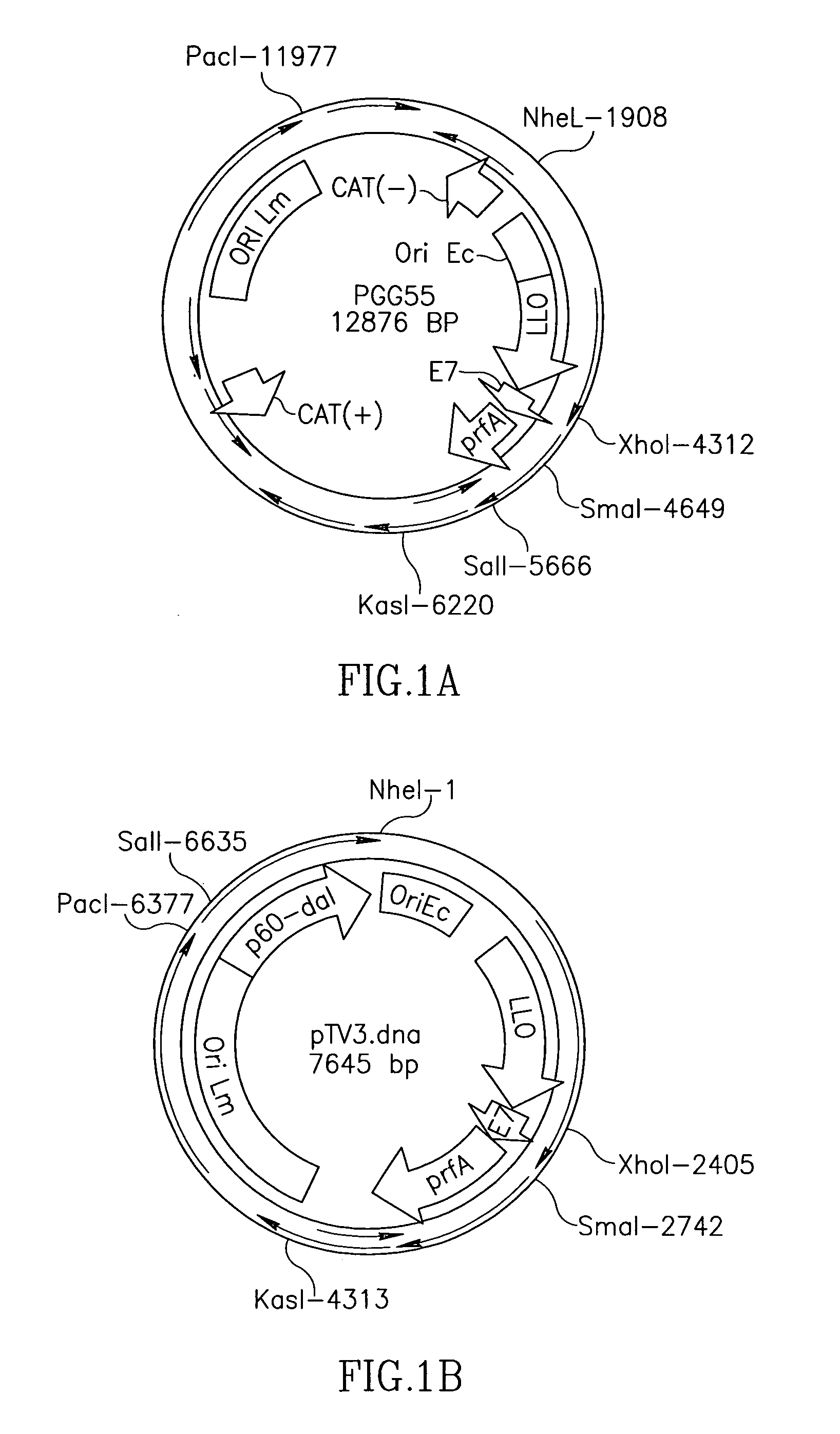
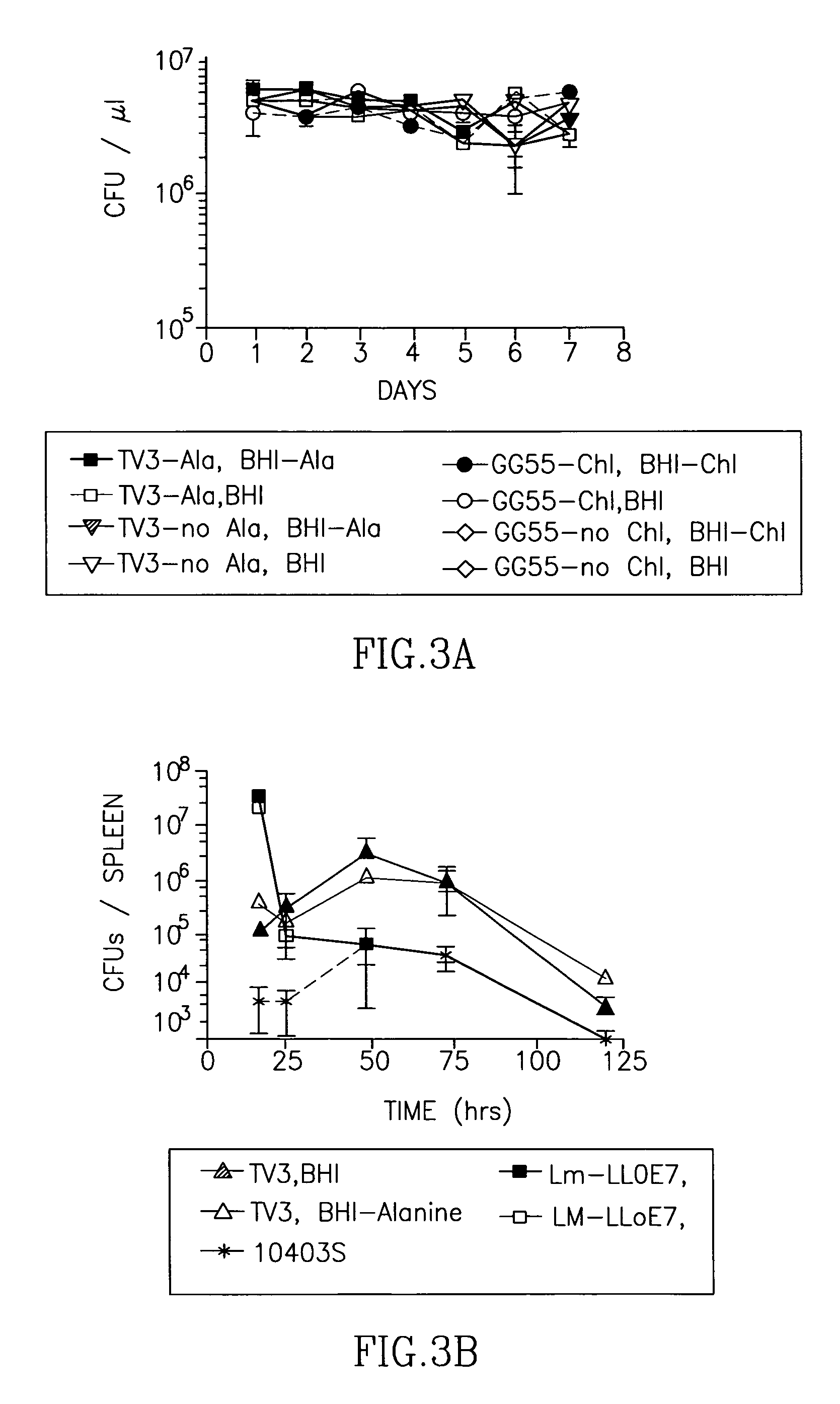
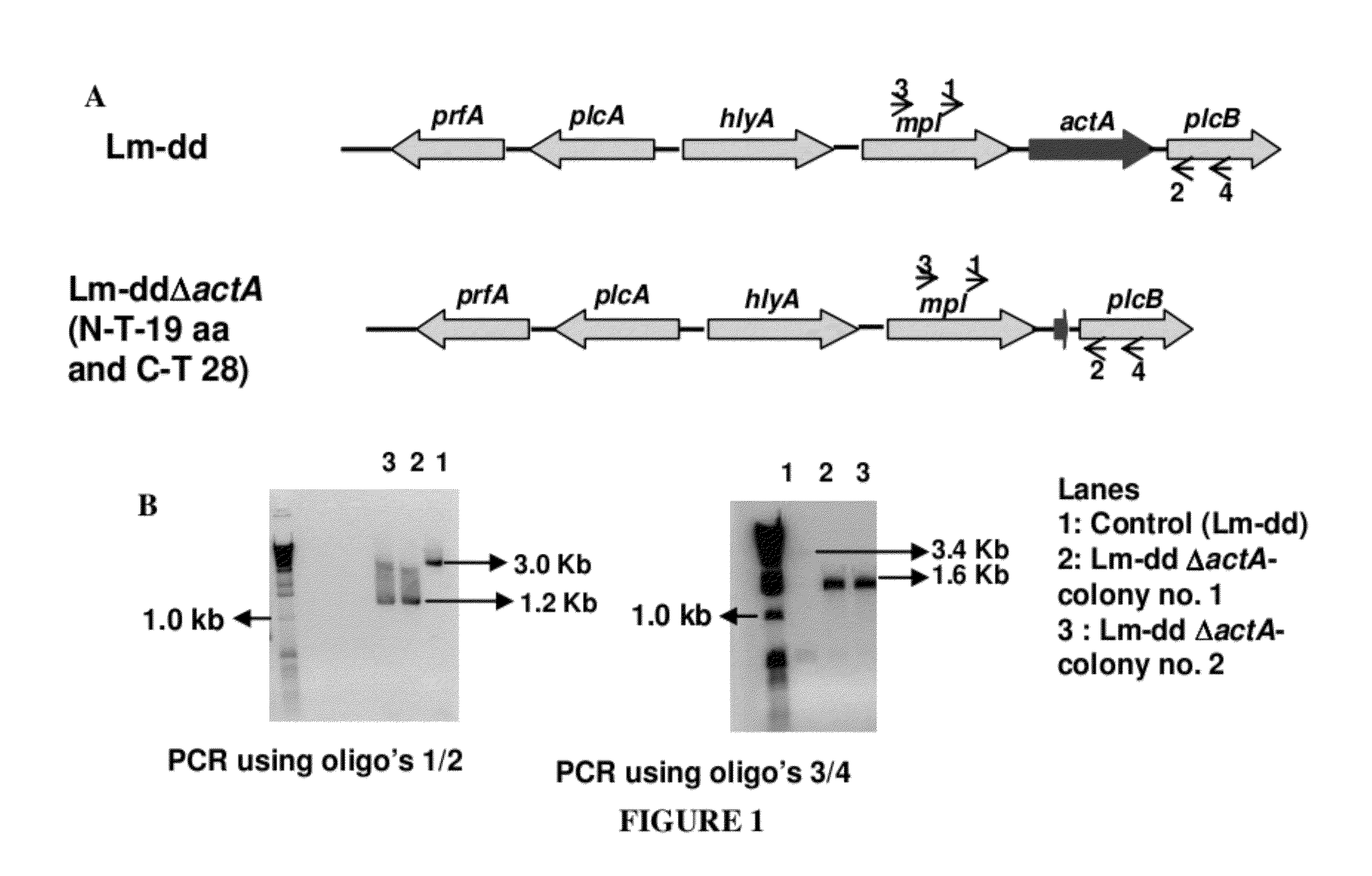
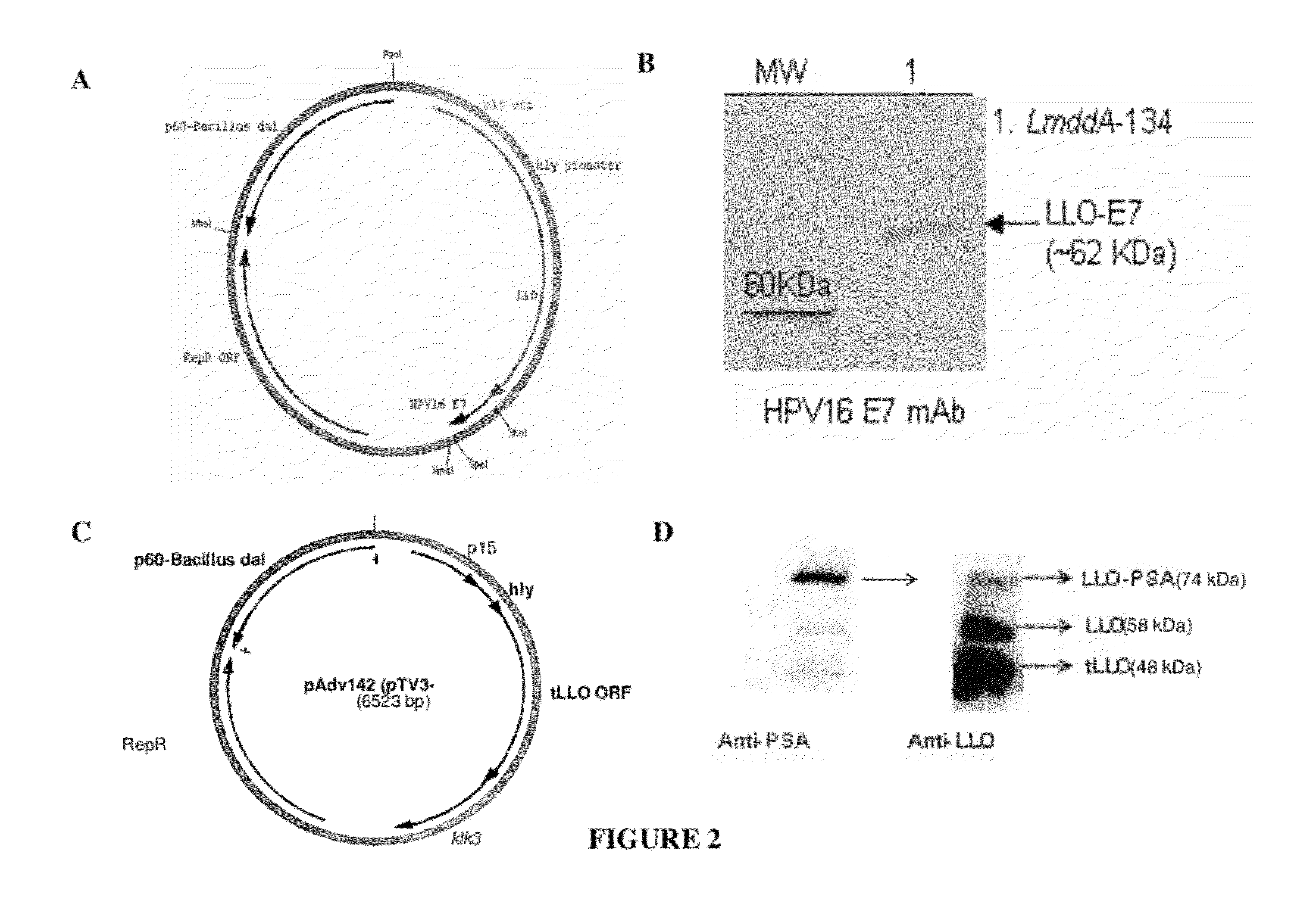
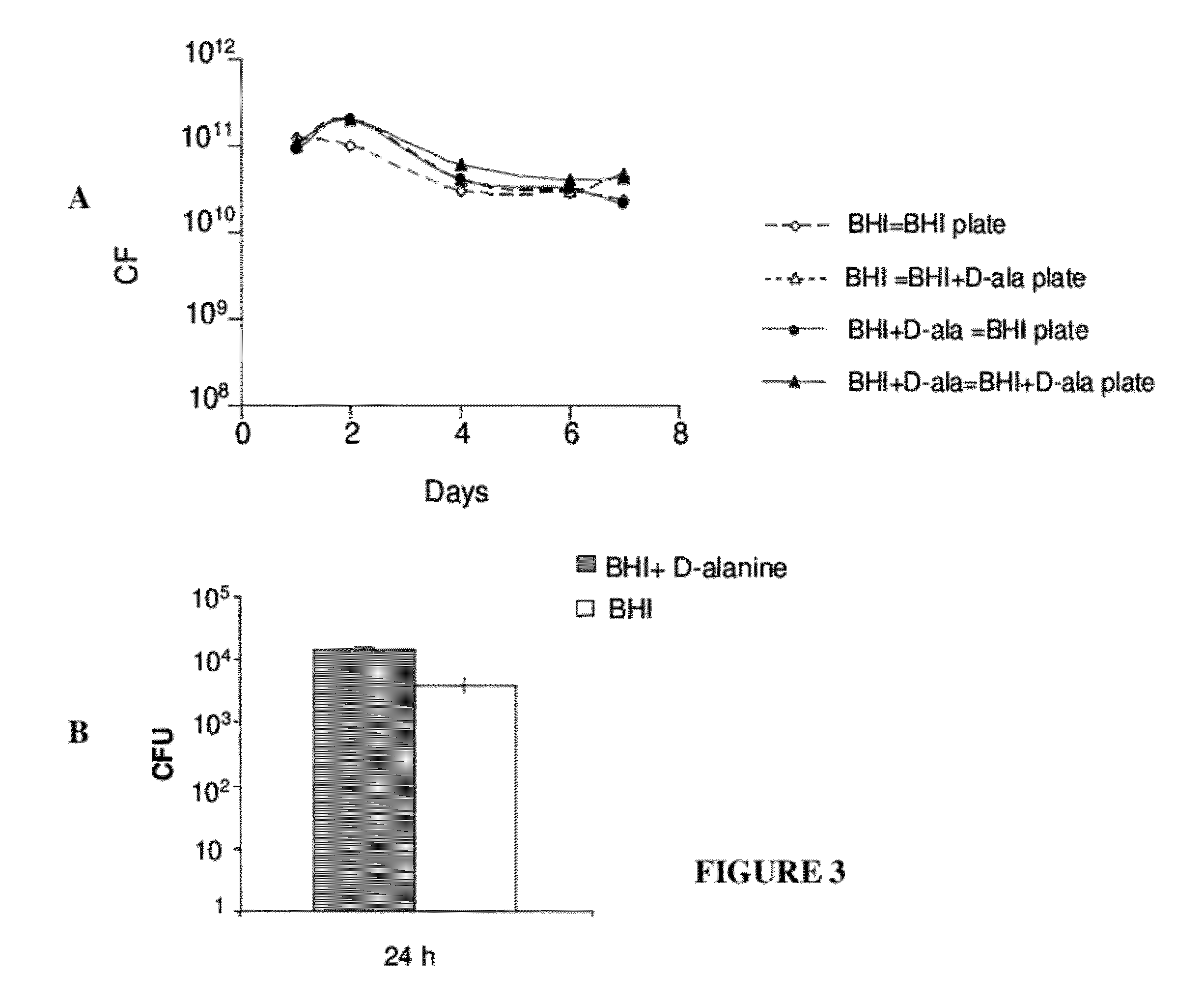
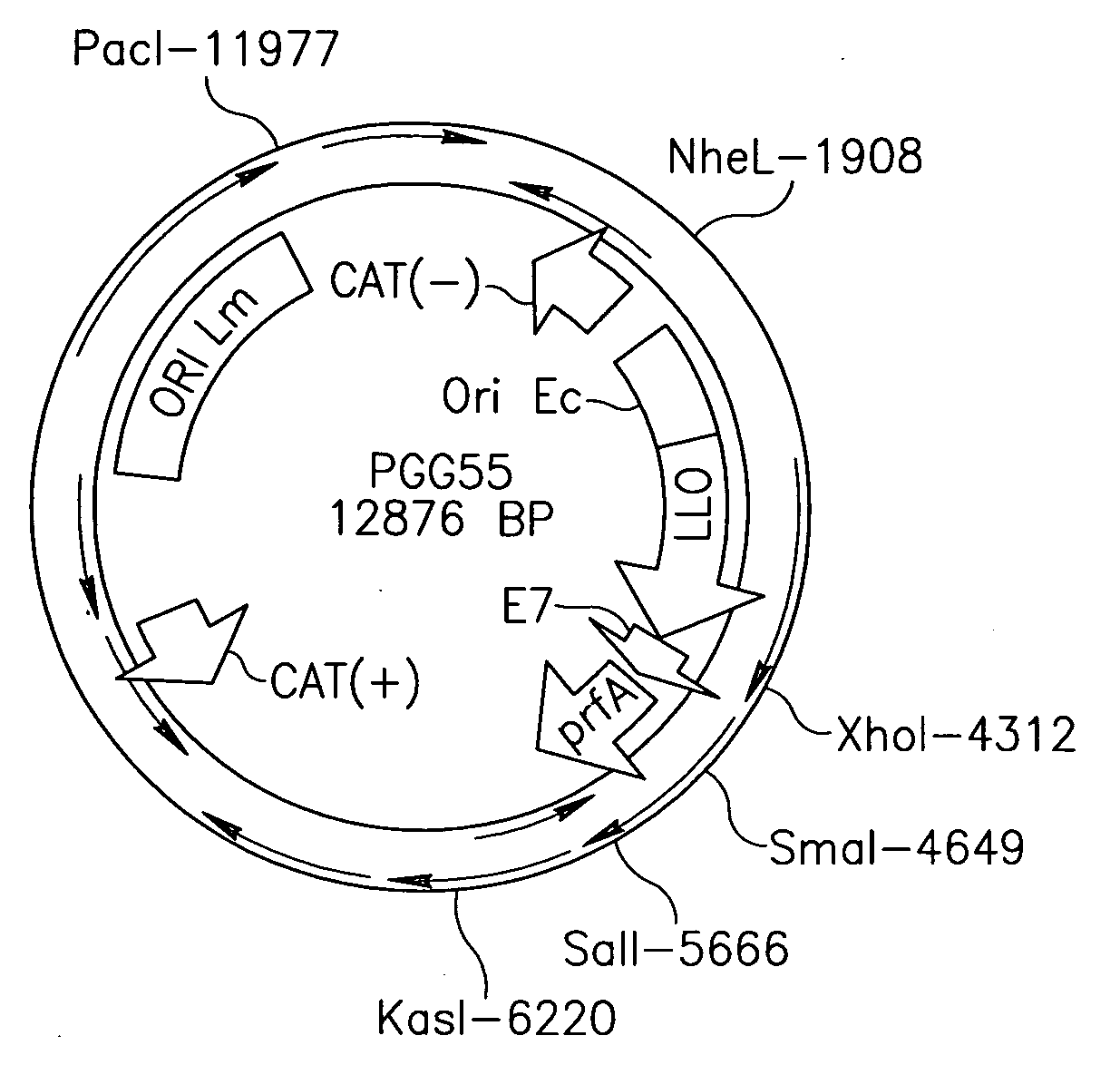
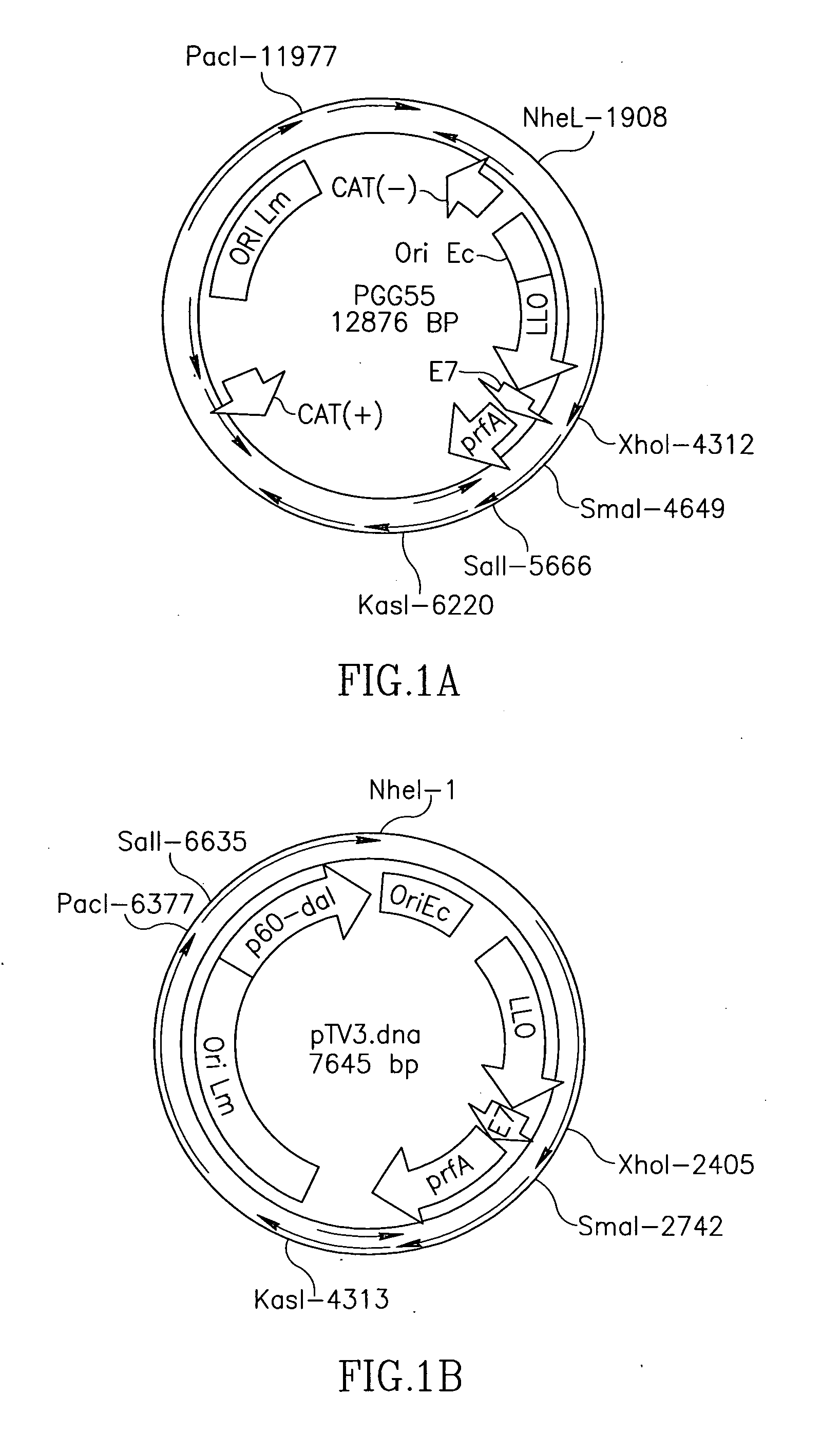
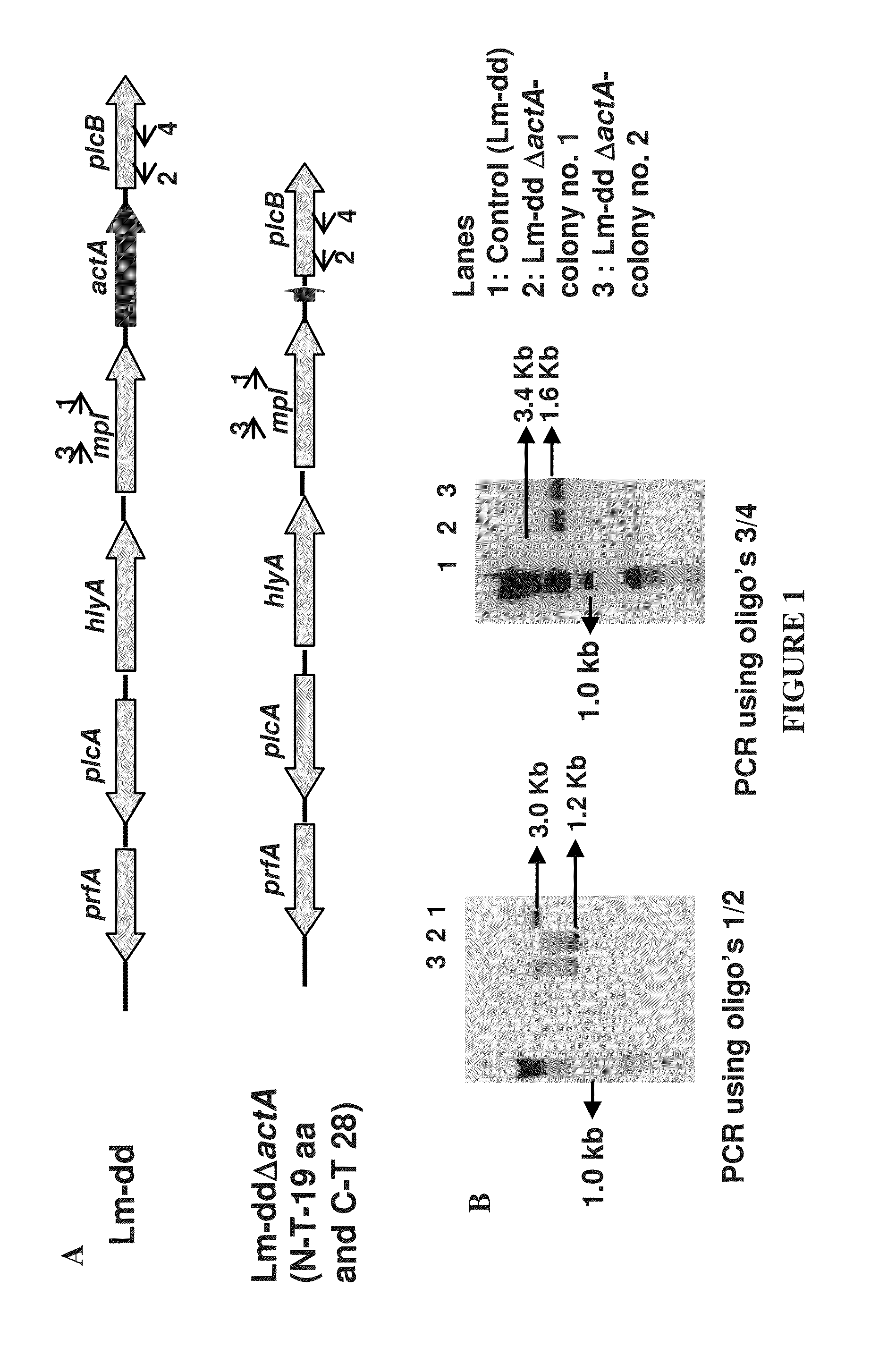
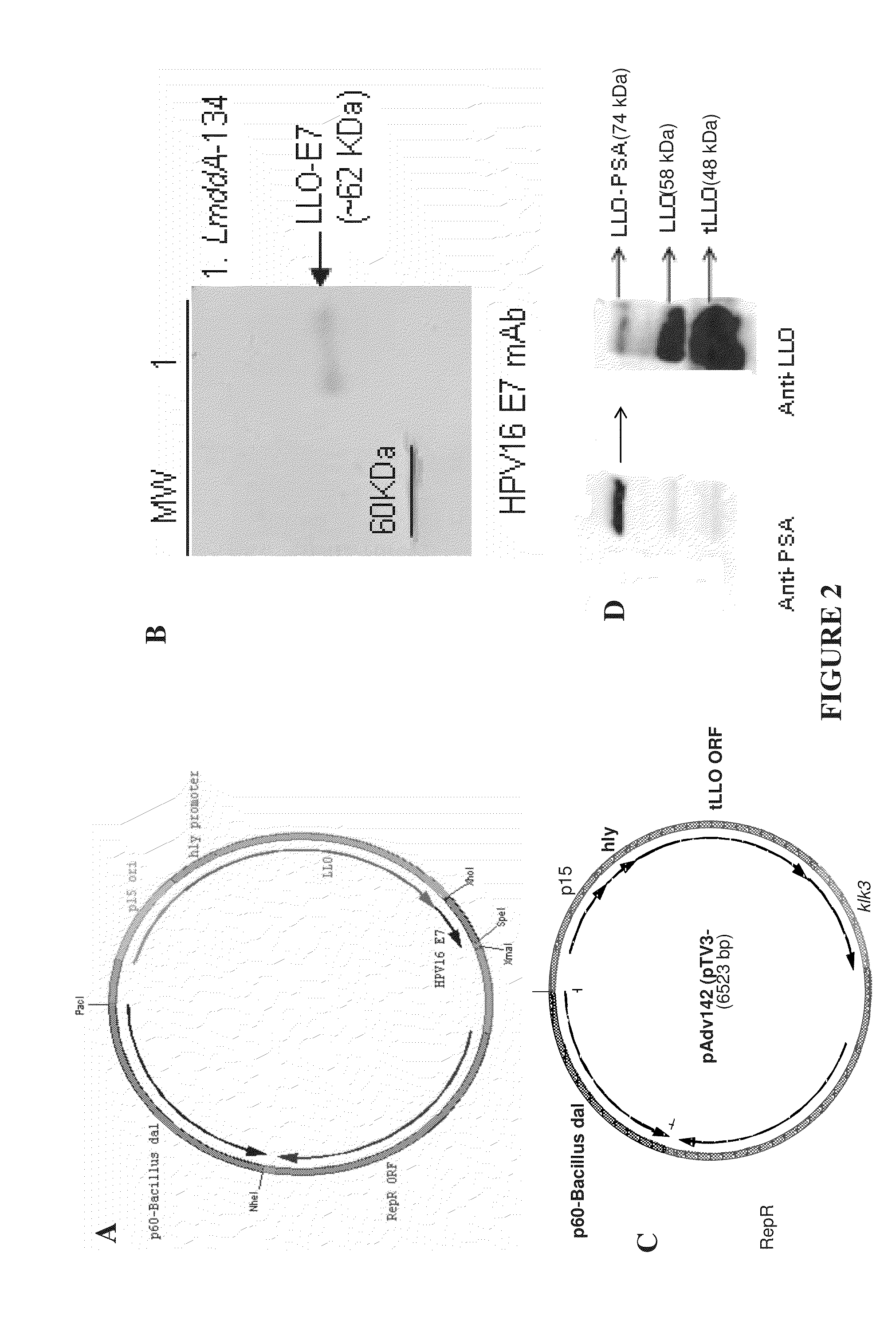
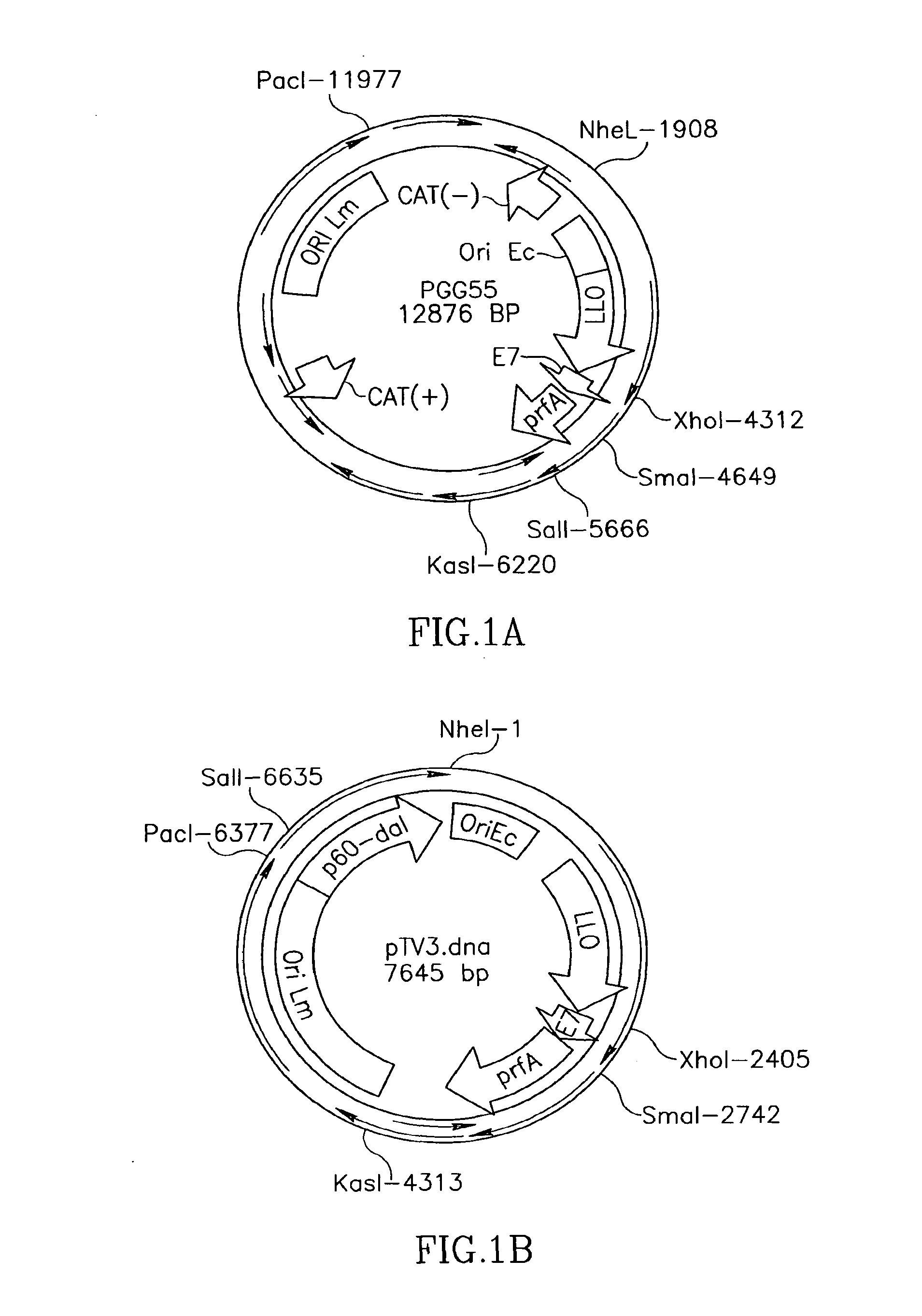
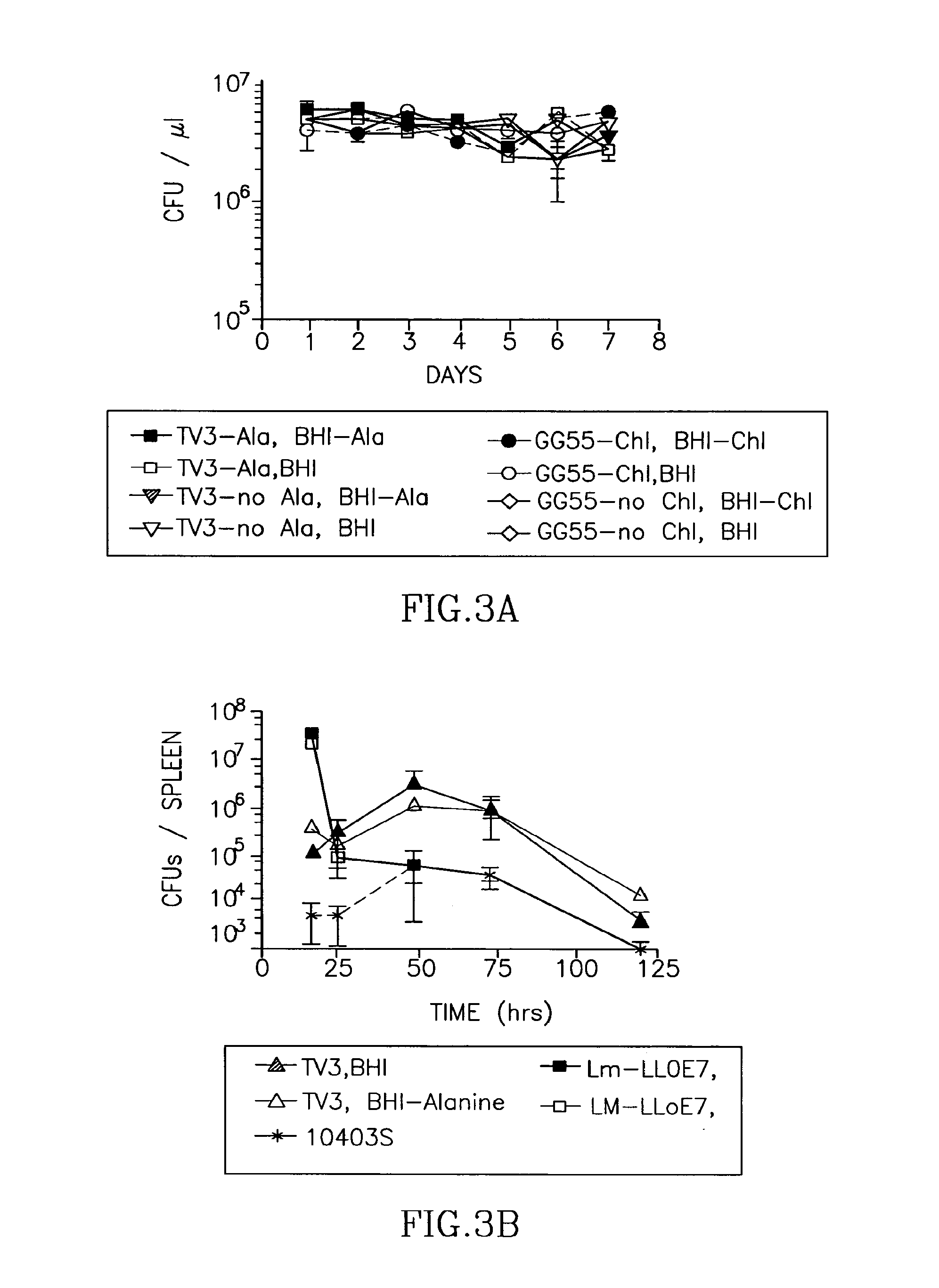
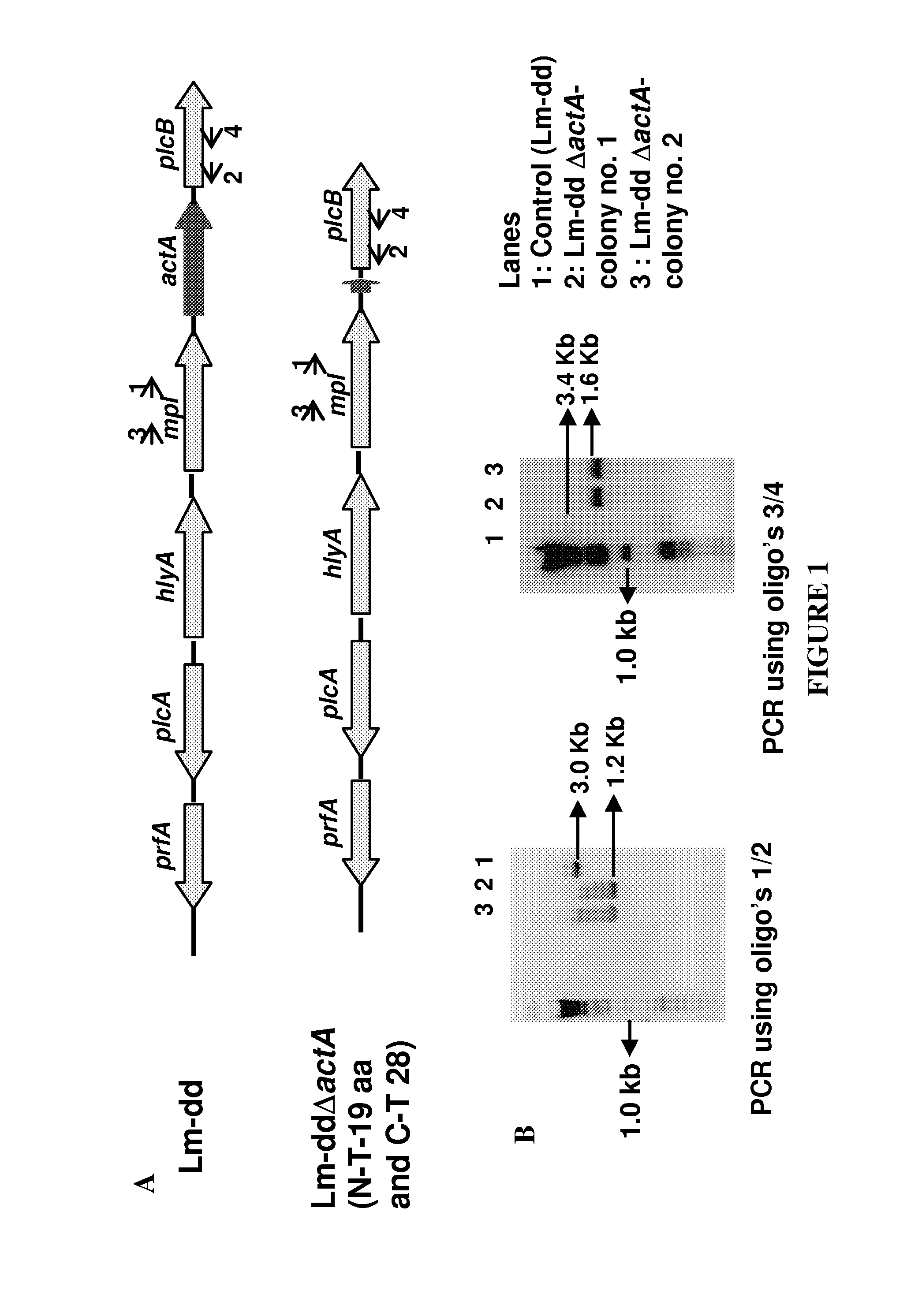
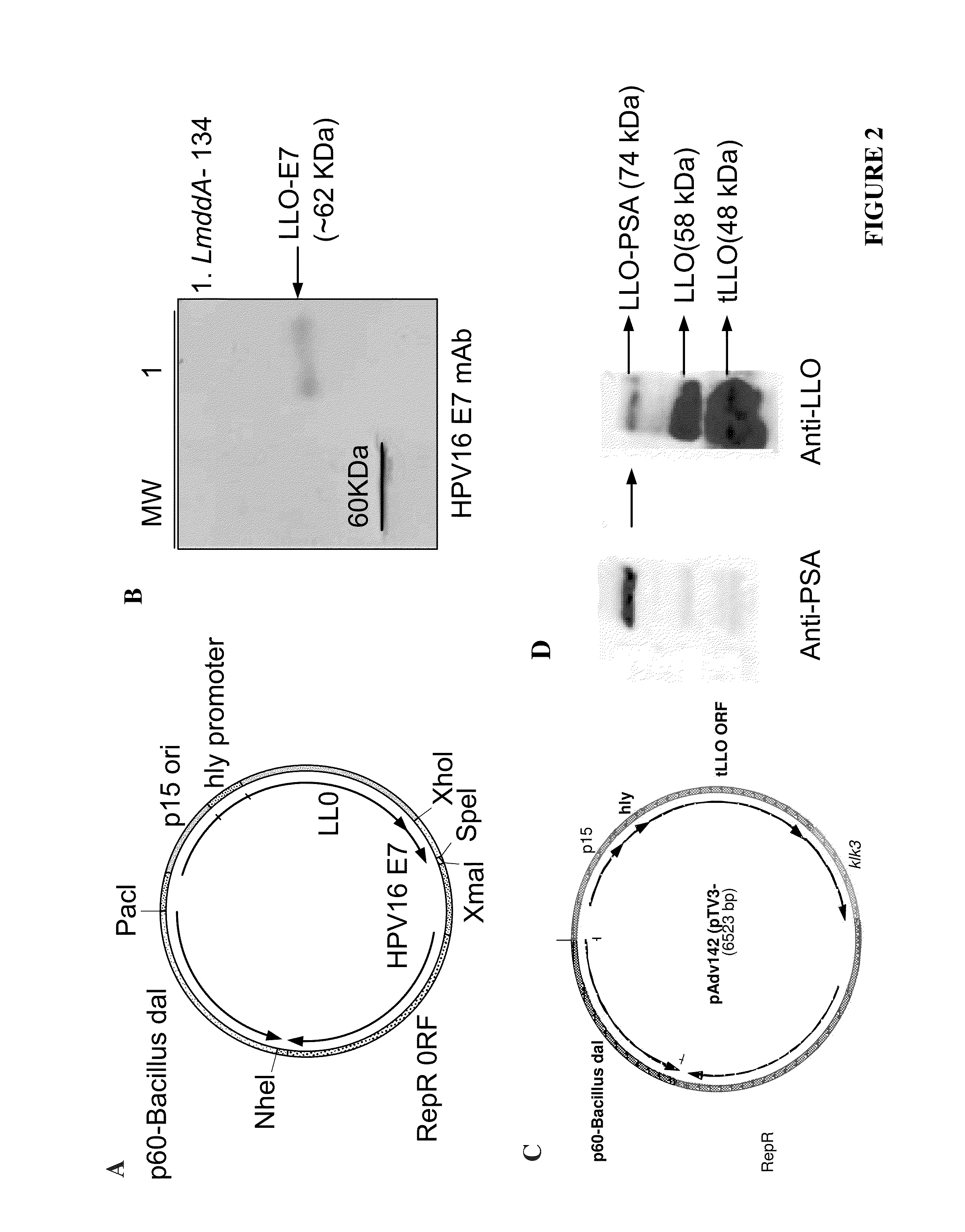
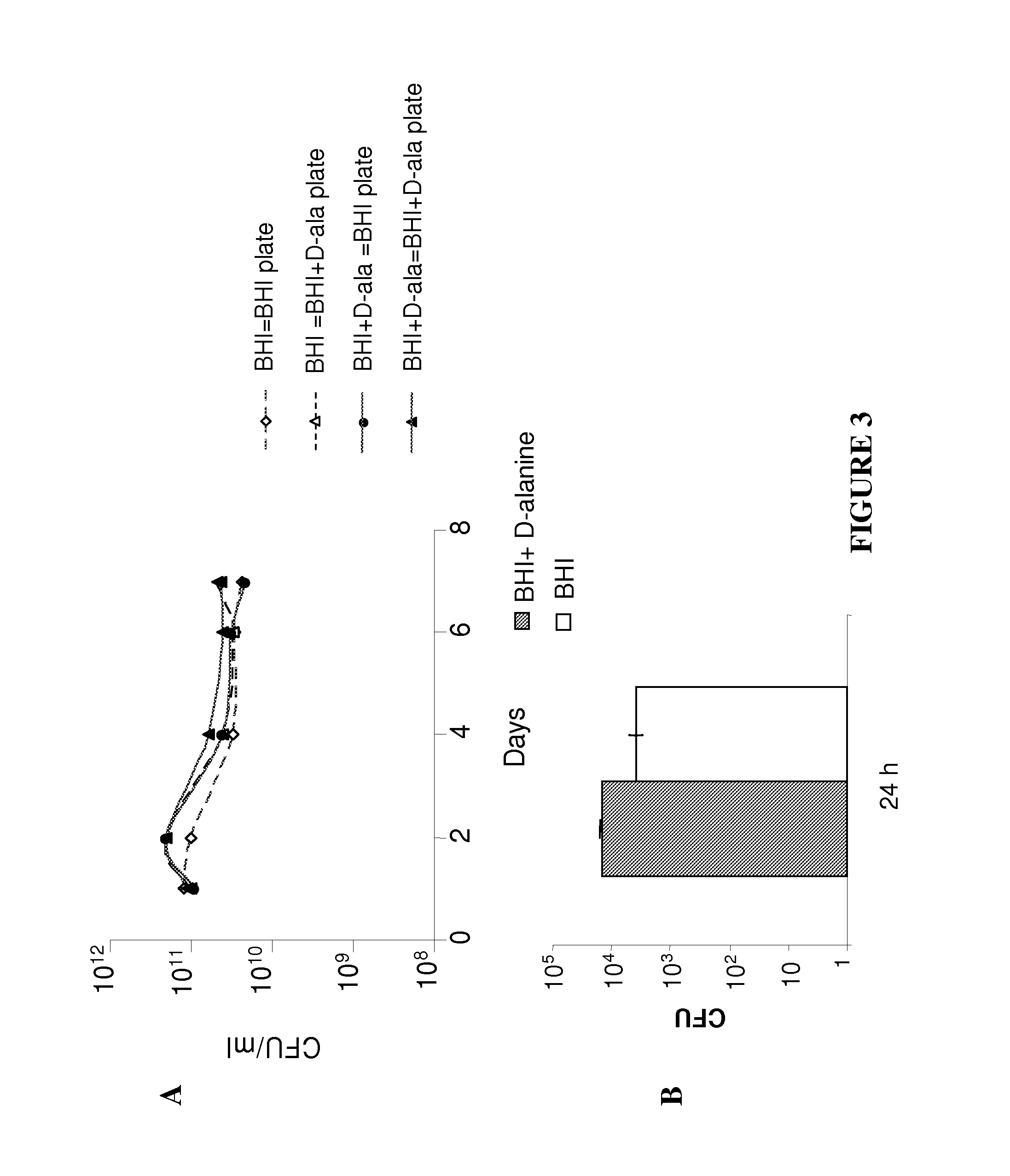
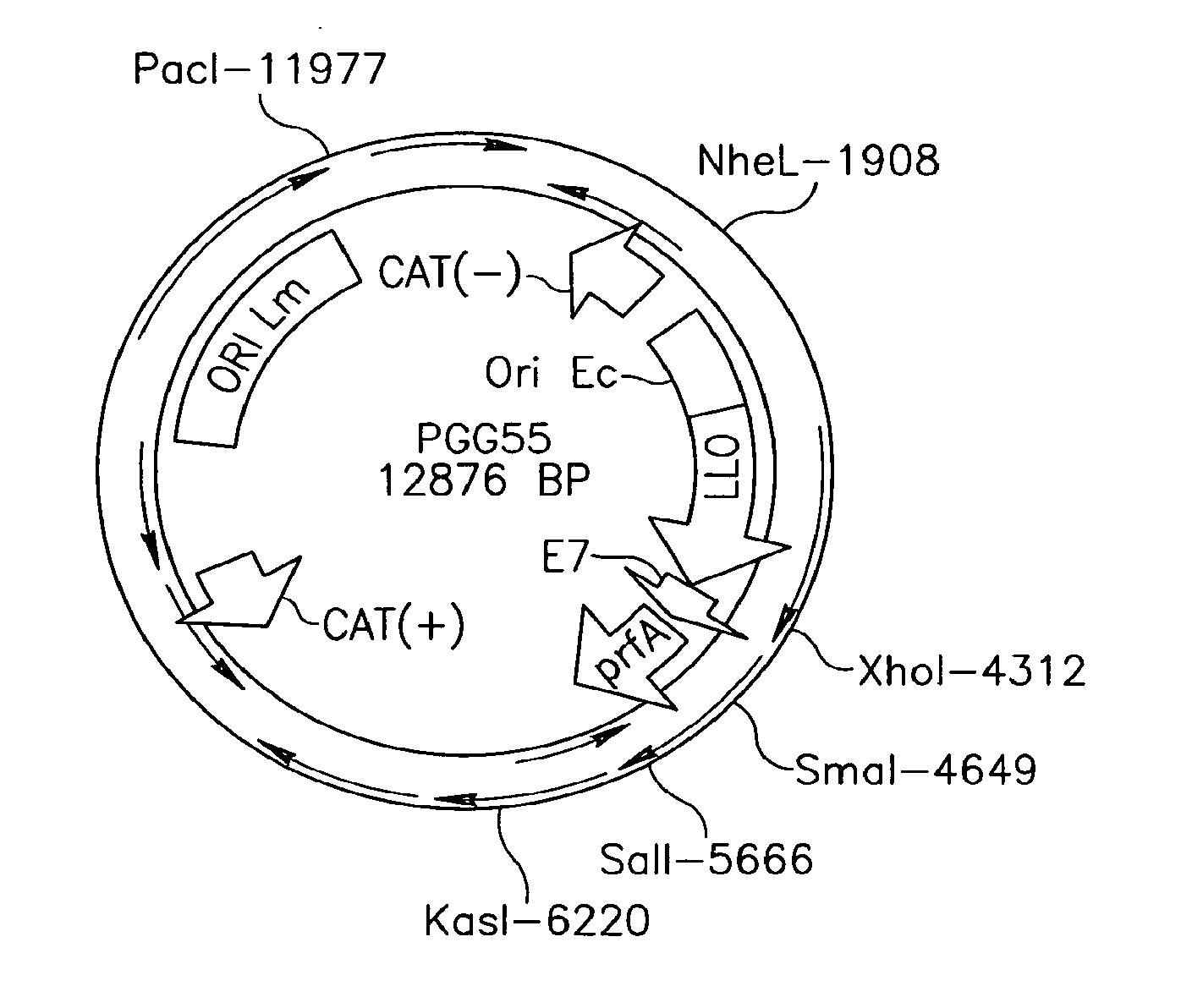
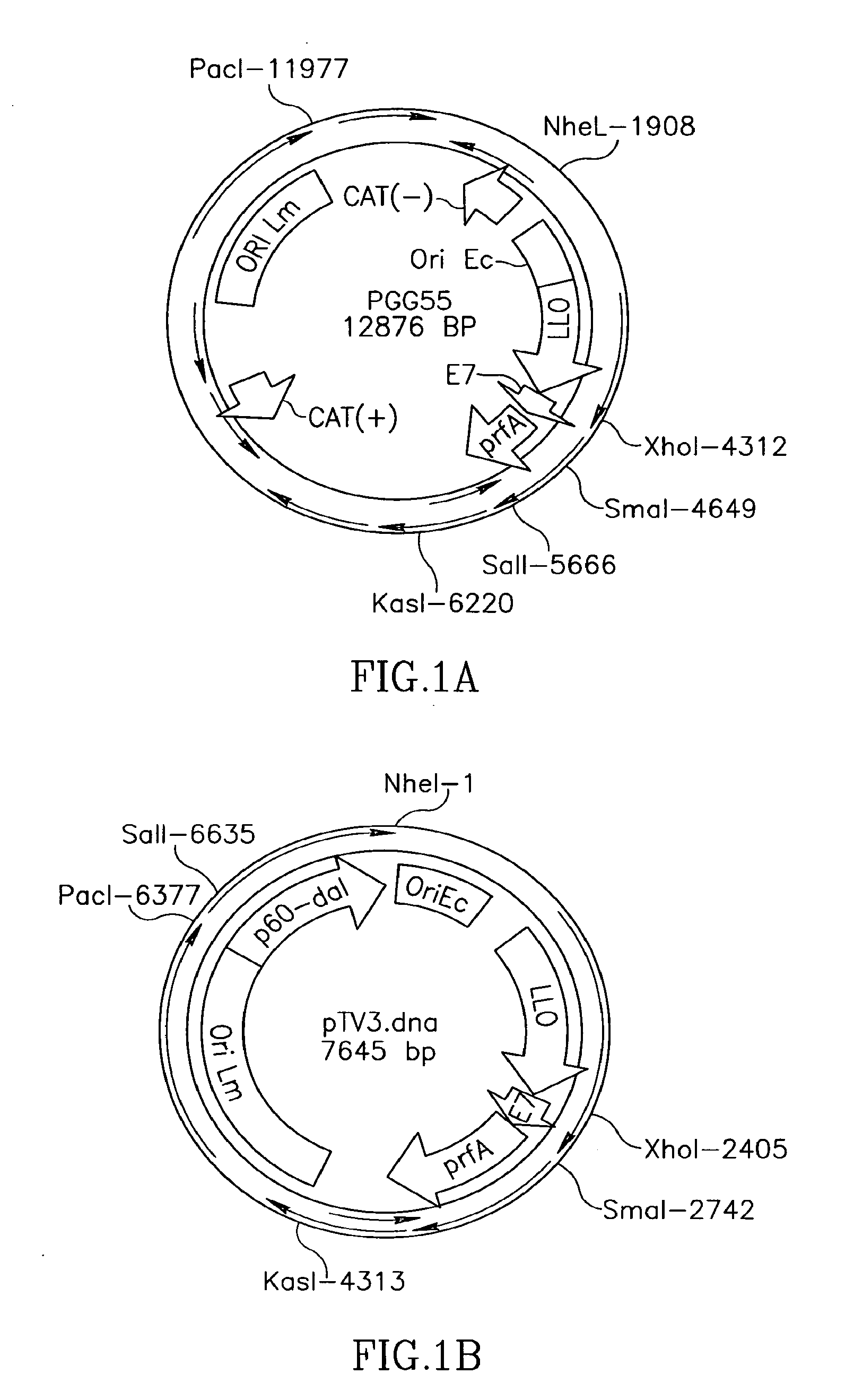
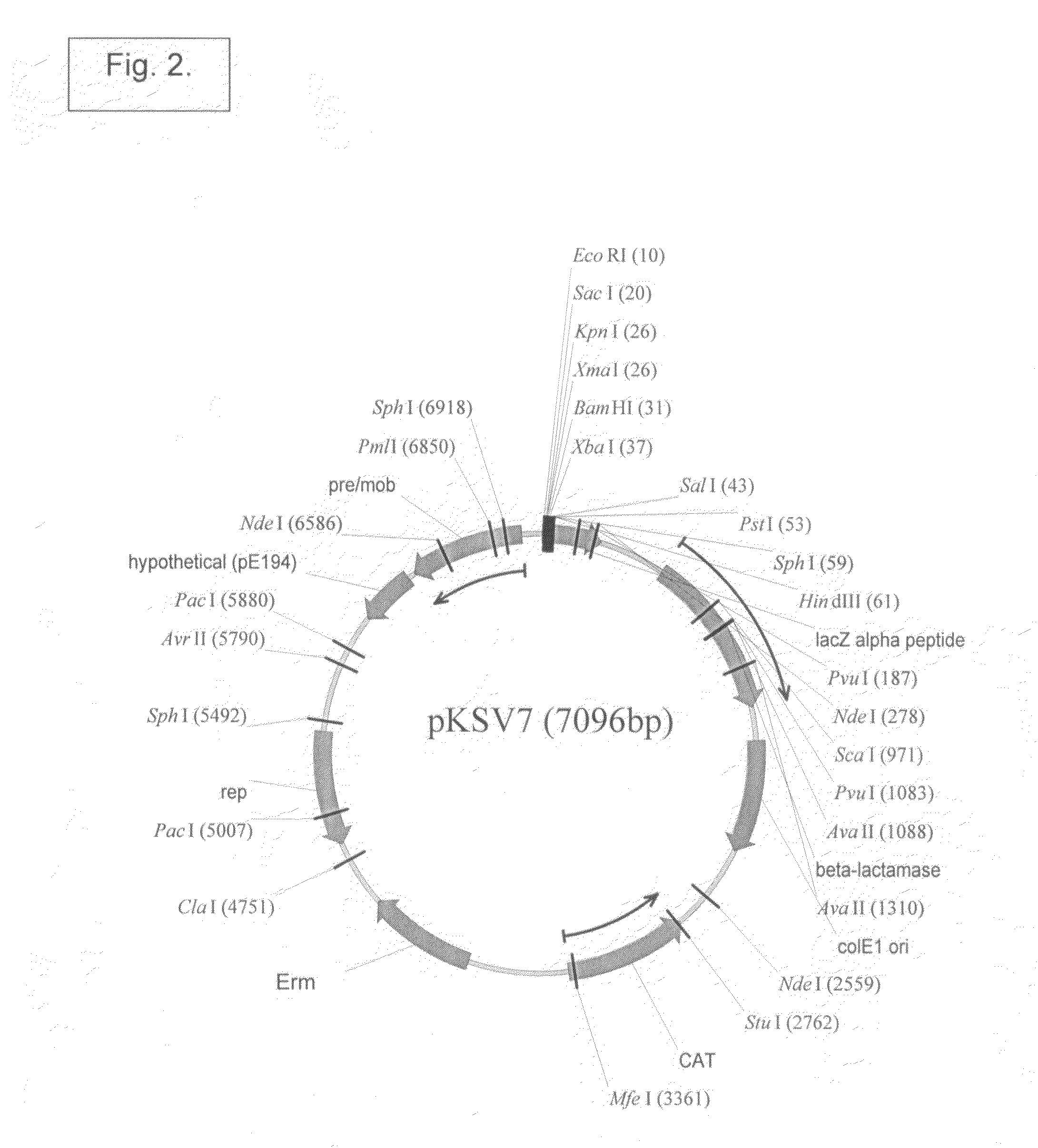
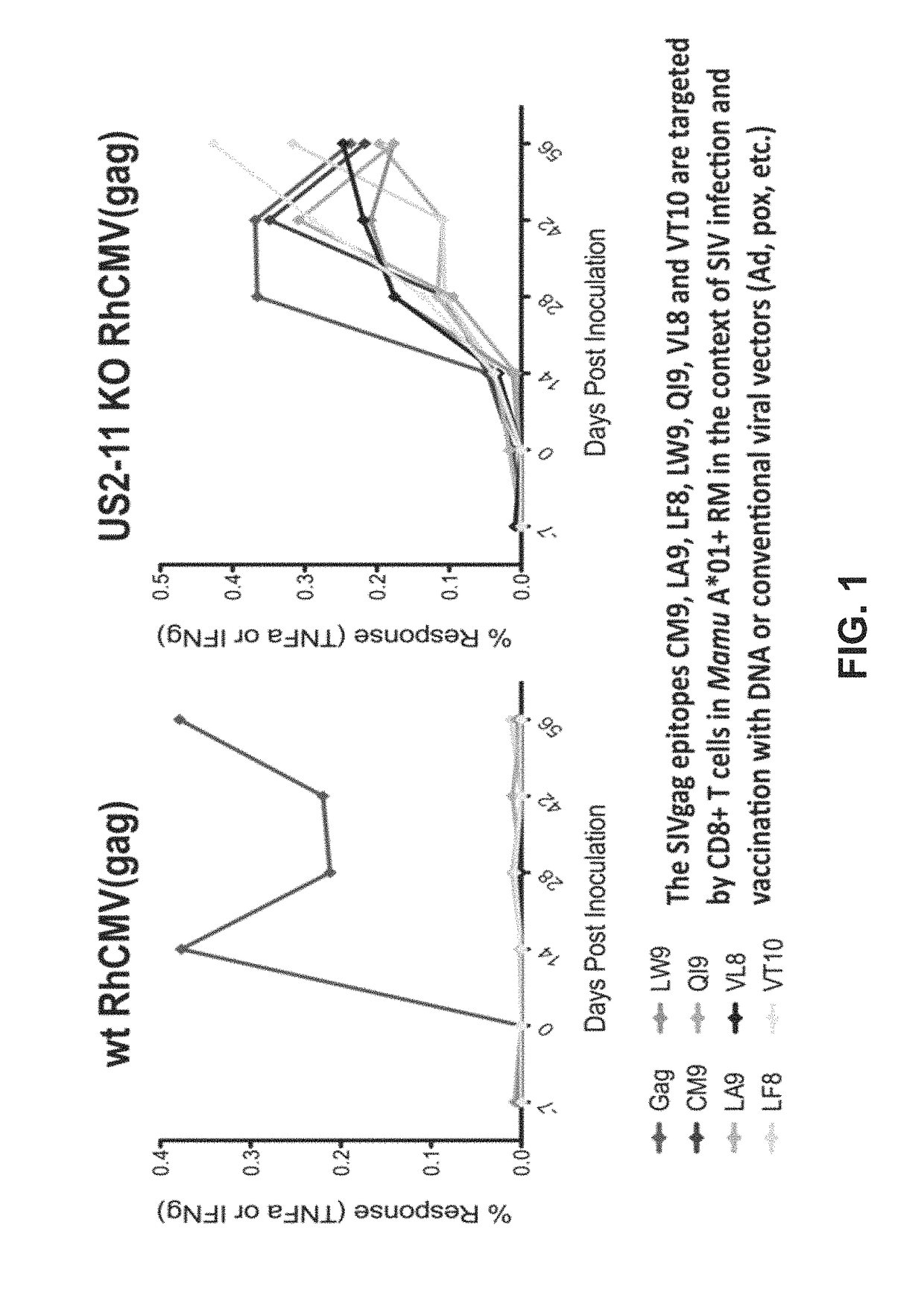
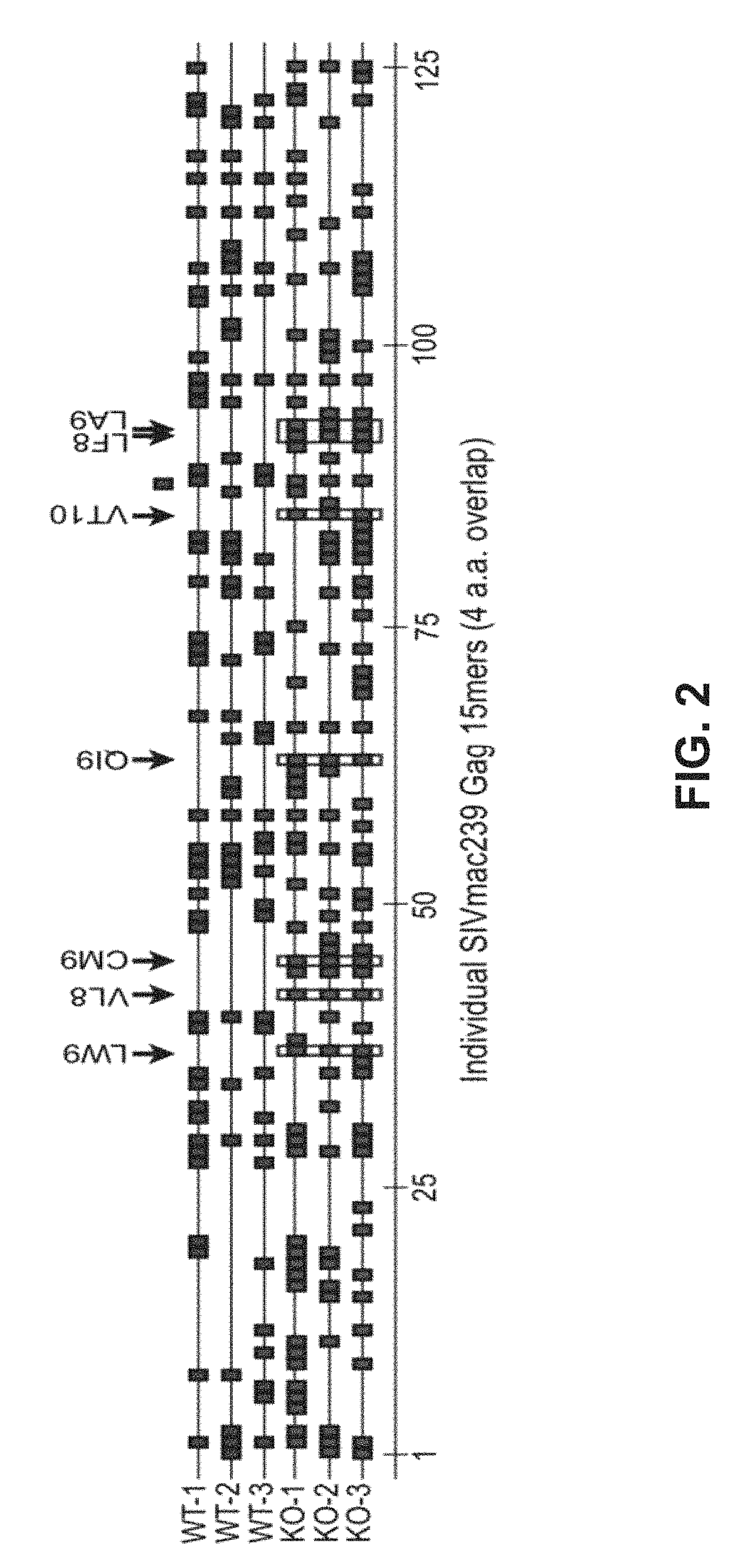
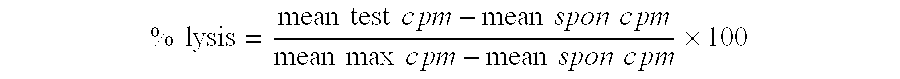Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
93 results about "Heterologous Antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
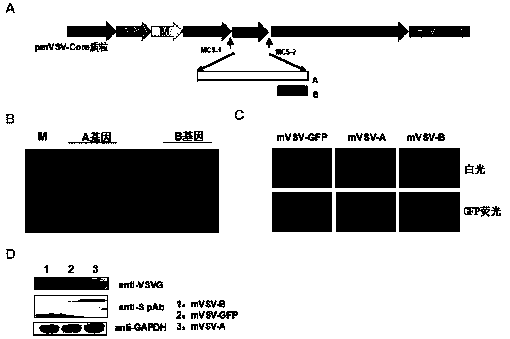
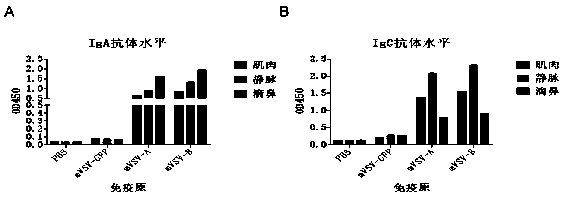
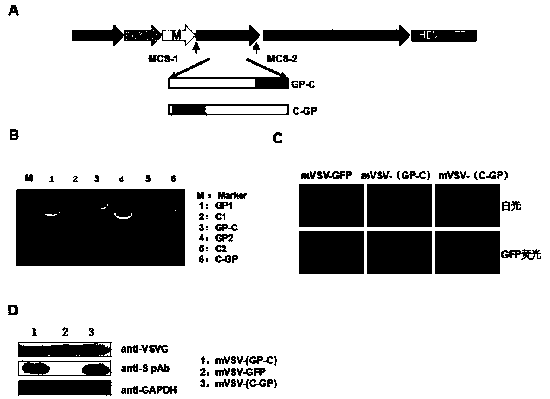
mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
ActiveCN111088283AEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
The invention provides an mVSV virus vector, i.e., attenuated mVSV obtained after multiple modification mutations occur to an M protein amino acid site of a wild Indiana strain VSV, and an optimized heterologous antigen gene is preferentially integrated to a double cloning site area of an mVSV packaging core plasmid pmVSV-Core at the same time. The mVSV virus vector vaccine comprises a heterologous antigen gene which fuses or embeds a target virus between G and L genes of an mVSV vector envelope, wherein the antigen gene comprises an enveloped and embedded antigen gene encoding the target virus, an embedded combination antigen gene or a fused antigen gene; the mVSV virus vector is embedded or fused with a dominant antigen of spike protein S of an SARS-CoV-2 pathogen; the dominant antigen is preferably selected from a receptor binding domain of spike protein S, namely RBD; and a COVID-19 vaccine based on mVSV mediation is formed. The vaccine has good prevention or treatment effect on COVID-19 infected people.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
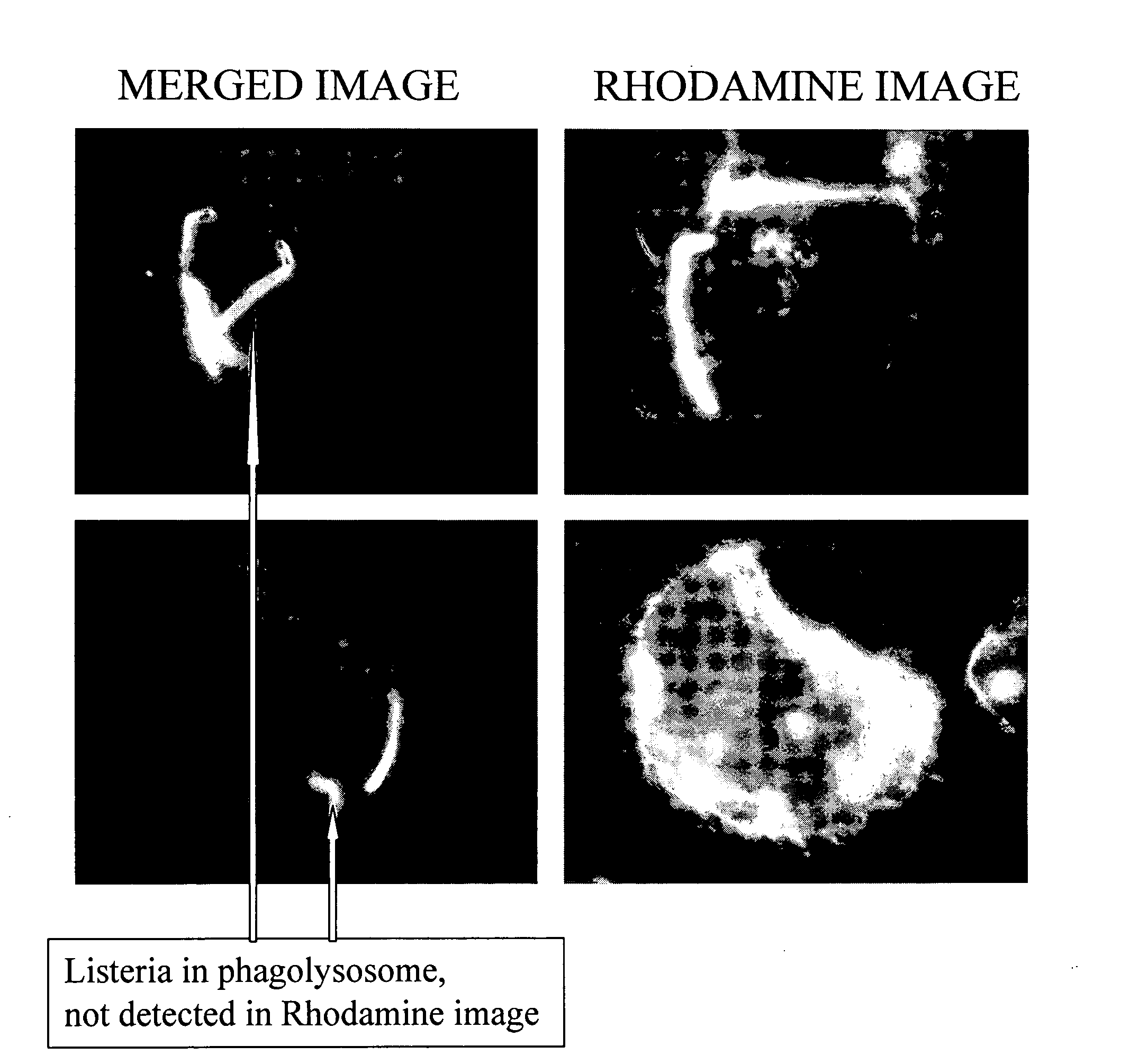
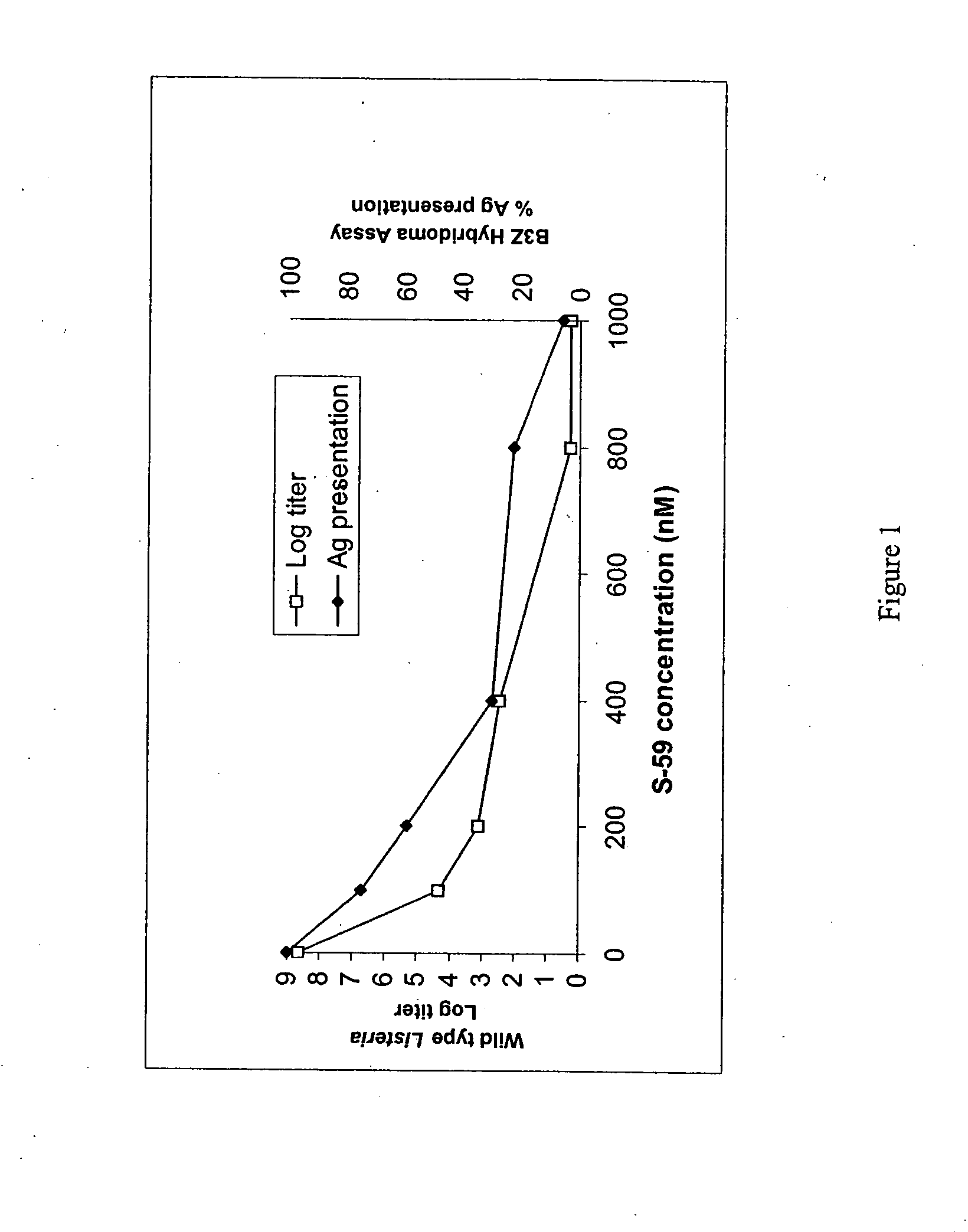
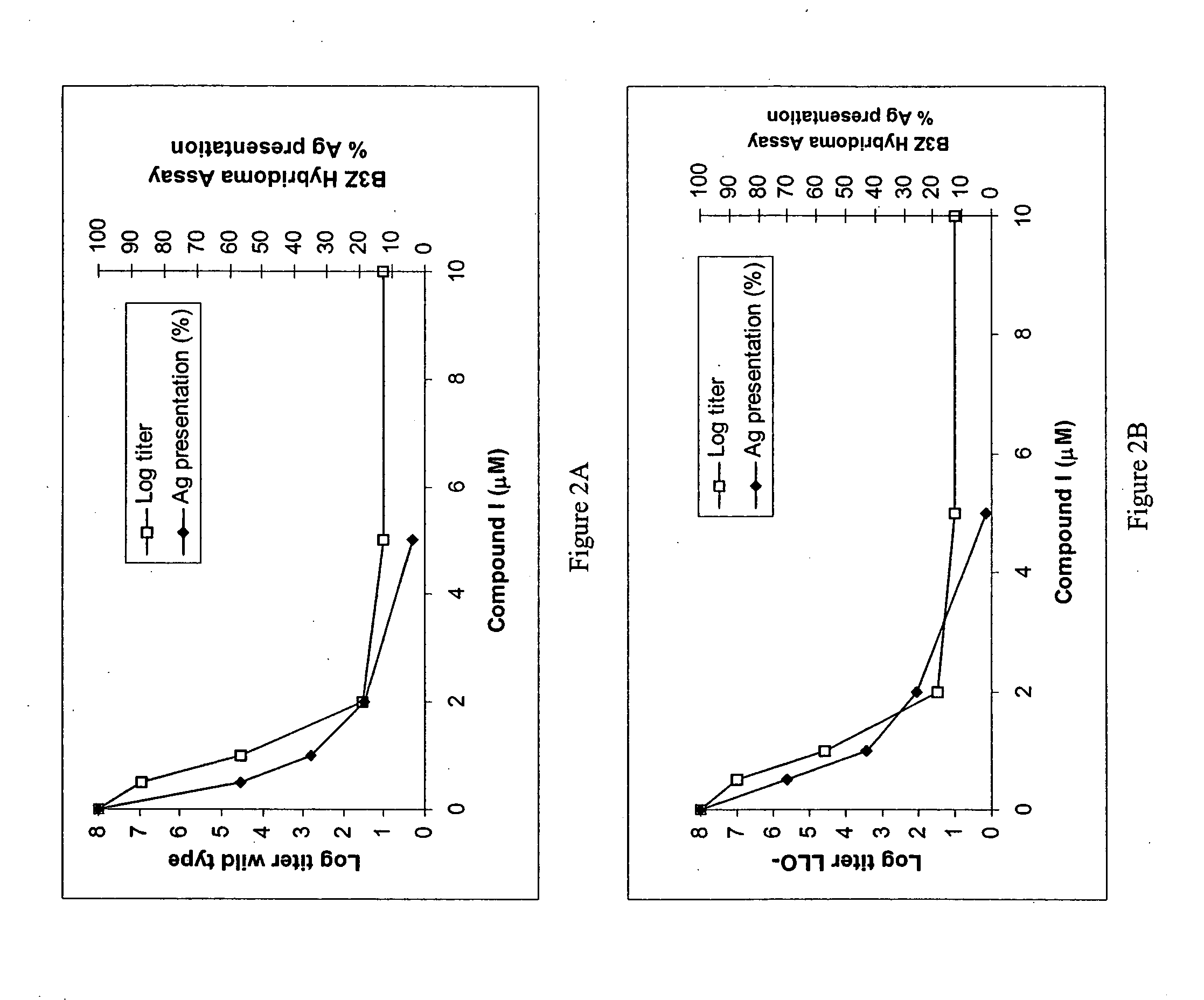
Engineered Listeria and methods of use thereof
InactiveUS20070207170A1Improve survivalIncreasing expression and secretionBiocideBacteriaHeterologous AntigensSite-specific recombination
The invention provides a bacterium containing a polynucleotide comprising a nucleic acid encoding a heterologous antigen, as well as fusion protein partners. Also provided are vectors for mediating site-specific recombination and vectors comprising removable antibiotic resistance genes.
Owner:ANZA THERAPEUTICS INC +1
Methods for constructing antibiotic resistance free vaccines
The present invention provides Listeria vaccine strains that express a heterologous antigen and a metabolic enzyme, and methods of generating same.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Modified free-living microbes, vaccine compositions and methods of use thereof
InactiveUS20080248066A1Reduce microbesReduce spreadBacterial antigen ingredientsBacteriaHeterologous AntigensMicroorganism
Free-living microbes are provided in which the nucleic acid has been modified so that the microbe is attenuated for proliferation and / or which comprise genetic mutations that attenuate the ability of the microbe to repair its nucleic acid. Methods of using the modified microbes for the loading, activation, and / or maturation of antigen-presenting cells are also provided. Vaccine compositions comprising the modified microbes and / or the antigen-presenting cells and methods of using the vaccines are also provided. The microbes may be further modified to include heterologous antigens, such as tumor antigens or infectious disease antigens, for use as a vaccine against cancer or infectious diseases.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
Owner:VIRONOVATIVE
Adenoviral vector-based malaria vaccines
The invention provides adenoviral vectors comprising an adenoviral genome comprising heterologous antigen-encoding nucleic acid sequences, such as Plasmodium nucleic acid sequences, operably linked to promoters. The invention further provides a method of inducing an immune response against malaria in a mammal comprising administering the adenoviral vectors to the mammal.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
Dual delivery system for heterologous antigens
Provided herein are recombinant Listeria strains expressing a tumor-specific antigenic polypeptide and, optionally, an angiogenic polypeptide wherein a nucleic acid molecule encoding at least one of the polypeptides is operably integrated into the Listeria genome in an open reading frame with a nucleic acid sequence encoding a PEST-containing polypeptide, methods of preparing same, and methods of inducing an immune response, and treating, inhibiting, or suppressing cancer or tumors comprising administering same.
Owner:ADVAXIS
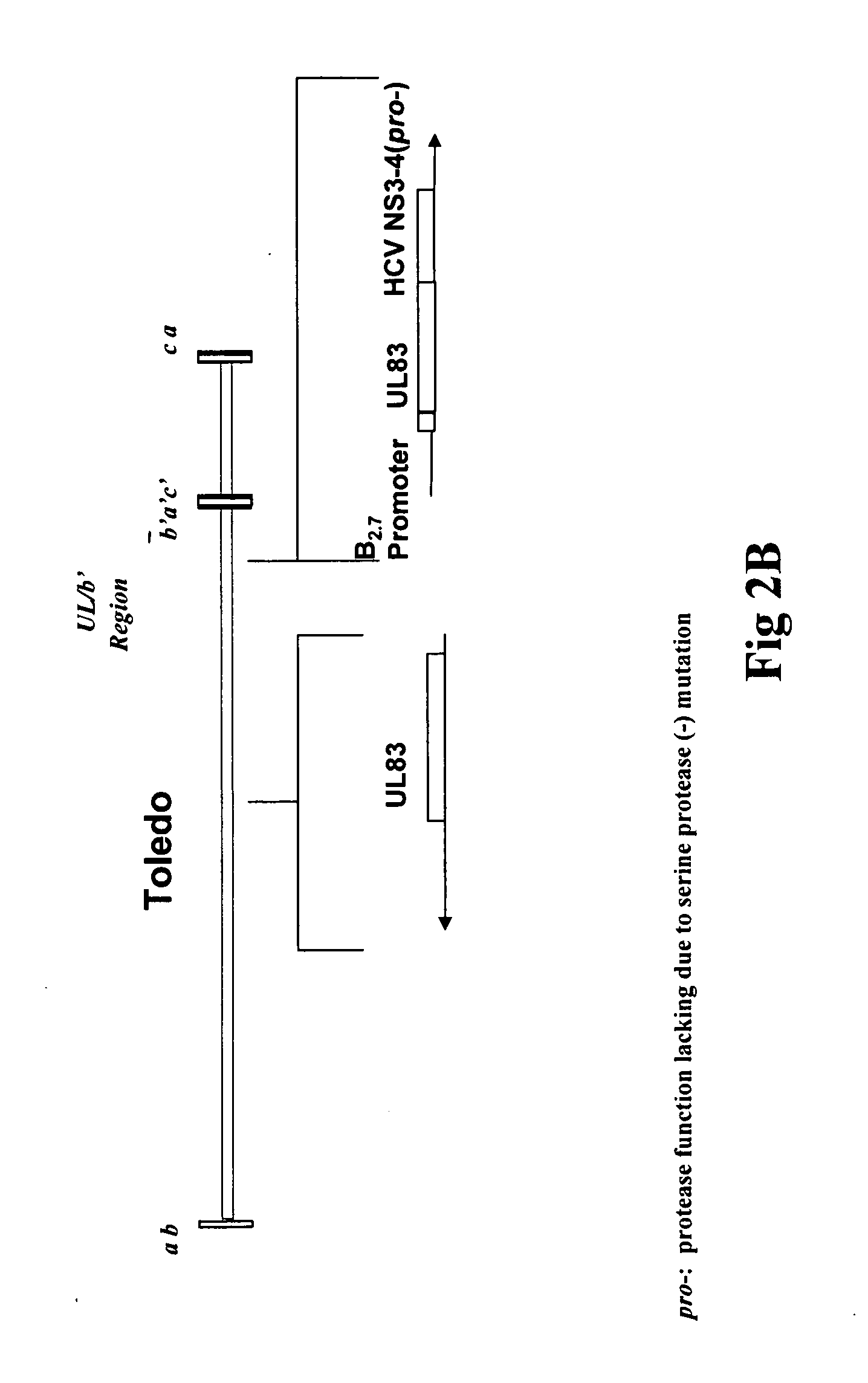
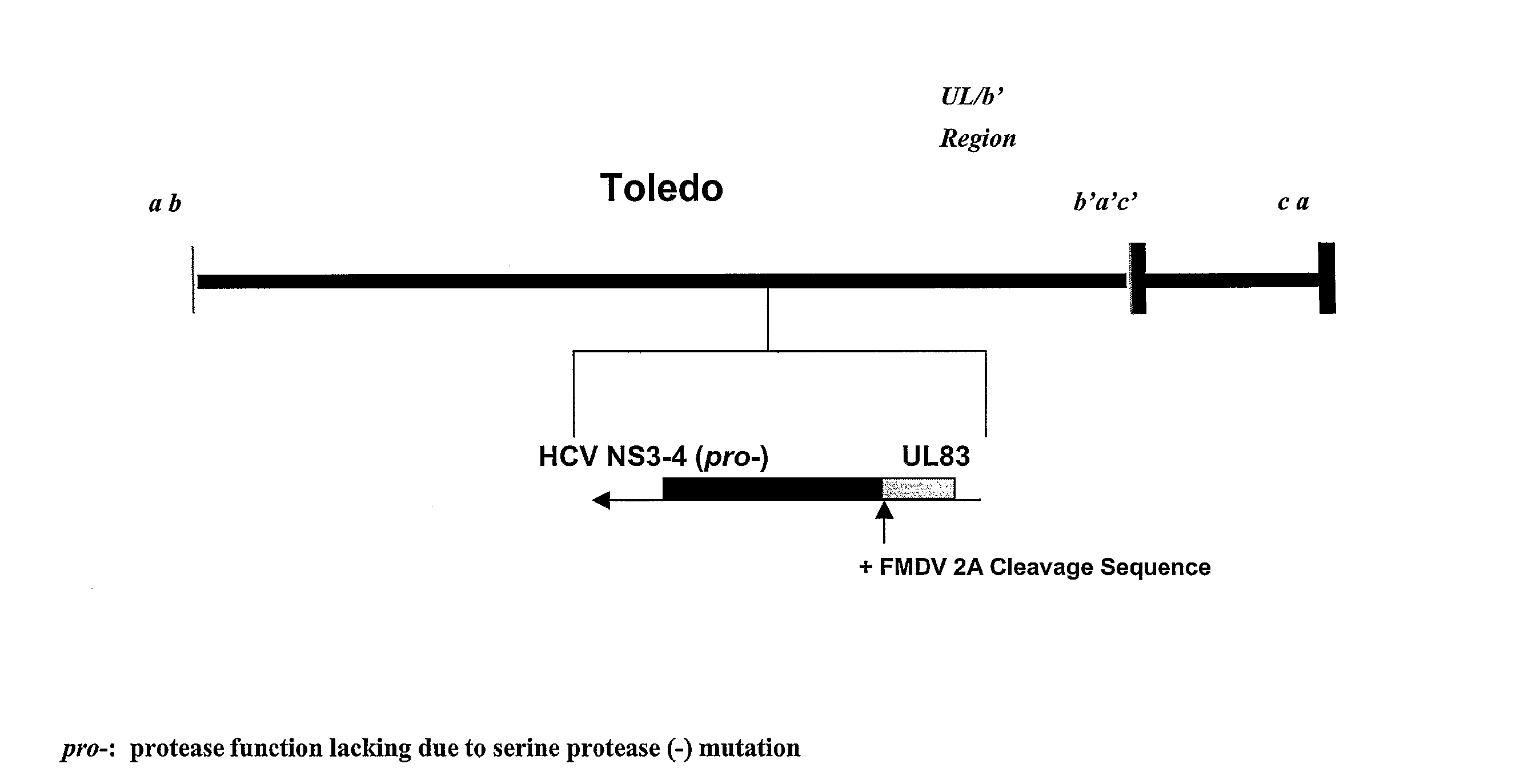
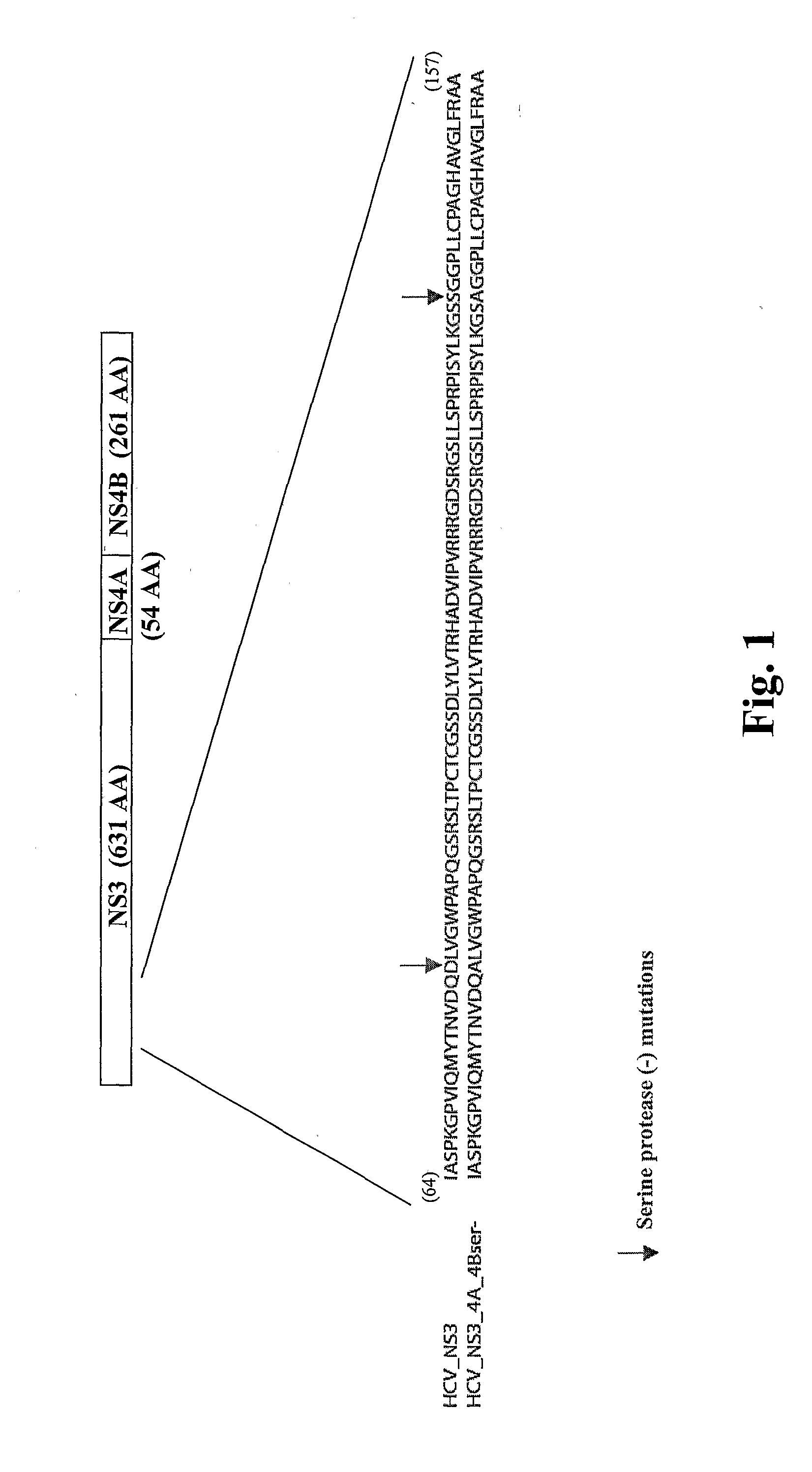
Recombinant human cytomegalovirus and vaccines comprising heterologous antigens
InactiveUS20090297555A1SsRNA viruses positive-senseViral antigen ingredientsHeterologousHeterologous Antigens
The present invention relates to recombinant HCMV (human cytomegalovirus) expressing a pp65 polypeptide or fragment thereof fused to a heterologous or non-native polypeptide, in particular immunogenic and / or antigenic polypeptides. In particular, the heterologous gene products include antigenic or immunogenic polypeptides from a variety of pathogens, cellular genes, tumor antigens, and viruses. The recombinant viruses may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Engineered Listeria and methods of use thereof
InactiveUS7935804B2Improve survivalIncreasing expression and secretionBiocideBacteriaHeterologousHeterologous Antigens
The invention provides a bacterium containing a polynucleotide comprising a nucleic acid encoding a heterologous antigen, as well as fusion protein partners. Also provided are vectors for mediating site-specific recombination and vectors comprising removable antibiotic resistance genes.
Owner:ANZA THERAPEUTICS INC +1
Antibiotic resistance free vaccines and methods for constructing and using same
ActiveUS7855064B2Reduce morbidityBacterial antigen ingredientsBacteriaHeterologousHeterologous Antigens
The present invention provides Listeria strains that express a heterologous antigen and a metabolic enzyme, and methods of generating same.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Multiple delivery system for heterologous antigens
InactiveUS20120135033A1Bacterial antigen ingredientsSugar derivativesHeterologousHeterologous Antigens
The invention is directed to an episomal recombinant nucleic acid encoding at least two heterologous antigens each fused to a PEST-endogenous polypeptide, vaccines comprising the same, methods of preparing same, and methods of inducing an immune response, and treating, inhibiting, or suppressing cancer or tumors comprising administering the same.
Owner:ADVAXIS
Methods and Compositions for Inducing an Immune Response Against Multiple Antigens
InactiveUS20090155297A1Effective and potent immune responseReduce and prevent infectionSugar derivativesAntibody mimetics/scaffoldsHeterologousHeterologous Antigens
Methods and compositions for inducing an immune response against multiple antigens are provided herein. In one aspect, the invention provides a chimeric immunogen, comprising a receptor binding domain, a translocation domain, and more than one non-contiguous heterologous antigen. In other aspects, the invention provides nucleic acids encoding chimeric immunogens of the invention, kits comprising chimeric immunogens of the invention, cells expressing chimeric immunogens of the invention, and methods or using chimeric immunogens of the invention. 1mggkwskssv igwptvrerm rraepaadrvgaasrdlekh gaitssntaa tnaacawlea 61qeeeevgfpv tpqvplrpmt ykaavdlshflkekgglegl ihsqrrqdil dlwiyhtqgy121fpdwqnytpg pgvrypltfg wcyklvpvepdkieeankge ntsllhpvsl hgmddperev181lewrfdsrla fhhvarelhp eyfknc
Owner:TRINITY BIOSYSTEMS INC
Antibiotic resistance free vaccines and methods for constructing and using same
ActiveUS20080131456A1Reduce morbidityBacterial antigen ingredientsBacteriaHeterologousHeterologous Antigens
The present invention provides Listeria strains that express a heterologous antigen and a metabolic enzyme, and methods of generating same.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Dual delivery system for heterologous antigens
ActiveUS20140335120A1Tumor rejection antigen precursorsBacteriaHeterologous AntigensOpen reading frame
Provided herein are recombinant Listeria strains expressing a tumor-specific antigenic polypeptide and, optionally, an angiogenic polypeptide wherein a nucleic acid molecule encoding at least one of the polypeptides is operably integrated into the Listeria genome in an open reading frame with a nucleic acid sequence encoding a PEST-containing polypeptide, methods of preparing same, and methods of inducing an immune response, and treating, inhibiting, or suppressing cancer or tumors comprising administering same.
Owner:ADVAXIS
Targeted heterologous antigen presentation on calicivirus virus-like particles
Owner:TAKEDA VACCINES INC
Antibiotic resistance free Listeria strains and methods for constructing and using same
ActiveUS7858097B2Reduce morbidityBacterial antigen ingredientsBacteriaHeterologousHeterologous Antigens
The present invention provides Listeria strains that express a heterologous antigen and a metabolic enzyme, and methods of generating same.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Dual delivery system for heterologous antigens
Provided herein are recombinant Listeria strains expressing a tumor-specific antigenic polypeptide and, optionally, an angiogenic polypeptide wherein a nucleic acid molecule encoding at least one of the polypeptides is operably integrated into the Listeria genome in an open reading frame with a nucleic acid sequence encoding a PEST-containing polypeptide, methods of preparing same, and methods of inducing an immune response, and treating, inhibiting, or suppressing cancer or tumors comprising administering same.
Owner:ADVAXIS
Antibiotic resistance free listeria strains and methods for constructing and using same
ActiveUS20100291140A1Reduce morbidityBacterial antigen ingredientsBacteriaHeterologousHeterologous Antigens
The present invention provides Listeria strains that express a heterologous antigen and a metabolic enzyme, and methods of generating same.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Pharmaceutical compositions comprising a polypeptide comprising at least one cxxc motif and heterologous antigens and uses thereof
ActiveUS20130095133A1Minimize occurrencePrecise deliverySsRNA viruses negative-senseAntibacterial agentsHeterologousHeterologous Antigens
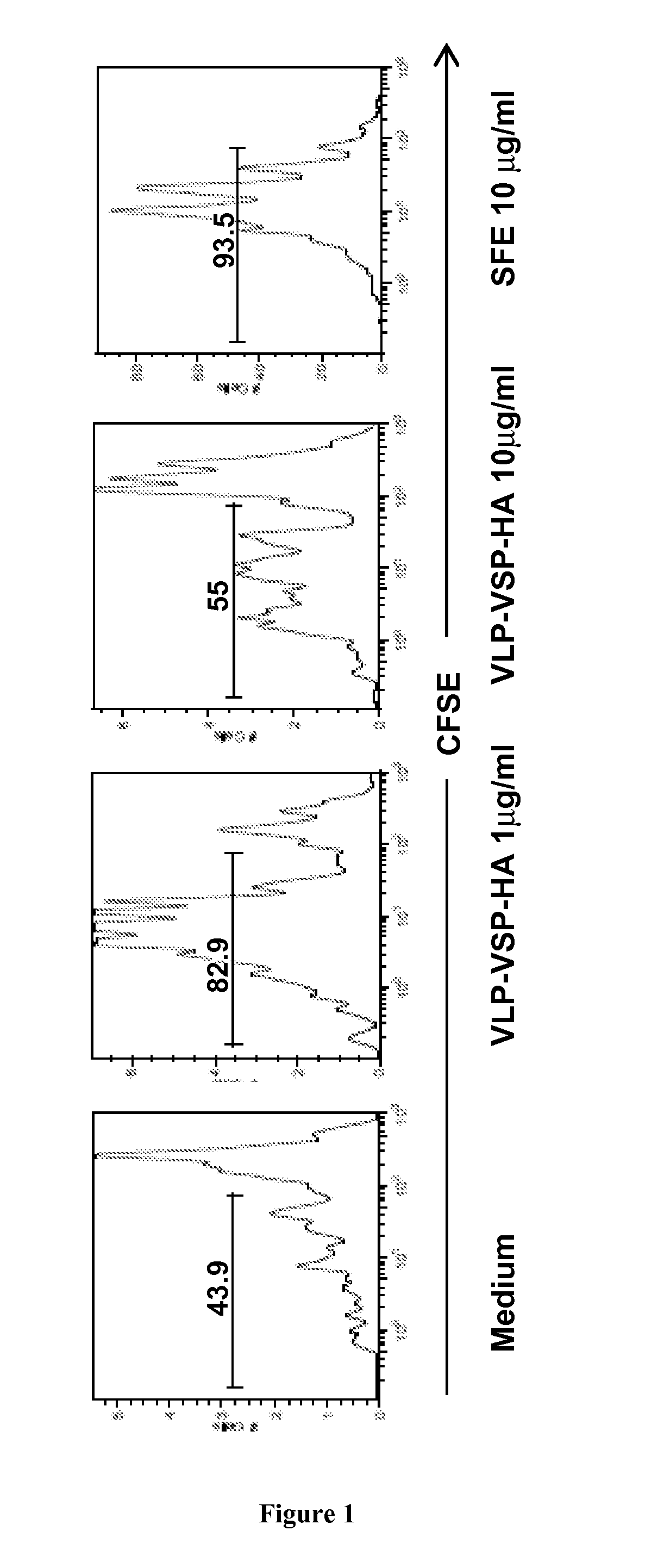
The invention relates to pharmaceutical compositions using a polypeptide comprising at least one CXXC motif, such as Giardia parasite's variable surface proteins (VSP) or a fragment thereof to raise by oral or mucosal vaccination an immune response against a heterologous selected antigen, such as tumor antigen, microbial antigen or other antigen.
Owner:CONSEJO NAT DE INVESTIGACIONES CIENTIFICAS Y TECH CONICET +4
Hepatitis b virus surface antigen as a mucosal immunostimulator and the resulting formulations
InactiveUS20050025780A1Enhance immune responseViral antigen ingredientsReverse transcribing DNA virusesHeterologous AntigensAdjuvant
The invention relates to a mucosal surface antigen which is used to promote and increase in the immune response against co-administered antigens in the formulations out line in the invention. Said novel formulations are obtained from the dual use of the surface antigen as an immunostimulatory agent and, at the same time, as a vaccine antigen. In this way it is possible to obtain multiple formulations of the hepatitis B surface antigen and heterologous antigens, with immunogenicity levels similar to those obtained following parenteral administration and with a reduction in components that can dispense with the use of nasal adjuvants, thereby converting same antigens into elements that can promote an increase in the response to the other co-administered antigens. Said novel use of the hepatitis B virus surface antigen and the resulting antigen formulations can be used in the pharmaceutical industry as therapeutic and preventive vaccine formulations.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
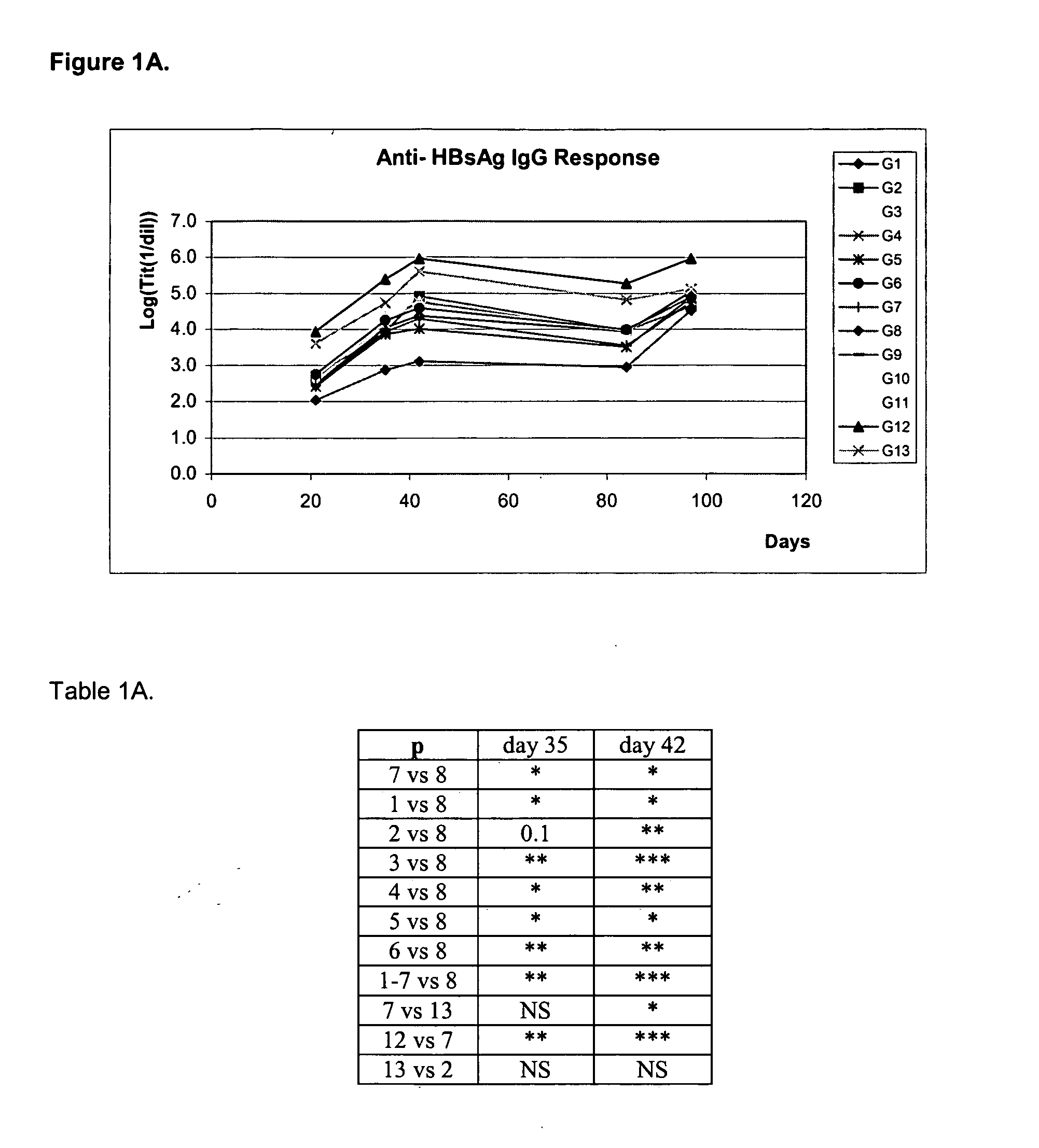
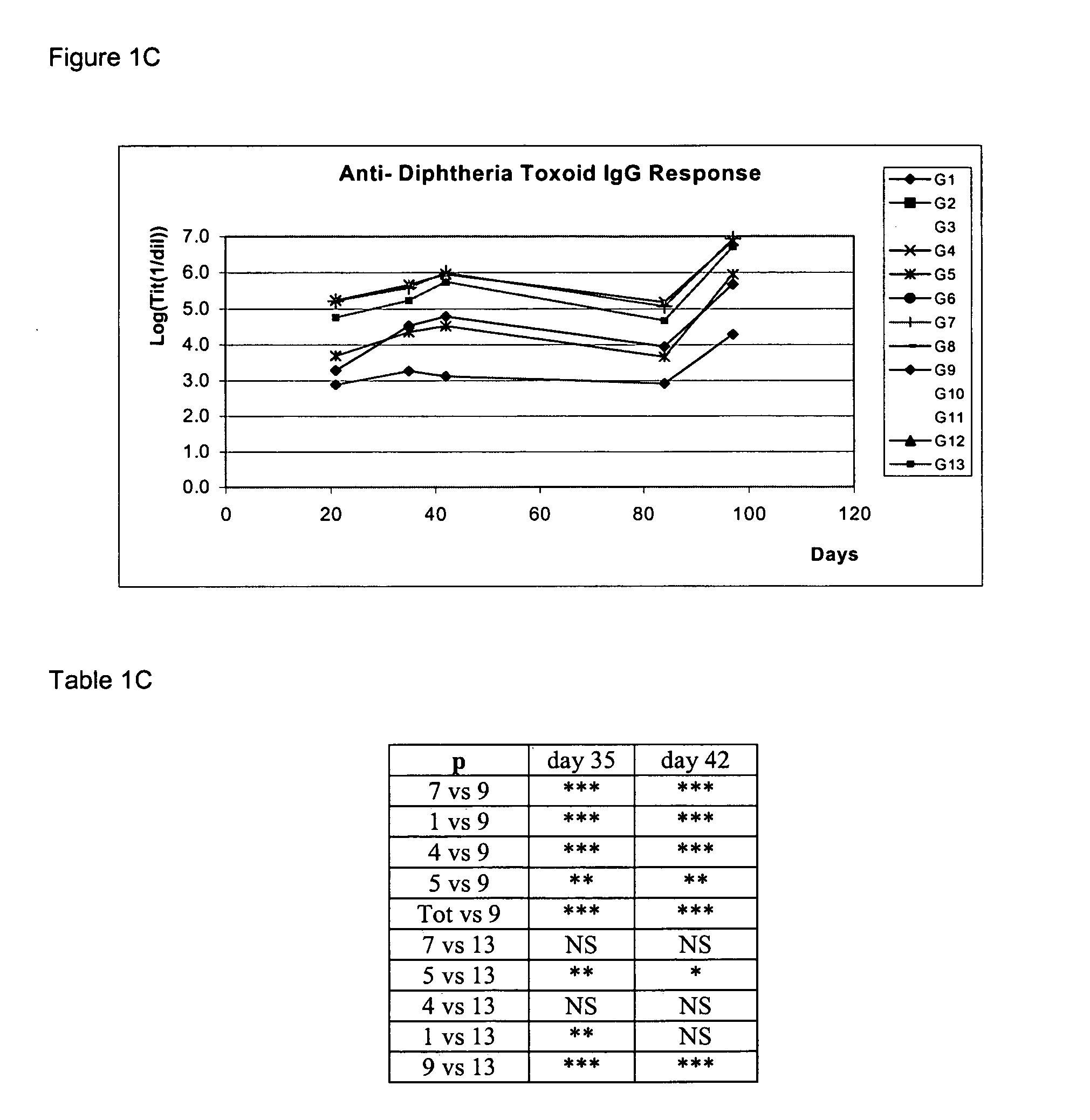
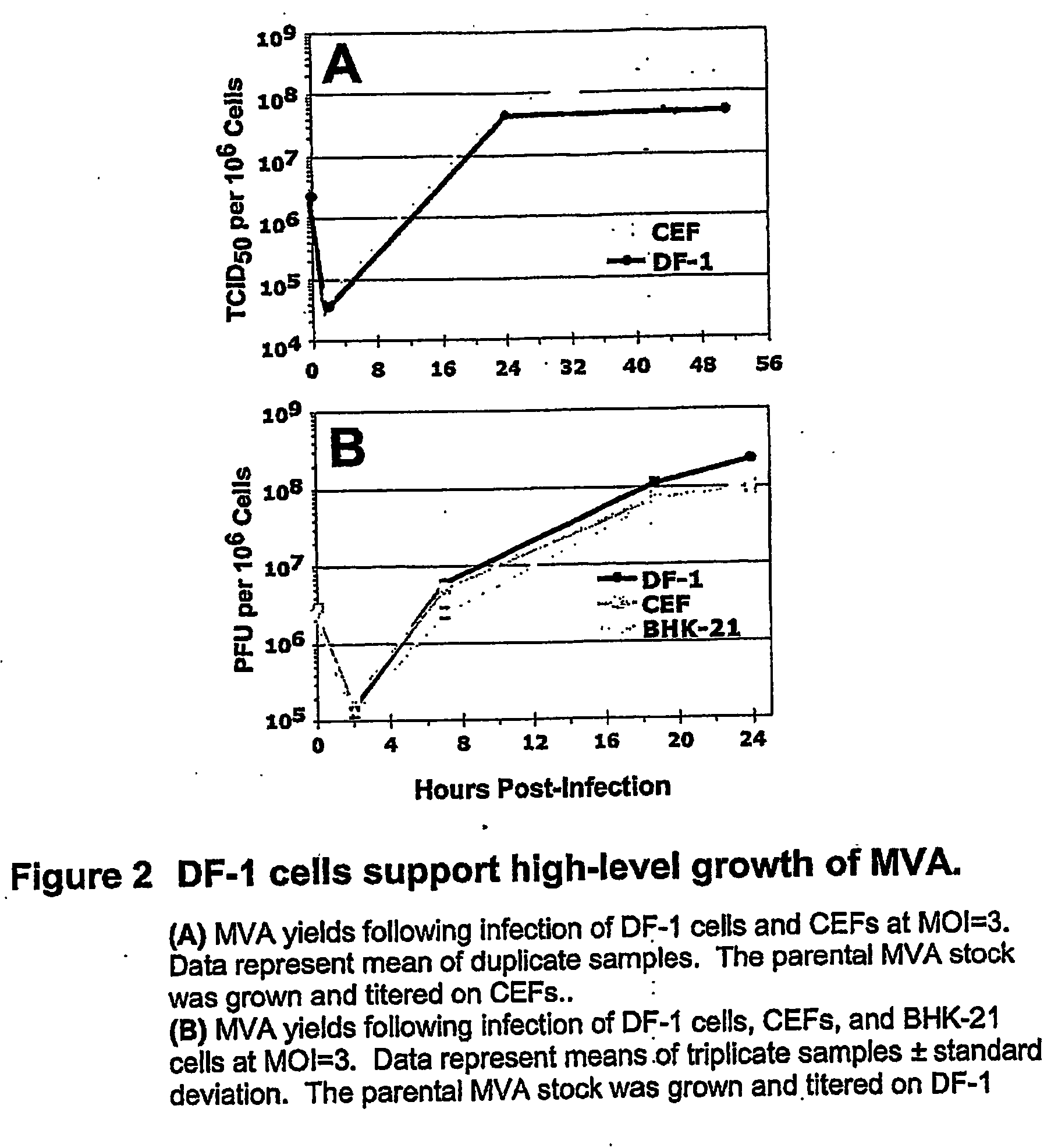
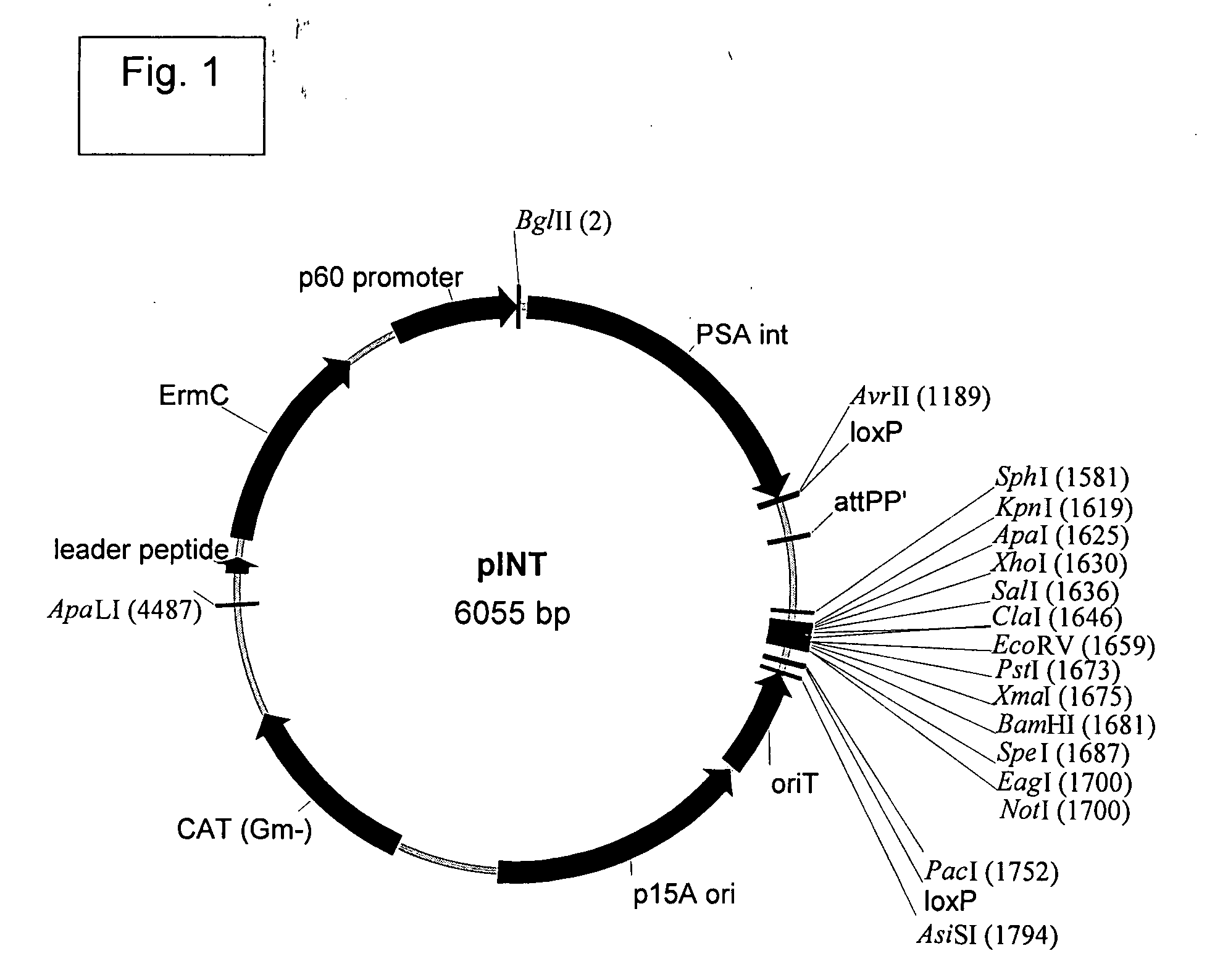
Mva Vaccines
Recombinant modified vaccinia Ankara vectors are provided having a null mutation in a gene necessary for replication of the recombinant modified vaccinia Ankara virus and at least one heterologous antigen. The disclosed vectors optionally encode at least one pro-apoptotic factor, at least one anti-apoptotic factor, at least one immunomodulator, and combinations thereof. Cells complementing the null mutation the disclosed vectors are also provided.
Owner:FEINBERG MARK +1
Engineered listeria and methods of use thereof
InactiveUS20120121643A1Improve survivalIncreasing expression and secretionBacteriaAntibody mimetics/scaffoldsHeterologous AntigensNucleotide
The invention provides a bacterium containing a polynucleotide comprising a nucleic acid encoding a heterologous antigen, as well as fusion protein partners. Also provided are vectors for mediating site-specific recombination and vectors comprising removable antibiotic resistance genes.
Owner:ANZA THERAPEUTICS INC
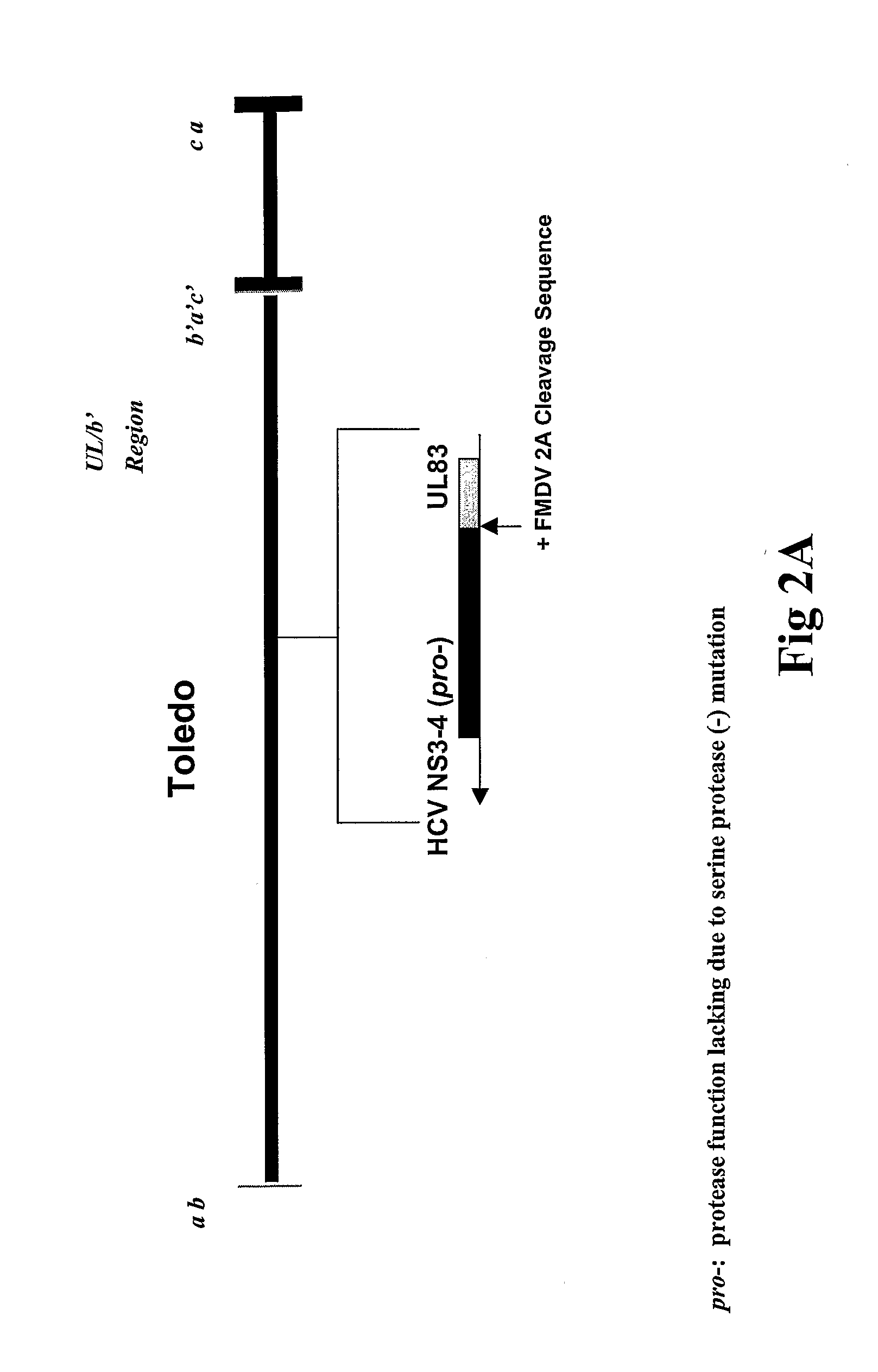
CMV glycoproteins and recombinant vectors
Disclosed herein are recombinant CMV vectors which may comprise a heterologous antigen that can repeatedly infect an organism while inducing a CD8+ T cell response to immunodominant epitopes of the heterologous antigen. The CMV vector may comprise a deleterious mutation in the US11 glycoprotein or a homolog thereof.
Owner:OREGON HEALTH & SCI UNIV
Ceramide-like glycolipid-associated bacterial vaccines and uses thereof
InactiveUS20130164325A1Improve responseAntibacterial agentsBacterial antigen ingredientsBacteroidesHeterologous Antigens
The invention is directed compositions and methods related to bacterial cells that are physically associated with ceramide-like glycolipids for use as antigen carriers for heterologous antigens. The invention further relates to methods of incorporating ceramide-like glycolipid to bacterial cell walls. The compositions and methods of the present invention are useful for the prevention and treatment of diseases.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE INC
Novel baculovirus display vectors and uses thereof
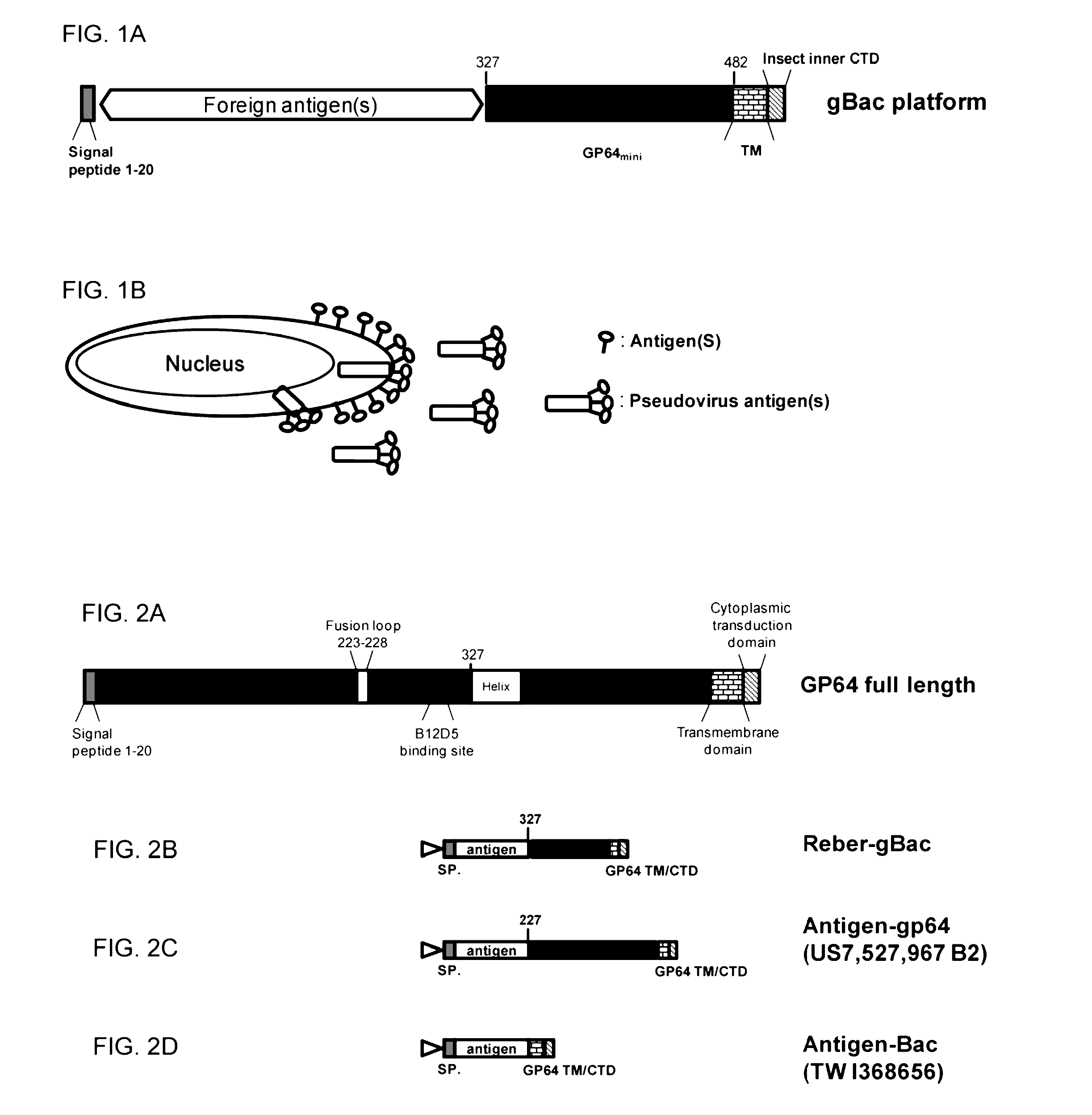
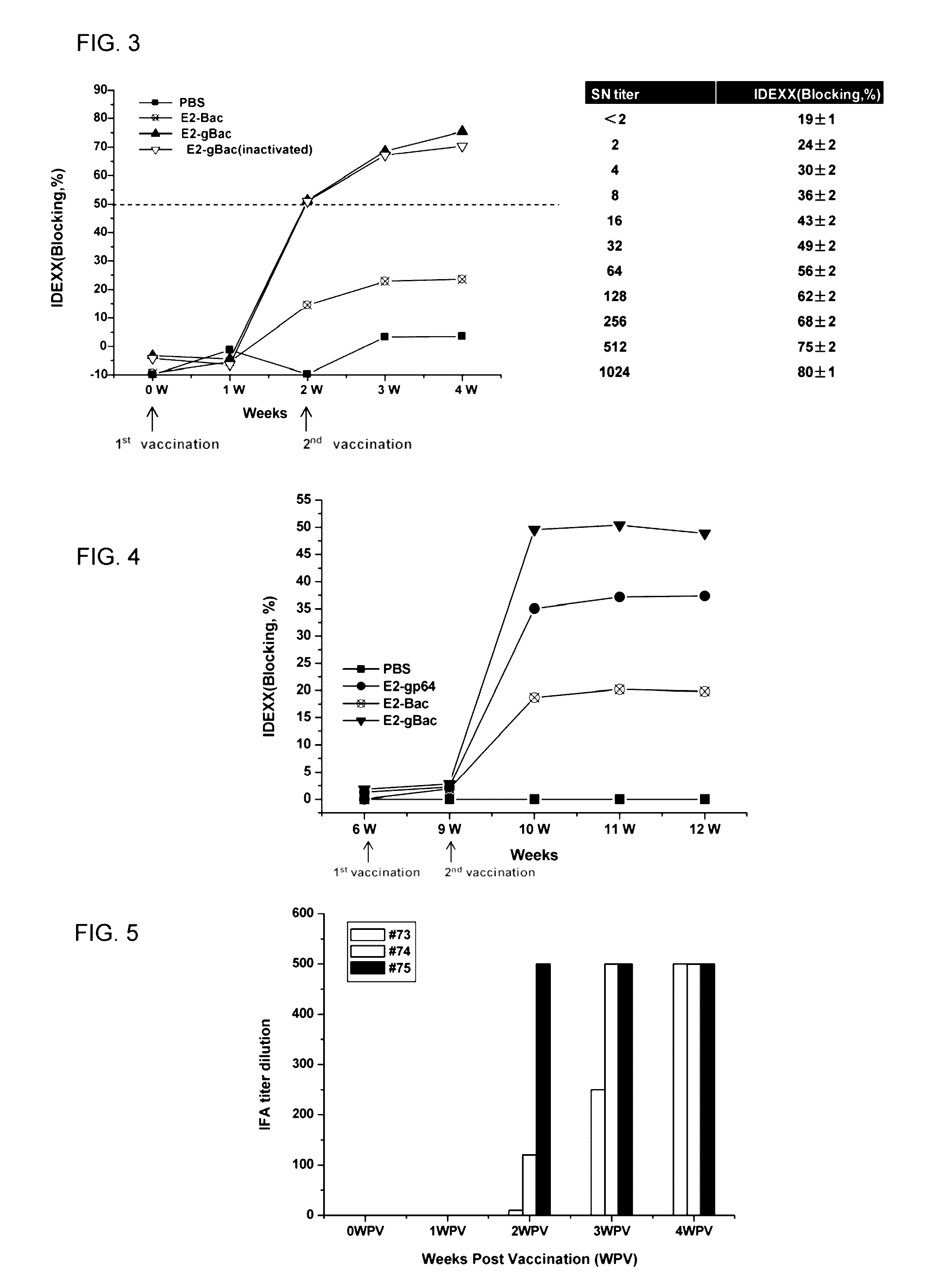
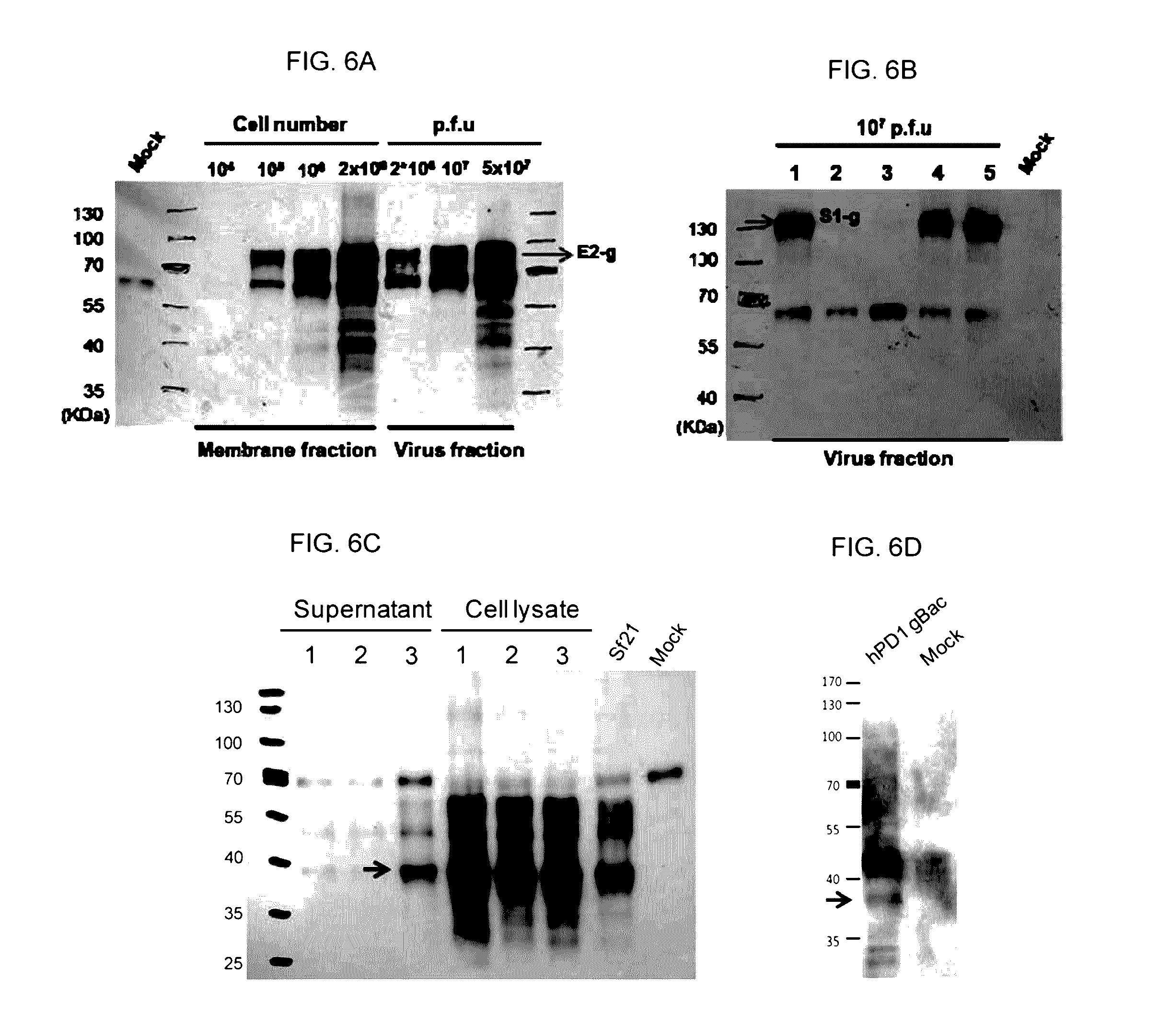
ActiveUS20160331826A1Polypeptide with localisation/targeting motifSsRNA viruses positive-senseSequence signalHeterologous
A recombinant baculovirus displaying on its envelop a fusion protein is disclosed. The fusion protein comprises a heterologous antigen, and a C-terminal region of baculovirus envelope GP64 protein, which has at least 100 amino acid residues in length and lacks a B12D5 binding epitope located within the central basic region of the GP64 protein. The genome of the recombinant baculovirus comprises a transgene encoding a fusion protein that comprises a signal peptide, the heterologous antigen, and the C-terminal region of the baculovirus envelope GP64 protein, in which the antigen is located between the signal peptide and the C-terminal region of the GP64 protein. Methods for eliciting an antigen-specific immunogenic response in a subject in need thereof are also disclosed.
Owner:REBER GENETICS CO LTD
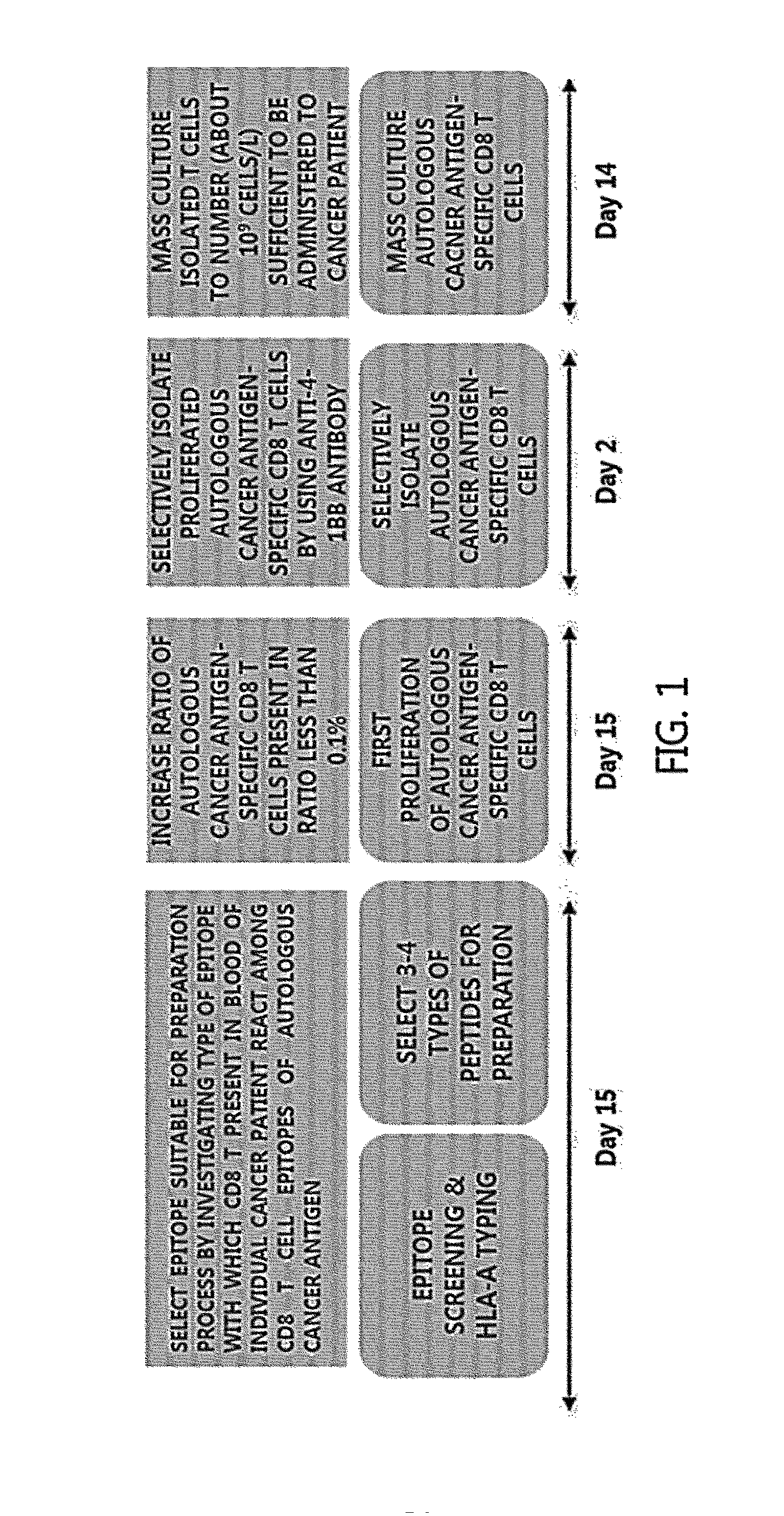
Methods for Isolating and Proliferating Autologous Cancer AntiGen-Specific CD8+ T Cells
InactiveUS20150259646A1Efficient selectionEliminate effectiveBlood/immune system cellsCell culture active agentsDiseaseHeterologous Antigens
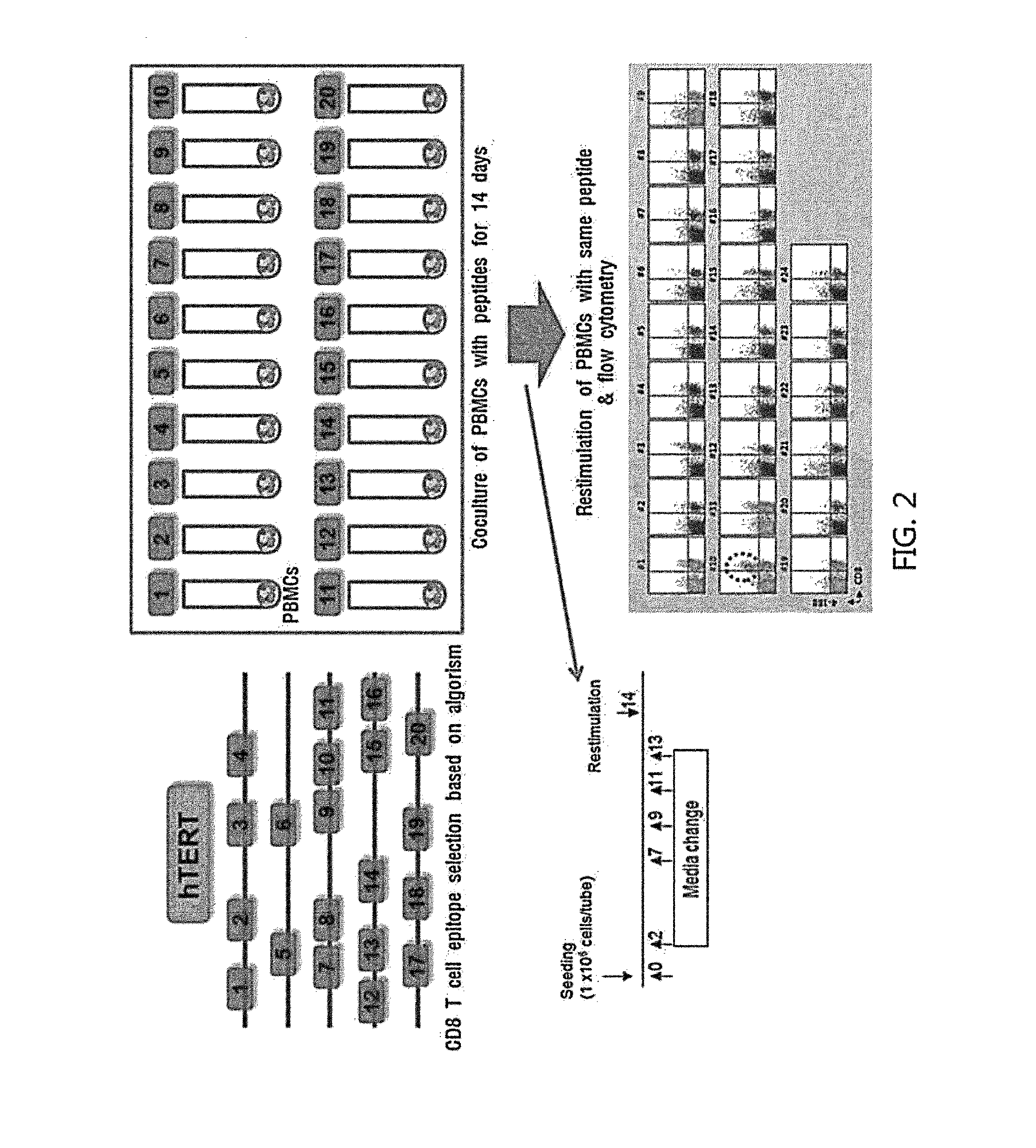
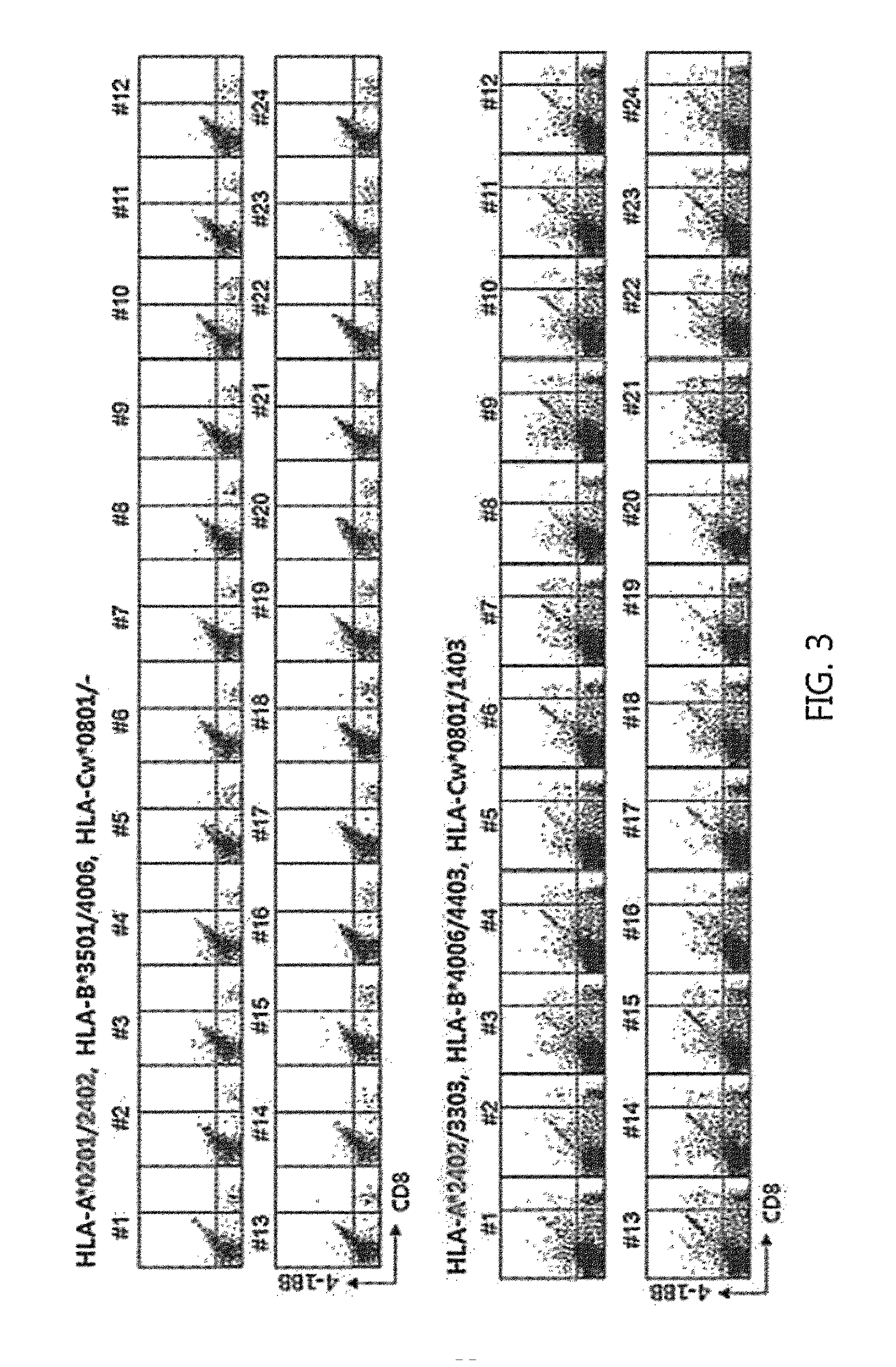
Provided is a method for isolating and proliferating autologous cancer antigen-specific CD8+T cells, and more particularly, a method for selecting an epitope recognized by CD8+ T cells from autologous cancer antigens present in blood of individual cancer patients; and isolating autologous cancer antigen-specific CD8+ T cells by using a peptide of the selected epitope, and a method of massively proliferating CD8+ T cells by using the method. According to the present invention, it is possible to isolate autologous cancer antigen-specific CD8+ T cells by using the peptide of the CD8 T cell epitope of the autologous cancer antigen present in blood of individual cancer patients instead of a heterologous antigen. Therefore, by using T cells recognizing the autologous cancer antigen, it is possible to effectively select and eliminate cancer cells derived from the cancer patient's own cells. Thus, T cells can be applied to treatment and alleviation of cancer diseases without side effects.
Owner:NAT CANCER CENT
Site specific listeria integration vectors and methods for using the same
Site-specific Listeria integration vectors and methods for their use are provided. The subject vectors include a bacteriophage integrase gene and a bacteriophage attachment site, where in many embodiments the bacteriophage that is the source of these elements is a listeriophage. In certain embodiments, the subject vectors further include a multiple cloning site, where the multiple cloning site may further include a polypeptide coding sequence, e.g., for a heterologous antigen. The subject vectors and methods find use in a variety of different applications, including the study of Listeria species and the preparation of Listeria vaccines.
Owner:RGT UNIV OF CALIFORNIA
Methods for constructing antibiotic resistance free vaccines
The present invention provides Listeria vaccine strains that express a heterologous antigen and a metabolic enzyme, and methods of generating same.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant Human Cytomegalovirus And Vaccines Comprising Heterologous Antigens
The present invention relates to recombinant HCMV (human cytomegalovirus) expressing a pp65 polypeptide or fragment thereof fused to a heterologous or non-native polypeptide, in particular immunogenic and / or antigenic polypeptides. In particular, the heterologous gene products include antigenic or immunogenic polypeptides from a variety of pathogens, cellular genes, tumor antigens, and viruses. The recombinant viruses may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com