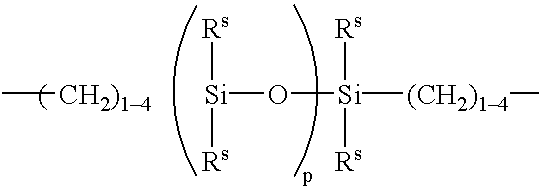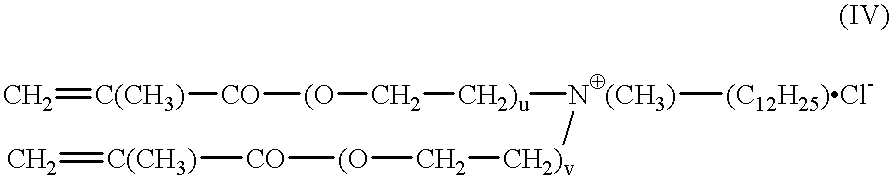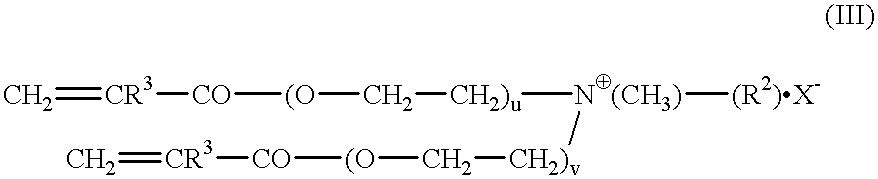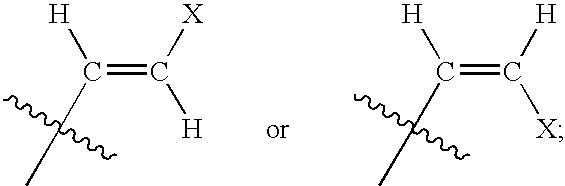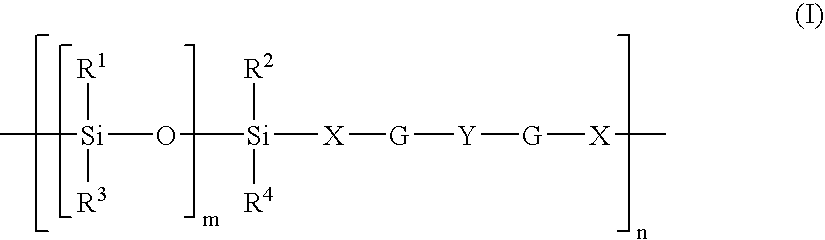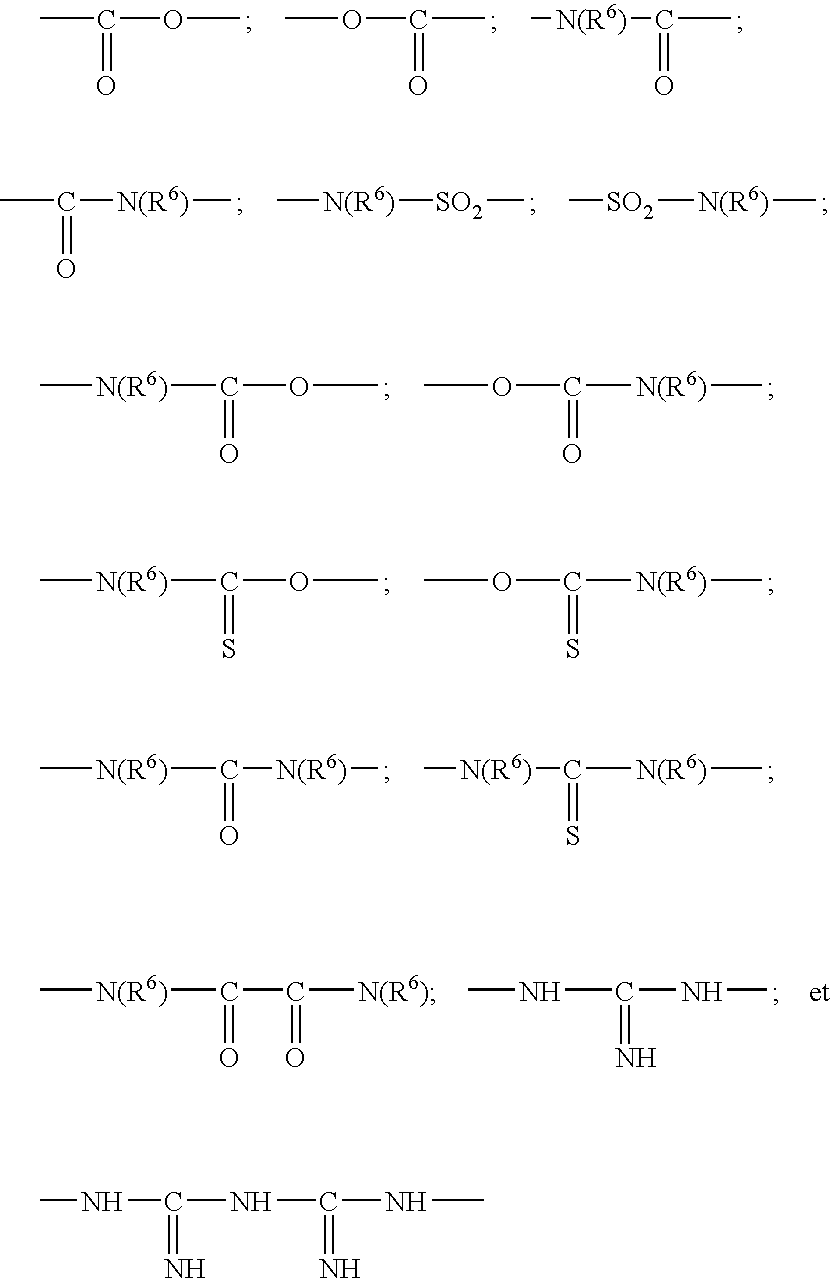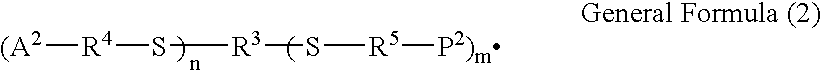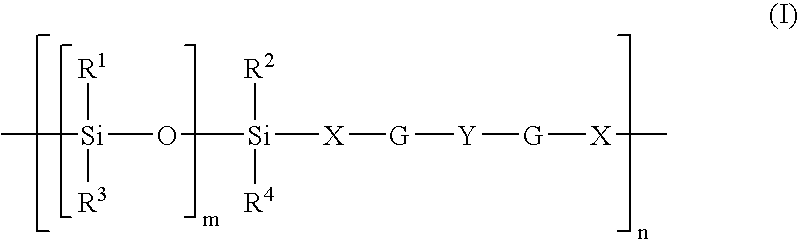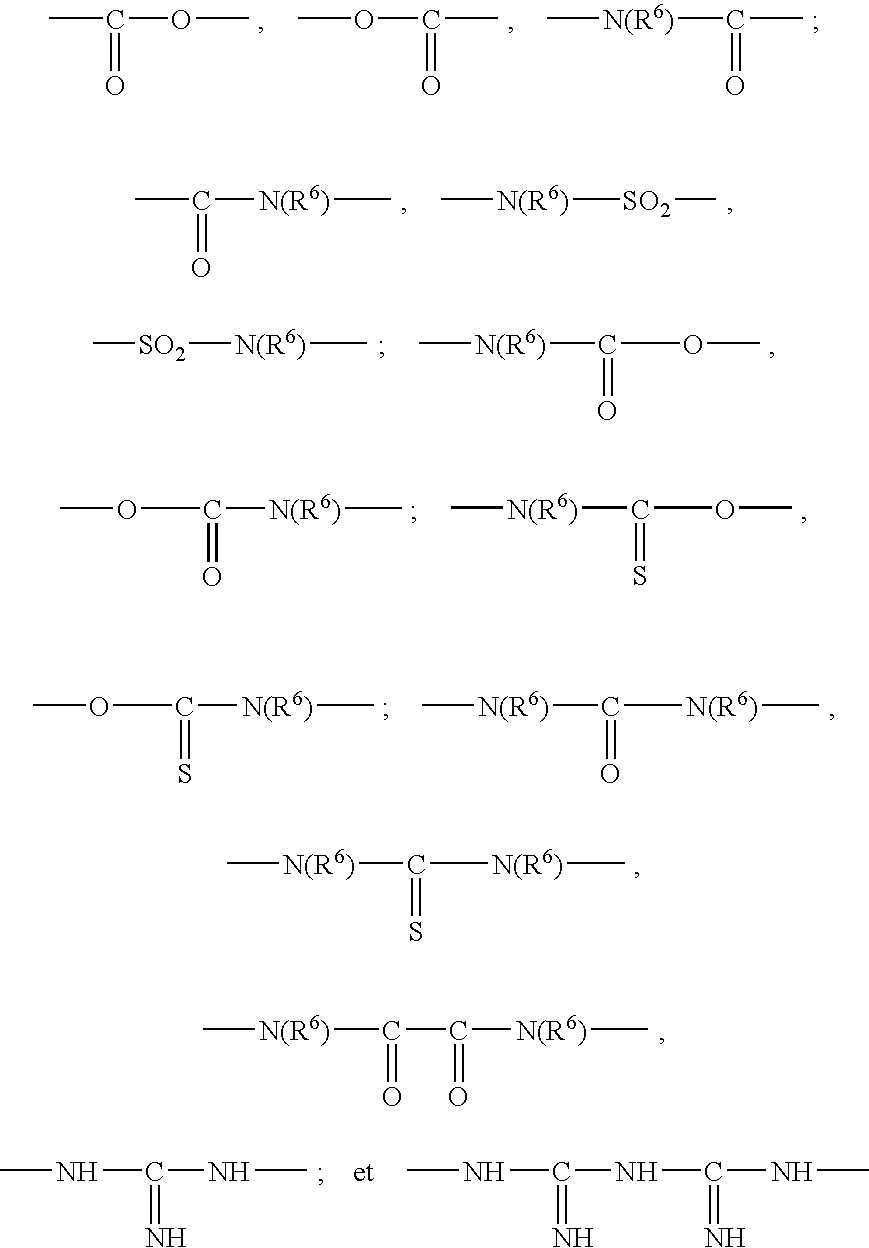Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4980 results about "Carbamate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
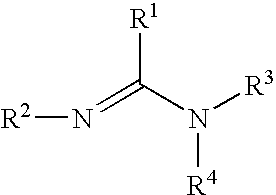
A carbamate is an organic compound derived from carbamic acid (NH₂COOH). A carbamate group, carbamate ester (e.g., ethyl carbamate), and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.
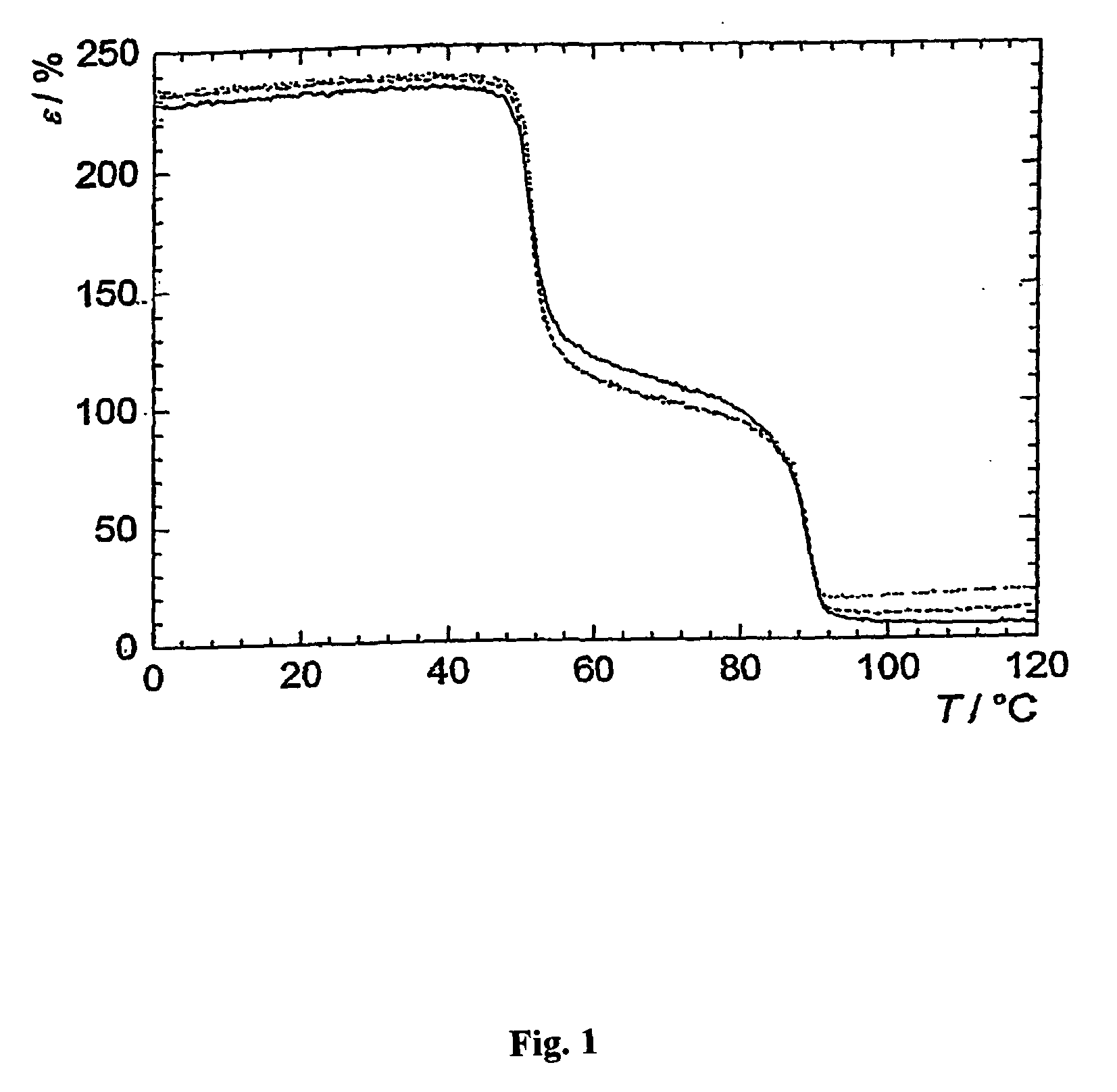
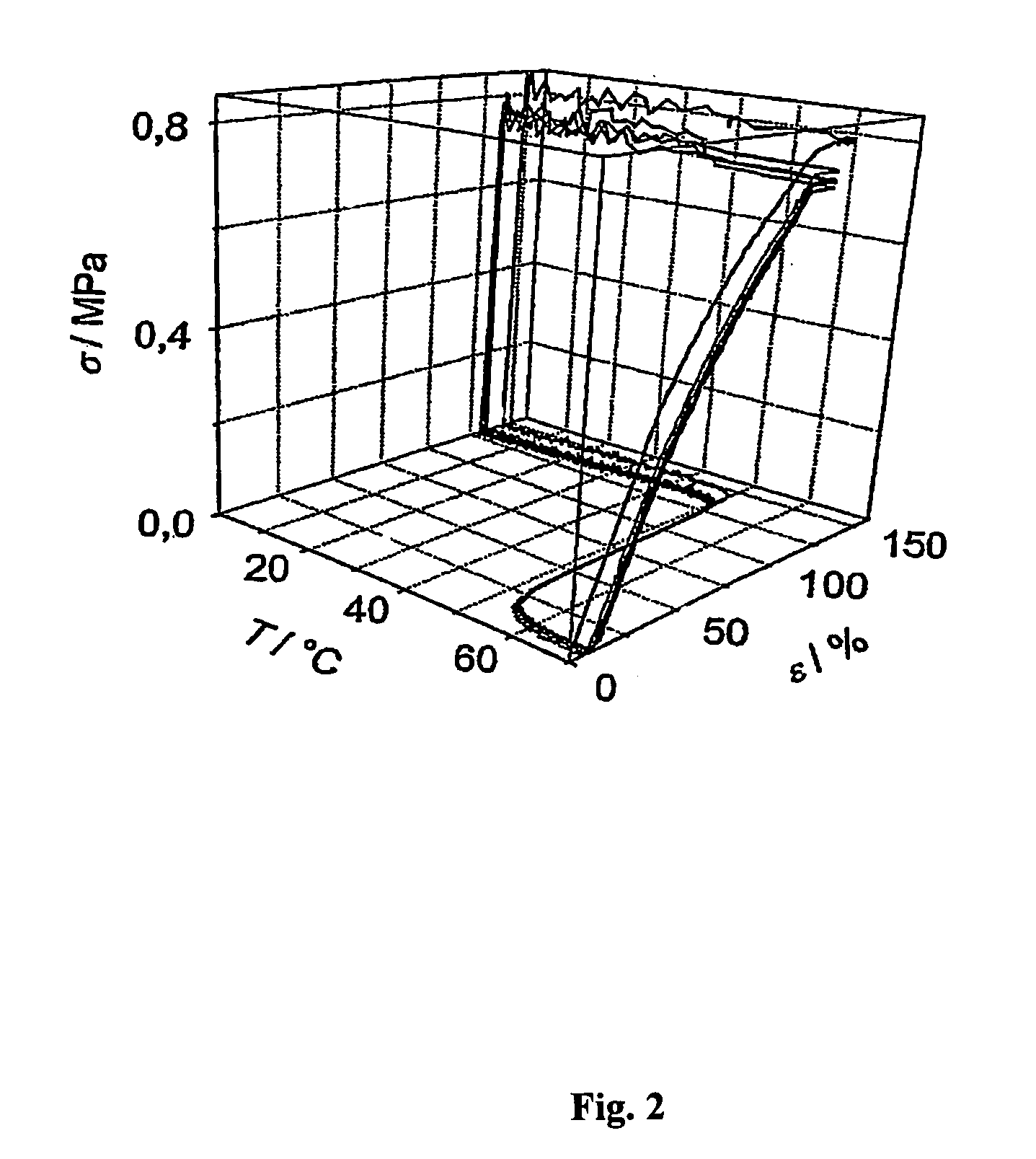
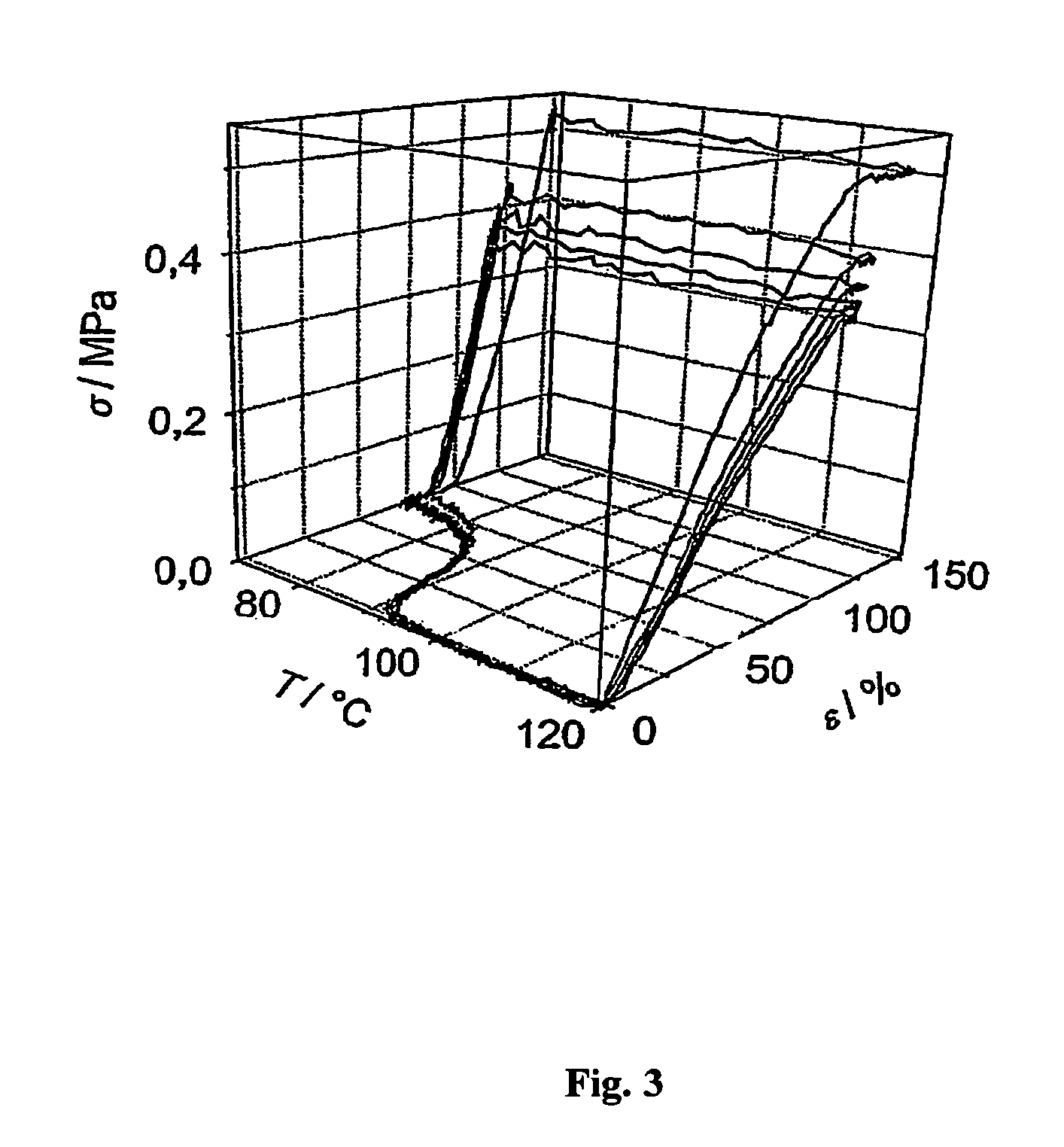
Fast curing compositions
Fast curing surgical adhesives and sealants contain an NCO-terminated hydrophilic urethane prepolymer derived from an aromatic diisocyanate and a polyol. Substantially all the aromatic diisocyanate used to prepare the NCO-terminated hydrophilic urethane prepolymer is in the para form. Optionally, the aromatic diisocyanate is substituted with at least one electron withdrawing group, such as, for example, a fluorine group.
Owner:TYCO HEALTHCARE GRP LP
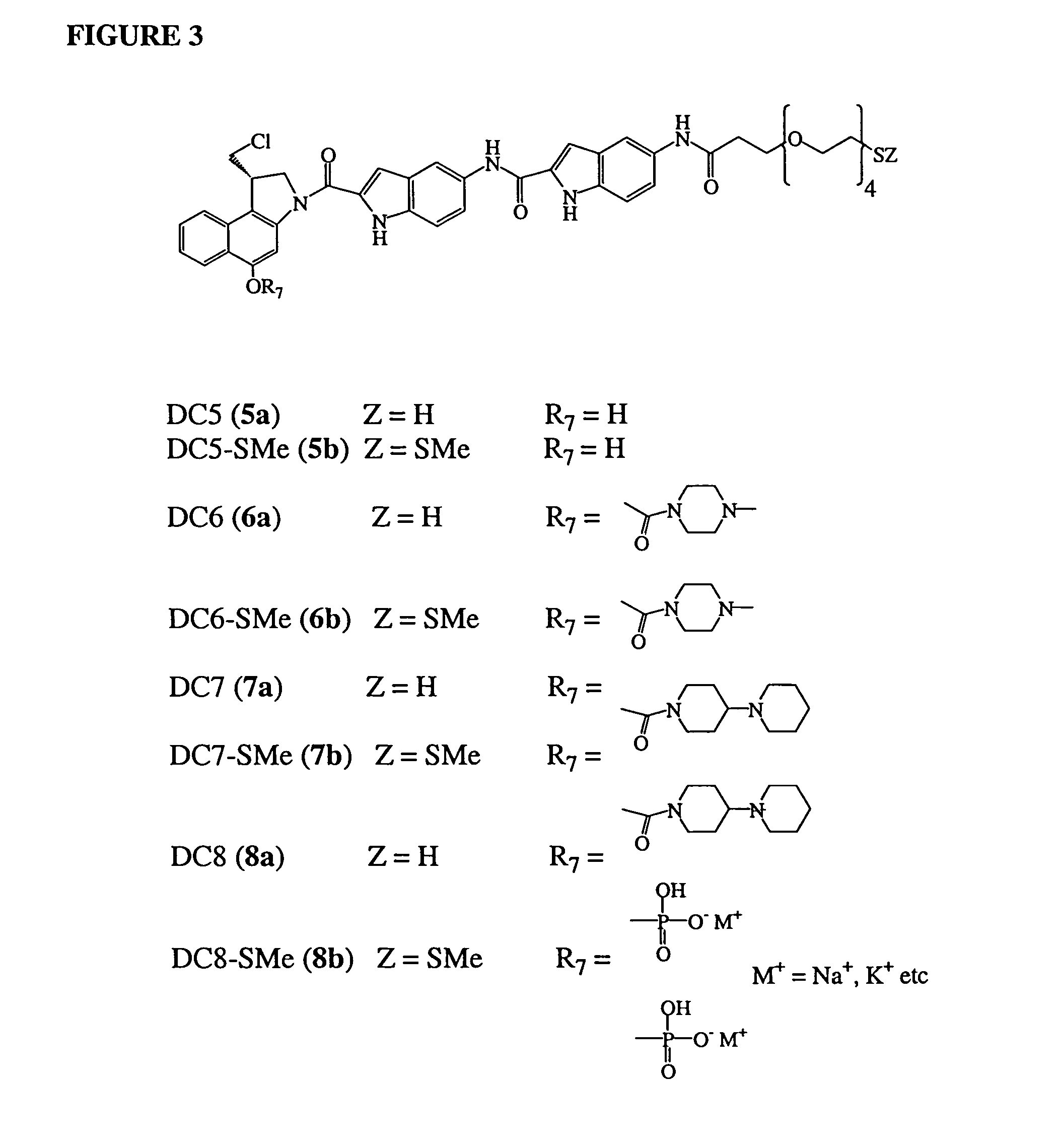
Pyrrolobenzodiazepines as key intermediates in the synthesis of dimeric cytotoxic pyrrolobenzodiazepines
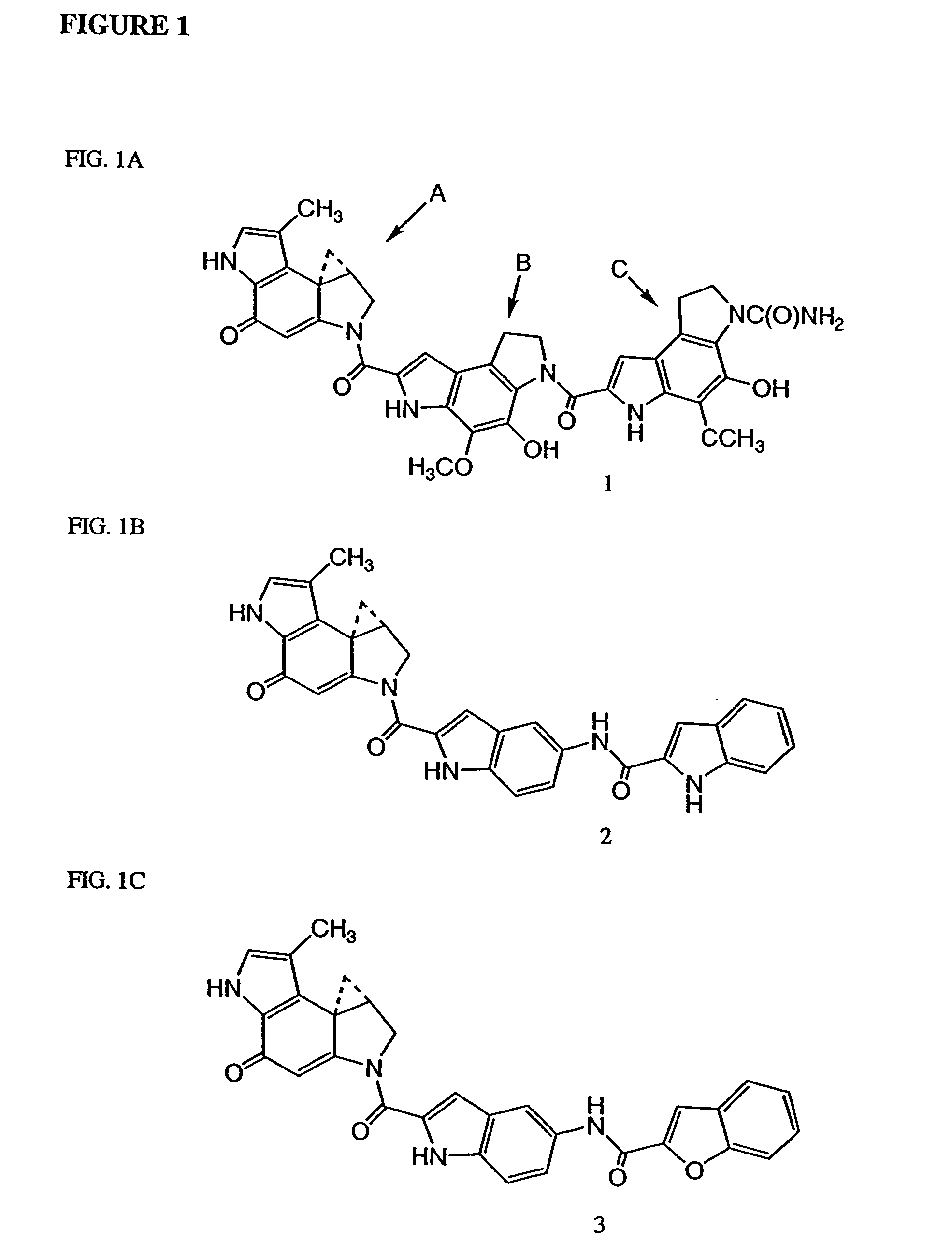
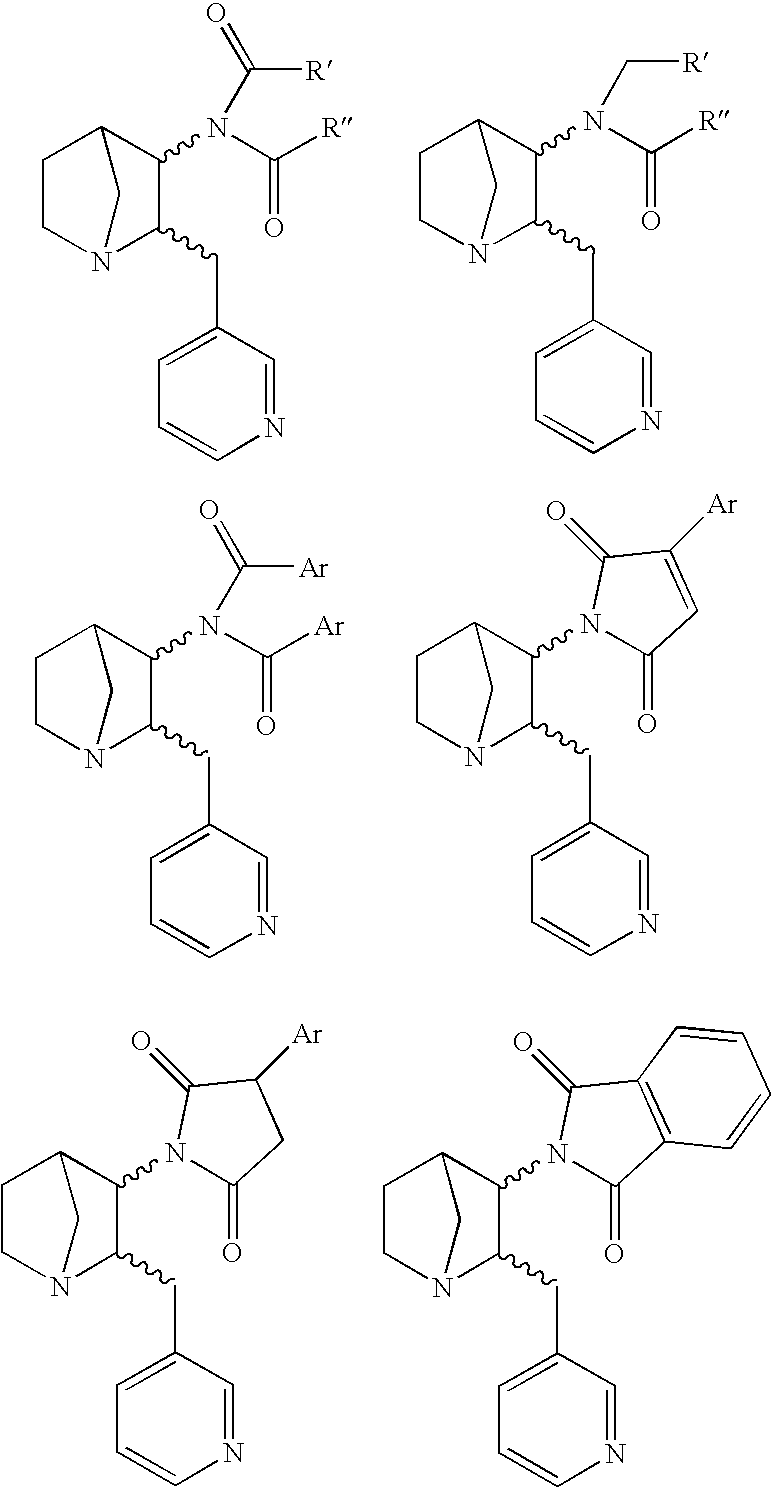
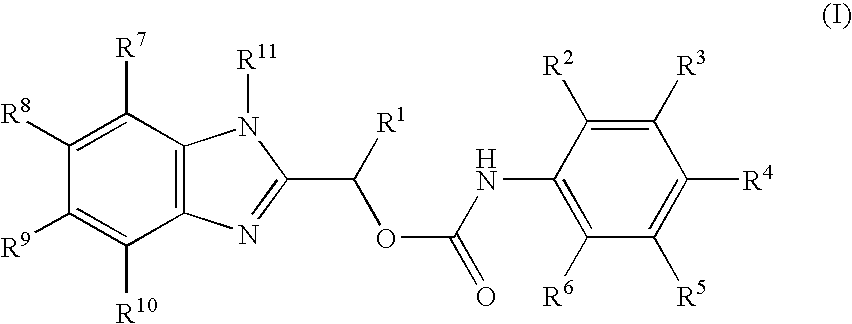
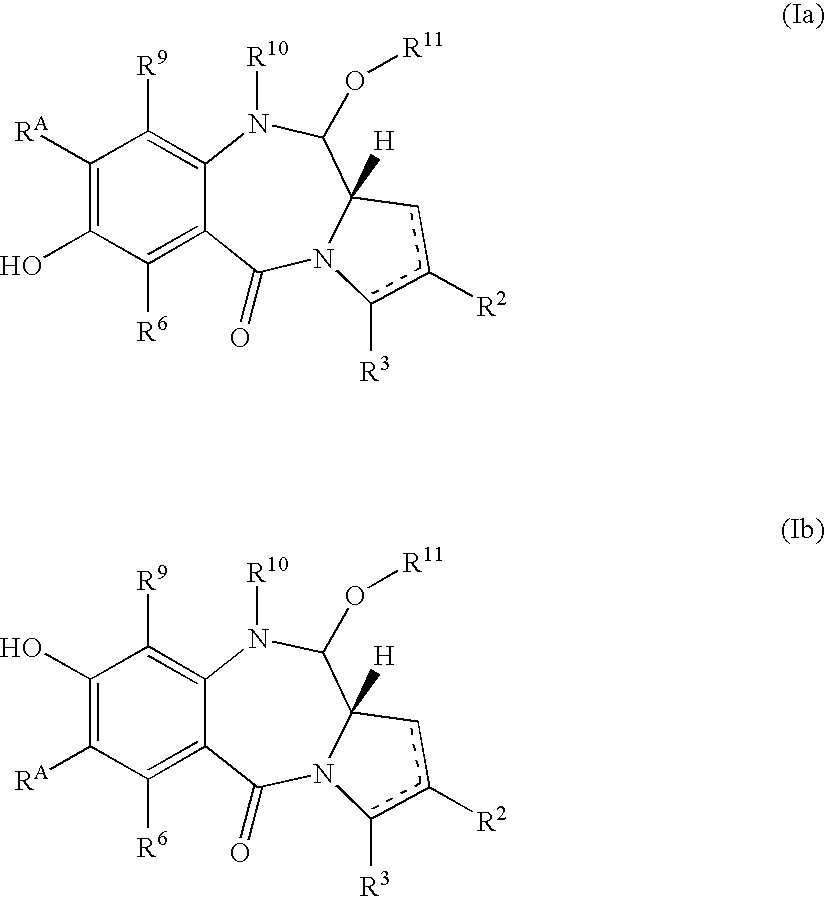
Compounds and a method of synthesis of compounds of formula (Ia) or (Ib): and salts, solvates, and chemically protected forms thereof, wherein the dotted lines indicate the optional presence of a double bond between C1 and C2 or C2 and C3; R2 and R3 are independently selected from —H, ═O, ═CH2, —CN, —R, OR, halo, ═CH—R, O—SO2—R, CO2R and COR; R10 is a carbamate-based nitrogen protecting group; and R11 is an oxygen protecting group.
Owner:MEDIMMUNE LTD
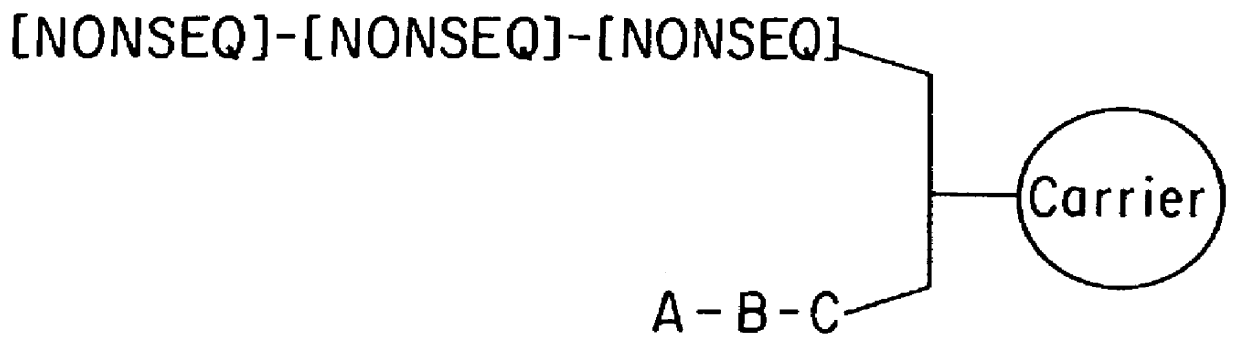
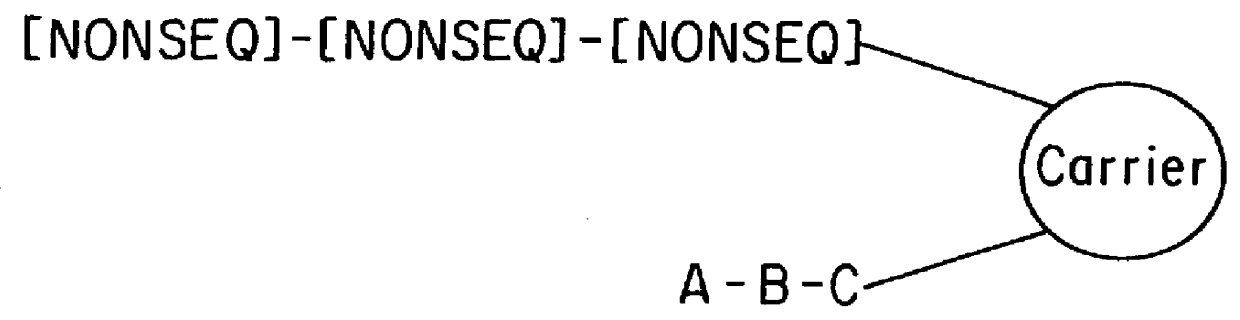
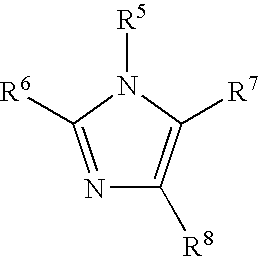
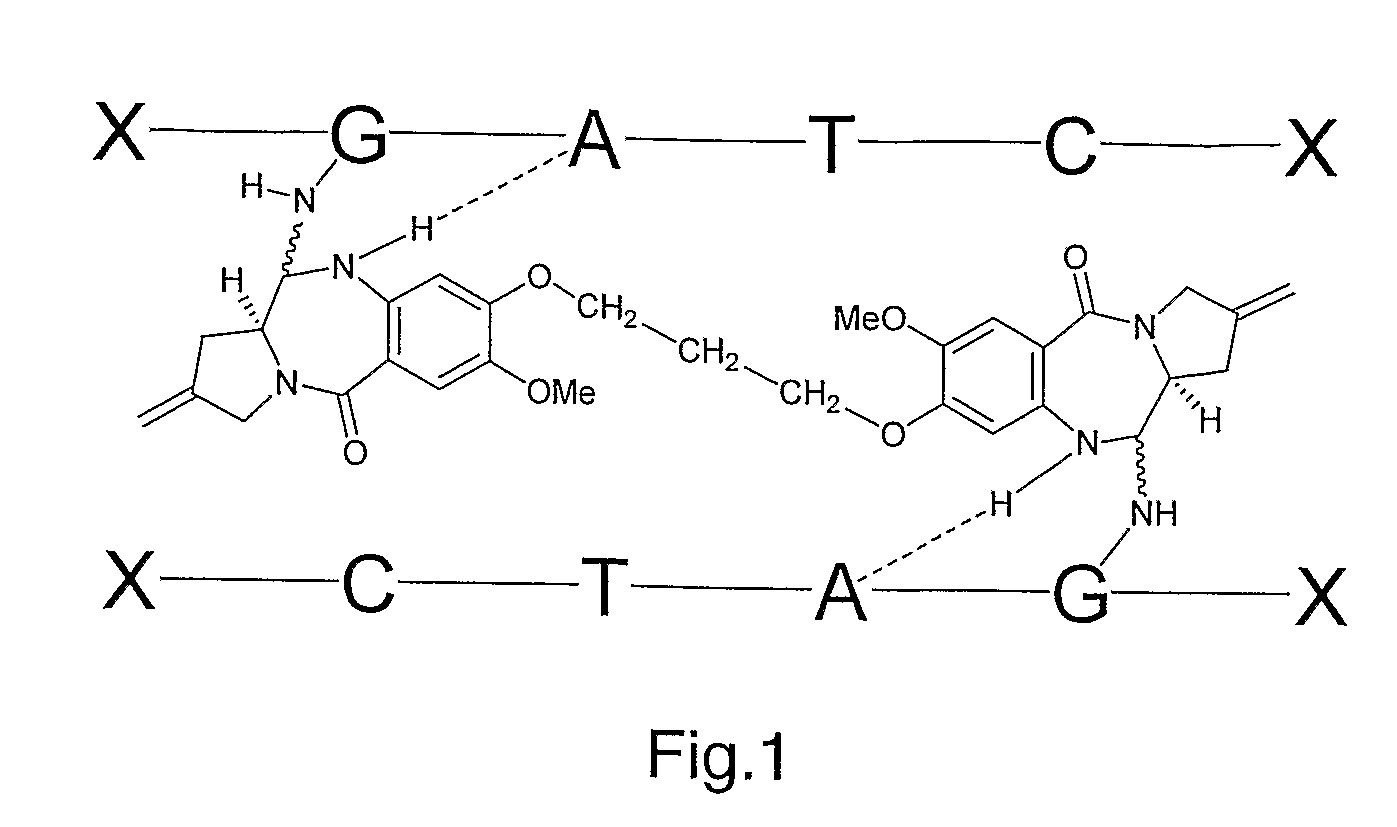
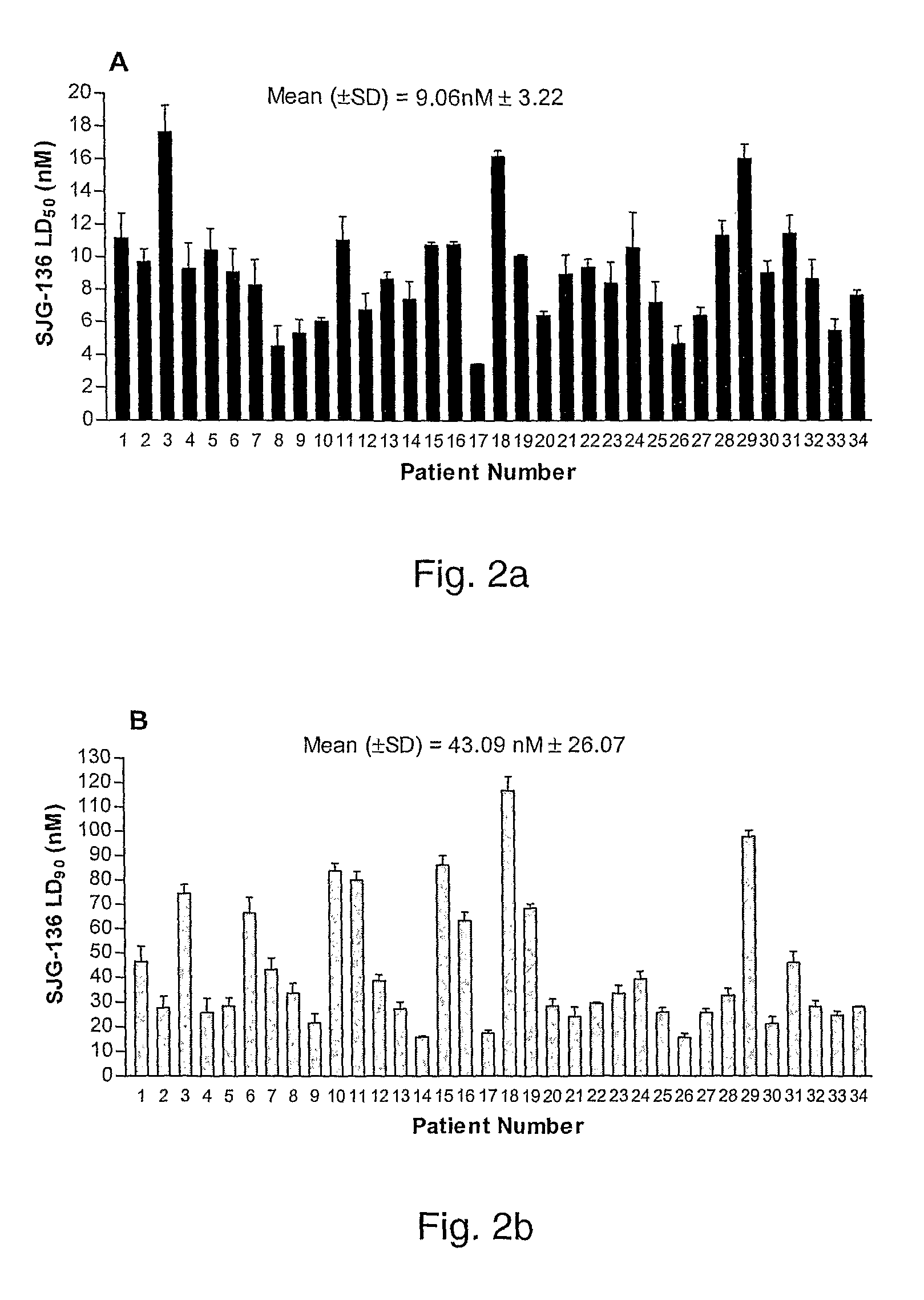
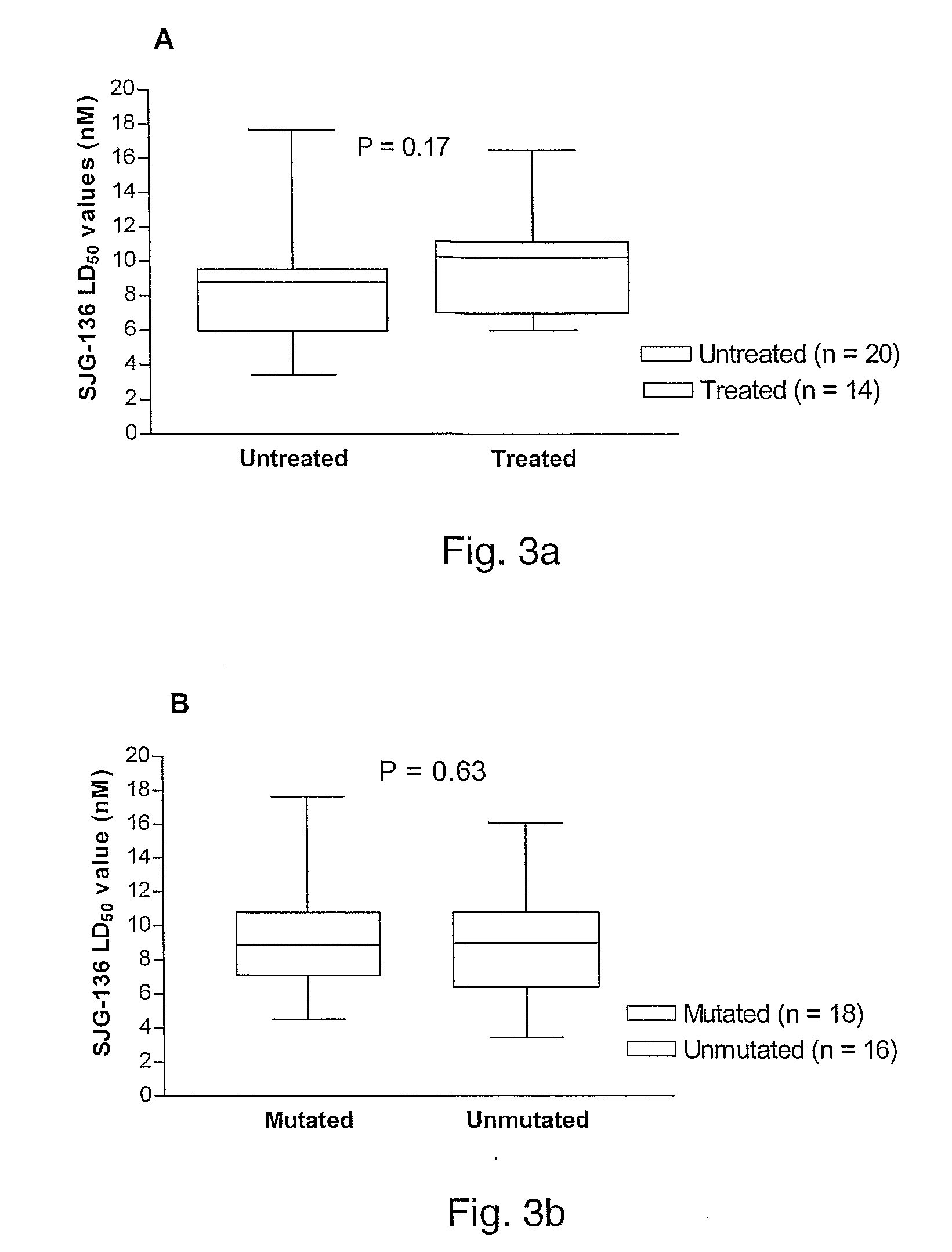
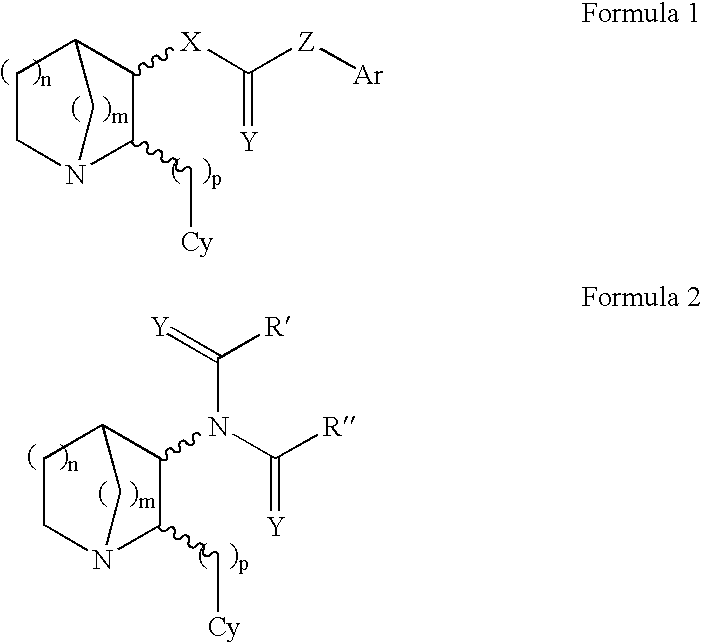
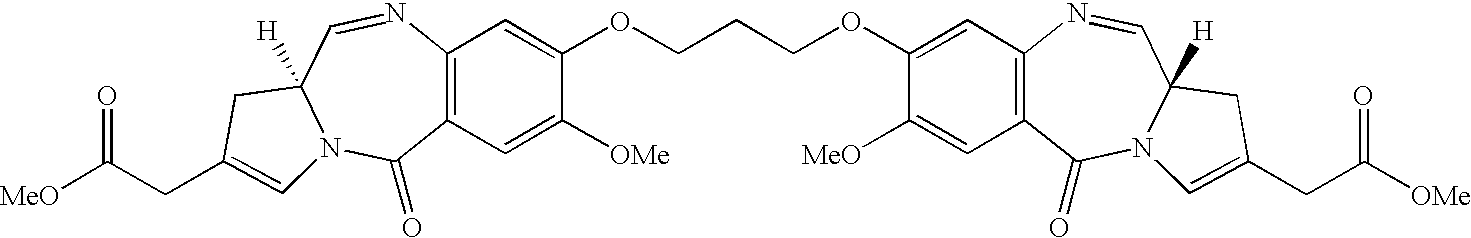
11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines
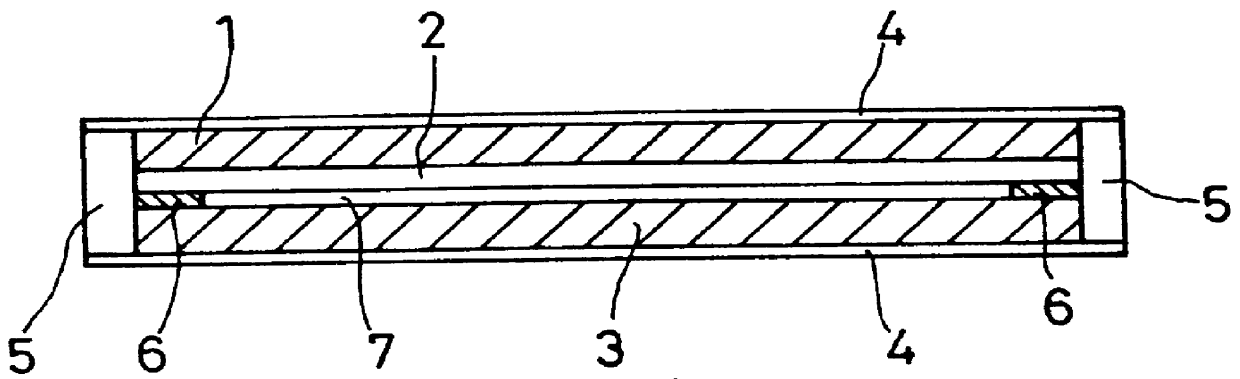
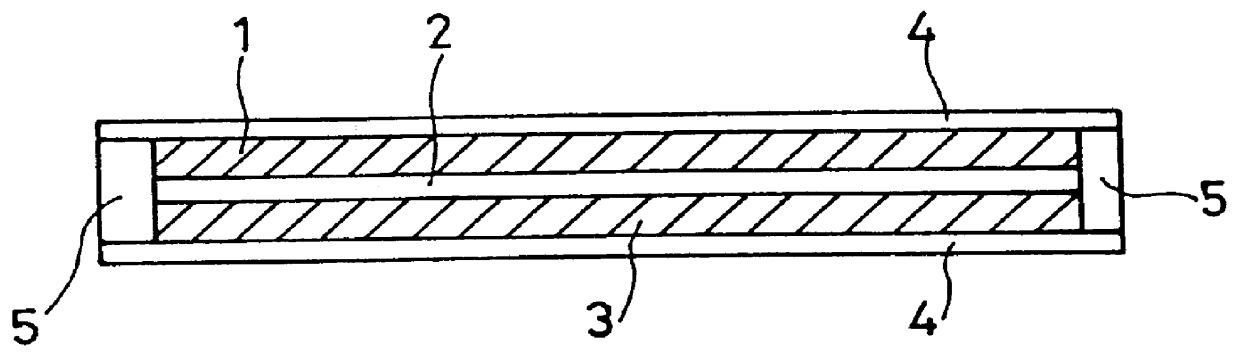
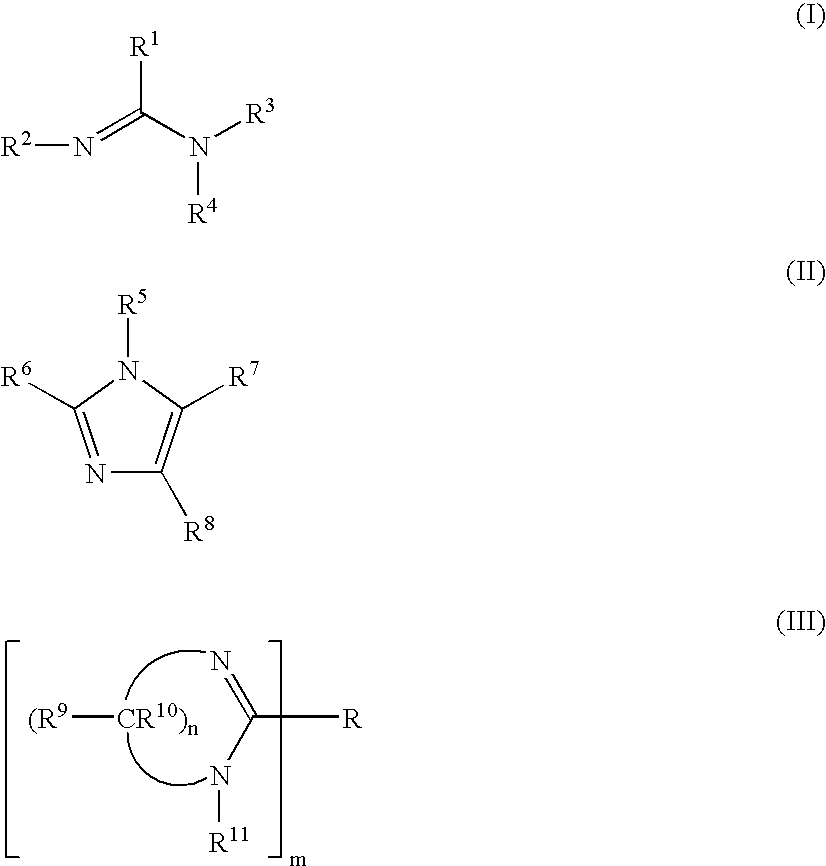
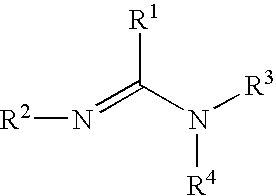
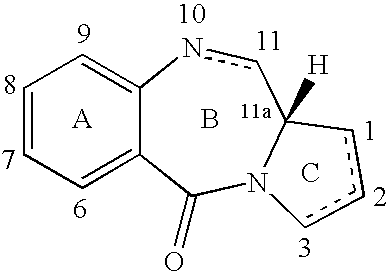
The present inventors have developed a key intermediate for the production of C2 substituted PBDs, which has a leaving group at the C2 position, a carbamate protecting group at the N10 position and a protected hydroxy group at the C11 position. In a first aspect, the present invention comprises a compound with a the formula (I), wherein: R10 is a carbamate-based nitrogen protecting group; R11 is an oxygen protecting group; and R2 is a labile leaving group. In a further aspect, the present invention comprises a method of synthesising a compound of formula (III), or a solvate thereof, from a compound of formula (I) as defined in the first aspect, R16 is either O—R11, wherein R11 is as defined in the first aspect, or OH, or R10 and R16 together form a double bond between N10 and C11; and R15 is R. The other substituents are defined in the claims. Further aspects of the present invention relate to compounds of formula (III) (including solvates thereof when R10 and R16 form a double bond between N10 and C11, and pharmaceutical salts thereof), pharmaceutical compositions comprising these, and their use in the manufacture of a medicament for the treatment of a proliferative disease.
Owner:MEDIMMUNE LTD
Wound healing polymer compositions and methods for use thereof
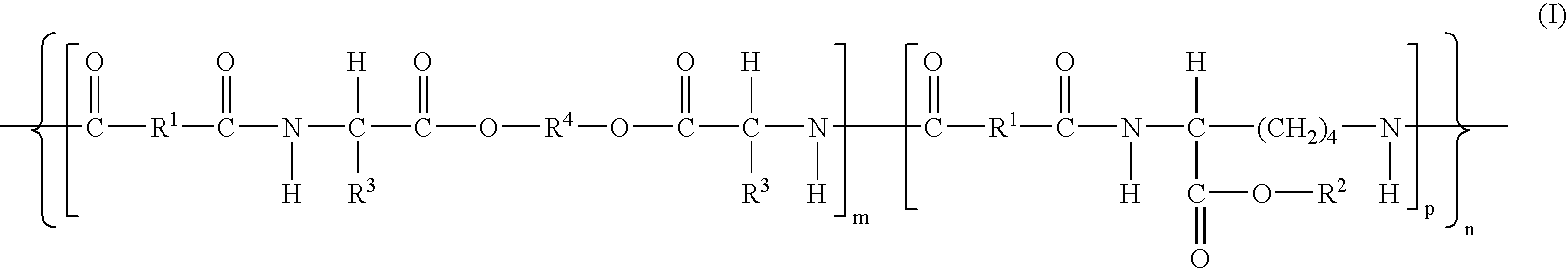
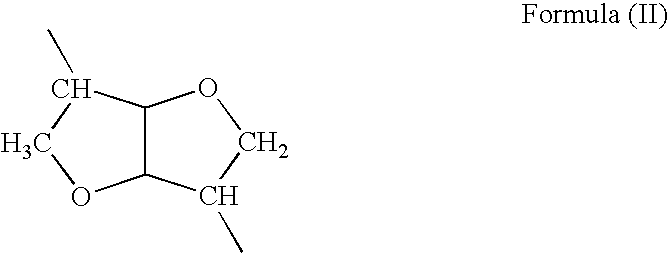
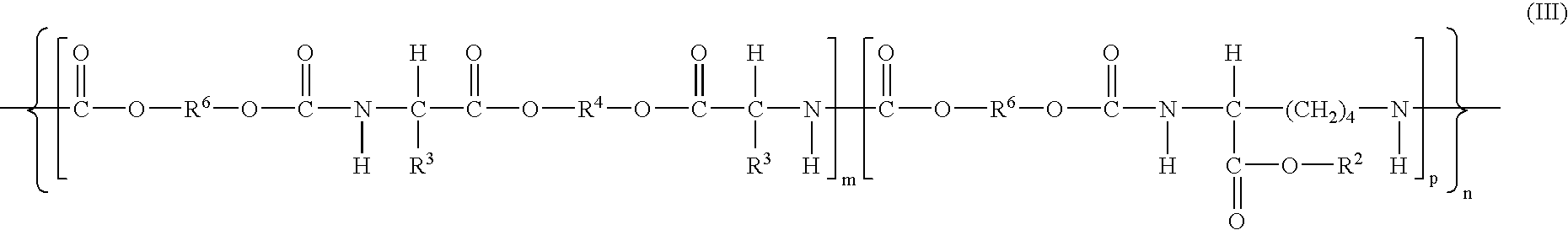
The present invention provides bioactive polymer compositions that can be formulated to release a wound healing agent at a controlled rate by adjusting the various components of the composition. The composition can be used in an external wound dressing, as a polymer implant for delivery of the wound healing agent to an internal body site, or as a coating on the surface of an implantable surgical device to deliver wound healing agents that are covalently attached to a biocompatible, biodegradable polymer and / or embedded within a hydrogel. Methods of using the invention bioactive polymer compositions to promote natural healing of wounds, especially chronic wounds, are also provided. Examples of biodegradable copolymer polyesters useful in forming the blood-compatible, hydrophilic layer or coating include copolyester amides, copolyester urethanes, glycolide-lactide copolymers, glycolide-caprolactone copolymers, poly-3-hydroxy butyrate-valerate copolymers, and copolymers of the cyclic diester monomer, 3-(S)[(alkyloxycarbonyl)methyl]-1,4-dioxane-2,5-dione, with L-lactide. The glycolide-lactide copolymers include poly(glycolide-L-lactide) copolymers formed utilizing a monomer mole ratio of glycolic acid to L-lactic acid ranging from 5:95 to 95:5 and preferably a monomer mole ratio of glycolic acid to L-lactic acid ranging from 45:65 to 95:5. The glycolide-caprolactone copolymers include glycolide and ε-caprolactone block copolymer, e.g., Monocryl or Poliglecaprone.
Owner:MEDIVAS LLC
Topologically segregated, encoded solid phase libraries comprising linkers having an enzymatically susceptible bond
The invention relates to libraries of synthetic test compound attached to separate phase synthesis supports. In particular, the invention relates to libraries of synthetic test compound attached to separate phase synthesis supports that also contain coding molecules that encode the structure of the synthetic test compound. The molecules may be polymers or multiple nonpolymeric molecules. Each of the solid phase synthesis support beads contains a single type of synthetic test compound. The synthetic test compound can have backbone structures with linkages such as amide, urea, carbamate (i.e., urethane), ester, amino, sulfide, disulfide, or carbon-carbon, such as alkane and alkene, or any combination thereof. Examples of subunits suited for the different linkage chemistries are provided. The synthetic test compound can also be molecular scaffolds, such as derivatives of monocyclic of bicyclic carbohydrates, steroids, sugars, heterocyclic structures, polyaromatic structures, or other structures capable of acting as a scaffolding. Examples of suitable molecular scaffolds are provided. The invention also relates to methods of synthesizing such libraries and the use of such libraries to identify and characterize molecules of interest from among the library of synthetic test compound.
Owner:AVENTIS PHARMA INC
Cellular plastic material
An improved cellular plastic material comprises a urethane foam that is the reaction product of soy oil, an isocyanate, and a cross linker. The soy oil replaces the polyol typically generally required in the production of urethanes. Because the replaced polyol is a petrochemical, use of a renewable and environmentally friendly material such as soy oil is most advantageous. Further, plastic materials of many final qualities may be formed using a single vegetable oil. In addition to cellular foams, solid plastic elastomers may be formed.
Owner:URETHANE SOY SYST +1
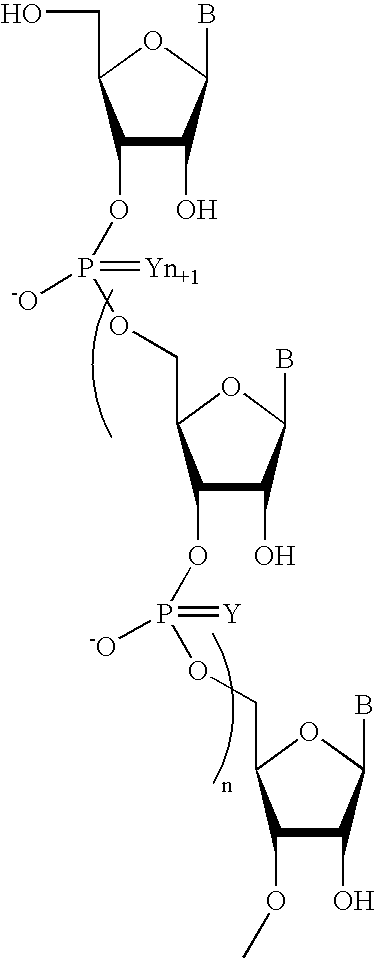
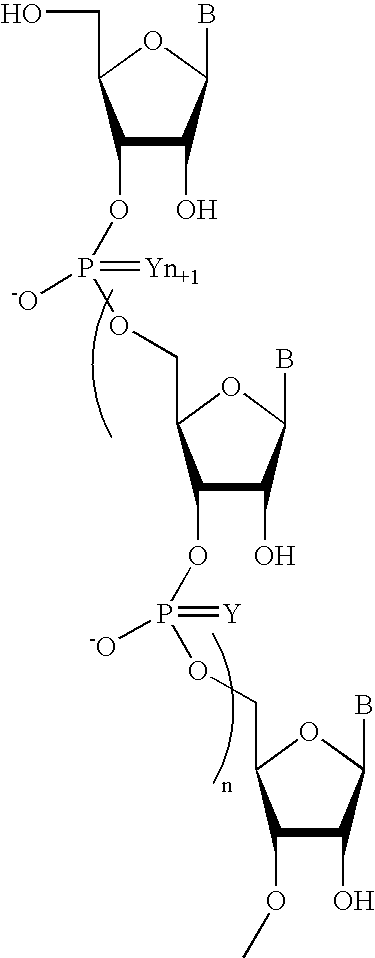
Oligonucleotides comprising a non-phosphate backbone linkage
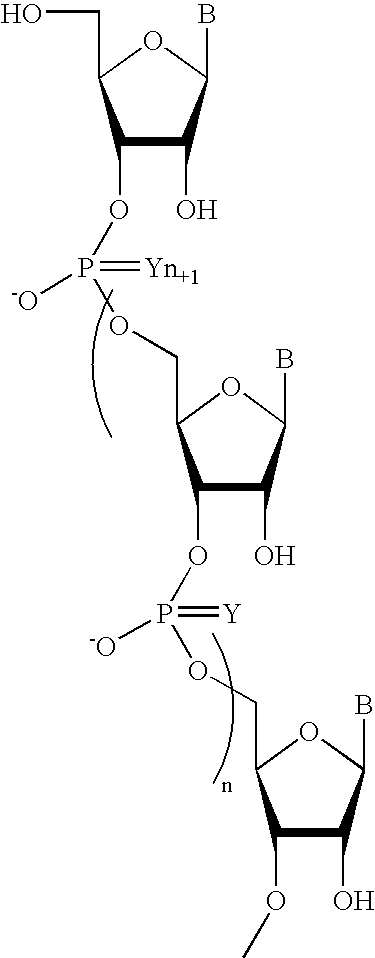
One aspect of the present invention relates to a ribonucleoside substituted with a phosphonamidite group at the 3′-position. In certain embodiments, the phosphonamidite is an alkyl phosphonamidite. Another aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one non-phosphate linkage. Representative non-phosphate linkages include phosphonate, hydroxylamine, hydroxylhydrazinyl, amide, and carbamate linkages. In certain embodiments, the non-phosphate linkage is a phosphonate linkage. In certain embodiments, a non-phosphate linkage occurs in only one strand. In certain embodiments, a non-phosphate linkage occurs in both strands. In certain embodiments, a ligand is bound to one of the oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, a ligand is bound to both of the oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one non-phosphate linkage. Representative non-phosphate linkages include phosphonate, hydroxylamine, hydroxylhydrazinyl, amide, and carbamate linkages. In certain embodiments, the non-phosphate linkage is a phosphonate linkage. In certain embodiments, a ligand is bound to the oligonucleotide strand. In certain embodiments, the oligonucleotide comprises at least one modified sugar moiety.
Owner:ALNYLAM PHARM INC
Prevention of Water and Condensate Blocks in Wells
InactiveUS20070029085A1Better placementReduce capillary pressureCleaning apparatusFluid removalBetaineCarbamate
Compositions and methods are given to prevent, alleviate and remedy water blocks and gas blocks (condensate block or condensate banking). Wettability modifiers are contacted with the formation to change the surfaces from water wet or oil wet to intermediate wet or gas wet. Preferred wettability modifiers include partially or completely fluorinated surfactants or polymers, for example fluorosilanes such as perfluorosilanes, urethane oligomers containing perfluoro alkyl moieties, fluoroacrylates, and fluoroalkyl containing terpolymers or their mixtures. Other examples include surfactants, for example viscoelastic surfactants such as cationic surfactants such as quaternary amines, and zwitterionic surfactants, such as betaines, optionally mixed with co-surfactants.
Owner:SCHLUMBERGER TECH CORP
Hydrogel from polysiloxane-containing urethane prepolymer, tris (trimethylsiloxy) silylpropyl methacrylate, and a hydrophilic comonomer
A polyurethane based prepolymer is provided and useful in biomedical devices which provides high oxygen permeability and superior physical properties. A hydrogel is produced from a comonomer mixture containing a polysiloxane-containing urethane prepolymer, tris(trimethylsiloxy)-silylpropyl methacrylate and a hydrophilic comonomer. The hydrogel is especially useful for biomedical materials such as contact lenses and implants.
Owner:BAUSCH & LOMB INC
Film for a separator of electrochemical apparatus, and production method and use thereof
InactiveUS6096456AHigh film strengthEasily and uniformly processedElectrolytic capacitorsSolid electrolyte cellsElectrolytic agentCarbamate
This invention provides a film comprising a cross-linked polymer having an oxyalkylene group or a cross-linked polymer having an oxyalkylene group through a urethane bond, as a constituent component, a production method of the film, and an electrochemical apparatus using the film as a separator. The film for separator of an electrochemical apparatus can be easily and uniformly processed, can include an electrolytic solution, exhibits good film thickness and ensures excellent safety and reliability. The electrochemical apparatus is free of leakage of the solution.
Owner:SHOWA DENKO KK
Radiation curable composition comprising a urethane oligomer having a polyester backbone
InactiveUS6023547AOptical fibre with multilayer core/claddingPolyurea/polyurethane coatingsPolyesterOligomer
A radiation curable composition comprising a urethane oligomer with a polyester backbone which composition, when cured, has improved hydrolytic stability. The composition comprises a urethane oligomer, having a polyester backbone with a number average molecular weight of less than about 1000, wherein the polyester backbone is at least in part based on a diol component wherein at least one carbon at the beta -position with respect to a hydroxyl group bears two carbon-containing substituents having a total of at least three carbon atoms. Alternatively, when the composition comprises a urethane oligomer with a number average molecular weight of less than about 2000, having a polyester polyol backbone with a number average molecular weight of less than about 1000, the polyester backbone is at least in part based on a diol component wherein at least one carbon at the beta -position with respect to a hydroxyl group bears at least one carbon-containing substituent.
Owner:DSM IP ASSETS BV
Organometallic compositions and coating compositions
InactiveUS20060247341A1Reduce yellowingImprove moisture resistanceOrganic-compounds/hydrides/coordination-complexes catalystsGroup 8/9/10/18 element organic compoundsCarbamateZinc
The present invention is directed to novel organometallic complexes as catalysts for the reaction of compounds with isocyanate and hydroxyl functional groups to form urethane and / or polyurethane and the process employing such catalysts. More particularly, the present invention is directed to novel complexes of zinc(II) with substituted amidines. These novel catalysts are useful for the production of urethanes and polyurethanes which are important in many industrial applications.
Owner:KING INDUSTRIES INC
Novel inhibitors of dipeptidyl peptidase I
The present invention relates to new compounds that act as specific inhibitors of the cysteine protease dipeptidyl peptidase I (DP I). Those compounds are represented by the general formula and the pharmaceutical salts thereof, in which R is an acyl-residue including urethane and peptide, or a branched or unbranched C1-C9 alkyl chain, a branched or unbranched C2-C9 alkenyl chain, a branched or unbranched C2-C9 alkynyl chain, a C3-C9 cycloalkyl, C4-C9 carbocyclic, C5-C14 aryl, C3-C9 heteroaryl, C3-C9 heterocyclic, all of the above residues may be optionally substituted, or is H, AS is an amino acid or a peptide mimetic thereof. The amino acid is peptide bound with R and R' is a branched or unbranched C1-C9 alkyl chain, a branched or unbranched C2-C9 alkenyl chain, a branched or unbranched C2-C9 alkynyl chain, a C3-C9 cycloalkyl, C4-C9 cycloalkenyl, C2-C9 heterocycloalkyl, C3-C9 heterocycloalkenyl, C5-C14 aryl, C3-C9 heteroaryl, C3-C9 heterocyclic, whereas the heterocycloalkyl, heterocycloalkenyl, heteroaryl, heterocyclic residue can have up to 6 hetero ring atoms, an amino acid or a peptide mimetic thereof, all of the above residues my be optionally substituted, or is H.
Owner:VIVORYON THERAPEUTICS NV
Method of down-regulating gene expression
InactiveUS6936593B1Down-regulated expressionOrganic active ingredientsBiocideCarbamateDithiophosphoric acid
Disclosed is a method of down-regulating the expression of a gene in an animal, wherein a pharmacological formulation comprising a chimeric oligonucleotide complementary to the gene is orally administered to an animal. The oligonucleotide administered has at least one phosphorothioate internucleotide linkage and at least one alkylphosphonate, phosphorodithioate, alkylphosphonothioate, phosphoramidate, phosphoramidite, phosphate ester, carbamate, carbonate, phosphate triester, acetamidate, or carboxymethyl ester internucleotide linkage.
Owner:IDERA PHARMA INC
Polymers with anti-microbial properties
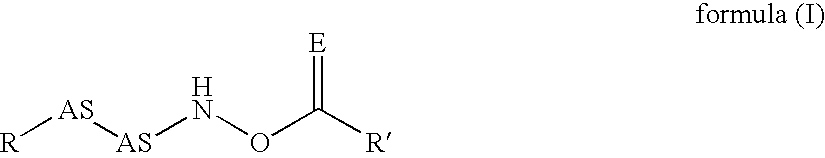
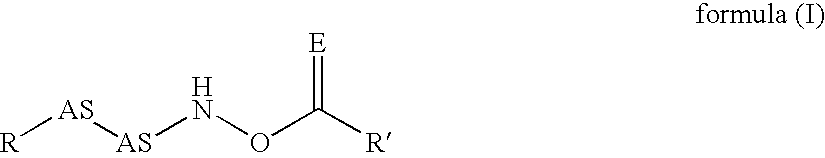
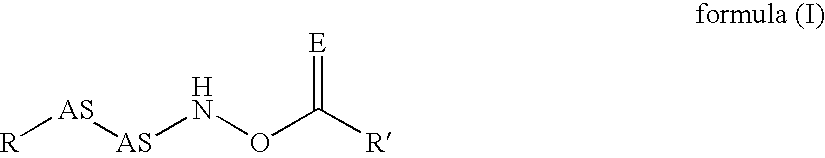
The invention relates to polymers with antimicrobial properties, consisting of: a) 99-40 wt % non functional vinylically polymerizable monomers and b) 1-60 wt % functional vinylically polymerizable monomers of general formula (I) wherein V=vinyl, (meth)acroyl, allyl or styryl, A=a possibly available linking unit, which can be alkyl, aryl, arylalkyl or hydroxy alkyl, which can also be interrupted by hetero atoms, e.g. by hetero atoms in urethane, carbonate, ester, amide or ether groups, wherein y=0 or is 1, Hsp=a hydrophilic spacer of general formula (i) -(O-CH2-CH2)r- and / or (ii) -(O-CH2-CH(CH3))s with r=0-40, s=0-40 and r+s=2-40, also m=1.2 or 3 and R1=CH3, ethyl or benzyl, R2=an alkyl radical with 8-20 C-atoms, wherein t=1, 2 or 3 and X-=Cl-, Br-, I- or alkyl sulphate.
Owner:EVONIK ROEHM GMBH
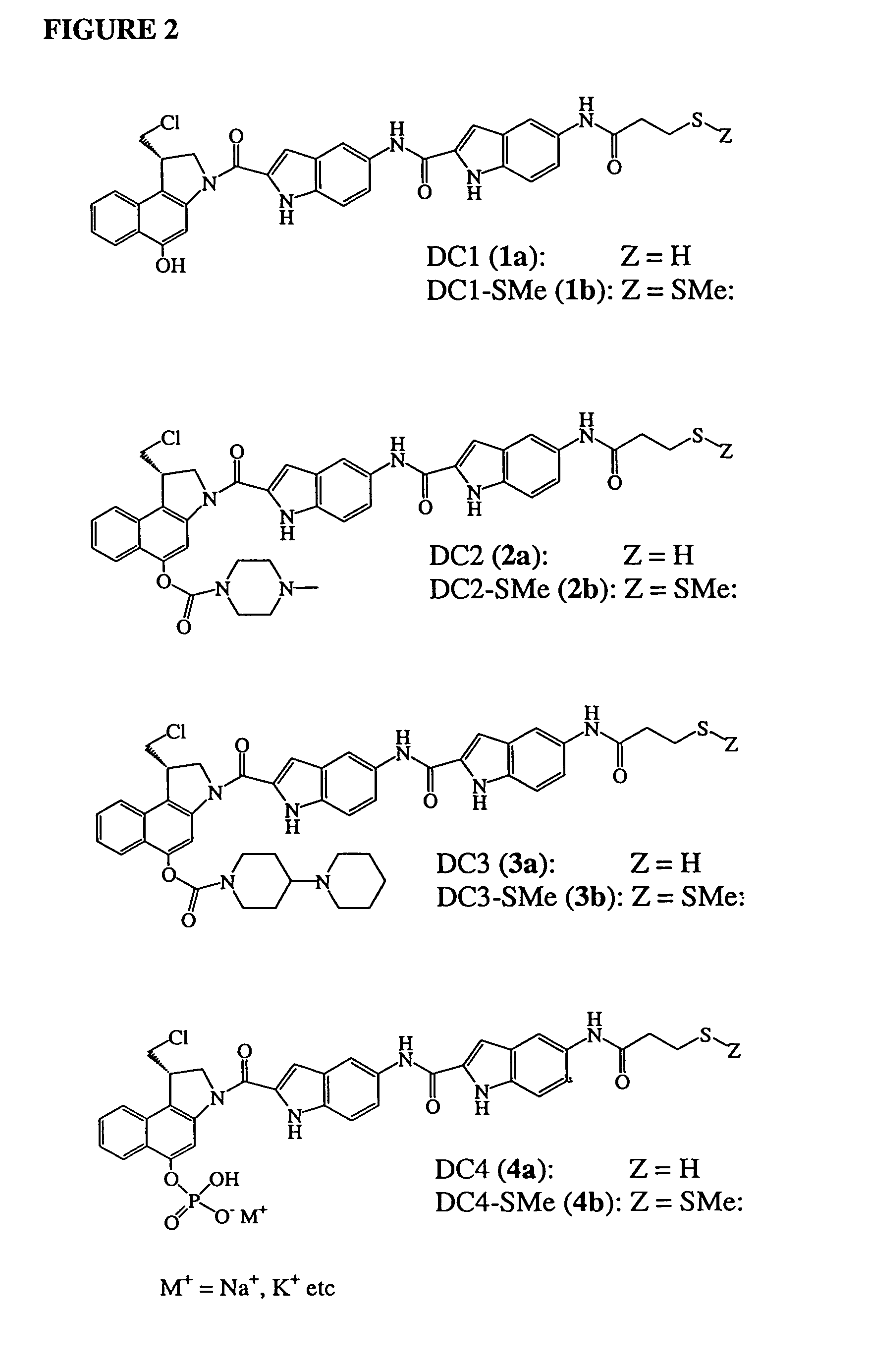
Prodrugs of CC-1065 analogs
InactiveUS7049316B2Improve stabilityGood water solubilityBiocideGroup 5/15 element organic compoundsSolubilityCarbamate
Prodrugs of analogs of the anti-tumor antibiotic CC-1065 having a cleavable protective group such as a piperazino carbamate, a 4-piperidino-piperidino carbamate or a phosphate, in which the protecting group confers enhanced water solubility and stability upon the prodrug, and in which the prodrug also has a moiety, such as a disulfide, that can conjugate to a cell binding reagent such as an antibody. The therapeutic use of such prodrug conjugates is also described; such prodrugs of cytotoxic agents have therapeutic use because they can deliver cytotoxic prodrugs to a specific cell population for enzymatic conversion to cytoxic drugs in a targeted fashion.
Owner:IMMUNOGEN INC
Ink jet receptive coating
InactiveUS6881458B2High color densityHigh densityDecorative surface effectsElectrography/magnetographyEngineeringPolymer
Owner:3M INNOVATIVE PROPERTIES CO
Olefin block copolymers, processes for producing the same and uses thereof
Olefin block copolymers (A-1) are disclosed represented by the formula (I) PO1-g1-B1 . . . (I) wherein PO1 is a segment comprised of repeating units derived from an olefin having 2 to 20 carbon atoms, g1 is an ester, ether, amide, imide, urethane, urea, silylether or carbonyl linkage, and B1 is a segment containing an unsaturated hydrocarbon or a hetero atom. The olefin block copolymers are suitable for uses in adhesives, various molded articles such as construction and civil engineering materials, automobile interior and exterior materials, gasoline tanks, electric and electronic parts, medical care and sanitation materials, materials of miscellaneous goods, resin materials having environmental degradation properties, films and sheets, modifiers and dispersions.
Owner:MITSUI CHEM INC
Anti-viral nucleoside analogs and methods for treating viral infections, especially HIV infections
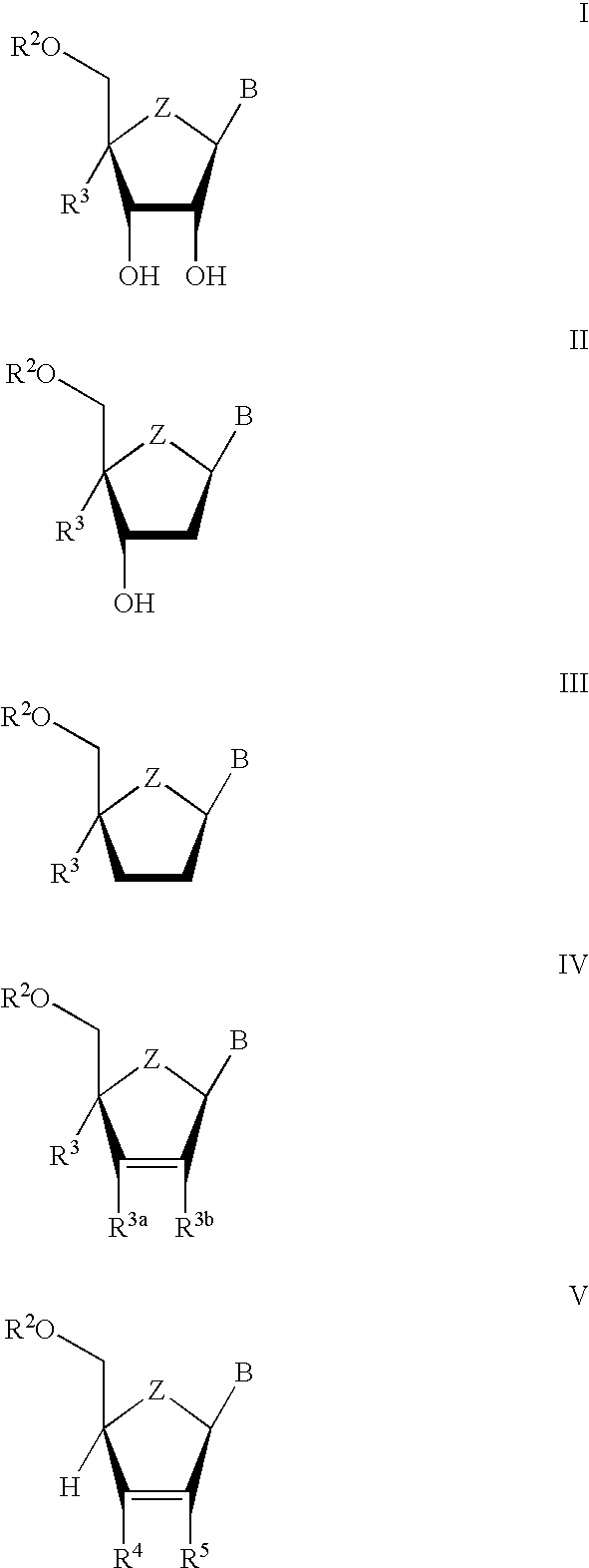
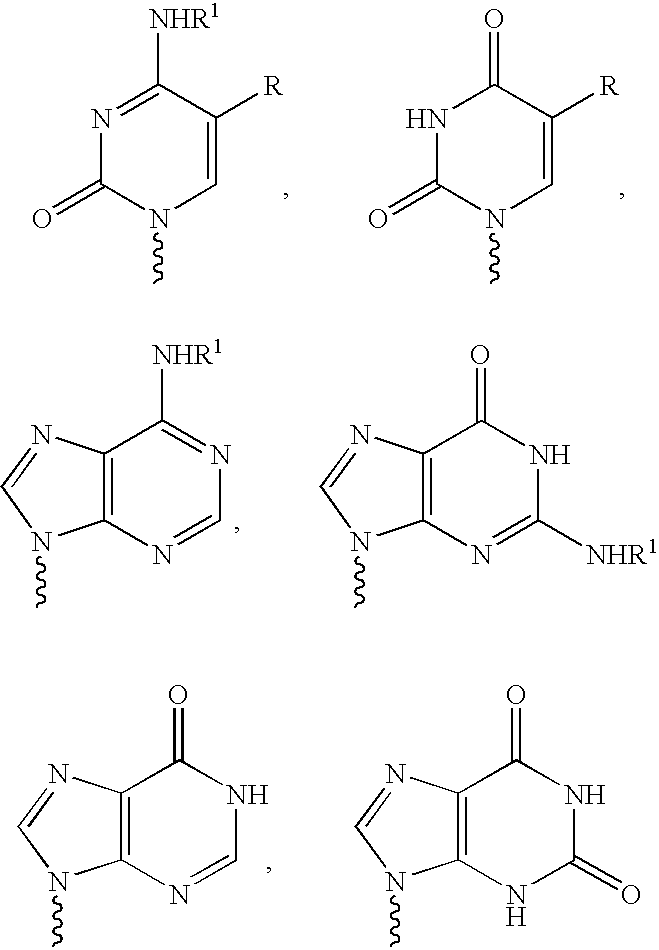
The present invention relates to novel compounds according to the to the general formulas I, II, III, IV or V: wherein B is nucleoside base according to the structure: R is H, F, Cl, Br, I, C1-C4 alkyl (preferably CH3), -C=N, -C=C-Ra, X is H, C1-C4 alkyl (preferably, CH3), F, Cl, Br or I; Z is O or CH2, with the proviso that Z is CH2 and not O when the compound is according to general formula II, R<3 >is -C=C-H and R<2 >is H or a phosphate, diphosphate, triphosphate or phosphotriester group; R<1 >is H, an acyl group, a C1-C20 alkyl or an ether group; R<2 >is H, an acyl group, a C1-C20 alkyl or ether group, a phosphate, diphosphate, triphosphate, phosphodiester group or a group; Nu is a radical of a biologically active antiviral compound such that an amino group or hydroxyl group from said biologically active antiviral compound forms a phosphate, phosphoramidate, carbonate or urethane group with the adjacent moiety; R<8 >is H, or a C1-C20 alkyl or ether group, preferably a C1-C12 alkyl group; k is 0-12, preferably, 0-2; R<3 >is selected from a C1-C4 alkyl (preferably, CH3), -(CH2)n-C=C-Ra, R<3a >and R<3b >are independently selected from H, F, Cl, Br or I; R<4 >and R<5 >are independently selected from H, F, Cl, Br, I, OH, C1-C4 alkyl (preferably, CH3), -(CH2)n-C=C-Ra, with the proviso that R<4 >and R<5 >are not both H; Ra is H, F, Cl, Br, I, or -C1-C4 alkyl, preferably H or CH3; Y is H, F, Cl, Br, I or -C1-C4 alkyl, preferably H or CH3; and n is 0, 1, 2, 3, 4 or 5, preferably 0, 1 or 2; and their anomers, pharmaceutically acceptable salts, solvates, or polymorphs thereof.
Owner:YALE UNIV
Solid golf ball
A solid golf ball with a polyurethane cover having satisfactory formability and ball properties. The solid golf ball has a solid core, and a polyurethane cover for covering the solid core, wherein the difference in Shore D hardness between a center portion and a surface portion of the solid core is at least 15; the polyurethane cover has a thickness (t) of not more than 1.0 mm, and is formed from a cured urethane composition having Shore D hardness (D) of from 35 to 60; a product of the thickness(t) and the Shore D Hardness (D) of the cured urethane composition is ranging from 10 to 45(10≦D×t≦45); and the urethane composition contains an isocyanate group-terminated urethane prepolymer having the residual polyisocyanate monomer content of not more than 0.1 mass %, and an aromatic polyamine compound.
Owner:SUMITOMO RUBBER IND LTD
Rigid gas permeable lens material
A copolymer useful as a rigid gas permeable contact lens material is the polymerization product of a monomeric mixture comprising a polysiloxane-containing urethane or urea prepolymer endcapped with polymerizable ethylenically unsaturated radicals.
Owner:BAUSCH & LOMB INC
Pyrrolobenzodiazepine Therapeutic Agents Useful in the Treatment of Leukemias
A pyrrolobenzodiazepine dimer compound of Formula (I): or pharmaceutically acceptable salt or solvate thereof is useful as a therapeutic agent for the treatment of leukaemias, especially B-cell leukaemias, that exhibit resistance to other chemotherapeutic drugs, wherein: the dotted lines indicate the optional presence of a double bond between C1 and C2 or C2 and C3; R2 and R3 are independently selected from —H, ═O, ═CH2, —CN, —R, OR, halo, ═CH—R, O—SO2—R, CO2R and COR; R6, R7 and R9 are independently selected from II, R, OII, OR, SII, SR, NII2, NIIR, NRR′, nitro, Me3Sn and halo; where R and R′ are independently selected from optionally substituted C1-12 alkyl, C3-20 heterocyclyl and C5-20 aryl groups; R10 is a carbamate-based nitrogen protecting group and R15 is either O—R11, wherein R is an oxygen protecting group, or OH, or R10 and R15 together form a double bond between N10 and C11; R″ is a C3-12 alkylene group, which chain may be interrupted by one or more heteroatoms and / or aromatic rings, and each X is independently selected from 0, S, or NH; R2′, R3′, R6′, R7′, R9′, R10′ and R15′ are all independently selected from the same lists as previously defined for R2, R3, R6, R7, R9, R10 and R15 respectively.
Owner:SPIROGEN
3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof
InactiveUS6953855B2Modulate activityWithout side effectAntibacterial agentsBiocideDiseaseThiocarbamate
The present invention relates to 3-substituted-2-(arylalkyl)-1-azabicycloalkanes, methods of preparing the compounds and methods of treatment using the compounds. The azabicycloalkanes generally are azabicycloheptanes, azabicyclooctanes, or azabicyclononanes. The aryl group in the arylalkyl moiety is a 5- or 6-membered ring heteroaromatic, preferably 3-pyridinyl and 5-pyrimidinyl moieties, and the alkyl group is typically a C1-4 alkyl. The substituent at the 3-position of the 1-azabicycloalkane is a carbonyl group-containing moiety, such as an amide, carbamate, urea, thioamide, thiocarbamate, thiourea or similar functionality. The compounds exhibit activity at nicotinic acetylcholine receptors (nAChRs), particularly the α7 nAChR subtype, and are useful towards modulating neurotransmission and the release of ligands involved in neurotransmission. Methods for preventing or treating conditions and disorders, including central nervous system (CNS) disorders, which are characterized by an alteration in normal neurotransmission, are also disclosed. Also disclosed are methods for treating inflammation, autoimmune disorders, pain and excess neovascularization, such as that associated with tumor growth.
Owner:ATTENUA INC
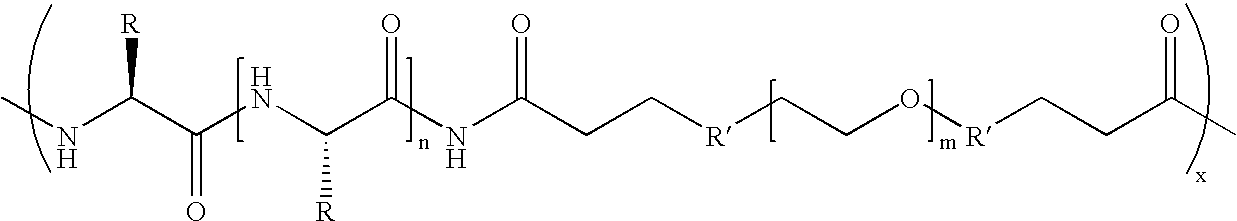
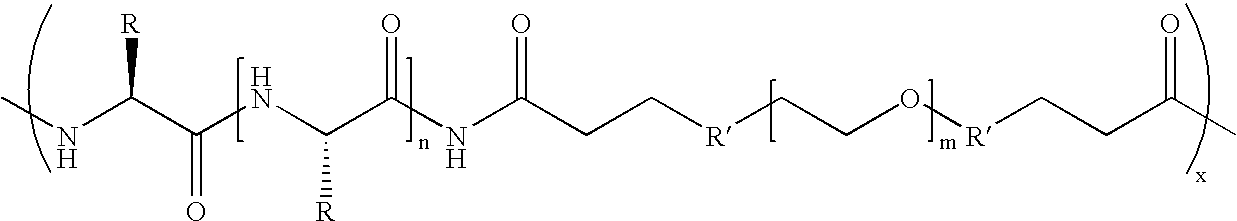
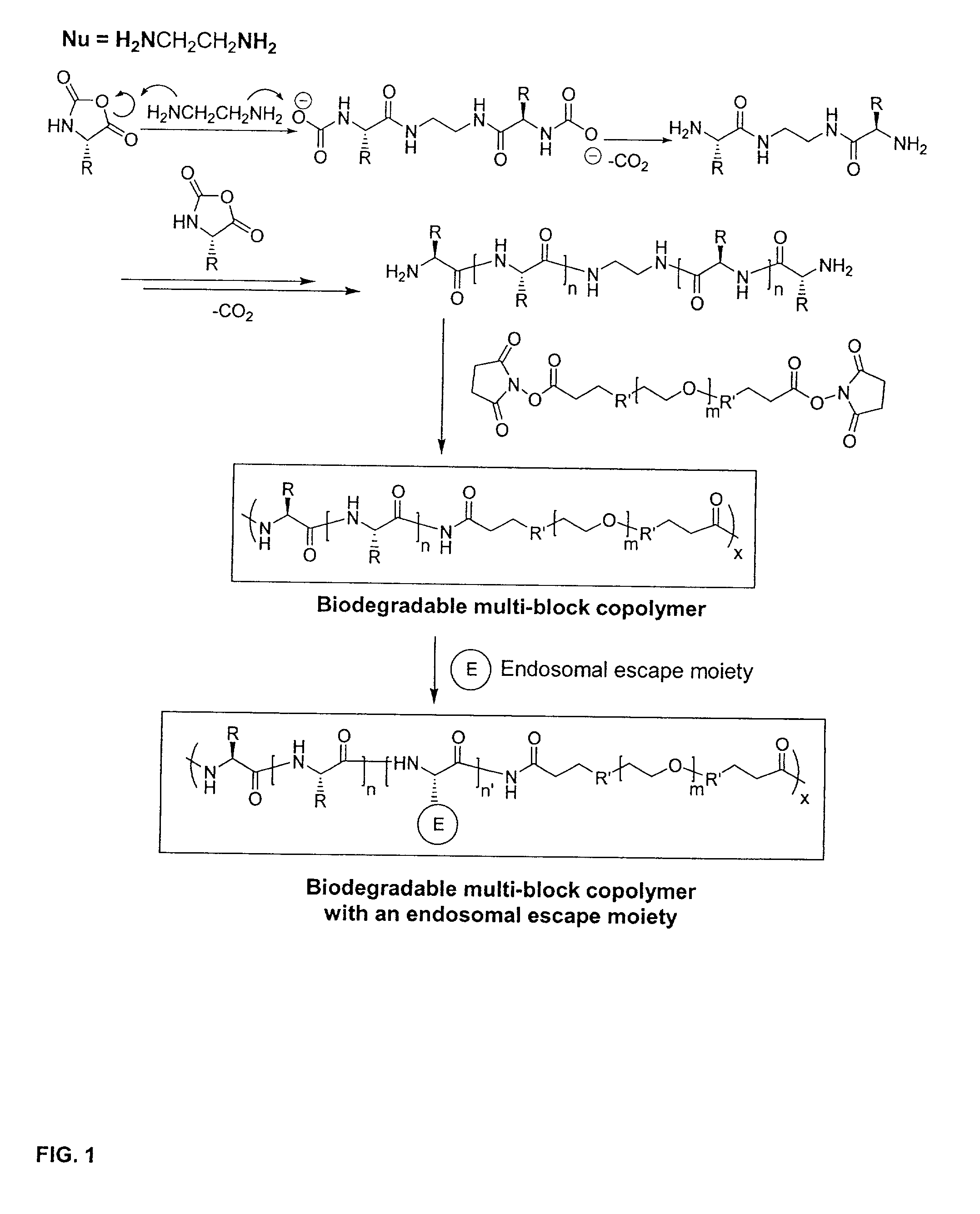
Biodegradable multi-block copolymers of poly(amino acid)s and poly(ethylene glycol) for the delivery of bioactive agents
InactiveUS20030147958A1Easy to chargeSmall sizePowder deliveryGenetic material ingredientsCarbamateHydrophilic polymers
This patent discloses the synthesis of a multi-block copolymer containing poly(amino acids) (PAA) and a hydrophilic polymer which are degradable under physiological conditions. Control over the degradation rate of the obtained copolymers is achieved by introducing ester, amide or urethane groups as a biodegradable linkage connecting the PAA and the hydrophilic polymer. The biodegradable multi-block copolymers display high transfection efficiency in plasmid delivery with low cytotoxicity.
Owner:EXPRESSION GENETICS INC
Cosmetic composition for care and/or makeup, structured with silicone polymers and film-forming silicone resins
InactiveUS20030235552A1Improved deposit staying-power propertyImprove propertiesCosmetic preparationsHair cosmeticsThiocarbamateCarbamate
Cosmetic composition for care and / or makeup, comprising: 1) a liquid fatty phase comprising at least one silicone oil, structured with a gelling system comprising at least one polymer (homopolymer or copolymer) with a weight-average molecular mass ranging from 500 to 500,000, containing at least one moiety comprising: at least one polyorganosiloxane group, composed of 1 to 1000 organosiloxane units in the chain of the moiety or in the form of a graft, and at least two groups capable of establishing hydrogen interactions chosen from among the ester, amide, sulfonamide, carbamate, thiocarbamate, urea, thiourea, oxamido, guanidino, biguanidino groups, and combinations thereof, on condition that at least one of the groups is other than an ester group, the polymer being solid at room temperature and soluble in the liquid fatty phase at a temperature of 25 to 250° C., and 2) at least one film-forming silicone resin, the liguid fatty phase, the gelling system and the film-forming silicone resin forming a physiologically acceptable medium.
Owner:LOREAL SA
Carbamate compositions and methods fo rmodulating the activity of the CHK1 enzyme
Described herein are carbamate compounds. Such compounds are capable of modulating the activity of a checkpoint kinase, and described herein are methods for utilizing such modulation to treat cell proliferative disorders. Also described are pharmaceutical compositions containing such compounds. Also described are the therapeutic or prophylactic use of such compounds and compositions, and methods of treating cancer as well as other diseases associated with unwanted cellular proliferation, by administering effective amounts of such compounds in combination with anti-neoplastic agents.
Owner:AGOURON PHARMA INC
Interpenetrating networks
The present invention relates to the penetrating networks comprising a covalently crosslinked polymer component and a polyester urethane component. The materials in accordance with the present invention are suitable in particular as materials for medicinal purposes, as implants, for target controlled stimuli-sensitive drug release, as ligament augmentation and as replacement material for inter-vertebrae disks.
Owner:GKSS FORSCHUNGSZENTRUM GEESTHACHT GMBH
Ink composition and image recording method and image recorded matter using same
ActiveUS20080241485A1Excels dispersivityExcels stabilityMeasurement apparatus componentsDecorative surface effectsEpoxyPolymer science
An ink composition containing at least a polymerizable composition, a pigment, and a polymer represented by General Formula (1):where R1 represents an (m+n)-valent organic linking group, R2 represents a single bond or divalent organic linking group; A1 represents a monovalent organic group having a pigment adsorption structure that contains at least one selected from organic pigment structure, heterocyclic structure, acidic group, group having a basic nitrogen atom, urea group, urethane group, group having a coordinating oxygen atom, hydrocarbon group having 4 or more carbon atoms, alkoxysilyl group, epoxy group, isocyanate group, and hydroxyl group; the n groups A1 and bonds or groups R2 may independently be the same or different; “m” is 1 to 8, “n” is 2 to 9, and m+n is 3 to 10; P1 represents a polymer skeleton; and the m skeletons P1 may be the same or different.
Owner:FUJIFILM CORP
Cosmetic composition for care and/or treatment and/or makeup of the emulsion type structured with silicone polymers
InactiveUS20030235548A1Outstanding propertyIncrease flexibilityCosmetic preparationsHair cosmeticsThiocarbamatePolymer science
Cosmetic emulsion for care and / or makeup, comprising an aqueous phase and a liquid fatty phase dispersed one within the other, said liquid fatty phase comprising at least one silicone oil and being structured with at least one gelling polymer (homopolymer or copolymer) with a weight-average molecular mass ranging from 500 to 500,000, containing at least one moiety comprising: at least one polyorganosiloxane group, composed of 1 to 1000 organosiloxane units in the chain of the moiety or in the form of a graft, and at least two groups capable of establishing hydrogen interactions chosen from among the ester, amide, sulfonamide, carbamate, thiocarbamate, urea, thiourea, oxamido, guanidino, biguanidino groups, and combinations thereof, on condition that at least one of the groups is other than an ester group, the polymer being solid at room temperature and soluble in the liquid fatty phase at a temperature of 25 to 250° C., the aqueous phase, the liquid fatty phase and the gelling polymer forming a physiologically acceptable medium.
Owner:LOREAL SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines 11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dfe0bdc2-9bfe-4984-a8af-93415b268a91/US07741319-20100622-C00001.png)
![11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines 11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dfe0bdc2-9bfe-4984-a8af-93415b268a91/US07741319-20100622-C00002.png)
![11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines 11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dfe0bdc2-9bfe-4984-a8af-93415b268a91/US07741319-20100622-C00003.png)