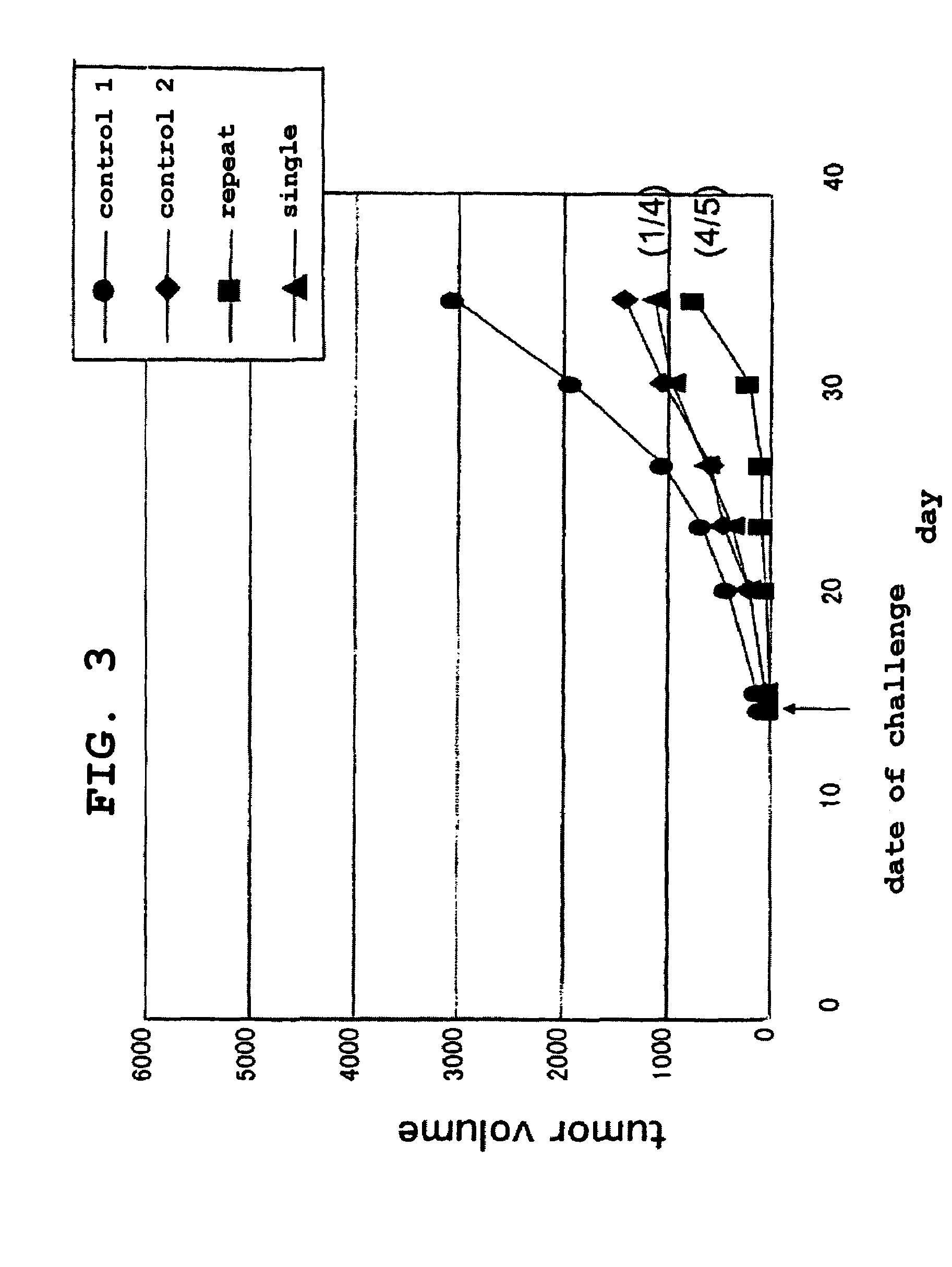
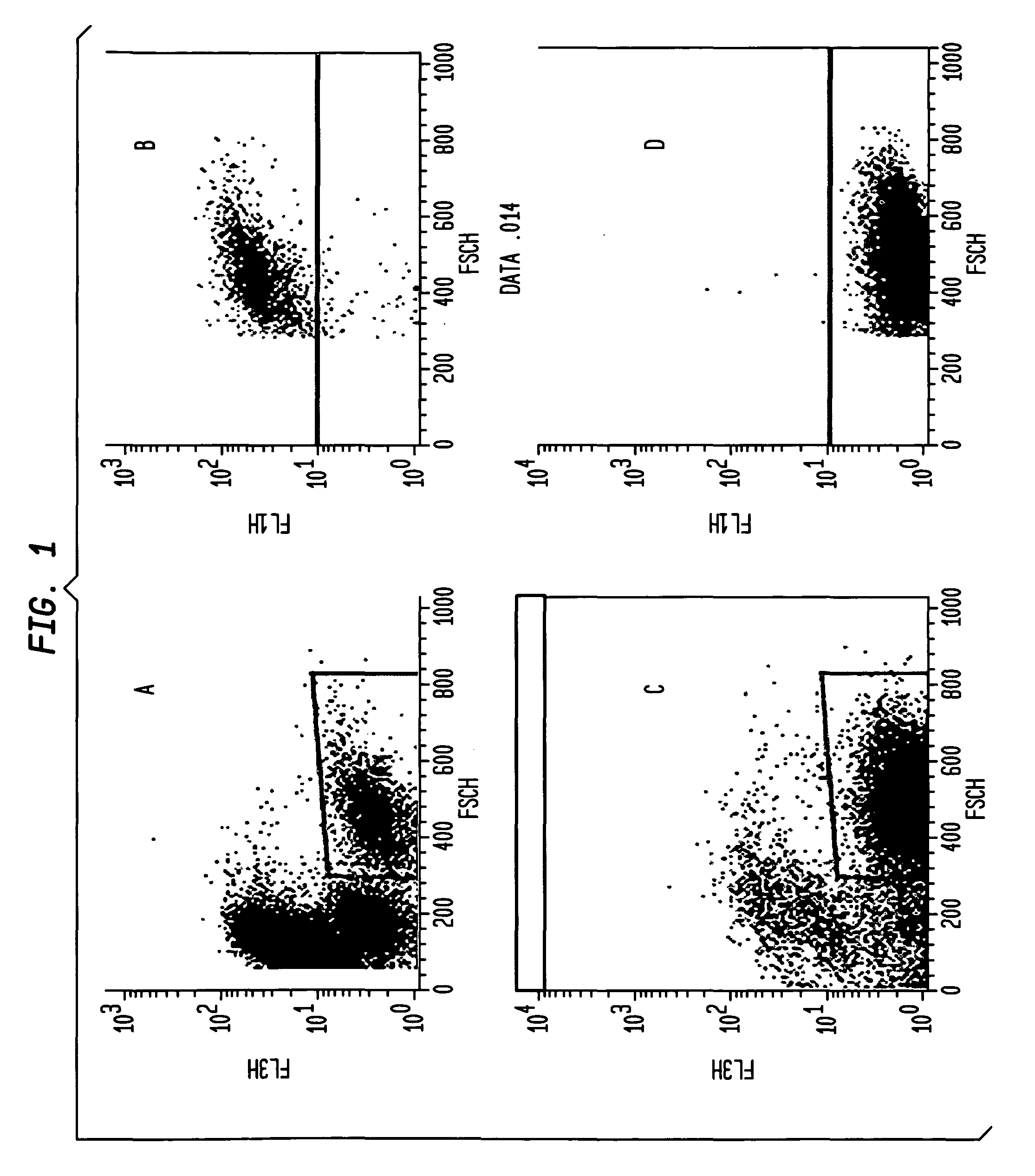
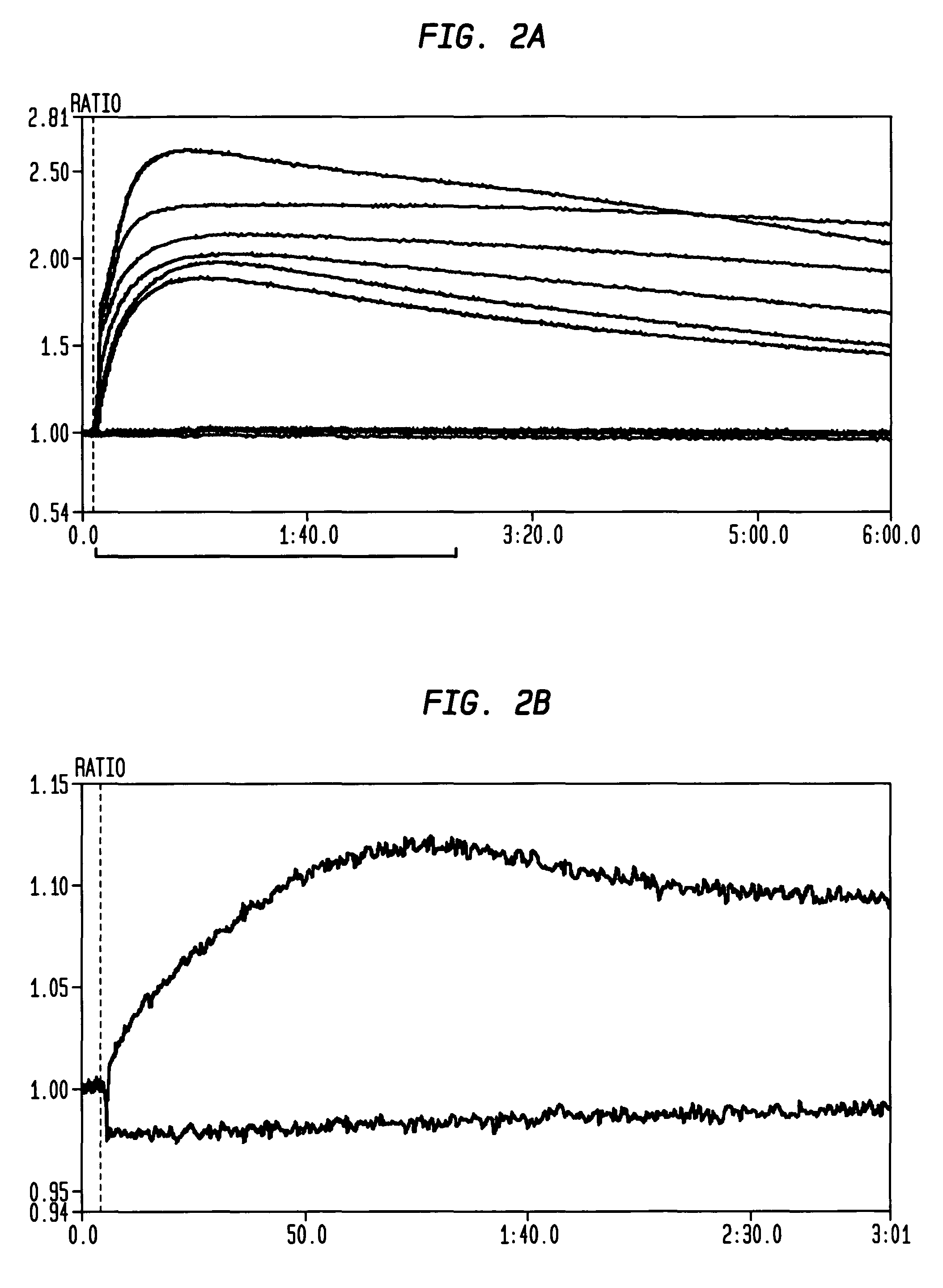
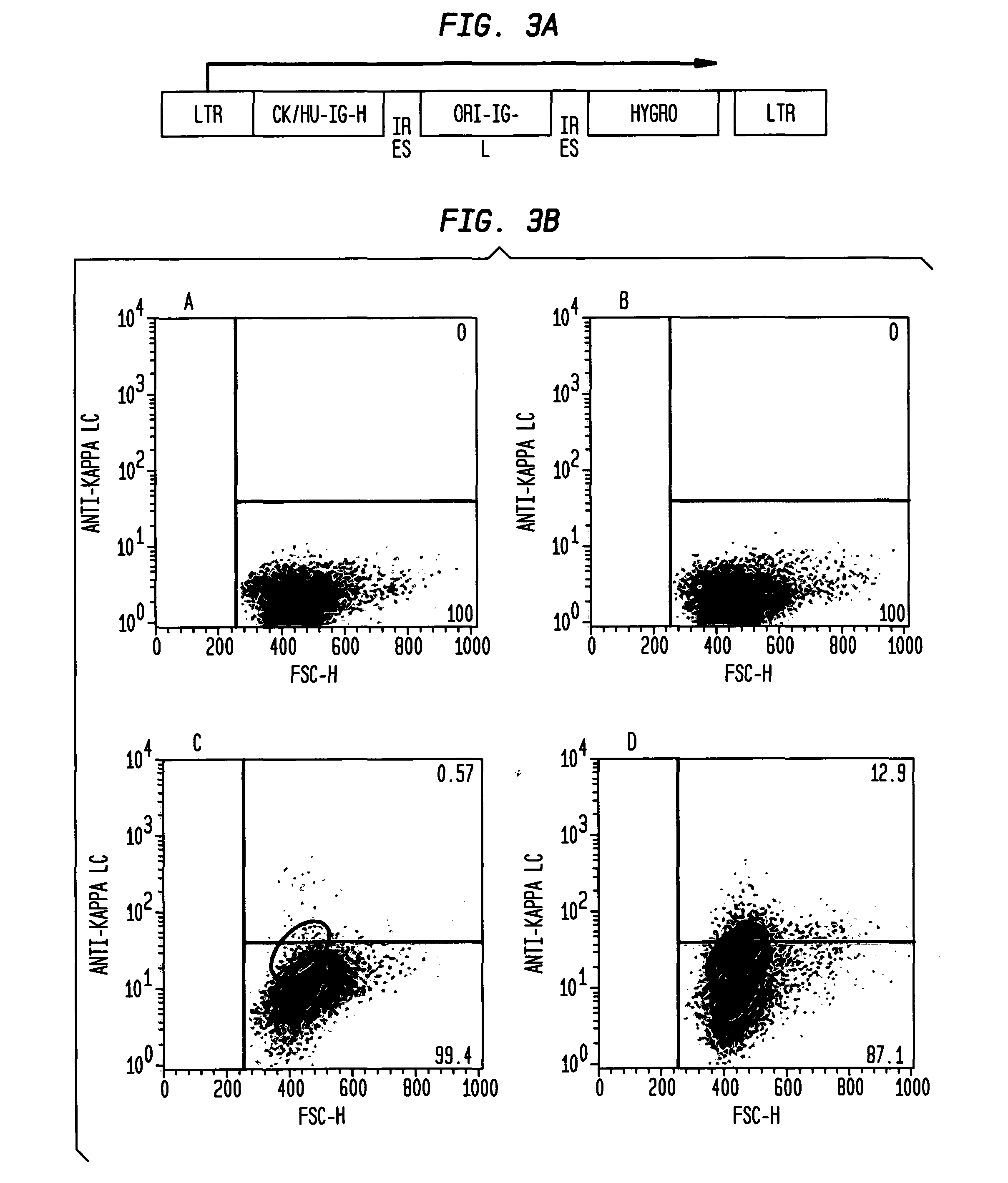
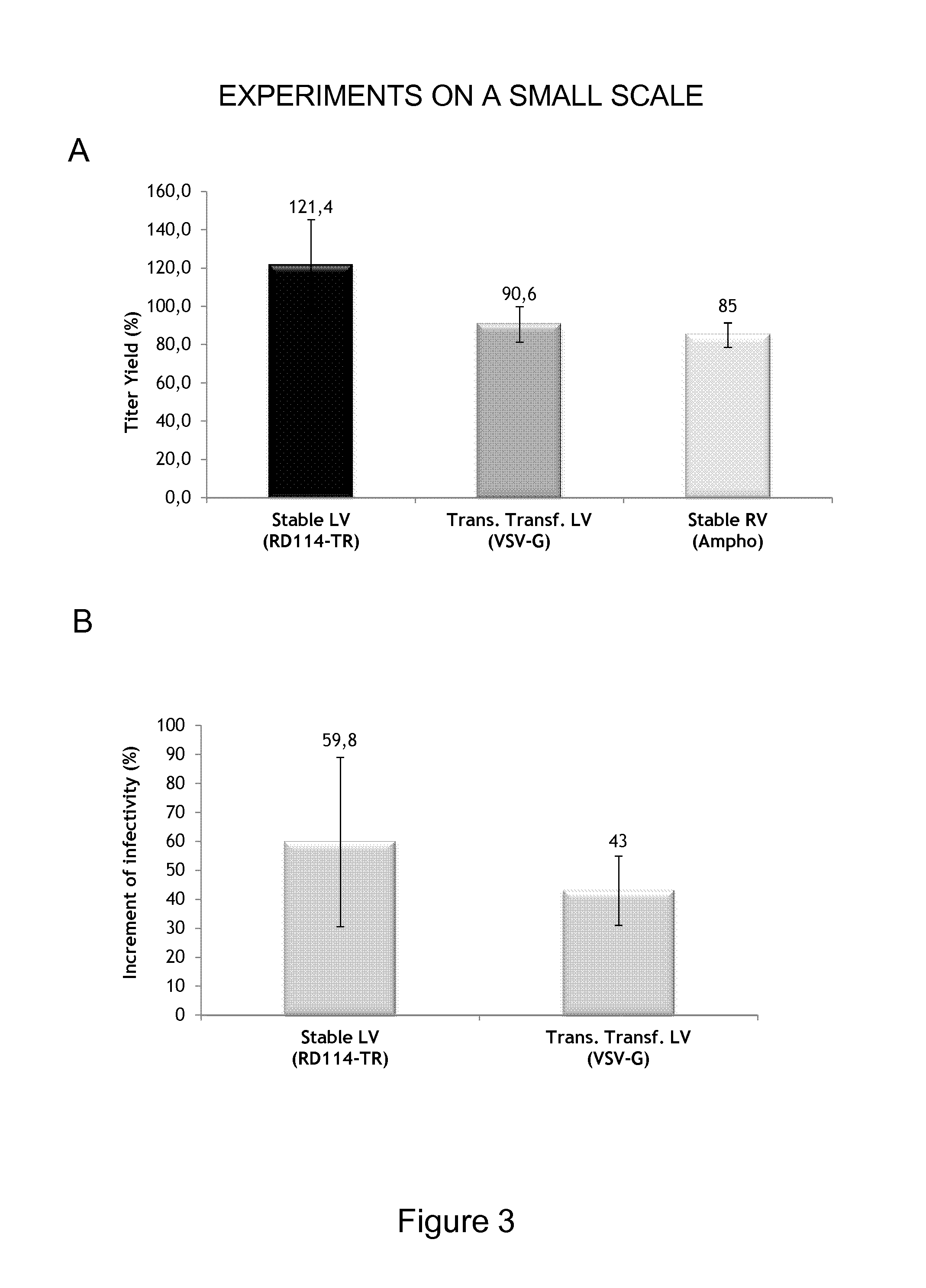
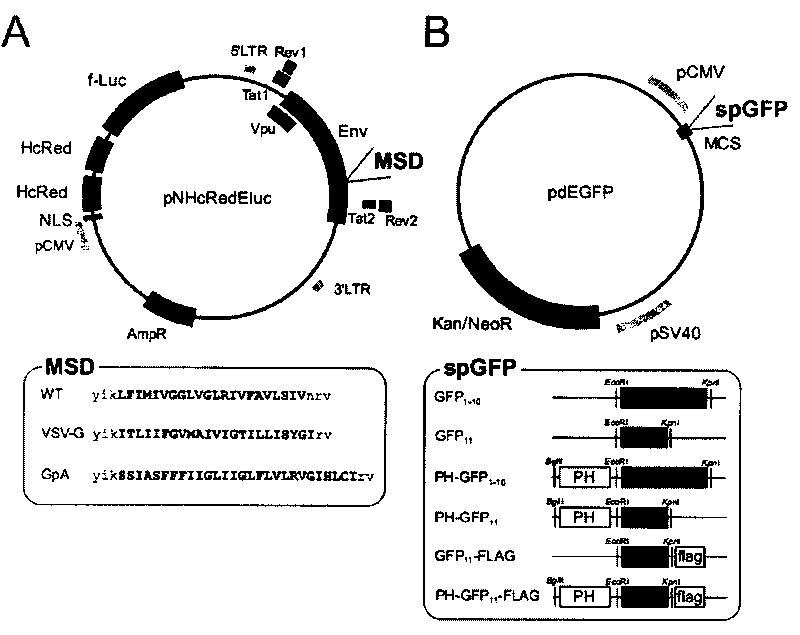
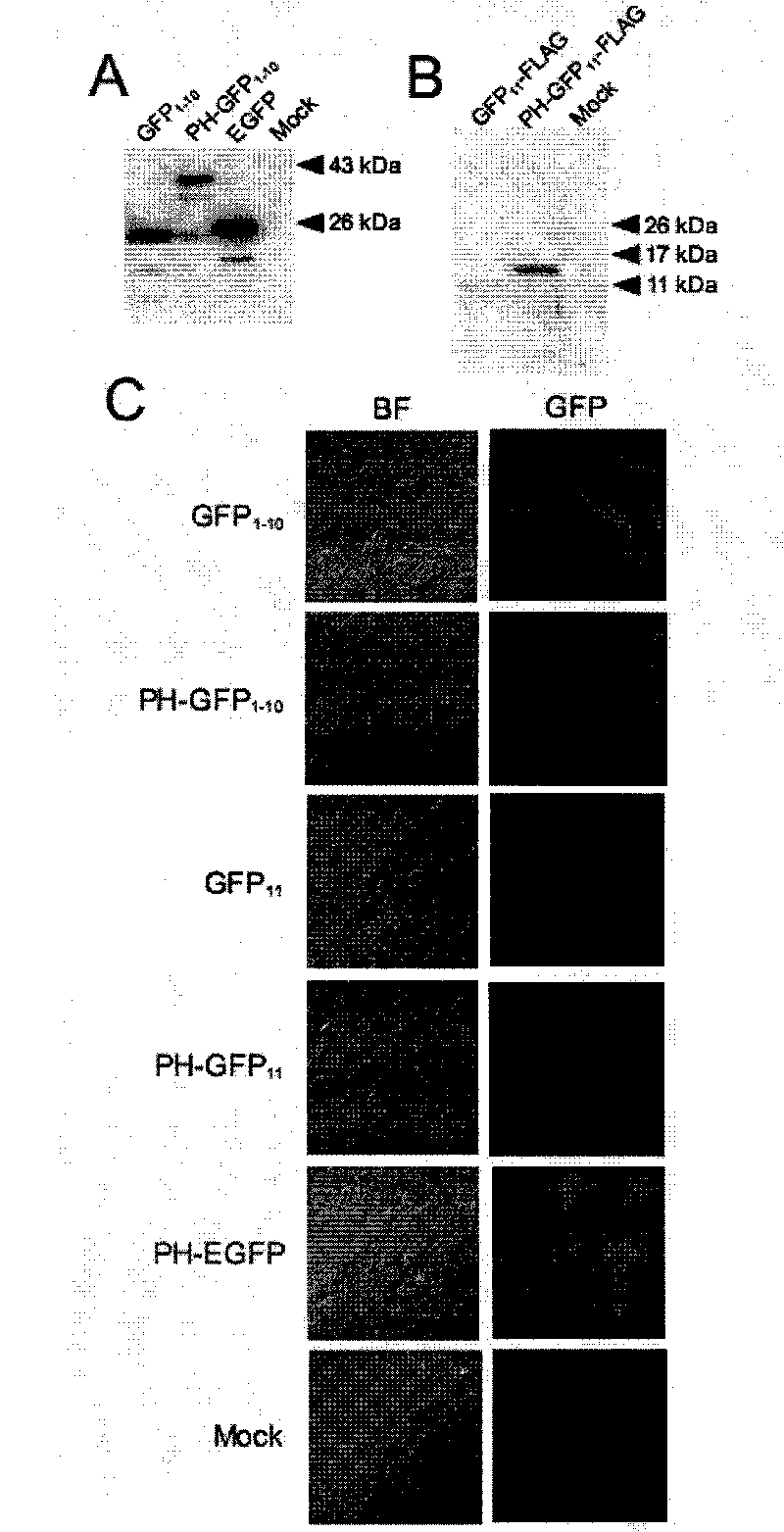
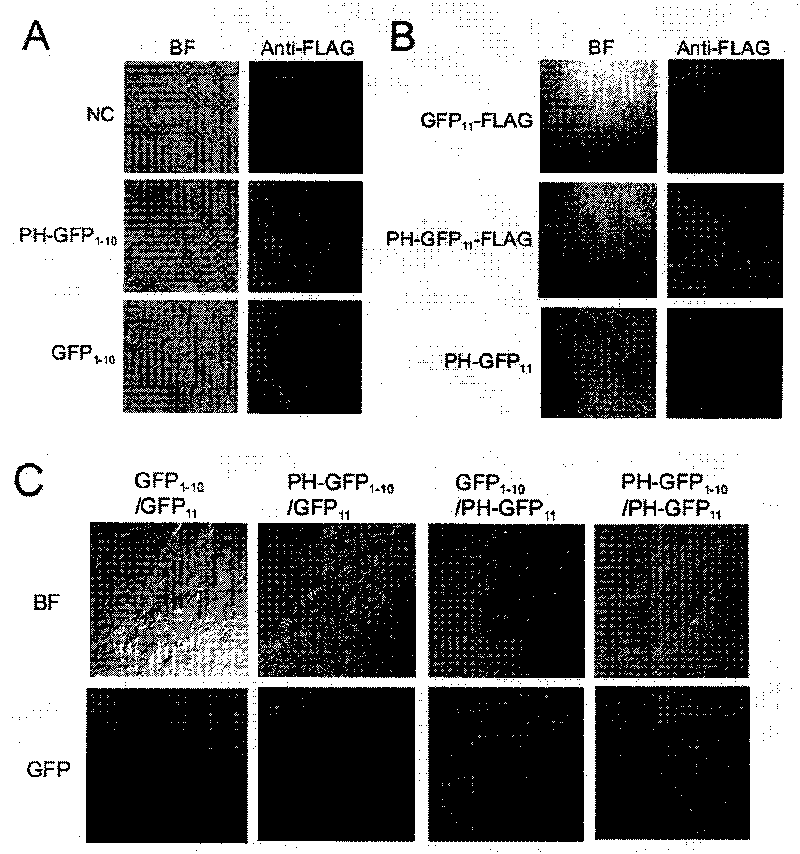
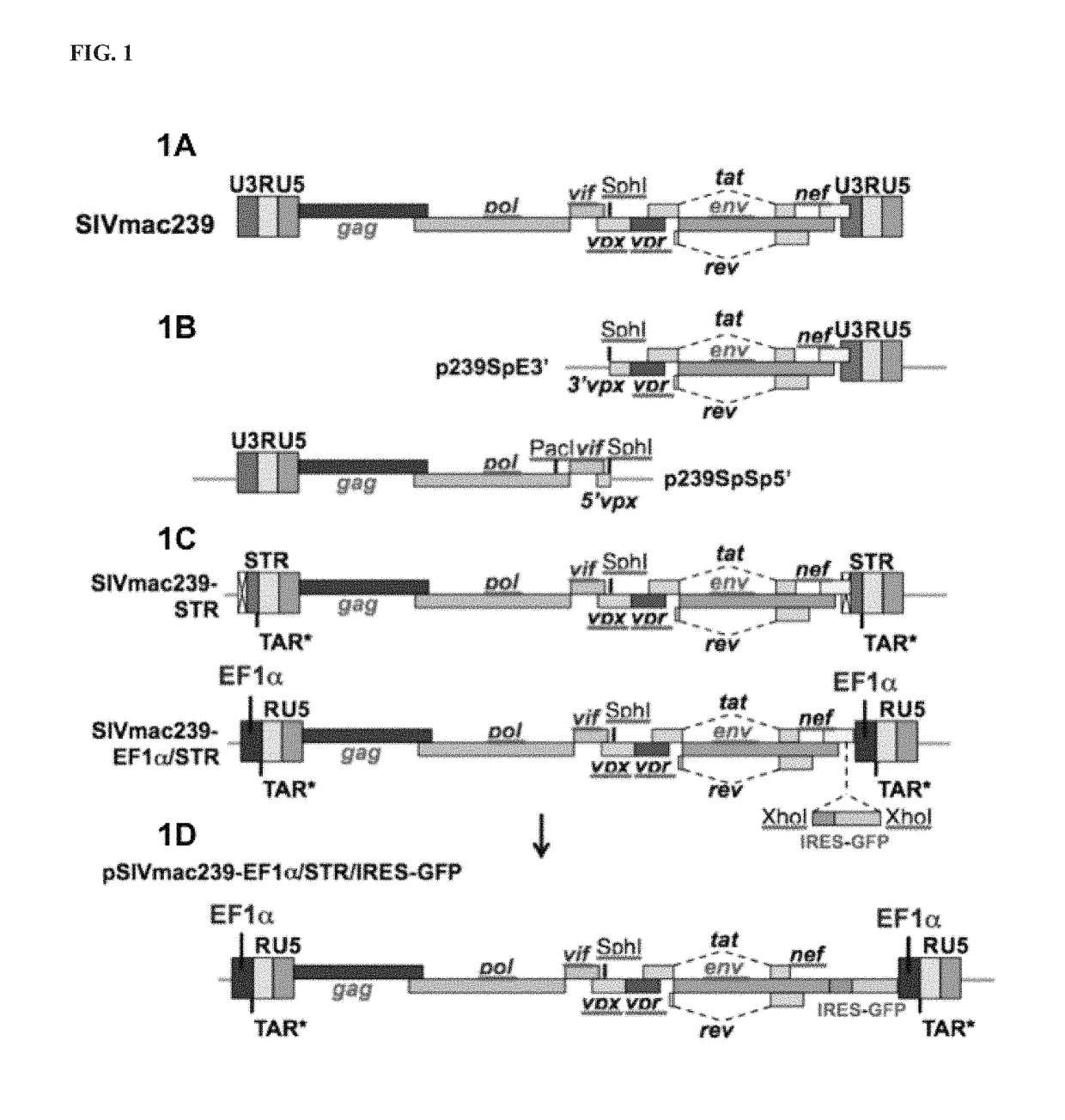
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Viral envelope" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
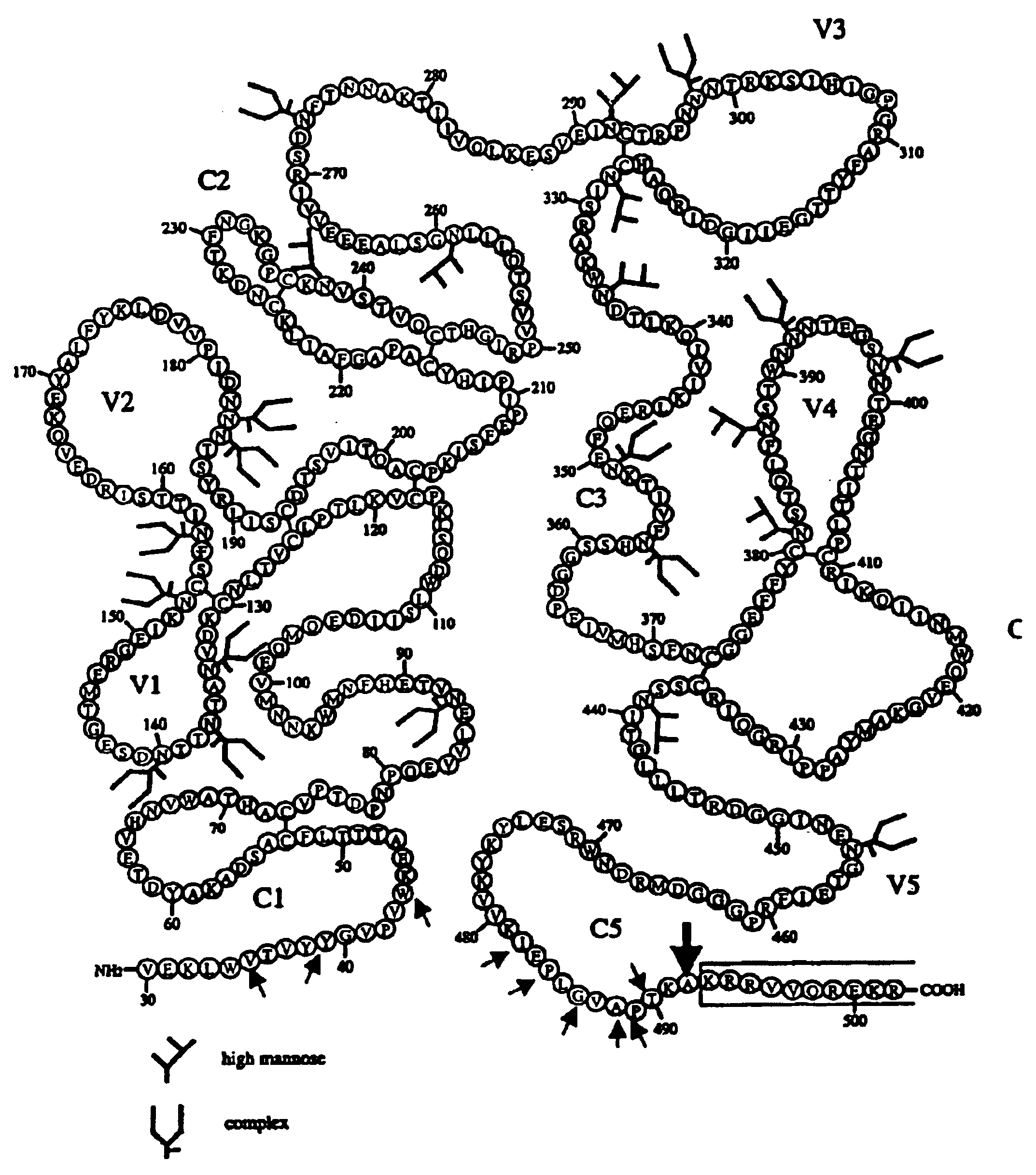
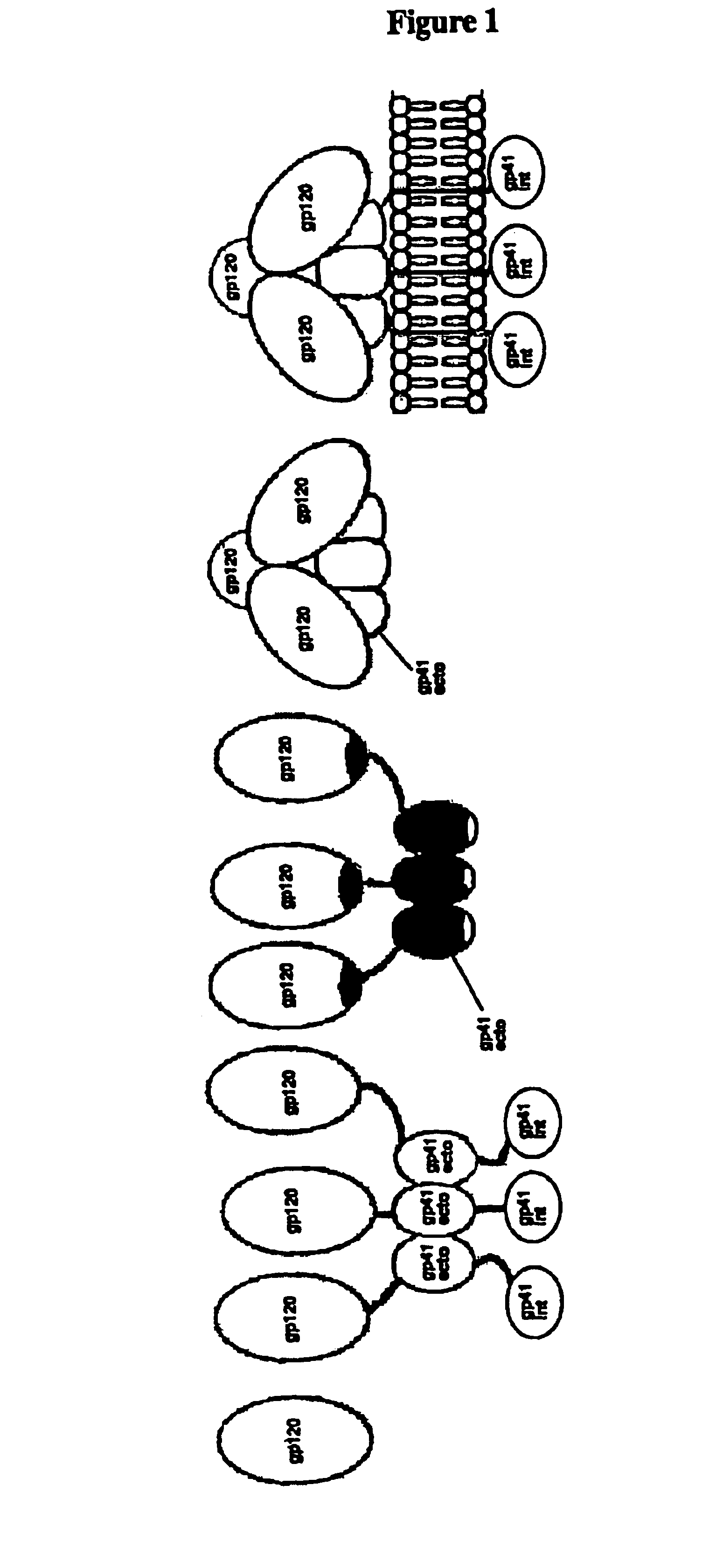
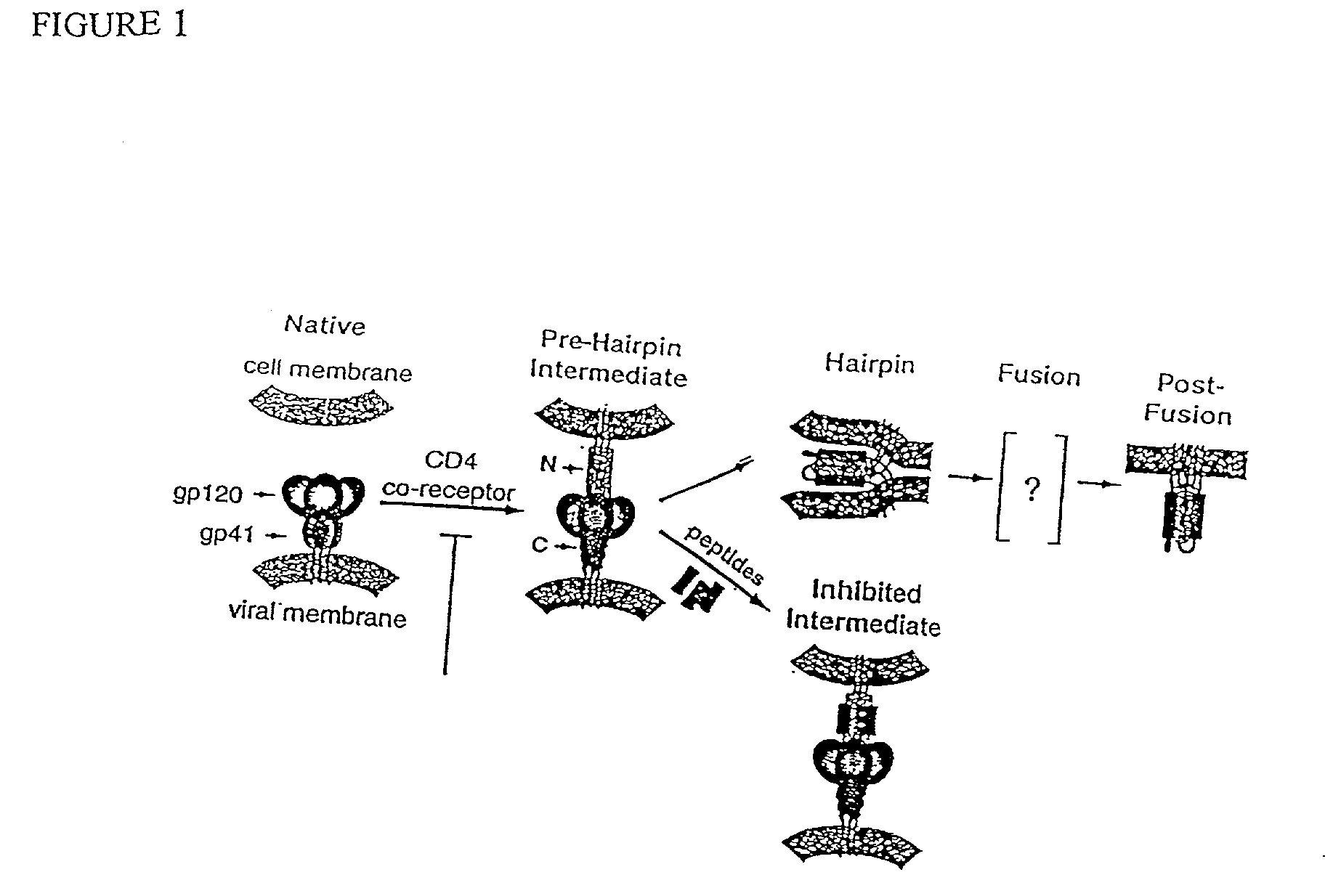
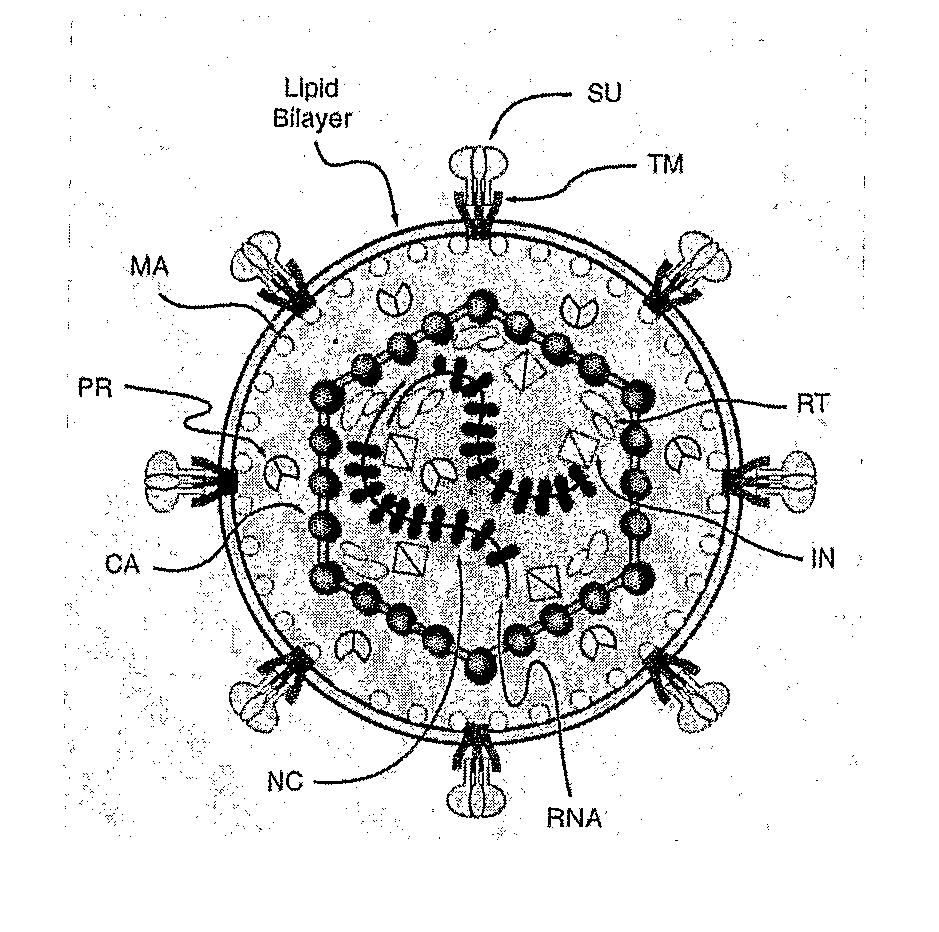
Some viruses (e.g. HIV and many animal viruses) have viral envelopes covering their protective protein capsids. The envelopes are typically derived from portions of the host cell membranes (phospholipids and proteins), but include some viral glycoproteins. They may help viruses avoid the host immune system. Glycoproteins on the surface of the envelope serve to identify and bind to receptor sites on the host's membrane. The viral envelope then fuses with the host's membrane, allowing the capsid and viral genome to enter and infect the host.
Recombinant viruses displaying a nonviral polypeptide on their external surface
InactiveUS6297004B1Genetic material ingredientsImmunological disordersGene deliveryAntibody fragments
We have made retrovirus particles displaying a functional antibody fragment. We fused the gene encoding an antibody fragment directed against a hapten with that encoding the viral envelope protein (Pr80env) of the ecotropic Moloney murine leukemia virus. The fusion gene was co-expressed in ecotropic retroviral packaging cells with a retroviral plasmid carrying the neomycin phosphotransferase gene (neo), and retroviral particles with specific hapten biding activities were recovered. Furthermore the hapten-binding particles were able to transfer the neo gene and the antibody-envelope fusion gene to mouse fibroblasts. In principle, the display of antibody fragments on the surface of recombinant retroviral particles could be used to target virus to cells for gene delivery, or to retain the virus in target tissues, or for the construction of libraries of viral display packages.
Owner:BIOFOCUS DICOVERY
Methods for the inhibition of epstein-barr virus transmission employing anti-viral peptides capable of abrogating viral fusion and transmission
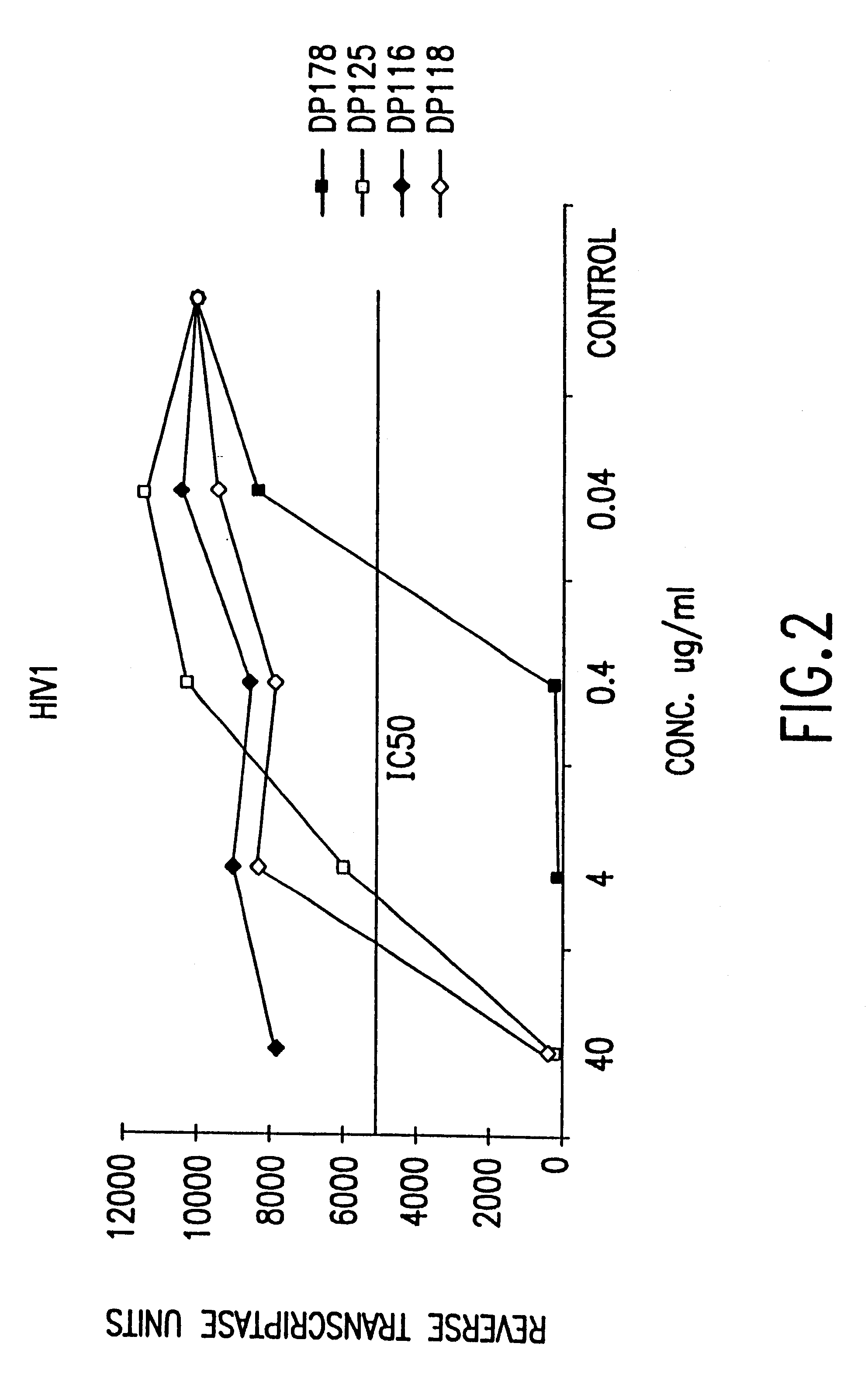
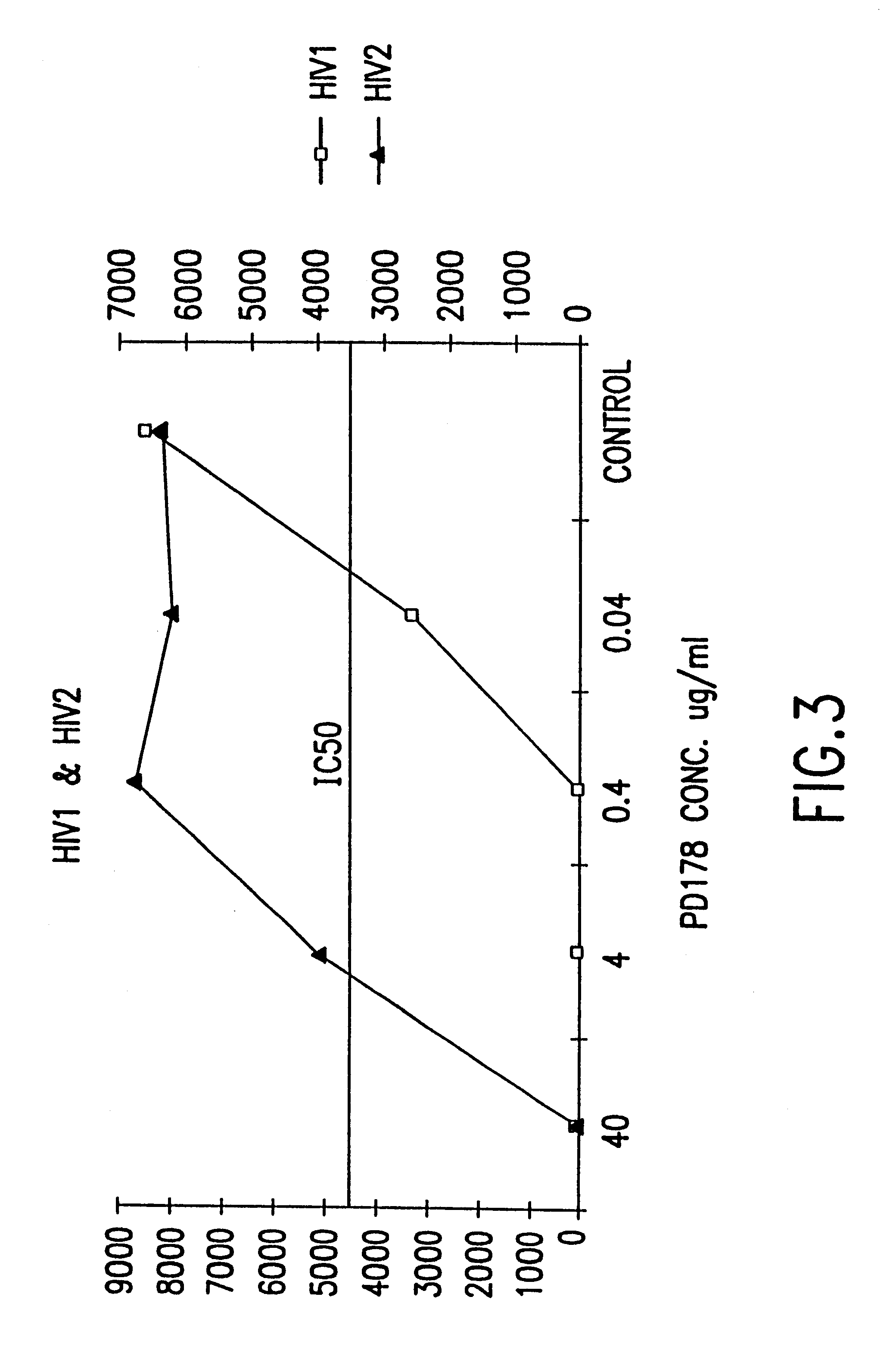
InactiveUS6518013B1SsRNA viruses negative-sensePeptide/protein ingredientsCell membraneViral life cycle
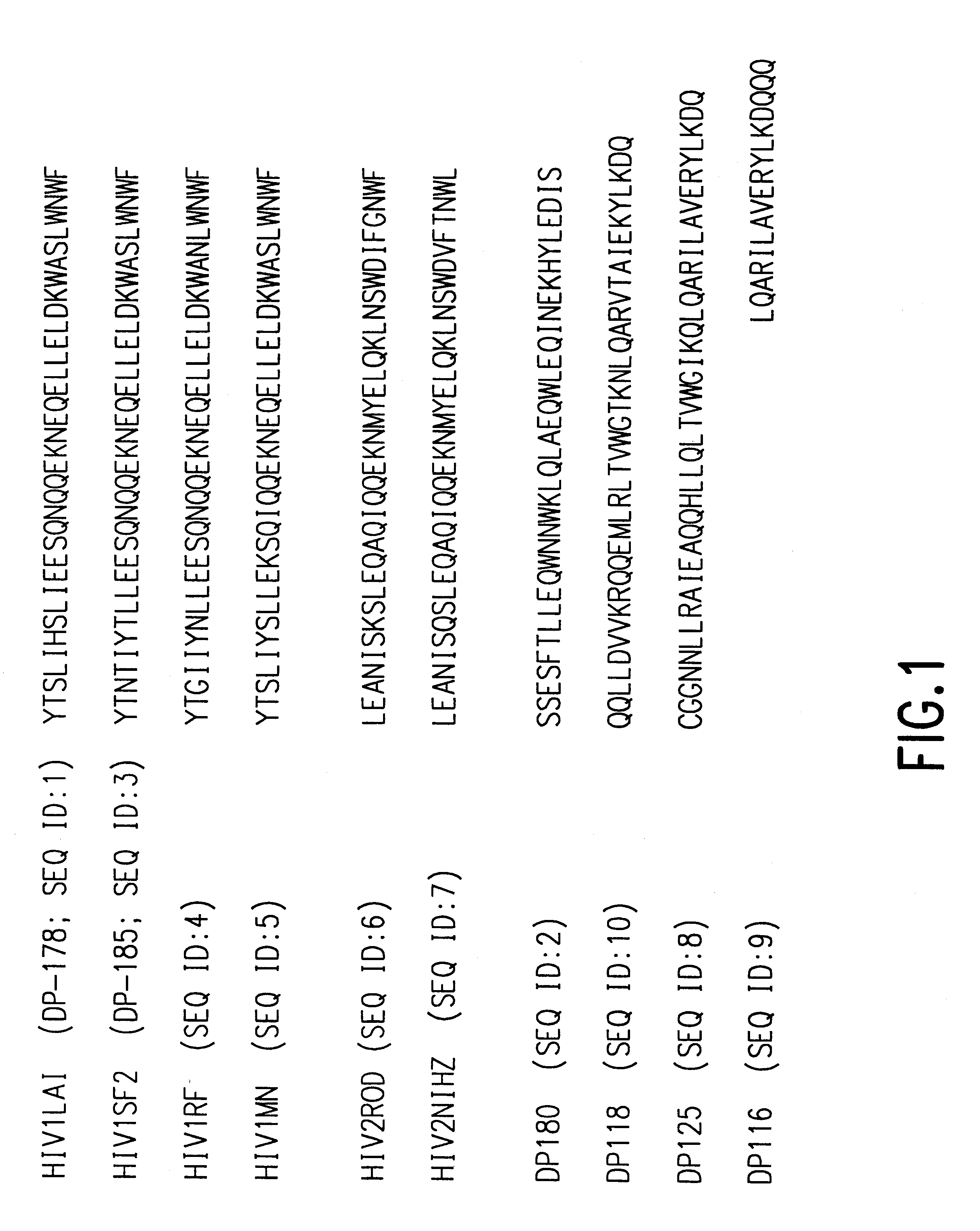
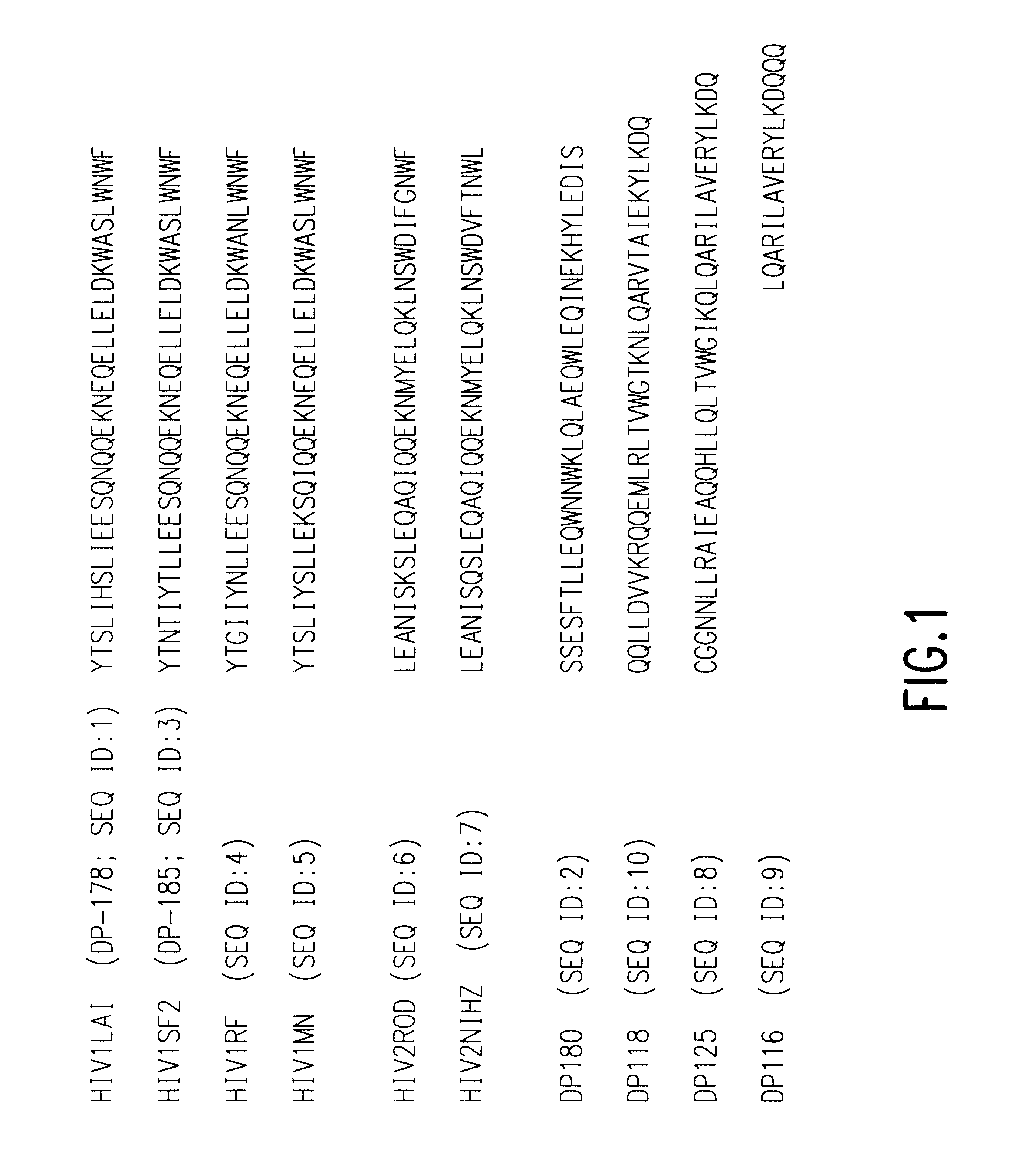
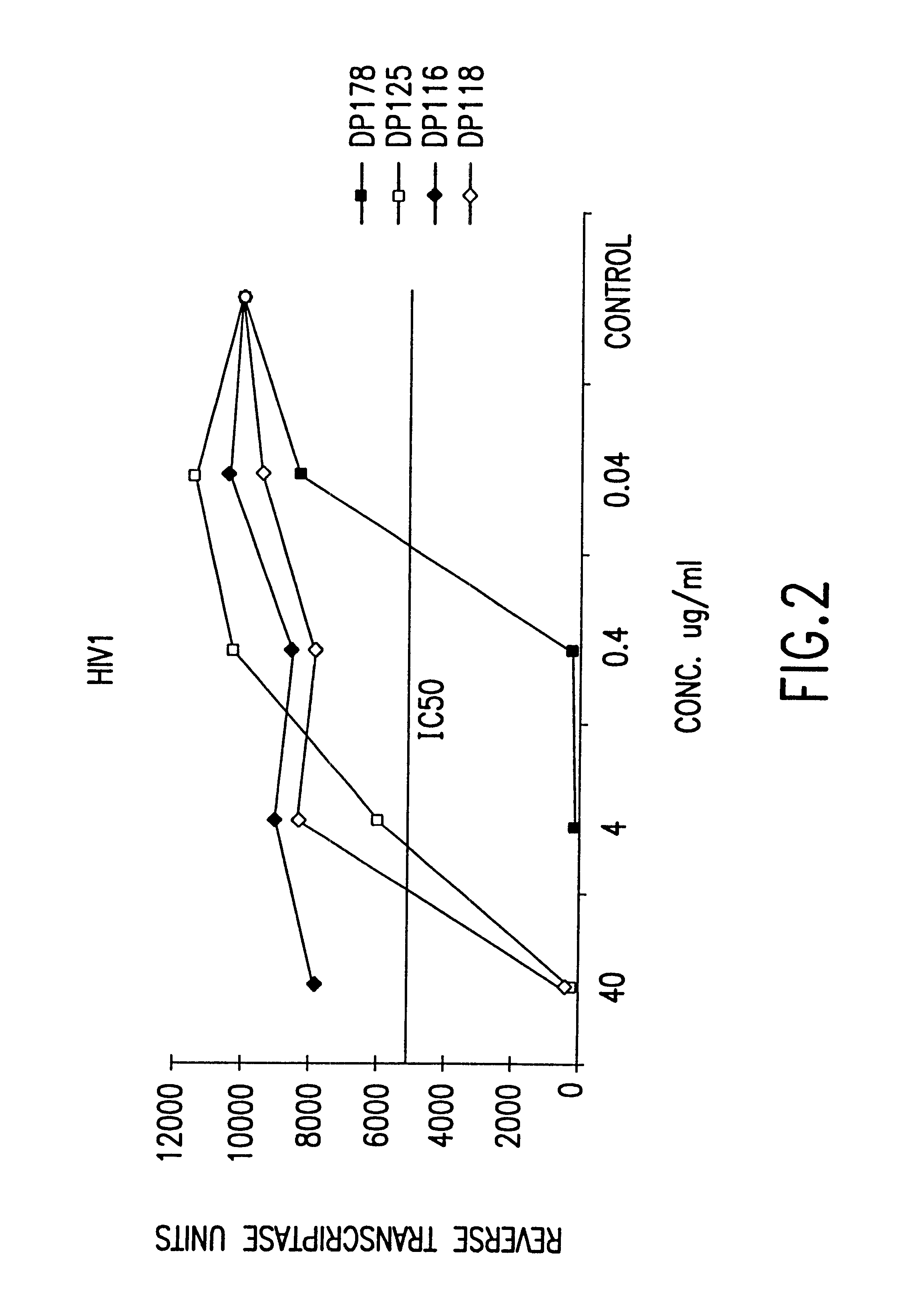
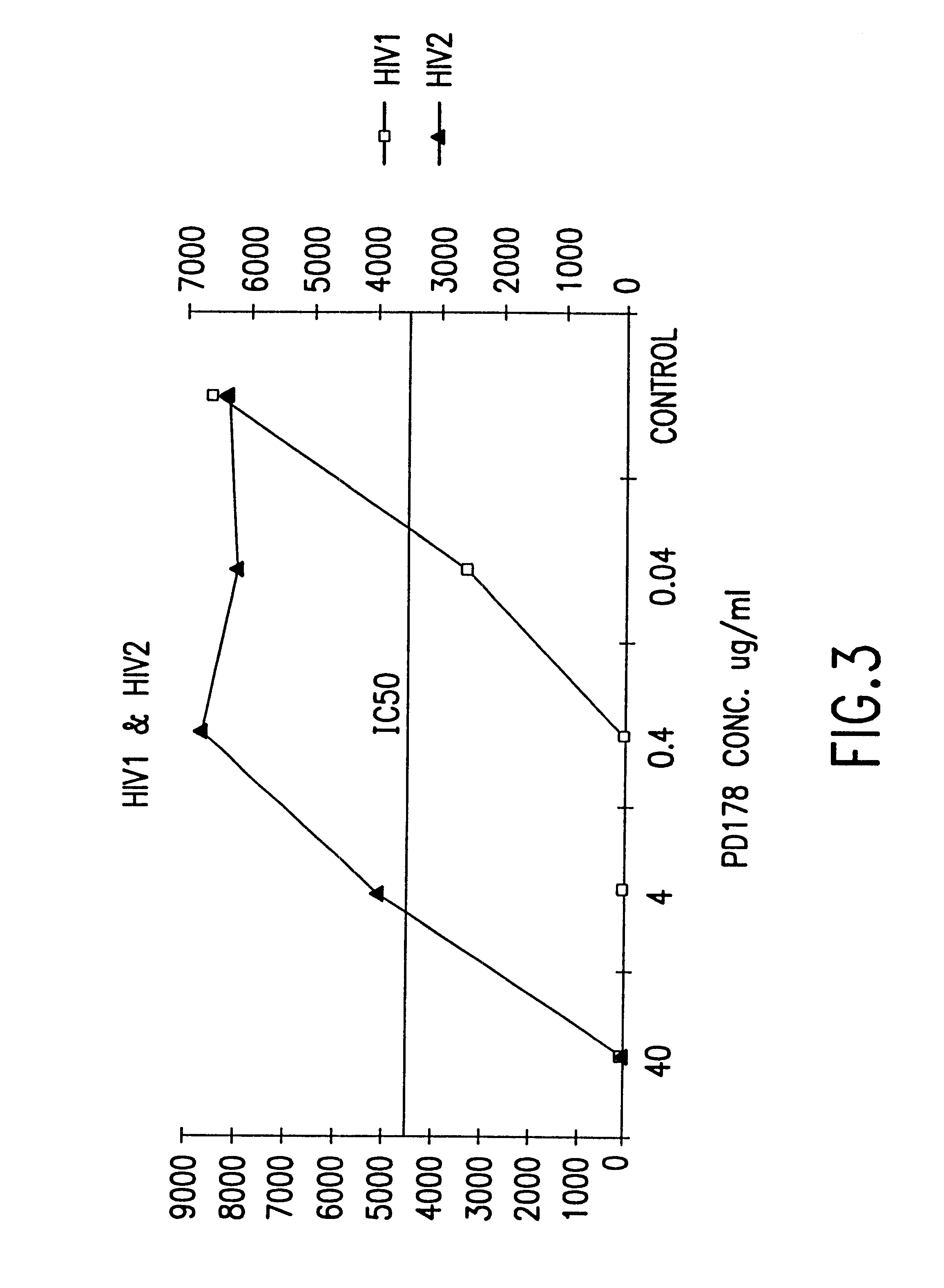
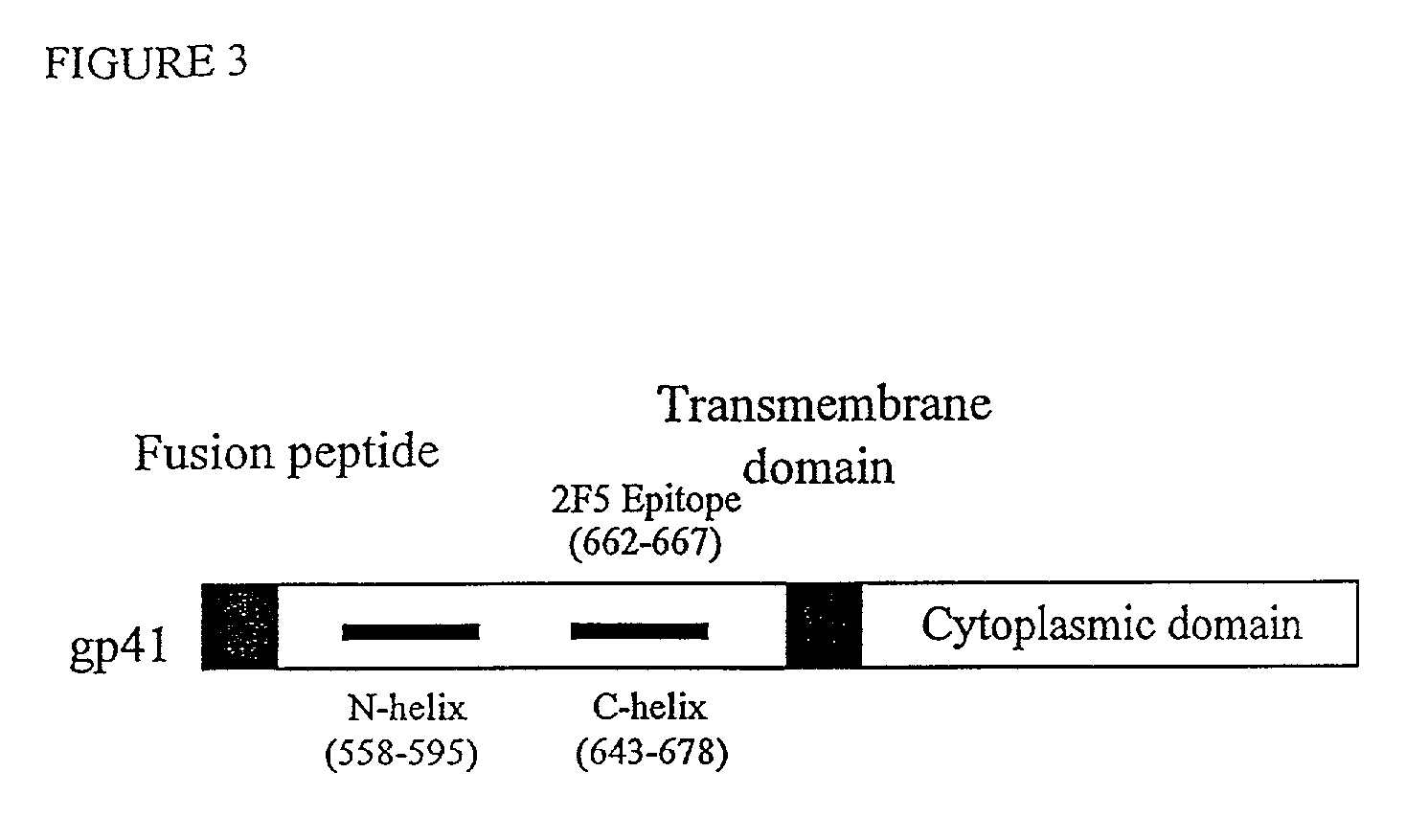
Fusion of the viral envelope, or infected cell membranes with uninfected cell membranes, is an essential step in the viral life cycle. Recent studies involving the human immunodeficiency virus type 1(HIV-1) demonstrated that synthetic peptides (designated DP-107 and DP-178) derived from potential helical regions of the transmembrane (TM) protein, gp41, were potent inhibitors of viral fusion and infection. A computerized antiviral searching technology (C.A.S.T.) that detects related structural motifs (e.g., ALLMOTI 5, 107x178x4, and PLZIP) in other viral proteins was employed to identify similar regions in the Epstein-Barr virus (EBV). Several conserved heptad repeat domains that are predicted to form coiled-coil structures with antiviral activity were identified in the EBV genome. Synthetic peptides of 16 to 39 amino acids derived from these regions were prepared and their antiviral activities assessed in a suitable in vitro screening assay. These peptides proved to be potent inhibitors of EBV fusion. Based upon their structural and functional equivalence to the known HIV-1 inhibitors DP-107 and DP-178, these peptides should provide a novel approach to the development of targeted therapies for the treatment of EBV infections.
Owner:TRIMERIS
Stabilized viral envelope proteins and uses thereof
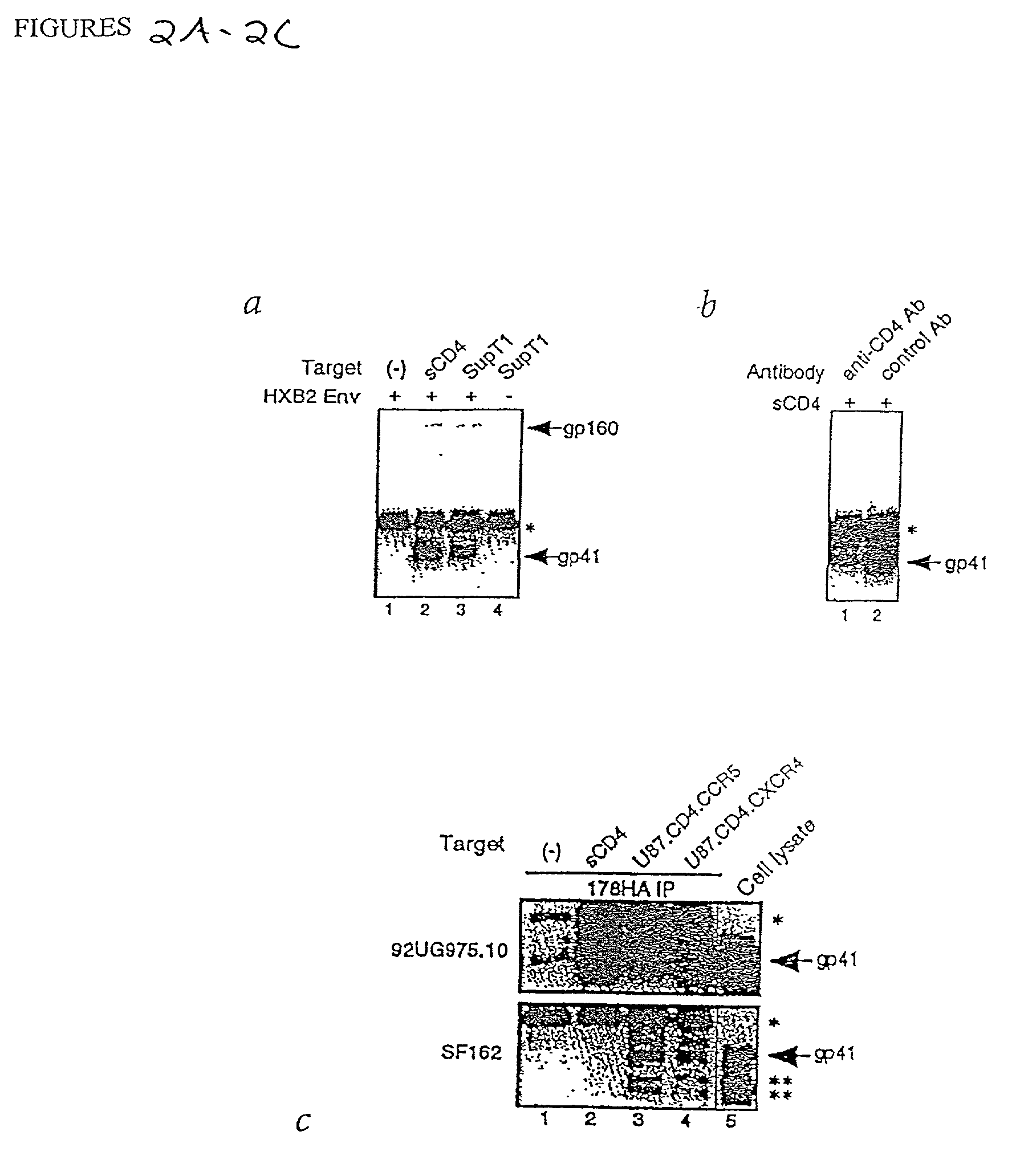
This invention provides an isolated nucleic acid which comprises a nucleotide segment having a sequence encoding a viral envelope protein comprising a viral surface protein and a corresponding viral transmembrane protein wherein the viral envelope protein contains one or more mutations in amino acid sequence that enhance the stability of the complex formed between the viral surface protein and transmembrane protein. This invention also provides a viral envelope protein comprising a viral surface protein and a corresponding viral transmembrane protein wherein the viral envelope protein contains one or more mutations in amino acid sequence that enhance the stability of the complex formed between the viral surface protein and transmembrane protein. This invention further provides methods of treating HIV-1 infection.
Owner:PROGENICS PHARMA INC
Methods for producing high titre vectors and compositions used in such methods
InactiveUS6969598B2Reduce chanceQuantity minimizationGenetic material ingredientsVirus peptidesNucleotideViral envelope
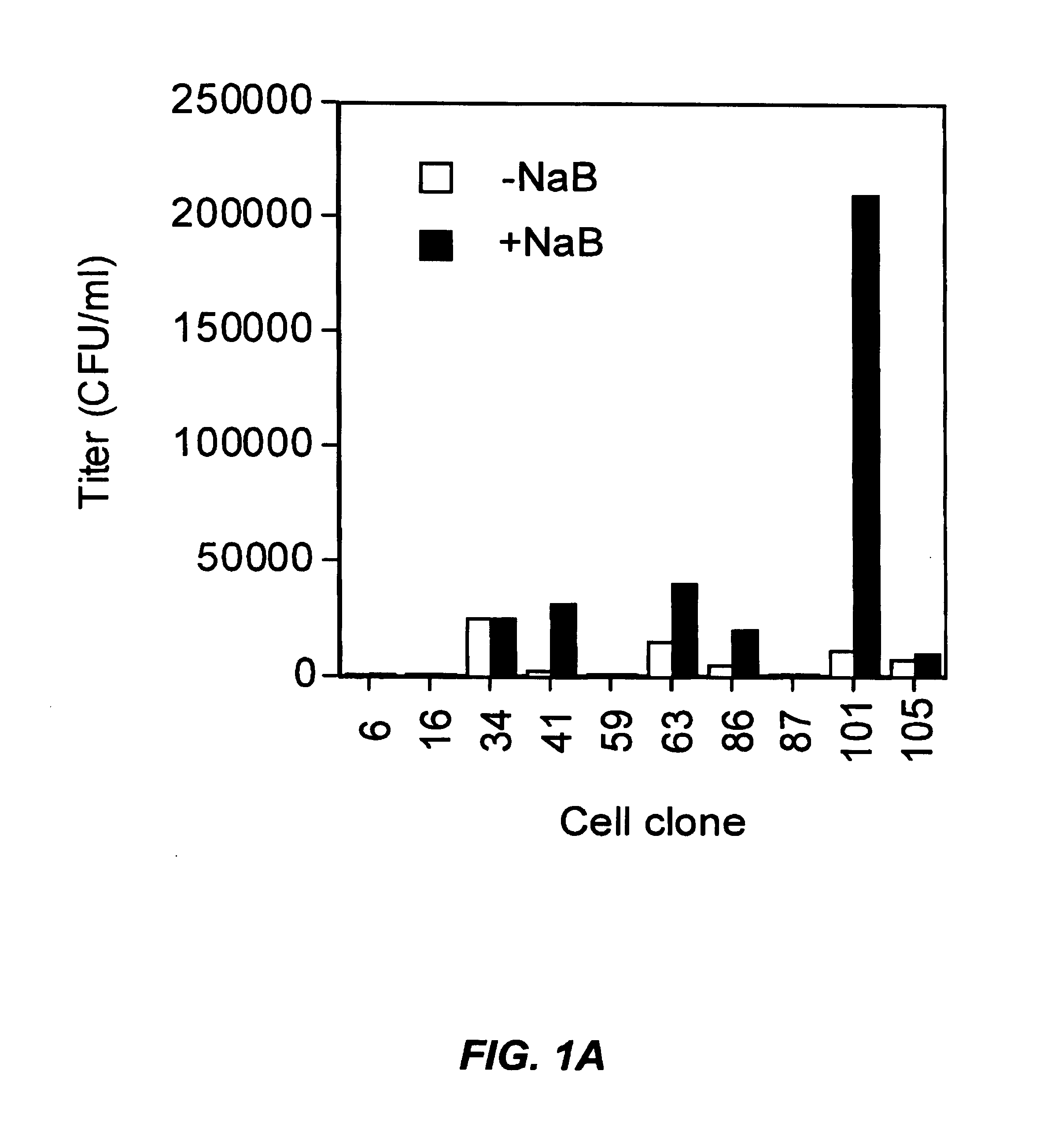
A method for producing viral vectors is described using packaging and producer cell lines is described. The producer cell comprises: (i) a first nucleotide sequence (NS) encoding a toxic viral envelope protein operably linked to a promoter; wherein the promoter is operably linked to at least one copy of a TRE; (ii) a second NS wherein the second NS comprises a sequence encoding a tetracycline modulator; (iii) a third NS encoding a retrovirus nucleocapsid protein; and (iv) a fourth NS comprising a retroviral sequence capable of being encapsidated in the nucleocapsid protein such that the retroviral vector particle titre obtainable from the producer cell is regulatable by tetracycline and an initial stimulus with sodium butyrate or functional analogues thereof.
Owner:OXFORD BIOMEDICA (UK) LTD +1
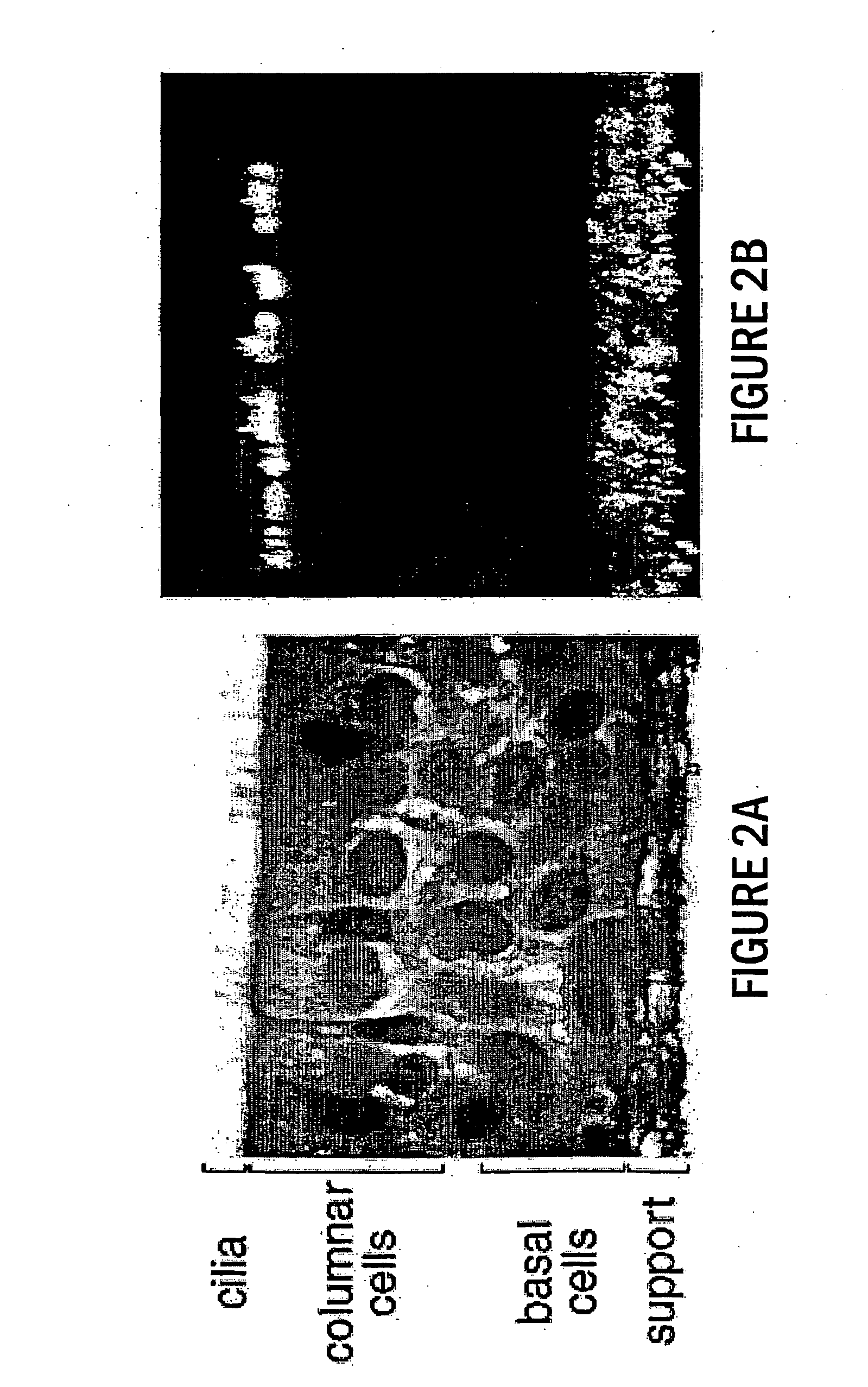
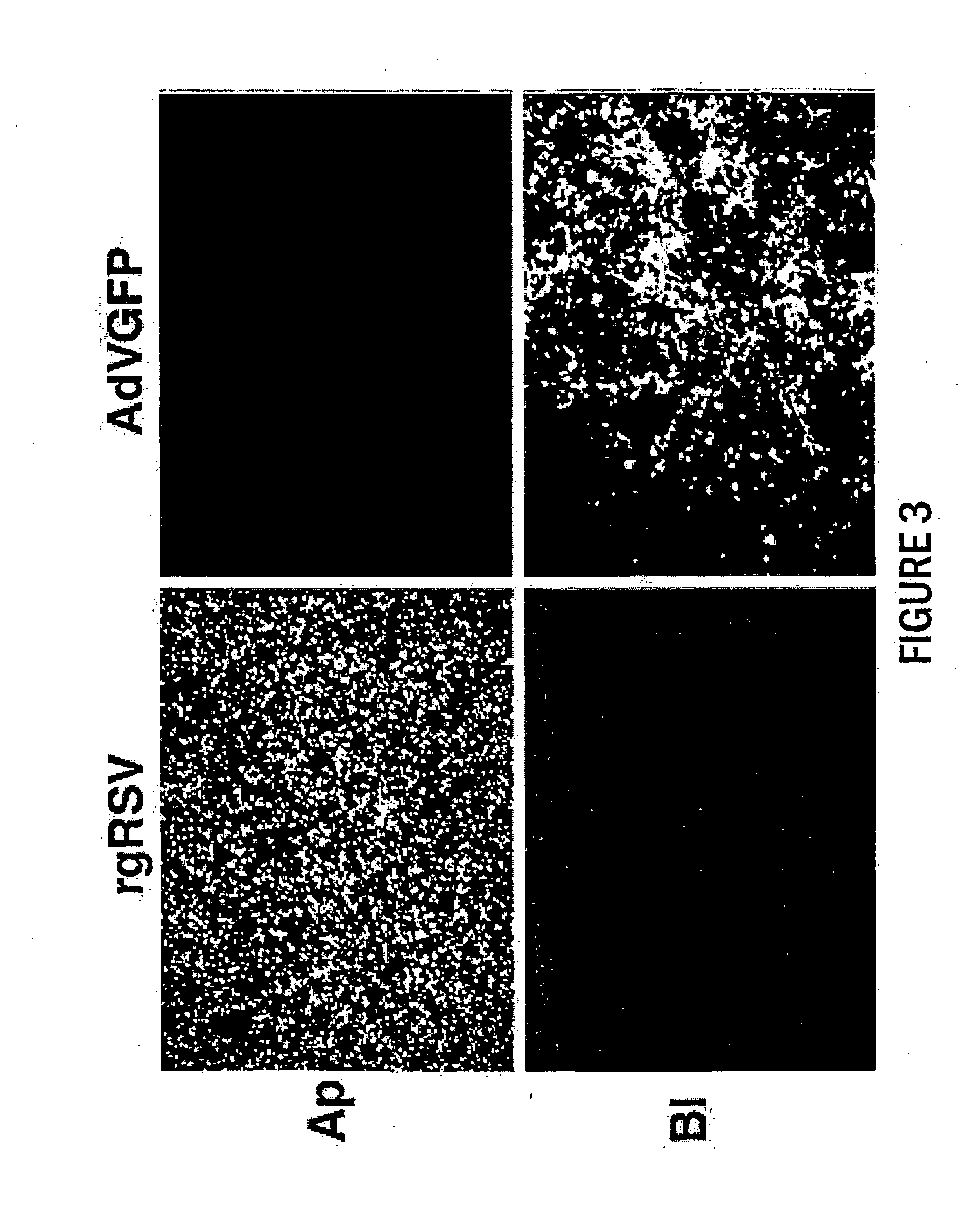
Paramyxoviruses as gene transfer vectors to lung cells
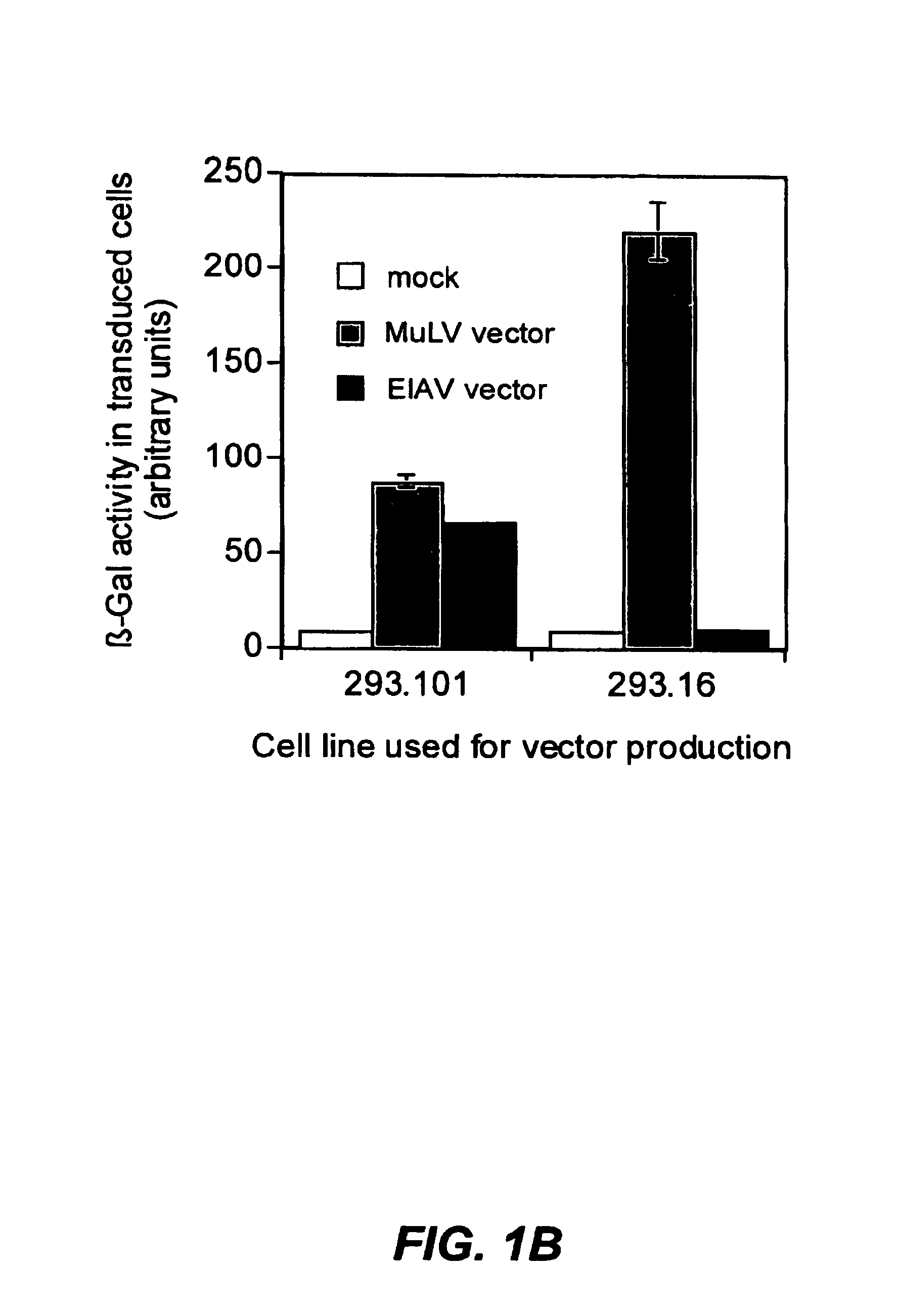
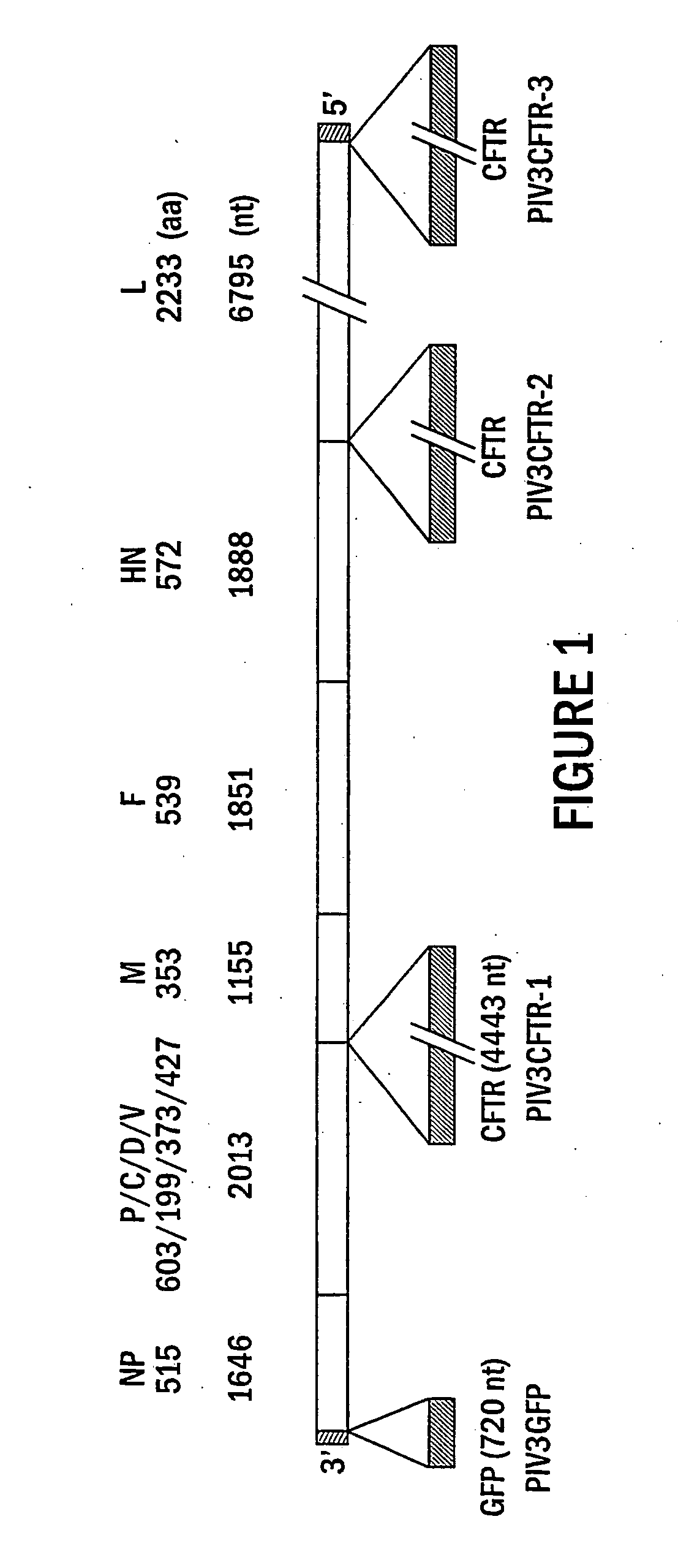
The present invention provides infectious recombinant viral vectors (e.g., parainfluenza virus (PIV) and a respiratory syncytial virus (RSV) vectors) comprising a viral genome comprising a heterologous nucleic acid of interest. Also provided are pseudotyped recombinant viral vectors comprising (i) a viral envelope and (ii) a viral genome comprising heterologous nucleic acids of interest. The viral envelope comprises a structural protein selected from the group consisting of envelope proteins from PIV and / or RSV. Further provided are methods of delivering heterologous nucleic acids of interest into airway epithelial cells comprising introducing viral vectors of the present invention comprising nucleic acids of interest into airway epithelial cells so that the nucleic acids of interest are expressed therein.
Owner:RUSH PRESBYTERIAN ST LUKES MEDICAL CENT +2
Production of virus and purification of viral envelope proteins for vaccine use
Immunogenic envelope glycoproteins are produced from enveloped virus, such as of the paramyxoviridae family, particularly PIV-3 and RSV, by culturing the virus in the substantial absence of exogenous serum proteins, isolating the virus from the tissue culture, solubilizing the envelope glycoproteins and isolating the solubilized envelope glycoproteins by chromatography.
Owner:CONNAUGHT LAB
Methods for the inhibition of respiratory syncytial virus transmission
Fusion of the viral envelope, or infected cell membranes with uninfected cell membranes, is an essential step in the viral life cycle. Recent studies involving the human immunodeficiency virus type 1 (HIV-1) demonstrated that synthetic peptides (designated DP-107 and DP-178) derived from potential helical regions of the transmembrane (TM) protein, gp41, were potent inhibitors of viral fusion and infection. A computerized antiviral searching technology (C.A.S.T.) that detects related structural motifs (e.g., ALLMOTI5, 107x178x4, and PLZIP) in other viral proteins was employed to identify similar regions in the respiratory syncytial virus (RSV). Several conserved heptad repeat domains that are predicted to form coiled-coil structures with antiviral activity were identified in the RSV genome. Synthetic peptides of 16 to 39 amino acids derived from these regions were prepared and their antiviral activities assessed in a suitable in vitro screening assay. These peptides proved to be potent inhibitors of RSV fusion. Based upon their structural and functional equivalence to the known HIV-1 inhibitors DP-107 and DP-178, these peptides should provide a novel approach to the development of targeted therapies for the treatment of RSV infections.
Owner:TRIMERIS
Composition Having Antitumor Effect
ActiveUS20080226674A1Improve immunityImproving tumor antigen-presenting capabilitySsRNA viruses negative-senseHeavy metal active ingredientsAntitumor immunityAdjuvanticity
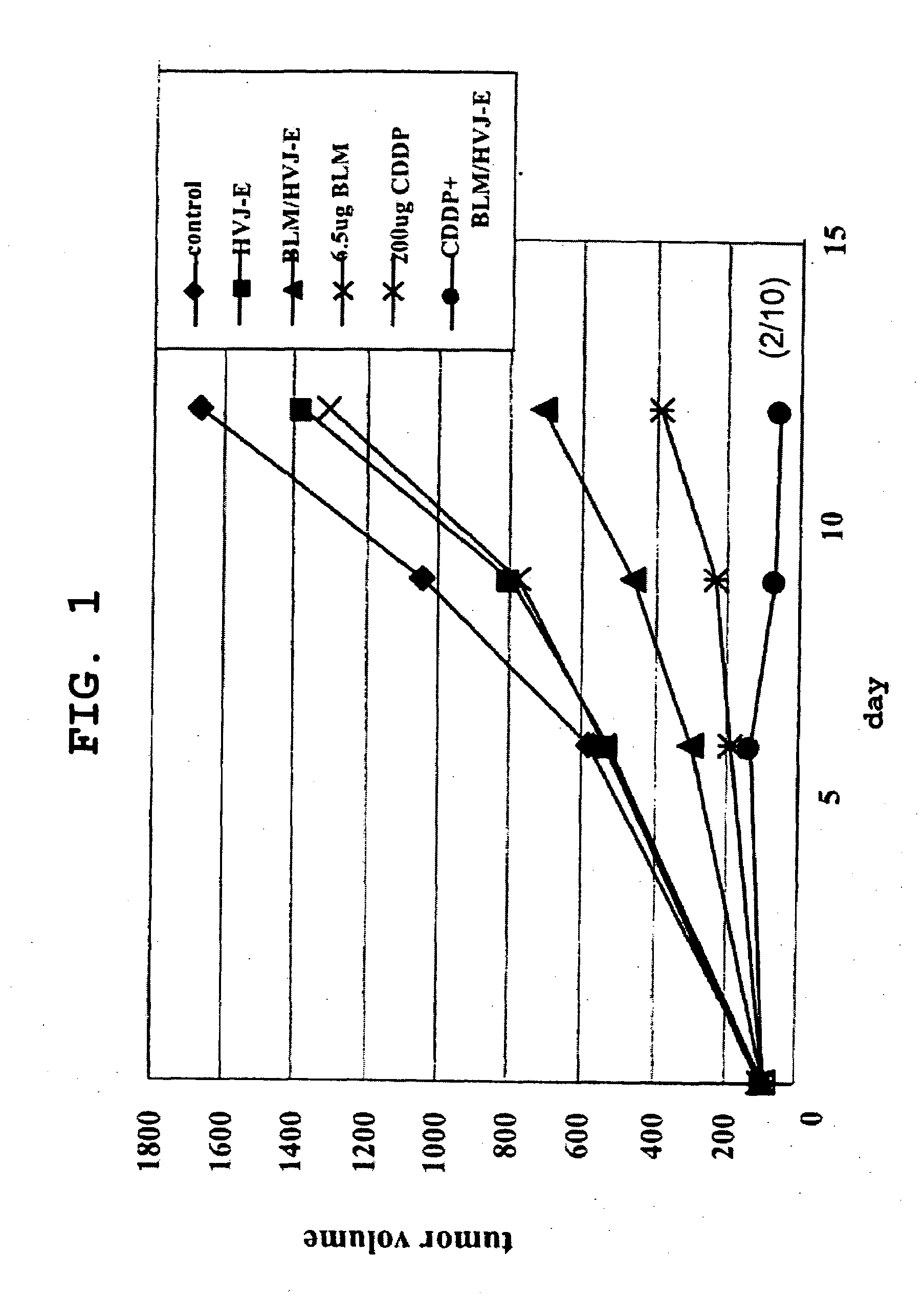
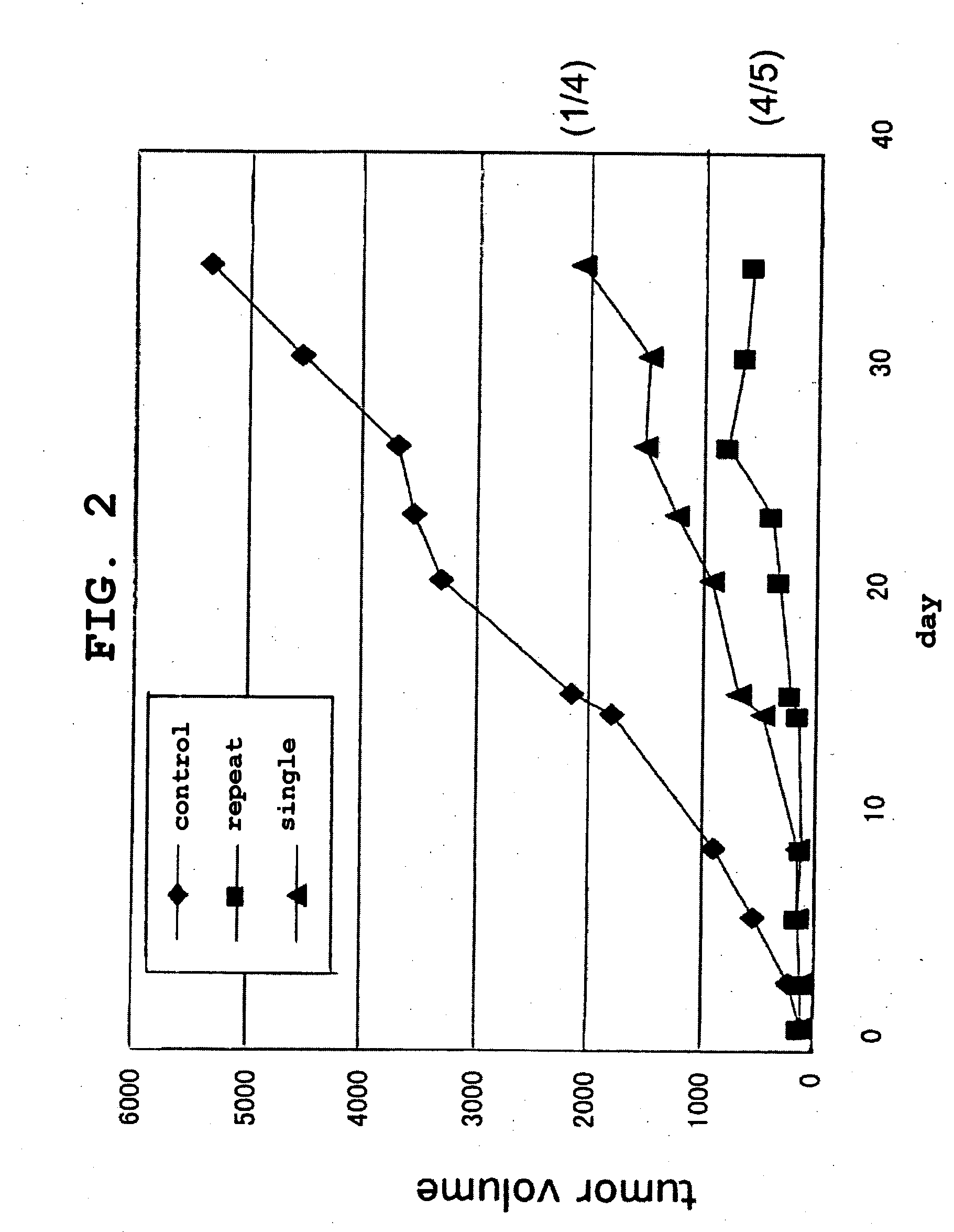
The present invention is intended to provide a pharmaceutical composition for delivering a chemotherapeutic, preferably an anticancer drug, into cells or into a living organism, using a viral envelope vector, and provides a pharmaceutical composition comprising a chemotherapeutic encapsulated in, or used in combination with, a viral envelope vector having an adjuvanticity as an active ingredient. Thereby it is possible to introduce an anticancer drug encapsulated in a viral envelope vector directly into a tumor, with coadministration of another anticancer drug so as to induce tumor cell-specific antitumor immunity also thanks to the adjuvant action of HVJ-E, and hence to regress the tumor. The present invention also provides a pharmaceutical composition comprising a viral envelope vector and a chemotherapeutic as active ingredients.
Owner:IMMUNOMEDICINE INC
Scavenger Receptor B1 (Cla-1) Targeting for the Treatment of Infection, Sepsis and Inflammation
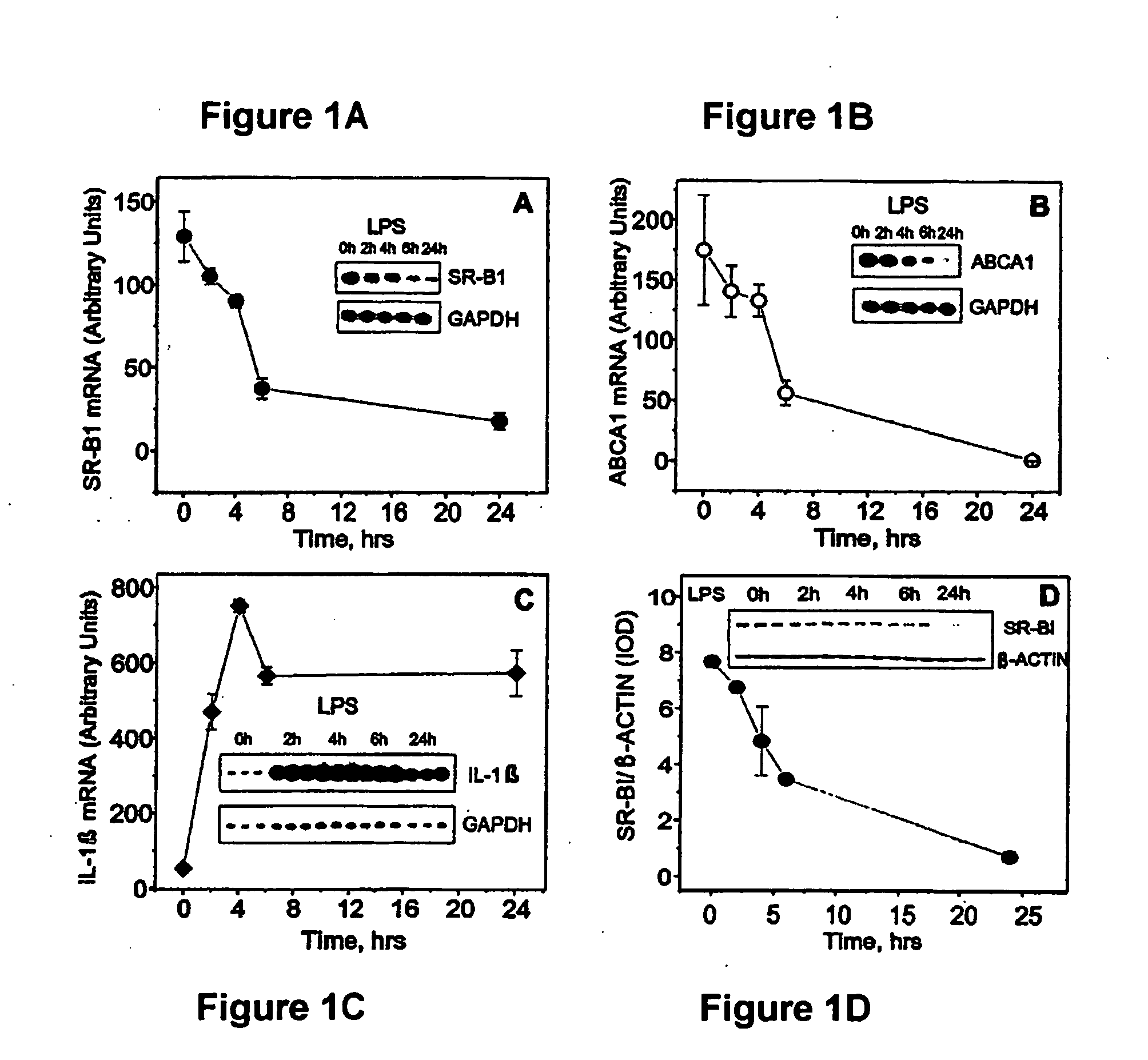
This invention relates to methods and compositions for the treatment of sepsis, inflammation or infection. In particular, the invention concerns the use of molecule(s) that target SR-BI, which is also referred to as CLA-1 (SR-BI / CLA-1), to treat sepsis, bacterial and viral infections, and inflammatory diseases. SRB I / CLA-1 ligands contributing to the pathogenesis of disease include LPS, LTA, viral envelope proteins, beta-amyloid, serum Amyloid A and / or heat shock proteins.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NIH
Virus-like particles (VLPs) comprising heterologous multiple membrane spanning proteins
Enveloped virus vectors are described which comprise a cellular virus receptor protein and which are capable of fusing with a cell which comprises a viral envelope protein to which the cellular virus receptor protein is cognate. Enveloped virus vectors comprising a plurality of cellular virus receptor proteins are also described. Methods for making the enveloped virus vectors are described, as are methods of using the enveloped virus vectors. The invention further relates to a lipoparticle comprising a membrane spanning protein, and the lipoparticle can be attached to a sensor surface. The invention relates to methods of producing and using the lipoparticle to, inter alia, assess protein binding interactions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Materials and methods relating to the transfer of nucleic acid into quiescent cells
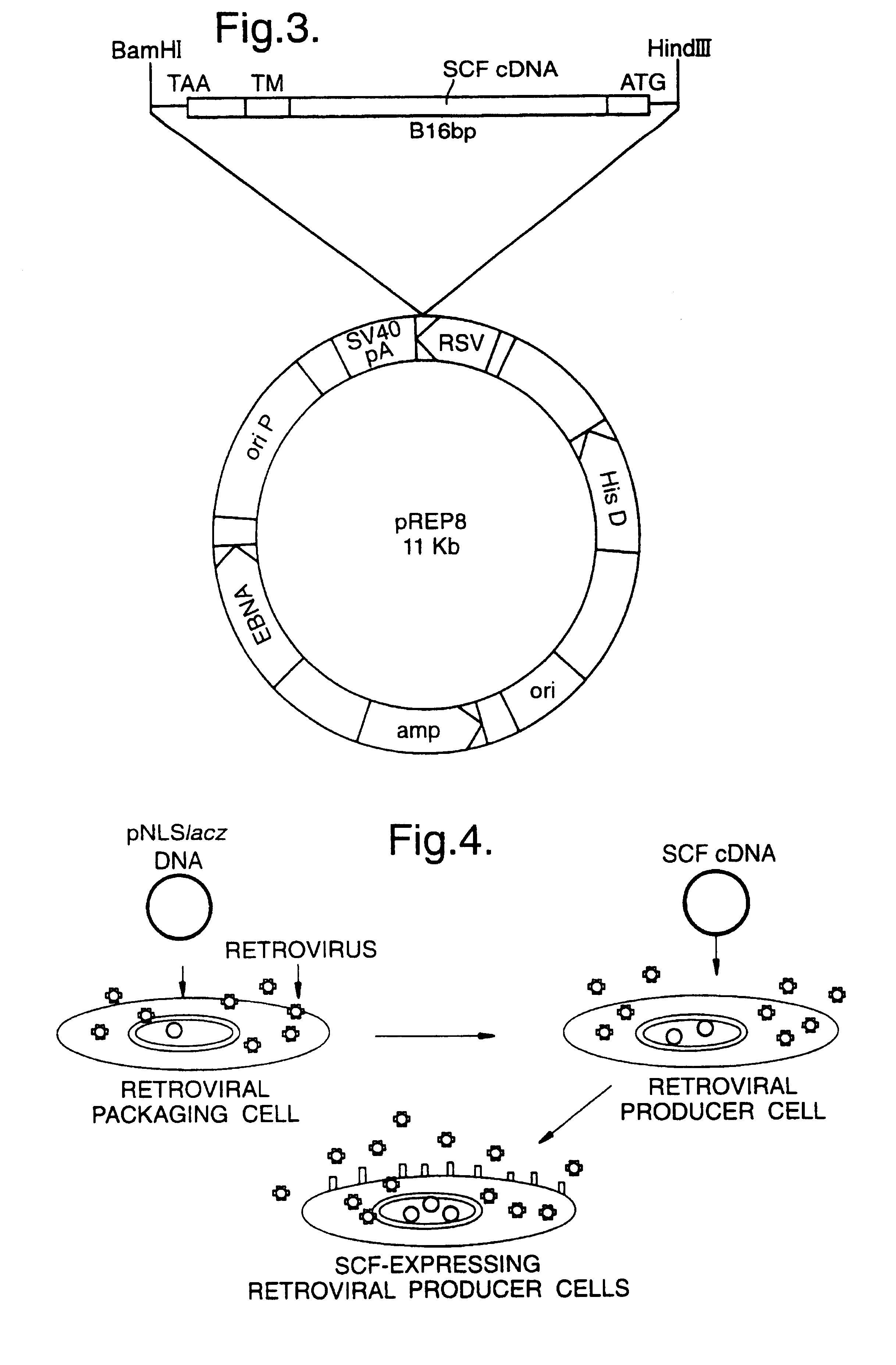
Materials and methods for transferring nucleic acid encoding a polypeptide for treating a disease or disorder into populations of quiescent cells such as haematopoietic stem cells (HSCs), using retroviral packaging cell lines and retroviral particles expressing and display a growth factor such as stem cell factor (SCF) on the cell surface or as a fusion with a viral envelope protein. The present invention also relates to compositions comprising the retroviral packaging cell lines and retroviral particles, and their use in methods of medical treatment, in vivo and ex vivo.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES +1
Virosome-like-particles
ActiveUS7901920B2Effective dissolutionAntibacterial agentsSsRNA viruses negative-senseVirosomePhospholipid
The invention relates to the production of virosome-like-particles. The invention provides a method for producing a virosome-like-particle comprising contacting an enveloped virus with a solution containing a short-chain phospholipid allowing solubilisation of the viral envelope of said virus further comprising removing short-chain phospholipid from said solution allowing formation of a functionally reconstituted viral envelope.
Owner:BESTEWIL HOLDING BV
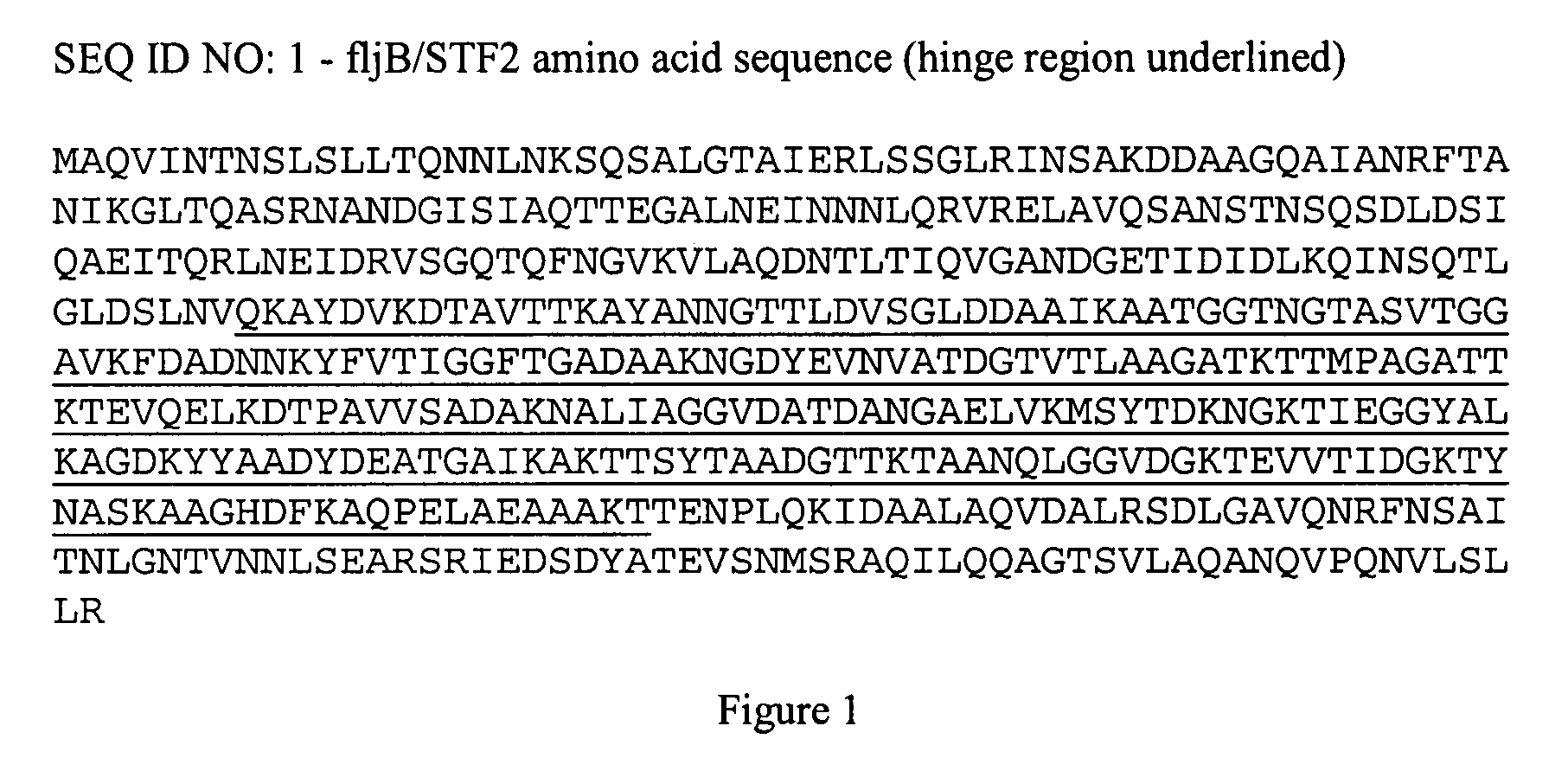
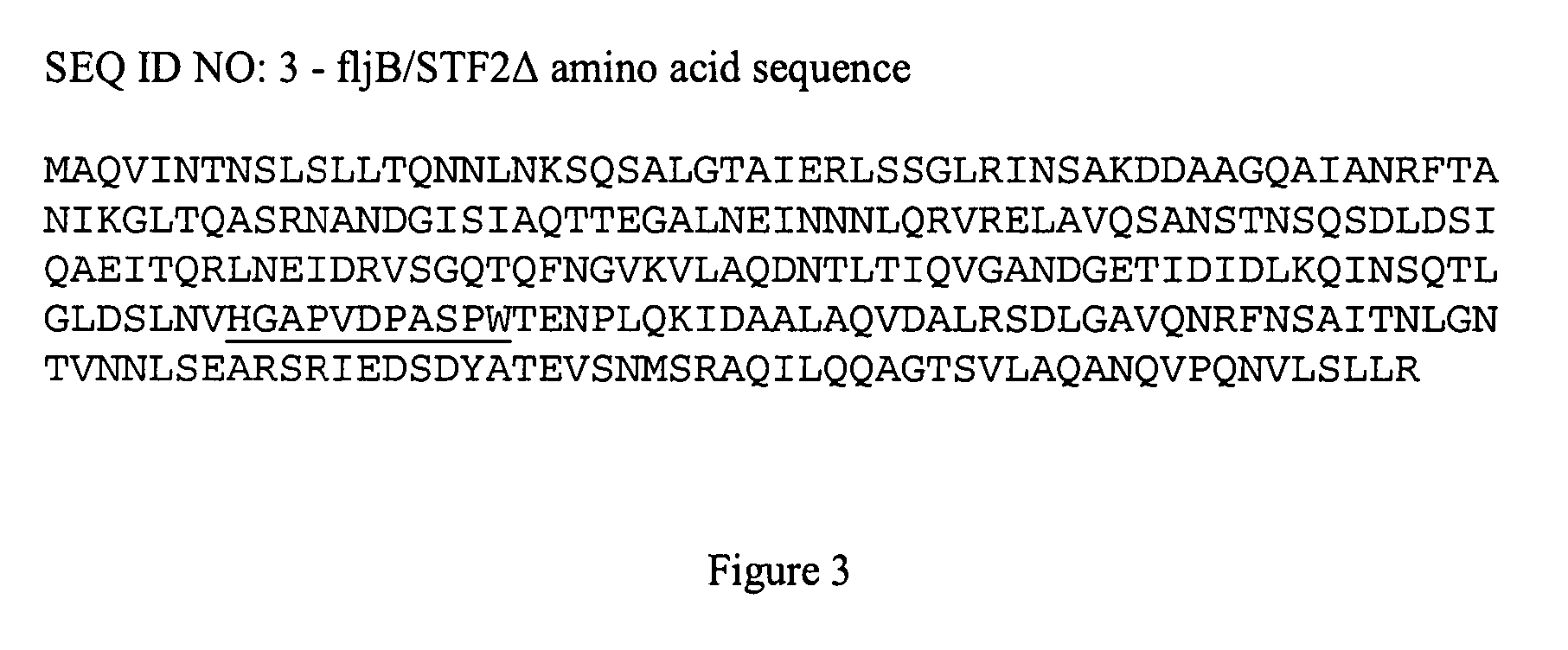
Fusion proteins comprising flagellin and dengue viral envelope proteins
InactiveUS8574588B2Effective immunogencityEliminate side effectsSsRNA viruses positive-sensePeptide/protein ingredientsViral envelopeProtective immunity
Fusion proteins comprise at least a portion of at least one flagellin and at least a portion of at least one Dengue viral envelope protein. The fusion proteins are used to stimulate an immune response and protective immunity in a subject.
Owner:VAXINNATE
Stabilized viral envelope proteins and uses thereof
InactiveUS20060094049A1Improve stabilityMicrobiological testing/measurementVirus peptidesNucleotideViral envelope
This invention provides an isolated nucleic acid which comprises a nucleotide segment having a sequence encoding a viral envelope protein comprising a viral surface protein and a corresponding viral transmembrane protein wherein the viral envelope protein contains one or more mutations in amino acid sequence that enhance the stability of the complex formed between the viral surface protein and transmembrane protein. This invention also provides a viral envelope protein comprising a viral surface protein and a corresponding viral transmembrane protein wherein the viral envelope protein contains one or more mutations in amino acid sequence that enhance the stability of the complex formed between the viral surface protein and transmembrane protein. This invention further provides methods of treating HIV-1 infection.
Owner:PROGENICS PHARMA INC +1
Virus coated nanoparticles and uses thereof
The present invention discloses method to coat nanoparticles with viral envelope containing specific proteins. The present invention also discloses that such viral envelope coated nanoparticles can be targeted to specific cells and cellular entry pathway, thereby permitting their use as vaccines, in targeted delivery of therapeutic products and in the study of virus adsorption, cell penetration and viral entry pathways.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
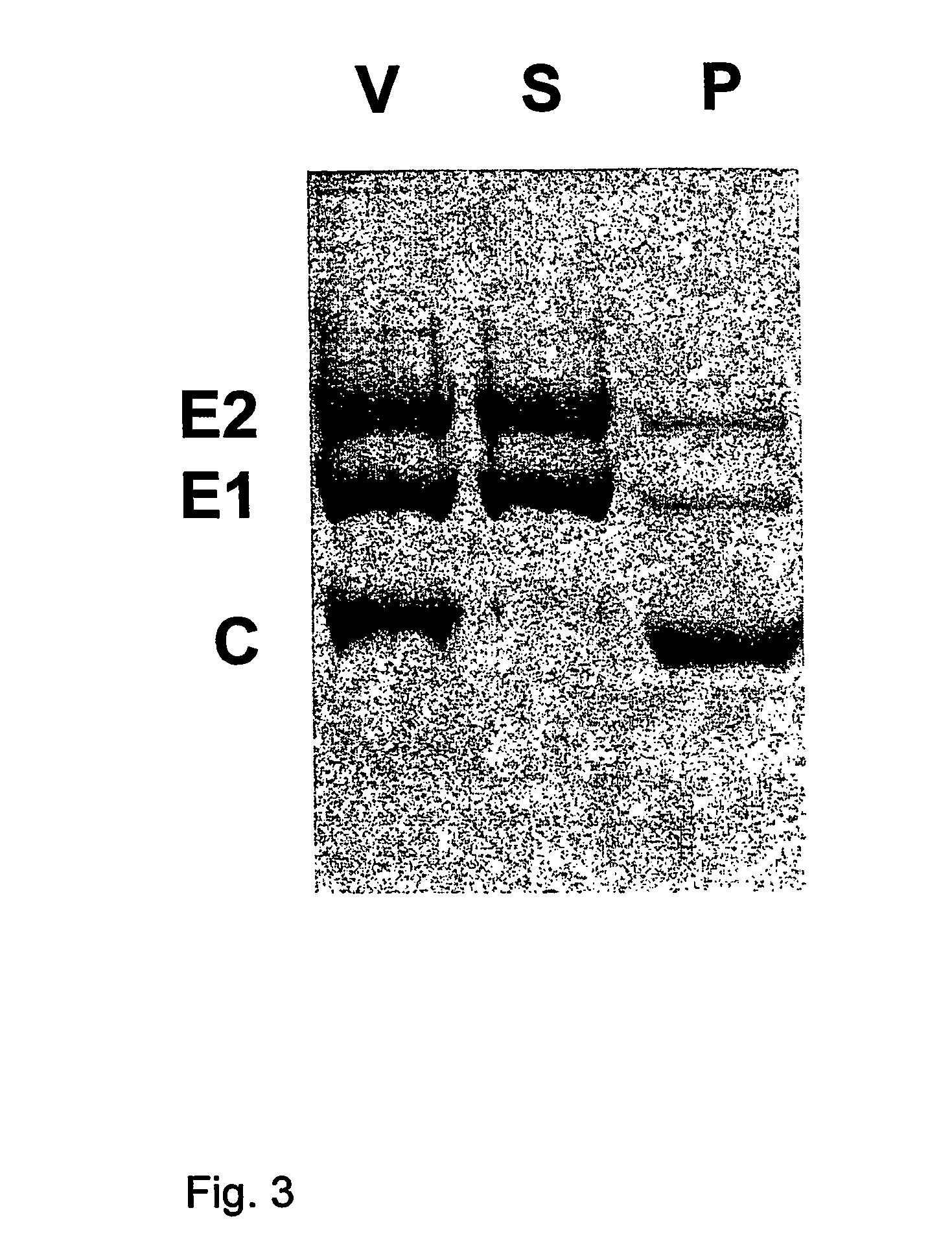
Epitopes in viral envelope proteins and specific antibodies directed against these epitopes: use for detection of HCV viral antigen in host tissue
Antibodies to two new epitopes on the HCV envelope proteins were identified which allow routine detection of native HCV envelope antigens, in tissue or cells derived from the host. The new epitopes are: the E1 region aa 307-326 and the N-terminal hyper variable region of E2 aa 395-415. Surprisingly, we characterised an antibody that reacts with various sequences of the hypervariable domain of E2. Specific monoclonal antibodies directed against these epitopes and allowing routine detection of viral antigen are described.
Owner:INNOGENETICS NV (NL)
Photoconductive antivirus fabric and manufacture method thereof
The invention discloses a photoconductive antivirus fabric and a manufacture method thereof. The photoconductive antivirus fabric prepared by the method comprises a photosensitive dye, a polyacrylic acid layer and a crosslinker and can absorb photons and act with surrounding oxygen to generate singlet oxygen under the illumination condition, the singlet oxygen can be oxidized to damage phospholipid and protein so as to damage a viral envelope and deactivate viral activity. For the protein, target amino acids to be damaged by the oxidized singlet oxygen comprise tryptophan, histidine, tyrosine, cysteine and methionine. The photoconductive antivirus fabric also acts on gram-positive bacteria and thus has the anti-virus and anti-bacterial action.
Owner:任浩明 +2
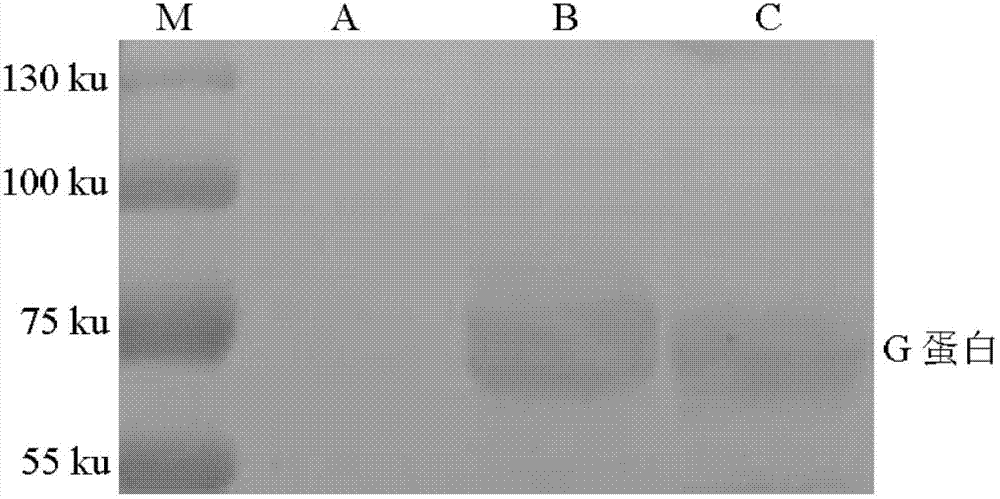
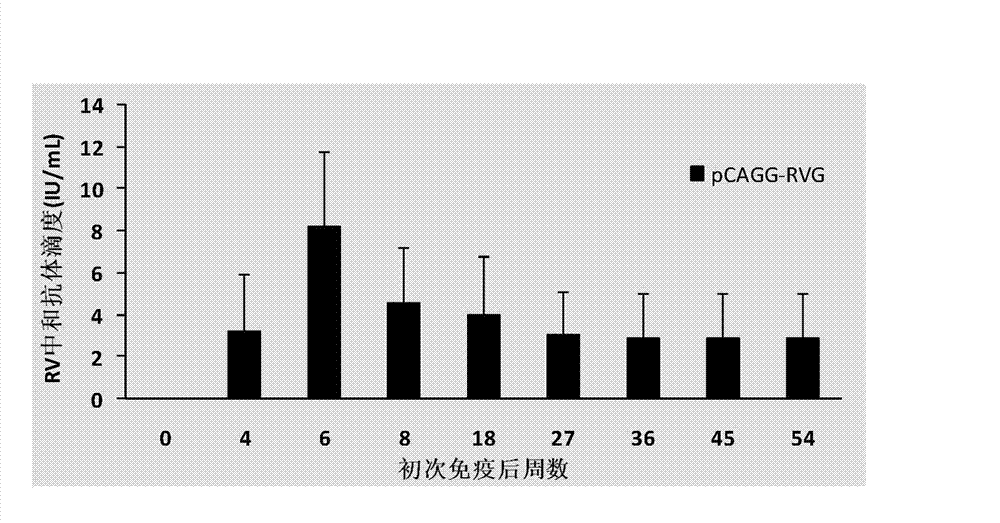
Building and application of hydrophobia DNA (deoxyribonucleic acid) vaccine
The invention provides recombinant eukaryotic expression plasmids of viral envelope glycoprotein (G protein) gene optimized according to mammal codon preference as expression of hydrophobia DNA (deoxyribonucleic acid) vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Anti-virus aluminum member and method for producing same
ActiveCN103781945AIncreased durabilityLong-term antiviralAnodisationAntiviralsAnti virusSecondary Infections
The present invention provides an anti-virus aluminum member capable of minimizing secondary infection by deactivating viruses in a short period of time even when viruses adhere thereto, regardless of whether a viral envelope is present, and useful for application in door knobs, handrails, air-conditioner fins or the like. An anti-virus aluminum member capable of deactivating viruses that adhere thereto is characterized in that an anti-virus inorganic compound is present in the pores of an anodized membrane provided with multiple pores and obtained by anodizing aluminum or an aluminum alloy.
Owner:NBC MESHTEC
Method for generating immunogens that elicit neutralizing antibodies against fusion-active regions of HIV envelope proteins
The current invention relates to methods of generating immunogens that elicit broadly neutralizing antibodies which target regions of viral envelope proteins such as the gp 120 / gp41 complex of HIV-1. More specifically, the current invention involves using stabilizing peptides modeling the alpha-helical regions of the ectodomain of the HIV-1 transmembrane protein to stabilize fusion-active intermediate structures which can be used as vaccine immunogens.
Owner:PANACOS PHARMACEUTICALS INC
Method for the preparation of virus-like particles (VLPS) comprising heterologous multiple membrane spanning proteins
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods of preparing lymphocytes that express interleukin-2 and their use in the treatment of cancer
A method of preparing autologus T-lymphocytes for re-introduction into a patient having cancer, which method comprises obtaining peripheral blood mononuclear cells (PBMCs) from a patient immunized with an antigen of the cancer, stimulating the PBMCs with the antigen of the cancer in vitro, transducing the PBMCs with a retrovial vector, which (a) comprises and expresses a human interleukin-2 (IL-2) coding sequence operably linked to a retroviral promoter, (b) does not comprise an exogenously introduced gene that enables phenotypic selection, and (c) comprises a viral envelope that efficiently transduces CD8+ T-lymphocytes; a method of preparing autologous tumor-infiltrating lymphocytes (TILs) for reintroduction into a patient having cancer, which method comprises obtaining TILs from a tumor of the patient, optionally immunized with an antigen of the cancer, optionally stimulating the TILs with the antigen of the cancer in vitro, and transducing the TILs with the aforementioned retroviral vector, compositions comprising cells obtained in accordance with such methods; and methods of treating a patient having cancer by administering to the patient cells obtained in accordance with such methods or compositions comprising same.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Composition having antitumor effect
ActiveUS7871765B2Improve immunityImproving tumor antigen-presenting capabilitySsRNA viruses negative-senseHeavy metal active ingredientsAntitumor immunityAdjuvanticity
The present invention is intended to provide a pharmaceutical composition for delivering a chemotherapeutic, preferably an anticancer drug, into cells or into a living organism, using a viral envelope vector, and provides a pharmaceutical composition comprising a chemotherapeutic encapsulated in, or used in combination with, a viral envelope vector having an adjuvanticity as an active ingredient. Thereby it is possible to introduce an anticancer drug encapsulated in a viral envelope vector directly into a tumor, with coadministration of another anticancer drug so as to induce tumor cell-specific antitumor immunity also thanks to the adjuvant action of HVJ-E, and hence to regress the tumor. The present invention also provides a pharmaceutical composition comprising a viral envelope vector and a chemotherapeutic as active ingredients.
Owner:IMMUNOMEDICINE INC
Assay for identifying antigens that activate b cell receptors comprising neutralizing antibodies
InactiveUS20130236905A1Polypeptide with localisation/targeting motifMicrobiological testing/measurementADAMTS ProteinsProtein-protein complex
The invention described herein provides a method for screening pathogenic viral envelope proteins and protein complexes to identify protein constructs with enhanced effectiveness as vaccine immunogens. The method is carried out by (i) expressing of a membrane-bound isotype of an antibody that has the same binding activity and specificity of an antibody that is known, or identified, to bind and neutralize the targeted virus, and that has the capacity to activate signaling pathways down-stream of B cell receptor ligand binding and activation—a modified neutralizing antibody-based B cell receptor; (ii) exposing the cell to antigen; and (iii) assay for signaling downstream of B cell receptor activation. The present invention also provides the antigens identified using the as say described herein, and neutralizing antibodies derived by immunization with the antigens identified using the assay described herein.
Owner:MARSHALL CHRISTOPHER
Viral Vectors Purification System
InactiveUS20140248695A1Promote resultsIncrease infectivityDepsipeptidesPeptide preparation methodsSurface markerViral envelope
The present invention relates to a new method for purification of viral vectors particularly those belonging to the Retroviridae family, which is based on the expression in the packaging cell line that produced such vectors of an exogenous gene encoding a cell surface marker. The incorporation of the cell surface marker in the viral envelope of the vector allows purification with immunological methods.
Owner:MOLMED SPA
Combination of fusion protein for separating fluorescent protein, expression vector and application thereof
InactiveCN101747438ADetection and monitoringEasy to detectMicrobiological testing/measurementFluorescence/phosphorescenceViral envelopeAccelerant
The invention relates to a combination and an expression vector of fusion protein for separating fluorescent protein, a method for screening receptors of viral envelope protein by detecting fluorescence of self re-combined fluorescent protein in the combination of the fusion protein for separating the fluorescent protein in cell fusion, and a method for screening accelerants or inhibitors in membrane fusion.
Owner:THE UNIV OF TOKYO +1
Involucrin-driven retroviral expression cassettes encoding human immunodeficiency virus envelope glycoproteins
ActiveUS9730996B2Improve barrier propertiesEnhance immune stimulationViral antigen ingredientsGenetic material ingredientsGene deliveryImmunodeficiency virus
The present invention provides for novel compositions and methods for delivering genes of interest to stem cells using vectors that contain differentiation-specific transcriptional regulatory elements. For example, stem cells in the internal epithelia could be transfected with a vaccine construct, which has an epithelial cell differentiation-specific promoter driving the expression of viral envelope proteins. When the promoter used is specific for terminally differentiated epithelial cells, then the viral envelope proteins will be expressed only in the upper part of the epithelia and therefore, stimulate the immune response. The infected epithelial stem cells in the basal layer will continue to produce new antigen-expressing cells, without being eliminated by the immune response. This invention will be useful in the development of vaccines against viral agents that target the internal mucosa like HIV.
Owner:TEXAS BIOMEDICAL RES INST
Chimeric Viral Envelopes
InactiveUS20090324553A1Prevent and reduce HIV infectionOrganic active ingredientsVirusesNucleotideCell membrane
The invention relates to chimeric polytropic viral envelope polypeptides and uses thereof, as well as to polynucleotides encoding said chimeric polypeptides and constructs comprising said polypeptides and / or polynucleotides. The invention also relates to chimeric retroviral envelope polypeptides, polynucleotides and vectors encoding said chimeric retroviral envelope polypeptides, virus particles and cells harbouring said chimeric envelope polypeptides. Said chimeric polypeptide comprise an envelope polypeptide, or fragment thereof, and a polypeptide sequence of a receptor binding region, ligand or polypeptide sequence of a ligand binding region, and optionally a linker sequence. The invention include methods of targeting receptors, methods of treatment and methods for delivery of agents using said chimeric retroviral envelope polypeptides. The invention is applicable for directed targeting and controlled fusion of virus particles with other cellular membranes.
Owner:AARHUS UNIV
Epitopes in viral envelope proteins and specific antibodies directed against these epitopes: use for detection of HCV viral antigen in host tissue
Antibodies to two new epitopes on the HCV envelope proteins were identified which allow routine detection of native HCV envelope antigens, in tissue or cells derived from the host. The new epitopes are: the E1 region aa 307-326 and the N-terminal hyper variable region of E2 aa 395-415. Surprisingly, we characterised an antibody that reacts with various sequences of the hypervariable domain of E2. Specific monoclonal antibodies directed against these epitopes and allowing routine detection of viral antigen are described.
Owner:INNOGENETICS NV
Viral vector nanocapsule for targeting gene therapy and its preparation
InactiveUS20150320693A1Improve stabilityResistance to serum inactivationSsRNA viruses negative-senseOrganic active ingredientsCyclic RGDViral envelope
The invention provides novel methods, materials and systems that can be used to generate viral vectors having altered tissue and cell targeting abilities. In illustrative embodiments of the invention, the specificity of lentiviral vectors was modulated by a thin polymer shell that synthesized and coupled to the viral envelope in situ. The polymer shell can confers such vectors with new targeting ability via agents such as cyclic RGD (cRGD) peptides that are coupled to the polymer shell. These polymer encapsulated viral vectors exhibit a number of highly desirable characteristics including a higher thermal stability, resistance to serum inactivation in vivo, and an ability to infect dividing and non-dividing cells with high efficiencies.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com