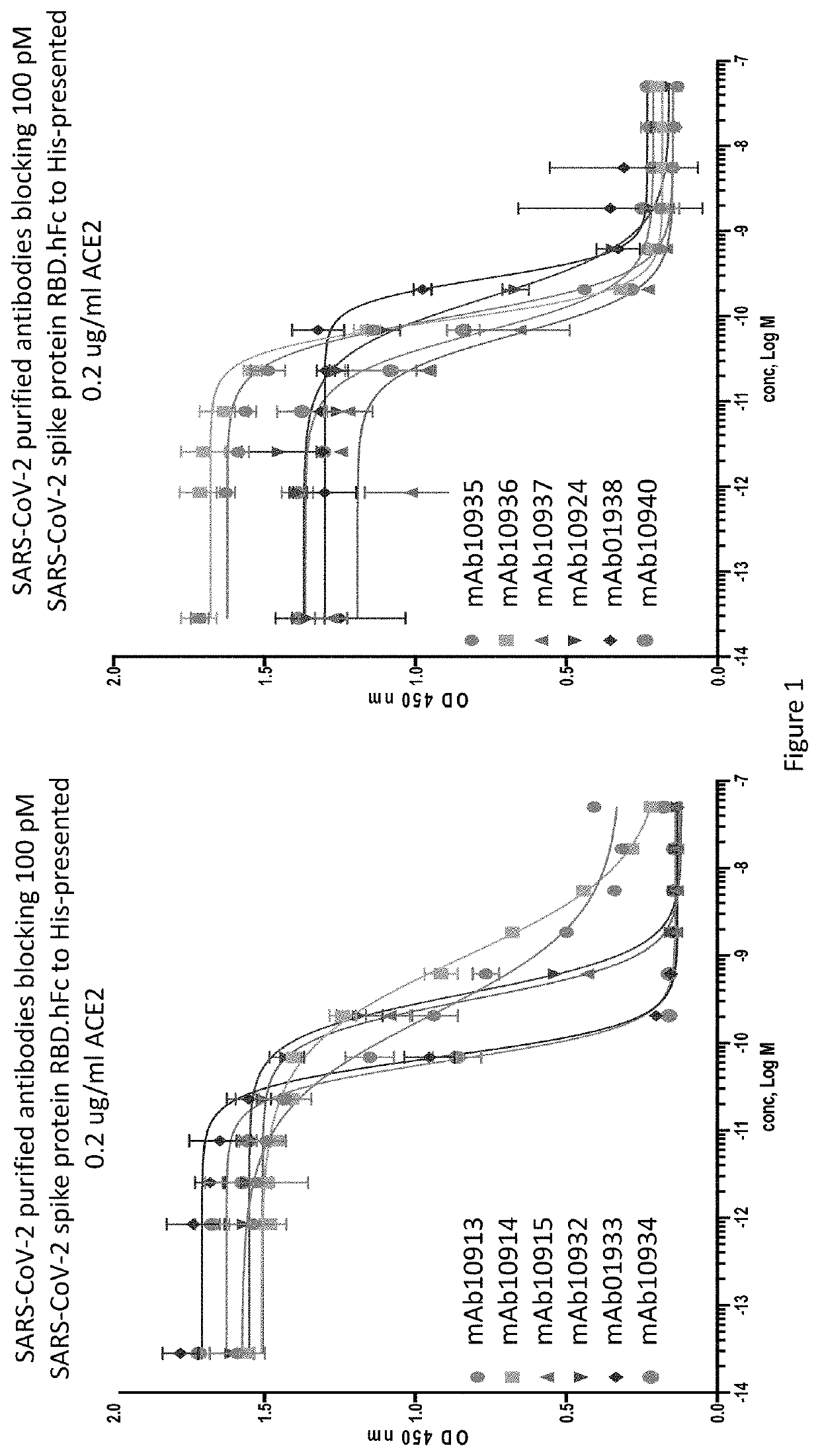
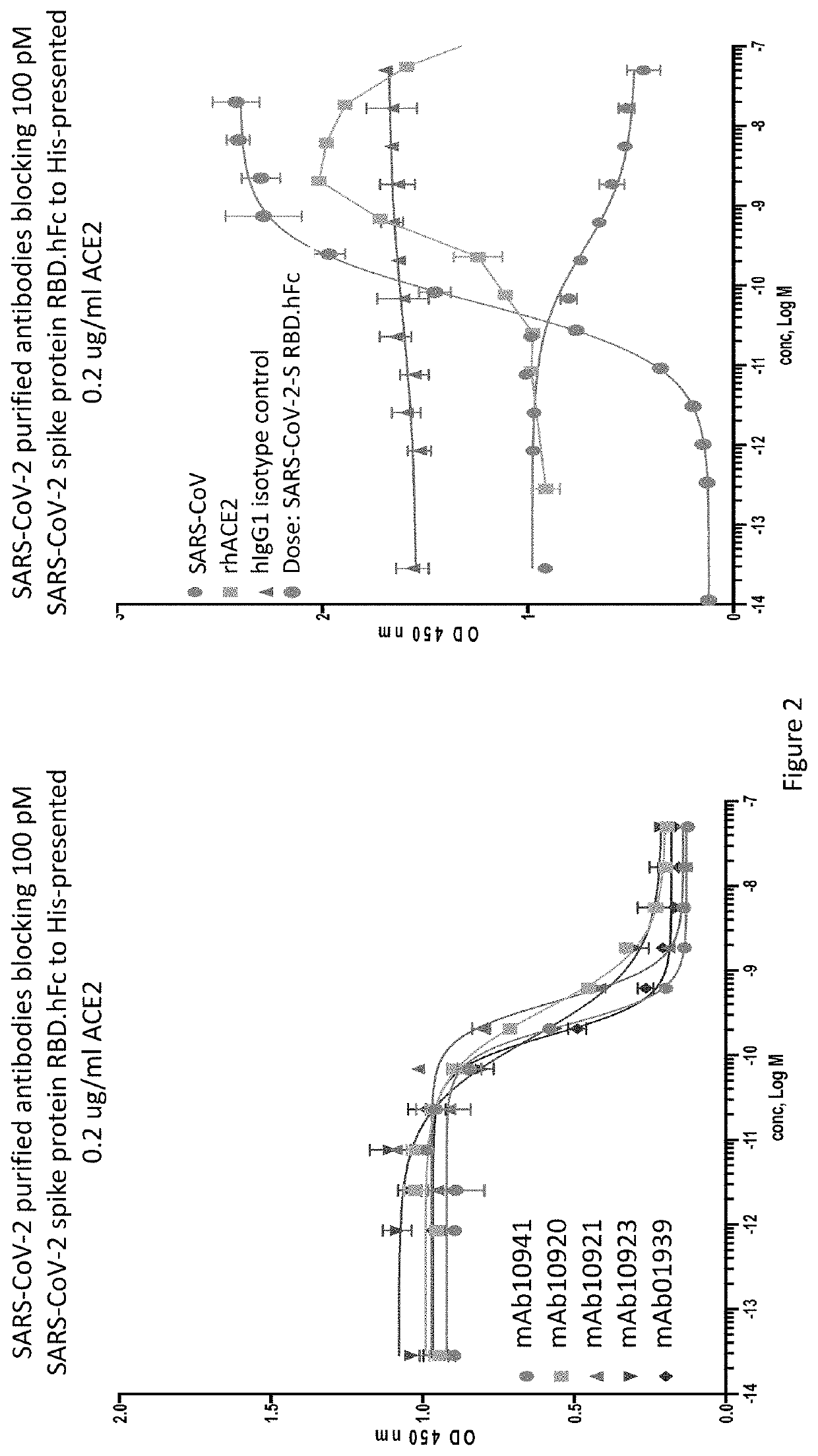
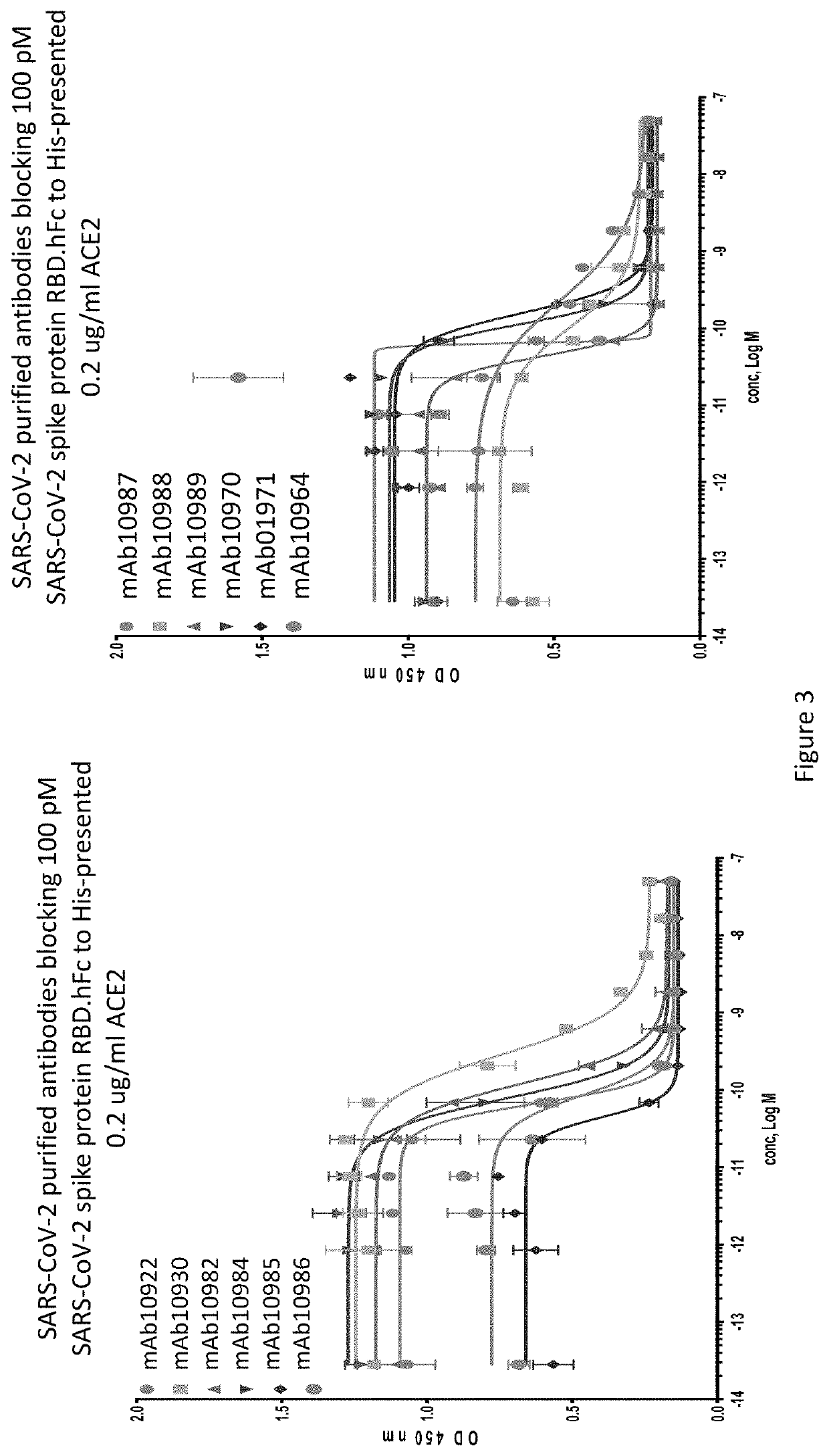
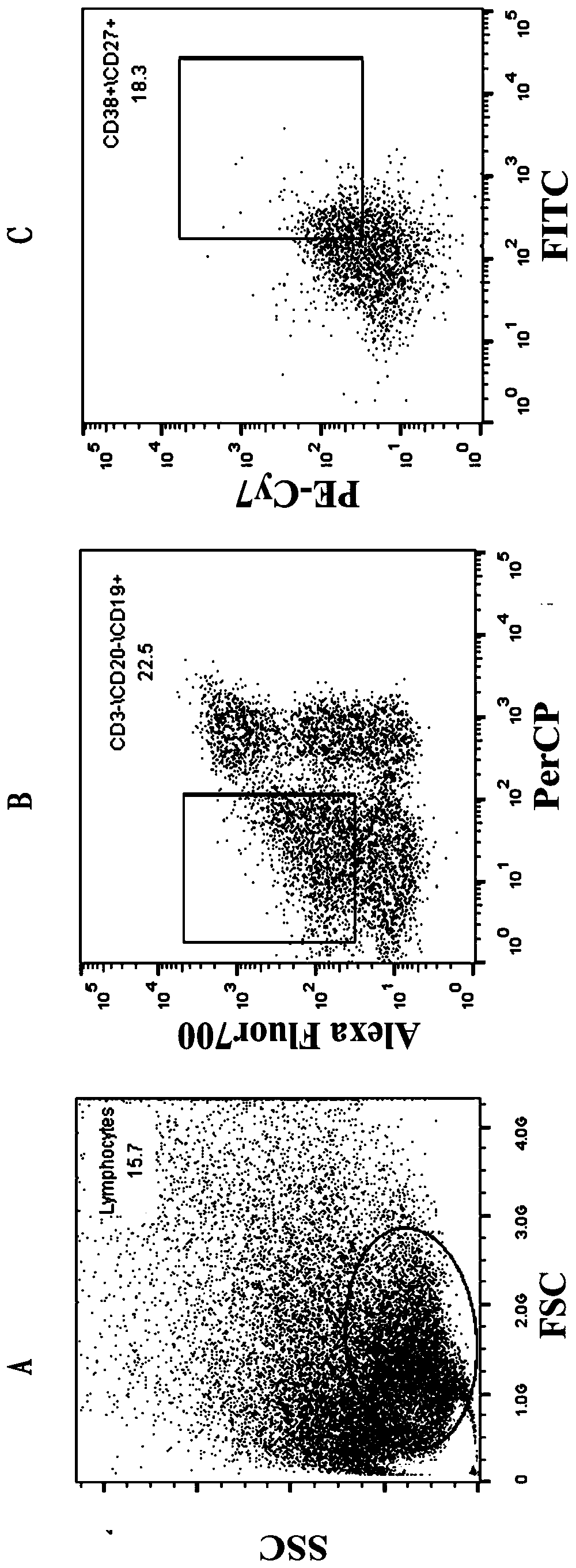
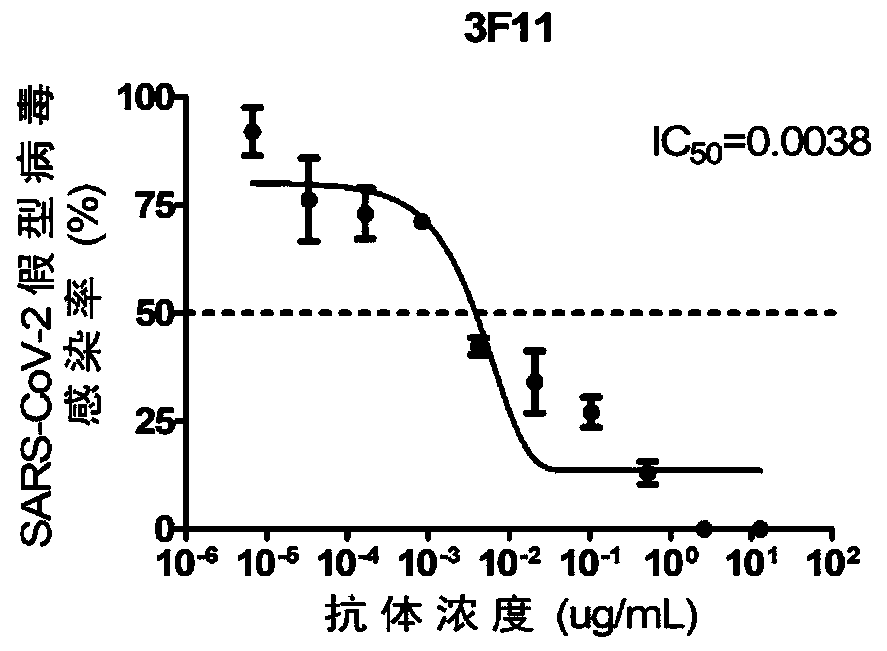
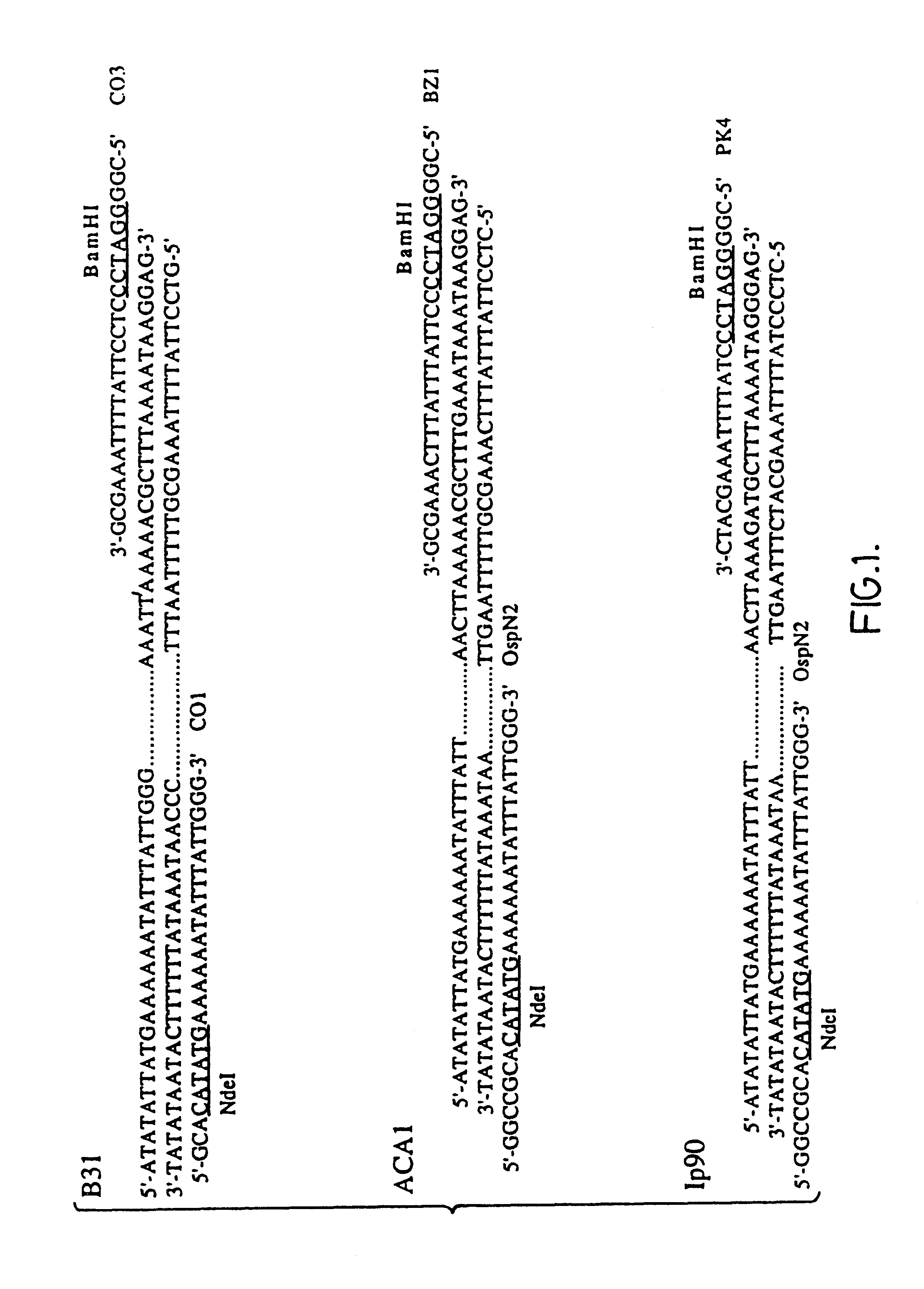
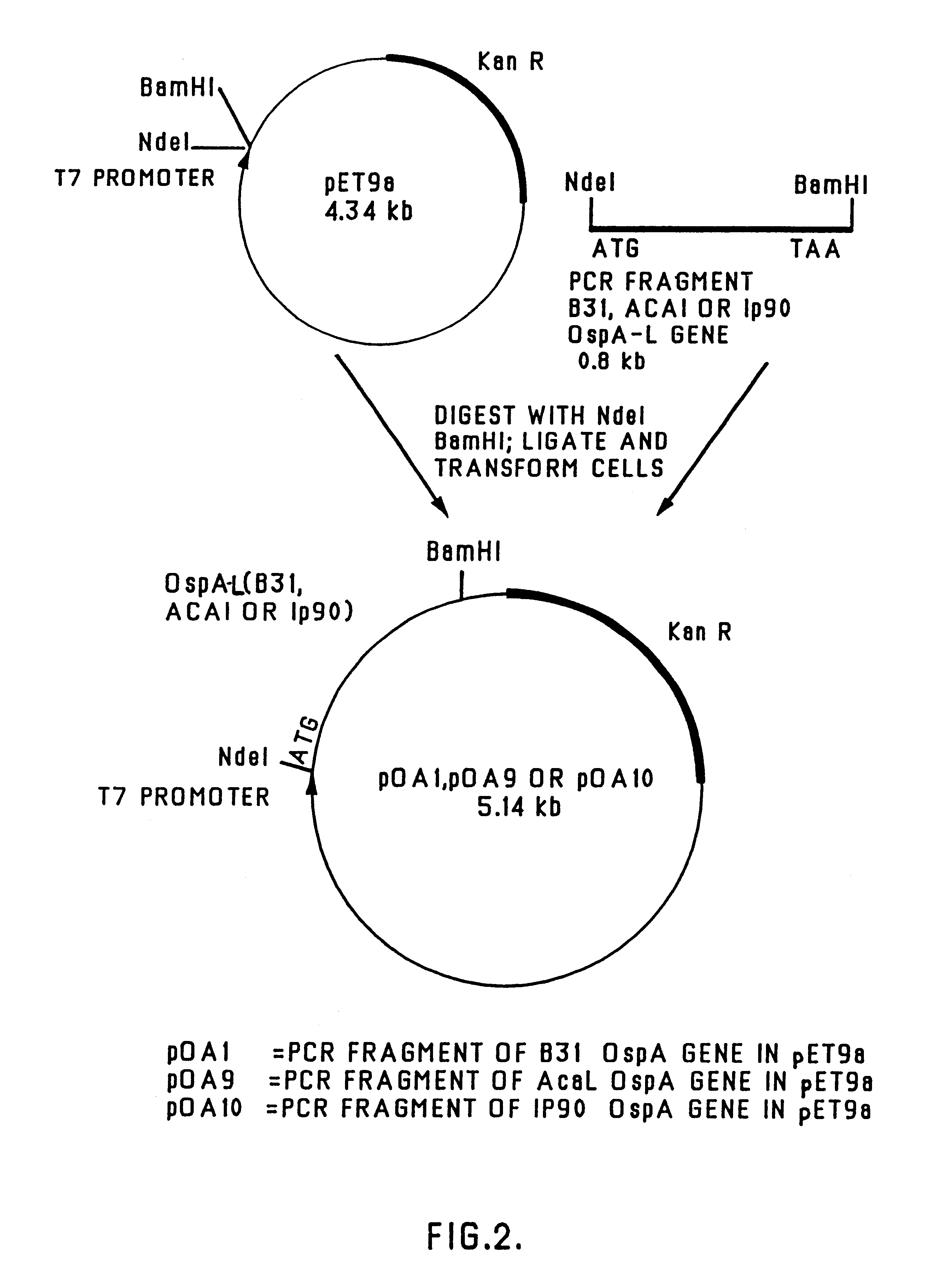
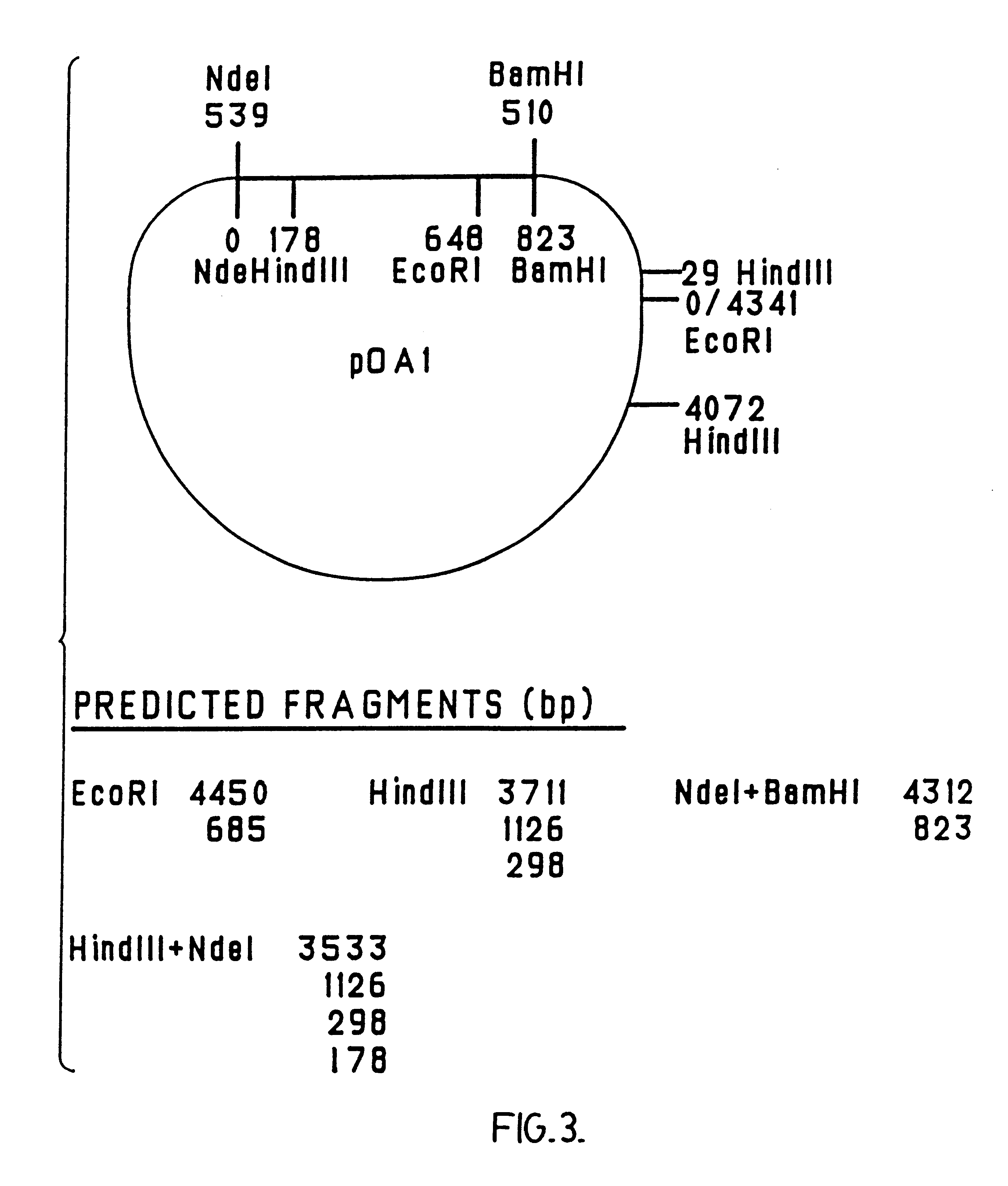
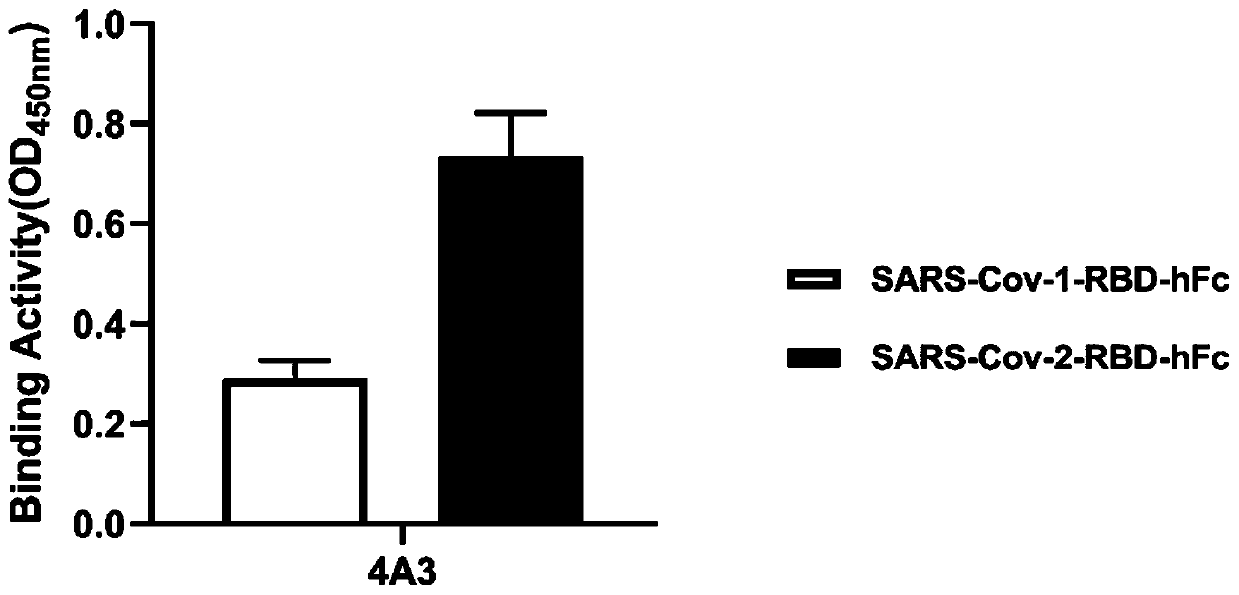
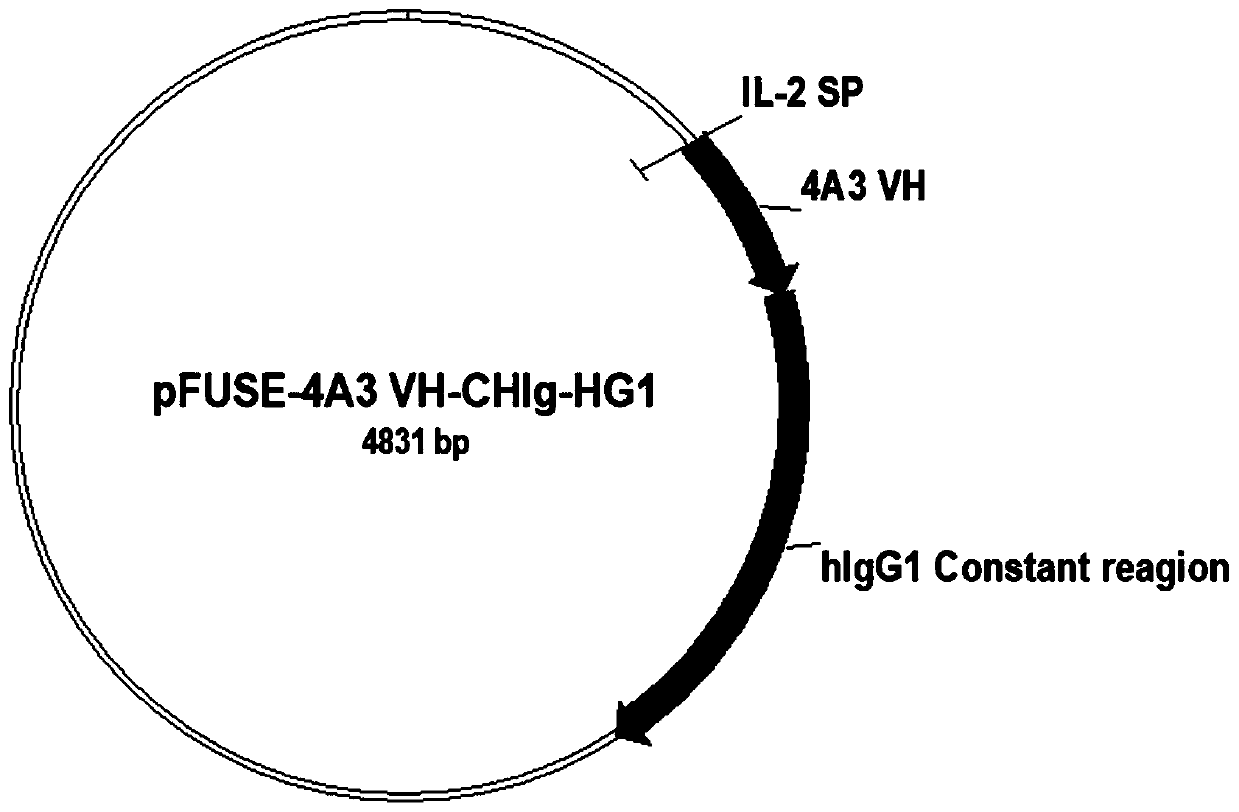
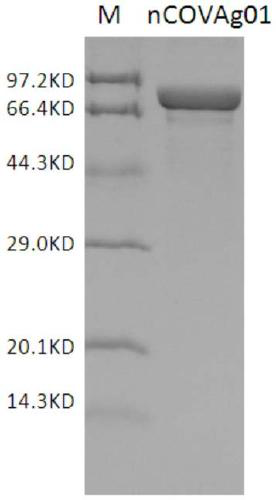
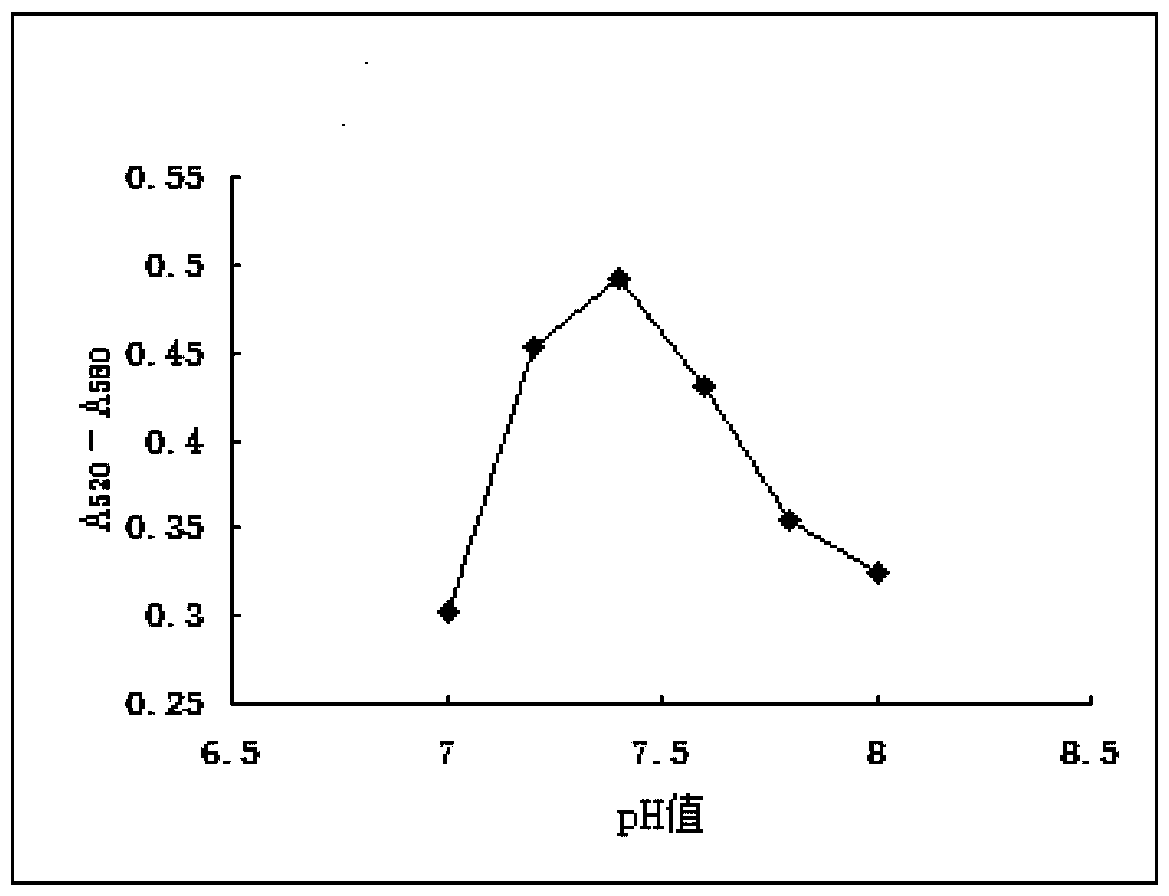
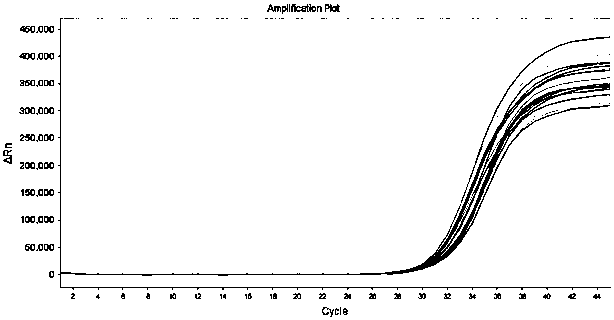
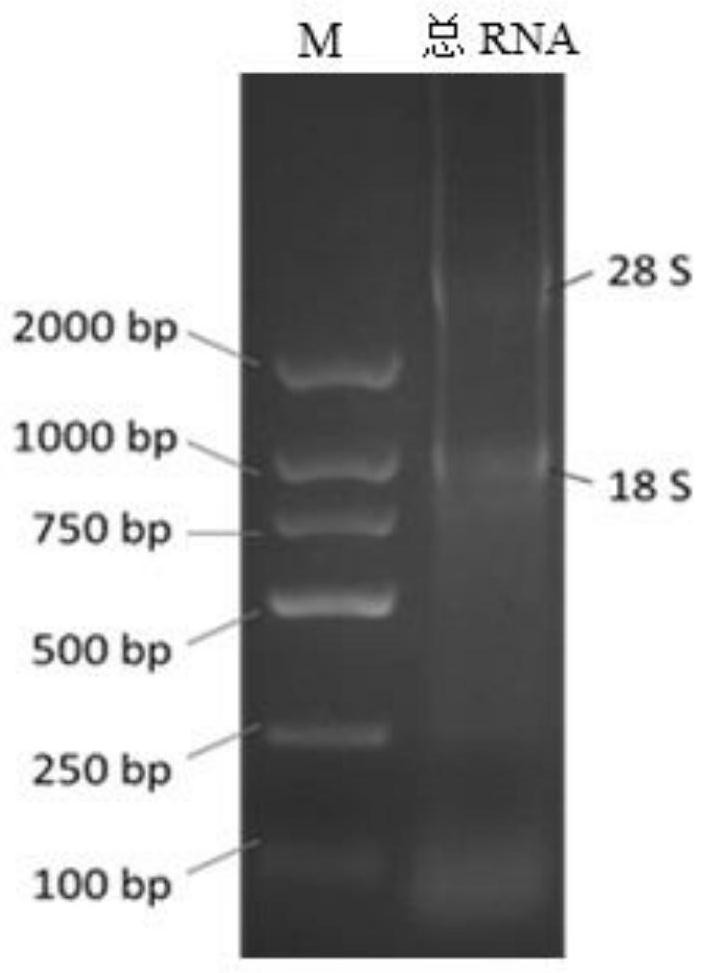
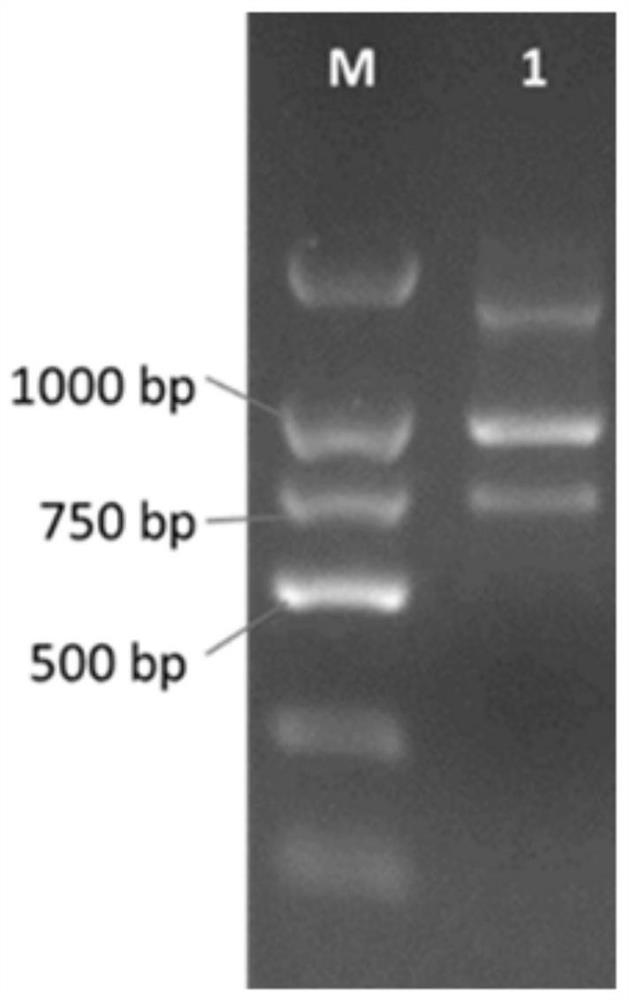
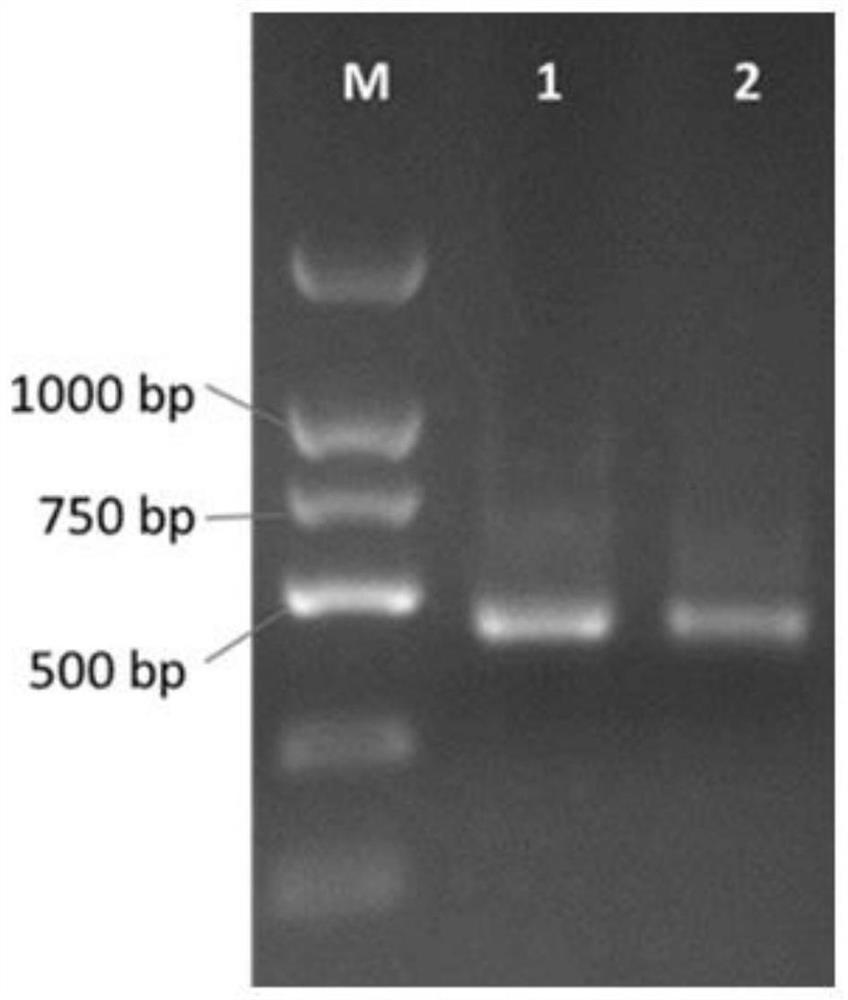
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3774 results about "Coronavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
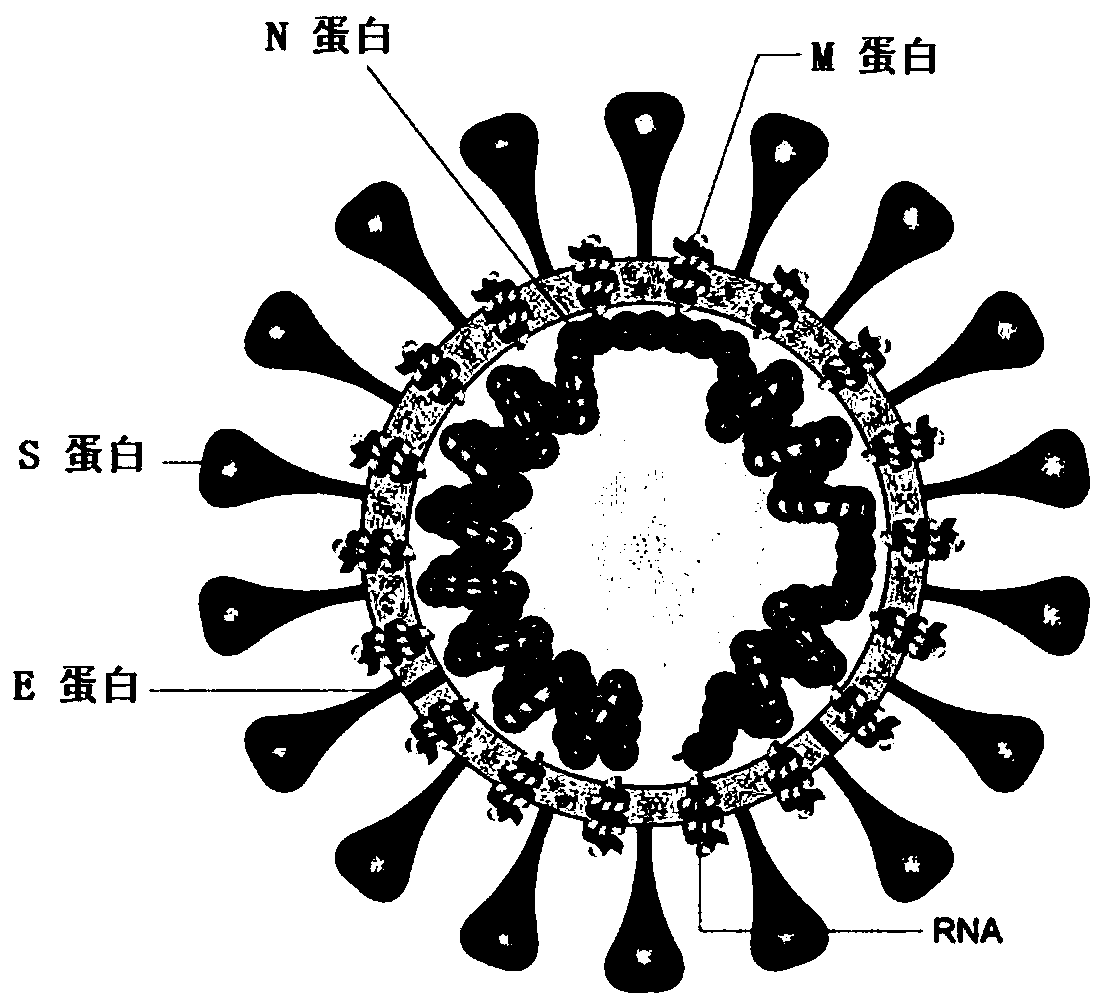
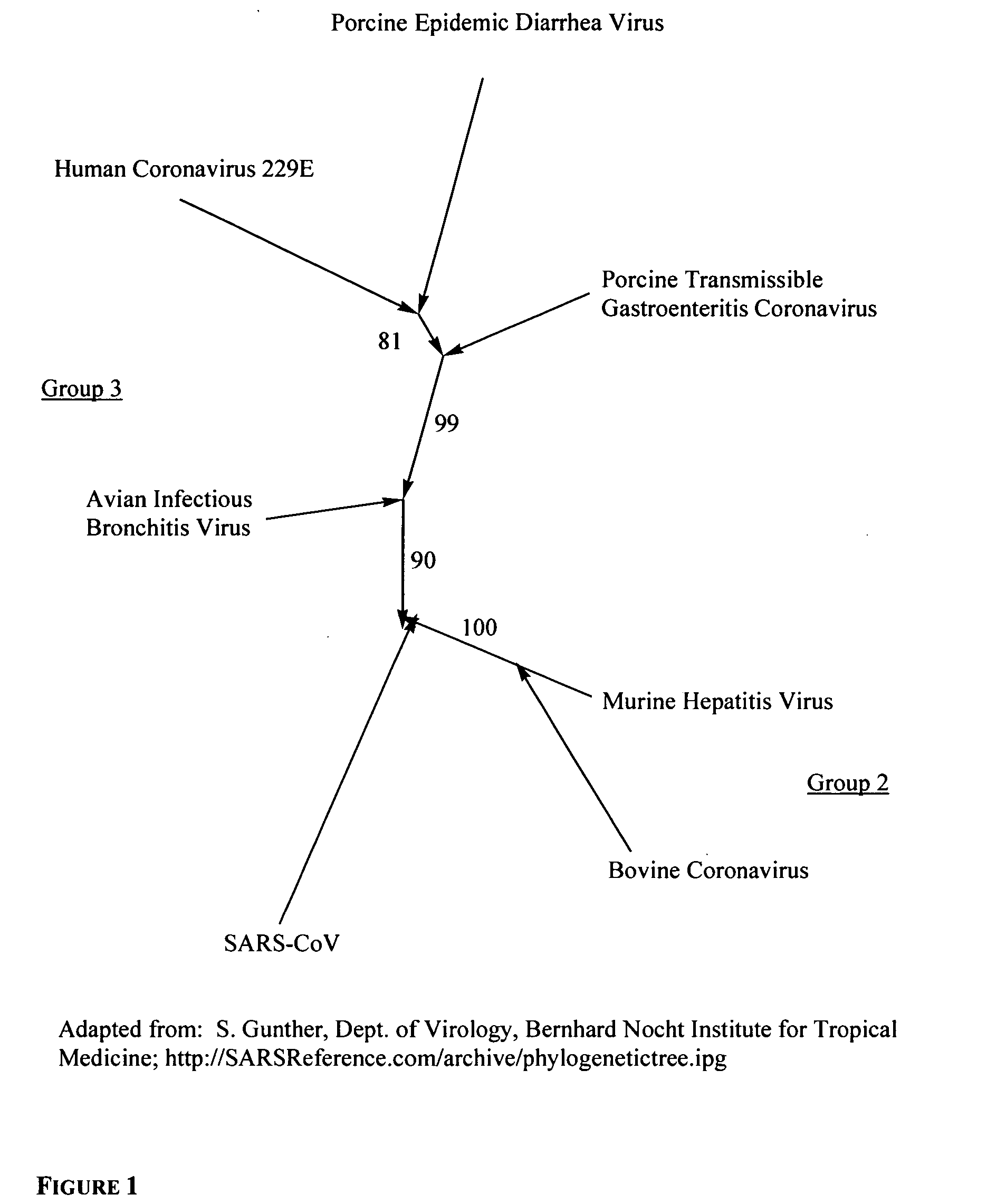
Coronaviruses are species of virus belonging to the subfamily Coronavirinae in the family Coronaviridae, in the order Nidovirales. Coronaviruses are enveloped viruses with a positive-sense single-stranded RNA genome and with a nucleocapsid of helical symmetry. The genomic size of coronaviruses ranges from approximately 26 to 32 kilobases, the largest for an RNA virus.
Anti-SARS-CoV-2-spike glycoprotein antibodies and antigen-binding fragments
ActiveUS10787501B1Growth inhibitionControl spreadViral antigen ingredientsImmunoglobulins against virusesAntigenAntiendomysial antibodies
The present disclosure provides antibodies and antigen-binding fragments thereof that bind specifically to a coronavirus spike protein and methods of using such antibodies and fragments for treating or preventing viral infections (e.g., coronavirus infections).
Owner:REGENERON PHARM INC
High-neutralizing-activity anti-SARS-CoV-2 fully-humanized monoclonal antibody and application thereof
ActiveCN111303280AGood neutralizing effectReduced responseImmunoglobulins against virusesAntiviralsDiseaseAntiendomysial antibodies
The invention discloses an anti-SARS-CoV-2 fully-humanized monoclonal antibody against SARS-CoV-2. The antibody is obtained by screening through a flow sorting-single cell PCR technology, and has a unique CDR partition. The invention further discloses an application of the antibody in preparation of medicines for treating the 2019-coronavirus disease. The monoclonal antibody disclosed by the invention has efficient and specific anti-SARS-COV-2 activity, also has the characteristics of high expression, complete humanization and good stability, and is suitable for industrial production.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Single-domain antibodies for novel coronavirus and application of single-domain antibodies
ActiveCN111303279AHigh affinityAnd high activityImmunoglobulins against virusesAntiviralsAntigenReceptor
The invention discloses humanized single-domain antibodies for a novel coronavirus SARS-CoV-2 and an application of the humanized single-domain antibodies. The invention protects the single-domain antibodies described in any one of SEQ ID No.1 to SEQ ID No.5. Experimental results show that the single-domain antibodies provided by the invention have good affinity with receptor binding domain (RBD)antigens and have high neutralization activity on SARS-CoV-2 pseudovirus. The humanized single-domain antibodies have important scientific significance and application prospect for prevention and clinical treatment of the new coronavirus SARS-CoV-2 and for development of diagnostic reagents of the new coronavirus SARS-CoV-2.
Owner:BEIJING KAWIN TECH SHARE HLDG
Lyme combination compositions and uses
InactiveUS6368603B1Safe and efficacious in dogNo exacerbation of diseaseAntibacterial agentsNanotechAntigenRabies
Disclosed and claimed are compositions containing a Borrelia burgdorferi antigen, and methods for making and using them. The antigen can be OspA. The compositions can contain at least one additional antigen from a pathogen other than Borrelia burgdorferi. The compositions are useful for eliciting an immunological response in a host mammal susceptible to Lyme Disease and to the mammalian pathogen other than Borrelia burgdorferi. Suitable host mammals include dogs, pups, horses, and, the additional antigen can be of a canine, equine or feline pathogen, such as rabies, canine distemper, adenovirus, coronavirus, parainfluenza and parvovirus. No significant efficacy interference is observed.
Owner:MERIAL LTD
SARS-CoV-2 vaccine and preparation method thereof
ActiveCN111217917AEnter to helpImproving immunogenicityPolypeptide with localisation/targeting motifSsRNA viruses positive-senseAntigenDisease
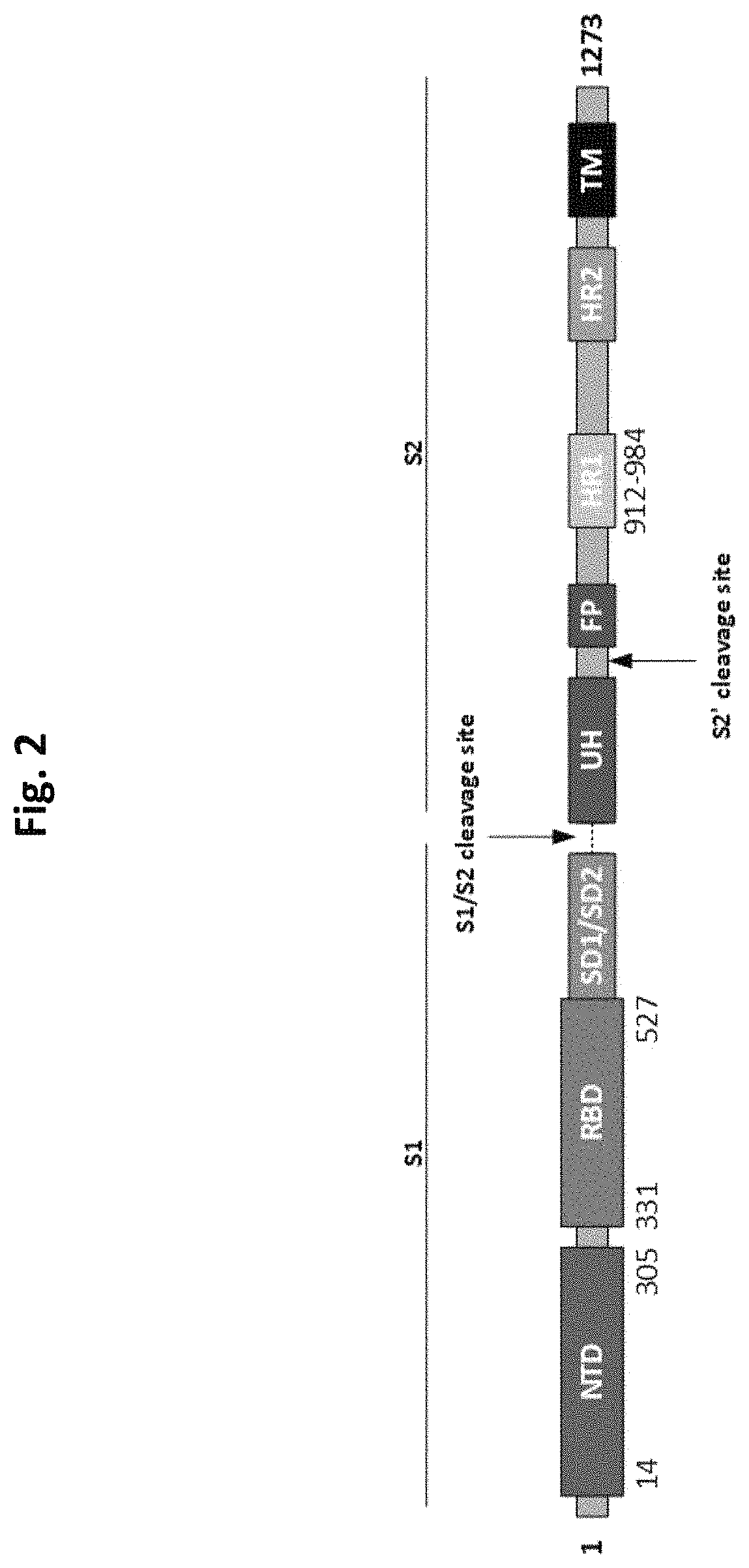
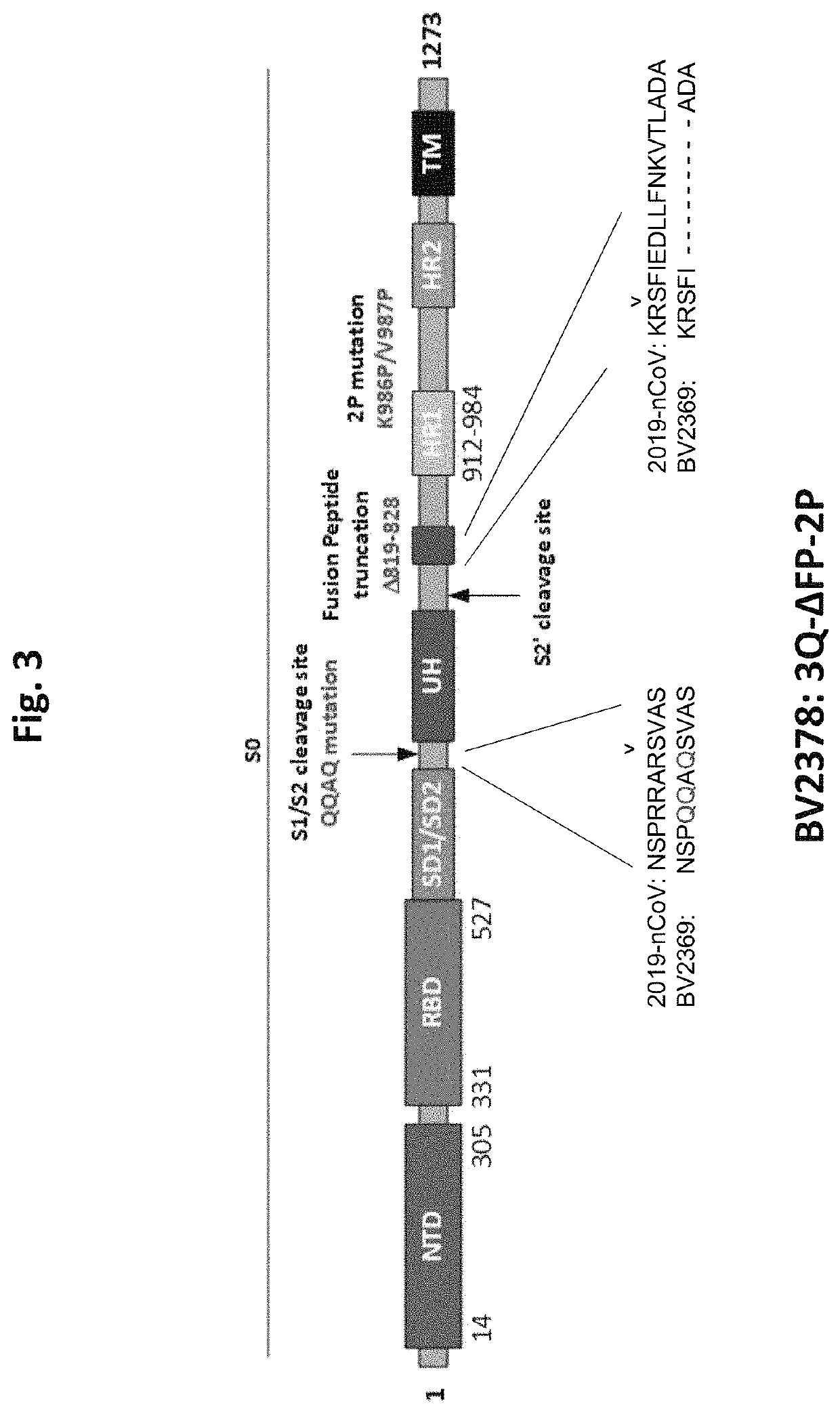
The invention relates to a preparation method for a vaccine capable of treating and / or preventing SARS-CoV-2 infection or COVID-19 diseases. The core antigen of the vaccine comprises the RBD (receptor binding zone) fusion protein of the SARS-CoV-2, and a vaccine form comprises an RBD fusion protein subunit vaccine, an RBD fusion protein mRNA vaccine or an RBD fusion protein adenovirus vector vaccine. The above vaccine immunizes an organism, and immune reaction for treating and / or preventing the SARS-CoV-2 infection can be generated so as to be used for treating and / or preventing COVID-19. The invention also relates to an RBD fusion gene, the RBD fusion protein, a carrier, a cell, a preparation method, a treatment method or a pharmacy purpose of the SARS-CoV-2.
Owner:CANSINO BIOLOGICS INC
Anti-novel coronavirus monoclonal antibody and application thereof
ActiveCN111592594AStrong neutralizing activityImmunoglobulins against virusesAntiviralsDiseaseInfection induced
The invention relates to the fields of immunology and molecular virology, in particular to the fields of diagnosis, prevention and treatment of novel coronaviruses. Specifically, the present inventionrelates to an anti-novel coronavirus monoclonal antibody, and a composition (e.g., a diagnostic agent and a therapeutic agent) comprising the antibody. Furthermore, the invention also relates to an application of the antibody. The antibody provided by the invention can be applied to diagnosis, prevention and / or treatment of infection of the novel coronavirus and / or diseases (e.g., novel coronavirus pneumonia) caused by the infection.
Owner:PEKING UNIV
Antisense antiviral compound and method for treating ssRNA viral infection
ActiveUS20060269911A1Promote absorptionSsRNA viruses positive-senseMicrobiological testing/measurementOligonucleotideAstroviridae
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Flaviviridae, Picomoviridae, Caliciviridae, Togaviridae, Arteriviridae, Coronaviridae, Astroviridae and Hepeviridae families in the treatment of a viral infection. The antisense antiviral compounds are substantially uncharged morpholino oligonucleotides having a sequence of 1240 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with stem-loop secondary structure within the 5′-terminal end 40 bases of the positive-sense RNA strand of the virus.
Owner:AVI BIOPHARMA
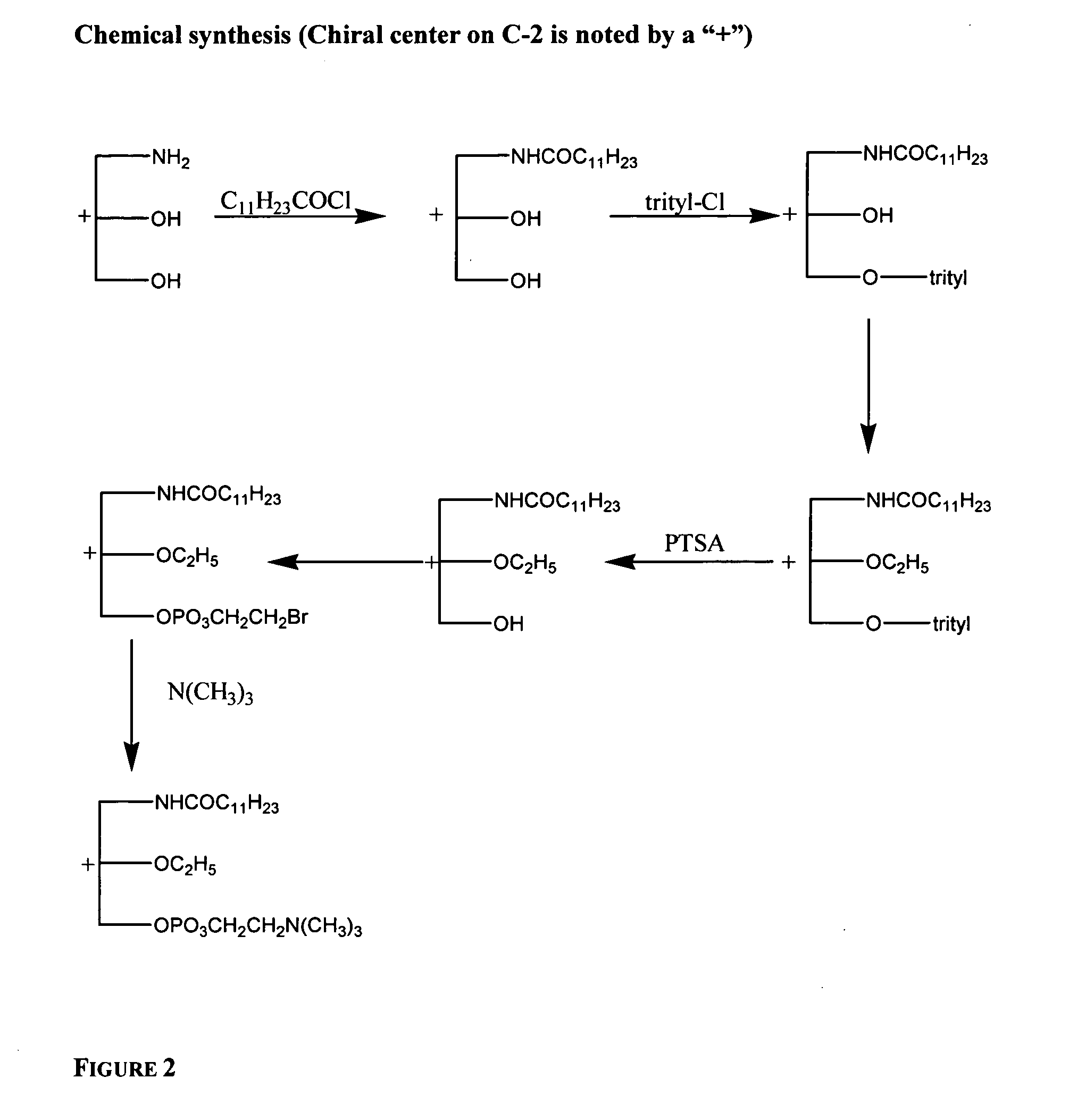
Phospholipids for the treatment of infection by togaviruses, herpes viruses and coronaviruses
InactiveUS20050187192A1BiocidePhosphorous compound active ingredientsHerpes simplex virus DNACompound (substance)
Provided are compounds, methods and pharmaceutical compositions for treating a host, especially a human, infected with a togavirus, herpes virus and / or coronavirus, and in particular SARS-CoV, cytomegalovirus or varicella-zoster virus. The method in one embodiment comprises administering to that host an effective amount of an anti-togavirus, anti-herpes virus and / or anti-coronavirus phospholipid or a pharmaceutically acceptable salt or prodrug thereof. The phospholipid compound is, e.g., a 3-alkylamido-2-alkoxypropylphosphocholine compound or salt thereof. The compound may be administered alone or in combination and / or alternation with one or more other anti-viral agents.
Owner:KUCERA PHARMA
Neutralizing antibody for resisting novel coronavirus SARS-Cov-2 and application thereof
ActiveCN111592595ABlock bindingGood effectImmunoglobulins against virusesAntiviralsNeutralizing antibodyPhage Display Techniques
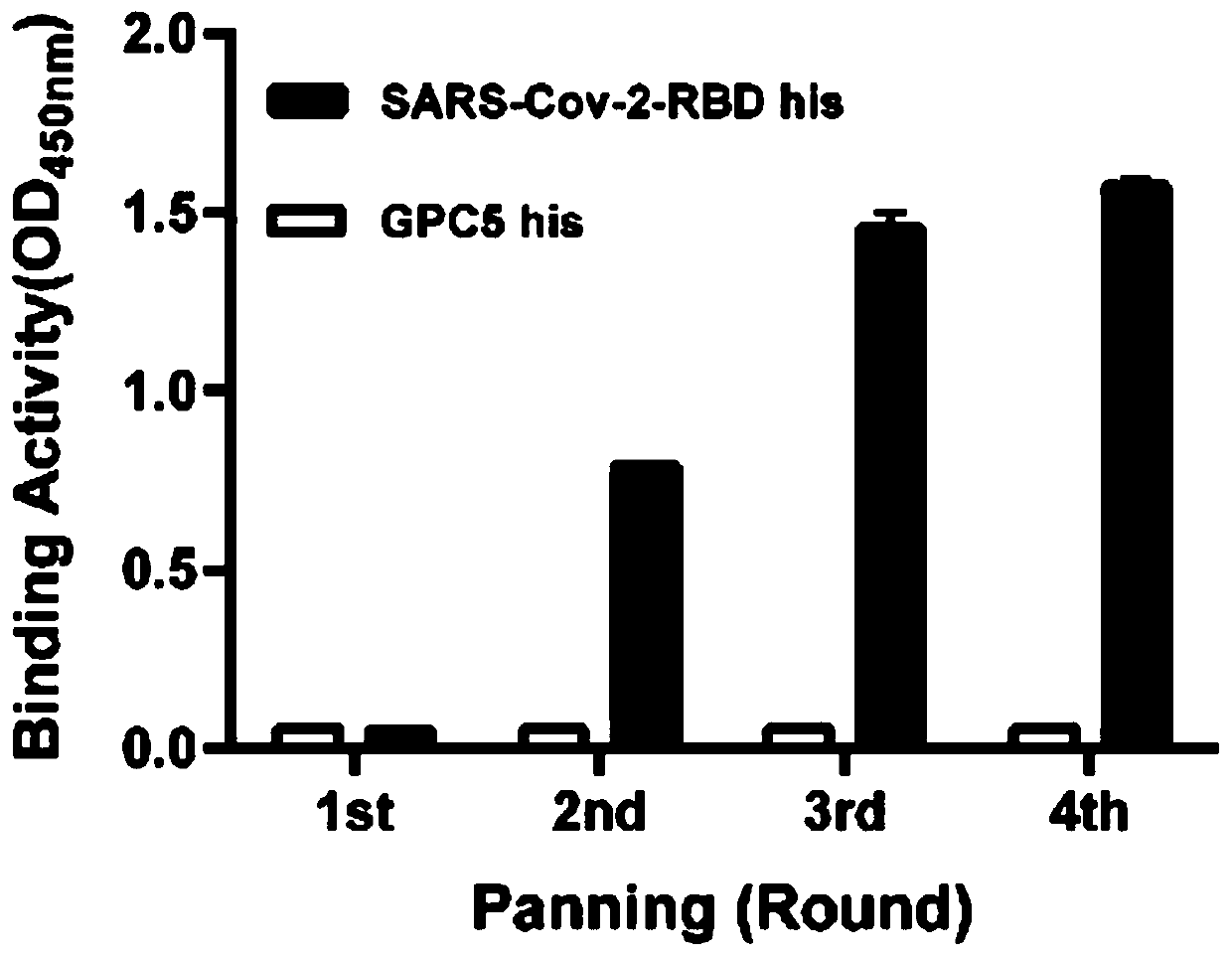
The invention relates to a neutralizing antibody for resisting a novel coronavirus SARS-Cov-2 and an application of the neutralizing antibody. The antibody at least comprises one of a heavy chain CDR1, a heavy chain CDR2, a heavy chain CDR3, a light chain CDR1, a light chain CDR2 and a light chain CDR3. The antibody can be used for preparing a diagnostic reagent or a diagnostic kit, a drug or a pharmaceutical composition for detecting, preventing and treating a COVID-19. According to the neutralizing antibody, differential antibody screening is carried out through a phage display technology ina manner of targeting SARS-Cov-2-RBD and SARS-Cov-1-RBD; the neutralizing antibody for resisting the novel coronavirus SARS-Cov-2 is obtained; binding of the SARS-Cov-2-RBD and ACE2 positive cells can be blocked; and the neutralizing antibody has a remarkable virus neutralizing effect on an SARS-Cov-2 pseudo virus and provides an effective alternative antibody drug for prevention and treatment ofthe COVID-19.
Owner:NANJING MEDICAL UNIV
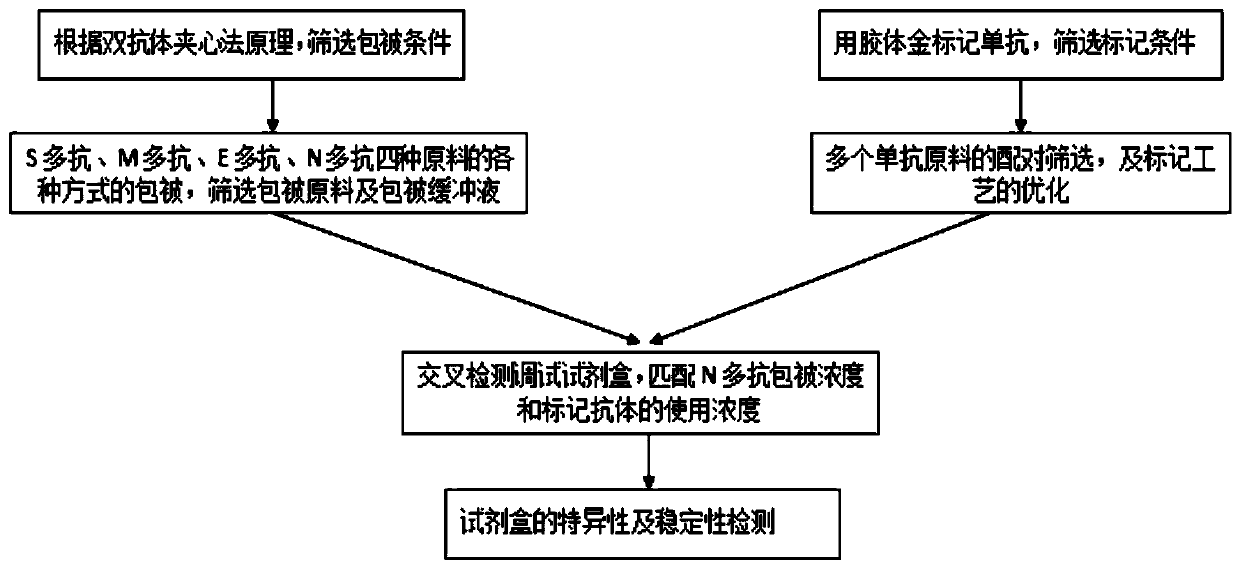
Detection kit of IgM/IgG antibodies of novel coronavirus (SARS-CoV-2)
ActiveCN111187354AImprove response accuracyOvercoming cumbersome detection operationsSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeCoronavirus antibody
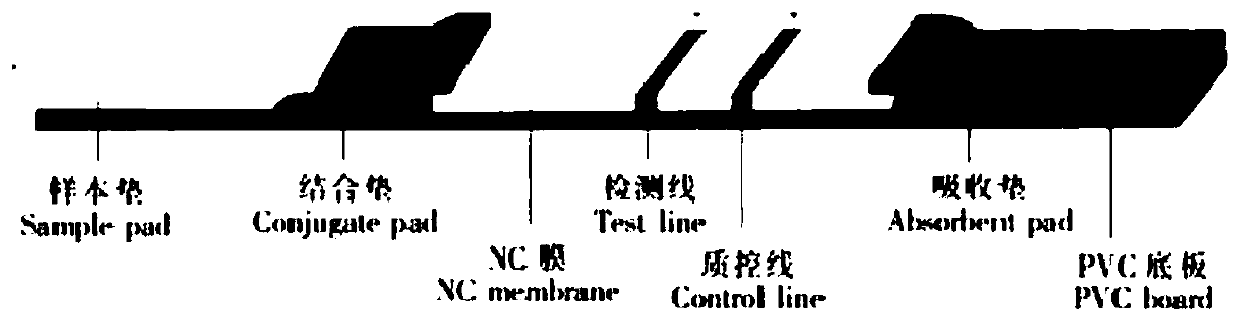
The invention provides a detection kit of IgM / IgG antibodies of novel coronavirus (SARS-CoV-2), and relates to the technical field of biology. According to the detection kit of IgM / IgG antibodies of novel coronavirus (SARS-CoV-2), the IgM / IgG antibodies of novel coronavirus (SARS-CoV-2) are detected through adoption of a colloidal gold capture method, a colloidal gold indirect method and a colloidal gold double antigen sandwich method, wherein adopted antigens are highly active recombinant antigens which are obtained through fusion expression of N protein fragments and S protein dominant epitope fragments, and colloidal gold is labeled indirectly, so that steric hindrance is reduced, and the activity, reaction consistency and accuracy of the antigens are increased. Since a sample pad is pretreated, the interference of complex components in a blood sample to a reaction can be reduced greatly, the detection kit has no requirements for instruments during detection, simple and flexible operation, direct and manual interpretation of results and a short test time, the results can be interpreted in only 15 minutes, and the test results are accurate.
Owner:北京新创生物工程有限公司
Colloidal gold kit for jointly detecting coronavirus IgM/IgG antibody, and preparation method thereof
ActiveCN111089962ASimple preparation processEasy to useMaterial analysisCoronavirus antibodyIgm antibody
The invention discloses a colloidal gold kit for jointly detecting coronavirus IgM / IgG antibody, and a preparation method thereof, and relates to the field of biological medicine. Whether anti-novel coronavirus nucleocapsid protein IgM antibody and / or anti-novel coronavirus nucleocapsid protein IgG antibody exists in human serum or plasma or not by adopting an antigen-antibody sandwich method anda colloidal gold immunochromatography method principle, the novel coronavirus nucleocapsid protein containing 6xHis mark is marked by applying colloidal gold, thereby forming gold-marked N protein tobe adsorbed on a gold-marked pad, the novel coronavirus nucleocapsid protein containing 6xHis mark is used as an indication marker, the mouse-anti-human u chain monoclonal antibody is coated on the IgM detection line of a NC membrane, the mouse-anti-human IgG monoclonal antibody is coated on the IgG detection line and the mouse-anti 6xHis monoclonal antibody is coated on a quality control line ofthe NC membrane, the qualitative detection of the anti-novel coronavirus nucleocapsid protein IgG antibody is realized, and the colloidal gold kit disclosed by the invention has the advantages of being convenient to use, high in sensitivity and short in detection time.
Owner:中山生物工程有限公司
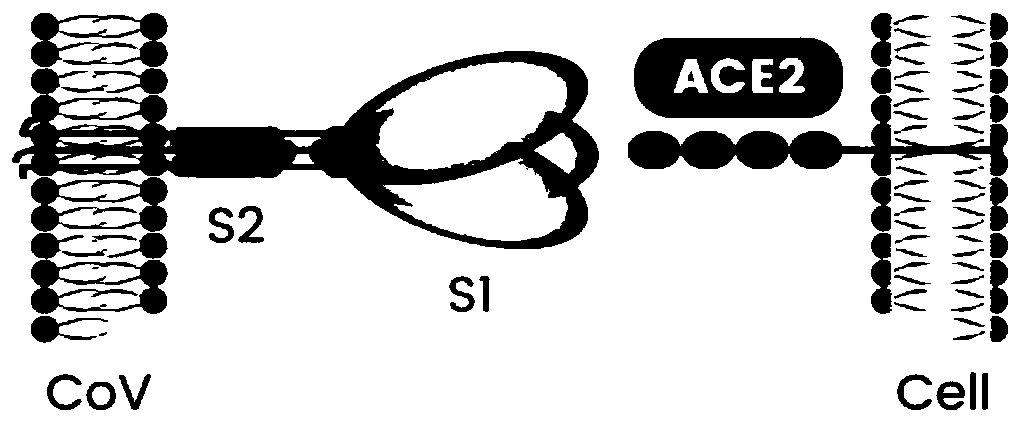
Coronavirus rapid detection kit based on S protein ligand and ACE2 receptor competitive chromatography
ActiveCN111273016AImmunochromatographic fastEasy immunochromatographyCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsReceptorBlood plasma
Owner:浙江诺迦生物科技有限公司 +1
SARS-CoV-2 (severe acute respiratory syndrome-corona virus disease-2) inhibitor and application thereof
InactiveCN111333722AHigh affinityRealize standardized productionBiological material analysisImmunoglobulins against virusesPhage antibodiesAntibody fragments
The invention relates to an SARS-CoV-2 (severe acute respiratory syndrome-corona virus disease-2) inhibitor and application thereof and in particularly relates to a neutralizing antibody for SARS-CoV-2 and application of the neutralizing antibody. According to the antibody, a phage display technology is adopted to construct a high-capacity human immunity phage antibody library, and an SARS-CoV-2 protein is adopted a target, human antibody single-chain antibody fragments are screened, and an antibody with a good neutralizing function on SARS-CoV-2 viruses is obtained. The antibody provided by the invention can be used for treating diseases caused by infection of novel corona viruses, and has significant clinical application value.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE CONTROL & PREVENTION PUBLIC HEALTH RES INST OF JIANGSU PROVINCE
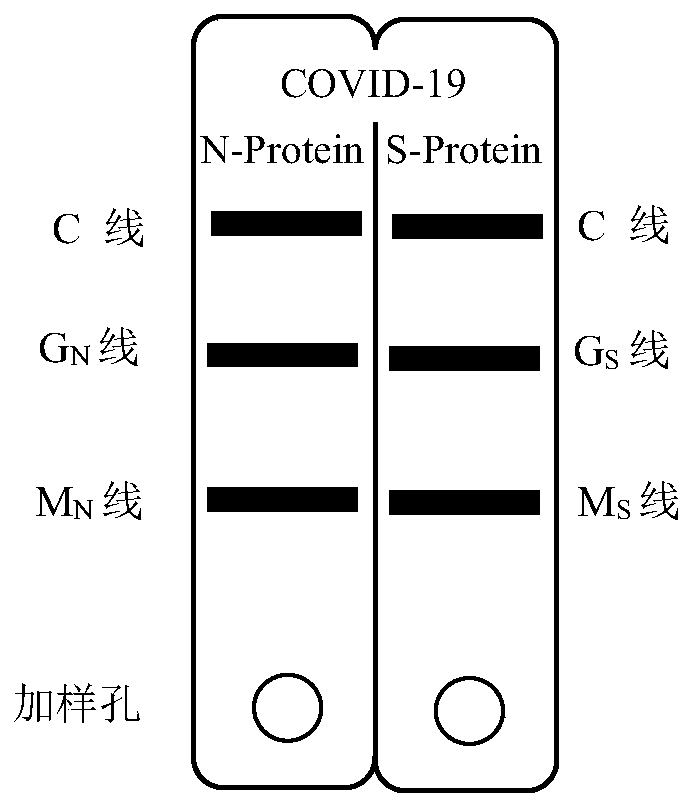
Novel coronavirus pneumonia (COVID-19) serological diagnosis kit
ActiveCN111239392AHigh detection sensitivityImprove detection accuracySsRNA viruses positive-senseVirus peptidesSerodiagnosesAntigen
The invention discloses a novel coronavirus pneumonia (COVID-19) serological diagnosis kit. The kit comprises an S-IgM / IgG test strip and an N-IgM / IgG test strip, the double-antigen quadruple detection kit can be used for simultaneously detecting four indexes of an IgM / IgG antibody for resisting novel coronavirus spinous process protein S and an IgM / IgG antibody for resisting novel coronavirus nucleocapsid protein N in serum of a patient suffering from novel coronavirus pneumonia COVID-19. According to the kit, the detection sensitivity is improved through quantum dot fluorescence labeling andmultistage coupling amplification signals, the detection accuracy is improved through double-antigen quadruple detection, and the biological safety in the detection process is guaranteed by establishing a virus inactivation system. The kit is suitable for whole blood, plasma and serum detection, and can be applied to novel COVID-19 serological diagnosis.
Owner:浙江诺迦生物科技有限公司 +1
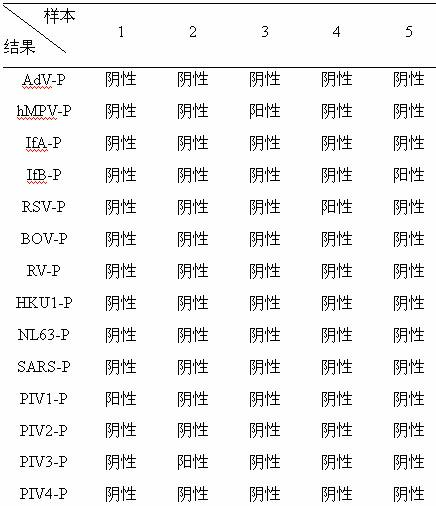
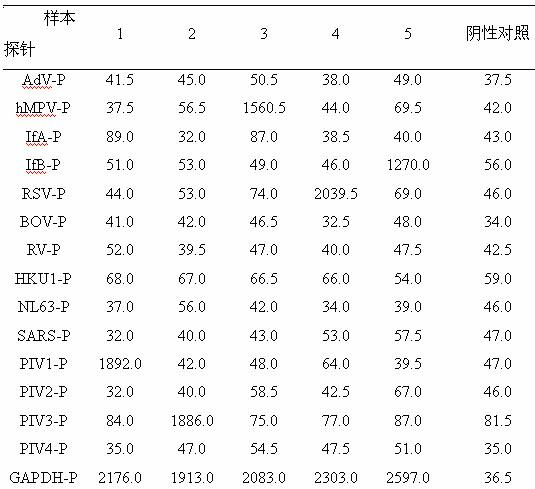
Method for detecting various respiratory viruses and primers and probes thereof
InactiveCN101985665AEasy to operateStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceMicrosphereNucleotide
The invention belongs to the technical fields of biochips and diagnostic reagents, and discloses a method for detecting various respiratory viruses, and primers and probes thereof. In the invention, nucleotide sequences of 14 respiratory viruses, namely adenovirus, human metapneumovirus, influenza virus A, influenza virus B, respiratory syncytial virus, bocavirus, rhinovirus, coronavirus (HKU1, NL63 and SARS), and parainfluenza virus (type I, type II, type III and type IV) are analyzed, and corresponding reverse transcription primers, PCR primers and specific probes are designed. Specific gene segments are amplified by reverse transcription and multiple asymmetric PCR methods; a fluorescence-coded microsphere group coupled with the virus specific probes and the PCR amplification product are incubated and hybridized by liquid phase chip technology; and finally the Bio-PlexTM200 is used for detection. The detection method has the advantages of high flux, high specificity and sensitivity, stable results and good repeatability, the detection method is easy to operate, and the detection speed is high.
Owner:FUDAN UNIV +1
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562369AGood repeatabilityStrong specificityImmunoassaysImmunodiagnosticsProtein s antigen
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, an S protein antigen of SARS-CoV-2 and acompetitive substance. The competitive substance is marked with a signal substance and can be specifically combined with the new coronavirus S protein antigen. Whether a tested person is infected bythe new coronavirus or not and whether infection risks exist or not are judged by detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
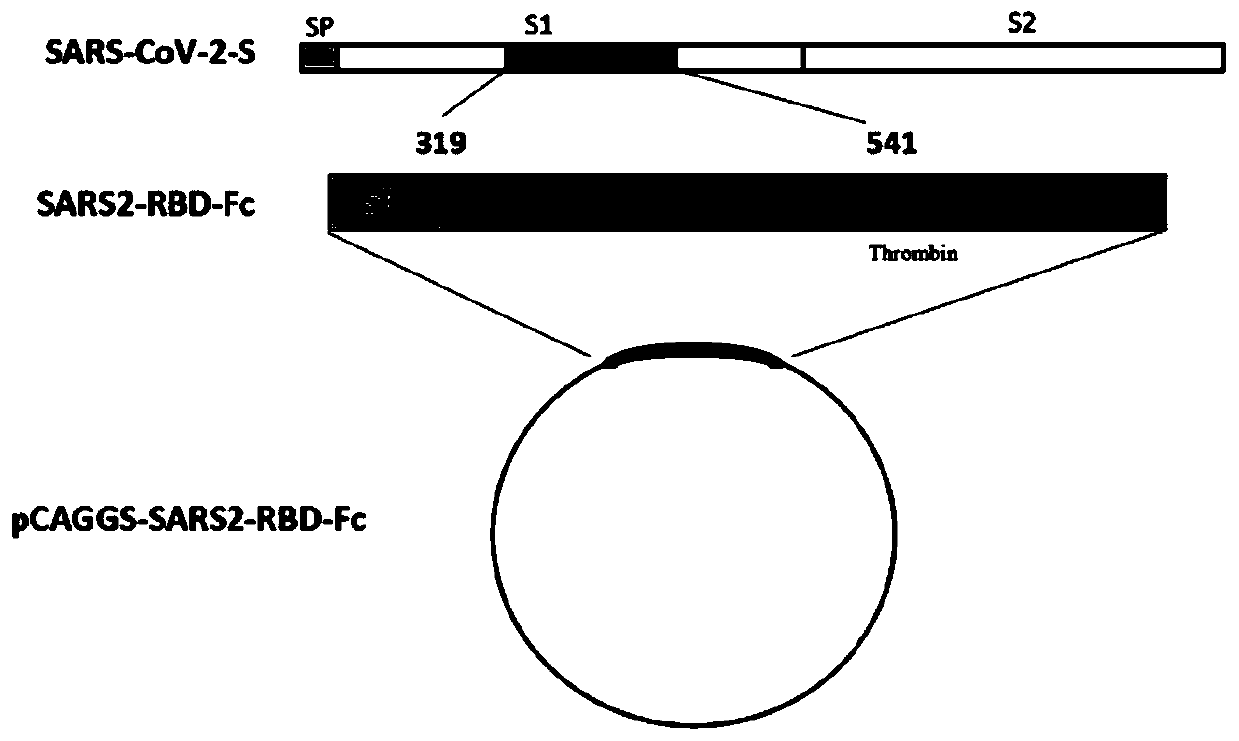
Subunit vaccine for novel coronavirus and application of subunit vaccine
InactiveCN111533809AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsAntibody fragmentsTGE VACCINE
The invention discloses a fusion protein of a novel coronavirus envelope protein and an application of the fusion protein. The fusion protein (SARS2-RBD-Fc) is obtained by fusing an RBD structural domain of a novel coronavirus envelope protein S with an antibody Fc fragment; and as a subunit vaccine, the fusion protein can induce an organism to generate an efficient neutralizing antibody through nasal drip immunization and intramuscular injection. It indicates that the SARS2-RBD-Fc can be used as a candidate vaccine for preventing and treating new coronavirus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Antimicrobial compositions, products and methods employing same
InactiveUS7569530B1Applied to skinImmediate and residual effectivenessAntibacterial agentsCosmetic preparationsOrganic acidGram-positive bacterium
Antimicrobial compositions that provide enhanced immediate and residual anti-viral and antibacterial efficacy against rhinovirus, rotavirus, coronovirus, respiratory syncytial virus, Gram-positive bacteria, Gram-negative bacteria and combinations thereof. More specifically, antimicrobial compositions comprising an organic acid or organic acid mixture and a short-chain anionic surfactant having at least one of a large head group; a branched alkyl chain and an unsaturated alkyl chain. Further, products incorporating the antimicrobial compositions of the present invention and methods of using the antimicrobial compositions and products are disclosed herein.
Owner:THE PROCTER & GAMBLE COMPANY
Coronavirus vaccine formulations
ActiveUS10953089B1SsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationNanoparticle
Disclosed herein are coronavirus Spike (S) proteins and nanoparticles comprising the same, which are suitable for use in vaccines. The nanoparticles present antigens from pathogens surrounded to and associated with a detergent core resulting in enhanced stability and good immunogenicity. Dosages, formulations, and methods for preparing the vaccines and nanoparticles are also disclosed.
Owner:NOVAVAX
Prescription capable of treating pneumonia caused by 2019-nCoV (a novel coronavirus) infection and application thereof
ActiveCN110870402AGood treatment effectDefinite curative effectAntiviralsFungi medical ingredientsBiotechnologyBelamcanda chinensis
The invention relates to traditional Chinese medicine compositions, and specifically discloses a prescription capable of treating pneumonia caused by 2019-nCoV (a novel coronavirus) infection as wellas application thereof. The prescription capable of treating the pneumonia caused by the 2019-nCoV infection comprises the following traditional Chinese medicine materials as raw materials in parts byweight: 9 parts of ephedra, 6 parts of roasted liquorice roots, 9 parts of apricot kernels, 15-30 parts of raw gypsum, 9 parts of cassia twigs, 9 parts of oriental waterplantain rhizome, 9 parts of zhuling, 9 parts of white atractylodes rhizomes, 15 parts of poria cocos, 16 parts of radix bupleuri, 6 parts of baical skullcap roots, 9 parts of ginger processed pinellia tubers, 9 parts of fresh ginger, 9 parts of aster, 9 parts of common coltsfoot flowers, 9 parts of blackberry lily rhizome, 6 parts of manchurian wildginger, 12 parts of Chinese yams, 6 parts of immature orange fruits, 6 parts of dried orange peel, and 9 parts of wrinkled gianthyssop herb. In combination with symptom manifestation of the pneumonia caused by the 2019-nCoV infection, the invention adopts an optimized and integrated traditional Chinese medicine prescription, thereby achieving synergistic treatment effects. According to current treatment results of diagnosed patients of different ages in many provinces and regions, the prescription capable of treating the pneumonia caused by the 2019-nCoV infection has true curative effects that the response rate is up to 95.12%.
Owner:葛又文
Detection kit for antigens of novel coronavirus (SARS-CoV-2)
InactiveCN111303254AThe test result is accurateHigh sensitivitySsRNA viruses positive-senseVirus peptidesAntigen testingColloidal au
The invention relates to the technical field of biology, and specifically provides a detection kit for a detection kit for antigens of a novel coronavirus (SARS-CoV-2). According to the detection kitprovided by the invention, the antigens of the SARS-CoV-2 are detected by virtue of a colloidal gold double antibody sandwich method, and two monoclonal antibodies and colloidal gold are mixed for labeling, so that a detection result of the antigens of the SARS-CoV-2 is accurate and high in sensitivity, and meanwhile, the detection rate can be remarkably increased. According to the detection kit,a blank in immunological detection of the SARS-CoV-2 is filled up, and field detection can be realized without a detection instrument, so that the time and the labor are saved, and operation is flexible.
Owner:北京新创生物工程有限公司
Therapeutic antimicrobial compositions and methods
InactiveUS20050271711A1Applied to skinImmediate and residual effectivenessAntibacterial agentsBiocideOrganic acidRotavirus RNA
Therapeutic antimicrobial compositions and methods for providing enhanced immediate and residual anti-viral and antibacterial efficacy against rhinovirus, rotavirus, coronovirus, respitory syricytial virus, Gram-positive bacteria, Gram-negative bacteria and combinations thereof. More specifically, therapeutic antimicrobial compositions comprising an organic acid or organic acid mixture and a short-chain anionic surfactant having at least one of a large head group; a branched alkyl chain and an unsaturated alkyl chain, and therapeutic methods of use thereof.
Owner:THE PROCTER & GAMBLE COMPANY
Novel coronavirus(2019-nCoV) ORF1ab gene nucleic acid detection kit
ActiveCN111020064AImprove efficiencyStrong specificityMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionGene
The invention provides a novel coronavirus ORF1ab gene nucleic acid detection kit, and particularly discloses a kit for detecting the ORF1ab gene of the novel coronavirus and a method thereof, which have extremely high sensitivity and specificity.
Owner:DAAN GENE CO LTD
Novel coronavirus (SARS-COV-2) spike protein binding molecule and applications thereof
ActiveCN111647077AAvoid infectionInhibition of amplificationComponent separationMaterial analysis by electric/magnetic meansReceptorIntravenous gammaglobulin
The invention relates to the technical field of medical biology, and specifically discloses a novel coronavirus (SARS-COV-2) spike protein binding molecule and applications thereof. The binding molecule can specifically bind the spike protein of SARS-COV-2 and includes at least one immunoglobulin single variable structural domain. The provided binding molecule can specifically bind the SARS-COV-2-Spike protein and effectively block the binding of the SARS-COV-2-Spike protein and human cell ACE2 receptors, so that the infection process of the SARS-COV-2 on cells can be further blocked, and theinfection and amplification of the SARS-COV-2 can be inhibited.
Owner:SHENZHEN IMMUNOTHERAPY BIOTECH CO LTD
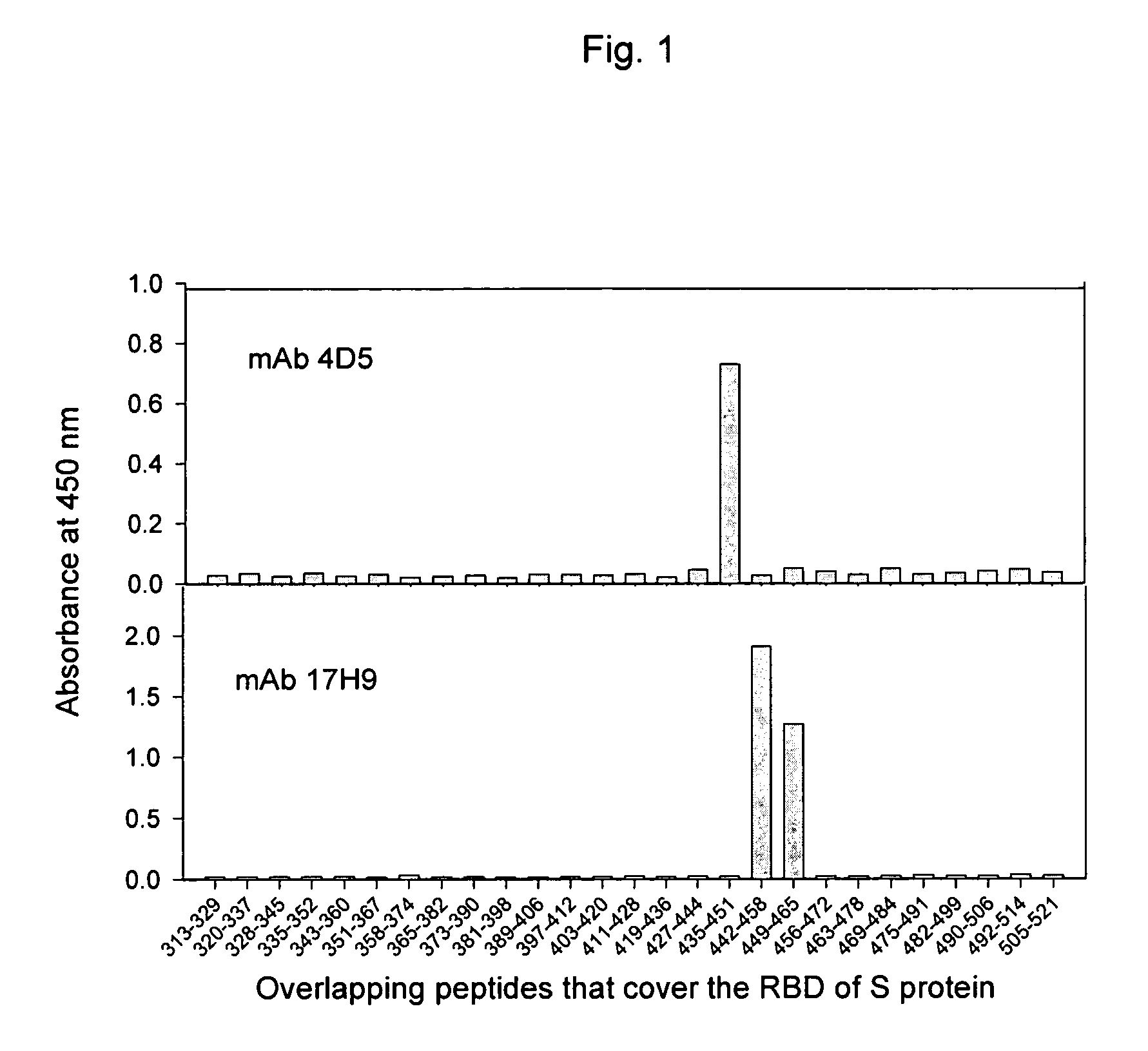
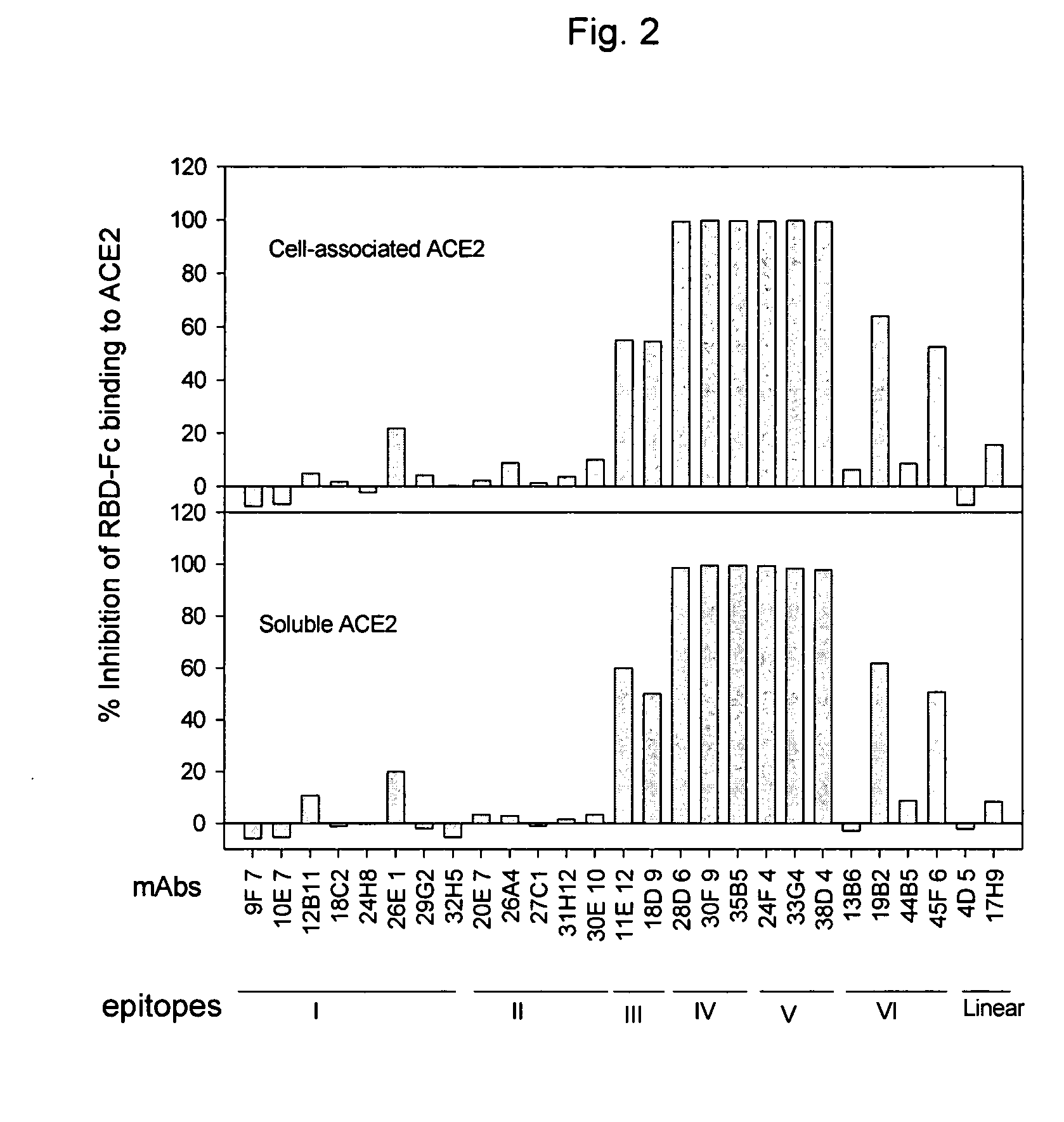
Neutralizing monoclonal antibodies against severe acute respiratory syndrome-associated coronavirus
InactiveUS20060240551A1Reduce the binding forceAvoid infectionAnimal cellsMicrobiological testing/measurementAntigenicitySpike Protein
The present invention provides an isolated antibody capable of binding to the receptor-binding domain of the spike protein of the severe acute respiratory syndrome-associated coronavirus (SARS-CoV) so as to competitively inhibit the binding of the SARS-CoV to host cells. These mAbs or substances can be used: 1) as passive-immunizing agents for prevention of SARS-CoV infection; 2) as biological reagents for diagnosis of SARS-CoV infection; 3) as immunotherapeutics for early treatment of SARS-CoV infection; and 4) as probes for studying the immunogenicity, antigenicity, structure, and function of the SARS-CoV S protein.
Owner:NEW YORK BLOOD CENT
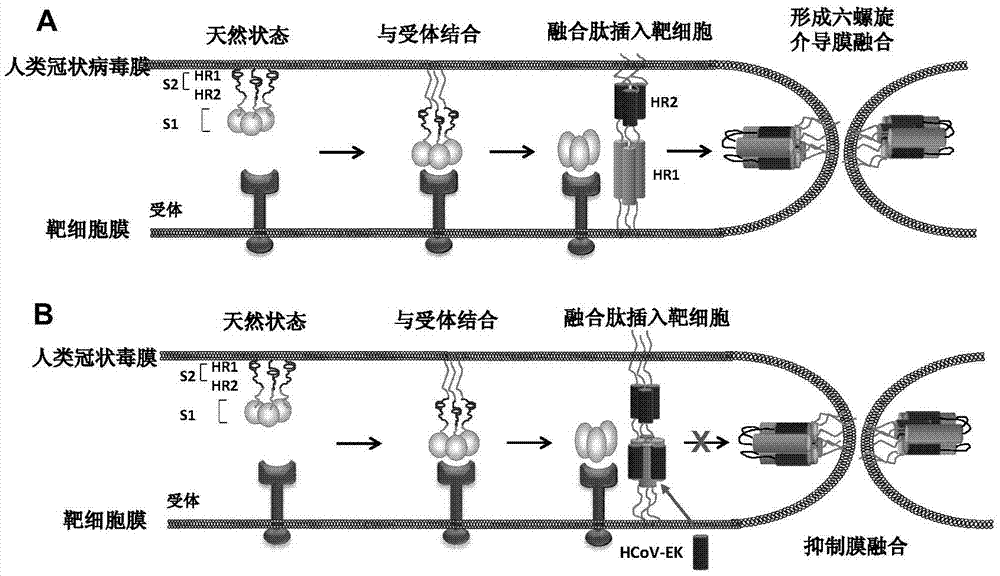
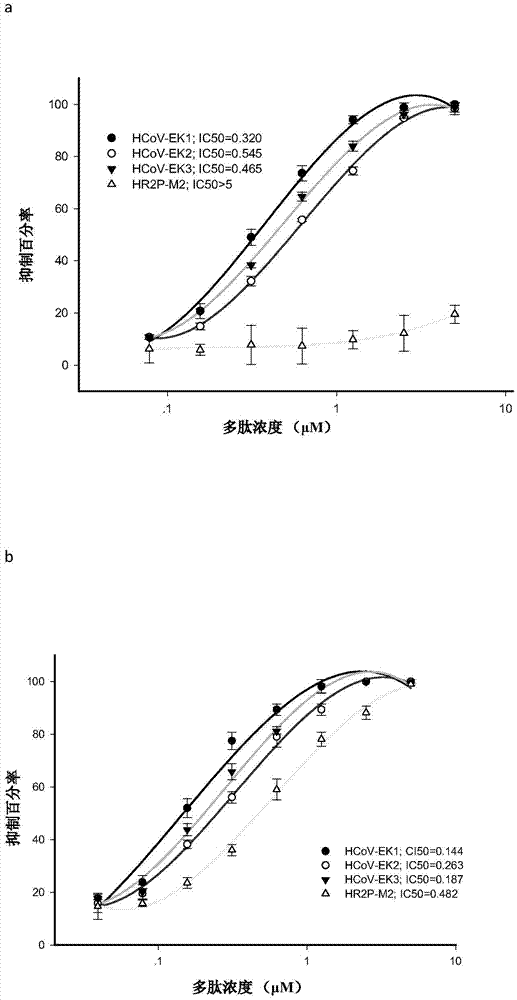
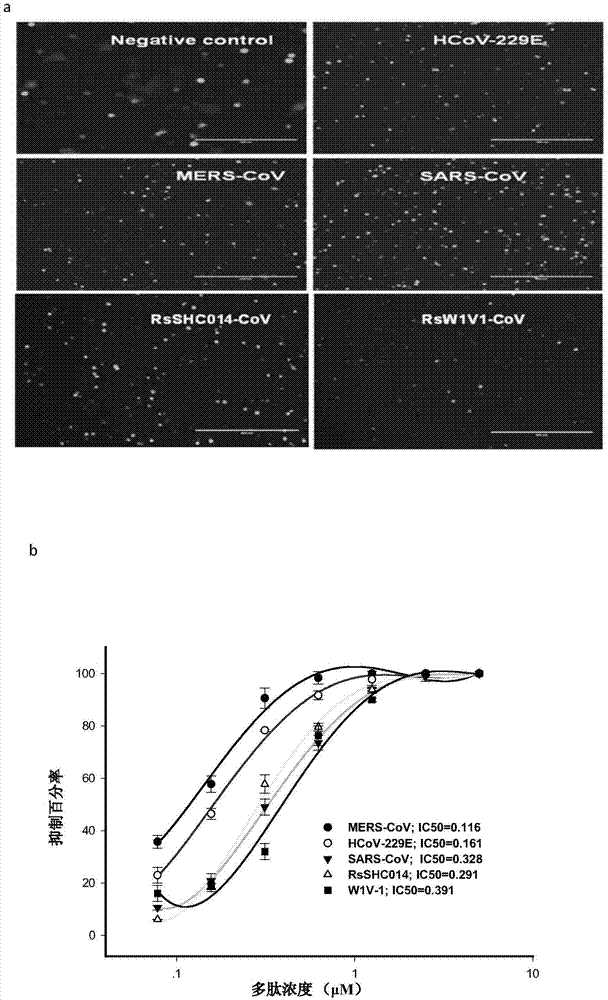
Polypeptides capable of inhibiting human coronavirus infections in a broad spectrum manner, and applications thereof
ActiveCN107022008AEnhanced inhibitory effectStrong inhibitory activitySsRNA viruses positive-sensePeptide/protein ingredientsHuman coronavirusFusion mechanism
The present invention belongs to the field of biomedicine, and relates to polypeptides capable of inhibiting human coronavirus infections, particularly to polypeptides capable of inhibiting human coronavirus infections in a broad spectrum manner, and applications thereof. According to the present invention, the polypeptides capable of providing broad spectrum inhibition effects for infections caused by more than 2 human coronaviruses are provided based on the conservation property of the S2 region of a coronavirus S protein and the similar fusion mechanism; the test results show that the polypeptides can achieve the commonality of the human coronavirus, ie., the similar HR region and the same fusion mechanism mediated by the coronavirus S protein so as to provide a series of HCoV-EK polypeptides, wherein the polypeptides provide good inhibition effects for currently popular human coronaviruses, and further provide good inhibition activity for SARS virus (RsSHC014-CoV or RsW1V1-CoV) possibly infecting human; and the polypeptides of the present invention can provide the prevention and treatment candidate drug for the currently popular human coronaviruses and the novel human coronavirus possibly emerging in the future.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Methods for treating arenaviridae and coronaviridae virus infections
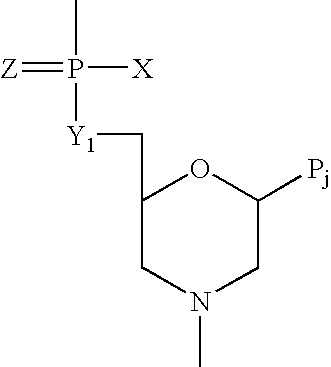
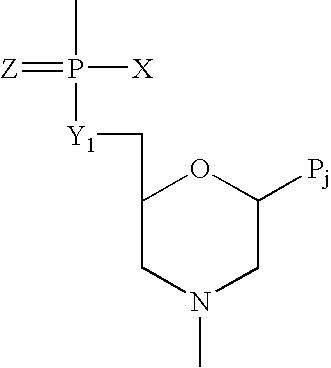
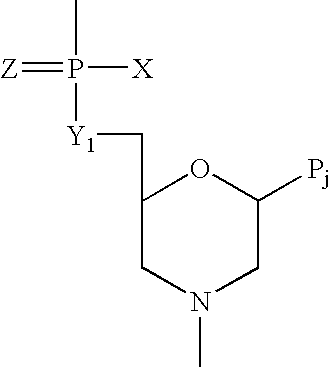
Provided are methods for treating Arenaviridae and Coronaviridae virus infections by administering nucleosides and prodrugs thereof, of Formula I:wherein the 1′ position of the nucleoside sugar is substituted. The compounds, compositions, and methods provided are particularly useful for the treatment of Lassa virus and Junin virus infections.
Owner:GILEAD SCI INC
Fluorescence immunochromatography device for detecting COVID-19 and using method thereof
InactiveCN111060691AImprove binding efficiencyHigh sensitivityBiological testingImmunoassaysVirologyDisease control
The invention discloses a fluorescence immunochromatography device for detecting a novel coronavirus COVID-19 and a using method of the fluorescence immunochromatography device. The fluorescence immunochromatography device disclosed by the invention is high in sensitivity and strong in specificity, the accuracy is high, the detection sensitivity can reach 10pg / ml, the detection speed is high, theoperation is simple, the device is portable, the requirement for personnel is low, the operation of professionals is not needed, the detection cost is low, the device can be applied to preliminary screening of various places such as hospitals, airports, customs and disease control centers, the detection time (10-15 min) is short, a simpler, more convenient and faster field detection means is provided for suspected patient investigation and asymptomatic infected person screening, and therefore epidemic spreading is prevented as soon as possible.
Owner:SHENZHEN BIOEASY BIOTECHNOLOGY CO LTD
Nucleic acid composition, kit and method for detecting 2019 novel coronaviruses
ActiveCN110982945AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesOrganic chemistryBiological organism
The invention discloses a nucleic acid composition, a kit and a method for detecting 2019 novel coronaviruses, belonging to the technical field of biology. The nucleic acid composition disclosed by the invention comprises at least one selected from the group consisting of a first nucleic acid combination and a second nucleic acid combination, wherein the first nucleic acid combination comprises nucleic acids as shown in SEQ ID NO.4-6, and the second nucleic acid combination comprises nucleic acids as shown in SEQ ID NO.7-9. When the nucleic acid composition, the kit or the method provided by the invention is used for detecting the 2019 novel coronaviruses, higher sensitivity and higher specificity are achieved.
Owner:ZHUHAI LIVZON DIAGNOSTICS
Novel coronavirus S protein two-region subunit nano vaccine based on pyrococcus furiosus ferritin
ActiveCN111217919AMultimerizationOvercoming the disadvantage of insufficient immunogenicitySsRNA viruses positive-senseViral antigen ingredientsEucaryotic cellNeutralising antibody
The invention discloses a novel coronavirus S protein double-region subunit nano vaccine based on pyrococcus furiosus ferritin. The virus receptor binding domain (RBD) and fusion peptide (FP) are usedtogether as a double antigen, and are connected with pyrococcus furiosus ferritin (PF_Ferritin) to from a fusion protein RBD-FP-PF_Ferritin to realize antigen multimerization; and then an eukaryoticcell expression system is used for expressing, and a 24-mer nano antigen can be formed through self-assembly of PF_Ferritin. The scheme can overcome the shortcoming of insufficient immunogenicity of RBD monomers, the obtained vaccine can significantly increase the level of a neutralizing antibody against the virus of a host, and the produced antibody has the ability to strongly block the virus from invading target cells. In addition, the vaccine of the invention is simple in preparation method, is easy to purify, and is high in safety, and the vaccine can be relatively quickly applied to clinical trials.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com