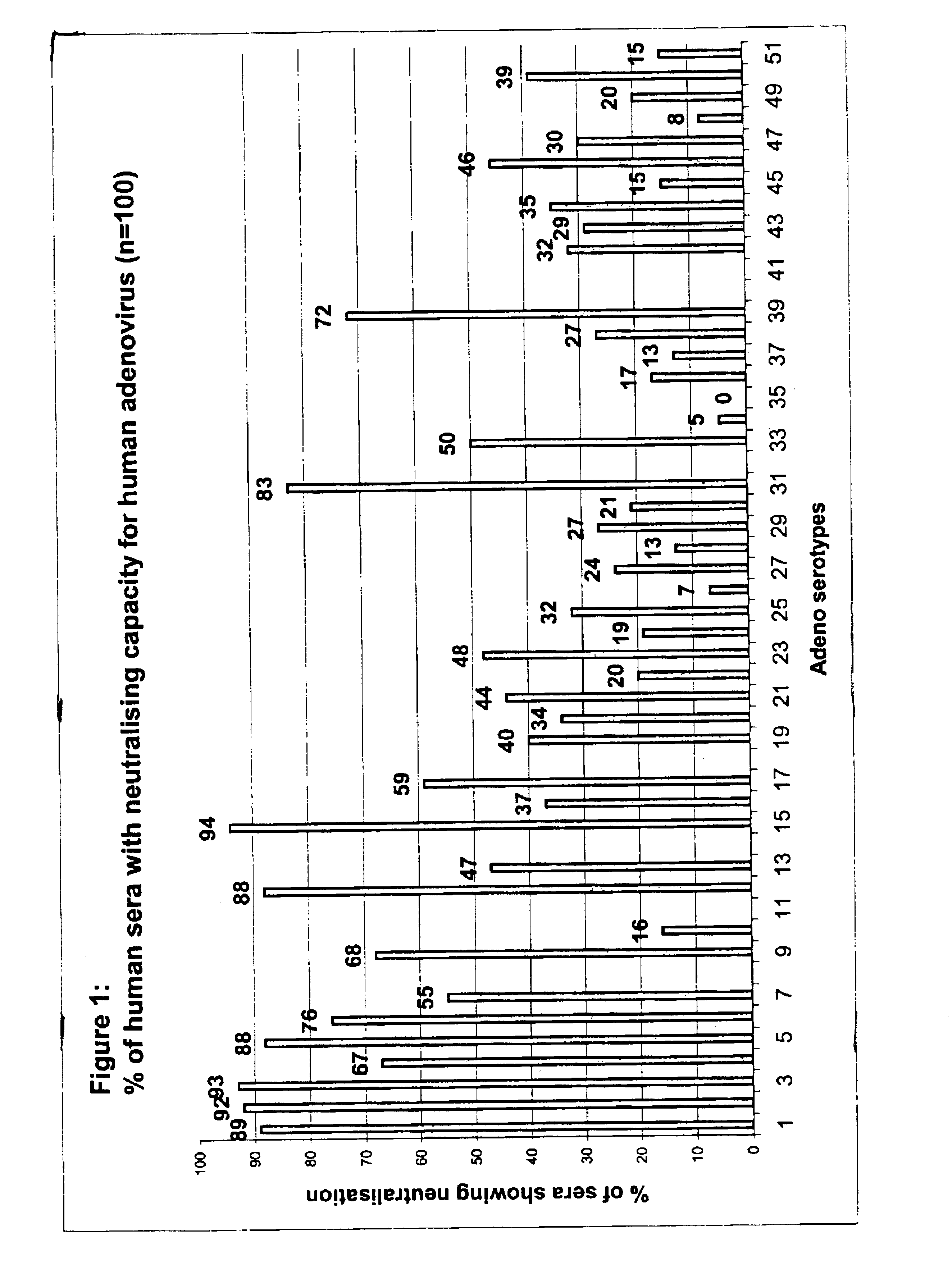
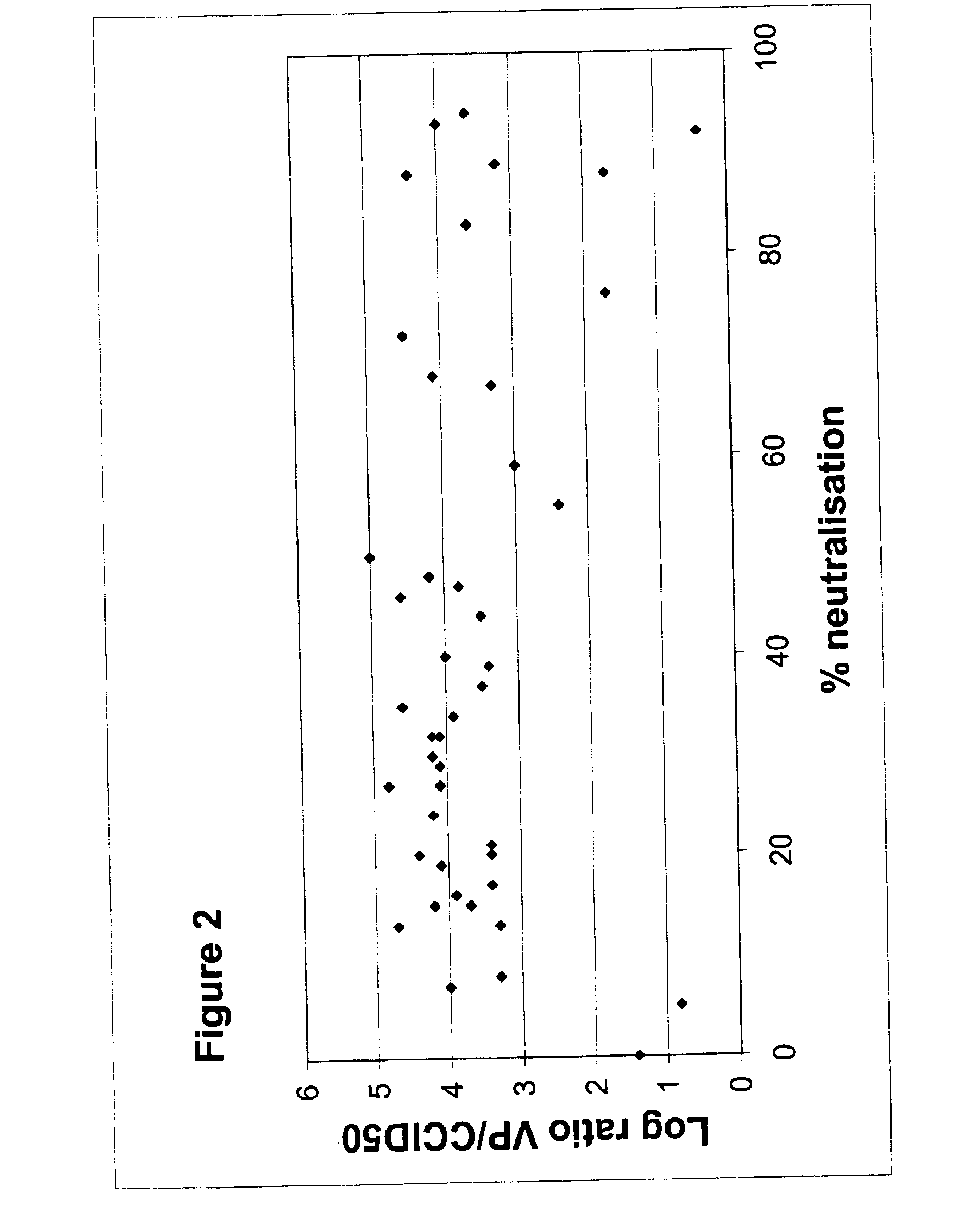
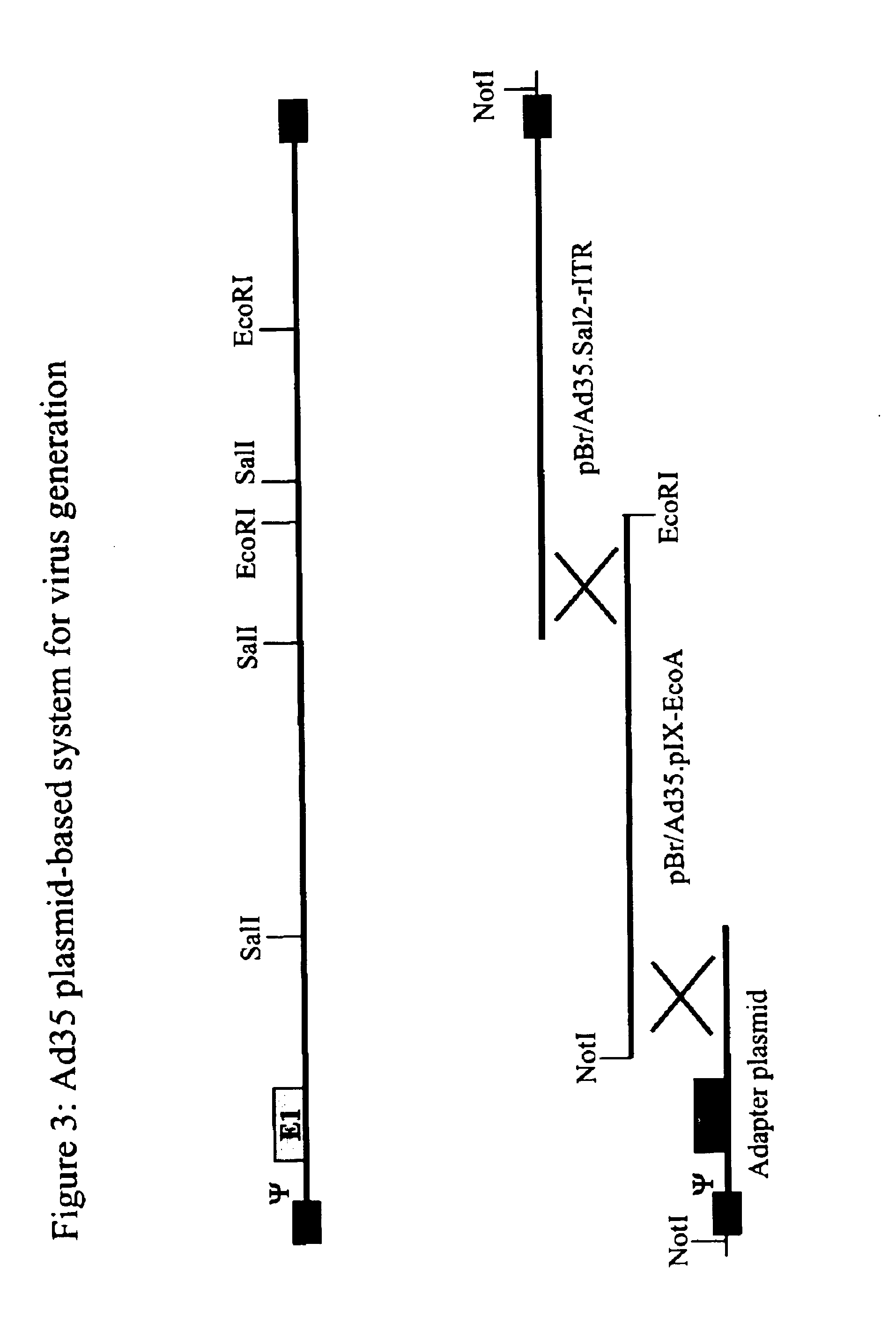
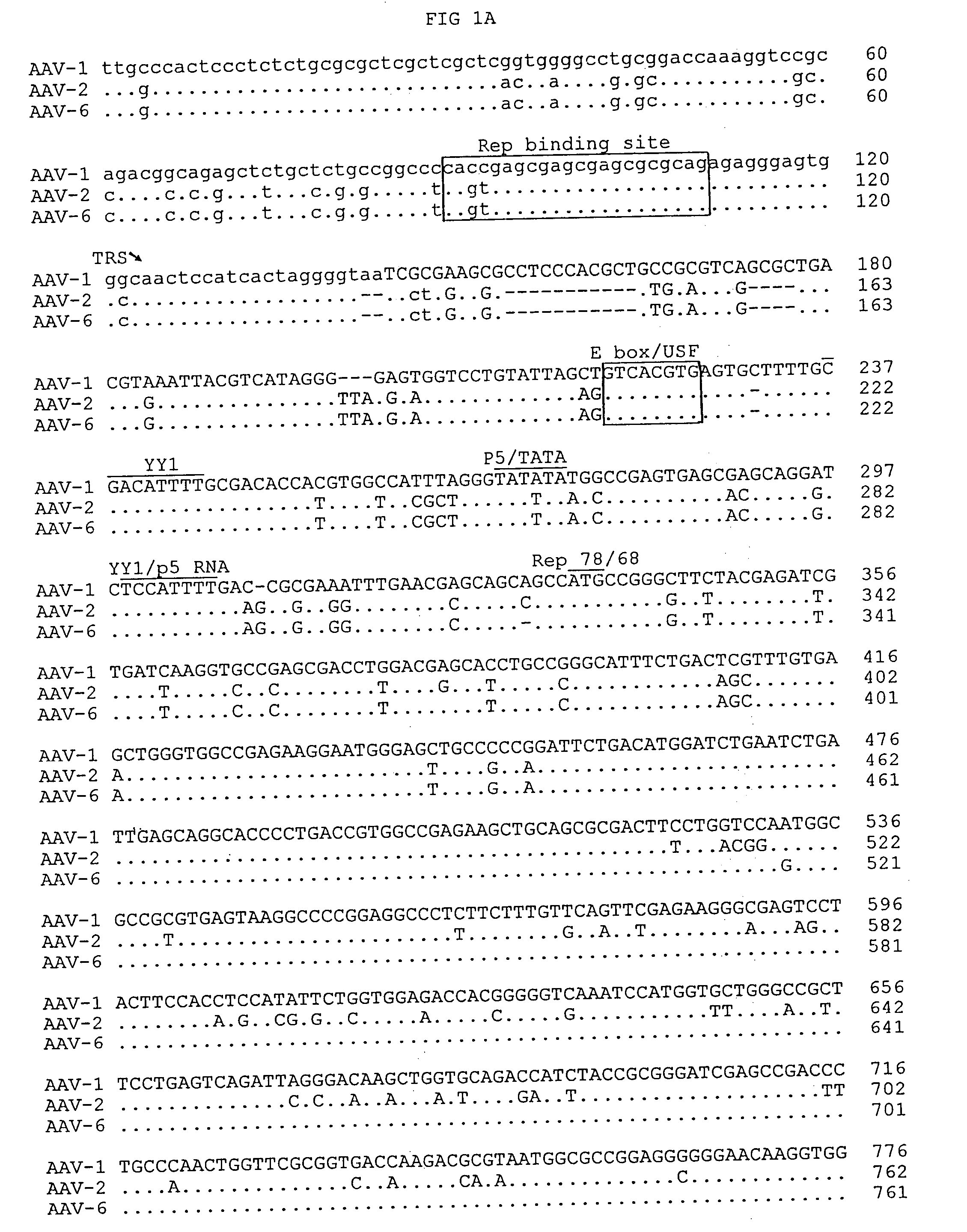
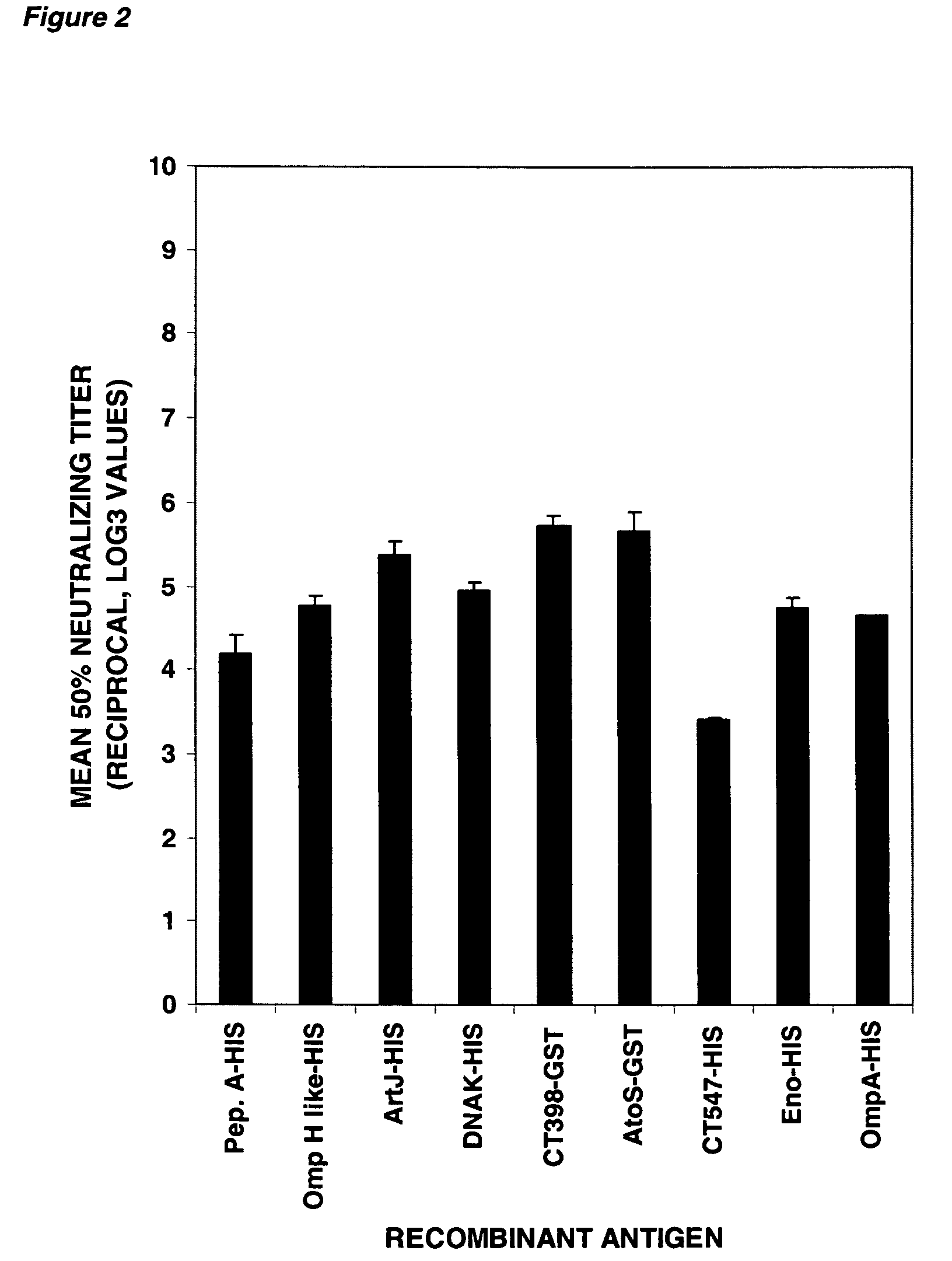
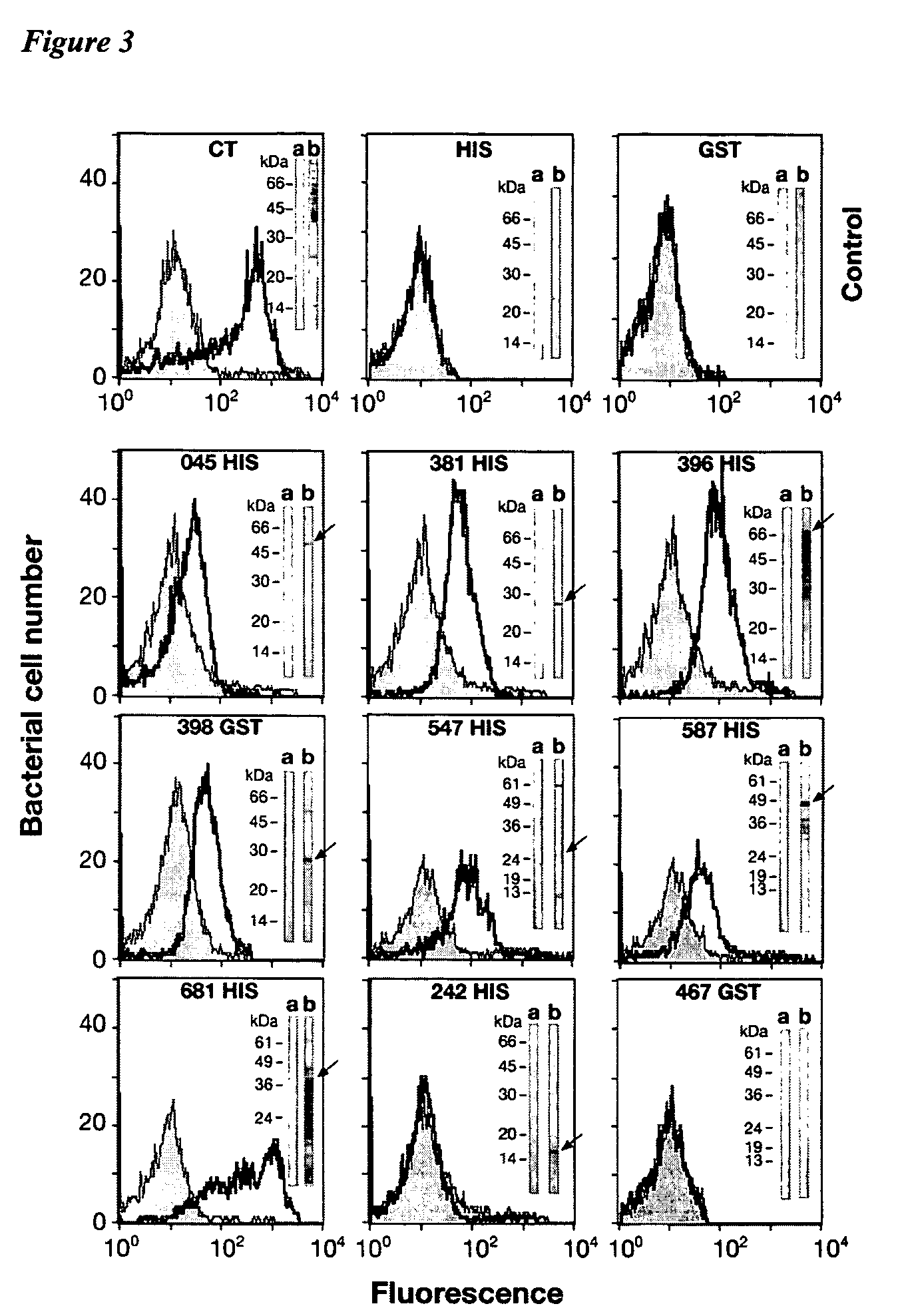
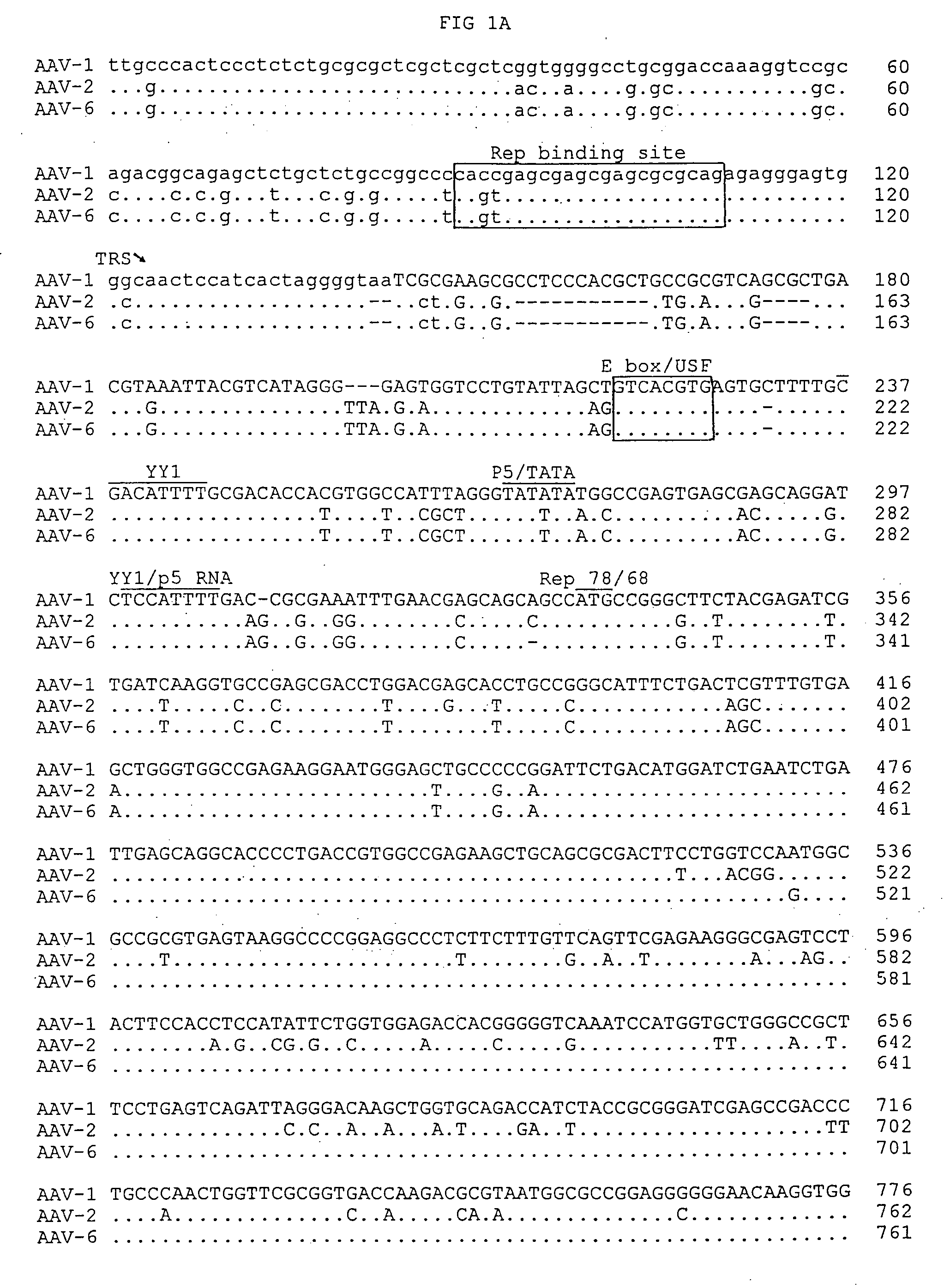
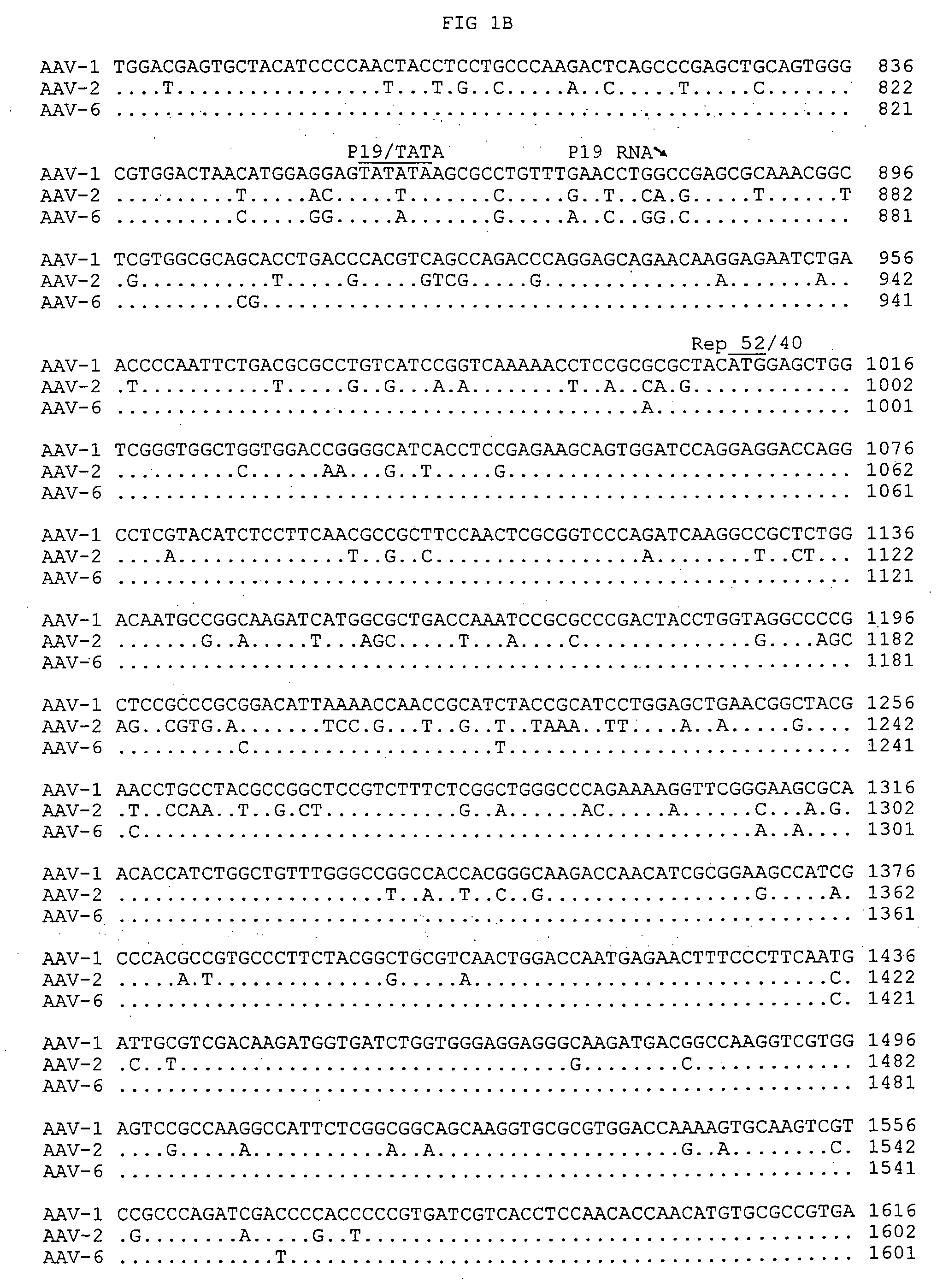
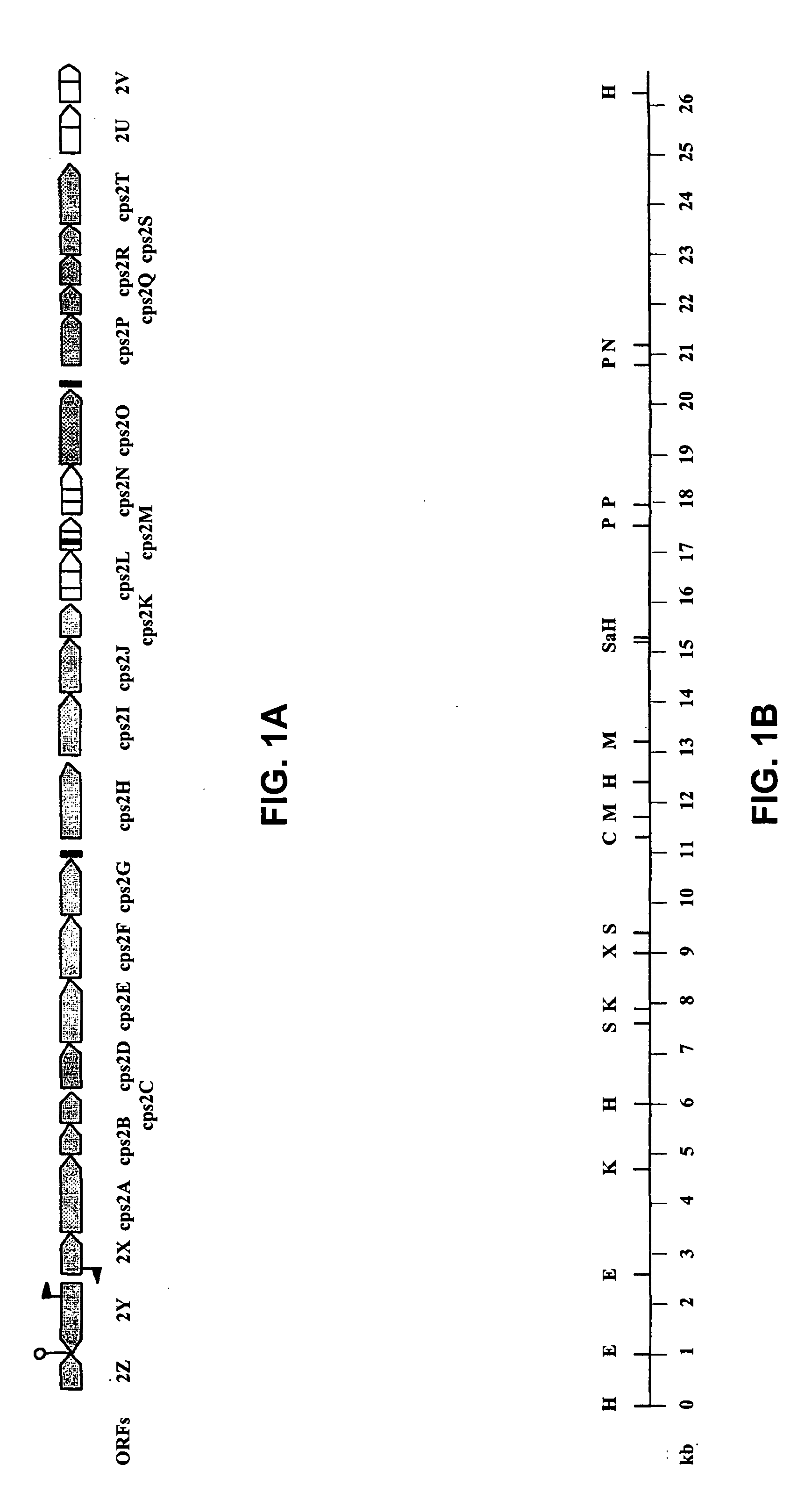
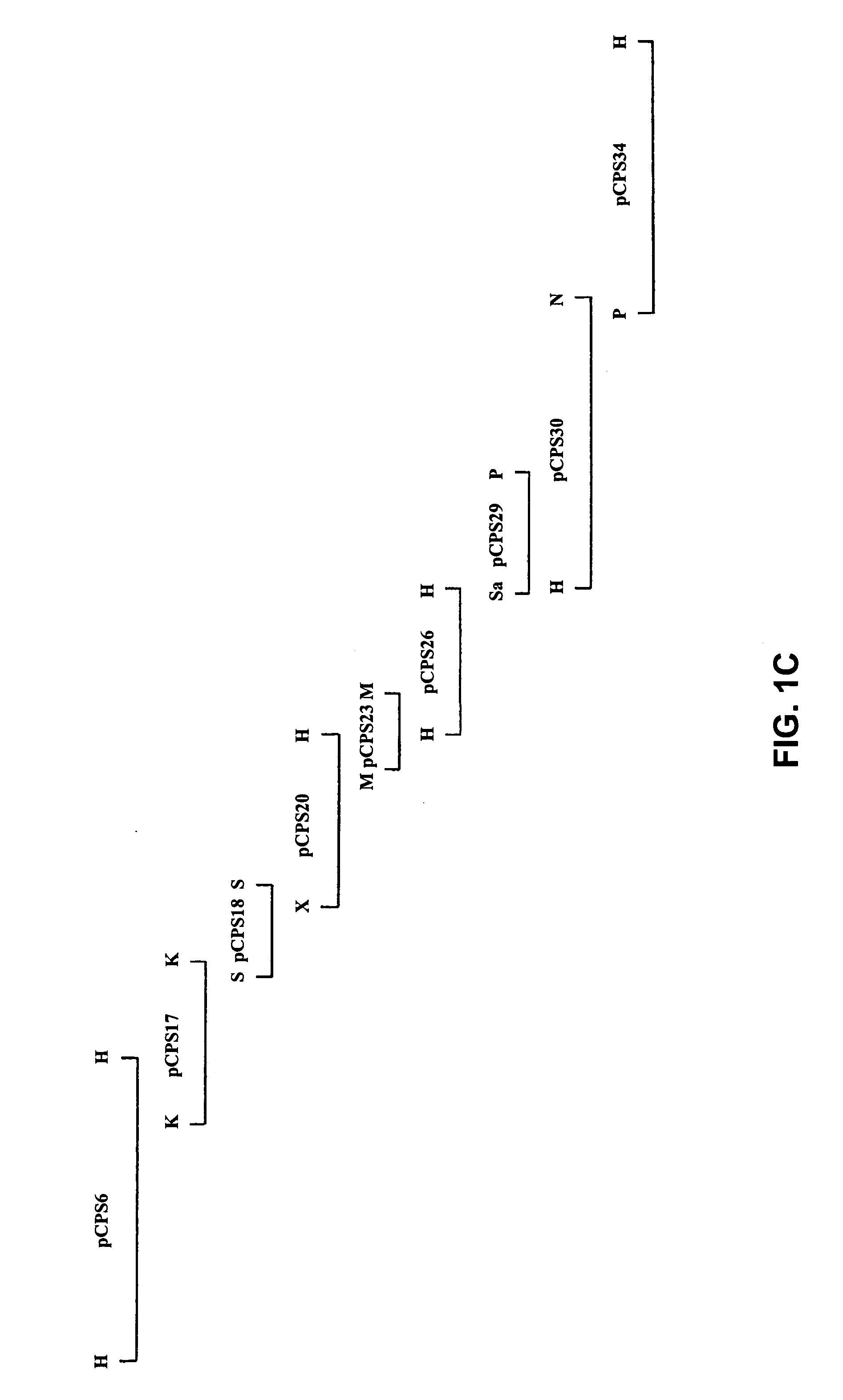
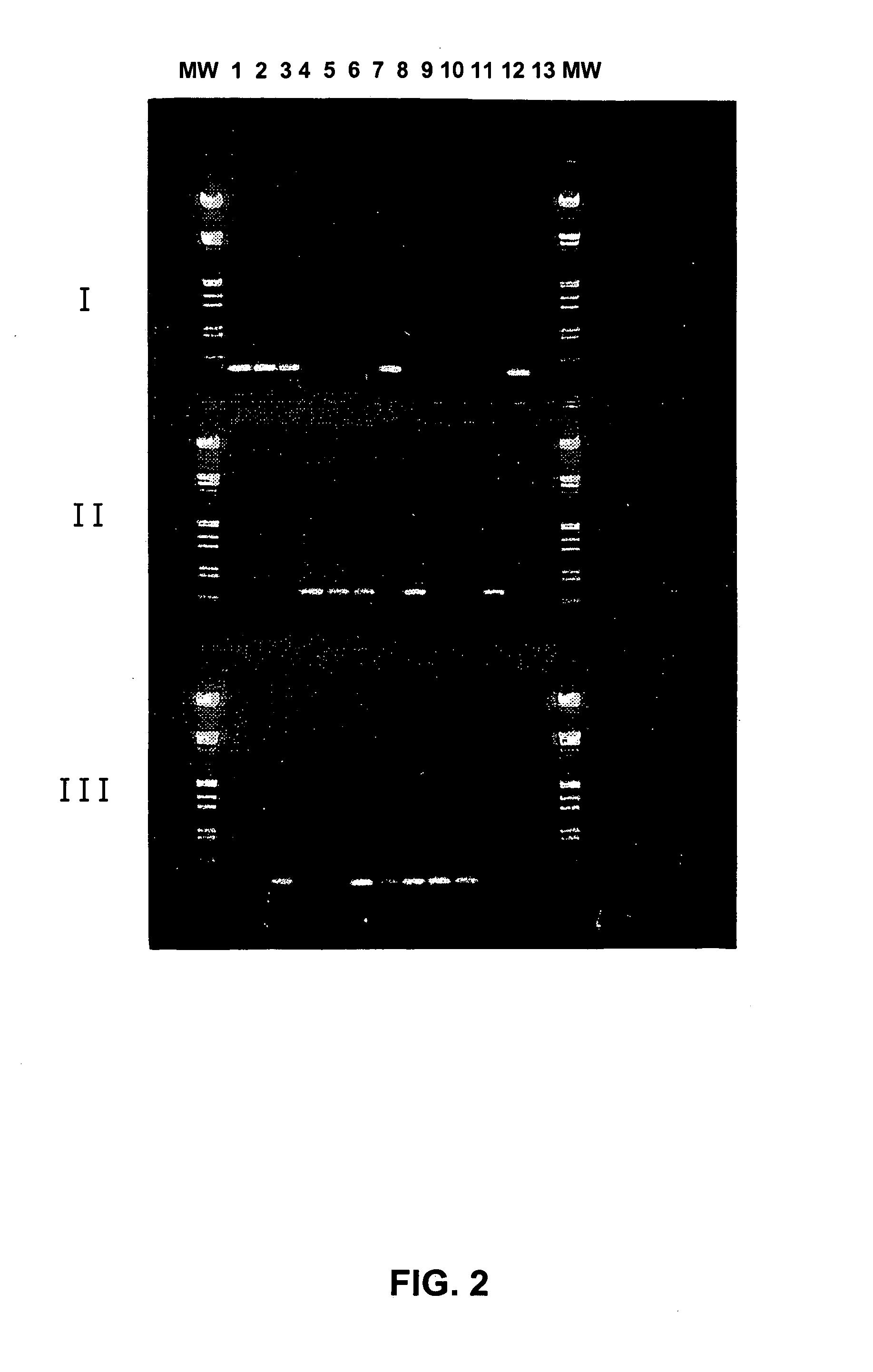
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1095 results about "Serotype" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A serotype or serovar is a distinct variation within a species of bacteria or virus or among immune cells of different individuals. These microorganisms, viruses, or cells are classified together based on their cell surface antigens, allowing the epidemiologic classification of organisms to the subspecies level. A group of serovars with common antigens is called a serogroup or sometimes serocomplex.
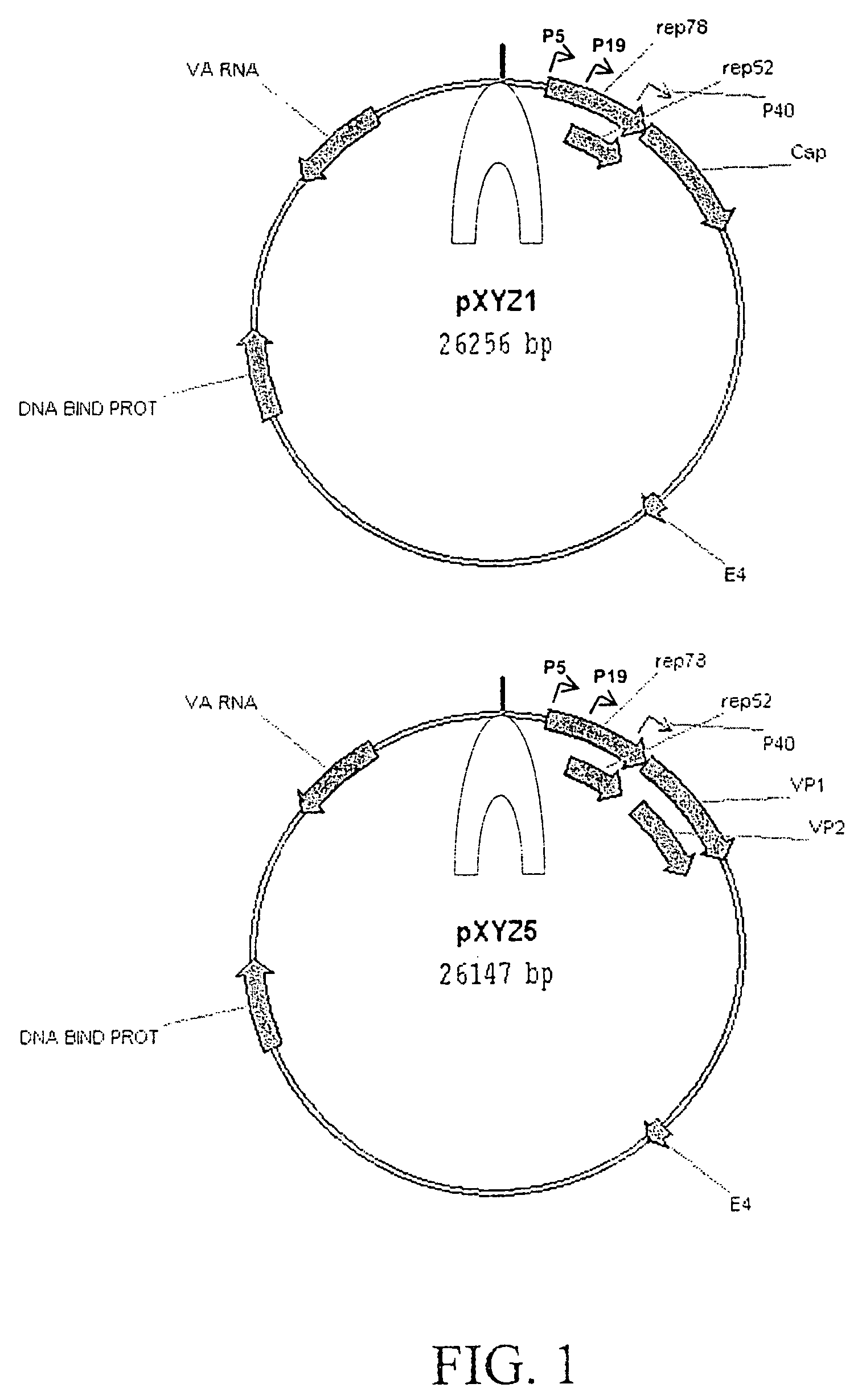
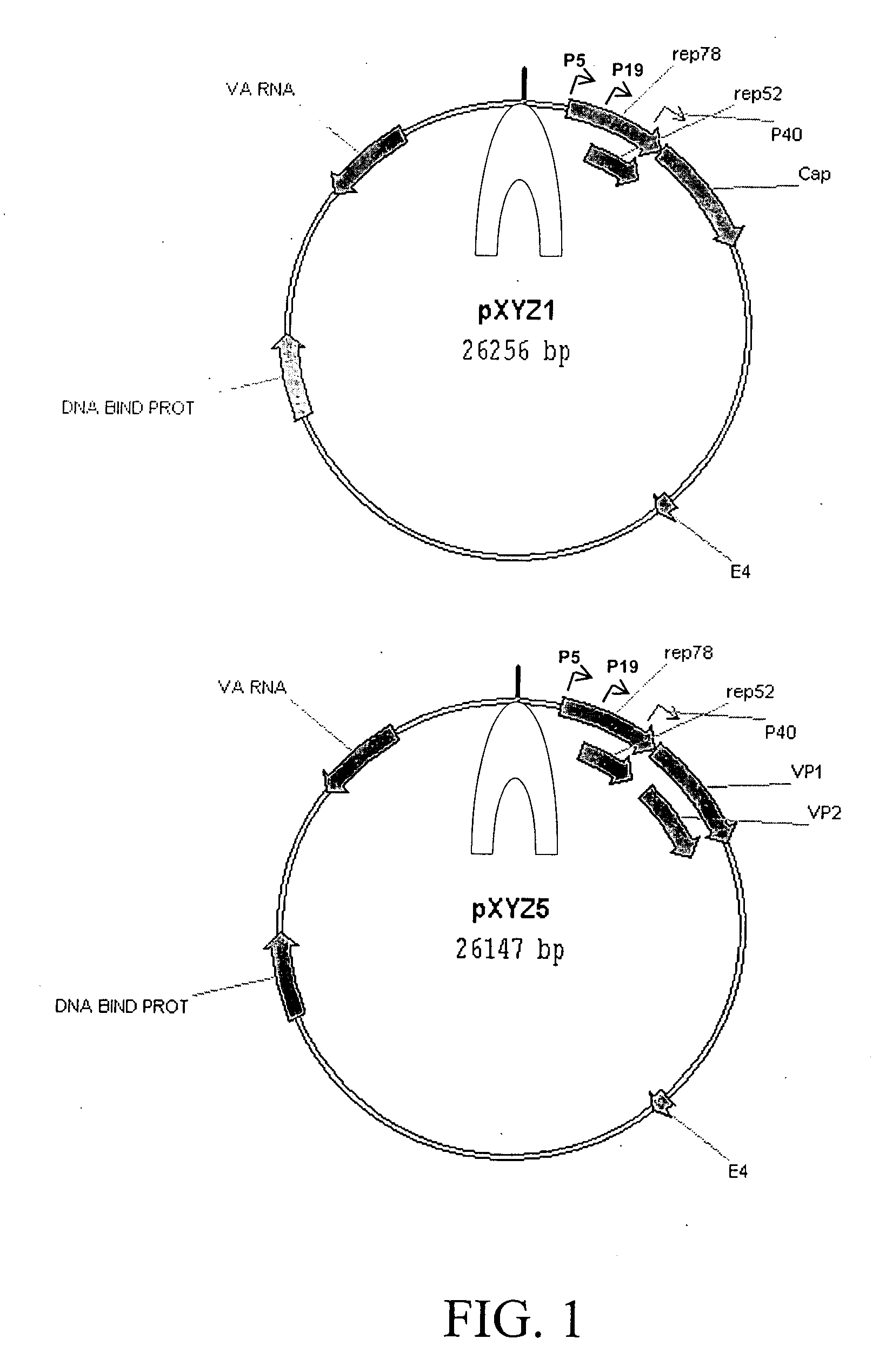
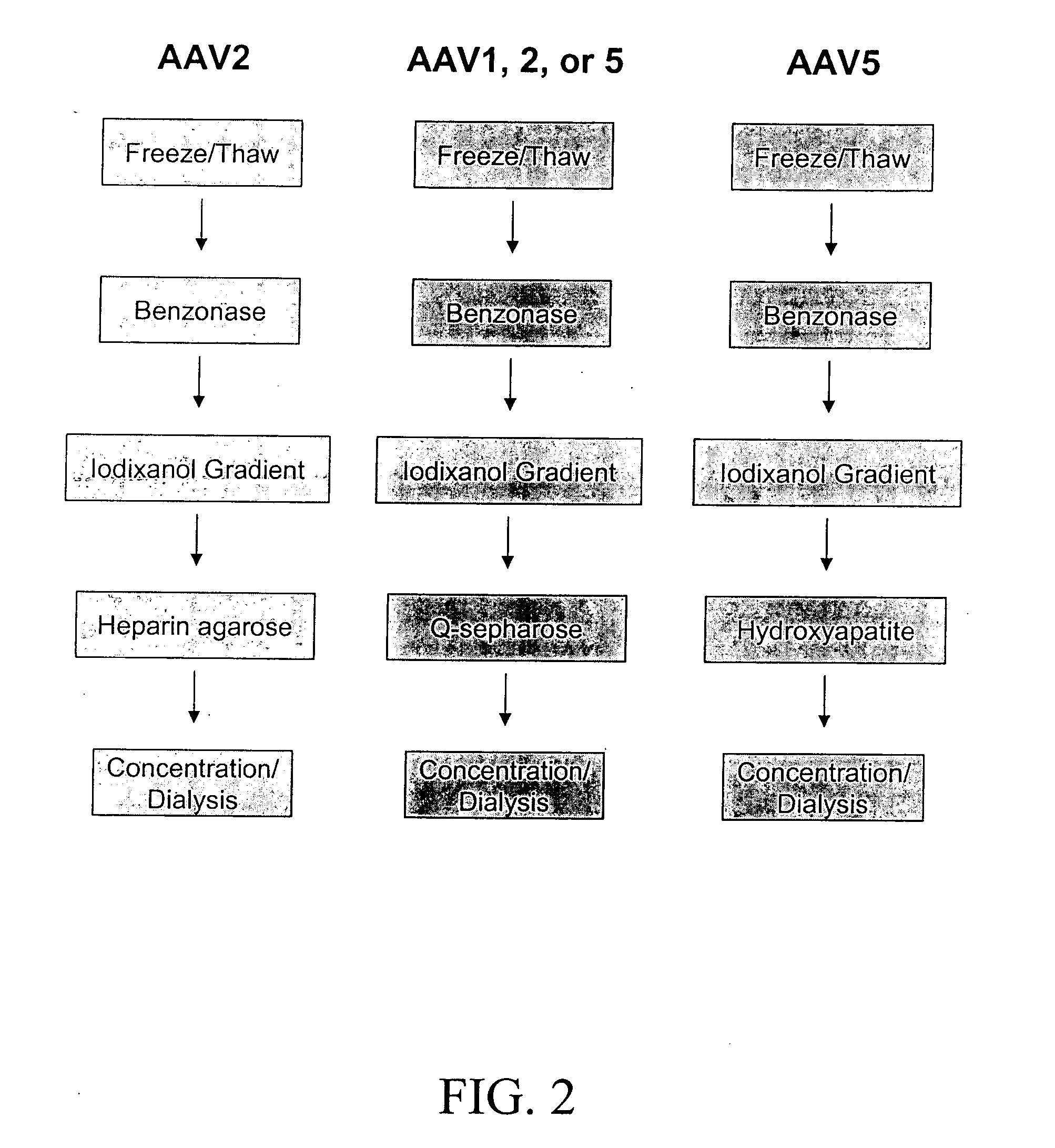
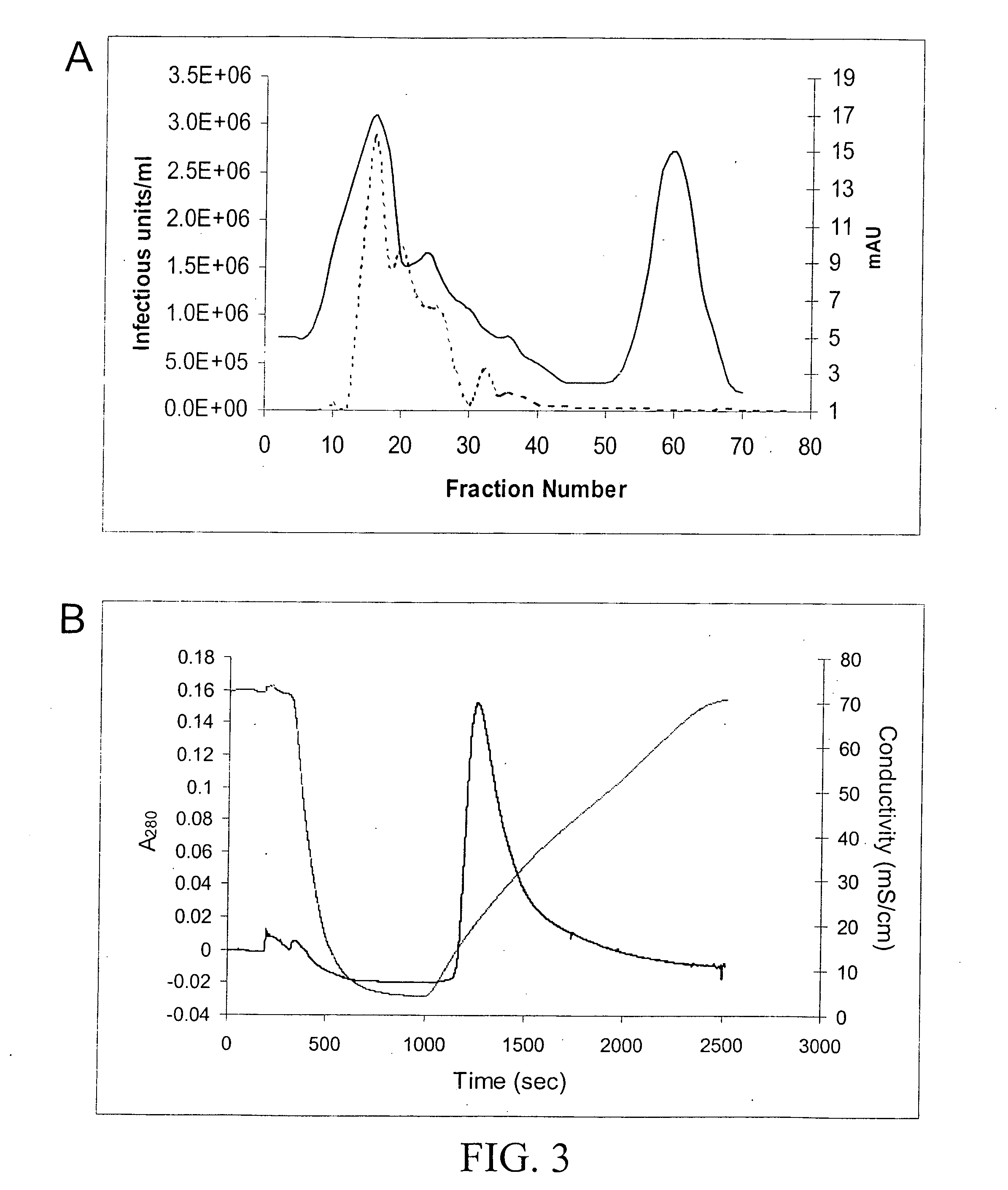
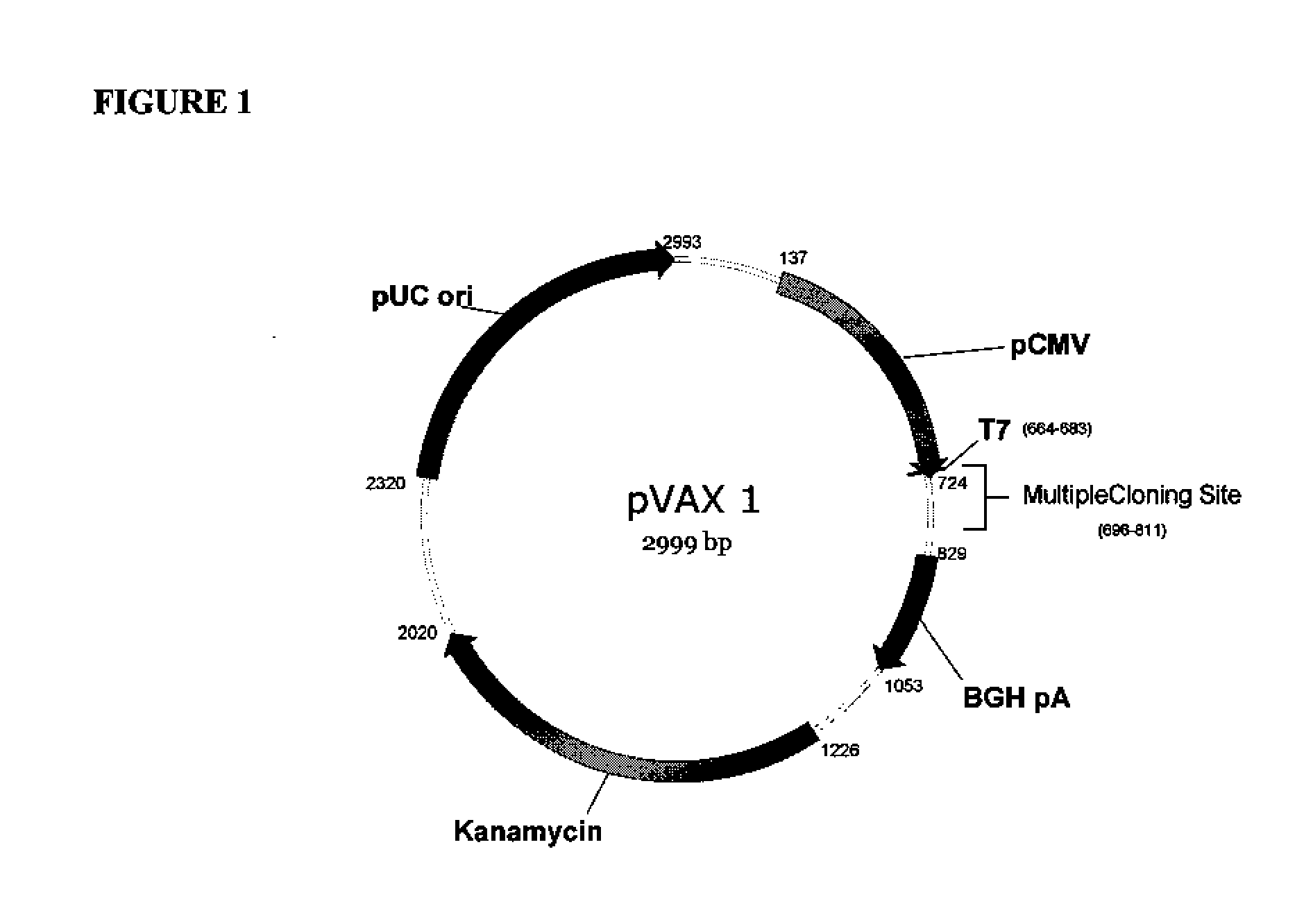
Production of pseudotyped recombinant AAV virions
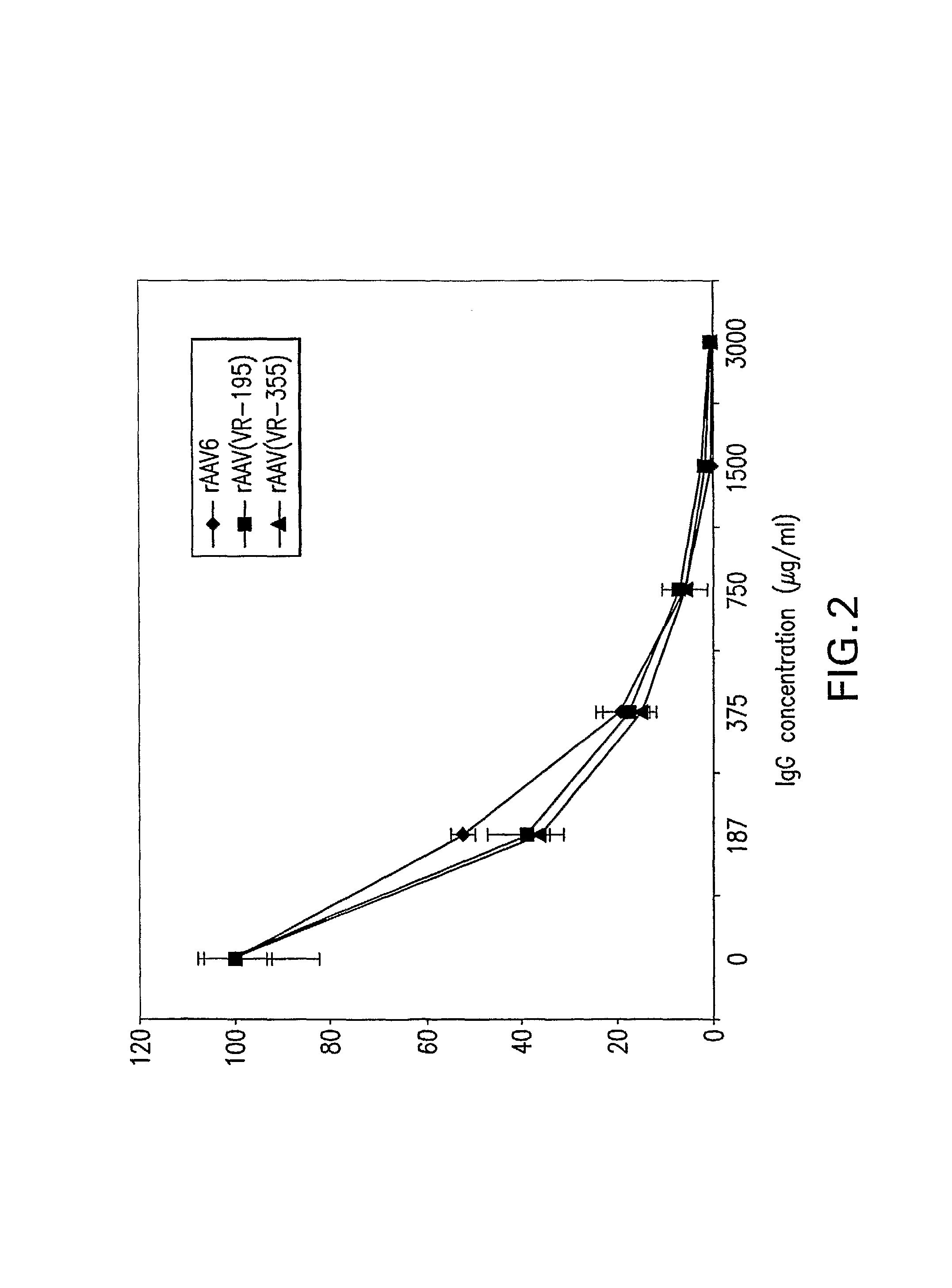
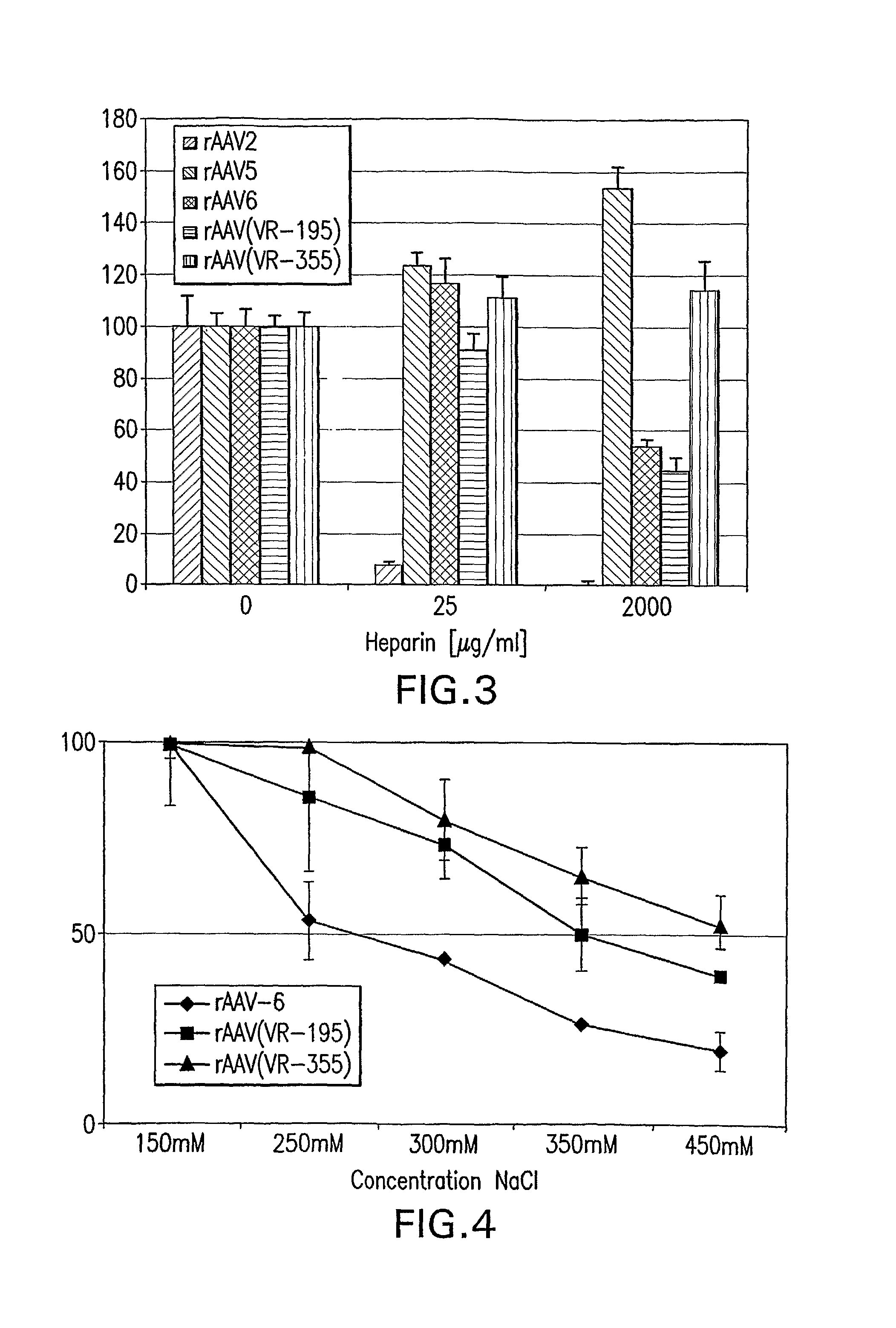
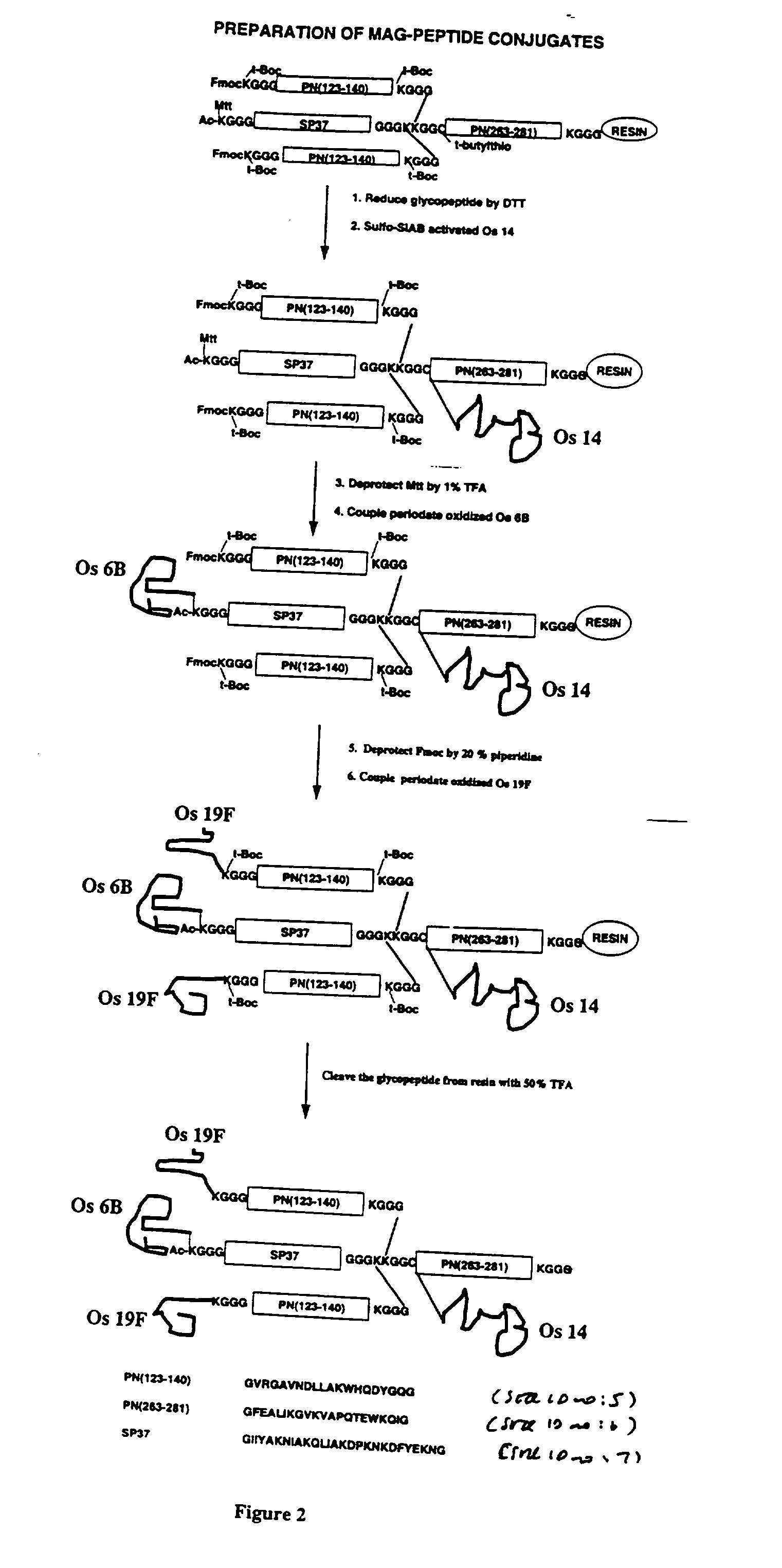
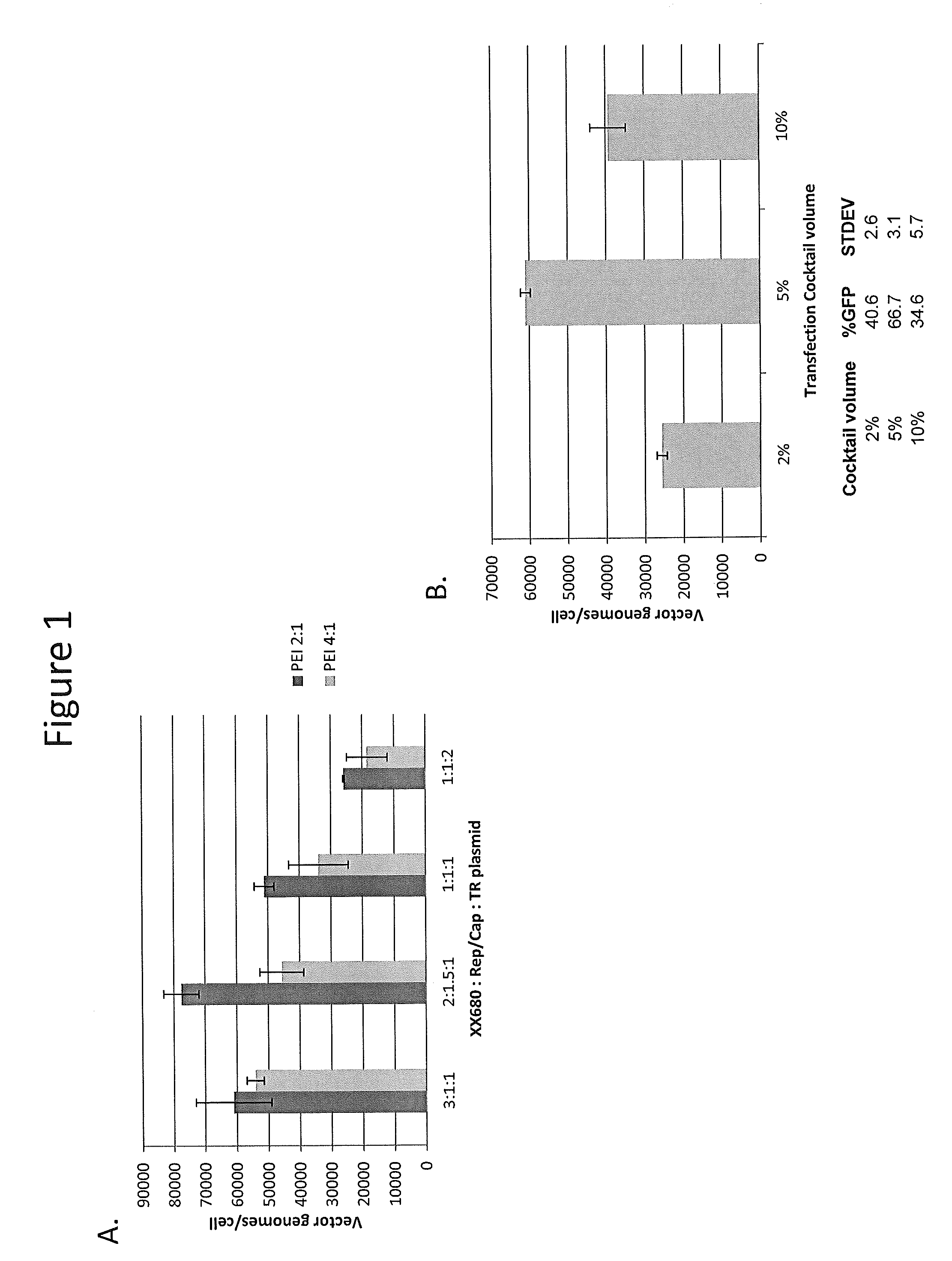
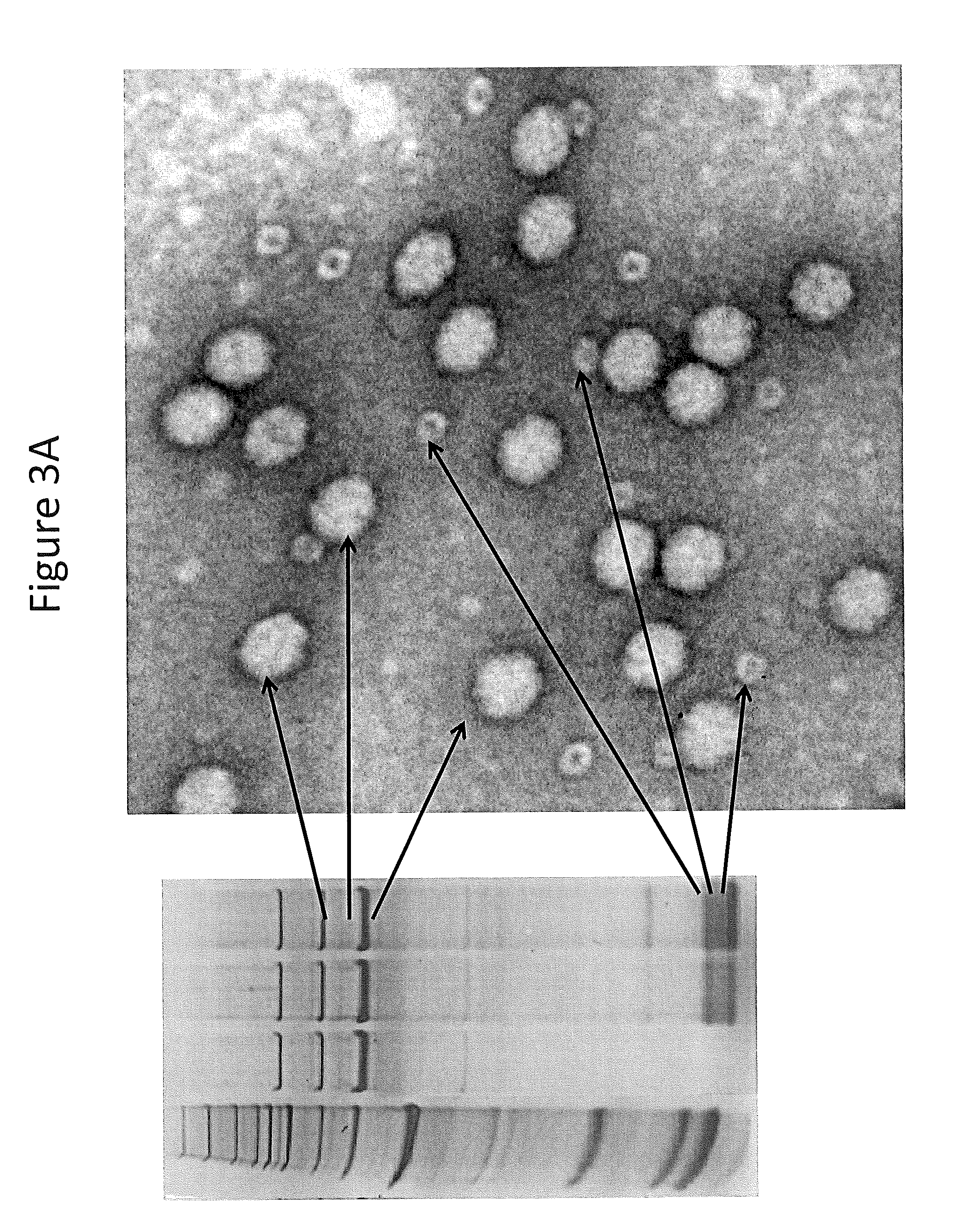
InactiveUS7094604B2Highly purified and concentratedEfficient and large-scale productionVectorsSugar derivativesPurification methodsSerotype
Vectors that encode Adeno-Associated Virus (AAV) Rep and Cap proteins of different serotypes and Adenovirus transcription products that provide helper functions were used to produce pseudotyped recombinant AAV (rAAV) virions. Purification methods generated pseudotyped rAAV virion stocks that were 99% pure with titers of 1×1012–1×1013 vector genomes / ml.
Owner:UNIVIRSITY OF FLORIDA RES FOUND INC
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
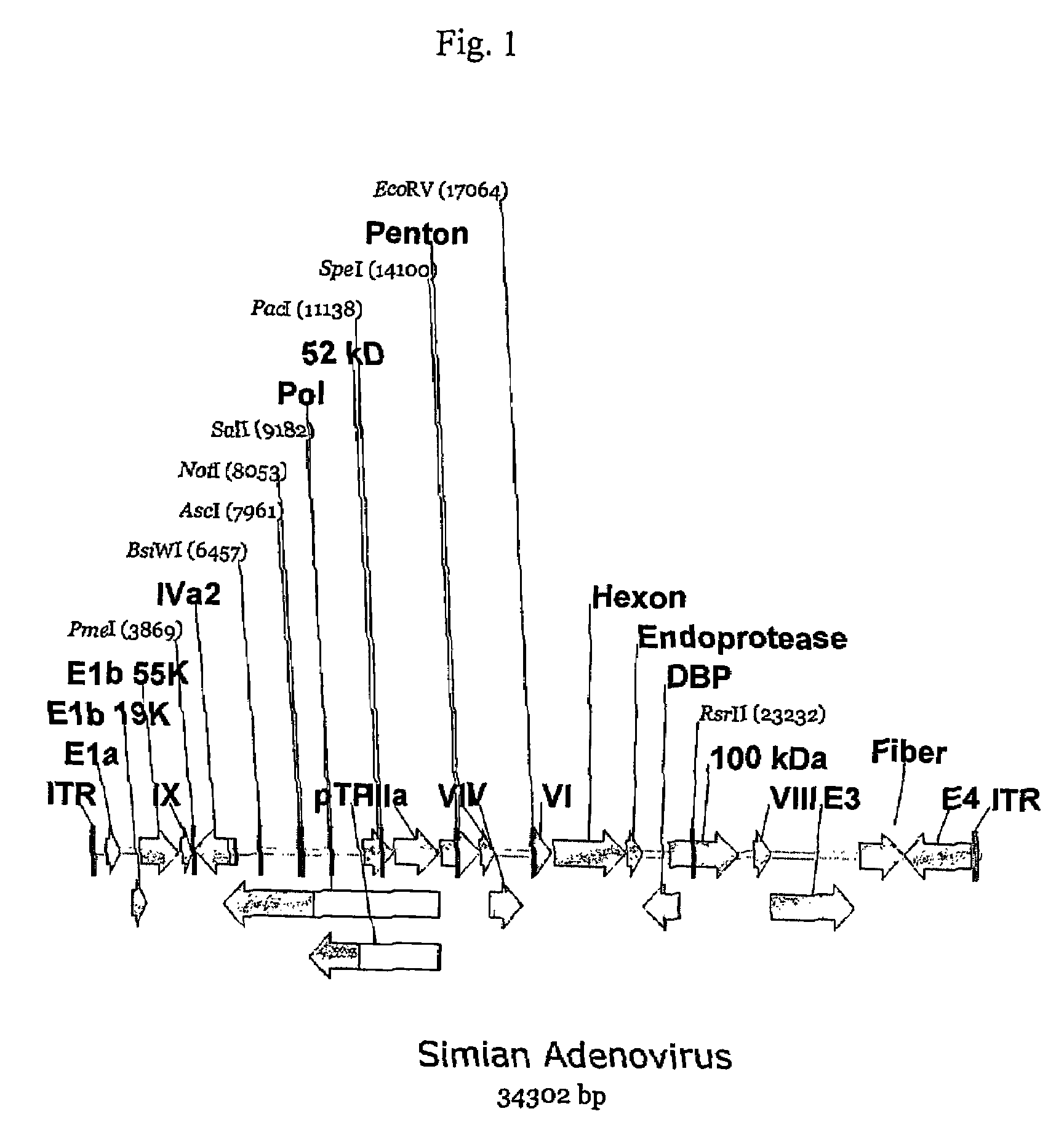
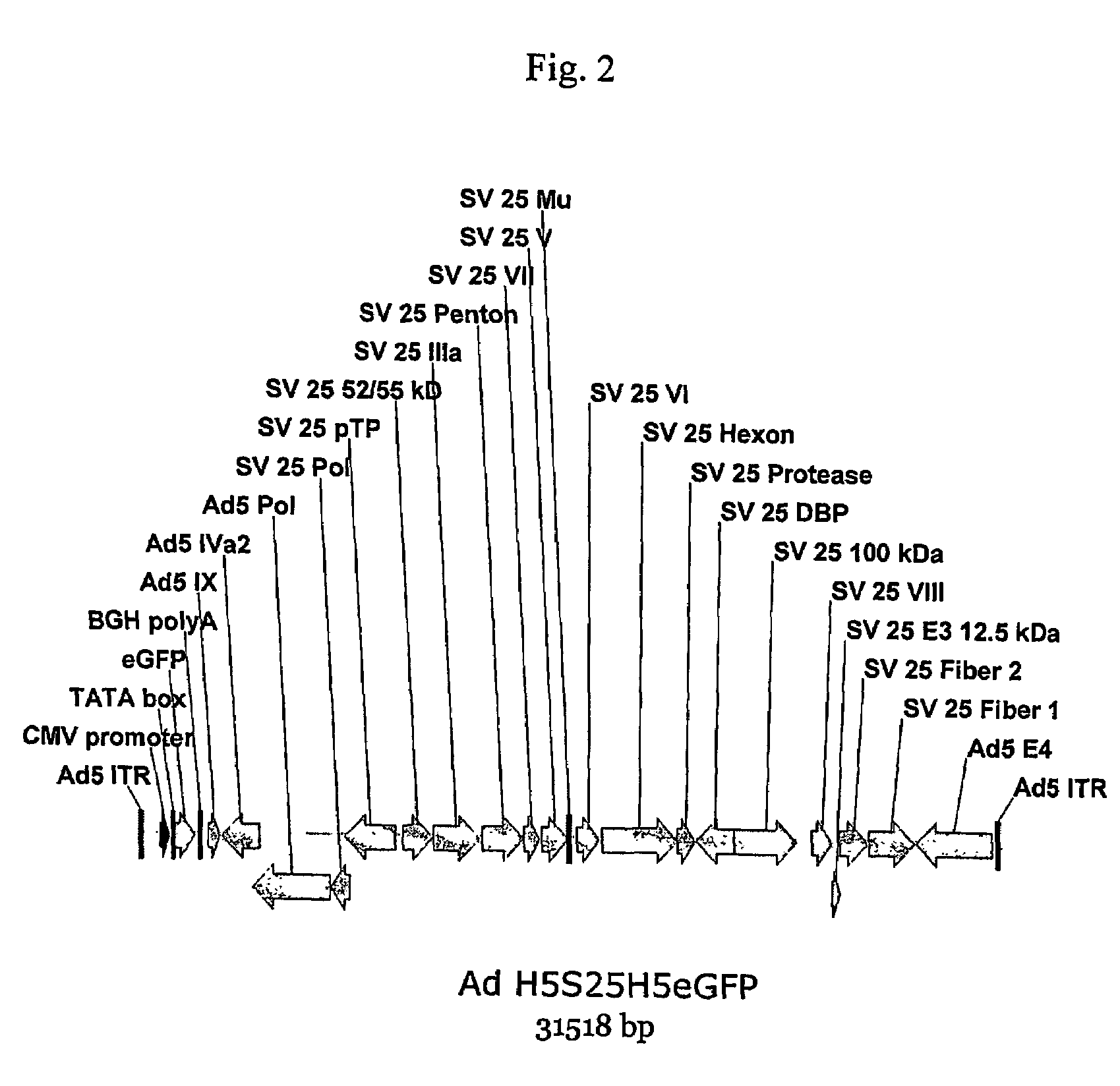
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
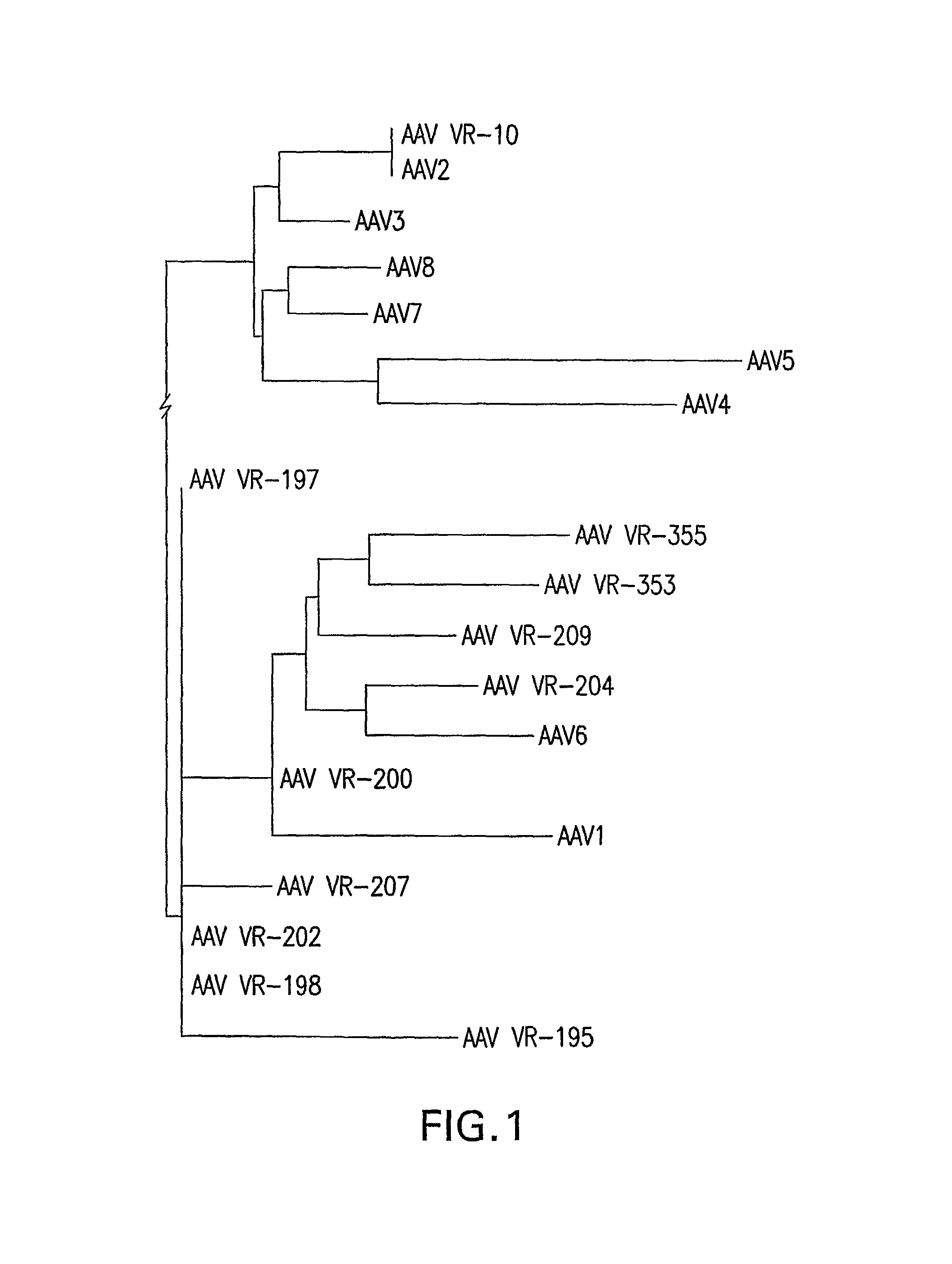
Isolation, cloning and characterization of new adeno-associated virus (AAV) serotypes
The present invention provides new adeno-associated virus (AAV) viruses and vectors, and particles derived therefrom. In addition, the present invention provides methods of delivering a nucleic acid to a cell using the AAV vectors and particles.
Owner:THE GOVERNMENT OF THE US SEC DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH OFFICE OF TECH TRANSFER
Method for treating neuromuscular disorders and conditions with botulinum toxin types A and B
A method of treating a patient suffering from a disease, disorder or condition includes the administration to the patient of a therapeutically effective amount of botulinum toxin of a selected serotype until the patient experiences loss of clinical response to the administered botulinum toxin and thereafter administering to the patient a therapeutically effective amount of another botulinum toxin of a different serotype.
Owner:SOLSTICE NEUROSCI
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the (5′) inverted terminal repeat, the left terminus spans the E4 region and the (3′) inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods for the separation of streptococcus pneumoniae type 3 polysaccharides
ActiveUS20080102498A1Reducing and removing impurityImprove filtering effectAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeLysis
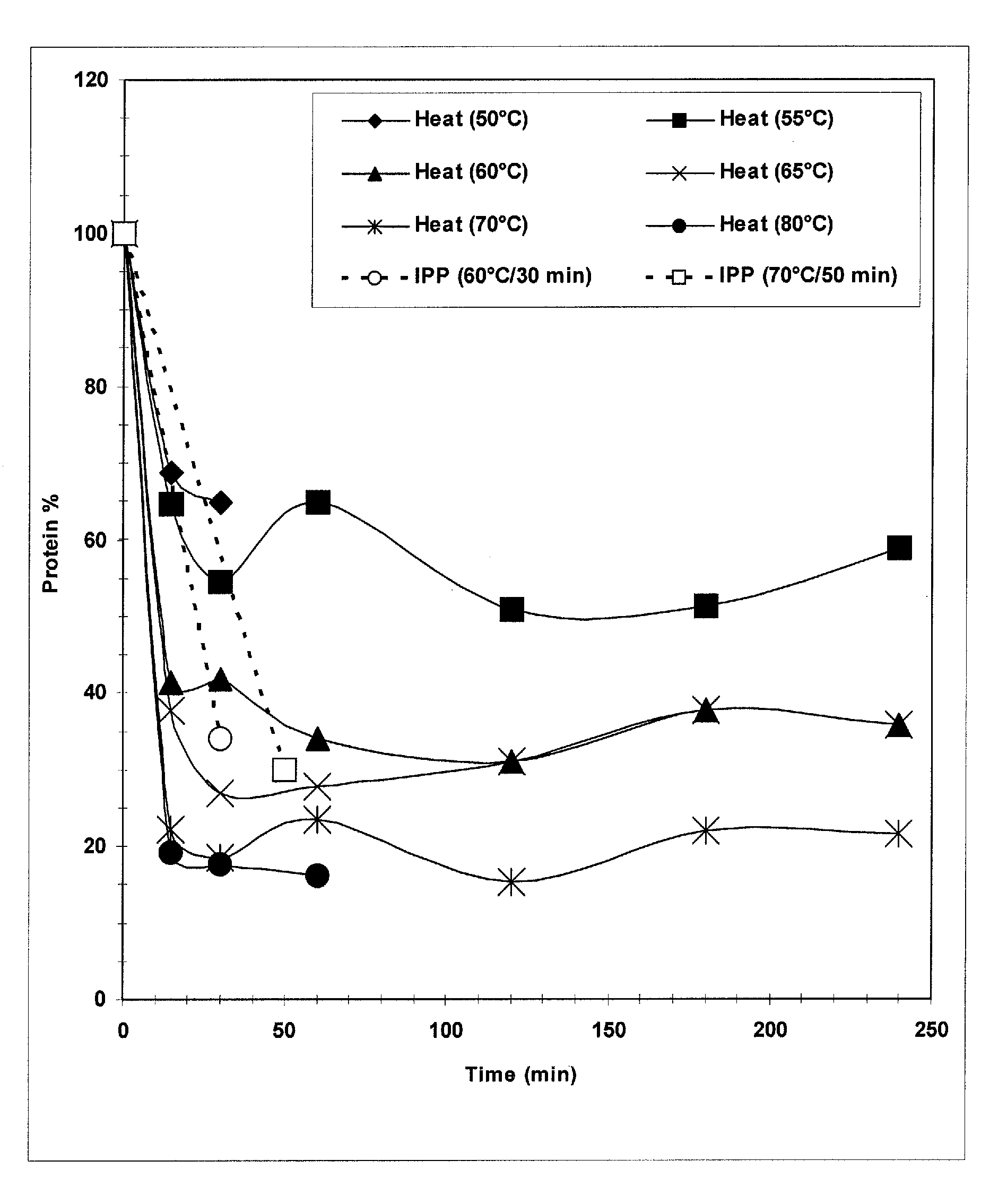
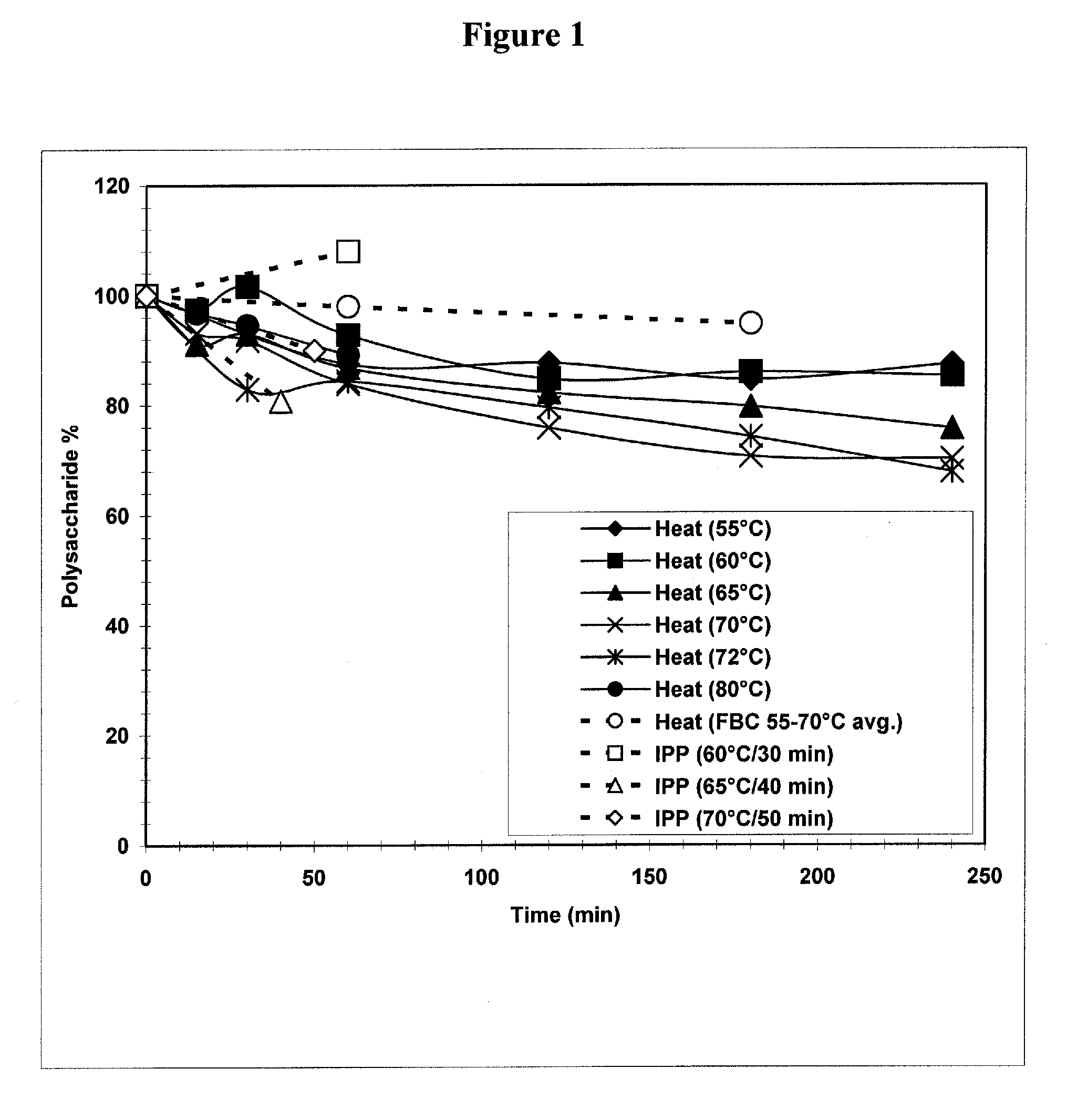
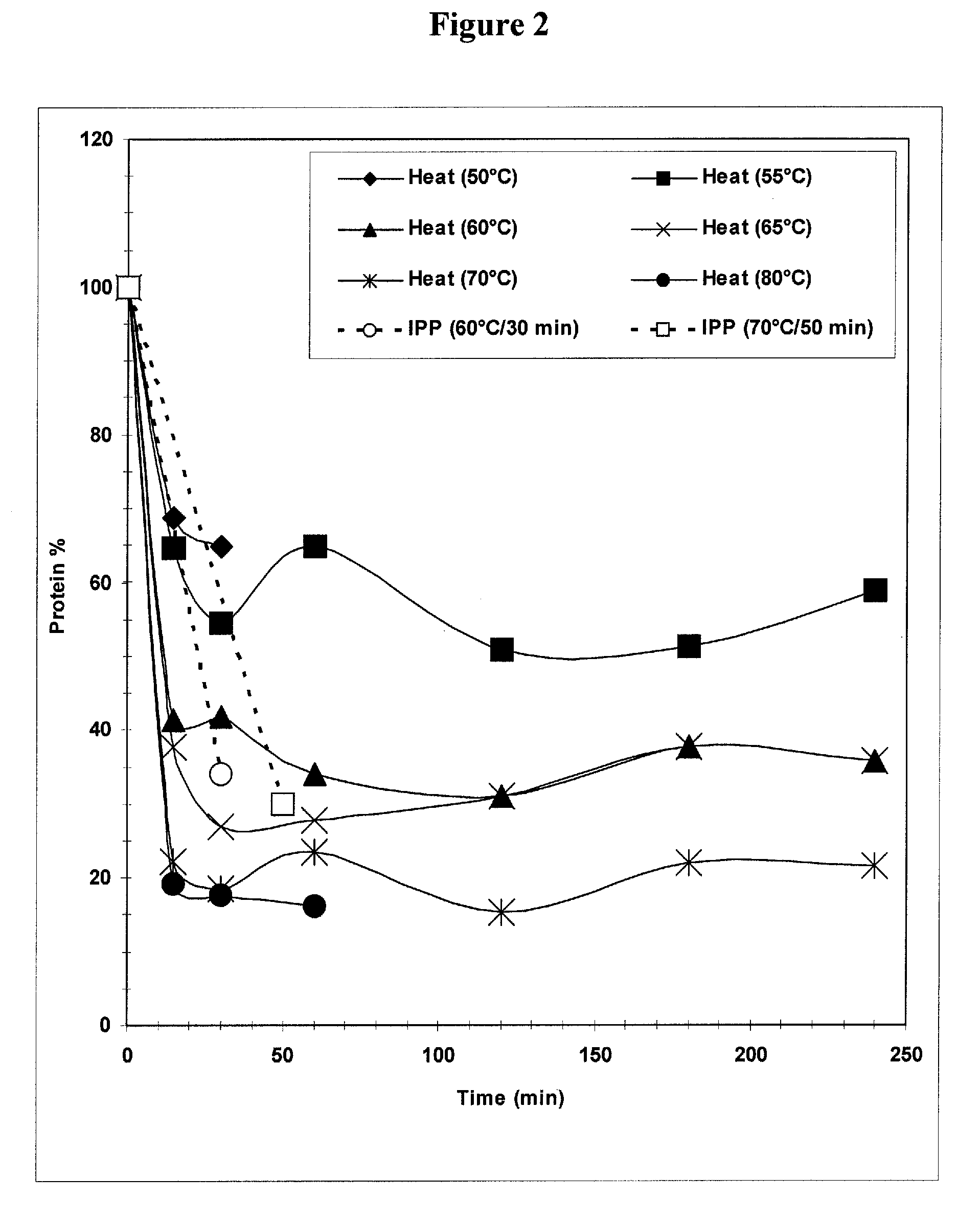
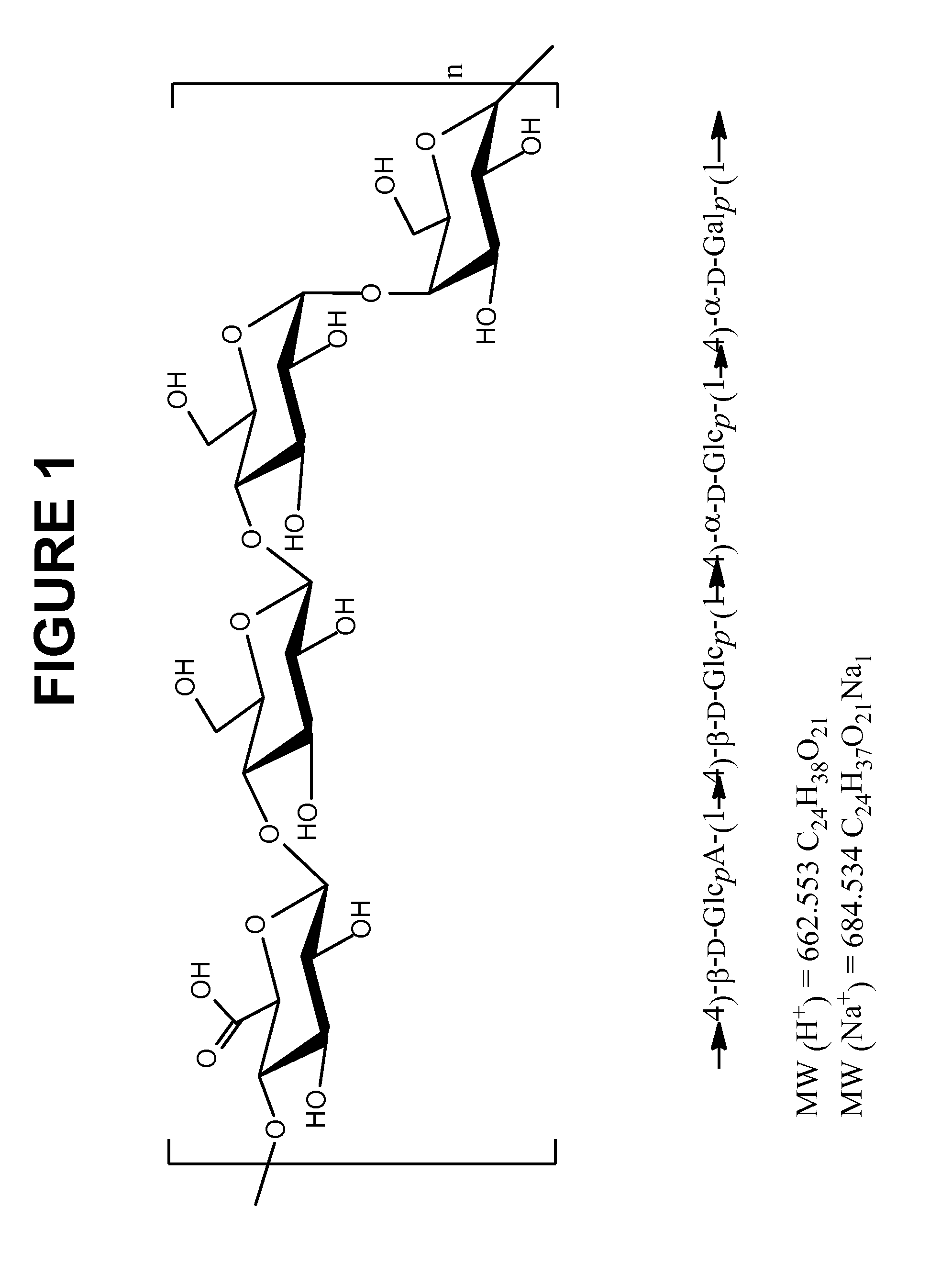
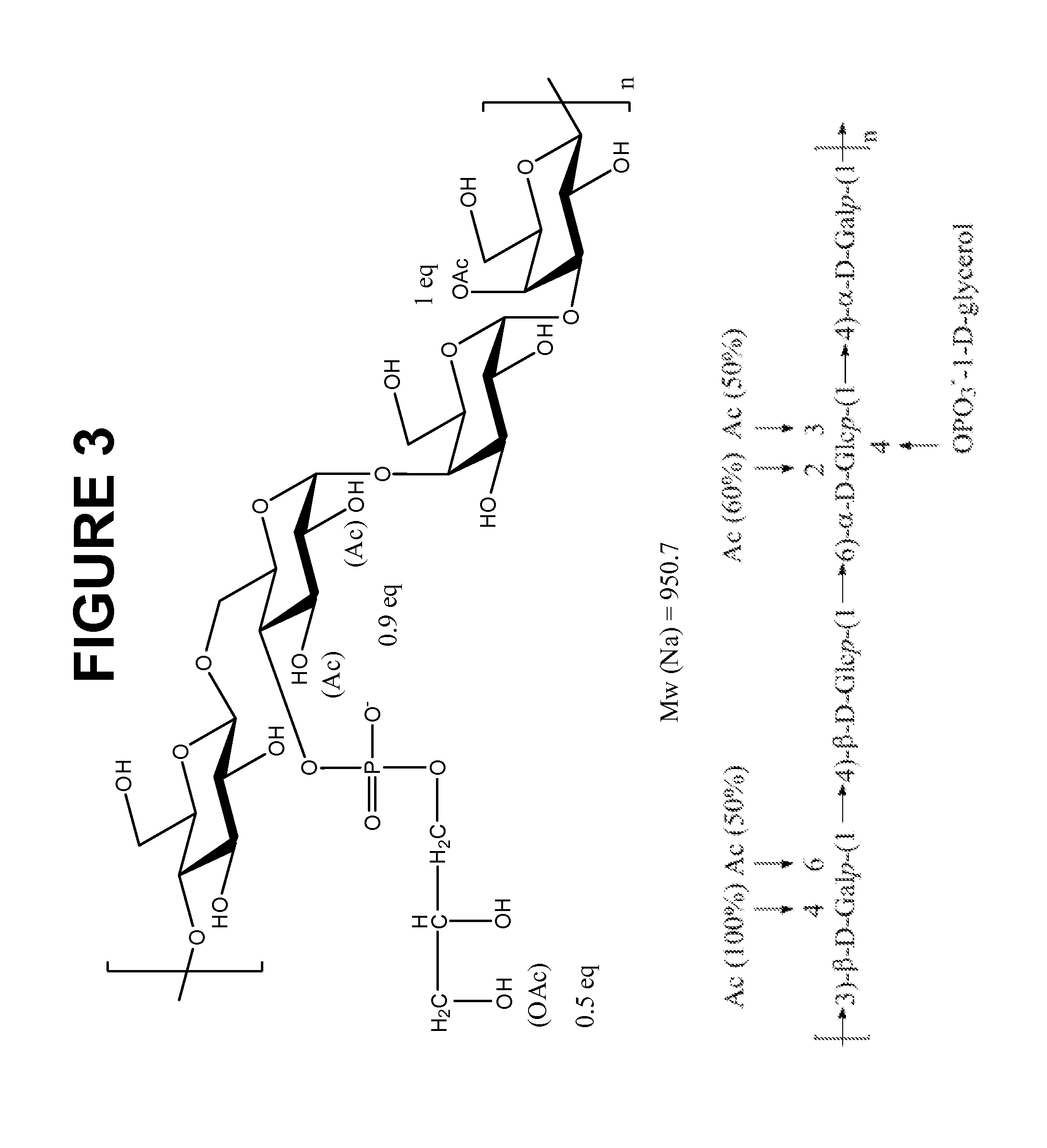
The present invention provides improved methods for the reduction or removal of protein impurities from a complex cellular Streptococcus pneumoniae lysate or centrate comprising serotype 3 polysaccharides involving steps relating to post-lysis heating or pH adjustment. In certain methods, the lysate is heated for a time and at a temperature sufficient to denature proteins present in the lysate and cause their aggregation and precipitation. In one embodiment, the lysate is heated to at least 60° C. for at least 30 minutes to cause protein aggregation and precipitation, more particularly about 60° C. to about 70° C. for about 30 to about 50 minutes, and even more particularly about 65° C. for about 40 minutes. In other methods, the pH of the lysate or centrate is increased to at least 8.0 to improve filterability, more particularly about 8.0 to 8.4, and even more particularly about 8.2. In further methods, heating and pH adjustment steps are combined to cause the aggregation and precipitation of proteins as well as to improve filterability of the lysates or centrates. In other methods, the pH of the lysate or centrate is lowered to about 3.0 to about 5.0 to cause protein aggregation and precipitation. Such methods allow for the production of substantially purified serotype 3 polysaccharide-containing lysates or centrates.
Owner:WYETH LLC
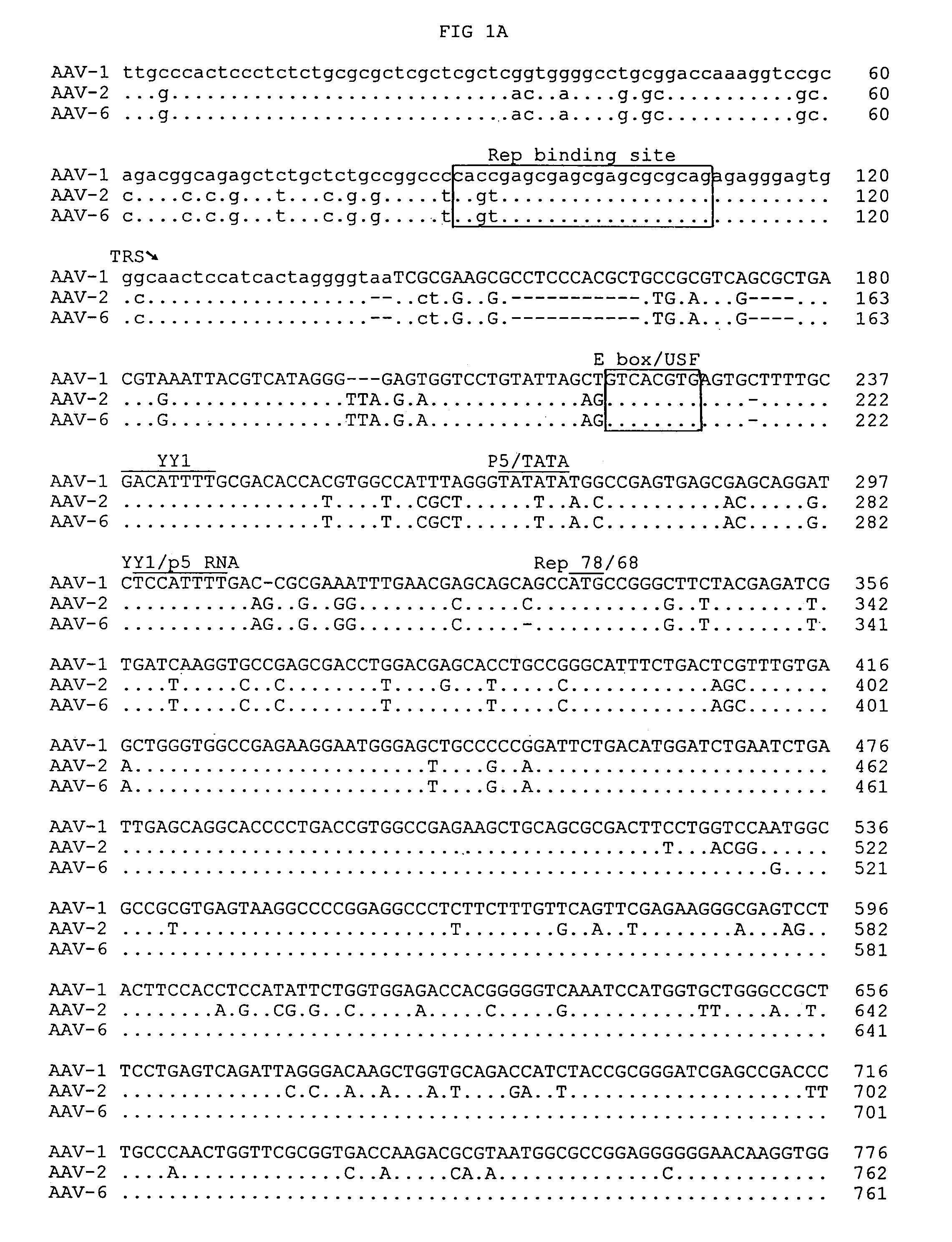
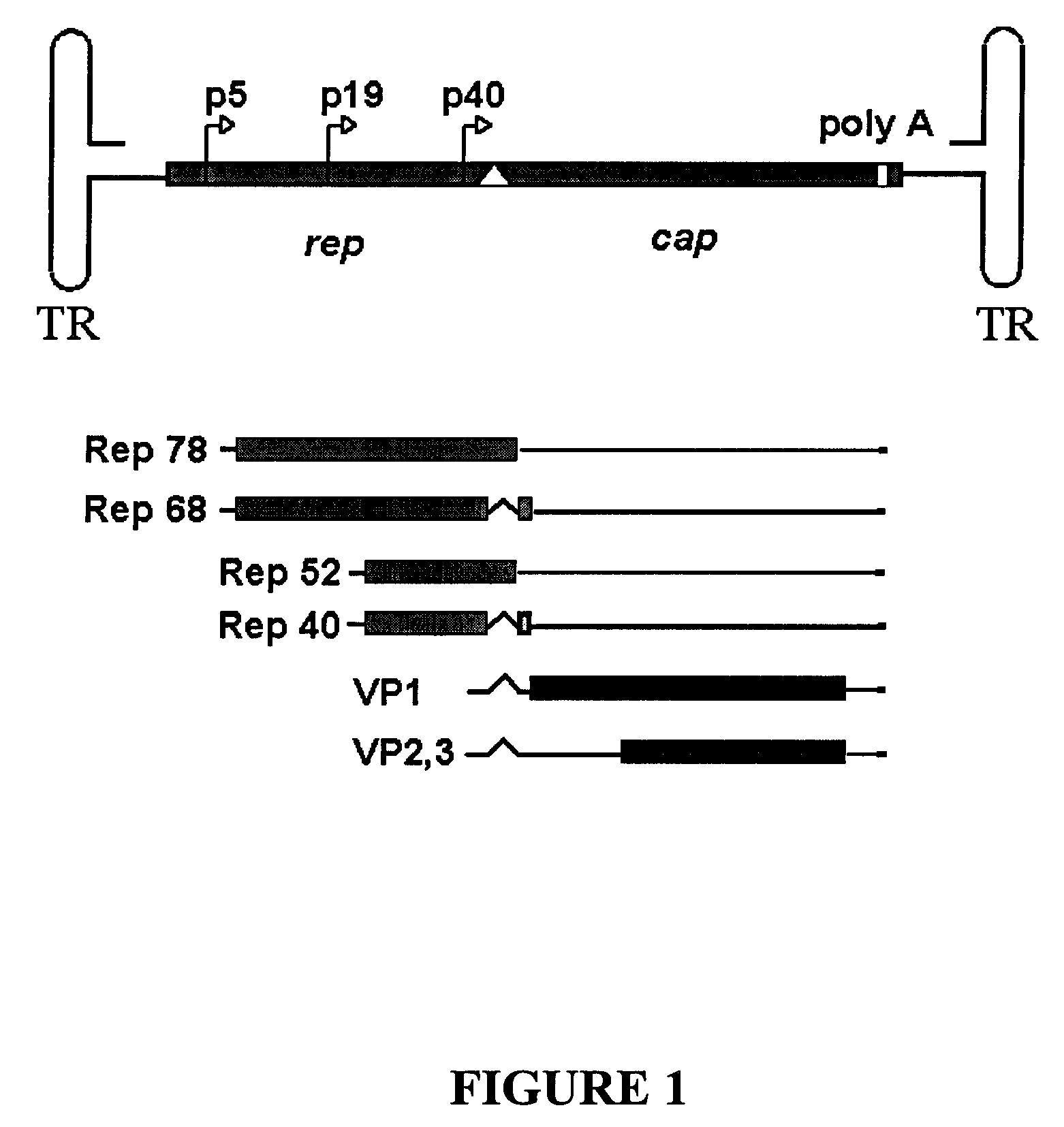
Adeno-associated virus serotype 1 nucleic acid sequences, vectors and host cells containing same
The nucleic acid sequences of adeno-associated virus (AAV) serotype 1 are provided, as are vectors and host cells containing these sequences and functional fragments thereof. Also provided are methods of delivering genes via AAV-1 derived vectors.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Serotype of adenovirus and uses thereof
InactiveUS6913922B1High infection efficiencyEasy to copyBiocideGenetic material ingredientsCell bindingSerotype
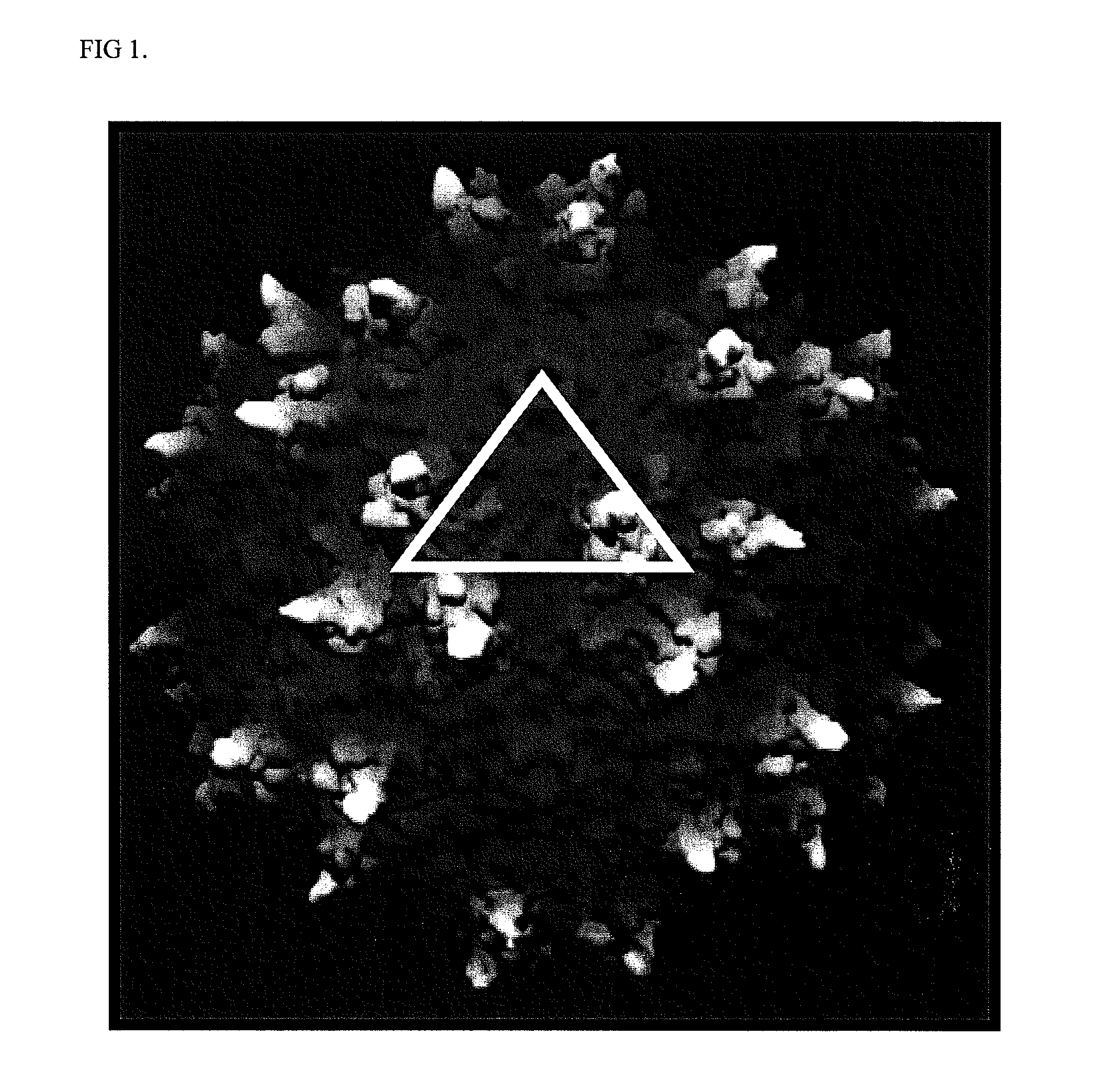
Adenovirus serotypes differ in their natural tropism. The adenovirus serotypes 2, 4, 5, and 7 all have a natural affiliation towards lung epithelia and other respiratory tissues. In contrast, serotypes 40 and 41 have a natural affiliation towards the gastrointestinal tract. The serotypes described, differ in at least capsid proteins (penton-base, hexon), proteins responsible for cell binding (fiber protein), and proteins involved in adenovirus replication. This difference in tropism and capsid protein among serotypes has led to the many research efforts aimed at redirecting the adenovirus tropism by modification of the capsid proteins.
Owner:JANSSEN VACCINES & PREVENTION BV
Adeno-associated virus serotype 1 nucleic acid sequences, vectors and host cells containing same
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
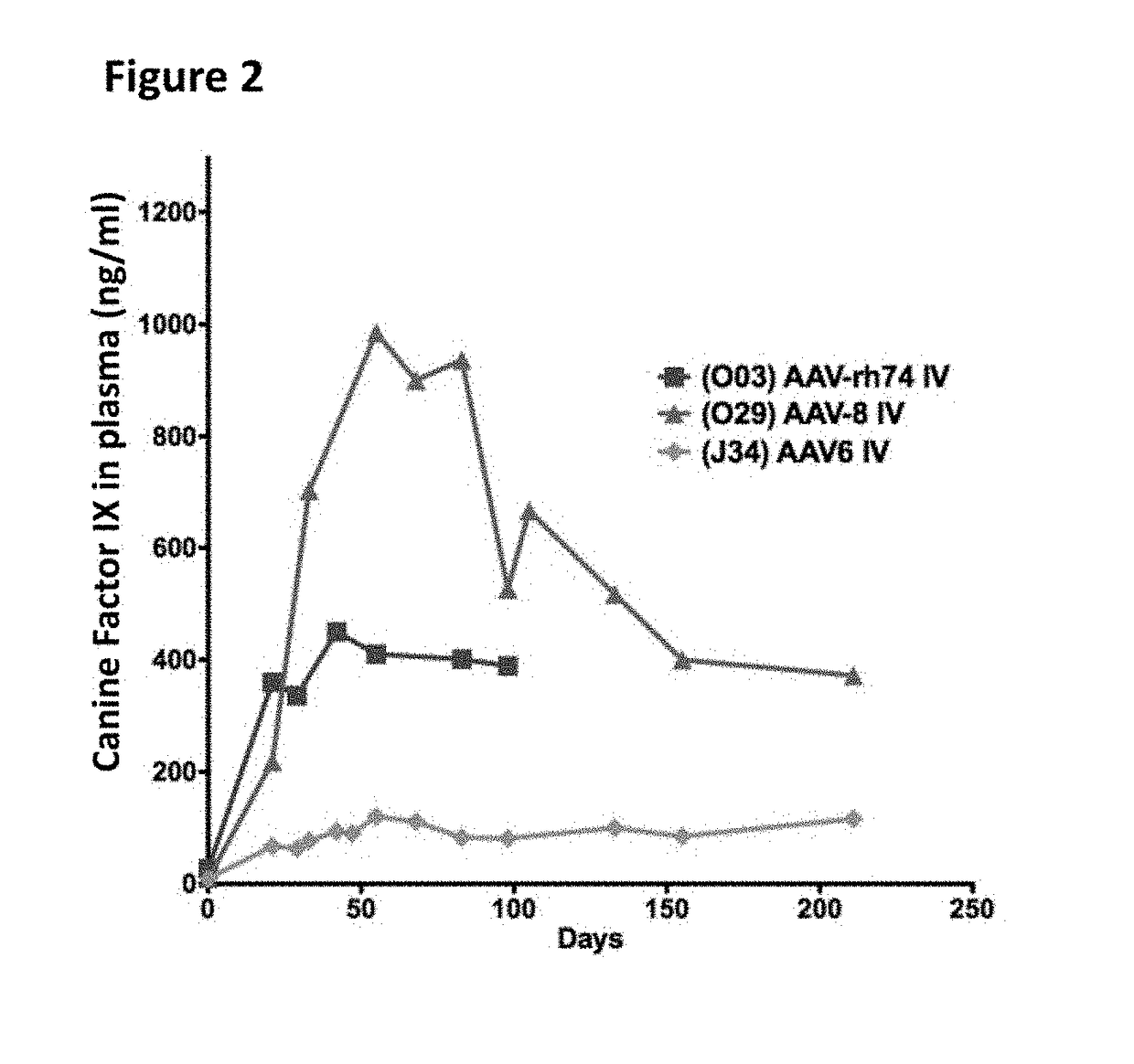
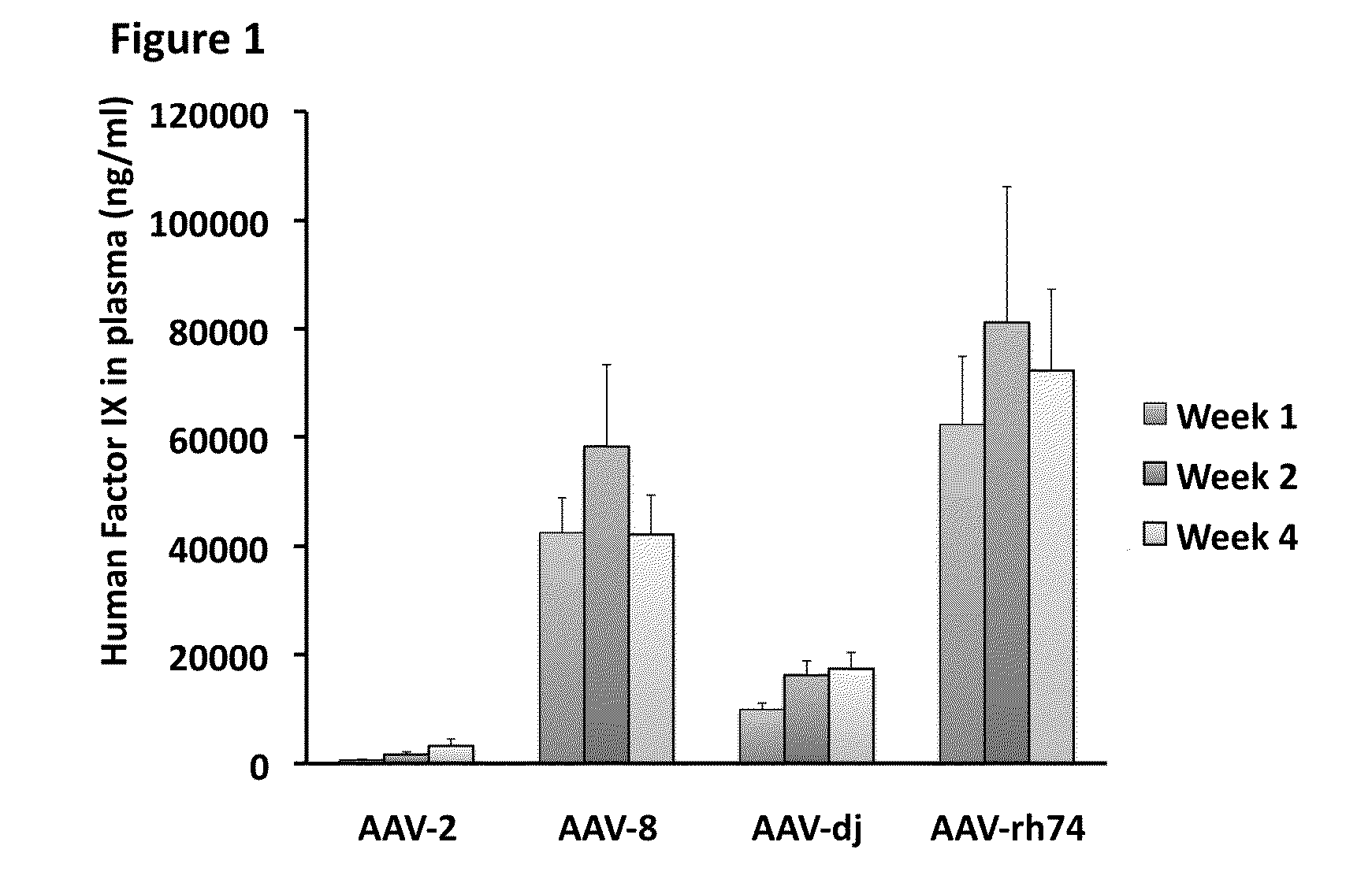
Variant AAV and compositions, methods and uses for gene transfer to cells, organs and tissues
ActiveUS9840719B2Reduce the possibilityCost of treatmentVectorsPeptide/protein ingredientsTherapeutic proteinNucleic acid sequencing
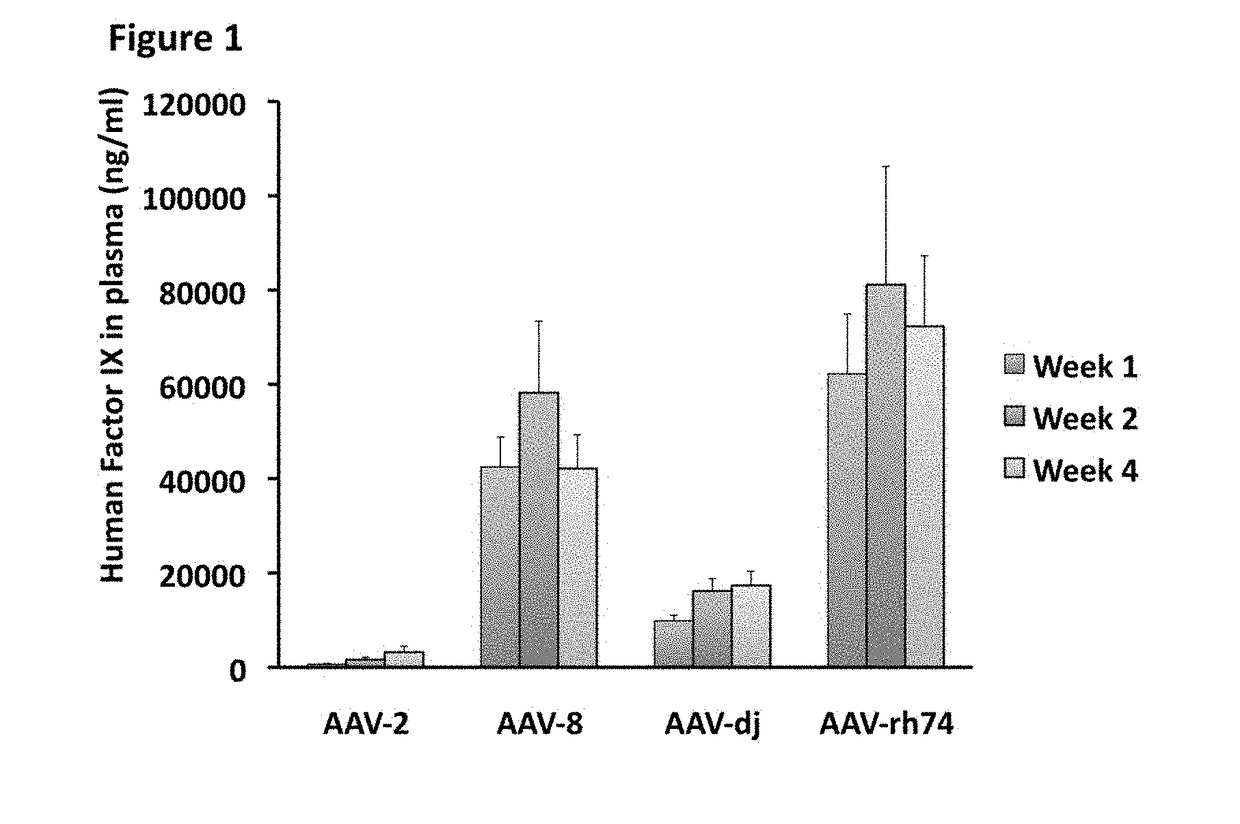
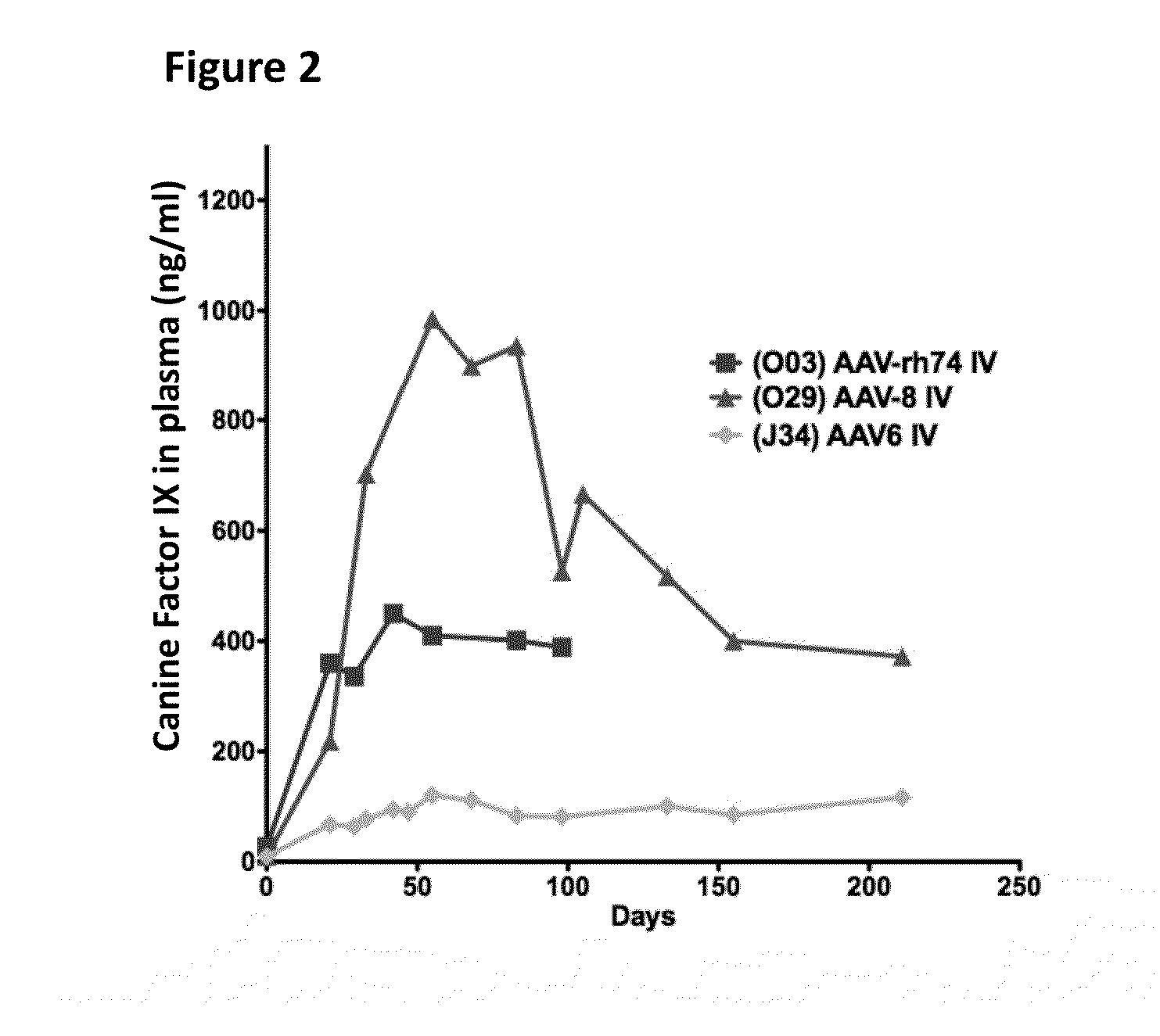
The invention relates to adeno-associated virus (AAV) serotype AAV-Rh74 and related AAV vectors, and AAV-Rh74 and related AAV vector mediated gene transfer methods and uses. In particular, AAV-Rh74 and related AAV vectors target polynucleotides to cells, tissues or organs for expression (transcription) of genes encoding therapeutic proteins and peptides, and polynucleotides that function as or are transcribed into inhibitory nucleic acid sequences.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Novel multi-oligosaccharide glycoconjugate bacterial meningitis vaccines
InactiveUS20010048929A1Inhibition effectWeight increaseAntibacterial agentsPeptide/protein ingredientsSerotypeTumor antigen
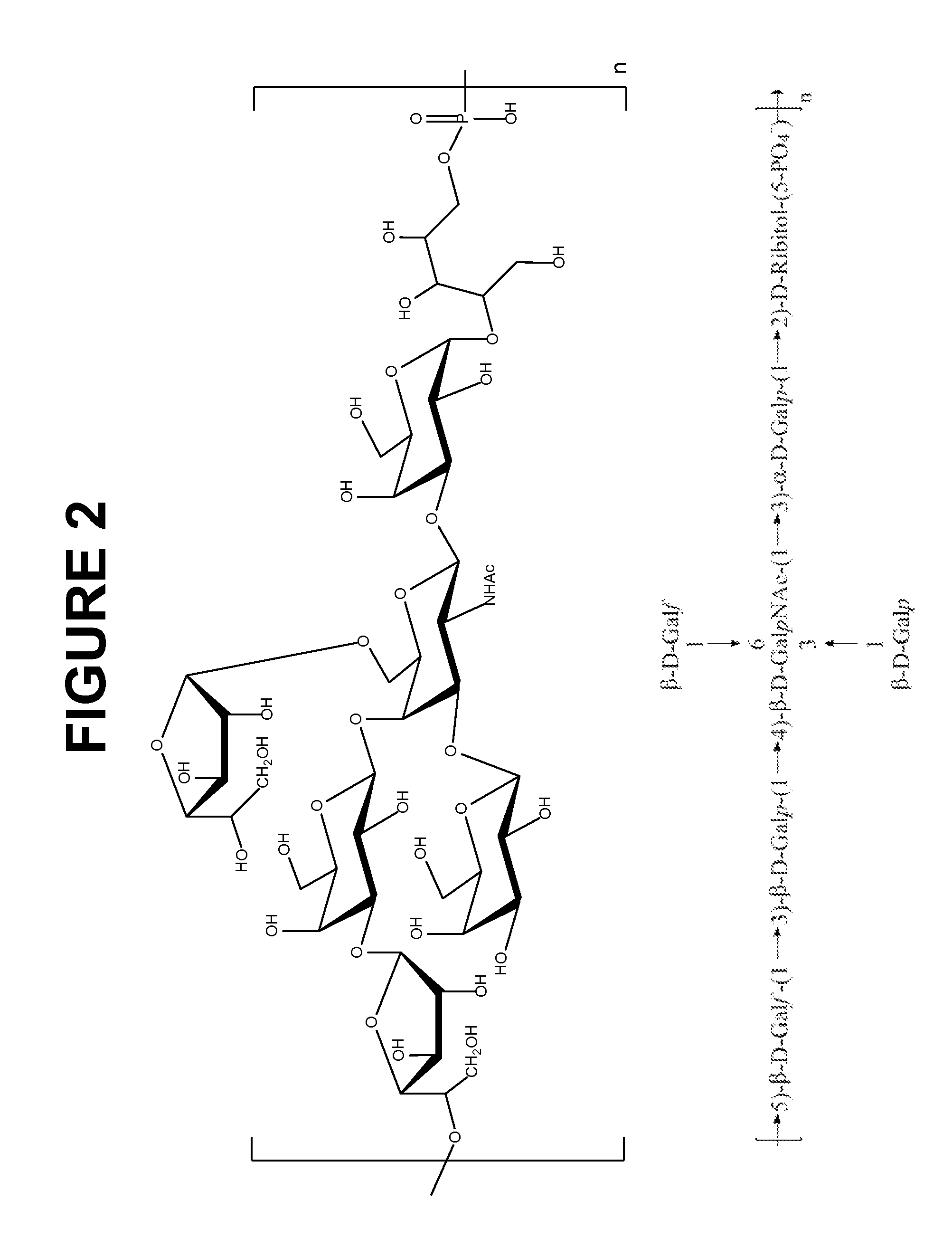
Multivalent immunogenic molecules comprise a carrier molecule containing at least one functional T-cell epitope and multiple different carbohydrate fragments each linker to the carrier molecule and each containing at least one functional B-cell epitope. The carrier molecule inputs enhanced immunogenicity to the multiple carbohydrate fragments. The carbohydrate fragments may be capsular oligosaccharide fragments from Streptococcus pneumoniae, which may be serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F or 23F, or Neisseria meningitidis, which may be serotype A, B, C, W-135 or Y. Such oligosaccharide fragments may be sized from 2 to 5 kDa. Alternatively, the carbohydrate fragments may be fragments of carbohydrate-based tumor antigens, such as Globo H, LeY or STn. The multivalent molecules may be produced by random conjugation or site-directed conjugation of the carbohydrate fragments to the carrier molecule. The multivalent molecules may be employed in vaccines or in the generation of antibodies for diagnostic application.
Owner:CONNAUGHT LAB
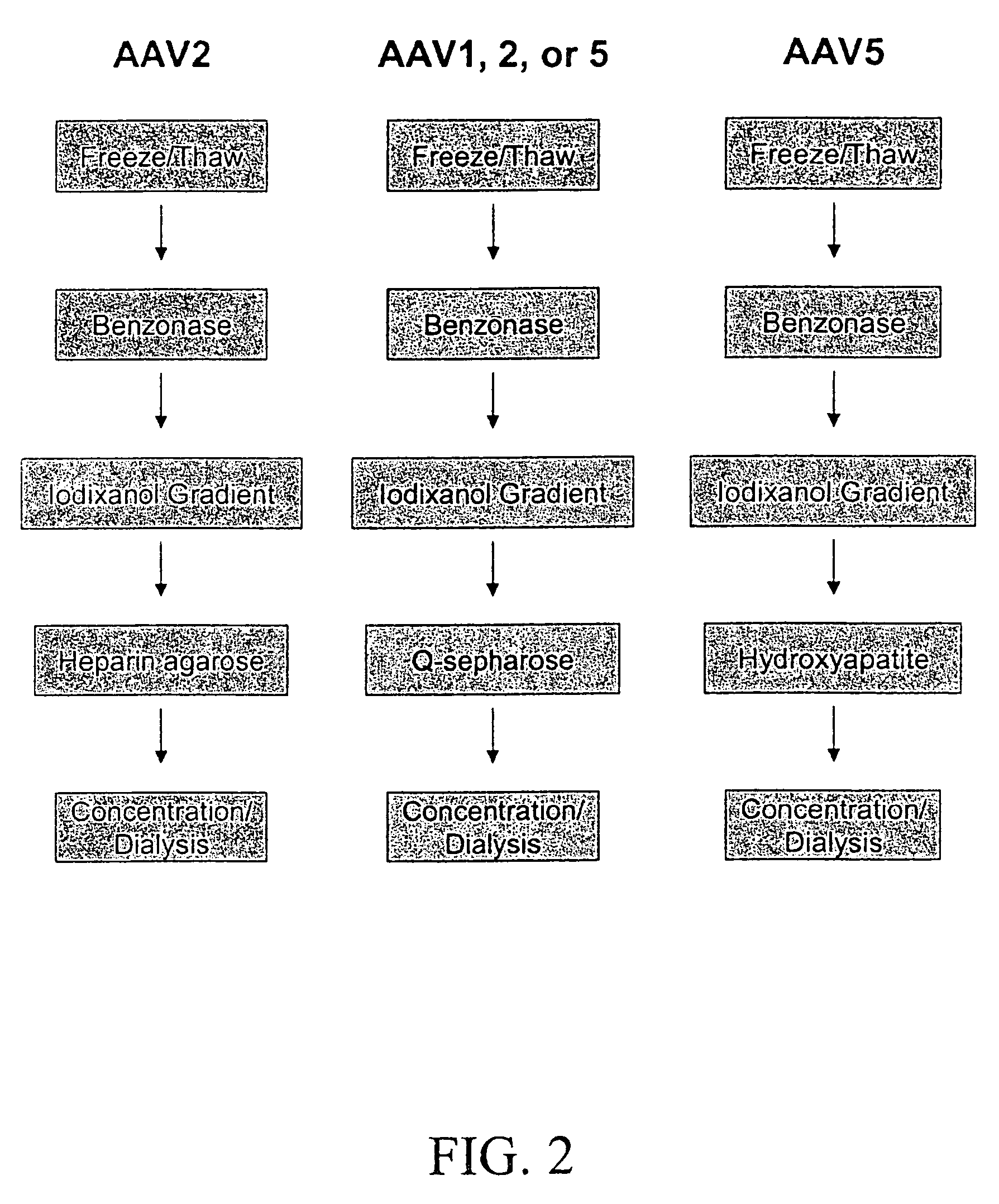
Production of recombinant AAV virions
ActiveUS7927585B2Simple working processCost effective productionBiocideSugar derivativesYeastVirosome
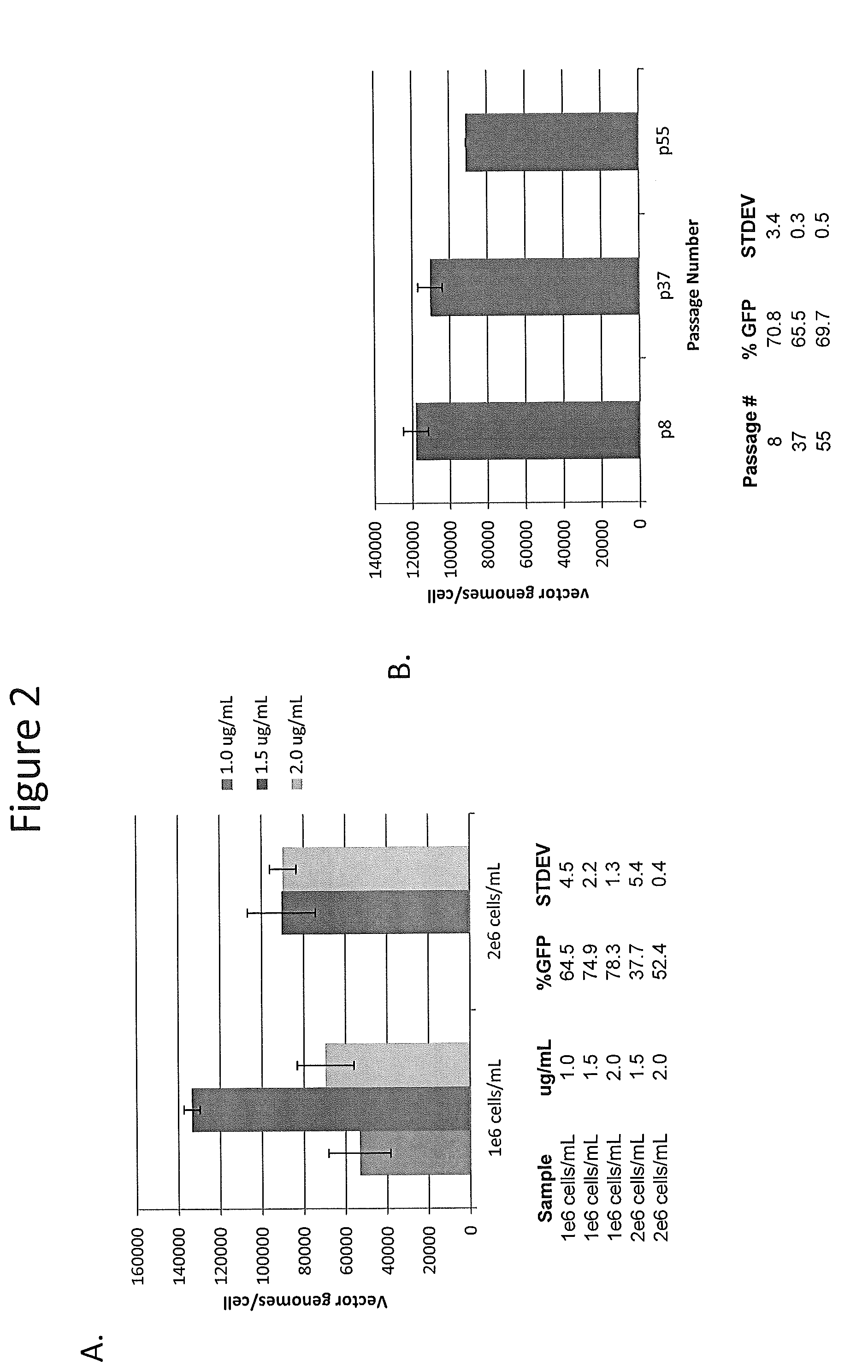
Stocks of infectious rAAV are generated using yeast strains, bacterial strains, and bacteriophages engineered to express the required AAV proteins and harboring rAAV vector sequences. Stocks of rAAV virions of all serotypes and pseudotypes can be generated in prokaryotic and eukaryotic cells using the methods described herein.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Production of pseudotyped recombinant AAV virions
InactiveUS20070015238A1Highly purified and concentratedEfficient and large-scale productionVectorsTissue culturePurification methodsSerotype
Vectors that encode Adeno-Associated Virus (AAV) Rep and Cap proteins of different serotypes and Adenovirus transcription products that provide helper functions were used to produce pseudotyped recombinant AAV (rAAV) virions. Purification methods generated pseudotyped rAAV virion stocks that were 99% pure with titers of 1×1012−1×1013 vector genomes / ml.
Owner:SNYDER RICHARD O +12
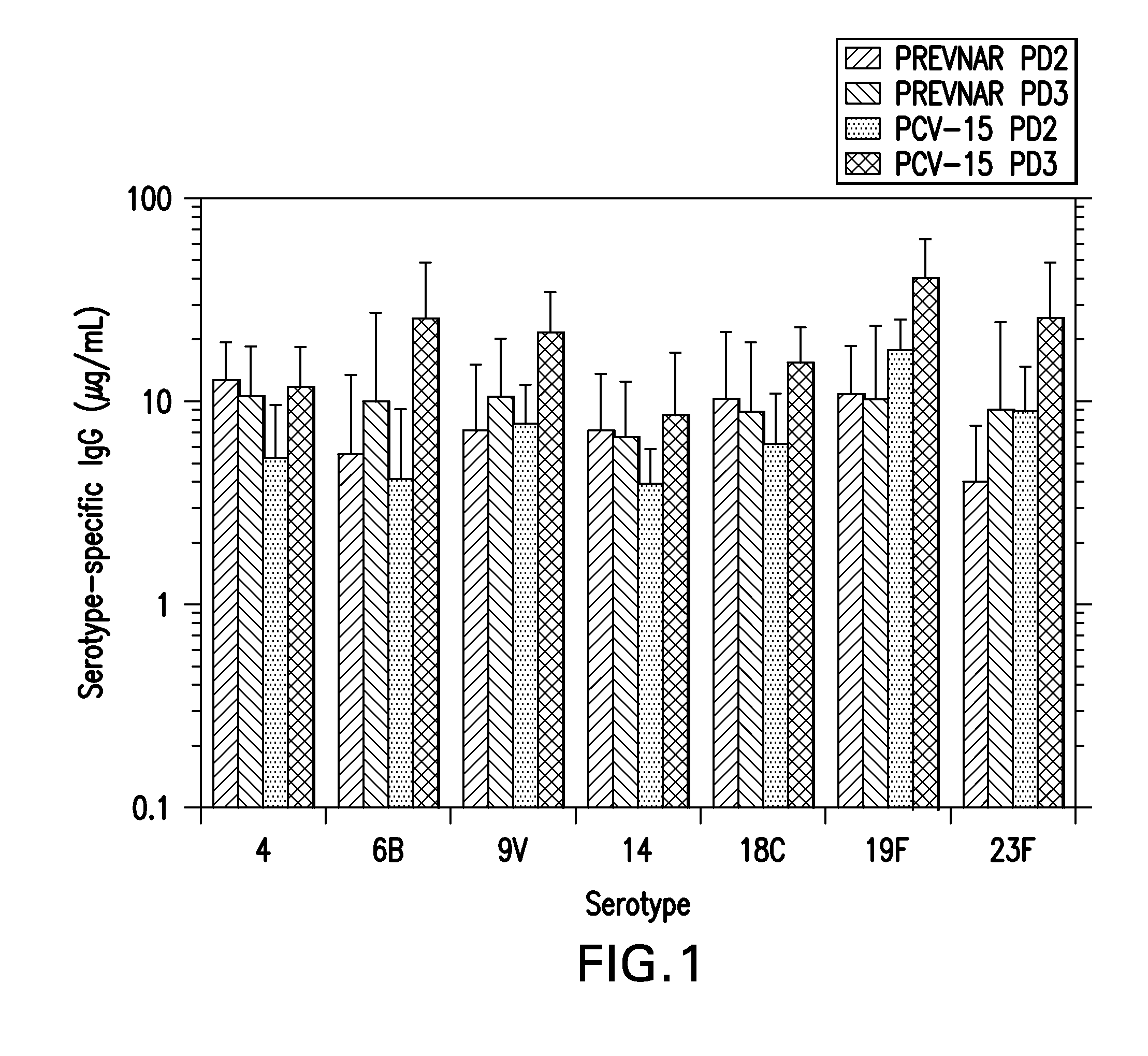
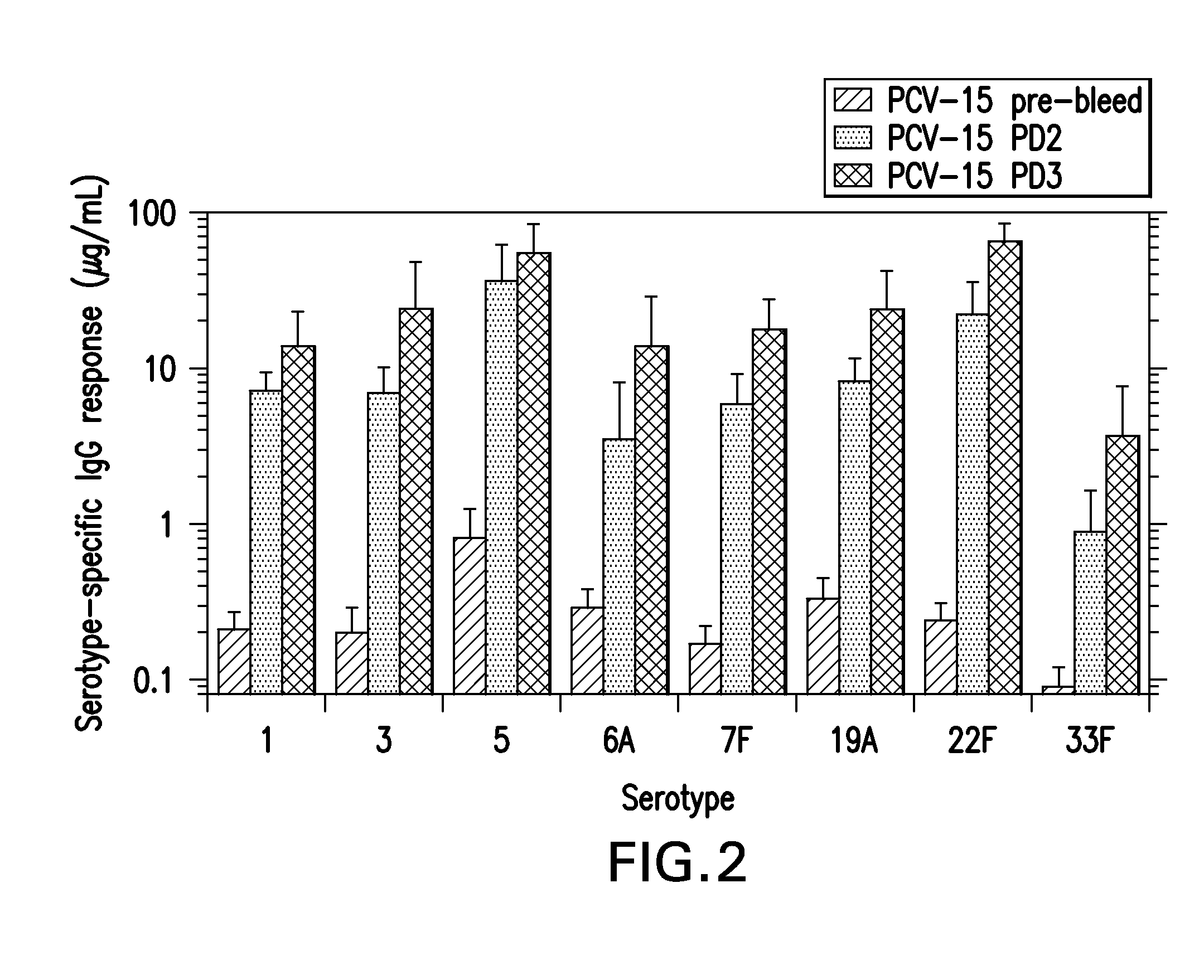
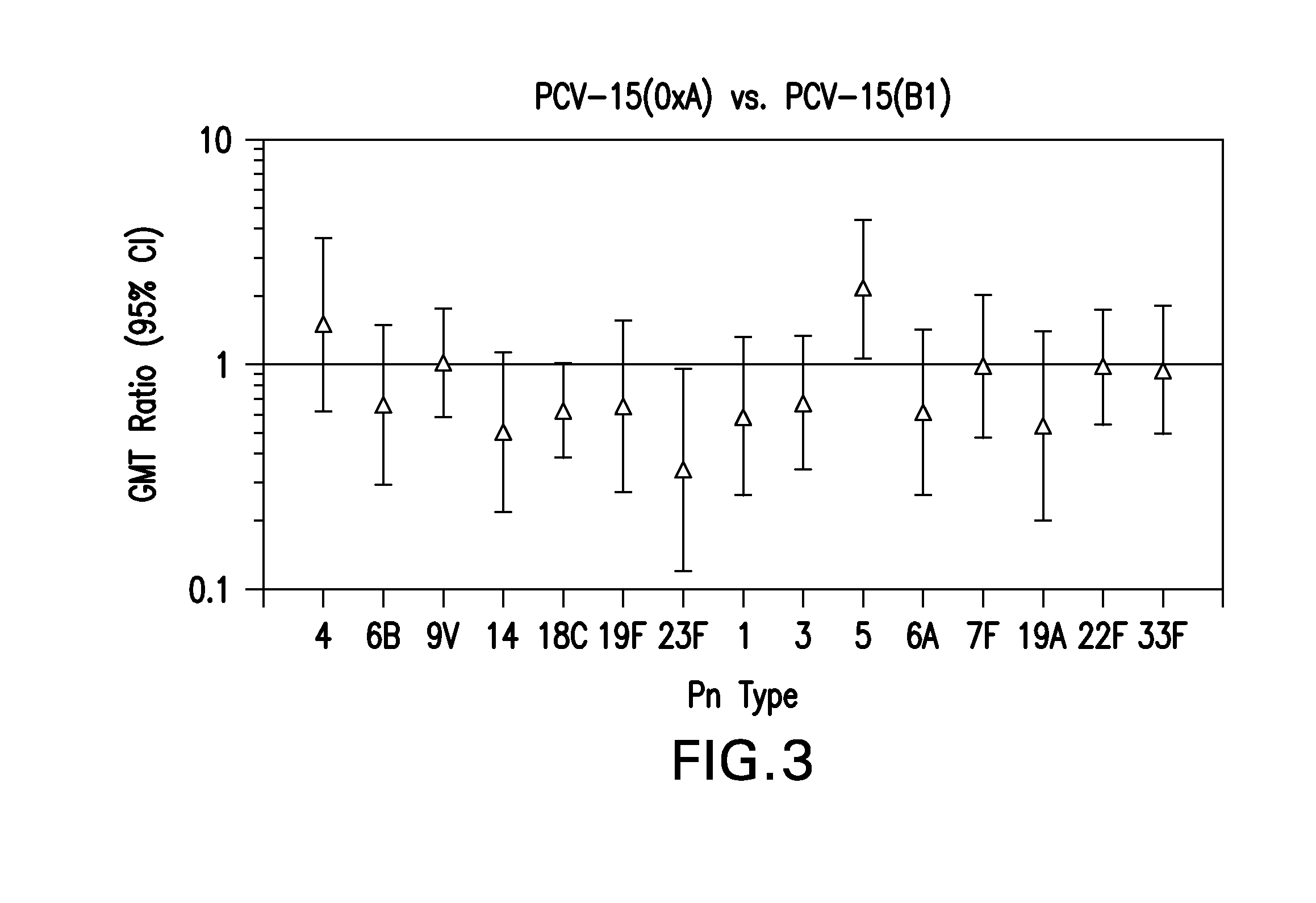
15-valent pneumococcal polysaccharide-protein conjugate vaccine composition
ActiveUS20110195086A1Antibacterial agentsBacteria material medical ingredientsConjugate vaccineDisease
The present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197. The immunogenic composition, preferably formulated as a vaccine on an aluminum-based adjuvant, provides broad coverage against pneumococcal disease, particularly in infants and young children.
Owner:MERCK SHARP & DOHME LLC
Cell line for production of adeno-associated virus
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Immunogenic compositions for gram positive bacteria such as streptococcus agalactiae
InactiveUS20060165716A1InhibitionEasy to eliminateAntibacterial agentsBacterial antigen ingredientsBacteroidesVirulent characteristics
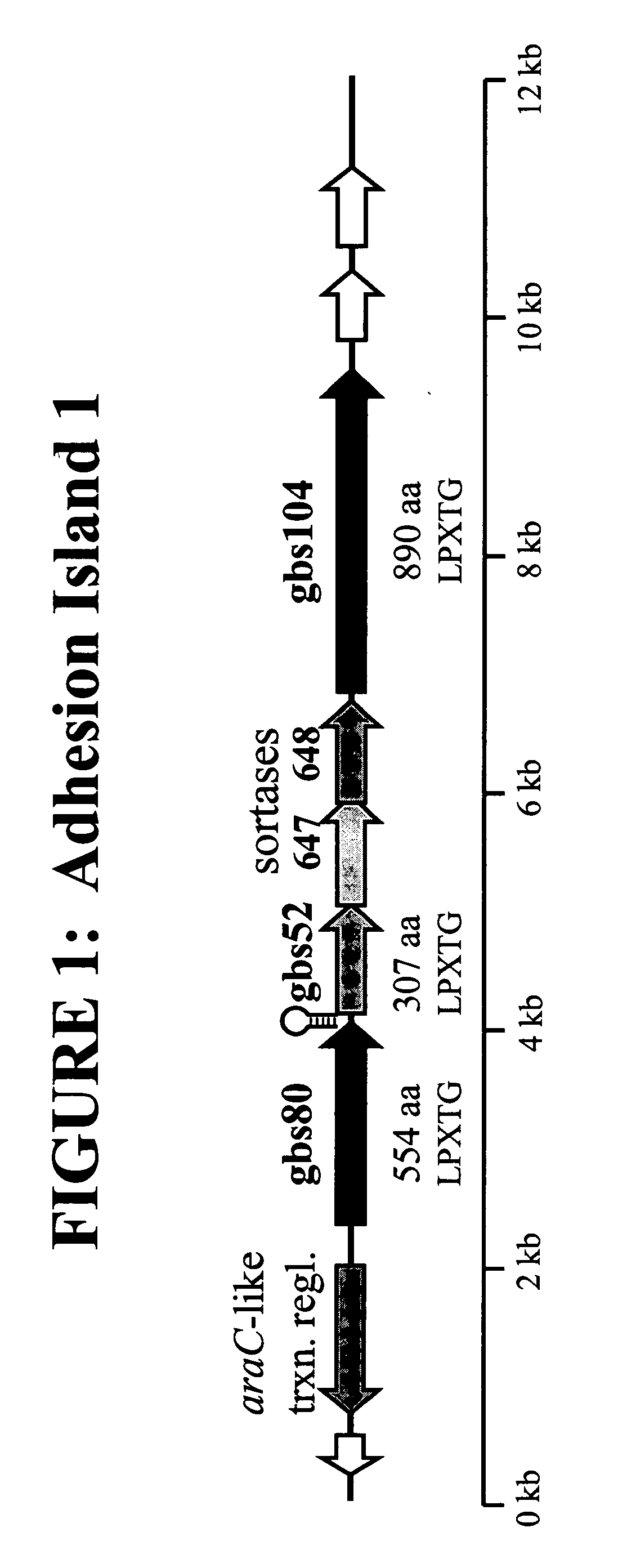
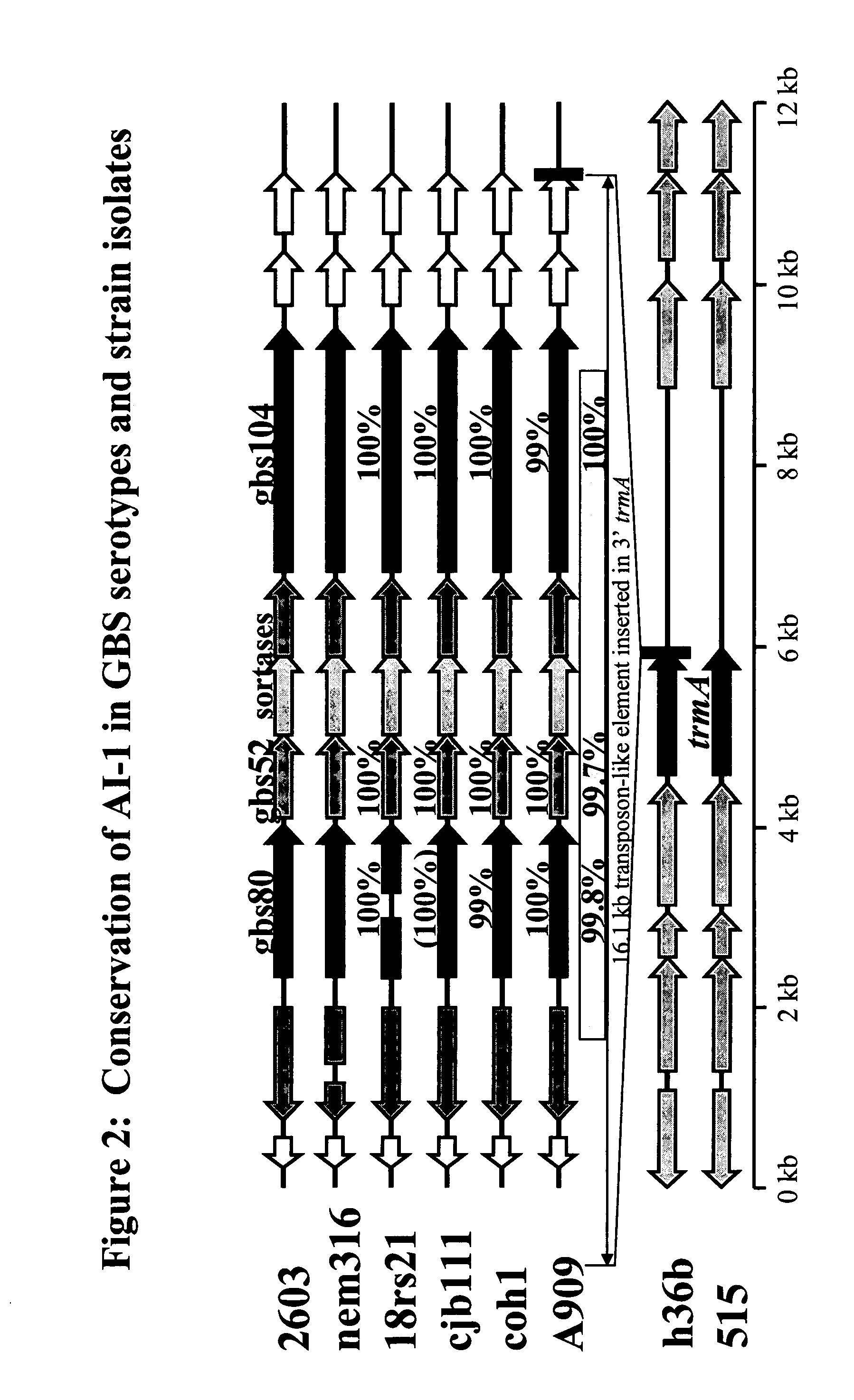
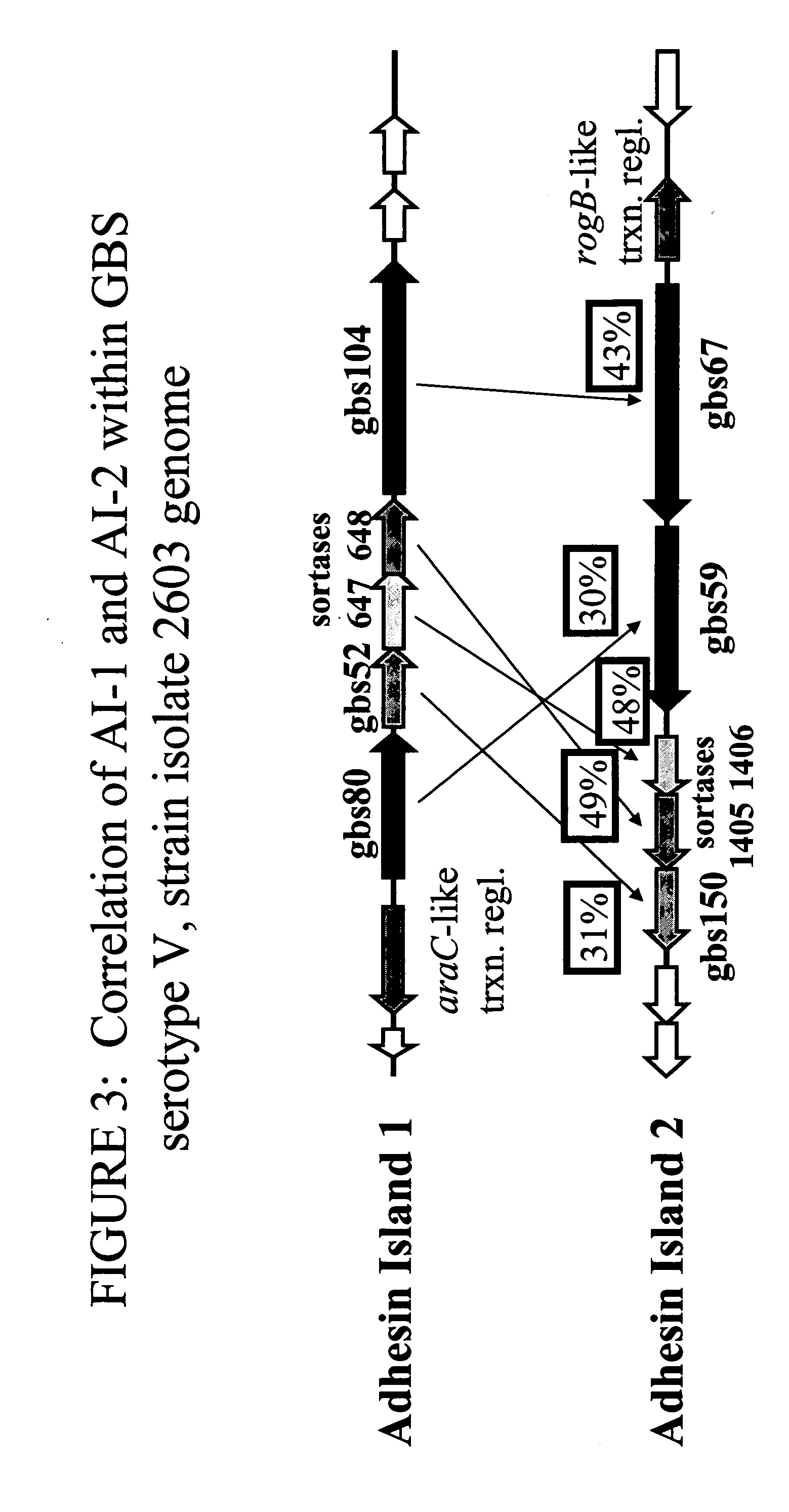
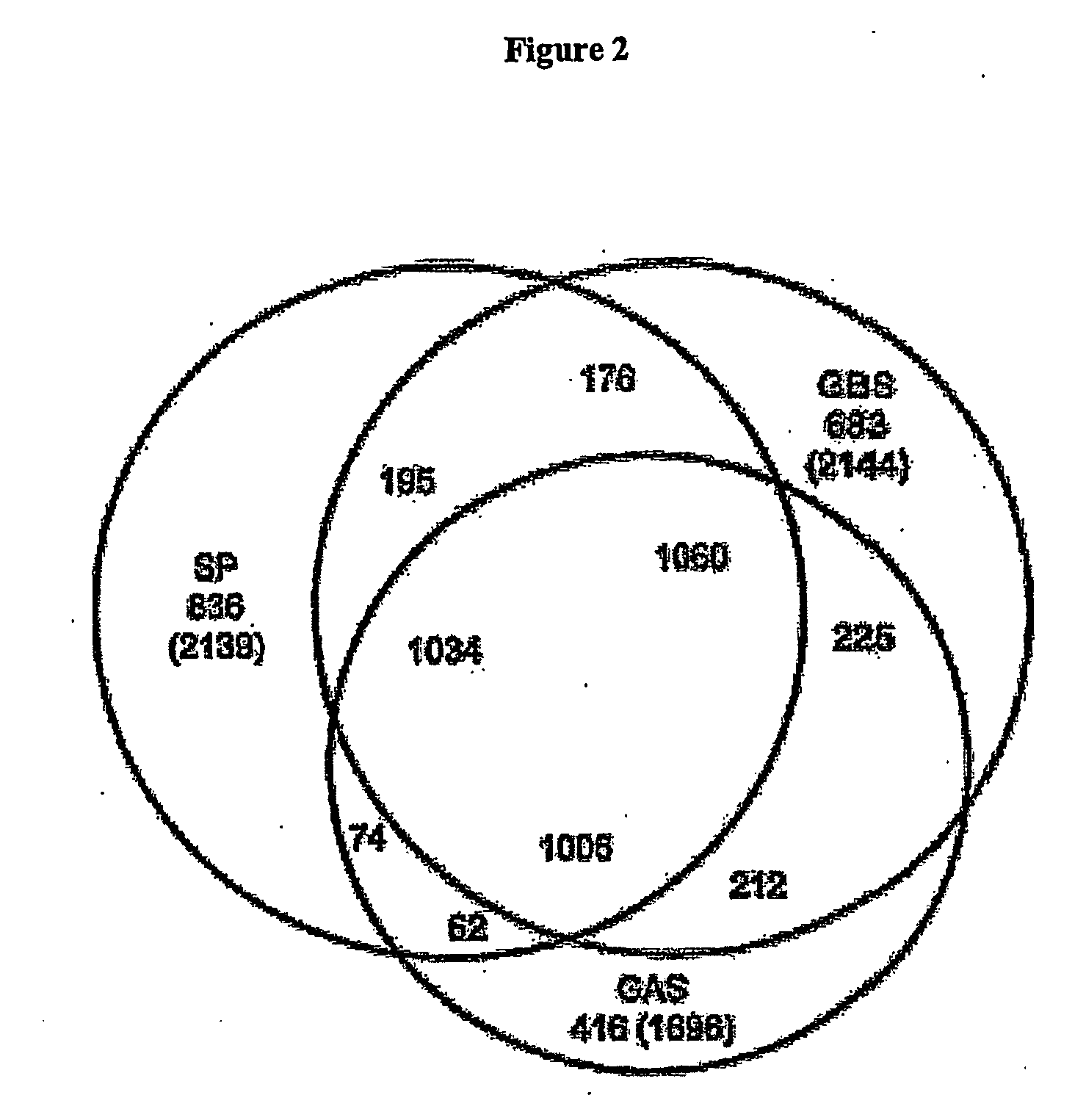
The invention relates to the identification of a new adhesin islands within the genomes of several Group A and Group B Streptococcus serotypes and isolates. The adhesin islands are thought to encode surface proteins which are important in the bacteria's virulence. Thus, the adhesin island proteins of the invention may be used in immunogenic compositions for prophylactic or therapeutic immunization against GAS or GBS infection. For example, the invention may include an immunogenic composition comprising one or more of the discovered adhesin island proteins.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens and Uses Thereof
ActiveUS20150202309A1Excellent characteristicsHigh yieldAntibacterial agentsMedical devicesStreptococcus pneumoniaeVaccination
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates) and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR®, SYNFLORIX® and / or PREVNAR 13®. The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
AAV virions with decreased immunoreactivity and uses therefor
ActiveUS7259151B2Decreased immunoreactivityEfficient transductionOrganic active ingredientsVirusesGeneticsSerotype
Owner:GENZYME CORP
Immunogenic compositions for Chlamydia trachomatis
PendingUS20060034871A1Enhance immune responseAntibacterial agentsBacterial antigen ingredientsDiseaseAdjuvant
The invention relates to immunogenic compositions comprising combinations of Chlamydia trachomatis antigens and their use in vaccines. The composition may comprise at least two components, one component of which comprises Chlamydia trachomatis antigens for eliciting a Chlamydia trachomatis specific TH1 immune response and another component of which comprises antigens for eliciting a Chlamydia trachomatis specific TH2 immune response. The invention further relates to an immunogenic composition comprising a Chlamydia trachomatis Type III secretion system (TTSS) regulatory protein and a Chlamydia trachomatis Type III secretion system (TTSS) secreted protein or a fragment thereof. The invention further relates to the use of combinations of adjuvants for use with antigens associated with a sexually transmissible disease, such as Chlamydia trachomatis antigens. Preferred adjuvant combinations include mineral salts, such as aluminium salts and oligonucleotides comprising a CpG motif. The invention further provides a combination of Chlamydia trachomatis antigens comprising a Chlamydia trachomatis antigen that is conserved over at least two serovars.
Owner:NOVARTIS AG
Adeno-associated virus serotype 1 nucleic acid sequences, vectors and host cells containing same
The nucleic acid sequences of adeno-associated virus (AAV) serotype 1 are provided, as are vectors and host cells containing these sequences and functional fragments thereof. Also provided are methods of delivering genes via AAV-1 derived vectors.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
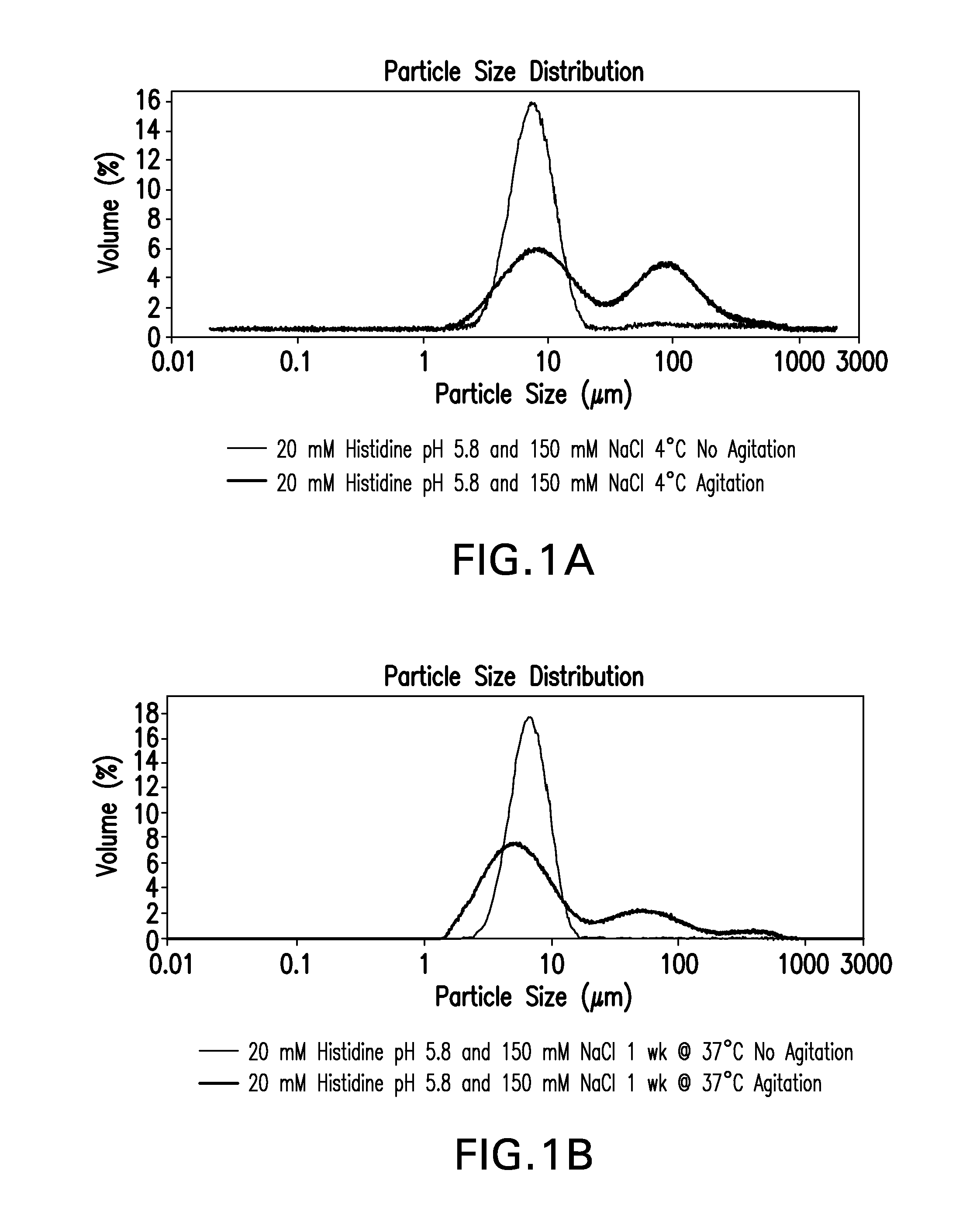
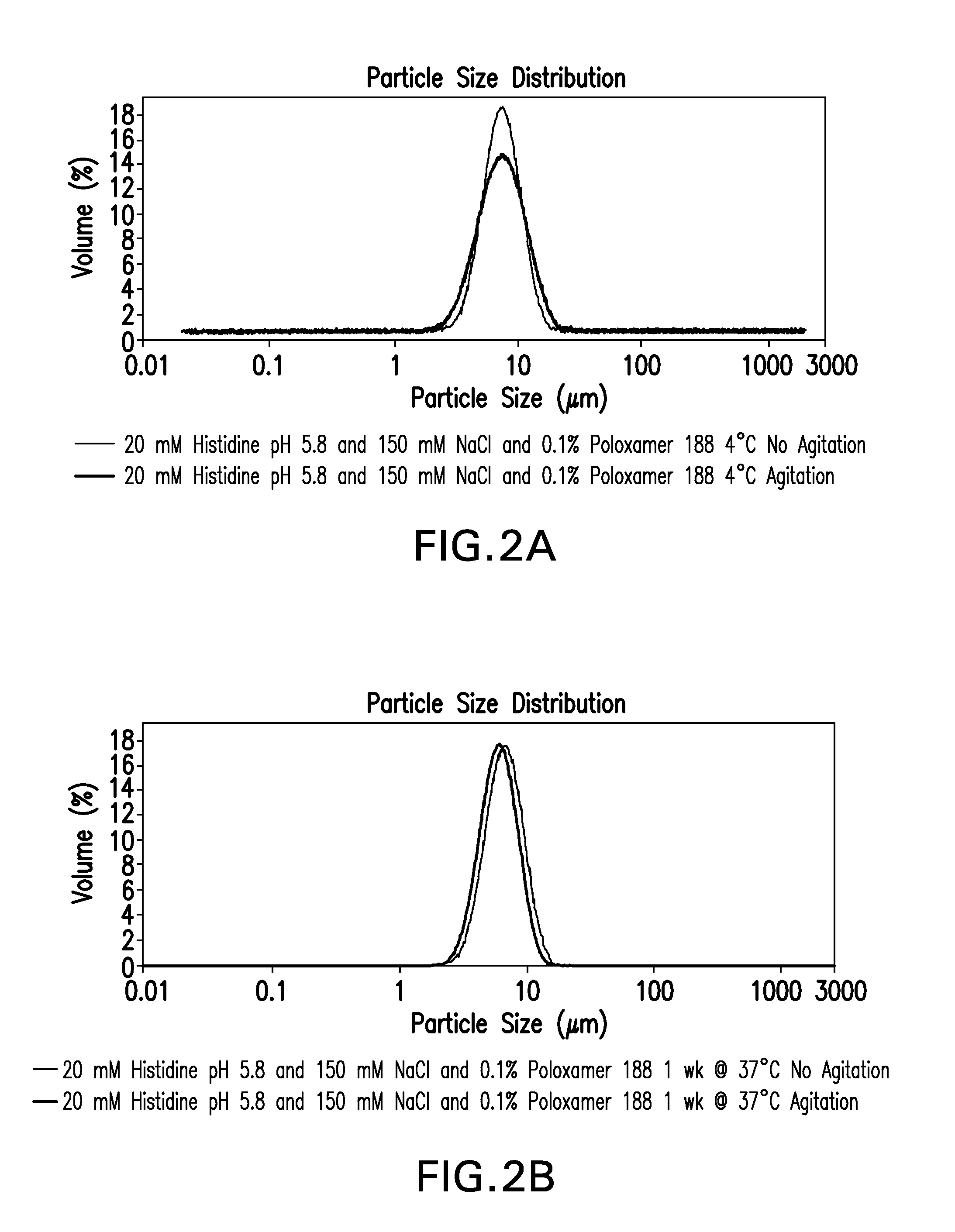
Novel formulations which mitigate agitation-induced aggregation of immunogenic compositions
InactiveUS20130273098A1Stabilize immunogenic compositionInhibit aggregationAntibacterial agentsBiocideStreptococcus pneumoniaeCarrier protein
The present invention provides novel formulations which mitigate agitation-induced aggregation of immunogenic compositions particularly those having polysaccharide-protein conjugates. Specifically, the novel formulations comprise a poloxamer within a molecular weight range of 1100 to 17,400 which provides significant advantages over previously used surfactants including polysorbate 80. In one embodiment, the present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates and a poloxamer. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197.
Owner:MERCK SHARP & DOHME CORP
Peptide vaccines against group A streptococci
Owner:HEALTH & HUMAN SERVICES DEPT OF THE GOVERNMENT OF THE UNITED STATES OF AS REPRESENTED BY THE SEC
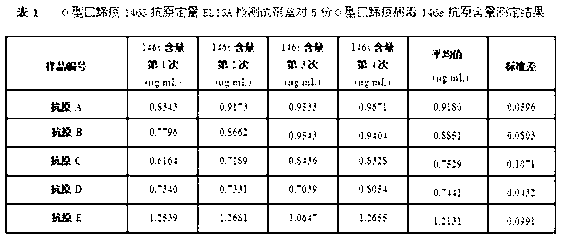
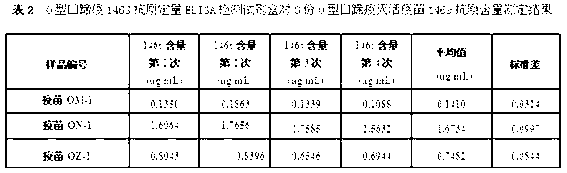
O type foot-and-mouth disease 146S antigen quantitative ELISA detection kit and method for using same
ActiveCN103076451ASolve efficiency problemsSolving the power test substitution problemMaterial analysisDiseaseVaccine Potency
The invention discloses an O type foot-and-mouth disease 146S antigen quantitative ELISA (enzyme-linked immuno sorbent assay) detection kit and a method for using the same. The kit comprises an ELISA plate, an O type foot-and-mouth disease standard reference antigen, a demulsifier, an O type foot-and-mouth disease rabbit antiserum, an O type foot-and-mouth disease guinea pig antiserum, a rabbit anti-guinea pig-horse radish peroxidase conjugate, a guinea pig antiserum dilute solution, a 25-fold PBST (phosphate buffer solution tween) concentrated solution, a carbonate buffer solution capsule, a citric acid-phosphate buffer solution tablet, an OPD (o-phenylenediamine) tablet, a stop solution, a plate sealing membrane, a moving liquid tank and a 96-mesh U-shaped dilution plate. The kit is an organic combination of a sucrose density gradient centrifugation method and an indirect sandwich ELISA method, integrates the advantages of the sucrose density gradient centrifugation method and the indirect sandwich ELISA method, is simple to operate and good in stability, is suitable for batch detection, can be used for distinguishing serum types, and is an ideal substitution method for antigen quantitative and vaccine efficacy detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Conserved and specific streptococcal genomes
InactiveUS20070053924A1Antibacterial agentsBacterial antigen ingredientsStreptococcus pyogenesCoccidia
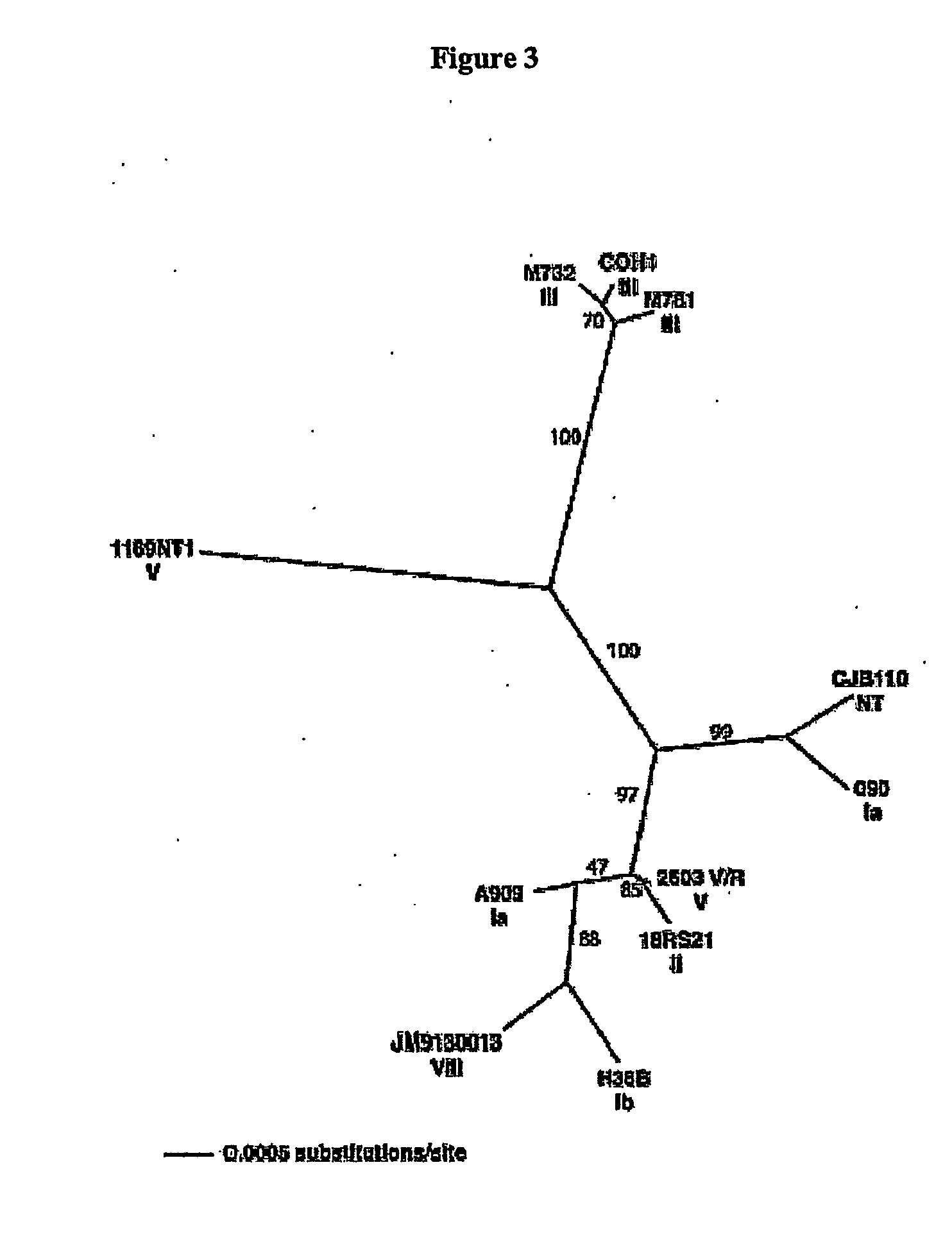
The invention relates to polynucleotides which are conserved or specific to one or more species of Streptococcus, Streptococcus species serotypes, and / or serotype isolates. In particular, the invention relates to polynucleotides from Streptococcus which are conserved or specific to one or more of the species of S. pneumoniae (“pneumococcus” or “S. pn.”), S. pyogenes (“group A streptococcus” or “GAS”), and S. agalactiae (“group B streptococcus” or “GBS”). The invention further relates to polynucleotides which are conserved or specific to one or more Streptococcal species serotypes, such as GBS serotypes Ia, Ib, II, III, IV, V, VI, VII, and VIII. The invention still further relates to polynucleotides which are conserved or specific to one or more clinical isolates of a Streptococcus species.
Owner:TETTELIN HERVE +1
Modified adenoviral vectors and methods of treatment using same
InactiveUS20140348791A1Improve abilitiesLow immunogenicityBiocideGenetic material ingredientsDiseaseFiber
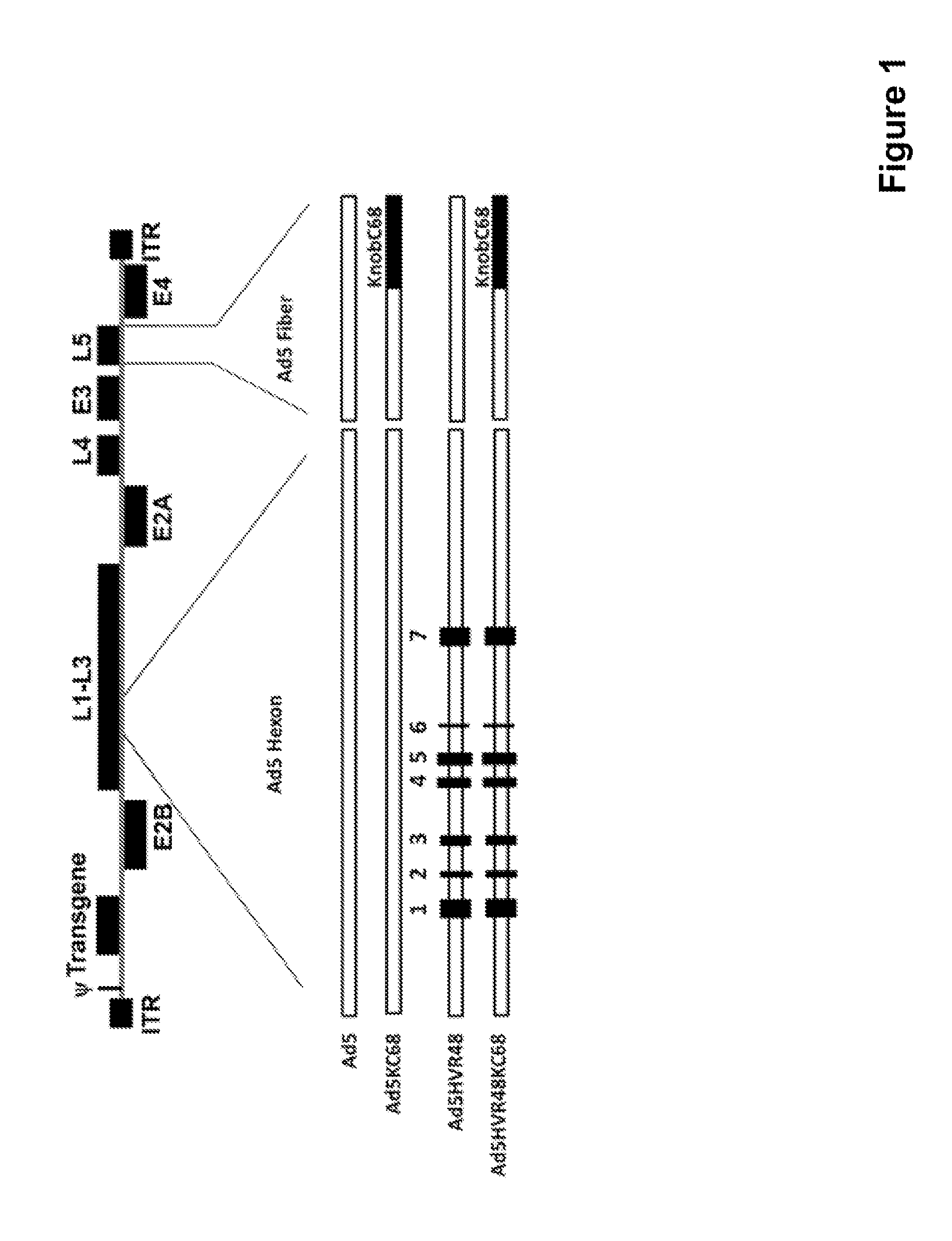
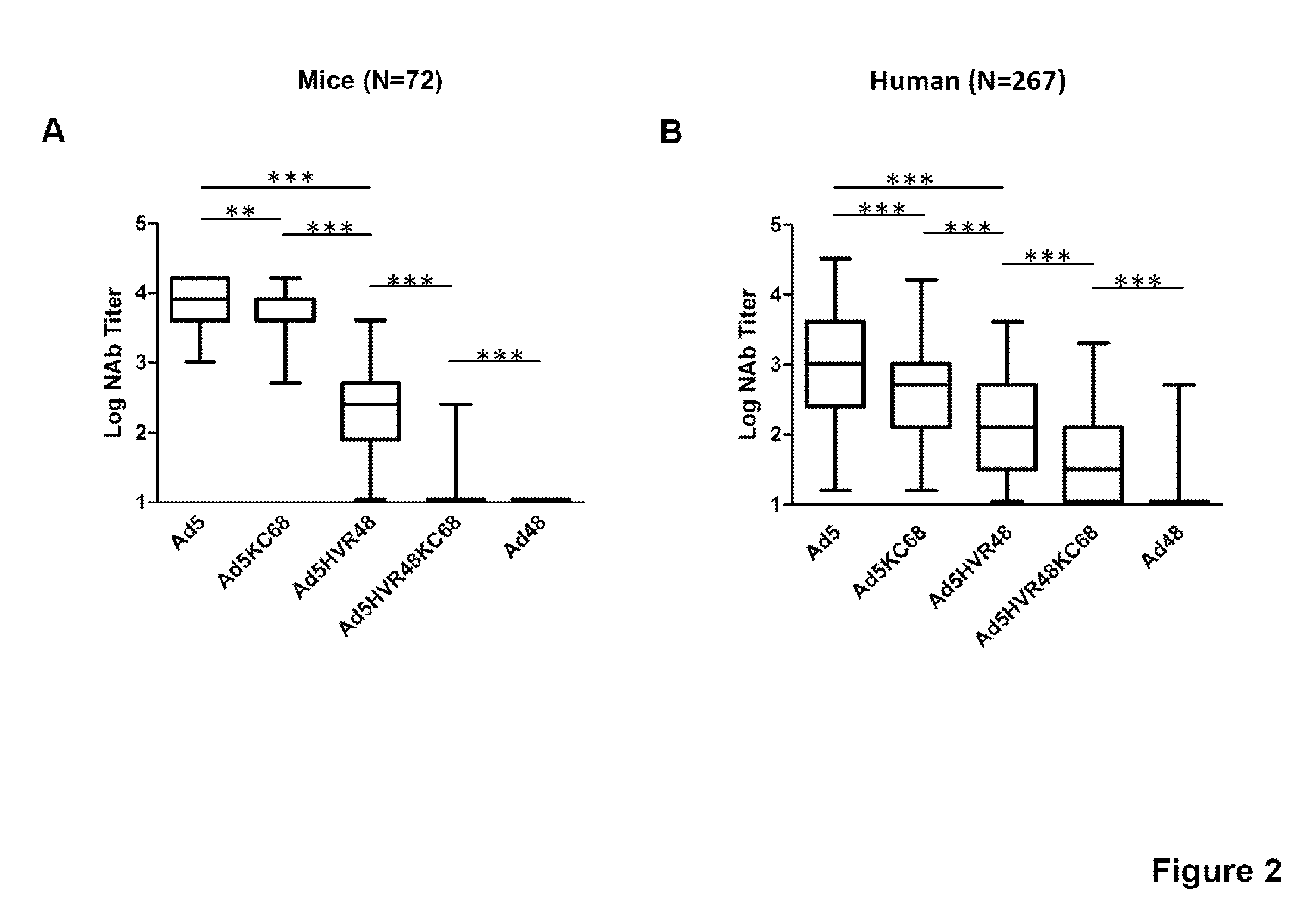
The present invention relates to recombinant adenovirus serotype 5 (Ad5) vectors which harbor chimeric capsid proteins including substitutions of the corresponding regions from adenovirus serotypes having a lower seroprevalence relative to Ad5. In particular, the chimeric capsid includes modifications of both the adenoviral hexon and fiber proteins. The invention also provides methods for the treatment of diseases or disorders caused by infective agent(s) by administering the adenoviral vector(s) to a subject (e.g., a mammal, such as a human).
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Glycan arrays for high throughput screening of viruses
ActiveUS20150160217A1Improve signal-to-noise ratioThe process is simple and fastSugar derivativesMicrobiological testing/measurementHemagglutininHigh-Throughput Screening Methods
Glycan arrays that can detect and distinguish between various sub-types and strains of influenza virus are provided. Methods for using the glycan arrays with assays using nanoparticle amplification technique are disclosed. Sandwich assays using gold nanoparticles conjugated to phage particles comprising influenza virus-specific antibodies for detecting multiple serotypes using a single reaction are provided. Plurality of glycans directed to specific target HA of influenza virus comprises the array. Detector molecules comprising noble metals conjugated to (a) phage display particles expressing antibodies against hemagglutinin and (b) neuraminidase binding agents are disclosed.
Owner:ACAD SINIC
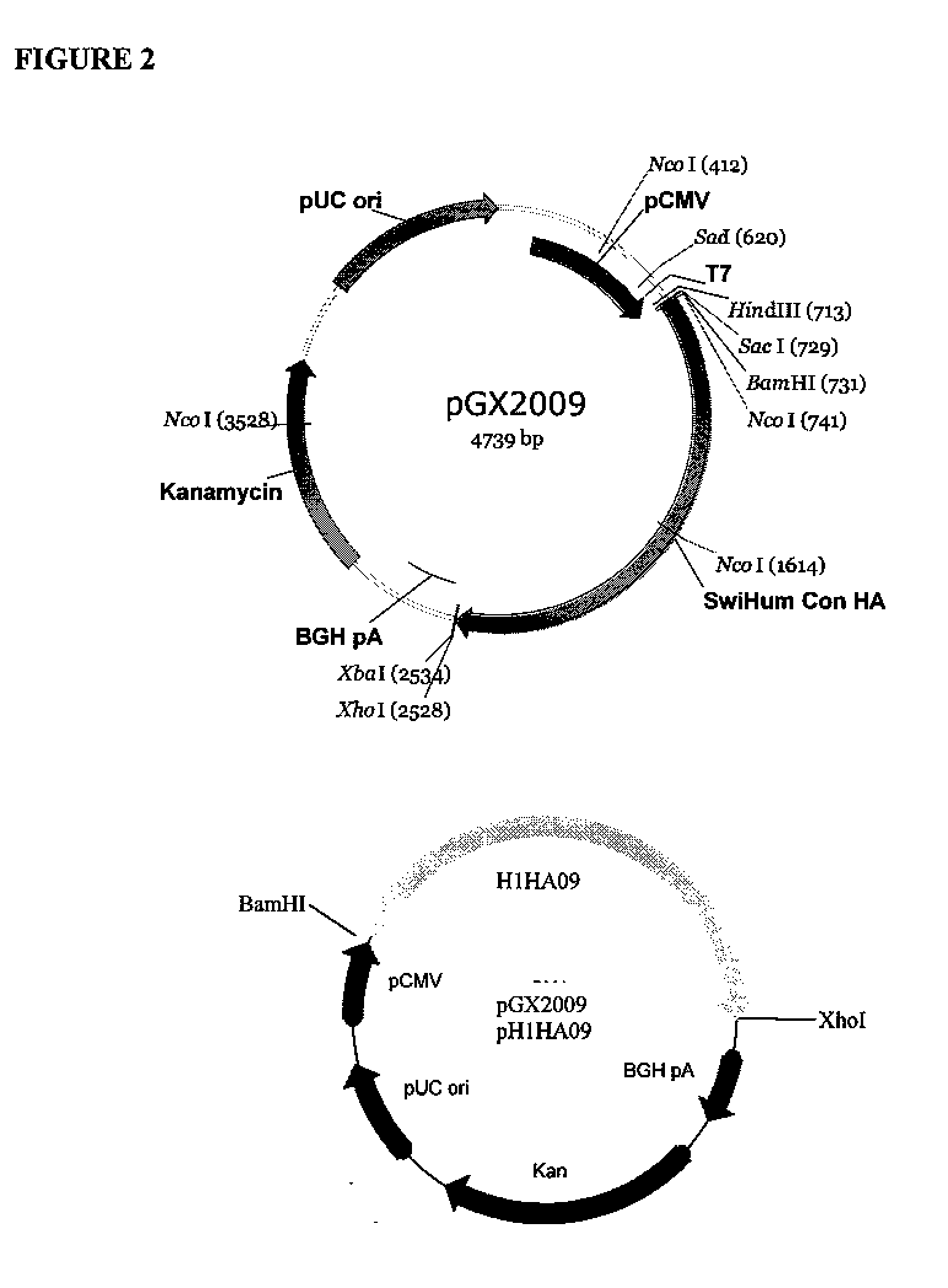
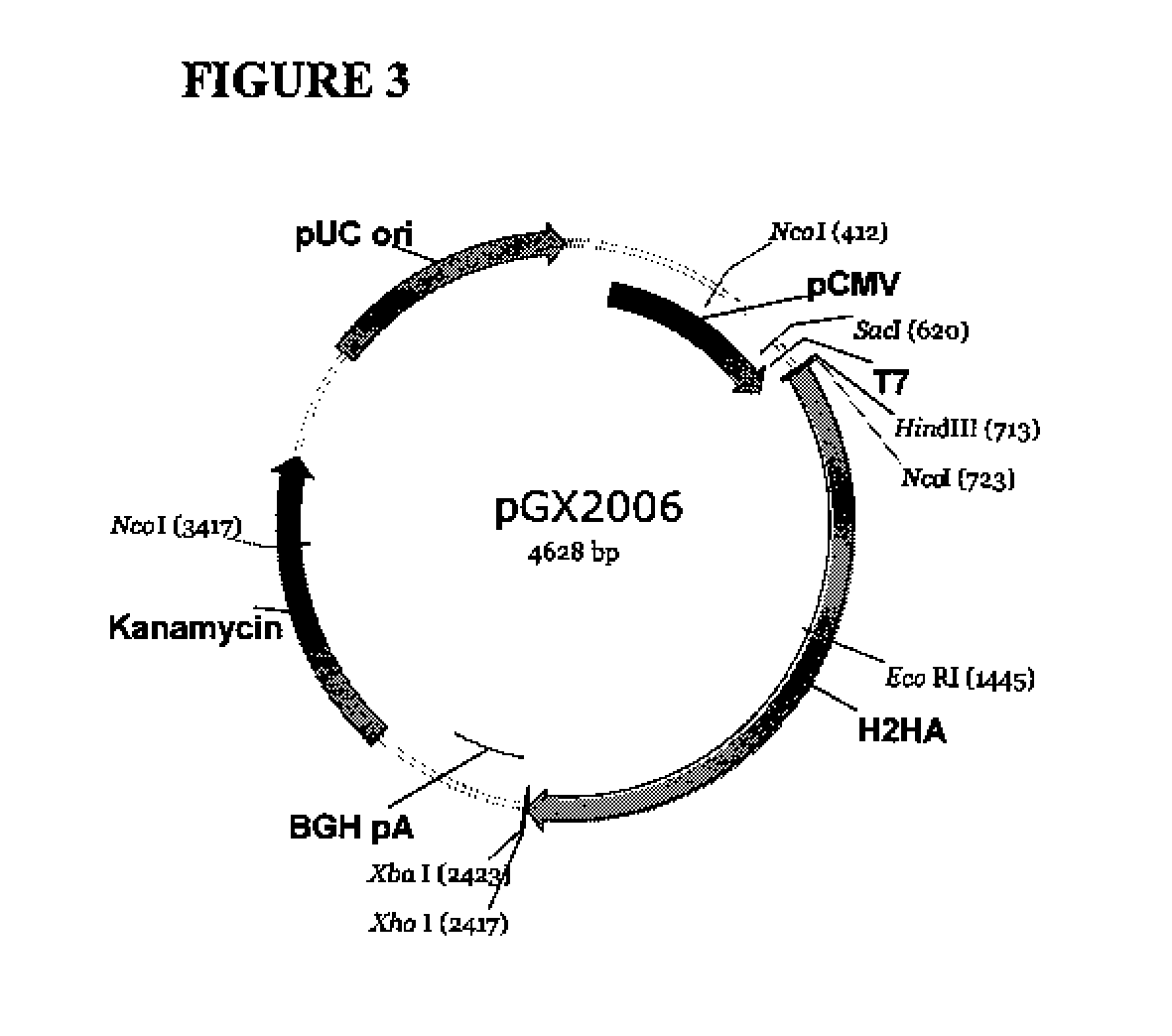
Influenza nucleic acid molecules and vaccines made therefrom
ActiveUS20110182938A1SsRNA viruses negative-senseOrganic active ingredientsHemagglutininInfluenza A antigen
Provided herein are nucleic acid sequences that encode novel consensus amino acid sequences of HA hemagglutinin, as well as genetic constructs / vectors and vaccines expressing the sequences. Also provided herein are methods for generating an immune response against one or more Influenza A serotypes using the vaccines that are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Aav vector compositions and methods for gene transfer to cells, organs and tissues
InactiveUS20150111955A1Reduce the possibilityCost of treatmentGenetic material ingredientsFermentationTherapeutic proteinSerotype
The invention relates to adeno-associated virus (AAV) serotype AAV-Rh74 and related AAV vectors, and AAV-Rh74 and related AAV vector mediated gene transfer methods and uses. In particular, AAV-Rh74 targets polynucleotides to cells, tissues or organs for expression (transcription) of genes encoding therapeutic proteins and peptides, and polynucleotides that function as or are transcribed into inhibitory nucleic acid sequences.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Vaccines for broad spectrum protection against diseases caused by Neisseria meningitidis
InactiveUS20060029621A1Wide applicabilityAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiSerotype
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Streptococcus suis vaccines and diagnostic tests
InactiveUS7125548B2ImmunogenicityImproving immunogenicityAntibacterial agentsBacteriaAntigenSpecific detection
The invention relates to Streptococcus suis infection in pigs, vaccines directed against those infections and tests for diagnosing Streptococcus suis infections. The invention provides an isolated or recombinant nucleic acid encoding a capsular gene cluster of Streptococcus suis or a gene or gene fragment derivated thereof. The invention further provides a nucleic acid probe or primer allowing species or serotype-specific detection of Streptococcus suis. The invention also provides a Streptococcus suis antigen and vaccine derived thereof.
Owner:STICHTING DIENST LANBOUWKUNDIG ONDERZOEK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com