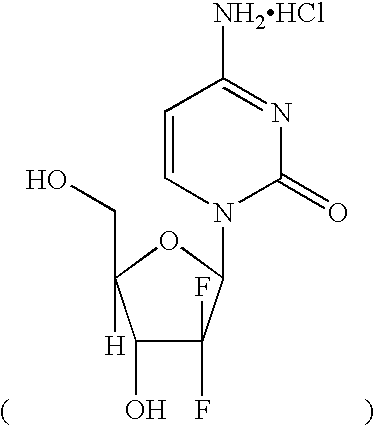
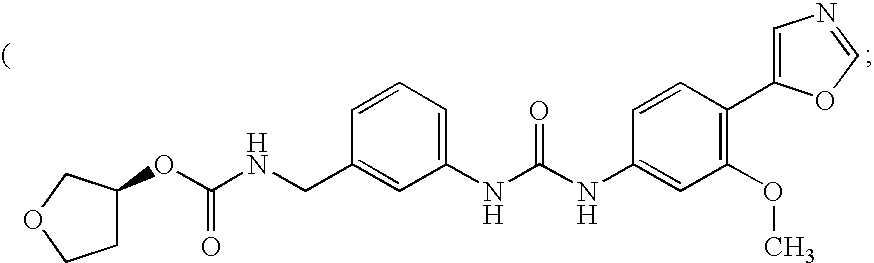
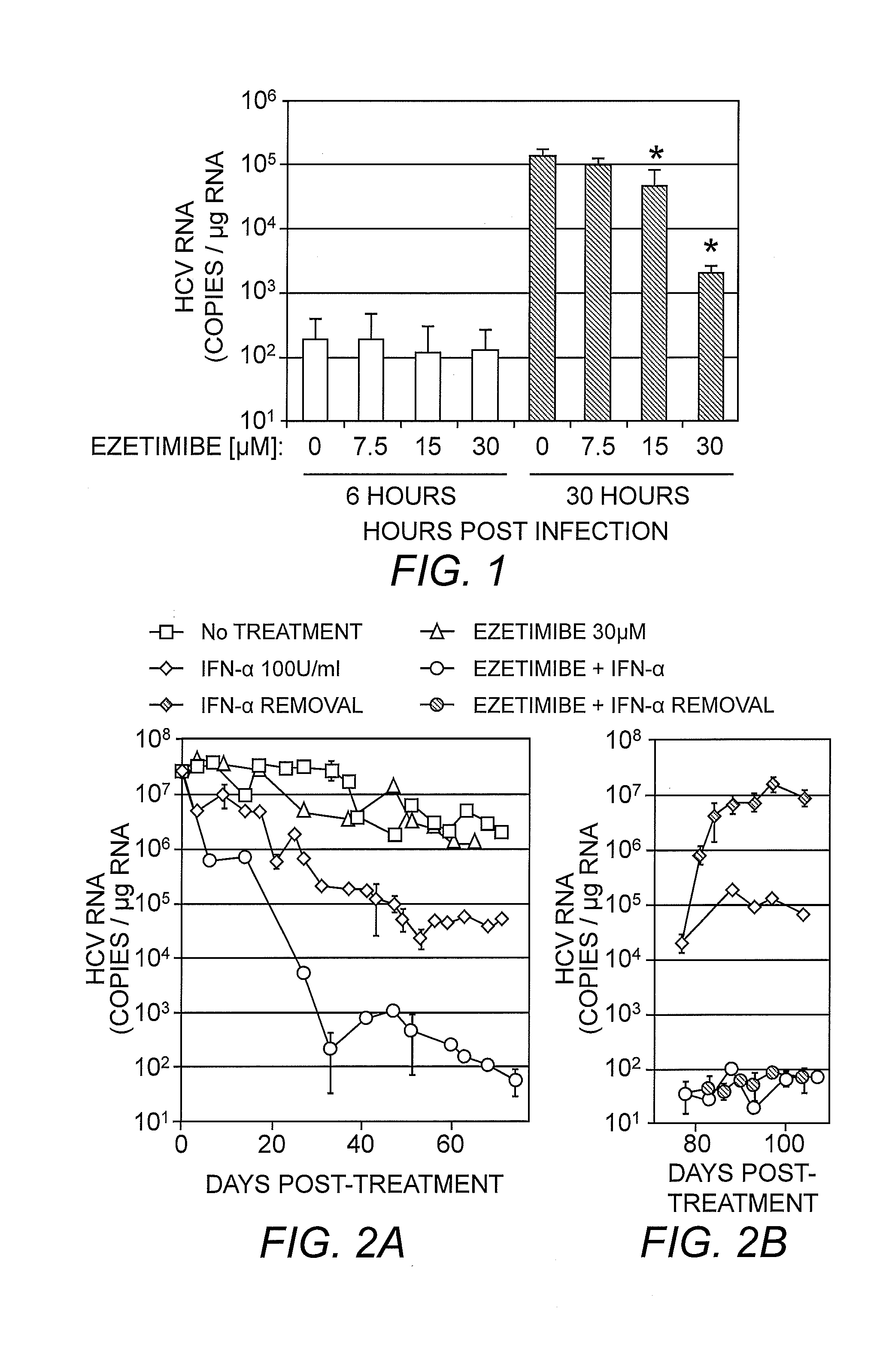
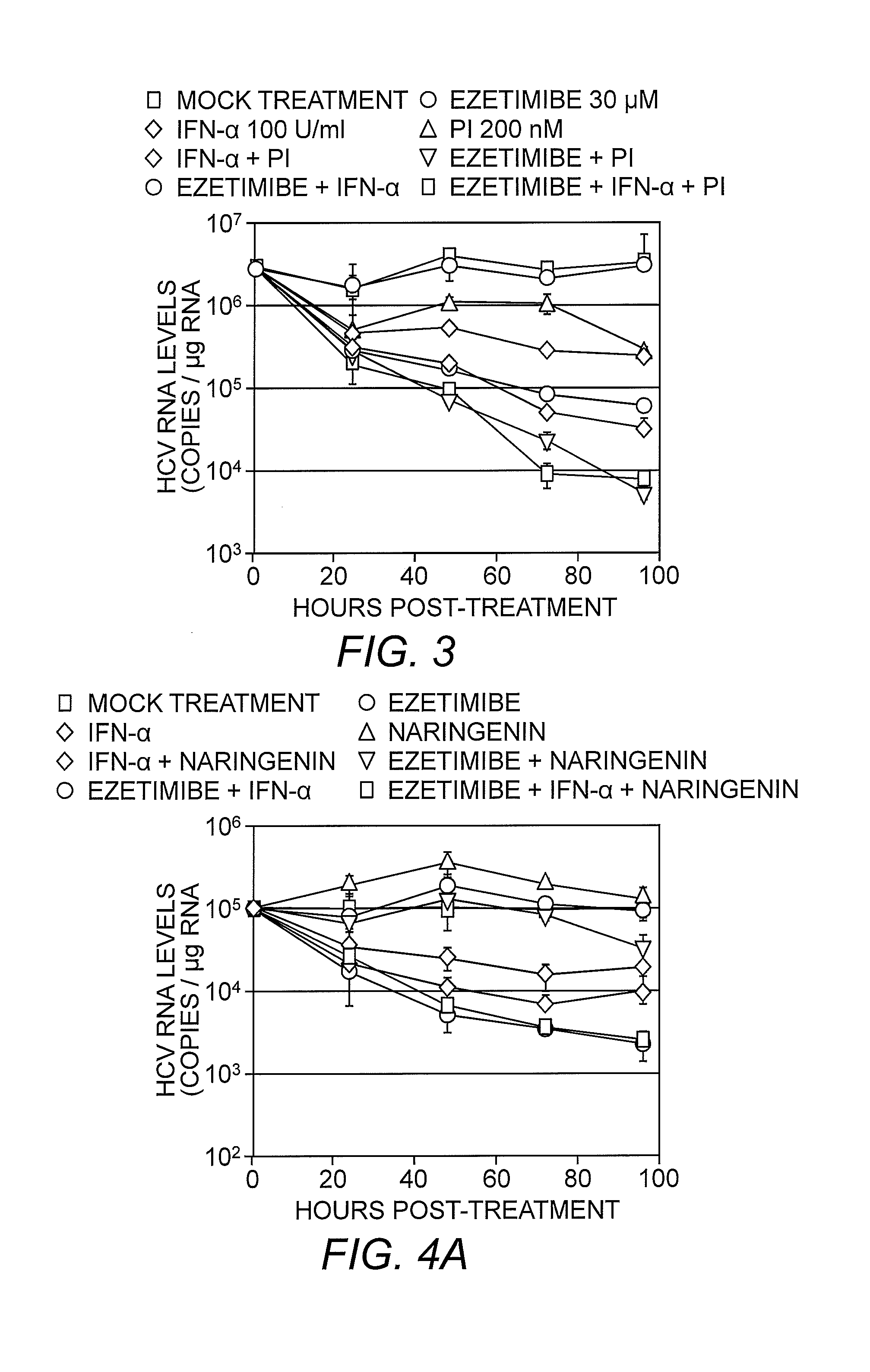
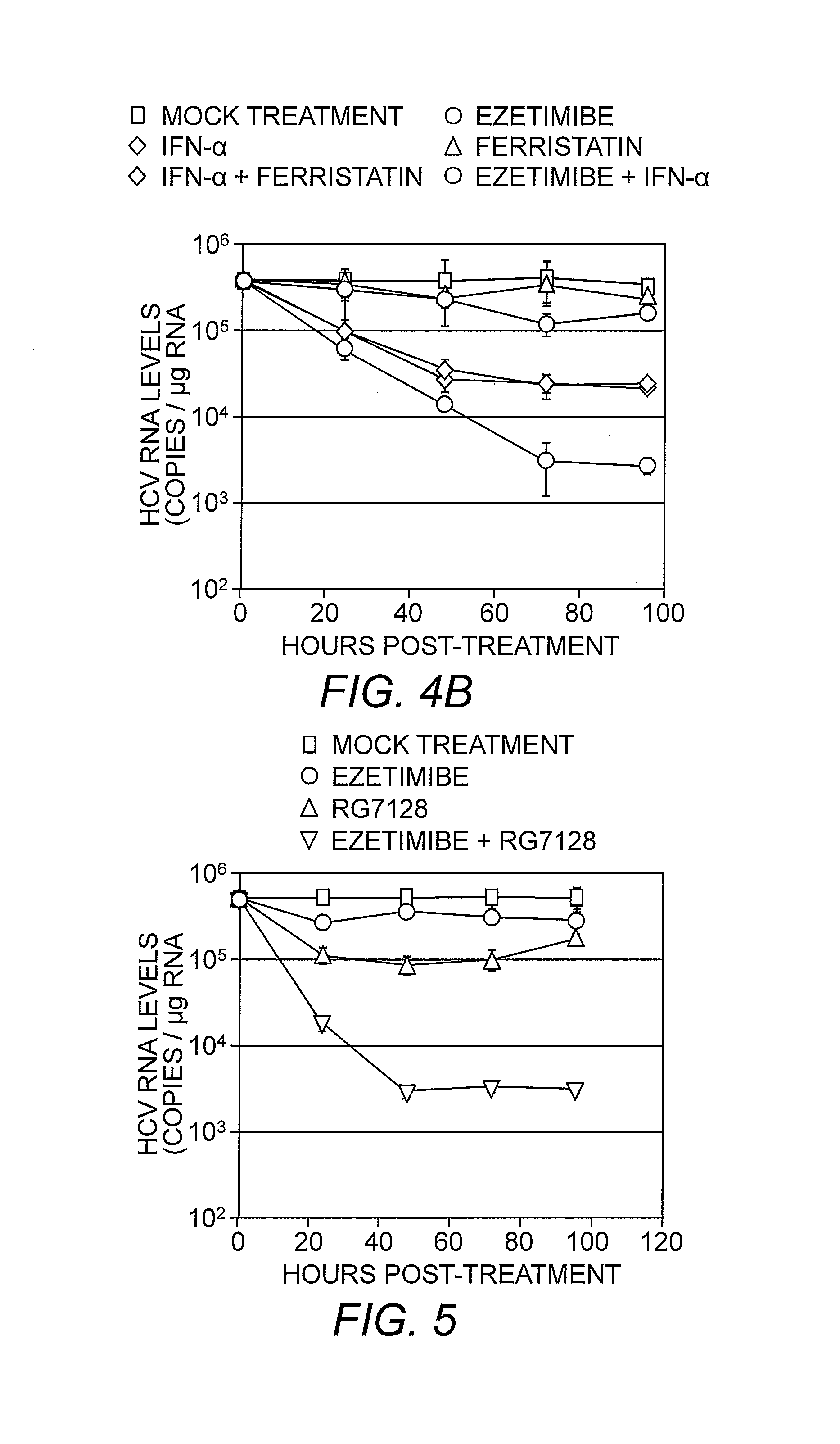
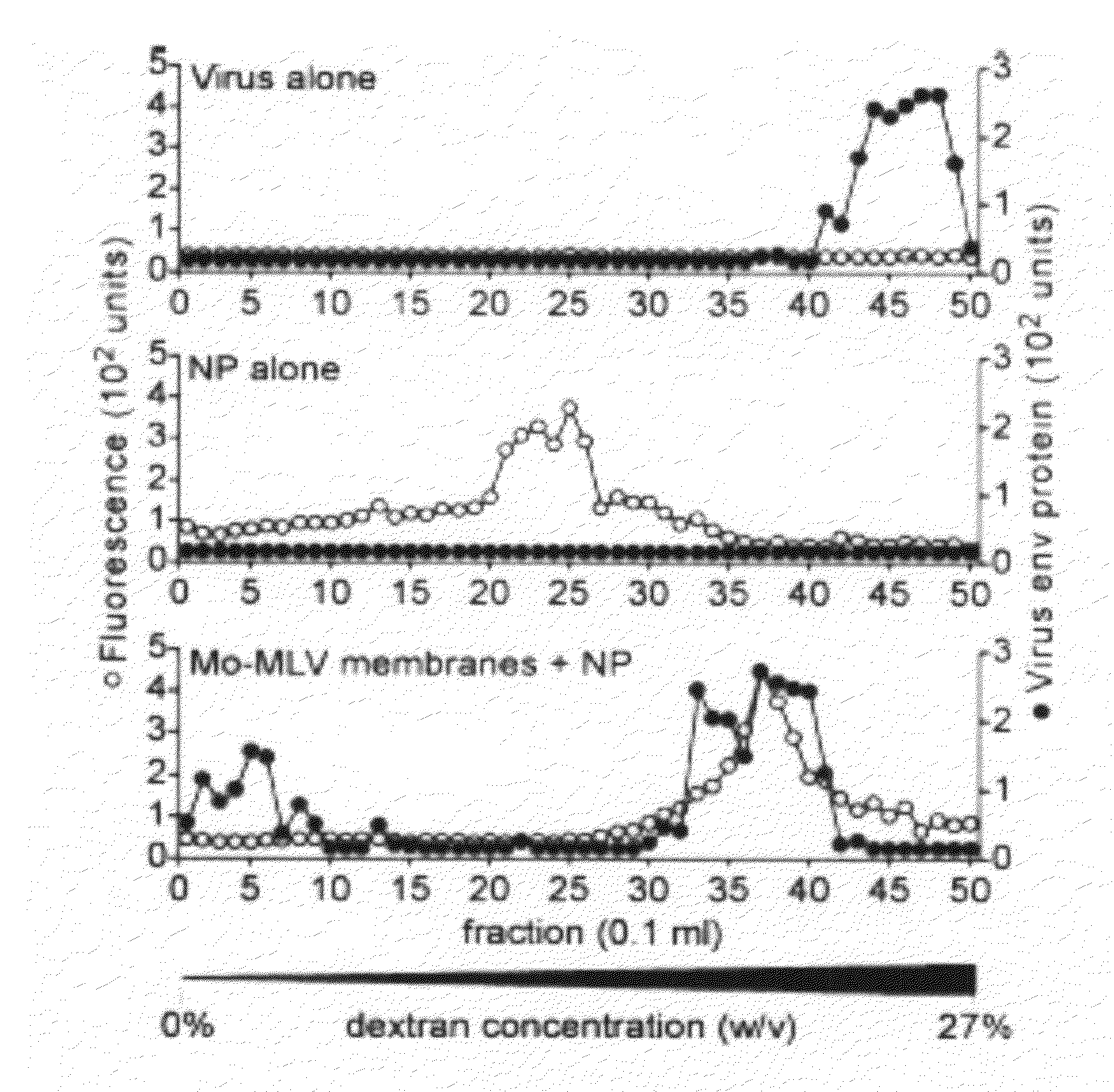
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
149 results about "Viral entry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
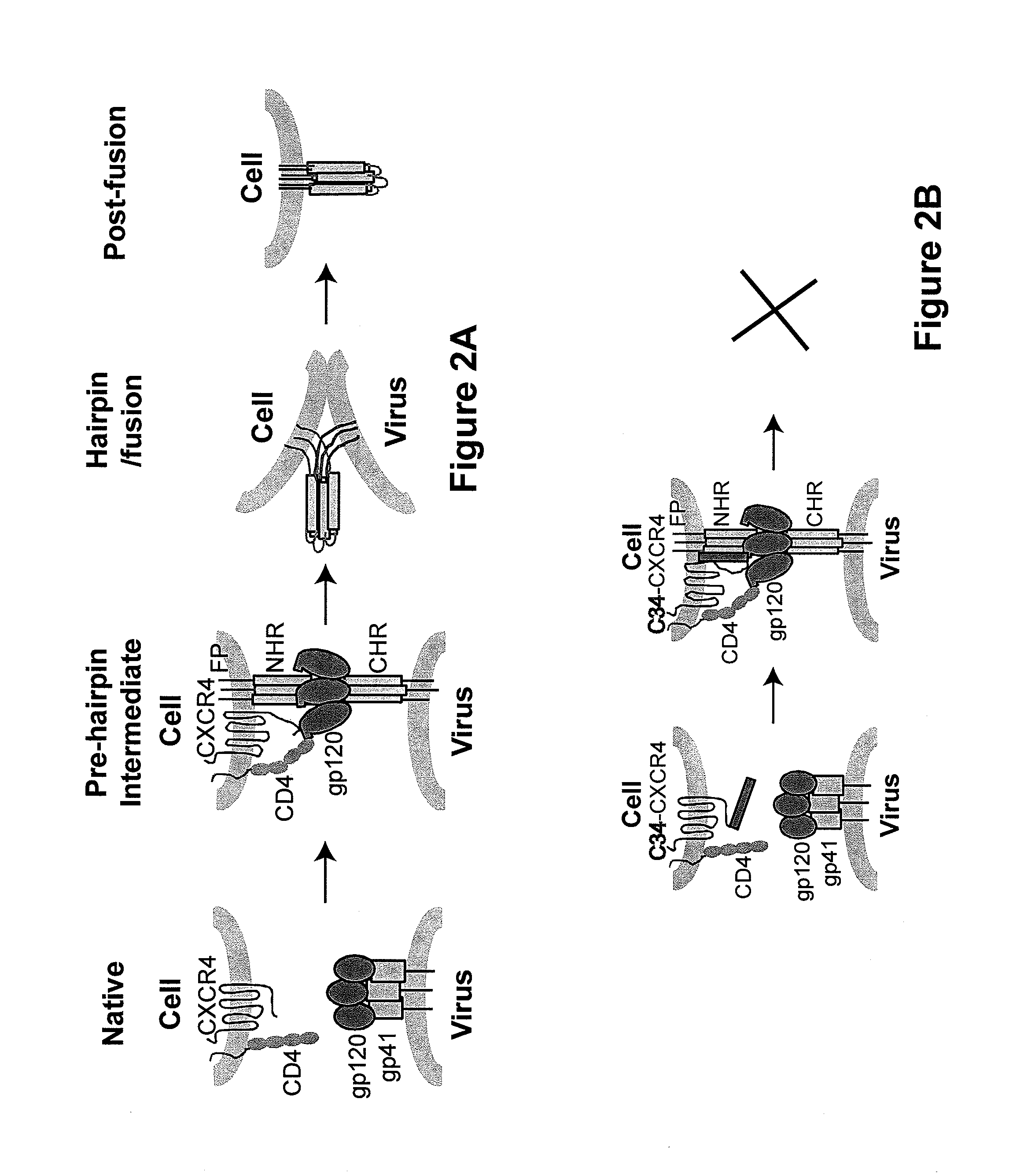
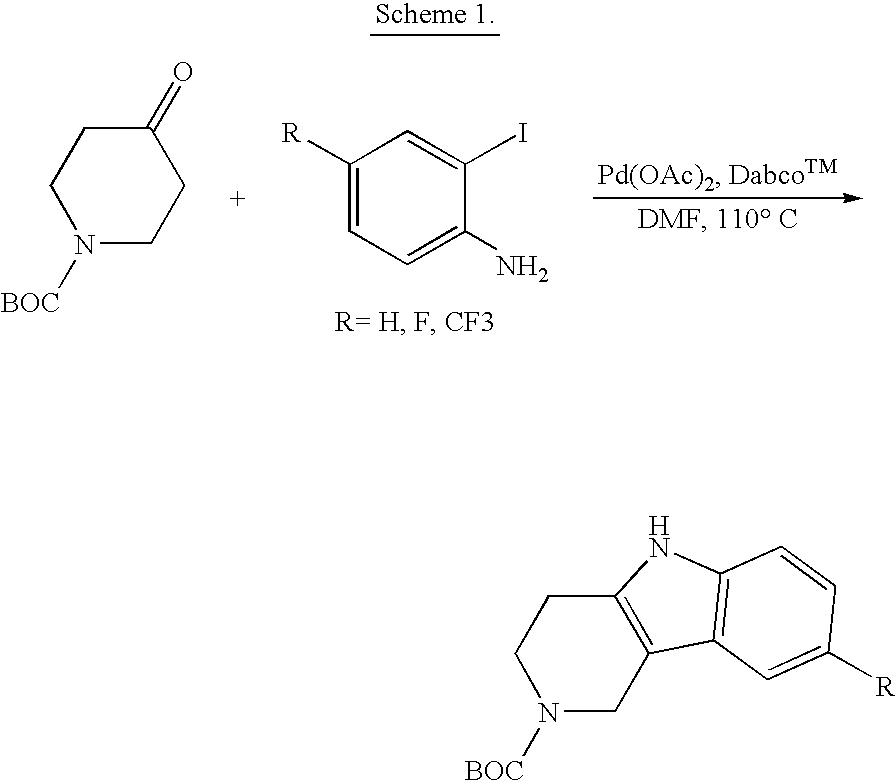
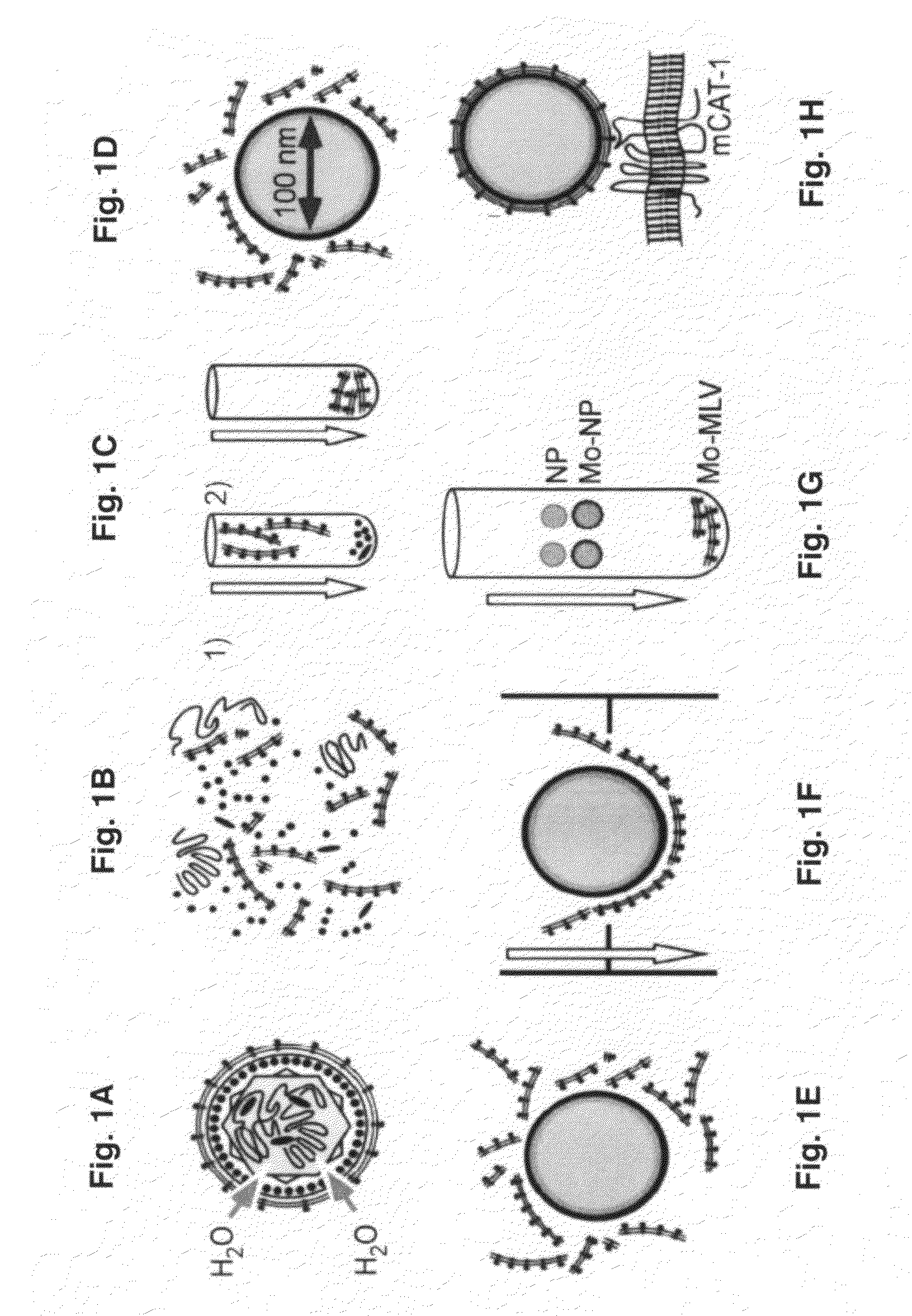
Viral entry is the earliest stage of infection in the viral life cycle, as the virus comes into contact with the host cell and introduces viral material into the cell. The major steps involved in viral entry are shown below. Despite the variation among viruses, there are several shared generalities concerning viral entry.
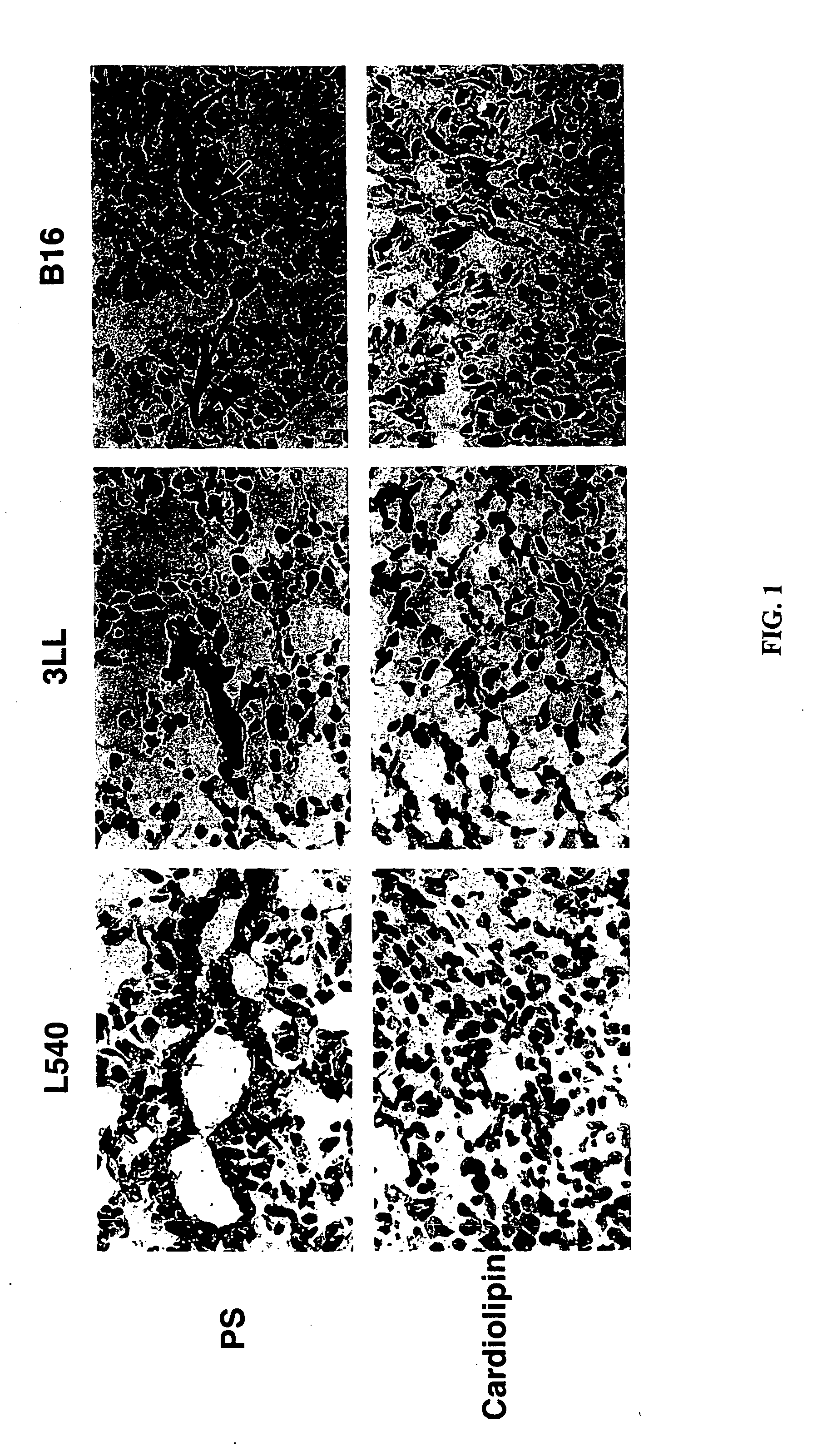
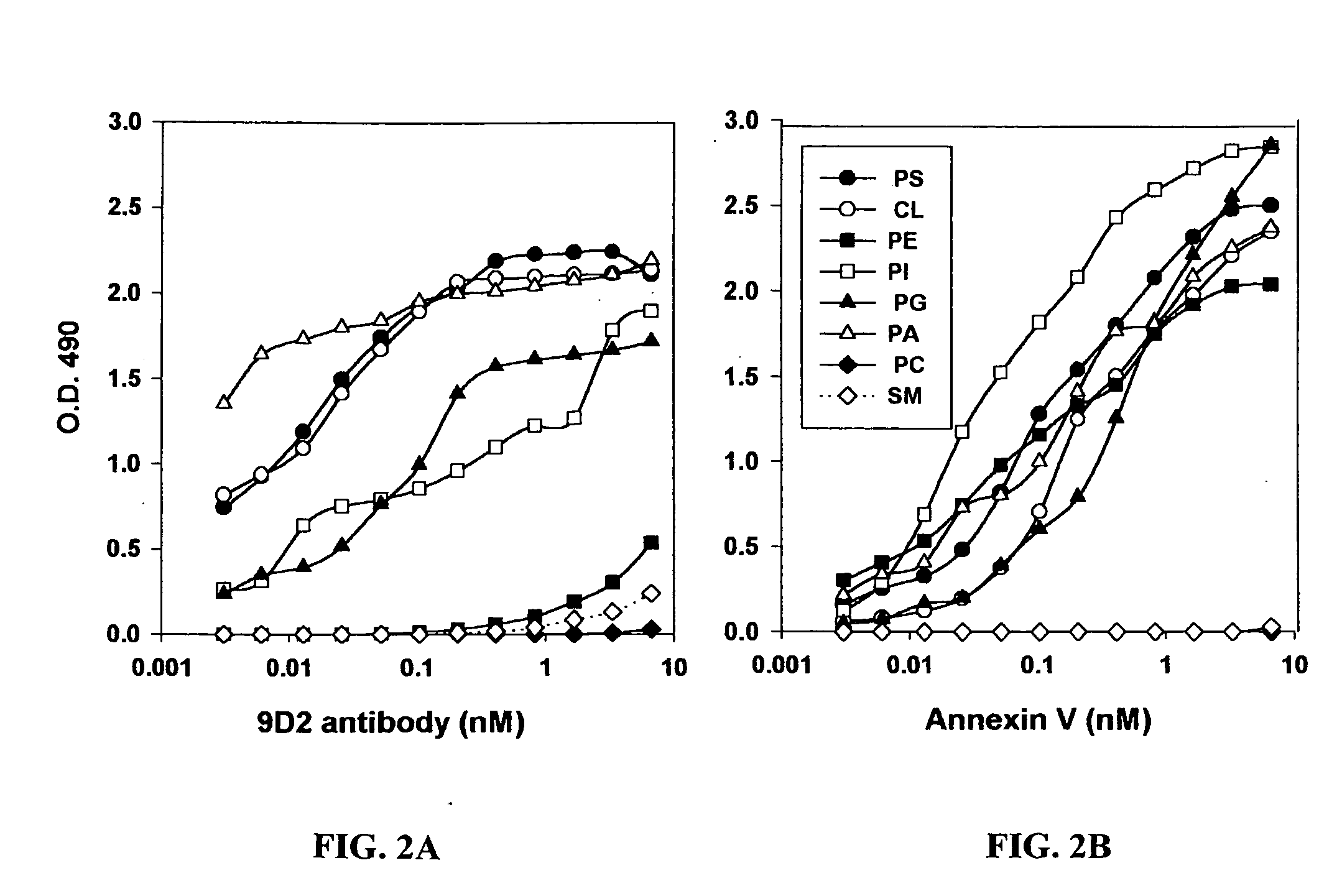
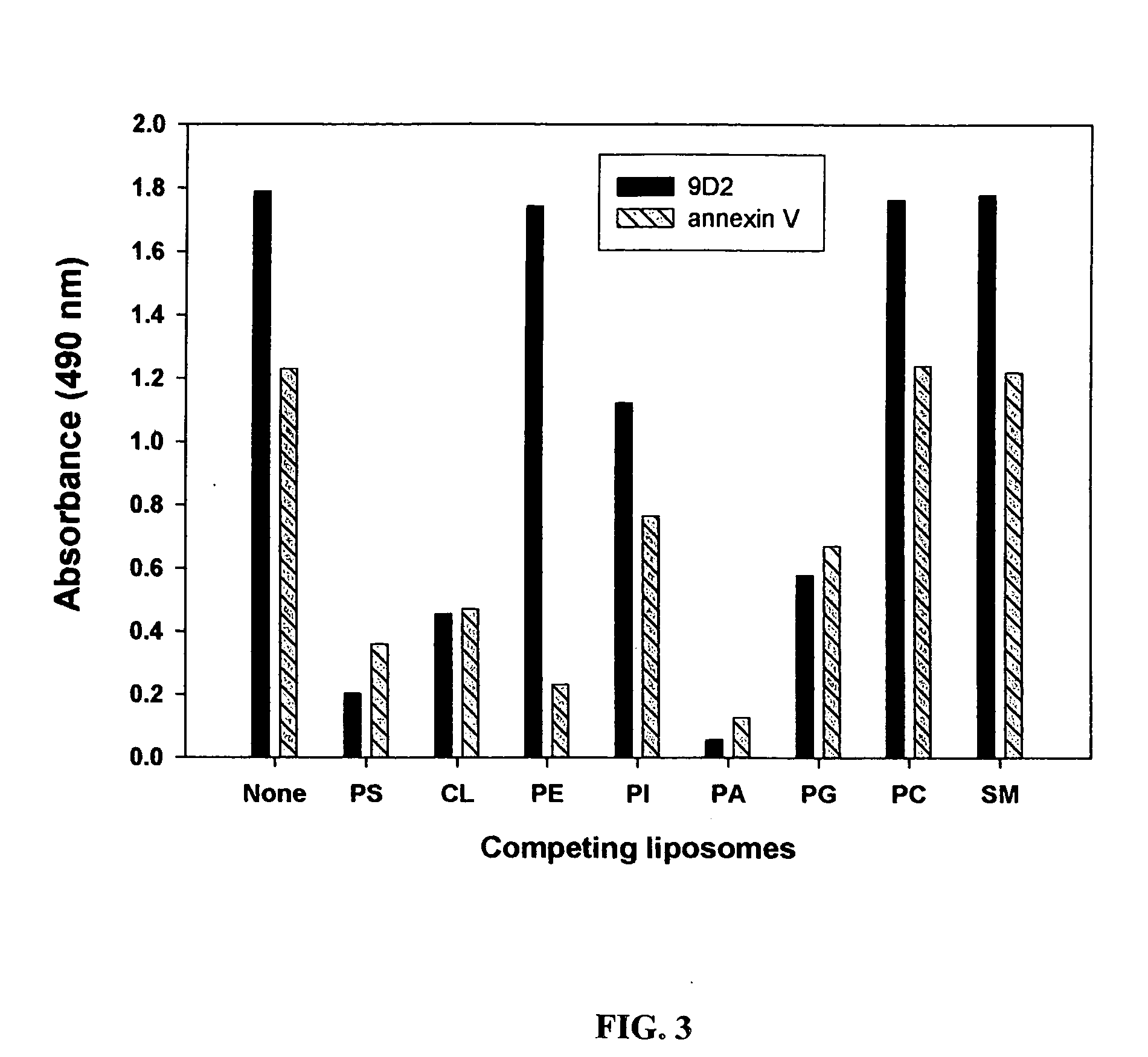
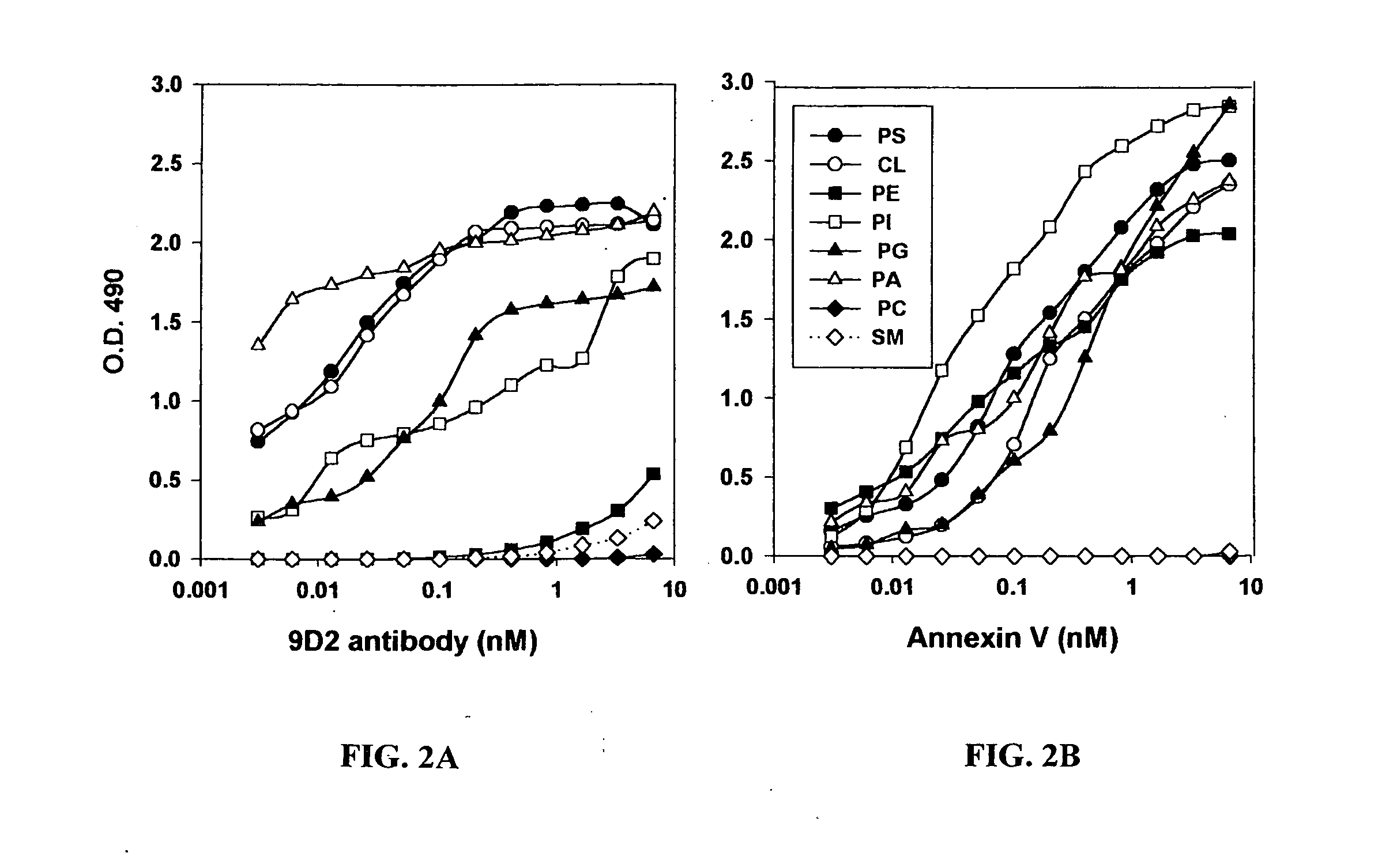
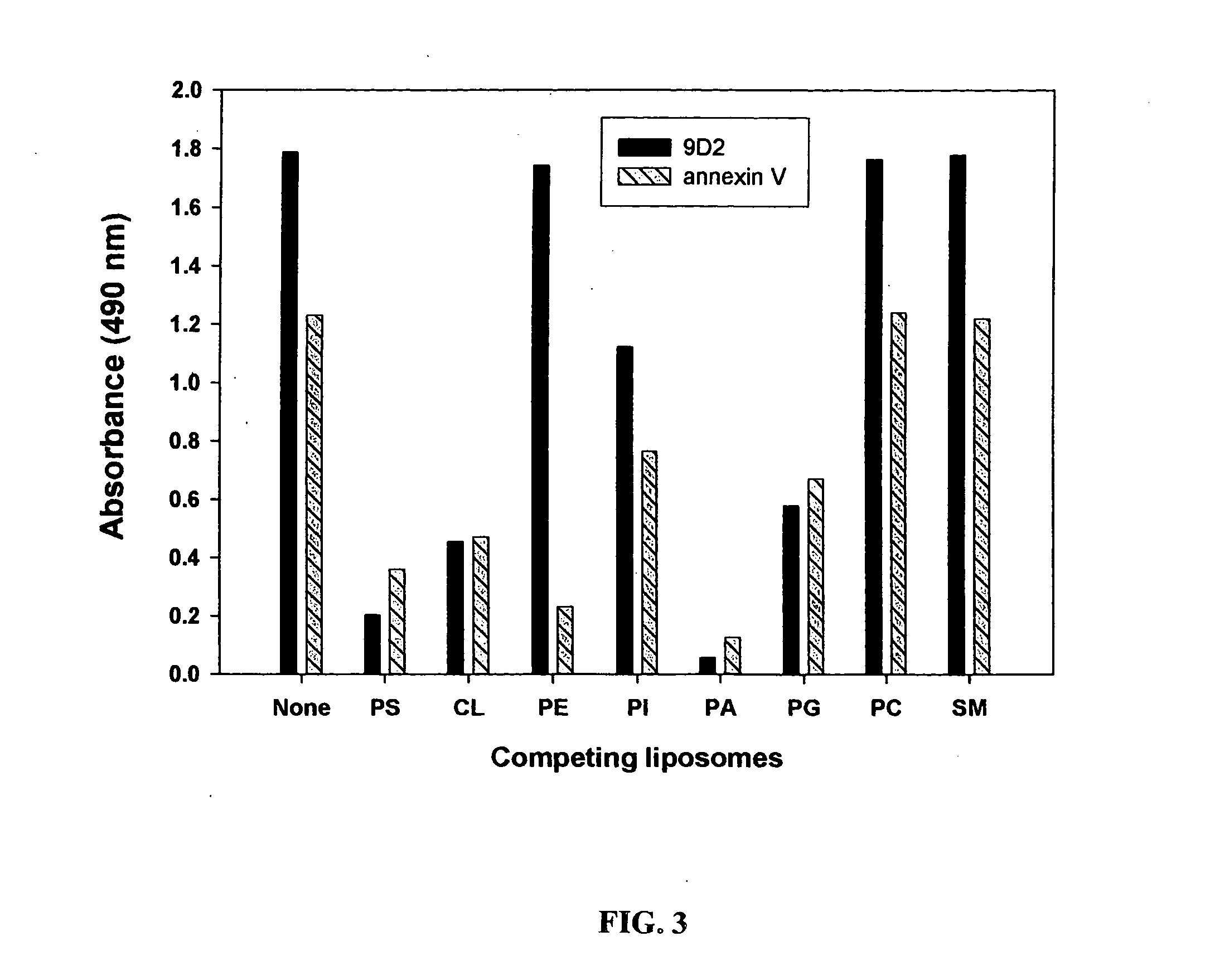
Cancer treatment methods using selected antibodies to aminophospholipids
ActiveUS20050136059A1Reduce control antibody bindingReduce the binding forceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPhospholipidTumor vasculature
Disclosed are surprising discoveries concerning the role of anionic phospholipids and aminophospholipids in tumor vasculature and in viral entry and spread, and compositions and methods for utilizing these findings in the treatment of cancer and viral infections. Also disclosed are advantageous antibody, immunoconjugate and duramycin-based compositions and combinations that bind and inhibit anionic phospholipids and aminophospholipids, for use in the safe and effective treatment of cancer, viral infections and related diseases.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Anti-viral compositions comprising heterocyclic substituted phenyl furans and related compounds
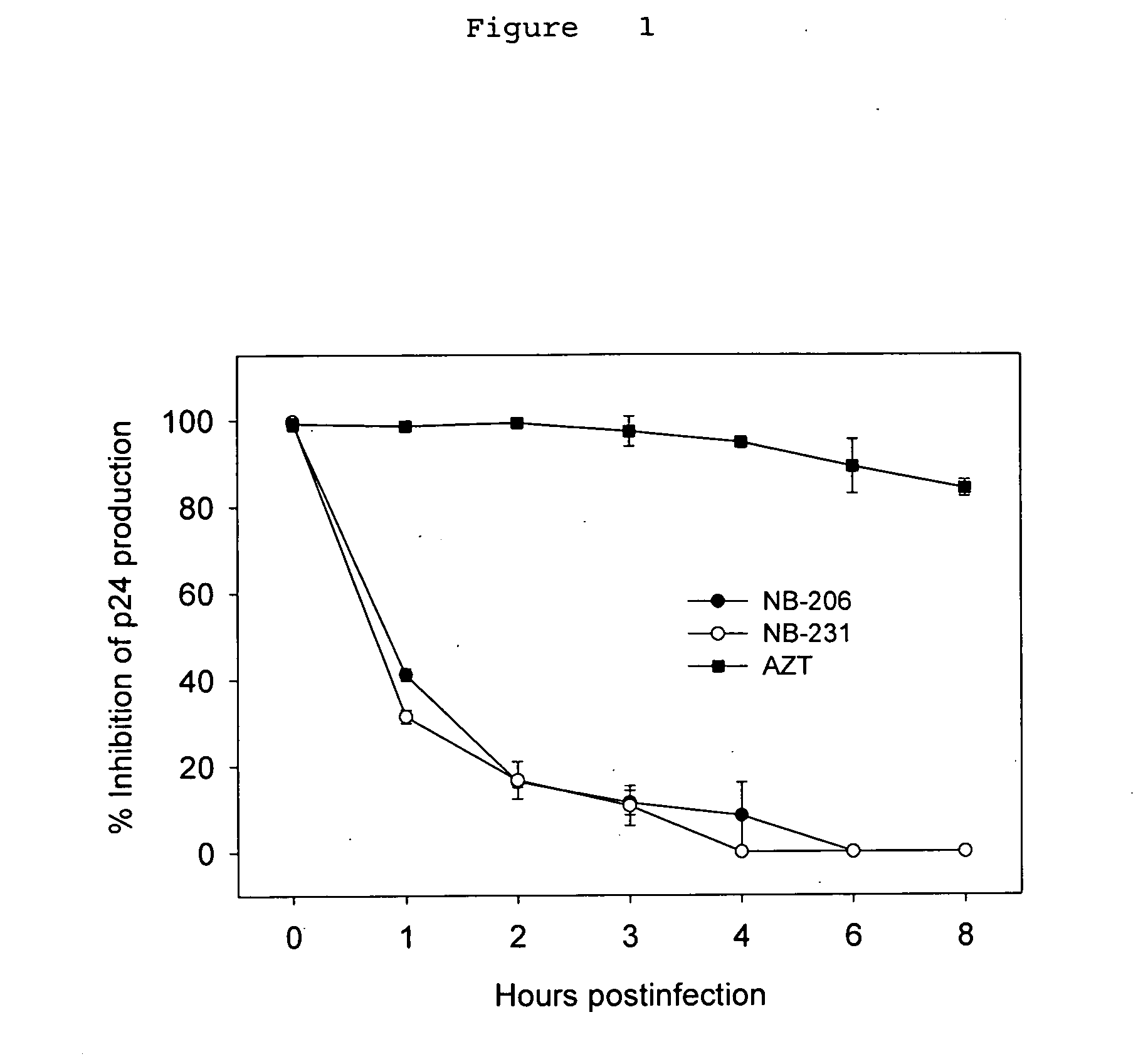
InactiveUS20060287319A1Potent anti-HIV activityInhibit HIV replicationBiocideAntiviralsFuranFluorescence
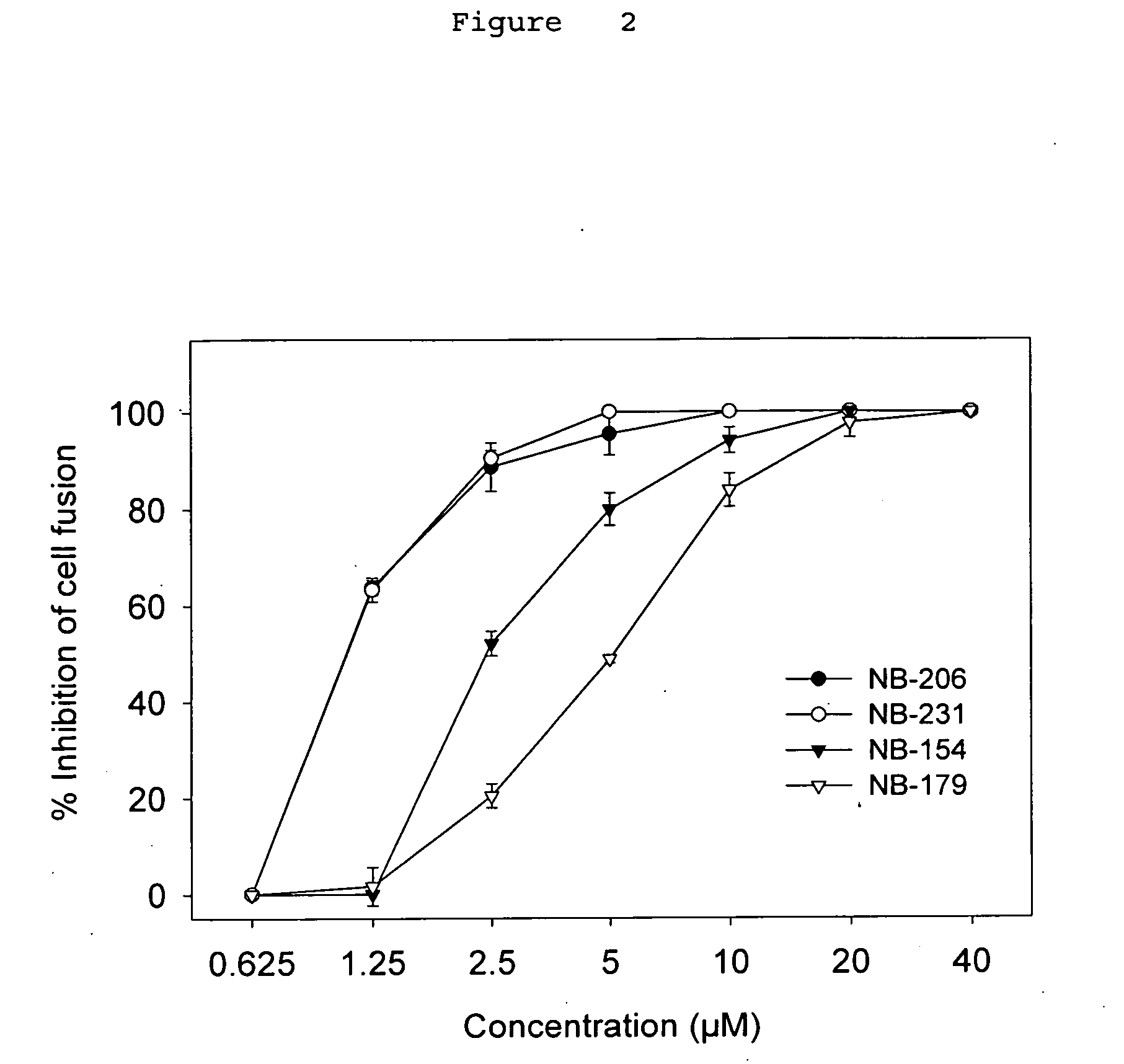
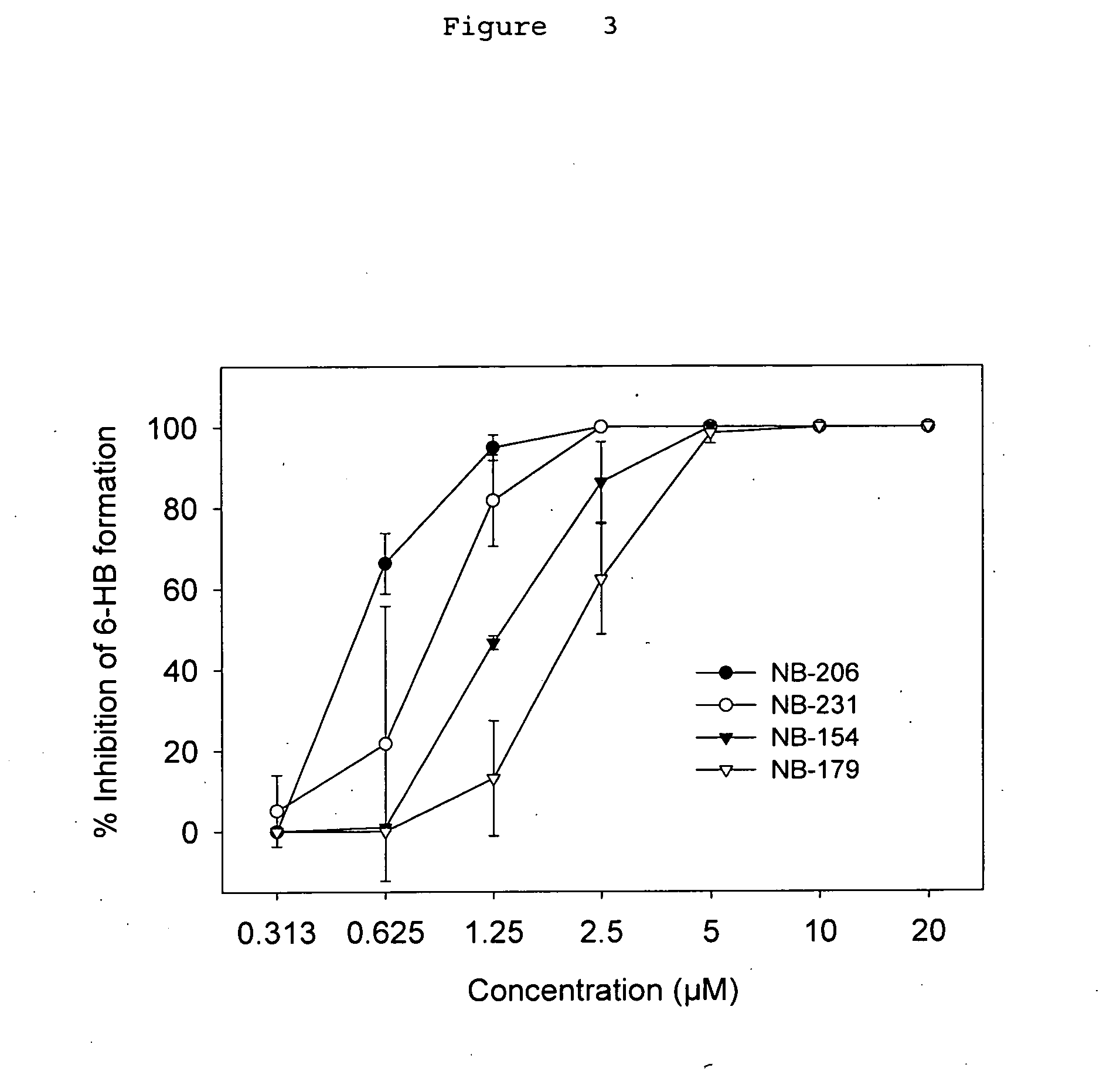
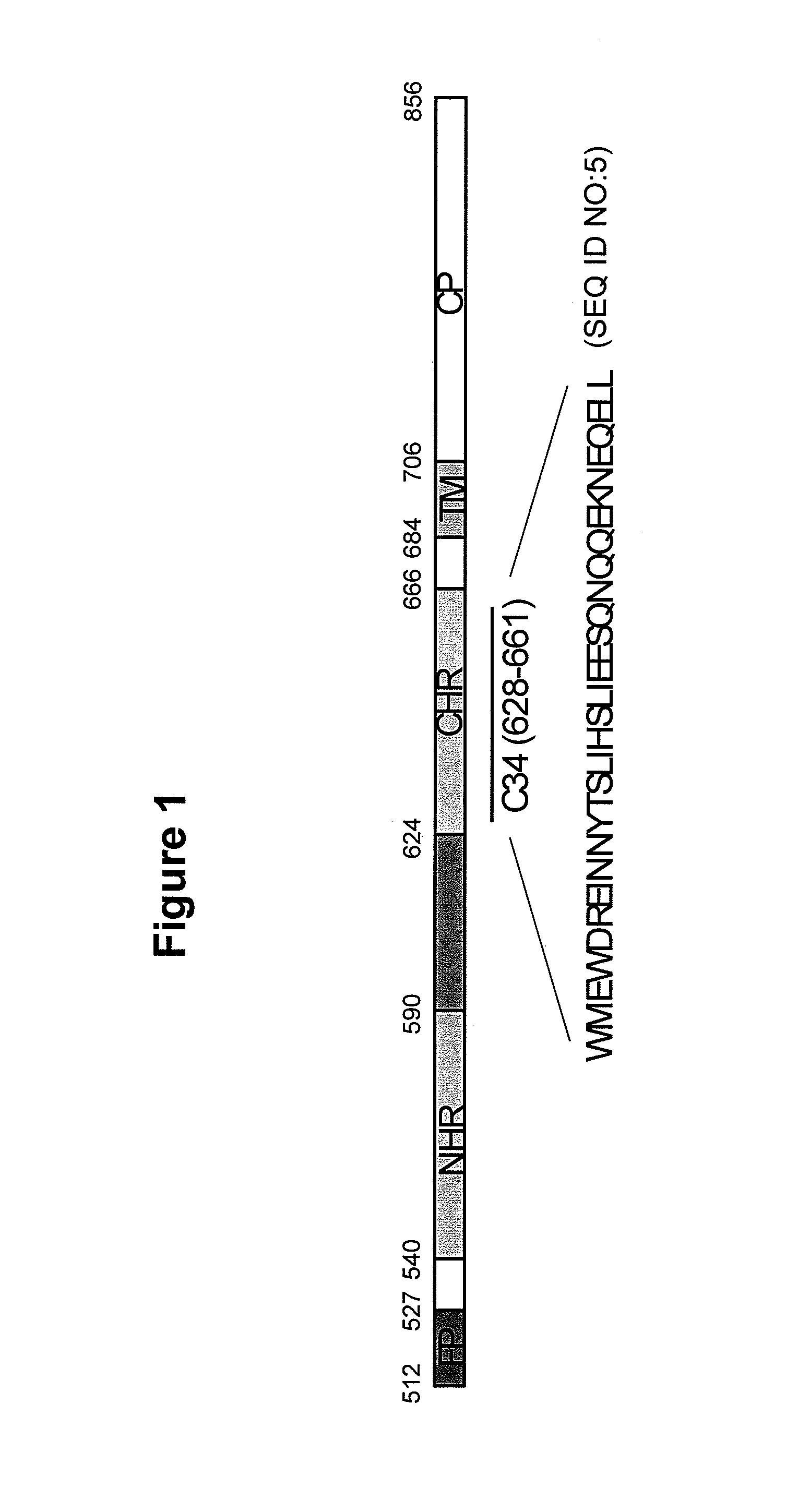
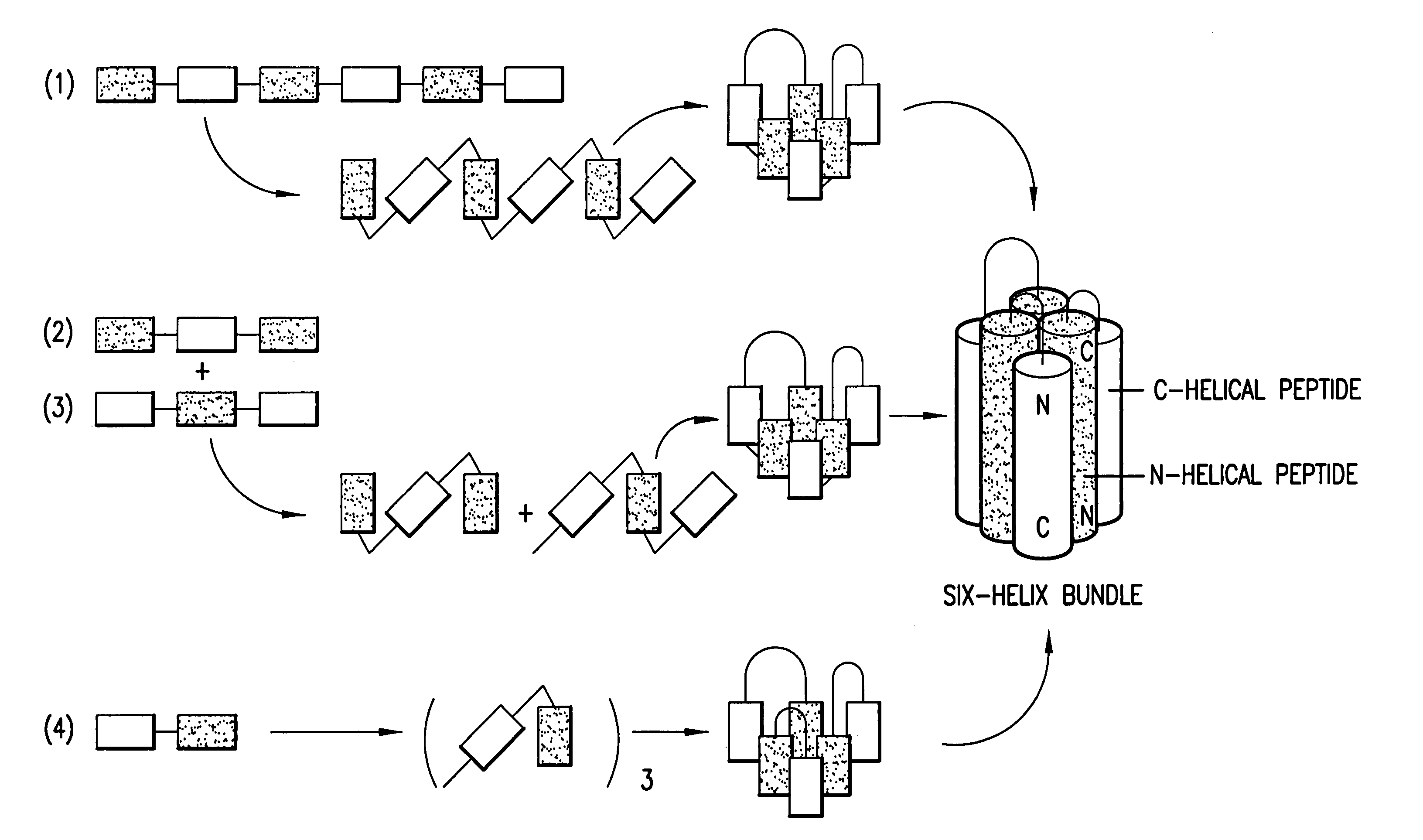
A group of compounds that inhibit HIV replication by blocking HIV entry was identified. One representative compound, designated NB-206, and its analogs inhibited HIV replication (p24 production) with IC50 values at nanomolar levels. It was proved that NB-206 and its analogs are HIV entry inhibitors by targeting the HIV gp41 since: 1) they inhibited HIV-mediated cell fusion; 2) they inhibited HIV replication only when they were added to the cells less than one hour after virus addition; 3) they blocked the formation of the gp41 core that is detected by sandwich enzyme linked immunosorbent assay (ELISA) using a conformation-specific MAb NC-1; and 4) they inhibited the formation of the gp41 six-helix bundle revealed by fluorescence native-polyacrylamide gel electrophoresis (FN-PAGE). These results suggested that NB-206 and its analogs may interact with the hydrophobic cavity and block the formation of the fusion-active gp41 coiled coil domain, resulting in inhibition of HIV-1 mediated membrane fusion and virus entry.
Owner:NEW YORK BLOOD CENT
Methods and compositions for inhibiting viral entry into cells
Owner:SANGAMO BIOSCIENCES INC
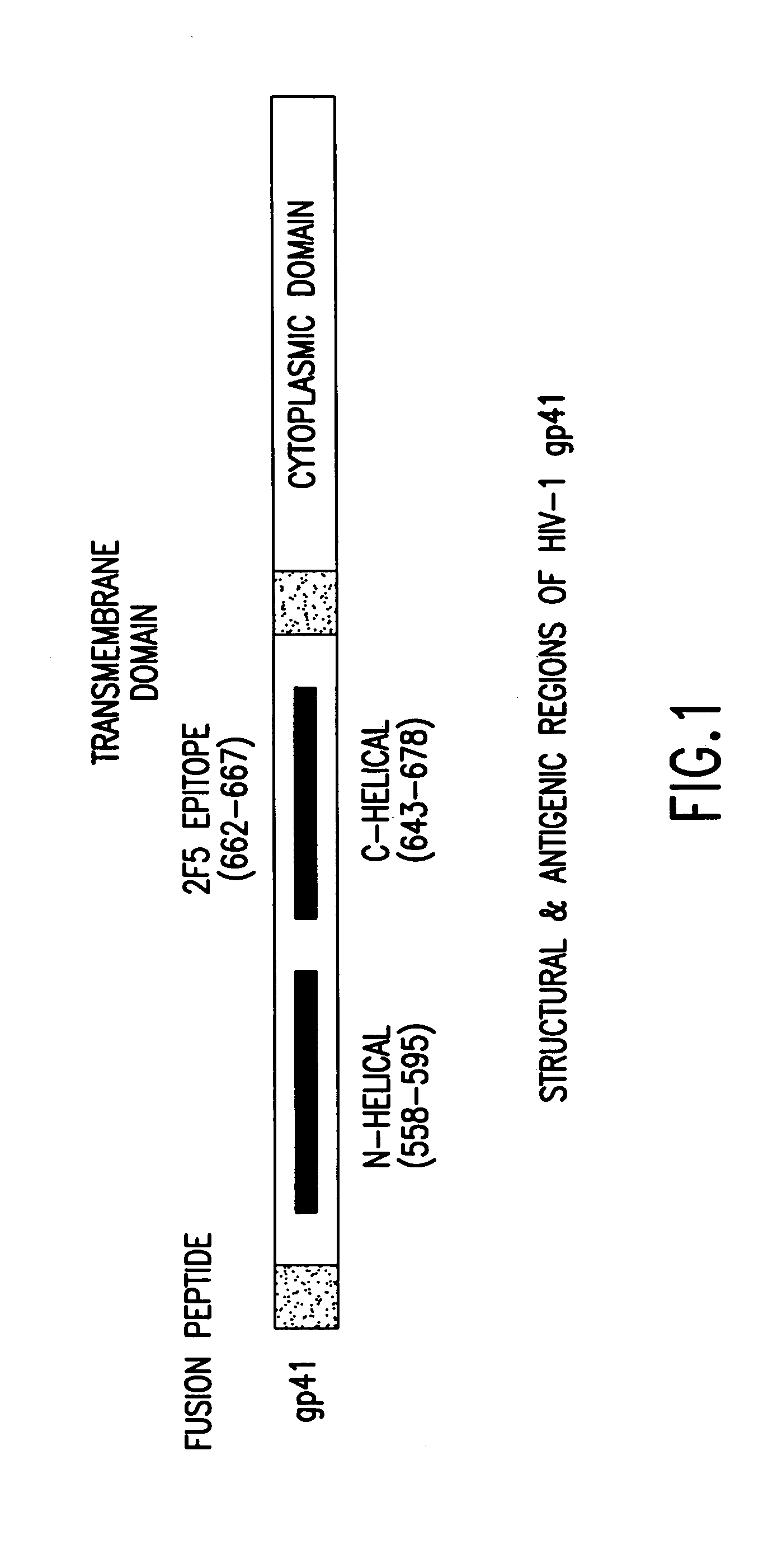
Methods of eliciting broadly neutralizing antibodies targeting HIV-1 gp41
The present invention is directed to the induction and characterization of a humoral immune response targeting "entry-relevant" gp41 structures. In its broadest aspect, the present invention is directed to methods of raising a neutralizing antibody response to a broad spectrum of HIV strains and isolates. The present invention targets particular molecular conformations or structures that occur at the cell surface of HIV during viral entry into host cells. Such a humoral response can be generated in vivo as a prophylactic measure in individuals to reduce or inhibit the ability of HIV to infect uninfected cells in the individual's body. Such a response can also be employed to raise antibodies against "entry relevant" gp41 structures. These antibodies can be employed for therapeutic uses, and as tools for further illuminating the mechanism of HIV cell entry.
Owner:UNITED STATES OF AMERICA +1
Modified erythrocytes and uses thereof
InactiveUS20070082392A1Genetically modified cellsArtificial cell constructsRed cell acanthocytosisHIV receptor
The present invention provides modified erythrocytes which comprise viral receptor proteins capable of mediating entry of respective viruses into the modified erythrocytes. The present invention also provides methods of using the modified erythrocytes for the treatment or prevention of viral infections. In one embodiment, the modified erythrocytes of the present invention comprise CD4 and at least one HIV coreceptor, such as CXCR4 or CCR5. The modified erythrocytes, when administered to an HIV patient, bind to the plasma virus and induce the injection of the HIV ribonucleoprotein complex into the cells. The entrapped viral content is either degraded or deactivated within the erythrocytes, or destroyed by erythrophagocytosis.
Owner:GLASER LAWRENCE F
Cancer treatment methods using selected immunoconjugates for binding to aminophospholipids
ActiveUS20050129696A1Safe and effectiveEfficient replicationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMedicinePhospholipid
Disclosed are surprising discoveries concerning the role of anionic phospholipids and aminophospholipids in tumor vasculature and in viral entry and spread, and compositions and methods for utilizing these findings in the treatment of cancer and viral infections. Also disclosed are advantageous antibody, immunoconjugate and duramycin-based compositions and combinations that bind and inhibit anionic phospholipids and aminophospholipids, for use in the safe and effective treatment of cancer, viral infections and related diseases.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods of eliciting broadly neutralizing antibodies targeting HIV-1 gp41
The present invention is directed to the induction and characterization of a humoral immune response targeting “entry-relevant” gp41 structures. In its broadest aspect, the present invention is directed to methods of raising a neutralizing antibody response to a broad spectrum of HIV strains and isolates. The present invention targets particular molecular conformations or structures that occur at the cell surface of HIV during viral entry into host cells. Such a humoral response can be generated in vivo as a prophylactic measure in individuals to reduce or inhibit the ability of HIV to infect uninfected cells in the individual's body. Such a response can also be employed to raise antibodies against “entry relevant” gp41 structures. These antibodies can be employed for therapeutic uses, and as tools for further illuminating the mechanism of HIV cell entry.
Owner:UNITED STATES OF AMERICA +1
Peptide viral entry inhibitors
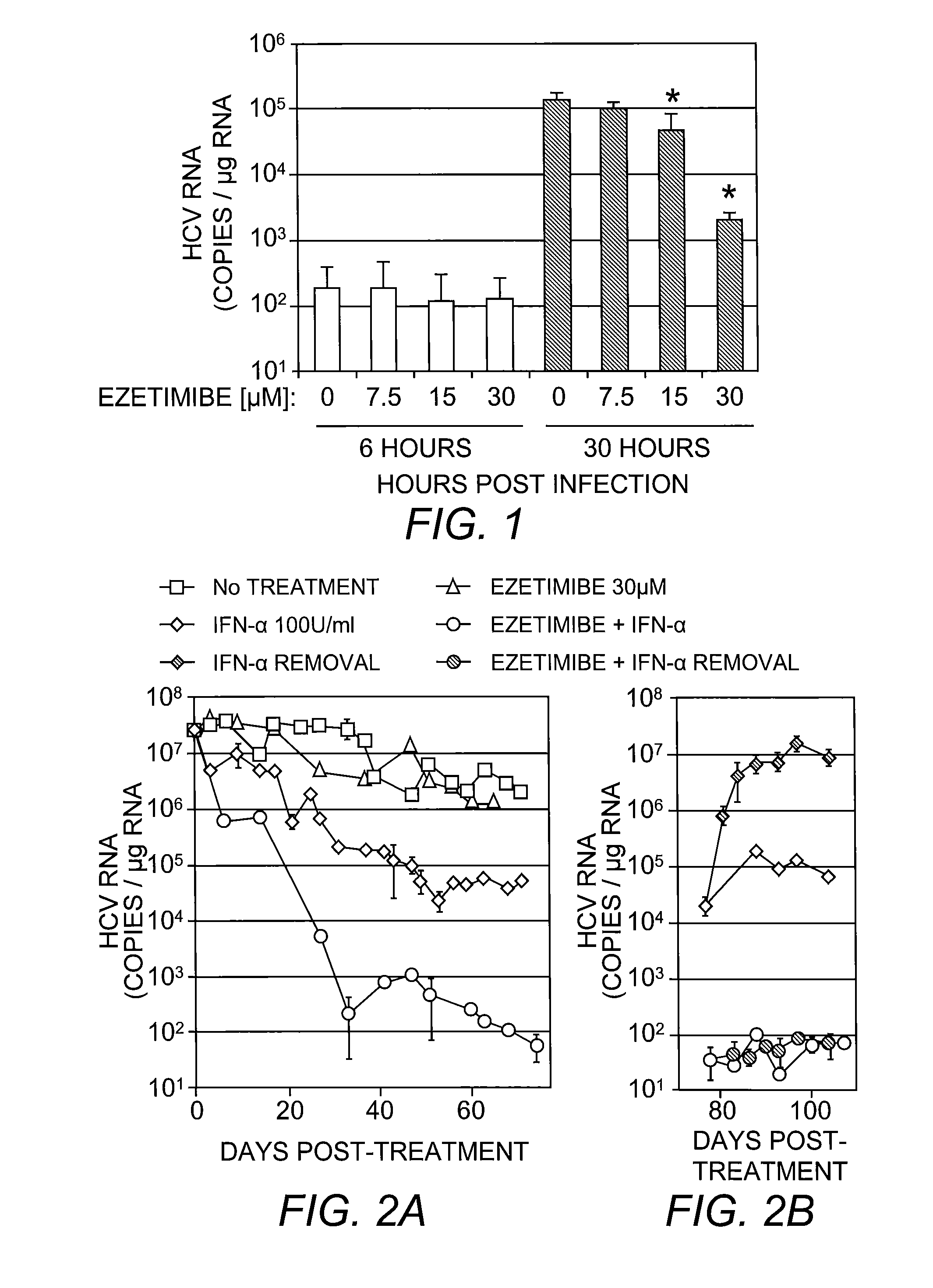
The present invention provides, inter alia, peptide compositions and methods for treating and preventing Flaviviridae virus (e.g., HCV) infections.
Owner:SCHERING CORP
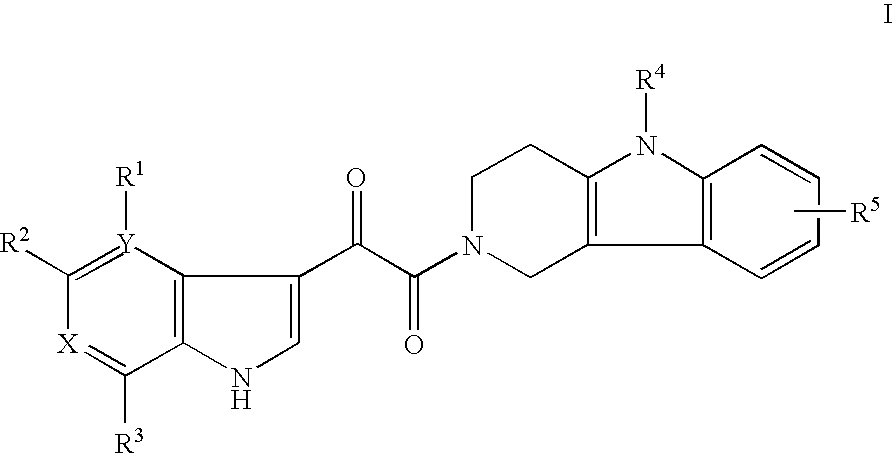
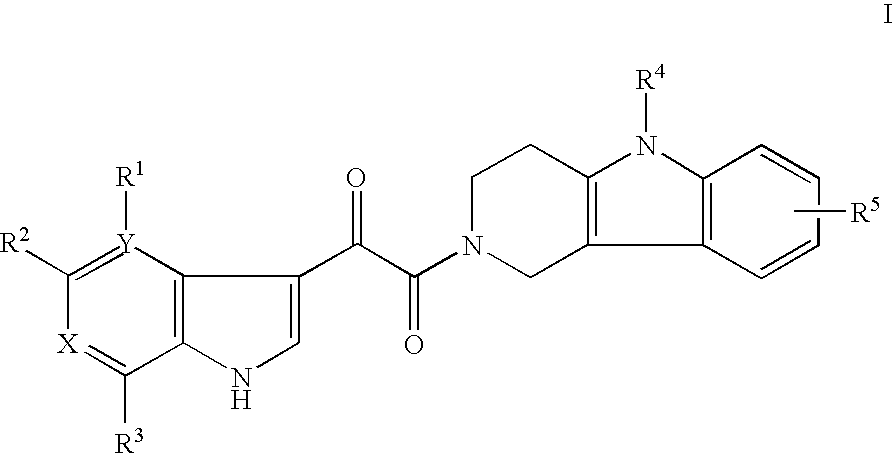
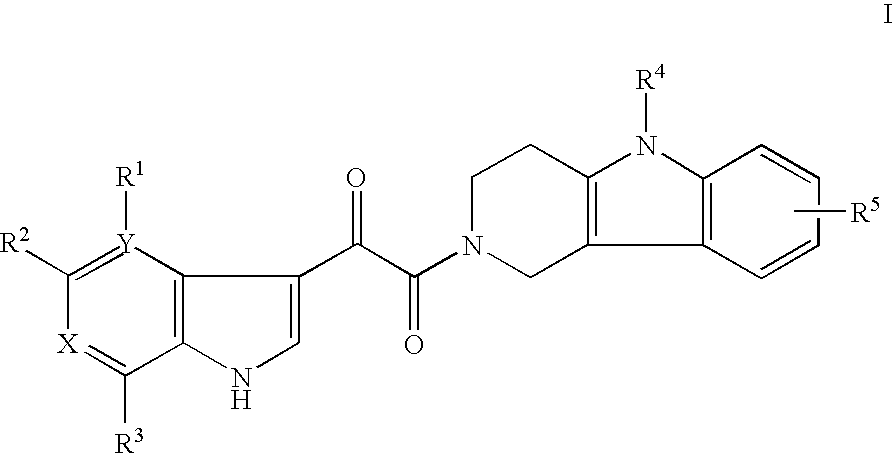
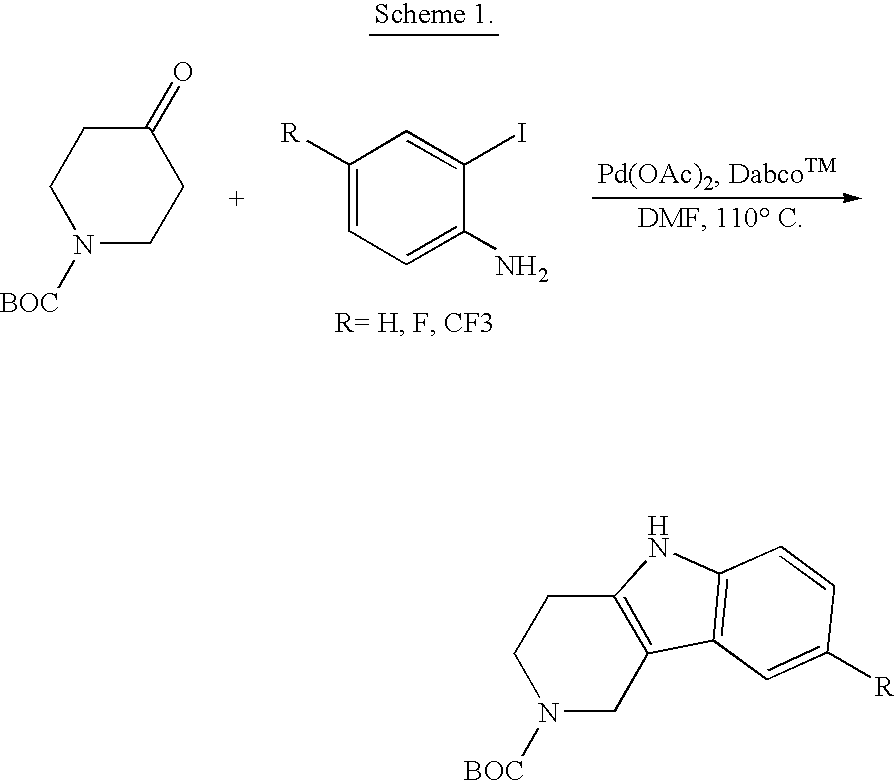
Tetrahydrocarboline antiviral agents
The invention encompasses a series of tetrahydrocarboline compounds of Formula I which inhibit HIV entry by attaching to the exterior viral envelop protein gp120 and interrupting the viral entry process, possibly by interfering with the cellular receptor CD4. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Modified erythrocytes and uses thereof
InactiveUS7462485B2Genetically modified cellsArtificial cell constructsViral ReceptorRed cell acanthocytosis
The present invention provides modified erythrocytes which comprise viral receptor proteins capable of mediating entry of respective viruses into the modified erythrocytes. The present invention also provides methods of using the modified erythrocytes for the treatment or prevention of viral infections. In one embodiment, the modified erythrocytes of the present invention comprise CD4 and at least one HIV coreceptor, such as CXCR4 or CCR5. The modified erythrocytes, when administered to an HIV patient, bind to the plasma virus and induce the injection of the HIV ribonucleoprotein complex into the cells. The entrapped viral content is either degraded or deactivated within the erythrocytes, or destroyed by erythrophagocytosis.
Owner:GLASER LAWRENCE F
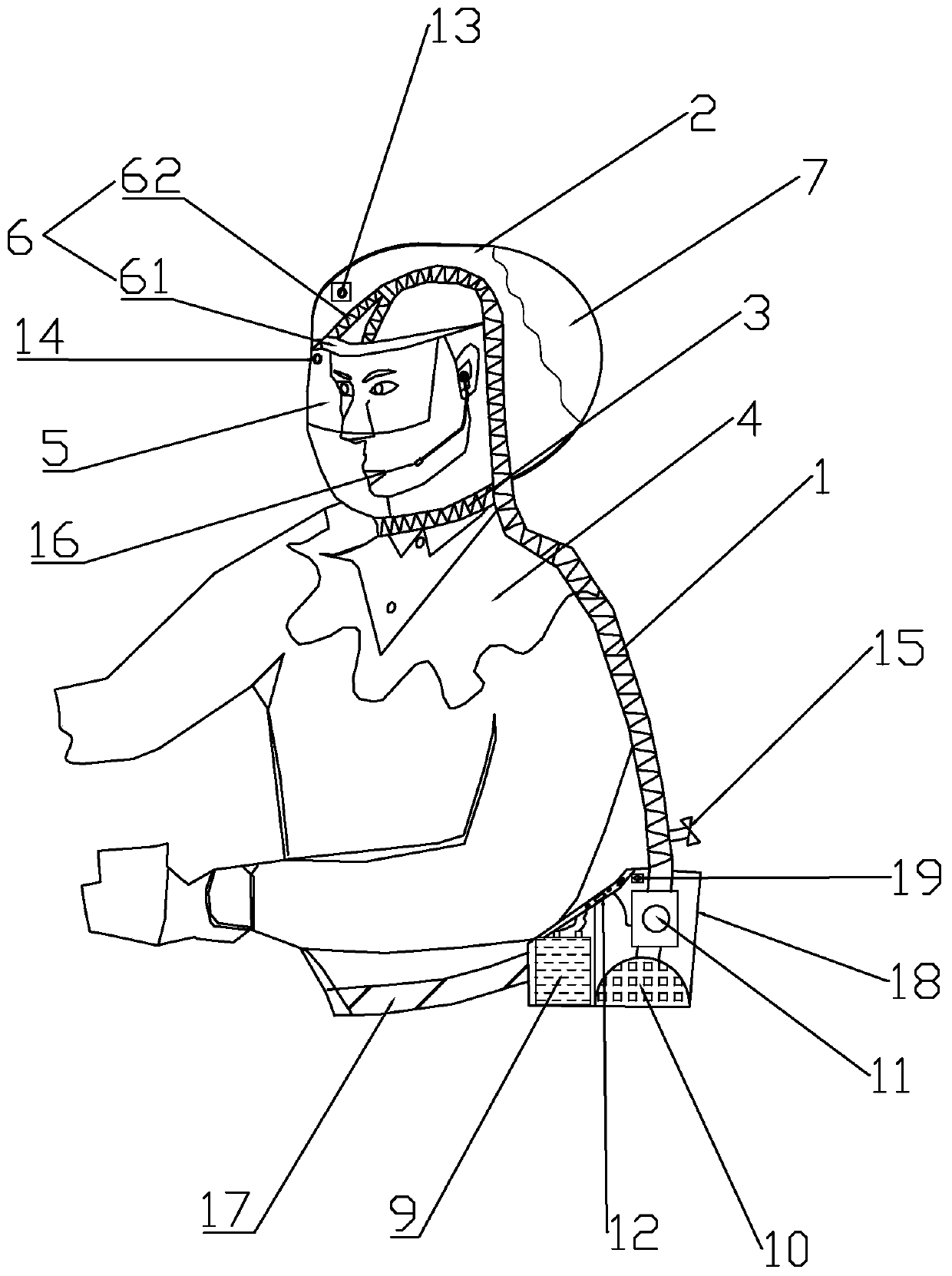
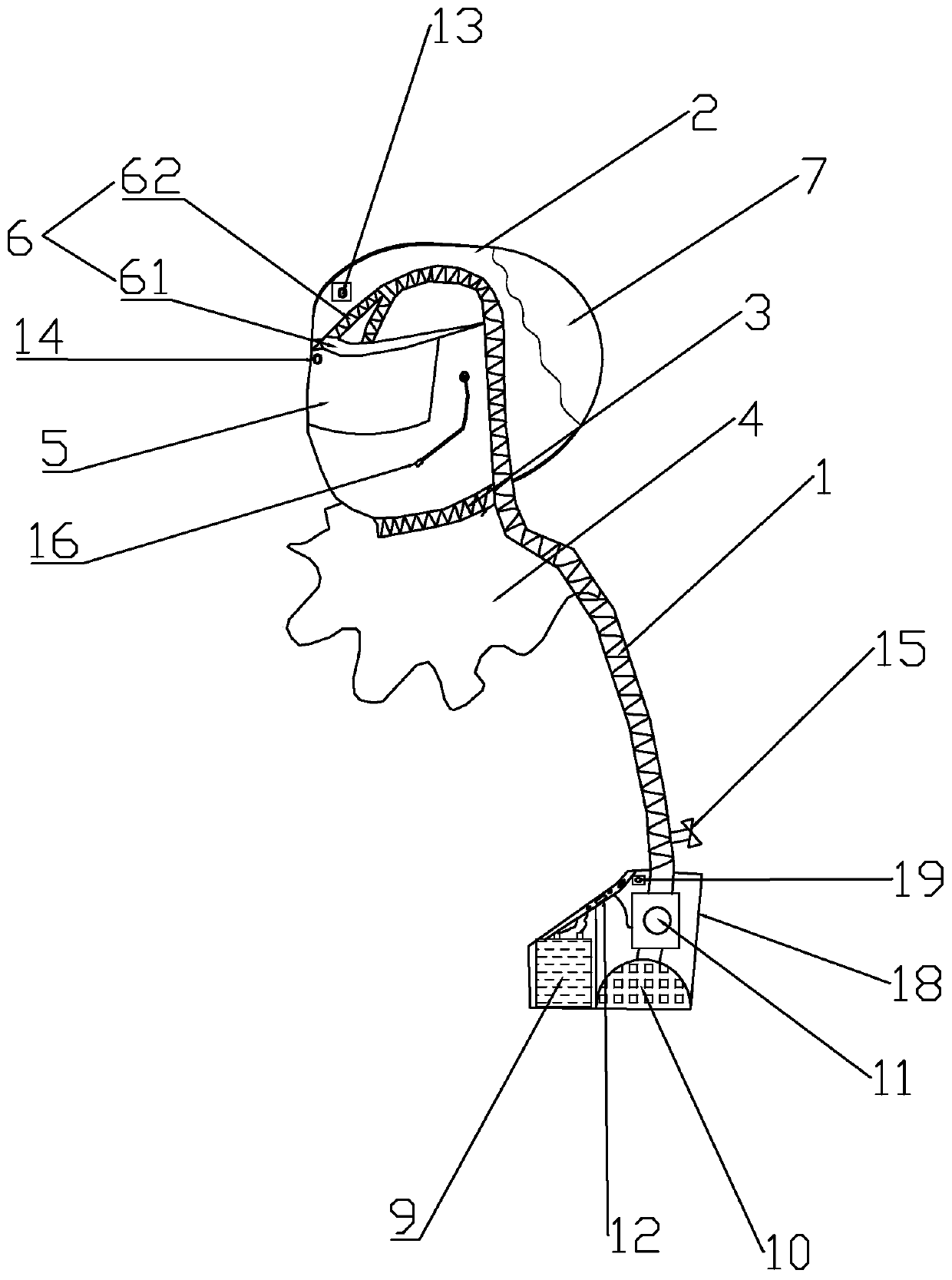
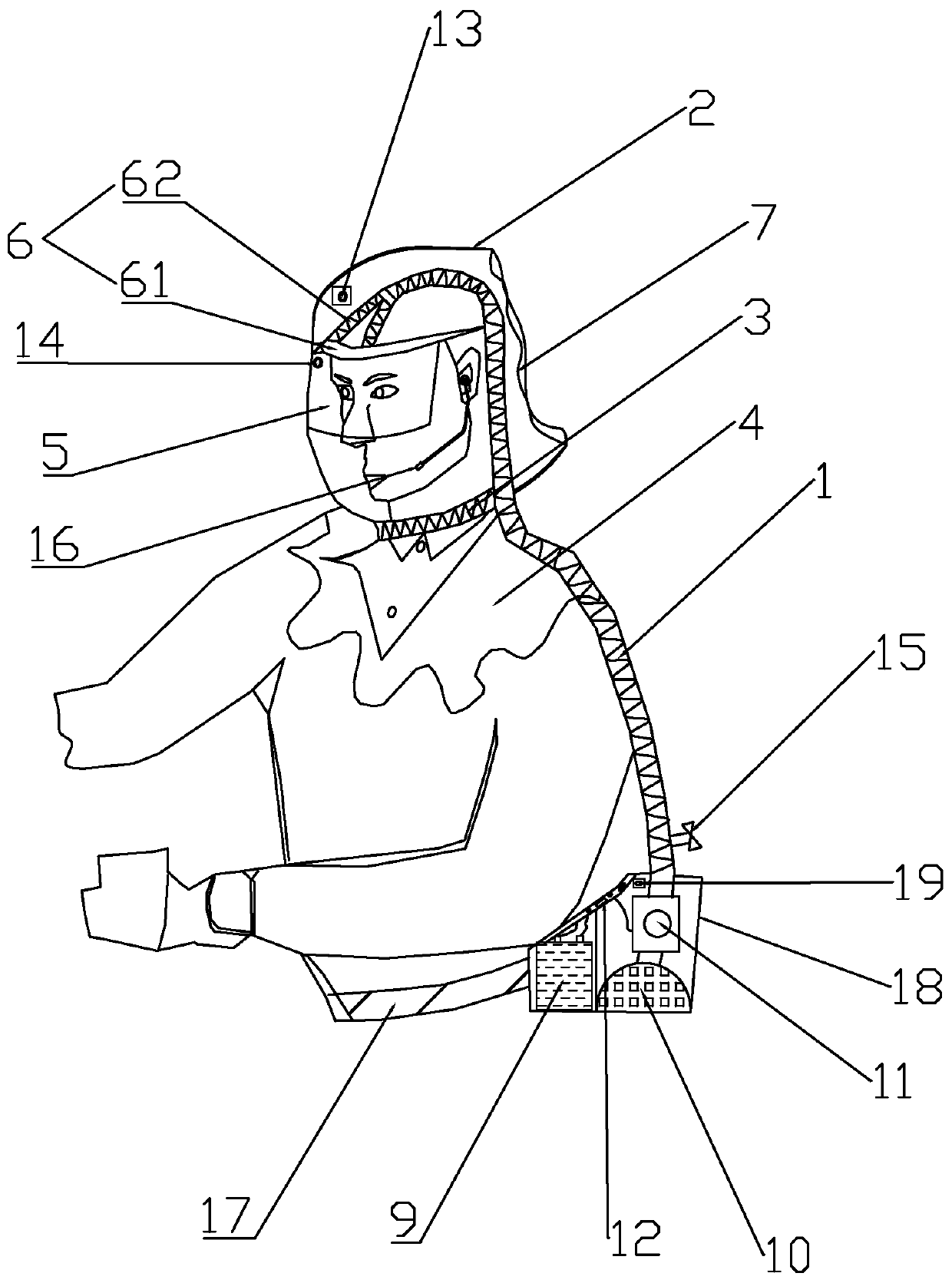
Positive pressure elastic protective head cover
PendingCN111195402AAvoid enteringAvoid tidal fluctuationsBreathing masksFire rescueEngineeringPlastic film
The invention provides a positive pressure elastic protective head cover. The positive pressure elastic protective head cover comprises a head cover body, an air guide pipe and an air pressurization and purification device, wherein the upper end of the air guide pipe is arranged in the head cover body; the lower end of the air guide pipe is connected to the air pressurization and purification device; the head cover body comprises a cover piece, a neck limiting sealing tape, a transparent lower hem, a visual window, a forehead fixed supporting part and a side transparent air storage bag; the whole surface of the side transparent air storage bag is made of a plastic film; the side transparent air storage bag is located on the side or rear of the cover piece; when the air pressure in an air storage chamber is reduced to a certain value, the side transparent air storage bag is collapsed inwards by the external atmospheric pressure; when the side transparent air storage bag is used, the resilience force of the elastic film of the side transparent air storage bag maintains the positive pressure in the head cover all the time, and the air pressure in the head cover is indicated through the change of the size of the air storage chamber; the air pressurization and purification device comprises a battery pack, a filter, an exhaust fan group and an electric control board; and the air guide pipe is used for supplying air into the head cover. Filtered clean air is injected through the mechanical pressurization in the head cover so as to prevent viruses from entering the respiratory tract to infect a user. The size change of the transparent air storage bag reliably indicates whether the air pressure in the head cover is appropriate, and thus, the safety is improved.
Owner:SUZHOU LIXIANG OPHTHALMIC HOSPITAL CO LTD +1
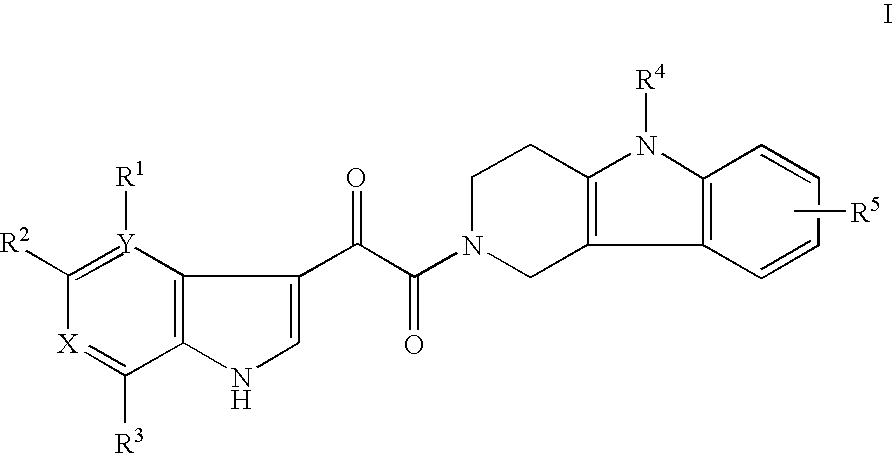
Tetrahydrocarboline antiviral agents
The invention encompasses a series of tetrahydrocarboline compounds of Formula I which inhibit HIV entry by attaching to the exterior viral envelop protein gp120 and interrupting the viral entry process, possibly by interfering with the cellular receptor CD4. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:VIIV HEALTHCARE UK (NO 5) LTD
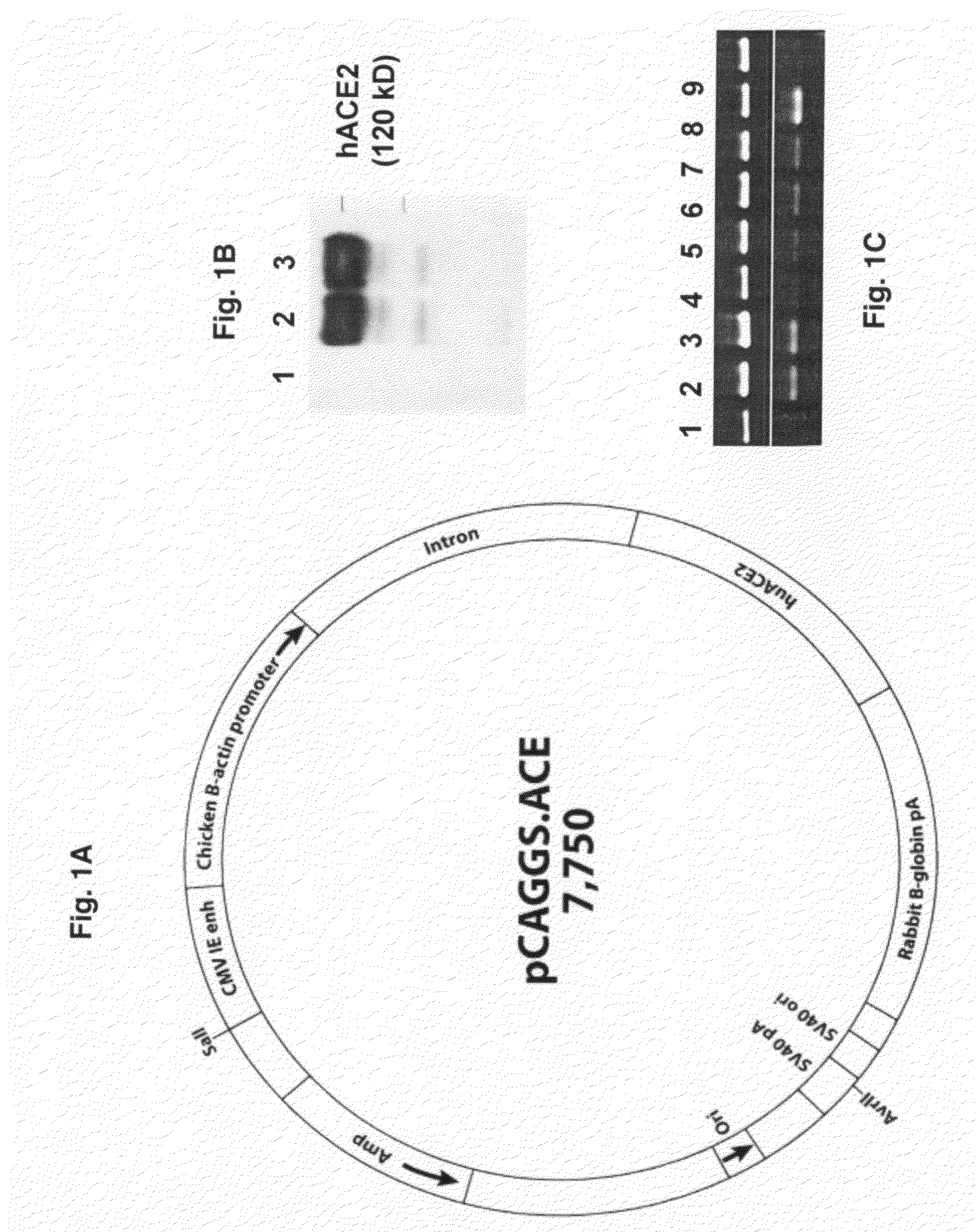
Transgenic Mouse Lines Expressing Human Ace2 and Uses Thereof
InactiveUS20090083865A1Prevents and alleviates symptomShortens courseHydrolasesBiological material analysisClinical manifestationMortality rate
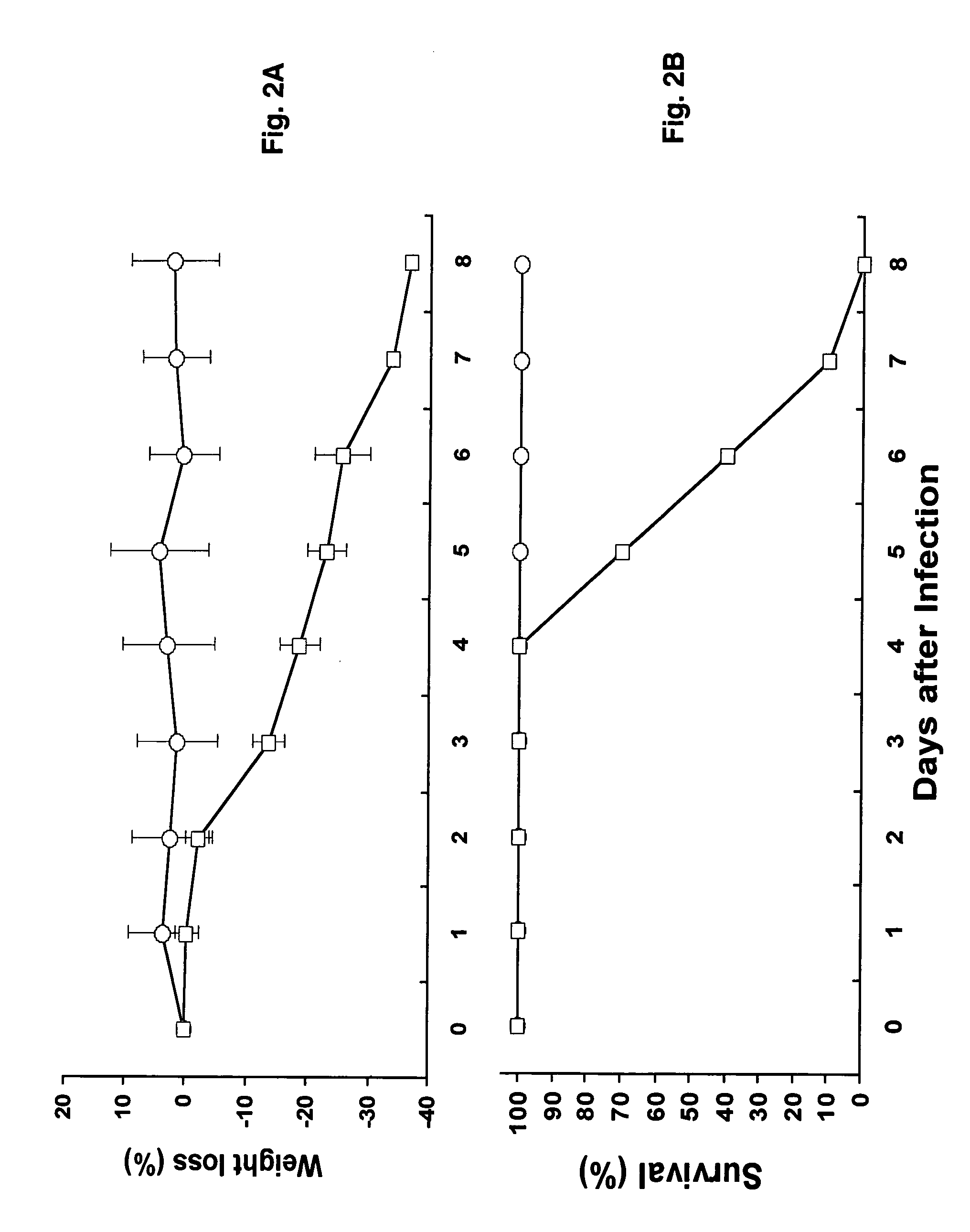
Animal models for severe acute respiratory syndrome-coronavirus infection of humans are needed to elucidate SARS pathogenesis and develop vaccines and antivirals. Transgenic mice were developed expressing human angiotensin-converting enzyme 2, a functional receptor for the virus, under the regulation of a global promoter. A transgenic lineage, designated AC70, was among the best characterized against SARS coronavirus infection, showing weight loss and other clinical manifestations before reaching 100% mortality within 8 days after intranasal infection. Inflammatory mediators were also detected in these tissues, coinciding with high levels of virus replication. In contrast, infected transgene-negative mice survived without showing any clinical illness. The severity of the disease developed in these transgenic mice, AC70 in particular, makes these mouse models valuable not only for evaluating the efficacy of antivirals and vaccines, but also for studying SARS coronavirus pathogenesis and infection by other coronaviruses utilizing human ACE2 for viral entry into cells.
Owner:CHAN TEH SHENG +3
Compositions and methods for inhibiting entry of a hepatic virus
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Solid- and solution-phase synthesis of heparin and other glycosaminoglycans
InactiveUS20050187381A1Sugar derivativesDisaccharidesHigh-Throughput Screening MethodsOligosaccharide
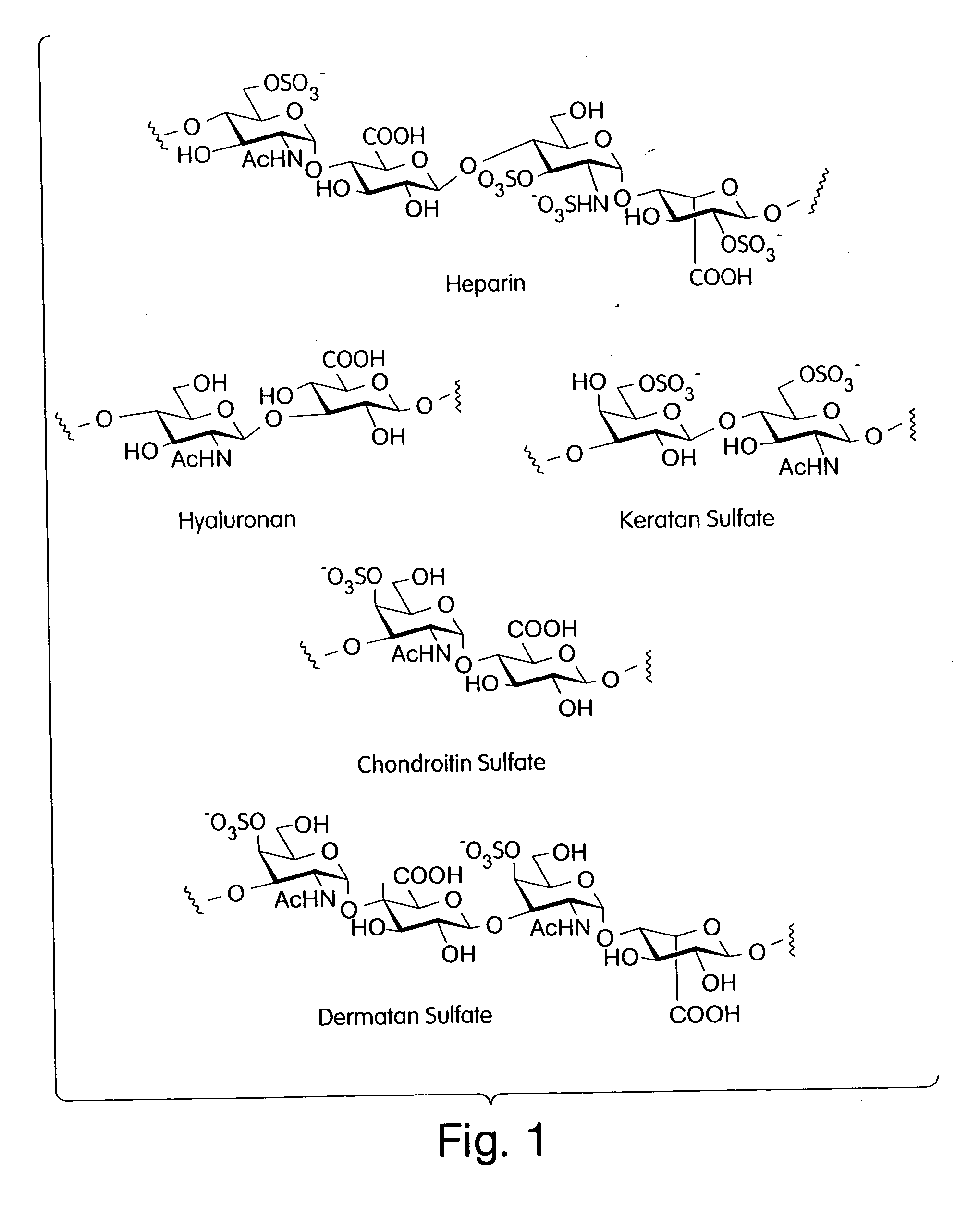
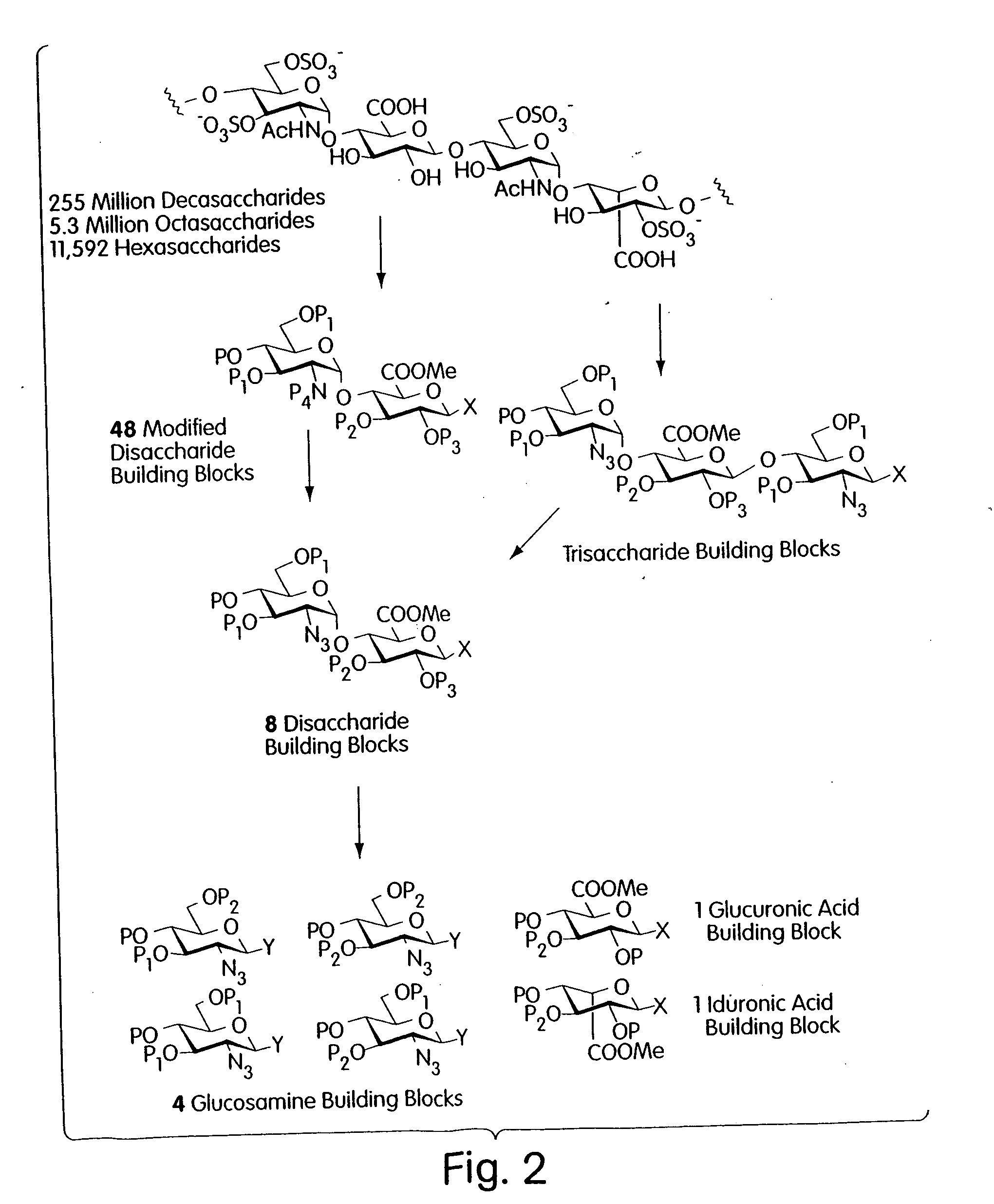
Described is a modular, general synthetic strategy for the preparation in solution and on a solid support of heparin, heparin-like glycosaminoglycans, glycosaminoglycans and non-natural analogs of each of them. Additionally, the modular strategy provides the basis for the preparation of combinatorial libraries and parallel libraries of defined glycosaminoglycan oligosaccharides. The defined glycosaminoglycan structures may be used in high-throughput screening experiments to identify carbohydrate sequences that regulate a host of recognition and signal-transduction processes. The determination of specific sequences involved in receptor binding holds great promise for the development of molecular tools which will allow modulation of processes underlying viral entry, angiogenesis, kidney diseases and diseases of the central nervous system. Notably, the present invention enables the automated synthesis of glycosaminoglycans in much the same fashion that peptides and oligonucleotides are currently assembled.
Owner:SEEBERGER PETER H +2
Compositions and methods for inhibiting entry of a hepatic virus
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
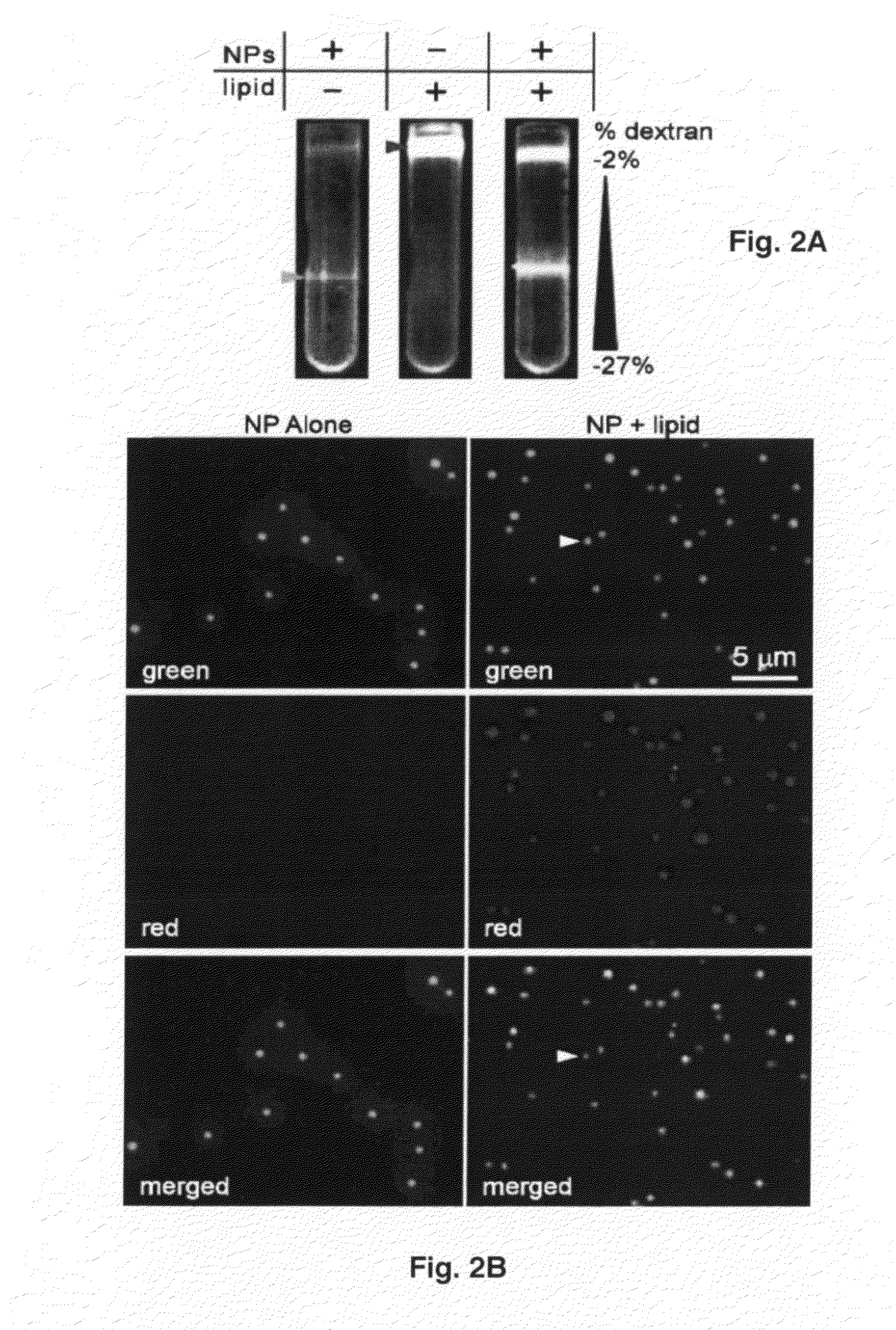
Virus coated nanoparticles and uses thereof
The present invention discloses method to coat nanoparticles with viral envelope containing specific proteins. The present invention also discloses that such viral envelope coated nanoparticles can be targeted to specific cells and cellular entry pathway, thereby permitting their use as vaccines, in targeted delivery of therapeutic products and in the study of virus adsorption, cell penetration and viral entry pathways.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
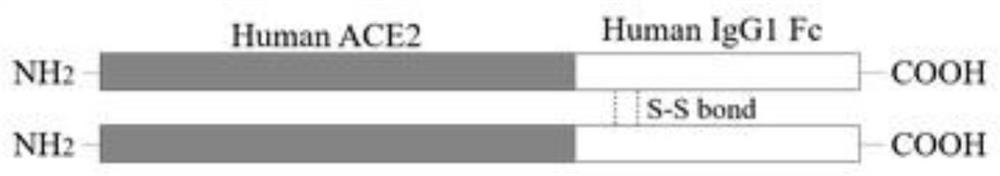
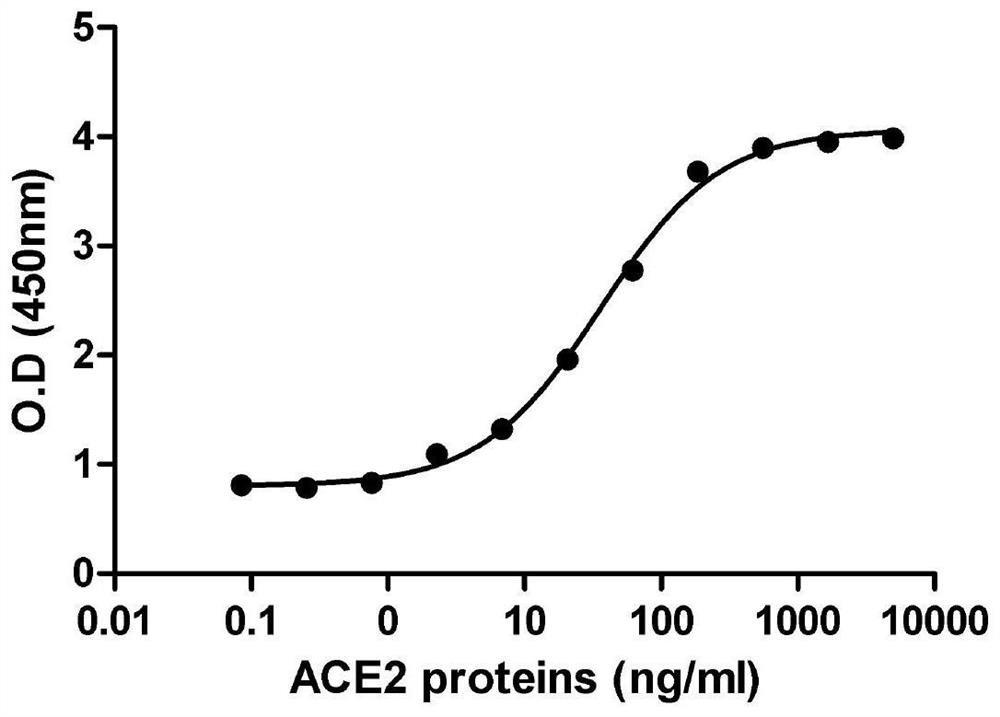
ACE2-Fc fusion protein function test method for treating COVID-19
PendingCN112226424APrevent or prevent accessSlow down the progress of the infectionAntibody mimetics/scaffoldsBiological material analysisWild typeA-DNA
The invention provides an ACE2-Fcfusion protein function test method for treating COVEID-19. The ACE2-Fc fusion protein function test method specifically comprises the following steps of expressing ahuman wild type ACE2-Fc fusion protein: cloning a DNA sequence of an extracellular domain (ECD) of human wild type ACE; enabling the ECD of the ACE2 to be fused with the Fc part of the human IgG1, sothat the half-life period of the soluble ACE2 is prolonged; expressing the ACE2-Fc fusion protein in CHO cells, and purifying the ACE2-Fc fusion protein in a cell culture supernatant by using proteinA / G agarose; and optimized an expression system. The invention effectively proves that the ACE2-Fc fusion protein can prevent or block viruses from entering cells, slow down the infection process andexpose the viruses to the outside of the cells so as to be recognized and eliminated by a human immune system.
Owner:苏州新格诺康生物技术有限公司
Compounds for inhibition of HIV infection by blocking HIV entry
ActiveUS7241803B2Potent anti-HIV activityInhibit HIV replicationBiocideOrganic chemistryFluorescenceCoiled coil
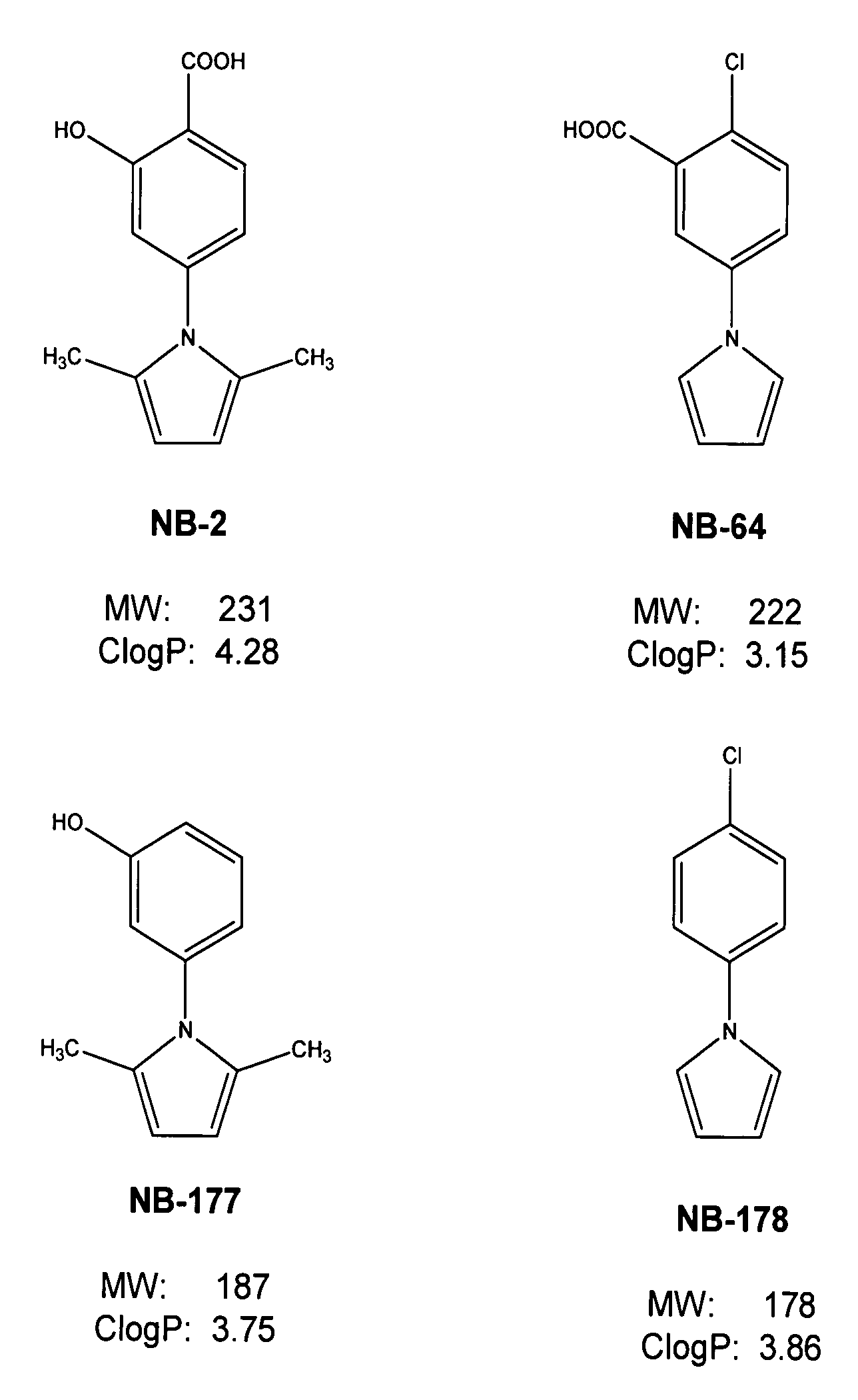
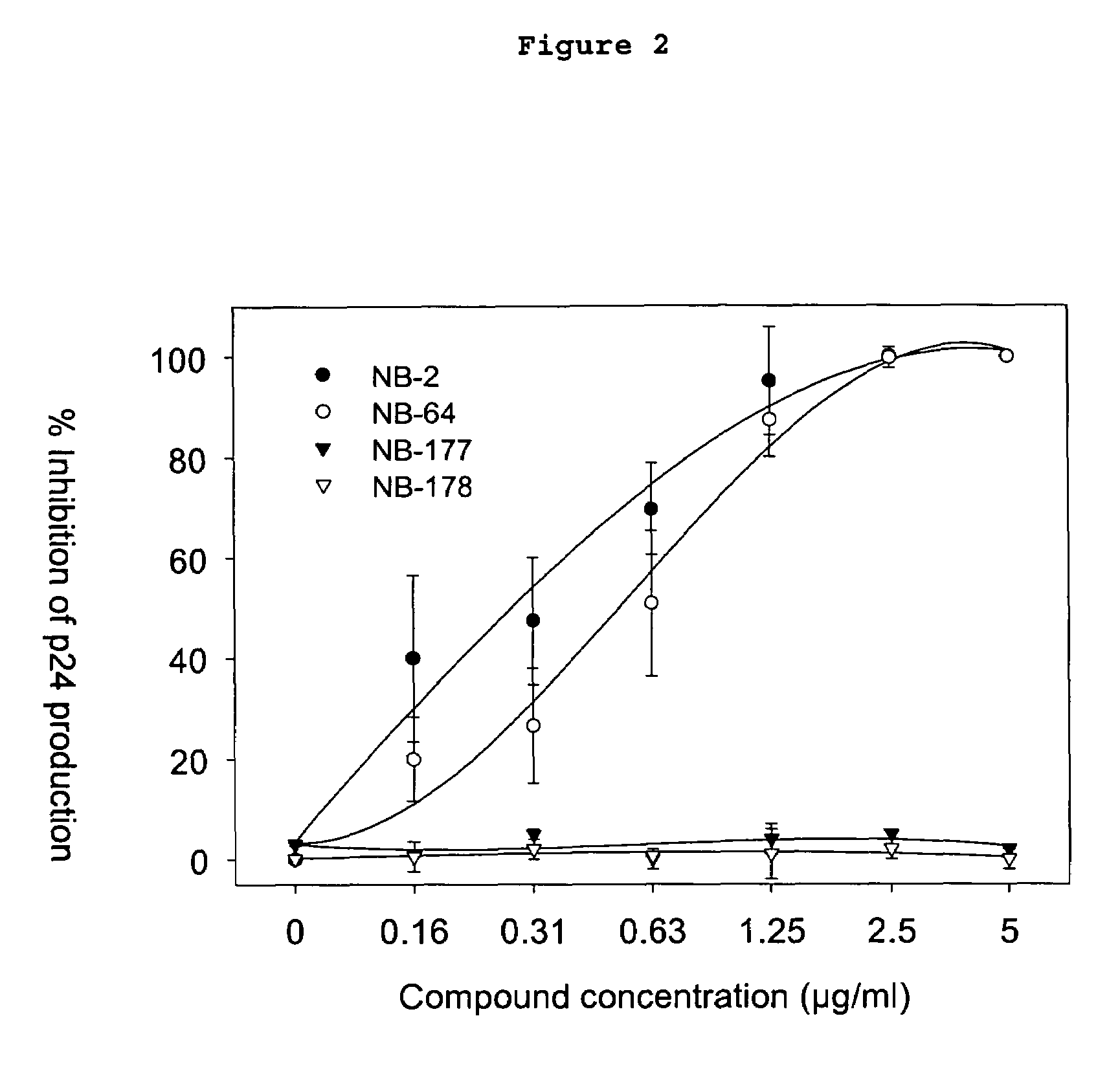
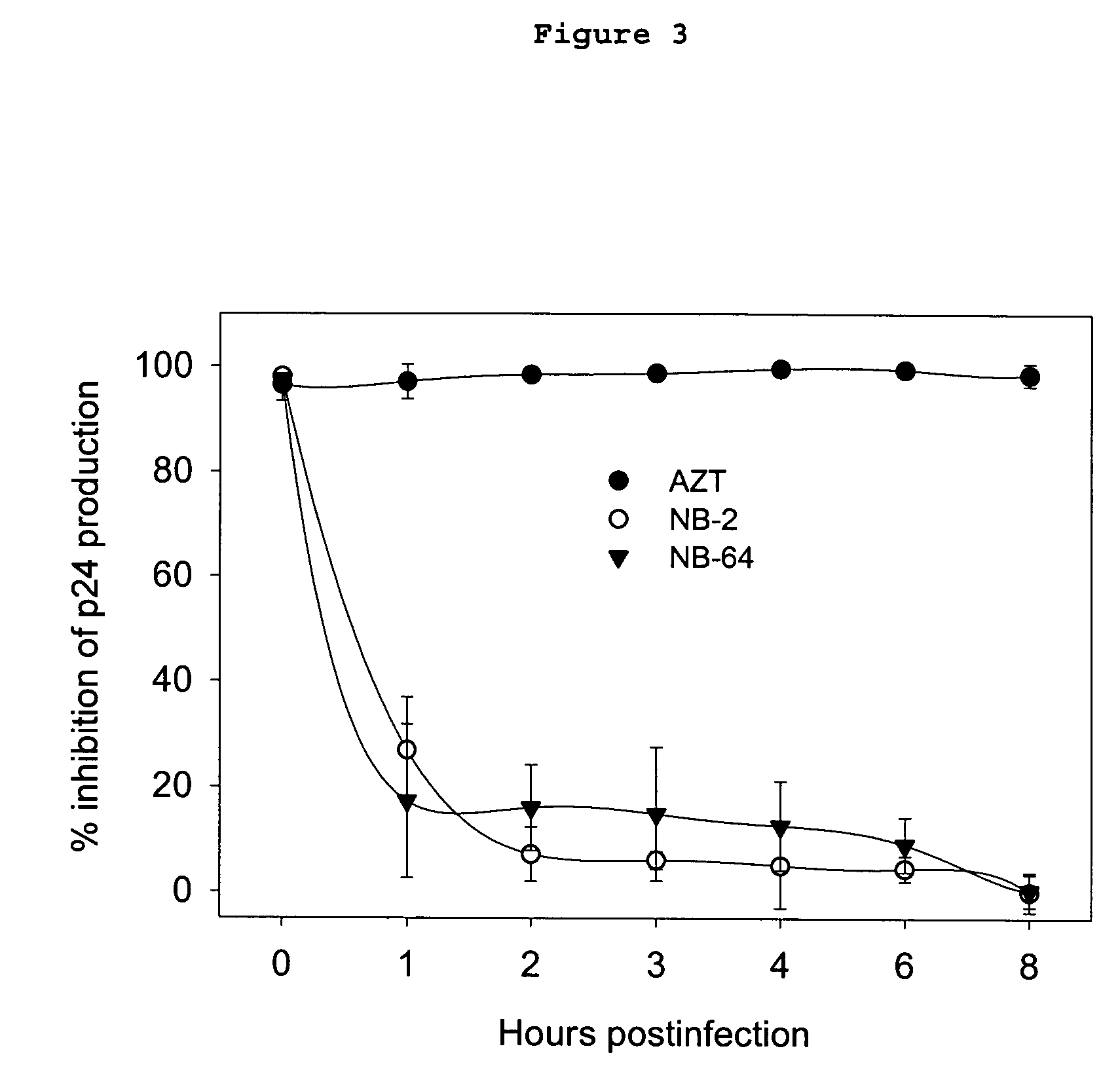
A group of compounds that inhibit HIV replication by blocking HIV entry was identified. Two representative compounds, designated NB-2 and NB-64, inhibited HIV replication (p24 production) with IC50 values <0.5 μg / ml. It was proved that NB-2 and NB-64 are HIV entry inhibitors by targeting the HIV gp41 since: 1) they inhibited HIV-mediated cell fusion; 2) they inhibited HIV replication only when they were added to the cells less than one hour after virus addition; 3) they did not block the gp120-CD4 binding; 4) they did not interact with the coreceptor CXCR4 since they failed to block anti-CXCR4 antibody binding to CXCR4-expressing cells; 5) they blocked the formation of the gp41 core that is detected by sandwich enzyme linked immunosorbent assay (ELISA) using a conformation-specific MAb NC-1; 6) they inhibited the formation of the gp41 six-helix bundle revealed by fluorescence native-polyacrylamide gel electrophoresis (FN-PAGE); and 7) they blocked binding of D-peptide to the hydrophobic cavity within gp41 coiled coil domain, modeled by peptide IQN17. These results suggested that NB-2 and NB-64 may interact with the hydrophobic cavity and block the formation of the fusion-active gp41 coiled coil domain, resulting in inhibition of HIV-1 mediated membrane fusion and virus entry.
Owner:NEW YORK BLOOD CENT
Modified and fusion enhanced erythrocytes, cells and uses thereof
InactiveUS20110091973A1Short half-lifeGenetically modified cellsBlood/immune system cellsADAMTS ProteinsChemokine receptor CCR5
Modified fusion enhanced erythrocytes (or other cell types and synthetic cells) including human viral receptor proteins, human viral coreceptor proteins and viral derived proteins capable of mediating entry of respective viruses into the modified erythrocytes, cells or pseudo-cells and the method of using the fusion enhanced modified erythrocytes, cells or pseudo-cells for the treatment or prevention of viral infections. The fusion enhanced modified erythrocytes comprises CD4 and at least one HIV coreceptor, such as CXCR4 or CCR5 and as well, at least one of cholesterol rafts, fusin, actin, a viral derived protein such as fusion peptide derived from HIV GP120 or HIV GP41 or a shorter protein derived from a long viral protein, such as a portion of HIV derived GP120, or HIV GP41 such as the 23 N-terminal peptide of the HIV-1 gp 41 protein (AVGIGALFLGFLGAAGSTMGARS) called FP23 (Fusion Peptide). These viral-fusion enhanced cells may also be electrostatic charge enhanced through further additions named in this invention. The modified erythrocytes, when administered to an HIV patient, bind to the plasma virus and induce the injection of the HIV ribonucleoprotein complex into the cells. The entrapped viral content is sequestered within said cell for at least the period of time that the cell maintains its outer membrane integrity. The virus is thereafter either degraded or deactivated within the erythrocytes, cells or pseudo-cells, or destroyed by erythrophagocytosis.
Owner:GLASER LARRY F
Method for preparing rabies vaccines for human use
InactiveCN104027800AIncreased toxin productionLess quantityAntiviralsRecovery/purificationHuman useVaccination
The invention provides a method for preparing rabies vaccines for human use, which is used for solving the problems of low virulence and low yield of viruses harvested after virus vaccination in the prior art. The method for preparing rabies vaccines for human use comprises the steps of 1) reviving and inheriting production cells, and then putting the inherited production cells into a microcarrier bioreactor for multiplication culture; 2) adding a treatment solution into the microcarrier bioreactor, adjusting the perfusion rate of the microcarrier bioreactor to be zero, and cleaning the surfaces of the production cells; 3) vaccinating rabies viruses to the cleaned production cells to fix strains; 4) harvesting a virus solution, and performing ultra-filtration concentration and inactivation; 5) separating and purifying. The method disclosed by the invention has the advantages that before the viruses are vaccinated, the cells are treated by using the treatment solution, so that the quantity of the viruses entering the cells is increased; after the viruses are vaccinated, the yield of the viruses is greatly improved, and the times of ultra-filtration concentration is reduced.
Owner:SHANDONG YIDU BIOTECH
Biological preparation for preventing and controlling human respiratory syncytial virus infection and preparation method
ActiveCN104353063AImprove stabilityLow costPeptide/protein ingredientsAntiviralsHuman respiratory virusViral infection
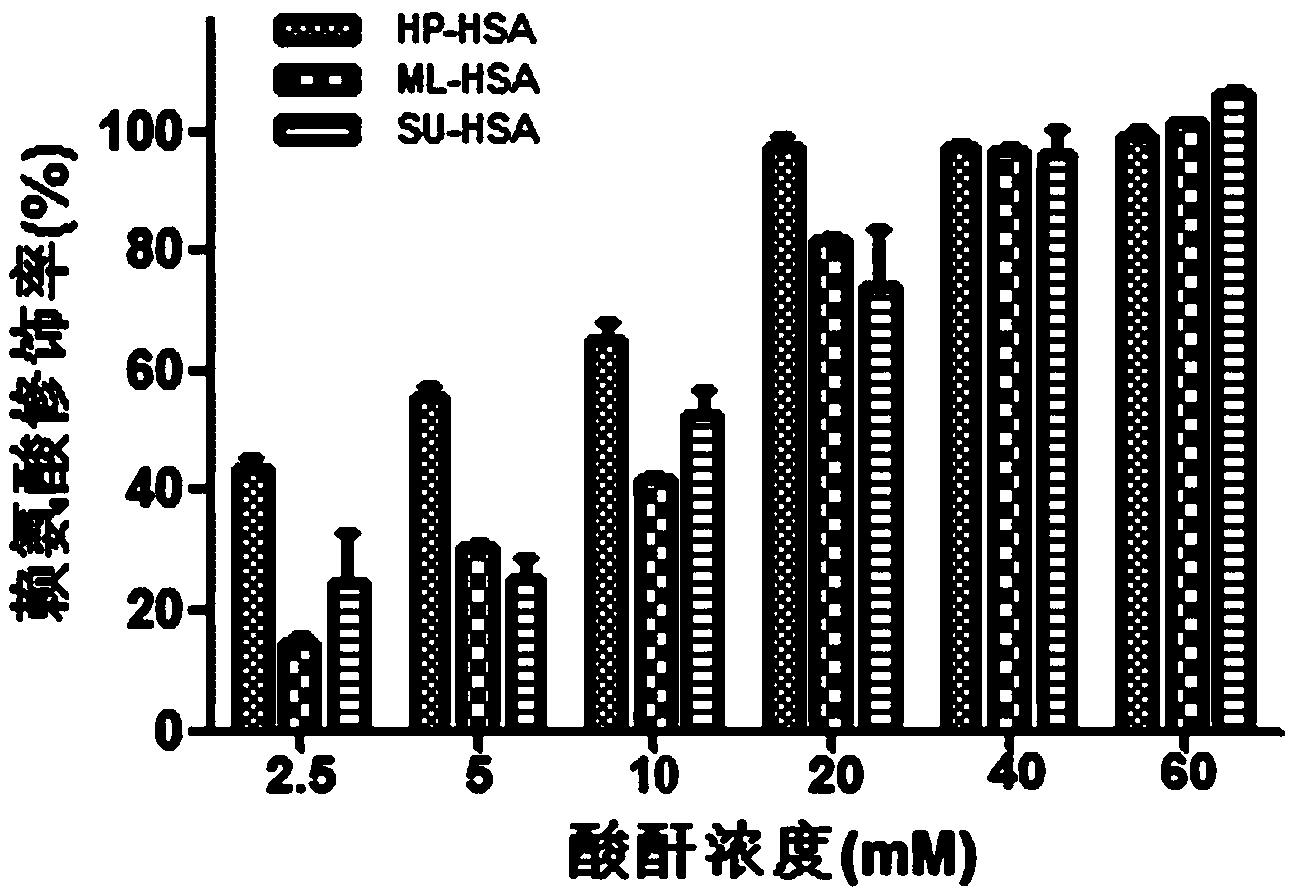
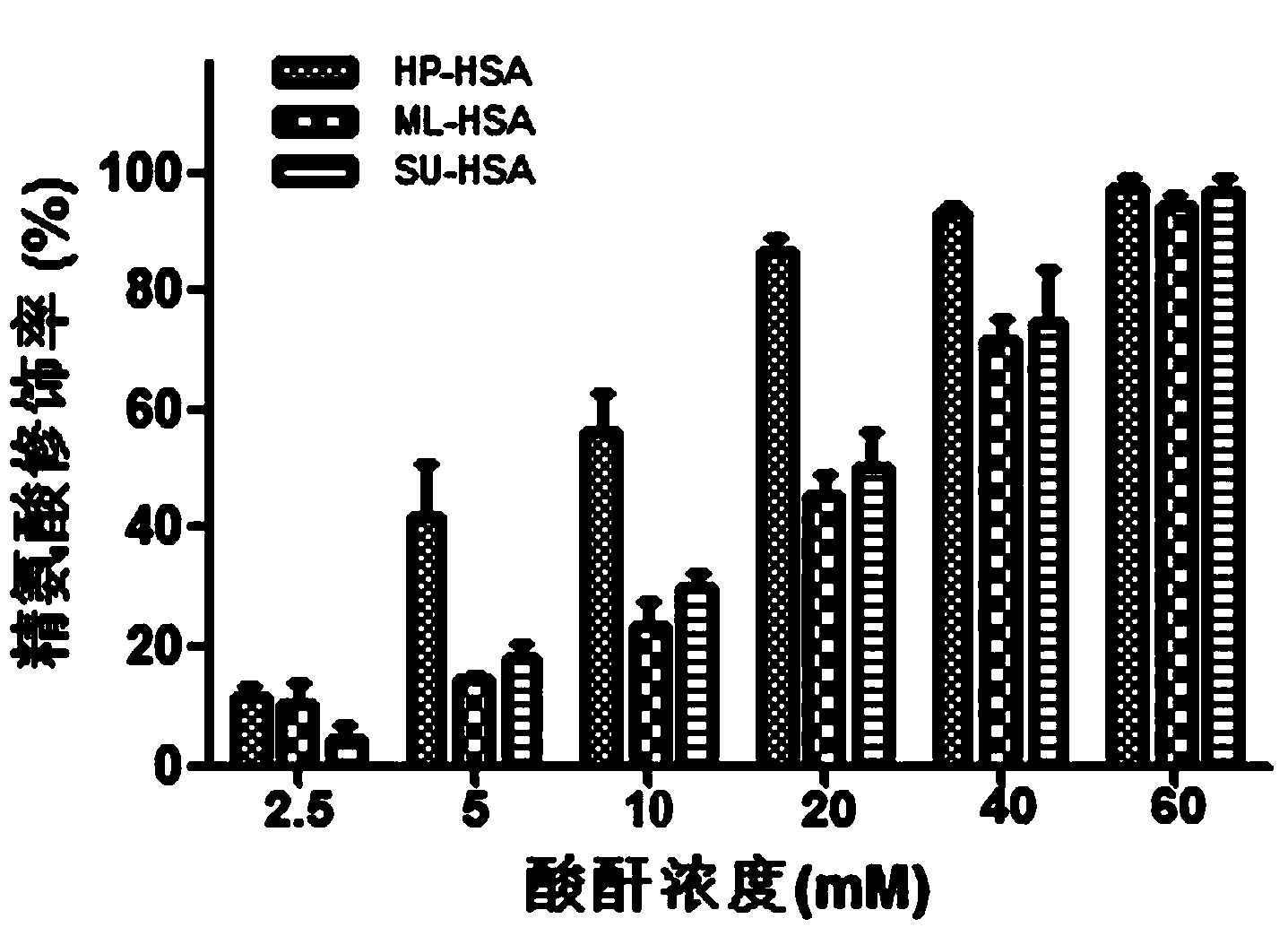
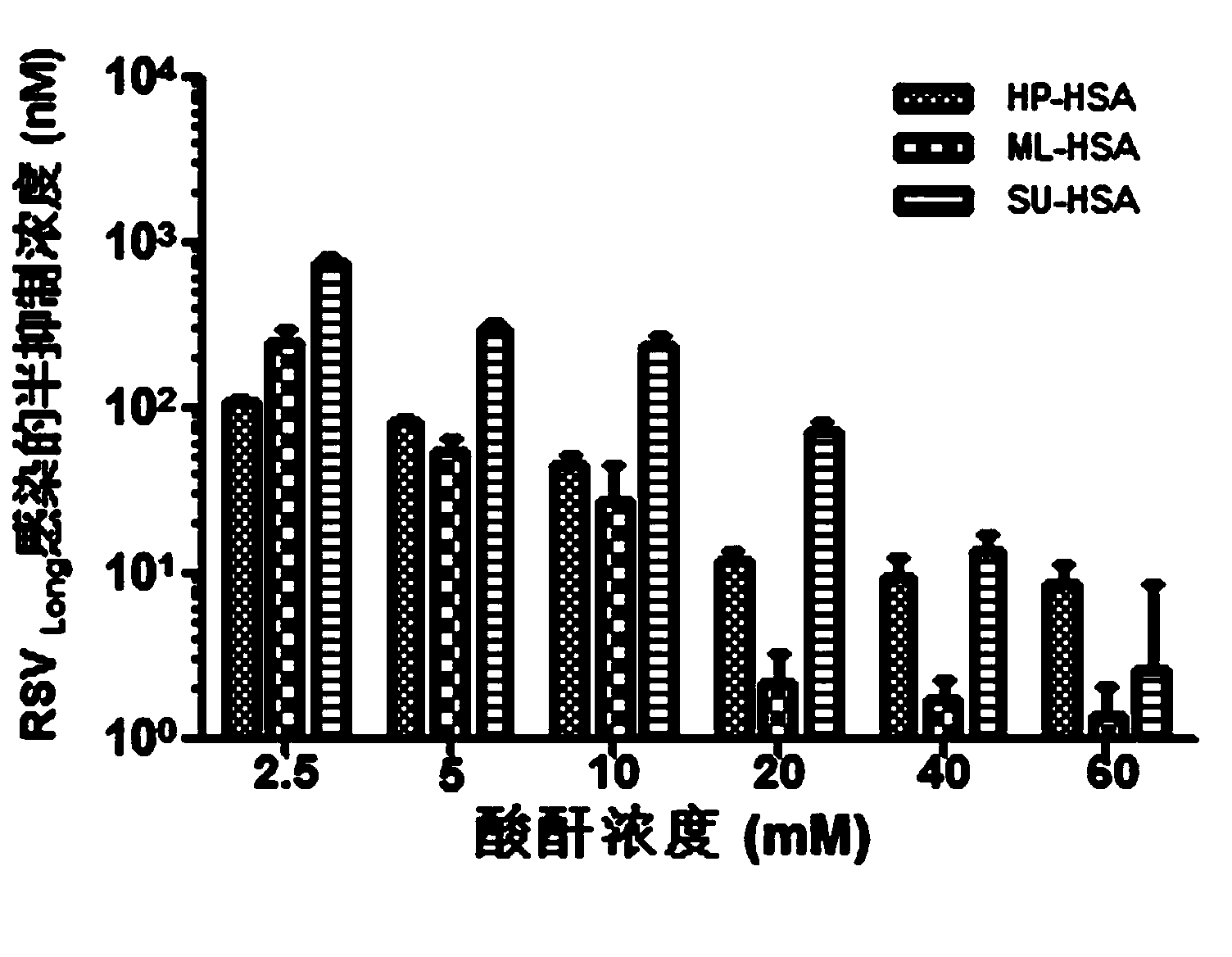
The invention relates to a novel antiviral biological preparation in the technical field of medicines, in particular to a biological preparation for preventing and controlling human respiratory syncytial virus infection and a preparation method. The biological preparation adopts globulin virus entry or fusion inhibitors. The biological preparation is applied to the first stage that virus invades target cells, and blocks the infection of virus to the cells, so as to achieve the effect of preventing and controlling the virus. The biological preparation adopts active anhydride to modify separated and purified amino acid with positive charge on the surface, thereby having the function of preventing the human RSV (respiratory syncytial virus) from entering and infecting the target cells.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Triterpene-amino acid derivative as well as preparation method and application thereof
The invention relates to application of a triterpene derivative in a formula in preparation of medicines for preventing or treating influenza diseases. The definition of substituent groups is shown ina description. The triterpene derivative has obvious inhibiting function on influenza viruses, and can obviously inhibit the influenza viruses from entering cells. The formula is shown in the description.
Owner:KUNMING UNIV OF SCI & TECH
Generation of virus-like particles and use as panfilovirus vaccine
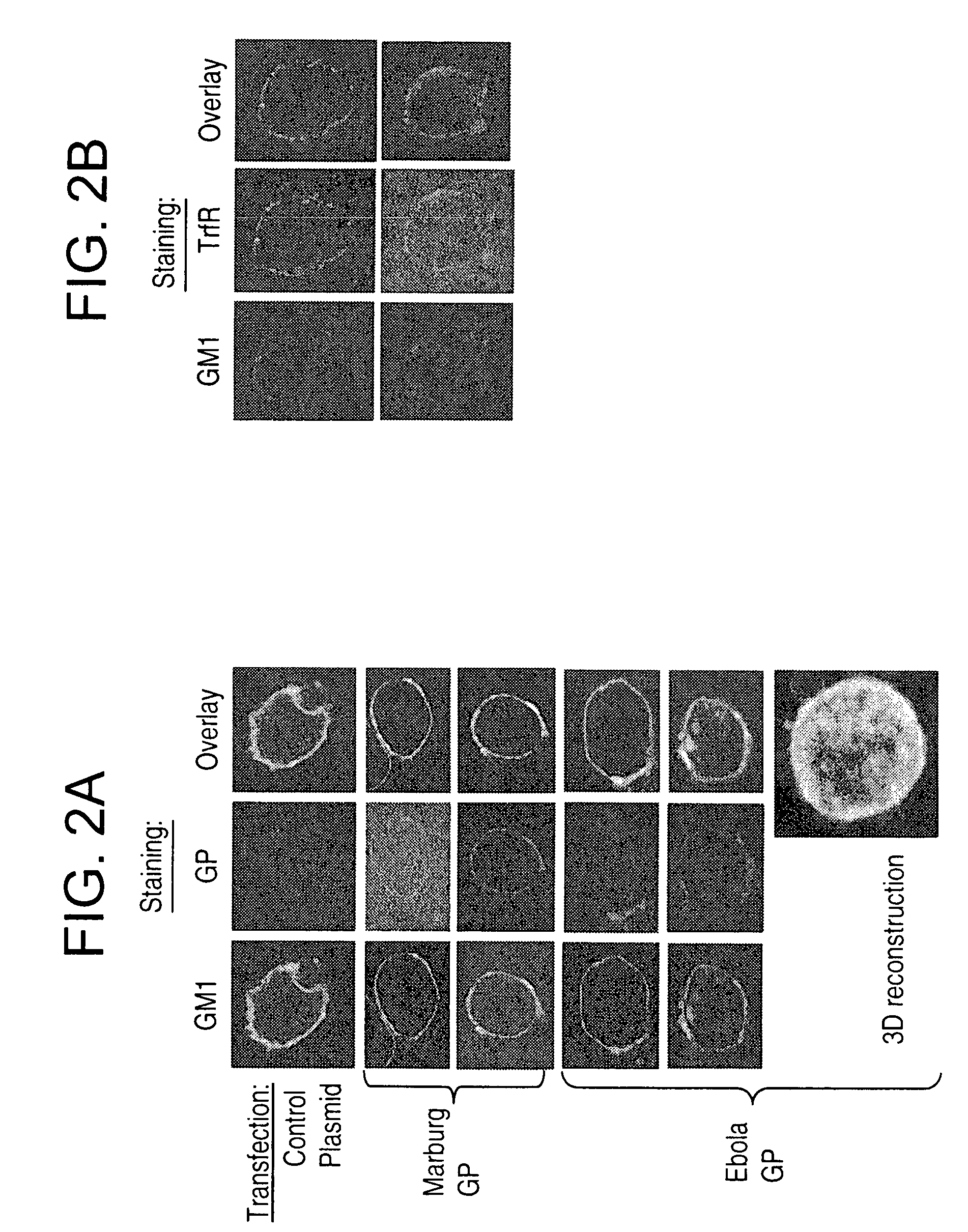
InactiveUS20060099225A1Avoid enteringReduced abilitySsRNA viruses negative-senseVectorsBuddingLipid formation
In this application are described filovirus-like particles for both Ebola and Marburg and their use as a diagnostic and therapeutic agent as well as a filovirus vaccine. Also described is the association of Ebola and Marburg with lipid rafts during assembly and budding, and the requirement of functional rafts for entry of filoviruses into cells.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Application of Yiqing compound preparation to preparation of drugs for preventing/treating novel coronavirus infection
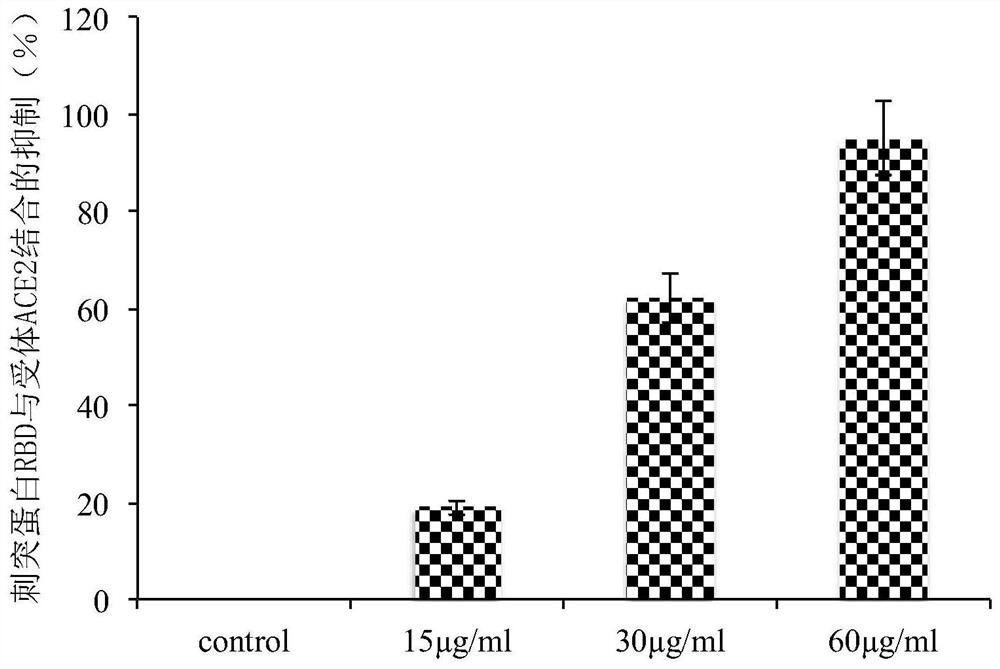
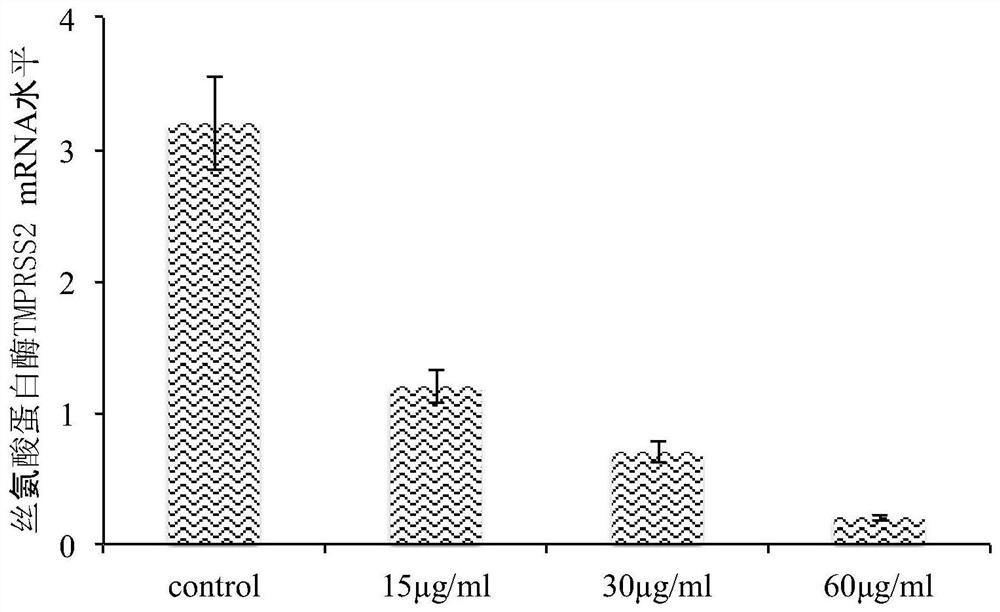
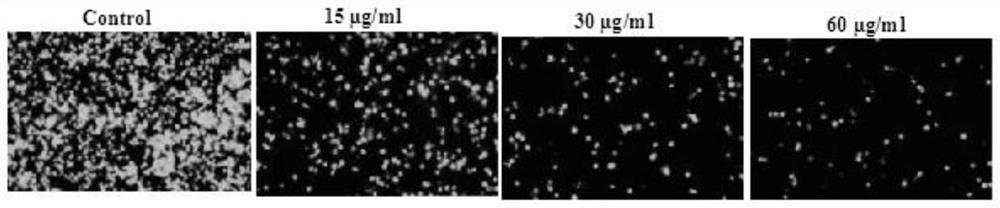
ActiveCN111773275AInhibit entryInhibitionAntiviralsAgainst vector-borne diseasesDiseaseInflammatory factors
The invention discloses an application of Yiqing compound preparation to preparation of drugs for preventing / treating novel coronavirus infection. The Yiqing compound preparation is prepared from bulkdrugs of rhizoma coptidis, radix et rhizoma rhei and radix scutellariae. A drug screening model for blocking novel coronavirus spike glycoprotein and target cell receptor angiotensin converting enzyme 2 (ACE2) is constructed, and the model shows that the Yiqing compound preparation has activity of inhibiting binding of novel coronavirus spike glycoprotein and target cell receptor ACE2 and inhibiting expression of TMPRSS2 (transmembrane protease serine 2) to prevent viruses from entering target cells, and can significantly inhibit production of inflammatory factors (IL-2, IL-6, etc.) by virus-infected cells. The Yiqing compound preparation can be applied to prevention and treatment of infectious diseases in novel coronavirus, provides a drug choice for novel coronavirus patients, and has great significance in current epidemic prevention and control.
Owner:THE KEY LAB OF CHEM FOR NATURAL PROD OF GUIZHOU PROVINCE & CHINESE ACADEMY OF SCI
Pseudotyped retroviruses and stable cell lines for their production
InactiveUS7033595B1Effective blockingSsRNA viruses positive-senseVectorsViral glycoproteinNucleotide
Cells that produce inventive pseudotyped retroviruses having a broad host range have been produced. In one aspect of the invention, the cells produce retroviruses pseudotyped with at least two different viral glycoproteins, such as togaviral glycoproteins. In alternative embodiments, the cells produce retroviruses pseudotyped with filoviral glycoproteins. Methods of producing the above-described cells, as well as the pseudotyped retroviruses thus produced, are also provided. In other embodiments, methods of screening agents effective in blocking viral entry into a cell, including filoviral entry or entry of viruses having at least two different viral glycoproteins disposed in their lipid bilayer, such as togaviruses, are provided. Moreover, methods of using the inventive pseudotyped retroviruses for introducing nucleotide sequences into target cells, and kits for forming the inventive pseudotyped retroviruses, are also provided.
Owner:PURDUE RES FOUND INC
Multi-objective transport path optimization method based on HIV epidemic dynamics
InactiveCN106126897AThe total number remains the sameImplement random searchEpidemiological alert systemsSpecial data processing applicationsCrowdsSexual intercourse
The invention discloses a multi-objective transport path optimization method based on HIV epidemic dynamics. It is assumed that an ecosystem consists of a number of crowds; an HIV virus spreads in the crowds, and people who are in effective contact with people with the HIV virus, are infected with the infectious disease; people who are not infected with the HIV virus are known as susceptible persons; after the susceptible persons are infected with the HIV virus, immediate onset does not occur, and the virus in the body enters an incubation period; people, whose HIV virus is in the incubation period, are known as HIV virus carriers, and such people are divided into two kinds, one kind is people who do not have the disease and have sexual intercourse, the kind of people become AIDS patients after a certain period of time, and ultimately die due to the AIDS; the other kind is people who have never had the disease and lose the sexual intercourse ability, and the kind of people eventually die a natural death; and under the action of the HIV virus, the growth state of each person is randomly changed, and by using the random variation and an HIV epidemic model, a global optimal solution of the multi-objective transport path combined optimization problem can be quickly obtained.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
Compositions and methods for inhibiting viral entry
ActiveUS20150297677A1Improve purification effectAvoid infectionBiocidePeptide/protein ingredientsT cell immunityPhosphatidylserine receptors
The invention provides methods, compositions, and kits featuring agents that inhibit viral entry mediated by T-cell Immunoglobulin and Mucin-domain containing proteins (TIM proteins) and other phosphatidylserine receptors.
Owner:DANA FARBER CANCER INST INC +1
Method of inhibiting infection by HCV, other flaviviridae viruses, and any other virus that complexes to low density lipoprotein or to very low density lipoprotein in blood preventing viral entry into a cell
InactiveUS20080213287A1Preventing cellular endocytosisBlocking binding of the lipoprotein complexCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsVery low-density lipoproteinFlaviviridae
A method of inhibiting infection by Flaviviridae viruses including HCV, GBC / HGV, and BVD in addition to VSV and any other virus capable of forming a complex with a lipoprotein strategies: preventing formation of a complex should one form, altering the conformation of such a complex to prevent its interaction with the cell receptor, blocking the cell receptor for the complex using an antibody to the receptor, blocking binding of the lipoprotein complex to the cell receptor using soluble lipoprotein receptor or fragments thereof, or downregulating the LDL receptor activity of the cells.
Owner:AGNELLO VINCENT
Application of Letemovir in preparation of SARS-CoV-2 novel coronavirus inhibitor
The invention belongs to the technical field of medicines, and particularly relates to application of Letemovir in preparation of an SARS-CoV-2 novel coronavirus inhibitor. Research finds that the Letemovir can be combined with an RBD region, combined with an ACE-2 receptor, on a spike protein S1 of SARS-CoV-2 novel coronavirus to prevent the virus from entering cells, so that virus replication isinhibited. Therefore, the Letemovir can be used as an inhibitor of the SARS-CoV-2 novel coronavirus, a new thought is provided for preventing and / or treating the SARS-CoV-2 novel coronavirus, a theoretical basis is provided for development of clinical tests, and the Letemovir has important medical research value.
Owner:南京双运生物技术有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com