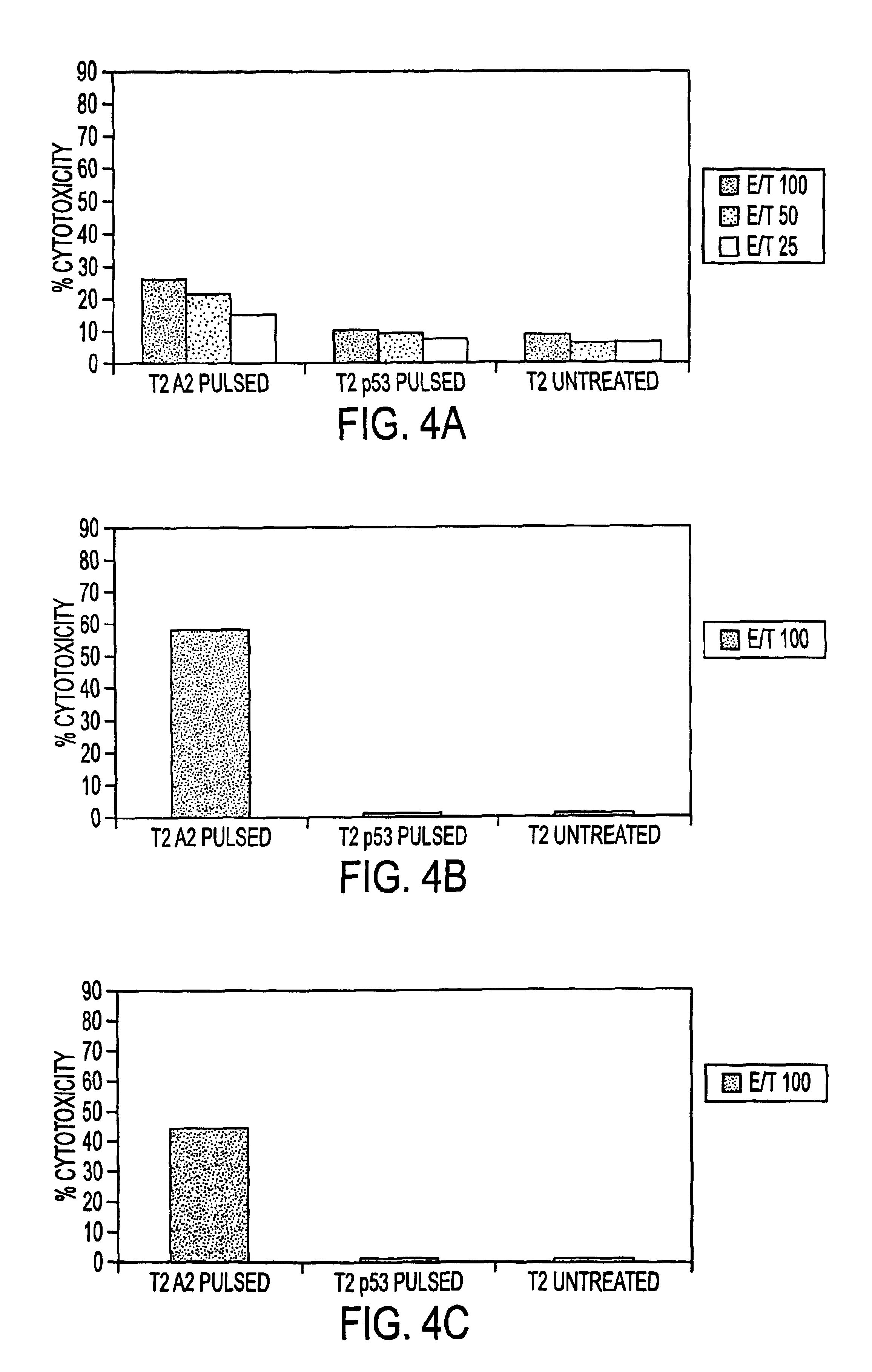
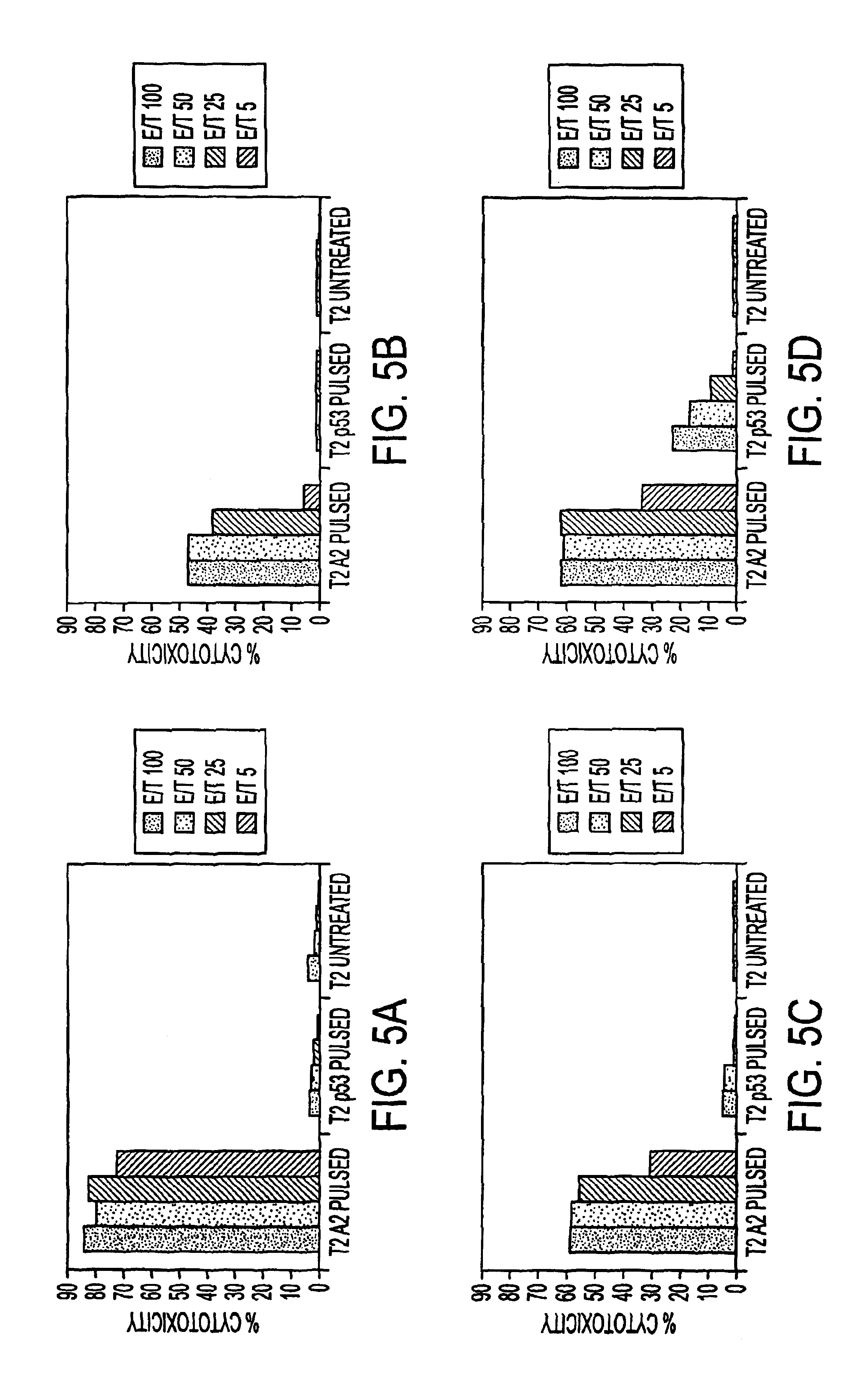
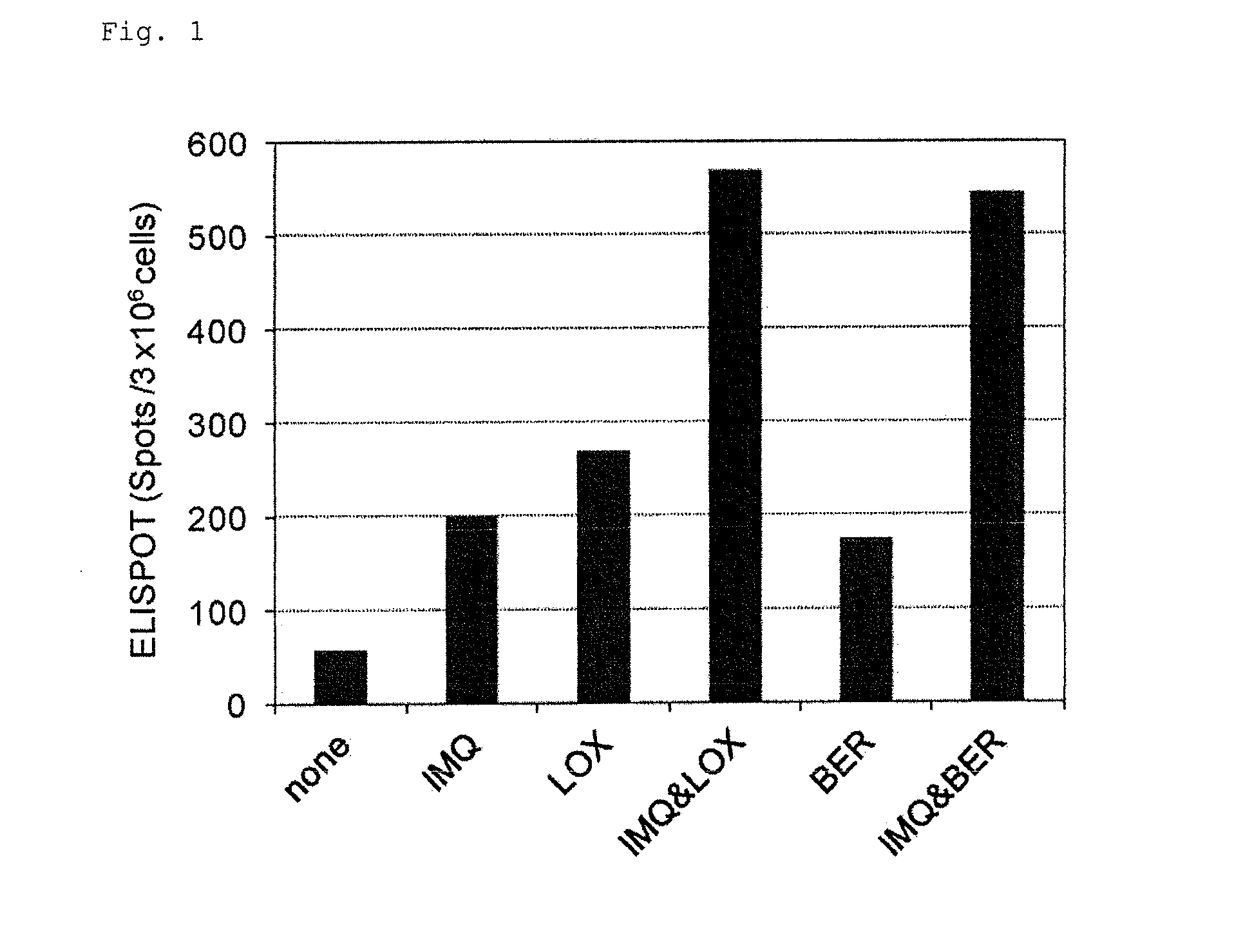
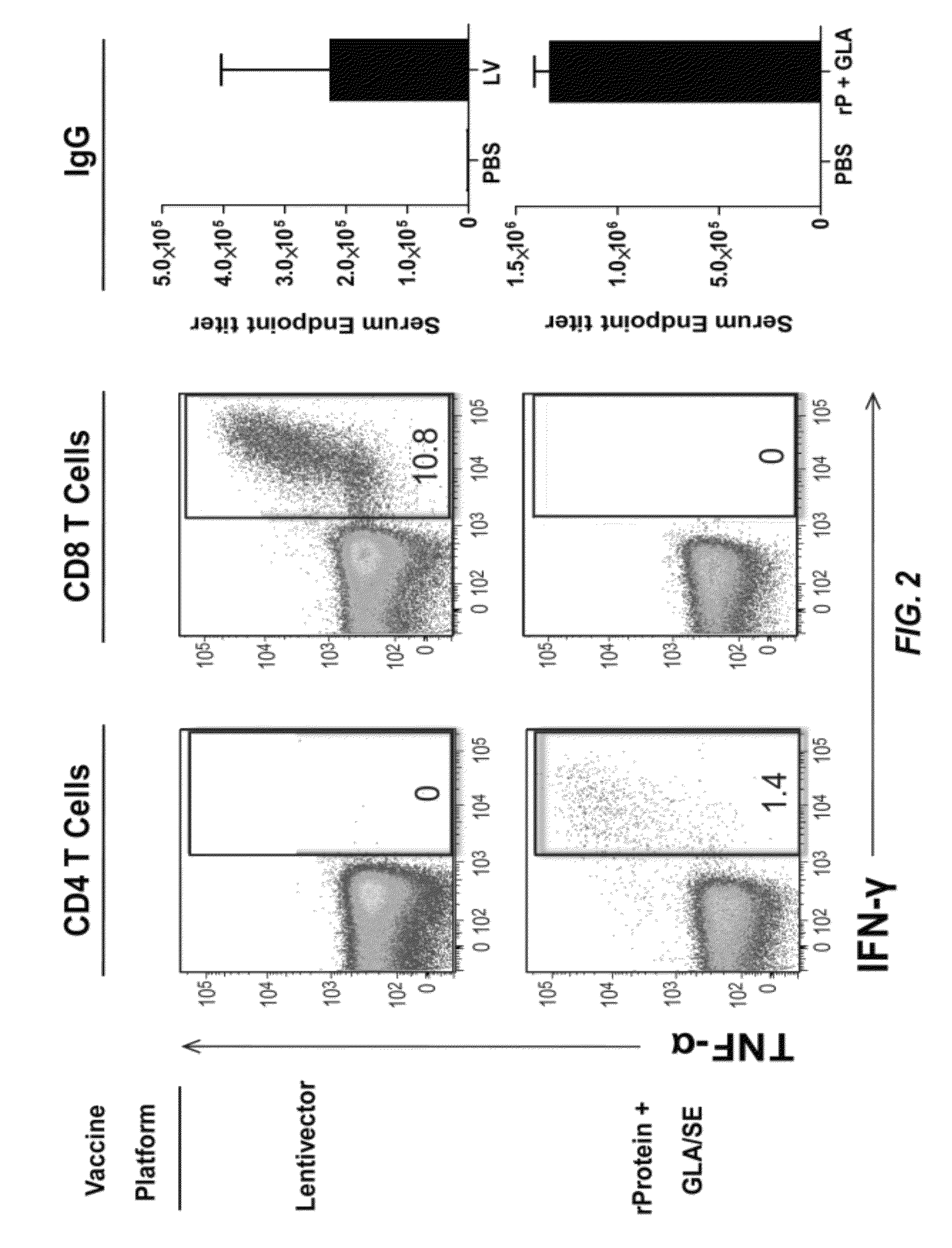
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
827 results about "Cellular immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cellular Immunity. Cellular immunity is defined as a response to a particular antigen that can be transferred to a naive (nonimmunized) individual via the lymphocytes (but not the plasma or serum) from another immunized subject.
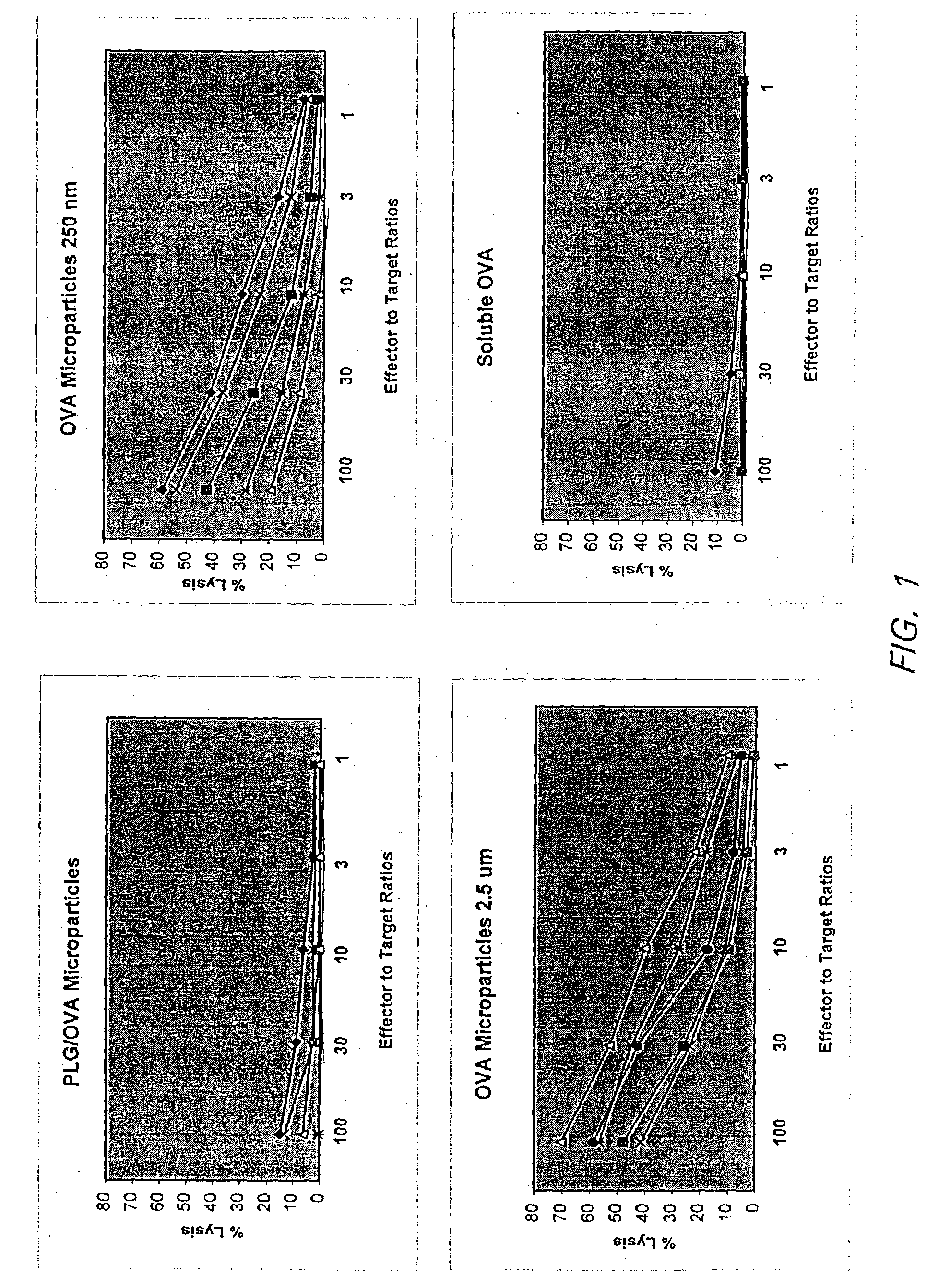
Nanoparticle Based Immunological Stimulation
A nanoparticle-based delivery system and methods for its use are disclosed. In one aspect, a nanoparticle-based delivery system comprising at least one molecule such as proteins, DNA / RNA or fragments thereof, carbohydrates, enzymes, chemicals, virus cells, bacteria, parts of a virus, parts of a bacteria, parts of a cell, part of a tissue, or a combination of one or more of these, which shall be referred to as immunogens, are chemically or physically combined with water soluble nanoparticles which, when administered to a living system, is capable of eliciting a desired immunological response. More particularly, the invention relates to nanoparticle-based delivery systems that are specifically engineered to enhance humoral or cellular immune response without the use of adjuvants.
Owner:UNIV OF HAWAII +1
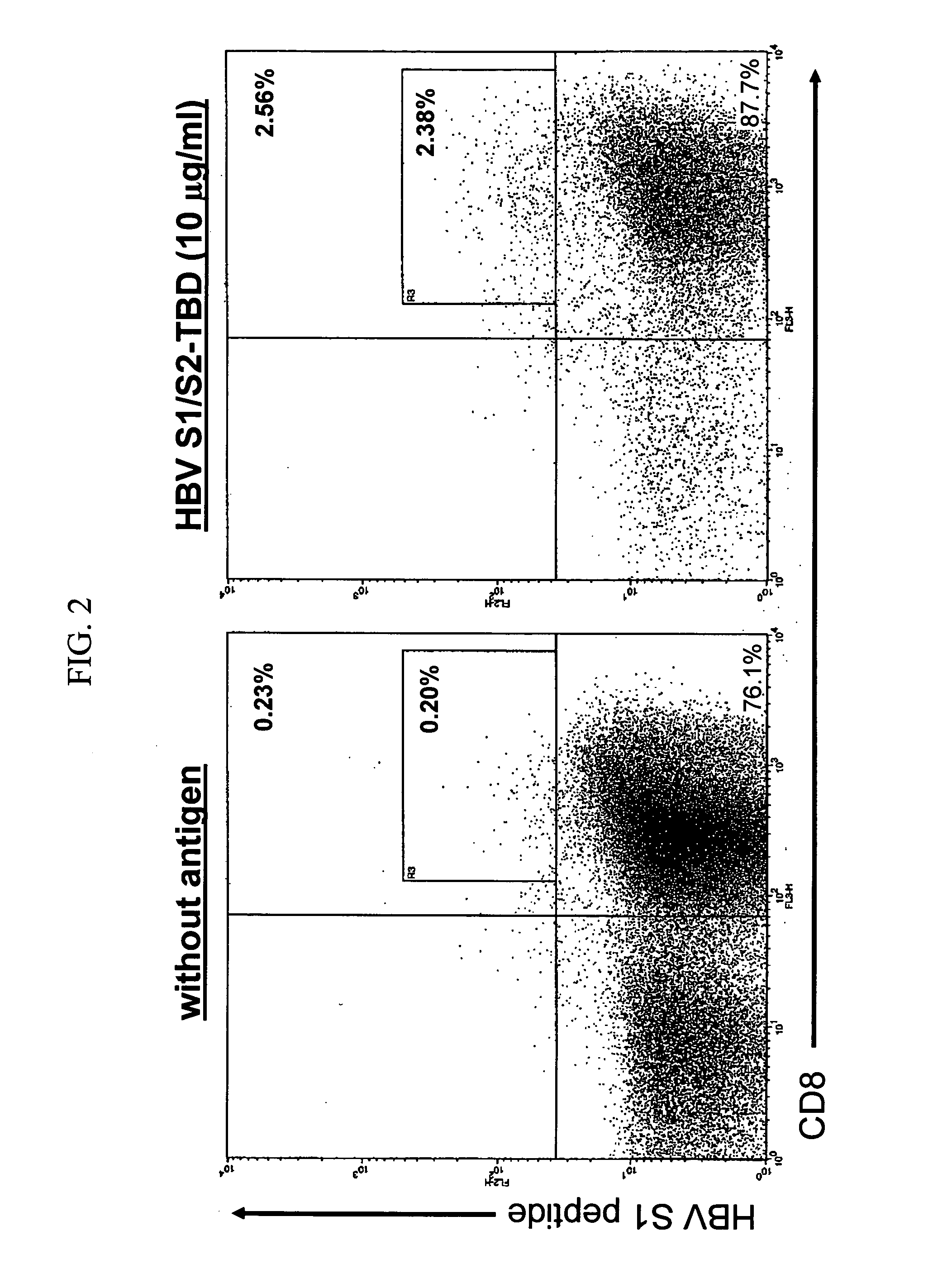
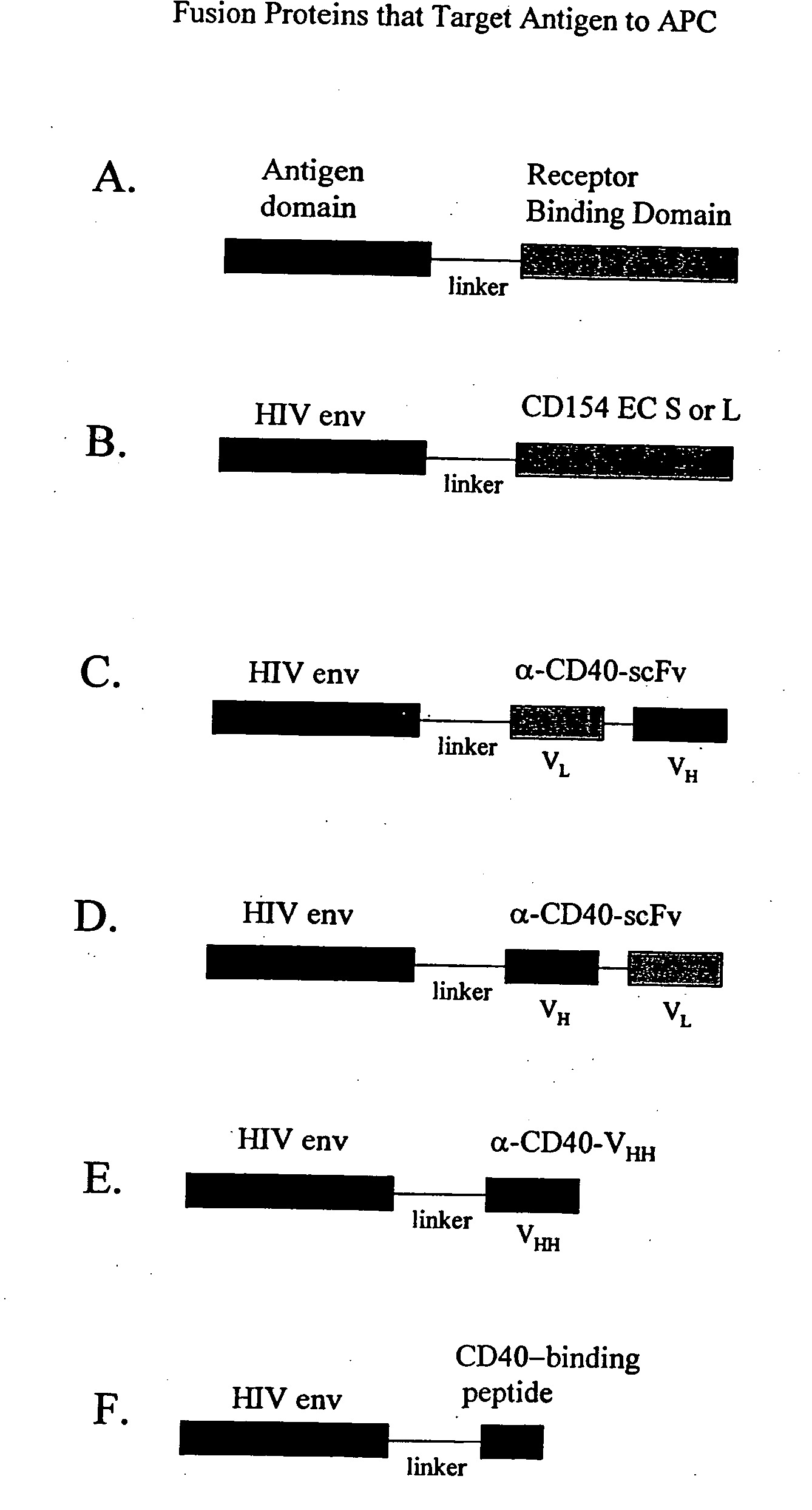
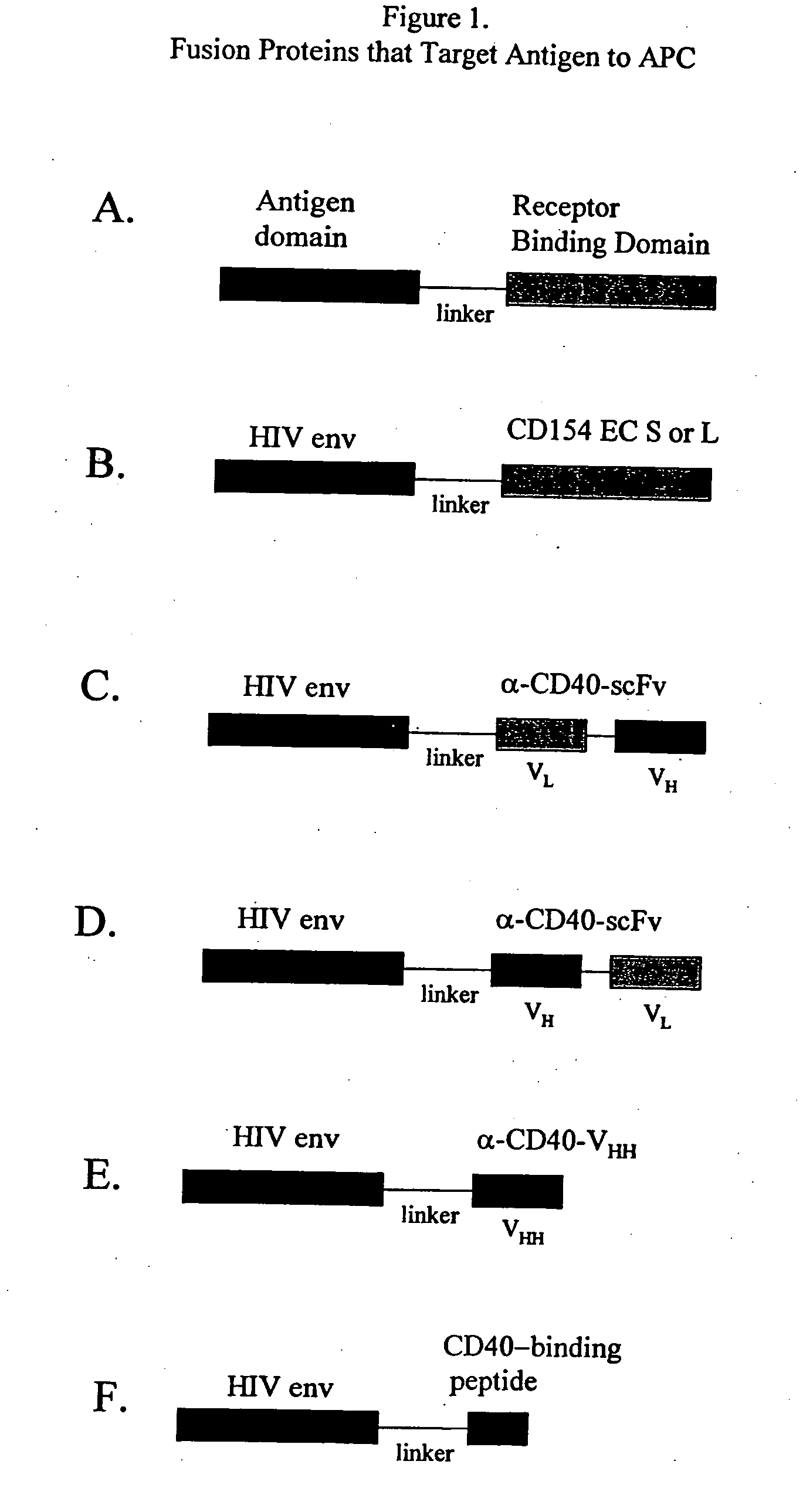
DNA vaccines encoding antigen linked to a domain that binds CD40
InactiveUS7118751B1Improve abilitiesEasy to demonstrateAntibody mimetics/scaffoldsVirus peptidesPeptide antigenEukaryotic plasmids
Vaccines that target one or more antigens to a cell surface receptor improve the antigen-specific humoral and cellular immune response. Antigen(s) linked to a domain that binds to a cell surface receptor are internalized, carrying antigen(s) into an intracellular compartment where the antigen(s) are digested into peptides and loaded onto MHC molecules. T cells specific for the peptide antigens are activated, leading to an enhanced immune response. The vaccine may comprise antigen(s) linked to a domain that binds at least one receptor or a DNA plasmid encoding antigen(s) linked to a domain that binds at least one receptor. A preferred embodiment of the invention targets HIV-1 env antigen to the CD40 receptor, resulting in delivery of antigen to CD40 positive cells, and selective activation of the CD40 receptor on cells presenting HIV-1 env antigens to T cells.
Owner:HAYDEN LEDBETTER MARTHA S +1
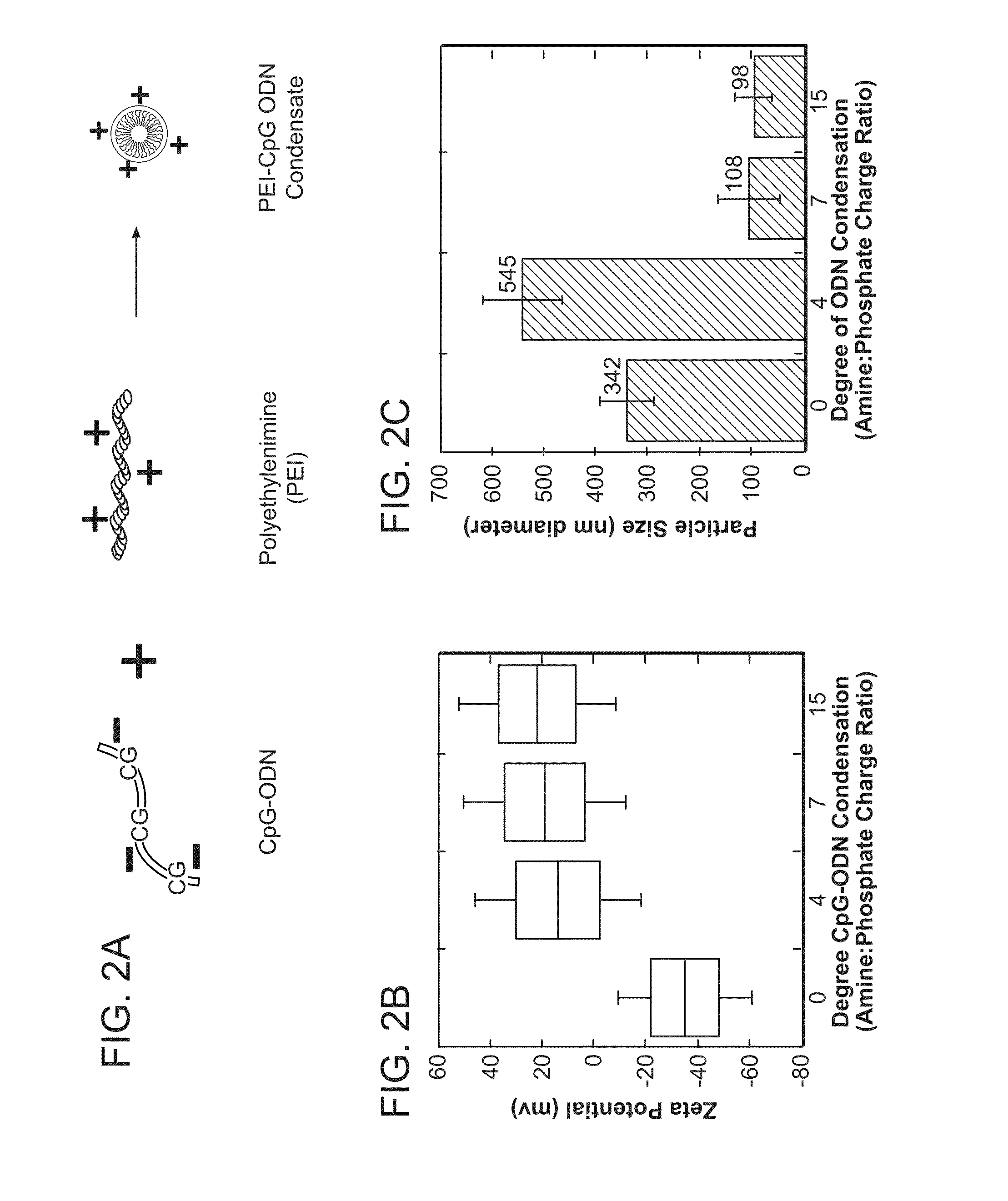
Method of inducing an antigen-specific immune response by administering a synergistic combination of adjuvants comprising unmethylated CpG-containing nucleic acids and a non-nucleic acid adjuvant
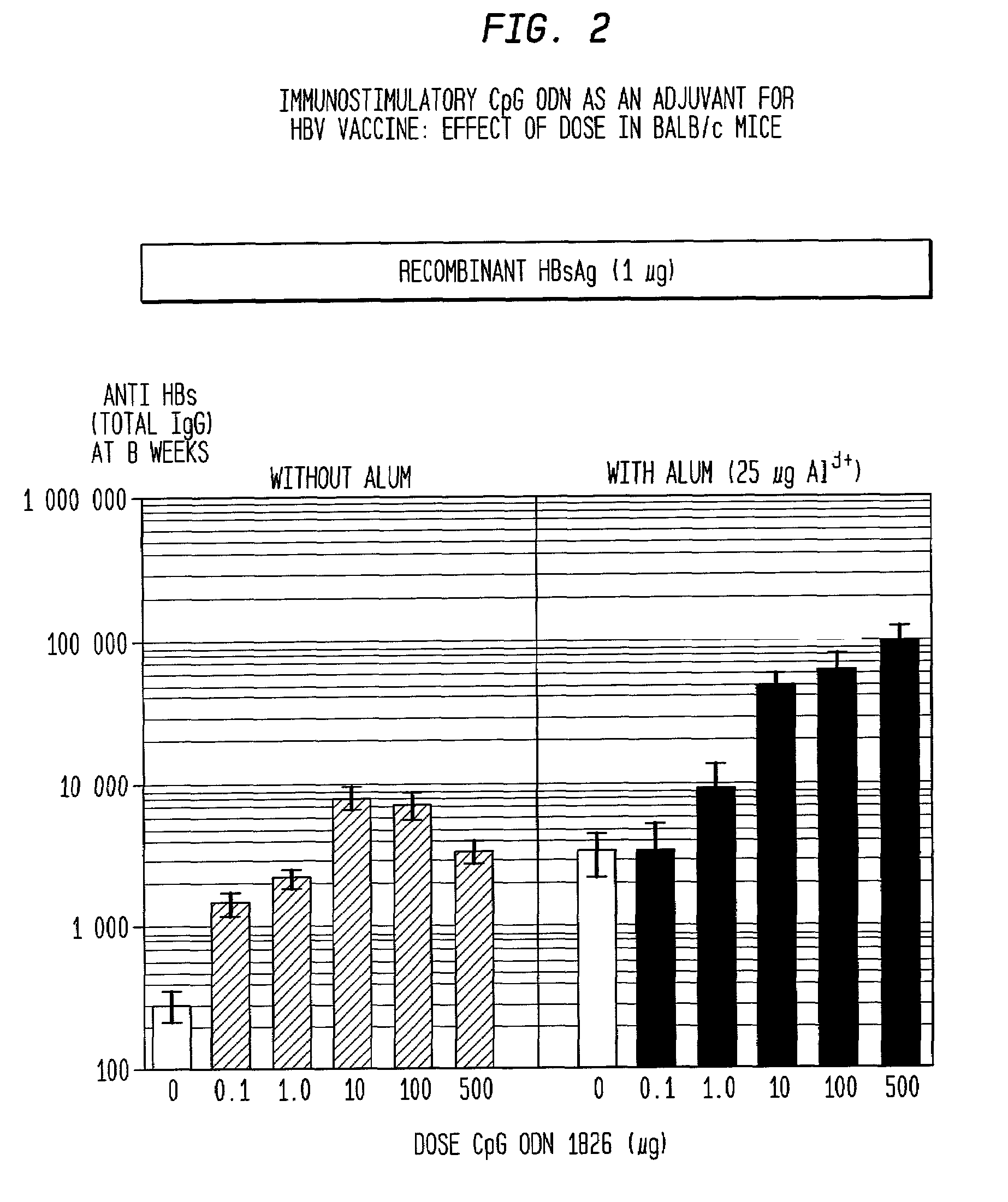
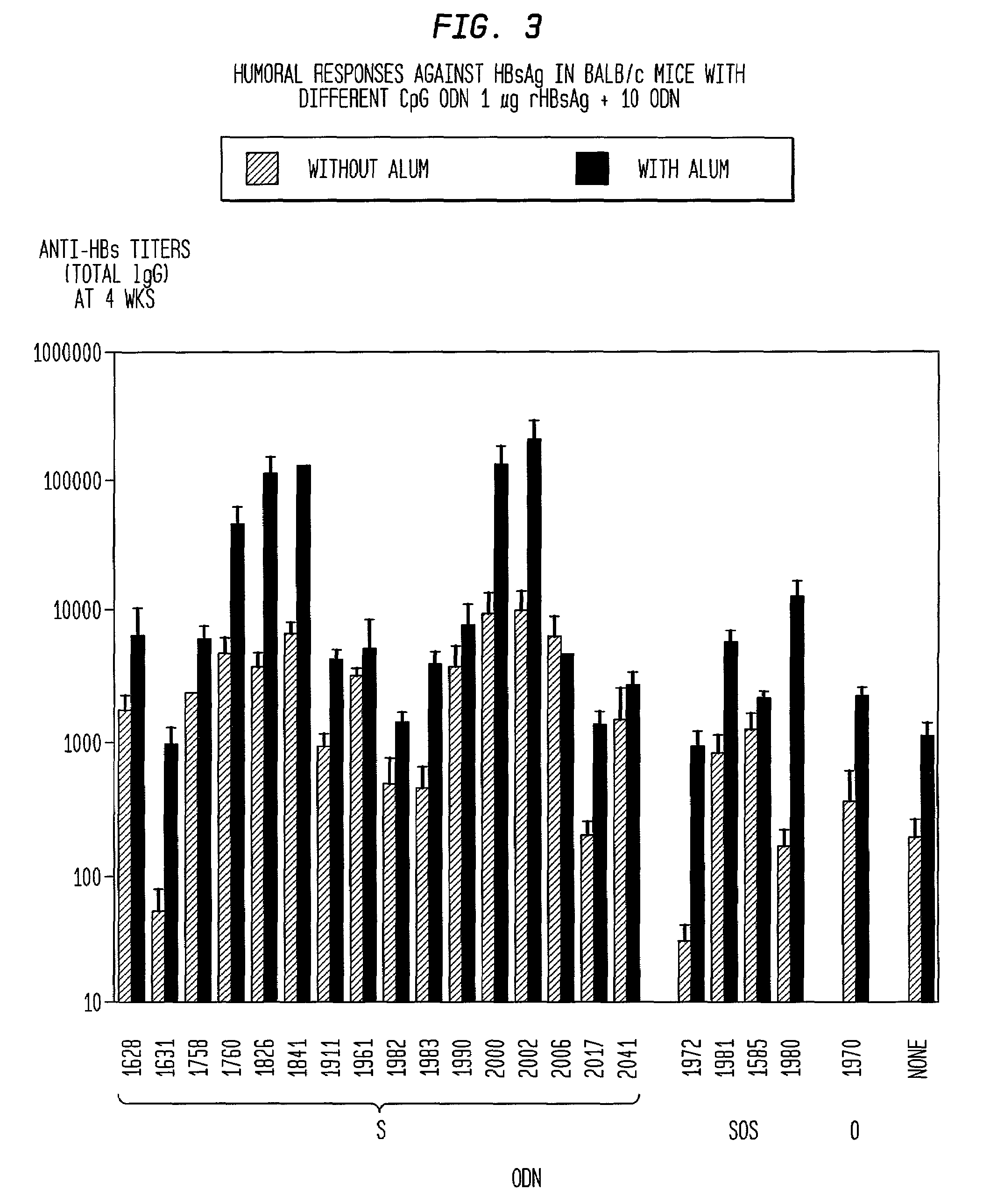
The present invention relates generally to adjuvants, and in particular to methods and products utilizing a synergistic combination of immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) and a non-nucleic acid adjuvant. Such combinations of adjuvants may be used with an antigen or alone. The present invention also relates to methods and products utilizing immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) for induction of cellular immunity in infants.
Owner:UNIV OF IOWA RES FOUND +2
Targeted cytolysis of HIV-infected cells by chimeric CD4 receptor-bearing cells
Disclosed is a method of directing a cellular immune response against an HIV-infected cell in a mammal involving administering to the mammal an effective amount of therapeutic cells which express a membrane-bound, proteinaceous chimeric receptor comprising (a) an extracellular portion which includes a fragment of CD4 which is capable of specifically recognizing and binding the HIV-infected cell but which does not mediate HIV infection and (b) an intracellular portion which is capable of signalling the therapeutic cell to destroy the receptor-bound HIV-infected cell. Also disclosed is a second method of treating HIV in a mammal involving administering to the mammal an effective amount of therapeutic cells expressing a membrane-bound, proteinaceous chimeric receptor comprising an extracellular portion which includes a fragment of CD4 which is capable of specifically recognizing and binding the HIV-infected cell but which does not mediate HIV infection. Also disclosed are cells which express the chimeric receptors and DNA and vectors encoding the chimeric receptors.
Owner:THE GENERAL HOSPITAL CORP
Immunostimulatory Combinations for Vaccine Adjuvants
This invention discloses immunostimulatory combinations of Tumor Necrosis Factor Receptor Superfamily (TN-FRSF) agonists, Toll-Like Receptor (TLR) agonists, “domain present in NAIP, CIITA, HET-E, TP-I (NACHT)-Leucine Rich Repeat (LRR)” or “NLR” agonists, RIG-I-Like Helicase or “RLH” agonists, purinergic receptor agonists and cytokine / chemokine receptor agonists, together with delivery methods. The combinations, when used alone at the site of pathology, provide immunostimulation that induces host humoral and cellular immunologic responses to eliminate pathogens or neoplasms. Alternatively, when the combinations are used with a defined antigens, these combinations can induce focused humoral and cellular immunologic responses useful as prophylactic and / or ameliorative therapeutic modalities for infections and the treatment of neoplastic disorders.
Owner:RGT UNIV OF CALIFORNIA
Redirection of cellular immunity by receptor chimeras
InactiveUS7049136B2Enhances immune system responseImprove responseVirusesPeptide/protein ingredientsAbnormal tissue growthCancer cell
Disclosed is a method of directing a cellular response in a mammal by expressing in a cell of the mammal a chimeric receptor which causes the cells to specifically recognize and destroy an infective agent, a cell infected with an infective agent, a tumor or cancerous cell, or an autoimmune-generated cell. Also disclosed are cells which express the chimeric receptors and DNA encoding the chimeric receptors.
Owner:THE GENERAL HOSPITAL CORP
Adjuvant combinations comprising a microbial tlr agonist, a cd40 or 4-1bb agonist, and optionally an antigen and the use thereof for inducing a synergistic enhancement in cellular immunity
InactiveUS20080241139A1Enhanced T cell responseImprove responseAntibacterial agentsAntimycoticsDiseaseYeast
Adjuvant combinations comprising at least one microbial TLR agonist such as a whole virus, bacterium or yeast or portion thereof such a membrane, spheroplast, cytoplast, or ghost, a CD40 or 4-1BB agonist and optionally an antigen wherein all 3 moieties may be separate or comprise the same recombinant microorganism or virus are disclosed. The use of these immune adjuvants for treatment of various chronic diseases such as cancers and HIV infection is also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
Treatment of B-cell associated diseases such as malignancies and autoimmune diseases using a cold anti-CD20 antibody/radiolabeled anti-CD22 antibody combination
InactiveUS20050112060A1Enhanced killing and depletionPrevent and inhibit relapseRadioactive preparation carriersAntibody ingredientsAutoimmune conditionRegimen
Owner:BIOGEN INC
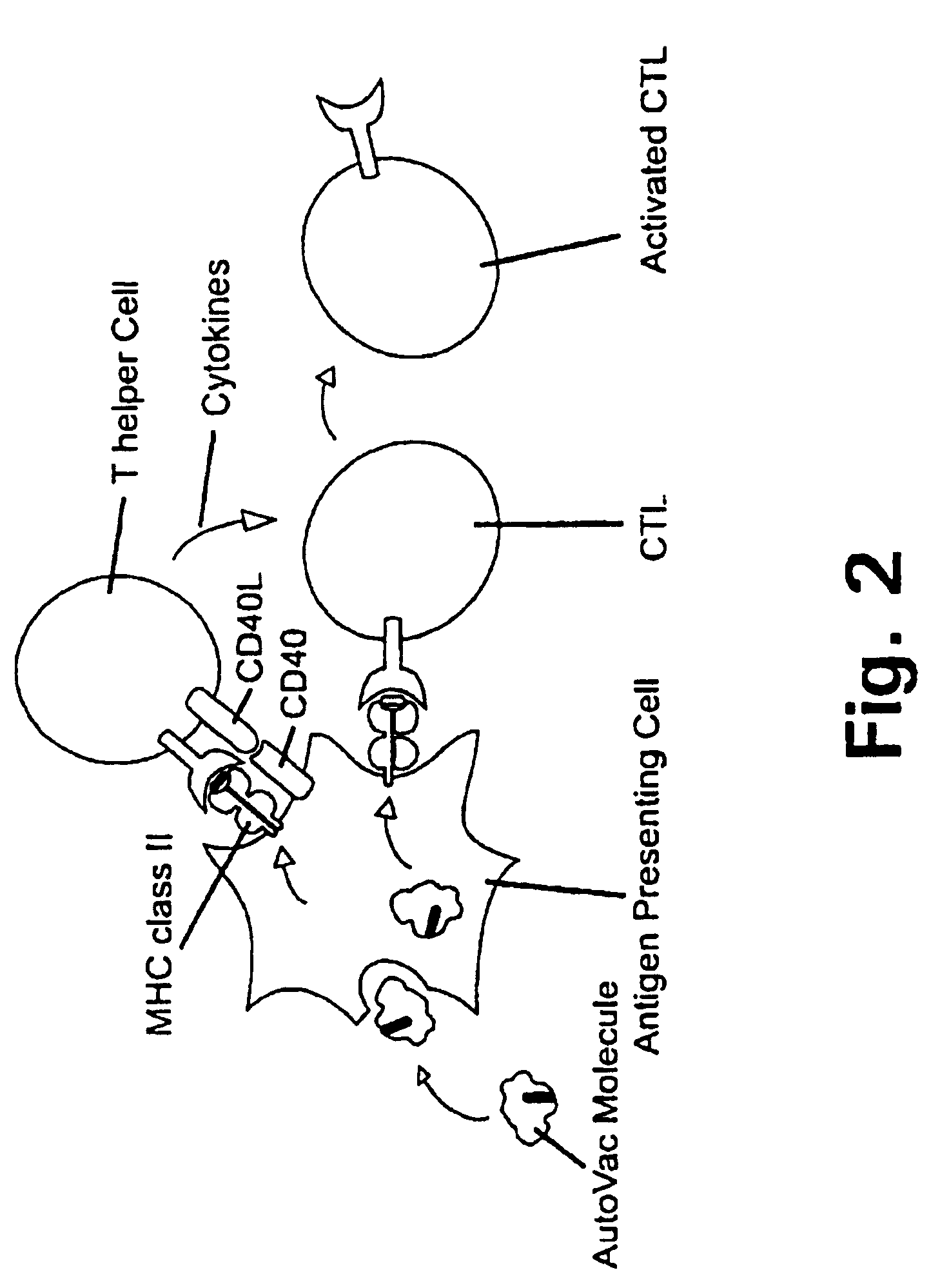
Novel methods for therapeutic vaccination
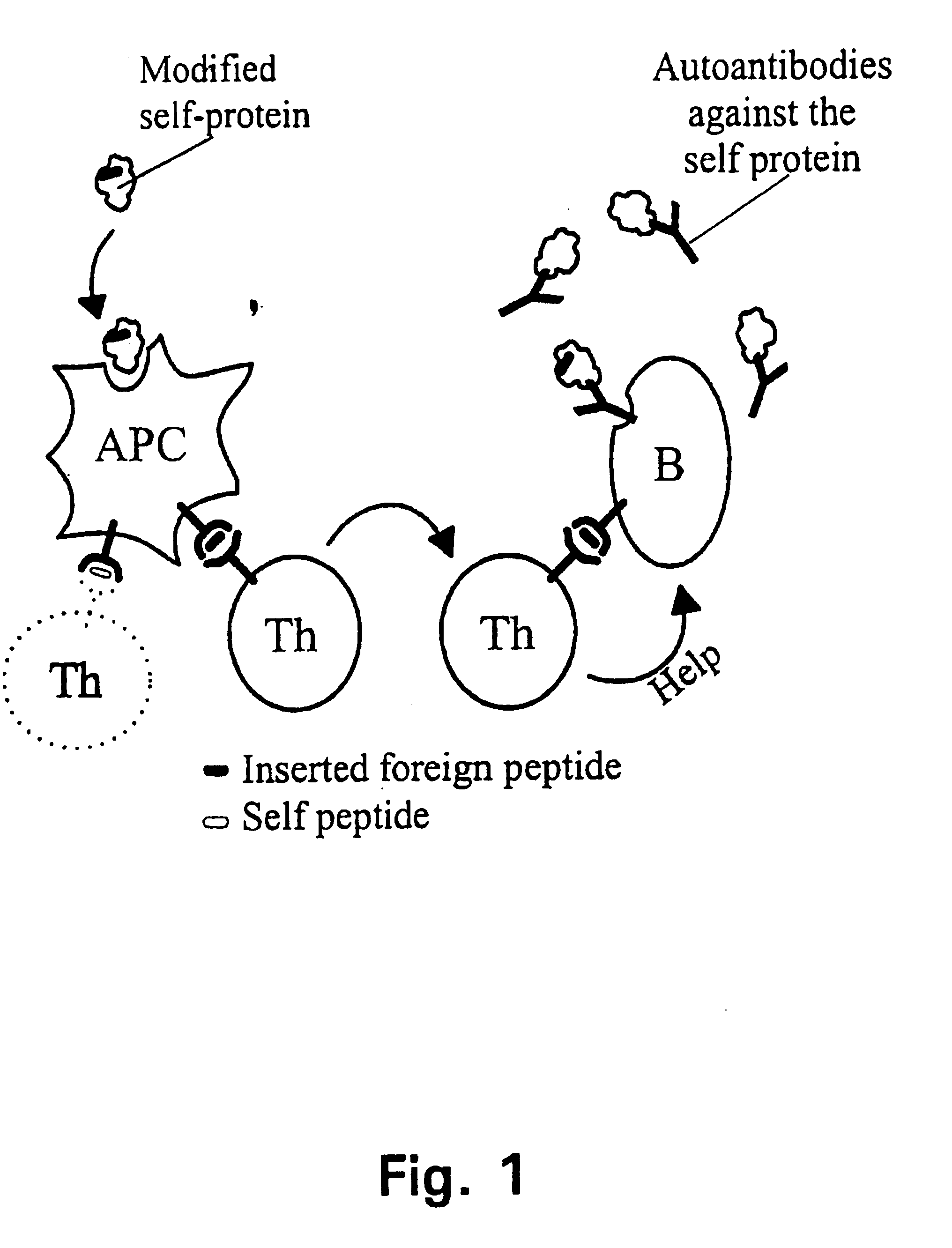
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
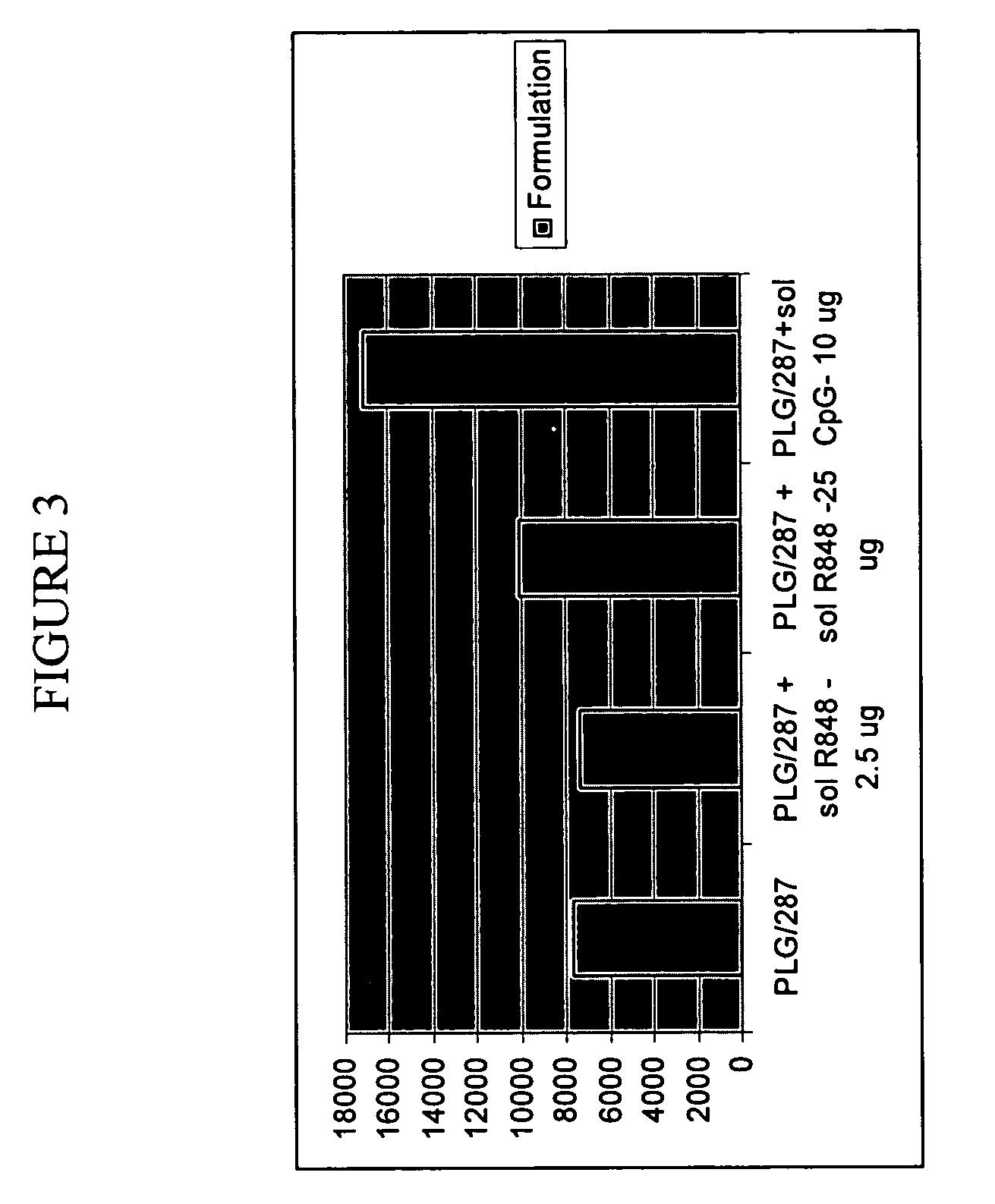
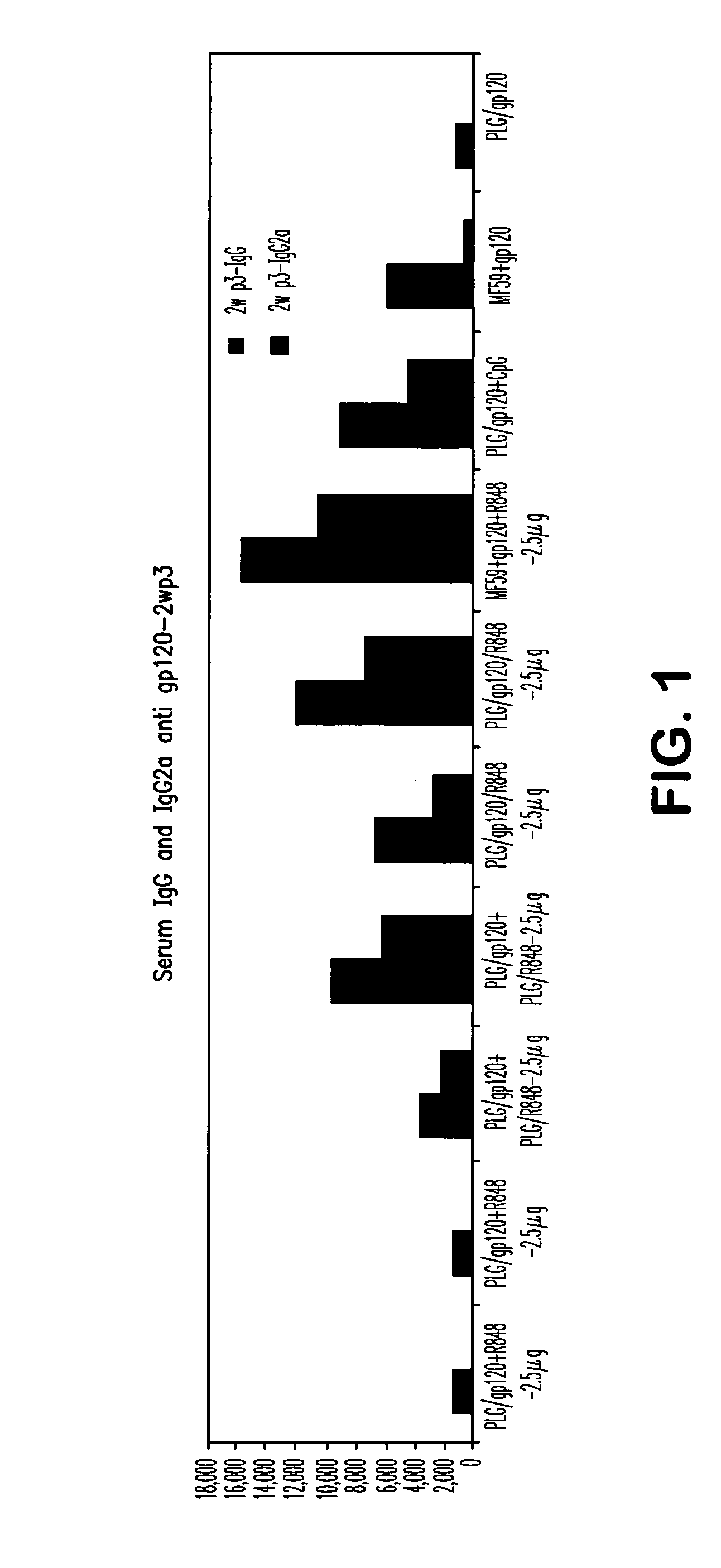
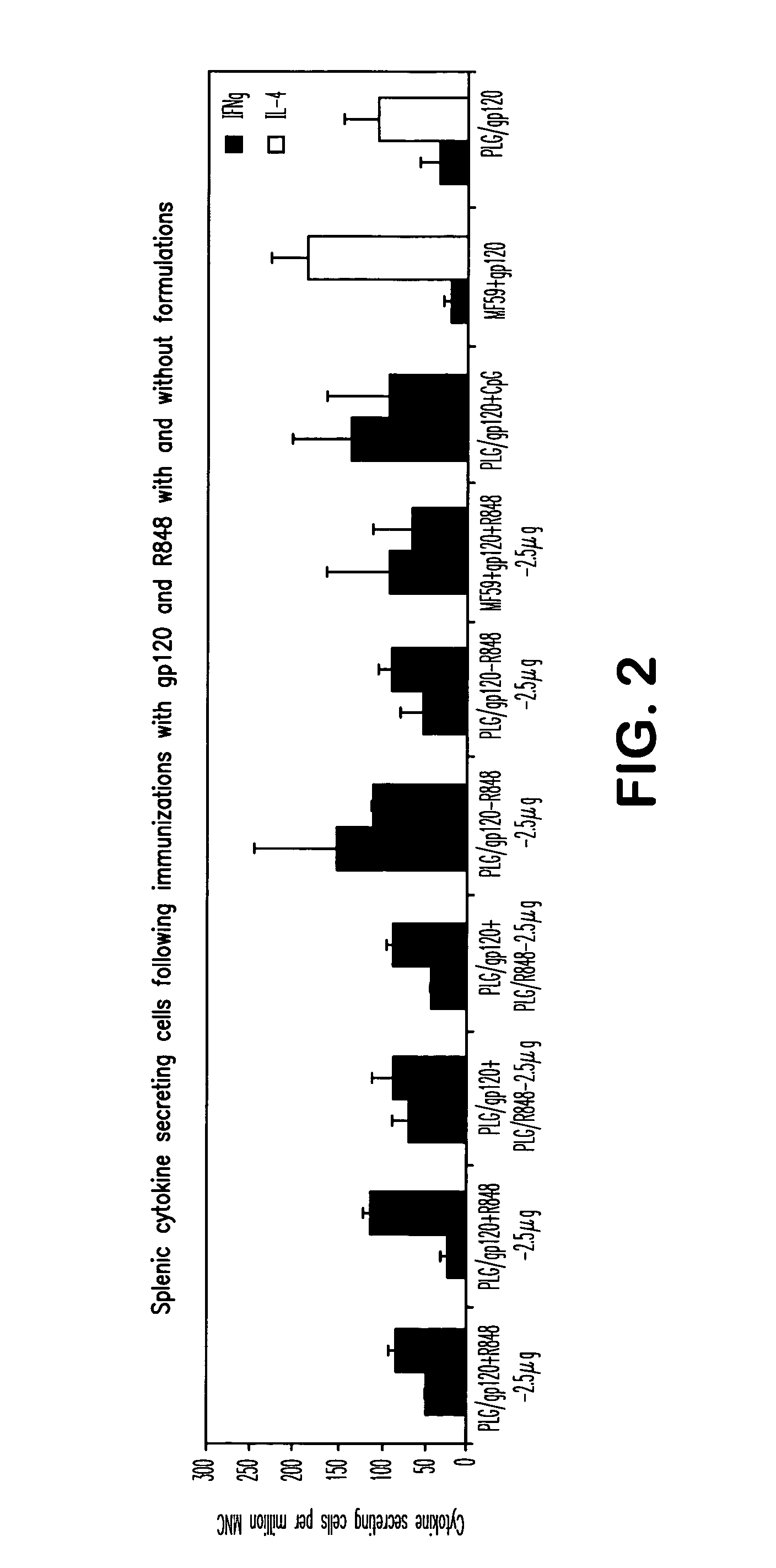
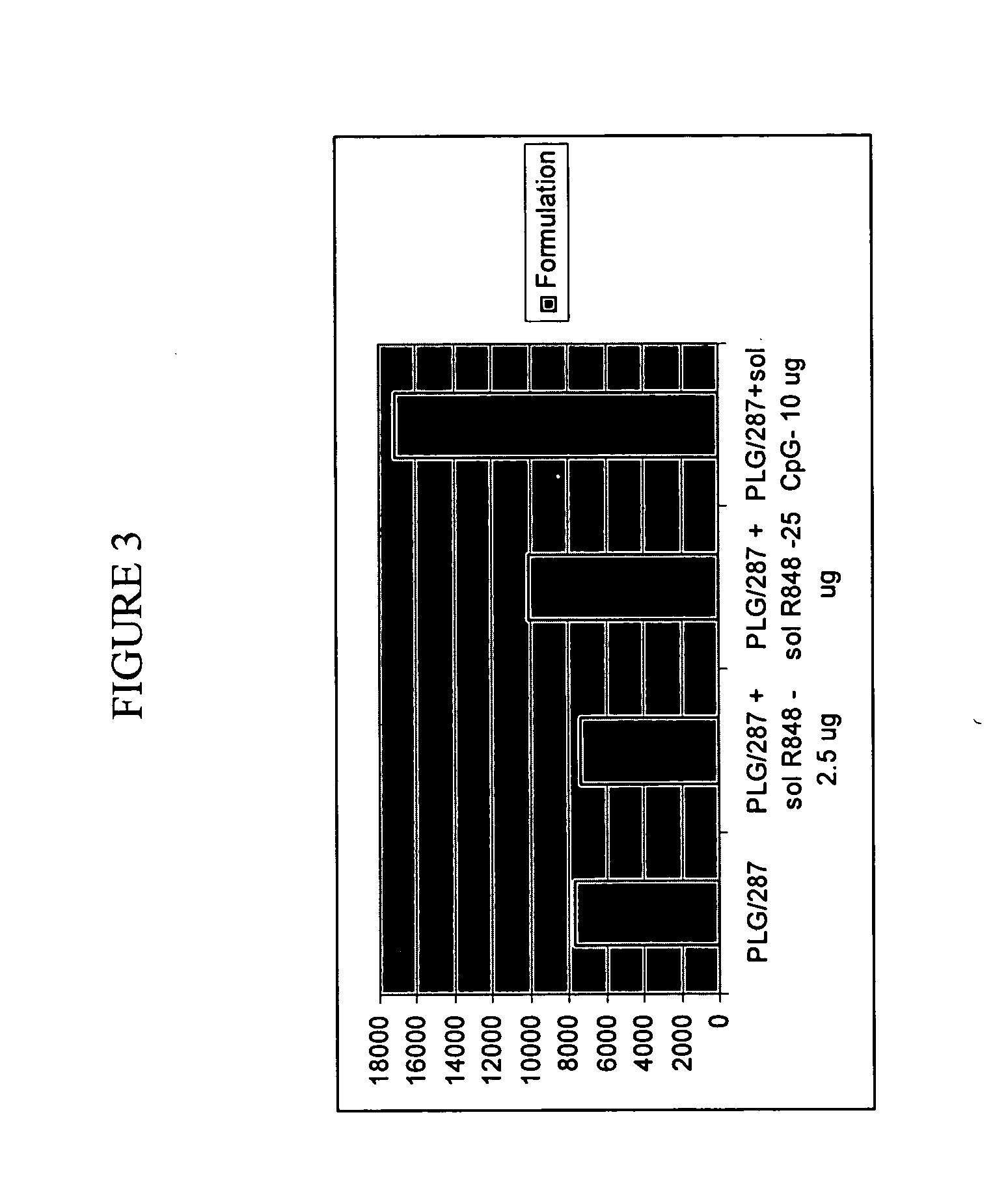
Controlled Delivery of TLR Agonists in Structural Polymeric Devices
ActiveUS20130202707A1Increase successStimulate immune responsePowder deliveryOrganic active ingredientsTlr agonistsDendritic cell
The present invention comprises compositions, methods, and devices for creating an stimulating an antigen-specific dendritic cell immune response. Devices and methods provide prophylactic and therapeutic immunity to subjects against cancer and infectious agents.
Owner:DANA FARBER CANCER INST INC +1
Inducing cellular immune responses to hepatitis B virus using peptide and nucleic acid compositions
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to develop epitope-based vaccines directed towards HBV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HBV infection.
Owner:PHARMEXA
Compositions for inducing immune responses
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions for inducing immune responses
ActiveUS20050107322A1Avoid inductionBacterial antigen ingredientsViral antigen ingredientsAdjuvantLactide
The invention provides, inter alia, immunogenic compositions comprising a first antigen, at least two adjuvants, wherein a first adjuvant comprises a polymer derived from poly(lactides) and / or poly(lactide-co-glycolides), and wherein a second adjuvant comprises an imidazoquinoline, wherein said first antigen is encapsulated within, adsorbed or conjugated to, co-lyophilized or mixed with said first adjuvant, and a pharmaceutically acceptable excipient, wherein said composition elicits a cellular immune response when administered to a vertebrate subject. The invention also provides methods of producing immunogenic compositions, methods for producing a cytotoxic-T lymphocyte (CTL) response in a vertebrate subject, and methods of immunization.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions and methods for eliciting an immune response to escape mutants of targeted therapies
InactiveUS20100034840A1Reduce resistanceSymptoms improvedAntibacterial agentsOrganic active ingredientsMolecular Targeted TherapiesMutant
Provided herein are cells, vectors and viruses in association with a mutant polypeptide that has emerged in response to a therapeutic or prophylactic agent; compositions comprising such cells, vectors and viruses and methods for their use in eliciting an immune response to the mutant polypeptide. In some examples, the immune response is a cellular immune response.
Owner:GLOBE IMMUNE INC
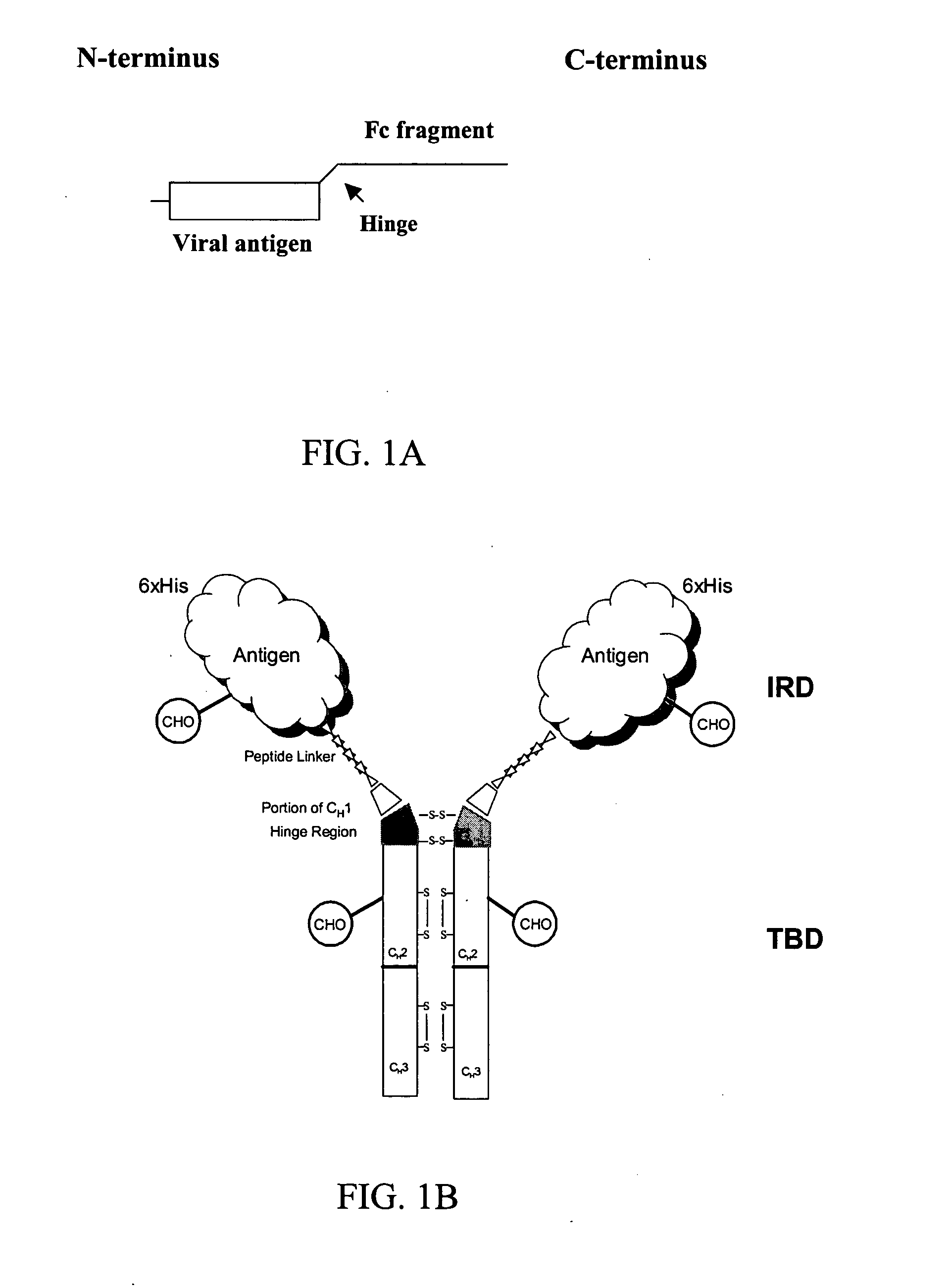
Chimeric antigens for eliciting an immune response
ActiveUS20050013828A1Effective presentationImprove efficiencyBiocideSsRNA viruses positive-senseHost immunityAntibody fragments
Disclosed herein are compositions and methods for eliciting immune responses against antigens. In particular embodiments, the compounds and methods elicit immune responses against antigens that are otherwise recognized by the host as “self” antigens. The immune response is enhanced by presenting the host immune system with a chimeric antigen comprising an immune response domain and a target binding domain, wherein the target binding domain comprises a xenotypic antibody fragment. By virtue of the target binding domain, antigen presenting cells take up, process, and present the chimeric antigen, eliciting both a humoral and cellular immune response.
Owner:KAIMI BIOMEDICINE (CHENGDU) CO LTD
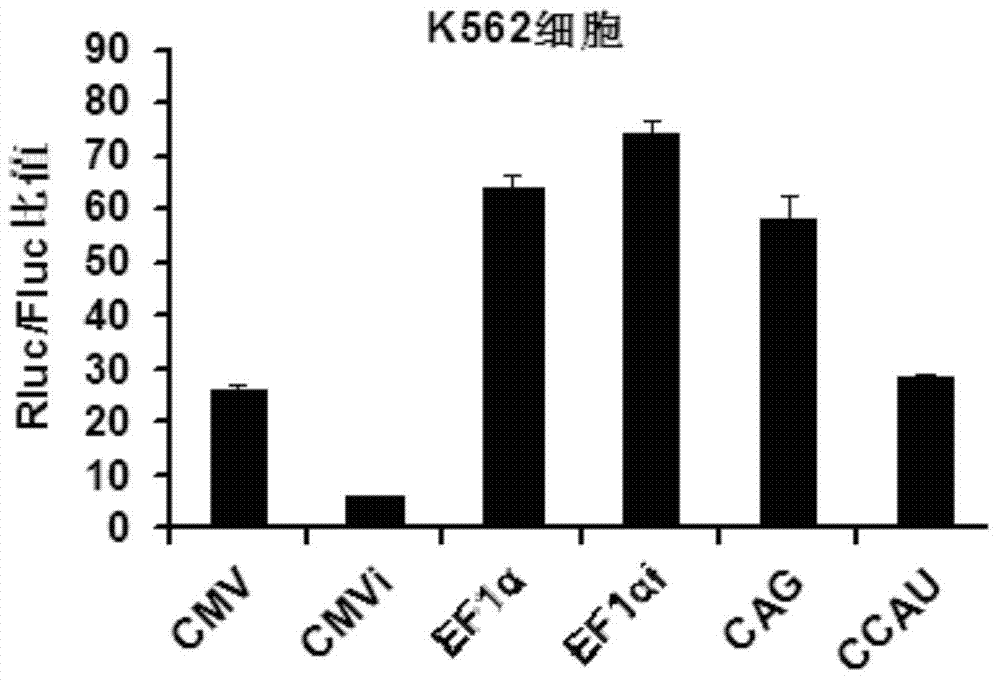
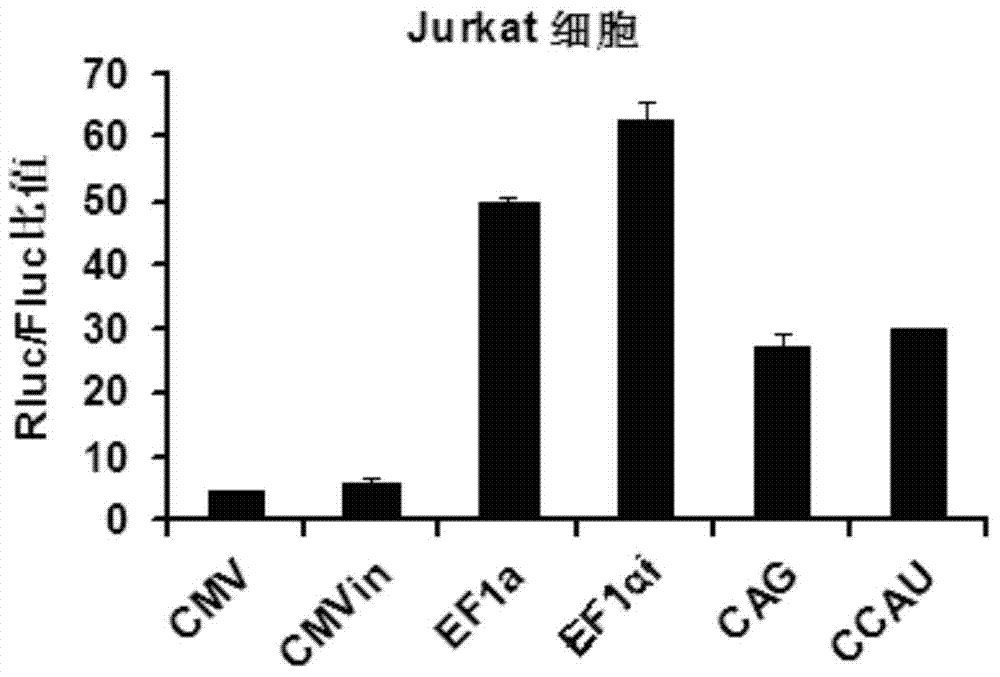
High-activity T-cell promoter and application thereof
ActiveCN104745581AEfficient expressionSequence stabilityGenetic material ingredientsAntibody ingredientsAdoptive cellular immunotherapyT cell
The invention belongs to the field of molecular biology and relates to a high-activity T-cell promoter and an application thereof. Particularly, the high-activity T-cell promoter comprises an element 1 and an element 2, wherein the element 1 is an EF1 alpha promoter and / or an EF1 alpha promoter containing an intron; and the element 2 is any one or more of an mCMV promoter, an hCMV promoter and a CD3e promoter. The high-activity T-cell promoter provided by the invention can be used for efficiently expressing a foreign gene in a T cell, has a stable sequence and is free from sequence loss during the transfer process of a prokaryotic cell and a eukaryotic cell. The high-activity T-cell promoter is applicable to driving the high-efficiency expression of the foreign gene, especially a full-length antibody gene, in the T cell during the process of adoptive cellular immunotherapy.
Owner:SHANGHAI CELL THERAPY RES INST +2
Chimeric antigens for breaking host tolerance to foreign antigens
ActiveUS20050031628A1Enhance immune responseAvoid infectionAntibacterial agentsSsRNA viruses positive-senseHost immunityAntibody fragments
Disclosed herein are compositions and methods for eliciting immune responses against antigens. In particular, the compounds and methods elicit immune responses against foreign antigens that are otherwise recognized by the host as "self" antigens, thus breaking host tolerance to those antigens. Presenting the host immune system with a chimeric antigen comprising an immune response domain and a target binding domain, wherein the target binding domain comprises an antibody fragment, enhances the immune response against the foreign or tolerated antigen. Antigen presenting cells take up, process, and present the chimeric antigen, eliciting both a humoral and cellular immune response against the desired antigen.
Owner:KAIMI BIOMEDICINE (CHENGDU) CO LTD
DNA vaccines encoding antigen linked to a domain that binds CD40
InactiveUS20070025982A1Improve abilitiesEasy to demonstrateAntibody mimetics/scaffoldsVirus peptidesPeptide antigenEukaryotic plasmids
Vaccines that target one or more antigens to a cell surface receptor improve the antigen-specific humoral and cellular immune response. Antigen(s) linked to a domain that binds to a cell surface receptor are internalized, carrying antigen(s) into an intracellular compartment where the antigen(s) are digested into peptides and loaded onto MHC molecules. T cells specific for the peptide antigens are activated, leading to an enhanced immune response. The vaccine may comprise antigen(s) linked to a domain that binds at least one receptor or a DNA plasmid encoding antigen(s) linked to a domain that binds at least one receptor. A preferred embodiment of the invention targets HIV-1 env antigen to the CD40 receptor, resulting in delivery of antigen to CD40 positive cells, and selective activation of the CD40 receptor on cells presenting HIV-1 env antigens to T cells.
Owner:LEDBETTER JEFFREY A +1
Preparation and application of exosome secreted by human derived blood or mesenchymal stem cell
InactiveCN103767985AAvoid blocking, not easily absorbed by cellsProtection stabilityCosmetic preparationsSenses disorderStem cell cultureSomatic cell
The invention discloses preparation and application of exosome secreted by human derived blood or mesenchymal stem cell. The preparation process of exosome comprises: extracting mesenchymal stem cells from health-human body blood or a stem cell culture supernatant, performing in-vitro culture and amplification, and utilizing means such as low-temperature separation, purification and the like to prepare high-purity high-bioactivity exosome. The blood or mesenchymal stem cell secreted exosome is added into medicines, health-care products and cosmetics according to ratios for developing of products with bioactivity, is capable of effectively regulating and controlling body cell signal transduction, activating cell regeneration, enhancing health cell proliferation and differentiation capacity, further regulating physiologic restoration or removing damaged, pathologic and aged cells of a body, fundamentally changing body physiologic functions, and helping to generate anti-tumor treatment effect by adjusting cellular immunity. The blood or mesenchymal stem cell secreted exosome is applicable to fields of medical science, health care and beauty treatment, and has wide application prospect.
Owner:珠海霍普金斯医药研究院股份有限公司
Inducing cellular immune responses to her2/neu using peptide and nucleic acid compositions
InactiveUS20040121946A9Improving immunogenicityReduce the possibilityAntibacterial agentsBiocideEpitopeT cell
This invention uses our knoeledge of the mechanisms by which antigen is recognized by T cells to identify and prepare HER2 / neu epitopes, and to develop epitope-based vaccines directed towards HERS2 / neu-bearing tumors. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of cancer.
Owner:BIOTECH SYNERGY
gRNA for knockout of wild type T cell TCR [beta] strand and method
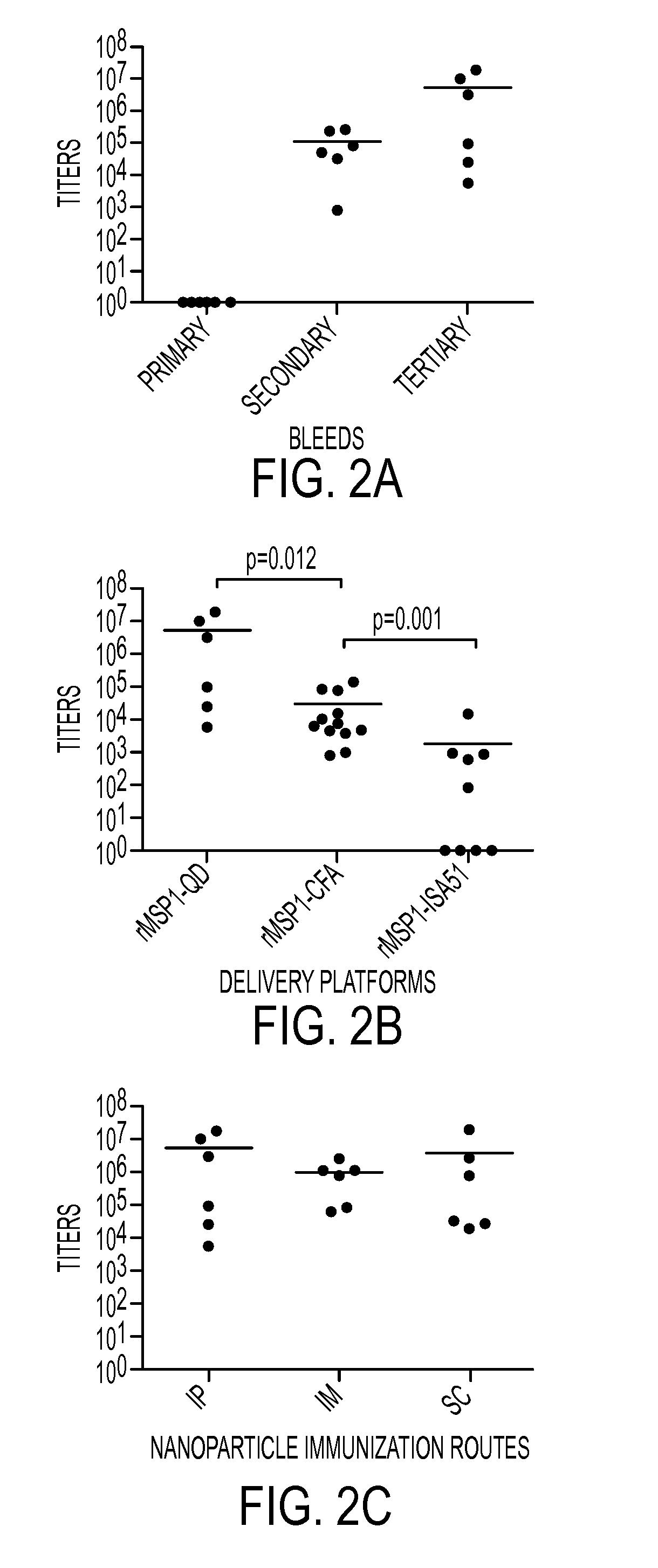
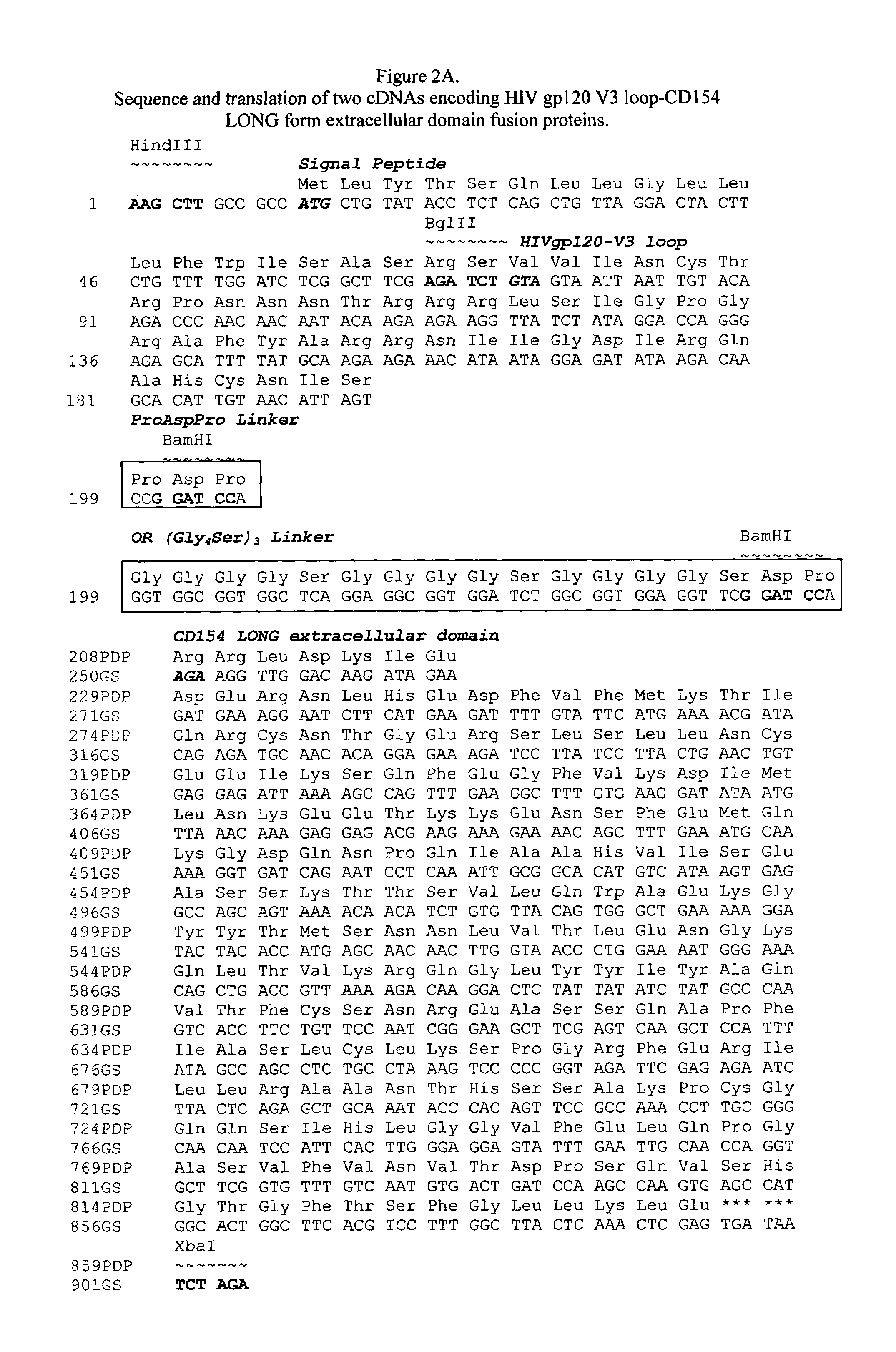
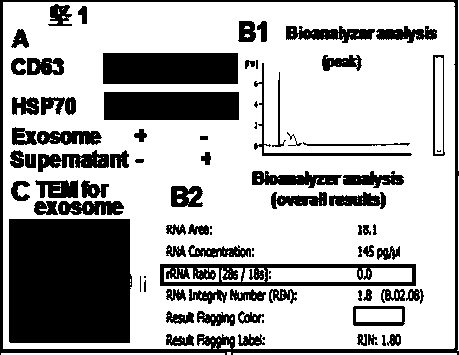
ActiveCN107354156AHigh knockout efficiencySimple manufacturing methodImmunoglobulin superfamilyStable introduction of DNAT cellCellular immunity
The invention discloses gRNA for knockout of a wild type T cell TCR [beta] strand and a method. The sequence of the gRNA is as shown in SEQ ID NO: 1, by utilizing a CRISPR / Cas9 technology, the gRNA and CRISPR / Cas9 jointly infect a T cell, the wild type T cell TCR [beta] strand is knocked out, and the T cell lacking the wild type TCR [beta] strand is constructed and used for CAR-T or TCR-T cellular immunotherapy. According to the gRNA for knockout of the wild type T cell TCR [beta] strand and the method, the knockout efficiency is high, the preparation method is relatively simple and easy, and T cells lacking wild type TCR [beta] strands can be provided for clinic rapidly and efficiently.
Owner:THE FIFTH AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
Method of obtaining cellular immune responses from proteins
InactiveUS7604802B2Improving immunogenicityPowerful toolNanotechViral antigen ingredientsExcipientCellular immunity
A method for producing a cellular immune response in a vertebrate subject comprising administering to the vertebrate subject a vaccine composition comprising a protein particle antigen and a pharmaceutically acceptable excipient is disclosed.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Bioactive polypeptide LPLP, and preparation and application thereof
ActiveCN103232526AReduce harmImprove immunityTetrapeptide ingredientsAntinoxious agentsReducing bodiesDrug biological activity
The invention relates to the field of protein, and specifically relates to a milk-derived bioactive polypeptide LPLP with in-vitro antioxidant activity and immunity improving function. An amino acid sequence of the polypeptide is Leu-Pro-Leu-Pro. In-vitro anti-oxidation and in-vitro immune function promotion experiments prove that the polypeptide LPLP has good anti-oxidation bioactivity and cellular immunity promotion activity. With the polypeptide LPLP, free radicals in bodies can be removed, such that harm of the free radicals to human bodies can be reduced; and the bioactive polypeptide LPLP can enhance macrophage phagocytic function, can improve body capacity for resisting external pathogens, can reduce body incidence rate, and does not cause body immunological rejection. The polypeptide has important significance in developing dairy products, health products and medicines with anti-oxidation function and immunity enhancing function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
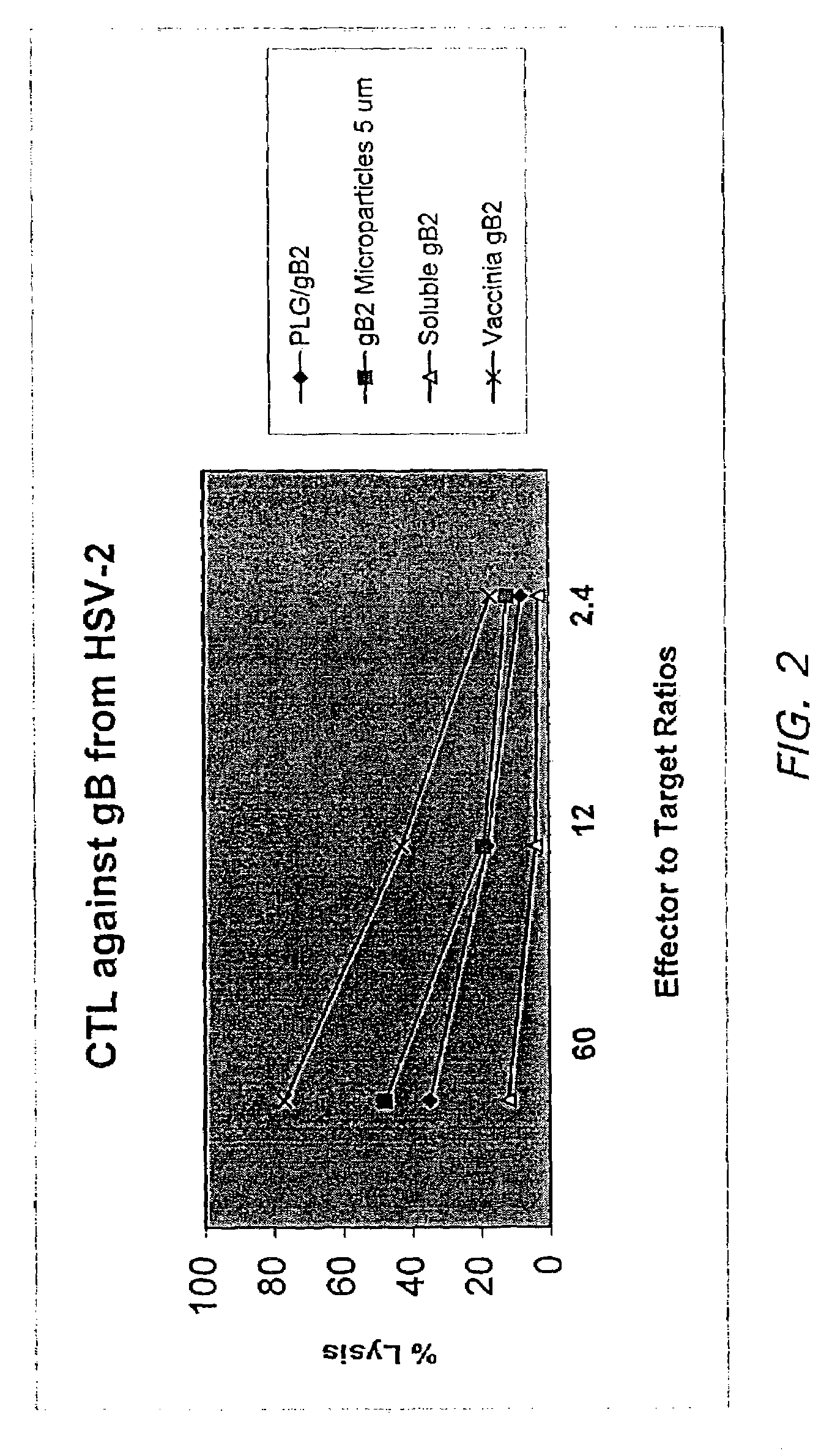
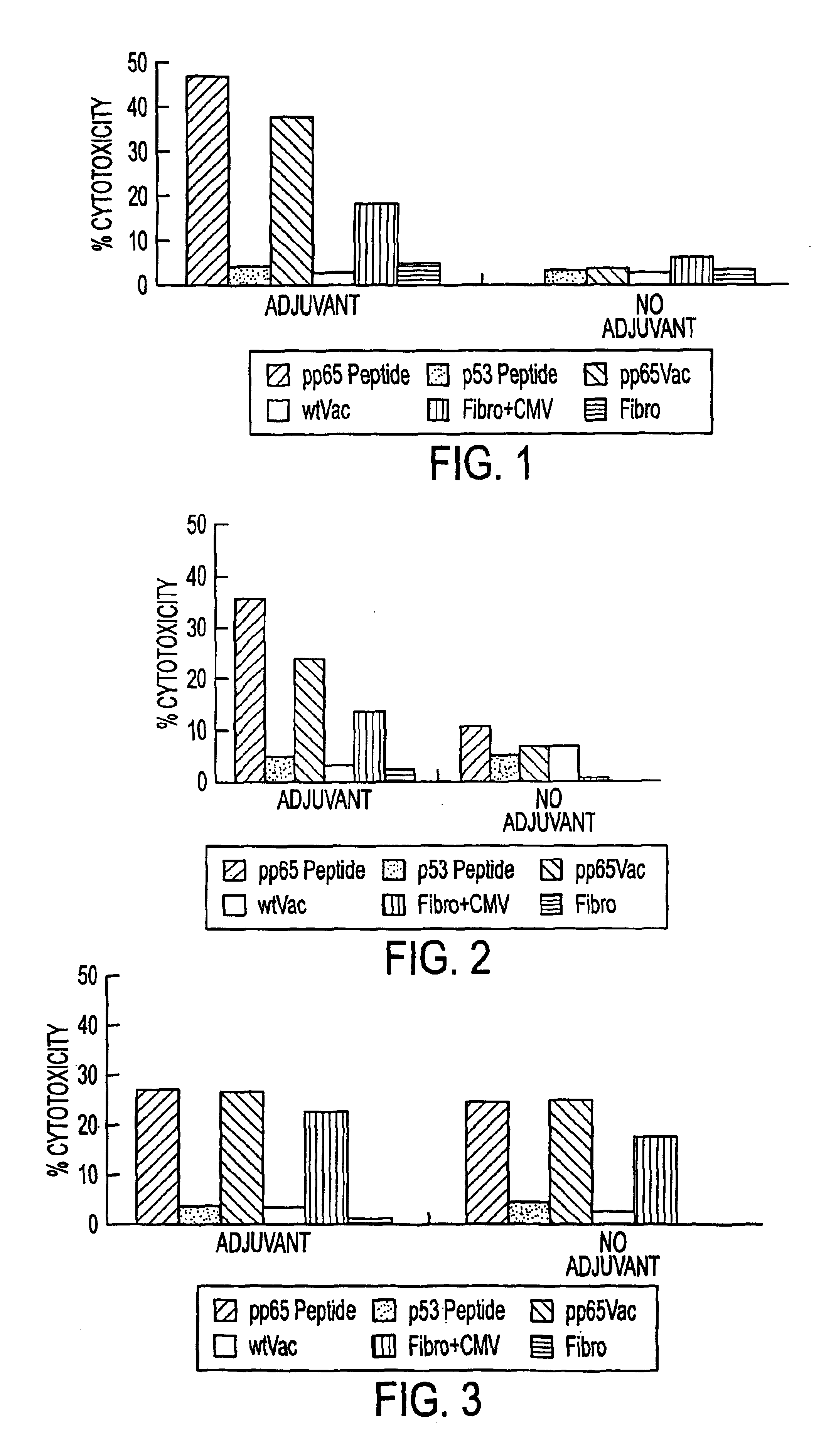
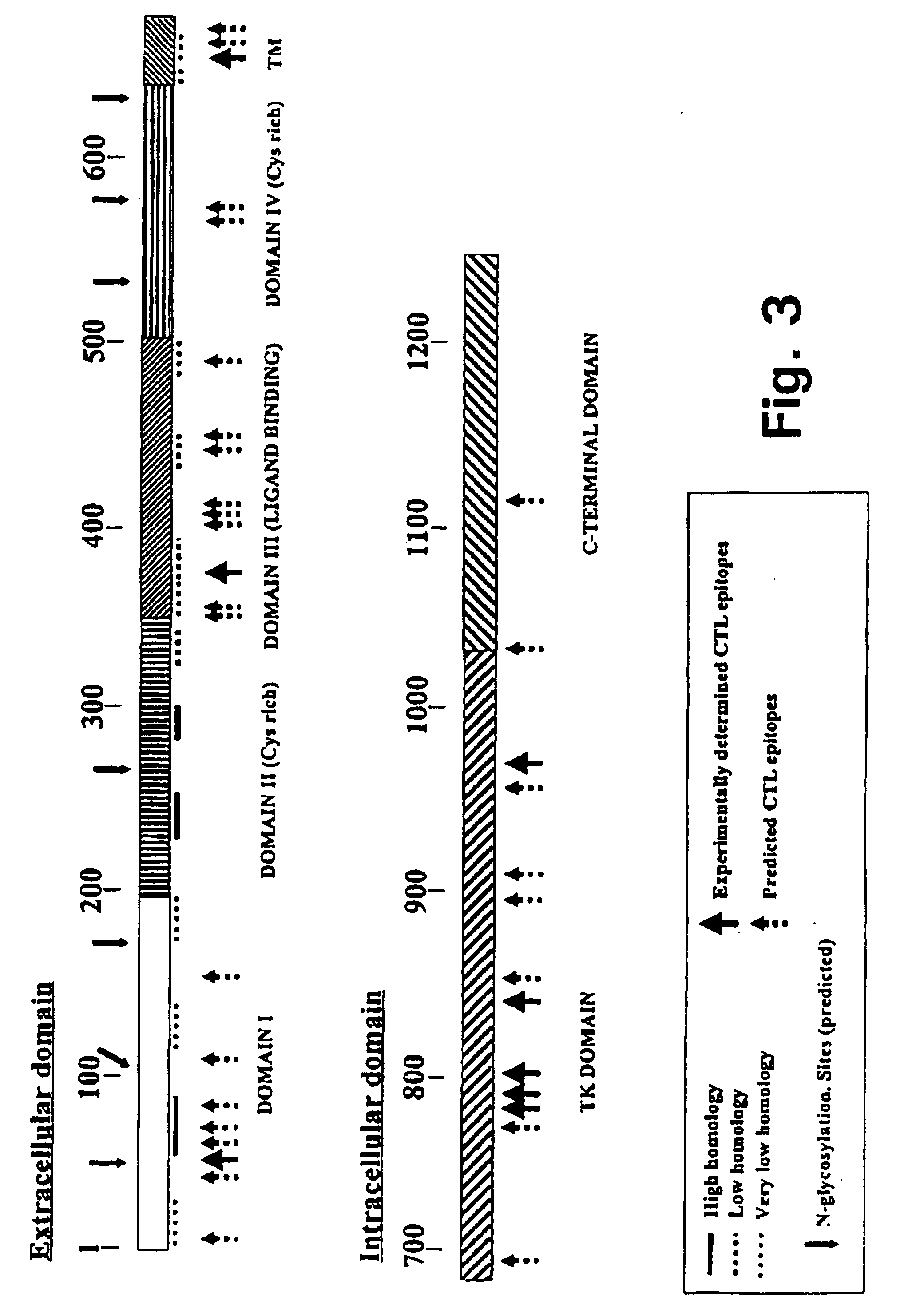
Immuno-reactive peptide CTL epitopes of human cytomegalovirus
The invention provides a plurality of peptides (and immunologically functional variants thereof) which are immunogenic epitopes recognized by CD8<+> class I MHC restricted cytotoxic T-lymphocytes of patients harboring latent human cytomegalovirus (HCMV) infection. The peptides are capable of activating CTLs and CTLps in the absence of active viral replication, and thus are useful for eliciting a cellular immune response against HCMV by normal and immunodeficient subjects. Peptide and lipopeptide vaccines, with and without adjuvants, also are disclosed. Cellular vaccines comprising the peptides form a further embodiment of this invention.
Owner:CITY OF HOPE
Vaccine composition
InactiveUS20140220063A1Cellular immunity effectivelyEasy to viewPeptide/protein ingredientsPharmaceutical delivery mechanismCellular immunity
The invention provides a vaccine composition containing an antigen for inducing cellular immunity, comprising at least one first cellular immunity induction promoter.
Owner:NITTO DENKO CORP
Preparation method of human cytokine-induced killer cells
InactiveCN102732481APromote proliferationRaise the ratioBlood/immune system cellsHybrid peptidesPeripheral blood mononuclear cellCytotoxicity
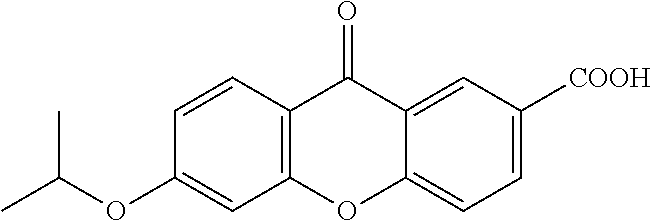
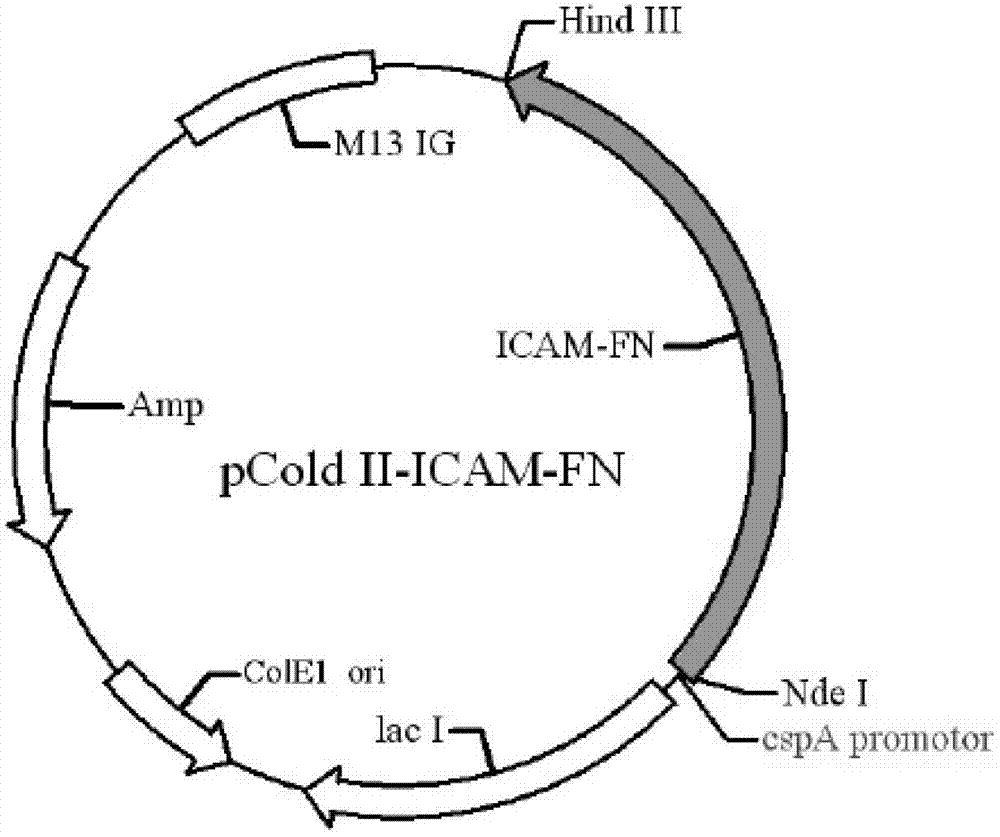
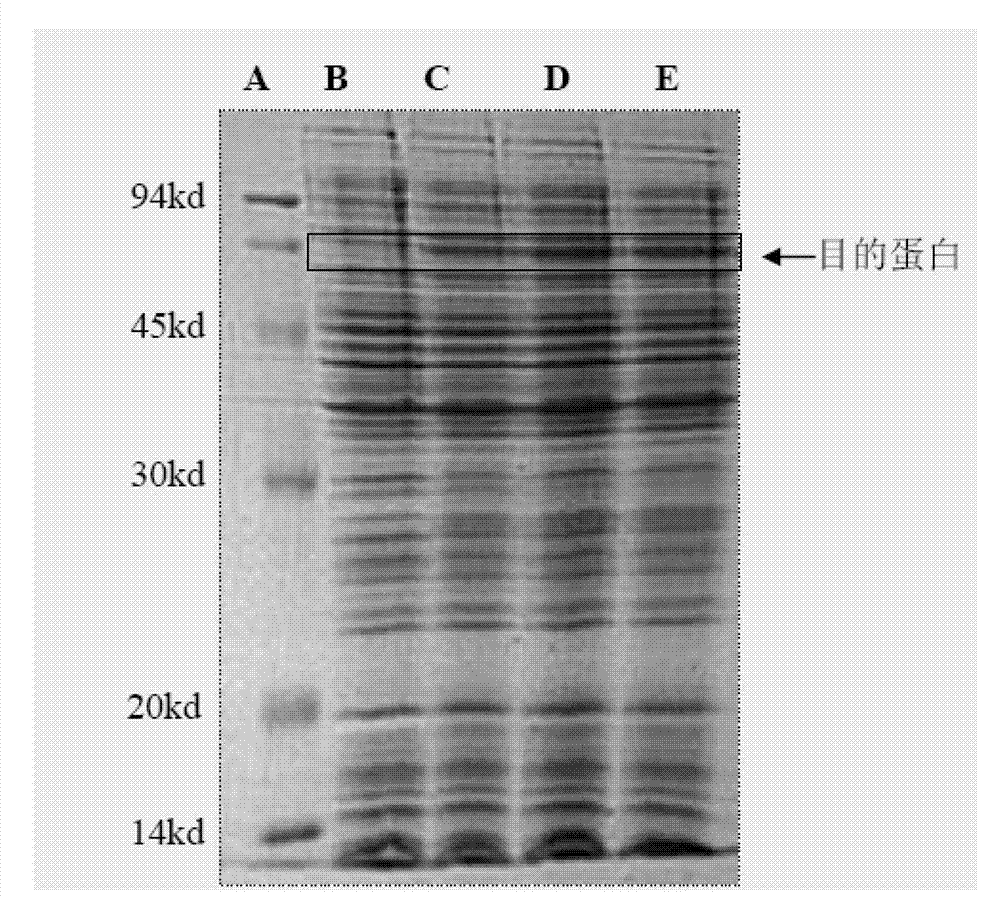
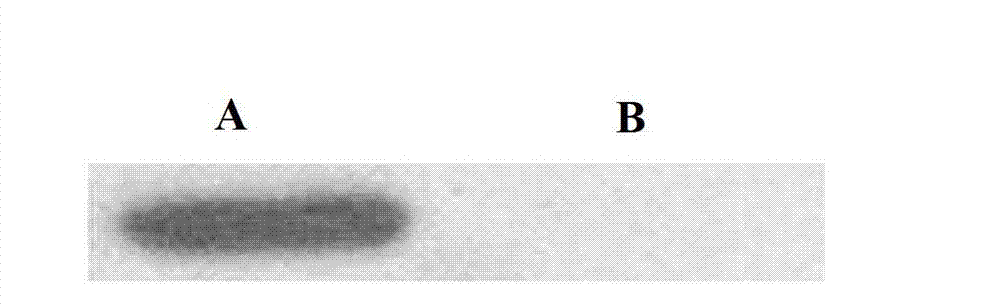
The invention discloses a preparation method of human cytokine-induced killer cells, comprising the following steps: coating a cell culture flask with a coating buffer containing effective amount of fusion protein and human CD3 monoclonal antibody before culturing precursor cells of human CIK cells, and adding the human CD3 monoclonal antibody in the whole process of inducing and culturing the human CIK cells, wherein the fusion protein is human intercellular adhesion molecule-1 functional domain and human fibronectin functional domain fusion protein, and the concentration of the human CD3 monoclonal antibody in the cell culture solution is lower than the concentration of the human CD3 monoclonal antibody in the coating buffer. According to the invention, ex-vivo expansion efficiency of peripheral blood mononuclear cells and the proportion of CD3 / CD56 double positive cells in the CIK cells are significantly raised, the cytotoxicity activity of the CIK cells is enhanced, thus the effect of cellular immunity treatment is raised.
Owner:SHENZHEN YOUNGCELL BIO TECH
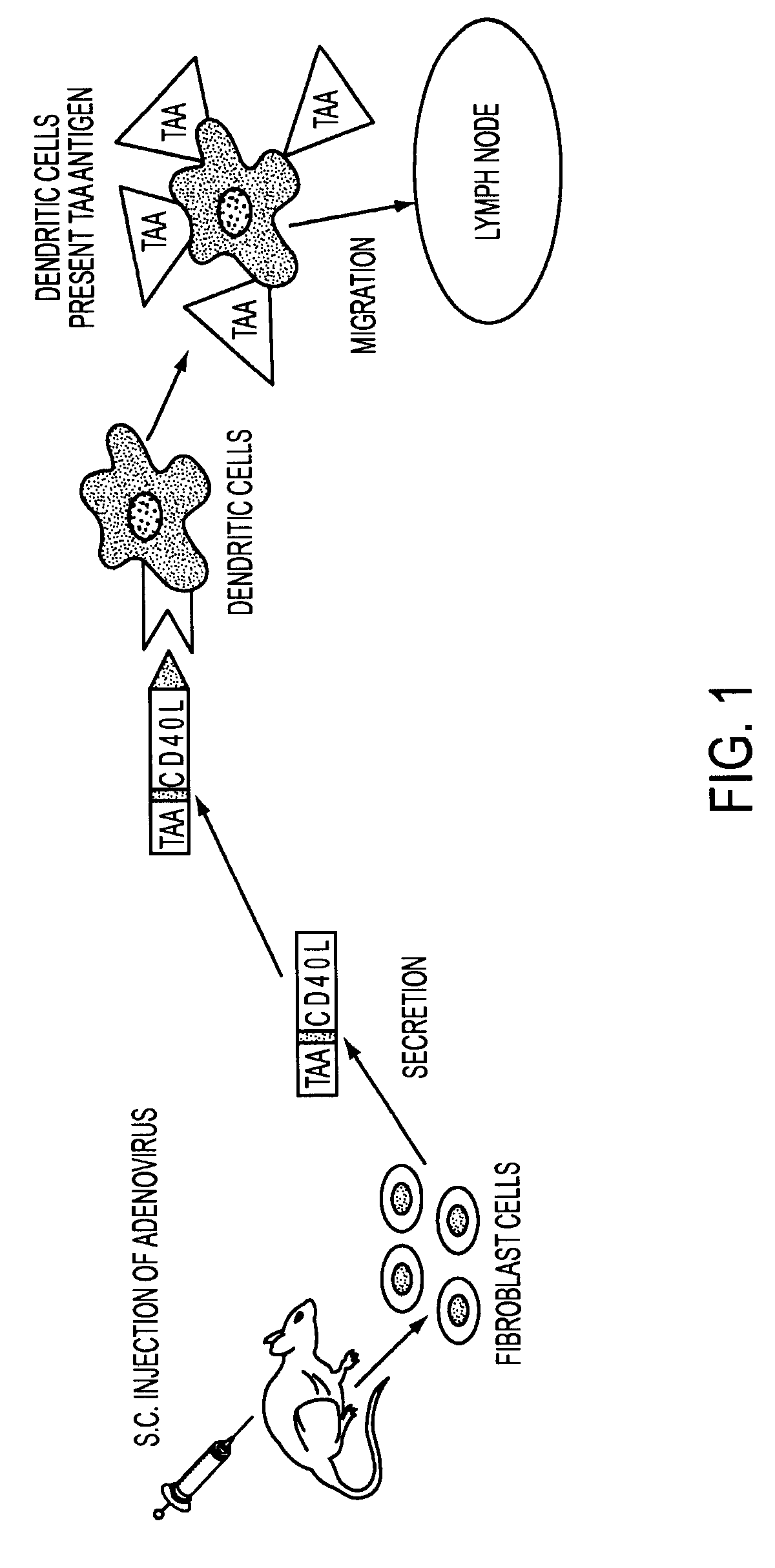
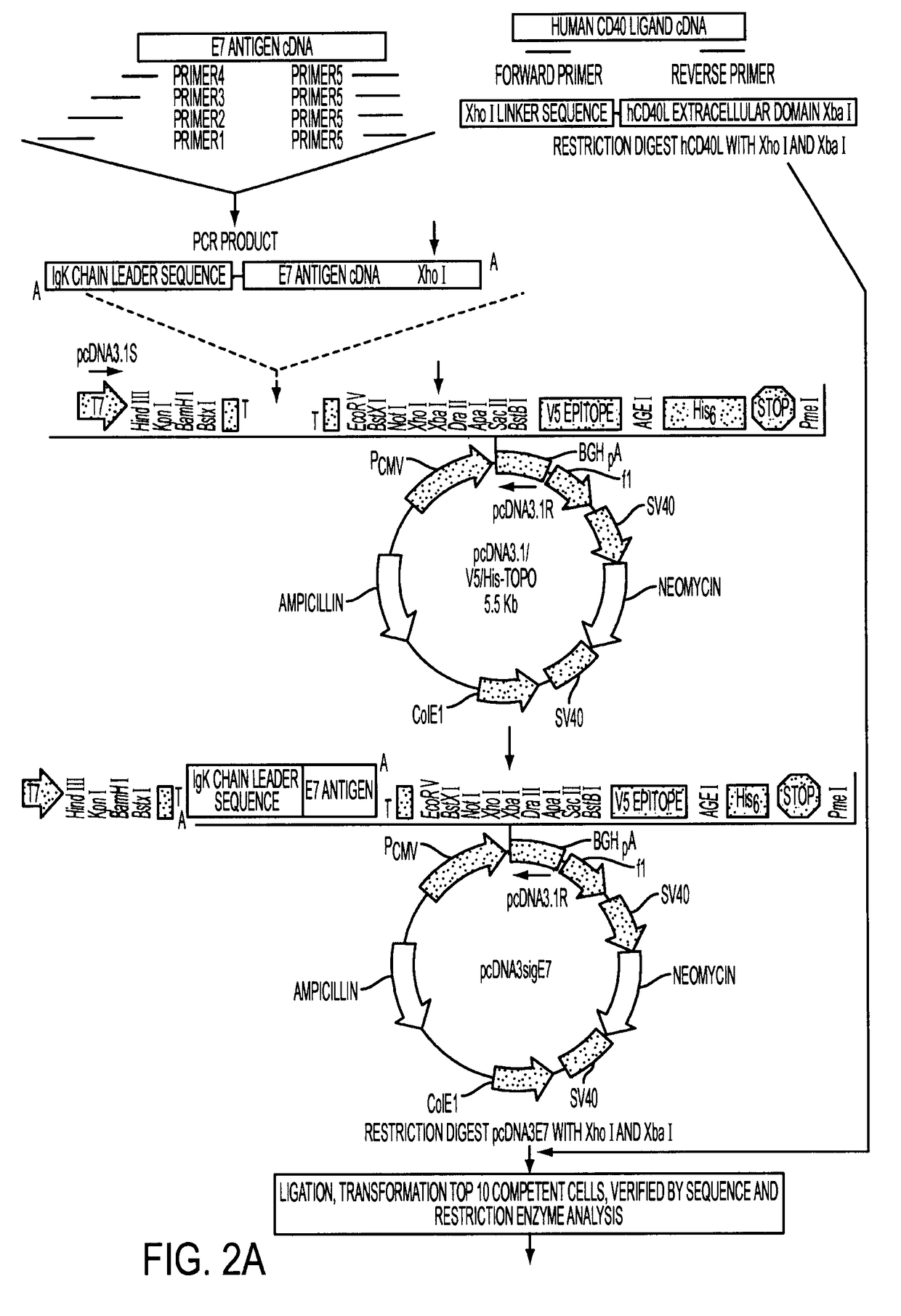
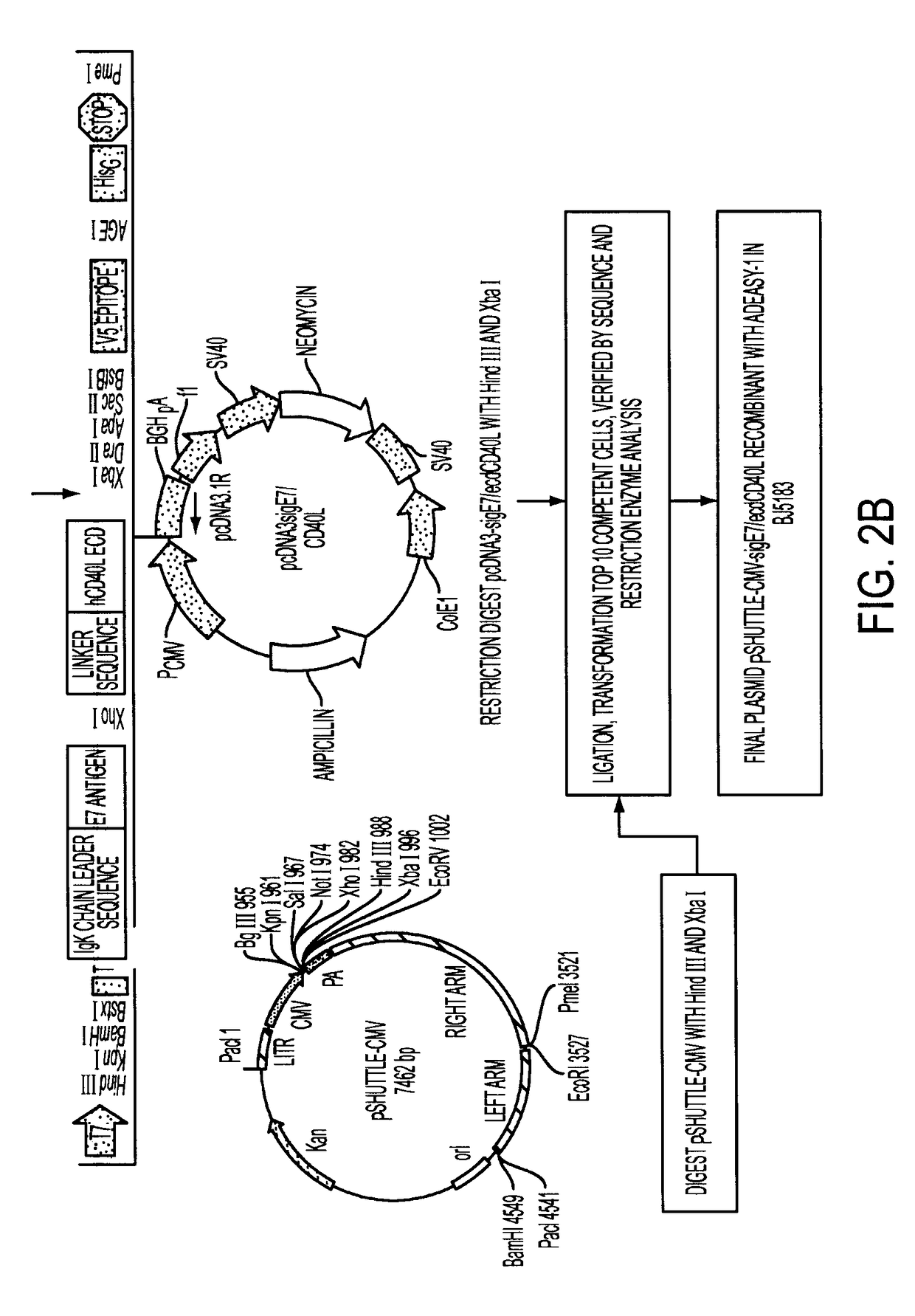
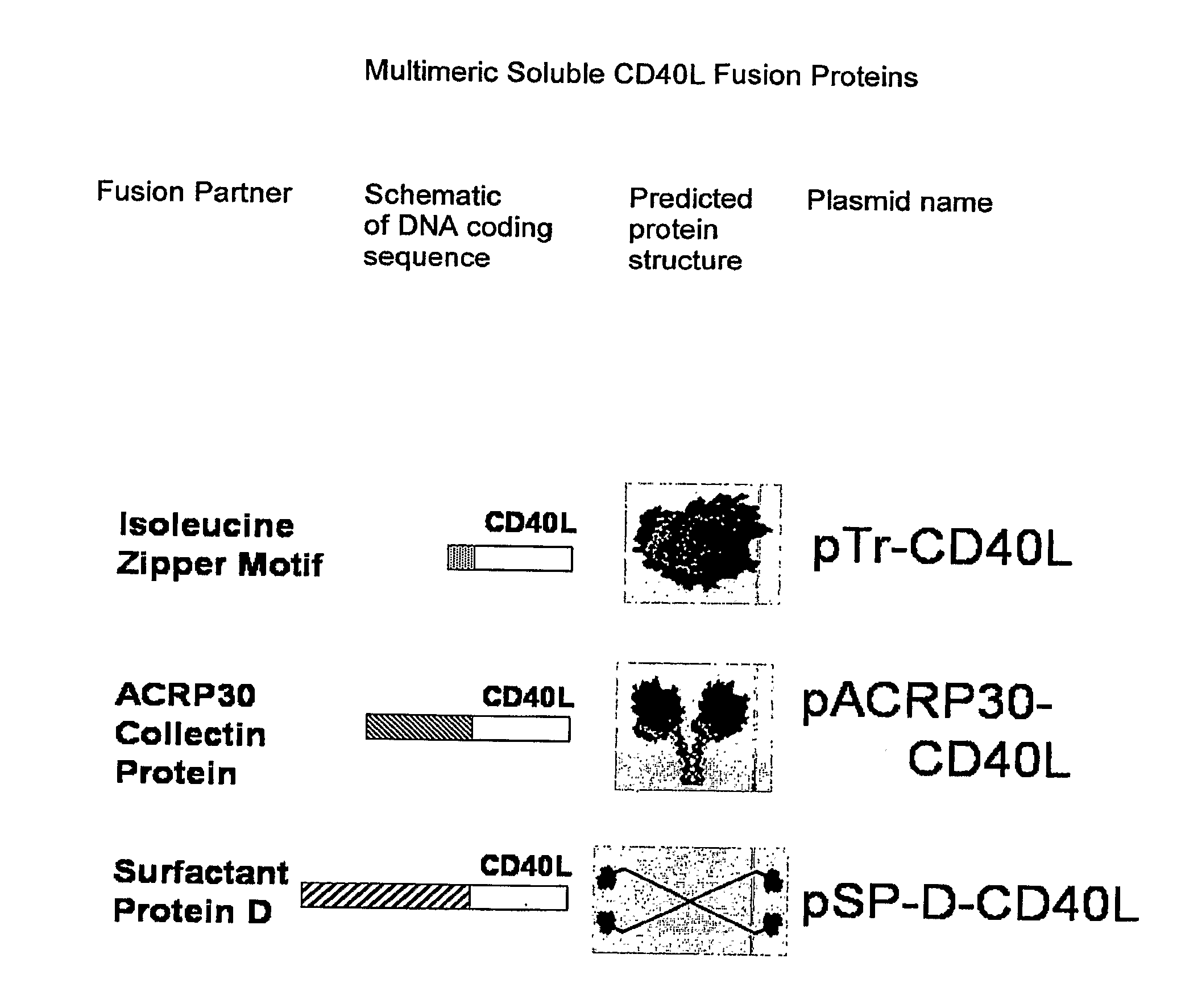
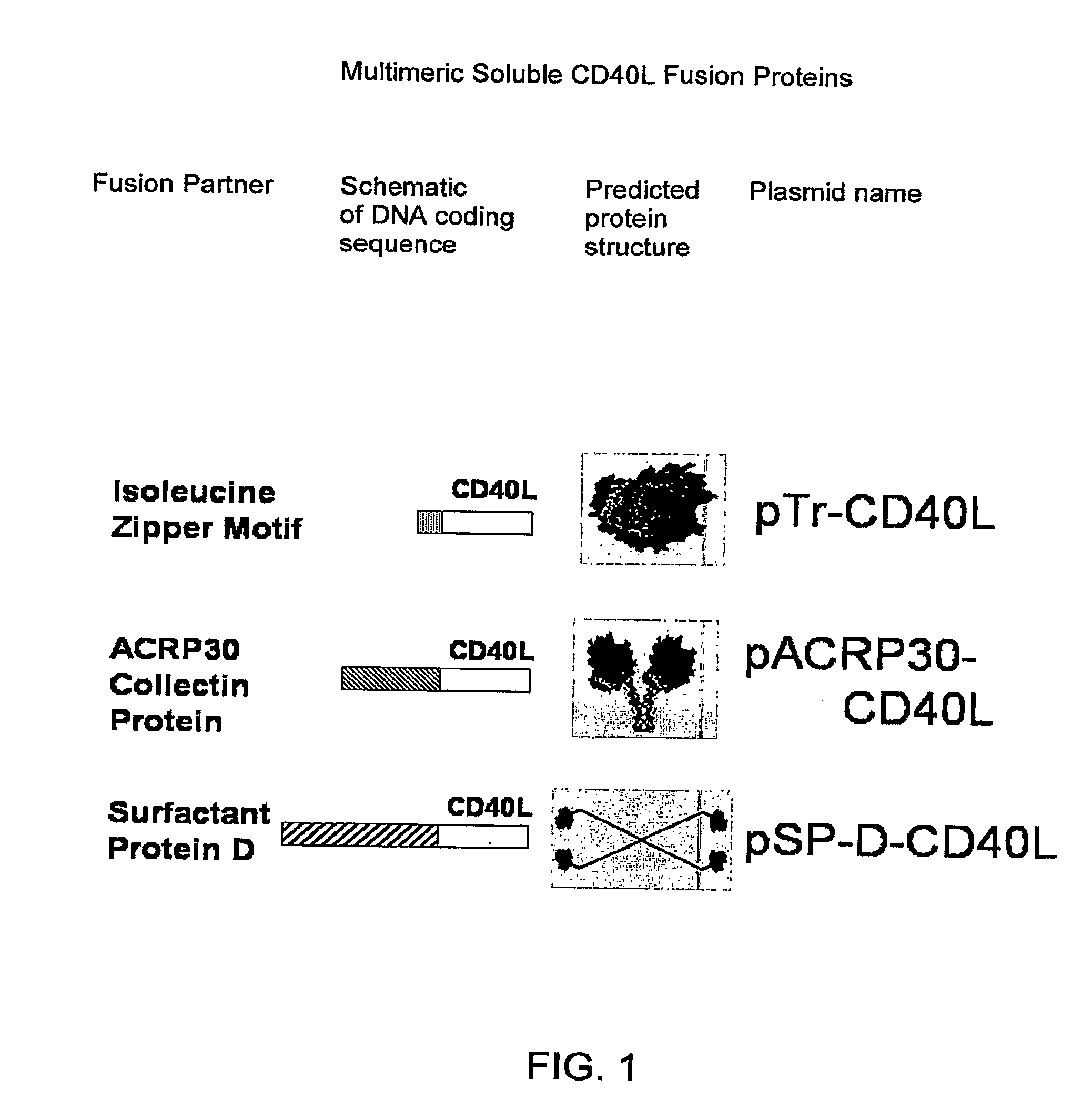
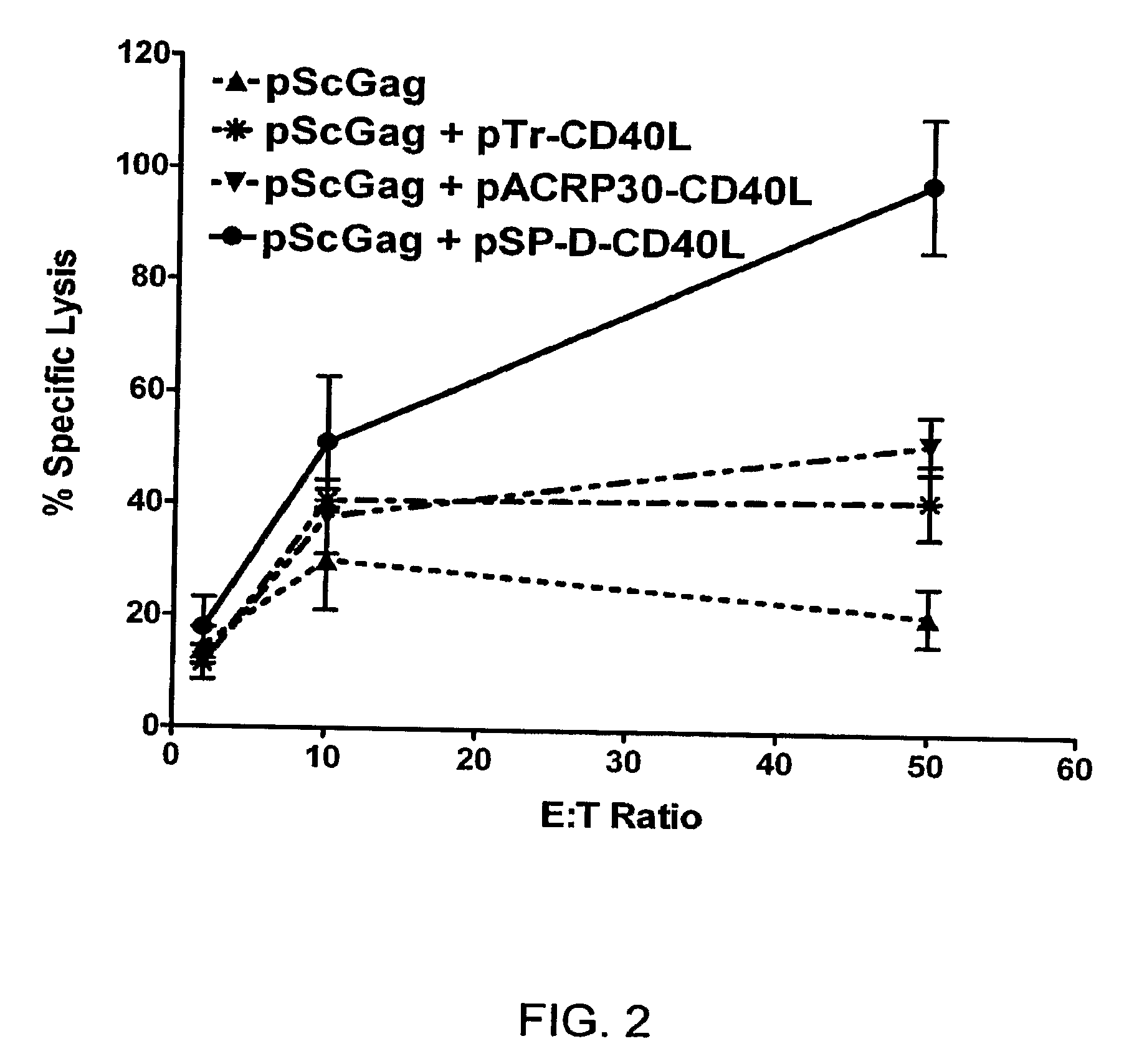
Adenoviral expression vector comprising a CD40L fusion protein adapted to elicit cellular immunity
ActiveUS8119117B2Enhance immune responseLong-lasting immunityVirusesAntibody mimetics/scaffoldsTransmembrane domainTumor antigen
Provided are adenoviral vectors for generating an immune response to antigen. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal sequence upstream of a tumor antigen upstream of CD40 ligand, which is missing all or substantially all of the transmembrane domain rendering CD40L secretable. Also provided are methods of generating an immune response against cells expressing a tumor antigen by administering an effective amount of the invention vector. Further provided are methods of generating an immune response against cancer expressing a tumor antigen in an individual by administering an effective amount of the invention vector. Still further provided are methods of generating immunity to infection by human papilloma virus (HPV) by administering an effective amount of the invention vector which enocodes the E6 or E7 protein of HPV. The immunity generated is long term.
Owner:VAXUM
Immunogenic compositions and methods of using the compositions for inducing humoral and cellular immune responses
ActiveUS9044420B2Reduce the possibilityAntibacterial agentsAntimycoticsImmunogenicityInfective disorder
Compositions and methods are provided herein for improved dual immunization strategies that induce in a subject an immune response that includes a humoral immune response and cellular immune response, both CD4 and CD8 T lymphocyte immune responses, thereby providing a complete adaptive immune response to one or more antigens. The methods described are therefore useful for treating and / or preventing (i.e., reducing the likelihood or risk of occurrence) different diseases, disorders, and conditions such as cancers and infectious diseases for which induction of both a humoral immune response and cellular immune response is desired and beneficial.
Owner:IMMUNE DESIGN CORP
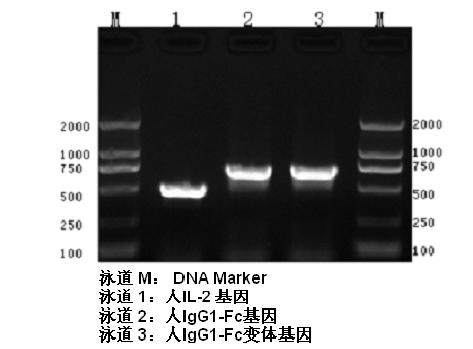
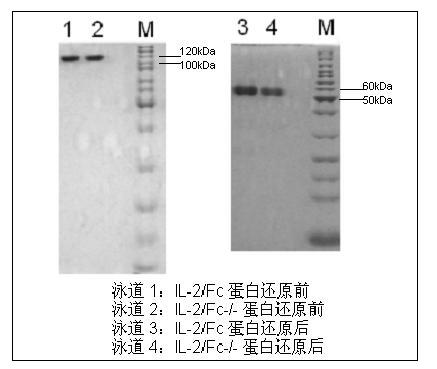
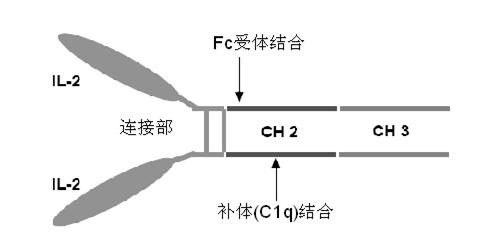
Human interleukin-2 (IL-2)/Fc fusion protein
ActiveCN102174111AEnhance humoral immune responseImprove immunityPeptide/protein ingredientsDigestive systemRegulatory T cellHalf-life
The invention provides human interleukin interleukin-2 (IL-2) / Fc fusion protein. The human IL-2 of the fusion protein comprises all sequences of a human IL-2 extracellular region; the Fc fragments comprise a hinge region, a CH2 region and a CH3 region; the human IL-2 / Fc sequences are fused directly or through a connection sequence; and the Fc fragments are human or animal IgG, IgM, IgD and IgA orsubtypes thereof. The ADCC and CDC effective factor action can be eliminated, and in addition, the human IL-2 / Fc fusion protein has the compatibility with a recombinant IL-2 receptor so that the half-life period is obviously prolonged and also has all the biological activity of the IL-2 receptor. The IL-2 / Fc obviously improves the humoral immune response stimulated by the hepatitis B vaccine and the immunity of the CD8+T cells targeted to the hepatitis B vaccine. Moreover, the balance immune (suppression) of the effective T cells and the regulatory t cells can be adjusted under the action of the cyclosporine A so that the pancreatic islet transplantation immune tolerance is induced.
Owner:上海百英生物科技股份有限公司
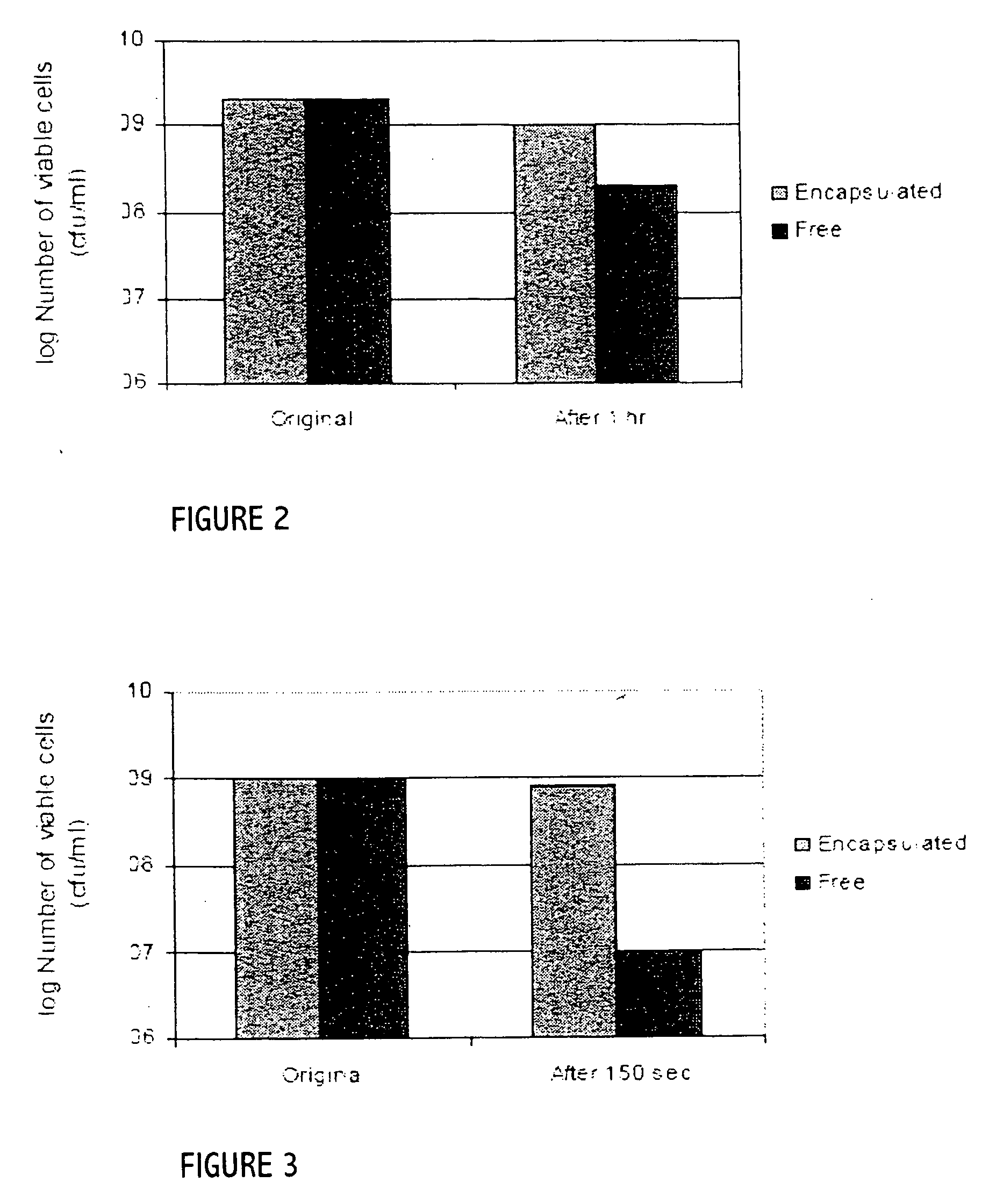
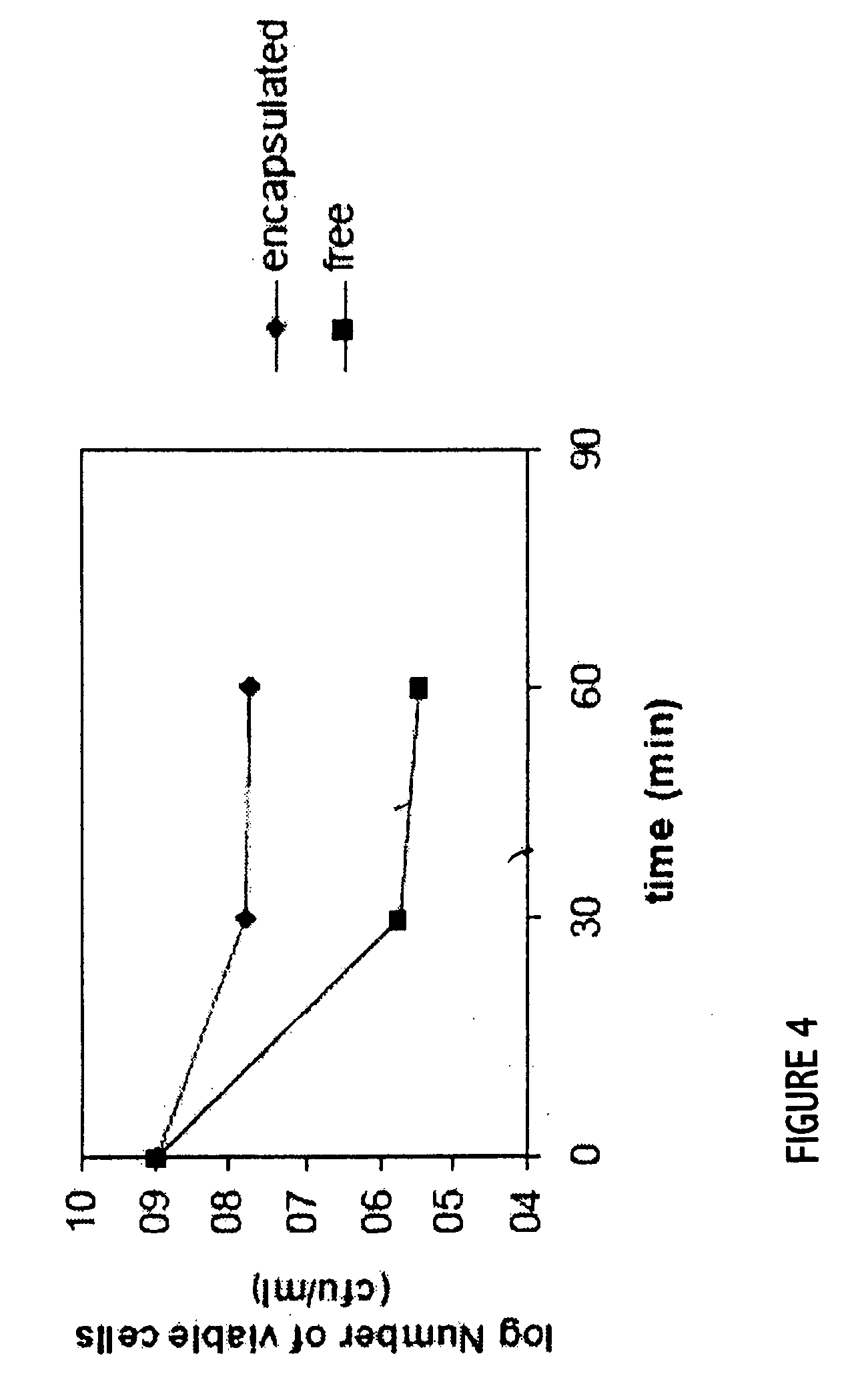
Probiotics as alternative medicines against infectious diseases
An exemplary embodiment providing one or more improvements includes feeding animals with probiotic microbes encapsulated in a mixture of xanthan gum and chitosan, or in gelatin, specifically Pediococcus acidilactici and Saccharomyces boulardii. Such encapsulation protects the viability of the probiotic microbes against unfavorable temperatures. An exemplary embodiment providing one or more improvements includes methods of using viable probiotics in therapy of birds and mammals infected with infectious diseases. Probiotics acted as adjuvants in stimulating antibody reaction and stimulated a cellular immunity response. In particular, probiotics were shown to reduce the number of viable oocytes from fecal samples, stimulate antibody production, and stimulate of proliferation of splenocytes in chickens infected with Elimeria. In addition, probiotics were shown to relieve symptoms of parvovirus infection in dogs.
Owner:IMAGILIN TECH LLC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![gRNA for knockout of wild type T cell TCR [beta] strand and method gRNA for knockout of wild type T cell TCR [beta] strand and method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f8b222e-5a7b-41ca-b86a-4b04c7497be5/DEST_PATH_HDA0001417539750000011.png)
![gRNA for knockout of wild type T cell TCR [beta] strand and method gRNA for knockout of wild type T cell TCR [beta] strand and method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f8b222e-5a7b-41ca-b86a-4b04c7497be5/DEST_PATH_HDA0001417539750000012.png)
![gRNA for knockout of wild type T cell TCR [beta] strand and method gRNA for knockout of wild type T cell TCR [beta] strand and method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f8b222e-5a7b-41ca-b86a-4b04c7497be5/DEST_PATH_HDA0001417539750000013.png)