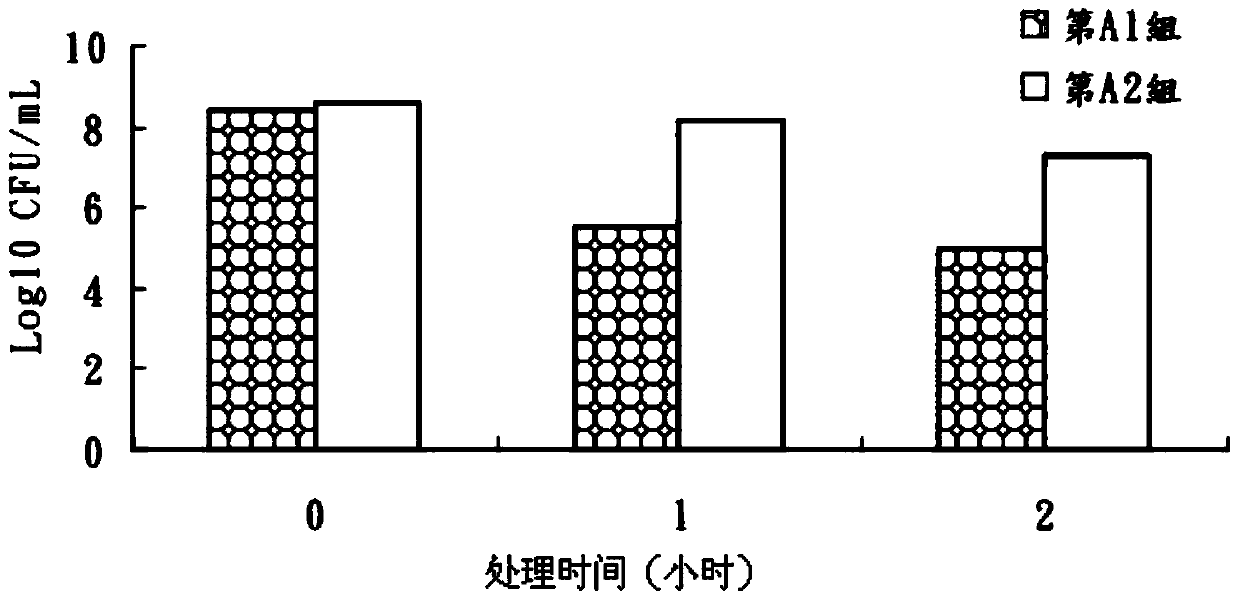
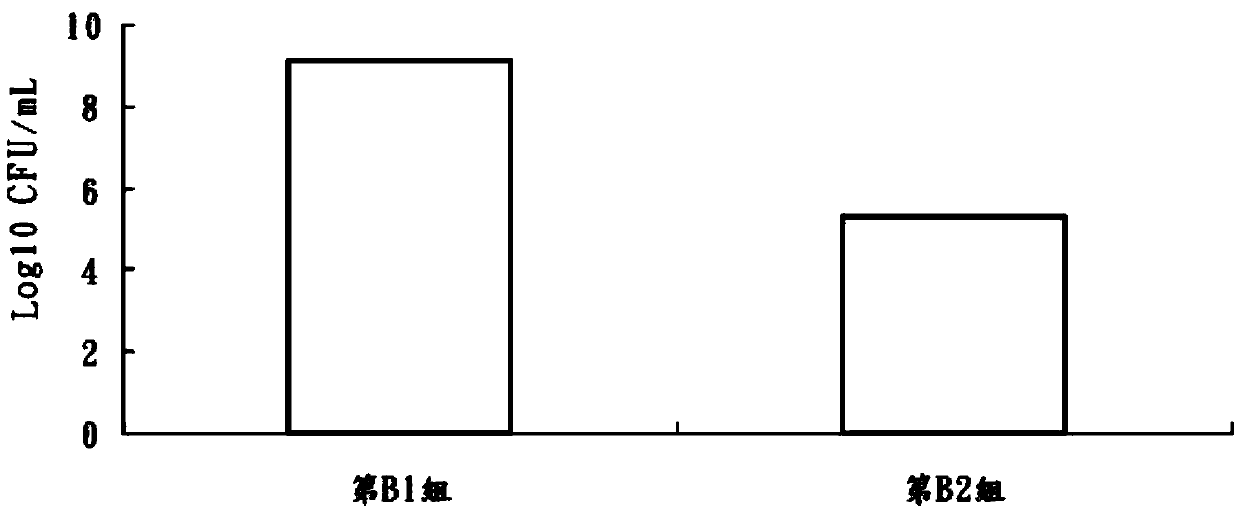
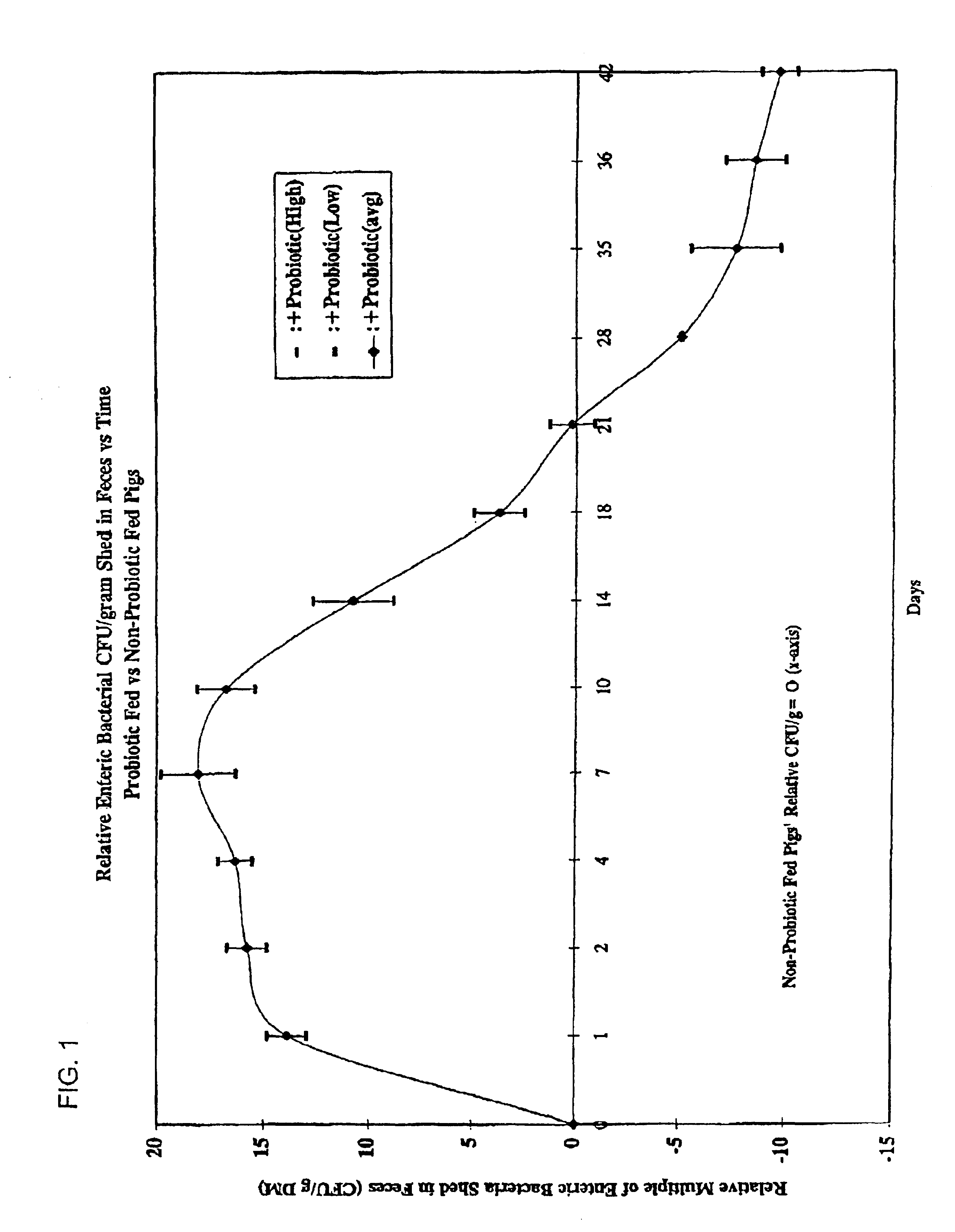
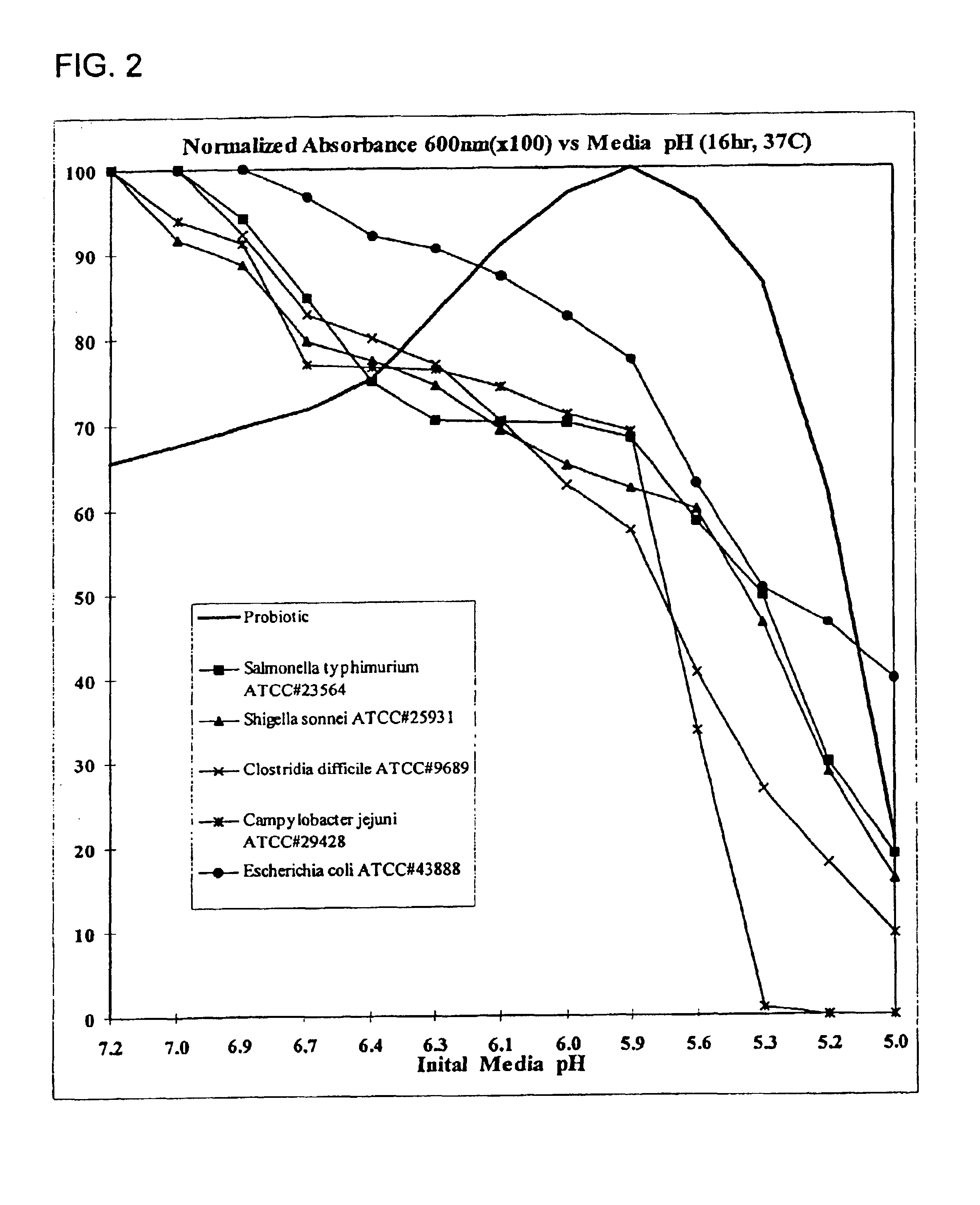
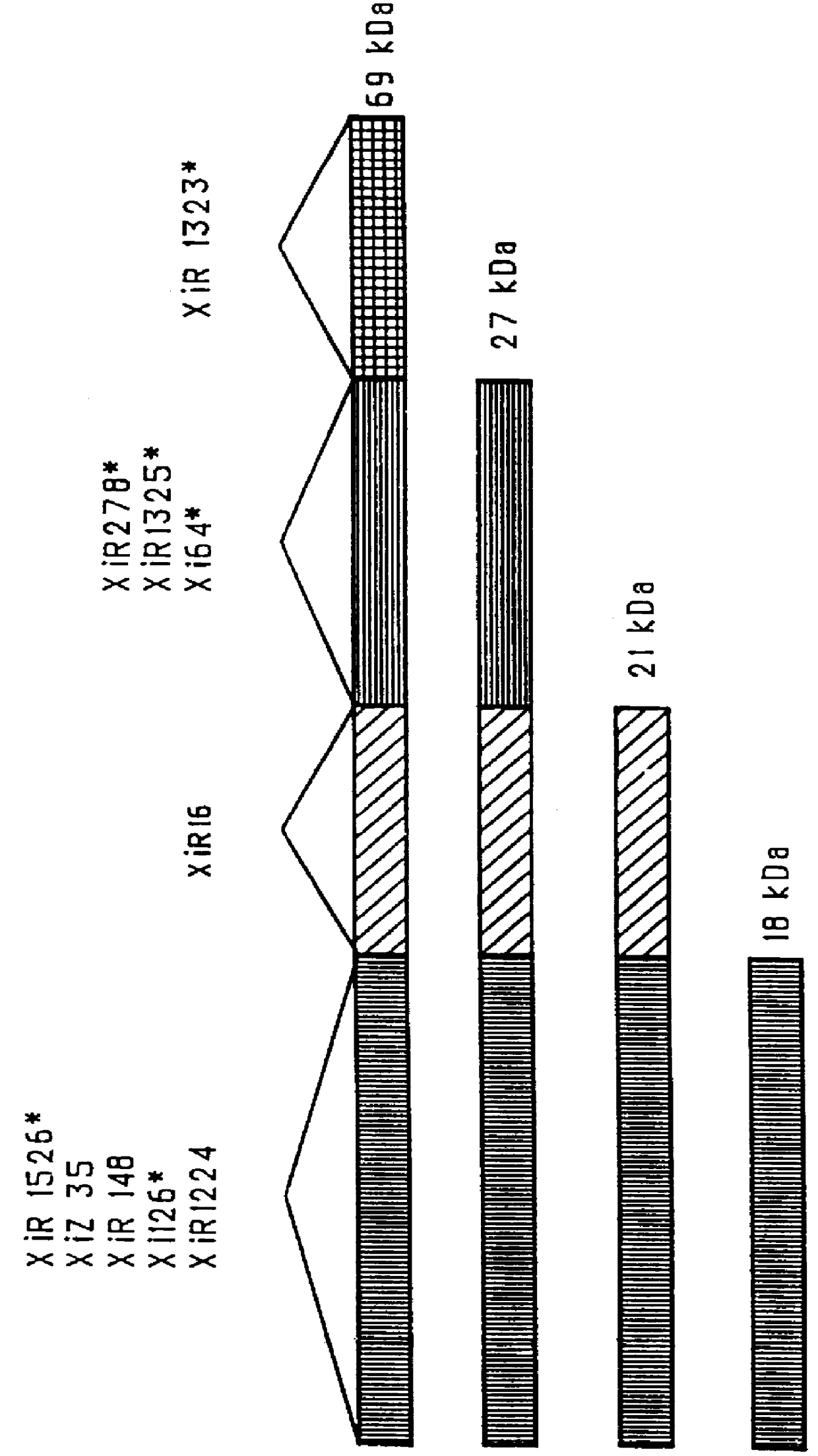
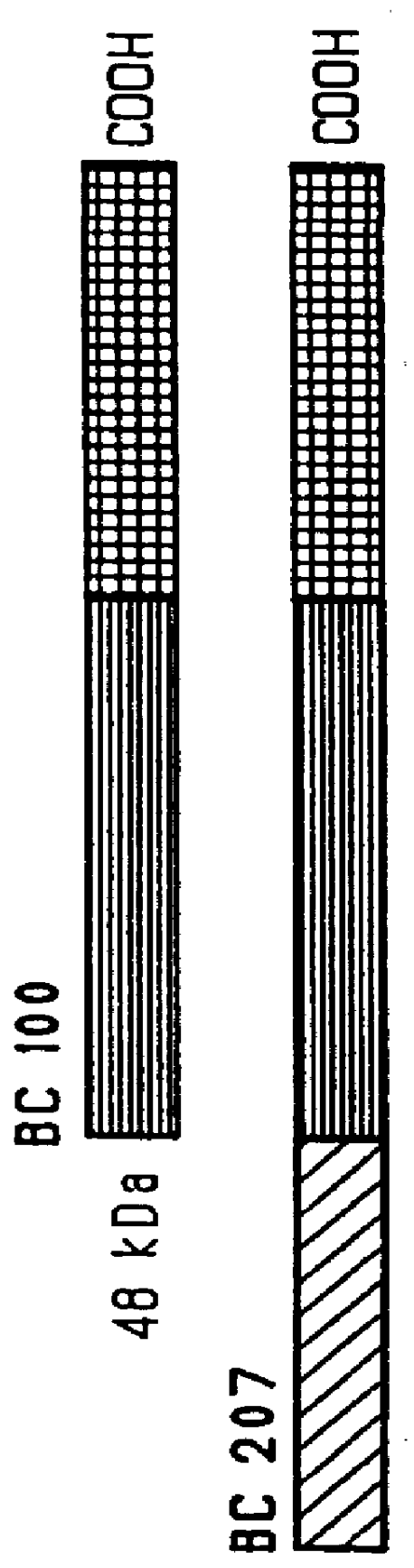
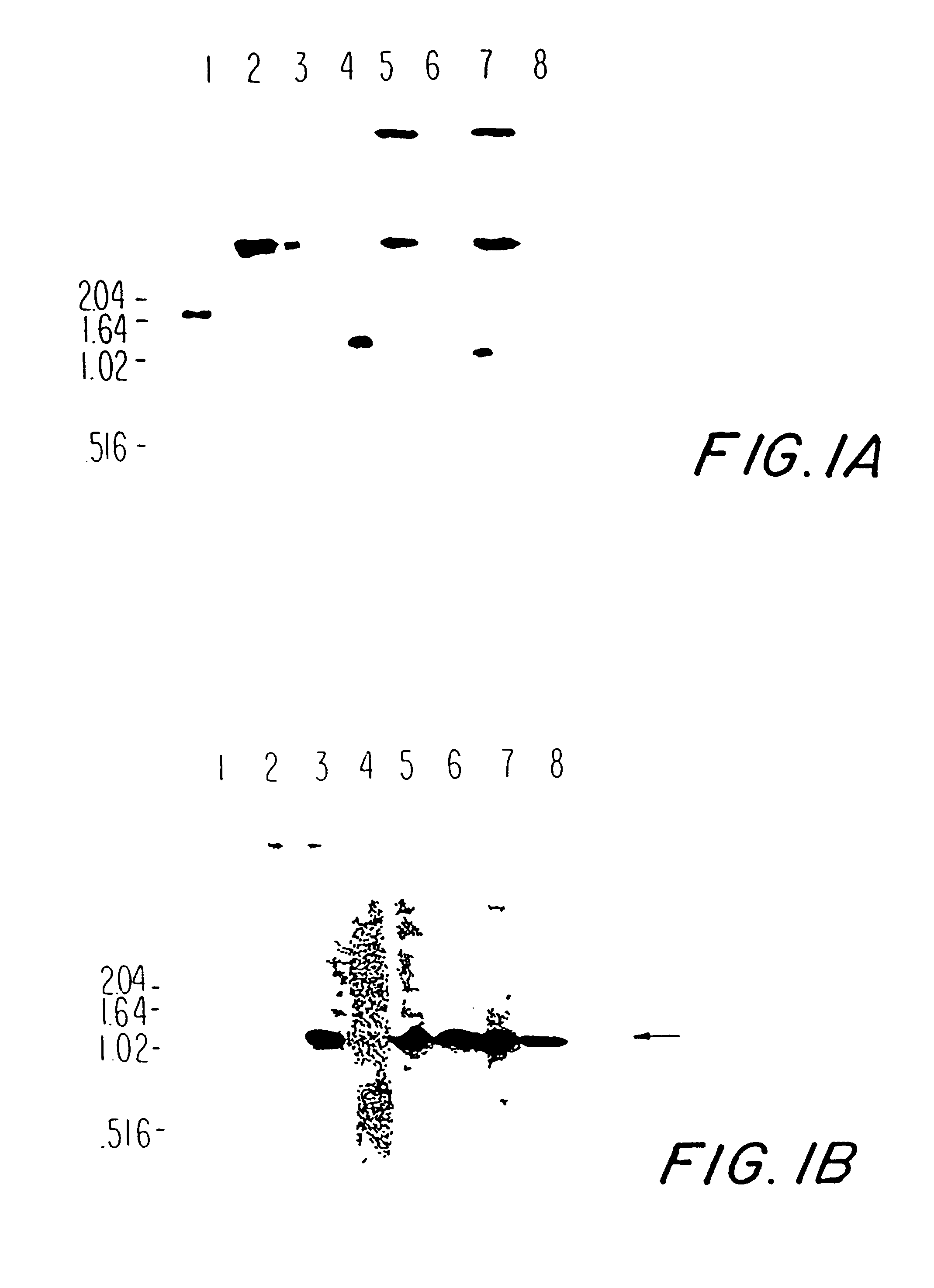
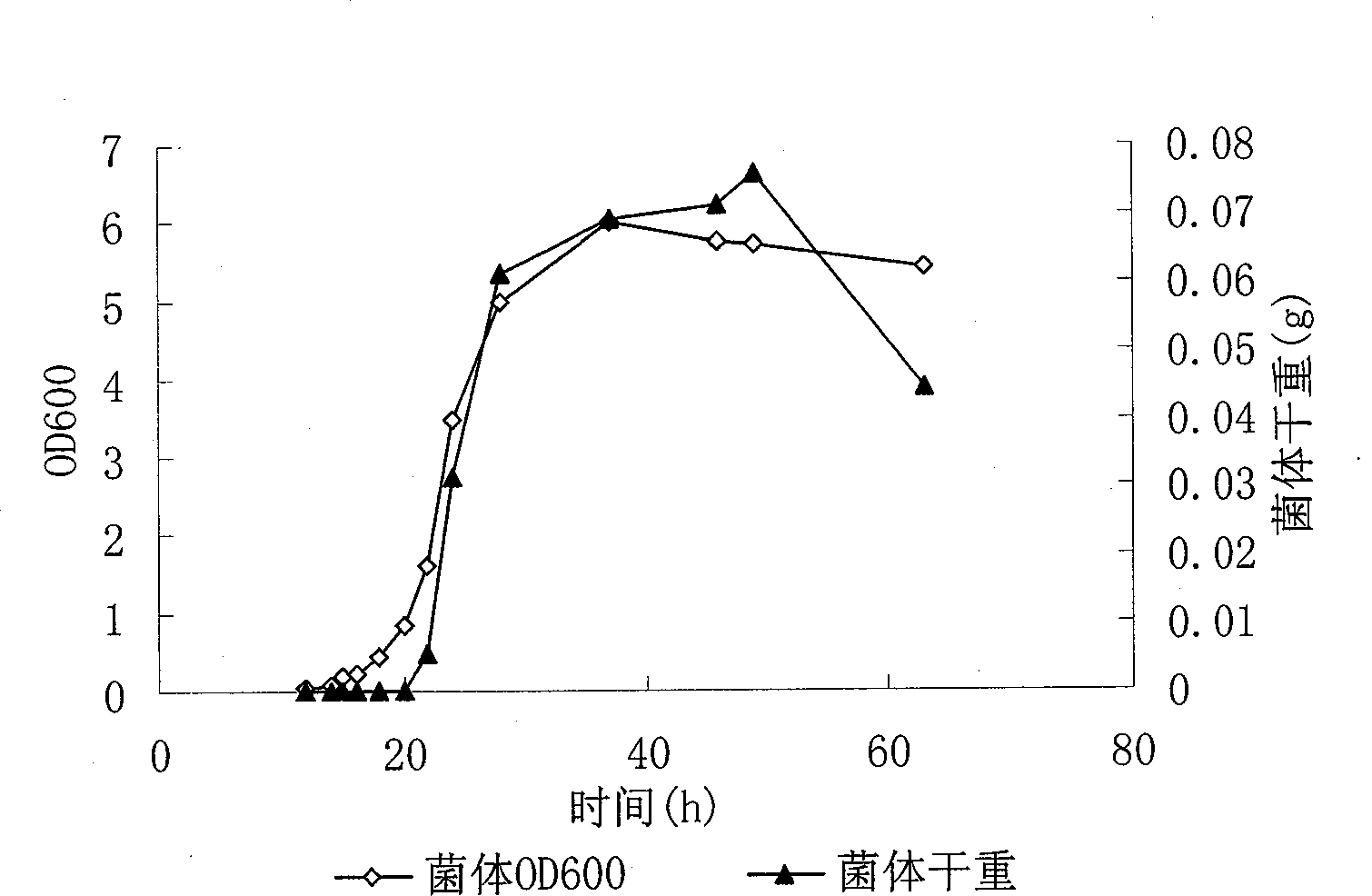
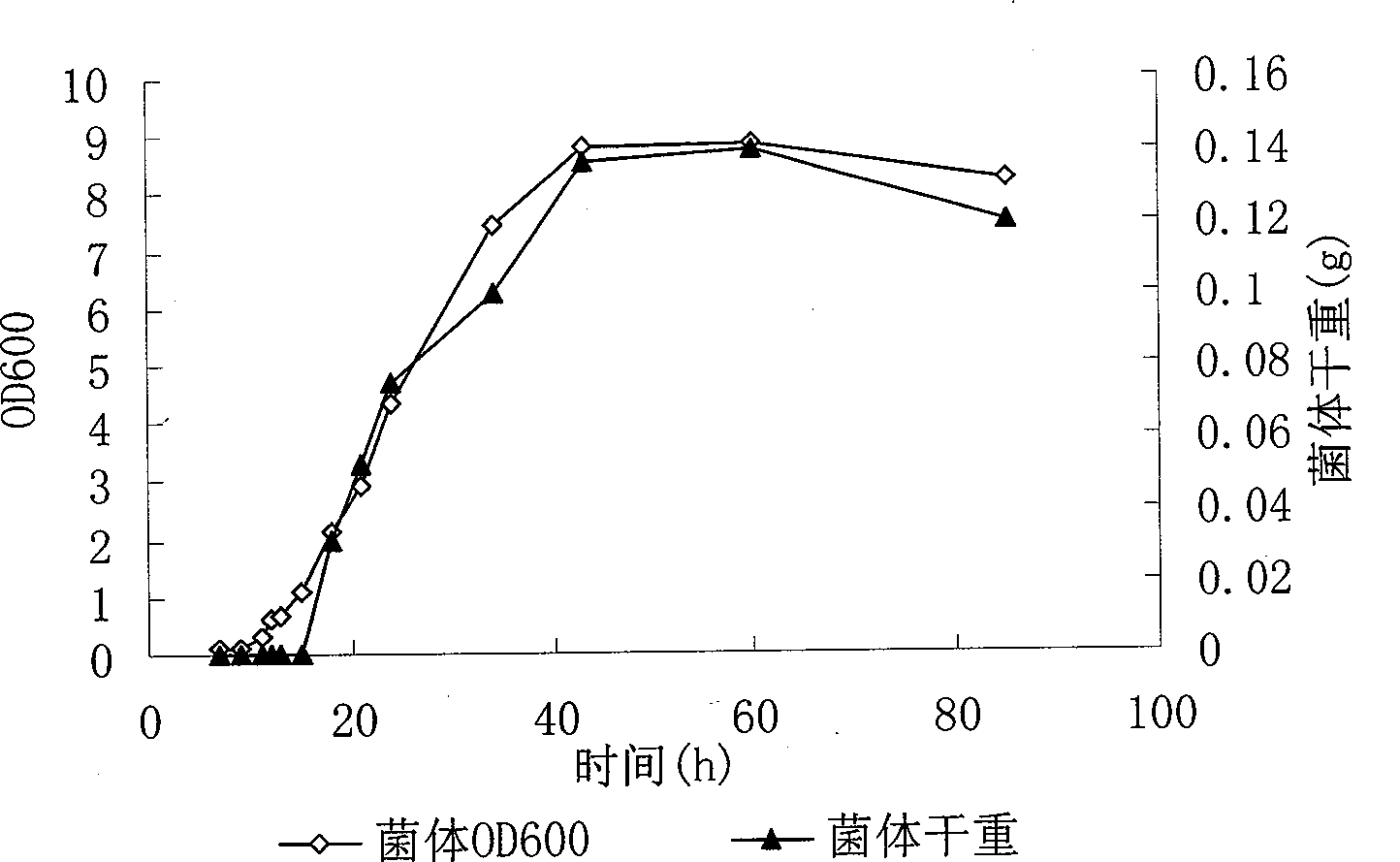
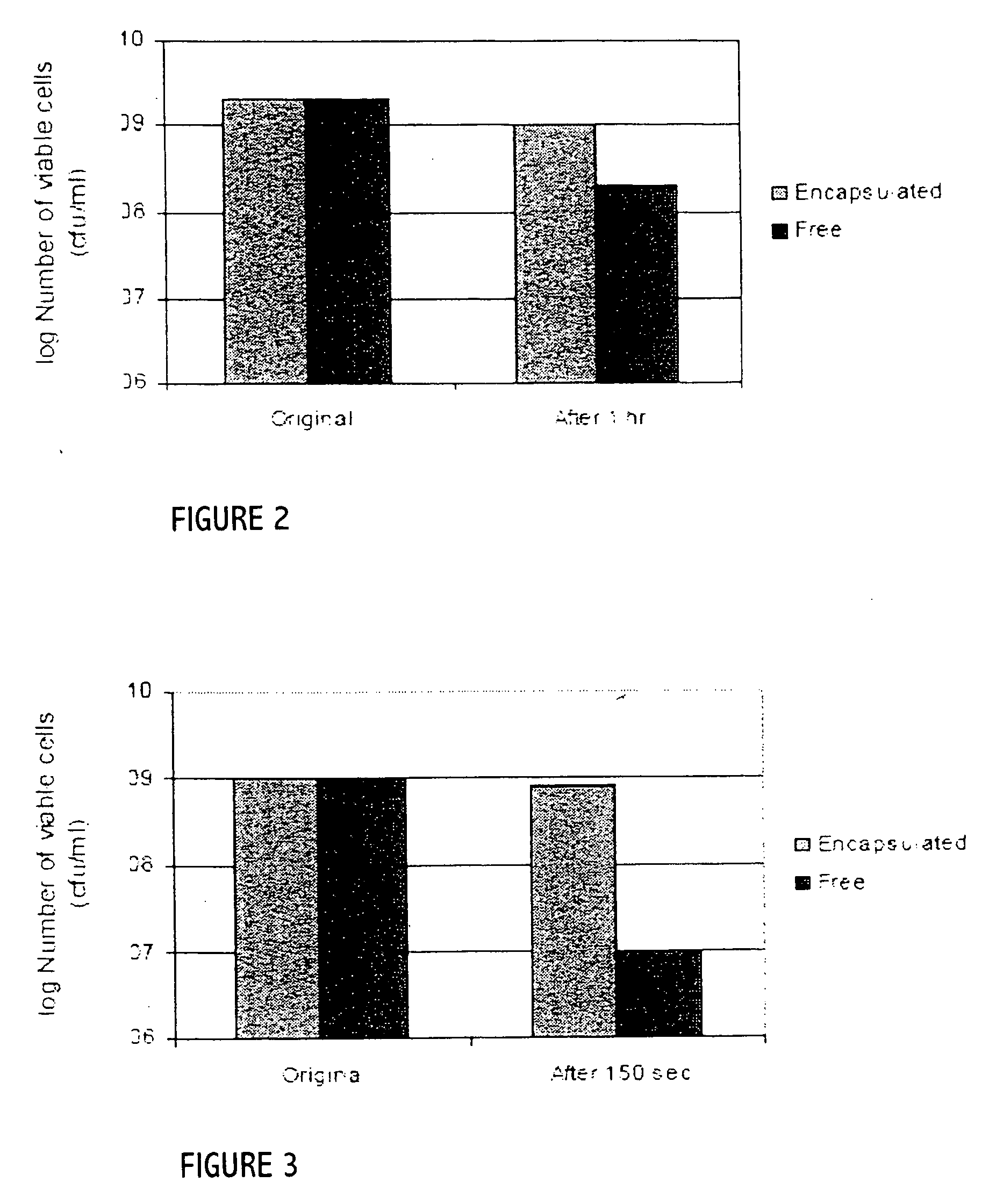
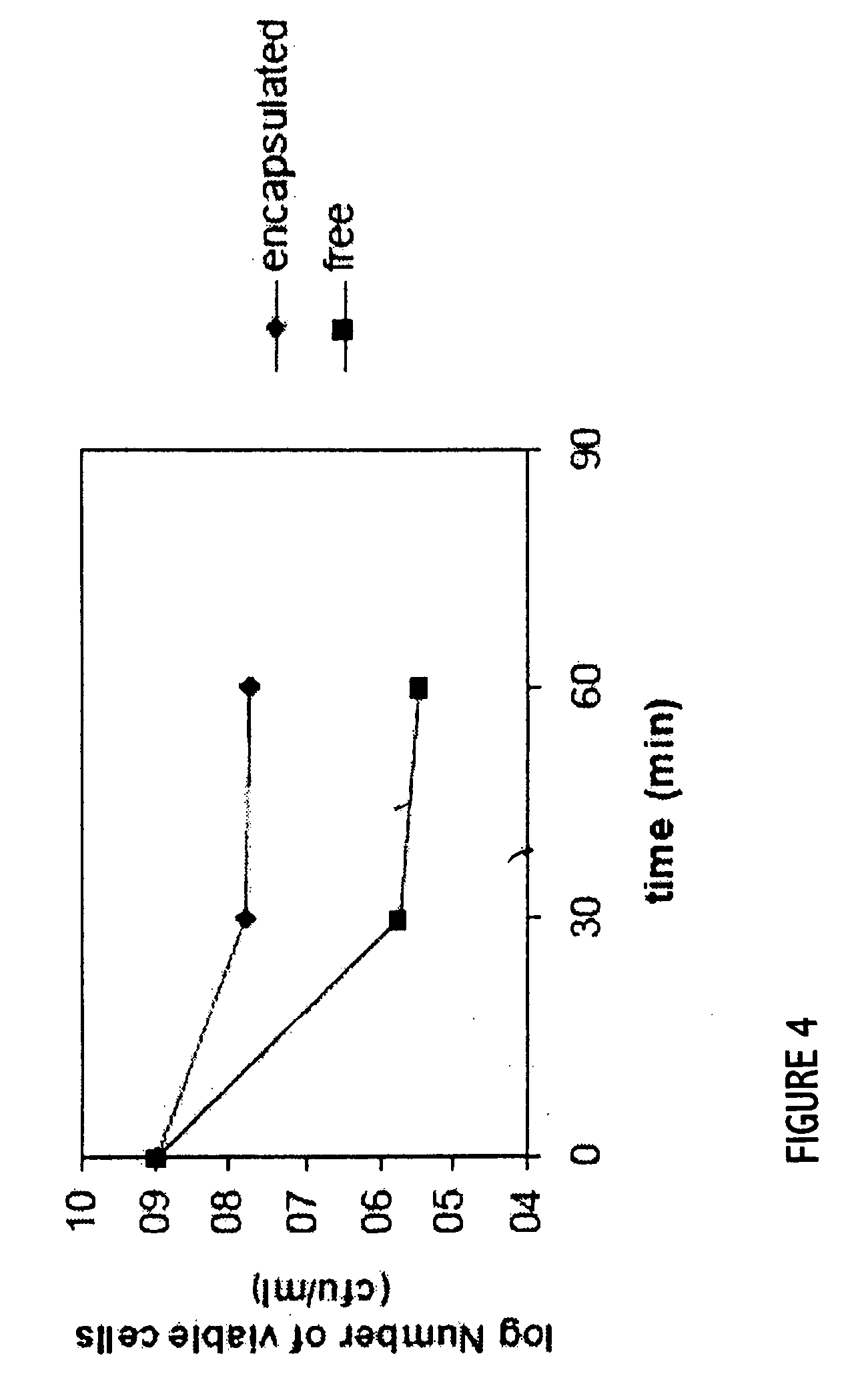
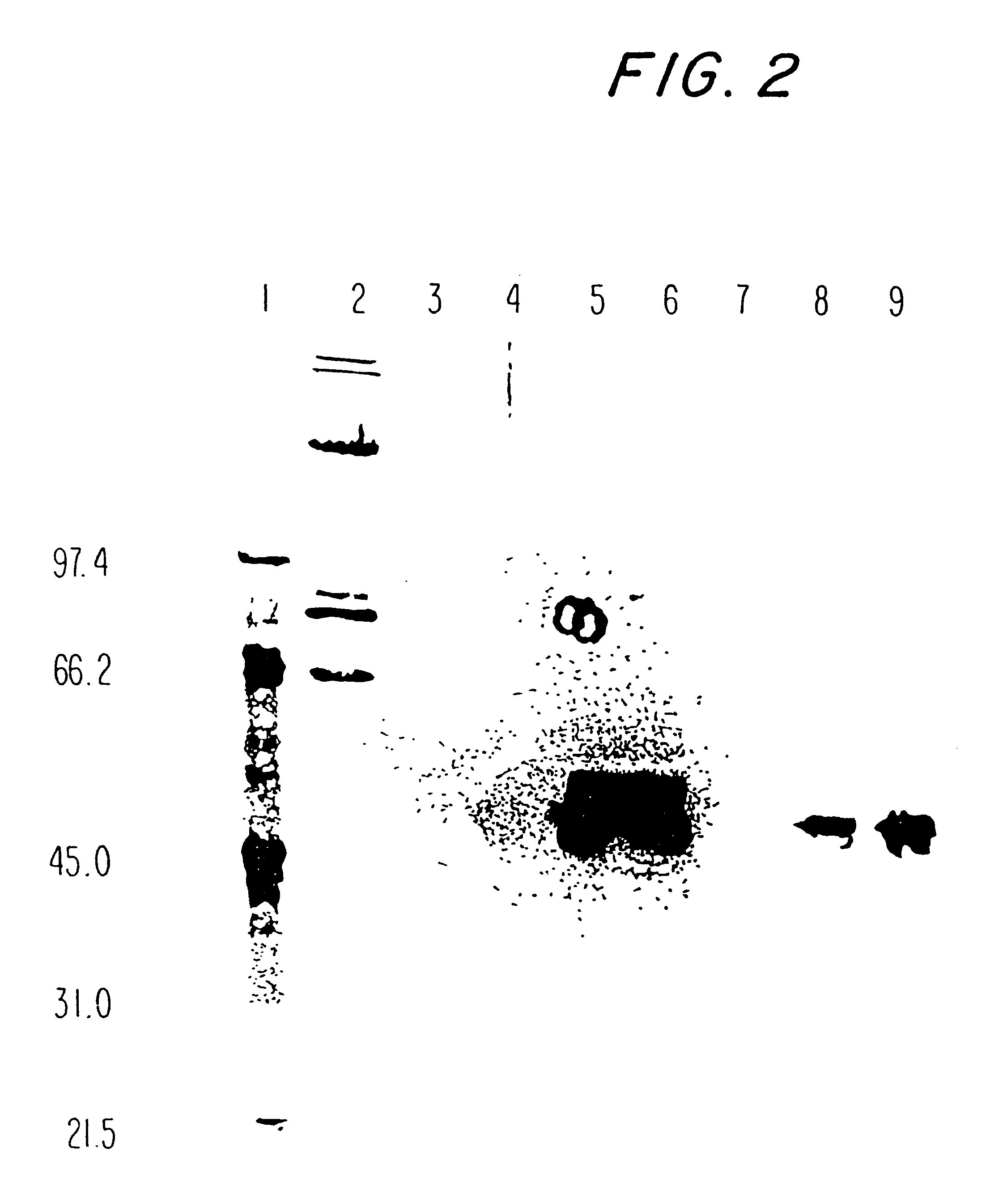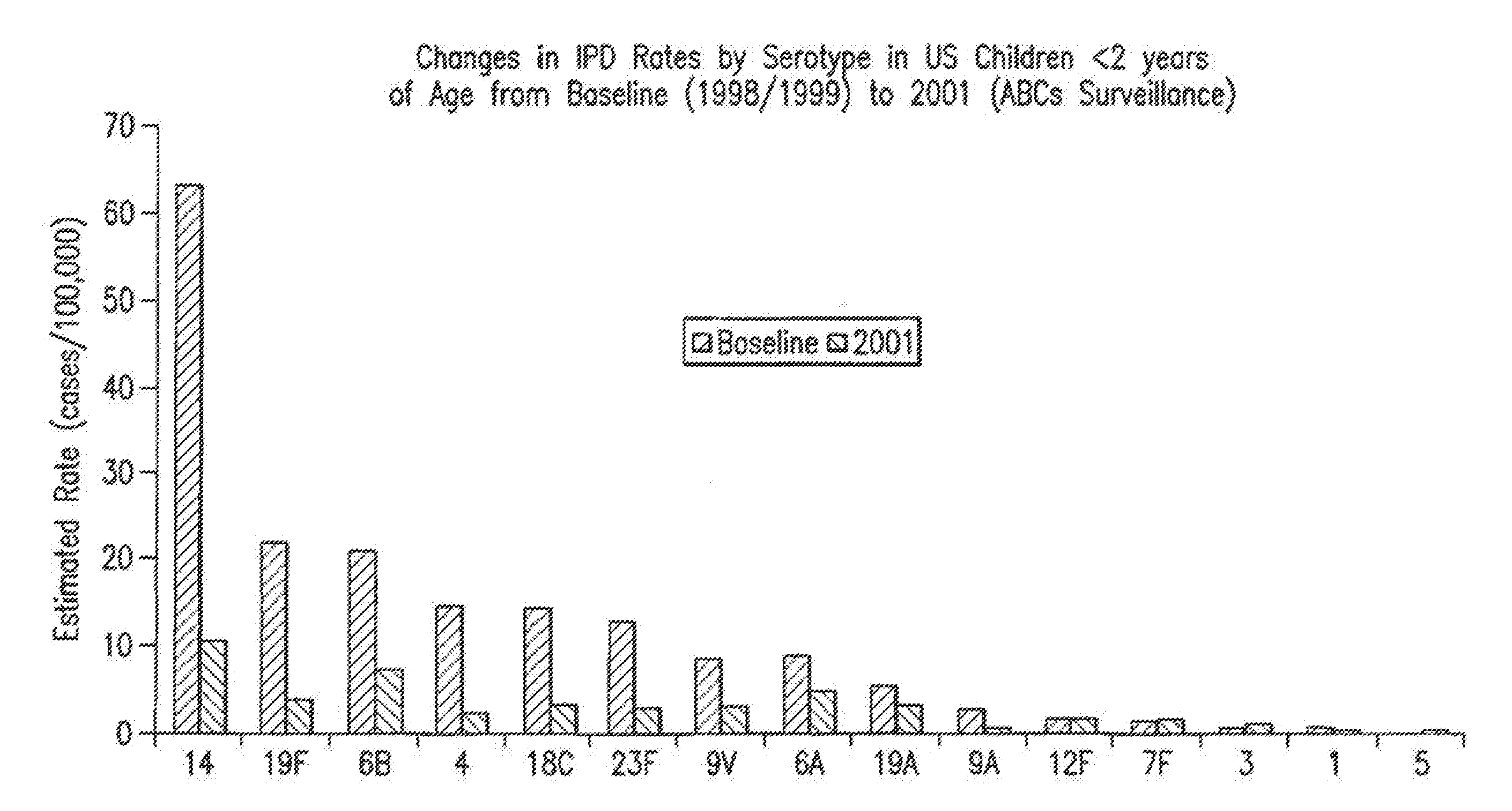Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
277 results about "Coccidia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Coccidia (Coccidiasina) are a subclass of microscopic, spore-forming, single-celled obligate intracellular parasites belonging to the apicomplexan class Conoidasida. As obligate intracellular parasites, they must live and reproduce within an animal cell. Coccidian parasites infect the intestinal tracts of animals, and are the largest group of apicomplexan protozoa.
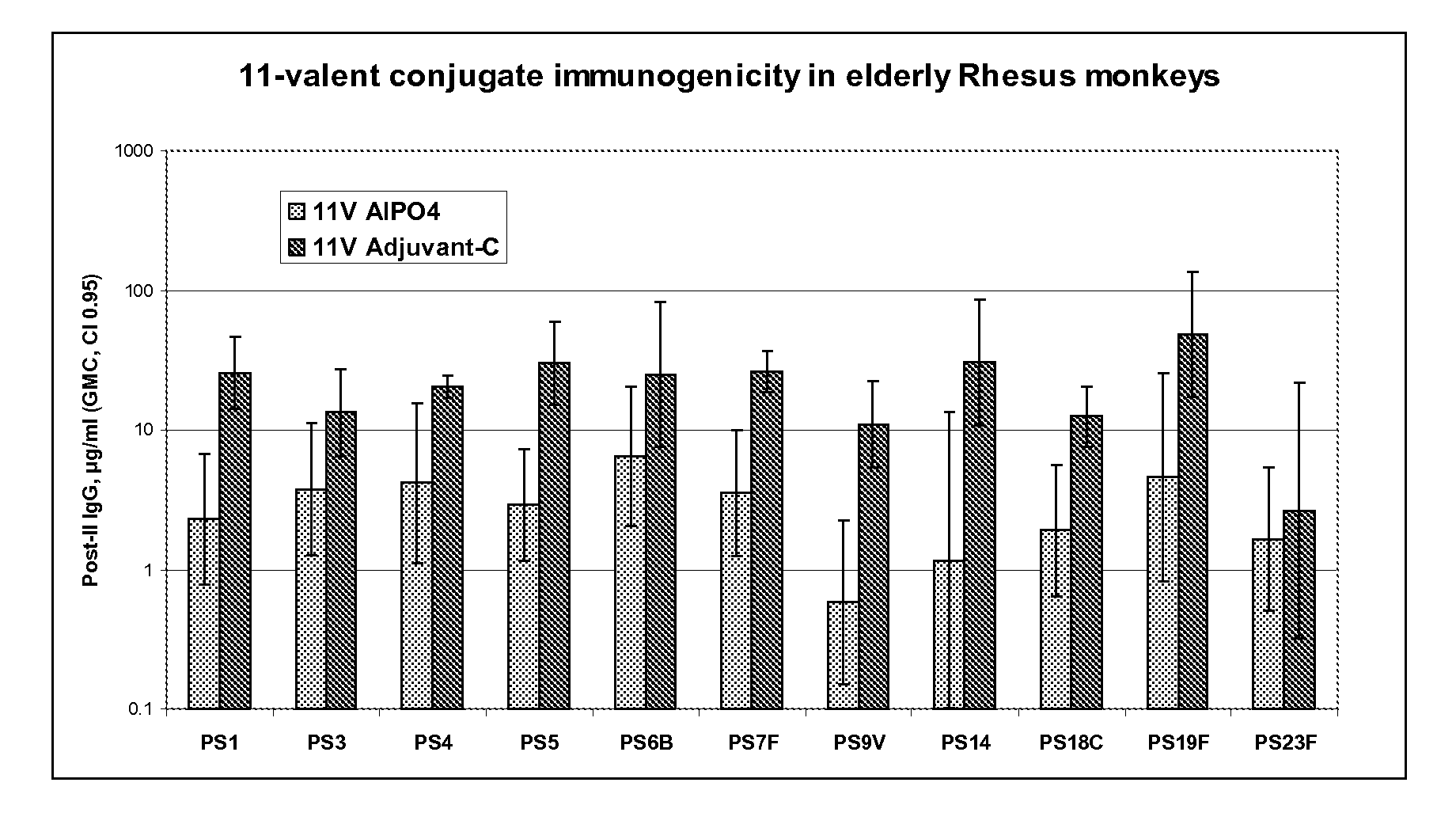
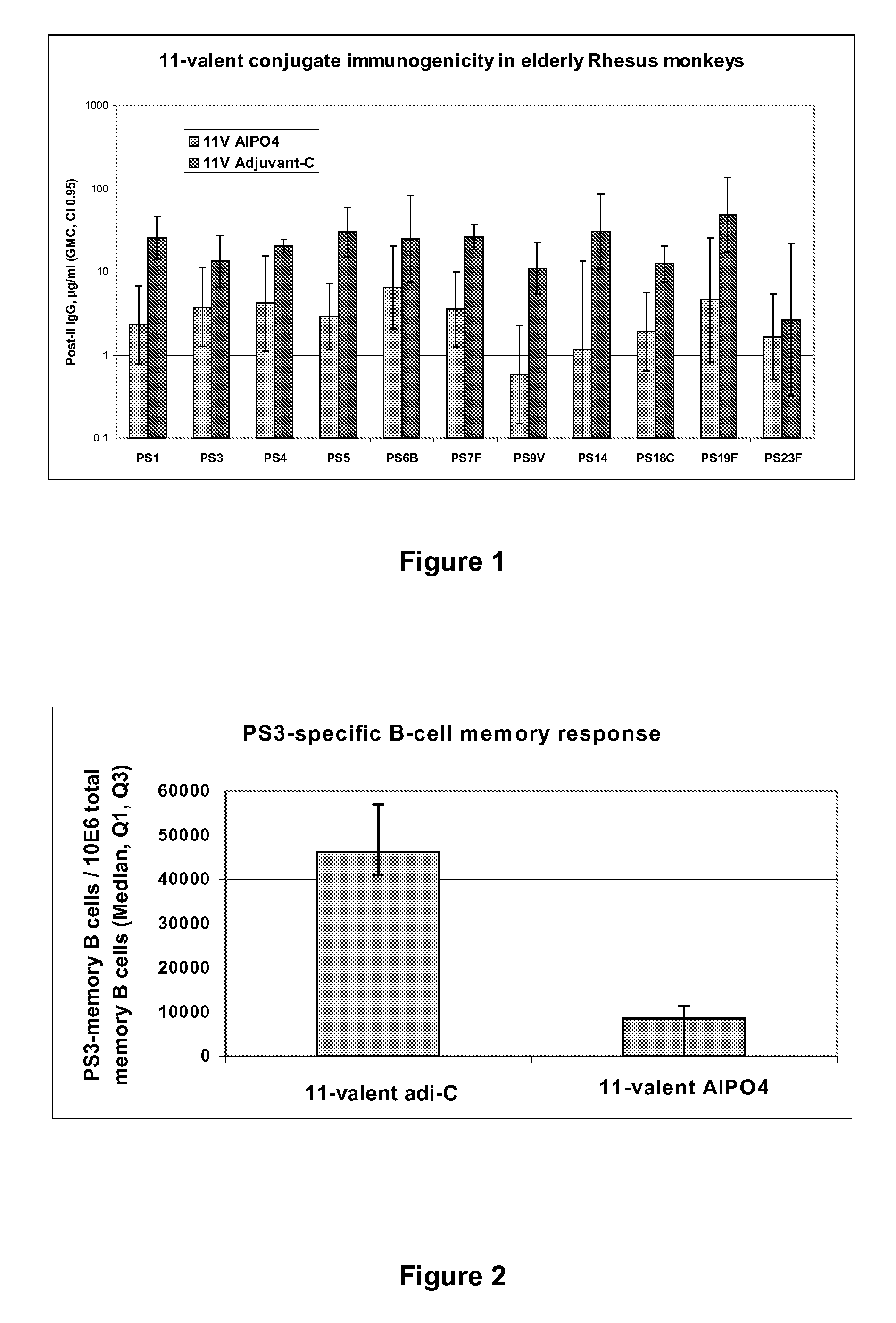
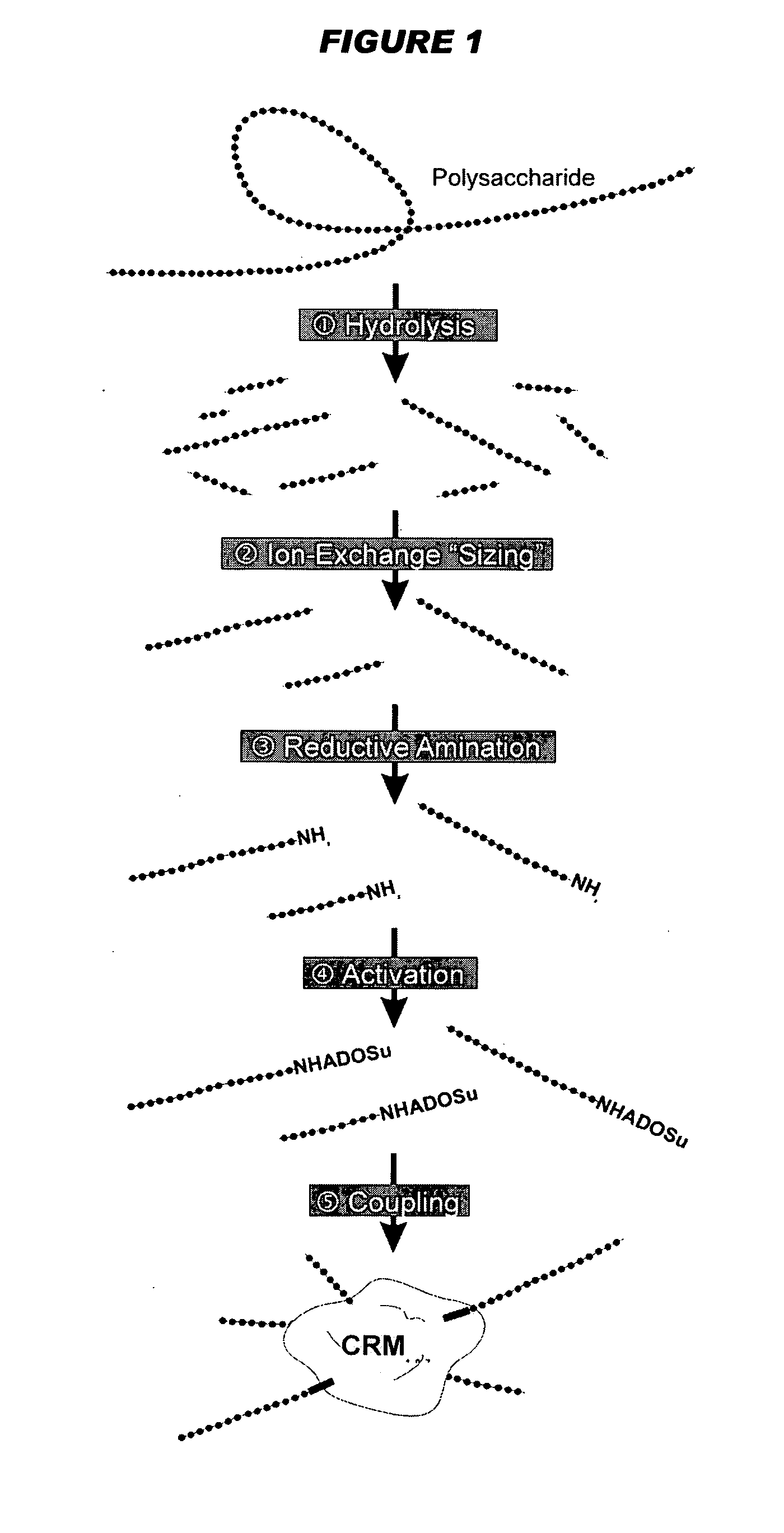
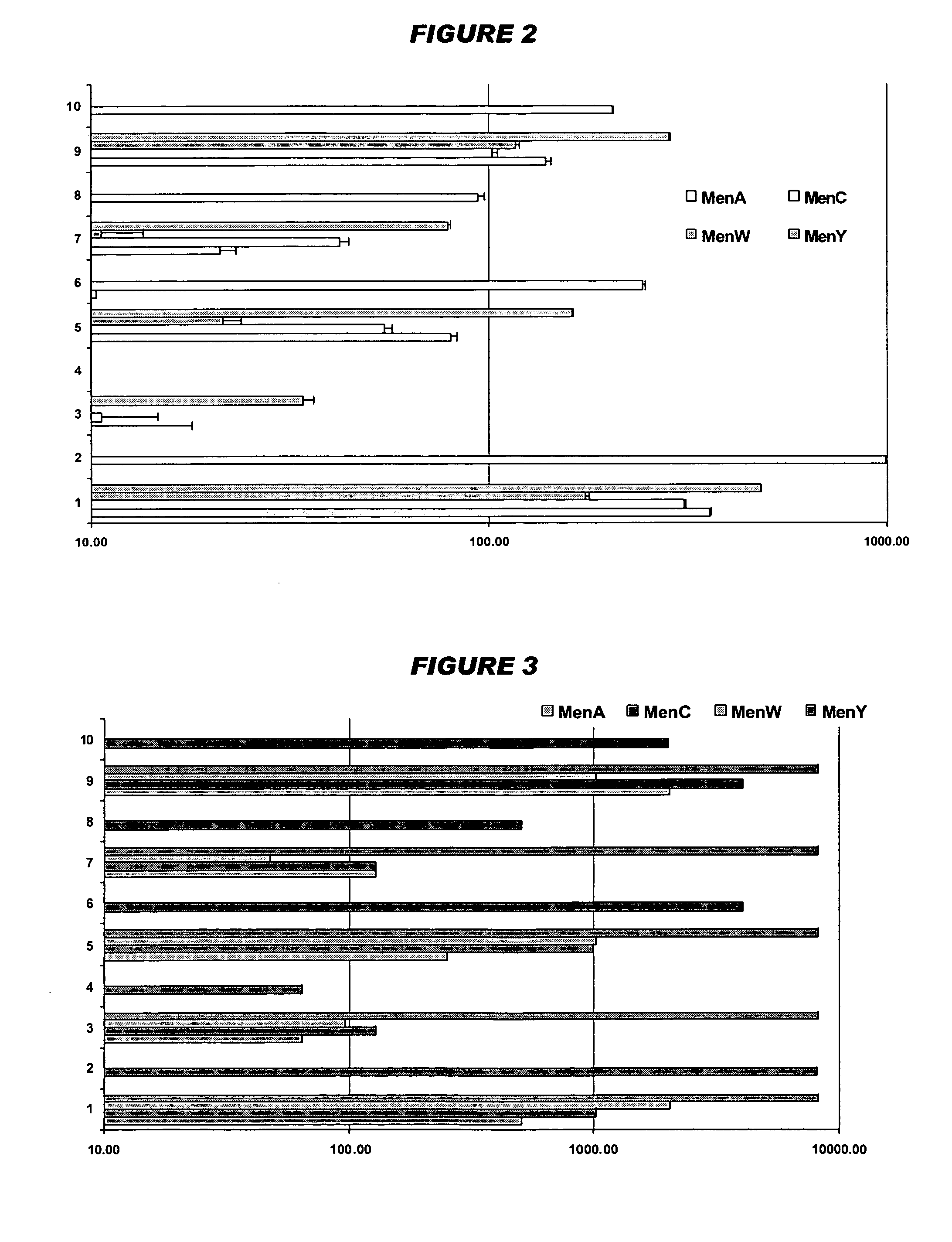
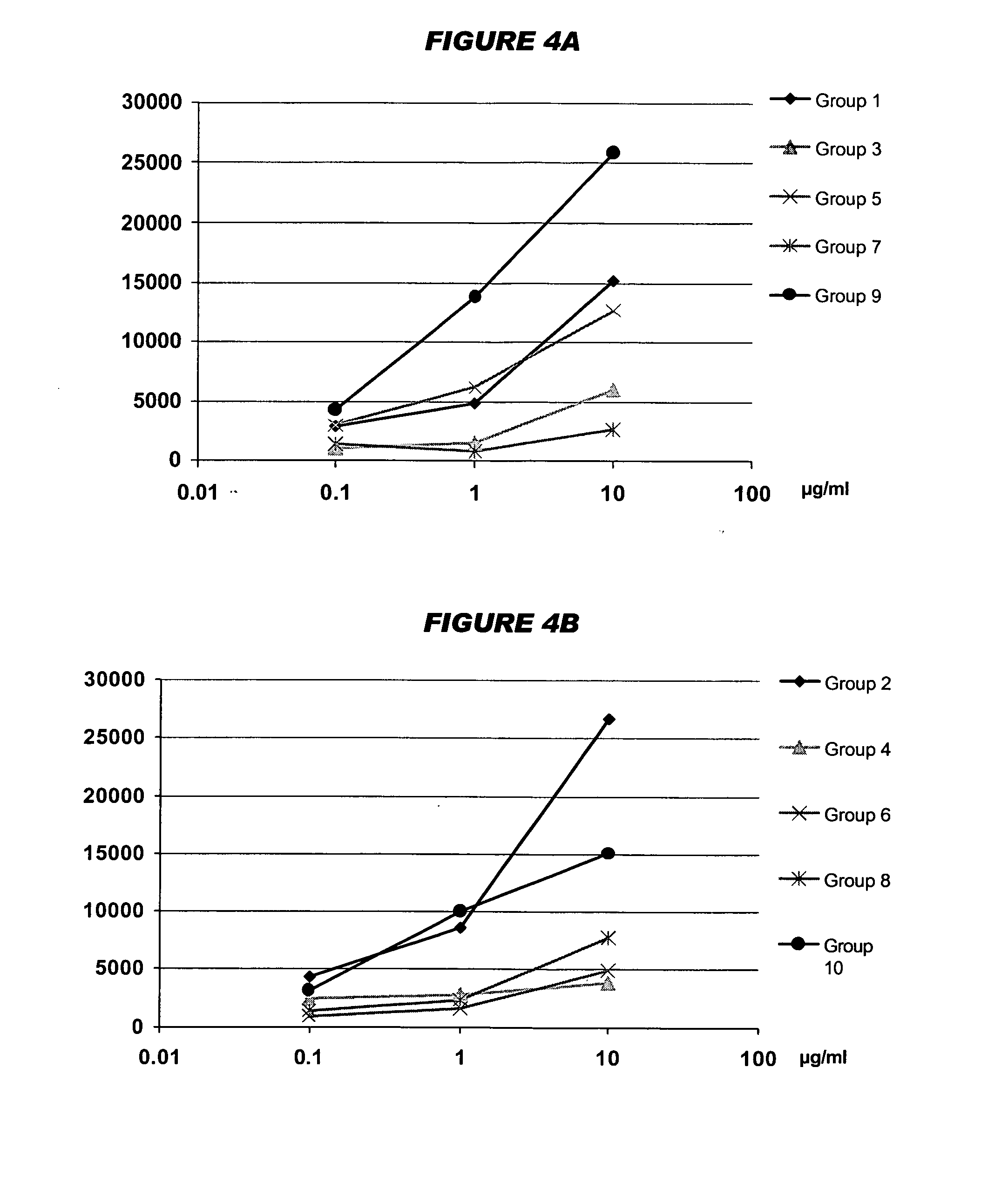
Multivalent pneumococcal polysaccharide-protein conjugate composition
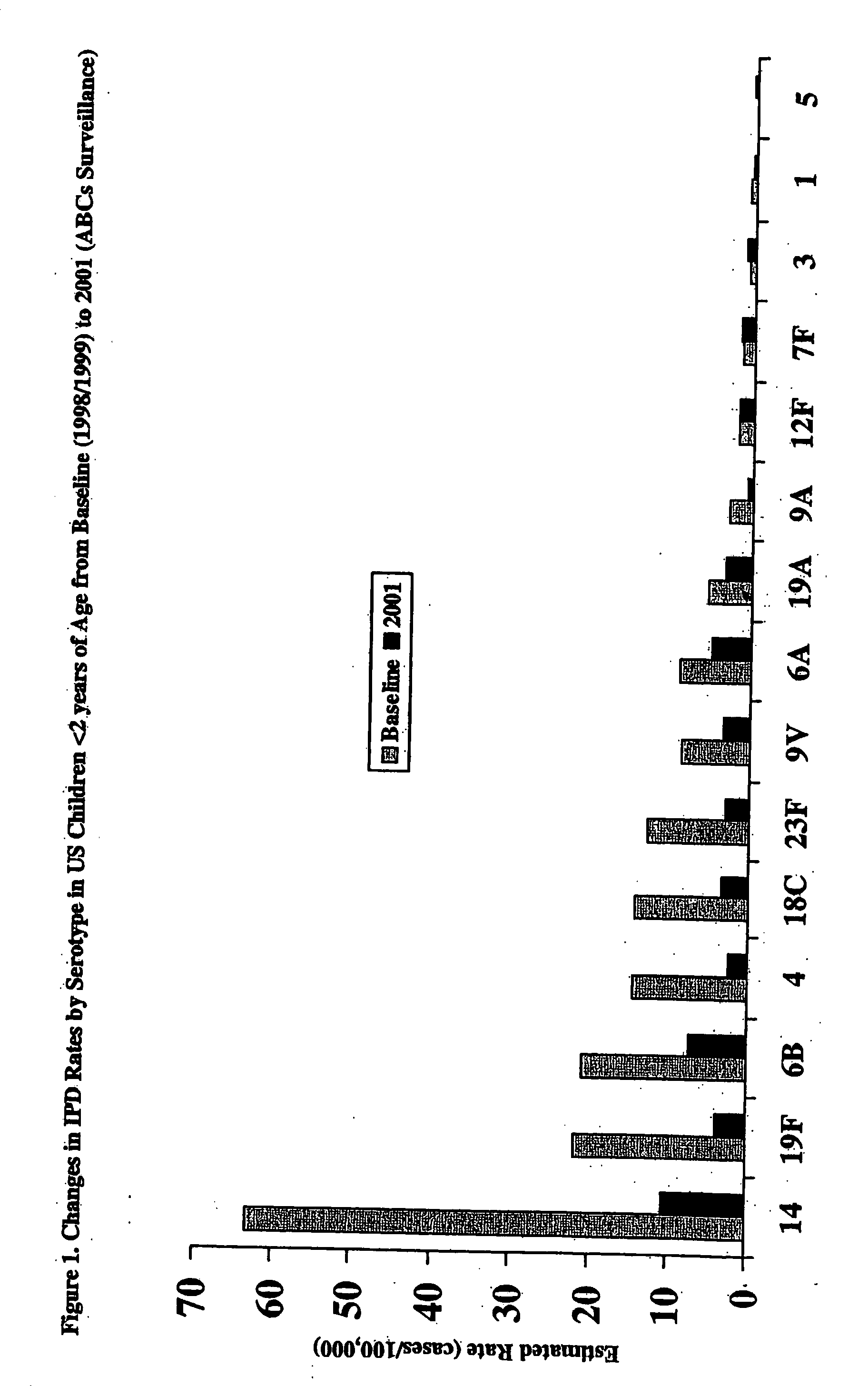
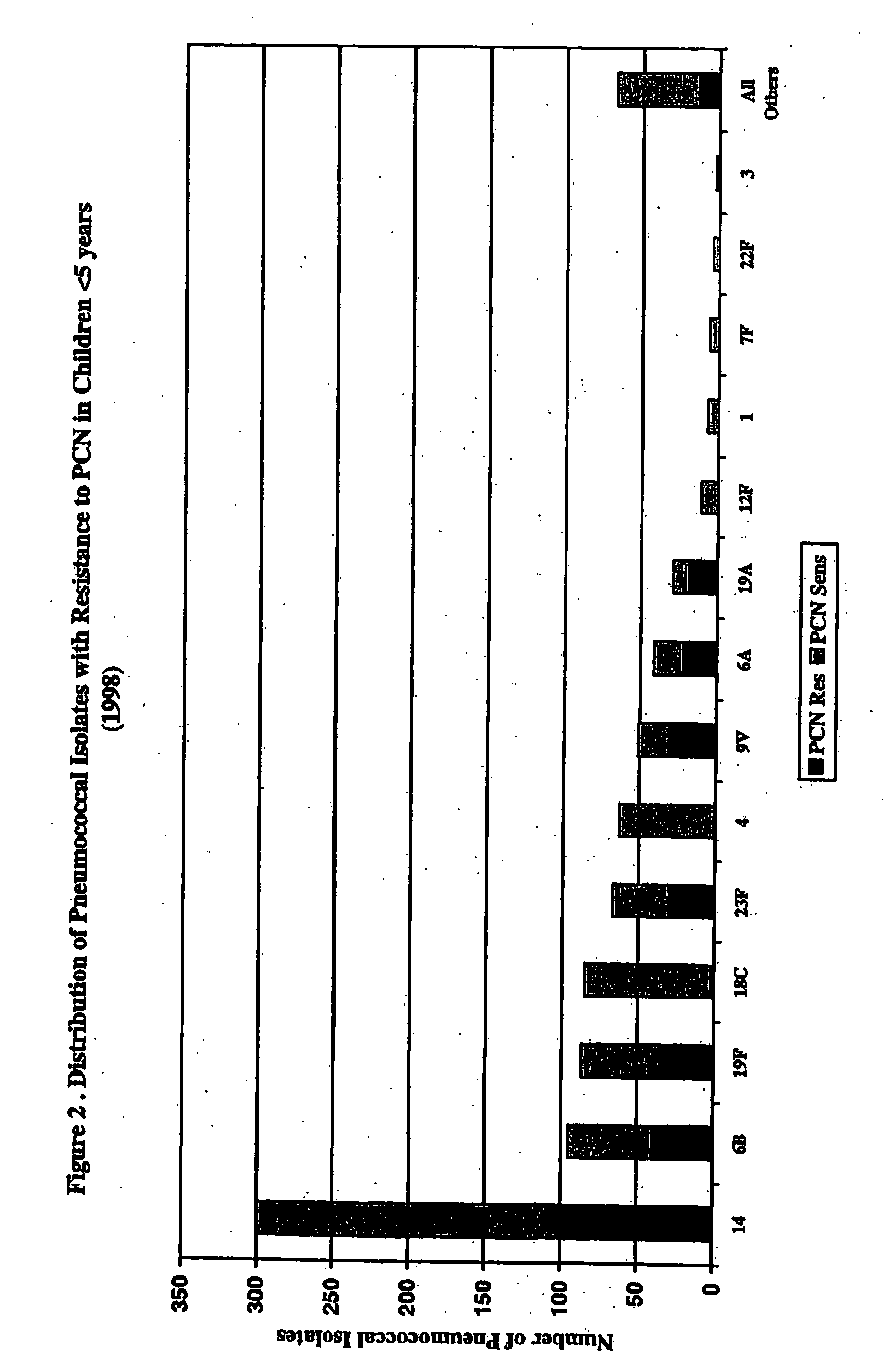
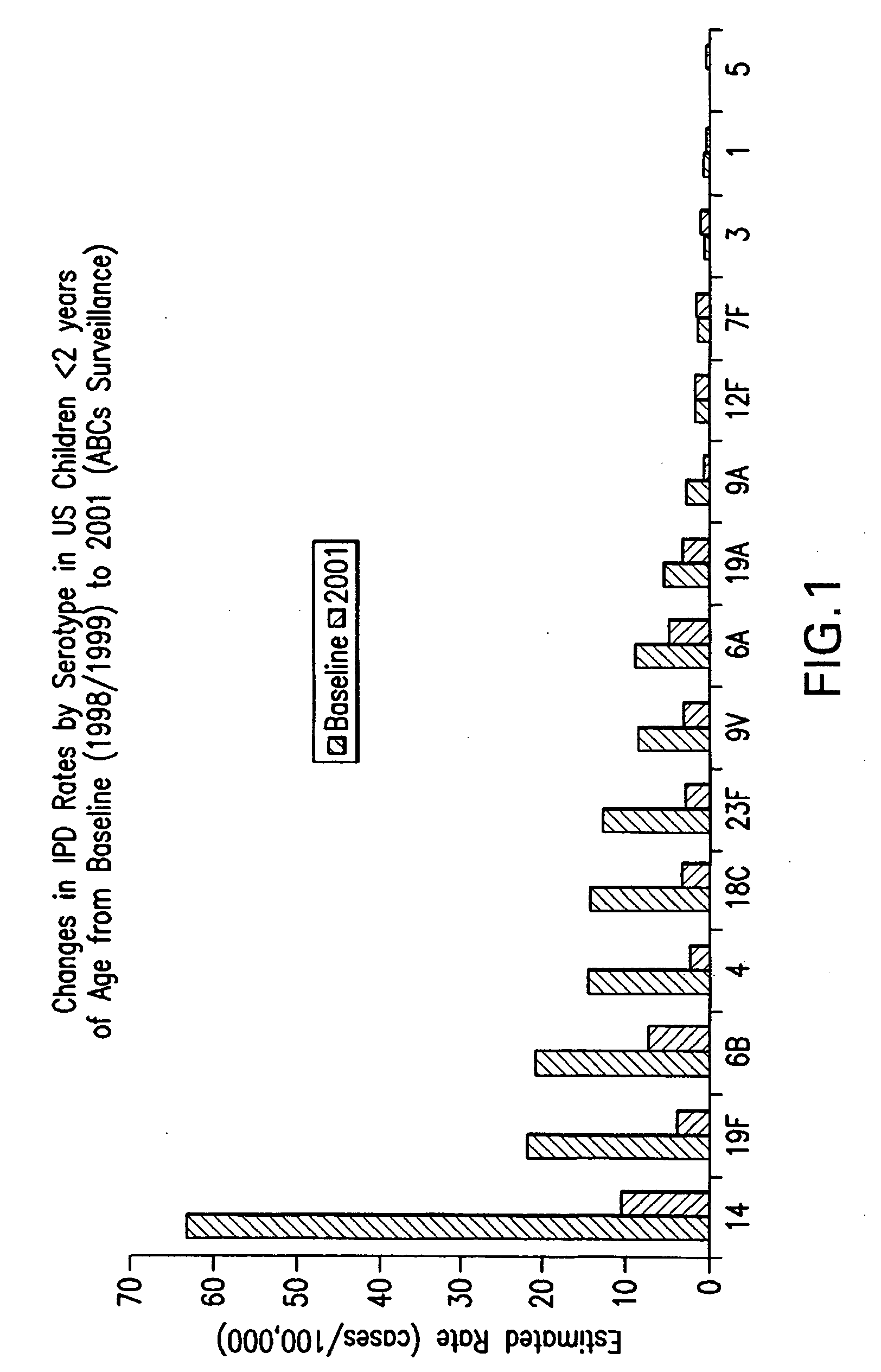
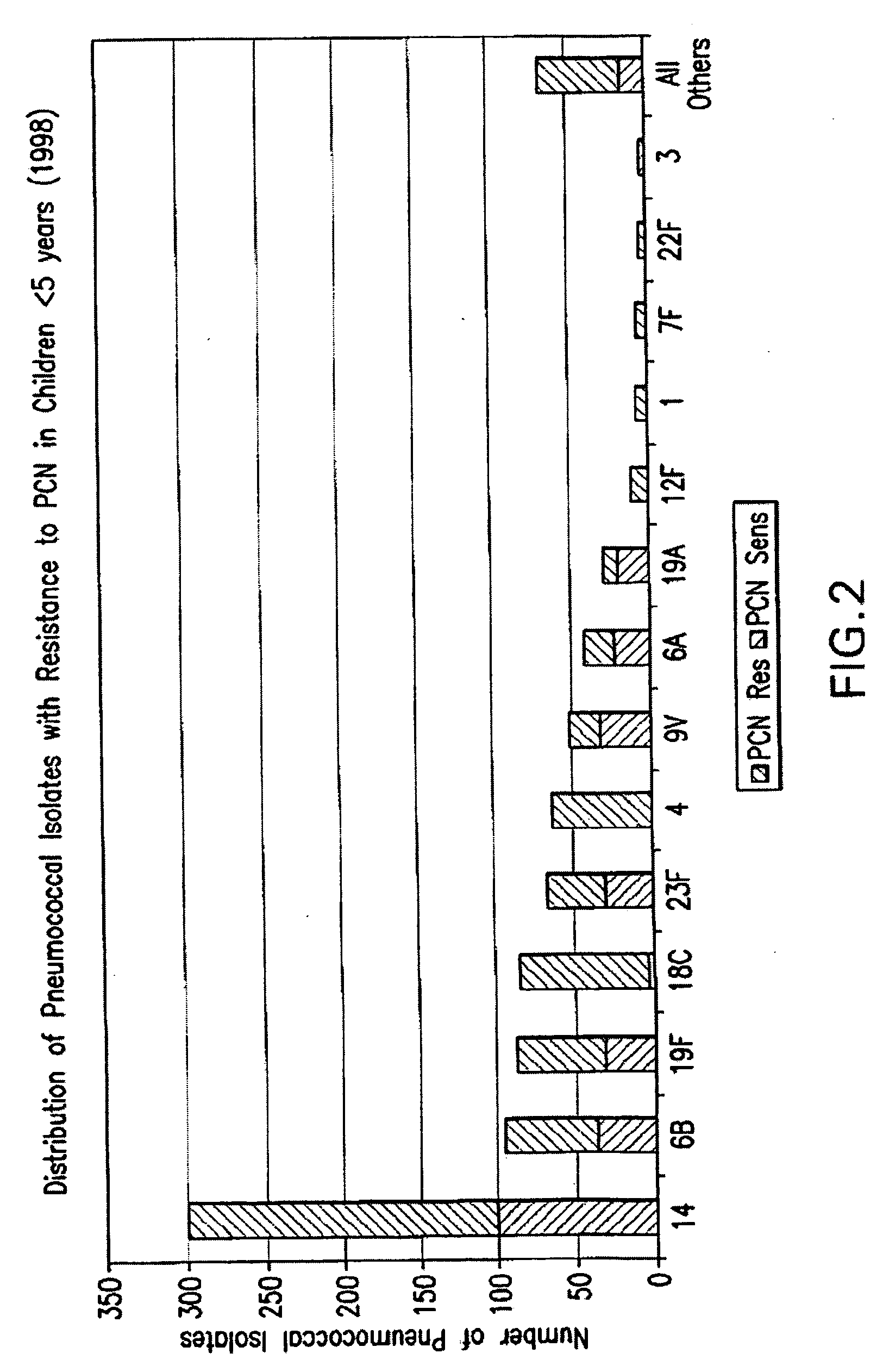
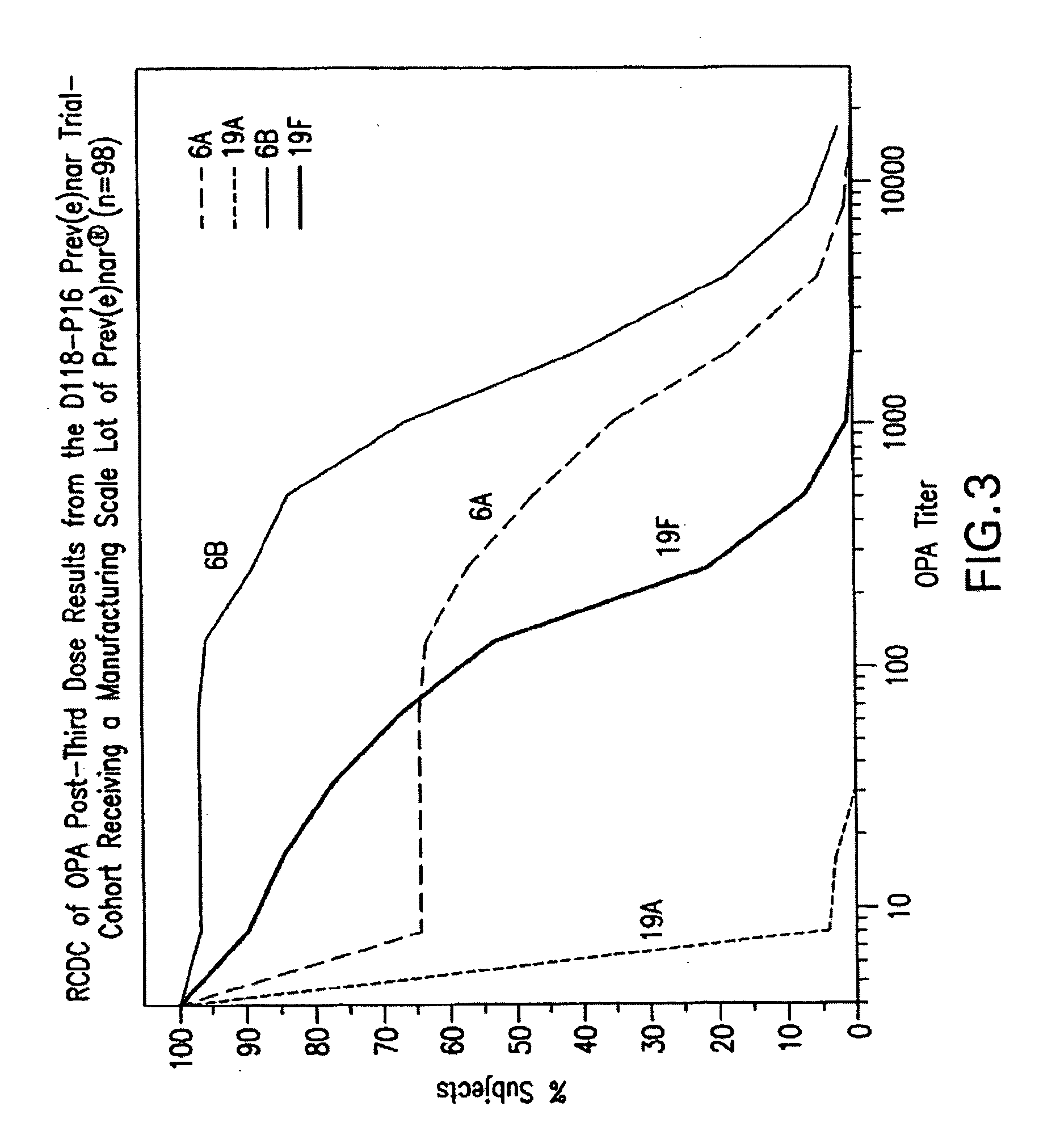
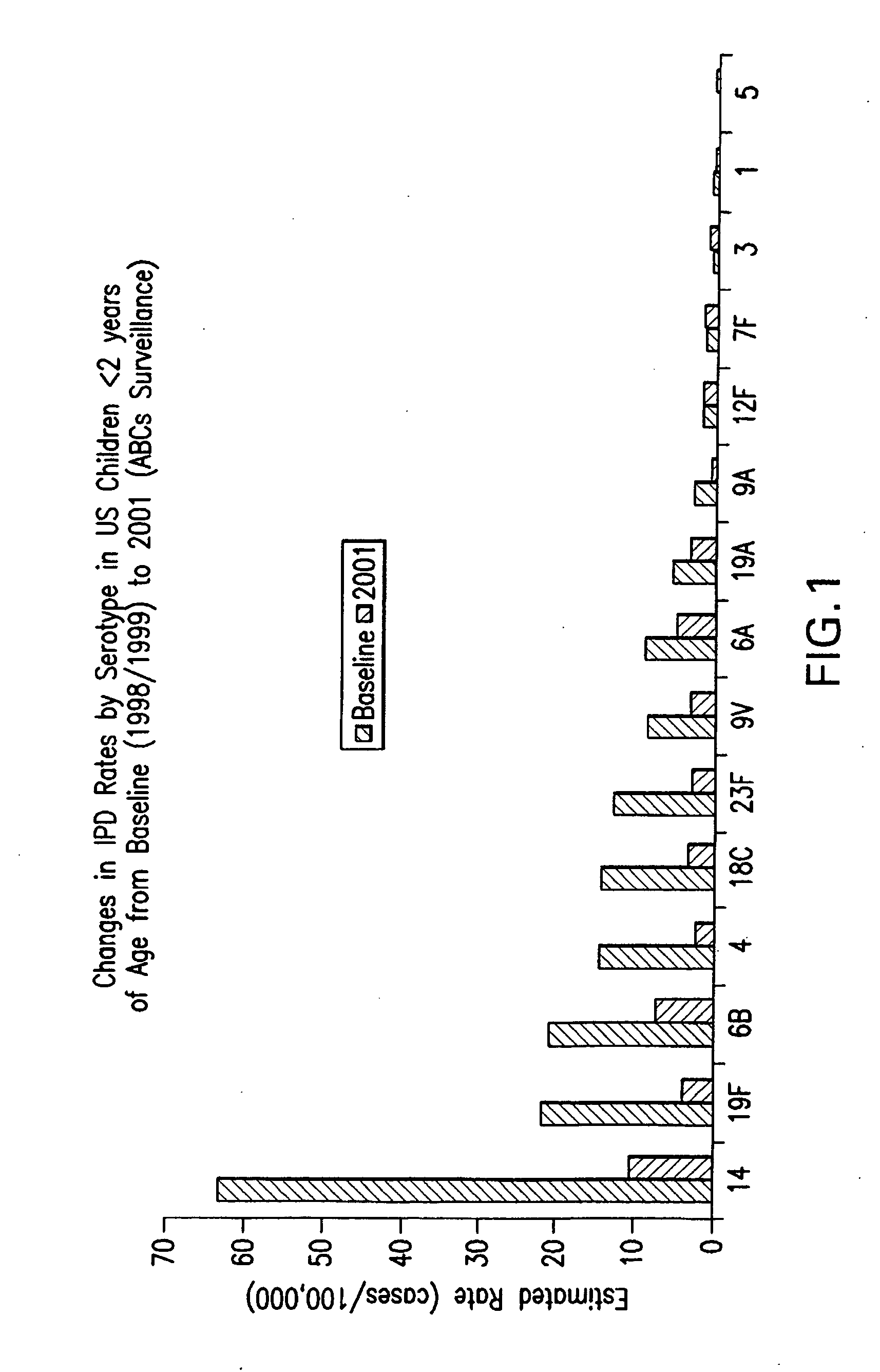
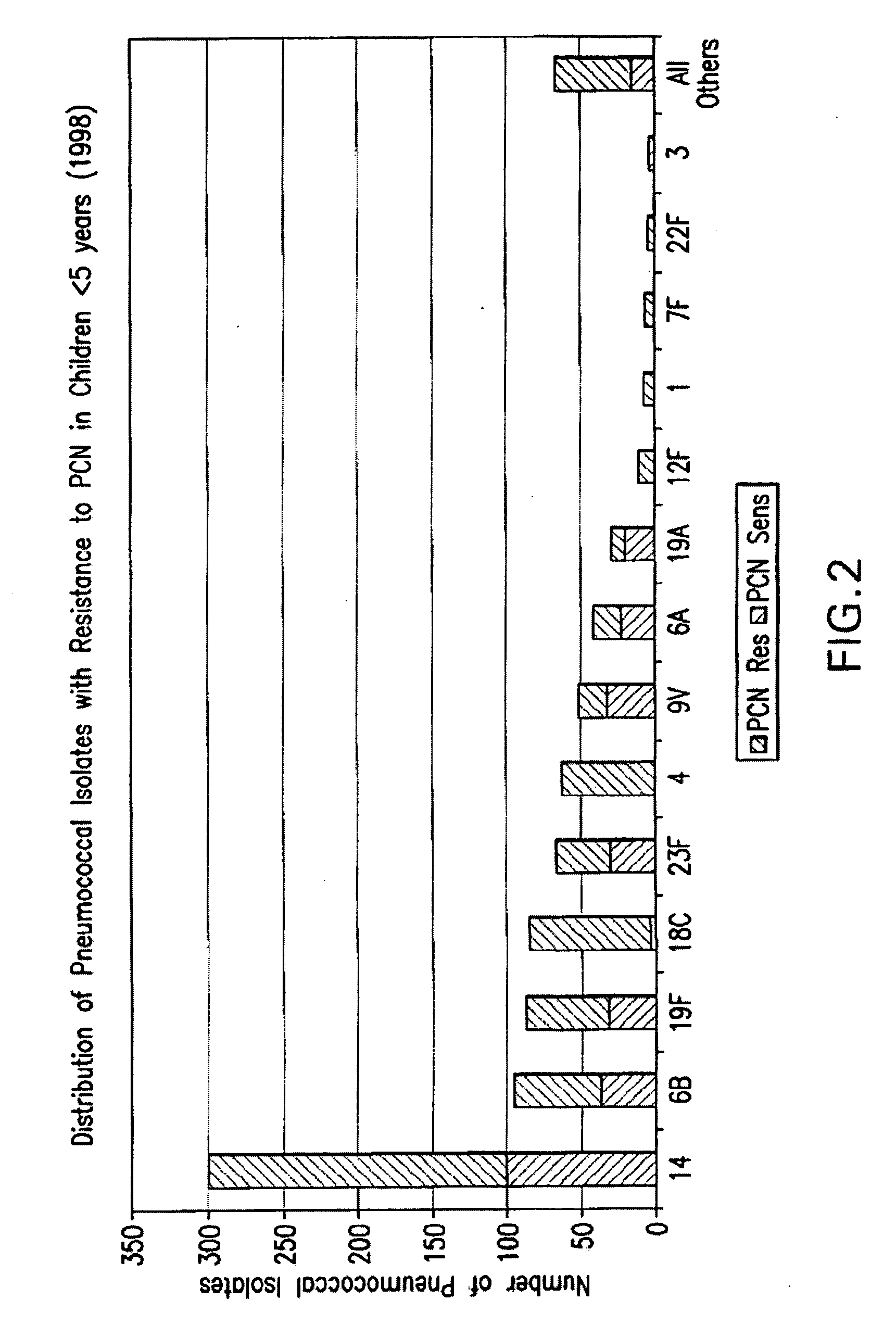
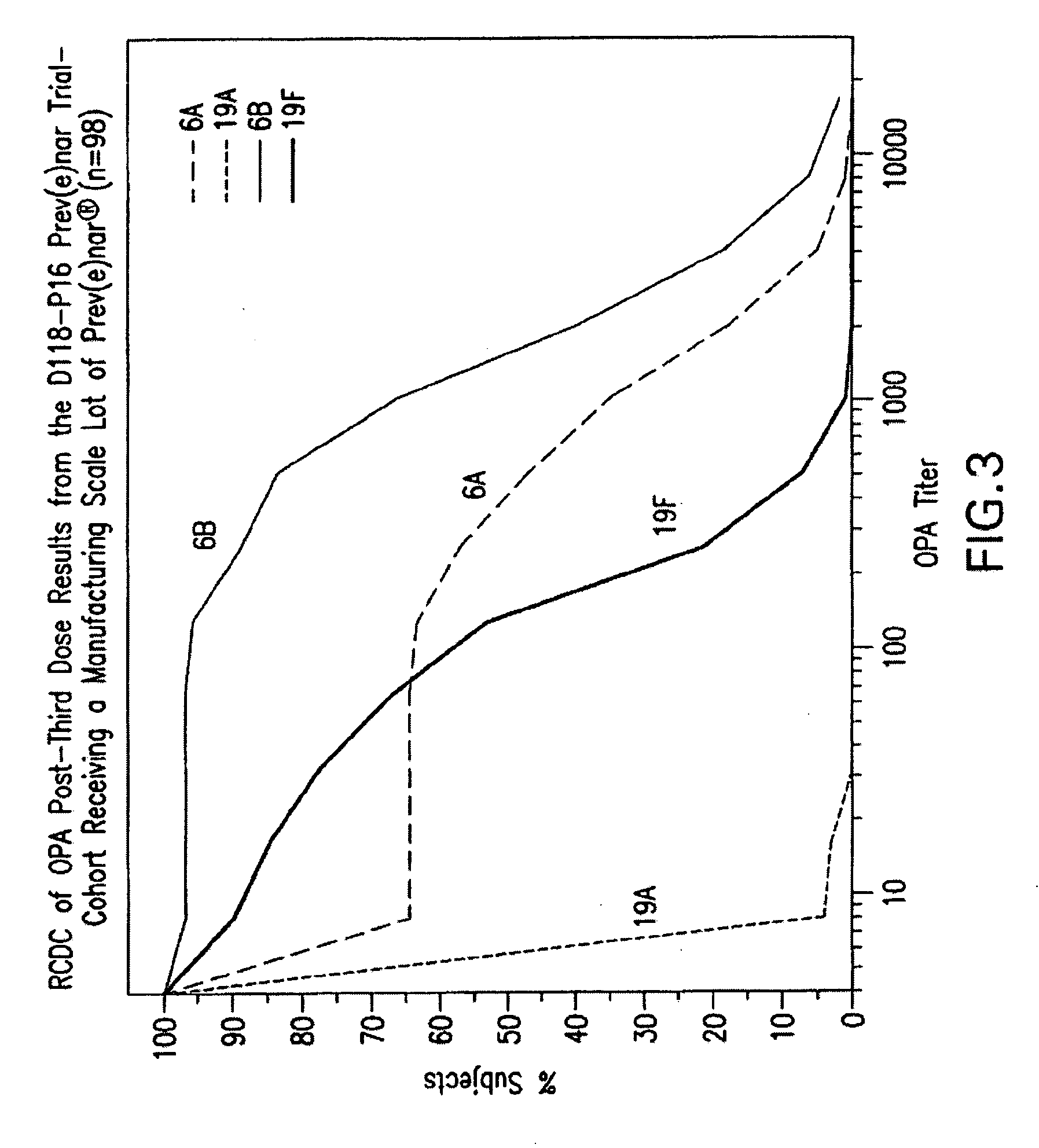
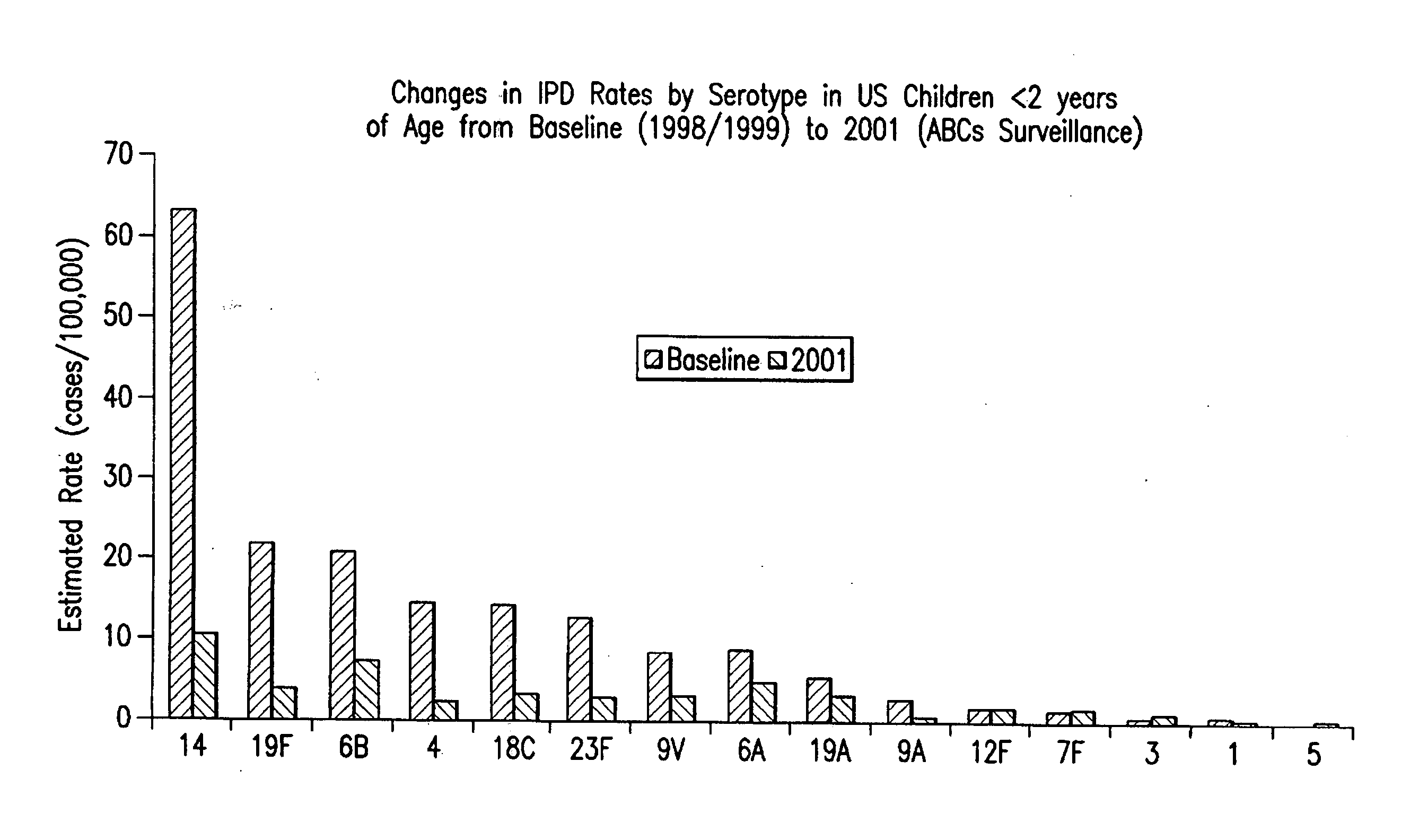
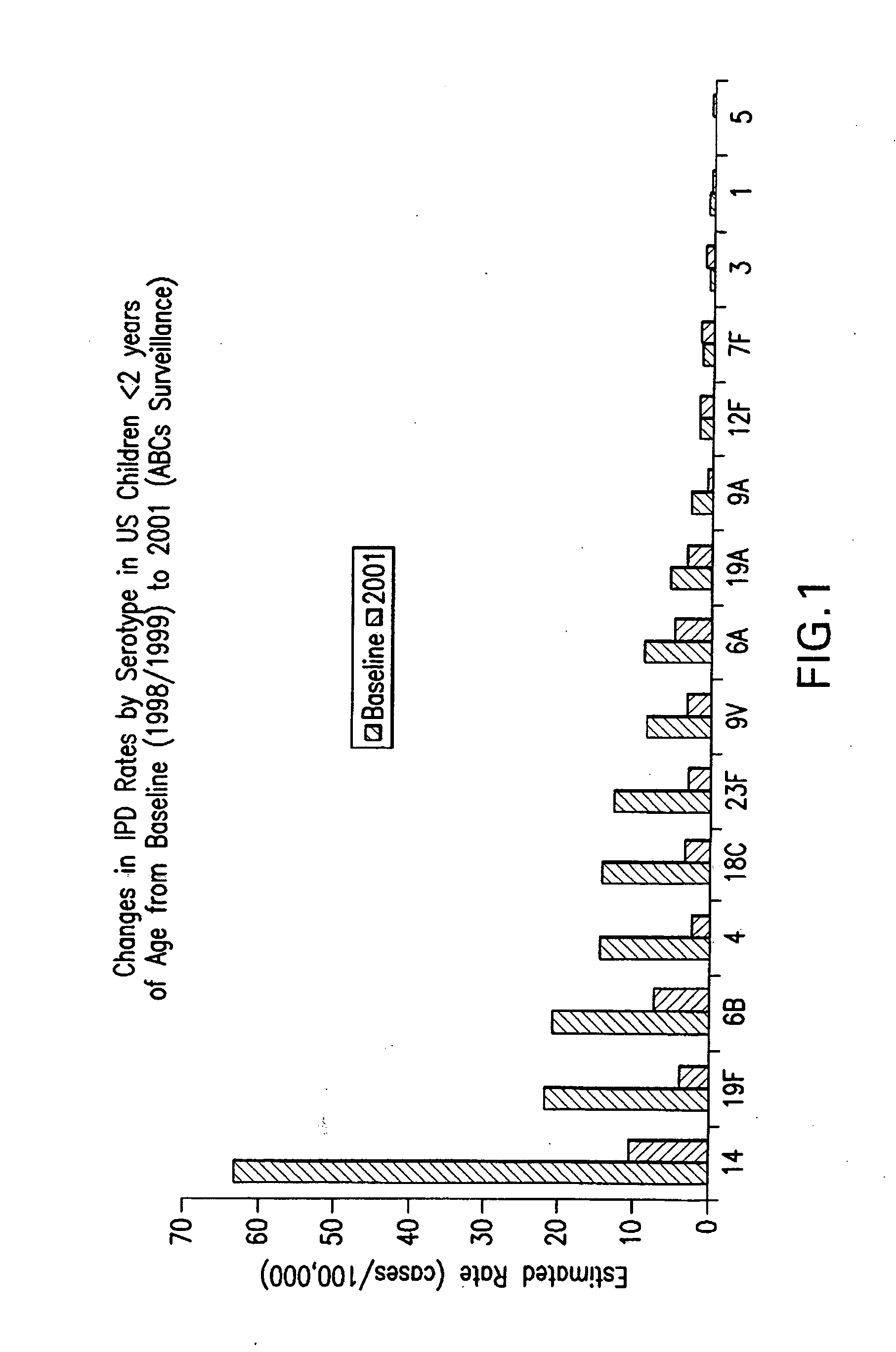
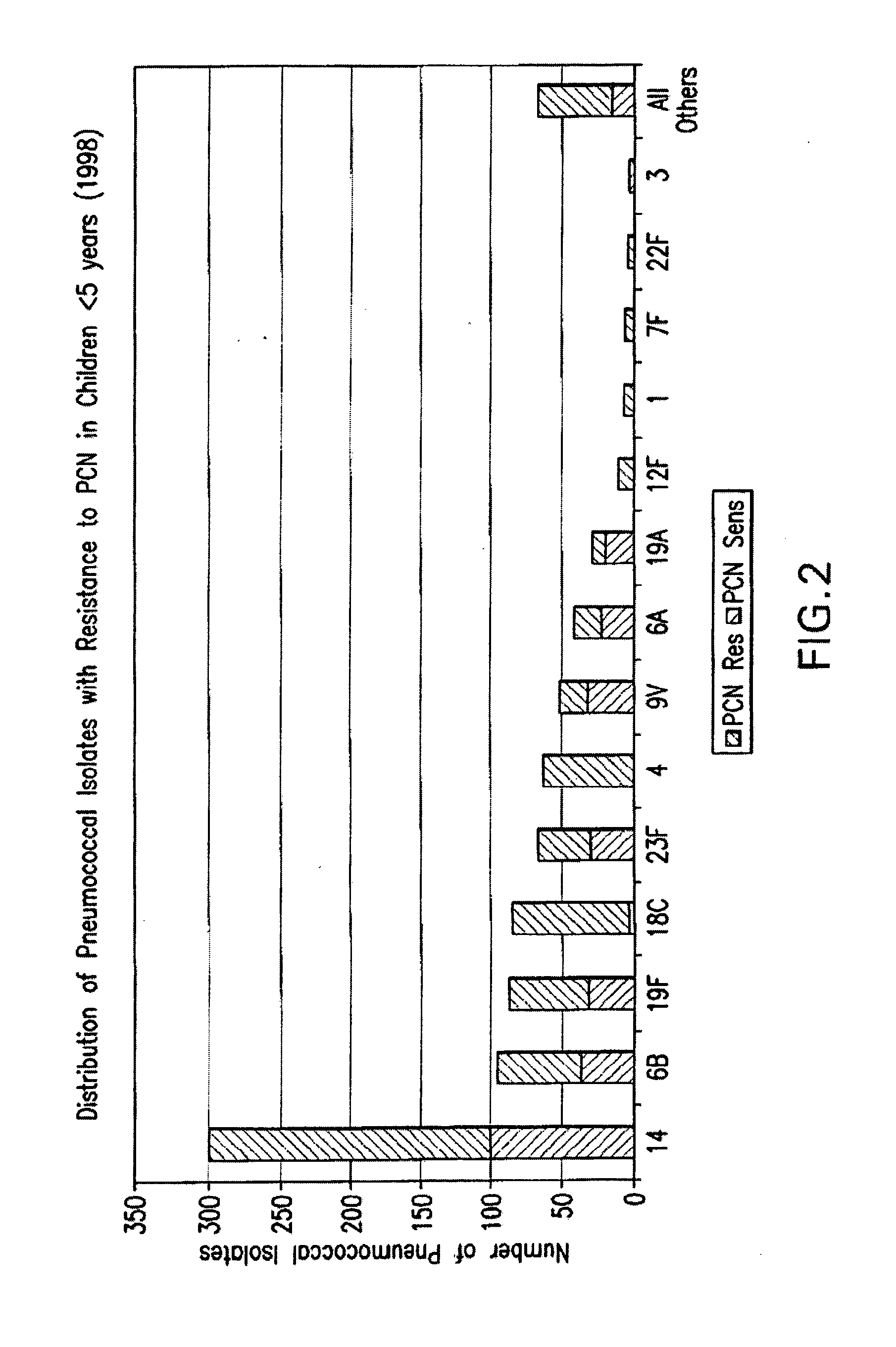
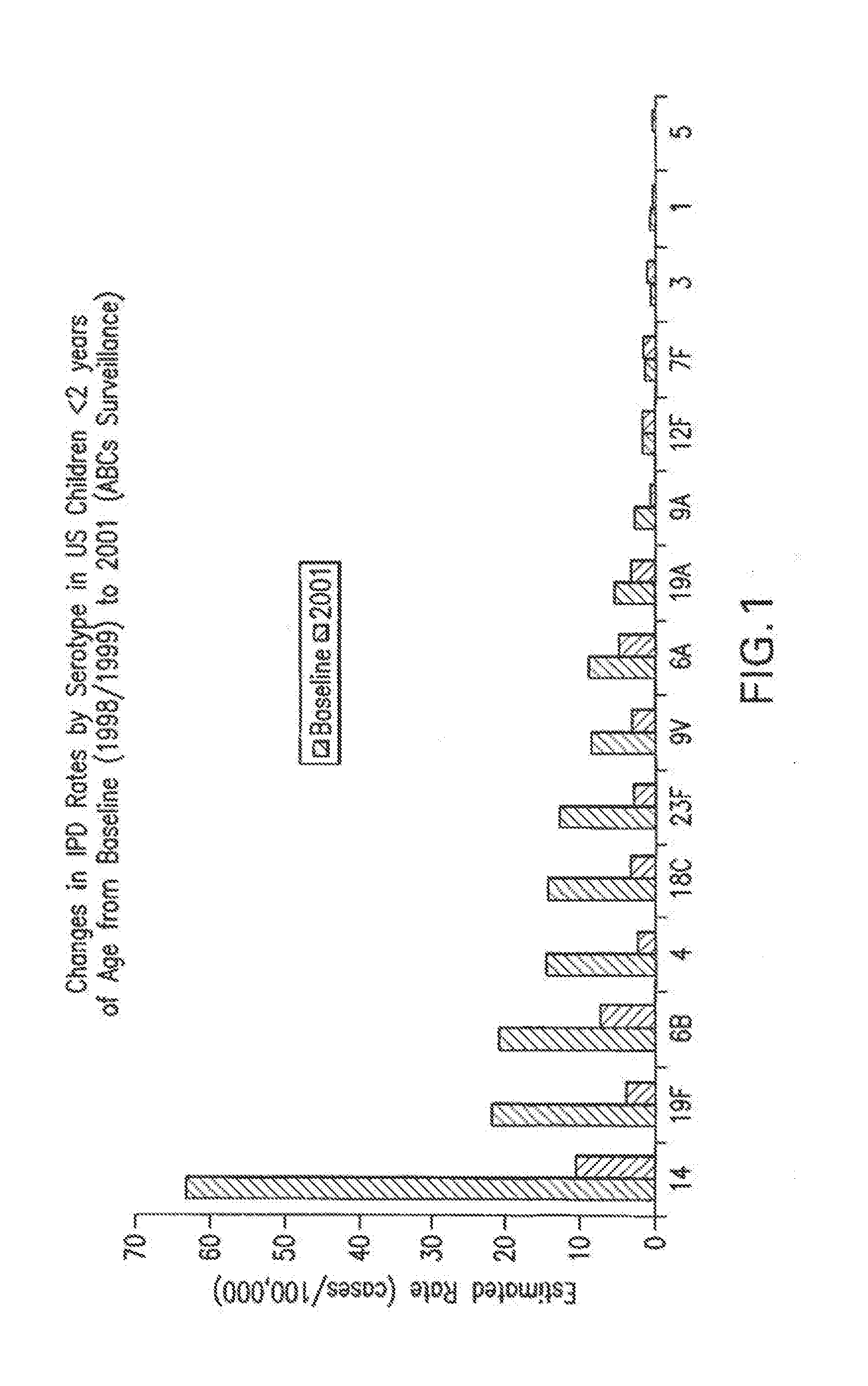
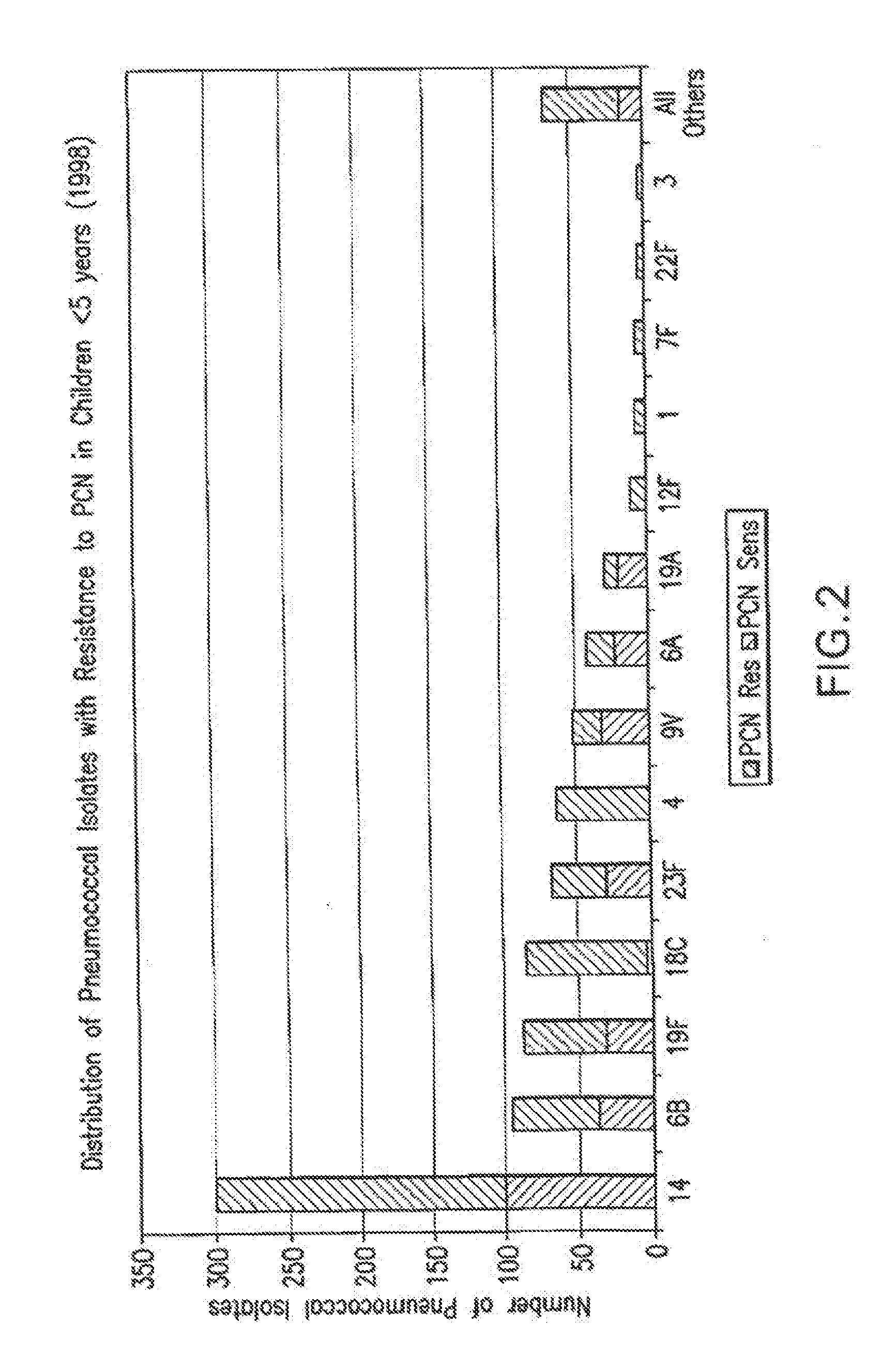
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
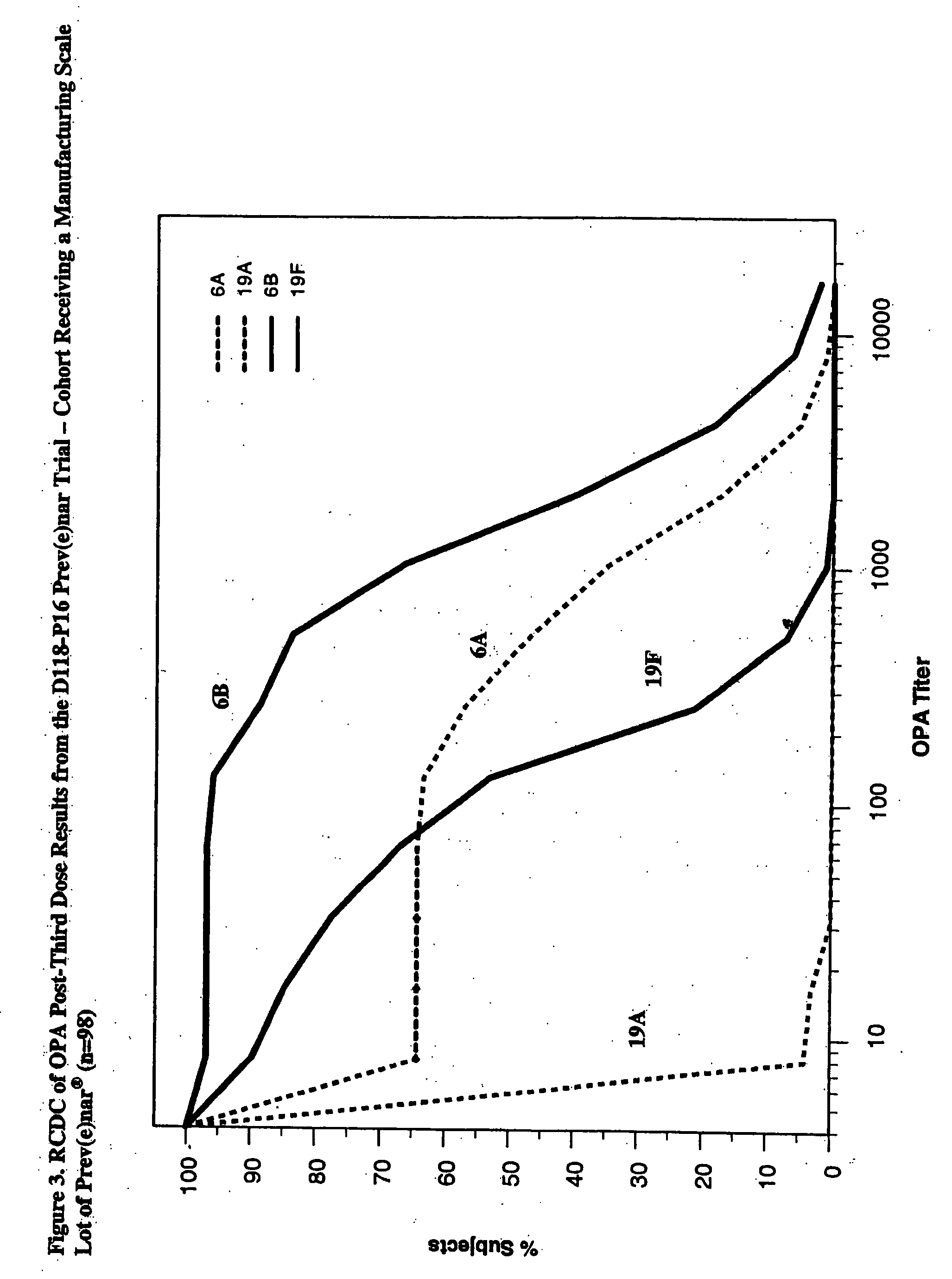
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 1 polysaccharide covalently linked to a carrier protein, the method including partial de-O-acetylation of the polysaccharide by mild hydrolysis in an alkaline pH buffer.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
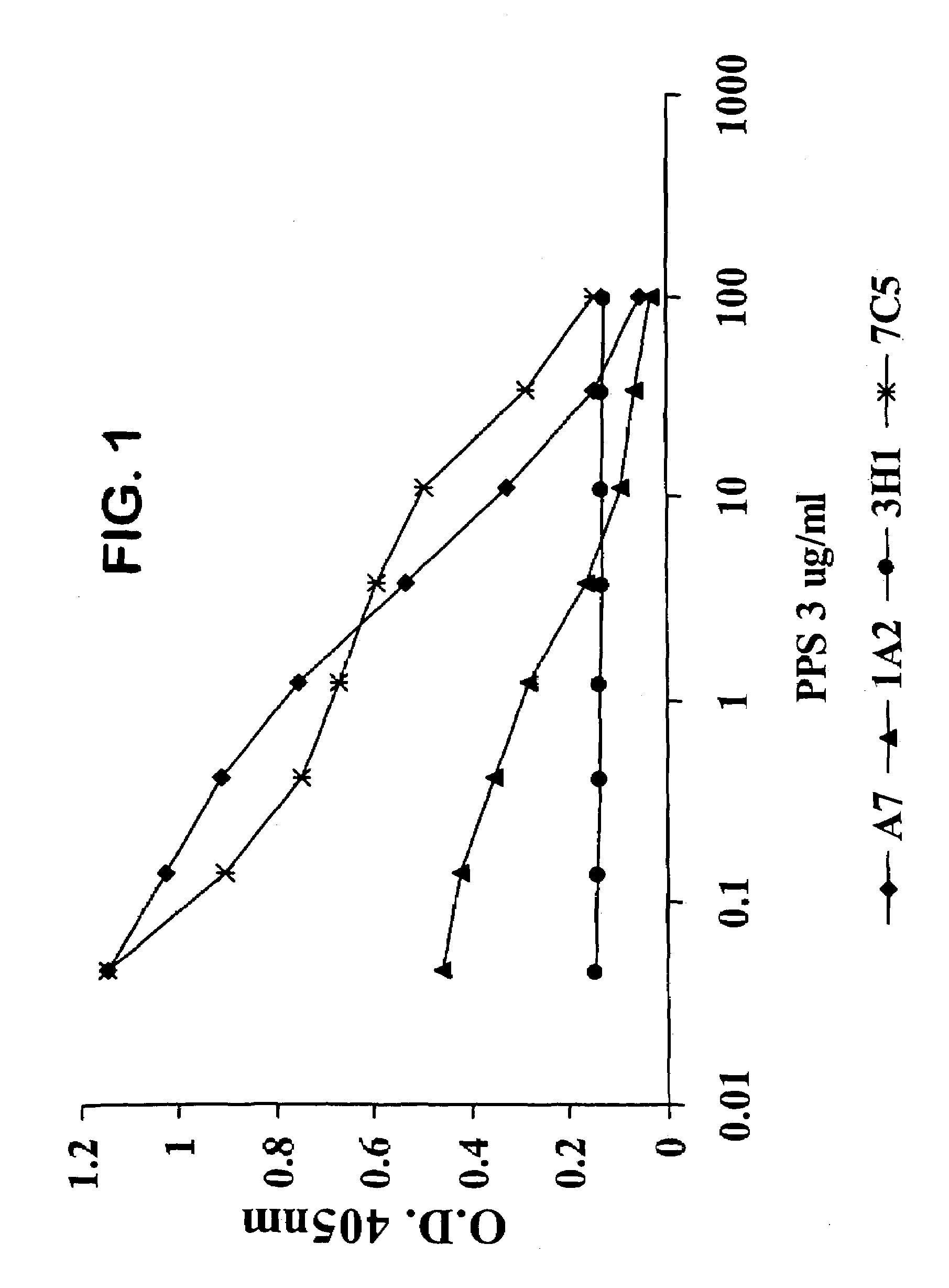
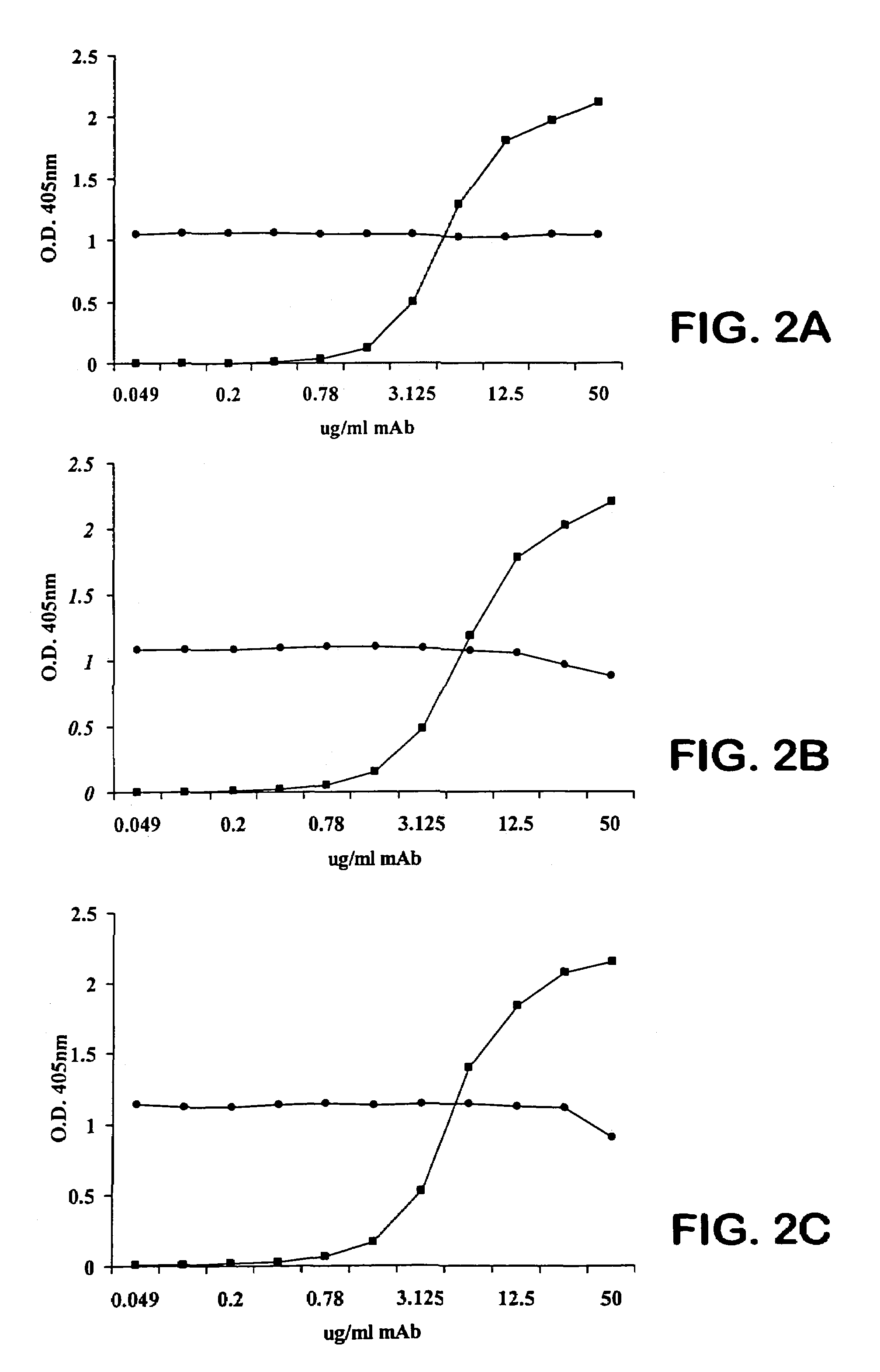
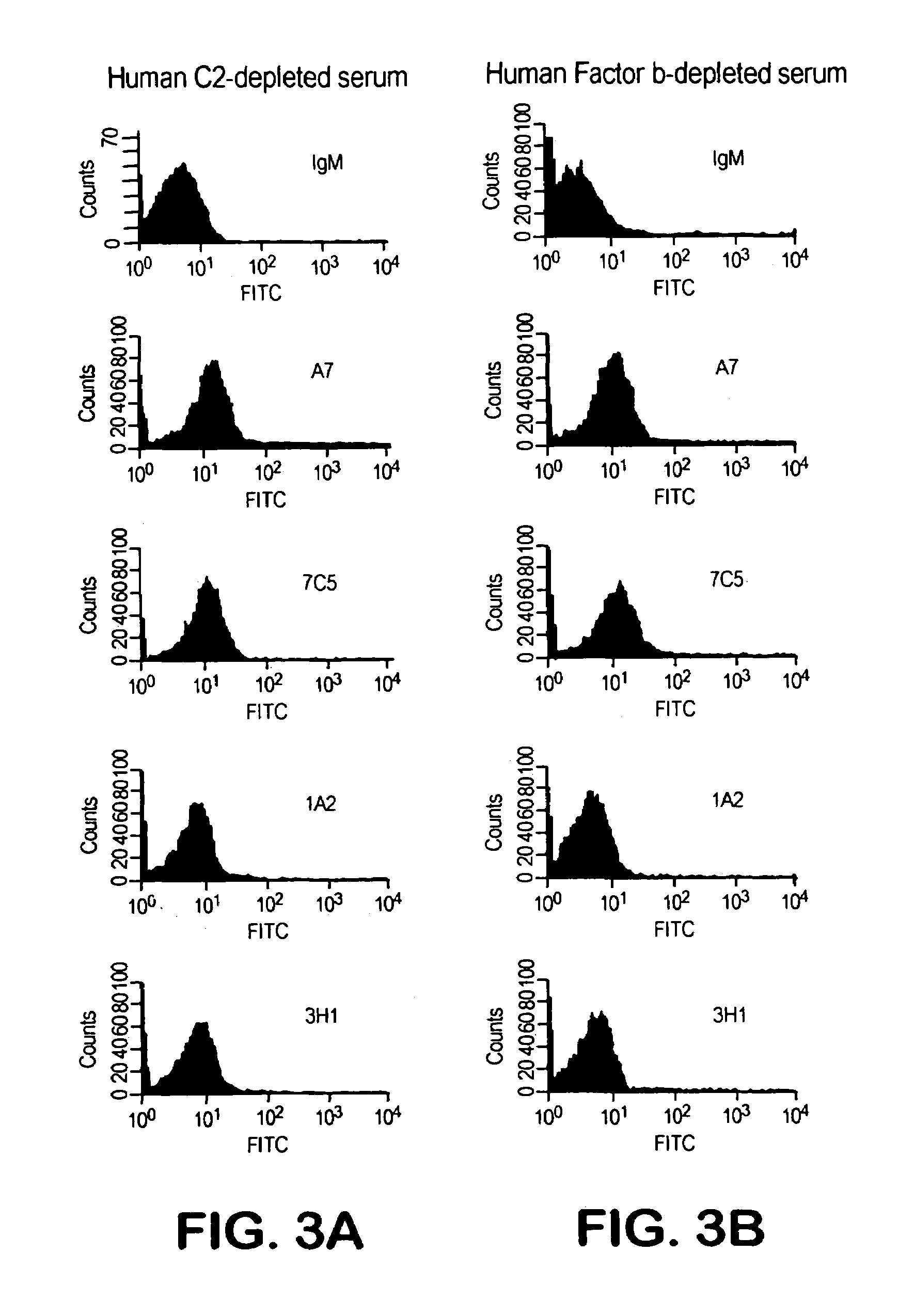
Human antipneumococcal antibodies from non-human animals
The invention described herein provides human antibodies produced in non-human animals that specifically bind to Streptococcus pneumoniae capsular polysaccharide (PPS-3). The invention further provides methods for making the antibodies in a non-human animal and for expressing the antibodies in cells including hybridomas and recombinant host cell systems. Kits and pharmaceutical compositions comprising the antibodies are also provided in addition to methods of treating, inhibitng or preventing S. pneumoniae infection or conditions or disorders caused by such infection by administering to a patient the pharmaceutical compositions described herein.
Owner:ABQENIX INC
Meningococcal antigens
InactiveUS6709660B1Easy to insertEfficient HarvestingAntibacterial agentsOrganic active ingredientsCoccidiaNucleotide
The invention provides proteins from Neisseria meningitidis (strains A & B), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics.
Owner:NOVARTIS AG
High-efficiency 14-valent pneumococcal conjugate vaccine
InactiveCN101590224AEffective protectionAntibacterial agentsBacterial antigen ingredientsCoccidiaHaemophilus
The invention relates to a high-efficiency 14-valent pneumococcal conjugate vaccine, which is formed by coupling and combining capsular polysaccharide extracted from fourteen types of serotype pneumococcuses and carrier protein; the fourteen types of the serotype pneumococcuses are 1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F; and the carrier protein is diphtherin non-toxic mutated protein CRM197, tetanus toxin protein and influenza haemophilus protein D. Compared with the prior pneumococcal conjugate vaccine, the high-efficiency 14-valent pneumococcal conjugate vaccine has the advantages that: (1) the cross-protection rate of the pneumococcuses of fourteen serotypes (1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F) reaches more than 90 percent; and (2) the high-efficiency 14-valent pneumococcal conjugate vaccine can generate effective protection on senior citizens which are susceptible to pneumonia and is suitable for children under two years old.
Owner:广州精达医学科技有限公司
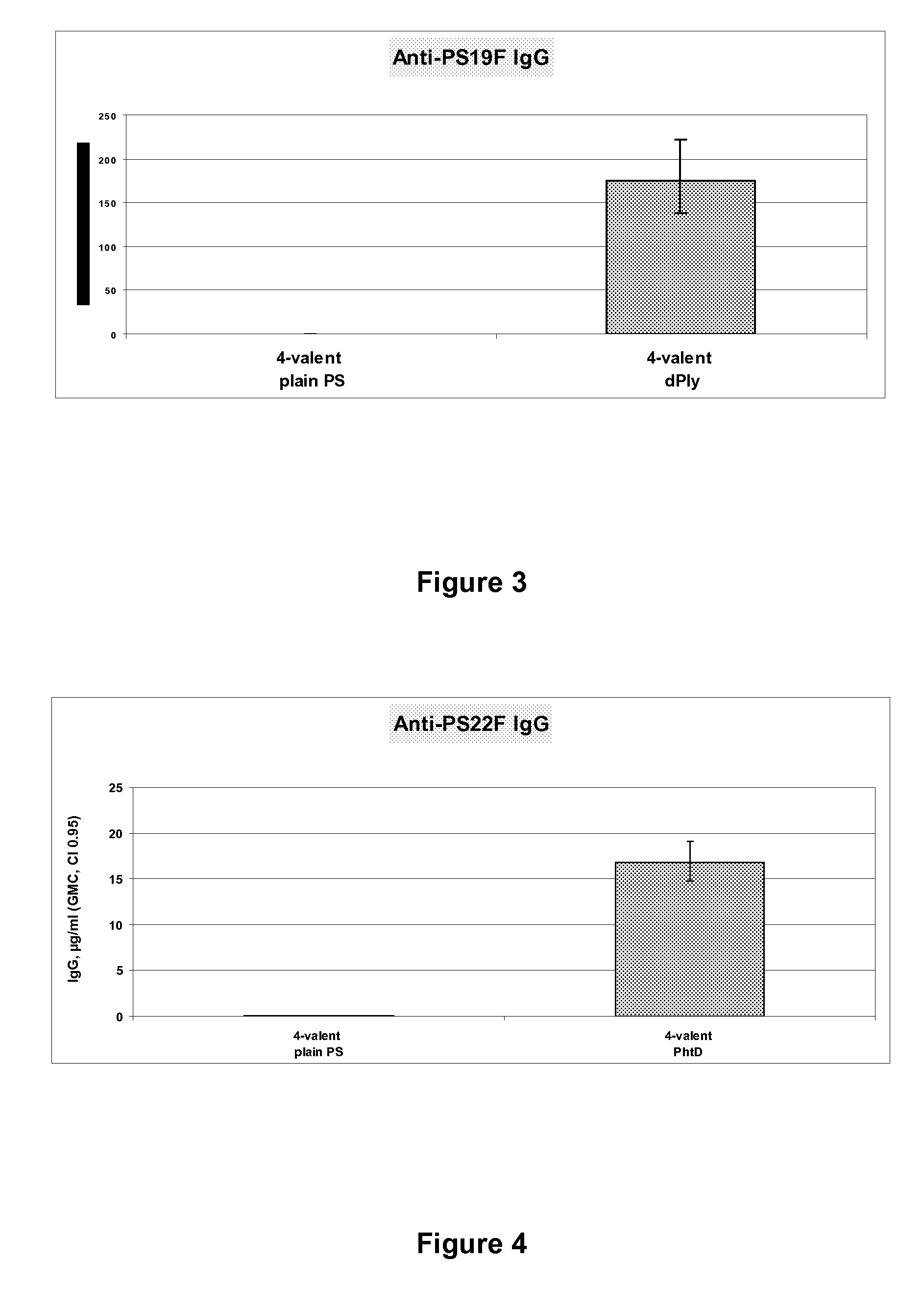
Vaccine comprising streptococcus pneumoniae capsular polysaccharide conjugates
InactiveUS20100209450A1Antibacterial agentsSenses disorderConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid. Methods of making and uses thereof are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
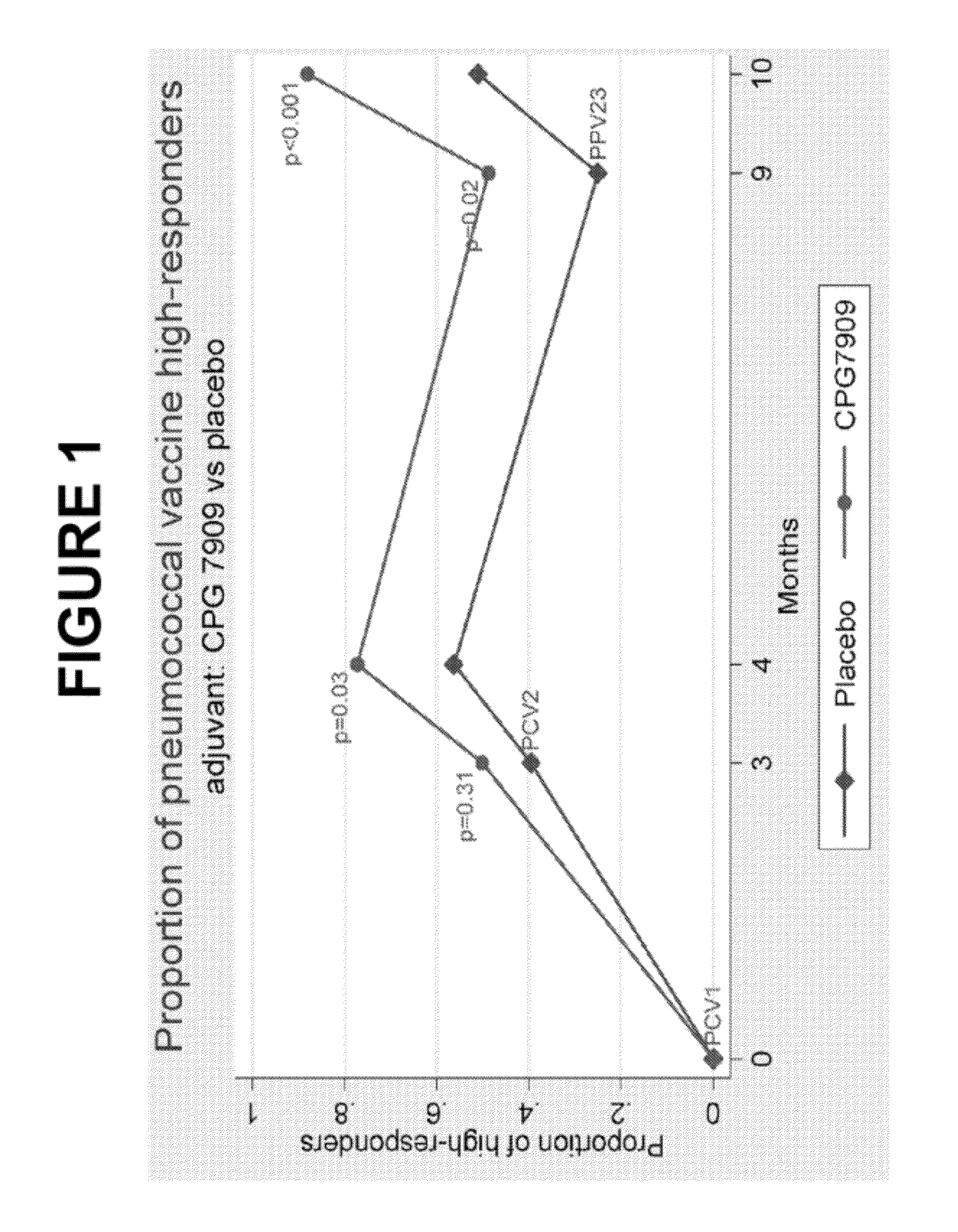
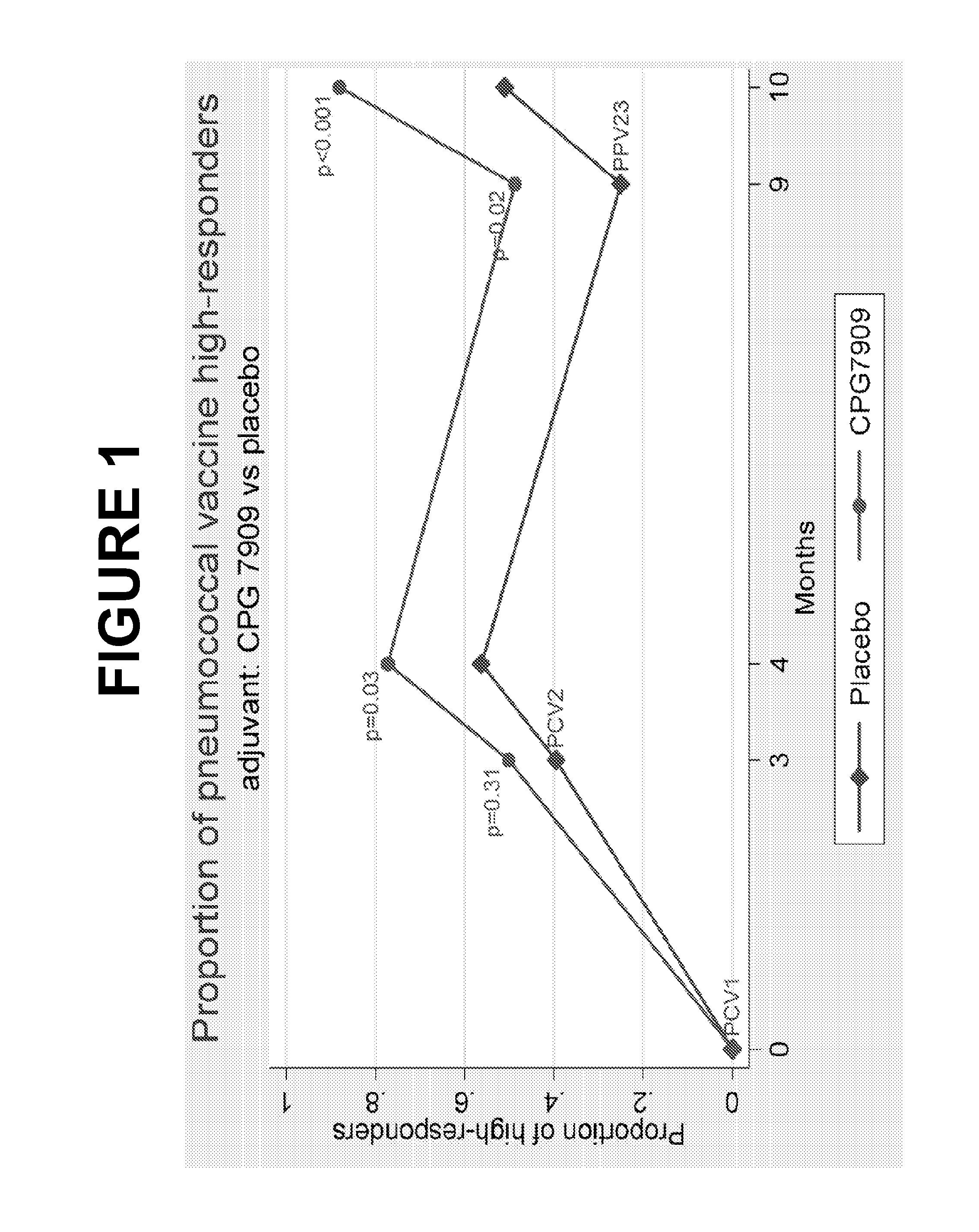
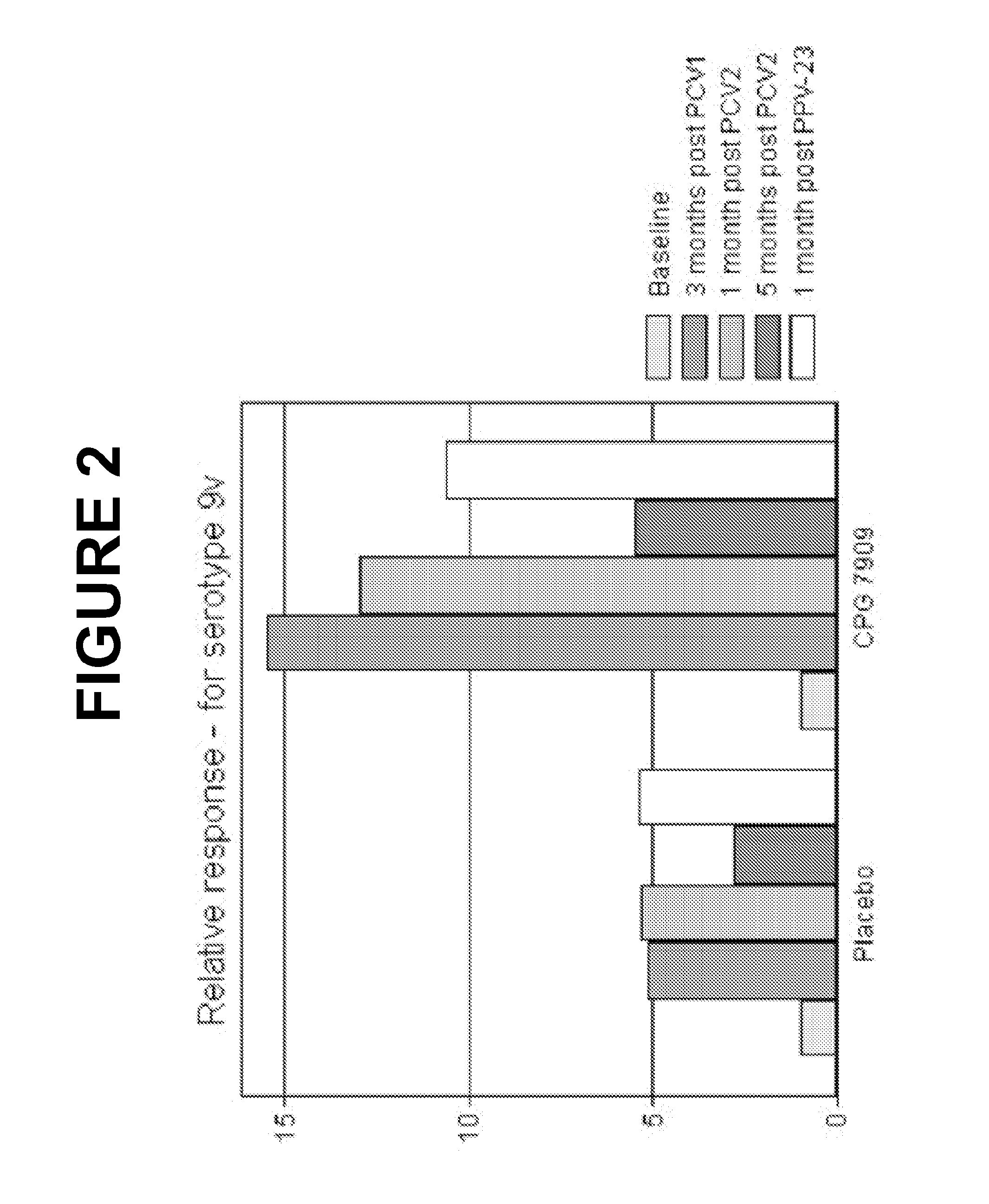
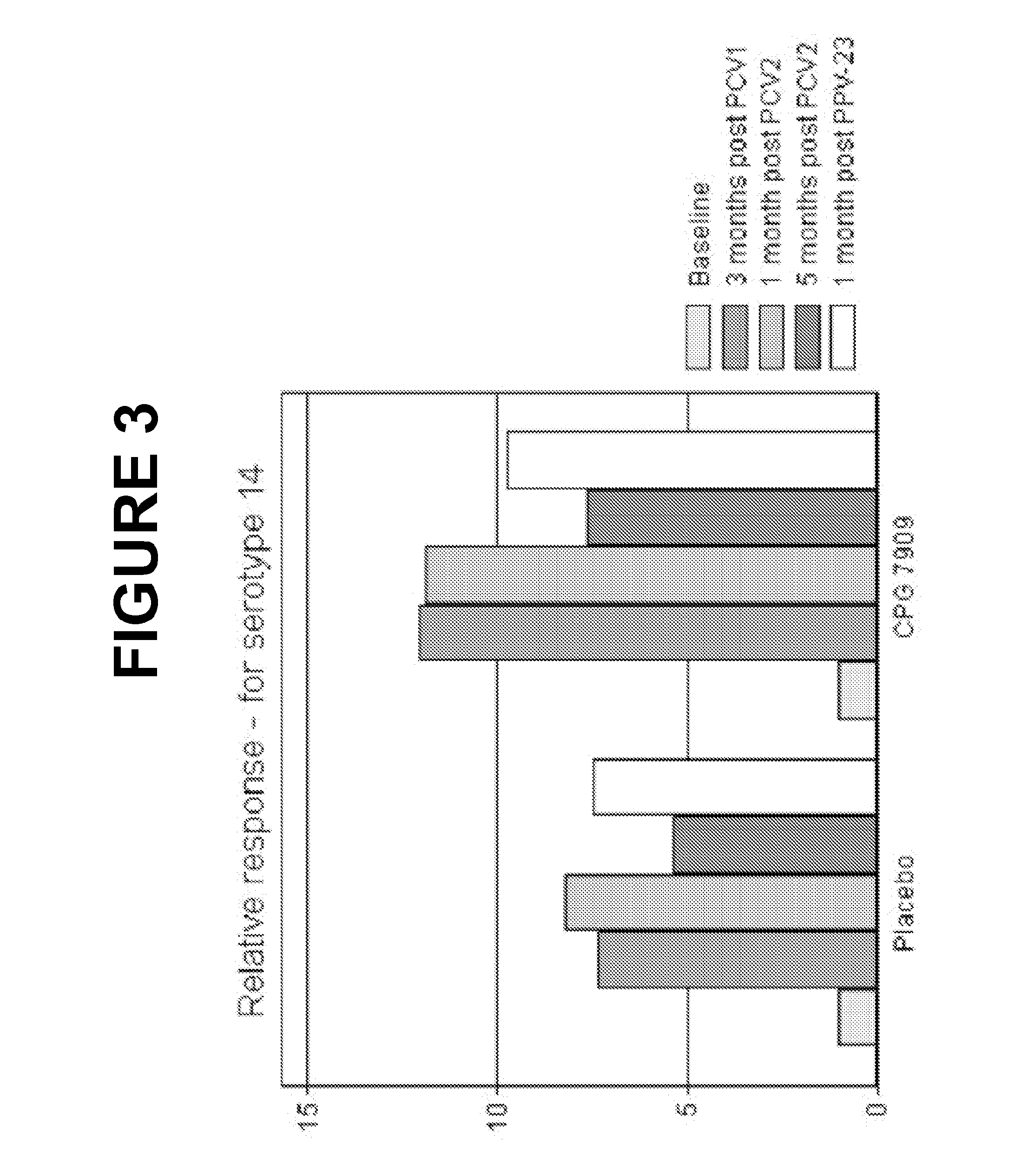
Pneumococcal vaccine and uses thereof
InactiveUS20120052088A1Low toxicitySafe and effective T-cell dependent carrierAntibacterial agentsNervous disorderCoccidiaSurgery
The present invention relates to new pneumococcal vaccines. The invention also relates to vaccination of subjects, in particular immunocompromised subjects, against pneumococcal infections using said novel pneumococcal vaccines.
Owner:COLEY PHARM GRP INC
immunogenic compositions for mucosal administration of pneumococcal surface protein A (PspA)
InactiveUS6042838ALittle or no tropism for the GALTGood mucosal immunogenAntibacterial agentsBacterial antigen ingredientsCoccidiaPneumococcal surface protein A
Mucosal administration, particularly intranasally, of killed whole pneumococci, lysate of pneumococci and isolated and purified PspA, as well as immunogenic fragments thereof, particularly when administered with cholera toxin B subunit, provides protection in animals against pneumococcal colonization and systemic infection. The ability to elicit protection against pneumococcal colonization in a host prevents carriage among immunized individuals, which can lead to elimination of disease from the population as a whole.
Owner:UNIVERSITY OF ALABAMA
Mucosal meningococcal vaccines
InactiveUS20070207090A1Improve securityReduced dosAntibacterial agentsBacterial antigen ingredientsMucosal Immune ResponsesAdjuvant
The invention provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred. The invention also provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The use of chitosan and / or detoxified ADP-ribosylating toxin adjuvants enhances anti-meningococcal mucosal immune responses and can shift the Th1 / Th2 bias of the responses.
Owner:NOVARTIS AG
Enterococcus faecium EF08 as well as feed additive and feed containing enterococcus faecium EF08
ActiveCN104195075AReduce excessive consumptionHigh feed conversionBacteriaMicroorganism based processesBiotechnologyAnimal product
The invention discloses an enterococcus faecium EF08 as well as a feed additive and a feed containing the enterococcus faecium EF08. The enterococcus faecium EF08 is collected with the number of CGMCC (China General Microbiological Culture Collection Center) No.5549 in CGMCC. The feed additive disclosed by the invention can be used for reducing the feed excessively consumed by harmful bacteria in intestines by virtue of an equalizing effect of bacteria for equalizing the intestinal environment, furthermore, the conversion rate of the feed for economic animals is increased, the feed for the economic animals can be effectively converted into a primary animal product with an economic value, and then, the effect of increasing the economic values of the economic animals can be improved.
Owner:SYNBIO TECH
Probiotic mixture intended for monogastric animals to control intestinal flora populations
InactiveUS6841149B1Good curative effectAssist in growth and activityBiocideBacteriaBiotechnologyBacteroides
A mixture of probiotics effective to reduce the contamination of enteric bacteria in humans and other monogastric animals. The mixture of probiotics includes one or more acid-producing bacteria strains and one or more yeast strains, and may advantageously be supplemented with a source of nutrients, such as prebiotics including fructo-oligosaccharides. In a preferred embodiment, said one or more bacteria strains contain Enterococcus faecium strain NCIMB #10415, and said one or more yeast strains contain NCYC #47 and CNCM I-1079.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES +1
Conserved and specific streptococcal genomes
InactiveUS20070053924A1Antibacterial agentsBacterial antigen ingredientsStreptococcus pyogenesCoccidia
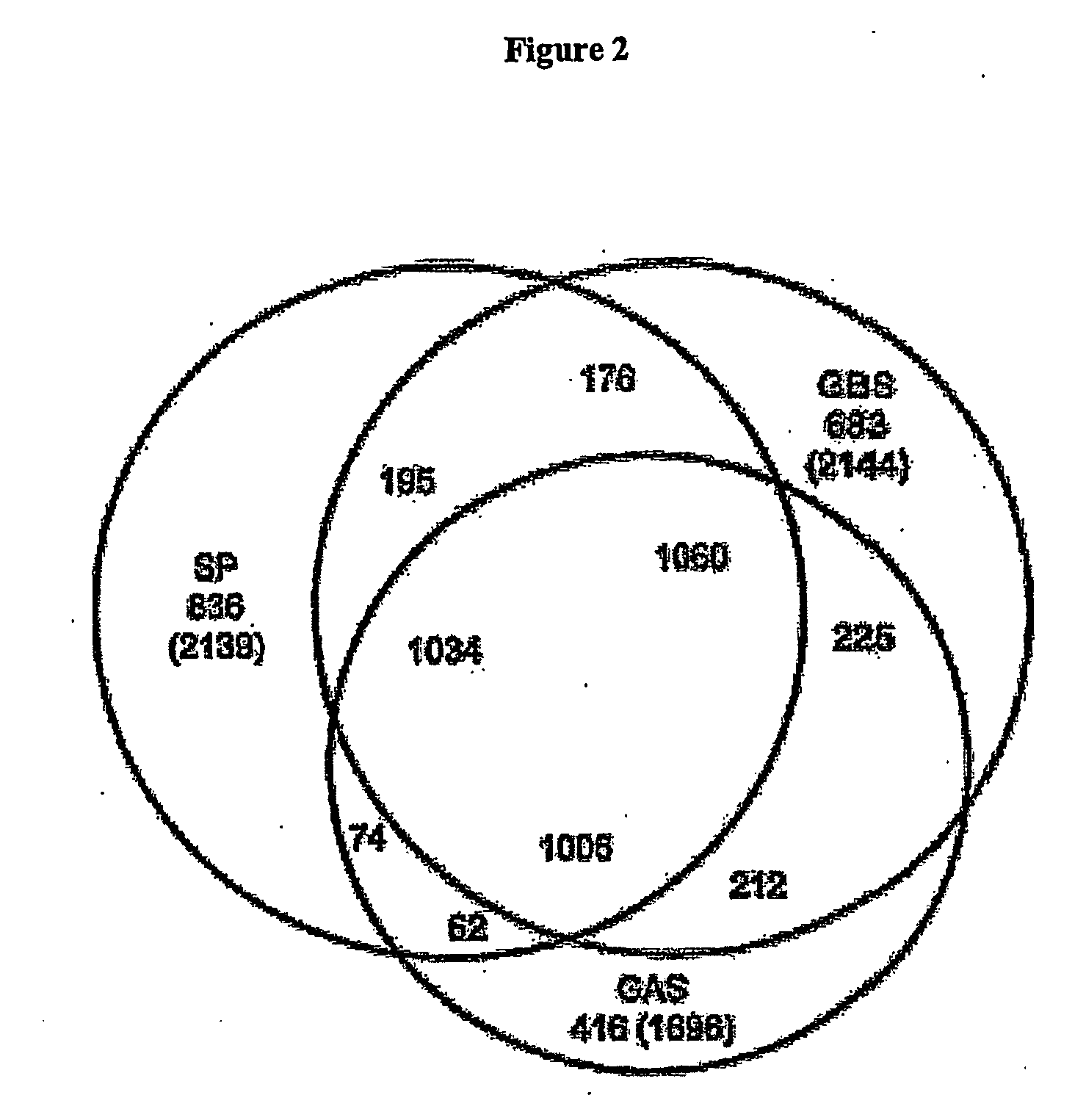
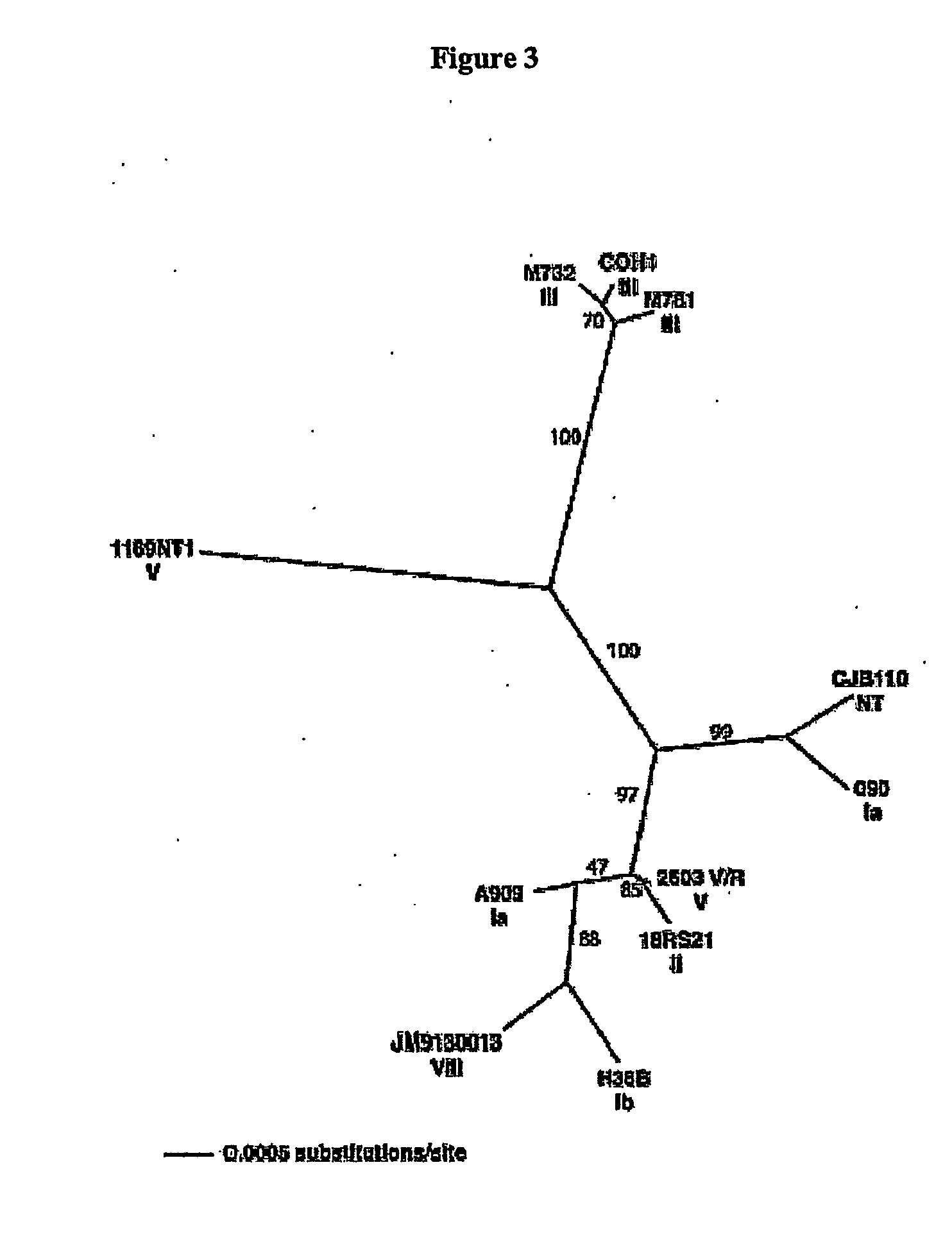
The invention relates to polynucleotides which are conserved or specific to one or more species of Streptococcus, Streptococcus species serotypes, and / or serotype isolates. In particular, the invention relates to polynucleotides from Streptococcus which are conserved or specific to one or more of the species of S. pneumoniae (“pneumococcus” or “S. pn.”), S. pyogenes (“group A streptococcus” or “GAS”), and S. agalactiae (“group B streptococcus” or “GBS”). The invention further relates to polynucleotides which are conserved or specific to one or more Streptococcal species serotypes, such as GBS serotypes Ia, Ib, II, III, IV, V, VI, VII, and VIII. The invention still further relates to polynucleotides which are conserved or specific to one or more clinical isolates of a Streptococcus species.
Owner:TETTELIN HERVE +1
Mucosal administration of pneumococcal antigens
InactiveUS6027734ALittle or no tropism for the GALTGood mucosal immunogenAntibacterial agentsBiocideDiseasePneumococcal antigen
Mucosal administration, particularly intranasally, of killed whole pneumococci, lysate of pneumococci and isolated and purified PspA, as well as immunogenic fragments thereof, particularly when administered with cholera toxin B subunit, provides protection in animals against pneumococcal colonization and systemic infection. The ability to elicit protection against pneumococcal colonization in a host prevents carriage among immunized individuals, which can lead to elimination of disease from the population as a whole.
Owner:UAB RES FOUND
Pneumococcal surface proteins and uses thereof
InactiveUS6500613B1Improving immunogenicityLow production costAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeCoccidia
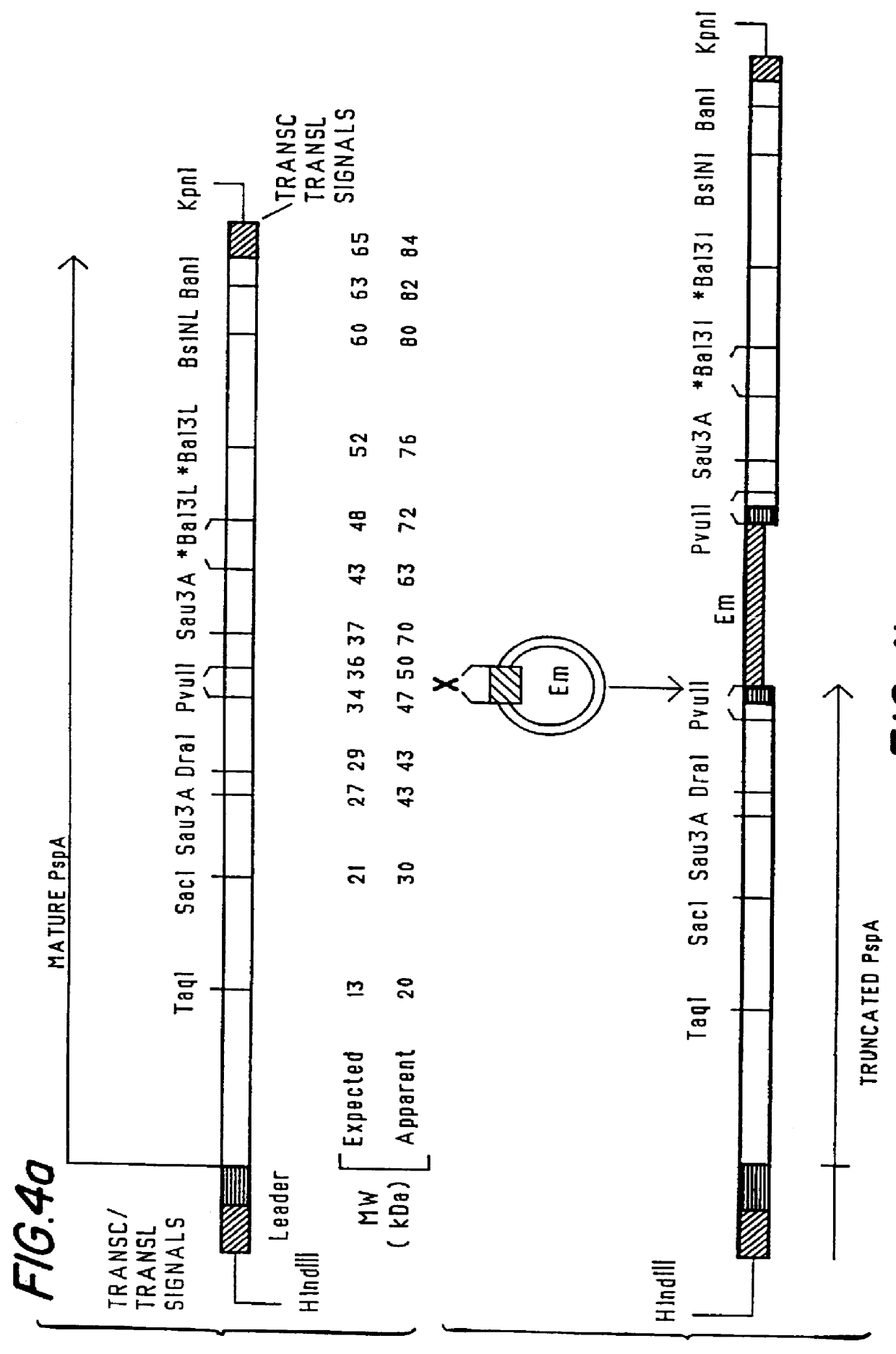
The present invention relates to pneumococcal genes, portions thereof, expression products therefrom and uses of such genes, portions and products; especially to genes of Streptococcus pneumoniae, e.g., the gene encoding pneumococcal surface protein A (PspA), i.e., the pspA gene, the gene encoding pneumococcal surface protein A-like proteins, such as pspA-like genes, e.g., the gene encoding pneumococcal surface protein C (PspC), i.e., the pspC gene, portions of such genes, expression products therefrom, and the uses of such genes, portions thereof and expression products therefrom.
Owner:ALABAMA AT BIRMINGHAM UNIVERISTY OF
Choline binding proteins for anti-pneumococcal vaccines
InactiveUS6245335B1Improve efficiencySufficient quantityAntibacterial agentsBacteriaBacteroidesCholine binding protein
The invention relates to bacterial choline binding proteins (CBPs) which bind choline. Such proteins are particularly desirable for vaccines against appropriate strains of Gram positive bacteria, particularly streptococcus, and more particularly pneumococcus. Also provided are DNA sequences encoding the bacterial choline binding proteins or fragment thereof, antibodies to the bacterial choline binding proteins, pharmaceutical compositions comprising the bacterial choline binding proteins, antibodies to the bacterial choline binding proteins suitable for use in passive immunization, and small molecule inhibitors of choline binding protein mediated adhesion. Methods for diagnosing the presence of the bacterial choline binding protein, or of the bacteria, are also provided. In a specific embodiment, a streptococcal choline binding protein is an enolase, which demonstrates strong affinity for fibronectin.
Owner:THE ROCKEFELLER UNIV
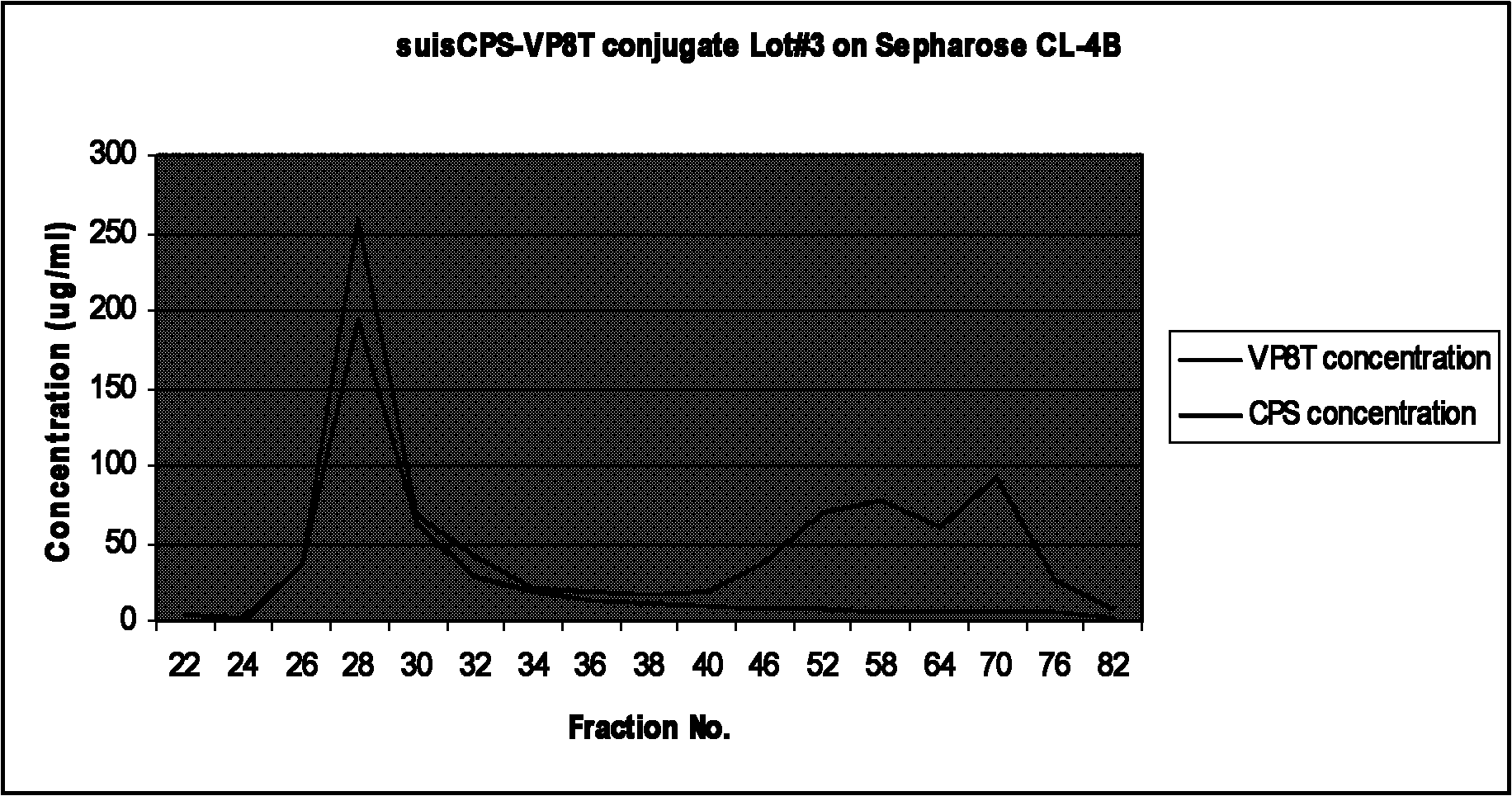
Bacterial polysaccharide-protein conjugate vaccine and preparation method thereof
The invention relates to a bacterial polysaccharide-protein conjugate vaccine with immunogenicity, in particular to a conjugate vaccine which is formed by connecting a recombinant rotavirus protein with a bacterial polysaccharide by using a covalent bond, a nucleotide sequence for coding the recombinant rotavirus protein, a recombinant expression system, a protein expressed by the recombinant expression system, a preparation method of the conjugate vaccine and a pneumococcus polysaccharide-recombinant rotavirus protein conjugate vaccine. The bacterial polysaccharide is connected with a recombinant rotavirus surface protein through a covalent bond. The recombinant rotavirus protein is selected from a partial or complete amino acid sequence of a P-gene rotavirus protein and a partial or complete amino acid sequence of a G-gene rotavirus protein.
Owner:普大生物科技(泰州)有限公司
Pyridine degradable bacteria, complex bacterial agent thereof, preparation and use
ActiveCN101481673AEfficient degradationEfficient metabolismBacteriaWater contaminantsSynechococcusHigh concentration
The invention discloses a pyridine-degrading bacterium as well as a composite bacterial agent, a preparation method and uses thereof. The newly-separated Rhodococcus pyridinovora KT-J002 CGMCC No.2789 and Cellulomonas sp KT-J007 CGMCC No.2788 not only can effectually degrade the high concentration pyridine, but also has tolerance or degradation ability for toxic substances such as benzene, phenol, dimethylbenzene, quinoline, cyanide and the like; the composite bacterial agent composed of Rhodococcus pyridinovora KT-J002 CGMCC No.2789, Cellulomonas sp KT-J007 CGMCC No.2788, Paracoccus d enitrificans W12 CGMCC No.1673, Micrococcus luteus ATCC 49442 and Arthrobacter crystallopoietes ATCC 15481 is used for environmental improvement, can effectually degrade the pyridine and is particularly suitable for the biological treatment of coking waste water.
Owner:SINOBIOWAY BIO AGRI GRP CO LTD
Probiotics as alternative medicines against infectious diseases
An exemplary embodiment providing one or more improvements includes feeding animals with probiotic microbes encapsulated in a mixture of xanthan gum and chitosan, or in gelatin, specifically Pediococcus acidilactici and Saccharomyces boulardii. Such encapsulation protects the viability of the probiotic microbes against unfavorable temperatures. An exemplary embodiment providing one or more improvements includes methods of using viable probiotics in therapy of birds and mammals infected with infectious diseases. Probiotics acted as adjuvants in stimulating antibody reaction and stimulated a cellular immunity response. In particular, probiotics were shown to reduce the number of viable oocytes from fecal samples, stimulate antibody production, and stimulate of proliferation of splenocytes in chickens infected with Elimeria. In addition, probiotics were shown to relieve symptoms of parvovirus infection in dogs.
Owner:IMAGILIN TECH LLC
Pneumococcal dosing regimen
ActiveUS20140322263A1Bacterial antigen ingredientsCarrier-bound antigen/hapten ingredientsDosing regimenCoccidia
Owner:WYETH LLC
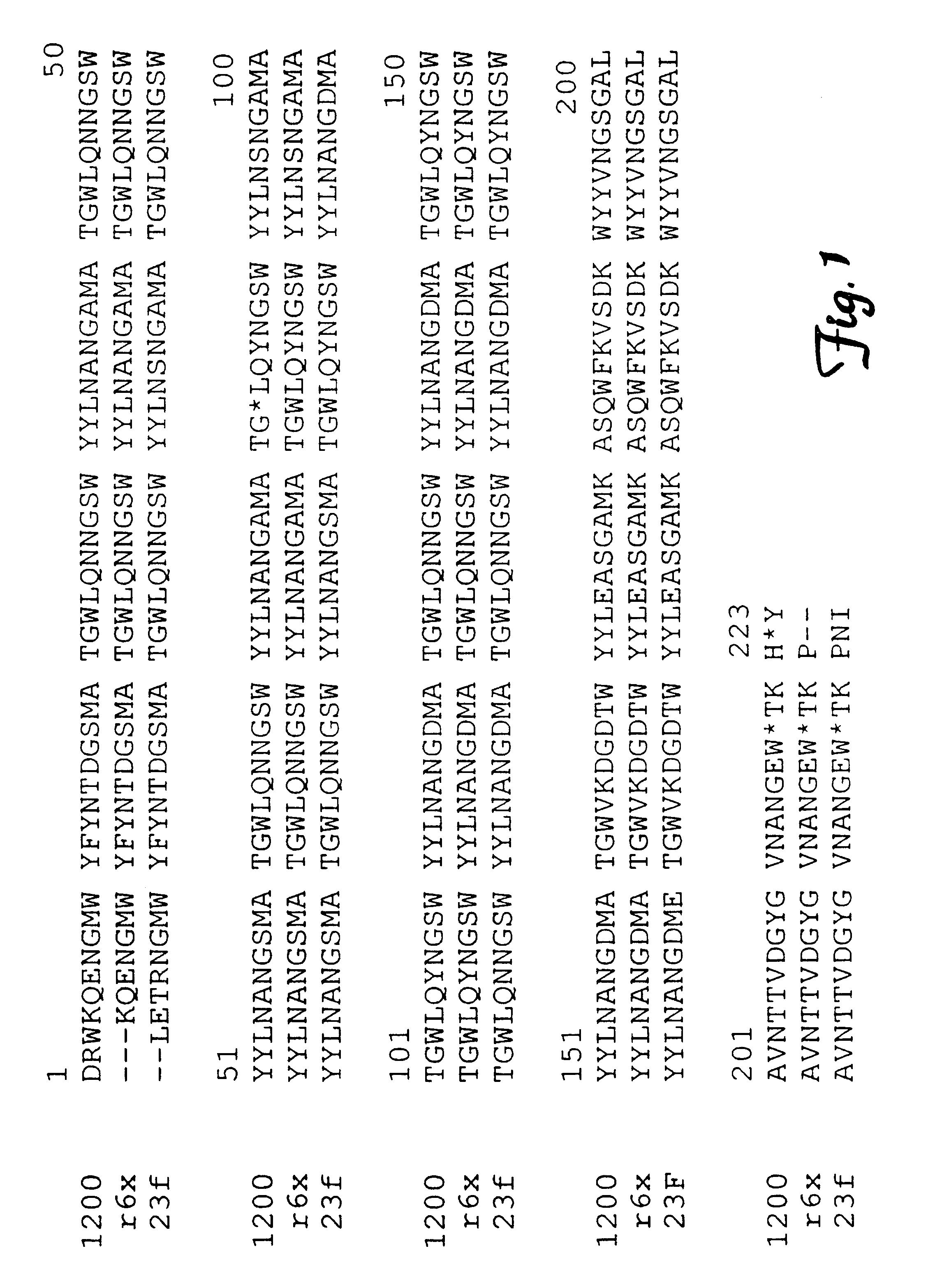
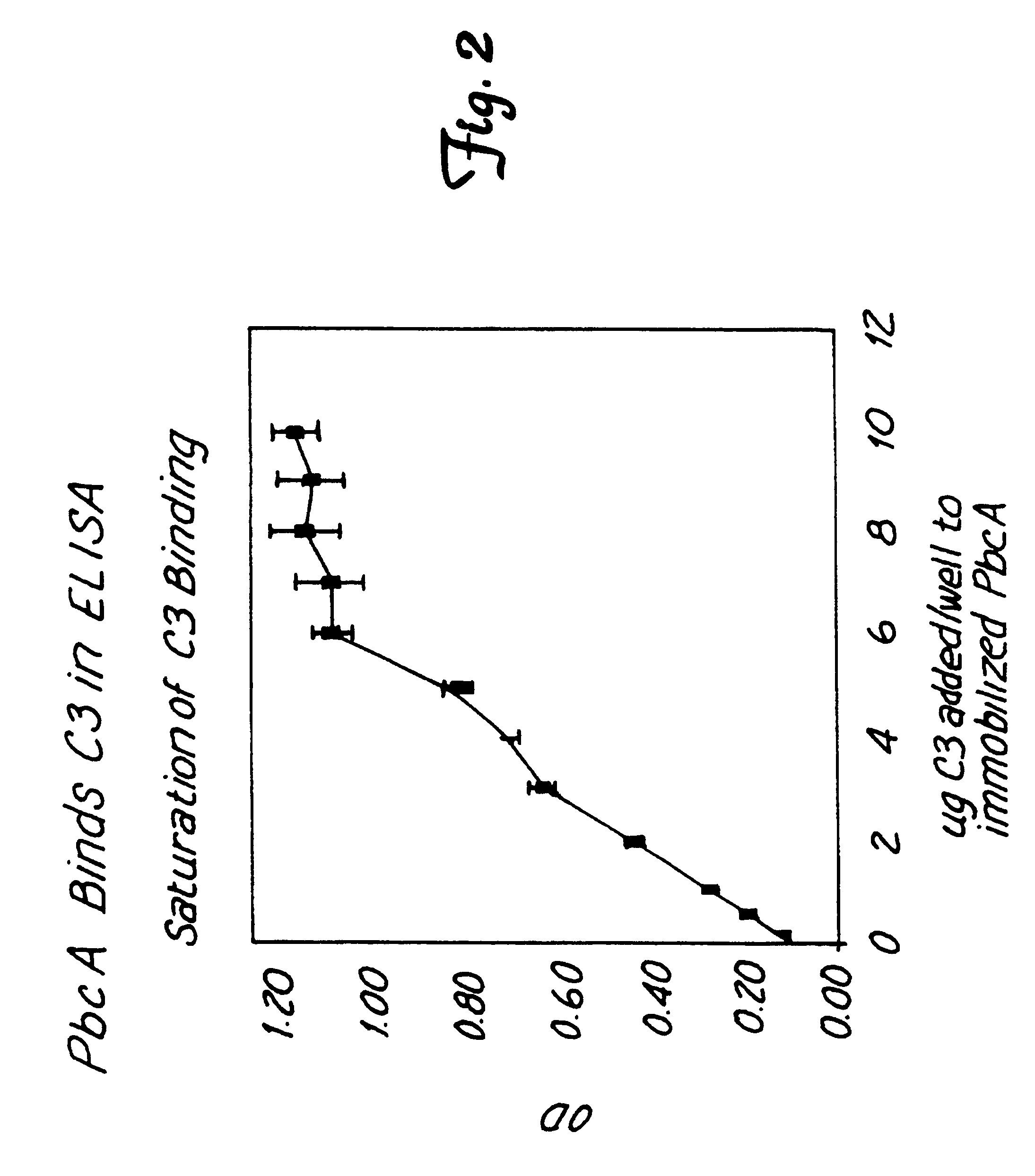
Method for isolating a C3 binding protein of streptococcus pneumoniae
This invention relates to the identification of a human complement C3 binding protein from Streptococcus pneumoniae and to its sequence and to methods for its purification and use. The protein binds but does not degrade or cleave C3 and is implicated in S. pneumoniae virulence. The protein is recognized by antibodies produced by humans recovering from pneumococcal infection.
Owner:RGT UNIV OF MINNESOTA
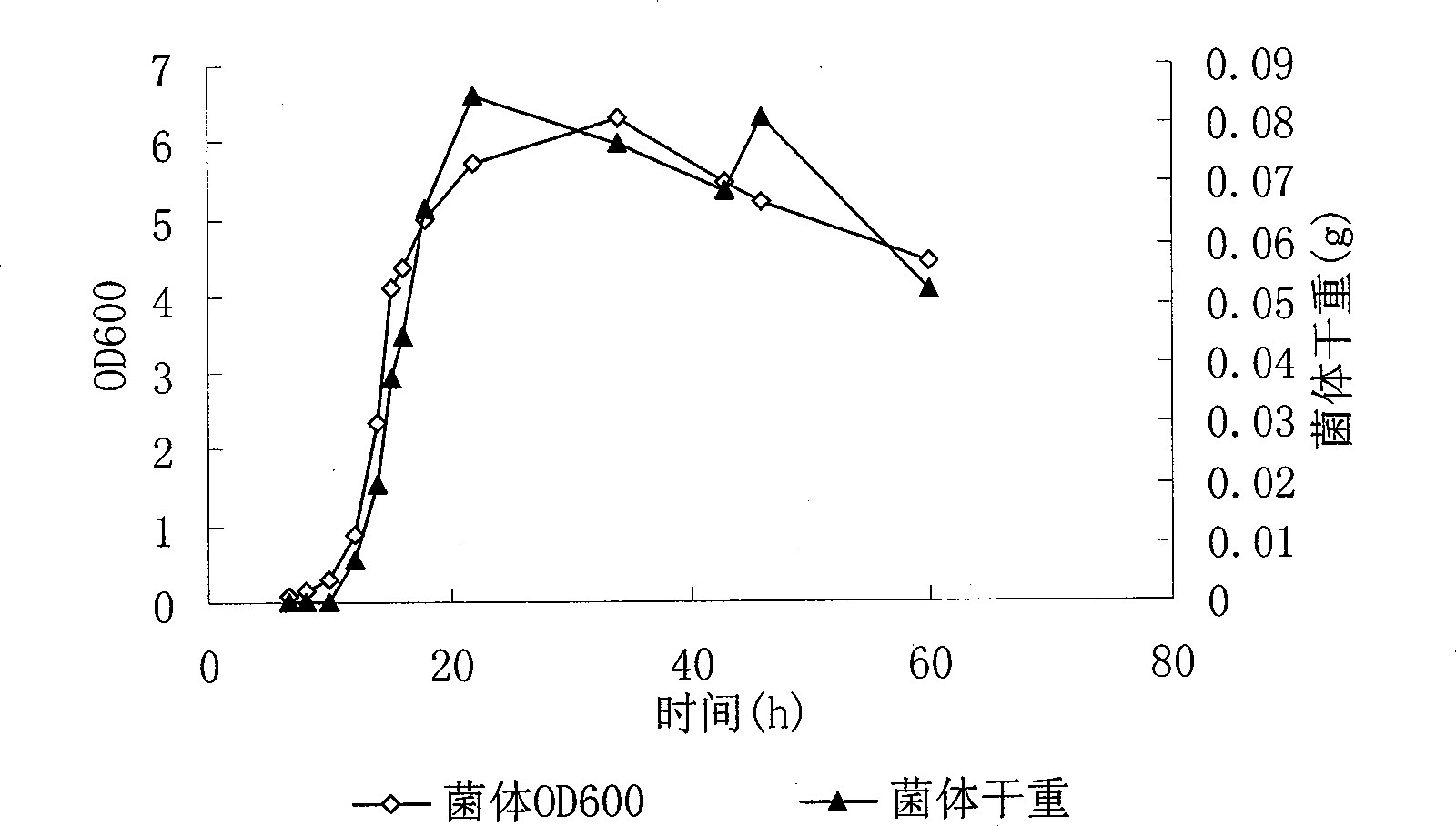
Method for producing bacterial strain of Pediococcus acidilactici, and bacterin of Pediococcus acidilactici
InactiveCN101050437AIncrease growth rateIncrease productionBacteriaFermentationSynechococcusCoccidia
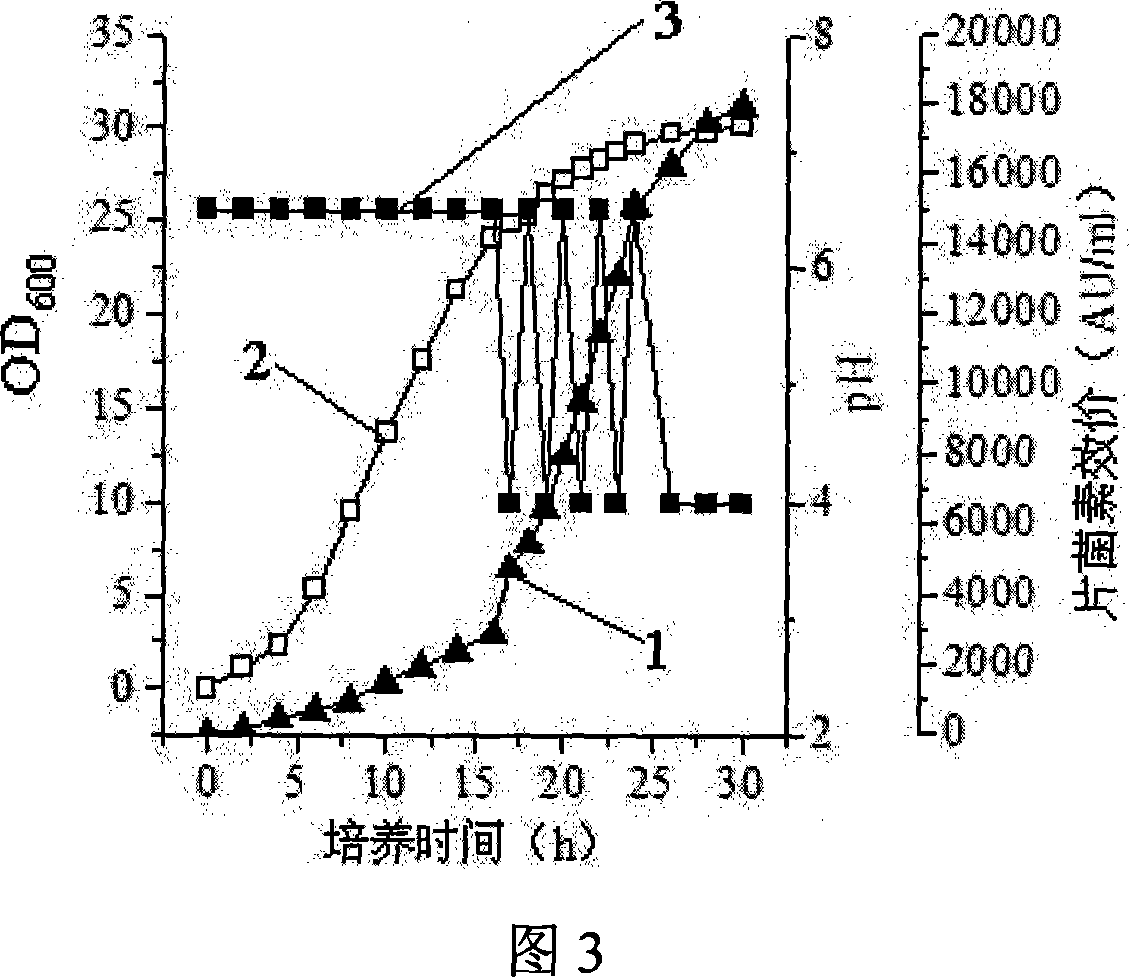
This invention discloses Pediococcus acidilactici LH31 (CCTCC M207013), and a method for producing pediocin from it. The method comprises: inoculating Pediococcus acidilactici LH31 in modified MRS liquid medium, culturing, activating, inoculating activated Pediococcus acidilactici LH31 in a fermentation tank, and performing feed-batch fermentation with three stages. Pediococcus acidilactici LH31 is proliferated in stages 1 and 2. The maximal bacterial density (OD600 nm) can reach 30. Pediocin is accumulated in stages 2 and 3. The maximal titer can reach 18000 AU / mL. The method is suitable for industrialization.
Owner:EAST CHINA UNIV OF SCI & TECH
Pneumococcal Vaccine and Uses Thereof
The present invention relates to new pneumococcal vaccines. The invention also relates to vaccination of subjects, in particular immunocompromised subjects, against pneumoccocal infections using said novel pneumococcal vaccines.
Owner:COLEY PHARMA GRP INC
Protein antigens that provide protection against pneumococcal colonization and/or disease
ActiveUS20150374811A1Reduce colonizationReduce the risk of infectionAntibacterial agentsBacterial antigen ingredientsDiseaseT cell
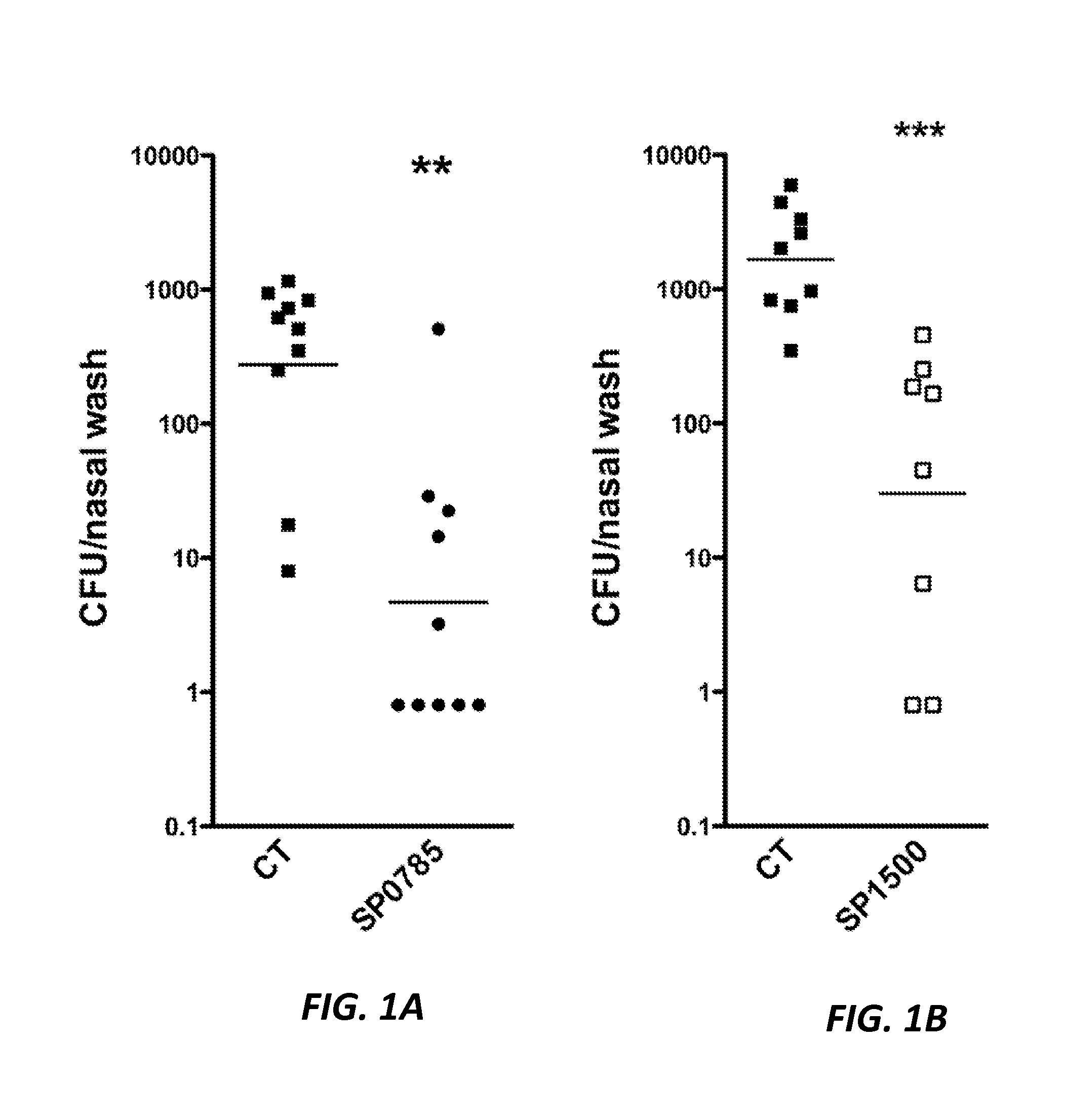
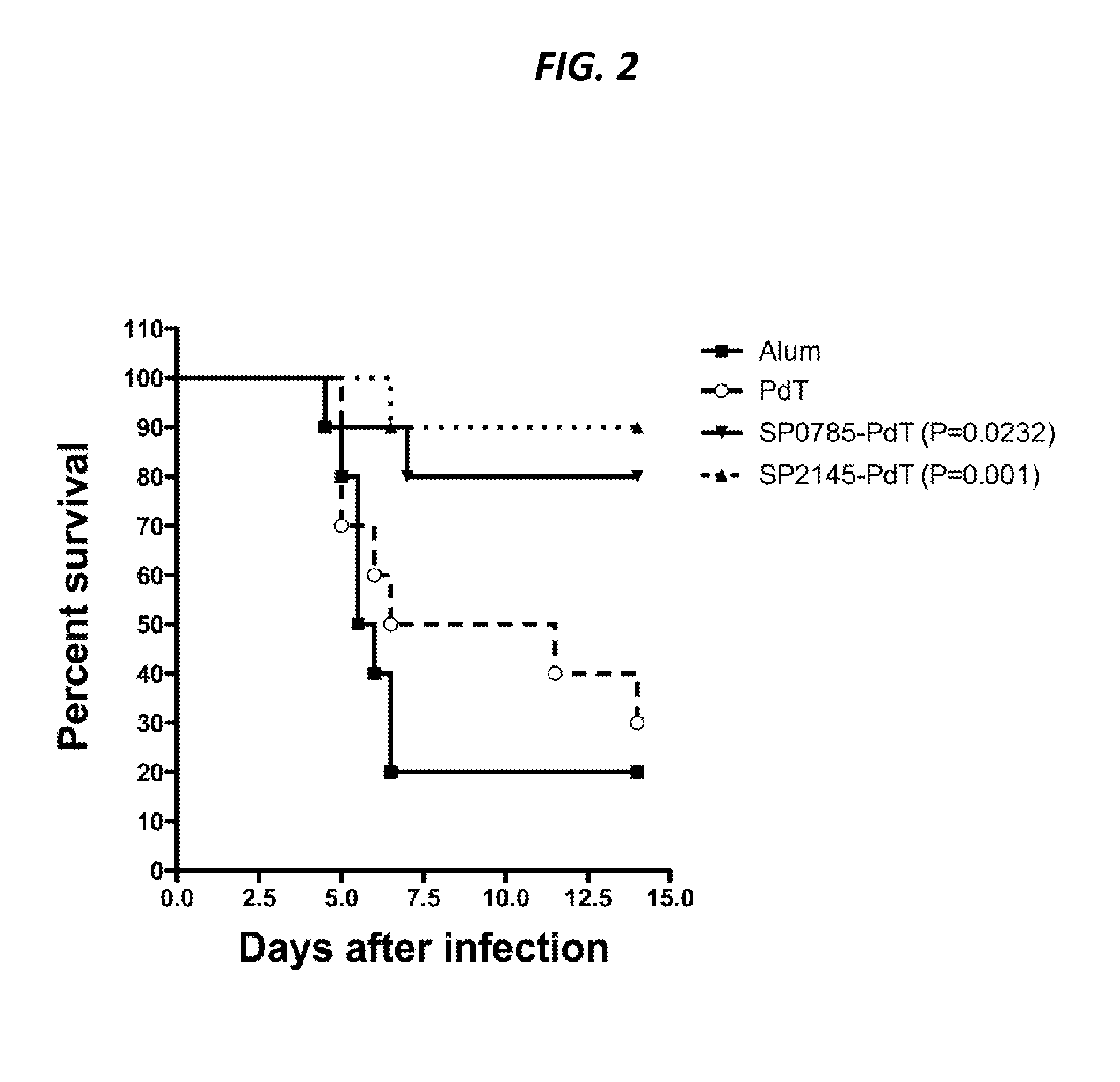
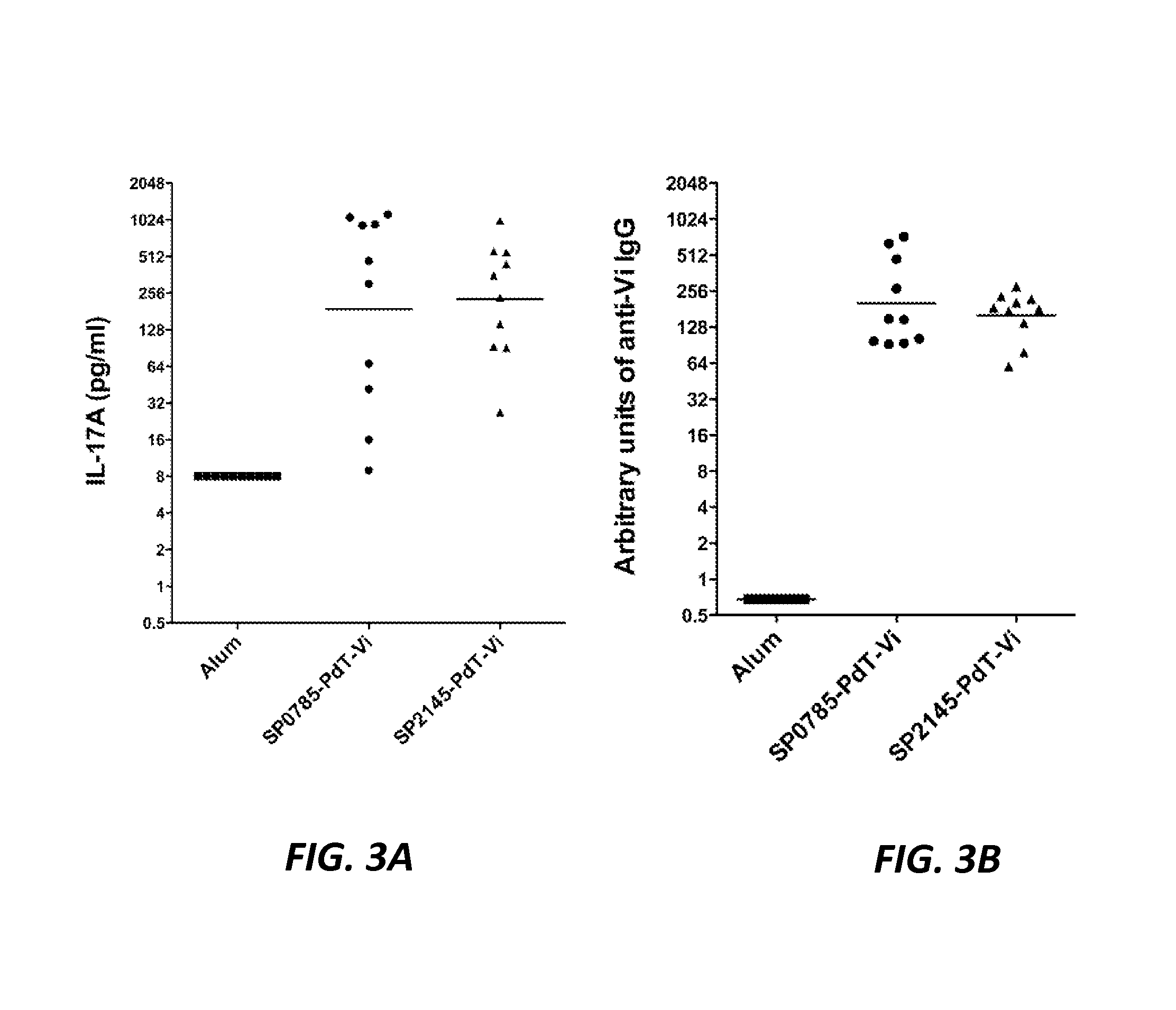
The present application is generally directed to novel pneumococcal polypeptide antigens and nucleic acids encoding such antigens, and immunogenic compositions comprising such antigens for treating and preventing pneumococcal infection. The present invention further provides method of using the antigens to elicits an immune response (e.g., IL-17A response, a T cell-mediated and / or B-cell-mediated immune responses). The present invention also provides methods of prophylaxis and / or treatment of pneumococcal-mediated diseases, such as sepsis, comprising administering an immunogenic composition including one or more of a combination of pneumococcal antigens or functional fragments thereof as disclosed herein. In some embodiments, one or more pneumococcal antigens can be present in a polysaccharide conjugate. The compositions induce an anti-pneumoccocus immune response when administered to a mammal. The compositions can be used prophylactically to vaccinate an individual and / or therapeutically to induce a thereapeutic immune response to an infected individual.
Owner:CHILDRENS MEDICAL CENT CORP
Multiple vaccination including serogroup C meningococcus
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminum phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
Polypeptides And Immunogenic Conjugates Capable of Inducing Antibodies Against Pathogens, and Uses Thereof
InactiveUS20080171053A1Efficient responseAntibacterial agentsBacterial antigen ingredientsKexinDNA construct
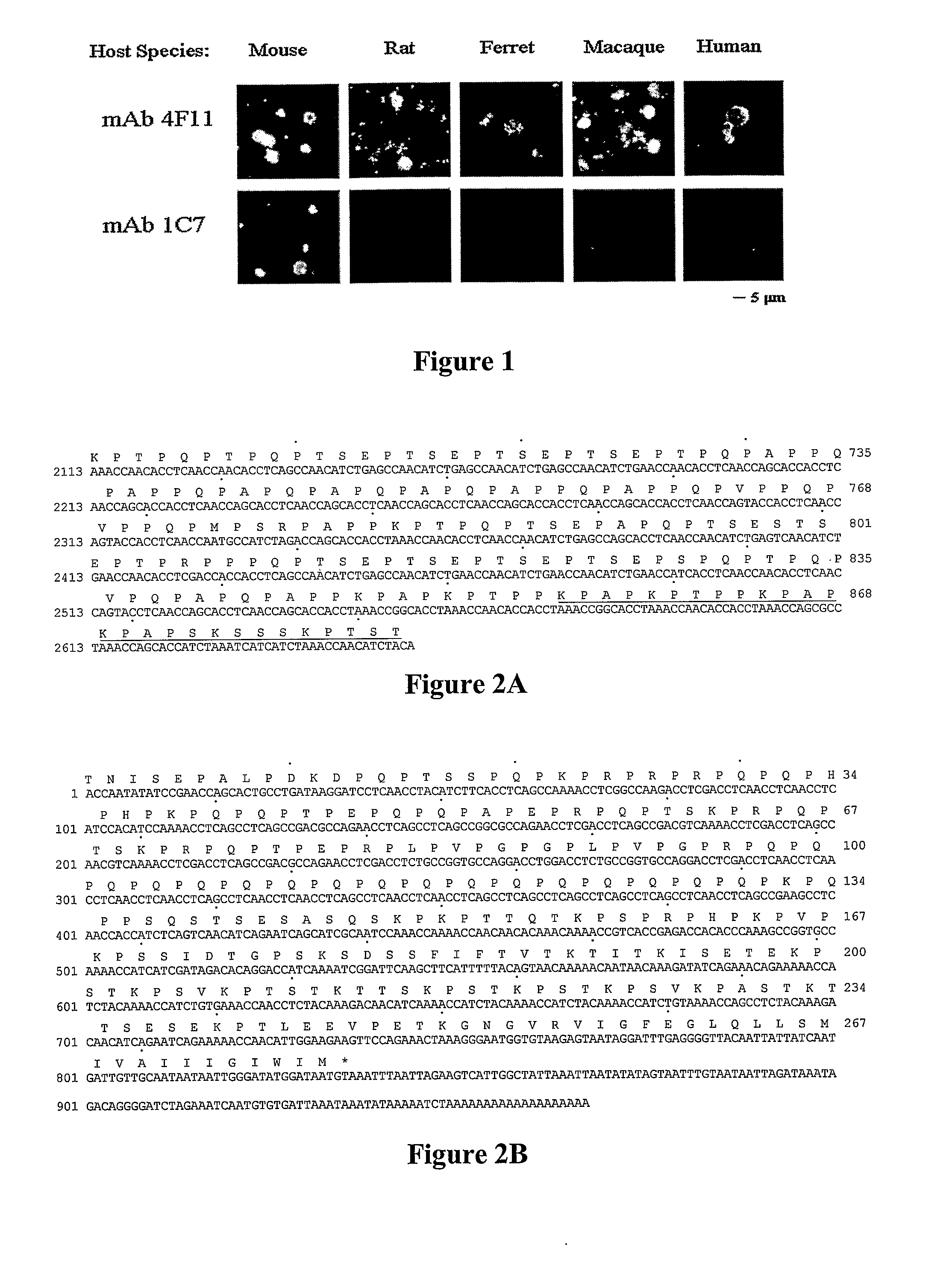
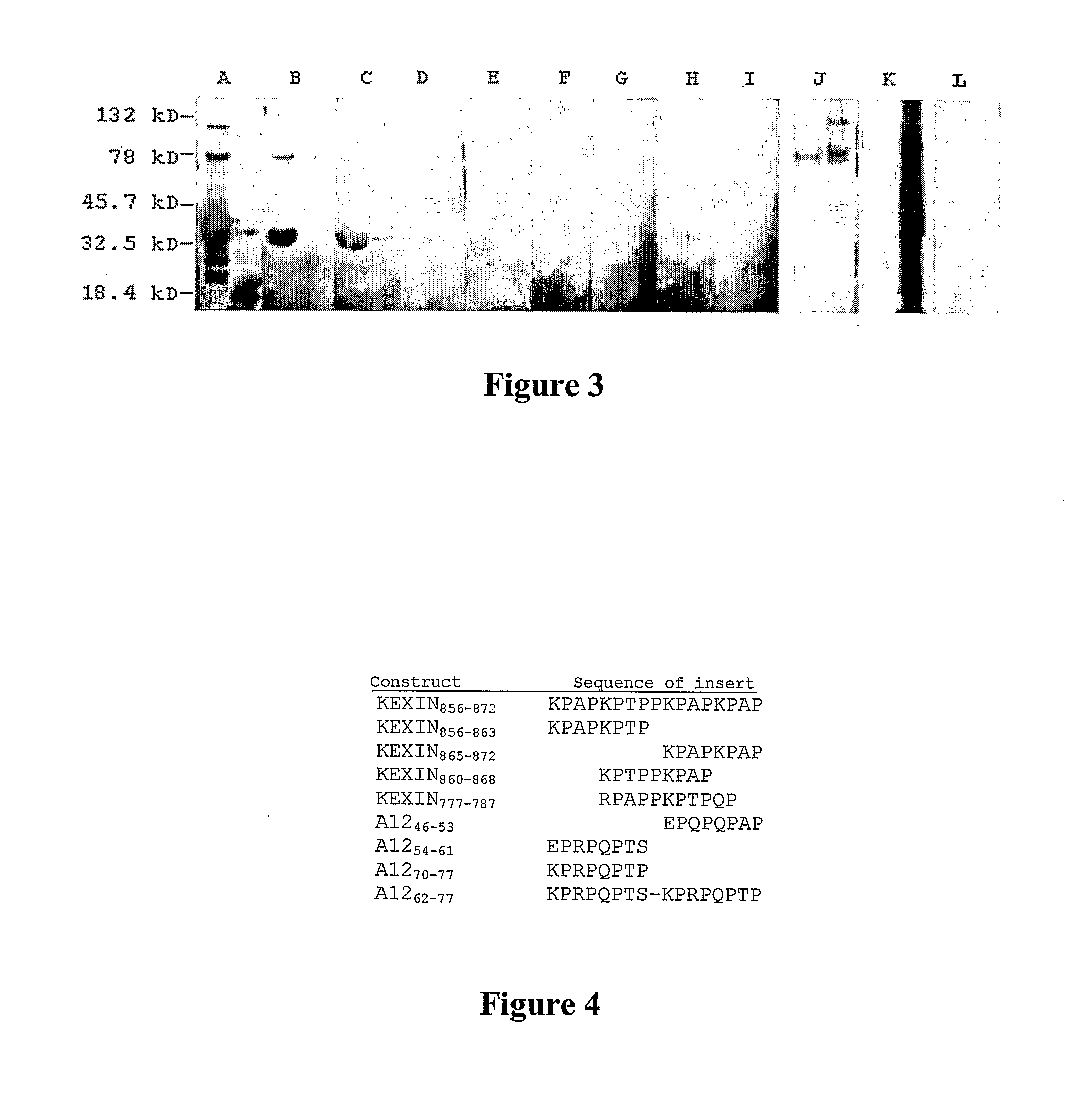
A number of immunologically active agents are described, including an isolated protein or polypeptide that includes the amino acid sequence of SEQ ID NO: 1, immunogenic conjugates containing either the protein or polypeptide, a full-length Pneumocystis kexin, or a full length Streptococcus pneumoniae pneumococcal surface protein A (PspA), antibodies recognizing the protein or polypeptide or the immunogenic conjugates (particularly the epitope of SEQ ID NO: 1), and nucleic acid molecules that encode the protein or polypeptide, as well as DNA constructs, expression vectors, and host cells that contain the nucleic acid molecules. Disclosed uses of the antibodies, immunogenic conjugates, and DNA constructs include inducing passive or active immunity to treat or prevent pathogen infections, particularly by a Pneumocystis organism, in a patient.
Owner:UNIVERSITY OF ROCHESTER
Formulation of meningitis vaccines
InactiveUS20100203137A1Improving immunogenicityWithout riskAntibacterial agentsPowder deliveryCoccidiaMeningococcal carriage
A liquid Hib component is used to reconstitute a lyophilised meningococcal component, thereby producing a combined meningitis vaccine. A lyophilised meningococcal component can also be reconstituted with an oil-in-water emulsion.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com