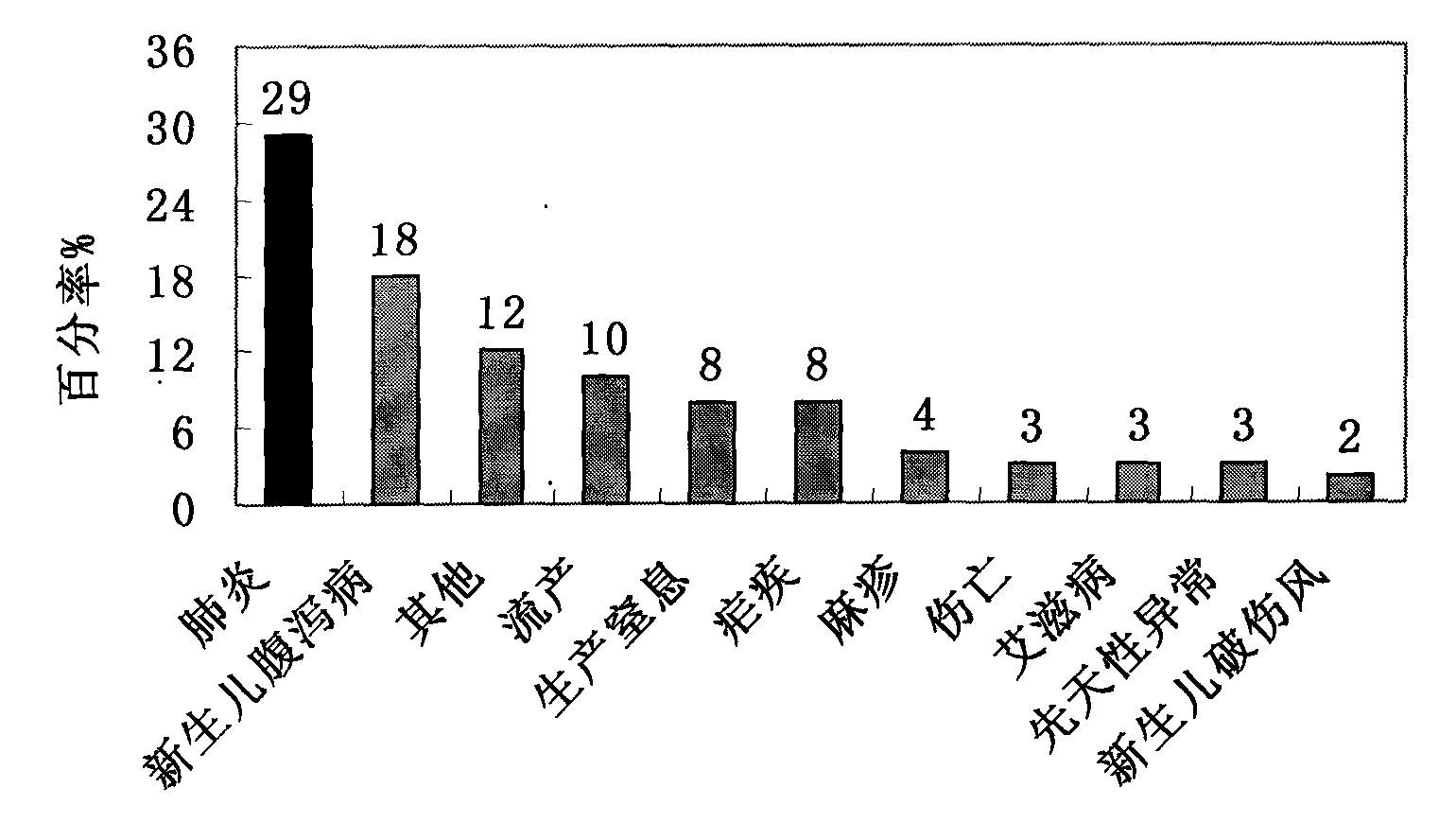
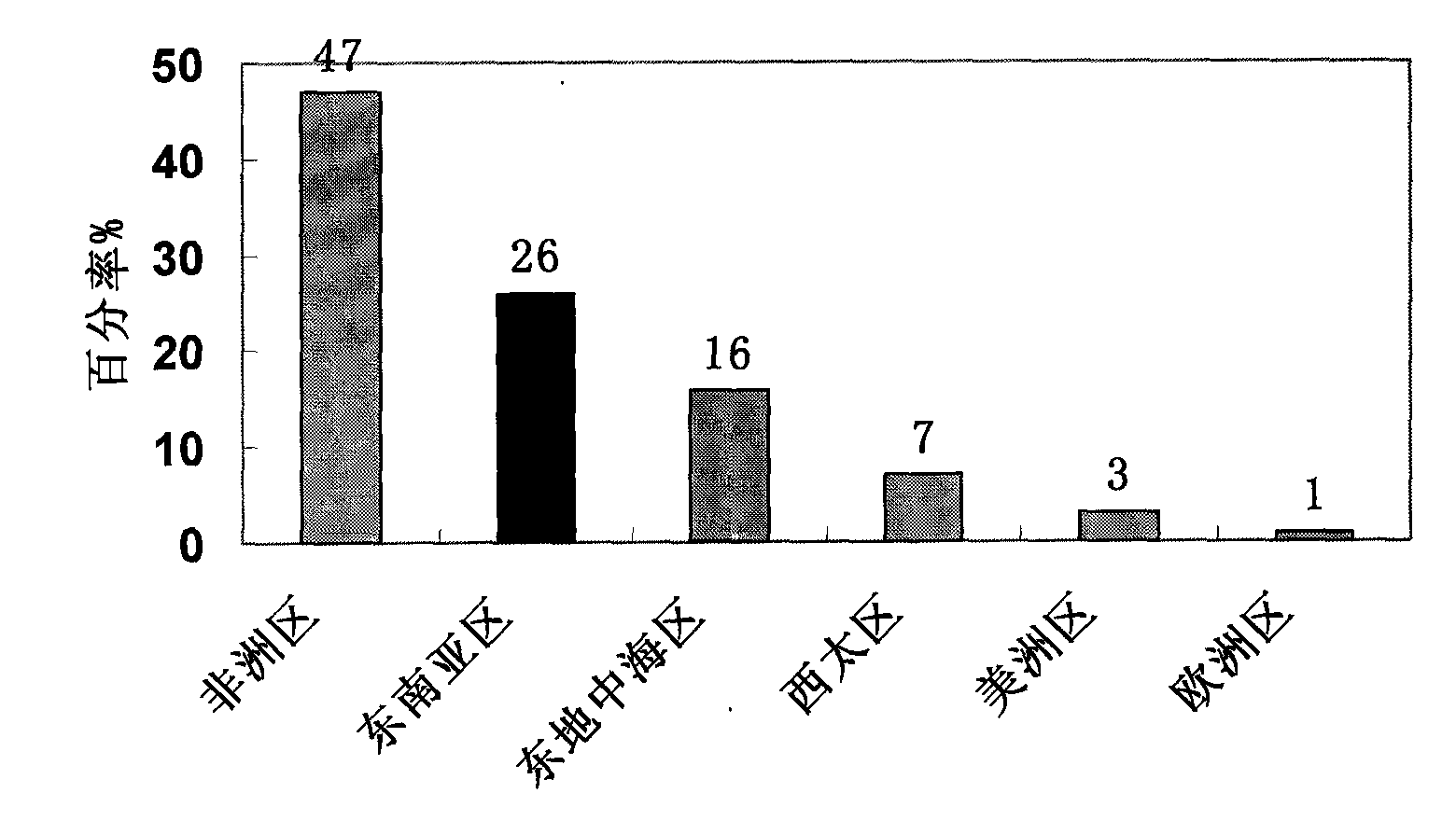
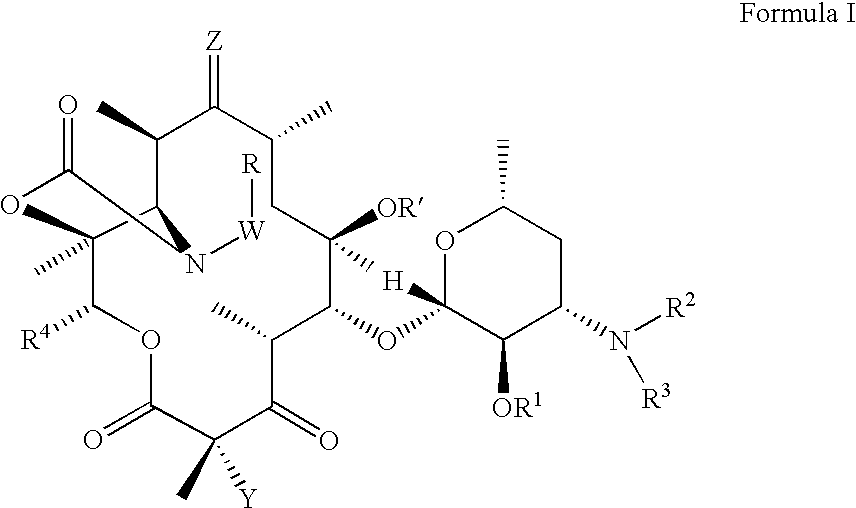
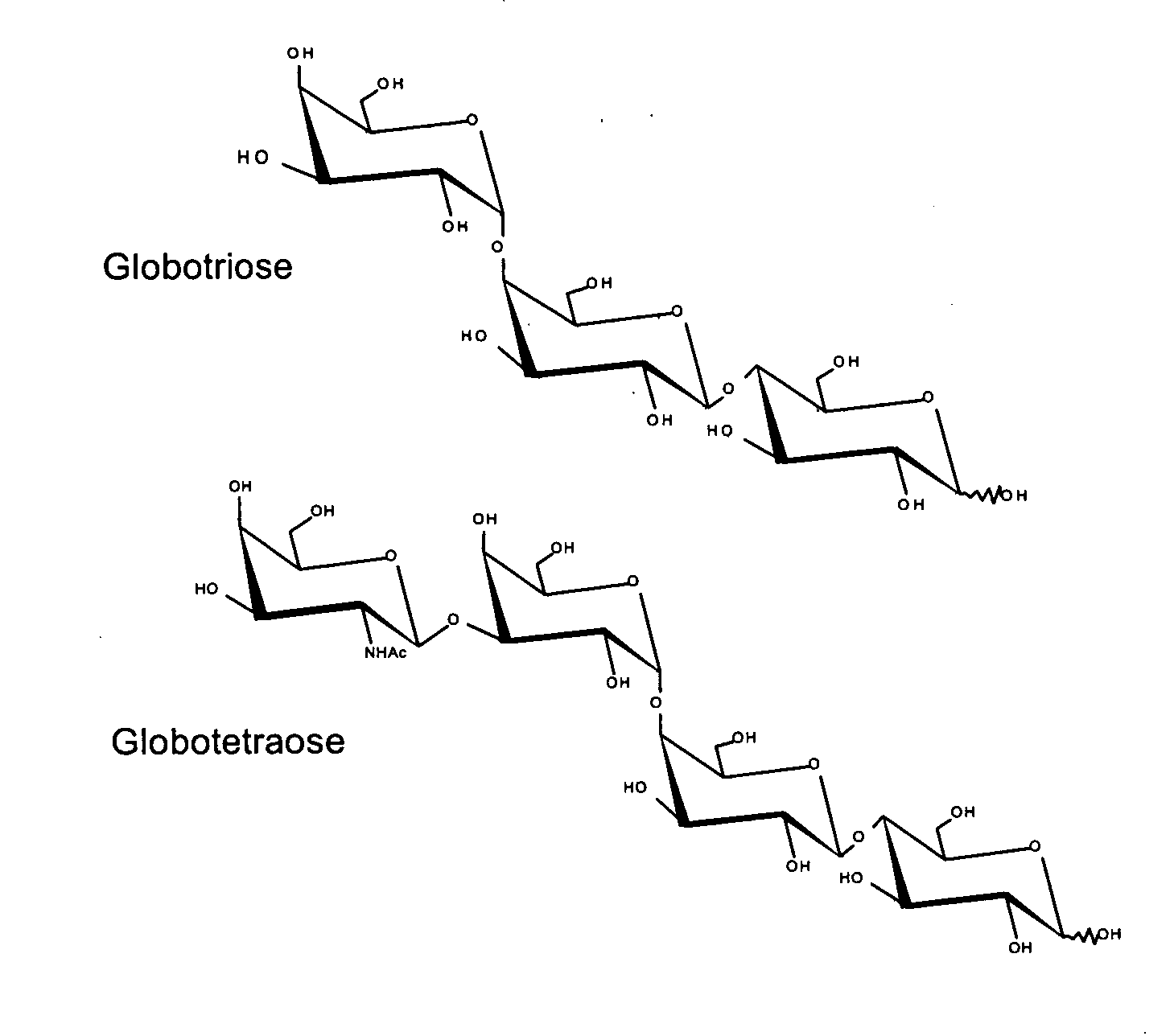
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
382 results about "Haemophilus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Haemophilus is a genus of Gram-negative, pleomorphic, coccobacilli bacteria belonging to the family Pasteurellaceae. While Haemophilus bacteria are typically small coccobacilli, they are categorized as pleomorphic bacteria because of the wide range of shapes they occasionally assume. These organisms inhabit the mucous membranes of the upper respiratory tract, mouth, vagina, and intestinal tract. The genus includes commensal organisms along with some significant pathogenic species such as H. influenzae—a cause of sepsis and bacterial meningitis in young children—and H. ducreyi, the causative agent of chancroid. All members are either aerobic or facultatively anaerobic. This genus has been found to be part of the salivary microbiome.
High-efficiency 14-valent pneumococcal conjugate vaccine
InactiveCN101590224AEffective protectionAntibacterial agentsBacterial antigen ingredientsCoccidiaHaemophilus
The invention relates to a high-efficiency 14-valent pneumococcal conjugate vaccine, which is formed by coupling and combining capsular polysaccharide extracted from fourteen types of serotype pneumococcuses and carrier protein; the fourteen types of the serotype pneumococcuses are 1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F; and the carrier protein is diphtherin non-toxic mutated protein CRM197, tetanus toxin protein and influenza haemophilus protein D. Compared with the prior pneumococcal conjugate vaccine, the high-efficiency 14-valent pneumococcal conjugate vaccine has the advantages that: (1) the cross-protection rate of the pneumococcuses of fourteen serotypes (1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F) reaches more than 90 percent; and (2) the high-efficiency 14-valent pneumococcal conjugate vaccine can generate effective protection on senior citizens which are susceptible to pneumonia and is suitable for children under two years old.
Owner:广州精达医学科技有限公司
Ketolide Derivatives as Antibacterial Agents
The present invention provides ketolide derivatives, which can be used as antibacterial agents. In particular, compounds described herein can be used for treating or preventing conditions caused by or contributed to by Gram-positive, Gram-negative or anaerobic bacteria, more particularly against, for example, Staphylococci, Streptococci, Enterococci, Haemophilus, Moraxalla spp. Chlamydia spp., Mycoplasm, Legionella spp., Mycobacterium, Helicobacter, Clostridium, Bacteroides, Corynebaclerium, Bacillus or Enterobactericeae. Also provided are processes for preparing such ketolide derivatives, pharmaceutical compositions thereof, and methods of treating bacterial infections.
Owner:RANBAXY LAB LTD
Specific and universal probes and amplification primers to rapidly detect and identify common bacterial pathogens and antibiotic resistance genes from clinical specimens for routine diagnosis in microbiology laboratories
InactiveUS20050042606A9Rapid bacterial identificationShorten the timeMicrobiological testing/measurementDepsipeptidesGenomic SegmentMoraxella catarrhalis
The present invention relates to DNA-based methods for universal bacterial detection, for specific detection of the common bacterial pathogens Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, Streptococcus pneumoniae, Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Staphylococcus saprophyticus, Streptococcus pyogenes, Haemophilus influenzae and Moraxella catarrhalis as well as for specific detection of commonly encountered and clinically relevant bacterial antibiotic resistance genes directly from clinical specimens or, alternatively, from a bacterial colony. The above bacterial species can account for as much as 80% of bacterial pathogens isolated in routine microbiology laboratories. The core of this invention consists primarily of the DNA sequences from all species-specific genomic DNA fragments selected by hybridization from genomic libraries or, alternatively, selected from data banks as well as any oligonucleotide sequences derived from these sequences which can be used as probes or amplification primers for PCR or any other nucleic acid amplification methods. This invention also includes DNA sequences from the selected clinically relevant antibiotic resistance genes. With these methods, bacteria can be detected (universal primers and / or probes) and identified (species-specific primers and / or probes) directly from the clinical specimens or from an isolated bacterial colony. Bacteria are further evaluated for their putative susceptibility to antibiotics by resistance gene detection (antibiotic resistance gene specific primers and / or probes). Diagnostic kits for the detection of the presence, for the bacterial identification of the above-mentioned bacterial species and for the detection of antibiotic resistance genes are also claimed. These kits for the rapid (one hour or less) and accurate diagnosis of bacterial infections and antibiotic resistance will gradually replace conventional methods currently used in clinical microbiology laboratories for routine diagnosis. They should provide tools to clinicians to help prescribe promptly optimal treatments when necessary. Consequently, these tests should contribute to saving human lives, rationalizing treatment, reducing the development of antibiotic resistance and avoid unnecessary hospitalizations.
Owner:GENEOHM SCI CANADA
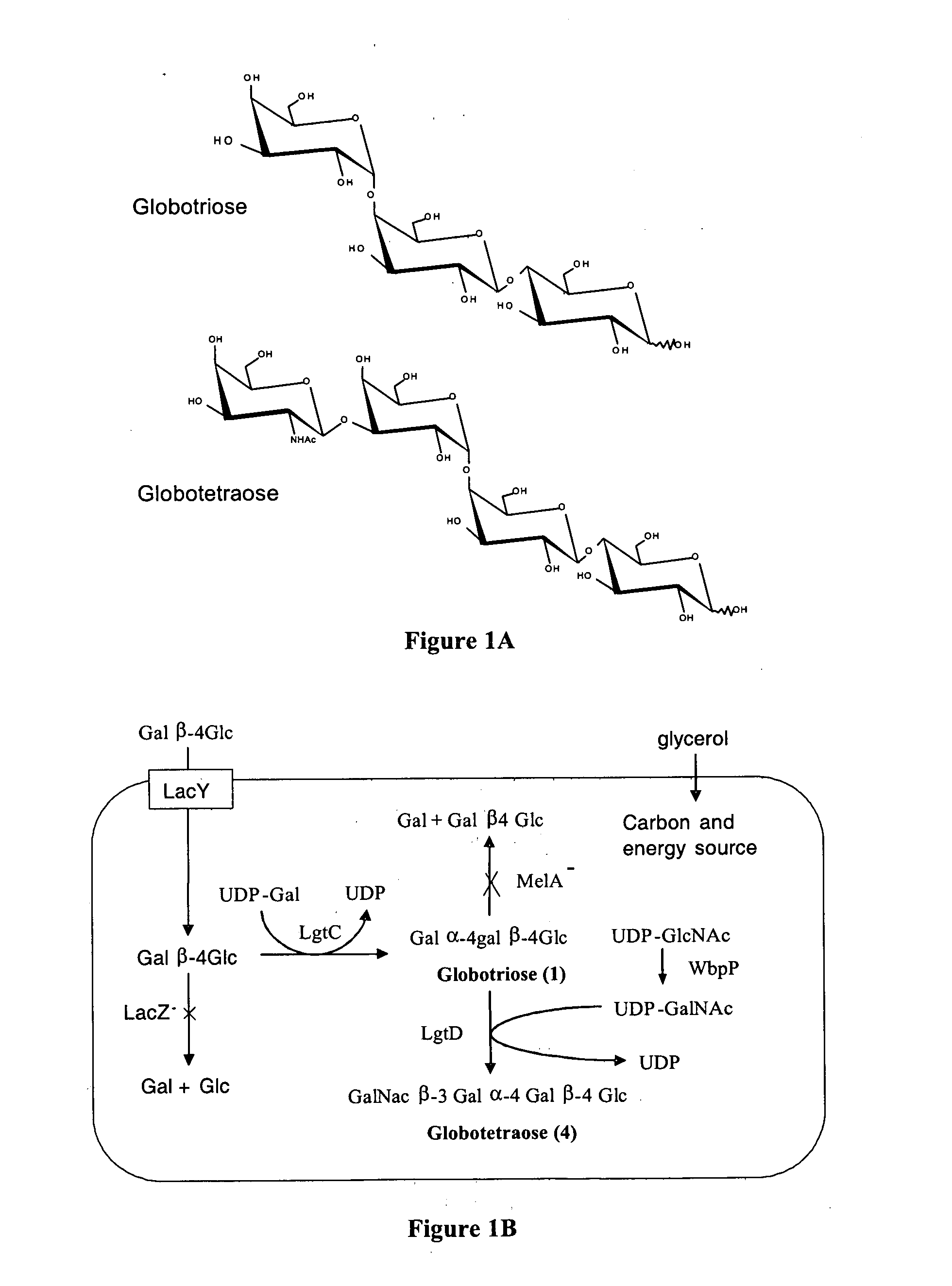
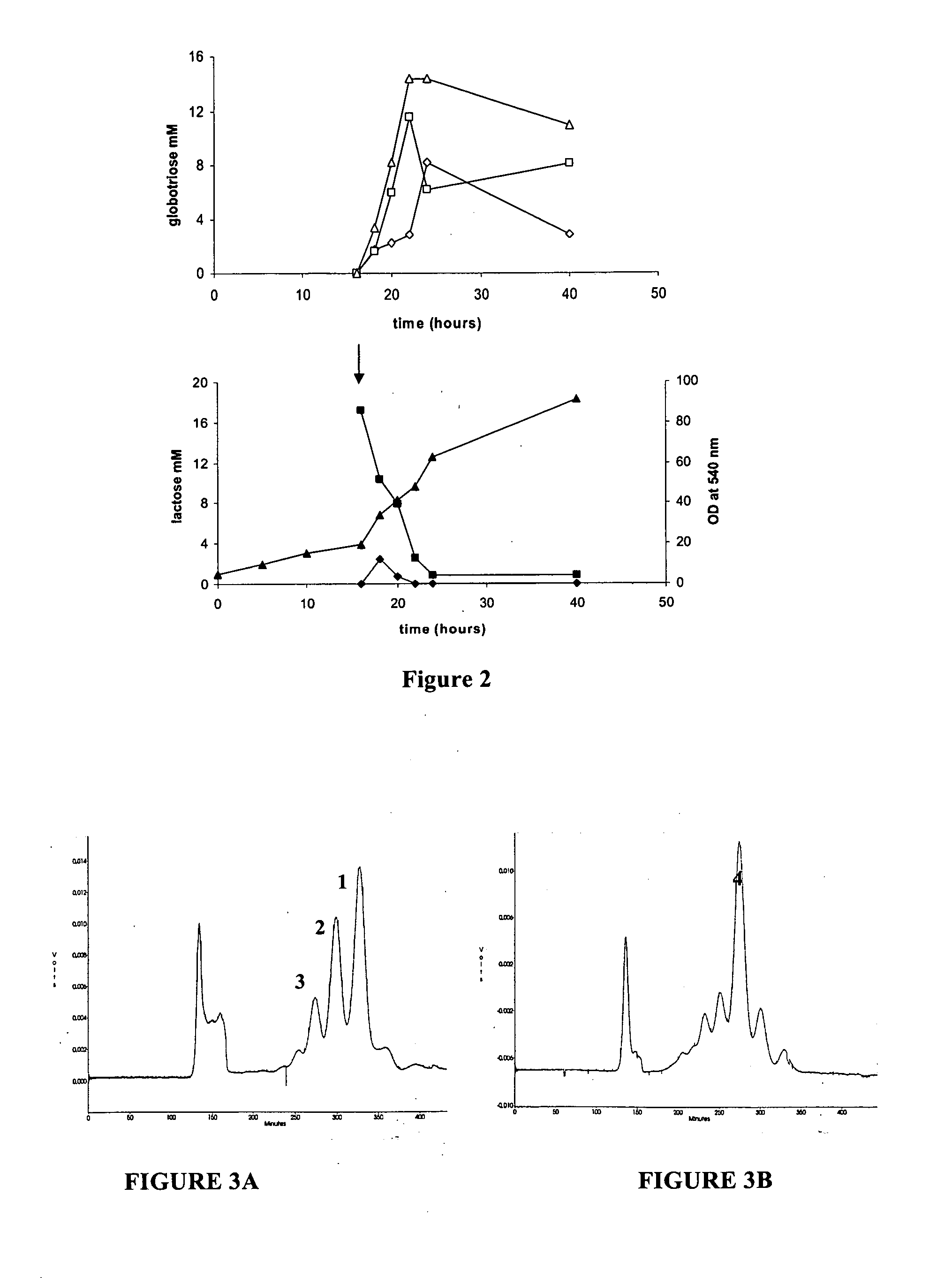
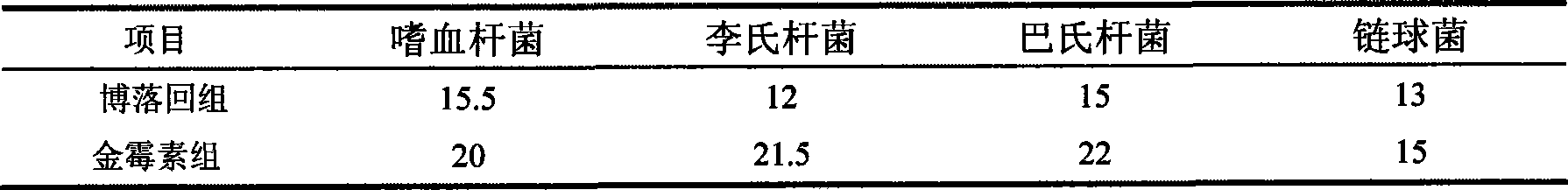
Production of globosides oligosaccharides using metabolically engineered microorganisms
The present invention relates to the large scale in vivo synthesis of globosides oligosaccharides; especially globotriose, globotetraose and globopentaose, which are the carbohydrate portions of globotriosylceramide (Gb3Cer), globotetraosylceramide (Gb4Cer) and globopentaosylceramide (Gb5Cer) respectively. It also relates to high yield production of potential anticancer vaccines of the globo-series glycosphingolipids, including the Globo-H. It also relates to the use of the glycosyltransferase encoded by the lgtD gene from Haemophilus influenzae as a beta1,3 galactosyl transferase to catalyze the transfer of a galactose moiety from UDP-Gal to an acceptor bearing the terminal non reducing structure GalNAcbeta-3-R to form the Galbeta-3GalNAcbeta-3-R structure
Owner:CENT NAT DE LA RECHERCHE SCI
Application of pink plumepoppy herb extract in veterinary drugs of economic animals
ActiveCN101530475APromote growthImprove conversion rateAntibacterial agentsAnimal feeding stuffFeed conversion ratioFeed additive
The invention relates to an application of pink plumepoppy herb extract in veterinary drugs of economic animals; the pink plumepoppy herb extract is used for preparing medical feed additives of the veterinary drugs which are used for enhancing the growth of the economic animals and increasing conversion rate of the feed or veterinary drugs which has better inhibiting action and disinfecting action to common pathogenic bacteria in livestock and poultry, such as listeria monocytogenes, haemophilus and pasteurella.
Owner:MICOLTA BIORESOURCE INC LTD
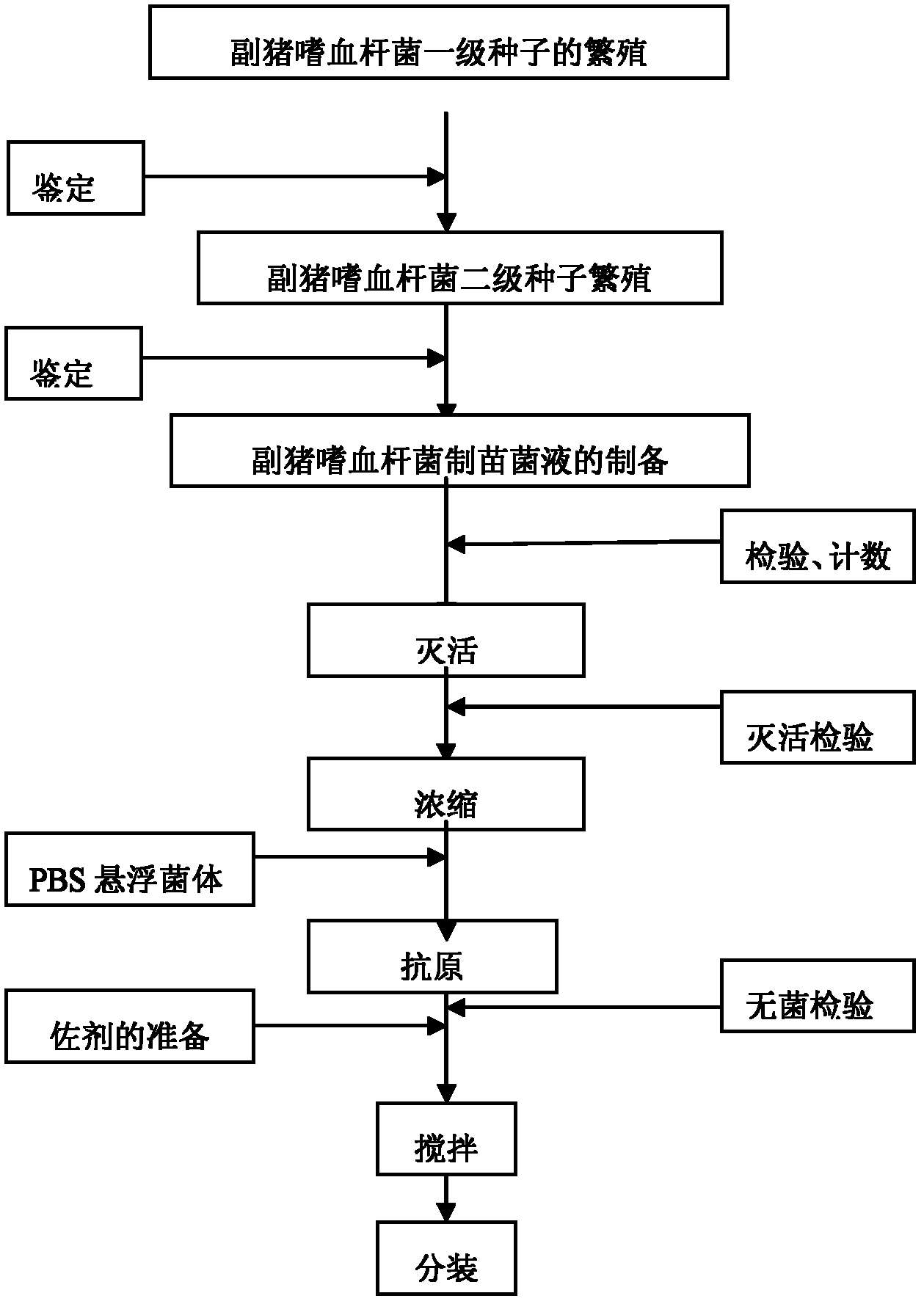
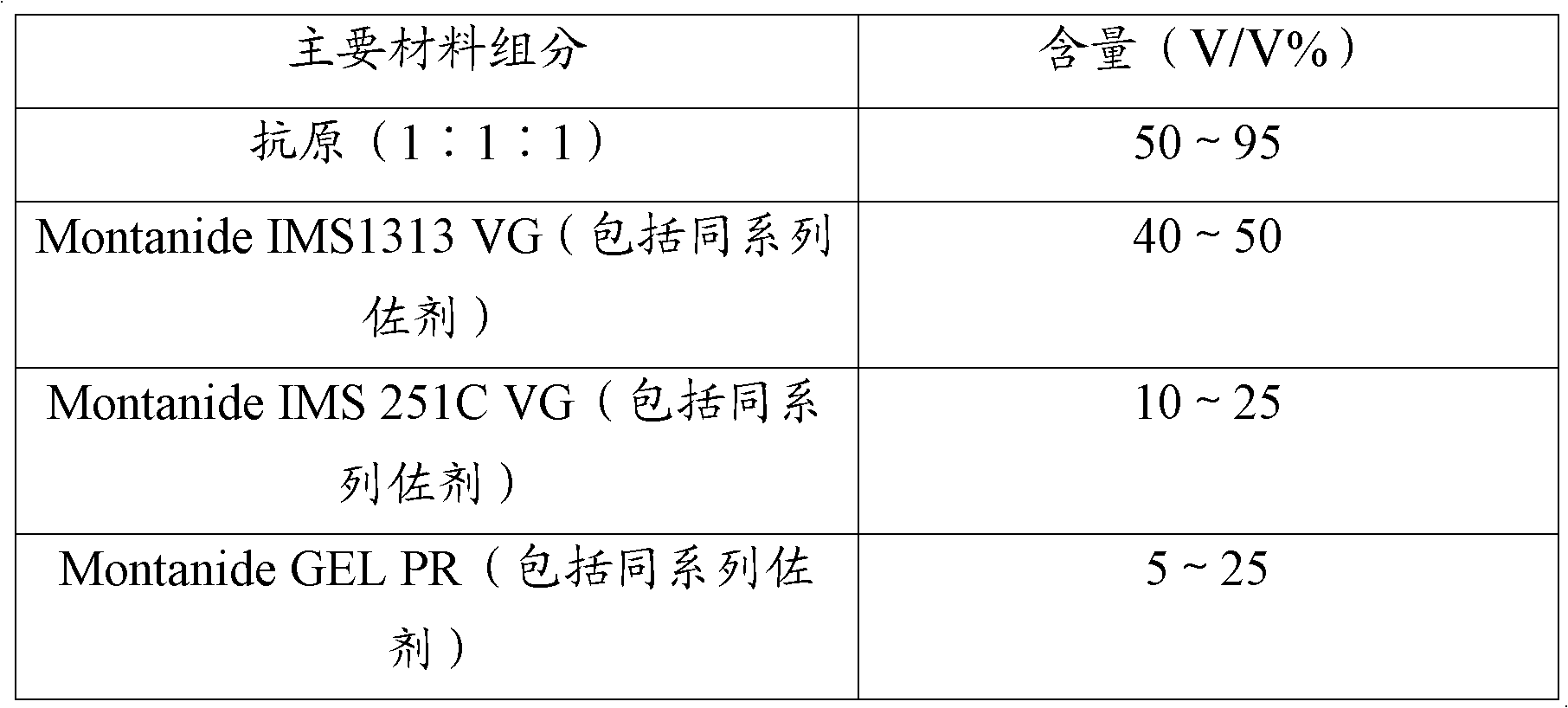
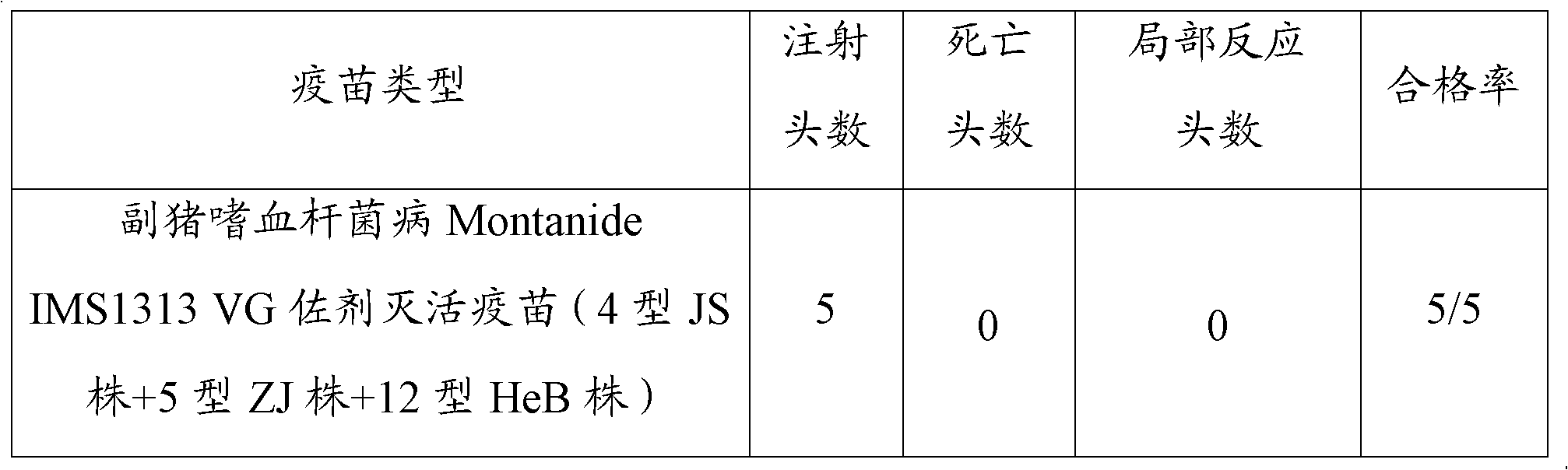
Novel haemophilus parasuis disease trivalent inactivated vaccine and preparation method thereof
ActiveCN102908615ASolve preparation difficultiesImprove immunityAntibacterial agentsBacterial antigen ingredientsAntigenDisease
The invention provides a novel haemophilus parasuis disease trivalent inactivated vaccine and a preparation method thereof. The vaccine comprises equal proportions of antigens of: inactivated haemophilus parasuis serotype 4 JS strain, inactivated haemophilus parasuis serotype 5 ZJ strain, and inactivated haemophilus parasuis serotype 12 HeB strain. According to the invention, the haemophilus parasuis serotype 4, serotype 5, and serotype 12 strains are obtained by separation. Concentration contents and relative proportions of the antigens prepared from the three strains are subjected to large amounts of woks and practices, such that the trivalent vaccine with an appropriate antigen ratio and an appropriate concentration is obtained. The trivalent vaccine has good preventing and treating effects against haemophilus parasuis diseases caused by various epidemic haemophilus parasuis serotypes in our nation. Especially, the trivalent vaccine can solve a problem of poor treatment effect of existing vaccines caused by novel haemophilus parasuis pathogens.
Owner:PU LIKE BIO ENG
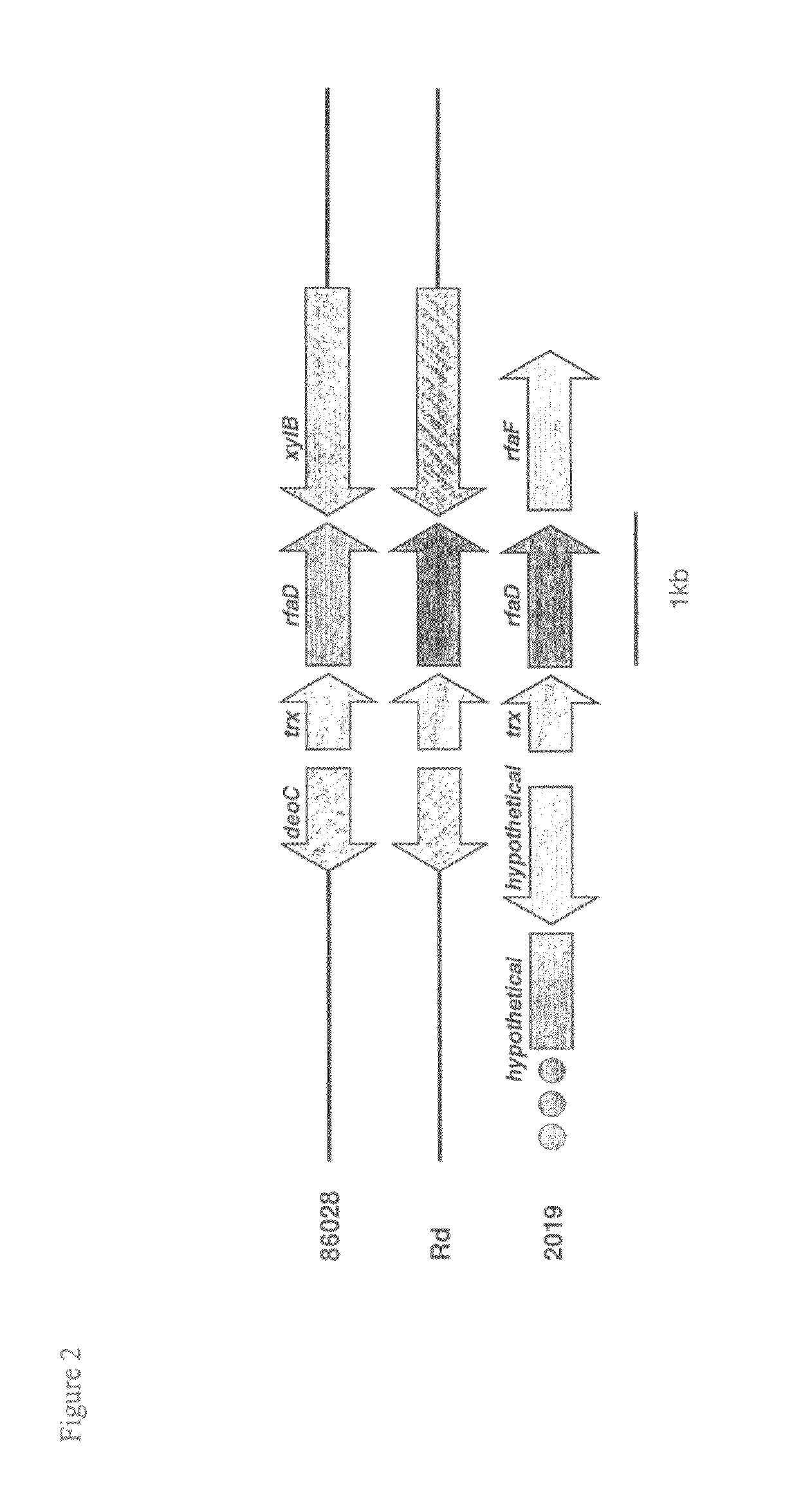
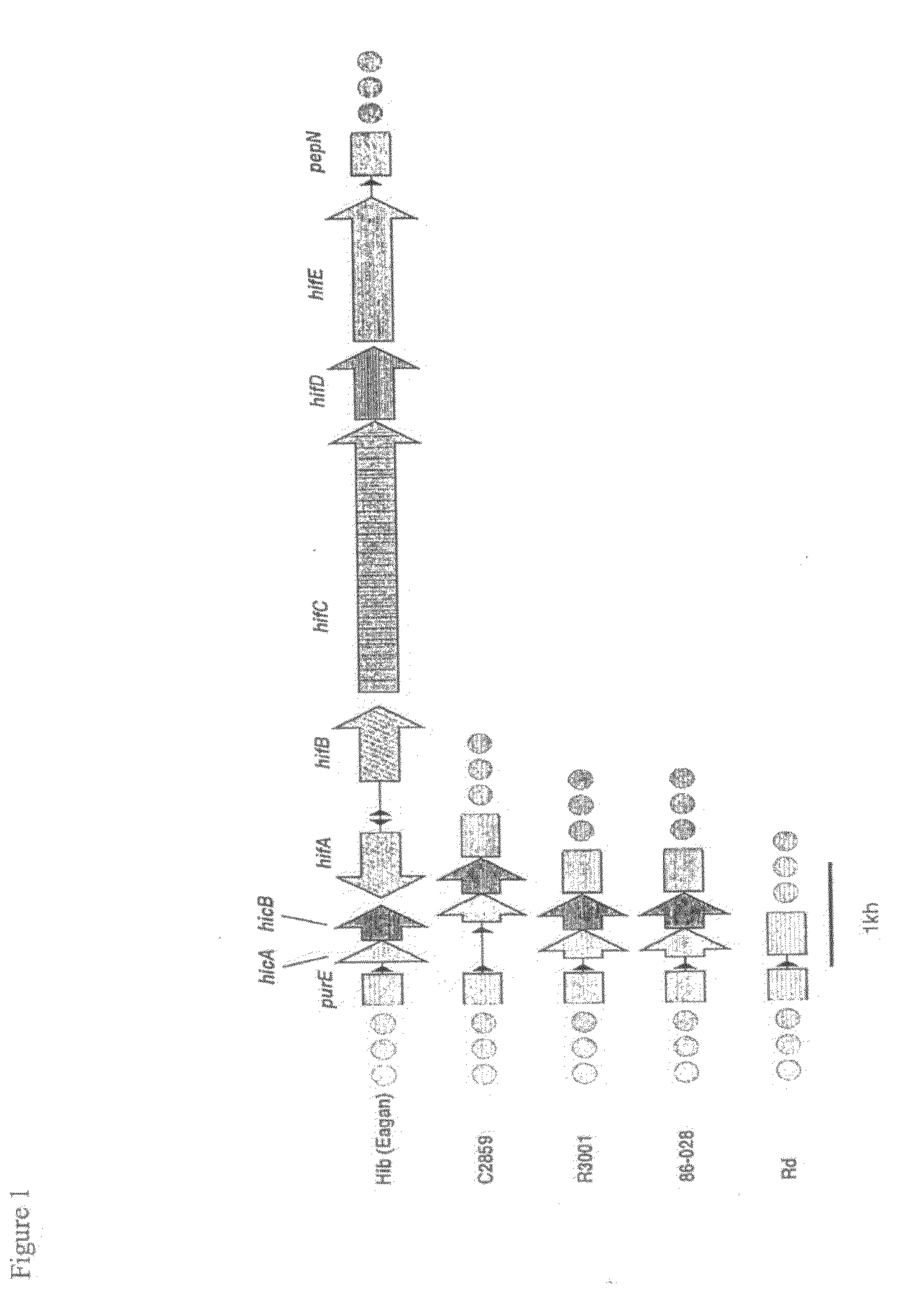
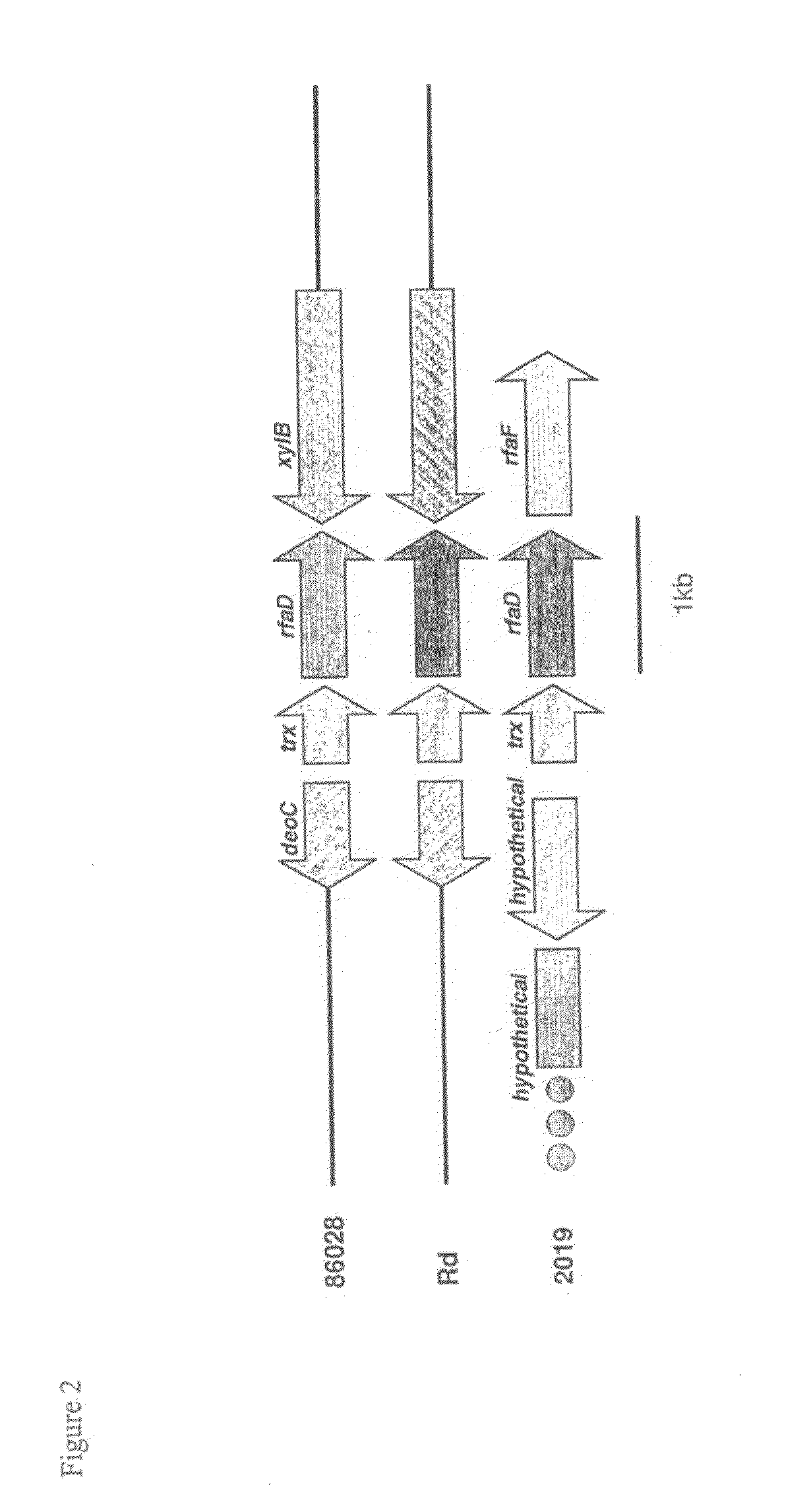
Genes of an otitis media isolate of nontypeable Haemophilus influenzae
InactiveUS8283114B2Good linkageEasy to modifyAntibacterial agentsSenses disorderBiotechnologyHaemophilus
The invention relates to the polynucleotide sequence of a nontypeable stain of Haemophilus influenzae (NTHi) and polypeptides encoded by the polynucleotides and uses thereof. The invention also relates to NTHi genes which are upregulated during or in response to NTHi infection of the middle ear and / or the nasopharynx.
Owner:NATIONWIDE CHILDRENS HOSPITAL +1
Vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection and preparation method thereof
ActiveCN103083655ASimplified immunization programReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsDiseaseCircovirus
The invention provides a vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection. The vaccine composition comprises an inactivated porcine circovirus type 2 antigen, inactivated haemophilus parasuis, inactivated mycoplasma hyopneumoniae and a vaccine adjuvant. The vaccine composition disclosed by the invention can realize the aim of preventing three diseases including a porcine circovirus disease, mycoplasma pneumonia, a haemophilus parasuis disease by one injection of the vaccine; the content of antigen is 1 / 2 of the content of a common single-vaccine antigen when the vaccine composition disclosed by the invention is prepared by mixing the three antigens; and compared with the existing condition that three injections of single vaccine are injected to prevent three infectious diseases, the technical scheme disclosed by the invention is economical and practical, reduces the production cost, simplifies an immune procedure and reduces the epidemic prevention cost.
Owner:PU LIKE BIO ENG
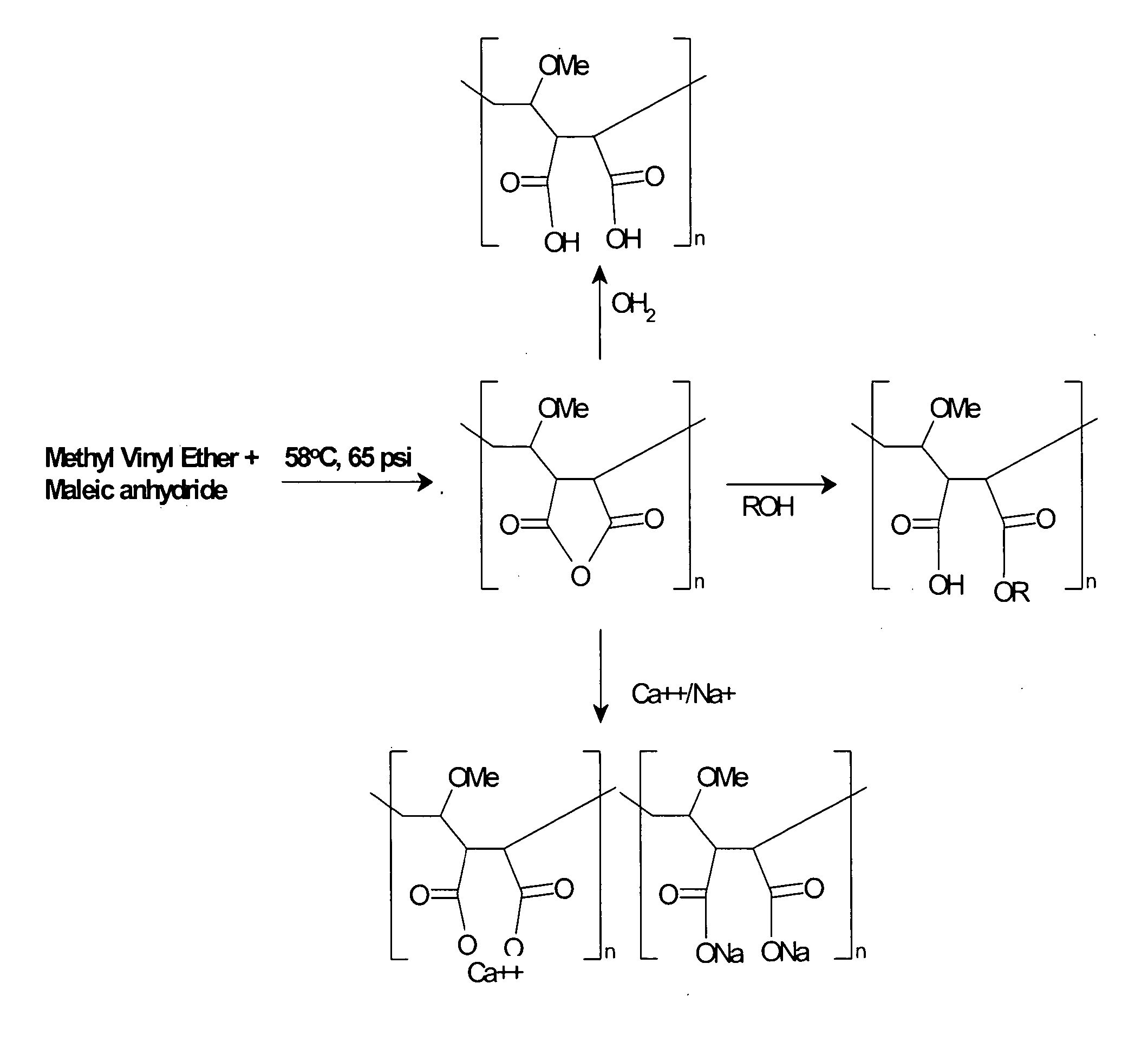
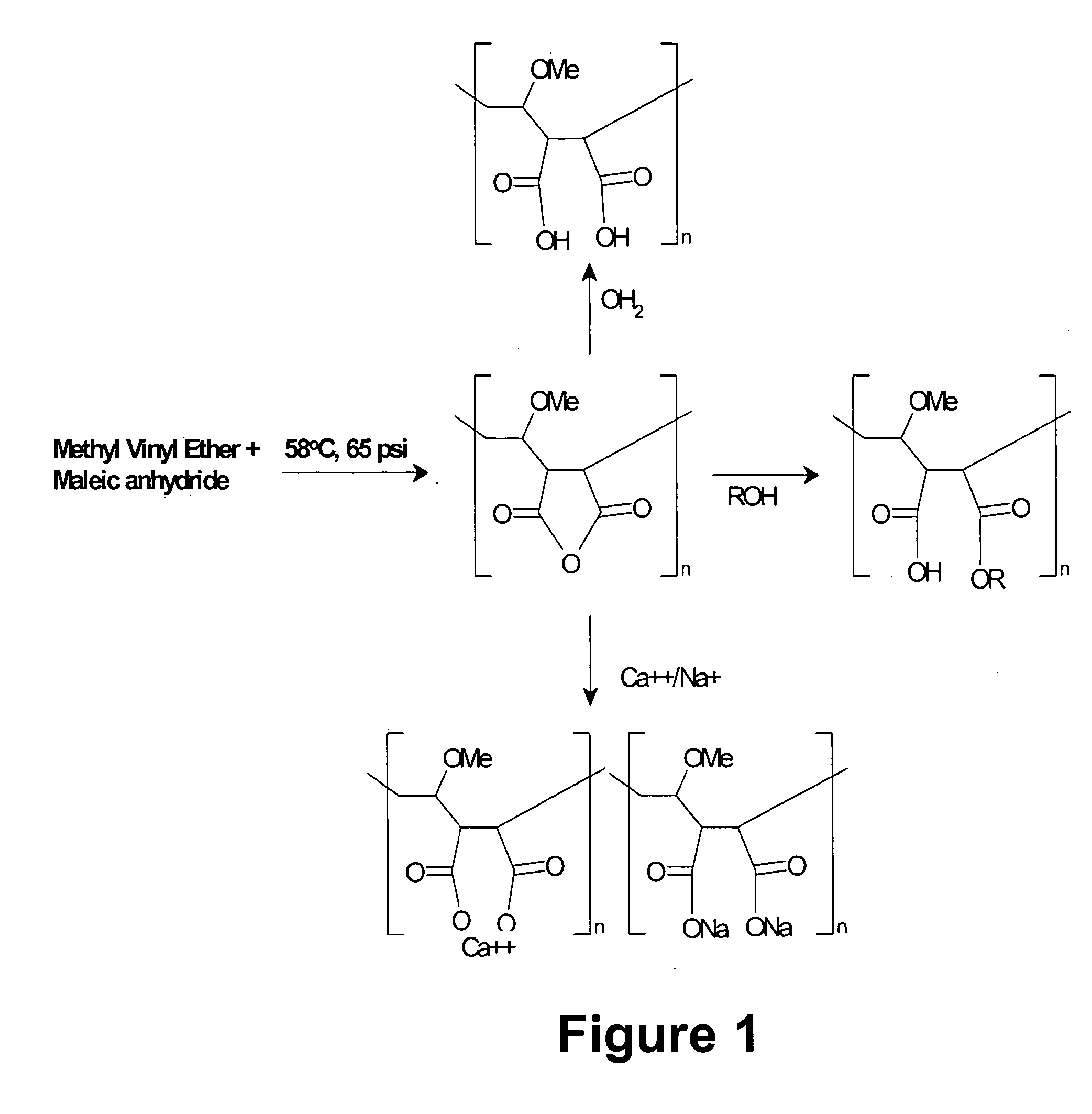
Methods, compositions, formulations, and uses of cellulose and acrylic-based polymers
InactiveUS20050244365A1Easy to chargeLow pKaAntibacterial agentsCosmetic preparationsDisinfectantReverse transcriptase
Compositions, formulations, and methods for the treatment or prevention, or decreasing the frequency of transmission of a virus (such as human immunodeficiency virus type 1 (HIV-1), Herpes Simplex virus type 1 (HSV1), or Herpes Simplex Virus Type 2 (HSV2), or other virus), or a bacterial infection (such as Trichomonas vaginalis, Neisseris gonorrhoeae Haemopholus ducreyl, or Chlamydia trachomatis, or other bacterial species), or a fungal infection, using an anionic cellulose- or acrylic-based oligomer, polymer, or copolymer. The present invention also includes administering a therapeutically effective amount of said oligomer, polymer, or copolymer, or a pharmaceutically acceptable salt thereof, or with a pharmaceutically acceptable carrier or diluent, thereof. The invention relies on the unique biochemical substitution of the cellulose or acrylic backbone such that the resultant molecule can remain molecularly dispersed in solution (or gel or other formulation) and mostly dissociated over a wide range of physiological microenvironments, such as the low pH found within the vaginal lumen, preferably from a pH of 14 to below 3.5. These specific substitutions also impart on the resultant molecule potent antiviral, anti-bacterial, and anti-fungal properties. In addition, these compositions can be used as general disinfectants for human use such as in contact lens solutions, mouthwashes, toothpastes, suppositories, or as more generalized disinfectants found in soaps, household cleaning products, paints, water treatments modalities, or can be incorporated into cosmetic, and can be used as vehicles for drug delivery, an adjuvant in a therapeutic formulation, or as a preservative. These compounds can be delivered in a liquid or solid dosage form and can be incorporated into barrier devices such as condoms, diaphragms, or cervical caps, to help prevent the transmission of STDs. The compounds of this invention can also be used in combination therapies with other classes of antiviral, antibacterial, or antifungal agent having similar or differing mechanisms of action including, but not limited to, anionic or cationic polymers, copolymers, or oligomers, surfactants, protease inhibitors, DNA or RNA polymerase inhibitors (including reverse transcriptase inhibitors), fusion inhibitors, cell wall biosynthesis inhibitors, integrase inhibitors, or virus or bacterial attachment inhibitors.
Owner:NOVAFLUX INC +1
Kit for jointly detecting respiratory tract pathogen through multiple fluorescent PCR method
ActiveCN107058622ANo further action requiredShorten the course of the diseaseMicrobiological testing/measurementAgainst vector-borne diseasesPositive controlFluorescence
The invention provides a kit for jointly detecting respiratory tract pathogen through a multiple fluorescent PCR method. The kit comprises six components: reaction liquid A, reaction liquid B, reaction liquid C, enzyme mixed liquid, positive control and negative control, and comprises 11 common respiratory tract pathogen detections (general type of influenza virus A, influenza virus B, respiratory syncytial virus, 1 / 2 / 3 type of human parainfluenza virus, adenovirus, mycoplasma pneumoniae, chlamydia pneumonia, legionella pneumophila, streptococcus pneumonia, haemophilus influenza, A streptococcal); the amplification is performed through three reaction buffers, and each reaction buffer contains four fluorescent channels, 90% pathogen infection on the clinic can be checked.
Owner:DEBIQI BIOTECH XIAMEN
Polyvalent bacteria capsule polysaccharide-protein conjugate combined vaccine
ActiveCN1709505AImproving immunogenicityReduce the number of vaccinationsBacterial antigen ingredientsHaemophilusMedicine
The present invention relates to a polyvalent bacterial capsule polysaccharide-protein conjugate combined vaccine preparation, in particular, it is a combined vaccine containing group A, group C, group Y and group W135 epidemic cerebrospinal meningitis coccal capsule polysaccharide-protein conjugate and b type haemophilus influenzal capsule polysaccharide-protein conjugate.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM
Genes of an Otitis Media Isolate of Nontypeable Haemophilus influenzae
InactiveUS20110135646A1Good linkageEasy to modifyAntibacterial agentsOrganic active ingredientsBiotechnologyHaemophilus
The invention relates to the polynucleotide sequence of a nontypeable stain of Haemophilus influenzae (NTHi) and polypeptides encoded by the polynucleotides and uses thereof. The invention also relates to NTHi genes which are upregulated during or in response to NTHi infection of the middle ear and / or the nasopharynx.
Owner:NATIONWIDE CHILDRENS HOSPITAL +1
Porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine and preparation method thereof
ActiveCN102329746AReduce stressEasy to useAntibacterial agentsBacterial antigen ingredientsHaemophilusAluminium stearate
The invention discloses a porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine and a preparation method thereof. The preparation method comprises the following steps of: a, respectively carrying out enrichment culture on a porcine streptococcus strain, a haemophilus parasuis strain and a haemophilus parasuis strain to obtain a porcine streptococcus strain bacterial solution, a haemophilus parasuis strain bacterial solution and a haemophilus parasuis strain bacterial solution; b, respectively adding a formaldehyde solution into the porcine streptococcus strain bacterial solution, the haemophilus parasuis strain bacterial solution and the haemophilus parasuis strain bacterial solution, and inactivating; c, mixing the collected porcine streptococcus strain bacterial solution, the haemophilus parasuis strain bacterial solution and the haemophilus parasuis strain bacterial solution, adding Tween-80 for preparing a water phase, preparing white oil, Span-80 and aluminium stearate into an oil phase, mixing the water phase with the oil phase to prepare a uniform emulsion, i.e. an oil emulsion inactivating vaccine; and 4, sub-packaging the oil emulsion inactivating vaccine. The porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine can effectively prevent the porcine streptococcus disease and haemophilus parasuis disease, does not have hidden danger of scattering viruses and is safe and reliable; and the immunization is realized by one vaccine, thus the cost is reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Method of creating antibodies and compositions used for same
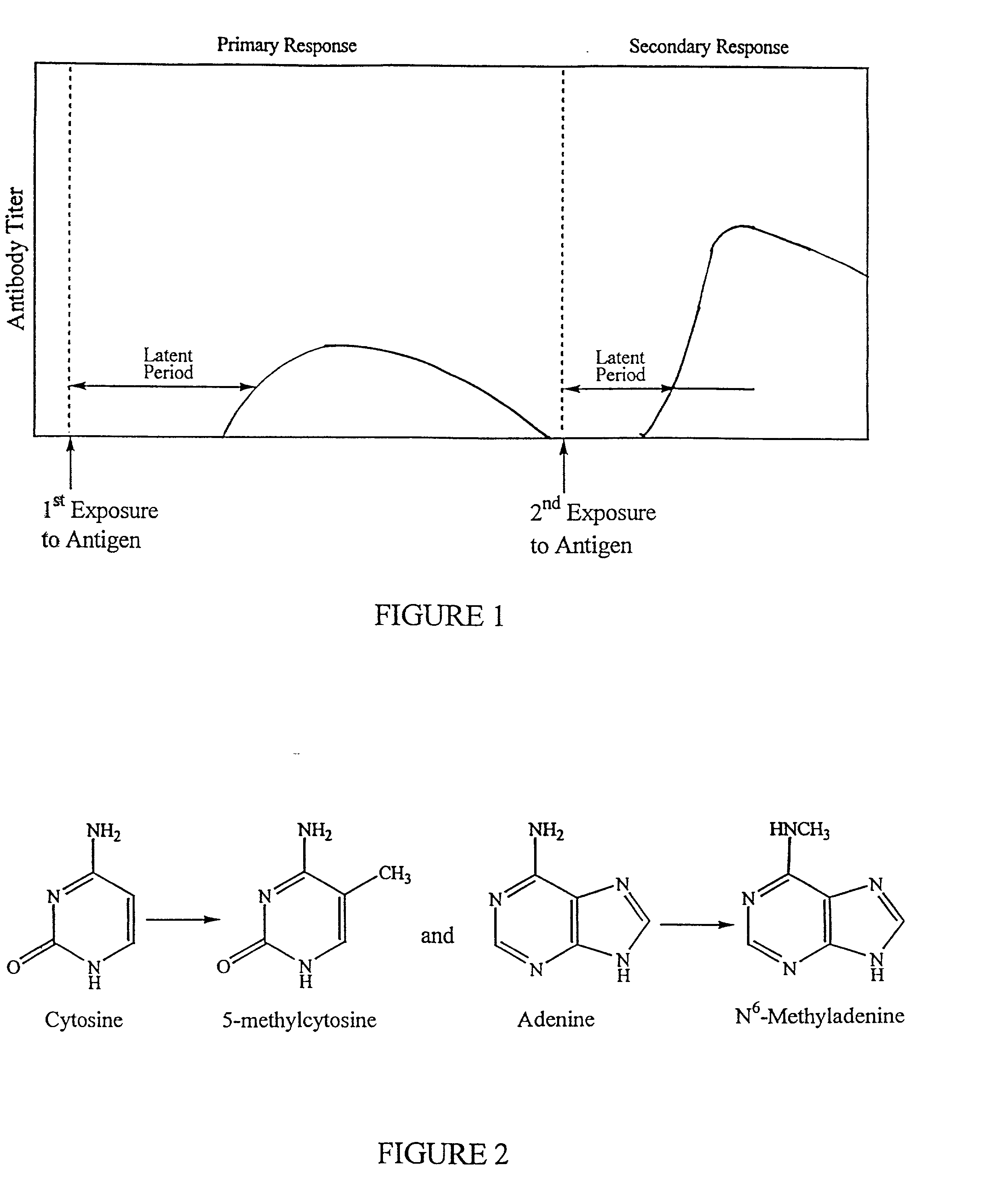
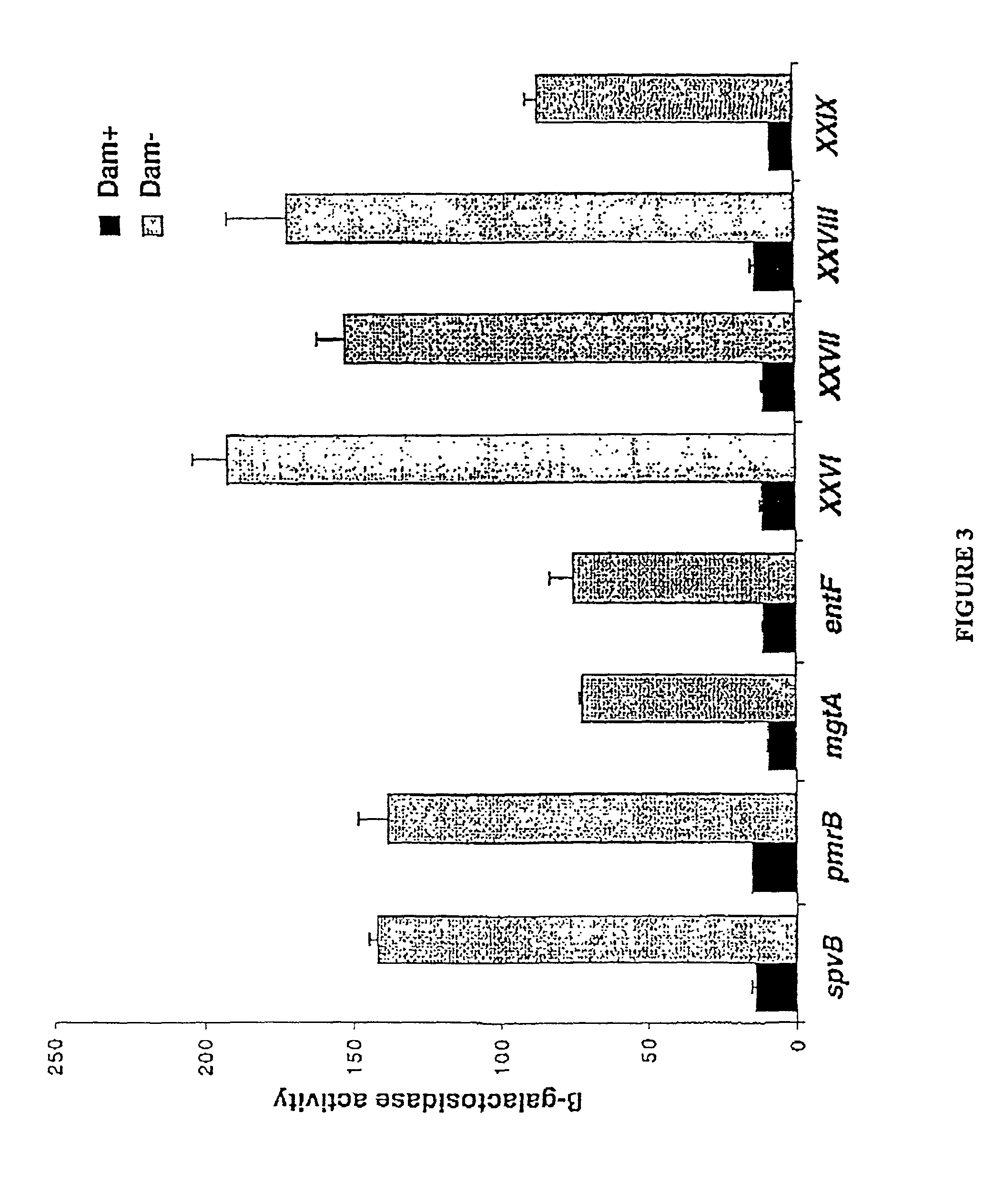
The present invention is directed towards compositions containing pathogenic bacteria (e.g. Haemophilus, E. Coli, and / or Salmonella) having non-reverting genetic mutations which alter activity of DNA adenine methylase (Dam) and methods using these compositions to elicit an immune response to produce highly specific antibodies. The invention also provides methods for preparing vaccines as well as screening methods to identify agents which may have anti-bacterial activity.
Owner:RGT UNIV OF CALIFORNIA
Vaccine
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
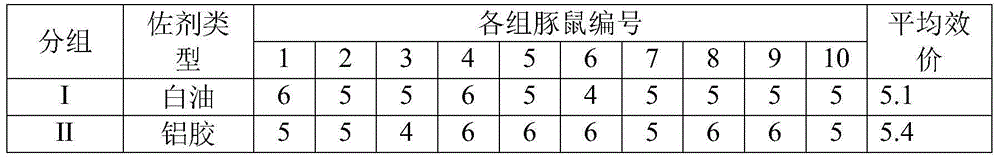
Immune protective antigen of haemophilus parasuis
ActiveCN102864157AGood protective antigen proteinAntibacterial agentsBacteriaProtective antigenEscherichia coli
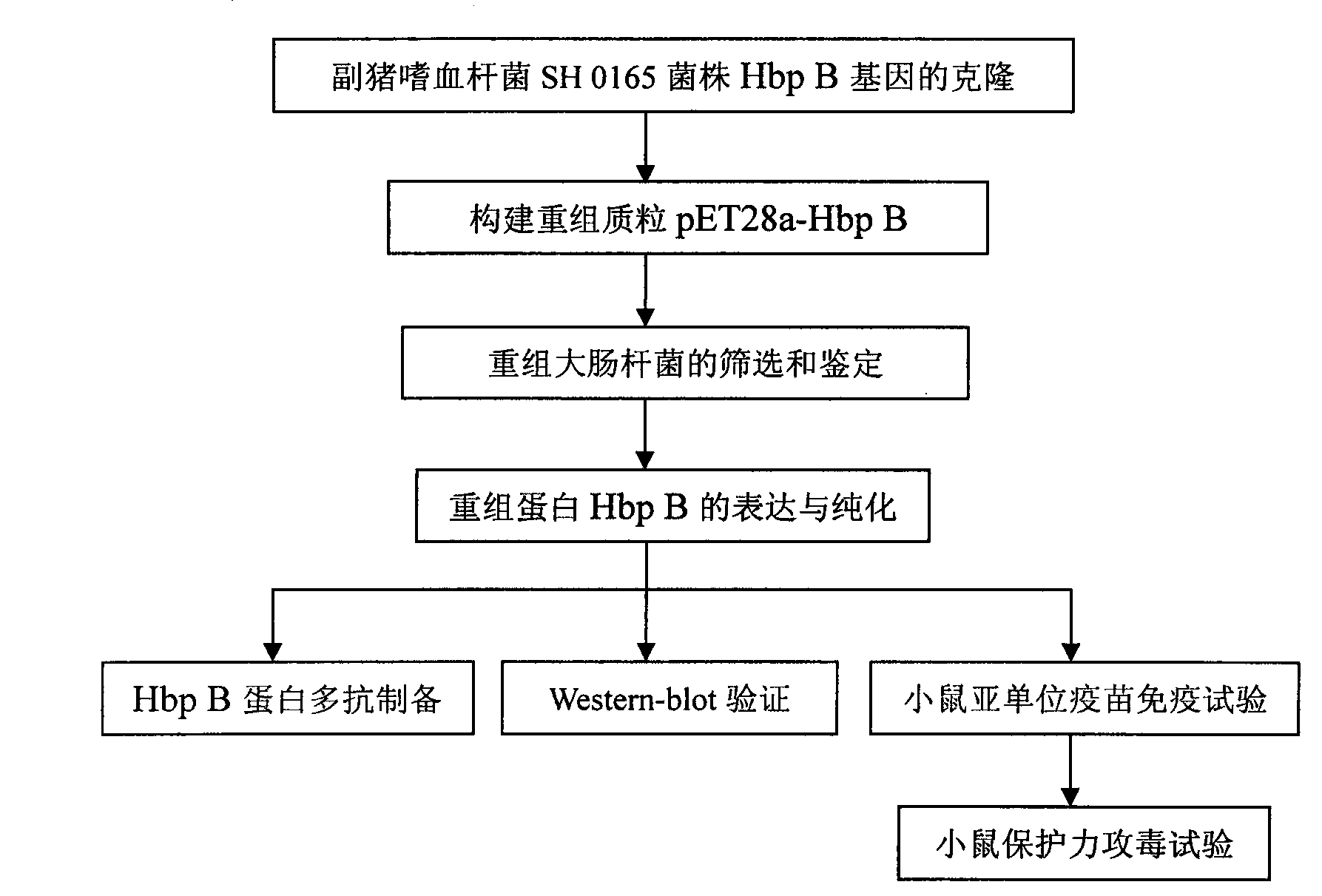
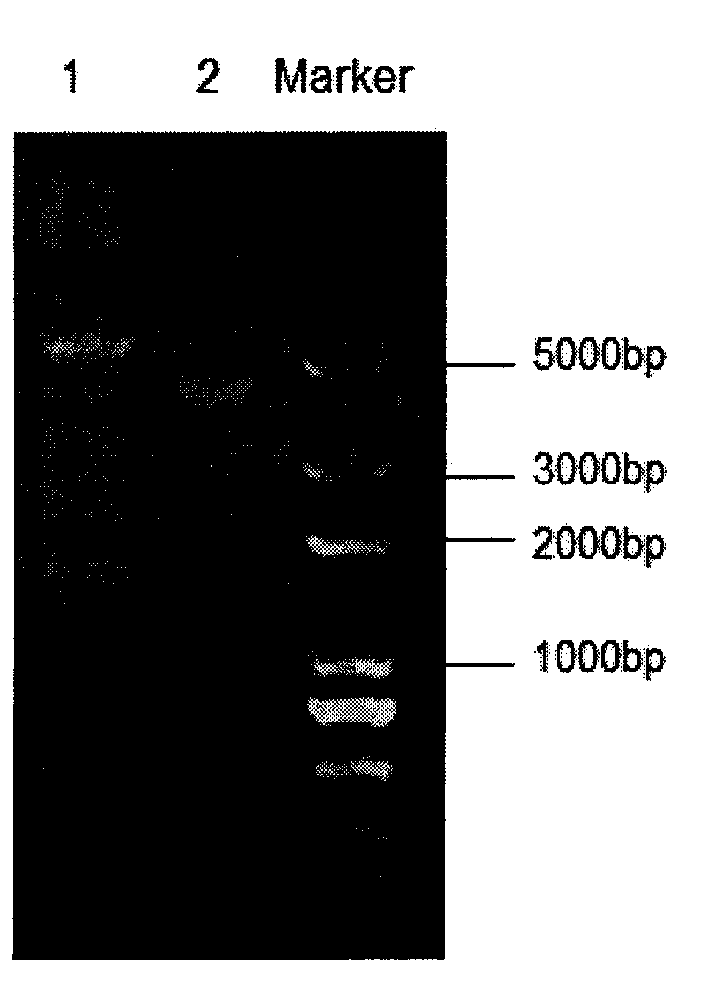
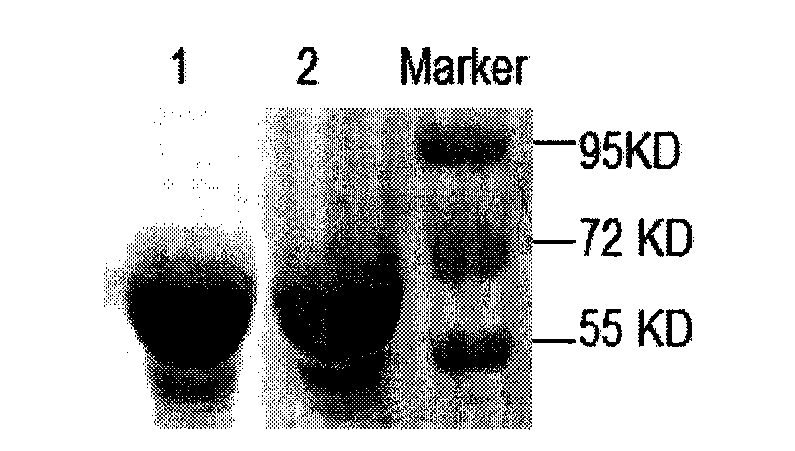
The invention belongs to the technical field of animal-borne disease subunit vaccine preparation and relates to preparation and application of the immune protective antigen of the haemophilus parasuis. Outer membrane protein Hbp B genes of the haemophilus parasuis are cloned, a nucleotide sequence is indicated as SEQID NO:1, and the sequence of gene code is indicated as SEQ ID NO:2. Recombination Escherichia coli BL21 / Pet-28a-Hbp B (preservation number is CCTCC NO:M2011228) is built and comprises genes in a sequence table SEQ ID NO:1. Antigen protein of the haemophilus parasuis is obtained and expressed through gene transformation Escherichia coli in the SEQ ID NO:1. The invention further discloses a preparation method and application of the recombination Escherichia coli. The haemophilus parasuis subunit vaccine has good safety, and an immune protection effect reaches 83%.
Owner:HUAZHONG AGRI UNIV +1
Application of Harmine derivative to preparation of antibacterial medicine
The invention discloses application of a Harmine derivative to preparation of antibacterial medicines. The bacteria is selected from Acinetobacter, Bacillus, Campylobacter, Chlamydia, Chlamydia trachomatis, Clostridium, Citrobacter, Escherichia, enterohemorrhagic escherichia coli, enteric bacteria, Enterococcus, Francisella, Haemophilus, helicobacter, Klebsiella Bacillus, Lester monocytogenes, Moraxella, Mycobacterium, Neisseria, proteus, Pseudomonas, Salmonella, shewanella oneidensis, Shigella, Stenotrophomonas, Staphylococcus, Streptococcus and Yersinia.
Owner:XINJIANG HUASHIDAN PHARMA RES
Water dispersible film
InactiveUS20050070501A1Avoid difficultyAntibacterial agentsOrganic active ingredientsDiseaseComposite film
Owner:NEW YORK BLOOD CENT
Rapidly dispersible vaginal tablet that provides a bioadhesive gel
InactiveUS20110159091A1Safe and relatively inexpensive methodAvoid spreadingAntibacterial agentsBiocideBacterial vaginosisSpiroplasma
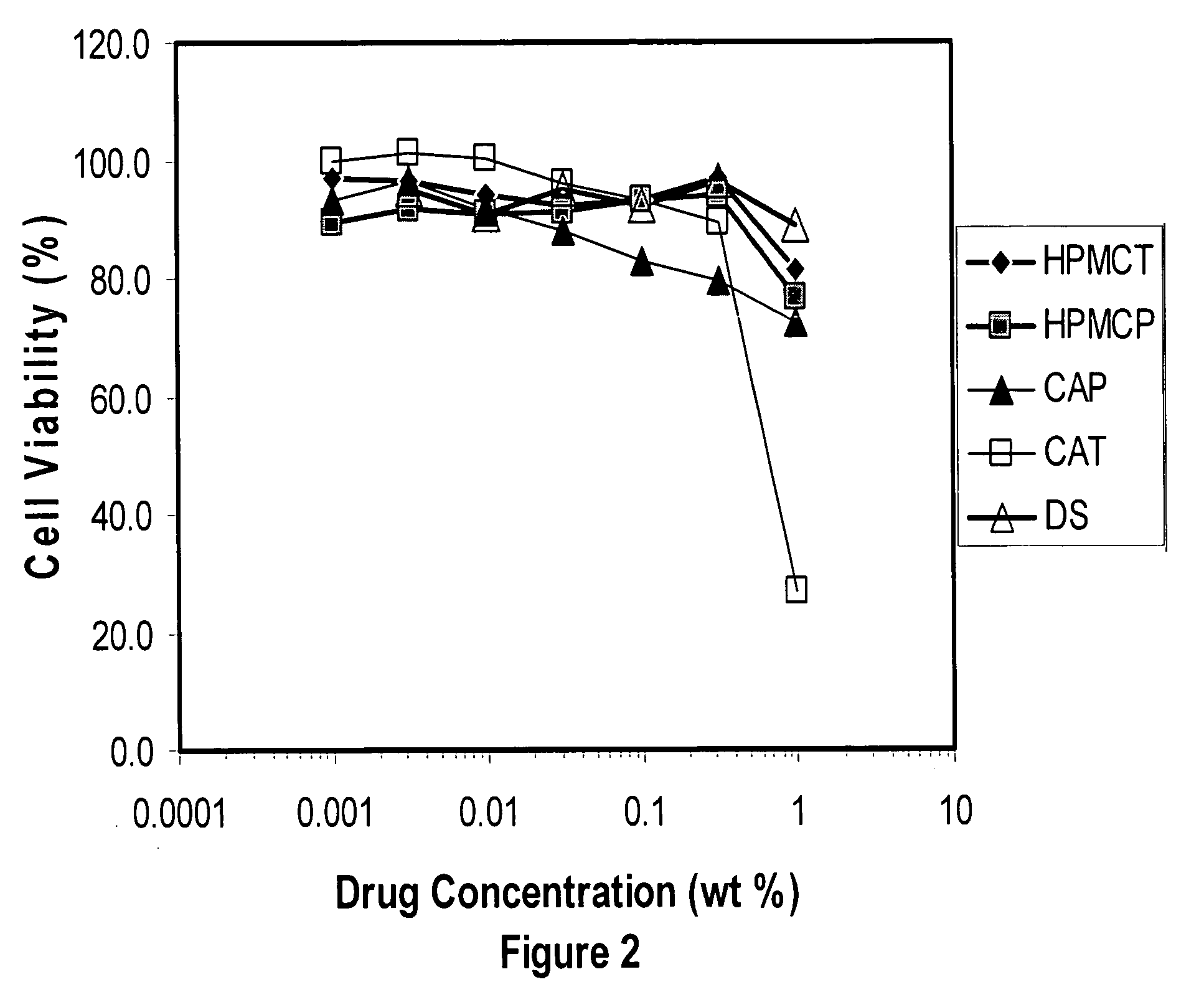
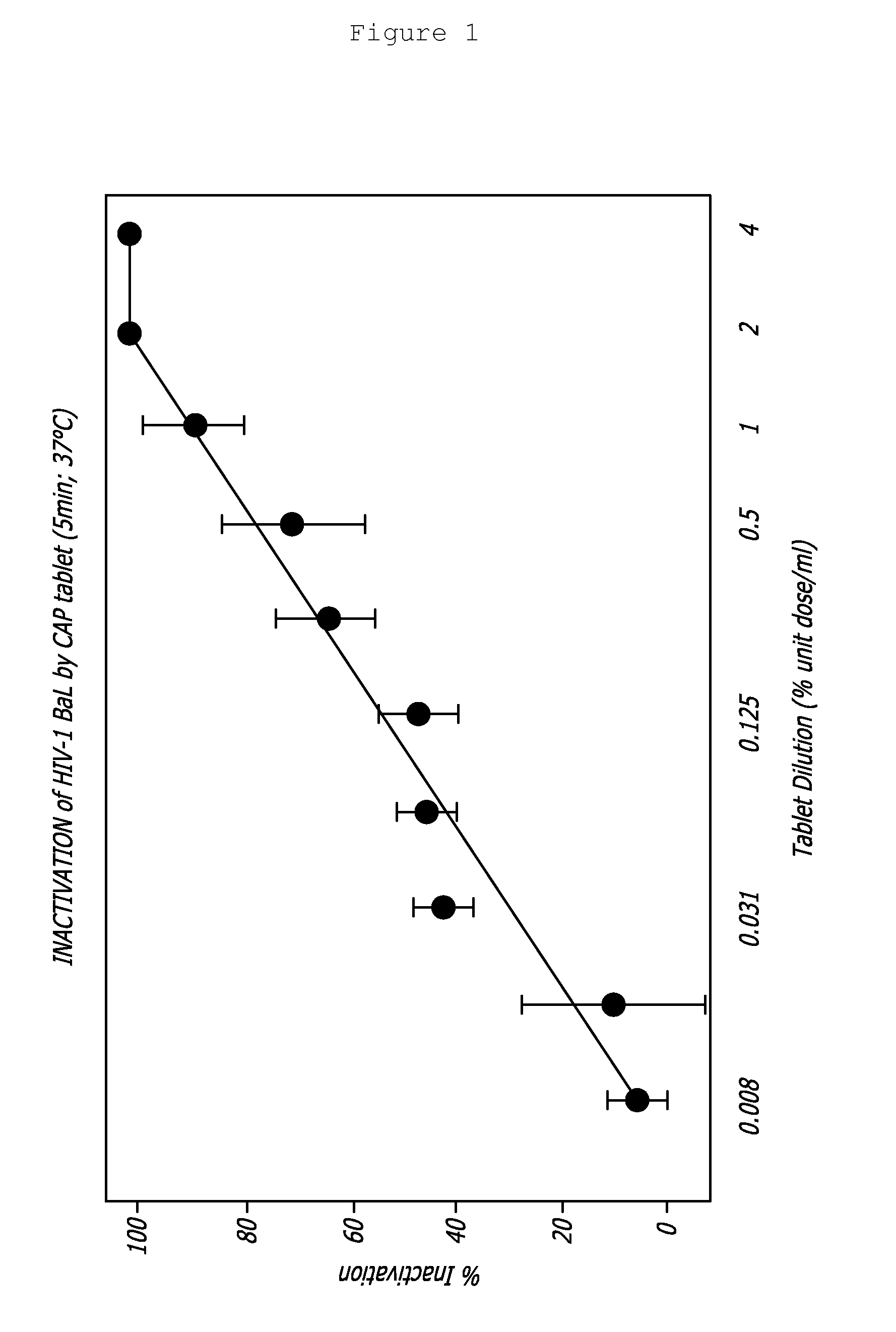
A tablet for insertion into a vagina including 0.01 to 500 mg of a vaginal medication, such as a microbicide, such as cellulose acetate 1,2-benzenedicarboxylate (CAP); 100 to 500 mg of mannitol powder; 50 to 300 mg of inert microcrystalline cellulose; 10 to 80 mg of hydroxypropyl methylcellulose; 50 to 250 mg of glycerol and optionally 2 to 4 mg of at least one preservative which protects against microbicidal contamination and discourages the growth of yeast in the vagina. The tablet which includes CAP as the vaginal medication is vaginally administered before coitus in methods for preventing the sexual transmission of HIV-1, HIV-2, herpesvirus, or an infection caused by Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Haemophilus ducreyi or Treponema pallidum. The tablet which includes CAP as the vaginal medication is vaginally administered to prevent or treat bacterial vaginosis.
Owner:NEW YORK BLOOD CENT
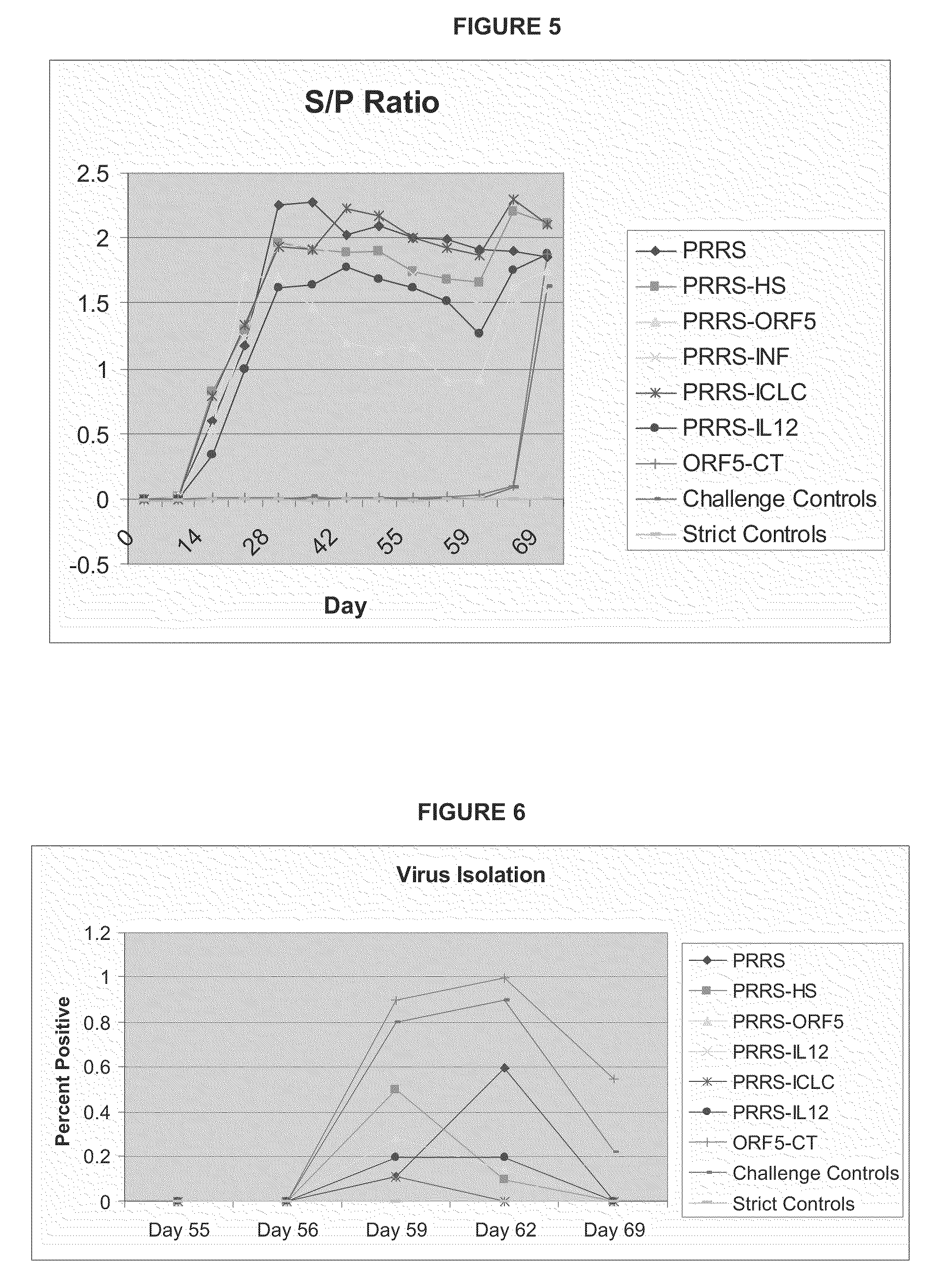
Immunological Compositions Effective for Lessening the Severity or Incidence of PRRSV Signs and Methods of Use Thereof
InactiveUS20100003278A1Reduce severityReduce percentageSsRNA viruses positive-senseViral antigen ingredientsAdjuvantHaemophilus
The present application describes improved an immunogenic compositions of virus vaccines wherein the virus vaccines comprise adjuvants selected from the group consisting of MCP-1, Haemophilus sonmus fractions, carbomer and combinations thereof. Methods and compositions using such improved compositions are described.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Primer probe combination and kit for combined inspection of 15 kinds of respiratory tract infection pathogens
ActiveCN107937578ARapid combined detectionGuaranteed matchMicrobiological testing/measurementMicroorganism based processesStreptococcus pyogenesStaphylococcus aureus
The invention provides a pathogen inspection reagent, and concretely discloses a primer probe combination and a kit for combined inspection of 15 kinds of respiratory tract infection pathogens. By aiming at specific target sequences of the gene sequence conserved region of klebsiella pneumoniae, haemophilus influenzae, streptococcus pyogenes, staphylococcus aureus, escherichia coli, chlamydia pneumoniae, mycobacterium tuberculosis, stenotrophomonas maltophilia, baumanii, mycoplasma pneumoniae, enterococcus faecalis, ligionella pneumohpillia, streptococcus pneumoniae, bacillus pyocyaneus and mycobacterium abscessus, primers and probes which do not have mutual crossed reaction are designed, so that the problem that detection probes of different pathogens can easily generate mutual influenceor interference is solved; the combination and matching of different primers and probes are ensured; the goal of simultaneously performing efficient specific inspection at the same temperature can also be achieved.
Owner:西安九安生物技术有限公司
Application of macleaya cordata extractive in veterinary drugs for economic animals
ActiveCN101972309APromote growthImprove conversion rateAntibacterial agentsDigestive systemPoultry diseaseHaemophilus
The invention relates to the application of a macleaya cordata extractive in veterinary drugs for economic animals, in particular to the application of the macleaya cordata extractive in the preparation of veterinary drugs with favorable inhibition effect and killing effect on common livestock and poultry diseases, such as protoblem listeria monocytogenes, haemophilus and pasteurella.
Owner:MICOLTA BIORESOURCE INC LTD
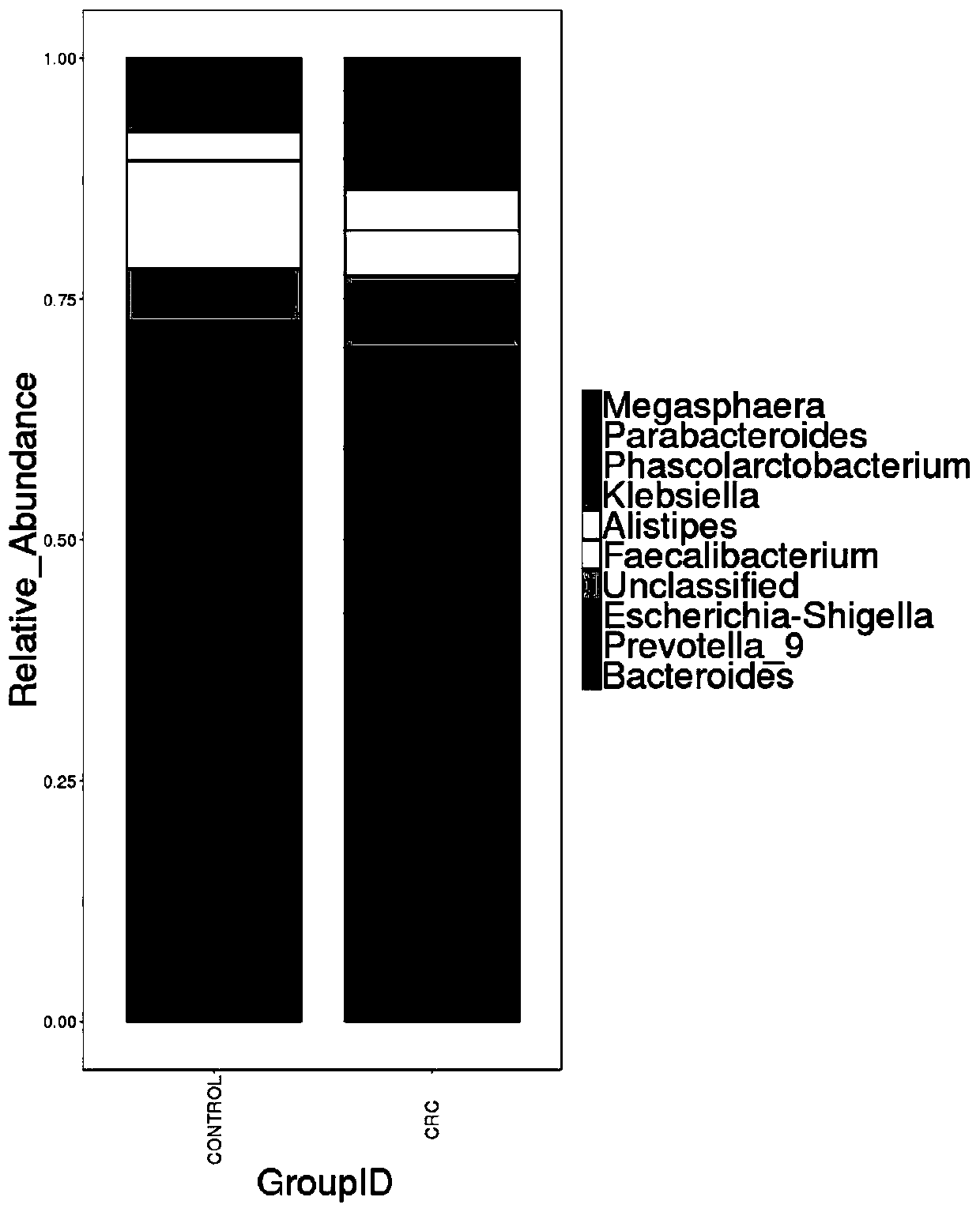
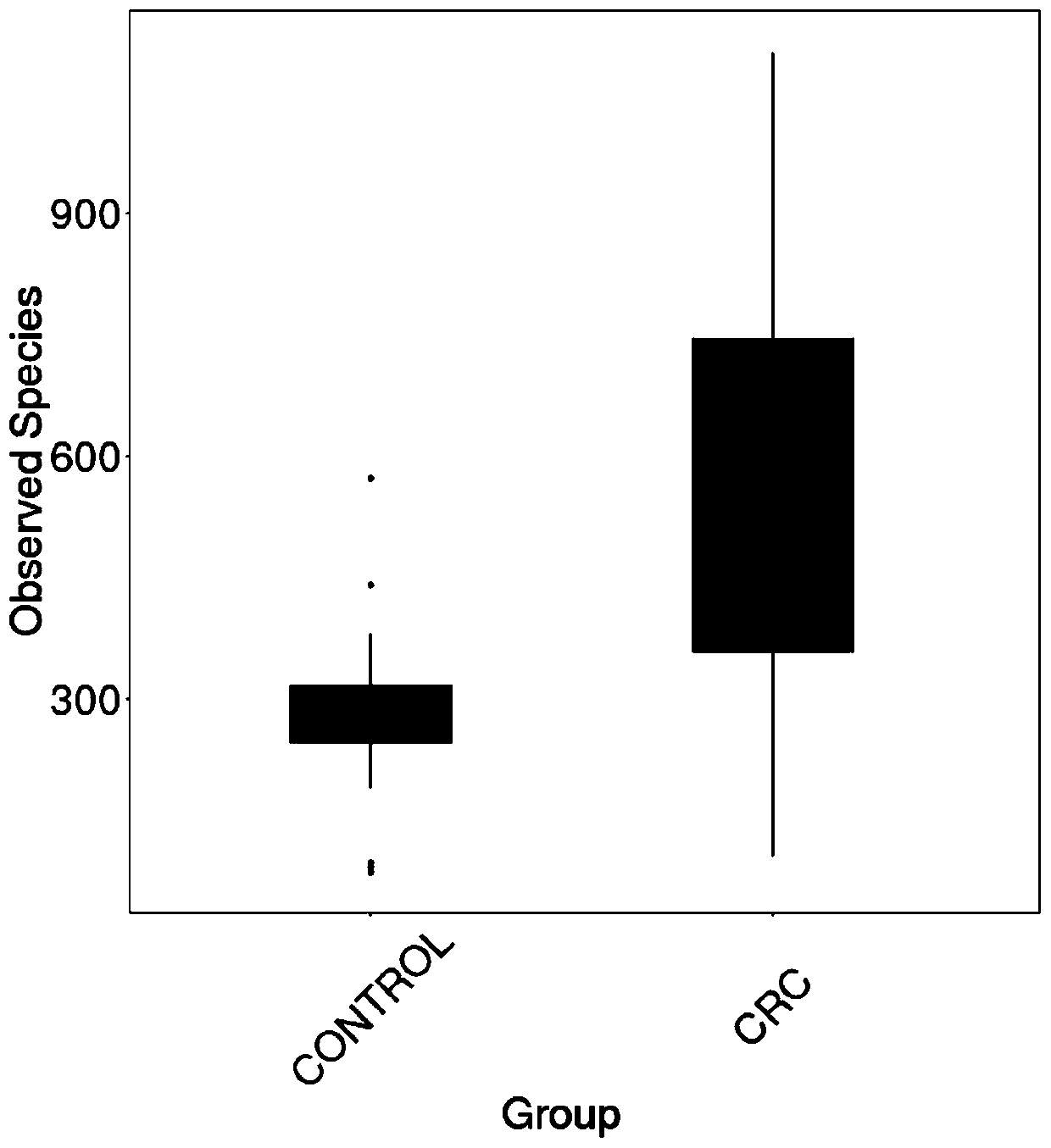
Intestinal flora maker for intestinal cancer and application of intestinal flora maker
InactiveCN110408699AGood forecastMicrobiological testing/measurementGut floraCorynebacterium amycolatum
The invention discloses an intestinal flora maker for intestinal cancer and an application of the intestinal flora maker, and belongs to the technical fields of microorganisms and genetic engineering.The intestinal flora maker comprises megasphaera, veillenella, streptococci, bilophila sp., haemophilus, corynebacterium or Escherichia-Shigella. Through detection by the intestinal flora maker, risks of individuals suffering from intestinal cancer can be assessed, and auxiliary reference can be provided for disease diagnosis and prognosis monitoring.
Owner:FUJIAN HEALTH COLLEGE
Benzothiazoles and aza-analogues thereof use as antibacterial agents
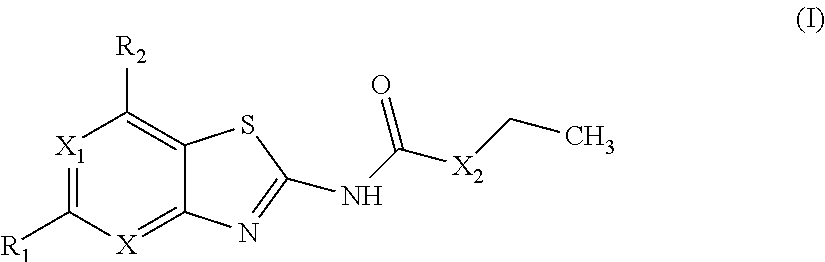
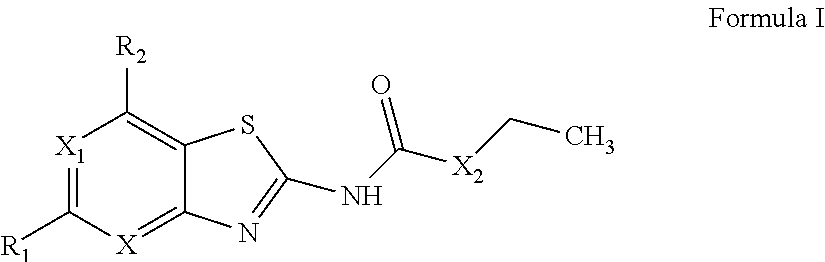
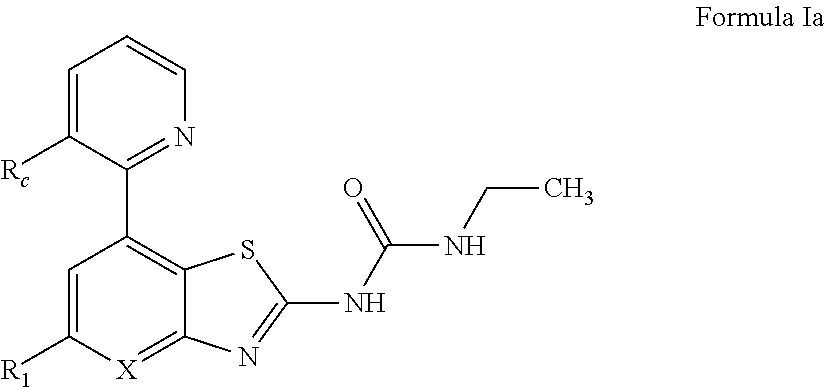
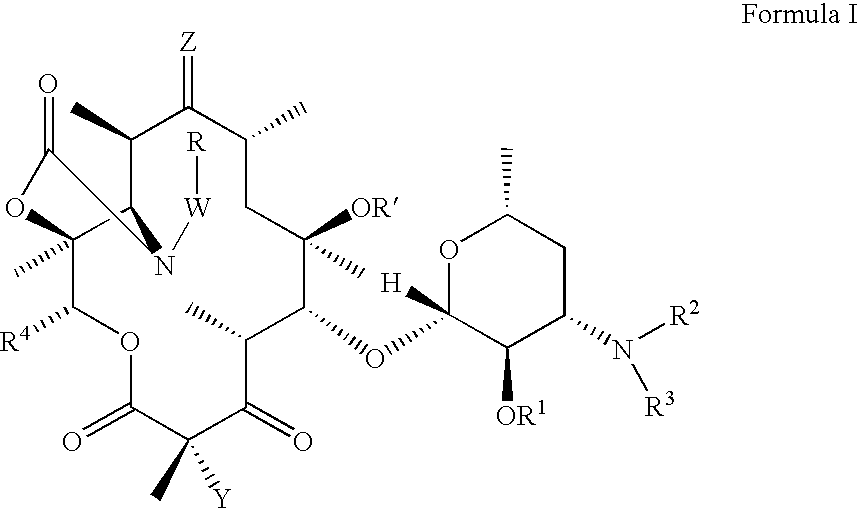
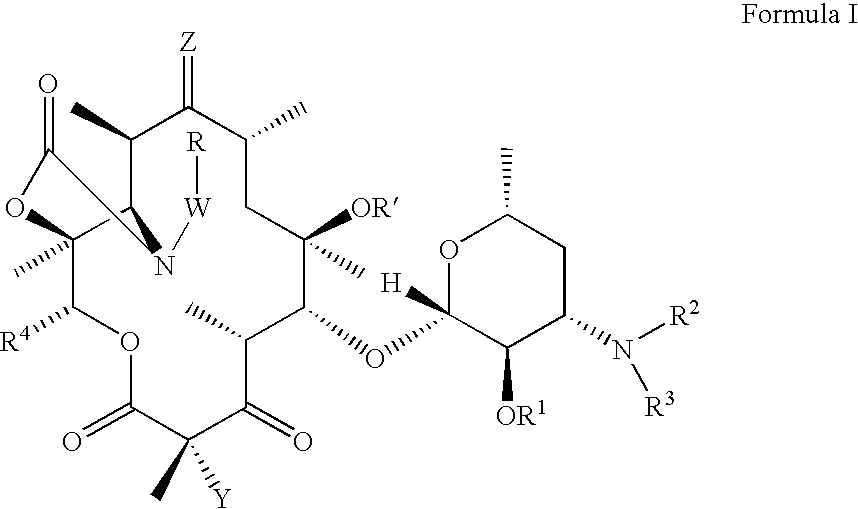
The present invention provides Gyrase B and / or Topoisomerase IV par E inhibitors, which can be used as antibacterial agents. Compounds disclosed herein can be used for treating or preventing conditions caused by or contributed by gram positive, gram negative and anaerobic bacteria, more particularly against, for example, Staphylococci, Streptococci, Enterococci, Haemophilus, Pseudomonas spp., Acenetobacter spp., Moraxalla spp., Chlamydia spp., Mycoplasma spp., Legionella spp., Ktycobacterium spp., Helicobacter, Clostridium spp., Bacteroides spp., Corynebacterium, Bacillus spp., Enterobactericeae,’ (E. coli, Klebsiella spp., Proteus spp., etc.) or any combination thereof. Also provided, are processes for preparing compounds disclosed herein, pharmaceutical compositions containing compounds disclosed herein, and methods of treating bacterial infections. (Formula)
Owner:RANBAXY LAB LTD
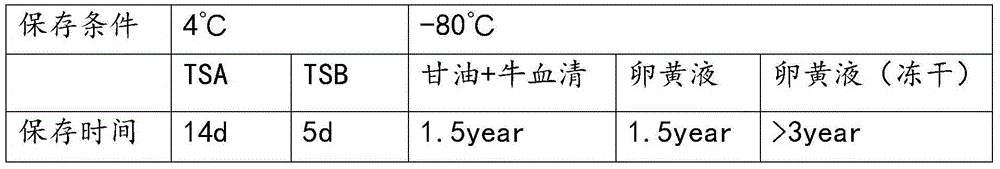
Haemophilus parasuis culture medium
ActiveCN103215208AMaintain integrityIncrease success rateBacteriaMicroorganism based processesSerum igeHaemophilus
The invention discloses a haemophilus parasuis culture medium. A preparation method of the haemophilus parasuis culture medium comprises the steps of preparing a basic culture solution, treating coenzyme A, and perfecting a culture medium, wherein the basic culture solution comprises peptone, tryptone, sodium chloride, dextrose, yeast extract, glycerin and distilled water, and the basic culture solution, the coenzyme A and fetal bovine serum are uniformly mixed so as to obtain the haemophilus parasuis culture medium. The haemophilus parasuis culture medium provided by the invention can keep haemophilus parasuis, thereby facilitating the transportation of suspicious haemophilus parasuis samples; and the death of haemophilus parasuis is not caused in the process of transportation, thereby increasing the separation probability of the haemophilus parasuis.
Owner:广西悦牧生物科技有限公司
Serum-12 type haemophilus lus paradis vaccine strain and application thereof
InactiveCN104450556AStrong pathogenicityLong durationAntibacterial agentsBacteriaSerum igeHaemophilus
The invention relates to a serum-12 type haemophilus lus paradis vaccine strain. The classified name of the vaccine strain is haemophilus lus paradis, the strain name is SHCM10 and the vaccine strain is preserved in the China Center for Type Culture Collection on June 15, 2014 with the preservation number of CCTCC NO:M 2014261. The invention further relates to an application of the serum-12 type haemophilus lus paradis vaccine strain in preparation of a haemophilus lus paradis inactivated vaccine. The serum-12 type haemophilus lus paradis SHCM10 strain is stable in biology, has a strong pathogenicity to a piglet, and has a good immunogenicity when being inactivated and vaccinated on the piglet. A univalent vaccine prepared from the vaccine strain serving as a vaccine candidate strain has good safety, can produce a relatively high antibody on the piglet, has long duration and good immune potency, and can be used for resisting attack of homotype wild strains. After a pig group is immunized, the morbidity and death rate are remarkably reduced, the economic loss of a piggery is reduced, and the immunizing effect of the vaccine strain is the same as or superior to that of existing commercial vaccines on the market.
Owner:扬州优邦生物药品有限公司
Ketolide derivatives as antibacterial agents
The present invention provides ketolide derivatives, which can be used as anti-bacterial agents. Compounds disclosed herein can be used for the treating or preventing conditions caused by or contributed to by gram positive, gram negative or anaerobic bacteria, more particularly against, for example, Staphylococci, Streptococci, Enterococci, Haemophilus, Moraxalla spp., Chlamydia spp., Mycoplasm, Legionella spp., Mycobacterium, Helicobacter, Clostridium, Bacteroides, Corynebacterium, Bacillus, Enterobactericeae or any combination thereof. Also provided are processes for preparing compounds disclosed herein, intermediates used in their synthesis, pharmaceutical compositions thereof, and methods of treating bacterial infections.
Owner:RANBAXY LAB LTD
Vaccine composition containing porcine circovirus type 2 antigen and haemophilus parasuis antigen, as well as preparation method and application thereof
ActiveCN102988978AChange cognitive biasImprove immunityAntibacterial agentsBacteriaAntigenHaemophilus
The invention relates to a polyvalent vaccine composition for porcine, and in particular relates to a vaccine composition capable of resisting infection of porcine circovirus type 2 (PCV 2) and haemophilus parasuis (HPS) at the same time. The vaccine composition comprises at least one PCV2 antigen, at least one HPS, as well as vector, an excipient and an adjuvant available in the field of veterinary medicine. The vaccine composition can be used for preventing and treating PCV2 related diseases and HPS diseases.
Owner:PU LIKE BIO ENG
Haemophilus outer membrane protein
InactiveUS6013514AImprove the protective effectHigh protection levelAntibacterial agentsBacteriaSerum igeHaemophilus
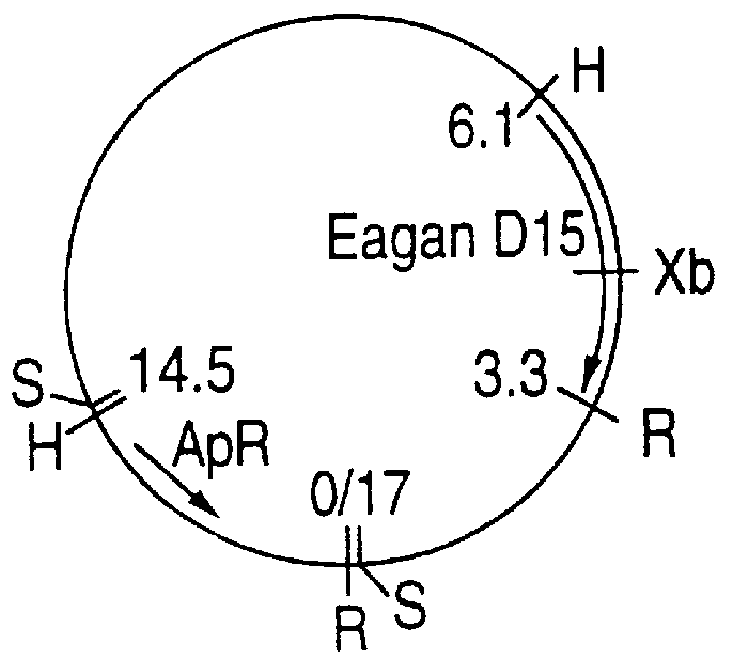
PCT No. PCT / CA93 / 00501 Sec. 371 Date Sep. 12, 1995 Sec. 102(e) Date Sep. 12, 1995 PCT Filed Nov. 23, 1993 PCT Pub. No. WO94 / 12641 PCT Pub. Date Jun. 9, 1994Purified and isolated nucleic acid from specific strains of Haemophilus influenzae is provided which encodes at least a portion of the D15 outer membrane protein of Haemophilus. The nucleic acid is used to produce peptides, polypeptides and proteins free of contaminant associated with Haemophilus for purposes of diagnosis and medical treatment. Furthermore, the nucleic acid may be used in the diagnosis of Haemophilus infection. Antisera obtained following immunization with the nucleic acid D15 outer membrane protein or peptides also may be used for the purpose of diagnosis and medical treatment.
Owner:COONNAUGHT LAB
Conjugation process of bacterial polysaccharides to carrier proteins
ActiveUS20120321660A1Size of it can retainAntibacterial agentsBacterial antigen ingredientsBacteroidesStreptococcus pneumoniae
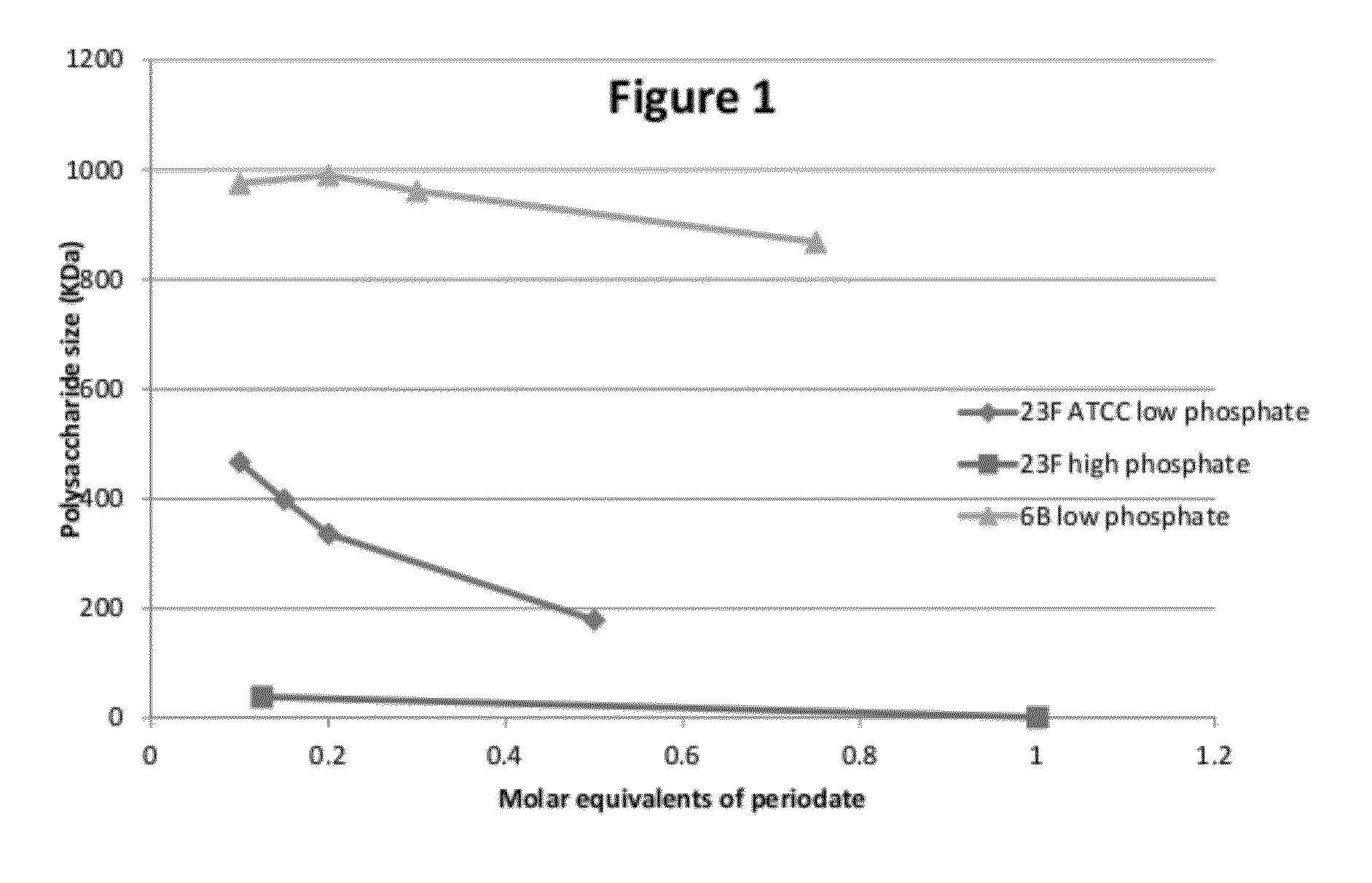
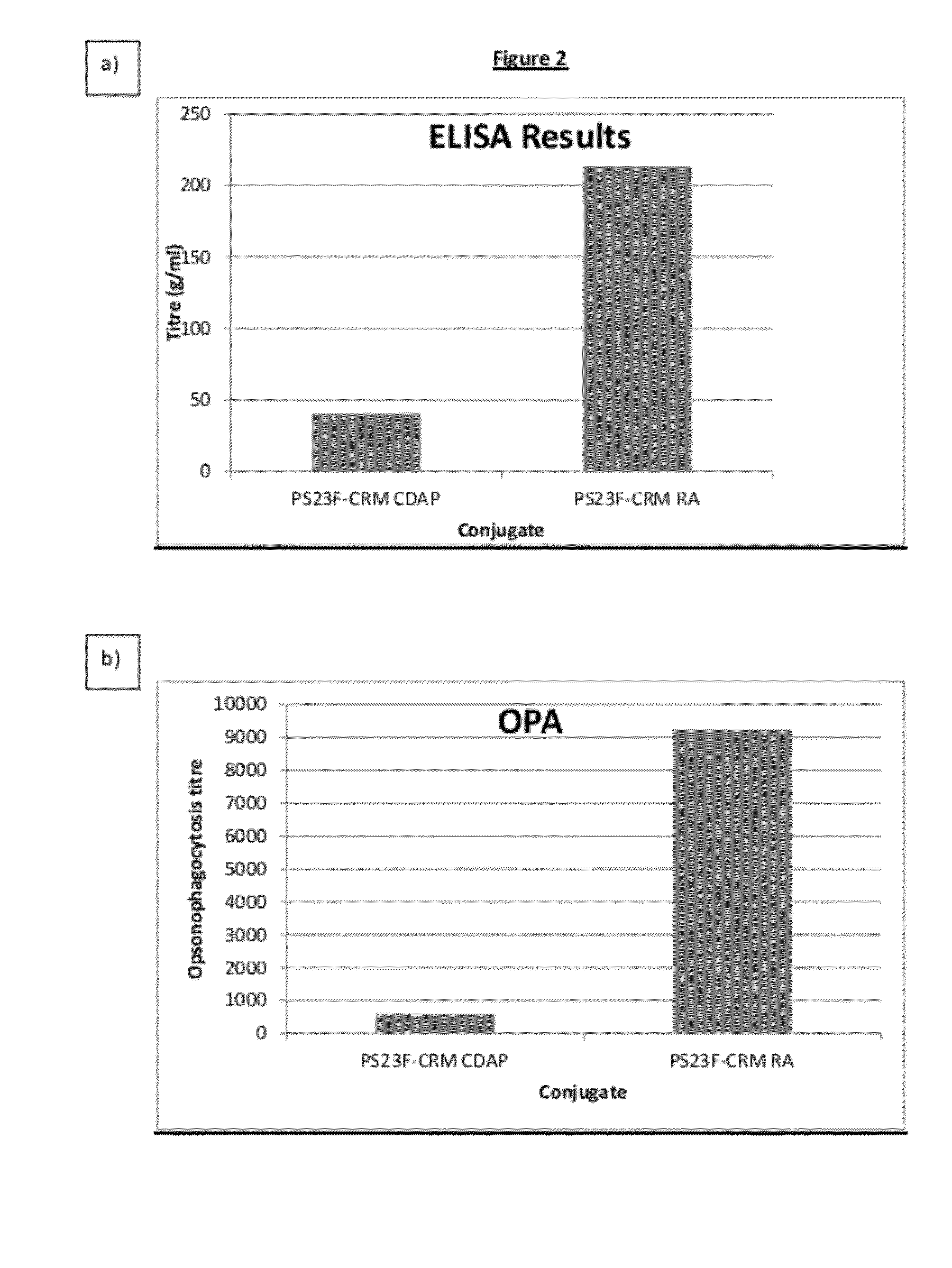
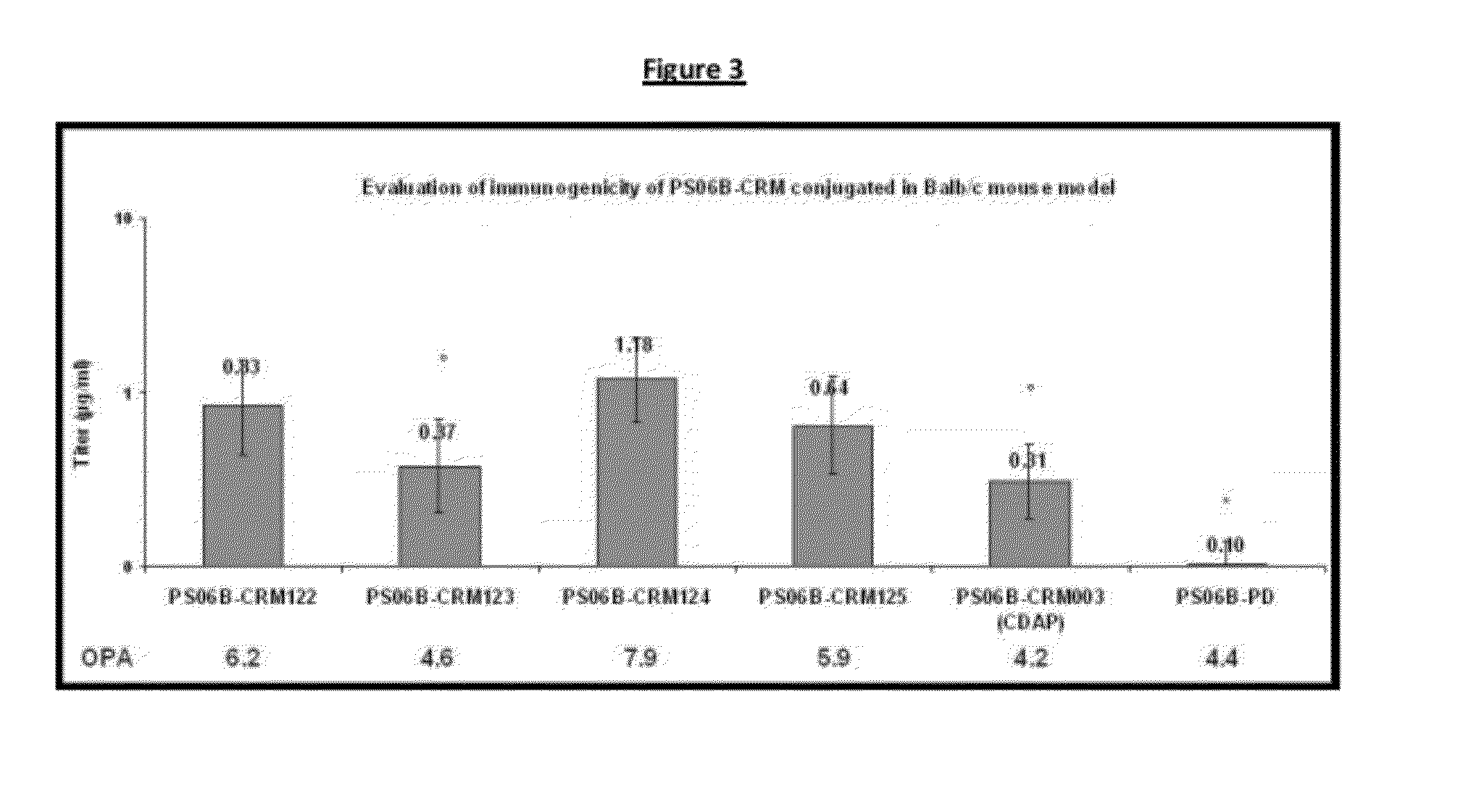
Process for conjugation of bacterial saccharides including Streptococcus pneumoniae and Haemophilus influenzae saccharides by reductive amination are provided herein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com