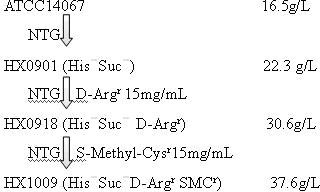Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Cystine knot" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
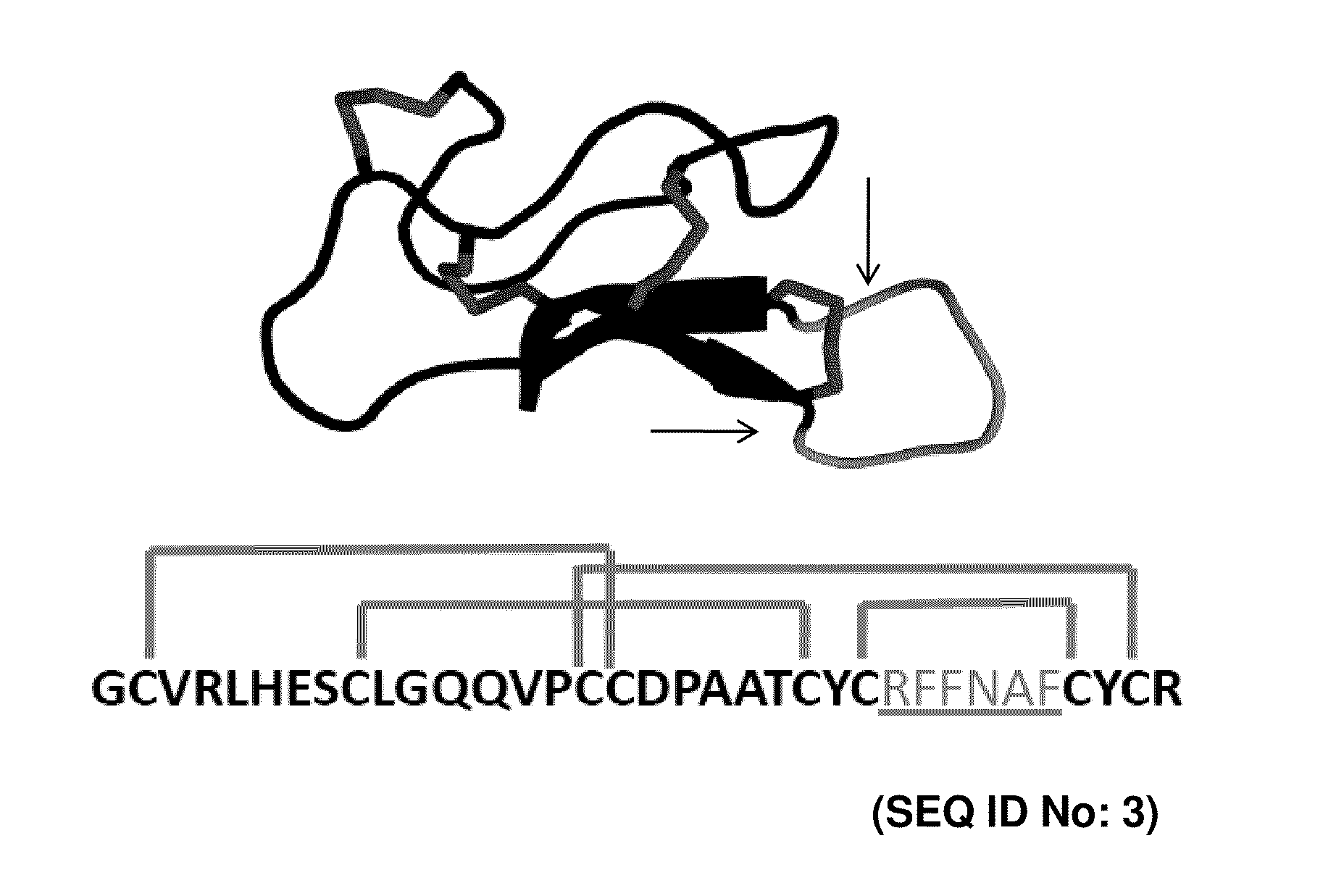
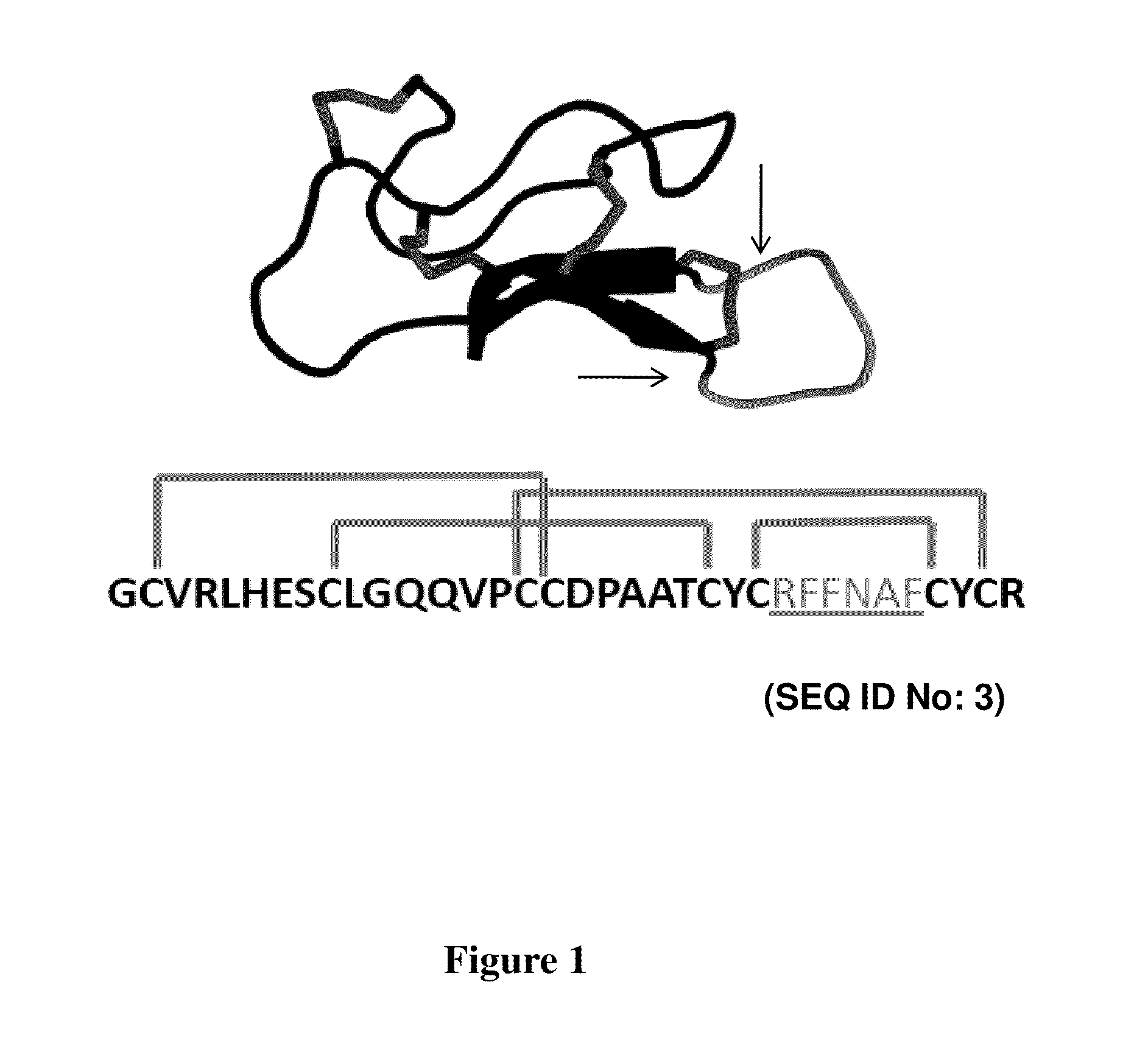
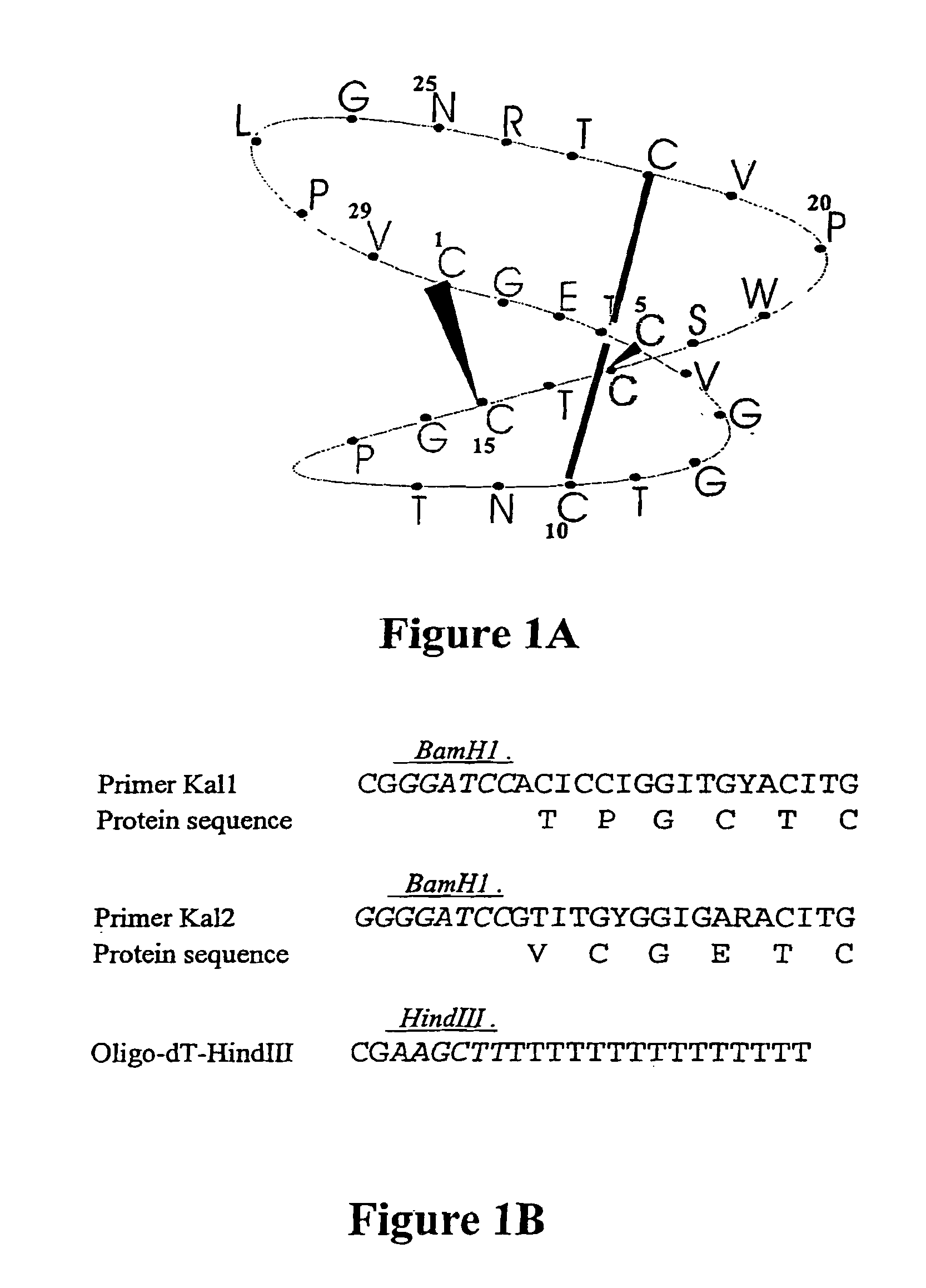
A cystine knot is a protein structural motif containing three disulfide bridges (formed from pairs of cysteine residues). The sections of polypeptide that occur between two of them form a loop through which a third disulfide bond passes, forming a rotaxane substructure. The cystine knot motif stabilizes protein structure and is conserved in proteins across various species. There are three types of cystine knot, which differ in the topology of the disulfide bonds...
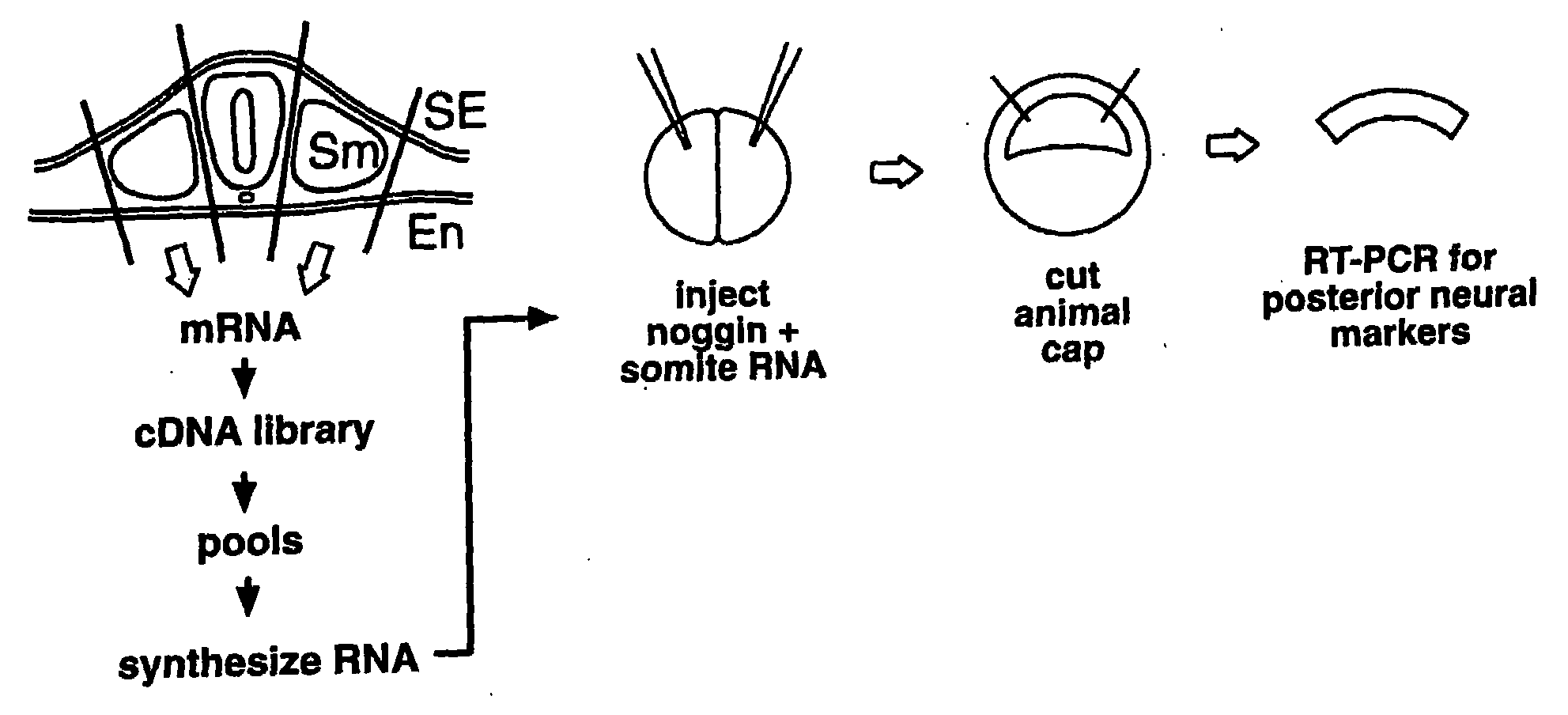
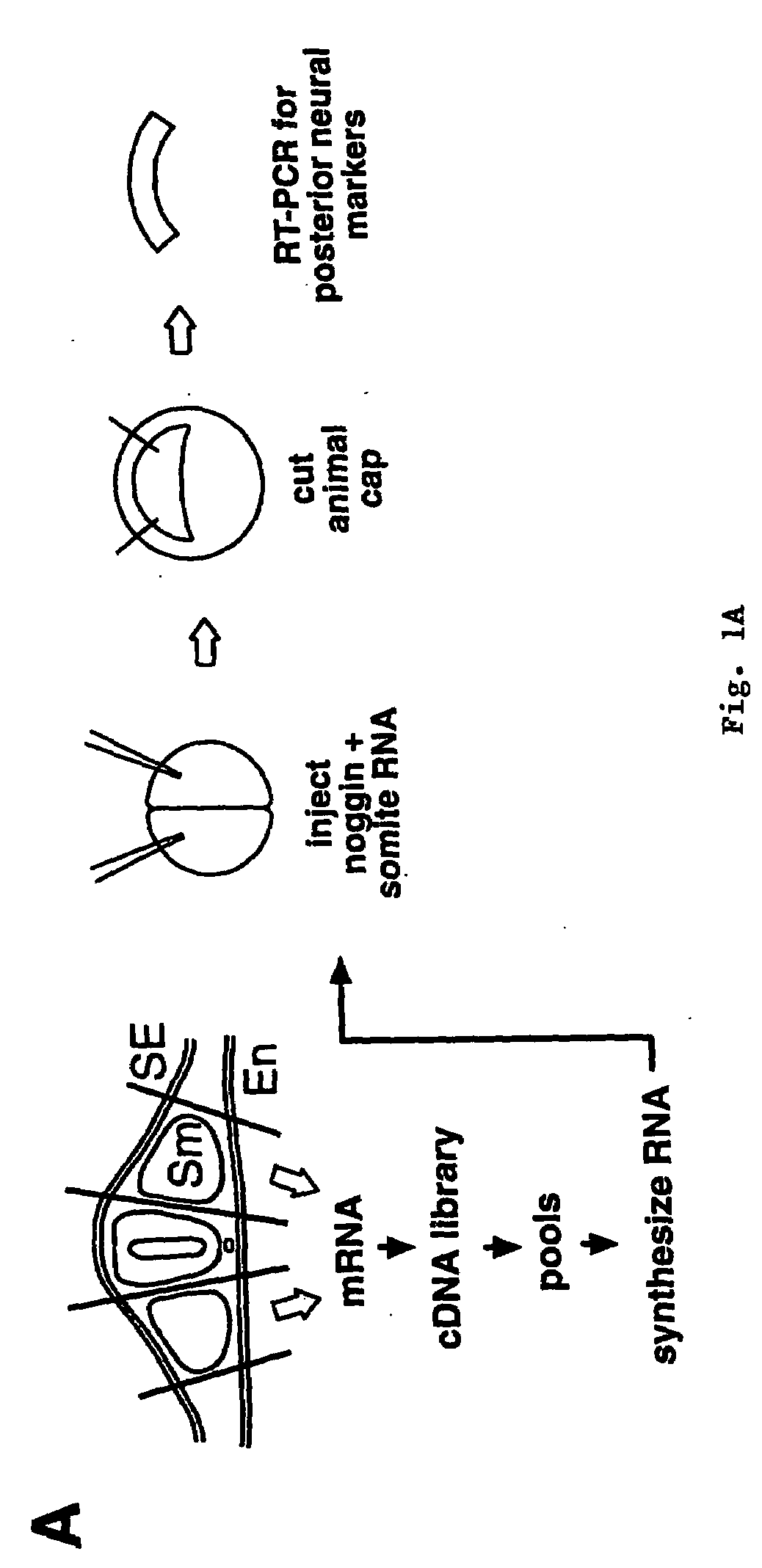
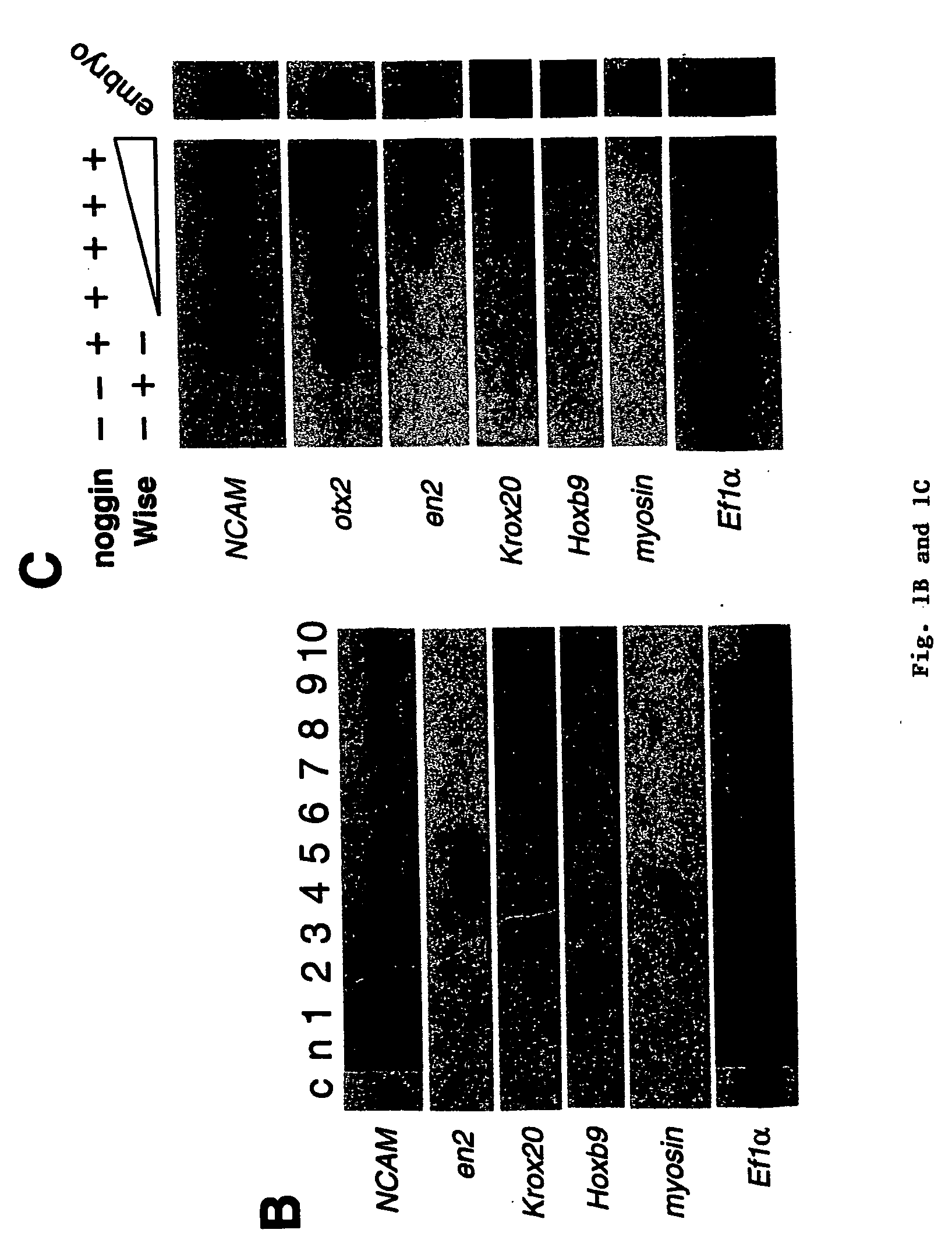
Peptides for treatment and diagnosis of bone diseases
ActiveUS20070292444A1Peptide/protein ingredientsGenetic material ingredientsCystine knotCysteine thiolate
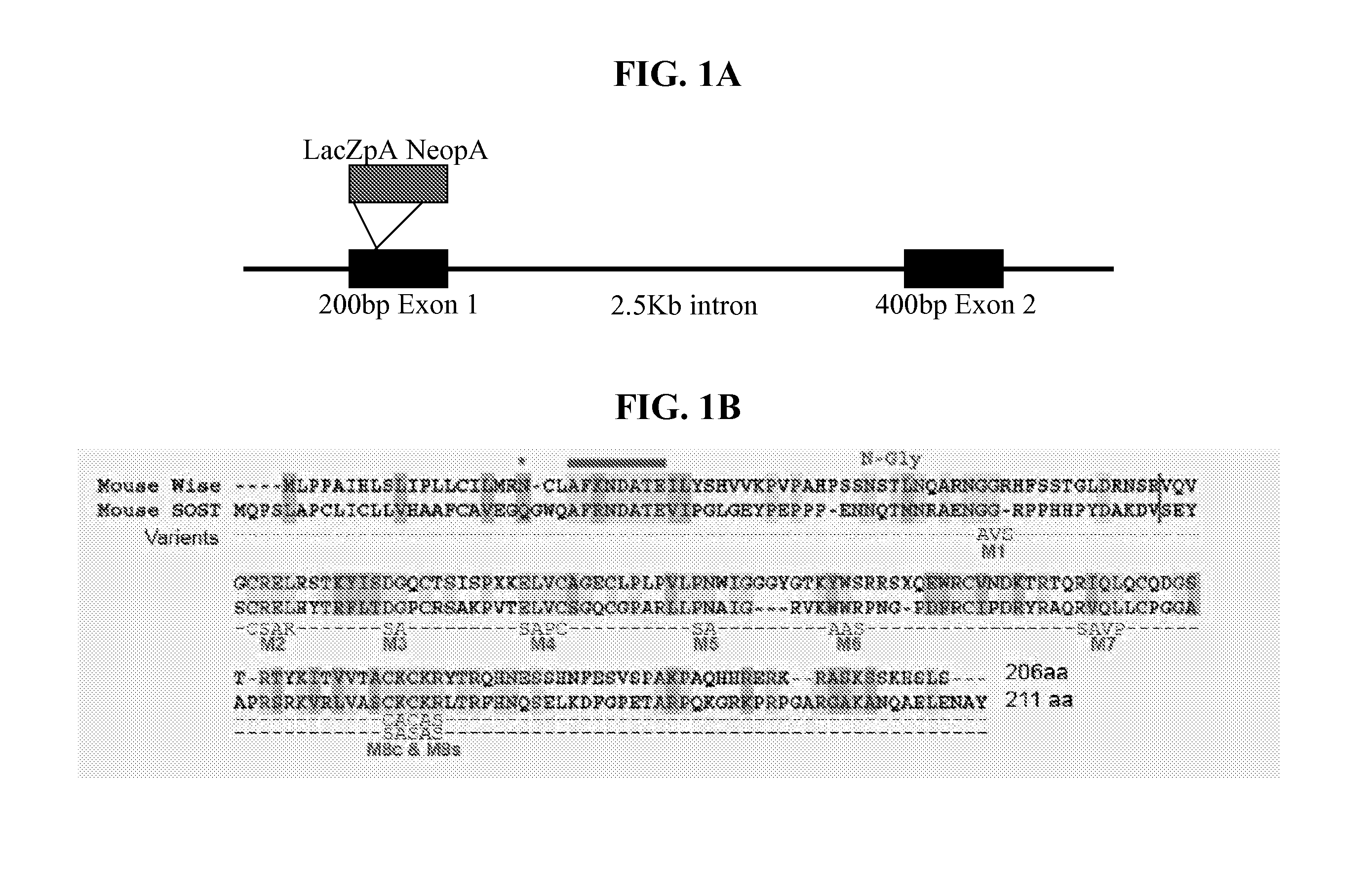
The present invention is directed to isolated polypeptides and antibodies suitable for producing therapeutic preparations, methods, and kits relating to bone deposition. One objective of the present invention is to provide compositions that improve bone deposition. Yet another objective of the present invention is to provide methods and compositions to be utilized in diagnosing bone dysregulation. The therapeutic compositions and methods of the present invention are related to the regulation of Wise, Sost, and closely related sequences. In particular, the nucleic acid sequences and polypeptides include Wise and Sost as well as a family of molecules that express a cysteine knot polypeptide.
Owner:STOWERS INST FOR MEDICAL RES
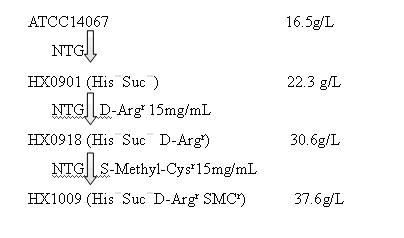
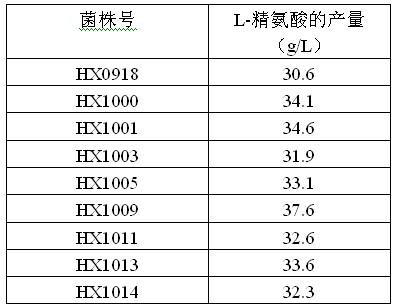
Strain capable of producing L-arginine and method for producing L-arginine by same
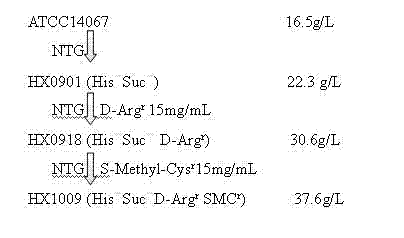
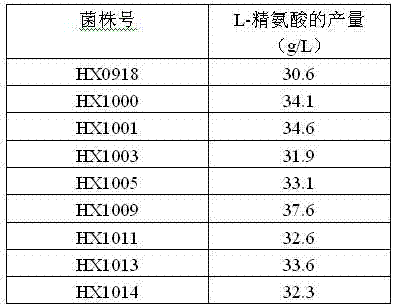
The invention relates to a strain capable of producing L-arginine and a method for producing the L-arginine by the strain and belongs to the technical field of biological engineering. For the strain, Brevibacterium flavum ATCC 14067 is used as a starting strain; nitrosoguanidine is adopted to carry out mutagenesis step by step; mutant strains with histidine and succinic acid auxotrophic strains are screened out so as to cut off a competitive metabolic pathway; and mutant strains (His-, Suc-, D-Argr, SMCr) with resistances of arginine structural analogs and cysteine structural analogs are screened out. The strain is named as Brevibacterium flavum HX1009, is preserved in the China general microbiological culture collection center, has the preservation number of CGMCC No.4464 and has the genetic characters of histidine auxotroph His-, succinic acid auxotroph Suc-, D-arginine resistance D-Argr and S-methyl cysteine resistance SMCr for improving the yield of the L-arginine. Under the optimized condition, the L-arginine is produced by fermentation on a fermentation tank with the volume of 5L to 5M3 and the arginine production level achieves 50 to 70g / L.
Owner:FUJIAN GUTIAN PHARMA
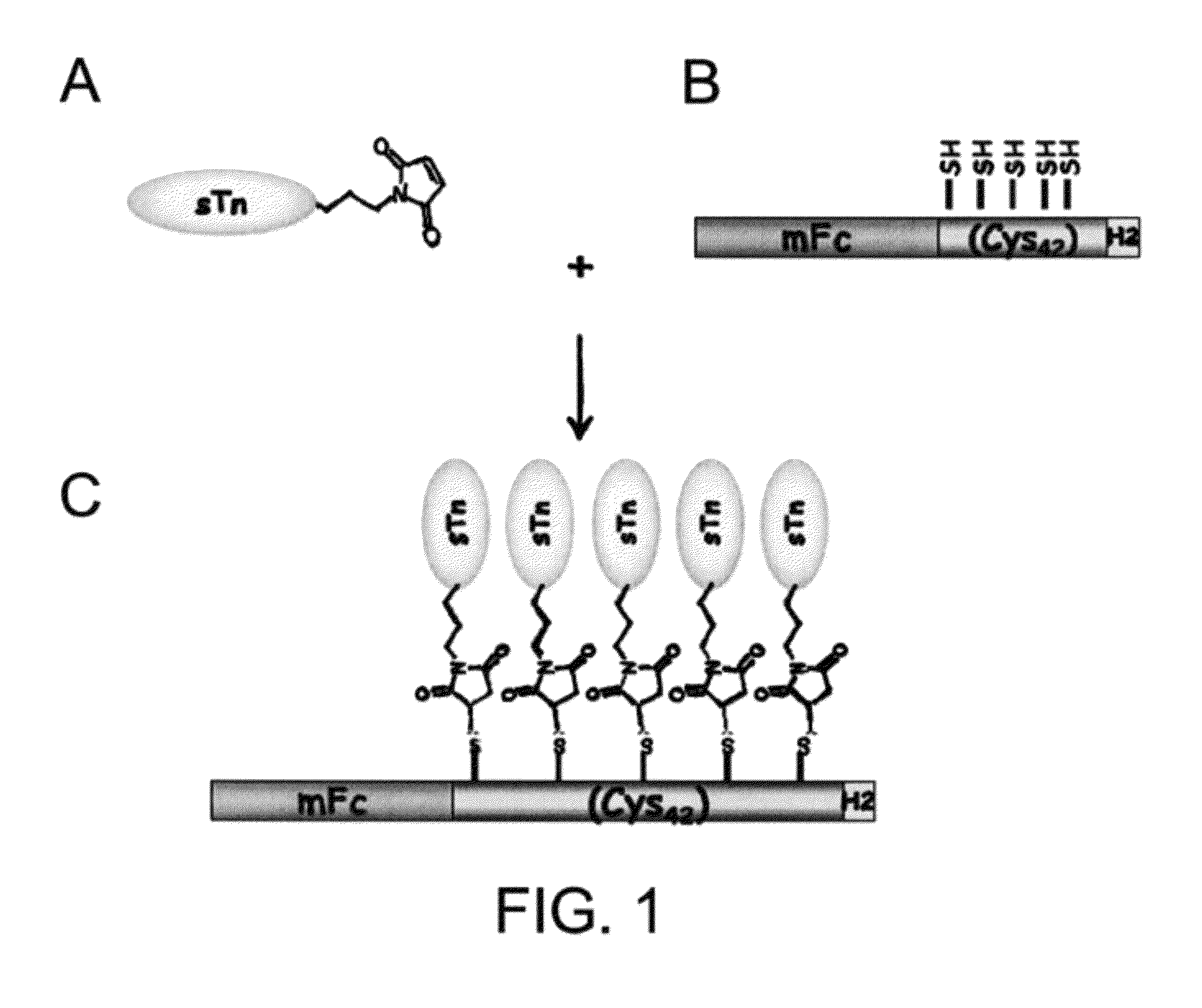
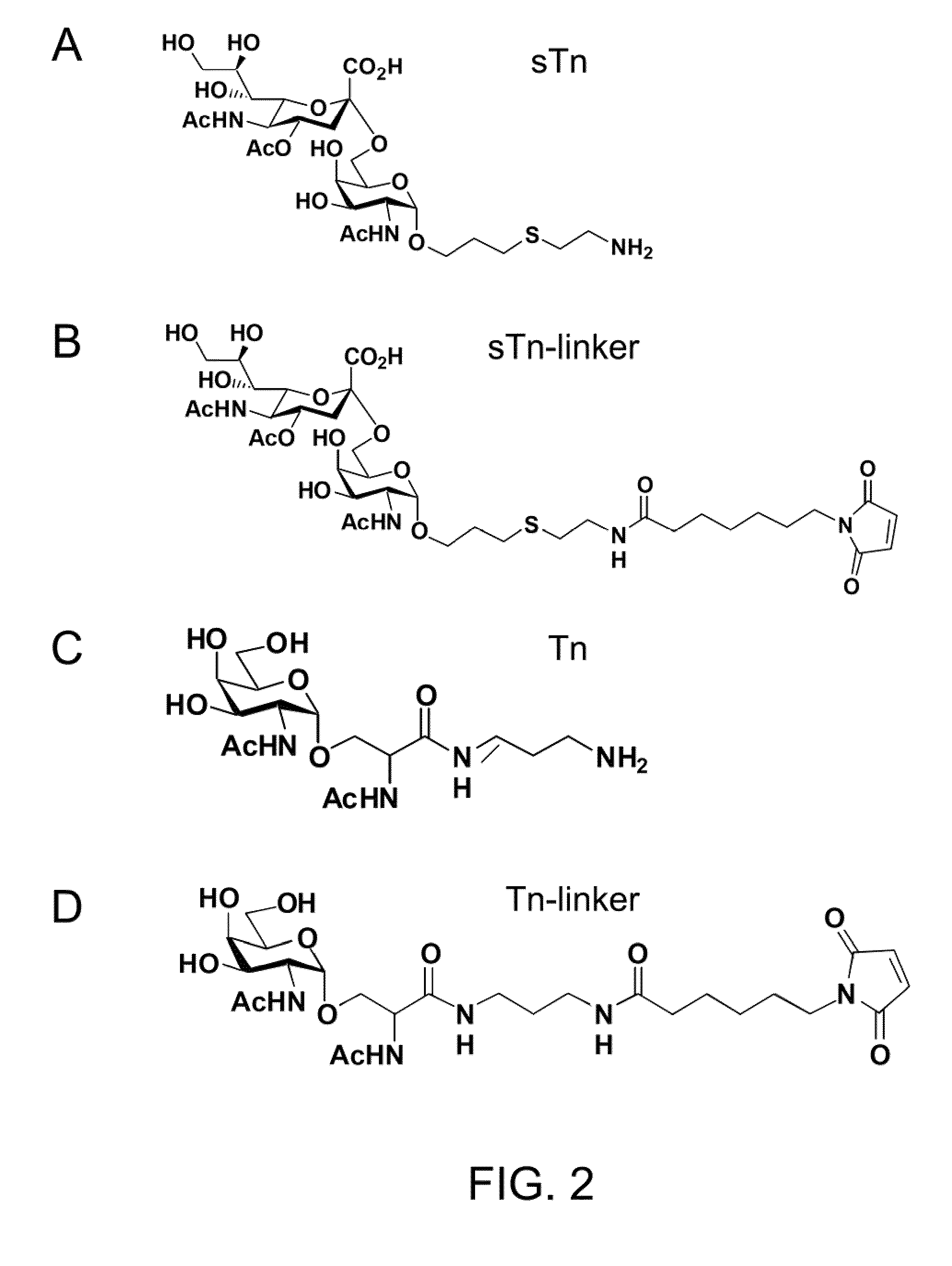
Immunogenic Protein Carrier Containing An Antigen Presenting Cell Binding Domain and A Cysteine-Rich Domain
ActiveUS20090324619A1Improved its antigenecitySugar derivativesPeptide/protein ingredientsCystine knotAdjuvant
A protein carrier containing an antigen presenting cell binding domain and a cysteine-rich domain. Also described herein is an immunoconjugate containing the protein carrier with an antigen conjugated to multiple cysteine residues in the cysteine-rich domain, and an immune composition containing the immunoconjugate and an adjuvant, as well as their uses in eliciting immune responses.
Owner:ACAD SINIC
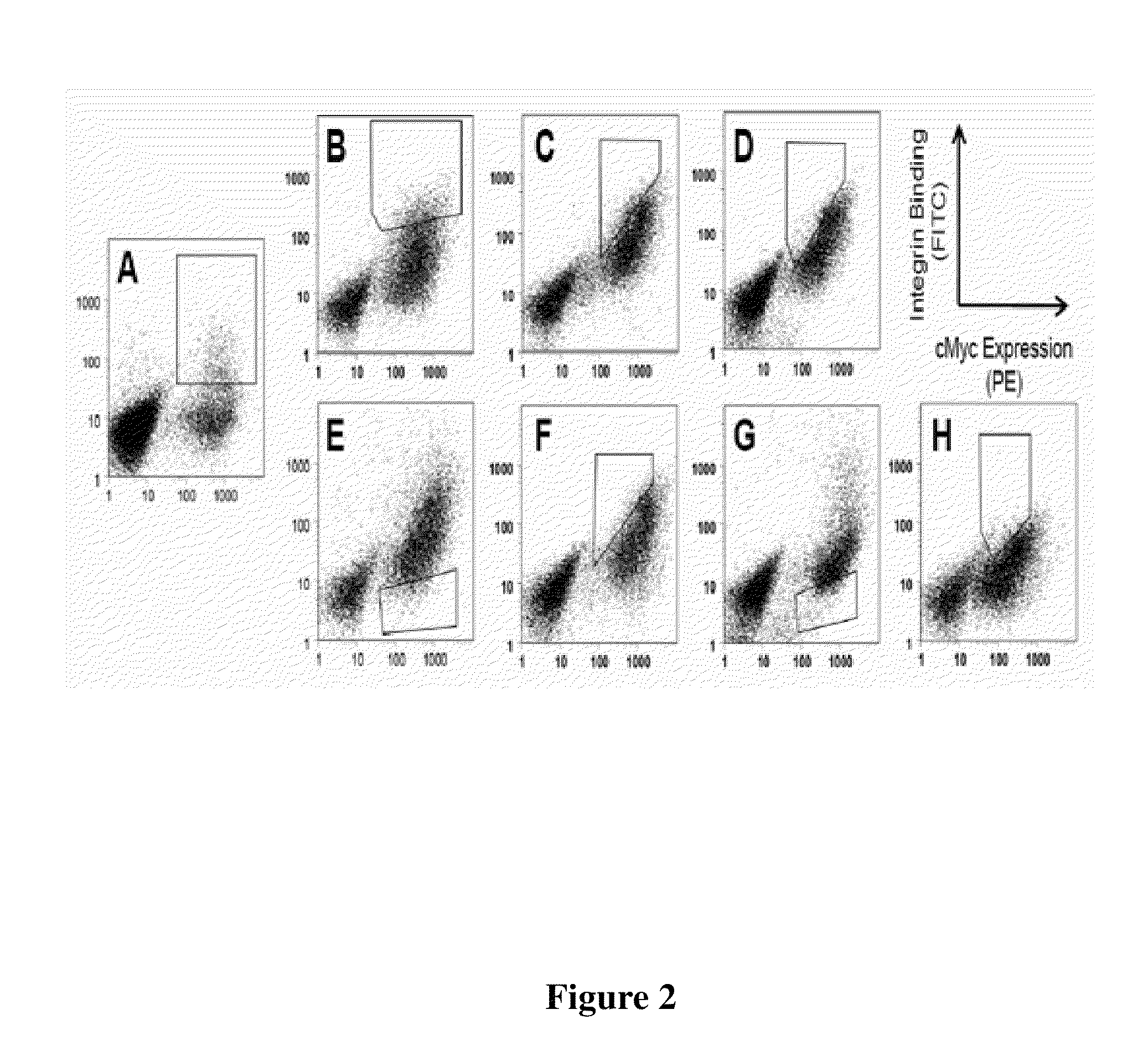
CYSTINE KNOT PEPTIDES BINDING TO ALPHA IIb BETA 3 INTEGRINS AND METHODS OF USE
InactiveUS20110136740A1Inhibits platelet aggregationImprove bindingDepsipeptidesPeptide preparation methodsCystine knotAlpha-IIb/Beta-3
Disclosed are peptides having a cystine knot structural motif and comprising a sequence engineered for specificity against αIIbβ3 integrin, found on platelets, and a method of using the same in anti-thrombotic therapies. The present peptides utilize a cystine knot scaffold derived from modified agouti-related protein or agatoxin, An alternate library screening strategy was used to isolate variants of peptides that selectively bound to αIIbβ3 integrin or to both αIIbβ3 and αVβ3 integrins. Unique consensus sequences were identified within the identified peptides suggesting alternative molecular recognition events that dictate different integrin binding specificities. In addition, the engineered peptides prevented human platelet aggregation in a plasma-based assay and showed high binding affinity for αIIbβ3 integrin.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Phosphorescence chemical sensor for qualitative homocysteine detection and use thereof
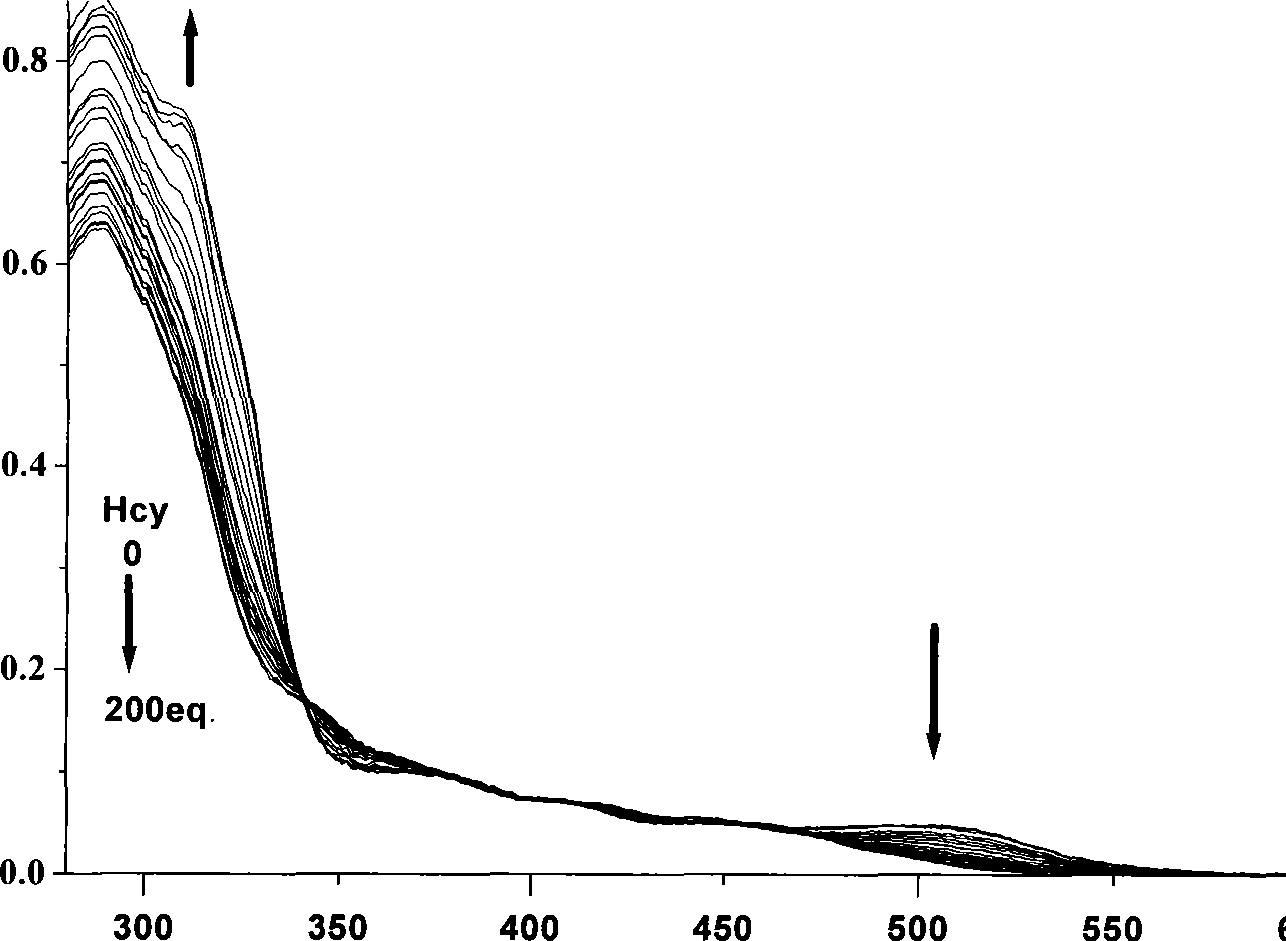
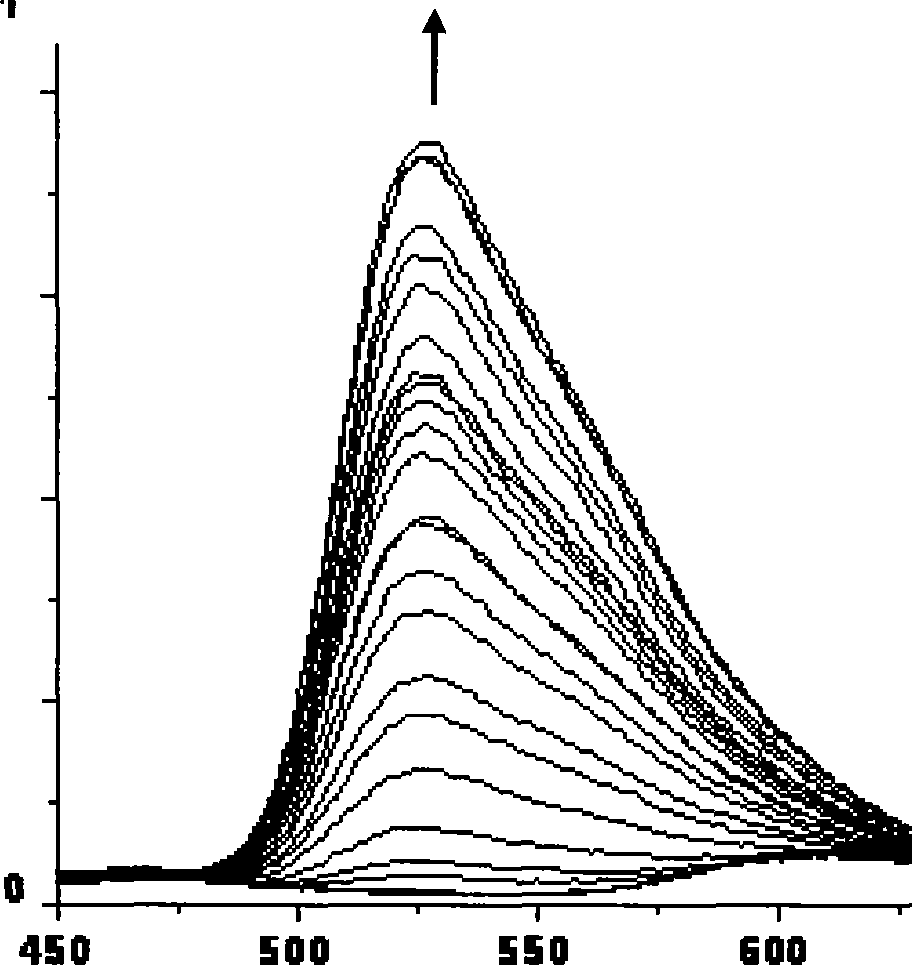
InactiveCN101430280ASpecificNo distractionGroup 8/9/10/18 element organic compoundsFluorescence/phosphorescenceCystine knotIridium
The invention relates to the technical field of chemical sensor, in particular to a phosphorescent chemical sensor for qualitatively detecting homocysteine and an application thereof. Aldehyde group of iridium complex can take cyclization with amido and hydrosulphonyl in the homocysteine to be transformed into hexahydroxy heterocycle and transformed into another compound. The original iridium complex is conjugated with a benzene ring and the aldehyde group and represents as weak red fluorescence while the compound obtained after reaction is not conjugated with the aldehyde group and represents as strong green fluorescence. Ultraviolet and phosphorescent spectrum is used for detecting the result that the complex identifies the homocysteine, thereby showing that the complex has specific response towards the homocysteine, has high sensitivity and can be identified by naked eye, and furthermore, the complex can distinguish cysteine which has a structure very similar as the homocysteine.
Owner:FUDAN UNIV
Anti-inflammatory agents and methods of their use
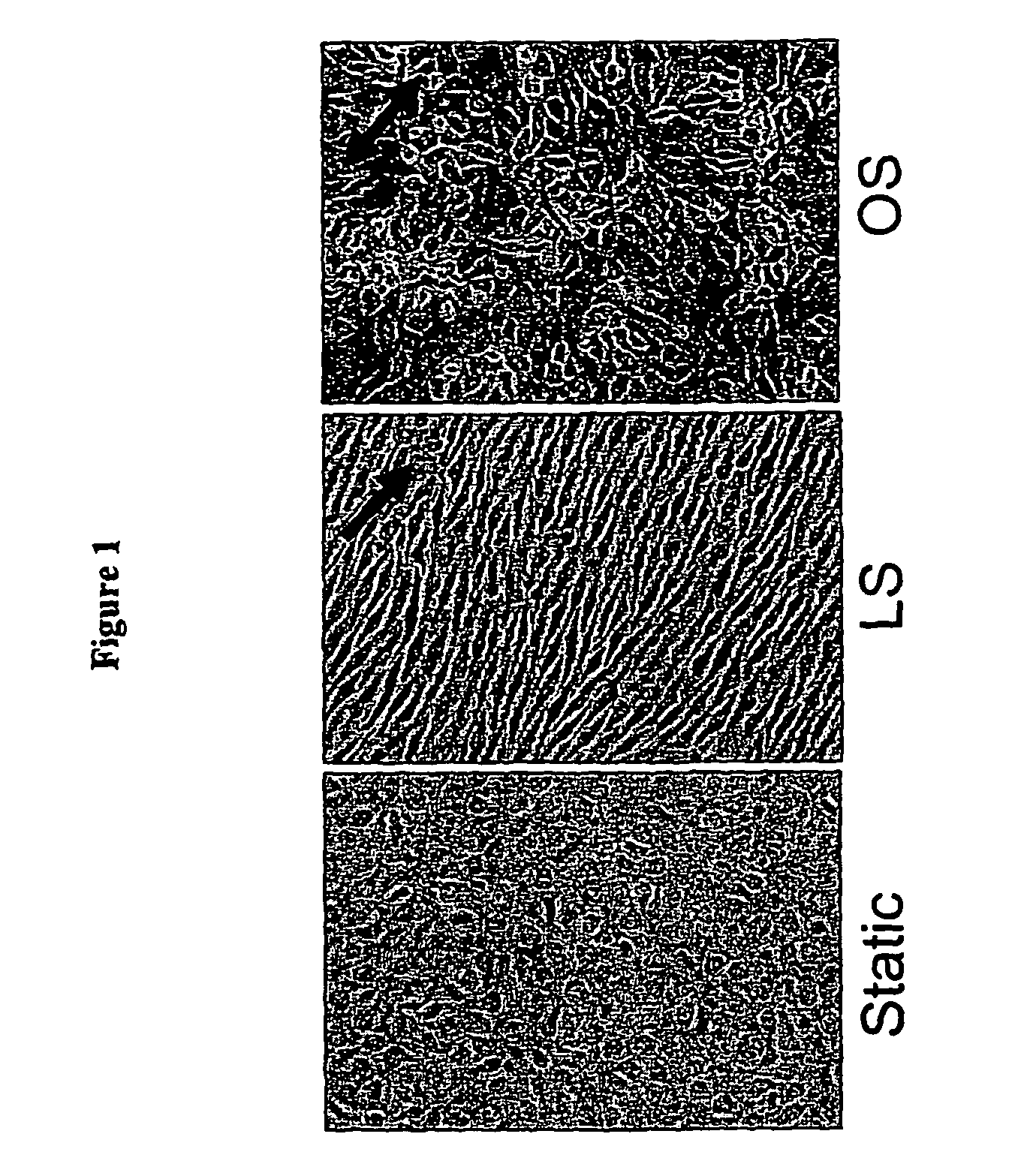
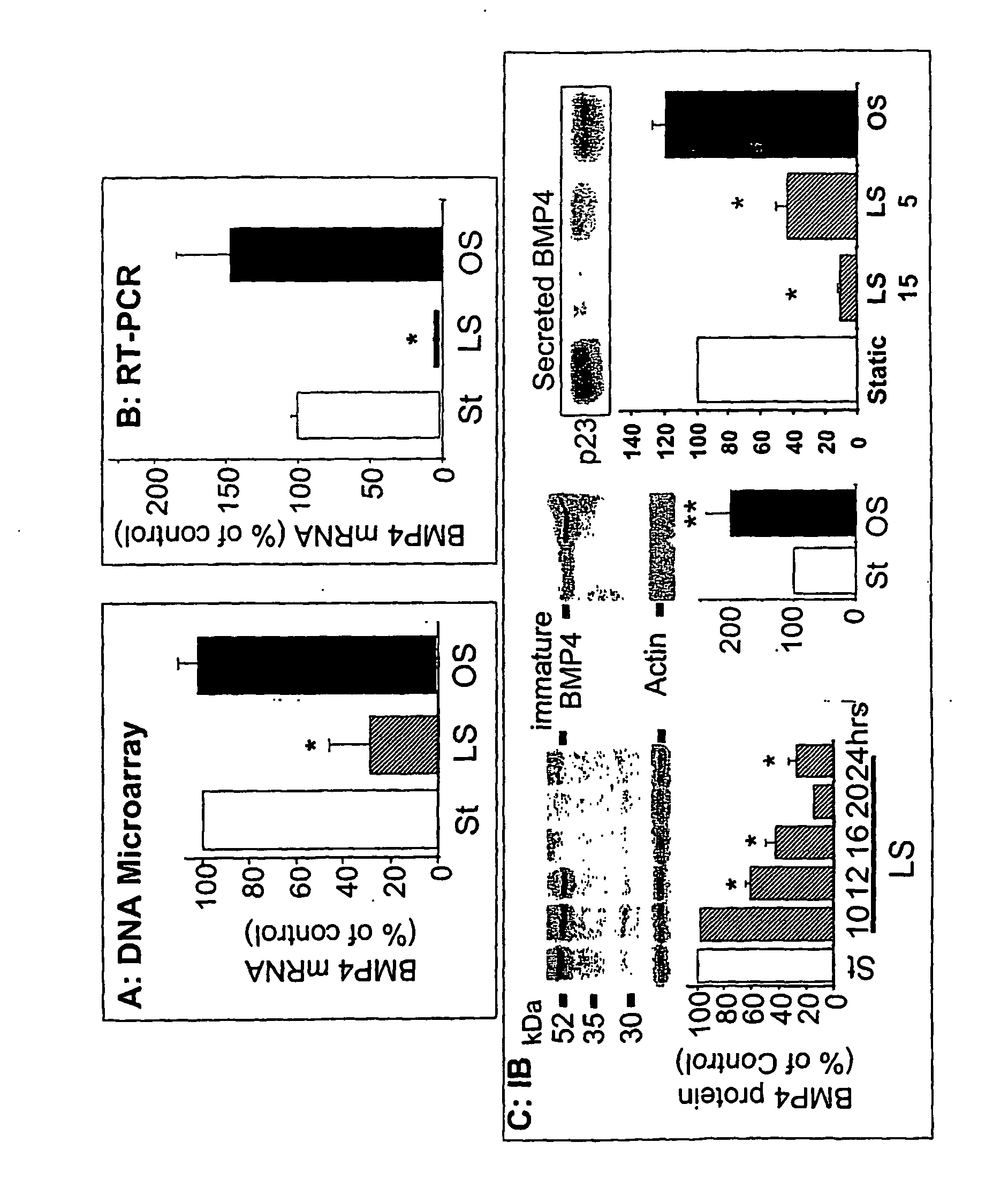
InactiveUS20060276385A1Reduce and prevent vascular inflammationPeptide/protein ingredientsAntipyreticCystine knotVascular inflammation
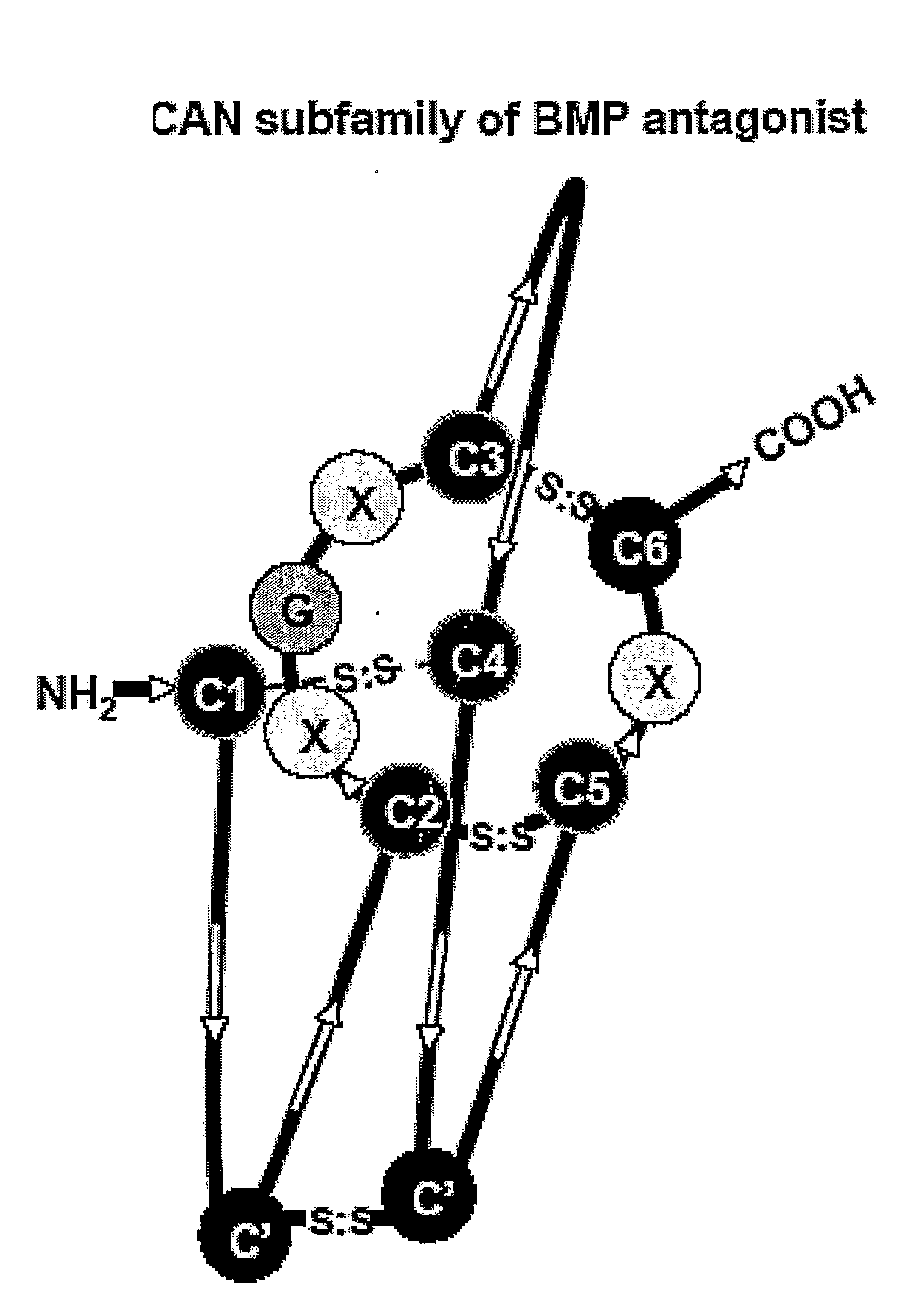
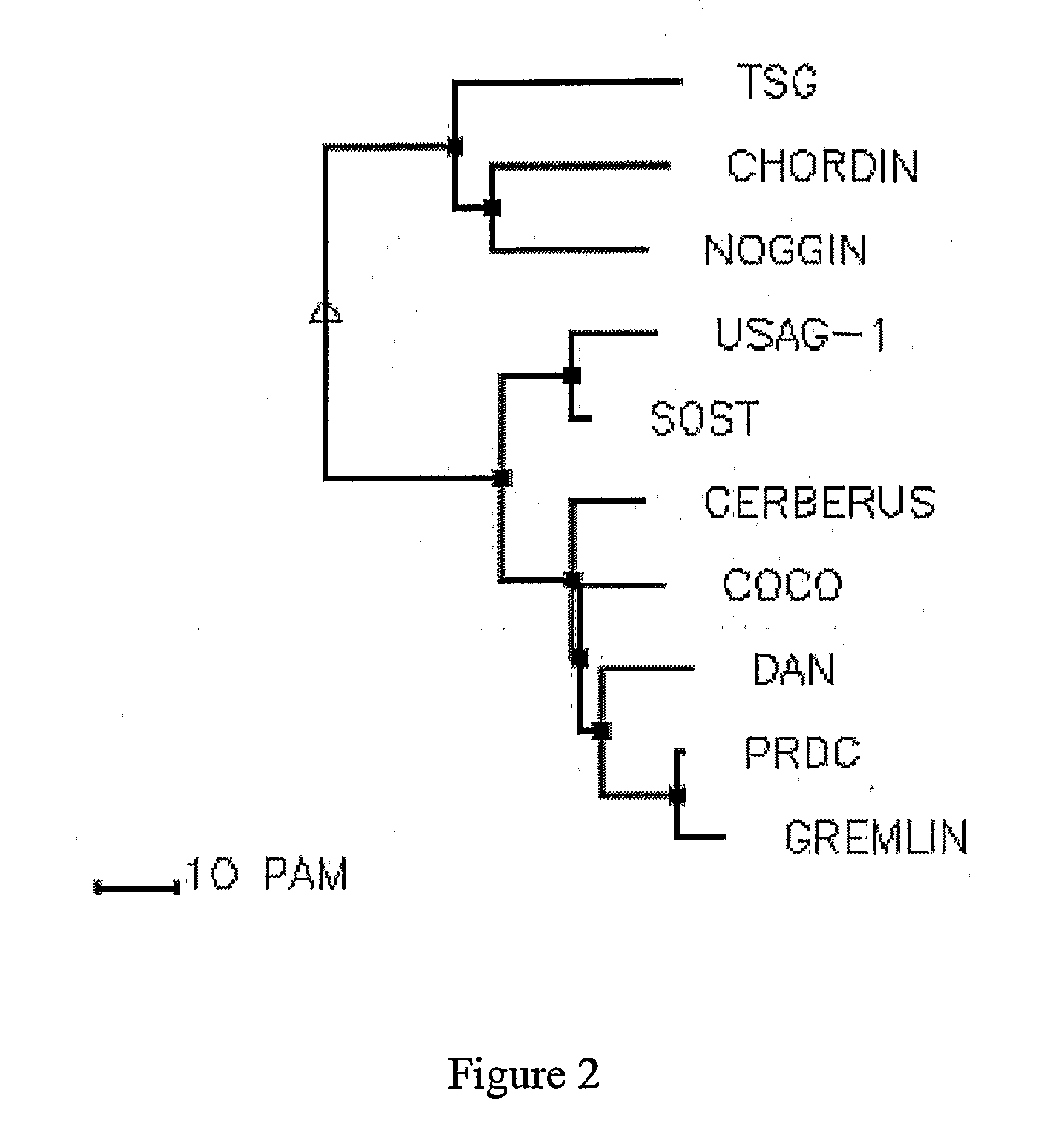
The present disclosure provides compositions and methods for reducing or inhibiting vascular inflammation, for example inflammation resulting from unstable blood flow conditions such as oscillatory shear. Representative compositions include an antagonist of BMPs, for example BMP4, or BMP receptors, for example BMPR-I and / or BMPR-II, in an amount sufficient to for inhibiting or reducing vascular inflammation by interfering with binding of bone morphogenic protein or a fragment thereof to bone morphogenic protein receptors. Exemplary BMP antagonists include polypeptides having an eight-, nine-, or ten-membered ring cystine knot structure. Representative BMP antagonists include, but are not limited to the CAN family of proteins, the chordin family that includes chordin and ventroptin, and noggin.
Owner:JO HANJOONG
Peptides as well as pharmaceutical composition and application thereof
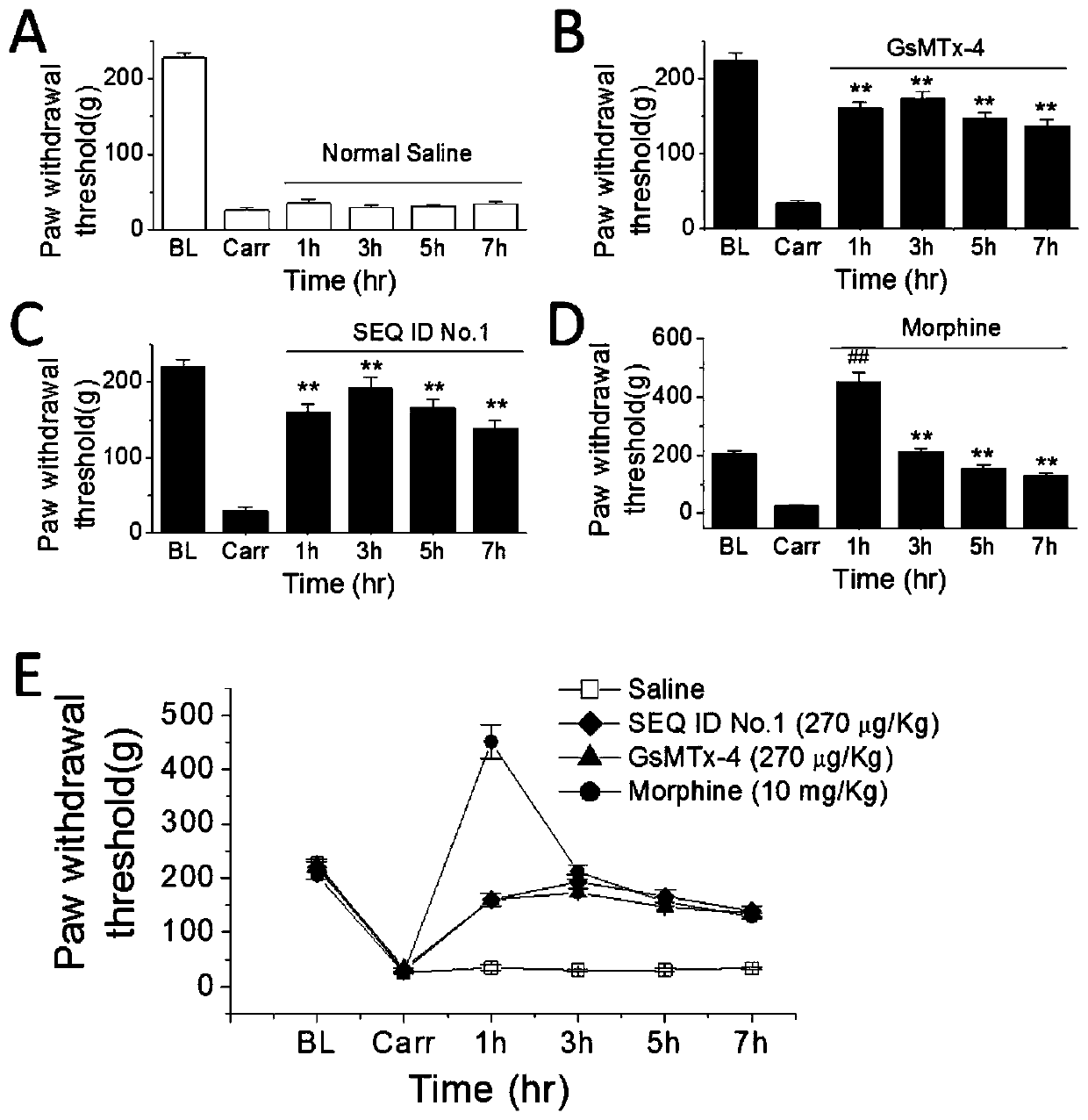
ActiveCN106632603AAnalgesic effect hasPeptide/protein ingredientsAntipyreticCystine knotPharmacophore
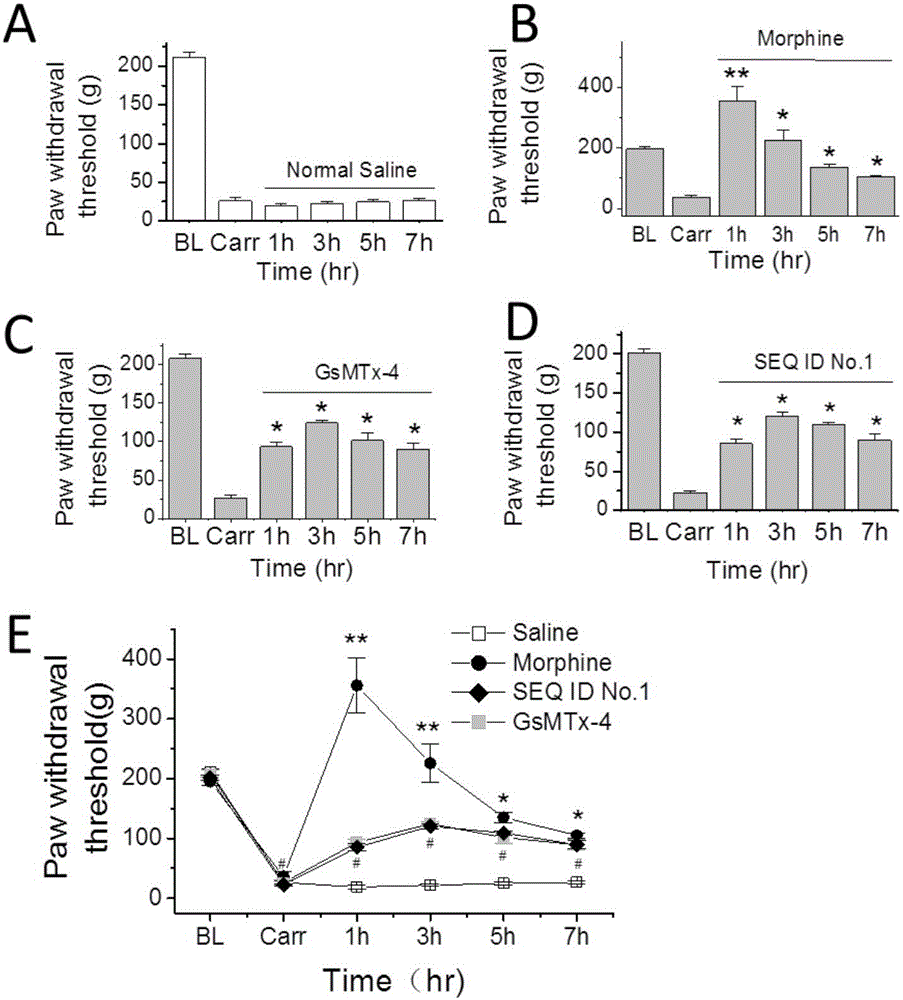
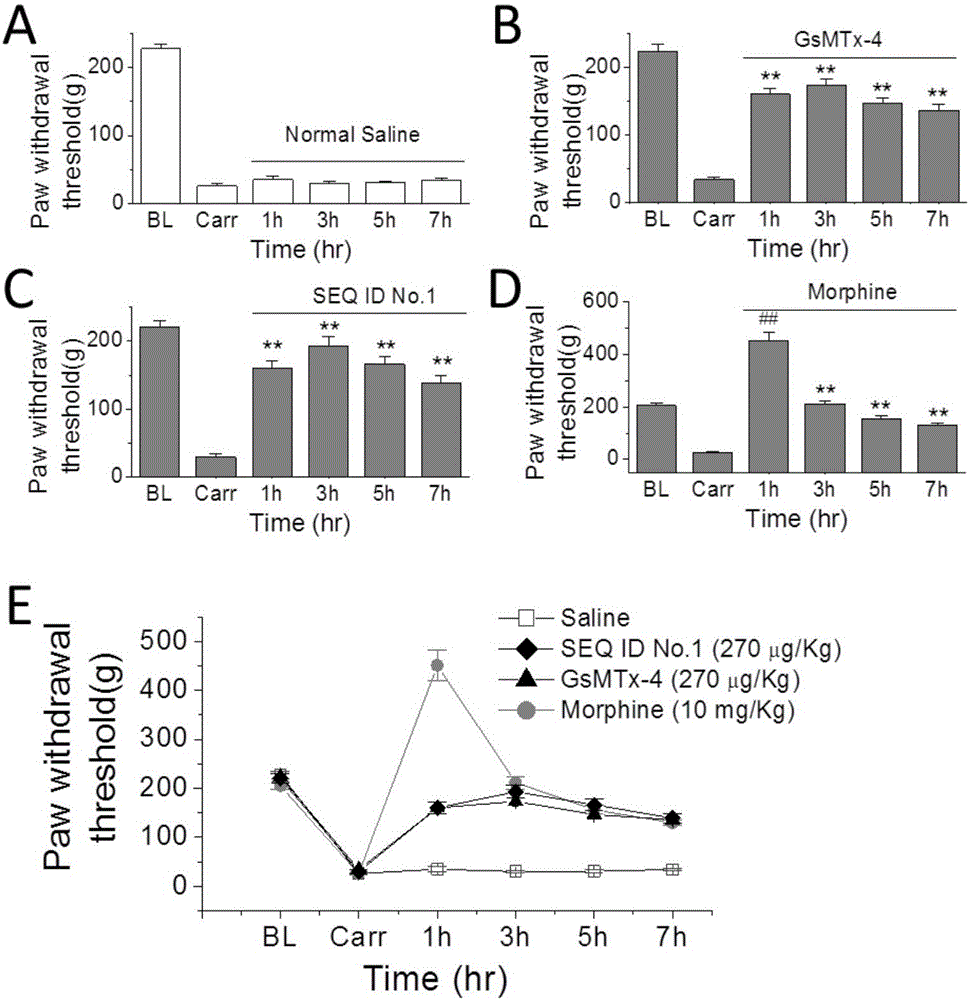
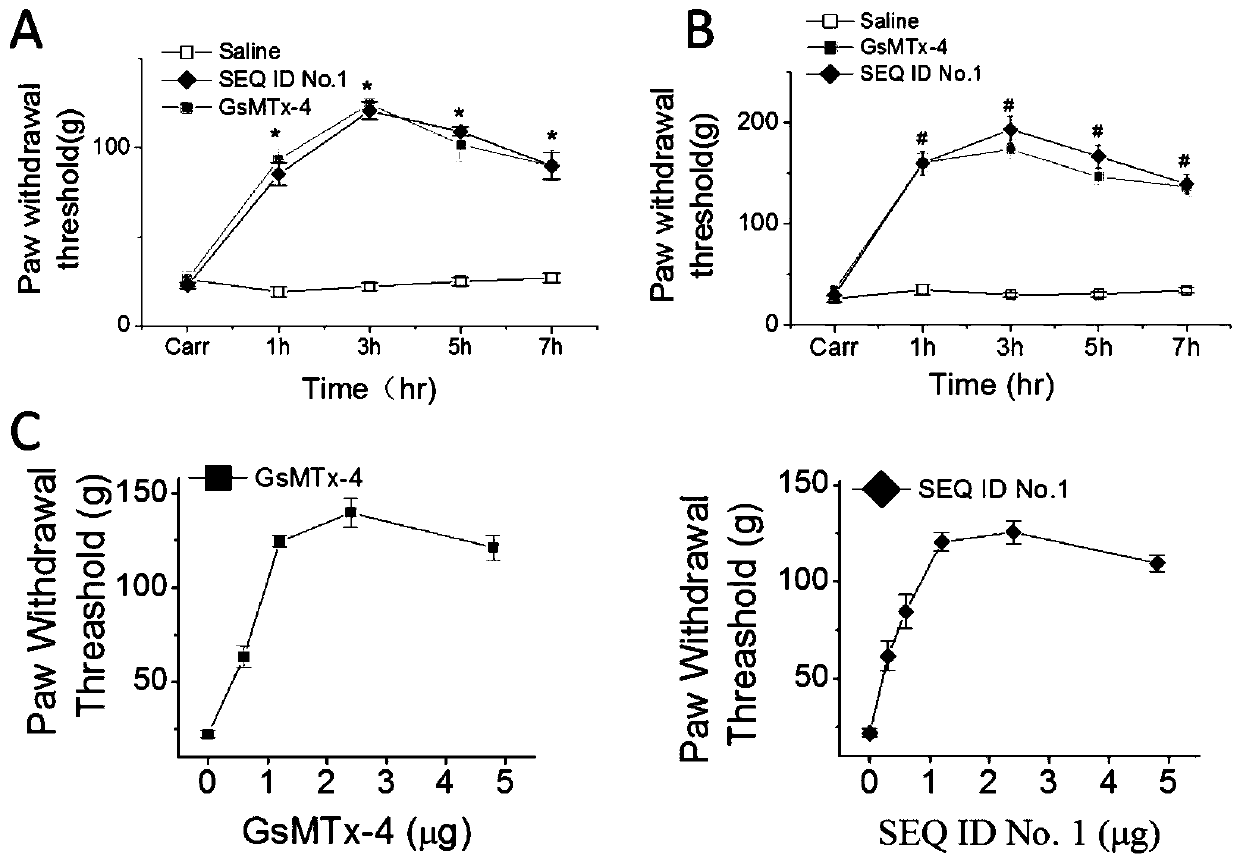
The invention provides a group of peptides as well as a pharmaceutical composition and application thereof. The peptides are as shown in sequence tables; the peptides have amino acid sequences with an analgesic effect, or are pharmacologically acceptable salts of the peptides. A ring is formed by two cysteines at the head end and the tail end of each peptide. A pharmacophore (a structural domain) which has abirritation in the GsMTx-4 peptide is determined by comparing multiple inhibitor cystein knot (ICK)-containing peptide sequences such as GsMTx-4 and GsMTX-2 and carrying out behavior test on a rat pain model.
Owner:唐琼瑶 +1
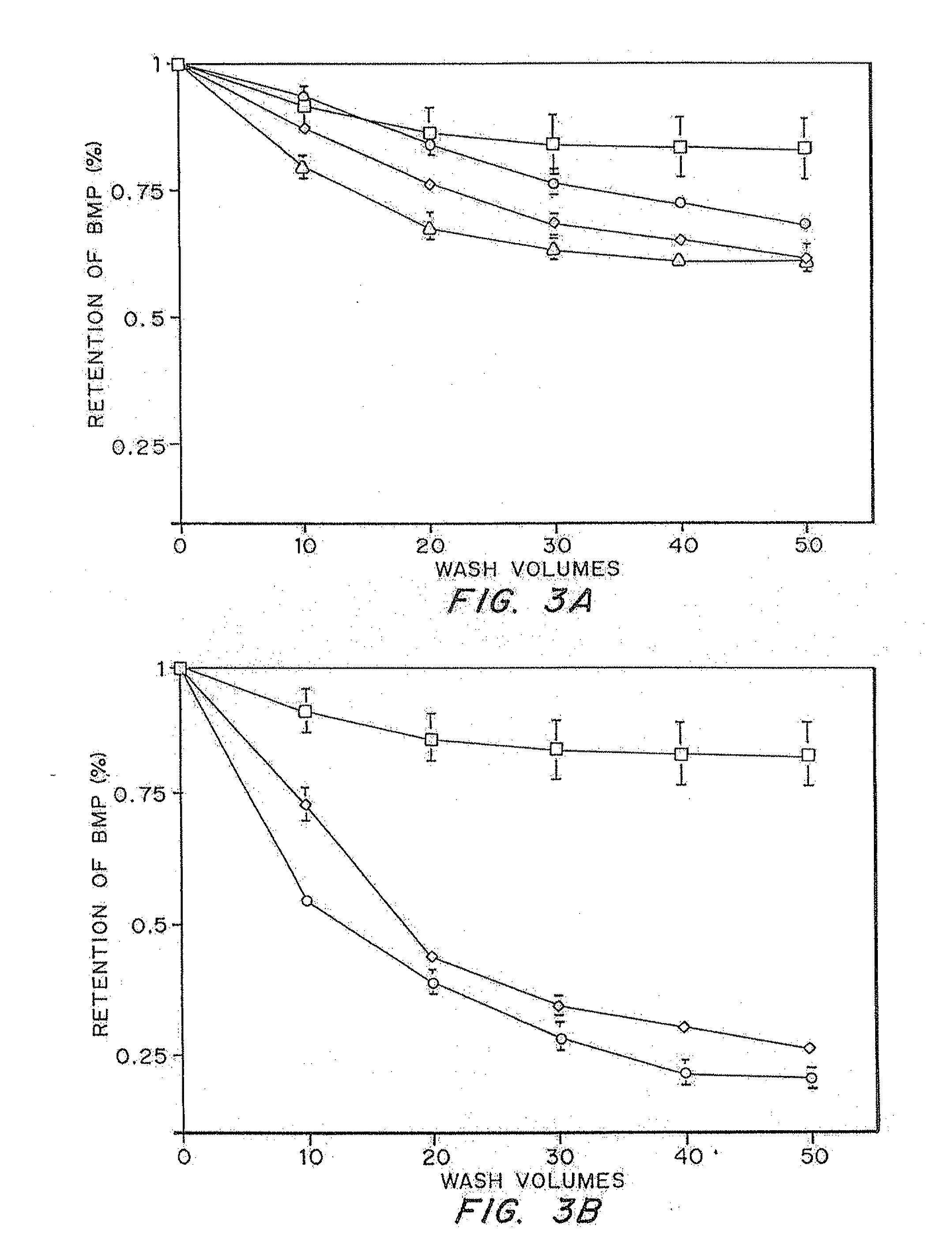
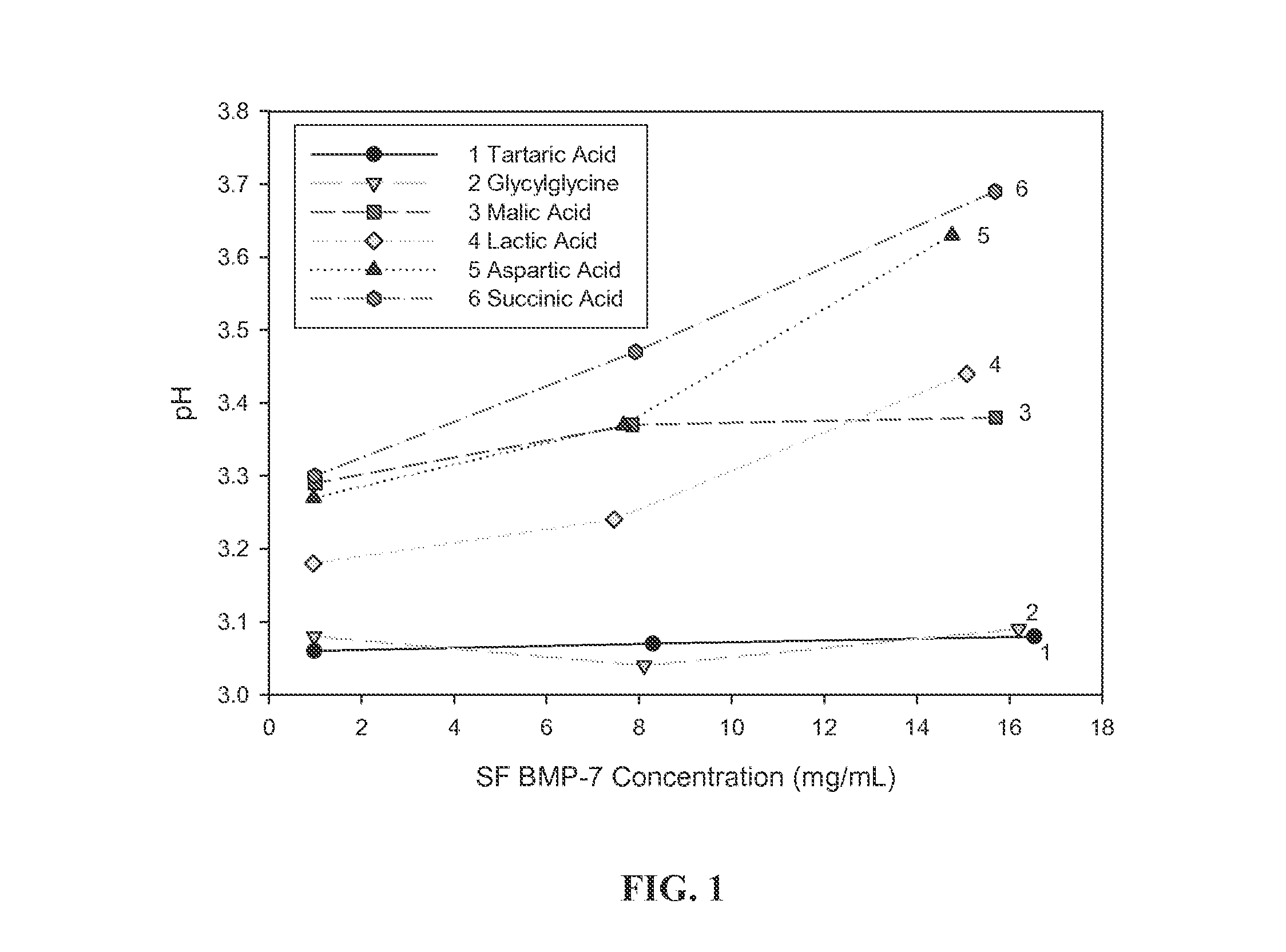
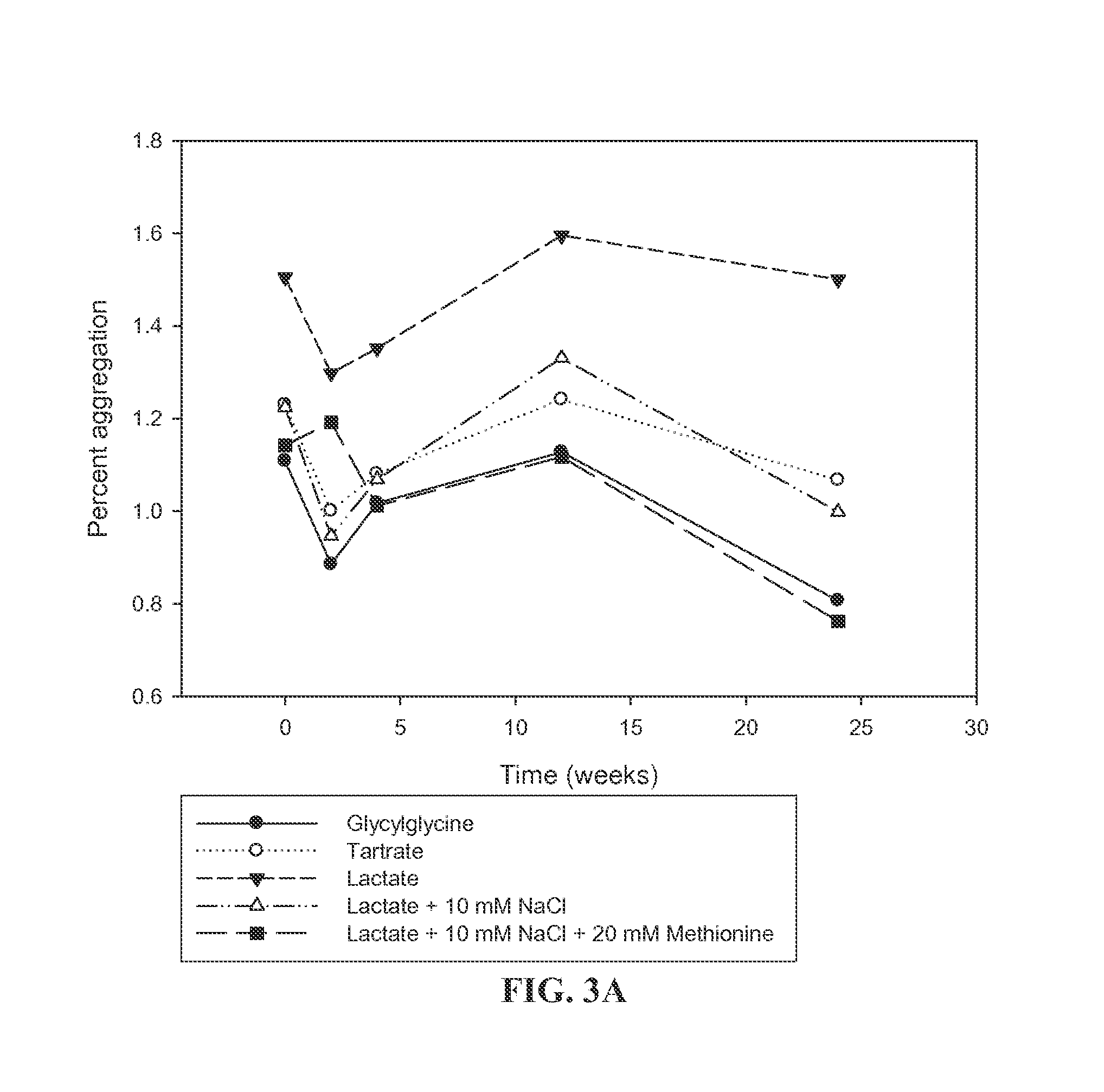
Buffers for Controlling the pH of Bone Morphogenetic Proteins
InactiveUS20110224410A1Good formulation stabilityIncrease ionic strengthPeptide/protein ingredientsSkeletal disorderCystine knotCrystallography
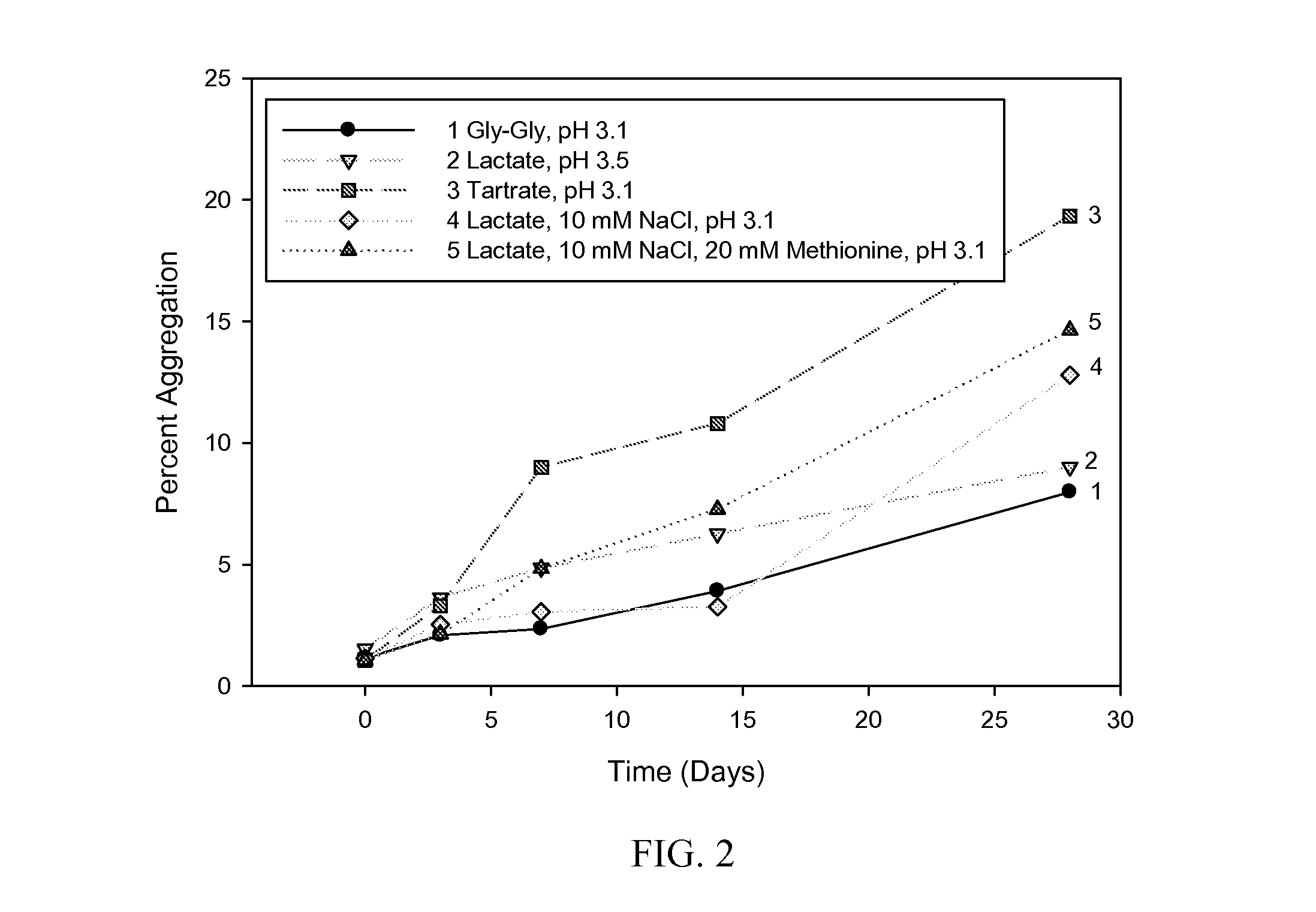
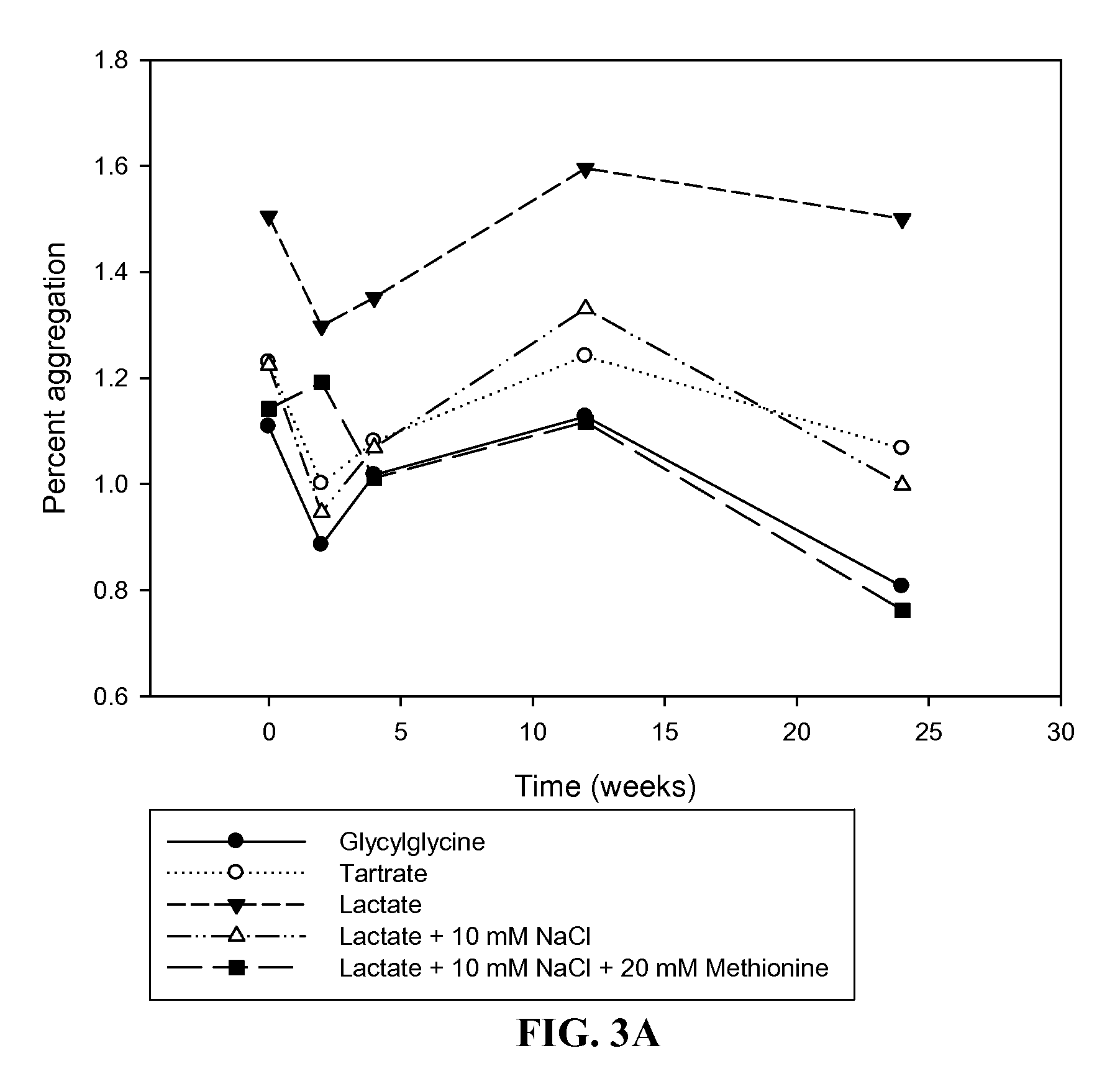
The present invention provides formulations of cysteine knot proteins, including TGF-β superfamily proteins and bone morphogenic proteins that are pH stabilized. In particular, the present invention relates to the observation that certain buffers enhance the stability of cysteine knot proteins, including TGF-β superfamily proteins and bone morphogenic proteins. In particular, disclosed herein are liquid and lyophilized formulations prepared with a glycylglycine and tartaric acid buffers to stabilize the pH of the formulation.
Owner:STRYKER CORP
Medicinal products incorporating bound organosulfur groups
Nutraceutical and dietary supplement formulations for delivering bioavailable thiols to a host, comprising certain organosulfur radicals, such as the allyl mercapto radical, bound to larger molecules such as proteins, resulting in the formation in the host's body of various allium-related compounds such as allicin. The formulations are produced by treating an ingestible material comprising a cysteine-containing protein with a thiol, disulfide, mixed disulfide, thiosufinate or mixed thiosulfinate so as to cause thiol residues to become disulfide bonded to cysteines contained in the protein.
Owner:ALLIUM VITALS
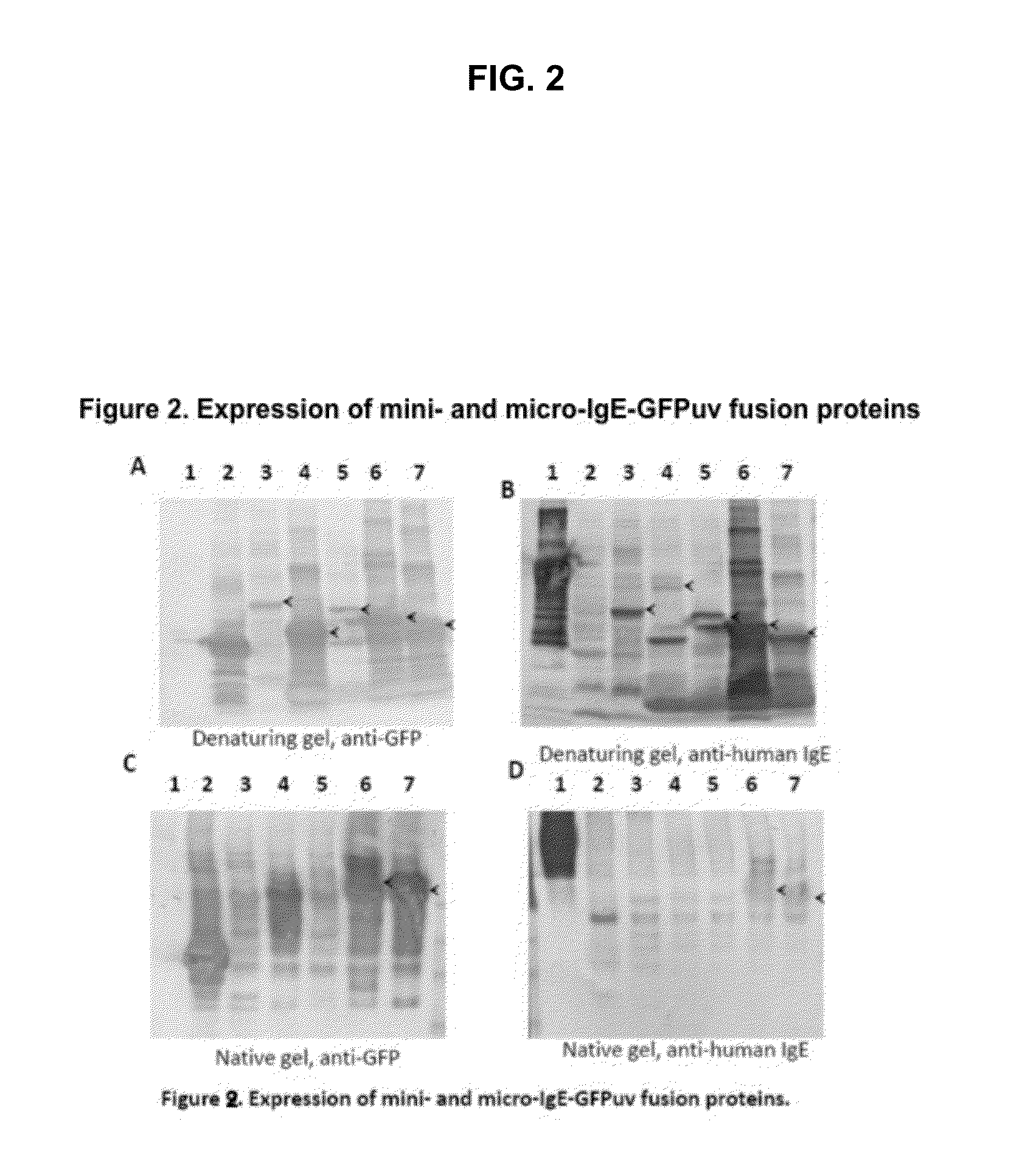
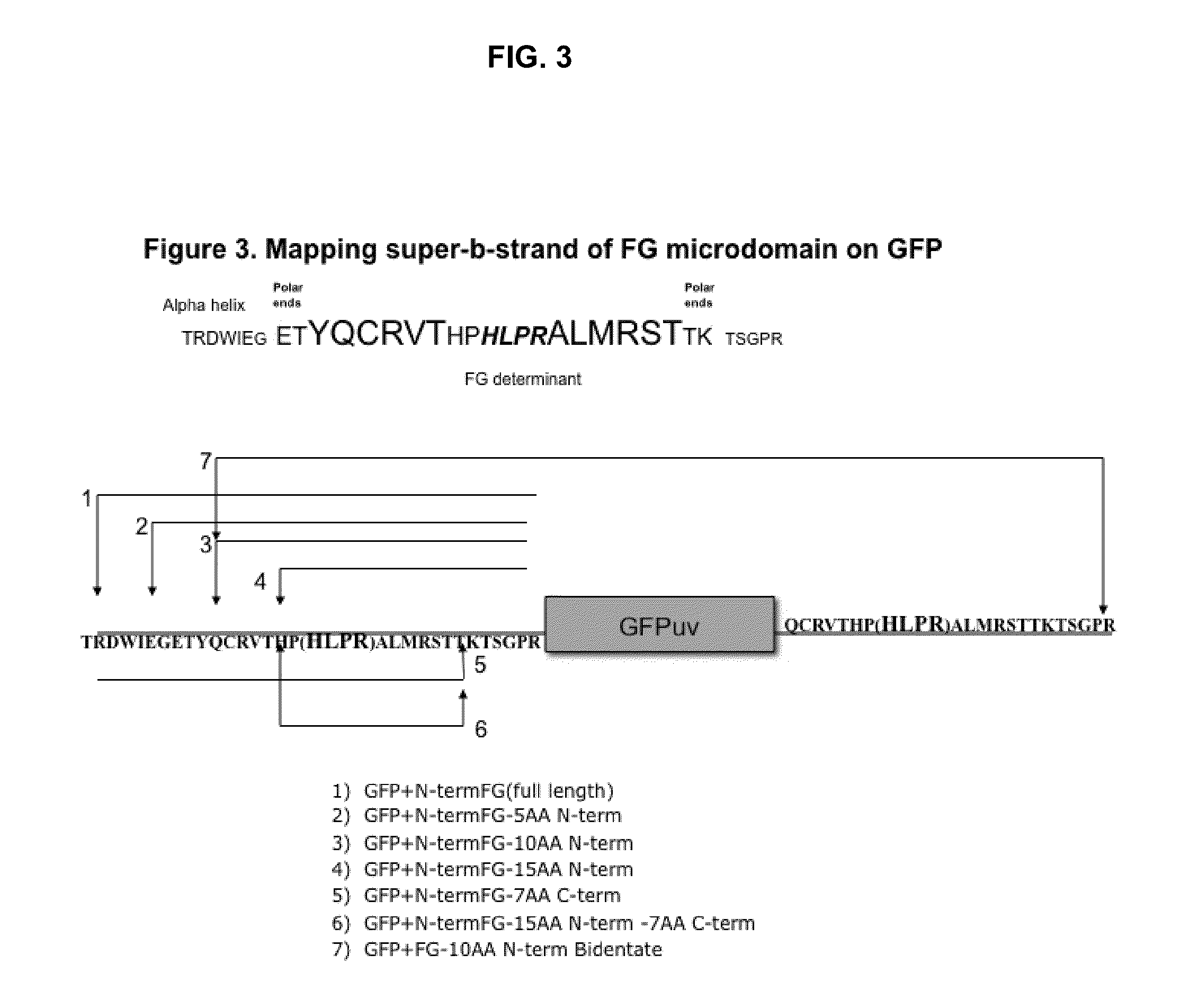
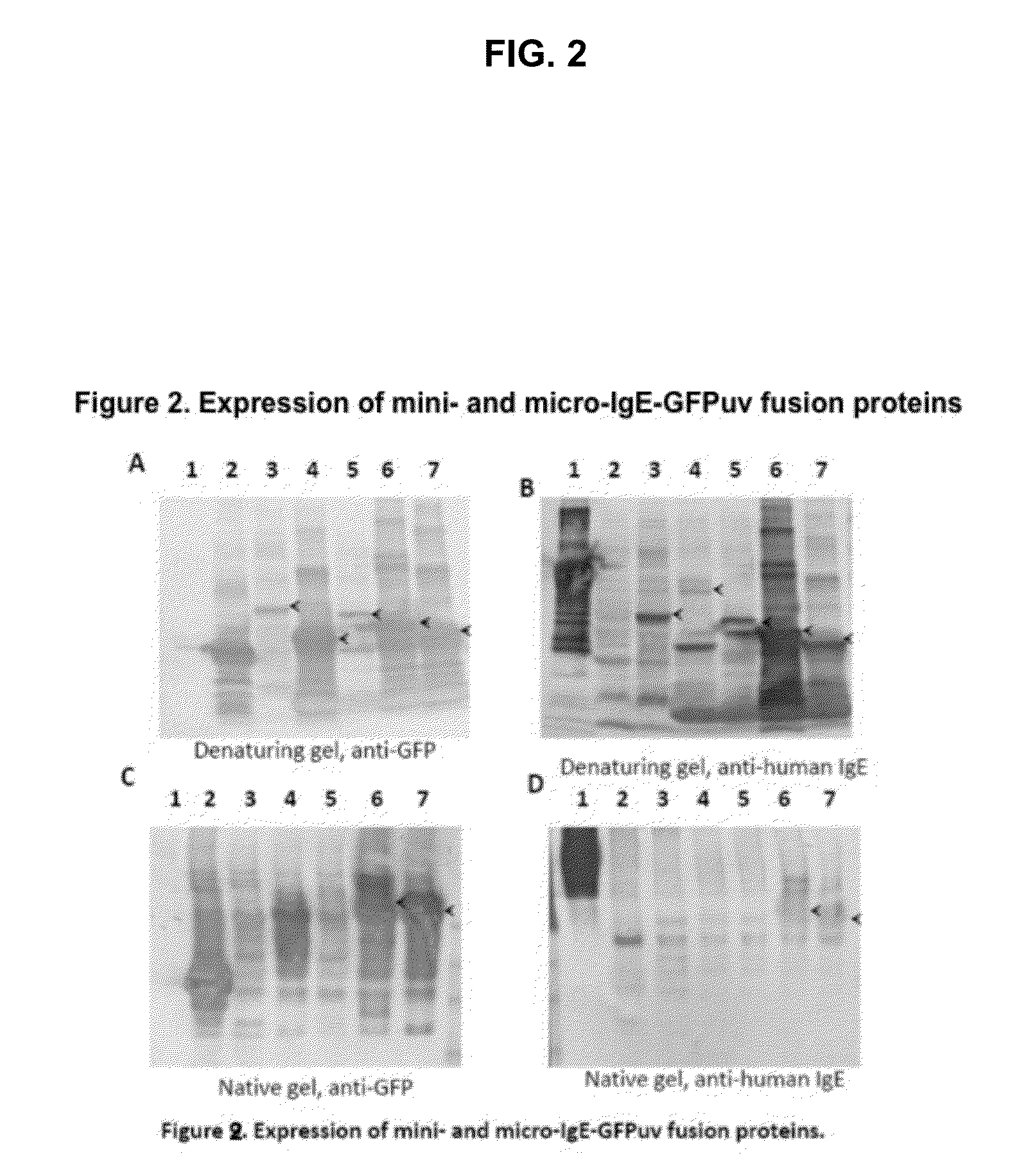
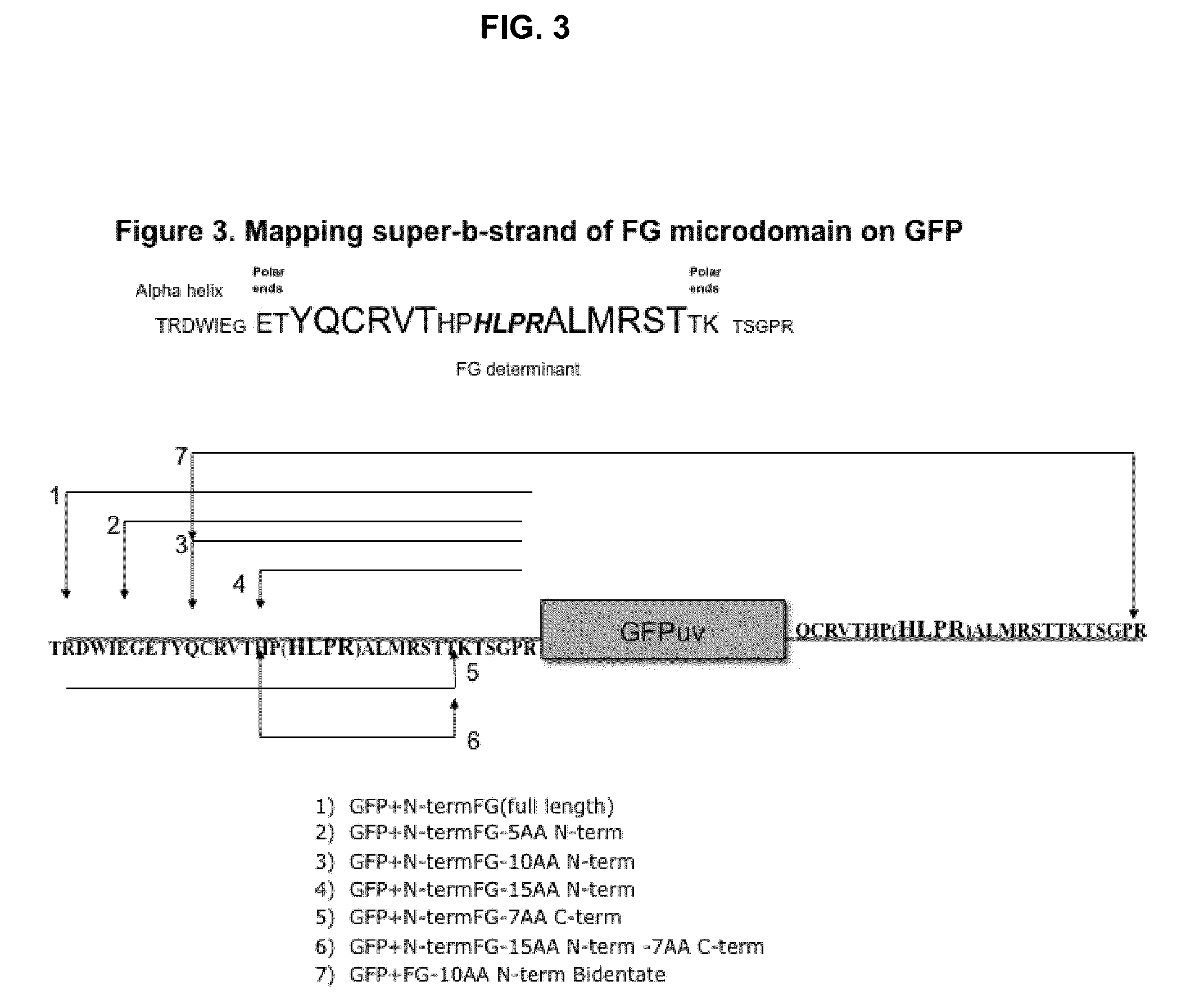
Displaying native human IgE neutralizing FceRla-contacting IgE B-cell epitopes by constraining super beta(b)-strands and cystine knots on thermostable protein scaffold
Vaccine displaying native antigenic loops of immunoglobulin E is critical for eliciting neutralizing anti-IgE antibodies. The embodiment of the invention enables the display of native antigenic IgE receptor-contacting loops as IgE B-cell vaccines via three steps of constraining methods. The loops of multiple antigenic B-cell epitopes can be molecularly grafted in, and conformationally constrained by the energy favorable flanking beta (b)-stands, i.e., the super b-strands identified in this invention. The constrained loops can be further stabilized in replacing a selective loop within the cystine knot peptide. These dual constrained antigenic loops are then integrated onto thermostable protein scaffolds, folded in the oxidative milieu that provides further conformational constraint and high yield.
Owner:CHEN SWEY SHEN
Tgf Derepressors and Uses Related Thereto
InactiveUS20080119396A1Reduced potencyReduce severityPeptide/protein ingredientsSkeletal disorderDiseaseCystine knot
The application is directed to TGF analogs / derepressors that bind to and neutralize cystine knot-containing BMP antagonists—such as the CAN subfamily of Cystine-knot proteins including sclerostin. The subject TGF derepressors can be prepared as substantially pyrogen-free pharmaceutical compositions for administration to mammals, in treating diseases such as bone diseases including osteoporosis, and any conditions with lesser-than-desired amount of BMP activity.
Owner:ACCELERON PHARMA INC
Construction method and application of electrochemical cell sensor for acetamiprid and imidacloprid joint toxicity evaluation
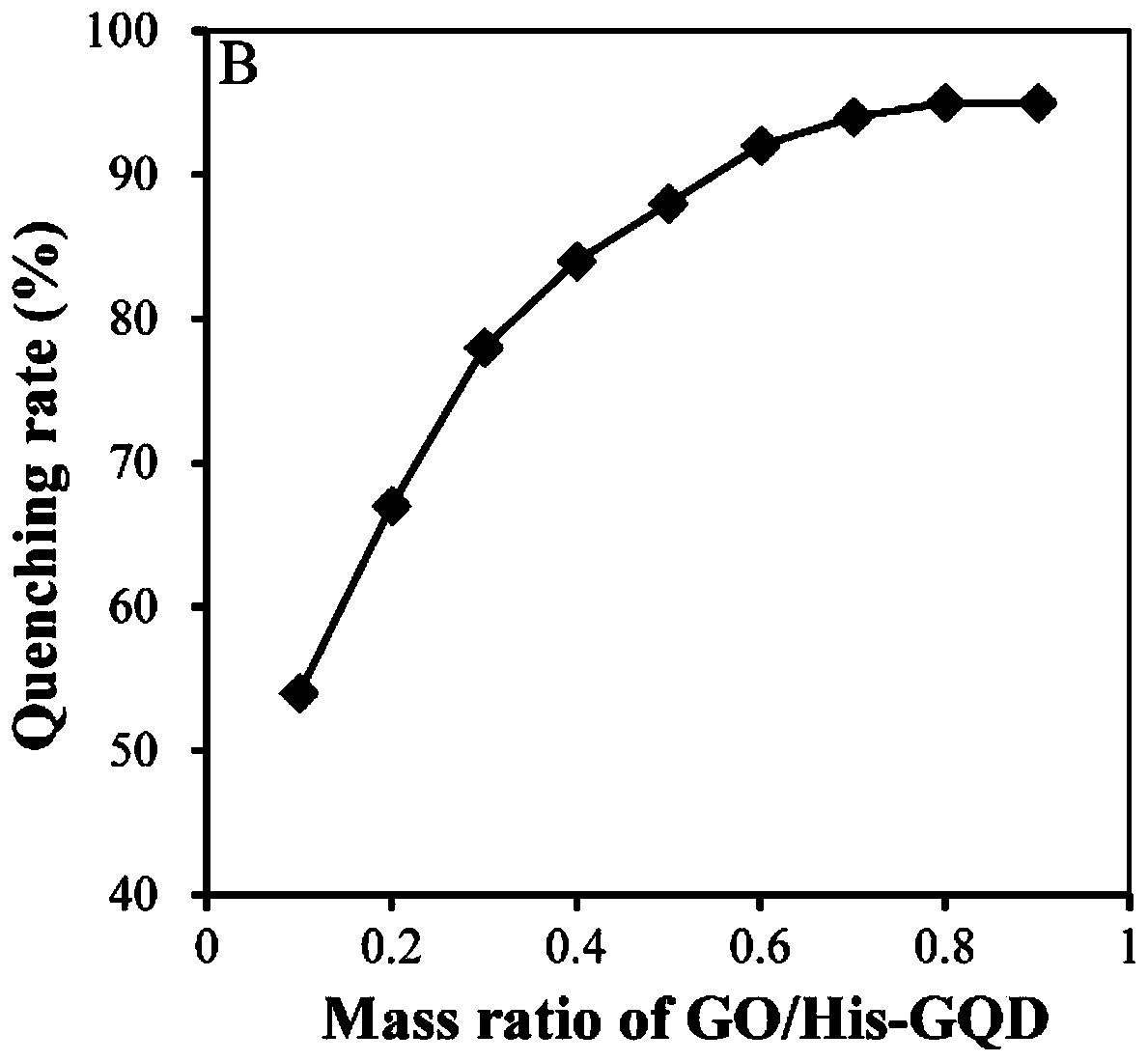
ActiveCN110568037AHigh sensitivityRapid determinationMaterial nanotechnologyCarbon compoundsCystine knotElectrochemical gas sensor
The invention relates to a construction method and application of an electrochemical cell sensor for acetamiprid and imidacloprid joint toxicity evaluation, and belongs to the technical field of celldetection. A size-controllable Ag / His-GQD / rGO three-dimensional composite material is prepared, after a glassy carbon electrode is modified with the composite material, L-cysteine is combined to the composite material through sulfydryl, activated folic acid is combined with L-cysteine through amido bonds, cells are fixed, determination is performed through an electrochemical impedance technology,and the electrochemical cell sensor is prepared. Through combination with a three-dimensional composite material, more binding sites can be provided, and the sensitivity of the sensor is improved. Combined with an electrochemical impedance technology, the constructed sensor can determine the Hep G2 cells quickly, and is relatively low in cost. The Hep G2 cell sensor can be used for evaluating thepesticide toxicity, and has a good application prospect in the aspect of pesticide joint toxicity evaluation.
Owner:JIANGNAN UNIV
Wise/sost nucleic acid sequences and amino acid sequences
InactiveUS20080181898A1Peptide/protein ingredientsGenetic material ingredientsNucleic acid sequencingFab Fragments
The present invention relates to nucleic acid sequences and amino acid sequences which influence bone deposition, the Wnt pathway, ocular development, tooth development, and may bind to LRP. The nucleic acid sequence and polypeptides include Wise and Sost as well as a family of molecules which express a cysteine knot polypeptide. Additionally, the present invention relates to various molecular tools derived from the nucleic acids and polypeptides including vectors, transfected host cells, monochronal antibodies, Fab fragments, and methods for impacting the pathways.
Owner:STOWERS INST FOR MEDICAL RES
Displaying native human IgE neutralizing FcepsilonRIa-contacting IgE B-cell epitopes by constraining super beta(b)-strands and cystine knots on thermostable protein scaffold
Vaccine displaying native antigenic loops of immunoglobulin E is critical for eliciting neutralizing anti-IgE antibodies. The embodiment of the invention enables the display of native antigenic IgE receptor-contacting loops as IgE B-cell vaccines via three steps of constraining methods. The loops of multiple antigenic B-cell epitopes can be molecularly grafted in, and conformationally constrained by the energy favorable flanking beta (b)-stands, i.e., the super b-strands identified in this invention. The constrained loops can be further stabilized in replacing a selective loop within the cystine knot peptide. These dual constrained antigenic loops are then integrated onto thermostable protein scaffolds, folded in the oxidative milieu that provides further conformational constraint and high yield.
Owner:CHEN SWEY SHEN
Cystine knot peptides binding to alpha IIb beta 3 integrins and methods of use
Disclosed are peptides having a cystine knot structural motif and comprising a sequence engineered for specificity against αIIbβ3 integrin, found on platelets, and a method of using the same in anti-thrombotic therapies. The present peptides utilize a cystine knot scaffold derived from modified agouti-related protein or agatoxin, An alternate library screening strategy was used to isolate variants of peptides that selectively bound to αIIbβ3 integrin or to both αIIbβ3 and αVβ3 integrins. Unique consensus sequences were identified within the identified peptides suggesting alternative molecular recognition events that dictate different integrin binding specificities. In addition, the engineered peptides prevented human platelet aggregation in a plasma-based assay and showed high binding affinity for αIIbβ3 integrin.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Oral spray preparation and preparation method thereof
ActiveCN108403637APrevent leakageImprove bioavailabilityTetracycline active ingredientsAerosol deliveryCystine knotCrystallography
The invention discloses a oral spray preparation and a preparation method thereof. The oral spray preparation is prepared from liquid crystal materials, alginate and mercapto polymer. The preparationmethod of the oral spray preparation comprises the following steps: firstly, dissolving and dispersing a medicine and the liquid crystal materials under an effect of a co-solvent so as to form medicine-carrier liquid crystal nanoparticles; then enveloping the medicine-carrier liquid crystal nanoparticles by adopting the alginate, so that the stability of the medicine-carrier liquid crystal nanoparticles can be greatly improved through the operations, a slow release effect is obvious, and deactivation of the medicine and aggregation and precipitation of the nanoparticles are prevented; the mercapto polymer and half-cystine in a body are combined on an affected part to form a film so as to play isolation and protection effects on the affected part, and therefore, the acting time of the medicine is prolonged, the concentration of the local medicines is obviously increased, development of the medicine is facilitated, the condition that a medicament is diluted due to retention of saliva inan oral cavity so as to lose efficacy is avoided.
Owner:武汉百纳礼康生物制药有限公司
Truncated cystine-knot proteins
InactiveUS20120231000A1Negligible hormonal activityMaintain good propertiesSenses disorderPeptide/protein ingredientsCystine knotImmunogenicity
The invention relates to the fields of protein chemistry, biology and medicine. More specifically, it relates to the design and preparation of proteinmimics of members of the cystine-knot growth factor superfamily. Further, the invention relates to the use of these proteinmimics as a medicament or prophylactic agent. The invention provides proteinmimics of members of the cystine-knot growth factor superfamily, preferably for use in immunogenic and / or therapeutic compositions.
Owner:PEPSCAN SYST +2
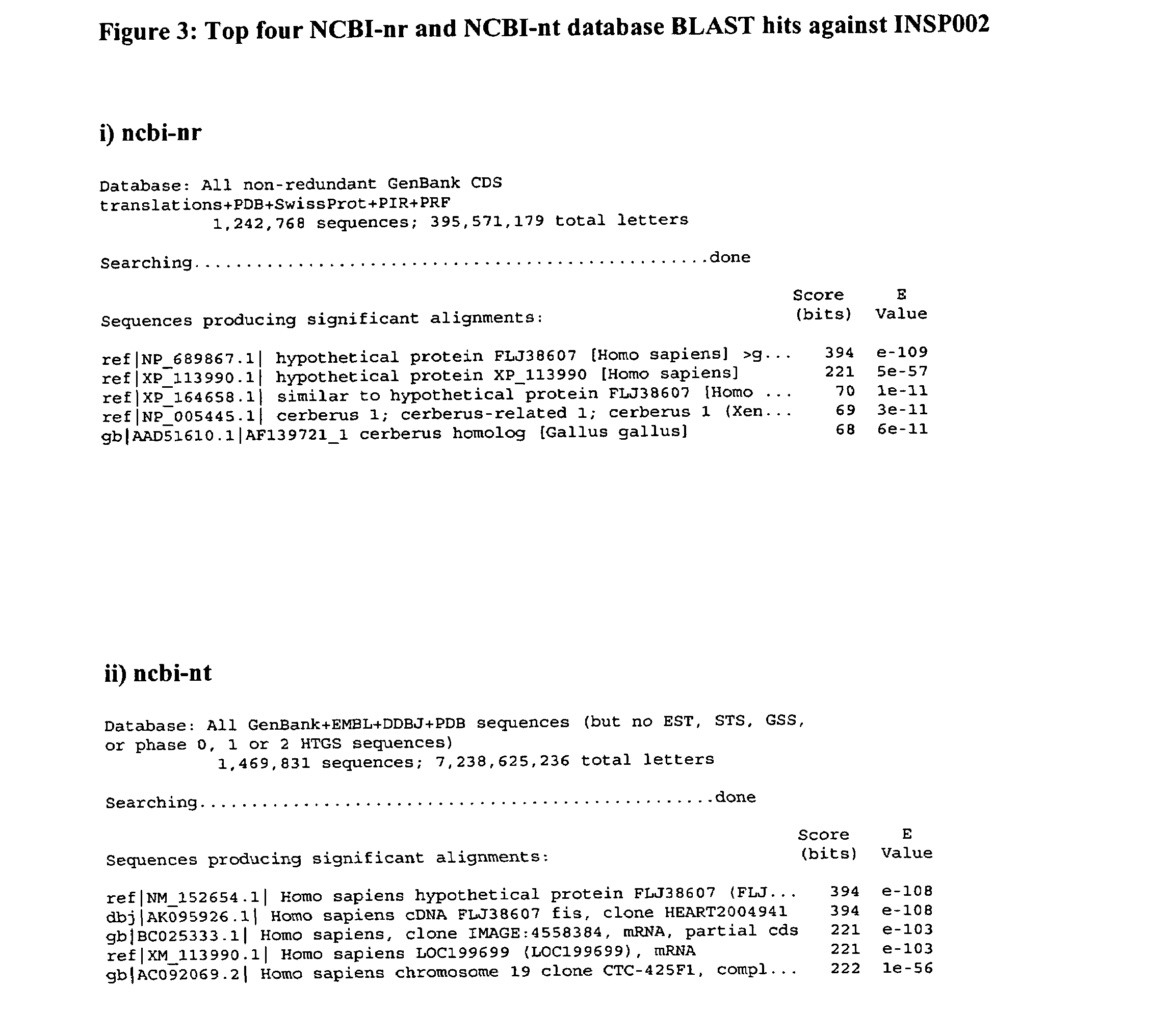
Cystine-knot fold protein
This invention relates to a novel protein (INSP002), herein identified as a secreted protein that is a member of the Dan family of the cystine-knot fold cytokine superfamily and to the use of this protein and nucleic acid sequences from the encoding genes in the diagnosis, prevention and treatment of disease.
Owner:ARES TRADING SA
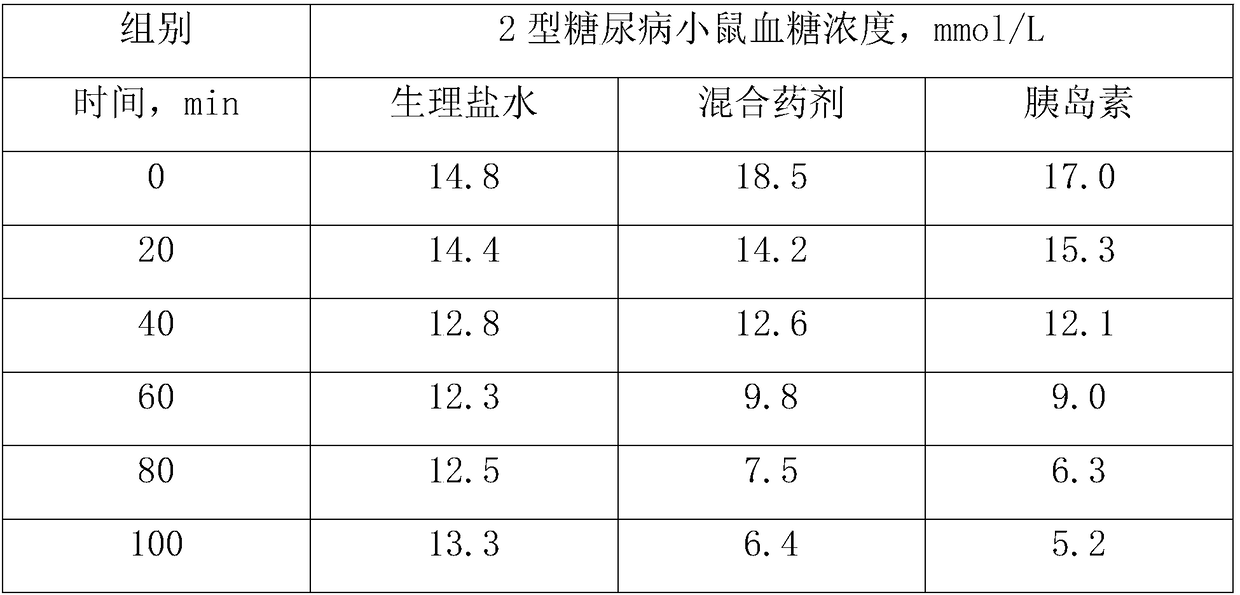
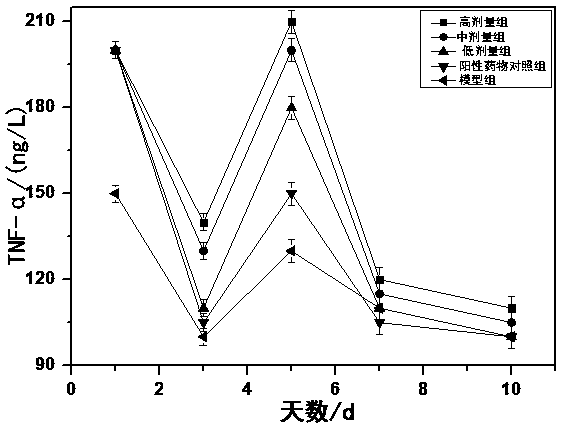
Polypeptide for preventing and treating diabetes, preparation method and application thereof
InactiveCN109384836ALow costSimple extraction processPeptide/protein ingredientsMetabolism disorderCystine knotSide effect
The invention discloses chickpea four-type 37-amino acids polypeptides, a preparation method thereof and an application thereof in preparing a medicine for treating diabetes or a functional food. Eachpeptide is composed of 37 amino acids, the six cysteines in the molecule and the formed three pairs of disulfide bridges are highly conserved, and the three pairs of disulfide bonds form a cysteine knot domain. The chickpea four-type 37-amino acids polypeptides can be dissolved in polar solvents such as water, which are stable to heat and resistant to hydrolysis of protease in human digestive tract. The preparation cost is lower than that of insulin and other peptide drugs; the product is not only can be used for treating symptomatic doeases, but also treats the root, and can be used for recovering the quality and function of diseased pancreas at varying degrees; the product reduces or eliminates the body's insulin resistance, can be developed to oral dosage form, and is easier to use than insulin and other peptide drugs; while in use, no risk of hypoglycemia is generated; and the product is derived from edible plant chickpeas and has no toxic side effects.
Owner:HUAZHONG UNIV OF SCI & TECH
Immunogenic protein carrier containing an antigen presenting cell binding domain and a cysteine-rich domain
ActiveUS8383767B2Improved its antigenecitySugar derivativesPeptide/protein ingredientsCystine knotAdjuvant
A protein carrier containing an antigen presenting cell binding domain and a cysteine-rich domain. Also described herein is an immunoconjugate containing the protein carrier with an antigen conjugated to multiple cysteine residues in the cysteine-rich domain, and an immune composition containing the immunoconjugate and an adjuvant, as well as their uses in eliciting immune responses.
Owner:ACAD SINIC
Capsaicin liquid crystal nano spray preparation for promoting skin wound healing and preparation method thereof
InactiveCN109820824AModerate viscosityImprove liquidityAntibacterial agentsOrganic active ingredientsCystine knotCapsaicin
The invention discloses a capsaicin liquid crystal nano spray preparation for promoting skin wound healing and a preparation method thereof. The capsaicin liquid crystal nano spray preparation is prepared from, by weight, 0.1-0.5% of capsaicin, 50-70% of a liquid crystal material, 5-10% of chitosan, 5-10% of a surfactant, 10-15% of a cosolvent and the balance water. The liquid crystal material isformed by mixing phosphatidyl glycerol and glyceryl dioleate in the weight ratio of 1:(1-3). The preparation method of the spray preparation comprises the steps that the capsaicin, the liquid crystalmaterial and the chitosan are dissolved and dispersed to form drug-loaded liquid crystal nanoparticles under the action of the cosolvent, the stability of the drug-loaded liquid crystal nanoparticlescan be greatly improved through the above operation, and the slow-release effect is obvious. The chitosan is combined with cysteine in the body to form a film on an affected part, the chitosan also has an antibacterial effect, the effects of isolating and protecting the affected part are achieved, and the action time of the medicine is prolonged, so that the local medicine concentration is obviously increased, the medicine takes effect easily, and wound healing is promoted.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Anticoagulant drug based on cobra venom PIII type metalloprotease and applications thereof
InactiveCN105567666AHas multiple anticoagulant effectsReduce the original levelPeptide/protein ingredientsBlood disorderCystine knotCobra venom
The invention discloses an anticoagulant drug based on cobra venom PIII type metalloprotease. The cobra venom PIII type metalloprotease is extracted from snake venom of naja atra and then purified. The metalloprotease is single chain glycoprotein. The structure analysis shows that metalloprotease has three structural domains: metalloprotease structural domain, disintegrin like structural domain, and cysteine enriched structural domain, and belongs to PIII type cobra venom metalloprotease. The metalloprotease is named as Atrase A and Atrase B; and the sequences of Atrase A and Atrase B are represented by SEQ ID No.1 and SEQ ID No.2 respectively. Target protein and anticoagulant drugs thereof have multiple anticoagulant effects: preventing multiple blood coagulation factors; reducing fibrinogen level; preventing platelet aggregation; and preventing complement so as to relieve abnormal blood coagulation caused by inflammation.
Owner:THE KEY LAB OF CHEM FOR NATURAL PROD OF GUIZHOU PROVINCE & CHINESE ACADEMY OF SCI
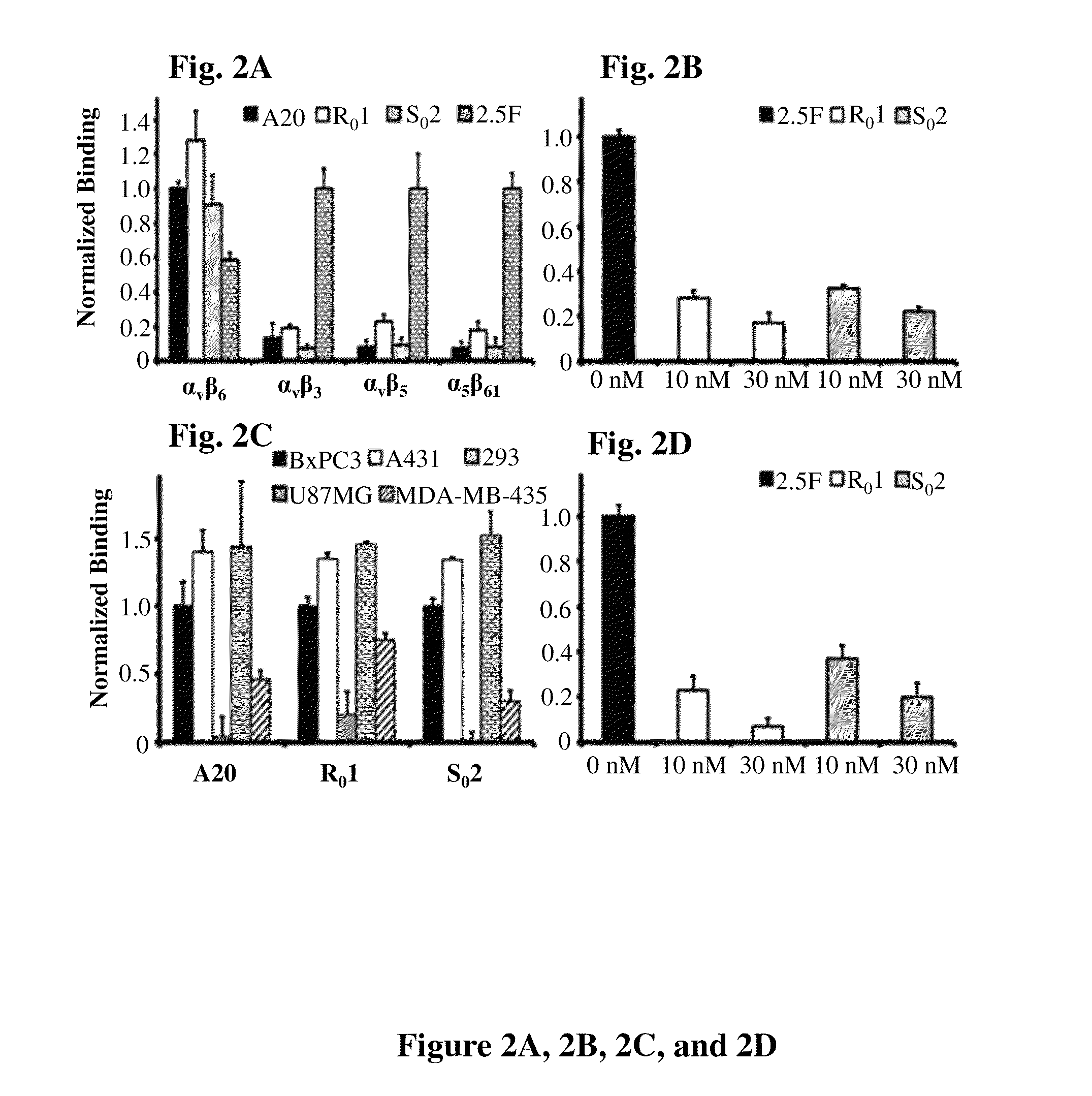
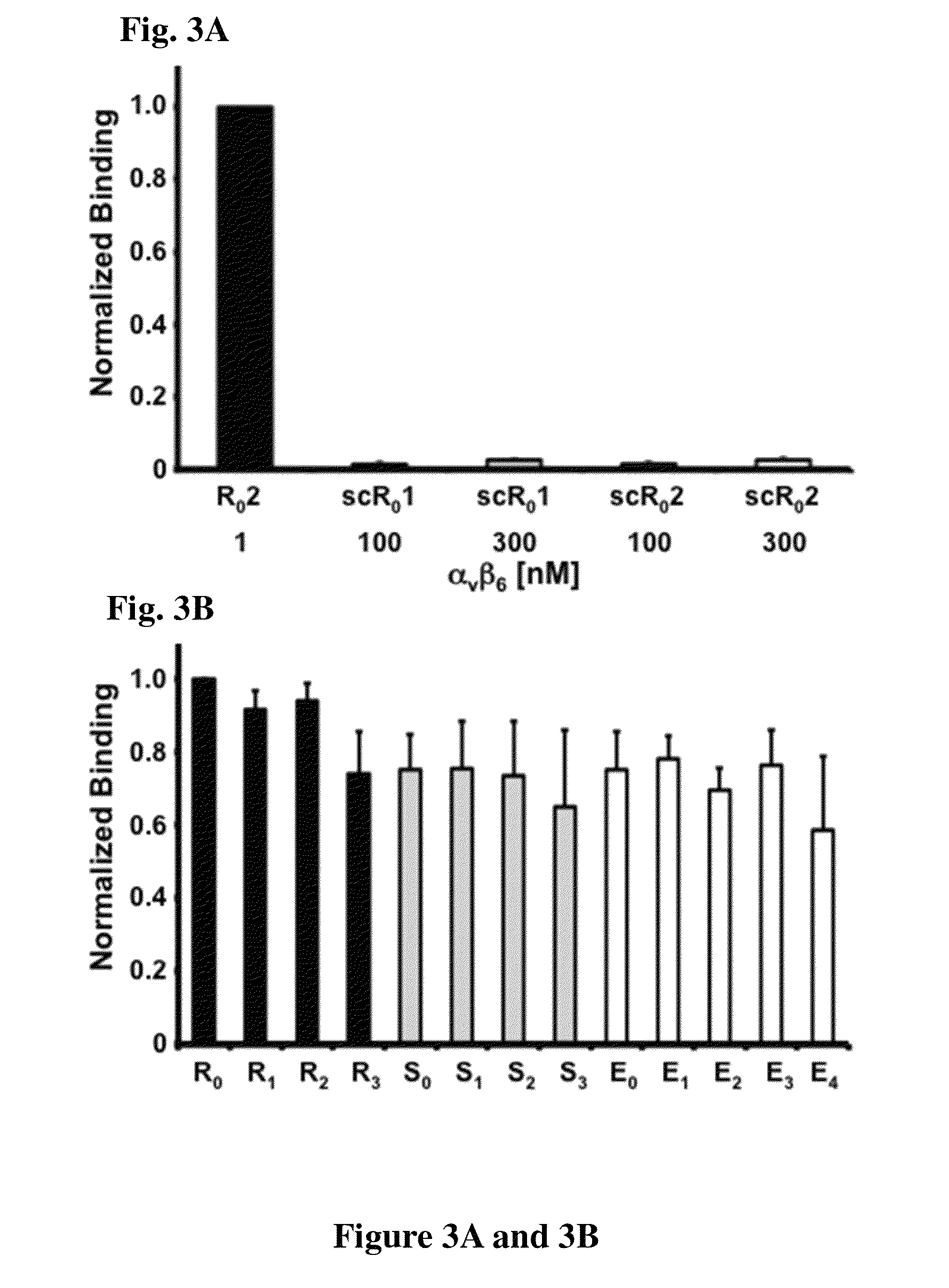
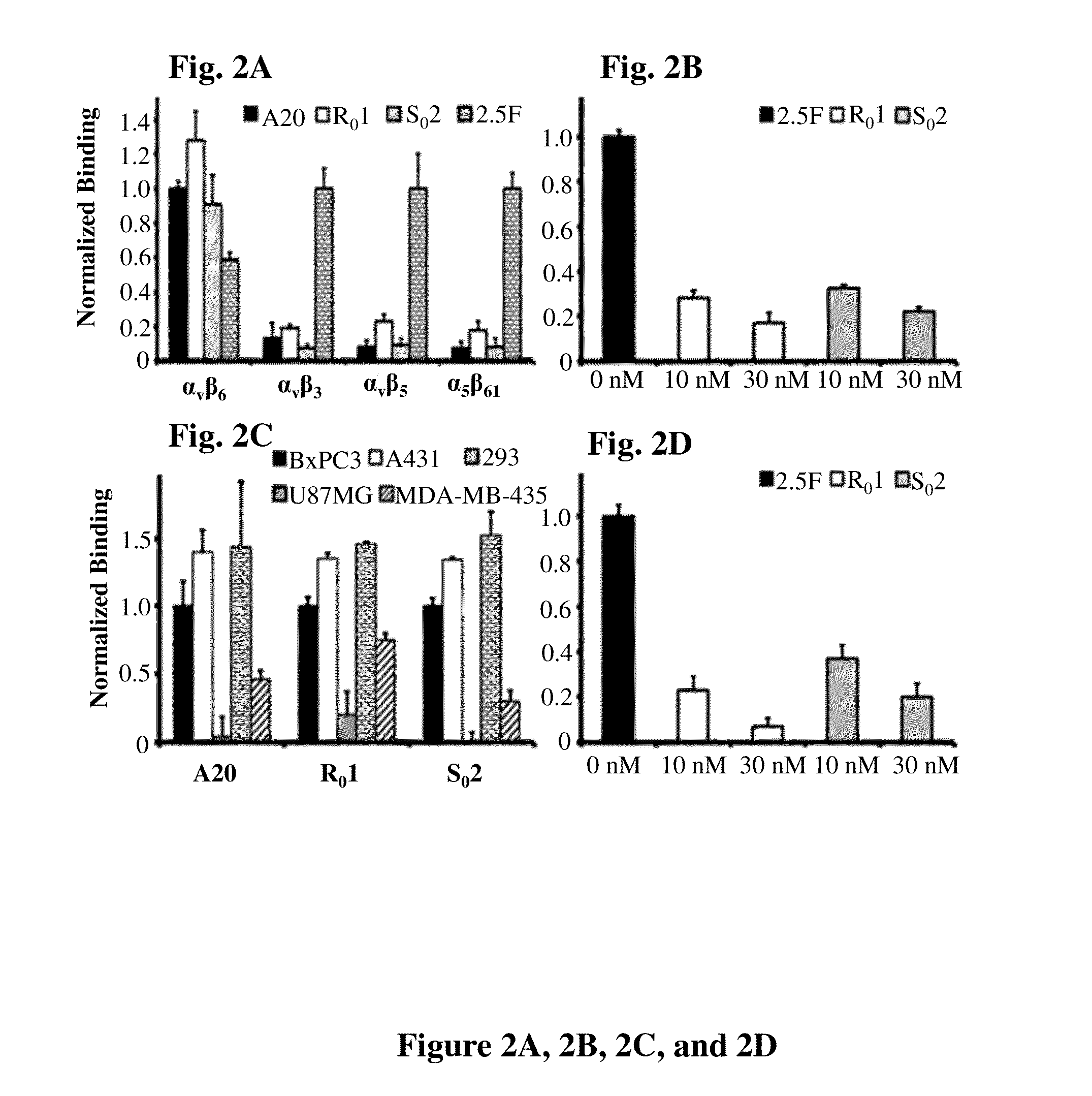
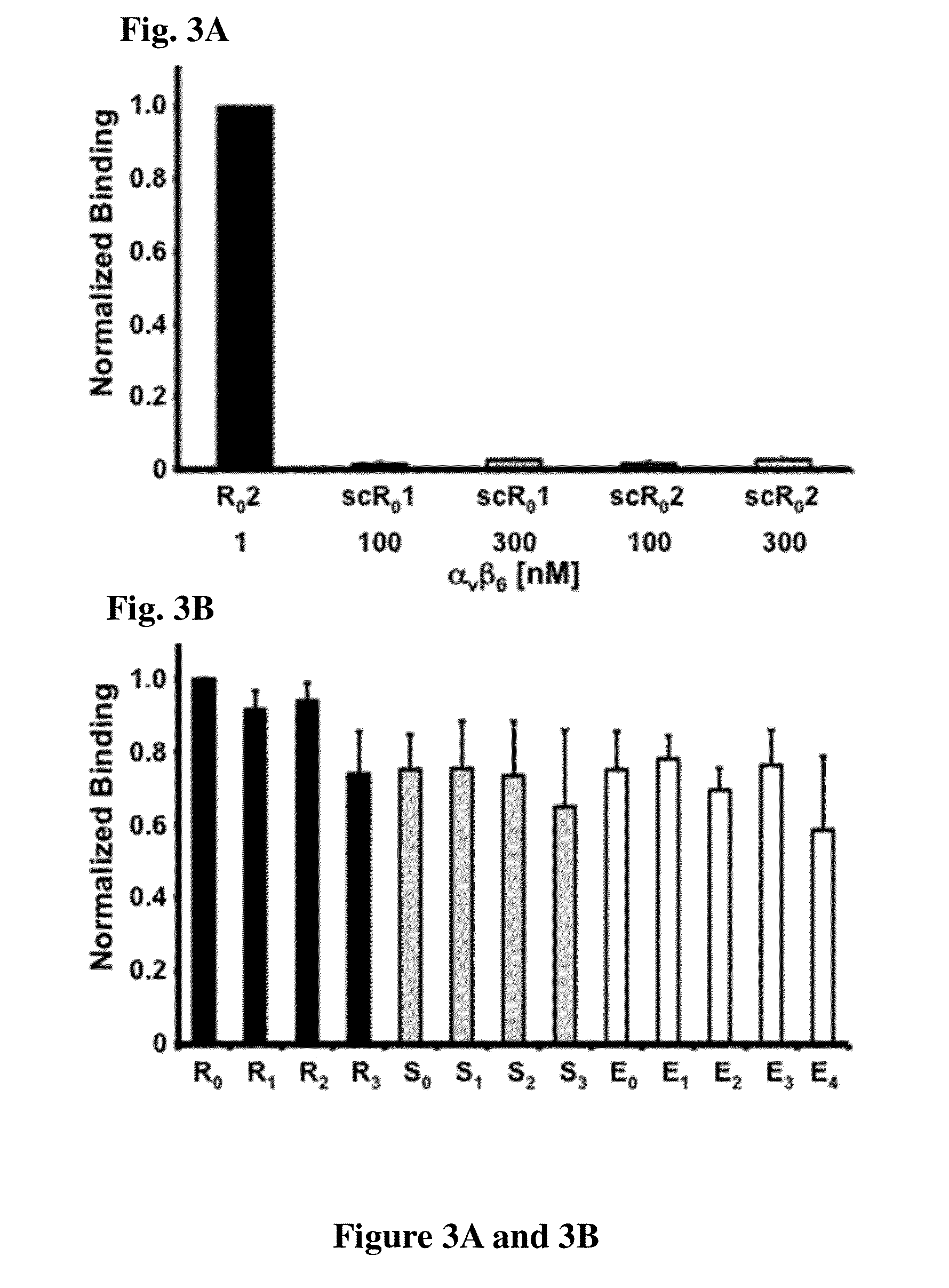
Cystine knot peptides that bind alpha-V-beta-6 integrin
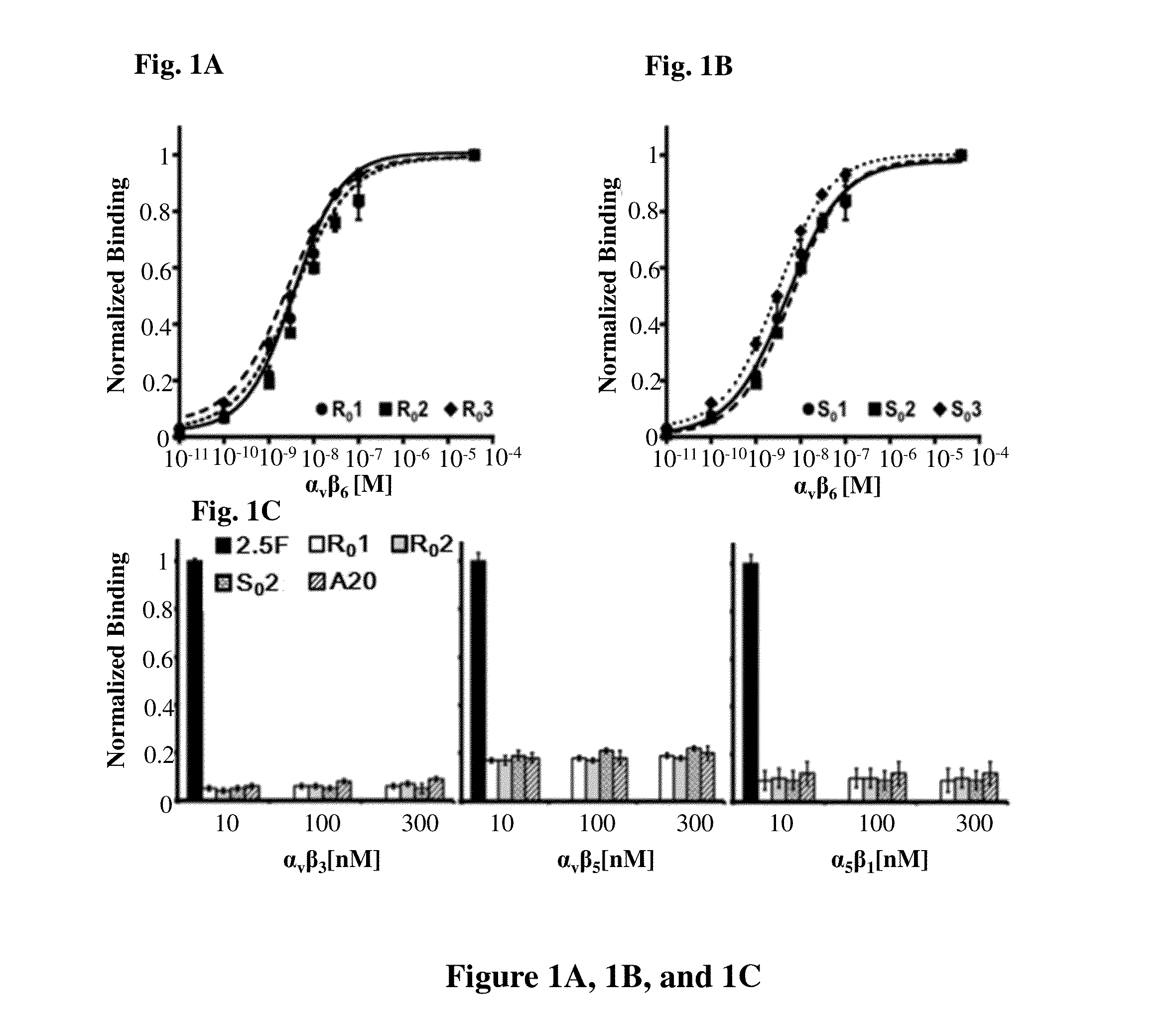
Disclosed are peptides comprising a molecular scaffold portion and a loop portion that binds to integrin αvβ6. This integrin is expressed on pancreatic tumors, making the peptides useful as imaging agents, among other uses. The peptides showed single-digit nanomolar dissociation constants similar to antibodies used clinically for imaging and therapy. The peptides rapidly accumulated in αvβ6-positive tumors, which led to excellent tumor-to-normal contrast. The peptides are specific for the targeted integrin αvβ6 receptors expressed on orthotopic pancreatic tumors and various xenografts used. Additionally, pharmacokinetic-stabilization strategies endowed knots with rapid renal clearance, which significantly reduced off-target dosing.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Drug delivery matrices to enhance wound healing
InactiveUS20100291215A1Retain it more effectively within the matrixReduce solubilityPowder deliverySurgical adhesivesCystine knotSolubility
Bioactive molecules are entrapped within a matrix for the controlled delivery of these compounds for therapeutic healing applications. The matrix may be formed of natural or synthetic compounds. The primary method of entrapment of the bioactive molecule is through precipitation of the bioactive molecule during gelation of the matrix, either in vitro or in vivo. The bioactive molecule may be modified to reduce its effective solubility in the matrix to retain it more effectively within the matrix, such as through the deglycosylation of members within the cystine knot growth factor superfamily and particularly within the TGFβ superfamily. The matrix may be modified to include sites with binding affinity for different bioactive molecules, for example, for heparin binding.
Owner:ETH ZZURICH +1
Buffers for Controlling the pH of Bone Morphogenetic Proteins
InactiveUS20130172254A1Increase ionic strengthEasy to controlPeptide/protein ingredientsBone-inducing factorCystine knotCrystallography
The present invention provides formulations of cysteine knot proteins, including TGF-β superfamily proteins and bone morphogenic proteins that are pH stabilized. In particular, the present invention relates to the observation that certain buffers enhance the stability of cysteine knot proteins, including TGF-β superfamily proteins and bone morphogenic proteins. In particular, disclosed herein are liquid and lyophilized formulations prepared with a glycylglycine and tartaric acid buffers to stabilize the pH of the formulation.
Owner:STRYKER CORP
Nucleic acid molecule encoding a cystine knot polypeptide
The present invention relates to a novel nucleic acid molecule encoding an amino acid sequence, which is capable of forming a cyclic structure. Cyclization may occur within a cell or cell membrane, or linear forms of the molecules may be circularised or partially circularised, in vitro using isolated enzyme systems or chemical means. The cyclised amino acid sequence is generally in the form of a stabilized folded structure such as acyclic knotted peptide, polypeptide or protein or functional equivalent. The nucleic acid molecules and cyclic and linear peptides are useful inter alia in the generation of molecules having animal or plant therapeutic properties, as well as in a range of diagnostic, industrial and agricultural, including horticultural, applications. Of particular importance is the use of these molecules in the protection of plants, such as crop plants, from pest and / or pathogen infestation.
Owner:THE UNIVERSITY OF QUEENSLAND +1
Thrombolytic drug based on cobra venom piii type metalloprotease and its application
ActiveCN105861476BObvious thrombolytic effectNo bleeding riskPowder deliveryPeptide/protein ingredientsCystine knotCobra venom
The invention a thrombolytic drug based on cobra venom PIIII type metalloproteinase which is obtained by separating and purifying venom of Chinese cobra or Naja cobra with a class name of Naja atra. The metalloproteinase is singe strand glycoprotein, structural analysis shows that the metalloproteinase has three structural domains, namely a metalloproteinase structural domain, a disintegrin structural domain and a cysteine-rich structural domain and belongs to PIIII type metalloproteinase with names of Atrase A and Atrase B. A sequence of the Atrase A is SEQ ID No.1, and a sequence of the Atrase B is SEQ ID No.2. Target protein and the thrombolytic drug can induce endogenous fibrinolytic enzyme activator tPA release so as to play a thrombolytic role, and the thrombolytic drug can be used for thrombolytic treatment of thrombotic diseases.
Owner:THE KEY LAB OF CHEM FOR NATURAL PROD OF GUIZHOU PROVINCE & CHINESE ACADEMY OF SCI
A group of peptides and their pharmaceutical compositions and applications
ActiveCN106632603BAnalgesic effect hasPeptide/protein ingredientsAntipyreticCystine knotPharmacophore
Provided are peptides, and pharmaceutical compositions and uses thereof. The peptides are as shown in the sequence listing, and are amino acid sequences having an analgesic effect, or pharmaceutically acceptable salts thereof. The two cysteine at the head end and the tail end of a peptide provided forms a ring. The pharmacophore (domain) having the analgesic effect in the GsMTx-4 polypeptide is determined by comparing the sequences of multiple polypeptides containing cysteine knots (ICK), such as GsMTx-4 and GsMTX-2, and by means of behavioral measurement on rat pain models.
Owner:唐琼瑶 +1
Strain capable of producing L-arginine and method for producing L-arginine by same
The invention relates to a strain capable of producing L-arginine and a method for producing the L-arginine by the strain and belongs to the technical field of biological engineering. For the strain, Brevibacterium flavum ATCC 14067 is used as a starting strain; nitrosoguanidine is adopted to carry out mutagenesis step by step; mutant strains with histidine and succinic acid auxotrophic strains are screened out so as to cut off a competitive metabolic pathway; and mutant strains (His-, Suc-, D-Argr, SMCr) with resistances of arginine structural analogs and cysteine structural analogs are screened out. The strain is named as Brevibacterium flavum HX1009, is preserved in the China general microbiological culture collection center, has the preservation number of CGMCC No.4464 and has the genetic characters of histidine auxotroph His-, succinic acid auxotroph Suc-, D-arginine resistance D-Argr and S-methyl cysteine resistance SMCr for improving the yield of the L-arginine. Under the optimized condition, the L-arginine is produced by fermentation on a fermentation tank with the volume of 5L to 5M3 and the arginine production level achieves 50 to 70g / L.
Owner:FUJIAN GUTIAN PHARMA
Cystine knot peptides that bind alpha-v-beta-6 integrin
Disclosed are peptides comprising a molecular scaffold portion and a loop portion that binds to integrin αvβ6. This integrin is expressed on pancreatic tumors, making the peptides useful as imaging agents, among other uses. The peptides showed single-digit nanomolar dissociation constants similar to antibodies used clinically for imaging and therapy. The peptides rapidly accumulated in αvβ6-positive tumors, which led to excellent tumor-to-normal contrast. The peptides are specific for the targeted integrin αvβ6 receptors expressed on orthotopic pancreatic tumors and various xenografts used. Additionally, pharmacokinetic-stabilization strategies endowed knots with rapid renal clearance, which significantly reduced off-target dosing.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com