Virus vectors and methods of making and administering the same
a virus and parvovirus technology, applied in the field of virus vectors, can solve the problems of inability to address the problem of packaging b19 genomes into altered capsids, inefficiency of vector transduction and packaging constraints, and recent light on obstacles to the application of raav vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
AAV Vectors
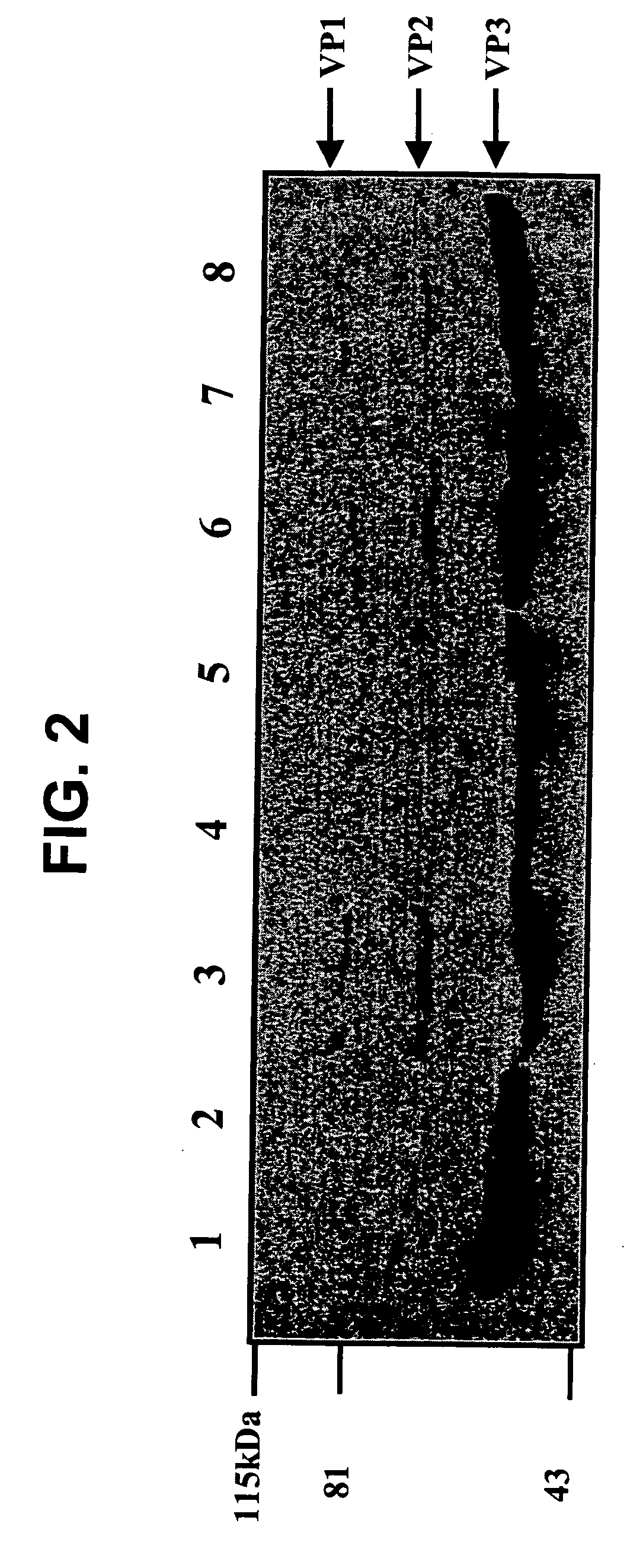
[0195] All production of AAV vectors used in these investigations utilized the vector production scheme as described in Ferrari et al., (1997) Nature Med. 3:1295 and Xiao et al., (1998) J. Virology 72:2224. Utilizing a transient transfection procedure, rAAV devoid of adenovirus has been generated. Id. This protocol utilizes an adenovirus DNA genome that has been incapacitated for viral replication and late gene expression. The mini Ad plasmid while unable to replicate and produce progeny, is still viable for adenovirus gene expression in 293 cells. Using this construct, the AAV packaging strategy involving new AAV helper plasmid (pAAV / Ad ACG) and AAV vector DNA (sub 201) has been successfully complemented (Samulski et al., (1989) J of Virology 63:3822). This new construct typically generates rAAV of 107-109 / 10 cm dish of 293 cells (Xiao et al., (1998) J. Virology 72:2224). Efficient gene delivery is observed in muscle, brain and liver with these vectors in the complete a...
example 2
Cells and Viruses
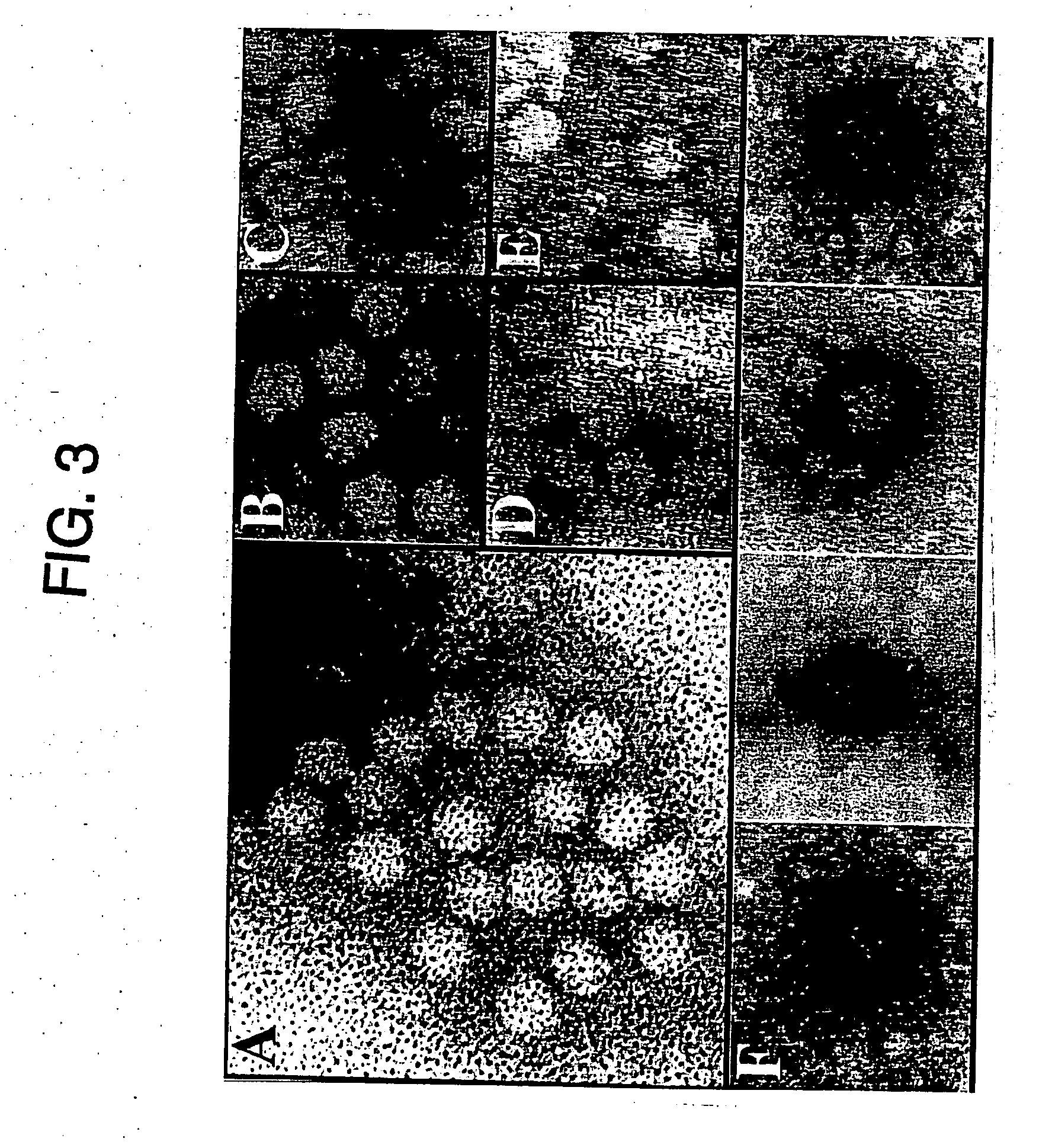
[0196] Human 293 and HeLa cells were maintained at 37° C. with 5% CO2 saturation in 10% fetal bovine serum (Hyclone) in Dulbecco's modified Eagles medium (Gibco BRL), with streptomycin and penicillin (Lineberger Comprehensive. Cancer Center, Chapel Hill, N.C.). Four×106293 cells were plated the day before transfection onto a 10 cm plate. Cells were transfected by both calcium phosphate (Gibco BRL) or Superfection (Qiagene) according to manufacturers specifications. The insertional mutant packaging plasmids, described below, were transfected along with pAB11 containing the CMV driven Lac Z gene with a nuclear localization signal. For each transfection the same amount of packaging plasmid (12 μg) and pAB11 (8 μg) were used for each 10 cm plate. For each transfection an additional plate was used containing the transgene plasmid only to assess transformation efficiencies. After transfection the cells were infected with helper virus Ad5 d / 309 at an MOI of 5, and 48 hour...
example 3
Construction of AAV Packaging Plasmids
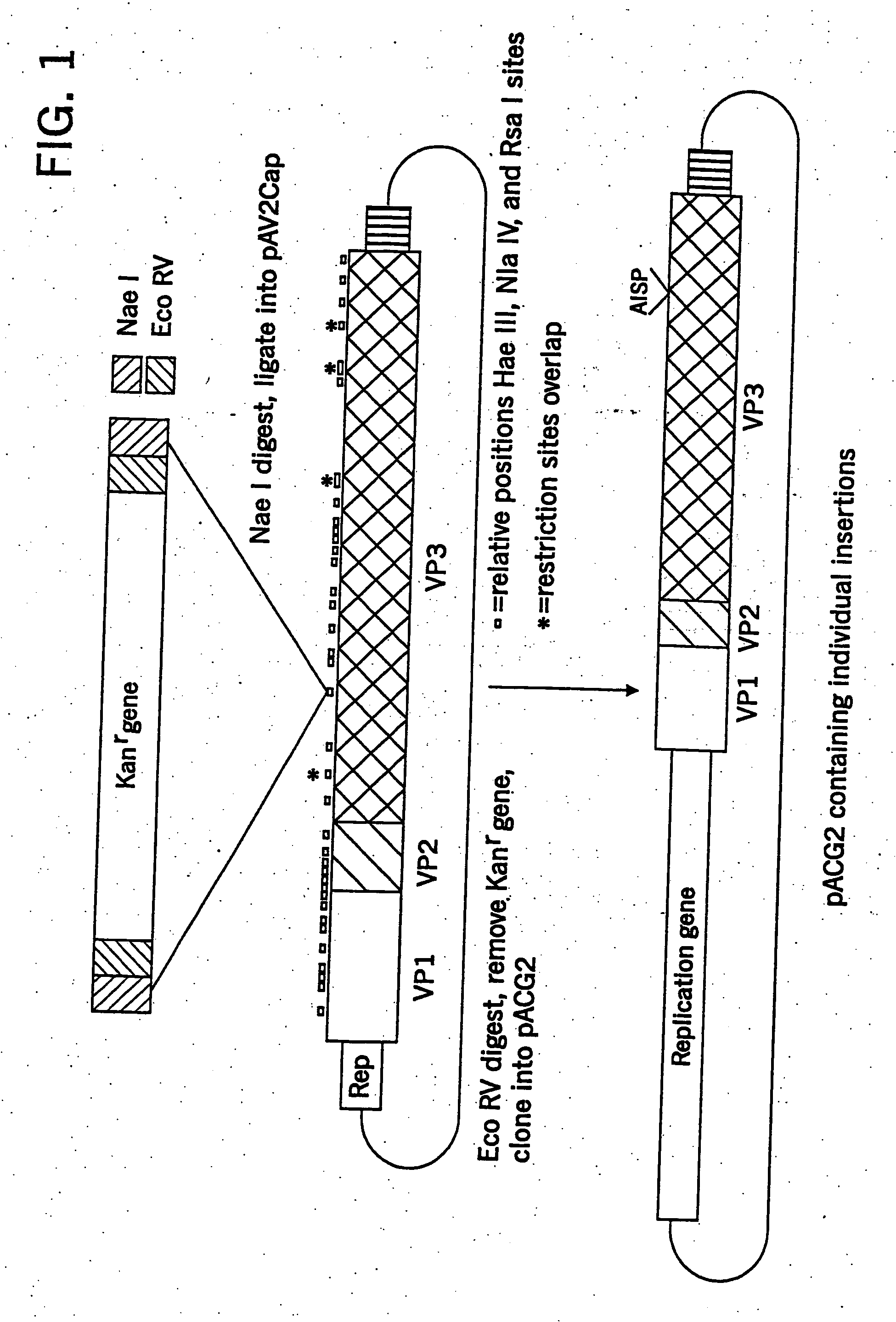
[0198] The capsid domain of pAAV / Ad was cloned into pBS+ (Stratagene) using Hind III, resulting in pAV2Cap. Partial digestion of pAV2Cap using the restriction enzymes Hae III, Nla IV, and Rsa I and gel purification of the unit length DNA fragment resulted in the isolation of the starting material for cloning. The aminoglycoside 3′-phosphotransferase gene, conferring kanamycin resistance (kanr), from pUC4K (Pharmacia) digested with Sal I was flanked by linkers containing Nae I and Eco RV sites, a Sal I overhang at one end and an Eco RI overhang at the other end (top 5′-MTTCGCCGGCGATATC-3′, SEQ ID NO:6, bottom 5′-TCGAGATATCGCCGGC-3′, SEQ ID NO:7). This fragment was cloned into the Eco RI site of pBluescript SK+ (Stratagene). Digestion with Nae I released the kanr gene, and this fragment was ligated into the pAV2Cap partials. The resulting plasmids were screened for insertion into the capsid domain and, then digested with Eco RV to remove the kanr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com