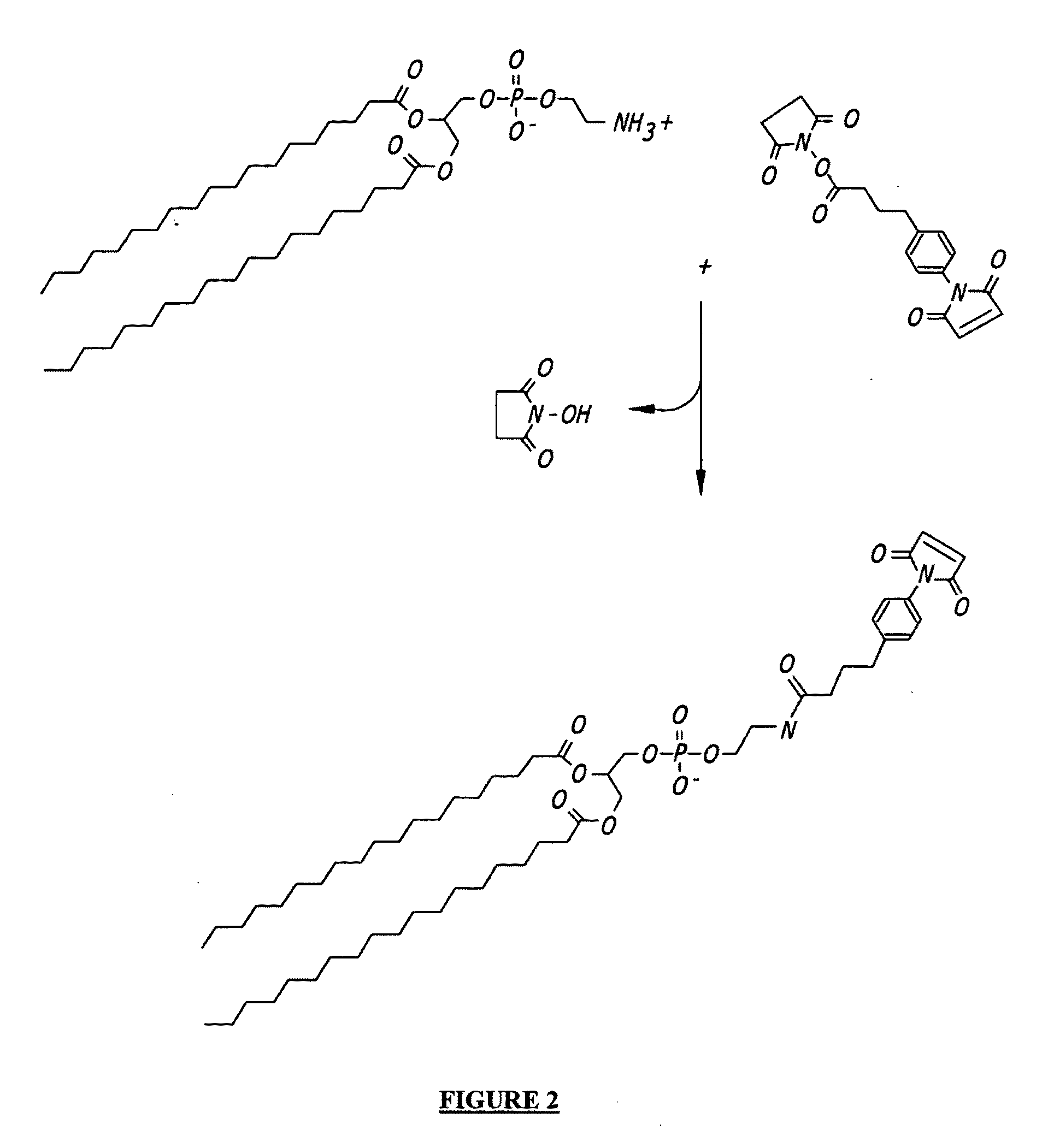
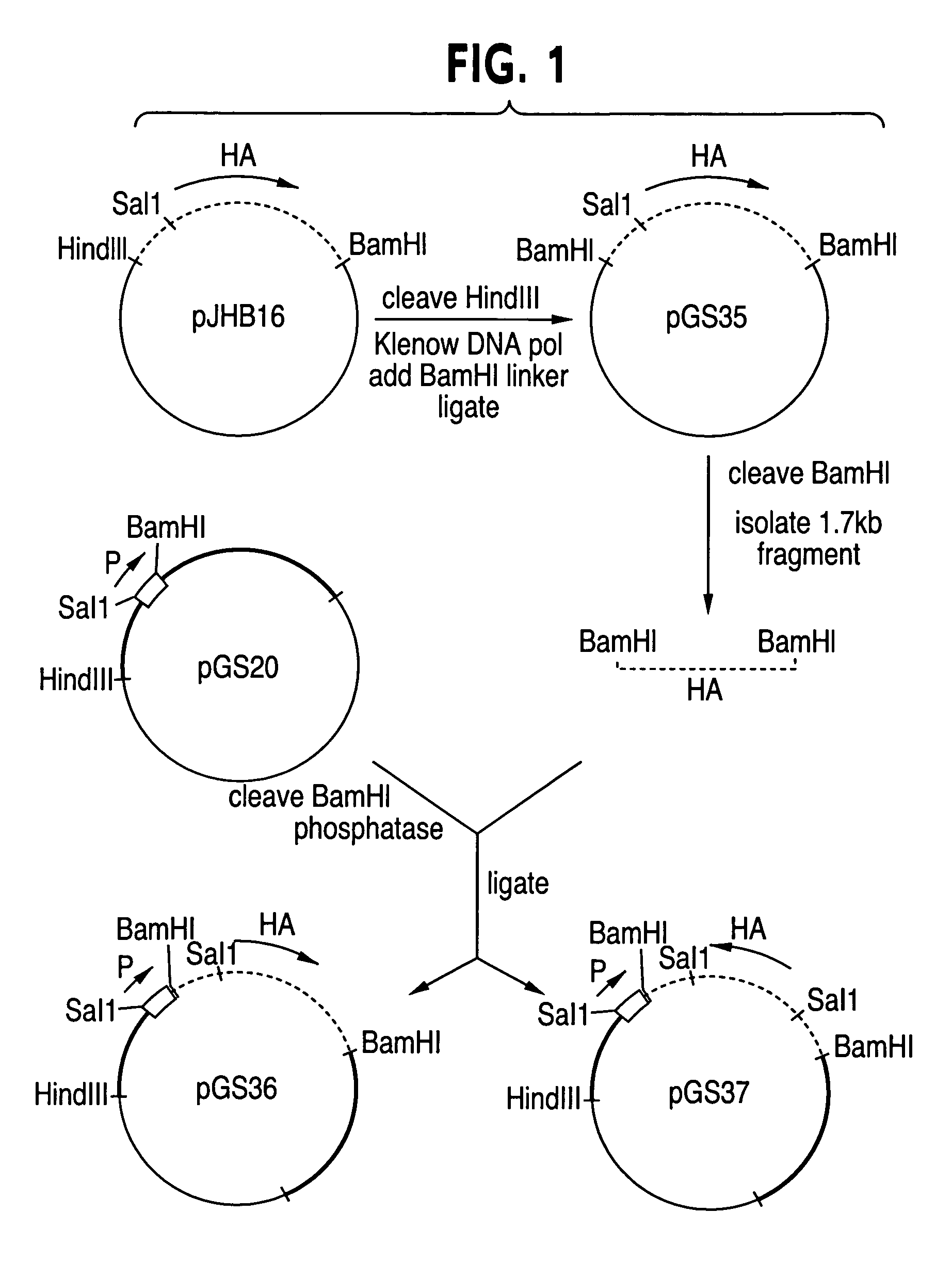
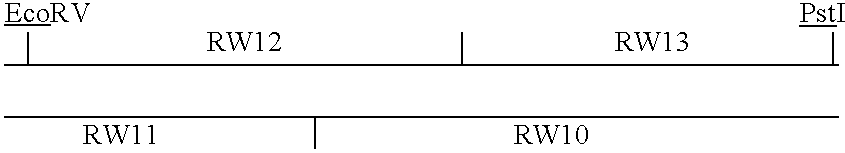
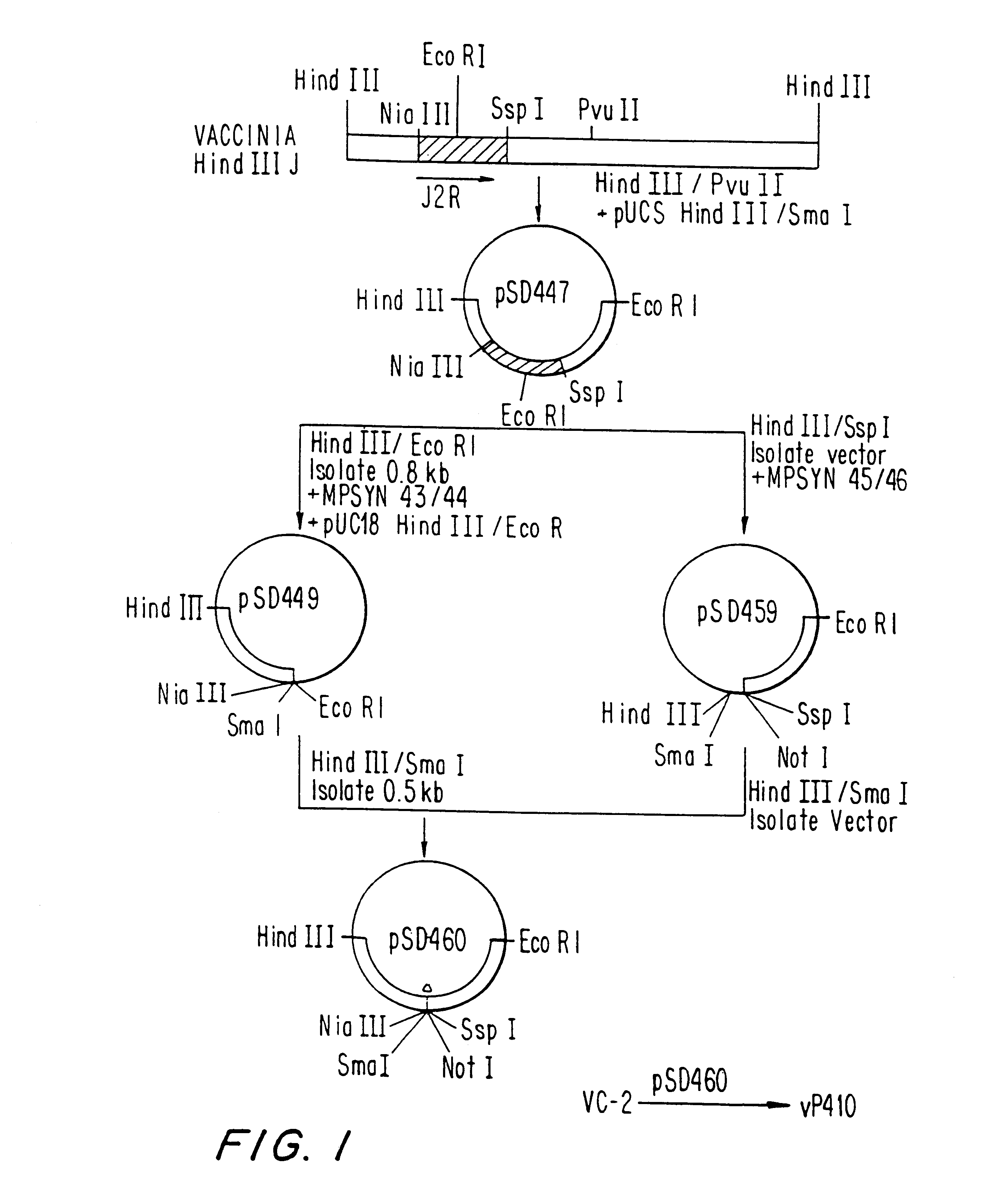
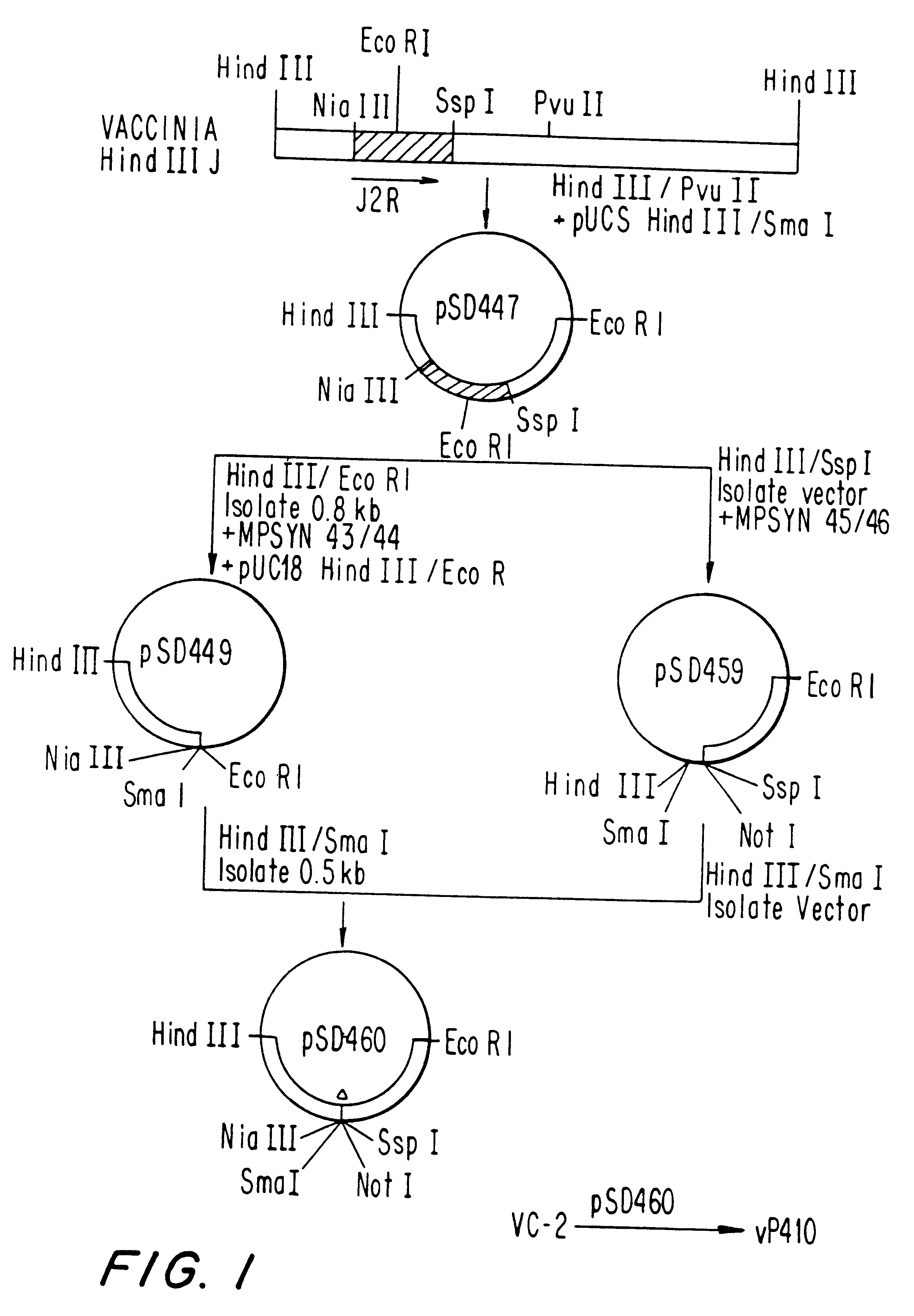
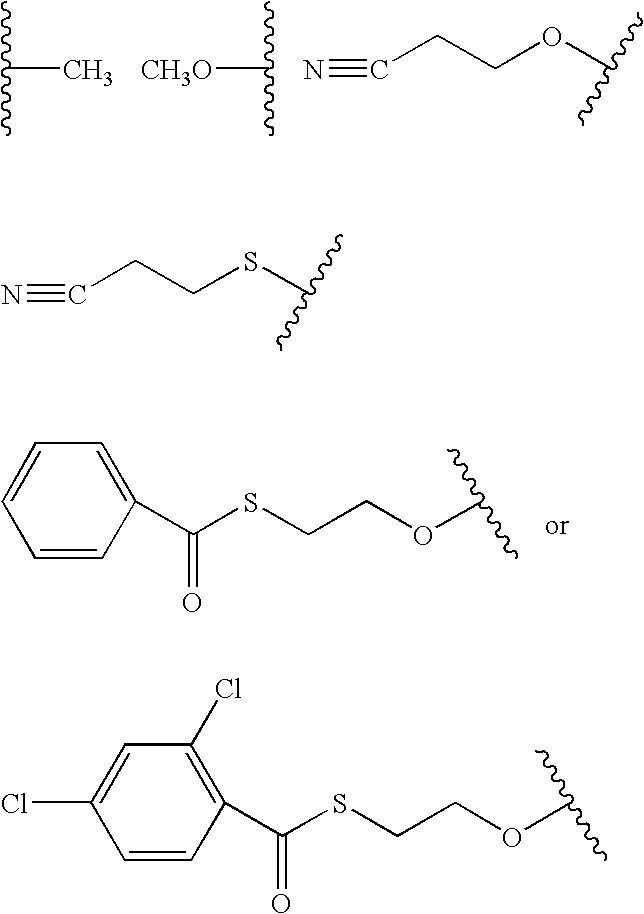
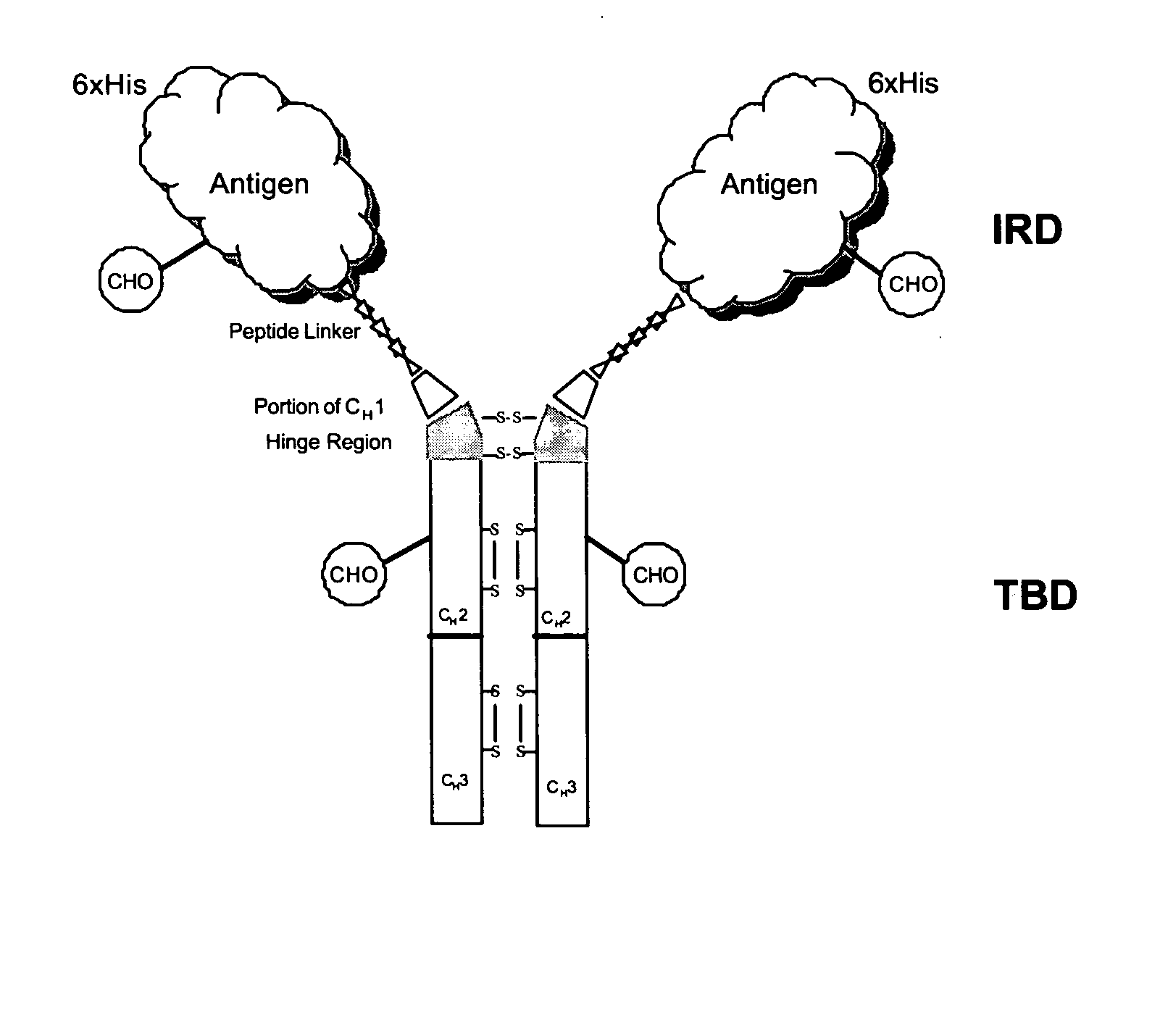
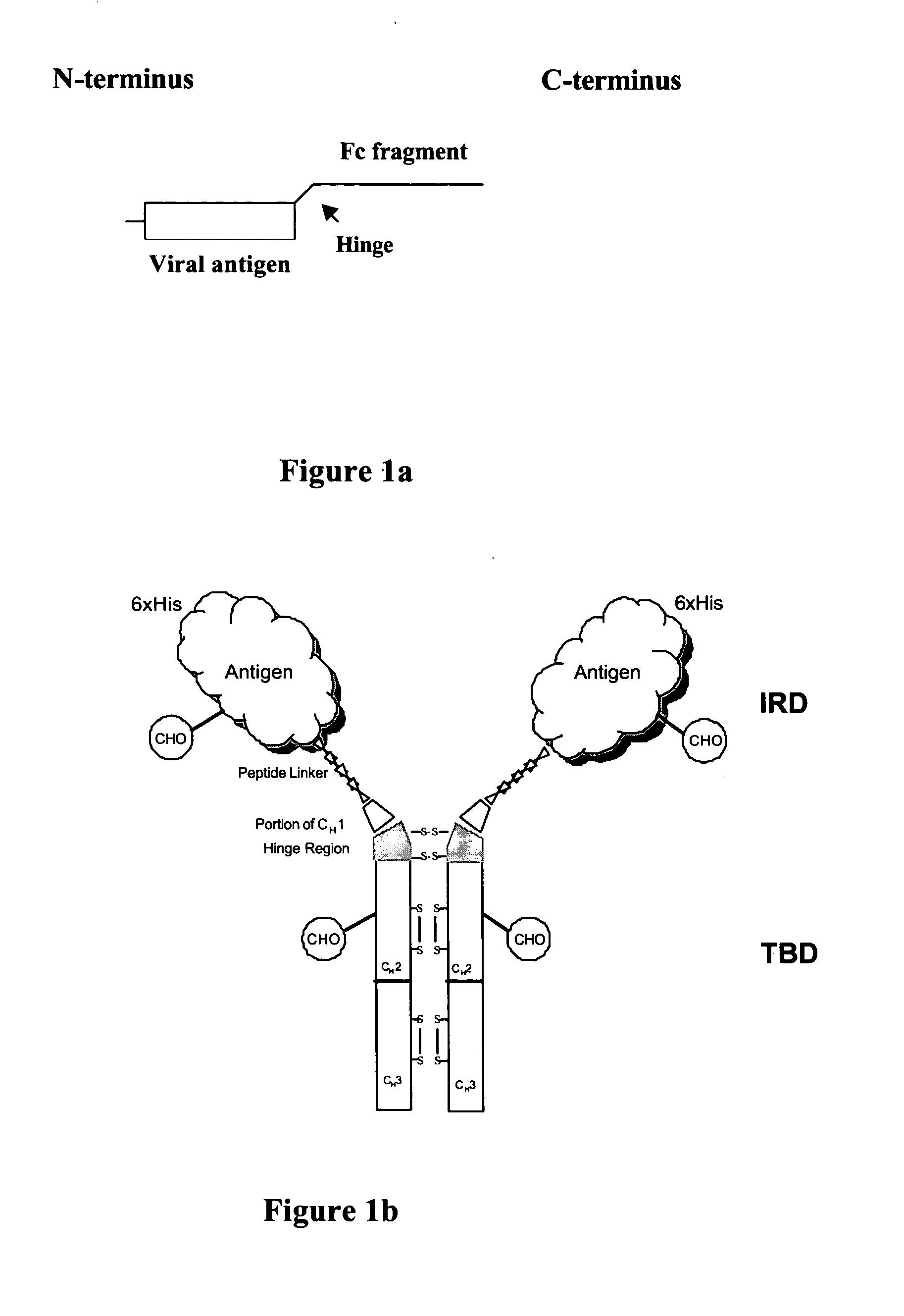
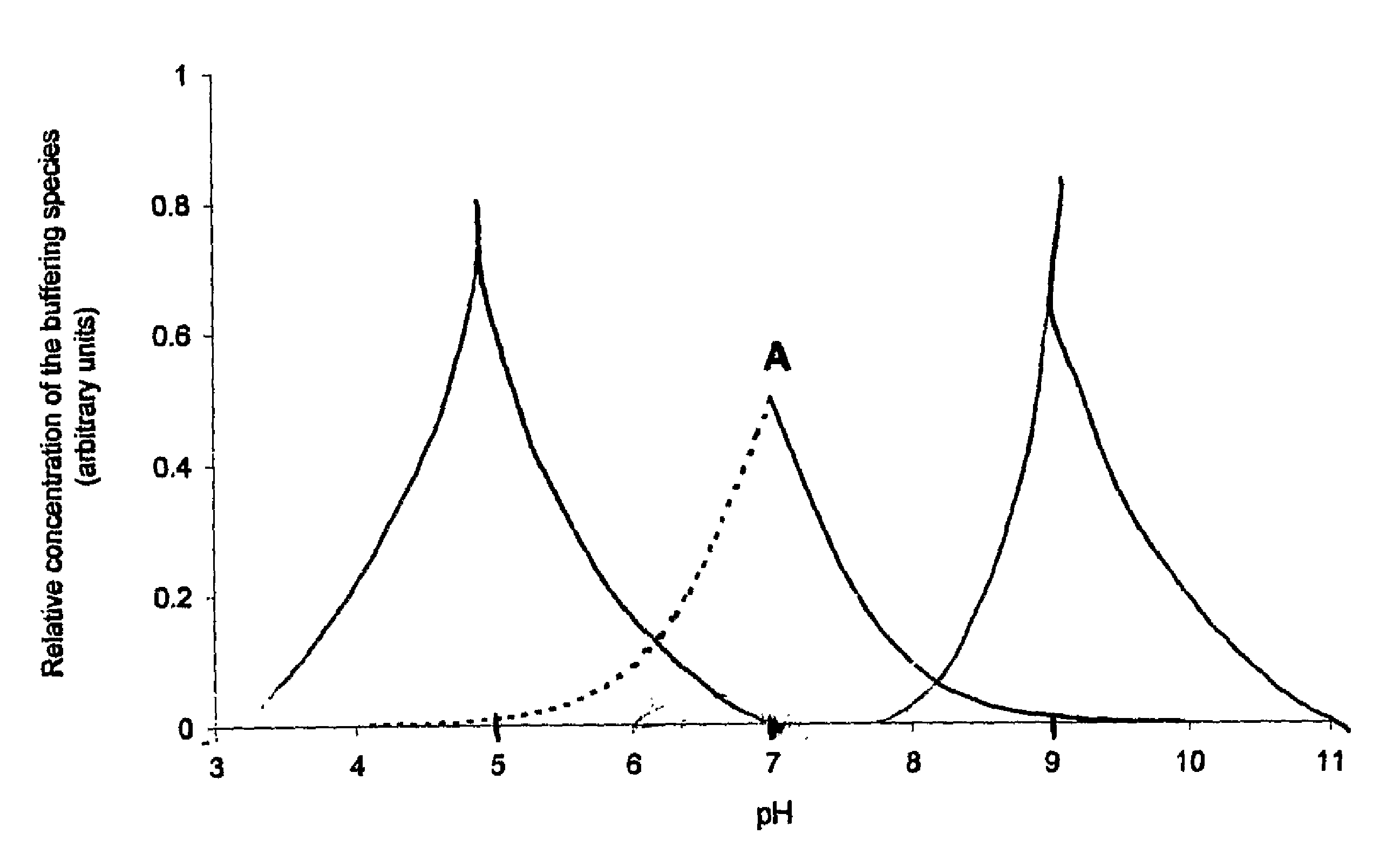
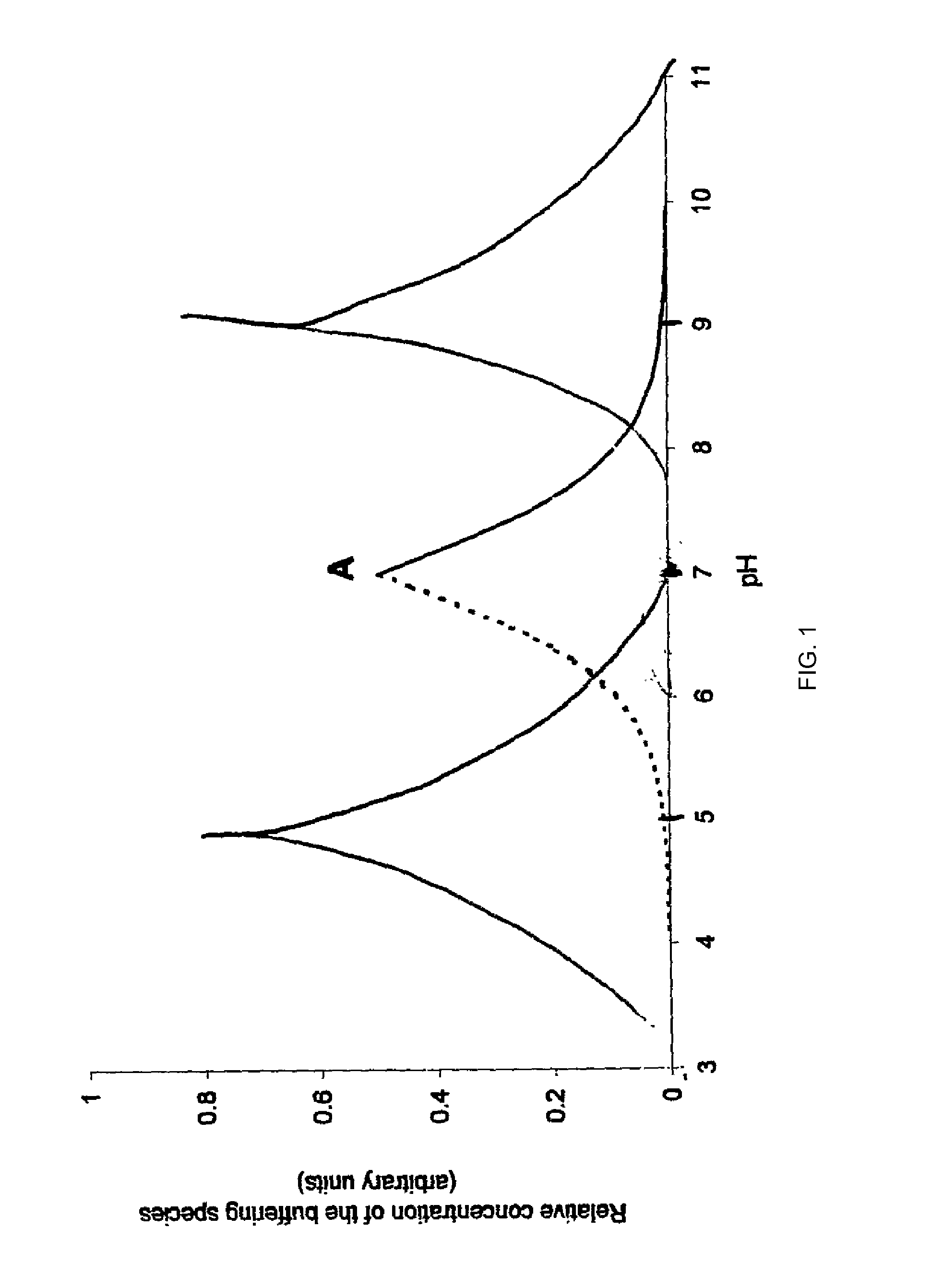
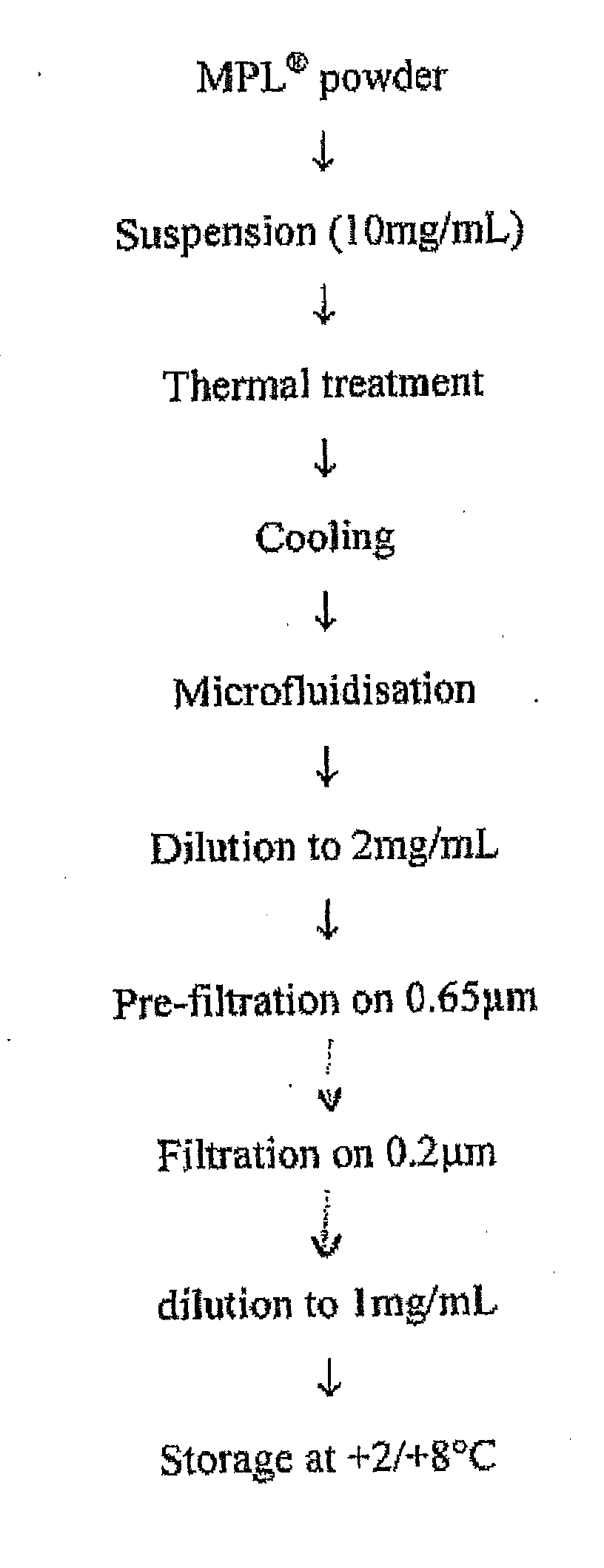
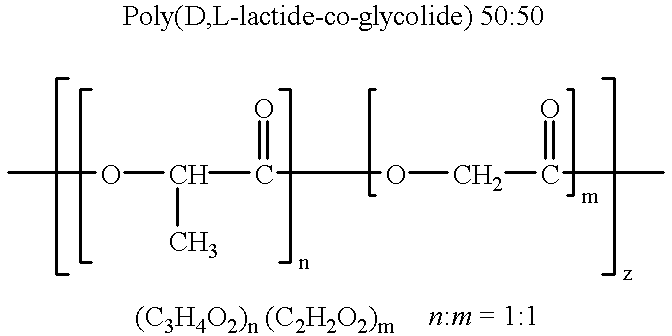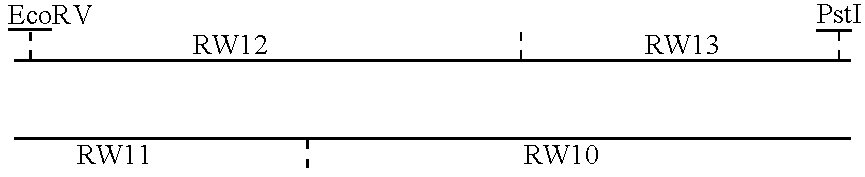Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
946results about "Reverse transcribing DNA viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
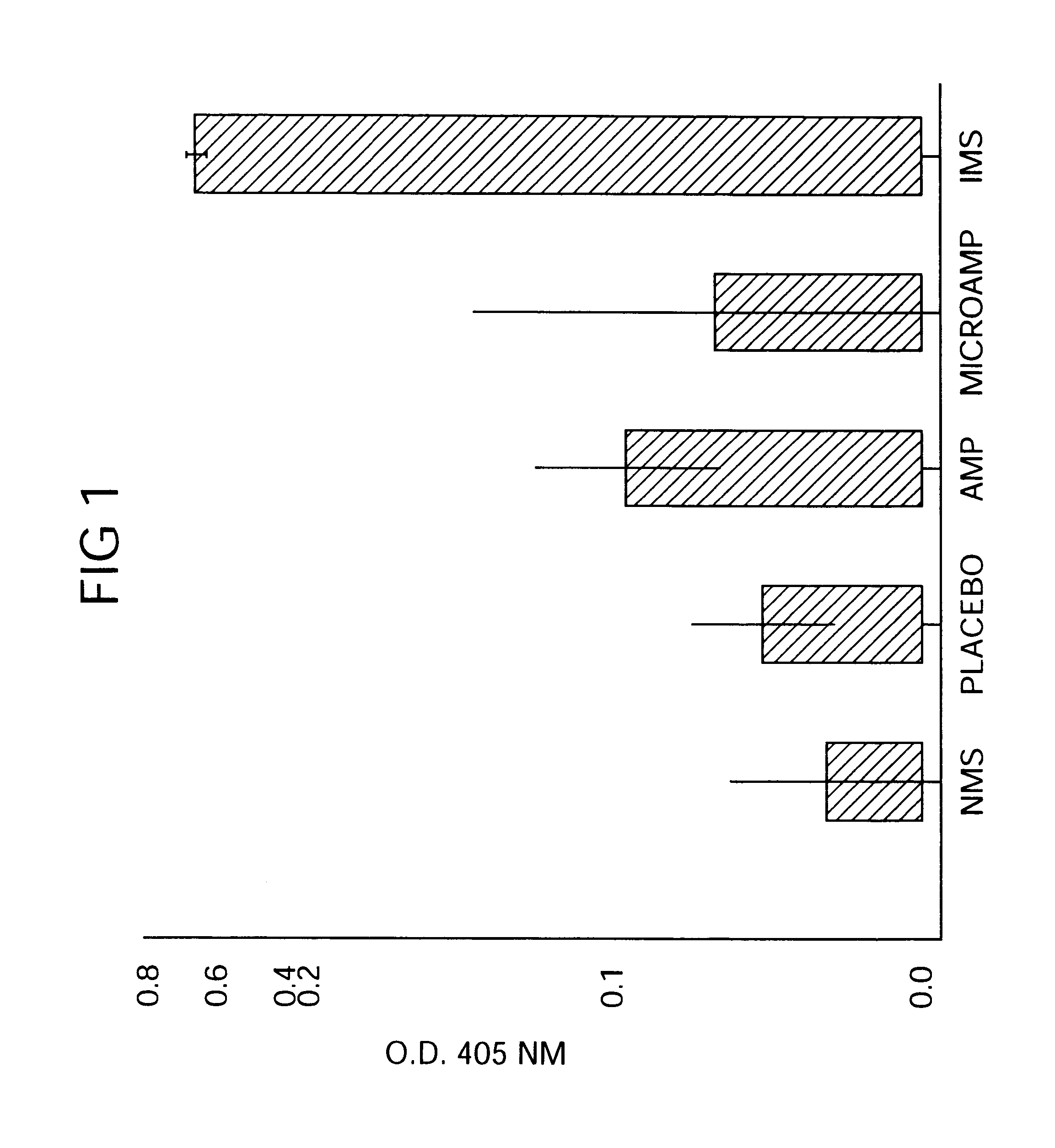
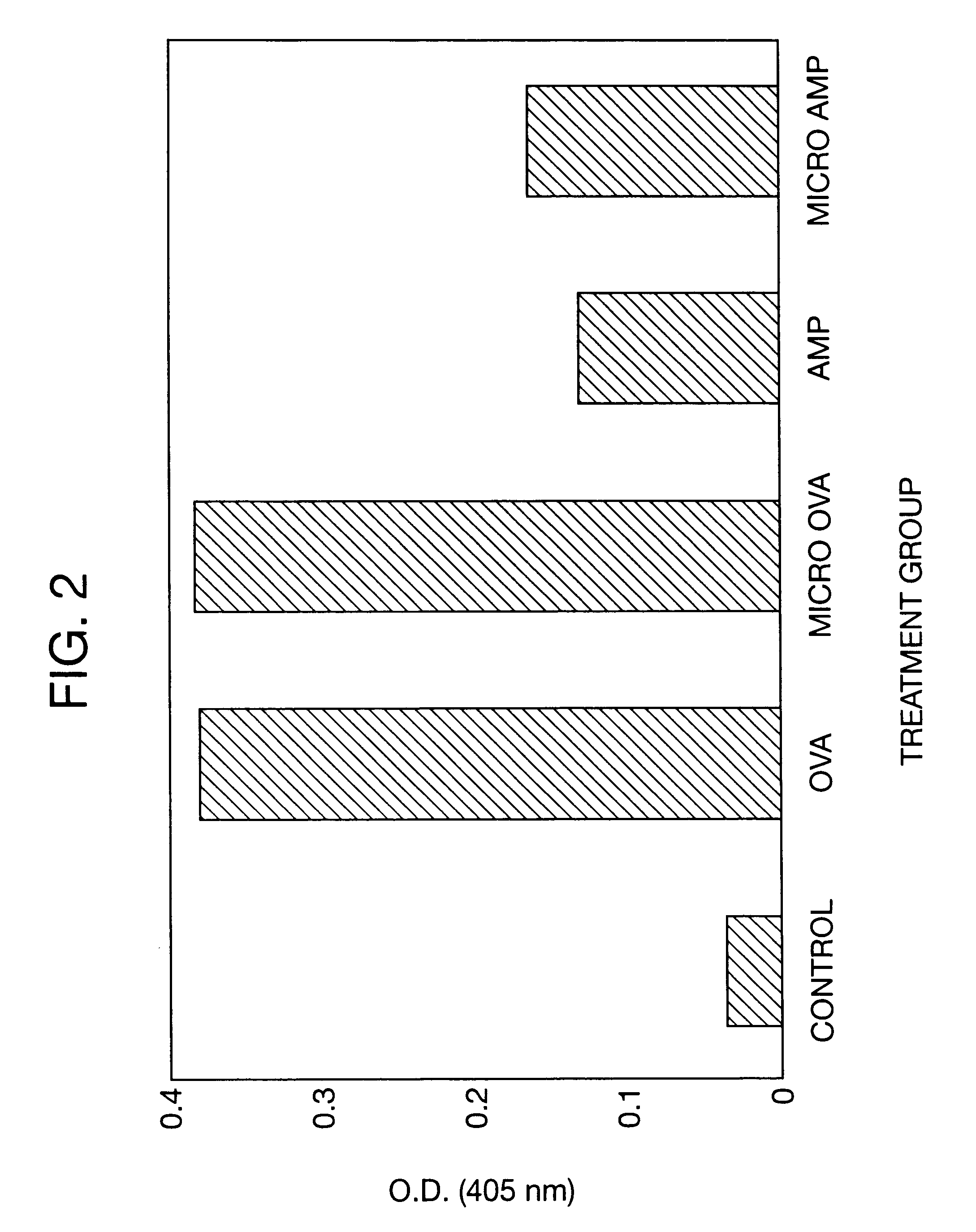
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Self-assembling nanoparticle drug delivery system
InactiveUS20090226525A1High binding affinityHigh affinityPowder deliveryMicroencapsulation basedLipid formationMedicine
A self-assembling nanoparticle drug delivery system for the delivery of various bioactive agents including peptides, proteins, nucleic acids or synthetic chemical drugs is provided. The self-assembling nanoparticle drug delivery system described herein includes viral capsid proteins, such as Hepatitis B Virus core protein, encapsulating the bioactive agent, a lipid layer or lipid / cholesterol layer coat and targeting or facilitating molecules anchored in the lipid layer. A method for construction of the self-assembling nanoparticle drug delivery system is also provided.
Owner:CHIMEROS
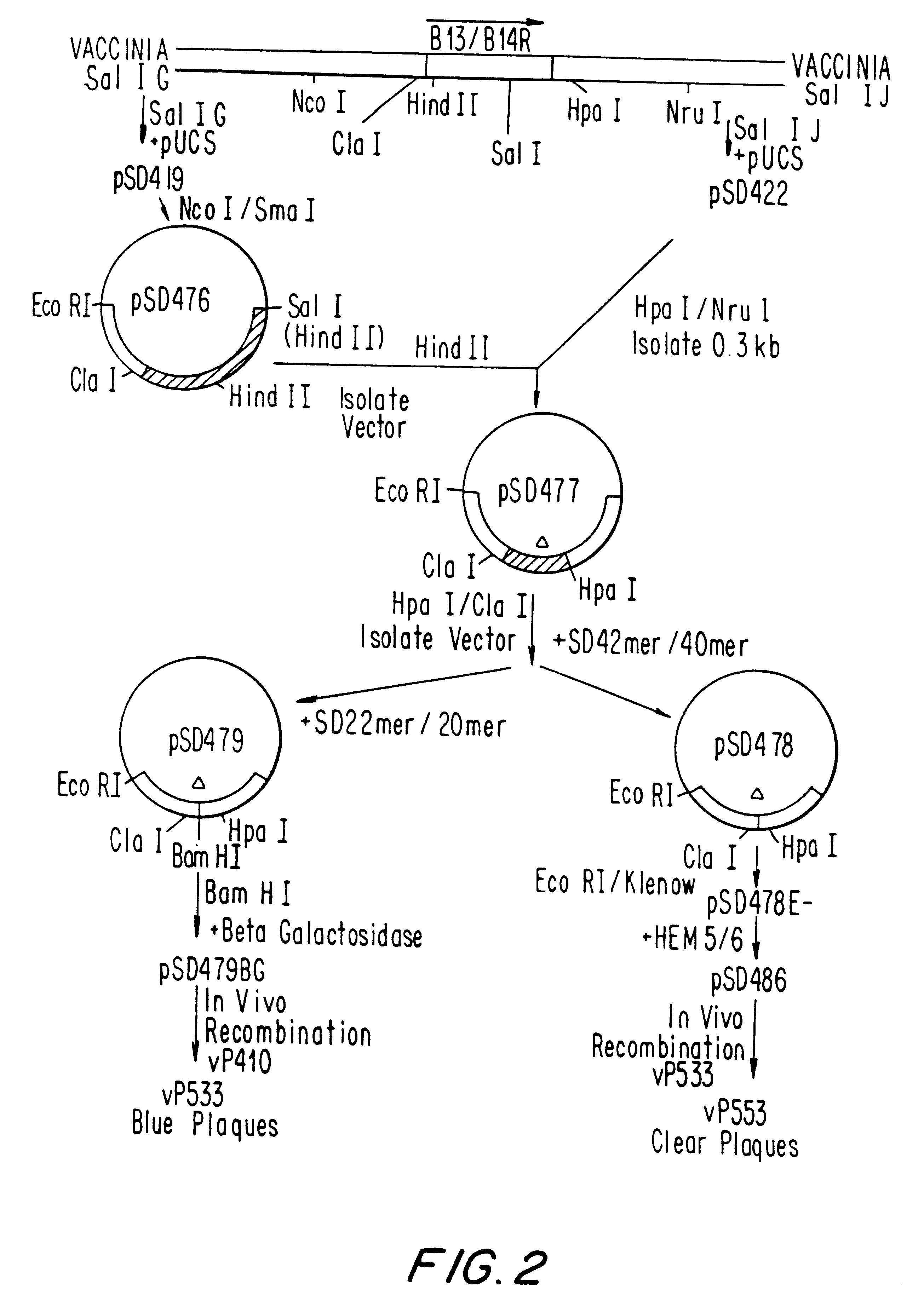
Recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS6998252B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses also are provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
Simian immunodeficiency virus peptides with antifusogenic and antiviral activities
InactiveUS6017536ASsRNA viruses negative-sensePeptide/protein ingredientsSimian immunodeficiency viruses SIVDefective virus
The present invention relates to peptides which exhibit antifusogenic and antiviral activities. The peptides of the invention consist of a 16 to 39 amino acid region of a simian immunodeficiency virus (SIV) protein. These regions were identified through computer algorithms capable of recognizing the ALLMOTI5, 107x178x4, or PLZIP amino acid motifs. These motifs are associated with the antifusogenic and antiviral activities of the claimed peptides.
Owner:TRIMERIS +1
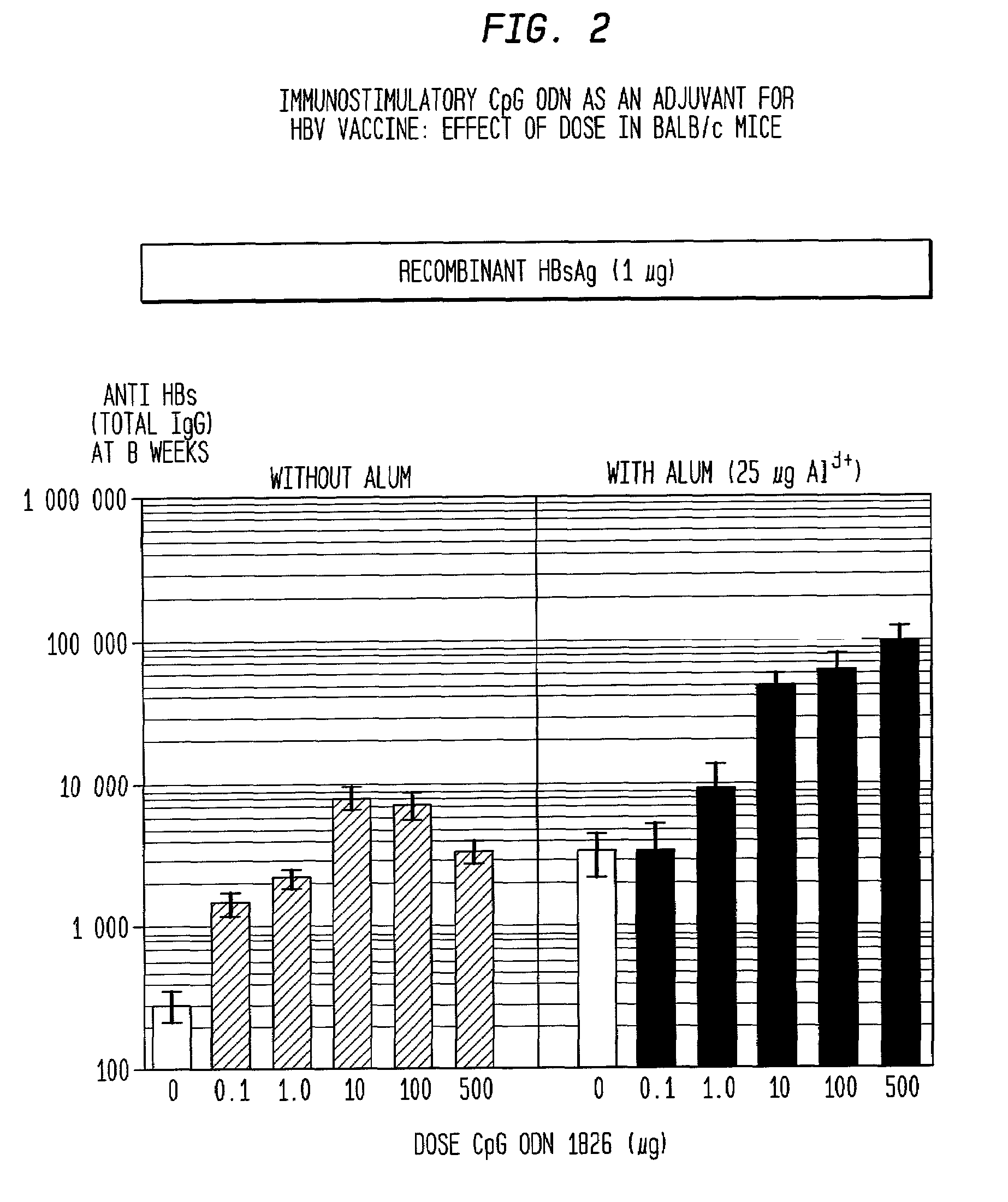
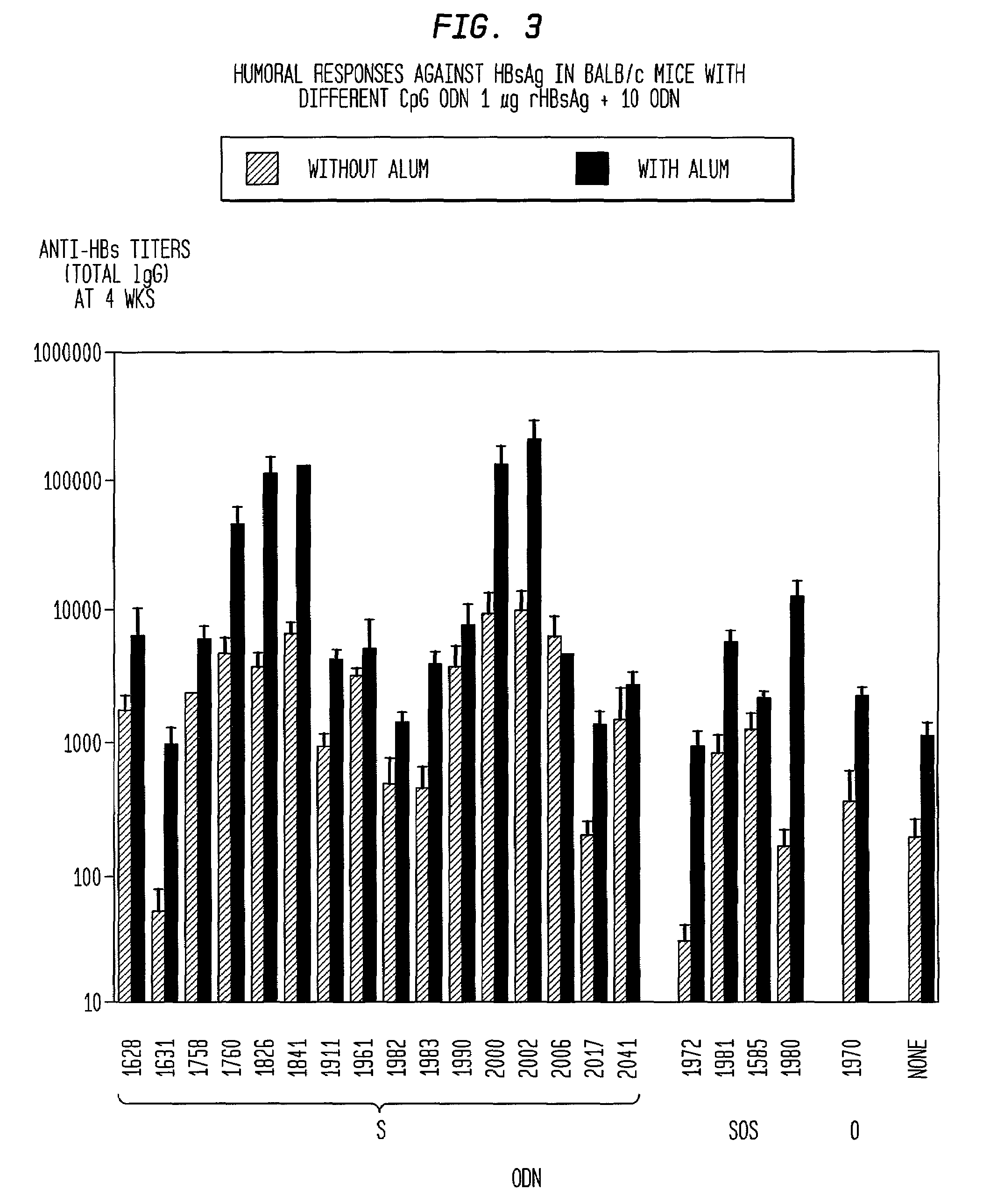
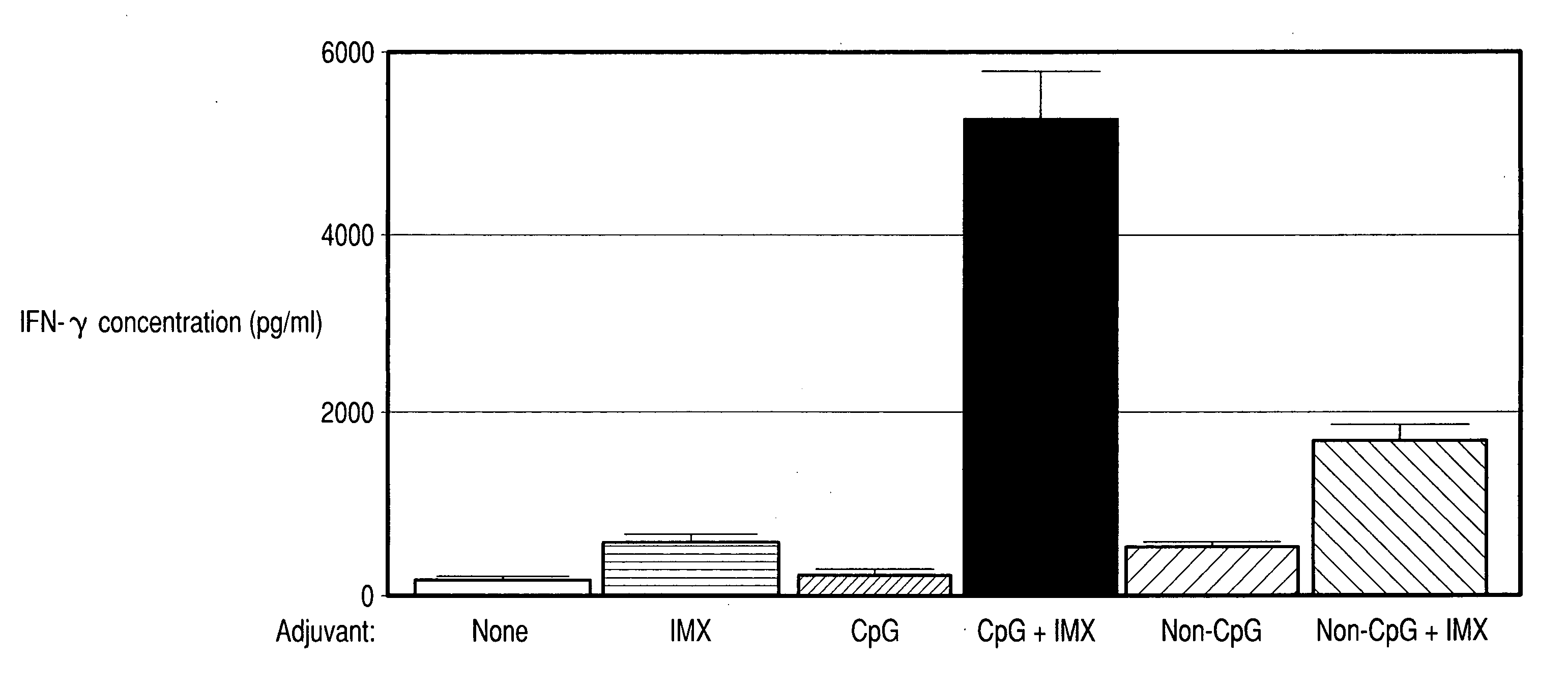
Method of inducing an antigen-specific immune response by administering a synergistic combination of adjuvants comprising unmethylated CpG-containing nucleic acids and a non-nucleic acid adjuvant
The present invention relates generally to adjuvants, and in particular to methods and products utilizing a synergistic combination of immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) and a non-nucleic acid adjuvant. Such combinations of adjuvants may be used with an antigen or alone. The present invention also relates to methods and products utilizing immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) for induction of cellular immunity in infants.
Owner:UNIV OF IOWA RES FOUND +2
Ultrasound assisted transdermal vaccine delivery method and system
InactiveUS20050112135A1Adequate buffering capacityHigh viscosityViral antigen ingredientsSurgeryVaccine deliveryBiocompatible coating
An apparatus and method for transdermally delivering a vaccine comprising a delivery system having (i) a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers and (ii) an ultrasonic device. In one embodiment, the vaccine is contained in a biocompatible coating that is applied to the microprojection member. In a further embodiment, the delivery system includes a gel pack having a vaccine-containing hydrogel formulation that is disposed on the microprojection member after application to the skin of a patient. In an alternative embodiment, the vaccine is contained in both the coating and the hydrogel formulation.
Owner:ALZA CORP
Hapten-carrier conjugates and uses thereof
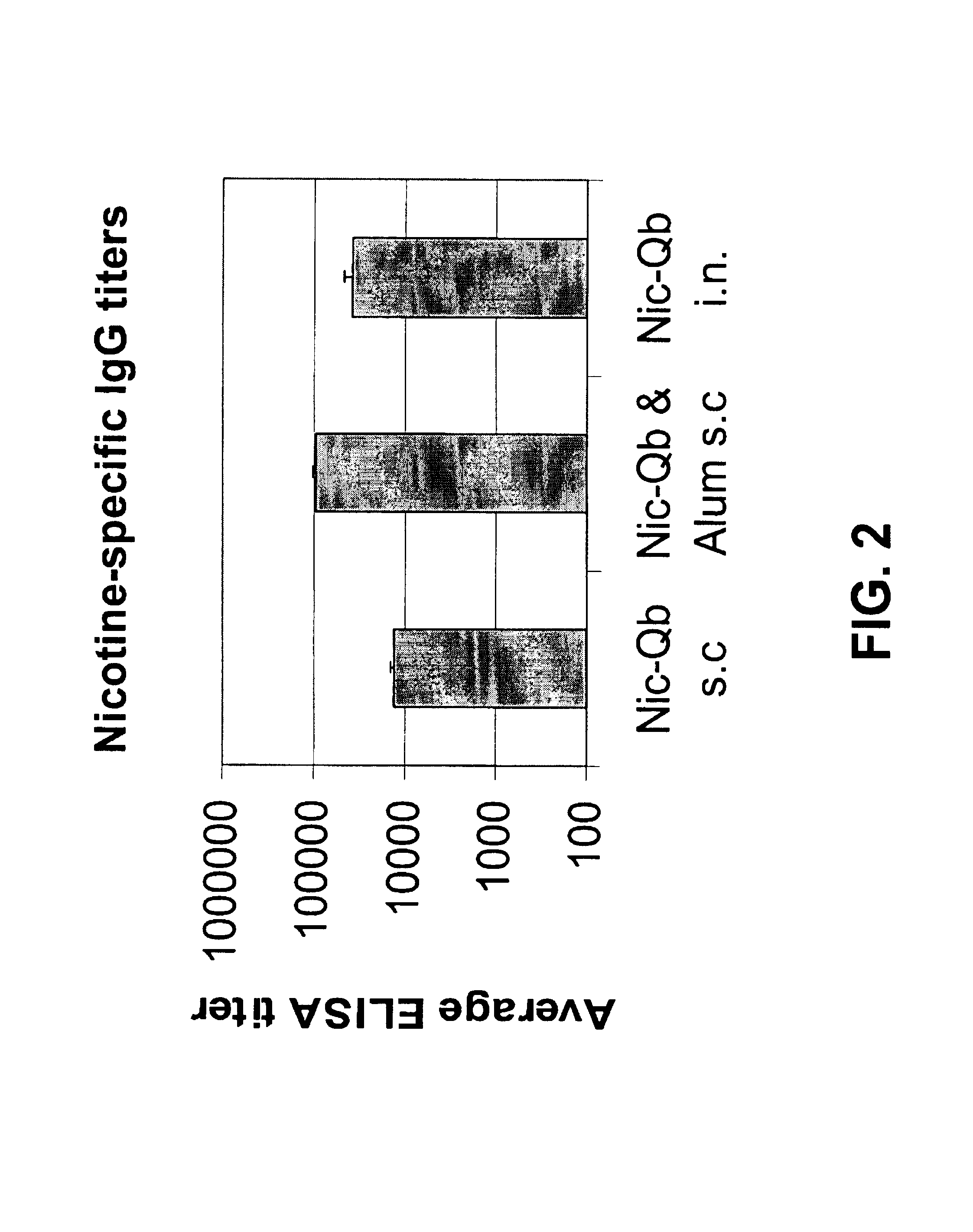
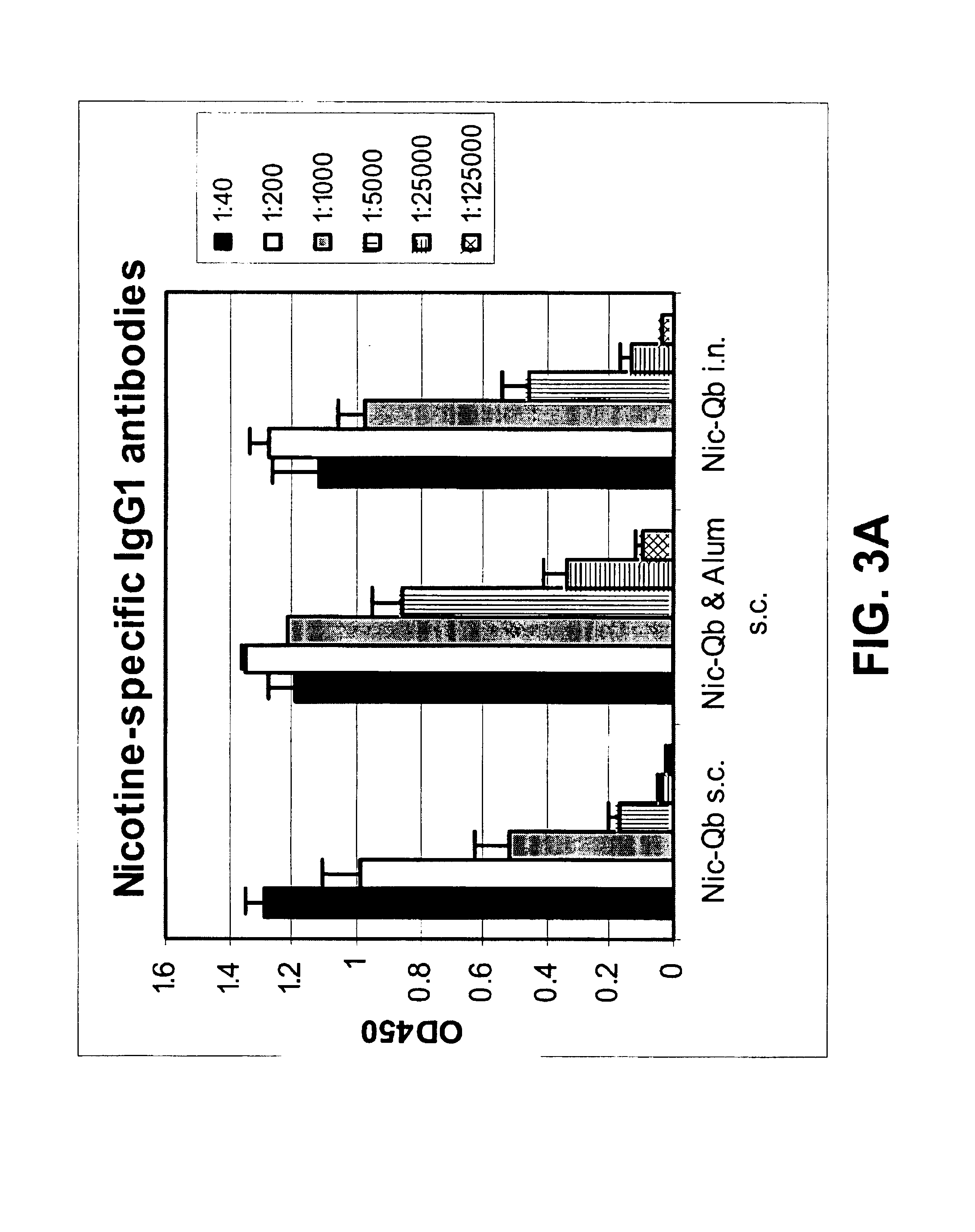
The present invention provides compositions comprising a conjugate of a hapten with a carrier in an ordered and repetitive array, and methods of making such compositions. The conjugates and compositions of the invention may comprise a variety of haptens, including hormones, toxins and drugs, especially drugs of addiction such as nicotine. Compositions and conjugates of the invention are useful for inducing immune responses against haptens, which can use useful in a variety of therapeutic, prophylactic and diagnostic regimens. In certain embodiments, immune responses generated using the conjugates, compositions and methods of the present invention are useful to prevent or treat addiction to drugs of abuse and the resultant diseases associated with drug addiction.
Owner:CYTOS BIOTECHNOLOGY AG
Immunodeficiency recombinant poxvirus
InactiveUS6596279B1SsRNA viruses negative-senseSsRNA viruses positive-senseFeline immunodeficiency virusCtl epitope
Attenuated recombinant viruses containing DNA encoding an immunodeficiency virus and / or CTL antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: HIV1gag(+pro)(IIIB), gp120(MN)(+transmembrane), nef(BRU)CTL, pol(IIIB)CTL, ELDKWA or LDKW epitopes, preferably HIV1gag(+pro)(IIIB), gp120(MN) (+transmembrane), two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL etpitopes; or two ELDKWA in gp120 V3 or another region or in gp160. The two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL epitopes are preferably CTL1, CTL2, pol1, pol2 and pol3. The recombinant viruses and gene products therefrom and antibodies generated by the viruses and gene products have several preventive, therapeutic and diagnostic uses. DNA from the recombinant viruses are useful as probes or, for generating PCR primers or for immunization. Also disclosed and claimed are HIV immunogens and modified gp160 and gp120.
Owner:VIROGENETICS
Poxvirus-canine distemper virus (CDV) or measles virus recombinants and compositions and methods employing the recombinants
InactiveUS6309647B1Improve securityImprove security levelSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininCanine distemper virus Antigen
Attenuated recombinant viruses containing DNA coding for a canine distemper virus antigen or measles M or N antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: canine distemper virus fusion protein, canine distemper virus hemagglutinin glycoprotein, canine distemper nucleocaspid protein, canine distemper matrix protein, measles virus nucleocaspid protein, and measles virus matrix protein. The recombinant viruses and gene products therefrom are useful for eliciting protection against canine distemper virus and / or measles virus, and, the gene products and antibodies elicited thereby are useful in assays. Additionally, DNA from the recombinants is used for probes or for generating PCR primers.
Owner:AVENTIS PASTEUR LTD
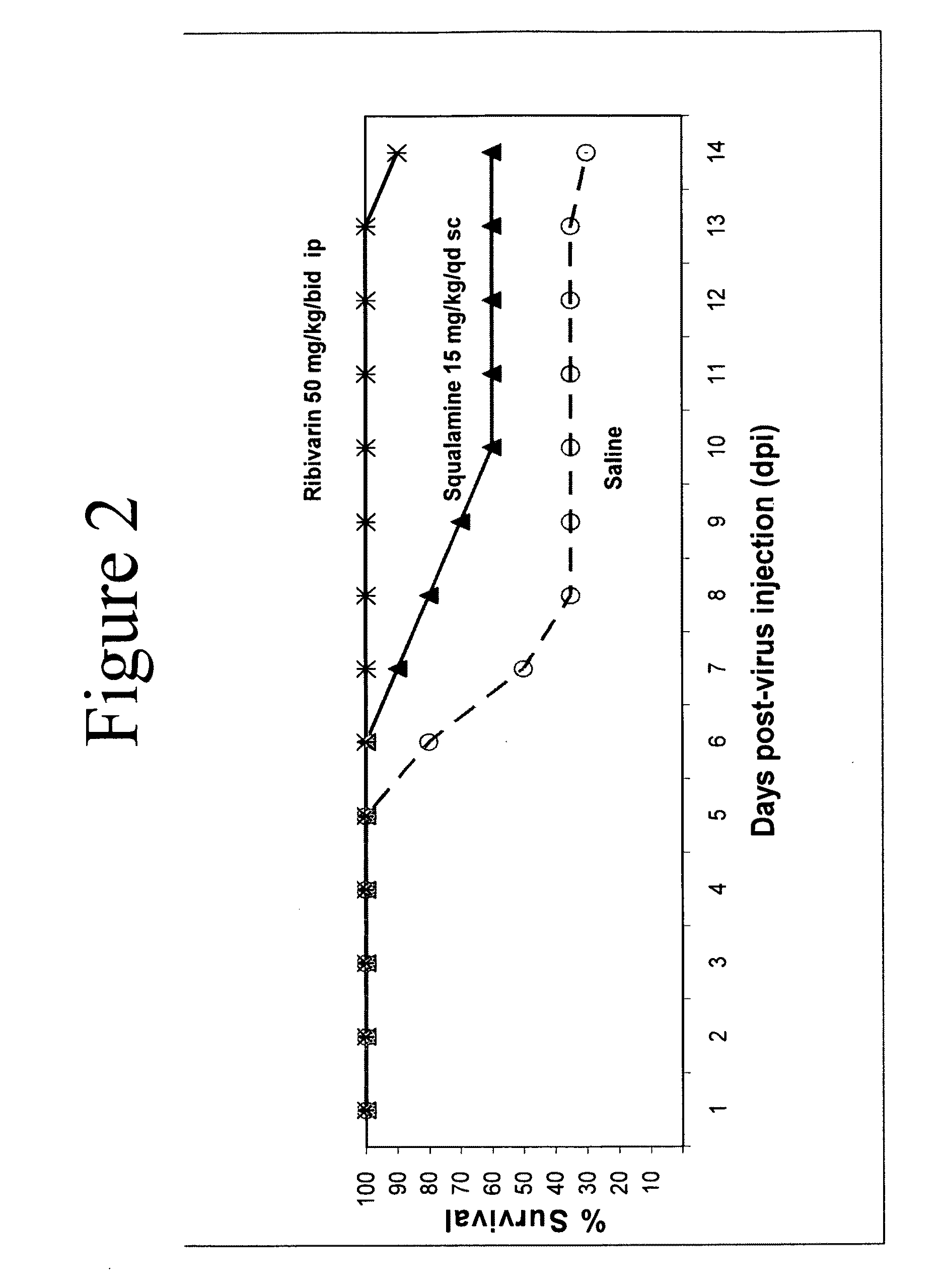
Formulations comprising aminosterols
ActiveUS20110123624A1Reduce local membrane damaging propertyReducing free concentrationSsRNA viruses negative-senseOrganic active ingredientsPhosphateEndocrinology
This invention relates to stable aminosterol phosphate compositions. The aminosterol phosphate compositions permit administration without associated tissue damage and achieve a sustained release effect.
Owner:ENTERIN INC
Influenza immunogen and vaccine
InactiveUS20060115489A1High antibody titerEasy to prepareSsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis B immunizationHepatitis B virus
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (HBc) protein is disclosed that contains an immunogen for inducing the production of antibodies to the influenza M2 protein. An immunogenic influenza sequence in two to four copies is preferably expressed at or near the N-terminus or in the HBc immunogenic loop sequence. The HBc chimer preferably contains an influenza-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:SANOFI PASTEUR BIOLOGICS CO +1
Chimeric immunomodulatory compounds and methods of using the same-I
Owner:DYNAVAX TECH CORP
Methods and compositions for inducing antigen-specific immune responses
InactiveUS20060287263A1Enhanced IFN-gamma productionIncrease productionAntibacterial agentsBiocideImmunostimulating ComplexesSpecific immunity
Vaccine compositions comprising (a) an oligonucleotide, (b) and immune stimulating complex and (c) an antigen induce a strong interferon-gamma immune response. Both oligonucleotides containing immune stimulatory motifs and oligonucleotides lacking immune stimulatory motifs contribute to an interferon-gamma response when administered with an immune stimulating complex.
Owner:COLEY PHARM GRP INC +1
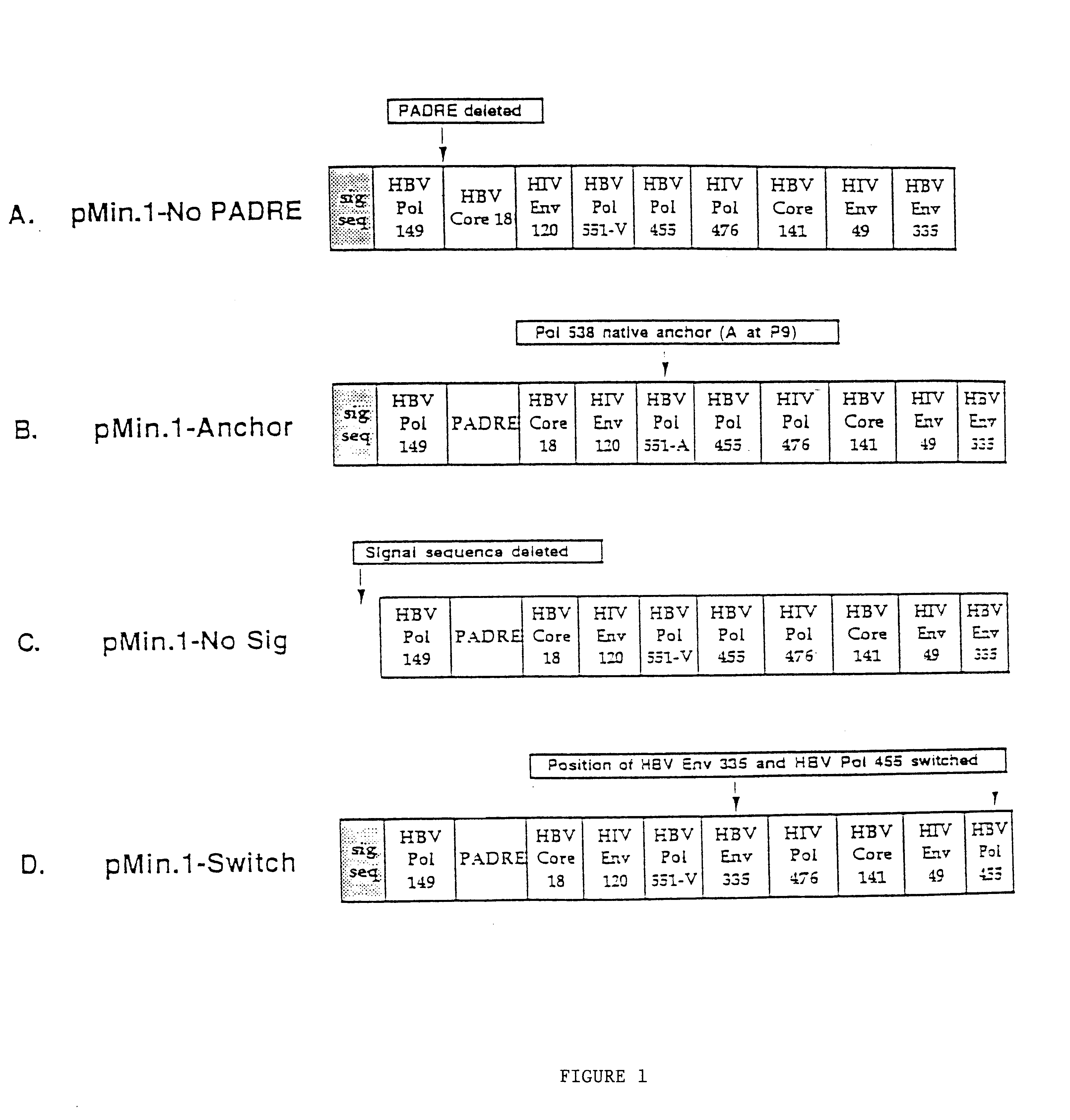
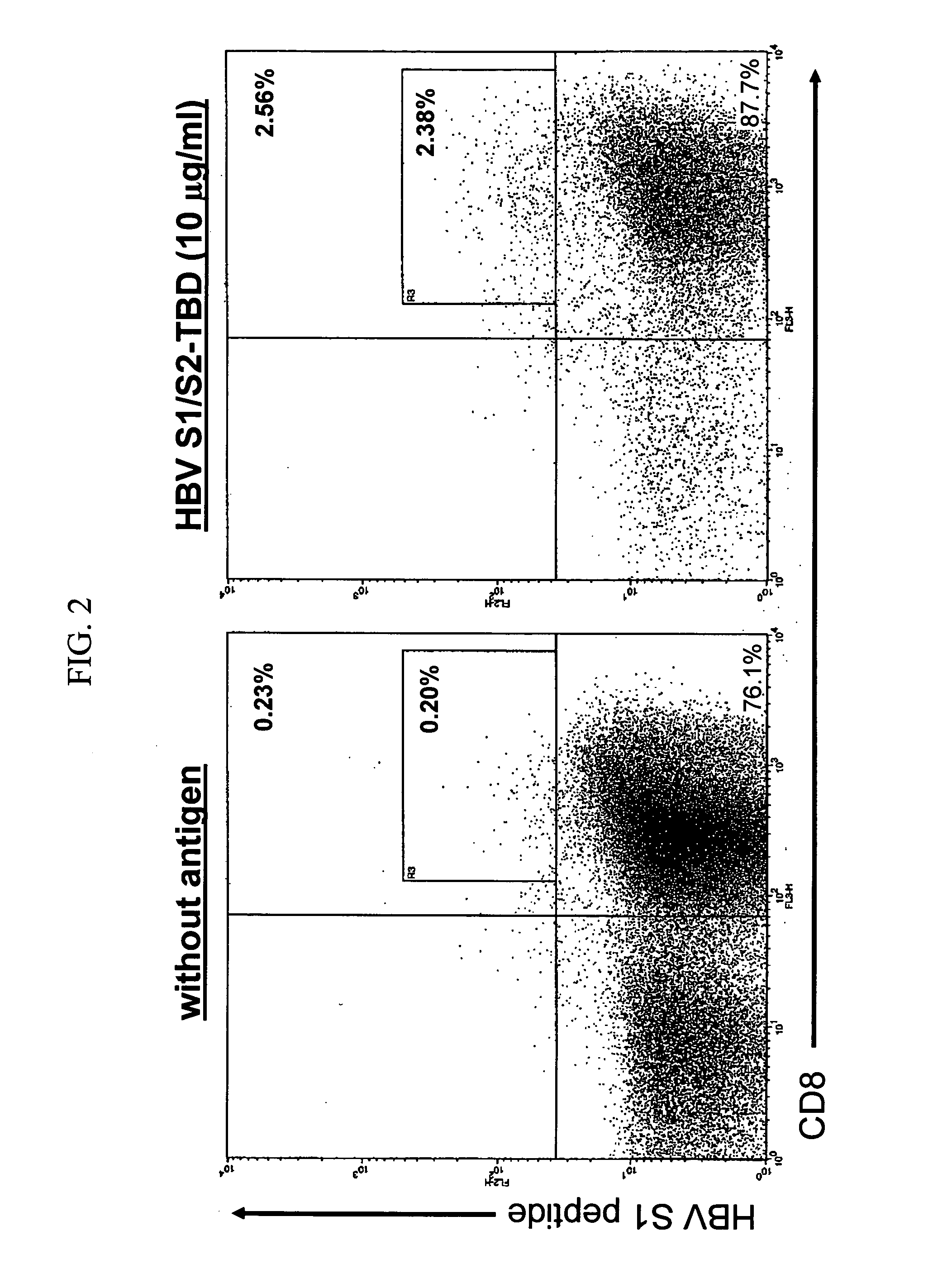
Inducing cellular immune responses to hepatitis B virus using peptide and nucleic acid compositions
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to develop epitope-based vaccines directed towards HBV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HBV infection.
Owner:PHARMEXA
Use of meganucleases for inducing homologous recombination ex vivo and in toto in vertebrate somatic tissues and application thereof
A single chain homing endonuclease, comprising a first variant of I-CreI having the amino acid sequence of accession number pdb 1g9y (SEQ ID NO: 23 is residues 1-153 of pdb Ig9y) and a second variant of I-CreI variant having the amino acid sequence of accession number pdb 1g9y (SEQ ID NO: 23 is residues 1-153 of pdb Ig9y) in a single polypeptide.
Owner:CELLECTIS SA
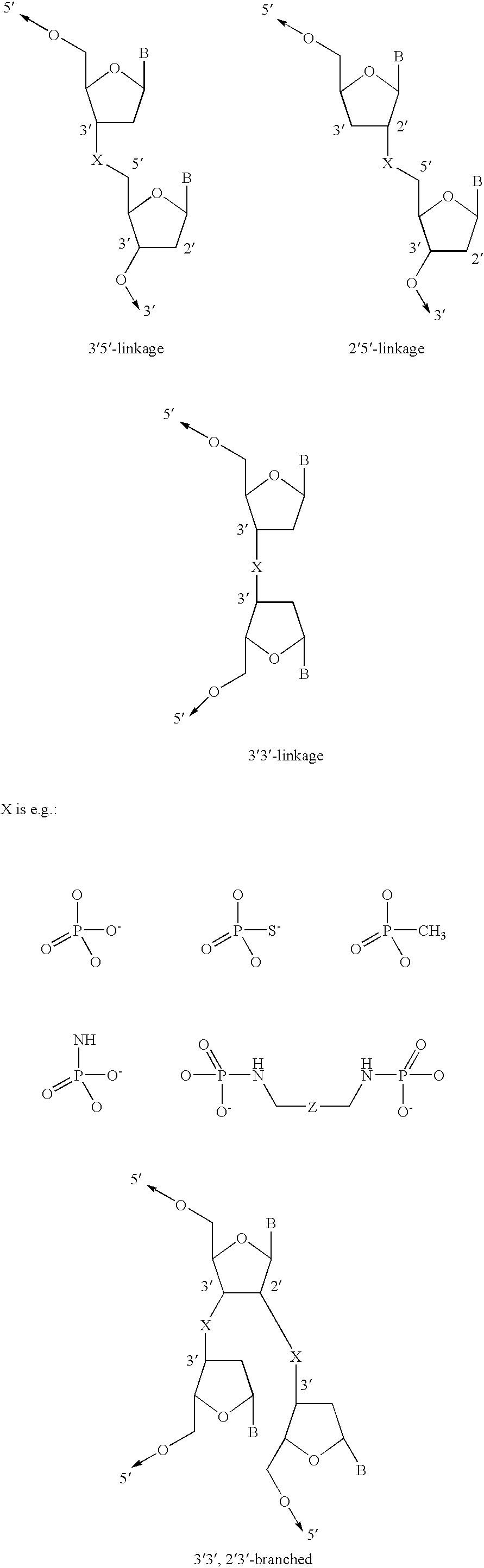
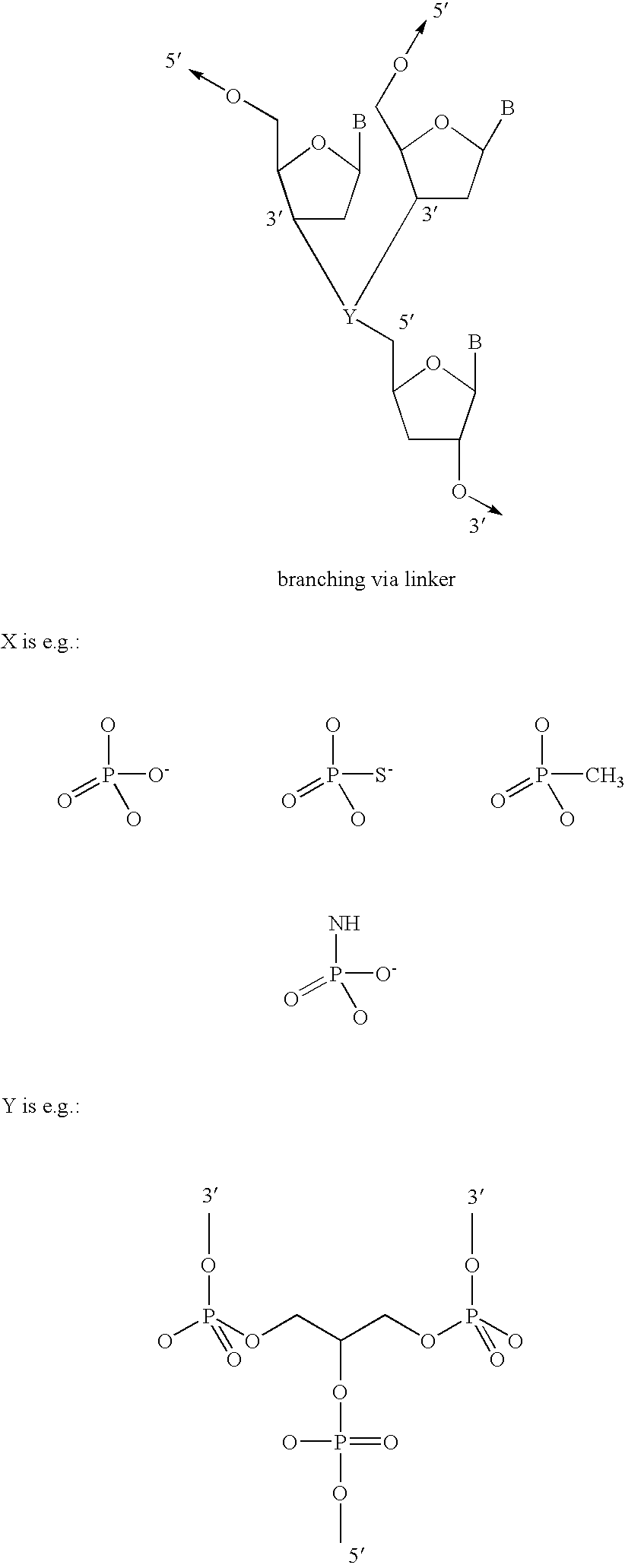
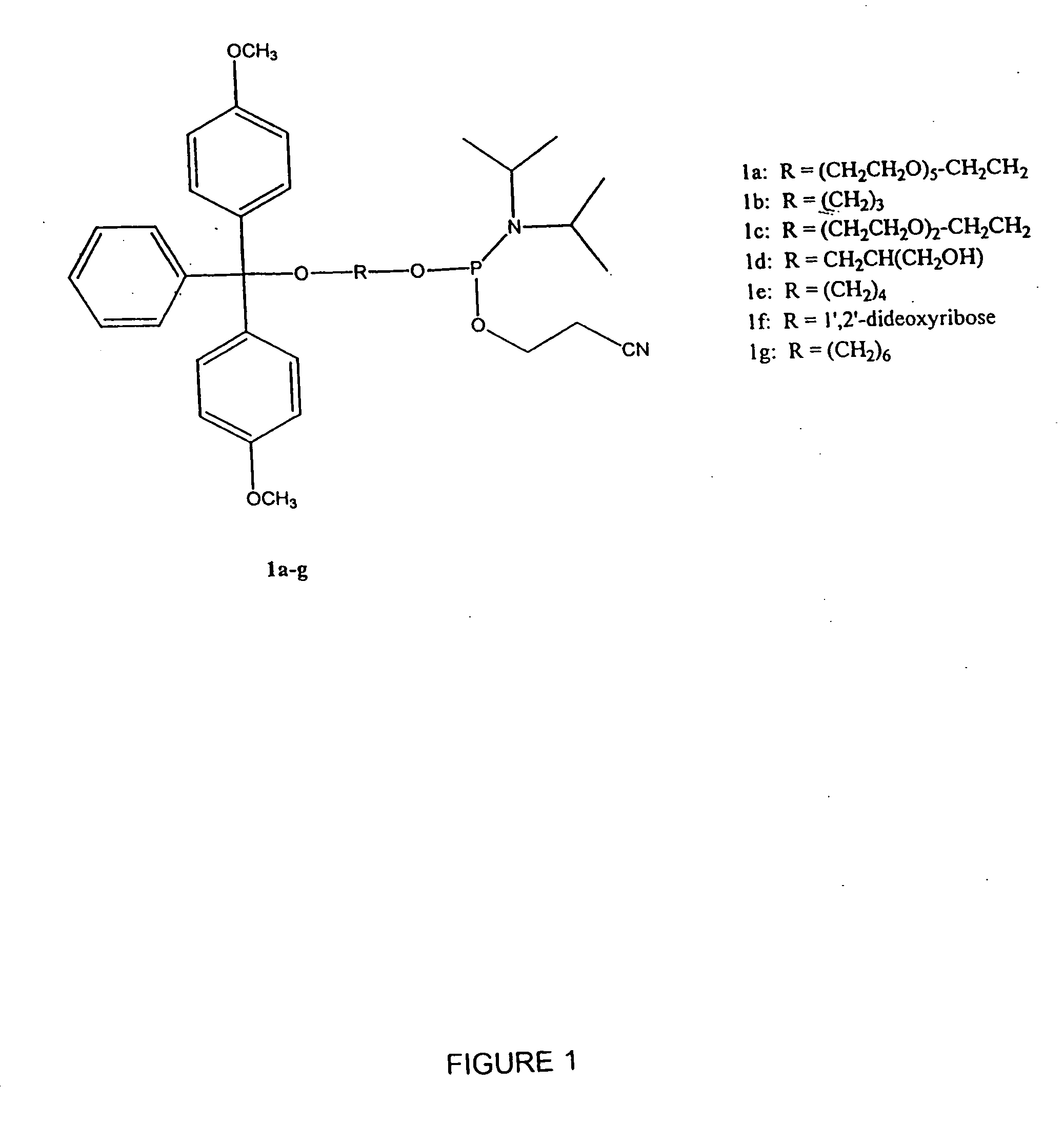
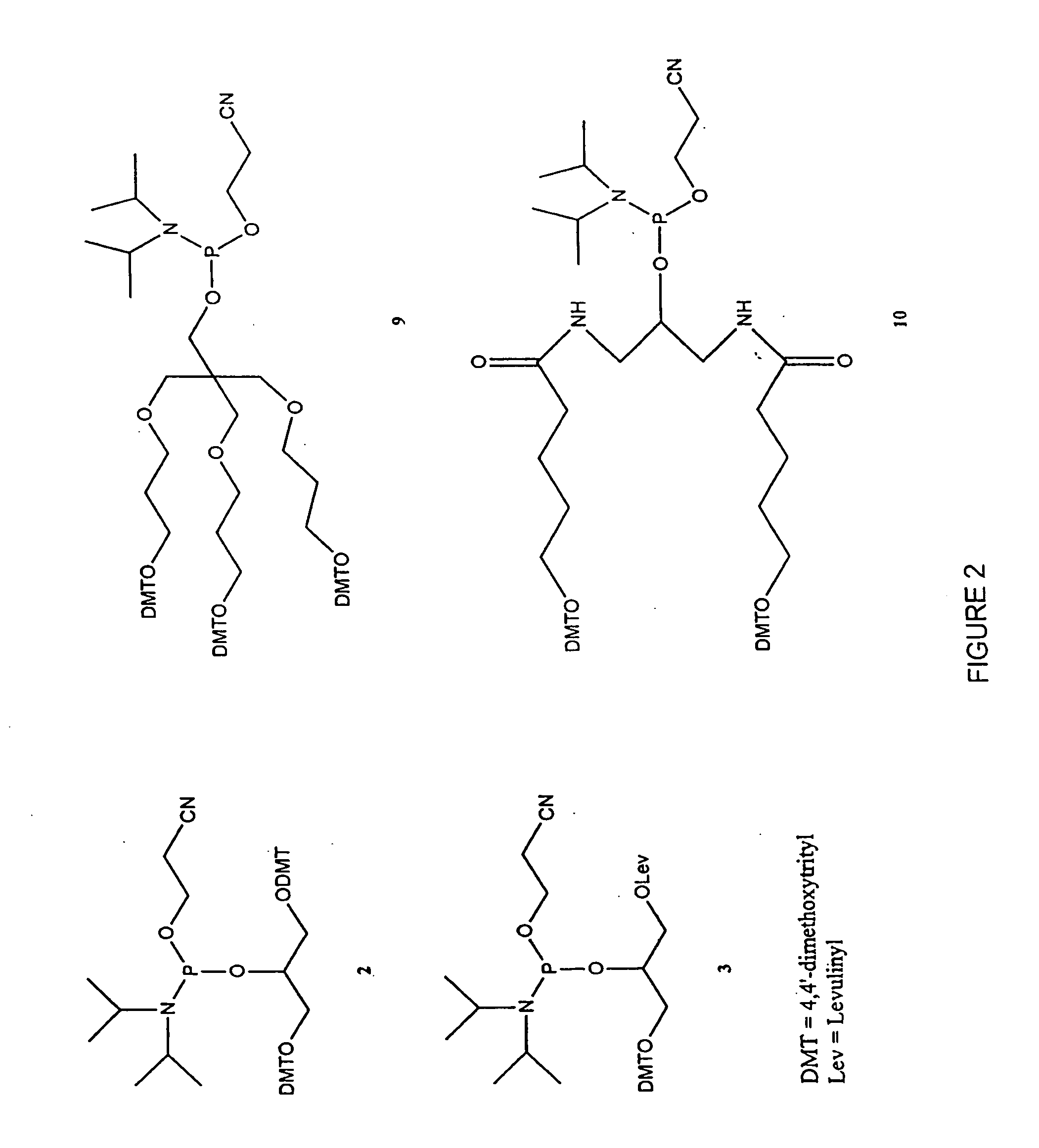
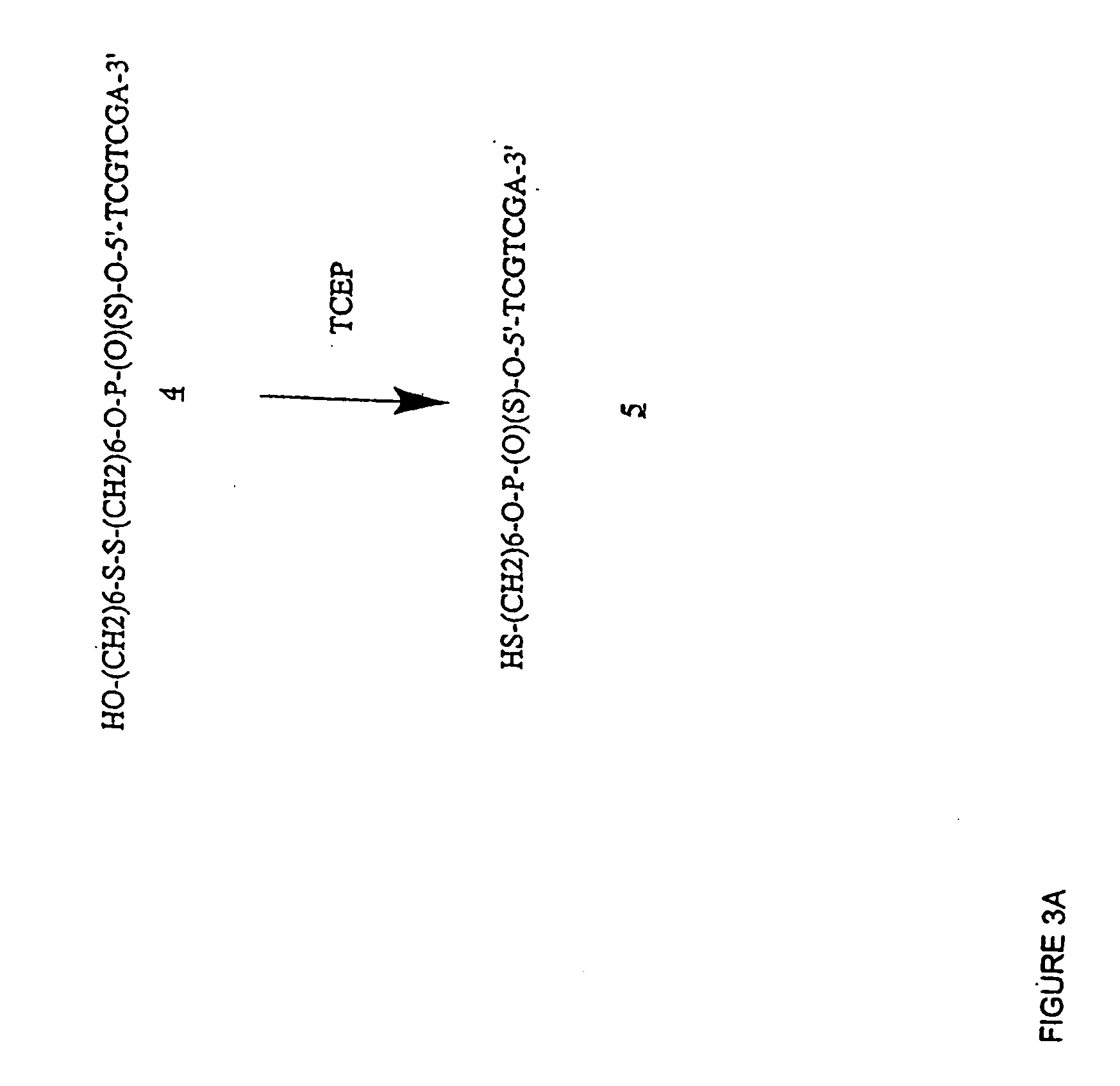
Fluoroalkoxy, nucleosides, nucleotides, and polynucleotides
InactiveUS20050266422A1Improves various propertyImprove the immunitySugar derivativesActivity regulationDiseaseDecoy
The present invention related to fluoroalkoxy (“—OCF3”) nucleosides, nucleotides, and polynucleotides comprising fluoroalkoxy nucleotides. The present invention also relates to methods of synthesizing fluoroalkoxy nucleosides, nucleotides, and polynucleotides comprising fluoroalkoxy nucleotides. The present invention also relates to compounds, compositions, and methods for the study, diagnosis, and treatment of traits, diseases and conditions that respond to the modulation of gene expression and / or activity. The invention also relates to fluoroalkoxy modified nucleic acid molecules, such as ribozymes, antisense, aptamers, decoys, triplex forming oligonucleotides (TFO), immune stimulatory oligonucleotides (ISO), immune modulatory oligonucleotides (IMO), and small nucleic acid molecules, including short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules capable of mediating RNA interference (RNAi) against polynucloetide targets. Such small nucleic acid molecules are useful, for example, in providing compositions to treat, prevent, inhibit, or reduce diseases, traits, or conditions in a subject or organism.
Owner:SIRNA THERAPEUTICS INC
Viruses and virus-like particles for multiple antigen and target display
InactiveUS20060121468A1Improve effectivenessImprove responseSsRNA viruses positive-senseVectorsTissue targetingVaccine Immunogenicity
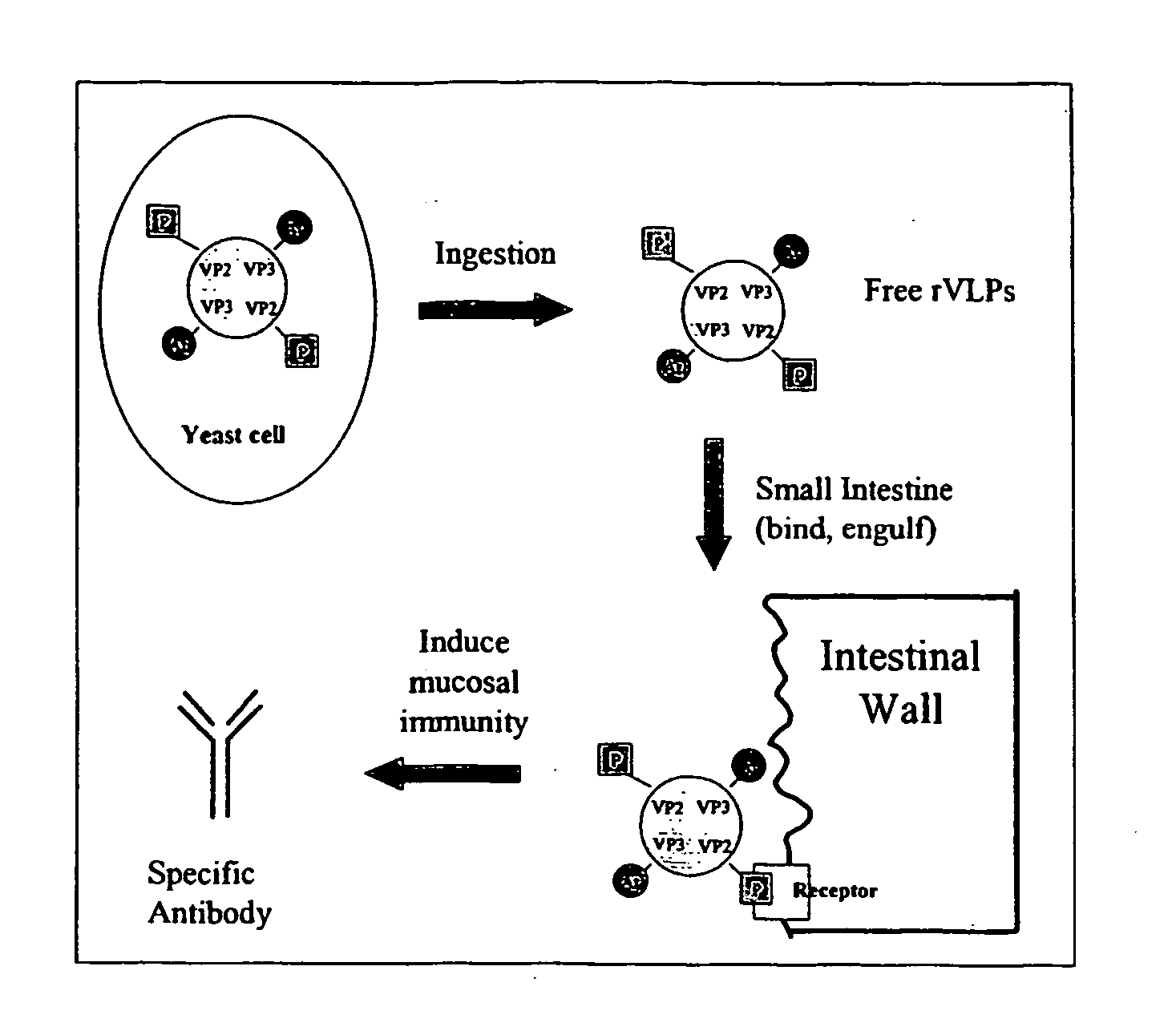
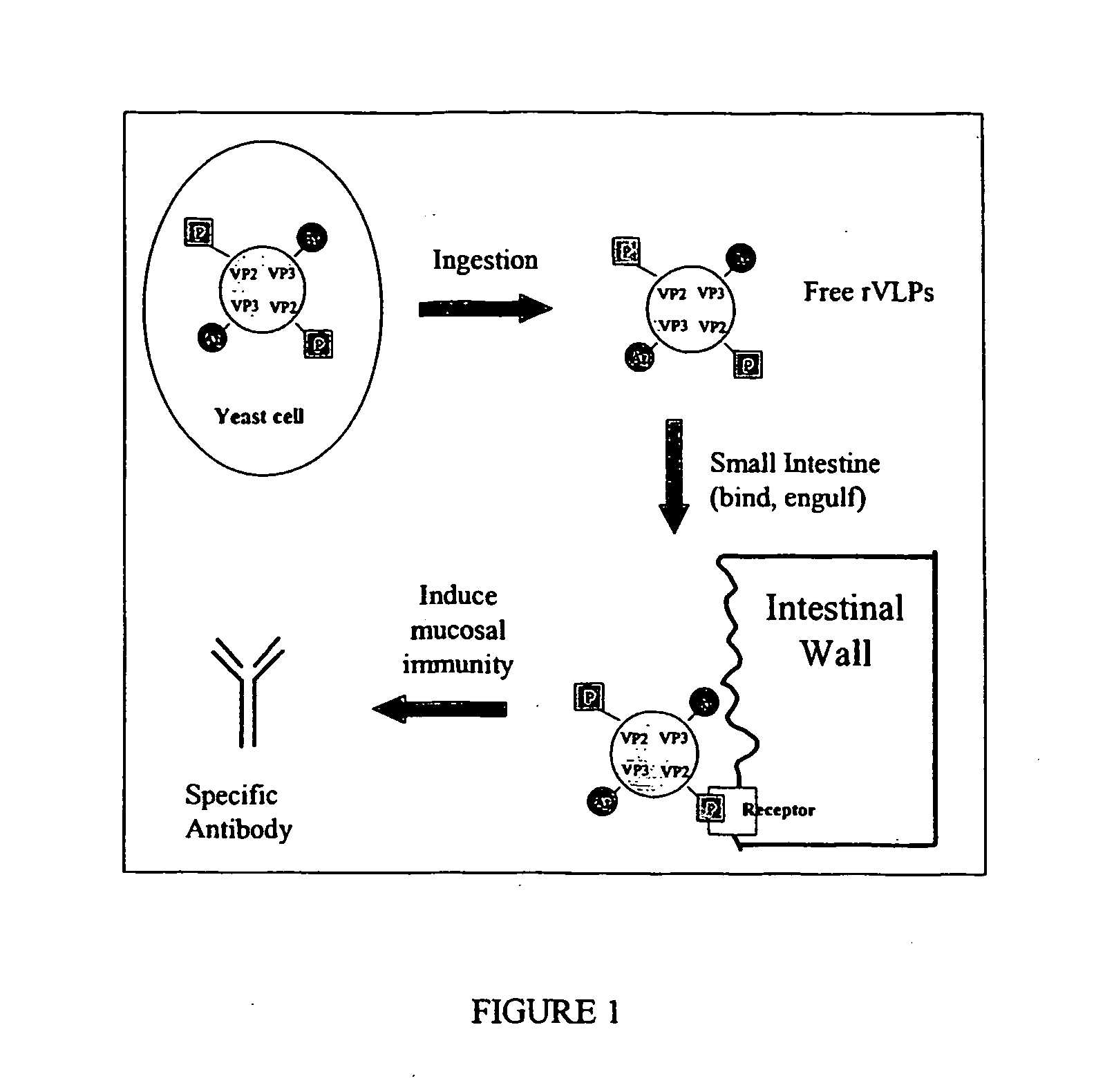
The present invention relates to the display of antigenic or allergenic components along with a tissue-targeting component on viruses or virus-like particles. Capsid protein genes are recombinantly modified to contain the specified components then expressed within a host organism, such as yeasts, bacteria, or algae, and allowed to spontaneously form active virus particles or virus-like particles. The recombinant complexes (virus or virus-like particle) can then be purified or used in situ as a therapeutic tool for disease or allergy prevention. The expression of multivalent and multifunctional components to increase the immunogenicity of the recombinant complexes, especially on oral administration, is provided.
Owner:ADVANCED BIONUTRITION CORP
Compositions and methods for eliciting an immune response to escape mutants of targeted therapies
InactiveUS20100034840A1Reduce resistanceSymptoms improvedAntibacterial agentsOrganic active ingredientsMolecular Targeted TherapiesMutant
Provided herein are cells, vectors and viruses in association with a mutant polypeptide that has emerged in response to a therapeutic or prophylactic agent; compositions comprising such cells, vectors and viruses and methods for their use in eliciting an immune response to the mutant polypeptide. In some examples, the immune response is a cellular immune response.
Owner:GLOBE IMMUNE INC
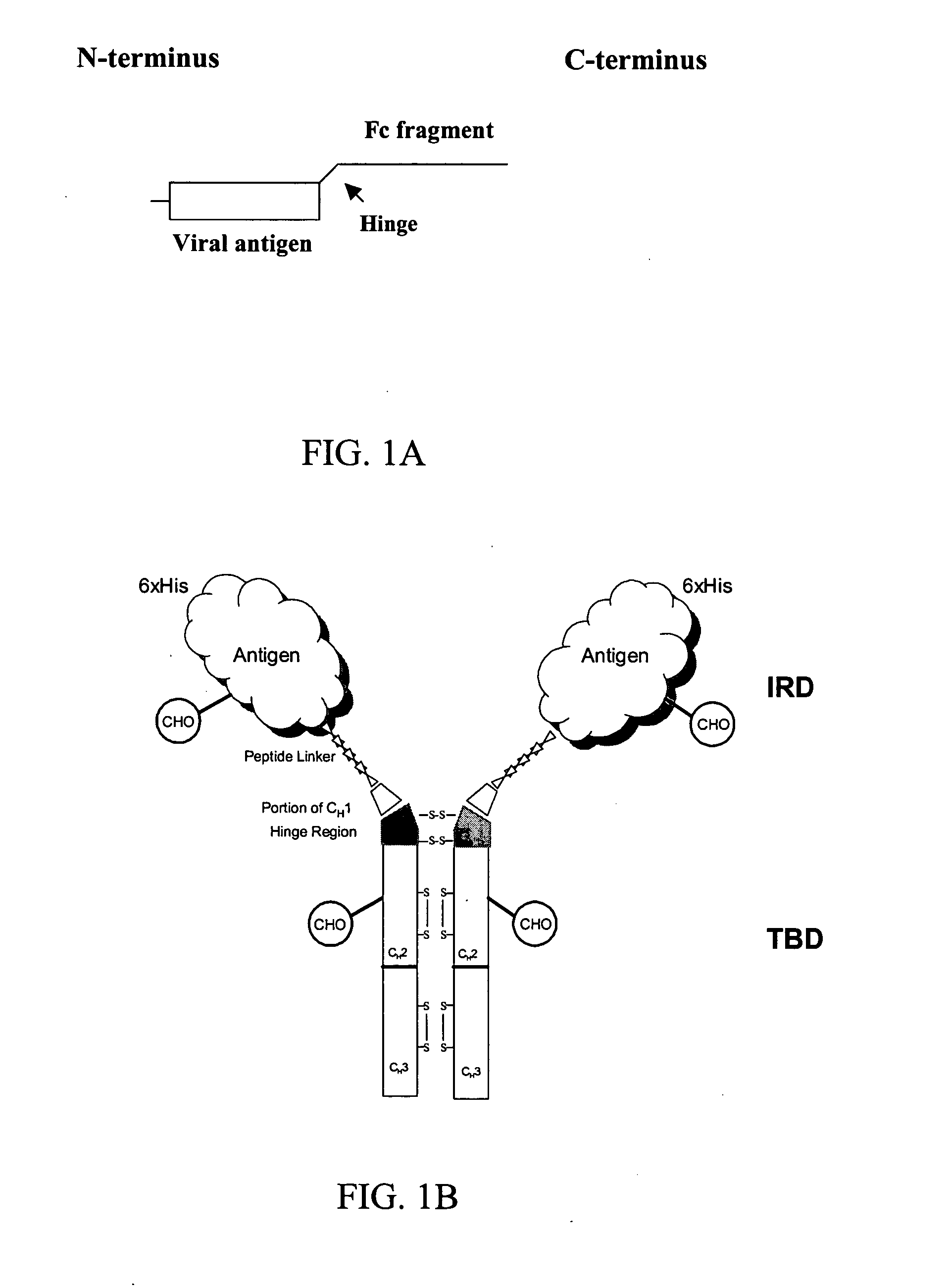
Chimeric antigens for eliciting an immune response
ActiveUS20050013828A1Effective presentationImprove efficiencyBiocideSsRNA viruses positive-senseHost immunityAntibody fragments
Disclosed herein are compositions and methods for eliciting immune responses against antigens. In particular embodiments, the compounds and methods elicit immune responses against antigens that are otherwise recognized by the host as “self” antigens. The immune response is enhanced by presenting the host immune system with a chimeric antigen comprising an immune response domain and a target binding domain, wherein the target binding domain comprises a xenotypic antibody fragment. By virtue of the target binding domain, antigen presenting cells take up, process, and present the chimeric antigen, eliciting both a humoral and cellular immune response.
Owner:KAIMI BIOMEDICINE (CHENGDU) CO LTD
Stabilization of aqueous compositions of proteins with displacement buffers
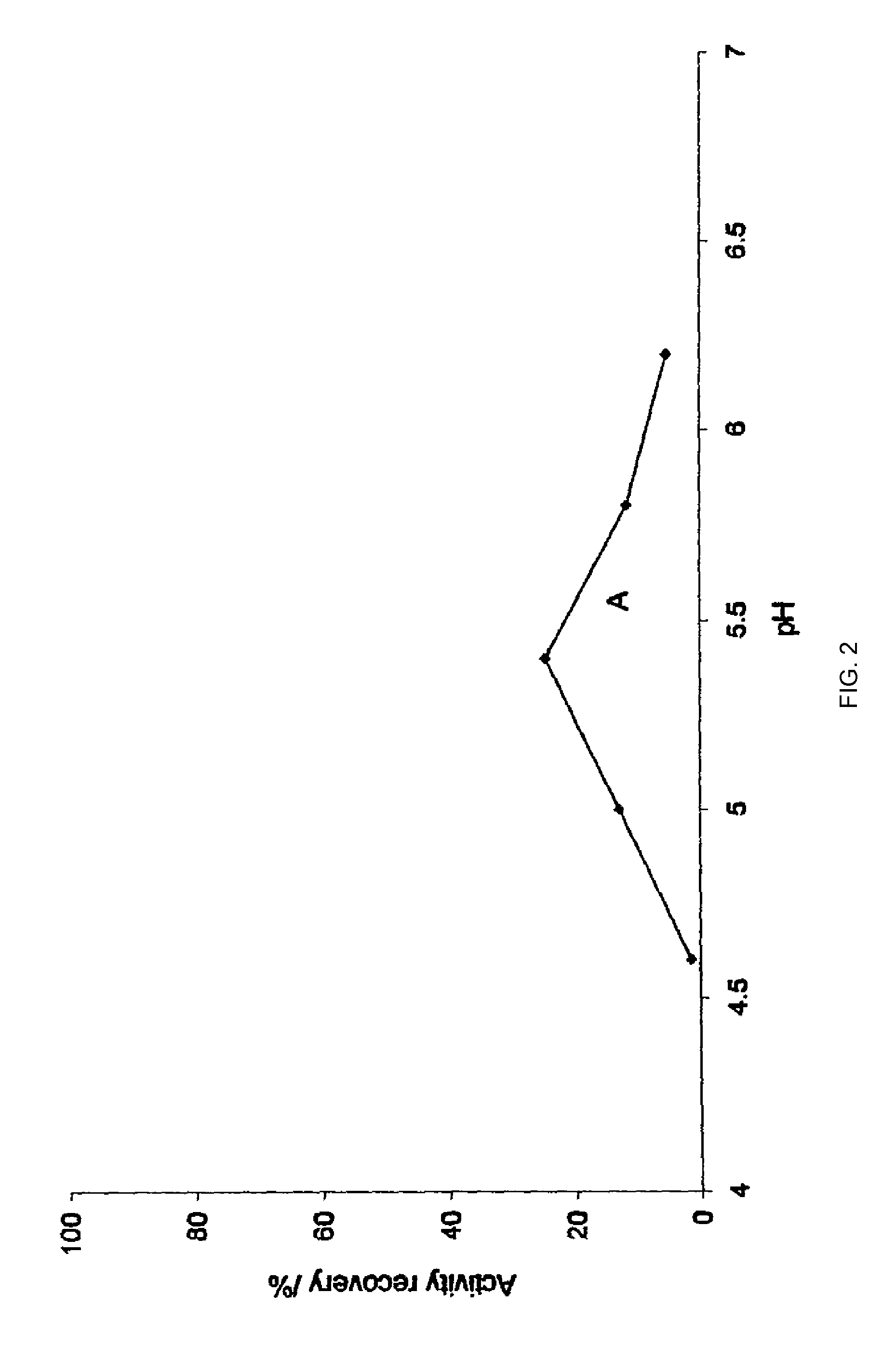
InactiveUS20100028372A1Improve stabilityImproves pH stabilityFactor VIIPeptide/protein ingredientsAnalytical chemistryProtein stability
An aqueous composition having increased protein stability is obtained by: a. determining a pH at which the protein has stability at the desired temperature; b. adding to the composition at least one displacement buffer wherein the displacement buffer has a pKa that is at least 1 unit greater or less than the pH of step (a); and c. adjusting the pH of the composition to the pH of step (a); wherein the aqueous composition does not comprise a conventional buffer at a concentration greater than about 2 mM and wherein the conventional buffer has a pKa that is within 1 unit of the pH of step (a).
Owner:ARECOR LTD
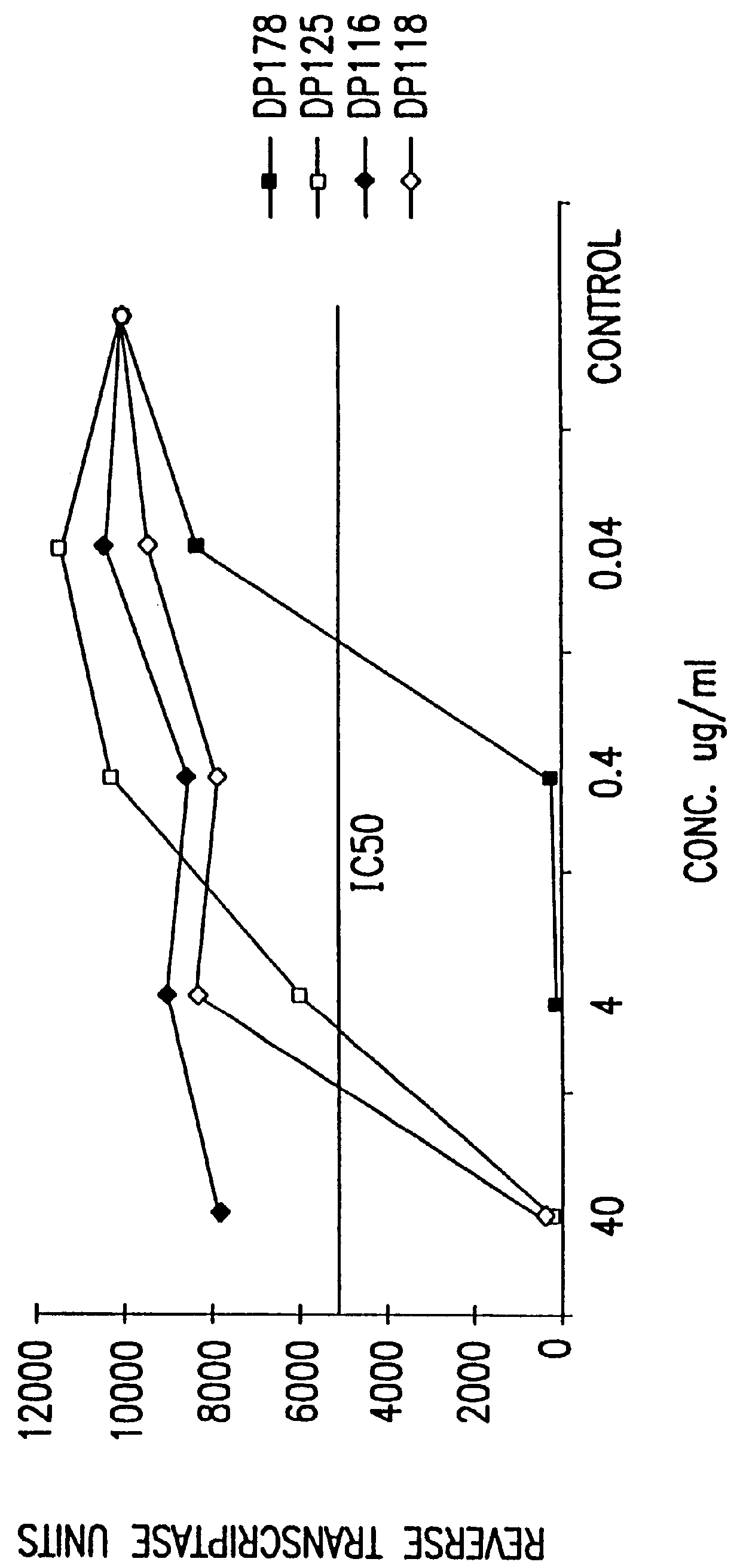
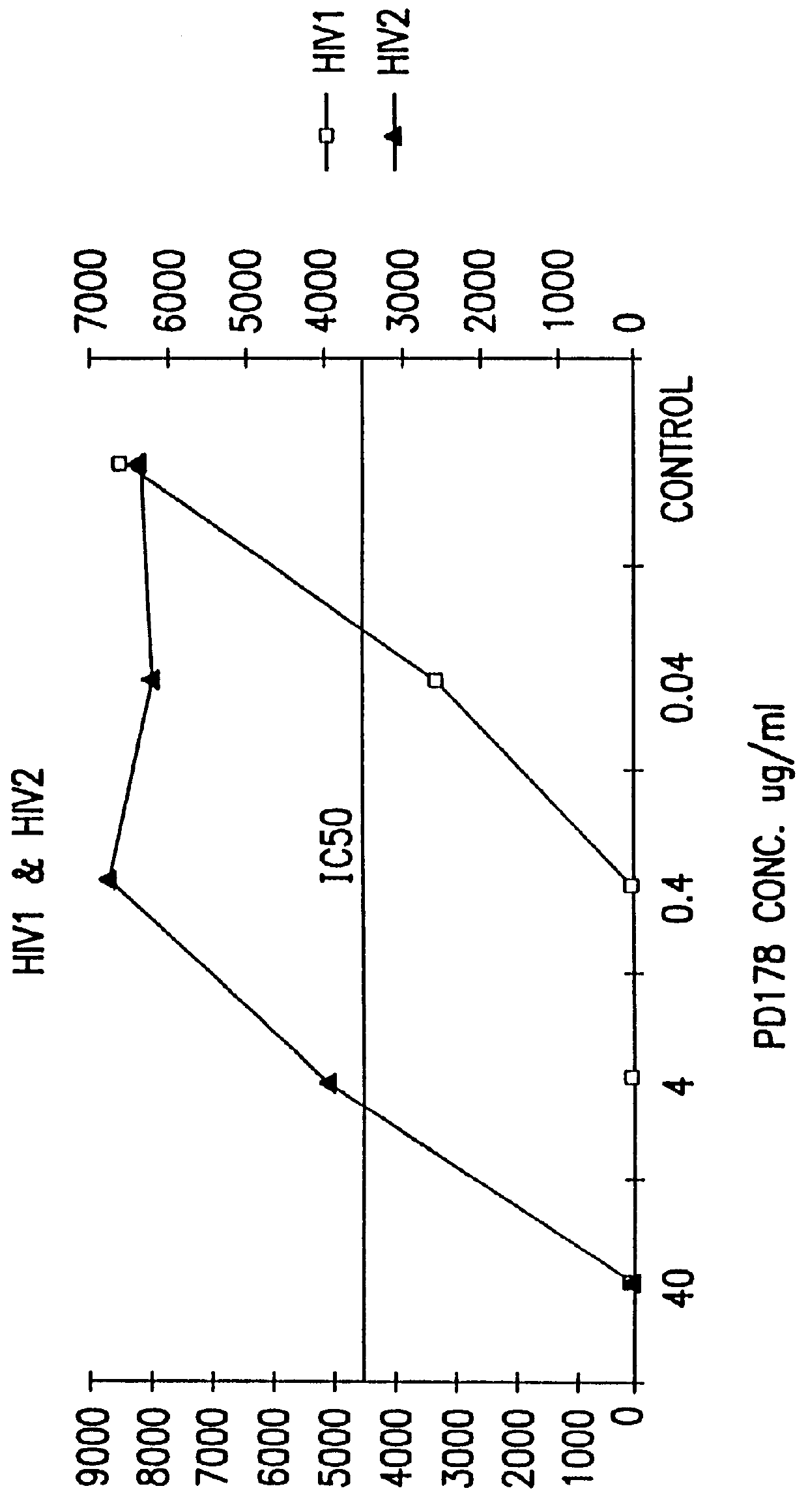
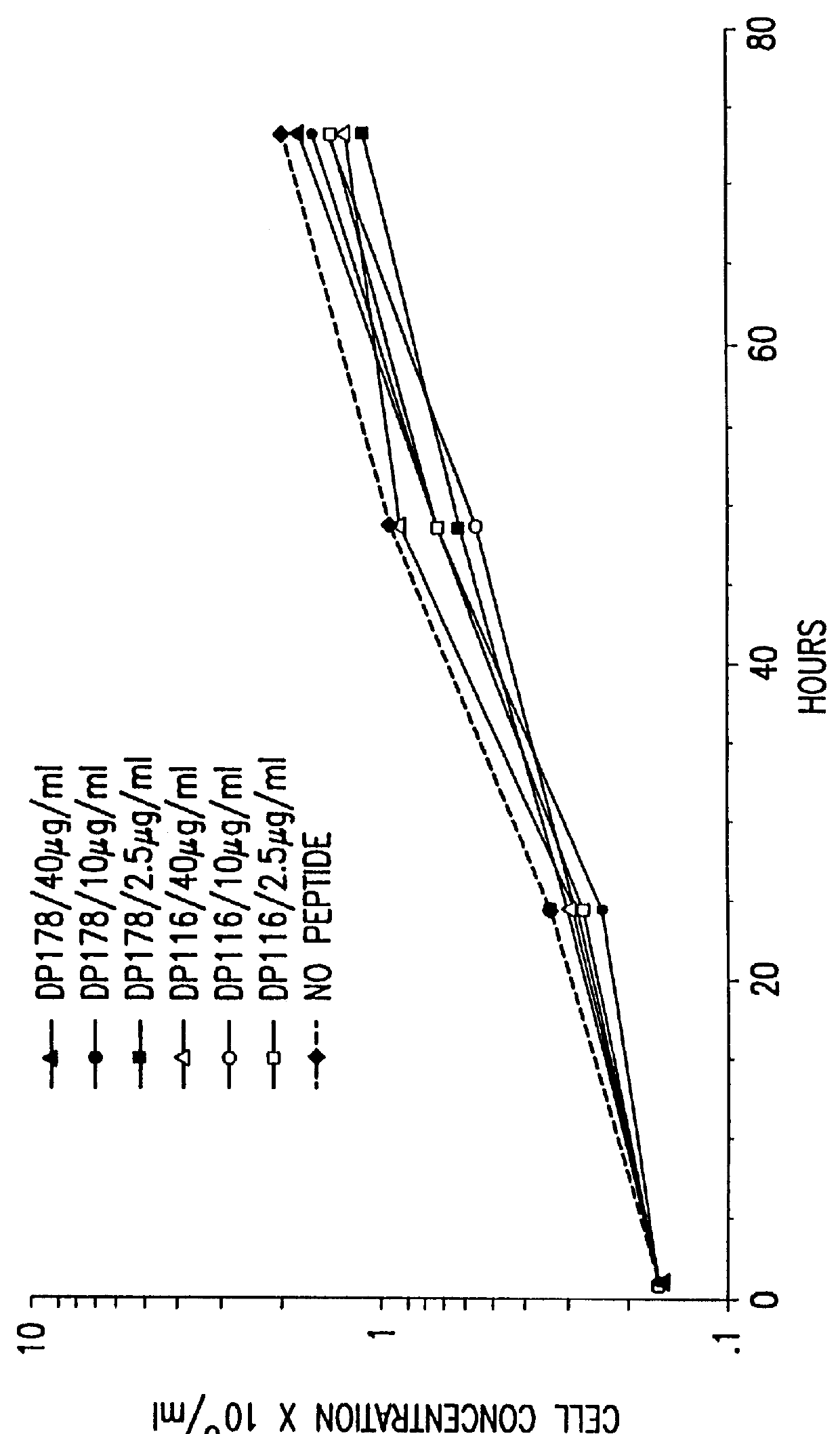
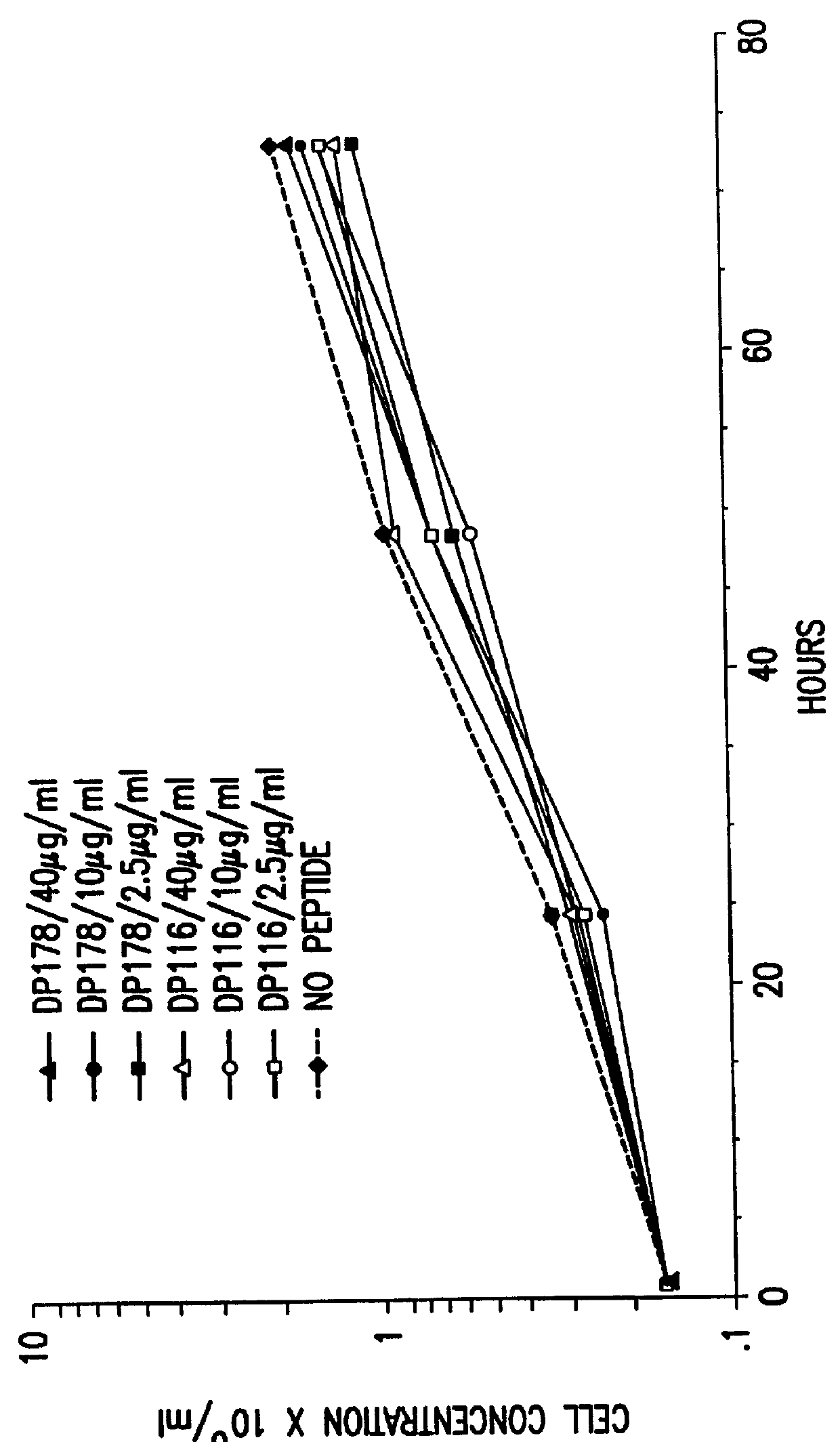
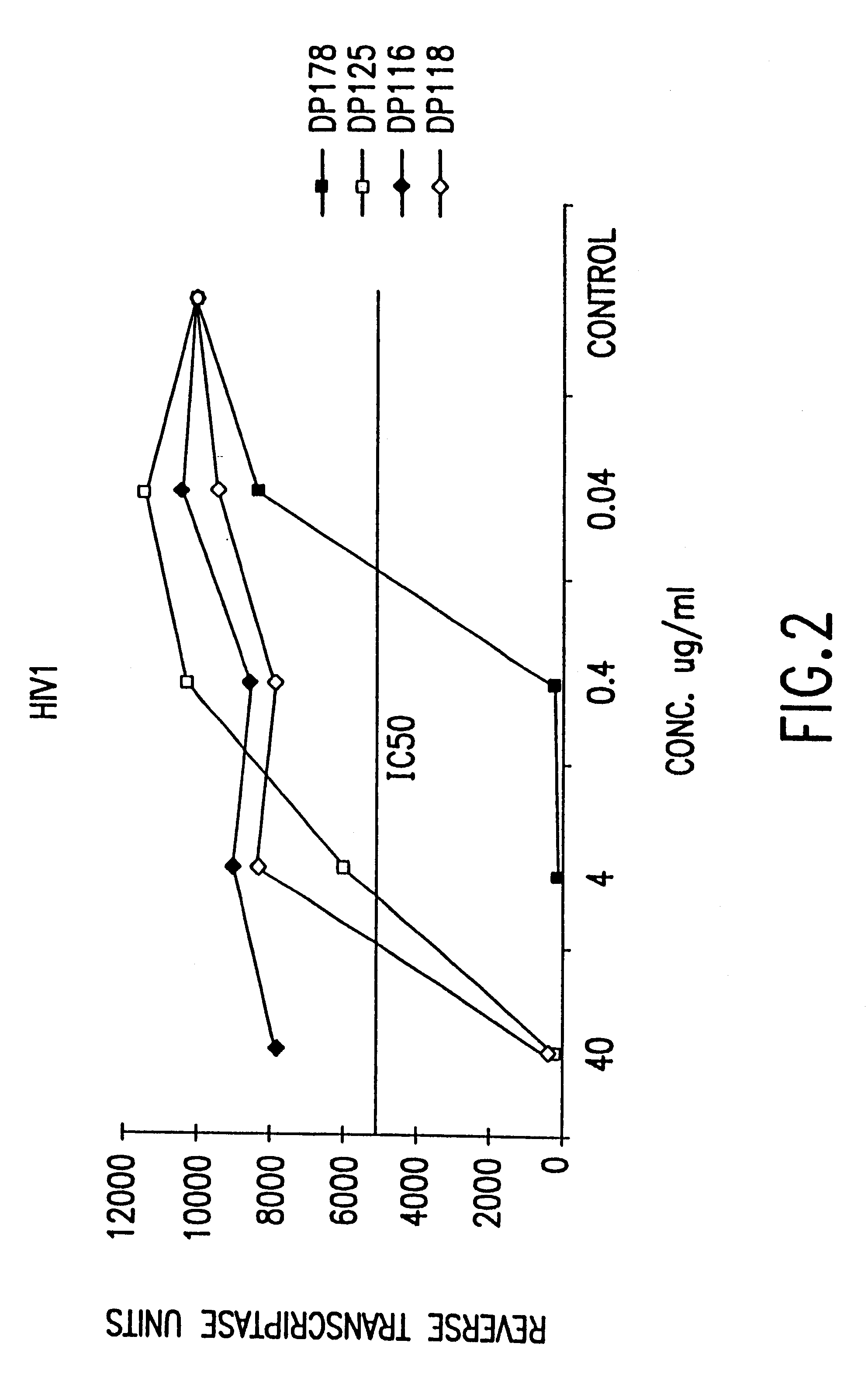
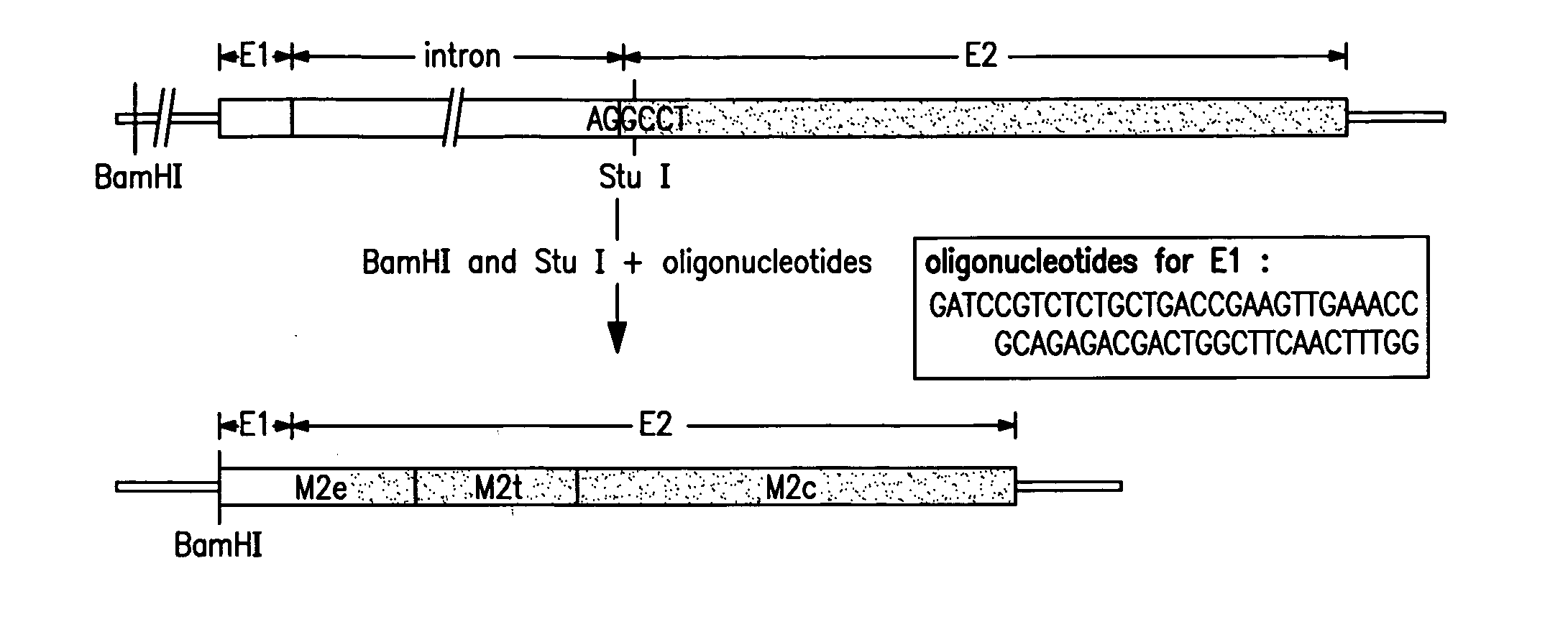
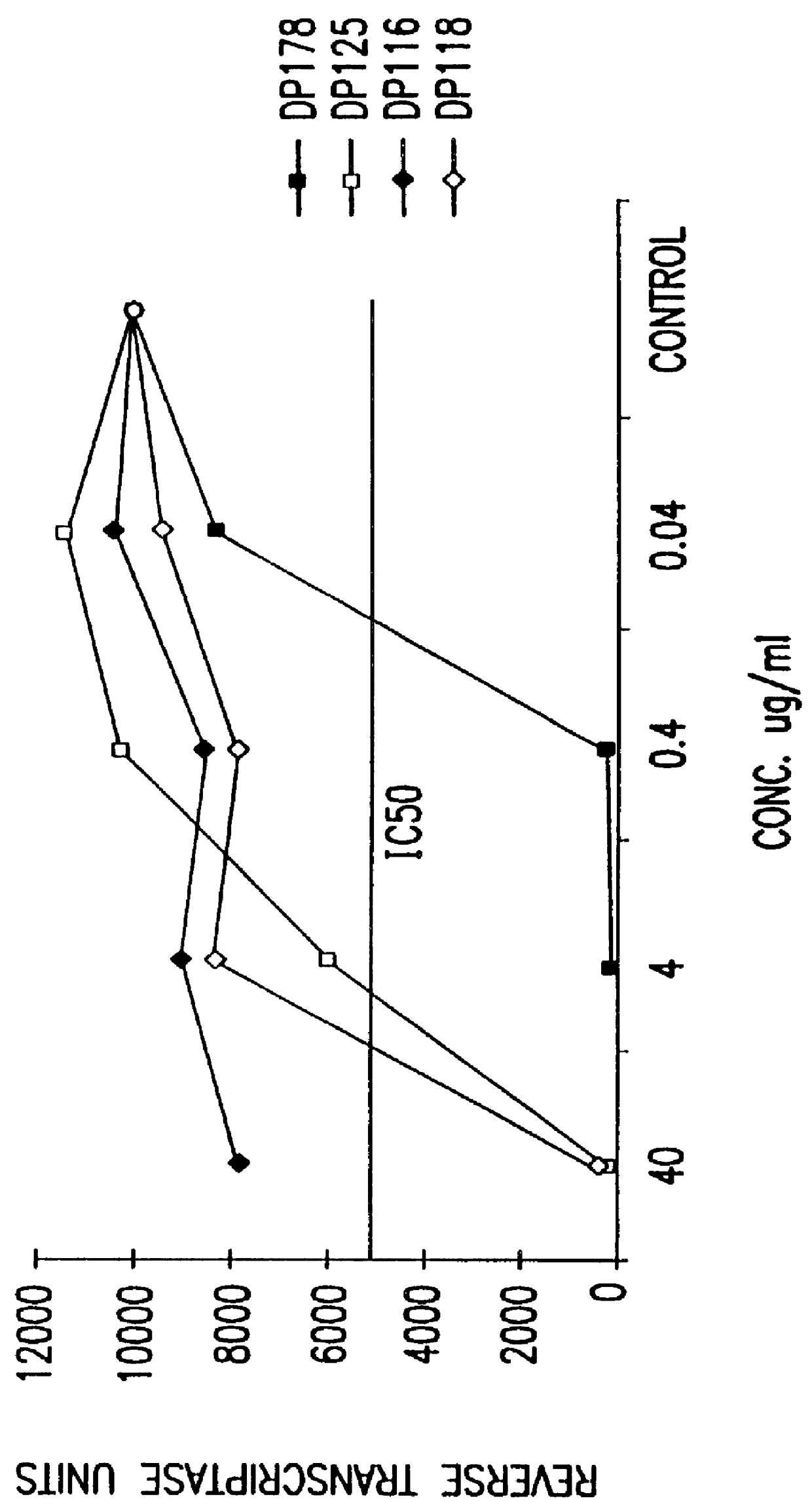
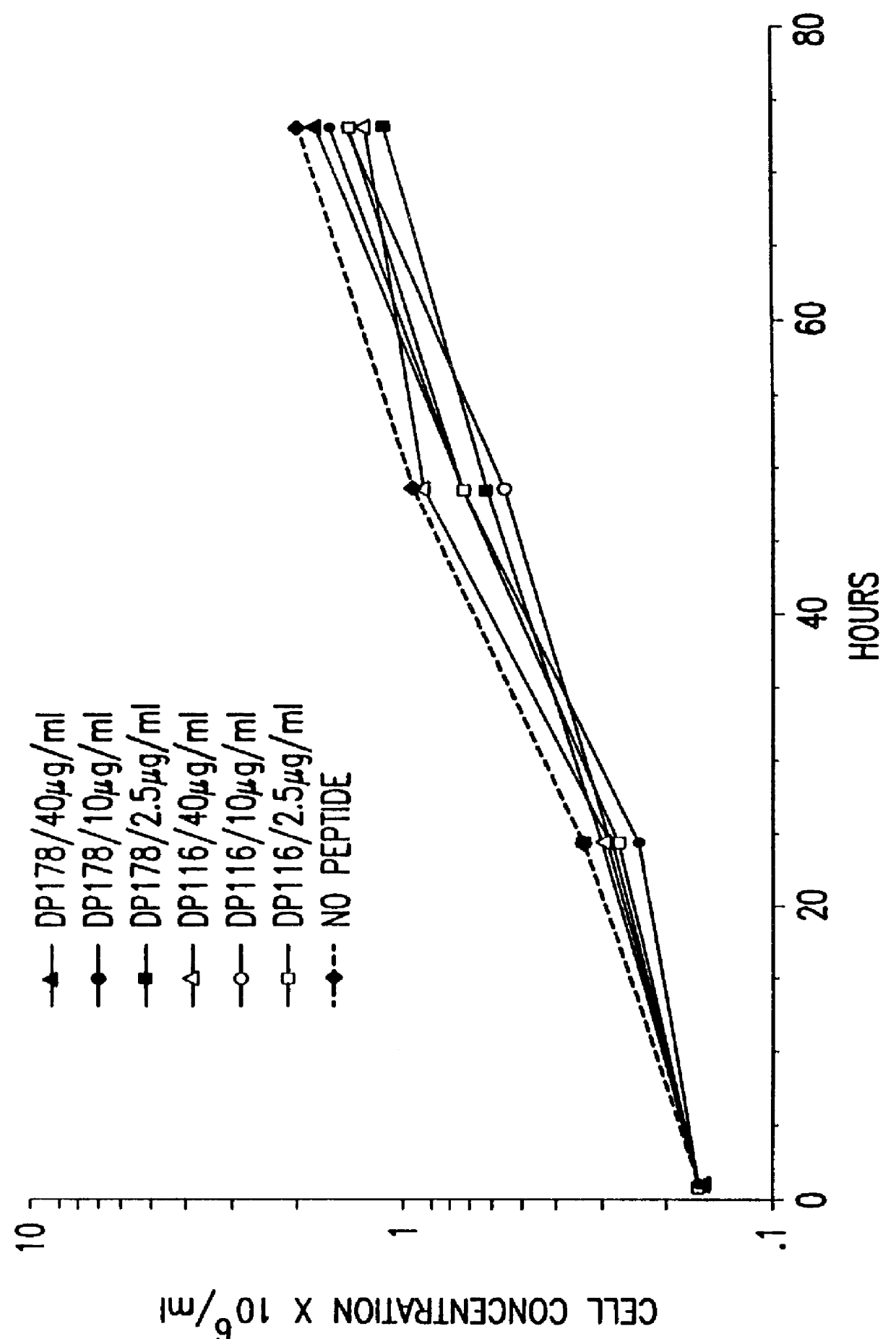
Synthetic peptide inhibitors of HIV transmission
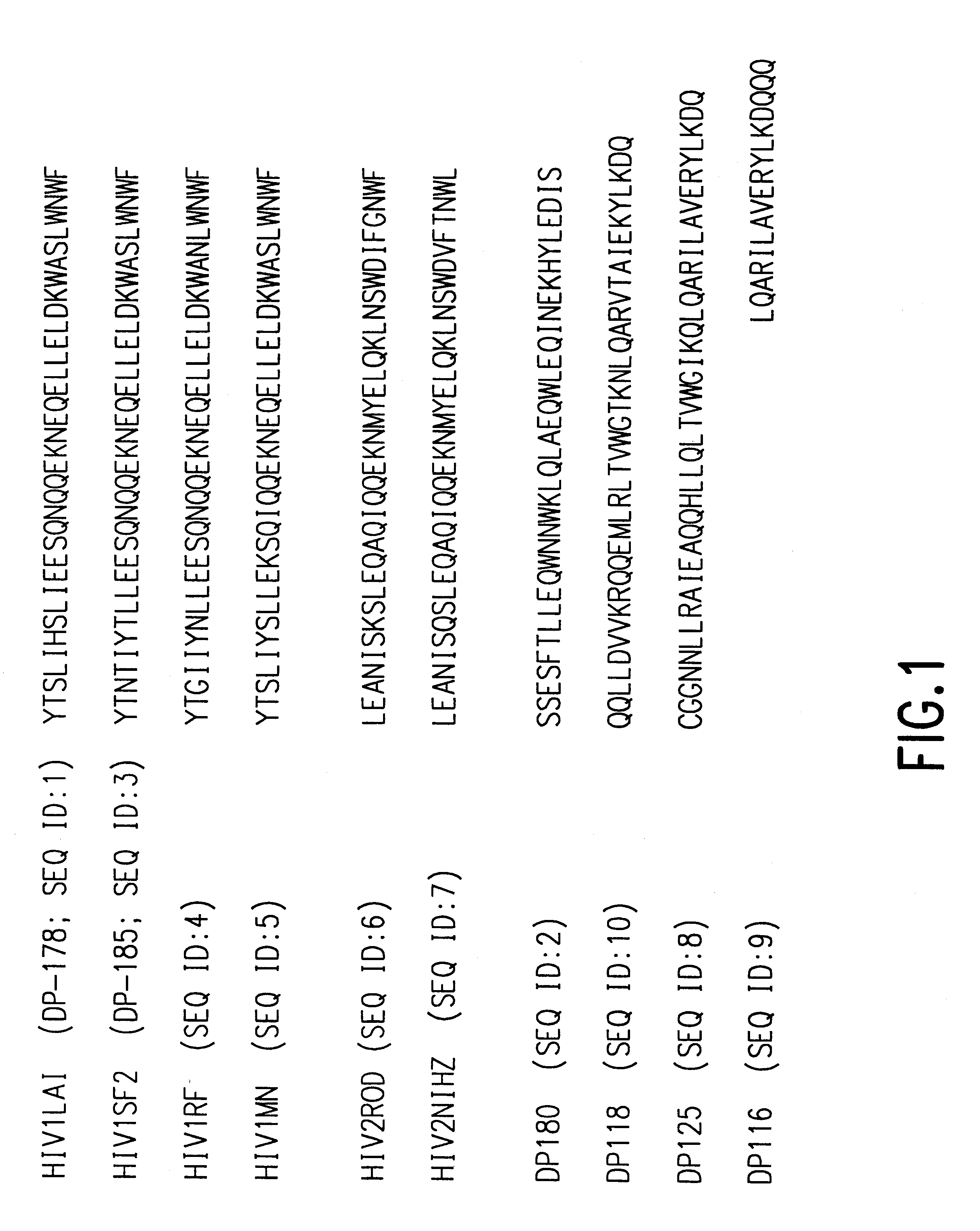
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP-178 (SEQ ID:1) ptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP-178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:DUKE UNIV
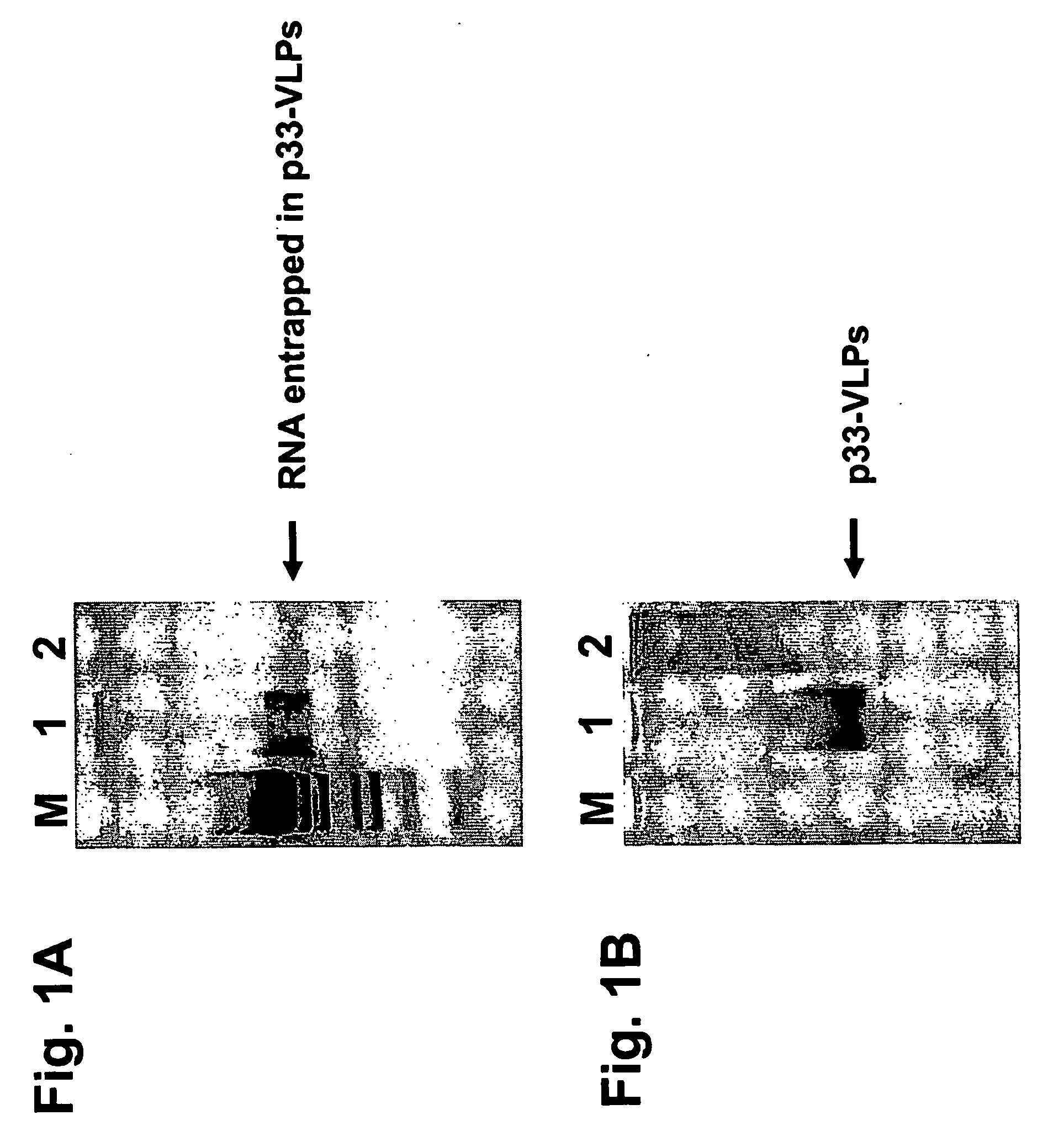
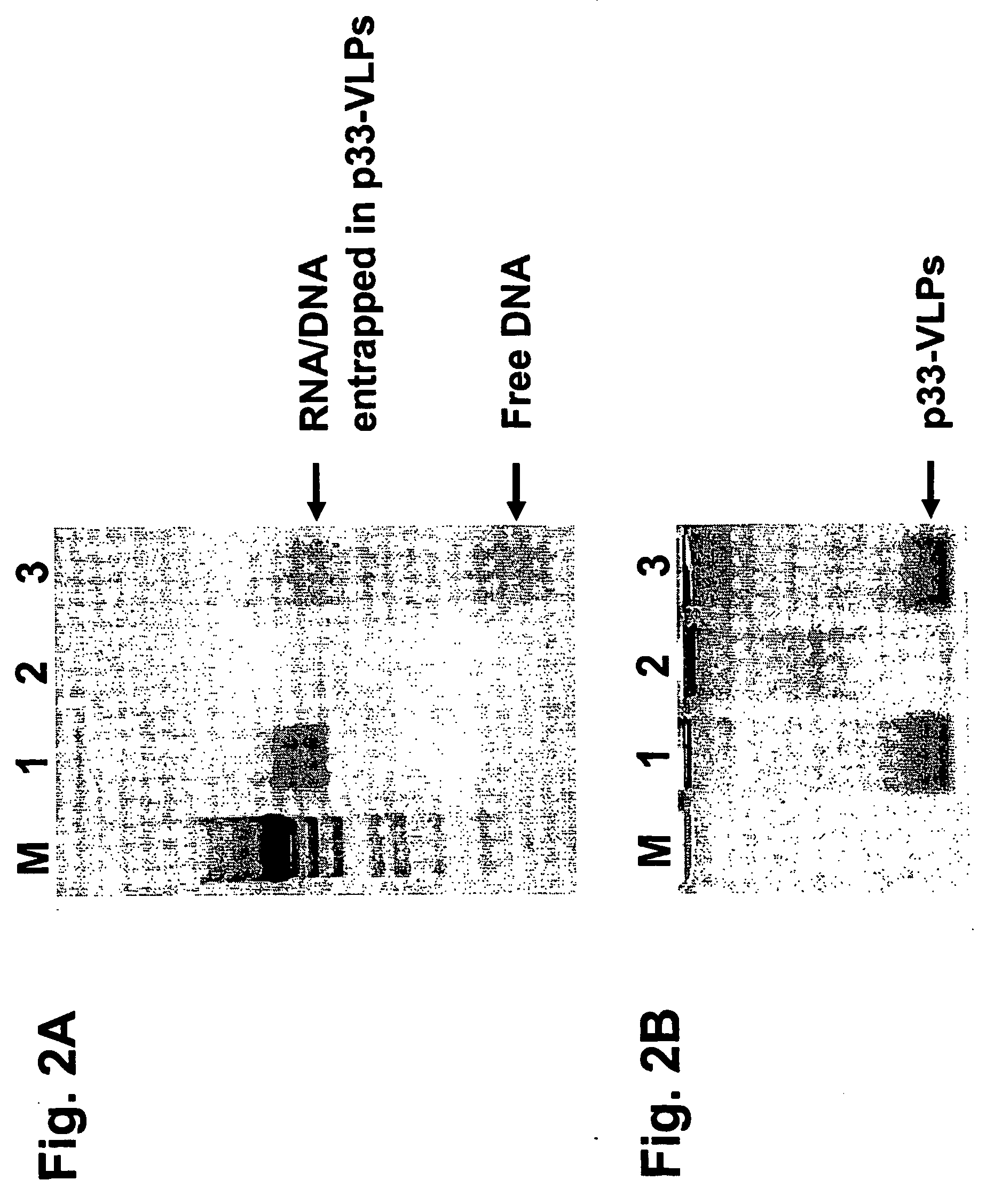
Packaged virus-like particles
InactiveUS20060251623A1Improve responseEnhanced T cell responseSsRNA viruses negative-senseBiocideAbnormal tissue growthDisease
The present invention is related to the fields of vaccinology, immunology and medicine. The invention provides compositions and methods for enhancing immunological responses against antigens coupled or fused to virus-like particles (VLPs) packaged with immunostimulatory nucleic acids, preferably oligonucleotides containing at least one non-methylated CpG sequence and a toll-like receptor (TLR) ligand. The invention can be used to induce strong antibody and T cell responses particularly useful for the treatment of allergies, tumors and chronic viral diseases as well as other chronic diseases.
Owner:CYTOS BIOTECHNOLOGY AG
Chimeric antigens for breaking host tolerance to foreign antigens
ActiveUS20050031628A1Enhance immune responseAvoid infectionAntibacterial agentsSsRNA viruses positive-senseHost immunityAntibody fragments
Disclosed herein are compositions and methods for eliciting immune responses against antigens. In particular, the compounds and methods elicit immune responses against foreign antigens that are otherwise recognized by the host as "self" antigens, thus breaking host tolerance to those antigens. Presenting the host immune system with a chimeric antigen comprising an immune response domain and a target binding domain, wherein the target binding domain comprises an antibody fragment, enhances the immune response against the foreign or tolerated antigen. Antigen presenting cells take up, process, and present the chimeric antigen, eliciting both a humoral and cellular immune response against the desired antigen.
Owner:KAIMI BIOMEDICINE (CHENGDU) CO LTD
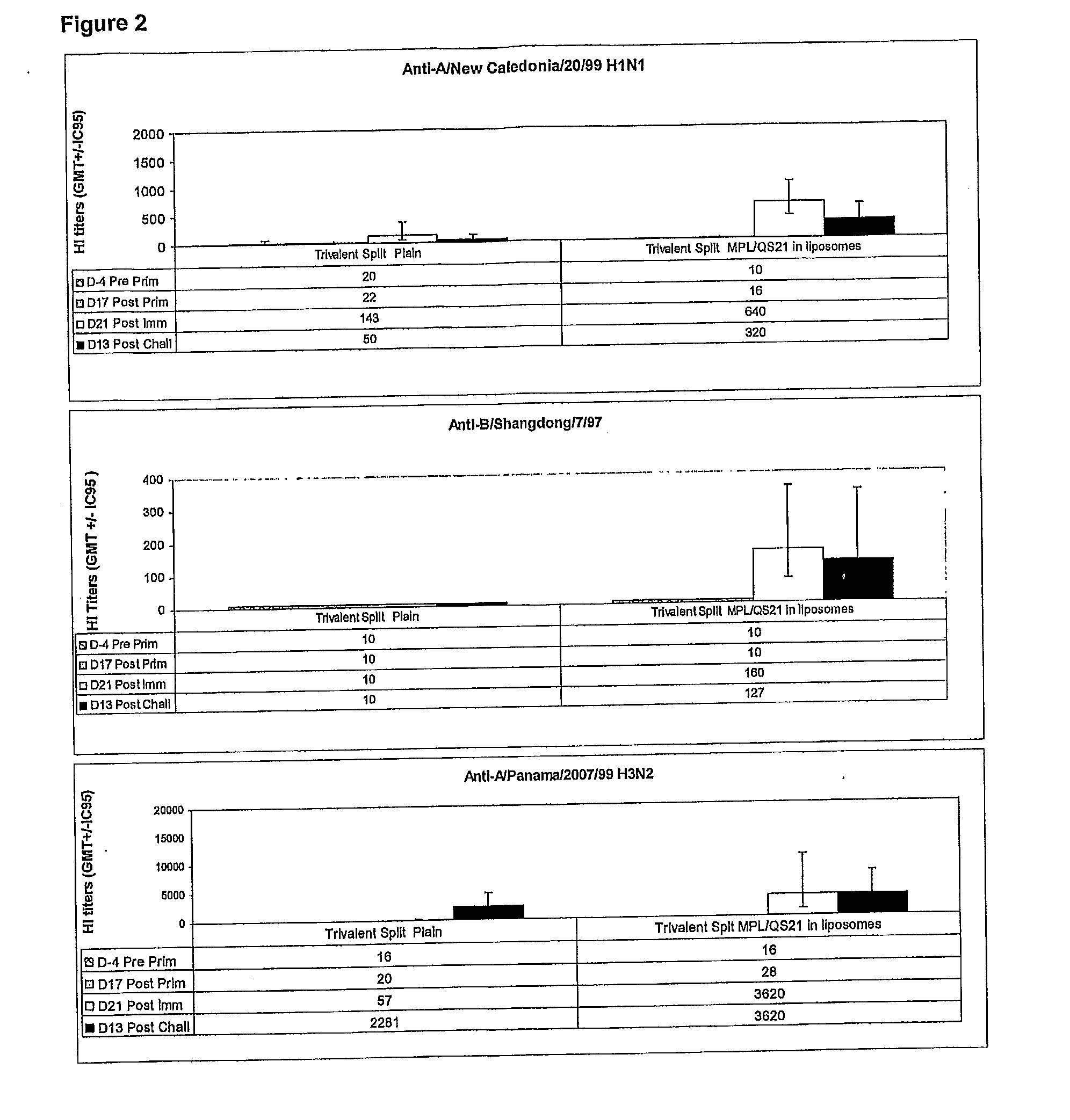
Vaccine Compositions Comprising a Saponin Adjuvant
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Methods for the inhibition of epstein-barr virus transmission employing anti-viral peptides capable of abrogating viral fusion and transmission
InactiveUS6518013B1SsRNA viruses negative-sensePeptide/protein ingredientsCell membraneViral life cycle
Fusion of the viral envelope, or infected cell membranes with uninfected cell membranes, is an essential step in the viral life cycle. Recent studies involving the human immunodeficiency virus type 1(HIV-1) demonstrated that synthetic peptides (designated DP-107 and DP-178) derived from potential helical regions of the transmembrane (TM) protein, gp41, were potent inhibitors of viral fusion and infection. A computerized antiviral searching technology (C.A.S.T.) that detects related structural motifs (e.g., ALLMOTI 5, 107x178x4, and PLZIP) in other viral proteins was employed to identify similar regions in the Epstein-Barr virus (EBV). Several conserved heptad repeat domains that are predicted to form coiled-coil structures with antiviral activity were identified in the EBV genome. Synthetic peptides of 16 to 39 amino acids derived from these regions were prepared and their antiviral activities assessed in a suitable in vitro screening assay. These peptides proved to be potent inhibitors of EBV fusion. Based upon their structural and functional equivalence to the known HIV-1 inhibitors DP-107 and DP-178, these peptides should provide a novel approach to the development of targeted therapies for the treatment of EBV infections.
Owner:TRIMERIS
Immunoprotective influenza antigen and its use in vaccination
The present invention relates to an influenza antigen, comprising a fusion product of at least the extracellular part of a conserved influenza membrane protein or a functional fragment thereof and a presenting carrier, which may be a presenting (poly)peptide or a non-peptidic structure, such as glycans, peptide mimetics, synthetic polymers. The invention further relates to a vaccine against influenza, comprising at least an antigen of the invention, optionally in the presence of one or more excipients. The invention also relates to use of the antigen, a method for preparing the antigen and acceptor cells expressing the antigen.
Owner:VLAAMS INTERUNIVIR INST VOORS BIOTECH
Screening assays for compounds that inhibit membrane fusion-associated events
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:TRIMERIS
Formulations comprising aminosterols
ActiveUS8623416B2Reduce local membrane damaging propertyReducing free concentrationSsRNA viruses negative-sensePowder deliveryPhosphateEndocrinology
Owner:ENTERIN INC
Oil-in-water emulsion free of surfactant and use thereof
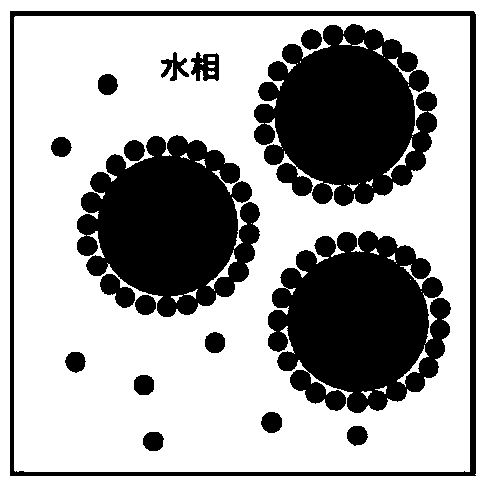
ActiveCN104013955AThe nature is easy to controlGood biocompatibilitySsRNA viruses negative-sensePowder deliveryActive agentGlycerol
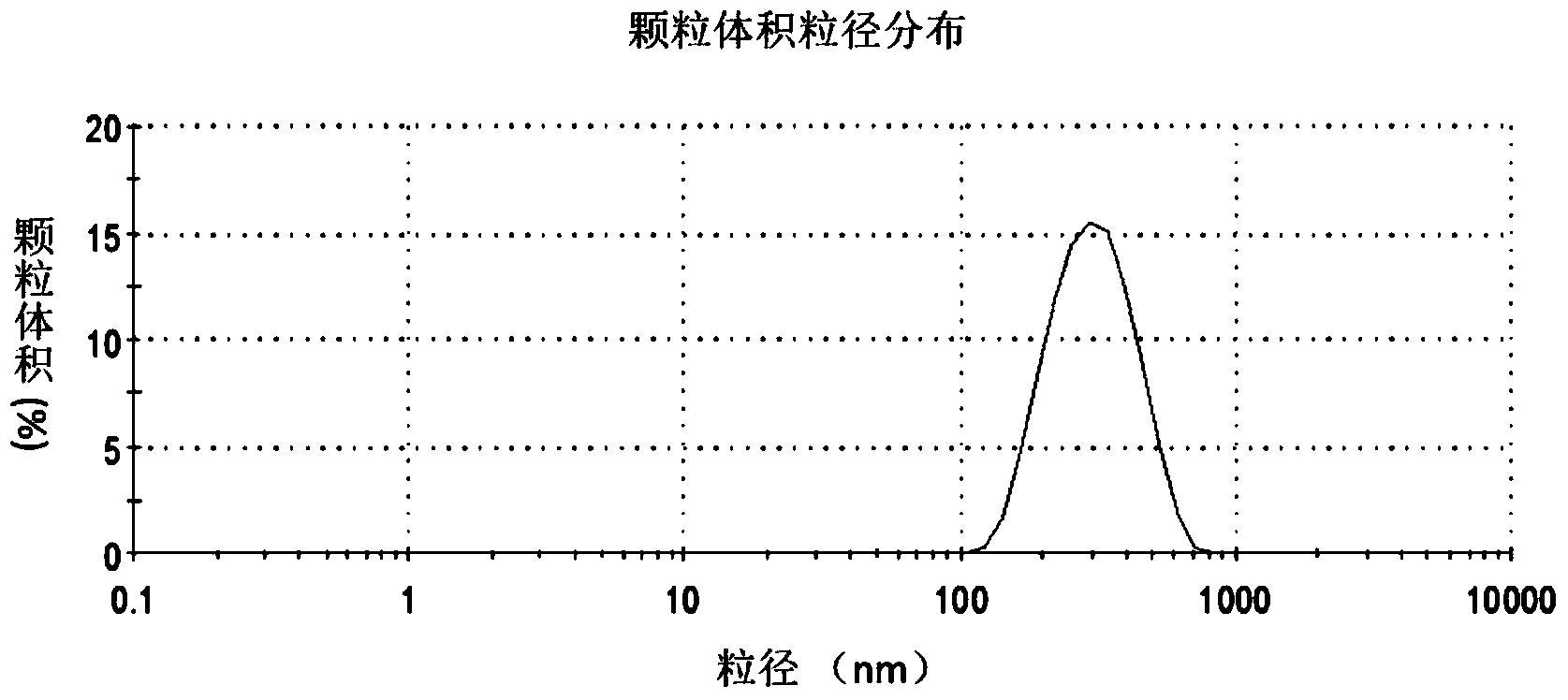
The invention discloses an oil-in-water emulsion free of surfactant. The emulsion comprises a metabolizable oil phase, a water phase and oil-water amphipathic solid particles dispersed in the water phase and having biocompatibility, wherein the oil phase comprises squalene or / and tocol; the water phase is any one or a combination of at least two of purified water, water for injection, aqueous liquid of glycerol, buffered saline liquid and clinically available infusion liquid; and the average grain diameter of the solid particles is at nanometer-to-micron grade. The emulsion can be used as a vaccine adjuvant or a medicine delivery or controlled release carrier; the properties of the emulsion can be controlled and regulated; the obtained emulsion is stable; the use of a surfactant is avoided; and harm to the human body and pollution on the environmental can be reduced.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Chimeric immunomodulatory compounds and methods of using the same - IV
Owner:DYNAVAX TECH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com