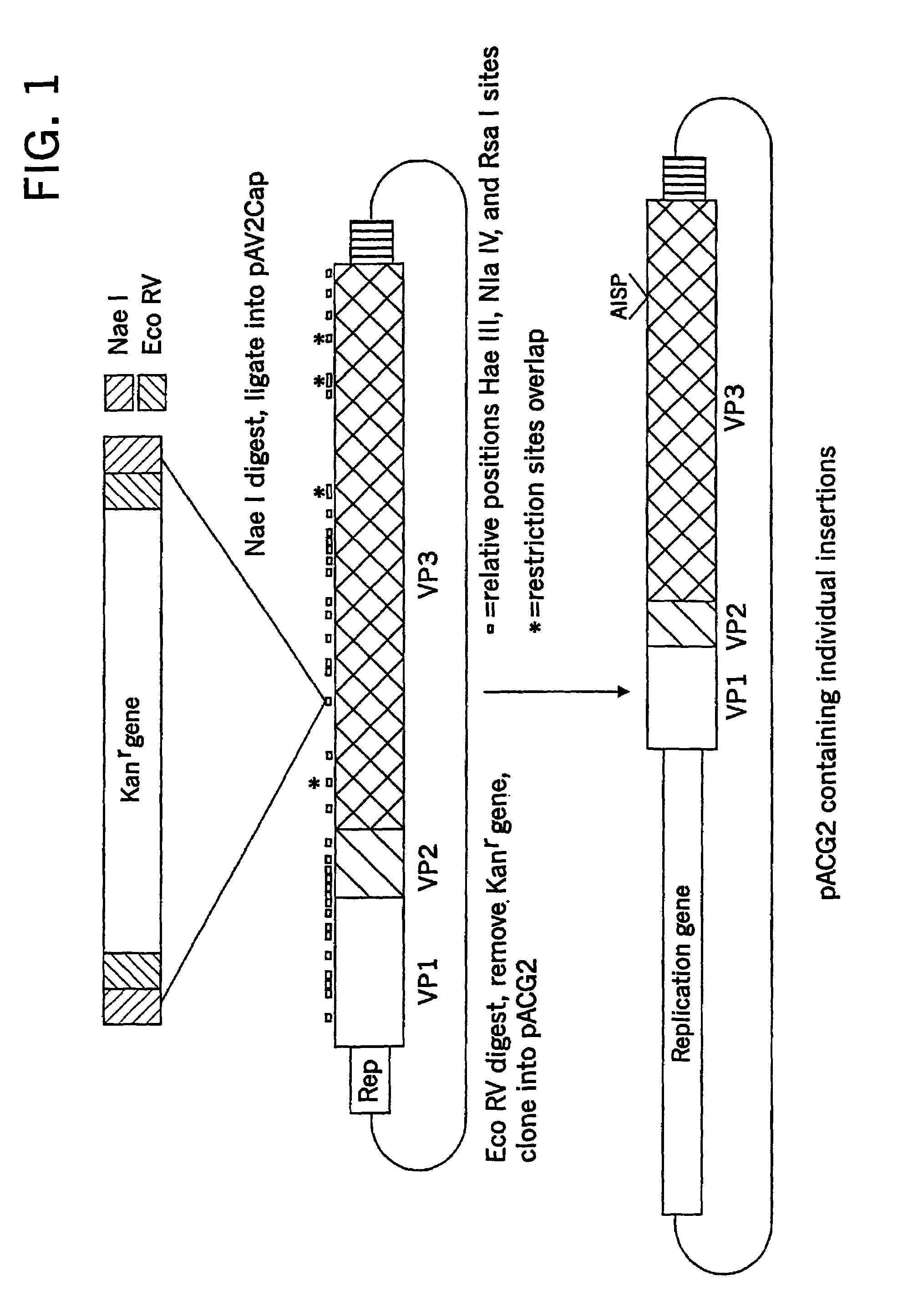
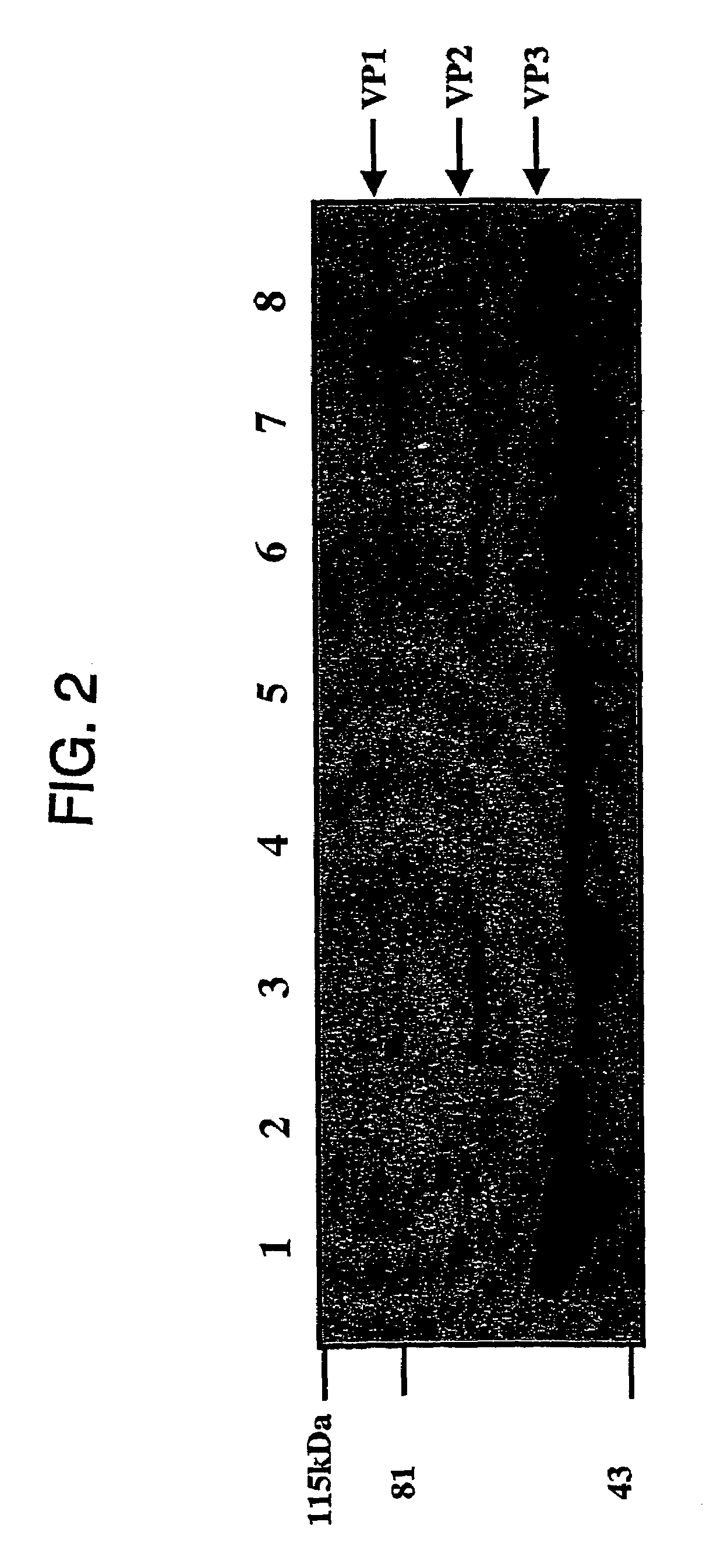
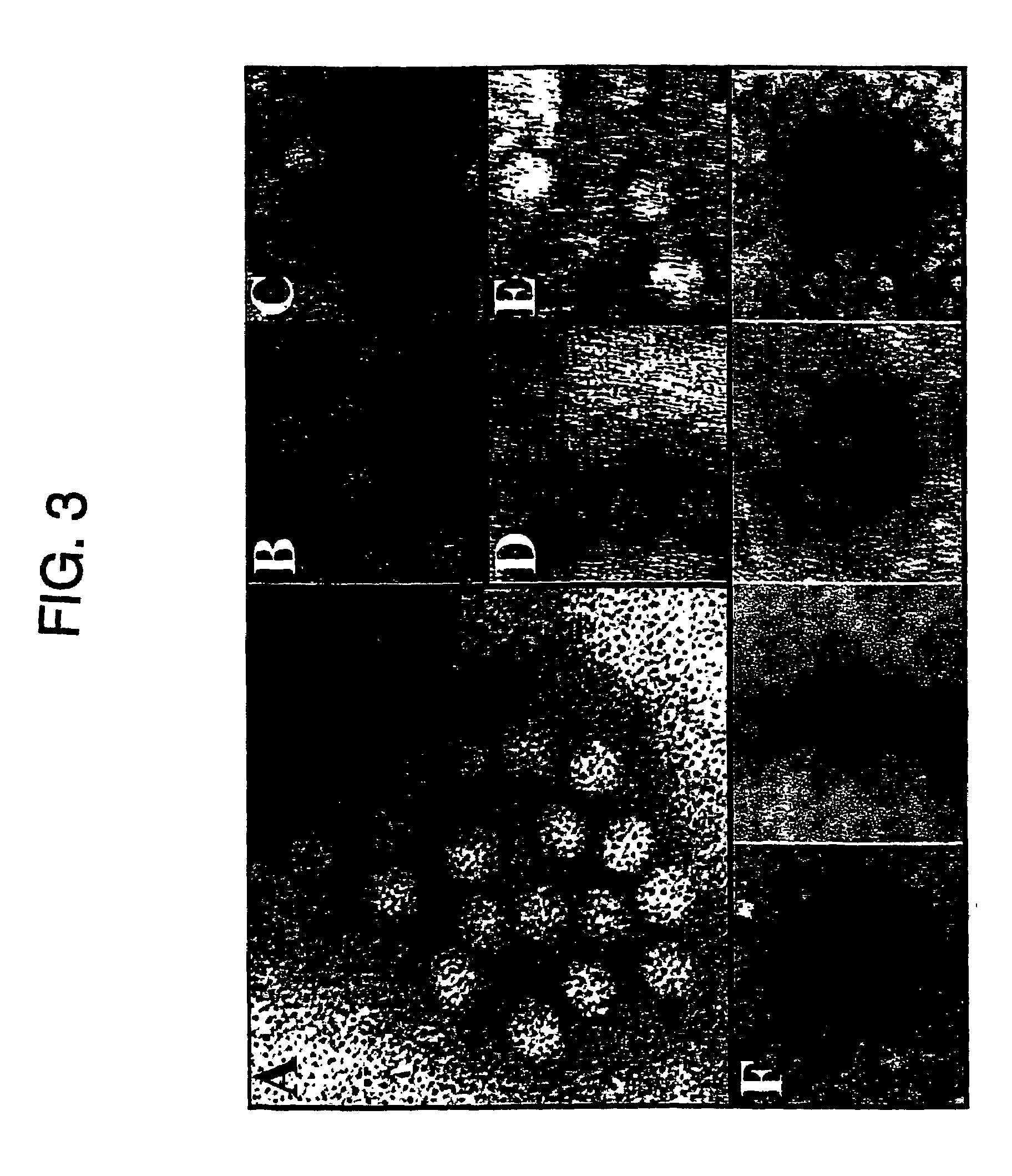
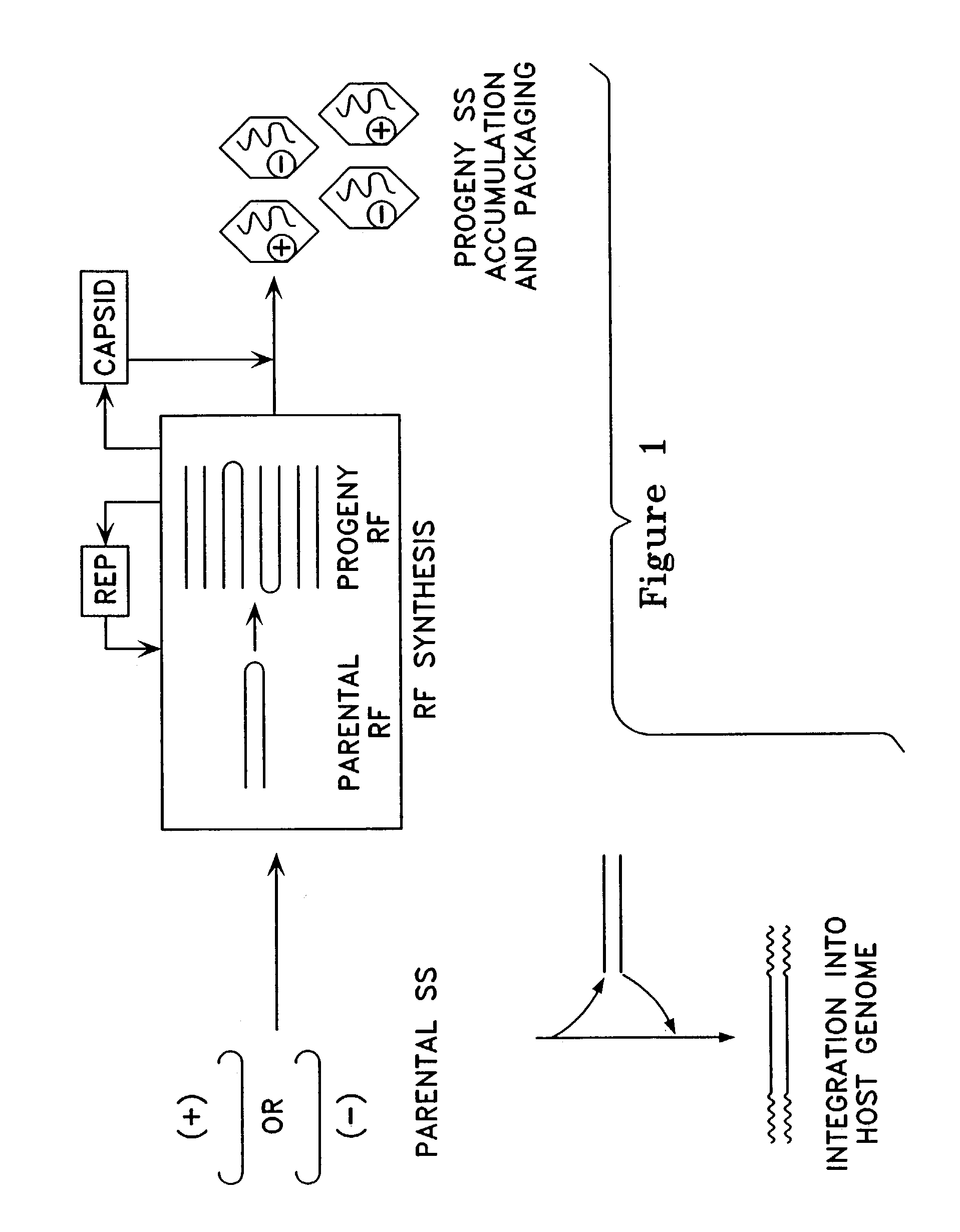
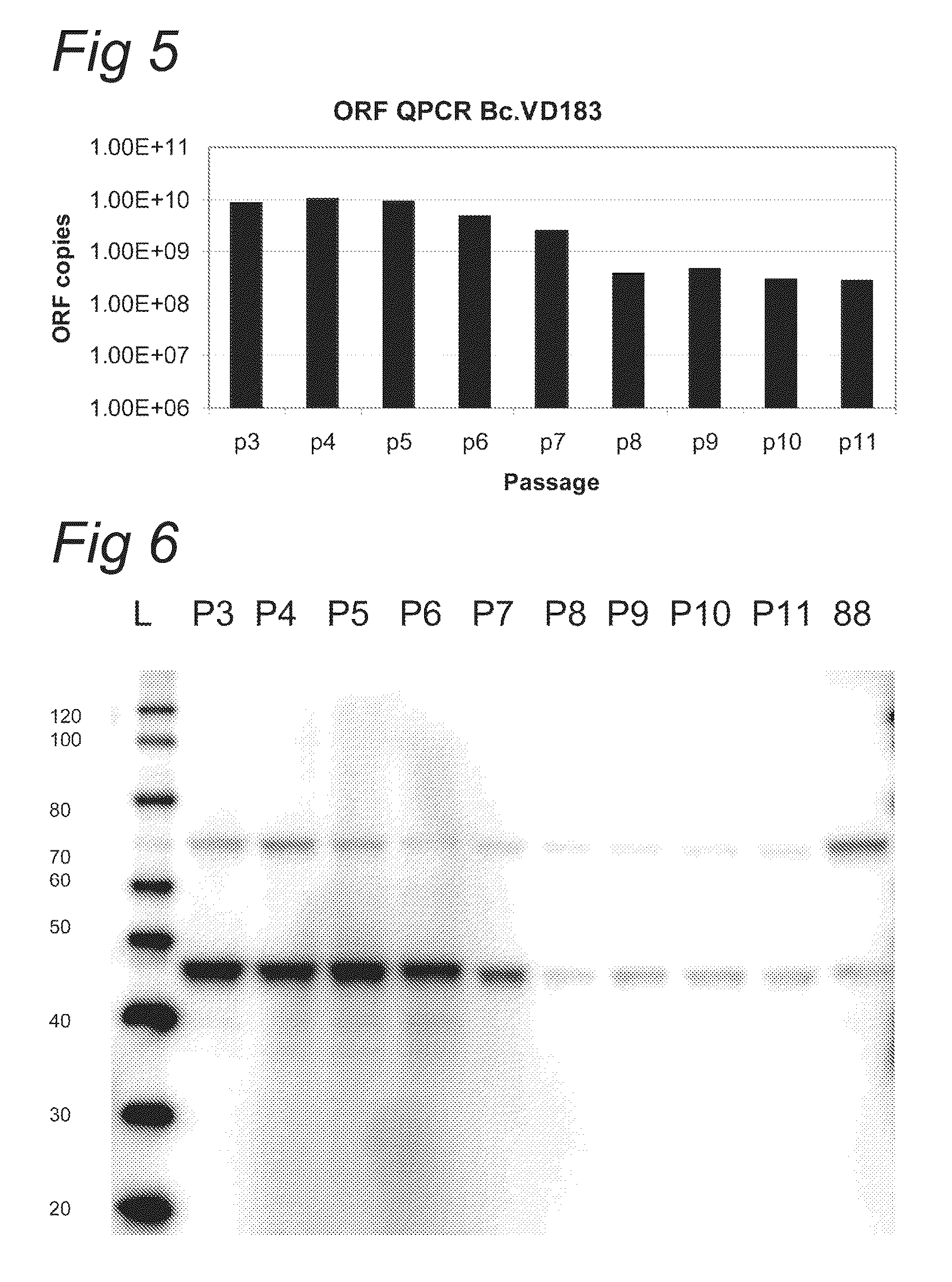
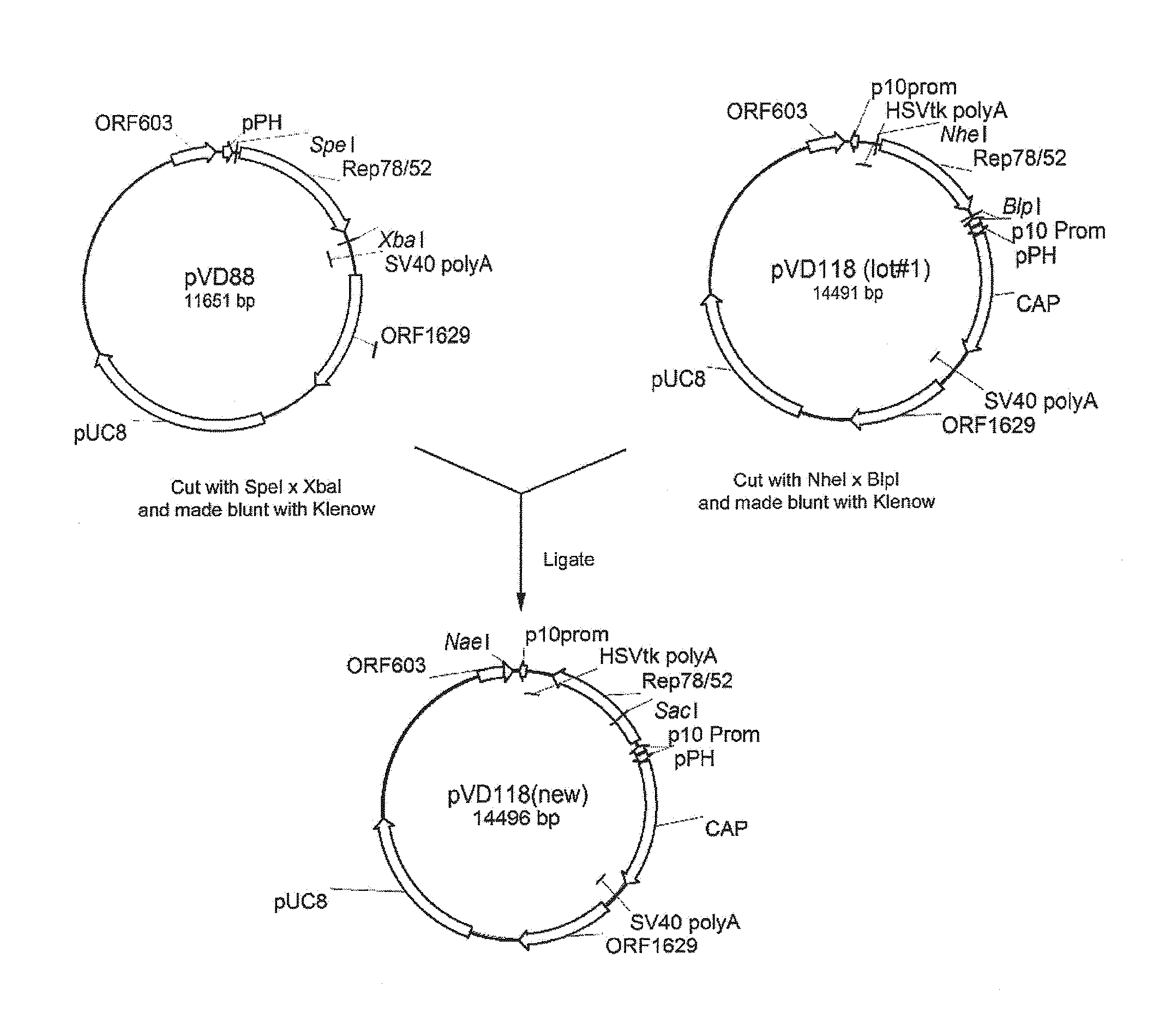
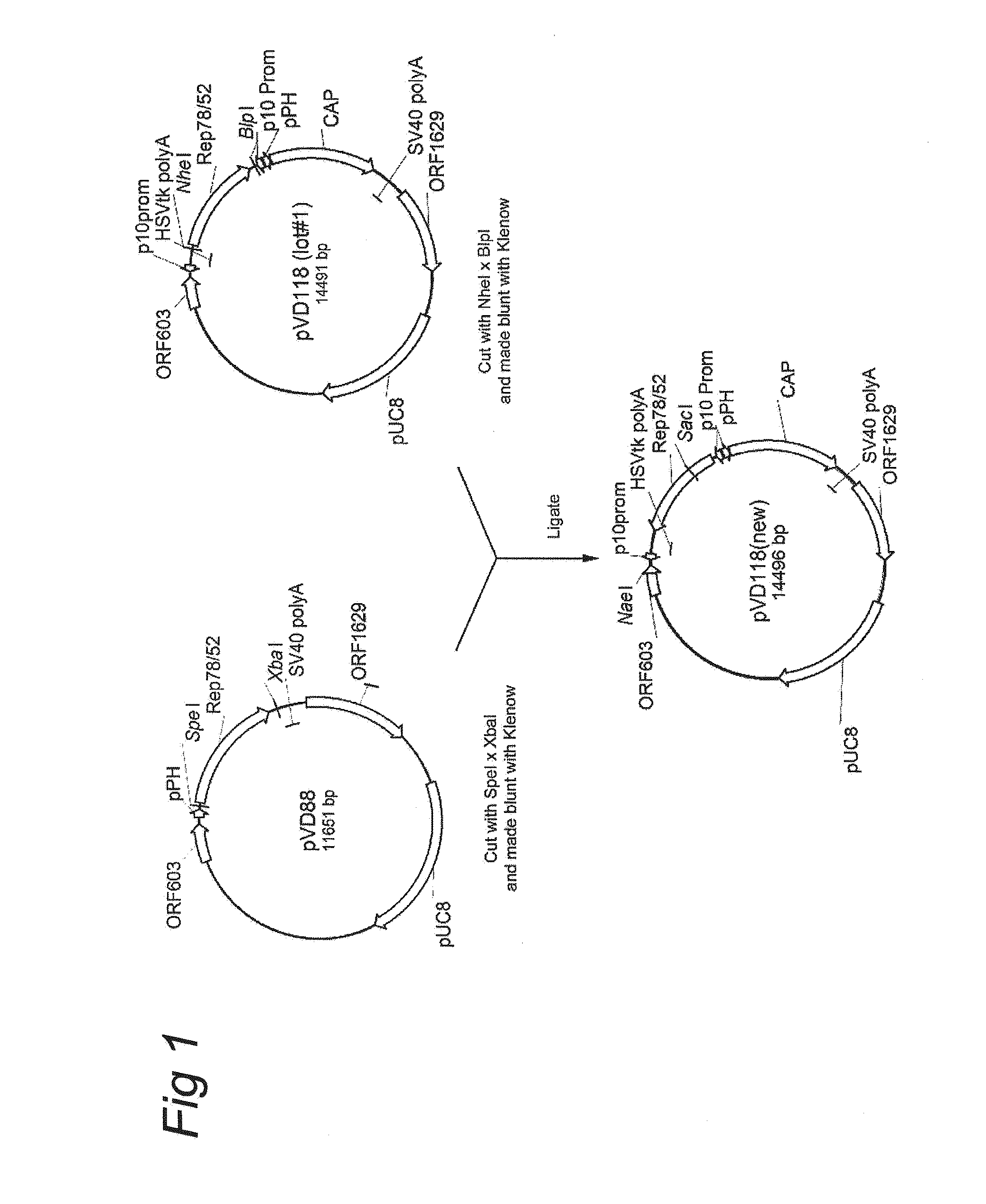
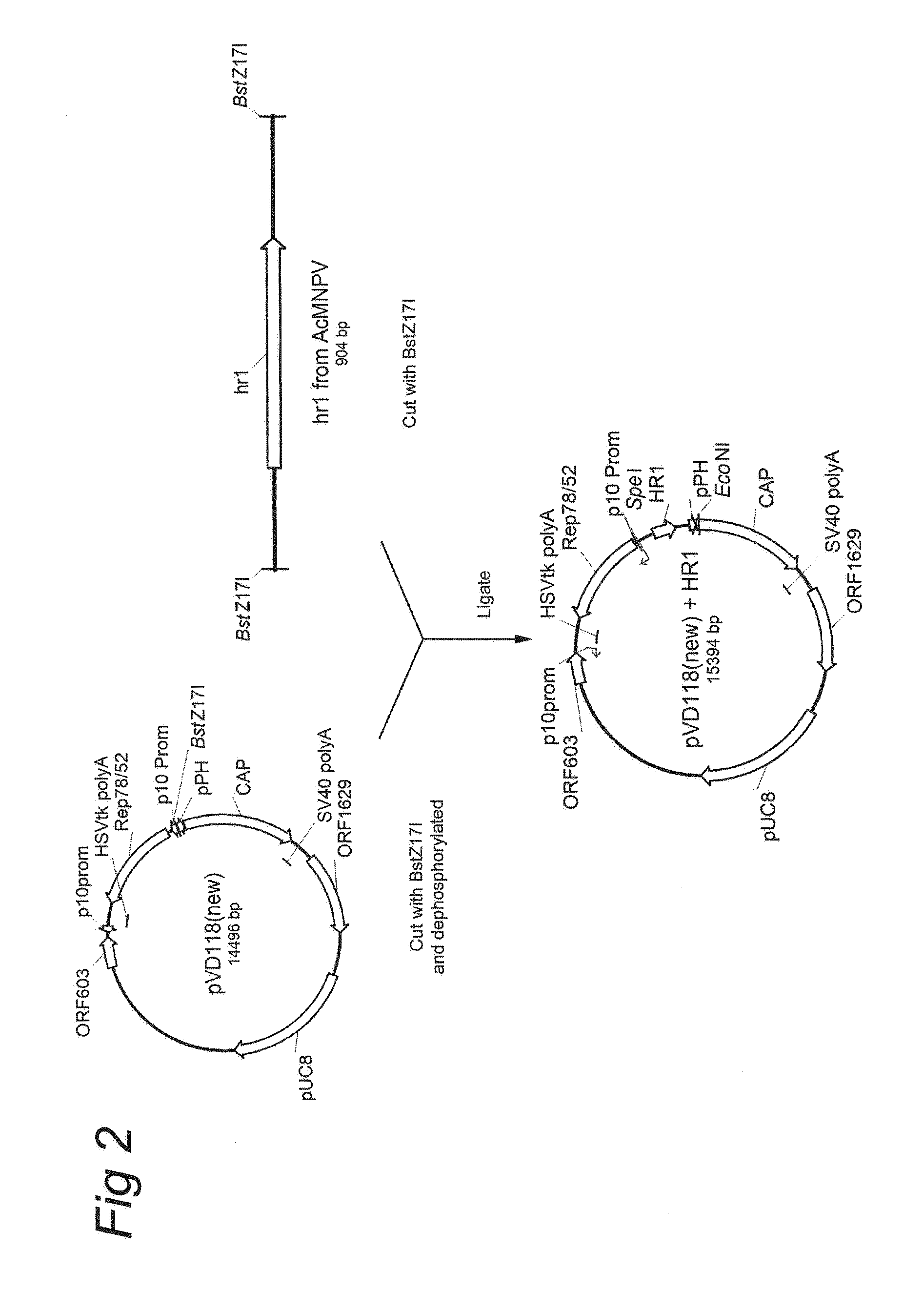
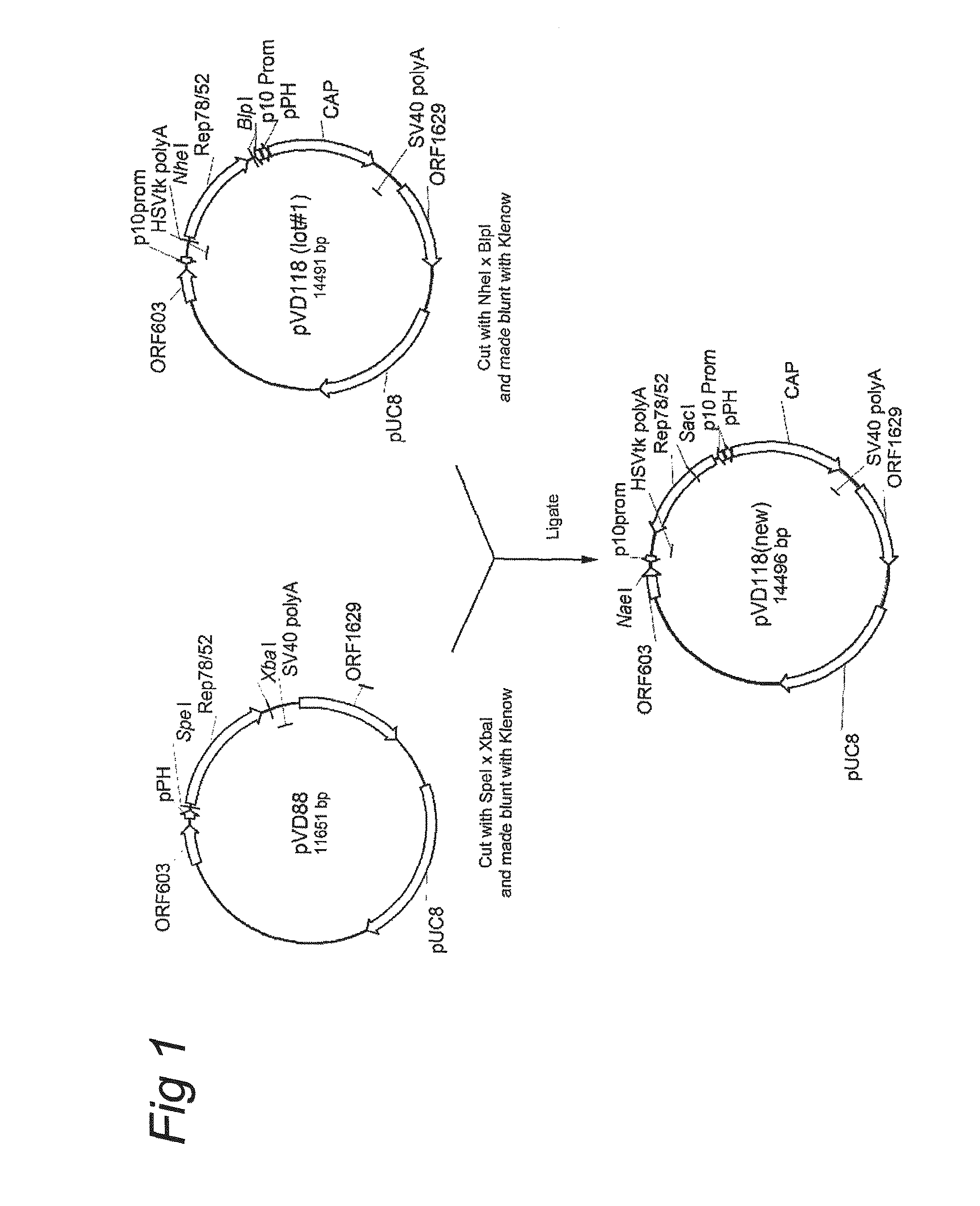
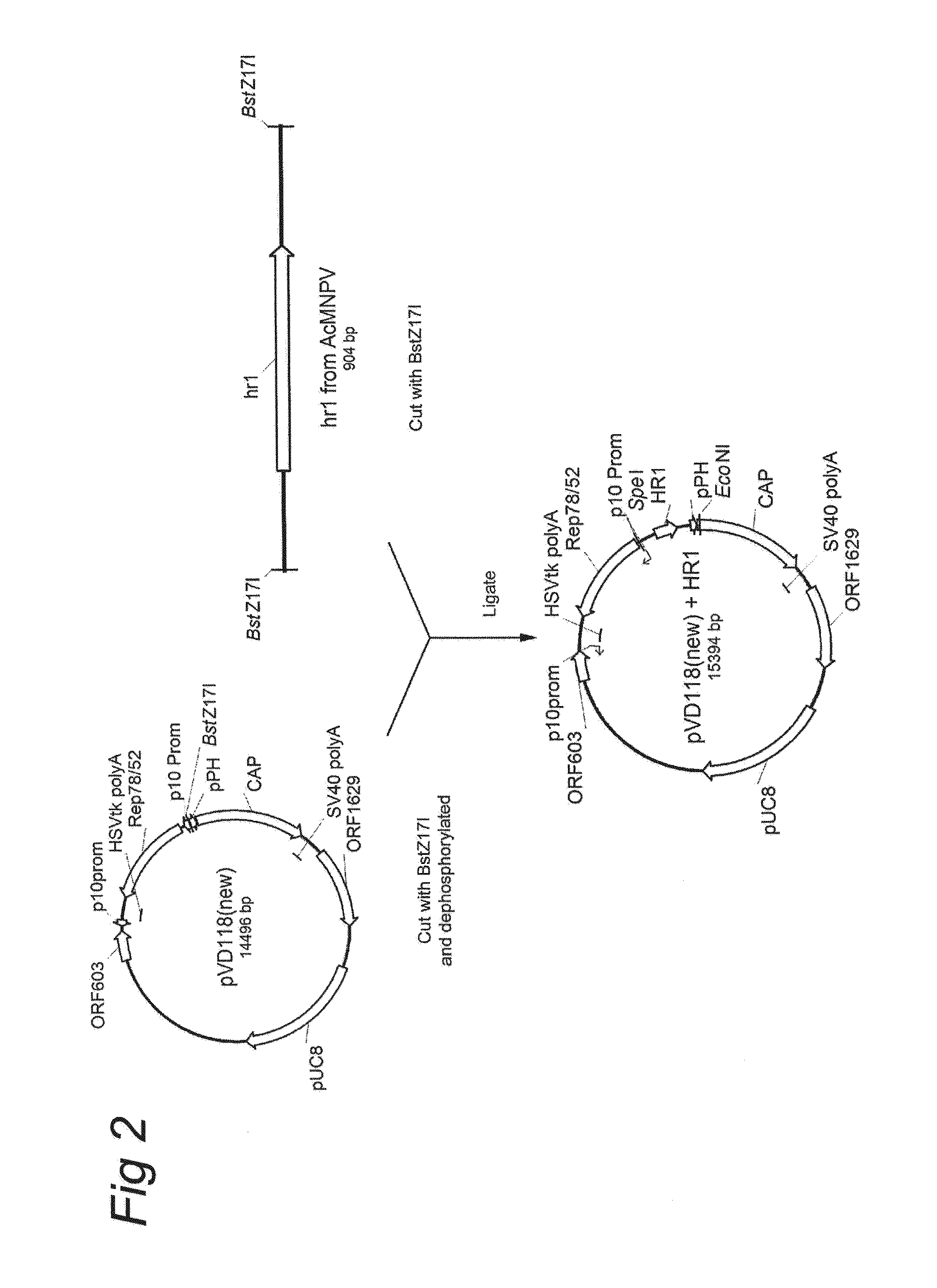
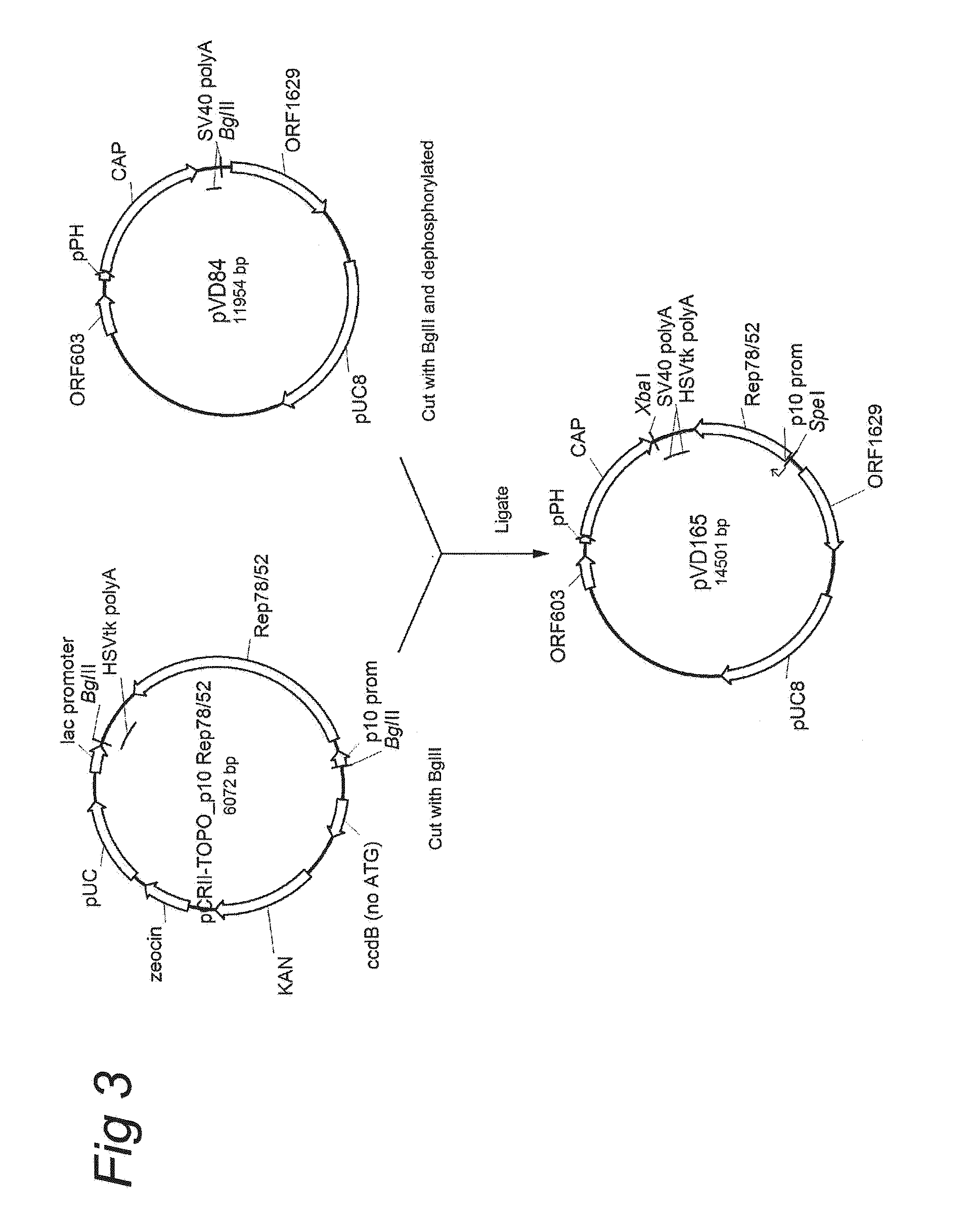
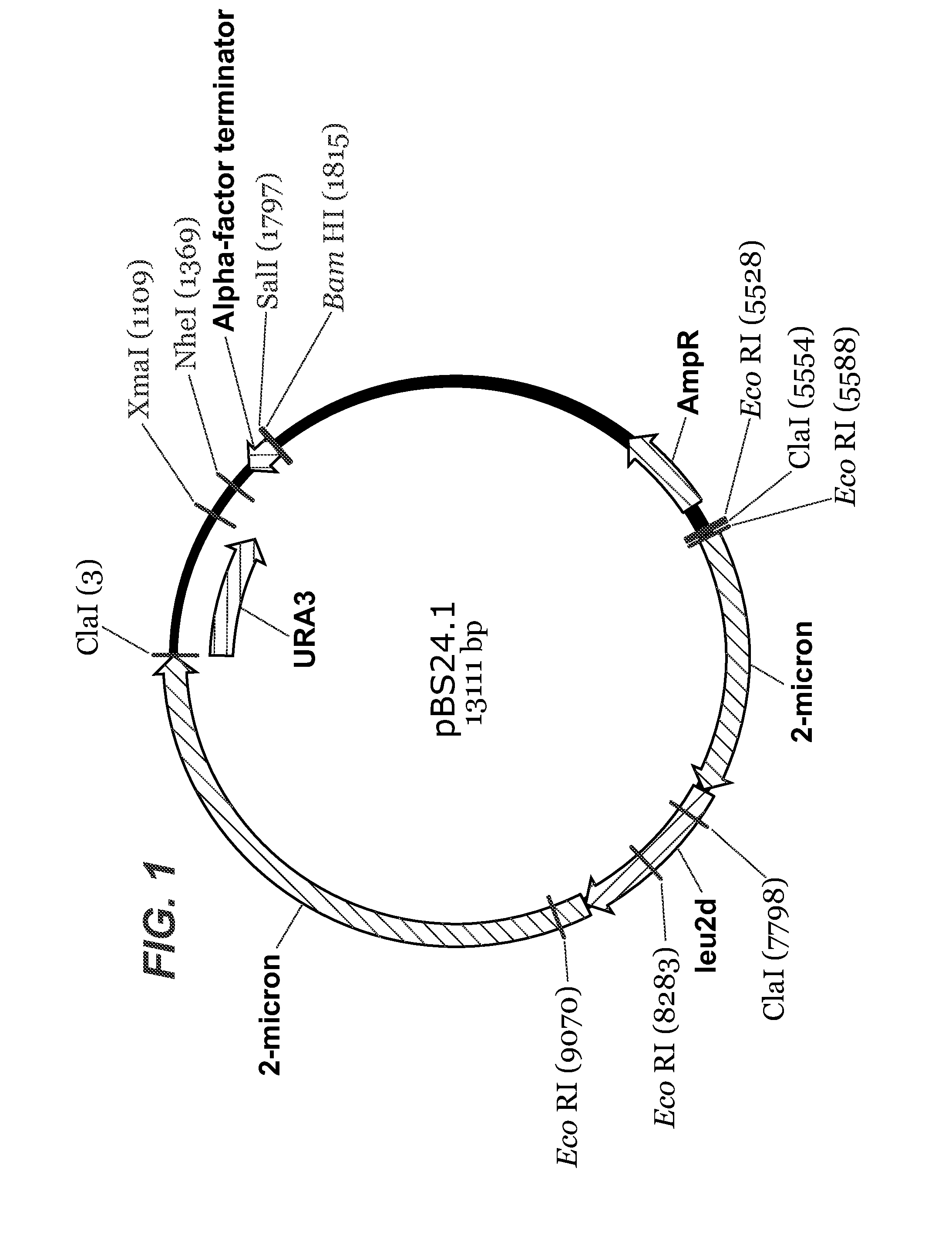
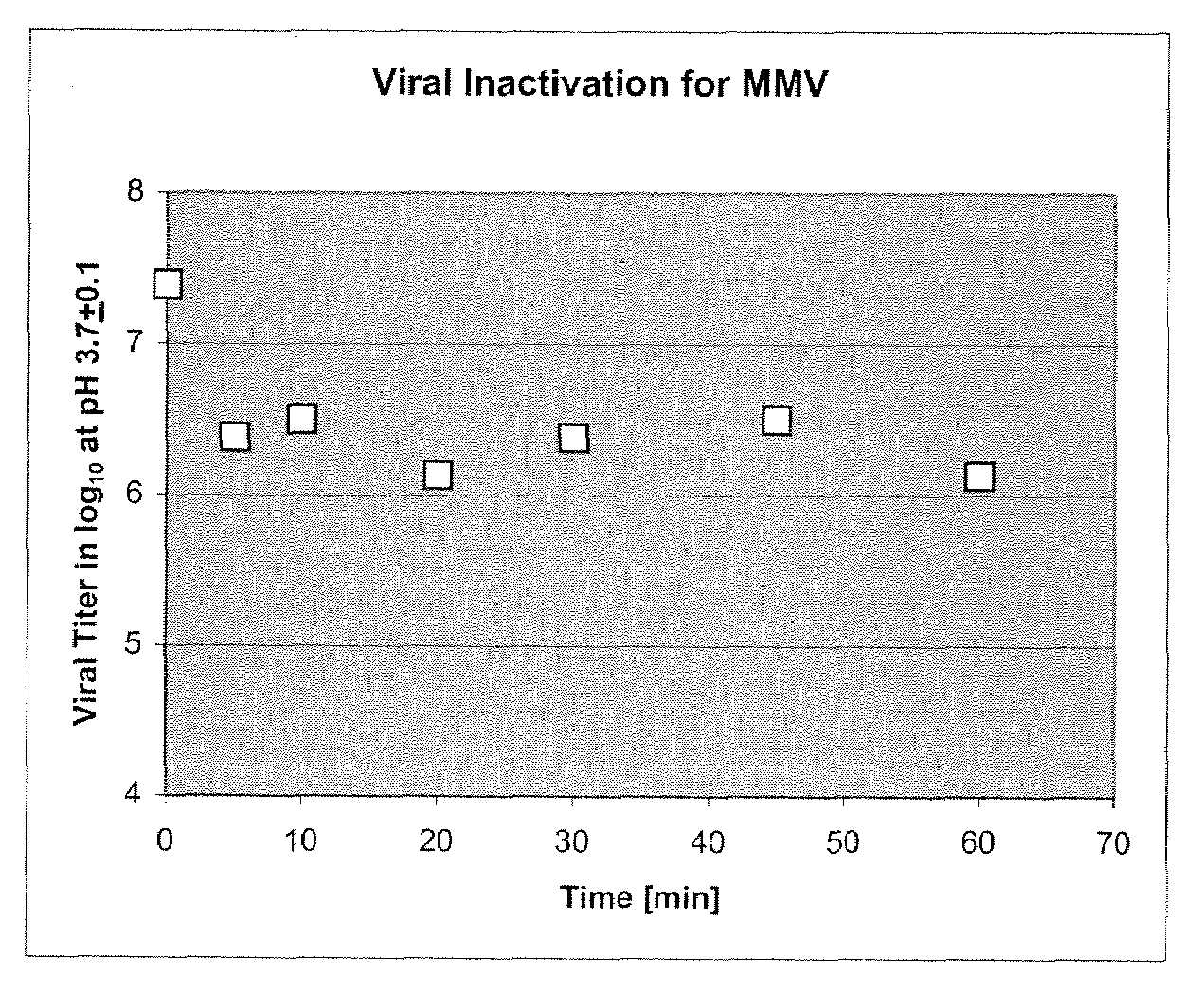
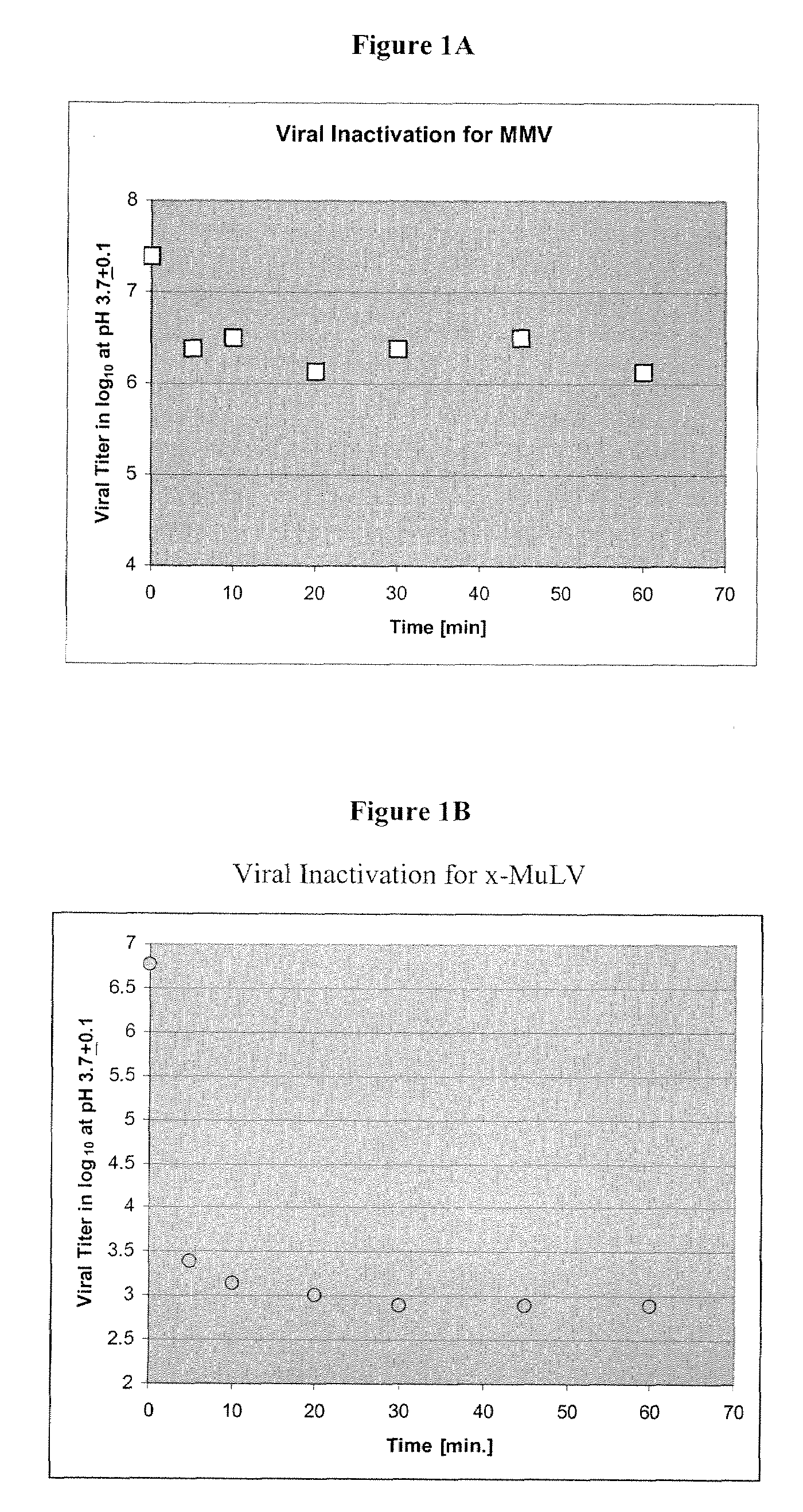
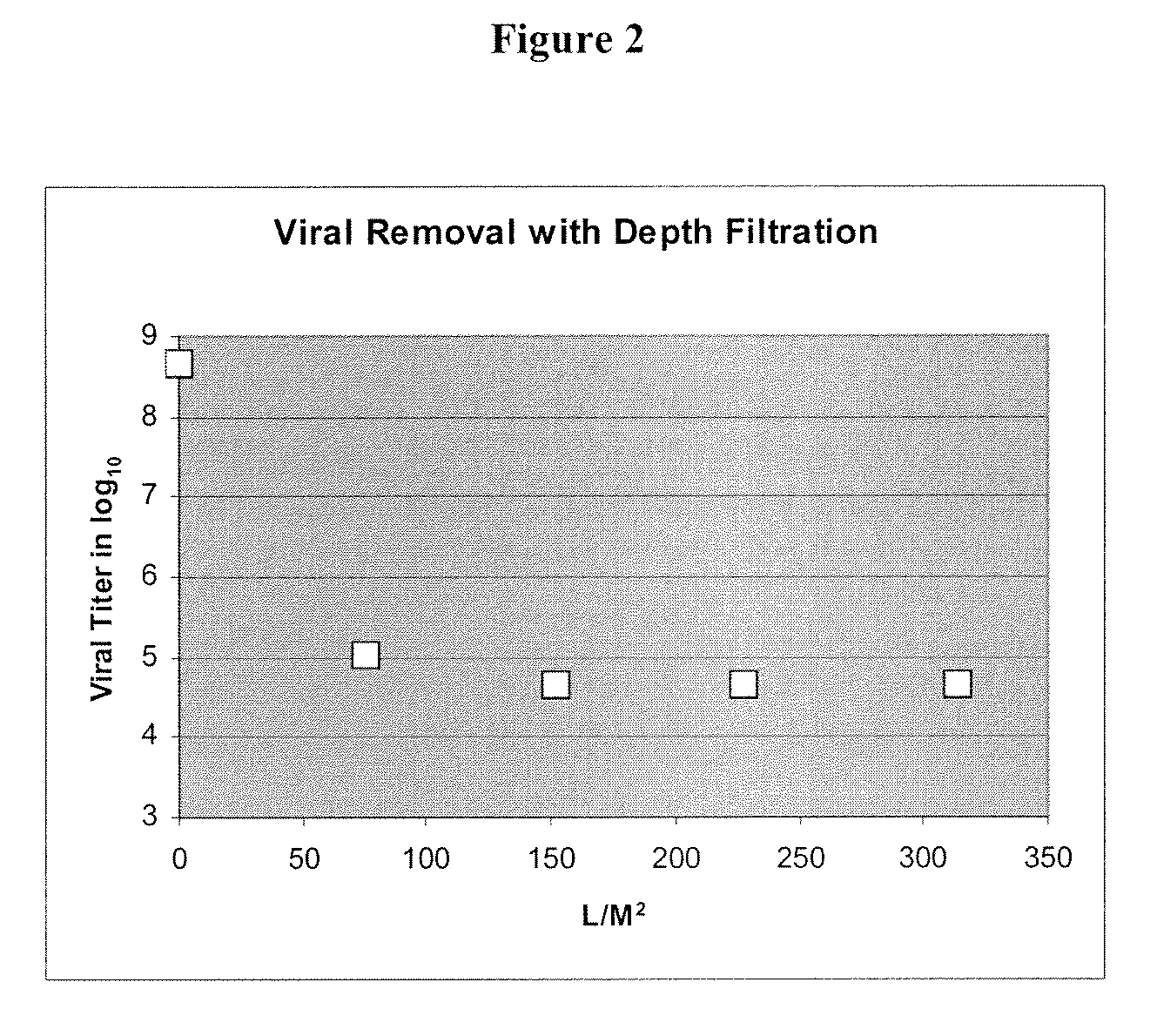
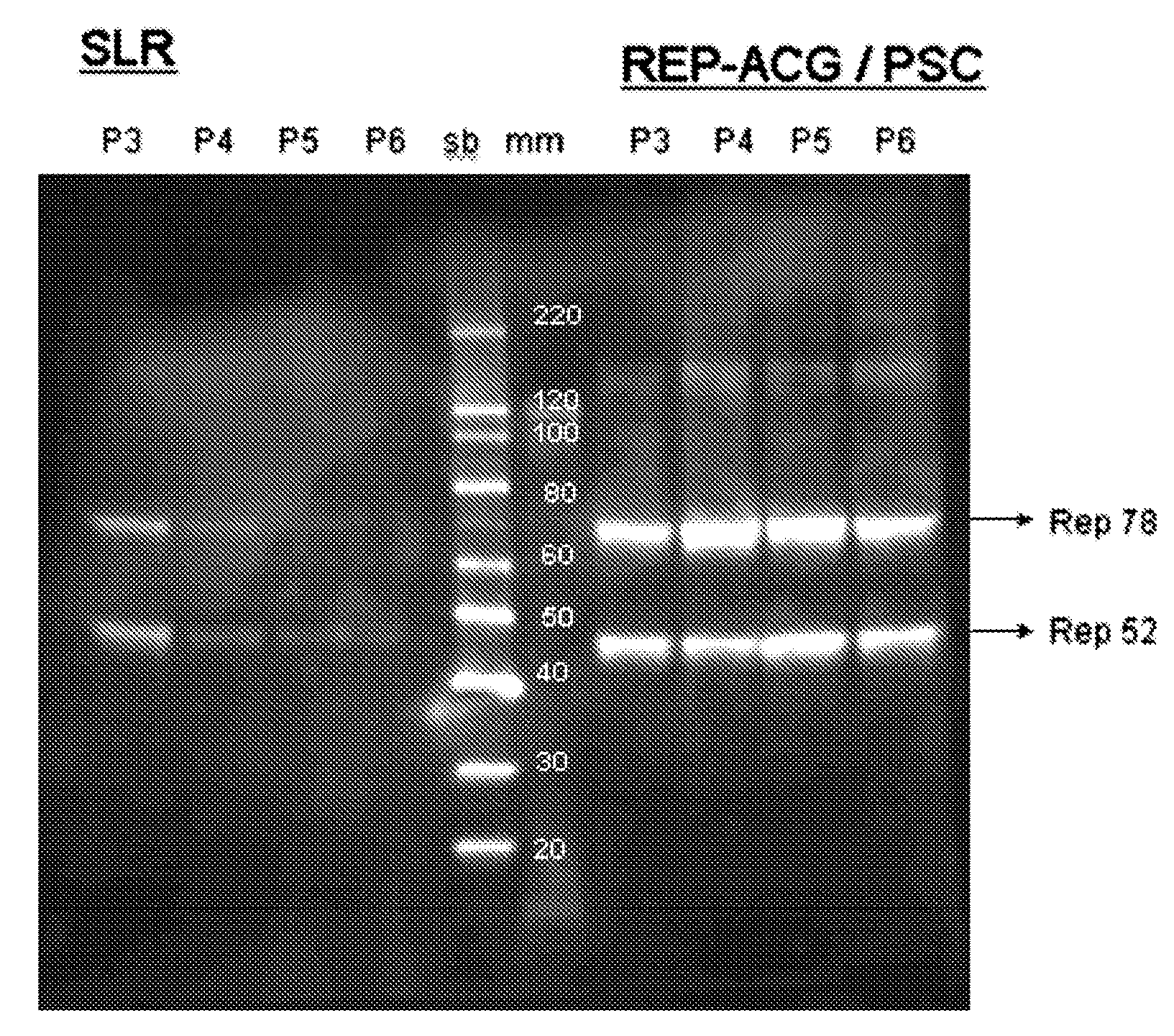
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
318 results about "Parvovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
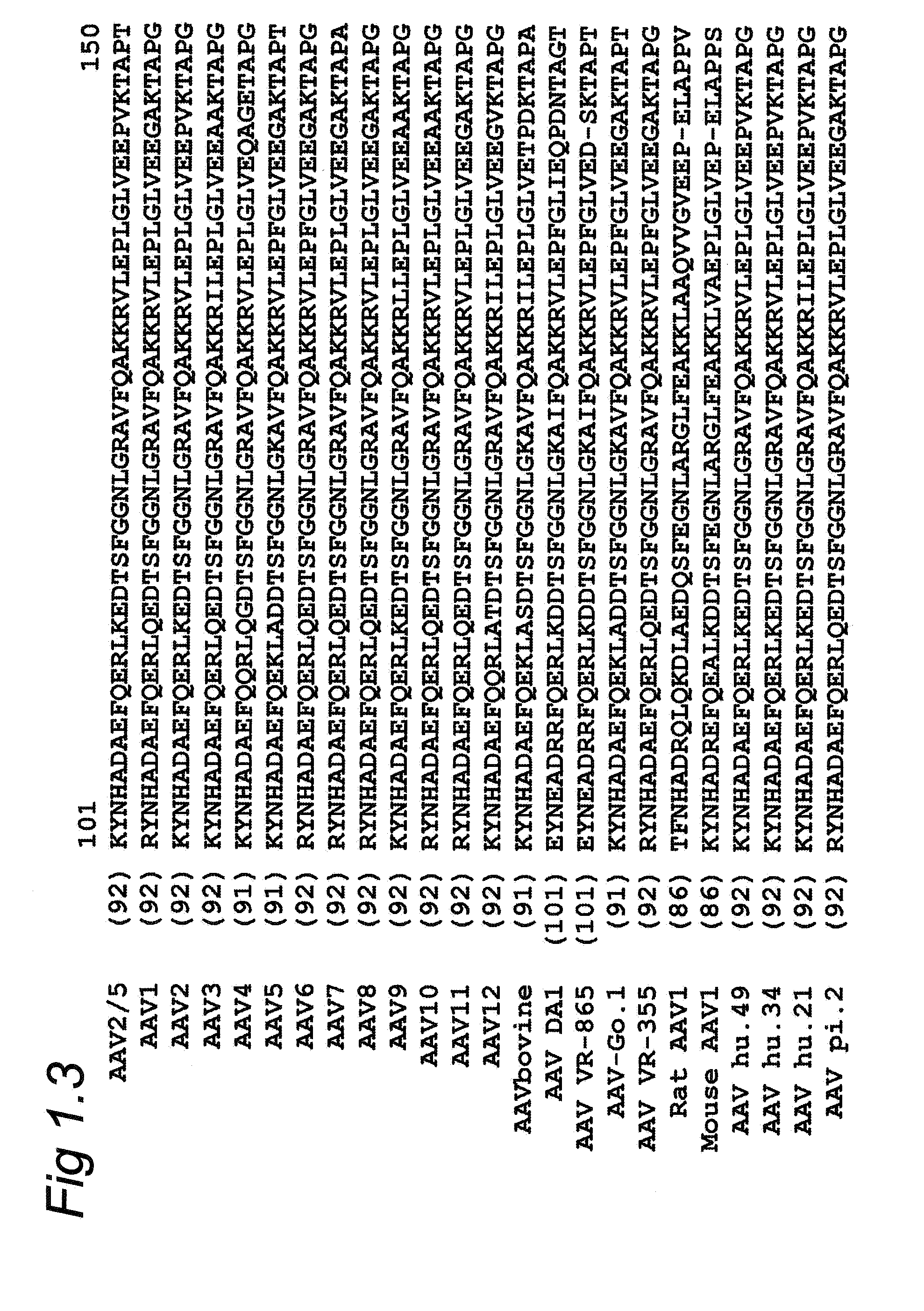
Parvovirus is the common name applied to all the viruses in the Parvoviridae taxonomic family, although it can also be used specifically for members of one of the two Parvoviridae subfamilies, the Parvovirinae, which infect vertebrate hosts. Members of the second subfamily, the Densovirinae, which infect invertebrate hosts, are more commonly referred to as densoviruses. In subfamily Parvovirinae there are eight genera, containing a total of 58 recognized species, while in subfamily Densovirinae there are 5 genera and a total of 21 species. These viruses have small genomes, encoding just two genes, and must rely on the synthetic machinery of their host cell for their own preferential replication. This means that many parvoviruses require host cells to enter S-phase before viral DNA replication can initiate, but they do not encode any gene products that can drive this transition. Parvoviruses overcome this problem in various ways: viruses in many genera simply wait within the cell for it to enter S-phase under its own cell cycle control, which means that they can only infect actively-dividing cell populations. In contrast, the so-called adeno-associated viruses (AAVs) from genus Dependoparvovirus must wait until the cell is co-infected by a helper DNA virus, commonly an adenovirus or herpes virus, which does encode gene products that can drive the cell into S-phase, allowing AAV infection to initiate and out-compete the helper virus. A third strategy is used by human bocavirus 1 (HBoV1) from genus Bocaparvovirus, which appears to invoke a specific DNA-damage response in its host cell that ultimately supports viral DNA amplification and progeny virus production.
Virus vectors and methods of making and administering the same
The present invention provides genetically-engineered parvovirus capsids and viruses designed to introduce a heterologous gene into a target cell. The parvoviruses of the invention provide a repertoire of vectors with altered antigenic properties, packaging capabilities, and / or cellular tropisms as compared with current AAV vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Metabolically activated recombinant viral vectors and methods for their preparation and use
Recombinant viral vectors, especially parvovirus vectors such as adeno-associated virus (AAV) vectors, capable of enhanced expression of heterologous sequences, and methods for their construction and use, are provided. The vectors have a structure, or are capable of rapidly adopting a structure, which involves intrastrand base pairing of at least one region in a heterologous sequence.
Owner:GENZYME CORP
Methods and compounds for controlled release of recombinant parvovirus vectors
InactiveUS7201898B2Prevent relapseImproved pulmonary mechanicsSuture equipmentsAntibacterial agentsControlled releaseSupport matrix
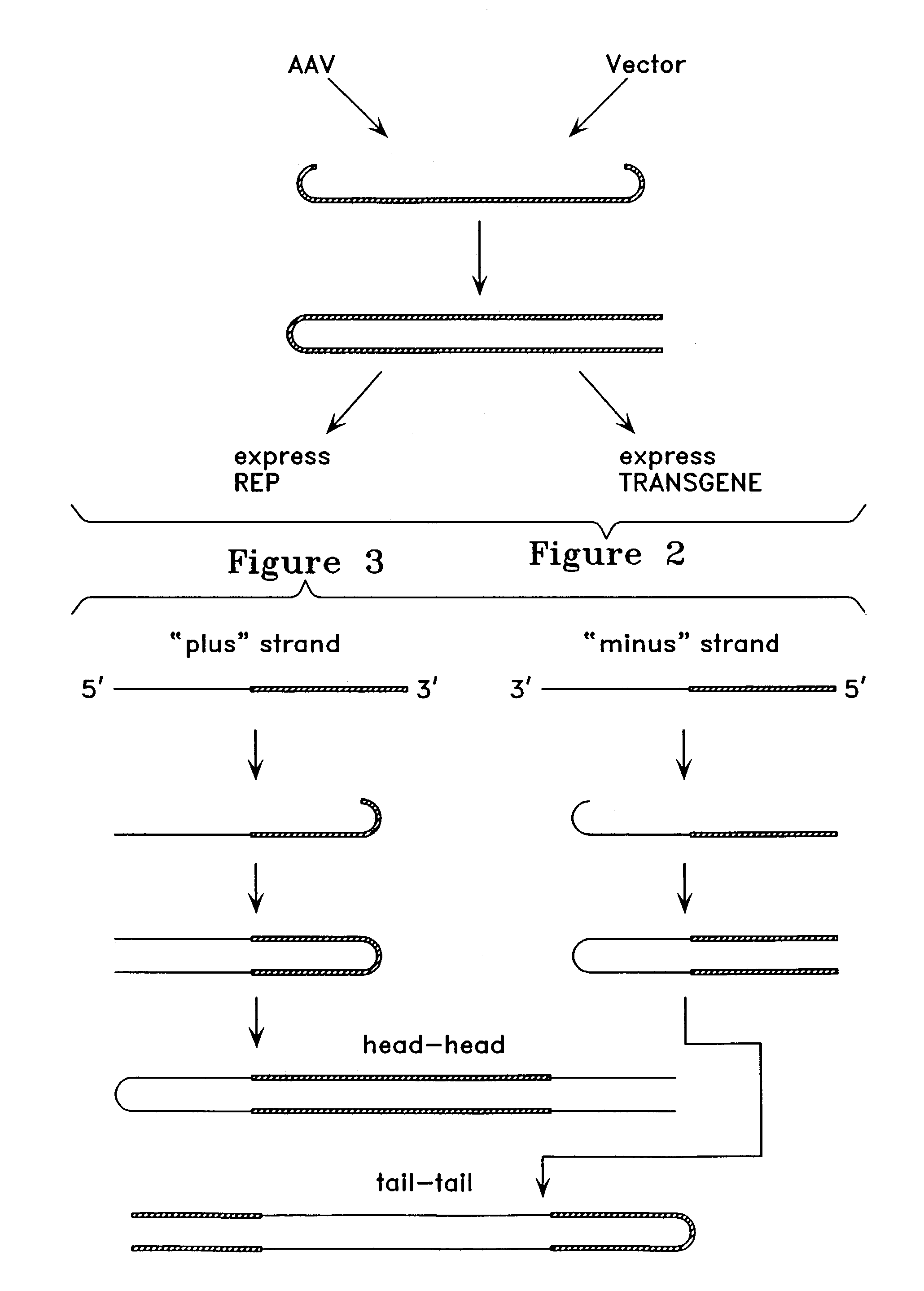
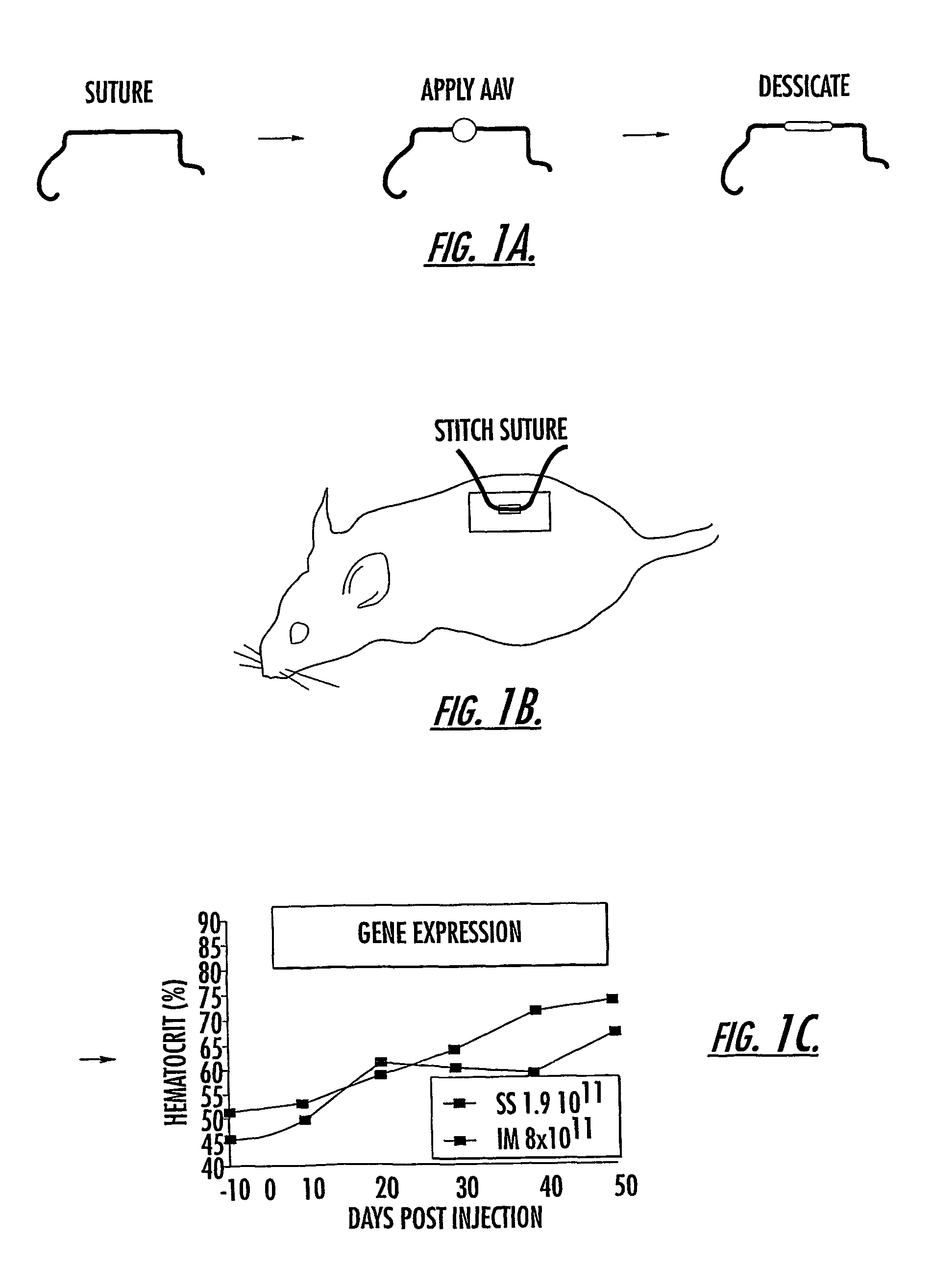
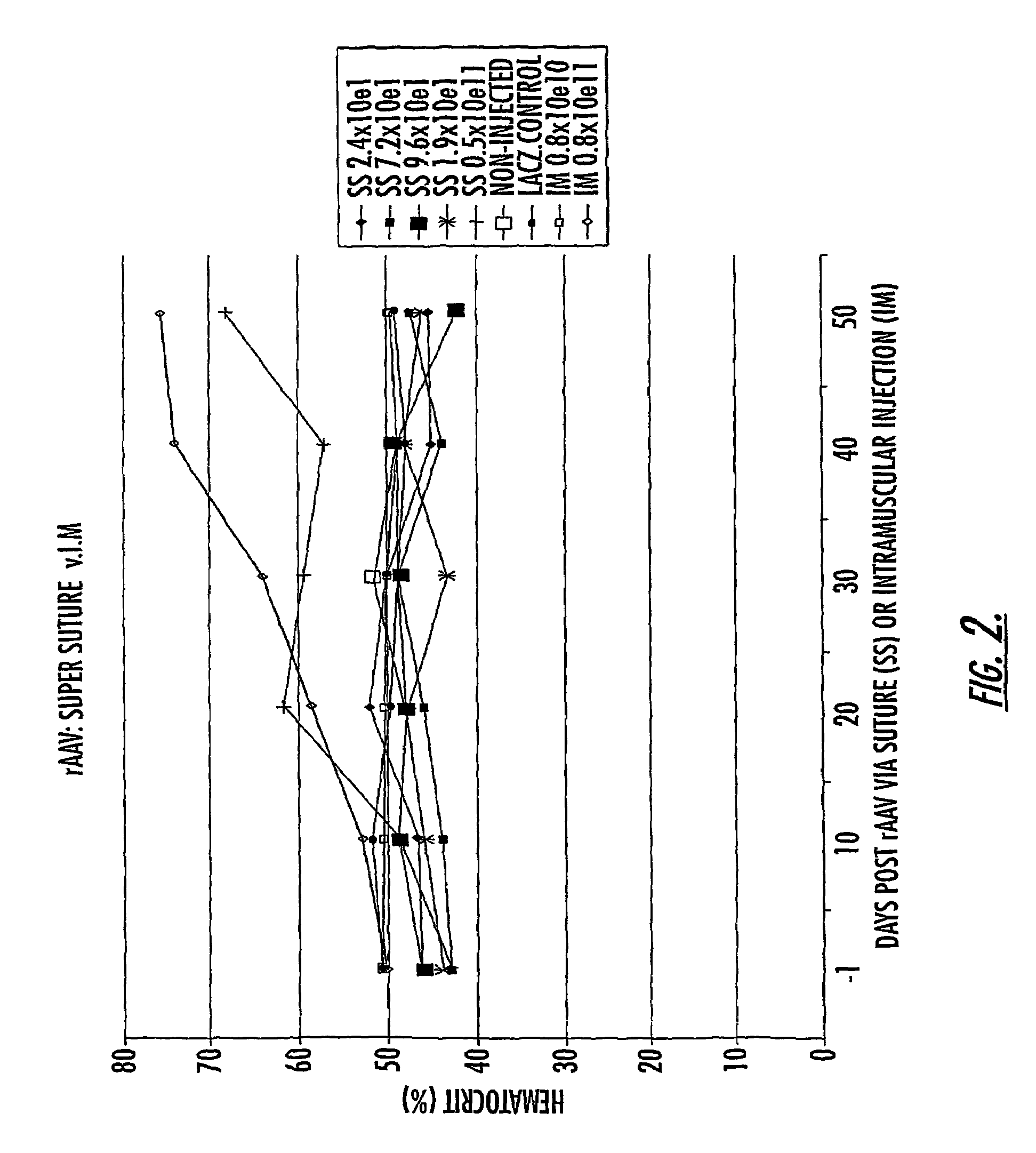
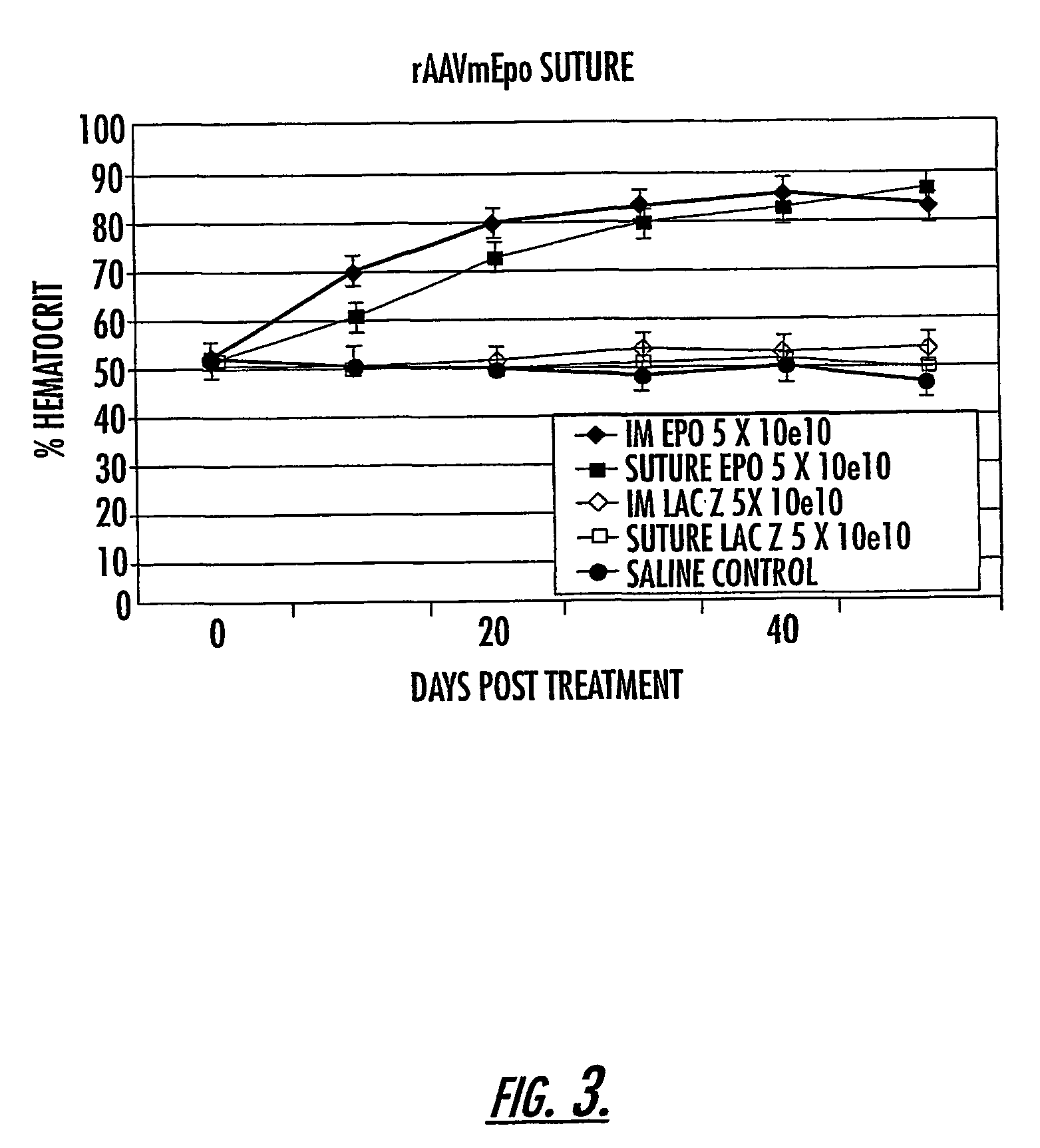
The invention uses recombinant parvoviruses, and particularly recombinant adeno-associated virus (rAAV) to deliver genes and DNA sequences for gene therapy following manipulation of the therapeutic virus for packaging and transport. The invention delivers therapeutic viral vectors via rAAV affixed to support matrixes (i.e., sutures, surgically implantable materials, grafts, and the like).
Owner:NORTH CAROLINA AT CHAPEL HILL THE UNIV OF
Duplexed parvovirus vectors
InactiveUS7465583B2High transduction efficiencyRapid onsetBiocidePeptide/protein ingredientsGeneticsViral vector
The present invention provides duplexed parvovirus vector genomes that are capable under appropriate conditions of forming a double-stranded molecule by intrastrand base-pairing. Also provided are duplexed parvovirus particles comprising the vector genome. Further disclosed are templates and methods for producing the duplexed vector genomes and duplexed parvovirus particles of the invention. Methods of administering these reagents to a cell or subject are also described. Preferably, the parvovirus capsid is an AAV capsid. It is further preferred that the vector genome comprises AAV terminal repeat sequences.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
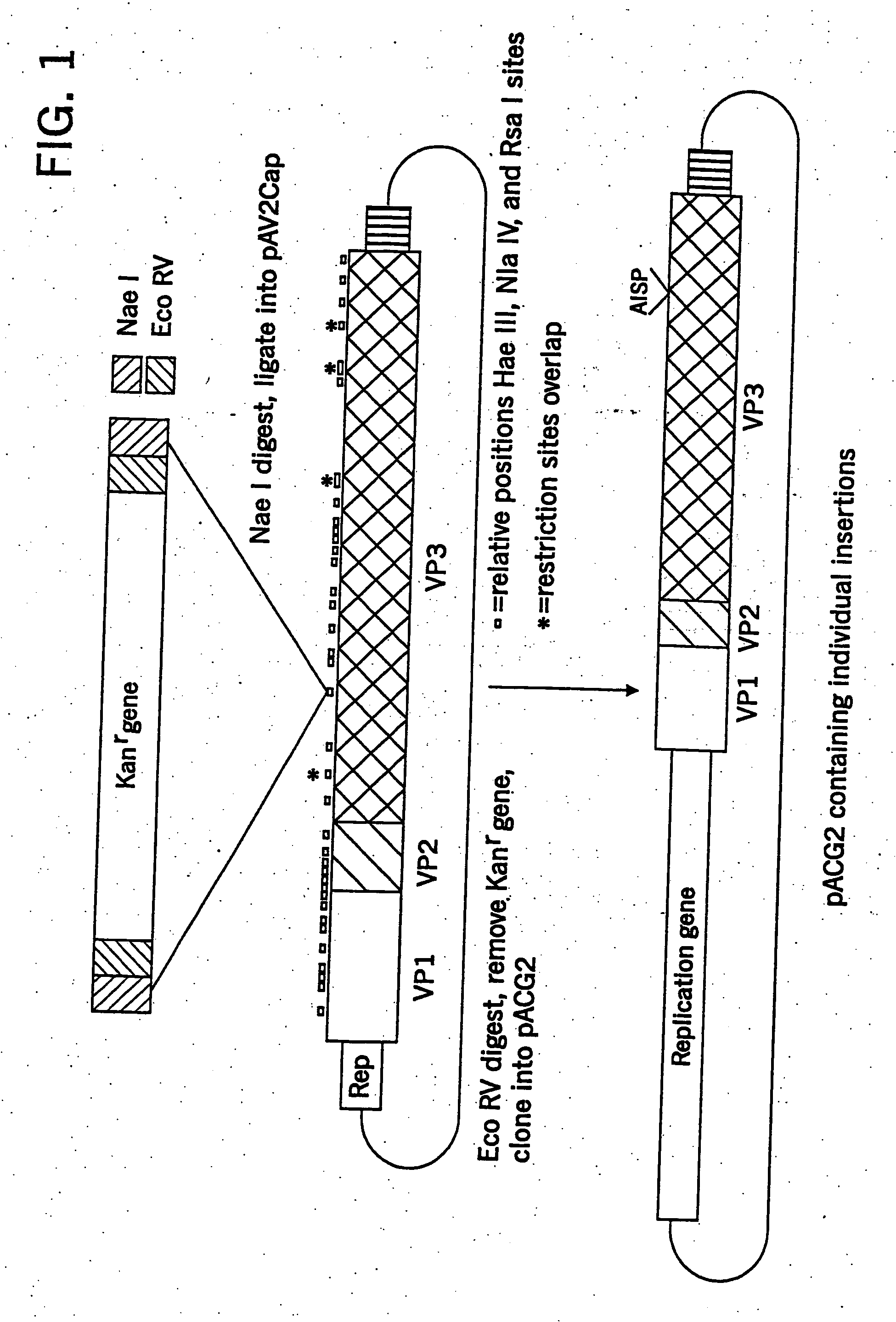
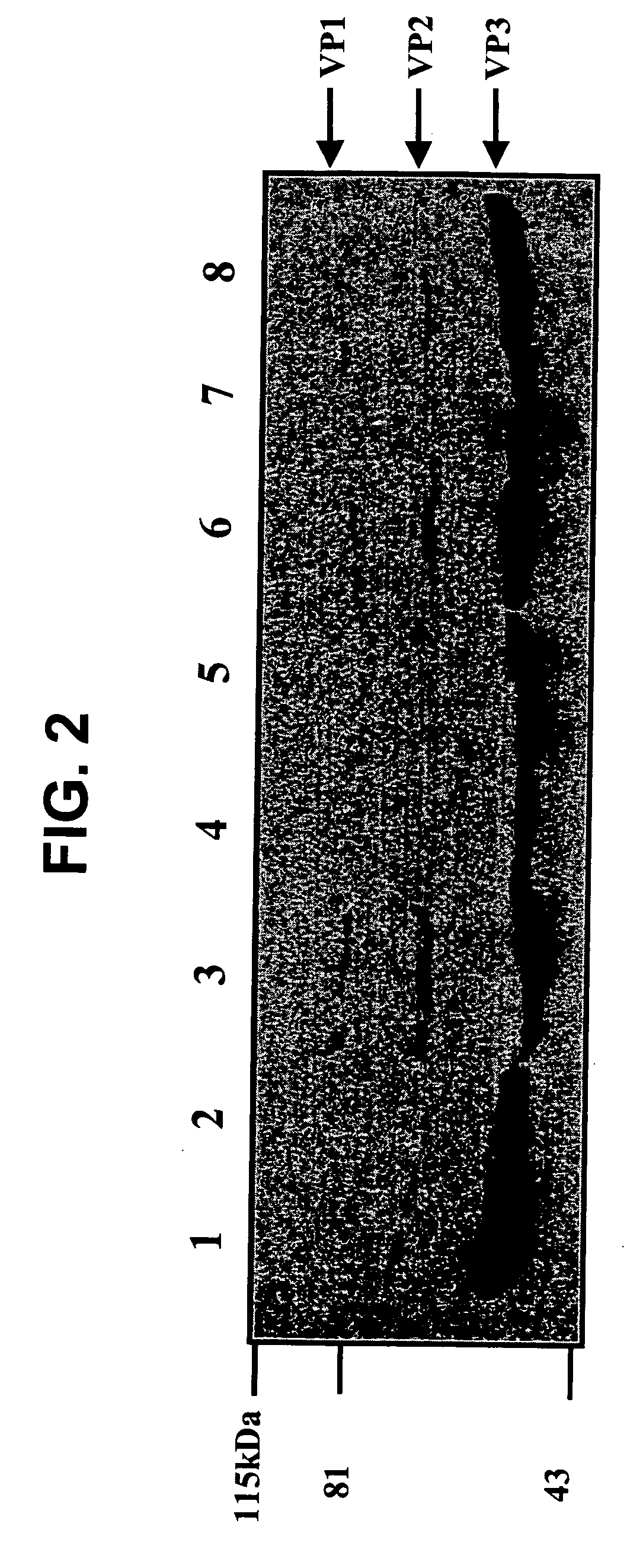
Parvoviral capsid with incorporated gly-ala repeat region
InactiveUS20110171262A1Improve stabilityReduced expression levelBiocideAntibody mimetics/scaffoldsNucleic acid sequencingCapsid
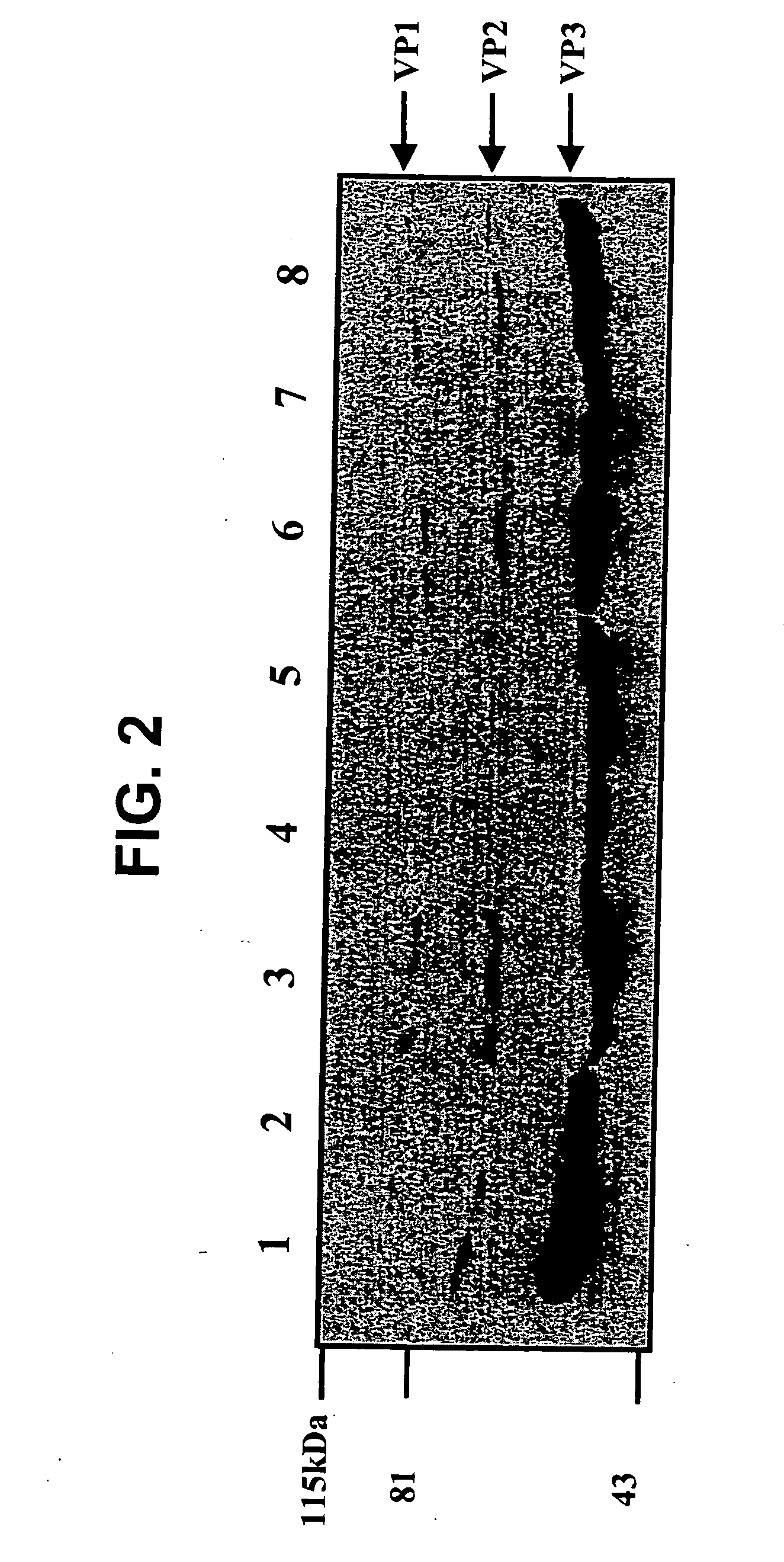
Parvoviral capsid with incorporated Gly-Ala repeat region The present invention provides a nucleic acid construct comprising a nucleic acid sequence encoding a parvoviral VP1, VP2 and VP3 capsid proteins comprising an immuno evasion repeat sequence. In addition, the present invention provides a cell comprising such construct, a parvoviral virion comprising a capsid protein that comprises an immune evasion repeat sequence, use of that parvoviral virion in gene therapy and a pharmaceutical composition comprising such parvoviral virion.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS
Lyme combination compositions and uses
InactiveUS6368603B1Safe and efficacious in dogNo exacerbation of diseaseAntibacterial agentsNanotechAntigenRabies
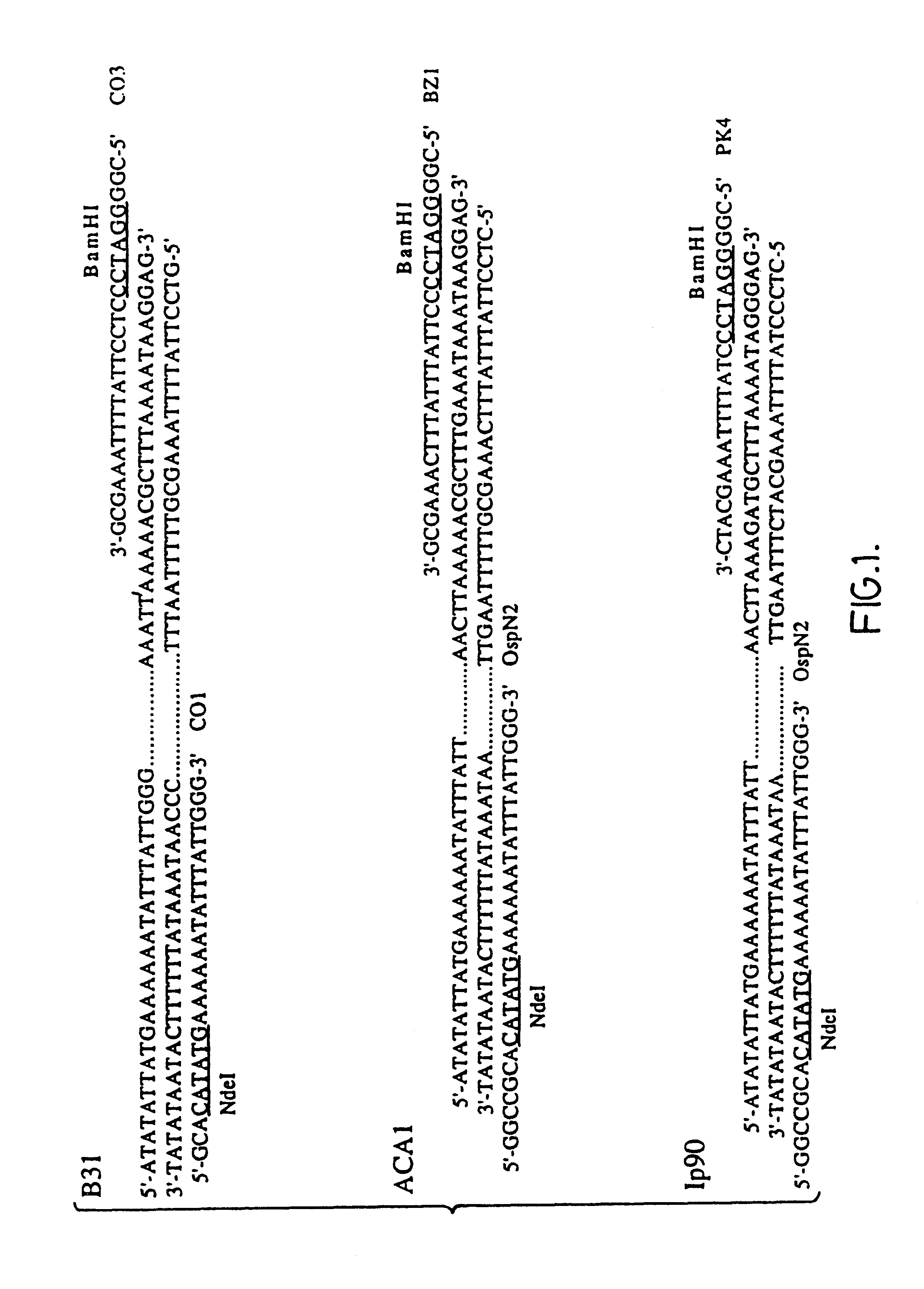
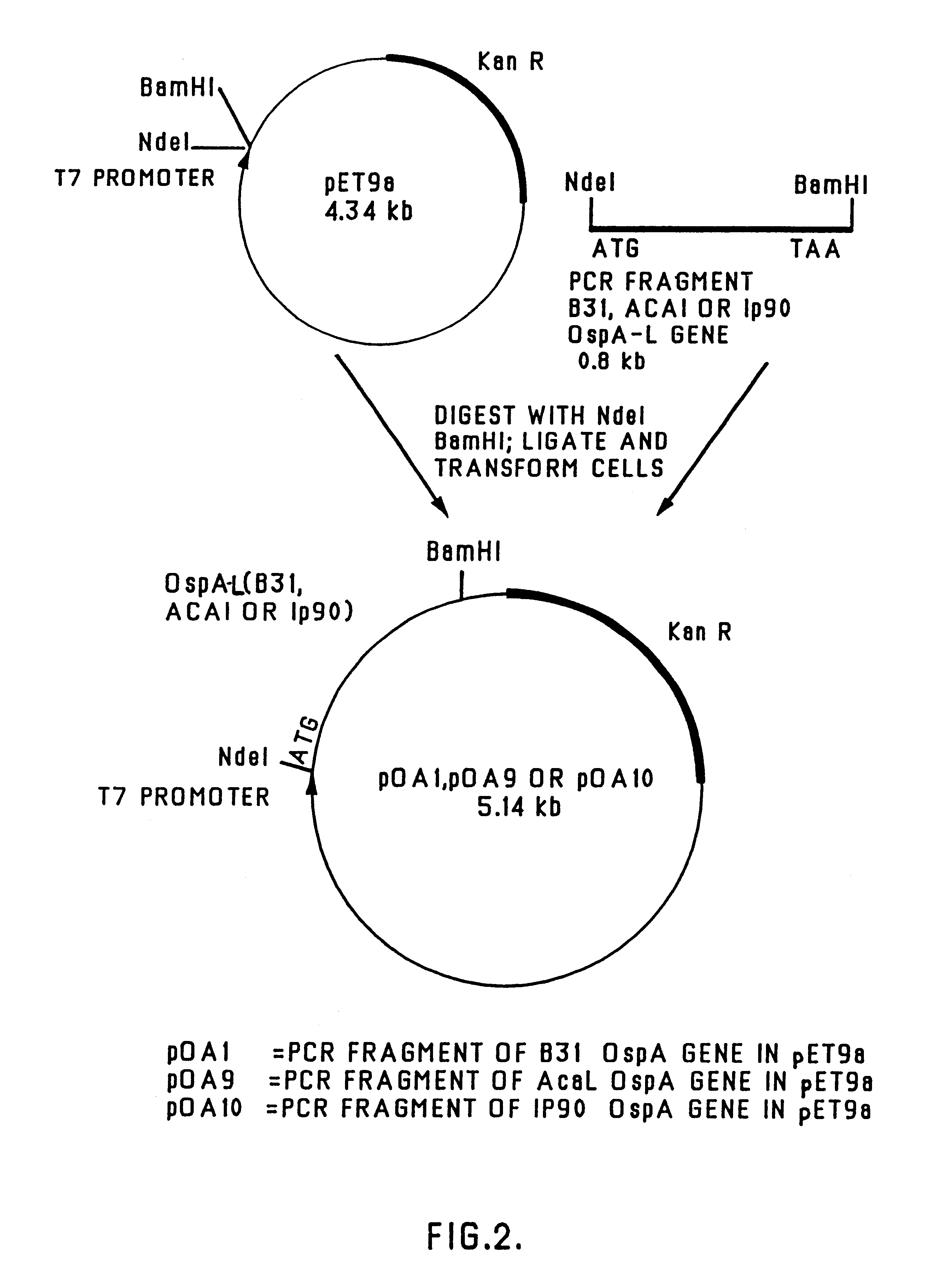
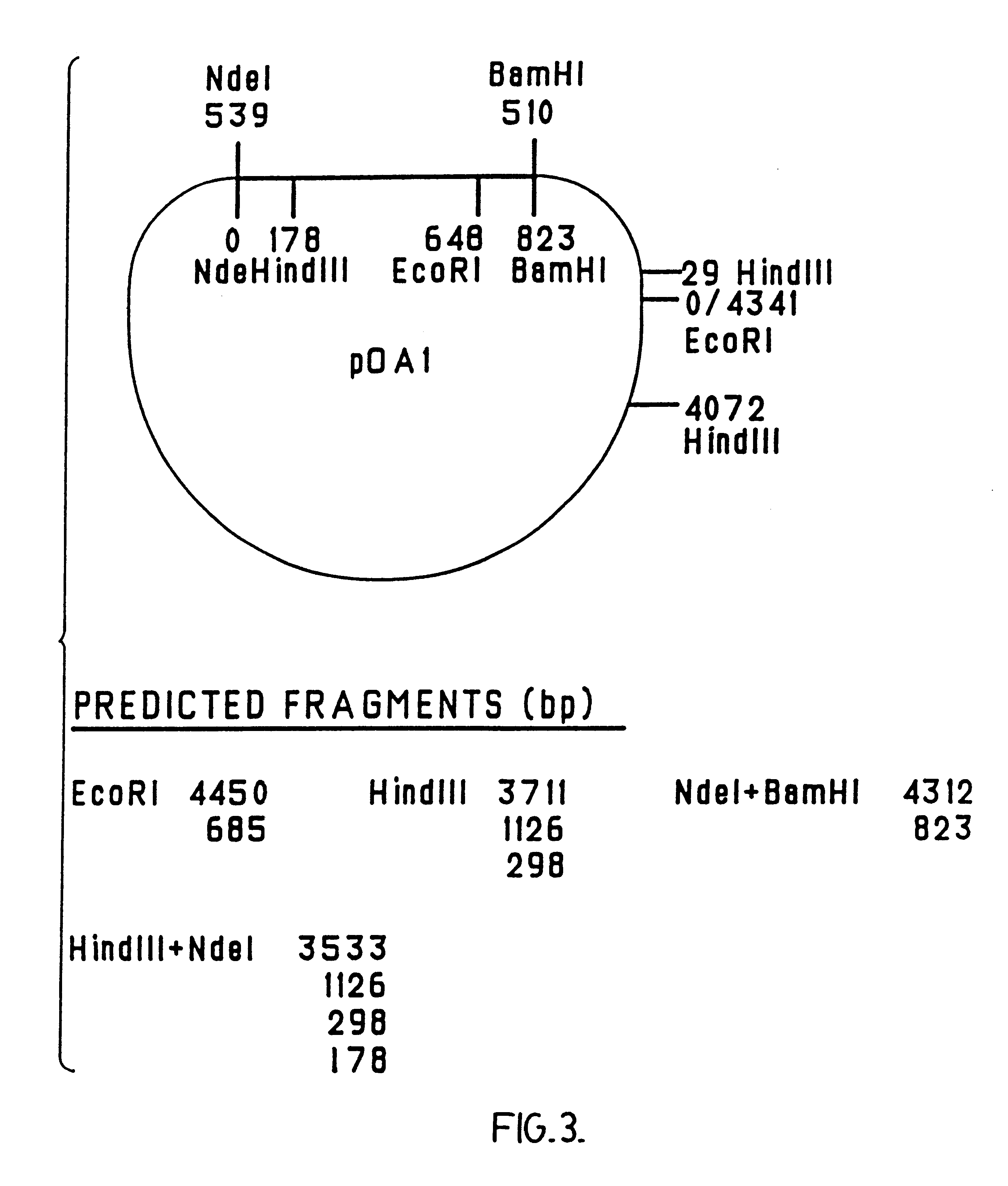
Disclosed and claimed are compositions containing a Borrelia burgdorferi antigen, and methods for making and using them. The antigen can be OspA. The compositions can contain at least one additional antigen from a pathogen other than Borrelia burgdorferi. The compositions are useful for eliciting an immunological response in a host mammal susceptible to Lyme Disease and to the mammalian pathogen other than Borrelia burgdorferi. Suitable host mammals include dogs, pups, horses, and, the additional antigen can be of a canine, equine or feline pathogen, such as rabies, canine distemper, adenovirus, coronavirus, parainfluenza and parvovirus. No significant efficacy interference is observed.
Owner:MERIAL LTD
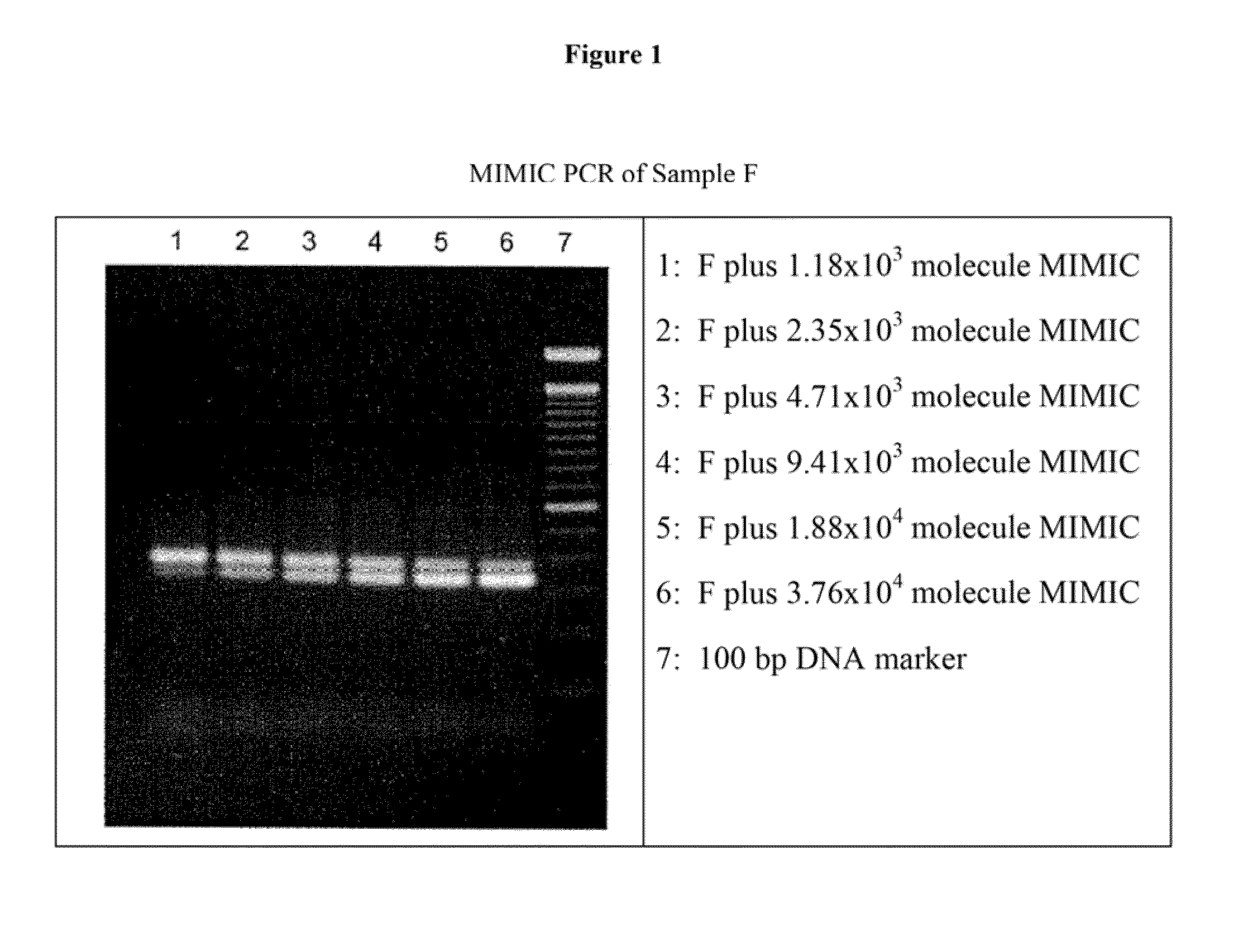
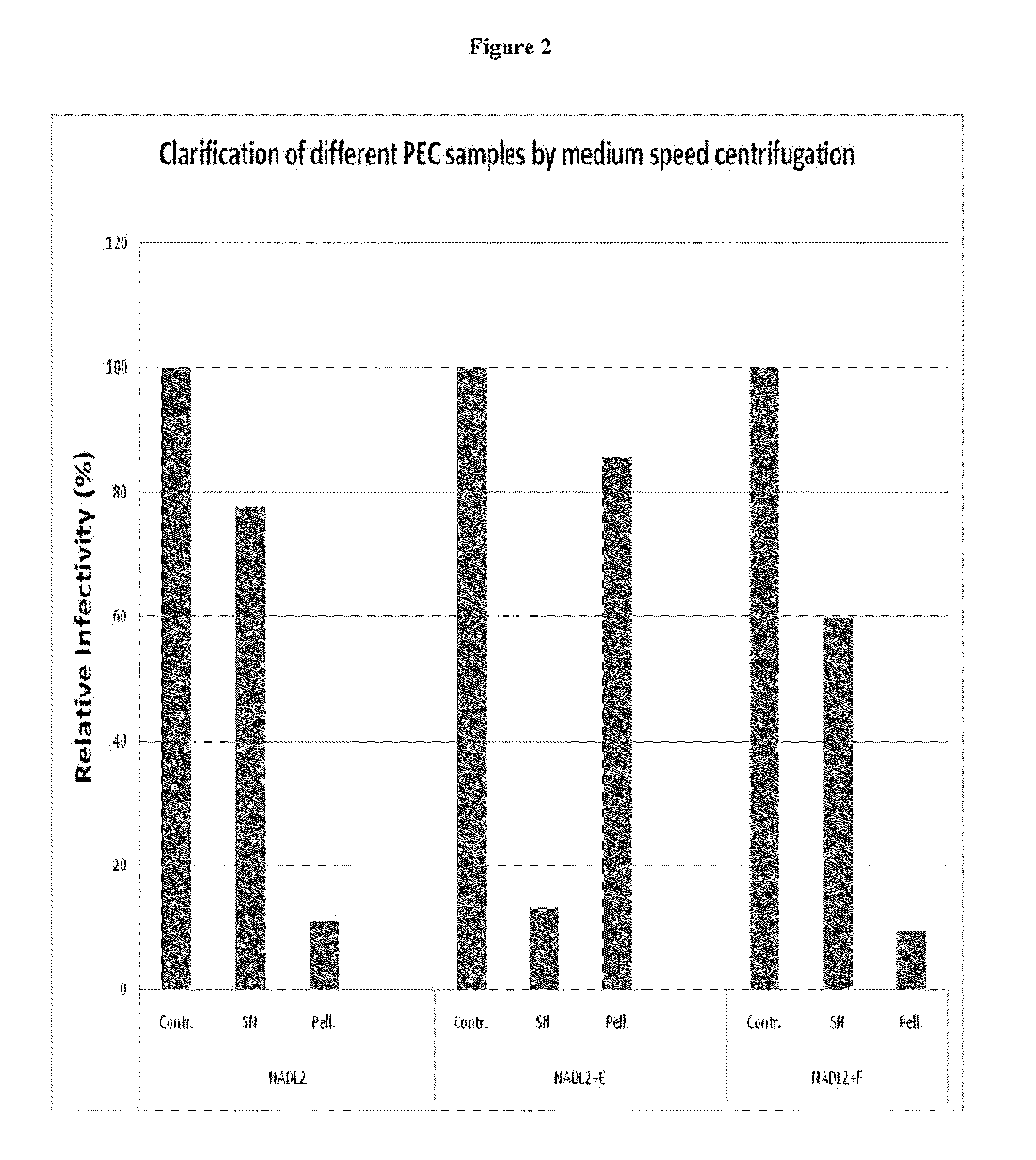
Method for detecting infectious parvovirus in pharmaceutical preparations
ActiveUS20090226414A1Peptide/protein ingredientsMicrobiological testing/measurementPancrelipaseViral infection
The present invention provides methods for detecting viral infectivity and content in an enzyme preparation. In certain embodiments, the invention relates to methods for producing a pharmaceutical pancreatic enzyme composition. In additional embodiments, the invention relates to detecting infectious porcine parvovirus (PPV) and determining PPV content in pancreatic enzyme preparations (PEPs), including pancrelipase preparations.
Owner:ALLERGAN SALES LLC
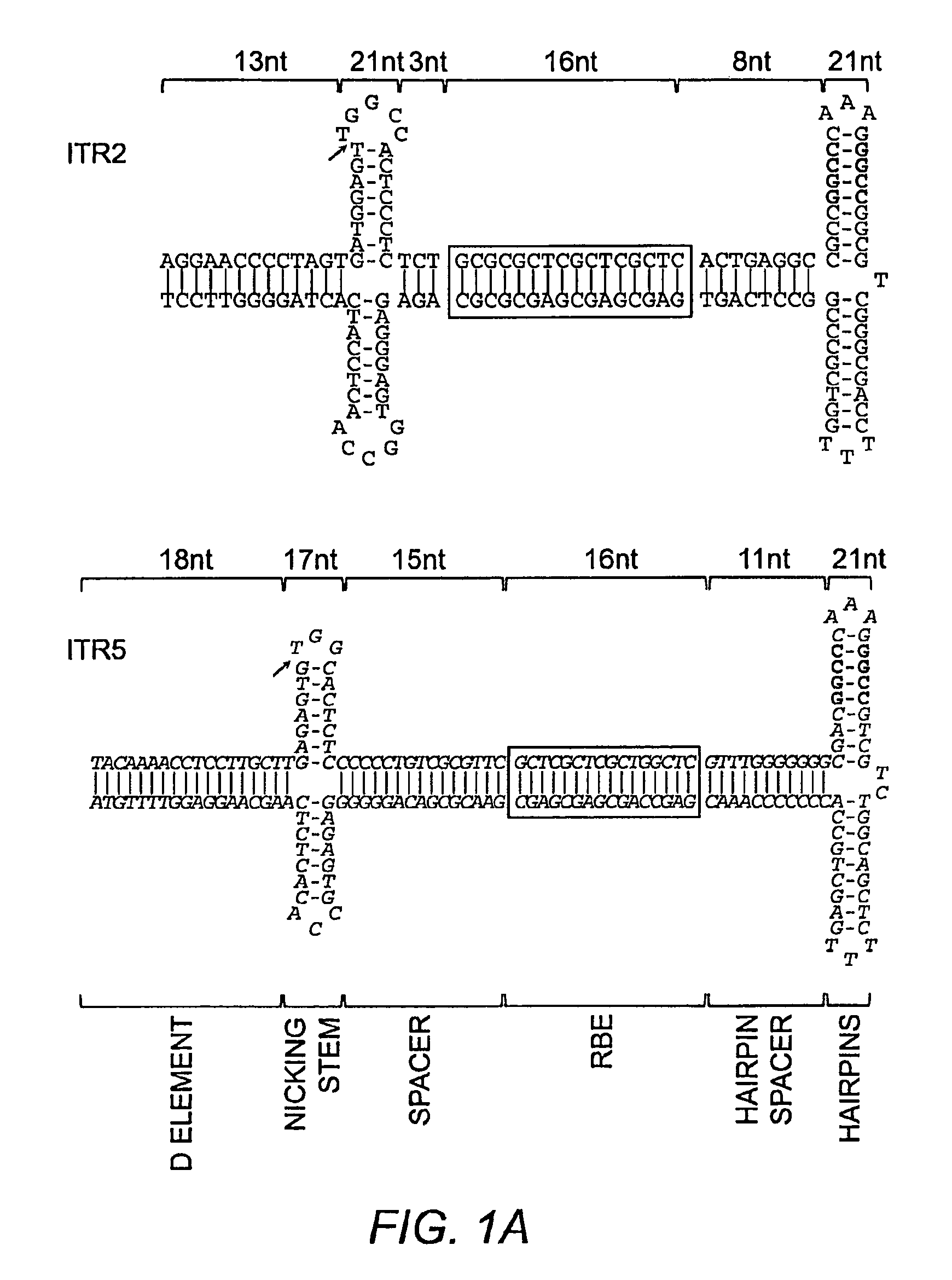
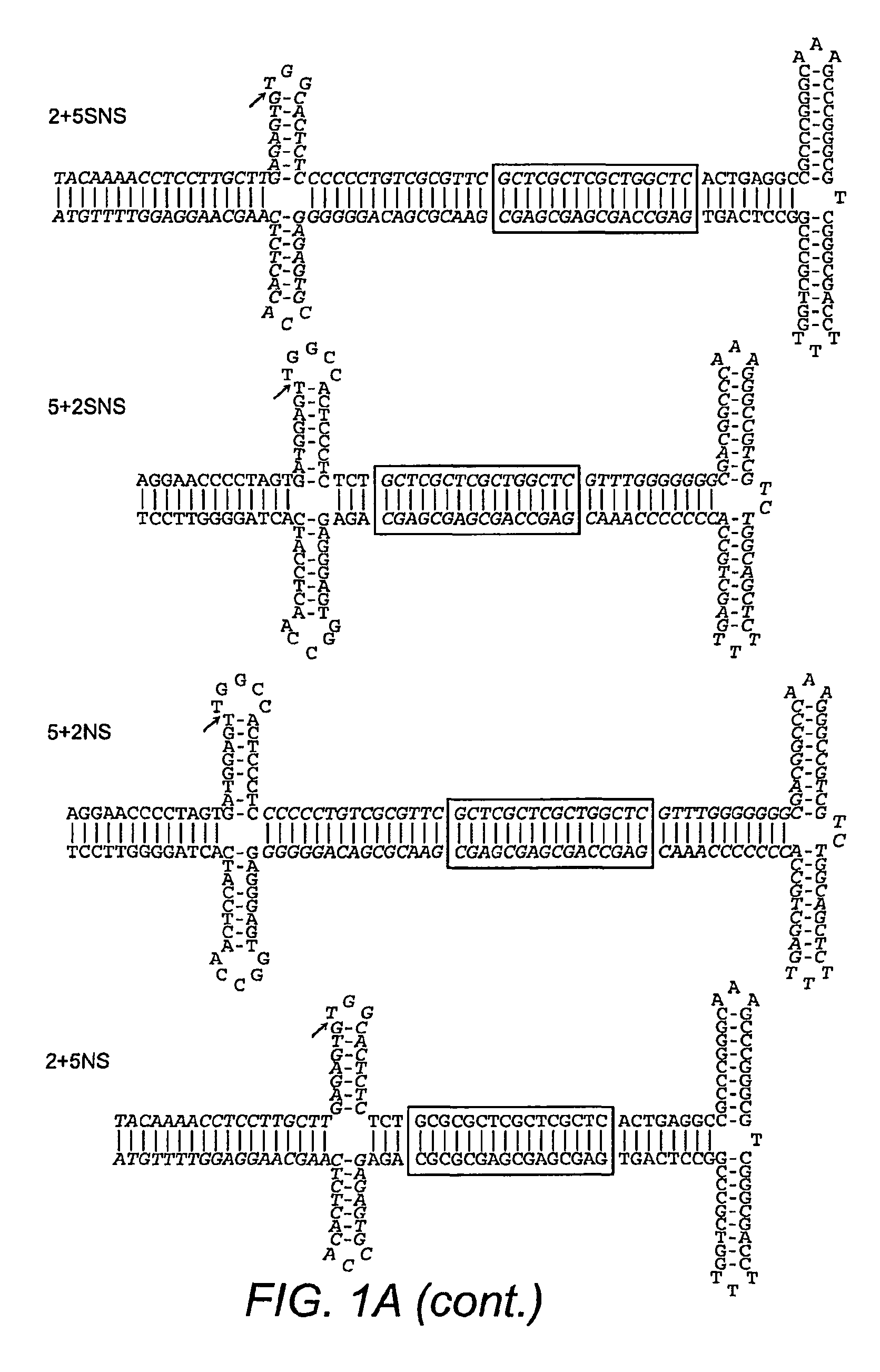
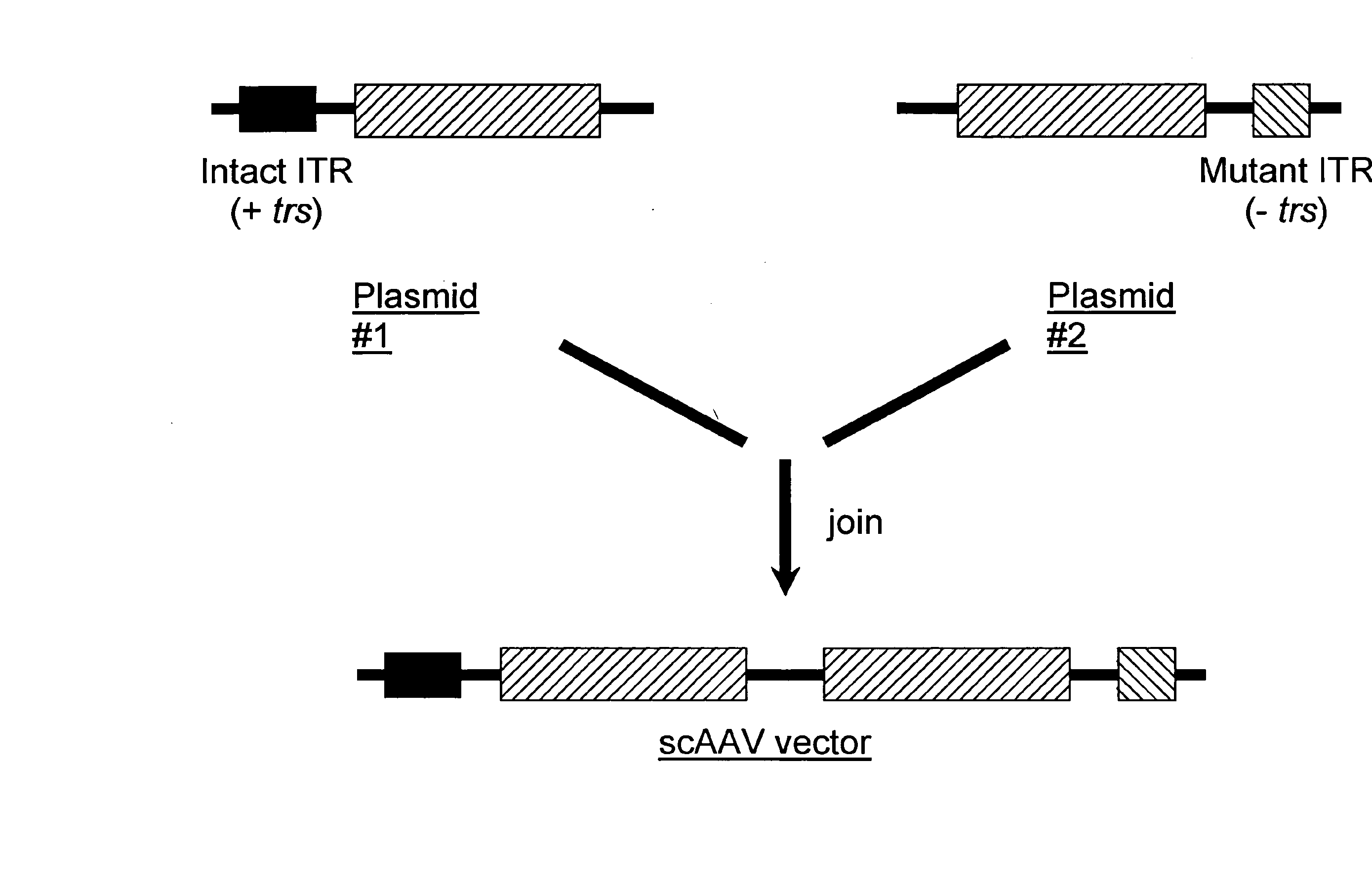
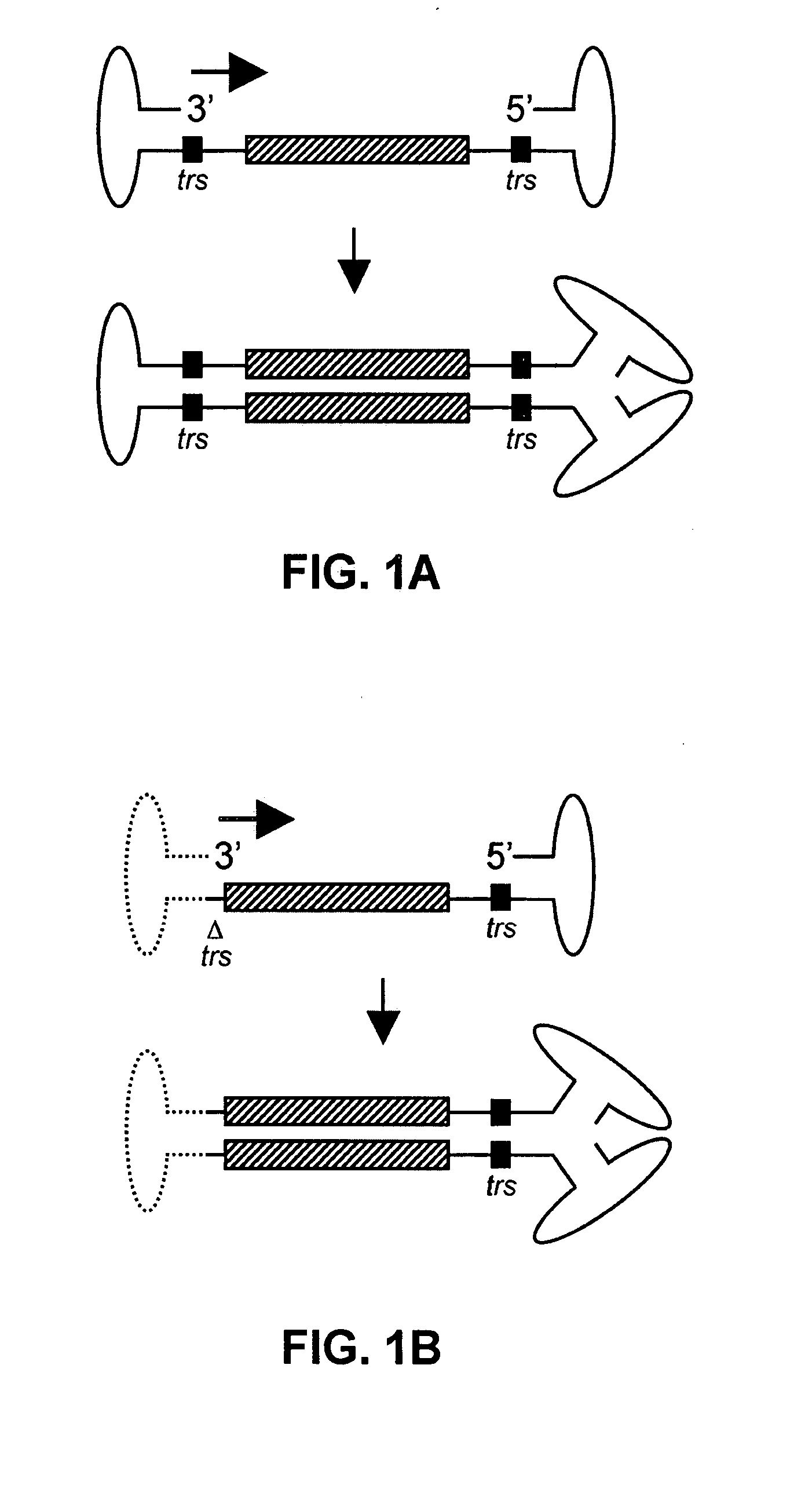
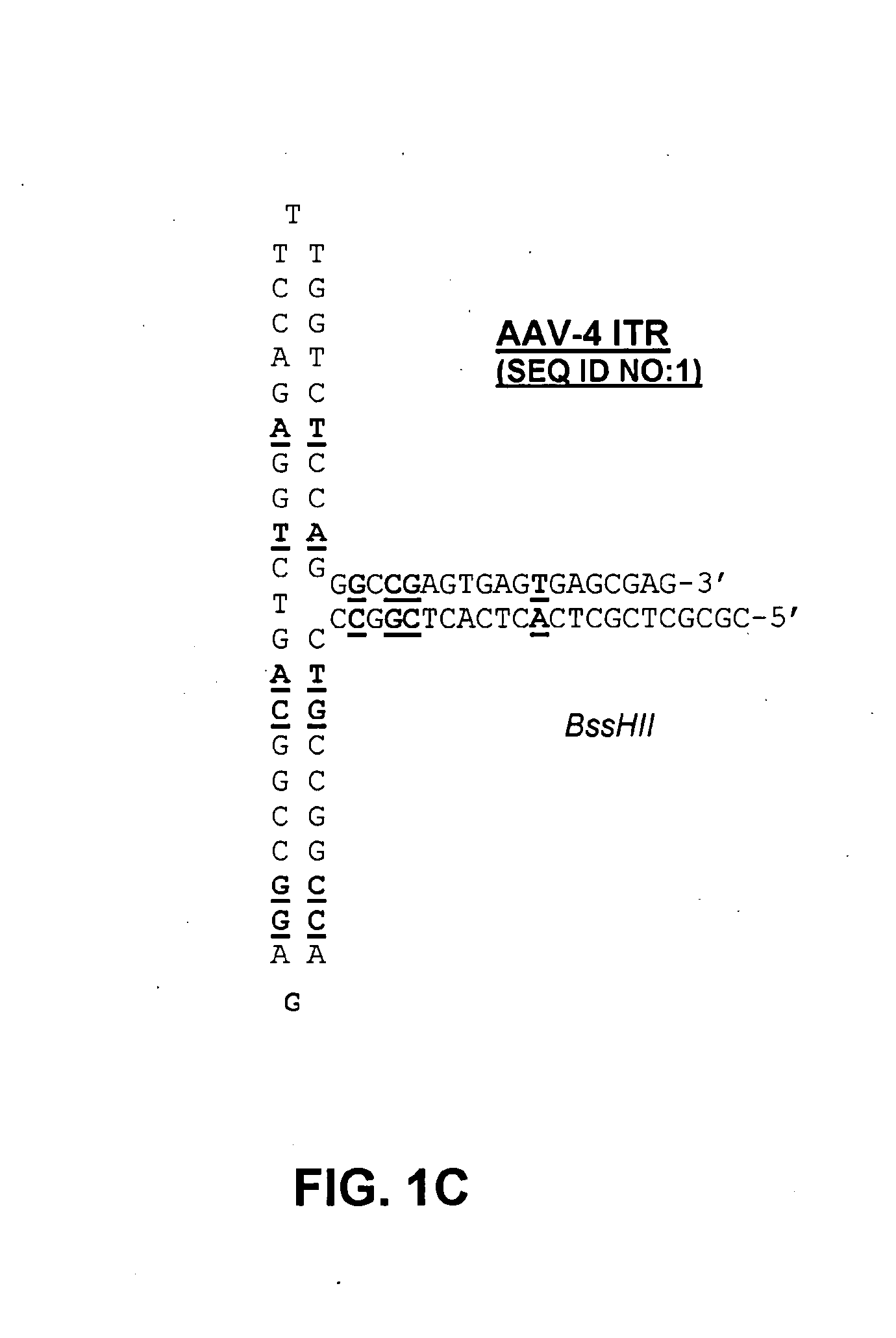
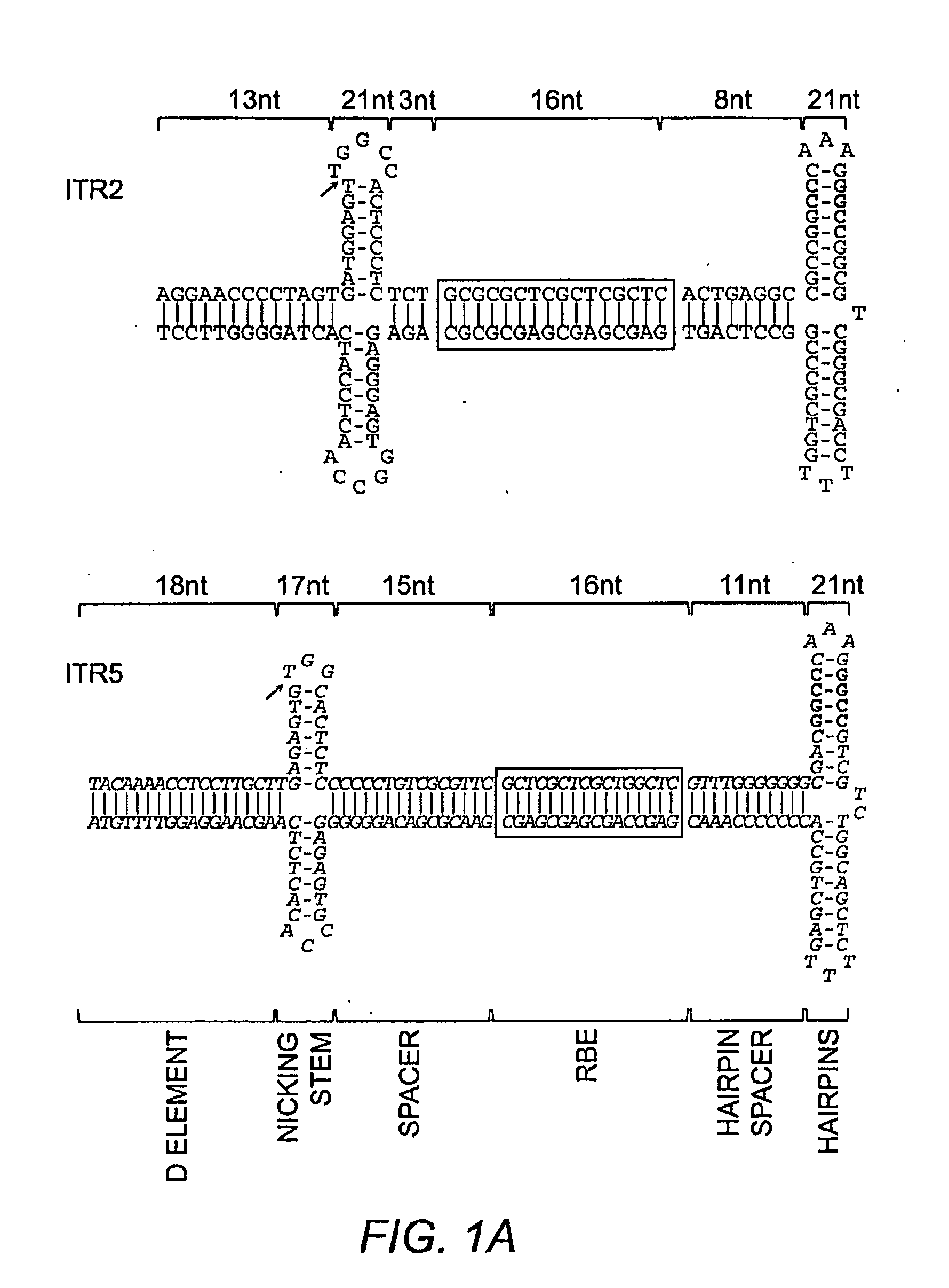
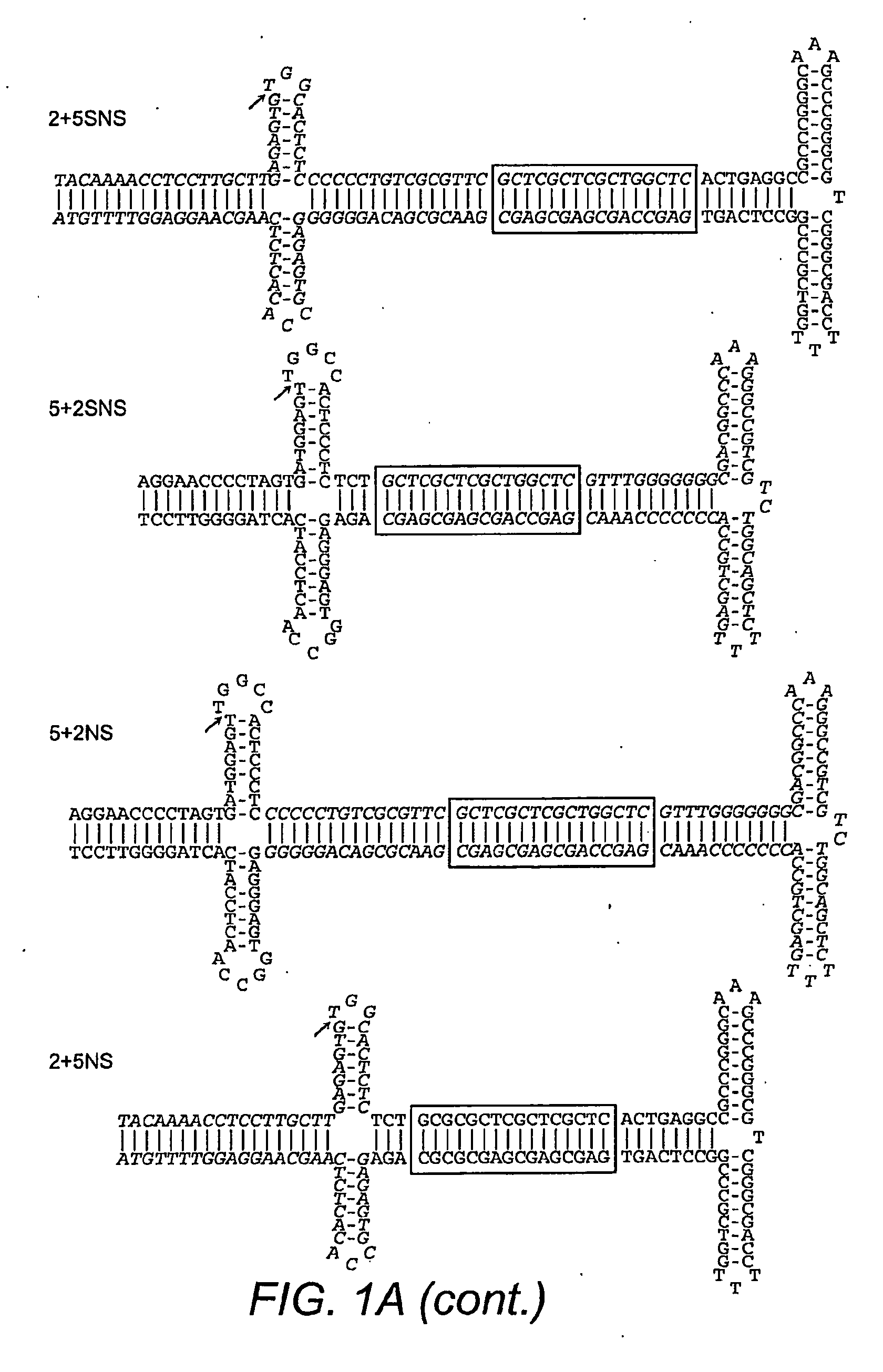
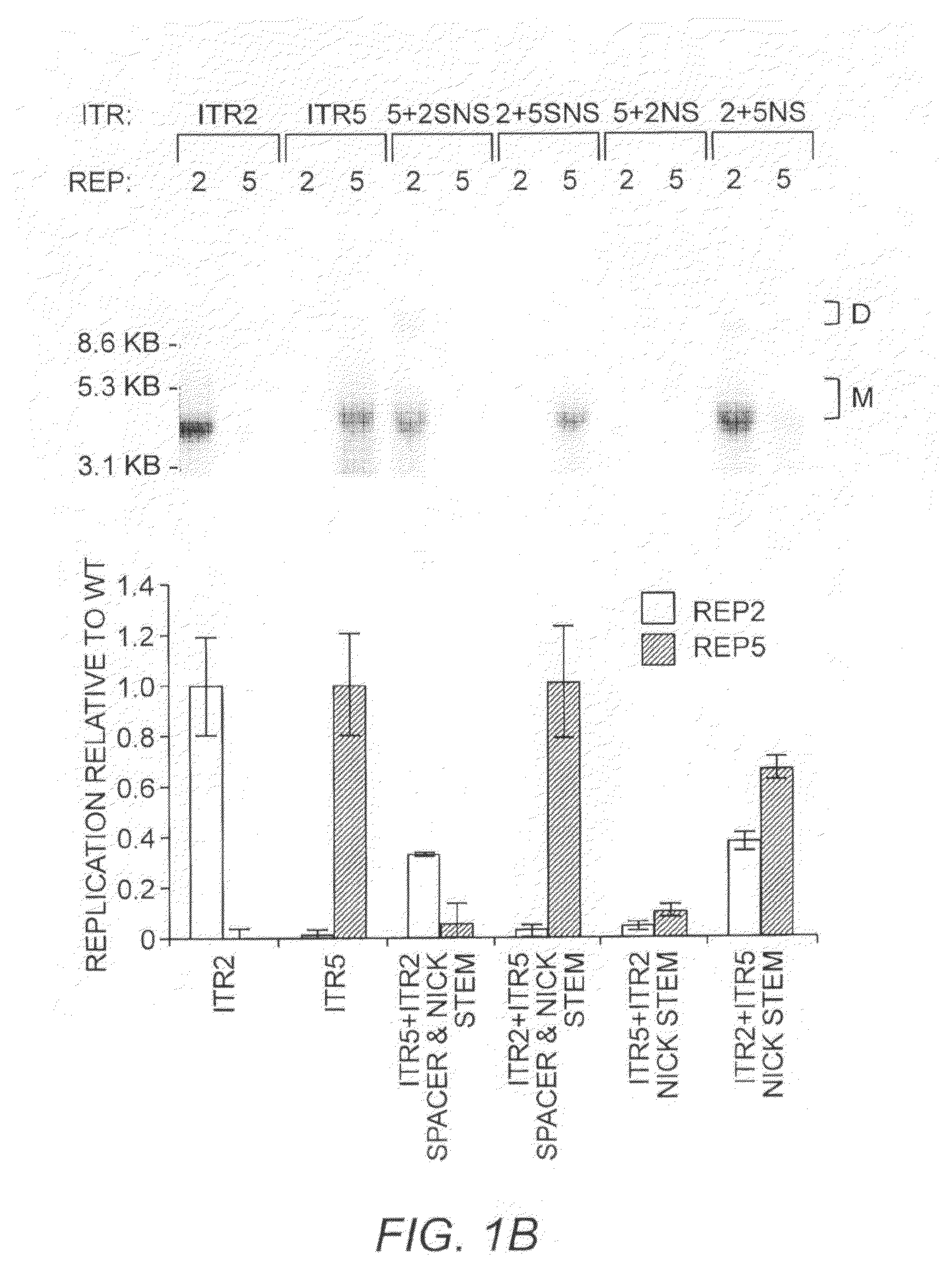
Restrictive inverted terminal repeats for viral vectors
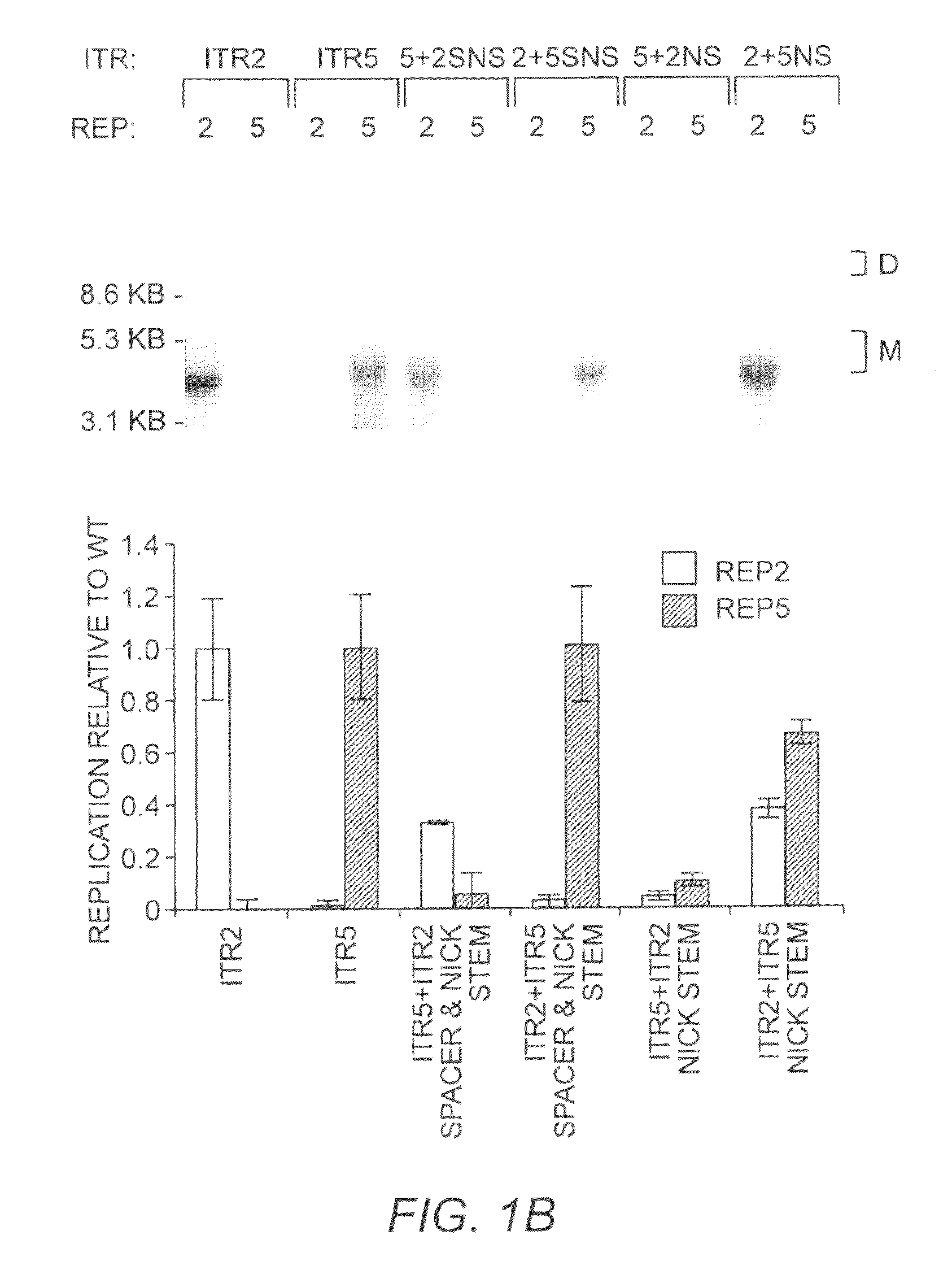
This invention relates to modified parvovirus inverted terminal repeats (ITRs) that do not functionally interact with wild-type large Rep proteins, synthetic Rep proteins that functionally interact with the modified ITRs, and methods of using the same for delivery of nucleic acids to a cell or a subject. The modifications provide a novel Rep-ITR interaction that limits vector mobilization, increasing the safety of viral vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
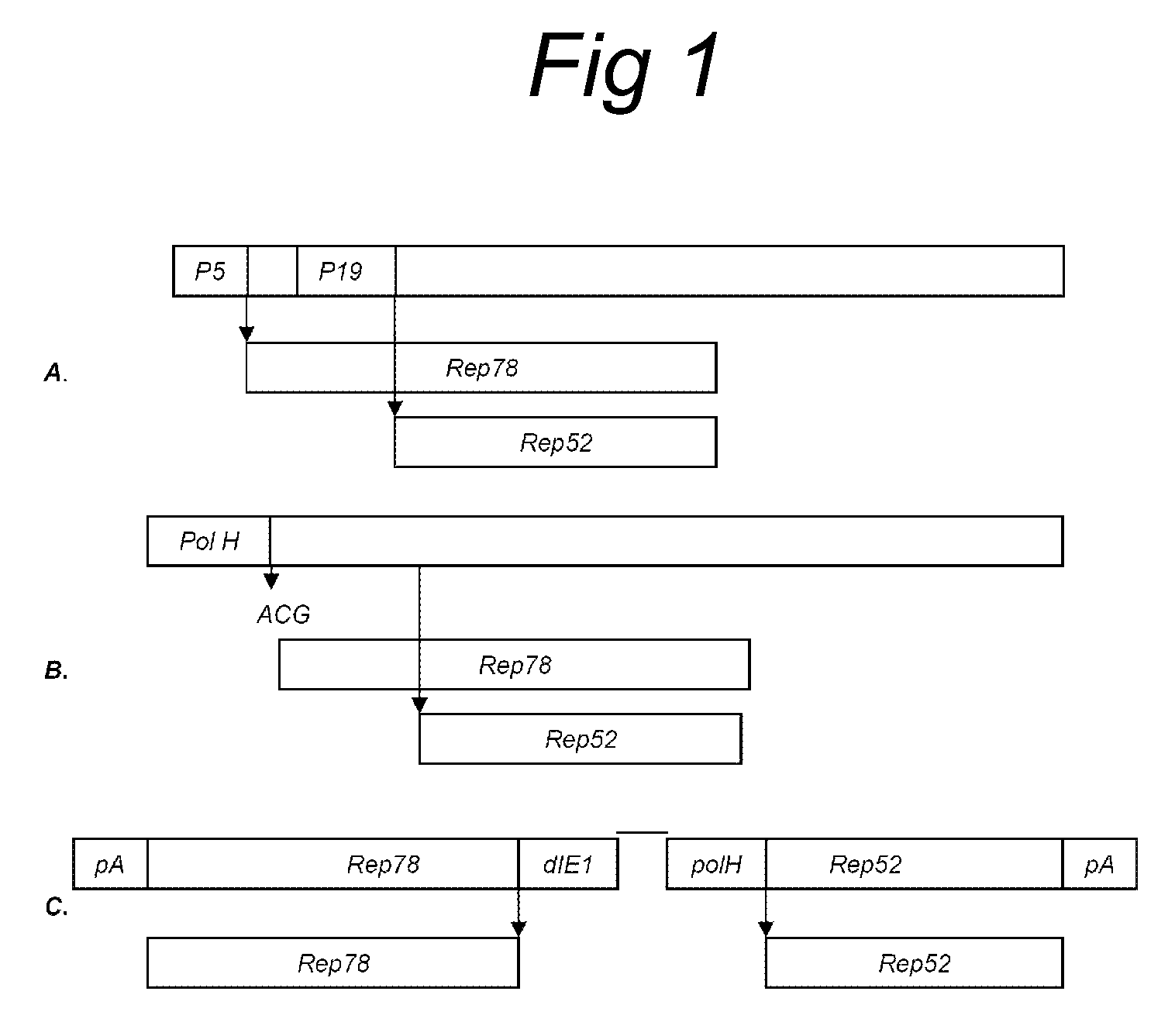
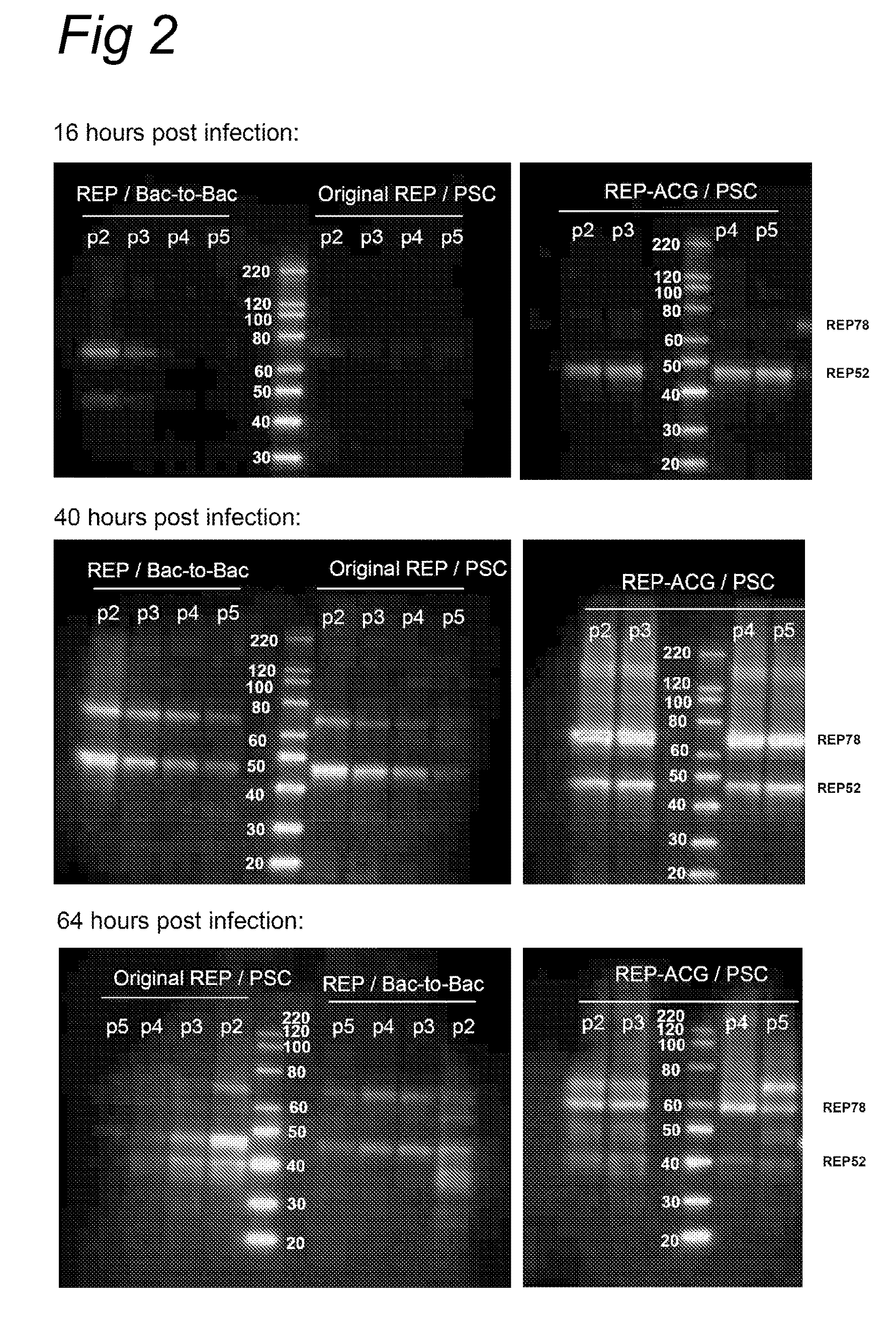
Vectors with modified initiation codon for the translation of AAV-Rep78 useful for production of AAV
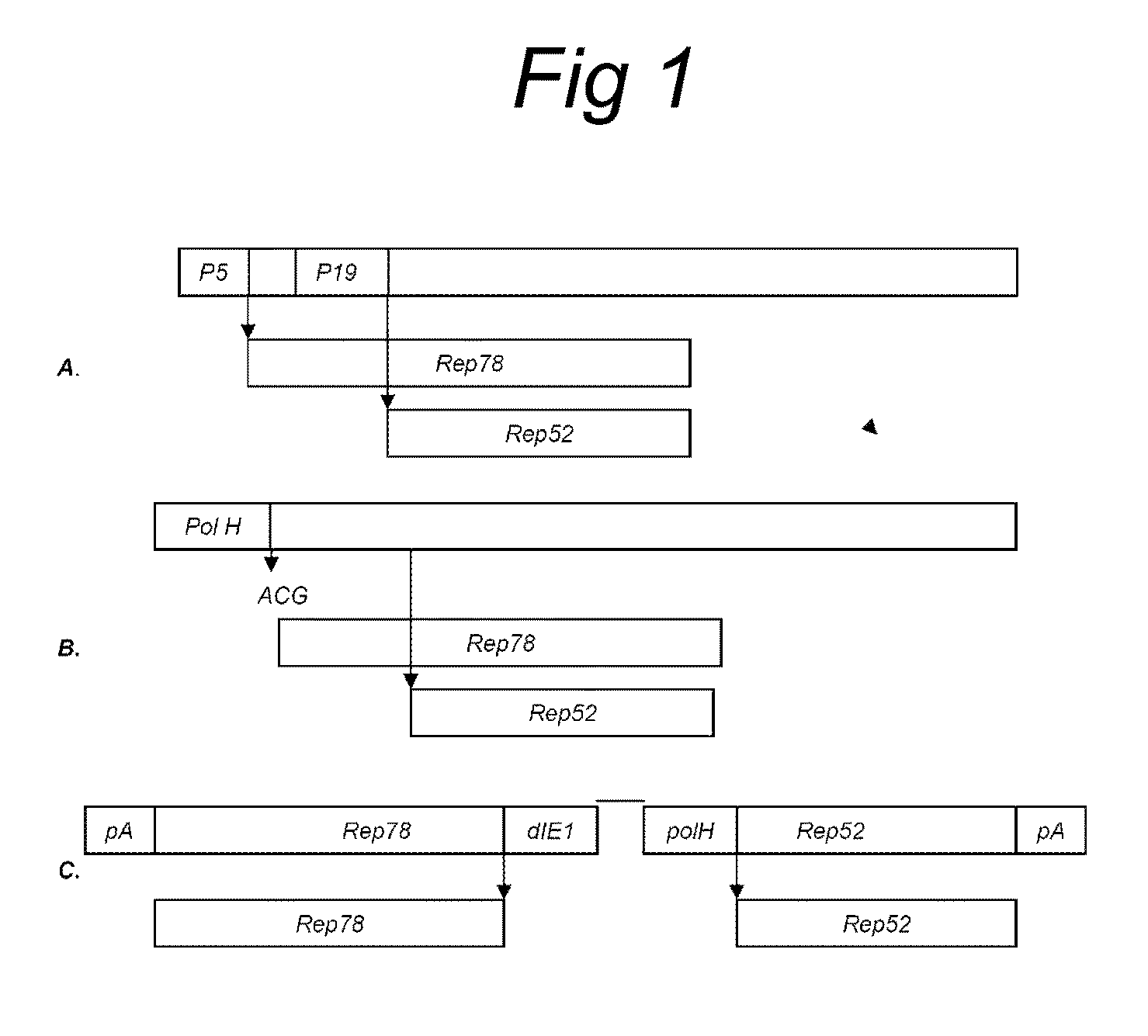
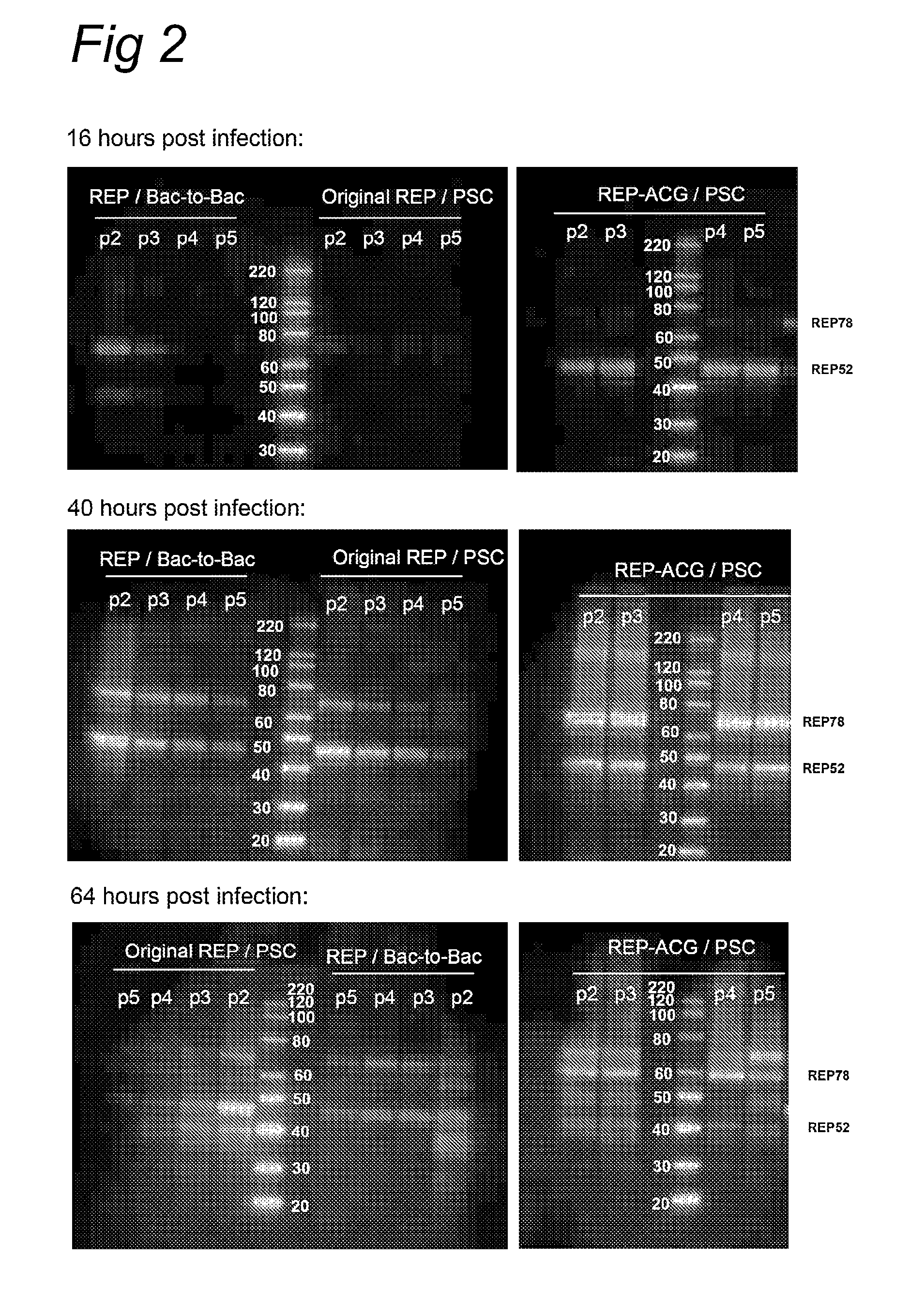
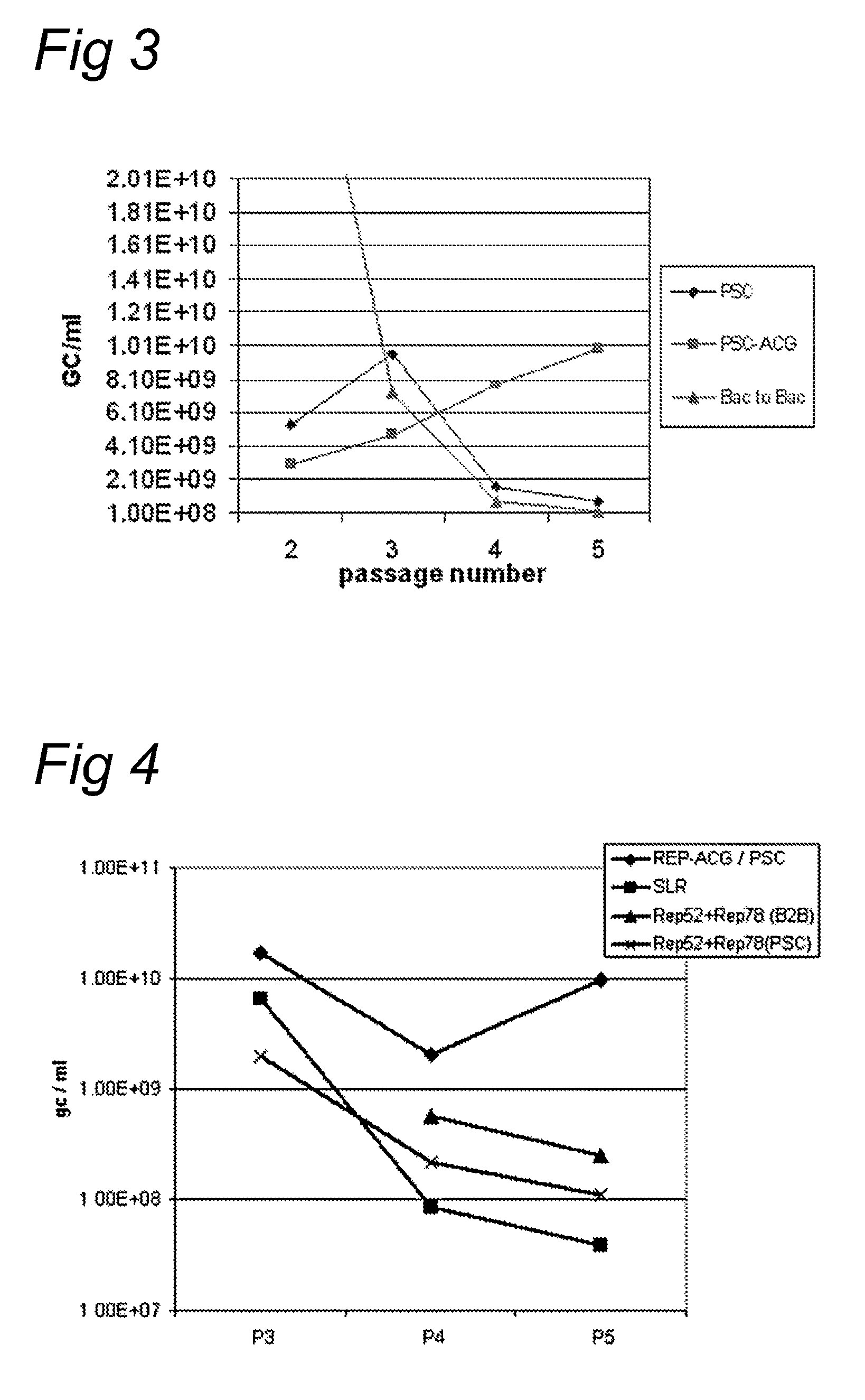
ActiveUS8512981B2Improve stabilityReduced expression levelVirus peptidesNucleic acid vectorStart codonNucleotide
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS +1
Self-complementary parvoviral vectors, and methods for making and using the same
ActiveUS20070253936A1High genetic stabilityStabilized parvovirus vector is more stableBiocideAnimal repellantsGene transferTiter
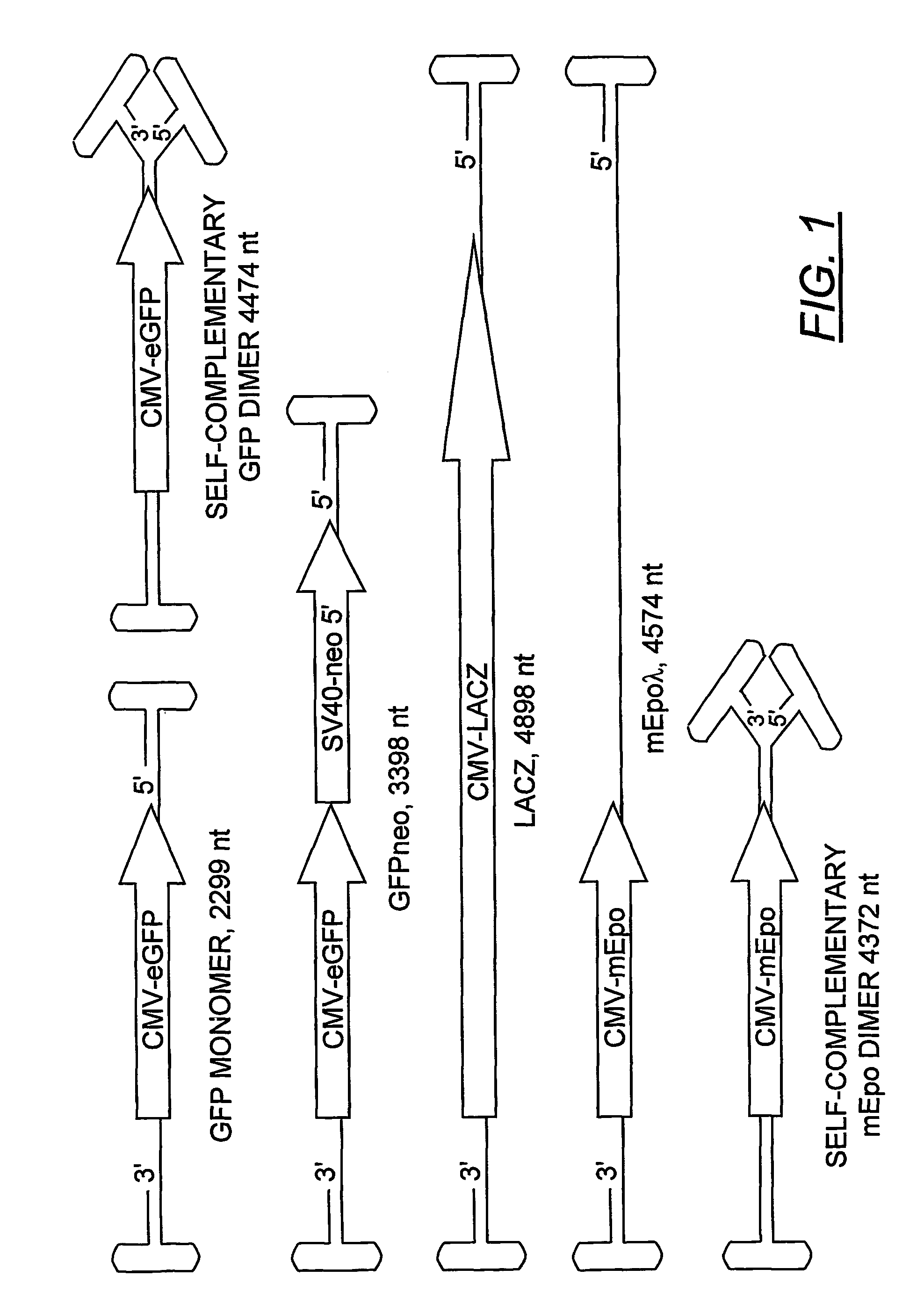
The teachings herein are generally directed to a method of enhancing the genetic stability of parvovirus vectors. The stability of conventional ss or dsAAV vector constructs can be enhanced, for example, to obtain a concurrent increase in vector titer and purity, as well as an improvement in vector safety, due at least in part to the elimination of stuffer DNA from the AAV vector. The method is broadly applicable to all gene transfer / therapy applications, such as those requiring delivery of foreign DNA containing recombinant gene expression cassettes. Such foreign DNA can range, for example, from about 0.2 up to about 5.2 kb in length. The enhanced vector constructs are highly flexible, user-friendly, and can be easily modified (via routine DNA cloning) and utilized (via standard AAV vector technology) by anyone skilled in the art.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Restrictive Inverted Terminal Repeats for Viral Vectors
This invention relates to modified parvovirus inverted terminal repeats (ITRs) that do not functionally interact with wild-type large Rep proteins, synthetic Rep proteins that functionally interact with the modified ITRs, and methods of using the same for delivery of nucleic acids to a cell or a subject. The modifications provide a novel Rep-ITR interaction that limits vector mobilization, increasing the safety of viral vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Baculoviral vectors comprising repeated coding sequences with differential codon biases
ActiveUS8697417B2Improve stabilityReduced expression levelAnimal cellsSugar derivativesViral vectorParvovirus
The present invention relates to production of proteins in insect cells whereby repeated coding sequences are used in baculoviral vectors. In particular the invention relates to the production of parvoviral vectors that may be used in gene therapy and to improvements in expression of the viral rep proteins that increase the productivity of parvoviral vectors.
Owner:UNIQURE IP BV
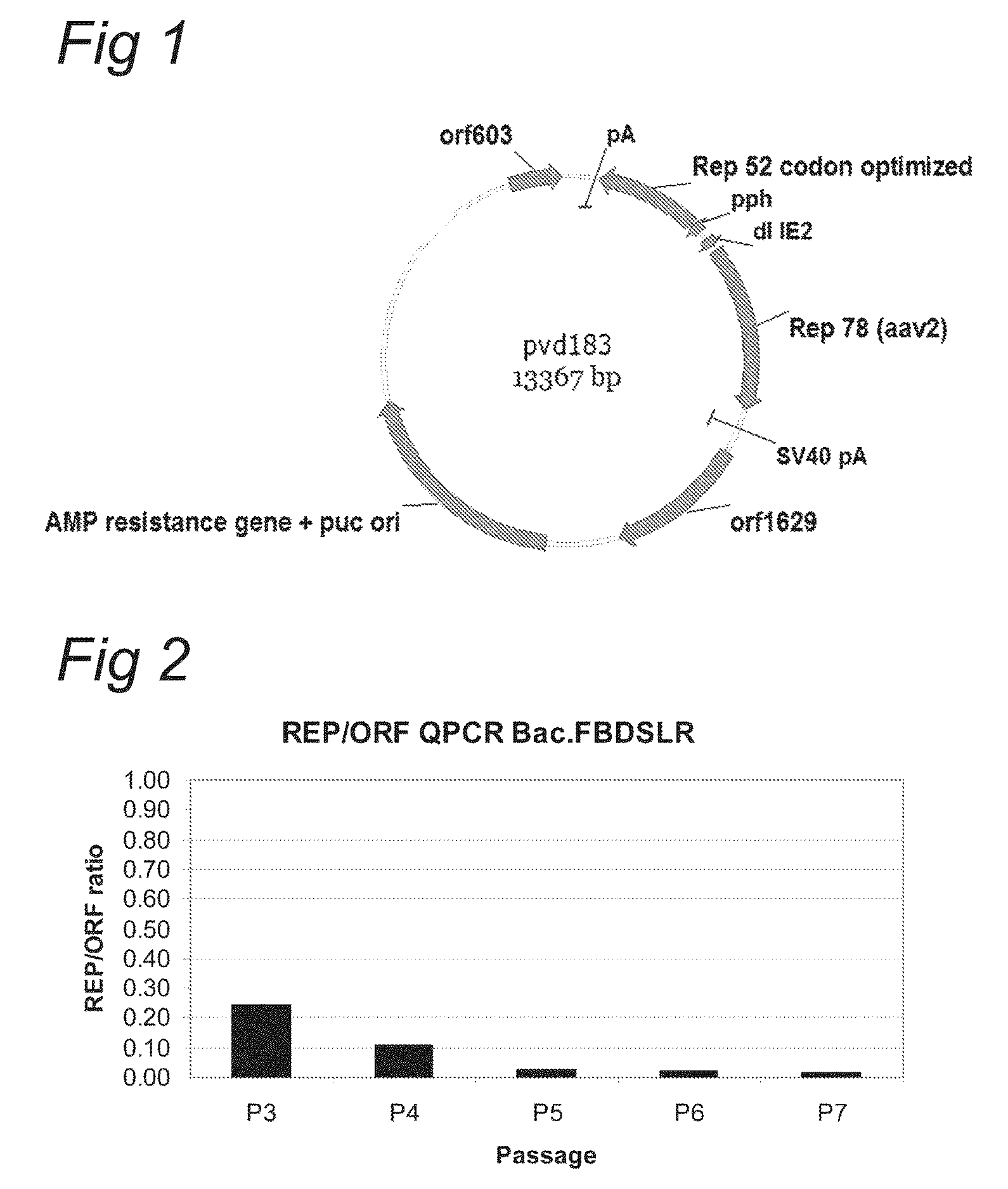
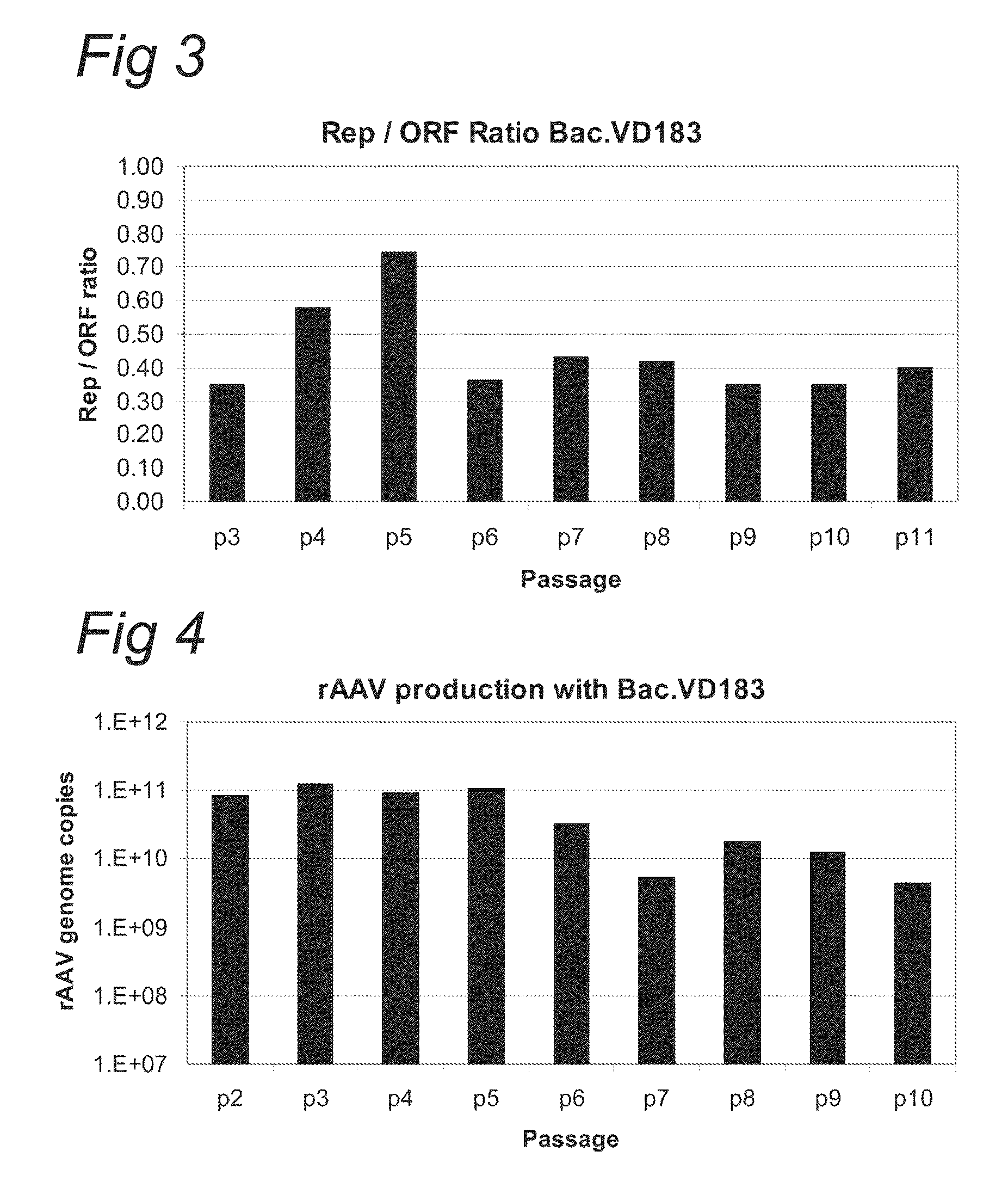
Optimisation of expression of parvoviral rep and cap proteins in insect cells
ActiveUS20110136227A1Increased production titreGreat proportionAnimal cellsVirus peptidesViral vectorParvovirus
The present invention relates to the improved production of recombinant parvoviral virions in insect cells. In particular, the invention relates to an improved process for the production of recombinant parvoviral virions in insect cells, wherein the full / empty parvoviral virion ratio is increased. The invention also relates to the production of parvoviral vectors that may be used in gene therapy and to improvements in expression of the viral Rep proteins that increase the productivity of parvoviral vectors.
Owner:UNIQURE IP BV
Optimization of expression of parvoviral rep and cap proteins in insect cells
The present invention relates to the improved production of recombinant parvoviral virions in insect cells. In particular, the invention relates to an improved process for the production of recombinant parvoviral virions in insect cells, wherein the full / empty parvoviral virion ratio is increased. The invention also relates to the production of parvoviral vectors that may be used in gene therapy and to improvements in expression of the viral Rep proteins that increase the productivity of parvoviral vectors.
Owner:UNIQURE IP BV
Method for generating a parvovirus b19 virus-like particle
InactiveUS20130273109A1Lower Level RequirementsPrevent correction of the anemiaBacteriaProtozoaParvovirusImmunogenicity
The invention provides a process for generating parvovirus VP1 / VP2 virus like particles (VLPs). The invention further provides methods for purification of the parvovirus VLPs and immunogenic compositions that contain the VLPs. The invention also includes recombinant nucleic acid molecules that encode parvovirus VP1 and VP2, and host cells that contain the recombinant nucleic acids.
Owner:NOVARTIS AG
Vectors with modified initiation codon for the translation of aav-rep78 useful for production of aav
ActiveUS20130296532A1Improve stabilityReduced expression levelVirus peptidesNucleic acid vectorNucleotideCapsid
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:UNIQURE IP BV
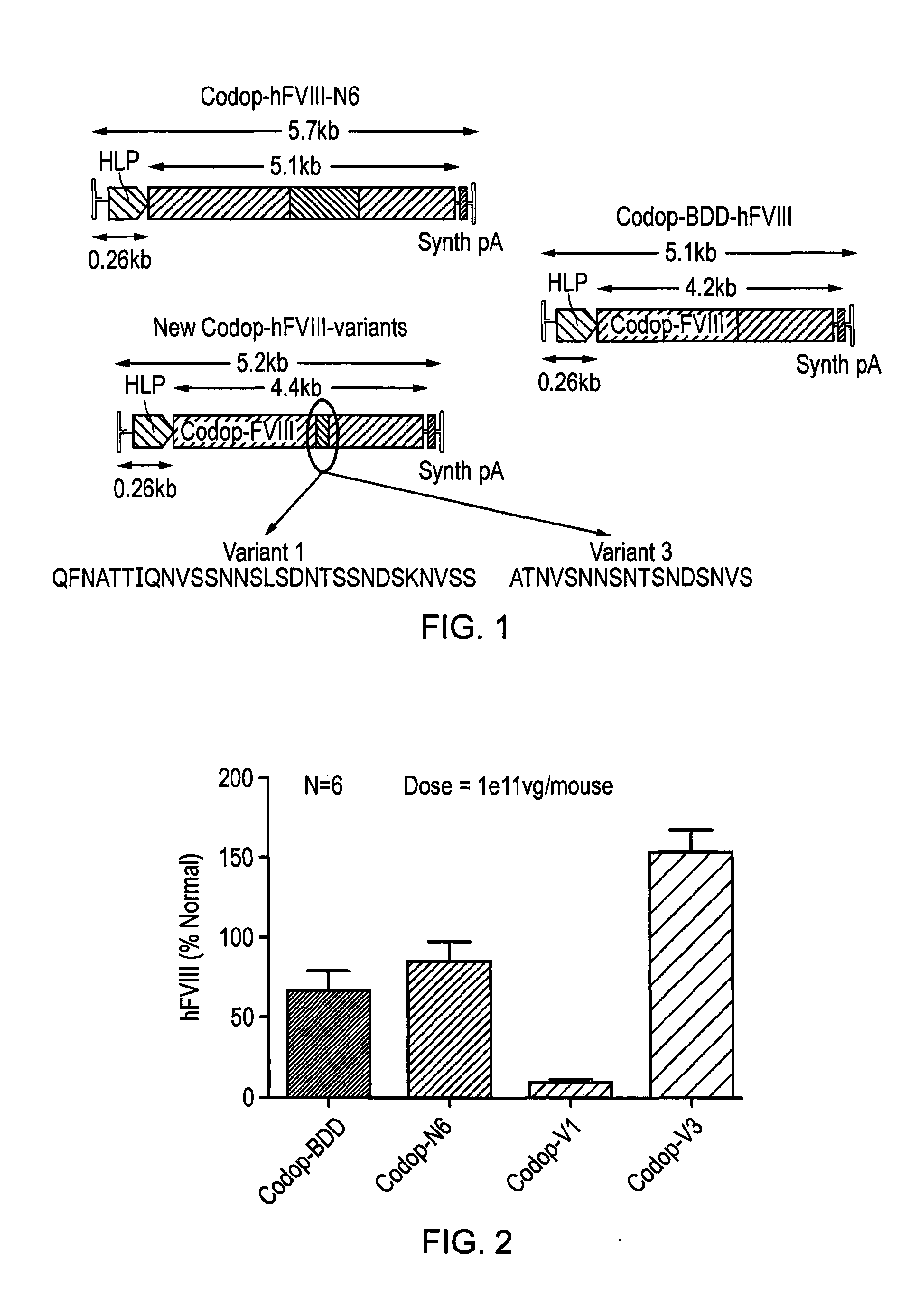
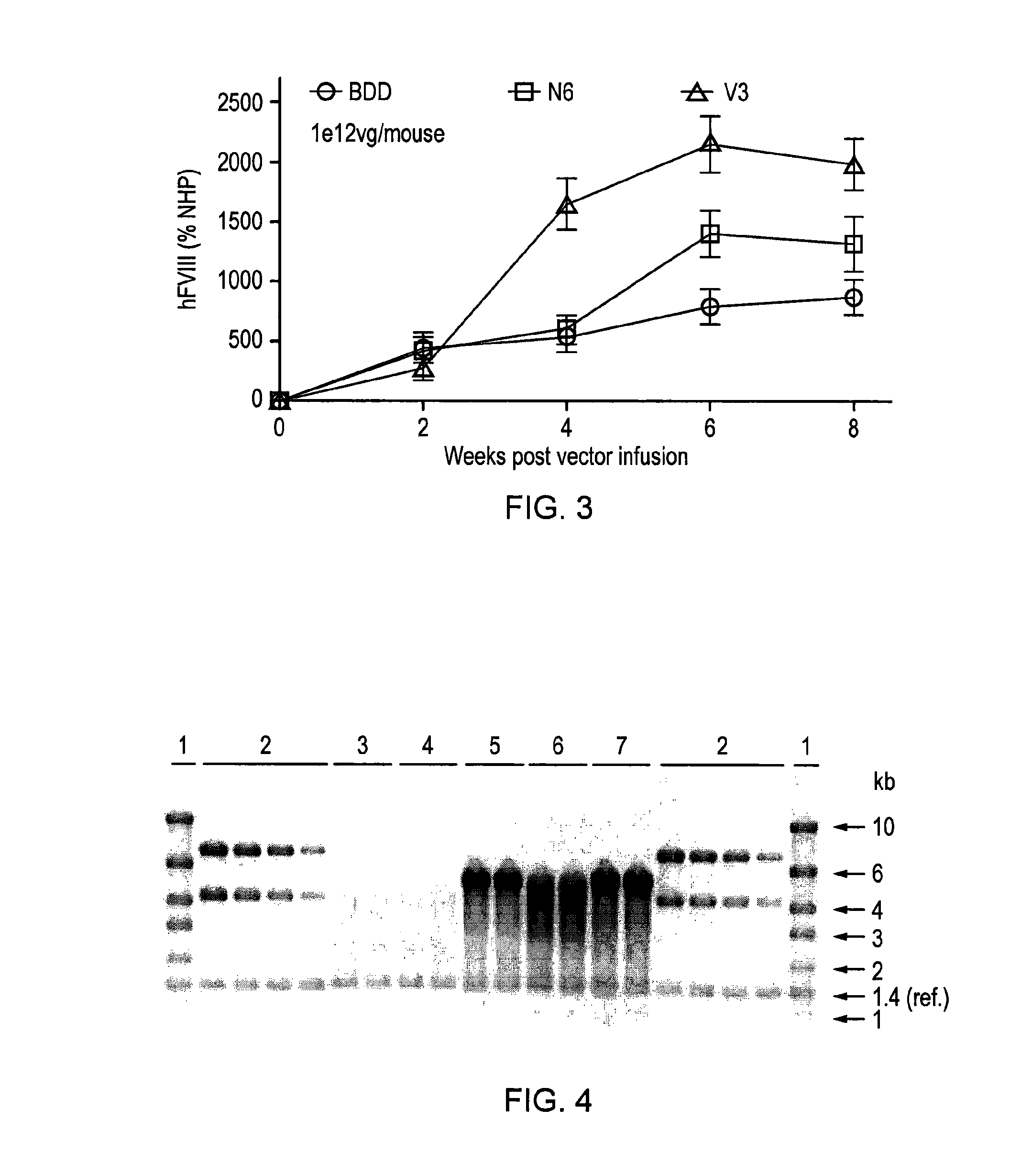
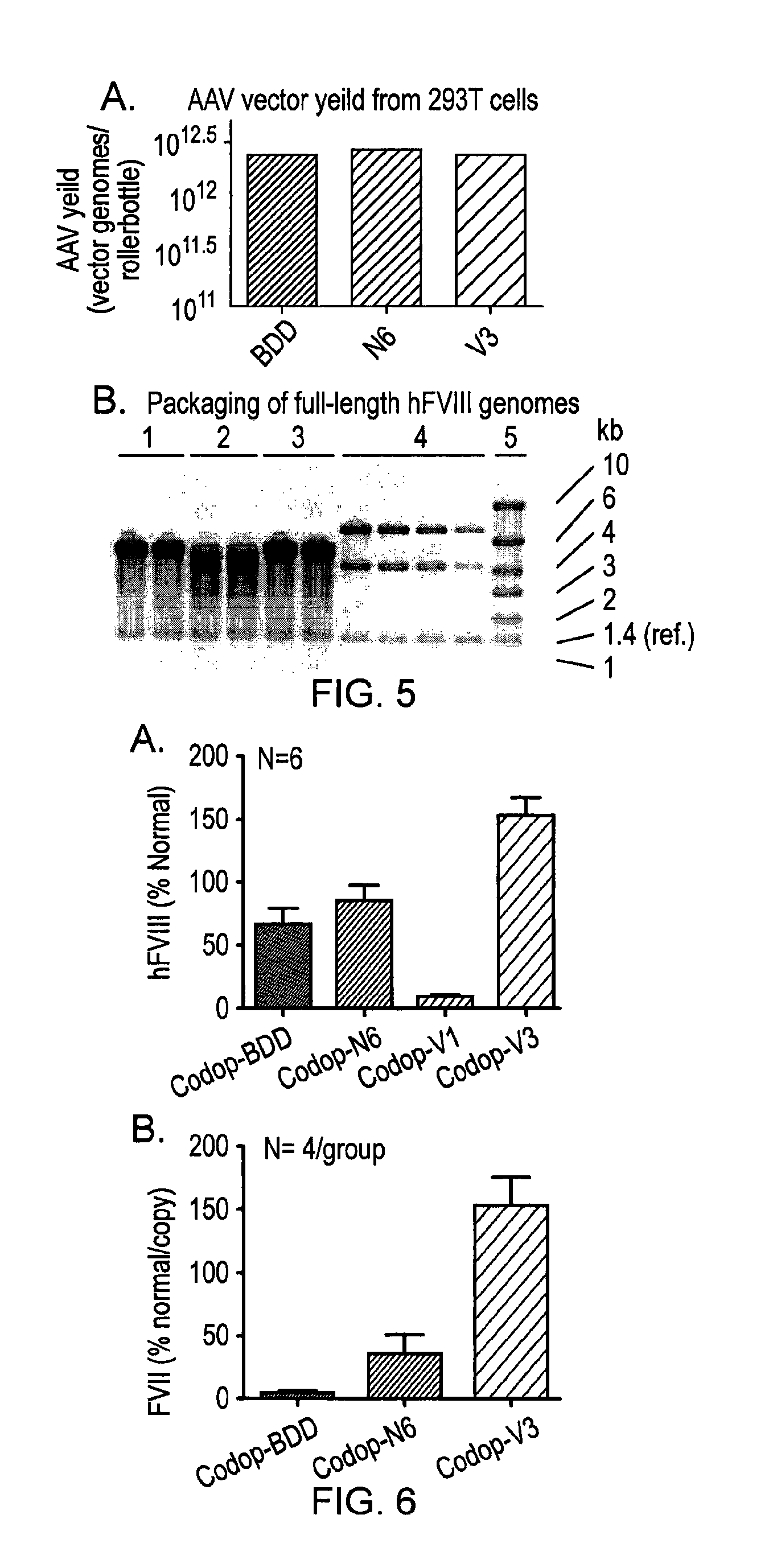
Factor viii sequences
ActiveUS20150158930A1Many symptomIncrease of functional factor VIIIFactor VIIBacteriaGene deliveryNucleotide
There is provided a nucleic acid molecule comprising a nucleotide sequence encoding for a functional factor VIII protein, wherein the portion of the nucleotide sequence encoding for the B domain of the factor VIII protein is between 90 and 111 nucleotides in length and encodes for an amino acid sequence comprising a sequence having at least 85% identity to SEQ ID NO: 4 and which comprises six asparagine residues. Also provided is a functional factor VIII protein, a vector comprising the above nucleic acid molecule, a host cell, a transgenic animal, a method of treating haemophilia, e.g. haemophilia A, and a method for the preparation of a parvoviral gene delivery vector.
Owner:UCL BUSINESS PLC
GeXP (Gene Expression Profiler) detection kit for differentiating 11 kinds of duck viral diseases
ActiveCN103773899AStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationDiseaseDuck hepatitis A virus
The invention discloses a GeXP (Gene Expression Profiler) detection kit for differentiating 11 kinds of duck viral diseases. The invention provides a GeXP detection primer group for identifying or assisting to identify duck infectious disease pathogens, wherein the primer group consists of a primer pair A, a primer pair B, a primer pair C, a primer pair D, a primer pair E, a primer pair F, a primer pair G, a primer pair H, a primer pair I, a primer pair J, a primer pair K and a primer pair L. According to the GeXP detection kit, shown by experiments, the primer group, a PCR (Polymerase Chain Reaction) reagent and the primer pairs, provided by the invention, are used for simultaneously differentiating and detecting avian influenza viruses, H5, H7 and H9 subtype avian influenza viruses, duck hepatitis viruses, duck plague viruses, duck flaviviruses, newcastle disease viruses, egg drop syndrome viruses, muscovy duck reoviruses, muscovy duck parvoviruses and duck circoviruses and are good in specificity and high in sensitivity. The detection kit, which is simple and convenient and is high in flux, and a detection system are provided for the detection on common major duck infectious disease pathogens, so that the practical needs are better met, and application prospects are broad.
Owner:GUANGXI VETERINARY RES INST
Mutated rep encoding sequences for use in aav production
ActiveUS20130023034A1Improve propertiesStable productionAnimal cellsSugar derivativesNucleotideWild type
The invention relates to a nucleic acid comprising a nucleotide sequence encoding a Parvoviral Rep protein, wherein a nuclear localization signal (NLS) in said Parvoviral Rep protein is mutated as compared with a corresponding wild type sequence. The invention also relates to a nucleic acid comprising a nucleotide sequence encoding a Parvoviral Rep protein, wherein the zinc finger domain in said Parvoviral Rep protein is mutated as compared with a corresponding wild type sequence. Further, the invention relates to a nucleic acid comprising a nucleotide sequence encoding a Parvoviral Rep protein, wherein an amino acid at position 43, 57, 79, 97, 120, 179, 305, 484, 493 or 571 of the said Parvoviral Rep protein is mutated in comparison to a corresponding wild type sequence, said amino acid position being defined with reference to SEQ ID NO: 2.
Owner:UNIQURE IP BV
Method for removing viruses from high concentration monoclonal antibody solution
ActiveUS20120077963A1High yieldHigh removal ratePeptide preparation methodsImmunoglobulinsHigh concentrationMonoclonal antibody
An object of the present invention is to provide a method for removing even small viruses from a high concentration monoclonal antibody solution using a membrane, and thus for recovering the antibody within a short time at high yield in the form of a filtrate. The present invention provides a method for producing a preparation containing a monoclonal antibody, which comprises a step of removing viruses by filtering viruses in a monoclonal antibody solution using a virus-removing membrane, wherein(1) the monomer content of the monoclonal antibody accounts for 90% or more;(2) the monoclonal antibody concentration in the monoclonal antibody solution ranges from 20 mg / ml to 100 mg / ml;(3) the monoclonal antibody solution contains at least a basic amino acid; and(4) the parvovirus removal rate of the virus-removing membrane satisfies the following conditions:LRV (Log Reduction Value: logarithmic reduction value) ≧4.
Owner:ASAHI KASEI MEDICAL CO LTD
Universal multi-variant detection system
InactiveUS7348164B2Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementParvovirusHepatitis C
The present invention provides a method to diagnostically detect the variants of a given pathogen, such as HIV, hepatitis C, hepatitis B (HBV), Parvovirus B19, etc., with the use of a single detection probe.
Owner:NEW YORK BLOOD CENT
Virus vectors and methods of making and administering the same
The present invention provides genetically-engineered parvovirus capsids and viruses designed to introduce a heterologous gene into a target cell. The parvoviruses of the invention provide a repertoire of vectors with altered antigenic properties, packaging capabilities, and / or cellular tropisms as compared with current AAV vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Ultraporous Nanofiber Mats And Uses Thereof
A porous electrospun polymeric nanofiber liquid filtration medium, such as an electrospun mats, used for the removal of viral particles (e.g., parvovirus) and other particles in the 18 nm to 30 nm size range from fluid streams, having a mean flow bubble point measured with perfluorohexane above 100 psi. The electrospun medium includes nanofibers having an average fiber diameter of about 6 nm to about 13 nm, and the nanofiber liquid filtration medium has a mean pore size ranging from about 0.01 um to about 0.03 um, a porosity ranging from about 80% to about 95%, a thickness ranging from about 1 um to about 100 um, and a liquid permeability greater than about 10 LMH / psi. The high porosity of the electro-spun mats enable much higher water fluxes, thus reducing the time required to complete virus filtration steps on a fluid stream.
Owner:MILLIPORE CORP
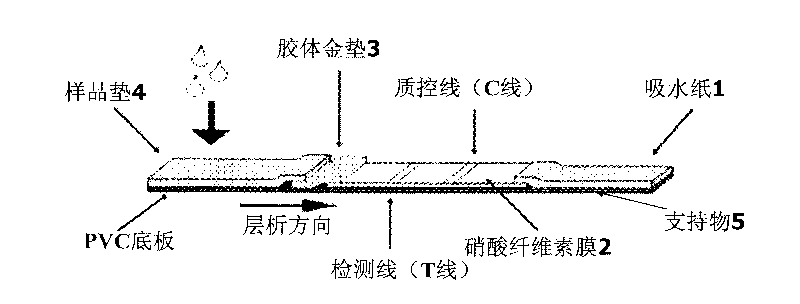
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Methods for Removing Viral Contaminants During Protein Purification
The present invention relates, in general, to methods for removing viral contaminants from therapeutic protein solutions to improve safety of therapeutic proteins administered to patients. Particularly contemplated is the removal of small non-enveloped viruses, such as parvovirus, from therapeutic protein solutions.
Owner:AMGEN INC +1
Vectors with modified initiation codon for the translation of aav-rep78 useful for production of aav
ActiveUS20090191588A1Improve stabilityReduced expression levelAnimal cellsSugar derivativesStart codonNucleotide
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS +1
Virus vectors and methods of making and administering the same
The present invention provides genetically-engineered parvovirus capsids and viruses designed to introduce a heterologous gene into a target cell. The parvoviruses of the invention provide a repertoire of vectors with altered antigenic properties, packaging capabilities, and / or cellular tropisms as compared with current AAV vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Mutated parvovirus structural proteins as vaccines
ActiveUS20160153992A1Easily be screened and producedHigh affinityAntibacterial agentsCompound screeningAntigenHeterologous
The present invention is related to a method for identifying a parvovirus mutated structural protein capable of specifically binding to a binder for an antigen, a parvovirus mutated structural protein which comprises at least one B-cell epitope heterologous to the parvovirus, a multimeric structure comprising the protein, a nucleic acid encoding the protein, a virus or cell comprising the protein, a method of preparing the protein, a medicament comprising the protein, nucleic acid or multimeric structure and its use.
Owner:MEDIGENE
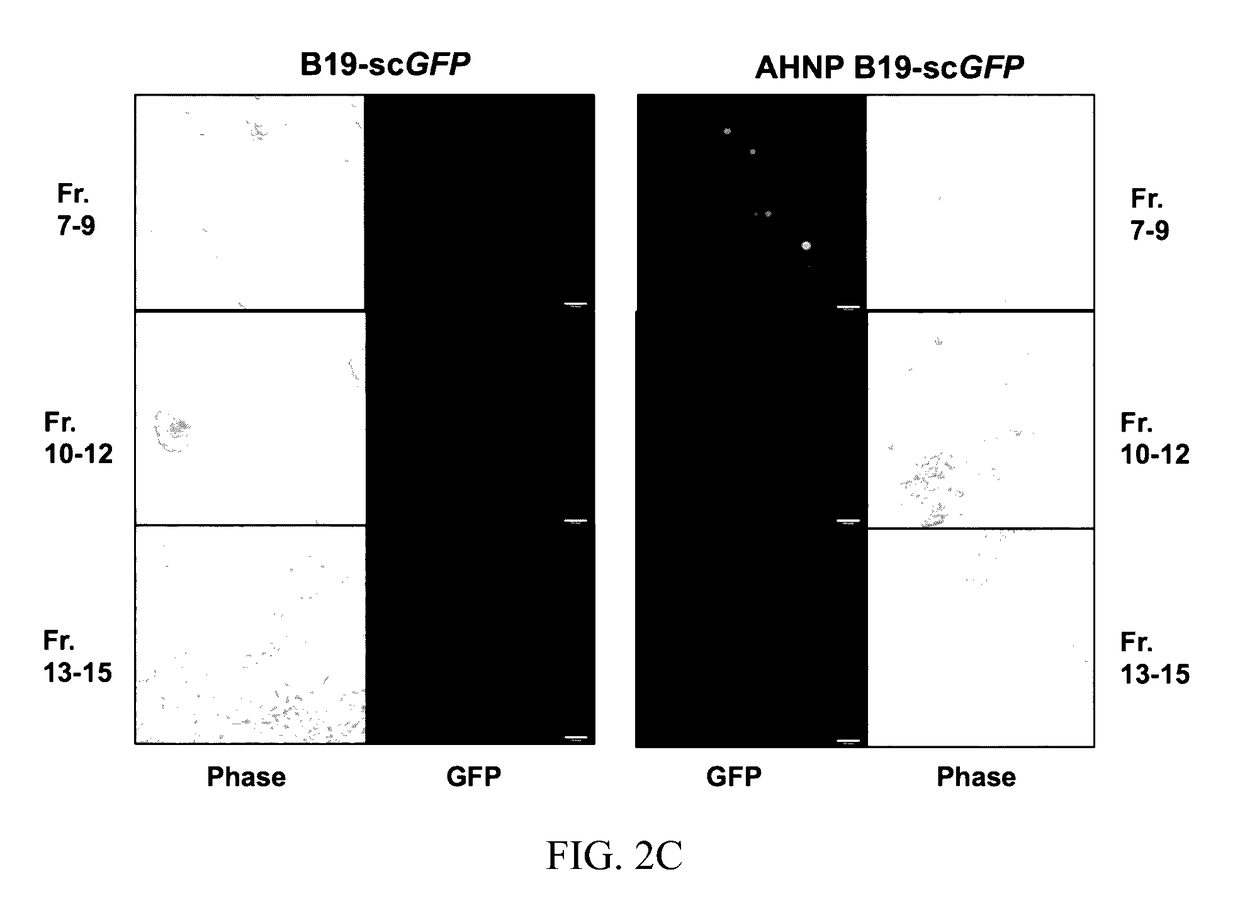
Polypeptides and vectors for targeting HER2/neu expressing cells and uses thereof
Various aspects of the invention provide for capsids, parvovirus capsids, hybrid parvovirus capsids, parvovirus vectors, hybrid parvovirus vectors, hybrid parvovirus particles and parvovirus particles containing polypeptides in which the sequence YCDGFYACYMDV (SEQ ID NO: 3) has been substituted into the VP2 loop of the B19 capsid protein. Polypeptides in which the sequence YCDGFYACYMDV (SEQ ID NO: 3) has been substituted into the VP2 loop of the B19 capsid protein are also provided (e.g., SEQ ID NO: 2). Other aspects of the invention provides capsids, parvovirus capsids, hybrid parvovirus capsids, parvovirus vectors, hybrid parvovirus vectors, hybrid parvovirus particles and parvovirus particles containing a polypeptide comprising SEQ ID NO: 2. Also provided in various aspects of the invention a pharmaceutical compositions and methods of delivering therapeutic agents and / or reporter peptides / proteins to target cells. Finally, methods of treating diseases characterized by cells expressing HER2 / neu receptors are also provided.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Method for producing porcine parvovirus antigen and its product
ActiveCN102382845AImprove immune activityReduce manufacturing costGenetic material ingredientsAntiviralsAntigenBaculovirus expression
The invention discloses a method for producing a porcine parvovirus antigen and its product. The method comprises the following steps: porcine parvovirus capsid protein VP2 gene or optimized VP2 gene is cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the constructed transfer expression carrier and baculovirus DNA are carried out cotransfection to obtain recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding porcine parvovirus capsid protein; and the expressed antigen is ingathered and purified so as to obtain the porcine parvovirus antigen. The method adopts a baculovirus expression system to make safe and efficient porcine parvovirus antigen capsid particles in a domestic silkworm bioreactor; the prepared purified antigen by the method has high safety, and can be directly produced to vaccines for animal immunity. The method for producing porcine parvovirus antigen has the advantages of high expression efficiency, high immunization activity of the expressed antigen, low production cost, large scale production realization and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com