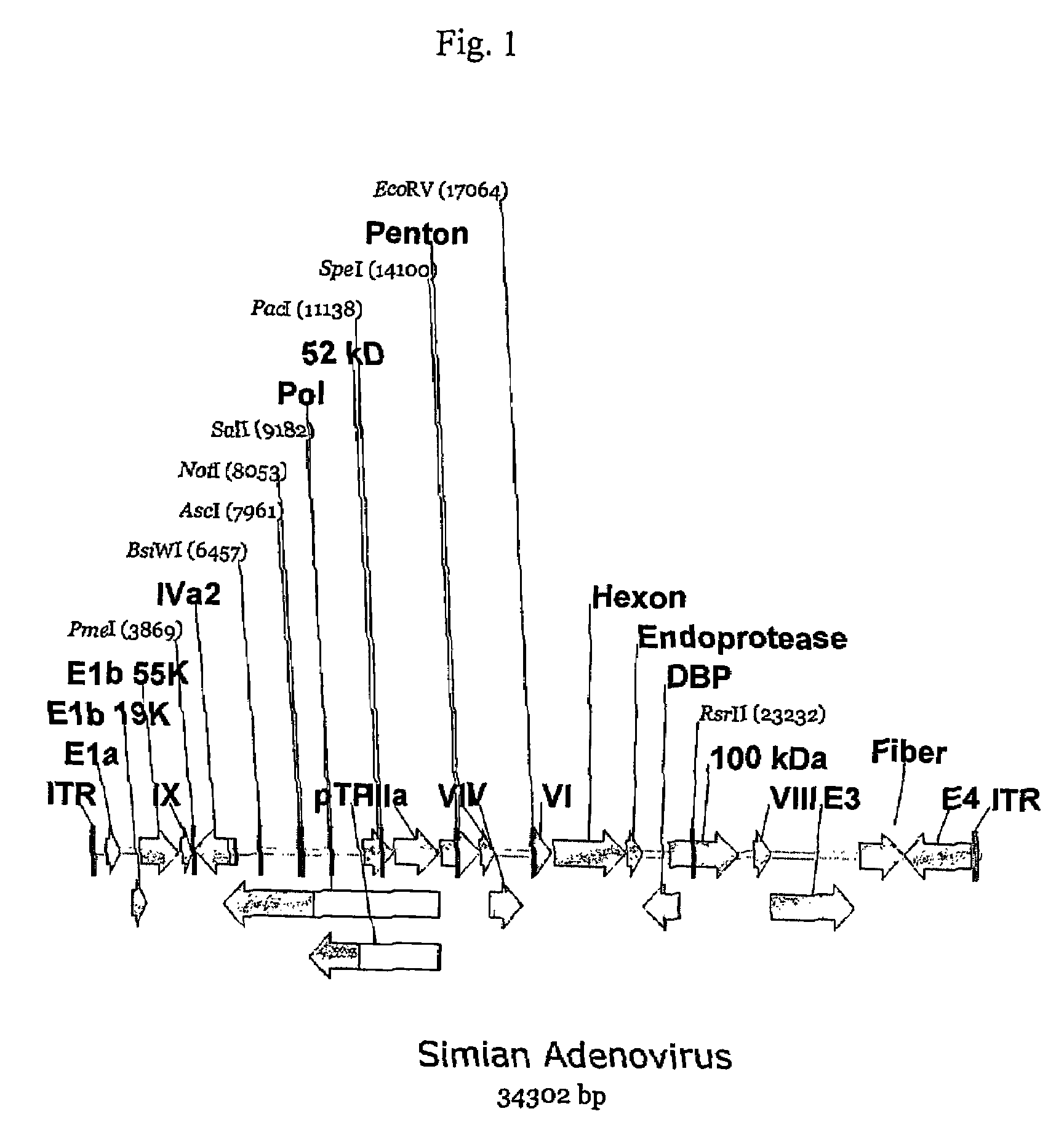
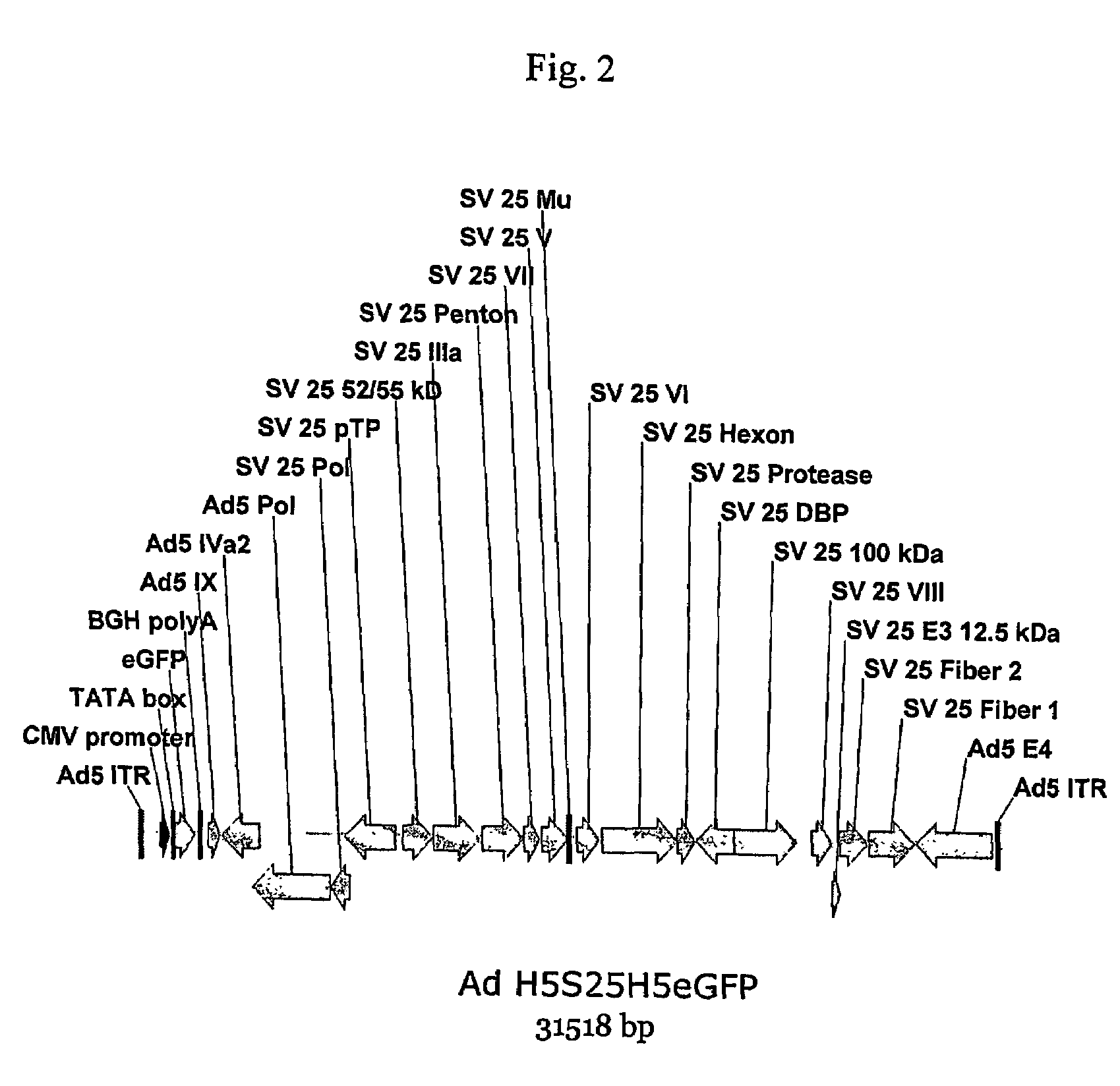
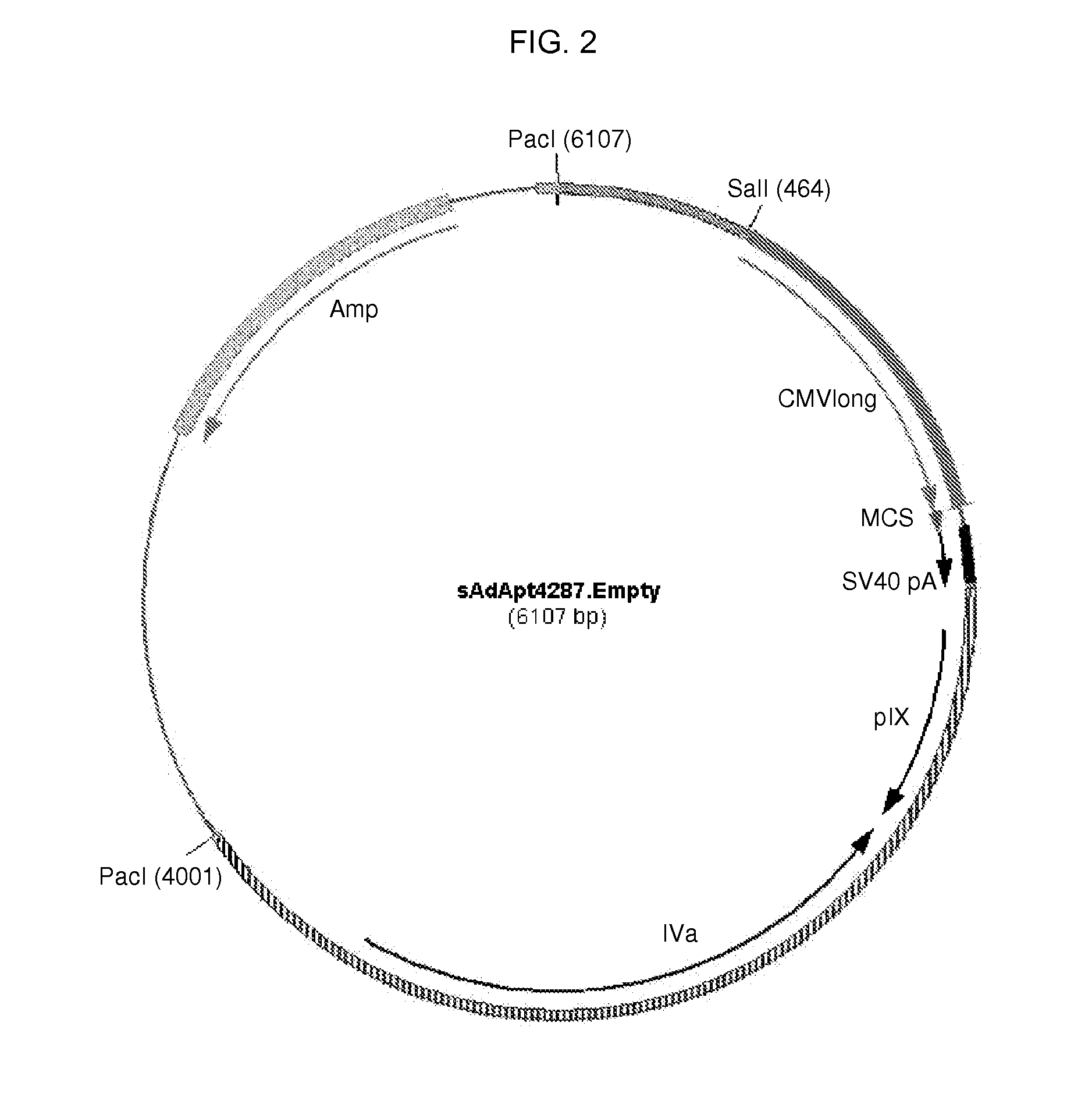
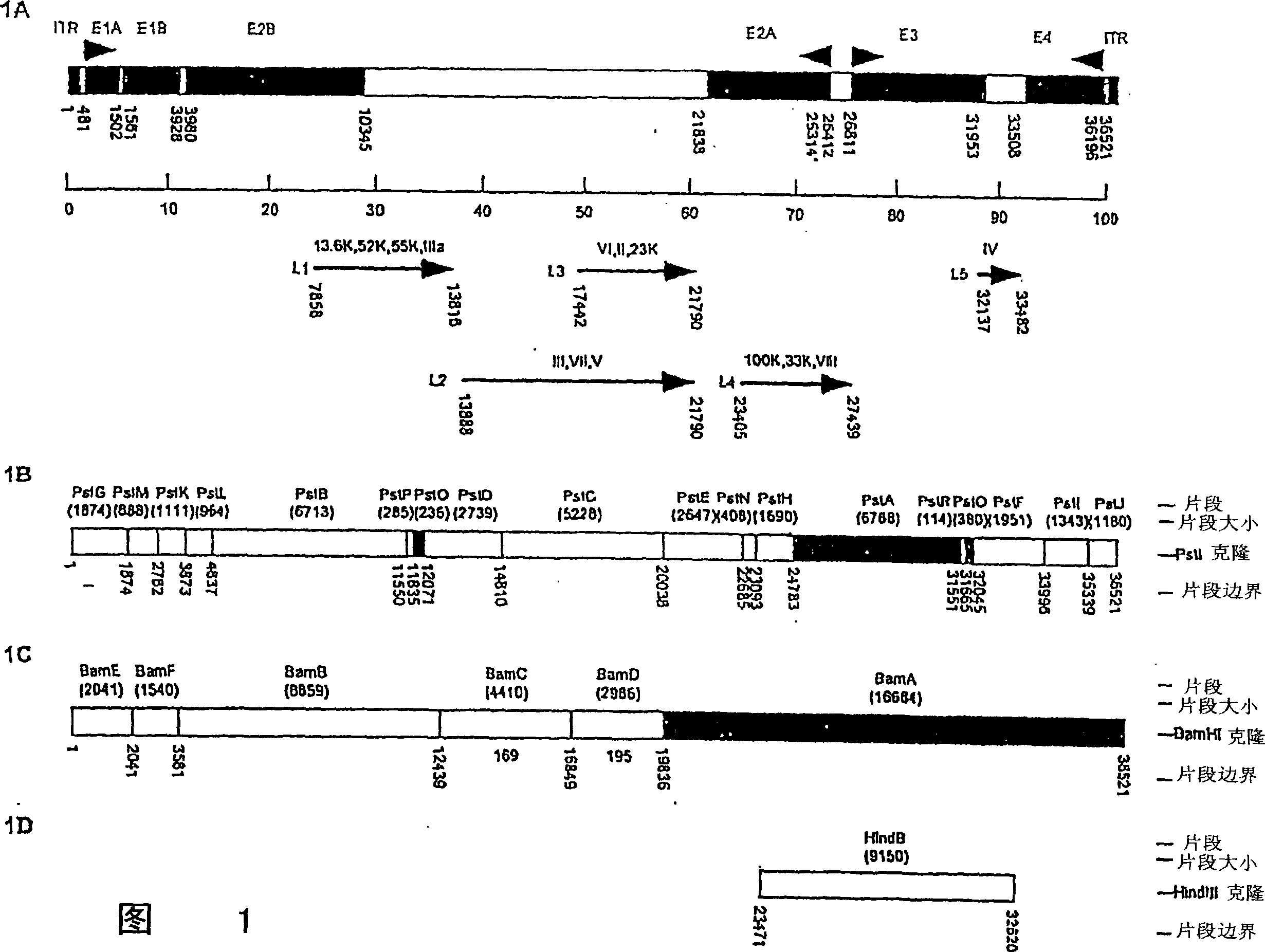
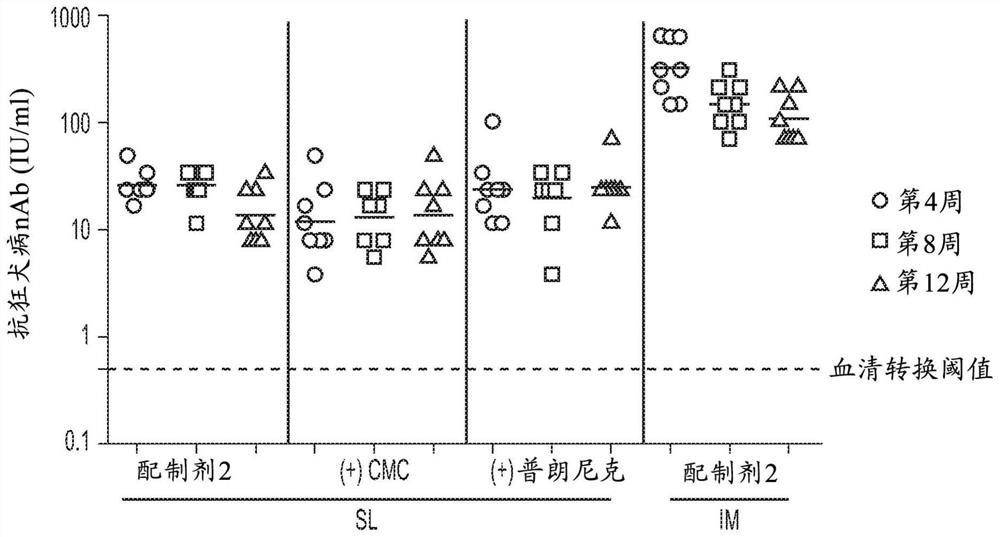
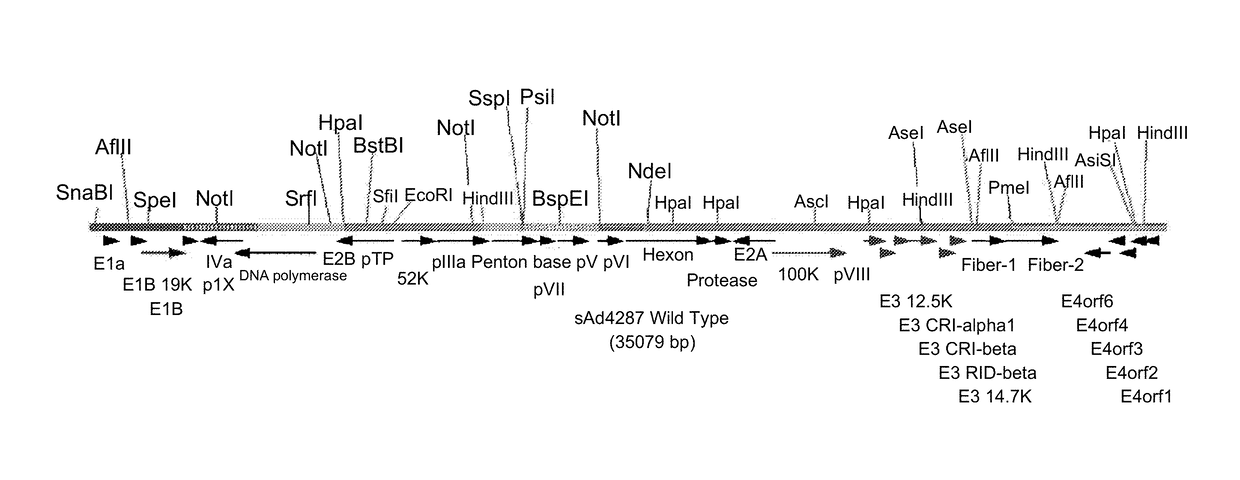
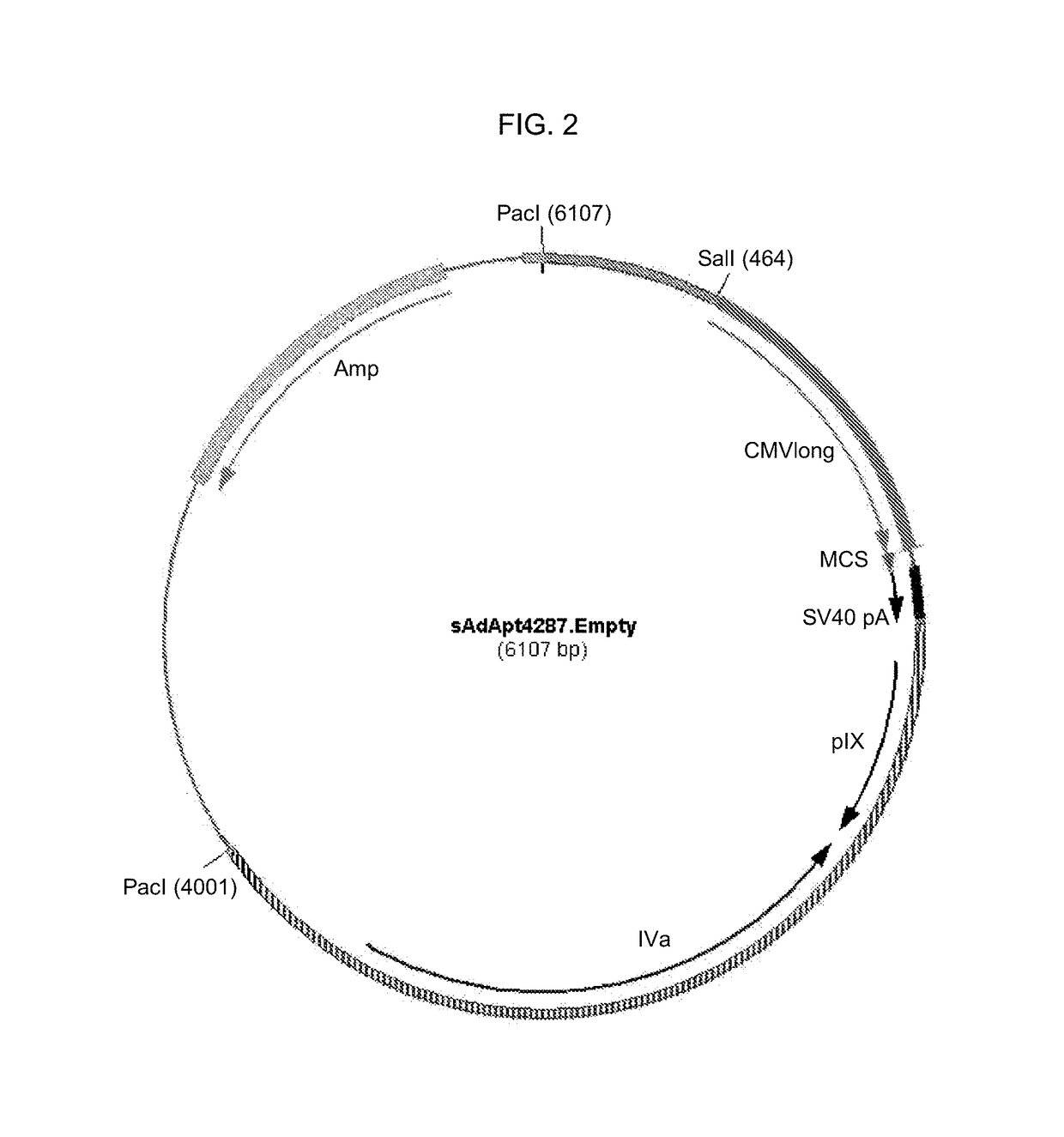
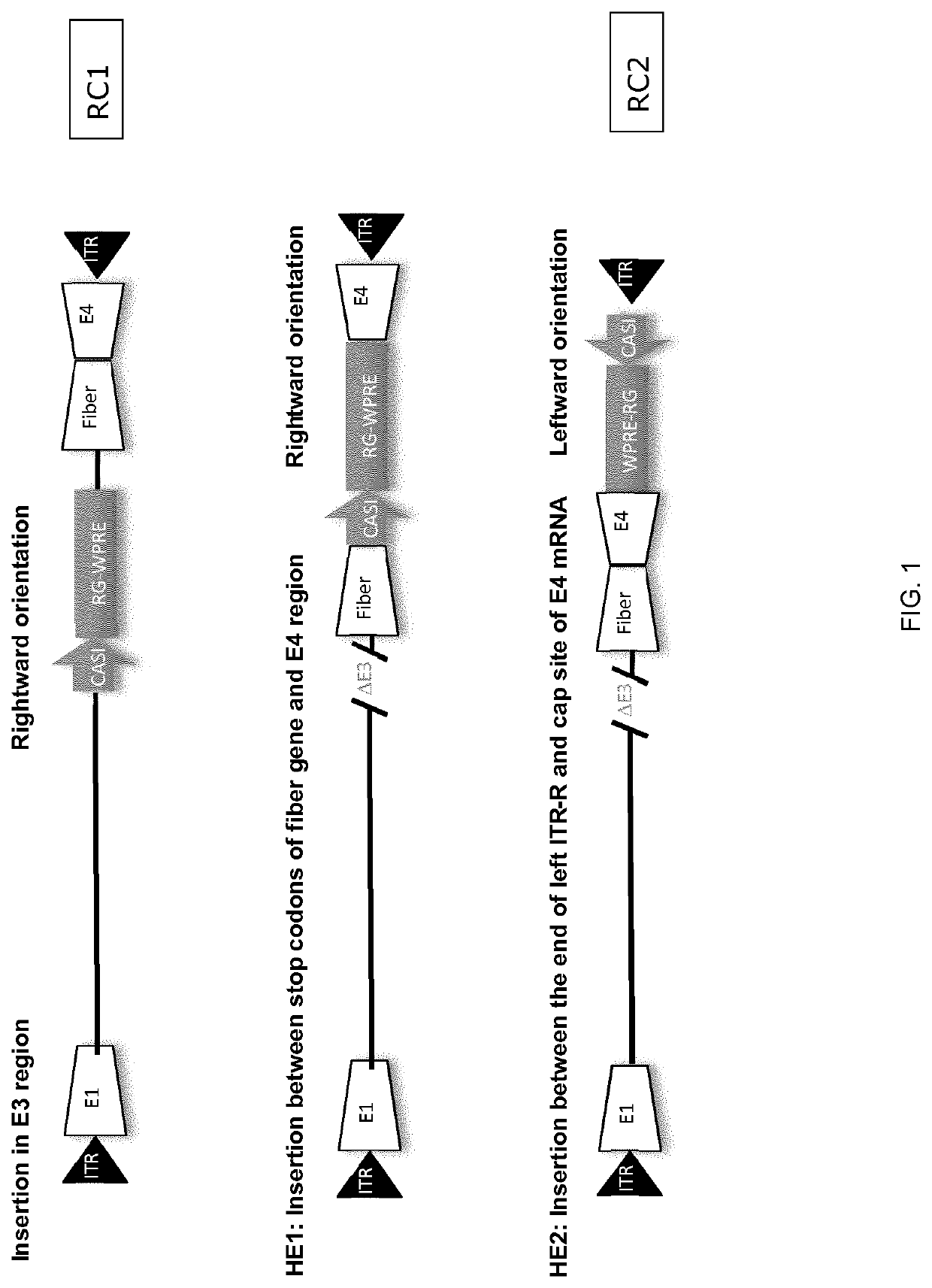
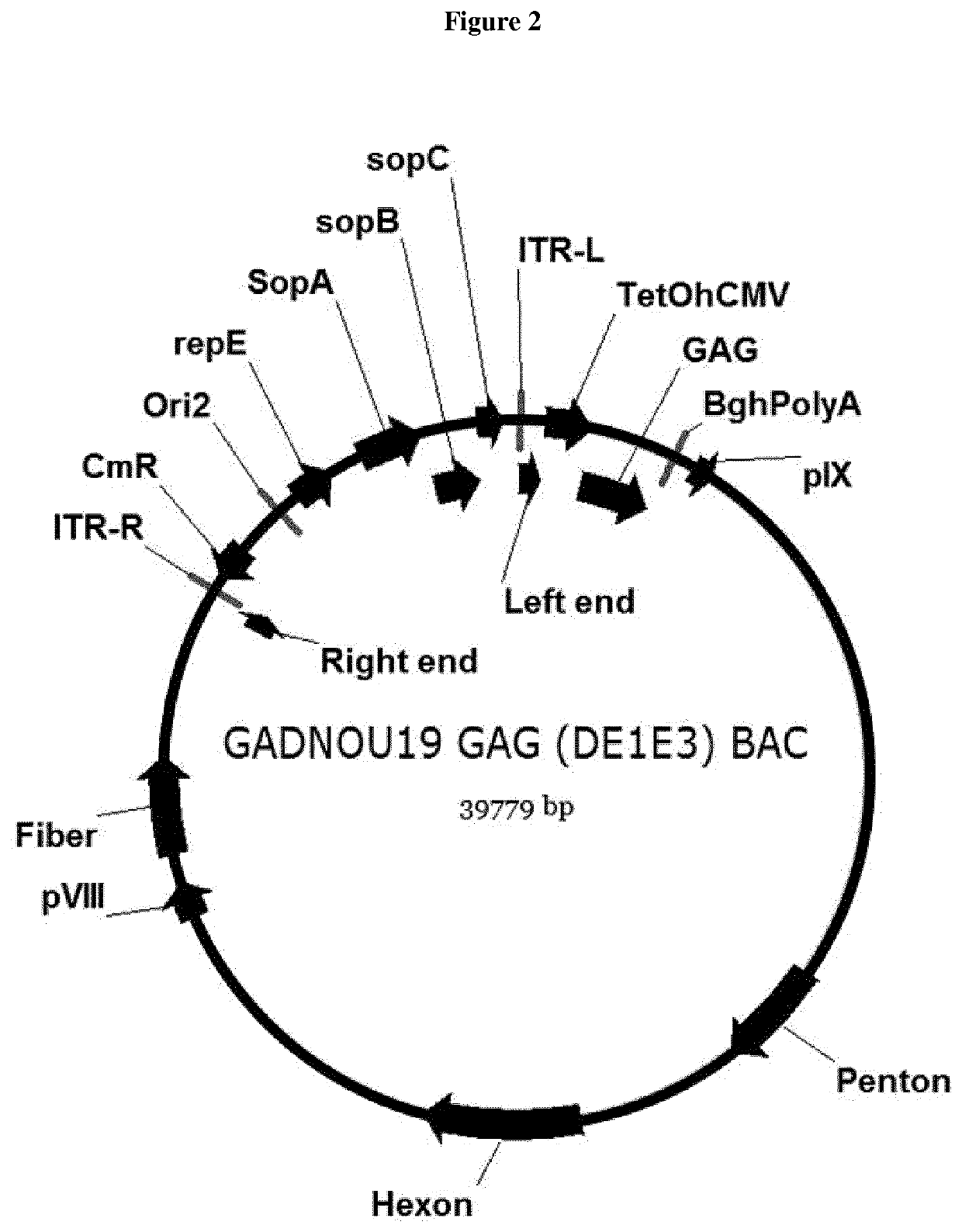
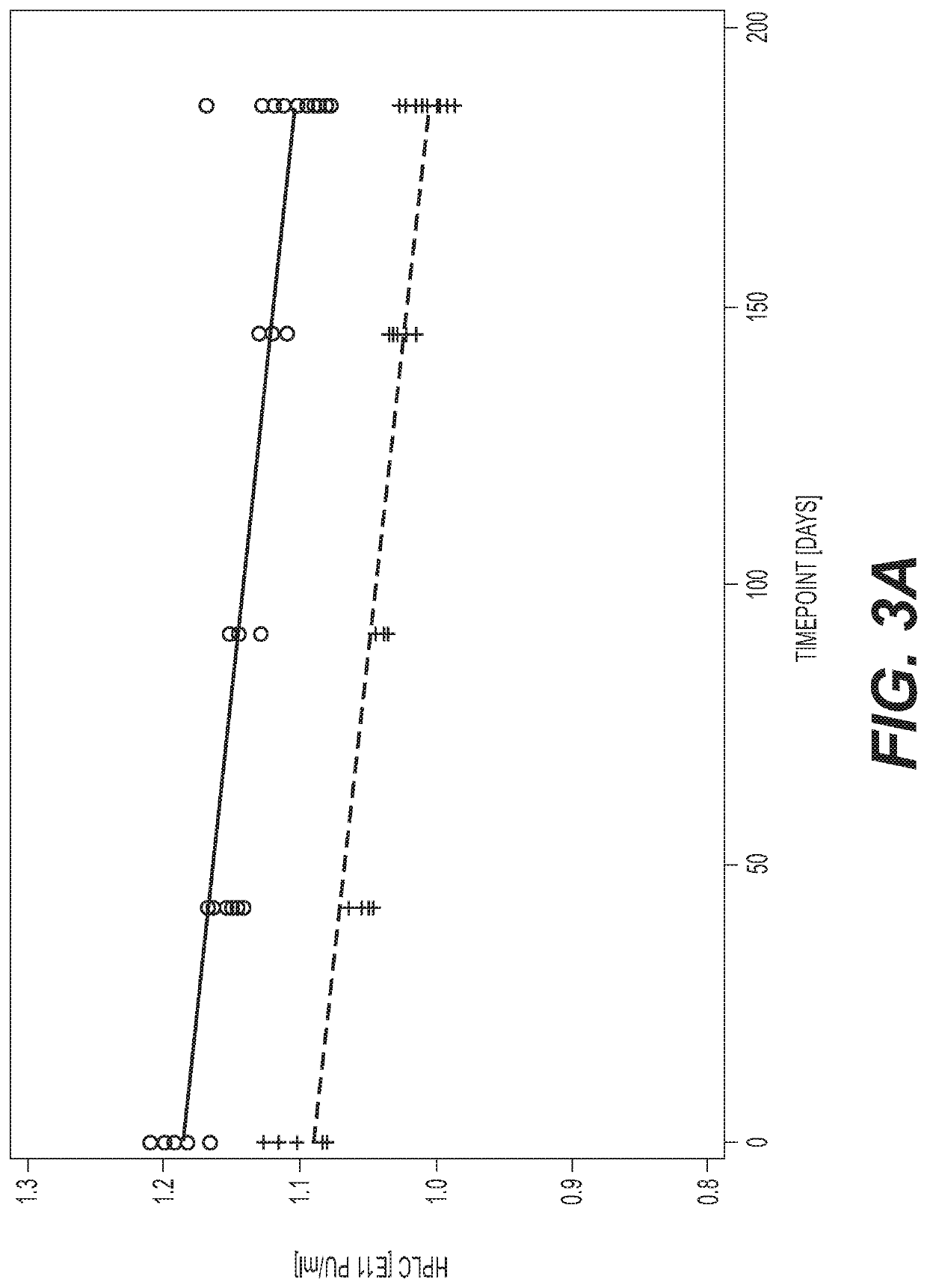
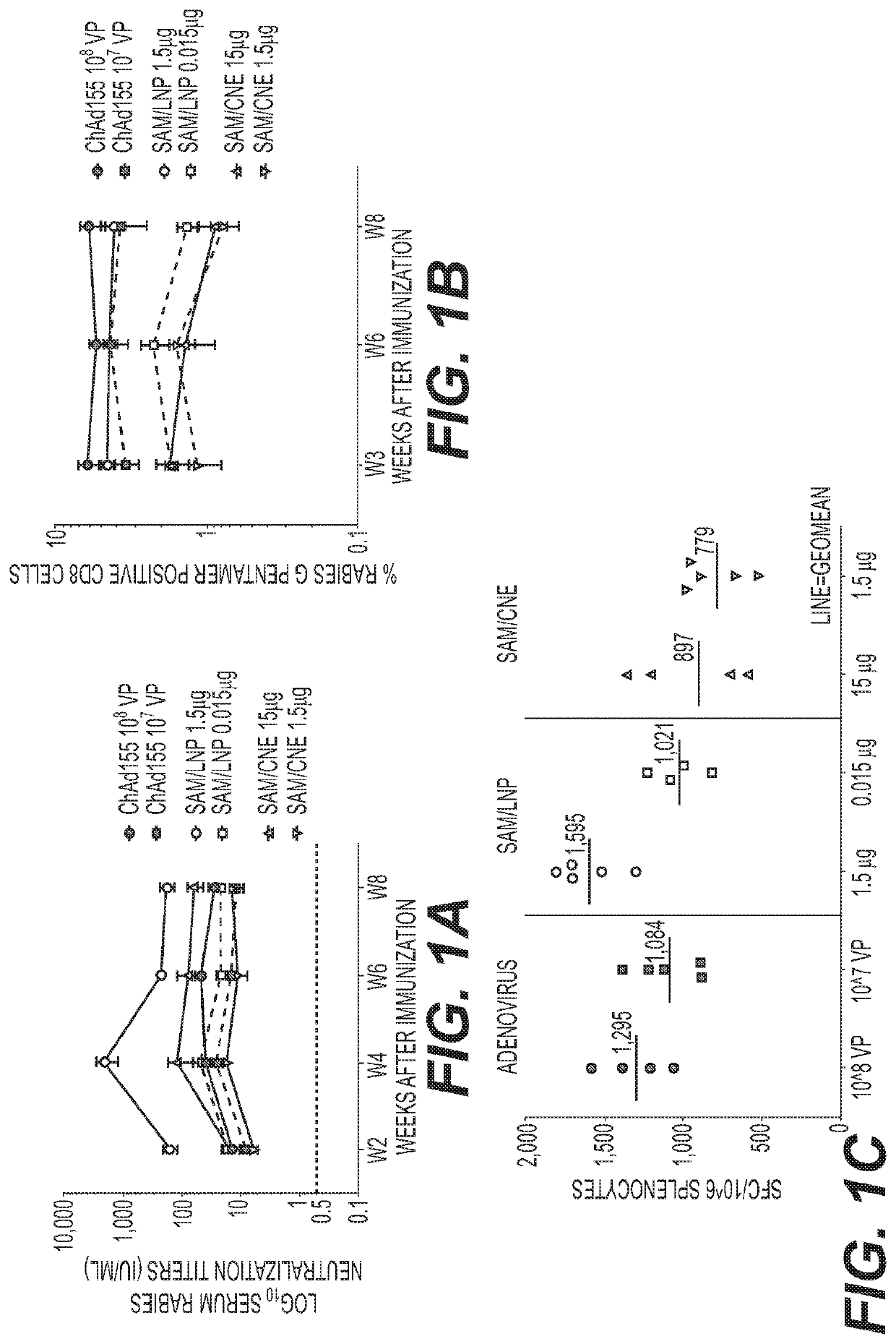
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Simian Adenoviruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
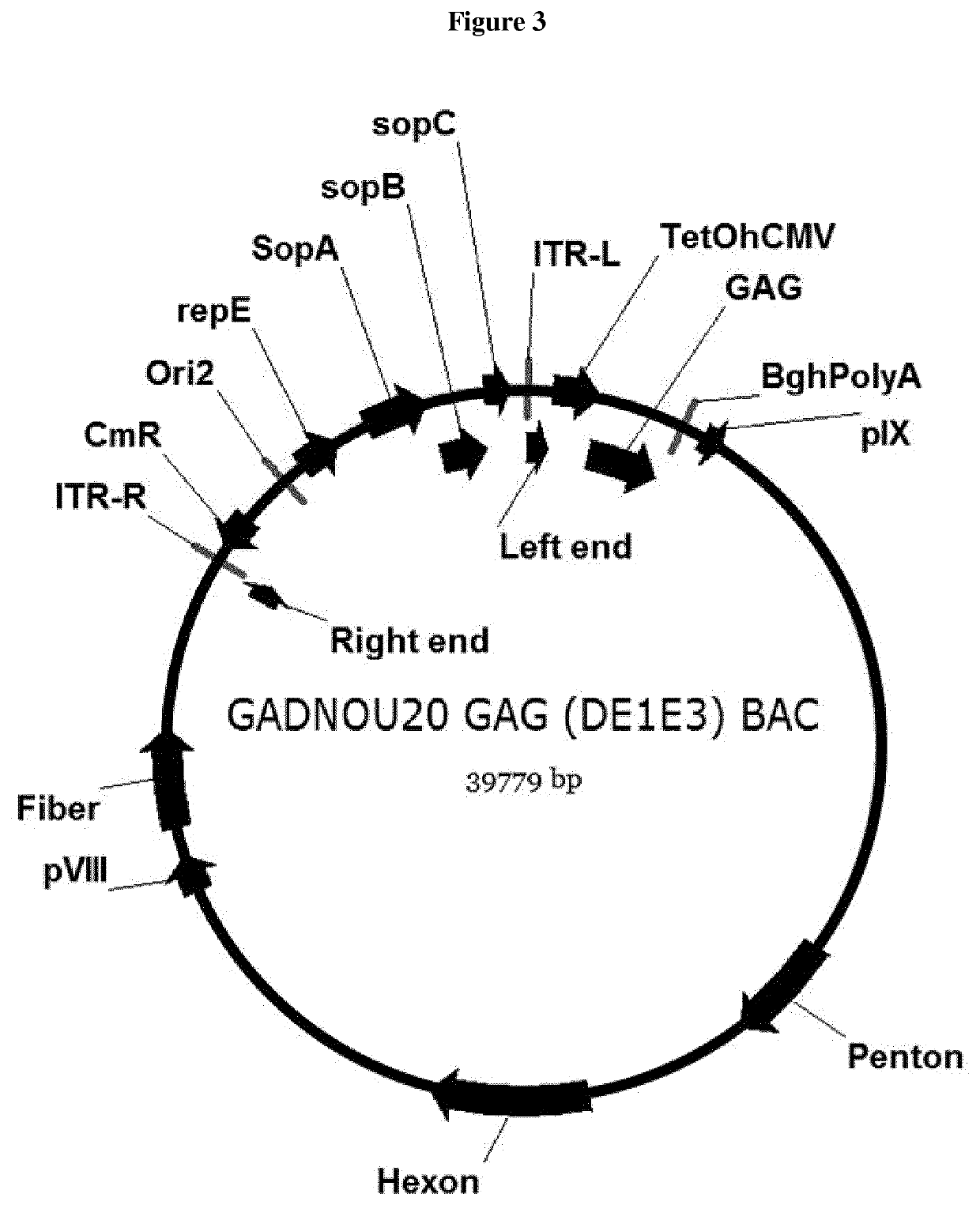
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the (5′) inverted terminal repeat, the left terminus spans the E4 region and the (3′) inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant adenoviruses and use thereof
ActiveUS20150291935A1Diminishment of extentAvoid spreadingAntibacterial agentsAntimycoticsSimian AdenovirusesDisease
The present invention relates to recombinant adenoviruses and vectors thereof. In particular, the adenoviruses are novel simian adenoviruses having a low seroprevalence and high immunogenicity relative to other adenoviruses and vectors thereof. The invention also provides methods for production of the adenoviruses and for the treatment of diseases by administering the adenoviral vector(s) to a subject (e.g., a human).
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Methods of generating chimeric adenoviruses and uses for such chimeric aden oviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the (5′) inverted terminal repeat, the left terminus spans the E4 region and the (3′) inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
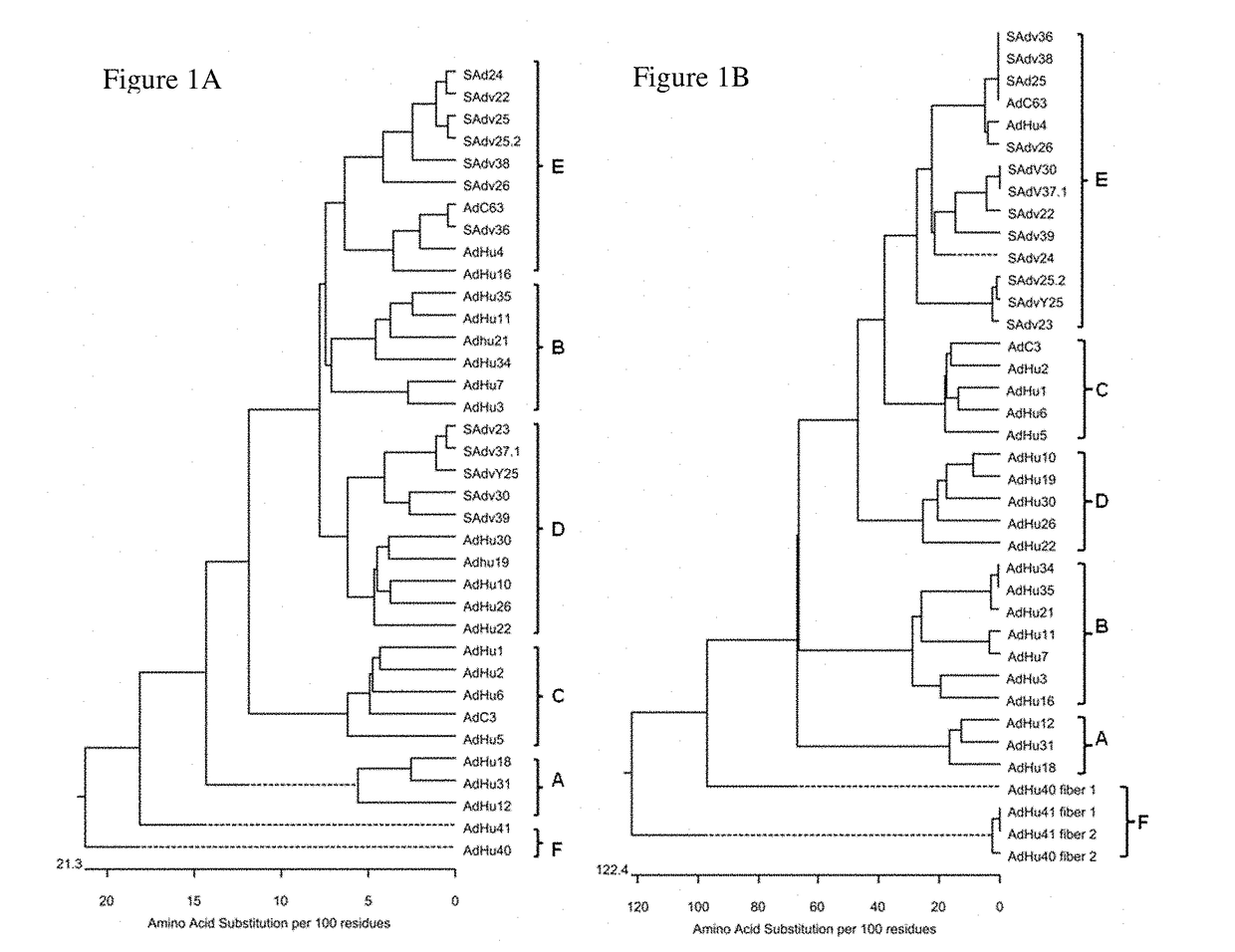
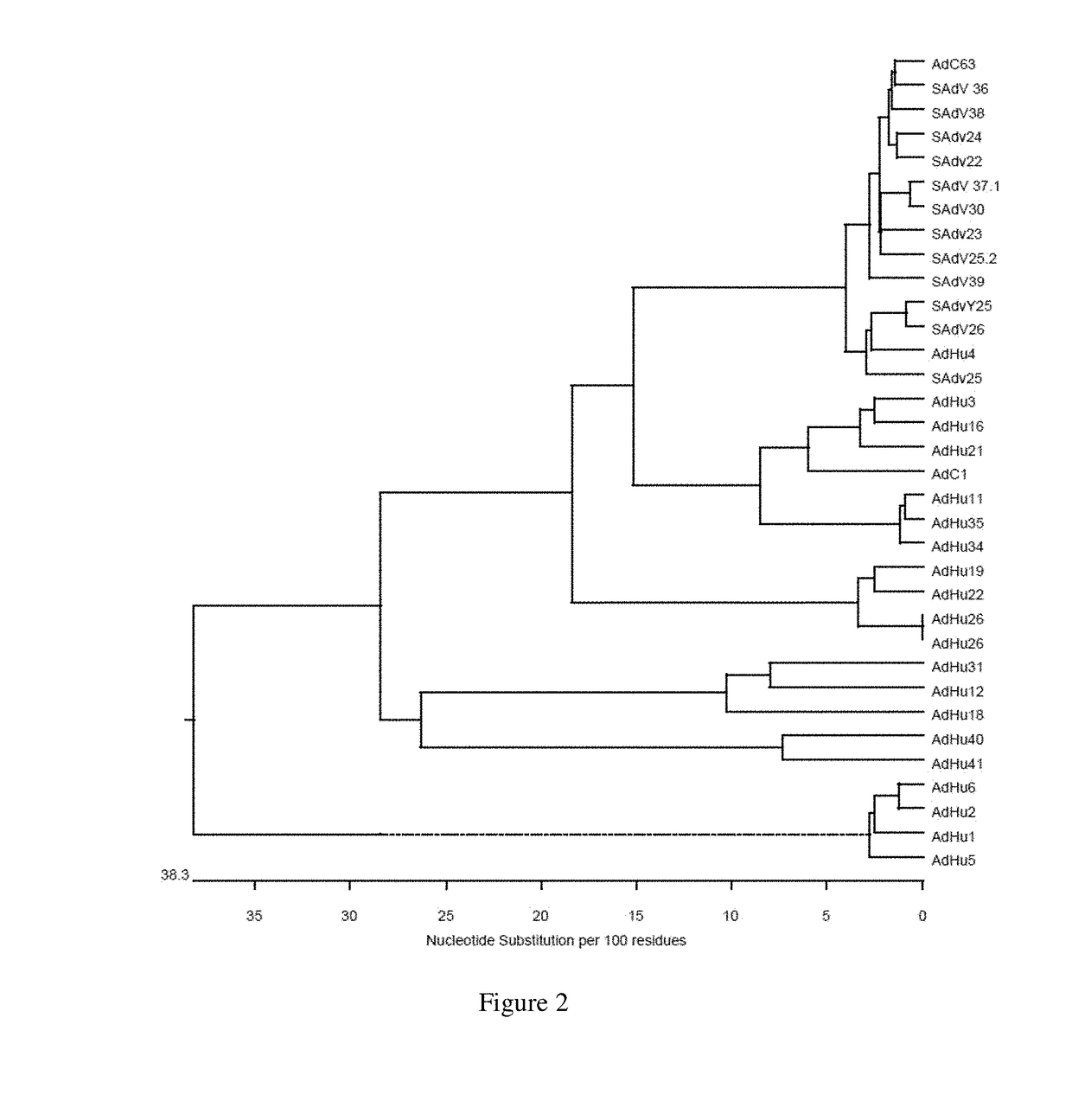
Simian adenovirus nucleic acid- and amino acid-sequences, vectors containing same, and uses thereof
ActiveUS20120027788A1Low cross-reactivityReduce adverse effectsFungiVirusesDiseaseSimian Adenoviruses
The present invention relates to novel adenovirus strains with an improved sero-prevalence. In one aspect, the present invention relates to isolated polypeptides of adenoviral capsid proteins such as hexon, penton and fiber protein and fragments thereof and polynucleotides encoding the same. Also provided is a vector comprising the isolated polynucleotide according to the invention and adenoviruses comprising the isolated polynucleotides or polypeptides according to the invention and a pharmaceutical composition comprising said vector, adenovirus, polypeptide and / or polynucleotide. The invention also relates to the use of the isolated polynucleotides, the isolated polypeptides, the vector, the adenoviruses and / or the pharmaceutical composition for the therapy or prophylaxis of a disease.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Simian adenovirus nucleic acid- and amino acid-sequences, vectors containing same, and uses thereof
ActiveUS9718863B2Low cross-reactivityReduce adverse effectsVirusesBacteriaSimian AdenovirusesDisease
The present invention relates to novel adenovirus strains with an improved seroprevalence. In one aspect, the present invention relates to isolated polypeptides of adenoviral capsid proteins such as hexon, penton and fiber protein and fragments thereof and polynucleotides encoding the same. Also provided is a vector comprising the isolated polynucleotide according to the invention and adenoviruses comprising the isolated polynucleotides or polypeptides according to the invention and a pharmaceutical composition comprising said vector, adenovirus, polypeptide and / or polynucleotide. The invention also relates to the use of the isolated polynucleotides, the isolated polypeptides, the vector, the adenoviruses and / or the pharmaceutical composition for the therapy or prophylaxis of a disease.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Simian adenovirus nucleic acid- and amino acid-sequences, vectors containing same, and uses thereof
The present invention relates to novel adenovirus strains with an improved seroprevalence in human. In one aspect, the present invention relates to isolated polypeptides of adenoviral capsid proteins such as hexon, penton and fiber protein and fragments thereof and polynucleotides encoding the same. Also provided is a vector comprising the isolated polynucleotide according to the invention and adenoviruses comprising the isolated polynucleotides or polypeptides according to the invention and a pharmaceutical composition comprising said vector, adenovirus, polypeptide and / or polynucleotide. The invention also relates to the use of the isolated polynucleotides, the isolated polypeptides, the vector, the adenoviruses and / or the pharmaceutical composition for the therapy or prophylaxis of a disease.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
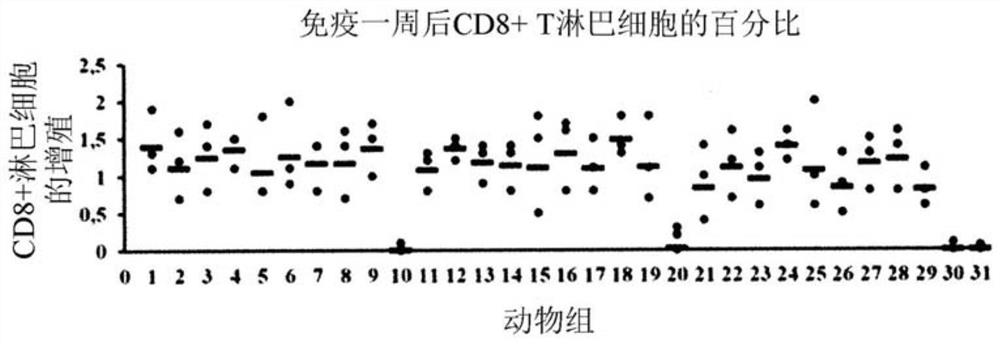
Methods of inducing cytotoxic immune response and recombinant simian ademovirus compositions useful therein
InactiveCN1518457ASsRNA viruses negative-senseGenetic material ingredientsSimian AdenovirusesImmunodeficiency virus
The present invention provides a method of inducing a CD8+ T cell response against a selected molecule by delivering the molecule via a recombinant simian adenovirus. The present invention also provides a method for inducing alpha interferon and beta interferon by delivering a recombinant simian adenovirus to a subject. The methods and compositions of the invention are particularly suitable for use in the prevention and treatment of human immunodeficiency virus, human papillomavirus infections and cancer treatment.
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY +1
Simian adenovirus and hybrid adenoviral vectors
Recombinant adenoviral vectors, immunogenic compositions thereof and their use in medicine, and methods for generating recombinant adenoviral vectors are provided. In particular, the an adenovirus vector having a capsid derived from chimpanzee adenovirus AdY25, wherein the capsid encapsidates a nucleic acid molecule comprising an exogeneous nucleotide sequence of interest are provided.
Owner:OXFORD UNIV INNOVATION LTD
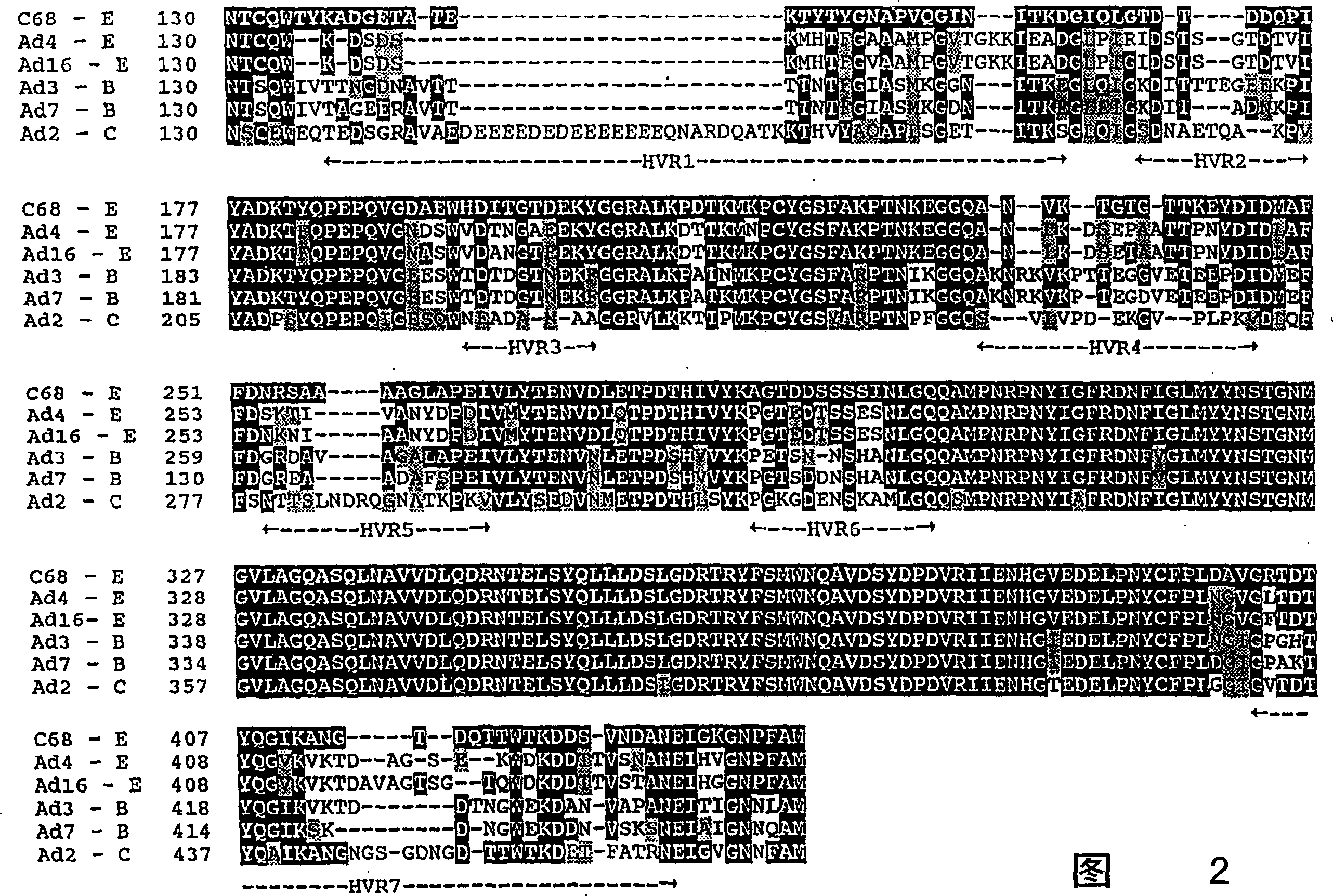
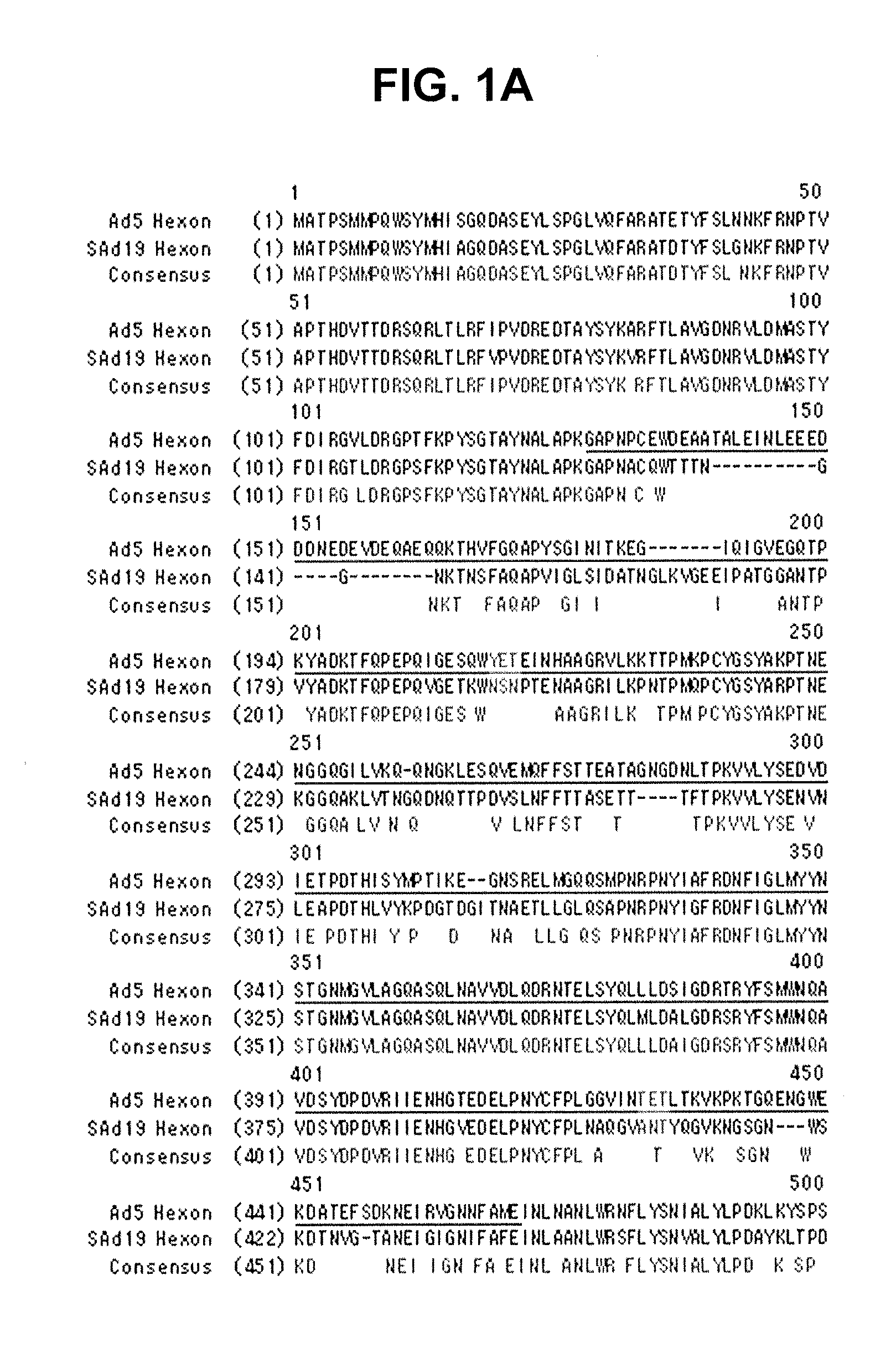
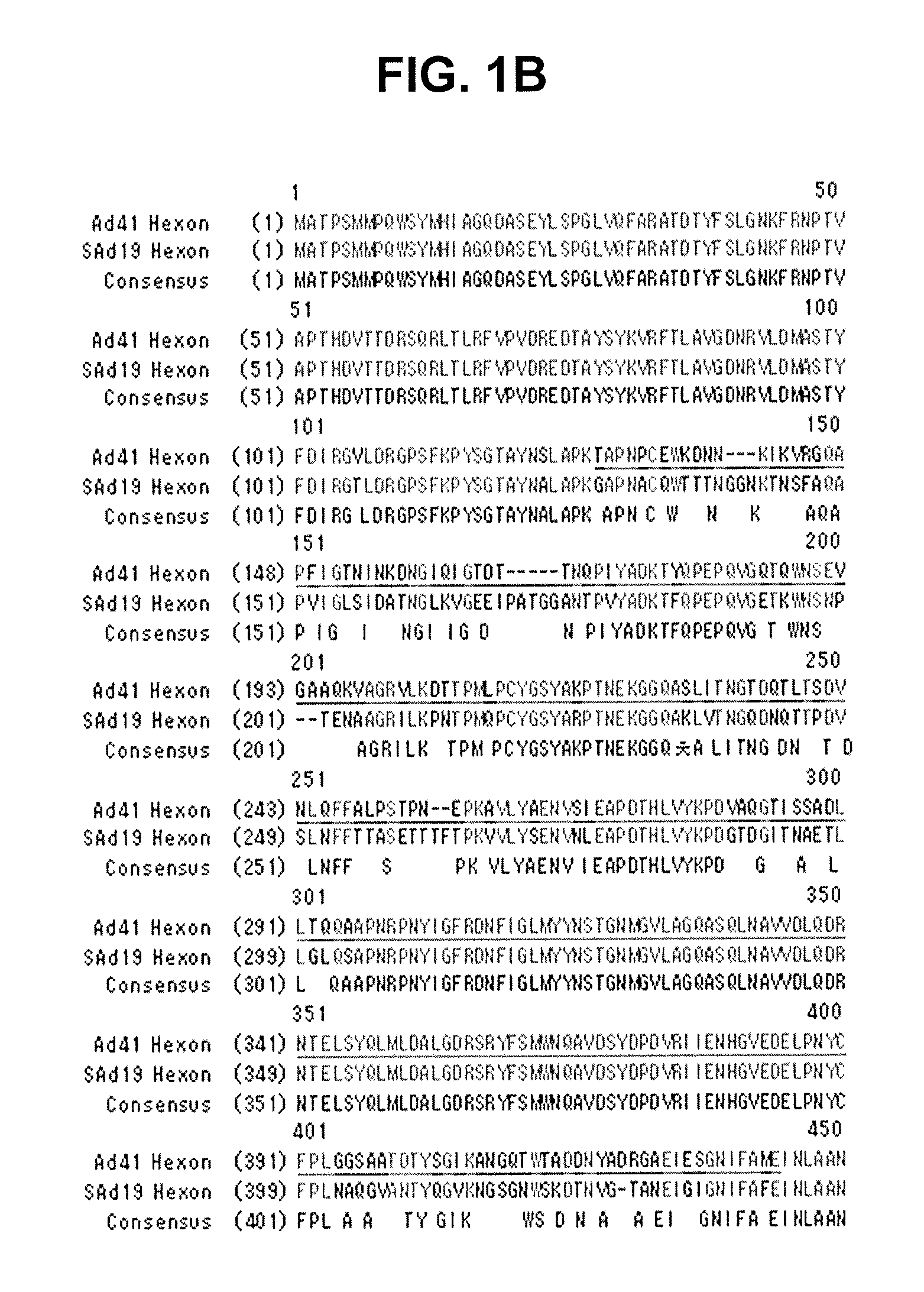
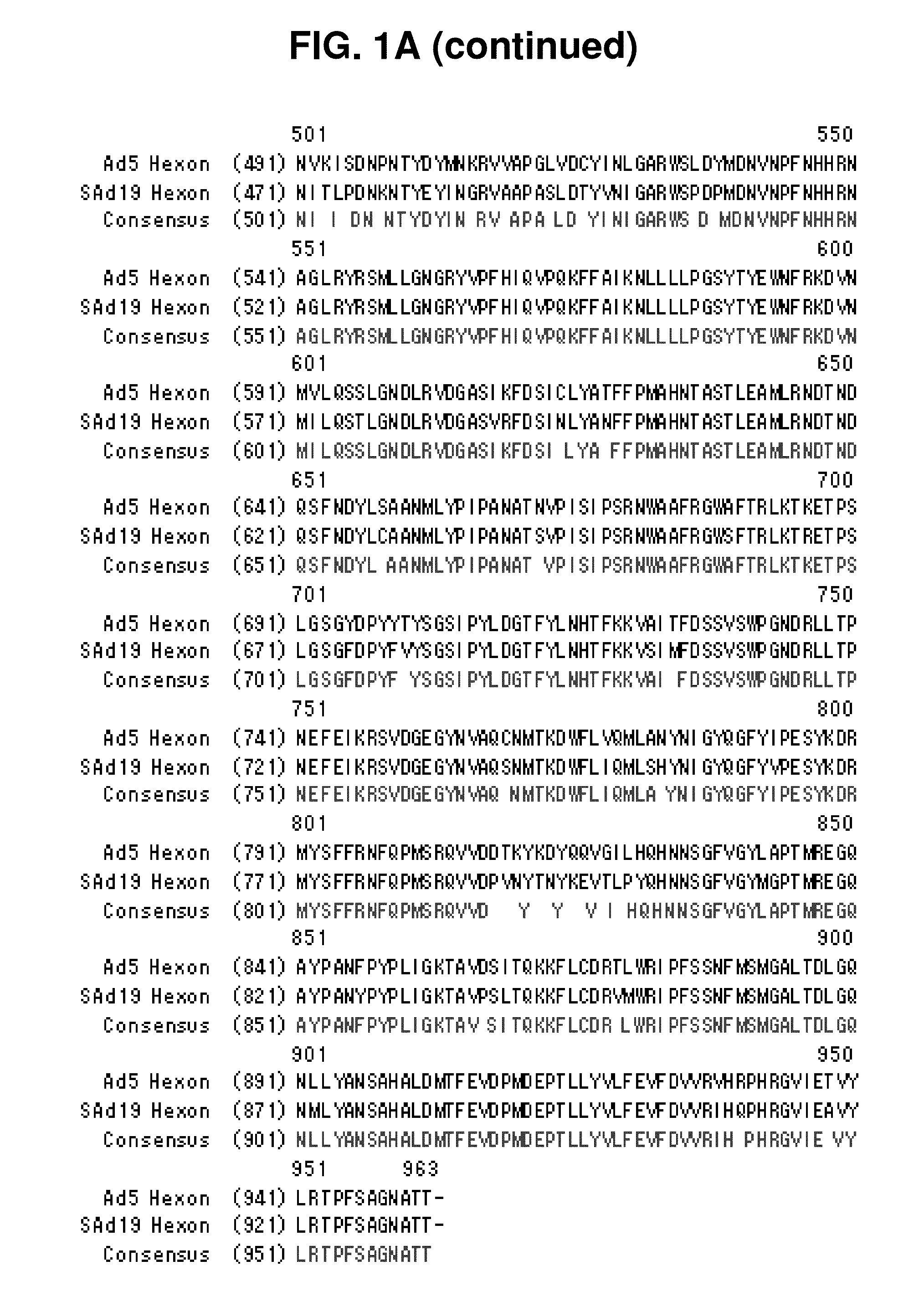
Hexon isolated from simian adenovirus serotype 19, hypervariable region thereof and chimeric adenovirus using the same
Novel hexon isolated from simian adenovirus serotype 19 encoded in the polynucleotide defined as SEQ ID NO: 3, hepervariable region thereof, chimeric adenovirus comprising the same, and therapeutic use thereof provides a solution to the problem of safety and effective systemic treatment for developing gene therapeutic agents using adenovirus.
Owner:MOGAM BIOTECH RES INST
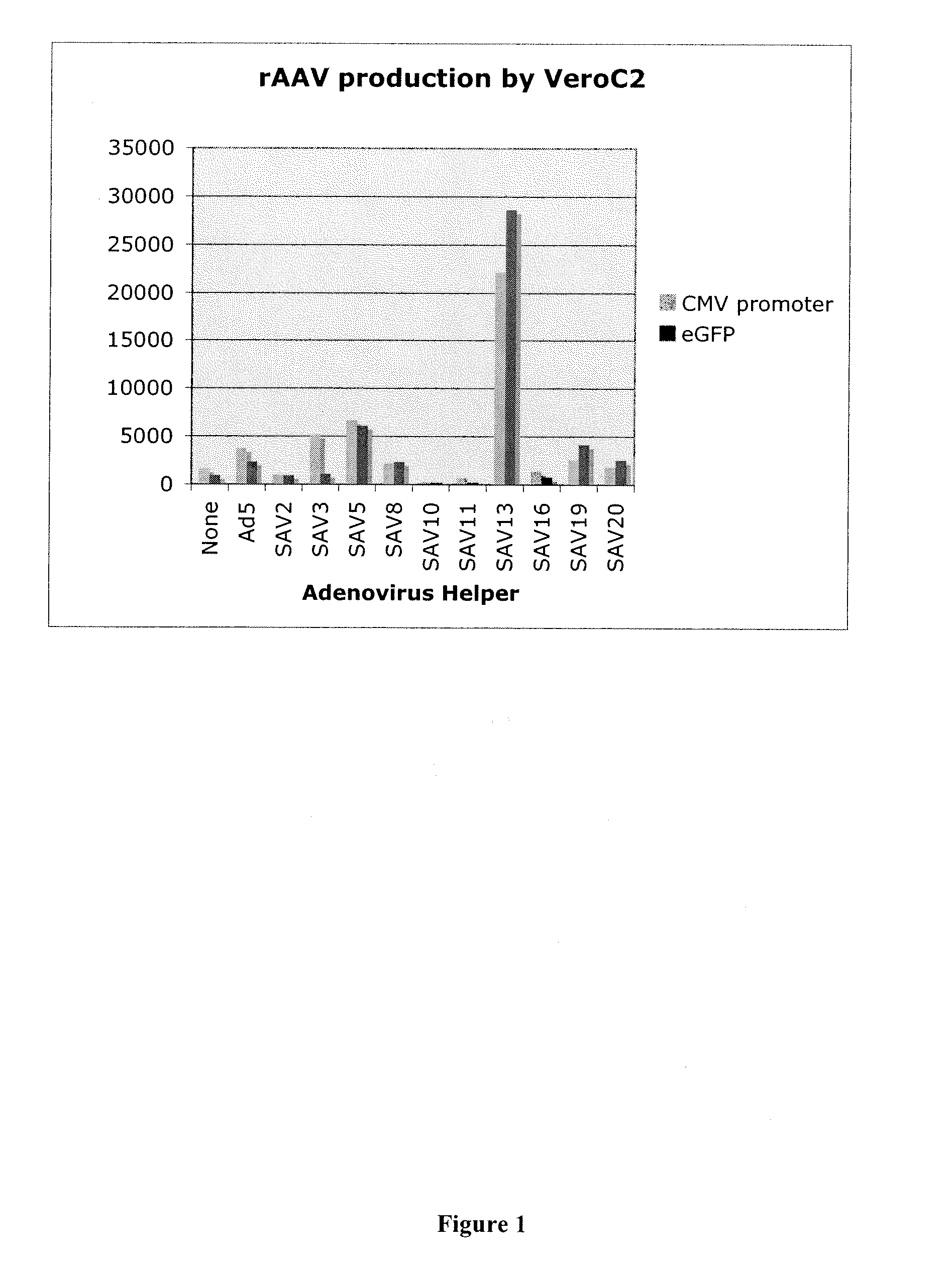
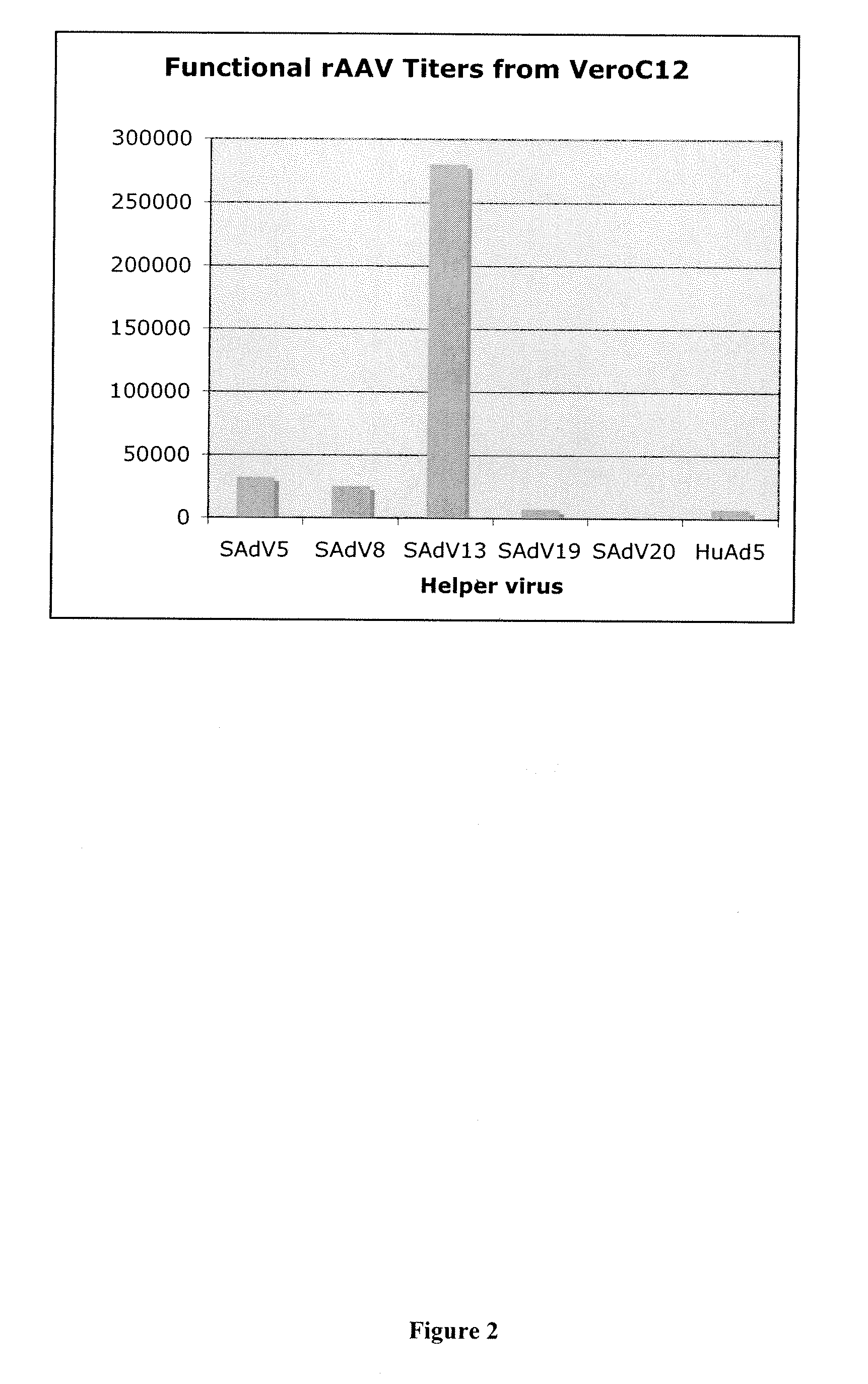
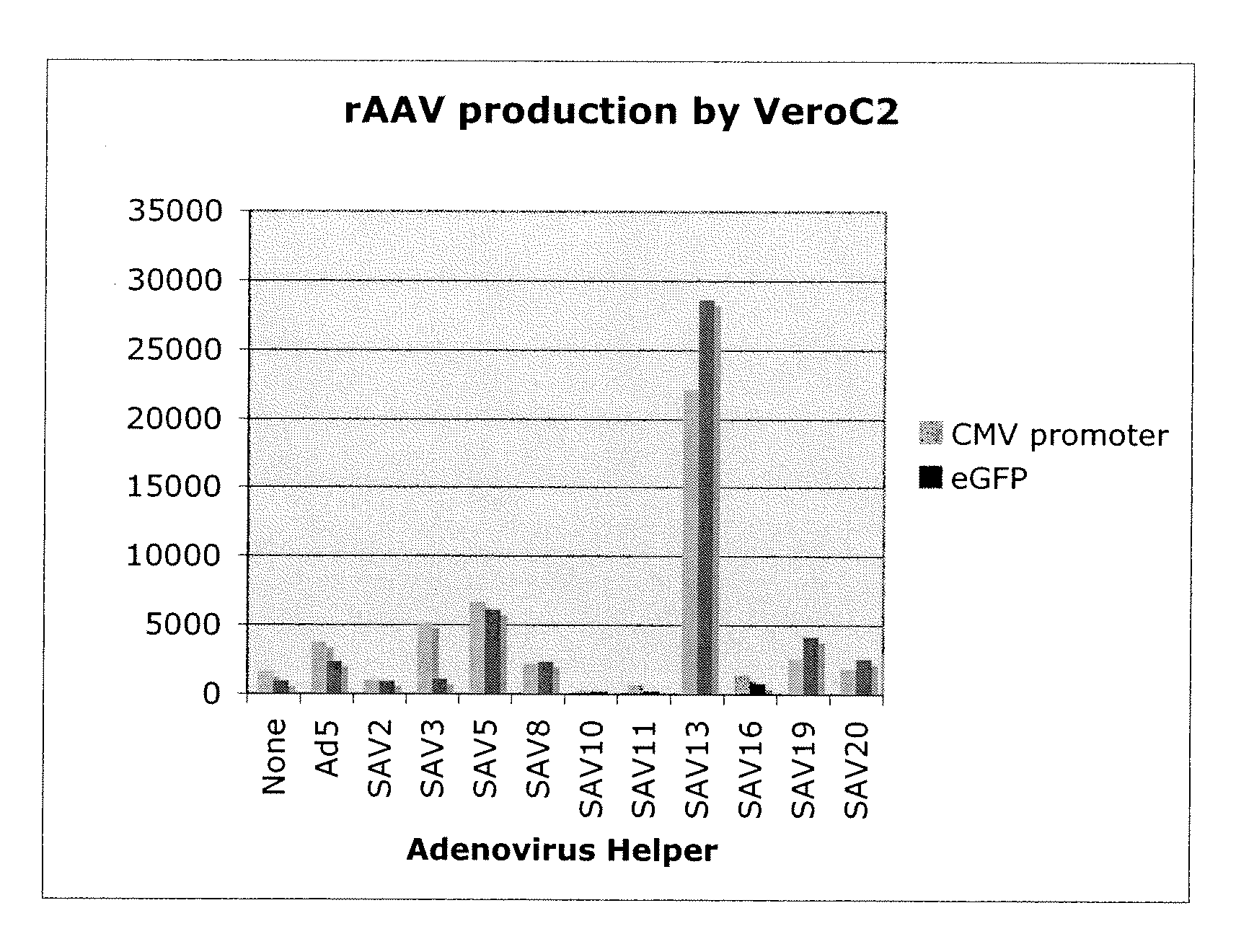
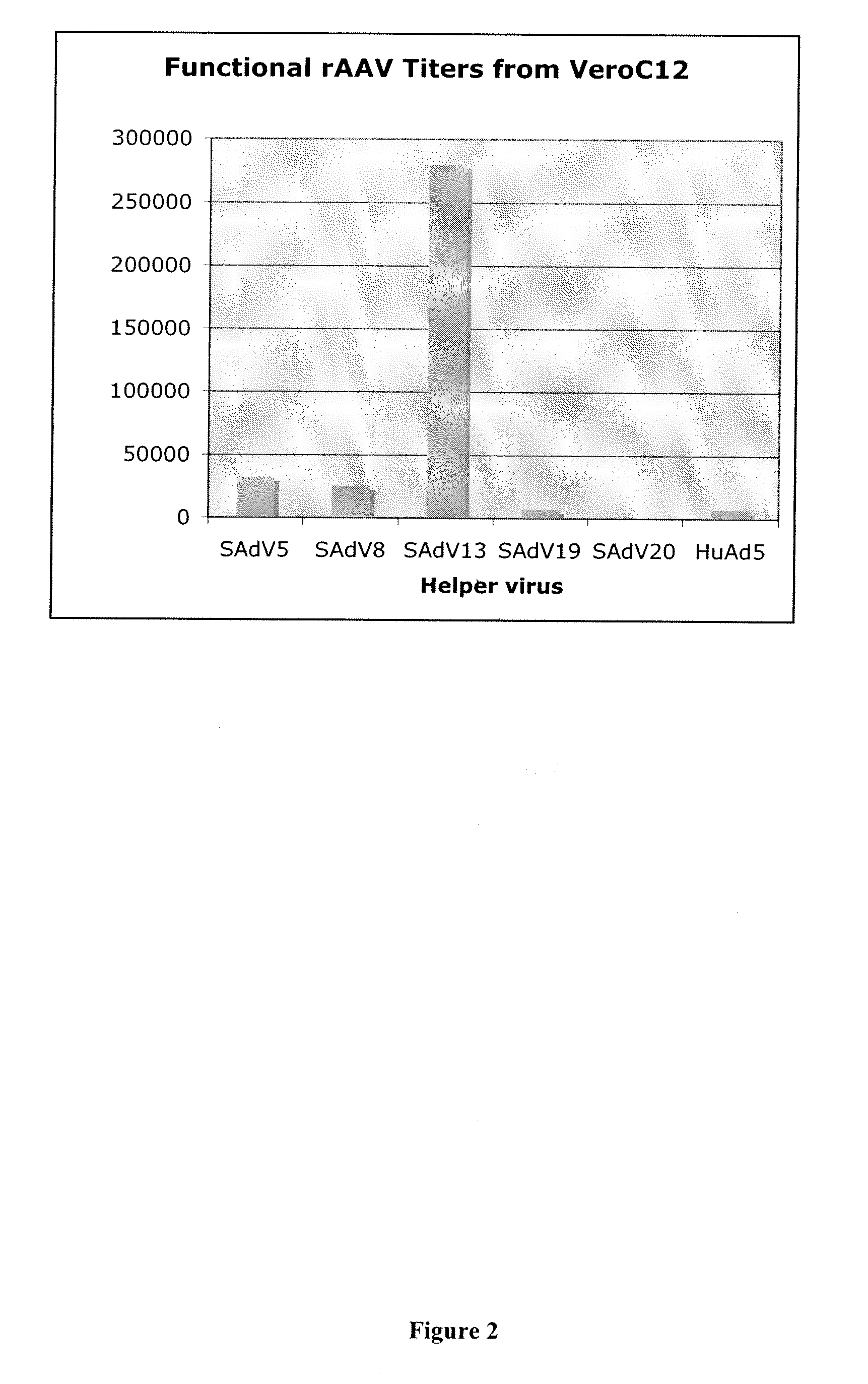
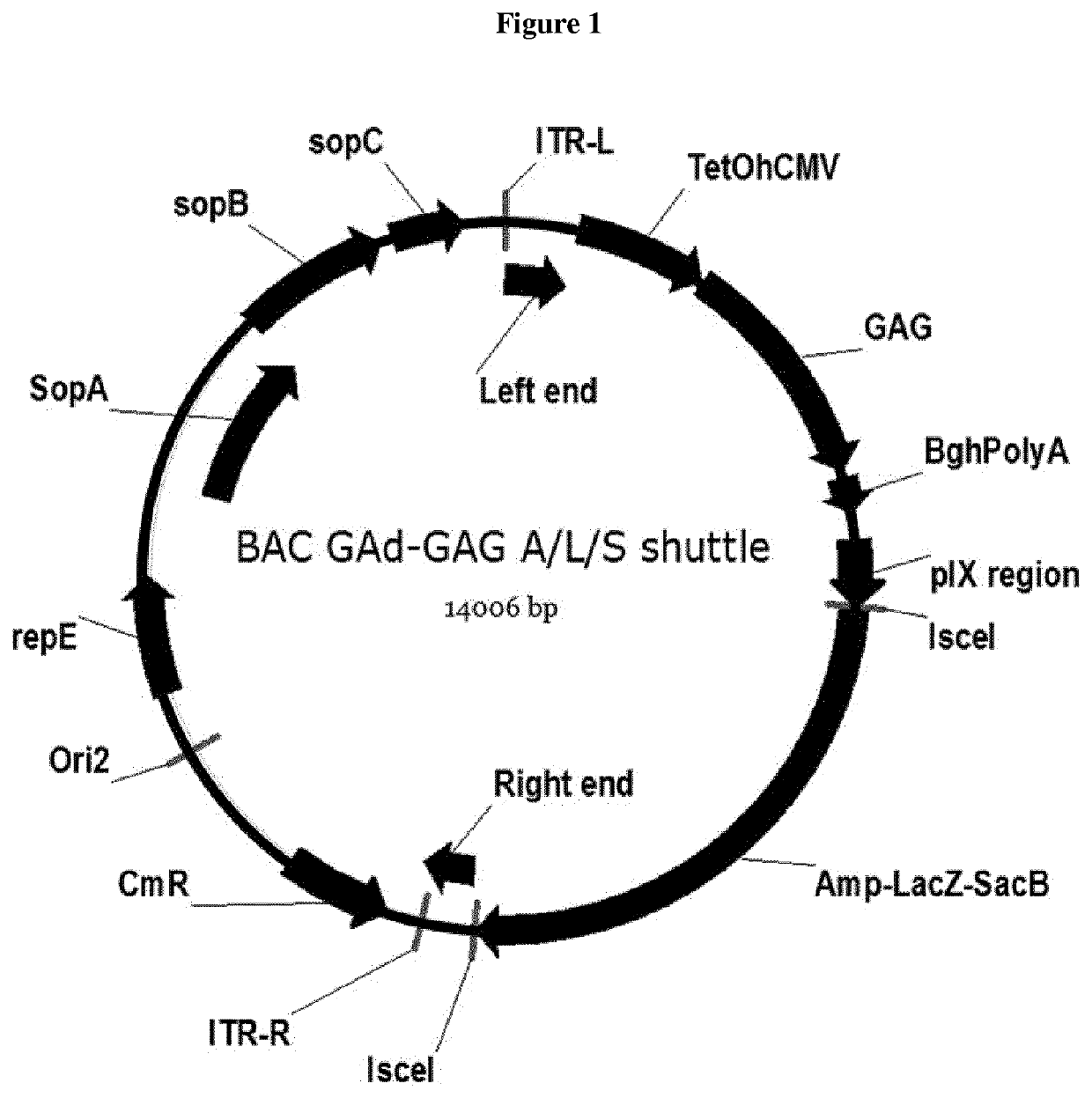
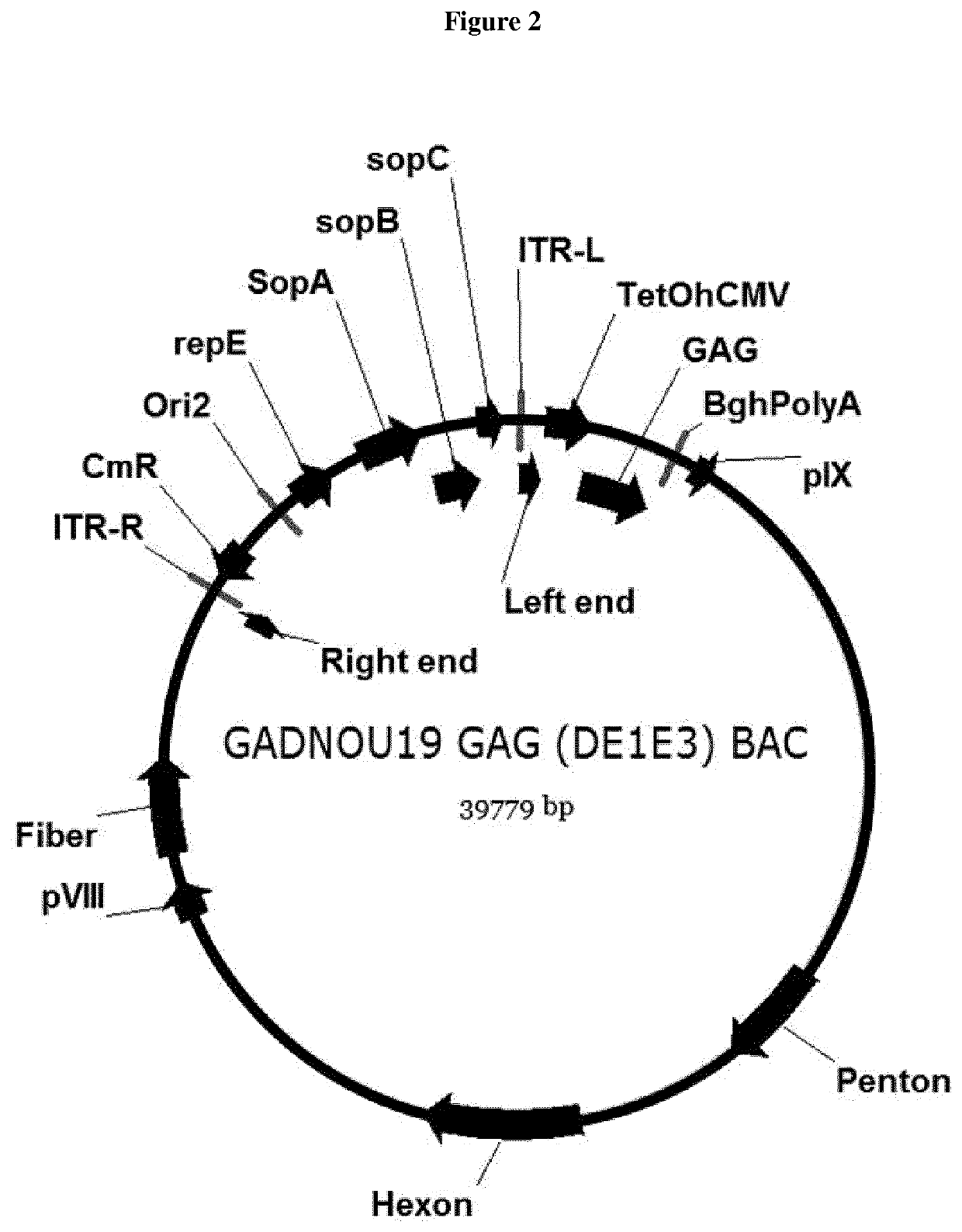
Production of rAAV in vero cells using particular adenovirus helpers
ActiveUS7943379B2High potencyHigh productMicrobiological testing/measurementTissue cultureSimian AdenovirusesAdeno associate virus
The present invention relates to methods and materials for recombinant adeno-associated virus production. More particularly, in some embodiments the invention contemplates the use of an adenovirus known as Simian Adenovirus 13 (SAdV-13) and Vero cells for production of recombinant adeno-associated virus (rAAV).
Owner:NATIONWIDE CHILDRENS HOSPITAL
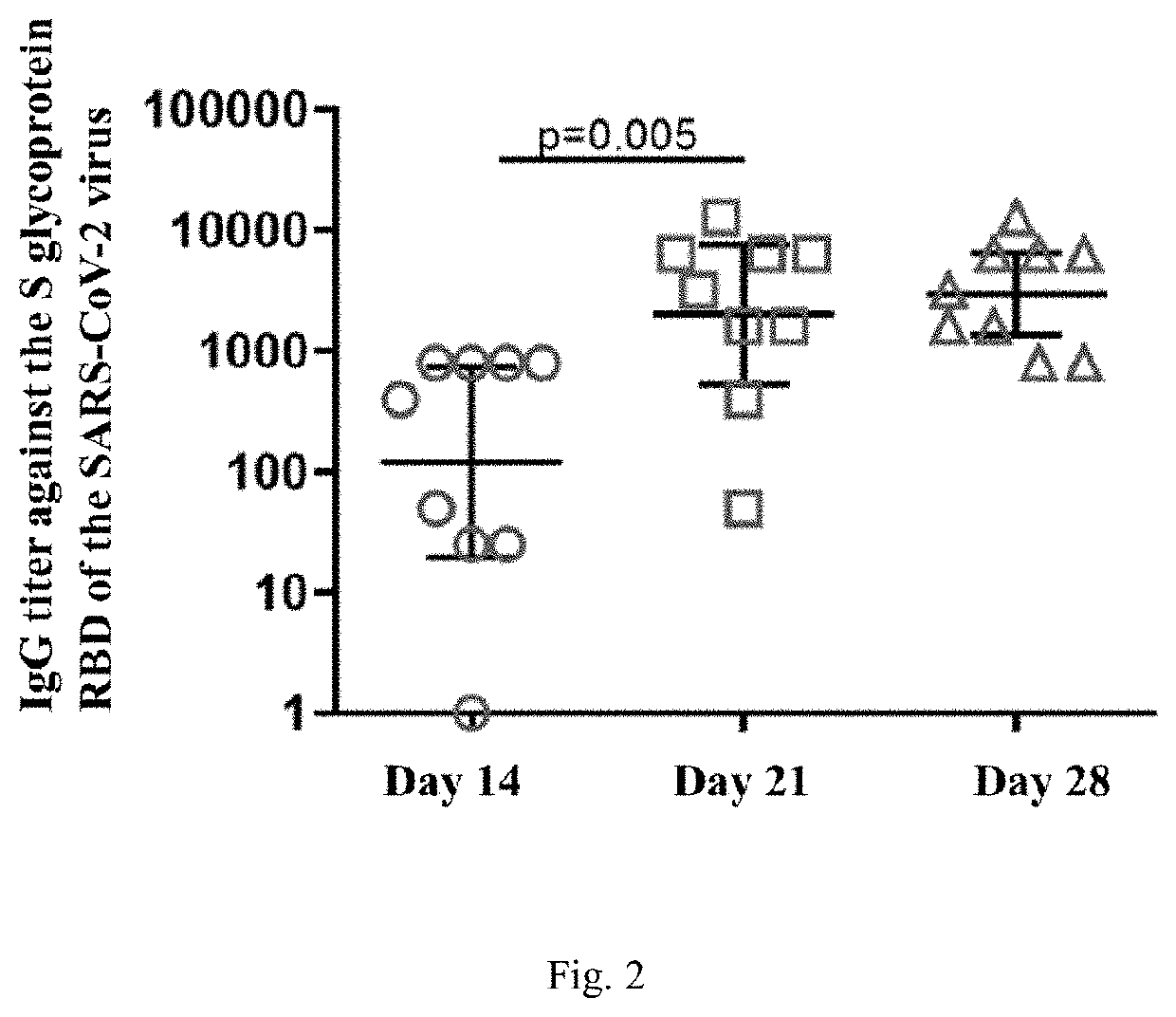
Agent for inducing specific immunity against severe acute respiratory syndrome virus sars-cov-2 in liquid form (variants)
InactiveUS20220259618A1High promotion rateSsRNA viruses positive-senseVectorsSimian AdenovirusesSpecific immunity
The invention relates to a biomolecule agent for inducing specific immunity against severe acute respiratory syndrome virus SARS-CoV-2, in liquid form, which contains a single active component, comprising the expression vector including either: the genome of the recombinant strain of human adenovirus serotype 26 or 5, wherein the E1 and E3 regions are deleted, the vector with an integrated expression cassette is selected from SEQ ID NO:1, SEQ ID NO:2, or SEQ ID NO:3; or the recombinant strain of simian adenovirus serotype 25, wherein the E1 and E3 regions are deleted, the vector with an integrated expression cassette selected from SEQ ID NO:4, SEQ ID NO:2, or SEQ ID NO:3. The recombinant strain of human adenovirus serotype 26 may include the ORF6-Ad26 region replaced by ORF6-Ad5.A buffer solution of the agent in liquid form contains the following, by mass %: tris from 0.1831 to 0.3432; sodium chloride from 0.3313-0.6212; sucrose from 3.7821-7.0915; magnesium chloride hexahydrate from 0.0154-0.0289; EDTA from 0.0029-0.0054; polysorbate-80 from 0.0378-0.0709; ethanol 95% from 0.0004-0.0007; and water to fill.The agent can be administered via intranasal and / or intramuscular routes. The invention promotes humoral and cell-mediated immune responses against SARS-CoV-2 virus among broad strata of the population.
Owner:FEDERAL STATE BUDGETARY INSTITUTION NAT RES CENT FOR EPIDEMIOLOGY & MICROBIOLOGY NAMED AFTER HONORARY ACADEMICIAN N F GAMALEYA OF THE MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION
Mucosal vaccine formulations
PendingCN113966228ASsRNA viruses negative-senseViral antigen ingredientsSimian AdenovirusesBioadhesive
Simian adenoviral vectors are formulated with bioadhesives and excipients that maintain immunogenicity. They can be administered mucosally to provide effective prophylaxis and therapy.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Recombinant adenoviruses and use thereof
The present invention relates to recombinant adenoviruses and vectors thereof. In particular, the adenoviruses are novel simian adenoviruses having a low seroprevalence and high immunogenicity relative to other adenoviruses and vectors thereof. The invention also provides methods for production of the adenoviruses and for the treatment of diseases by administering the adenoviral vector(s) to a subject (e.g., a human).
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
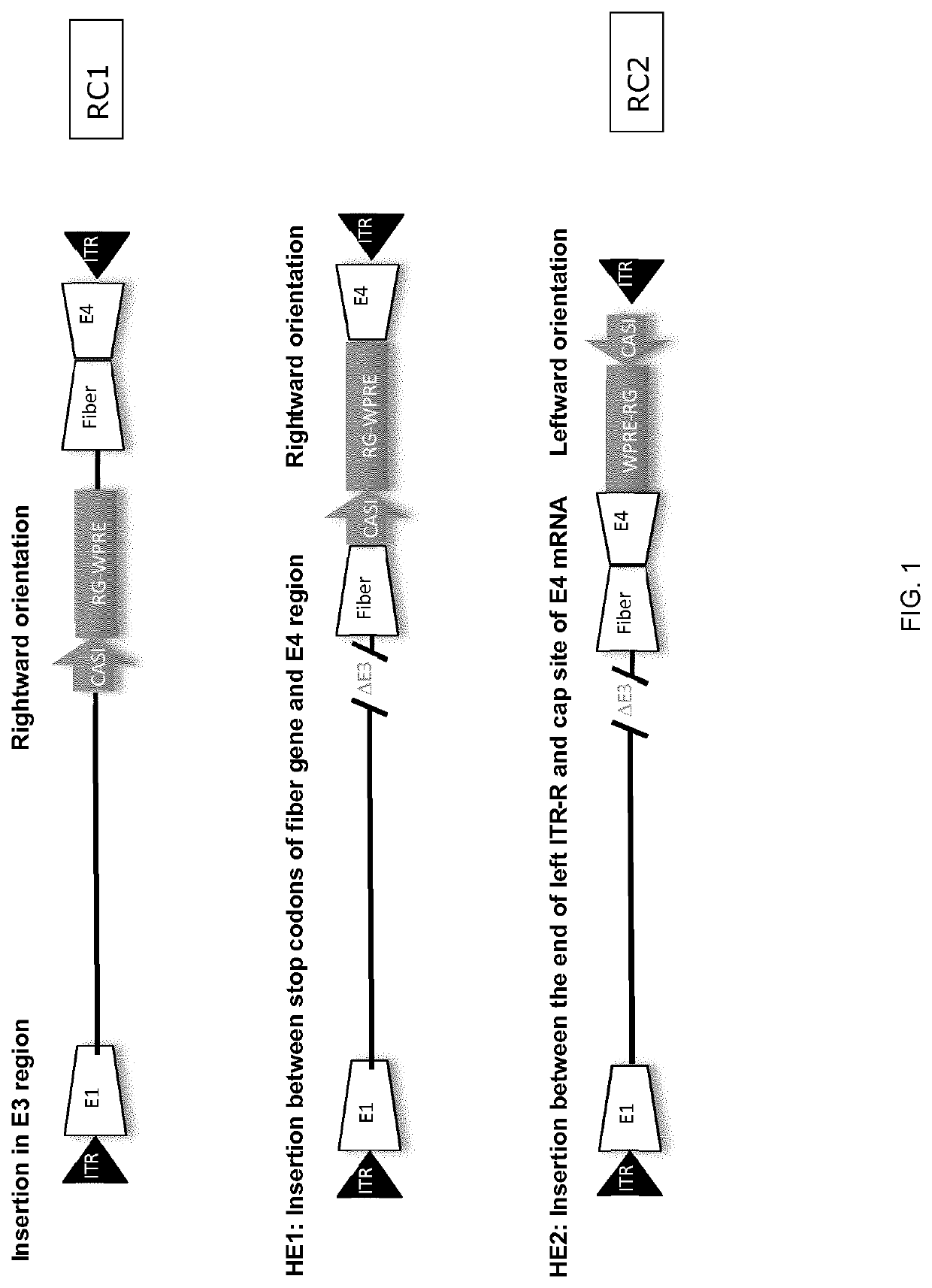
Replication competent adenoviral vectors
ActiveUS20210189421A1StrongImproved in vivo potencySsRNA viruses negative-senseViral antigen ingredientsSimian AdenovirusesTGE VACCINE
Replication competent simian adenoviral vectors are provided for the delivery of exogenous immunogens. Vectors of the invention demonstrate superior replication and expression of exogenous immunogens. They are useful as prophylactic and therapeutic vaccines as well as in gene therapy.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Non human great apes adenovirus nucleic acid- and amino acid-sequences, vectors containing same, and uses thereof
The present invention relates to novel adenovirus strains with a high immunogenicity and no pre-existing immunity in the general human population. The lack of pre-existing immunity is due to novel hypervariable regions in the adenoviral capsid protein hexon. The novel adenovirus strains also have an improved capacity for reproduction. The present invention provides nucleotide and amino acid sequences of these novel adenovirus strains, as well as recombinant viruses, virus-like particles and vectors based on these strains. Further provided are pharmaceutical compositions and medical uses in the therapy or prophylaxis of a disease, and methods for producing an adenovirus or virus-like particles utilizing the novel sequences, recombinant viruses, virus-like particles and vectors.
Owner:NOUSCOM AG
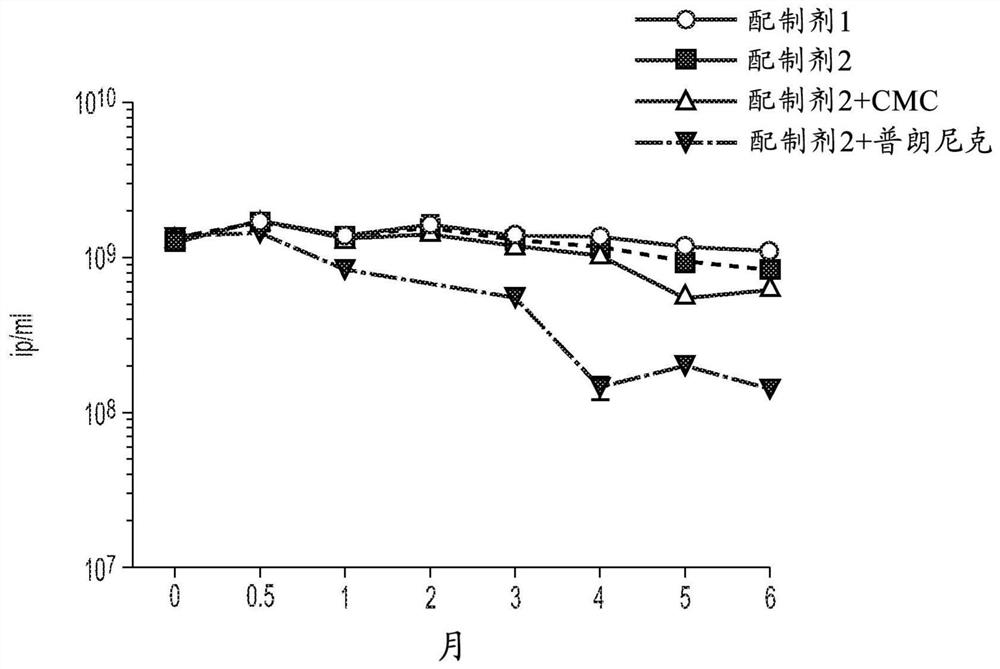
Formulations for simian adenoviral vectors having enhanced stability
PendingUS20210207171A1Improve stabilitySerum albuminUnknown materialsSimian AdenovirusesSuccinic acid
The invention relates to liquid formulations of simian adenoviruses and methods for obtaining the formulations. The formulations comprise an amorphous sugar, Vitamin E succinate and recombinant human serum albumin in a buffered solution.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Dengue virus vaccine
InactiveCN108159410AOvercome limitationsAvoid double digestionSsRNA viruses positive-senseViral antigen ingredientsSimian AdenovirusesEnzyme digestion
The invention provides a dengue virus vaccine. The invention starts from the currently rarely reported T-cell immunity of the dengue virus, a simian adenovirus carrier is used to construct the vaccineto break through the limitations of the human adenovirus carrier in the practical application, an Ad carrier vaccine C9-NS1 for the dengue virus II is successfully constructed, the carrier clones a PCR (Polymerase Chain Reaction) product rapidly through the clone technology, the double enzyme digestion operations of a plasmid and a target fragment are avoided, and the carrier is relatively convenient and efficient. A histidine tag is arranged at the end C, expressing the fusion protein, of the carrier, so that the affinity chromatography and purification of the recombinant protein is facilitated, and the protein of the histidine tag is relatively small and barely produces effects on the activity and the function of the expression protein. The immunologic function of the vaccine from the protein NS 1 of the dengue virus is evaluated comprehensively from multiple aspects, and a foundation is laid for the further research and development of the efficient spectrum dengue gene vaccine.
Owner:重庆卓诺生物技术有限公司
Heterologous prime boost vaccine compositions and methods
PendingUS20220062409A1Strong and long-lasting immunitySsRNA viruses negative-senseViral antigen ingredientsSimian AdenovirusesMedicine
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Pharmaceutical agent for inducing specific immunity against sars-cov-2
InactiveUS20220226466A1Powder deliverySsRNA viruses positive-senseSimian AdenovirusesSpecific immunity
The invention relates to biotechnology. The claimed agent can be used for the prevention of SARS-CoV-2.A pharmaceutical agent may contain component (1), and contains a recombinant human adenovirus serotype genome (26), with an expression cassette selected from SEQ ID NO: 1, SEQ ID NO: 2, or SEQ ID NO: 3, and also contains component (2), comprising an agent selected from (i) a recombinant human adenovirus serotype genome (5), with an expression cassette selected from SEQ ID NO: 1, SEQ ID NO: 2, or SEQ ID NO: 3; or (ii) a recombinant simian adenovirus serotype genome (25), with an expression cassette selected from SEQ ID NO: 4, SEQ ID NO: 2, or SEQ ID NO: 3.Furthermore, a pharmaceutical agent may contain component (1), comprising an agent comprising a recombinant simian adenovirus serotype genome (25), with an expression cassette selected from SEQ ID NO: 4, SEQ ID NO: 2, or SEQ ID NO: 3, and also contains component (2), comprising an agent comprising a recombinant human adenovirus serotype genome (5), with an expression cassette selected from SEQ ID NO: 1, SEQ ID NO: 2, or SEQ ID NO: 3.
Owner:FEDERATION FEDERAL STATE BUDGETARY INSTITUTION NAT RES CENT FOR EPIDEMIOLOGY & MICROBIOLOGY R
PRODUCTION OF rAAV IN VERO CELLS USING PARTICULAR ADENOVIRUS HELPERS
ActiveUS20090275138A1High potencyHigh productMicrobiological testing/measurementTissue cultureSimian AdenovirusesVero cell
The present invention relates to methods and materials for recombinant adeno-associated virus production. More particularly, in some embodiments the invention contemplates the use of an adenovirus known as Simian Adenovirus 13 (SAdV-13) and Vero cells for production of recombinant adeno-associated virus (rAAV).
Owner:NATIONWIDE CHILDRENS HOSPITAL
Non human great apes adenovirus nucleic acid- and amino acid-sequences, vectors containing same, and uses thereof
ActiveUS11098324B2Genetic material ingredientsMicrobiological testing/measurementSimian AdenovirusesDisease
The present invention relates to novel adenovirus strains with a high immunogenicity and no pre-existing immunity in the general human population. The lack of pre-existing immunity is due to novel hypervariable regions in the adenoviral capsid protein hexon. The novel adenovirus strains also have an improved capacity for reproduction. The present invention provides nucleotide and amino acid sequences of these novel adenovirus strains, as well as recombinant viruses, virus-like particles and vectors based on these strains. Further provided are pharmaceutical compositions and medical uses in the therapy or prophylaxis of a disease, and methods for producing an adenovirus or virus-like particles utilizing the novel sequences, recombinant viruses, virus-like particles and vectors.
Owner:NOUSCOM AG
Agent for inducing specific immunity against severe acute respiratory syndrome virus sars-cov-2 in lyophilized form (variants)
InactiveUS20220249655A1Reduce in quantityEnsure protectionPowder deliverySsRNA viruses positive-senseSimian AdenovirusesSpecific immunity
The invention relates to a biomolecule agent for inducing specific immunity against severe acute respiratory syndrome virus SARS-CoV-2, in lyophilized (freeze-dried) form, which contains a single active component, comprising the expression vector including either the genome of the recombinant strain of human adenovirus serotype 26 or 5, wherein the E1 and E3 regions are deleted, and an integrated expression cassette selected from SEQ ID NO:1, SEQ ID NO:2, or SEQ ID NO:3; or the genome of the recombinant strain of simian adenovirus serotype 25, wherein the E1 and E3 regions are deleted, and an integrated expression cassette is selected from SEQ ID NO:4, SEQ ID NO:2, or SEQ ID NO:3. The genome of the recombinant strain of human adenovirus serotype 26 may include the ORF6-Ad26 region replaced by ORF6-Ad5.A buffer solution for reconstitution of the lyophilized form of the agent may contain the following, mass %: tris from 0.0180-0.0338; sodium chloride from 0.1044-0.1957; sucrose from 5.4688-10.2539; magnesium chloride hexahydrate from 0.0015-0.0028; EDTA from 0.0003-0.0005; polysorbate-80 from 0.0037-0.0070; and water to fill.The agent can be administered via intranasal and / or intramuscular routes. The invention promotes humoral and cell-mediated immune responses against SARS-CoV-2 virus among broad strata of the population.
Owner:FEDERATION FEDERAL STATE BUDGETARY INSTITUTION NAT RES CENT FOR EPIDEMIOLOGY & MICROBIOLOGY R
Simian adenovirus nucleic acid and amino acid sequences, vectors containing same and uses thereof
The present invention relates to simian adenovirus nucleic acid and amino acid sequences, vectors containing them and uses thereof. In particular, the present invention relates to novel adenovirus strains with improved seropositivity. In one aspect, the invention relates to isolated polypeptides of adenoviral capsid proteins such as hexon, penton and fibrin, fragments thereof and polynucleotides encoding the same. Also provided are vectors comprising the isolated polynucleotides according to the invention and adenoviruses comprising the isolated polynucleotides or polypeptides according to the invention and pharmaceutical combinations comprising said vectors, adenoviruses, polypeptides and / or polynucleotides things. The present invention also relates to the use of isolated polynucleotides, isolated polypeptides, vectors, adenoviruses and / or pharmaceutical compositions for the treatment or prevention of diseases.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Expression vector against severe acute respiratory syndrome virus sars-cov-2
InactiveUS20220235376A1SsRNA viruses positive-senseViral antigen ingredientsSimian AdenovirusesSpecific immunity
The invention relates to preparing and using recombinant expression vectors for inducing specific immunity against severe acute respiratory syndrome virus SARS-CoV-2. One expression vector contains the recombinant human adenovirus serotype26 genome, wherein the E1 and E3 regions are deleted, and the ORF6-Ad26 region is replaced by ORF6-Ad5, with an integrated expression cassette of SEQ ID NO:1, 2, or 3 (variant 1). Therein, SEQ ID NO:5 was a parental sequence of human adenovirus serotype 26.Another expression vector contains the recombinant simian adenovirus serotype25 genome, wherein the E1 and E3 regions are deleted, with an integrated expression cassette of SEQ ID NO:4, 2, or 3 (variant 2). Therein, SEQ ID NO:6 was a parental sequence of simian adenovirus serotype 25.Further, the recombinant human adenovirus serotype5 genome is disclosed, wherein the E1 and E3 regions are deleted, with an integrated expression cassette of SEQ ID NO:1, 2, or 3 (variant 3). Therein, SEQ ID NO:7 was a parental sequence of human adenovirus serotype 5.
Owner:FEDERATION FEDERAL STATE BUDGETARY INSTITUTION NAT RES CENT FOR EPIDEMIOLOGY & MICROBIOLOGY R
Pharmaceutical formulations for inducing specific immunity against SARS-COV-2
PendingCN114728055ASsRNA viruses positive-senseViral antigen ingredientsSimian AdenovirusesSpecific immunity
The present invention relates to biotechnology. The claimed agents can be used for the prevention of SARS-CoV-2. A pharmaceutical formulation is created which contains a component (1) comprising an agent in the genomic form of a recombinant human adenovirus serotype (26) in which an expression cassette selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3 is placed, and which further contains a component (2) comprising an agent in the genomic form of a recombinant human adenovirus serotype (5) in which an expression cassette selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3 is placed. Furthermore, a pharmaceutical formulation is created which contains a component 1 comprising an agent in the genomic form of a recombinant human adenovirus serotype (26) in which an expression cassette selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3 is placed, and which further contains a component (2) comprising an agent in the genomic form of a recombinant simian adenovirus serotype (25) in which an expression cassette selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3 is placed, wherein an expression cassette selected from SEQ ID NO: 4, SEQ ID NO: 2 and SEQ ID NO: 3 is placed. Furthermore, a pharmaceutical formulation is created which contains component 1 comprising an agent in the genomic form of a recombinant simian adenovirus serotype (25) in which an expression cassette selected from the group consisting of SEQ ID NO: 4, SEQ ID NO: 2, SEQ ID NO: 3 is placed, and which further contains component (2) comprising an agent in the genomic form of a recombinant human adenovirus serotype (5) in which an expression cassette selected from the group consisting of SEQ ID NO: 4, SEQ ID NO: 2, SEQ ID NO: 3 is placed, wherein an expression cassette selected from SEQ ID NO: 1, SEQ ID NO: 2 and SEQ ID NO: 3 is placed.
Use of the agent for induction of specific immunity against severe acute respiratory syndrome virus sars-cov-2 for revaccination of population (variants)
The disclosed invention relates to safe and efficacious methods of extending postvaccinal immunity against severe acute respiratory syndrome virus SARS-CoV-2 and revaccinating a population against diseases caused by SARS-CoV-2. The disclosed methods include administration of an agent to a person or population. The agent may include a first component comprising an expression vector based on a genome of recombinant strain of (i) human adenovirus serotype 26 with the E1 and E3 sites deleted from the genome and the site ORF6-Ad26 substituted for ORF6-Ad5 with an integrated expression cassette selected from SEQ ID NO:1, SEQ ID NO:2, or SEQ ID NO:3, or (ii) a simian adenovirus serotype 25 with the E1 and E3 sites deleted from the genome with an integrated expression cassette selected from SEQ ID NO:4, SEQ ID NO:2, or SEQ ID NO:3. The first component of the agent is combined or replaced with a second component in the form of an expression vector based on a genome of recombinant strain of human adenovirus serotype 5 with the E1 and E3 sites deleted from the genome with an integrated expression cassette selected from SEQ ID NO:1, SEQ ID NO:2, or SEQ ID NO:3. In addition to the above, the method may include administering an agent as disclosed herein, wherein the first component or the second component has the form of an expression vector based on genome of recombinant strain of human adenovirus serotype 5 with E1 and E3 sites deleted from the genome with the integrated expression cassette selected from SEQ ID NO:1, SEQ ID NO:2, or SEQ ID NO:3.
Owner:FEDERATION FEDERAL STATE BUDGETARY INSTITUTION NAT RES CENT FOR EPIDEMIOLOGY & MICROBIOLOGY R
Hexon isolated from simian adenovirus serotype 19, hypervariable region thereof and chimeric adenovirus using the same
Novel hexon isolated from simian adenovirus serotype 19 encoded in the polynucleotide defined as SEQ ID NO: 3, hepervariable region thereof, chimeric adenovirus comprising the same, and therapeutic use thereof provides a solution to the problem of safety and effective systemic treatment for developing gene therapeutic agents using adenovirus.
Owner:MOGAM BIOTECH RES INST
Replication competent adenoviral vectors
ActiveUS11268108B2Free from painEase of convenienceSsRNA viruses negative-senseViral antigen ingredientsSimian AdenovirusesTGE VACCINE
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com