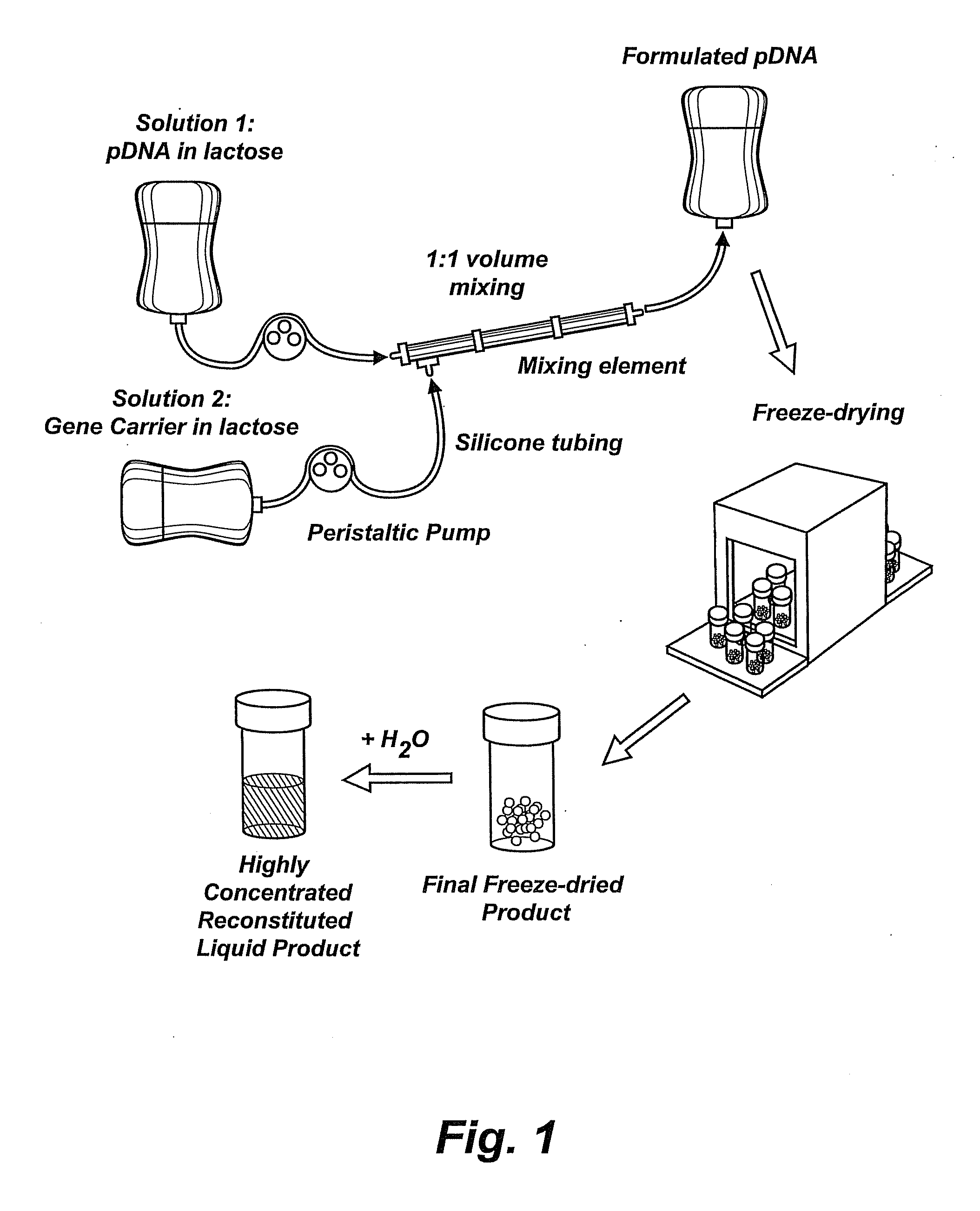
Composition, method of preparation & application of concentrated formulations of condensed nucleic acids with a cationic lipopolymer
UndeterminedUS20090042825A1Increase efficiency and dosing flexibilitySpecial deliveryPeptide/protein ingredientsFiller ExcipientCholesterol
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:EXPRESSION GENETICS INC
Novel lipids and compositions for the delivery of therapeutics
ActiveUS20110311583A1Adequate therapeutic indexSimple compositionAntibacterial agentsOrganic active ingredientsLipid formationAryl
(A1) Translate this text The present invention provides lipids that are advantageously used in lipid particles for the in vivo delivery of therapeutic agents to cells. In particular, the invention provides lipids having the following structure (I) wherein R1 and R2 are each independently for each occurrence optionally substituted C10-C30 alkyl, optionally substituted C10-C30 alkenyl, optionally substituted C10-C30 alkynyl, optionally substituted C10-C30 acyl, or -linker-ligand; R3 is H, optionally substituted C1-C10 alkyl, optionally substituted C2-C10 alkenyl, optionally substituted C2-C10 alkynyl, alkylhetrocycle, alkylphosphate, alkylphosphorothioate, alkylphosphorodithioate, alkylphosphonates, alkylamines, hydroxyalkyls, ?-aminoalkyls, ?-(substituted)aminoalkyls, ?-phosphoalkyls, ?-thiophosphoalkyls, optionally substituted polyethylene glycol (PEG, mw 100-40K), optionally substituted mPEG (mw 120-40K), heteroaryl, heterocycle, or linker-ligand; E is O, S, N(Q), C(O), N(Q)C(O), C(0)N(Q), (Q)N(CO)O, O(CO)N(Q), S(O), NS(O)2N(Q), S(O)2, N(Q)S(O)2, SS, O═N, aryl, heteroaryl, cyclic or heterocycle; and, Q is H, alkyl, ?-aminoalkyl, ?-(substituted)aminoalky, ?-phosphoalkyl or ?-thiophosphoalkyl.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Sweetener containing d-psicose and foods and drinks obtained by using the same
Providing a D-psicose-containing sweetener with the modification of the taste of D-psicose, comprising D-psicose, a sugar alcohol and / or a high intensity sweetener, preferably containing D-psicose as the main component, particularly a low-calorie sweetener and / or a sweetener giving refreshing feel in the oral cavity, as well as foods and drinks obtained by using the D-psicose-containing sweetener with the modification of the taste of D-psicose, and other products given with sweetness. The sugar alcohol is one or more sugar alcohols selected from the group consisting of sorbitol, mannitol, lactitol, maltitol, xylitol and erythritol, while the high intensity sweetener is one or more high intensity sweeteners as selected from aspartame, acesulfame K, sodium cyclamate, sodium saccharin, Sucralose (under trade name), stevia sweetener, dulcin, taumatin, neotame and monellin.
Owner:MATSUTANI CHEM INDS CO LTD +1
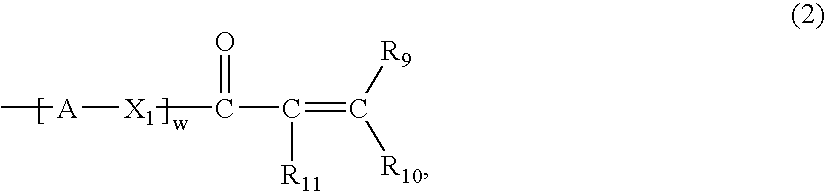
Amphiphilic siloxane-containing (meth)acrylamides and uses thereof
The invention provides an amphiphilic siloxane-containing (meth)acrylamide which comprises one sole (meth)acrylamido group, one sole tris(trimethylsiloxy)silyl group, and one polyethylene glycol segment which is either dangling polymer chain or a hydrophilic linker between the (meth)acrylamido group and the tris(trimethylsiloxy)silyl group. The present invention is also related to a polymer, an actinically-crosslinkable silicone-containing prepolymer, a silicone hydrogel polymeric material, or a silicone hydrogel contact lens, which comprises monomeric units derived from an amphiphilic siloxane-containing (meth)acrylamido group, one tris(trimethylsiloxy)silyl group of the invention. In addition, the invention provides a method for making silicone hydrogel contact lenses using a water-based lens-forming formulation comprising an amphiphilic siloxane-containing (meth)acrylamido group, one tris(trimethylsiloxy)silyl group of the invention and / or an actinically-crosslinkable silicone-containing prepolymer of the invention.
Owner:ALCON INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com