Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
422 results about "Cysteine thiolate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cysteine (symbol Cys or C; /ˈsɪstiiːn/) is a semi-essential proteinogenic amino acid with the formula HO2CCH(NH2)CH2SH. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile.
Cysteine engineered antibodies and conjugates
ActiveUS20070092940A1Sugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkCysteine thiolate
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab), optionally with an albumin-binding peptide (ABP) sequence, are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered antibody-drug conjugates having Formula I: Ab-(L-D)p I where p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Methods for making proteins containing free cysteine residues
The present invention relates to novel methods of making soluble proteins having free cysteines in which a host cell is exposed to a cysteine blocking agent. The soluble proteins produced by the methods can then be modified to increase their effectiveness. Such modifications include attaching a PEG moiety to form pegylated proteins.
Owner:BOLDER BIOTECH
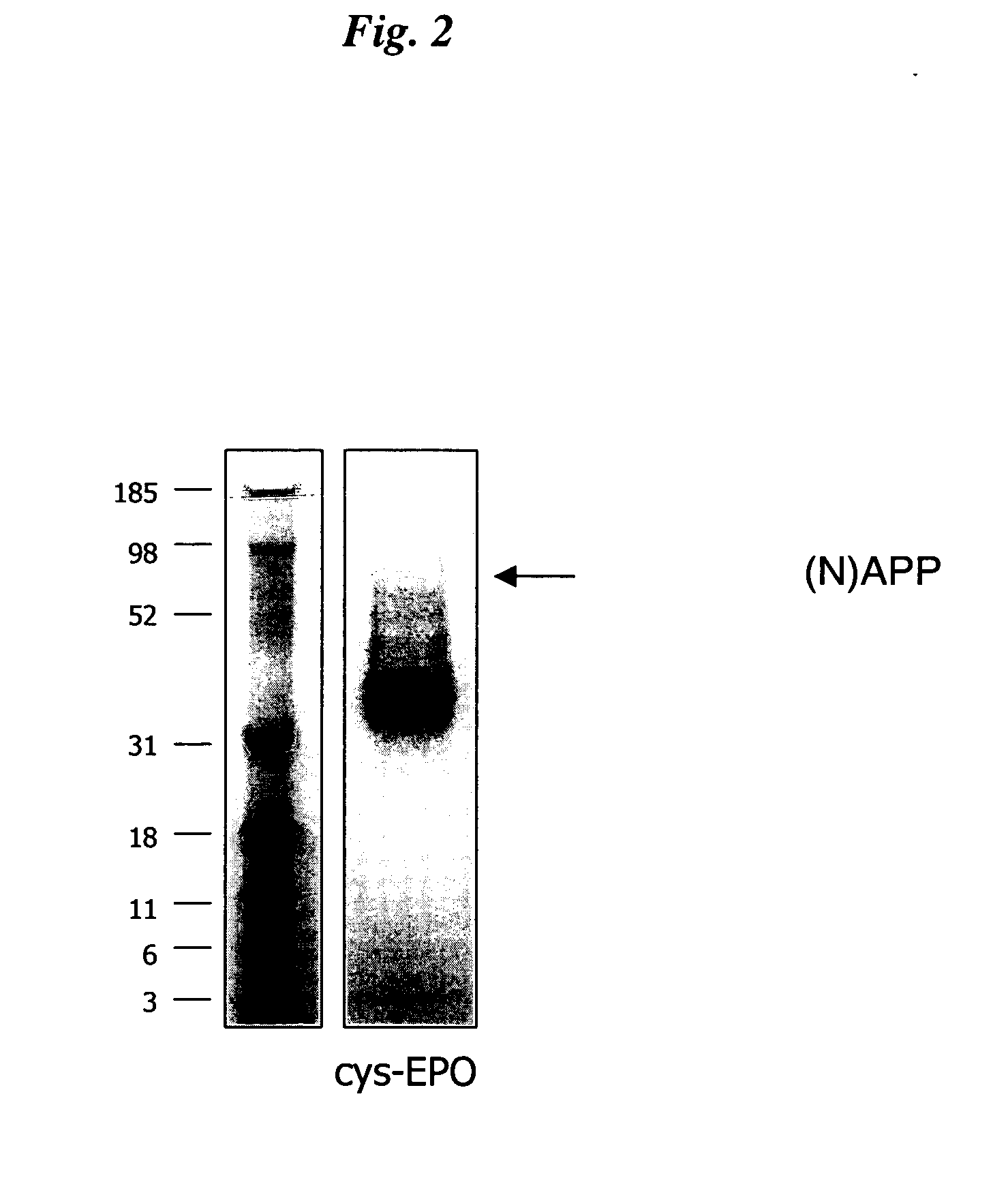
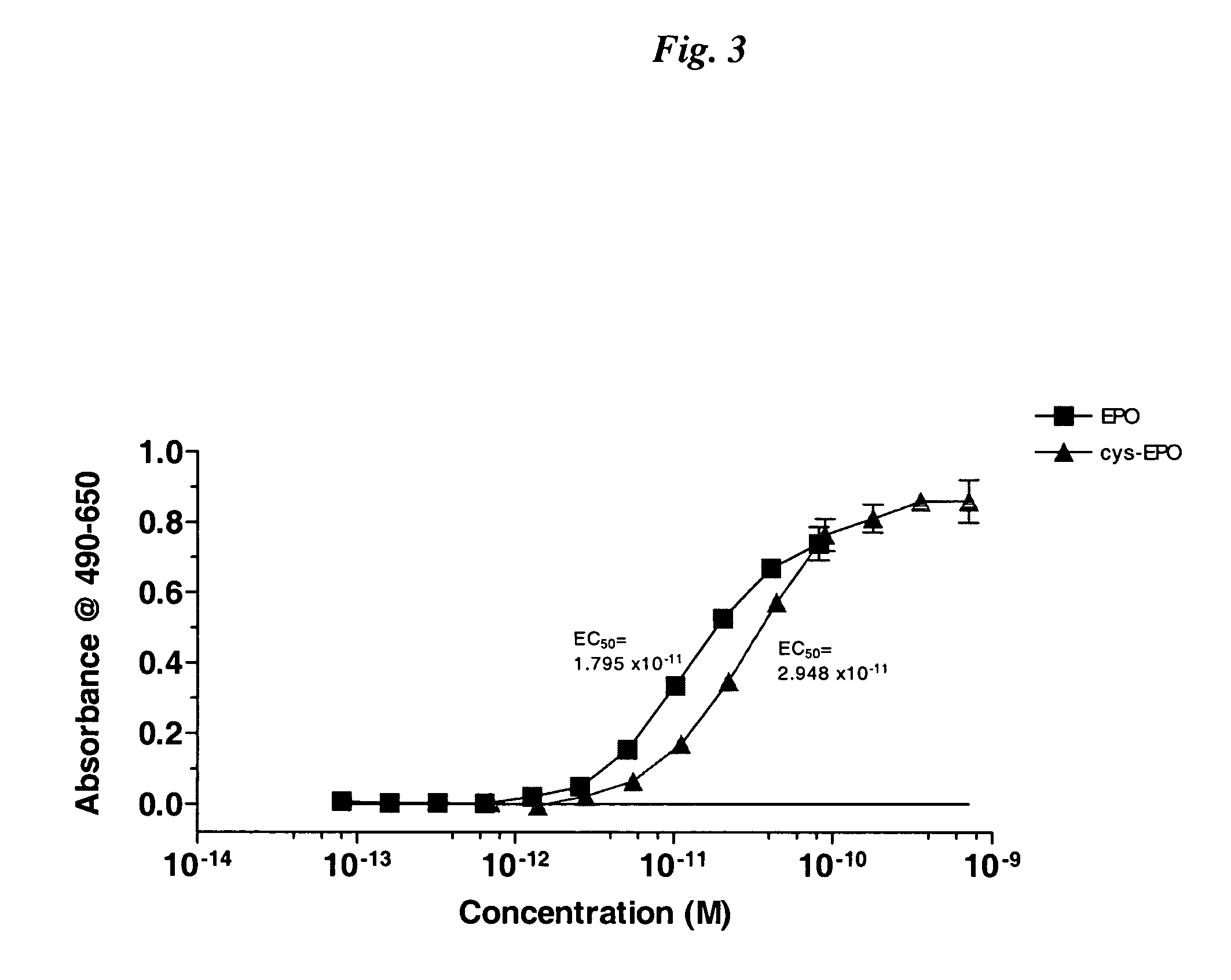
Cysteine variants of erythropoietin
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
Binding domain-immunoglobulin fusion proteins
InactiveUS20050175614A1Reduced ability to dimerizeHybrid immunoglobulinsAntipyreticCrystallographyAntigen
The invention relates to novel binding domain-immunoglobulin fusion proteins that feature a binding domain for a cognate structure such as an antigen, a counterreceptor or the like, a hinge region polypeptide having either zero or one cysteine residue, and immunoglobulin CH2 and CH3 domains, and that are capable of ADCC and / or CDC while occurring predominantly as monomeric polypeptides. The fusion proteins can be recombinantly produced at high expression levels. Also provided are related compositions and methods, including immunotherapeutic applications.
Owner:TRUBION PHARM INC
Novel recombinant proteins with N-terminal free thiol
InactiveUS20050170457A1Extended half-lifeIncreases circulating serum half-lifePeptide/protein ingredientsTissue cultureCysteine thiolateHalf-life
The present invention relates to novel modified proteins having N-terminal free thiols that can be produced by recombinant methods and are ready for further chemical derivatization. In particular, the invention relates to erythropoietin conjugate compounds having altered biochemical, physiochemical and pharmacokinetic properties. More particularly, one embodiment of the invention relates to erythropoietin conjugate compounds of the formula: (M)n-X-A-cys-EPO (I) where EPO is an erythropoeitin moiety selected from erythropoietin or an erythropoietin variant having at least one amino acid different from the wild-type human EPO, or any pharmaceutical acceptable derivatives thereof having biological properties of causing bone marrow cells to increase production of red blood cells; cys represents the amino acid cysteine and occurs at position −1 relative to the amino acid sequence of the erythropoietin moiety; A indicates the structure of the residual moiety used to chemically attach X to the thiol group of −1Cys; X is a water soluble polymer such as a polyalkylene glycol or other polymer; M is an organic molecule (including peptides and proteins) that increases the circulating half-life of the construct; and N is an integer from 0 to 15.
Owner:CENTOCOR
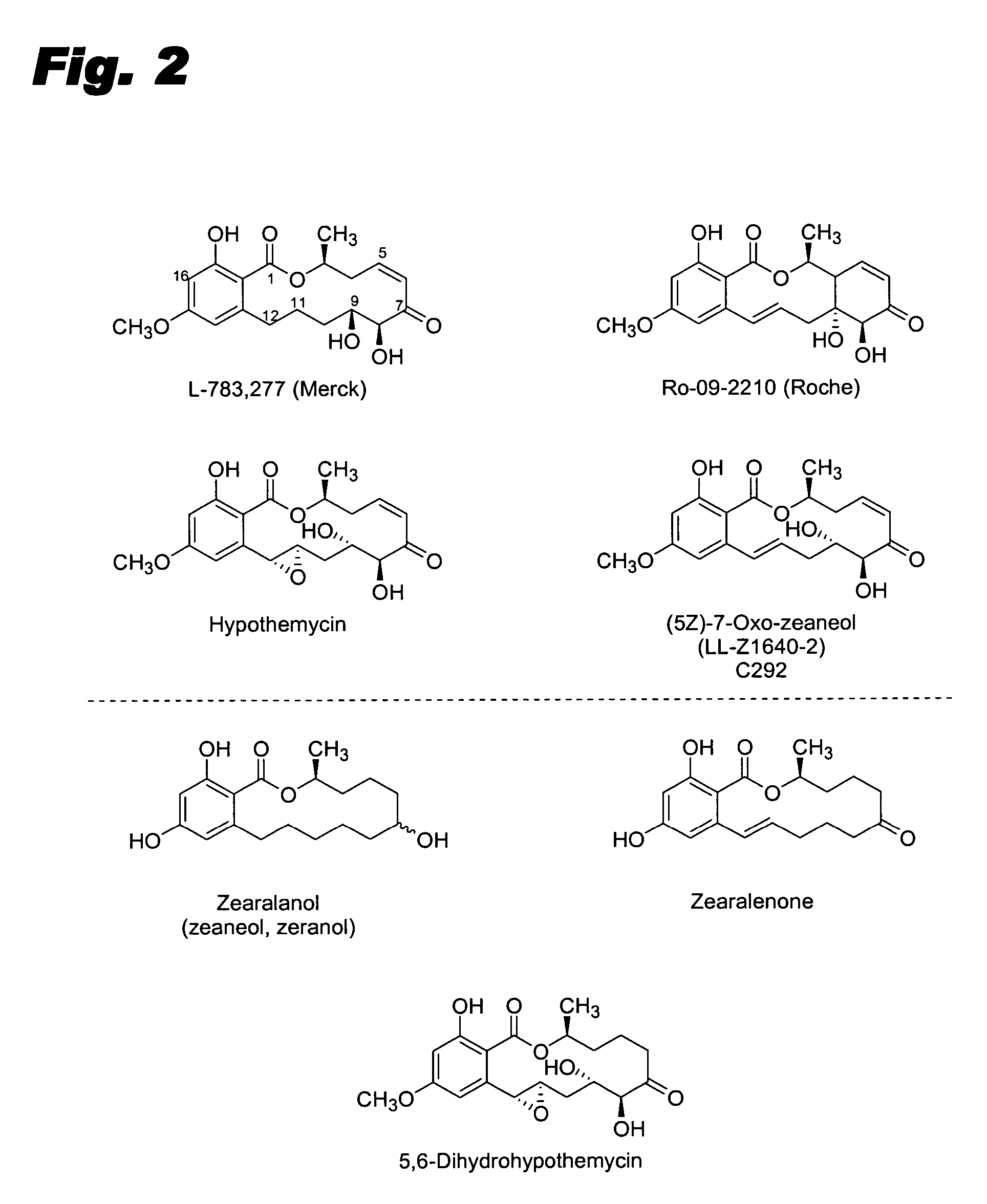
Specific kinase inhibitors
Resorcylic acid lactones having a C5-C6 cis double bond and a ketone at C7 and other compounds capable of Michael adduct formation are potent and stable inhibitors of a subset of protein kinases having a specific cysteine residue in the ATP binding site.
Owner:KOSAN BIOSCI
Cysteine variants of erythropoietin
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
Binding domain-immunoglobulin fusion proteins
The invention relates to novel binding domain-immunoglobulin fusion proteins that feature a binding domain for a cognate structure such as an antigen, a counterreceptor or the like, a hinge region polypeptide having either zero or one cysteine residue, and immunoglobulin CH2 and CH3 domains, and that are capable of ADCC and / or CDC while occurring predominantly as monomeric polypeptides. The fusion proteins can be recombinantly produced at high expression levels. Also provided are related compositions and methods, including immunotherapeutic applications.
Owner:TRUBION PHARM INC
Proteinaceous pharmaceuticals and uses thereof
InactiveUS20070191272A1High disulfide densityReduce molecular weightPeptide librariesNervous disorderCysteine thiolateBiology
The present invention provides cysteine-containing scaffolds and / or proteins, expression vectors, host cell and display systems harboring and / or expressing such cysteine-containing products. The present invention also provides methods of designing libraries of such products, methods of screening such libraries to yield entities exhibiting binding specificities towards a target molecule. Further provided by the invention are pharmaceutical compositions comprising the cysteine-containing products of the present invention.
Owner:AMUNIX OPERATING INC
Crosslinked gels comprising polyalkyleneimines, and their uses as medical devices
ActiveUS20070196454A1Promote cell growthSoft tissue growthIn-vivo radioactive preparationsSurgical adhesivesCross-linkCysteine thiolate
One aspect of the present invention generally relates to methods of sealing a wound or tissue plane or filling a void splace. In a preferred embodiment, the wound is an ophthalmic, pleural or dural wound. In certain instances, the compositions used to seal the wound or tissue plane comprises a polyalkyleneamine. In a preferred embodiment, the polyalkyleneamine is polyethyleneimine. Treatment of the polyethyleneimine with a cross-linking reagent causes the polyethyleneimine polymers to polymerize forming a seal. In certain instances, the cross-linking reagent is a polyethylene glycol having reactive terminal groups. In certain instances, the reactive terminal groups are activated esters, such as N-hydroxy succinimide ester. In certain instances, the reactive terminal groups are isocyanates. In certain instances, the polyethyleneimine has a lysine, cysteine, isocysteine or other nucleophilic group attached to the periphery of the polymer. In certain instances, the polyethyleneimine is mixed with a second polymer, such as a polyethylene glycol containing nucleophilic groups. In certain instances, the compositions used to seal the wound or tissue plane are formed by reacting a polyalkyleneamine bearing electrophilic groups with a cross-linking reagent containing nucleophilic groups. In certain instances, the electrophilic groups on the polyalkyleneamine are activated esters, such as N-hydroxy succinimide ester. In certain instances, the compositions used to seal the wound or tissue plane are formed by reacting a polyalkyleneamine bearing photopolymerizable groups with ultraviolet or visibile light. Compositions used to seal the wound which contain PEI or a derivative of PEI are found to adhere tightly to the tissue. Other aspects of the present invention relate to methods of filling a void of a patient or adhering tissue. In certain instances, the methods use a polyalkyleneamine. In a preferred embodiment, the polyalkyleneamine is polyethyleneimine. Another aspect of the present invention relates to a polymeric composition formed by exposing a polyalkyleneamine to an activated polyalkylene glycol. In certain instances, the composition is attached to mammalian tissue.
Owner:SQUARE 1 BANK
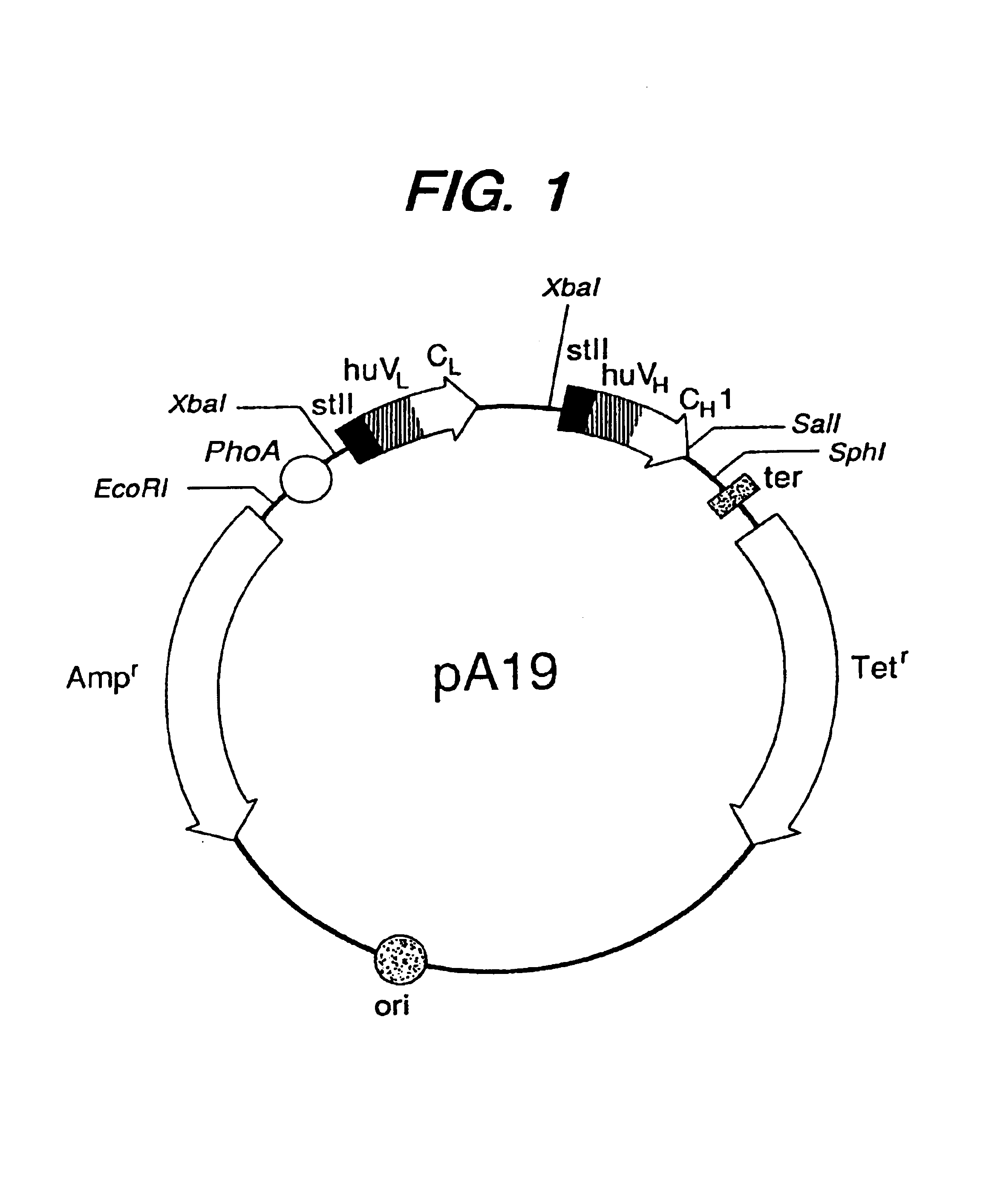
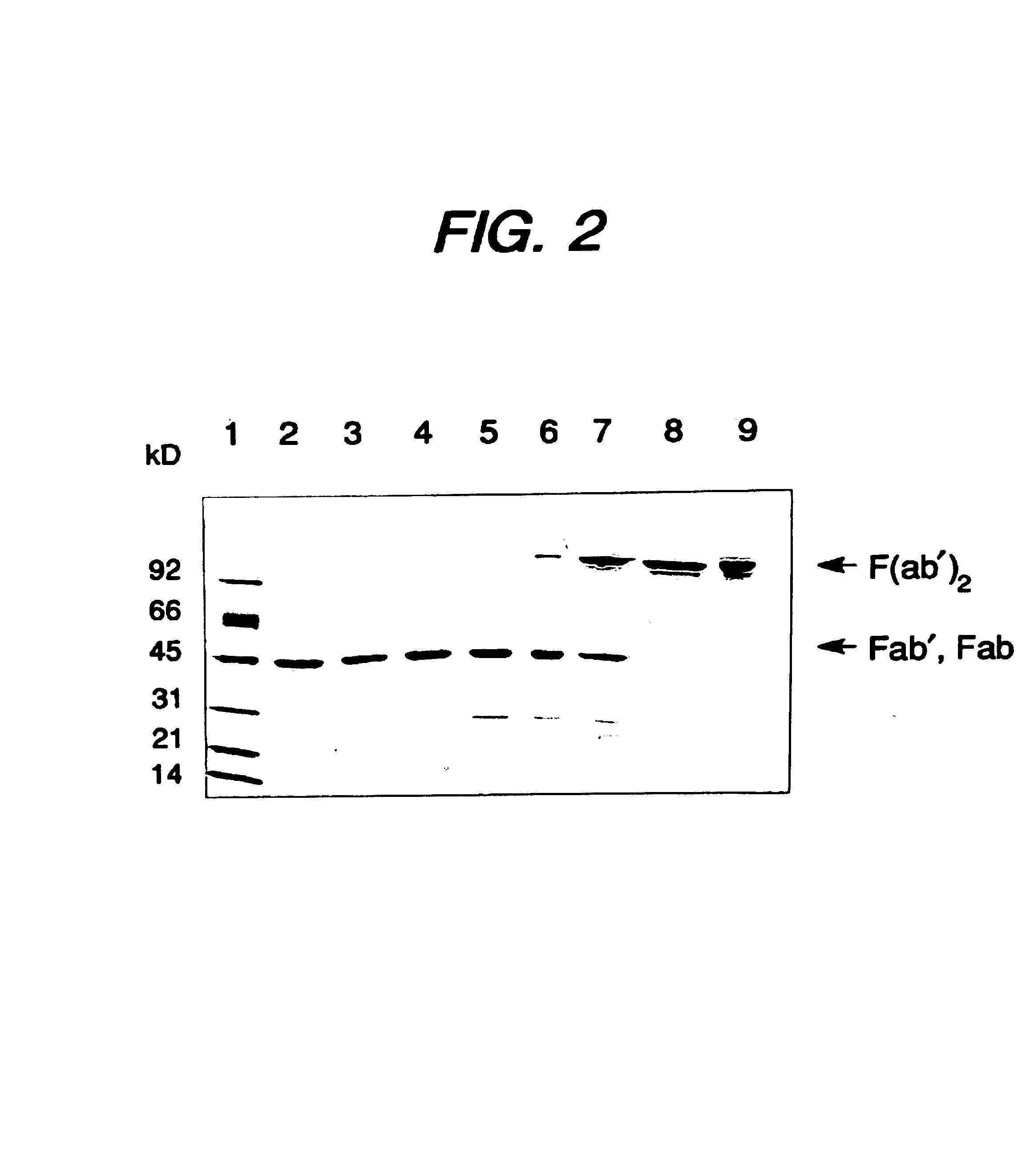
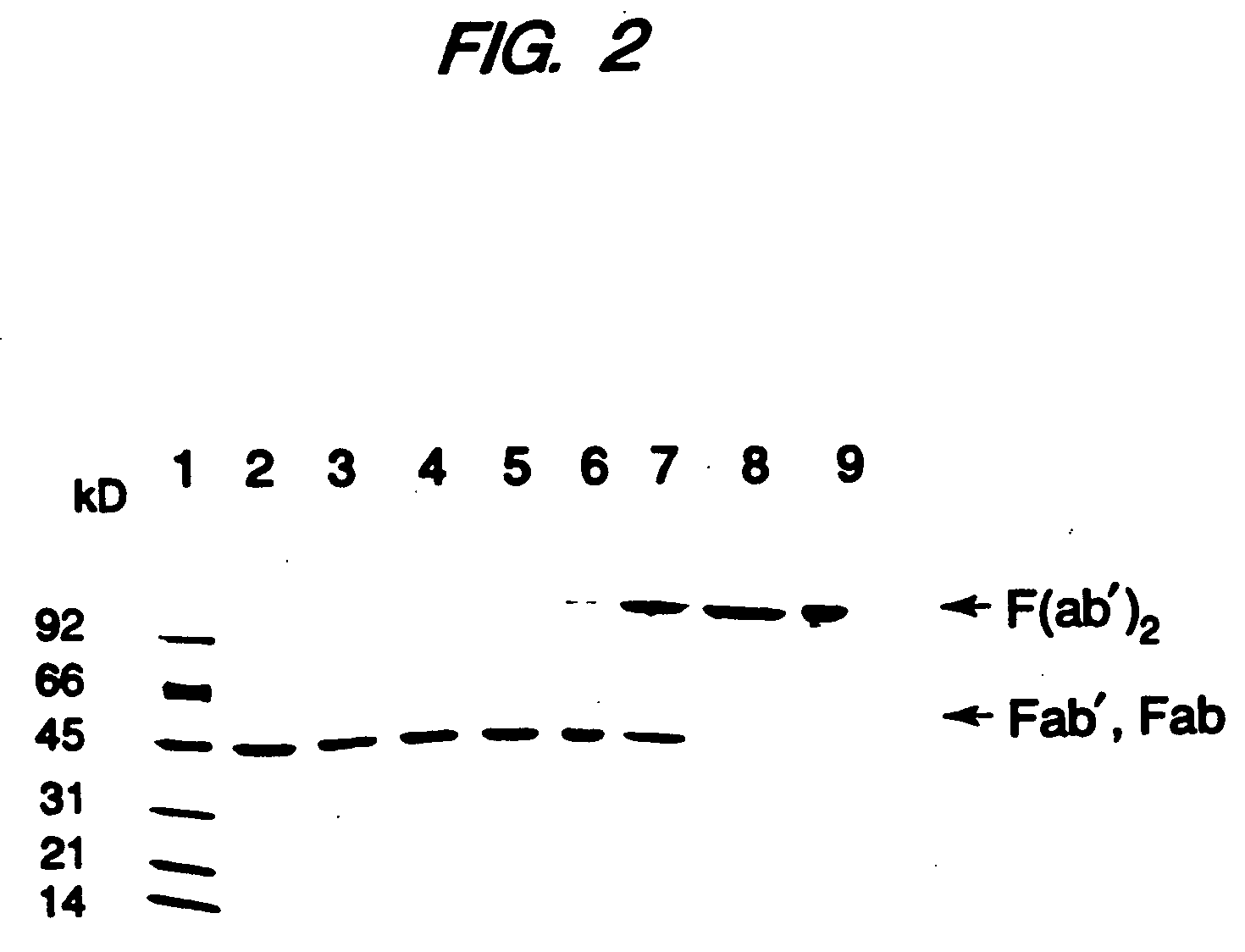
Expression of functional antibody fragments
InactiveUS7018809B1Easy to prepareFacilitated releaseHybrid immunoglobulinsSerum immunoglobulinsMicroorganismHeavy chain
Methods for the high yield production of antibody Fv-containing polypeptides, especially Fab′ and F(ab′)2 antibody fragments are provided. Expression of heavy and light chain Fv in a microbial secretory system is followed by recovery of Fv from the periplasm under conditions that maintain a cysteine residue as a free thiol. The free thiol is reacted with free thiol of an antibody fragment of the same or differing specificity, or with agents such as diagnostic labels or therapeutic moieties. The products offer advantages of homogeneity and purity not available through the use of known methods for preparing such derivatives.
Owner:GENENTECH INC
L-cysteine producing microorganism and method for producing L-cysteine
InactiveUS20050221453A1High expressionBacteriaRecombinant DNA-technologyMicroorganismCysteine thiolate
L-Cysteine is produced by culturing a microorganism having an ability to produce L-cysteine and modified so that expression of emrAB, emrKY, yojIH, acrEF, bcr, or cusA gene should be enhanced in a medium to produce and accumulate L-cysteine in the medium and collecting the L-cysteine from the medium. Genes coding for novel L-cysteine-excreting proteins are identified, and utilized for breeding of L-cysteine-producing microorganism to provide a novel method of producing L-cysteine.
Owner:AJINOMOTO CO INC
Mutant serine acetyltransferase
O-acetylserine, L-cysteine and sulphurous compounds derived therefrom may be produced using a bacterium belonging to the genus Escherichia which harbors a mutant feedback-resistant serine acetyltransferases in which the amino acid sequence corresponding to positions from 89 to 96 in a wild-type serine acetyltransferase is replaced with any one of the amino acid sequences shown in SEQ ID NOS: 4 to 9, and feedback inhibition by L-cysteine in the bacterium is desensitized.
Owner:AJINOMOTO CO INC
Homogeneous preparations of IL-31
Homogeneous preparations of human and murine IL-31 have been produced by mutating one or more of the cysteine residues in the polynucleotide sequences encoding the mature proteins. The cysteine mutant proteins can be shown to either bind to their cognate receptor or exhibit biological activity.
Owner:ZYMOGENETICS INC
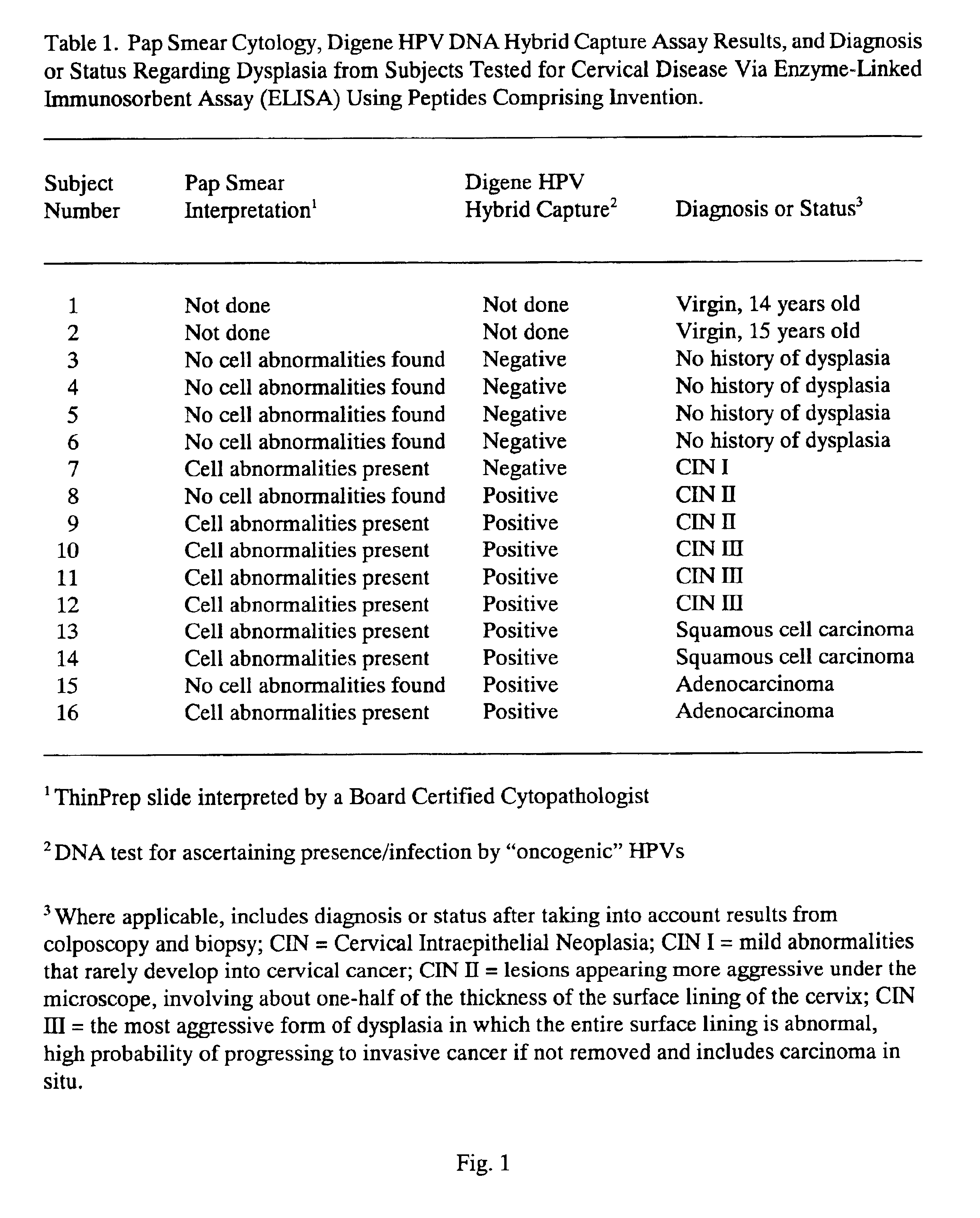
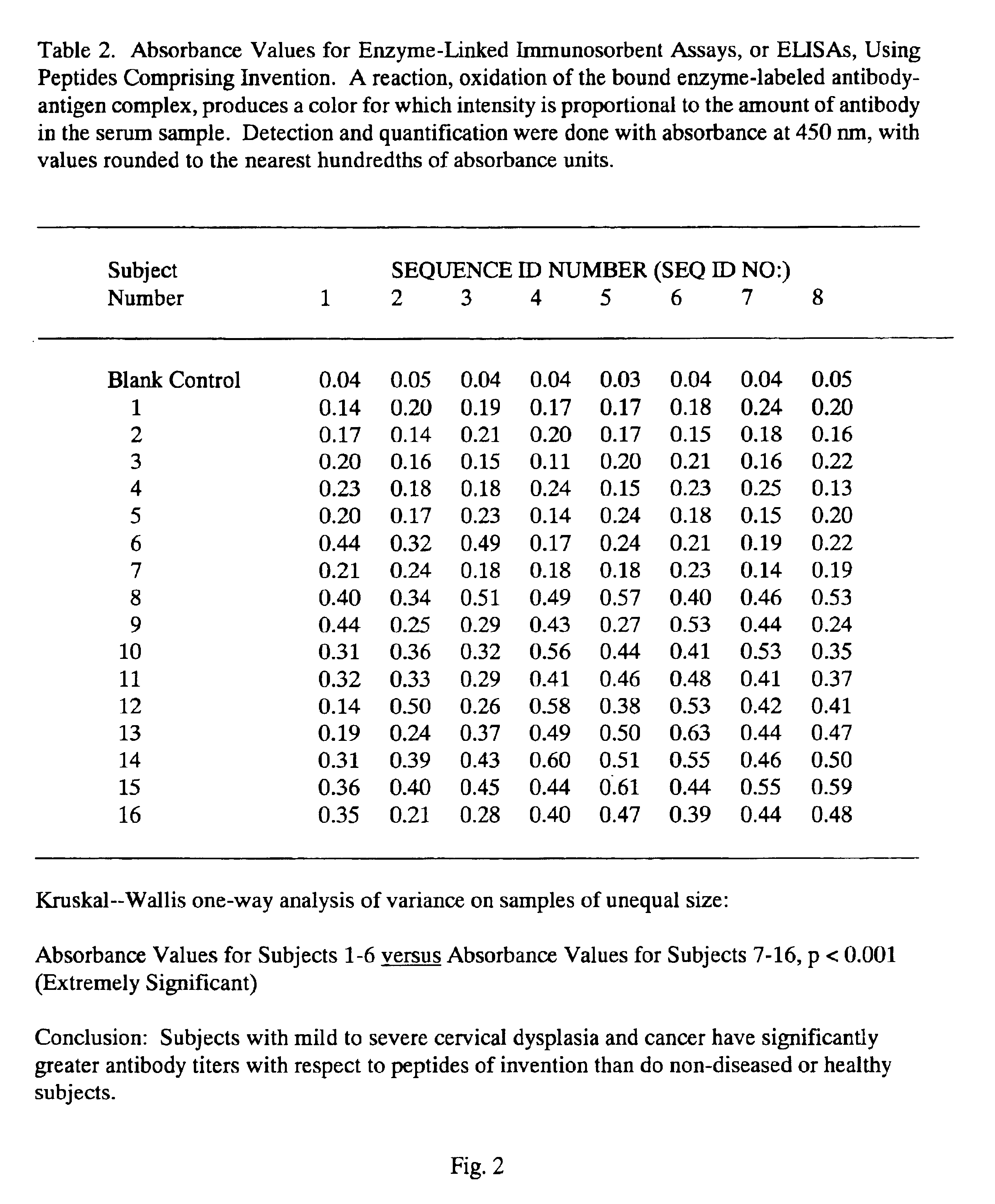
Peptides from the E2, E6, and E7 proteins of human papilloma viruses 16 and 18 for detecting and/or diagnosing cervical and other human papillomavirus associated cancers
InactiveUS6933123B2Simple and rapid and and more testMicrobiological testing/measurementVirus peptidesCysteine thiolateTryptophan
Owner:HU YAO XIONG
Adding photoregulated amino acids to the genetic code
Compositions and methods of producing components of protein biosynthetic machinery that include orthogonal leucyl-tRNAs, orthogonal leucyl-aminoacyl-tRNA synthetases, and orthogonal pairs of leucyl-tRNAs / synthetases, which incorporate photoregulated amino acids, OMe-L-tyrosine, α-aminocaprylic acid, or o-nitrobenzyl cysteine into proteins are provided in response to an amber selector codon. Methods for identifying these orthogonal pairs are also provided along with methods of producing proteins with a photoregulated amino acid, OMe-L-tyrosine, α-aminocaprylic acid, or o-nitrobenzyl cysteine using these orthogonal pairs.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
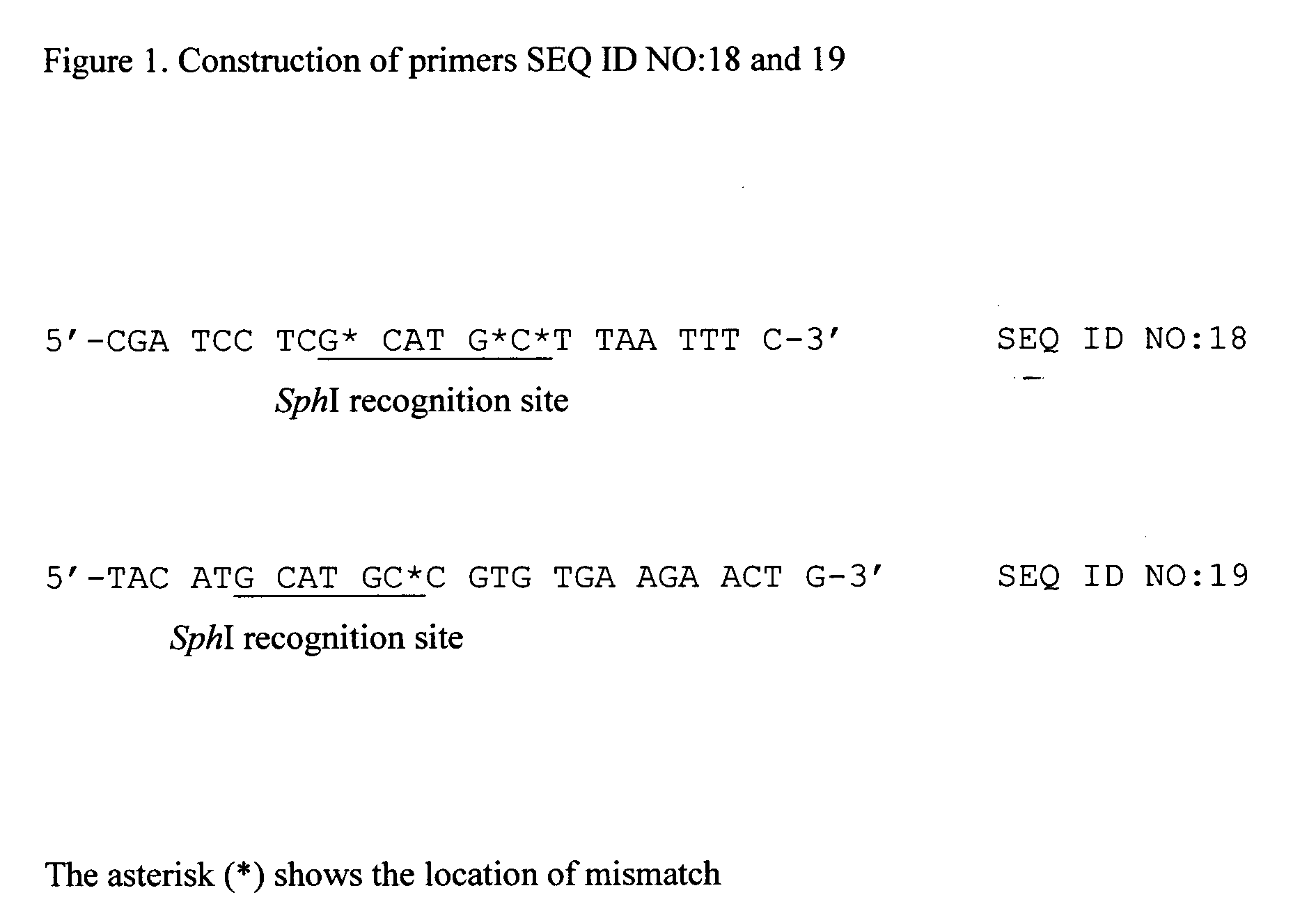
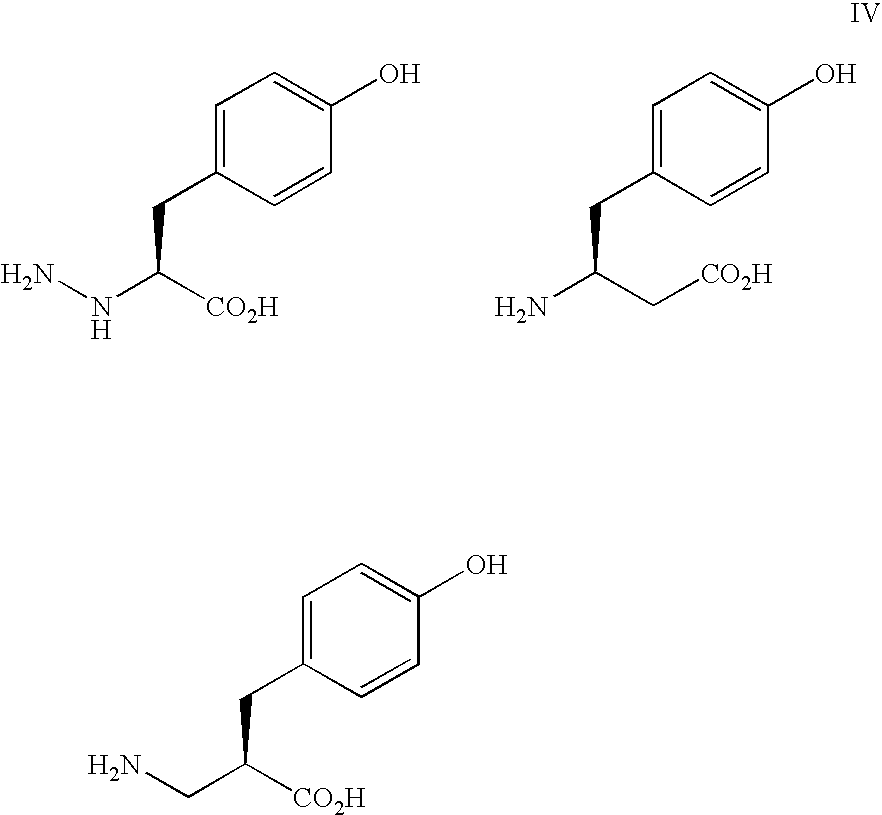
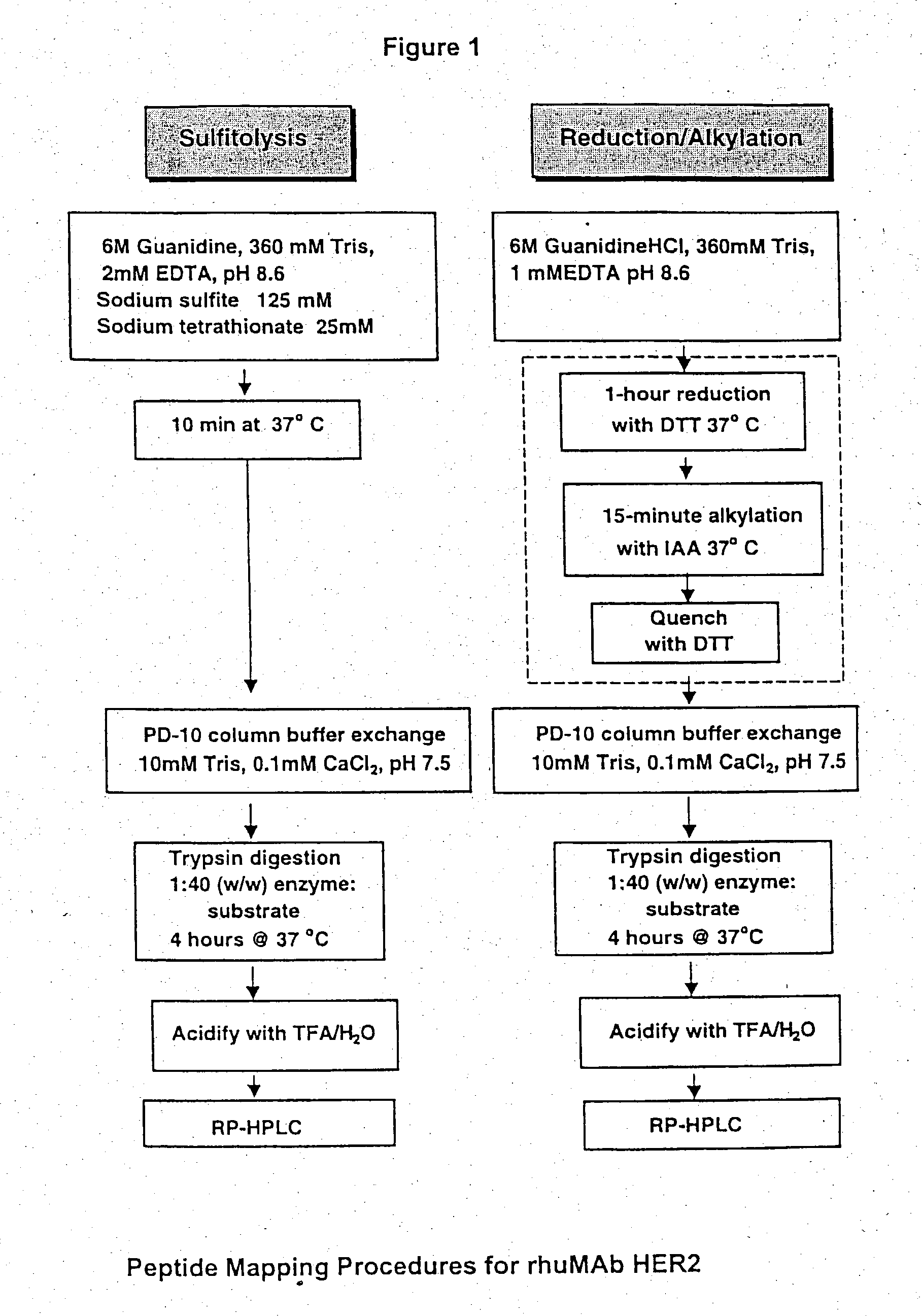
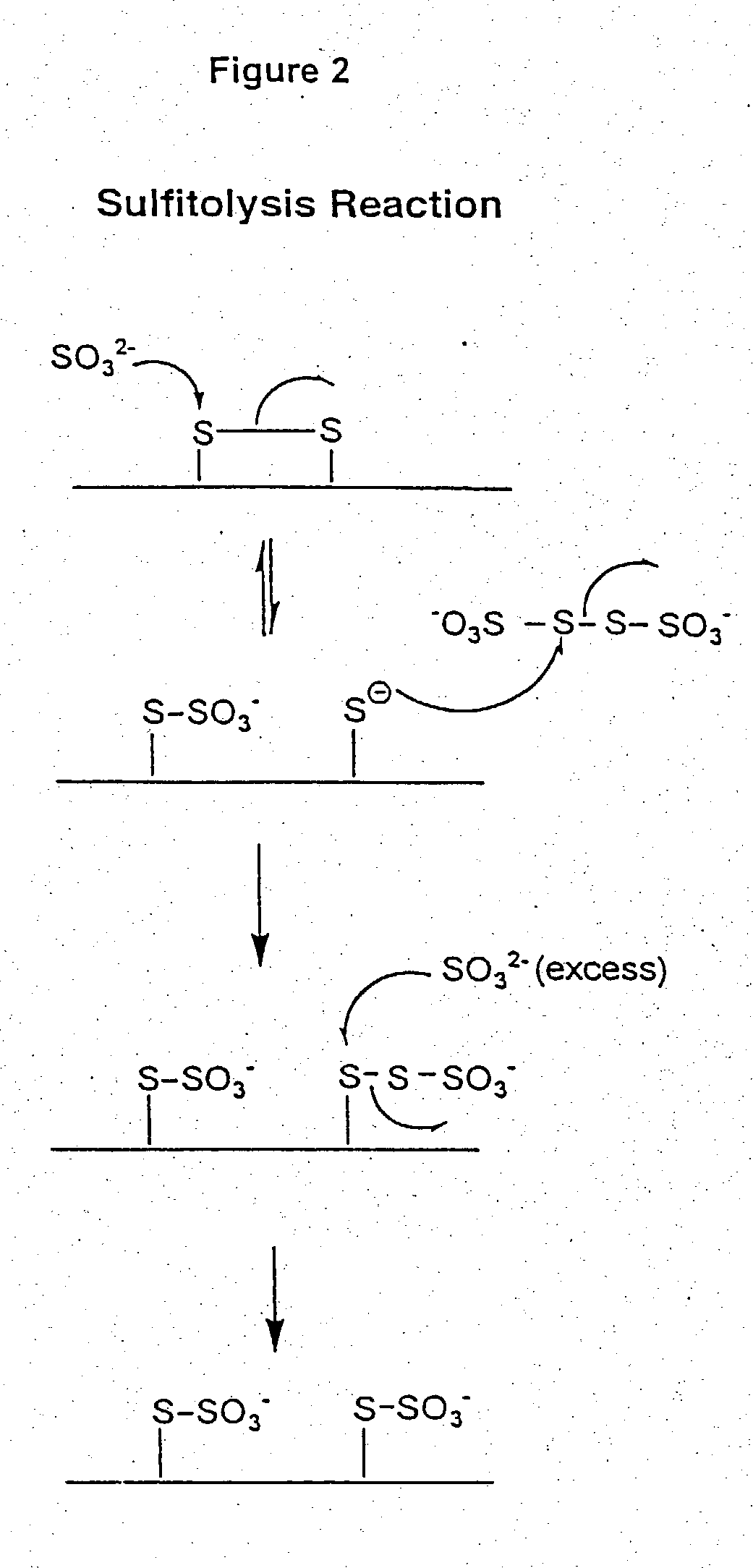
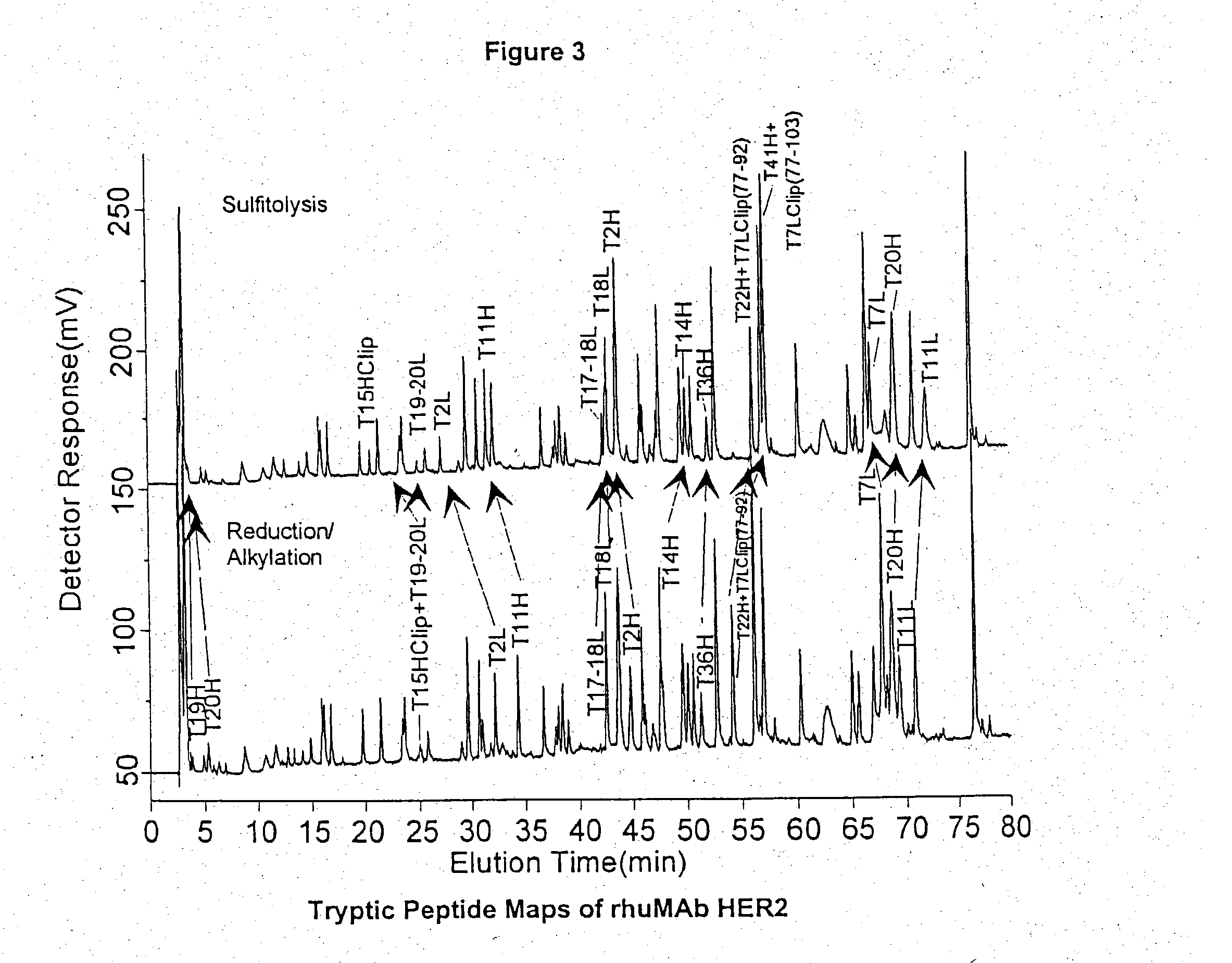
Use of sulfitolysis in high performance peptide mapping
InactiveUS20030175845A1Microbiological testing/measurementBiological testingCrystallographyCysteine thiolate
Owner:GENENTECH INC
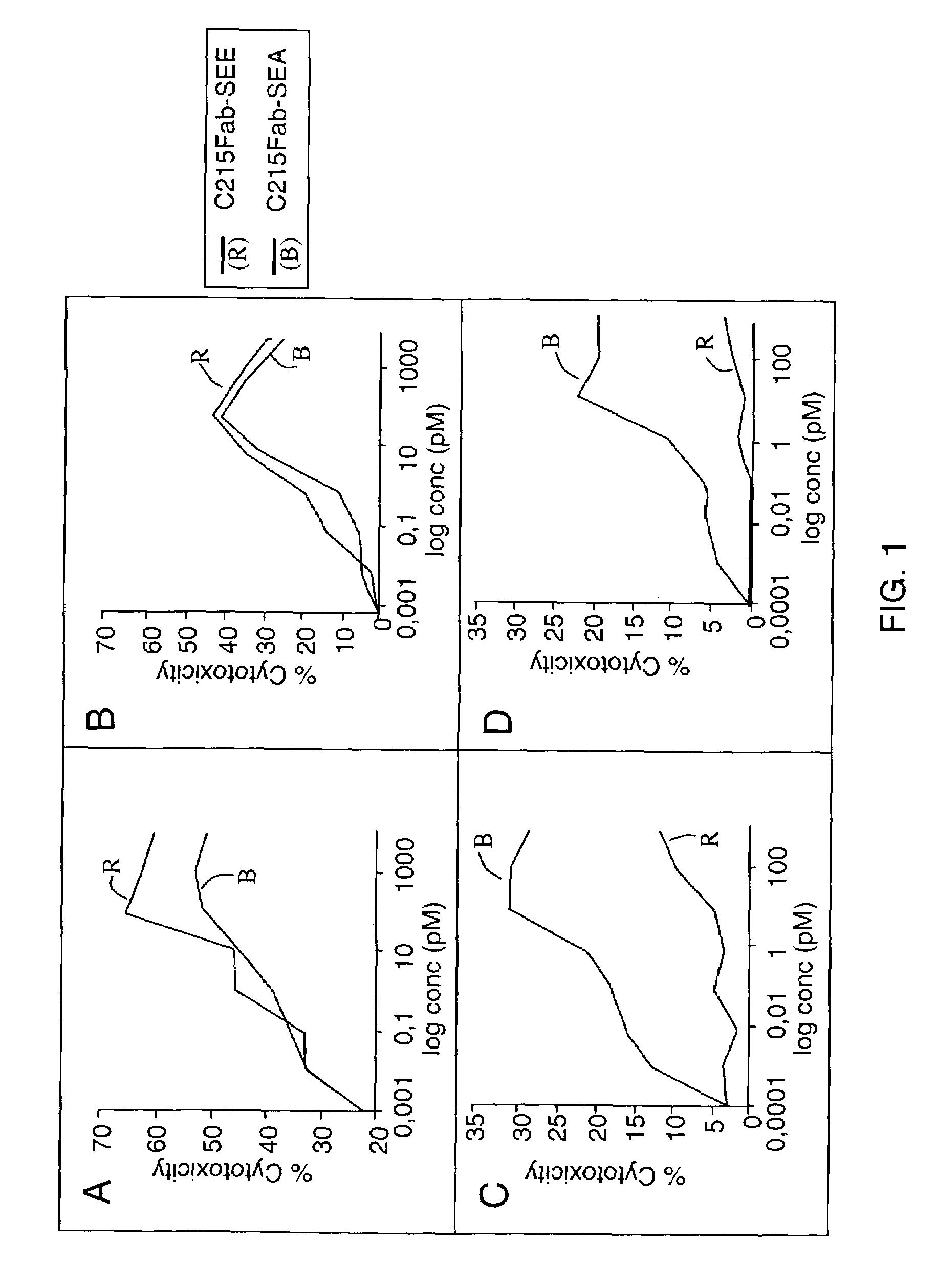
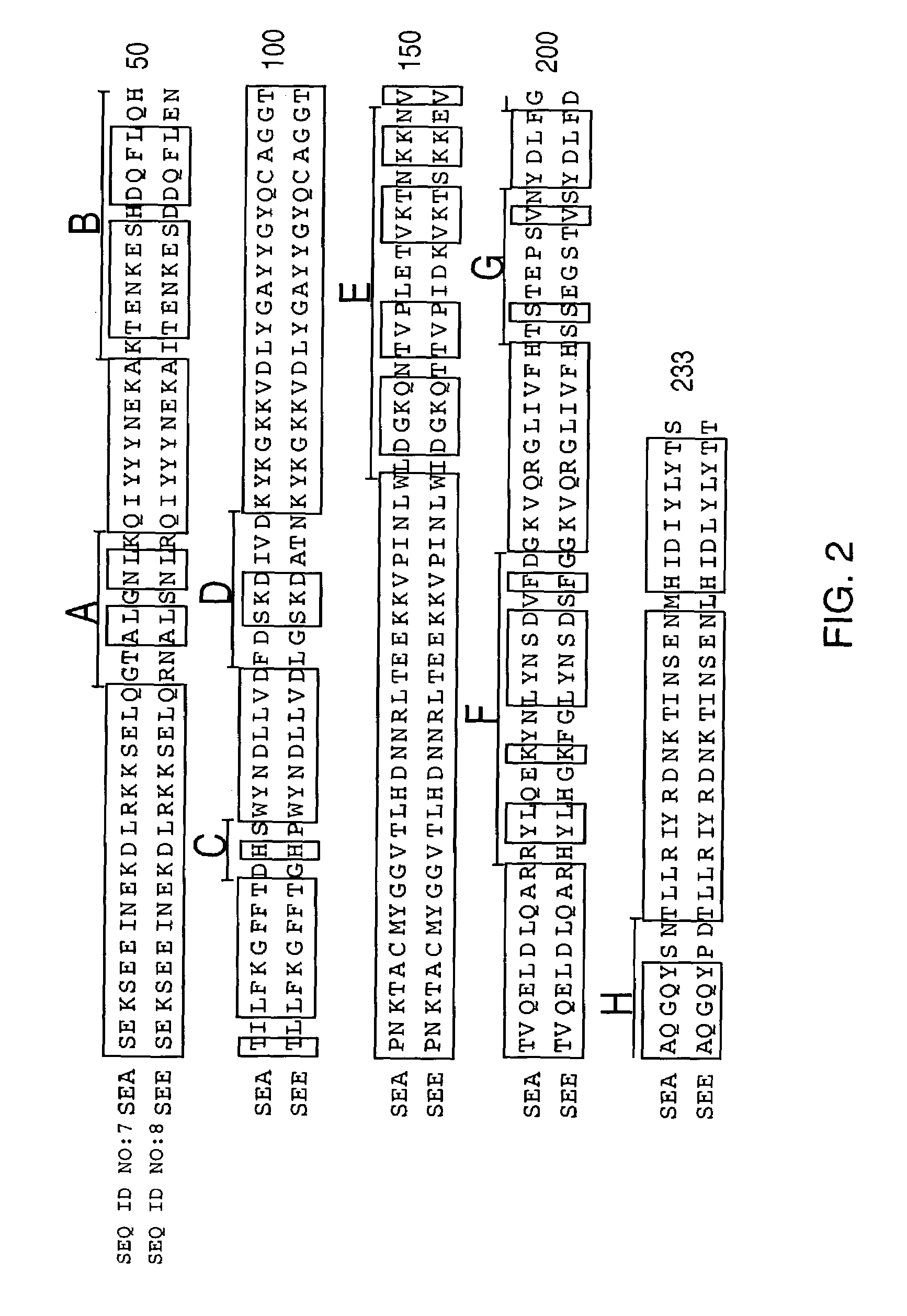
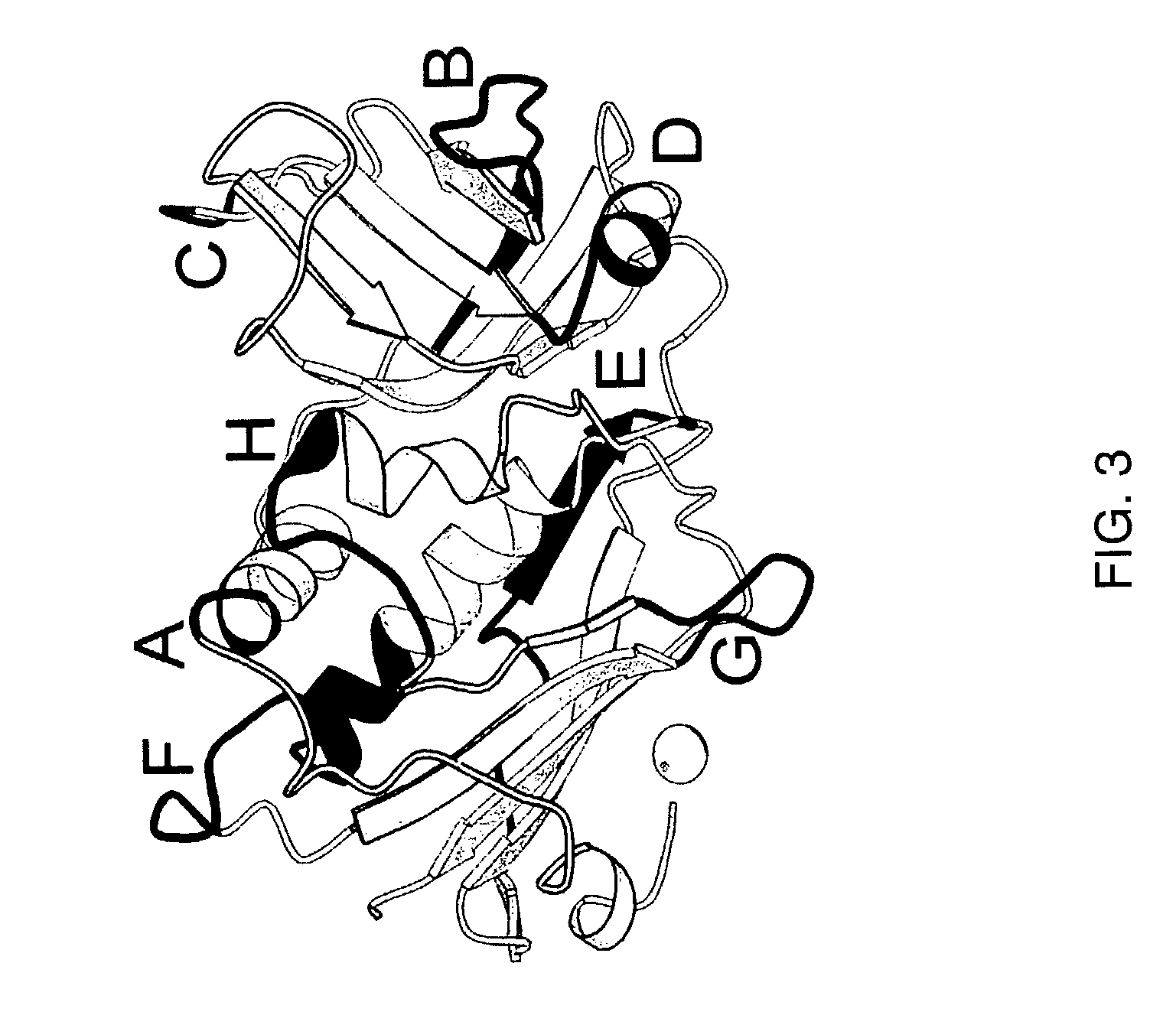
Modified Chimeric superantigens and their use
InactiveUS7226595B2Low immunogenicityReduce reduction reactionPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenCysteine thiolate
A conjugate between a target-seeking moiety and a modified superantigen, characterized in that the superantigen is a wild-type superantigen (SA I) in which an amino acid residue in a superantigen region (region I) determining binding to TCR, preferably TCRVβ, and T cell activation have been replaced by another amino acid residue while retaining the ability to activate a subset of T cells.In preferred embodiment the modified superantigen is a chimer between at least two wild-type superantigens (SA I, SA II etc) characterized in that one or more amino acid residues in a region determining binding to TCR and T cell activation have been interchanged between various wild-type superantigens.A therapeutic method making use of modified / chimeric superantigens as defined in the preceding paragraphs.An antibody preparation in which the cysteine residues that provide for interchain disulfide bonds have been mutated so as to forbid interchain disulfide bridges, preferably to serine residues, for use as pharmaceutical.
Owner:ACTIVE BIOTECH AB
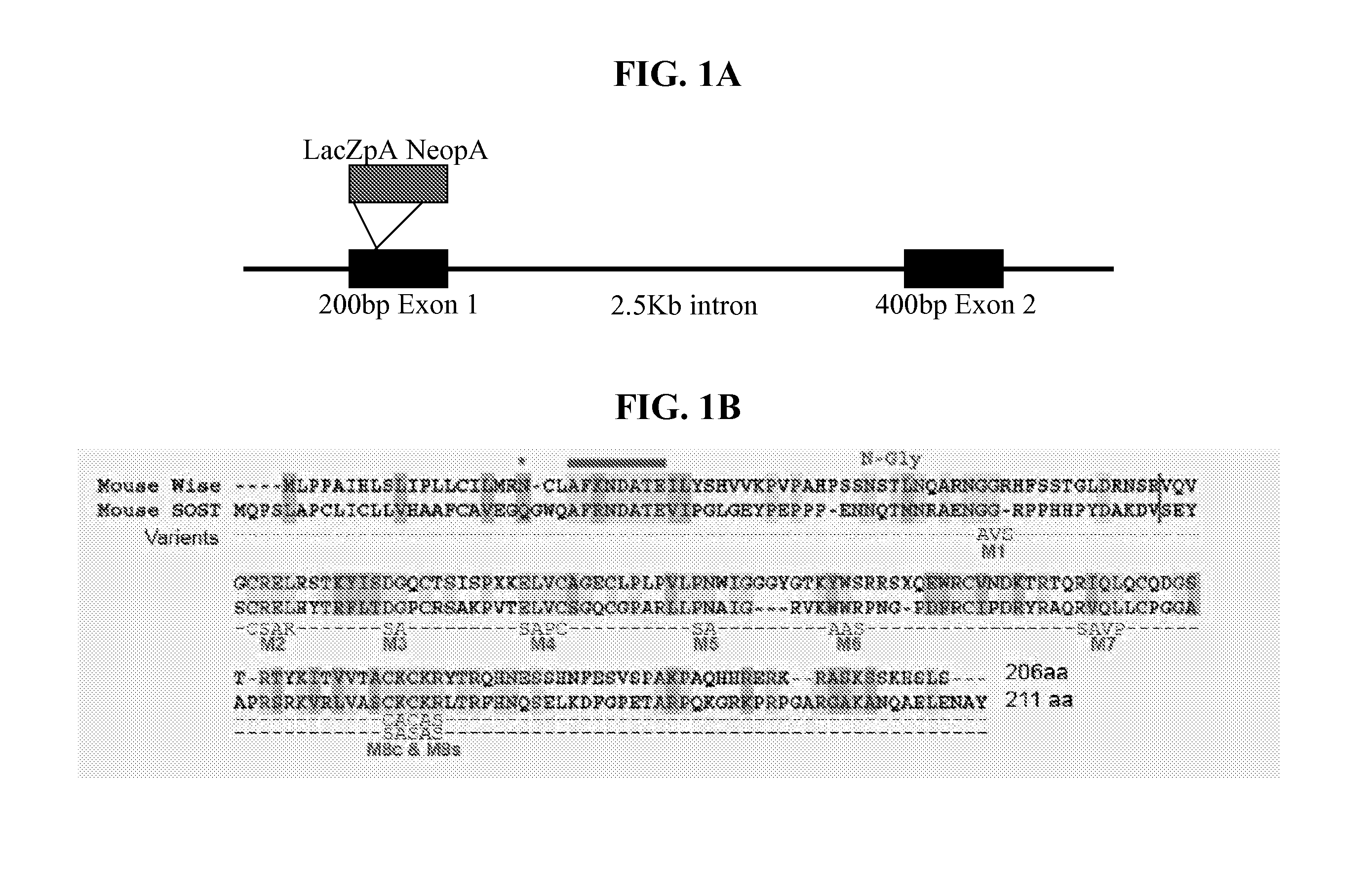
Peptides for treatment and diagnosis of bone diseases
ActiveUS20070292444A1Peptide/protein ingredientsGenetic material ingredientsCystine knotCysteine thiolate
The present invention is directed to isolated polypeptides and antibodies suitable for producing therapeutic preparations, methods, and kits relating to bone deposition. One objective of the present invention is to provide compositions that improve bone deposition. Yet another objective of the present invention is to provide methods and compositions to be utilized in diagnosing bone dysregulation. The therapeutic compositions and methods of the present invention are related to the regulation of Wise, Sost, and closely related sequences. In particular, the nucleic acid sequences and polypeptides include Wise and Sost as well as a family of molecules that express a cysteine knot polypeptide.
Owner:STOWERS INST FOR MEDICAL RES
Expression of functional antibody fragments
InactiveUS20050244929A1Easy to prepareFacilitated releaseHybrid immunoglobulinsBacteriaMicroorganismHeavy chain
Methods for the high yield production of antibody Fv-containing polypeptides, especially Fab′ and F(ab′)2 antibody fragments are provided. Expression of heavy and light chain Fv in a microbial secretory system is followed by recovery of Fv from the periplasm under conditions that maintain a cysteine residue as a free thiol. The free thiol is reacted with free thiol of an antibody fragment of the same or differing specificity, or with agents such as diagnostic labels or therapeutic moieties. The products offer advantages of homogeneity and purity not available through the use of known methods for preparing such derivatives.
Owner:GENENTECH INC
N-acetylcysteine compositions and methods for the treatment and prevention of cysteine/glutathione deficiency in diseases and conditions
InactiveUS20050070607A1Low toxicityAllow administrationBiocideOrganic active ingredientsCysteine thiolateClinical settings
Life-threatening hepatotoxicity in the setting of acetaminophen overdose is due to depletion of glutathione (GSH), a vital cysteine-containing tripeptide that protects cells and organs against oxidant injury. Rapid administration of N-acetylcysteine (NAC), which provides the cysteine necessary to replenish the depleted GSH, is the standard of care for preventing injury in acetaminophen overdose. Beneficial effects of NAC treatment have also been demonstrated in respiratory, cardiovascular, endocrine and infectious and other diseases. In fact, over fifty randomized placebo-controlled trials conducted in diverse clinical settings document positive responses to NAC treatment. The present invention relates to cysteine / glutathione (GSH) deficiency as a previously unrecognized clinical entity that can complicate the course of commonly encountered diseases and methods of treatment of this generalized deficiency involving administering N-acetylcysteine (NAC) or a pharmaceutically acceptable salt or derivative to a subject in need thereof and monitoring the subjects appropriate glutathione blood levels as needed.
Owner:ANDRUS JAMES +4
Methods and compositions for delivering siRNA into mammalian cells
ActiveUS20050239687A1Quick releaseRobust downregulation of target mRNABiocidePeptide/protein ingredientsLipid formationCysteine thiolate
Complex comprising a peptide carrier of SEQ ID NO:1 GALFLGFLGAAGSTMGAWSQPKR1KRKVR2 and an appropriate siRNA, wherein R1 represents any amino acid residue and more preferably K or S, R2 is null or represents one of the following groups: cysteamide, cysteine, thiol, amide, linear or ramified C1-C6 alkyl optionally substituted, primary or secondary amine, osidic derivative, lipid, phospholipid or cholesterol and said siRNA is selected to silence a target mRNA.
Owner:CENT NAT DE LA RECHERCHE SCI
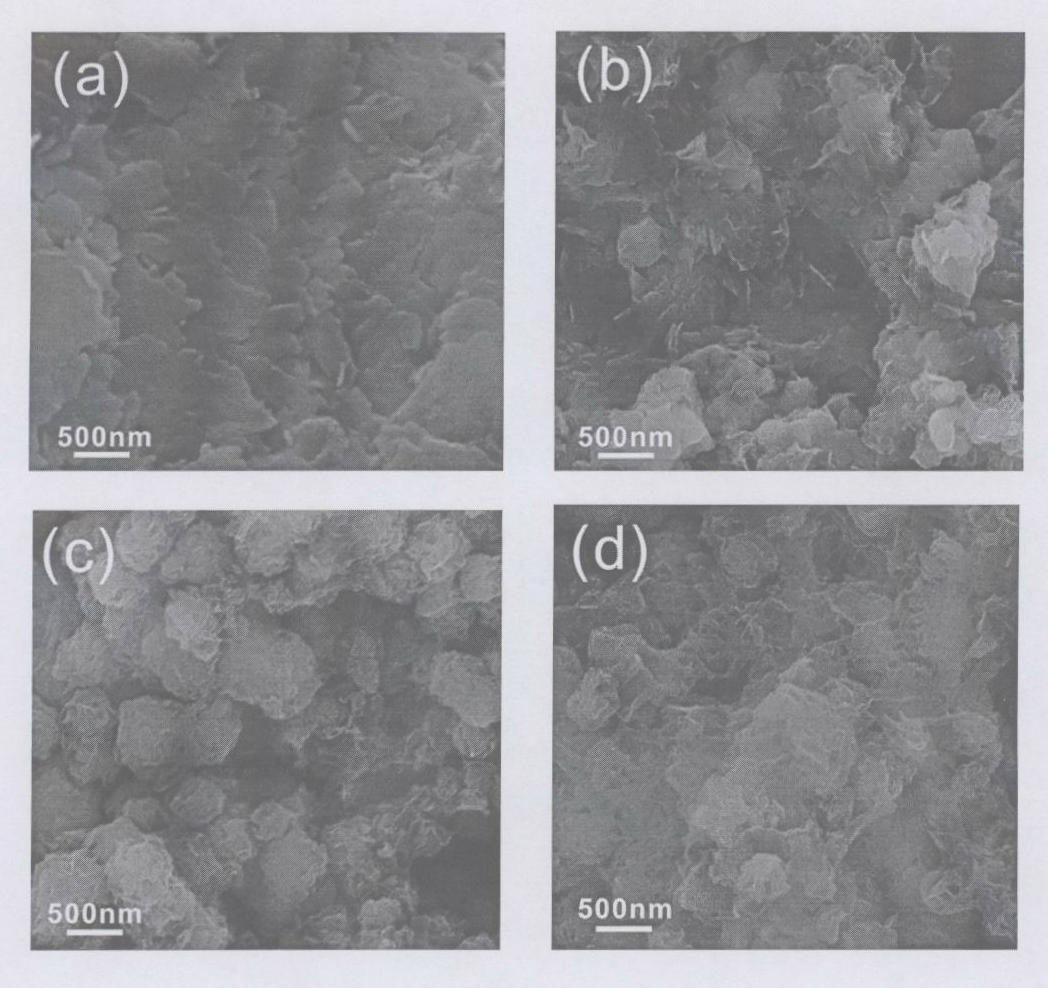
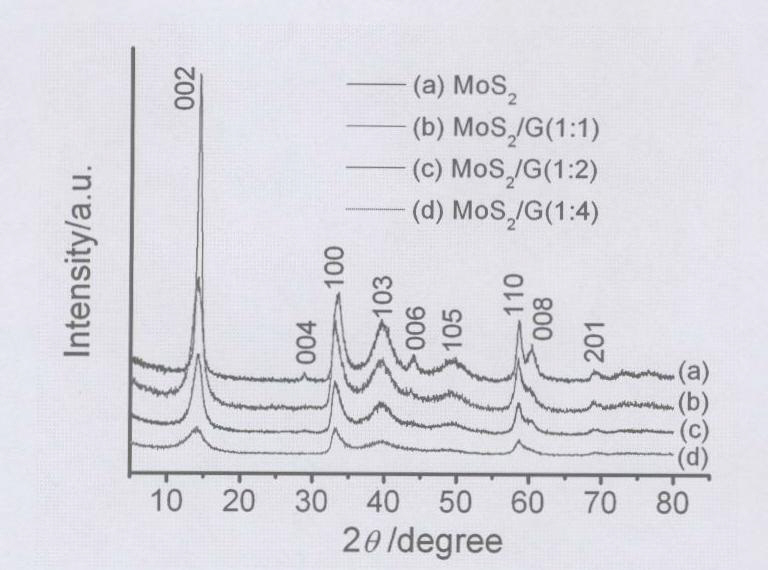
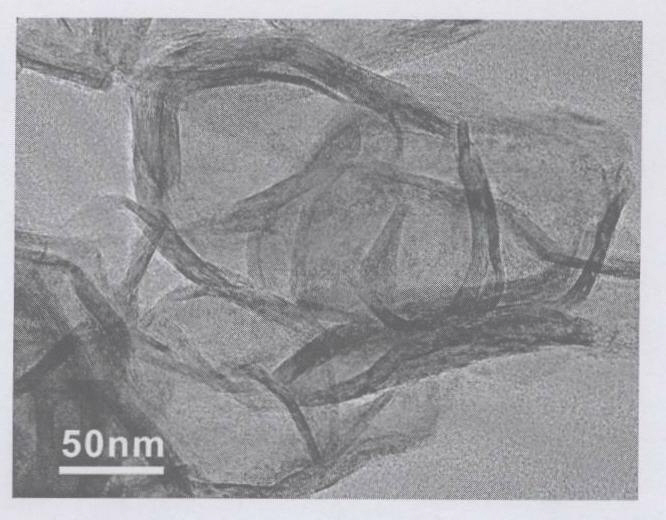
Compound nano material of graphene and MoS2 and preparation method thereof
InactiveCN102142548AMild reaction conditionsSimple preparation processMaterial nanotechnologyCell electrodesCysteine thiolateNew energy
The invention discloses a compound nano material of graphene and molybdenum disulfide (MoS2) and a preparation method thereof. The compound material is formed by mixing graphene and a MoS2 nano material in a mass ratio of (1 to 1)-(4 to 1). The preparation method comprises the following steps of: preparing an oxidized graphite nano slice from graphite by a chemical oxidization method; then dissolving molybdate into deionized water so as to form 0.02 to 0.07M of solution; adding L-cysteine serving as a sulfur source and a reduction agent, wherein the mass ratio of the L-cysteine to the molybdate is (5 to 1)-(12 to 1); adding the oxidized graphite nano slice into the solution, and ultrasonically treating so that the oxidized graphite nano slice can be fully dispersed in the hydrothermal reaction solution; transferring the mixture into a hydrothermal reaction kettle and sealing; and synthesizing by a one-step hydrothermal method to obtain the compound nano material of graphene and MoS2, wherein the mass ratio of the graphene nano slice to the MoS2 is (1 to 1)-(4 to 1). The method has the characteristics of mild reaction condition and simple process. The compound nano material of graphene and MoS2 synthesized by the method can be widely used as electrode materials of new energy batteries, high-performance national lubricants, catalyst carriers and the like.
Owner:ZHEJIANG UNIV
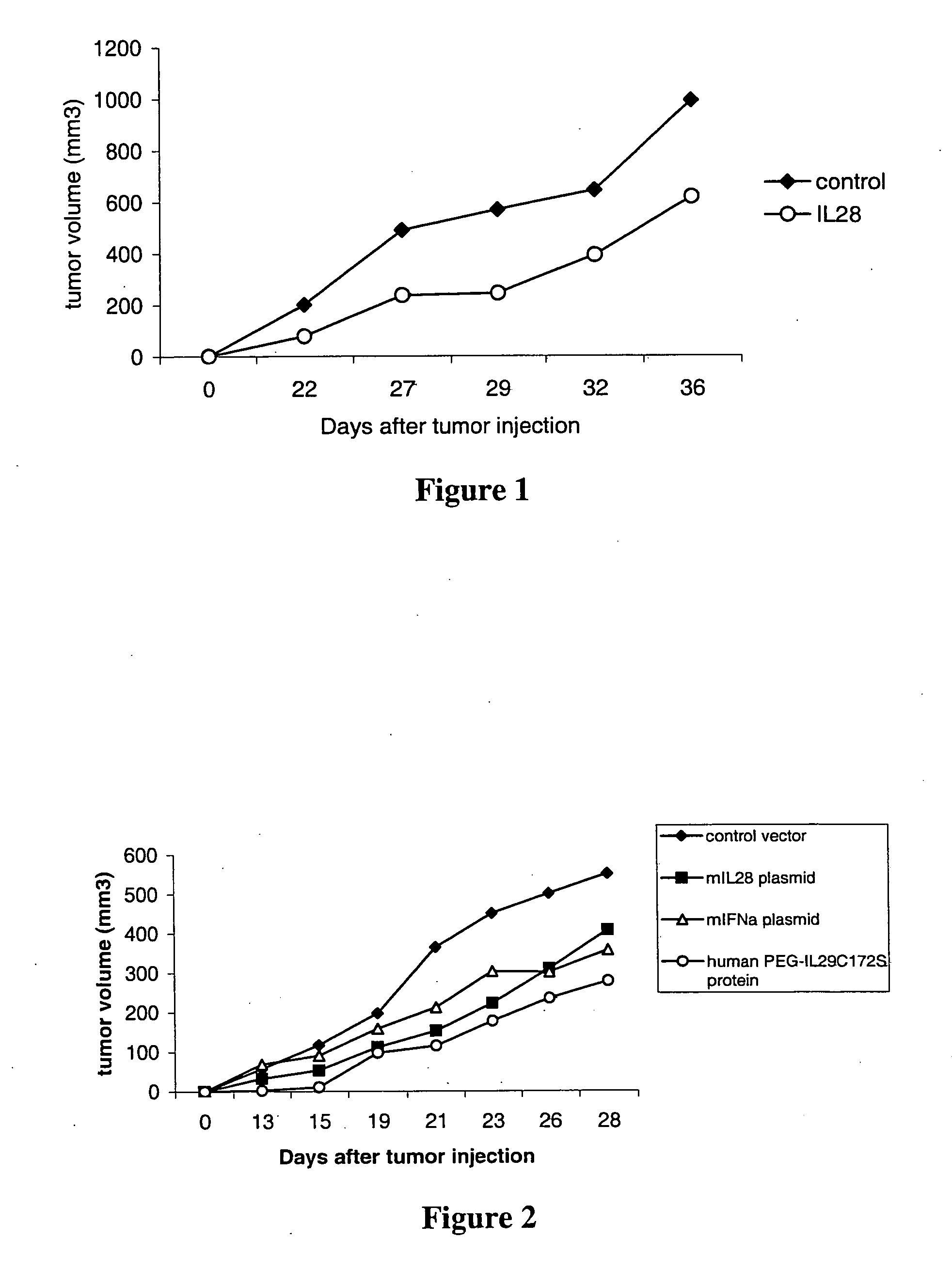
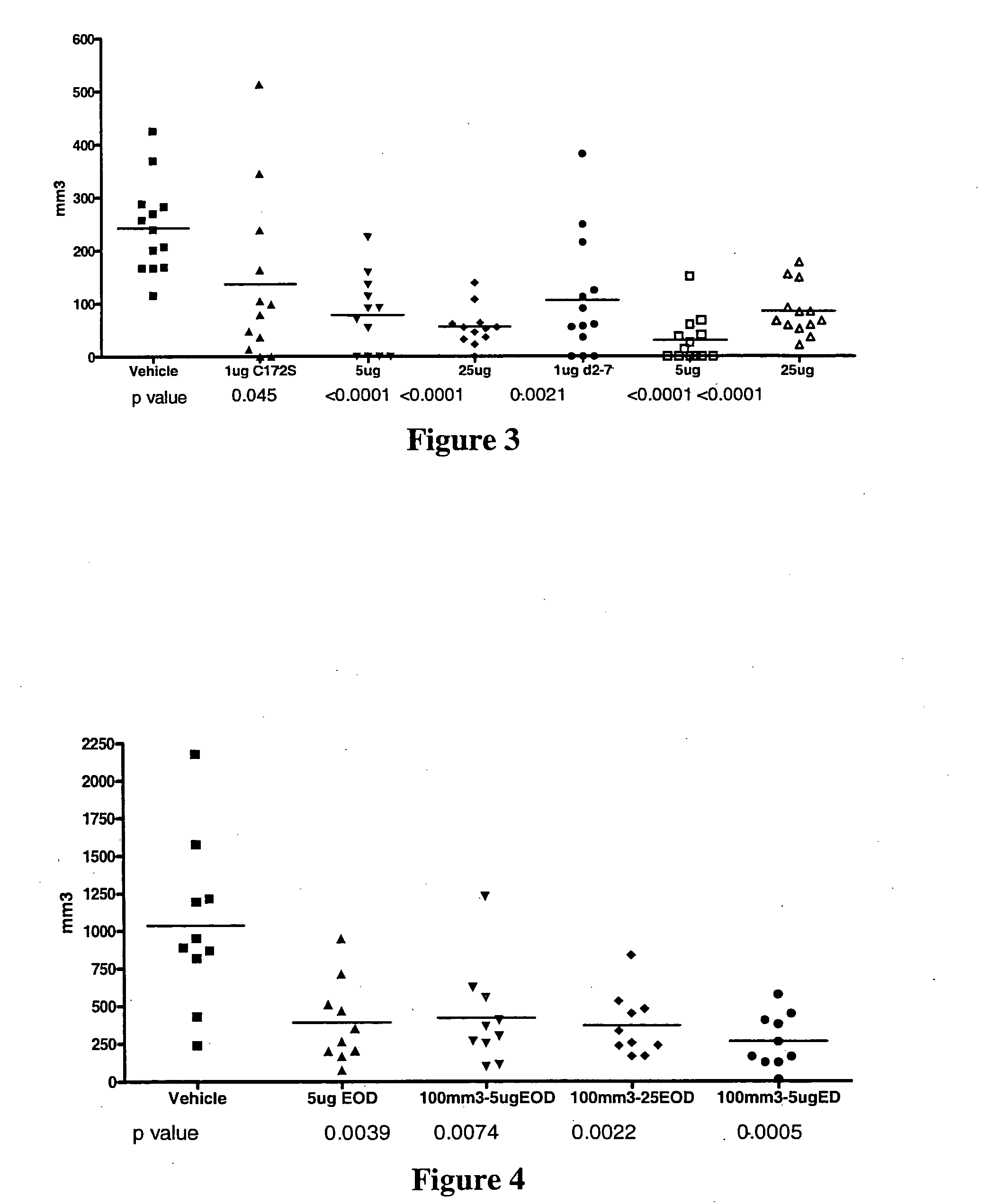
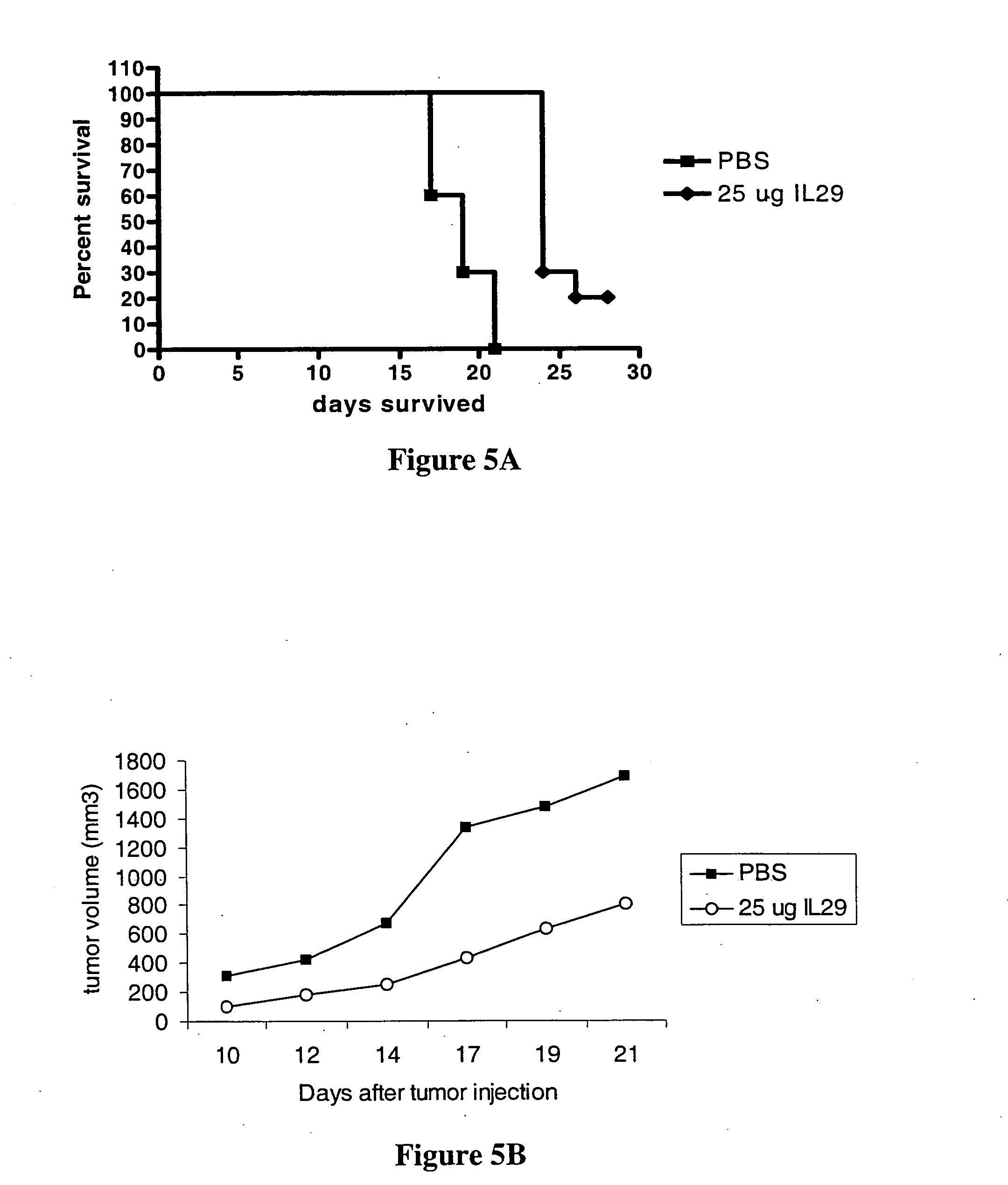
IL28 and IL29 TRUNCATED CYSTEINE MUTANTS AND ANTIVIRAL METHODS OF USING SAME
InactiveUS20070053933A1Organic active ingredientsPeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Use of truncated cysteine IL28 and IL29 mutants to treat cancers and autoimmune disorders
InactiveUS20070020227A1Improve expression levelIncrease productionNervous disorderPeptide/protein ingredientsDiseaseCysteine thiolate
Methods for treating patients with cancer and autoimmune disorders using IL-28 and IL-29 molecules. The IL-28 and IL-29 molecules include polypeptides that have homology to the human IL-28 or IL-29 polypeptide sequence and proteins fused to a polypeptide with IL-28 and IL-29 functional activity. The molecules can be used as a monotherapy or in combination with other known cancer and / or autoimmune therapeutics.
Owner:ZYMOGENETICS INC
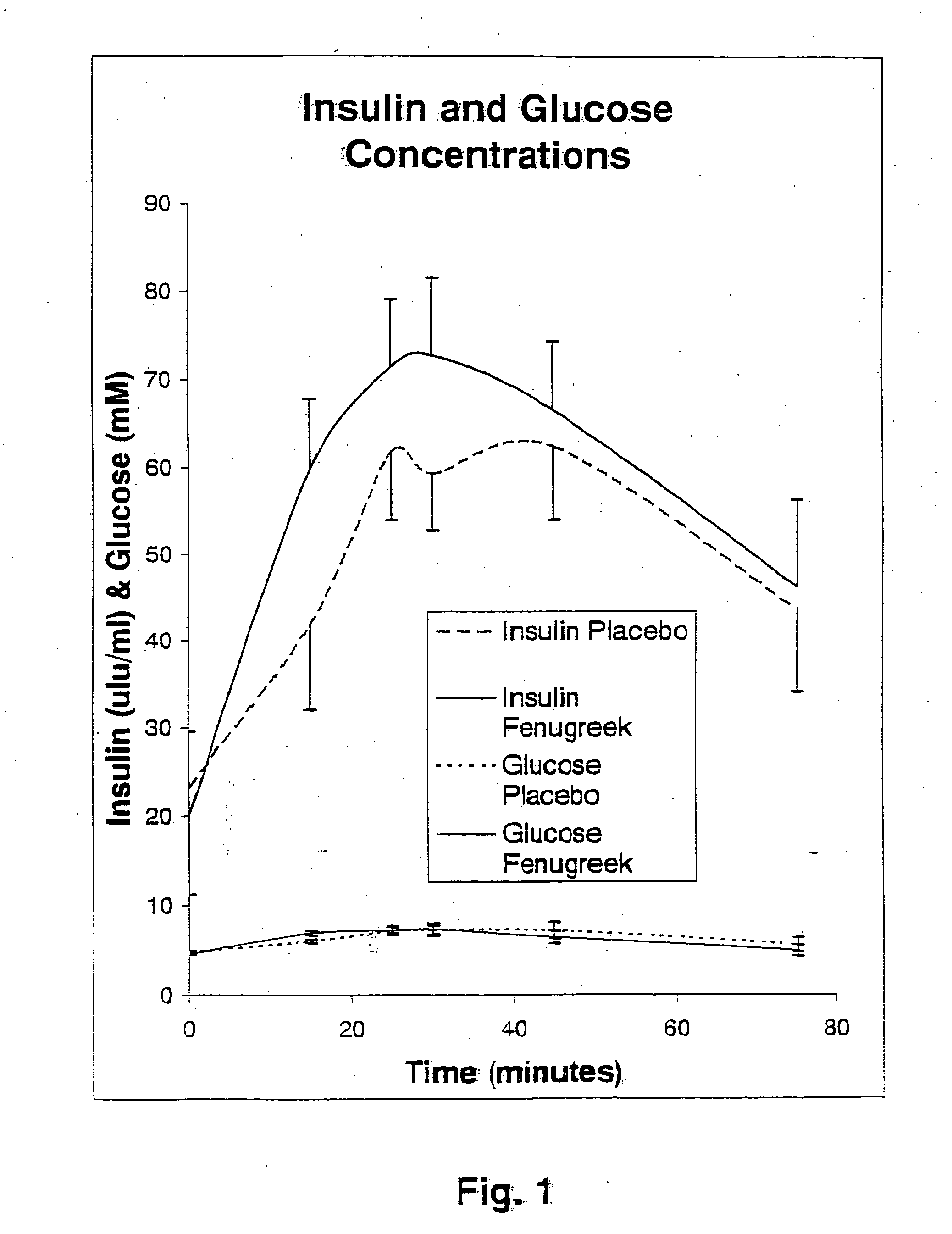
Compositions and methods for glycogen synthesis
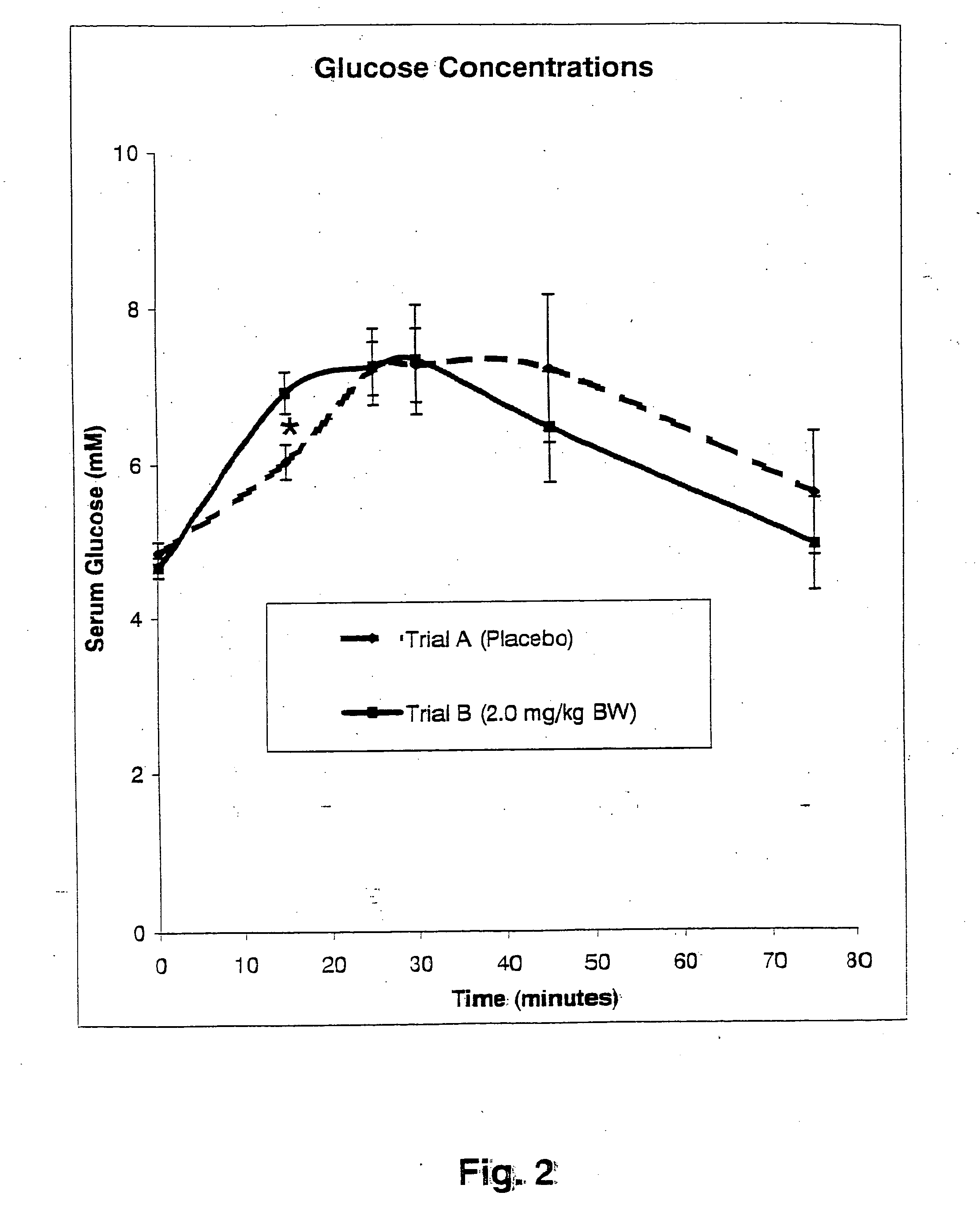
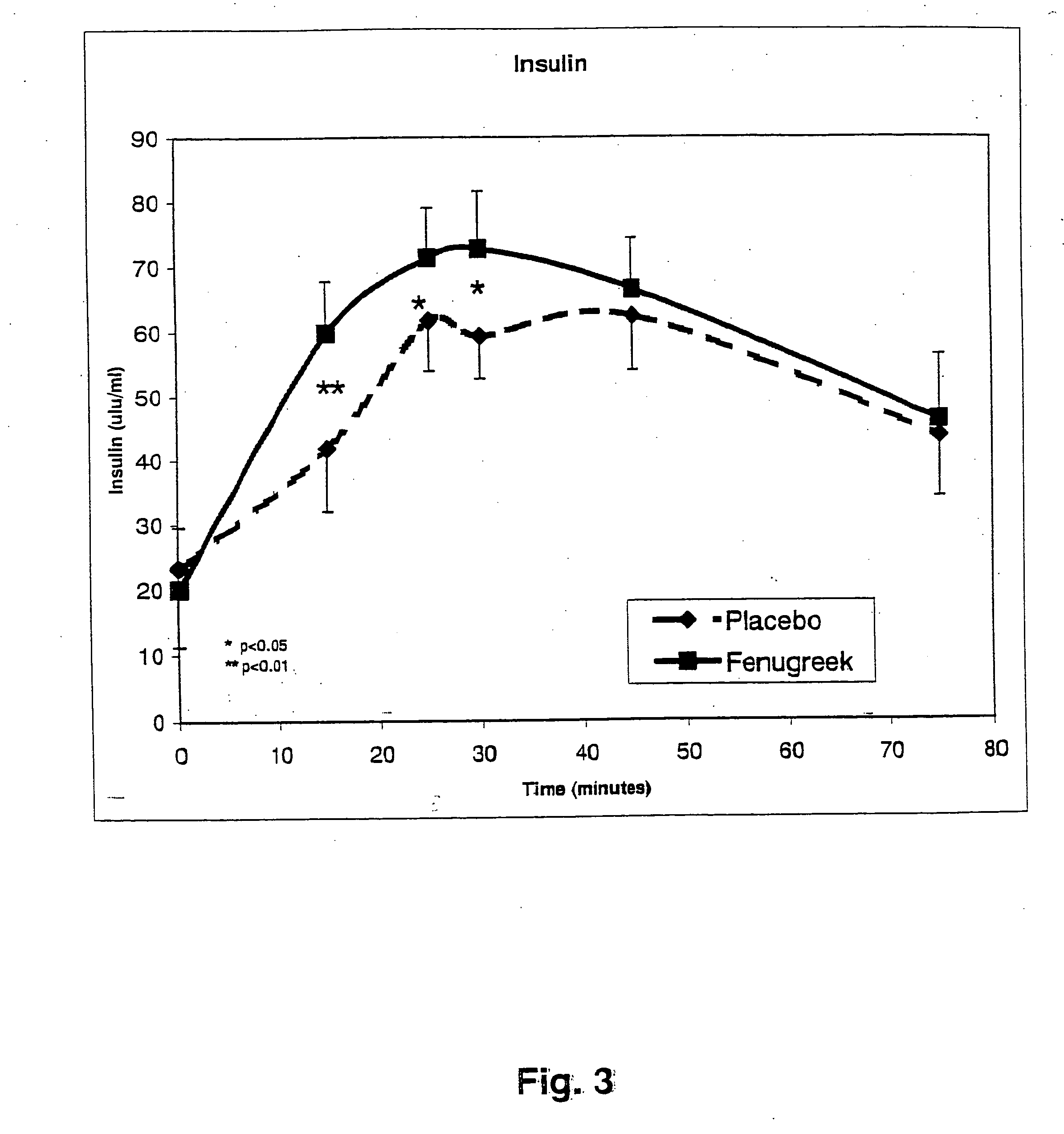
InactiveUS20050176827A1Function increaseIncrease insulin sensitivityBiocideOrganic active ingredientsCysteine thiolateTryptophan
A composition of bio-active compounds and methods for facilitating and supporting the metabolism and transport of glucose and carbohydrates into muscle cells, promoting muscle function and growth, promoting glycogen synthesis, enhancing glucose disposal, stimulating pancreatic beta cells, promoting metabolic recovery, promoting muscle recovery, promoting lean body mass, and promoting fat burning. Preferably, the composition of bio-active compounds includes a combination of 4-hydroxyisoleucine with at least one amino acid selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, lysine, histidine, gamma-amino butyrate and tyrosine. In one presently preferred embodiment of the present invention, the combination is derived, isolated, and / or extracted from fenugreek seeds. Methods for using a novel composition of bio-active compounds from fenugreek seed for facilitating and supporting the metabolism and transport of glucose and carbohydrates into muscle cells, promoting muscle function and growth, promoting glycogen synthesis, enhancing glucose disposal, stimulating pancreatic beta cells, promoting metabolic recovery, promoting muscle recovery, promoting lean body mass, and promoting fat burning are also disclosed, wherein methods comprise the steps of: (1) providing an effective amount of a composition of bio-active compounds derived, isolated, and / or extracted from fenugreek seeds; and (2) administering the composition to a human or animal.
Owner:TSI INC
Homogeneous preparations of il-28 and il-29
ActiveUS20070042471A1Improve expression levelIncrease productionPeptide/protein ingredientsAntipyreticMutated proteinCysteine thiolate
Owner:ZYMOGENETICS INC
Methods for treating viral infection using il-28 and il-29 cysteine mutants
InactiveUS20080075693A1Prolonged Circulatory Half-LifeLow immunogenicityPeptide/protein ingredientsAntipyreticInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Medical Products and Parenteral Formulations
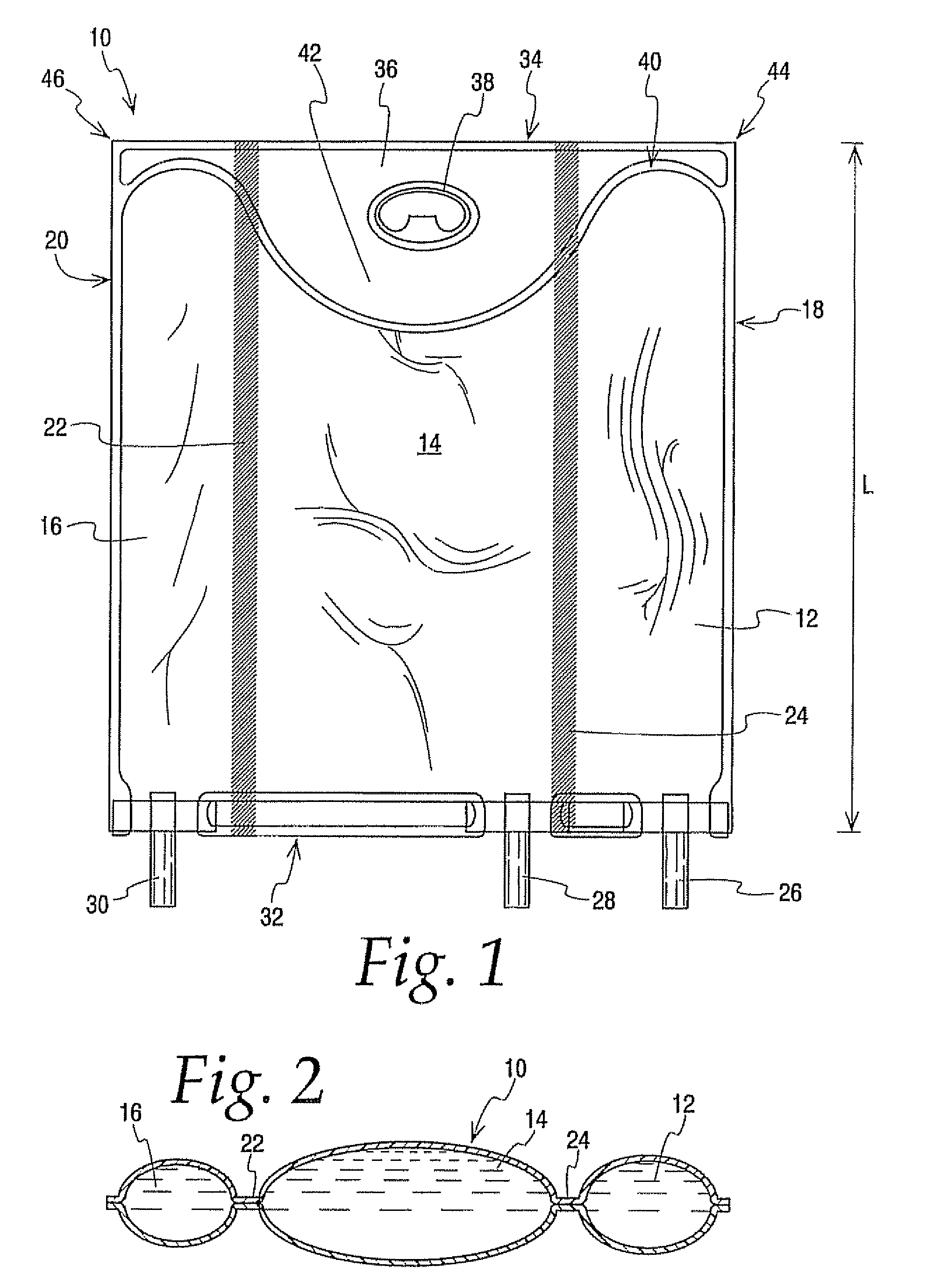
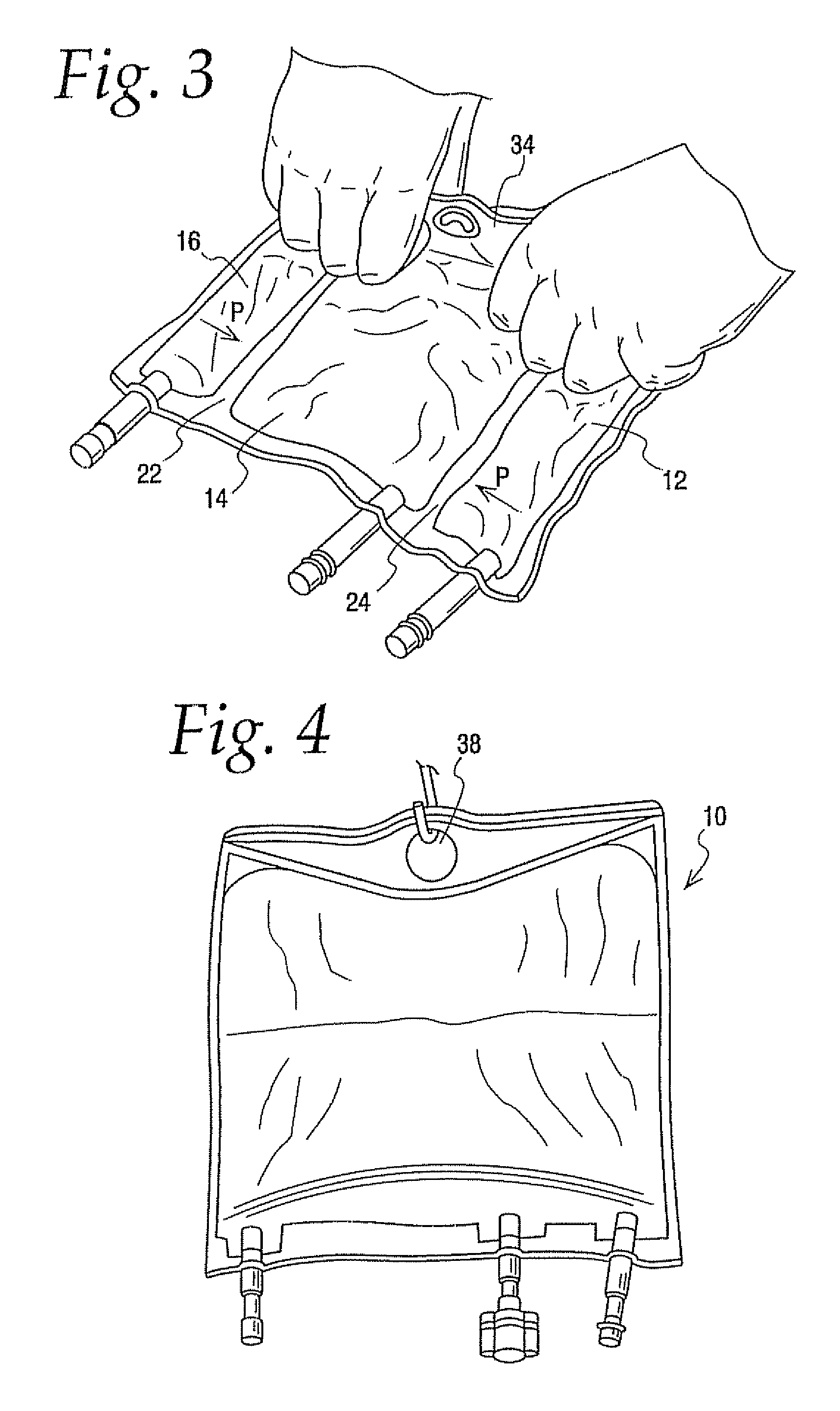
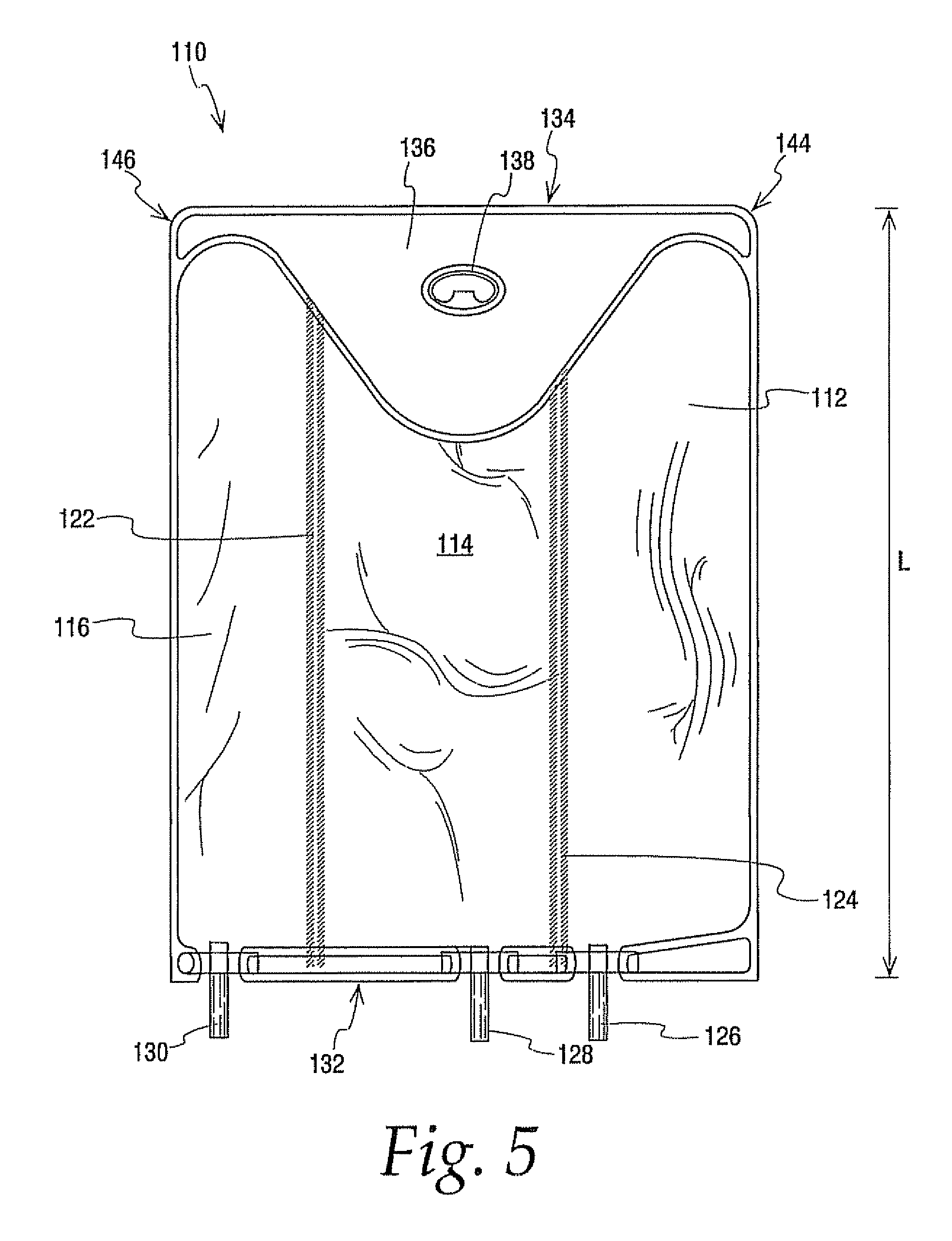
A medical product and parenteral formulation are provided. The parenteral formulation is preferably for fluid restricted patients such as pediatric patients and can include amino acids, lipids, carbohydrates and electrolytes. The parenteral formulation can be housed in a multi-chamber container for separately storing a amino acid component, a lipid component and a carbohydrate component to provide a medical product. Frangible barriers preferably peelable seals separate the components and allow for admix to form a just prior to administration. Electrolytes can also be included in one or both of the carbohydrate and amino acid components; preferably the electrolytes are included in the amino acid component and the amino acid component includes cysteine. The multi-chamber container preferably facilitates the selective activation of the peelable seals to permit the admixing of less than all the separately stored components. The medical product can include a parenteral formulation for supplying the mean nutritional requirements for separate patient populations, such as for preterm infants, term to two year olds, and two to eighteen year olds.
Owner:BAXTER INT INC +1
Homogeneous preparations of il-28 and il-29
ActiveUS20070042470A1Improve expression levelIncrease productionPeptide/protein ingredientsAntipyreticMutated proteinCysteine thiolate
Homogeneous preparations of IL-28A, IL-28B, and IL-29 have been produced by mutating one or more of the cysteine residues in the polynucleotide sequences encoding the mature proteins. The cysteine mutant proteins can be shown to either bind to their cognate receptor or exhibit biological activity. One type of biological activity that is shown is an antiviral activity.
Owner:ZYMOGENETICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com