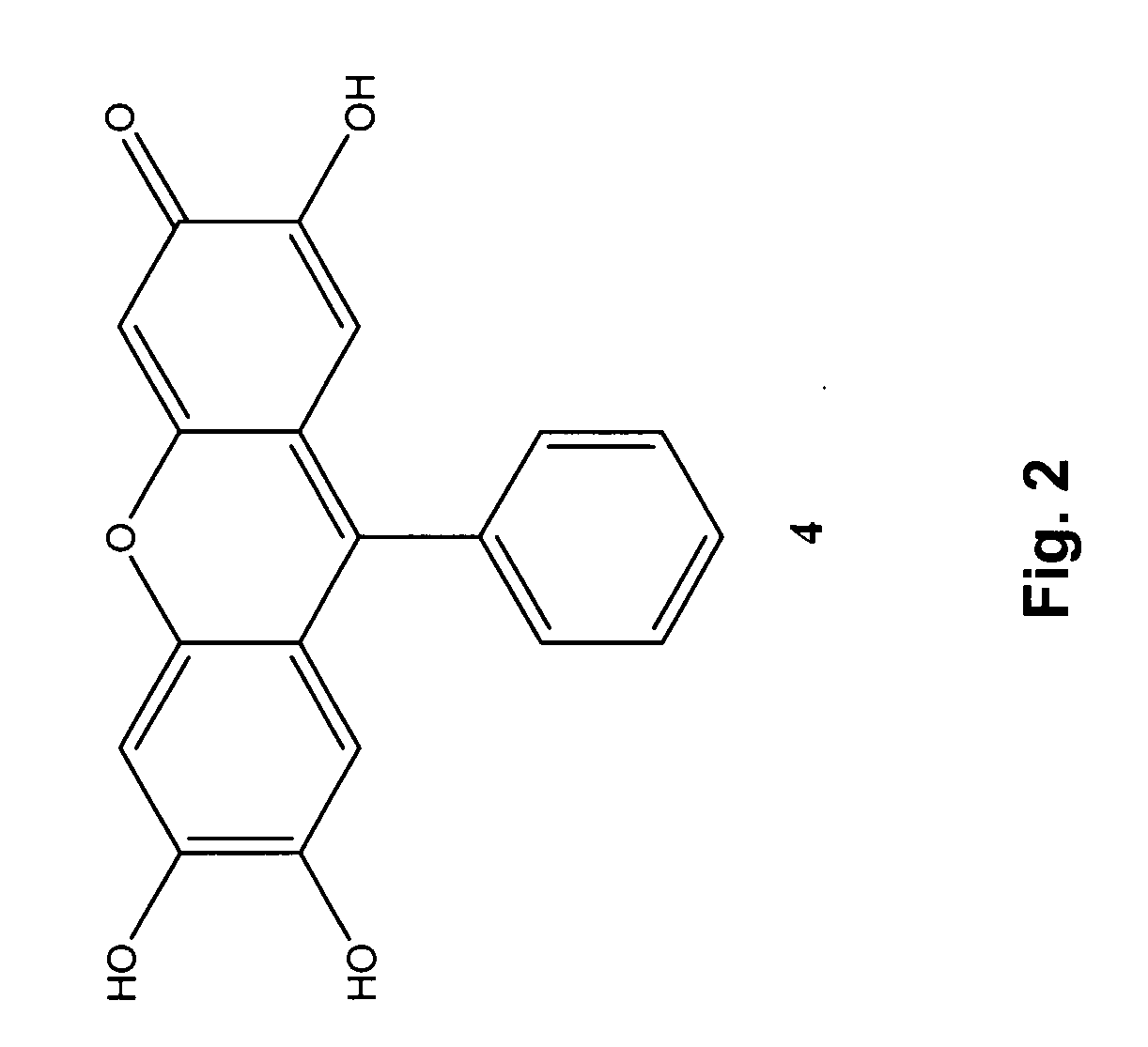
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1397 results about "Cystine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
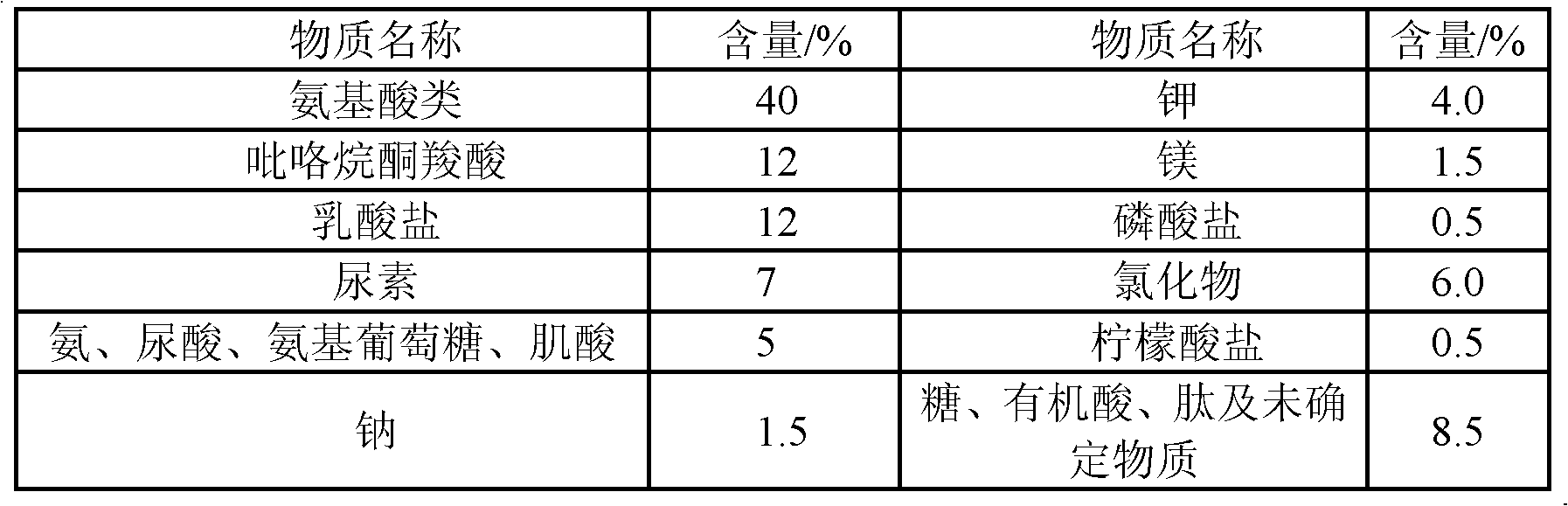
Cystine is the oxidized dimer form of the amino acid cysteine and has the formula (SCH₂CH(NH₂)CO₂H)₂. It is a white solid that is slightly soluble in water. It serves two biological functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure.
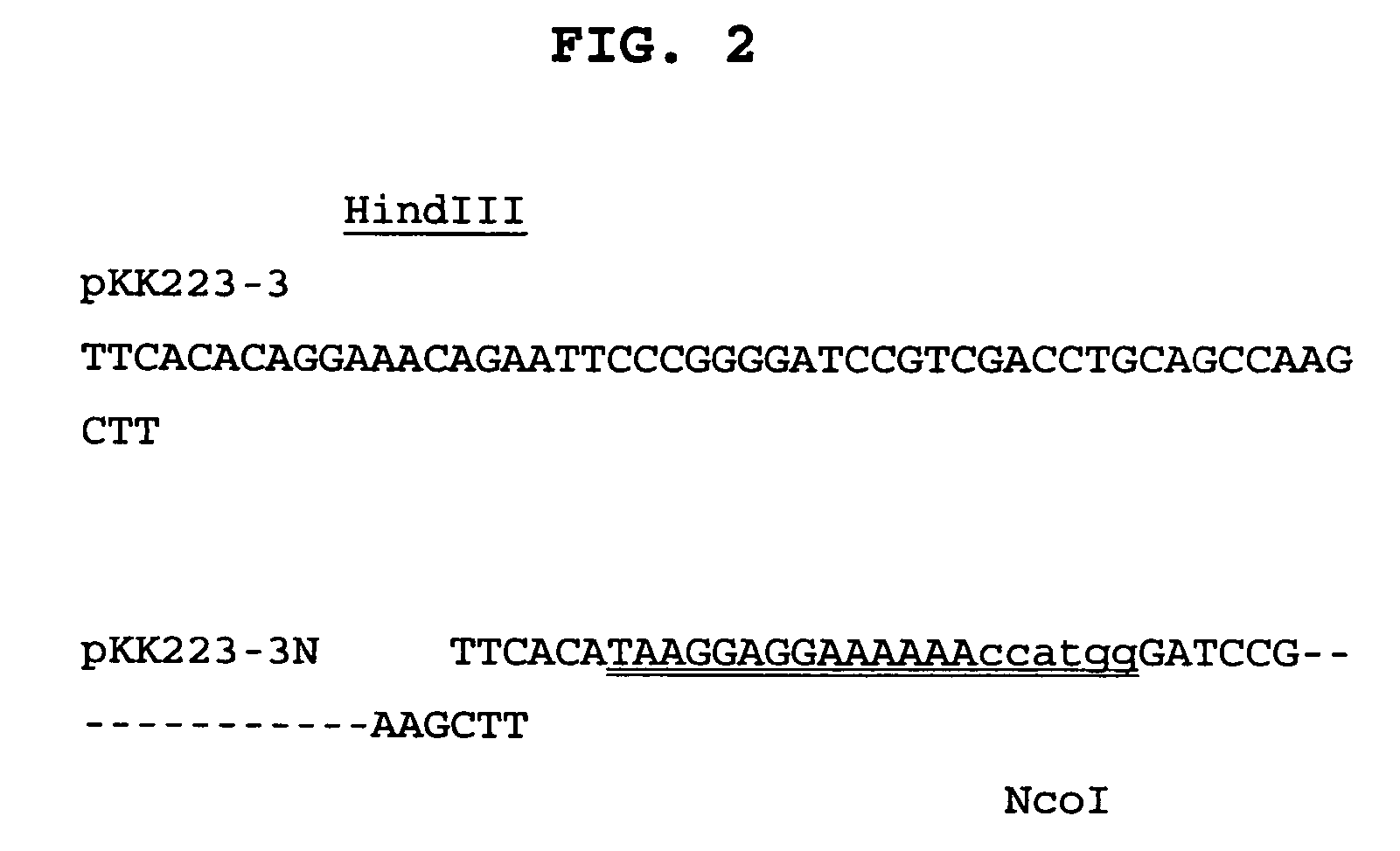
Novel recombinant proteins with N-terminal free thiol
InactiveUS20050170457A1Extended half-lifeIncreases circulating serum half-lifePeptide/protein ingredientsTissue cultureCysteine thiolateHalf-life
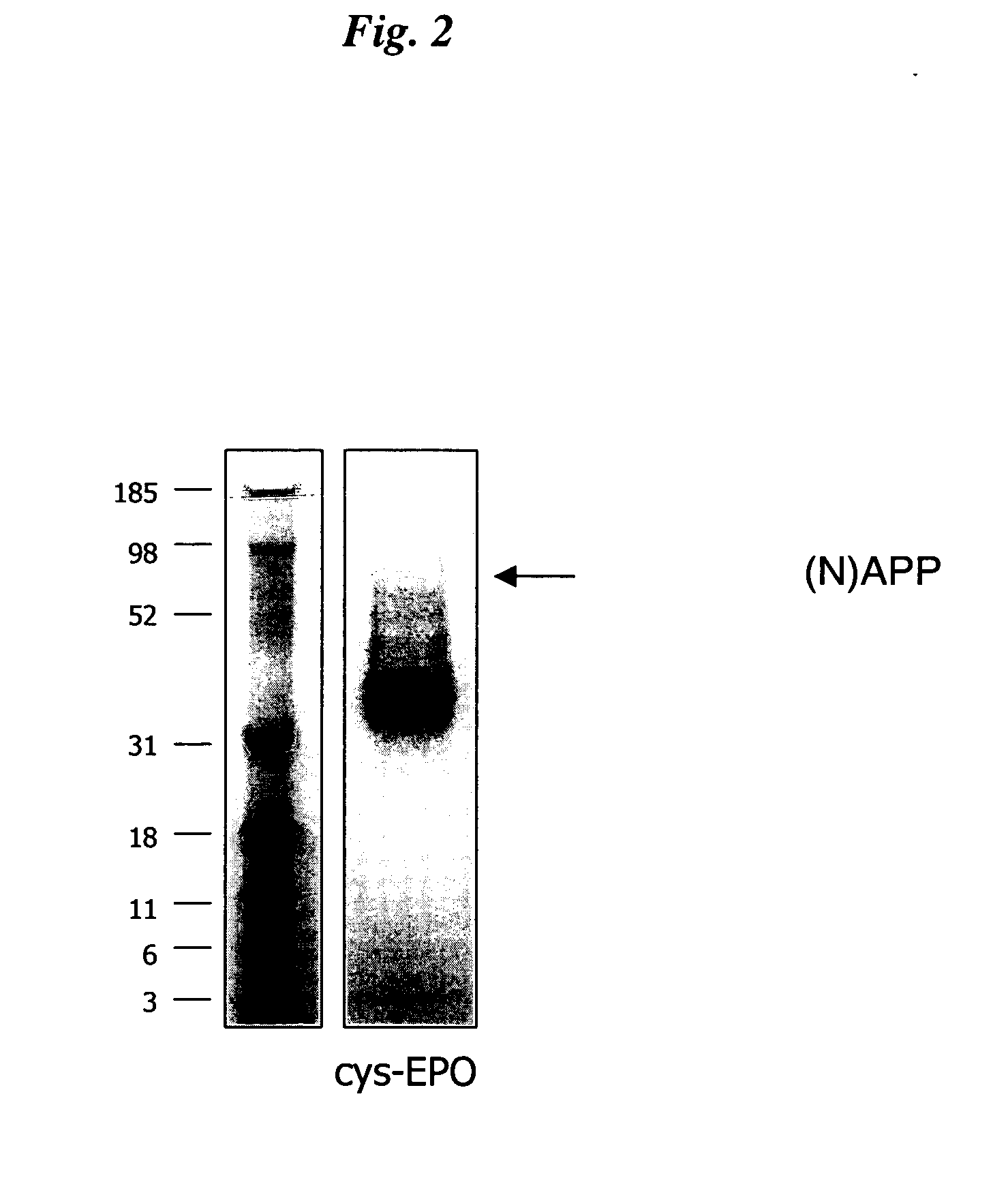
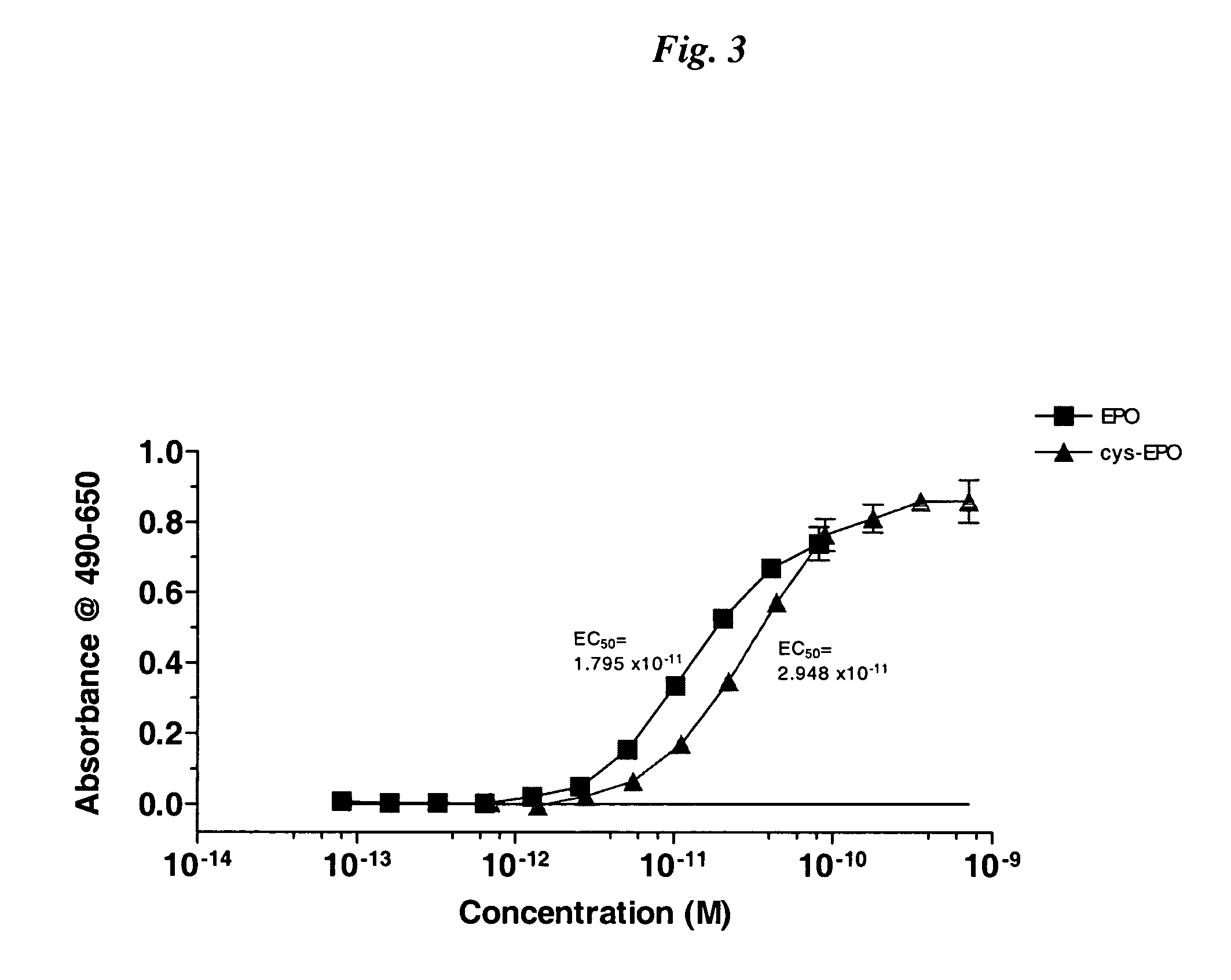
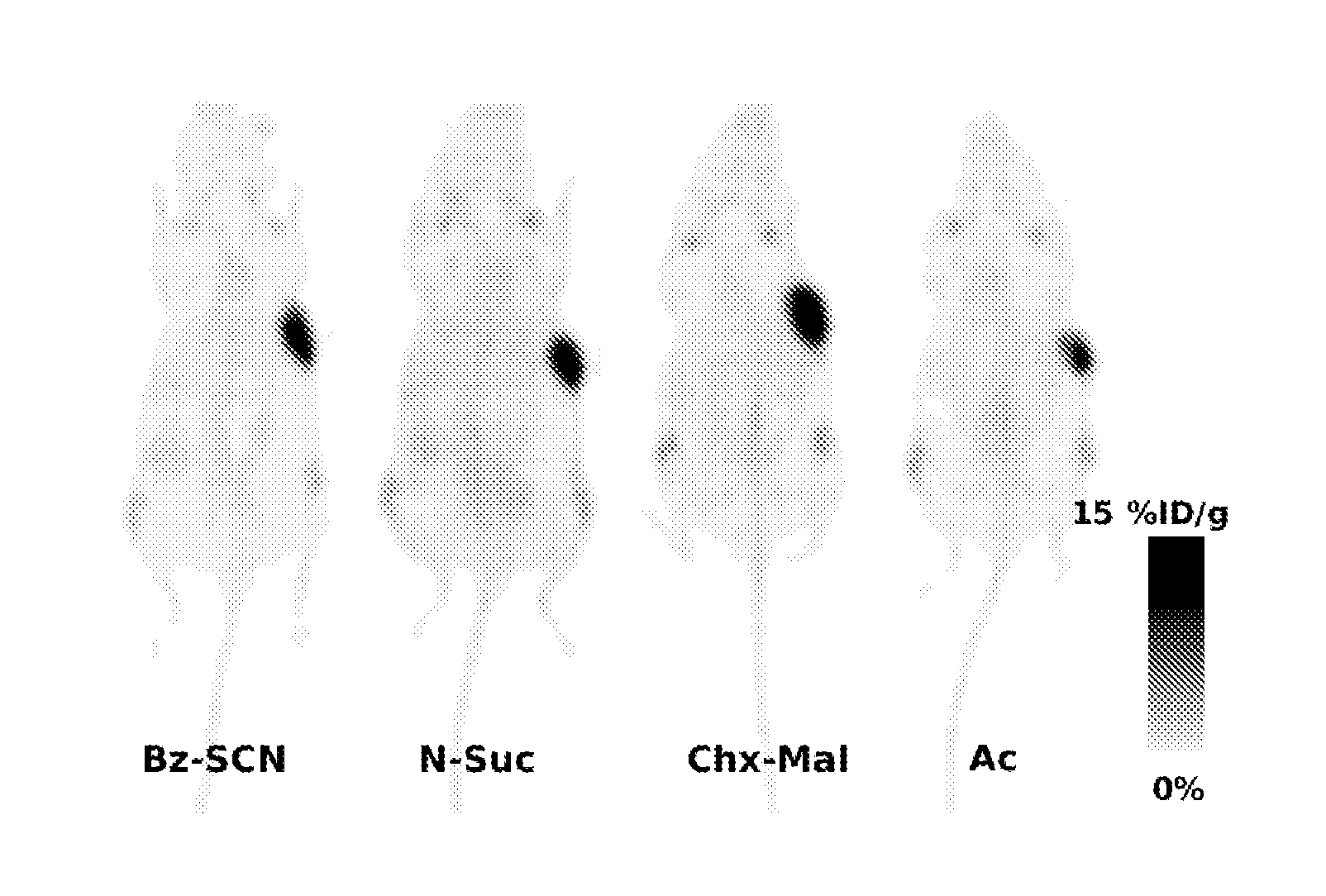
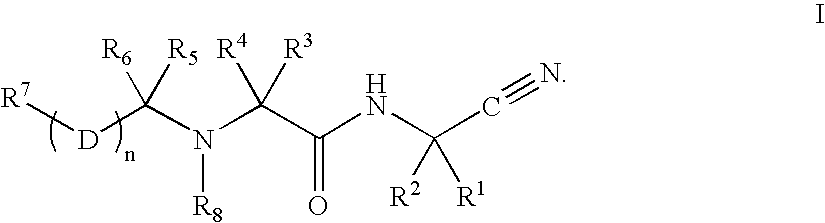
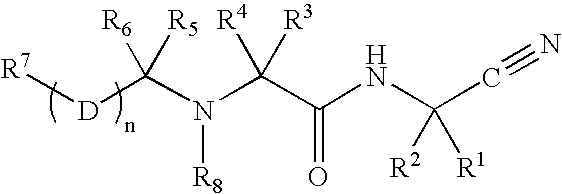
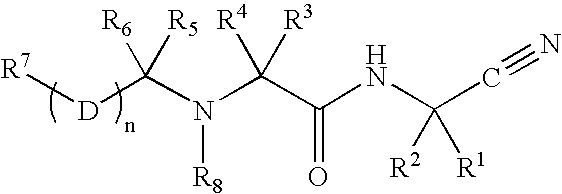
The present invention relates to novel modified proteins having N-terminal free thiols that can be produced by recombinant methods and are ready for further chemical derivatization. In particular, the invention relates to erythropoietin conjugate compounds having altered biochemical, physiochemical and pharmacokinetic properties. More particularly, one embodiment of the invention relates to erythropoietin conjugate compounds of the formula: (M)n-X-A-cys-EPO (I) where EPO is an erythropoeitin moiety selected from erythropoietin or an erythropoietin variant having at least one amino acid different from the wild-type human EPO, or any pharmaceutical acceptable derivatives thereof having biological properties of causing bone marrow cells to increase production of red blood cells; cys represents the amino acid cysteine and occurs at position −1 relative to the amino acid sequence of the erythropoietin moiety; A indicates the structure of the residual moiety used to chemically attach X to the thiol group of −1Cys; X is a water soluble polymer such as a polyalkylene glycol or other polymer; M is an organic molecule (including peptides and proteins) that increases the circulating half-life of the construct; and N is an integer from 0 to 15.
Owner:CENTOCOR
Zirconium-radiolabeled, cysteine engineered antibody conjugates
InactiveUS20100111856A1Peptide/protein ingredientsGenetic material ingredientsAntibody conjugateAntibody fragments
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab) are conjugated with one or more zirconium complex (Z) labels through a linker (L) to form cysteine engineered zirconium-labeled antibody conjugates having Formula I:Ab-(L-Z)p Iwhere p is 1 to 4. Imaging methods and diagnostic uses for zirconium-radiolabeled, cysteine engineered antibody conjugate compositions are disclosed.
Owner:F HOFFMANN LA ROCHE & CO AG
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
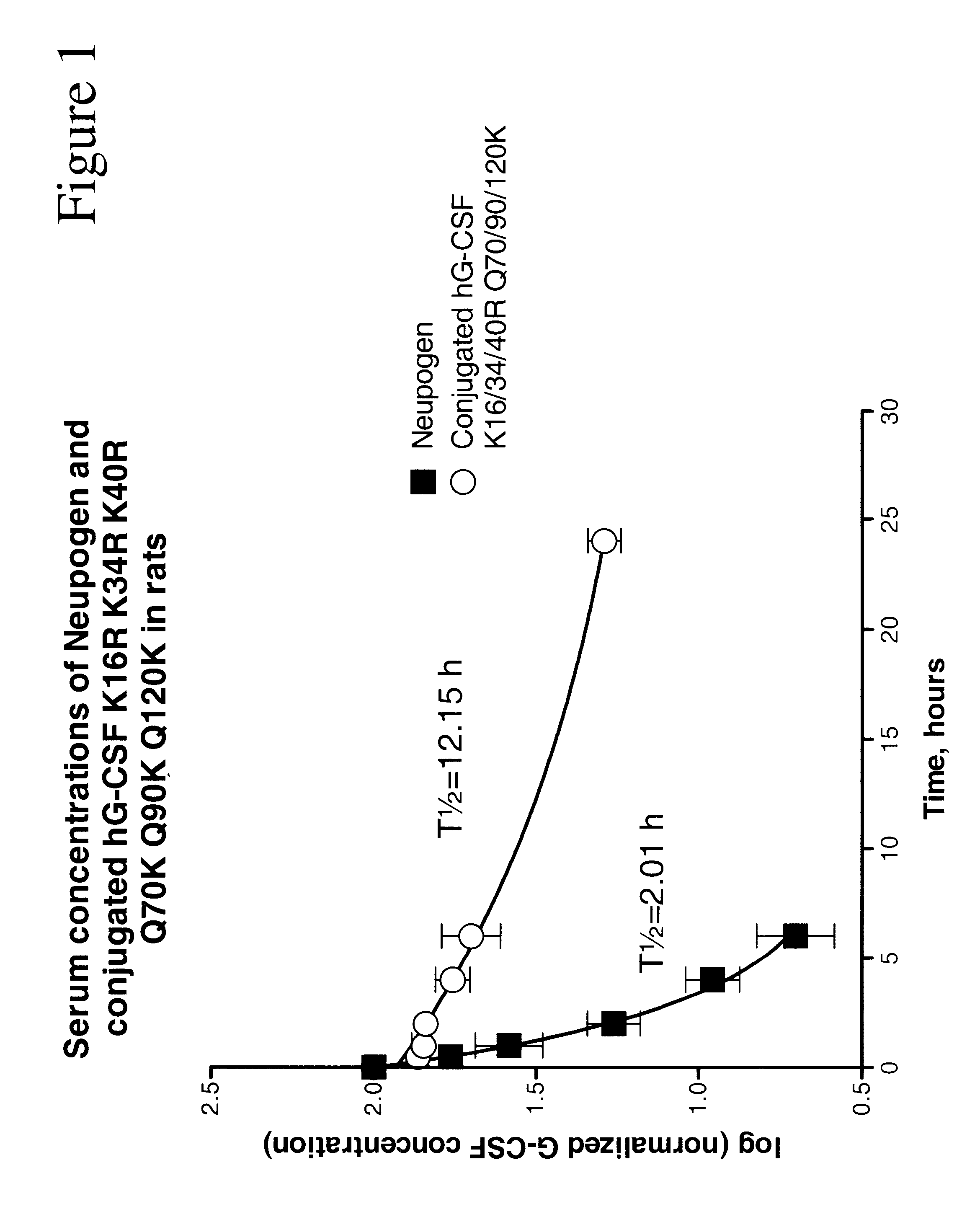
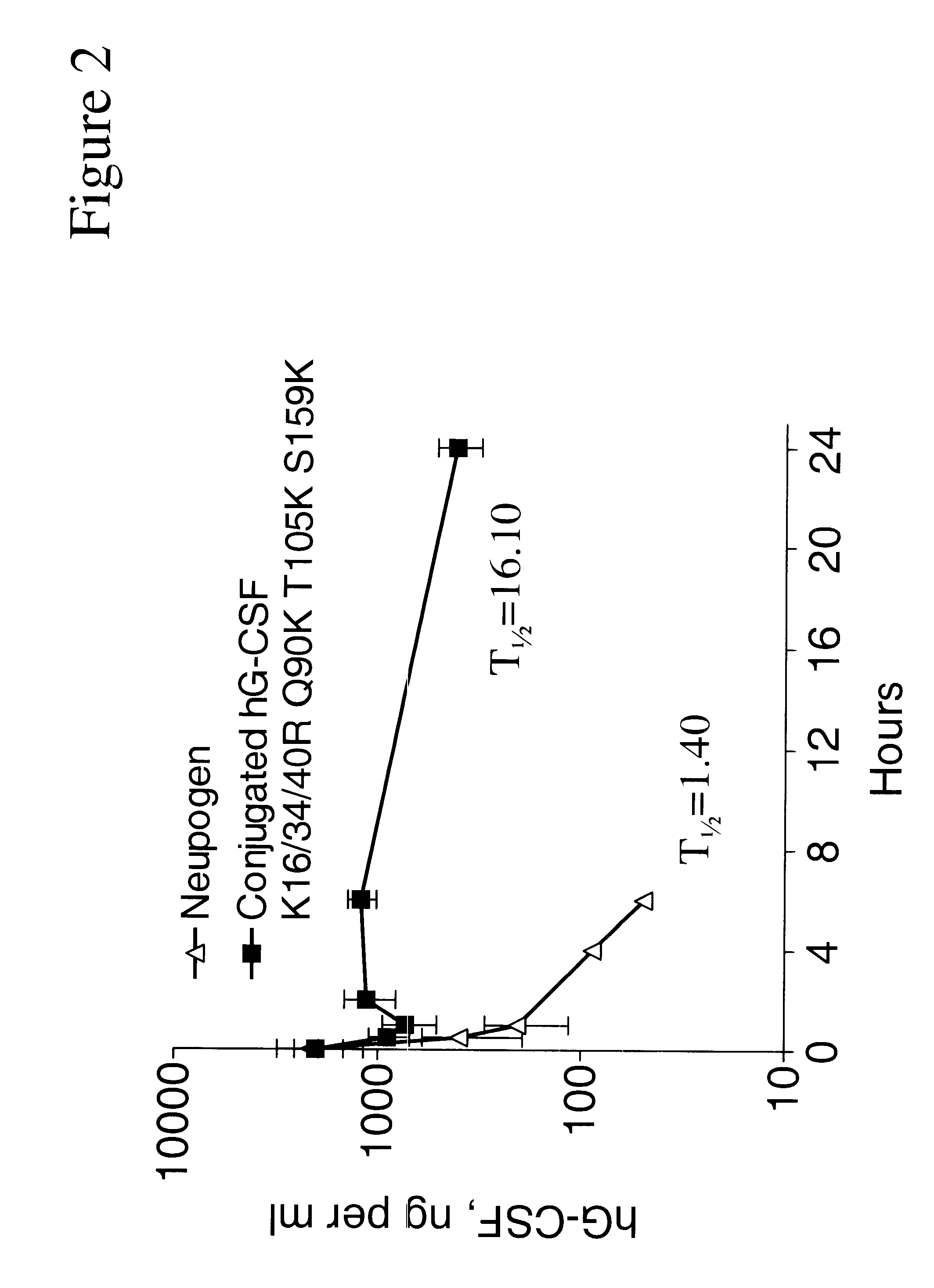
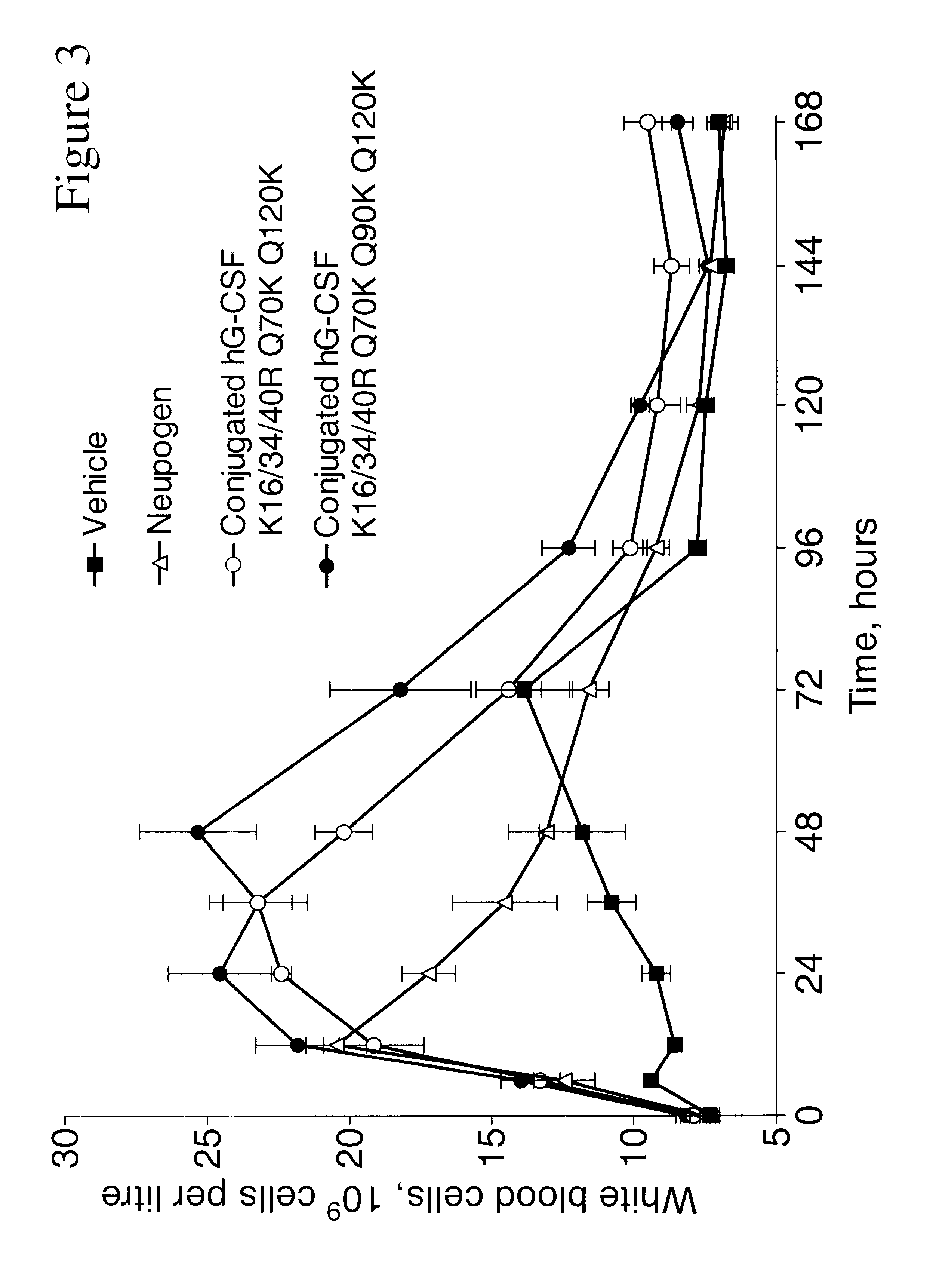
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
L-cysteine producing microorganism and method for producing L-cysteine
InactiveUS20050221453A1High expressionBacteriaRecombinant DNA-technologyMicroorganismCysteine thiolate
L-Cysteine is produced by culturing a microorganism having an ability to produce L-cysteine and modified so that expression of emrAB, emrKY, yojIH, acrEF, bcr, or cusA gene should be enhanced in a medium to produce and accumulate L-cysteine in the medium and collecting the L-cysteine from the medium. Genes coding for novel L-cysteine-excreting proteins are identified, and utilized for breeding of L-cysteine-producing microorganism to provide a novel method of producing L-cysteine.
Owner:AJINOMOTO CO INC
Sensor with improved shelf life
InactiveUS20080121533A1Weather/light/corrosion resistanceVolume/mass flow measurementMetal electrodesEthylamine
Owner:LIFESCAN INC
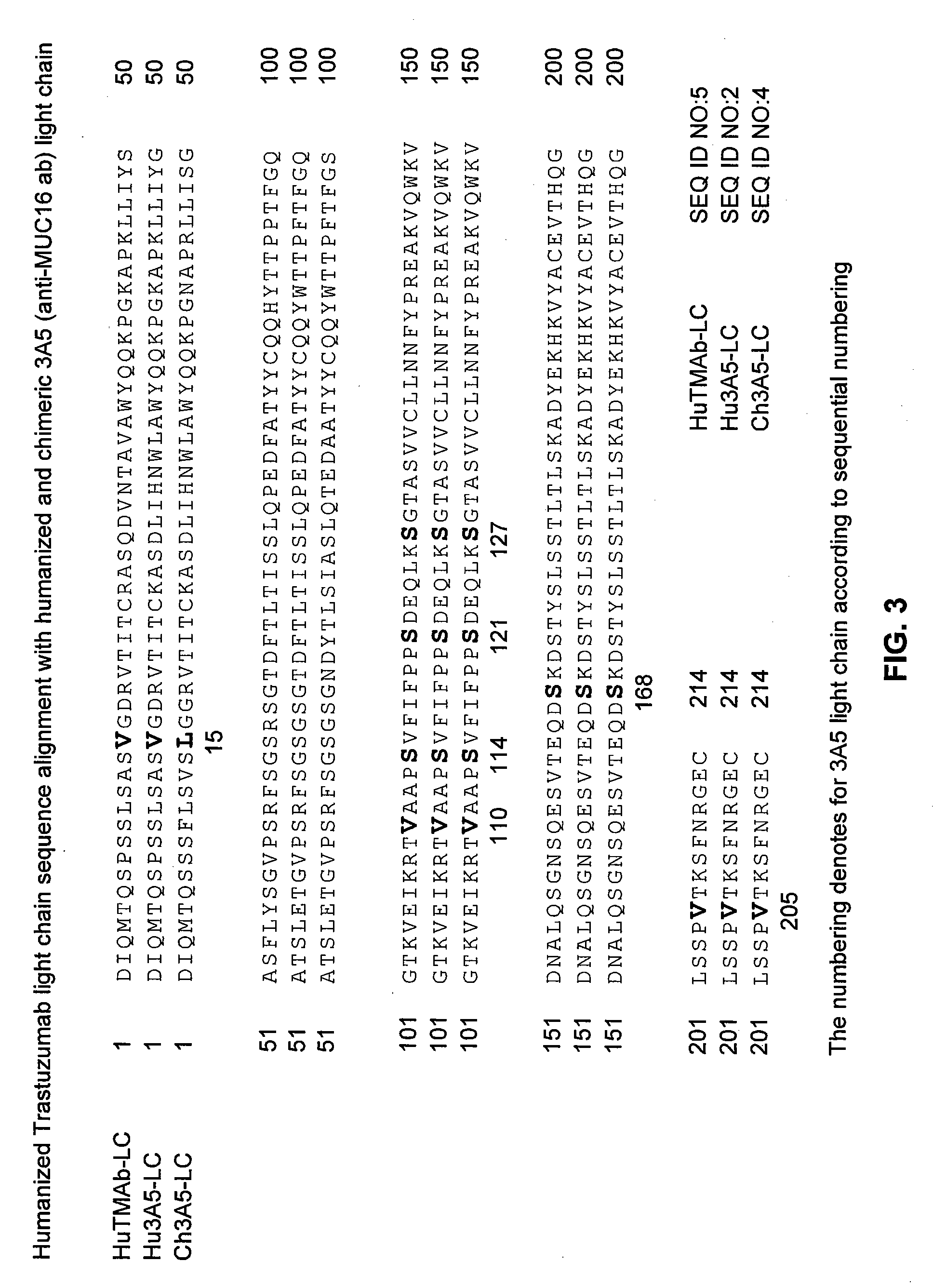
Cysteine engineered Anti-muc16 antibodies and antibody drug conjugates
ActiveUS20080311134A1In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsChemistryAntibody
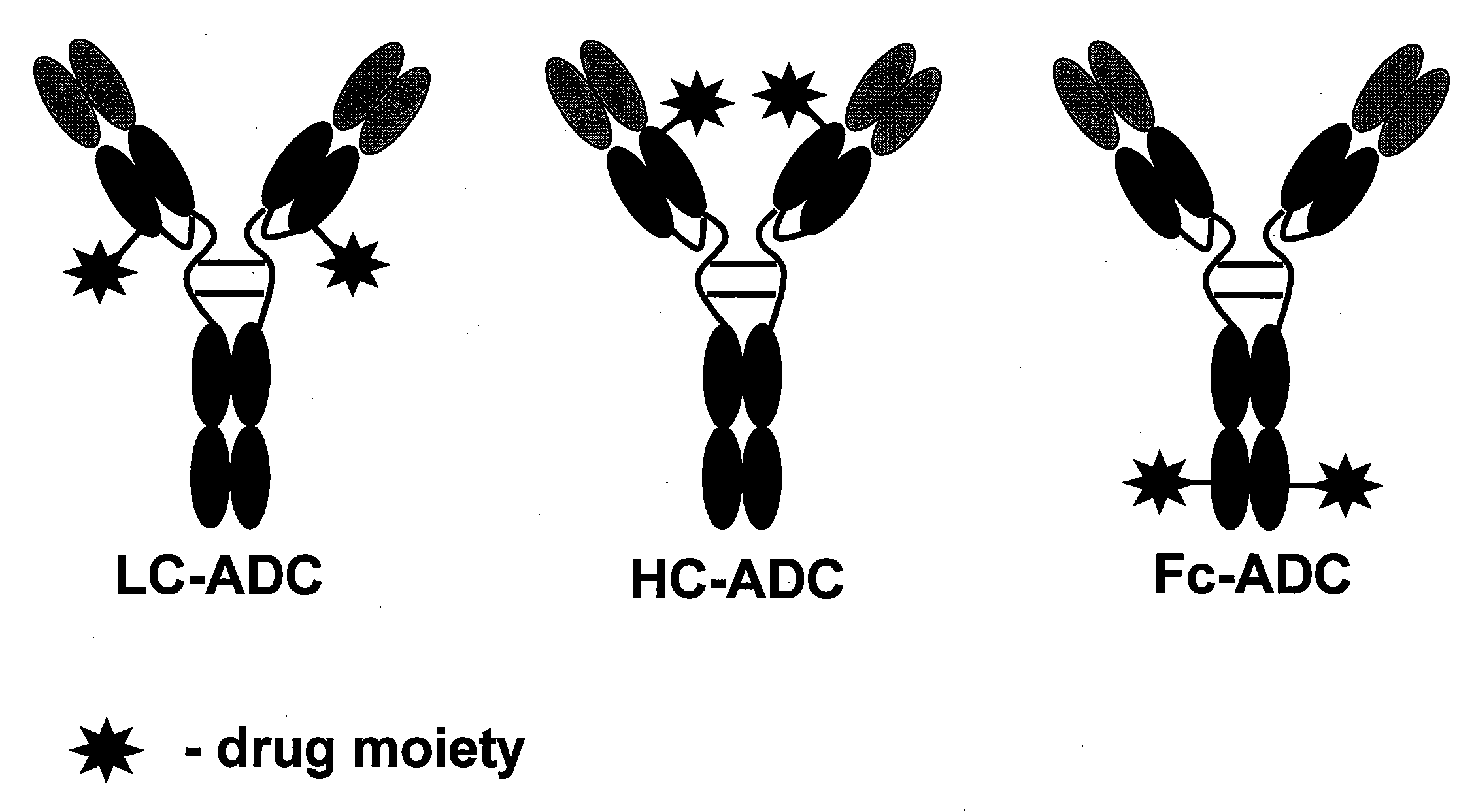
Cysteine engineered anti-MUC16 antibodies are engineered by replacing one or more amino acids of a parent anti-MUC16 antibody with non cross-linked, reactive cysteine amino acids. Methods of design, preparation, screening, and selection of the cysteine engineered anti-MUC16 antibodies are provided. Cysteine engineered anti-MUC16 antibodies (Ab) are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered anti-MUC16 antibody-drug conjugates having Formula I:Ab-(L-D)p Iwhere p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Composition for an in vitro fertilization medium
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
Self-molding permanent agent and method for proceeding free-rod and free-band type permanent
The present invention relates to a self-molding permanent agent and a method for proceeding free-rod and free-band type permanent, more particularly to a self-molding permanent agent comprising (a) a reducing composition containing a reducing agent reducing a disulfide bond of cystine on the hair and a molding stimulant spontaneously molding to fix a hair design; (b) a molding composition inducing to mold after reacting with the molding stimulant; and (c) a softening composition releasing the action of a molding stimulant, and a method for pressing a free-rod and free-band type permanent, which overcomes a disadvantage in the conventional method for pressing a permanent that needs to wear a curling device such as rods for a permanent (perm rod) or rubber band and improves to apply a wave set without a hair-curling device for a short time, since it has a self-molding feature.
Owner:KOREA RES INST OF CHEM TECH
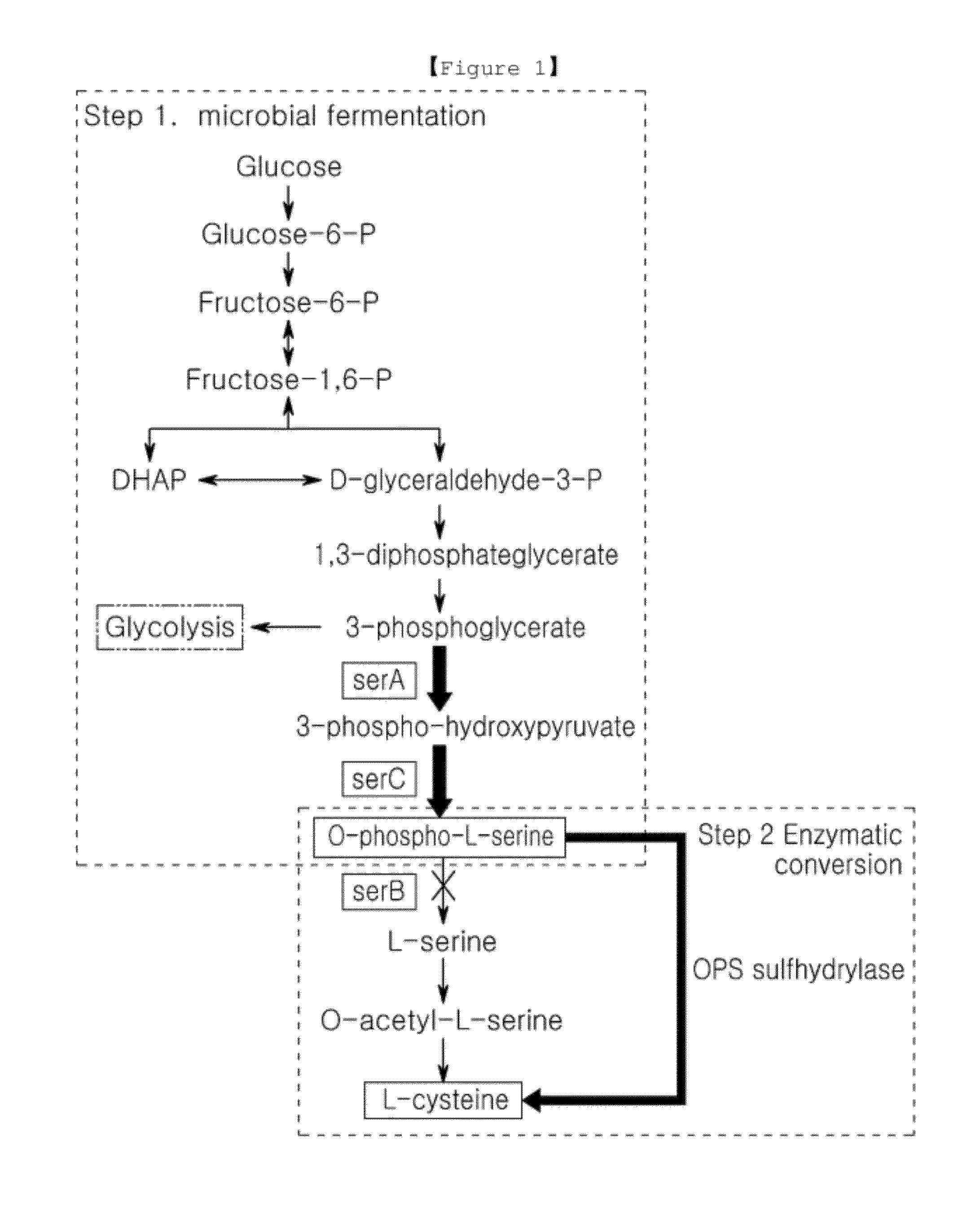
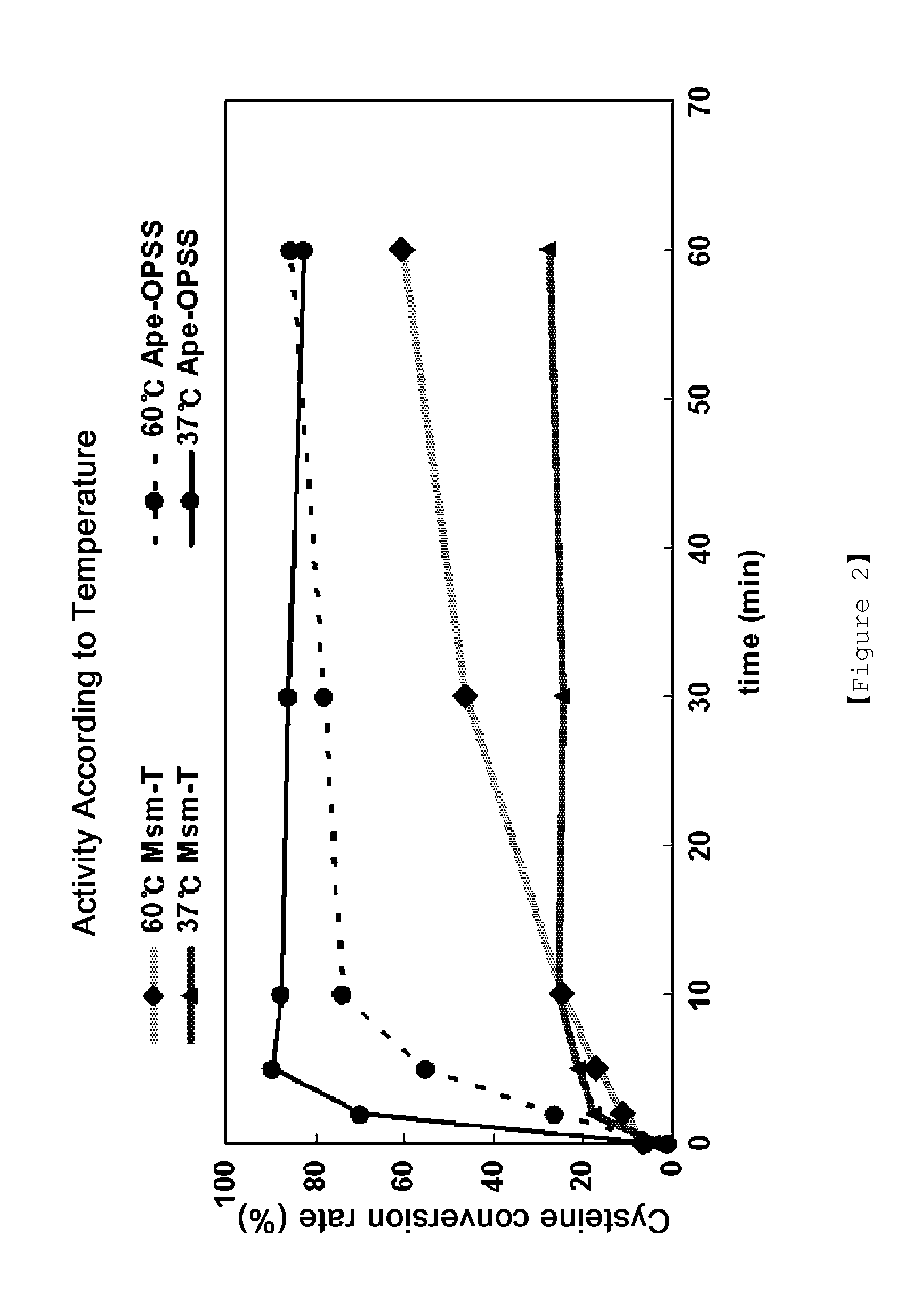
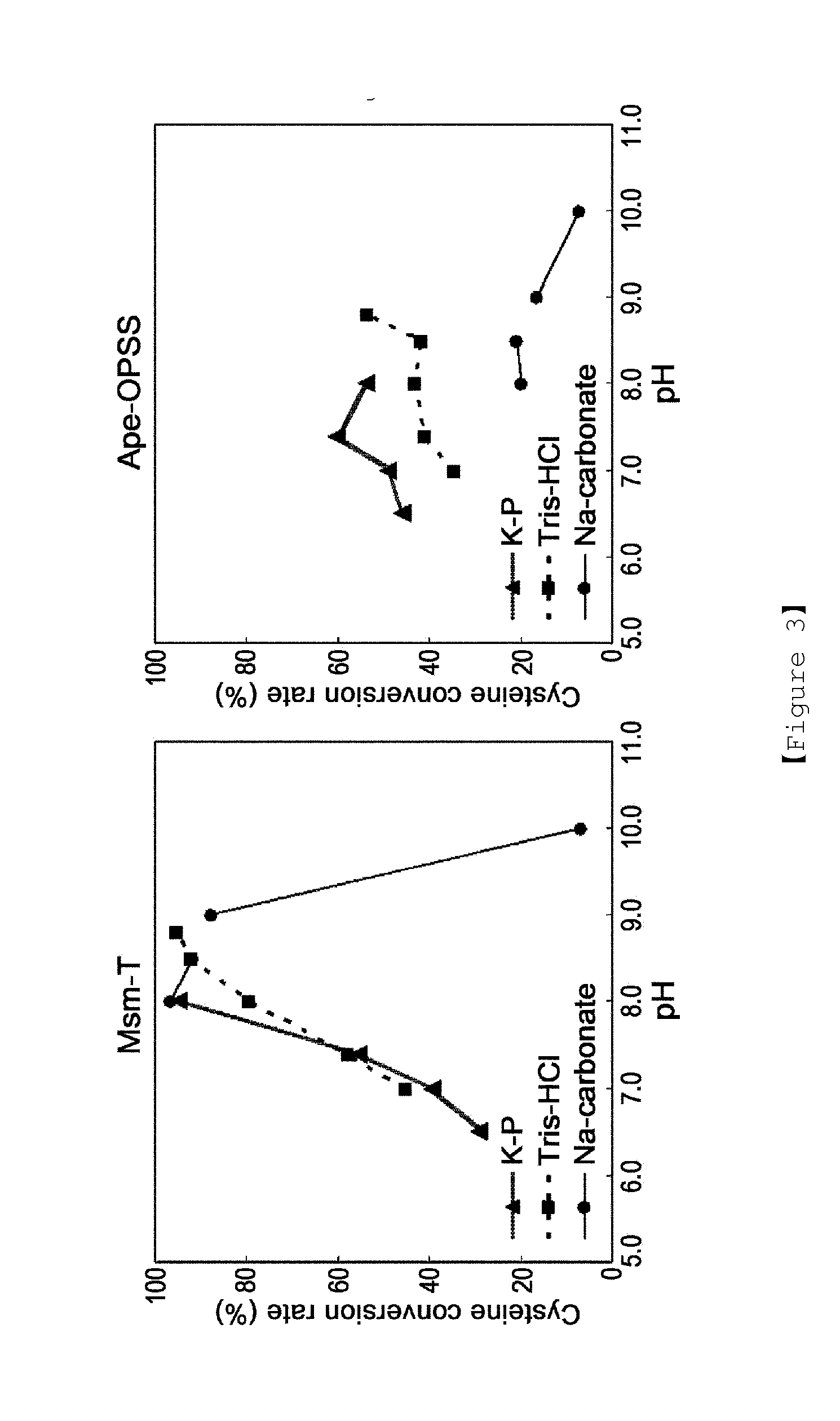
Microorganism producing o-phosphoserine and method of producing l-cysteine or derivatives thereof from o-phosphoserine using the same
InactiveUS20120190081A1Improve efficiencyHigh yieldBacteriaHydrolasesPhosphoserine phosphatase activityMicroorganism
The present invention provides methods for the production of cysteine or derivates thereof by culturing a microorganism having reduced activity of endogenous phosphoserine phosphatase. The O-phosphoserine produced by such an organism can then be reacted with a sulfide in the presence of a sulfydrylase or a microorganism expressing a sulfhydrylase to produce cysteine or a derivative thereof. Microorganisms having the properties noted above are also provided herein.
Owner:CJ CHEILJEDANG CORP
High-efficacy environment-friendly compound feed for layer hens
InactiveCN101606634AIncrease profitEmission reductionFood processingAnimal feeding stuffEconomic benefitsNitrogen
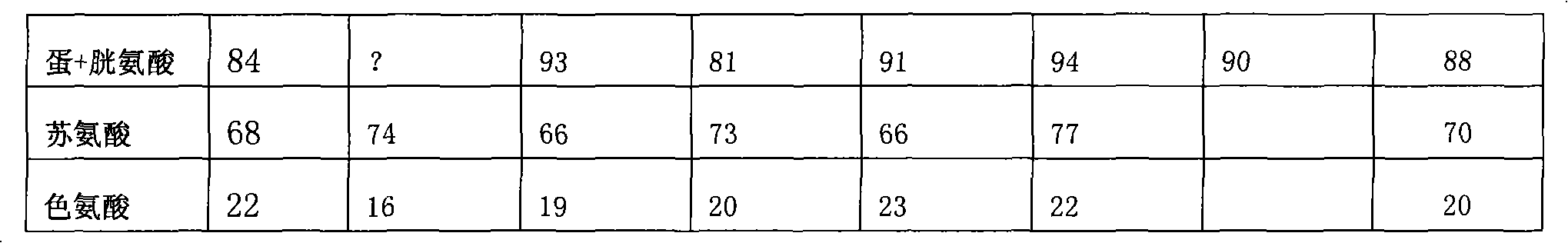
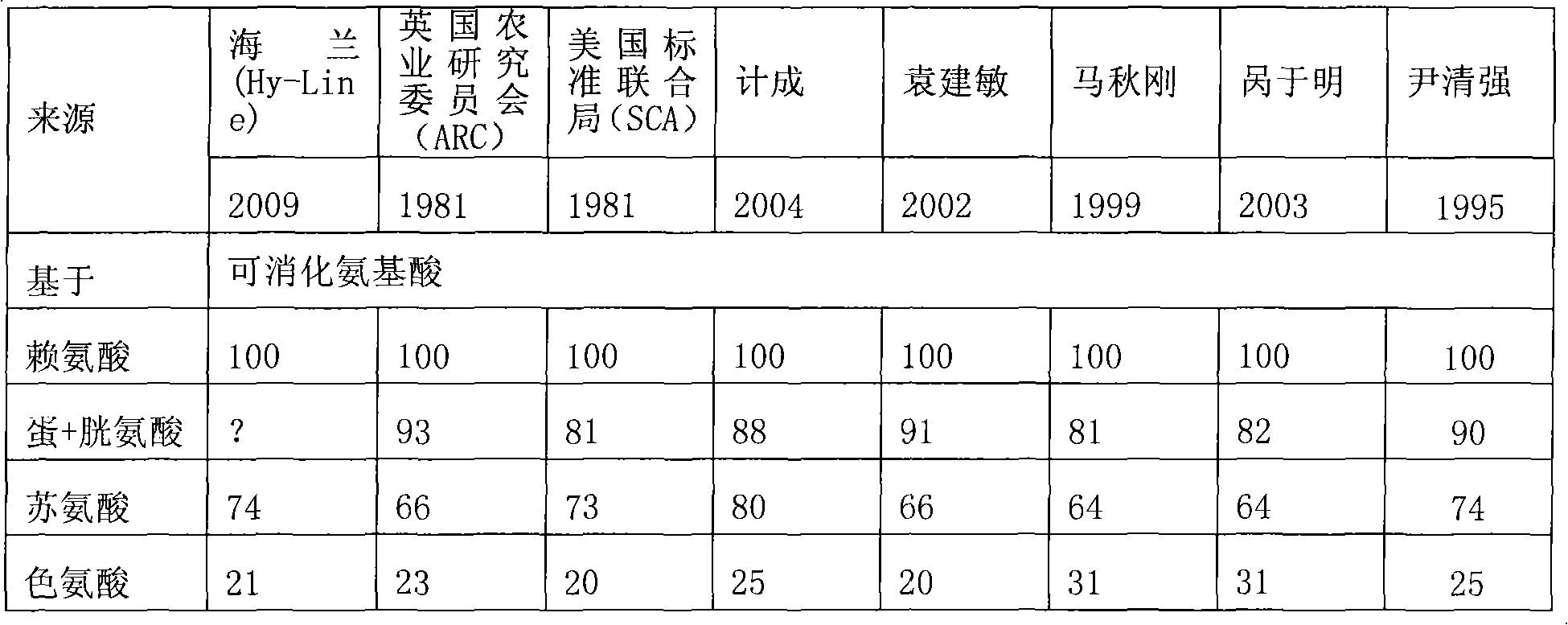
The invention relates to a high-efficacy environment-friendly compound feed for layer hens, fundamentally solving the problems of high content of crude protein, poor quality of protein and large discharge of nitrogen and phosphorus in the prior feed formulation. In the material composition of the compound feed, the balance model of digestible lysine: digestible methionine+ digestible cystine: digestible threonine: digestible tryptophan is 100:87:71:18. The invention has the advantages of reasonable design, environment protection, energy saving, improvement of laying rate, reduction of feed-egg ratio, reduction of the discharge of fecal nitrogen and fecal phosphorus and improvement of the economic benefits of enterprises.
Owner:LIAONING ZHONGYOU FEED
Target sequences for synthetic molecules
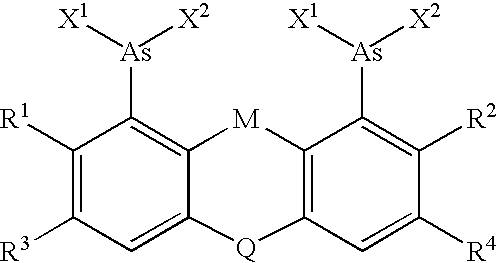
The invention is based on the discovery that certain biarsenical molecules react with specified target sequences, thereby providing a facile means for labeling polypeptides containing the target sequence. The invention is useful in creating stable mammalian cell lines expressing a certain tetracysteine tagged polypeptides, thereby overcoming toxicity associated with native tetracysteine. In addition, the invention allows for orthogonal labeling of polypeptides, thereby allowing for the observation of protein-protein interactions and conformational changes in proteins, for example.
Owner:LIFE TECH CORP
Cysteine protease inhibitor
InactiveUS6162828ALow toxicitySafety and toxicityBiocideCarbamic acid derivatives preparationHydrogen atomCysteine Proteinase Inhibitors
PCT No. PCT / JP96 / 00840 Sec. 371 Date May 20, 1996 Sec. 102(e) Date May 20, 1996 PCT Filed Mar. 29, 1996 PCT Pub. No. WO96 / 30395 PCT Pub. Date Oct. 3, 1996A pharmaceutical composition for inhibiting cysteine protease which comprises a compound of the formula: wherein R1 is a hydrogen atom or an acyl group; R2, R3 and R4, same or different, are a bond, an amino acid residue or a group of the formula:-Y-R5-in which R5 is a group resulting from imino group removal from an amino acid residue; Y is -O-, -S- or -NR6- in which R6 is a hydrogen atom or a lower alkyl group; A is Z is a hydrogen atom, an acyl group or an optionally substituted hydrocarbon group; n is 1 or 2; provided that when n is 1, then A is and Y is -S- or -NR6-, and, at least one of R2, R3 and R4 is the formula -Y-R5-, provided that when further all Y are -NR6-, at least one of the amino acid residues is not bound to amhydrogen atom at the alpha -carbon thereof but substituted via carbon; provided that when n is 2 and Z is an aldehyde group, then R1 is an acyl group having 6 or more carbon atoms; provided that when n is 2 and A is, then at least one of R2, R3 and R4 is the formula -Y-R5-; or an ester or a salt thereof, and a pharmaceutically acceptable carrier.
Owner:TAKEDA PHARMACEUTICALS CO LTD
Black garlic, process and device for fermenting same
InactiveCN101518319AEasy to peel offNo irritating odorClimate change adaptationFruits/vegetable preservation using acidsS-Allyl cysteinePesticide residue
The invention discloses black garlic, a process and a device for fermenting the same, which overcomes technical defects of the prior product, processing method and processing device. The black garlic is integral in bulk, dry in outer peel and easy to peel. Garlic cloves are in preserved fruit paste shape, are free from pungent taste, have jelly taste, taste good and are easy to preserve. Every 100 grams of ripening black garlic contains 140 milligrams of isoleucine, 250 milligrams of leucine, 170 milligrams of lysine, 160 milligrams of cystine, S-allyl cysteine and polyphenol of which the content is 22 times that of raw garlic, 36 milligrams of sodium, 930 milligrams of potassium, 52 milligrams of magnesium and 13 milligrams of calcium. The black garlic has the advantages of low heavy-metal content, no pesticide residue and no additive. The fermenting process comprises the steps of taking integral garlic as raw material, initially washing the raw material with clear water, soaking the raw material in brine, placing the raw material in a tray, fermenting, steaming and baking the raw material, performing aging treatment, sterilizing and disinfecting the obtained product, and packing the obtained product in vacuum or with nitrogen. The fermenting process has the advantages of adopting mineral water vapor and discharging air in the whole process, along with few links and low cost. The fermenting device is heated by a steam boiler, has a humidifying vapor pipe, and uses all-wooden fermentation frame and a container.
Owner:DALIAN HUAGU GARLIC
Compositions for potentiating glutathione
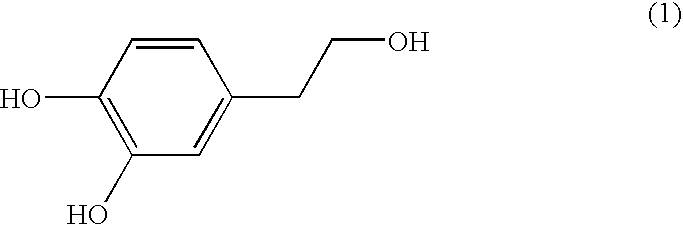
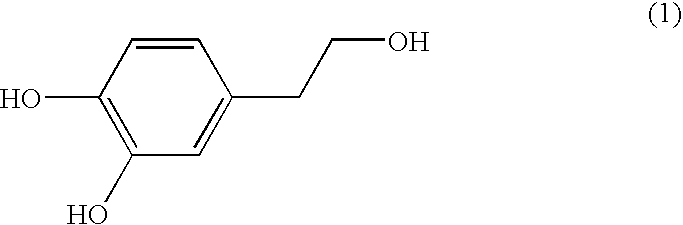
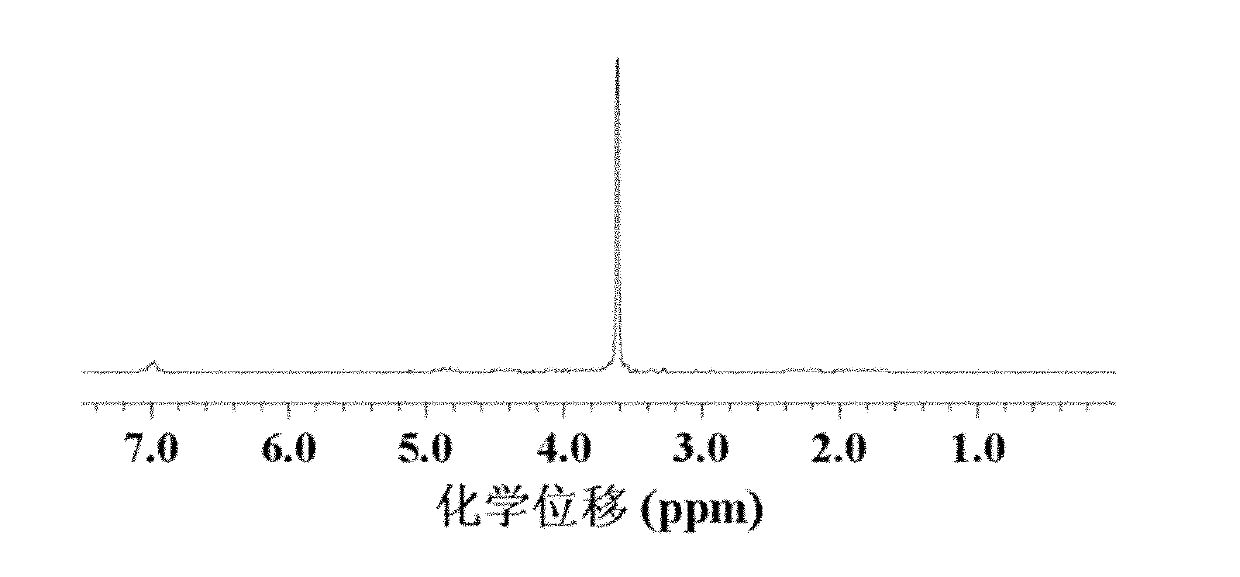
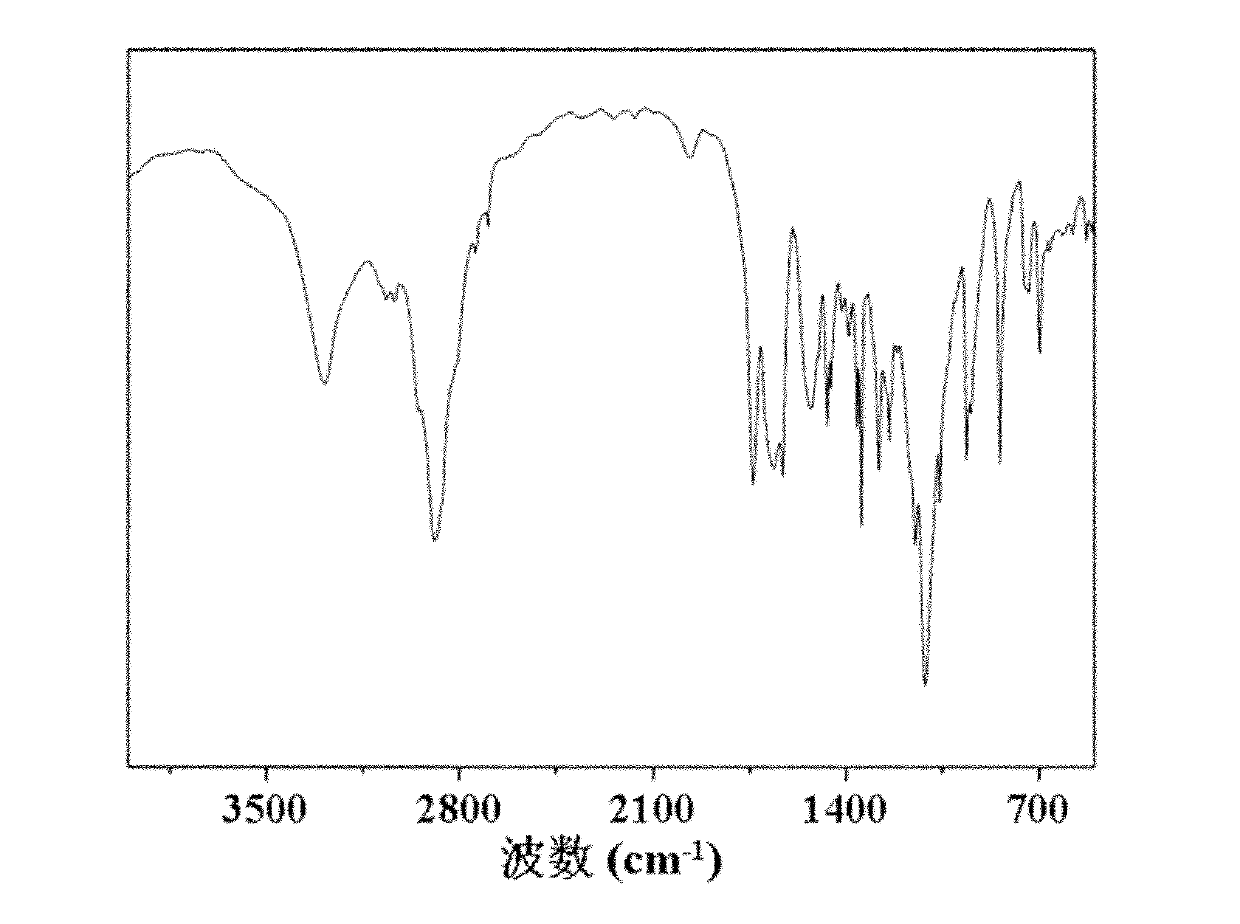
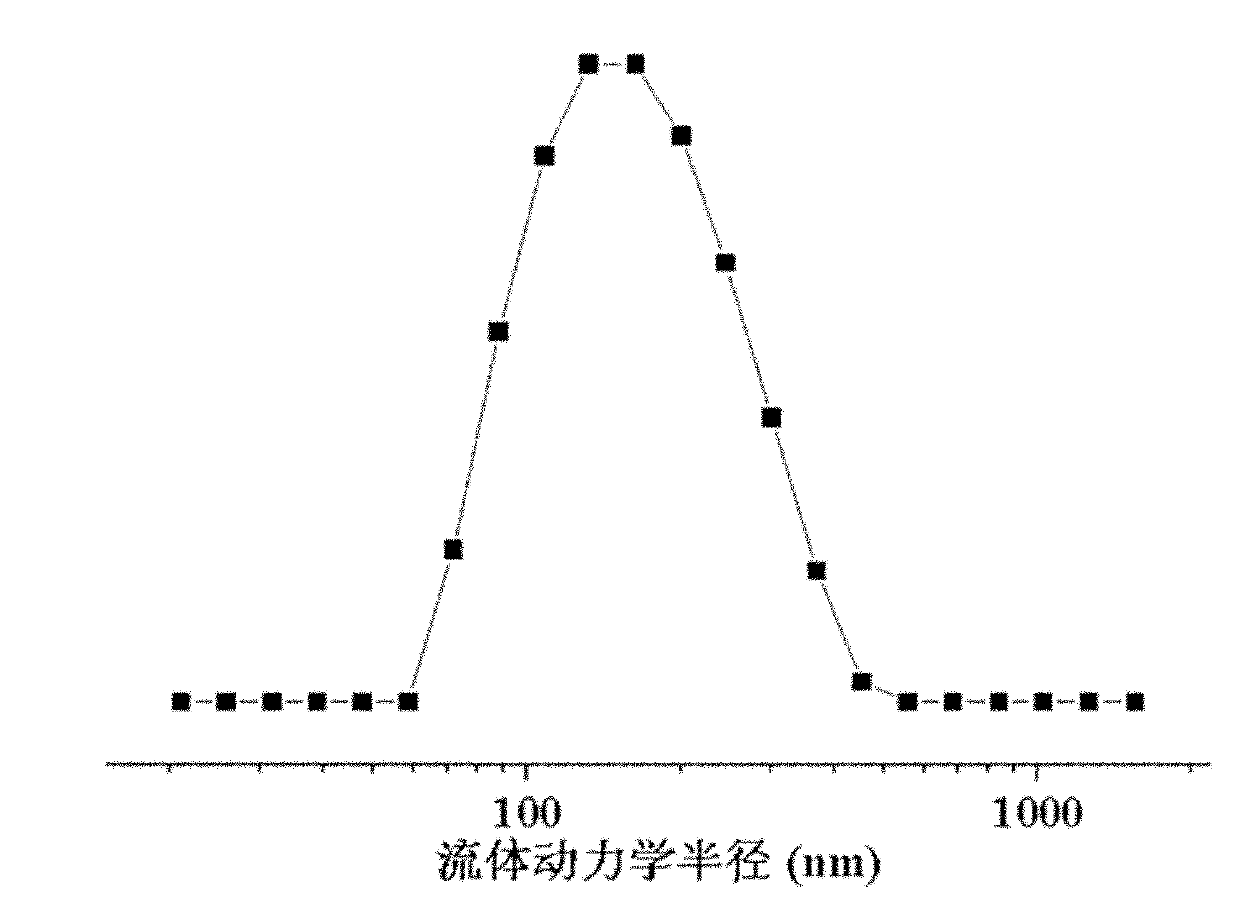
A composition for potentiating glutathione, which contains at least one member selected from the group consisting of 2-(3,4-dihydroxyphenyl)ethanol or a glycoside thereof, a plant containing 2-(3,4-dihydroxyphenyl)ethanol or a glycoside thereof, an extract of said plant, a hydrolysate of said plant, and a hydrolysate of the extract of said plant (excluding Olea europaea and an extract thereof), and which further contains at least one member selected from among an S-containing compound that is a supply source of cysteine, a protein that contains cysteine and / or cystine, a yeast that contains cysteine and / or cystine, and a vitamin.
Owner:FUAN KERU
Compositions for potentiating glutatthione
A composition for potentiating glutathione, which contains at least one member selected from the group consisting of 2-(3,4-dihydroxyphenyl)ethanol or a glycoside thereof, a plant containing 2-(3,4-dihydroxyphenyl)ethanol or a glycoside thereof, an extract of said plant, a hydrolysate of said plant, and a hydrolysate of the extract of said plant (excluding Olea europaea and an extract thereof), and which further contains at least one member selected from among an S-containing compound that is a supply source of cysteine, a protein that contains cysteine and / or cystine, a yeast that contains cysteine and / or cystine, and a vitamin.
Owner:FUAN KERU
Preparation method of polyamino acid and polyamino acid nano-hydrogel
ActiveCN102167817ASimple stepsGood biocompatibilityPharmaceutical non-active ingredientsCross-linkControlled release
The invention provides a preparation method of polyamino acid, which comprises the following steps: dissolving a terminal-aminated hydrophilic polymer, L-cystine-N-endo-carboxylic acid anhydride and amino acid-N-endo-carboxylic acid anhydride in an organic solvent, stirring and reacting, thus obtaining the polyamino acid. The invention further provides polyamino acid nano-hydrogel which comprises the polyamino acid prepared by the method adopting the technical scheme, and water. The polyamino acid which can form the nano-hydrogel can be prepared by only one step, and the step is simple, convenient and fast. The obtained polyamino acid comprises a hydrophilic polymer and a cross-linked polyamino acid, the nano-hydrogel can be formed by dissolving the polyamino acid in the water, and the nano-hydrogel can be used as carrier materials for medicament transmission, and medicament control and release. The terminal-aminated hydrophilic polymer, the L-cystine-N-endo-carboxylic acid anhydrideand the amino acid-N-endo-carboxylic acid anhydride are used as raw materials, and the nano-hydrogel formed by the obtained polyamino acid has good biocompatibility and biodegradability.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Detergent composition containing amino acid component for washing fruits, vegetables and dishes
InactiveCN102492571APromote degradationMaintain stain releaseNon-ionic surface-active compoundsOrganic detergent compounding agentsAntioxidantArginine
The present invention relates to a detergent composition containing an amino acid component for washing fruits, vegetables and dishes, which comprises the following components of an anionic surfactant, a non-ionic surfactant, an amphoteric surfactant, amino acid, a viscosity modifier, a chelating agent, an antiseptic, a light stabilizer, an antioxidant, a flavor and deionized water. The amino acid composition is one or a mixture with more than two of alanine, arginine, asparagine, aspartic acid, cystine, glutamine, glutamic acid, leucine, lysine, methionine, phenylalanine, prolinol acid, serine, threonine, glycine, histidine, isoleucine, tryptophan, tyrosine and valine. The detergent of the invention can maintain good decontamination capability, improve the feeling when the detergent composition is used, reduce the hands skin irritation and enhance the hand skin moisturizing ability to improve dry feeling.
Owner:GUANGZHOU LIBY
Cysteine engineered anti-TENB2 antibodies and antibody drug conjugates
Cysteine engineered anti-TENB2 antibodies are engineered by replacing one or more amino acids of a parent anti-TENB2 antibody with non cross-linked, reactive cysteine amino acids. Methods of design, preparation, screening, and selection of the cysteine engineered anti-TENB2 antibodies are provided. Cysteine engineered anti-TENB2 antibodies (Ab) are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered anti-TENB2 antibody-drug conjugates having Formula I:Ab-(L-D)p Iwhere p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
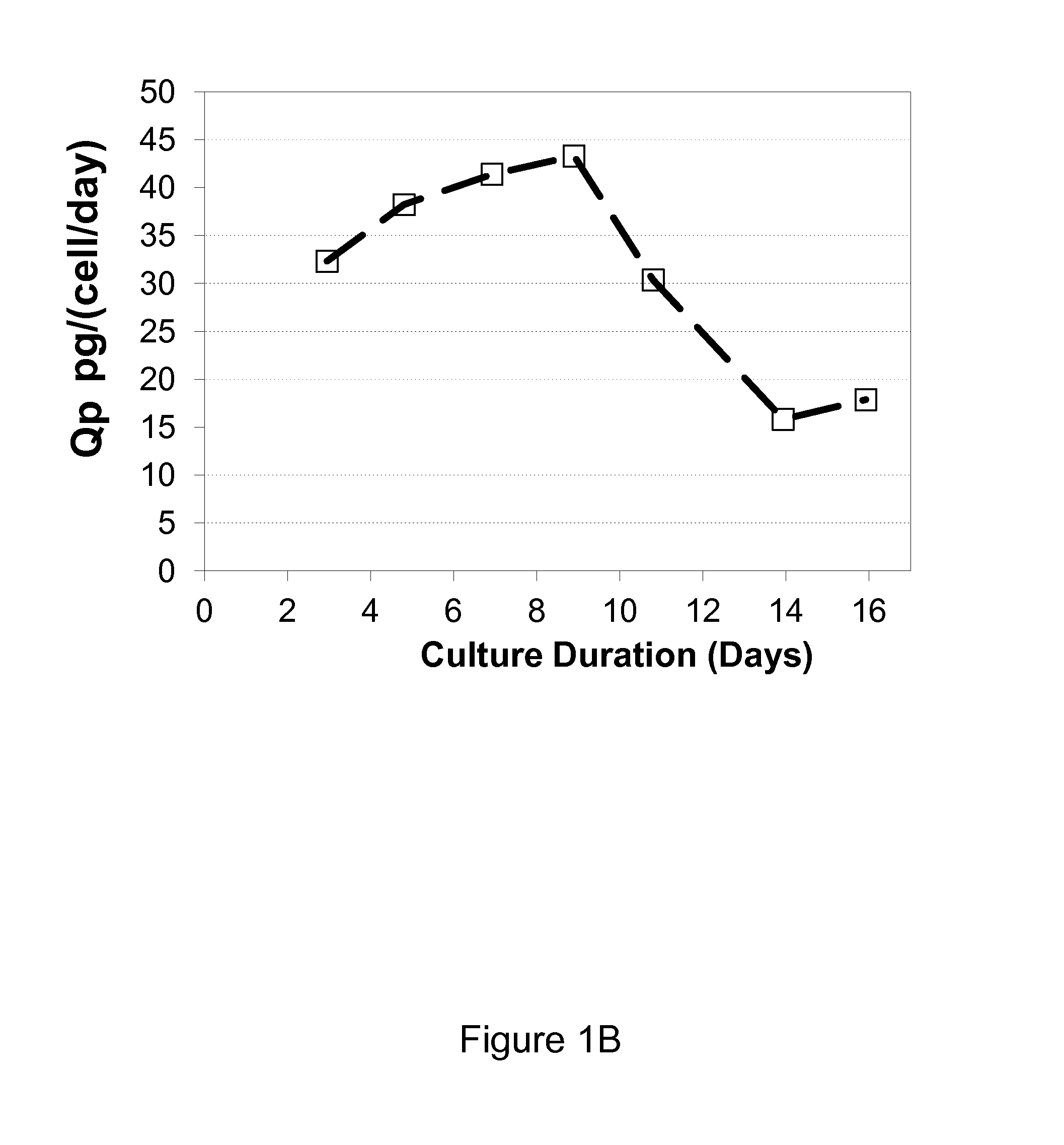
Method for culturing mammalian cells to improve recombinant protein production
ActiveUS20140178984A1Increase productivityImprove viabilityAnimal cellsGenetically modified cellsBiotechnologyTyrosine
The present invention relates to methods for mammalian cell culture. The methods make use of independent tyrosine and cystine feed streams.
Owner:AMGEN INC
Composition for prevention or treatment of an alcohol hangover
InactiveUS20050271754A1Relieve symptomsLittle and no toxicityBiocideDipeptide ingredientsNutritionSuccinic acid
The present invention represents a formulation and method for preventing or reducing the effects of veisalgia or alcohol “hangover.” More particularly, the present invention relates to a treatment which provides nutritional requirements in the form of L-glutamine, L-cysteine, fumaric acid, succinic acid, young barley grass juice powder, vitamin B-12, vitamin B-1, calcium ascorbate, and dextrose. The composition may be ingested before, before and during, or after consumption of ethyl alcohol. The composition of the present invention is made available in several forms including, but not limited to, capsule, tablet or liquid form suitable for administration for amelioration of alcohol induced hangover symptoms. This treatment enables rapid relief of symptoms in affected individuals by slowing or reducing the conversion of ethanol into acetaldehyde, a toxic substance resulting from alcohol metabolism. Additionally, the present invention also incorporates nutritional elements which are directed toward assisting in the conversion of acetaldehyde into acetic acid and the oxidative processes in the mitochondria responsible for decomposition of acetaldehyde.
Owner:CHEERZ USA
Preparation method and application of selenium-enriched cadmium-resistant foliar fertilizer special for rice
InactiveCN105819986AReduce usageEliminate the risk of poisoningFertilising methodsNitrogenous fertilisersSucroseSilicic acid
The invention provides a preparation method and application of a selenium-enriched cadmium-resistant foliar fertilizer special for rice .The preparation method of the selenium-enriched cadmium-resistant foliar fertilizer special for the rice comprises the following steps that sodium selenite, zinc sulfate, manganese sulfate and ferrous sulfate are dissolved in water and prepared into an original mother liquid, wherein the concentration of sodium selenite ranges from 30 g / l to 60 g / l; the original mother liquid is mixed with an amino acid solution of which the mass concentration ranges from 15% to 20%, shaking chelating is conducted, and a chelating liquid is obtained; the chelating liquid and a single silicic acid aqueous solution are mixed to be uniform to be prepared into a mixed liquid; sodium alkyl benzene sulfonate is added, the mixture and filtrate obtained through saccharose alcohol fermentation are stirred at 25 DEG C to 35 DEG C for secondary chelating, and the selenium-enriched cadmium-resistant foliar fertilizer special for the rice is obtained .By means of the preparation method and application of the selenium-enriched cadmium-resistant foliar fertilizer special for the rice, 90% or above of inorganic selenium is converted into organic selenium such as selenomethionine and selenocystine, the poisoning risk is eliminated, the absorption conversion rate of selenium is increased, the raw material amount is reduced, the cost is reduced, and the rice has a good cadmium-resistant property.
Owner:AGRI RESOURCE & ENVIRONMENT RES INST GUANGXI ACADEMY OF AGRI SCI
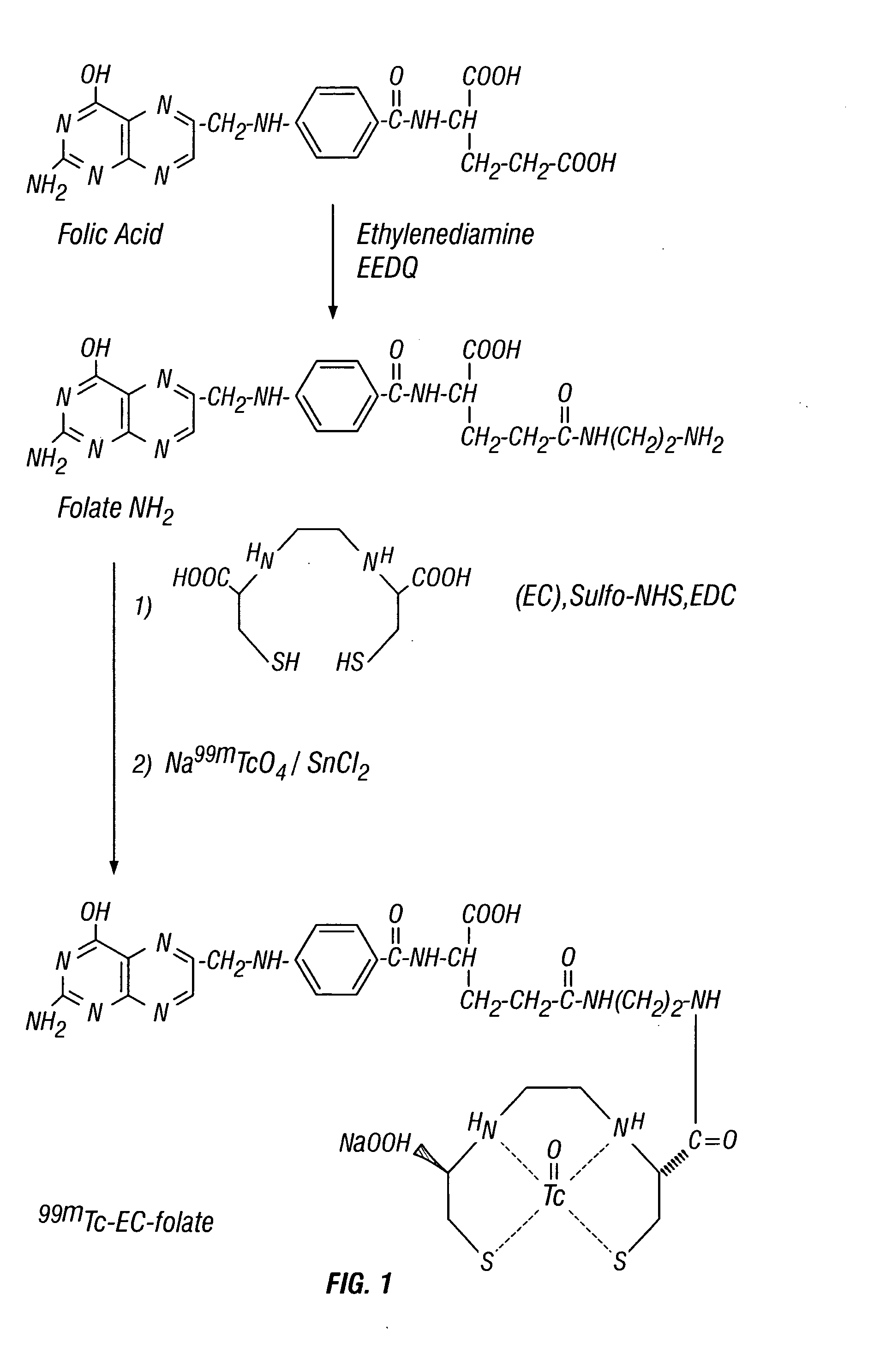
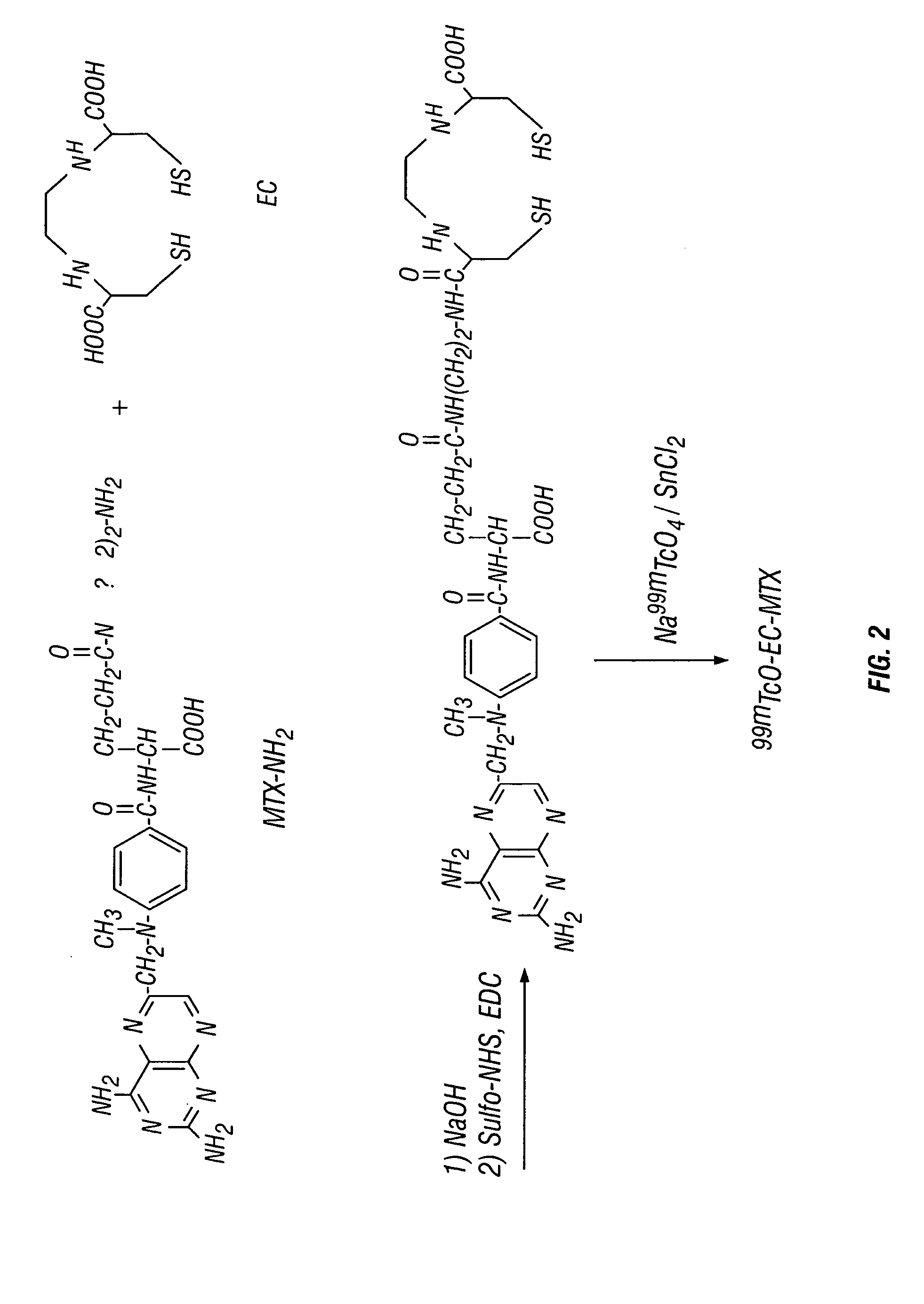
Ethylenedicysteine (EC)-drug conjugates, compositions and methods for tissue specific disease imaging
InactiveUS20050079133A1Reacts more completelyRadioactive preparation carriersRadiation therapyImaging agentTissue specific
The invention provides, in a general sense, a new labeling strategy employing 99mTc chelated with ethylenedicysteine (EC). EC is conjugated with a variety of ligands and chelated to 99mTc for use as an imaging agent for tissue-specific diseases. The drug conjugates of the invention may also be used as a prognostic tool or as a tool to deliver therapeutics to specific sites within a mammalian body. Kits for use in tissue-specific disease imaging are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
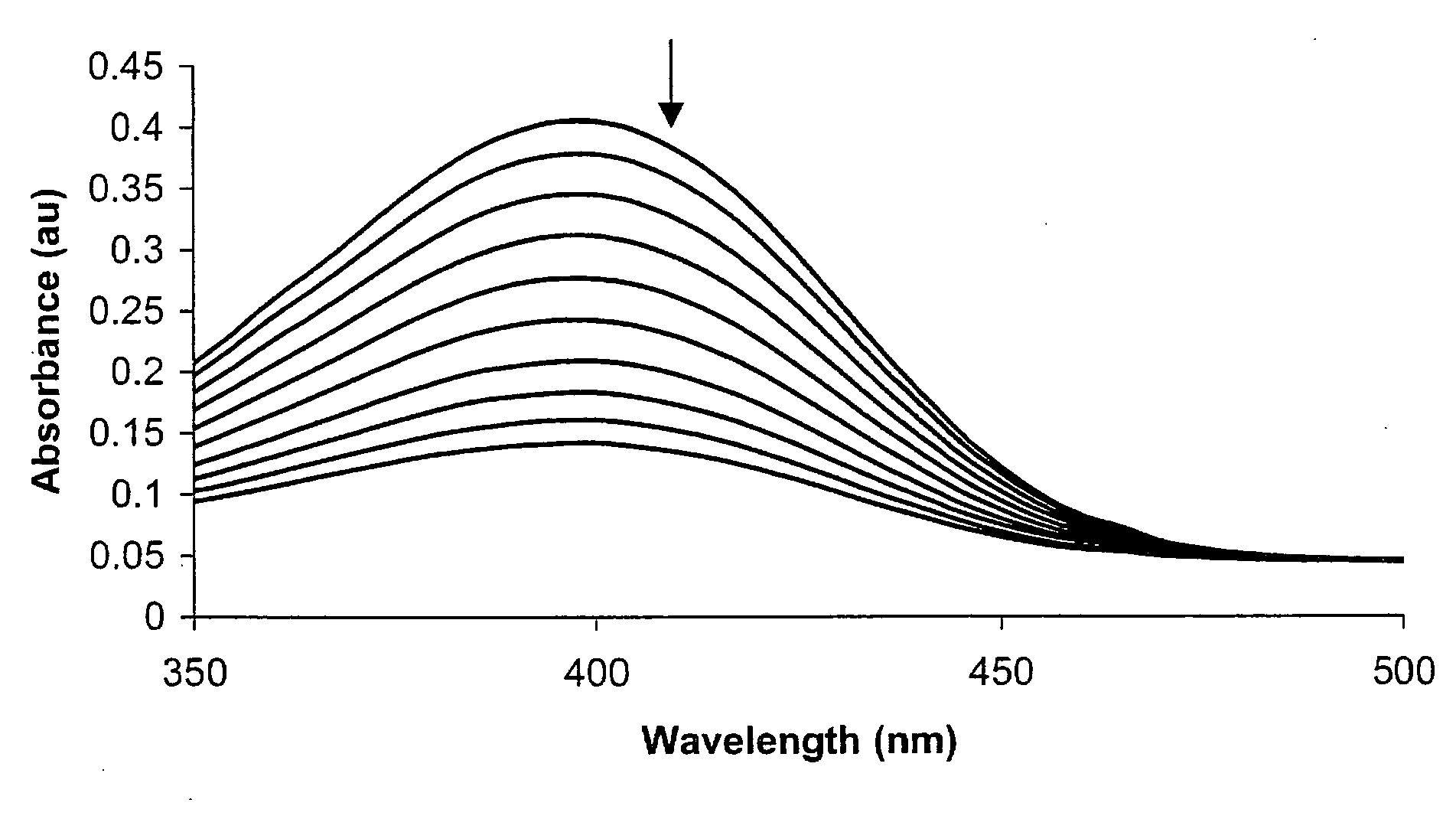
Colorimetric and Fluorometric Determination of Homocysteine and Cysteine
ActiveUS20080261315A1Material analysis by observing effect on chemical indicatorBiological testingFluorescenceCysteine thiolate
Colorimetric and fluorometric methods are disclosed for the rapid, accurate, selective, and inexpensive detection of homocysteine, or of homocysteine and cysteine, or of cysteine. The methods may be employed with materials that are readily available commercially. The novel methods are selective for homocysteine, for cysteine, or for total homocysteine and cysteine, and do not cross-react substantially with chemically-related species such as glutathione. The homocysteine-selective method does not have substantial cross-reactivity to the very closely related species cysteine. The cysteine-selective method does not have substantial cross-reactivity to the very closely related species homocysteine. The methods may be used, for example, in a direct assay of human blood plasma for homocysteine levels.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Formaldehyde fluorescent probe, and preparation method and application thereof
InactiveCN105372217AImprove detection accuracyImprove accuracyFluorescence/phosphorescenceLuminescent compositionsFluoProbesArginine
The invention relates to a preparation method and application of a formaldehyde fluorescent probe, and belongs to the technical field of analytical chemistry. The formaldehyde fluorescent probe is a ratio-type fluorescent probe with double emission bands. The excitation wavelength is 318 nm; and the fluorescence peak at 359nm decreases gradually with the increasing of the concentration of formaldehyde, and at the same time a new emission band is produced at 451 nm and the fluorescence peak gradually increases. The formaldehyde fluorescent probe can resist the interference of acetaldehyde, glyoxal, methylglyoxal, benzaldehyde, alanine, glycine, serine, arginine, cysteine, glutathione and glucose, has high detection accuracy, high sensitivity and strong anti-interference capability. In addition, the formaldehyde fluorescent probe of the invention can detect the formaldehyde in a biological sample (cell environment), realizes formaldehyde fluorescence imaging in living cell level, and has potential practical application value. The synthesis of the formaldehyde fluorescent probe only needs a one-step reaction, which is simple and high in yield.
Owner:UNIV OF JINAN
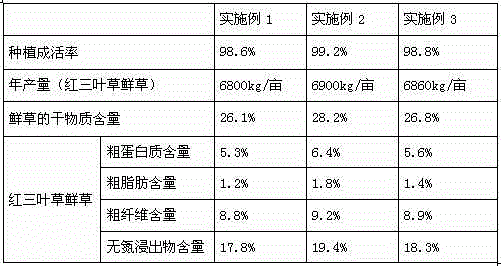
Brackish water and fresh water rotation irrigation method for saline and alkaline land and application thereof to red clover planting
InactiveCN104956886AImprove the survival rate of plantingIncrease productionWatering devicesPlant cultivationAlkali soilMicrobial agent
The invention provides a brackish water and fresh water rotation irrigation method for a saline and alkaline land. Irrigation includes irrigation in the seedling period, irrigation in the growing period and irrigation in the cutting period. According to irrigation in the growing period, the ratio of rotation irrigation number of times of the organic-matter-enriched brackish water to that of the fresh water is 1:2, the land is irrigated once every 8-10 days, and the one-time irrigation volume is 450-500 m<3> / hectare. A preparing method for the organic-matter-enriched brackish water includes the step that organic-matter-enriched fertilizer is dissolved in the brackish water. The organic-matter-enriched fertilizer comprises, by weight, 24-26 parts of seaweed residues, 10-12 parts of chicken manure, 1-3 parts of microbial agent, 2-4 parts of magnesium-containing phosphate, 5-8 parts of fulvic acid and 2-3 parts of glutelin cystine. The irrigation method is adopted to plant red clover, the planting survival rate is 98.6-99.2%, and the annual output of red clover fresh grass is 6800-6900 kg / mu.
Owner:WEIFANG YOURONG IND
Nutritional or therapeutic compositions and methods to increase bodily glutathione levels
Nutritional or therapeutic compositions containing glutamic acid, cystine, glycine and a selenium precursor and methods for their utilization to increase glutathione synthesis and thereby enhance the immune system are described.
Owner:THE PROIMMUNE
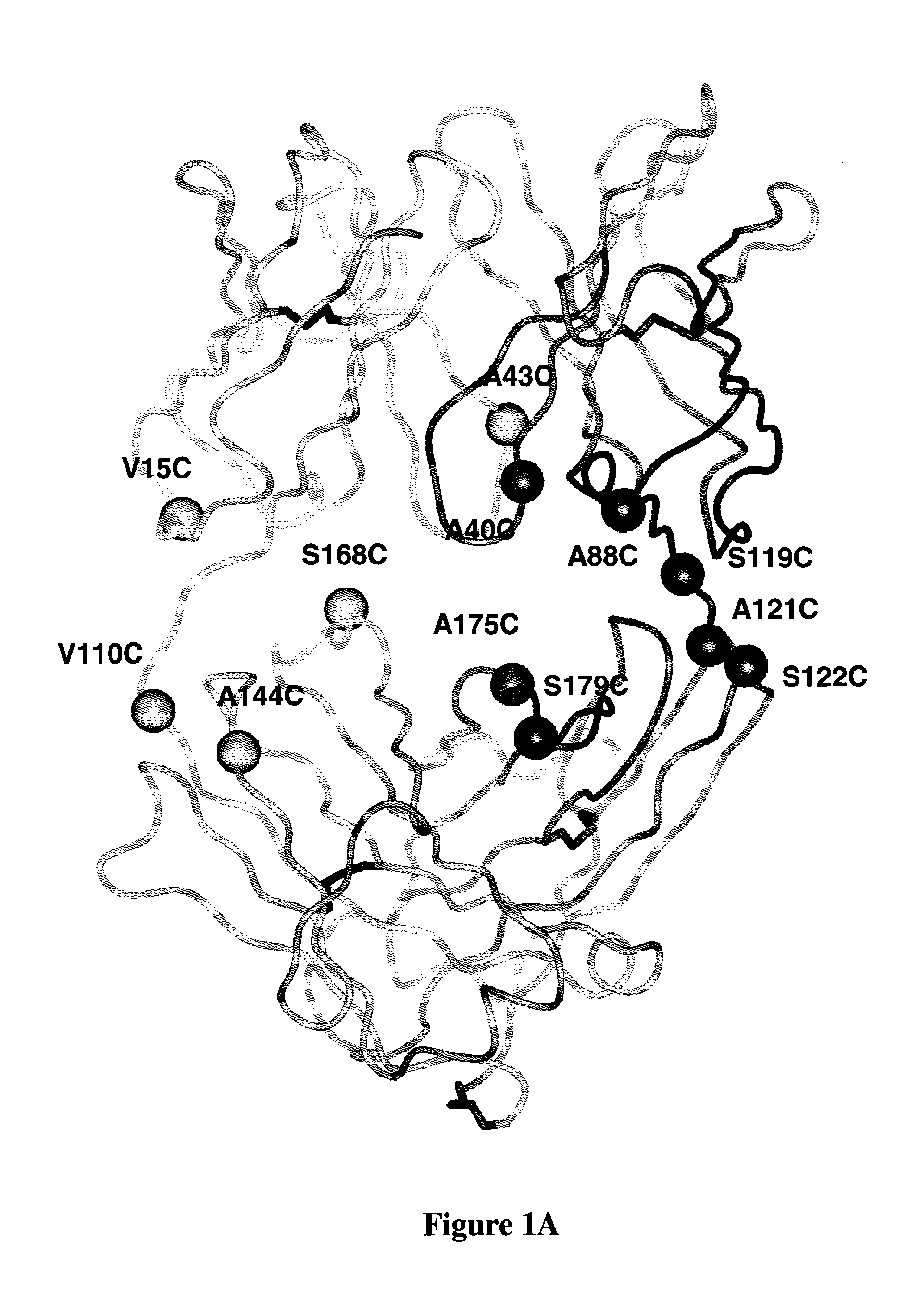
Cysteine engineered antibodies and conjugates
Cysteine engineered antibodies comprising a free cysteine amino acid in the heavy chain or light chain are prepared by mutagenizing a nucleic acid sequence of a parent antibody and replacing one or more amino acid residues by cysteine to encode the cysteine engineered antibody; expressing the cysteine engineered antibody; and isolating the cysteine engineered antibody. Certain highly reactive cysteine engineered antibodies were identified by the PHESELECTOR assay. Isolated cysteine engineered antibodies may be covalently attached to a capture label, a detection label, a drug moiety, or a solid support.
Owner:GENENTECH INC
Stabilized HBc chimer particles as immunogens for chronic hepatitis
InactiveUS7351413B2Easy to prepareImprove stabilityAntibody mimetics/scaffoldsAntipyreticProtein moleculesChronic hepatitis
A method of treating chronic hepatitis B is disclosed that comprises administering a T cell-stimulating amount of a vaccine to a patient. The vaccine comprises an immunogenic amount of chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (core) protein (HBc) that is engineered for both enhanced stability of self-assembled particles and the substantial absence of nucleic acid binding by those particles. The chimeric protein molecule can include one or more immunogenic epitopes peptide-bonded to one or more of the N-terminus, the immunogenic loop or the C-terminus of HBc. The enhanced stability of self-assembled particles is obtained by the presence of at least one heterologous cysteine residue near one or both of the amino-terminus and carboxy-terminus of the chimer molecule.
Owner:LORANTIS
Cathepsin cysteine protease inhibitors
Owner:AXYX PHARMA INC +1
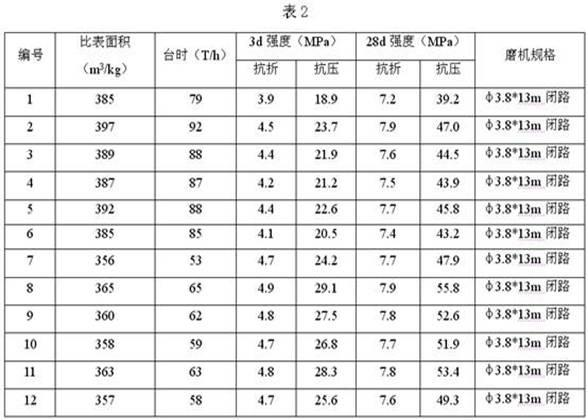
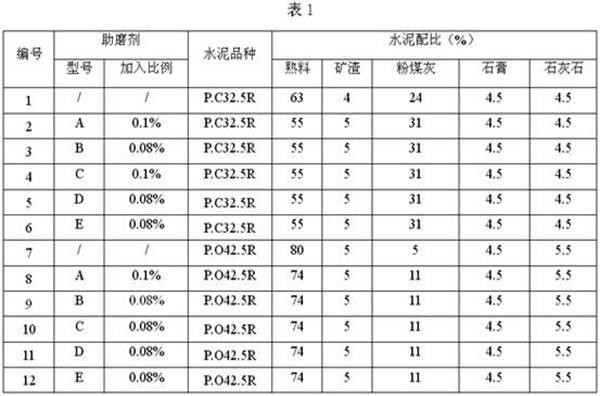
Cement grinding aid and preparation method thereof
ActiveCN102126843AImprove performanceNo adverse side effectsSolid waste managementCelluloseCarbamate
The invention discloses a cement grinding aid. The cement grinding aid comprises the following components in percentage by weight: 8 to 20 percent of triethanolamine, 0 to 10 percent of triisopropanolamine, 8 to 20 percent of diol byproduct, 0 to 10 percent of synthetic glycerine, 0 to 25 percent of molasses, 0 to 5 percent of calcium lignosulfonate, 0 to 15 percent of polyurethane, 0 to 10 percent of cystine, 3 to 5 percent of sorbitol, 5 to 10 percent of sodium carboxymethylcellulose, 0 to 5 percent of citric acid, 0 to 5 percent of silica powder, 0 to 5 percent of fly ash and the balance of water. The invention also discloses a preparation method of the cement grinding aid. The grinding aid can effectively improve the equipment-hour yield of a cement grinding mill, reduce energy consumption and remarkably improve cement performance, does not have adverse side effects on cement, reduces the using amount of cement clinkers by 4 to 10 percent, improves the equipment-hour yield of cement production by 6 to 10 percent, and has a good effect. The method has a unique process, a part of raw materials are treated by ultrasound, and the prepared grinding aid has high performance.
Owner:CHINA UNITED CEMENT LUNAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com