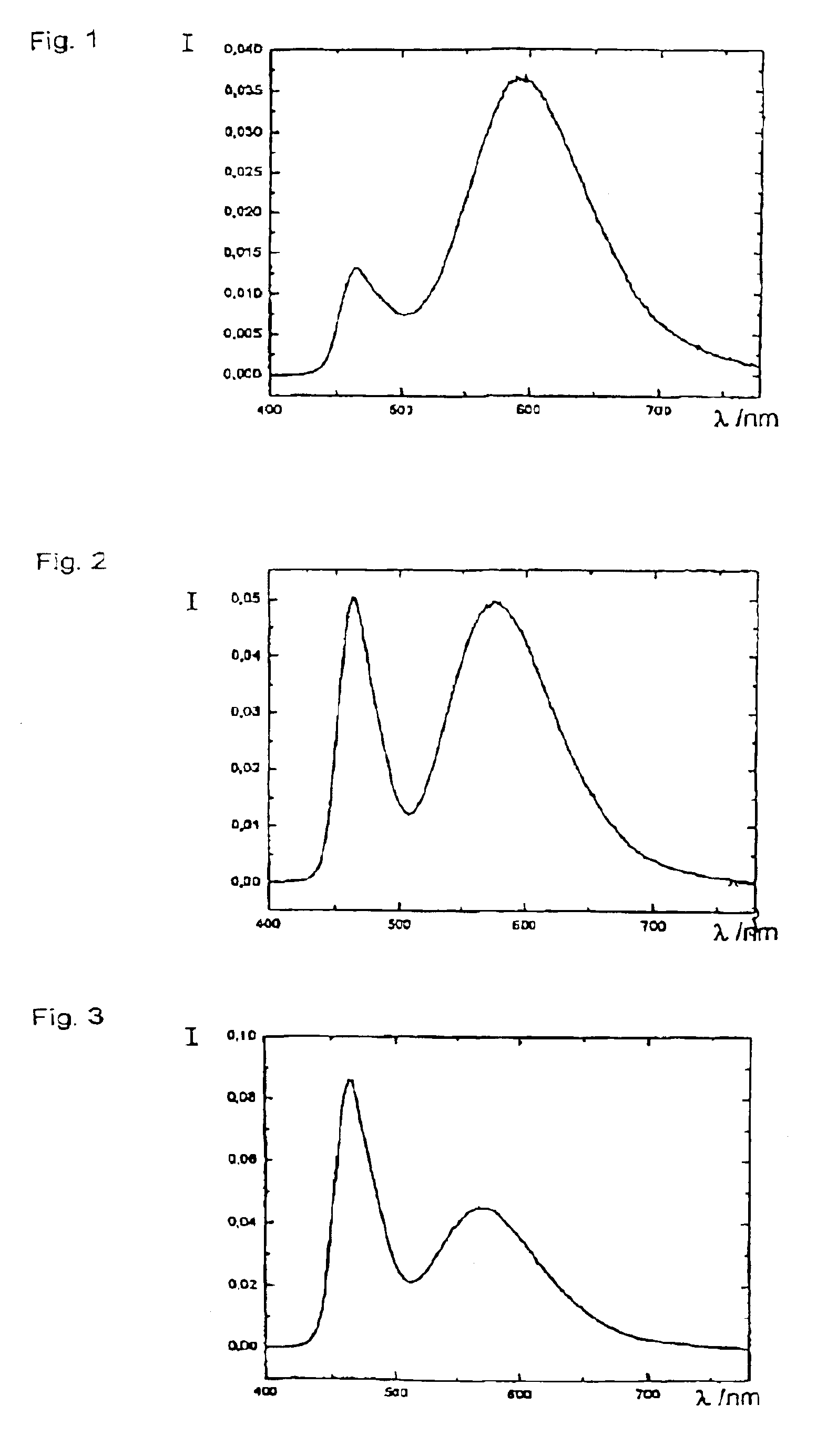
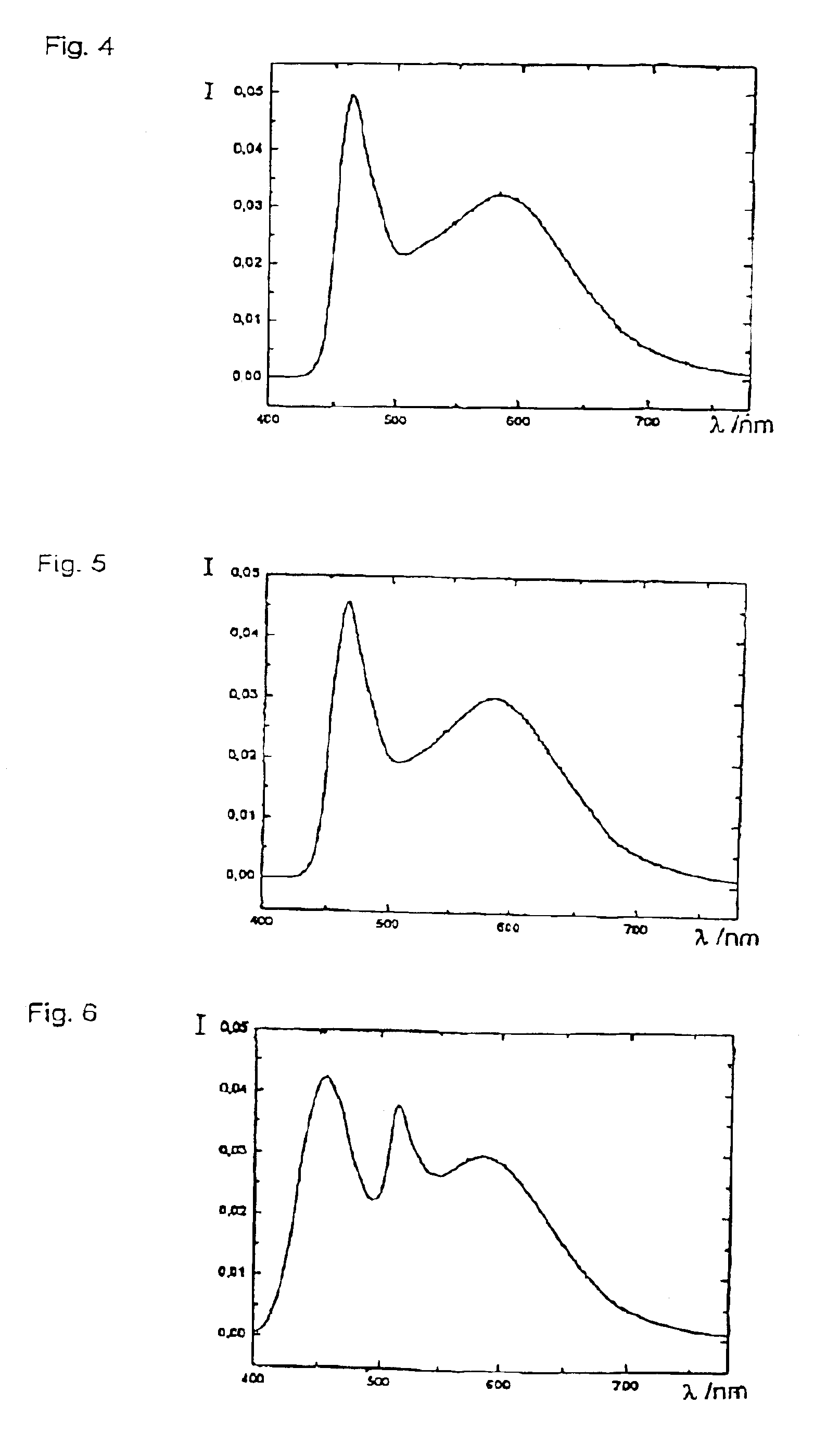
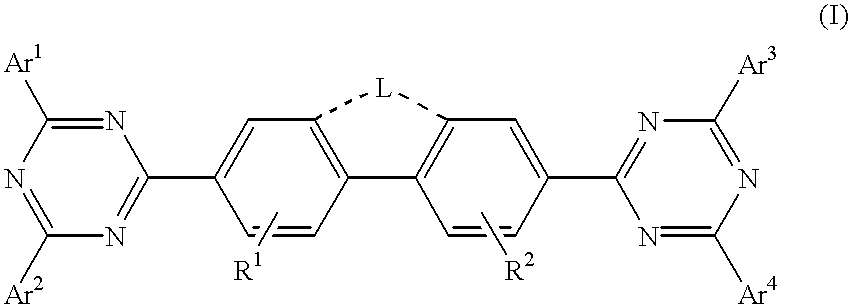
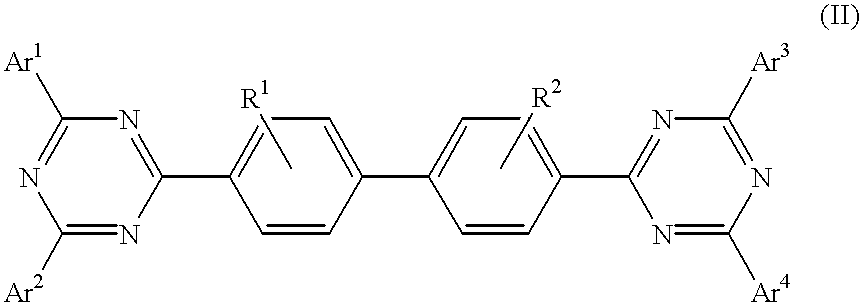
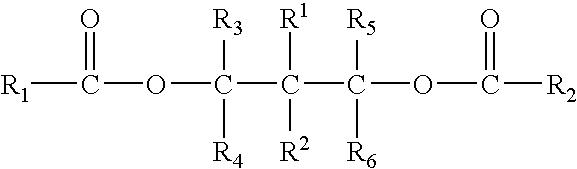
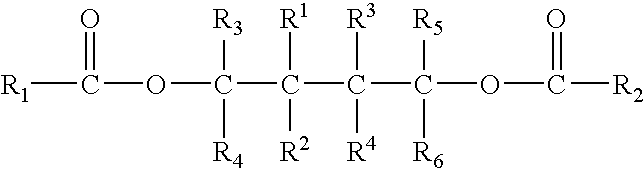
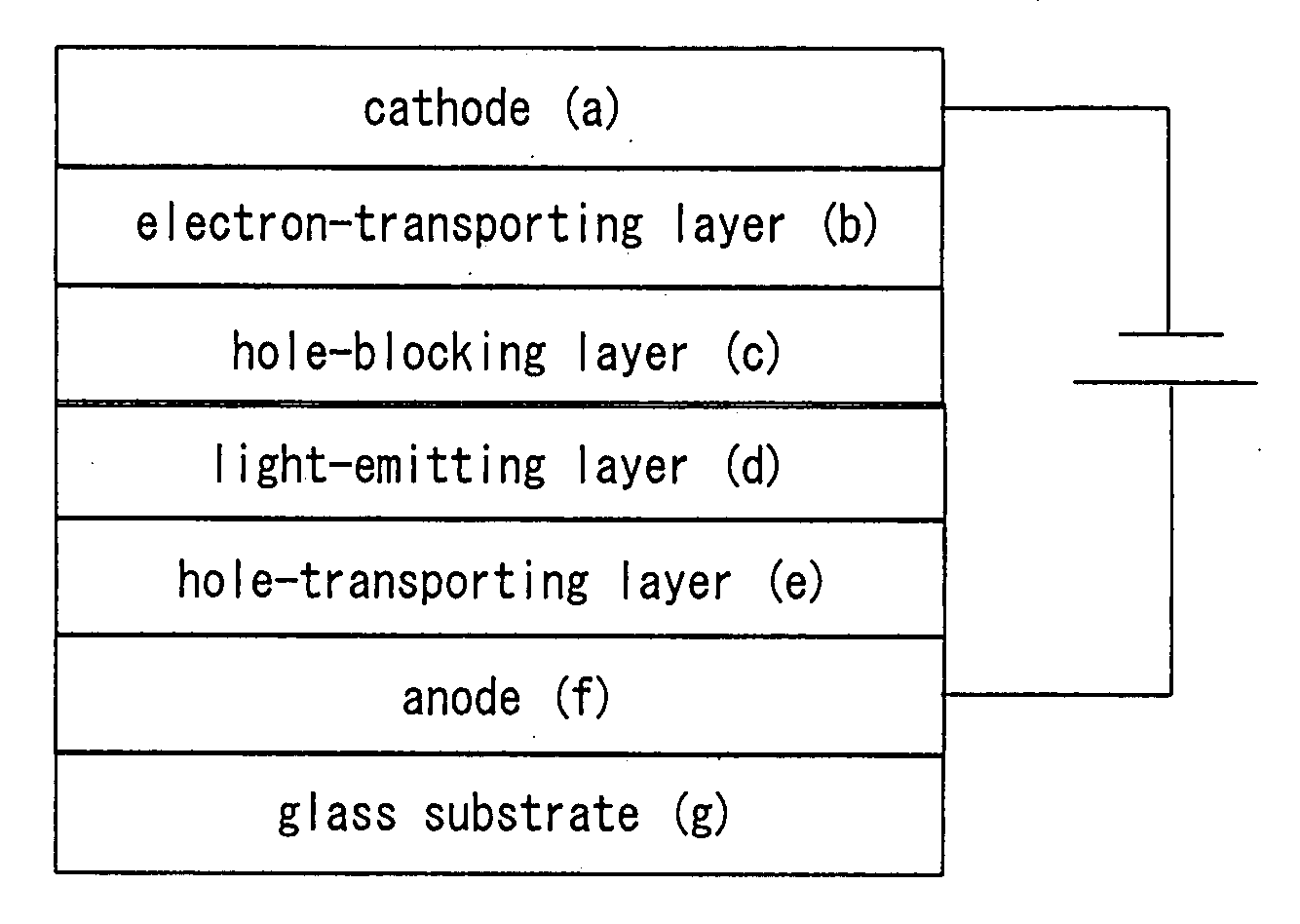
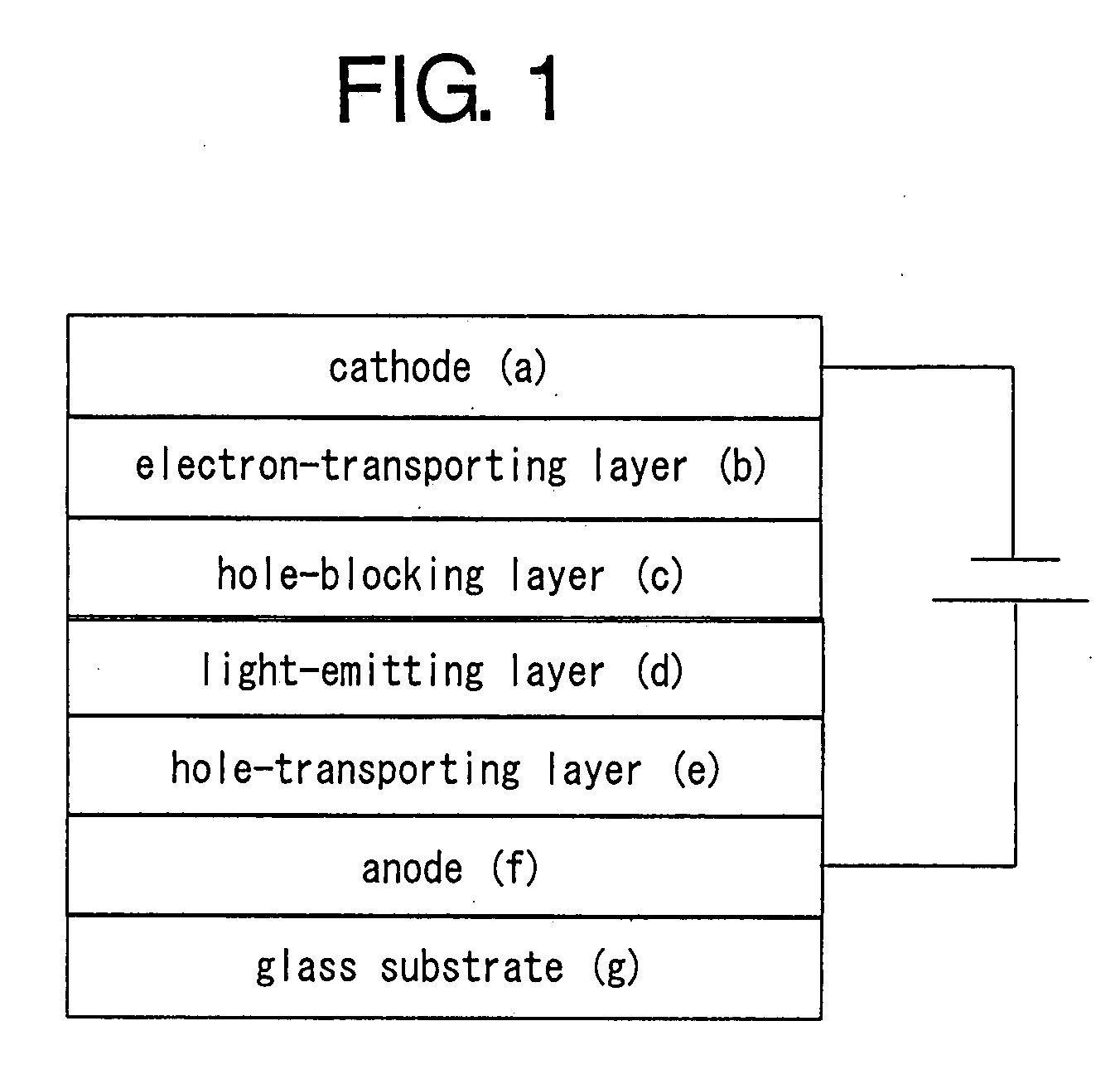
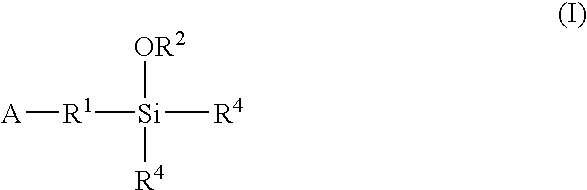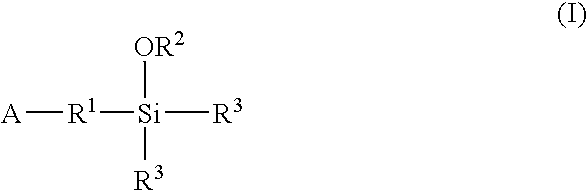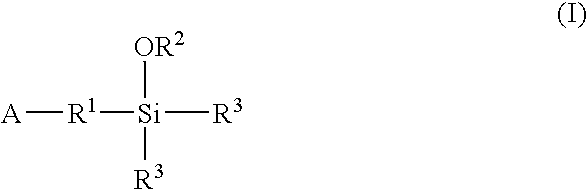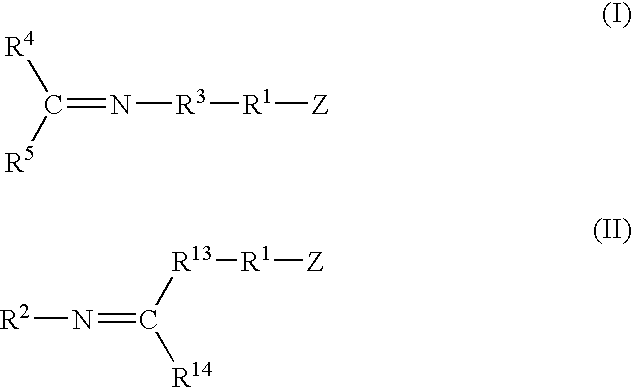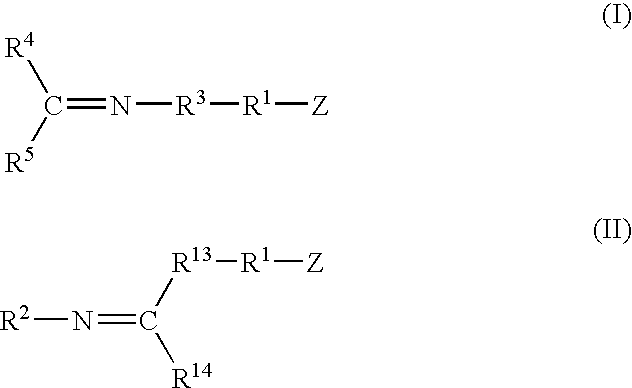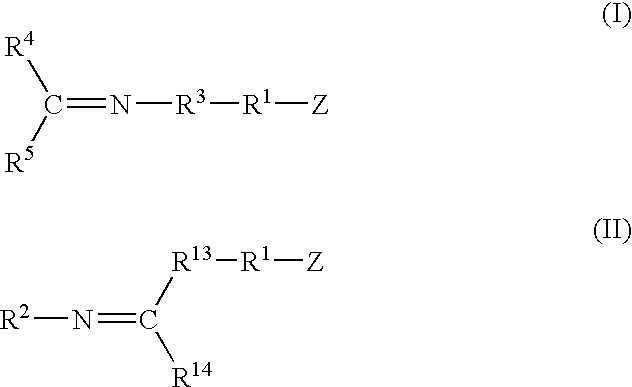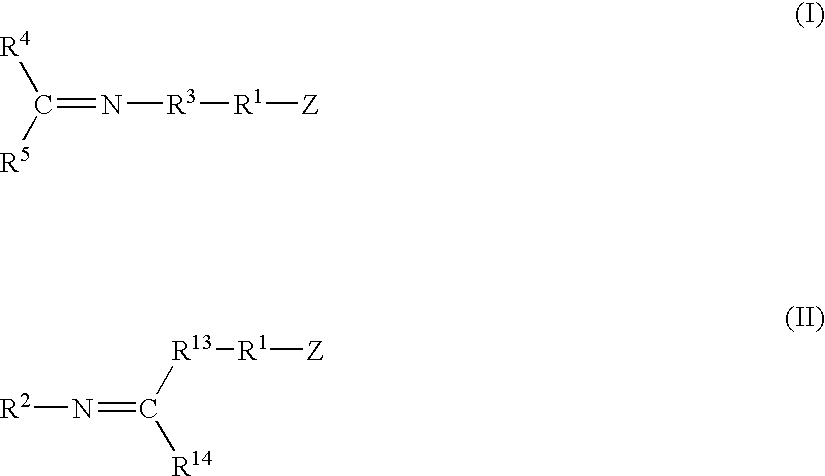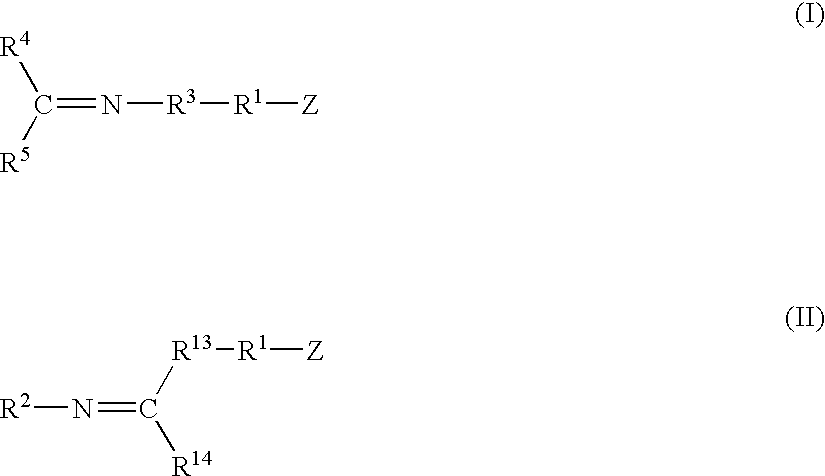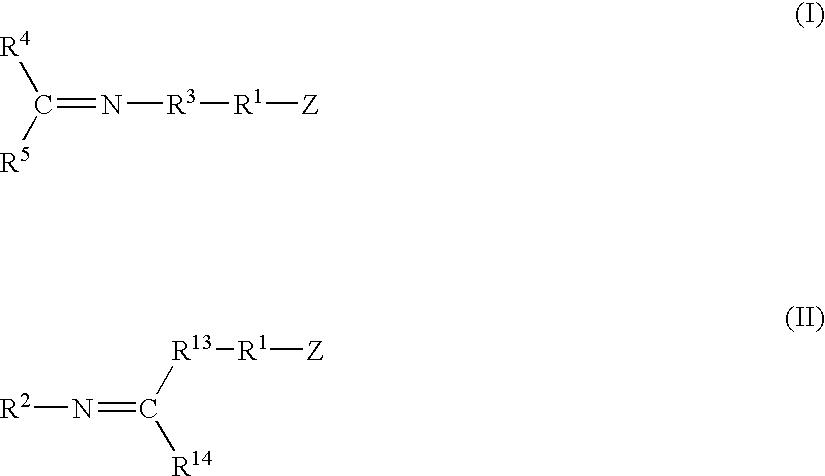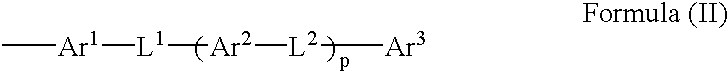Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6658 results about "Divalent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, a divalent element, ion, functional group or molecule has a valence of two. Valency is the number of chemical bonds formed, which may be covalent, polar covalent or ionic. Examples: Divalent elements include calcium and sulfur. While the bonds formed by calcium are ionic; sulfur can form covalent bonds as in H₂S or ionic bonds as in Na₂S. Divalent anions have a charge of -2, for example S2– and SO₄2–. Divalent cations have a charge of +2, for example Fe²⁺, Ca²⁺ and Hg₂²⁺. Divalent functional groups include the imino, =NH, and carbonyl, =O.
Organic electroluminescent device
ActiveUS20060263635A1Group 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsPlatinumNitrogen
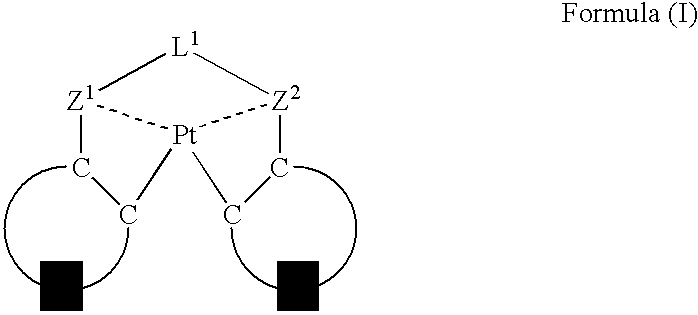
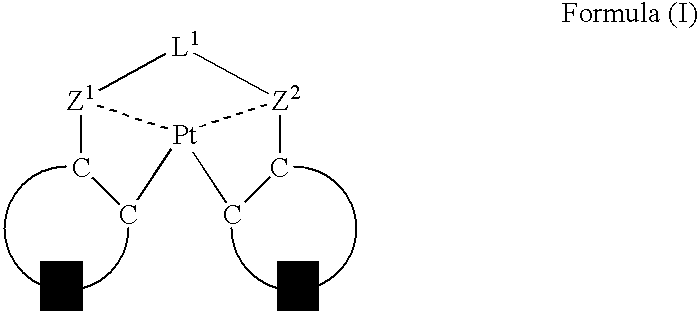
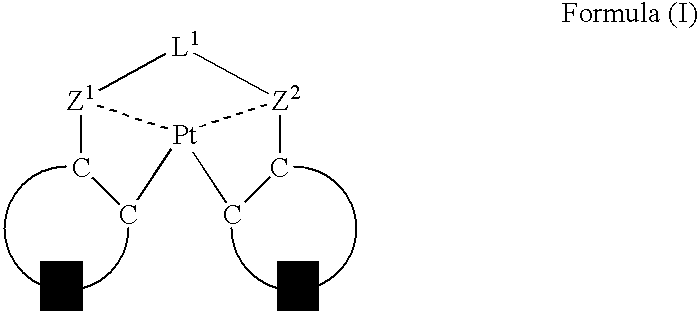
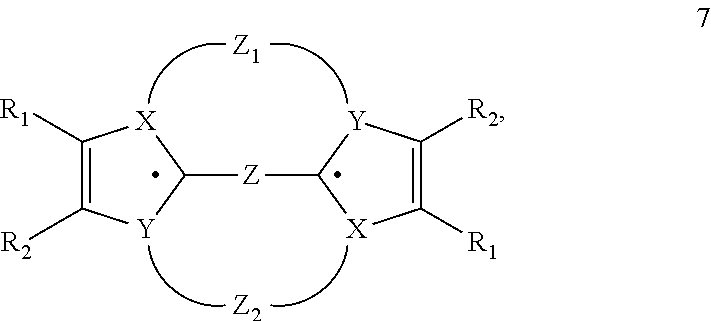
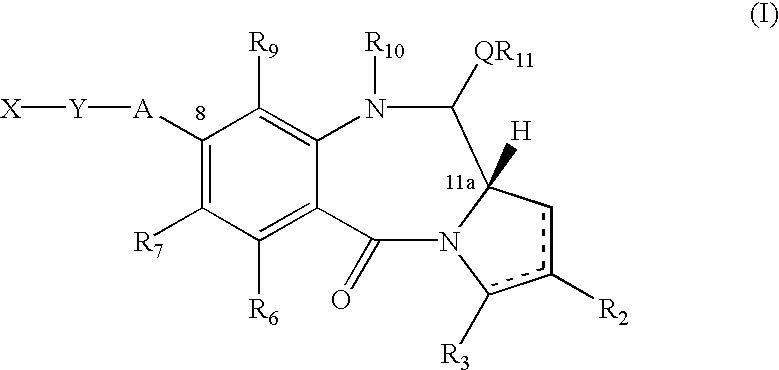
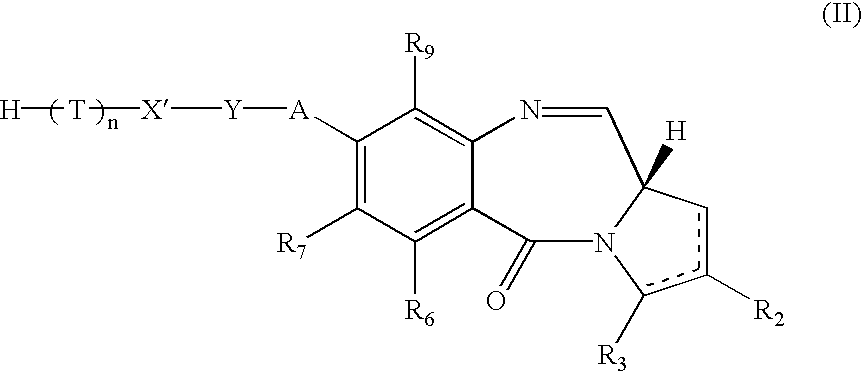
An organic electroluminescent device having a pair of electrodes and at least one organic layer interposed between the pair of electrodes, in which the at least one organic layer contains at least one compound represented by formula (I): wherein, Z1 and Z2 each independently represent a nitrogen-containing aromatic six-membered ring coordinated to the platinum through a nitrogen atom; Q1 represents a group of atoms necessary for forming, together with the —C—C—, a nitrogen-containing aromatic five-membered ring; L1 represents a single bond or a divalent linking group; and n is 0 or 1.
Owner:UDC IRELAND +1
Platinum complex and light emitting device
ActiveUS20070103060A1Enhanced glowSolve low luminous efficiencyDischarge tube luminescnet screensElectroluminescent light sourcesOxygenLight emission
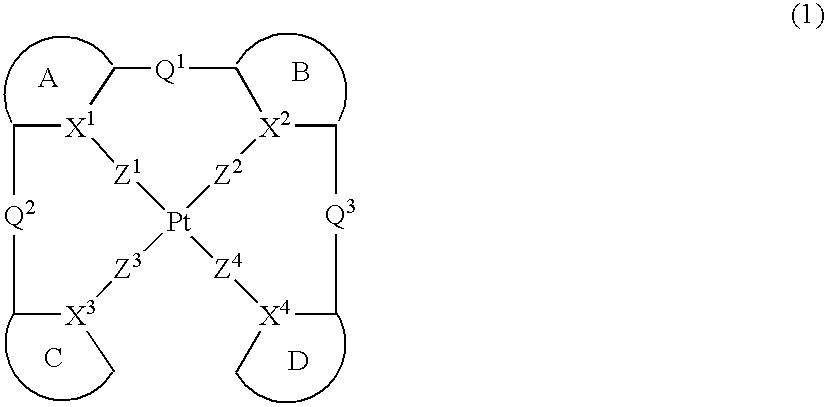
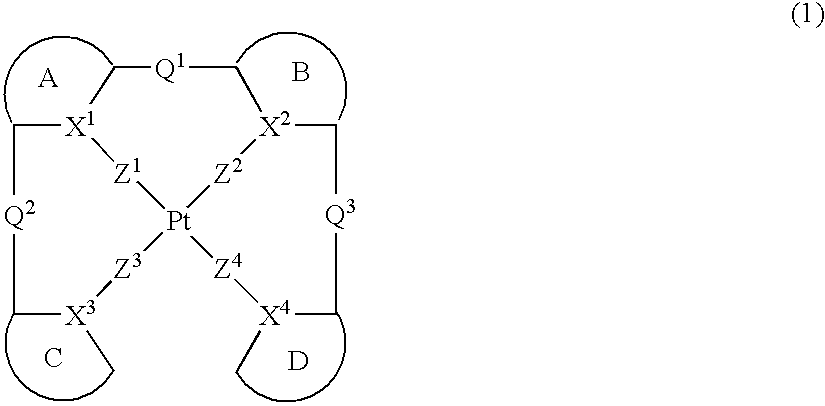
Provision of a novel platinum complex which is useful as a material for a light-emitting device of good light emission characteristic and light emission efficiency, and a novel light-emitting material that may be utilized in various fields. A platinum complex represented by the following general formula (1): (in which two rings of ring A, ring B, ring C, and ring D represent nitrogen-containing heterocyclic rings which may have a substituent and the remaining two rings of them represent aryl rings or hetero aryl rings which may have a substituent, the ring A and the ring B, the ring A and the ring C or / and the ring B and the rind D may form condensed rings. Two of X1, X2, X3, and X4 represent nitrogen atoms coordination bonded to a platinum atom and the remaining two of them represent carbon atoms or nitrogen atoms. Q1, Q2, and Q3 each represents a bond, oxygen atom, sulfur atom or bivalent group, two of Z1, Z2, Z3, and Z4 represent coordination bonds, and the remaining two of them represent covalent bonds, oxygen atoms or sulfur atoms), and a light-emitting device containing the platinum complex.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Aqueous emulsion polymerization of fluorinated monomers using a perfluoropolyether surfactant
The invention relates to an aqueous emulsion polymerization of fluorinated monomers using perfluoropolyethers of the following formula (I) or (II). In particular, the perfluoropolyether surfactants correspond to formula (I) or (II) CF3—(OCF2)m—O—CF2—X (I) wherein m has a value of 1 to 6 and X represents a carboxylic acid group or salt thereof, CF3—O—(CF2)3—(OCF(CF3)—CF2)z—O-L-Y (II) wherein z has a value of 0, 1, 2 or 3, L represents a divalent linking group selected from —CF(CF3)—, —CF2— and —CF2CF2— and Y represents a carboxylic acid group or salt thereof. The invention further relates to an aqueous dispersion of a fluoropolymer having the aforementioned perfluoropolyether surfactant(s).
Owner:3M INNOVATIVE PROPERTIES CO
Heterocyclic Radical or Diradical, The Dimers, Oligomers, Polymers, Dispiro Compounds and Polycycles Thereof, the Use Thereof, Organic Semiconductive Material and Electronic or Optoelectronic Component
ActiveUS20120146012A1Improve stabilityOrganic chemistrySolid-state devicesOligomerOrganic semiconductor
Owner:NOVALED GMBH
Modified polymers prepared with lanthanide-based catalysts
A method for preparing a functionalized polymer comprising the steps of preparing a pseudo-living polymer by polymerizing conjugated diene monomer with a lanthanide-based catalyst, and reacting the pseudo-living polymer with at least one functionalizing agent defined by the formula (I) where A is a substituent that will undergo an addition reaction with a pseudo-living polymer, R1 is a divalent organic group, R2 is a monovalent organic group, and each R4, which may be the same or different, is a monovalent organic group or a substituent defined by —OR5 where R5 is a monovalent organic group, with the proviso that A, R1, R2, R4, and R5 are substituents that will not protonate a pseudo-living polymer. Also, the functionalized polymer and a vulcanizable composition containing the polymer.
Owner:BRIDGESTONE CORP
Modified polymers prepared with lanthanide-based catalysts
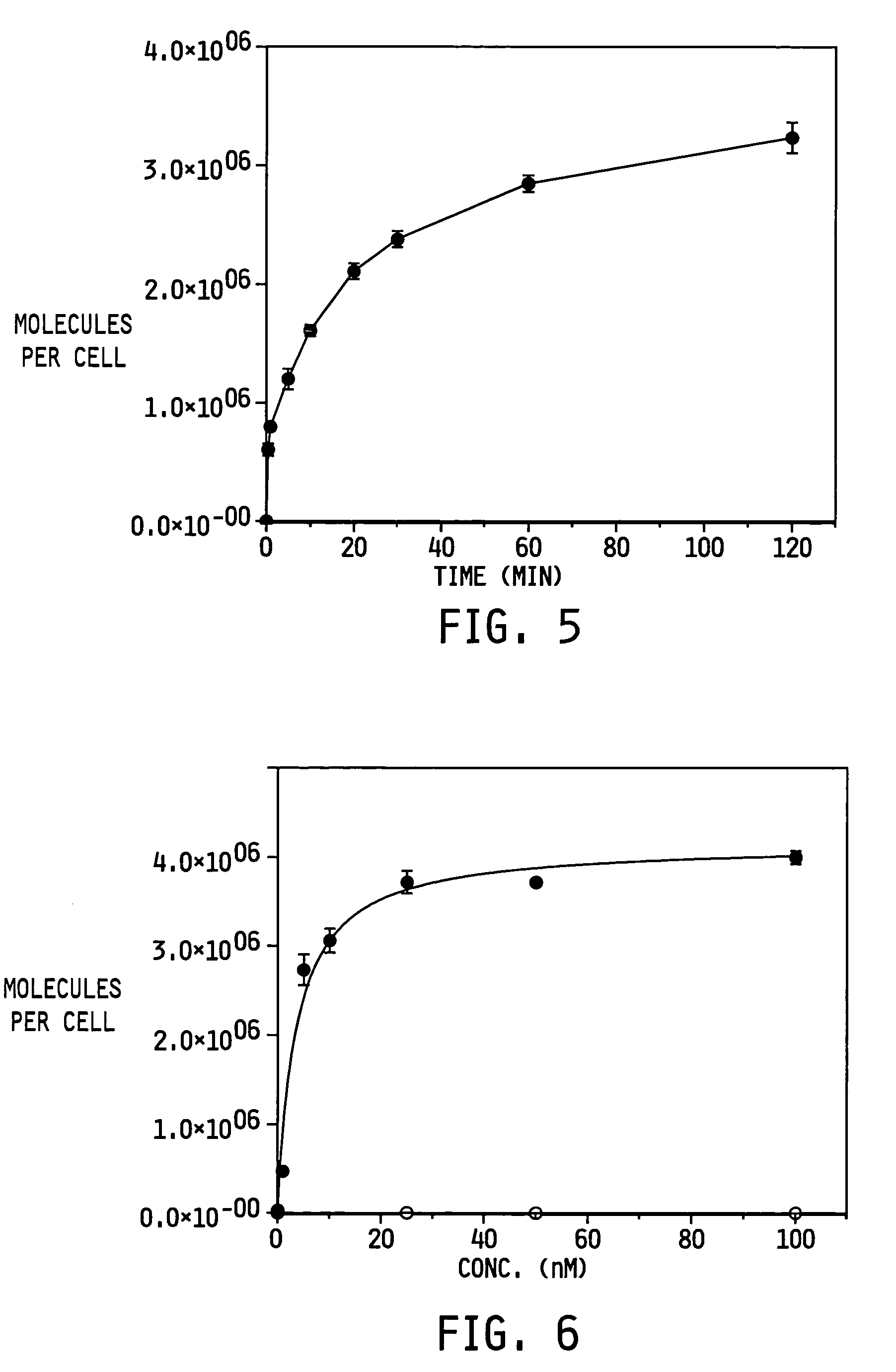
A method for preparing a functionalized polymer comprising the steps of preparing a pseudo-living polymer by polymerizing conjugated diene monomer with a lanthanide-based catalyst, where said pseudo-living polymer is characterized by having greater than about 85 percent of the polymer in the cis microstructure and less than about 3 percent of the polymer is in the 1,2- or 3,4-microstructure, and reacting the pseudo-living polymer with at least one functionalizing agent defined by the formula (I) or (II) where Z is a substituent that will react or interact with organic or inorganic fillers; R1 is a single bond or a divalent organic group; R2 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R13 or R14; R3 is a single bond, a divalent organic group, or a trivalent organic group that forms a cyclic organic group with R4 or R5; R13 is a single bond, a divalent organic group, or a trivalent organic group that forms a cyclic organic group with R2 or R14; R4 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R3 or R5; R14 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R2 or R13; and R5 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R3 or R4; with the proviso that each group attached to the imino carbon is attached via a carbon atom and R1, R2, R3, R4, R5, R13, R14 and Z are substituents that will not protonate a pseudo-living polymer.
Owner:BRIDGESTONE CORP
Light source comprising a light-emitting element
InactiveUS6809347B2High luminous efficiencyHigh degreePlanar light sourcesPoint-like light sourceLuminophoreManganese
Owner:TOYODA GOSEI CO LTD
Triazine compositions
A triazine compound of the formulawherein Ar1, Ar2, Ar3, and Ar4 are each an aryl, aliphatic, or a mixture of aryl and aliphatic; R1 and R2 are selected from the group consisting of hydrogen, alkyl, aryl, alkoxy, halogen, and cyano; and L is L(n) wherein n is zero or 1, said L being a divalent group.
Owner:LG DISPLAY CO LTD
Modified polymers prepared with lanthanide-based catalysts
A method for preparing a functionalized polymer comprising the steps of preparing a pseudo-living polymer by polymerizing conjugated diene monomer with a lanthanide-based catalyst, and reacting the pseudo-living polymer with at least one functionalizing agent defined by the formula (I) where A is a substituent that will undergo an addition reaction with a pseudo-living polymer, R1 is a divalent organic group, R2 is a monovalent organic group, and each R4, which may be the same or different, is a monovalent organic group or a substituent defined by —OR5 where R5 is a monovalent organic group, with the proviso that A, R1, R2, R4, and R5 are substituents that will not protonate a pseudo-living polymer. Also, the functionalized polymer and a vulcanizable composition containing the polymer.
Owner:ENEOS MATERIALS CORP
Liquid crystal composition comprising liquid crystal molecules and alignment promoter
InactiveUS20020039627A1Easy alignmentLiquid crystal compositionsPolarising elementsSingle bondCarbon atom
A liquid crystal composition comprises liquid crystal molecules and an alignment promoter. The alignment promoter is represented by the formula (I). <paragraph lvl="0"><in-line-formula>(Hb-L1-)nBl (I) < / in-line-formula>In the formula (I), Hb is an aliphatic group having 4 to 40 carbon atoms, an aromatic group having 6 to 40 carbon atoms or an aliphatic substituted oligosiloxanoxy group having 1 to 40 carbon atoms. L1 is a single bond or a divalent linking group, and n is an integer of 2 to 12. Bl is an n-valent group comprising at least two rings.
Owner:FUJIFILM CORP
Vitamin-targeted imaging agents
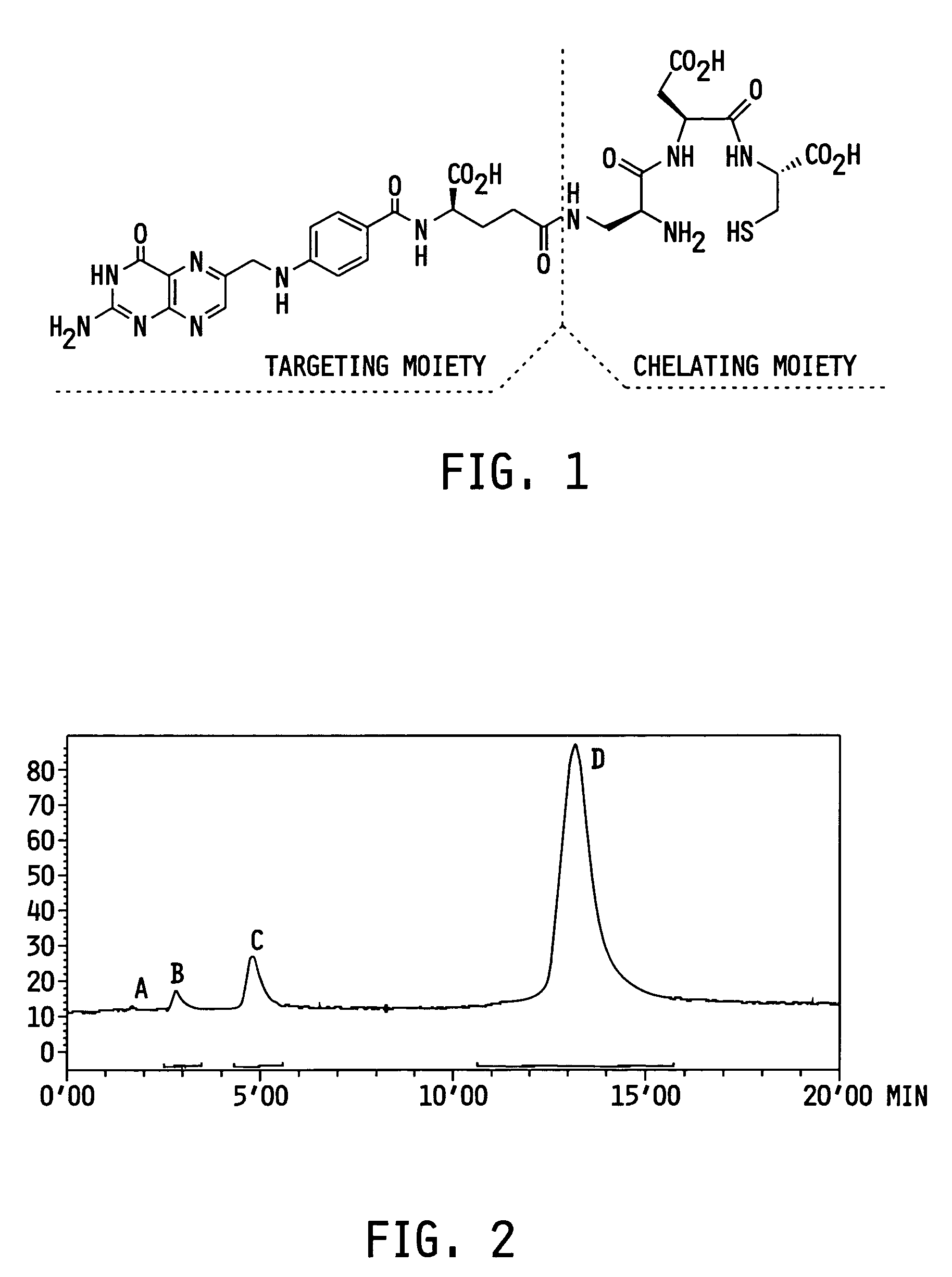
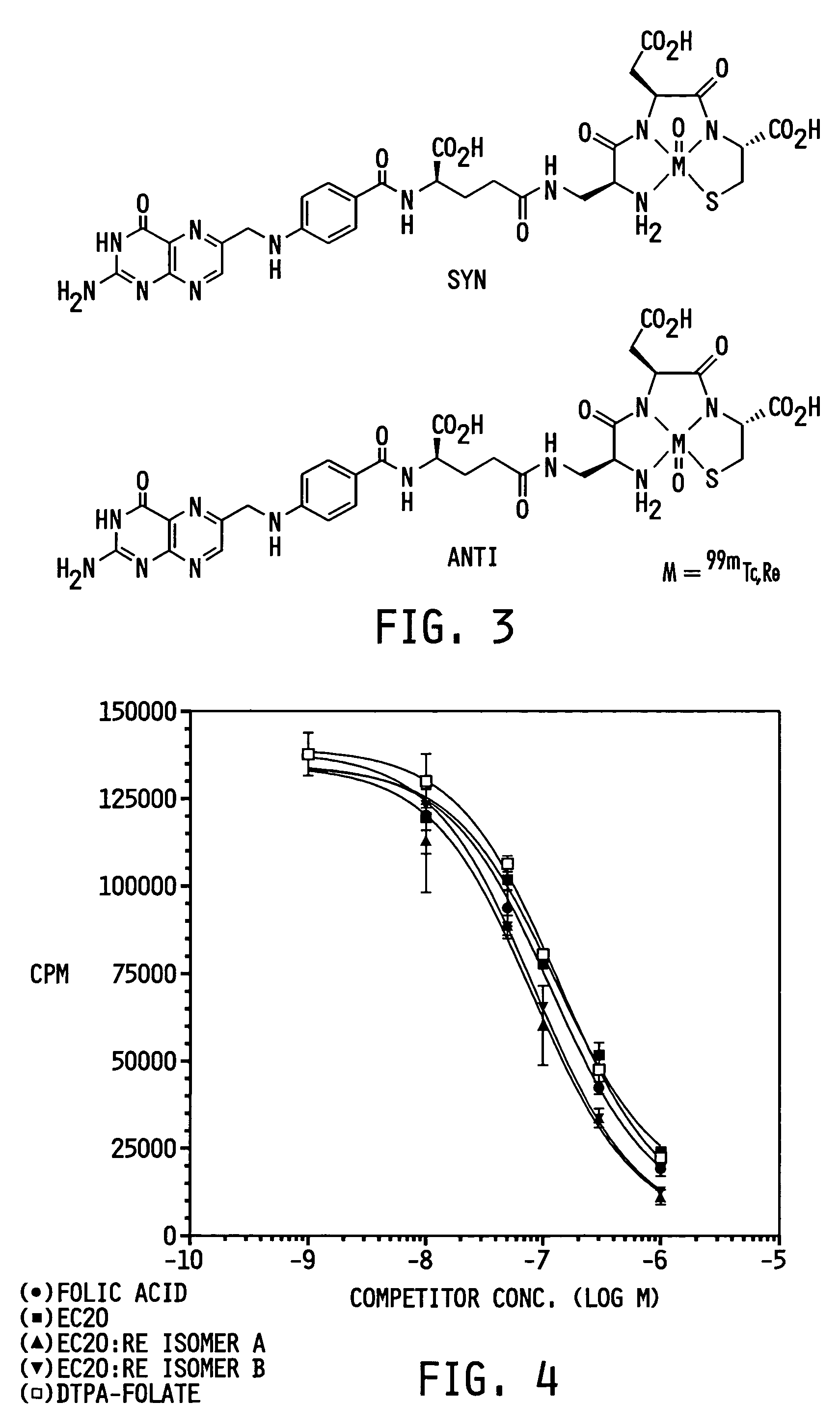
ActiveUS7128893B2Easy to getProducing cost is highPeptide/protein ingredientsRadioactive preparation carriersVitamin receptorSide chain
The invention relates to compounds and methods for targeting radionuclide-based imaging agents to cells having receptors for a vitamin, or vitamin receptor binding derivative or analog thereof, by using such a vitamin as the targeting ligand for the imaging agent. The invention provides a compound of the formulafor use in such methods. In the compound, V is a vitamin that is a substrate for receptor-mediated transmembrane transport in vivo, or a vitamin receptor binding derivative or analog thereof, L is a divalent linker, R is a side chain of an amino acid, M is a cation of a radionuclide, n is 1 or 0, K is 1 or 0, and the compound can be in a pharmaceutically acceptable carrier therefor. The vitamin-based compounds can be used to target radionuclides to cells, such as a variety of tumor cell types, for use in diagnostic imaging of the targeted cells.
Owner:ENDOCTYE INC
Modified polymers prepared with lanthanide-based catalysts
A method for preparing a functionalized polymer comprising the steps of preparing a pseudo-living polymer by polymerizing conjugated diene monomer with a lanthanide-based catalyst, where said pseudo-living polymer is characterized by having greater than about 85 percent of the polymer in the cis microstructure and less than about 3 percent of the polymer is in the 1,2- or 3,4-microstructure, and reacting the pseudo-living polymer with at least one functionalizing agent defined by the formula (I) or (II) where Z is a substituent that will react or interact with organic or inorganic fillers; R1 is a single bond or a divalent organic group; R2 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R13 or R14; R3 is a single bond, a divalent organic group, or a trivalent organic group that forms a cyclic organic group with R4 or R5; R13 is a single bond, a divalent organic group, or a trivalent organic group that forms a cyclic organic group with R2 or R14; R4 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R3 or R5; R14 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R2 or R13; and R5 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R3 or R4; with the proviso that each group attached to the imino carbon is attached via a carbon atom and R1, R2 R3, R4, R5, R13, R14 and Z are substituents that will not protonate a pseudo-living polymer.
Owner:BRIDGESTONE CORP
Cosmetic and Pharmaceutical Masks Based on Ion-Pair Delivery System
InactiveUS20040219124A1Easy to disassembleMaximum absorption and bioavailabilityCosmetic preparationsToilet preparationsCross-linkBiopolymer
The present invention discloses a novel ion-pair delivery system based mask compositions for face, hair, skin, and body applications. These compositions come off from the site of their application essentially in one piece with the appearance, for example, of a piece of sea-weed or a continuous film. These mask compositions are suitable for a variety of delivery system methods, such as peel-off mask, moisturizing mask, exfoliating mask, prosthetic mask, soaking mask, depilatory mask, rub-off mask, two-phase mask, two-compartment mask, heat-releasing mask, and such. These mask compositions are made from the biopolymer based films that are cross-linked with divalent or trivalent metal cations. During the cross-linking process, such divalent and trivalent metal cations may also act as release agents for other face, hair, skin, and body beneficial compositions in their enhanced bioavailable forms by an ion-pair activation mechanism.
Owner:GUPTA SHYAM K
Water absorbent resin composition and production method thereof
InactiveUS20050288182A1Promote absorptionInhibitionOther chemical processesBaby linensCross-linkAbsorption capacity
The water absorbent resin composition and the production method thereof according to the present invention are characterized by including: water absorbent resin particles having an internal cross-linked structure obtained by polymerizing a water-soluble unsaturated monomer; a nitrogenous ketone compound (A) (containing no carboxyl group) having a structure represented by formula (1); and a bivalent and / or trivalent and / or tetravalent water-soluble metal salt, wherein a total amount of the nitrogenous ketone compound (A) and the bivalent and / or trivalent and / or tetravalent water-soluble metal salt ranges from 0.01 to 100 parts by mass with respect to 100 parts by mass of the water absorbent resin particles, thereby providing a water absorbent resin composition, having an excellent absorption capacity represented by a centrifuge retention capacity (CRC), an absorbency against pressure of 4.83 kPa (AAP) etc., having excellent liquid permeability and liquid diffusion properties, having excellent fluidity at the time of moisture absorption, having an excellent damage resistance property, effectively suppressing occurrence of dusts, hardly bringing about permeation of added metal compounds into water absorbent resin particles, hardly bringing about segregation of added metal compounds.
Owner:NIPPON SHOKUBAI CO LTD
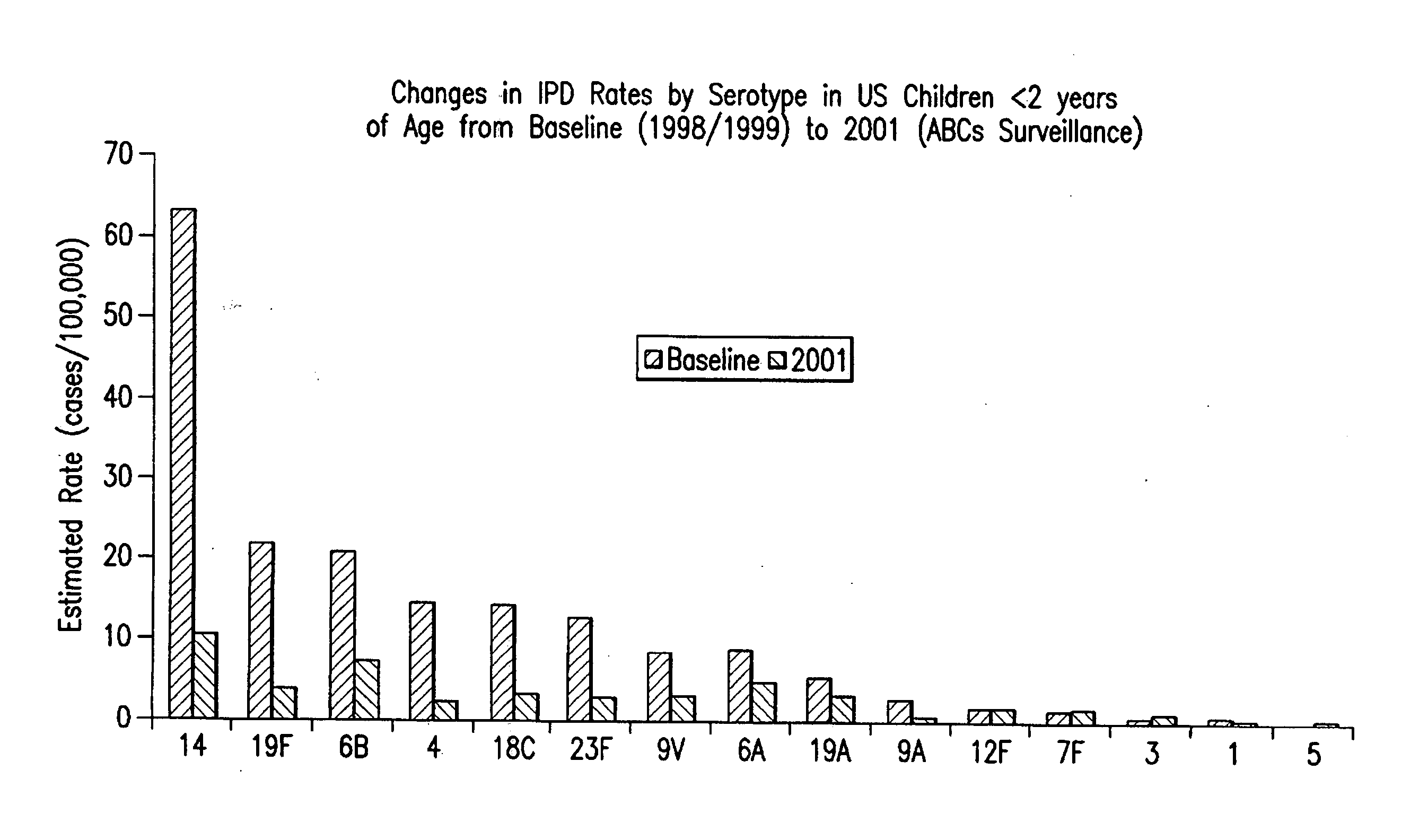
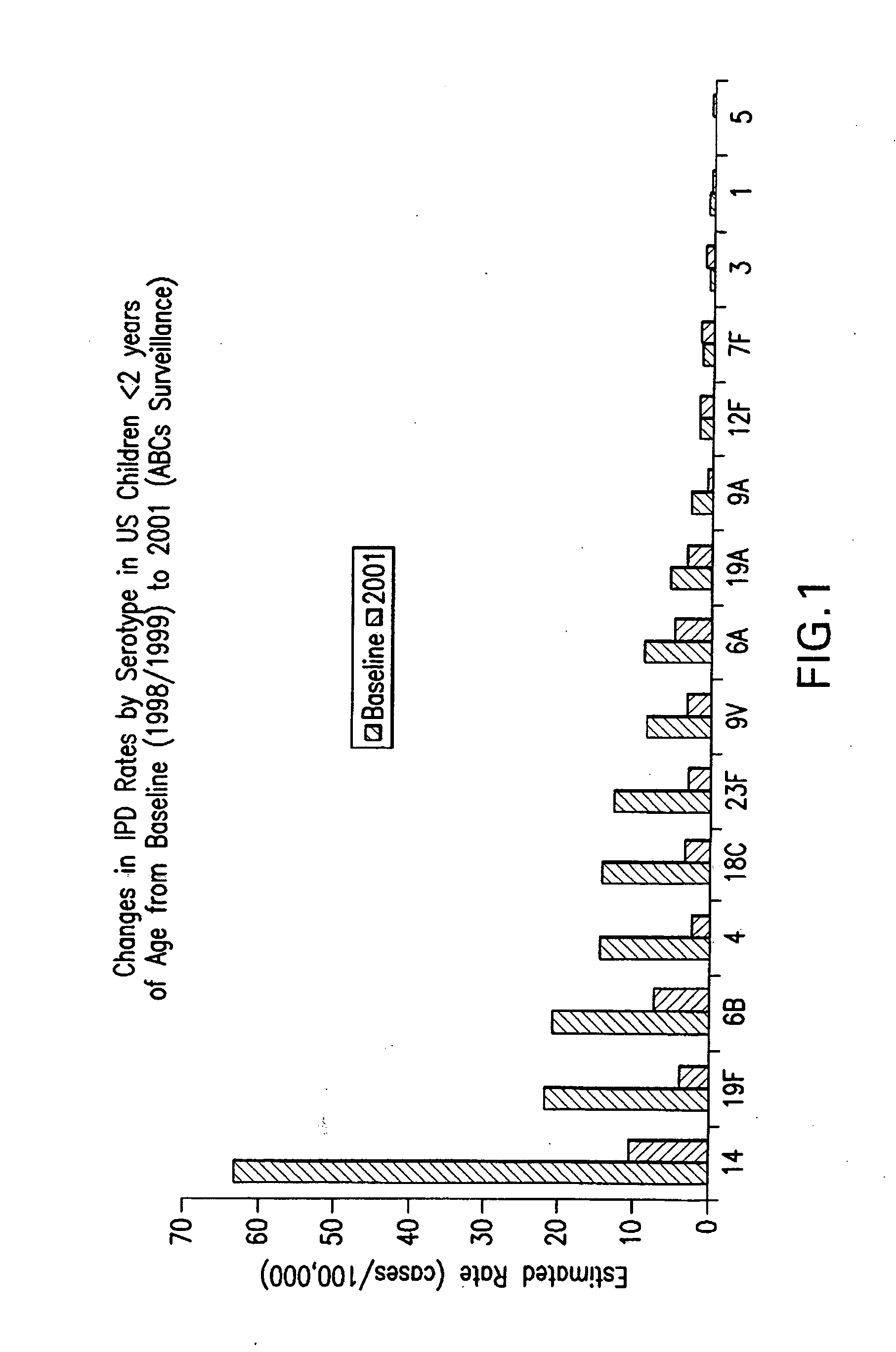
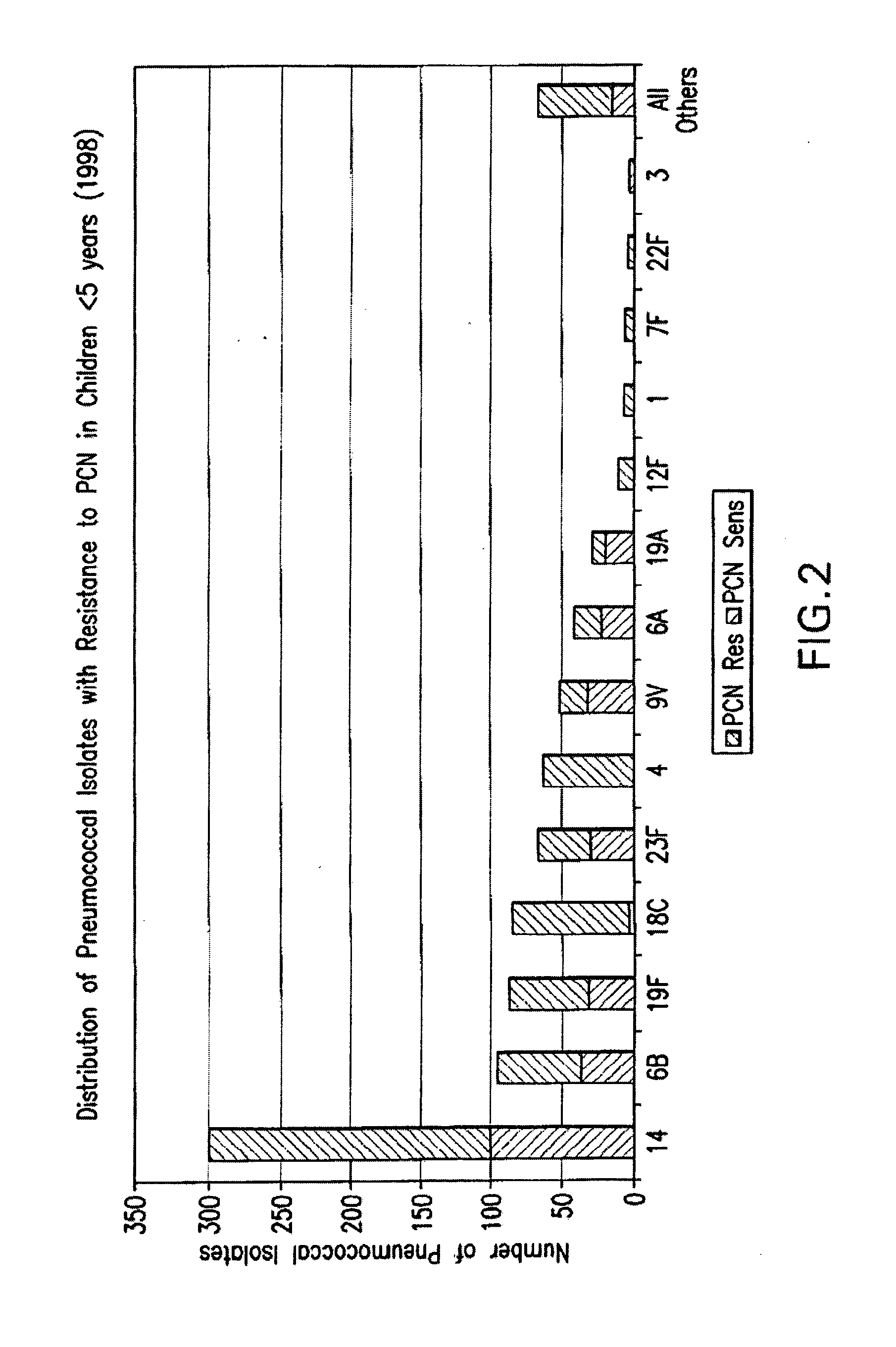
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
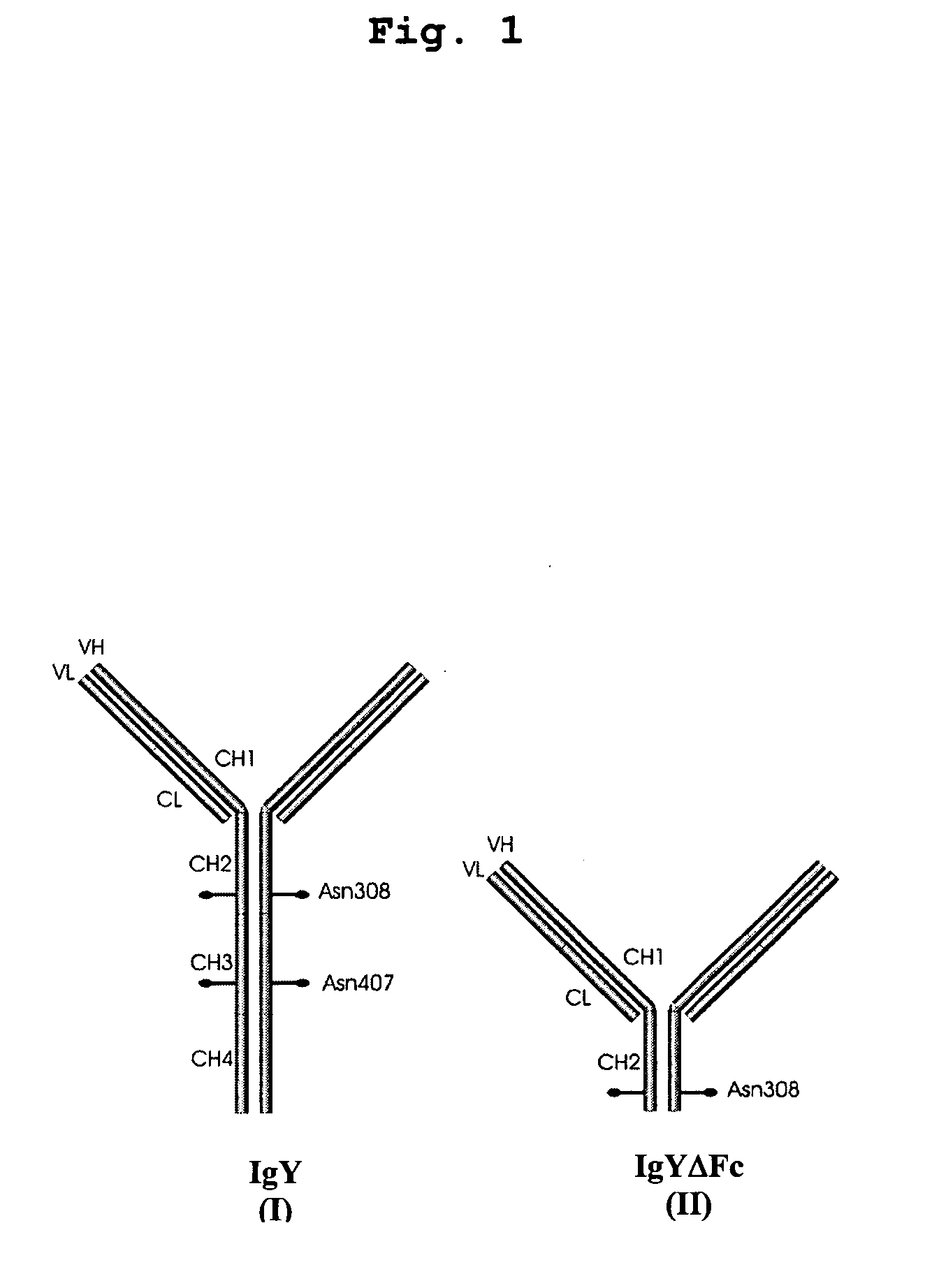
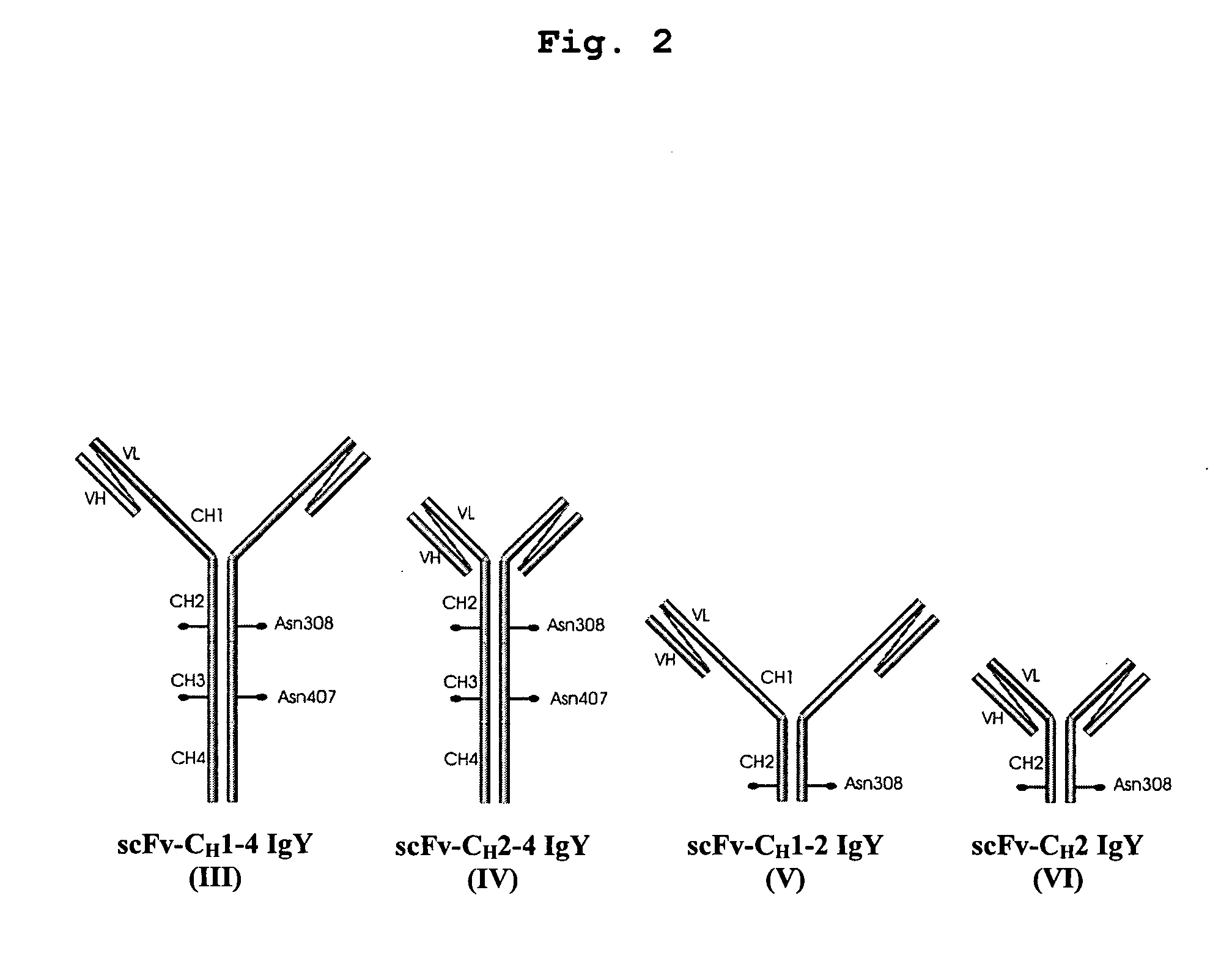
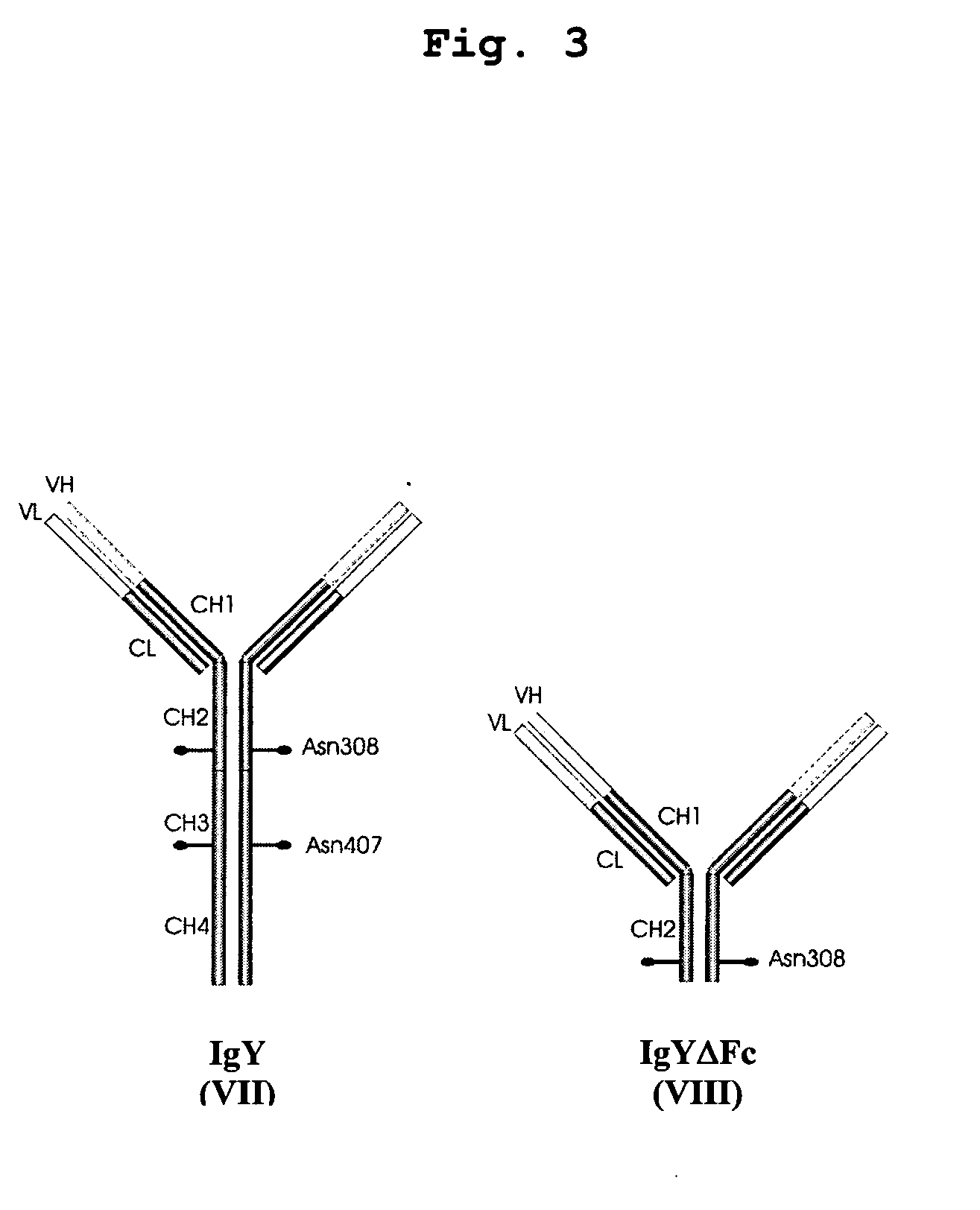
Bivalent IgY antibody constructs for diagnostic and therapeutic applications
InactiveUS20070141049A1Improve the immunityIncrease contentImmunoglobulins against virusesAntibody ingredientsHeavy chainMammal
This invention relates to the field of recombinant antibody technology. It provides novel recombinant IgY antibody constructs for diagnostic and therapeutical applications. The bivalent antibody constructs display a heterotetrameric or homodimeric format stabilized by disulfide bonds. The constant heavy chain domains CH2-CH4 are partly or completely of avian origin, whereas the VH, VL, CL, and CH1 domains as well as the hinge region may be of avian origin or derived from any other species. The invention allows to combine the advantages of IgY antibodies with those of established mammalian monoclonal antibodies. IgY antibody constructs comprising nonglycosylated IgY constant heavy chain domains allow to reduce unwanted interactions with C-type lectins, e.g., in human sera. Furthermore, chimeric IgY antibody containing mammalian VH, VL, CL, and CH1 domains as well as a mammalian hinge region provide a higher molecular stability than IgY antibodies in acidic conditions and, thereby, are especially suited for peroral therapeutic applications.
Owner:PLS DESIGN
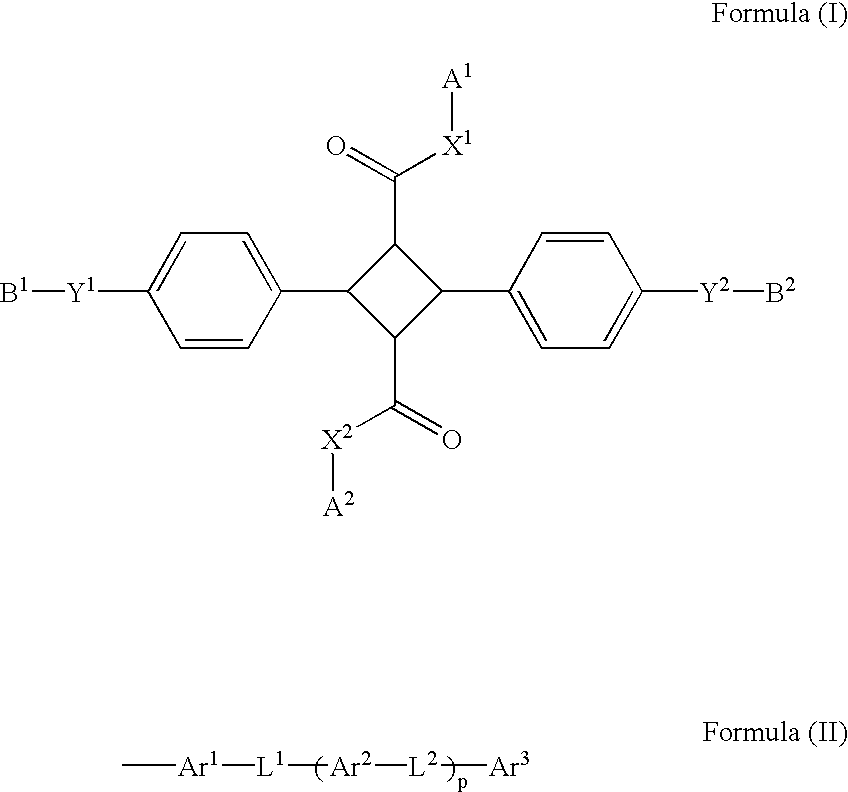
4-Membered ring compound and optical phase optical retardation plate using the same
InactiveUS20030102458A1Liquid crystal compositionsCarbamic acid derivatives preparationSulfurSingle bond
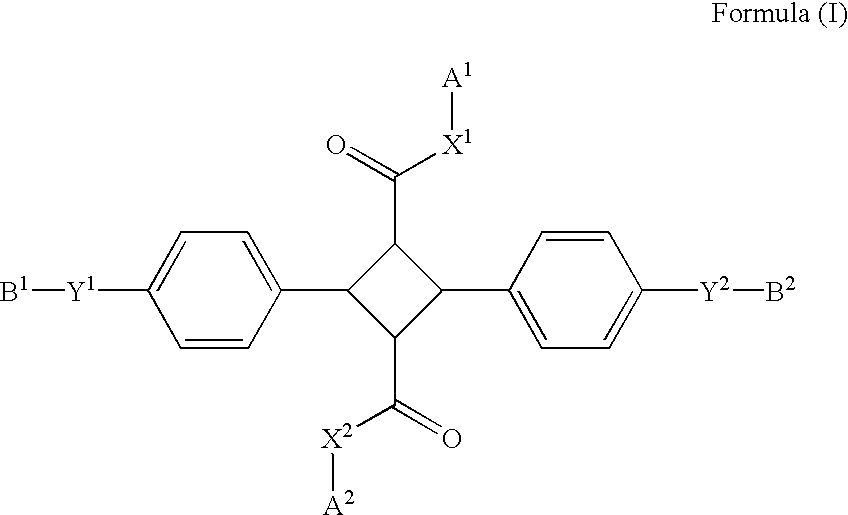
A 4-membered compound is represented by the following formula (I) is disclosed (in the formula, X1 and X2 each independently represent an oxygen atom, a sulfur atom or a substituted or unsubstituted imino group, Y1 and Y2 each independently represent a single bond, an oxygen atom or a substituted or unsubstituted imino group, B1 and B2 each independently represent an optionally substituted aliphatic, aliphatic carbonyl, aromatic or aromatic carbonyl group having 1-20 carbon atoms, and A1 and A2 each independently represent a group represented by the following formula (II) (Ar1, Ar2 and Ar3 each independently represent a cyclic group having 5-14 carbon atoms, L1 and L2 each independently represent a single bond or a divalent linking group, and p represents an integer of 0-2)). There is also disclosed a birefringence medium containing a 4-membered compound represented by the formula (I) and an optical element comprising the birefringence medium.
Owner:FUJIFILM CORP
Vitamin receptor binding drug delivery conjugates
The invention describes a vitamin receptor binding drug delivery conjugate, and preparations therefor. The drug delivery conjugate consists of a vitamin receptor binding moiety, a bivalent linker (L), and a drug. The vitamin receptor binding moiety includes vitamins, and vitamin receptor binding analogs and derivatives thereof, and the drug includes analogs and derivatives thereof. The vitamin receptor binding moiety is covalently linked to the bivalent linker, and the drug, or the analog or the derivative thereof, is covalently linked to the bivalent linker, wherein the bivalent linker (L) includes components such as spacer linkers, releasable linkers, and heteroatom linkers, and combinations thereof. Methods and pharmaceutical compositions for eliminating pathogenic cell populations using the drug delivery conjugate are also described.
Owner:ENDOCTYE INC
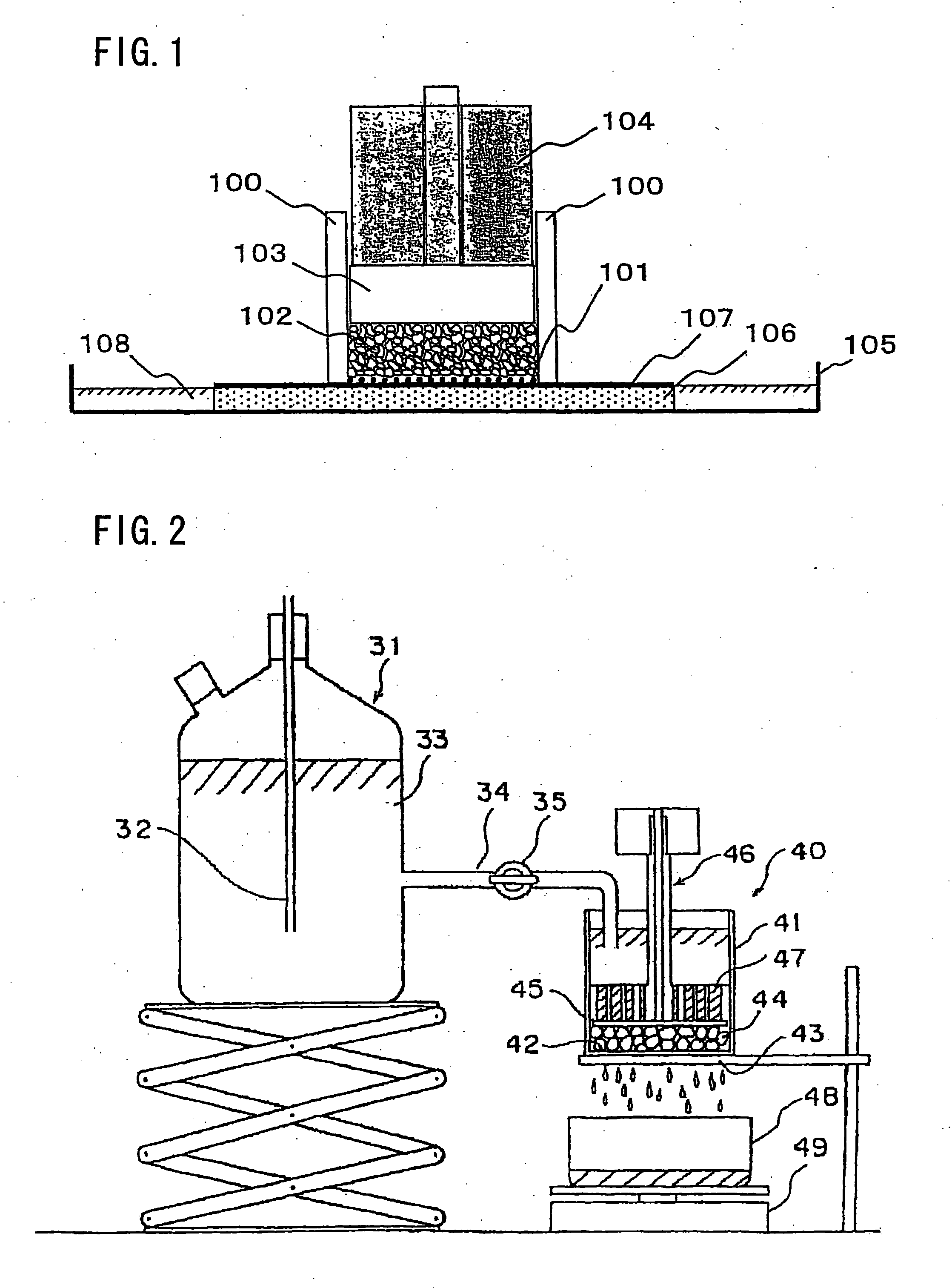
Water desalination process and apparatus
ActiveUS20060060532A1Electrolysis componentsGeneral water supply conservationSolubilityWater desalination
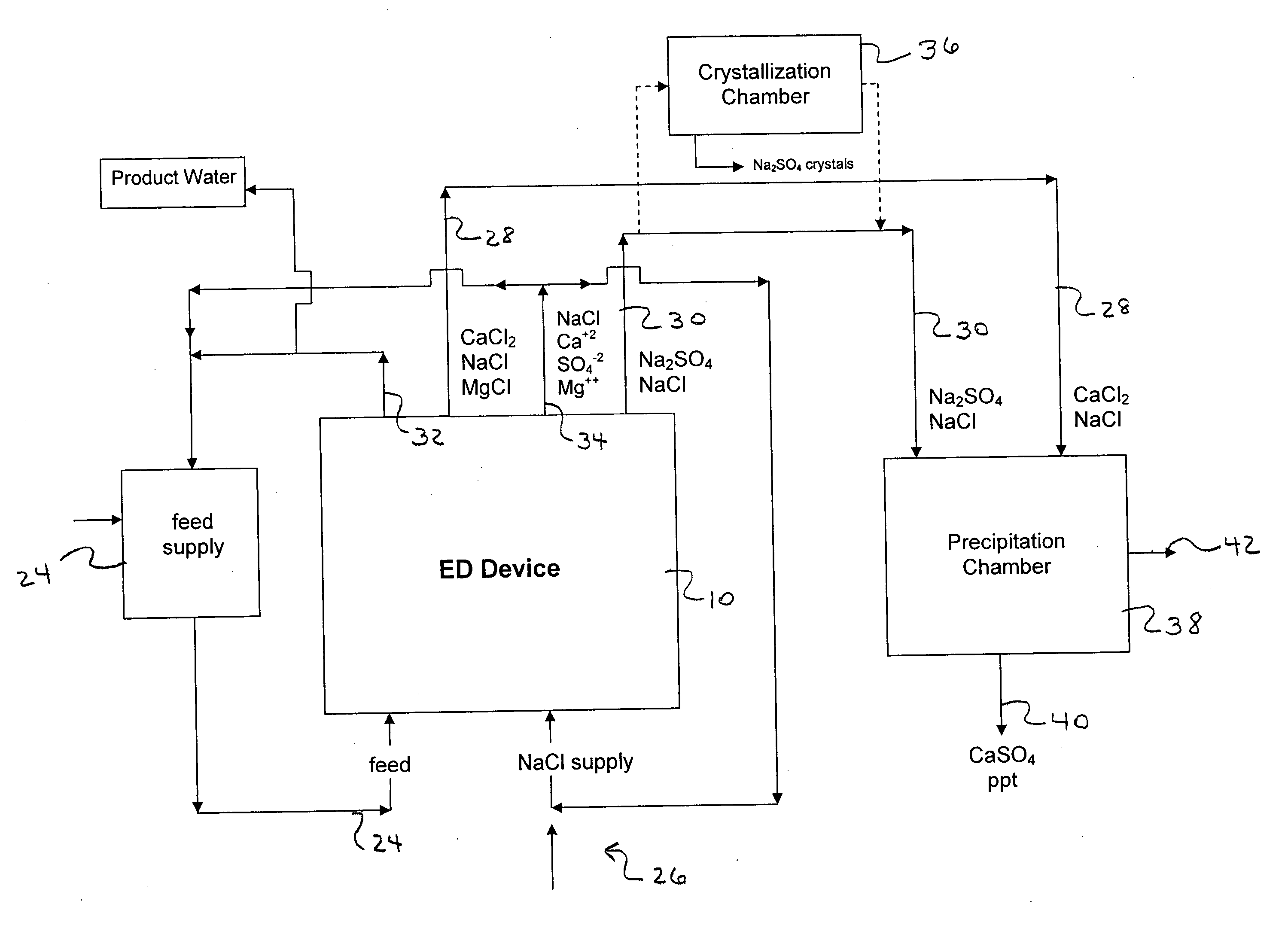
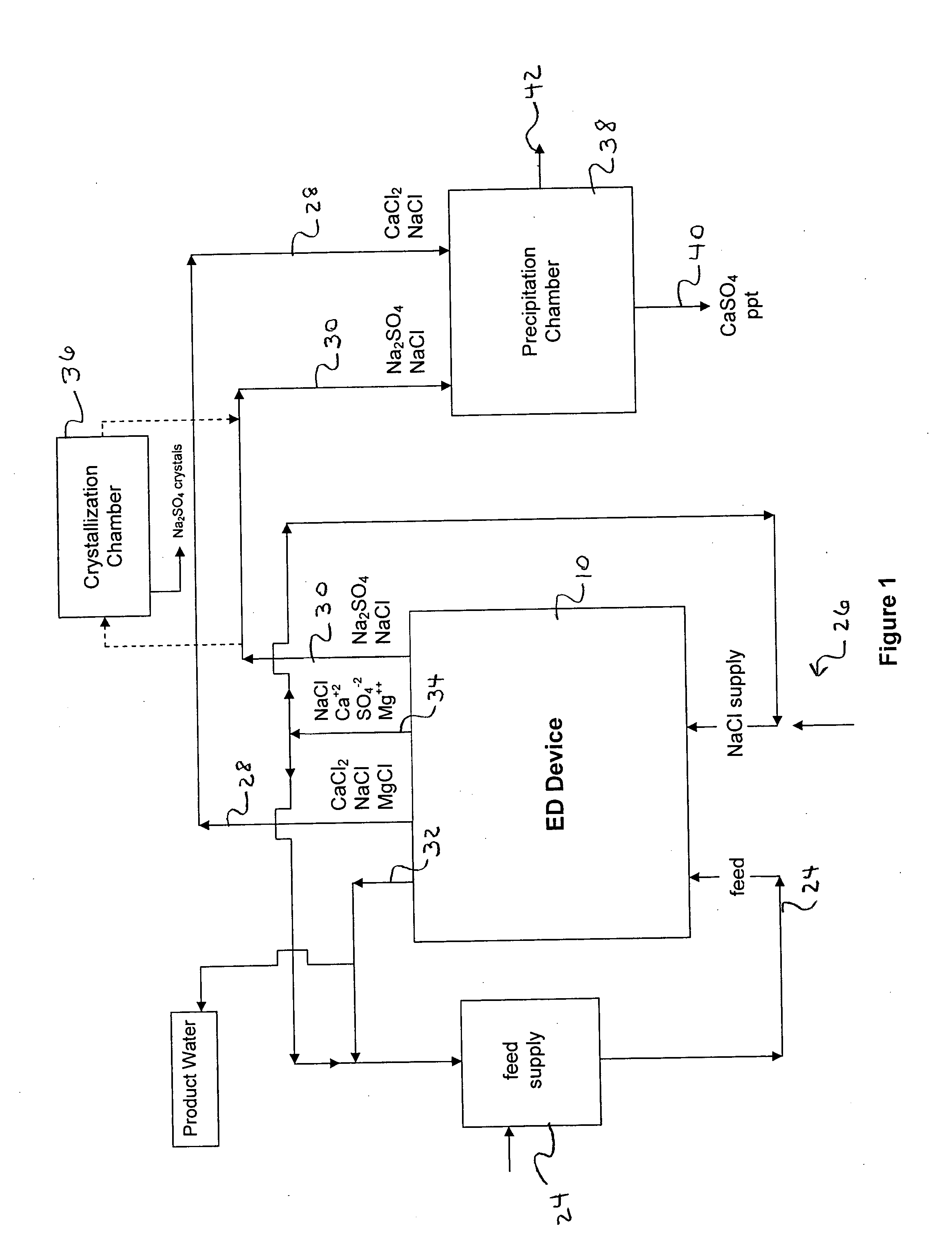
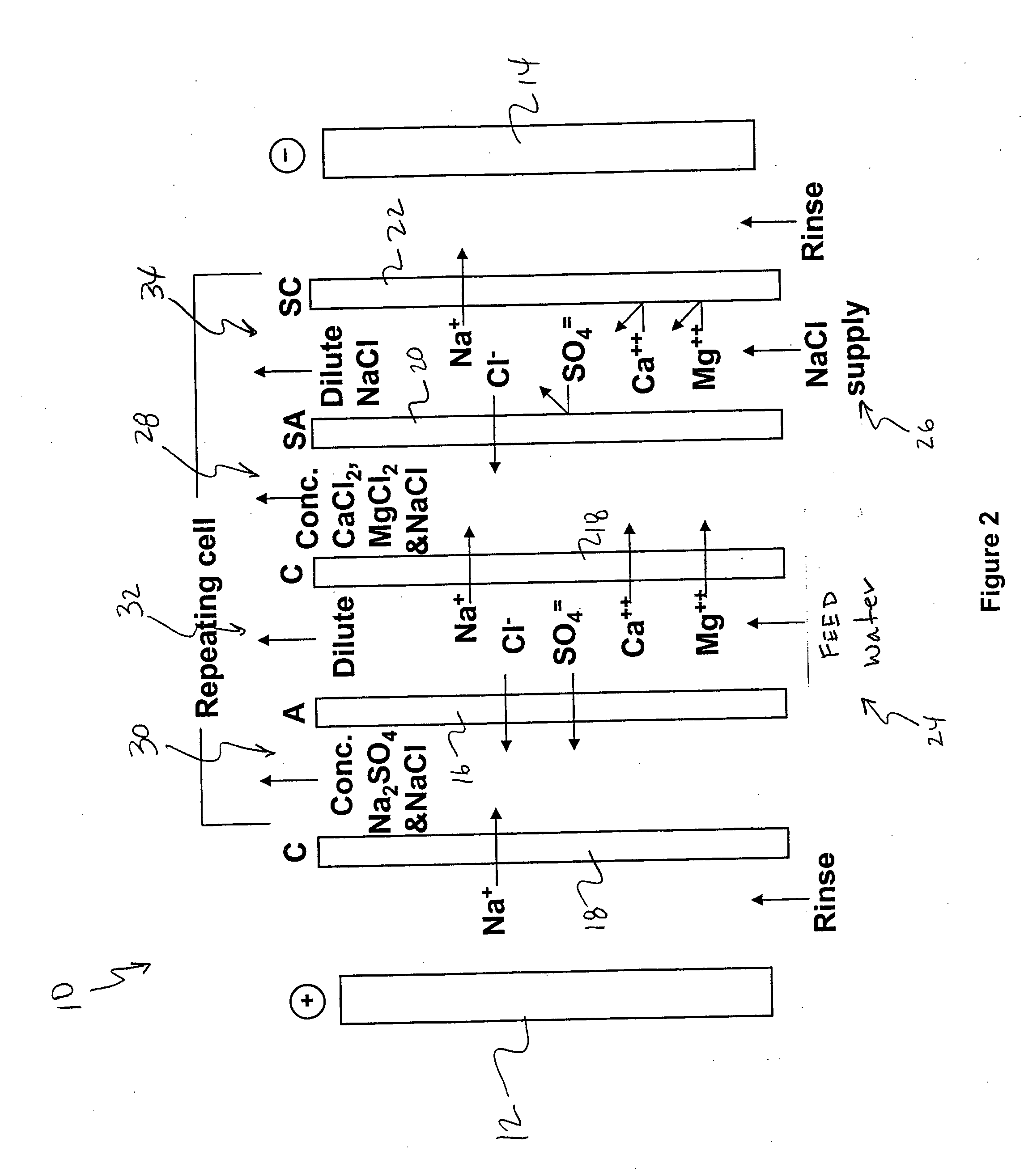
A process and system for purifying water is disclosed. For example, in one embodiment, the process may be used to remove a divalent salt, such as calcium sulfate, from a water source in order to prevent the divalent salt from precipitating during the process. The water source, for instance, may be fed to an ion separating device, such as an electrodialysis device. In the electrodialysis device, an ion exchange takes place between the divalent salt and another salt, such as a monovalent salt to produce two concentrated salt streams that contain salts having greater solubility in water than the divalent salt. In one embodiment, the two salt streams that are produced may then be combined to precipitate the divalent salt in a controlled manner. During the process, various other components contained within the water feed stream may also be removed from the stream and converted into useful products. In one particular embodiment, the process is configured to receive a byproduct stream from a reverse osmosis process.
Owner:SOUTH CAROLINA THE UNIV OF
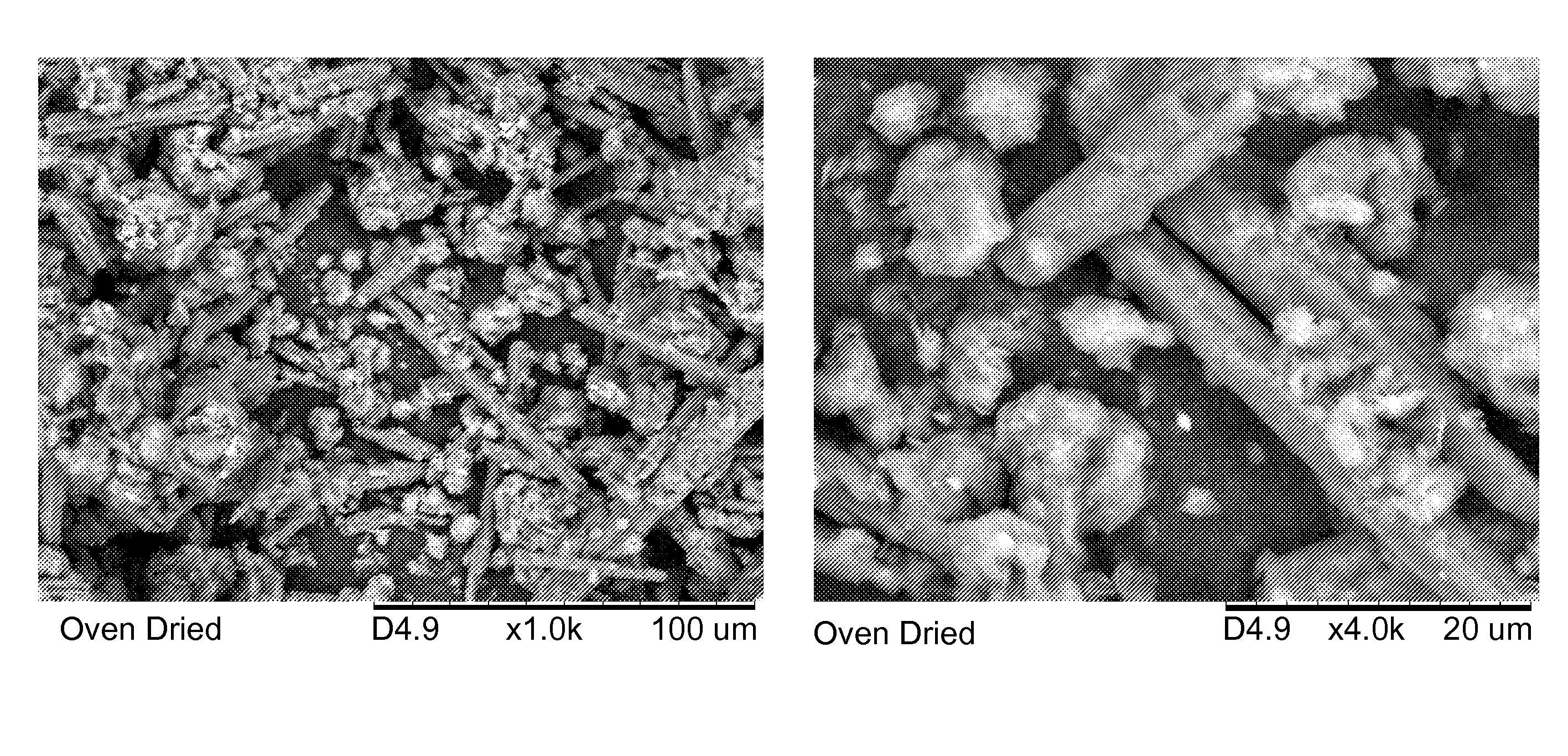
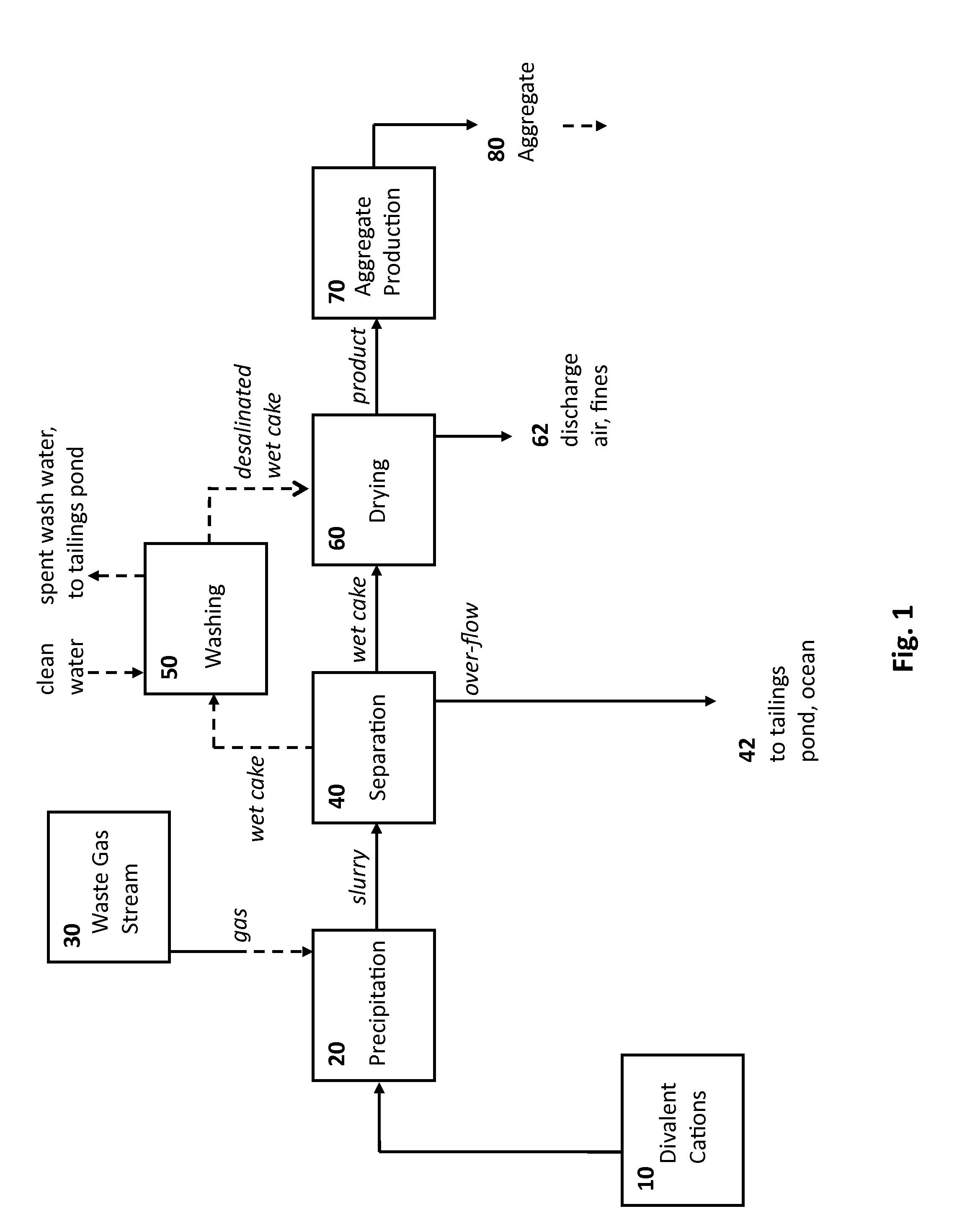
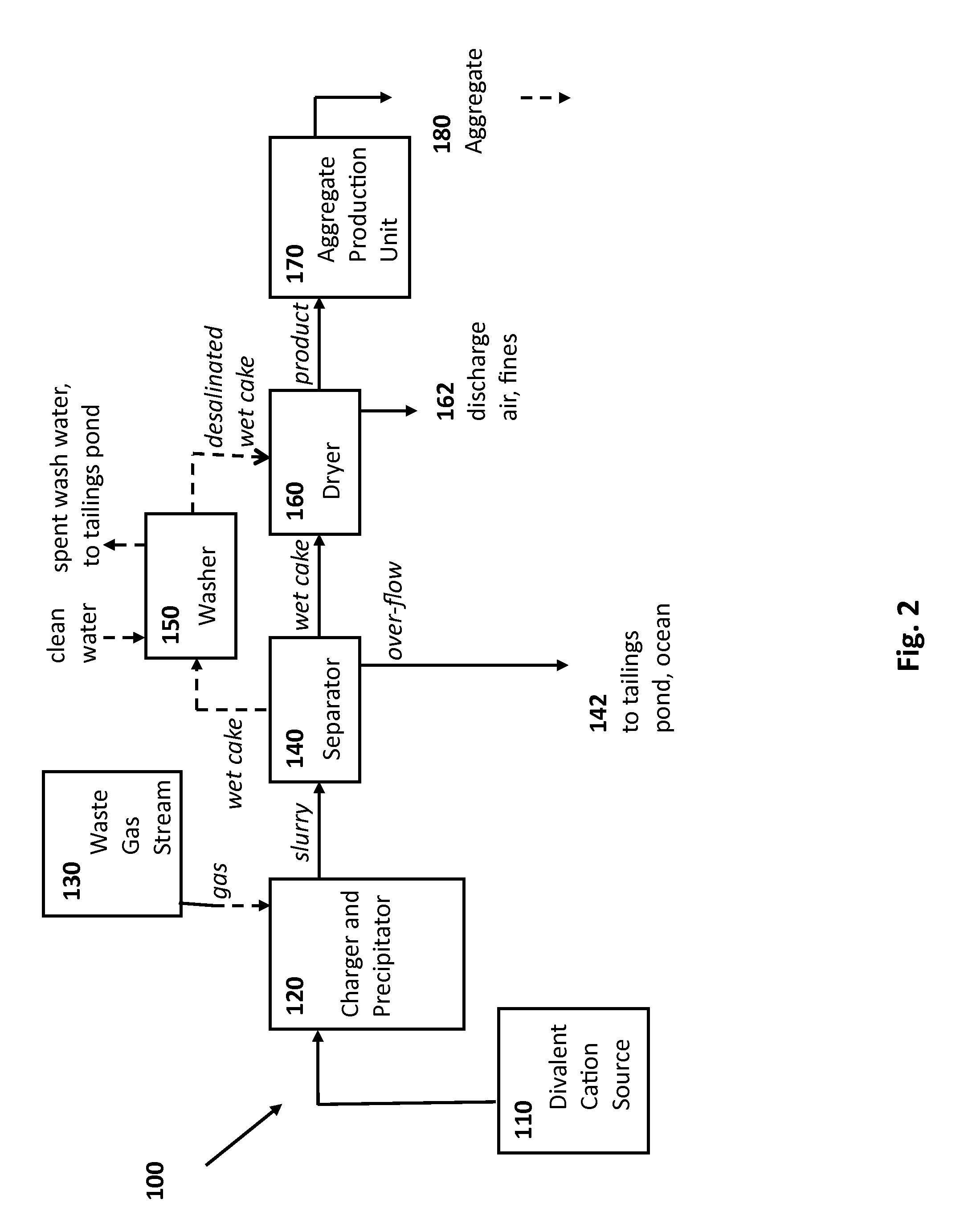
Rocks and aggregate, and methods of making and using the same
ActiveUS20100024686A1Calcium/strontium/barium carbonatesPigmenting treatmentCarbonateToxic industrial waste
Compositions comprising synthetic rock, e.g., aggregate, and methods of producing and using them are provided. The rock, e.g., aggregate, contains CO2 and / or other components of an industrial waste stream. The CO2 may be in the form of divalent cation carbonates, e.g., magnesium and calcium carbonates. Aspects of the invention include contacting a CO2 containing gaseous stream with a water to dissolve CO2, and placing the water under precipitation conditions sufficient to produce a carbonate containing precipitate product, e.g., a divalent cation carbonate.
Owner:ARELAC INC
Solid catalyst component for polymerization of olefins, catalyst comprising the same and use thereof
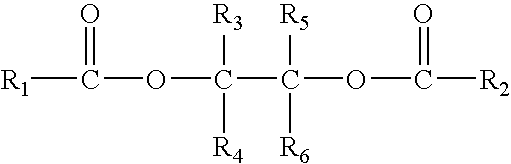
InactiveUS7388061B2High stereospecificitySatisfactory polymerization yieldOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationPolymer sciencePtru catalyst
The present invention provides a solid catalyst component for the polymerization of olefins, comprising magnesium, titanium, a halogen and an electron donor, wherein said electron donor comprises at least one selected from the group consisting of ester of polyol of the formula (I):R1CO—O—CR3R4—A—CR5R6—O—CO—R2 (I)wherein, R1 and R2 groups, which may be identical or different, can be substituted or unsubstituted hydrocarbyl having 1 to 20 carbon atoms, R3-R6 groups, which may be identical or different, can be selected from the group consisting of hydrogen, halogen or substituted or unsubstituted hydrocarbyl having 1 to 20 carbon atoms, R1-R6 groups optionally contain one or more hetero-atoms replacing carbon, hydrogen atom or the both, said hetero-atom is selected from the group consisting of nitrogen, oxygen, sulfur, silicon, phosphorus and halogen atom, two or more of R3-R6 groups can be linked to form saturated or unsaturated monocyclic or polycyclic ring A is a single bond or bivalent linking group with chain length between two free radicals being 1-10 atoms, wherein said bivalent linking group is selected from the group consisting of aliphatic, alicyclic and aromatic bivalent radicals, and can carry C1-C20 linear or branched substituents one or more of carbon atom and / or hydrogen atom on above-mentioned bivalent linking group and substituents can be replaced by a hetero-atom selected from the group consisting of nitrogen, oxygen, sulfur, silicon, phosphorus, and halogen atom, and two or more said substituents on the linking group as well as above-mentioned R3-R6 groups can be linked to form saturated or unsaturated monocyclic or polycyclic ring.
Owner:CHINA PETROCHEMICAL CORP +1
Platinum complex and light-emitting device
InactiveUS20080036373A1Superior in short wavelengthEasy to useDischarge tube luminescnet screensLamp detailsPlatinum complexAtomic group
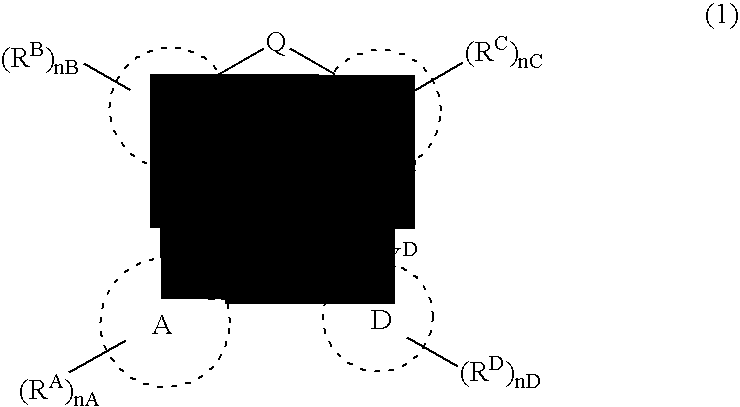
A platinum complex represented by the general formula (1) below, useful as a phosphorescence emission material, a tetradentate ligand useful for synthesizing the platinum complex, and a light-emitting device containing at least one of the platinum complex.wherein ring B represents a nitrogen-containing aromatic heterocyclic ring; rings A, C and D each independently represent an aromatic or aromatic heterocyclic ring, while either of the rings C and D represents five-membered ring, the other represents a five- or six-membered ring; RA, RB, RC, and RD respectively represent substituents on the rings A, B, C, and D; two of XA, XB, XC, and XD represent a nitrogen atom that may be bound with the platinum atom by a coordinate bond, the others each independently represent a carbon atom or nitrogen atom that may be bound with the platinum atom by a covalent bond; Q represents a bivalent atom or atomic group bridging the rings B and C; and YC and YD each independently represent a carbon atom or nitrogen atom.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Materials for multicolor light emitting diodes
InactiveUS6045930ADischarge tube luminescnet screensElectroluminescent light sourcesIndiumOrganic light emitting device
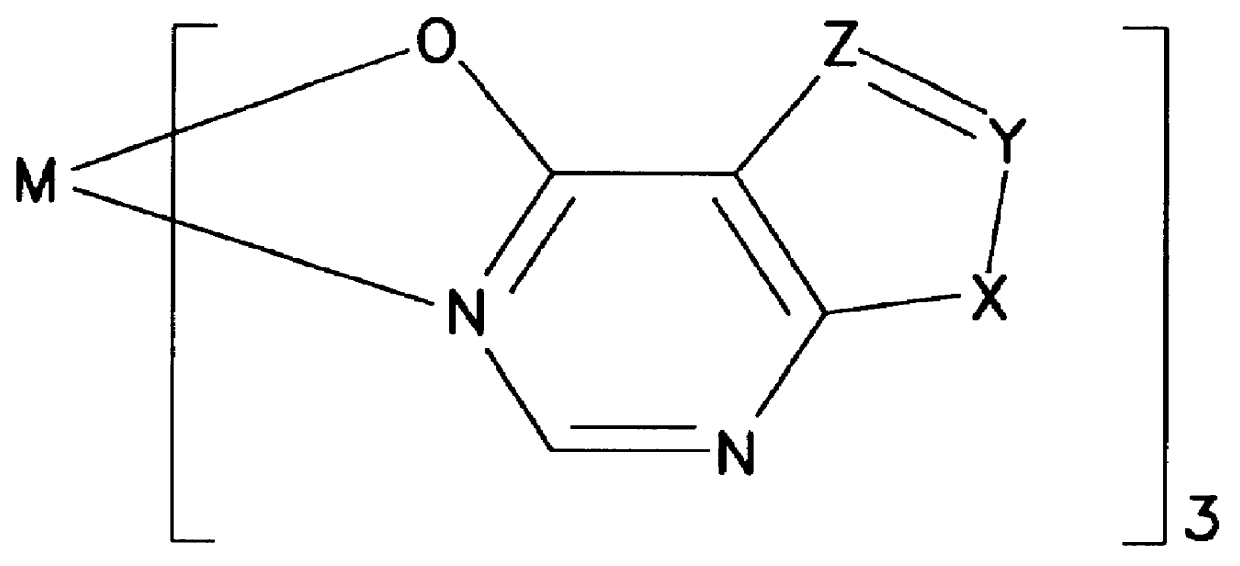
Disclosed herein are organic light emitting devices in which emitting layers (EL) comprise a suitable receiving compound according to Formulas I and II: ##STR1## wherein M is an ion of a divalent or trivalent metal atom, wherein n=3 when M is trivalent, and n=2 when M is divalent, wherein the metal atom is selected from the group consisting of aluminum, gallium, indium, and zinc, and wherein X, Y, and Z are each individually and independently C or N, such that at least two of X, Y and Z are N; and ##STR2## wherein R is alkyl, phenyl, substituted alkyl, substituted phenyl, trimethylsilyl, or substituted trimethylsilyl. Also disclosed are OLED's utilizing device elements comprising the above compounds and display devices based on those OLED's.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Vitamin receptor binding drug delivery conjugates
The invention describes a vitamin receptor binding drug delivery conjugate, and preparations therefor. The drug delivery conjugate consists of a vitamin receptor binding moiety, a bivalent linker (L), and a drug. The vitamin receptor binding moiety includes vitamins, and vitamin receptor binding analogs and derivatives thereof, and the drug includes analogs and derivatives thereof. The vitamin receptor binding moiety is covalently linked to the bivalent linker, and the drug, or the analog or the derivative thereof, is covalently linked to the bivalent linker, wherein the bivalent linker (L) includes components such as spacer linkers, releasable linkers, and heteroatom linkers, and combinations thereof. Methods and pharmaceutical compositions for eliminating pathogenic cell populations using the drug delivery conjugate are also described.
Owner:ENDOCTYE INC
Ansa group 4 metal bis ( mu -substituted) aluminum complexes
InactiveUS6140521AOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationBridging ligandAluminum metal
Ansa bis( mu -substituted) Group 4 metal and aluminum compounds comprising a single Group 4 metal atom and two aluminum metal atoms corresponding to the formula: wherein: L' is a pi -bonded group, M is a Group 4 metal, J is nitrogen or phosphorus; Z is a divalent bridging group causing the complex to have an ansa structure, R' is an inert monovalent ligand; r is one or two; X independently each occurrence is a Lewis basic ligand group able to form a mu -bridging ligand group, and optionally the two X groups may be joined together, and A' independently each occurrence is an aluminum containing Lewis acid compound that forms an adduct with the metal complex by means of the mu -bridging groups, and optionally two A' groups may be joined together thereby forming a single difunctional Lewis acid containing compound, and a method of preparation comprising contacting a charge-neutral Group IV metal coordination complex having at least two Lewis basic groups with at least two molar equivalents of charge-neutral aluminum coordination complexes having Lewis acidic aluminum atoms such that at least two of the aluminum atoms of the aluminum coordination complexes bond to at least two of the Lewis basic groups of the Group IV coordination complex.
Owner:THE DOW CHEM CO
Collections of compounds
A compound of formula (IV): O is a solid support; L is a linking group or a single bond; X' is selected from CO, NH, S, or O; A is O, S, NH, or a single bond; R2 and R3 are independently selected from: H, R, OH, OR, =O, =CH-R, =CH2, CH2-CO2R', CH2-CO2H, CH2-SO2R, O-SO2R, CO2R, COR, CN and there is optionally a double bond between C1 and C2 or C2 and C3; R6, R7, and R9 are independently selected from H, R, OH, OR, halo, nitro, amino, Me3Sn; R11 is either H or R; Q is S, O or NH; R10 is a nitrogen protecting group; and Y is a divalent group such that HY=R, and other related compounds and collections of compounds.
Owner:MEDIMMUNE LTD
Thermal treatment process for tobacco materials
ActiveUS20100300463A1Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Method for producing carbon coated nano stage lithium iron phosphate by precipitation
InactiveCN101393982AAvoid synthetic stepsEasy to controlElectrode manufacturing processesIron saltsPhosphate
The invention discloses a precipitation method for preparing nanometer level iron phosphate lithium coated with carbon. The method comprises the following steps: firstly, weighing iron salt, deionized water and a compound of metallic elements; after the stirring and the mixing are performed, adding a phosphorous compound and citric acid diluted with water to the mixture; after the stirring is performed again, adding a precipitation agent to the mixture and controlling to the neutrality; stirring to react in a container, and after the static placement, respectively adding the deionized water, a carbon source and lithium salt to mix uniformly after the precipitate is filtered and washed; stirring again to react, and drying the water at 30 to 160 DEG C and warming up at the heating rate under the protection of non-oxidized gas after a product is crashed; baking at a constant temperature of 450 to 850 DEG C, cooling down to a room temperature at a cooling rate or with a stove, and finally obtaining the nanometer level ferric phosphate lithium coated with the carbon after crashing is performed. The precipitation method has the advantage that the raw material cost and the processing cost are low because bivalent iron is taken as the raw material. The iron phosphate lithium prepared by using the process has the characteristics of good physical processing performance and good electrochemistry performance, and is suitable for industrialized production.
Owner:南京海泰纳米材料有限公司
New compositions containing quinoline compounds
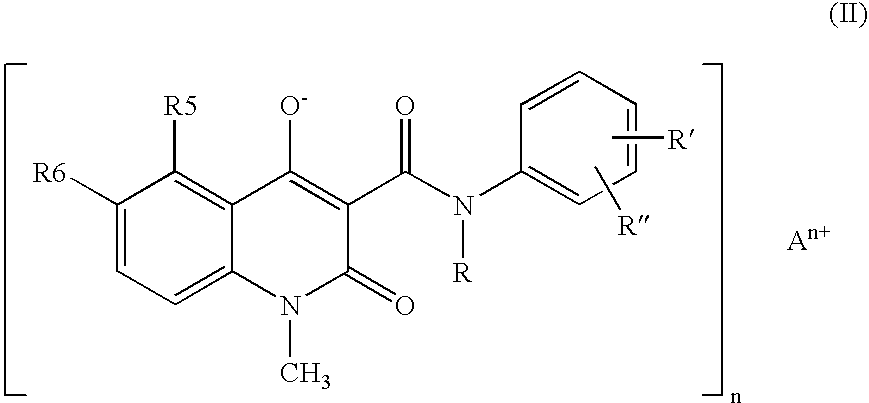
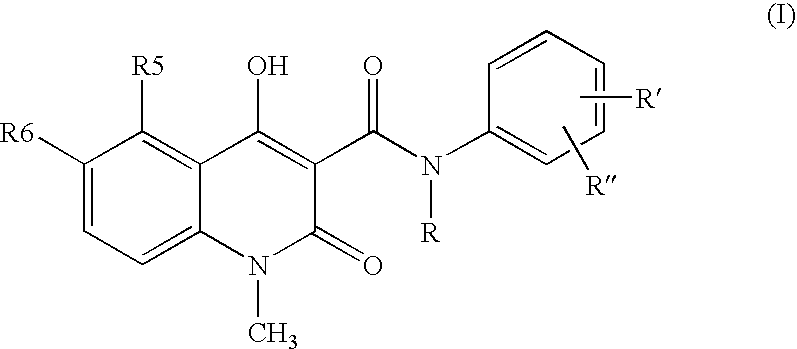
A stable solid pharmaceutical composition consisting essentially of an effective amount of a salt of formula (II) together with an alkaline-reacting component maintaining the pH preferably above 8, or a salt with a divalent metal cation; and at least one pharmaceutical excipient; said salt of formula (II) being essentially stable during storage at room temperature for a period of at least 3 years. A process for stabilizing the salt of formula (II). A crystalline salt of formula (II) and a process for preparing said salt.
Owner:ACTIVE BIOTECH AB
Two-phase silicate-based yellow phosphor
InactiveUS20060261309A1Efficient at fluorescingSolve low luminous efficiencyDischarge tube luminescnet screensLamp detailsOxygenSilicon dioxide
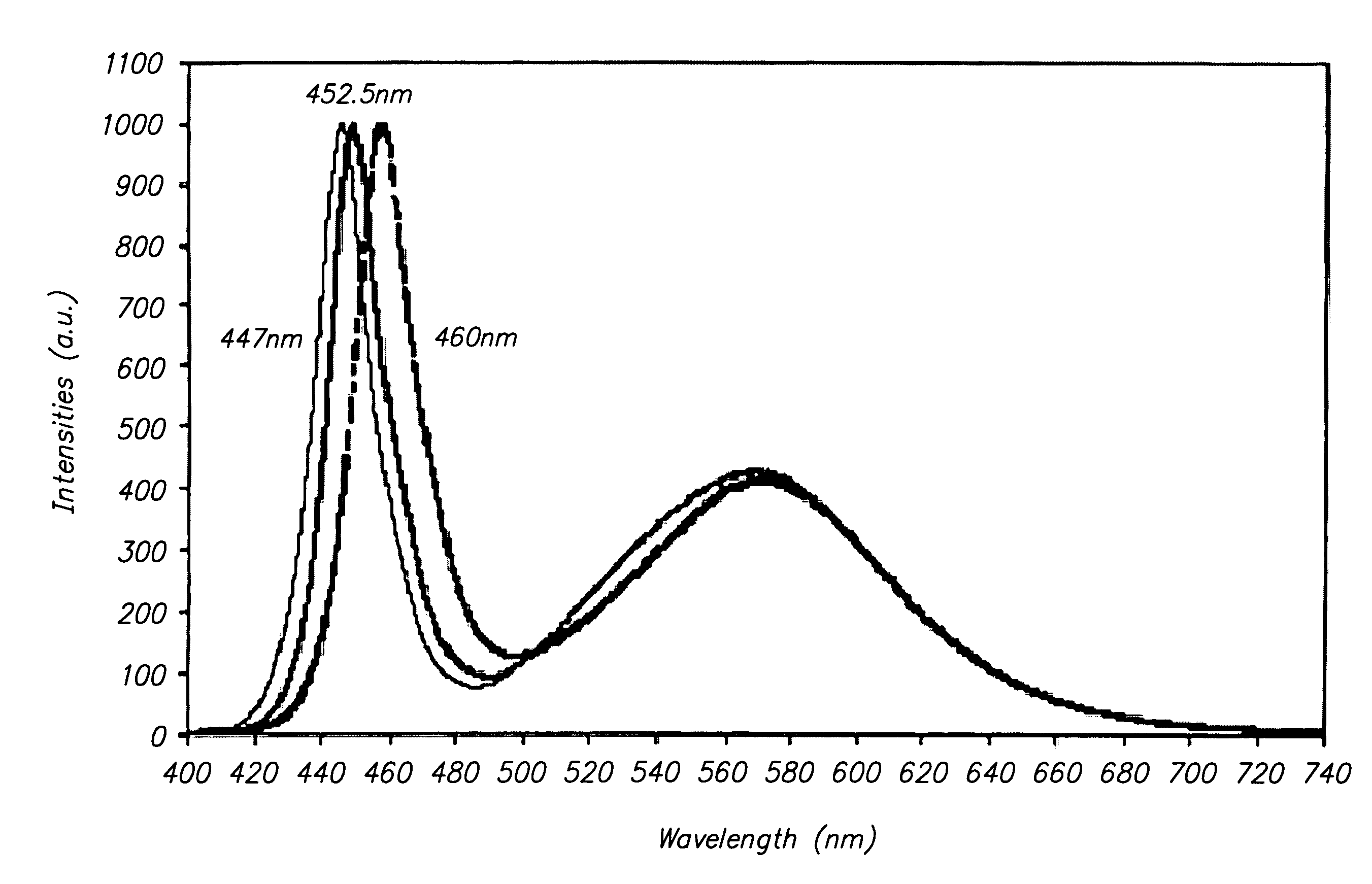
Novel two-phase yellow phosphors are disclosed having a peak emission intensity at wavelengths ranging from about 555 nm to about 580 nm when excited by a radiation source having a wavelength ranging from 220 nm to 530 nm. The present phosphors may be represented by the formula a[Srx(M1)1-x]zSiO4●(1-a)[Sry(M2)1-y]uSiO5:Eu2+D, wherein M1 and M2 are at least one of a divalent metal such as Ba, Mg, Ca, and Zn, the values of a, x, y, z and u follow the following relationships: 0.6≦a≦0.85; 0.3≦x≦0.6; 0.85≦y≦1; 1.5≦z≦2.5; 2.6≦u≦3.3; and Eu and D each range from 0.001 to about 0.5. D is an anion selected from the group consisting of F, Cl, Br, S, and N, and at least some of the D anion replaces oxygen in the host silicate lattice of the phosphor. The present yellow phosphors have applications in high brightness white LED illumination systems, LCD display panels, plasma display panels, and yellow LEDs and illumination systems.
Owner:INTEMATIX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com