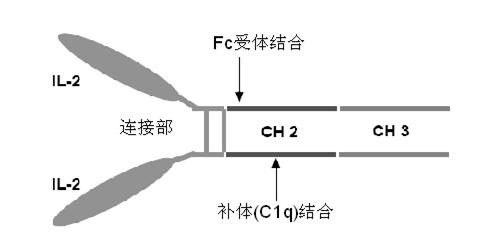
Human interleukin-2 (IL-2)/Fc fusion protein
A fusion protein and human interleukin technology, applied in the field of DNA recombination, can solve the problems of limited antiviral treatment effect, deterioration of virus disease, difficult treatment, etc., and achieve the effect of inducing immune tolerance and prolonging half-life of islet transplantation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
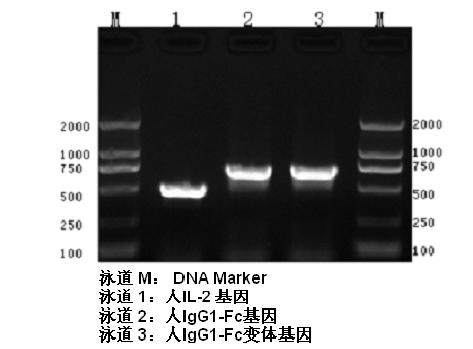
[0045] Example 1 Construction of Human Interleukin 2 and Fc Fusion Protein Expression Plasmid
[0046] 1. Cloning of human interleukin-2 functional gene
[0047] Adherent mononuclear cells were isolated from peripheral blood of normal people, stimulated by adding LPS for 4 hours, total RNA was extracted by one-step method of guanidine isothiocyanate, the first strand of cDNA was synthesized by MMLV reverse transcriptase, and then used as a template The entire sequence of the extracellular region of human interleukin 2 (GeneBank: NM_000586.3) was amplified, including the secretion signal peptide sequence, and the 145th amino acid Cys was changed to Ser by using the downstream primers, and the upstream and downstream primers were respectively introduced into the NotI and BamHI sites. The primer sequences used are as follows:
[0048] Upstream primers:
[0049] P1:5'ATATGGCGGCCGCTAACCTCAACTCCTGCCACA3'
[0050] Downstream primers:
[0051] P2: 5'CTCTGGGATCCGTCAGTGTTGAGATGATGCT...
Embodiment 2
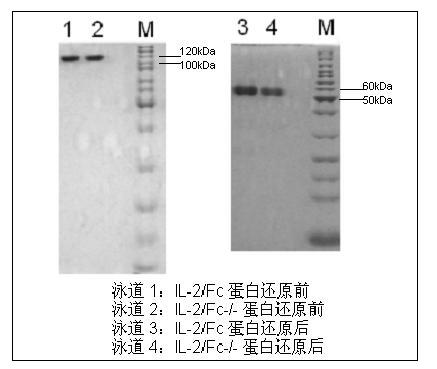
[0078] Example 2. Production of Human Interleukin 2 and Fc Fusion Protein
[0079] 1. Screening of stable high-expression cell lines
[0080] Dilute CHO cells in logarithmic phase to 8 x 10 with cold 1 x PBS 6 After / ml,
[0081] Take 0.4ml of the suspension, add it to the electric shock cup, then add 15ug of linearized expression plasmid, mix and let stand on ice for 10-15min. Set the voltage of the electroporator to 800V, and the capacitance to 25uF. After spotting, the electric shock cup was placed on ice for 10 minutes, and the cells were transferred to a 10cm culture dish containing 5% 1×FBS culture solution with a pipette for culture. Two days later, when the growth state of the cells was restored, negative cells were screened with 400 ug / ml of G418. Observe the state of the cells, and change the medium every 4 days. After two weeks of culture, use the limiting dilution method to screen high-expression cell lines with 96-well culture plates. Finally, a stable high-ex...
Embodiment 3
[0088] Example 3. Detection of biological function of human interleukin 2 and Fc fusion protein
[0089] 1. Detection of biological activity of IL-2 in human interleukin 2 and Fc fusion protein
[0090] The detection method is as follows (reference, Nature, 1977: 268, 154-156): IL-2 receptor-positive CTLL-2 cells are the target cells of the receptor combination, and the CTLL-2 cells are treated with PH 3 RPMI for 20 seconds to remove the binding IL-2 on the receptor. Cells were washed twice with RPMI (PH 7) and then mixed with 125 I (Dupont, Boston, MA) was co-cultured for 60 minutes in a 37°C incubator, and the radiolabeled CTLL-2 cells were washed twice, and incubated with different concentrations of rIL-2 or IL-2 / Fc for 60 minutes on ice , and the supernatant was transferred to a glass test tube for radioactivity determination.
[0091] Test results:
[0092] 1) if Figure 4 As shown, in the classic assay for detecting IL-2 activity, IL-2 / Fc and recombinant IL-2 (recom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com