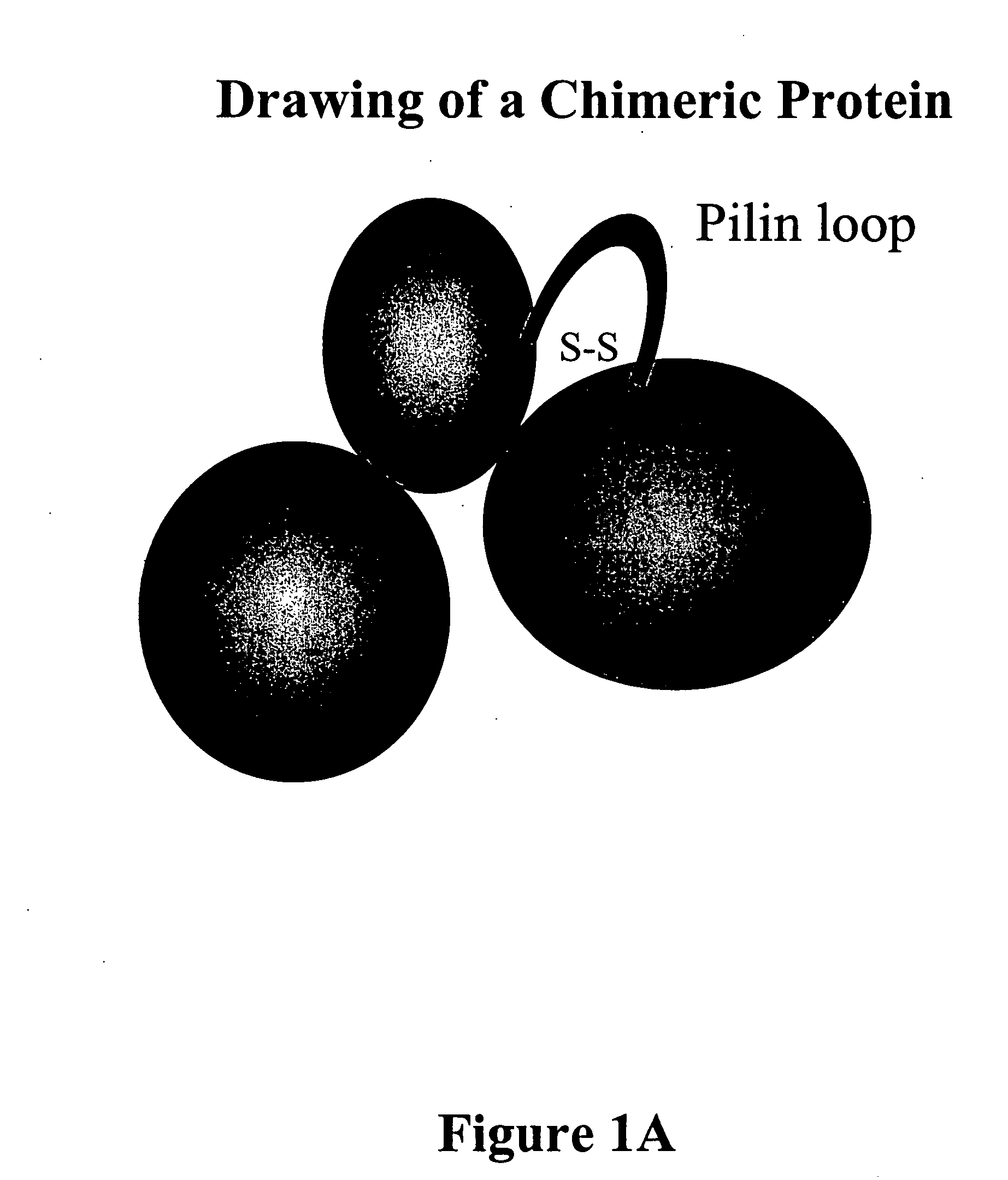Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Bacterial exotoxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host.
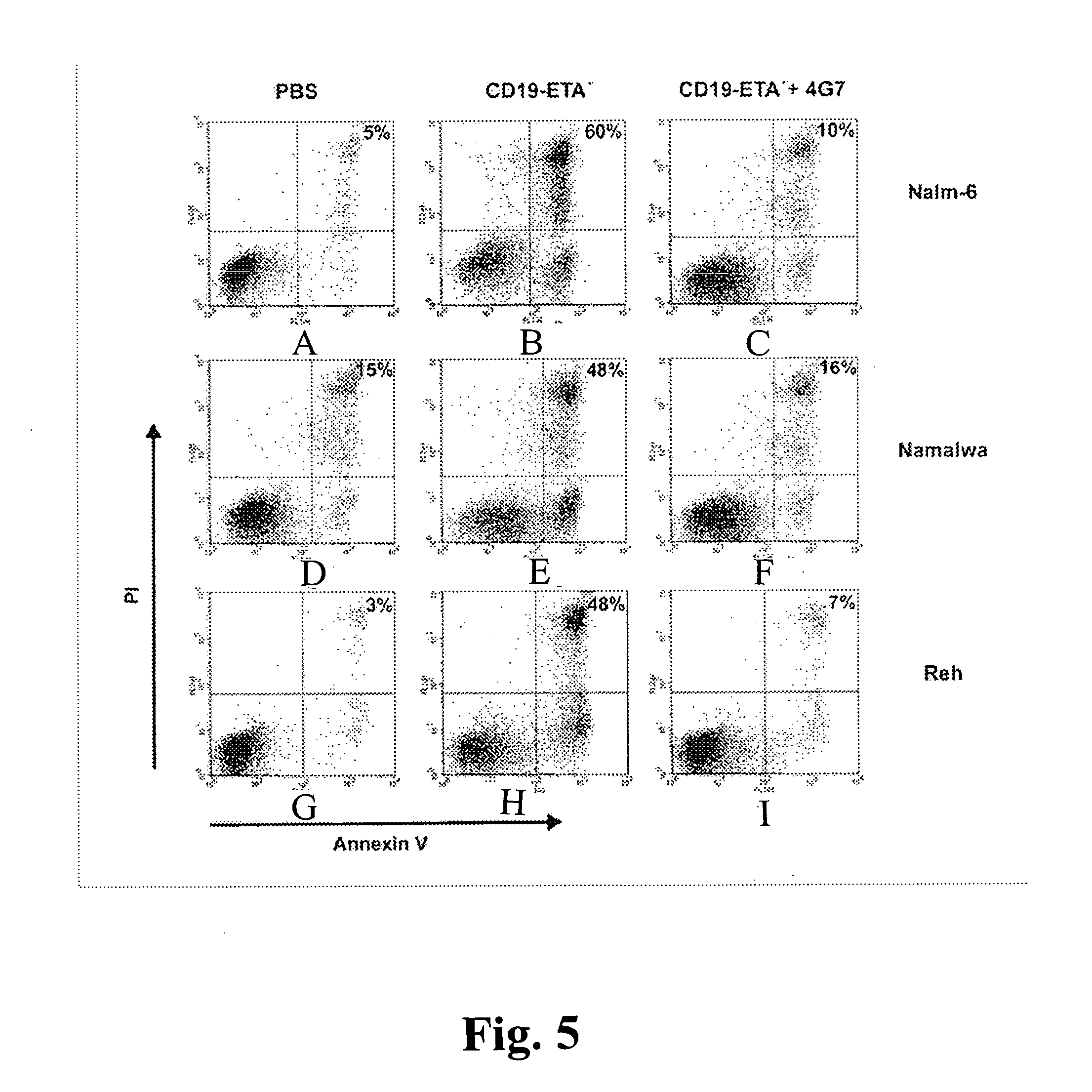
CD19-specific immunotoxin and treatment method
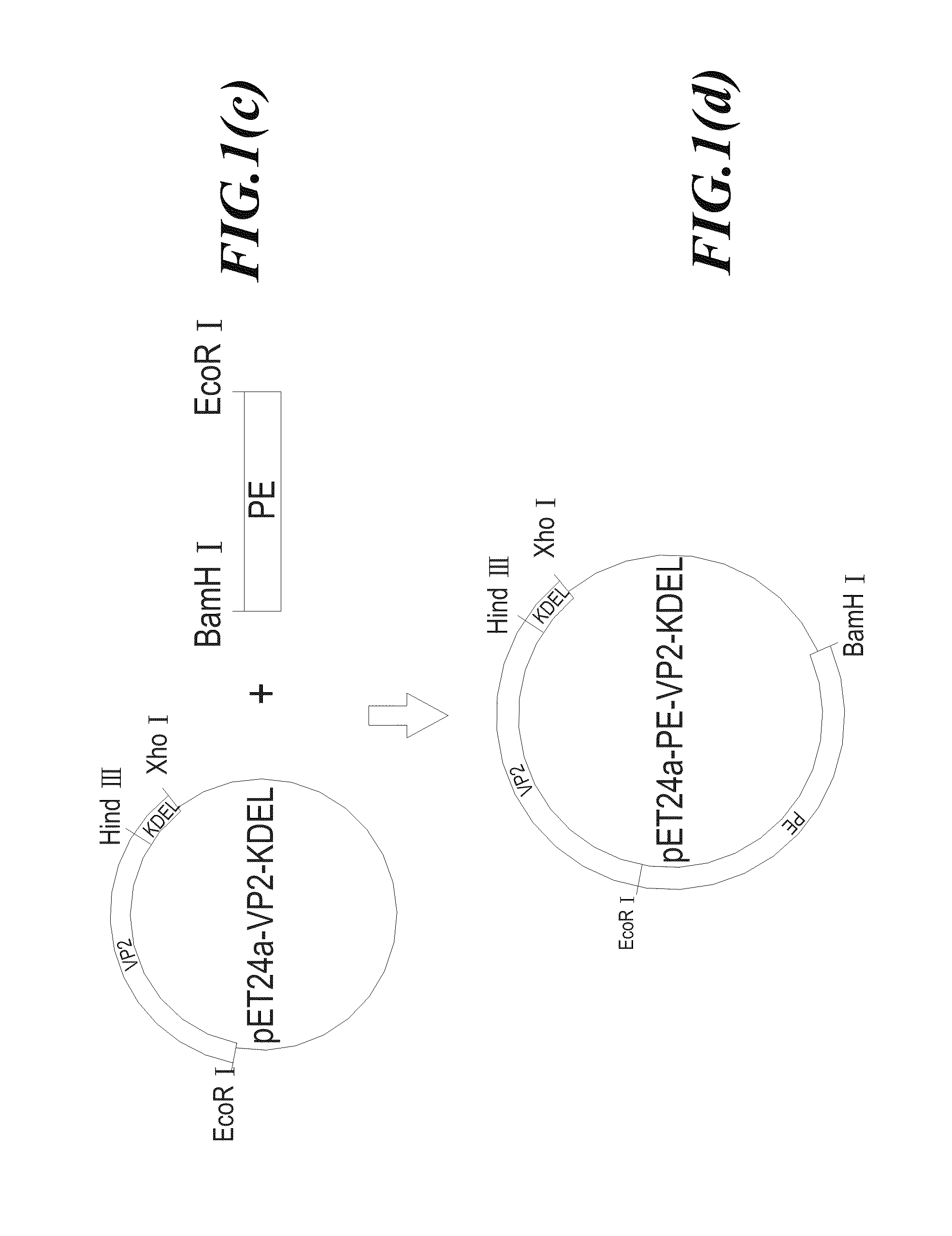
InactiveUS20070178103A1Easy to transportResistant to extracellular cleavagePolypeptide with localisation/targeting motifHybrid immunoglobulinsAutoimmune conditionBacterial exotoxin
An immunotoxin for use in, and a method for treating a subject having a cancer associated with malignant B-lineage cells or an autoimmune condition, are disclosed. The immunotoxin includes (a) an anti-CD19 antibody lacking an Fc fragment, (b) a modified exotoxin A protein having both Domains II and III, but lacking Domain I, and (c) a peptide linker joining the C-terminal end of the antibody to the N-terminal end of the modified exotoxin A protein. The linker is substantially resistant to extracellular cleavage. The modified exotoxin A protein may be further modified to include a C-terminal KDEL sequence (SEQ ID NO: 6) that promotes transport of the protein to the endoplasmic reticulum of cells that have taken up the immunotoxin.
Owner:FRIEDRICH ALEXANDER UNIV ERLANGEN NURNBERG
Dry Formulation for Transcutaneous Immunization
InactiveUS20090081244A1Easy to identifyEfficient deliverySsRNA viruses negative-senseAntibacterial agentsAdjuvantBacterial exotoxin
A transcutaneous immunization system delivers antigen to immune cells through the skin, and induces an immune response in an animal or human. For example, skin-active adjuvant (e.g., an ADP-ribosylating exotoxin) can be used to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a dry formulation containing antigen and adjuvant to skin of the animal or human. The dry formulation may be a powder or a unit-dose patch. Use of adjuvant is not required if the antigen is sufficiently antigenic. Transcutaneous immunization may be induced with or without penetration enhancement
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Adjuvant for transcutaneous immunization
A transcutaneous immunization system delivers antigen to immune cells without perforation of the skin, and induces an immune response in an animal or human. The system uses an adjuvant, preferably an ADP-ribosylating exotoxin, to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a formulation containing antigen and adjuvant to intact skin of the animal or human. The efficiency of immunization may be enhanced by adding hydrating agents (e.g., liposomes), penetration enhancers, or occlusive dressings to the transcutaneous delivery system. This system may allow activation of Langerhans cells in the skin, migration of the Langerhans cells to lymph nodes, and antigen presentation.
Owner:UNITED STATES OF AMERICA
GM1 binding deficient exotoxins for use as immunoadjuvants
InactiveUS20060002960A1Low toxicityRetaining adjuvant activityBacterial antigen ingredientsPharmaceutical delivery mechanismBacterial exotoxinGanglioside binding
Addition of a bacterial ADP-ribosylating exotoxin (bARE) to a formulation (e.g., immunogen or vaccine) or a system (e.g., patch or kit) for immunization enhances the immune response in a subject to one or more components of the formulation. Binding of the B subunit of a bARE to ganglioside GM1 of the subject in vivo, however, mediates toxicity and limits the use of native bARE as adjuvants. Mutation or in vitro coupling of the B subunit to ligands such as GM1 inhibits binding to GM1 in vivo, thereby eliminating toxicity but retaining desired adjuvant activity. The use of such detoxified, GM-1 binding deficient exotoxins provides a safe and potent new strategy for development of effective formulation for immunization.
Owner:INTERCELL USA
Dry formulation for transcutaneous immunization
A transcutaneous immunization system delivers antigen to immune cells through the skin, and induces an immune response in an animal or human. For example, a skin-active adjuvant (e.g., an ADP-ribosylating exotoxin) can be used to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a dry formulation containing antigen and adjuvant to skin of the animal or human. The dry formulation may be a powder or a unit-dose patch. Use of adjuvant is not required if the antigen is sufficiently antigenic. Transcutaneous immunization may be induced with or without penetration enhancement.
Owner:IOMAI CORP
Systems and methods of delivery of bioactive agents using bacterial toxin-derived transport sequences
ActiveUS9090691B2Efficient transportPowder deliveryOrganic active ingredientsDendrimerBacterial exotoxin
The field of the present invention relates, in part, to a strategy for novel pharmaceutical applications. More specifically, the present invention relates to a genetically detoxified form of Vibrio cholera exotoxin (cholix) and the use of cholix-derived polypeptide sequences to enhance intestinal delivery of biologically-active therapeutics. Importantly, the systems and methods described herein provide for the following: the ability to deliver macromolecule doses without injections; the ability to deliver cargo, such as (but not limited to) siRNA or antisense molecules into intracellular compartments where their activity is required; and the delivery of nanoparticles and dendrimer-based carriers across biological membranes, which otherwise would have been impeded due to the barrier properties of most such membranes.
Owner:APPLIED MOLECULAR TRANSPORT INC
Fusion antigen used as vaccine
InactiveUS7595054B2SsRNA viruses positive-sensePeptide/protein ingredientsBacterial exotoxinReticulum cell
Fusion antigen used as vaccine. The invention relates to a fusion antigen specific for a target cell. The fusion antigen contains a ligand moiety, a Pseudomonas exotoxin A translocation domain II, an antigenic moiety, and a carboxyl terminal moiety. The ligand moiety is capable of reacting, recognizing or binding to receptors on the target cell. The carboxyl terminal moiety permits retention and processing of the fusion antigen in the endoplasmic reticulum (ER) membrane of the target cell. Pharmaceutical compositions and methods of inducing an immune response using the same are also disclosed.
Owner:AGRI TECH RES INST
Anionic polymers as toxin binders and antibacterial agents
InactiveUS6890523B2Avoid developmentHighly effectiveAntibacterial agentsDigestive systemPathogenic microorganismBacterial exotoxin
The present invention relates to a method of inhibiting a toxin in an animal, such as a human, by administering to the animal a therapeutically effective amount of a polymer having a plurality of pendant acid functional groups which are directly attached to the polymer backbone or attached to the polymer backbone by a spacer group. The spacer group can have a length in the range from 0 to about 20 atoms. The toxin is, typically, an exotoxin secreted by a pathogenic microorganism, such as a bacterium.
Owner:GENZYME CORP
Chimeric protein comprising non-toxic pseudomonas exotoxin and type IV pilin sequences
InactiveUS20070003578A1Reduce adhesionAntibacterial agentsSenses disorderPseudomonas aeruginosa exotoxin ABacterial exotoxin
The invention provides chimeric proteins comprising a non-toxic Pseudomonas exotoxin A sequence and a Type IV pilin loop sequence, wherein the Type IV loop sequence is inserted within the non-toxic Pseudomonas exotoxin A. The invention also provides polynucleotides encoding the chimeric proteins, and compositions comprising the polynucleotides or the chimeric proteins. The invention also provides methods for using the chimeric proteins, polynucleotides and compositions of the invention.
Owner:THE GOV OF THE US REPRESENTED BY THE SEC OF THE DEP OF HEALTH & HUAMN SERVICES
Dry formulation for transcutaneous immunization
A transcutaneous immunization system delivers antigen to immune cells through the skin, and induces an immune response in an animal or human. For example, a skin-active adjuvant (e.g., an ADP-ribosylating exotoxin) can be used to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a dry formulation containing antigen and adjuvant to skin of the animal or human. The dry formulation may be a powder or a unit-dose patch. Use of adjuvant is not required if the antigen is sufficiently antigenic. Transcutaneous immunization may be induced with or without penetration enhancement.
Owner:IOMAI CORP
Broad-spectrum in-vivo effective superantigen toxin antagonists based on the interaction between CD28 and the superantigen and uses thereof
ActiveUS20050191296A1Facilitates the binding of a B7-2 ligandEliciting protective immunity against toxic shockCell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenBacterial exotoxin
Disclosed are methods and compositions for the inhibition of modulation of T cell costimulatory pathway by a pathogenic agent, particularly, the inhibition of activation of a T cell costimulatory pathway, preferably, the CD28 / B7 pathway, by a pyrogenic exotoxin. The method of the invention is based on the inhibition of the direct interaction of a superantigen with a specific site within the dimer interface of a CD28 family member, using immunomodulatory peptides. Further disclosed are specific antagonist immunomodulatory peptides comprising an amino acid sequence derived from a dimer interface of a T cell co-stimulatory pathway member, or peptides which comprise an amino acid sequence which specifically binds to an amino acid sequence within the dimer interface of a T cell co-stimulatory pathway member. Compositions comprising said peptides and methods for the treatment of immune-related disorders are also disclosed. Also disclosed is the use of the CD28 molecule or any fragment thereof comprising the sAg binding site in a method of screening for a test substance which specifically binds to the CD28 molecule and is capable of antagonizing pyrogenic exotoxin-mediated activation of Th1 lymphocytes.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Methods of delivery of exogenous proteins to the cytosol and uses thereof
InactiveUS20050220807A1Effective immune responseEvaluated effectively and efficientlyAntibacterial agentsVirusesProtective antigenAbnormal tissue growth
The present invention is directed to a method for delivering exogenous proteins to the cytosol, by binding a target antigen (such as a protein) to a transport factor that contains a fragment of a bipartite protein exotoxin, but not the corresponding protective antigen. Preferably, the target antigen is fused to the transport factor. Preferred transport factors include the protective antigen binding domain of lethal factor (LFn) from B. anthracis, consisting of amino acids 1-255, preferably a fragment of at least 80 amino acids that shows at least 80% homology to LFn, and a fragment of about 105 amino acids from the carboxy portion that does not bind PA. The target antigen can include any molecule for which it would be desirable to elicit a CMI response, including viral antigens and tumor antigens.
Owner:THE GENERAL HOSPITAL CORP +1
ExPEC glycoconjugate vaccine formulations
ActiveUS10159751B2Improve stabilityImproved stability against thermal stressInorganic non-active ingredientsCarrier-bound antigen/hapten ingredientsPseudomonas exotoxinEscherichia coli
Compositions and methods for inducing an immune response against extra-intestinal pathogenic Escherichia coli (ExPEC) are described. In particular, multivalent vaccines containing E. coli antigen polysaccharide covalently bound to a exotoxin A of Pseudomonas aeruginosa (EPA) carrier protein that can withstand multiple environmental stresses are described.
Owner:JANSSEN PHARMA INC
Clostridium difficile exotoxin A carboxy-terminal gene sequence with optimized codon and nucleic acid vaccine thereof
InactiveCN102199611AStimulus expression levelGood immune protectionAntibacterial agentsBacterial antigen ingredientsPseudomonas exotoxinEscherichia coli
Owner:王世霞 +3
Clostridium difficile exotoxin A carboxy-terminal gene sequence with optimized codon and nucleic acid vaccine thereof
InactiveCN101870978AStimulus expression levelGood immune protectionAntibacterial agentsBacterial antigen ingredientsPseudomonas exotoxinEscherichia coli
The invention belongs to the technical field of biomedicine, relating to a clostridium difficile exotoxin A carboxy-terminal gene sequence with optimized codon and a nucleic acid vaccine thereof. The codon optimized gene sequence gives consideration to use preferences of mammalian cells and Esherichia coli codon, and the related clostridium difficile vaccine is composed of exotoxin A carboxy-terminal gene sequence with optimized codon and eukaryon expression vector pJW4303. The clostridium difficile exotoxin A carboxy-terminal gene segment optimized by codon can not only be used for constructing nucleic acid vaccines, effectively stimulating host immune system after immunizing mammals, generating better humoral immunity reaction, being applicable to conduct pronucleus expression in esherichia coli, and laying a foundation for acquiring protein in great quantities.
Owner:王世霞 +3
Anionic polymers as toxin binders and antibacterial agents
InactiveUS20060029568A1Avoid developmentHighly effectiveAntibacterial agentsDigestive systemPathogenic microorganismBacterial exotoxin
The present invention relates to a method of inhibiting a toxin in an animal, such as a human, by administering to the animal a therapeutically effective amount of a polymer having a plurality of pendant acid functional groups which are directly attached to the polymer backbone or attached to the polymer backbone by a spacer group. The spacer group can have a length in the range from 0 to about 20 atoms. The toxin is, typically, an exotoxin secreted by a pathogenic microorganism, such as a bacterium.
Owner:GENZYME CORP
Subunit Vaccine for Aquaculture
ActiveUS20100316663A1Simple processReduce manufacturing costPolypeptide with localisation/targeting motifBacterial antigen ingredientsDiseaseBacterial exotoxin
A subunit vaccine for aquaculture comprises an antigen fusion protein, and appropriate carriers or adjuvants. From amino terminal to carboxyl terminal, the antigen fusion protein comprises domain I and domain II of Pseudomonas aeruginosa exotoxin A; a viral antigenic protein producing a fish disease; and a signal peptide of a protein.
Owner:SBC VIRBAC LTD
Methods and compositions relating to anthrax pathogenesis
InactiveUS20080124746A1Peptide/protein ingredientsMicrobiological testing/measurementBacterial exotoxinFactor ii
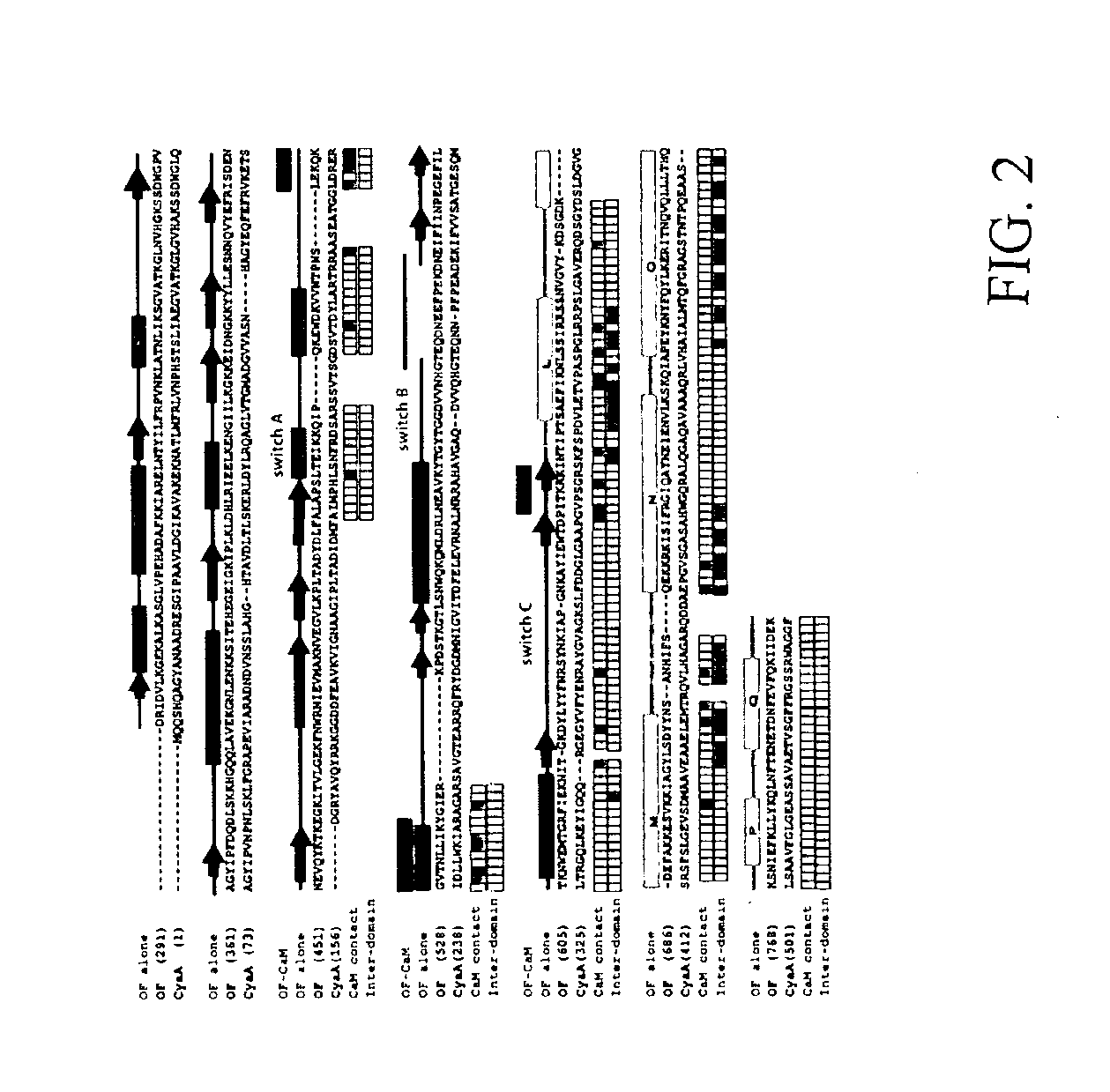
The structures of Edema Factor alone and Edema Factor bound to calmodulin without substrate has been crystallized and its structure determined by x-ray crystallography. Based upon these crystal structures, a method assaying for inhibitors of infection by a bacteria is presented which comprises obtaining a potential inhibitor, obtaining a calmodulin activated adenylyl cyclase exotoxin, obtaining calmodulin, admixing the potential inhibitor, the exotoxin, and the calmodulin, and assaying to determine whether or not the potential inhibitor inhibits production of cAMP by exotoxin.
Owner:BOSTON BIOMEDICAL RES INST +1
Use of non-antibacterial tetracycline analogs and formulations thereof for the treatment of bacterial exotoxins
The invention relates to methods for protecting and / or treating a mammal at risk of acquiring a condition associated with bacteria that produce a calmodulin exotoxin, a metalloproteinase exotoxin, or both, by administering a non-antibacterial tetracycline formulation.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Skin immunization using lt-sta fusion proteins
InactiveUS20090017056A1Enhance immune responseBacterial antigen ingredientsAntibody mimetics/scaffoldsBacterial exotoxinRibose
This invention includes fusion proteins comprising a bacterial ADP-ribosylating exotoxin (bARE), or a variant or portion thereof, fused to a STa exotoxin, or a portion or variant thereof. Optionally, the exotoxins are fused via a peptide linker. The invention also includes compositions formulated for transcutaneous immunizations and / or induction of an immune response by epicutaneous administration comprising an effective amount of a fusion protein comprising a bacterial ADP-ribosylating exotoxin fused to a STa exotoxin. Optionally, the exotoxins are fused via a peptide linker.
Owner:INTERCELL USA
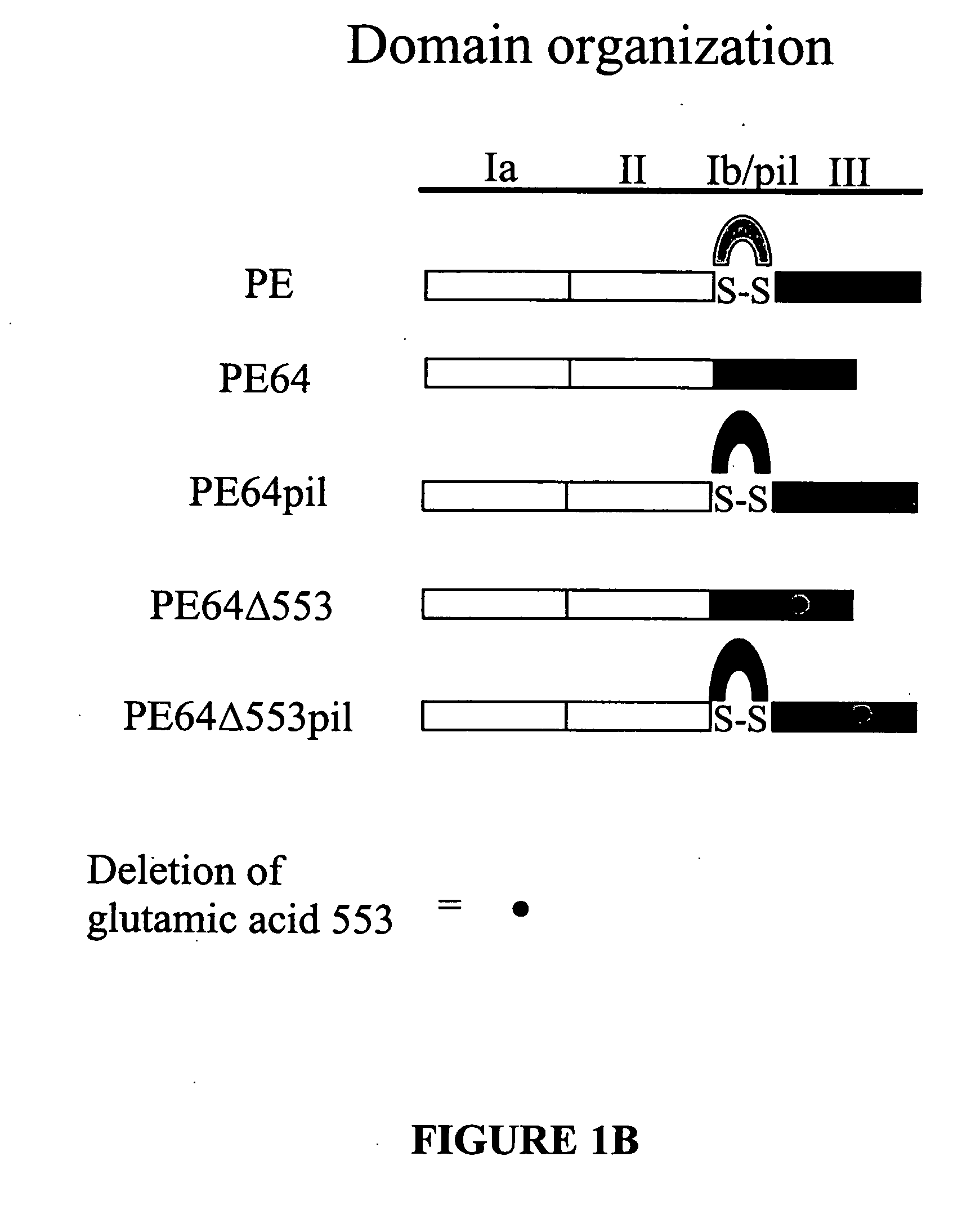
Modified Forms of Pseudomonas Exotoxin A
InactiveUS20130121983A1Reduce and prevent PE38-induced immunogenicityBacteriaPeptide/protein ingredientsHeterologousPseudomonas exotoxin
Pseudomonas exotoxin A or “PE” is a 66 kD, highly potent, cytotoxic protein secreted by the bacterium Pseudomonas aeruginosa. Various forms of PE have been coupled to other proteins, such as antibodies, to generate therapeutically useful cytotoxin conjugates that selectively target cells of a desired phenotype (such as tumor cells). In the present invention, peptides spanning the sequence of an approximately 38 kD form of Pseudomonas exotoxin A protein were analyzed for the presence of immunogenic CD4+ T cell epitopes. Six immunogenic T cell epitopes were identified. Residues were identified within each epitope for introduction of targeted amino acid substitutions to reduce or prevent immunogenic T-cell responses in PE molecules which may be administered to a heterologous host.
Owner:PRECIGEN INC
Broad spectrum pyrogenic antagonists and vaccines directed against pyrogenic exotoxins
InactiveUS20060222650A1Avoid problemsBiocidePeptide/protein ingredientsBacterial exotoxinBiological activation
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Production method of bacterial exotoxin
ActiveCN101619339AImprove the growing environmentWiden the breeding spacePeptide preparation methodsBacteria peptidesDialysis membranesBacteroides
The invention discloses a production method of bacterial exotoxin, comprising the procedures of bacterial toxin production culture, bacterial exotoxin extraction, bacterial exotoxin purification and bacterial exotoxin preservation, wherein the bacterial toxin production culture is carried out in a microorganism dialysis incubator which comprises a culture medium chamber and a culture chamber, a dialysis membrane and a dialysis membrane supporting and protecting device are arranged between the culture medium chamber and the culture chamber, the ratio of the volume of the culture medium chamber to the total volume of the culture chamber is selected between 3:1 and 1:6 according to a microorganism culture time, and facilities needed by microorganism growth are arranged in the culture medium chamber. The production method comprises the procedures of toxin production culture, sterilization, filtration, concentration, precipitation, percrystallization and preservation. The production method of bacterial exotoxin is adopted to produce toxin, optimizes bacterial growth environment, simplifies the technologies of concentration and purification of bacterial exotoxin, increases the yield to 90 percent from 17-33 percent, and shortens the purification time to about 12-24 hours from the original natural dialysis of 12-15 days.
Owner:河北施坦纳生物科技有限公司
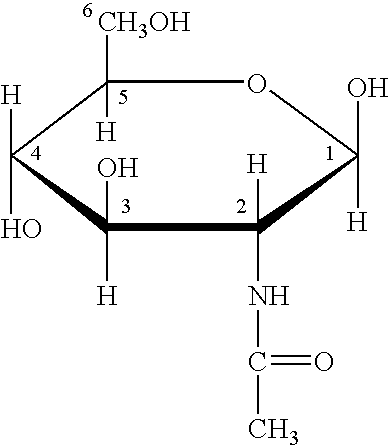
Use of acetyl-d-aminoglycosamine in treatment of local lesions and systematic symptoms related to infections of virus or bacteria
InactiveUS20070042995A1Reducing local inflammationAlleviating systematic toxic symptomAntibacterial agentsBiocideToxic symptomBacterial exotoxin
The present invention discloses a use of N-acetyl-D-glucosamine or pharmaceutically acceptable salts thereof in the manufacture of a medicament for treating local lesions or systematic symptoms caused by infections of virus or bacteria. A parenteral preparation comprising N-acetyl-D-glucosamine or pharmaceutically acceptable salts thereof as active component is capable of controlling systematic toxic symptoms caused by infections of virus and bacteria and local and systematic lesions caused by endotoxins and exotoxins, and exhibits an excellence rate of 90%.
Owner:ARMY MEDICAL UNIV +2
Bispecific molecule comprising an anti-cr1 antibody cross-linked to an antigen-binding antibody fragment
InactiveUS20060140931A1Undesirable conditionAntibacterial agentsHybrid immunoglobulinsProtective antigenCross-link
The invention provides a bispecific molecule comprising an antibody that binds a C3b-like to one or more antigen-binding antibody fragments, each of which binds an antigenic molecule. The invention also provides methods of producing such bispecific molecules and to therapeutic uses of such bispecific molecules. The invention further provides bispecific molecules in which the antigen-binding antibody fragment binds the protective antigen protein of Bacillus anthracis (Anthrax) exotoxin for treatment of Anthrax infection.
Owner:ELUSYS THERAPEUTICS
Methods and compositions for promoting a cell-mediated immune response
ActiveUS9968666B2Good curative effectEasy to displayAntibacterial agentsPeptide librariesProtective antigenBacterial exotoxin
The present invention is directed to a method for promoting or stimulating a cell-mediated immune response to an antigen, by administering a target antigen (such as a protein) with a transport factor that contains a fragment of a bipartite protein exotoxin, but not the corresponding protective antigen. Preferred transport factors include the protective antigen binding domain of lethal factor (LFn) from B. anthracis, consisting of amino acids 1-255, preferably a fragment of at least 80 amino acids that shows at least 80% homology to LFn, and a fragment of about 105 amino acids from the carboxy portion that does not bind PA. The target antigen can include any molecule for which it would be desirable to elicit a CMI response, including viral antigens and tumor antigens.
Owner:VACCINE TECH
Adjuvant for transcutaneous immunization
A transcutaneous immunization system delivers antigen to immune cells without perforation of the skin, and induces an immune response in an animal or human. The system uses an adjuvant, preferably an ADP-ribosylating exotoxin, to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a formulation containing antigen and adjuvant to intact skin of the animal or human. The efficiency of immunization may be enhanced by adding hydrating agents (e.g., liposomes), penetration enhancers, or occlusive dressings to the transcutaneous delivery system. This system may allow activation of Langerhans cells in the skin, migration of the Langerhans cells to lymph nodes, and antigen presentation.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Nucleic acid aptamer eta01 of Pseudomonas aeruginosa exotoxin a and its application
ActiveCN111793629BStrong specificityHigh affinityAntibacterial agentsOrganic active ingredientsPseudomonas aeruginosa exotoxin AAptamer
The present invention relates to a nucleic acid aptamer ETA01 of Pseudomonas aeruginosa exotoxin A and its application. The sequence of the nucleic acid aptamer ETA01 includes: 5'-TGCAACTCCAACTAACCATGTGTTCAT CTGAGGGTGGTCTTCGCA-3'. The nucleic acid aptamer ETA01 of the present invention can bind to Pseudomonas aeruginosa exotoxin A with high affinity and high specificity, and use the nucleic acid aptamer ETA01 as the recognition element of Pseudomonas aeruginosa exotoxin A to establish Pseudomonas aeruginosa The detection method of bacterial exotoxin A, the preparation of detection reagents, and the development of exotoxin A antagonistic drugs can be used in the diagnosis and treatment of Pseudomonas aeruginosa infection and the research on the biological function of Pseudomonas aeruginosa exotoxin A. The method has the advantages of simplicity, rapidity and good characteristics.
Owner:丽水君弘生物科技有限公司
Subunit vaccine for aquaculture
ActiveUS8343505B2High puritySimple processPolypeptide with localisation/targeting motifBacterial antigen ingredientsDiseasePseudomonas aeruginosa exotoxin A
Owner:SBC VIRBAC LTD
Quick-dry hand disinfection fluid for viruses on children
InactiveCN108703921AImprove disinfection effectInhibitory activityCosmetic preparationsToilet preparationsGlycerolHand disinfection
The invention discloses quick-dry hand disinfection fluid for viruses on children. The quick-dry hand disinfection fluid comprises, by weight, 0.1%-0.2% of hydrogen peroxide, 50%-60% of medicinal alcohol, 1.0%-1.5% of PEG-7 (polypropylene glycol-7) glyceryl coconate, 0.01%-0.05% of sodium hyaluronate, 0.001%-0.005% of green tea extract, 0.2%-0.5% of sorbitan monostearate, 1.0%-1.5% of medical glycerin, an appropriate quantity of citric acid and the balance purified water. The quick-dry hand disinfection fluid has the advantages that the green tea extract of the quick-dry hand disinfection fluid contains abundant tea polyphenol, accordingly, clostridium botulinum and spores can be killed, the activity of bacterial exotoxin can be inhibited, and antibacterial effects can be realized for diversified pathogens which can cause diarrhea and respiratory tract and skin infection; the quick-dry hand disinfection fluid comprises the sodium hyaluronate which is a natural moisture retention lubricant, and accordingly skin injury due to the quick-dry hand disinfection fluid can be reduced to a great extent; the viruses can be quickly killed by the quick-dry hand disinfection fluid in a formulaafter hands are dried, bacteria can be inhibited in a long-acting manner for 6 hours, skin can be efficiently protected, and the quick-dry hand disinfection fluid in the weak acidity formula is good in fusion property with the skin.
Owner:山东卓健医疗科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com