Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
372 results about "Protective antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protective antigens are specifically targeted by the acquired immune response of the host and are able to induce protection in the host against infectious and non-infectious diseases.
rPA optimization
An optimized synthetic polynucleotide encoding a Bacillus anthracis protective antigen and an anthrax vaccine based on the encoded protective antigen. Furthermore, heterologous expression in a host Pseudomonas fluorescens bacteria of an optimized polynucleotide sequence encoding a Bacillus anthracis protective antigen.
Owner:PELICAN TECH HLDG INC
Outer membrane vesicle (OMV) vaccine comprising N. meningitidis serogroup B outer membrane proteins
ActiveUS8273360B2Broadens their efficacyAntibacterial agentsNervous disorderProtective antigenNeisseria weaveri
A composition comprising (a) Neisseria meningitidis serogroup B outer membrane vesicles (OMVs), and (b) an immunogenic component selected from other Neisseria proteins, or immunogenic fragments thereof. Component (b) preferably includes a protein from a different NmB strain from that from which the OMV of component (a) is derived. The OMVs are preferably obtained by deoxycholate extraction. Optionally, the composition may also comprise a protective antigen against other pathogens.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Recombinant SARS-CoV-2 vaccine using human replication-defective adenovirus as vector
ActiveCN111218459AReduce loadSimple manufacturing methodSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
The invention provides a SARS-CoV-2 vaccine using human type-5 replication-defective adenovirus as a vector. The vaccine uses E1 and E3 to be combined with replication-defective human type-5 adenovirus as the vector and HEK293 cells integrating adenovirus E1 gene as a packaging cell line, and a protective antigen gene carried is the 2019 SARS-CoV-2 S protein gene (Ad5-nCoV) which is subjected to optimization design. After the S protein gene is optimized, the expression level in transfected cells is increased significantly. The vaccine has good immunogenicity in mouse and guinea pig models, andcan induce a body to produce a strong cellular and humoral immune response in a short time. Studies on the protective effect of hACE2 transgenic mice show that after 14 days of single immunization ofAd5-nCoV, the viral load in lung tissue can be significantly reduced, and it is indicated that the vaccine has a good immunoprotective effect on the 2019 SARS-CoV-2. In addition, the vaccine is quick, simple and convenient to prepare, and can be mass-produced in a short period of time to respond to sudden outbreaks.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Antibodies against protective antigen
ActiveUS7601351B1Efficient killingIncreased activationAntibacterial agentsAntibody ingredientsAntigenProtective antigen
The present invention relates to antibodies and related molecules that specifically bind to protective antigen of Bacillus anthracis (PA). Such antibodies have uses, for example, in the prevention and treatment of anthrax and anthrax toxin poisoning. The invention also relates to nucleic acid molecules encoding anti-PA antibodies, vectors and host cells containing these nucleic acids, and methods for producing the same.
Owner:EMERGENT MFG OPERATIONS BALTIMORE LLC
Attenuated chimeric respiratory syncytial virus
InactiveUS7846455B2Reduce the possibilityDesired characteristicSsRNA viruses negative-senseViral antigen ingredientsHeterologousProtective antigen
Owner:UNITED STATES OF AMERICA
Diagnostic and protective antigen gene sequences of ichthyophthirius
A novel i-antigen protein from Ichthyophthirius multifiliis is effective to induce a protective immune response in fish. The invention includes antigenic and membrane-targeting sequences of i-antigen proteins, nucleic acid molecules that encode antigenic or membrane-targeting portions of i-antigen proteins, DNA and protein subunit vaccines, methods of inducing an immune response in fish and methods for detection and characterization of I. multifiliis.
Owner:GEORGIA UNIV OF RES FOUND INC THE +1
Helicobacter pylori vaccine based on urease B subunit active segment and its prepn process
InactiveCN1887349AGood immune protectionAntibacterial agentsBacterial antigen ingredientsProtective antigenAntigen
The present invention provides one kind of genetic engineering multivalent subunit vaccine for preventing and treating human helicobacter pylori infection and its preparation process. The vaccine consists of active helicobacter pylori UreB fragment UreB414 as the central antigen component, other combined protective antigens, and intramolecular or extramolecular adjuvant. Compared with univalent vaccine, the multivalent combined vaccine can stimulate the body to generate more powerful and more comprehensive helicobacter pylori resisting specific immune reaction.
Owner:ARMY MEDICAL UNIV
Use of a live attenuated Mycoplasma gallisepticum strain as a vaccine and vector for the protection of chickens and turkeys from respiratory disease
The present invention relates to a live cytadherence-deficient M. gallisepticum strain that does not express at least two of three proteins, the Gap-A molecule, crnA protein, and the 45 kDa protein, expressed by wildtype M. gallisepticum Strain R and its use as a vaccine for preventing and protecting birds, especially chickens and turkeys against the respiratory diseases attendant with wildtype Mycoplasma gallispeticum infection. The invention also relates to the use of the vaccine as a vector for the delivery of genes encoding protective antigens from other bacterial and viral avian pathogens, such as avian influenza virus. There is also disclosed a method for identifying the attenuated cytadherence-deficient M. gallisepticum Rhigh or a strain thereof.
Owner:UNIV OF CONNECTICUT
Codon-optimized polynucleotide-based vaccines against Bacillus anthracis infection
The invention is related to polynucleotide-based anthrax vaccines. In particular, the invention is plasmids operably encoding Bacillus anthracis antigens, in which the naturally-occurring coding regions for the B. anthracis antigens have been modified for improved translation in human or other mammalian cells through codon optimization. In certain embodiments, the coding regions are also modified so as to remove potential N-linked glycosylation sites. B. anthracis antigens which are useful in the invention include, but are not limited to protective antigen (PA), lethal factor (LF), and fragments, variants or derivatives of either of these antigens. The invention is further directed to methods to induce an immune response to B. anthracis in a mammal, for example, a human, comprising delivering a plasmid encoding a codon-optimized B. anthracis antigen as described above. The invention is also directed to pharmaceutical compositions comprising plasmids encoding a codon-optimized B. anthracis antigen as described above, and further comprising adjuvants, excipients, or immune modulators.
Owner:VICAL INC
Modified Bacillus anthracis, vaccine compositions and methods of use thereof
InactiveUS20070031457A1Low toxicityReduce maintenanceBacterial antigen ingredientsBacteriaAntigenProtective antigen
A variety of modified Bacillus anthracis bacteria useful in vaccines are provided. For instance, asporogenic strains of Bacillus anthracis are provided. In addition, Bacillus anthracis strains attenuated in their ability to repair their nucleic acid, such as in their nucleic acid excision repair ability or recombination repair ability, are provided. Strains expressing an antigen, such as protective antigen, under the control of a heterologous promoter and / or an inducible promoter are also provided. Bacillus anthracis bacteria comprising mutations in toxin genes are further provided. Vaccine compositions comprising the bacteria, methods of making the modified strains, and methods of using the vaccines are also provided.
Owner:ANZA THERAPEUTICS INC +1
Immune protective antigen of haemophilus parasuis
ActiveCN102864157AGood protective antigen proteinAntibacterial agentsBacteriaProtective antigenEscherichia coli
The invention belongs to the technical field of animal-borne disease subunit vaccine preparation and relates to preparation and application of the immune protective antigen of the haemophilus parasuis. Outer membrane protein Hbp B genes of the haemophilus parasuis are cloned, a nucleotide sequence is indicated as SEQID NO:1, and the sequence of gene code is indicated as SEQ ID NO:2. Recombination Escherichia coli BL21 / Pet-28a-Hbp B (preservation number is CCTCC NO:M2011228) is built and comprises genes in a sequence table SEQ ID NO:1. Antigen protein of the haemophilus parasuis is obtained and expressed through gene transformation Escherichia coli in the SEQ ID NO:1. The invention further discloses a preparation method and application of the recombination Escherichia coli. The haemophilus parasuis subunit vaccine has good safety, and an immune protection effect reaches 83%.
Owner:HUAZHONG AGRI UNIV +1
Recombinant low-virulent vaccine strain of chicken infectious bursal disease viruses (IBDV) and application thereof
ActiveCN101935637ANon-pathogenicGood spiritsViral antigen ingredientsMicroorganism based processesProtective antigenOrganism
The invention discloses a recombinant low-virulent vaccine strain of chicken infectious bursal disease viruses (IBDV) and application thereof. In the invention, a major protective antigen gene VP2 of an epidemic superhigh virulent strain is cloned, the nucleotide of the gene VP2 is modified by mutation and then used for replacing a corresponding segment of a Gt genome of a low-virulent strain of the IBDV, so that the infectious clone of a recombinant genome of the IBDV is constructed, and the recombinant low-virulent vaccine strain is saved and identified by using an IBDV reverse genetic operation system. The microbial collection number of the vaccine strain is CGMCC No.3749. The recombinant low-virulent vaccine strain of the invention has high replicability, genetic stability and safety. The immune effect of the low-virulent vaccine strain of the invention is as good as that of the medium-virulent vaccine strain, but is superior to that of the low-virulent vaccine strain. The biological safety of the low-virulent vaccine strain of the invention is superior to that of the medium-virulent vaccine strain. As the vaccine strain, the recombinant low-virulent vaccine strain of the invention has the characteristics of high efficiency and low toxicity, is a good candidate vaccine strain and can be used for controlling chicken infectious bursal disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant modified Bacillus anthracis protective antigen for use in vaccines
The invention relates to improved methods of producing and recovering sporulation-deficient B. anthracis mutant stains, and for producing and recovering recombinant B. anthracis protective antigen (PA), especially modified PA which is protease resistant, and to methods of using of these PAs or nucleic acids encoding these PAs for eliciting an immunogenic response in humans, including responses which provide protection against, or reduce the severity of, B. anthracis bacterial infections and which are useful to prevent and / or treat illnesses caused by B. anthracis, such as inhalation anthrax, cutaneous anthrax and gastrointestinal anthrax.
Owner:DEPT OF HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE +1
Vaccine composition
InactiveUS20060216307A1UpregulationReducing lipid A toxicityAntibacterial agentsSenses disorderProtective antigenBacteroides
The present invention relates to an immuno-protective and non-toxic Gram-negative bleb vaccine suitable for paediatric use. Examples of the Gram-negative strains from which the blebs are made are N. meningitidis, M. catarrhalis and H. influenzae. The blebs of the invention are improved by one or more genetic changes to the chromosome of the bacterium, including up-regulation of protective antigens, down-regulation of immunodominant non-protective antigens, and detoxification of the Lipid A moiety of LPS.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Recombinant immunogenic compositions and methods for protecting against lethal infections from Bacillus anthracis
ActiveUS7201912B2Reduce in quantityLow costBacterial antigen ingredientsSnake antigen ingredientsProtective antigenFusion Protein Expression
Recombinant immunogenic compositions and methods for protecting against lethal infections from Bacillus anthracis having a variant of recombinant Bacillus anthracis protective antigen (rPA) and a variant of recombinant Bacillus anthracis lethal factor (rLF). These proteins may be expressed separately or as a fusion protein. The recombinant proteins are produced in an avirulent strain of Bacillus anthracis that overproduces the desired antigens. The compositions and methods induce the animal host to produce antibodies against a virulent strain of Bacillus anthracis.
Owner:EMERGENT BIODEFENSE OPERATIONS LANSING
Adjuvant for enhancing fish vaccine immunization effect and application thereof
ActiveCN102430120AImprove featuresImprove protectionImmunological disordersAntibody medical ingredientsProtective antigenAstragaloside
The invention relates to an adjuvant for enhancing a fish vaccine immunization effect and an application thereof. The adjuvant for enhancing the fish vaccine immunization effect is characterized in that an immune potentiator is extracted from Astragalus mongholicus as a leguminous plant or effective components of the Astragalus mongholicus, comprising Astragaloside and Astragalus polysacharin, and natural products or manually modified products or manually synthetic products comprising the Astragaloside and the Astragalus polysacharin can be adopted to serve as the effective components. The adjuvant is capable of increasing the specific immune protection rate after vaccine component immunization is finished. When the adjuvant is applied, a vaccine can comprise the following components: any one or more than one of expression products of inactivated pathogens, bacterial ghost components, hypotoxic pathogens, attenuated pathogens, protective antigens, antigen subunits, antigen determinants or antigen gene expression vectors of bacteria, viruses and parasites. When in use, the adjuvant can be mixed with the components of the vaccine for application, also cannot be mixed with the components of the vaccine for application and also can be applied with the vaccine at different time.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Expression of protective antigens in transgenic chloroplasts and the production of improved vaccines
InactiveUS20050108792A1Bacterial antigen ingredientsAntibody mimetics/scaffoldsAntigenDevelopmental stage
Vaccines for conferring immunity in mammals to infective pathogens are provided, as well as vectors and methods for plastid transformation of plants to produce protective antigens and vaccines for oral delivery. The invention further provides transformed plastids having the ability to survive selection in both the light and the dark, at different developmental stages by using genes coding for two different enzymes capable of detoxifying the same selectable marker, driven by regulatory signals that are functional in proplastids as well as in mature chloroplasts. The invention utilizes antibiotic-free selectable markers to provide edible vaccines for conferring immunity to a mammal against Bacillus anthracis, as well as Yersina pestis. The vaccines are operative by parenteral administration as well. The invention also extends to the transformed plants, plant parts, and seeds and progeny thereof. The invention is applicable to monocot and dicot plants.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Construction of lactobacillus acidophilus S-layer protein surface display system
InactiveCN101638660AReduce degradationEasy to participateBiological testingFluorescence/phosphorescenceSurface displayAntigen
The invention provides construction of a lactobacillus acidophilus S-layer protein surface display system, which belongs to the field of biotechnology. The construction is characterized in that gene acquisition comprises (1) PCR amplification, (2) the connection of a SlpA gene and a pMD18-TVector system and (3) the identification of cloning plasmid pMD18T-S, and the construction comprises (1) theacquisition of a target gene and a double-labeling expression vector, (2) the connection and transformation of the double-labeling expression vector and SlpA, (3) the identification of a surface display vector system and (4) the detection of the anchored expression situation of S-layer protein on recombinant surface. The construction has the advantages that: 1, the constructed vector can be used to be transformed into S-layer protein gene defect lactobacillus to study the formation mechanism of lactobacillus S-layer protein; and 2, by utilizing a DNA recombination technique, protective antigengenes of pathogens are fused with lactobacillus S-layer protein on the surface display vector.
Owner:CHANGCHUN UNIV OF SCI & TECH
Recombinant porcine epidemic diarrhea virus (PEDV) lactococcus lactis and its construction method and use
InactiveCN103074291AGood immune protectionPromote absorptionBacteriaMicroorganism based processesProtective antigenWestern blot
Owner:DALIAN UNIVERSITY
Velogenic Edwardsiella tarda vaccine strain and application thereof
ActiveCN103255089AStrong drug resistanceReduce lossesAntibacterial agentsBacterial antigen ingredientsBacteroidesProtective antigen
The invention relates to an Edwardsiella tarda strain and an application method thereof. The Edwardsiella tarda strain is separated from a turbot adult fish body and is a wild strain with strong virulence, and the preservation number of the Edwardsiella tarda strain is CGMCC No.7197. Preparation modes of an antigen of the Edwardsiella tarda strain comprise any one or more than one of an inactivated thallus, a bacteruak ghost ingredient, an attenuated strain, a protective antigen, an antigen subunit and an expression product of an antigen determinant or an antigen gene expression carrier; the produced vaccine can be a single ingredient of the antigen prepared by utilizing the Edwardsiella tarda strain and can also be a combined vaccine produced by mixing the antigen prepared by utilizing the Edwardsiella tarda strain with antigens of other bacteria, and the prepared single or combined vaccine antigen is added with an adjuvant to produce the vaccine; and an inoculation mode of the vaccine in immunization application can adopt injection immunization, wound immunization, immersion bath immunization or oral administration immunization.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Identification of protective antigenic determinants of porcine reproductive and respiratory syndrome virus and uses thereof
ActiveUS20080019912A1Effective protectionAvoid infectionVirusesPeptide/protein ingredientsAntigenProtective antigen
The invention relates to a polypeptide of a protective antigenic determinant (PAD polypeptide) of porcine reproductive and respiratory syndrome virus (PRRSV) and nucleic acids encoding a PAD polypeptide. The PAD polypeptide and nucleic acids encoding a PAD polypeptide are useful in the development of antibodies directed to PAD, vaccines effective in providing protection against PRRSV infection, and diagnostic assays detecting the presence of PAD antibodies generated by a PAD-specific vaccine. The invention also discloses methods of generating antibodies to PAD, for vaccinating a pig to provide protection from PRRSV infections, a method of preparing the vaccine, a method of treating PRRSV infections in a pig, and a method of detecting antibodies to PAD of PRRSV.
Owner:IOWA STATE UNIV RES FOUND
Methods of delivery of exogenous proteins to the cytosol and uses thereof
InactiveUS20050220807A1Effective immune responseEvaluated effectively and efficientlyAntibacterial agentsVirusesProtective antigenAbnormal tissue growth
The present invention is directed to a method for delivering exogenous proteins to the cytosol, by binding a target antigen (such as a protein) to a transport factor that contains a fragment of a bipartite protein exotoxin, but not the corresponding protective antigen. Preferably, the target antigen is fused to the transport factor. Preferred transport factors include the protective antigen binding domain of lethal factor (LFn) from B. anthracis, consisting of amino acids 1-255, preferably a fragment of at least 80 amino acids that shows at least 80% homology to LFn, and a fragment of about 105 amino acids from the carboxy portion that does not bind PA. The target antigen can include any molecule for which it would be desirable to elicit a CMI response, including viral antigens and tumor antigens.
Owner:THE GENERAL HOSPITAL CORP +1
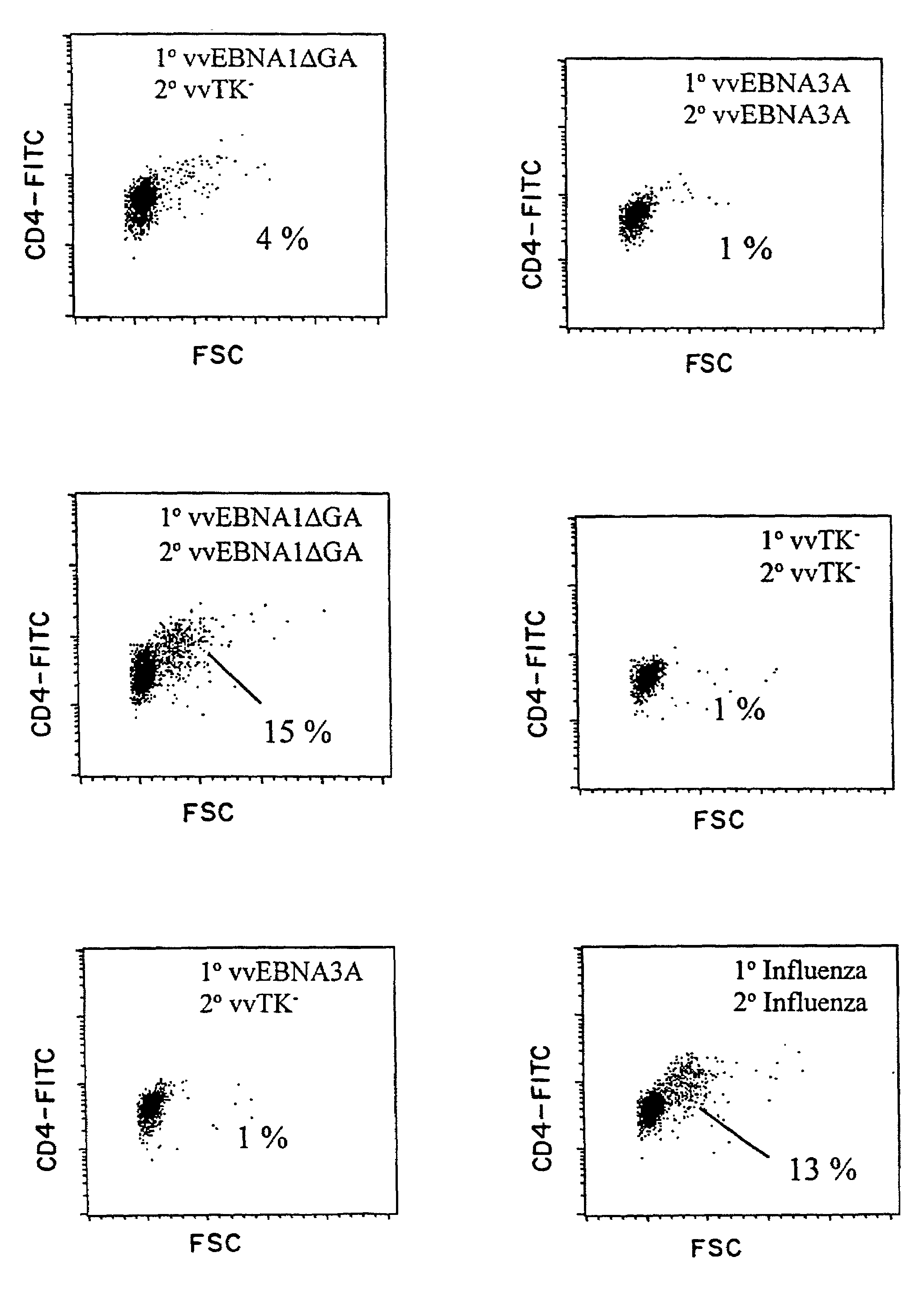
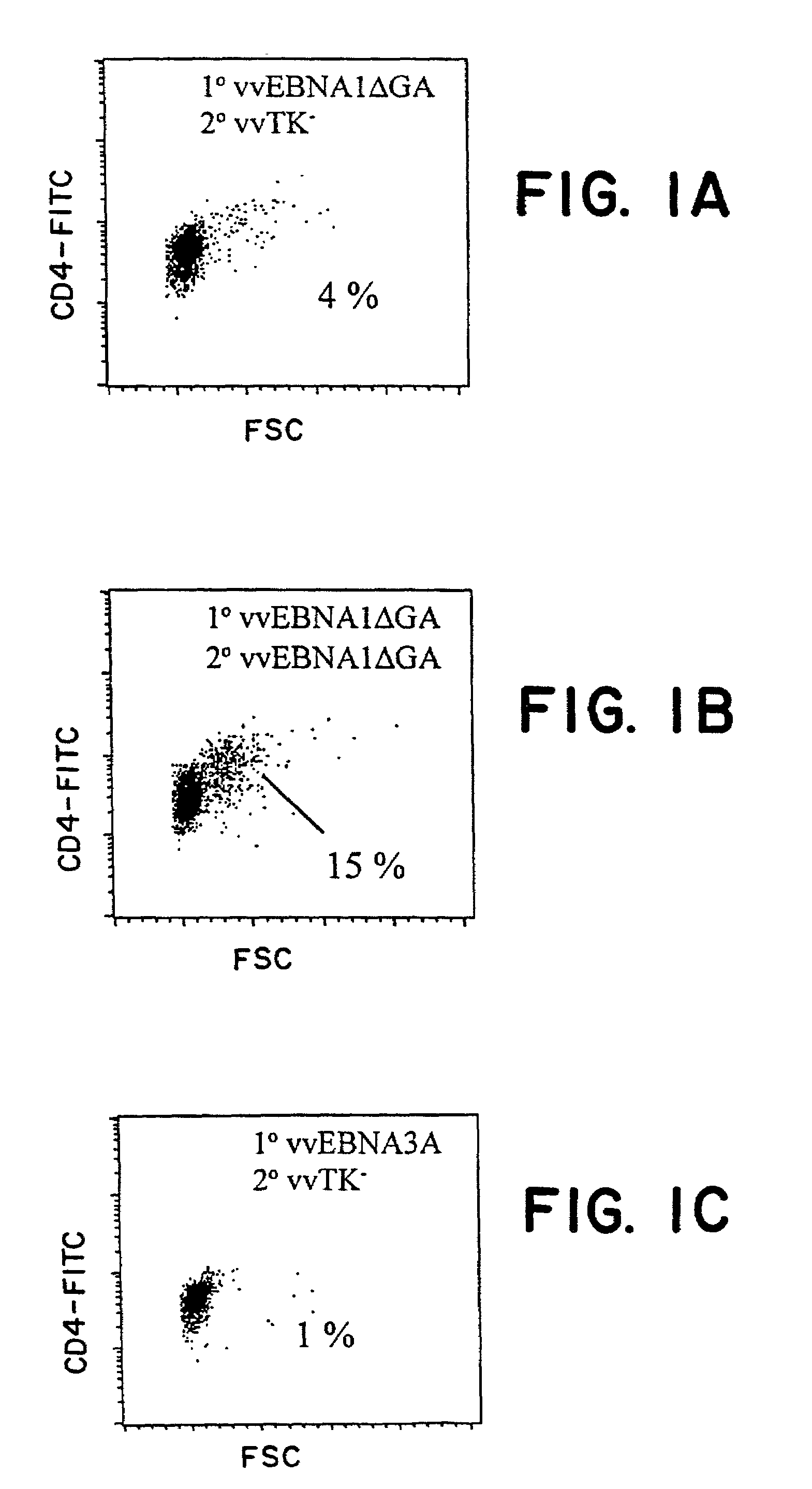
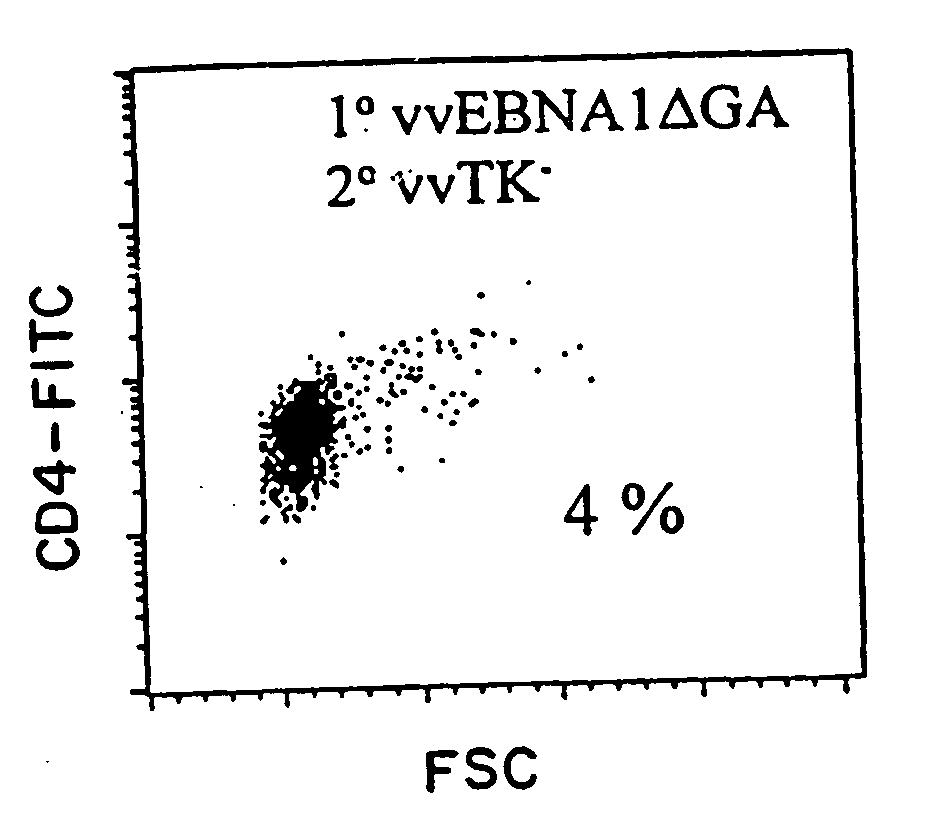
Protective antigen of Epstein Barr Virus
The present invention relates to the identification of a subunit vaccine to prevent or treat infection of Epstein Barr Virus. In particular, EBNA-1 was identified as a vaccine antigen. In a specific embodiment, a purified protein corresponding to EBNA-1 elicited a strong CD4+ T cell response. The responsive CD4+ T cell are primarily TH1 in function. EBNA-1 is an attractive candidate for a protective vaccine against EBV, and for immunotherapy of EBV infection and neoplasms, particularly with dendritic cells charged with EBNA-1.
Owner:THE ROCKEFELLER UNIV
Protective antigen of epstein barr virus
The present invention relates to the identification of a subunit vaccine to prevent or treat infection of Epstein Barr Virus. In particular, EBNA-1 was identified as a vaccine antigen. In a specific embodiment, a purified protein corresponding to EBNA-1 elicited a strong CD4+ T cell response. The responsive CD4+ T cell are primarily TH1 in function. EBNA-1 is an attractive candidate for a protective vaccine against EBV, and for immunotherapy of EBV infection and neoplasms, particularly with dendritic cells charged with EBNA-1.
Owner:THE ROCKEFELLER UNIV
Respiratory syncytial virus vaccines expressing protective antigens from promoter-proximal genes
InactiveUS6923971B2Altered immunogenicitySmall sizeSsRNA viruses negative-senseSugar derivativesProtective antigenGene Position
Recombinant respiratory syncytial virus (RSV) having the position of genes shifted within the genome or antigenome of the recombinant virus are constructed by insertion, deletion or rearrangement of genes or genome segments within the recombinant genome or antigenome and are useful for eliciting an anti-RSV immune response. Shifting the position of genes in this manner provides for a selected increase or decrease in expression of the gene. In one embodiment, expression of RSV glycoproteins is upregulated by shifting one or more glycoprotein-encoding genes to a more promoter-proximal position. Genes of interest for manipulation to create gene position-shifted RSV include any of the NS1, NS2, N, P, M, SH, M2(ORF1), M2(ORF2), L, F or G genes or a genome segment that may be part of a gene or extragenic. Additional mutations and nucleotide modifications are provided within gene position-shifted RSV to yield desired phenotypic and structural effects.
Owner:DEPT OF HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE +1
Production of attenuated chimeric respiratory syncytial virus vaccines from cloned nucleotide sequences
InactiveUS20050100557A1Satisfactory level of attenuationReduce the possibilitySsRNA viruses negative-senseViral antigen ingredientsHeterologousProtective antigen
Chimeric respiratory syncytial virus (RSV) and vaccine compositions thereof are produced by introducing one or more heterologous gene(s) or gene segment(s) from one RSV subgroup or strain into a recipient RSV backround of a different subgroup or strain. The resulting chimeric RSV virus or subviral particle is infectious and attenuated, preferably by introduction of selected mutations specifying attenuated phenotypes into a chimeric genome or antigenome to yield, for example, temperature sensitive (ts) and / or cold adapted (ca) vaccine strains. Alternatively, chimeric RSV and vaccine compositions thereof incorporate other mutations specifying desired structural and / or phenotypic characteristics in an infectious chimeric RSV. Such chimeric RSV incorporate desired mutations specified by insertion, deletion, substitution or rearrangement of one or more selected nucleotide sequence(s), gene(s), or gene segment(s) in a chimeric RSV clone. This provides a method for development of novel vaccines against diverse RSV strains by using a common attenuated backbone as a vector to express protective antigens of heterologous strains. The immune system of an individual is stimulated to induce protection against natural RSV infection, preferably in a multivalent manner to achieve protection against multiple RSV strains and / or subgroups.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Adjuvant for improving immunization effect of Edwardsiella vaccine and use method of adjuvant
ActiveCN102988981AGood immune protectionSafe to useImmunological disordersAntibody medical ingredientsProtective antigenAdjuvant
The invention relates to an adjuvant for improving the immunization effect of Edwardsiella vaccine and a use method of the adjuvant. The adjuvant is characterized by being extracted from raw materials including grain, yeast and a part of fungi or algae. The effective component of the adjuvant is beta-1,3-glucan or the natural, artificially modified or synthetic product of the beta-1,3-glucan. The adjuvant can be used for increasing the specific immune protection ratio of any one or more inculcated Edwardsiella vaccine antigens including the inactivated bacteria, the bacteria disintegration component, the less-virulent strain, the attenuated strain, the protective antigen, the antigen subunit, the antigenic determinant clusters or the expression product of the antigen cell expression vector of the Edwardsiella, can be used together or not together with the vaccine antigen and can be prepared into a single preparation which is used together with the vaccine antigen.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Pig viral infectious disease gene recombined live vaccine using canine II type adenovirus as carrier and preparation process thereof
InactiveCN1827172AGood genetic stabilityEasy to storePowder deliveryGenetic material ingredientsAntigenVp4 gene
This invention supplies a series of production techniques of gene recombination live vaccine of swine virus contagion with canine ó� adenovirus as carrier and finished goods. The viral live vectors vaccine takes swine important virus zymad protective antigens gene as object gene, which are chosen from HCV-E1, E2 gene, FMDV-VP1íóVP2íóVP3íóVP4 gene, TGEV-SíóNíóM gene, PEDV-SíóNíóM geneú¼SIV-HAíóNA geneú¼RV-GíóN gene, etc. The produced vaccines contain recombined swine influenza virus HA gene adenovirus carrier live vaccine, swine plague virus E2 gene adenovirus carrier live vaccine and recombination swine AsiaI foot-and-mouth disease virus VPI gene adenovirus carrier live vaccine. Recombination virus has good inheritance stability, and vaccine immunization can induct pig develop differential antiviral neutralization antibody. It has good immune protection effect and has no toxic side effect. The goods are facilitating for preserve and transportú”it has long storage life and simple technics, and it fits for commercial manufacture.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Application of protein Rh054_01780 to rickettsia heilongjiangensis-resistant immune protection
InactiveCN102716474AEasy to prepareLow costAntibacterial agentsBacterial antigen ingredientsProtective antigenAntigen
The invention discloses application of protein Rh054_01780 to rickettsia heilongjiangensis-resistant immune protection, and provides application of the protein Rh054_01780 to preparation of a vaccine for far eastern spotted fever, a vaccine for rickettsia heilongjiangensis, a protective antigen for far eastern spotted fever, or a protective antigen for rickettsia heilongjiangensis. The protein Rh054_01780 is (a) or (b), wherein (a) is a protein which has an amino acid sequence shown as a sequence 1 in a sequence table; and (b) is a protein which is formed by substituting and / or deleting and / or adding one or more amino acid residues in the amino acid sequence shown as the sequence 1 in the sequence table, has the same function and is derived from the sequence 1. Compared with a mycoproteinvaccine, a vaccine prepared from the protein Rh054_01780 has the advantages of simple and convenient preparation method, low cost, high yield, safety and the like; and the protein Rh054_01780 has significant value in the aspect of epidemic prevention and treatment of far eastern spotted fever.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
RPA optimization
An optimized synthetic polynucleotide encoding a Bacillus anthracis protective antigen and an anthrax vaccine based on the encoded protective antigen. Furthermore, heterologous expression in a host Pseudomonas fluorescens bacteria of an optimized polynucleotide sequence encoding a Bacillus anthracis protective antigen.
Owner:PELICAN TECH HLDG INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com