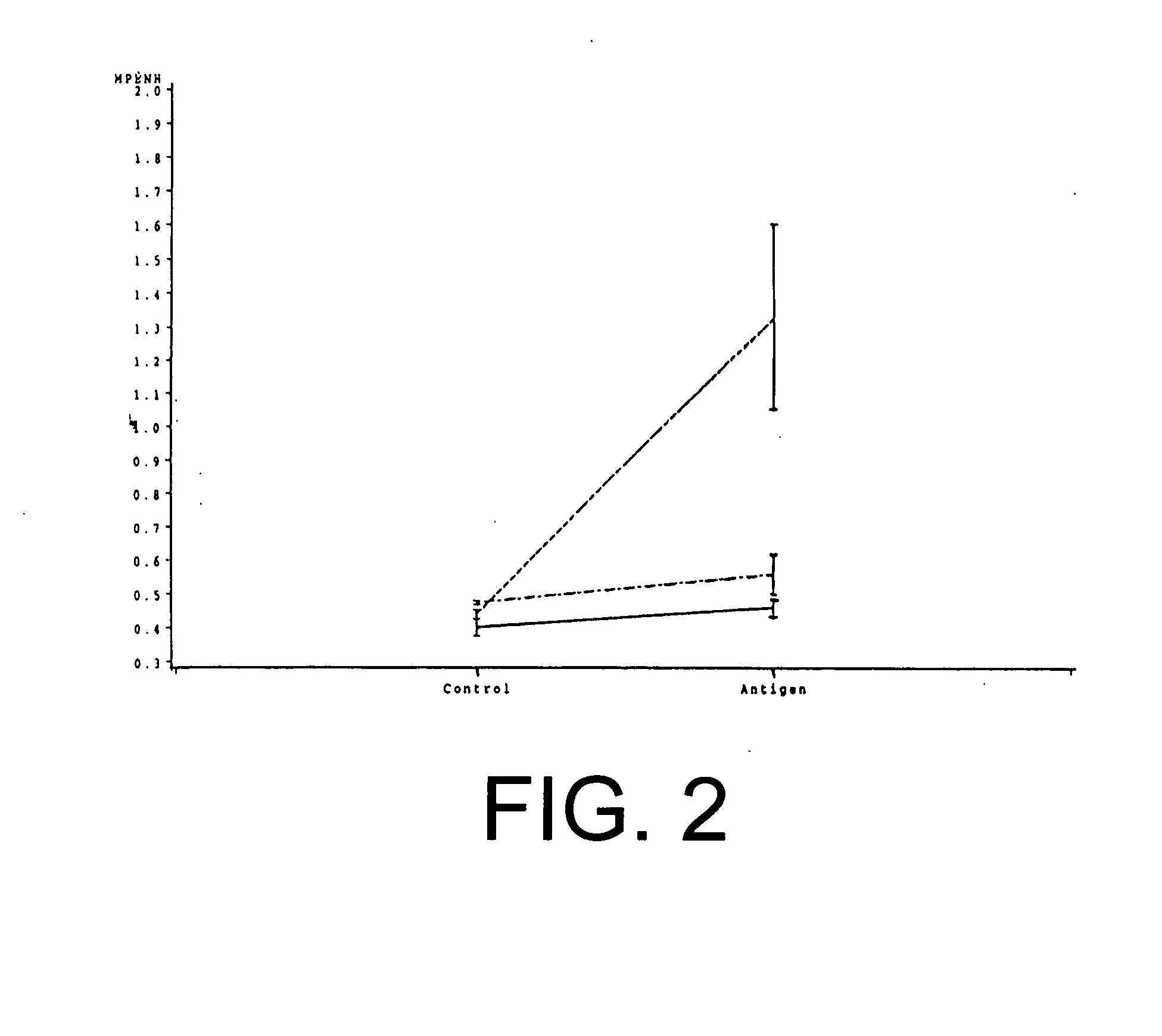
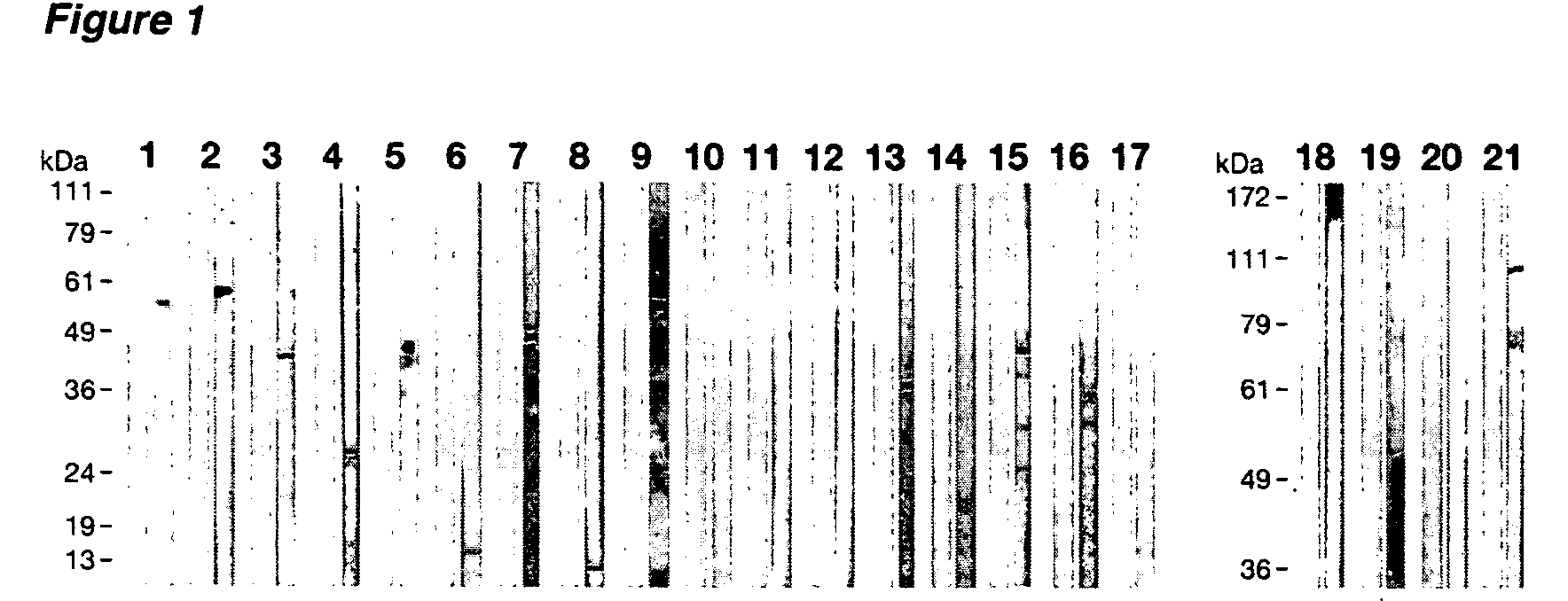
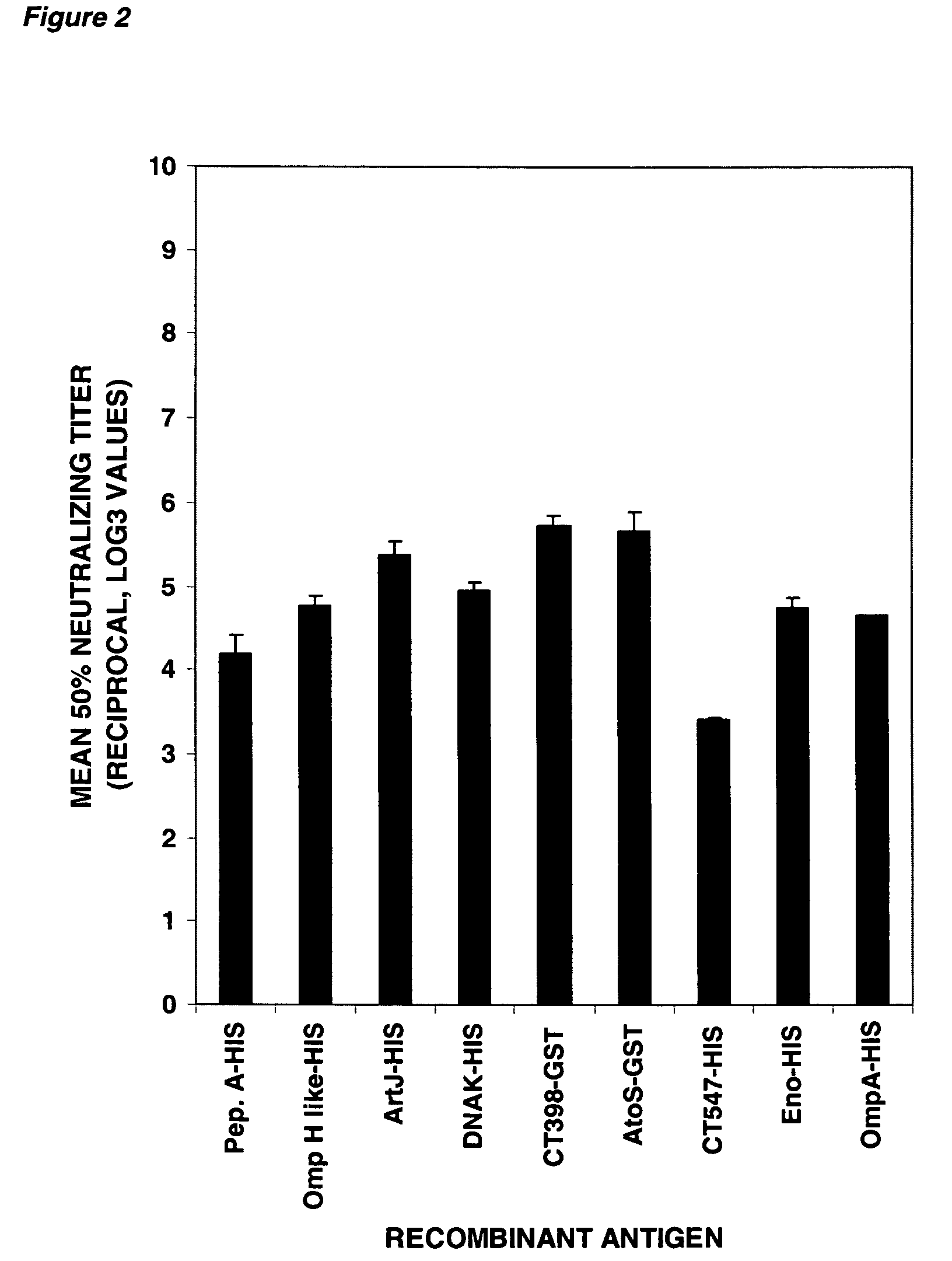
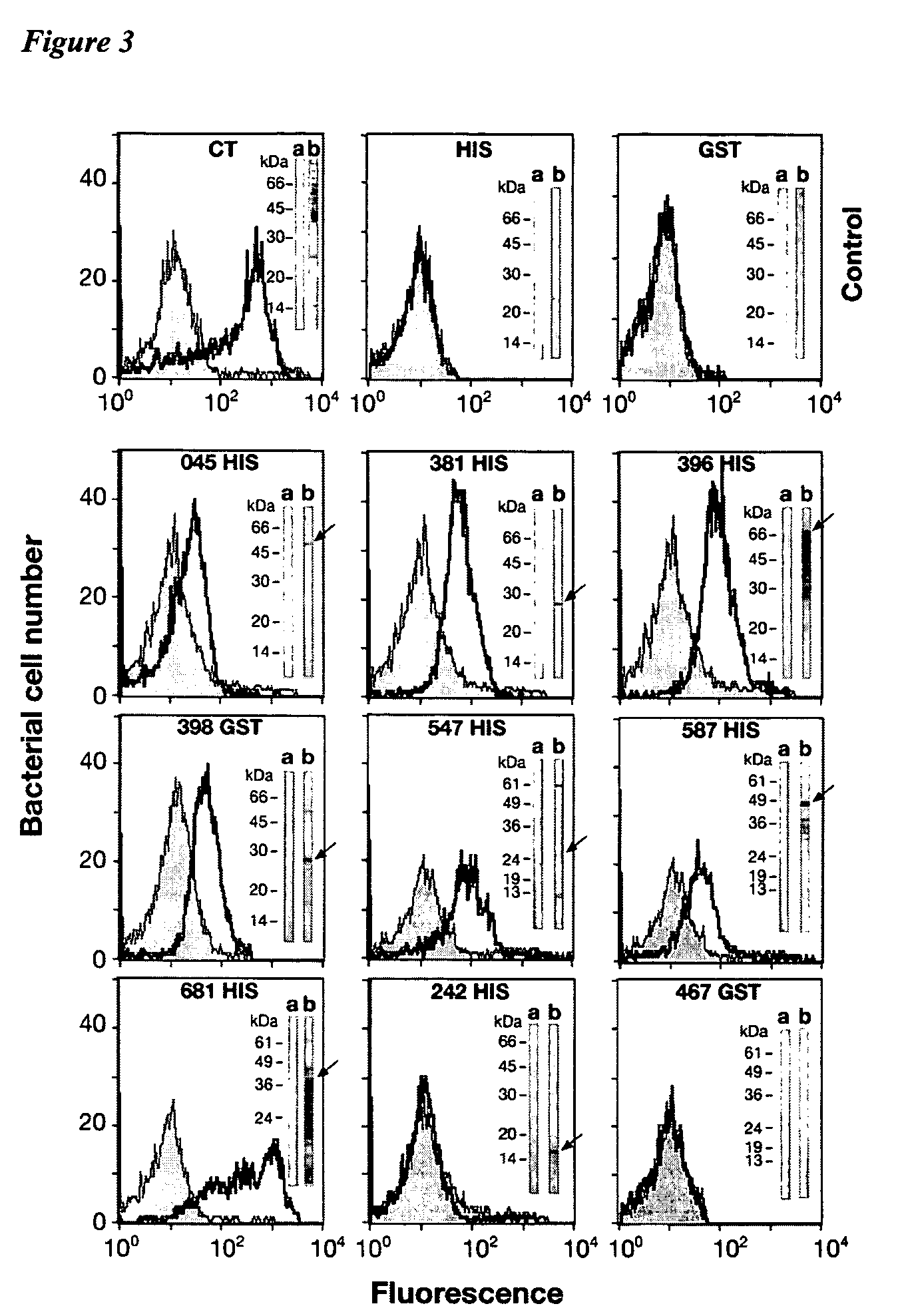
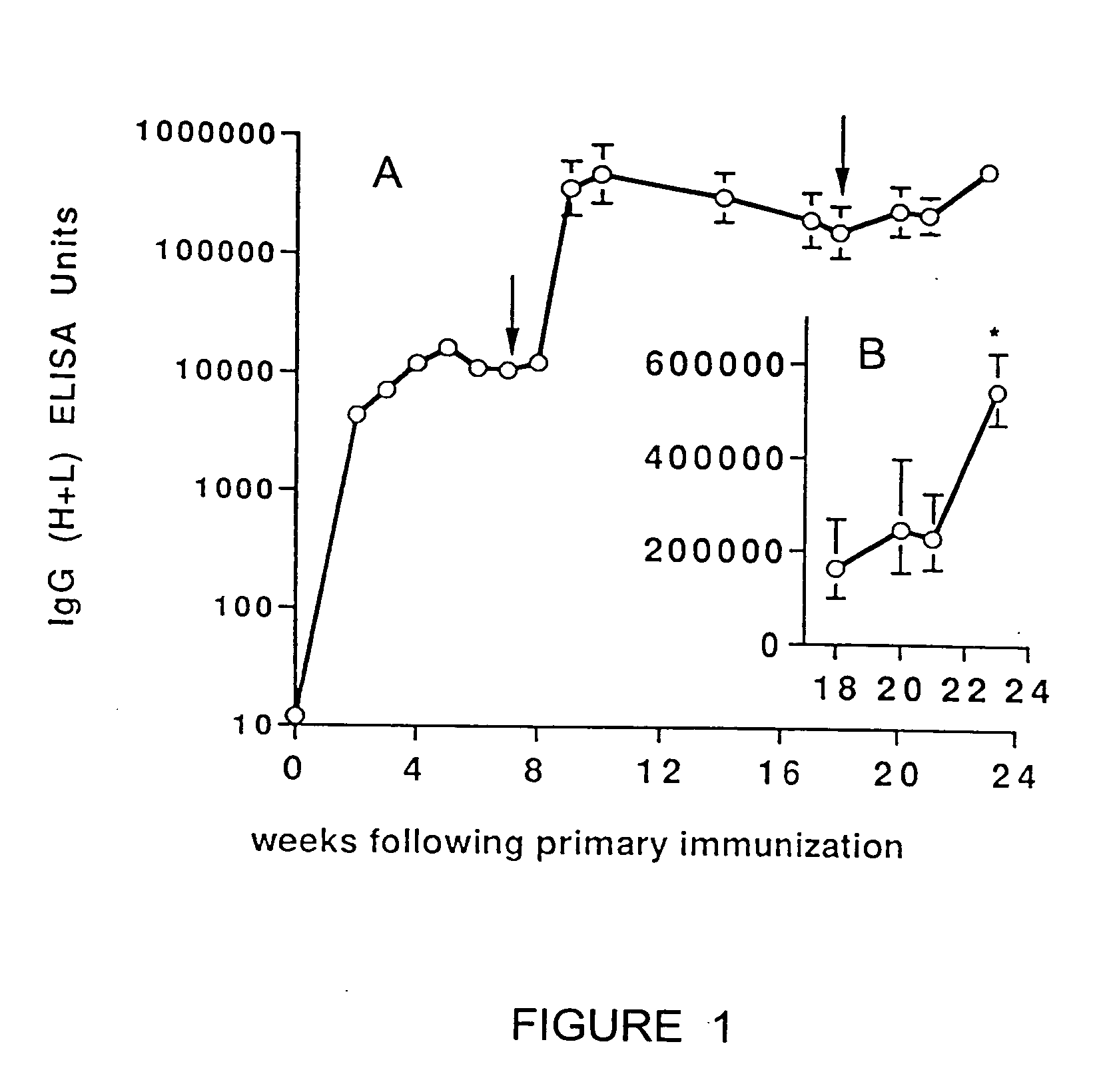
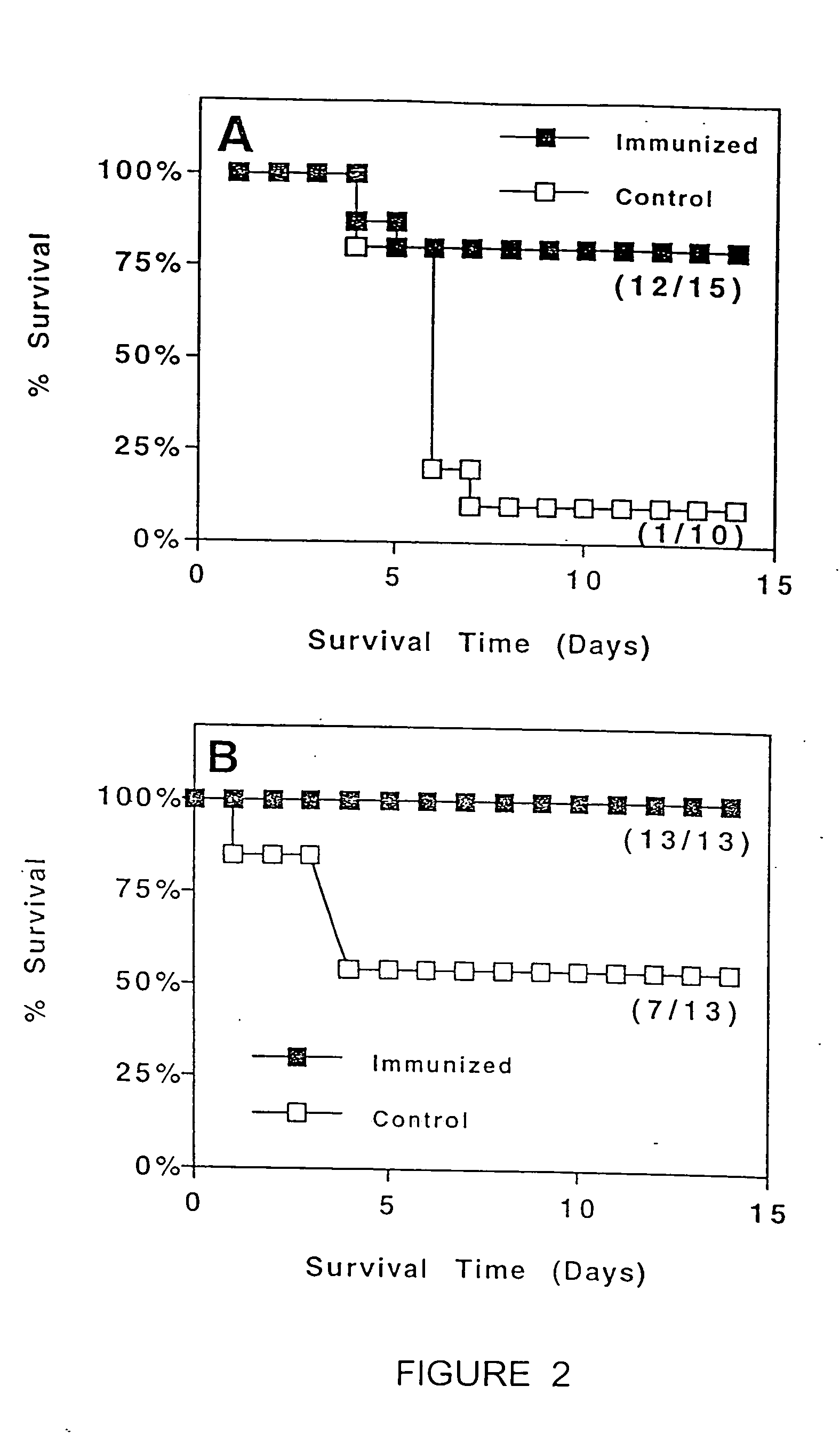
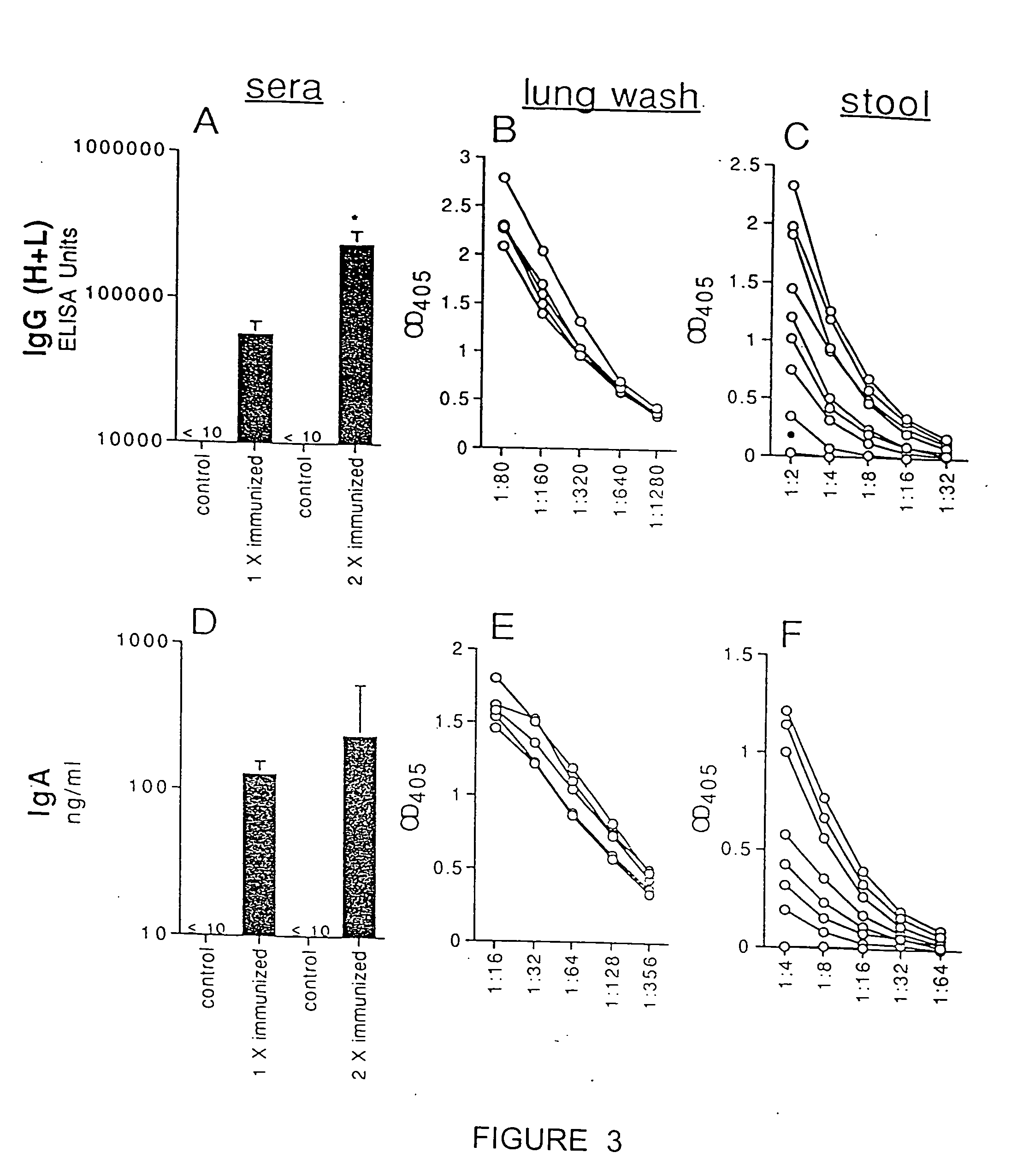
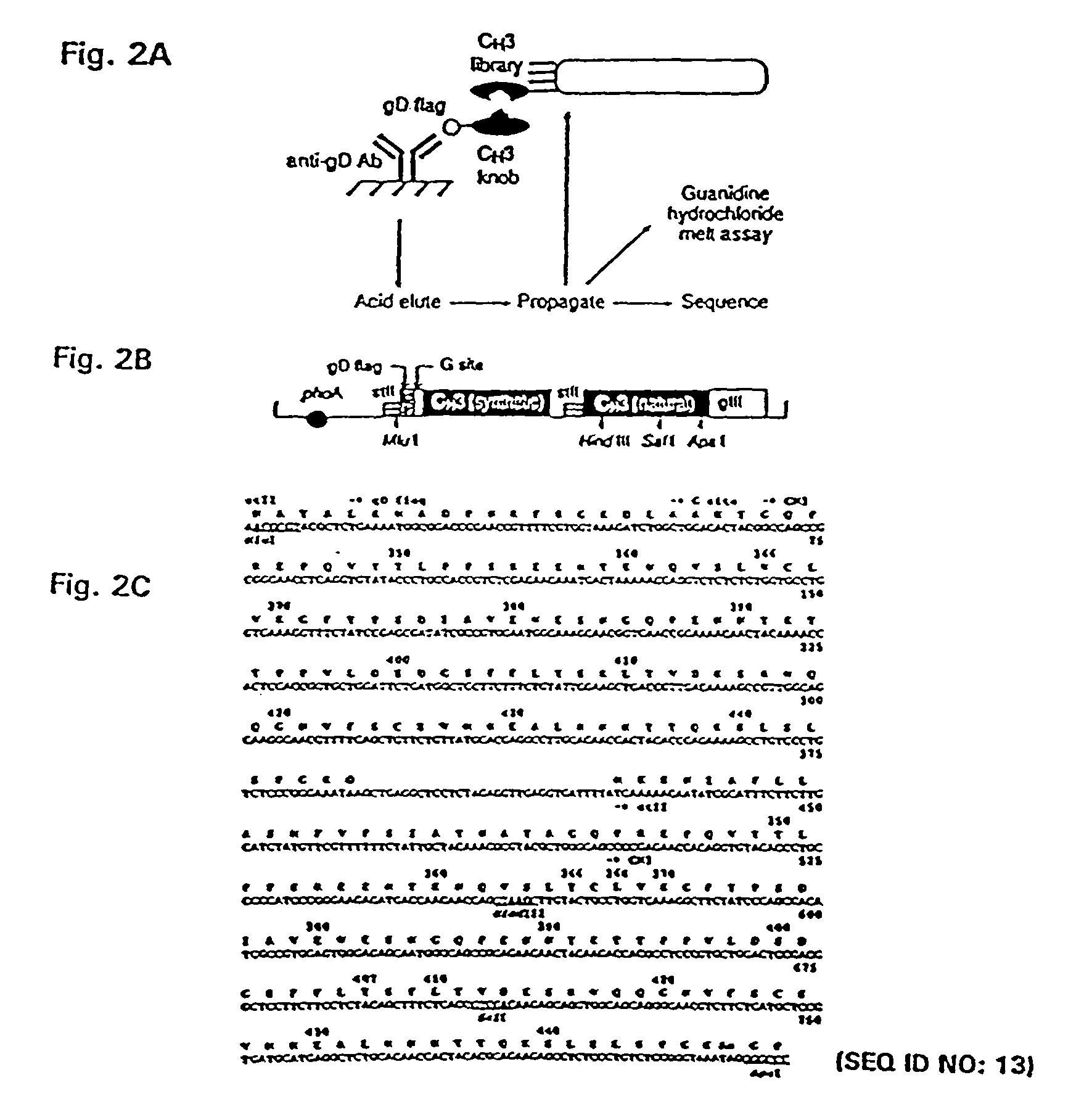
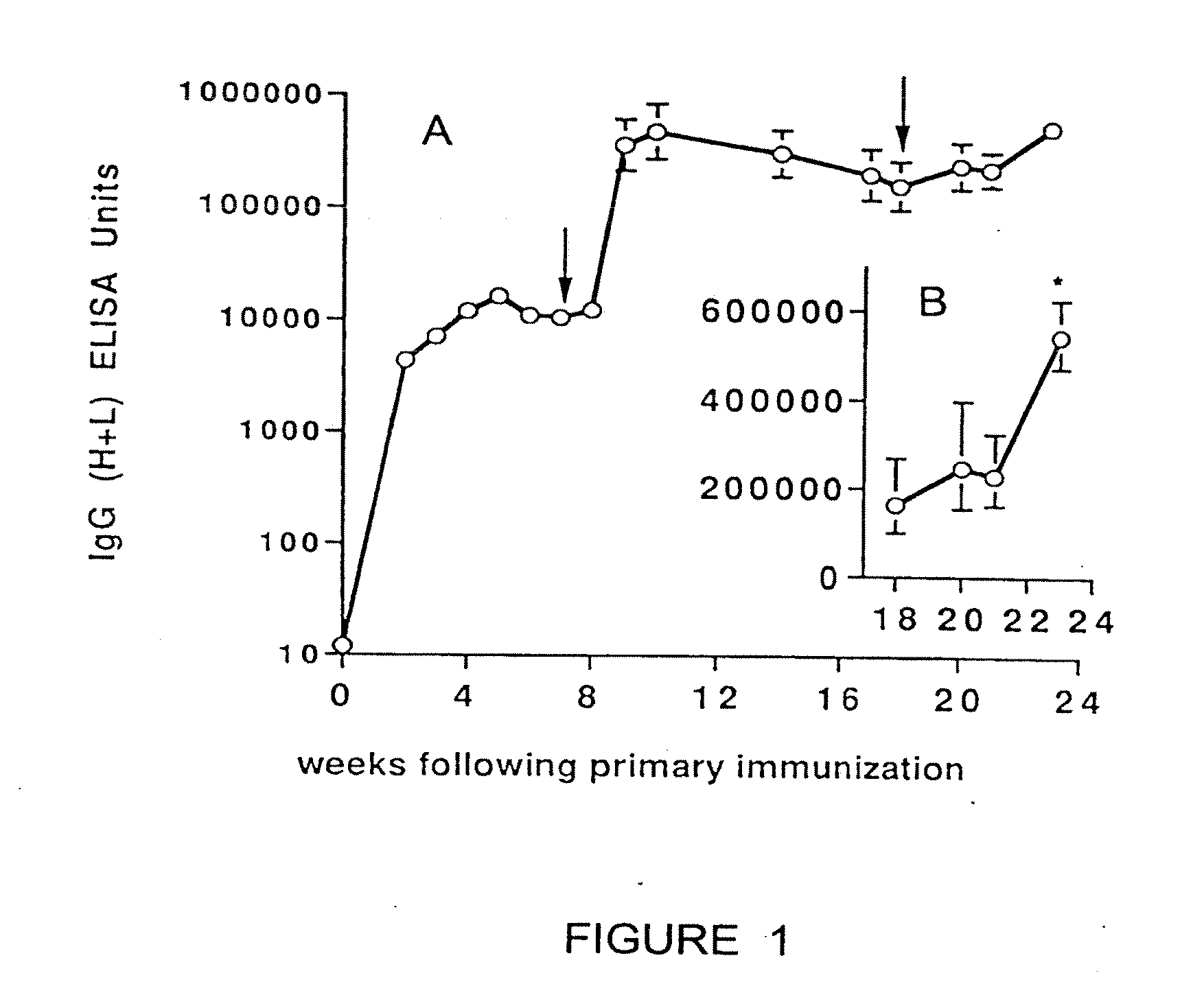
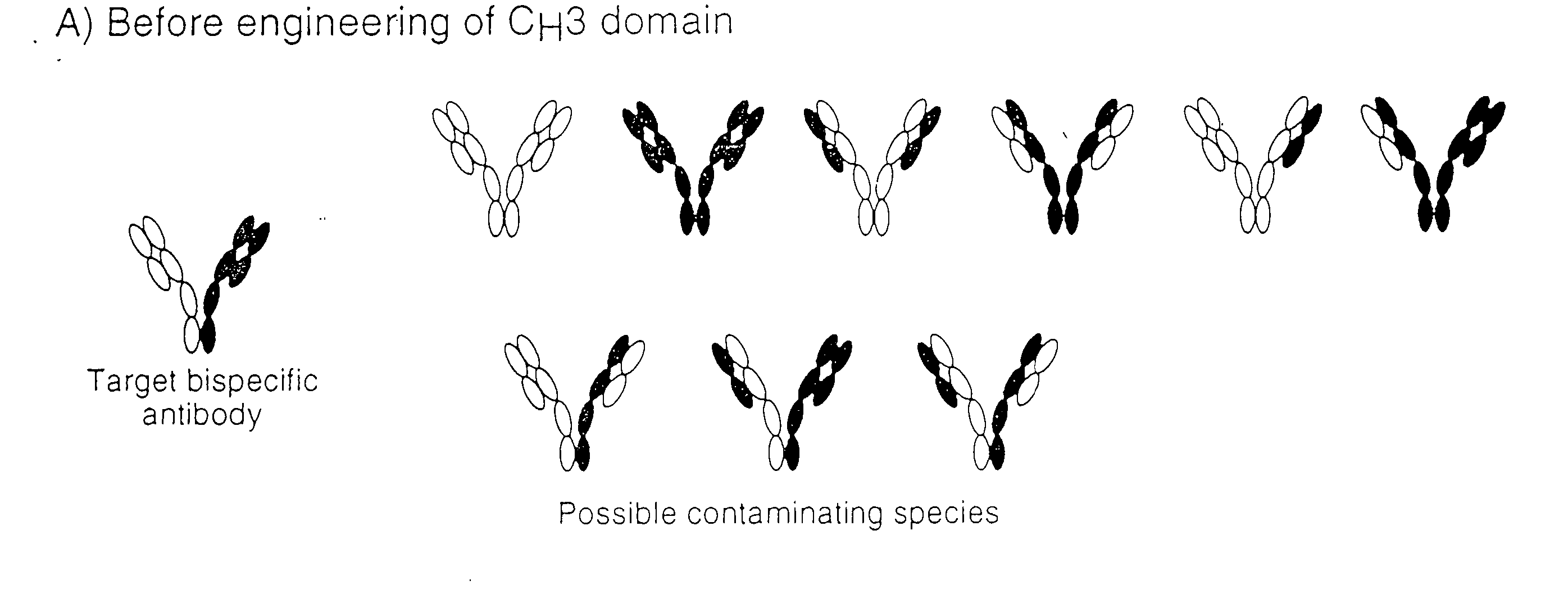Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
950 results about "Specific immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Specific immunity is the body's learned immune response to disease-causing foreign substances, also referred to as pathogens or antigens. It is also commonly called acquired immunity or adaptive immunity.
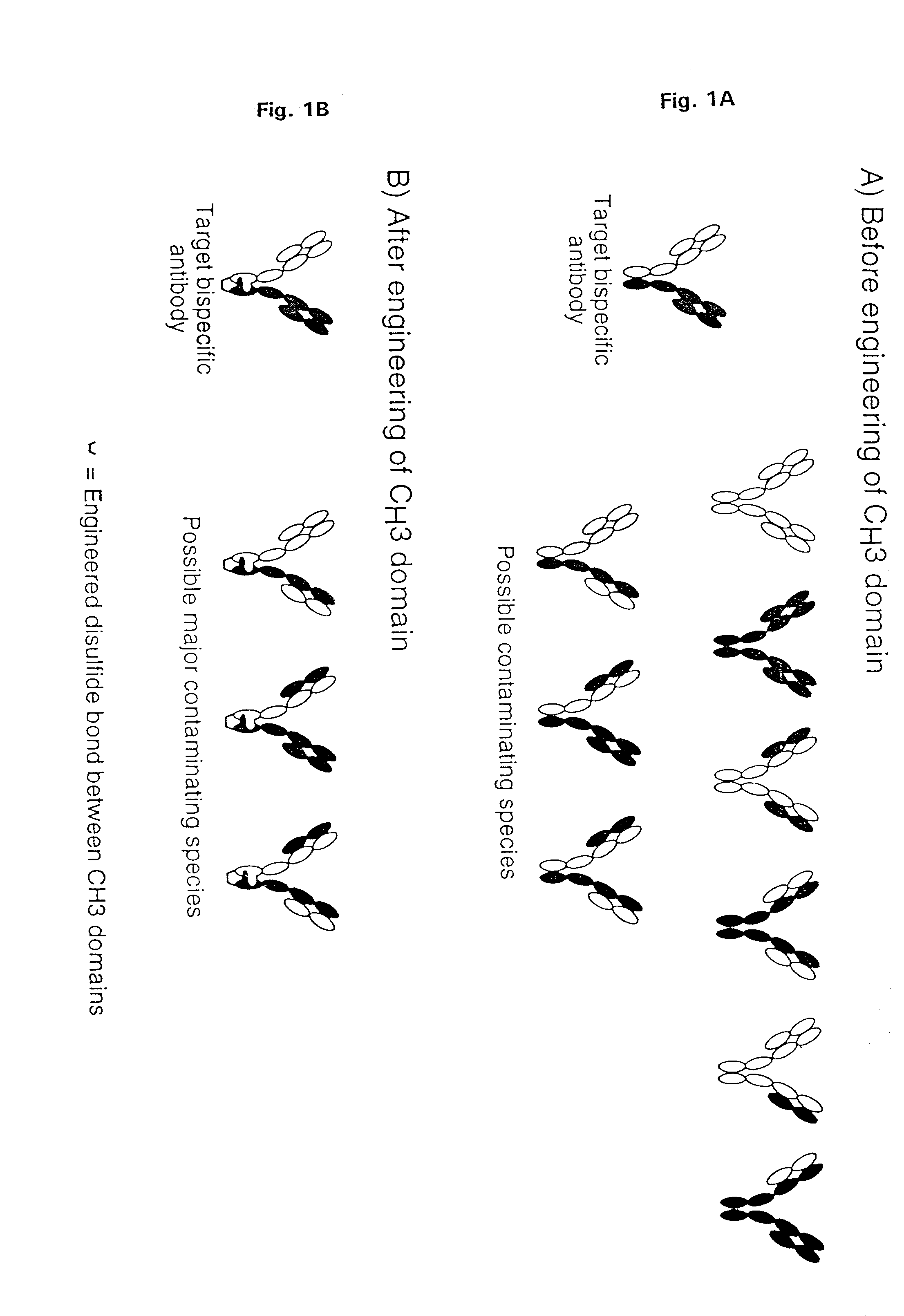
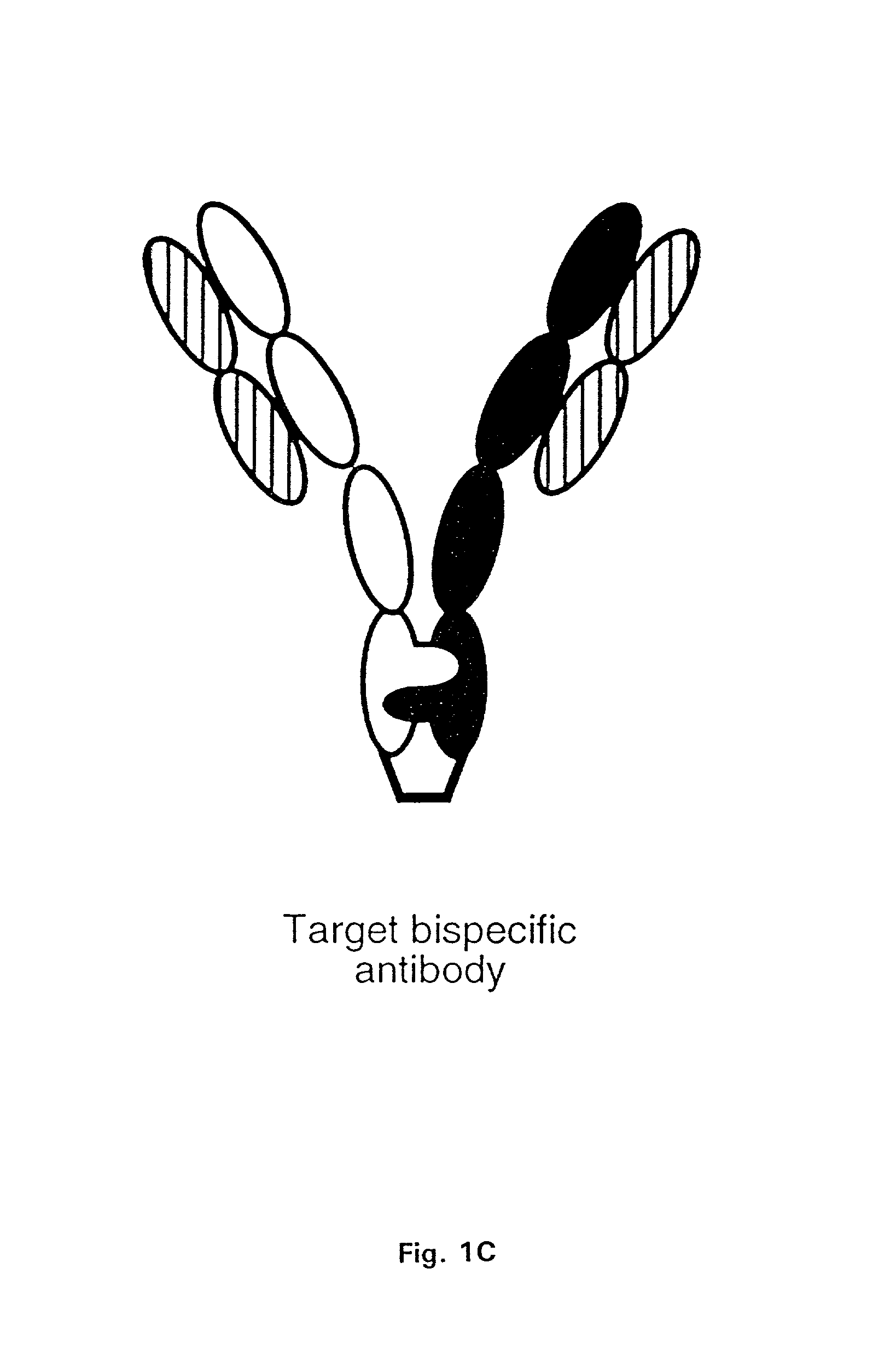
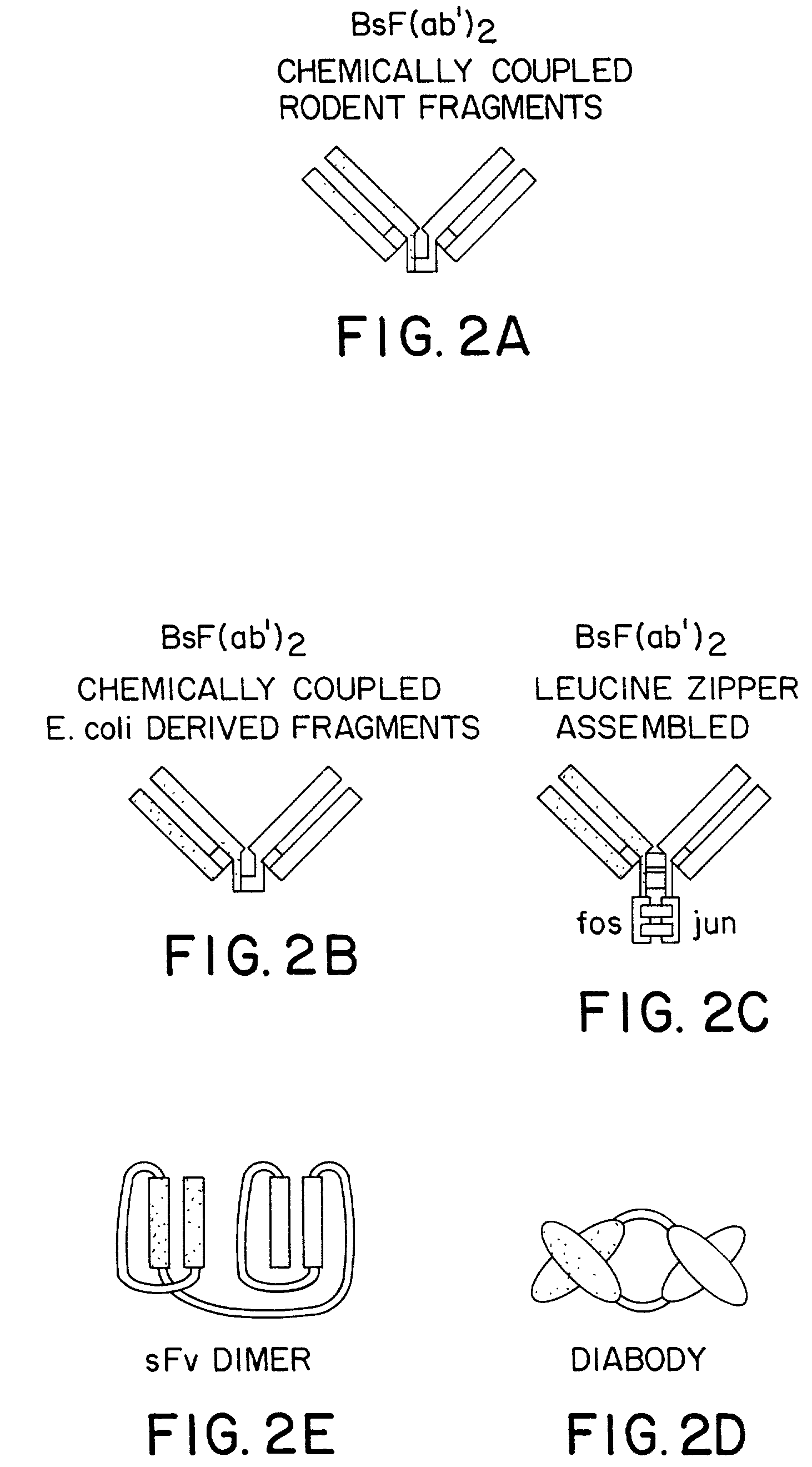
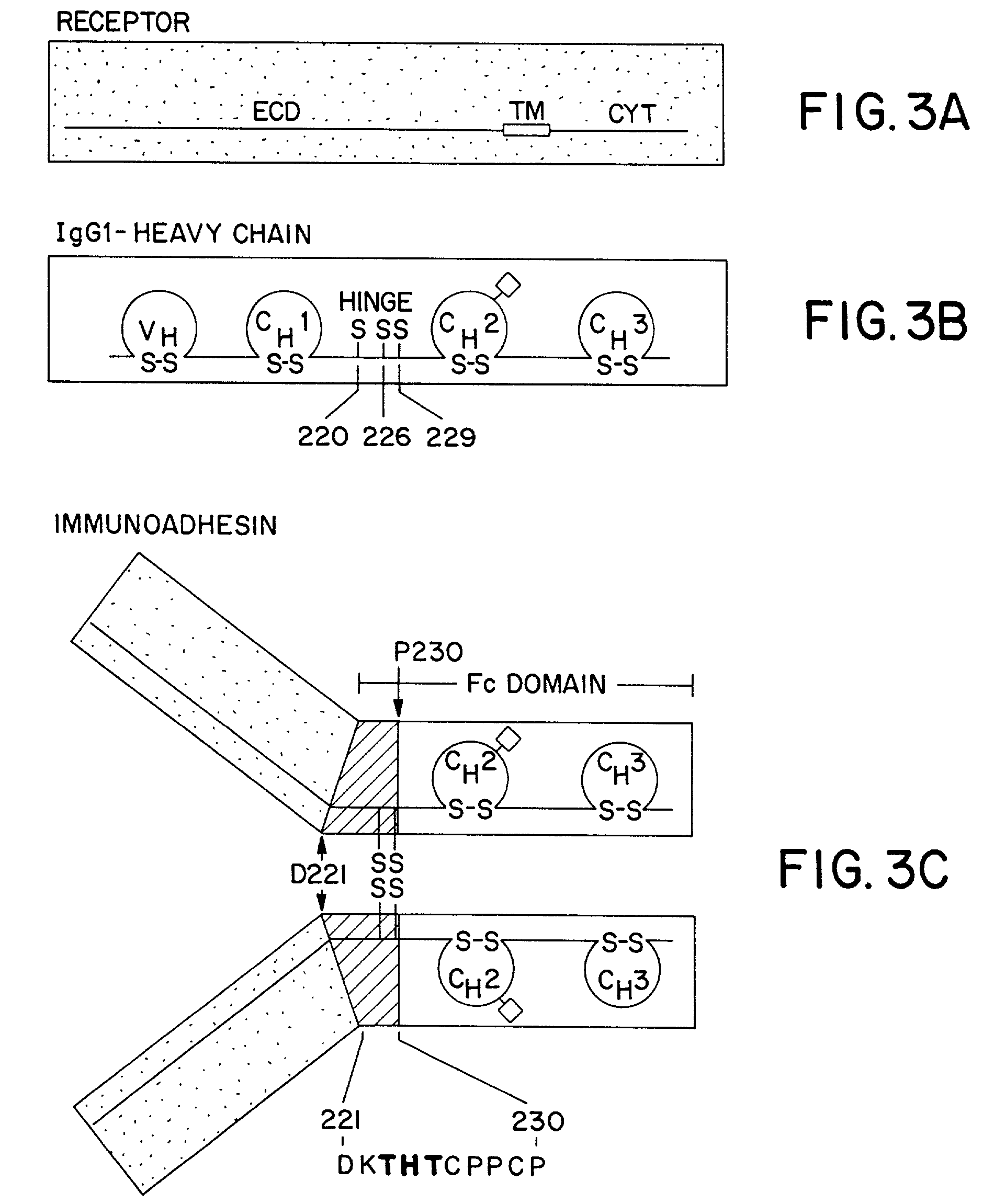
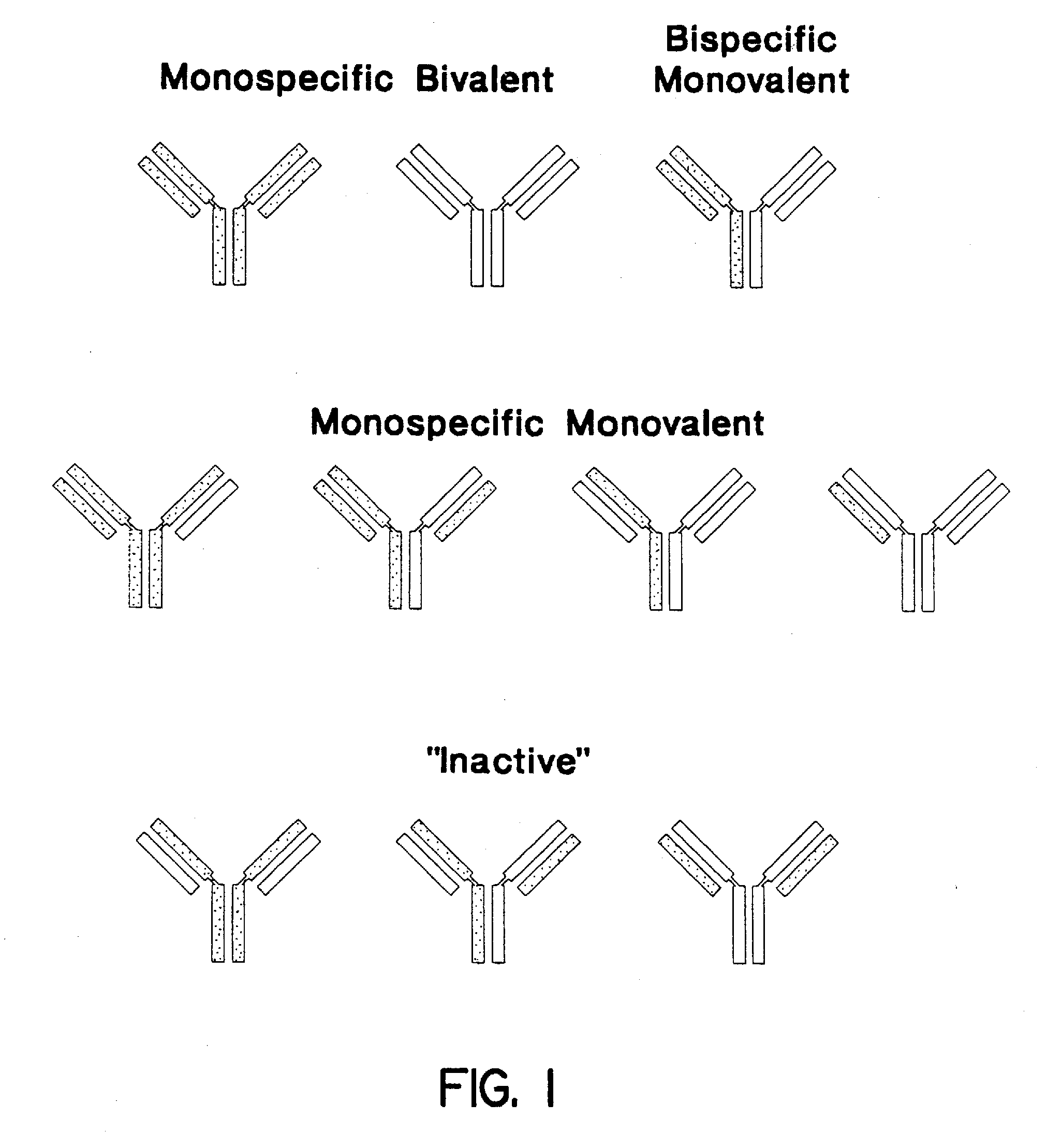
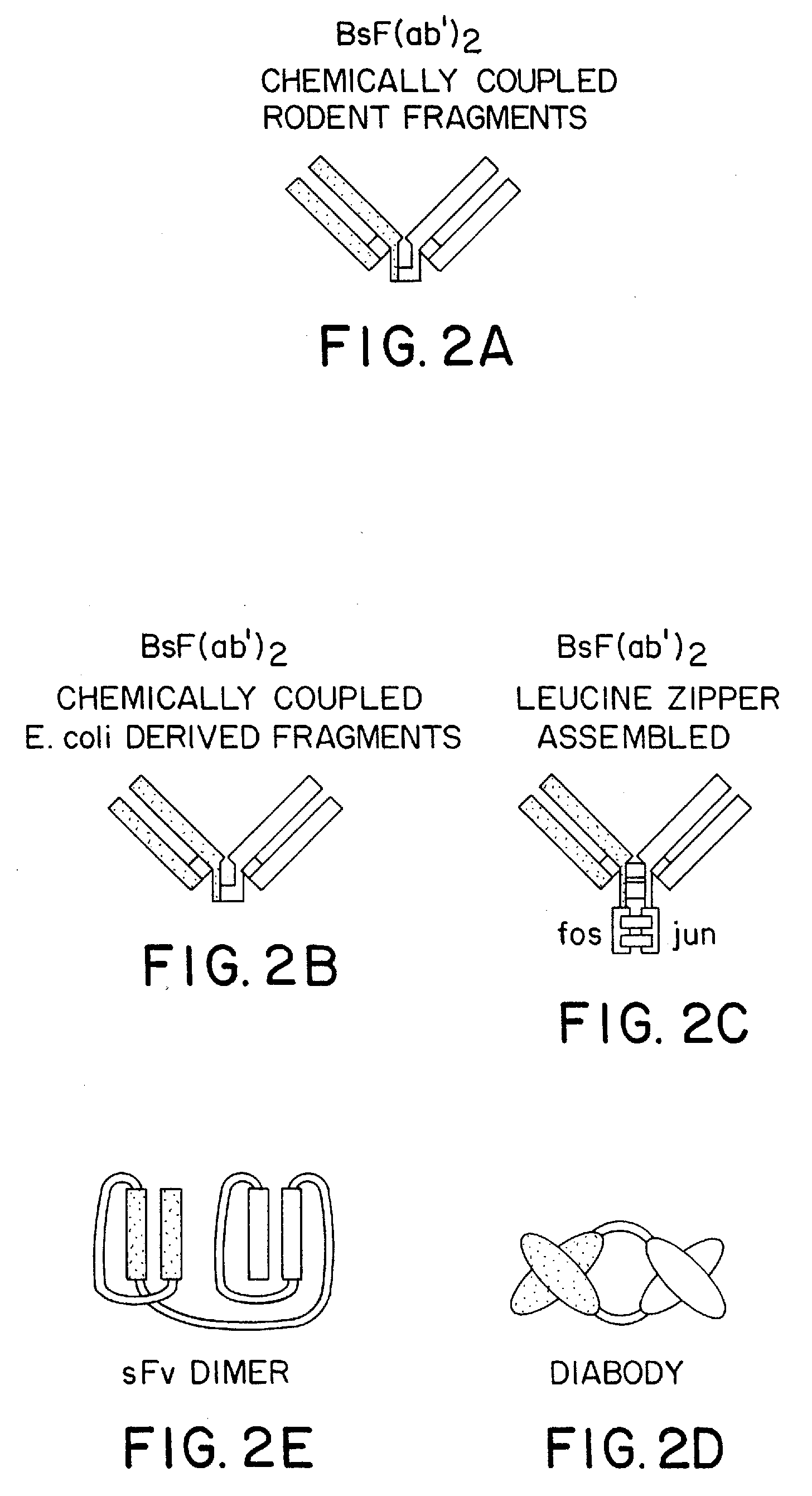
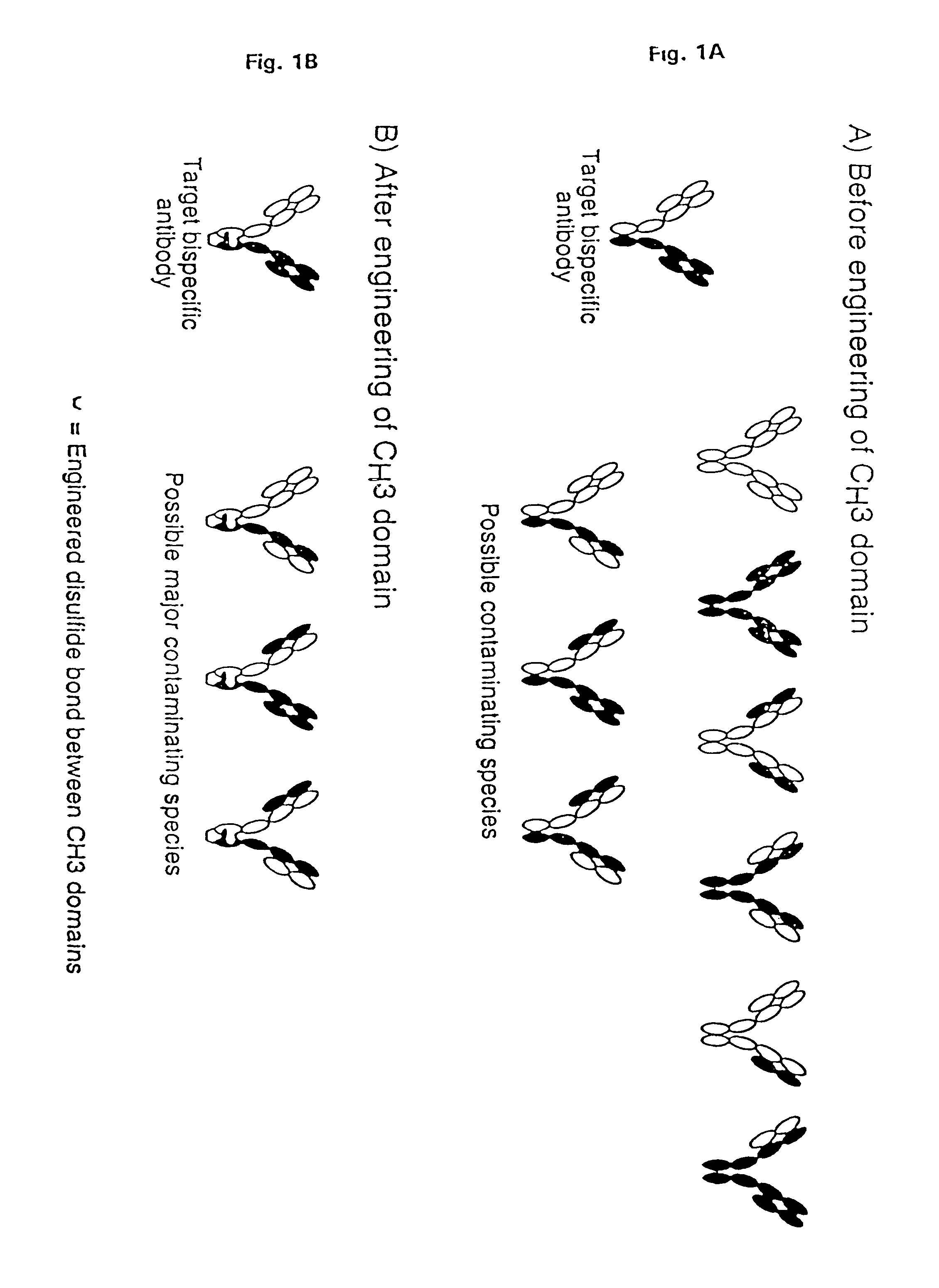
Method for making multispecific antibodies having heteromultimeric and common components
InactiveUS7183076B2Increased formationIncrease productionAnimal cellsAntibody mimetics/scaffoldsSpecific immunityBispecific antibody
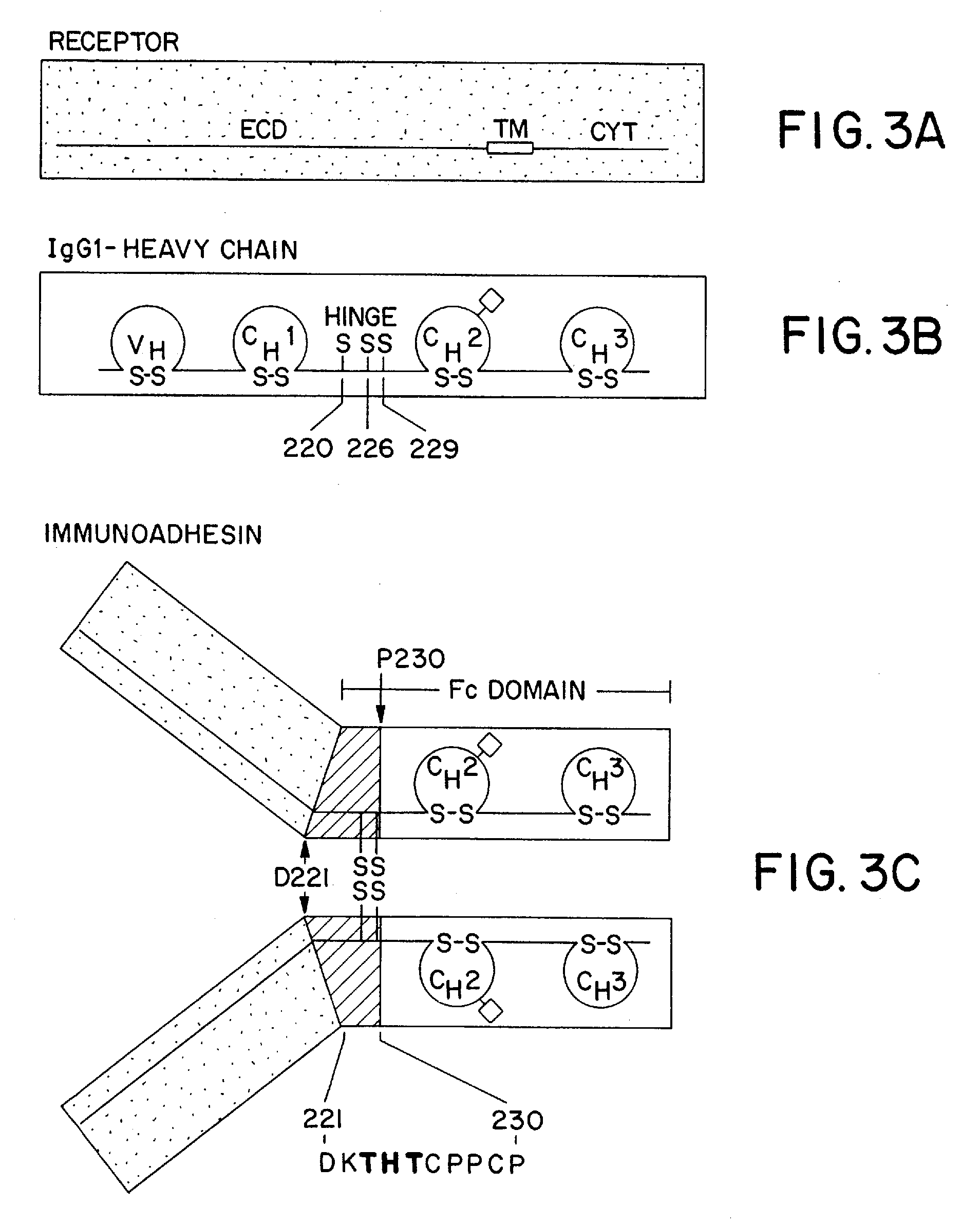
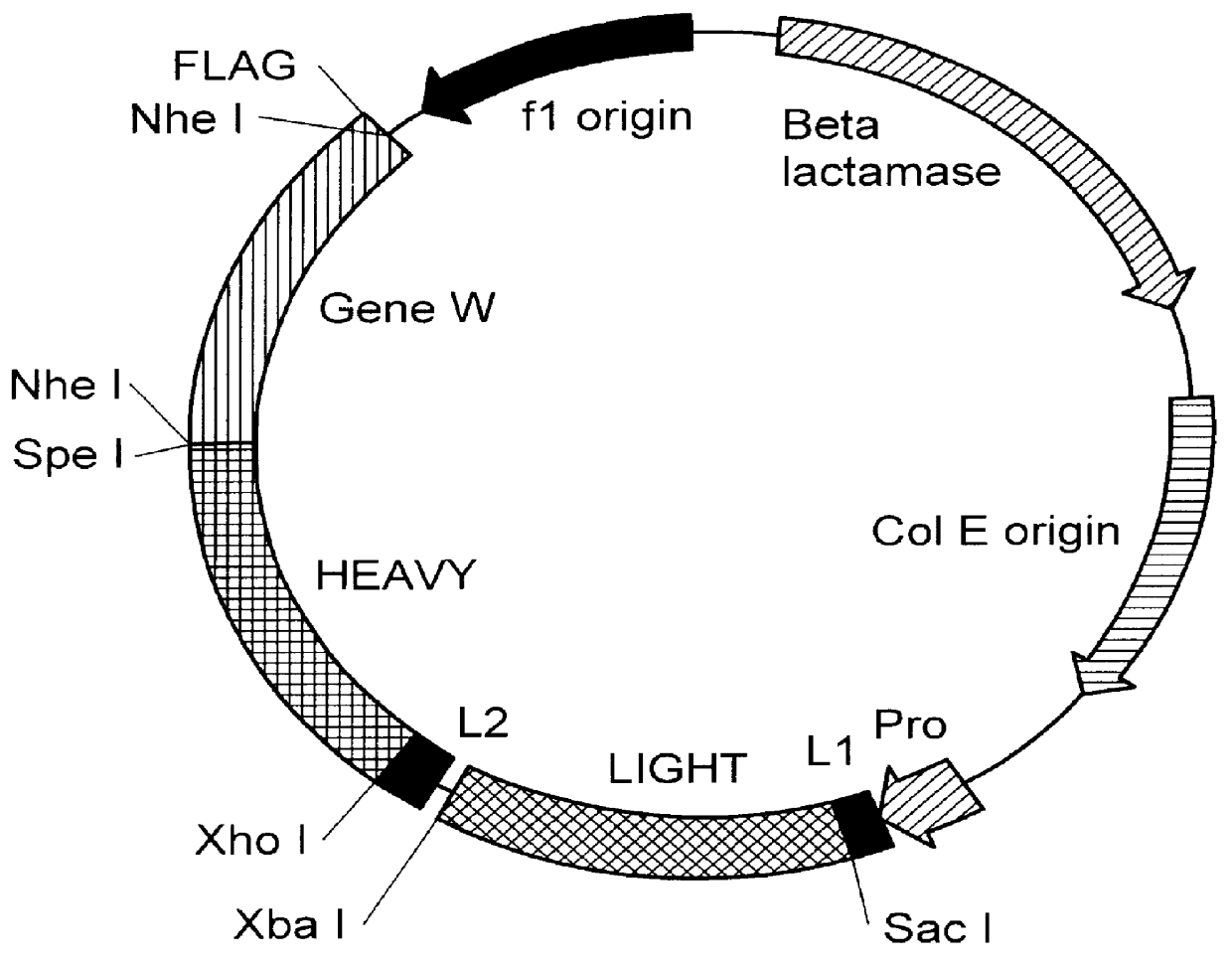
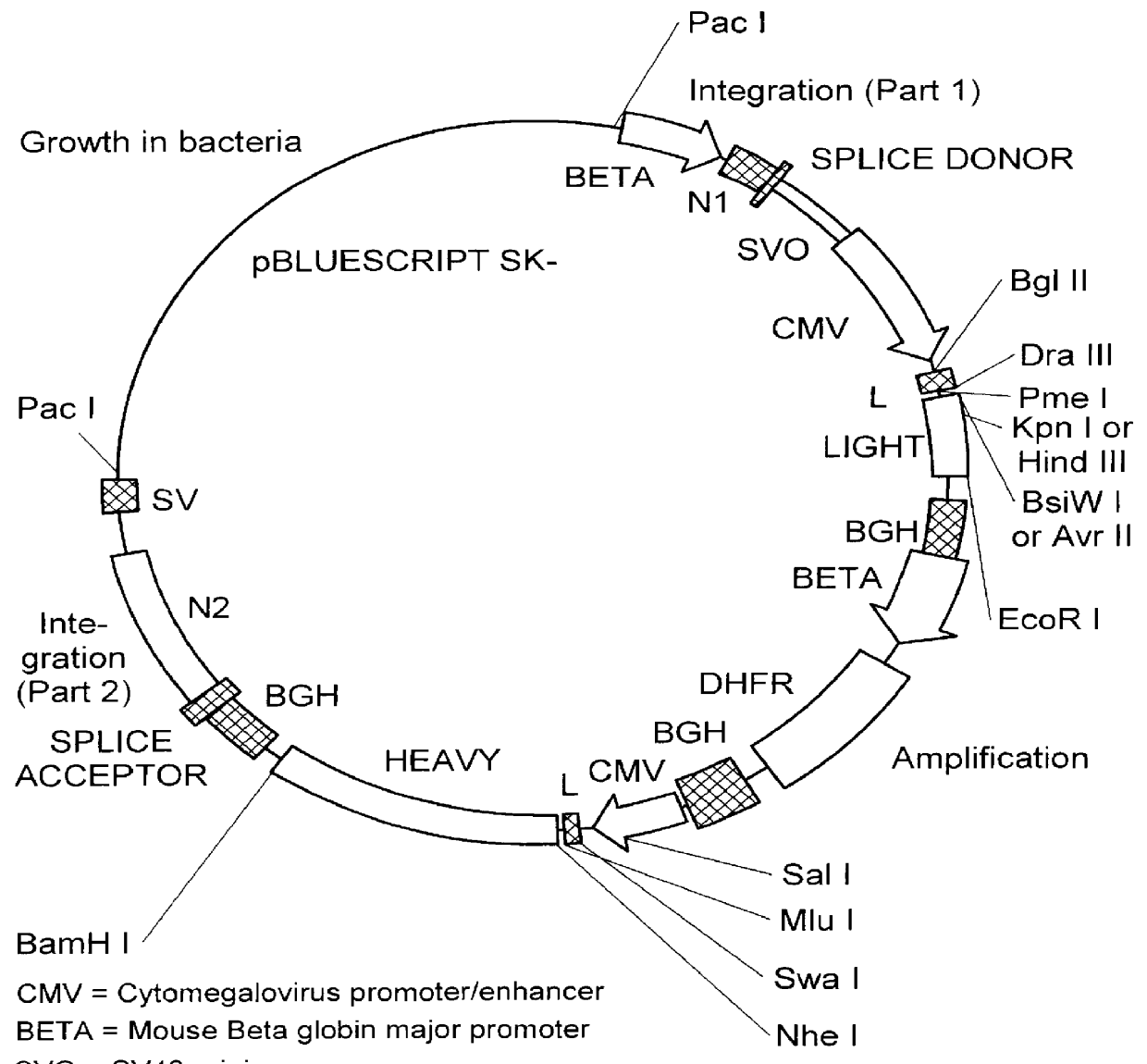
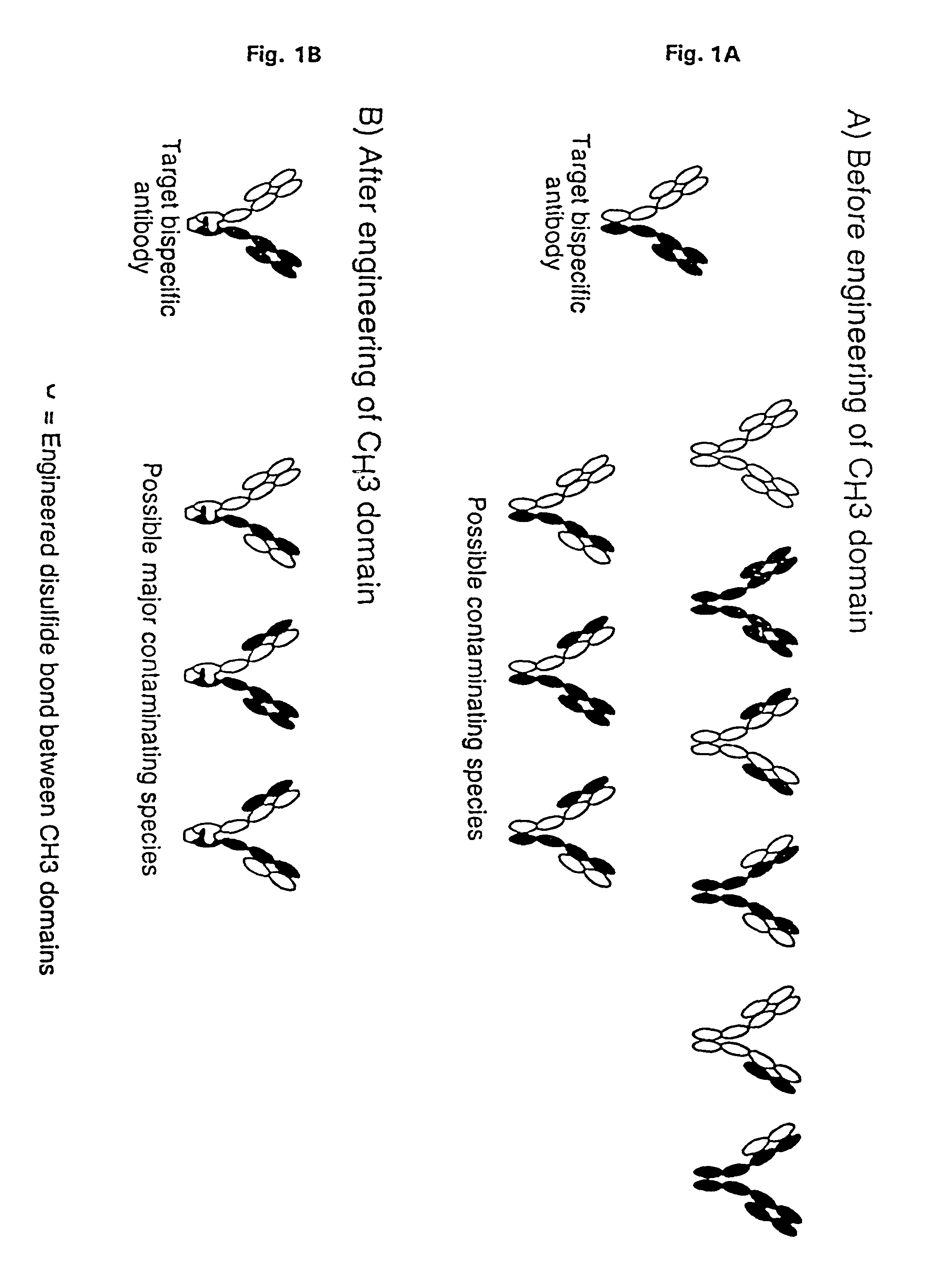
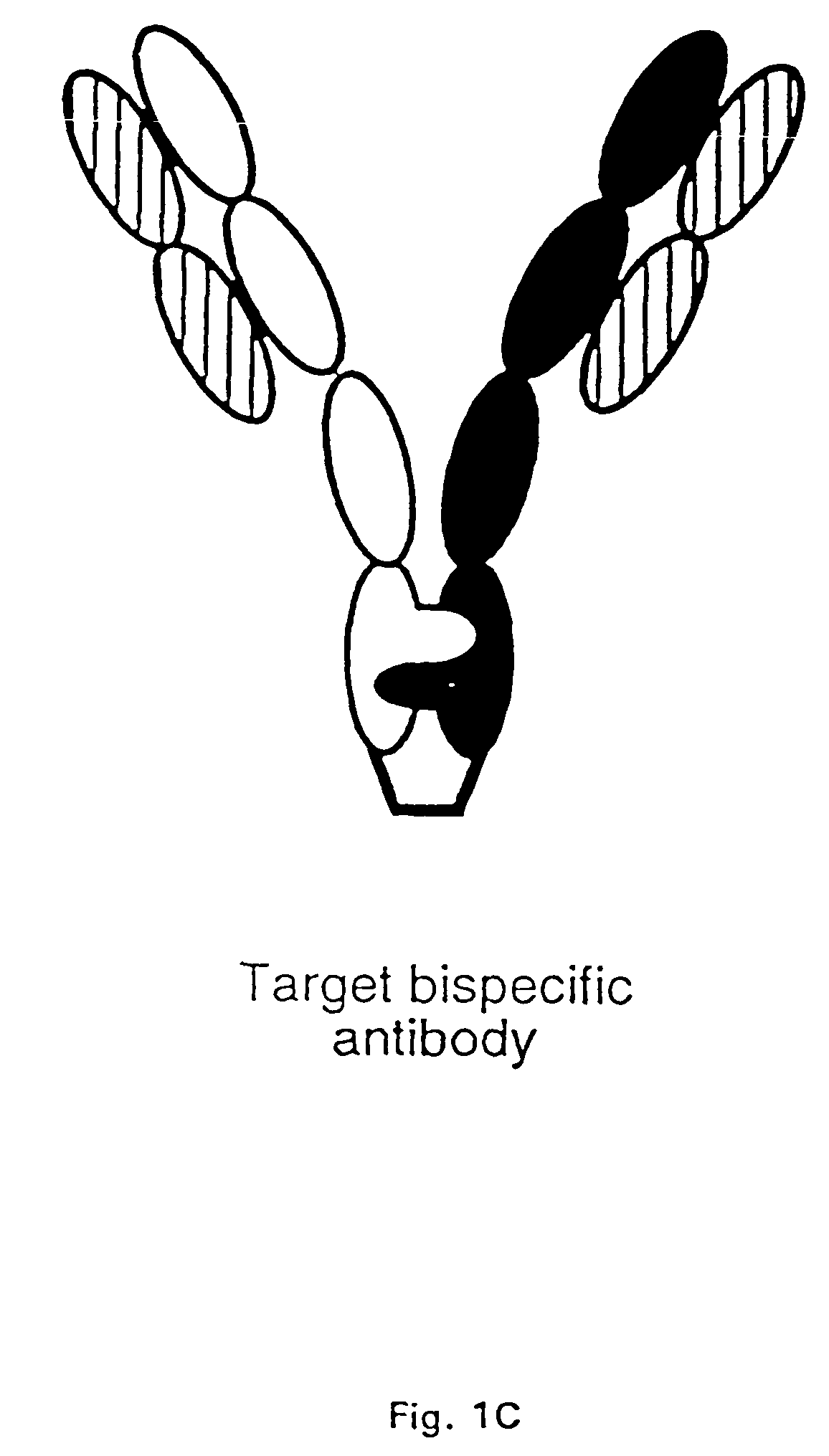
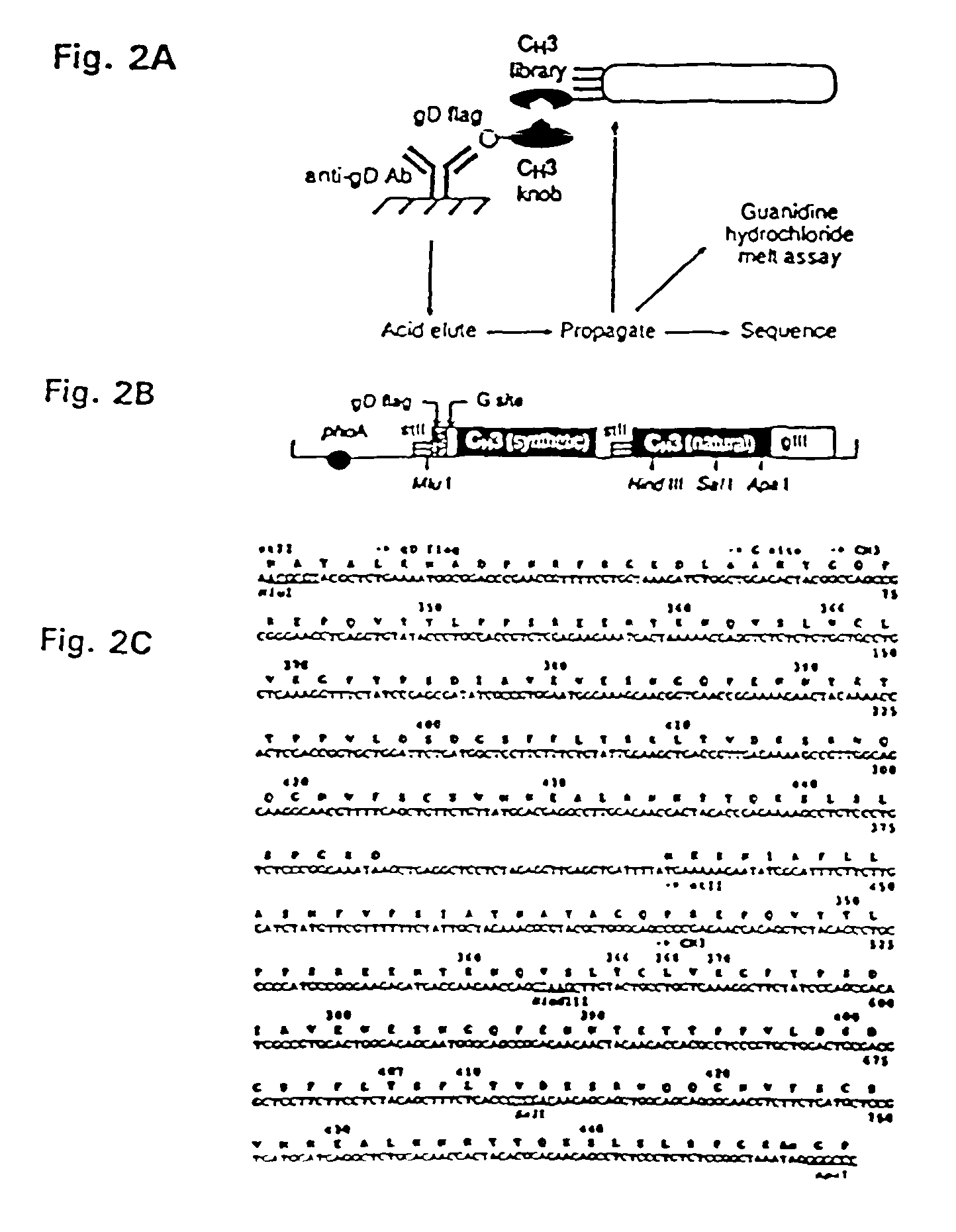
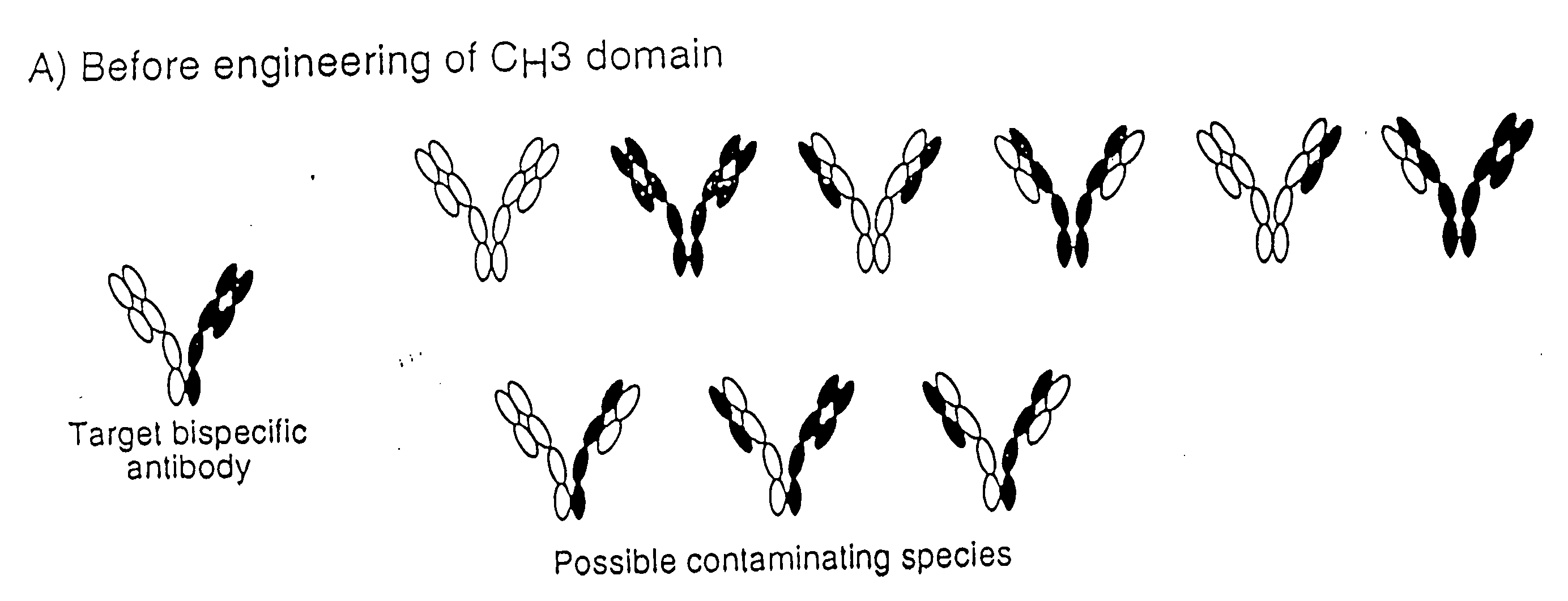
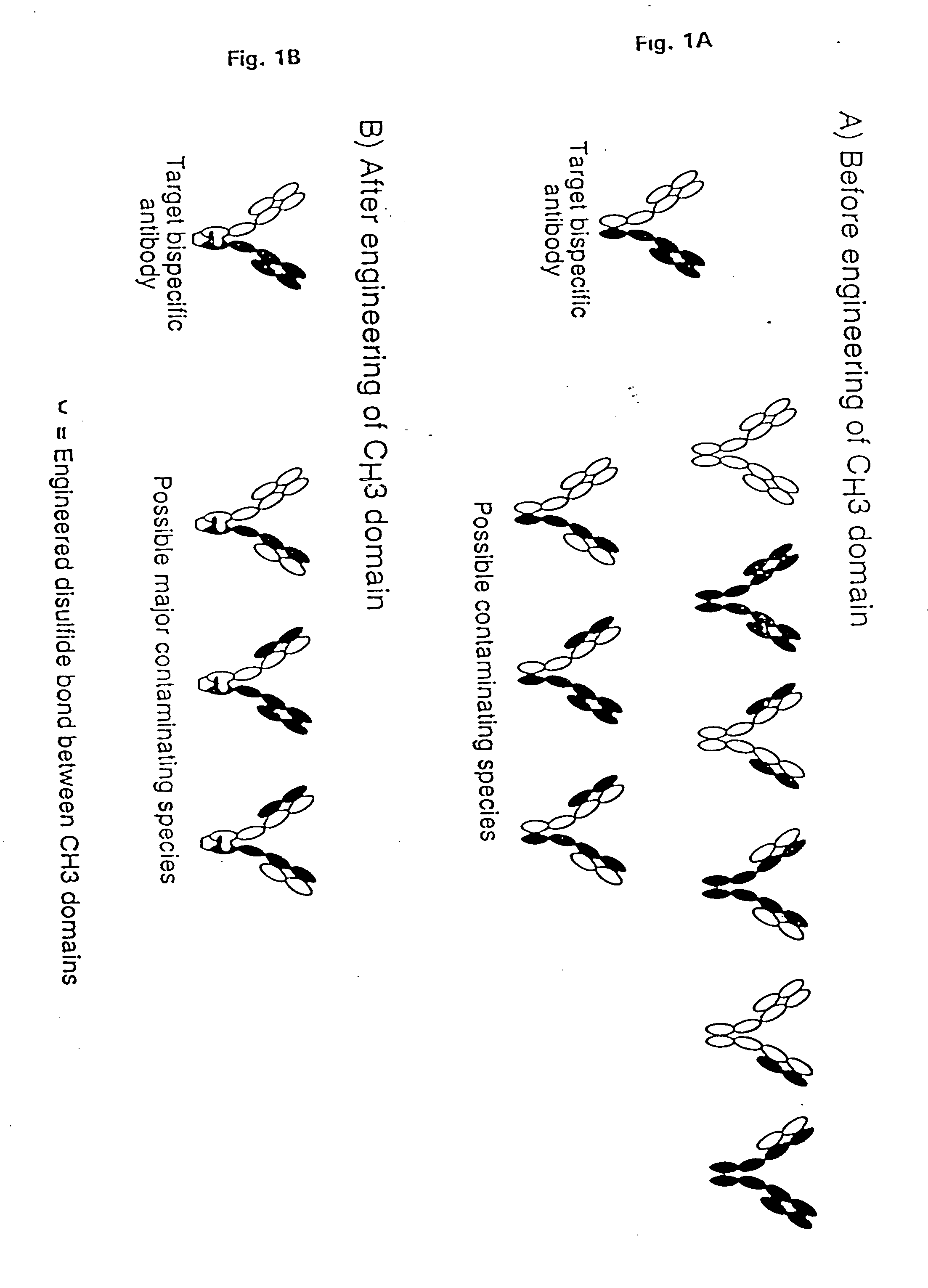
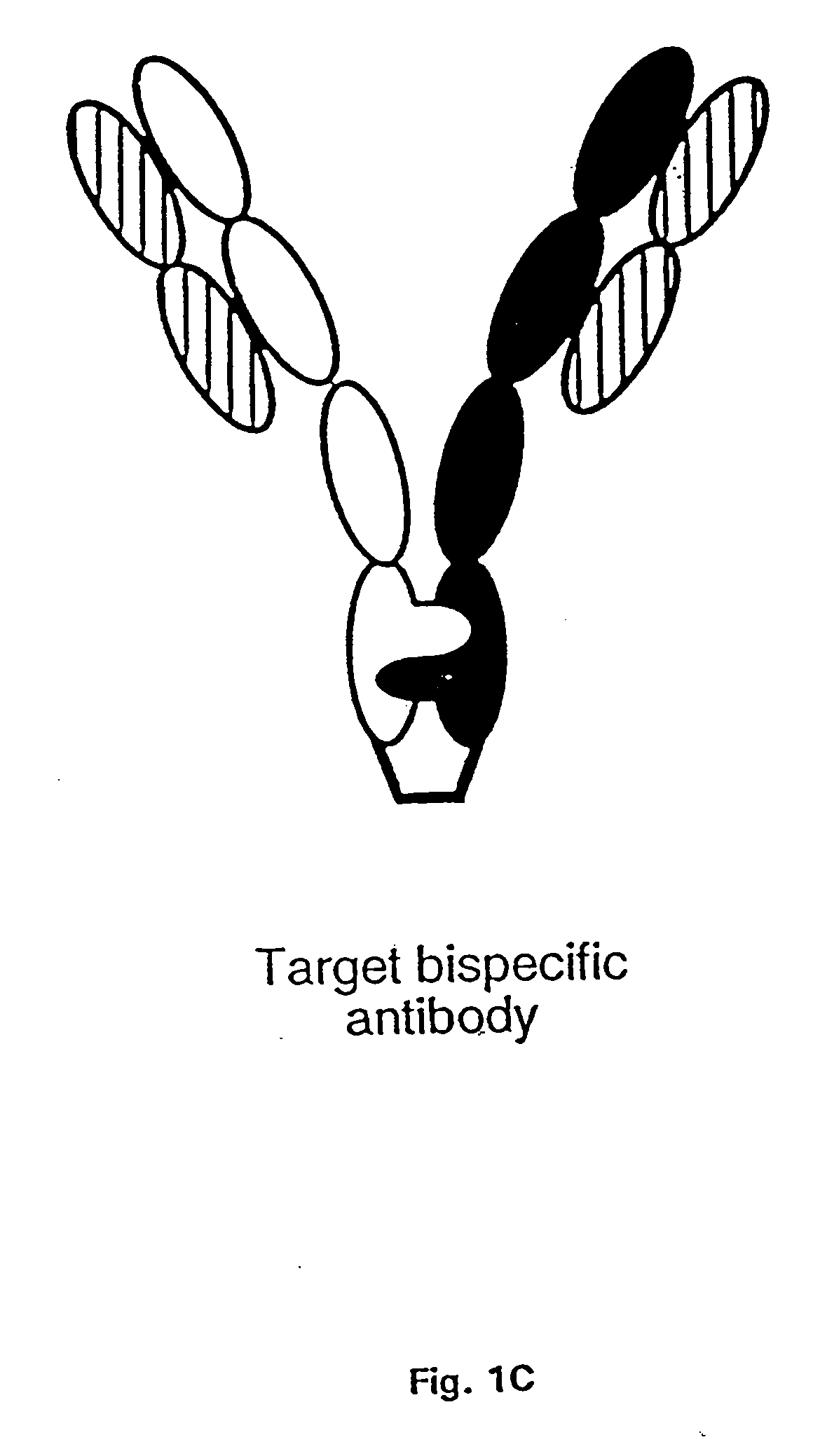
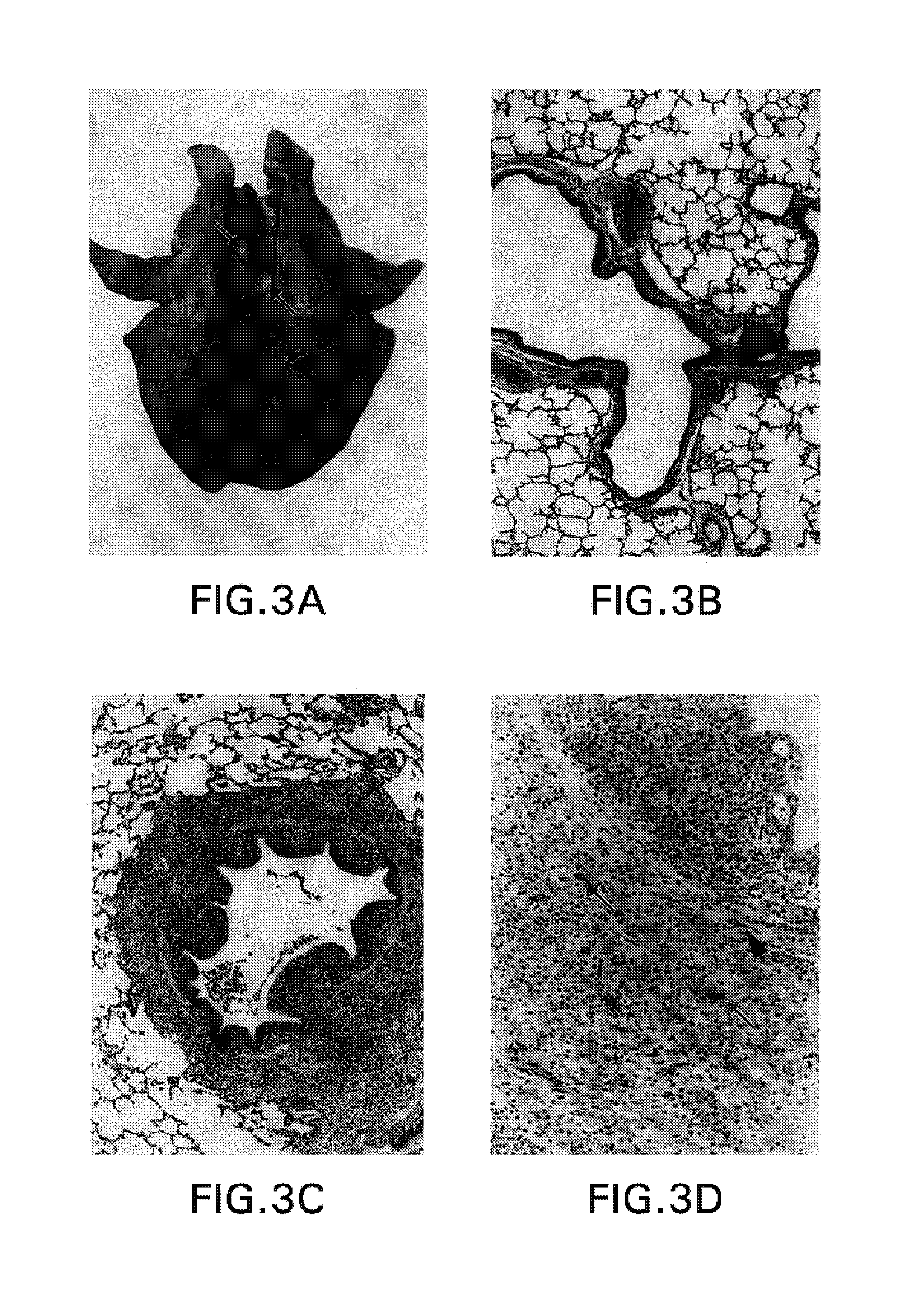
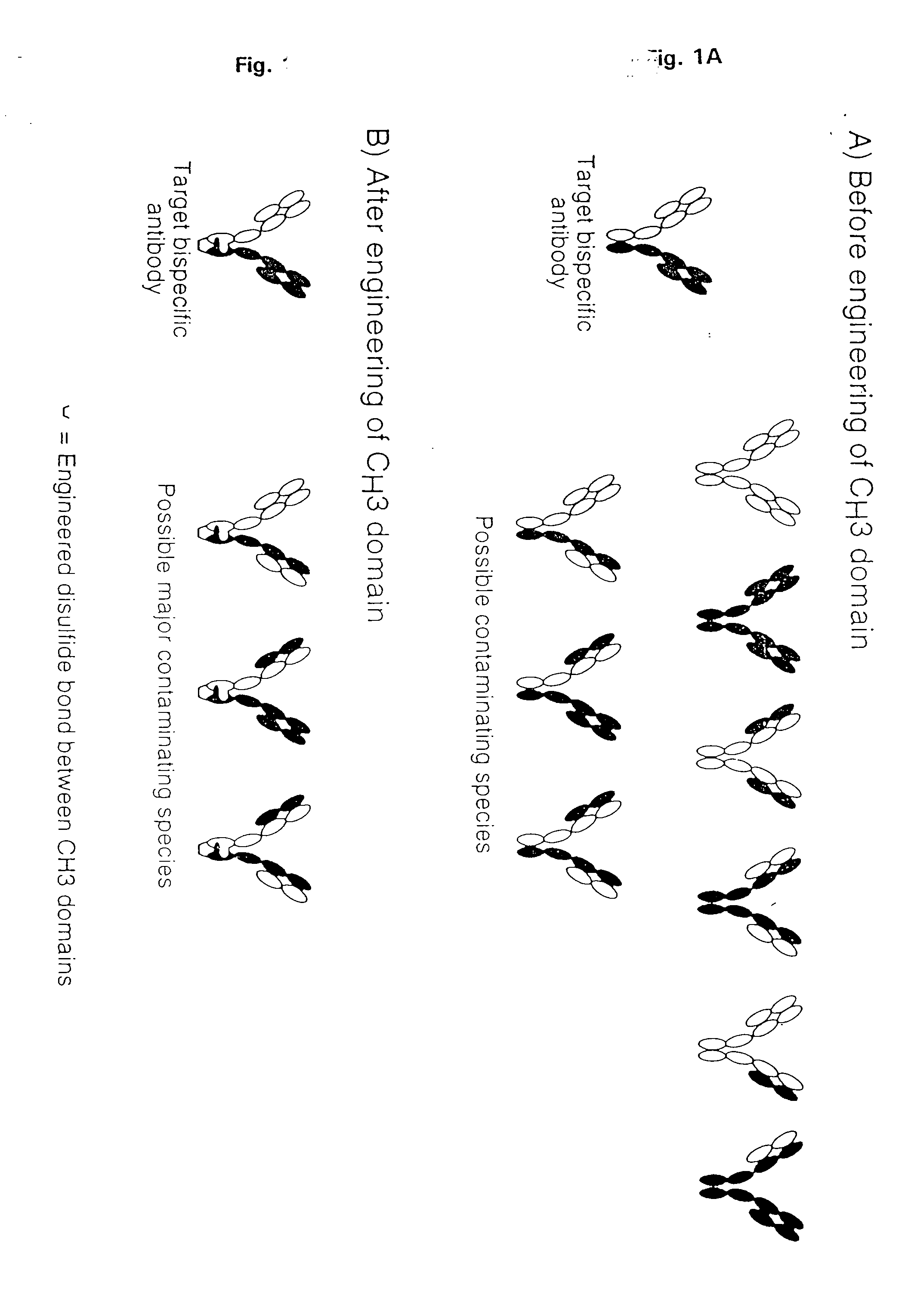
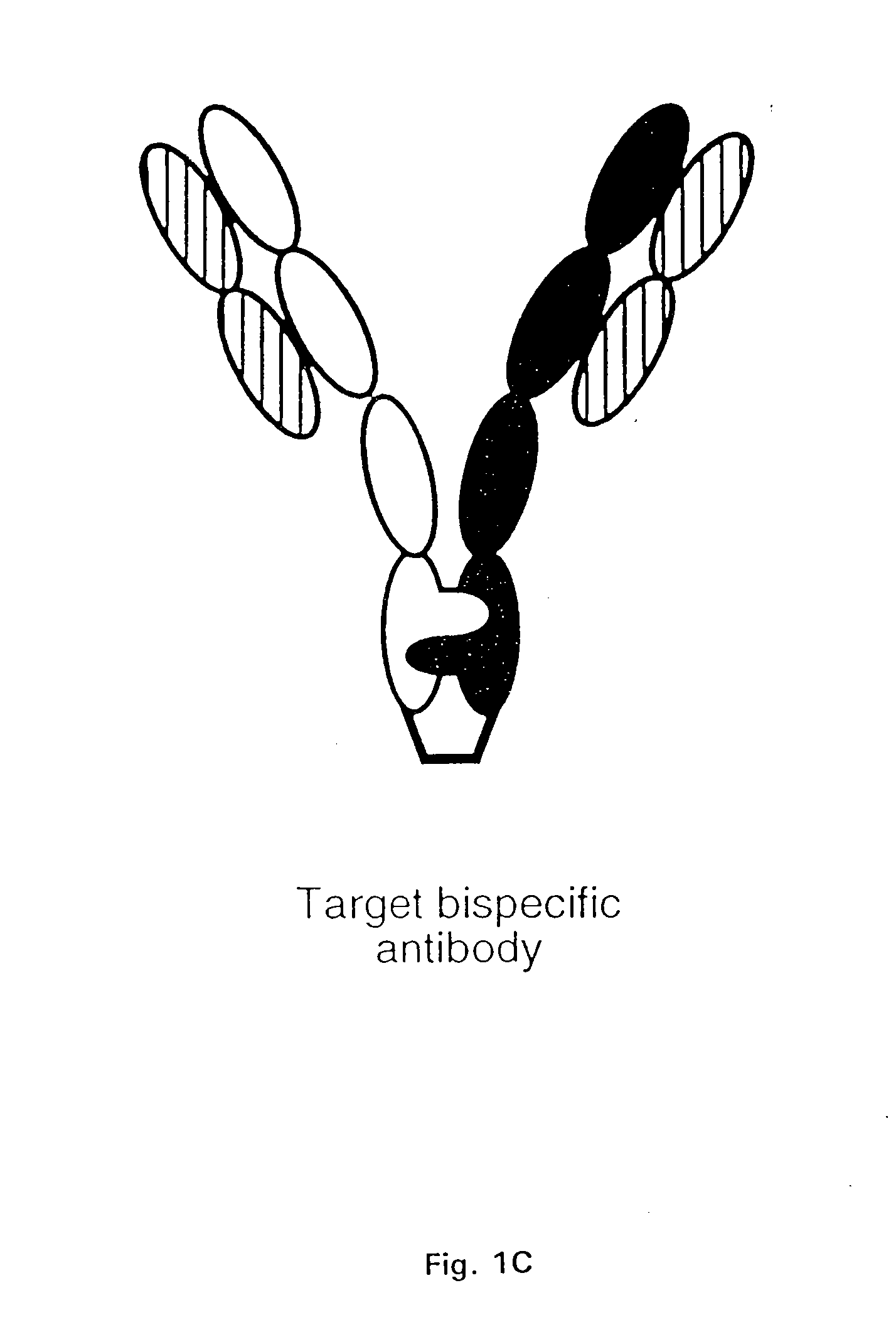
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method provides a multispecific antibody having a common light chain associated with each heteromeric polypeptide having an antibody binding domain. Additionally the method futher involves introducing into the multispecific antibody a specific and complementary interaction at the interface of a first polypeptide and the interface of a second polypeptide, so as to promote heteromultimer formation and hinder homomultimer formation; and / or a free thiol-containing residue at the interface of a first polypeptide and a corresponding free thiol-containing residue in the interface of a second polypeptide, such that a non-naturally occurring disulfide bond is formed between the first and second polypeptide. The method allows for the enhanced formation of the desired heteromultimer relative to undesired heteromultimers and homomultimers.
Owner:GENENTECH INC
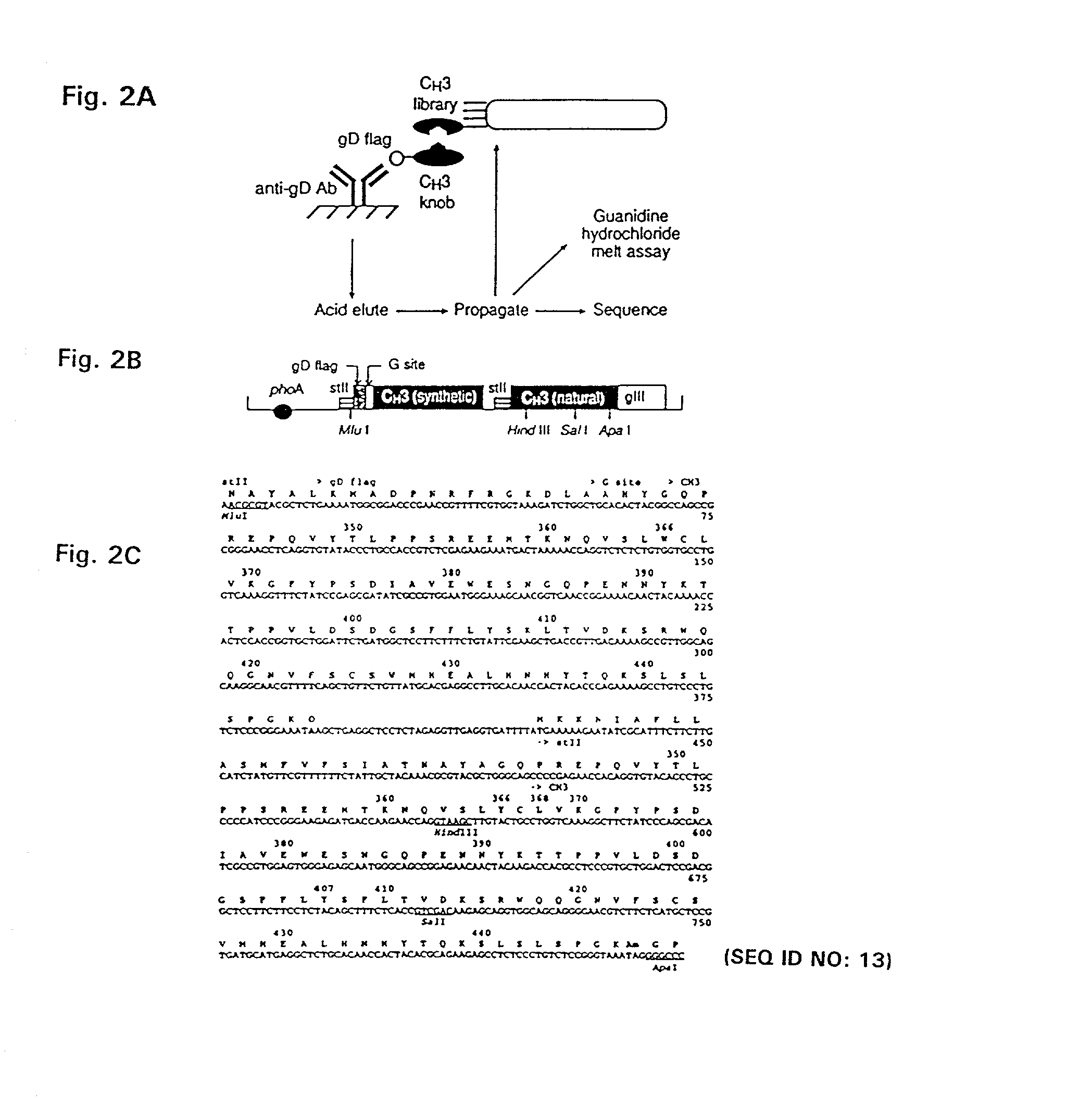
Method for making heteromultimeric polypeptides
InactiveUS7642228B2Increase productionAntibacterial agentsPeptide/protein ingredientsCrystallographyAmino acid side chain
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method involves introducing a protuberance at the interface of a first polypeptide and a corresponding cavity in the interface of a second polypeptide, such that the protuberance can be positioned in the cavity so as to promote heteromultimer formation and hinder homomultimer formation. “Protuberances” are constructed by replacing small amino acid side chains from the interface of the first polypeptide with larger side chains (e.g. tyrosine or tryptophan). Compensatory “cavities” of identical or similar size to the protuberances are created in the interface of the second polypeptide by replacing large amino acid side chains with smaller ones (e.g. alanine or threonine). The protuberance and cavity can be made by synthetic means such as altering the nucleic acid encoding the polypeptides or by peptide synthesis.
Owner:GENENTECH INC
Method for making heteromultimeric polypeptides
InactiveUS20070014794A1Increase productionAntibacterial agentsPeptide/protein ingredientsCrystallographyAmino acid side chain
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method involves introducing a protuberance at the interface of a first polypeptide and a corresponding cavity in the interface of a second polypeptide, such that the protuberance can be positioned in the cavity so as to promote heteromultimer formation and hinder homomultimer formation. “Protuberances” are constructed by replacing small amino acid side chains from the interface of the first polypeptide with larger side chains (e.g. tyrosine or tryptophan). Compensatory “cavities” of identical or similar size to the protuberances are created in the interface of the second polypeptide by replacing large amino acid side chains with smaller ones (e.g. alanine or threonine). The protuberance and cavity can be made by synthetic means such as altering the nucleic acid encoding the polypeptides or by peptide synthesis.
Owner:GENENTECH INC
Human B7.1-specific primatized antibodies and transfectomas expressing said antibodies
InactiveUS6113898AShrink tumorInhibit tumor growthPeptide/protein ingredientsAntipyreticDiseaseOrgan transplant rejection
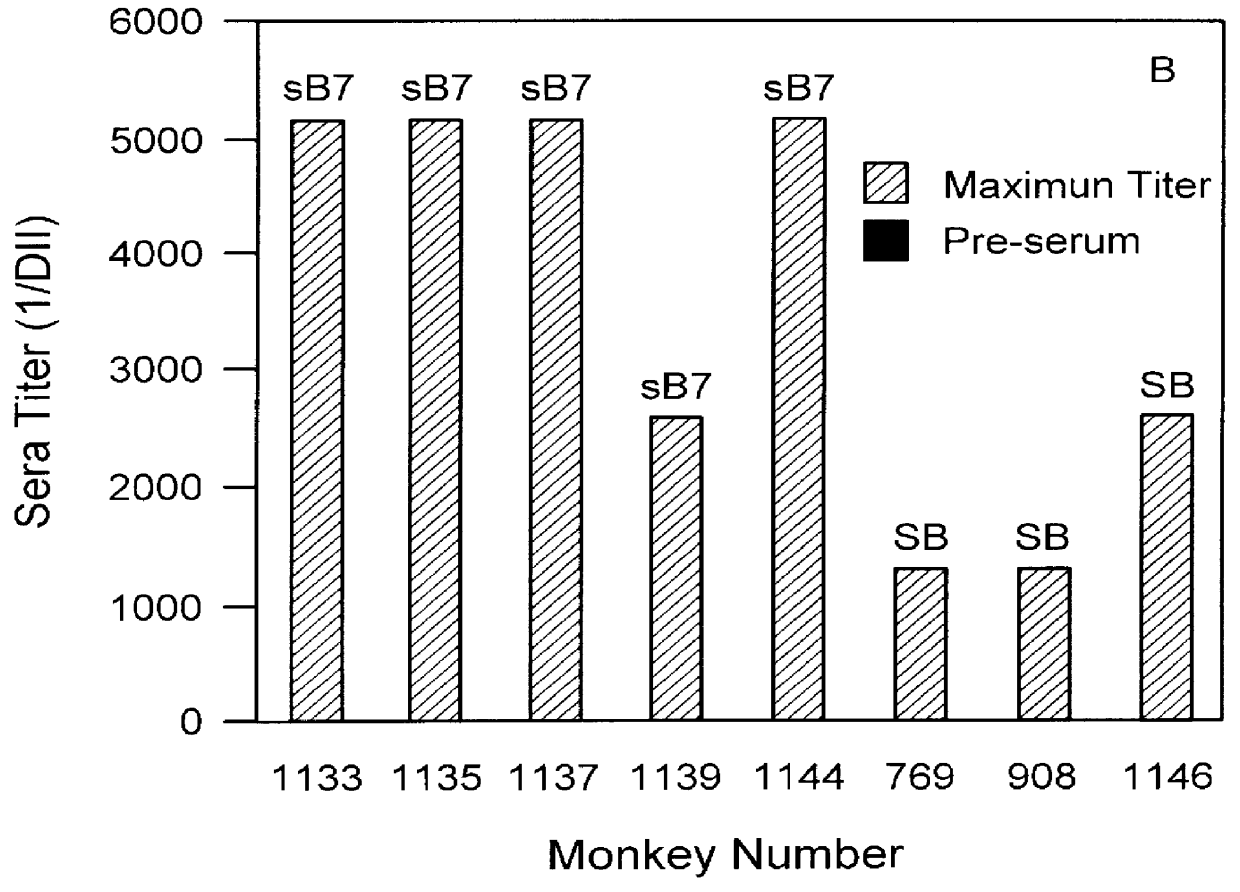
The present invention relates to the identification of macaque antibodies to human B7.1 and B7.2 by screening of phage display libraries or monkey heterohybridomas obtained using B lymphocytes from B7.1 and / or B7.2 immunized monkeys. More specifically, the invention provides four monkey monoclonal antibodies 7B6, 16C10, 7C10 and 20C9 which inhibit the B7:CD28 pathway and thereby function as effective immunosuppressants. The invention further provides the complete DNA and amino acid sequences of the light and heavy chain of three primatized antibodies derived from those monkey monoclonal antibodies which bind B7.1 and possibly B7.2, primatized 7C10, primatized 7B6 and primatized 16C10. These primatized and monkey antibodies may be used as specific immunosuppressants, e.g., for the treatment of autoimmune diseases and to prevent organ transplant rejection.
Owner:BIOGEN INC
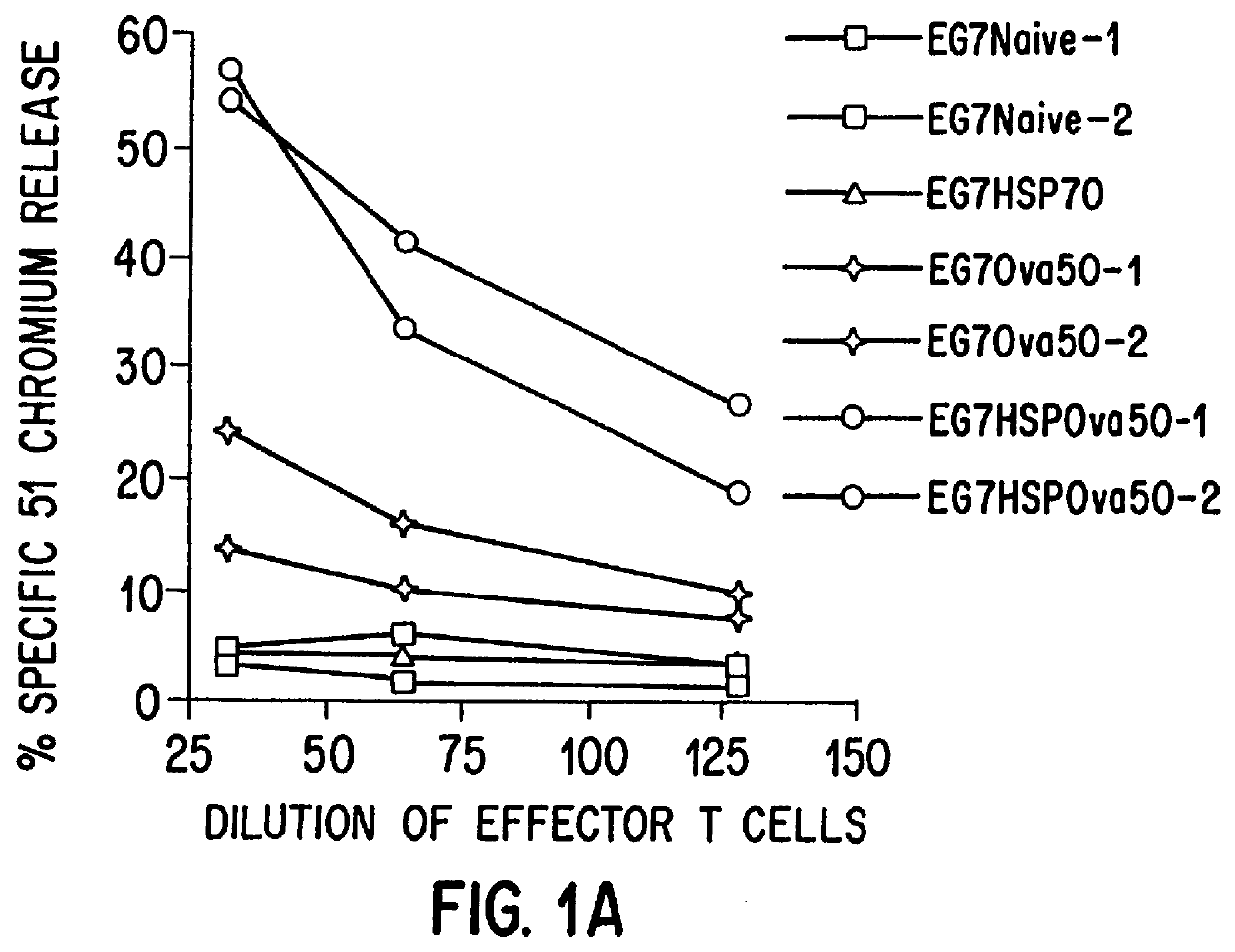
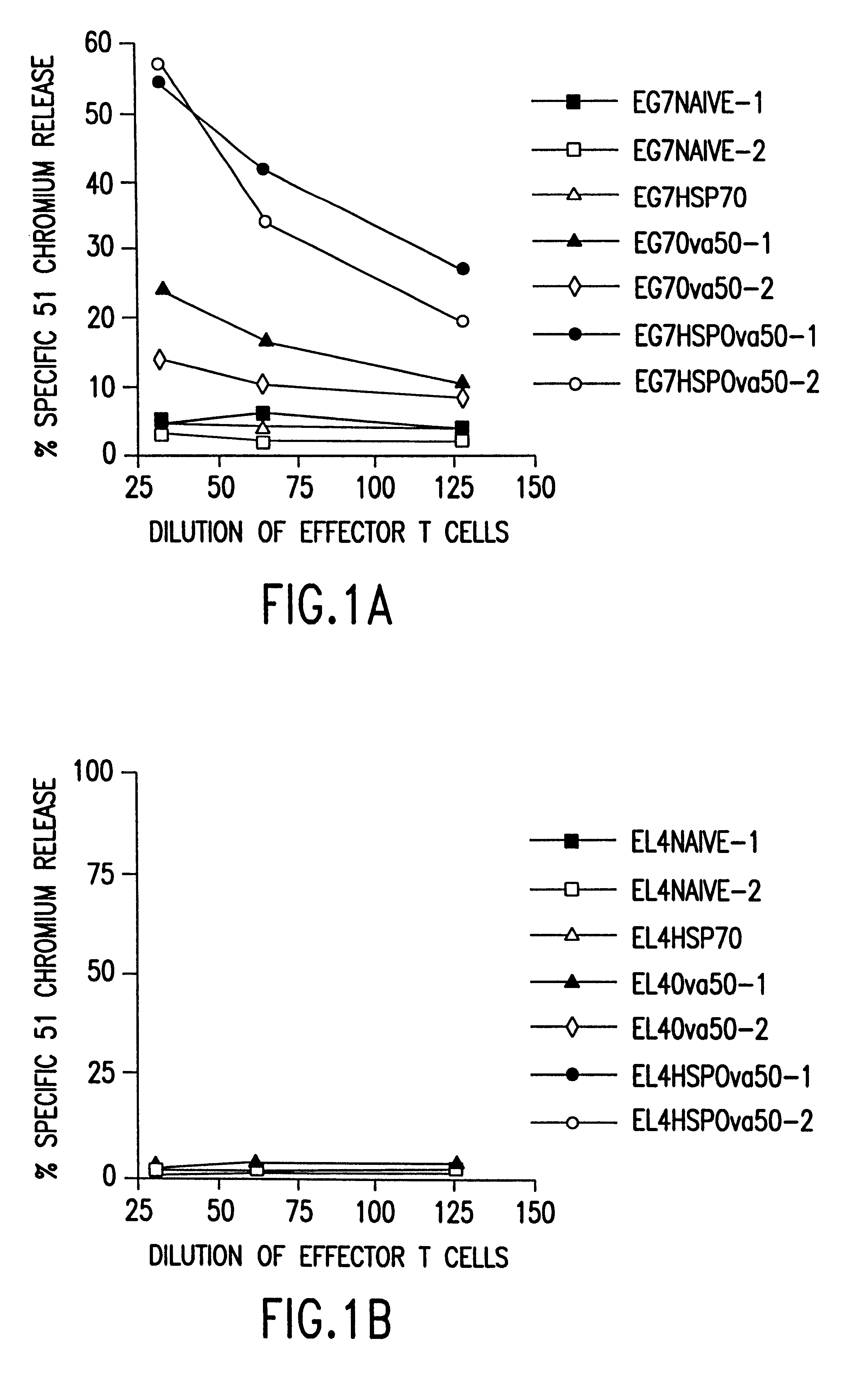
Therapeutic and prophylactic methods using heat shock proteins
The present invention relates to immunogenic complexes of heat shock proteins (hsp) noncovalently bound to exogenous antigenic molecules which when administered to an individual elicit specific immunological responses in the host. Methods of prevention and treatment of cancer and infectious disease are provided.
Owner:FORDHAM UNIVERSITY
Method for making multispecific antibodies having heteromultimeric and common components
InactiveUS7951917B1Increased formationIncrease productionHybrid immunoglobulinsAntibody ingredientsSpecific immunityBispecific antibody
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method provides a multispecific antibody having a common light chain associated with each heteromeric polypeptide having an antibody binding domain. Additionally the method further involves introducing into the multispecific antibody a specific and complementary interaction at the interface of a first polypeptide and the interface of a second polypeptide, so as to promote heteromultimer formation and hinder homomultimer formation; and / or a free thiol-containing residue at the interface of a first polypeptide and a corresponding free thiol-containing residue in the interface of a second polypeptide, such that a non-naturally occurring disulfide bond is formed between the first and second polypeptide. The method allows for the enhanced formation of the desired heteromultimer relative to undesired heteromultimers and homomultimers.
Owner:GENENTECH INC
Method for Making Multispecific Antibodies Having Heteromultimeric and Common Components
InactiveUS20070178552A1Increased formationIncrease productionAnimal cellsHybrid immunoglobulinsSpecific immunityBiology
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method provides a multispecific antibody having a common light chain associated with each heteromeric polypeptide having an antibody binding domain. Additionally the method further involves introducing into the multispecific antibody a specific and complementary interaction at the interface of a first polypeptide and the interface of a second polypeptide, so as to promote heteromultimer formation and hinder homomultimer formation; and / or a free thiol-containing residue at the interface of a first polypeptide and a corresponding free thiol-containing residue in the interface of a second polypeptide, such that a non-naturally occurring disulfide bond is formed between the first and second polypeptide. The method allows for the enhanced formation of the desired heteromultimer relative to undesired heteromultimers and homomultimers.
Owner:GENENTECH INC
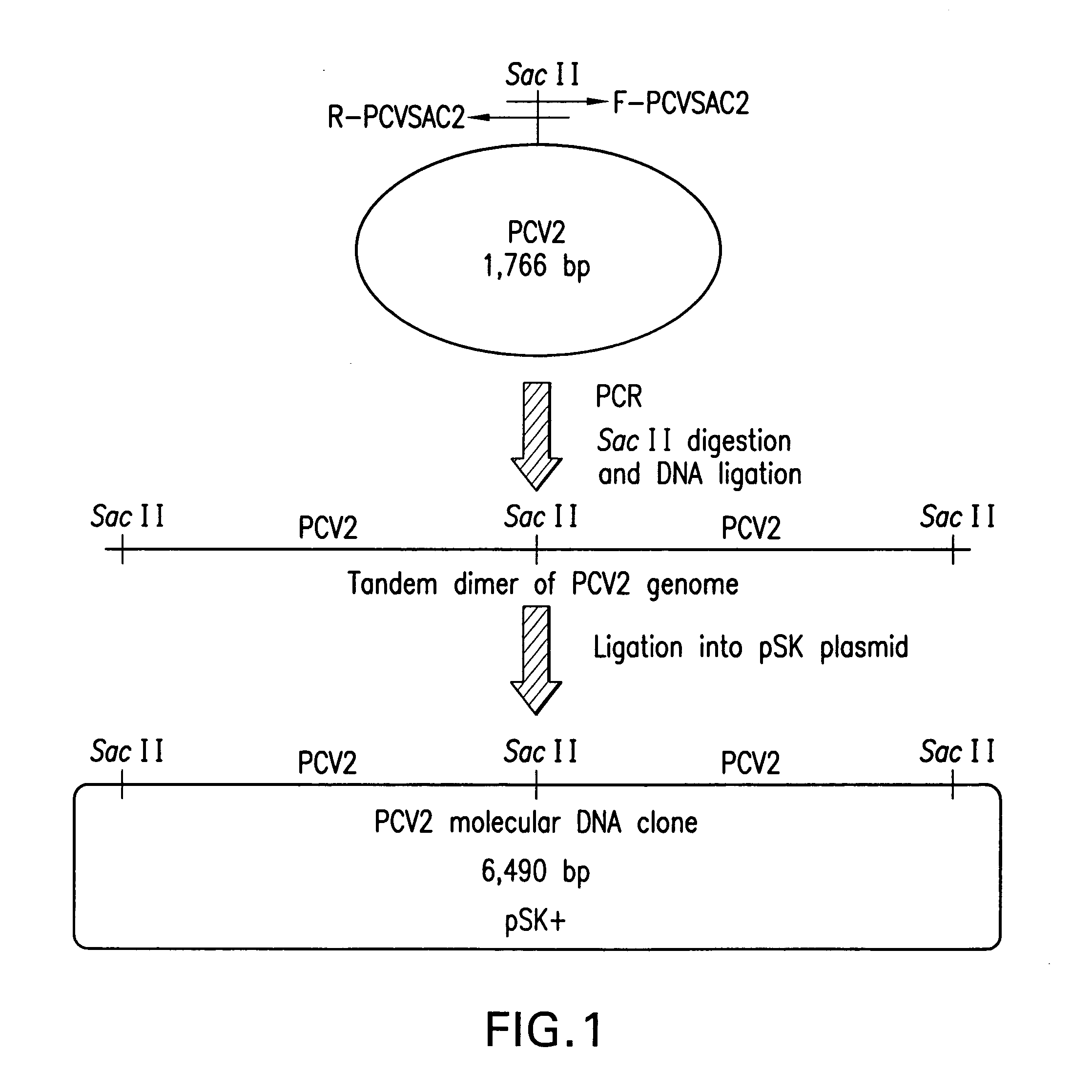
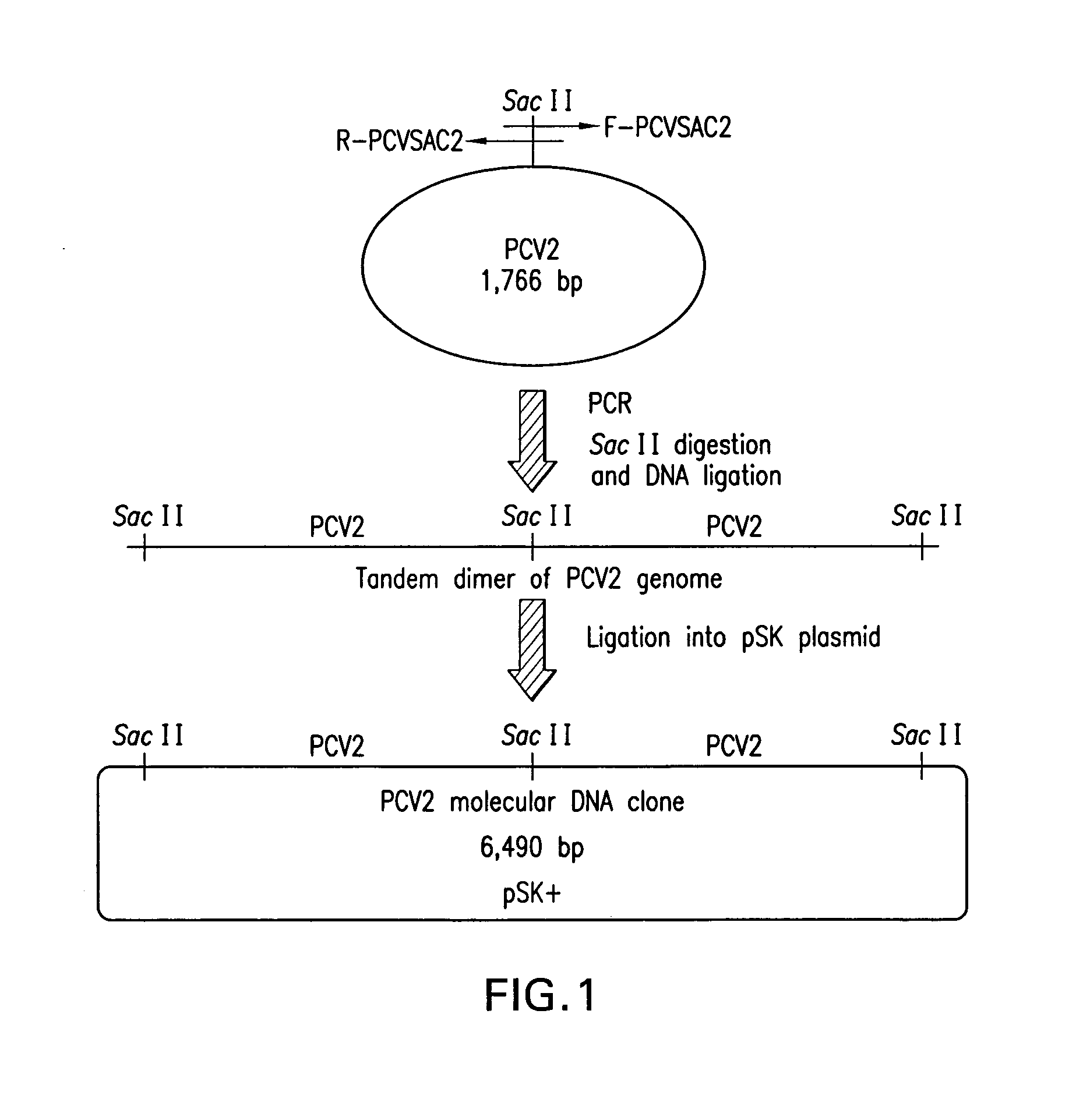
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
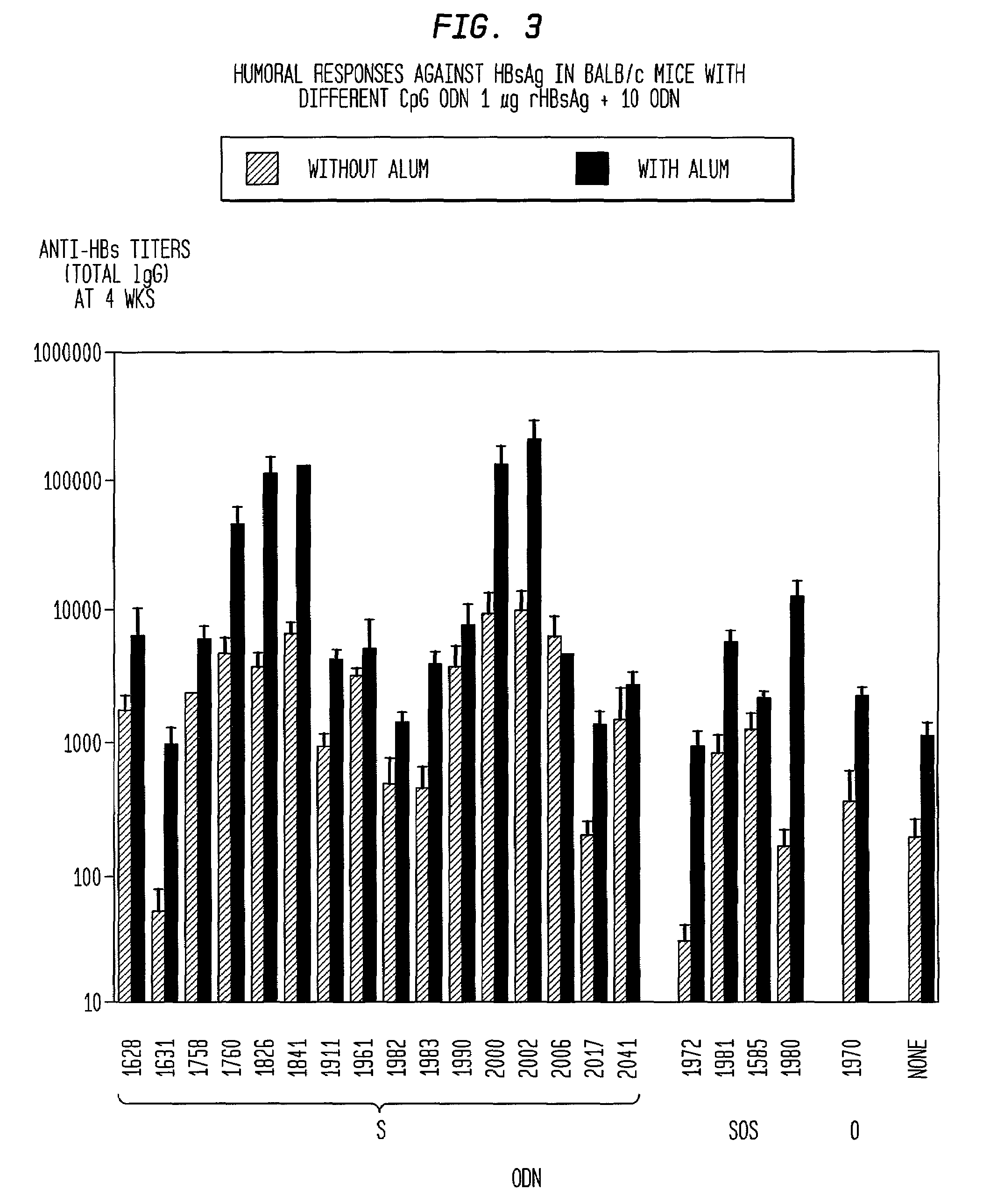
Method of inducing an antigen-specific immune response by administering a synergistic combination of adjuvants comprising unmethylated CpG-containing nucleic acids and a non-nucleic acid adjuvant
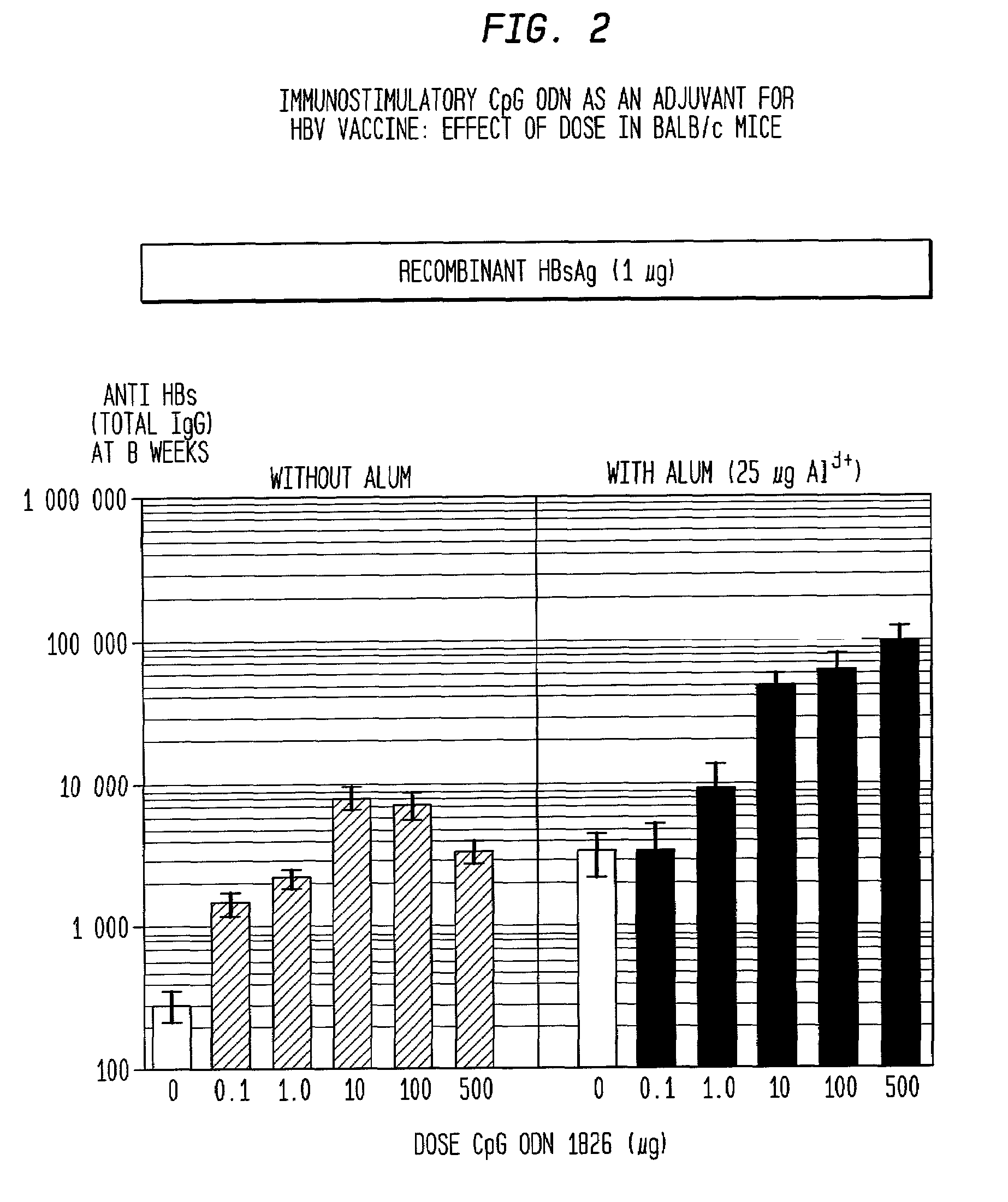
The present invention relates generally to adjuvants, and in particular to methods and products utilizing a synergistic combination of immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) and a non-nucleic acid adjuvant. Such combinations of adjuvants may be used with an antigen or alone. The present invention also relates to methods and products utilizing immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) for induction of cellular immunity in infants.
Owner:UNIV OF IOWA RES FOUND +2
Mycoplasma hyopneumoniae bacterin vaccine
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably CARBOPOL®, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polyoxypropylene block copolymer such as PLURONIC®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another embodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
Compositions and Methods for Targeted Immunomodulatory Antibodies and Fusion Proteins
ActiveUS20130039911A1Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antobodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antobodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immuno-suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β)) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By conteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
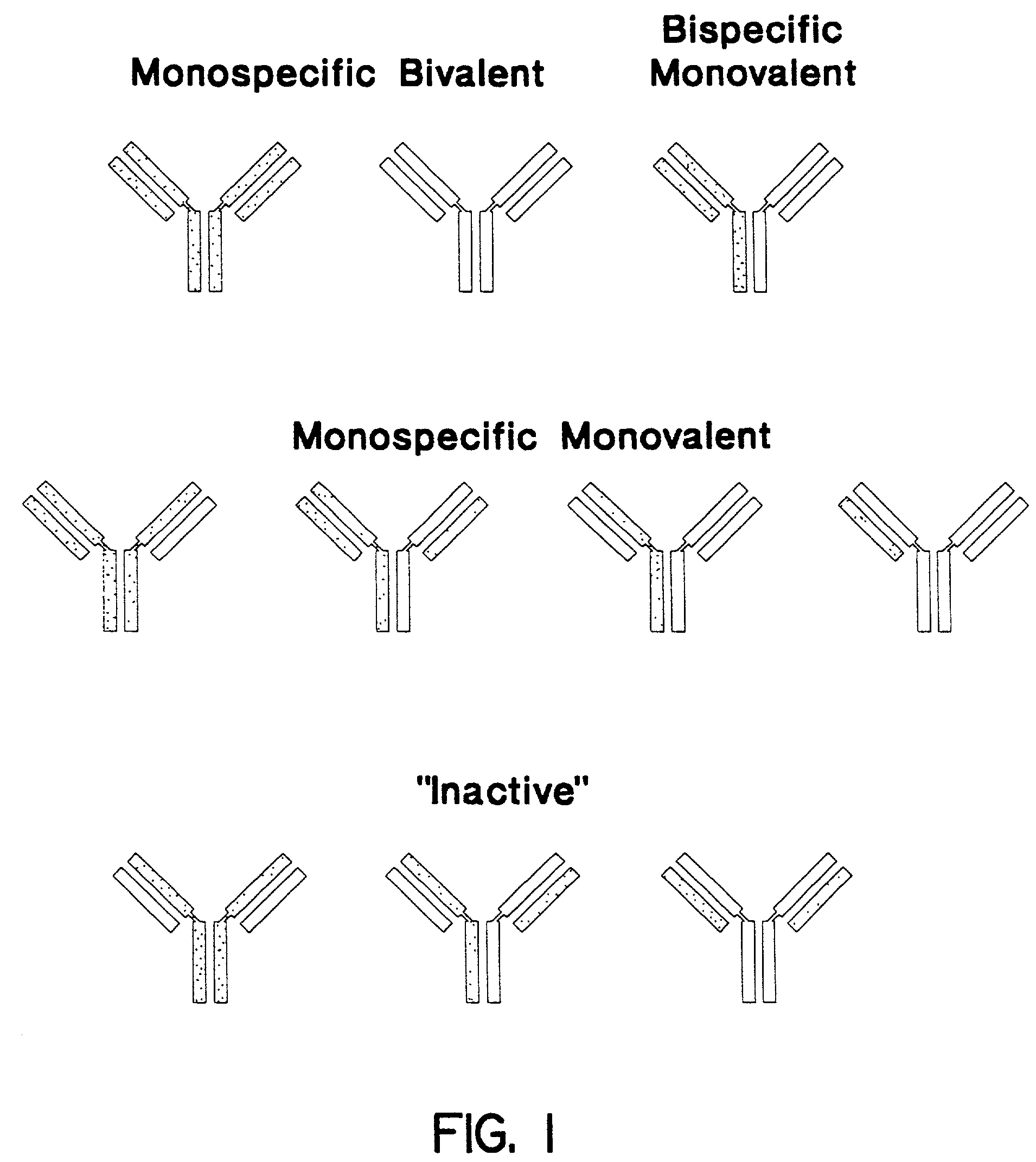
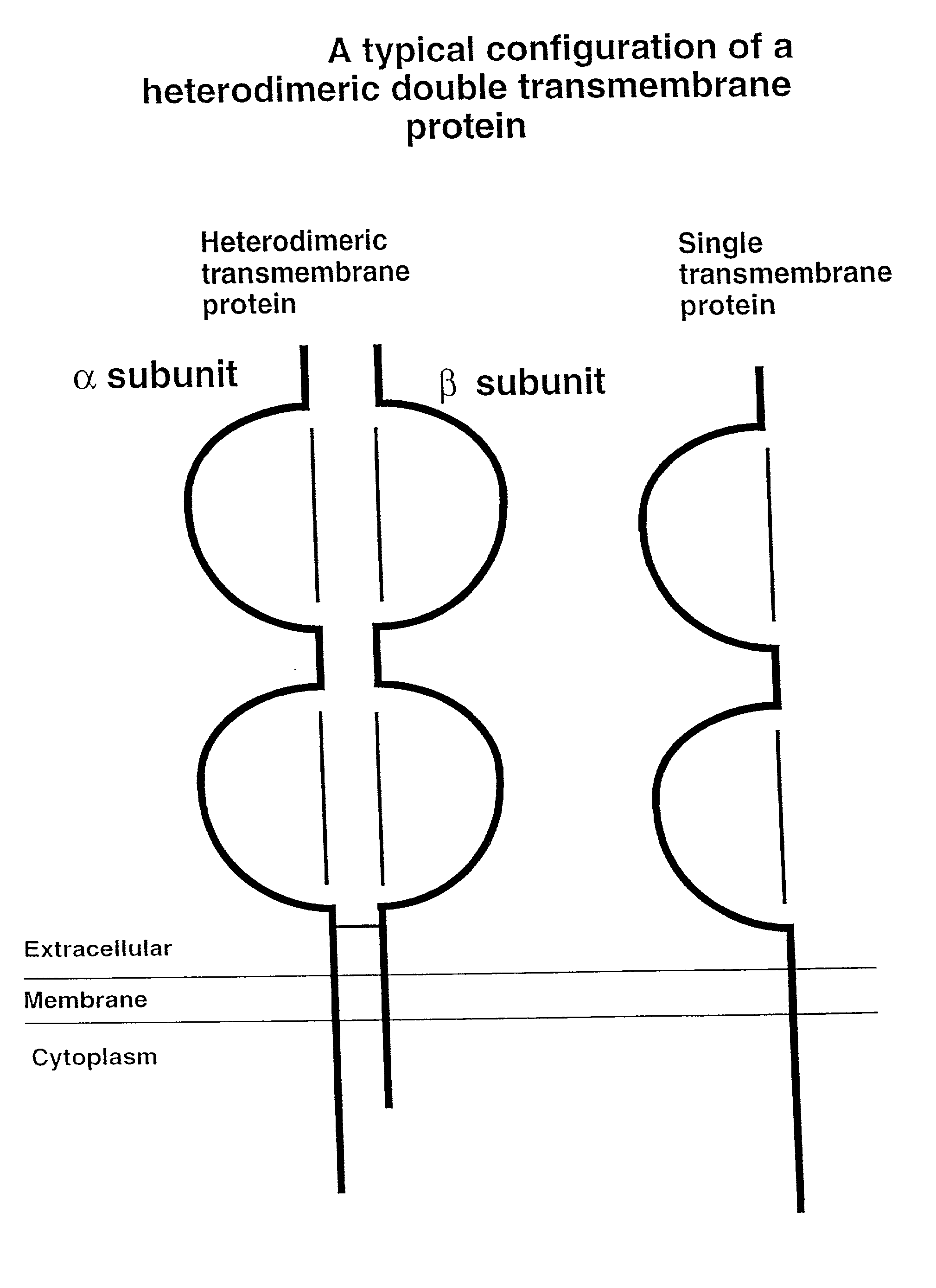
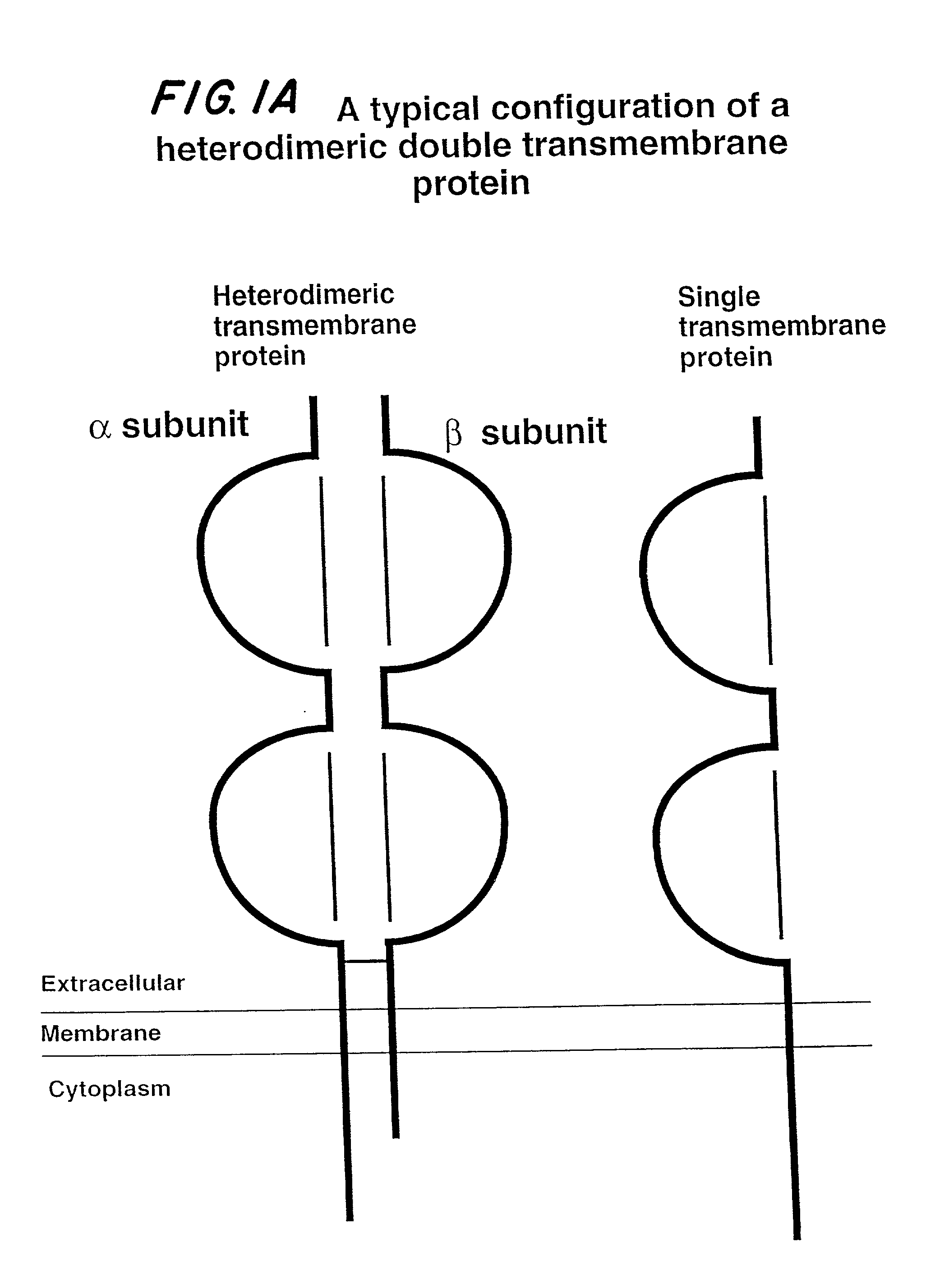
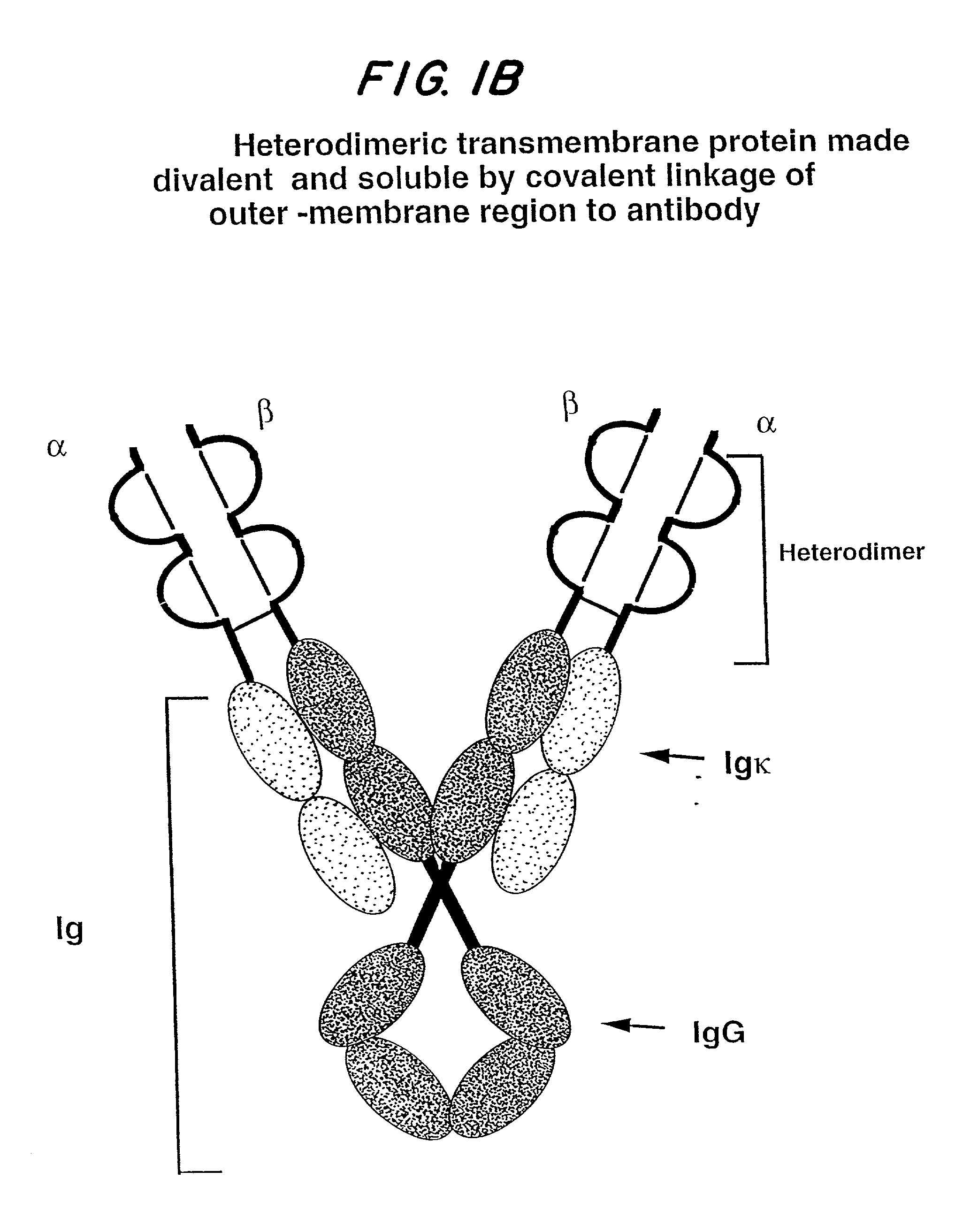
Soluble divalent and multivalent heterodimeric analogs of proteins
InactiveUS20020127231A1Well representedHigh affinityVirusesPeptide/protein ingredientsADAMTS ProteinsSpecific immunity
Specificity in immune responses is in part controlled by the selective interaction of T cell receptors with their cognate ligands, peptide / MHC molecules. The discriminating nature of this interaction makes these molecules, in soluble form, good candidates for selectively regulating immune responses. Attempts to exploit soluble analogs of these proteins has been hampered by the intrinsic low avidity of these molecules for their ligands. To increase the avidity of soluble analogs for their cognates to biologically relevant levels, divalent peptide / MHC complexes or T cell receptors (superdimers) were constructed. Using a recombinant DNA strategy, DNA encoding either the MHC class II / peptide or TCR heterodimers was ligated to DNA coding for murine Ig heavy and light chains. These constructs were subsequently expressed in a baculovirus expression system. Enzyme-linked immunosorbant assays (ELISA) specific for the Ig and polymorphic determinants of either the TCR or MHC fraction of the molecule indicated that infected insect cells secreted approximately 1 .mu.g / ml of soluble, conformnationally intact chimeric superdimers. SDS PAGE gel analysis of purified protein showed that expected molecular weight species. The results of flow cytometry demonstrated that the TCR and class II chimeras bound specifically with high avidity to cells bearing their cognate receptors. These superdimers will be useful for studying TCR / MHC interactions, lymphocyte tracking, identifying new antigens, and have possible uses as specific regulators of immune responses.
Owner:SCHNECK JONATHAN +1
Methods and compositions for inducing antigen-specific immune responses
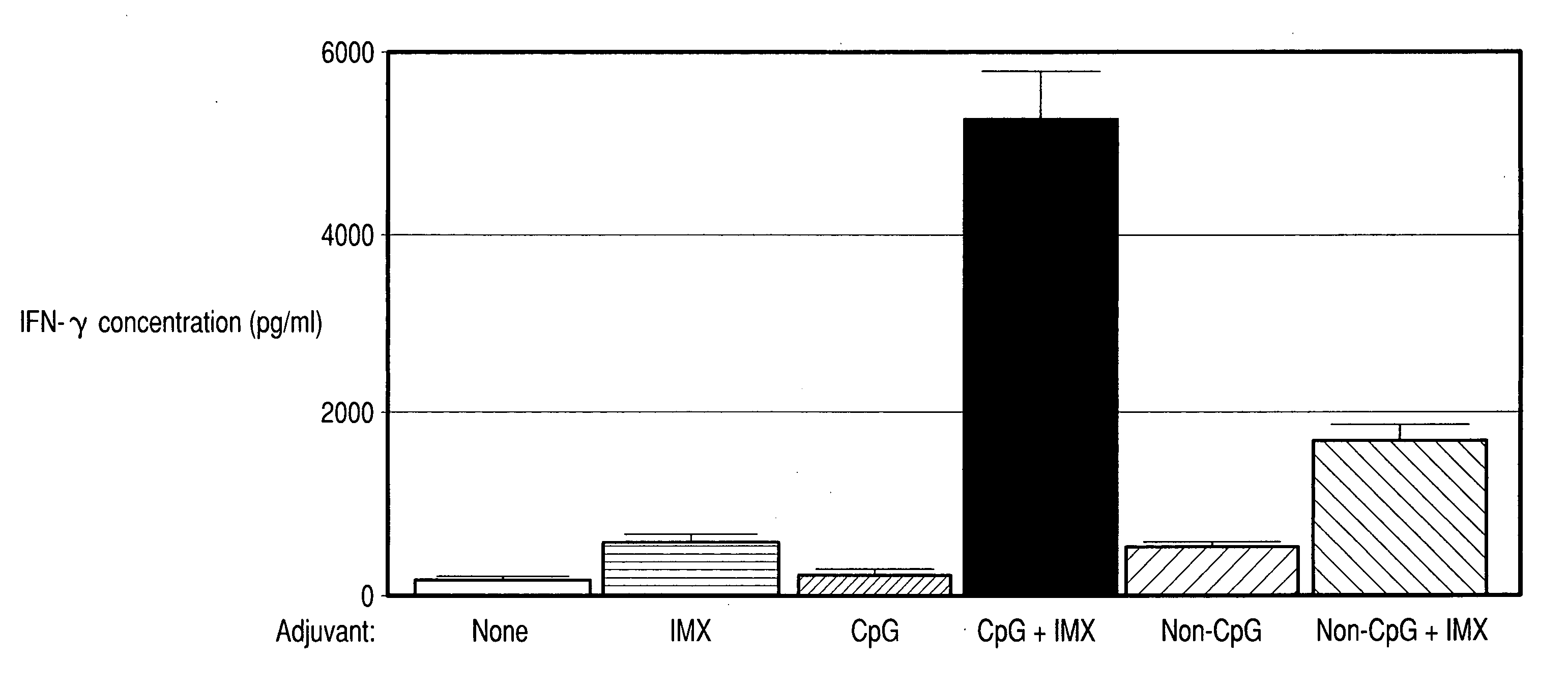
InactiveUS20060287263A1Enhanced IFN-gamma productionIncrease productionAntibacterial agentsBiocideImmunostimulating ComplexesSpecific immunity
Vaccine compositions comprising (a) an oligonucleotide, (b) and immune stimulating complex and (c) an antigen induce a strong interferon-gamma immune response. Both oligonucleotides containing immune stimulatory motifs and oligonucleotides lacking immune stimulatory motifs contribute to an interferon-gamma response when administered with an immune stimulating complex.
Owner:COLEY PHARM GRP INC +1
Compositions and methods for targeted immunomodulatory antibodies and fusion proteins
ActiveUS8993524B2Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antibodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antibodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immune suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By counteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
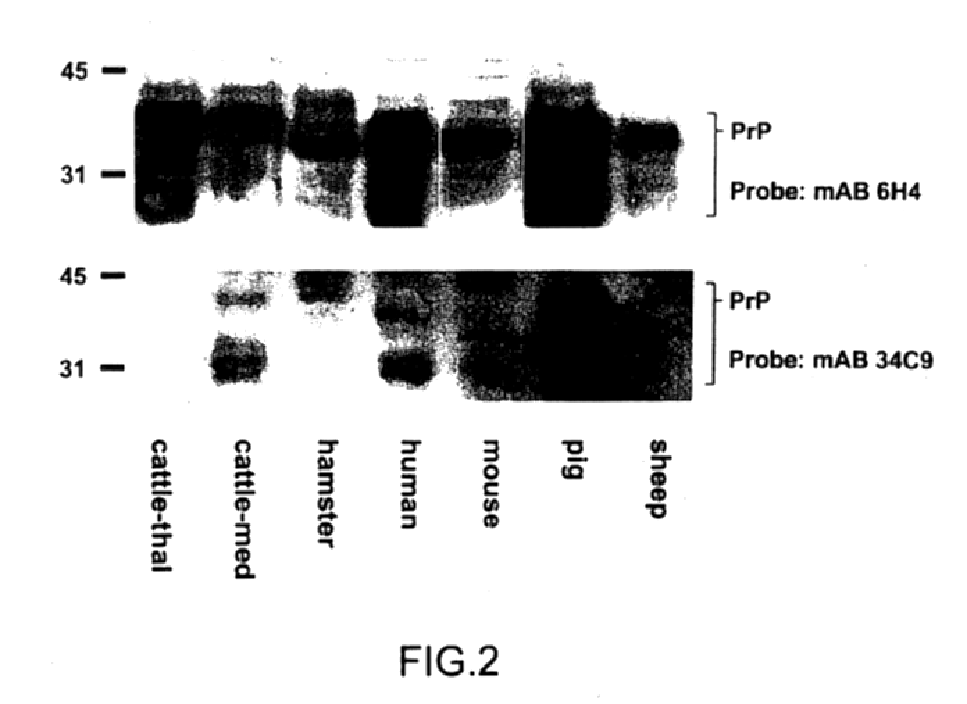
Immunological detection of prions
The presented invention relates to monoclonal antibodies useful in sensitive and specific immunological assays for the identification of prions in various tissues and body fluids, the production of such monoclonal antibodies by means of immunization of PrP<0 / 0 >mice by means of a new recombinant fragment of PrP and the use of the antibodies, e.g. for therapeutic and preventive treatments of humans and animals suffering from prion diseases.
Owner:UNIV ZURICH
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20090257994A1Negligible toxicityPotent and selectiveBiocidePeptide/protein ingredientsIntracellular signallingMalignancy
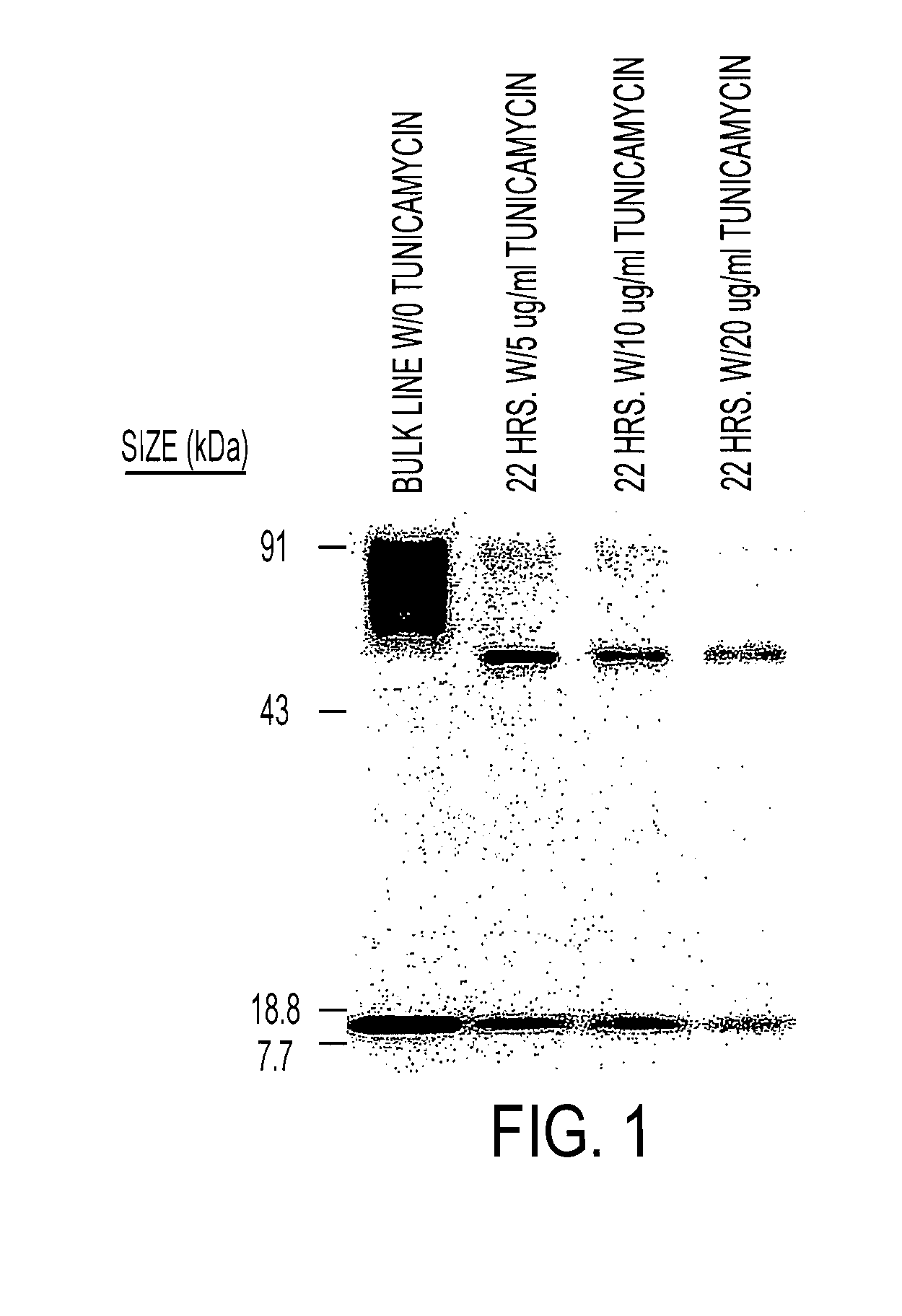
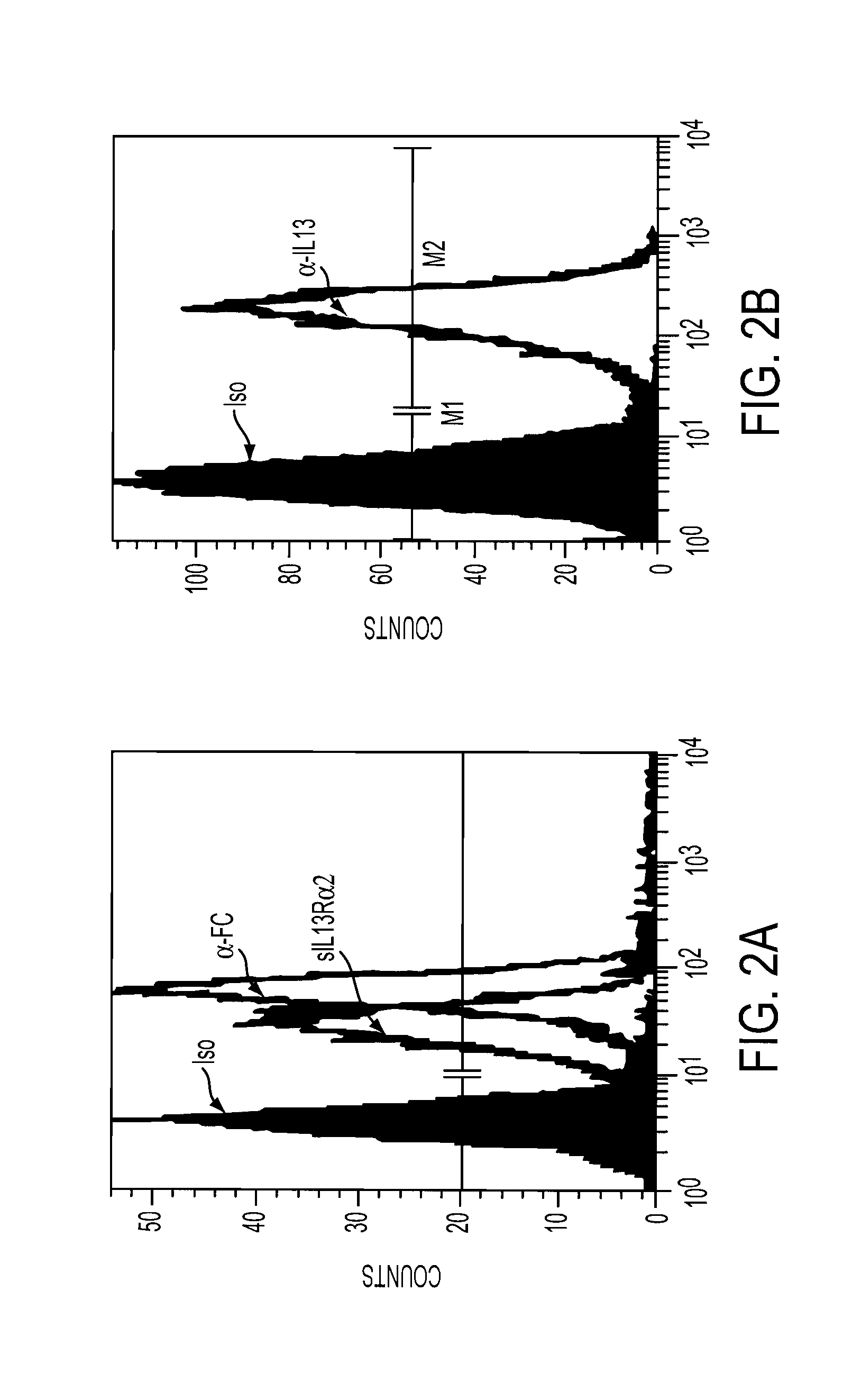
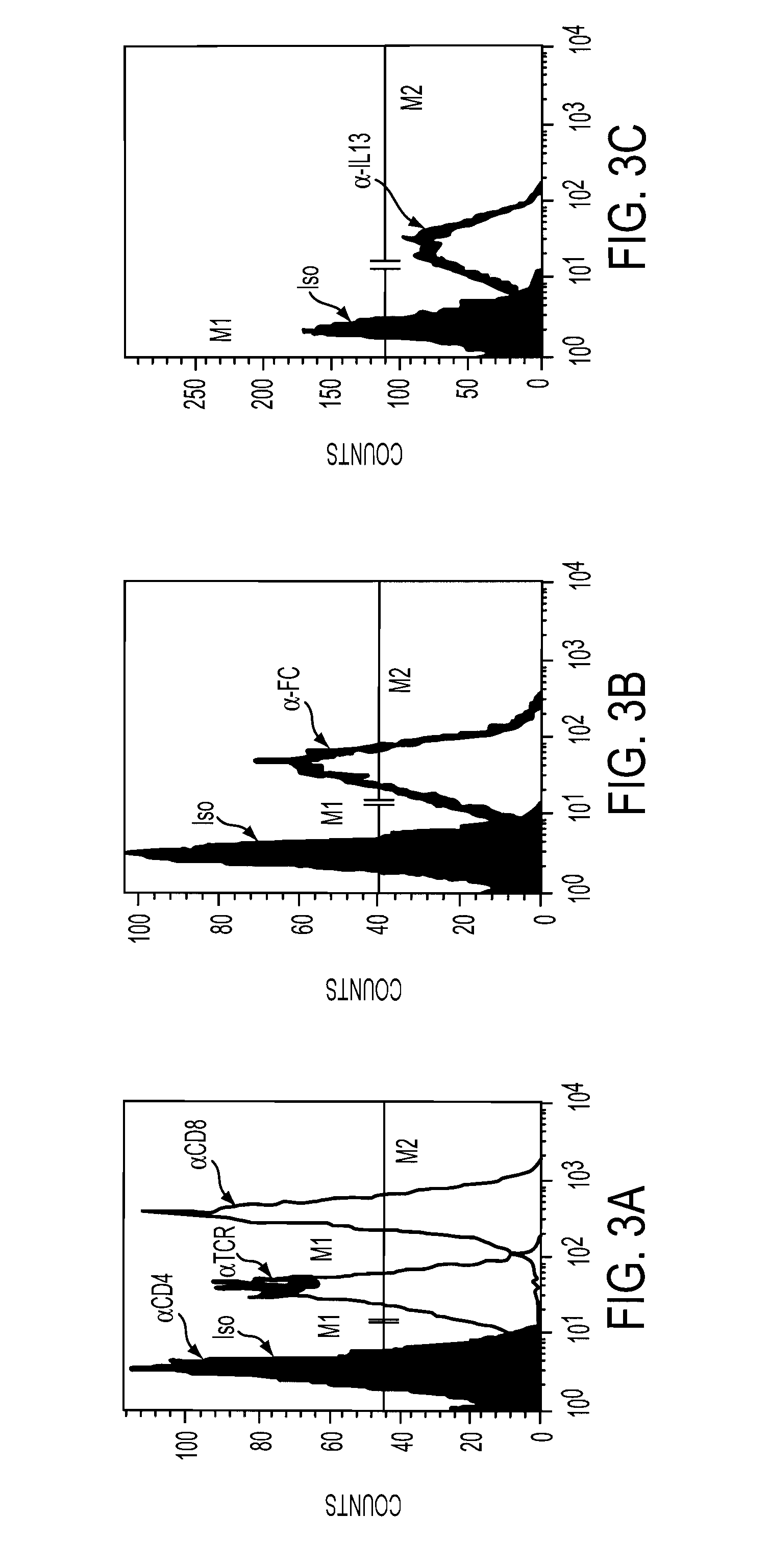
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human malignancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Potent and specific immunoproteasome inhibitors
InactiveUS20060241056A1BiocideCarbamic acid derivatives preparationAutoimmune conditionAutoimmune disease
Compounds and methods of selectively inhibiting an immunoproteasome are described. Also described are methods of treating a cancer, an inflammation, and / or an autoimmune disease and methods of suppressing endogenous antigenic peptide generation by administering to a subject in need of treatment thereof a therapeutic amount of an immunoproteasome specific inhibitor.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products.
Owner:IOWA STATE UNIV RES FOUND +1
Method for Making Multispecific Antibodies Having Heteromultimeric and Common Components
InactiveUS20070196363A1Increased formationIncrease productionAnimal cellsSugar derivativesSpecific immunityBispecific antibody
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method provides a multispecific antibody having a common light chain associated with each heteromeric polypeptide having an antibody binding domain. Additionally the method further involves introducing into the multispecific antibody a specific and complementary interaction at the interface of a first polypeptide and the interface of a second polypeptide, so as to promote heteromultimer formation and hinder homomultimer formation; and / or a free thiol-containing residue at the interface of a first polypeptide and a corresponding free thiol-containing residue in the interface of a second polypeptide, such that a non-naturally occurring disulfide bond is formed between the first and second polypeptide. The method allows for the enhanced formation of the desired heteromultimer relative to undesired heteromultimers and homomultimers.
Owner:GENENTECH INC
Methods for preparation of vaccines against cancer
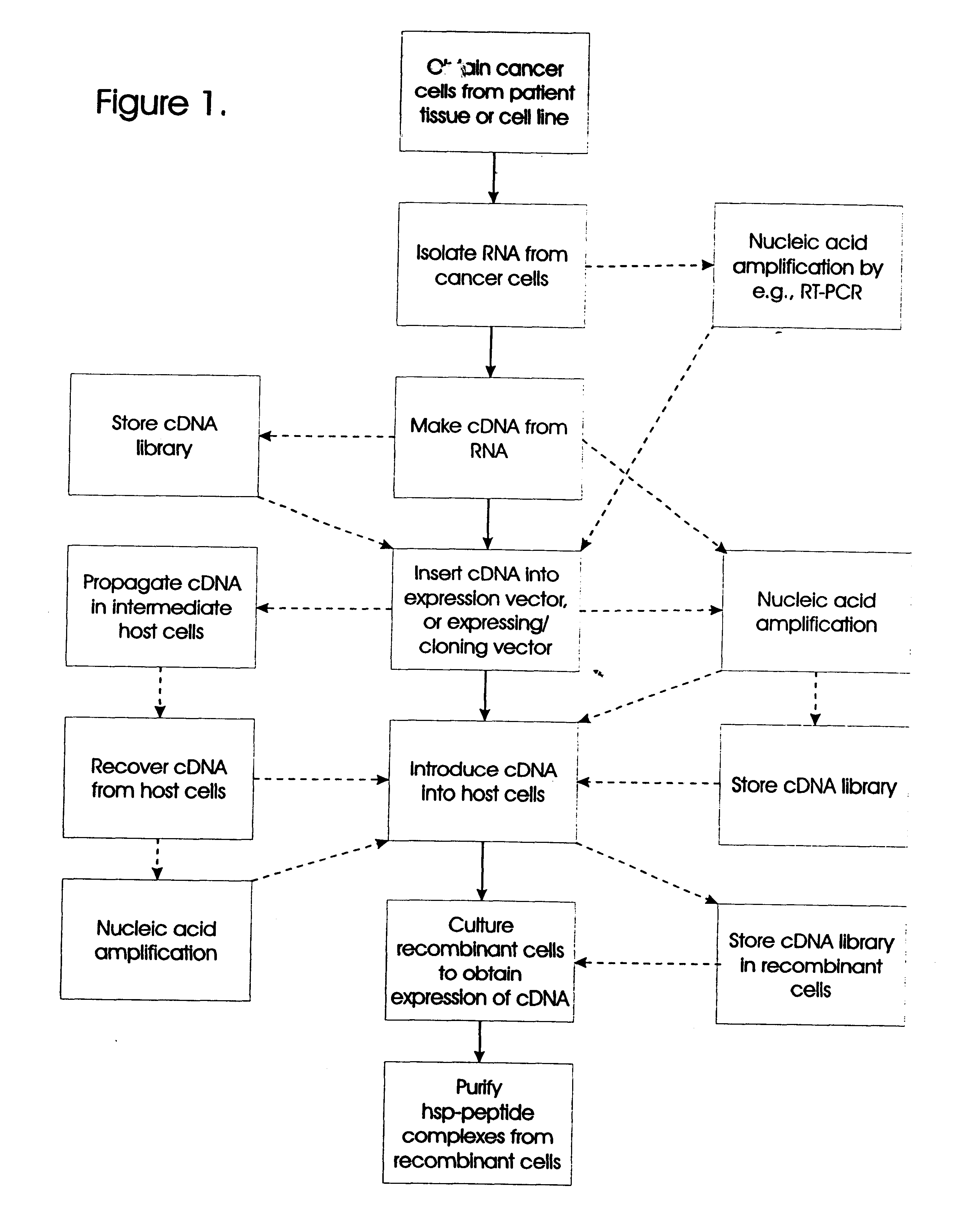
The present invention relates to methods for preparing immunogenic, prophylactically and therapeutically effective complexes of heat shock proteins noncovalently associated with antigenic peptides of cancer cells. The claimed methods comprise the constructing of a cDNA library from cancer or preneoplastic cell RNA, expressing the cDNA library in an appropriate host cell, and recovering the immunogenic complexes from the cells. Large amounts of such immunogenic complexes can be obtained by large-scale culturing of host cells containing the cDNA library. The complexes can be used as a vaccine to elicit specific immune responses against cancer or preneoplastic cells, and to treat or prevent cancer.
Owner:FORDHAM UNIVERSITY
Recombinant multiple domain fusion protein mitogens and use thereof for inducing enhancement or repression of antigen-specific immunity.
ActiveUS20100303811A1Increase heightVirusesPeptide/protein ingredientsIMMUNE SUPPRESSANTSAutoimmune responses
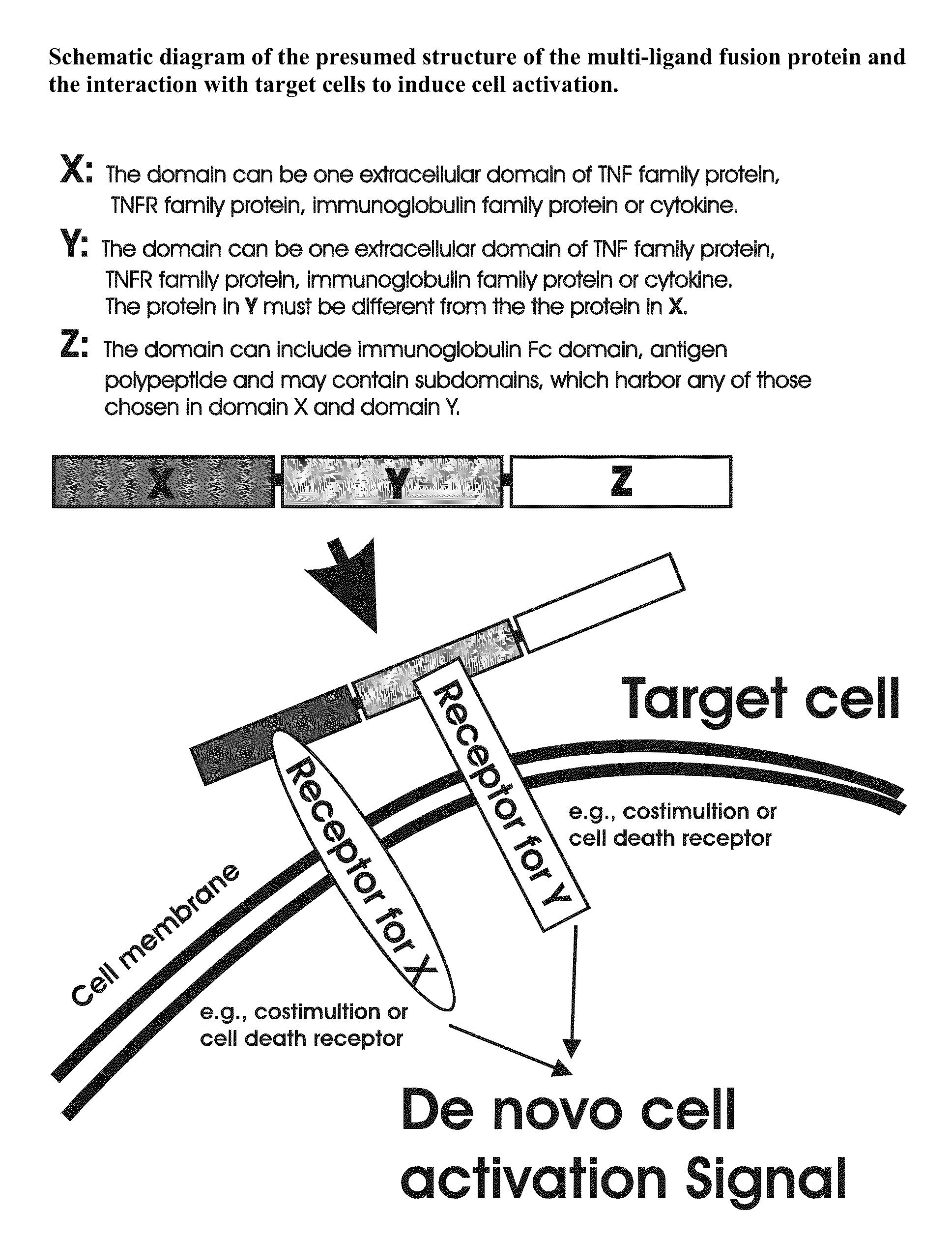
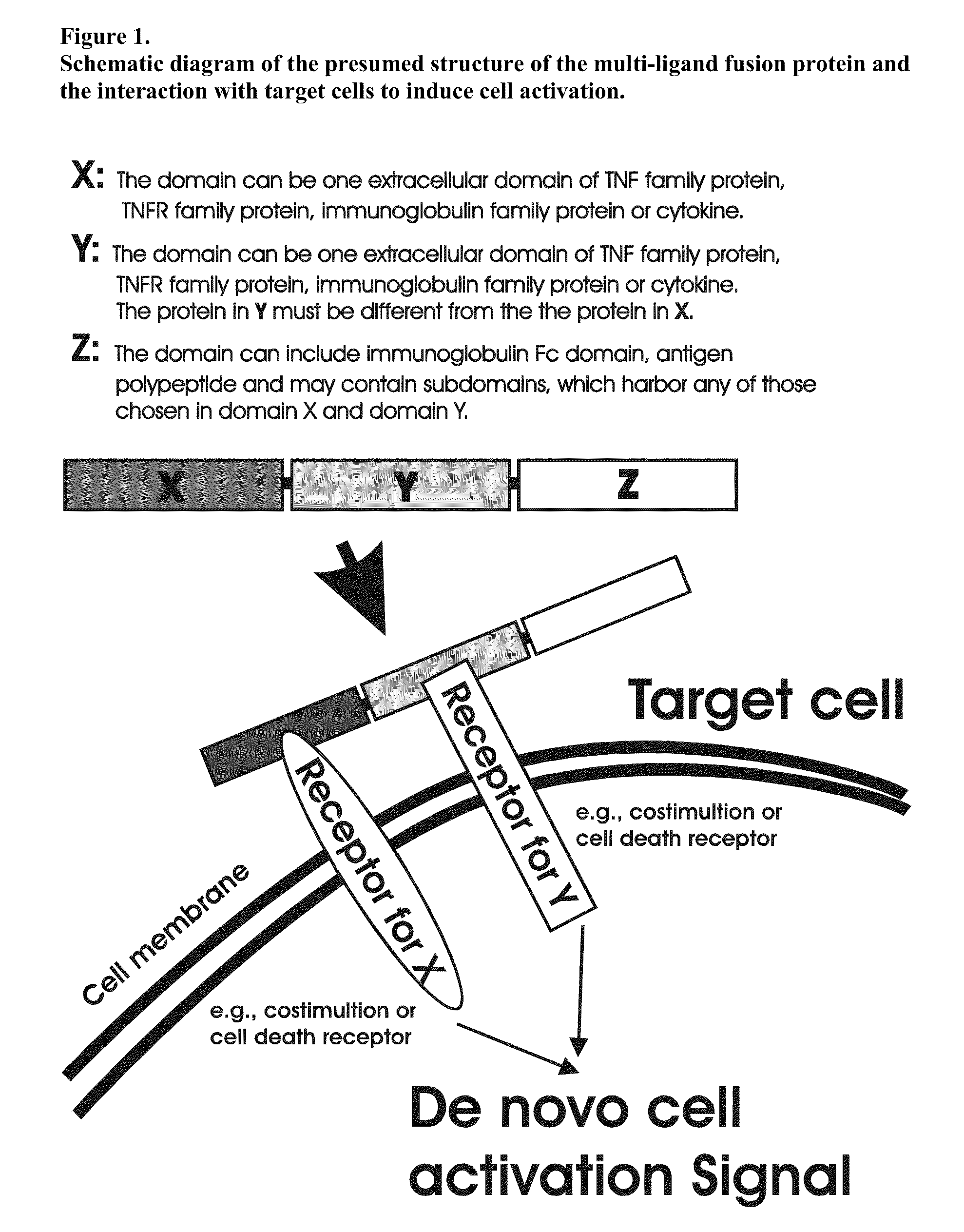
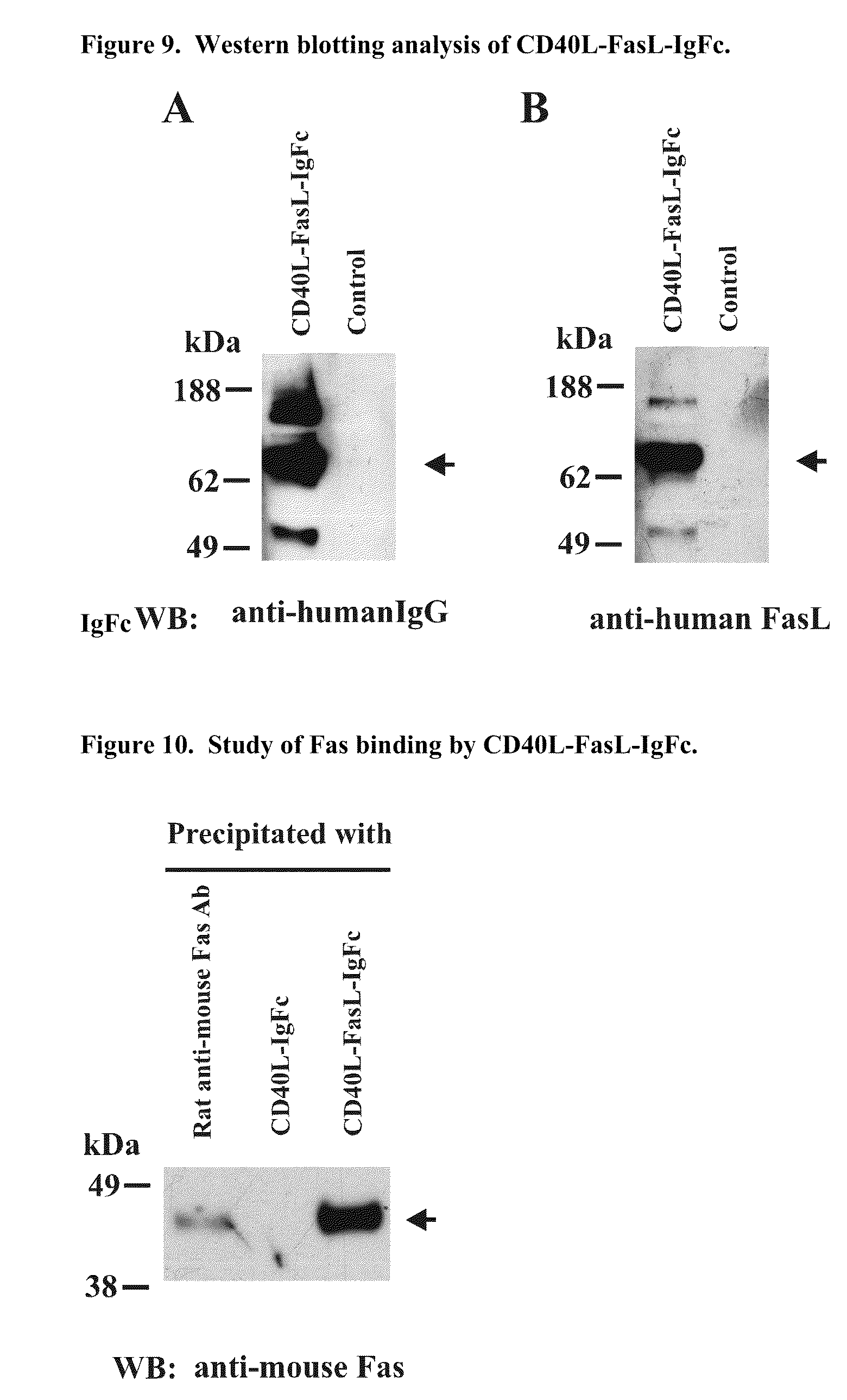
The invention relates to cell stimulatory fusion proteins and DNA sequences, vectors comprising at least two agonists of TNF / TNFR super family, immunoglobulin super family, cytokine family proteins and optional antigen combination. Instructions for use of these proteins and DNA constructs as immune adjuvants and vaccines for treatment of various chronic diseases such as viral infection are also provided. Additionally, the use of these protein and DNA constructs as immune suppressant for treatment of various chronic diseases, such as autoimmunity and organ transplant rejection, is also illustrated.
Owner:OCHI ATSUO
Therapeutic and prophylactic methods using heat shock proteins
The present invention relates to immunogenic complexes of heat shock proteins (hsp) noncovalently bound to exogenous antigenic molecules which when administered to an individual elicit specific immunological responses in the host. Methods of prevention and treatment of cancer and infectious disease are provided.
Owner:FORDHAM UNIVERSITY
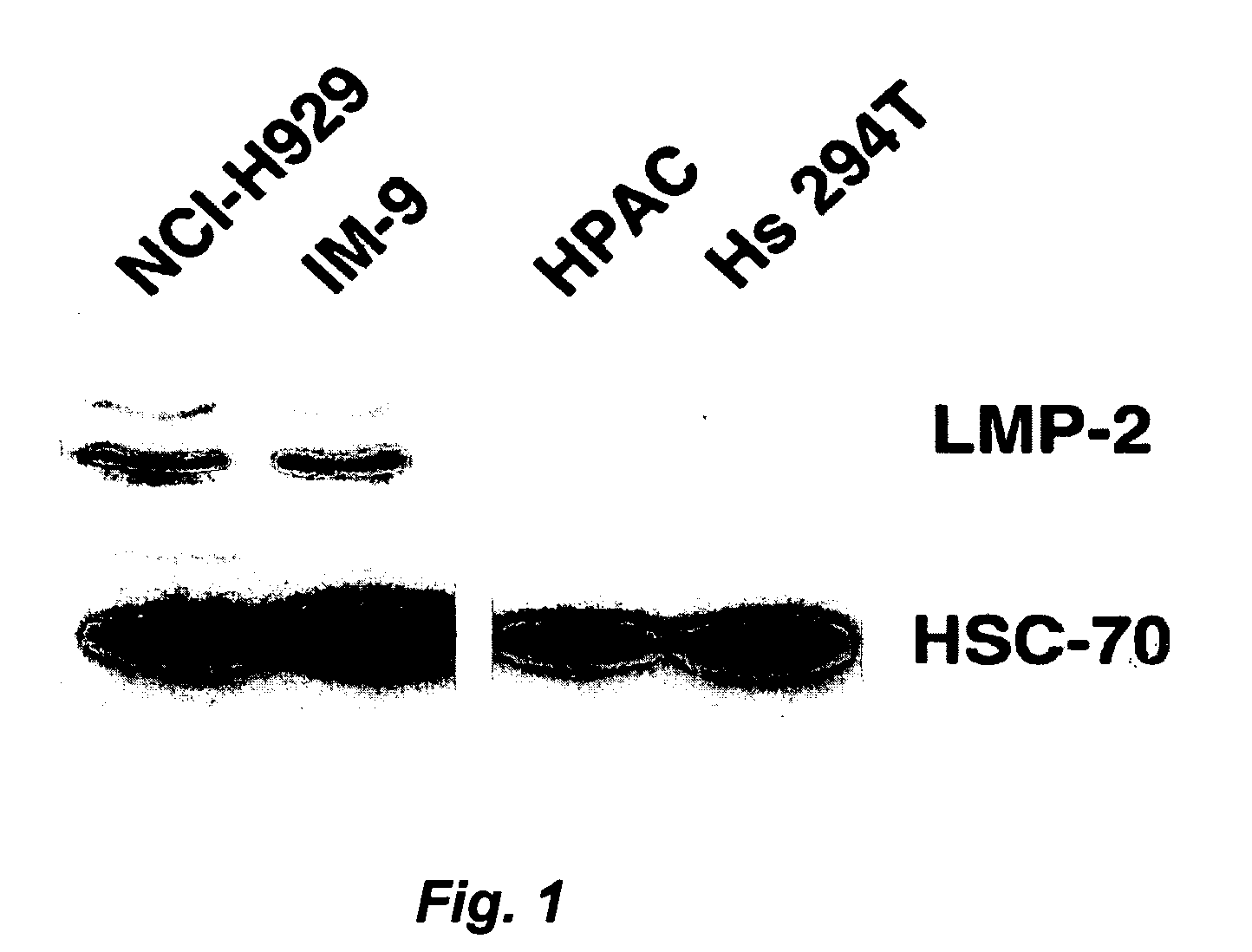
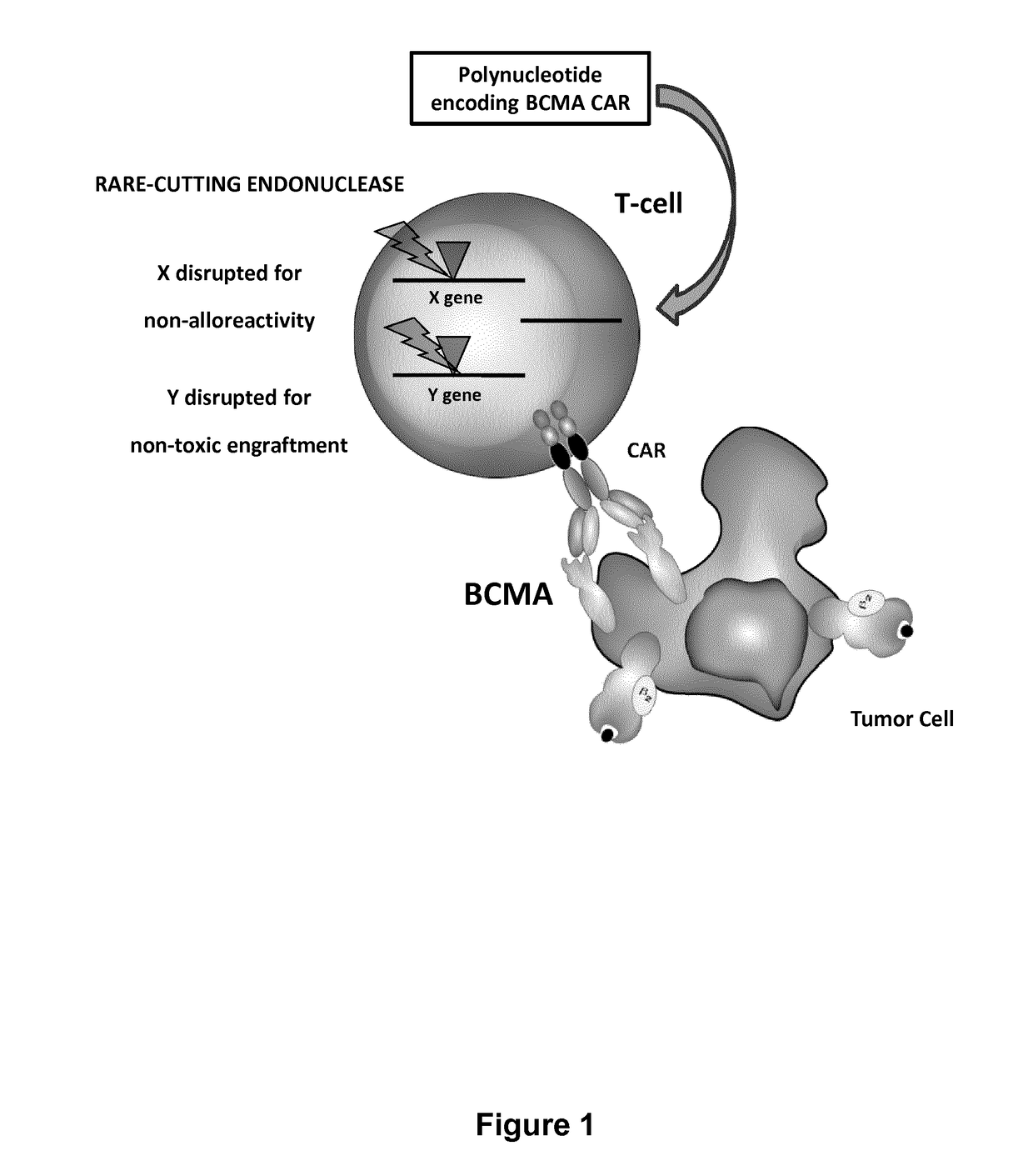
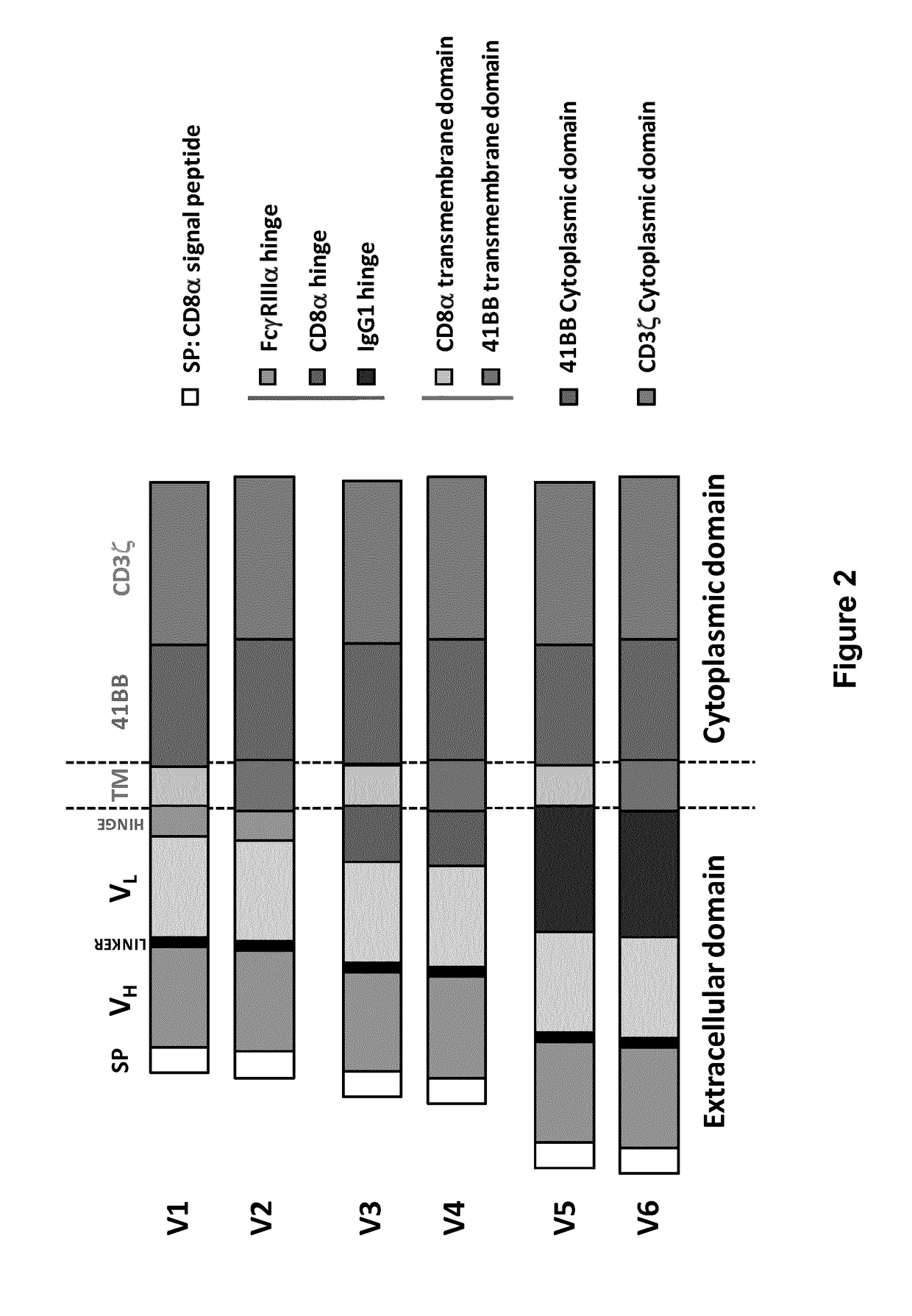
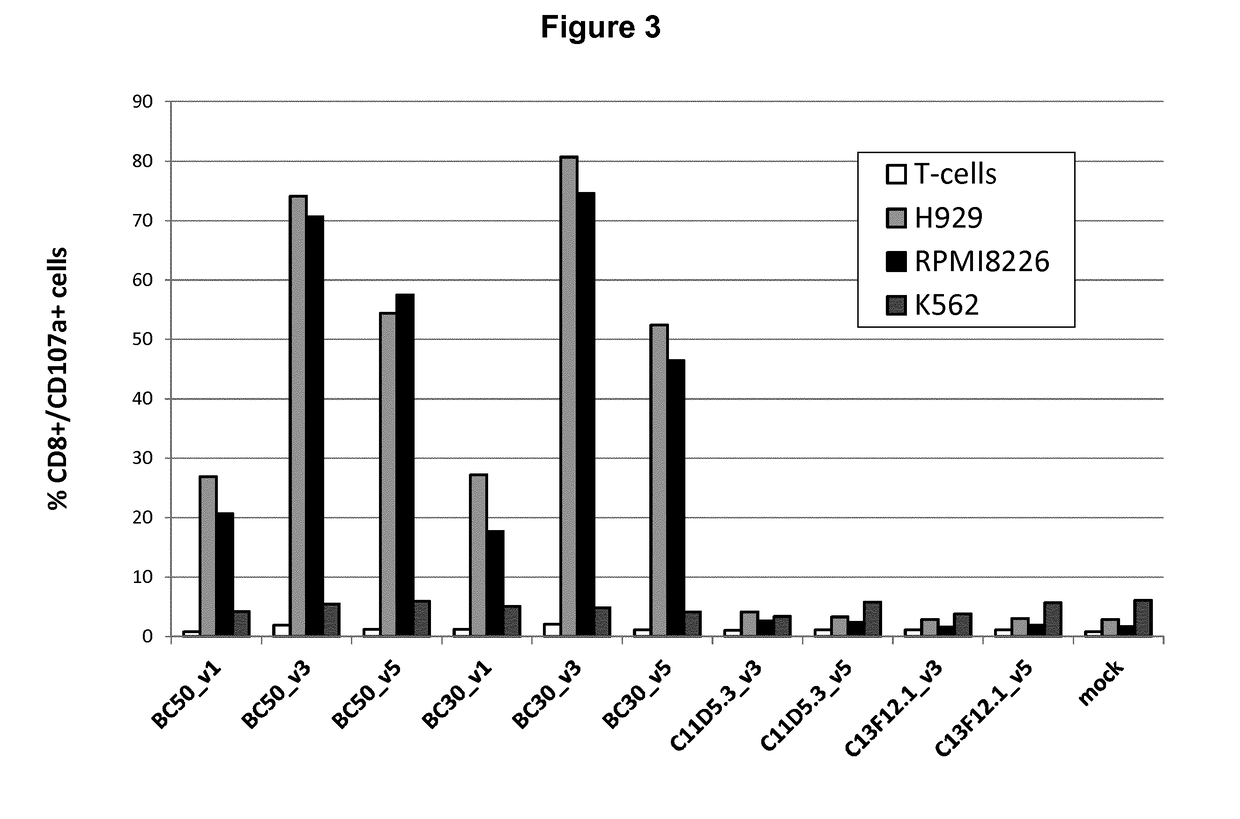
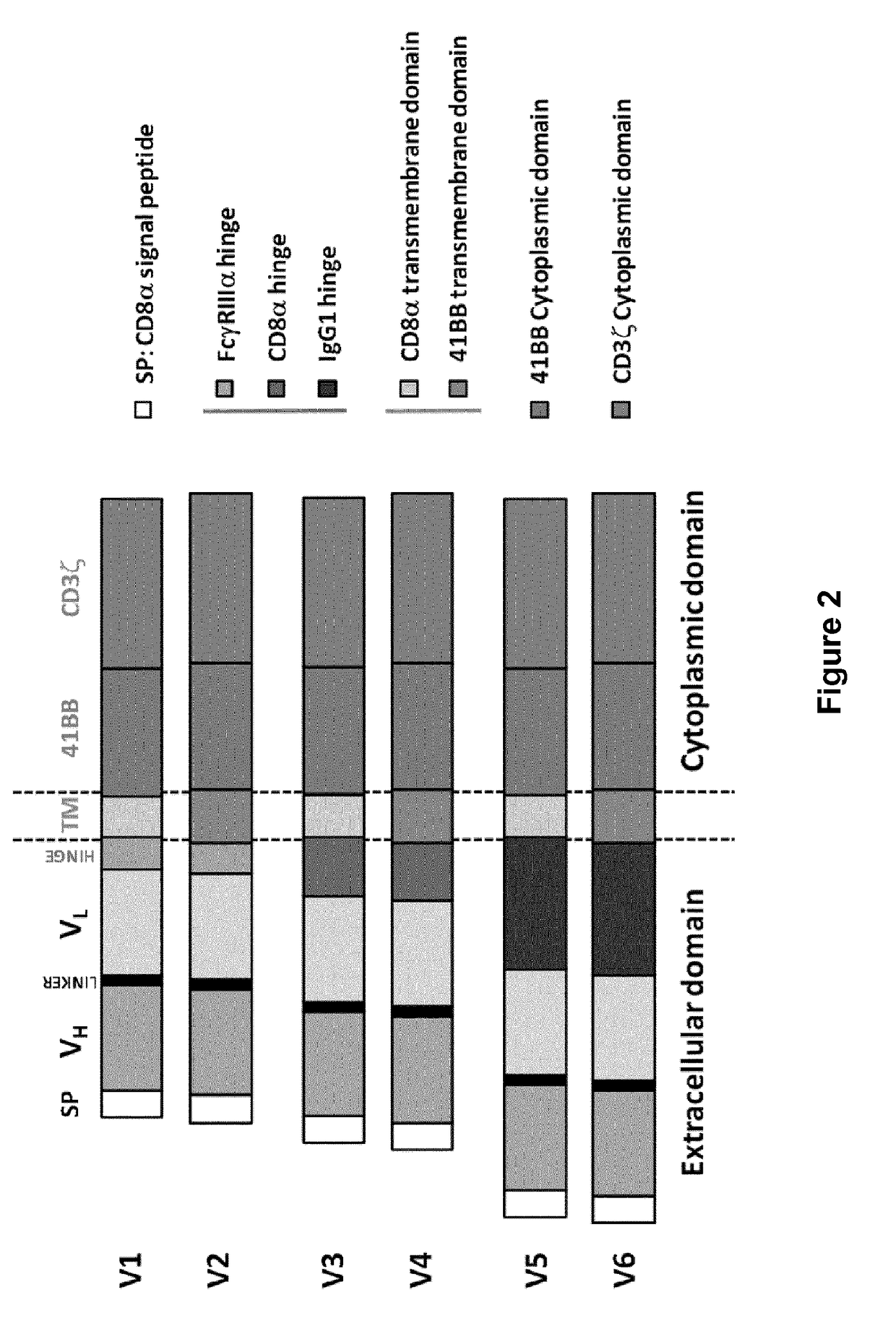
Bcma (CD269) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170183418A1Useful for immunotherapyHigh selectivityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a BCMA monoclonal antibody, conferring specific immunity against BCMA positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas, multiple myeloma and leukemia.
Owner:CELLECTIS SA
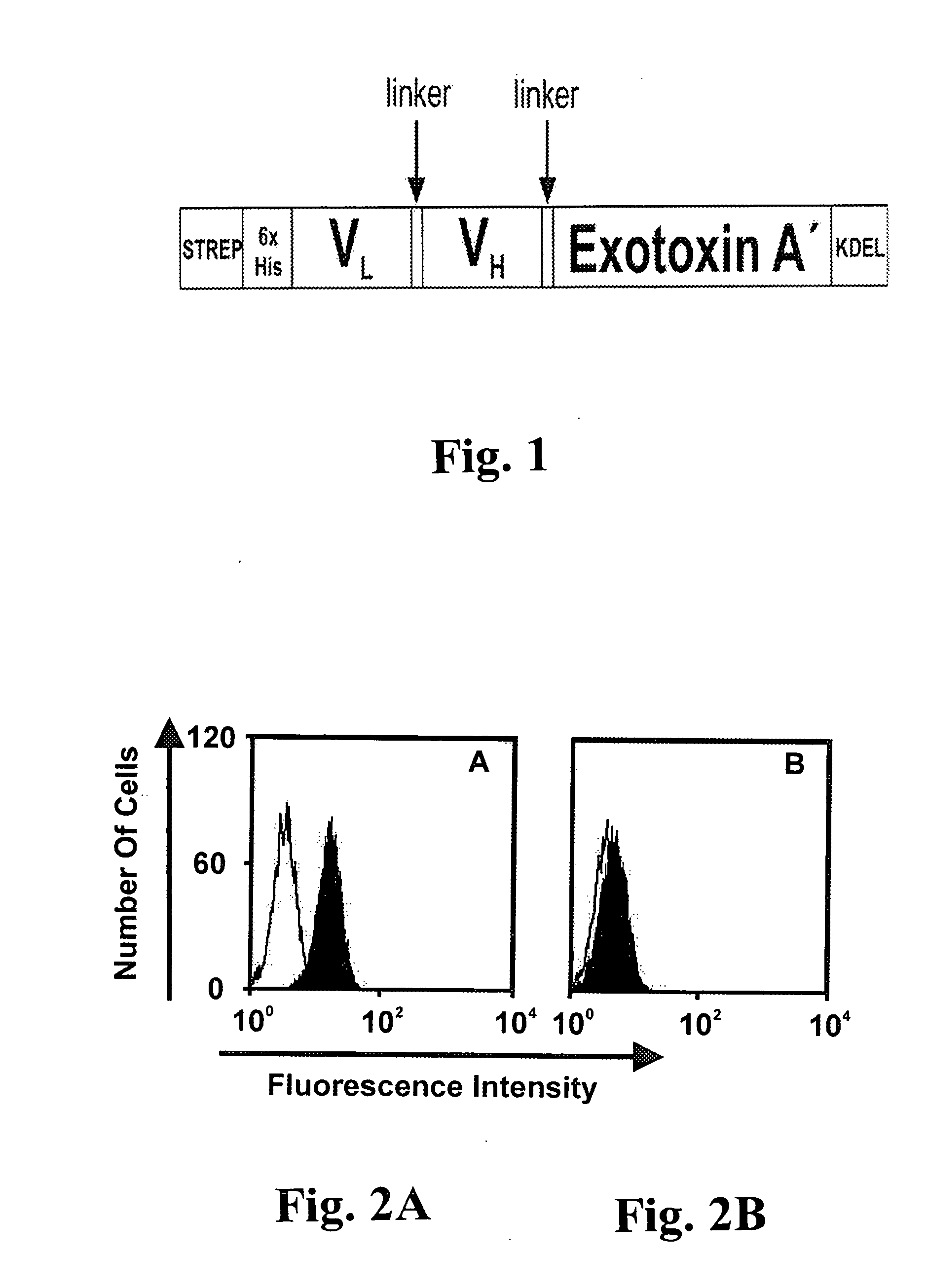
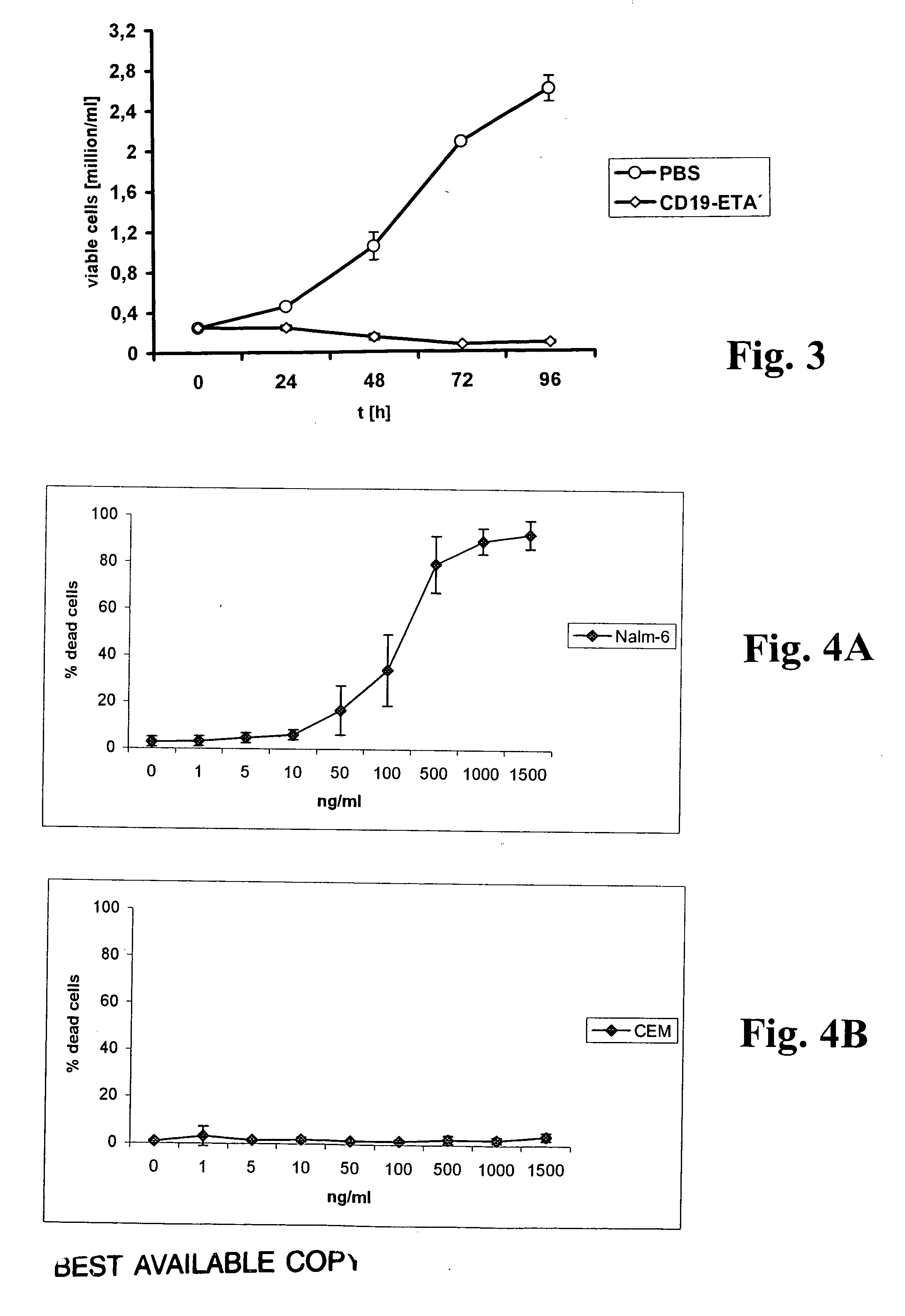
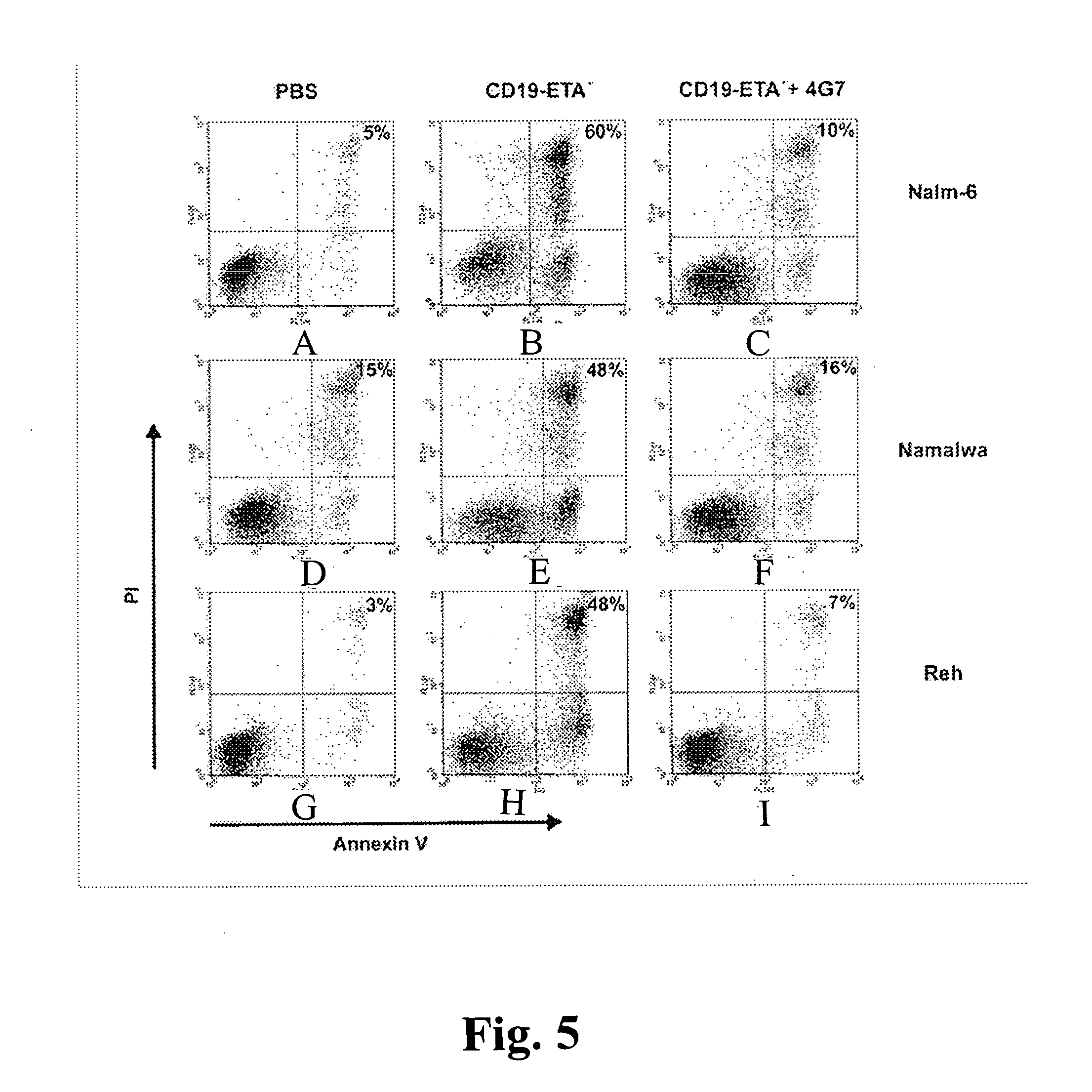
CD19-specific immunotoxin and treatment method
InactiveUS20070178103A1Easy to transportResistant to extracellular cleavagePolypeptide with localisation/targeting motifHybrid immunoglobulinsAutoimmune conditionBacterial exotoxin
An immunotoxin for use in, and a method for treating a subject having a cancer associated with malignant B-lineage cells or an autoimmune condition, are disclosed. The immunotoxin includes (a) an anti-CD19 antibody lacking an Fc fragment, (b) a modified exotoxin A protein having both Domains II and III, but lacking Domain I, and (c) a peptide linker joining the C-terminal end of the antibody to the N-terminal end of the modified exotoxin A protein. The linker is substantially resistant to extracellular cleavage. The modified exotoxin A protein may be further modified to include a C-terminal KDEL sequence (SEQ ID NO: 6) that promotes transport of the protein to the endoplasmic reticulum of cells that have taken up the immunotoxin.
Owner:FRIEDRICH ALEXANDER UNIV ERLANGEN NURNBERG
Methods of manufacture and use of calcium phosphate particles containing allergens
The present invention relates to the use of calcium phosphate particles in formulation with allergens for allergic desensitization. Particularly, the invention relates to novel calcium phosphate core particles, particularly nano- and micron-sized particles, as allergen adjuvants and in compositions for inducing allergic desensitization. Methods of making such particles and to methods of inducing a specific immune response using the particles of this invention are also provided.
Owner:CAPTIVATE PHARMACEUTICALS LLC
Immunogenic compositions for Chlamydia trachomatis
PendingUS20060034871A1Enhance immune responseAntibacterial agentsBacterial antigen ingredientsDiseaseAdjuvant
The invention relates to immunogenic compositions comprising combinations of Chlamydia trachomatis antigens and their use in vaccines. The composition may comprise at least two components, one component of which comprises Chlamydia trachomatis antigens for eliciting a Chlamydia trachomatis specific TH1 immune response and another component of which comprises antigens for eliciting a Chlamydia trachomatis specific TH2 immune response. The invention further relates to an immunogenic composition comprising a Chlamydia trachomatis Type III secretion system (TTSS) regulatory protein and a Chlamydia trachomatis Type III secretion system (TTSS) secreted protein or a fragment thereof. The invention further relates to the use of combinations of adjuvants for use with antigens associated with a sexually transmissible disease, such as Chlamydia trachomatis antigens. Preferred adjuvant combinations include mineral salts, such as aluminium salts and oligonucleotides comprising a CpG motif. The invention further provides a combination of Chlamydia trachomatis antigens comprising a Chlamydia trachomatis antigen that is conserved over at least two serovars.
Owner:NOVARTIS AG
Transcutaneous immunization without heterologous adjuvant
InactiveUS20060002949A1Increase skin hydrationIncrease local concentrationSsRNA viruses negative-senseAntibacterial agentsDendritic cellSpecific immunity
Transcutaneous immunization can deliver antigen to the immune system through the stratum corneum without physical or chemical penetration to the dermis layer of the skin. This delivery system induces an antigen-specific immune response without the use of a heterologous adjuvant. Although perforation of intact skin is not required, superficial penetration or micropenetration of the skin can act as an enhancer; similarly, hydration may enhance the immune response. This system can induce antigen-specific immune effectors after epicutaneous application of a formulation containing one or more antigens. The formulation may initiate processes such as antigen uptake, processing, and presentation; Langerhans cell activation, migration from the skin to other immune organs, and differentiation to mature dendritic cells; contacting antigen with lymphocytes bearing cognate antigen receptors on the cell surface and their stimulation; and combinations thereof. Systemic and / or regional immunity may be induced. Immune responses that provide prophylactic and / or therapeutic treatments are preferred. Antigenic activities in the formulation may be found in the same molecule, two or more different molecules dissociated from each other, or multiple molecules in a complex formed by covalent or non-covalent bonds. For antigens which are proteinaceous, they may be provided in the formulation as a polynucleotide for transcutaneous genetic immunization. Besides simple application of a dry or liquid formulation to the skin, patches and other medical devices may be used to deliver antigen for immunization.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE OFFICE OF THE COMMAND JUDGE ADVOCATE
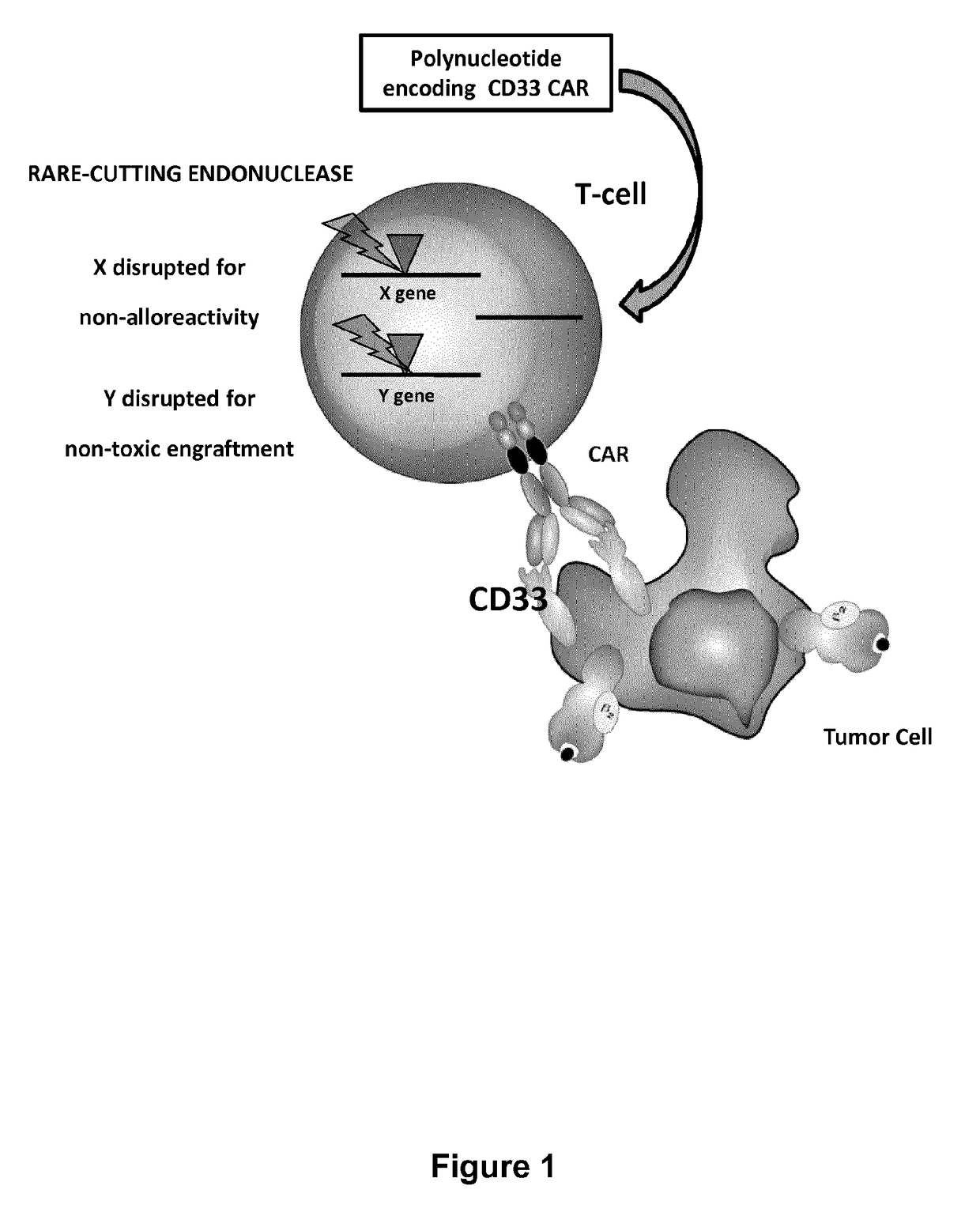
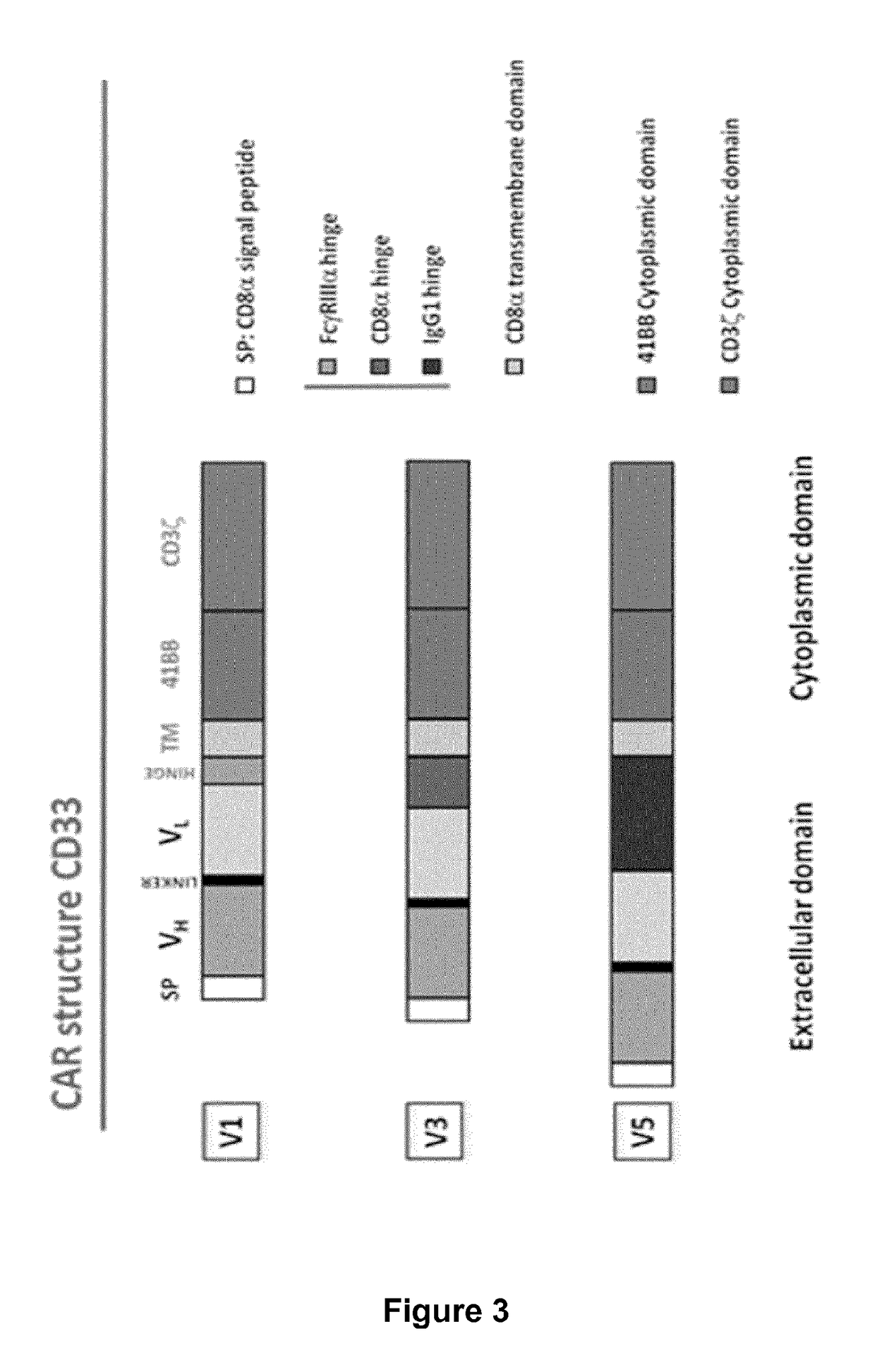
Cd33 specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170145094A1Useful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenCD33
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a CD33 monoclonal antibody, conferring specific immunity against CD33 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia.
Owner:CELLECTIS SA
Method for making multispecific antibodies having heteromultimeric and common components
InactiveUS8642745B2Increased formationIncrease productionHybrid immunoglobulinsPeptide/protein ingredientsHeterologousSpecific immunity
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method provides a multispecific antibody having a common light chain associated with each heteromeric polypeptide having an antibody binding domain. Additionally the method further involves introducing into the multispecific antibody a specific and complementary interaction at the interface of a first polypeptide and the interface of a second polypeptide, so as to promote heteromultimer formation and hinder homomultimer formation; and / or a free thiol-containing residue at the interface of a first polypeptide and a corresponding free thiol-containing residue in the interface of a second polypeptide, such that a non-naturally occurring disulfide bond is formed between the first and second polypeptide. The method allows for the enhanced formation of the desired heteromultimer relative to undesired heteromultimers and homomultimers.
Owner:GENENTECH INC
Dry Formulation for Transcutaneous Immunization
InactiveUS20090081244A1Easy to identifyEfficient deliverySsRNA viruses negative-senseAntibacterial agentsAdjuvantBacterial exotoxin
A transcutaneous immunization system delivers antigen to immune cells through the skin, and induces an immune response in an animal or human. For example, skin-active adjuvant (e.g., an ADP-ribosylating exotoxin) can be used to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a dry formulation containing antigen and adjuvant to skin of the animal or human. The dry formulation may be a powder or a unit-dose patch. Use of adjuvant is not required if the antigen is sufficiently antigenic. Transcutaneous immunization may be induced with or without penetration enhancement
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com